User login
Help Patients Avoid Weight Gain After Stopping GLP-1s
Weight loss drugs have surged in popularity — in part because they work. Patients on glucagon-like peptide 1 (GLP-1) agonists like liraglutide, semaglutide, and tirzepatide (which is technically also a glucose-dependent insulinotropic polypeptide agonist) can lose 10%, 20%, or even 25% of their body weight.
But if those patients stop taking GLP-1s, they tend to regain most of that weight within a year, studies showed.
“These drugs work inside the person from a biologic point of view to alter appetite,” said Robert Kushner, MD, an endocrinologist and professor at Northwestern University Feinberg School of Medicine, Chicago, Illinois, who specializes in obesity medicine. “And when the drug is gone, that disease comes back.”
Often, “patients are told by their insurers that they are no longer going to cover a GLP-1 for obesity,” said Carolyn Bramante, MD, MPH, an assistant professor at the University of Minnesota Medical School, Minneapolis, Minnesota, who sees patients at the M Health Fairview weight management clinic.
Other barriers include side effects like nausea, diarrhea, stomach pain, and vomiting. Some patients simply don’t want to take a medication forever, instead choosing to take their chances keeping the weight off sans drug.
If your patient must stop GLP-1s, or really wants to, here’s how to help.
Find out why the patient wants to go off the GLP-1. Ask them to help you understand, suggested Jaime Almandoz, MD, associate professor of internal medicine and medical director of the University of Texas Southwestern Medical Center’s Weight Wellness Program. Sometimes, the patient or family members worry about safety, Dr. Almandoz said. “They may be concerned about the risks and may not have had an opportunity to ask questions.” Dr. Almandoz reviews the drug safety data and tells patients that studies show, on average, people gain back two-thirds of the weight they’ve lost within a year. You’re not trying to persuade them, only to equip them to make a well-informed choice.
Don’t let bias affect treatment decisions. Patients on GLP-1s often ask: How long will I have to take this? The reason: “We’re biased to believe that this is not a disease state, that this is a character flaw,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for weight management in Burlington, Ontario, Canada. Remind your patient that obesity is not a personal failure but rather a complex mix of genetic and biological factors.
Give patients a primer on the biology of obesity. Science shows that when we lose weight, our bodies fight back, trying to return to our highest-ever fat mass. Changes in neurohormones, gut hormones, satiety mechanisms, metabolism, and muscle function all converge to promote weight recurrence, Dr. Almandoz said. To explain this to patients, Dr. Almandoz compares gaining fat to depositing money in a savings account. “When we try to lose weight, it isn’t as simple as withdrawing this money,” he’ll tell them. “It is almost like the money that we put into the savings account is now tied up in investments that we can’t liquidate easily.”
Prepare patients for an uptick in appetite. When patients stop GLP-1s, their hunger and food cravings tend to increase. “I explain that GLP-1 medications mimic a hormone that is released from our intestines when they sense we have eaten,” said Dr. Almandoz. This signals the brain and body that food is on board, decreasing appetite and cravings. Ask patients what hungry and full feel like on the medication, Dr. Almandoz suggested. “Many will report that their hunger and cravings are low, that they now have an indifference to foods,” said Dr. Almandoz. Such probing questions can help patients be more aware of the medication’s effects. “This positions a more informed conversation if medications are to be discontinued,” Dr. Almandoz said.
Help their body adjust. “Slowly wean down on the dose, if possible, to avoid a big rebound in hunger,” said Dr. Bramante. If your patient has the time — say, they received a letter from their insurance that coverage will end in 3 months — use it to taper the dose as low as possible before stopping. The slower and more gradual, the better. Dr. Almandoz checks in with patients every 4-8 weeks. If they›re maintaining weight well, he considers decreasing the dose again and repeating with follow-up visits.
Substitute one intervention for another. In general, maintaining weight loss requires some intervention, Dr. Wharton said. “But that intervention does not need to be the same as the intervention that got the weight down.” If the patient can›t continue a GLP-1, consider an alternate medication, cognitive behavioral therapy, or a combination of the two. When patients lose coverage for GLP-1s, Dr. Bramante sometimes prescribes an older, less-expensive weight loss drug, such as phentermine, topiramate, or metformin. And sometimes, insurers that don’t cover GLP-1s (like Medicare), do cover bariatric surgery, a potential option depending on the patient›s body mass index, overall health, and comorbidities, said Dr. Almandoz.
Create a habit template. Dr. Kushner asks patients who have successfully lost weight to take an inventory of everything they’re doing to support their efforts. He’ll have them describe how they plan their diet, what types of food they’re eating, how much they eat, and when they eat it. He’ll also ask about physical activity, exercise patterns, and sleep. He logs all the habits into a bulleted list in the patient’s after-visit summary and hands them a printout before they leave. “That’s your template,” he’ll tell them. “That’s what you’re going to try to maintain to the best of your ability because it’s working for you.”
Prescribe exercise. “Increasing exercise is not usually effective for initial weight loss, but it is important for maintaining weight loss,” said Dr. Bramante. Tell patients to start right away, ideally while they’re still on the drug. In a study published last month, patients on liraglutide (Saxenda) who exercised 4 days a week were much more likely to keep weight off after stopping the drug than those who didn’t work out. (The study was partially funded by Novo Nordisk Foundation, the charitable arm of Saxenda’s maker, also the maker of semaglutide meds Ozempic and Wegovy.) By establishing strong exercise habits while on the medication, they were able to sustain higher physical activity levels after they stopped. Ask your patient to identify someone or something to help them stick to their plan, “whether it’s seeing a personal trainer or being accountable to a friend or family member or to themselves through record keeping,” said Dr. Kushner. Learn more about how to prescribe exercise to patients here.
Help them create a “microenvironment” for success. Dr. Kushner asks patients which of the recommended dietary habits for weight loss are hardest to follow: Eating more plant-based foods? Cutting back on ultra-processed foods, fatty foods, fast foods, and/or sugary beverages? Depending on the patient’s answers, he tries to recommend strategies — maybe going meatless a few days a week or keeping tempting foods out of the house. “If you go off medication, food may become more enticing, and you may not feel as content eating less,” Dr. Kushner said. “Make sure your own what we call microenvironment, your home environment, is filled with healthy foods.”
Rely on multidisciplinary expertise. Obesity is a complex, multifactorial disease, so call in reinforcements. “When I see someone, I’m always evaluating what other team members they would benefit from,” said Dr. Kushner. If the patient lacks nutrition knowledge, he refers them to a registered dietitian. If they struggle with self-blame, low self-esteem, and emotional eating, he’ll refer them to a psychologist. It can make a difference: A 2023 study showed that people who lost weight and received support from professionals like trainers, dietitians, and mental health therapists regained less weight over 2 years than those who did not receive the same help.
Reassure patients you will help them no matter what. Ask patients to follow-up within the first month of quitting medication or to call back sooner if they gain 5 pounds. People who stop taking GLP-1s often report less satisfaction with eating, or that they think about food more. That’s when Dr. Kushner asks whether they want to go back on the medication or focus on other strategies. Sometimes, patients who gain weight feel embarrassed and delay their follow-up visits. If that happens, welcome them back and let them know that all chronic conditions ebb and flow. “I constantly remind them that I am here to help you, and there are many tools or resources that will help you,” Dr. Kushner said. “And dispel the notion that it’s somehow your fault.”
Dr. Kushner reported participation on the medical advisory board or consultancy with Novo Nordisk, WeightWatchers, Eli Lilly and Company, Boehringer Ingelheim, Structure Therapeutics, and Altimmune. He added he does not own stock or participate in any speaker’s bureau. Dr. Almandoz reported participation on advisory boards with Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and Company. Dr. Wharton reported participation on advisory boards and honoraria for academic talks and clinical research with Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Amgen, Regeneron, and BioHaven.
A version of this article appeared on Medscape.com.
Weight loss drugs have surged in popularity — in part because they work. Patients on glucagon-like peptide 1 (GLP-1) agonists like liraglutide, semaglutide, and tirzepatide (which is technically also a glucose-dependent insulinotropic polypeptide agonist) can lose 10%, 20%, or even 25% of their body weight.
But if those patients stop taking GLP-1s, they tend to regain most of that weight within a year, studies showed.
“These drugs work inside the person from a biologic point of view to alter appetite,” said Robert Kushner, MD, an endocrinologist and professor at Northwestern University Feinberg School of Medicine, Chicago, Illinois, who specializes in obesity medicine. “And when the drug is gone, that disease comes back.”
Often, “patients are told by their insurers that they are no longer going to cover a GLP-1 for obesity,” said Carolyn Bramante, MD, MPH, an assistant professor at the University of Minnesota Medical School, Minneapolis, Minnesota, who sees patients at the M Health Fairview weight management clinic.
Other barriers include side effects like nausea, diarrhea, stomach pain, and vomiting. Some patients simply don’t want to take a medication forever, instead choosing to take their chances keeping the weight off sans drug.
If your patient must stop GLP-1s, or really wants to, here’s how to help.
Find out why the patient wants to go off the GLP-1. Ask them to help you understand, suggested Jaime Almandoz, MD, associate professor of internal medicine and medical director of the University of Texas Southwestern Medical Center’s Weight Wellness Program. Sometimes, the patient or family members worry about safety, Dr. Almandoz said. “They may be concerned about the risks and may not have had an opportunity to ask questions.” Dr. Almandoz reviews the drug safety data and tells patients that studies show, on average, people gain back two-thirds of the weight they’ve lost within a year. You’re not trying to persuade them, only to equip them to make a well-informed choice.
Don’t let bias affect treatment decisions. Patients on GLP-1s often ask: How long will I have to take this? The reason: “We’re biased to believe that this is not a disease state, that this is a character flaw,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for weight management in Burlington, Ontario, Canada. Remind your patient that obesity is not a personal failure but rather a complex mix of genetic and biological factors.
Give patients a primer on the biology of obesity. Science shows that when we lose weight, our bodies fight back, trying to return to our highest-ever fat mass. Changes in neurohormones, gut hormones, satiety mechanisms, metabolism, and muscle function all converge to promote weight recurrence, Dr. Almandoz said. To explain this to patients, Dr. Almandoz compares gaining fat to depositing money in a savings account. “When we try to lose weight, it isn’t as simple as withdrawing this money,” he’ll tell them. “It is almost like the money that we put into the savings account is now tied up in investments that we can’t liquidate easily.”
Prepare patients for an uptick in appetite. When patients stop GLP-1s, their hunger and food cravings tend to increase. “I explain that GLP-1 medications mimic a hormone that is released from our intestines when they sense we have eaten,” said Dr. Almandoz. This signals the brain and body that food is on board, decreasing appetite and cravings. Ask patients what hungry and full feel like on the medication, Dr. Almandoz suggested. “Many will report that their hunger and cravings are low, that they now have an indifference to foods,” said Dr. Almandoz. Such probing questions can help patients be more aware of the medication’s effects. “This positions a more informed conversation if medications are to be discontinued,” Dr. Almandoz said.
Help their body adjust. “Slowly wean down on the dose, if possible, to avoid a big rebound in hunger,” said Dr. Bramante. If your patient has the time — say, they received a letter from their insurance that coverage will end in 3 months — use it to taper the dose as low as possible before stopping. The slower and more gradual, the better. Dr. Almandoz checks in with patients every 4-8 weeks. If they›re maintaining weight well, he considers decreasing the dose again and repeating with follow-up visits.
Substitute one intervention for another. In general, maintaining weight loss requires some intervention, Dr. Wharton said. “But that intervention does not need to be the same as the intervention that got the weight down.” If the patient can›t continue a GLP-1, consider an alternate medication, cognitive behavioral therapy, or a combination of the two. When patients lose coverage for GLP-1s, Dr. Bramante sometimes prescribes an older, less-expensive weight loss drug, such as phentermine, topiramate, or metformin. And sometimes, insurers that don’t cover GLP-1s (like Medicare), do cover bariatric surgery, a potential option depending on the patient›s body mass index, overall health, and comorbidities, said Dr. Almandoz.
Create a habit template. Dr. Kushner asks patients who have successfully lost weight to take an inventory of everything they’re doing to support their efforts. He’ll have them describe how they plan their diet, what types of food they’re eating, how much they eat, and when they eat it. He’ll also ask about physical activity, exercise patterns, and sleep. He logs all the habits into a bulleted list in the patient’s after-visit summary and hands them a printout before they leave. “That’s your template,” he’ll tell them. “That’s what you’re going to try to maintain to the best of your ability because it’s working for you.”
Prescribe exercise. “Increasing exercise is not usually effective for initial weight loss, but it is important for maintaining weight loss,” said Dr. Bramante. Tell patients to start right away, ideally while they’re still on the drug. In a study published last month, patients on liraglutide (Saxenda) who exercised 4 days a week were much more likely to keep weight off after stopping the drug than those who didn’t work out. (The study was partially funded by Novo Nordisk Foundation, the charitable arm of Saxenda’s maker, also the maker of semaglutide meds Ozempic and Wegovy.) By establishing strong exercise habits while on the medication, they were able to sustain higher physical activity levels after they stopped. Ask your patient to identify someone or something to help them stick to their plan, “whether it’s seeing a personal trainer or being accountable to a friend or family member or to themselves through record keeping,” said Dr. Kushner. Learn more about how to prescribe exercise to patients here.
Help them create a “microenvironment” for success. Dr. Kushner asks patients which of the recommended dietary habits for weight loss are hardest to follow: Eating more plant-based foods? Cutting back on ultra-processed foods, fatty foods, fast foods, and/or sugary beverages? Depending on the patient’s answers, he tries to recommend strategies — maybe going meatless a few days a week or keeping tempting foods out of the house. “If you go off medication, food may become more enticing, and you may not feel as content eating less,” Dr. Kushner said. “Make sure your own what we call microenvironment, your home environment, is filled with healthy foods.”
Rely on multidisciplinary expertise. Obesity is a complex, multifactorial disease, so call in reinforcements. “When I see someone, I’m always evaluating what other team members they would benefit from,” said Dr. Kushner. If the patient lacks nutrition knowledge, he refers them to a registered dietitian. If they struggle with self-blame, low self-esteem, and emotional eating, he’ll refer them to a psychologist. It can make a difference: A 2023 study showed that people who lost weight and received support from professionals like trainers, dietitians, and mental health therapists regained less weight over 2 years than those who did not receive the same help.
Reassure patients you will help them no matter what. Ask patients to follow-up within the first month of quitting medication or to call back sooner if they gain 5 pounds. People who stop taking GLP-1s often report less satisfaction with eating, or that they think about food more. That’s when Dr. Kushner asks whether they want to go back on the medication or focus on other strategies. Sometimes, patients who gain weight feel embarrassed and delay their follow-up visits. If that happens, welcome them back and let them know that all chronic conditions ebb and flow. “I constantly remind them that I am here to help you, and there are many tools or resources that will help you,” Dr. Kushner said. “And dispel the notion that it’s somehow your fault.”
Dr. Kushner reported participation on the medical advisory board or consultancy with Novo Nordisk, WeightWatchers, Eli Lilly and Company, Boehringer Ingelheim, Structure Therapeutics, and Altimmune. He added he does not own stock or participate in any speaker’s bureau. Dr. Almandoz reported participation on advisory boards with Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and Company. Dr. Wharton reported participation on advisory boards and honoraria for academic talks and clinical research with Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Amgen, Regeneron, and BioHaven.
A version of this article appeared on Medscape.com.
Weight loss drugs have surged in popularity — in part because they work. Patients on glucagon-like peptide 1 (GLP-1) agonists like liraglutide, semaglutide, and tirzepatide (which is technically also a glucose-dependent insulinotropic polypeptide agonist) can lose 10%, 20%, or even 25% of their body weight.
But if those patients stop taking GLP-1s, they tend to regain most of that weight within a year, studies showed.
“These drugs work inside the person from a biologic point of view to alter appetite,” said Robert Kushner, MD, an endocrinologist and professor at Northwestern University Feinberg School of Medicine, Chicago, Illinois, who specializes in obesity medicine. “And when the drug is gone, that disease comes back.”
Often, “patients are told by their insurers that they are no longer going to cover a GLP-1 for obesity,” said Carolyn Bramante, MD, MPH, an assistant professor at the University of Minnesota Medical School, Minneapolis, Minnesota, who sees patients at the M Health Fairview weight management clinic.
Other barriers include side effects like nausea, diarrhea, stomach pain, and vomiting. Some patients simply don’t want to take a medication forever, instead choosing to take their chances keeping the weight off sans drug.
If your patient must stop GLP-1s, or really wants to, here’s how to help.
Find out why the patient wants to go off the GLP-1. Ask them to help you understand, suggested Jaime Almandoz, MD, associate professor of internal medicine and medical director of the University of Texas Southwestern Medical Center’s Weight Wellness Program. Sometimes, the patient or family members worry about safety, Dr. Almandoz said. “They may be concerned about the risks and may not have had an opportunity to ask questions.” Dr. Almandoz reviews the drug safety data and tells patients that studies show, on average, people gain back two-thirds of the weight they’ve lost within a year. You’re not trying to persuade them, only to equip them to make a well-informed choice.
Don’t let bias affect treatment decisions. Patients on GLP-1s often ask: How long will I have to take this? The reason: “We’re biased to believe that this is not a disease state, that this is a character flaw,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for weight management in Burlington, Ontario, Canada. Remind your patient that obesity is not a personal failure but rather a complex mix of genetic and biological factors.
Give patients a primer on the biology of obesity. Science shows that when we lose weight, our bodies fight back, trying to return to our highest-ever fat mass. Changes in neurohormones, gut hormones, satiety mechanisms, metabolism, and muscle function all converge to promote weight recurrence, Dr. Almandoz said. To explain this to patients, Dr. Almandoz compares gaining fat to depositing money in a savings account. “When we try to lose weight, it isn’t as simple as withdrawing this money,” he’ll tell them. “It is almost like the money that we put into the savings account is now tied up in investments that we can’t liquidate easily.”
Prepare patients for an uptick in appetite. When patients stop GLP-1s, their hunger and food cravings tend to increase. “I explain that GLP-1 medications mimic a hormone that is released from our intestines when they sense we have eaten,” said Dr. Almandoz. This signals the brain and body that food is on board, decreasing appetite and cravings. Ask patients what hungry and full feel like on the medication, Dr. Almandoz suggested. “Many will report that their hunger and cravings are low, that they now have an indifference to foods,” said Dr. Almandoz. Such probing questions can help patients be more aware of the medication’s effects. “This positions a more informed conversation if medications are to be discontinued,” Dr. Almandoz said.
Help their body adjust. “Slowly wean down on the dose, if possible, to avoid a big rebound in hunger,” said Dr. Bramante. If your patient has the time — say, they received a letter from their insurance that coverage will end in 3 months — use it to taper the dose as low as possible before stopping. The slower and more gradual, the better. Dr. Almandoz checks in with patients every 4-8 weeks. If they›re maintaining weight well, he considers decreasing the dose again and repeating with follow-up visits.
Substitute one intervention for another. In general, maintaining weight loss requires some intervention, Dr. Wharton said. “But that intervention does not need to be the same as the intervention that got the weight down.” If the patient can›t continue a GLP-1, consider an alternate medication, cognitive behavioral therapy, or a combination of the two. When patients lose coverage for GLP-1s, Dr. Bramante sometimes prescribes an older, less-expensive weight loss drug, such as phentermine, topiramate, or metformin. And sometimes, insurers that don’t cover GLP-1s (like Medicare), do cover bariatric surgery, a potential option depending on the patient›s body mass index, overall health, and comorbidities, said Dr. Almandoz.
Create a habit template. Dr. Kushner asks patients who have successfully lost weight to take an inventory of everything they’re doing to support their efforts. He’ll have them describe how they plan their diet, what types of food they’re eating, how much they eat, and when they eat it. He’ll also ask about physical activity, exercise patterns, and sleep. He logs all the habits into a bulleted list in the patient’s after-visit summary and hands them a printout before they leave. “That’s your template,” he’ll tell them. “That’s what you’re going to try to maintain to the best of your ability because it’s working for you.”
Prescribe exercise. “Increasing exercise is not usually effective for initial weight loss, but it is important for maintaining weight loss,” said Dr. Bramante. Tell patients to start right away, ideally while they’re still on the drug. In a study published last month, patients on liraglutide (Saxenda) who exercised 4 days a week were much more likely to keep weight off after stopping the drug than those who didn’t work out. (The study was partially funded by Novo Nordisk Foundation, the charitable arm of Saxenda’s maker, also the maker of semaglutide meds Ozempic and Wegovy.) By establishing strong exercise habits while on the medication, they were able to sustain higher physical activity levels after they stopped. Ask your patient to identify someone or something to help them stick to their plan, “whether it’s seeing a personal trainer or being accountable to a friend or family member or to themselves through record keeping,” said Dr. Kushner. Learn more about how to prescribe exercise to patients here.
Help them create a “microenvironment” for success. Dr. Kushner asks patients which of the recommended dietary habits for weight loss are hardest to follow: Eating more plant-based foods? Cutting back on ultra-processed foods, fatty foods, fast foods, and/or sugary beverages? Depending on the patient’s answers, he tries to recommend strategies — maybe going meatless a few days a week or keeping tempting foods out of the house. “If you go off medication, food may become more enticing, and you may not feel as content eating less,” Dr. Kushner said. “Make sure your own what we call microenvironment, your home environment, is filled with healthy foods.”
Rely on multidisciplinary expertise. Obesity is a complex, multifactorial disease, so call in reinforcements. “When I see someone, I’m always evaluating what other team members they would benefit from,” said Dr. Kushner. If the patient lacks nutrition knowledge, he refers them to a registered dietitian. If they struggle with self-blame, low self-esteem, and emotional eating, he’ll refer them to a psychologist. It can make a difference: A 2023 study showed that people who lost weight and received support from professionals like trainers, dietitians, and mental health therapists regained less weight over 2 years than those who did not receive the same help.
Reassure patients you will help them no matter what. Ask patients to follow-up within the first month of quitting medication or to call back sooner if they gain 5 pounds. People who stop taking GLP-1s often report less satisfaction with eating, or that they think about food more. That’s when Dr. Kushner asks whether they want to go back on the medication or focus on other strategies. Sometimes, patients who gain weight feel embarrassed and delay their follow-up visits. If that happens, welcome them back and let them know that all chronic conditions ebb and flow. “I constantly remind them that I am here to help you, and there are many tools or resources that will help you,” Dr. Kushner said. “And dispel the notion that it’s somehow your fault.”
Dr. Kushner reported participation on the medical advisory board or consultancy with Novo Nordisk, WeightWatchers, Eli Lilly and Company, Boehringer Ingelheim, Structure Therapeutics, and Altimmune. He added he does not own stock or participate in any speaker’s bureau. Dr. Almandoz reported participation on advisory boards with Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and Company. Dr. Wharton reported participation on advisory boards and honoraria for academic talks and clinical research with Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Amgen, Regeneron, and BioHaven.
A version of this article appeared on Medscape.com.
Ginger, Cinnamon, Cumin Improve Glycemic Control
TOPLINE:
The spices and aromatic herbs of the Mediterranean diet with significant benefits in improving glycemic health in type 2 diabetes are limited to ginger, cinnamon, black cumin, turmeric, and saffron, with ginger, black cumin, and cinnamon having the strongest effects on fasting glucose, according to a systematic review and meta-analysis of research.
The meta-analysis also evaluated clove, thyme, turmeric, and various other spices and herbs common in the diet but showed no other correlations with glycemic benefits.
METHODOLOGY:
- In the analysis of 77 studies, 45, involving 3050 participants, were included in the meta-analysis and 32 studies in the systematic review.
- The studies’ inclusion criteria included adult patients with type 2 diabetes, with data on fasting glucose and/or A1c and/or , and involving any supplementation with black cumin, clove, , saffron, thyme, ginger, black pepper, , curcumin, cinnamon, basil, and/or oregano.
- The number of studies involving clove, parsley, thyme, black pepper, rosemary, basil, or oregano and their association with glycemic factors in people with type 2 diabetes was insufficient, hence the analysis primarily focused on the remaining five ingredients of cinnamon, curcumin, ginger, black cumin, saffron, and rosemary.
TAKEAWAY:
- However, the most significant decreases in fasting glucose, between 17 mg/dL and 27 mg/dL, occurred after supplementation with black cumin, followed by cinnamon and ginger.
- Notably, only ginger and black cumin were associated with a significant improvement in A1c.
- Only cinnamon and ginger were associated with a significant decrease in insulin values.
- Of the 11 studies including cinnamon in the meta-analysis, 6 reported significant differences in fasting glucose, while 4 had differences in A1c after the supplementation.
- However, ginger was the only component associated with a significant decrease in each of the 3 outcomes examined of fasting glucose, A1c, and insulin.
IN PRACTICE:
“The Mediterranean Diet is the dietary pattern par excellence for managing and preventing metabolic diseases, such as type 2 diabetes,” the authors reported.
“As far as we are aware, this is the first systematic review and meta-analysis aiming to evaluate the effect of aromatic herbs and spices included in the Mediterranean Diet, such as black cumin, clove [and others], on the glycemic profile of individuals with type 2 diabetes,” they added.
“When focusing on HbA1c, only ginger and black cumin demonstrated therapeutic effects,” the authors noted. “However, our meta-analysis highlights ginger as an herb with substantial translational potential for diabetes treatment, impacting all three glycemic parameters.”
“Regarding clove, parsley, thyme, black pepper, rosemary, basil, and oregano, more studies are needed to analyze the effect of these herbs on the glycemic profile in type 2 diabetes subjects,” the authors concluded.
SOURCE:
The study was published on March 7, 2024, in Nutrients. The first author was Maria Carmen Garza, PhD, of the Department of Human Anatomy and Histology, School Medicine, University of Zaragoza, Zaragoza, Spain.
LIMITATIONS:
Despite the results, a variety of other factors can affect fasting glucose levels, including changes in body weight or body mass index, as well as the combination of spice or aromatic herb supplementation with physical activity or lifestyle changes, the authors noted.
Due to the studies’ differences, the determination of effective dosages of the herbs and spices was not possible.
Furthermore, the studies had wide variations in quality, with few studies including adequate statistical analysis.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
The spices and aromatic herbs of the Mediterranean diet with significant benefits in improving glycemic health in type 2 diabetes are limited to ginger, cinnamon, black cumin, turmeric, and saffron, with ginger, black cumin, and cinnamon having the strongest effects on fasting glucose, according to a systematic review and meta-analysis of research.
The meta-analysis also evaluated clove, thyme, turmeric, and various other spices and herbs common in the diet but showed no other correlations with glycemic benefits.
METHODOLOGY:
- In the analysis of 77 studies, 45, involving 3050 participants, were included in the meta-analysis and 32 studies in the systematic review.
- The studies’ inclusion criteria included adult patients with type 2 diabetes, with data on fasting glucose and/or A1c and/or , and involving any supplementation with black cumin, clove, , saffron, thyme, ginger, black pepper, , curcumin, cinnamon, basil, and/or oregano.
- The number of studies involving clove, parsley, thyme, black pepper, rosemary, basil, or oregano and their association with glycemic factors in people with type 2 diabetes was insufficient, hence the analysis primarily focused on the remaining five ingredients of cinnamon, curcumin, ginger, black cumin, saffron, and rosemary.
TAKEAWAY:
- However, the most significant decreases in fasting glucose, between 17 mg/dL and 27 mg/dL, occurred after supplementation with black cumin, followed by cinnamon and ginger.
- Notably, only ginger and black cumin were associated with a significant improvement in A1c.
- Only cinnamon and ginger were associated with a significant decrease in insulin values.
- Of the 11 studies including cinnamon in the meta-analysis, 6 reported significant differences in fasting glucose, while 4 had differences in A1c after the supplementation.
- However, ginger was the only component associated with a significant decrease in each of the 3 outcomes examined of fasting glucose, A1c, and insulin.
IN PRACTICE:
“The Mediterranean Diet is the dietary pattern par excellence for managing and preventing metabolic diseases, such as type 2 diabetes,” the authors reported.
“As far as we are aware, this is the first systematic review and meta-analysis aiming to evaluate the effect of aromatic herbs and spices included in the Mediterranean Diet, such as black cumin, clove [and others], on the glycemic profile of individuals with type 2 diabetes,” they added.
“When focusing on HbA1c, only ginger and black cumin demonstrated therapeutic effects,” the authors noted. “However, our meta-analysis highlights ginger as an herb with substantial translational potential for diabetes treatment, impacting all three glycemic parameters.”
“Regarding clove, parsley, thyme, black pepper, rosemary, basil, and oregano, more studies are needed to analyze the effect of these herbs on the glycemic profile in type 2 diabetes subjects,” the authors concluded.
SOURCE:
The study was published on March 7, 2024, in Nutrients. The first author was Maria Carmen Garza, PhD, of the Department of Human Anatomy and Histology, School Medicine, University of Zaragoza, Zaragoza, Spain.
LIMITATIONS:
Despite the results, a variety of other factors can affect fasting glucose levels, including changes in body weight or body mass index, as well as the combination of spice or aromatic herb supplementation with physical activity or lifestyle changes, the authors noted.
Due to the studies’ differences, the determination of effective dosages of the herbs and spices was not possible.
Furthermore, the studies had wide variations in quality, with few studies including adequate statistical analysis.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
The spices and aromatic herbs of the Mediterranean diet with significant benefits in improving glycemic health in type 2 diabetes are limited to ginger, cinnamon, black cumin, turmeric, and saffron, with ginger, black cumin, and cinnamon having the strongest effects on fasting glucose, according to a systematic review and meta-analysis of research.
The meta-analysis also evaluated clove, thyme, turmeric, and various other spices and herbs common in the diet but showed no other correlations with glycemic benefits.
METHODOLOGY:
- In the analysis of 77 studies, 45, involving 3050 participants, were included in the meta-analysis and 32 studies in the systematic review.
- The studies’ inclusion criteria included adult patients with type 2 diabetes, with data on fasting glucose and/or A1c and/or , and involving any supplementation with black cumin, clove, , saffron, thyme, ginger, black pepper, , curcumin, cinnamon, basil, and/or oregano.
- The number of studies involving clove, parsley, thyme, black pepper, rosemary, basil, or oregano and their association with glycemic factors in people with type 2 diabetes was insufficient, hence the analysis primarily focused on the remaining five ingredients of cinnamon, curcumin, ginger, black cumin, saffron, and rosemary.
TAKEAWAY:
- However, the most significant decreases in fasting glucose, between 17 mg/dL and 27 mg/dL, occurred after supplementation with black cumin, followed by cinnamon and ginger.
- Notably, only ginger and black cumin were associated with a significant improvement in A1c.
- Only cinnamon and ginger were associated with a significant decrease in insulin values.
- Of the 11 studies including cinnamon in the meta-analysis, 6 reported significant differences in fasting glucose, while 4 had differences in A1c after the supplementation.
- However, ginger was the only component associated with a significant decrease in each of the 3 outcomes examined of fasting glucose, A1c, and insulin.
IN PRACTICE:
“The Mediterranean Diet is the dietary pattern par excellence for managing and preventing metabolic diseases, such as type 2 diabetes,” the authors reported.
“As far as we are aware, this is the first systematic review and meta-analysis aiming to evaluate the effect of aromatic herbs and spices included in the Mediterranean Diet, such as black cumin, clove [and others], on the glycemic profile of individuals with type 2 diabetes,” they added.
“When focusing on HbA1c, only ginger and black cumin demonstrated therapeutic effects,” the authors noted. “However, our meta-analysis highlights ginger as an herb with substantial translational potential for diabetes treatment, impacting all three glycemic parameters.”
“Regarding clove, parsley, thyme, black pepper, rosemary, basil, and oregano, more studies are needed to analyze the effect of these herbs on the glycemic profile in type 2 diabetes subjects,” the authors concluded.
SOURCE:
The study was published on March 7, 2024, in Nutrients. The first author was Maria Carmen Garza, PhD, of the Department of Human Anatomy and Histology, School Medicine, University of Zaragoza, Zaragoza, Spain.
LIMITATIONS:
Despite the results, a variety of other factors can affect fasting glucose levels, including changes in body weight or body mass index, as well as the combination of spice or aromatic herb supplementation with physical activity or lifestyle changes, the authors noted.
Due to the studies’ differences, the determination of effective dosages of the herbs and spices was not possible.
Furthermore, the studies had wide variations in quality, with few studies including adequate statistical analysis.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
New Research Dissects Transgenerational Obesity and Diabetes
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
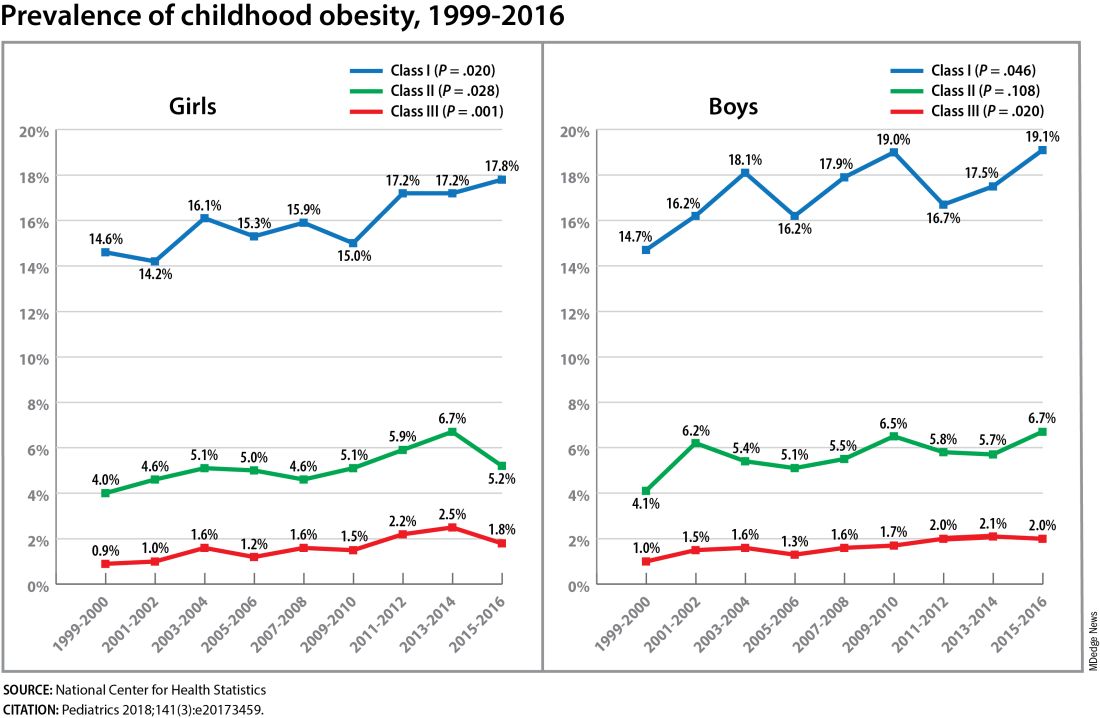
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1).
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1).

Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1).

Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FROM DPSG-NA 2023
Hormones and Viruses Influence Each Other: Consider These Connections in Your Patients
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Tirzepatide Weight Loss Consistent Regardless of BMI
Tirzepatide (Zepbound for weight loss; Mounjaro for type 2 diabetes; Eli Lilly) consistently reduced body weight regardless of pretreatment body mass index (BMI) and reduced body weight and waist circumference regardless of duration of overweight or obesity.
The analyses — firstly of the impact of baseline BMI and secondly investigating the impact of the duration of overweight/obesity — are drawn from combined findings from the SURMOUNT 1-4 studies that examined the efficacy and safety of tirzepatide vs placebo. They are scheduled to be presented at May’s European Congress on Obesity (ECO) by Carel Le Roux, MD, University College Dublin, Ireland, and Giovanna Dr. Muscogiuri, MD, endocrinologist from the University of Naples Federico II, Naples, Italy, respectively.
The first analysis of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, aimed to analyze the impact of baseline BMI category on weight reduction across the series of phase 3 trials.
More participants on tirzepatide than on placebo achieved the body weight reduction targets of 5%, 10%, and 15%. “Across the SURMOUNT 1-4 trials, treatment with tirzepatide, along with a reduced-calorie diet and increased physical activity, consistently resulted in clinically significant weight reductions of 5% or more, 10% or more, or 15% or more, as compared to placebo, regardless of baseline BMI subgroup, in adults with obesity or overweight (BMI of 27 and above),” said obesity specialist, Louis J. Aronne, MD, from the Comprehensive Weight Control Center, Weill Cornell Medicine, New York City, and coauthor of the BMI-related analysis.
Dr. Muscogiuri, who is first author of the second analysis that looked at the impact of duration of adiposity, and her coauthors concluded that, “Tirzepatide consistently reduced body weight and waist circumference in people living with obesity or overweight with weight-related comorbidities regardless of the duration of disease. These results are consistent with the overall findings from each study in the SURMOUNT program.”
Weight Loss Consistent Regardless of BMI
The SURMOUNT series of trials involved people with a BMI of 30 kg/m2 and above, or 27 kg/m2 with at least one weight-related comorbidity without type 2 diabetes (SURMOUNT-1, 72 weeks), with type 2 diabetes (SURMOUNT-2, 72 weeks), and without type 2 diabetes after a 12-week intensive lifestyle intervention (SURMOUNT-3, 72 weeks from randomization) or after an 88 week intervention (SURMOUNT-4, 36-week open label tirzepatide lead-in and 52 weeks following randomization).
BMI subgroups were defined by 27-30 (overweight), 30-35 (obesity class I), 35-40 (obesity class II), and 40 kg/m2 and above (obesity class III). Percentage change in body weight from randomization to week 72 (SURMOUNT-1, -2, and -3) or to week 52 (SURMOUNT-4) was determined, as well as the proportions of participants achieving the weight reduction targets of 5%, 10%, and 15%. The per protocol analyses included all participants who received at least one dose of tirzepatide or placebo.
Across these BMI levels, up to 100% of tirzepatide-treated participants achieved weight reduction of 5% or more compared with 30% on placebo in SURMOUNT-1, up to 93% vs 43% in SURMOUNT-2, and up to 97% vs 15%, respectively, in SURMOUNT-3.
At least 10% weight reduction was achieved by up to 93% vs 16%, respectively, in SURMOUNT-1, up to 76% vs 14% in SURMOUNT-2, and up to 92% vs 8% in SURMOUNT-3.
Weight reduction of 15% was achieved by up to 85% compared with 7% of patients on tirzepatide and placebo, respectively, in SURMOUNT-1; up to 60% vs 3%, respectively, in SURMOUNT-2; and up to 78% vs 4% in SURMOUNT-3.
In SURMOUNT-4, during the 36-week open-label tirzepatide treatment, the mean body weight % or more reduction was 21%. Following this lead-in period, further weight reductions of 5% or more, 10%, and 15% or more were achieved by up to 70%, 39%, and 22%, respectively, of participants treated with tirzepatide compared with 2%, 2%, and 0% of patients on placebo.
Body Weight and Waist Circumference Reduced Regardless of Disease Duration
In this second presentation, participants were categorized based on duration with overweight/obesity at baseline (10 years or less, between 10 and 20 years, and above 20 years). Percentage body weight change; the proportions achieving weight loss targets of 5%, 10%, 15%, 20%, and 25%; and the change in waist circumference were analyzed.
Greater weight reductions were found in participants who took tirzepatide than in those who took placebo across the SURMOUNT 1-4 study endpoints, including weight reduction targets of 5%, 10%, 15%, 20%, and 25% compared with placebo-treated participants, regardless of disease duration, reported the authors in an early press release from ECO. The magnitude of weight reductions was generally similar across the disease duration categories.
For example, in the SURMOUNT-1 trial, for patients given 10-mg dose of tirzepatide, those with disease duration under 10 years lost 21% of their weight after 72 weeks compared with 20% body weight loss for those with 10-20 years disease duration and 23% for those with over 20 years disease duration.
In the SURMOUNT-2 trial (where all participants were also living with type 2 diabetes), for patients given the 10-mg dose of tirzepatide, those with disease duration under 10 years lost 12.6% of their body weight, while those with disease duration of 10-20 years lost 12.5%; in people living with overweight or obesity for over 20 years, 14.4% of body weight was lost.
Waist circumference also reduced to a greater extent than placebo for each disease duration category across the four studies, and again, these reductions were consistent across disease duration subgroups.
A difference between patients with and without type 2 diabetes was evident and requires further analysis to explore and understand why patients with type 2 diabetes have less weight loss in these trials than those without type 2 diabetes.
Asked to comment on the findings, Jens Juul Holst, MD, from the Department of Biomedical Sciences and Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, said that the results were as expected.
“The first abstract is said to show that there is the same effect regardless of the baseline BMI, but this is the expected outcome — nothing exciting there,” he told this news organization. “The second deals with the effects in people with different duration of adiposity. Again, it was equally effective in all groups and that was also the expected outcome, although important.”
“One question is whether one should treat people with BMI < 30 at all, and that depends on preexisting comorbidities — in particular metabolic syndrome, where treatment could be lifesaving and prevent complications,” added Dr. Holst.
This news organization also asked Jason Halford, ECO president, for his view on the findings. He remarked that with these weight loss drugs overall, “Usually weight loss tends to be proportional and actually greater in the lower BMI categories. This is partly because dosing is not done by body weight, and everyone gets the same doses irrespective of how they weigh. There is an argument that doses should be adjusted. The data suggests these drugs are so potent this does not occur for some reason.”
Dr. Holst added that, “In principle, for a given reduction in food intake, one would expect a similar reduction in body mass, and these agents should be dosed according to the size of the individual — since energy expenditure depends linearly on body weight, this is probably a reasonable measure. But what actually happens is dosing is according to the occurrence of side effects, which is a good pragmatic principle.”
Dr. Holst pointed out that the interesting question here is whether the very obese would somehow be resistant to the GLP-1 RAs (like leptin) — “they are not,” he noted.
He added that to his knowledge, the question around the role played by duration of the adiposity had not been explicitly looked at before. “However, the many individuals with obesity studied after GLP-1 RA treatment have varied widely with respect to duration and weight loss has not previously been known to depend on this, but there is no known physiological mechanism underpinning this.”
Tirzepatide (Mounjaro) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes in 2022. In November 2023, the FDA approved tirzepatide (Zepbound) for chronic weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight-related comorbidity. Also in November 2023, the EMA Committee for Medicinal Products for Human Use offered a positive opinion on extension of the Mounjaro label to include weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 and at least one weight-related comorbid condition.
Dr. Holst had no conflicting interest with Eli Lilly but is a member of advisory boards for Novo Nordisk. This work (abstract 014) was funded by Eli Lilly and Company. Dr. Le Roux reported grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He served on advisory boards and speaker panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. Dr. Le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Dr. Le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. Dr. Le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Dr. Le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. Dr. Aronne reported receiving grants or personal fees from Altimmune, AstraZeneca, Boehringer Ingelheim, Eli Lilly, ERX, Gelesis, Intellihealth, Jamieson Wellness, Janssen, Novo Nordisk, Optum, Pfizer, Senda Biosciences, and Versanis and being a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, and Jamieson Wellness. FJ, TF, MM, LG, and LN are employees and shareholders of Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Tirzepatide (Zepbound for weight loss; Mounjaro for type 2 diabetes; Eli Lilly) consistently reduced body weight regardless of pretreatment body mass index (BMI) and reduced body weight and waist circumference regardless of duration of overweight or obesity.
The analyses — firstly of the impact of baseline BMI and secondly investigating the impact of the duration of overweight/obesity — are drawn from combined findings from the SURMOUNT 1-4 studies that examined the efficacy and safety of tirzepatide vs placebo. They are scheduled to be presented at May’s European Congress on Obesity (ECO) by Carel Le Roux, MD, University College Dublin, Ireland, and Giovanna Dr. Muscogiuri, MD, endocrinologist from the University of Naples Federico II, Naples, Italy, respectively.
The first analysis of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, aimed to analyze the impact of baseline BMI category on weight reduction across the series of phase 3 trials.
More participants on tirzepatide than on placebo achieved the body weight reduction targets of 5%, 10%, and 15%. “Across the SURMOUNT 1-4 trials, treatment with tirzepatide, along with a reduced-calorie diet and increased physical activity, consistently resulted in clinically significant weight reductions of 5% or more, 10% or more, or 15% or more, as compared to placebo, regardless of baseline BMI subgroup, in adults with obesity or overweight (BMI of 27 and above),” said obesity specialist, Louis J. Aronne, MD, from the Comprehensive Weight Control Center, Weill Cornell Medicine, New York City, and coauthor of the BMI-related analysis.
Dr. Muscogiuri, who is first author of the second analysis that looked at the impact of duration of adiposity, and her coauthors concluded that, “Tirzepatide consistently reduced body weight and waist circumference in people living with obesity or overweight with weight-related comorbidities regardless of the duration of disease. These results are consistent with the overall findings from each study in the SURMOUNT program.”
Weight Loss Consistent Regardless of BMI
The SURMOUNT series of trials involved people with a BMI of 30 kg/m2 and above, or 27 kg/m2 with at least one weight-related comorbidity without type 2 diabetes (SURMOUNT-1, 72 weeks), with type 2 diabetes (SURMOUNT-2, 72 weeks), and without type 2 diabetes after a 12-week intensive lifestyle intervention (SURMOUNT-3, 72 weeks from randomization) or after an 88 week intervention (SURMOUNT-4, 36-week open label tirzepatide lead-in and 52 weeks following randomization).
BMI subgroups were defined by 27-30 (overweight), 30-35 (obesity class I), 35-40 (obesity class II), and 40 kg/m2 and above (obesity class III). Percentage change in body weight from randomization to week 72 (SURMOUNT-1, -2, and -3) or to week 52 (SURMOUNT-4) was determined, as well as the proportions of participants achieving the weight reduction targets of 5%, 10%, and 15%. The per protocol analyses included all participants who received at least one dose of tirzepatide or placebo.
Across these BMI levels, up to 100% of tirzepatide-treated participants achieved weight reduction of 5% or more compared with 30% on placebo in SURMOUNT-1, up to 93% vs 43% in SURMOUNT-2, and up to 97% vs 15%, respectively, in SURMOUNT-3.
At least 10% weight reduction was achieved by up to 93% vs 16%, respectively, in SURMOUNT-1, up to 76% vs 14% in SURMOUNT-2, and up to 92% vs 8% in SURMOUNT-3.
Weight reduction of 15% was achieved by up to 85% compared with 7% of patients on tirzepatide and placebo, respectively, in SURMOUNT-1; up to 60% vs 3%, respectively, in SURMOUNT-2; and up to 78% vs 4% in SURMOUNT-3.
In SURMOUNT-4, during the 36-week open-label tirzepatide treatment, the mean body weight % or more reduction was 21%. Following this lead-in period, further weight reductions of 5% or more, 10%, and 15% or more were achieved by up to 70%, 39%, and 22%, respectively, of participants treated with tirzepatide compared with 2%, 2%, and 0% of patients on placebo.
Body Weight and Waist Circumference Reduced Regardless of Disease Duration
In this second presentation, participants were categorized based on duration with overweight/obesity at baseline (10 years or less, between 10 and 20 years, and above 20 years). Percentage body weight change; the proportions achieving weight loss targets of 5%, 10%, 15%, 20%, and 25%; and the change in waist circumference were analyzed.
Greater weight reductions were found in participants who took tirzepatide than in those who took placebo across the SURMOUNT 1-4 study endpoints, including weight reduction targets of 5%, 10%, 15%, 20%, and 25% compared with placebo-treated participants, regardless of disease duration, reported the authors in an early press release from ECO. The magnitude of weight reductions was generally similar across the disease duration categories.
For example, in the SURMOUNT-1 trial, for patients given 10-mg dose of tirzepatide, those with disease duration under 10 years lost 21% of their weight after 72 weeks compared with 20% body weight loss for those with 10-20 years disease duration and 23% for those with over 20 years disease duration.
In the SURMOUNT-2 trial (where all participants were also living with type 2 diabetes), for patients given the 10-mg dose of tirzepatide, those with disease duration under 10 years lost 12.6% of their body weight, while those with disease duration of 10-20 years lost 12.5%; in people living with overweight or obesity for over 20 years, 14.4% of body weight was lost.
Waist circumference also reduced to a greater extent than placebo for each disease duration category across the four studies, and again, these reductions were consistent across disease duration subgroups.
A difference between patients with and without type 2 diabetes was evident and requires further analysis to explore and understand why patients with type 2 diabetes have less weight loss in these trials than those without type 2 diabetes.
Asked to comment on the findings, Jens Juul Holst, MD, from the Department of Biomedical Sciences and Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, said that the results were as expected.
“The first abstract is said to show that there is the same effect regardless of the baseline BMI, but this is the expected outcome — nothing exciting there,” he told this news organization. “The second deals with the effects in people with different duration of adiposity. Again, it was equally effective in all groups and that was also the expected outcome, although important.”
“One question is whether one should treat people with BMI < 30 at all, and that depends on preexisting comorbidities — in particular metabolic syndrome, where treatment could be lifesaving and prevent complications,” added Dr. Holst.
This news organization also asked Jason Halford, ECO president, for his view on the findings. He remarked that with these weight loss drugs overall, “Usually weight loss tends to be proportional and actually greater in the lower BMI categories. This is partly because dosing is not done by body weight, and everyone gets the same doses irrespective of how they weigh. There is an argument that doses should be adjusted. The data suggests these drugs are so potent this does not occur for some reason.”
Dr. Holst added that, “In principle, for a given reduction in food intake, one would expect a similar reduction in body mass, and these agents should be dosed according to the size of the individual — since energy expenditure depends linearly on body weight, this is probably a reasonable measure. But what actually happens is dosing is according to the occurrence of side effects, which is a good pragmatic principle.”
Dr. Holst pointed out that the interesting question here is whether the very obese would somehow be resistant to the GLP-1 RAs (like leptin) — “they are not,” he noted.
He added that to his knowledge, the question around the role played by duration of the adiposity had not been explicitly looked at before. “However, the many individuals with obesity studied after GLP-1 RA treatment have varied widely with respect to duration and weight loss has not previously been known to depend on this, but there is no known physiological mechanism underpinning this.”
Tirzepatide (Mounjaro) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes in 2022. In November 2023, the FDA approved tirzepatide (Zepbound) for chronic weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight-related comorbidity. Also in November 2023, the EMA Committee for Medicinal Products for Human Use offered a positive opinion on extension of the Mounjaro label to include weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 and at least one weight-related comorbid condition.
Dr. Holst had no conflicting interest with Eli Lilly but is a member of advisory boards for Novo Nordisk. This work (abstract 014) was funded by Eli Lilly and Company. Dr. Le Roux reported grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He served on advisory boards and speaker panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. Dr. Le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Dr. Le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. Dr. Le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Dr. Le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. Dr. Aronne reported receiving grants or personal fees from Altimmune, AstraZeneca, Boehringer Ingelheim, Eli Lilly, ERX, Gelesis, Intellihealth, Jamieson Wellness, Janssen, Novo Nordisk, Optum, Pfizer, Senda Biosciences, and Versanis and being a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, and Jamieson Wellness. FJ, TF, MM, LG, and LN are employees and shareholders of Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Tirzepatide (Zepbound for weight loss; Mounjaro for type 2 diabetes; Eli Lilly) consistently reduced body weight regardless of pretreatment body mass index (BMI) and reduced body weight and waist circumference regardless of duration of overweight or obesity.
The analyses — firstly of the impact of baseline BMI and secondly investigating the impact of the duration of overweight/obesity — are drawn from combined findings from the SURMOUNT 1-4 studies that examined the efficacy and safety of tirzepatide vs placebo. They are scheduled to be presented at May’s European Congress on Obesity (ECO) by Carel Le Roux, MD, University College Dublin, Ireland, and Giovanna Dr. Muscogiuri, MD, endocrinologist from the University of Naples Federico II, Naples, Italy, respectively.
The first analysis of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, aimed to analyze the impact of baseline BMI category on weight reduction across the series of phase 3 trials.
More participants on tirzepatide than on placebo achieved the body weight reduction targets of 5%, 10%, and 15%. “Across the SURMOUNT 1-4 trials, treatment with tirzepatide, along with a reduced-calorie diet and increased physical activity, consistently resulted in clinically significant weight reductions of 5% or more, 10% or more, or 15% or more, as compared to placebo, regardless of baseline BMI subgroup, in adults with obesity or overweight (BMI of 27 and above),” said obesity specialist, Louis J. Aronne, MD, from the Comprehensive Weight Control Center, Weill Cornell Medicine, New York City, and coauthor of the BMI-related analysis.
Dr. Muscogiuri, who is first author of the second analysis that looked at the impact of duration of adiposity, and her coauthors concluded that, “Tirzepatide consistently reduced body weight and waist circumference in people living with obesity or overweight with weight-related comorbidities regardless of the duration of disease. These results are consistent with the overall findings from each study in the SURMOUNT program.”
Weight Loss Consistent Regardless of BMI
The SURMOUNT series of trials involved people with a BMI of 30 kg/m2 and above, or 27 kg/m2 with at least one weight-related comorbidity without type 2 diabetes (SURMOUNT-1, 72 weeks), with type 2 diabetes (SURMOUNT-2, 72 weeks), and without type 2 diabetes after a 12-week intensive lifestyle intervention (SURMOUNT-3, 72 weeks from randomization) or after an 88 week intervention (SURMOUNT-4, 36-week open label tirzepatide lead-in and 52 weeks following randomization).
BMI subgroups were defined by 27-30 (overweight), 30-35 (obesity class I), 35-40 (obesity class II), and 40 kg/m2 and above (obesity class III). Percentage change in body weight from randomization to week 72 (SURMOUNT-1, -2, and -3) or to week 52 (SURMOUNT-4) was determined, as well as the proportions of participants achieving the weight reduction targets of 5%, 10%, and 15%. The per protocol analyses included all participants who received at least one dose of tirzepatide or placebo.
Across these BMI levels, up to 100% of tirzepatide-treated participants achieved weight reduction of 5% or more compared with 30% on placebo in SURMOUNT-1, up to 93% vs 43% in SURMOUNT-2, and up to 97% vs 15%, respectively, in SURMOUNT-3.
At least 10% weight reduction was achieved by up to 93% vs 16%, respectively, in SURMOUNT-1, up to 76% vs 14% in SURMOUNT-2, and up to 92% vs 8% in SURMOUNT-3.
Weight reduction of 15% was achieved by up to 85% compared with 7% of patients on tirzepatide and placebo, respectively, in SURMOUNT-1; up to 60% vs 3%, respectively, in SURMOUNT-2; and up to 78% vs 4% in SURMOUNT-3.
In SURMOUNT-4, during the 36-week open-label tirzepatide treatment, the mean body weight % or more reduction was 21%. Following this lead-in period, further weight reductions of 5% or more, 10%, and 15% or more were achieved by up to 70%, 39%, and 22%, respectively, of participants treated with tirzepatide compared with 2%, 2%, and 0% of patients on placebo.
Body Weight and Waist Circumference Reduced Regardless of Disease Duration
In this second presentation, participants were categorized based on duration with overweight/obesity at baseline (10 years or less, between 10 and 20 years, and above 20 years). Percentage body weight change; the proportions achieving weight loss targets of 5%, 10%, 15%, 20%, and 25%; and the change in waist circumference were analyzed.
Greater weight reductions were found in participants who took tirzepatide than in those who took placebo across the SURMOUNT 1-4 study endpoints, including weight reduction targets of 5%, 10%, 15%, 20%, and 25% compared with placebo-treated participants, regardless of disease duration, reported the authors in an early press release from ECO. The magnitude of weight reductions was generally similar across the disease duration categories.
For example, in the SURMOUNT-1 trial, for patients given 10-mg dose of tirzepatide, those with disease duration under 10 years lost 21% of their weight after 72 weeks compared with 20% body weight loss for those with 10-20 years disease duration and 23% for those with over 20 years disease duration.
In the SURMOUNT-2 trial (where all participants were also living with type 2 diabetes), for patients given the 10-mg dose of tirzepatide, those with disease duration under 10 years lost 12.6% of their body weight, while those with disease duration of 10-20 years lost 12.5%; in people living with overweight or obesity for over 20 years, 14.4% of body weight was lost.
Waist circumference also reduced to a greater extent than placebo for each disease duration category across the four studies, and again, these reductions were consistent across disease duration subgroups.
A difference between patients with and without type 2 diabetes was evident and requires further analysis to explore and understand why patients with type 2 diabetes have less weight loss in these trials than those without type 2 diabetes.
Asked to comment on the findings, Jens Juul Holst, MD, from the Department of Biomedical Sciences and Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, said that the results were as expected.
“The first abstract is said to show that there is the same effect regardless of the baseline BMI, but this is the expected outcome — nothing exciting there,” he told this news organization. “The second deals with the effects in people with different duration of adiposity. Again, it was equally effective in all groups and that was also the expected outcome, although important.”
“One question is whether one should treat people with BMI < 30 at all, and that depends on preexisting comorbidities — in particular metabolic syndrome, where treatment could be lifesaving and prevent complications,” added Dr. Holst.
This news organization also asked Jason Halford, ECO president, for his view on the findings. He remarked that with these weight loss drugs overall, “Usually weight loss tends to be proportional and actually greater in the lower BMI categories. This is partly because dosing is not done by body weight, and everyone gets the same doses irrespective of how they weigh. There is an argument that doses should be adjusted. The data suggests these drugs are so potent this does not occur for some reason.”
Dr. Holst added that, “In principle, for a given reduction in food intake, one would expect a similar reduction in body mass, and these agents should be dosed according to the size of the individual — since energy expenditure depends linearly on body weight, this is probably a reasonable measure. But what actually happens is dosing is according to the occurrence of side effects, which is a good pragmatic principle.”
Dr. Holst pointed out that the interesting question here is whether the very obese would somehow be resistant to the GLP-1 RAs (like leptin) — “they are not,” he noted.
He added that to his knowledge, the question around the role played by duration of the adiposity had not been explicitly looked at before. “However, the many individuals with obesity studied after GLP-1 RA treatment have varied widely with respect to duration and weight loss has not previously been known to depend on this, but there is no known physiological mechanism underpinning this.”
Tirzepatide (Mounjaro) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes in 2022. In November 2023, the FDA approved tirzepatide (Zepbound) for chronic weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight-related comorbidity. Also in November 2023, the EMA Committee for Medicinal Products for Human Use offered a positive opinion on extension of the Mounjaro label to include weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 and at least one weight-related comorbid condition.
Dr. Holst had no conflicting interest with Eli Lilly but is a member of advisory boards for Novo Nordisk. This work (abstract 014) was funded by Eli Lilly and Company. Dr. Le Roux reported grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He served on advisory boards and speaker panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. Dr. Le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Dr. Le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. Dr. Le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Dr. Le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. Dr. Aronne reported receiving grants or personal fees from Altimmune, AstraZeneca, Boehringer Ingelheim, Eli Lilly, ERX, Gelesis, Intellihealth, Jamieson Wellness, Janssen, Novo Nordisk, Optum, Pfizer, Senda Biosciences, and Versanis and being a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, and Jamieson Wellness. FJ, TF, MM, LG, and LN are employees and shareholders of Eli Lilly and Company.
A version of this article appeared on Medscape.com.
During Global GLP-1 Shortage, Doctors Prioritize Certain Patients
Glucagon-like peptide 1 (GLP-1) receptor agonists are the latest blockbuster drugs — thanks to their potent ability to help patients lose weight. But
Semaglutide for weight loss (Wegovy) has launched in eight countries, namely, Denmark, Germany, Iceland, Norway, the United Arab Emirates, the United Kingdom, the United States, and Switzerland, and was released in Japan in February. Semaglutide for type 2 diabetes (Ozempic) is approved in 82 countries and often is prescribed off-label to treat obesity.
The dual glucose-dependent insulinotropic polypeptide/GLP-1 agonist tirzepatide — sold as Mounjaro for type 2 diabetes — started rolling out in 2022. It’s approved for chronic weight management in the European Union and the United Kingdom, sold in the United States as the weight loss drug Zepbound, and is currently under review in China.
As shortages continue, some governments are asking clinicians not to prescribe the drugs for obesity and instead reserve them for people with type 2 diabetes. But governments are limited in how to enforce this request, and some providers disagree with the guidance. Here’s a look at various countries’ approaches to handling these blockbuster drugs.
Sweden
Ylva Trolle Lagerros, MD, said it’s common for the Swedish Medicines Agency to post guidance for drugs on their website, and occasionally, the agency will send letters to physicians if a drug is recalled or found to have new side effects. In December, Dr. Lagerros, along with physicians throughout Sweden, received a letter at her home address requesting that they not prescribe GLP-1 receptor agonists to people for weight loss alone, over concerns the drugs wouldn’t be available for patients with type 2 diabetes.
Given the shortages, Dr. Lagerros, an obesity medicine specialist and associate professor at the Karolinska Institutet in Stockholm, Sweden, expected the guidance but said it was reinforced with the letters mailed to physicians’ homes.
“It’s not forbidden to go off-label. It is a right you have as a physician, but we are clearly told not to,” said Dr. Lagerros, who is also a senior physician at the Center for Obesity in Stockholm, Sweden’s largest obesity clinic.
Providers are being forced to prioritize some patients above others, she added.
“Yes, GLP-1 [agonists] are good for people with type 2 diabetes, but given this global shortage, I think the people who are most severely sick should be prioritized,” she said. “With this principle, we are walking away from that, saying only people with type 2 diabetes should get it.”
Dr. Lagerros said she does not prescribe Ozempic, the only injectable GLP-1 currently available in Sweden, off-label because she works closely with the government on national obesity guidelines and feels unable to, but she understands why some of her colleagues at other clinics do.
In Sweden, some companies are importing and selling Wegovy, which is typically not available, at different price points, said Dr. Lagerros. She said she knows of at least three telehealth apps operating in Sweden through which patients are prescribed semaglutide for weight loss without being seen by a doctor.
“That adds to the ethical problem that if you prescribe it as a diabetes medication, the patient doesn’t have to pay, but if you prescribe it as an obesity medication, the patient has to pay a lot of money,” Dr. Lagerros said.
United Kingdom
Last summer, health officials in the United Kingdom took a similar approach to Sweden’s, urging providers to stop prescribing appetite-suppressing medications for weight loss due to shortages for patients with diabetes. The notice also asked providers to hold off on writing new prescriptions for GLP-1 agonists, as well as the drug Trulicity, for patients with type 2 diabetes.
In the United Kingdom, Wegovy, Mounjaro, and Saxenda, an oral semaglutide, have been approved for weight loss and are covered by the National Health System. People must have a body mass index (BMI) of 30 or more with one weight-related condition, or a BMI of at least 35, to qualify for Wegovy. Because Ozempic, only approved for treating type 2 diabetes, is used off-label but is not specifically indicated for weight loss, physicians typically use the same parameters when prescribing it off-label as they do Wegovy.
Naresh Dr. Kanumilli, MD, a general practitioner and diabetes specialist in the Northenden Group Practice in Manchester, England, said he believes GLP-1 agonists should not be used off-label for weight loss.
“The global shortage was probably exacerbated because a lot of the drugs were going toward obesity when they should be going to diabetes,” he said.
Dr. Kanumilli, who is also a National Health Service England Clinical Network lead for diabetes, said he hopes more doctors in the United Kingdom offer their patients other drugs for weight loss before reaching for Wegovy.
He said doctors in the United Kingdom are allowing patients to jump from a metformin-only regimen to GLP-1 plus metformin, without trying an intermediate group of drugs called sodium-glucose transport protein 2 inhibitors. “We want to reinforce that these drugs should be tried prior to GLP-1 agonists [for obesity treatment],” he said.
United States
Despite widespread shortages, the US government has not asked clinicians to reserve GLP-1 agonists for patients with type 2 diabetes, but patients are experiencing additional restrictions related to cost and insurance coverage.
In the United States, where these types of medications already cost more than they do in other countries, private insurers rarely cover the drugs for obesity. Medicare is forbidden to cover any type of weight loss drug, although proposed legislation could change that.
According to August 2023 data from KFF, formerly The Kaiser Family Foundation, a month’s supply of a 1.7-mg or 2.4-mg dose of Wegovy costs an average of $1349 in the United States, which is considerably higher than other countries. In Germany, that same supply runs about $328. In the Netherlands, it’s $296. A 1-month supply of Rybelsus or Ozempic costs about four times as much in the United States as it does in the Netherlands. Eli Lilly’s list price for 1 month of Mounjaro in the United States is $1069.08 compared to about $319 in Japan, according to the report.
On the rare occasion a private insurer in the United States does cover a GLP-1 agonist prescribed for weight loss — only about 27% of insurance companies did in 2023 — people may need to prove other interventions, including lifestyle changes, did not produce results.
Beverly Tchang, MD, an assistant professor of clinical medicine at Weill Comprehensive Weight Control Center in New York, said she takes a patient-by-patient approach when considering prescribing these medications.
The BMI thresholds for Wegovy are 27 if a person has at least one weight-related comorbidity, and 30 if they do not, in the United States. Dr. Tchang said these rules are strict, but some exceptions are made for ethnicities such as those of South or East Asian descent where a BMI of 25 can be used as they have a lower threshold for overweight or obesity.
If Dr. Tchang feels a patient would benefit from significant weight loss, she is comfortable prescribing the drugs for weight loss to a patient who doesn’t have type 2 diabetes.
“Most people I see would benefit from that 10%-15% or more weight loss threshold, so I often do reach for the tirzepatide and semaglutide,” she said.
For patients who need to lose closer to 5% of their body weight to manage or prevent comorbidities, Dr. Tchang said she would likely try another medication that does not produce as extreme results.
Canada
The Canadian government has not directed clinicians to reserve GLP-1 agonists for certain patients. Instead, access is limited by cost, said Ehud Ur, MD, a professor of medicine at the University of British Columbia and consulting endocrinologist at St. Paul’s Hospital in Vancouver, British Columbia, Canada.
About 67% of Canadians have private insurance, according to The Commonwealth Fund. Most private insurers cover GLP-1 agonists for weight loss, but Canada’s public healthcare system only covers the drugs for type 2 diabetes, not for weight loss alone.
He agreed that people with type 2 diabetes should not be favored over those with obesity for prescriptions of GLP-1 agonists. Rather, he said, physicians should focus on what is the best treatment is for each patient. For some people with obesity, these medications can elicit the same weight loss as surgery, which no other medication currently can.
Dr. Ur said some clinicians in Canada prescribe GLP-1 agonists to people who do not need to lose a significant amount of weight, but the drugs are also being taken by people who do.
“The drive for the drugs is largely due to their efficacy,” he said. “You have physicians that have more confidence in this drug than they have for any other antiobesity agent, so you have a big drive for prescriptions.”
What Are the Alternatives?
In the face of shortages, physicians including Dr. Lagerros, Dr. Tchang, and Dr. Ur are resorting to other drugs when necessary to get patients the care they need.
“We have been in the business of treating obesity for decades,” Dr. Tchang said. “Before the GLP-1s were invented.”
Dr. Lagerros does not believe all her patients need GLP-1 agonists but does want them more widely available for those who overeat because they are unable to control their appetite, who she said are prime candidates for the drugs.
“I’m telling my patients, ‘yes, we don’t have semaglutide right now, but we just have to hang in there and work with what we have right now,’” she said.
A version of this article appeared on Medscape.com.
Glucagon-like peptide 1 (GLP-1) receptor agonists are the latest blockbuster drugs — thanks to their potent ability to help patients lose weight. But
Semaglutide for weight loss (Wegovy) has launched in eight countries, namely, Denmark, Germany, Iceland, Norway, the United Arab Emirates, the United Kingdom, the United States, and Switzerland, and was released in Japan in February. Semaglutide for type 2 diabetes (Ozempic) is approved in 82 countries and often is prescribed off-label to treat obesity.
The dual glucose-dependent insulinotropic polypeptide/GLP-1 agonist tirzepatide — sold as Mounjaro for type 2 diabetes — started rolling out in 2022. It’s approved for chronic weight management in the European Union and the United Kingdom, sold in the United States as the weight loss drug Zepbound, and is currently under review in China.
As shortages continue, some governments are asking clinicians not to prescribe the drugs for obesity and instead reserve them for people with type 2 diabetes. But governments are limited in how to enforce this request, and some providers disagree with the guidance. Here’s a look at various countries’ approaches to handling these blockbuster drugs.
Sweden
Ylva Trolle Lagerros, MD, said it’s common for the Swedish Medicines Agency to post guidance for drugs on their website, and occasionally, the agency will send letters to physicians if a drug is recalled or found to have new side effects. In December, Dr. Lagerros, along with physicians throughout Sweden, received a letter at her home address requesting that they not prescribe GLP-1 receptor agonists to people for weight loss alone, over concerns the drugs wouldn’t be available for patients with type 2 diabetes.
Given the shortages, Dr. Lagerros, an obesity medicine specialist and associate professor at the Karolinska Institutet in Stockholm, Sweden, expected the guidance but said it was reinforced with the letters mailed to physicians’ homes.
“It’s not forbidden to go off-label. It is a right you have as a physician, but we are clearly told not to,” said Dr. Lagerros, who is also a senior physician at the Center for Obesity in Stockholm, Sweden’s largest obesity clinic.
Providers are being forced to prioritize some patients above others, she added.
“Yes, GLP-1 [agonists] are good for people with type 2 diabetes, but given this global shortage, I think the people who are most severely sick should be prioritized,” she said. “With this principle, we are walking away from that, saying only people with type 2 diabetes should get it.”
Dr. Lagerros said she does not prescribe Ozempic, the only injectable GLP-1 currently available in Sweden, off-label because she works closely with the government on national obesity guidelines and feels unable to, but she understands why some of her colleagues at other clinics do.
In Sweden, some companies are importing and selling Wegovy, which is typically not available, at different price points, said Dr. Lagerros. She said she knows of at least three telehealth apps operating in Sweden through which patients are prescribed semaglutide for weight loss without being seen by a doctor.
“That adds to the ethical problem that if you prescribe it as a diabetes medication, the patient doesn’t have to pay, but if you prescribe it as an obesity medication, the patient has to pay a lot of money,” Dr. Lagerros said.
United Kingdom
Last summer, health officials in the United Kingdom took a similar approach to Sweden’s, urging providers to stop prescribing appetite-suppressing medications for weight loss due to shortages for patients with diabetes. The notice also asked providers to hold off on writing new prescriptions for GLP-1 agonists, as well as the drug Trulicity, for patients with type 2 diabetes.
In the United Kingdom, Wegovy, Mounjaro, and Saxenda, an oral semaglutide, have been approved for weight loss and are covered by the National Health System. People must have a body mass index (BMI) of 30 or more with one weight-related condition, or a BMI of at least 35, to qualify for Wegovy. Because Ozempic, only approved for treating type 2 diabetes, is used off-label but is not specifically indicated for weight loss, physicians typically use the same parameters when prescribing it off-label as they do Wegovy.
Naresh Dr. Kanumilli, MD, a general practitioner and diabetes specialist in the Northenden Group Practice in Manchester, England, said he believes GLP-1 agonists should not be used off-label for weight loss.
“The global shortage was probably exacerbated because a lot of the drugs were going toward obesity when they should be going to diabetes,” he said.
Dr. Kanumilli, who is also a National Health Service England Clinical Network lead for diabetes, said he hopes more doctors in the United Kingdom offer their patients other drugs for weight loss before reaching for Wegovy.
He said doctors in the United Kingdom are allowing patients to jump from a metformin-only regimen to GLP-1 plus metformin, without trying an intermediate group of drugs called sodium-glucose transport protein 2 inhibitors. “We want to reinforce that these drugs should be tried prior to GLP-1 agonists [for obesity treatment],” he said.
United States
Despite widespread shortages, the US government has not asked clinicians to reserve GLP-1 agonists for patients with type 2 diabetes, but patients are experiencing additional restrictions related to cost and insurance coverage.
In the United States, where these types of medications already cost more than they do in other countries, private insurers rarely cover the drugs for obesity. Medicare is forbidden to cover any type of weight loss drug, although proposed legislation could change that.
According to August 2023 data from KFF, formerly The Kaiser Family Foundation, a month’s supply of a 1.7-mg or 2.4-mg dose of Wegovy costs an average of $1349 in the United States, which is considerably higher than other countries. In Germany, that same supply runs about $328. In the Netherlands, it’s $296. A 1-month supply of Rybelsus or Ozempic costs about four times as much in the United States as it does in the Netherlands. Eli Lilly’s list price for 1 month of Mounjaro in the United States is $1069.08 compared to about $319 in Japan, according to the report.
On the rare occasion a private insurer in the United States does cover a GLP-1 agonist prescribed for weight loss — only about 27% of insurance companies did in 2023 — people may need to prove other interventions, including lifestyle changes, did not produce results.
Beverly Tchang, MD, an assistant professor of clinical medicine at Weill Comprehensive Weight Control Center in New York, said she takes a patient-by-patient approach when considering prescribing these medications.
The BMI thresholds for Wegovy are 27 if a person has at least one weight-related comorbidity, and 30 if they do not, in the United States. Dr. Tchang said these rules are strict, but some exceptions are made for ethnicities such as those of South or East Asian descent where a BMI of 25 can be used as they have a lower threshold for overweight or obesity.
If Dr. Tchang feels a patient would benefit from significant weight loss, she is comfortable prescribing the drugs for weight loss to a patient who doesn’t have type 2 diabetes.
“Most people I see would benefit from that 10%-15% or more weight loss threshold, so I often do reach for the tirzepatide and semaglutide,” she said.
For patients who need to lose closer to 5% of their body weight to manage or prevent comorbidities, Dr. Tchang said she would likely try another medication that does not produce as extreme results.
Canada
The Canadian government has not directed clinicians to reserve GLP-1 agonists for certain patients. Instead, access is limited by cost, said Ehud Ur, MD, a professor of medicine at the University of British Columbia and consulting endocrinologist at St. Paul’s Hospital in Vancouver, British Columbia, Canada.
About 67% of Canadians have private insurance, according to The Commonwealth Fund. Most private insurers cover GLP-1 agonists for weight loss, but Canada’s public healthcare system only covers the drugs for type 2 diabetes, not for weight loss alone.
He agreed that people with type 2 diabetes should not be favored over those with obesity for prescriptions of GLP-1 agonists. Rather, he said, physicians should focus on what is the best treatment is for each patient. For some people with obesity, these medications can elicit the same weight loss as surgery, which no other medication currently can.
Dr. Ur said some clinicians in Canada prescribe GLP-1 agonists to people who do not need to lose a significant amount of weight, but the drugs are also being taken by people who do.
“The drive for the drugs is largely due to their efficacy,” he said. “You have physicians that have more confidence in this drug than they have for any other antiobesity agent, so you have a big drive for prescriptions.”
What Are the Alternatives?
In the face of shortages, physicians including Dr. Lagerros, Dr. Tchang, and Dr. Ur are resorting to other drugs when necessary to get patients the care they need.
“We have been in the business of treating obesity for decades,” Dr. Tchang said. “Before the GLP-1s were invented.”
Dr. Lagerros does not believe all her patients need GLP-1 agonists but does want them more widely available for those who overeat because they are unable to control their appetite, who she said are prime candidates for the drugs.
“I’m telling my patients, ‘yes, we don’t have semaglutide right now, but we just have to hang in there and work with what we have right now,’” she said.
A version of this article appeared on Medscape.com.
Glucagon-like peptide 1 (GLP-1) receptor agonists are the latest blockbuster drugs — thanks to their potent ability to help patients lose weight. But
Semaglutide for weight loss (Wegovy) has launched in eight countries, namely, Denmark, Germany, Iceland, Norway, the United Arab Emirates, the United Kingdom, the United States, and Switzerland, and was released in Japan in February. Semaglutide for type 2 diabetes (Ozempic) is approved in 82 countries and often is prescribed off-label to treat obesity.
The dual glucose-dependent insulinotropic polypeptide/GLP-1 agonist tirzepatide — sold as Mounjaro for type 2 diabetes — started rolling out in 2022. It’s approved for chronic weight management in the European Union and the United Kingdom, sold in the United States as the weight loss drug Zepbound, and is currently under review in China.
As shortages continue, some governments are asking clinicians not to prescribe the drugs for obesity and instead reserve them for people with type 2 diabetes. But governments are limited in how to enforce this request, and some providers disagree with the guidance. Here’s a look at various countries’ approaches to handling these blockbuster drugs.
Sweden
Ylva Trolle Lagerros, MD, said it’s common for the Swedish Medicines Agency to post guidance for drugs on their website, and occasionally, the agency will send letters to physicians if a drug is recalled or found to have new side effects. In December, Dr. Lagerros, along with physicians throughout Sweden, received a letter at her home address requesting that they not prescribe GLP-1 receptor agonists to people for weight loss alone, over concerns the drugs wouldn’t be available for patients with type 2 diabetes.
Given the shortages, Dr. Lagerros, an obesity medicine specialist and associate professor at the Karolinska Institutet in Stockholm, Sweden, expected the guidance but said it was reinforced with the letters mailed to physicians’ homes.
“It’s not forbidden to go off-label. It is a right you have as a physician, but we are clearly told not to,” said Dr. Lagerros, who is also a senior physician at the Center for Obesity in Stockholm, Sweden’s largest obesity clinic.
Providers are being forced to prioritize some patients above others, she added.
“Yes, GLP-1 [agonists] are good for people with type 2 diabetes, but given this global shortage, I think the people who are most severely sick should be prioritized,” she said. “With this principle, we are walking away from that, saying only people with type 2 diabetes should get it.”
Dr. Lagerros said she does not prescribe Ozempic, the only injectable GLP-1 currently available in Sweden, off-label because she works closely with the government on national obesity guidelines and feels unable to, but she understands why some of her colleagues at other clinics do.
In Sweden, some companies are importing and selling Wegovy, which is typically not available, at different price points, said Dr. Lagerros. She said she knows of at least three telehealth apps operating in Sweden through which patients are prescribed semaglutide for weight loss without being seen by a doctor.
“That adds to the ethical problem that if you prescribe it as a diabetes medication, the patient doesn’t have to pay, but if you prescribe it as an obesity medication, the patient has to pay a lot of money,” Dr. Lagerros said.
United Kingdom
Last summer, health officials in the United Kingdom took a similar approach to Sweden’s, urging providers to stop prescribing appetite-suppressing medications for weight loss due to shortages for patients with diabetes. The notice also asked providers to hold off on writing new prescriptions for GLP-1 agonists, as well as the drug Trulicity, for patients with type 2 diabetes.
In the United Kingdom, Wegovy, Mounjaro, and Saxenda, an oral semaglutide, have been approved for weight loss and are covered by the National Health System. People must have a body mass index (BMI) of 30 or more with one weight-related condition, or a BMI of at least 35, to qualify for Wegovy. Because Ozempic, only approved for treating type 2 diabetes, is used off-label but is not specifically indicated for weight loss, physicians typically use the same parameters when prescribing it off-label as they do Wegovy.
Naresh Dr. Kanumilli, MD, a general practitioner and diabetes specialist in the Northenden Group Practice in Manchester, England, said he believes GLP-1 agonists should not be used off-label for weight loss.
“The global shortage was probably exacerbated because a lot of the drugs were going toward obesity when they should be going to diabetes,” he said.
Dr. Kanumilli, who is also a National Health Service England Clinical Network lead for diabetes, said he hopes more doctors in the United Kingdom offer their patients other drugs for weight loss before reaching for Wegovy.
He said doctors in the United Kingdom are allowing patients to jump from a metformin-only regimen to GLP-1 plus metformin, without trying an intermediate group of drugs called sodium-glucose transport protein 2 inhibitors. “We want to reinforce that these drugs should be tried prior to GLP-1 agonists [for obesity treatment],” he said.
United States
Despite widespread shortages, the US government has not asked clinicians to reserve GLP-1 agonists for patients with type 2 diabetes, but patients are experiencing additional restrictions related to cost and insurance coverage.
In the United States, where these types of medications already cost more than they do in other countries, private insurers rarely cover the drugs for obesity. Medicare is forbidden to cover any type of weight loss drug, although proposed legislation could change that.
According to August 2023 data from KFF, formerly The Kaiser Family Foundation, a month’s supply of a 1.7-mg or 2.4-mg dose of Wegovy costs an average of $1349 in the United States, which is considerably higher than other countries. In Germany, that same supply runs about $328. In the Netherlands, it’s $296. A 1-month supply of Rybelsus or Ozempic costs about four times as much in the United States as it does in the Netherlands. Eli Lilly’s list price for 1 month of Mounjaro in the United States is $1069.08 compared to about $319 in Japan, according to the report.
On the rare occasion a private insurer in the United States does cover a GLP-1 agonist prescribed for weight loss — only about 27% of insurance companies did in 2023 — people may need to prove other interventions, including lifestyle changes, did not produce results.
Beverly Tchang, MD, an assistant professor of clinical medicine at Weill Comprehensive Weight Control Center in New York, said she takes a patient-by-patient approach when considering prescribing these medications.
The BMI thresholds for Wegovy are 27 if a person has at least one weight-related comorbidity, and 30 if they do not, in the United States. Dr. Tchang said these rules are strict, but some exceptions are made for ethnicities such as those of South or East Asian descent where a BMI of 25 can be used as they have a lower threshold for overweight or obesity.
If Dr. Tchang feels a patient would benefit from significant weight loss, she is comfortable prescribing the drugs for weight loss to a patient who doesn’t have type 2 diabetes.
“Most people I see would benefit from that 10%-15% or more weight loss threshold, so I often do reach for the tirzepatide and semaglutide,” she said.
For patients who need to lose closer to 5% of their body weight to manage or prevent comorbidities, Dr. Tchang said she would likely try another medication that does not produce as extreme results.
Canada
The Canadian government has not directed clinicians to reserve GLP-1 agonists for certain patients. Instead, access is limited by cost, said Ehud Ur, MD, a professor of medicine at the University of British Columbia and consulting endocrinologist at St. Paul’s Hospital in Vancouver, British Columbia, Canada.
About 67% of Canadians have private insurance, according to The Commonwealth Fund. Most private insurers cover GLP-1 agonists for weight loss, but Canada’s public healthcare system only covers the drugs for type 2 diabetes, not for weight loss alone.
He agreed that people with type 2 diabetes should not be favored over those with obesity for prescriptions of GLP-1 agonists. Rather, he said, physicians should focus on what is the best treatment is for each patient. For some people with obesity, these medications can elicit the same weight loss as surgery, which no other medication currently can.
Dr. Ur said some clinicians in Canada prescribe GLP-1 agonists to people who do not need to lose a significant amount of weight, but the drugs are also being taken by people who do.
“The drive for the drugs is largely due to their efficacy,” he said. “You have physicians that have more confidence in this drug than they have for any other antiobesity agent, so you have a big drive for prescriptions.”
What Are the Alternatives?
In the face of shortages, physicians including Dr. Lagerros, Dr. Tchang, and Dr. Ur are resorting to other drugs when necessary to get patients the care they need.
“We have been in the business of treating obesity for decades,” Dr. Tchang said. “Before the GLP-1s were invented.”
Dr. Lagerros does not believe all her patients need GLP-1 agonists but does want them more widely available for those who overeat because they are unable to control their appetite, who she said are prime candidates for the drugs.
“I’m telling my patients, ‘yes, we don’t have semaglutide right now, but we just have to hang in there and work with what we have right now,’” she said.
A version of this article appeared on Medscape.com.
Diet and Exercise in a Pill Are Real: How Mimetics Work
If couch-potato lab mice had beach-body dreams and if they could speak, they might tell you they’re thrilled by advances in the science of exercise and calorie-restriction (CR) mimetics.
In recent studies conducted at research centers across the United States, mice have chowed down, fattened up, exercised only if they felt like it, and still managed to lose body fat, improve their blood lipids, increase muscle power, avoid blood sugar problems, and boost heart function.
How did these mice get so lucky? They were given mimetics, experimental drugs that “mimic” the effects of exercise and calorie reduction in the body without the need to break a sweat or eat less.
“The mice looked like they’d done endurance training,” said Thomas Burris, PhD, chair of the Department of Pharmacodynamics at the University of Florida, Gainesville, Florida, and coauthor of a September 2023 study of the exercise mimetic SLU-PP-332, published in The Journal of Pharmacology and Experimental Therapeutics.
Meanwhile, the CR mimetic mannoheptulose (MH) “was incredibly effective at stopping the negative effects of a high-fat diet in mice,” said Donald K. Ingram, PhD, an adjunct professor at Louisiana State University’s Pennington Biomedical Research Center, Baton Rouge, Louisiana, who began studying CR mimetics at the National Institute on Aging in the 1980s. In a 2022 study published in Nutrients, MH also increased insulin sensitivity.
These “have your cake and eat it, too” drugs aren’t on the market for human use — but they’re edging closer. Several have moved into human trials with encouraging results. The National Institutes of Health and the pharmaceutical industry are taking notice, anteing up big research dollars. At the earliest, one could win US Food and Drug Administration (FDA) approval in 4-5 years, Dr. Burris said.
The medical appeal is clear: Mimetics could one day prevent and treat serious conditions such as age- and disease-related muscle loss, diabetes, heart failure, and even neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease, said the scientists studying them.
The commercial appeal is unavoidable: Mimetics have the potential to help nondieters avoid weight gain and allow dieters to build and/or preserve more calorie-burning muscle — a boon because losing weight can reduce muscle, especially with rapid loss.
How do these drugs work? What’s their downside? Like the “miracle” glucagon-like peptide 1 (GLP-1) weight-loss drugs that are now ubiquitous, are mimetics an effective pharmaceutical way to replicate two of society’s biggest lifestyle sticking points — diet and exercise?
It’s possible…
CR Mimetics: The Healthspan Drug?
From nematodes and fruit flies to yeast, Labrador Retrievers, and people, plenty of research shows that reducing calorie intake may improve health and prolong life. By how much? Cutting calories by 25% for 2 years slowed the pace of aging 2%-3% in the landmark CALERIE study of 197 adults, according to a 2023 study in Nature Aging. Sounds small, but the researchers said that equals a 10%-15% lower risk for an early death — on par with the longevity bonus you’d get from quitting smoking.
Trouble is low-cal living isn’t easy. “Diets work,” said George Roth, PhD, of GeroScience, Inc., in Pylesville, MD, who began studying CR at the National Institute on Aging in the 1980s with Ingram. “But it’s hard to sustain.”
That’s where CR mimetics come in. They activate the same health-promoting genes switched on by dieting, fasting, and extended periods of hunger, Dr. Roth said. The end result isn’t big weight loss. Instead, CR mimetics may keep us healthier and younger as we age. “Calorie restriction shifts metabolic processes in the body to protect against damage and stress,” he said.
Dr. Roth and Dr. Ingram are currently focused on the CR mimetic mannoheptulose (MH), a sugar found in unripe avocados. “It works at the first step in carbohydrate metabolism in cells throughout the body, so less energy goes through that pathway,” he said. “Glucose metabolism is reduced by 10%-15%. It’s the closest thing to actually eating less food.”
Their 2022 study found that while mice on an all-you-can-eat high-fat diet gained weight and body fat and saw blood lipids increase while insulin sensitivity decreased, mice that also got MH avoided these problems. A 2023 human study in Nutrients coauthored by Dr. Roth and Dr. Ingram found that a group consuming freeze-dried avocado had lower insulin levels than a placebo group.
Other researchers are looking at ways to stimulate the CR target nicotinamide adenine dinucleotide (NAD+). NAD+ assists sirtuins — a group of seven enzymes central to the beneficial effects of CR on aging — but levels drop with age. University of Colorado researchers are studying the effects of nicotinamide riboside (NR), an NAD+ precursor, in older adults with a $2.5 million National Institute on Aging grant. Small, preliminary human studies have found the compound reduced indicators of insulin resistance in the brain, in a January 2023 study in Aging Cell, and reduced blood pressure and arterial stiffness in a 2018 study published in Nature Communications.
Another NAD+ precursor, nicotinamide mononucleotide, reduced low-density lipoprotein cholesterol, diastolic blood pressure, and body weight in a Harvard Medical School study of 30 midlife and older adults with overweight and obesity, published in August 2023 in The Journal of Clinical Endocrinology & Metabolism. And in an April 2022 study published in Hepatology of people with nonalcoholic fatty liver disease, a proprietary supplement that included NR didn’t reduce liver fat but had a significant (vs placebo) reduction in ceramide and the liver enzyme alanine aminotransferase, a marker of inflammation.
“I think it was a pretty interesting result,” said lead researcher Leonard Guarente, PhD, professor of biology at Massachusetts Institute of Technology and founder of the supplement company Elysium. “Fatty liver progressively damages the liver. This has the potential to slow that down.”
Exercise Mimetics: Fitness in a Pill?
Physical activity builds muscle and fitness, helps keeps bones strong, sharpens thinking and memory, guards against depression, and helps discourage a slew of health concerns from weight gain and high blood pressure to diabetes and heart disease. Muscle becomes more dense, more powerful and may even burn more calories, said Dr. Burris. The problem: That pesky part about actually moving. Fewer than half of American adults get recommended amounts of aerobic exercise and fewer than a quarter fit in strength training, according to the Centers for Disease Control and Prevention.
Enter the exercise mimetics. Unlike CR mimetics, exercise mimetics affect mitochondria — the tiny power plants in muscle and every other cell in the body. They switch on genes that encourage the growth of more mitochondria and encourage them to burn fatty acids, not just glucose, for fuel.
In mice, this can keep them from gaining weight, increase insulin sensitivity, and boost exercise endurance. “We can use a drug to activate the same networks that are activated by physical activity,” said Ronald Evans, PhD, professor and director of the Gene Expression Laboratory at the Salk Institute for Biological Studies in La Jolla, California.
Among notable mimetics moving into human studies is ASP0367, a drug in a class called PPAR delta modulators first developed in Evans’ lab. ASP0367 was licensed to the pharmaceutical company Mitobridge, later acquired by Astellas. Astellas is currently running a phase 2/3 human trial of the investigational drug in people with the rare genetic disorder primary mitochondrial myopathy.
At the University of Florida, Dr. Burris and team hope to soon move the exercise mimetic SLU-PP-332 into human studies. “It targets a receptor called ERR that I’ve been working on since the 1980s,” Dr. Burris said. “We knew from genetic studies that ERR has a role in exercise’s effects on mitochondrial function in muscle.” The calorie mimetics he’s studying also activate genes for making more mitochondria and driving them to burn fatty acids. “This generates a lot of energy,” he said. In a January 2024 study in Circulation, Dr. Burris found the drug restores heart function in mice experiencing heart failure. “Very little heart function was lost,” he said. It’s had no serious side effects.
The Future of Exercise and CR Pills
The field has hit some bumps. Some feel inevitable — such as otherwise healthy people misusing the drugs. GW1516, an early experimental exercise mimetic studied by Dr. Evans and abandoned because it triggered tumor growth in lab studies, is used illegally by elite athletes as a performance-enhancing drug despite warnings from the US Anti-Doping Agency. Dr. Burris worries that future CR mimetics could be misused the same way.
But he and others see plenty of benefits in future, FDA-approved drugs. Exercise mimetics like SLU-PP-332 might one day be given to people alongside weight-loss drugs, such as Mounjaro (tirzepatide) or Ozempic (semaglutide) to prevent muscle loss. “SLU-PP-332 doesn’t affect hunger or food intake the way those drugs do,” he said. “It changes muscle.”
Mimetics may one day help older adults and people with muscle disorders rebuild muscle even when they cannot exercise and to delay a range of age-related diseases without onerous dieting. “The chance to intervene and provide a longer healthspan and lifespan — that’s been the moon shot,” Dr. Roth said.
Dr. Guarente noted that CR mimetics may work best for people who aren’t carrying extra pounds but want the health benefits of slashing calories without sacrificing meals and snacks. “Fat is still going to be a problem for joints, cholesterol, inflammation,” he said. “Calorie mimetics are not a panacea for obesity but could help preserve overall health and vitality.”
And what about the billion-dollar question: What happens when these drugs become available to a general public that has issues with actual exercise and healthy diet?
Evans sees only positives. “Our environment is designed to keep people sitting down and consuming high-calorie foods,” he said. “In the absence of people getting motivated to exercise — and there’s no evidence the country is moving in that direction on its own — a pill is an important option to have.”
A version of this article appeared on Medscape.com.
If couch-potato lab mice had beach-body dreams and if they could speak, they might tell you they’re thrilled by advances in the science of exercise and calorie-restriction (CR) mimetics.
In recent studies conducted at research centers across the United States, mice have chowed down, fattened up, exercised only if they felt like it, and still managed to lose body fat, improve their blood lipids, increase muscle power, avoid blood sugar problems, and boost heart function.
How did these mice get so lucky? They were given mimetics, experimental drugs that “mimic” the effects of exercise and calorie reduction in the body without the need to break a sweat or eat less.
“The mice looked like they’d done endurance training,” said Thomas Burris, PhD, chair of the Department of Pharmacodynamics at the University of Florida, Gainesville, Florida, and coauthor of a September 2023 study of the exercise mimetic SLU-PP-332, published in The Journal of Pharmacology and Experimental Therapeutics.
Meanwhile, the CR mimetic mannoheptulose (MH) “was incredibly effective at stopping the negative effects of a high-fat diet in mice,” said Donald K. Ingram, PhD, an adjunct professor at Louisiana State University’s Pennington Biomedical Research Center, Baton Rouge, Louisiana, who began studying CR mimetics at the National Institute on Aging in the 1980s. In a 2022 study published in Nutrients, MH also increased insulin sensitivity.
These “have your cake and eat it, too” drugs aren’t on the market for human use — but they’re edging closer. Several have moved into human trials with encouraging results. The National Institutes of Health and the pharmaceutical industry are taking notice, anteing up big research dollars. At the earliest, one could win US Food and Drug Administration (FDA) approval in 4-5 years, Dr. Burris said.
The medical appeal is clear: Mimetics could one day prevent and treat serious conditions such as age- and disease-related muscle loss, diabetes, heart failure, and even neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease, said the scientists studying them.
The commercial appeal is unavoidable: Mimetics have the potential to help nondieters avoid weight gain and allow dieters to build and/or preserve more calorie-burning muscle — a boon because losing weight can reduce muscle, especially with rapid loss.
How do these drugs work? What’s their downside? Like the “miracle” glucagon-like peptide 1 (GLP-1) weight-loss drugs that are now ubiquitous, are mimetics an effective pharmaceutical way to replicate two of society’s biggest lifestyle sticking points — diet and exercise?
It’s possible…
CR Mimetics: The Healthspan Drug?
From nematodes and fruit flies to yeast, Labrador Retrievers, and people, plenty of research shows that reducing calorie intake may improve health and prolong life. By how much? Cutting calories by 25% for 2 years slowed the pace of aging 2%-3% in the landmark CALERIE study of 197 adults, according to a 2023 study in Nature Aging. Sounds small, but the researchers said that equals a 10%-15% lower risk for an early death — on par with the longevity bonus you’d get from quitting smoking.
Trouble is low-cal living isn’t easy. “Diets work,” said George Roth, PhD, of GeroScience, Inc., in Pylesville, MD, who began studying CR at the National Institute on Aging in the 1980s with Ingram. “But it’s hard to sustain.”
That’s where CR mimetics come in. They activate the same health-promoting genes switched on by dieting, fasting, and extended periods of hunger, Dr. Roth said. The end result isn’t big weight loss. Instead, CR mimetics may keep us healthier and younger as we age. “Calorie restriction shifts metabolic processes in the body to protect against damage and stress,” he said.
Dr. Roth and Dr. Ingram are currently focused on the CR mimetic mannoheptulose (MH), a sugar found in unripe avocados. “It works at the first step in carbohydrate metabolism in cells throughout the body, so less energy goes through that pathway,” he said. “Glucose metabolism is reduced by 10%-15%. It’s the closest thing to actually eating less food.”
Their 2022 study found that while mice on an all-you-can-eat high-fat diet gained weight and body fat and saw blood lipids increase while insulin sensitivity decreased, mice that also got MH avoided these problems. A 2023 human study in Nutrients coauthored by Dr. Roth and Dr. Ingram found that a group consuming freeze-dried avocado had lower insulin levels than a placebo group.
Other researchers are looking at ways to stimulate the CR target nicotinamide adenine dinucleotide (NAD+). NAD+ assists sirtuins — a group of seven enzymes central to the beneficial effects of CR on aging — but levels drop with age. University of Colorado researchers are studying the effects of nicotinamide riboside (NR), an NAD+ precursor, in older adults with a $2.5 million National Institute on Aging grant. Small, preliminary human studies have found the compound reduced indicators of insulin resistance in the brain, in a January 2023 study in Aging Cell, and reduced blood pressure and arterial stiffness in a 2018 study published in Nature Communications.
Another NAD+ precursor, nicotinamide mononucleotide, reduced low-density lipoprotein cholesterol, diastolic blood pressure, and body weight in a Harvard Medical School study of 30 midlife and older adults with overweight and obesity, published in August 2023 in The Journal of Clinical Endocrinology & Metabolism. And in an April 2022 study published in Hepatology of people with nonalcoholic fatty liver disease, a proprietary supplement that included NR didn’t reduce liver fat but had a significant (vs placebo) reduction in ceramide and the liver enzyme alanine aminotransferase, a marker of inflammation.
“I think it was a pretty interesting result,” said lead researcher Leonard Guarente, PhD, professor of biology at Massachusetts Institute of Technology and founder of the supplement company Elysium. “Fatty liver progressively damages the liver. This has the potential to slow that down.”
Exercise Mimetics: Fitness in a Pill?
Physical activity builds muscle and fitness, helps keeps bones strong, sharpens thinking and memory, guards against depression, and helps discourage a slew of health concerns from weight gain and high blood pressure to diabetes and heart disease. Muscle becomes more dense, more powerful and may even burn more calories, said Dr. Burris. The problem: That pesky part about actually moving. Fewer than half of American adults get recommended amounts of aerobic exercise and fewer than a quarter fit in strength training, according to the Centers for Disease Control and Prevention.
Enter the exercise mimetics. Unlike CR mimetics, exercise mimetics affect mitochondria — the tiny power plants in muscle and every other cell in the body. They switch on genes that encourage the growth of more mitochondria and encourage them to burn fatty acids, not just glucose, for fuel.
In mice, this can keep them from gaining weight, increase insulin sensitivity, and boost exercise endurance. “We can use a drug to activate the same networks that are activated by physical activity,” said Ronald Evans, PhD, professor and director of the Gene Expression Laboratory at the Salk Institute for Biological Studies in La Jolla, California.
Among notable mimetics moving into human studies is ASP0367, a drug in a class called PPAR delta modulators first developed in Evans’ lab. ASP0367 was licensed to the pharmaceutical company Mitobridge, later acquired by Astellas. Astellas is currently running a phase 2/3 human trial of the investigational drug in people with the rare genetic disorder primary mitochondrial myopathy.
At the University of Florida, Dr. Burris and team hope to soon move the exercise mimetic SLU-PP-332 into human studies. “It targets a receptor called ERR that I’ve been working on since the 1980s,” Dr. Burris said. “We knew from genetic studies that ERR has a role in exercise’s effects on mitochondrial function in muscle.” The calorie mimetics he’s studying also activate genes for making more mitochondria and driving them to burn fatty acids. “This generates a lot of energy,” he said. In a January 2024 study in Circulation, Dr. Burris found the drug restores heart function in mice experiencing heart failure. “Very little heart function was lost,” he said. It’s had no serious side effects.
The Future of Exercise and CR Pills
The field has hit some bumps. Some feel inevitable — such as otherwise healthy people misusing the drugs. GW1516, an early experimental exercise mimetic studied by Dr. Evans and abandoned because it triggered tumor growth in lab studies, is used illegally by elite athletes as a performance-enhancing drug despite warnings from the US Anti-Doping Agency. Dr. Burris worries that future CR mimetics could be misused the same way.
But he and others see plenty of benefits in future, FDA-approved drugs. Exercise mimetics like SLU-PP-332 might one day be given to people alongside weight-loss drugs, such as Mounjaro (tirzepatide) or Ozempic (semaglutide) to prevent muscle loss. “SLU-PP-332 doesn’t affect hunger or food intake the way those drugs do,” he said. “It changes muscle.”
Mimetics may one day help older adults and people with muscle disorders rebuild muscle even when they cannot exercise and to delay a range of age-related diseases without onerous dieting. “The chance to intervene and provide a longer healthspan and lifespan — that’s been the moon shot,” Dr. Roth said.
Dr. Guarente noted that CR mimetics may work best for people who aren’t carrying extra pounds but want the health benefits of slashing calories without sacrificing meals and snacks. “Fat is still going to be a problem for joints, cholesterol, inflammation,” he said. “Calorie mimetics are not a panacea for obesity but could help preserve overall health and vitality.”
And what about the billion-dollar question: What happens when these drugs become available to a general public that has issues with actual exercise and healthy diet?
Evans sees only positives. “Our environment is designed to keep people sitting down and consuming high-calorie foods,” he said. “In the absence of people getting motivated to exercise — and there’s no evidence the country is moving in that direction on its own — a pill is an important option to have.”
A version of this article appeared on Medscape.com.
If couch-potato lab mice had beach-body dreams and if they could speak, they might tell you they’re thrilled by advances in the science of exercise and calorie-restriction (CR) mimetics.
In recent studies conducted at research centers across the United States, mice have chowed down, fattened up, exercised only if they felt like it, and still managed to lose body fat, improve their blood lipids, increase muscle power, avoid blood sugar problems, and boost heart function.
How did these mice get so lucky? They were given mimetics, experimental drugs that “mimic” the effects of exercise and calorie reduction in the body without the need to break a sweat or eat less.
“The mice looked like they’d done endurance training,” said Thomas Burris, PhD, chair of the Department of Pharmacodynamics at the University of Florida, Gainesville, Florida, and coauthor of a September 2023 study of the exercise mimetic SLU-PP-332, published in The Journal of Pharmacology and Experimental Therapeutics.
Meanwhile, the CR mimetic mannoheptulose (MH) “was incredibly effective at stopping the negative effects of a high-fat diet in mice,” said Donald K. Ingram, PhD, an adjunct professor at Louisiana State University’s Pennington Biomedical Research Center, Baton Rouge, Louisiana, who began studying CR mimetics at the National Institute on Aging in the 1980s. In a 2022 study published in Nutrients, MH also increased insulin sensitivity.
These “have your cake and eat it, too” drugs aren’t on the market for human use — but they’re edging closer. Several have moved into human trials with encouraging results. The National Institutes of Health and the pharmaceutical industry are taking notice, anteing up big research dollars. At the earliest, one could win US Food and Drug Administration (FDA) approval in 4-5 years, Dr. Burris said.
The medical appeal is clear: Mimetics could one day prevent and treat serious conditions such as age- and disease-related muscle loss, diabetes, heart failure, and even neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease, said the scientists studying them.
The commercial appeal is unavoidable: Mimetics have the potential to help nondieters avoid weight gain and allow dieters to build and/or preserve more calorie-burning muscle — a boon because losing weight can reduce muscle, especially with rapid loss.
How do these drugs work? What’s their downside? Like the “miracle” glucagon-like peptide 1 (GLP-1) weight-loss drugs that are now ubiquitous, are mimetics an effective pharmaceutical way to replicate two of society’s biggest lifestyle sticking points — diet and exercise?
It’s possible…
CR Mimetics: The Healthspan Drug?
From nematodes and fruit flies to yeast, Labrador Retrievers, and people, plenty of research shows that reducing calorie intake may improve health and prolong life. By how much? Cutting calories by 25% for 2 years slowed the pace of aging 2%-3% in the landmark CALERIE study of 197 adults, according to a 2023 study in Nature Aging. Sounds small, but the researchers said that equals a 10%-15% lower risk for an early death — on par with the longevity bonus you’d get from quitting smoking.
Trouble is low-cal living isn’t easy. “Diets work,” said George Roth, PhD, of GeroScience, Inc., in Pylesville, MD, who began studying CR at the National Institute on Aging in the 1980s with Ingram. “But it’s hard to sustain.”
That’s where CR mimetics come in. They activate the same health-promoting genes switched on by dieting, fasting, and extended periods of hunger, Dr. Roth said. The end result isn’t big weight loss. Instead, CR mimetics may keep us healthier and younger as we age. “Calorie restriction shifts metabolic processes in the body to protect against damage and stress,” he said.
Dr. Roth and Dr. Ingram are currently focused on the CR mimetic mannoheptulose (MH), a sugar found in unripe avocados. “It works at the first step in carbohydrate metabolism in cells throughout the body, so less energy goes through that pathway,” he said. “Glucose metabolism is reduced by 10%-15%. It’s the closest thing to actually eating less food.”
Their 2022 study found that while mice on an all-you-can-eat high-fat diet gained weight and body fat and saw blood lipids increase while insulin sensitivity decreased, mice that also got MH avoided these problems. A 2023 human study in Nutrients coauthored by Dr. Roth and Dr. Ingram found that a group consuming freeze-dried avocado had lower insulin levels than a placebo group.
Other researchers are looking at ways to stimulate the CR target nicotinamide adenine dinucleotide (NAD+). NAD+ assists sirtuins — a group of seven enzymes central to the beneficial effects of CR on aging — but levels drop with age. University of Colorado researchers are studying the effects of nicotinamide riboside (NR), an NAD+ precursor, in older adults with a $2.5 million National Institute on Aging grant. Small, preliminary human studies have found the compound reduced indicators of insulin resistance in the brain, in a January 2023 study in Aging Cell, and reduced blood pressure and arterial stiffness in a 2018 study published in Nature Communications.
Another NAD+ precursor, nicotinamide mononucleotide, reduced low-density lipoprotein cholesterol, diastolic blood pressure, and body weight in a Harvard Medical School study of 30 midlife and older adults with overweight and obesity, published in August 2023 in The Journal of Clinical Endocrinology & Metabolism. And in an April 2022 study published in Hepatology of people with nonalcoholic fatty liver disease, a proprietary supplement that included NR didn’t reduce liver fat but had a significant (vs placebo) reduction in ceramide and the liver enzyme alanine aminotransferase, a marker of inflammation.
“I think it was a pretty interesting result,” said lead researcher Leonard Guarente, PhD, professor of biology at Massachusetts Institute of Technology and founder of the supplement company Elysium. “Fatty liver progressively damages the liver. This has the potential to slow that down.”
Exercise Mimetics: Fitness in a Pill?
Physical activity builds muscle and fitness, helps keeps bones strong, sharpens thinking and memory, guards against depression, and helps discourage a slew of health concerns from weight gain and high blood pressure to diabetes and heart disease. Muscle becomes more dense, more powerful and may even burn more calories, said Dr. Burris. The problem: That pesky part about actually moving. Fewer than half of American adults get recommended amounts of aerobic exercise and fewer than a quarter fit in strength training, according to the Centers for Disease Control and Prevention.
Enter the exercise mimetics. Unlike CR mimetics, exercise mimetics affect mitochondria — the tiny power plants in muscle and every other cell in the body. They switch on genes that encourage the growth of more mitochondria and encourage them to burn fatty acids, not just glucose, for fuel.
In mice, this can keep them from gaining weight, increase insulin sensitivity, and boost exercise endurance. “We can use a drug to activate the same networks that are activated by physical activity,” said Ronald Evans, PhD, professor and director of the Gene Expression Laboratory at the Salk Institute for Biological Studies in La Jolla, California.
Among notable mimetics moving into human studies is ASP0367, a drug in a class called PPAR delta modulators first developed in Evans’ lab. ASP0367 was licensed to the pharmaceutical company Mitobridge, later acquired by Astellas. Astellas is currently running a phase 2/3 human trial of the investigational drug in people with the rare genetic disorder primary mitochondrial myopathy.
At the University of Florida, Dr. Burris and team hope to soon move the exercise mimetic SLU-PP-332 into human studies. “It targets a receptor called ERR that I’ve been working on since the 1980s,” Dr. Burris said. “We knew from genetic studies that ERR has a role in exercise’s effects on mitochondrial function in muscle.” The calorie mimetics he’s studying also activate genes for making more mitochondria and driving them to burn fatty acids. “This generates a lot of energy,” he said. In a January 2024 study in Circulation, Dr. Burris found the drug restores heart function in mice experiencing heart failure. “Very little heart function was lost,” he said. It’s had no serious side effects.
The Future of Exercise and CR Pills
The field has hit some bumps. Some feel inevitable — such as otherwise healthy people misusing the drugs. GW1516, an early experimental exercise mimetic studied by Dr. Evans and abandoned because it triggered tumor growth in lab studies, is used illegally by elite athletes as a performance-enhancing drug despite warnings from the US Anti-Doping Agency. Dr. Burris worries that future CR mimetics could be misused the same way.
But he and others see plenty of benefits in future, FDA-approved drugs. Exercise mimetics like SLU-PP-332 might one day be given to people alongside weight-loss drugs, such as Mounjaro (tirzepatide) or Ozempic (semaglutide) to prevent muscle loss. “SLU-PP-332 doesn’t affect hunger or food intake the way those drugs do,” he said. “It changes muscle.”
Mimetics may one day help older adults and people with muscle disorders rebuild muscle even when they cannot exercise and to delay a range of age-related diseases without onerous dieting. “The chance to intervene and provide a longer healthspan and lifespan — that’s been the moon shot,” Dr. Roth said.
Dr. Guarente noted that CR mimetics may work best for people who aren’t carrying extra pounds but want the health benefits of slashing calories without sacrificing meals and snacks. “Fat is still going to be a problem for joints, cholesterol, inflammation,” he said. “Calorie mimetics are not a panacea for obesity but could help preserve overall health and vitality.”
And what about the billion-dollar question: What happens when these drugs become available to a general public that has issues with actual exercise and healthy diet?
Evans sees only positives. “Our environment is designed to keep people sitting down and consuming high-calorie foods,” he said. “In the absence of people getting motivated to exercise — and there’s no evidence the country is moving in that direction on its own — a pill is an important option to have.”
A version of this article appeared on Medscape.com.
Look Beyond BMI: Metabolic Factors’ Link to Cancer Explained
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
FROM CANCER
Semaglutide Curbs MASLD Severity in People Living With HIV
Semaglutide improved metabolic dysfunction–associated steatotic liver disease (MASLD) among people living with HIV, and in some cases resolved it completely, according to results from the SLIM LIVER study presented by the AIDS Clinical Trials Group (ACTG) at this year’s Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver.
Furthermore, although muscle volume decreased with weight loss, participants did not experience significant changes in muscle quality or physical function.
‘A First’
SLIM LIVER is the first study evaluating semaglutide as a treatment of MASLD among people living with HIV.
The phase 2b, single-arm pilot study enrolled adults living with HIV who were virally suppressed and had central adiposity, insulin resistance or prediabetes, and steatotic liver disease.
Participants self-injected semaglutide weekly at increasing doses until they reached a 1-mg dose at week 4. At 24 weeks, the study team assessed changes in participants’ intra-hepatic triglyceride content using magnetic resonance imaging-proton density fat fraction.
The primary analysis results from SLIM LIVER were reported in an oral presentation, “Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER Study,” on March 5 by Jordan E. Lake, MD, MSc, of UTHealth Houston.
A subgroup analysis of the study was provided in a poster, “Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study,” presented on March 4 by Grace L. Ditzenberger, PT, DPT, of the University of Colorado Anschutz Medical Campus in Aurora.
In the primary analysis, the median age of the 49 participants was 52 years, 43% were women (cisgender and transgender), the mean body mass index was 35, 39% were Hispanic and 33% were Black/African American, and 82% were taking antiretroviral therapy that included an integrase inhibitor.
Liver fat was reduced by an average of 31%, with 29% of participants experiencing a complete resolution (5% or less liver fat) of MASLD. They also experienced weight loss, reduced fasting blood glucose, and reduced fasting triglycerides, consistent with effects observed in studies of semaglutide in people without HIV.
The sub-analysis of the 46 participants for whom muscle measurements were available showed that muscle volume (measured in the psoas) decreased but with no significant change in physical function.
Semaglutide was generally well tolerated, with an adverse event profile similar to that seen in individuals without HIV.
The most common adverse events were gastrointestinal (ie, nausea, diarrhea, vomiting, and abdominal pain). Two participants experienced more significant adverse events possibly related to semaglutide but were able to continue in the study.
All participants completed the full 24 weeks of therapy at the originally prescribed dose.
Potential Impact
“Even at the low dose of 1 mg every week, most participants lost significant weight, and weight loss was closely associated with improvements in MASLD,” Dr. Lake said. “Additional research will assess the secondary effects of semaglutide on systemic inflammation and metabolism and determine whether semaglutide may have unique risks or benefits for people living with HIV.”
“These findings have the potential to have a significant impact on the health and quality of life of people living with HIV,” added ACTG Chair Judith Currier, MD, MSc, University of California Los Angeles.
The SLIM LIVER study was sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), with additional funding from UTHealth Houston McGovern School of Medicine. ACTG is a clinical trials network focused on HIV and other infectious diseases, funded by NIAID and collaborating institutes of the US National Institutes of Health.
No conflicts of interest were reported.
A version of this article appeared on Medscape.com.
Semaglutide improved metabolic dysfunction–associated steatotic liver disease (MASLD) among people living with HIV, and in some cases resolved it completely, according to results from the SLIM LIVER study presented by the AIDS Clinical Trials Group (ACTG) at this year’s Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver.
Furthermore, although muscle volume decreased with weight loss, participants did not experience significant changes in muscle quality or physical function.
‘A First’
SLIM LIVER is the first study evaluating semaglutide as a treatment of MASLD among people living with HIV.
The phase 2b, single-arm pilot study enrolled adults living with HIV who were virally suppressed and had central adiposity, insulin resistance or prediabetes, and steatotic liver disease.
Participants self-injected semaglutide weekly at increasing doses until they reached a 1-mg dose at week 4. At 24 weeks, the study team assessed changes in participants’ intra-hepatic triglyceride content using magnetic resonance imaging-proton density fat fraction.
The primary analysis results from SLIM LIVER were reported in an oral presentation, “Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER Study,” on March 5 by Jordan E. Lake, MD, MSc, of UTHealth Houston.
A subgroup analysis of the study was provided in a poster, “Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study,” presented on March 4 by Grace L. Ditzenberger, PT, DPT, of the University of Colorado Anschutz Medical Campus in Aurora.
In the primary analysis, the median age of the 49 participants was 52 years, 43% were women (cisgender and transgender), the mean body mass index was 35, 39% were Hispanic and 33% were Black/African American, and 82% were taking antiretroviral therapy that included an integrase inhibitor.
Liver fat was reduced by an average of 31%, with 29% of participants experiencing a complete resolution (5% or less liver fat) of MASLD. They also experienced weight loss, reduced fasting blood glucose, and reduced fasting triglycerides, consistent with effects observed in studies of semaglutide in people without HIV.
The sub-analysis of the 46 participants for whom muscle measurements were available showed that muscle volume (measured in the psoas) decreased but with no significant change in physical function.
Semaglutide was generally well tolerated, with an adverse event profile similar to that seen in individuals without HIV.
The most common adverse events were gastrointestinal (ie, nausea, diarrhea, vomiting, and abdominal pain). Two participants experienced more significant adverse events possibly related to semaglutide but were able to continue in the study.
All participants completed the full 24 weeks of therapy at the originally prescribed dose.
Potential Impact
“Even at the low dose of 1 mg every week, most participants lost significant weight, and weight loss was closely associated with improvements in MASLD,” Dr. Lake said. “Additional research will assess the secondary effects of semaglutide on systemic inflammation and metabolism and determine whether semaglutide may have unique risks or benefits for people living with HIV.”
“These findings have the potential to have a significant impact on the health and quality of life of people living with HIV,” added ACTG Chair Judith Currier, MD, MSc, University of California Los Angeles.
The SLIM LIVER study was sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), with additional funding from UTHealth Houston McGovern School of Medicine. ACTG is a clinical trials network focused on HIV and other infectious diseases, funded by NIAID and collaborating institutes of the US National Institutes of Health.
No conflicts of interest were reported.
A version of this article appeared on Medscape.com.
Semaglutide improved metabolic dysfunction–associated steatotic liver disease (MASLD) among people living with HIV, and in some cases resolved it completely, according to results from the SLIM LIVER study presented by the AIDS Clinical Trials Group (ACTG) at this year’s Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver.
Furthermore, although muscle volume decreased with weight loss, participants did not experience significant changes in muscle quality or physical function.
‘A First’
SLIM LIVER is the first study evaluating semaglutide as a treatment of MASLD among people living with HIV.
The phase 2b, single-arm pilot study enrolled adults living with HIV who were virally suppressed and had central adiposity, insulin resistance or prediabetes, and steatotic liver disease.
Participants self-injected semaglutide weekly at increasing doses until they reached a 1-mg dose at week 4. At 24 weeks, the study team assessed changes in participants’ intra-hepatic triglyceride content using magnetic resonance imaging-proton density fat fraction.
The primary analysis results from SLIM LIVER were reported in an oral presentation, “Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER Study,” on March 5 by Jordan E. Lake, MD, MSc, of UTHealth Houston.
A subgroup analysis of the study was provided in a poster, “Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study,” presented on March 4 by Grace L. Ditzenberger, PT, DPT, of the University of Colorado Anschutz Medical Campus in Aurora.
In the primary analysis, the median age of the 49 participants was 52 years, 43% were women (cisgender and transgender), the mean body mass index was 35, 39% were Hispanic and 33% were Black/African American, and 82% were taking antiretroviral therapy that included an integrase inhibitor.
Liver fat was reduced by an average of 31%, with 29% of participants experiencing a complete resolution (5% or less liver fat) of MASLD. They also experienced weight loss, reduced fasting blood glucose, and reduced fasting triglycerides, consistent with effects observed in studies of semaglutide in people without HIV.
The sub-analysis of the 46 participants for whom muscle measurements were available showed that muscle volume (measured in the psoas) decreased but with no significant change in physical function.
Semaglutide was generally well tolerated, with an adverse event profile similar to that seen in individuals without HIV.
The most common adverse events were gastrointestinal (ie, nausea, diarrhea, vomiting, and abdominal pain). Two participants experienced more significant adverse events possibly related to semaglutide but were able to continue in the study.
All participants completed the full 24 weeks of therapy at the originally prescribed dose.
Potential Impact
“Even at the low dose of 1 mg every week, most participants lost significant weight, and weight loss was closely associated with improvements in MASLD,” Dr. Lake said. “Additional research will assess the secondary effects of semaglutide on systemic inflammation and metabolism and determine whether semaglutide may have unique risks or benefits for people living with HIV.”
“These findings have the potential to have a significant impact on the health and quality of life of people living with HIV,” added ACTG Chair Judith Currier, MD, MSc, University of California Los Angeles.
The SLIM LIVER study was sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), with additional funding from UTHealth Houston McGovern School of Medicine. ACTG is a clinical trials network focused on HIV and other infectious diseases, funded by NIAID and collaborating institutes of the US National Institutes of Health.
No conflicts of interest were reported.
A version of this article appeared on Medscape.com.
FROM CROI 2024
The Role of Growth Hormone Mediators in Youth-Onset T2D
TOPLINE:
Changes in plasma growth hormone mediators such as growth hormone receptor (GHR) and insulin-like growth factor-binding protein 1 (IGFBP-1) were associated with glycemic failure in youth-onset type 2 diabetes (T2D), an analysis of the TODAY trial showed.
METHODOLOGY:
- In youth, T2D often occurs during or after puberty, hinting at hormonal influences in the development and/or progression of the disease.
- This secondary analysis assessed the role of growth hormone mediators including insulin-like growth factor-1 (IGF-1), GHR, and IGFBP-1 in glycemic failure in a subset of 398 youths, aged 10-17 years, with a T2D duration of less than 2 years (62% girls; 21% White).
- The participants were followed up for a mean of 3.9 years.
- The primary outcomes included glycemic failure, defined as an A1c level of 8% or more for 6 months, or acute metabolic decompensation requiring insulin.
- Other assessments included baseline and 36-month measures of glycemia, insulin sensitivity, high molecular weight adiponectin, and beta cell function.
TAKEAWAY:
- Of 398 participants, 182 (46%) experienced glycemic failure, while 216 (54%) retained glycemic control.
- At 36 months, youths with glycemic failure had lower IGF-1 levels (P < .001) and higher log2 GHR (P = .03) and log2 IGFBP-1 (P = .009) levels than those who maintained glycemic control.
- A greater increase in IGF-1 level at 36 months was associated with lower odds of glycemic failure (odds ratio [OR], 0.995; P < .001).
- Increased levels of log2 GHR and log2 IGFBP-1 were associated with higher odds of glycemic failure (OR, 1.75; P = .04 and OR, 1.37; P = .007, respectively). Results were adjusted for body mass index (BMI), suggesting that associations between GHR level and glycemic outcomes exist independent of BMI.
- Interhormonal correlations suggested an association between glucose metabolism and growth hormone signaling or a shared process leading to changes in both processes.
IN PRACTICE:
“Our study has identified GHR level as a novel biomarker of decrease in glycemic control in youths with T2D,” the study authors wrote. Future research is needed, with an emphasis on assessing alterations in growth hormone mediators which may contribute to diabetes complications in youth.
SOURCE:
The study, published online in JAMA Network Open, was led by Chang Lu, MD, Division of Endocrinology, Boston Children’s Hospital, and Joslin Diabetes Center at Harvard Medical School, Boston, Massachusetts.
LIMITATIONS:
The study did not include a control group (individuals without diabetes). The cohort largely included youth in late puberty or after puberty, affecting subgroup analysis. Moreover, only circulating growth hormone mediators were measured, limiting the identity of the source tissue of the hormone and the target organs.
DISCLOSURES:
Some authors reported receiving grants from the National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases while conducting the study. Also, certain authors reported receiving grants and personal fees from various trusts as well as pharmaceutical, healthcare, and medical technology companies outside the submitted work.
TOPLINE:
Changes in plasma growth hormone mediators such as growth hormone receptor (GHR) and insulin-like growth factor-binding protein 1 (IGFBP-1) were associated with glycemic failure in youth-onset type 2 diabetes (T2D), an analysis of the TODAY trial showed.
METHODOLOGY:
- In youth, T2D often occurs during or after puberty, hinting at hormonal influences in the development and/or progression of the disease.
- This secondary analysis assessed the role of growth hormone mediators including insulin-like growth factor-1 (IGF-1), GHR, and IGFBP-1 in glycemic failure in a subset of 398 youths, aged 10-17 years, with a T2D duration of less than 2 years (62% girls; 21% White).
- The participants were followed up for a mean of 3.9 years.
- The primary outcomes included glycemic failure, defined as an A1c level of 8% or more for 6 months, or acute metabolic decompensation requiring insulin.
- Other assessments included baseline and 36-month measures of glycemia, insulin sensitivity, high molecular weight adiponectin, and beta cell function.
TAKEAWAY:
- Of 398 participants, 182 (46%) experienced glycemic failure, while 216 (54%) retained glycemic control.
- At 36 months, youths with glycemic failure had lower IGF-1 levels (P < .001) and higher log2 GHR (P = .03) and log2 IGFBP-1 (P = .009) levels than those who maintained glycemic control.
- A greater increase in IGF-1 level at 36 months was associated with lower odds of glycemic failure (odds ratio [OR], 0.995; P < .001).
- Increased levels of log2 GHR and log2 IGFBP-1 were associated with higher odds of glycemic failure (OR, 1.75; P = .04 and OR, 1.37; P = .007, respectively). Results were adjusted for body mass index (BMI), suggesting that associations between GHR level and glycemic outcomes exist independent of BMI.
- Interhormonal correlations suggested an association between glucose metabolism and growth hormone signaling or a shared process leading to changes in both processes.
IN PRACTICE:
“Our study has identified GHR level as a novel biomarker of decrease in glycemic control in youths with T2D,” the study authors wrote. Future research is needed, with an emphasis on assessing alterations in growth hormone mediators which may contribute to diabetes complications in youth.
SOURCE:
The study, published online in JAMA Network Open, was led by Chang Lu, MD, Division of Endocrinology, Boston Children’s Hospital, and Joslin Diabetes Center at Harvard Medical School, Boston, Massachusetts.
LIMITATIONS:
The study did not include a control group (individuals without diabetes). The cohort largely included youth in late puberty or after puberty, affecting subgroup analysis. Moreover, only circulating growth hormone mediators were measured, limiting the identity of the source tissue of the hormone and the target organs.
DISCLOSURES:
Some authors reported receiving grants from the National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases while conducting the study. Also, certain authors reported receiving grants and personal fees from various trusts as well as pharmaceutical, healthcare, and medical technology companies outside the submitted work.
TOPLINE:
Changes in plasma growth hormone mediators such as growth hormone receptor (GHR) and insulin-like growth factor-binding protein 1 (IGFBP-1) were associated with glycemic failure in youth-onset type 2 diabetes (T2D), an analysis of the TODAY trial showed.
METHODOLOGY:
- In youth, T2D often occurs during or after puberty, hinting at hormonal influences in the development and/or progression of the disease.
- This secondary analysis assessed the role of growth hormone mediators including insulin-like growth factor-1 (IGF-1), GHR, and IGFBP-1 in glycemic failure in a subset of 398 youths, aged 10-17 years, with a T2D duration of less than 2 years (62% girls; 21% White).
- The participants were followed up for a mean of 3.9 years.
- The primary outcomes included glycemic failure, defined as an A1c level of 8% or more for 6 months, or acute metabolic decompensation requiring insulin.
- Other assessments included baseline and 36-month measures of glycemia, insulin sensitivity, high molecular weight adiponectin, and beta cell function.
TAKEAWAY:
- Of 398 participants, 182 (46%) experienced glycemic failure, while 216 (54%) retained glycemic control.
- At 36 months, youths with glycemic failure had lower IGF-1 levels (P < .001) and higher log2 GHR (P = .03) and log2 IGFBP-1 (P = .009) levels than those who maintained glycemic control.
- A greater increase in IGF-1 level at 36 months was associated with lower odds of glycemic failure (odds ratio [OR], 0.995; P < .001).
- Increased levels of log2 GHR and log2 IGFBP-1 were associated with higher odds of glycemic failure (OR, 1.75; P = .04 and OR, 1.37; P = .007, respectively). Results were adjusted for body mass index (BMI), suggesting that associations between GHR level and glycemic outcomes exist independent of BMI.
- Interhormonal correlations suggested an association between glucose metabolism and growth hormone signaling or a shared process leading to changes in both processes.
IN PRACTICE:
“Our study has identified GHR level as a novel biomarker of decrease in glycemic control in youths with T2D,” the study authors wrote. Future research is needed, with an emphasis on assessing alterations in growth hormone mediators which may contribute to diabetes complications in youth.
SOURCE:
The study, published online in JAMA Network Open, was led by Chang Lu, MD, Division of Endocrinology, Boston Children’s Hospital, and Joslin Diabetes Center at Harvard Medical School, Boston, Massachusetts.
LIMITATIONS:
The study did not include a control group (individuals without diabetes). The cohort largely included youth in late puberty or after puberty, affecting subgroup analysis. Moreover, only circulating growth hormone mediators were measured, limiting the identity of the source tissue of the hormone and the target organs.
DISCLOSURES:
Some authors reported receiving grants from the National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases while conducting the study. Also, certain authors reported receiving grants and personal fees from various trusts as well as pharmaceutical, healthcare, and medical technology companies outside the submitted work.