User login
In the Story of the Rubella Virus as a Source of Granulomas, the Plot Is Still Thickening
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
FROM AAD 2024
Gene Therapy for Dystrophic EB: Extension Study Results Reported
and no new safety signals were identified.
The results were presented by Amy S. Paller, MD, during a late-breaking session at the annual meeting of the American Academy of Dermatology.
In May 2023, beremagene geperpavec, marketed as Vyjuvek (formerly known as B-VEC) was approved by the US Food and Drug Administration (FDA) for the treatment of wounds in patients 6 months of age and older with DEB, a rare genetic blistering disorder caused by COL7A1 gene variants. The therapy uses a nonreplicating herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells, restoring the COL7 protein fibrils that stabilize skin structure. It is designed to be used repetitively, to heal a single wound, or on more than one wound.
In the pivotal study of patients with DEB, the gene therapy, delivered in a topical gel, was administered once a week for 6 months to one wound and placebo was applied to another wound for each participant. The proportion of wounds treated with beremagene geperpavec that healed was significantly higher than among placebo-treated wounds at 3 and 6 months (68% vs. 23% at 3 months, P = .003) and 65% vs. 26% at 6 months (P = .012), with no serious adverse events related to treatment.
The prospective, open label, uncontrolled extension study included 24 patients from the phase 3 study and 23 treatment-naive patients from five US sites. Their mean age was 16 years (range, 6 months to 46 years).
Of the 47 patients, 29 (62%) were on treatment for more than 1 year (the longest was about 2 years), and the mean duration of treatment was 475 days; 5 patients withdrew from the study for reasons not related to treatment.
Their types of adverse events (AEs) were similar to those seen in the phase 3 study and were consistent with what would be expected in patients with DEB, said Dr. Paller, professor and chair of dermatology, Northwestern University, Chicago. One patient experienced two wound hemorrhages that were possibly related to treatment, but there were no treatment-related AEs, no deaths or treatment discontinuations because of an AE, and no serious AEs thought to be related to treatment.
Wounds that were evaluated in the phase 3 study showed “a high durability of closure with continued treatment,” according to Dr. Paller. There were enough data on 19 of the 24 patients who had been in the phase 3 trial to evaluate wound closure, defined as “complete wound closure based on comparison to the exact wound area selected at baseline” at the beginning of the phase 3 study.
In the extension study, wound closure rates were almost 90% at baseline, 84.2% at 3 months, 61.1% at 6 months, 82.4% at 9 months, and 62.5% at 12 months, which was comparable to the rates observed in the third (86.4%) and sixth (73.7%) months of the phase 3 study, Dr. Paller said.
Patient-reported outcomes indicated that quality of life and satisfaction with treatment were preserved with continued treatment.The extension study was terminated in July 2023, after FDA approval, when patients could be transitioned to the commercially available treatment.Dr. Paller disclosed being an investigator (funds to institution) for multiple pharmaceutical companies, including the manufacturer of beremagene geperpavec, Krystal Biotech, which funded the study.
and no new safety signals were identified.
The results were presented by Amy S. Paller, MD, during a late-breaking session at the annual meeting of the American Academy of Dermatology.
In May 2023, beremagene geperpavec, marketed as Vyjuvek (formerly known as B-VEC) was approved by the US Food and Drug Administration (FDA) for the treatment of wounds in patients 6 months of age and older with DEB, a rare genetic blistering disorder caused by COL7A1 gene variants. The therapy uses a nonreplicating herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells, restoring the COL7 protein fibrils that stabilize skin structure. It is designed to be used repetitively, to heal a single wound, or on more than one wound.
In the pivotal study of patients with DEB, the gene therapy, delivered in a topical gel, was administered once a week for 6 months to one wound and placebo was applied to another wound for each participant. The proportion of wounds treated with beremagene geperpavec that healed was significantly higher than among placebo-treated wounds at 3 and 6 months (68% vs. 23% at 3 months, P = .003) and 65% vs. 26% at 6 months (P = .012), with no serious adverse events related to treatment.
The prospective, open label, uncontrolled extension study included 24 patients from the phase 3 study and 23 treatment-naive patients from five US sites. Their mean age was 16 years (range, 6 months to 46 years).
Of the 47 patients, 29 (62%) were on treatment for more than 1 year (the longest was about 2 years), and the mean duration of treatment was 475 days; 5 patients withdrew from the study for reasons not related to treatment.
Their types of adverse events (AEs) were similar to those seen in the phase 3 study and were consistent with what would be expected in patients with DEB, said Dr. Paller, professor and chair of dermatology, Northwestern University, Chicago. One patient experienced two wound hemorrhages that were possibly related to treatment, but there were no treatment-related AEs, no deaths or treatment discontinuations because of an AE, and no serious AEs thought to be related to treatment.
Wounds that were evaluated in the phase 3 study showed “a high durability of closure with continued treatment,” according to Dr. Paller. There were enough data on 19 of the 24 patients who had been in the phase 3 trial to evaluate wound closure, defined as “complete wound closure based on comparison to the exact wound area selected at baseline” at the beginning of the phase 3 study.
In the extension study, wound closure rates were almost 90% at baseline, 84.2% at 3 months, 61.1% at 6 months, 82.4% at 9 months, and 62.5% at 12 months, which was comparable to the rates observed in the third (86.4%) and sixth (73.7%) months of the phase 3 study, Dr. Paller said.
Patient-reported outcomes indicated that quality of life and satisfaction with treatment were preserved with continued treatment.The extension study was terminated in July 2023, after FDA approval, when patients could be transitioned to the commercially available treatment.Dr. Paller disclosed being an investigator (funds to institution) for multiple pharmaceutical companies, including the manufacturer of beremagene geperpavec, Krystal Biotech, which funded the study.
and no new safety signals were identified.
The results were presented by Amy S. Paller, MD, during a late-breaking session at the annual meeting of the American Academy of Dermatology.
In May 2023, beremagene geperpavec, marketed as Vyjuvek (formerly known as B-VEC) was approved by the US Food and Drug Administration (FDA) for the treatment of wounds in patients 6 months of age and older with DEB, a rare genetic blistering disorder caused by COL7A1 gene variants. The therapy uses a nonreplicating herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells, restoring the COL7 protein fibrils that stabilize skin structure. It is designed to be used repetitively, to heal a single wound, or on more than one wound.
In the pivotal study of patients with DEB, the gene therapy, delivered in a topical gel, was administered once a week for 6 months to one wound and placebo was applied to another wound for each participant. The proportion of wounds treated with beremagene geperpavec that healed was significantly higher than among placebo-treated wounds at 3 and 6 months (68% vs. 23% at 3 months, P = .003) and 65% vs. 26% at 6 months (P = .012), with no serious adverse events related to treatment.
The prospective, open label, uncontrolled extension study included 24 patients from the phase 3 study and 23 treatment-naive patients from five US sites. Their mean age was 16 years (range, 6 months to 46 years).
Of the 47 patients, 29 (62%) were on treatment for more than 1 year (the longest was about 2 years), and the mean duration of treatment was 475 days; 5 patients withdrew from the study for reasons not related to treatment.
Their types of adverse events (AEs) were similar to those seen in the phase 3 study and were consistent with what would be expected in patients with DEB, said Dr. Paller, professor and chair of dermatology, Northwestern University, Chicago. One patient experienced two wound hemorrhages that were possibly related to treatment, but there were no treatment-related AEs, no deaths or treatment discontinuations because of an AE, and no serious AEs thought to be related to treatment.
Wounds that were evaluated in the phase 3 study showed “a high durability of closure with continued treatment,” according to Dr. Paller. There were enough data on 19 of the 24 patients who had been in the phase 3 trial to evaluate wound closure, defined as “complete wound closure based on comparison to the exact wound area selected at baseline” at the beginning of the phase 3 study.
In the extension study, wound closure rates were almost 90% at baseline, 84.2% at 3 months, 61.1% at 6 months, 82.4% at 9 months, and 62.5% at 12 months, which was comparable to the rates observed in the third (86.4%) and sixth (73.7%) months of the phase 3 study, Dr. Paller said.
Patient-reported outcomes indicated that quality of life and satisfaction with treatment were preserved with continued treatment.The extension study was terminated in July 2023, after FDA approval, when patients could be transitioned to the commercially available treatment.Dr. Paller disclosed being an investigator (funds to institution) for multiple pharmaceutical companies, including the manufacturer of beremagene geperpavec, Krystal Biotech, which funded the study.
FROM AAD 2024
FDA Requests More Information for RDEB Rx Under Review
The Food and Drug Administration (RDEB), requesting more information from the manufacturer.
Pz-cel, which comprises autologous, COL7A1 gene–corrected epidermal sheets, is being evaluated for its ability to enable normal type VII collagen expression in a patient’s skin cells and to facilitate wound healing and pain reduction in wounds in patients with RDEB after a one-time application procedure. The cause of RDEB is a defect in the COL7A1 gene that “results in the inability to produce type VII collagen,” a press release from the manufacturer noted.
On April 22, 2024, the manufacturer Abeona Therapeutics announced that following a meeting with the FDA in March and in a subsequent request for information, the agency requires additional information to satisfy certain Chemistry Manufacturing and Controls requirements before the BLA for pz-cel can be approved. According to a press release from the company, the information pertains to validation requirements for certain manufacturing and release testing methods, including some that were observed during the FDA’s pre-licensing inspection.
The complete response letter did not identify any issues related to the clinical efficacy or safety data in the BLA, and the FDA did not request any new clinical trials or clinical data to support approval, according to the company.
The company anticipates completing the BLA resubmission in the third quarter of 2024. The application is supported by clinical efficacy and safety data from the pivotal phase 3 VIITAL study and a phase 1/2a study in patients with RDEB.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (RDEB), requesting more information from the manufacturer.
Pz-cel, which comprises autologous, COL7A1 gene–corrected epidermal sheets, is being evaluated for its ability to enable normal type VII collagen expression in a patient’s skin cells and to facilitate wound healing and pain reduction in wounds in patients with RDEB after a one-time application procedure. The cause of RDEB is a defect in the COL7A1 gene that “results in the inability to produce type VII collagen,” a press release from the manufacturer noted.
On April 22, 2024, the manufacturer Abeona Therapeutics announced that following a meeting with the FDA in March and in a subsequent request for information, the agency requires additional information to satisfy certain Chemistry Manufacturing and Controls requirements before the BLA for pz-cel can be approved. According to a press release from the company, the information pertains to validation requirements for certain manufacturing and release testing methods, including some that were observed during the FDA’s pre-licensing inspection.
The complete response letter did not identify any issues related to the clinical efficacy or safety data in the BLA, and the FDA did not request any new clinical trials or clinical data to support approval, according to the company.
The company anticipates completing the BLA resubmission in the third quarter of 2024. The application is supported by clinical efficacy and safety data from the pivotal phase 3 VIITAL study and a phase 1/2a study in patients with RDEB.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (RDEB), requesting more information from the manufacturer.
Pz-cel, which comprises autologous, COL7A1 gene–corrected epidermal sheets, is being evaluated for its ability to enable normal type VII collagen expression in a patient’s skin cells and to facilitate wound healing and pain reduction in wounds in patients with RDEB after a one-time application procedure. The cause of RDEB is a defect in the COL7A1 gene that “results in the inability to produce type VII collagen,” a press release from the manufacturer noted.
On April 22, 2024, the manufacturer Abeona Therapeutics announced that following a meeting with the FDA in March and in a subsequent request for information, the agency requires additional information to satisfy certain Chemistry Manufacturing and Controls requirements before the BLA for pz-cel can be approved. According to a press release from the company, the information pertains to validation requirements for certain manufacturing and release testing methods, including some that were observed during the FDA’s pre-licensing inspection.
The complete response letter did not identify any issues related to the clinical efficacy or safety data in the BLA, and the FDA did not request any new clinical trials or clinical data to support approval, according to the company.
The company anticipates completing the BLA resubmission in the third quarter of 2024. The application is supported by clinical efficacy and safety data from the pivotal phase 3 VIITAL study and a phase 1/2a study in patients with RDEB.
A version of this article first appeared on Medscape.com.
Children With Chronic Skin Disorders Face Substantial Stigma
TOPLINE:
METHODOLOGY:
- Stigmatization has been addressed for several chronic medical conditions, such as HIV/AIDS, obesity, and mental illness; however, it has received limited attention in children living with chronic skin disorders.
- This cross-sectional, single-visit study examined the prevalence of stigma, its dependence on disease visibility and severity, and its association with mental health and QoL in children with chronic skin disorders.
- A total of 1671 children aged 8-17 years (57.9% girls; mean age, 13.7 years) were recruited from 32 pediatric dermatology centers in the United States and Canada from November 2018 to November 2021. The most common conditions were acne, atopic dermatitis/eczematous disorders, alopecia, and psoriasis, but rare genetic disorders were also represented.
- The primary outcome was the extent of stigmatization in relation to disease visibility, assessed using the Patient-Reported Outcomes Measurement Instrumentation System Pediatric Stigma-Skin.
- Secondary outcomes were the extent of stigmatization in relation to disease severity, along with QoL, depression, anxiety, and poor peer relationships.
TAKEAWAY:
- Approximately half (56.4%) of the children self-reported their skin condition as highly visible; 50.5% reported their disease severity as moderate, while 21.3% reported it as severe.
- Stigma was experienced by 73% of children and adolescents with chronic skin disease, with 43.8% reporting moderate stigma.
- Stigma scores correlated strongly with impaired QOL (Spearman’s rank correlation coefficient = 0.73) and child-reported scores for depression (Spearman’s rank correlation coefficient = 0.61) and moderately with anxiety (Spearman’s rank correlation coefficient = 0.54) and peer relationships (Spearman’s rank correlation coefficient = −0.49; all P < .001).
- Although stigma is increased for children with higher disease visibility and severity, the relatively weak correlation between child-assessed disease visibility and stigma (Spearman’s rank correlation coefficient = 0.22) showed that stigma is common in children even when diseases are not highly visible.
IN PRACTICE:
“Better treatment approaches for chronic skin diseases in children remain an unmet need. Increased awareness and instituting medical and psychological interventions to identify and reduce stigma and disease severity are important directions for improving QOL,” the authors concluded.
SOURCE:
Amy S. Paller, MD, professor of pediatrics and dermatology, Northwestern University, Chicago, led the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Stigmatization needs to be assessed in children from low- and middle-income countries. Investigators enrolled children who had physician-assessed moderate to severe disease severity and/or at least some visibility of skin disease while wearing clothing, which resulted in exclusion of children with mild chronic disease, and the pandemic limited enrollment.
DISCLOSURES:
This study was funded through a grant from the Pediatric Dermatology Research Alliance (PeDRA). The authors declared receiving grants, personal fees, and honorarium and having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Stigmatization has been addressed for several chronic medical conditions, such as HIV/AIDS, obesity, and mental illness; however, it has received limited attention in children living with chronic skin disorders.
- This cross-sectional, single-visit study examined the prevalence of stigma, its dependence on disease visibility and severity, and its association with mental health and QoL in children with chronic skin disorders.
- A total of 1671 children aged 8-17 years (57.9% girls; mean age, 13.7 years) were recruited from 32 pediatric dermatology centers in the United States and Canada from November 2018 to November 2021. The most common conditions were acne, atopic dermatitis/eczematous disorders, alopecia, and psoriasis, but rare genetic disorders were also represented.
- The primary outcome was the extent of stigmatization in relation to disease visibility, assessed using the Patient-Reported Outcomes Measurement Instrumentation System Pediatric Stigma-Skin.
- Secondary outcomes were the extent of stigmatization in relation to disease severity, along with QoL, depression, anxiety, and poor peer relationships.
TAKEAWAY:
- Approximately half (56.4%) of the children self-reported their skin condition as highly visible; 50.5% reported their disease severity as moderate, while 21.3% reported it as severe.
- Stigma was experienced by 73% of children and adolescents with chronic skin disease, with 43.8% reporting moderate stigma.
- Stigma scores correlated strongly with impaired QOL (Spearman’s rank correlation coefficient = 0.73) and child-reported scores for depression (Spearman’s rank correlation coefficient = 0.61) and moderately with anxiety (Spearman’s rank correlation coefficient = 0.54) and peer relationships (Spearman’s rank correlation coefficient = −0.49; all P < .001).
- Although stigma is increased for children with higher disease visibility and severity, the relatively weak correlation between child-assessed disease visibility and stigma (Spearman’s rank correlation coefficient = 0.22) showed that stigma is common in children even when diseases are not highly visible.
IN PRACTICE:
“Better treatment approaches for chronic skin diseases in children remain an unmet need. Increased awareness and instituting medical and psychological interventions to identify and reduce stigma and disease severity are important directions for improving QOL,” the authors concluded.
SOURCE:
Amy S. Paller, MD, professor of pediatrics and dermatology, Northwestern University, Chicago, led the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Stigmatization needs to be assessed in children from low- and middle-income countries. Investigators enrolled children who had physician-assessed moderate to severe disease severity and/or at least some visibility of skin disease while wearing clothing, which resulted in exclusion of children with mild chronic disease, and the pandemic limited enrollment.
DISCLOSURES:
This study was funded through a grant from the Pediatric Dermatology Research Alliance (PeDRA). The authors declared receiving grants, personal fees, and honorarium and having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Stigmatization has been addressed for several chronic medical conditions, such as HIV/AIDS, obesity, and mental illness; however, it has received limited attention in children living with chronic skin disorders.
- This cross-sectional, single-visit study examined the prevalence of stigma, its dependence on disease visibility and severity, and its association with mental health and QoL in children with chronic skin disorders.
- A total of 1671 children aged 8-17 years (57.9% girls; mean age, 13.7 years) were recruited from 32 pediatric dermatology centers in the United States and Canada from November 2018 to November 2021. The most common conditions were acne, atopic dermatitis/eczematous disorders, alopecia, and psoriasis, but rare genetic disorders were also represented.
- The primary outcome was the extent of stigmatization in relation to disease visibility, assessed using the Patient-Reported Outcomes Measurement Instrumentation System Pediatric Stigma-Skin.
- Secondary outcomes were the extent of stigmatization in relation to disease severity, along with QoL, depression, anxiety, and poor peer relationships.
TAKEAWAY:
- Approximately half (56.4%) of the children self-reported their skin condition as highly visible; 50.5% reported their disease severity as moderate, while 21.3% reported it as severe.
- Stigma was experienced by 73% of children and adolescents with chronic skin disease, with 43.8% reporting moderate stigma.
- Stigma scores correlated strongly with impaired QOL (Spearman’s rank correlation coefficient = 0.73) and child-reported scores for depression (Spearman’s rank correlation coefficient = 0.61) and moderately with anxiety (Spearman’s rank correlation coefficient = 0.54) and peer relationships (Spearman’s rank correlation coefficient = −0.49; all P < .001).
- Although stigma is increased for children with higher disease visibility and severity, the relatively weak correlation between child-assessed disease visibility and stigma (Spearman’s rank correlation coefficient = 0.22) showed that stigma is common in children even when diseases are not highly visible.
IN PRACTICE:
“Better treatment approaches for chronic skin diseases in children remain an unmet need. Increased awareness and instituting medical and psychological interventions to identify and reduce stigma and disease severity are important directions for improving QOL,” the authors concluded.
SOURCE:
Amy S. Paller, MD, professor of pediatrics and dermatology, Northwestern University, Chicago, led the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Stigmatization needs to be assessed in children from low- and middle-income countries. Investigators enrolled children who had physician-assessed moderate to severe disease severity and/or at least some visibility of skin disease while wearing clothing, which resulted in exclusion of children with mild chronic disease, and the pandemic limited enrollment.
DISCLOSURES:
This study was funded through a grant from the Pediatric Dermatology Research Alliance (PeDRA). The authors declared receiving grants, personal fees, and honorarium and having other ties with various sources.
A version of this article appeared on Medscape.com.
New British Behçet’s Disease Guidelines Emphasize Multidisciplinary Management
LIVERPOOL, ENGLAND — The British Society for Rheumatology (BSR) and the British Association of Dermatologists (BAD) have joined forces for the first time to develop the first British guidelines for the management of people living with Behçet’s disease.
The guidelines will also be the first “living guidelines” produced by either society, which means they will be regularly revised and updated when new evidence emerges that warrants inclusion.
With more than 90 recommendations being made, the new guidelines promise to be the most comprehensive and most up-to-date yet for what is regarded as a rare disease. Robert Moots, MBBS, PhD, provided a “sneak peek” of the guidelines at the annual meeting of the British Society for Rheumatology.
Dr. Moots, professor of rheumatology at the University of Liverpool and a consultant rheumatologist for Liverpool University Hospitals NHS Foundation Trust in England, noted that while the European Alliance of Associations for Rheumatology has produced a guideline for Behçet’s disease, this was last updated in 2018 and is not specific for the population for patients that is seen in the United Kingdom.
The British recommendations will cover all possible manifestations of Behçet’s disease and give practical advice on how to manage everything from the most common presentations such as skin lesions, mouth ulcers, and genital ulcers, as well as the potentially more serious eye, neurological, and vascular involvement.
Importance of Raising Awareness
“Joint and musculoskeletal problems are actually one of the least complained of symptoms in people with Behçet’s, and they often can’t understand why a rheumatologist is seeing them,” Dr. Moot said. “But of course, people do get joint problems, they can get enthesitis and arthralgia.”
Dr. Moots has been leading one of the three National Health Service (NHS) Centres of Excellence for Behçet’s Syndrome in England for more than a decade and told this news organization that diagnosing patients could be challenging. It can take up to 10 years from the first symptoms appearing to getting a diagnosis, so part of the job of the NHS Centres of Excellence is to raise awareness among both the healthcare profession and the general public.
“It’s a condition that people learn about at medical school. Most doctors will have come across it, but because it was thought to be really rare in the UK, nobody perhaps really expects to see it,” Dr. Moot said.
“But we all have these patients,” he added. “In Liverpool, we’re commissioned to be looking after an anticipated 150 people with Behçet’s — we’ve got 700. With more awareness, there’s more diagnoses being made, and people are being looked after better.”
Patient Perspective
Tony Thornburn, OBE, chair of the patient advocacy group Behçet’s UK, agreed in a separate interview that raising awareness of the syndrome was key to improving its management.
“Patients have said that it is a bit like having arthritis, lupus, MS [multiple sclerosis], and Crohn’s [disease] all at once,” Mr. Thorburn said. “So what we need is a guideline to ensure that people know what they’re looking at.”
Mr. Thorburn added, “Guidelines are important for raising awareness but also providing the detailed information that clinicians and GPs [general practitioners] need to have to treat a patient when they come in with this multifaceted condition.”
Multifaceted Means Multidisciplinary Management
Because there can be so many different aspects to managing someone with Behçet’s disease, a multispecialty team that was convened to develop the guidelines agreed that multidisciplinary management should be an overarching theme.
“The guideline development group consisted of all the specialties that you would need for a complex multisystem disease like Behçet’s,” Dr. Moot said. He highlighted that working alongside the consultants in adult and pediatric rheumatology were specialists in dermatology, gastroenterology, neurology, ophthalmology, obstetrics and gynecology, and psychology.
“We’re actually looking at psychological interactions and their impact for the first time,” Dr. Moot said, noting that clinicians needed to “take it seriously, and ask about it.”
Management of Manifestations
One of the general principles of the guidelines is to assess the involvement of each organ system and target treatment accordingly.
“One of the problems is that the evidence base to tell us what to do is pretty low,” Dr. Moots acknowledged. There have been few good quality randomized trials, so “treatment tends to be eminence-based rather than evidence-based.”
The recommendation wording bears this in mind, stating whether a treatment should or should not be offered, or just considered if there is no strong evidence to back up its use.
With regard to musculoskeletal manifestations, the recommendations say that colchicine should be offered, perhaps as a first-line option, or an intraarticular steroid injection in the case of monoarthritis. An intramuscular depot steroid may also be appropriate to offer, and there was good evidence to offer azathioprine or, as an alternative in refractory cases, a tumor necrosis factor (TNF) inhibitor. Nonsteroidal anti-inflammatory drugs, methotrexate, apremilast, secukinumab, and referral to a physiotherapist could only be considered, however, based on weaker levels of evidence for their use.
To treat mucocutaneous disease, the guidelines advise offering topical steroids in the form of ointment for genital ulcers or mouthwash or ointment for oral ulcers. For skin lesions, it is recommended to offer colchicine, azathioprine, mycophenolate mofetil, or TNF inhibitor and to consider the use of apremilast, secukinumab, or dapsone.
Future Work and Revision
“One of the key things we would like to see developing is a national registry,” Dr. Moots said. This would include biobanking samples for future research and possible genomic and phenotyping studies.
More work needs to be done in conducting clinical trials in children and young people with Behçet’s disease, studies to find prognostic factors for neurological disease, and clinical trials of potential new drug approaches such as Janus kinase inhibitors. Importantly, an auditing process needs to be set up to see what effect, if any, the guidelines will actually have onpatient management.
“It’s taken 5 years to today” to develop the guidelines, Dr. Moot said. What form the process of updating them will take still has to be decided, he said in the interview. It is likely that the necessary literature searches will be performed every 6 months or so, but it will be a compromise between the ideal situation and having the staffing time to do it.
“It’s a big ask,” Dr. Moot acknowledged, adding that even if updates were only once a year, it would still be much faster than the 5- or 6-year cycle that it traditionally takes for most guidelines to be updated.
The BSR and BAD’s processes for developing guidelines are accredited by the National Institute for Health and Care Excellence in England. Dr. Moots is the chief investigator for the Secukinumab in Behçet’s trial, which is sponsored by the Liverpool University Hospitals NHS Foundation Trust via grant funding from Novartis.
A version of this article appeared on Medscape.com.
LIVERPOOL, ENGLAND — The British Society for Rheumatology (BSR) and the British Association of Dermatologists (BAD) have joined forces for the first time to develop the first British guidelines for the management of people living with Behçet’s disease.
The guidelines will also be the first “living guidelines” produced by either society, which means they will be regularly revised and updated when new evidence emerges that warrants inclusion.
With more than 90 recommendations being made, the new guidelines promise to be the most comprehensive and most up-to-date yet for what is regarded as a rare disease. Robert Moots, MBBS, PhD, provided a “sneak peek” of the guidelines at the annual meeting of the British Society for Rheumatology.
Dr. Moots, professor of rheumatology at the University of Liverpool and a consultant rheumatologist for Liverpool University Hospitals NHS Foundation Trust in England, noted that while the European Alliance of Associations for Rheumatology has produced a guideline for Behçet’s disease, this was last updated in 2018 and is not specific for the population for patients that is seen in the United Kingdom.
The British recommendations will cover all possible manifestations of Behçet’s disease and give practical advice on how to manage everything from the most common presentations such as skin lesions, mouth ulcers, and genital ulcers, as well as the potentially more serious eye, neurological, and vascular involvement.
Importance of Raising Awareness
“Joint and musculoskeletal problems are actually one of the least complained of symptoms in people with Behçet’s, and they often can’t understand why a rheumatologist is seeing them,” Dr. Moot said. “But of course, people do get joint problems, they can get enthesitis and arthralgia.”
Dr. Moots has been leading one of the three National Health Service (NHS) Centres of Excellence for Behçet’s Syndrome in England for more than a decade and told this news organization that diagnosing patients could be challenging. It can take up to 10 years from the first symptoms appearing to getting a diagnosis, so part of the job of the NHS Centres of Excellence is to raise awareness among both the healthcare profession and the general public.
“It’s a condition that people learn about at medical school. Most doctors will have come across it, but because it was thought to be really rare in the UK, nobody perhaps really expects to see it,” Dr. Moot said.
“But we all have these patients,” he added. “In Liverpool, we’re commissioned to be looking after an anticipated 150 people with Behçet’s — we’ve got 700. With more awareness, there’s more diagnoses being made, and people are being looked after better.”
Patient Perspective
Tony Thornburn, OBE, chair of the patient advocacy group Behçet’s UK, agreed in a separate interview that raising awareness of the syndrome was key to improving its management.
“Patients have said that it is a bit like having arthritis, lupus, MS [multiple sclerosis], and Crohn’s [disease] all at once,” Mr. Thorburn said. “So what we need is a guideline to ensure that people know what they’re looking at.”
Mr. Thorburn added, “Guidelines are important for raising awareness but also providing the detailed information that clinicians and GPs [general practitioners] need to have to treat a patient when they come in with this multifaceted condition.”
Multifaceted Means Multidisciplinary Management
Because there can be so many different aspects to managing someone with Behçet’s disease, a multispecialty team that was convened to develop the guidelines agreed that multidisciplinary management should be an overarching theme.
“The guideline development group consisted of all the specialties that you would need for a complex multisystem disease like Behçet’s,” Dr. Moot said. He highlighted that working alongside the consultants in adult and pediatric rheumatology were specialists in dermatology, gastroenterology, neurology, ophthalmology, obstetrics and gynecology, and psychology.
“We’re actually looking at psychological interactions and their impact for the first time,” Dr. Moot said, noting that clinicians needed to “take it seriously, and ask about it.”
Management of Manifestations
One of the general principles of the guidelines is to assess the involvement of each organ system and target treatment accordingly.
“One of the problems is that the evidence base to tell us what to do is pretty low,” Dr. Moots acknowledged. There have been few good quality randomized trials, so “treatment tends to be eminence-based rather than evidence-based.”
The recommendation wording bears this in mind, stating whether a treatment should or should not be offered, or just considered if there is no strong evidence to back up its use.
With regard to musculoskeletal manifestations, the recommendations say that colchicine should be offered, perhaps as a first-line option, or an intraarticular steroid injection in the case of monoarthritis. An intramuscular depot steroid may also be appropriate to offer, and there was good evidence to offer azathioprine or, as an alternative in refractory cases, a tumor necrosis factor (TNF) inhibitor. Nonsteroidal anti-inflammatory drugs, methotrexate, apremilast, secukinumab, and referral to a physiotherapist could only be considered, however, based on weaker levels of evidence for their use.
To treat mucocutaneous disease, the guidelines advise offering topical steroids in the form of ointment for genital ulcers or mouthwash or ointment for oral ulcers. For skin lesions, it is recommended to offer colchicine, azathioprine, mycophenolate mofetil, or TNF inhibitor and to consider the use of apremilast, secukinumab, or dapsone.
Future Work and Revision
“One of the key things we would like to see developing is a national registry,” Dr. Moots said. This would include biobanking samples for future research and possible genomic and phenotyping studies.
More work needs to be done in conducting clinical trials in children and young people with Behçet’s disease, studies to find prognostic factors for neurological disease, and clinical trials of potential new drug approaches such as Janus kinase inhibitors. Importantly, an auditing process needs to be set up to see what effect, if any, the guidelines will actually have onpatient management.
“It’s taken 5 years to today” to develop the guidelines, Dr. Moot said. What form the process of updating them will take still has to be decided, he said in the interview. It is likely that the necessary literature searches will be performed every 6 months or so, but it will be a compromise between the ideal situation and having the staffing time to do it.
“It’s a big ask,” Dr. Moot acknowledged, adding that even if updates were only once a year, it would still be much faster than the 5- or 6-year cycle that it traditionally takes for most guidelines to be updated.
The BSR and BAD’s processes for developing guidelines are accredited by the National Institute for Health and Care Excellence in England. Dr. Moots is the chief investigator for the Secukinumab in Behçet’s trial, which is sponsored by the Liverpool University Hospitals NHS Foundation Trust via grant funding from Novartis.
A version of this article appeared on Medscape.com.
LIVERPOOL, ENGLAND — The British Society for Rheumatology (BSR) and the British Association of Dermatologists (BAD) have joined forces for the first time to develop the first British guidelines for the management of people living with Behçet’s disease.
The guidelines will also be the first “living guidelines” produced by either society, which means they will be regularly revised and updated when new evidence emerges that warrants inclusion.
With more than 90 recommendations being made, the new guidelines promise to be the most comprehensive and most up-to-date yet for what is regarded as a rare disease. Robert Moots, MBBS, PhD, provided a “sneak peek” of the guidelines at the annual meeting of the British Society for Rheumatology.
Dr. Moots, professor of rheumatology at the University of Liverpool and a consultant rheumatologist for Liverpool University Hospitals NHS Foundation Trust in England, noted that while the European Alliance of Associations for Rheumatology has produced a guideline for Behçet’s disease, this was last updated in 2018 and is not specific for the population for patients that is seen in the United Kingdom.
The British recommendations will cover all possible manifestations of Behçet’s disease and give practical advice on how to manage everything from the most common presentations such as skin lesions, mouth ulcers, and genital ulcers, as well as the potentially more serious eye, neurological, and vascular involvement.
Importance of Raising Awareness
“Joint and musculoskeletal problems are actually one of the least complained of symptoms in people with Behçet’s, and they often can’t understand why a rheumatologist is seeing them,” Dr. Moot said. “But of course, people do get joint problems, they can get enthesitis and arthralgia.”
Dr. Moots has been leading one of the three National Health Service (NHS) Centres of Excellence for Behçet’s Syndrome in England for more than a decade and told this news organization that diagnosing patients could be challenging. It can take up to 10 years from the first symptoms appearing to getting a diagnosis, so part of the job of the NHS Centres of Excellence is to raise awareness among both the healthcare profession and the general public.
“It’s a condition that people learn about at medical school. Most doctors will have come across it, but because it was thought to be really rare in the UK, nobody perhaps really expects to see it,” Dr. Moot said.
“But we all have these patients,” he added. “In Liverpool, we’re commissioned to be looking after an anticipated 150 people with Behçet’s — we’ve got 700. With more awareness, there’s more diagnoses being made, and people are being looked after better.”
Patient Perspective
Tony Thornburn, OBE, chair of the patient advocacy group Behçet’s UK, agreed in a separate interview that raising awareness of the syndrome was key to improving its management.
“Patients have said that it is a bit like having arthritis, lupus, MS [multiple sclerosis], and Crohn’s [disease] all at once,” Mr. Thorburn said. “So what we need is a guideline to ensure that people know what they’re looking at.”
Mr. Thorburn added, “Guidelines are important for raising awareness but also providing the detailed information that clinicians and GPs [general practitioners] need to have to treat a patient when they come in with this multifaceted condition.”
Multifaceted Means Multidisciplinary Management
Because there can be so many different aspects to managing someone with Behçet’s disease, a multispecialty team that was convened to develop the guidelines agreed that multidisciplinary management should be an overarching theme.
“The guideline development group consisted of all the specialties that you would need for a complex multisystem disease like Behçet’s,” Dr. Moot said. He highlighted that working alongside the consultants in adult and pediatric rheumatology were specialists in dermatology, gastroenterology, neurology, ophthalmology, obstetrics and gynecology, and psychology.
“We’re actually looking at psychological interactions and their impact for the first time,” Dr. Moot said, noting that clinicians needed to “take it seriously, and ask about it.”
Management of Manifestations
One of the general principles of the guidelines is to assess the involvement of each organ system and target treatment accordingly.
“One of the problems is that the evidence base to tell us what to do is pretty low,” Dr. Moots acknowledged. There have been few good quality randomized trials, so “treatment tends to be eminence-based rather than evidence-based.”
The recommendation wording bears this in mind, stating whether a treatment should or should not be offered, or just considered if there is no strong evidence to back up its use.
With regard to musculoskeletal manifestations, the recommendations say that colchicine should be offered, perhaps as a first-line option, or an intraarticular steroid injection in the case of monoarthritis. An intramuscular depot steroid may also be appropriate to offer, and there was good evidence to offer azathioprine or, as an alternative in refractory cases, a tumor necrosis factor (TNF) inhibitor. Nonsteroidal anti-inflammatory drugs, methotrexate, apremilast, secukinumab, and referral to a physiotherapist could only be considered, however, based on weaker levels of evidence for their use.
To treat mucocutaneous disease, the guidelines advise offering topical steroids in the form of ointment for genital ulcers or mouthwash or ointment for oral ulcers. For skin lesions, it is recommended to offer colchicine, azathioprine, mycophenolate mofetil, or TNF inhibitor and to consider the use of apremilast, secukinumab, or dapsone.
Future Work and Revision
“One of the key things we would like to see developing is a national registry,” Dr. Moots said. This would include biobanking samples for future research and possible genomic and phenotyping studies.
More work needs to be done in conducting clinical trials in children and young people with Behçet’s disease, studies to find prognostic factors for neurological disease, and clinical trials of potential new drug approaches such as Janus kinase inhibitors. Importantly, an auditing process needs to be set up to see what effect, if any, the guidelines will actually have onpatient management.
“It’s taken 5 years to today” to develop the guidelines, Dr. Moot said. What form the process of updating them will take still has to be decided, he said in the interview. It is likely that the necessary literature searches will be performed every 6 months or so, but it will be a compromise between the ideal situation and having the staffing time to do it.
“It’s a big ask,” Dr. Moot acknowledged, adding that even if updates were only once a year, it would still be much faster than the 5- or 6-year cycle that it traditionally takes for most guidelines to be updated.
The BSR and BAD’s processes for developing guidelines are accredited by the National Institute for Health and Care Excellence in England. Dr. Moots is the chief investigator for the Secukinumab in Behçet’s trial, which is sponsored by the Liverpool University Hospitals NHS Foundation Trust via grant funding from Novartis.
A version of this article appeared on Medscape.com.
FROM BSR 2024
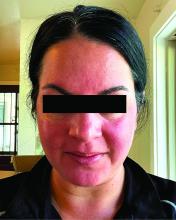
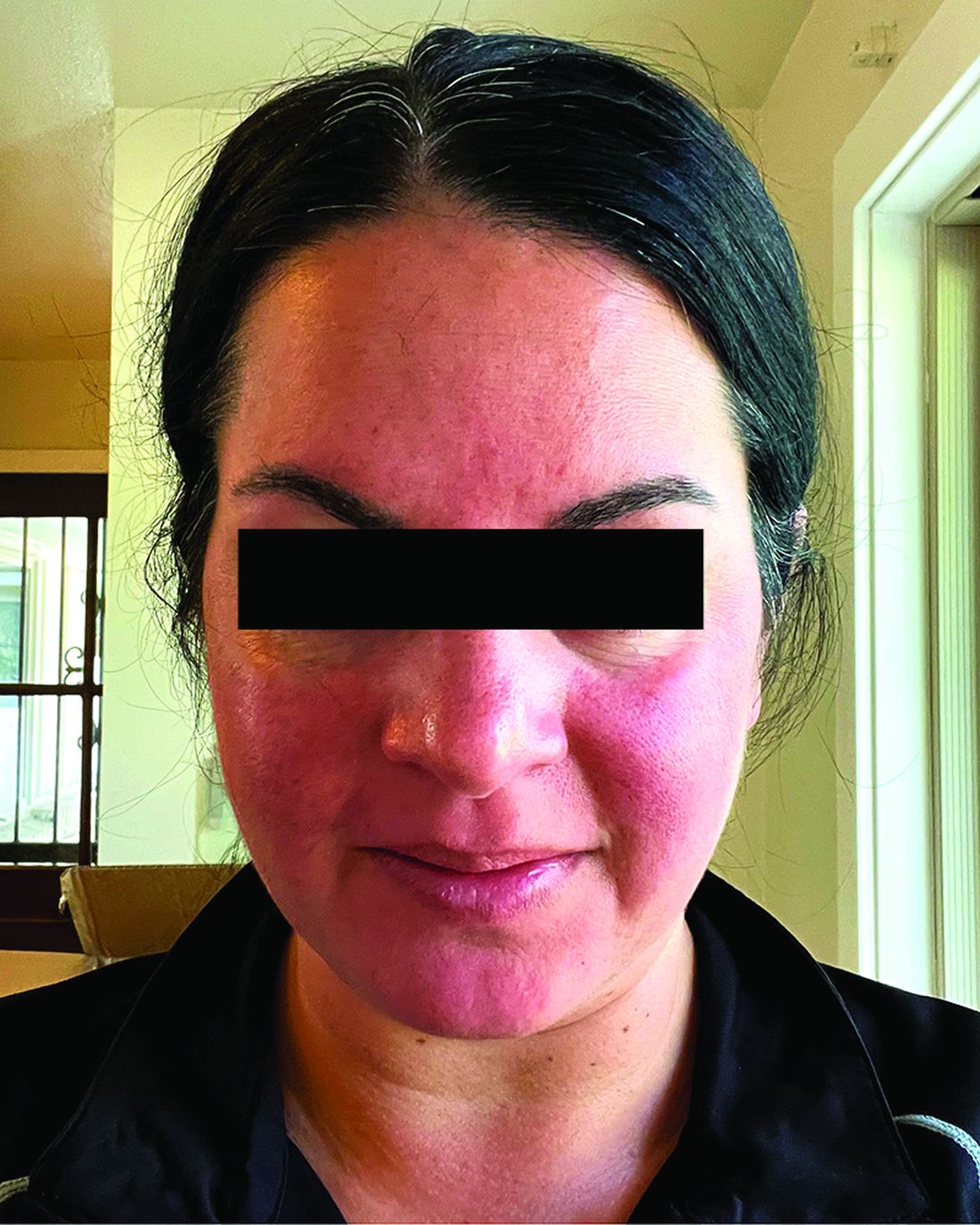
Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
How Does Moderate to Severe Eczema Affect Growth in Children?
FROM AAD 2024
SAN DIEGO — , results from an ongoing 10-year observational study showed.
“We need to sort out whether this is reversed by newer treatments, especially in the 6- to 11-year-olds, as well as the factors that underlie it in atopic dermatitis,” said the study’s first author Amy S. Paller, MD, chair of dermatology, Northwestern University, Chicago, Illinois, following the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
Atopic Dermatitis Impacts Growth
In the ongoing international study called PEDISTAD, researchers enrolled 1326 children younger than 12 years with moderate to severe atopic dermatitis inadequately controlled by topical therapies who were candidates to receive systemic medications. They assessed the percentage of patients above the 50th percentile and the mean percentiles for height, weight, and body mass index (BMI) at baseline against the Centers for Disease Control and Prevention’s (CDC’s) Learning Management System reference healthy population, by age in months, and compared results to the CDC’s standardized growth curves for healthy children aged 0-12 years.
The investigators found that at baseline, compared with the age-specific population norms, 50% of men and 51% of women in the PEDISTAD study were above the 50th percentile for weight, but only 38% and 52%, respectively, were above the 50th percentile for height. Among patients aged 5-12 years, only 28% of men and 47% of women were above the 50th percentile for height, while 69% of men and 71% of women were above the 50th percentile for BMI.
Dr. Paller said that she was “not really surprised by the reduction in linear growth, since there are so many factors that may contribute,” including chronic inflammation, poor sleep, and the use of topical and systemic steroids. “But [it’s] good to have this data as an opportunity to see if our improved therapies can reverse this.”
She said that she was “a bit surprised by the increase in weight and body mass index, but this could reflect less physical activity/sports [participation and] deserves more investigation,” and added that the findings “mesh nicely with new attention on bone growth with good control of atopic dermatitis in this age group.”
Dr. Paller acknowledged certain limitations of the study, including the fact that those enrolled are a heterogeneous cohort with variable treatment regimens.
Some Answers, More Questions
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the findings, said that atopic dermatitis “should be considered the cutaneous manifestations of a systemic inflammatory disease, though even if it were not, the impact on daily and nightly activities [such as sleep] could indirectly have systemic medical consequences.”
The data presented “highlights that children with moderate to severe disease have higher BMIs and shorter height than matched counterparts, likely owing to the treasure trove of direct and indirect consequences of uncontrolled type 2 inflammation,” he said. “What I would like to know, and as the authors astutely noted, could treatment, and especially early intervention, prevent or even alter this impact?”
Dr. Paller disclosed that she is a consultant for several pharmaceutical companies, including Sanofi and Regeneron, the study sponsor. She is also an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, LEO Pharma, and UCB and is a member of the data monitoring safety board for AbbVie, Abeona, Catawba, Galderma, and InMed. Dr. Friedman, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , results from an ongoing 10-year observational study showed.
“We need to sort out whether this is reversed by newer treatments, especially in the 6- to 11-year-olds, as well as the factors that underlie it in atopic dermatitis,” said the study’s first author Amy S. Paller, MD, chair of dermatology, Northwestern University, Chicago, Illinois, following the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
Atopic Dermatitis Impacts Growth
In the ongoing international study called PEDISTAD, researchers enrolled 1326 children younger than 12 years with moderate to severe atopic dermatitis inadequately controlled by topical therapies who were candidates to receive systemic medications. They assessed the percentage of patients above the 50th percentile and the mean percentiles for height, weight, and body mass index (BMI) at baseline against the Centers for Disease Control and Prevention’s (CDC’s) Learning Management System reference healthy population, by age in months, and compared results to the CDC’s standardized growth curves for healthy children aged 0-12 years.
The investigators found that at baseline, compared with the age-specific population norms, 50% of men and 51% of women in the PEDISTAD study were above the 50th percentile for weight, but only 38% and 52%, respectively, were above the 50th percentile for height. Among patients aged 5-12 years, only 28% of men and 47% of women were above the 50th percentile for height, while 69% of men and 71% of women were above the 50th percentile for BMI.
Dr. Paller said that she was “not really surprised by the reduction in linear growth, since there are so many factors that may contribute,” including chronic inflammation, poor sleep, and the use of topical and systemic steroids. “But [it’s] good to have this data as an opportunity to see if our improved therapies can reverse this.”
She said that she was “a bit surprised by the increase in weight and body mass index, but this could reflect less physical activity/sports [participation and] deserves more investigation,” and added that the findings “mesh nicely with new attention on bone growth with good control of atopic dermatitis in this age group.”
Dr. Paller acknowledged certain limitations of the study, including the fact that those enrolled are a heterogeneous cohort with variable treatment regimens.
Some Answers, More Questions
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the findings, said that atopic dermatitis “should be considered the cutaneous manifestations of a systemic inflammatory disease, though even if it were not, the impact on daily and nightly activities [such as sleep] could indirectly have systemic medical consequences.”
The data presented “highlights that children with moderate to severe disease have higher BMIs and shorter height than matched counterparts, likely owing to the treasure trove of direct and indirect consequences of uncontrolled type 2 inflammation,” he said. “What I would like to know, and as the authors astutely noted, could treatment, and especially early intervention, prevent or even alter this impact?”
Dr. Paller disclosed that she is a consultant for several pharmaceutical companies, including Sanofi and Regeneron, the study sponsor. She is also an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, LEO Pharma, and UCB and is a member of the data monitoring safety board for AbbVie, Abeona, Catawba, Galderma, and InMed. Dr. Friedman, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , results from an ongoing 10-year observational study showed.
“We need to sort out whether this is reversed by newer treatments, especially in the 6- to 11-year-olds, as well as the factors that underlie it in atopic dermatitis,” said the study’s first author Amy S. Paller, MD, chair of dermatology, Northwestern University, Chicago, Illinois, following the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
Atopic Dermatitis Impacts Growth
In the ongoing international study called PEDISTAD, researchers enrolled 1326 children younger than 12 years with moderate to severe atopic dermatitis inadequately controlled by topical therapies who were candidates to receive systemic medications. They assessed the percentage of patients above the 50th percentile and the mean percentiles for height, weight, and body mass index (BMI) at baseline against the Centers for Disease Control and Prevention’s (CDC’s) Learning Management System reference healthy population, by age in months, and compared results to the CDC’s standardized growth curves for healthy children aged 0-12 years.
The investigators found that at baseline, compared with the age-specific population norms, 50% of men and 51% of women in the PEDISTAD study were above the 50th percentile for weight, but only 38% and 52%, respectively, were above the 50th percentile for height. Among patients aged 5-12 years, only 28% of men and 47% of women were above the 50th percentile for height, while 69% of men and 71% of women were above the 50th percentile for BMI.
Dr. Paller said that she was “not really surprised by the reduction in linear growth, since there are so many factors that may contribute,” including chronic inflammation, poor sleep, and the use of topical and systemic steroids. “But [it’s] good to have this data as an opportunity to see if our improved therapies can reverse this.”
She said that she was “a bit surprised by the increase in weight and body mass index, but this could reflect less physical activity/sports [participation and] deserves more investigation,” and added that the findings “mesh nicely with new attention on bone growth with good control of atopic dermatitis in this age group.”
Dr. Paller acknowledged certain limitations of the study, including the fact that those enrolled are a heterogeneous cohort with variable treatment regimens.
Some Answers, More Questions
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the findings, said that atopic dermatitis “should be considered the cutaneous manifestations of a systemic inflammatory disease, though even if it were not, the impact on daily and nightly activities [such as sleep] could indirectly have systemic medical consequences.”
The data presented “highlights that children with moderate to severe disease have higher BMIs and shorter height than matched counterparts, likely owing to the treasure trove of direct and indirect consequences of uncontrolled type 2 inflammation,” he said. “What I would like to know, and as the authors astutely noted, could treatment, and especially early intervention, prevent or even alter this impact?”
Dr. Paller disclosed that she is a consultant for several pharmaceutical companies, including Sanofi and Regeneron, the study sponsor. She is also an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, LEO Pharma, and UCB and is a member of the data monitoring safety board for AbbVie, Abeona, Catawba, Galderma, and InMed. Dr. Friedman, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
Time to Lung Disease in Patients With Dermatomyositis Subtype Estimated
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Keratoacanthoma, SCC Relatively Rare With PD-1/PD-L1 Inhibitors, Study Suggests
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
Combined Pediatric Derm-Rheum Clinics Supported by Survey Respondents
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.