User login
‘Alarming’ worldwide decline in mental health
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
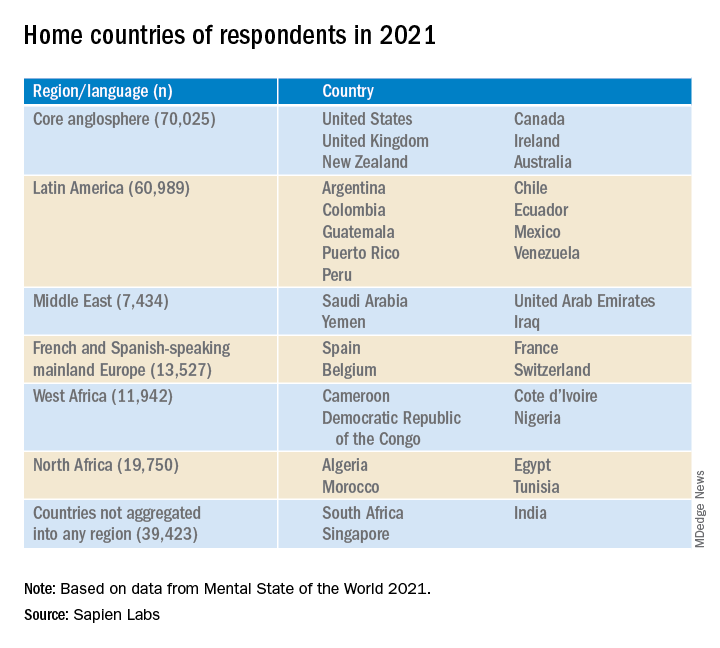
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.
Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.
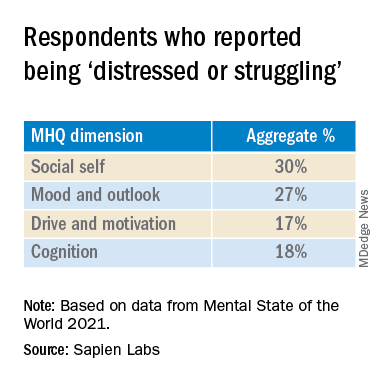
In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.

Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.

In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.

Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.

In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
Navigating patient requests for an emotional support animal
When Serena-Lian Sakheim-Devine’s best friend from childhood died of cancer, she felt sad and lonely while away at college. Wanting something warm to snuggle, she got a guinea pig and named her Basil. Then she got two more and called them Nutmeg and Paprika. The three became her Spice Girls.
“They were of great comfort to me, but also to others at times of need,” said Ms. Sakheim-Devine, 26, who lived with them in a dormitory at Smith College, an all-women’s institution in Northampton, Mass.
Her therapist wrote a letter and sent it to the disability office at Smith, which permitted the guinea pigs as emotional support animals (ESAs). Eventually, though, she wanted a dog to help manage her PTSD, depression, anxiety, and panic attacks. So, she adopted a beagle from a shelter.
Once again, a therapist provided a letter, and Ms. Sakheim-Devine was allowed to keep the beagle, Finnian, then about 13 years old, in her dorm room on the condition that she give up the guinea pigs, which she did.
She and Finnian bonded almost instantly. When she woke up drenched in sweat, unable to move or speak, the dog sensed how tense she was. Finnian licked her hands, got her fingers moving, and helped ground her.
“I didn’t really teach her that. She just knew,” said Ms. Sakheim-Devine, now a safety engineer who lives in New Haven, Conn. “It was incredible how well connected we were, even from the get-go.”
The therapeutic benefits of four-legged friends
Although there is limited scientific literature on the therapeutic use of ESAs, there are well-established benefits of having pets that also apply in these situations. Animals can provide distraction from stress, alleviate loneliness, and instill a sense of responsibility, said Rachel A. Davis, MD, associate professor of psychiatry and neurosurgery at the University of Colorado at Denver, Aurora.
They add structure to a person’s day by needing to be fed at specific times, and they can help the human get exercise. “Patients have reported improved sense of meaning in life and purpose,” Dr. Davis said.
Examples include depression, anxiety, obsessive-compulsive disorder, panic attacks, and PTSD.
ESAs differ from psychiatric service animals, which are trained to perform specific tasks, such as applying deep pressure that calms the owner. By their mere existence, ESAs provide emotional benefits to a person with a mental health disability.
“Social support, even from an animal, can really help people feel less alone, better about themselves, and safer from unpleasantness or even a physical attack,” said David Spiegel, MD, professor and associate chair of psychiatry and behavioral sciences and director of the Center on Stress and Health at Stanford (Calif.) University.
Writing a letter on your patient’s behalf
Writing a letter that serves as proof of a person’s need for an ESA is a request that mental health professionals sometimes receive from patients. The letter can grant access to housing without additional cost regardless of no-pet polices, and some employers may allow an ESA at work as a reasonable accommodation for a psychological disability. Until recently, an ESA could accompany its owner on a plane, but most airlines no longer permit this, partly because some passengers falsely claim their pets as ESAs.
Before crafting a letter for someone with an ESA, Dr. Spiegel asks for the patient’s permission to elaborate on the clinical condition that merits professional help and to explain how the animal relieves associated symptoms.
The Fair Housing Act, a federal law, requires a landlord to grant a reasonable accommodation involving an emotional support or other assistance animal. Such an accommodation honors a request to live on the property despite a no-pets policy. It also waives a pet deposit, fee, or other rules involving animals on the premises.
Landlords are usually supportive of a request to permit an ESA, said Jonathan Betlinski, MD, associate professor and director of the public psychiatry division at Oregon Health and Science University, Portland. None of his patients have experienced any difficulties once they obtained a letter from him.
However, “anytime somebody asks me about a letter for an ESA, that’s the time to have a conversation. It’s not automatic,” Dr. Betlinski said. The discussion involves learning about the type of animal a patient has and how it helps his or her emotional state.
Because of privacy concerns, Dr. Betlinski doesn’t disclose the specific diagnosis in the letter unless the patient signs a release of information. The laws pertaining to ESAs only require his letter to note that an individual has a qualifying diagnosis and that an ESA helps improve symptoms, but it’s not necessary to explain how.
“You can see where writing the letter is a fine balancing act,” he said. But he finds it helpful to mention any training the animal has completed, such as the Canine Good Citizen course sponsored by the American Kennel Club.
Most of the letters Luis Anez, PsyD, a clinical psychologist and associate professor of psychiatry at Yale University, New Haven, Conn., has written for this purpose were in support of ESAs in housing. But he also recalled providing a letter for a patient who was flying to Puerto Rico with an ESA. The letters are generally provided only to established patients with psychiatric diagnoses.
Without a letter, “we’ve seen people say: ‘I’d rather be homeless than part with my dog,’ ”said Dr. Anez, who is also director of Hispanic services at Connecticut Mental Health Center in New Haven, a partnership between Yale and the Connecticut Department of Mental Health and Addiction Services. Before getting an ESA, Dr. Anez recommends that individuals become aware of their landlord’s policies on possible restrictions relating to dog sizes and breeds.
Additional considerations
An ESA doesn’t necessarily have to be a dog. “It certainly could be a cat. It could be a parrot, too,” said Stephen Stern, MD, a psychiatrist in private practice in Mount Kisco, N.Y. But, “if they say that their emotional support animal is an earthworm, that would make you wonder,” he added half-jokingly.
Dr. Stern only writes an ESA letter for a patient with whom he has an ongoing professional relationship. For instance, if he’s treating someone for depression and that patient tells him how the animal helps relieve symptoms, then that is sufficient justification to write a letter.
“Because you know them, you’ve assessed that what they’re saying is plausible,” said Dr. Stern, who is also an adjunct professor of psychiatry at the University of Texas Health Science in San Antonio, where he conducted research on companion dogs for veterans with PTSD and continues to collaborate with colleagues via email and Zoom.
While veterans benefit from ESAs, some live in housing that doesn’t permit animals, said Beth Zimmerman, founder and executive director of Pets for Patriots, a nationally operating nonprofit organization in Long Beach, N.Y., that partners with shelters and animal welfare groups to adopt dogs and cats for companionship and emotional support. She said an ESA can be “a wonderful complement to other forms of therapy that a veteran may undertake.
“Most of the time when the veteran encounters a problem, it’s because the landlord is ill-informed of the law,” Dr. Zimmerman said. “We provide information to the veteran to share with the landlord or building management, and always recommend taking a very amicable approach. In our experience, with very few exceptions, once the landlord understands his or her responsibilities under the law, they will permit the veteran to have that emotional support animal in their dwelling.”
For Kristin Lowe, a chocolate Labrador-Weimaraner mix named Lola provided emotional support from her puppy days until her death at age 12 in May 2021. Ms. Lowe’s psychiatrist provided letters that allowed Lola to live in her apartment and to travel on commercial airline flights.
“She was so connected to me,” said Ms. Lowe, 34, who lives in Denver and works as an administrative office worker in physical therapy. “She was a part of me. She could read every emotion that I had.”
Now, Ms. Lowe relies on Henry, an Australian shepherd puppy, to help her cope with obsessive-compulsive disorder, major depressive disorder, and an eating disorder. She described him as “a very happy little guy and a constant tail wagger – and that lights up something in me.”
More information, which is provided by the U.S. Department of Housing and Urban Development, can be found here.
A version of this article first appeared on Medscape.com.
When Serena-Lian Sakheim-Devine’s best friend from childhood died of cancer, she felt sad and lonely while away at college. Wanting something warm to snuggle, she got a guinea pig and named her Basil. Then she got two more and called them Nutmeg and Paprika. The three became her Spice Girls.
“They were of great comfort to me, but also to others at times of need,” said Ms. Sakheim-Devine, 26, who lived with them in a dormitory at Smith College, an all-women’s institution in Northampton, Mass.
Her therapist wrote a letter and sent it to the disability office at Smith, which permitted the guinea pigs as emotional support animals (ESAs). Eventually, though, she wanted a dog to help manage her PTSD, depression, anxiety, and panic attacks. So, she adopted a beagle from a shelter.
Once again, a therapist provided a letter, and Ms. Sakheim-Devine was allowed to keep the beagle, Finnian, then about 13 years old, in her dorm room on the condition that she give up the guinea pigs, which she did.
She and Finnian bonded almost instantly. When she woke up drenched in sweat, unable to move or speak, the dog sensed how tense she was. Finnian licked her hands, got her fingers moving, and helped ground her.
“I didn’t really teach her that. She just knew,” said Ms. Sakheim-Devine, now a safety engineer who lives in New Haven, Conn. “It was incredible how well connected we were, even from the get-go.”
The therapeutic benefits of four-legged friends
Although there is limited scientific literature on the therapeutic use of ESAs, there are well-established benefits of having pets that also apply in these situations. Animals can provide distraction from stress, alleviate loneliness, and instill a sense of responsibility, said Rachel A. Davis, MD, associate professor of psychiatry and neurosurgery at the University of Colorado at Denver, Aurora.
They add structure to a person’s day by needing to be fed at specific times, and they can help the human get exercise. “Patients have reported improved sense of meaning in life and purpose,” Dr. Davis said.
Examples include depression, anxiety, obsessive-compulsive disorder, panic attacks, and PTSD.
ESAs differ from psychiatric service animals, which are trained to perform specific tasks, such as applying deep pressure that calms the owner. By their mere existence, ESAs provide emotional benefits to a person with a mental health disability.
“Social support, even from an animal, can really help people feel less alone, better about themselves, and safer from unpleasantness or even a physical attack,” said David Spiegel, MD, professor and associate chair of psychiatry and behavioral sciences and director of the Center on Stress and Health at Stanford (Calif.) University.
Writing a letter on your patient’s behalf
Writing a letter that serves as proof of a person’s need for an ESA is a request that mental health professionals sometimes receive from patients. The letter can grant access to housing without additional cost regardless of no-pet polices, and some employers may allow an ESA at work as a reasonable accommodation for a psychological disability. Until recently, an ESA could accompany its owner on a plane, but most airlines no longer permit this, partly because some passengers falsely claim their pets as ESAs.
Before crafting a letter for someone with an ESA, Dr. Spiegel asks for the patient’s permission to elaborate on the clinical condition that merits professional help and to explain how the animal relieves associated symptoms.
The Fair Housing Act, a federal law, requires a landlord to grant a reasonable accommodation involving an emotional support or other assistance animal. Such an accommodation honors a request to live on the property despite a no-pets policy. It also waives a pet deposit, fee, or other rules involving animals on the premises.
Landlords are usually supportive of a request to permit an ESA, said Jonathan Betlinski, MD, associate professor and director of the public psychiatry division at Oregon Health and Science University, Portland. None of his patients have experienced any difficulties once they obtained a letter from him.
However, “anytime somebody asks me about a letter for an ESA, that’s the time to have a conversation. It’s not automatic,” Dr. Betlinski said. The discussion involves learning about the type of animal a patient has and how it helps his or her emotional state.
Because of privacy concerns, Dr. Betlinski doesn’t disclose the specific diagnosis in the letter unless the patient signs a release of information. The laws pertaining to ESAs only require his letter to note that an individual has a qualifying diagnosis and that an ESA helps improve symptoms, but it’s not necessary to explain how.
“You can see where writing the letter is a fine balancing act,” he said. But he finds it helpful to mention any training the animal has completed, such as the Canine Good Citizen course sponsored by the American Kennel Club.
Most of the letters Luis Anez, PsyD, a clinical psychologist and associate professor of psychiatry at Yale University, New Haven, Conn., has written for this purpose were in support of ESAs in housing. But he also recalled providing a letter for a patient who was flying to Puerto Rico with an ESA. The letters are generally provided only to established patients with psychiatric diagnoses.
Without a letter, “we’ve seen people say: ‘I’d rather be homeless than part with my dog,’ ”said Dr. Anez, who is also director of Hispanic services at Connecticut Mental Health Center in New Haven, a partnership between Yale and the Connecticut Department of Mental Health and Addiction Services. Before getting an ESA, Dr. Anez recommends that individuals become aware of their landlord’s policies on possible restrictions relating to dog sizes and breeds.
Additional considerations
An ESA doesn’t necessarily have to be a dog. “It certainly could be a cat. It could be a parrot, too,” said Stephen Stern, MD, a psychiatrist in private practice in Mount Kisco, N.Y. But, “if they say that their emotional support animal is an earthworm, that would make you wonder,” he added half-jokingly.
Dr. Stern only writes an ESA letter for a patient with whom he has an ongoing professional relationship. For instance, if he’s treating someone for depression and that patient tells him how the animal helps relieve symptoms, then that is sufficient justification to write a letter.
“Because you know them, you’ve assessed that what they’re saying is plausible,” said Dr. Stern, who is also an adjunct professor of psychiatry at the University of Texas Health Science in San Antonio, where he conducted research on companion dogs for veterans with PTSD and continues to collaborate with colleagues via email and Zoom.
While veterans benefit from ESAs, some live in housing that doesn’t permit animals, said Beth Zimmerman, founder and executive director of Pets for Patriots, a nationally operating nonprofit organization in Long Beach, N.Y., that partners with shelters and animal welfare groups to adopt dogs and cats for companionship and emotional support. She said an ESA can be “a wonderful complement to other forms of therapy that a veteran may undertake.
“Most of the time when the veteran encounters a problem, it’s because the landlord is ill-informed of the law,” Dr. Zimmerman said. “We provide information to the veteran to share with the landlord or building management, and always recommend taking a very amicable approach. In our experience, with very few exceptions, once the landlord understands his or her responsibilities under the law, they will permit the veteran to have that emotional support animal in their dwelling.”
For Kristin Lowe, a chocolate Labrador-Weimaraner mix named Lola provided emotional support from her puppy days until her death at age 12 in May 2021. Ms. Lowe’s psychiatrist provided letters that allowed Lola to live in her apartment and to travel on commercial airline flights.
“She was so connected to me,” said Ms. Lowe, 34, who lives in Denver and works as an administrative office worker in physical therapy. “She was a part of me. She could read every emotion that I had.”
Now, Ms. Lowe relies on Henry, an Australian shepherd puppy, to help her cope with obsessive-compulsive disorder, major depressive disorder, and an eating disorder. She described him as “a very happy little guy and a constant tail wagger – and that lights up something in me.”
More information, which is provided by the U.S. Department of Housing and Urban Development, can be found here.
A version of this article first appeared on Medscape.com.
When Serena-Lian Sakheim-Devine’s best friend from childhood died of cancer, she felt sad and lonely while away at college. Wanting something warm to snuggle, she got a guinea pig and named her Basil. Then she got two more and called them Nutmeg and Paprika. The three became her Spice Girls.
“They were of great comfort to me, but also to others at times of need,” said Ms. Sakheim-Devine, 26, who lived with them in a dormitory at Smith College, an all-women’s institution in Northampton, Mass.
Her therapist wrote a letter and sent it to the disability office at Smith, which permitted the guinea pigs as emotional support animals (ESAs). Eventually, though, she wanted a dog to help manage her PTSD, depression, anxiety, and panic attacks. So, she adopted a beagle from a shelter.
Once again, a therapist provided a letter, and Ms. Sakheim-Devine was allowed to keep the beagle, Finnian, then about 13 years old, in her dorm room on the condition that she give up the guinea pigs, which she did.
She and Finnian bonded almost instantly. When she woke up drenched in sweat, unable to move or speak, the dog sensed how tense she was. Finnian licked her hands, got her fingers moving, and helped ground her.
“I didn’t really teach her that. She just knew,” said Ms. Sakheim-Devine, now a safety engineer who lives in New Haven, Conn. “It was incredible how well connected we were, even from the get-go.”
The therapeutic benefits of four-legged friends
Although there is limited scientific literature on the therapeutic use of ESAs, there are well-established benefits of having pets that also apply in these situations. Animals can provide distraction from stress, alleviate loneliness, and instill a sense of responsibility, said Rachel A. Davis, MD, associate professor of psychiatry and neurosurgery at the University of Colorado at Denver, Aurora.
They add structure to a person’s day by needing to be fed at specific times, and they can help the human get exercise. “Patients have reported improved sense of meaning in life and purpose,” Dr. Davis said.
Examples include depression, anxiety, obsessive-compulsive disorder, panic attacks, and PTSD.
ESAs differ from psychiatric service animals, which are trained to perform specific tasks, such as applying deep pressure that calms the owner. By their mere existence, ESAs provide emotional benefits to a person with a mental health disability.
“Social support, even from an animal, can really help people feel less alone, better about themselves, and safer from unpleasantness or even a physical attack,” said David Spiegel, MD, professor and associate chair of psychiatry and behavioral sciences and director of the Center on Stress and Health at Stanford (Calif.) University.
Writing a letter on your patient’s behalf
Writing a letter that serves as proof of a person’s need for an ESA is a request that mental health professionals sometimes receive from patients. The letter can grant access to housing without additional cost regardless of no-pet polices, and some employers may allow an ESA at work as a reasonable accommodation for a psychological disability. Until recently, an ESA could accompany its owner on a plane, but most airlines no longer permit this, partly because some passengers falsely claim their pets as ESAs.
Before crafting a letter for someone with an ESA, Dr. Spiegel asks for the patient’s permission to elaborate on the clinical condition that merits professional help and to explain how the animal relieves associated symptoms.
The Fair Housing Act, a federal law, requires a landlord to grant a reasonable accommodation involving an emotional support or other assistance animal. Such an accommodation honors a request to live on the property despite a no-pets policy. It also waives a pet deposit, fee, or other rules involving animals on the premises.
Landlords are usually supportive of a request to permit an ESA, said Jonathan Betlinski, MD, associate professor and director of the public psychiatry division at Oregon Health and Science University, Portland. None of his patients have experienced any difficulties once they obtained a letter from him.
However, “anytime somebody asks me about a letter for an ESA, that’s the time to have a conversation. It’s not automatic,” Dr. Betlinski said. The discussion involves learning about the type of animal a patient has and how it helps his or her emotional state.
Because of privacy concerns, Dr. Betlinski doesn’t disclose the specific diagnosis in the letter unless the patient signs a release of information. The laws pertaining to ESAs only require his letter to note that an individual has a qualifying diagnosis and that an ESA helps improve symptoms, but it’s not necessary to explain how.
“You can see where writing the letter is a fine balancing act,” he said. But he finds it helpful to mention any training the animal has completed, such as the Canine Good Citizen course sponsored by the American Kennel Club.
Most of the letters Luis Anez, PsyD, a clinical psychologist and associate professor of psychiatry at Yale University, New Haven, Conn., has written for this purpose were in support of ESAs in housing. But he also recalled providing a letter for a patient who was flying to Puerto Rico with an ESA. The letters are generally provided only to established patients with psychiatric diagnoses.
Without a letter, “we’ve seen people say: ‘I’d rather be homeless than part with my dog,’ ”said Dr. Anez, who is also director of Hispanic services at Connecticut Mental Health Center in New Haven, a partnership between Yale and the Connecticut Department of Mental Health and Addiction Services. Before getting an ESA, Dr. Anez recommends that individuals become aware of their landlord’s policies on possible restrictions relating to dog sizes and breeds.
Additional considerations
An ESA doesn’t necessarily have to be a dog. “It certainly could be a cat. It could be a parrot, too,” said Stephen Stern, MD, a psychiatrist in private practice in Mount Kisco, N.Y. But, “if they say that their emotional support animal is an earthworm, that would make you wonder,” he added half-jokingly.
Dr. Stern only writes an ESA letter for a patient with whom he has an ongoing professional relationship. For instance, if he’s treating someone for depression and that patient tells him how the animal helps relieve symptoms, then that is sufficient justification to write a letter.
“Because you know them, you’ve assessed that what they’re saying is plausible,” said Dr. Stern, who is also an adjunct professor of psychiatry at the University of Texas Health Science in San Antonio, where he conducted research on companion dogs for veterans with PTSD and continues to collaborate with colleagues via email and Zoom.
While veterans benefit from ESAs, some live in housing that doesn’t permit animals, said Beth Zimmerman, founder and executive director of Pets for Patriots, a nationally operating nonprofit organization in Long Beach, N.Y., that partners with shelters and animal welfare groups to adopt dogs and cats for companionship and emotional support. She said an ESA can be “a wonderful complement to other forms of therapy that a veteran may undertake.
“Most of the time when the veteran encounters a problem, it’s because the landlord is ill-informed of the law,” Dr. Zimmerman said. “We provide information to the veteran to share with the landlord or building management, and always recommend taking a very amicable approach. In our experience, with very few exceptions, once the landlord understands his or her responsibilities under the law, they will permit the veteran to have that emotional support animal in their dwelling.”
For Kristin Lowe, a chocolate Labrador-Weimaraner mix named Lola provided emotional support from her puppy days until her death at age 12 in May 2021. Ms. Lowe’s psychiatrist provided letters that allowed Lola to live in her apartment and to travel on commercial airline flights.
“She was so connected to me,” said Ms. Lowe, 34, who lives in Denver and works as an administrative office worker in physical therapy. “She was a part of me. She could read every emotion that I had.”
Now, Ms. Lowe relies on Henry, an Australian shepherd puppy, to help her cope with obsessive-compulsive disorder, major depressive disorder, and an eating disorder. She described him as “a very happy little guy and a constant tail wagger – and that lights up something in me.”
More information, which is provided by the U.S. Department of Housing and Urban Development, can be found here.
A version of this article first appeared on Medscape.com.
Why is there an increased risk of cancer in depressed patients?
LAS VEGAS – Is the relationship between major depressive disorder and the development of cancer, cardiovascular disease, and other medical conditions a coincidence, or is there more at play?
According to Charles B. Nemeroff, MD, PhD, a host of circumstances potentially underlies this association, including treatment of the medical disorder itself.
“The best example of that is probably the use of interferon-alpha for the treatment of malignant melanoma,” Dr. Nemeroff, professor and chair of the department of psychiatry and behavioral sciences at the University of Texas at Austin, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “Many patients treated with interferon-alpha ended up with very severe depression, including several documented suicides. Another possibility of the relationship between depression and medical disorders is that treating a patient for depression could result in a medical disorder. The best example of this is the use of 20 mg of olanzapine to augment the effects of an antidepressant, resulting in a 50-pound weight gain and the development of type 2 diabetes and metabolic syndrome. Both of those scenarios are well understood.”
Then there’s the behavioral aspects of the relationship, he continued, in which patients adopt the mindset that “I’m depressed. I don’t want to exercise. I’m a couch potato. I have been gaining a lot of weight. It’s bad for my heart.”
Converging biology is another possibility. “Is it possible that the biology of depression is linked to the biology of other disorders?” asked Dr. Nemeroff, who directs the university’s Institute for Early Life Adversity Research. “We can talk about this in relation to thyroid disease, a well known cause of depression, but we can also talk about the relationship to other disorders. There’s amazing epidemiologic evidence that patients with PTSD are much more likely to develop Alzheimer’s disease than patients without PTSD.”
Psychosocial issues also play a role. He recalled seeing patient in a clinic for the underserved who had underlying severe ulcerative colitis and anemia and couldn’t afford medical treatment. “The patient had a low hemoglobin, so it was impossible to distinguish between that and whether they had a primary depressive disorder or not,” he said.
In a study that explored the relationship between major depression and cancer, Dr. Nemeroff and colleagues found that the prevalence was highest in those with pancreatic cancer (50%), followed by oropharyngeal (40%), colon (13-25%), breast (18-25%), and gynecologic (23%), and Hodgkin’s lymphoma (17%) (Arch Gen Psychiatry 1995;52[2]:89-99). “Not all cancers have the same rate of depression,” he said. “One of the central questions is, not so much is the cancer patient depressed, but is depression a risk factor for developing cancer? The answer is a resounding yes. But what we don’t know is if you treat the depression aggressively, can you reduce that risk of either developing cancer or the progression of cancer?”
Dr. Nemeroff spotlighted several studies largely from the oncology literature, including a prospective survival analysis of 578 women with early-stage breast cancer (Lancet 1999;354:1331-6). After 5 years, 395 were alive and without relapse, 50 were alive with relapse, and 133 had died. The researchers found a significantly increased risk of death from all causes by 5 years in women with a high depression score (HR 3.59). There was a significantly increased risk of relapse or death at 5 years in women with high scores on helplessness and hopelessness measures.
In an analysis of the association between breast cancer and traumatic events, women who had severe stress or a traumatic event had lower rates of disease-free intervals (J Psychosomatic Res 2007;63:233-9). Another study by the same investigators found that a decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer (J Clin Oncol 2010;29:413-20). The median survival was 53.6 months for women with decreasing depression scores over 1 year and 25.1 months for women with increasing depression scores.
A more recent study of cervical cancer patients found that those exposed to psychological stress had an increased risk of cancer-specific mortality (HR 1.33) (Cancer Res 2019;79:3965-72). The association was mainly driven by distress experienced within 1 year before or after diagnosis (HR 1.30) but not afterward (HR 1.12). In addition, data from the large longitudinal Nurses’ Health Study II found that women with high PTSD symptoms had a twofold greater risk of ovarian cancer compared with women who had no trauma exposure (Cancer Res 2019;79:5113-20).
Authors of a separate study analyzed data from the Women’s Health Initiative to examine if depression precedes the development of a cancer diagnosis. They found that depression 3 years before a diagnosis of breast cancer was associated with all-cause mortality (HR 1.35) (Cancer 2017;123[16]:3107-15). Meanwhile, among women with late-stage breast cancer, newly developed depression at year 3 was significantly associated with all-cause mortality (HR 2.0) and breast cancer-specific mortality (HR 2.42). “That’s a pretty amazing finding,” Dr. Nemeroff said. “We have to think about depression as a systemic illness. What is depression doing that’s creating a fertile environment for cancer or worsening of cancer?”
He then discussed the risk of suicide in patients who are newly diagnosed with cancer. “No one ever talks about this, and I can’t get anybody to support research in this area,” he said. In one of the first studies on the topic, researchers conducted a case-control study of Medicare patients and determined risk of suicide among those with cancer was 2.3-fold higher compared with controls, even after adjustment for psychiatric illness and the risk of dying within a year (J Clin Oncol 2008;26[29]:4720-4). More recently, authors of a large population-based study in England found that the overall standardized mortality ratio for suicide was 1.20 (JAMA Psychiatry 2019;76[1]51-60). The risk was highest among patients with mesothelioma, with a 4.51-fold risk, followed by pancreatic (3.89-fold), esophageal (2.65-fold), lung (2.57-fold), and stomach cancer (2.20-fold). “They reported that the first 6 months after the diagnosis is associated with an increased risk of suicide – unrelated to prognosis,” Dr. Nemeroff said.
A separate analysis of SEER data from 1973-2014 and comprising more than 8.6 million cancer patients found that newly diagnosed cancer patients are 4.4 times more likely to die from suicide than patients in the same age group without cancer (Nat Commun 2019;10[1]:207). The highest risk was in lung cancer, followed by head and neck, testes, bladder, and Hodgkin’s lymphoma.
According to Dr. Nemeroff, For example, he said, if the depressed environment is associated with a marked increase in tumor necrosis factor, interleukin 6, and other inflammatory markers, “that probably contributes to the body’s ability to fight disease. Ironically, depression is associated with an increase in inflammation but a decreased in T cell function. Remember, there are two fundamental types of immunity: the antibody response and the cellular response. What’s odd about depression is that there’s an increase in inflammatory markers but a decrease in the ability of T cells to function in terms of cellular immunity.”
Dr. Nemeroff disclosed that he has served as a consultant and/or scientific adviser for numerous pharmaceutical companies. He has received research and grant support from the National Institutes of Health.
LAS VEGAS – Is the relationship between major depressive disorder and the development of cancer, cardiovascular disease, and other medical conditions a coincidence, or is there more at play?
According to Charles B. Nemeroff, MD, PhD, a host of circumstances potentially underlies this association, including treatment of the medical disorder itself.
“The best example of that is probably the use of interferon-alpha for the treatment of malignant melanoma,” Dr. Nemeroff, professor and chair of the department of psychiatry and behavioral sciences at the University of Texas at Austin, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “Many patients treated with interferon-alpha ended up with very severe depression, including several documented suicides. Another possibility of the relationship between depression and medical disorders is that treating a patient for depression could result in a medical disorder. The best example of this is the use of 20 mg of olanzapine to augment the effects of an antidepressant, resulting in a 50-pound weight gain and the development of type 2 diabetes and metabolic syndrome. Both of those scenarios are well understood.”
Then there’s the behavioral aspects of the relationship, he continued, in which patients adopt the mindset that “I’m depressed. I don’t want to exercise. I’m a couch potato. I have been gaining a lot of weight. It’s bad for my heart.”
Converging biology is another possibility. “Is it possible that the biology of depression is linked to the biology of other disorders?” asked Dr. Nemeroff, who directs the university’s Institute for Early Life Adversity Research. “We can talk about this in relation to thyroid disease, a well known cause of depression, but we can also talk about the relationship to other disorders. There’s amazing epidemiologic evidence that patients with PTSD are much more likely to develop Alzheimer’s disease than patients without PTSD.”
Psychosocial issues also play a role. He recalled seeing patient in a clinic for the underserved who had underlying severe ulcerative colitis and anemia and couldn’t afford medical treatment. “The patient had a low hemoglobin, so it was impossible to distinguish between that and whether they had a primary depressive disorder or not,” he said.
In a study that explored the relationship between major depression and cancer, Dr. Nemeroff and colleagues found that the prevalence was highest in those with pancreatic cancer (50%), followed by oropharyngeal (40%), colon (13-25%), breast (18-25%), and gynecologic (23%), and Hodgkin’s lymphoma (17%) (Arch Gen Psychiatry 1995;52[2]:89-99). “Not all cancers have the same rate of depression,” he said. “One of the central questions is, not so much is the cancer patient depressed, but is depression a risk factor for developing cancer? The answer is a resounding yes. But what we don’t know is if you treat the depression aggressively, can you reduce that risk of either developing cancer or the progression of cancer?”
Dr. Nemeroff spotlighted several studies largely from the oncology literature, including a prospective survival analysis of 578 women with early-stage breast cancer (Lancet 1999;354:1331-6). After 5 years, 395 were alive and without relapse, 50 were alive with relapse, and 133 had died. The researchers found a significantly increased risk of death from all causes by 5 years in women with a high depression score (HR 3.59). There was a significantly increased risk of relapse or death at 5 years in women with high scores on helplessness and hopelessness measures.
In an analysis of the association between breast cancer and traumatic events, women who had severe stress or a traumatic event had lower rates of disease-free intervals (J Psychosomatic Res 2007;63:233-9). Another study by the same investigators found that a decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer (J Clin Oncol 2010;29:413-20). The median survival was 53.6 months for women with decreasing depression scores over 1 year and 25.1 months for women with increasing depression scores.
A more recent study of cervical cancer patients found that those exposed to psychological stress had an increased risk of cancer-specific mortality (HR 1.33) (Cancer Res 2019;79:3965-72). The association was mainly driven by distress experienced within 1 year before or after diagnosis (HR 1.30) but not afterward (HR 1.12). In addition, data from the large longitudinal Nurses’ Health Study II found that women with high PTSD symptoms had a twofold greater risk of ovarian cancer compared with women who had no trauma exposure (Cancer Res 2019;79:5113-20).
Authors of a separate study analyzed data from the Women’s Health Initiative to examine if depression precedes the development of a cancer diagnosis. They found that depression 3 years before a diagnosis of breast cancer was associated with all-cause mortality (HR 1.35) (Cancer 2017;123[16]:3107-15). Meanwhile, among women with late-stage breast cancer, newly developed depression at year 3 was significantly associated with all-cause mortality (HR 2.0) and breast cancer-specific mortality (HR 2.42). “That’s a pretty amazing finding,” Dr. Nemeroff said. “We have to think about depression as a systemic illness. What is depression doing that’s creating a fertile environment for cancer or worsening of cancer?”
He then discussed the risk of suicide in patients who are newly diagnosed with cancer. “No one ever talks about this, and I can’t get anybody to support research in this area,” he said. In one of the first studies on the topic, researchers conducted a case-control study of Medicare patients and determined risk of suicide among those with cancer was 2.3-fold higher compared with controls, even after adjustment for psychiatric illness and the risk of dying within a year (J Clin Oncol 2008;26[29]:4720-4). More recently, authors of a large population-based study in England found that the overall standardized mortality ratio for suicide was 1.20 (JAMA Psychiatry 2019;76[1]51-60). The risk was highest among patients with mesothelioma, with a 4.51-fold risk, followed by pancreatic (3.89-fold), esophageal (2.65-fold), lung (2.57-fold), and stomach cancer (2.20-fold). “They reported that the first 6 months after the diagnosis is associated with an increased risk of suicide – unrelated to prognosis,” Dr. Nemeroff said.
A separate analysis of SEER data from 1973-2014 and comprising more than 8.6 million cancer patients found that newly diagnosed cancer patients are 4.4 times more likely to die from suicide than patients in the same age group without cancer (Nat Commun 2019;10[1]:207). The highest risk was in lung cancer, followed by head and neck, testes, bladder, and Hodgkin’s lymphoma.
According to Dr. Nemeroff, For example, he said, if the depressed environment is associated with a marked increase in tumor necrosis factor, interleukin 6, and other inflammatory markers, “that probably contributes to the body’s ability to fight disease. Ironically, depression is associated with an increase in inflammation but a decreased in T cell function. Remember, there are two fundamental types of immunity: the antibody response and the cellular response. What’s odd about depression is that there’s an increase in inflammatory markers but a decrease in the ability of T cells to function in terms of cellular immunity.”
Dr. Nemeroff disclosed that he has served as a consultant and/or scientific adviser for numerous pharmaceutical companies. He has received research and grant support from the National Institutes of Health.
LAS VEGAS – Is the relationship between major depressive disorder and the development of cancer, cardiovascular disease, and other medical conditions a coincidence, or is there more at play?
According to Charles B. Nemeroff, MD, PhD, a host of circumstances potentially underlies this association, including treatment of the medical disorder itself.
“The best example of that is probably the use of interferon-alpha for the treatment of malignant melanoma,” Dr. Nemeroff, professor and chair of the department of psychiatry and behavioral sciences at the University of Texas at Austin, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “Many patients treated with interferon-alpha ended up with very severe depression, including several documented suicides. Another possibility of the relationship between depression and medical disorders is that treating a patient for depression could result in a medical disorder. The best example of this is the use of 20 mg of olanzapine to augment the effects of an antidepressant, resulting in a 50-pound weight gain and the development of type 2 diabetes and metabolic syndrome. Both of those scenarios are well understood.”
Then there’s the behavioral aspects of the relationship, he continued, in which patients adopt the mindset that “I’m depressed. I don’t want to exercise. I’m a couch potato. I have been gaining a lot of weight. It’s bad for my heart.”
Converging biology is another possibility. “Is it possible that the biology of depression is linked to the biology of other disorders?” asked Dr. Nemeroff, who directs the university’s Institute for Early Life Adversity Research. “We can talk about this in relation to thyroid disease, a well known cause of depression, but we can also talk about the relationship to other disorders. There’s amazing epidemiologic evidence that patients with PTSD are much more likely to develop Alzheimer’s disease than patients without PTSD.”
Psychosocial issues also play a role. He recalled seeing patient in a clinic for the underserved who had underlying severe ulcerative colitis and anemia and couldn’t afford medical treatment. “The patient had a low hemoglobin, so it was impossible to distinguish between that and whether they had a primary depressive disorder or not,” he said.
In a study that explored the relationship between major depression and cancer, Dr. Nemeroff and colleagues found that the prevalence was highest in those with pancreatic cancer (50%), followed by oropharyngeal (40%), colon (13-25%), breast (18-25%), and gynecologic (23%), and Hodgkin’s lymphoma (17%) (Arch Gen Psychiatry 1995;52[2]:89-99). “Not all cancers have the same rate of depression,” he said. “One of the central questions is, not so much is the cancer patient depressed, but is depression a risk factor for developing cancer? The answer is a resounding yes. But what we don’t know is if you treat the depression aggressively, can you reduce that risk of either developing cancer or the progression of cancer?”
Dr. Nemeroff spotlighted several studies largely from the oncology literature, including a prospective survival analysis of 578 women with early-stage breast cancer (Lancet 1999;354:1331-6). After 5 years, 395 were alive and without relapse, 50 were alive with relapse, and 133 had died. The researchers found a significantly increased risk of death from all causes by 5 years in women with a high depression score (HR 3.59). There was a significantly increased risk of relapse or death at 5 years in women with high scores on helplessness and hopelessness measures.
In an analysis of the association between breast cancer and traumatic events, women who had severe stress or a traumatic event had lower rates of disease-free intervals (J Psychosomatic Res 2007;63:233-9). Another study by the same investigators found that a decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer (J Clin Oncol 2010;29:413-20). The median survival was 53.6 months for women with decreasing depression scores over 1 year and 25.1 months for women with increasing depression scores.
A more recent study of cervical cancer patients found that those exposed to psychological stress had an increased risk of cancer-specific mortality (HR 1.33) (Cancer Res 2019;79:3965-72). The association was mainly driven by distress experienced within 1 year before or after diagnosis (HR 1.30) but not afterward (HR 1.12). In addition, data from the large longitudinal Nurses’ Health Study II found that women with high PTSD symptoms had a twofold greater risk of ovarian cancer compared with women who had no trauma exposure (Cancer Res 2019;79:5113-20).
Authors of a separate study analyzed data from the Women’s Health Initiative to examine if depression precedes the development of a cancer diagnosis. They found that depression 3 years before a diagnosis of breast cancer was associated with all-cause mortality (HR 1.35) (Cancer 2017;123[16]:3107-15). Meanwhile, among women with late-stage breast cancer, newly developed depression at year 3 was significantly associated with all-cause mortality (HR 2.0) and breast cancer-specific mortality (HR 2.42). “That’s a pretty amazing finding,” Dr. Nemeroff said. “We have to think about depression as a systemic illness. What is depression doing that’s creating a fertile environment for cancer or worsening of cancer?”
He then discussed the risk of suicide in patients who are newly diagnosed with cancer. “No one ever talks about this, and I can’t get anybody to support research in this area,” he said. In one of the first studies on the topic, researchers conducted a case-control study of Medicare patients and determined risk of suicide among those with cancer was 2.3-fold higher compared with controls, even after adjustment for psychiatric illness and the risk of dying within a year (J Clin Oncol 2008;26[29]:4720-4). More recently, authors of a large population-based study in England found that the overall standardized mortality ratio for suicide was 1.20 (JAMA Psychiatry 2019;76[1]51-60). The risk was highest among patients with mesothelioma, with a 4.51-fold risk, followed by pancreatic (3.89-fold), esophageal (2.65-fold), lung (2.57-fold), and stomach cancer (2.20-fold). “They reported that the first 6 months after the diagnosis is associated with an increased risk of suicide – unrelated to prognosis,” Dr. Nemeroff said.
A separate analysis of SEER data from 1973-2014 and comprising more than 8.6 million cancer patients found that newly diagnosed cancer patients are 4.4 times more likely to die from suicide than patients in the same age group without cancer (Nat Commun 2019;10[1]:207). The highest risk was in lung cancer, followed by head and neck, testes, bladder, and Hodgkin’s lymphoma.
According to Dr. Nemeroff, For example, he said, if the depressed environment is associated with a marked increase in tumor necrosis factor, interleukin 6, and other inflammatory markers, “that probably contributes to the body’s ability to fight disease. Ironically, depression is associated with an increase in inflammation but a decreased in T cell function. Remember, there are two fundamental types of immunity: the antibody response and the cellular response. What’s odd about depression is that there’s an increase in inflammatory markers but a decrease in the ability of T cells to function in terms of cellular immunity.”
Dr. Nemeroff disclosed that he has served as a consultant and/or scientific adviser for numerous pharmaceutical companies. He has received research and grant support from the National Institutes of Health.
FROM NPA 2022
Depression, suicidal ideation continue to plague physicians: Survey
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
What is the psychological impact of type 1 diabetes?
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
DSM-5 update: What’s new?
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Self-care tips for clinicians as COVID-19 lingers
LAS VEGAS – according to Jon A. Levenson, MD.
“There are those who will need mental health treatment, so creating an easy way to reach out for help and facilitate linkage with care is critically important,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The vast majority of our workforce will thrive with proper support. But what can each of us do to take care of ourselves?”
Step one is to recognize common stress reactions as well as signs of distress. He offered the oxygen mask metaphor, the idea that before we can take care of and support anyone else, we must first take care of ourselves. “When people are stressed, they don’t always think about the oxygen mask metaphor,” Dr. Levenson said. Step two is to practice and model self-care by adopting principles often discussed in acceptance and commitment therapy: to focus on what you can control, not on what you can’t control.
“We can’t control the amount of toilet paper at the grocery store, how long the pandemic will last, or how others have reacted,” Dr. Levenson said. “We also can’t control other people’s motives, predict what will happen, or the actions of others, including whether they will follow social distancing guidelines or not.”
How about what we can control? One is a positive attitude, “which can sustain people during times of intense stress,” he said. “Other things that we can do include turn off the news and find fun and enriching activities to do at home, whether it be playing a game with family or reaching out to friends through an iPad or a smartphone. You can also follow [Centers for Disease Control and Prevention] recommendations, control your own social distancing, and limit social media activity, which can be stressful. We can also control our kindness and grace.” He added that resilience does not mean “snapping back” to how you were before the pandemic, but rather “learning to integrate the adverse experiences into who you are and growing with them, which is sometimes known as posttraumatic growth.”
Dr. Levenson encouraged health care workers to use their coping resources, connect to others, and cultivate their values and purpose in life as they navigate these challenging times. “You also want to promote realistic optimism; find a way to stay positive,” he said. “We emphasize to our staff that while you won’t forget this time, focus on what you can control – your positive relationships – and remind yourself of your values and sources of gratitude. Figure out, and reflect on, what you care about, and then care about it. Remind yourself in a deliberate, purposeful way what anchors you to your job, which in the health care setting tends to be a desire to care for others, to assist those in need, and to work in teams. We also encourage staff to refrain from judgment. Guilt is a normal and near-universal response to this stressor, but there are many ways to contribute without a judgmental or guilty tone.”
Other tips for self-support are to remind yourself that it is not selfish to take breaks. “The needs of your patients are not more important than your own needs,” Dr. Levenson said. “Working nonstop can put you at higher risk for stress, exhaustion, and illness. You may need to give yourself more time to step back and recover from workplace challenges or extended coverage for peers; this is important. We remind our staff that your work may feel more emotionally draining than usual because everything is more intense overall during the COVID-19 pandemic. This reminder helps staff normalize what they already may be experiencing, and in turn, to further support each other.”
Soothing activities to relieve stress include meditation, prayer, deep and slow breathing, relaxation exercises, yoga, mindfulness, stretching, staying hydrated, eating healthfully, exercise, and getting sufficient sleep. Other stress management tips include avoiding excessive alcohol intake, reaching out to others, asking for assistance, and delegating when possible. “We want to promote psychological flexibility: the ability to stay in contact with the present moment,” he said. “We encourage our peers to be aware of unpleasant thoughts and feelings, and to try to redirect negative thought patterns to a proactive problem-solving approach; this includes choosing one’s behaviors based on the situation and personal values.”
Dr. Levenson reported having no disclosures related to his presentation.
LAS VEGAS – according to Jon A. Levenson, MD.
“There are those who will need mental health treatment, so creating an easy way to reach out for help and facilitate linkage with care is critically important,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The vast majority of our workforce will thrive with proper support. But what can each of us do to take care of ourselves?”
Step one is to recognize common stress reactions as well as signs of distress. He offered the oxygen mask metaphor, the idea that before we can take care of and support anyone else, we must first take care of ourselves. “When people are stressed, they don’t always think about the oxygen mask metaphor,” Dr. Levenson said. Step two is to practice and model self-care by adopting principles often discussed in acceptance and commitment therapy: to focus on what you can control, not on what you can’t control.
“We can’t control the amount of toilet paper at the grocery store, how long the pandemic will last, or how others have reacted,” Dr. Levenson said. “We also can’t control other people’s motives, predict what will happen, or the actions of others, including whether they will follow social distancing guidelines or not.”
How about what we can control? One is a positive attitude, “which can sustain people during times of intense stress,” he said. “Other things that we can do include turn off the news and find fun and enriching activities to do at home, whether it be playing a game with family or reaching out to friends through an iPad or a smartphone. You can also follow [Centers for Disease Control and Prevention] recommendations, control your own social distancing, and limit social media activity, which can be stressful. We can also control our kindness and grace.” He added that resilience does not mean “snapping back” to how you were before the pandemic, but rather “learning to integrate the adverse experiences into who you are and growing with them, which is sometimes known as posttraumatic growth.”
Dr. Levenson encouraged health care workers to use their coping resources, connect to others, and cultivate their values and purpose in life as they navigate these challenging times. “You also want to promote realistic optimism; find a way to stay positive,” he said. “We emphasize to our staff that while you won’t forget this time, focus on what you can control – your positive relationships – and remind yourself of your values and sources of gratitude. Figure out, and reflect on, what you care about, and then care about it. Remind yourself in a deliberate, purposeful way what anchors you to your job, which in the health care setting tends to be a desire to care for others, to assist those in need, and to work in teams. We also encourage staff to refrain from judgment. Guilt is a normal and near-universal response to this stressor, but there are many ways to contribute without a judgmental or guilty tone.”
Other tips for self-support are to remind yourself that it is not selfish to take breaks. “The needs of your patients are not more important than your own needs,” Dr. Levenson said. “Working nonstop can put you at higher risk for stress, exhaustion, and illness. You may need to give yourself more time to step back and recover from workplace challenges or extended coverage for peers; this is important. We remind our staff that your work may feel more emotionally draining than usual because everything is more intense overall during the COVID-19 pandemic. This reminder helps staff normalize what they already may be experiencing, and in turn, to further support each other.”
Soothing activities to relieve stress include meditation, prayer, deep and slow breathing, relaxation exercises, yoga, mindfulness, stretching, staying hydrated, eating healthfully, exercise, and getting sufficient sleep. Other stress management tips include avoiding excessive alcohol intake, reaching out to others, asking for assistance, and delegating when possible. “We want to promote psychological flexibility: the ability to stay in contact with the present moment,” he said. “We encourage our peers to be aware of unpleasant thoughts and feelings, and to try to redirect negative thought patterns to a proactive problem-solving approach; this includes choosing one’s behaviors based on the situation and personal values.”
Dr. Levenson reported having no disclosures related to his presentation.
LAS VEGAS – according to Jon A. Levenson, MD.
“There are those who will need mental health treatment, so creating an easy way to reach out for help and facilitate linkage with care is critically important,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The vast majority of our workforce will thrive with proper support. But what can each of us do to take care of ourselves?”
Step one is to recognize common stress reactions as well as signs of distress. He offered the oxygen mask metaphor, the idea that before we can take care of and support anyone else, we must first take care of ourselves. “When people are stressed, they don’t always think about the oxygen mask metaphor,” Dr. Levenson said. Step two is to practice and model self-care by adopting principles often discussed in acceptance and commitment therapy: to focus on what you can control, not on what you can’t control.
“We can’t control the amount of toilet paper at the grocery store, how long the pandemic will last, or how others have reacted,” Dr. Levenson said. “We also can’t control other people’s motives, predict what will happen, or the actions of others, including whether they will follow social distancing guidelines or not.”
How about what we can control? One is a positive attitude, “which can sustain people during times of intense stress,” he said. “Other things that we can do include turn off the news and find fun and enriching activities to do at home, whether it be playing a game with family or reaching out to friends through an iPad or a smartphone. You can also follow [Centers for Disease Control and Prevention] recommendations, control your own social distancing, and limit social media activity, which can be stressful. We can also control our kindness and grace.” He added that resilience does not mean “snapping back” to how you were before the pandemic, but rather “learning to integrate the adverse experiences into who you are and growing with them, which is sometimes known as posttraumatic growth.”
Dr. Levenson encouraged health care workers to use their coping resources, connect to others, and cultivate their values and purpose in life as they navigate these challenging times. “You also want to promote realistic optimism; find a way to stay positive,” he said. “We emphasize to our staff that while you won’t forget this time, focus on what you can control – your positive relationships – and remind yourself of your values and sources of gratitude. Figure out, and reflect on, what you care about, and then care about it. Remind yourself in a deliberate, purposeful way what anchors you to your job, which in the health care setting tends to be a desire to care for others, to assist those in need, and to work in teams. We also encourage staff to refrain from judgment. Guilt is a normal and near-universal response to this stressor, but there are many ways to contribute without a judgmental or guilty tone.”
Other tips for self-support are to remind yourself that it is not selfish to take breaks. “The needs of your patients are not more important than your own needs,” Dr. Levenson said. “Working nonstop can put you at higher risk for stress, exhaustion, and illness. You may need to give yourself more time to step back and recover from workplace challenges or extended coverage for peers; this is important. We remind our staff that your work may feel more emotionally draining than usual because everything is more intense overall during the COVID-19 pandemic. This reminder helps staff normalize what they already may be experiencing, and in turn, to further support each other.”
Soothing activities to relieve stress include meditation, prayer, deep and slow breathing, relaxation exercises, yoga, mindfulness, stretching, staying hydrated, eating healthfully, exercise, and getting sufficient sleep. Other stress management tips include avoiding excessive alcohol intake, reaching out to others, asking for assistance, and delegating when possible. “We want to promote psychological flexibility: the ability to stay in contact with the present moment,” he said. “We encourage our peers to be aware of unpleasant thoughts and feelings, and to try to redirect negative thought patterns to a proactive problem-solving approach; this includes choosing one’s behaviors based on the situation and personal values.”
Dr. Levenson reported having no disclosures related to his presentation.
AT NPA 2022
Psychoses: The 5 comorbidity-defined subtypes
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
Nonpsychiatric indications for antidepressants and antipsychotics
Ms. A, age 45, is hospitalized for abdominal pain. She is noted to have hiccups, the onset of which she reports was >1 month ago and did not have a clear precipitant. Abdominal and head imaging return no acute findings, and data from a serum electrolyte test, hepatic function test, and thyroid function test are within normal limits. The medical team notices that Ms. A’s speech is pressured, she hardly sleeps, and she appears animated, full of ideas and energy.
Ms. A has a history of bipolar I disorder, hypertension, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. Her present medications include hydrochlorothiazide 25 mg/d; levothyroxine 25 mcg/d; omeprazole 20 mg/d; and lovastatin 20 mg/d. She states that she was remotely treated for bipolar disorder, but she was cured by a shamanic healer, and therefore no longer needs treatment.
Approximately 35% of adults in the United States age 60 to 79 reported taking ≥5 prescription medications in 2016, compared to 15% of adults age 40 to 59.1 In a study of 372 patients with advanced, life-limiting illness, Schenker et al2 found that those who took multiple medications (mean: 11.6 medications) had a lower quality of life and worse symptoms. Optimizing medications to patients’ specific needs and diagnoses in order to reduce pill burden can be a favorable intervention. In addition, some patients—approximately 30% of those with schizophrenia and 20% of those with bipolar disorder—may not have insight into their mental illness as they do with their medical conditions, and may be more accepting of treatment for the latter.3 Dual-indication prescribing may be a useful way to decrease polypharmacy, reduce potential drug-drug interactions (DDIs), increase patient acceptance and adherence, and improve a patient’s overall health.
Continue on for: Multiple uses for antidepressants and antipsychotics...
Multiple uses for antidepressants and antipsychotics
One of the first medications discovered to have antidepressant effects was iproniazid, a monoamine oxidase inhibitor (MAOI) initially used to treat tuberculosis.4 Since then, numerous classes of antidepressant medications have been developed that capitalize on monoamine reuptake through several different mechanisms of action. These drugs can be grouped into subclasses that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, MAOIs, and others. True to their roots in iproniazid, these medications can have a myriad of effects not limited to mental health and can therefore be beneficial for a variety of comorbid conditions.
As was the case with antidepressants, the first medication approved in the antipsychotic class, chlorpromazine, was serendipitously discovered to treat psychosis and agitation after being approved and used to treat presurgical apprehension.5 The term “antipsychotic” is almost a misnomer given these agents’ broad pharmacology profiles and impact on various mental illnesses, including bipolar disorder, depressive disorders, anxiety disorders, and many other mental conditions. First-generation antipsychotics (FGAs) were the first to enter the market; they work primarily by blocking dopamine-2 (D2) receptors. Second-generation antipsychotics have less movement-based adverse effects than FGAs by having higher affinity for serotonin 5-HT2A receptors than for D2 receptors. However, they tend to carry a higher risk for weight gain and metabolic syndrome.
Antidepressants and antipsychotics are widely utilized in psychiatry. Many have been found to have additional uses beyond their original FDA-approved indication and can therefore be beneficial for a variety of comorbid conditions.
One limitation of using psychiatric medications for nonpsychiatric indications is that different doses of antidepressants and antipsychotics are typically targeted for different indications based on receptor binding affinity. A common example of this is trazodone, where doses below 100 mg are used as needed for insomnia, but higher doses ranging from 200 to 600 mg/d are used for depression. Another important consideration is DDIs. For example, the possibility of adding an agent such as fluoxetine to a complex pain regimen for fibromyalgia could impact the clearance of other agents that are cytochrome P450 (CYP) 2D6 substrates due to fluoxetine’s potent inhibition of the enzyme.6,7 Table 16-51, Table 252-68, Table 369-107, and Table 4108-123 provide information on select antidepressants, while Table 5124-140 and Table 6141-171 provide information on select antipsychotics. Each table lists psychiatric and nonpsychiatric indications for the respective medications, including both FDA-approved (where applicable) and common off-label uses. Most of the indications listed are for adult use only, unless otherwise noted.
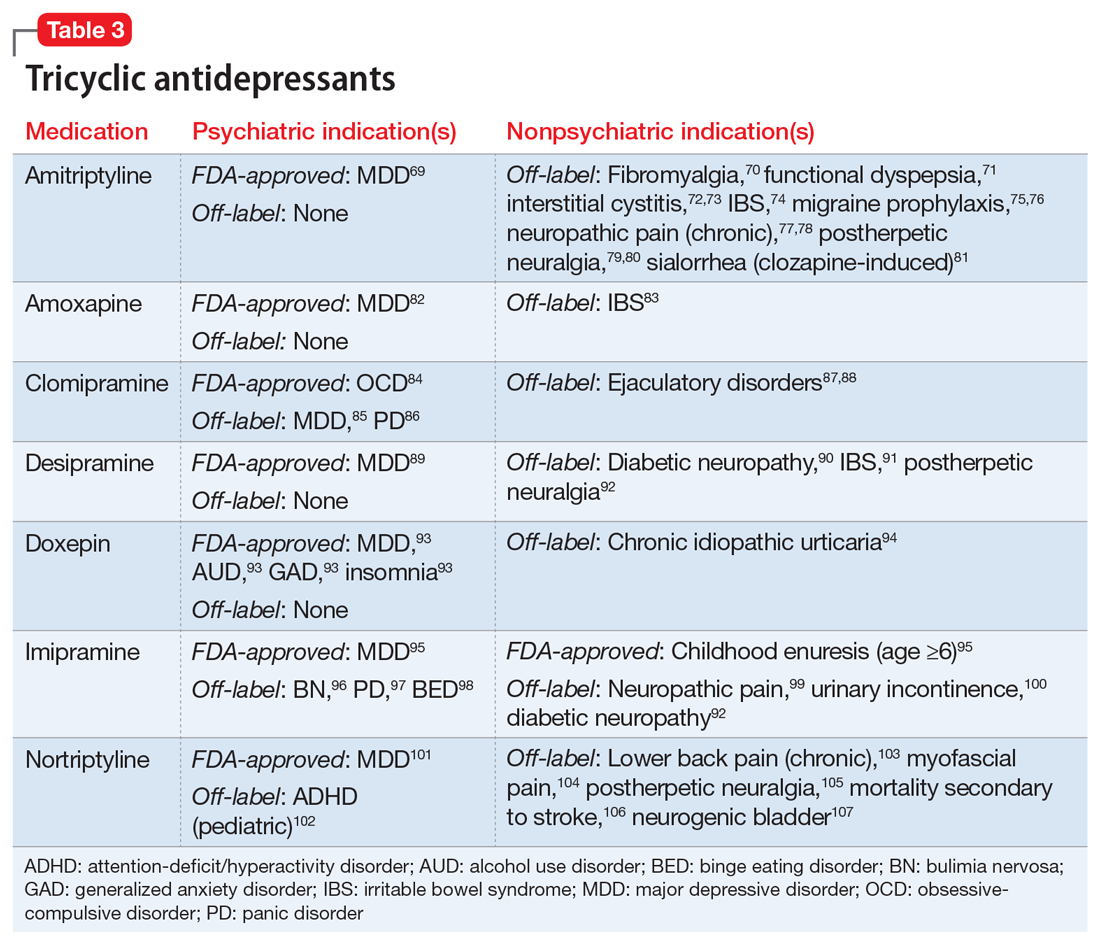
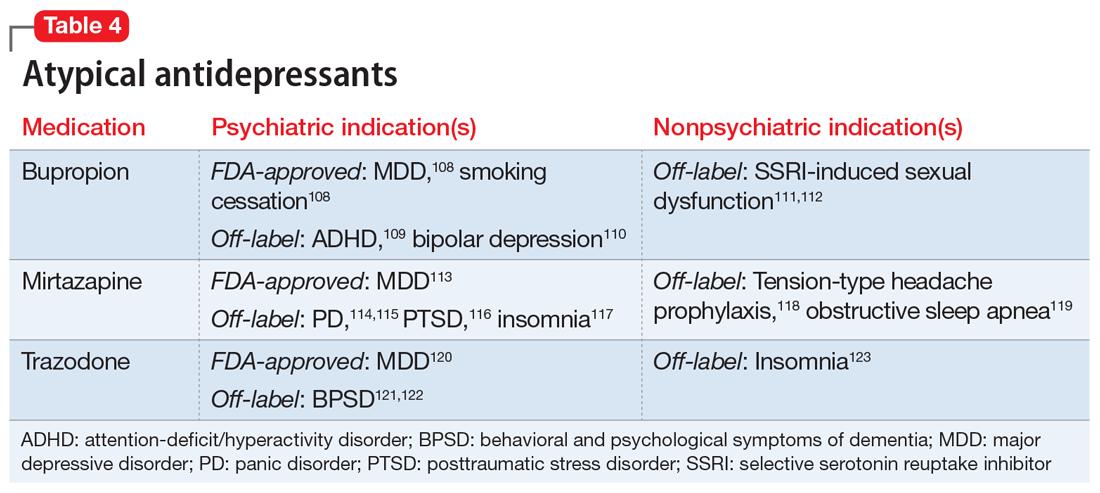
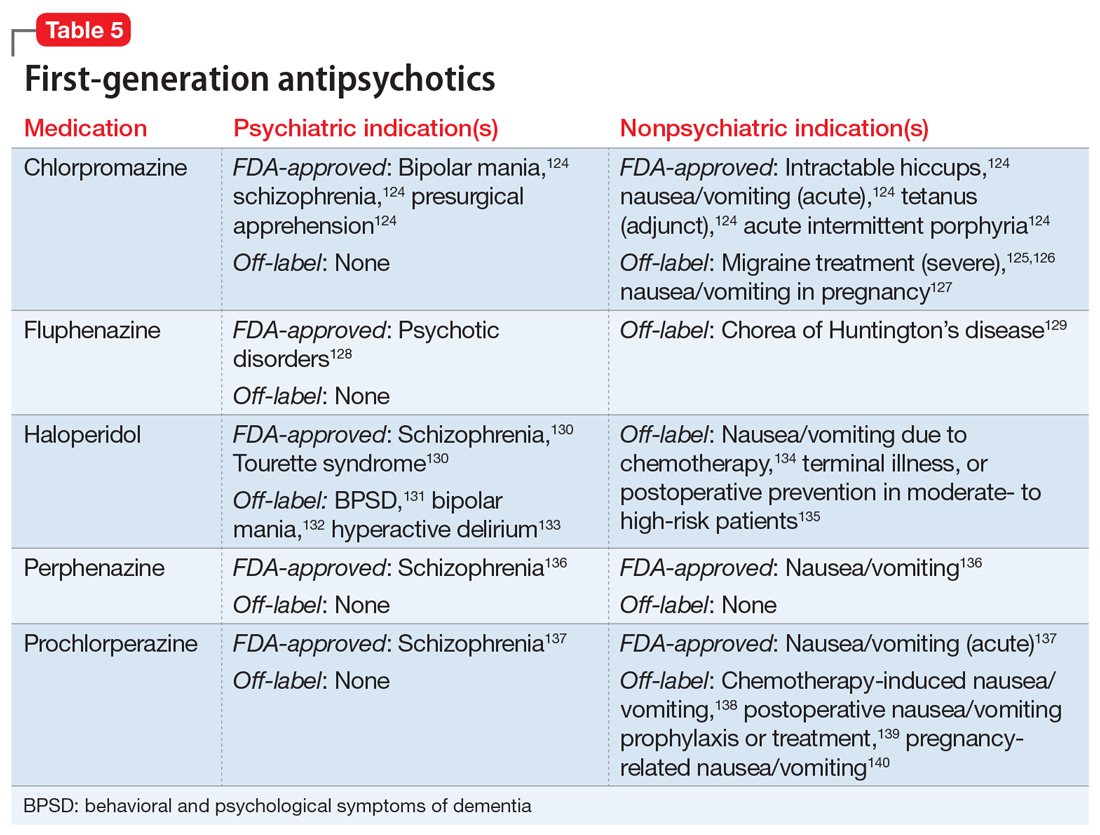
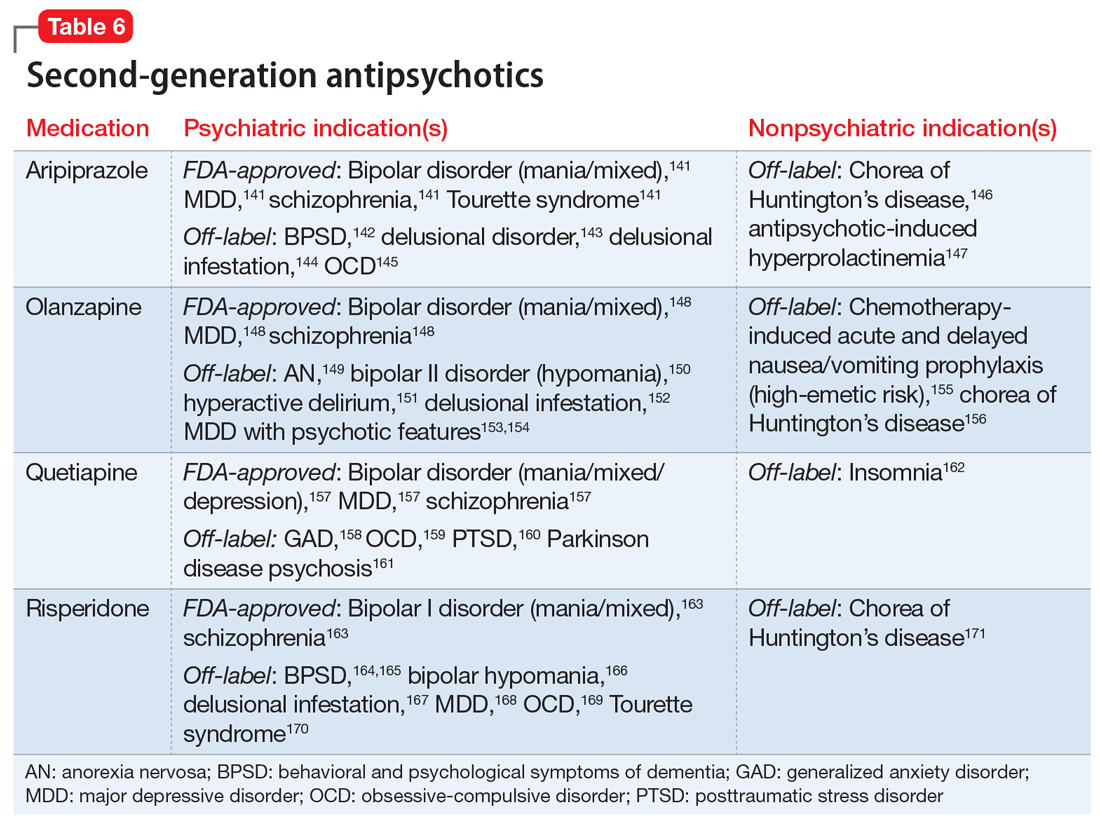
Continue on to: Case Continued...
CASE CONTINUED
After reviewing Ms. A’s medical history, the treatment team initiates chlorpromazine, 25 mg 3 times a day, for intractable hiccups, and increases the dosage to 50 mg 3 times a day after 3 days. Chlorpromazine is FDA-approved for treating bipolar mania, and also for treating intractable hiccups. Shortly thereafter, Ms. A’s hiccups subside, she sleeps for longer periods, and her manic symptoms resolve.
1. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. National Center for Health Statistics (Centers for Disease Control and Prevention). 2019. NCHS Data Brief No. 347. https://www.cdc.gov/nchs/products/databriefs/db347.htm
2. Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566.
3. National Alliance on Mental Illness. Anosognosia. 2021. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
4. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
5. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
6. Prozac [package insert]. Indianapolis, IN: Eli Lilly and Company; 2009.
7. Arnold LM, Hess EV, Hudson JI, et al. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112(3):191-197.
8. Celexa [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2009.
9. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
10. McElroy SL, Hudson JI, Malhotra S, et al. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64(7):807-813.
11. Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67(3):468-472.
12. Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146-150.
13. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86.
14. Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25(1):24-32.
15. Perna G, Bertani A, Caldirola D, et al. A comparison of citalopram and paroxetine in the treatment of panic disorder: a randomized, single-blind study. Pharmacopsychiatry. 2001;34(3):85-90.
16. Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. 1998;18(5):390-398.
17. English BA, Jewell M, Jewell G, et al. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram: an open trial. J Clin Psychopharmacol. 2006;26(1):84-88.
18. Furmark T, Appel L, Michelgård A, et al. Cerebral blood flow changes after treatment of social phobia with neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58(2):132-142.
19. Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51(6):729-739.
20. Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18(2):164-169.
21. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204-213.
22. Lexapro [package insert]. Irvine, CA: Allergan USA, Inc; 2016.
23. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
24. Aigner M, Treasure J, Kaye W, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443.
25. Fineberg NA, Tonnoir B, Lemming O, et al. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6-7):430-439.
26. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
27. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64(11):1322-1327.
28. Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769-773.
29. Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37(1):89-93.
30. Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399-1404.
31. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
32. Arnold LM, McElroy SL, Hudson JI, et al. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. 2002;63(11):1028-1033.
33. Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in posttraumatic stress disorder. Randomized, double-blind study. Br J Psychiatry. 1999;175:17-22.
34. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63(3):199-206.
35. Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61(10):1005-1013.
36. Kara H, Aydin S, Yücel M, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo-controlled study. J Urol. 1996;156(5):1631-1632.
37. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578-1583.
38. Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40(9):1038-1043.
39. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
40. Zhang D, Cheng Y, Wu K, et al. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2.
41. Walitt B, Urrútia G, Nishishinya MB. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
42. Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587-589.
43. Zylicz Z, Krajnik M, Sorge A, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26(3):1105-1112.
44. Zoloft [package insert]. New York, NY: Pfizer; 2016.
45. Leombruni P, Pierò A, Lavagnino L, et al. A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1599-1605.
46. McElroy SL, Casuto LS, Nelson EB, et al. Placebo-controlled trial of sertraline in the treatment of binge eating disorder. Am J Psychiatry. 2000;157(6):1004-1006.
47. Milano W, Petrella C, Sabatino C, et al. Treatment of bulimia nervosa with sertraline: a randomized controlled trial. Adv Ther. 2004;21(4):232-237.
48. Brawman-Mintzer O, Knapp RG, Rynn M, et al. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874-881.
49. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo-controlled crossover study. J Urol. 1998;159(6):1935-1938.
50. Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore). 2019;98(23):e15989.
51. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279-1298.
52. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2011.
53. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75(4):255-262.
54. Cymbalta [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
55. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systemic review and meta-analysis. Int Urol Nephrol. 2013;45(3):679-686.
56. Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559-1564.
57. Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2017.
58. Denys D, Van der Wee N, Van Megen HJ, et al. A double-blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
59. Albert U, Aguglia E, Maina G, et al. Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry. 2002;63(11):1004-1009.
60. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158-1165.
61. Zarinara AR, Mohammad MR, Hazrati N, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol. 2010;25(7-8):530-535.
62. Mukaddes NM, Abali O. Venlafaxine in children and adolescents with attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):92-95.
63. Cohen LS, Soares CN, Lyster A, et al. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540-543.
64. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152.
65. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258.
66. Kadiroglu AK, Sit D, Kayabasi H, et al. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241-245.
67. Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161-166.
68. Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82(5):787-793.
69. Amitriptyline Hydrochloride [package insert]. Princeton, NJ: Sandoz Inc; 2014.
70. Hauser W, Wolfe F, Tolle T, et al. The role of antidepressants in the management of fibromyalgia syndrome: a systemic review and meta-analysis. CNS Drugs. 2012;26(4):297-307.
71. Braak B, Klooker T, Lei A, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(6):638-648.
72. Van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
73. Foster HE Jr, Hanno P, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
74. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominent irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678-684.
75. Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: a randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107(1):44-48.
76. Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110(10):979-984.
77. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
78. Boyle J, Eriksson M, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451-2458.
79. Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16(3):188-192.
80. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671-673.
81. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf. 2016;11(3):262-263.
82. Amoxapine [package insert]. Parsippany, NJ: Watson Pharma, Inc; 2014.
83. Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1146-1148.
84. Anafranil (clomipramine hydrochloride) [package insert]. Whitby, Ontario: Patheon Inc; 2012.
85. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Danish University Antidepressant Group (DUAG). Clin Pharmacol Ther. 1999;66(2):152-165.
86. Caillard V, Rouillon F, Viel J, et al. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatr Scand. 1999;99(1):51-58.
87. Segraves RT, Saran A, Segraves K, et al. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Therap. 1993;19(3):198-200.
88. Rowland DL, de Gouveia Brazao CA, Koos Slob A. Effective daily treatment with clomipramine in men with premature ejaculation when 25 mg (as required) is ineffective. BJU Int. 2001;87(4):357-360.
89. Norpramin (desipramine hydrochloride) [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2014.
90. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3-9.
91. Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19-31.
92. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systemic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
93. Doxepin hydrochloride [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2014.
94. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867-873.
95. Imipramine hydrochloride [package insert]. Fairfield, NJ: Excellium Pharmaceutical, Inc; 2012.
96. Pope HG Jr, Hudson JI, Jonas JM, et al. Bulimia treated with imipramine: a placebo-controlled, double-blind study. Am J Psychiatry. 1983;140(5):554-558.
97. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
98. Laederach-Hofmann K, Graf C, Horber F, et al. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. Int J Eat Disord. 1999;26(3):231-244.
99. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284-1289.
100. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes of imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
101. Pamelor (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2007.
102. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
103. Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287-296.
104. Desai MJ, Saini V, Saini S. Myofacial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21-36.
105. Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial – the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363.
106. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
107. Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double-blind placebo-controlled study. Br J Urol. 1984;56(2):178-179.
108. Wellbutrin (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
109. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
110. Li DJ, Tseng PT, Chen YW, et al. Significant treatment effect of bupropion in patients with bipolar disorder but similar phase-shifting rate as other antidepressants: a meta-analysis following the PRISMA guidelines. Medicine (Baltimore). 2016;95(13):e3165.
111. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65(1):62-67.
112. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25(3):370-378.
113. Remeron (mirtazapine) [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2020.
114. Boshuisen ML, Slaap BR, Vester-Blokland ED, et al. The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363-368.
115. Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol. 2003;18(1):35-38.
116. Connor KM, Davidson JR, Weisler RH, et al. A pilot study of mirtazapine in post-traumatic stress disorder. Int Clin Psychopharmacol. 1999;14(1):29-31.
117. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
118. Bedtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706-1711.
119. AbdelFattah MR, Jung SW, Greenspan MA, et al. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. A systemic review with meta-analysis. Sleep Breath. 2020;24(2):443-453.
120. Desyrel [package insert]. Locust Valley, NY: Pragma Pharmaceuticals, LLC; 2017.
121. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359.
122. Sultzer DL, Gray KF, Gunay I, et al. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997;5(1):60-69.
123. Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32.
124. Chlorpromazine hydrochloride [package insert]. Minneapolis, MN: Upsher-Smith Laboratories, Inc; 2010.
125. Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
126. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
127. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-e30.
128. Fluphenazine hydrochloride [package insert]. Philadelphia, PA: Lannett Company, Inc; 2019.
129. Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701-2720.
130. Haldol [package insert]. Columbus, OH: American Health Packaging; 2020.
131. MacDonald K, Wilson M, Minassian A, et al. A naturalistic study for intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317-322.
132. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
133. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
134. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
135. Büttner M, Walder B, von Elm E, et al. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454-1463.
136. Perphenazine [package insert]. Princeton, NJ: Sandoz Inc; 2010.
137. Compazine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
138. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
139. Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158(19):2124-2128.
140. Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137.
141. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
142. Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601-607.
143. Iannuzzi GL, Patel AA, Stewart JT. Aripiprazole and delusional disorder. J Psychiatr Pract. 2019;25(2):132-134.
144. Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434.
145. Sayyah M, Sayyah M, Boostani H, et al. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double-blind clinical trial). Depress Anxiety. 2012;29(10):850-854.
146. Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
147. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179.
148. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
149. Attia E, Steinglass JE, Walsh BT, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449-456.
150. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol. 2003;18(3):143-145.
151. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277-281.
152. Bosmans A, Verbanck P. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry. 2008;41(3):121-122.
153. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
154. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
155. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-195.
156. Bonelli RM, Mahnert FA, Niederwieser G. Olanzapine for Huntington’s disease: an open label study. Clin Neuropharmacol. 2002;25(5):263-265.
157. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013.
158. Khan A, Atkinson S, Mezhebovsky I, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with generalized anxiety disorder and a history of inadequate treatment response: a randomized, double-blind study. Ann Clin Psychiatry. 2014;26(1):3-18.
159. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
160. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
161. Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14(3):484-487.
162. Doroudgar S, Chou T, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):PCC.13m01558. doi: 10.4088/PCC.13m01558
163. Risperdal [package insert]. Titusville, NJ: Janssen Pharamceuticals, Inc; 2007.
164. Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81-86.
165. Currier GW, Chou J, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
166. Bahk WM, Yoon JS, Kim YH, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol. 2004;19(5):299-303.
167. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508.
168. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9): 980-991.
169. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794-801.
170. Scahill L, Leckman JF, Schulz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
171. Dallocchio C, Buffa C, Tinelli C, et al. Effectiveness of risperidone in Huntington Chorea patients. J Clin Psychopharmacol. 1999;19(1):101-103.
Ms. A, age 45, is hospitalized for abdominal pain. She is noted to have hiccups, the onset of which she reports was >1 month ago and did not have a clear precipitant. Abdominal and head imaging return no acute findings, and data from a serum electrolyte test, hepatic function test, and thyroid function test are within normal limits. The medical team notices that Ms. A’s speech is pressured, she hardly sleeps, and she appears animated, full of ideas and energy.
Ms. A has a history of bipolar I disorder, hypertension, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. Her present medications include hydrochlorothiazide 25 mg/d; levothyroxine 25 mcg/d; omeprazole 20 mg/d; and lovastatin 20 mg/d. She states that she was remotely treated for bipolar disorder, but she was cured by a shamanic healer, and therefore no longer needs treatment.
Approximately 35% of adults in the United States age 60 to 79 reported taking ≥5 prescription medications in 2016, compared to 15% of adults age 40 to 59.1 In a study of 372 patients with advanced, life-limiting illness, Schenker et al2 found that those who took multiple medications (mean: 11.6 medications) had a lower quality of life and worse symptoms. Optimizing medications to patients’ specific needs and diagnoses in order to reduce pill burden can be a favorable intervention. In addition, some patients—approximately 30% of those with schizophrenia and 20% of those with bipolar disorder—may not have insight into their mental illness as they do with their medical conditions, and may be more accepting of treatment for the latter.3 Dual-indication prescribing may be a useful way to decrease polypharmacy, reduce potential drug-drug interactions (DDIs), increase patient acceptance and adherence, and improve a patient’s overall health.
Continue on for: Multiple uses for antidepressants and antipsychotics...
Multiple uses for antidepressants and antipsychotics
One of the first medications discovered to have antidepressant effects was iproniazid, a monoamine oxidase inhibitor (MAOI) initially used to treat tuberculosis.4 Since then, numerous classes of antidepressant medications have been developed that capitalize on monoamine reuptake through several different mechanisms of action. These drugs can be grouped into subclasses that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, MAOIs, and others. True to their roots in iproniazid, these medications can have a myriad of effects not limited to mental health and can therefore be beneficial for a variety of comorbid conditions.
As was the case with antidepressants, the first medication approved in the antipsychotic class, chlorpromazine, was serendipitously discovered to treat psychosis and agitation after being approved and used to treat presurgical apprehension.5 The term “antipsychotic” is almost a misnomer given these agents’ broad pharmacology profiles and impact on various mental illnesses, including bipolar disorder, depressive disorders, anxiety disorders, and many other mental conditions. First-generation antipsychotics (FGAs) were the first to enter the market; they work primarily by blocking dopamine-2 (D2) receptors. Second-generation antipsychotics have less movement-based adverse effects than FGAs by having higher affinity for serotonin 5-HT2A receptors than for D2 receptors. However, they tend to carry a higher risk for weight gain and metabolic syndrome.
Antidepressants and antipsychotics are widely utilized in psychiatry. Many have been found to have additional uses beyond their original FDA-approved indication and can therefore be beneficial for a variety of comorbid conditions.
One limitation of using psychiatric medications for nonpsychiatric indications is that different doses of antidepressants and antipsychotics are typically targeted for different indications based on receptor binding affinity. A common example of this is trazodone, where doses below 100 mg are used as needed for insomnia, but higher doses ranging from 200 to 600 mg/d are used for depression. Another important consideration is DDIs. For example, the possibility of adding an agent such as fluoxetine to a complex pain regimen for fibromyalgia could impact the clearance of other agents that are cytochrome P450 (CYP) 2D6 substrates due to fluoxetine’s potent inhibition of the enzyme.6,7 Table 16-51, Table 252-68, Table 369-107, and Table 4108-123 provide information on select antidepressants, while Table 5124-140 and Table 6141-171 provide information on select antipsychotics. Each table lists psychiatric and nonpsychiatric indications for the respective medications, including both FDA-approved (where applicable) and common off-label uses. Most of the indications listed are for adult use only, unless otherwise noted.






Continue on to: Case Continued...
CASE CONTINUED
After reviewing Ms. A’s medical history, the treatment team initiates chlorpromazine, 25 mg 3 times a day, for intractable hiccups, and increases the dosage to 50 mg 3 times a day after 3 days. Chlorpromazine is FDA-approved for treating bipolar mania, and also for treating intractable hiccups. Shortly thereafter, Ms. A’s hiccups subside, she sleeps for longer periods, and her manic symptoms resolve.
Ms. A, age 45, is hospitalized for abdominal pain. She is noted to have hiccups, the onset of which she reports was >1 month ago and did not have a clear precipitant. Abdominal and head imaging return no acute findings, and data from a serum electrolyte test, hepatic function test, and thyroid function test are within normal limits. The medical team notices that Ms. A’s speech is pressured, she hardly sleeps, and she appears animated, full of ideas and energy.
Ms. A has a history of bipolar I disorder, hypertension, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. Her present medications include hydrochlorothiazide 25 mg/d; levothyroxine 25 mcg/d; omeprazole 20 mg/d; and lovastatin 20 mg/d. She states that she was remotely treated for bipolar disorder, but she was cured by a shamanic healer, and therefore no longer needs treatment.
Approximately 35% of adults in the United States age 60 to 79 reported taking ≥5 prescription medications in 2016, compared to 15% of adults age 40 to 59.1 In a study of 372 patients with advanced, life-limiting illness, Schenker et al2 found that those who took multiple medications (mean: 11.6 medications) had a lower quality of life and worse symptoms. Optimizing medications to patients’ specific needs and diagnoses in order to reduce pill burden can be a favorable intervention. In addition, some patients—approximately 30% of those with schizophrenia and 20% of those with bipolar disorder—may not have insight into their mental illness as they do with their medical conditions, and may be more accepting of treatment for the latter.3 Dual-indication prescribing may be a useful way to decrease polypharmacy, reduce potential drug-drug interactions (DDIs), increase patient acceptance and adherence, and improve a patient’s overall health.
Continue on for: Multiple uses for antidepressants and antipsychotics...
Multiple uses for antidepressants and antipsychotics
One of the first medications discovered to have antidepressant effects was iproniazid, a monoamine oxidase inhibitor (MAOI) initially used to treat tuberculosis.4 Since then, numerous classes of antidepressant medications have been developed that capitalize on monoamine reuptake through several different mechanisms of action. These drugs can be grouped into subclasses that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, MAOIs, and others. True to their roots in iproniazid, these medications can have a myriad of effects not limited to mental health and can therefore be beneficial for a variety of comorbid conditions.
As was the case with antidepressants, the first medication approved in the antipsychotic class, chlorpromazine, was serendipitously discovered to treat psychosis and agitation after being approved and used to treat presurgical apprehension.5 The term “antipsychotic” is almost a misnomer given these agents’ broad pharmacology profiles and impact on various mental illnesses, including bipolar disorder, depressive disorders, anxiety disorders, and many other mental conditions. First-generation antipsychotics (FGAs) were the first to enter the market; they work primarily by blocking dopamine-2 (D2) receptors. Second-generation antipsychotics have less movement-based adverse effects than FGAs by having higher affinity for serotonin 5-HT2A receptors than for D2 receptors. However, they tend to carry a higher risk for weight gain and metabolic syndrome.
Antidepressants and antipsychotics are widely utilized in psychiatry. Many have been found to have additional uses beyond their original FDA-approved indication and can therefore be beneficial for a variety of comorbid conditions.
One limitation of using psychiatric medications for nonpsychiatric indications is that different doses of antidepressants and antipsychotics are typically targeted for different indications based on receptor binding affinity. A common example of this is trazodone, where doses below 100 mg are used as needed for insomnia, but higher doses ranging from 200 to 600 mg/d are used for depression. Another important consideration is DDIs. For example, the possibility of adding an agent such as fluoxetine to a complex pain regimen for fibromyalgia could impact the clearance of other agents that are cytochrome P450 (CYP) 2D6 substrates due to fluoxetine’s potent inhibition of the enzyme.6,7 Table 16-51, Table 252-68, Table 369-107, and Table 4108-123 provide information on select antidepressants, while Table 5124-140 and Table 6141-171 provide information on select antipsychotics. Each table lists psychiatric and nonpsychiatric indications for the respective medications, including both FDA-approved (where applicable) and common off-label uses. Most of the indications listed are for adult use only, unless otherwise noted.






Continue on to: Case Continued...
CASE CONTINUED
After reviewing Ms. A’s medical history, the treatment team initiates chlorpromazine, 25 mg 3 times a day, for intractable hiccups, and increases the dosage to 50 mg 3 times a day after 3 days. Chlorpromazine is FDA-approved for treating bipolar mania, and also for treating intractable hiccups. Shortly thereafter, Ms. A’s hiccups subside, she sleeps for longer periods, and her manic symptoms resolve.
1. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. National Center for Health Statistics (Centers for Disease Control and Prevention). 2019. NCHS Data Brief No. 347. https://www.cdc.gov/nchs/products/databriefs/db347.htm
2. Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566.
3. National Alliance on Mental Illness. Anosognosia. 2021. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
4. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
5. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
6. Prozac [package insert]. Indianapolis, IN: Eli Lilly and Company; 2009.
7. Arnold LM, Hess EV, Hudson JI, et al. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112(3):191-197.
8. Celexa [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2009.
9. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
10. McElroy SL, Hudson JI, Malhotra S, et al. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64(7):807-813.
11. Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67(3):468-472.
12. Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146-150.
13. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86.
14. Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25(1):24-32.
15. Perna G, Bertani A, Caldirola D, et al. A comparison of citalopram and paroxetine in the treatment of panic disorder: a randomized, single-blind study. Pharmacopsychiatry. 2001;34(3):85-90.
16. Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. 1998;18(5):390-398.
17. English BA, Jewell M, Jewell G, et al. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram: an open trial. J Clin Psychopharmacol. 2006;26(1):84-88.
18. Furmark T, Appel L, Michelgård A, et al. Cerebral blood flow changes after treatment of social phobia with neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58(2):132-142.
19. Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51(6):729-739.
20. Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18(2):164-169.
21. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204-213.
22. Lexapro [package insert]. Irvine, CA: Allergan USA, Inc; 2016.
23. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
24. Aigner M, Treasure J, Kaye W, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443.
25. Fineberg NA, Tonnoir B, Lemming O, et al. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6-7):430-439.
26. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
27. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64(11):1322-1327.
28. Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769-773.
29. Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37(1):89-93.
30. Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399-1404.
31. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
32. Arnold LM, McElroy SL, Hudson JI, et al. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. 2002;63(11):1028-1033.
33. Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in posttraumatic stress disorder. Randomized, double-blind study. Br J Psychiatry. 1999;175:17-22.
34. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63(3):199-206.
35. Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61(10):1005-1013.
36. Kara H, Aydin S, Yücel M, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo-controlled study. J Urol. 1996;156(5):1631-1632.
37. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578-1583.
38. Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40(9):1038-1043.
39. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
40. Zhang D, Cheng Y, Wu K, et al. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2.
41. Walitt B, Urrútia G, Nishishinya MB. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
42. Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587-589.
43. Zylicz Z, Krajnik M, Sorge A, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26(3):1105-1112.
44. Zoloft [package insert]. New York, NY: Pfizer; 2016.
45. Leombruni P, Pierò A, Lavagnino L, et al. A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1599-1605.
46. McElroy SL, Casuto LS, Nelson EB, et al. Placebo-controlled trial of sertraline in the treatment of binge eating disorder. Am J Psychiatry. 2000;157(6):1004-1006.
47. Milano W, Petrella C, Sabatino C, et al. Treatment of bulimia nervosa with sertraline: a randomized controlled trial. Adv Ther. 2004;21(4):232-237.
48. Brawman-Mintzer O, Knapp RG, Rynn M, et al. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874-881.
49. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo-controlled crossover study. J Urol. 1998;159(6):1935-1938.
50. Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore). 2019;98(23):e15989.
51. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279-1298.
52. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2011.
53. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75(4):255-262.
54. Cymbalta [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
55. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systemic review and meta-analysis. Int Urol Nephrol. 2013;45(3):679-686.
56. Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559-1564.
57. Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2017.
58. Denys D, Van der Wee N, Van Megen HJ, et al. A double-blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
59. Albert U, Aguglia E, Maina G, et al. Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry. 2002;63(11):1004-1009.
60. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158-1165.
61. Zarinara AR, Mohammad MR, Hazrati N, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol. 2010;25(7-8):530-535.
62. Mukaddes NM, Abali O. Venlafaxine in children and adolescents with attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):92-95.
63. Cohen LS, Soares CN, Lyster A, et al. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540-543.
64. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152.
65. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258.
66. Kadiroglu AK, Sit D, Kayabasi H, et al. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241-245.
67. Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161-166.
68. Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82(5):787-793.
69. Amitriptyline Hydrochloride [package insert]. Princeton, NJ: Sandoz Inc; 2014.
70. Hauser W, Wolfe F, Tolle T, et al. The role of antidepressants in the management of fibromyalgia syndrome: a systemic review and meta-analysis. CNS Drugs. 2012;26(4):297-307.
71. Braak B, Klooker T, Lei A, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(6):638-648.
72. Van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
73. Foster HE Jr, Hanno P, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
74. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominent irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678-684.
75. Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: a randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107(1):44-48.
76. Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110(10):979-984.
77. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
78. Boyle J, Eriksson M, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451-2458.
79. Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16(3):188-192.
80. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671-673.
81. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf. 2016;11(3):262-263.
82. Amoxapine [package insert]. Parsippany, NJ: Watson Pharma, Inc; 2014.
83. Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1146-1148.
84. Anafranil (clomipramine hydrochloride) [package insert]. Whitby, Ontario: Patheon Inc; 2012.
85. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Danish University Antidepressant Group (DUAG). Clin Pharmacol Ther. 1999;66(2):152-165.
86. Caillard V, Rouillon F, Viel J, et al. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatr Scand. 1999;99(1):51-58.
87. Segraves RT, Saran A, Segraves K, et al. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Therap. 1993;19(3):198-200.
88. Rowland DL, de Gouveia Brazao CA, Koos Slob A. Effective daily treatment with clomipramine in men with premature ejaculation when 25 mg (as required) is ineffective. BJU Int. 2001;87(4):357-360.
89. Norpramin (desipramine hydrochloride) [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2014.
90. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3-9.
91. Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19-31.
92. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systemic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
93. Doxepin hydrochloride [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2014.
94. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867-873.
95. Imipramine hydrochloride [package insert]. Fairfield, NJ: Excellium Pharmaceutical, Inc; 2012.
96. Pope HG Jr, Hudson JI, Jonas JM, et al. Bulimia treated with imipramine: a placebo-controlled, double-blind study. Am J Psychiatry. 1983;140(5):554-558.
97. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
98. Laederach-Hofmann K, Graf C, Horber F, et al. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. Int J Eat Disord. 1999;26(3):231-244.
99. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284-1289.
100. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes of imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
101. Pamelor (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2007.
102. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
103. Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287-296.
104. Desai MJ, Saini V, Saini S. Myofacial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21-36.
105. Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial – the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363.
106. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
107. Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double-blind placebo-controlled study. Br J Urol. 1984;56(2):178-179.
108. Wellbutrin (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
109. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
110. Li DJ, Tseng PT, Chen YW, et al. Significant treatment effect of bupropion in patients with bipolar disorder but similar phase-shifting rate as other antidepressants: a meta-analysis following the PRISMA guidelines. Medicine (Baltimore). 2016;95(13):e3165.
111. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65(1):62-67.
112. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25(3):370-378.
113. Remeron (mirtazapine) [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2020.
114. Boshuisen ML, Slaap BR, Vester-Blokland ED, et al. The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363-368.
115. Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol. 2003;18(1):35-38.
116. Connor KM, Davidson JR, Weisler RH, et al. A pilot study of mirtazapine in post-traumatic stress disorder. Int Clin Psychopharmacol. 1999;14(1):29-31.
117. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
118. Bedtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706-1711.
119. AbdelFattah MR, Jung SW, Greenspan MA, et al. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. A systemic review with meta-analysis. Sleep Breath. 2020;24(2):443-453.
120. Desyrel [package insert]. Locust Valley, NY: Pragma Pharmaceuticals, LLC; 2017.
121. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359.
122. Sultzer DL, Gray KF, Gunay I, et al. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997;5(1):60-69.
123. Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32.
124. Chlorpromazine hydrochloride [package insert]. Minneapolis, MN: Upsher-Smith Laboratories, Inc; 2010.
125. Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
126. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
127. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-e30.
128. Fluphenazine hydrochloride [package insert]. Philadelphia, PA: Lannett Company, Inc; 2019.
129. Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701-2720.
130. Haldol [package insert]. Columbus, OH: American Health Packaging; 2020.
131. MacDonald K, Wilson M, Minassian A, et al. A naturalistic study for intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317-322.
132. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
133. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
134. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
135. Büttner M, Walder B, von Elm E, et al. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454-1463.
136. Perphenazine [package insert]. Princeton, NJ: Sandoz Inc; 2010.
137. Compazine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
138. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
139. Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158(19):2124-2128.
140. Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137.
141. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
142. Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601-607.
143. Iannuzzi GL, Patel AA, Stewart JT. Aripiprazole and delusional disorder. J Psychiatr Pract. 2019;25(2):132-134.
144. Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434.
145. Sayyah M, Sayyah M, Boostani H, et al. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double-blind clinical trial). Depress Anxiety. 2012;29(10):850-854.
146. Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
147. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179.
148. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
149. Attia E, Steinglass JE, Walsh BT, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449-456.
150. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol. 2003;18(3):143-145.
151. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277-281.
152. Bosmans A, Verbanck P. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry. 2008;41(3):121-122.
153. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
154. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
155. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-195.
156. Bonelli RM, Mahnert FA, Niederwieser G. Olanzapine for Huntington’s disease: an open label study. Clin Neuropharmacol. 2002;25(5):263-265.
157. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013.
158. Khan A, Atkinson S, Mezhebovsky I, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with generalized anxiety disorder and a history of inadequate treatment response: a randomized, double-blind study. Ann Clin Psychiatry. 2014;26(1):3-18.
159. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
160. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
161. Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14(3):484-487.
162. Doroudgar S, Chou T, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):PCC.13m01558. doi: 10.4088/PCC.13m01558
163. Risperdal [package insert]. Titusville, NJ: Janssen Pharamceuticals, Inc; 2007.
164. Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81-86.
165. Currier GW, Chou J, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
166. Bahk WM, Yoon JS, Kim YH, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol. 2004;19(5):299-303.
167. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508.
168. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9): 980-991.
169. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794-801.
170. Scahill L, Leckman JF, Schulz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
171. Dallocchio C, Buffa C, Tinelli C, et al. Effectiveness of risperidone in Huntington Chorea patients. J Clin Psychopharmacol. 1999;19(1):101-103.
1. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. National Center for Health Statistics (Centers for Disease Control and Prevention). 2019. NCHS Data Brief No. 347. https://www.cdc.gov/nchs/products/databriefs/db347.htm
2. Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566.
3. National Alliance on Mental Illness. Anosognosia. 2021. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
4. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
5. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
6. Prozac [package insert]. Indianapolis, IN: Eli Lilly and Company; 2009.
7. Arnold LM, Hess EV, Hudson JI, et al. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am J Med. 2002;112(3):191-197.
8. Celexa [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2009.
9. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
10. McElroy SL, Hudson JI, Malhotra S, et al. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64(7):807-813.
11. Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67(3):468-472.
12. Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146-150.
13. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86.
14. Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25(1):24-32.
15. Perna G, Bertani A, Caldirola D, et al. A comparison of citalopram and paroxetine in the treatment of panic disorder: a randomized, single-blind study. Pharmacopsychiatry. 2001;34(3):85-90.
16. Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. 1998;18(5):390-398.
17. English BA, Jewell M, Jewell G, et al. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram: an open trial. J Clin Psychopharmacol. 2006;26(1):84-88.
18. Furmark T, Appel L, Michelgård A, et al. Cerebral blood flow changes after treatment of social phobia with neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58(2):132-142.
19. Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51(6):729-739.
20. Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res. 2006;18(2):164-169.
21. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204-213.
22. Lexapro [package insert]. Irvine, CA: Allergan USA, Inc; 2016.
23. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
24. Aigner M, Treasure J, Kaye W, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443.
25. Fineberg NA, Tonnoir B, Lemming O, et al. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6-7):430-439.
26. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
27. Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64(11):1322-1327.
28. Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769-773.
29. Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37(1):89-93.
30. Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399-1404.
31. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
32. Arnold LM, McElroy SL, Hudson JI, et al. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. 2002;63(11):1028-1033.
33. Connor KM, Sutherland SM, Tupler LA, et al. Fluoxetine in posttraumatic stress disorder. Randomized, double-blind study. Br J Psychiatry. 1999;175:17-22.
34. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63(3):199-206.
35. Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61(10):1005-1013.
36. Kara H, Aydin S, Yücel M, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo-controlled study. J Urol. 1996;156(5):1631-1632.
37. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578-1583.
38. Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40(9):1038-1043.
39. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
40. Zhang D, Cheng Y, Wu K, et al. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2.
41. Walitt B, Urrútia G, Nishishinya MB. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
42. Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587-589.
43. Zylicz Z, Krajnik M, Sorge A, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26(3):1105-1112.
44. Zoloft [package insert]. New York, NY: Pfizer; 2016.
45. Leombruni P, Pierò A, Lavagnino L, et al. A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1599-1605.
46. McElroy SL, Casuto LS, Nelson EB, et al. Placebo-controlled trial of sertraline in the treatment of binge eating disorder. Am J Psychiatry. 2000;157(6):1004-1006.
47. Milano W, Petrella C, Sabatino C, et al. Treatment of bulimia nervosa with sertraline: a randomized controlled trial. Adv Ther. 2004;21(4):232-237.
48. Brawman-Mintzer O, Knapp RG, Rynn M, et al. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874-881.
49. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo-controlled crossover study. J Urol. 1998;159(6):1935-1938.
50. Yi ZM, Chen SD, Tang QY, et al. Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore). 2019;98(23):e15989.
51. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59(9):1279-1298.
52. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2011.
53. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75(4):255-262.
54. Cymbalta [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
55. Li J, Yang L, Pu C, et al. The role of duloxetine in stress urinary incontinence: a systemic review and meta-analysis. Int Urol Nephrol. 2013;45(3):679-686.
56. Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559-1564.
57. Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2017.
58. Denys D, Van der Wee N, Van Megen HJ, et al. A double-blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
59. Albert U, Aguglia E, Maina G, et al. Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry. 2002;63(11):1004-1009.
60. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158-1165.
61. Zarinara AR, Mohammad MR, Hazrati N, et al. Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol. 2010;25(7-8):530-535.
62. Mukaddes NM, Abali O. Venlafaxine in children and adolescents with attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58(1):92-95.
63. Cohen LS, Soares CN, Lyster A, et al. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540-543.
64. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152.
65. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258.
66. Kadiroglu AK, Sit D, Kayabasi H, et al. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241-245.
67. Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005;105(1):161-166.
68. Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82(5):787-793.
69. Amitriptyline Hydrochloride [package insert]. Princeton, NJ: Sandoz Inc; 2014.
70. Hauser W, Wolfe F, Tolle T, et al. The role of antidepressants in the management of fibromyalgia syndrome: a systemic review and meta-analysis. CNS Drugs. 2012;26(4):297-307.
71. Braak B, Klooker T, Lei A, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(6):638-648.
72. Van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
73. Foster HE Jr, Hanno P, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
74. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominent irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678-684.
75. Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: a randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107(1):44-48.
76. Keskinbora K, Aydinli I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. 2008;110(10):979-984.
77. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
78. Boyle J, Eriksson M, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451-2458.
79. Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16(3):188-192.
80. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32(6):671-673.
81. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf. 2016;11(3):262-263.
82. Amoxapine [package insert]. Parsippany, NJ: Watson Pharma, Inc; 2014.
83. Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1146-1148.
84. Anafranil (clomipramine hydrochloride) [package insert]. Whitby, Ontario: Patheon Inc; 2012.
85. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Danish University Antidepressant Group (DUAG). Clin Pharmacol Ther. 1999;66(2):152-165.
86. Caillard V, Rouillon F, Viel J, et al. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatr Scand. 1999;99(1):51-58.
87. Segraves RT, Saran A, Segraves K, et al. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Therap. 1993;19(3):198-200.
88. Rowland DL, de Gouveia Brazao CA, Koos Slob A. Effective daily treatment with clomipramine in men with premature ejaculation when 25 mg (as required) is ineffective. BJU Int. 2001;87(4):357-360.
89. Norpramin (desipramine hydrochloride) [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2014.
90. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3-9.
91. Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19-31.
92. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systemic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
93. Doxepin hydrochloride [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2014.
94. Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867-873.
95. Imipramine hydrochloride [package insert]. Fairfield, NJ: Excellium Pharmaceutical, Inc; 2012.
96. Pope HG Jr, Hudson JI, Jonas JM, et al. Bulimia treated with imipramine: a placebo-controlled, double-blind study. Am J Psychiatry. 1983;140(5):554-558.
97. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
98. Laederach-Hofmann K, Graf C, Horber F, et al. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. Int J Eat Disord. 1999;26(3):231-244.
99. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284-1289.
100. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes of imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089-1092.
101. Pamelor (nortriptyline) [package insert]. Hazelwood, MO: Mallinckrodt Inc; 2007.
102. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
103. Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76(3):287-296.
104. Desai MJ, Saini V, Saini S. Myofacial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21-36.
105. Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial – the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363.
106. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
107. Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double-blind placebo-controlled study. Br J Urol. 1984;56(2):178-179.
108. Wellbutrin (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
109. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
110. Li DJ, Tseng PT, Chen YW, et al. Significant treatment effect of bupropion in patients with bipolar disorder but similar phase-shifting rate as other antidepressants: a meta-analysis following the PRISMA guidelines. Medicine (Baltimore). 2016;95(13):e3165.
111. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65(1):62-67.
112. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25(3):370-378.
113. Remeron (mirtazapine) [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2020.
114. Boshuisen ML, Slaap BR, Vester-Blokland ED, et al. The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363-368.
115. Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol. 2003;18(1):35-38.
116. Connor KM, Davidson JR, Weisler RH, et al. A pilot study of mirtazapine in post-traumatic stress disorder. Int Clin Psychopharmacol. 1999;14(1):29-31.
117. Wichniak A, Wierzbicka A, Walecka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
118. Bedtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706-1711.
119. AbdelFattah MR, Jung SW, Greenspan MA, et al. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. A systemic review with meta-analysis. Sleep Breath. 2020;24(2):443-453.
120. Desyrel [package insert]. Locust Valley, NY: Pragma Pharmaceuticals, LLC; 2017.
121. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359.
122. Sultzer DL, Gray KF, Gunay I, et al. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997;5(1):60-69.
123. Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32.
124. Chlorpromazine hydrochloride [package insert]. Minneapolis, MN: Upsher-Smith Laboratories, Inc; 2010.
125. Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
126. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
127. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):e15-e30.
128. Fluphenazine hydrochloride [package insert]. Philadelphia, PA: Lannett Company, Inc; 2019.
129. Bonelli RM, Wenning GK. Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701-2720.
130. Haldol [package insert]. Columbus, OH: American Health Packaging; 2020.
131. MacDonald K, Wilson M, Minassian A, et al. A naturalistic study for intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317-322.
132. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
133. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
134. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
135. Büttner M, Walder B, von Elm E, et al. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454-1463.
136. Perphenazine [package insert]. Princeton, NJ: Sandoz Inc; 2010.
137. Compazine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2004.
138. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
139. Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158(19):2124-2128.
140. Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137.
141. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
142. Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601-607.
143. Iannuzzi GL, Patel AA, Stewart JT. Aripiprazole and delusional disorder. J Psychiatr Pract. 2019;25(2):132-134.
144. Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434.
145. Sayyah M, Sayyah M, Boostani H, et al. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double-blind clinical trial). Depress Anxiety. 2012;29(10):850-854.
146. Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
147. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179.
148. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
149. Attia E, Steinglass JE, Walsh BT, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449-456.
150. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol. 2003;18(3):143-145.
151. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277-281.
152. Bosmans A, Verbanck P. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry. 2008;41(3):121-122.
153. Meyers BS, Flint AJ, Rothschild AJ, et al; STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
154. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
155. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-195.
156. Bonelli RM, Mahnert FA, Niederwieser G. Olanzapine for Huntington’s disease: an open label study. Clin Neuropharmacol. 2002;25(5):263-265.
157. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013.
158. Khan A, Atkinson S, Mezhebovsky I, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with generalized anxiety disorder and a history of inadequate treatment response: a randomized, double-blind study. Ann Clin Psychiatry. 2014;26(1):3-18.
159. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
160. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
161. Fernandez HH, Friedman JH, Jacques C, et al. Quetiapine for the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 1999;14(3):484-487.
162. Doroudgar S, Chou T, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):PCC.13m01558. doi: 10.4088/PCC.13m01558
163. Risperdal [package insert]. Titusville, NJ: Janssen Pharamceuticals, Inc; 2007.
164. Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81-86.
165. Currier GW, Chou J, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
166. Bahk WM, Yoon JS, Kim YH, et al. Risperidone in combination with mood stabilizers for acute mania: a multicentre, open study. Int Clin Psychopharmacol. 2004;19(5):299-303.
167. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508.
168. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9): 980-991.
169. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794-801.
170. Scahill L, Leckman JF, Schulz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
171. Dallocchio C, Buffa C, Tinelli C, et al. Effectiveness of risperidone in Huntington Chorea patients. J Clin Psychopharmacol. 1999;19(1):101-103.
Lumateperone for major depressive episodes in bipolar I or bipolar II disorder
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8
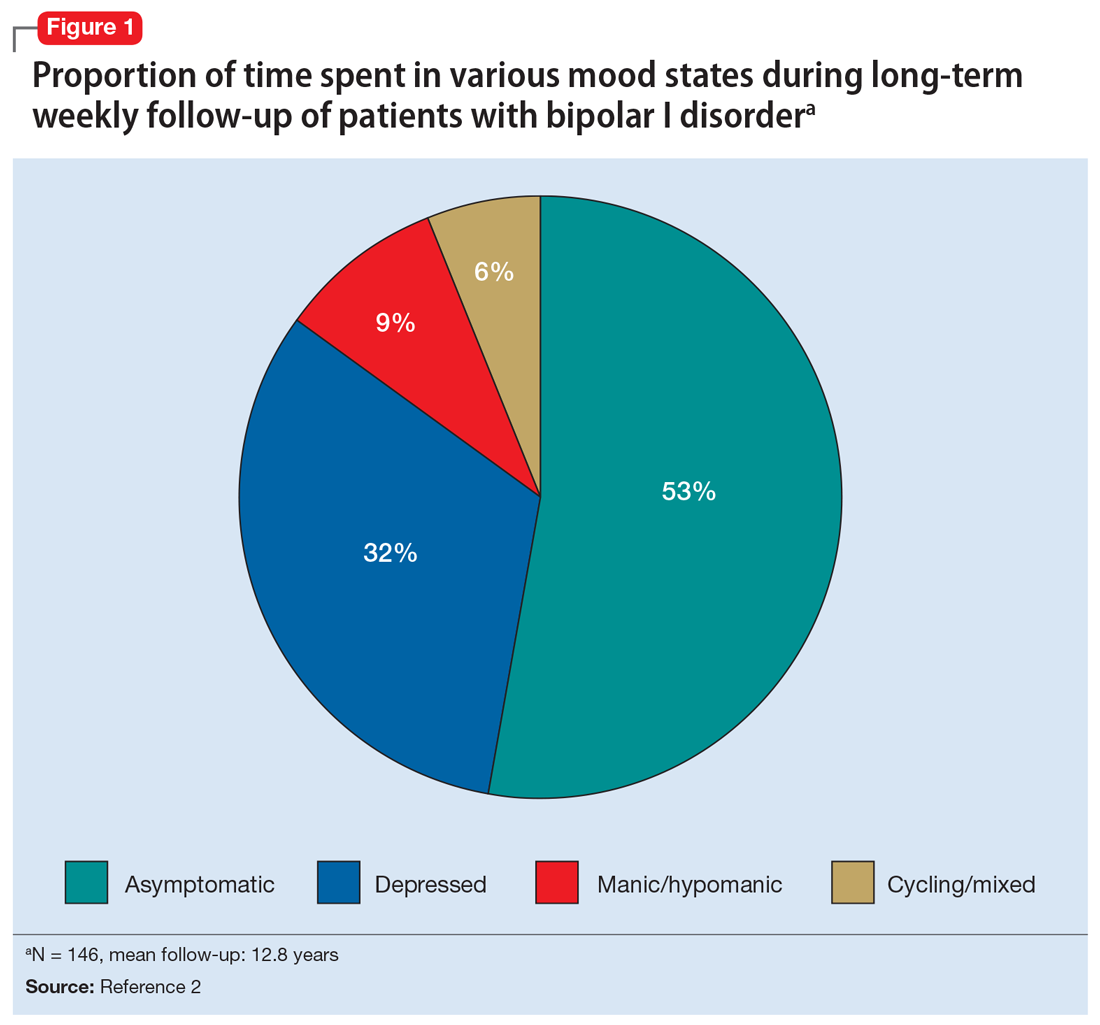
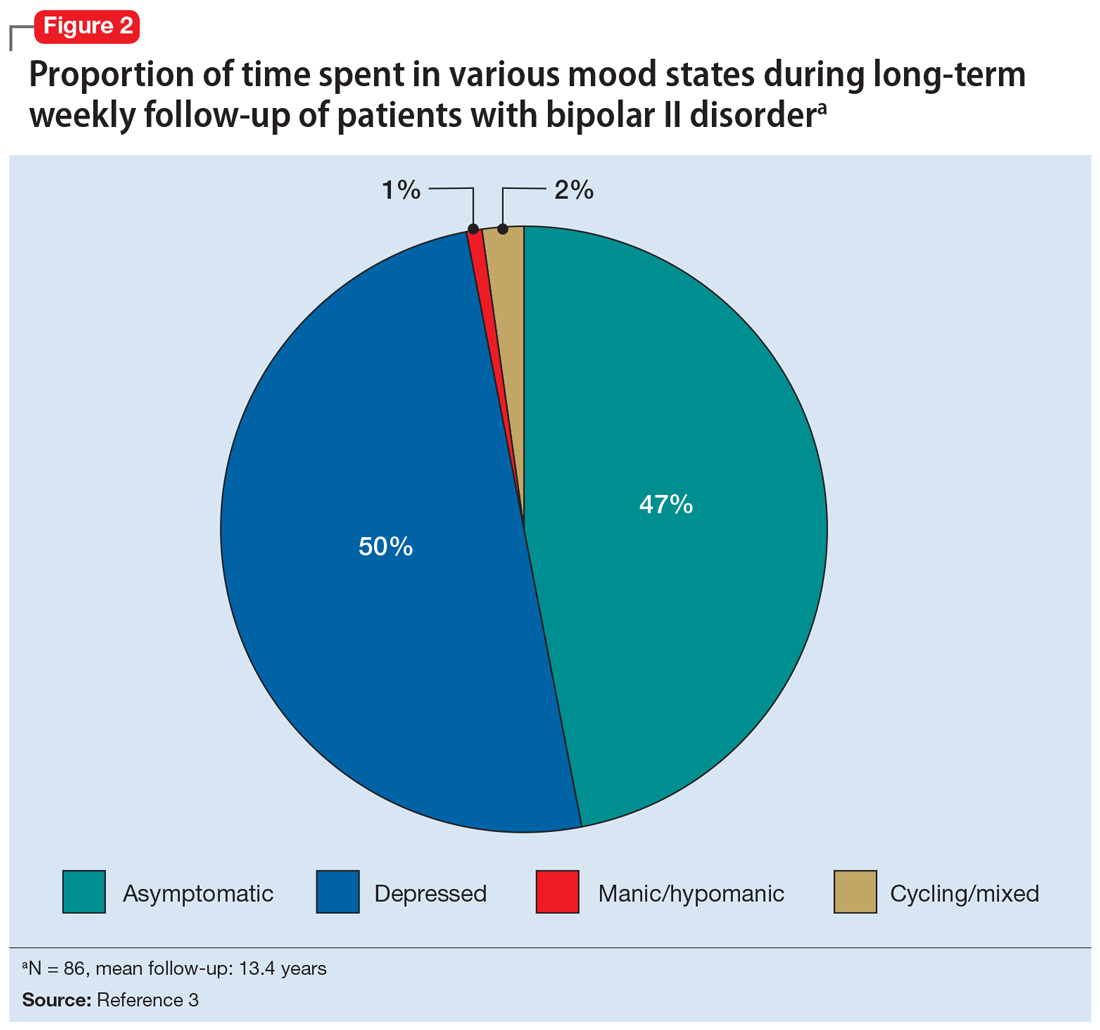
Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...
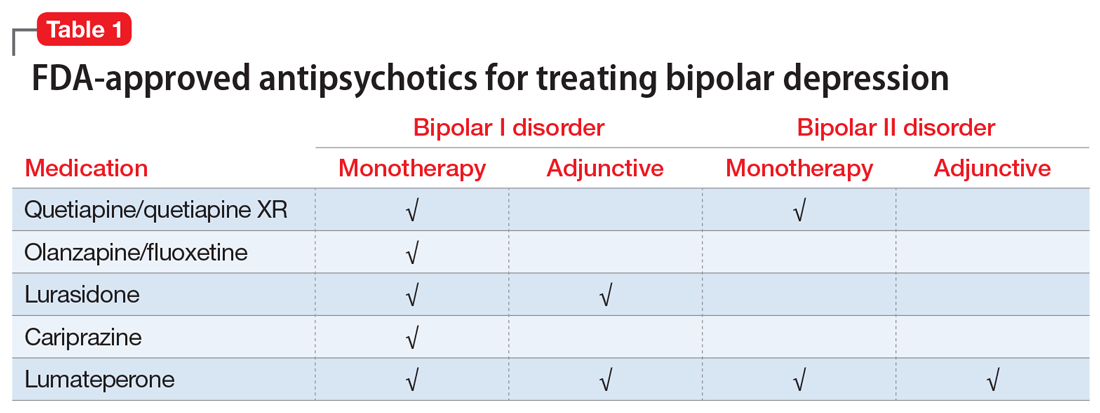
Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13
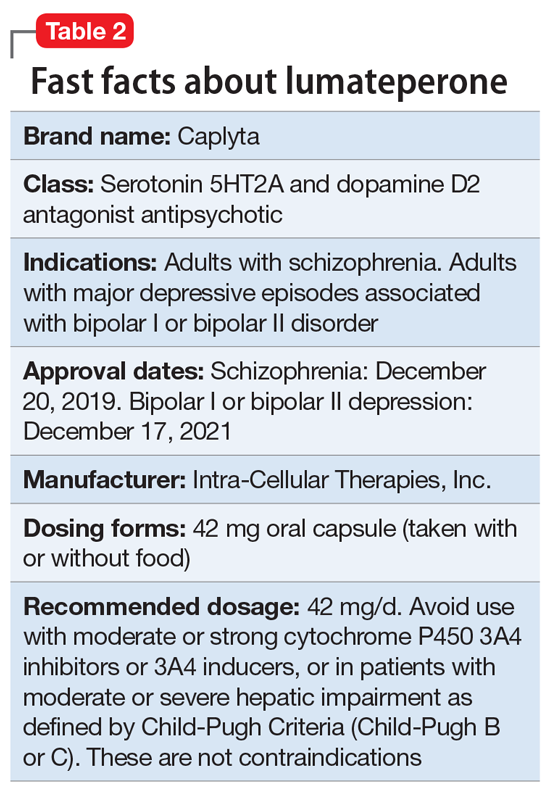
In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.
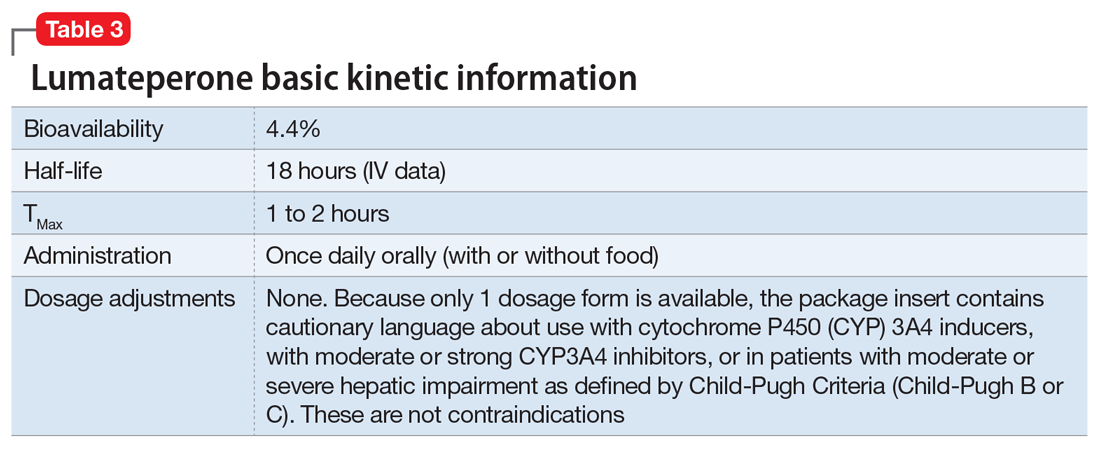
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8


Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...

Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13

In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.

Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8


Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...

Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13

In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.

Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.















