User login
LARC prolongs interpregnancy intervals but doesn’t cut preterm birth risk
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
REPORTING FROM ACOG 2019
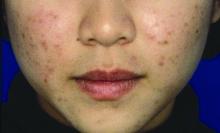
Ovarian reserve markers fall on isotretinoin, but rebound after stopping treatment
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
REPORTING FROM WCD2019
Judge bars contraceptive mandate from being enforced
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
Postpartum LARC uptake increased with separate payment
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
FROM JAMA
Pharmacist-prescribed hormonal contraception safe, effective
Pharmacists in Oregon with the authority to prescribe hormonal contraceptive therapy have improved access to and continuation of contraceptive therapy, based on two retrospective studies of Medicaid patients published in Obstetrics & Gynecology.
Additionally, the safety profile associated with pharmacist prescribing of hormonal contraceptive therapy was on par with that of other prescribing clinicians.
“In the first 2 years of program implementation, we found evidence that pharmacists were safely reaching new contraceptive users and [helping to meet national goals in reducing unwanted pregnancy],” Lorinda Anderson, PharmD, and colleagues, the authors of one of the studies, wrote.
In 2016, Oregon became the first state to grant pharmacists authority to prescribe hormonal contraception without requiring consultation. The findings suggest that expanding prescribing authority for contraceptive therapy to pharmacists in other states could limit barriers to access, as 90% of United States residents live within 5 miles of a pharmacy.
In one of the two studies, Maria I. Rodriguez, MD, MPH, and colleagues conducted a claims-based review of the primary outcomes of pharmacist-initiated and non-pharmacist-initiated prescriptions on unintended pregnancies in Oregon’s Medicaid program. They also evaluated secondary outcomes, such as costs and quality-adjusted life years (QALYs).
In the first 2 years after the Oregon law went into effect, 248 pharmacists wrote 1,313, or 10%, of all hormonal contraception prescriptions for women who were Medicaid recipients and were prescribed hormonal contraception by any legally allowed healthcare provider. Pharmacists prescribed hormonal contraception for 367 of the 3,614 women studied.
Based on an economic model, pharmacist-initiated hormonal contraceptive therapy prevented an estimated 51 unintended pregnancies and saved $1.6 million in the first two years following the program’s inception in Oregon. Quality of life improved with 158 QALYs per 198,100 women.
Additionally, pharmacist-provided services cost less per patient than non pharmacist health care provider-services, $28 vs. $81.
“We believe our findings to be conservative given that our model was based on use 24 months after implementation. We expect over time that knowledge of and use of contraceptive access from pharmacists will increase,” Dr. Rodriguez, of Oregon Health & Science University, Portland, and colleagues wrote.
In the second study, Dr. Anderson, of the Oregon State University, Corvallis, and colleagues pooled Oregon Medicaid pharmacy claims, eligibility, medical, diagnostic, and demographic data over the 2-year period for the 3,614 patients who received new prescriptions for transdermal and oral contraception, and the 1,313 claims filed for 367 women prescribed contraception by 162 pharmacists.
Within the first 4 months following the program’s inception in Oregon, pharmacists averaged 40 contraceptive claims per month. Over the next 7 months, claims increased to 61 and peaked at 80 claims after 18 months. Chain community pharmacies accounted for 94% of the claims; 71% of claims were in metropolitan areas.
Based on demographics, 73.8% of the women who were prescribed contraception by a pharmacist were first-time recipients. Combined oral contraception was prescribed for 90.5% of the women, and 82% of the women were 18-35 years of age. In the 180-day period prior to receiving pharmacist-prescribed contraception, 61.5% of patients were not using contraception but were attempting to engage in pharmacy-provided hormonal contraceptive care.
The researchers also examined contraceptive safety by looking at whether patients with medical contraindications (Medical Eligibility Criteria Category 3 or 4) were receiving contraindicated methods. “We found that overall adherence to the clinical algorithm for prescribing pharmacists was high. Only 12 (5%) patients were identified as having Medical Eligibility Criteria Category 3 or 4 medical conditions, and two (less than 1%) patients with medications contraindicating OC use received a prescription,” Dr. Anderson and her colleagues wrote.
They noted that the initial legislation passed in Oregon only included oral and transdermal hormonal contraception as methods pharmacists could prescribe. In 2017, with implementation in 2018, this was amended to include the vaginal ring and injection. “As the program matures, and contracts with additional insurers are implemented at pharmacies, we expect the number of pharmacist prescriptions to increase,” the authors wrote.
Dr. Rodriguez reported financial compensation from Merck, the World Health Organization, CooperSurgical, and a previous relationship with Merck. Dr. Anderson reports no conflicts of interest.
SOURCES: Rodriguez M et al. Obstet Gynecol. 2019 Jun;133(6):1238-46; Anderson A et al. Obstet Gynecol. 2019 Jun;133(6):1231-7.
Pharmacists in Oregon with the authority to prescribe hormonal contraceptive therapy have improved access to and continuation of contraceptive therapy, based on two retrospective studies of Medicaid patients published in Obstetrics & Gynecology.
Additionally, the safety profile associated with pharmacist prescribing of hormonal contraceptive therapy was on par with that of other prescribing clinicians.
“In the first 2 years of program implementation, we found evidence that pharmacists were safely reaching new contraceptive users and [helping to meet national goals in reducing unwanted pregnancy],” Lorinda Anderson, PharmD, and colleagues, the authors of one of the studies, wrote.
In 2016, Oregon became the first state to grant pharmacists authority to prescribe hormonal contraception without requiring consultation. The findings suggest that expanding prescribing authority for contraceptive therapy to pharmacists in other states could limit barriers to access, as 90% of United States residents live within 5 miles of a pharmacy.
In one of the two studies, Maria I. Rodriguez, MD, MPH, and colleagues conducted a claims-based review of the primary outcomes of pharmacist-initiated and non-pharmacist-initiated prescriptions on unintended pregnancies in Oregon’s Medicaid program. They also evaluated secondary outcomes, such as costs and quality-adjusted life years (QALYs).
In the first 2 years after the Oregon law went into effect, 248 pharmacists wrote 1,313, or 10%, of all hormonal contraception prescriptions for women who were Medicaid recipients and were prescribed hormonal contraception by any legally allowed healthcare provider. Pharmacists prescribed hormonal contraception for 367 of the 3,614 women studied.
Based on an economic model, pharmacist-initiated hormonal contraceptive therapy prevented an estimated 51 unintended pregnancies and saved $1.6 million in the first two years following the program’s inception in Oregon. Quality of life improved with 158 QALYs per 198,100 women.
Additionally, pharmacist-provided services cost less per patient than non pharmacist health care provider-services, $28 vs. $81.
“We believe our findings to be conservative given that our model was based on use 24 months after implementation. We expect over time that knowledge of and use of contraceptive access from pharmacists will increase,” Dr. Rodriguez, of Oregon Health & Science University, Portland, and colleagues wrote.
In the second study, Dr. Anderson, of the Oregon State University, Corvallis, and colleagues pooled Oregon Medicaid pharmacy claims, eligibility, medical, diagnostic, and demographic data over the 2-year period for the 3,614 patients who received new prescriptions for transdermal and oral contraception, and the 1,313 claims filed for 367 women prescribed contraception by 162 pharmacists.
Within the first 4 months following the program’s inception in Oregon, pharmacists averaged 40 contraceptive claims per month. Over the next 7 months, claims increased to 61 and peaked at 80 claims after 18 months. Chain community pharmacies accounted for 94% of the claims; 71% of claims were in metropolitan areas.
Based on demographics, 73.8% of the women who were prescribed contraception by a pharmacist were first-time recipients. Combined oral contraception was prescribed for 90.5% of the women, and 82% of the women were 18-35 years of age. In the 180-day period prior to receiving pharmacist-prescribed contraception, 61.5% of patients were not using contraception but were attempting to engage in pharmacy-provided hormonal contraceptive care.
The researchers also examined contraceptive safety by looking at whether patients with medical contraindications (Medical Eligibility Criteria Category 3 or 4) were receiving contraindicated methods. “We found that overall adherence to the clinical algorithm for prescribing pharmacists was high. Only 12 (5%) patients were identified as having Medical Eligibility Criteria Category 3 or 4 medical conditions, and two (less than 1%) patients with medications contraindicating OC use received a prescription,” Dr. Anderson and her colleagues wrote.
They noted that the initial legislation passed in Oregon only included oral and transdermal hormonal contraception as methods pharmacists could prescribe. In 2017, with implementation in 2018, this was amended to include the vaginal ring and injection. “As the program matures, and contracts with additional insurers are implemented at pharmacies, we expect the number of pharmacist prescriptions to increase,” the authors wrote.
Dr. Rodriguez reported financial compensation from Merck, the World Health Organization, CooperSurgical, and a previous relationship with Merck. Dr. Anderson reports no conflicts of interest.
SOURCES: Rodriguez M et al. Obstet Gynecol. 2019 Jun;133(6):1238-46; Anderson A et al. Obstet Gynecol. 2019 Jun;133(6):1231-7.
Pharmacists in Oregon with the authority to prescribe hormonal contraceptive therapy have improved access to and continuation of contraceptive therapy, based on two retrospective studies of Medicaid patients published in Obstetrics & Gynecology.
Additionally, the safety profile associated with pharmacist prescribing of hormonal contraceptive therapy was on par with that of other prescribing clinicians.
“In the first 2 years of program implementation, we found evidence that pharmacists were safely reaching new contraceptive users and [helping to meet national goals in reducing unwanted pregnancy],” Lorinda Anderson, PharmD, and colleagues, the authors of one of the studies, wrote.
In 2016, Oregon became the first state to grant pharmacists authority to prescribe hormonal contraception without requiring consultation. The findings suggest that expanding prescribing authority for contraceptive therapy to pharmacists in other states could limit barriers to access, as 90% of United States residents live within 5 miles of a pharmacy.
In one of the two studies, Maria I. Rodriguez, MD, MPH, and colleagues conducted a claims-based review of the primary outcomes of pharmacist-initiated and non-pharmacist-initiated prescriptions on unintended pregnancies in Oregon’s Medicaid program. They also evaluated secondary outcomes, such as costs and quality-adjusted life years (QALYs).
In the first 2 years after the Oregon law went into effect, 248 pharmacists wrote 1,313, or 10%, of all hormonal contraception prescriptions for women who were Medicaid recipients and were prescribed hormonal contraception by any legally allowed healthcare provider. Pharmacists prescribed hormonal contraception for 367 of the 3,614 women studied.
Based on an economic model, pharmacist-initiated hormonal contraceptive therapy prevented an estimated 51 unintended pregnancies and saved $1.6 million in the first two years following the program’s inception in Oregon. Quality of life improved with 158 QALYs per 198,100 women.
Additionally, pharmacist-provided services cost less per patient than non pharmacist health care provider-services, $28 vs. $81.
“We believe our findings to be conservative given that our model was based on use 24 months after implementation. We expect over time that knowledge of and use of contraceptive access from pharmacists will increase,” Dr. Rodriguez, of Oregon Health & Science University, Portland, and colleagues wrote.
In the second study, Dr. Anderson, of the Oregon State University, Corvallis, and colleagues pooled Oregon Medicaid pharmacy claims, eligibility, medical, diagnostic, and demographic data over the 2-year period for the 3,614 patients who received new prescriptions for transdermal and oral contraception, and the 1,313 claims filed for 367 women prescribed contraception by 162 pharmacists.
Within the first 4 months following the program’s inception in Oregon, pharmacists averaged 40 contraceptive claims per month. Over the next 7 months, claims increased to 61 and peaked at 80 claims after 18 months. Chain community pharmacies accounted for 94% of the claims; 71% of claims were in metropolitan areas.
Based on demographics, 73.8% of the women who were prescribed contraception by a pharmacist were first-time recipients. Combined oral contraception was prescribed for 90.5% of the women, and 82% of the women were 18-35 years of age. In the 180-day period prior to receiving pharmacist-prescribed contraception, 61.5% of patients were not using contraception but were attempting to engage in pharmacy-provided hormonal contraceptive care.
The researchers also examined contraceptive safety by looking at whether patients with medical contraindications (Medical Eligibility Criteria Category 3 or 4) were receiving contraindicated methods. “We found that overall adherence to the clinical algorithm for prescribing pharmacists was high. Only 12 (5%) patients were identified as having Medical Eligibility Criteria Category 3 or 4 medical conditions, and two (less than 1%) patients with medications contraindicating OC use received a prescription,” Dr. Anderson and her colleagues wrote.
They noted that the initial legislation passed in Oregon only included oral and transdermal hormonal contraception as methods pharmacists could prescribe. In 2017, with implementation in 2018, this was amended to include the vaginal ring and injection. “As the program matures, and contracts with additional insurers are implemented at pharmacies, we expect the number of pharmacist prescriptions to increase,” the authors wrote.
Dr. Rodriguez reported financial compensation from Merck, the World Health Organization, CooperSurgical, and a previous relationship with Merck. Dr. Anderson reports no conflicts of interest.
SOURCES: Rodriguez M et al. Obstet Gynecol. 2019 Jun;133(6):1238-46; Anderson A et al. Obstet Gynecol. 2019 Jun;133(6):1231-7.
FROM OBSTETRICS & GYNECOLOGY
Addressing the sexual and reproductive health needs of trans and gender nonconforming patients
Separating gender identity from sexual identity to allow for more comprehensive history-taking
Grouping the term “transgender” in the abbreviation LGBT (lesbian, gay, bisexual, transgender) has historically been empowering for trans and gender nonconforming (GNC) persons. However, it also has contributed to the misunderstanding that gender identity is interchangeable with sexual identity. This common misconception can be a barrier to trans and GNC patients seeking care from ob.gyns. for their reproductive health needs.
By definition, gender identity refers to an internal experience of one’s gender, of one’s self.1 While gender identity has social implications, it ultimately is something that a person experiences independently of interactions with others. By contrast, sexual orientation has an explicitly relational underpinning because sexual orientation involves attraction to others. The distinction between gender identity and sexual orientation is similar to an internal-versus-external, or a self-versus-other dichotomy. A further nuance to add is that sexual behavior does not always reflect sexual orientation, and sexual behavior can vary along a wide spectrum when gender identity is added to the equation.
Overall, When approaching a sexual history with any patient, but especially a transgender or GNC patient, providers should think deeply about what information is medically relevant.2 The purpose of a sexual history is to identify behaviors that contribute to health risk, including pregnancy, sexually transmitted infection, and social problems such as sex-trafficking or intimate partner violence. The health care provider’s job is to ask questions that will uncover these risk factors.
With the advent of a more inclusive attitude toward gay and lesbian partnership, many providers already have learned to collect the sexual history without assuming the gender of a person’s sexual contacts. Still, when a provider is taking the sexual history, gender often is inappropriately used as proxy for the type of sex that a patient may be having. For example, a provider asking a cisgender woman about her sexual activity may ask, “how many sexual partners have you had in the last year?” But then, the provider may follow-up her response of “three sexual partners in the last year” by asking “men, women, or both?” By asking a patient if the patient’s sexual partners are “men, women, or both,” providers fail to accurately elucidate the risk factors that they are actually seeking when taking a sexual history. The cisgender woman from the above scenario may reply that she has been sleeping only with women for the last year, but if the sexual partners are transgender women, aka a woman who was assigned male at birth and therefore still may use her penis/testes for sexual purposes, then the patient actually may be at risk for pregnancy and may also have a different risk factor profile for sexually transmitted infections than if the patient were sexually active with cisgender women.
A different approach to using gender in taking the sexual history is to speak plainly about which sex organs come into contact during sexual activity. When patients identify as transgender or GNC, a provider first should start by asking them what language they would like providers to use when discussing sex organs.3 One example is that many trans men, both those who have undergone mastectomy as well as those who have not, may not use the word “breasts” to describe their “chests.” This distinction may make the difference between gaining and losing the trust of a trans/GNC patient in your clinic. After identifying how a patient would like to refer to sex organs, a provider can continue by asking which of the patient’s sex partners’ organs come into contact with the patient’s organs during sexual activity. Alternatively, starting with an even more broad line of questioning may be best for some patients, such as “how do you like to have sex?”
Carefully identifying the type of sex and what sex organs are involved has concrete medical implications. Patients assigned female at birth who are on hormone therapy with testosterone may need supportive care if they continue to use their vaginas in sexual encounters because testosterone can lead to a relatively hypoestrogenic state. Patients assigned male at birth who have undergone vaginoplasty procedures may need counseling about how to use and support their neovaginas as well as adjusted testing for dysplasia. Patients assigned female at birth who want to avoid pregnancy may need a nuanced consultation regarding contraception. These are just a few examples of how obstetrician-gynecologists can better support the sexual health of their trans/GNC patients by having an accurate understanding of how a trans/GNC person has sex.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Bahng and Dr. Joyner reported no relevant financial disclosures
References
1. Sexual orientation and gender identity definitions. Human Rights Campaign.
2. Taking a sexual history from transgender people. Transforming Health at the Centers for Disease Control and Prevention.
3. Sexual health history: Talking sex with gender non-conforming and trans patients. National LGBT Health Education Center at The Fenway Institute.
Separating gender identity from sexual identity to allow for more comprehensive history-taking
Separating gender identity from sexual identity to allow for more comprehensive history-taking
Grouping the term “transgender” in the abbreviation LGBT (lesbian, gay, bisexual, transgender) has historically been empowering for trans and gender nonconforming (GNC) persons. However, it also has contributed to the misunderstanding that gender identity is interchangeable with sexual identity. This common misconception can be a barrier to trans and GNC patients seeking care from ob.gyns. for their reproductive health needs.
By definition, gender identity refers to an internal experience of one’s gender, of one’s self.1 While gender identity has social implications, it ultimately is something that a person experiences independently of interactions with others. By contrast, sexual orientation has an explicitly relational underpinning because sexual orientation involves attraction to others. The distinction between gender identity and sexual orientation is similar to an internal-versus-external, or a self-versus-other dichotomy. A further nuance to add is that sexual behavior does not always reflect sexual orientation, and sexual behavior can vary along a wide spectrum when gender identity is added to the equation.
Overall, When approaching a sexual history with any patient, but especially a transgender or GNC patient, providers should think deeply about what information is medically relevant.2 The purpose of a sexual history is to identify behaviors that contribute to health risk, including pregnancy, sexually transmitted infection, and social problems such as sex-trafficking or intimate partner violence. The health care provider’s job is to ask questions that will uncover these risk factors.
With the advent of a more inclusive attitude toward gay and lesbian partnership, many providers already have learned to collect the sexual history without assuming the gender of a person’s sexual contacts. Still, when a provider is taking the sexual history, gender often is inappropriately used as proxy for the type of sex that a patient may be having. For example, a provider asking a cisgender woman about her sexual activity may ask, “how many sexual partners have you had in the last year?” But then, the provider may follow-up her response of “three sexual partners in the last year” by asking “men, women, or both?” By asking a patient if the patient’s sexual partners are “men, women, or both,” providers fail to accurately elucidate the risk factors that they are actually seeking when taking a sexual history. The cisgender woman from the above scenario may reply that she has been sleeping only with women for the last year, but if the sexual partners are transgender women, aka a woman who was assigned male at birth and therefore still may use her penis/testes for sexual purposes, then the patient actually may be at risk for pregnancy and may also have a different risk factor profile for sexually transmitted infections than if the patient were sexually active with cisgender women.
A different approach to using gender in taking the sexual history is to speak plainly about which sex organs come into contact during sexual activity. When patients identify as transgender or GNC, a provider first should start by asking them what language they would like providers to use when discussing sex organs.3 One example is that many trans men, both those who have undergone mastectomy as well as those who have not, may not use the word “breasts” to describe their “chests.” This distinction may make the difference between gaining and losing the trust of a trans/GNC patient in your clinic. After identifying how a patient would like to refer to sex organs, a provider can continue by asking which of the patient’s sex partners’ organs come into contact with the patient’s organs during sexual activity. Alternatively, starting with an even more broad line of questioning may be best for some patients, such as “how do you like to have sex?”
Carefully identifying the type of sex and what sex organs are involved has concrete medical implications. Patients assigned female at birth who are on hormone therapy with testosterone may need supportive care if they continue to use their vaginas in sexual encounters because testosterone can lead to a relatively hypoestrogenic state. Patients assigned male at birth who have undergone vaginoplasty procedures may need counseling about how to use and support their neovaginas as well as adjusted testing for dysplasia. Patients assigned female at birth who want to avoid pregnancy may need a nuanced consultation regarding contraception. These are just a few examples of how obstetrician-gynecologists can better support the sexual health of their trans/GNC patients by having an accurate understanding of how a trans/GNC person has sex.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Bahng and Dr. Joyner reported no relevant financial disclosures
References
1. Sexual orientation and gender identity definitions. Human Rights Campaign.
2. Taking a sexual history from transgender people. Transforming Health at the Centers for Disease Control and Prevention.
3. Sexual health history: Talking sex with gender non-conforming and trans patients. National LGBT Health Education Center at The Fenway Institute.
Grouping the term “transgender” in the abbreviation LGBT (lesbian, gay, bisexual, transgender) has historically been empowering for trans and gender nonconforming (GNC) persons. However, it also has contributed to the misunderstanding that gender identity is interchangeable with sexual identity. This common misconception can be a barrier to trans and GNC patients seeking care from ob.gyns. for their reproductive health needs.
By definition, gender identity refers to an internal experience of one’s gender, of one’s self.1 While gender identity has social implications, it ultimately is something that a person experiences independently of interactions with others. By contrast, sexual orientation has an explicitly relational underpinning because sexual orientation involves attraction to others. The distinction between gender identity and sexual orientation is similar to an internal-versus-external, or a self-versus-other dichotomy. A further nuance to add is that sexual behavior does not always reflect sexual orientation, and sexual behavior can vary along a wide spectrum when gender identity is added to the equation.
Overall, When approaching a sexual history with any patient, but especially a transgender or GNC patient, providers should think deeply about what information is medically relevant.2 The purpose of a sexual history is to identify behaviors that contribute to health risk, including pregnancy, sexually transmitted infection, and social problems such as sex-trafficking or intimate partner violence. The health care provider’s job is to ask questions that will uncover these risk factors.
With the advent of a more inclusive attitude toward gay and lesbian partnership, many providers already have learned to collect the sexual history without assuming the gender of a person’s sexual contacts. Still, when a provider is taking the sexual history, gender often is inappropriately used as proxy for the type of sex that a patient may be having. For example, a provider asking a cisgender woman about her sexual activity may ask, “how many sexual partners have you had in the last year?” But then, the provider may follow-up her response of “three sexual partners in the last year” by asking “men, women, or both?” By asking a patient if the patient’s sexual partners are “men, women, or both,” providers fail to accurately elucidate the risk factors that they are actually seeking when taking a sexual history. The cisgender woman from the above scenario may reply that she has been sleeping only with women for the last year, but if the sexual partners are transgender women, aka a woman who was assigned male at birth and therefore still may use her penis/testes for sexual purposes, then the patient actually may be at risk for pregnancy and may also have a different risk factor profile for sexually transmitted infections than if the patient were sexually active with cisgender women.
A different approach to using gender in taking the sexual history is to speak plainly about which sex organs come into contact during sexual activity. When patients identify as transgender or GNC, a provider first should start by asking them what language they would like providers to use when discussing sex organs.3 One example is that many trans men, both those who have undergone mastectomy as well as those who have not, may not use the word “breasts” to describe their “chests.” This distinction may make the difference between gaining and losing the trust of a trans/GNC patient in your clinic. After identifying how a patient would like to refer to sex organs, a provider can continue by asking which of the patient’s sex partners’ organs come into contact with the patient’s organs during sexual activity. Alternatively, starting with an even more broad line of questioning may be best for some patients, such as “how do you like to have sex?”
Carefully identifying the type of sex and what sex organs are involved has concrete medical implications. Patients assigned female at birth who are on hormone therapy with testosterone may need supportive care if they continue to use their vaginas in sexual encounters because testosterone can lead to a relatively hypoestrogenic state. Patients assigned male at birth who have undergone vaginoplasty procedures may need counseling about how to use and support their neovaginas as well as adjusted testing for dysplasia. Patients assigned female at birth who want to avoid pregnancy may need a nuanced consultation regarding contraception. These are just a few examples of how obstetrician-gynecologists can better support the sexual health of their trans/GNC patients by having an accurate understanding of how a trans/GNC person has sex.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Bahng and Dr. Joyner reported no relevant financial disclosures
References
1. Sexual orientation and gender identity definitions. Human Rights Campaign.
2. Taking a sexual history from transgender people. Transforming Health at the Centers for Disease Control and Prevention.
3. Sexual health history: Talking sex with gender non-conforming and trans patients. National LGBT Health Education Center at The Fenway Institute.
Immediate postpartum LARC: ‘Agony and ecstasy’
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
2019 Update: Contraceptives and unintended pregnancy rates
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Liletta IUD efficacy extends to 6 years
NASHVILLE, TENN. – The 52 mg levonorgestrel intrauterine system Liletta, which is currently approved as a contraceptive for up to 5 years of use, remains highly effective and safe for an additional year of use, according to findings from an ongoing 10-year trial.
Of 1,714 women enrolled in the multicenter phase 3 trial and for whom demographic information is available, 379 have completed 6 years of use. Nine pregnancies have occurred to date, including 2 in year 1, 4 in year 2, and 1 each in years 3-5, for a cumulative life-table pregnancy rate through year 6 of 0.87, Carolyn L. Westhoff, MD, MSc, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Six (67%) of the pregnancies were ectopic, noted Dr. Westhoff of Columbia University, N.Y.
Study subjects include 1,568 women aged 16-35 years at enrollment (986 were nulliparous and 433 were obese) and 146 aged 36 to 45 years. All were followed after device placement, with 138 having completed 8 years of use.
Two perforations occurred following device placement–both within the first year, and none have occurred since, Dr. Westhoff noted.
Other adverse events included expulsion in 68 women (4.0%), with 50 of those (73.5%) occurring in the first year of use, and pelvic infection in 15 women (0.9%), with 11 (73.3%) of those occurring after 6 or more months of use.
Only 40 women (2.3%) discontinued the study due to bleeding, and 30 of those (75%) did so in the first 2 years.
in both nulliparous and parous women, and in both non-obese and obese women, Dr. Westhoff concluded. She added that “there were no additional pregnancies in 6 years, and this study will be continuing to look all the way to 10-year effectiveness.”
This study is funded by Medicines360, a non-profit pharmaceutical company. Dr. Westhoff is a consultant or advisory board member for Agile and Cooper Surgical, and a Data and Safety Monitoring Board member for phase 4 studies for Bayer and Merck.
SOURCE: Westhoff C et al., ACOG 2019: Abstract 13M.
NASHVILLE, TENN. – The 52 mg levonorgestrel intrauterine system Liletta, which is currently approved as a contraceptive for up to 5 years of use, remains highly effective and safe for an additional year of use, according to findings from an ongoing 10-year trial.
Of 1,714 women enrolled in the multicenter phase 3 trial and for whom demographic information is available, 379 have completed 6 years of use. Nine pregnancies have occurred to date, including 2 in year 1, 4 in year 2, and 1 each in years 3-5, for a cumulative life-table pregnancy rate through year 6 of 0.87, Carolyn L. Westhoff, MD, MSc, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Six (67%) of the pregnancies were ectopic, noted Dr. Westhoff of Columbia University, N.Y.
Study subjects include 1,568 women aged 16-35 years at enrollment (986 were nulliparous and 433 were obese) and 146 aged 36 to 45 years. All were followed after device placement, with 138 having completed 8 years of use.
Two perforations occurred following device placement–both within the first year, and none have occurred since, Dr. Westhoff noted.
Other adverse events included expulsion in 68 women (4.0%), with 50 of those (73.5%) occurring in the first year of use, and pelvic infection in 15 women (0.9%), with 11 (73.3%) of those occurring after 6 or more months of use.
Only 40 women (2.3%) discontinued the study due to bleeding, and 30 of those (75%) did so in the first 2 years.
in both nulliparous and parous women, and in both non-obese and obese women, Dr. Westhoff concluded. She added that “there were no additional pregnancies in 6 years, and this study will be continuing to look all the way to 10-year effectiveness.”
This study is funded by Medicines360, a non-profit pharmaceutical company. Dr. Westhoff is a consultant or advisory board member for Agile and Cooper Surgical, and a Data and Safety Monitoring Board member for phase 4 studies for Bayer and Merck.
SOURCE: Westhoff C et al., ACOG 2019: Abstract 13M.
NASHVILLE, TENN. – The 52 mg levonorgestrel intrauterine system Liletta, which is currently approved as a contraceptive for up to 5 years of use, remains highly effective and safe for an additional year of use, according to findings from an ongoing 10-year trial.
Of 1,714 women enrolled in the multicenter phase 3 trial and for whom demographic information is available, 379 have completed 6 years of use. Nine pregnancies have occurred to date, including 2 in year 1, 4 in year 2, and 1 each in years 3-5, for a cumulative life-table pregnancy rate through year 6 of 0.87, Carolyn L. Westhoff, MD, MSc, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Six (67%) of the pregnancies were ectopic, noted Dr. Westhoff of Columbia University, N.Y.
Study subjects include 1,568 women aged 16-35 years at enrollment (986 were nulliparous and 433 were obese) and 146 aged 36 to 45 years. All were followed after device placement, with 138 having completed 8 years of use.
Two perforations occurred following device placement–both within the first year, and none have occurred since, Dr. Westhoff noted.
Other adverse events included expulsion in 68 women (4.0%), with 50 of those (73.5%) occurring in the first year of use, and pelvic infection in 15 women (0.9%), with 11 (73.3%) of those occurring after 6 or more months of use.
Only 40 women (2.3%) discontinued the study due to bleeding, and 30 of those (75%) did so in the first 2 years.
in both nulliparous and parous women, and in both non-obese and obese women, Dr. Westhoff concluded. She added that “there were no additional pregnancies in 6 years, and this study will be continuing to look all the way to 10-year effectiveness.”
This study is funded by Medicines360, a non-profit pharmaceutical company. Dr. Westhoff is a consultant or advisory board member for Agile and Cooper Surgical, and a Data and Safety Monitoring Board member for phase 4 studies for Bayer and Merck.
SOURCE: Westhoff C et al., ACOG 2019: Abstract 13M.
REPORTING FROM ACOG 2019
Courts temporarily block Title X changes
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
[email protected]
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
[email protected]
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
[email protected]