User login
Benzodiazepine reduction without withdrawal symptoms possible in patients with schizophrenia, bipolar disorder
COLORADO SPRINGS – Patients with schizophrenia and bipolar disorder can reduce or even discontinue their reliance on benzodiazepines when kept to a gradually tapered titration, results of a Danish study have shown.
Benzodiazepines often are prescribed to patients with severe mental illnesses to help relieve comorbid anxiety and insomnia. According to Dr. Lone Baandrup, a researcher at the Center for Neuropsychiatric Schizophrenia Research at the Copenhagen University Hospital in Glostrup, Denmark, the treatment is usually meant as a temporary measure during acute episodes, but patients often become addicted.
Dr. Baandrup said in an interview at the biennial meeting of the 15th International Congress on Schizophrenia Research that because it is well established that patients with severe mental illness often suffer from a diminished ability to secrete endogenous melatonin, “we wanted to see if we could facilitate tapering patients off their benzos using a prolonged-release melatonin.”
Dr. Baandrup and her colleagues randomly assigned 86 adults with a diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder who had been on a daily regimen of an antipsychotic and at least one benzodiazepine derivative for at least 3 months, to receive either prolonged-release melatonin 2 mg daily or matching placebo. In the intention-to-treat analysis, each group was guided by a caregiver to gradually taper their respective daily benzodiazepine dosage.
Participants were examined at baseline and at 8, 16, and 24 weeks, and were monitored weekly by telephone. All participants, researchers, treating clinicians, and outcome assessors were blinded to group assignment.
No significant difference was found between the groups as to mean benzodiazepine dosage at 24 weeks, the study’s primary outcome, but the placebo arm had a greater reduction in benzodiazepine dosage (8.01 mg in the study arm vs. 5.72 mg in placebo; difference between means, –2.29; 95% confidence interval, –5.78-1.21; P = .20).
Nearly half of the placebo group achieved complete cessation of benzodiazepine use, compared with more than one-third of the study group (21 out of 44 vs. 16 out of 42; odds ratio, 0.64; 95% confidence interval, 0.26-1.56; P = .32).
Serious or nonserious adverse events were similar across the groups.
A separate analysis of the data not presented at the conference as to whether melatonin had any effect on subjective sleep measures showed that those in the melatonin group had improved sleep according to the Pittsburgh Sleep Quality Index. When compared with the control group, “the difference was about 2 points, so it was only on the border of clinical significance, but their sleep did not get worse,” Dr. Baandrup said.
The investigators also used the Pittsburgh Sleep Diary to measure whether there were any objective differences in sleep between the melatonin and placebo groups, and found that reducing benzodiazepine use did not result in insomnia. “It remained the same from baseline to follow-up," Dr. Baandrup said. “Many patients are afraid to taper off their benzos because they fear their sleep will worsen, but we didn’t find that.”
Dr. Baandrup did not have any relevant disclosures.
On Twitter @whitneymcknight
COLORADO SPRINGS – Patients with schizophrenia and bipolar disorder can reduce or even discontinue their reliance on benzodiazepines when kept to a gradually tapered titration, results of a Danish study have shown.
Benzodiazepines often are prescribed to patients with severe mental illnesses to help relieve comorbid anxiety and insomnia. According to Dr. Lone Baandrup, a researcher at the Center for Neuropsychiatric Schizophrenia Research at the Copenhagen University Hospital in Glostrup, Denmark, the treatment is usually meant as a temporary measure during acute episodes, but patients often become addicted.
Dr. Baandrup said in an interview at the biennial meeting of the 15th International Congress on Schizophrenia Research that because it is well established that patients with severe mental illness often suffer from a diminished ability to secrete endogenous melatonin, “we wanted to see if we could facilitate tapering patients off their benzos using a prolonged-release melatonin.”
Dr. Baandrup and her colleagues randomly assigned 86 adults with a diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder who had been on a daily regimen of an antipsychotic and at least one benzodiazepine derivative for at least 3 months, to receive either prolonged-release melatonin 2 mg daily or matching placebo. In the intention-to-treat analysis, each group was guided by a caregiver to gradually taper their respective daily benzodiazepine dosage.
Participants were examined at baseline and at 8, 16, and 24 weeks, and were monitored weekly by telephone. All participants, researchers, treating clinicians, and outcome assessors were blinded to group assignment.
No significant difference was found between the groups as to mean benzodiazepine dosage at 24 weeks, the study’s primary outcome, but the placebo arm had a greater reduction in benzodiazepine dosage (8.01 mg in the study arm vs. 5.72 mg in placebo; difference between means, –2.29; 95% confidence interval, –5.78-1.21; P = .20).
Nearly half of the placebo group achieved complete cessation of benzodiazepine use, compared with more than one-third of the study group (21 out of 44 vs. 16 out of 42; odds ratio, 0.64; 95% confidence interval, 0.26-1.56; P = .32).
Serious or nonserious adverse events were similar across the groups.
A separate analysis of the data not presented at the conference as to whether melatonin had any effect on subjective sleep measures showed that those in the melatonin group had improved sleep according to the Pittsburgh Sleep Quality Index. When compared with the control group, “the difference was about 2 points, so it was only on the border of clinical significance, but their sleep did not get worse,” Dr. Baandrup said.
The investigators also used the Pittsburgh Sleep Diary to measure whether there were any objective differences in sleep between the melatonin and placebo groups, and found that reducing benzodiazepine use did not result in insomnia. “It remained the same from baseline to follow-up," Dr. Baandrup said. “Many patients are afraid to taper off their benzos because they fear their sleep will worsen, but we didn’t find that.”
Dr. Baandrup did not have any relevant disclosures.
On Twitter @whitneymcknight
COLORADO SPRINGS – Patients with schizophrenia and bipolar disorder can reduce or even discontinue their reliance on benzodiazepines when kept to a gradually tapered titration, results of a Danish study have shown.
Benzodiazepines often are prescribed to patients with severe mental illnesses to help relieve comorbid anxiety and insomnia. According to Dr. Lone Baandrup, a researcher at the Center for Neuropsychiatric Schizophrenia Research at the Copenhagen University Hospital in Glostrup, Denmark, the treatment is usually meant as a temporary measure during acute episodes, but patients often become addicted.
Dr. Baandrup said in an interview at the biennial meeting of the 15th International Congress on Schizophrenia Research that because it is well established that patients with severe mental illness often suffer from a diminished ability to secrete endogenous melatonin, “we wanted to see if we could facilitate tapering patients off their benzos using a prolonged-release melatonin.”
Dr. Baandrup and her colleagues randomly assigned 86 adults with a diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder who had been on a daily regimen of an antipsychotic and at least one benzodiazepine derivative for at least 3 months, to receive either prolonged-release melatonin 2 mg daily or matching placebo. In the intention-to-treat analysis, each group was guided by a caregiver to gradually taper their respective daily benzodiazepine dosage.
Participants were examined at baseline and at 8, 16, and 24 weeks, and were monitored weekly by telephone. All participants, researchers, treating clinicians, and outcome assessors were blinded to group assignment.
No significant difference was found between the groups as to mean benzodiazepine dosage at 24 weeks, the study’s primary outcome, but the placebo arm had a greater reduction in benzodiazepine dosage (8.01 mg in the study arm vs. 5.72 mg in placebo; difference between means, –2.29; 95% confidence interval, –5.78-1.21; P = .20).
Nearly half of the placebo group achieved complete cessation of benzodiazepine use, compared with more than one-third of the study group (21 out of 44 vs. 16 out of 42; odds ratio, 0.64; 95% confidence interval, 0.26-1.56; P = .32).
Serious or nonserious adverse events were similar across the groups.
A separate analysis of the data not presented at the conference as to whether melatonin had any effect on subjective sleep measures showed that those in the melatonin group had improved sleep according to the Pittsburgh Sleep Quality Index. When compared with the control group, “the difference was about 2 points, so it was only on the border of clinical significance, but their sleep did not get worse,” Dr. Baandrup said.
The investigators also used the Pittsburgh Sleep Diary to measure whether there were any objective differences in sleep between the melatonin and placebo groups, and found that reducing benzodiazepine use did not result in insomnia. “It remained the same from baseline to follow-up," Dr. Baandrup said. “Many patients are afraid to taper off their benzos because they fear their sleep will worsen, but we didn’t find that.”
Dr. Baandrup did not have any relevant disclosures.
On Twitter @whitneymcknight
AT THE INTERNATIONAL CONGRESS ON SCHIZOPHRENIA RESEARCH
Key clinical point: Patients with schizophrenia or bipolar disorder can reduce, or even discontinue, reliance on benzodiazepines when tapered gradually but steadily.
Major finding: No significant difference was found in benzodiazepine reduction rates between patients with psychosis given prolonged-release melatonin and placebo.
Data source: Negative, single-center, blinded parallel study of 86 patients with schizophrenia or bipolar disorder randomly assigned to long-acting melatonin or placebo while reducing their benzodiazepine dosage over 6 months.
Disclosures: Dr. Baandrup did not have any relevant disclosures.
FDA calls study of unexplained olanzapine deaths ‘inconclusive’
The Food and Drug Administration’s review of a study that looked into the unexplained deaths of two patients days after receiving injections of the long-acting injectable formulation of the atypical antipsychotic olanzapine are “inconclusive,” and no labeling changes are currently recommended, the agency has announced.
“We are unable to exclude the possibility that the deaths were caused by rapid, but delayed, entry of the drug into the bloodstream following intramuscular injection,” according to the drug safety communication issued March 23 by the FDA.
Patients who receive olanzapine pamoate must be monitored for 3 hours after an injection because of the risk of post-injection delirium sedation (PDSS) associated with the injection. These patients, however, died 3 to 4 days after having received appropriate IM doses of olanzapine pamoate (Zyprexa Relprevv) and were found to have very high levels of the drug. The deaths and subsequent investigation were announced by the FDA in 2013.
The March 23 statement said an animal study, conducted by olanzapine manufacturer Eli Lilly at the FDA’s request to determine whether an IM injection could result in “postmortem redistribution” of olanzapine pamoate, “suggested that much of the drug level increase could have occurred after death, a finding that could explain the extremely high blood levels” in the two patients.
Based on its review, the FDA is not recommending any changes to the prescribing information of olanzapine pamoate. However, the agency is advising health care professionals to continue to follow the Risk Evaluation and Mitigation Strategy in place for this drug, which includes mandatory enrollment of patients, prescribers, health care facilities, and pharmacies in the Zyprexa Relprevv Patient Care program. Patients “should not stop receiving treatment without first talking to their health care professionals,” the statement adds.
The REMS requirements include continuous monitoring of patients for 3 hours after the injection, which must be administered at a REMS-certified health care facility that has quick access to emergency response services. A medication guide explaining these and other risks associated with the drug are provided to patients.
The increased risk for severe sedation, including coma, and/or delirium after each olanzapine pamoate injection, also is described in a boxed warning in the prescribing information.
Adverse events associated with Zyprexa Relprevv should be reported to the FDA’s MedWatch program at 800-332-1088 or https://www.accessdata.fda.gov/scripts/medwatch/
The Food and Drug Administration’s review of a study that looked into the unexplained deaths of two patients days after receiving injections of the long-acting injectable formulation of the atypical antipsychotic olanzapine are “inconclusive,” and no labeling changes are currently recommended, the agency has announced.
“We are unable to exclude the possibility that the deaths were caused by rapid, but delayed, entry of the drug into the bloodstream following intramuscular injection,” according to the drug safety communication issued March 23 by the FDA.
Patients who receive olanzapine pamoate must be monitored for 3 hours after an injection because of the risk of post-injection delirium sedation (PDSS) associated with the injection. These patients, however, died 3 to 4 days after having received appropriate IM doses of olanzapine pamoate (Zyprexa Relprevv) and were found to have very high levels of the drug. The deaths and subsequent investigation were announced by the FDA in 2013.
The March 23 statement said an animal study, conducted by olanzapine manufacturer Eli Lilly at the FDA’s request to determine whether an IM injection could result in “postmortem redistribution” of olanzapine pamoate, “suggested that much of the drug level increase could have occurred after death, a finding that could explain the extremely high blood levels” in the two patients.
Based on its review, the FDA is not recommending any changes to the prescribing information of olanzapine pamoate. However, the agency is advising health care professionals to continue to follow the Risk Evaluation and Mitigation Strategy in place for this drug, which includes mandatory enrollment of patients, prescribers, health care facilities, and pharmacies in the Zyprexa Relprevv Patient Care program. Patients “should not stop receiving treatment without first talking to their health care professionals,” the statement adds.
The REMS requirements include continuous monitoring of patients for 3 hours after the injection, which must be administered at a REMS-certified health care facility that has quick access to emergency response services. A medication guide explaining these and other risks associated with the drug are provided to patients.
The increased risk for severe sedation, including coma, and/or delirium after each olanzapine pamoate injection, also is described in a boxed warning in the prescribing information.
Adverse events associated with Zyprexa Relprevv should be reported to the FDA’s MedWatch program at 800-332-1088 or https://www.accessdata.fda.gov/scripts/medwatch/
The Food and Drug Administration’s review of a study that looked into the unexplained deaths of two patients days after receiving injections of the long-acting injectable formulation of the atypical antipsychotic olanzapine are “inconclusive,” and no labeling changes are currently recommended, the agency has announced.
“We are unable to exclude the possibility that the deaths were caused by rapid, but delayed, entry of the drug into the bloodstream following intramuscular injection,” according to the drug safety communication issued March 23 by the FDA.
Patients who receive olanzapine pamoate must be monitored for 3 hours after an injection because of the risk of post-injection delirium sedation (PDSS) associated with the injection. These patients, however, died 3 to 4 days after having received appropriate IM doses of olanzapine pamoate (Zyprexa Relprevv) and were found to have very high levels of the drug. The deaths and subsequent investigation were announced by the FDA in 2013.
The March 23 statement said an animal study, conducted by olanzapine manufacturer Eli Lilly at the FDA’s request to determine whether an IM injection could result in “postmortem redistribution” of olanzapine pamoate, “suggested that much of the drug level increase could have occurred after death, a finding that could explain the extremely high blood levels” in the two patients.
Based on its review, the FDA is not recommending any changes to the prescribing information of olanzapine pamoate. However, the agency is advising health care professionals to continue to follow the Risk Evaluation and Mitigation Strategy in place for this drug, which includes mandatory enrollment of patients, prescribers, health care facilities, and pharmacies in the Zyprexa Relprevv Patient Care program. Patients “should not stop receiving treatment without first talking to their health care professionals,” the statement adds.
The REMS requirements include continuous monitoring of patients for 3 hours after the injection, which must be administered at a REMS-certified health care facility that has quick access to emergency response services. A medication guide explaining these and other risks associated with the drug are provided to patients.
The increased risk for severe sedation, including coma, and/or delirium after each olanzapine pamoate injection, also is described in a boxed warning in the prescribing information.
Adverse events associated with Zyprexa Relprevv should be reported to the FDA’s MedWatch program at 800-332-1088 or https://www.accessdata.fda.gov/scripts/medwatch/
What to tell your bipolar disorder patient who wants to breast-feed
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
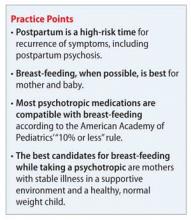
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
FDA green lights sublingual antipsychotic to treat bipolar I in children
For the first time in 5 years, the Food and Drug Administration has approved an application to market an antipsychotic to clinicians for treating bipolar I disorder in children aged 10-17 years.
Actavis, the Dublin-based pharmaceutical manufacturer, announced March 13 that the FDA has approved the company’s supplemental new drug application for asenapine (Saphris), a second-generation atypical antipsychotic, as monotherapy for the acute treatment of manic or mixed episodes associated with bipolar I disorder in this pediatric population.
The FDA approved asenapine in 2009 for treating adults with manic or mixed episodes of bipolar I, either as monotherapy or adjunctive to either lithium or valproate. It also is indicated for the acute or maintenance treatment of schizophrenia in adults.
Asenapine is administered as a sublingual tablet, and the company announced in the statement that it would begin marketing black cherry–flavored sublingual asenapine tablets in 2.5 mg, 5 mg, and 10 mg doses beginning in the second quarter of this year.
According to the manufacturer, the agency’s approval was granted based on the results of a 3-week monotherapy trial in 403 pediatric patients, aged 10-17 years, 302 of whom were treated twice daily with either 2.5 mg, 5 mg, or 10 mg of asenapine. The drug maker also said asenapine demonstrated improvement in the Young Mania Rating Scale total score and the Clinical Global Impressions-Bipolar Scale overall score, compared with placebo.
When asked about the approval, Dr. David Fassler said it's helpful to have a range of medications to treat pediatric patients with bipolar disorder. "However, the current approval is based on results of a relatively short-term clinical trial," said Dr. Fassler, clinical professor of psychiatry at the University of Vermont, Burlington. "In the real world, many young people ultimately take atypical antipsychotics for an extended period of time. Physicians and parents need data on the safety and efficacy of these medications when used on a more long-term or ongoing basis in order to make fully informed decisions about treatment options."
The most common side effects the company said it recorded in the pediatric clinical trial were sleepiness, dizziness, strange sense of taste, numbing of the mouth, nausea, increased appetite, feeling tired, and weight gain.
On Twitter @whitneymcknight
For the first time in 5 years, the Food and Drug Administration has approved an application to market an antipsychotic to clinicians for treating bipolar I disorder in children aged 10-17 years.
Actavis, the Dublin-based pharmaceutical manufacturer, announced March 13 that the FDA has approved the company’s supplemental new drug application for asenapine (Saphris), a second-generation atypical antipsychotic, as monotherapy for the acute treatment of manic or mixed episodes associated with bipolar I disorder in this pediatric population.
The FDA approved asenapine in 2009 for treating adults with manic or mixed episodes of bipolar I, either as monotherapy or adjunctive to either lithium or valproate. It also is indicated for the acute or maintenance treatment of schizophrenia in adults.
Asenapine is administered as a sublingual tablet, and the company announced in the statement that it would begin marketing black cherry–flavored sublingual asenapine tablets in 2.5 mg, 5 mg, and 10 mg doses beginning in the second quarter of this year.
According to the manufacturer, the agency’s approval was granted based on the results of a 3-week monotherapy trial in 403 pediatric patients, aged 10-17 years, 302 of whom were treated twice daily with either 2.5 mg, 5 mg, or 10 mg of asenapine. The drug maker also said asenapine demonstrated improvement in the Young Mania Rating Scale total score and the Clinical Global Impressions-Bipolar Scale overall score, compared with placebo.
When asked about the approval, Dr. David Fassler said it's helpful to have a range of medications to treat pediatric patients with bipolar disorder. "However, the current approval is based on results of a relatively short-term clinical trial," said Dr. Fassler, clinical professor of psychiatry at the University of Vermont, Burlington. "In the real world, many young people ultimately take atypical antipsychotics for an extended period of time. Physicians and parents need data on the safety and efficacy of these medications when used on a more long-term or ongoing basis in order to make fully informed decisions about treatment options."
The most common side effects the company said it recorded in the pediatric clinical trial were sleepiness, dizziness, strange sense of taste, numbing of the mouth, nausea, increased appetite, feeling tired, and weight gain.
On Twitter @whitneymcknight
For the first time in 5 years, the Food and Drug Administration has approved an application to market an antipsychotic to clinicians for treating bipolar I disorder in children aged 10-17 years.
Actavis, the Dublin-based pharmaceutical manufacturer, announced March 13 that the FDA has approved the company’s supplemental new drug application for asenapine (Saphris), a second-generation atypical antipsychotic, as monotherapy for the acute treatment of manic or mixed episodes associated with bipolar I disorder in this pediatric population.
The FDA approved asenapine in 2009 for treating adults with manic or mixed episodes of bipolar I, either as monotherapy or adjunctive to either lithium or valproate. It also is indicated for the acute or maintenance treatment of schizophrenia in adults.
Asenapine is administered as a sublingual tablet, and the company announced in the statement that it would begin marketing black cherry–flavored sublingual asenapine tablets in 2.5 mg, 5 mg, and 10 mg doses beginning in the second quarter of this year.
According to the manufacturer, the agency’s approval was granted based on the results of a 3-week monotherapy trial in 403 pediatric patients, aged 10-17 years, 302 of whom were treated twice daily with either 2.5 mg, 5 mg, or 10 mg of asenapine. The drug maker also said asenapine demonstrated improvement in the Young Mania Rating Scale total score and the Clinical Global Impressions-Bipolar Scale overall score, compared with placebo.
When asked about the approval, Dr. David Fassler said it's helpful to have a range of medications to treat pediatric patients with bipolar disorder. "However, the current approval is based on results of a relatively short-term clinical trial," said Dr. Fassler, clinical professor of psychiatry at the University of Vermont, Burlington. "In the real world, many young people ultimately take atypical antipsychotics for an extended period of time. Physicians and parents need data on the safety and efficacy of these medications when used on a more long-term or ongoing basis in order to make fully informed decisions about treatment options."
The most common side effects the company said it recorded in the pediatric clinical trial were sleepiness, dizziness, strange sense of taste, numbing of the mouth, nausea, increased appetite, feeling tired, and weight gain.
On Twitter @whitneymcknight
Dimensional aspects of DSM-5 personality disorder criteria discussed
HUNTINGTON BEACH, CALIF. – In the opinion of Dr. John M. Oldham, clinicians who deem the alternative personality disorder model of the DSM-5 as too confusing are misguided.
“If you’re going to compare DSM-5 alternative personality disorder model with the DSM-IV model, you have to do a fair comparison,” Dr. Oldham told attendees at the annual meeting of the American College of Psychiatrists. ”In fact, we reduced the number of items that you have to measure by 43%.”
So when people describe the DSM-5’s personality disorders criteria as more complicated, he continued, “what they really mean is, ‘it’s more complicated than what I do,’ not that it’s more complicated than [the] DSM-IV.”
Along with Dr. Andrew E. Skodol, Dr. Oldham cochaired a work group of experts convened by the American Psychiatric Association to update diagnostic criteria related to personality and personality disorders for the DSM-5. “We took our work and our charge seriously,” recalled Dr. Oldham, senior vice president and chief of staff at the Menninger Clinic, Houston. “It was not easy. We had many challenges. A great deal of research has been done in the factor analytic research psychology world around things like the five-factor model of personality. Such terms are not always terribly familiar in clinical medicine, so there was a problem with the lack of familiarity. Then there were vested interests different groups had that were influential in some ways.”
Ultimately, the alternative personality disorder model was placed in section III of the DSM-5. The model enables clinicians “to individually portray the dimensions of the patient’s pathology in a thorough and broad way,” Dr. Oldham explained. “We emphasize impairment in functioning. That’s an important new requirement. So you have to determine, by using the level of functioning scale, whether the person does or doesn’t have moderate or greater impairment. If you have a patient with mild impairment, you can describe what you’re concerned about, but you’re not putting that patient into a diagnostic box of pathology. There is a dimensional scope that enables you to capture many types of patients.”
An empirical study of 337 clinicians demonstrated that in 14 of 18 comparisons, respondents deemed the DSM-5 pathological personality traits as more clinical useful, compared with the DSM-IV, with respect to ease of use, communication of clinical information to other professionals, communication of clinical information to patients, comprehensiveness in describing pathology, and treatment planning (J. Abnorm. Psychol. 2013;122:836-41). “In fact, this was a preference to the new model, which was unfamiliar, compared to the model that these clinicians had been using for 20 years,” Dr. Oldham said.
The study also found that the new DSM-5 personality disorder model was more strongly related to clinical decision making in areas of global functioning, risk assessment, recommended treatment type and intensity, and prognosis.
According to unpublished data from the DSM-5 field trials conducted in the United States and Canada, more than 80% of clinicians in academic and routine clinical practice fields found the new personality disorder criteria “moderately” to “extremely” useful, compared with the DSM-IV. In fact, the respondents rated the new criteria as more useful than other changes to the DSM-5, including those related to bipolar and related disorders, schizophrenia spectrum and other psychotic disorders, and other conditions.
In addition, a test-retest reliability study conducted at 11 academic medical centers found that the new model for borderline personality disorder had a good test-retest reliability (.054), in the same ballpark as that for bipolar I disorder (0.56) and schizophrenia (.50) (Am. J. Psychiatry 2013;170:43-58). “This surprised a lot of people,” Dr. Oldham said.
About 1 year after the DSM-5’s release, Medscape Psychiatry surveyed almost 3,000 clinicians about their impressions of the new guidelines. Of the 2,828 respondents, nearly one-third (28%) were psychiatrists, 22% were psychologists, 13% were family medicine clinicians, and the rest were from other medical fields. The researchers found that 39% of survey respondents were considering the dimensional approaches offered in the new personality disorder criteria of the DSM-5.
“That’s not bad,” Dr. Oldham said.
He reported having no relevant financial conflicts.
On Twitter @dougbrunk
HUNTINGTON BEACH, CALIF. – In the opinion of Dr. John M. Oldham, clinicians who deem the alternative personality disorder model of the DSM-5 as too confusing are misguided.
“If you’re going to compare DSM-5 alternative personality disorder model with the DSM-IV model, you have to do a fair comparison,” Dr. Oldham told attendees at the annual meeting of the American College of Psychiatrists. ”In fact, we reduced the number of items that you have to measure by 43%.”
So when people describe the DSM-5’s personality disorders criteria as more complicated, he continued, “what they really mean is, ‘it’s more complicated than what I do,’ not that it’s more complicated than [the] DSM-IV.”
Along with Dr. Andrew E. Skodol, Dr. Oldham cochaired a work group of experts convened by the American Psychiatric Association to update diagnostic criteria related to personality and personality disorders for the DSM-5. “We took our work and our charge seriously,” recalled Dr. Oldham, senior vice president and chief of staff at the Menninger Clinic, Houston. “It was not easy. We had many challenges. A great deal of research has been done in the factor analytic research psychology world around things like the five-factor model of personality. Such terms are not always terribly familiar in clinical medicine, so there was a problem with the lack of familiarity. Then there were vested interests different groups had that were influential in some ways.”
Ultimately, the alternative personality disorder model was placed in section III of the DSM-5. The model enables clinicians “to individually portray the dimensions of the patient’s pathology in a thorough and broad way,” Dr. Oldham explained. “We emphasize impairment in functioning. That’s an important new requirement. So you have to determine, by using the level of functioning scale, whether the person does or doesn’t have moderate or greater impairment. If you have a patient with mild impairment, you can describe what you’re concerned about, but you’re not putting that patient into a diagnostic box of pathology. There is a dimensional scope that enables you to capture many types of patients.”
An empirical study of 337 clinicians demonstrated that in 14 of 18 comparisons, respondents deemed the DSM-5 pathological personality traits as more clinical useful, compared with the DSM-IV, with respect to ease of use, communication of clinical information to other professionals, communication of clinical information to patients, comprehensiveness in describing pathology, and treatment planning (J. Abnorm. Psychol. 2013;122:836-41). “In fact, this was a preference to the new model, which was unfamiliar, compared to the model that these clinicians had been using for 20 years,” Dr. Oldham said.
The study also found that the new DSM-5 personality disorder model was more strongly related to clinical decision making in areas of global functioning, risk assessment, recommended treatment type and intensity, and prognosis.
According to unpublished data from the DSM-5 field trials conducted in the United States and Canada, more than 80% of clinicians in academic and routine clinical practice fields found the new personality disorder criteria “moderately” to “extremely” useful, compared with the DSM-IV. In fact, the respondents rated the new criteria as more useful than other changes to the DSM-5, including those related to bipolar and related disorders, schizophrenia spectrum and other psychotic disorders, and other conditions.
In addition, a test-retest reliability study conducted at 11 academic medical centers found that the new model for borderline personality disorder had a good test-retest reliability (.054), in the same ballpark as that for bipolar I disorder (0.56) and schizophrenia (.50) (Am. J. Psychiatry 2013;170:43-58). “This surprised a lot of people,” Dr. Oldham said.
About 1 year after the DSM-5’s release, Medscape Psychiatry surveyed almost 3,000 clinicians about their impressions of the new guidelines. Of the 2,828 respondents, nearly one-third (28%) were psychiatrists, 22% were psychologists, 13% were family medicine clinicians, and the rest were from other medical fields. The researchers found that 39% of survey respondents were considering the dimensional approaches offered in the new personality disorder criteria of the DSM-5.
“That’s not bad,” Dr. Oldham said.
He reported having no relevant financial conflicts.
On Twitter @dougbrunk
HUNTINGTON BEACH, CALIF. – In the opinion of Dr. John M. Oldham, clinicians who deem the alternative personality disorder model of the DSM-5 as too confusing are misguided.
“If you’re going to compare DSM-5 alternative personality disorder model with the DSM-IV model, you have to do a fair comparison,” Dr. Oldham told attendees at the annual meeting of the American College of Psychiatrists. ”In fact, we reduced the number of items that you have to measure by 43%.”
So when people describe the DSM-5’s personality disorders criteria as more complicated, he continued, “what they really mean is, ‘it’s more complicated than what I do,’ not that it’s more complicated than [the] DSM-IV.”
Along with Dr. Andrew E. Skodol, Dr. Oldham cochaired a work group of experts convened by the American Psychiatric Association to update diagnostic criteria related to personality and personality disorders for the DSM-5. “We took our work and our charge seriously,” recalled Dr. Oldham, senior vice president and chief of staff at the Menninger Clinic, Houston. “It was not easy. We had many challenges. A great deal of research has been done in the factor analytic research psychology world around things like the five-factor model of personality. Such terms are not always terribly familiar in clinical medicine, so there was a problem with the lack of familiarity. Then there were vested interests different groups had that were influential in some ways.”
Ultimately, the alternative personality disorder model was placed in section III of the DSM-5. The model enables clinicians “to individually portray the dimensions of the patient’s pathology in a thorough and broad way,” Dr. Oldham explained. “We emphasize impairment in functioning. That’s an important new requirement. So you have to determine, by using the level of functioning scale, whether the person does or doesn’t have moderate or greater impairment. If you have a patient with mild impairment, you can describe what you’re concerned about, but you’re not putting that patient into a diagnostic box of pathology. There is a dimensional scope that enables you to capture many types of patients.”
An empirical study of 337 clinicians demonstrated that in 14 of 18 comparisons, respondents deemed the DSM-5 pathological personality traits as more clinical useful, compared with the DSM-IV, with respect to ease of use, communication of clinical information to other professionals, communication of clinical information to patients, comprehensiveness in describing pathology, and treatment planning (J. Abnorm. Psychol. 2013;122:836-41). “In fact, this was a preference to the new model, which was unfamiliar, compared to the model that these clinicians had been using for 20 years,” Dr. Oldham said.
The study also found that the new DSM-5 personality disorder model was more strongly related to clinical decision making in areas of global functioning, risk assessment, recommended treatment type and intensity, and prognosis.
According to unpublished data from the DSM-5 field trials conducted in the United States and Canada, more than 80% of clinicians in academic and routine clinical practice fields found the new personality disorder criteria “moderately” to “extremely” useful, compared with the DSM-IV. In fact, the respondents rated the new criteria as more useful than other changes to the DSM-5, including those related to bipolar and related disorders, schizophrenia spectrum and other psychotic disorders, and other conditions.
In addition, a test-retest reliability study conducted at 11 academic medical centers found that the new model for borderline personality disorder had a good test-retest reliability (.054), in the same ballpark as that for bipolar I disorder (0.56) and schizophrenia (.50) (Am. J. Psychiatry 2013;170:43-58). “This surprised a lot of people,” Dr. Oldham said.
About 1 year after the DSM-5’s release, Medscape Psychiatry surveyed almost 3,000 clinicians about their impressions of the new guidelines. Of the 2,828 respondents, nearly one-third (28%) were psychiatrists, 22% were psychologists, 13% were family medicine clinicians, and the rest were from other medical fields. The researchers found that 39% of survey respondents were considering the dimensional approaches offered in the new personality disorder criteria of the DSM-5.
“That’s not bad,” Dr. Oldham said.
He reported having no relevant financial conflicts.
On Twitter @dougbrunk
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PSYCHIATRISTS
Schizophrenia, but not bipolar disorder, linked to social cognition deficits
Significant deficits in social cognition were found in patients with schizophrenia spectrum disorders (SSDs), but not in patients with bipolar disorder (BD), Dr. George C. Nitzburg and his associates reported.
In a study of 537 SSD patients, 85 BD patients with psychotic features, 37 BD patients without psychotic features, and 309 controls, SSD patients had significant social cognition deficits, compared with controls. Bipolar patients did not have these deficits, the investigators reported (F (2,964) = 24.85, P < .001). Social cognition was assessed using scores on the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT).
The results of this study “highlight the importance of developing standardized social-cognitive batteries for use across BD and SSD and emphasize the need for future work on social brain development in clinical populations,” Dr. Nitzburg and his coauthors wrote in the report.
Read the full article here: doi:10.1016/j.scog.2014.12.003.
Significant deficits in social cognition were found in patients with schizophrenia spectrum disorders (SSDs), but not in patients with bipolar disorder (BD), Dr. George C. Nitzburg and his associates reported.
In a study of 537 SSD patients, 85 BD patients with psychotic features, 37 BD patients without psychotic features, and 309 controls, SSD patients had significant social cognition deficits, compared with controls. Bipolar patients did not have these deficits, the investigators reported (F (2,964) = 24.85, P < .001). Social cognition was assessed using scores on the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT).
The results of this study “highlight the importance of developing standardized social-cognitive batteries for use across BD and SSD and emphasize the need for future work on social brain development in clinical populations,” Dr. Nitzburg and his coauthors wrote in the report.
Read the full article here: doi:10.1016/j.scog.2014.12.003.
Significant deficits in social cognition were found in patients with schizophrenia spectrum disorders (SSDs), but not in patients with bipolar disorder (BD), Dr. George C. Nitzburg and his associates reported.
In a study of 537 SSD patients, 85 BD patients with psychotic features, 37 BD patients without psychotic features, and 309 controls, SSD patients had significant social cognition deficits, compared with controls. Bipolar patients did not have these deficits, the investigators reported (F (2,964) = 24.85, P < .001). Social cognition was assessed using scores on the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT).
The results of this study “highlight the importance of developing standardized social-cognitive batteries for use across BD and SSD and emphasize the need for future work on social brain development in clinical populations,” Dr. Nitzburg and his coauthors wrote in the report.
Read the full article here: doi:10.1016/j.scog.2014.12.003.
Delusional and aggressive, while playing the lottery
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
Outpatient mania
Treating bipolar mania in the outpatient setting: Risk vs reward
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
Manic episodes, by definition, are associated with significant social or occupational impairment.1 Some manic patients are violent or engage in reckless behaviors that can harm themselves or others, such as speeding, disrupting traffic, or playing with fire. When these patients present to a psychiatrist’s outpatient practice, involuntary hospitalization might be justified.
However, some manic patients, in spite of their elevated, expansive, or irritable mood state, never behave dangerously and might not meet legal criteria for involuntary hospitalization, although these criteria differ from state to state. These patients might see a psychiatrist because manic symptoms such as irritability, talkativeness, and impulsivity are bothersome to their family members but pose no serious danger (Box). In this situation, the psychiatrist can strongly encourage the patient to seek voluntary hospitalization or attend a partial hospitalization program. If the patient declines, the psychiatrist is left with 2 choices: initiate treatment in the outpatient setting or refuse to treat the patient and refer to another provider.
Treating “non-dangerous” mania in the outpatient setting is fraught with challenges:
• the possibility that the patient’s condition will progress to dangerousness
• poor adherence to treatment because of the patient’s limited insight
• the large amount of time required from the psychiatrist and care team to adequately manage the manic episode (eg, time spent with family members, frequent patient visits, and managing communications from the patient).
There are no guidelines to assist the office-based practitioner in treating mania in the outpatient setting. When considering dosing and optimal medication combinations for treating mania, clinical trials may be of limited value because most of these studies only included hospitalized manic patients.
Because of this dearth of knowledge, we provide recommendations based on our review of the literature and from our experience working with manic patients who refuse voluntary hospitalization and could not be hospitalized against their will. These recommendations are organized into 3 sections: diagnostic approach, treatment strategy, and family involvement.
Diagnostic approach
Making a diagnosis of mania might seem straightforward for clinicians who work in inpatient settings; however, mania might not present with classic florid symptoms among outpatients. Patients might have a chief concern of irritability, dysphoria, anxiety, or “insomnia,” which may lead clinicians to focus initially on non-bipolar conditions.2
During the interview, it is important to assess for any current DSM-5 symptoms of a manic episode, while being careful not to accept a patient’s denial of symptoms. Patients with mania often have poor insight and are unaware of changes from their baseline state when manic.3 Alternatively, manic patients may want you to believe that they are well and could minimize or deny all symptoms. Therefore, it is important to pay attention to mental status examination findings, such as hyperverbal speech, elated affect, psychomotor agitation, a tangential thought process, or flight of ideas.
Countertransference feelings of diagnostic confusion or frustration after long patient monologues or multiple interruptions by the patient should be incorporated into the diagnostic assessment. Family members or friends often can provide objective observations of behavioral changes necessary to secure the diagnosis.
Treatment strategy
Decision points. When treating manic outpatients, assess the need for hospitalization at each visit. Advantages of the inpatient setting include:
• the possibility of rapid medication adjustments
• continuous observation to ensure the patient’s safety
• keeping the patient temporarily removed from his community to prevent irreversible social and economic harms.
However, a challenge with hospitalization is third-party payers’ influence on a patient’s length of stay, which may lead to rapid medication changes that may not be clinically ideal.
At each outpatient visit, explore with the patient and family emerging symptoms that could justify involuntary hospitalization. Document whether you recommended inpatient hospitalization, the patient’s response to the recommendation, that you are aware and have considered the risks associated with outpatient care, and that you have discussed these risks with the patient and family.
For patients well-known to the psychiatrist, a history of dangerous mania may lead him (her) to strongly recommend hospitalization, whereas a pre-existing therapeutic alliance and no current or distant history of dangerous mania may lead the clinician to look for alternatives to inpatient care. Concomitant drug or alcohol use may increase the likelihood of mania becoming dangerous, making outpatient treatment ill-advised and riskier for everyone involved.
In exchange for agreeing to provide outpatient care for mania, it often is helpful to negotiate with the patient and family a threshold level of symptoms or behavior that will result in the patient agreeing to voluntary hospitalization (Table 1). Such an agreement can include stopping outpatient treatment if the patient does not improve significantly after 2 or 3 weeks or develops psychotic symptoms. The negotiation also can include partial hospitalization as an option, so long as the patient’s mania continues to be non-dangerous.
Obtaining pretreatment blood work can help a clinician determine whether a medication is safe to prescribe and establish causality if laboratory abnormalities arise after treatment begins. Ideally, the psychiatrist should follow consensus guidelines developed by the International Society for Bipolar Disorders4 or the American Psychiatric Association (APA)5 and order appropriate laboratory tests before prescribing anti-manic medications. Determine the pregnancy status of female patients of child-bearing age before prescribing a potentially teratogenic medication, especially because mania is associated with increased libido.6
Manic patients might be too disorganized to follow up with recommendations for laboratory testing, or could wait several days before completing blood work. Although not ideal, to avoid delaying treatment, a clinician might need to prescribe medication at the initial office visit, without pretreatment laboratory results. When the patient is more organized, complete the blood work. Keeping home pregnancy tests in the office can help rule out pregnancy before prescribing medication.
Medication. Meta-analyses have established the efficacy of mood stabilizers and antipsychotics for treating mania,7,8 and several consensus guidelines have incorporated these findings into treatment algorithms.9
For a patient already taking medications recommended by the guidelines, assess treatment adherence during the initial interview by questioning the patient and family. When the logistics of phlebotomy permit, obtaining the blood level of psychotropics can show the presence of any detectable drug concentration, which demonstrates that the patient has taken the medication recently.
If there is no evidence of nonadherence, an initial step might be to increase the dosage of the antipsychotic or mood stabilizer that the patient is already taking, ensuring that the dosage is optimized based on FDA indications and clinical trials data. The recommended rate of dosage adjustments differs among medications; however, optimal dosing should be reached quickly because a World Federation of Societies of Biological Psychiatry task force recommends that a mania treatment trial not exceed 2 weeks.10
Dosage increases can be made at weekly visits or sooner, based on treatment response and tolerability. If there is no benefit after optimizing the dosage, the next step would be to add a mood stabilizer to a second-generation antipsychotic (SGA), or vice versa to promote additive or synergistic medication effects.11 Switching one medication for the other should be avoided unless there are tolerability concerns.
For a patient who is not taking any medications, select a treatment that balances rapid stabilization with long-term efficacy and tolerability. Table 2 lists FDA-approved treatments for mania. Lamotrigine provides prophylactic efficacy with few associated risks, but it has no anti-manic effects and would be a poor choice for most actively manic patients. Most studies indicate that antipsychotics work faster than lithium at the 1-week mark; however, this may be a function of the lithium titration schedule followed in the protocols, the severity of mania among enrolled patients, the inclusion of typically non-responsive manic patients (eg, mixed) in the analysis, and the antipsychotic’s sedative potential relative to lithium. Although the anti-manic and prophylactic potential of lithium and valproate might make them an ideal first-line option, antipsychotics could stabilize a manic patient faster, especially if agitation is present.12,13
Breaking mania quickly is important when treating patients in the outpatient setting. In these situations, a reasonable choice is to prescribe a SGA, because of their rapid onset of effect, low potential for switch to depression, and utility in treating classic, mixed, or psychotic mania.10 Oral loading of valproate (20 mg/kg) is another option. An inpatient study that used an oral-loading strategy demonstrated a similar time to response as olanzapine,14 in contrast to an inpatient15 and an outpatient study16 that employed a standard starting dosage for each patient and led to slower improvement compared with olanzapine.
SGAs should be dosed moderately and lower than if the patient were hospitalized, to avoid alienating the patient from treatment by causing intolerable side effects. In particular, patients and their families should be warned about immediate risks, such as orthostasis or extrapyramidal symptoms. Although treatment guidelines recommend combination therapy as a possible first-line option,9 in the outpatient setting, monotherapy with an optimally dosed, rapid-acting agent is preferred to promote medication adherence and avoid potentially dangerous sedation. Manic patients experience increased distractibility and verbal memory and executive function impairments that can interfere with medication adherence.17 Therefore, patients are more likely to follow a simpler regimen. If SGA or valproate monotherapy does not control mania, begin combination treatment with a mood stabilizer and SGA. If the patient experiences remission with SGA monotherapy, the risks and benefits of maintaining the SGA vs switching to a mood stabilizer can be discussed.
Provide medication “as needed” for agitation—additional SGA dosing or a benzodiazepine—and explain to family members when their use is warranted. Benzodiazepines can provide short-term benefits for manic patients: anxiety relief, sedation, and anti-manic efficacy as monotherapy18-20 and in combination with other medications.21 Studies showing monotherapy efficacy employed high dosages of benzodiazepines (lorazepam mean dosage, 14 mg/d; clonazepam mean dosage, 13 mg/d)19 and high dosages of antipsychotics as needed,18,20 and often were associated with excessive sedation and ataxia.18,19 This makes benzodiazepine monotherapy a potentially dangerous approach for outpatient treatment of mania. IM lorazepam treated manic agitation less quickly than IM olanzapine, suggesting that SGAs are preferable in the outpatient setting because rapid control of agitation is crucial.22 If prescribed, a trusted family member should dispense benzodiazepines to the patient to minimize misuse because of impulsivity, distractibility, desperation to sleep, or pleasure seeking.
SGAs have the benefit of sedation but occasionally additional sleep medications are required. Benzodiazepine receptor agonists (BzRAs), such as zolpidem, eszopiclone, and zaleplon, should be used with caution. Although these medicines are effective in treating insomnia in individuals with primary insomnia23 and major depression,24 they have not been studied in manic patients. The decreased need for sleep in mania is phenomenologically25 and perhaps biologically different than insomnia in major depression.26 Therefore, mania-associated sleep disturbance might not respond to BZRAs. BzRAs also might induce somnambulism and other parasomnias,27 especially when used in combination with psychotropics, such as valproate28; it is unclear if the manic state itself increases this risk further. Sedating antihistamines with anticholinergic blockade, such as diphenhydramine and low dosages (<100 mg/d) of quetiapine, are best used only in combination with anti-manic medications because of putative link between anticholinergic blockade and manic induction.29 Less studied but safer options include novel anticonvulsants (gabapentin, pregabalin), melatonin, and melatonin receptor agonists. Sedating antidepressants, such as mirtazapine and trazodone, should be avoided.25
Important adjunctive treatment steps include discontinuing all pro-manic agents, including antidepressants, stimulants, and steroids, and discouraging use of caffeine, energy drinks, illicit drugs, and alcohol. The patient should return for office visits at least weekly, and possibly more frequently, depending on severity. Telephone check-in calls between scheduled visits may be necessary until the mania is broken.
Psychotherapy. Other than supportive therapy and psychoeducation, other forms of psychotherapy during mania are not indicated. Psychotherapy trials in bipolar disorder do not inform anti-manic efficacy because few have enrolled acutely manic patients and most report long-term benefits rather than short-term efficacy for the index manic episode.30 Educate patients about the importance of maintaining regular social rhythms and taking medication as prescribed. Manic patients might not be aware that they are acting differently during manic episodes, therefore efforts to improve the patient’s insight are unlikely to succeed. More time should be spent emphasizing the importance of adherence to treatment and taking anti-manic medications as prescribed. This discussion can be enhanced by focusing on the medication’s potential to reduce the unpleasant symptoms of mania, including irritability, insomnia, anxiety, and racing thoughts. At the first visit, discuss setting boundaries with the patient to reduce mania-driven, intrusive phone calls. A patient might develop insight after mania has resolved and he (she) can appreciate social or economic harm that occurred while manic. This discussion might foster adherence to maintenance treatment. Advise your patient to limit activities that may increase stimulation and perpetuate the mania, such as exercise, parties, concerts, or crowded shopping malls. Also, recommend that your patient stop working temporarily, to reduce stress and prevent any manic-driven interactions that could result in job loss.
If your patient has an established relationship with a psychotherapist, discuss with the therapist the plan to initiate mania treatment in the outpatient setting and work as a collaborative team, assuming that the patient has granted permission to share information. Encourage the therapist to increase the frequency of sessions with the patient to enable greater monitoring of changes in the patient’s manic symptoms.
Family involvement
Family support is crucial when treating mania in the outpatient setting. Lacking insight and organization, manic patients require the “auxiliary” judgment of trusted family members to ensure treatment success. The family should identify a single person to act as the liaison between the family and the psychiatrist. The psychiatrist should instruct this individual to accompany the patient to each clinic visit and provide regular updates on the patient’s adherence to treatment, changes in symptoms, and any new behaviors that would justify involuntary hospitalization. The treatment plan should be clearly communicated to this individual to ensure that it is implemented correctly. Ideally, this individual would be someone who understands that bipolar disorder is a mental illness, who can tolerate the patient’s potential resentment of them for taking on this role, and who can influence the patient and the other family members to adhere to the treatment plan.
This family member also should watch the patient take medication to rule out nonadherence if the patient’s condition does not improve.
Provide extensive psychoeducation to the family (Table 3). Discuss these teaching points and their implications at length during the first visit and reinforce them at subsequent visits. Advise spouses that the acute manic period is not the time to make major decisions about their marriage or to engage in couple’s therapy. These options are better explored after the patient recovers from the manic episode.
Encourage the family to engage in mania harm-reduction techniques to the extent that the patient will allow (Table 4). In particular, they should hold onto their loved one’s credit cards and checkbook, and discourage the patient from making any major financial decisions until the mania has resolved. Additionally, patients should be relieved of childcare responsibilities during this period. If there are any child welfare safety concerns, the clinician will need to report this to authorities as required by local laws.
Advise family members or roommates to call emergency services and request a crisis intervention team, or to take the patient to an emergency room if he (she) makes verbal threats to harm themselves or others, is violent, or demonstrates behaviors that indicate that he is no longer able to care for himself. The psychiatrist should assist with completing Family and Medical Leave Act paperwork for family members who will monitor the patient at home, a work-excuse letter for the patient so he does not lose his job, and short-term disability paperwork to ensure income for the patient during the manic period.
These interventions can be challenging for the entire family system because they place family members in a paternalistic role and reduce the patient’s autonomy within the family. This is problematic when these role changes occur between spouses or between a patient-parent and his (her) children. Such changes typically need to be reversed over time and may require the help of a family or couple’s therapist. To support the psychological health of the patient’s family, refer them to the National Alliance on Mental Illness for family support groups or to individual psychotherapists.
Outpatient management can be rewarding
For “non-dangerous” manic patients who cannot be hospitalized involuntarily and refuse full or partial hospitalization, a psychiatrist must choose between beginning treatment in the clinic and referring the patient to another provider. The latter option is consistent with the APA’s ethical guidelines,31 but must be done appropriately to avoid legal liability.32 This decision may disappoint a family desperate to see their loved one recover quickly and may leave them feeling betrayed by the mental health system. On the other hand, choosing to treat mania in the outpatient setting can be rewarding when resolution of mania restores the family’s homeostasis.
To achieve this outcome, the outpatient psychiatrist must engage the patient’s family to ensure that the patient adheres to the treatment plan and monitor for potentially dangerous behavior. The psychiatrist also must use his knowledge of mood symptoms, cognitive impairments, and the psychological experience of manic patients to create a safe and effective treatment strategy that the patient and family can implement.
Because of mania’s unpredictability and destructive potential, psychiatrists who agree to treat manic patients as outpatients should be familiar with their state’s statutes and case law that pertain to the refusal to accept a new patient, patient abandonment, involuntary hospitalization, confidentiality, and mandatory reporting. They also should seek clinical or legal consultation if they feel overwhelmed or uncertain about the safest and most legally sound approach.
Bottom Line
Treating mania in the outpatient setting is risky but can be accomplished in select patients with the help of the patient’s family and a strategy that integrates evidence-based pharmacotherapeutic and psychotherapeutic strategies. Because manic patients could display dangerous behavior, be familiar with your state’s laws regarding involuntary commitment, patient abandonment, and mandatory reporting.
Related Resources
• National Alliance on Mental Illness. www.NAMI.org.
• Depression and Bipolar Support Alliance. www.DBSAlliance.org.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Asenapine • Saphris Olanzapine • Zyprexa
Carbamazepine • Equetro, Tegretol Pregabalin • Lyrica
Chlorpromazine • Thorazine Quetiapine • Seroquel
Clonazepam • Klonopin Risperidone • Risperdal
Diphrenhydramine • Benadryl Trazodone • Desyrel
Eszopiclone • Lunesta Valproate • Divalproex
Gabapentin • Neurontin Zaleplon • Sonata
Lamotrigine • Lamictal Ziprasidone • Geodon
Lithium • Eskalith, Lithobid Zolpidem • Ambien
Lorazepam • Ativan
Acknowledgement
The authors thank Peter Ash, MD, for carefully reviewing this manuscript and providing feedback.
Disclosures
Dr. Rakofsky receives research or grant support from Takeda. Dr. Dunlop receives research or grant support from Forest, GlaxoSmithKline, and Otsuka.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Cassidy F, Murry E, Forest K, et al. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50(2-3):187-201.
3. Yen CF, Chen CS, Ko CH, et al. Changes in insight among patients with bipolar I disorder: a 2-year prospective study. Bipolar Disord. 2007;9(3):238-242.
4. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
5. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
6. Allison JB, Wilson WP. Sexual behavior of manic patients: a preliminary report. South Med J. 1960;53:870-874.
7. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011; 378(9799):1306-1315.
8. Yildiz A, Vieta E, Leucht S, et al. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36(2):375-389.
9. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
10. Grunze H, Vieta E, Goodwin GM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
11. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59(1):62-69.
12. Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(3 pt 2):261-268.
13. Goikolea JM, Colom F, Capapey J, et al. Faster onset of antimanic action with haloperidol compared to second-generation antipsychotics. A meta-analysis of randomized clinical trials in acute mania. Eur Neuropsychopharmacol. 2013;23(4):305-316.
14. Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63(12):1148-1155.
15. Tohen M, Baker RW, Altshuler LL, et al. Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry. 2002;159(6):1011-1017.
16. Tohen M, Vieta E, Goodwin GM, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. 2008;69(11):1776-1789.
17. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004; 161(2):262-270.
18. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry. 1991;25(2):238-242.
19. Bradwejn J, Shriqui C, Koszycki D, et al. Double-blind comparison of the effects of clonazepam and lorazepam in acute mania. J Clin Psychopharmacol. 1990;10(6):403-408.
20. Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology: Clinical and Experimental. 1997;12(4):325-328.
21. Lenox RH, Newhouse PA, Creelman WL, et al. Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry. 1992;53(2):47-52.
22. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389-397.
23. Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343.
24. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928.
25. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
26. Linkowski P, Kerkhofs M, Rielaert C, et al. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339-341.
27. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
28. Sattar SP, Ramaswamy S, Bhatia SC, et al. Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother. 2003;37(10):1429-1433.
29. Rybakowski JK, Koszewska I, Puzynski S. Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder [Comment on: The neurolobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010]. J Clin Psychiatry. 2010;71(12):1698-1699; author reply 1699-1700.
30. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008;165(11):1408-1419.
31. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry. Arlington, VA: American Psychiatric Association; 2013.
32. Simon RI, Shuman DW. Clinical manual of psychiatry and law. Arlington, VA: American Psychiatric Publishing; 2007:17-36.
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.