User login
Bipolar and seizure medication linked with serious immune system reaction
The Food and Drug Administration has issued a warning that the seizure and bipolar medication Lamictal (lamotrigine) can cause a rare but potentially life-threatening immune response.
This life-threatening immune response, known as hemophagocytic lymphohistiocytosis (HLH), causes an uncontrolled immune response and can present as a persistent fever greater than 101° F. HLH can also lead to severe issues with blood cells and organs like the liver, kidneys, and lungs.
Lamotrigine is commonly used as a maintenance treatment for patients with bipolar disorder to help manage depression and mood episodes of mania and hypomania. Patients who abruptly stop taking lamotrigine before talking to their physician can suffer seizures, as well as new or worsening mental health issues.
The FDA is recommending that health care providers be aware of the connection between lamotrigine and HLH and be able to recognize and treat the immune response promptly to improve outcomes and decrease mortality. This can be difficult because of the nonspecific nature of HLH symptoms like fever and rash. HLH is commonly confused with another immune-related reaction known as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Patients should be evaluated if they develop fever or rash and immediately discontinue use of lamotrigine if HLH is suspected.
The basis for the new warning is eight cases worldwide of confirmed or suspected HLH involving “reasonable evidence that lamotrigine was the cause of HLH ... based on the timing of events and the order in which they occurred,” the agency said, noting that this number includes only reports submitted to the FDA and found in the medical literature during the 24-year approval history of the drug, so there are likely additional cases about which we are unaware. The eight patients were all hospitalized and received drug and other medical treatments, with one dying.
HLH can be diagnosed if a patient has at least five of the following eight signs or symptoms: fever and rash; enlarged spleen; cytopenias; elevated blood triglycerides and high levels of ferritin or low levels of fibrinogen; hemophagocytosis confirmed via bone marrow, spleen, or lymph node biopsy; decreased or absent natural killer (NK) cell activity; and elevated levels of CD25 in the blood.
Other signs and symptoms may include: enlarged liver, swollen lymph nodes, yellowing of the skin or eyes, unusual bleeding, disturbances in vision, and trouble walking.
The FDA encourages health care providers and patients to report adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration has issued a warning that the seizure and bipolar medication Lamictal (lamotrigine) can cause a rare but potentially life-threatening immune response.
This life-threatening immune response, known as hemophagocytic lymphohistiocytosis (HLH), causes an uncontrolled immune response and can present as a persistent fever greater than 101° F. HLH can also lead to severe issues with blood cells and organs like the liver, kidneys, and lungs.
Lamotrigine is commonly used as a maintenance treatment for patients with bipolar disorder to help manage depression and mood episodes of mania and hypomania. Patients who abruptly stop taking lamotrigine before talking to their physician can suffer seizures, as well as new or worsening mental health issues.
The FDA is recommending that health care providers be aware of the connection between lamotrigine and HLH and be able to recognize and treat the immune response promptly to improve outcomes and decrease mortality. This can be difficult because of the nonspecific nature of HLH symptoms like fever and rash. HLH is commonly confused with another immune-related reaction known as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Patients should be evaluated if they develop fever or rash and immediately discontinue use of lamotrigine if HLH is suspected.
The basis for the new warning is eight cases worldwide of confirmed or suspected HLH involving “reasonable evidence that lamotrigine was the cause of HLH ... based on the timing of events and the order in which they occurred,” the agency said, noting that this number includes only reports submitted to the FDA and found in the medical literature during the 24-year approval history of the drug, so there are likely additional cases about which we are unaware. The eight patients were all hospitalized and received drug and other medical treatments, with one dying.
HLH can be diagnosed if a patient has at least five of the following eight signs or symptoms: fever and rash; enlarged spleen; cytopenias; elevated blood triglycerides and high levels of ferritin or low levels of fibrinogen; hemophagocytosis confirmed via bone marrow, spleen, or lymph node biopsy; decreased or absent natural killer (NK) cell activity; and elevated levels of CD25 in the blood.
Other signs and symptoms may include: enlarged liver, swollen lymph nodes, yellowing of the skin or eyes, unusual bleeding, disturbances in vision, and trouble walking.
The FDA encourages health care providers and patients to report adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration has issued a warning that the seizure and bipolar medication Lamictal (lamotrigine) can cause a rare but potentially life-threatening immune response.
This life-threatening immune response, known as hemophagocytic lymphohistiocytosis (HLH), causes an uncontrolled immune response and can present as a persistent fever greater than 101° F. HLH can also lead to severe issues with blood cells and organs like the liver, kidneys, and lungs.
Lamotrigine is commonly used as a maintenance treatment for patients with bipolar disorder to help manage depression and mood episodes of mania and hypomania. Patients who abruptly stop taking lamotrigine before talking to their physician can suffer seizures, as well as new or worsening mental health issues.
The FDA is recommending that health care providers be aware of the connection between lamotrigine and HLH and be able to recognize and treat the immune response promptly to improve outcomes and decrease mortality. This can be difficult because of the nonspecific nature of HLH symptoms like fever and rash. HLH is commonly confused with another immune-related reaction known as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Patients should be evaluated if they develop fever or rash and immediately discontinue use of lamotrigine if HLH is suspected.
The basis for the new warning is eight cases worldwide of confirmed or suspected HLH involving “reasonable evidence that lamotrigine was the cause of HLH ... based on the timing of events and the order in which they occurred,” the agency said, noting that this number includes only reports submitted to the FDA and found in the medical literature during the 24-year approval history of the drug, so there are likely additional cases about which we are unaware. The eight patients were all hospitalized and received drug and other medical treatments, with one dying.
HLH can be diagnosed if a patient has at least five of the following eight signs or symptoms: fever and rash; enlarged spleen; cytopenias; elevated blood triglycerides and high levels of ferritin or low levels of fibrinogen; hemophagocytosis confirmed via bone marrow, spleen, or lymph node biopsy; decreased or absent natural killer (NK) cell activity; and elevated levels of CD25 in the blood.
Other signs and symptoms may include: enlarged liver, swollen lymph nodes, yellowing of the skin or eyes, unusual bleeding, disturbances in vision, and trouble walking.
The FDA encourages health care providers and patients to report adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Lurasidone approved for bipolar I depression for children aged 10-17
The Food and Drug Administration has approved lurasidone HCI (Latuda) for treating bipolar I depression in children and adolescents, according to a March 6 statement from the drug’s manufacturer.
“We know that children who have been diagnosed with bipolar depression can be at risk for poor school performance and impairments in social functioning,” said Robert L. Findling, MD, professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, in the statement.
Approval of the atypical antipsychotic is based on results of a 6-week, randomized placebo-controlled phase 3 study of 347 children and adolescents diagnosed with bipolar I depression. Patients received either 20-80 mg/day of lurasidone or placebo.
Patients who received lurasidone reportedly experienced improved bipolar depression symptoms, compared with placebo, based on “the primary efficacy endpoint of change from baseline to week 6 on the Children’s Depression Rating Scale–Revised total score (–21.0 vs. –15.3; effect size = 0.45; P less than .0001),” the statement said. Clinically relevant changes also were found among patients who took the medication on other measures, including the Clinical Global Impressions-Bipolar Scale.
The most common adverse effects were nausea (16% vs. 5.8%), weight gain (6.9% vs. 1.7%), and insomnia (5.1% vs. 2.3%).
Lurasidone also has been approved for treating schizophrenia and bipolar I depression in adults. Last year, the drug was approved for treating schizophrenia in adolescents.
The Food and Drug Administration has approved lurasidone HCI (Latuda) for treating bipolar I depression in children and adolescents, according to a March 6 statement from the drug’s manufacturer.
“We know that children who have been diagnosed with bipolar depression can be at risk for poor school performance and impairments in social functioning,” said Robert L. Findling, MD, professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, in the statement.
Approval of the atypical antipsychotic is based on results of a 6-week, randomized placebo-controlled phase 3 study of 347 children and adolescents diagnosed with bipolar I depression. Patients received either 20-80 mg/day of lurasidone or placebo.
Patients who received lurasidone reportedly experienced improved bipolar depression symptoms, compared with placebo, based on “the primary efficacy endpoint of change from baseline to week 6 on the Children’s Depression Rating Scale–Revised total score (–21.0 vs. –15.3; effect size = 0.45; P less than .0001),” the statement said. Clinically relevant changes also were found among patients who took the medication on other measures, including the Clinical Global Impressions-Bipolar Scale.
The most common adverse effects were nausea (16% vs. 5.8%), weight gain (6.9% vs. 1.7%), and insomnia (5.1% vs. 2.3%).
Lurasidone also has been approved for treating schizophrenia and bipolar I depression in adults. Last year, the drug was approved for treating schizophrenia in adolescents.
The Food and Drug Administration has approved lurasidone HCI (Latuda) for treating bipolar I depression in children and adolescents, according to a March 6 statement from the drug’s manufacturer.
“We know that children who have been diagnosed with bipolar depression can be at risk for poor school performance and impairments in social functioning,” said Robert L. Findling, MD, professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, in the statement.
Approval of the atypical antipsychotic is based on results of a 6-week, randomized placebo-controlled phase 3 study of 347 children and adolescents diagnosed with bipolar I depression. Patients received either 20-80 mg/day of lurasidone or placebo.
Patients who received lurasidone reportedly experienced improved bipolar depression symptoms, compared with placebo, based on “the primary efficacy endpoint of change from baseline to week 6 on the Children’s Depression Rating Scale–Revised total score (–21.0 vs. –15.3; effect size = 0.45; P less than .0001),” the statement said. Clinically relevant changes also were found among patients who took the medication on other measures, including the Clinical Global Impressions-Bipolar Scale.
The most common adverse effects were nausea (16% vs. 5.8%), weight gain (6.9% vs. 1.7%), and insomnia (5.1% vs. 2.3%).
Lurasidone also has been approved for treating schizophrenia and bipolar I depression in adults. Last year, the drug was approved for treating schizophrenia in adolescents.
Mental health apps: What to tell patients
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.
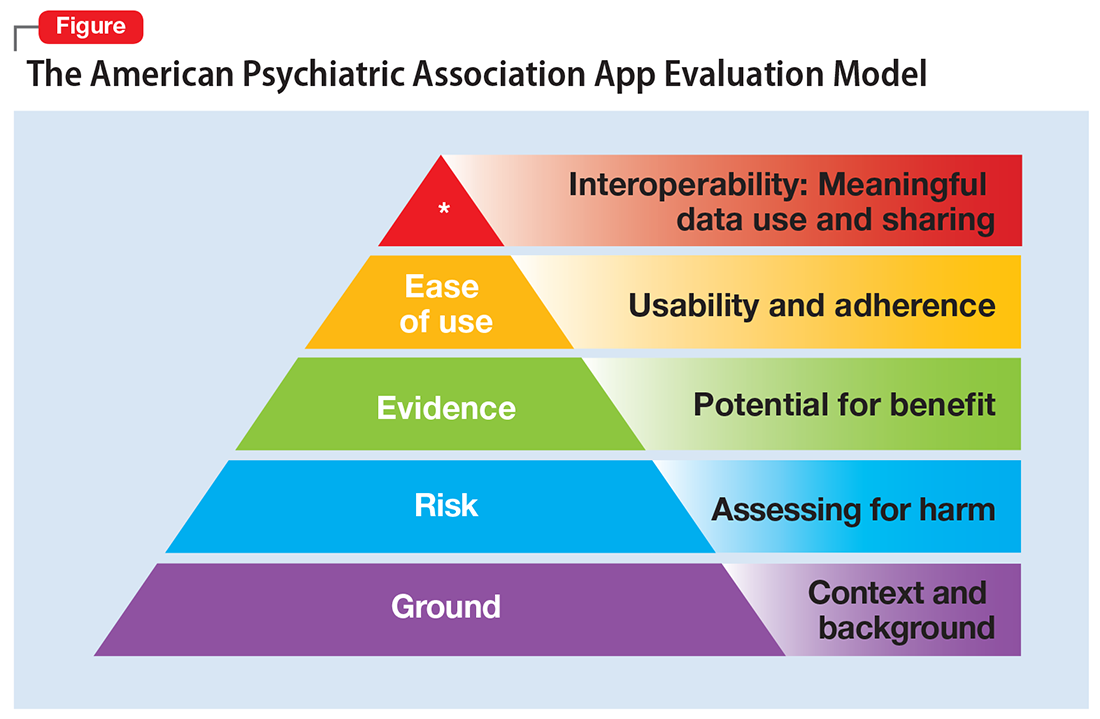
Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
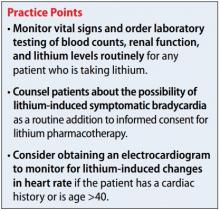
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.

Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.

Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
Can mood stabilizers reduce chronic pain in patients with bipolar disorder?
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
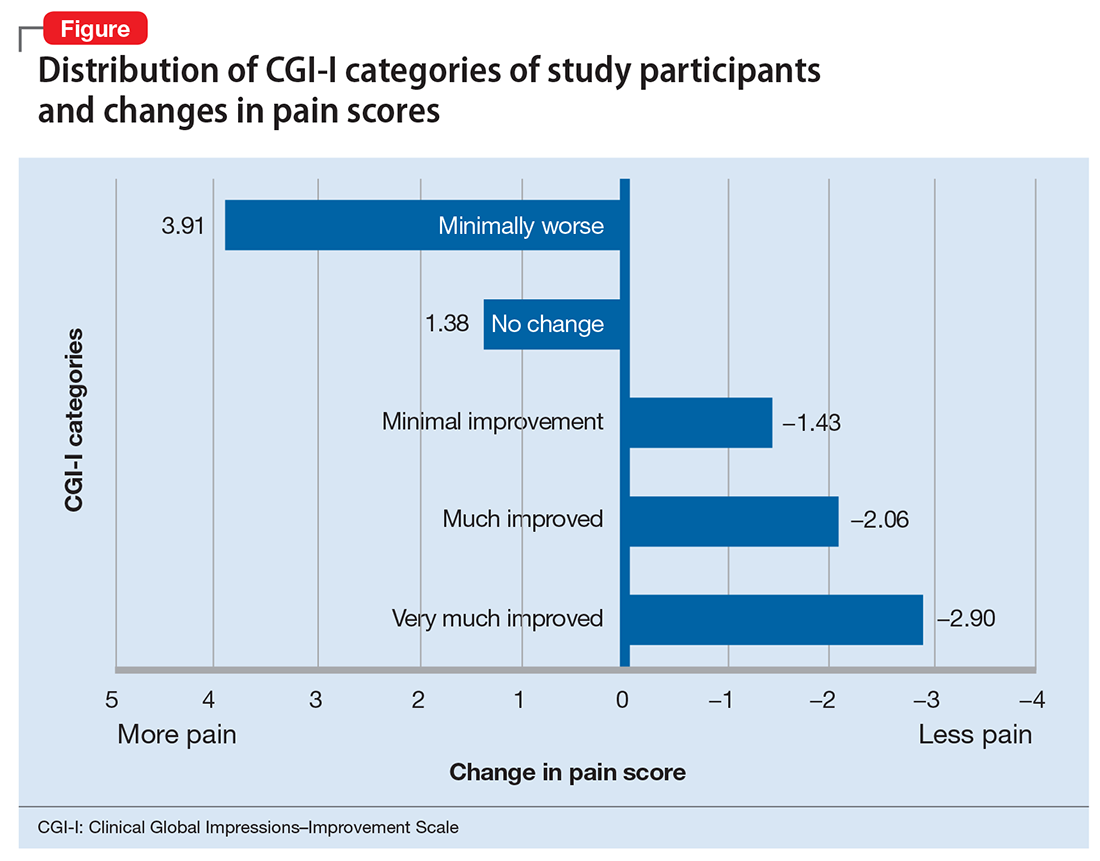
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).
Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).

Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).

Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
RA associated with higher risk of psychiatric disorders
The incidence and prevalence of anxiety disorder, depression, and bipolar disorder are higher among patients with rheumatoid arthritis than individuals from the general population, according to findings from a Canadian retrospective matched cohort study.
In the study of 10,206 rheumatoid arthritis (RA) patients and 50,960 individuals matched from the general population of Manitoba between 1989 and 2012, depression incidence was higher in the RA group, compared with the matched group, when adjusted for factors including age, sex, year, region of residence, and socioeconomic status (incidence rate ratio = 1.46; 95% confidence interval, 1.35-1.58). Incidence of anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34) and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were also higher in the RA group. The incidence of schizophrenia did not differ between groups (IRR = 0.96; 95% CI, 0.61-1.50), Ruth Ann Marrie, MD, PhD, of the University of Manitoba, Winnipeg, and her coauthors reported in Arthritis Care & Research.
To estimate psychiatric disorder incidence after RA diagnosis (or the index date in the matched population), the first claim had to occur after the index date, and had to be preceded by a 5-year period with no claims for that psychiatric disorder. To estimate lifetime prevalence, once a patient met the case definition for a disorder, he or she was considered affected in all subsequent years if alive and a Manitoba resident. To account for varying periods of remission, however, annual period prevalence was defined as a patient having one or more hospital claims or two or more physician claims for the disorder in that year, Dr. Marrie and her colleagues wrote.
The adjusted lifetime prevalence was also higher in the RA group for both depression (PR = 1.35; 95% CI, 1.26-1.45) and anxiety disorder (PR = 1.20; 95% CI, 1.13-1.27), as was the annual period prevalence of depression (PR = 1.36; 95% CI, 1.26-1.47) and anxiety disorder (PR = 1.30; 95% CI, 1.19-1.41). Neither lifetime prevalence of bipolar disorder (PR = 1.13; 95% CI, 0.95-1.36) and schizophrenia (PR = 1.02; 95% CI, 0.72-1.43) nor annual period prevalence of bipolar disorder (PR = 1.06; 95% CI, 0.86-1.31) and schizophrenia (PR = 0.68; 95% CI, 0.40-1.15) differed between the RA and matched cohorts, the authors reported.
Female sex was associated with risk of psychiatric disease, as was lower socioeconomic status and living in an urban area, the authors reported.
Although the study had strengths including a large study population and use of population-based data, it did not evaluate psychiatric multimorbidity, a “common and clinically relevant issue which may affect outcomes,” Dr. Marrie and her coauthors said in the report. Additionally, the use of administrative data makes it difficult to account for care provided by nonphysician providers, such as psychologists, and for conditions that cause symptoms but do not meet diagnostic criteria, the authors noted.
“Future studies should explore these issues in population-based clinical cohorts which comprehensively evaluate multiple psychiatric disorders,” they concluded.
The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
SOURCE: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539.
The incidence and prevalence of anxiety disorder, depression, and bipolar disorder are higher among patients with rheumatoid arthritis than individuals from the general population, according to findings from a Canadian retrospective matched cohort study.
In the study of 10,206 rheumatoid arthritis (RA) patients and 50,960 individuals matched from the general population of Manitoba between 1989 and 2012, depression incidence was higher in the RA group, compared with the matched group, when adjusted for factors including age, sex, year, region of residence, and socioeconomic status (incidence rate ratio = 1.46; 95% confidence interval, 1.35-1.58). Incidence of anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34) and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were also higher in the RA group. The incidence of schizophrenia did not differ between groups (IRR = 0.96; 95% CI, 0.61-1.50), Ruth Ann Marrie, MD, PhD, of the University of Manitoba, Winnipeg, and her coauthors reported in Arthritis Care & Research.
To estimate psychiatric disorder incidence after RA diagnosis (or the index date in the matched population), the first claim had to occur after the index date, and had to be preceded by a 5-year period with no claims for that psychiatric disorder. To estimate lifetime prevalence, once a patient met the case definition for a disorder, he or she was considered affected in all subsequent years if alive and a Manitoba resident. To account for varying periods of remission, however, annual period prevalence was defined as a patient having one or more hospital claims or two or more physician claims for the disorder in that year, Dr. Marrie and her colleagues wrote.
The adjusted lifetime prevalence was also higher in the RA group for both depression (PR = 1.35; 95% CI, 1.26-1.45) and anxiety disorder (PR = 1.20; 95% CI, 1.13-1.27), as was the annual period prevalence of depression (PR = 1.36; 95% CI, 1.26-1.47) and anxiety disorder (PR = 1.30; 95% CI, 1.19-1.41). Neither lifetime prevalence of bipolar disorder (PR = 1.13; 95% CI, 0.95-1.36) and schizophrenia (PR = 1.02; 95% CI, 0.72-1.43) nor annual period prevalence of bipolar disorder (PR = 1.06; 95% CI, 0.86-1.31) and schizophrenia (PR = 0.68; 95% CI, 0.40-1.15) differed between the RA and matched cohorts, the authors reported.
Female sex was associated with risk of psychiatric disease, as was lower socioeconomic status and living in an urban area, the authors reported.
Although the study had strengths including a large study population and use of population-based data, it did not evaluate psychiatric multimorbidity, a “common and clinically relevant issue which may affect outcomes,” Dr. Marrie and her coauthors said in the report. Additionally, the use of administrative data makes it difficult to account for care provided by nonphysician providers, such as psychologists, and for conditions that cause symptoms but do not meet diagnostic criteria, the authors noted.
“Future studies should explore these issues in population-based clinical cohorts which comprehensively evaluate multiple psychiatric disorders,” they concluded.
The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
SOURCE: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539.
The incidence and prevalence of anxiety disorder, depression, and bipolar disorder are higher among patients with rheumatoid arthritis than individuals from the general population, according to findings from a Canadian retrospective matched cohort study.
In the study of 10,206 rheumatoid arthritis (RA) patients and 50,960 individuals matched from the general population of Manitoba between 1989 and 2012, depression incidence was higher in the RA group, compared with the matched group, when adjusted for factors including age, sex, year, region of residence, and socioeconomic status (incidence rate ratio = 1.46; 95% confidence interval, 1.35-1.58). Incidence of anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34) and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were also higher in the RA group. The incidence of schizophrenia did not differ between groups (IRR = 0.96; 95% CI, 0.61-1.50), Ruth Ann Marrie, MD, PhD, of the University of Manitoba, Winnipeg, and her coauthors reported in Arthritis Care & Research.
To estimate psychiatric disorder incidence after RA diagnosis (or the index date in the matched population), the first claim had to occur after the index date, and had to be preceded by a 5-year period with no claims for that psychiatric disorder. To estimate lifetime prevalence, once a patient met the case definition for a disorder, he or she was considered affected in all subsequent years if alive and a Manitoba resident. To account for varying periods of remission, however, annual period prevalence was defined as a patient having one or more hospital claims or two or more physician claims for the disorder in that year, Dr. Marrie and her colleagues wrote.
The adjusted lifetime prevalence was also higher in the RA group for both depression (PR = 1.35; 95% CI, 1.26-1.45) and anxiety disorder (PR = 1.20; 95% CI, 1.13-1.27), as was the annual period prevalence of depression (PR = 1.36; 95% CI, 1.26-1.47) and anxiety disorder (PR = 1.30; 95% CI, 1.19-1.41). Neither lifetime prevalence of bipolar disorder (PR = 1.13; 95% CI, 0.95-1.36) and schizophrenia (PR = 1.02; 95% CI, 0.72-1.43) nor annual period prevalence of bipolar disorder (PR = 1.06; 95% CI, 0.86-1.31) and schizophrenia (PR = 0.68; 95% CI, 0.40-1.15) differed between the RA and matched cohorts, the authors reported.
Female sex was associated with risk of psychiatric disease, as was lower socioeconomic status and living in an urban area, the authors reported.
Although the study had strengths including a large study population and use of population-based data, it did not evaluate psychiatric multimorbidity, a “common and clinically relevant issue which may affect outcomes,” Dr. Marrie and her coauthors said in the report. Additionally, the use of administrative data makes it difficult to account for care provided by nonphysician providers, such as psychologists, and for conditions that cause symptoms but do not meet diagnostic criteria, the authors noted.
“Future studies should explore these issues in population-based clinical cohorts which comprehensively evaluate multiple psychiatric disorders,” they concluded.
The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
SOURCE: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Incidence of depression (IRR = 1.46; 95% CI, 1.35-1.58), anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34), and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were higher in the RA group than in the matched group.
Data source: A retrospective matched cohort study of 10,206 RA patients and 50,960 matched individuals from the general population between 1989 and 2012.
Disclosures: The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
Source: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539
Adherence boon, or Big Brother loom?
The Food and Drug Administration has approved the first drug in the United States with a digital ingestion tracking system. Abilify MyCite (aripiprazole tablets with sensor) has an ingestible sensor embedded in the pill that records that the medication was taken. The product is approved for the treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar I disorder, and for use as an add-on treatment for depression in adults.
The system works by sending a message from the pill’s sensor to a wearable patch, according to a statement issued by the FDA. The patch transmits the information to a mobile application so that patients can track the ingestion of the medication on their smartphones. Patients can also permit their caregivers and physician to access the information through a web-based portal.
[polldaddy:9874958]
The Food and Drug Administration has approved the first drug in the United States with a digital ingestion tracking system. Abilify MyCite (aripiprazole tablets with sensor) has an ingestible sensor embedded in the pill that records that the medication was taken. The product is approved for the treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar I disorder, and for use as an add-on treatment for depression in adults.
The system works by sending a message from the pill’s sensor to a wearable patch, according to a statement issued by the FDA. The patch transmits the information to a mobile application so that patients can track the ingestion of the medication on their smartphones. Patients can also permit their caregivers and physician to access the information through a web-based portal.
[polldaddy:9874958]
The Food and Drug Administration has approved the first drug in the United States with a digital ingestion tracking system. Abilify MyCite (aripiprazole tablets with sensor) has an ingestible sensor embedded in the pill that records that the medication was taken. The product is approved for the treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar I disorder, and for use as an add-on treatment for depression in adults.
The system works by sending a message from the pill’s sensor to a wearable patch, according to a statement issued by the FDA. The patch transmits the information to a mobile application so that patients can track the ingestion of the medication on their smartphones. Patients can also permit their caregivers and physician to access the information through a web-based portal.
[polldaddy:9874958]
Dopamine synthesis capacity appears linked to psychosis in bipolar disorder
Increased dopamine synthesis – a feature classically associated with schizophrenia – might underlie bipolar psychosis, a new study suggests.
To conduct the cross-sectional case-control study, Sameer Jauhar, MRCPsych, and his associates recruited 60 people from first-episode psychosis services in London – 22 with bipolar psychosis, 16 with schizophrenia, and 22 matched controls. Of the 22 with bipolar psychosis, 18 were antipsychotic-naïve or free, and of the 16 patients with schizophrenia, 14 were antipsychotic-naïve (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2943). The researchers used fluorodihydroxyphenyl-L-alanine ([18F]-DOPA) positron emission tomography to study dopamine synthesis capacity in the participants.
The study showed that mean dopamine synthesis capacity in the striatum was significantly higher both in the bipolar group and the schizophrenia group, compared with controls – even after excluding individuals taking antipsychotic medication.
“These results extend previous findings that dopamine synthesis capacity is elevated in schizophrenia and psychosis associated with temporal lobe epilepsy and increases with the onset of psychosis, suggesting that presynaptic dopamine dysfunction is associated with psychosis across diagnostic categories,” wrote Dr. Jauhar, who is affiliated with the Institute of Psychiatry, Psychology & Neuroscience at King’s College, London, and his coauthors.
In addition, Dr. Jauhar and his coauthors found a significant relationship between mean whole striatal dopamine synthesis capacity and Positive and Negative Syndrome Scale (PANSS) scores in the group of patients who was experiencing a psychotic episode at the time of the study, which overall explained 27% of the variance.
In the bipolar disorder group, there was a nonsignificant relationship between whole striatal dopamine synthesis capacity and the PANSS positive subscale symptom severity score. However, this became significant when the analysis was restricted to patients who were experiencing a current psychotic episode and accounted for 36% of the variance in psychotic symptoms.
But this effect was not seen in patients with schizophrenia – all of whom were experiencing a psychotic episode at the time.
“Our finding of a relationship between positive psychotic symptoms and dopamine synthesis capacity in the combined bipolar and schizophrenia sample but not in the schizophrenia group could be due to a lack of power or inclusion of more patients with longer illness durations in the schizophrenia group,” the researchers reported.
Overall, no significant difference was found in mean dopamine synthesis capacity between patients with bipolar disorder and those with schizophrenia.
The authors also controlled for duration of illness, given that those in the schizophrenia group had a longer duration of illness than those in the bipolar group, with no effect on mean dopamine synthesis capacity differences between the two. The effect was seen in the whole striatum, the associative striatum, the limbic striatum, and the sensorimotor striatum.
“Relative to controls, the subregional analyses showed significant elevations in all three functional striatal subdivisions in the bipolar group but only a suggestion in the associative striatum in the schizophrenia group, with no differences in the substantia nigra for either group,” the authors wrote.
The authors acknowledged one concern with using patients experiencing a first episode of psychosis was that their diagnoses may change over time. But even after a minimum of 18 months’ follow-up, none of the original diagnoses had changed, and they had even been strengthened by the difference in negative but not positive symptoms.
The outcome measure used – KiCER – was an index for the uptake of [18F]-DOPA into dopamine neurons, and its conversion into [18F]-dopamine and storage in terminals. “Therefore, the increased KiCER we report likely reflects an increase in one or more of these processes, as well as a net increase in dopamine synthesis capacity,” they wrote.
“This finding provides a potential neurobiological explanation for why antipsychotic drugs, which are all dopamine D2/D3 receptor blockers, are effective in bipolar psychosis and schizophrenia and identifies the regulation of dopamine synthesis as a potential novel drug target for bipolar disorder and schizophrenia.” Furthermore, they said, the findings suggest that dopamine synthesis capacity might be a drug target for bipolar disorder and schizophrenia.
The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
Some empirical evidence suggests that “schizophrenia and bipolar disorder exist along a psychosis continuum, with some patients having more affective features and others having more psychotic features,” Dost Öngür, MD, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2330). However, the study by Dr. Jauhar and his associates show that both illnesses are similar when it comes to dopaminergic dysfunction.
“Clinical experience is perhaps more consistent with a model including not one but two dimensions, namely, affective and psychotic,” Dr. Öngür wrote. “This is because more severe psychosis does not always indicate less severe affective illness; affective syndromes of variable intensity are seen among patients with severe psychosis and vice versa.”
Future studies could seek to quantify the psychosis dimension with the ultimate goal of “developing a more valid classification system for psychotic disorders,” he wrote.
Dr. Öngür is affiliated with the department of psychiatry at McLean Hospital and Harvard Medical School, both in Boston. He reported serving on a scientific advisory board for Neurocrine Biosciences.
Some empirical evidence suggests that “schizophrenia and bipolar disorder exist along a psychosis continuum, with some patients having more affective features and others having more psychotic features,” Dost Öngür, MD, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2330). However, the study by Dr. Jauhar and his associates show that both illnesses are similar when it comes to dopaminergic dysfunction.
“Clinical experience is perhaps more consistent with a model including not one but two dimensions, namely, affective and psychotic,” Dr. Öngür wrote. “This is because more severe psychosis does not always indicate less severe affective illness; affective syndromes of variable intensity are seen among patients with severe psychosis and vice versa.”
Future studies could seek to quantify the psychosis dimension with the ultimate goal of “developing a more valid classification system for psychotic disorders,” he wrote.
Dr. Öngür is affiliated with the department of psychiatry at McLean Hospital and Harvard Medical School, both in Boston. He reported serving on a scientific advisory board for Neurocrine Biosciences.
Some empirical evidence suggests that “schizophrenia and bipolar disorder exist along a psychosis continuum, with some patients having more affective features and others having more psychotic features,” Dost Öngür, MD, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2330). However, the study by Dr. Jauhar and his associates show that both illnesses are similar when it comes to dopaminergic dysfunction.
“Clinical experience is perhaps more consistent with a model including not one but two dimensions, namely, affective and psychotic,” Dr. Öngür wrote. “This is because more severe psychosis does not always indicate less severe affective illness; affective syndromes of variable intensity are seen among patients with severe psychosis and vice versa.”
Future studies could seek to quantify the psychosis dimension with the ultimate goal of “developing a more valid classification system for psychotic disorders,” he wrote.
Dr. Öngür is affiliated with the department of psychiatry at McLean Hospital and Harvard Medical School, both in Boston. He reported serving on a scientific advisory board for Neurocrine Biosciences.
Increased dopamine synthesis – a feature classically associated with schizophrenia – might underlie bipolar psychosis, a new study suggests.
To conduct the cross-sectional case-control study, Sameer Jauhar, MRCPsych, and his associates recruited 60 people from first-episode psychosis services in London – 22 with bipolar psychosis, 16 with schizophrenia, and 22 matched controls. Of the 22 with bipolar psychosis, 18 were antipsychotic-naïve or free, and of the 16 patients with schizophrenia, 14 were antipsychotic-naïve (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2943). The researchers used fluorodihydroxyphenyl-L-alanine ([18F]-DOPA) positron emission tomography to study dopamine synthesis capacity in the participants.
The study showed that mean dopamine synthesis capacity in the striatum was significantly higher both in the bipolar group and the schizophrenia group, compared with controls – even after excluding individuals taking antipsychotic medication.
“These results extend previous findings that dopamine synthesis capacity is elevated in schizophrenia and psychosis associated with temporal lobe epilepsy and increases with the onset of psychosis, suggesting that presynaptic dopamine dysfunction is associated with psychosis across diagnostic categories,” wrote Dr. Jauhar, who is affiliated with the Institute of Psychiatry, Psychology & Neuroscience at King’s College, London, and his coauthors.
In addition, Dr. Jauhar and his coauthors found a significant relationship between mean whole striatal dopamine synthesis capacity and Positive and Negative Syndrome Scale (PANSS) scores in the group of patients who was experiencing a psychotic episode at the time of the study, which overall explained 27% of the variance.
In the bipolar disorder group, there was a nonsignificant relationship between whole striatal dopamine synthesis capacity and the PANSS positive subscale symptom severity score. However, this became significant when the analysis was restricted to patients who were experiencing a current psychotic episode and accounted for 36% of the variance in psychotic symptoms.
But this effect was not seen in patients with schizophrenia – all of whom were experiencing a psychotic episode at the time.
“Our finding of a relationship between positive psychotic symptoms and dopamine synthesis capacity in the combined bipolar and schizophrenia sample but not in the schizophrenia group could be due to a lack of power or inclusion of more patients with longer illness durations in the schizophrenia group,” the researchers reported.
Overall, no significant difference was found in mean dopamine synthesis capacity between patients with bipolar disorder and those with schizophrenia.
The authors also controlled for duration of illness, given that those in the schizophrenia group had a longer duration of illness than those in the bipolar group, with no effect on mean dopamine synthesis capacity differences between the two. The effect was seen in the whole striatum, the associative striatum, the limbic striatum, and the sensorimotor striatum.
“Relative to controls, the subregional analyses showed significant elevations in all three functional striatal subdivisions in the bipolar group but only a suggestion in the associative striatum in the schizophrenia group, with no differences in the substantia nigra for either group,” the authors wrote.
The authors acknowledged one concern with using patients experiencing a first episode of psychosis was that their diagnoses may change over time. But even after a minimum of 18 months’ follow-up, none of the original diagnoses had changed, and they had even been strengthened by the difference in negative but not positive symptoms.
The outcome measure used – KiCER – was an index for the uptake of [18F]-DOPA into dopamine neurons, and its conversion into [18F]-dopamine and storage in terminals. “Therefore, the increased KiCER we report likely reflects an increase in one or more of these processes, as well as a net increase in dopamine synthesis capacity,” they wrote.
“This finding provides a potential neurobiological explanation for why antipsychotic drugs, which are all dopamine D2/D3 receptor blockers, are effective in bipolar psychosis and schizophrenia and identifies the regulation of dopamine synthesis as a potential novel drug target for bipolar disorder and schizophrenia.” Furthermore, they said, the findings suggest that dopamine synthesis capacity might be a drug target for bipolar disorder and schizophrenia.
The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
Increased dopamine synthesis – a feature classically associated with schizophrenia – might underlie bipolar psychosis, a new study suggests.
To conduct the cross-sectional case-control study, Sameer Jauhar, MRCPsych, and his associates recruited 60 people from first-episode psychosis services in London – 22 with bipolar psychosis, 16 with schizophrenia, and 22 matched controls. Of the 22 with bipolar psychosis, 18 were antipsychotic-naïve or free, and of the 16 patients with schizophrenia, 14 were antipsychotic-naïve (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2943). The researchers used fluorodihydroxyphenyl-L-alanine ([18F]-DOPA) positron emission tomography to study dopamine synthesis capacity in the participants.
The study showed that mean dopamine synthesis capacity in the striatum was significantly higher both in the bipolar group and the schizophrenia group, compared with controls – even after excluding individuals taking antipsychotic medication.
“These results extend previous findings that dopamine synthesis capacity is elevated in schizophrenia and psychosis associated with temporal lobe epilepsy and increases with the onset of psychosis, suggesting that presynaptic dopamine dysfunction is associated with psychosis across diagnostic categories,” wrote Dr. Jauhar, who is affiliated with the Institute of Psychiatry, Psychology & Neuroscience at King’s College, London, and his coauthors.
In addition, Dr. Jauhar and his coauthors found a significant relationship between mean whole striatal dopamine synthesis capacity and Positive and Negative Syndrome Scale (PANSS) scores in the group of patients who was experiencing a psychotic episode at the time of the study, which overall explained 27% of the variance.
In the bipolar disorder group, there was a nonsignificant relationship between whole striatal dopamine synthesis capacity and the PANSS positive subscale symptom severity score. However, this became significant when the analysis was restricted to patients who were experiencing a current psychotic episode and accounted for 36% of the variance in psychotic symptoms.
But this effect was not seen in patients with schizophrenia – all of whom were experiencing a psychotic episode at the time.
“Our finding of a relationship between positive psychotic symptoms and dopamine synthesis capacity in the combined bipolar and schizophrenia sample but not in the schizophrenia group could be due to a lack of power or inclusion of more patients with longer illness durations in the schizophrenia group,” the researchers reported.
Overall, no significant difference was found in mean dopamine synthesis capacity between patients with bipolar disorder and those with schizophrenia.
The authors also controlled for duration of illness, given that those in the schizophrenia group had a longer duration of illness than those in the bipolar group, with no effect on mean dopamine synthesis capacity differences between the two. The effect was seen in the whole striatum, the associative striatum, the limbic striatum, and the sensorimotor striatum.
“Relative to controls, the subregional analyses showed significant elevations in all three functional striatal subdivisions in the bipolar group but only a suggestion in the associative striatum in the schizophrenia group, with no differences in the substantia nigra for either group,” the authors wrote.
The authors acknowledged one concern with using patients experiencing a first episode of psychosis was that their diagnoses may change over time. But even after a minimum of 18 months’ follow-up, none of the original diagnoses had changed, and they had even been strengthened by the difference in negative but not positive symptoms.
The outcome measure used – KiCER – was an index for the uptake of [18F]-DOPA into dopamine neurons, and its conversion into [18F]-dopamine and storage in terminals. “Therefore, the increased KiCER we report likely reflects an increase in one or more of these processes, as well as a net increase in dopamine synthesis capacity,” they wrote.
“This finding provides a potential neurobiological explanation for why antipsychotic drugs, which are all dopamine D2/D3 receptor blockers, are effective in bipolar psychosis and schizophrenia and identifies the regulation of dopamine synthesis as a potential novel drug target for bipolar disorder and schizophrenia.” Furthermore, they said, the findings suggest that dopamine synthesis capacity might be a drug target for bipolar disorder and schizophrenia.
The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
FROM JAMA PSYCHIATRY
Key clinical point: Dopamine synthesis capacity is elevated in individuals with bipolar disorder, particularly those experiencing a psychotic episode.
Major finding: Individuals with bipolar disorder have a similarly elevated dopamine synthesis capacity as individuals with schizophrenia.
Data source: Positron emission tomography study in 22 individuals with bipolar disorder, 16 with schizophrenia, and 22 controls.
Disclosures: The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
Lithium-induced bradycardia: A rare but serious adverse effect
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.

Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.

1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
Lumateperone shows broad phase 3 potential for psychiatric disorders
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Add aggressiveness to mixed features specifier for major depressive episode
PARIS – Aggressiveness deserves to be incorporated in the next Diagnostic and Statistical Manual of Mental Disorders update as a new clinical criterion triggering application of the “with mixed features” specifier in patients diagnosed with a major depressive episode, Norma Verdolini, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Aggressiveness might be a trait component of bipolarity and a diagnostic indicator of ‘mixicity’ in patients with a major depressive episode. This has implications for the therapeutic strategy,” said Dr. Verdolini of the bipolar disorders unit at the University of Barcelona Institute of Neurosciences.
The BRIDGE-II-MIX study was a cross-sectional observational study of 2,811 adults with MDE at 239 centers in eight European countries (J Clin Psychiatry. 2015 Mar;76[3]:e351-8). Three hundred ninety-nine participants (14.2%) met the operational definition of physical or verbal aggressiveness used in Dr. Verdolini’s new post-hoc analysis.
Statistically significant and clinically meaningful differences were found between MDE patients with aggressiveness (MDE-aggro) and MDE without aggressiveness. For example, the MDE-aggro group was twice as likely to meet DSM-IV-TR criteria for bipolar disorder I. Twenty-seven percent of the MDE-aggro group met DSM-5 criteria for a mixed state, meaning both depressed mood and mania in the same episode, compared with just 4% of the MDE-no-aggro group.
The MDE-aggro patients also had a strikingly greater prevalence of comorbid borderline personality disorder, by a margin of 20% versus 4%. They had a younger mean age at their first depressive episode: 29.9 years old, compared with 36.1 in the MDE-no-aggro group. The MDE-aggro patients had more prior mood episodes and a greater number of lifetime suicide attempts. In addition, they had significantly more severe depression, mania, and bipolar disorder scores on the Clinical Global Impression Scale for Bipolar Disorder.
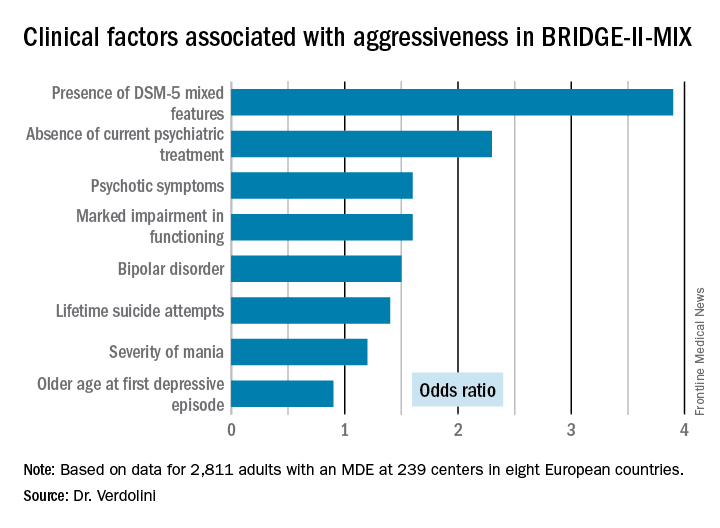
“Our results should prompt reconsideration of the diagnostic criteria for the mixed features specifier. The detection of aggression in MDE could represent a therapeutic target in personalized pharmacological treatment for bipolar disorder,” Dr. Verdolini concluded.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Verdolini reported receiving research funding from the company.
PARIS – Aggressiveness deserves to be incorporated in the next Diagnostic and Statistical Manual of Mental Disorders update as a new clinical criterion triggering application of the “with mixed features” specifier in patients diagnosed with a major depressive episode, Norma Verdolini, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Aggressiveness might be a trait component of bipolarity and a diagnostic indicator of ‘mixicity’ in patients with a major depressive episode. This has implications for the therapeutic strategy,” said Dr. Verdolini of the bipolar disorders unit at the University of Barcelona Institute of Neurosciences.
The BRIDGE-II-MIX study was a cross-sectional observational study of 2,811 adults with MDE at 239 centers in eight European countries (J Clin Psychiatry. 2015 Mar;76[3]:e351-8). Three hundred ninety-nine participants (14.2%) met the operational definition of physical or verbal aggressiveness used in Dr. Verdolini’s new post-hoc analysis.
Statistically significant and clinically meaningful differences were found between MDE patients with aggressiveness (MDE-aggro) and MDE without aggressiveness. For example, the MDE-aggro group was twice as likely to meet DSM-IV-TR criteria for bipolar disorder I. Twenty-seven percent of the MDE-aggro group met DSM-5 criteria for a mixed state, meaning both depressed mood and mania in the same episode, compared with just 4% of the MDE-no-aggro group.
The MDE-aggro patients also had a strikingly greater prevalence of comorbid borderline personality disorder, by a margin of 20% versus 4%. They had a younger mean age at their first depressive episode: 29.9 years old, compared with 36.1 in the MDE-no-aggro group. The MDE-aggro patients had more prior mood episodes and a greater number of lifetime suicide attempts. In addition, they had significantly more severe depression, mania, and bipolar disorder scores on the Clinical Global Impression Scale for Bipolar Disorder.

“Our results should prompt reconsideration of the diagnostic criteria for the mixed features specifier. The detection of aggression in MDE could represent a therapeutic target in personalized pharmacological treatment for bipolar disorder,” Dr. Verdolini concluded.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Verdolini reported receiving research funding from the company.
PARIS – Aggressiveness deserves to be incorporated in the next Diagnostic and Statistical Manual of Mental Disorders update as a new clinical criterion triggering application of the “with mixed features” specifier in patients diagnosed with a major depressive episode, Norma Verdolini, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Aggressiveness might be a trait component of bipolarity and a diagnostic indicator of ‘mixicity’ in patients with a major depressive episode. This has implications for the therapeutic strategy,” said Dr. Verdolini of the bipolar disorders unit at the University of Barcelona Institute of Neurosciences.
The BRIDGE-II-MIX study was a cross-sectional observational study of 2,811 adults with MDE at 239 centers in eight European countries (J Clin Psychiatry. 2015 Mar;76[3]:e351-8). Three hundred ninety-nine participants (14.2%) met the operational definition of physical or verbal aggressiveness used in Dr. Verdolini’s new post-hoc analysis.
Statistically significant and clinically meaningful differences were found between MDE patients with aggressiveness (MDE-aggro) and MDE without aggressiveness. For example, the MDE-aggro group was twice as likely to meet DSM-IV-TR criteria for bipolar disorder I. Twenty-seven percent of the MDE-aggro group met DSM-5 criteria for a mixed state, meaning both depressed mood and mania in the same episode, compared with just 4% of the MDE-no-aggro group.
The MDE-aggro patients also had a strikingly greater prevalence of comorbid borderline personality disorder, by a margin of 20% versus 4%. They had a younger mean age at their first depressive episode: 29.9 years old, compared with 36.1 in the MDE-no-aggro group. The MDE-aggro patients had more prior mood episodes and a greater number of lifetime suicide attempts. In addition, they had significantly more severe depression, mania, and bipolar disorder scores on the Clinical Global Impression Scale for Bipolar Disorder.

“Our results should prompt reconsideration of the diagnostic criteria for the mixed features specifier. The detection of aggression in MDE could represent a therapeutic target in personalized pharmacological treatment for bipolar disorder,” Dr. Verdolini concluded.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Verdolini reported receiving research funding from the company.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Patients who fulfilled the DSM-5 criteria for a major depressive episode with mixed features were 3.9-fold more likely to meet investigators’ operational definition of aggressiveness.
Data source: This was a post-hoc analysis of the BRIDGE-II-MIX study, an observational cross-sectional study of 2,811 adults experiencing a major depressive episode.
Disclosures: The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. The presenter reported receiving research funding from the company.