User login
Discordant Antibiotic Use in Pediatric UTIs Associated with Higher LOS
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Guidelines for Pneumonia Call for Decreased Use of Broad-Spectrum Antibiotics
Clinical question: What is the impact of a clinical practice guideline for hospitalized children with community-acquired pneumonia (CAP) on antibiotic selection?
Background: CAP is one of the most common reasons for hospitalizations in children. Broad-spectrum antibiotics frequently are prescribed for presumed bacterial pneumonia in children. Recent guidelines for CAP in children have emphasized that ampicillin is an appropriate empiric inpatient treatment option.
Study design: Retrospective review.
Setting: Tertiary referral children’s hospital.
Synopsis: Patients older than two months old with acute, uncomplicated CAP and without significant secondary illness were identified in the 12-month periods preceding and following the implementation of a clinical practice guideline (CPG) that recommended empiric treatment with ampicillin upon admission, and amoxicillin upon discharge.
A total of 1,033 patients were identified, 530 pre-CPG and 503 post-CPG, and the groups were similar. After the CPG, there was a significant increase in empiric ampicillin use (13% to 63%) and concomitant decrease in ceftriaxone use (72% to 21%). Rates of outpatient narrow-spectrum antibiotic prescribing increased as well, and the rate of treatment failure was similar between the groups.
Complex regression analysis was used to analyze the impact of a concomitant antibiotic stewardship program (ASP), implemented three months prior to the initiation of the CPG and demonstrating a separate and additive effect of both initiatives. Thus, changes in antibiotic prescribing were multifactorial over this time period.
The outcomes remain impressive in the context of two increasingly popular QI efforts—CPGs and ASPs. This study represents a meaningful contribution toward demonstration of outcomes-based quality improvement (QI).
Bottom line: In the context of a CPG, antibiotic spectrum may be safely narrowed in pediatric CAP.
Citation: Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597-604.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the impact of a clinical practice guideline for hospitalized children with community-acquired pneumonia (CAP) on antibiotic selection?
Background: CAP is one of the most common reasons for hospitalizations in children. Broad-spectrum antibiotics frequently are prescribed for presumed bacterial pneumonia in children. Recent guidelines for CAP in children have emphasized that ampicillin is an appropriate empiric inpatient treatment option.
Study design: Retrospective review.
Setting: Tertiary referral children’s hospital.
Synopsis: Patients older than two months old with acute, uncomplicated CAP and without significant secondary illness were identified in the 12-month periods preceding and following the implementation of a clinical practice guideline (CPG) that recommended empiric treatment with ampicillin upon admission, and amoxicillin upon discharge.
A total of 1,033 patients were identified, 530 pre-CPG and 503 post-CPG, and the groups were similar. After the CPG, there was a significant increase in empiric ampicillin use (13% to 63%) and concomitant decrease in ceftriaxone use (72% to 21%). Rates of outpatient narrow-spectrum antibiotic prescribing increased as well, and the rate of treatment failure was similar between the groups.
Complex regression analysis was used to analyze the impact of a concomitant antibiotic stewardship program (ASP), implemented three months prior to the initiation of the CPG and demonstrating a separate and additive effect of both initiatives. Thus, changes in antibiotic prescribing were multifactorial over this time period.
The outcomes remain impressive in the context of two increasingly popular QI efforts—CPGs and ASPs. This study represents a meaningful contribution toward demonstration of outcomes-based quality improvement (QI).
Bottom line: In the context of a CPG, antibiotic spectrum may be safely narrowed in pediatric CAP.
Citation: Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597-604.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the impact of a clinical practice guideline for hospitalized children with community-acquired pneumonia (CAP) on antibiotic selection?
Background: CAP is one of the most common reasons for hospitalizations in children. Broad-spectrum antibiotics frequently are prescribed for presumed bacterial pneumonia in children. Recent guidelines for CAP in children have emphasized that ampicillin is an appropriate empiric inpatient treatment option.
Study design: Retrospective review.
Setting: Tertiary referral children’s hospital.
Synopsis: Patients older than two months old with acute, uncomplicated CAP and without significant secondary illness were identified in the 12-month periods preceding and following the implementation of a clinical practice guideline (CPG) that recommended empiric treatment with ampicillin upon admission, and amoxicillin upon discharge.
A total of 1,033 patients were identified, 530 pre-CPG and 503 post-CPG, and the groups were similar. After the CPG, there was a significant increase in empiric ampicillin use (13% to 63%) and concomitant decrease in ceftriaxone use (72% to 21%). Rates of outpatient narrow-spectrum antibiotic prescribing increased as well, and the rate of treatment failure was similar between the groups.
Complex regression analysis was used to analyze the impact of a concomitant antibiotic stewardship program (ASP), implemented three months prior to the initiation of the CPG and demonstrating a separate and additive effect of both initiatives. Thus, changes in antibiotic prescribing were multifactorial over this time period.
The outcomes remain impressive in the context of two increasingly popular QI efforts—CPGs and ASPs. This study represents a meaningful contribution toward demonstration of outcomes-based quality improvement (QI).
Bottom line: In the context of a CPG, antibiotic spectrum may be safely narrowed in pediatric CAP.
Citation: Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597-604.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
ONLINE EXCLUSIVE: Listen to an ID specialist explains why de-escalation of antibiotics isn't a simple proposition
Click here to listen to Dr. Allen
Click here to listen to Dr. Allen
Click here to listen to Dr. Allen
What Is the Appropriate Use of Antibiotics In Acute Exacerbations of COPD?
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
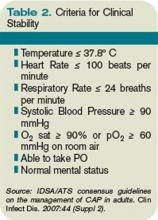
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
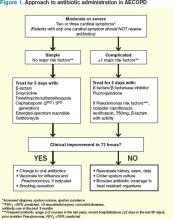
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
New Mindset on Antibiotics
Hospitalists should consider the arena of antimicrobial stewardship one of the newest frontiers of clinical efficiency and cost savings, according to the author of a study in a supplement to this month's Journal of Hospital Medicine.
David Rosenberg, MD, MPH, FACP, SFHM, of the Department of Medicine, Section of Hospital Medicine at North Shore University Hospital in Manhasset, N.Y., says that changing the mindset on antibiotic resistance might seem like a daunting task, but it dovetails neatly with HM's current focus on quality and safety, particularly when it can help reduce length of stay (LOS).
"Think different about antibiotics and build that into your practice," he says.
The supplement highlights four related papers tackling the issues of appropriate initiation and selection of antibiotics, antimicrobial de-escalation strategies, duration and cessation of treatment, and Dr. Rosenberg's paper, "The Emerging Role of Hospitalists." The research includes an online CME component.
Dr. Rosenberg writes that hospitalists "are positioned as excellent champions of the principles and practices of antimicrobial stewardship." That means revamping the use of antibiotics both for individual patients and on an institutional level. That leadership means accepting that "culture change is slow" and physicians often feel "trapped" in letting an antibiotic treatment run its course rather than reassessing midstream.
Still, Dr. Rosenberg says, national guidelines on antibiotic overuse are likely to be developed in the coming years, and hospitalists would do well to get ahead of that curve.
"We're talking about the optimal treatment of patients we are already taking care of," he says. "Stewardship is a natural step forward."
Hospitalists should consider the arena of antimicrobial stewardship one of the newest frontiers of clinical efficiency and cost savings, according to the author of a study in a supplement to this month's Journal of Hospital Medicine.
David Rosenberg, MD, MPH, FACP, SFHM, of the Department of Medicine, Section of Hospital Medicine at North Shore University Hospital in Manhasset, N.Y., says that changing the mindset on antibiotic resistance might seem like a daunting task, but it dovetails neatly with HM's current focus on quality and safety, particularly when it can help reduce length of stay (LOS).
"Think different about antibiotics and build that into your practice," he says.
The supplement highlights four related papers tackling the issues of appropriate initiation and selection of antibiotics, antimicrobial de-escalation strategies, duration and cessation of treatment, and Dr. Rosenberg's paper, "The Emerging Role of Hospitalists." The research includes an online CME component.
Dr. Rosenberg writes that hospitalists "are positioned as excellent champions of the principles and practices of antimicrobial stewardship." That means revamping the use of antibiotics both for individual patients and on an institutional level. That leadership means accepting that "culture change is slow" and physicians often feel "trapped" in letting an antibiotic treatment run its course rather than reassessing midstream.
Still, Dr. Rosenberg says, national guidelines on antibiotic overuse are likely to be developed in the coming years, and hospitalists would do well to get ahead of that curve.
"We're talking about the optimal treatment of patients we are already taking care of," he says. "Stewardship is a natural step forward."
Hospitalists should consider the arena of antimicrobial stewardship one of the newest frontiers of clinical efficiency and cost savings, according to the author of a study in a supplement to this month's Journal of Hospital Medicine.
David Rosenberg, MD, MPH, FACP, SFHM, of the Department of Medicine, Section of Hospital Medicine at North Shore University Hospital in Manhasset, N.Y., says that changing the mindset on antibiotic resistance might seem like a daunting task, but it dovetails neatly with HM's current focus on quality and safety, particularly when it can help reduce length of stay (LOS).
"Think different about antibiotics and build that into your practice," he says.
The supplement highlights four related papers tackling the issues of appropriate initiation and selection of antibiotics, antimicrobial de-escalation strategies, duration and cessation of treatment, and Dr. Rosenberg's paper, "The Emerging Role of Hospitalists." The research includes an online CME component.
Dr. Rosenberg writes that hospitalists "are positioned as excellent champions of the principles and practices of antimicrobial stewardship." That means revamping the use of antibiotics both for individual patients and on an institutional level. That leadership means accepting that "culture change is slow" and physicians often feel "trapped" in letting an antibiotic treatment run its course rather than reassessing midstream.
Still, Dr. Rosenberg says, national guidelines on antibiotic overuse are likely to be developed in the coming years, and hospitalists would do well to get ahead of that curve.
"We're talking about the optimal treatment of patients we are already taking care of," he says. "Stewardship is a natural step forward."
Antibiotic Overuse Linked to C. Diff Infections
A new study that shows cumulative antibiotic exposures appear to be associated with Clostridium difficile infections (CDI) should be seen as another reason to reduce the use of antibiotics to minimum levels, according to the paper's lead author.
"In terms of prevention, it's really important for us to start delineating [shortened antibiotic courses] in treating the primary infection," says Vanessa Stevens, PhD, a fellow at the Center for Health Outcomes, Pharmacoinformatics, and Epidemiology at the State University of New York at Buffalo. "What are the minimums that are necessary to accomplish the job?"
Dr. Stevens says CDI's growing incidence is clear; however, there is little research linking the risk to the total dose, duration, or number of antibiotics a patient receives. So her team set out to provide one of the first links. They found that compared to patients who received one antibiotic, the adjusted hazard ratios for those receiving two to five antibiotics were 2.5 (95% confidence interval [CI] 1.6-4.0), 3.3 (CI 2.2-5.2), and 9.6 (CI 6.1-15.1), respectively (Clin Infect Dis. 2011;53(1):42-48). Patients exposed to fluoroquinolones were associated with higher risk, while those given metronidazole saw reduced risk.
Dr. Stevens says she expected the research would confirm her suspicions that continued exposure to antibiotics increased risk of infection. Still, she says, the more difficult question is when to balance a minimalistic approach to antibiotic use with the need to aggressively deal with more acute primary infections.
"The risk of C. diff might be an acceptable risk in a case where you're treating a life-threatening infection," Dr. Stevens adds. "If you're treating acne or something that isn't a life-threatening condition to the patient, there has to be a balance."
A new study that shows cumulative antibiotic exposures appear to be associated with Clostridium difficile infections (CDI) should be seen as another reason to reduce the use of antibiotics to minimum levels, according to the paper's lead author.
"In terms of prevention, it's really important for us to start delineating [shortened antibiotic courses] in treating the primary infection," says Vanessa Stevens, PhD, a fellow at the Center for Health Outcomes, Pharmacoinformatics, and Epidemiology at the State University of New York at Buffalo. "What are the minimums that are necessary to accomplish the job?"
Dr. Stevens says CDI's growing incidence is clear; however, there is little research linking the risk to the total dose, duration, or number of antibiotics a patient receives. So her team set out to provide one of the first links. They found that compared to patients who received one antibiotic, the adjusted hazard ratios for those receiving two to five antibiotics were 2.5 (95% confidence interval [CI] 1.6-4.0), 3.3 (CI 2.2-5.2), and 9.6 (CI 6.1-15.1), respectively (Clin Infect Dis. 2011;53(1):42-48). Patients exposed to fluoroquinolones were associated with higher risk, while those given metronidazole saw reduced risk.
Dr. Stevens says she expected the research would confirm her suspicions that continued exposure to antibiotics increased risk of infection. Still, she says, the more difficult question is when to balance a minimalistic approach to antibiotic use with the need to aggressively deal with more acute primary infections.
"The risk of C. diff might be an acceptable risk in a case where you're treating a life-threatening infection," Dr. Stevens adds. "If you're treating acne or something that isn't a life-threatening condition to the patient, there has to be a balance."
A new study that shows cumulative antibiotic exposures appear to be associated with Clostridium difficile infections (CDI) should be seen as another reason to reduce the use of antibiotics to minimum levels, according to the paper's lead author.
"In terms of prevention, it's really important for us to start delineating [shortened antibiotic courses] in treating the primary infection," says Vanessa Stevens, PhD, a fellow at the Center for Health Outcomes, Pharmacoinformatics, and Epidemiology at the State University of New York at Buffalo. "What are the minimums that are necessary to accomplish the job?"
Dr. Stevens says CDI's growing incidence is clear; however, there is little research linking the risk to the total dose, duration, or number of antibiotics a patient receives. So her team set out to provide one of the first links. They found that compared to patients who received one antibiotic, the adjusted hazard ratios for those receiving two to five antibiotics were 2.5 (95% confidence interval [CI] 1.6-4.0), 3.3 (CI 2.2-5.2), and 9.6 (CI 6.1-15.1), respectively (Clin Infect Dis. 2011;53(1):42-48). Patients exposed to fluoroquinolones were associated with higher risk, while those given metronidazole saw reduced risk.
Dr. Stevens says she expected the research would confirm her suspicions that continued exposure to antibiotics increased risk of infection. Still, she says, the more difficult question is when to balance a minimalistic approach to antibiotic use with the need to aggressively deal with more acute primary infections.
"The risk of C. diff might be an acceptable risk in a case where you're treating a life-threatening infection," Dr. Stevens adds. "If you're treating acne or something that isn't a life-threatening condition to the patient, there has to be a balance."
Hospitalized Patients Take MRSA Home
A new report on how hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) spreads after patients are discharged has at least one hospitalist wondering whether HM could, or should, take a leading role in reducing MRSA transfers.
The study, "Carriage of Methicillin-Resistant Staphylococcus aureus in Home Care Settings," identified MRSA in 191 of the 1,501 patients (12.7%) who were screened before discharge from French hospitals in 2003 and 2004. Researchers reported that 19% of relatives and caretakers who came into contact with the patients identified with MRSA also acquired the bacteria (Arch Intern Med. 2009;169(15)1372-1378).
Hospitalist and infectious-disease specialist James Pile, MD, FACP, FHM, interim director of the Division of Hospital Medicine at CWRU/MetroHealth Medical Center in Cleveland, says the study might be most important for the questions it raises regarding the degree to which community-acquired MRSA (CA-MRSA) is colonizing household contacts of discharged patients, as the burden of clinical disease in those individuals is likely to be greater than in those colonized with traditional, healthcare-associated MRSA (HA-MRSA). CA-MRSA appears to be supplanting HA-MRSA in many hospitals, Dr. Pile says, and the simple intervention of more rigorous hand washing by caregivers and other household contacts of patients discharged with MRSA infections could help limit the associated fallout.
“This is a chance for healthcare professionals, and hospitalists specifically, to recognize that and to counsel that as patients leave the hospital,” Dr. Pile says.
The authors note that “because none of the household contacts who acquired MRSA developed an infection, it is unclear whether this transmission represents a serious health problem.”
To that end, Dr. Pile says HM should wait for more definitive studies before committing to potentially time-consuming QI projects focused on MRSA transmissions to the home. “Before hospitalists galvanize their resources to try to tackle this problem,” Dr. Pile says, “we want to make sure there is enough bang for the buck.”
A new report on how hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) spreads after patients are discharged has at least one hospitalist wondering whether HM could, or should, take a leading role in reducing MRSA transfers.
The study, "Carriage of Methicillin-Resistant Staphylococcus aureus in Home Care Settings," identified MRSA in 191 of the 1,501 patients (12.7%) who were screened before discharge from French hospitals in 2003 and 2004. Researchers reported that 19% of relatives and caretakers who came into contact with the patients identified with MRSA also acquired the bacteria (Arch Intern Med. 2009;169(15)1372-1378).
Hospitalist and infectious-disease specialist James Pile, MD, FACP, FHM, interim director of the Division of Hospital Medicine at CWRU/MetroHealth Medical Center in Cleveland, says the study might be most important for the questions it raises regarding the degree to which community-acquired MRSA (CA-MRSA) is colonizing household contacts of discharged patients, as the burden of clinical disease in those individuals is likely to be greater than in those colonized with traditional, healthcare-associated MRSA (HA-MRSA). CA-MRSA appears to be supplanting HA-MRSA in many hospitals, Dr. Pile says, and the simple intervention of more rigorous hand washing by caregivers and other household contacts of patients discharged with MRSA infections could help limit the associated fallout.
“This is a chance for healthcare professionals, and hospitalists specifically, to recognize that and to counsel that as patients leave the hospital,” Dr. Pile says.
The authors note that “because none of the household contacts who acquired MRSA developed an infection, it is unclear whether this transmission represents a serious health problem.”
To that end, Dr. Pile says HM should wait for more definitive studies before committing to potentially time-consuming QI projects focused on MRSA transmissions to the home. “Before hospitalists galvanize their resources to try to tackle this problem,” Dr. Pile says, “we want to make sure there is enough bang for the buck.”
A new report on how hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) spreads after patients are discharged has at least one hospitalist wondering whether HM could, or should, take a leading role in reducing MRSA transfers.
The study, "Carriage of Methicillin-Resistant Staphylococcus aureus in Home Care Settings," identified MRSA in 191 of the 1,501 patients (12.7%) who were screened before discharge from French hospitals in 2003 and 2004. Researchers reported that 19% of relatives and caretakers who came into contact with the patients identified with MRSA also acquired the bacteria (Arch Intern Med. 2009;169(15)1372-1378).
Hospitalist and infectious-disease specialist James Pile, MD, FACP, FHM, interim director of the Division of Hospital Medicine at CWRU/MetroHealth Medical Center in Cleveland, says the study might be most important for the questions it raises regarding the degree to which community-acquired MRSA (CA-MRSA) is colonizing household contacts of discharged patients, as the burden of clinical disease in those individuals is likely to be greater than in those colonized with traditional, healthcare-associated MRSA (HA-MRSA). CA-MRSA appears to be supplanting HA-MRSA in many hospitals, Dr. Pile says, and the simple intervention of more rigorous hand washing by caregivers and other household contacts of patients discharged with MRSA infections could help limit the associated fallout.
“This is a chance for healthcare professionals, and hospitalists specifically, to recognize that and to counsel that as patients leave the hospital,” Dr. Pile says.
The authors note that “because none of the household contacts who acquired MRSA developed an infection, it is unclear whether this transmission represents a serious health problem.”
To that end, Dr. Pile says HM should wait for more definitive studies before committing to potentially time-consuming QI projects focused on MRSA transmissions to the home. “Before hospitalists galvanize their resources to try to tackle this problem,” Dr. Pile says, “we want to make sure there is enough bang for the buck.”
What is the best antibiotic treatment for C.difficile-associated diarrhea?
Case
An 84-year-old woman presents with watery diarrhea. She recently received a fluoroquinolone antibiotic during a hospitalization for pneumonia. Her temperature is 101 degrees, her heart rate is 110 beats per minute, and her respiratory rate is 22 breaths per minute. Her abdominal exam is significant for mild distention, hyperactive bowel sounds, and diffuse, mild tenderness without rebound or guarding. Her white blood cell count is 18,200 cells/mm3. You suspect C. difficile infection. Should you treat empirically with antibiotics and, if so, which antibiotic should you prescribe?
Overview
C. difficile is an anaerobic gram-positive bacillus that produces spores and toxins. In 1978, C. difficile was identified as the causative agent for antibiotic-associated diarrhea.1 The portal of entry is via the fecal-oral route.
Some patients carry C. difficile in their intestinal flora and show no signs of infection. Patients who develop symptoms commonly present with profuse, watery diarrhea. Nausea, vomiting, and abdominal pain also can be seen. Severe cases of C. difficile-associated diarrhea (CDAD) can present with significant abdominal pain and multisystem organ failure, with toxic megacolon resulting from toxin production and ileus.2 In severe cases due to ileus, diarrhea may be absent. Risk of mortality in severe cases is high, with some reviews citing death rates of 57% in patients requiring total colectomy.3 Risk factors for developing CDAD include the prior or current use of antibiotics, advanced age, hospitalization, and prior gastrointestinal surgery or procedures.4
The initial CDAD treatment involves removal of the agent that incited the infection. In most cases, this means discontinuation of an antimicrobial agent. Removal of the inciting agent allows restoration of the normal bowel flora. In mild CDAD cases, this may be sufficient therapy. However, most CDAD cases require treatment. Although many antimicrobial and probiotic agents have been used in CDAD treatment, metronidazole and vancomycin are the most commonly prescribed agents. There is an ongoing debate as to which should be considered the first-line agent.
Review of the Data
Metronidazole and vancomycin have the longest histories of use and are the most studied agents in CDAD. Metronidazole is prescribed 250 mg four times daily (or 500 mg twice daily) for 14 days. It is reasonably tolerated, although it can cause a metallic taste in the mouth. Vancomycin is given 125 mg four times daily (or 500 mg three times daily) for 10 to 14 days. Unlike metronidazole, which can be given by mouth or intravenously, only oral vancomycin is effective in CDAD.
Historically, metronidazole has been prescribed more frequently as the first-line agent in CDAD. Proponents of the drug tout its low cost and the importance of minimizing the development of vancomycin-resistant enteric pathogens. There are two small, prospective, randomized studies comparing the efficacy of the agents against one another in the treatment of C. difficile infection, with similar efficacy demonstrated in both studies. In the early 1980s, Teasley and colleagues randomized 94 patients with C. difficile infection to either metronidazole or vancomycin.5 All the patients receiving vancomycin resolved their disease; 95% of patients receiving metronidazole were cured. The differences were not statistically significant.
In the mid-1990s, Wenisch and colleagues randomized patients with C. difficile infection to receive vancomycin, metronidazole, fusidic acid, or teicoplanin therapy.6 Ninety-four percent of patients in both the vancomycin and metronidazole groups were cured.
However, since 2000, investigators have reported higher failure rates with metronidazole therapy in C. difficile infections. For example, in 2005, Pepin and colleagues reviewed cases of C. difficile infections at a hospital in Quebec.7 They determined the number of patients with C. difficile infection initially treated with metronidazole who required additional therapy had markedly increased. Between 1991 and 2002, 9.6% of patients who initially were treated with metronidazole required a switch to vancomycin (or the addition of vancomycin) because of a disappointing response. This figure doubled to 25.7% in 2003-2004. The 60-day probability of recurrence also increased in the 2003-2004 test group (47.2%), compared with the 1991-2002 group (20.8%). Both results were statistically significant. Such data contributed to the debate regarding whether metronidazole or vancomycin is the superior agent in the treatment of C. difficile infections.
In 2007, Zar and colleagues studied the efficacy of metronidazole and vancomycin in the treatment of CDAD patients, but the study stratified patients according to disease severity.8 This allowed the authors to investigate whether one agent was superior in treating mild or severe CDAD. They determined disease severity by assigning points to individual patient characteristics. Patients with two or more points were deemed to have “severe” CDAD.
The investigators assigned one point for each of the following patient characteristics: temperature >38.3 degrees Celsius, age >60 years, albumin level <2.5 mg/dL, and white blood cell count >15,000 cells/mm3 within 48 hours of enrolling in the study. Any patient with endoscopic evidence of pseudomembrane formation or admission to the intensive-care unit (ICU) for CDAD treatment was considered to have severe disease.
This was a prospective, randomized controlled trial of 150 patients. Patients were randomly prescribed 500 mg metronidazole by mouth three times daily or 125 mg of vancomycin by mouth four times daily. Patients with mild CDAD had similar cure rates: 90% metronidazole versus 98% vancomycin (P=0.36). However, patients with severe CDAD fared statistically better when treated with oral vancomycin. Ninety-seven percent of severe CDAD patients treated with oral vancomycin had a clinical cure, while only 76% of those treated with metronidazole were cured (P=0.02). Recurrence of the disease was similar in each treatment group.
Based on this study, metronidazole and vancomycin appear equally effective in the treatment of mild CDAD, but vancomycin is the superior agent in the treatment of patients with severe CDAD.
Back to the Case
Our patient had several risk factors predisposing her to developing CDAD. She was of advanced age and took a fluoroquinolone antibiotic during a recent hospitalization. She also presented with signs consistent with a severe case of CDAD. She had a fever, a white blood cell count >15,000 cells/mm3, and was older than 60. Thus, she should be treated with supportive care, placed on contact precautions, and administered oral vancomycin 125 mg by mouth every six hours for 10 days as empiric therapy for CDAD. Stool cultures should be sent to confirm the presence of the C. difficile toxin.
Bottom Line
The appropriate antibiotic choice to treat CDAD in any patient depends upon the clinical severity of the disease. Treat patients with mild CDAD with metronidazole; prescribe oral vancomycin for patients with severe CDAD. TH
Dr. Mattison, instructor of medicine at Harvard Medical School, is a hospitalist and co-director of the Inpatient Geriatrics Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston. Dr. Li, assistant professor of medicine at Harvard Medical School, is director of hospital medicine and associate chief of BIDMC’s Division of General Medicine and Primary Care.
References
1.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of C. difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778-782.
2.Poutanen SM, Simor AE. C. difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
3.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant C. difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372.
4.Bartlett JG. Narrative review: the new epidemic of C. difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for C. difficile-associated diarrhea and colitis. Lancet. 1983;2:1043-1046.
6.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of C. difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
7.Pepin J, Alary M, Valiquette L, et al. Increasing risk of relapse after treatment of C. difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of C. difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
Case
An 84-year-old woman presents with watery diarrhea. She recently received a fluoroquinolone antibiotic during a hospitalization for pneumonia. Her temperature is 101 degrees, her heart rate is 110 beats per minute, and her respiratory rate is 22 breaths per minute. Her abdominal exam is significant for mild distention, hyperactive bowel sounds, and diffuse, mild tenderness without rebound or guarding. Her white blood cell count is 18,200 cells/mm3. You suspect C. difficile infection. Should you treat empirically with antibiotics and, if so, which antibiotic should you prescribe?
Overview
C. difficile is an anaerobic gram-positive bacillus that produces spores and toxins. In 1978, C. difficile was identified as the causative agent for antibiotic-associated diarrhea.1 The portal of entry is via the fecal-oral route.
Some patients carry C. difficile in their intestinal flora and show no signs of infection. Patients who develop symptoms commonly present with profuse, watery diarrhea. Nausea, vomiting, and abdominal pain also can be seen. Severe cases of C. difficile-associated diarrhea (CDAD) can present with significant abdominal pain and multisystem organ failure, with toxic megacolon resulting from toxin production and ileus.2 In severe cases due to ileus, diarrhea may be absent. Risk of mortality in severe cases is high, with some reviews citing death rates of 57% in patients requiring total colectomy.3 Risk factors for developing CDAD include the prior or current use of antibiotics, advanced age, hospitalization, and prior gastrointestinal surgery or procedures.4
The initial CDAD treatment involves removal of the agent that incited the infection. In most cases, this means discontinuation of an antimicrobial agent. Removal of the inciting agent allows restoration of the normal bowel flora. In mild CDAD cases, this may be sufficient therapy. However, most CDAD cases require treatment. Although many antimicrobial and probiotic agents have been used in CDAD treatment, metronidazole and vancomycin are the most commonly prescribed agents. There is an ongoing debate as to which should be considered the first-line agent.
Review of the Data
Metronidazole and vancomycin have the longest histories of use and are the most studied agents in CDAD. Metronidazole is prescribed 250 mg four times daily (or 500 mg twice daily) for 14 days. It is reasonably tolerated, although it can cause a metallic taste in the mouth. Vancomycin is given 125 mg four times daily (or 500 mg three times daily) for 10 to 14 days. Unlike metronidazole, which can be given by mouth or intravenously, only oral vancomycin is effective in CDAD.
Historically, metronidazole has been prescribed more frequently as the first-line agent in CDAD. Proponents of the drug tout its low cost and the importance of minimizing the development of vancomycin-resistant enteric pathogens. There are two small, prospective, randomized studies comparing the efficacy of the agents against one another in the treatment of C. difficile infection, with similar efficacy demonstrated in both studies. In the early 1980s, Teasley and colleagues randomized 94 patients with C. difficile infection to either metronidazole or vancomycin.5 All the patients receiving vancomycin resolved their disease; 95% of patients receiving metronidazole were cured. The differences were not statistically significant.
In the mid-1990s, Wenisch and colleagues randomized patients with C. difficile infection to receive vancomycin, metronidazole, fusidic acid, or teicoplanin therapy.6 Ninety-four percent of patients in both the vancomycin and metronidazole groups were cured.
However, since 2000, investigators have reported higher failure rates with metronidazole therapy in C. difficile infections. For example, in 2005, Pepin and colleagues reviewed cases of C. difficile infections at a hospital in Quebec.7 They determined the number of patients with C. difficile infection initially treated with metronidazole who required additional therapy had markedly increased. Between 1991 and 2002, 9.6% of patients who initially were treated with metronidazole required a switch to vancomycin (or the addition of vancomycin) because of a disappointing response. This figure doubled to 25.7% in 2003-2004. The 60-day probability of recurrence also increased in the 2003-2004 test group (47.2%), compared with the 1991-2002 group (20.8%). Both results were statistically significant. Such data contributed to the debate regarding whether metronidazole or vancomycin is the superior agent in the treatment of C. difficile infections.
In 2007, Zar and colleagues studied the efficacy of metronidazole and vancomycin in the treatment of CDAD patients, but the study stratified patients according to disease severity.8 This allowed the authors to investigate whether one agent was superior in treating mild or severe CDAD. They determined disease severity by assigning points to individual patient characteristics. Patients with two or more points were deemed to have “severe” CDAD.
The investigators assigned one point for each of the following patient characteristics: temperature >38.3 degrees Celsius, age >60 years, albumin level <2.5 mg/dL, and white blood cell count >15,000 cells/mm3 within 48 hours of enrolling in the study. Any patient with endoscopic evidence of pseudomembrane formation or admission to the intensive-care unit (ICU) for CDAD treatment was considered to have severe disease.
This was a prospective, randomized controlled trial of 150 patients. Patients were randomly prescribed 500 mg metronidazole by mouth three times daily or 125 mg of vancomycin by mouth four times daily. Patients with mild CDAD had similar cure rates: 90% metronidazole versus 98% vancomycin (P=0.36). However, patients with severe CDAD fared statistically better when treated with oral vancomycin. Ninety-seven percent of severe CDAD patients treated with oral vancomycin had a clinical cure, while only 76% of those treated with metronidazole were cured (P=0.02). Recurrence of the disease was similar in each treatment group.
Based on this study, metronidazole and vancomycin appear equally effective in the treatment of mild CDAD, but vancomycin is the superior agent in the treatment of patients with severe CDAD.
Back to the Case
Our patient had several risk factors predisposing her to developing CDAD. She was of advanced age and took a fluoroquinolone antibiotic during a recent hospitalization. She also presented with signs consistent with a severe case of CDAD. She had a fever, a white blood cell count >15,000 cells/mm3, and was older than 60. Thus, she should be treated with supportive care, placed on contact precautions, and administered oral vancomycin 125 mg by mouth every six hours for 10 days as empiric therapy for CDAD. Stool cultures should be sent to confirm the presence of the C. difficile toxin.
Bottom Line
The appropriate antibiotic choice to treat CDAD in any patient depends upon the clinical severity of the disease. Treat patients with mild CDAD with metronidazole; prescribe oral vancomycin for patients with severe CDAD. TH
Dr. Mattison, instructor of medicine at Harvard Medical School, is a hospitalist and co-director of the Inpatient Geriatrics Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston. Dr. Li, assistant professor of medicine at Harvard Medical School, is director of hospital medicine and associate chief of BIDMC’s Division of General Medicine and Primary Care.
References
1.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of C. difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778-782.
2.Poutanen SM, Simor AE. C. difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
3.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant C. difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372.
4.Bartlett JG. Narrative review: the new epidemic of C. difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for C. difficile-associated diarrhea and colitis. Lancet. 1983;2:1043-1046.
6.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of C. difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
7.Pepin J, Alary M, Valiquette L, et al. Increasing risk of relapse after treatment of C. difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of C. difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
Case
An 84-year-old woman presents with watery diarrhea. She recently received a fluoroquinolone antibiotic during a hospitalization for pneumonia. Her temperature is 101 degrees, her heart rate is 110 beats per minute, and her respiratory rate is 22 breaths per minute. Her abdominal exam is significant for mild distention, hyperactive bowel sounds, and diffuse, mild tenderness without rebound or guarding. Her white blood cell count is 18,200 cells/mm3. You suspect C. difficile infection. Should you treat empirically with antibiotics and, if so, which antibiotic should you prescribe?
Overview
C. difficile is an anaerobic gram-positive bacillus that produces spores and toxins. In 1978, C. difficile was identified as the causative agent for antibiotic-associated diarrhea.1 The portal of entry is via the fecal-oral route.
Some patients carry C. difficile in their intestinal flora and show no signs of infection. Patients who develop symptoms commonly present with profuse, watery diarrhea. Nausea, vomiting, and abdominal pain also can be seen. Severe cases of C. difficile-associated diarrhea (CDAD) can present with significant abdominal pain and multisystem organ failure, with toxic megacolon resulting from toxin production and ileus.2 In severe cases due to ileus, diarrhea may be absent. Risk of mortality in severe cases is high, with some reviews citing death rates of 57% in patients requiring total colectomy.3 Risk factors for developing CDAD include the prior or current use of antibiotics, advanced age, hospitalization, and prior gastrointestinal surgery or procedures.4
The initial CDAD treatment involves removal of the agent that incited the infection. In most cases, this means discontinuation of an antimicrobial agent. Removal of the inciting agent allows restoration of the normal bowel flora. In mild CDAD cases, this may be sufficient therapy. However, most CDAD cases require treatment. Although many antimicrobial and probiotic agents have been used in CDAD treatment, metronidazole and vancomycin are the most commonly prescribed agents. There is an ongoing debate as to which should be considered the first-line agent.
Review of the Data
Metronidazole and vancomycin have the longest histories of use and are the most studied agents in CDAD. Metronidazole is prescribed 250 mg four times daily (or 500 mg twice daily) for 14 days. It is reasonably tolerated, although it can cause a metallic taste in the mouth. Vancomycin is given 125 mg four times daily (or 500 mg three times daily) for 10 to 14 days. Unlike metronidazole, which can be given by mouth or intravenously, only oral vancomycin is effective in CDAD.
Historically, metronidazole has been prescribed more frequently as the first-line agent in CDAD. Proponents of the drug tout its low cost and the importance of minimizing the development of vancomycin-resistant enteric pathogens. There are two small, prospective, randomized studies comparing the efficacy of the agents against one another in the treatment of C. difficile infection, with similar efficacy demonstrated in both studies. In the early 1980s, Teasley and colleagues randomized 94 patients with C. difficile infection to either metronidazole or vancomycin.5 All the patients receiving vancomycin resolved their disease; 95% of patients receiving metronidazole were cured. The differences were not statistically significant.
In the mid-1990s, Wenisch and colleagues randomized patients with C. difficile infection to receive vancomycin, metronidazole, fusidic acid, or teicoplanin therapy.6 Ninety-four percent of patients in both the vancomycin and metronidazole groups were cured.
However, since 2000, investigators have reported higher failure rates with metronidazole therapy in C. difficile infections. For example, in 2005, Pepin and colleagues reviewed cases of C. difficile infections at a hospital in Quebec.7 They determined the number of patients with C. difficile infection initially treated with metronidazole who required additional therapy had markedly increased. Between 1991 and 2002, 9.6% of patients who initially were treated with metronidazole required a switch to vancomycin (or the addition of vancomycin) because of a disappointing response. This figure doubled to 25.7% in 2003-2004. The 60-day probability of recurrence also increased in the 2003-2004 test group (47.2%), compared with the 1991-2002 group (20.8%). Both results were statistically significant. Such data contributed to the debate regarding whether metronidazole or vancomycin is the superior agent in the treatment of C. difficile infections.
In 2007, Zar and colleagues studied the efficacy of metronidazole and vancomycin in the treatment of CDAD patients, but the study stratified patients according to disease severity.8 This allowed the authors to investigate whether one agent was superior in treating mild or severe CDAD. They determined disease severity by assigning points to individual patient characteristics. Patients with two or more points were deemed to have “severe” CDAD.
The investigators assigned one point for each of the following patient characteristics: temperature >38.3 degrees Celsius, age >60 years, albumin level <2.5 mg/dL, and white blood cell count >15,000 cells/mm3 within 48 hours of enrolling in the study. Any patient with endoscopic evidence of pseudomembrane formation or admission to the intensive-care unit (ICU) for CDAD treatment was considered to have severe disease.
This was a prospective, randomized controlled trial of 150 patients. Patients were randomly prescribed 500 mg metronidazole by mouth three times daily or 125 mg of vancomycin by mouth four times daily. Patients with mild CDAD had similar cure rates: 90% metronidazole versus 98% vancomycin (P=0.36). However, patients with severe CDAD fared statistically better when treated with oral vancomycin. Ninety-seven percent of severe CDAD patients treated with oral vancomycin had a clinical cure, while only 76% of those treated with metronidazole were cured (P=0.02). Recurrence of the disease was similar in each treatment group.
Based on this study, metronidazole and vancomycin appear equally effective in the treatment of mild CDAD, but vancomycin is the superior agent in the treatment of patients with severe CDAD.
Back to the Case
Our patient had several risk factors predisposing her to developing CDAD. She was of advanced age and took a fluoroquinolone antibiotic during a recent hospitalization. She also presented with signs consistent with a severe case of CDAD. She had a fever, a white blood cell count >15,000 cells/mm3, and was older than 60. Thus, she should be treated with supportive care, placed on contact precautions, and administered oral vancomycin 125 mg by mouth every six hours for 10 days as empiric therapy for CDAD. Stool cultures should be sent to confirm the presence of the C. difficile toxin.
Bottom Line
The appropriate antibiotic choice to treat CDAD in any patient depends upon the clinical severity of the disease. Treat patients with mild CDAD with metronidazole; prescribe oral vancomycin for patients with severe CDAD. TH
Dr. Mattison, instructor of medicine at Harvard Medical School, is a hospitalist and co-director of the Inpatient Geriatrics Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston. Dr. Li, assistant professor of medicine at Harvard Medical School, is director of hospital medicine and associate chief of BIDMC’s Division of General Medicine and Primary Care.
References
1.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of C. difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778-782.
2.Poutanen SM, Simor AE. C. difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
3.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant C. difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372.
4.Bartlett JG. Narrative review: the new epidemic of C. difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for C. difficile-associated diarrhea and colitis. Lancet. 1983;2:1043-1046.
6.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of C. difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
7.Pepin J, Alary M, Valiquette L, et al. Increasing risk of relapse after treatment of C. difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of C. difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
What is the proper duration of antibiotic treatment in adults hospitalized with community-acquired pneumonia?
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Bacterial Meningitis, Non-Specific Troponin Elevation, Antibiotics for ECOPD, VTE Update, and More
Treatment of Bacterial Meningitis with Vancomycin
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007 Jan 15;44(2):250-255. Epub 2006 Dec 15.
In 2002, van de Beek and de Gans published a study demonstrating that adjuvant dexamethasone decreased mortality and improved neurological disability when given to patients with bacterial meningitis. Their results changed our treatment paradigm for this disease but left us with several questions. At what point in the treatment course does giving corticosteroids become ineffective? Do their results apply to all bacterial pathogens? Can the results be applied to the use of vancomycin in treating penicillin-resistant strains of Streptococcus pneumoniae? This final question arises from the disturbing ability of vancomycin to penetrate the cerebrospinal fluid (CSF). Previous data support this concern; thus, bactericidal titers may be inadequate within the CSF. Because meningeal inflammation exerts a strong influence over whether or not vancomycin enters the CSF, administering steroids may decrease its ability to do so. This study brings some clarity to the issue.
In this observational open multicenter trial from France, 14 adults were admitted to intensive care units with suspected pneumococcal meningitis. They were treated with intravenous cefotaxime, vancomycin, and dexamethasone. The vancomycin was given as a loading dose of 15 mg per kg of body weight followed by administration of a continuous infusion of 60 mg per kg of body weight per day. The diagnosis of pneumococcal meningitis was made using a CSF pleocytosis as well as one or more of the following: a positive culture from either the blood or CSF, a Gram stain showing Gram-positive diplococci, or pneumococcal antigens in the CSF as demonstrated by latex agglutination. Patients had a second lumbar puncture on either day two or three to measure vancomycin levels—among other markers of disease activity—in the CSF. Serum levels of vancomycin were drawn simultaneously.
Thirteen of the 14 patients had pneumococcal meningitis; one patient was found to have meningitis from Neisseria meningitidis. Seven patients had pneumococcal strains resistant to penicillin. Ten of the 14 patients required mechanical intubation. The second lumbar puncture demonstrated marked improvements in leukocyte counts, protein levels, and glucose levels. All subsequent cultures from the CSF were negative. Three patients died, two had neurological sequelae, and the remainder were discharged from the hospital without complications. Vancomycin concentrations in the serum ranged from 14.2 to 39.0 mg/L, with a mean of 25.2 mg/L; concentrations in the CSF ranged from 3.1 to 22.3 mg/L, with a mean of 7.9 mg/L. There was a significant correlation between vancomycin levels in the serum and those in the CSF (r = 0.68; P = 0.01). The concentration of vancomycin in the CSF was between four and 10 times the mean inhibitory concentrations (MICs). A linear correlation exists between penetration of vancomycin into CSF and serum levels. No evidence of drug toxicities was observed.
The results demonstrate that a therapeutic concentration of vancomycin can be achieved in the CSF. The continuous infusion of vancomycin with a loading dose, which has not been standard practice, has previously been shown to achieve targeted serum levels more quickly than intermittent dosing. Levels of serum vancomycin were likely higher in this study than when troughs of 15-20 mg/L are the goal. This data strongly suggests, however, that this same treatment regimen can obtain adequate vancomycin levels in the CSF while treating pneumococcal meningitis with adjunctive steroids.
Nonspecific elevations in troponins
Alcalai R, Planer D, Culhaoqlu A, et al. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007 Feb 12;167(3):276-281.
In 2000, the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) jointly produced a recommendation for a new definition of myocardial infarction. This proposal based the diagnosis primarily on the elevation of biomarkers specific to cardiac tissue, troponin T and troponin I. Since that time, as use of these blood tests has escalated, it is apparent that elevations in these biomarkers do not always translate into thrombotic coronary artery occlusion. Instead, we have seen that they are positive in a variety of clinical settings. These include sepsis, renal failure, pulmonary embolism, and atrial fibrillation. This investigation attempts to characterize the differences among patients presenting with acute coronary syndrome (ACS) and nonthrombotic troponin elevation (NTTE), to report on outcomes for each, and to note the positive predictive values (PPV) for elevated troponins across clinical settings.
Two hospitals in Israel collected data on all adult patients who experienced an elevation in troponin T (defined as at least 0.1 ng/mL) at any time during their hospital stay. Six hundred and fifteen patients were evaluated by age, sex, cardiovascular risk factors, history of ischemic heart disease, left ventricular function (LVF) by echocardiogram, serum creatine phosphokinase (CPK), and creatinine levels, as well as by which hospital service each had been admitted under. The highest troponin T value was used in the analysis, along with the creatinine level taken on the same day. Two physicians, one a specialist in internal medicine and the other a specialist in cardiology, independently determined the principal diagnosis in accordance with the ACC/ESC guidelines for thrombotic ACS and used other diagnostic studies for alternative diagnosis for conditions known to cause NTTE.
Patients were followed up for causes of mortality for up to two-and-a-half years. Kappa (k) was calculated for physician agreement regarding the principal diagnosis. To assess independent odds ratios and their 95% confidence intervals (CIs) of predictor variables for ACS, an unconditional multiple logistic regression analysis was used. The PPV for troponin T in the diagnosis of ACS was calculated. In-house mortality rates were measured. Long-term risk of death was assessed using Cox proportional hazard models.
The diagnosis of ACS was made in only 53% (326) of the patients. Forty-one percent (254) had NTTE, and the diagnosis was not determined in 6% (35). The diagnoses comprising NTTE included—in order from most to least common—cardiac non-ischemic conditions, sepsis, pulmonary diseases, and neurologic diseases. Using the multivariate analysis, the diagnostic predictors for ACS were history of hypertension or ischemic heart disease, age between 40 and 70 years, higher troponin levels (greater than 1.0 ng/mL), and normal renal function. Extreme age and admission to a surgical team were negative predictors for ACS. Gender, presence of diabetes, and LVF did not appear to make a difference.
The PPV of an elevated troponin T for ACS among all patients was only 56% (95% CI, 52%-60%). It became lower (27%) in those older than 70 with abnormal renal function and higher (90%) in those with a troponin T greater than 1.0 ng/mL and normal renal function. In-house mortality for all patients was 8%; for those with ACS, it was 3%, while for those with NTTE, it was—at 21%—almost eight times higher than the ACS group (P<0.001). Patients were followed up for mortality for a median of 22 months. The long-term mortality was also significantly better (P<0.001) for those with a diagnosis of ACS than for those with NTTE.
Since the incorporation of the ACC/ESC guidelines, the diagnosis of ACS has substantially increased. It is critical to distinguish between ACS and NTTE when using these very sensitive biomarkers, because the underlying cause of NTTE usually requires a drastically different therapy than that of ACS; in addition, misdiagnosing a myocardial infarction may lead to potentially harmful diagnostic studies and therapies in the form of coronary angiography, antithrombotics, and antiplatelet agents. Hospitalists should look for ACS when troponin T levels exceed 1.0 ng/mL in the face of normal renal function. Based on their data, the authors present an algorithm for working up ACS and NTTE that takes into consideration the clinical presentation, age, renal function, electrocardiographic changes, and troponin T levels. Though this is a retrospective trial, it provides guidance for a very common clinical scenario. We should be concerned about a patient’s prognosis when we encounter an elevated troponin in a setting of NTTE.
Guiding Antibiotic Therapy for COPD Exacerbations
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007 Jan;131(1):9-19.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States. Exacerbations of COPD (ECOPD) that require hospitalization are both common and costly. Though recent literature suggests that antibiotic therapy during exacerbations reduces morbidity and mortality and lowers the lack of response to treatment, controversy persists concerning whether or not these results are applicable to all patients with this condition. Procalcitonin is a protein not typically measurable in plasma. Levels of this protein rise with bacterial infections, but appear to be unaffected by inflammation from other etiologies such as autoimmune processes or viral infections. Measuring procalcitonin levels has already been shown to safely decrease the use of antibiotics in lower respiratory infections.
This single-center trial from Switzerland evaluated consecutive patients admitted from the emergency department with ECOPD. For 226 enrolled patients, symptoms were quantified, sputum was collected, spirometry was measured, and procalcitonin levels were evaluated. Attending physicians chose antibiotics, using current guidelines, for patients randomized to the standard therapy group. In the group randomized to procalcitonin guidance, antibiotics were given according to serum levels. No antibiotics were administered for levels below 0.1 micrograms (mcg)/L; antibiotics were encouraged for levels greater than 0.25 mcg/L. For levels between 0.1 and .25 mcg/L, antibiotics were encouraged or discouraged based on the clinical condition of the patient. The primary outcomes evaluated were total antibiotics used during hospitalization and up to six months following hospitalization. Secondary endpoints included clinical and laboratory data and six-month follow-up for exacerbation rate and time to the next ECOPD.
Procalcitonin guidance significantly decreased antibiotic administration compared with the standard-therapy arm (40% versus 72% respectively; P<0.0001) and antibiotic exposure (RR, 0.56; 95% CI, 0.43 to 0.73; P<0.0001). The absolute risk reduction was 31.5% (95% CI, 18.7 to 44.3%; p<0.0001). No difference in the mean time to the next exacerbation was noticed between the two groups. Clinical and laboratory measures at baseline and through the six-month follow-up demonstrated no significant differences.
Using procalcitonin levels to guide antibiotic therapy for ECOPD is a practice that is exciting and full of promise. Not only could costs be cut by omitting antibiotics for this treatment regimen in select patients, but some pressure will be relieved in terms of decreasing emerging bacterial resistance. Because procalcitonin levels have a lab turn-around time of approximately one hour, this test becomes even more attractive: decisions for treatment can be made while patients are still in the emergency department. On a cautionary note, there is more than one method of testing for procalcitonin levels, and this trial was done at only one center. Before widespread use of this test is applied, these results should be validated in a multicenter trial. In addition, one test should be used consistently for measuring procalcitonin levels.
Community-Associated MRSA and MSSA: Clinical and Epidemiologic Characteristics
Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007 Feb 15;44(4):471-482. Epub 2007 Jan 19.
Methicillin-susceptible Staphylococcus aureus (MSSA) was, until very recently, the predominant strain seen in community-associated (CA) S. aureus infections. Now methicillin-resistant S aureus (MRSA) is a concern around the world. Deciding whether or not to treat empirically for MRSA in those patients who do not have risk factors for healthcare-associated (HCA) infections is difficult.
Investigators at the University of California-Los Angeles Medical Center (Torrance) prospectively evaluated consecutive patients admitted to the county hospital with S. aureus infections. Daily cultures of wounds, urine, blood, and sputum were taken. An extensive questionnaire was completed by 280 patients who provided information on exposures, demographic characteristics, and clinical characteristics. CA infections were defined as those not having a positive culture from a surgical site in a patient who, in the past year, had not lived in an extended living facility, had any indwelling devices, visited an infusion center, or received dialysis.
Of those evaluated, 202 patients (78%) had CA S. aureus and 78 (28%) had HCA S. aureus. Of those with the CA infections, 108 (60%) had MRSA and 72 (40%) had MSSA. Sensitivity, specificity, predictive values, and likelihood ratios for the risk factors evaluated were unable to distinguish CA-MRSA from CA-MSSA. For example, the sensitivities for most MRSA risk factors were less than 30%, and all the positive likelihood ratios were lower than three.
This study has very important consequences. Given the data presented, there is currently no way to consistently distinguish between CA-MRSA and CA-MSSA prior to culture results. It would be very reasonable in this population to treat for MRSA empirically. One limitation is that the information comes from a single center in an area that has a very diverse patient population. Also, because this was done at a county hospital, the resources for treating patients who would be cared for in the outpatient arena at other centers might not otherwise be available, thus generalizing this data to potential outpatients. Because the morbidity and mortality from a delay in treatment of MRSA infections is significant, however, it appears sensible to treat CA S. aureus empirically in areas where CA-MRSA is common, regardless of patients’ risk factors.
Venous Thromboembolism Update
King CS, Holley AB, Jackson JL, et al. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007 Feb;131(2):507-516.
Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007 Feb;131(2):517-523.
Segal JB, Streiff MB, Hoffman LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007 Feb 6;146(3):211-222.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146 (3):204-210. Epub 2007 Jan 29.
The prevention and treatment of venous thromboembolism (VTE) is a skill set required for all hospitalists given the prevalence of this condition in hospitalized patients as well as the significant morbidity and mortality associated with the condition. Several articles that help to guide our decisions in managing VTE have been published recently.
We have no randomized controlled trials (RCT) comparing twice-daily (bid) with three-times-daily (tid) dosing of unfractionated heparin (UFH) for the prevention of VTE in medically ill patient populations. It is unlikely that such a study, involving an adequate number of patients, will ever be conducted. Though low molecular weight heparins (LMWH) are used more frequently for VTE prevention, many hospitalists still use UFH to prevent VTE in patients who are morbidly obese or who have profound renal insufficiency. King and colleagues have done a meta-analysis to find out whether or not tid dosing is superior to bid dosing for VTE prevention. Twelve studies, including almost 8,000 patients from 1966 to 2004, were reviewed. All patients were hospitalized for medical rather than surgical conditions.
Tid heparin significantly decreased the incidence of the combined outcome of pulmonary embolism (PE) and proximal deep vein thrombosis (DVT). There was a trend toward significance in decreasing the incidence of PE. There was a significant increase in the number of major bleeds with tid dosing compared with bid dosing. There are many limitations to this study: It is retrospective, the population is extremely heterogeneous, and varying methods have been employed to diagnosis VTE across the many studies from which data were pooled. This is likely the best data we will have for UFH in VTE prevention, however. In summary, tid dosing is preferred for high-risk patients, but bid dosing should be considered for those at risk for bleeding complications.
Data are limited for the clinical course of PE. Outpatient treatment of PE with LMWH is not uncommon in select patients, but choosing who is safe to treat in this arena is uncertain. Nijkeuter and colleagues assessed the incidence of recurrent VTE, hemorrhagic complications from therapy, mortality, risk factors for recurrence, and the course of these events from the time of diagnosis through a three-month follow-up period.
Six hundred and seventy-three patients completed the three-month follow-up. Twenty of them (3%) had recurrent VTE; 14 of these had recurrent PE. Recurrence predominantly transpired in the first three weeks of therapy. Of those with recurrent PE, 11 (79%) were fatal, and most of these occurred within the first week of diagnosis. Major bleeding occurred in 1.5% of the patients. Immobilization for more than three days was a significant risk factor for recurrence. Inpatient status, a diagnosis of COPD, and malignancy were independent risk factors for bleeding complications. Fifty-five patients (8.2%) died over the three-month period. Twenty percent died of fatal recurrent PE, while 4% suffered fatal hemorrhage.
Multivariate analysis revealed four characteristics as independent risk factors for mortality in patients with PE. These include age, inpatient status, immobilization for more than three days, and malignancy. It appears that the majority of recurrent and fatal PE occurs during the first week of therapy. Physicians should not discharge patients to home with LMWH for PE without considering these risk factors for hemorrhage, recurrence, and mortality.
Annals of Internal Medicine has published a systematic review of management issues in VTE to provide the framework for the American College of Physicians practice guidelines. These guidelines pool data from more than 100 randomized controlled trials and comment on six areas in VTE management. The following are quotes from this document.
Recommendation #1: Use low molecular-weight heparin (LMWH) rather than unfractionated heparin whenever possible for the initial inpatient treatment of deep vein thrombosis (DVT). Either unfractionated heparin or LMWH is appropriate for the initial treatment of pulmonary embolism.
Recommendation #2: Outpatient treatment of DVT, and possibly pulmonary embolism, with LMWH is safe and cost-effective for carefully selected patients and should be considered if the required support services are in place.
Recommendation #3: Compression stockings should be used routinely to prevent post-thrombotic syndrome, beginning within one month of diagnosis of proximal DVT and continuing for a minimum of one year after diagnosis.
Recommendation #4: There is insufficient evidence to make specific recommendations for types of anticoagulation management of VTE in pregnant women.
Recommendation #5: Anticoagulation should be maintained for three to six months for VTE secondary to transient risk factors and for more than 12 months for recurrent VTE. While the appropriate duration of anticoagulation for idiopathic or recurrent VTE is not definitively known, there is evidence of substantial benefit for extended-duration therapy.
Recommendation #6: LMWH is safe and efficacious for the long-term treatment of VTE in selected patients (and may be preferable for patients with cancer).
All of these seem reasonable and appropriate with a possible exception in the second recommendation. Using LMWH to treat patients diagnosed with PE in the outpatient setting is not well supported by data. The vast majority of trials involving the treatment of VTE with LMWH have been conducted on those with DVT; the number of patients in the trials with PE has been very small. The Food and Drug Administration has not approved LMWH for outpatient treatment of PE; LMWH is FDA approved in the outpatient setting only for the treatment of DVT. We know that the hemodynamic changes that can accompany PE may not occur for at least 24 hours. In addition, we now have data from the Nijkeuter study that point to dangers that may result from treating PE outside the hospital setting. At this time, we should treat PE with LMWH in the outpatient setting only with patients whose risk factors, clinical characteristics, and outpatient resources have been carefully scrutinized. TH
Treatment of Bacterial Meningitis with Vancomycin
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007 Jan 15;44(2):250-255. Epub 2006 Dec 15.
In 2002, van de Beek and de Gans published a study demonstrating that adjuvant dexamethasone decreased mortality and improved neurological disability when given to patients with bacterial meningitis. Their results changed our treatment paradigm for this disease but left us with several questions. At what point in the treatment course does giving corticosteroids become ineffective? Do their results apply to all bacterial pathogens? Can the results be applied to the use of vancomycin in treating penicillin-resistant strains of Streptococcus pneumoniae? This final question arises from the disturbing ability of vancomycin to penetrate the cerebrospinal fluid (CSF). Previous data support this concern; thus, bactericidal titers may be inadequate within the CSF. Because meningeal inflammation exerts a strong influence over whether or not vancomycin enters the CSF, administering steroids may decrease its ability to do so. This study brings some clarity to the issue.
In this observational open multicenter trial from France, 14 adults were admitted to intensive care units with suspected pneumococcal meningitis. They were treated with intravenous cefotaxime, vancomycin, and dexamethasone. The vancomycin was given as a loading dose of 15 mg per kg of body weight followed by administration of a continuous infusion of 60 mg per kg of body weight per day. The diagnosis of pneumococcal meningitis was made using a CSF pleocytosis as well as one or more of the following: a positive culture from either the blood or CSF, a Gram stain showing Gram-positive diplococci, or pneumococcal antigens in the CSF as demonstrated by latex agglutination. Patients had a second lumbar puncture on either day two or three to measure vancomycin levels—among other markers of disease activity—in the CSF. Serum levels of vancomycin were drawn simultaneously.
Thirteen of the 14 patients had pneumococcal meningitis; one patient was found to have meningitis from Neisseria meningitidis. Seven patients had pneumococcal strains resistant to penicillin. Ten of the 14 patients required mechanical intubation. The second lumbar puncture demonstrated marked improvements in leukocyte counts, protein levels, and glucose levels. All subsequent cultures from the CSF were negative. Three patients died, two had neurological sequelae, and the remainder were discharged from the hospital without complications. Vancomycin concentrations in the serum ranged from 14.2 to 39.0 mg/L, with a mean of 25.2 mg/L; concentrations in the CSF ranged from 3.1 to 22.3 mg/L, with a mean of 7.9 mg/L. There was a significant correlation between vancomycin levels in the serum and those in the CSF (r = 0.68; P = 0.01). The concentration of vancomycin in the CSF was between four and 10 times the mean inhibitory concentrations (MICs). A linear correlation exists between penetration of vancomycin into CSF and serum levels. No evidence of drug toxicities was observed.
The results demonstrate that a therapeutic concentration of vancomycin can be achieved in the CSF. The continuous infusion of vancomycin with a loading dose, which has not been standard practice, has previously been shown to achieve targeted serum levels more quickly than intermittent dosing. Levels of serum vancomycin were likely higher in this study than when troughs of 15-20 mg/L are the goal. This data strongly suggests, however, that this same treatment regimen can obtain adequate vancomycin levels in the CSF while treating pneumococcal meningitis with adjunctive steroids.
Nonspecific elevations in troponins
Alcalai R, Planer D, Culhaoqlu A, et al. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007 Feb 12;167(3):276-281.
In 2000, the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) jointly produced a recommendation for a new definition of myocardial infarction. This proposal based the diagnosis primarily on the elevation of biomarkers specific to cardiac tissue, troponin T and troponin I. Since that time, as use of these blood tests has escalated, it is apparent that elevations in these biomarkers do not always translate into thrombotic coronary artery occlusion. Instead, we have seen that they are positive in a variety of clinical settings. These include sepsis, renal failure, pulmonary embolism, and atrial fibrillation. This investigation attempts to characterize the differences among patients presenting with acute coronary syndrome (ACS) and nonthrombotic troponin elevation (NTTE), to report on outcomes for each, and to note the positive predictive values (PPV) for elevated troponins across clinical settings.
Two hospitals in Israel collected data on all adult patients who experienced an elevation in troponin T (defined as at least 0.1 ng/mL) at any time during their hospital stay. Six hundred and fifteen patients were evaluated by age, sex, cardiovascular risk factors, history of ischemic heart disease, left ventricular function (LVF) by echocardiogram, serum creatine phosphokinase (CPK), and creatinine levels, as well as by which hospital service each had been admitted under. The highest troponin T value was used in the analysis, along with the creatinine level taken on the same day. Two physicians, one a specialist in internal medicine and the other a specialist in cardiology, independently determined the principal diagnosis in accordance with the ACC/ESC guidelines for thrombotic ACS and used other diagnostic studies for alternative diagnosis for conditions known to cause NTTE.
Patients were followed up for causes of mortality for up to two-and-a-half years. Kappa (k) was calculated for physician agreement regarding the principal diagnosis. To assess independent odds ratios and their 95% confidence intervals (CIs) of predictor variables for ACS, an unconditional multiple logistic regression analysis was used. The PPV for troponin T in the diagnosis of ACS was calculated. In-house mortality rates were measured. Long-term risk of death was assessed using Cox proportional hazard models.
The diagnosis of ACS was made in only 53% (326) of the patients. Forty-one percent (254) had NTTE, and the diagnosis was not determined in 6% (35). The diagnoses comprising NTTE included—in order from most to least common—cardiac non-ischemic conditions, sepsis, pulmonary diseases, and neurologic diseases. Using the multivariate analysis, the diagnostic predictors for ACS were history of hypertension or ischemic heart disease, age between 40 and 70 years, higher troponin levels (greater than 1.0 ng/mL), and normal renal function. Extreme age and admission to a surgical team were negative predictors for ACS. Gender, presence of diabetes, and LVF did not appear to make a difference.
The PPV of an elevated troponin T for ACS among all patients was only 56% (95% CI, 52%-60%). It became lower (27%) in those older than 70 with abnormal renal function and higher (90%) in those with a troponin T greater than 1.0 ng/mL and normal renal function. In-house mortality for all patients was 8%; for those with ACS, it was 3%, while for those with NTTE, it was—at 21%—almost eight times higher than the ACS group (P<0.001). Patients were followed up for mortality for a median of 22 months. The long-term mortality was also significantly better (P<0.001) for those with a diagnosis of ACS than for those with NTTE.
Since the incorporation of the ACC/ESC guidelines, the diagnosis of ACS has substantially increased. It is critical to distinguish between ACS and NTTE when using these very sensitive biomarkers, because the underlying cause of NTTE usually requires a drastically different therapy than that of ACS; in addition, misdiagnosing a myocardial infarction may lead to potentially harmful diagnostic studies and therapies in the form of coronary angiography, antithrombotics, and antiplatelet agents. Hospitalists should look for ACS when troponin T levels exceed 1.0 ng/mL in the face of normal renal function. Based on their data, the authors present an algorithm for working up ACS and NTTE that takes into consideration the clinical presentation, age, renal function, electrocardiographic changes, and troponin T levels. Though this is a retrospective trial, it provides guidance for a very common clinical scenario. We should be concerned about a patient’s prognosis when we encounter an elevated troponin in a setting of NTTE.
Guiding Antibiotic Therapy for COPD Exacerbations
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007 Jan;131(1):9-19.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States. Exacerbations of COPD (ECOPD) that require hospitalization are both common and costly. Though recent literature suggests that antibiotic therapy during exacerbations reduces morbidity and mortality and lowers the lack of response to treatment, controversy persists concerning whether or not these results are applicable to all patients with this condition. Procalcitonin is a protein not typically measurable in plasma. Levels of this protein rise with bacterial infections, but appear to be unaffected by inflammation from other etiologies such as autoimmune processes or viral infections. Measuring procalcitonin levels has already been shown to safely decrease the use of antibiotics in lower respiratory infections.
This single-center trial from Switzerland evaluated consecutive patients admitted from the emergency department with ECOPD. For 226 enrolled patients, symptoms were quantified, sputum was collected, spirometry was measured, and procalcitonin levels were evaluated. Attending physicians chose antibiotics, using current guidelines, for patients randomized to the standard therapy group. In the group randomized to procalcitonin guidance, antibiotics were given according to serum levels. No antibiotics were administered for levels below 0.1 micrograms (mcg)/L; antibiotics were encouraged for levels greater than 0.25 mcg/L. For levels between 0.1 and .25 mcg/L, antibiotics were encouraged or discouraged based on the clinical condition of the patient. The primary outcomes evaluated were total antibiotics used during hospitalization and up to six months following hospitalization. Secondary endpoints included clinical and laboratory data and six-month follow-up for exacerbation rate and time to the next ECOPD.
Procalcitonin guidance significantly decreased antibiotic administration compared with the standard-therapy arm (40% versus 72% respectively; P<0.0001) and antibiotic exposure (RR, 0.56; 95% CI, 0.43 to 0.73; P<0.0001). The absolute risk reduction was 31.5% (95% CI, 18.7 to 44.3%; p<0.0001). No difference in the mean time to the next exacerbation was noticed between the two groups. Clinical and laboratory measures at baseline and through the six-month follow-up demonstrated no significant differences.
Using procalcitonin levels to guide antibiotic therapy for ECOPD is a practice that is exciting and full of promise. Not only could costs be cut by omitting antibiotics for this treatment regimen in select patients, but some pressure will be relieved in terms of decreasing emerging bacterial resistance. Because procalcitonin levels have a lab turn-around time of approximately one hour, this test becomes even more attractive: decisions for treatment can be made while patients are still in the emergency department. On a cautionary note, there is more than one method of testing for procalcitonin levels, and this trial was done at only one center. Before widespread use of this test is applied, these results should be validated in a multicenter trial. In addition, one test should be used consistently for measuring procalcitonin levels.
Community-Associated MRSA and MSSA: Clinical and Epidemiologic Characteristics
Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007 Feb 15;44(4):471-482. Epub 2007 Jan 19.
Methicillin-susceptible Staphylococcus aureus (MSSA) was, until very recently, the predominant strain seen in community-associated (CA) S. aureus infections. Now methicillin-resistant S aureus (MRSA) is a concern around the world. Deciding whether or not to treat empirically for MRSA in those patients who do not have risk factors for healthcare-associated (HCA) infections is difficult.
Investigators at the University of California-Los Angeles Medical Center (Torrance) prospectively evaluated consecutive patients admitted to the county hospital with S. aureus infections. Daily cultures of wounds, urine, blood, and sputum were taken. An extensive questionnaire was completed by 280 patients who provided information on exposures, demographic characteristics, and clinical characteristics. CA infections were defined as those not having a positive culture from a surgical site in a patient who, in the past year, had not lived in an extended living facility, had any indwelling devices, visited an infusion center, or received dialysis.
Of those evaluated, 202 patients (78%) had CA S. aureus and 78 (28%) had HCA S. aureus. Of those with the CA infections, 108 (60%) had MRSA and 72 (40%) had MSSA. Sensitivity, specificity, predictive values, and likelihood ratios for the risk factors evaluated were unable to distinguish CA-MRSA from CA-MSSA. For example, the sensitivities for most MRSA risk factors were less than 30%, and all the positive likelihood ratios were lower than three.
This study has very important consequences. Given the data presented, there is currently no way to consistently distinguish between CA-MRSA and CA-MSSA prior to culture results. It would be very reasonable in this population to treat for MRSA empirically. One limitation is that the information comes from a single center in an area that has a very diverse patient population. Also, because this was done at a county hospital, the resources for treating patients who would be cared for in the outpatient arena at other centers might not otherwise be available, thus generalizing this data to potential outpatients. Because the morbidity and mortality from a delay in treatment of MRSA infections is significant, however, it appears sensible to treat CA S. aureus empirically in areas where CA-MRSA is common, regardless of patients’ risk factors.
Venous Thromboembolism Update
King CS, Holley AB, Jackson JL, et al. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007 Feb;131(2):507-516.
Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007 Feb;131(2):517-523.
Segal JB, Streiff MB, Hoffman LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007 Feb 6;146(3):211-222.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146 (3):204-210. Epub 2007 Jan 29.
The prevention and treatment of venous thromboembolism (VTE) is a skill set required for all hospitalists given the prevalence of this condition in hospitalized patients as well as the significant morbidity and mortality associated with the condition. Several articles that help to guide our decisions in managing VTE have been published recently.
We have no randomized controlled trials (RCT) comparing twice-daily (bid) with three-times-daily (tid) dosing of unfractionated heparin (UFH) for the prevention of VTE in medically ill patient populations. It is unlikely that such a study, involving an adequate number of patients, will ever be conducted. Though low molecular weight heparins (LMWH) are used more frequently for VTE prevention, many hospitalists still use UFH to prevent VTE in patients who are morbidly obese or who have profound renal insufficiency. King and colleagues have done a meta-analysis to find out whether or not tid dosing is superior to bid dosing for VTE prevention. Twelve studies, including almost 8,000 patients from 1966 to 2004, were reviewed. All patients were hospitalized for medical rather than surgical conditions.
Tid heparin significantly decreased the incidence of the combined outcome of pulmonary embolism (PE) and proximal deep vein thrombosis (DVT). There was a trend toward significance in decreasing the incidence of PE. There was a significant increase in the number of major bleeds with tid dosing compared with bid dosing. There are many limitations to this study: It is retrospective, the population is extremely heterogeneous, and varying methods have been employed to diagnosis VTE across the many studies from which data were pooled. This is likely the best data we will have for UFH in VTE prevention, however. In summary, tid dosing is preferred for high-risk patients, but bid dosing should be considered for those at risk for bleeding complications.
Data are limited for the clinical course of PE. Outpatient treatment of PE with LMWH is not uncommon in select patients, but choosing who is safe to treat in this arena is uncertain. Nijkeuter and colleagues assessed the incidence of recurrent VTE, hemorrhagic complications from therapy, mortality, risk factors for recurrence, and the course of these events from the time of diagnosis through a three-month follow-up period.
Six hundred and seventy-three patients completed the three-month follow-up. Twenty of them (3%) had recurrent VTE; 14 of these had recurrent PE. Recurrence predominantly transpired in the first three weeks of therapy. Of those with recurrent PE, 11 (79%) were fatal, and most of these occurred within the first week of diagnosis. Major bleeding occurred in 1.5% of the patients. Immobilization for more than three days was a significant risk factor for recurrence. Inpatient status, a diagnosis of COPD, and malignancy were independent risk factors for bleeding complications. Fifty-five patients (8.2%) died over the three-month period. Twenty percent died of fatal recurrent PE, while 4% suffered fatal hemorrhage.
Multivariate analysis revealed four characteristics as independent risk factors for mortality in patients with PE. These include age, inpatient status, immobilization for more than three days, and malignancy. It appears that the majority of recurrent and fatal PE occurs during the first week of therapy. Physicians should not discharge patients to home with LMWH for PE without considering these risk factors for hemorrhage, recurrence, and mortality.
Annals of Internal Medicine has published a systematic review of management issues in VTE to provide the framework for the American College of Physicians practice guidelines. These guidelines pool data from more than 100 randomized controlled trials and comment on six areas in VTE management. The following are quotes from this document.
Recommendation #1: Use low molecular-weight heparin (LMWH) rather than unfractionated heparin whenever possible for the initial inpatient treatment of deep vein thrombosis (DVT). Either unfractionated heparin or LMWH is appropriate for the initial treatment of pulmonary embolism.
Recommendation #2: Outpatient treatment of DVT, and possibly pulmonary embolism, with LMWH is safe and cost-effective for carefully selected patients and should be considered if the required support services are in place.
Recommendation #3: Compression stockings should be used routinely to prevent post-thrombotic syndrome, beginning within one month of diagnosis of proximal DVT and continuing for a minimum of one year after diagnosis.
Recommendation #4: There is insufficient evidence to make specific recommendations for types of anticoagulation management of VTE in pregnant women.
Recommendation #5: Anticoagulation should be maintained for three to six months for VTE secondary to transient risk factors and for more than 12 months for recurrent VTE. While the appropriate duration of anticoagulation for idiopathic or recurrent VTE is not definitively known, there is evidence of substantial benefit for extended-duration therapy.
Recommendation #6: LMWH is safe and efficacious for the long-term treatment of VTE in selected patients (and may be preferable for patients with cancer).
All of these seem reasonable and appropriate with a possible exception in the second recommendation. Using LMWH to treat patients diagnosed with PE in the outpatient setting is not well supported by data. The vast majority of trials involving the treatment of VTE with LMWH have been conducted on those with DVT; the number of patients in the trials with PE has been very small. The Food and Drug Administration has not approved LMWH for outpatient treatment of PE; LMWH is FDA approved in the outpatient setting only for the treatment of DVT. We know that the hemodynamic changes that can accompany PE may not occur for at least 24 hours. In addition, we now have data from the Nijkeuter study that point to dangers that may result from treating PE outside the hospital setting. At this time, we should treat PE with LMWH in the outpatient setting only with patients whose risk factors, clinical characteristics, and outpatient resources have been carefully scrutinized. TH
Treatment of Bacterial Meningitis with Vancomycin
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007 Jan 15;44(2):250-255. Epub 2006 Dec 15.
In 2002, van de Beek and de Gans published a study demonstrating that adjuvant dexamethasone decreased mortality and improved neurological disability when given to patients with bacterial meningitis. Their results changed our treatment paradigm for this disease but left us with several questions. At what point in the treatment course does giving corticosteroids become ineffective? Do their results apply to all bacterial pathogens? Can the results be applied to the use of vancomycin in treating penicillin-resistant strains of Streptococcus pneumoniae? This final question arises from the disturbing ability of vancomycin to penetrate the cerebrospinal fluid (CSF). Previous data support this concern; thus, bactericidal titers may be inadequate within the CSF. Because meningeal inflammation exerts a strong influence over whether or not vancomycin enters the CSF, administering steroids may decrease its ability to do so. This study brings some clarity to the issue.
In this observational open multicenter trial from France, 14 adults were admitted to intensive care units with suspected pneumococcal meningitis. They were treated with intravenous cefotaxime, vancomycin, and dexamethasone. The vancomycin was given as a loading dose of 15 mg per kg of body weight followed by administration of a continuous infusion of 60 mg per kg of body weight per day. The diagnosis of pneumococcal meningitis was made using a CSF pleocytosis as well as one or more of the following: a positive culture from either the blood or CSF, a Gram stain showing Gram-positive diplococci, or pneumococcal antigens in the CSF as demonstrated by latex agglutination. Patients had a second lumbar puncture on either day two or three to measure vancomycin levels—among other markers of disease activity—in the CSF. Serum levels of vancomycin were drawn simultaneously.
Thirteen of the 14 patients had pneumococcal meningitis; one patient was found to have meningitis from Neisseria meningitidis. Seven patients had pneumococcal strains resistant to penicillin. Ten of the 14 patients required mechanical intubation. The second lumbar puncture demonstrated marked improvements in leukocyte counts, protein levels, and glucose levels. All subsequent cultures from the CSF were negative. Three patients died, two had neurological sequelae, and the remainder were discharged from the hospital without complications. Vancomycin concentrations in the serum ranged from 14.2 to 39.0 mg/L, with a mean of 25.2 mg/L; concentrations in the CSF ranged from 3.1 to 22.3 mg/L, with a mean of 7.9 mg/L. There was a significant correlation between vancomycin levels in the serum and those in the CSF (r = 0.68; P = 0.01). The concentration of vancomycin in the CSF was between four and 10 times the mean inhibitory concentrations (MICs). A linear correlation exists between penetration of vancomycin into CSF and serum levels. No evidence of drug toxicities was observed.
The results demonstrate that a therapeutic concentration of vancomycin can be achieved in the CSF. The continuous infusion of vancomycin with a loading dose, which has not been standard practice, has previously been shown to achieve targeted serum levels more quickly than intermittent dosing. Levels of serum vancomycin were likely higher in this study than when troughs of 15-20 mg/L are the goal. This data strongly suggests, however, that this same treatment regimen can obtain adequate vancomycin levels in the CSF while treating pneumococcal meningitis with adjunctive steroids.
Nonspecific elevations in troponins
Alcalai R, Planer D, Culhaoqlu A, et al. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007 Feb 12;167(3):276-281.
In 2000, the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) jointly produced a recommendation for a new definition of myocardial infarction. This proposal based the diagnosis primarily on the elevation of biomarkers specific to cardiac tissue, troponin T and troponin I. Since that time, as use of these blood tests has escalated, it is apparent that elevations in these biomarkers do not always translate into thrombotic coronary artery occlusion. Instead, we have seen that they are positive in a variety of clinical settings. These include sepsis, renal failure, pulmonary embolism, and atrial fibrillation. This investigation attempts to characterize the differences among patients presenting with acute coronary syndrome (ACS) and nonthrombotic troponin elevation (NTTE), to report on outcomes for each, and to note the positive predictive values (PPV) for elevated troponins across clinical settings.
Two hospitals in Israel collected data on all adult patients who experienced an elevation in troponin T (defined as at least 0.1 ng/mL) at any time during their hospital stay. Six hundred and fifteen patients were evaluated by age, sex, cardiovascular risk factors, history of ischemic heart disease, left ventricular function (LVF) by echocardiogram, serum creatine phosphokinase (CPK), and creatinine levels, as well as by which hospital service each had been admitted under. The highest troponin T value was used in the analysis, along with the creatinine level taken on the same day. Two physicians, one a specialist in internal medicine and the other a specialist in cardiology, independently determined the principal diagnosis in accordance with the ACC/ESC guidelines for thrombotic ACS and used other diagnostic studies for alternative diagnosis for conditions known to cause NTTE.
Patients were followed up for causes of mortality for up to two-and-a-half years. Kappa (k) was calculated for physician agreement regarding the principal diagnosis. To assess independent odds ratios and their 95% confidence intervals (CIs) of predictor variables for ACS, an unconditional multiple logistic regression analysis was used. The PPV for troponin T in the diagnosis of ACS was calculated. In-house mortality rates were measured. Long-term risk of death was assessed using Cox proportional hazard models.
The diagnosis of ACS was made in only 53% (326) of the patients. Forty-one percent (254) had NTTE, and the diagnosis was not determined in 6% (35). The diagnoses comprising NTTE included—in order from most to least common—cardiac non-ischemic conditions, sepsis, pulmonary diseases, and neurologic diseases. Using the multivariate analysis, the diagnostic predictors for ACS were history of hypertension or ischemic heart disease, age between 40 and 70 years, higher troponin levels (greater than 1.0 ng/mL), and normal renal function. Extreme age and admission to a surgical team were negative predictors for ACS. Gender, presence of diabetes, and LVF did not appear to make a difference.
The PPV of an elevated troponin T for ACS among all patients was only 56% (95% CI, 52%-60%). It became lower (27%) in those older than 70 with abnormal renal function and higher (90%) in those with a troponin T greater than 1.0 ng/mL and normal renal function. In-house mortality for all patients was 8%; for those with ACS, it was 3%, while for those with NTTE, it was—at 21%—almost eight times higher than the ACS group (P<0.001). Patients were followed up for mortality for a median of 22 months. The long-term mortality was also significantly better (P<0.001) for those with a diagnosis of ACS than for those with NTTE.
Since the incorporation of the ACC/ESC guidelines, the diagnosis of ACS has substantially increased. It is critical to distinguish between ACS and NTTE when using these very sensitive biomarkers, because the underlying cause of NTTE usually requires a drastically different therapy than that of ACS; in addition, misdiagnosing a myocardial infarction may lead to potentially harmful diagnostic studies and therapies in the form of coronary angiography, antithrombotics, and antiplatelet agents. Hospitalists should look for ACS when troponin T levels exceed 1.0 ng/mL in the face of normal renal function. Based on their data, the authors present an algorithm for working up ACS and NTTE that takes into consideration the clinical presentation, age, renal function, electrocardiographic changes, and troponin T levels. Though this is a retrospective trial, it provides guidance for a very common clinical scenario. We should be concerned about a patient’s prognosis when we encounter an elevated troponin in a setting of NTTE.
Guiding Antibiotic Therapy for COPD Exacerbations
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007 Jan;131(1):9-19.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States. Exacerbations of COPD (ECOPD) that require hospitalization are both common and costly. Though recent literature suggests that antibiotic therapy during exacerbations reduces morbidity and mortality and lowers the lack of response to treatment, controversy persists concerning whether or not these results are applicable to all patients with this condition. Procalcitonin is a protein not typically measurable in plasma. Levels of this protein rise with bacterial infections, but appear to be unaffected by inflammation from other etiologies such as autoimmune processes or viral infections. Measuring procalcitonin levels has already been shown to safely decrease the use of antibiotics in lower respiratory infections.
This single-center trial from Switzerland evaluated consecutive patients admitted from the emergency department with ECOPD. For 226 enrolled patients, symptoms were quantified, sputum was collected, spirometry was measured, and procalcitonin levels were evaluated. Attending physicians chose antibiotics, using current guidelines, for patients randomized to the standard therapy group. In the group randomized to procalcitonin guidance, antibiotics were given according to serum levels. No antibiotics were administered for levels below 0.1 micrograms (mcg)/L; antibiotics were encouraged for levels greater than 0.25 mcg/L. For levels between 0.1 and .25 mcg/L, antibiotics were encouraged or discouraged based on the clinical condition of the patient. The primary outcomes evaluated were total antibiotics used during hospitalization and up to six months following hospitalization. Secondary endpoints included clinical and laboratory data and six-month follow-up for exacerbation rate and time to the next ECOPD.
Procalcitonin guidance significantly decreased antibiotic administration compared with the standard-therapy arm (40% versus 72% respectively; P<0.0001) and antibiotic exposure (RR, 0.56; 95% CI, 0.43 to 0.73; P<0.0001). The absolute risk reduction was 31.5% (95% CI, 18.7 to 44.3%; p<0.0001). No difference in the mean time to the next exacerbation was noticed between the two groups. Clinical and laboratory measures at baseline and through the six-month follow-up demonstrated no significant differences.
Using procalcitonin levels to guide antibiotic therapy for ECOPD is a practice that is exciting and full of promise. Not only could costs be cut by omitting antibiotics for this treatment regimen in select patients, but some pressure will be relieved in terms of decreasing emerging bacterial resistance. Because procalcitonin levels have a lab turn-around time of approximately one hour, this test becomes even more attractive: decisions for treatment can be made while patients are still in the emergency department. On a cautionary note, there is more than one method of testing for procalcitonin levels, and this trial was done at only one center. Before widespread use of this test is applied, these results should be validated in a multicenter trial. In addition, one test should be used consistently for measuring procalcitonin levels.
Community-Associated MRSA and MSSA: Clinical and Epidemiologic Characteristics
Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007 Feb 15;44(4):471-482. Epub 2007 Jan 19.
Methicillin-susceptible Staphylococcus aureus (MSSA) was, until very recently, the predominant strain seen in community-associated (CA) S. aureus infections. Now methicillin-resistant S aureus (MRSA) is a concern around the world. Deciding whether or not to treat empirically for MRSA in those patients who do not have risk factors for healthcare-associated (HCA) infections is difficult.
Investigators at the University of California-Los Angeles Medical Center (Torrance) prospectively evaluated consecutive patients admitted to the county hospital with S. aureus infections. Daily cultures of wounds, urine, blood, and sputum were taken. An extensive questionnaire was completed by 280 patients who provided information on exposures, demographic characteristics, and clinical characteristics. CA infections were defined as those not having a positive culture from a surgical site in a patient who, in the past year, had not lived in an extended living facility, had any indwelling devices, visited an infusion center, or received dialysis.
Of those evaluated, 202 patients (78%) had CA S. aureus and 78 (28%) had HCA S. aureus. Of those with the CA infections, 108 (60%) had MRSA and 72 (40%) had MSSA. Sensitivity, specificity, predictive values, and likelihood ratios for the risk factors evaluated were unable to distinguish CA-MRSA from CA-MSSA. For example, the sensitivities for most MRSA risk factors were less than 30%, and all the positive likelihood ratios were lower than three.
This study has very important consequences. Given the data presented, there is currently no way to consistently distinguish between CA-MRSA and CA-MSSA prior to culture results. It would be very reasonable in this population to treat for MRSA empirically. One limitation is that the information comes from a single center in an area that has a very diverse patient population. Also, because this was done at a county hospital, the resources for treating patients who would be cared for in the outpatient arena at other centers might not otherwise be available, thus generalizing this data to potential outpatients. Because the morbidity and mortality from a delay in treatment of MRSA infections is significant, however, it appears sensible to treat CA S. aureus empirically in areas where CA-MRSA is common, regardless of patients’ risk factors.
Venous Thromboembolism Update
King CS, Holley AB, Jackson JL, et al. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007 Feb;131(2):507-516.
Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007 Feb;131(2):517-523.
Segal JB, Streiff MB, Hoffman LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007 Feb 6;146(3):211-222.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146 (3):204-210. Epub 2007 Jan 29.
The prevention and treatment of venous thromboembolism (VTE) is a skill set required for all hospitalists given the prevalence of this condition in hospitalized patients as well as the significant morbidity and mortality associated with the condition. Several articles that help to guide our decisions in managing VTE have been published recently.
We have no randomized controlled trials (RCT) comparing twice-daily (bid) with three-times-daily (tid) dosing of unfractionated heparin (UFH) for the prevention of VTE in medically ill patient populations. It is unlikely that such a study, involving an adequate number of patients, will ever be conducted. Though low molecular weight heparins (LMWH) are used more frequently for VTE prevention, many hospitalists still use UFH to prevent VTE in patients who are morbidly obese or who have profound renal insufficiency. King and colleagues have done a meta-analysis to find out whether or not tid dosing is superior to bid dosing for VTE prevention. Twelve studies, including almost 8,000 patients from 1966 to 2004, were reviewed. All patients were hospitalized for medical rather than surgical conditions.
Tid heparin significantly decreased the incidence of the combined outcome of pulmonary embolism (PE) and proximal deep vein thrombosis (DVT). There was a trend toward significance in decreasing the incidence of PE. There was a significant increase in the number of major bleeds with tid dosing compared with bid dosing. There are many limitations to this study: It is retrospective, the population is extremely heterogeneous, and varying methods have been employed to diagnosis VTE across the many studies from which data were pooled. This is likely the best data we will have for UFH in VTE prevention, however. In summary, tid dosing is preferred for high-risk patients, but bid dosing should be considered for those at risk for bleeding complications.
Data are limited for the clinical course of PE. Outpatient treatment of PE with LMWH is not uncommon in select patients, but choosing who is safe to treat in this arena is uncertain. Nijkeuter and colleagues assessed the incidence of recurrent VTE, hemorrhagic complications from therapy, mortality, risk factors for recurrence, and the course of these events from the time of diagnosis through a three-month follow-up period.
Six hundred and seventy-three patients completed the three-month follow-up. Twenty of them (3%) had recurrent VTE; 14 of these had recurrent PE. Recurrence predominantly transpired in the first three weeks of therapy. Of those with recurrent PE, 11 (79%) were fatal, and most of these occurred within the first week of diagnosis. Major bleeding occurred in 1.5% of the patients. Immobilization for more than three days was a significant risk factor for recurrence. Inpatient status, a diagnosis of COPD, and malignancy were independent risk factors for bleeding complications. Fifty-five patients (8.2%) died over the three-month period. Twenty percent died of fatal recurrent PE, while 4% suffered fatal hemorrhage.
Multivariate analysis revealed four characteristics as independent risk factors for mortality in patients with PE. These include age, inpatient status, immobilization for more than three days, and malignancy. It appears that the majority of recurrent and fatal PE occurs during the first week of therapy. Physicians should not discharge patients to home with LMWH for PE without considering these risk factors for hemorrhage, recurrence, and mortality.
Annals of Internal Medicine has published a systematic review of management issues in VTE to provide the framework for the American College of Physicians practice guidelines. These guidelines pool data from more than 100 randomized controlled trials and comment on six areas in VTE management. The following are quotes from this document.
Recommendation #1: Use low molecular-weight heparin (LMWH) rather than unfractionated heparin whenever possible for the initial inpatient treatment of deep vein thrombosis (DVT). Either unfractionated heparin or LMWH is appropriate for the initial treatment of pulmonary embolism.
Recommendation #2: Outpatient treatment of DVT, and possibly pulmonary embolism, with LMWH is safe and cost-effective for carefully selected patients and should be considered if the required support services are in place.
Recommendation #3: Compression stockings should be used routinely to prevent post-thrombotic syndrome, beginning within one month of diagnosis of proximal DVT and continuing for a minimum of one year after diagnosis.
Recommendation #4: There is insufficient evidence to make specific recommendations for types of anticoagulation management of VTE in pregnant women.
Recommendation #5: Anticoagulation should be maintained for three to six months for VTE secondary to transient risk factors and for more than 12 months for recurrent VTE. While the appropriate duration of anticoagulation for idiopathic or recurrent VTE is not definitively known, there is evidence of substantial benefit for extended-duration therapy.
Recommendation #6: LMWH is safe and efficacious for the long-term treatment of VTE in selected patients (and may be preferable for patients with cancer).
All of these seem reasonable and appropriate with a possible exception in the second recommendation. Using LMWH to treat patients diagnosed with PE in the outpatient setting is not well supported by data. The vast majority of trials involving the treatment of VTE with LMWH have been conducted on those with DVT; the number of patients in the trials with PE has been very small. The Food and Drug Administration has not approved LMWH for outpatient treatment of PE; LMWH is FDA approved in the outpatient setting only for the treatment of DVT. We know that the hemodynamic changes that can accompany PE may not occur for at least 24 hours. In addition, we now have data from the Nijkeuter study that point to dangers that may result from treating PE outside the hospital setting. At this time, we should treat PE with LMWH in the outpatient setting only with patients whose risk factors, clinical characteristics, and outpatient resources have been carefully scrutinized. TH