User login
Mortality, outcomes good in AAA repair in octogenarians
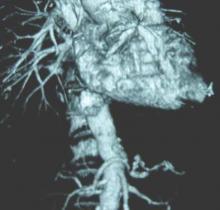
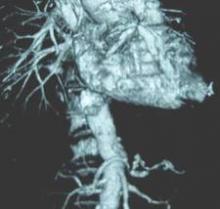
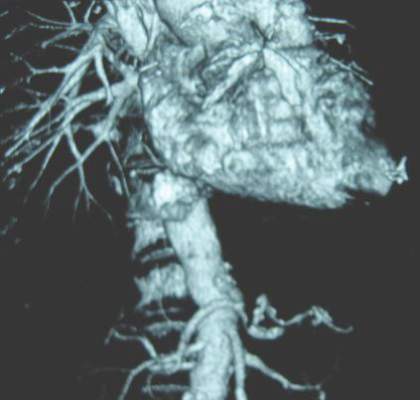
SCOTTSDALE, ARIZ.– Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting.
“However, whether we should be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria; there were nearly seven men for every woman, all with a median age of 83 years. Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation–related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data were warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost-effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within 6 months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
On Twitter @whitneymcknight
This discussion is provocative and raises some interesting points. Obviously cost-effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost-effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost-effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
SCOTTSDALE, ARIZ.– Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting.
“However, whether we should be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria; there were nearly seven men for every woman, all with a median age of 83 years. Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation–related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data were warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost-effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within 6 months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ.– Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting.
“However, whether we should be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria; there were nearly seven men for every woman, all with a median age of 83 years. Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation–related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data were warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost-effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within 6 months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
On Twitter @whitneymcknight
Low mortality, good outcomes in octogenarian AAA repair sparks QOL vs. utility debate
SCOTTSDALE, ARIZ. – Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting. “However, whether should we be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”
Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria, there were nearly seven men for every woman, all with a median age of 83 years.
Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)
Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation-related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data was warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.
Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within six months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
[email protected]
On Twitter @whitneymcknight
This discussion is provocative and raises some interesting points. Obviously cost effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
SCOTTSDALE, ARIZ. – Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting. “However, whether should we be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”
Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria, there were nearly seven men for every woman, all with a median age of 83 years.
Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)
Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation-related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data was warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.
Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within six months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting. “However, whether should we be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”
Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria, there were nearly seven men for every woman, all with a median age of 83 years.
Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)
Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation-related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data was warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.
Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within six months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
[email protected]
On Twitter @whitneymcknight
AT THE SAVS ANNUAL MEETING 2015
Key clinical point: EVAR and OSR outcomes for AAA were both shown safe and effective at 30 days and 6 months in patients 80 years and older.
Major finding: Perioperative mortality in elective and emergent AAA repair was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
Data source: Prospective study of 847 consecutive AAA-repair patients at a single site between May 2005 and February 2014.
Disclosures: Dr. Lamb did not have any relevant disclosures.
Acute renal failure biggest short-term risk in I-EVAR explantation
SCOTTSDALE, ARIZ. – Acute renal failure occurred postoperatively in one-third of patients who underwent endograft explantation after endovascular abdominal aortic aneurysm repair (EVAR), according to the results of a small retrospective study.
The perioperative infected EVAR (I-EVAR) mortality across the study’s 36 patient records (83% male patients, average age 69 years), culled from four surgery centers’ data from 1997 to 2014, was 8%. The overall mortality was 25%, according to Dr. Victor J. Davila of Mayo Clinic Arizona, Phoenix, and his colleagues. Dr. Davila presented the findings at the Southern Association for Vascular Surgery annual meeting.
“These data show that I-EVAR explantation can be performed safely, with acceptable morbidity and mortality,” said Dr. Davila, who noted that while acceptable, the rates were still high, particularly for acute renal failure.
“We did not find any difference between the patients who developed renal failure and the type of graft, whether or not there was suprarenal fixation, and an incidence of postoperative acute renal failure,” Dr. Davila said, “However, because acute renal failure is multifactorial, we need to minimize aortic clamp time, as well as minimize the aortic intimal disruption around the renal arteries.”
Three deaths occurred within 30 days post operation, all from anastomotic dehiscence. Additional short-term morbidities included respiratory failure that required tracheostomy in three patients, and bleeding and sepsis in two patients each. Six patients required re-exploration because of infected hematoma, lymphatic leak, small-bowel perforation, open abdomen at initial operation, and anastomotic bleeding. Six more deaths occurred at a mean follow-up of 402 days. One death was attributable to a ruptured aneurysm, another to a progressive inflammatory illness, and four deaths were of indeterminate cause.
Only three of the explantations reviewed by Dr. Davila and his colleagues were considered emergent. The rest (92%) were either elective or urgent. Infected patients tended to present with leukocytosis (63%), pain (58%), and fever (56%), usually about 65 days prior to explantation. The average time between EVAR and presentation with infection was 589 days.
Although most underwent total graft excision, two patients underwent partial excision, including one with a distal iliac limb infection that showed no sign of infection within the main portion of the endograft. Nearly three-quarters of patients had in situ reconstruction.
While nearly a third of patients had positive preoperative blood cultures indicating infection, 81% of intraoperative cultures taken from the explanted graft, aneurysm wall, or sac contents indicated infection.
The gram-positive Staphylococcus and Streptococcus were the most common organisms found in cultures (33% and 17%, respectively), although anaerobics were found in a third of patients, gram negatives in a quarter of patients, and fungal infections in 14%. A majority (58%) of patients received long-term suppressive antibiotic therapy.
Surgeons should reserve the option to keep a graft in situ only in infected EVAR patients who likely would not survive surgical explantation and reconstruction, Dr. Davila said. “Although I believe [medical management] is an alternative, the best course of action is to remove the endograft.”
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Acute renal failure occurred postoperatively in one-third of patients who underwent endograft explantation after endovascular abdominal aortic aneurysm repair (EVAR), according to the results of a small retrospective study.
The perioperative infected EVAR (I-EVAR) mortality across the study’s 36 patient records (83% male patients, average age 69 years), culled from four surgery centers’ data from 1997 to 2014, was 8%. The overall mortality was 25%, according to Dr. Victor J. Davila of Mayo Clinic Arizona, Phoenix, and his colleagues. Dr. Davila presented the findings at the Southern Association for Vascular Surgery annual meeting.
“These data show that I-EVAR explantation can be performed safely, with acceptable morbidity and mortality,” said Dr. Davila, who noted that while acceptable, the rates were still high, particularly for acute renal failure.
“We did not find any difference between the patients who developed renal failure and the type of graft, whether or not there was suprarenal fixation, and an incidence of postoperative acute renal failure,” Dr. Davila said, “However, because acute renal failure is multifactorial, we need to minimize aortic clamp time, as well as minimize the aortic intimal disruption around the renal arteries.”
Three deaths occurred within 30 days post operation, all from anastomotic dehiscence. Additional short-term morbidities included respiratory failure that required tracheostomy in three patients, and bleeding and sepsis in two patients each. Six patients required re-exploration because of infected hematoma, lymphatic leak, small-bowel perforation, open abdomen at initial operation, and anastomotic bleeding. Six more deaths occurred at a mean follow-up of 402 days. One death was attributable to a ruptured aneurysm, another to a progressive inflammatory illness, and four deaths were of indeterminate cause.
Only three of the explantations reviewed by Dr. Davila and his colleagues were considered emergent. The rest (92%) were either elective or urgent. Infected patients tended to present with leukocytosis (63%), pain (58%), and fever (56%), usually about 65 days prior to explantation. The average time between EVAR and presentation with infection was 589 days.
Although most underwent total graft excision, two patients underwent partial excision, including one with a distal iliac limb infection that showed no sign of infection within the main portion of the endograft. Nearly three-quarters of patients had in situ reconstruction.
While nearly a third of patients had positive preoperative blood cultures indicating infection, 81% of intraoperative cultures taken from the explanted graft, aneurysm wall, or sac contents indicated infection.
The gram-positive Staphylococcus and Streptococcus were the most common organisms found in cultures (33% and 17%, respectively), although anaerobics were found in a third of patients, gram negatives in a quarter of patients, and fungal infections in 14%. A majority (58%) of patients received long-term suppressive antibiotic therapy.
Surgeons should reserve the option to keep a graft in situ only in infected EVAR patients who likely would not survive surgical explantation and reconstruction, Dr. Davila said. “Although I believe [medical management] is an alternative, the best course of action is to remove the endograft.”
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Acute renal failure occurred postoperatively in one-third of patients who underwent endograft explantation after endovascular abdominal aortic aneurysm repair (EVAR), according to the results of a small retrospective study.
The perioperative infected EVAR (I-EVAR) mortality across the study’s 36 patient records (83% male patients, average age 69 years), culled from four surgery centers’ data from 1997 to 2014, was 8%. The overall mortality was 25%, according to Dr. Victor J. Davila of Mayo Clinic Arizona, Phoenix, and his colleagues. Dr. Davila presented the findings at the Southern Association for Vascular Surgery annual meeting.
“These data show that I-EVAR explantation can be performed safely, with acceptable morbidity and mortality,” said Dr. Davila, who noted that while acceptable, the rates were still high, particularly for acute renal failure.
“We did not find any difference between the patients who developed renal failure and the type of graft, whether or not there was suprarenal fixation, and an incidence of postoperative acute renal failure,” Dr. Davila said, “However, because acute renal failure is multifactorial, we need to minimize aortic clamp time, as well as minimize the aortic intimal disruption around the renal arteries.”
Three deaths occurred within 30 days post operation, all from anastomotic dehiscence. Additional short-term morbidities included respiratory failure that required tracheostomy in three patients, and bleeding and sepsis in two patients each. Six patients required re-exploration because of infected hematoma, lymphatic leak, small-bowel perforation, open abdomen at initial operation, and anastomotic bleeding. Six more deaths occurred at a mean follow-up of 402 days. One death was attributable to a ruptured aneurysm, another to a progressive inflammatory illness, and four deaths were of indeterminate cause.
Only three of the explantations reviewed by Dr. Davila and his colleagues were considered emergent. The rest (92%) were either elective or urgent. Infected patients tended to present with leukocytosis (63%), pain (58%), and fever (56%), usually about 65 days prior to explantation. The average time between EVAR and presentation with infection was 589 days.
Although most underwent total graft excision, two patients underwent partial excision, including one with a distal iliac limb infection that showed no sign of infection within the main portion of the endograft. Nearly three-quarters of patients had in situ reconstruction.
While nearly a third of patients had positive preoperative blood cultures indicating infection, 81% of intraoperative cultures taken from the explanted graft, aneurysm wall, or sac contents indicated infection.
The gram-positive Staphylococcus and Streptococcus were the most common organisms found in cultures (33% and 17%, respectively), although anaerobics were found in a third of patients, gram negatives in a quarter of patients, and fungal infections in 14%. A majority (58%) of patients received long-term suppressive antibiotic therapy.
Surgeons should reserve the option to keep a graft in situ only in infected EVAR patients who likely would not survive surgical explantation and reconstruction, Dr. Davila said. “Although I believe [medical management] is an alternative, the best course of action is to remove the endograft.”
On Twitter @whitneymcknight
AT THE SAVS ANNUAL MEETING
Key clinical point: Minimizing cross-clamp time may reduce the rate of acute renal failure 30 days post op in infected EVAR explantation patients.
Major finding: One-third of I-EVAR patients had postoperative acute renal failure; perioperative mortality in I-EVAR was 8%, and overall mortality was 25%.
Data source: Retrospective analysis of 36 patients with infected EVAR explants performed between 1997 and 2014 across four surgical centers.
Disclosures: Dr. Davila reported he had no relevant disclosures.
Meticulous planning, creativity key to management of EVAR infections
CHICAGO – Successful management of infected aortic endovascular grafts requires careful operative planning and execution, meticulous postoperative care, and a fair bit of creativity.
“Each patient is different, so surgeons have to tailor the reconstructions to the individual patient and with these specific infections, have to be creative,” Dr. Thomas C. Bower, chair of vascular and endovascular surgery at Mayo Clinic, Rochester, Minn., said. “I’ve found the operations to be more challenging and more difficult than explanting portions or total graft excision when the infection has occurred in a hand-sewn graft.”
Unlike the typical bimodal distribution seen with hand-sewn graft infections, infection following endovascular repair of aortic aneurysms (EVAR) occurs from days up to 3 years after implantation. At the Mayo Clinic, a 79-year-old man presented with an infected endograft, psoas abscess, and Salmonella septicemia 4 years after EVAR.
“These infections are uncommon, but we are seeing more of them,” Dr. Bower said at a symposium on vascular surgery sponsored by Northwestern University.
Roughly two-thirds of patients will present with fever, nonspecific abdominal or back pain, malaise, weight loss or night sweats. If time permits, preoperative assessments include echocardiography for left ventricular function, arterial blood gases for pulmonary function since many patients are smokers, and renal ultrasound if creatinine is ≥ 1.5 mg/dL after rehydration. These tests are important because preoperative chronic obstructive pulmonary disease and renal dysfunction correlate with worse postoperative outcomes, he said.
Computed tomography angiography (CTA), however, stands as the single most important step of preoperative preparation, with the sine qua non of infection being air around the graft. Unlike hand-sewn grafts where infections can be localized, typically there is total graft involvement in these cases because the device is left inside the aneurysm sac. Aneurysms or pseudoaneurysms also have been seen above the infected device, including at the top end of suprarenal stents.
“This clearly has an impact on how we approach patients, but what’s become very apparent to me is that CTA often underestimates the amount of periaortic inflammation, especially at the juxta- and pararenal locations,” Dr. Bower said.
The Mayo group initially used in situ antibiotic-soaked prosthetic grafts for explanting EVAR devices, which yielded “acceptable mortality and reinfection rates, but primarily outstanding patency rates.” However, cryopreserved aortoiliac grafts have now become their first choice, Dr. Bower said. An ABO match is not imperative, preparation takes roughly 45 minutes, branch closures done in the lab are buttressed with sutures, and the graft is turned over to keep the lumbar arteries anterior, which offers an easy fix if there is bleeding, rather than having it on the posterior wall. Cryopreserved grafts, however, can dilate 40% and lengthen 10% under pressure.
“I’ve been burned more than once where the graft elongates more than I think, and I end up having to cut a small piece out to foreshorten it,” he said.
Reconstructions are tailored to patient anatomy. Surgeons should have several plans for reconstruction, including routing a graft through a remote path, remembering that CTA will underestimate the amount of periaortic inflammation. Separate bypasses of the renal or visceral arteries are performed first before the aortic clamp is applied to reduce physiologic stress. This requires knowledge of the supraceliac and pararenal aorta exposures, which really begins with the correct choice of incisions, Dr. Bower said. This is based on the aortic segment to be treated, position of the new graft, the aortic clamp site, and patient body habitus.
Most patients with EVAR infections are approached with a midline abdominal incision extended along the xiphoid process, which is the lynch pin for allowing upward and lateral retraction of the abdominal wall, he said. Choosing an incision that allows a more vertical orientation to where the new aortic anastomosis and clamp site will be, rather than operating in a keyhole, is important.
The second step is to open up the pararenal space by moving the viscera out of the way. This begins by ligating the inferior mesenteric vein and adjacent lymphatics, which allows incision of an avascular plane along the base of the left transverse colon. Retractor blades are set to allow the upward and lateral retraction of the small bowel, the left colon, and pancreas. Exposure of the suprarenal or supramesenteric aorta requires mobilization of the left renal vein after ligation and division of its branches.
“If that vein is intensely involved in inflammation, don’t ligate the branches in case you have to divide that vein at the caval confluence. Otherwise, you’ll run into some dysfunction of that left kidney,” Dr. Bower cautioned.
To have a secure place for the aortic cross clamp, the crura must be divided on either side of the diaphragm at or above the supramesenteric aorta, he added.
Key steps in total graft explantation are to drain abscesses prior to surgery to lower the bacterial burden and thus reduce the postoperative inflammatory response, bypass renal/visceral arteries first, if needed, remove the infected graft, debride the aorta to healthy tissue, place the new graft and cover it with omentum, and repair the bowel, if needed.
A piece of the proximal aortic wall should be sent to pathology to ensure the absence of bacteria or microabscesses. Organism-specific antibiotics are administered intravenously for 6-8 weeks followed by lifelong oral antibiotics, he said.
An earlier report involving 24 patients with infected aortic endografts (21 EVARs and 3 thoracic EVARs) treated at Mayo Clinic between 1997 and 2012 revealed polymicrobial infection in 11 patients, with methicillin-resistant Staphylococcus aureus being common. Potential contributors to infection were endovascular reintervention in eight, aortoenteric fistula/erosion in four, and various remote infections (J. Vasc. Surg. 2013;58:371-9).
Rifampin-soaked grafts were used in 15 patients, cryopreserved grafts in 4, femoral vein in 2, and axillofemoral grafts in 3. At a median of 14 months follow-up, patient survival, graft-related complications, and reinfection rates were 79%, 13%, and 4%, respectively, Dr. Bower said.
Dr. Bower reported having no financial disclosures.
The expert opinion from the Northwestern Vascular Symposium regarding the management of EVAR infections reminds us of the importance of appropriate patient selection, proper performance of the planned procedure, and long-term follow-up. As EVAR has become the treatment of choice for more than 80% of patients with infrarenal AAAs in the United States, the rate of patients that return with EVAR infections, although rare, is increasing and their management can be more challenging than that of a primary or aortic graft infection as suggested by Dr. Thomas C. Bower in this opinion. The planning for these cases is critical with multiple options for treatment currently available and endorsed by a variety of investigators. From an evaluation standpoint, CTA is critical for diagnosis and case planning. Air around the graft is considered the “sine qua non” of infection but if it presents in the first month after EVAR it can be due to trapped air introduced into the sac during the intervention.

|
Dr. Luis A. Sanchez |
Patients with air in the sac at the initial postprocedure evaluation should be considered for early follow-up to make sure this finding resolves. Further assessment that will change the management of the patient includes the type of EVAR device, infra- or suprarenal, since the entire removal of a suprarenal device usually requires supraceliac cross-clamping with its associated morbidity and mortality. Drainage of the infected cavity, as suggested by Dr. Bower, can help lower the bacterial burden and provide information regarding the offending organism. That information will help the vascular surgeon decide if an in-line reconstruction or an extra-anatomical one is more appropriate in the patient’s situation as more virulent organisms tend to be associated with higher reinfection and complication rates when in-line reconstructions are performed.
The different options for aortic access need to be evaluated based on the anatomy of the patient. A transabdominal approach is best for most patients as it allows access to the iliac arteries bilaterally for removal of the entire graft, debridement of the infected bed, aortic and/or visceral reconstruction, and omental coverage of the in-line graft or aortic stump if an extra-anatomical reconstruction is selected. The retroperitoneal approach should be considered for patients that will require extensive perivisceral work, as may be necessary from suprarenal or fenestrated devices, but limitations exist accessing the right iliac system and potentially intraabdominal targets for visceral or renal reconstructions.
The best configuration to reconstruct these patients remains largely undetermined based on the literature. The published experience from the Mayo Clinic (J. Vasc. Surg. 2013;58:371-9), in which some of the opinions of Dr. Bower are based, suggested excellent results in 24 patients mostly treated with rifampin-soaked in-line reconstructions with a periprocedural mortality of 4%. Cryopreserved aortic grafts “have become the conduit of choice for the group at this time,” stated Dr. Bower, to try to further decrease the reinfection rates in their patient population. There are limited data regarding the use of cryopreserved aortoiliac segments for aortic infections and less for EVAR infections. The most recent and largest series (J. Vasc. Surg. 2014;59:669-74) included 220 patients with aortic infections with a perioperative mortality of 9% and cryopreserved graft complications in another 12%-15% of patients.
In summary, aortic infections associated with EVAR are challenging problems that should be addressed in regional centers with experience. Renal and visceral reconstructions as well as supravisceral clamping are associated with significantly higher periprocedural morbidity and mortality based on the extensive experience at the Cleveland Clinic with EVAR explants (J. Vasc. Surg. 2014;59:886-93). The choice of the reconstruction and the material used should be based on the offending organism, type of EVAR device, extent of the infectious process, and the expertise of the treating physician.
Dr. Luis A. Sanchez is chief, section of vascular surgery and Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Washington University, St. Louis, and an associate medical editor for Vascular Specialist. He had no relevant disclosures.
The expert opinion from the Northwestern Vascular Symposium regarding the management of EVAR infections reminds us of the importance of appropriate patient selection, proper performance of the planned procedure, and long-term follow-up. As EVAR has become the treatment of choice for more than 80% of patients with infrarenal AAAs in the United States, the rate of patients that return with EVAR infections, although rare, is increasing and their management can be more challenging than that of a primary or aortic graft infection as suggested by Dr. Thomas C. Bower in this opinion. The planning for these cases is critical with multiple options for treatment currently available and endorsed by a variety of investigators. From an evaluation standpoint, CTA is critical for diagnosis and case planning. Air around the graft is considered the “sine qua non” of infection but if it presents in the first month after EVAR it can be due to trapped air introduced into the sac during the intervention.

|
Dr. Luis A. Sanchez |
Patients with air in the sac at the initial postprocedure evaluation should be considered for early follow-up to make sure this finding resolves. Further assessment that will change the management of the patient includes the type of EVAR device, infra- or suprarenal, since the entire removal of a suprarenal device usually requires supraceliac cross-clamping with its associated morbidity and mortality. Drainage of the infected cavity, as suggested by Dr. Bower, can help lower the bacterial burden and provide information regarding the offending organism. That information will help the vascular surgeon decide if an in-line reconstruction or an extra-anatomical one is more appropriate in the patient’s situation as more virulent organisms tend to be associated with higher reinfection and complication rates when in-line reconstructions are performed.
The different options for aortic access need to be evaluated based on the anatomy of the patient. A transabdominal approach is best for most patients as it allows access to the iliac arteries bilaterally for removal of the entire graft, debridement of the infected bed, aortic and/or visceral reconstruction, and omental coverage of the in-line graft or aortic stump if an extra-anatomical reconstruction is selected. The retroperitoneal approach should be considered for patients that will require extensive perivisceral work, as may be necessary from suprarenal or fenestrated devices, but limitations exist accessing the right iliac system and potentially intraabdominal targets for visceral or renal reconstructions.
The best configuration to reconstruct these patients remains largely undetermined based on the literature. The published experience from the Mayo Clinic (J. Vasc. Surg. 2013;58:371-9), in which some of the opinions of Dr. Bower are based, suggested excellent results in 24 patients mostly treated with rifampin-soaked in-line reconstructions with a periprocedural mortality of 4%. Cryopreserved aortic grafts “have become the conduit of choice for the group at this time,” stated Dr. Bower, to try to further decrease the reinfection rates in their patient population. There are limited data regarding the use of cryopreserved aortoiliac segments for aortic infections and less for EVAR infections. The most recent and largest series (J. Vasc. Surg. 2014;59:669-74) included 220 patients with aortic infections with a perioperative mortality of 9% and cryopreserved graft complications in another 12%-15% of patients.
In summary, aortic infections associated with EVAR are challenging problems that should be addressed in regional centers with experience. Renal and visceral reconstructions as well as supravisceral clamping are associated with significantly higher periprocedural morbidity and mortality based on the extensive experience at the Cleveland Clinic with EVAR explants (J. Vasc. Surg. 2014;59:886-93). The choice of the reconstruction and the material used should be based on the offending organism, type of EVAR device, extent of the infectious process, and the expertise of the treating physician.
Dr. Luis A. Sanchez is chief, section of vascular surgery and Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Washington University, St. Louis, and an associate medical editor for Vascular Specialist. He had no relevant disclosures.
The expert opinion from the Northwestern Vascular Symposium regarding the management of EVAR infections reminds us of the importance of appropriate patient selection, proper performance of the planned procedure, and long-term follow-up. As EVAR has become the treatment of choice for more than 80% of patients with infrarenal AAAs in the United States, the rate of patients that return with EVAR infections, although rare, is increasing and their management can be more challenging than that of a primary or aortic graft infection as suggested by Dr. Thomas C. Bower in this opinion. The planning for these cases is critical with multiple options for treatment currently available and endorsed by a variety of investigators. From an evaluation standpoint, CTA is critical for diagnosis and case planning. Air around the graft is considered the “sine qua non” of infection but if it presents in the first month after EVAR it can be due to trapped air introduced into the sac during the intervention.

|
Dr. Luis A. Sanchez |
Patients with air in the sac at the initial postprocedure evaluation should be considered for early follow-up to make sure this finding resolves. Further assessment that will change the management of the patient includes the type of EVAR device, infra- or suprarenal, since the entire removal of a suprarenal device usually requires supraceliac cross-clamping with its associated morbidity and mortality. Drainage of the infected cavity, as suggested by Dr. Bower, can help lower the bacterial burden and provide information regarding the offending organism. That information will help the vascular surgeon decide if an in-line reconstruction or an extra-anatomical one is more appropriate in the patient’s situation as more virulent organisms tend to be associated with higher reinfection and complication rates when in-line reconstructions are performed.
The different options for aortic access need to be evaluated based on the anatomy of the patient. A transabdominal approach is best for most patients as it allows access to the iliac arteries bilaterally for removal of the entire graft, debridement of the infected bed, aortic and/or visceral reconstruction, and omental coverage of the in-line graft or aortic stump if an extra-anatomical reconstruction is selected. The retroperitoneal approach should be considered for patients that will require extensive perivisceral work, as may be necessary from suprarenal or fenestrated devices, but limitations exist accessing the right iliac system and potentially intraabdominal targets for visceral or renal reconstructions.
The best configuration to reconstruct these patients remains largely undetermined based on the literature. The published experience from the Mayo Clinic (J. Vasc. Surg. 2013;58:371-9), in which some of the opinions of Dr. Bower are based, suggested excellent results in 24 patients mostly treated with rifampin-soaked in-line reconstructions with a periprocedural mortality of 4%. Cryopreserved aortic grafts “have become the conduit of choice for the group at this time,” stated Dr. Bower, to try to further decrease the reinfection rates in their patient population. There are limited data regarding the use of cryopreserved aortoiliac segments for aortic infections and less for EVAR infections. The most recent and largest series (J. Vasc. Surg. 2014;59:669-74) included 220 patients with aortic infections with a perioperative mortality of 9% and cryopreserved graft complications in another 12%-15% of patients.
In summary, aortic infections associated with EVAR are challenging problems that should be addressed in regional centers with experience. Renal and visceral reconstructions as well as supravisceral clamping are associated with significantly higher periprocedural morbidity and mortality based on the extensive experience at the Cleveland Clinic with EVAR explants (J. Vasc. Surg. 2014;59:886-93). The choice of the reconstruction and the material used should be based on the offending organism, type of EVAR device, extent of the infectious process, and the expertise of the treating physician.
Dr. Luis A. Sanchez is chief, section of vascular surgery and Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Washington University, St. Louis, and an associate medical editor for Vascular Specialist. He had no relevant disclosures.
CHICAGO – Successful management of infected aortic endovascular grafts requires careful operative planning and execution, meticulous postoperative care, and a fair bit of creativity.
“Each patient is different, so surgeons have to tailor the reconstructions to the individual patient and with these specific infections, have to be creative,” Dr. Thomas C. Bower, chair of vascular and endovascular surgery at Mayo Clinic, Rochester, Minn., said. “I’ve found the operations to be more challenging and more difficult than explanting portions or total graft excision when the infection has occurred in a hand-sewn graft.”
Unlike the typical bimodal distribution seen with hand-sewn graft infections, infection following endovascular repair of aortic aneurysms (EVAR) occurs from days up to 3 years after implantation. At the Mayo Clinic, a 79-year-old man presented with an infected endograft, psoas abscess, and Salmonella septicemia 4 years after EVAR.
“These infections are uncommon, but we are seeing more of them,” Dr. Bower said at a symposium on vascular surgery sponsored by Northwestern University.
Roughly two-thirds of patients will present with fever, nonspecific abdominal or back pain, malaise, weight loss or night sweats. If time permits, preoperative assessments include echocardiography for left ventricular function, arterial blood gases for pulmonary function since many patients are smokers, and renal ultrasound if creatinine is ≥ 1.5 mg/dL after rehydration. These tests are important because preoperative chronic obstructive pulmonary disease and renal dysfunction correlate with worse postoperative outcomes, he said.
Computed tomography angiography (CTA), however, stands as the single most important step of preoperative preparation, with the sine qua non of infection being air around the graft. Unlike hand-sewn grafts where infections can be localized, typically there is total graft involvement in these cases because the device is left inside the aneurysm sac. Aneurysms or pseudoaneurysms also have been seen above the infected device, including at the top end of suprarenal stents.
“This clearly has an impact on how we approach patients, but what’s become very apparent to me is that CTA often underestimates the amount of periaortic inflammation, especially at the juxta- and pararenal locations,” Dr. Bower said.
The Mayo group initially used in situ antibiotic-soaked prosthetic grafts for explanting EVAR devices, which yielded “acceptable mortality and reinfection rates, but primarily outstanding patency rates.” However, cryopreserved aortoiliac grafts have now become their first choice, Dr. Bower said. An ABO match is not imperative, preparation takes roughly 45 minutes, branch closures done in the lab are buttressed with sutures, and the graft is turned over to keep the lumbar arteries anterior, which offers an easy fix if there is bleeding, rather than having it on the posterior wall. Cryopreserved grafts, however, can dilate 40% and lengthen 10% under pressure.
“I’ve been burned more than once where the graft elongates more than I think, and I end up having to cut a small piece out to foreshorten it,” he said.
Reconstructions are tailored to patient anatomy. Surgeons should have several plans for reconstruction, including routing a graft through a remote path, remembering that CTA will underestimate the amount of periaortic inflammation. Separate bypasses of the renal or visceral arteries are performed first before the aortic clamp is applied to reduce physiologic stress. This requires knowledge of the supraceliac and pararenal aorta exposures, which really begins with the correct choice of incisions, Dr. Bower said. This is based on the aortic segment to be treated, position of the new graft, the aortic clamp site, and patient body habitus.
Most patients with EVAR infections are approached with a midline abdominal incision extended along the xiphoid process, which is the lynch pin for allowing upward and lateral retraction of the abdominal wall, he said. Choosing an incision that allows a more vertical orientation to where the new aortic anastomosis and clamp site will be, rather than operating in a keyhole, is important.
The second step is to open up the pararenal space by moving the viscera out of the way. This begins by ligating the inferior mesenteric vein and adjacent lymphatics, which allows incision of an avascular plane along the base of the left transverse colon. Retractor blades are set to allow the upward and lateral retraction of the small bowel, the left colon, and pancreas. Exposure of the suprarenal or supramesenteric aorta requires mobilization of the left renal vein after ligation and division of its branches.
“If that vein is intensely involved in inflammation, don’t ligate the branches in case you have to divide that vein at the caval confluence. Otherwise, you’ll run into some dysfunction of that left kidney,” Dr. Bower cautioned.
To have a secure place for the aortic cross clamp, the crura must be divided on either side of the diaphragm at or above the supramesenteric aorta, he added.
Key steps in total graft explantation are to drain abscesses prior to surgery to lower the bacterial burden and thus reduce the postoperative inflammatory response, bypass renal/visceral arteries first, if needed, remove the infected graft, debride the aorta to healthy tissue, place the new graft and cover it with omentum, and repair the bowel, if needed.
A piece of the proximal aortic wall should be sent to pathology to ensure the absence of bacteria or microabscesses. Organism-specific antibiotics are administered intravenously for 6-8 weeks followed by lifelong oral antibiotics, he said.
An earlier report involving 24 patients with infected aortic endografts (21 EVARs and 3 thoracic EVARs) treated at Mayo Clinic between 1997 and 2012 revealed polymicrobial infection in 11 patients, with methicillin-resistant Staphylococcus aureus being common. Potential contributors to infection were endovascular reintervention in eight, aortoenteric fistula/erosion in four, and various remote infections (J. Vasc. Surg. 2013;58:371-9).
Rifampin-soaked grafts were used in 15 patients, cryopreserved grafts in 4, femoral vein in 2, and axillofemoral grafts in 3. At a median of 14 months follow-up, patient survival, graft-related complications, and reinfection rates were 79%, 13%, and 4%, respectively, Dr. Bower said.
Dr. Bower reported having no financial disclosures.
CHICAGO – Successful management of infected aortic endovascular grafts requires careful operative planning and execution, meticulous postoperative care, and a fair bit of creativity.
“Each patient is different, so surgeons have to tailor the reconstructions to the individual patient and with these specific infections, have to be creative,” Dr. Thomas C. Bower, chair of vascular and endovascular surgery at Mayo Clinic, Rochester, Minn., said. “I’ve found the operations to be more challenging and more difficult than explanting portions or total graft excision when the infection has occurred in a hand-sewn graft.”
Unlike the typical bimodal distribution seen with hand-sewn graft infections, infection following endovascular repair of aortic aneurysms (EVAR) occurs from days up to 3 years after implantation. At the Mayo Clinic, a 79-year-old man presented with an infected endograft, psoas abscess, and Salmonella septicemia 4 years after EVAR.
“These infections are uncommon, but we are seeing more of them,” Dr. Bower said at a symposium on vascular surgery sponsored by Northwestern University.
Roughly two-thirds of patients will present with fever, nonspecific abdominal or back pain, malaise, weight loss or night sweats. If time permits, preoperative assessments include echocardiography for left ventricular function, arterial blood gases for pulmonary function since many patients are smokers, and renal ultrasound if creatinine is ≥ 1.5 mg/dL after rehydration. These tests are important because preoperative chronic obstructive pulmonary disease and renal dysfunction correlate with worse postoperative outcomes, he said.
Computed tomography angiography (CTA), however, stands as the single most important step of preoperative preparation, with the sine qua non of infection being air around the graft. Unlike hand-sewn grafts where infections can be localized, typically there is total graft involvement in these cases because the device is left inside the aneurysm sac. Aneurysms or pseudoaneurysms also have been seen above the infected device, including at the top end of suprarenal stents.
“This clearly has an impact on how we approach patients, but what’s become very apparent to me is that CTA often underestimates the amount of periaortic inflammation, especially at the juxta- and pararenal locations,” Dr. Bower said.
The Mayo group initially used in situ antibiotic-soaked prosthetic grafts for explanting EVAR devices, which yielded “acceptable mortality and reinfection rates, but primarily outstanding patency rates.” However, cryopreserved aortoiliac grafts have now become their first choice, Dr. Bower said. An ABO match is not imperative, preparation takes roughly 45 minutes, branch closures done in the lab are buttressed with sutures, and the graft is turned over to keep the lumbar arteries anterior, which offers an easy fix if there is bleeding, rather than having it on the posterior wall. Cryopreserved grafts, however, can dilate 40% and lengthen 10% under pressure.
“I’ve been burned more than once where the graft elongates more than I think, and I end up having to cut a small piece out to foreshorten it,” he said.
Reconstructions are tailored to patient anatomy. Surgeons should have several plans for reconstruction, including routing a graft through a remote path, remembering that CTA will underestimate the amount of periaortic inflammation. Separate bypasses of the renal or visceral arteries are performed first before the aortic clamp is applied to reduce physiologic stress. This requires knowledge of the supraceliac and pararenal aorta exposures, which really begins with the correct choice of incisions, Dr. Bower said. This is based on the aortic segment to be treated, position of the new graft, the aortic clamp site, and patient body habitus.
Most patients with EVAR infections are approached with a midline abdominal incision extended along the xiphoid process, which is the lynch pin for allowing upward and lateral retraction of the abdominal wall, he said. Choosing an incision that allows a more vertical orientation to where the new aortic anastomosis and clamp site will be, rather than operating in a keyhole, is important.
The second step is to open up the pararenal space by moving the viscera out of the way. This begins by ligating the inferior mesenteric vein and adjacent lymphatics, which allows incision of an avascular plane along the base of the left transverse colon. Retractor blades are set to allow the upward and lateral retraction of the small bowel, the left colon, and pancreas. Exposure of the suprarenal or supramesenteric aorta requires mobilization of the left renal vein after ligation and division of its branches.
“If that vein is intensely involved in inflammation, don’t ligate the branches in case you have to divide that vein at the caval confluence. Otherwise, you’ll run into some dysfunction of that left kidney,” Dr. Bower cautioned.
To have a secure place for the aortic cross clamp, the crura must be divided on either side of the diaphragm at or above the supramesenteric aorta, he added.
Key steps in total graft explantation are to drain abscesses prior to surgery to lower the bacterial burden and thus reduce the postoperative inflammatory response, bypass renal/visceral arteries first, if needed, remove the infected graft, debride the aorta to healthy tissue, place the new graft and cover it with omentum, and repair the bowel, if needed.
A piece of the proximal aortic wall should be sent to pathology to ensure the absence of bacteria or microabscesses. Organism-specific antibiotics are administered intravenously for 6-8 weeks followed by lifelong oral antibiotics, he said.
An earlier report involving 24 patients with infected aortic endografts (21 EVARs and 3 thoracic EVARs) treated at Mayo Clinic between 1997 and 2012 revealed polymicrobial infection in 11 patients, with methicillin-resistant Staphylococcus aureus being common. Potential contributors to infection were endovascular reintervention in eight, aortoenteric fistula/erosion in four, and various remote infections (J. Vasc. Surg. 2013;58:371-9).
Rifampin-soaked grafts were used in 15 patients, cryopreserved grafts in 4, femoral vein in 2, and axillofemoral grafts in 3. At a median of 14 months follow-up, patient survival, graft-related complications, and reinfection rates were 79%, 13%, and 4%, respectively, Dr. Bower said.
Dr. Bower reported having no financial disclosures.
EXPERT OPINION FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Clinical follow-up data promising for EVAR in AAA with angulated aortic neck
SCOTTSDALE, ARIZ. – Endovascular abdominal aortic aneurysm repair using a flexible endovascular stent graft in patients with infrarenal aortic neck angles of sixty degrees or greater had more favorable survival and major adverse event rates when compared with open repair, although the difference was not statistically significant, according to clinical, 2-year, postmarketing data.
At this year’s annual Southern Association for Vascular Surgery meeting, Dr. Mahmoud B. Malas presented 2-year safety and efficacy follow-up data from the PYTHAGORAS trial to evaluate the Aorfix (Lombard Medical, U.K.). The device, approved in 2013 by the Food and Drug Administration, is an endovascular stent graft for use in patients whose aortic neck angulation of between 60 and 90 degrees typically has disqualified them from having endovascular aneurysm repair (EVAR) for AAA.
The device is placed within the aneurysm, where it conforms to the individual patient’s anatomy, creating an internal bypass of the aneurysm to reduce the risk of rupture.
“The freedom from major adverse events, despite this hostile neck anatomy, was excellent,” Dr. Malas said of the data.
The PYTHAGORAS study enrolled and treated 151 patients with aortic neck angles of 60 degrees or greater, and 67 patients with necks less than 60 degrees using EVAR. The primary control group consisted of 67 patients undergoing actual open surgical repair (OSR). A secondary control group was a meta-analysis of 323 patients taken from other U.S. EVAR studies (SVS Lifeline).
There were no statistically significant differences between major adverse event rates, nor 30-day and 1-year mortality rates between low- or high-angle EVAR groups when compared with controls. There also was no difference between low- and high-angle EVAR patients sac shrinkage, type I/III endoleaks, and endograft migration, according to Dr. Malas.
The median neck angle in the EVAR group was 71 degrees (standard deviation of ±23 degrees; P < .05), compared with 48 degrees (SD, ± 23 degrees; P < .05) in the OSR control group. There were twice as many women in the EVAR group (35% vs.17%; P < .0001). Patient demographics and comorbidities were similar between the entire EVAR cohort and control group, with the exception of age (76 years vs. 70 years, respectively; P < .05) and heart failure (13% vs. 7%, P = .015). Operative data favored EVAR for procedure duration, blood loss, and hospital length of stay (P < .05 for all).
Dr. Malas said that in the combined EVAR cohort, there was a tendency for the infrarenal area to dilate more rapidly than the suprarenal aorta. “If the neck dilated more than 10%, there was a significant increase in risk of migration and sac expansion, especially close to the renal, but it was not true as you went beyond 7 mm distal to the renal.”
He also noted that the suprarenal aorta does change in association with migration and that there is a “clear association between the degree of oversizing and neck dilation.”
The presentation’s discussant, Dr. Jean M. Panneton, a vascular surgeon at Sentara Heart Hospital in Norfolk, Va., challenged the findings.
“Unfortunately, this trial did suffer from a slow accrual. As a result, only a small proportion of patients have reached the 5-year follow-up, and any subanalysis of such a small study population divided into three groups reduces the n value to the point that a type II error can easily be introduced into your analysis.”
Among the issues he raised was that the mortality data at 30 days, 1 year, and 2 years for the patients with the highest neck angulations could be misleading. “The patients in this group had a threefold increase [in mortality] compared with the standard group. Could this difference have been significant with a larger number?”
To overcome the lack of follow-up time, Dr. Malas said he and his colleagues used statistical modeling that gave them 500 data points on which they based their analysis.
Dr. Panneton also wondered if in the realm of EVAR AAA outside of the study, patients whose aortic neck lengths he said would average between 10 and 15 mm, would enjoy the same success rates as those EVAR patients in the study whose median aortic neck size was 20 mm-25 mm. “Do you think that this long seal zone accounted partially for the performance of the Aorfix? And will this performance hold up in real life?”
Dr. Malas responded that the investigators mandated at least a 15-mm neck for patients in the EVAR arms “because the way the seal zone is in a severely angulated neck means the effective seal zone will be on the inner curve of the neck, which might end up being only 4 or 5 mm, even if you have a 15-mm neck. So, it is very important to get the message out that if you’re going to use the Aorfix in a standard neck – less than 60 degrees – that you have zero migration. If you’re going to place this at a 90-degree angle, it’s very important you do not put it in a patient who doesn’t have a 15-mm neck.”
Dr. Mahmoud was one of the lead site investigators for the PYTHAGORAS trial, sponsored by Lombard Medical.
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Endovascular abdominal aortic aneurysm repair using a flexible endovascular stent graft in patients with infrarenal aortic neck angles of sixty degrees or greater had more favorable survival and major adverse event rates when compared with open repair, although the difference was not statistically significant, according to clinical, 2-year, postmarketing data.
At this year’s annual Southern Association for Vascular Surgery meeting, Dr. Mahmoud B. Malas presented 2-year safety and efficacy follow-up data from the PYTHAGORAS trial to evaluate the Aorfix (Lombard Medical, U.K.). The device, approved in 2013 by the Food and Drug Administration, is an endovascular stent graft for use in patients whose aortic neck angulation of between 60 and 90 degrees typically has disqualified them from having endovascular aneurysm repair (EVAR) for AAA.
The device is placed within the aneurysm, where it conforms to the individual patient’s anatomy, creating an internal bypass of the aneurysm to reduce the risk of rupture.
“The freedom from major adverse events, despite this hostile neck anatomy, was excellent,” Dr. Malas said of the data.
The PYTHAGORAS study enrolled and treated 151 patients with aortic neck angles of 60 degrees or greater, and 67 patients with necks less than 60 degrees using EVAR. The primary control group consisted of 67 patients undergoing actual open surgical repair (OSR). A secondary control group was a meta-analysis of 323 patients taken from other U.S. EVAR studies (SVS Lifeline).
There were no statistically significant differences between major adverse event rates, nor 30-day and 1-year mortality rates between low- or high-angle EVAR groups when compared with controls. There also was no difference between low- and high-angle EVAR patients sac shrinkage, type I/III endoleaks, and endograft migration, according to Dr. Malas.
The median neck angle in the EVAR group was 71 degrees (standard deviation of ±23 degrees; P < .05), compared with 48 degrees (SD, ± 23 degrees; P < .05) in the OSR control group. There were twice as many women in the EVAR group (35% vs.17%; P < .0001). Patient demographics and comorbidities were similar between the entire EVAR cohort and control group, with the exception of age (76 years vs. 70 years, respectively; P < .05) and heart failure (13% vs. 7%, P = .015). Operative data favored EVAR for procedure duration, blood loss, and hospital length of stay (P < .05 for all).
Dr. Malas said that in the combined EVAR cohort, there was a tendency for the infrarenal area to dilate more rapidly than the suprarenal aorta. “If the neck dilated more than 10%, there was a significant increase in risk of migration and sac expansion, especially close to the renal, but it was not true as you went beyond 7 mm distal to the renal.”
He also noted that the suprarenal aorta does change in association with migration and that there is a “clear association between the degree of oversizing and neck dilation.”
The presentation’s discussant, Dr. Jean M. Panneton, a vascular surgeon at Sentara Heart Hospital in Norfolk, Va., challenged the findings.
“Unfortunately, this trial did suffer from a slow accrual. As a result, only a small proportion of patients have reached the 5-year follow-up, and any subanalysis of such a small study population divided into three groups reduces the n value to the point that a type II error can easily be introduced into your analysis.”
Among the issues he raised was that the mortality data at 30 days, 1 year, and 2 years for the patients with the highest neck angulations could be misleading. “The patients in this group had a threefold increase [in mortality] compared with the standard group. Could this difference have been significant with a larger number?”
To overcome the lack of follow-up time, Dr. Malas said he and his colleagues used statistical modeling that gave them 500 data points on which they based their analysis.
Dr. Panneton also wondered if in the realm of EVAR AAA outside of the study, patients whose aortic neck lengths he said would average between 10 and 15 mm, would enjoy the same success rates as those EVAR patients in the study whose median aortic neck size was 20 mm-25 mm. “Do you think that this long seal zone accounted partially for the performance of the Aorfix? And will this performance hold up in real life?”
Dr. Malas responded that the investigators mandated at least a 15-mm neck for patients in the EVAR arms “because the way the seal zone is in a severely angulated neck means the effective seal zone will be on the inner curve of the neck, which might end up being only 4 or 5 mm, even if you have a 15-mm neck. So, it is very important to get the message out that if you’re going to use the Aorfix in a standard neck – less than 60 degrees – that you have zero migration. If you’re going to place this at a 90-degree angle, it’s very important you do not put it in a patient who doesn’t have a 15-mm neck.”
Dr. Mahmoud was one of the lead site investigators for the PYTHAGORAS trial, sponsored by Lombard Medical.
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Endovascular abdominal aortic aneurysm repair using a flexible endovascular stent graft in patients with infrarenal aortic neck angles of sixty degrees or greater had more favorable survival and major adverse event rates when compared with open repair, although the difference was not statistically significant, according to clinical, 2-year, postmarketing data.
At this year’s annual Southern Association for Vascular Surgery meeting, Dr. Mahmoud B. Malas presented 2-year safety and efficacy follow-up data from the PYTHAGORAS trial to evaluate the Aorfix (Lombard Medical, U.K.). The device, approved in 2013 by the Food and Drug Administration, is an endovascular stent graft for use in patients whose aortic neck angulation of between 60 and 90 degrees typically has disqualified them from having endovascular aneurysm repair (EVAR) for AAA.
The device is placed within the aneurysm, where it conforms to the individual patient’s anatomy, creating an internal bypass of the aneurysm to reduce the risk of rupture.
“The freedom from major adverse events, despite this hostile neck anatomy, was excellent,” Dr. Malas said of the data.
The PYTHAGORAS study enrolled and treated 151 patients with aortic neck angles of 60 degrees or greater, and 67 patients with necks less than 60 degrees using EVAR. The primary control group consisted of 67 patients undergoing actual open surgical repair (OSR). A secondary control group was a meta-analysis of 323 patients taken from other U.S. EVAR studies (SVS Lifeline).
There were no statistically significant differences between major adverse event rates, nor 30-day and 1-year mortality rates between low- or high-angle EVAR groups when compared with controls. There also was no difference between low- and high-angle EVAR patients sac shrinkage, type I/III endoleaks, and endograft migration, according to Dr. Malas.
The median neck angle in the EVAR group was 71 degrees (standard deviation of ±23 degrees; P < .05), compared with 48 degrees (SD, ± 23 degrees; P < .05) in the OSR control group. There were twice as many women in the EVAR group (35% vs.17%; P < .0001). Patient demographics and comorbidities were similar between the entire EVAR cohort and control group, with the exception of age (76 years vs. 70 years, respectively; P < .05) and heart failure (13% vs. 7%, P = .015). Operative data favored EVAR for procedure duration, blood loss, and hospital length of stay (P < .05 for all).
Dr. Malas said that in the combined EVAR cohort, there was a tendency for the infrarenal area to dilate more rapidly than the suprarenal aorta. “If the neck dilated more than 10%, there was a significant increase in risk of migration and sac expansion, especially close to the renal, but it was not true as you went beyond 7 mm distal to the renal.”
He also noted that the suprarenal aorta does change in association with migration and that there is a “clear association between the degree of oversizing and neck dilation.”
The presentation’s discussant, Dr. Jean M. Panneton, a vascular surgeon at Sentara Heart Hospital in Norfolk, Va., challenged the findings.
“Unfortunately, this trial did suffer from a slow accrual. As a result, only a small proportion of patients have reached the 5-year follow-up, and any subanalysis of such a small study population divided into three groups reduces the n value to the point that a type II error can easily be introduced into your analysis.”
Among the issues he raised was that the mortality data at 30 days, 1 year, and 2 years for the patients with the highest neck angulations could be misleading. “The patients in this group had a threefold increase [in mortality] compared with the standard group. Could this difference have been significant with a larger number?”
To overcome the lack of follow-up time, Dr. Malas said he and his colleagues used statistical modeling that gave them 500 data points on which they based their analysis.
Dr. Panneton also wondered if in the realm of EVAR AAA outside of the study, patients whose aortic neck lengths he said would average between 10 and 15 mm, would enjoy the same success rates as those EVAR patients in the study whose median aortic neck size was 20 mm-25 mm. “Do you think that this long seal zone accounted partially for the performance of the Aorfix? And will this performance hold up in real life?”
Dr. Malas responded that the investigators mandated at least a 15-mm neck for patients in the EVAR arms “because the way the seal zone is in a severely angulated neck means the effective seal zone will be on the inner curve of the neck, which might end up being only 4 or 5 mm, even if you have a 15-mm neck. So, it is very important to get the message out that if you’re going to use the Aorfix in a standard neck – less than 60 degrees – that you have zero migration. If you’re going to place this at a 90-degree angle, it’s very important you do not put it in a patient who doesn’t have a 15-mm neck.”
Dr. Mahmoud was one of the lead site investigators for the PYTHAGORAS trial, sponsored by Lombard Medical.
On Twitter @whitneymcknight
AT THE SAVS ANNUAL MEETING 2015
Key clinical point: AAA patients with 60 degree or greater aortic neck angles may benefit from EVAR with flexible stent graft instead of open surgical repair.
Major finding: There was no statistical difference in rates of major adverse events between open repair and EVAR in AAA patients with a 60-90 degree aortic neck angulation .
Data source: Postmarketing safety and efficacy data from the controlled, prospective, nonrandomized, multicenter PYTHAGORAS study of 218 patients.
Disclosures: Dr. Mahmoud was one of the lead site investigators for the PYTHAGORAS trial, sponsored by Lombard Medical.
Rethinking the ABCs of EVAR
CHICAGO – Real-world experience with novel endografts like the Ovation Prime abdominal endograft system is prompting some vascular specialists to rethink such central abdominal aortic aneurysm tenets as aortic neck dilation and minimum neck size.
“We started using this in our worst cases, patients with small caliber access vessels and very short aortic necks, to test this device, but over time we’ve pretty much made this our workhorse graft based on our outcomes,” Dr. Syed Hussain of the University of Illinois at Champaign-Urbana, said at a vascular surgery symposium sponsored by Northwestern University.
Among 67 patients with AAAs treated since the team’s first implant in November 2012, the technical success rate is 100%. At baseline, 35% of patients had access vessels < 7 mm, 45% had short aortic neck (< 15 mm), 60% had moderate to severe calcification (> 25% circumferential), and half had moderate to severe thrombus (> 25% circumferential).
The Ovation Prime (TriVascular Technologies) device is relatively quick and easy to put in, with an average procedure time of only 33 minutes, he said. Access was percutaneous in 27%, average blood loss was minimal at 60 mL, and average hospital stay was 1.7 days.
Two patients with severe comorbidities were admitted to the ICU and two patients experienced intraoperative type 1a endoleaks, both successfully treated with a Palmaz stent.
After an average follow-up of 12 months, there have been no type 1, III or IV endoleaks, graft migration, aneurysm enlargement, conversions, ruptures, limb occlusions, or secondary procedures, said Dr. Hussain, who disclosed serving as a consultant for Trivascular and national principal investigator of the PostMarket Ovation Trial. There were 12 type II endoleaks (17%) and all have been clinically irrelevant.
Because of the Ovation’s novel O-ring sealing mechanism, “you get a pretty watertight seal ring on these patients,” he said. More importantly, shear stress is distributed evenly along the entire O-ring, which creates very minimal outward stress on the aorta, “maybe 2 or 3 atmospheres at best.”
Evidence continues to build that self-expandable stents place chronic outward stress on the aorta that causes degeneration of the aortic wall, resulting in eventual aortic neck dilation and endograft migration. While it’s been argued that disease progression leads to aortic dilation, the phenomenon took off after the arrival of endovascular stents, not during decades of open AAA repair, Dr. Hussain, also of the Vein & Vascular Center at the Christie Clinic in Champaign, said.
In the Ovation approval trial, proximal neck dilation at 2 years followed a similar curve in the Ovation and open repair cohorts, compared with those for the more traditional endografts, he noted.
The Ovation Prime system was approved in 2012 and in mid-2014, the Food and Drug Administration approved changes to the indication statement that eliminated the requirement for a minimal aortic neck length.
Essentially, the Ovation device can be placed in any patient if the diameter at 13 mm below the lowest renal artery (the site of the most proximal sealing ring) is within the treatable diameter range of the device (15.8 mm-30.4 mm), Dr. Hussain said.
“The idea of having a neck length is completely starting to go away,” he said. “And even though the trial by Endologix is looking at 1 centimeter as the current requirement for enrolling patients, I think eventually it’s going to get to the point where you’re not going to need a neck for the Nellix device either. You’re going to be able to treat patients who have very short, 1 to 2 millimeter necks, basically perirenal aneurysms, and get a seal on.”
The Nellix endovascular aneurysm sealing system (Endologix) is not commercially available in the U.S., but is the being evaluated in at least three studies. It consists of dual balloon-expandable end-frames surrounded by polymer-filled endobags and is designed to completely fill and seal the aortic aneurysm sac. Anatomical requirements for patients to be enrolled in clinical studies include a nonaneurysmal aortic neck length of ≥ 10 mm, nonaneurysmal aortic neck diameter of 18 mm-32 mm, maximum aortic blood flow lumen diameter of ≤ 60 mm, and common iliac artery diameter of 8 mm-35 mm, according to the company’s website.
CHICAGO – Real-world experience with novel endografts like the Ovation Prime abdominal endograft system is prompting some vascular specialists to rethink such central abdominal aortic aneurysm tenets as aortic neck dilation and minimum neck size.
“We started using this in our worst cases, patients with small caliber access vessels and very short aortic necks, to test this device, but over time we’ve pretty much made this our workhorse graft based on our outcomes,” Dr. Syed Hussain of the University of Illinois at Champaign-Urbana, said at a vascular surgery symposium sponsored by Northwestern University.
Among 67 patients with AAAs treated since the team’s first implant in November 2012, the technical success rate is 100%. At baseline, 35% of patients had access vessels < 7 mm, 45% had short aortic neck (< 15 mm), 60% had moderate to severe calcification (> 25% circumferential), and half had moderate to severe thrombus (> 25% circumferential).
The Ovation Prime (TriVascular Technologies) device is relatively quick and easy to put in, with an average procedure time of only 33 minutes, he said. Access was percutaneous in 27%, average blood loss was minimal at 60 mL, and average hospital stay was 1.7 days.
Two patients with severe comorbidities were admitted to the ICU and two patients experienced intraoperative type 1a endoleaks, both successfully treated with a Palmaz stent.
After an average follow-up of 12 months, there have been no type 1, III or IV endoleaks, graft migration, aneurysm enlargement, conversions, ruptures, limb occlusions, or secondary procedures, said Dr. Hussain, who disclosed serving as a consultant for Trivascular and national principal investigator of the PostMarket Ovation Trial. There were 12 type II endoleaks (17%) and all have been clinically irrelevant.
Because of the Ovation’s novel O-ring sealing mechanism, “you get a pretty watertight seal ring on these patients,” he said. More importantly, shear stress is distributed evenly along the entire O-ring, which creates very minimal outward stress on the aorta, “maybe 2 or 3 atmospheres at best.”
Evidence continues to build that self-expandable stents place chronic outward stress on the aorta that causes degeneration of the aortic wall, resulting in eventual aortic neck dilation and endograft migration. While it’s been argued that disease progression leads to aortic dilation, the phenomenon took off after the arrival of endovascular stents, not during decades of open AAA repair, Dr. Hussain, also of the Vein & Vascular Center at the Christie Clinic in Champaign, said.
In the Ovation approval trial, proximal neck dilation at 2 years followed a similar curve in the Ovation and open repair cohorts, compared with those for the more traditional endografts, he noted.
The Ovation Prime system was approved in 2012 and in mid-2014, the Food and Drug Administration approved changes to the indication statement that eliminated the requirement for a minimal aortic neck length.
Essentially, the Ovation device can be placed in any patient if the diameter at 13 mm below the lowest renal artery (the site of the most proximal sealing ring) is within the treatable diameter range of the device (15.8 mm-30.4 mm), Dr. Hussain said.
“The idea of having a neck length is completely starting to go away,” he said. “And even though the trial by Endologix is looking at 1 centimeter as the current requirement for enrolling patients, I think eventually it’s going to get to the point where you’re not going to need a neck for the Nellix device either. You’re going to be able to treat patients who have very short, 1 to 2 millimeter necks, basically perirenal aneurysms, and get a seal on.”
The Nellix endovascular aneurysm sealing system (Endologix) is not commercially available in the U.S., but is the being evaluated in at least three studies. It consists of dual balloon-expandable end-frames surrounded by polymer-filled endobags and is designed to completely fill and seal the aortic aneurysm sac. Anatomical requirements for patients to be enrolled in clinical studies include a nonaneurysmal aortic neck length of ≥ 10 mm, nonaneurysmal aortic neck diameter of 18 mm-32 mm, maximum aortic blood flow lumen diameter of ≤ 60 mm, and common iliac artery diameter of 8 mm-35 mm, according to the company’s website.
CHICAGO – Real-world experience with novel endografts like the Ovation Prime abdominal endograft system is prompting some vascular specialists to rethink such central abdominal aortic aneurysm tenets as aortic neck dilation and minimum neck size.
“We started using this in our worst cases, patients with small caliber access vessels and very short aortic necks, to test this device, but over time we’ve pretty much made this our workhorse graft based on our outcomes,” Dr. Syed Hussain of the University of Illinois at Champaign-Urbana, said at a vascular surgery symposium sponsored by Northwestern University.
Among 67 patients with AAAs treated since the team’s first implant in November 2012, the technical success rate is 100%. At baseline, 35% of patients had access vessels < 7 mm, 45% had short aortic neck (< 15 mm), 60% had moderate to severe calcification (> 25% circumferential), and half had moderate to severe thrombus (> 25% circumferential).
The Ovation Prime (TriVascular Technologies) device is relatively quick and easy to put in, with an average procedure time of only 33 minutes, he said. Access was percutaneous in 27%, average blood loss was minimal at 60 mL, and average hospital stay was 1.7 days.
Two patients with severe comorbidities were admitted to the ICU and two patients experienced intraoperative type 1a endoleaks, both successfully treated with a Palmaz stent.
After an average follow-up of 12 months, there have been no type 1, III or IV endoleaks, graft migration, aneurysm enlargement, conversions, ruptures, limb occlusions, or secondary procedures, said Dr. Hussain, who disclosed serving as a consultant for Trivascular and national principal investigator of the PostMarket Ovation Trial. There were 12 type II endoleaks (17%) and all have been clinically irrelevant.
Because of the Ovation’s novel O-ring sealing mechanism, “you get a pretty watertight seal ring on these patients,” he said. More importantly, shear stress is distributed evenly along the entire O-ring, which creates very minimal outward stress on the aorta, “maybe 2 or 3 atmospheres at best.”
Evidence continues to build that self-expandable stents place chronic outward stress on the aorta that causes degeneration of the aortic wall, resulting in eventual aortic neck dilation and endograft migration. While it’s been argued that disease progression leads to aortic dilation, the phenomenon took off after the arrival of endovascular stents, not during decades of open AAA repair, Dr. Hussain, also of the Vein & Vascular Center at the Christie Clinic in Champaign, said.
In the Ovation approval trial, proximal neck dilation at 2 years followed a similar curve in the Ovation and open repair cohorts, compared with those for the more traditional endografts, he noted.
The Ovation Prime system was approved in 2012 and in mid-2014, the Food and Drug Administration approved changes to the indication statement that eliminated the requirement for a minimal aortic neck length.
Essentially, the Ovation device can be placed in any patient if the diameter at 13 mm below the lowest renal artery (the site of the most proximal sealing ring) is within the treatable diameter range of the device (15.8 mm-30.4 mm), Dr. Hussain said.
“The idea of having a neck length is completely starting to go away,” he said. “And even though the trial by Endologix is looking at 1 centimeter as the current requirement for enrolling patients, I think eventually it’s going to get to the point where you’re not going to need a neck for the Nellix device either. You’re going to be able to treat patients who have very short, 1 to 2 millimeter necks, basically perirenal aneurysms, and get a seal on.”
The Nellix endovascular aneurysm sealing system (Endologix) is not commercially available in the U.S., but is the being evaluated in at least three studies. It consists of dual balloon-expandable end-frames surrounded by polymer-filled endobags and is designed to completely fill and seal the aortic aneurysm sac. Anatomical requirements for patients to be enrolled in clinical studies include a nonaneurysmal aortic neck length of ≥ 10 mm, nonaneurysmal aortic neck diameter of 18 mm-32 mm, maximum aortic blood flow lumen diameter of ≤ 60 mm, and common iliac artery diameter of 8 mm-35 mm, according to the company’s website.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: Requirement for an specified aortic neck for placement diminishing for new endografts.
Major finding: No type I, III or IV endoleaks, graft migration, aneurysm enlargement, conversions, ruptures, limb occlusions, or secondary procedures occurred after 12 months follow-up.
Data source: Retrospective analysis of 67 patients with AAA treated with Ovation Prime.
Disclosures: Dr. Hussain disclosed serving as a consultant for TriVascular and a national principal investigator for the PostMarket Ovation Trial.
FEVAR radiation injury reexamined
CORONADO, CALIF. – Skin injury following fenestrated endovascular aortic stent grafting is less prevalent than expected, results from a single-center retrospective study showed.
“Radiation-induced skin injury is a serious potential complication of fluoroscopically guided interventions,” Dr. Melissa L. Kirkwood said at the annual meeting of the Western Vascular Society. “These injuries are associated with a threshold radiation dose, above which the severity of injury increases with increasing dose. Instances of these injuries are mostly limited to case reports of coronary interventions, TIPS procedures, and neuroembolizations.”
These radiation-induced skin lesions can be classified as prompt, early, mid-term, or late depending on when they present following the fluoroscopically guided intervention. “The National Cancer Institute has defined four grades of skin injury, with the most frequent being transient erythema, a prompt reaction within the first 24 hours occurring at skin doses as low as 2 Gy,” said Dr. Kirkwood of the division of vascular and endovascular surgery at the University of Texas Southwestern Medical Center, Dallas. “With increasing skin doses, more severe effects present themselves. Atrophy, ulceration, and necrosis are possibilities.”
She went on to note that fenestrated endovascular aneurysm repair often requires high doses of radiation, yet the prevalence of deterministic skin injury following these cases is unknown. In a recent study, Dr. Kirkwood and her associates retrospectively reviewed 61 complex fluoroscopically guided interventions that met substantial radiation dose level (SRDL) criteria, which is defined by the National Council on Radiation and Protection Measurements as a reference air kerma (RAK) greater than or equal to 5 Gy (J. Vasc. Surg. 2014; 60:742-8).
“Despite mean peak skin doses as high as 6.5 Gy, ranging up to 18.5 Gy, we did not detect any skin injuries in this cohort,” Dr. Kirkwood said. “That study, however, was limited by its retrospective design. There was no postoperative protocol in place to ensure that a thorough skin exam was performed on each patient at every follow-up visit. Therefore, we hypothesized that a more thorough postoperative follow-up of patients would detect some skin injury following these cases.”For the current study, she and her associates sought to examine the prevalence of deterministic effects after FEVAR as well as any patient characteristics that may predispose patients to skin injury.
In June 2013, the researchers implemented a new policy regarding the follow-up of FEVAR patients, which involved a full skin exam at postoperative week 2 and 4, and at 3 and 6 months, as well as questioning patients about any skin-related complaints. For the current study, they retrospectively reviewed all FEVARs over a 7-month period after the change in policy and highlighted all the cases that reached a RAK of 5 Gy or greater.
Peak skin dose, a dose index, and simulated skin dose maps were calculated using customized software employing input data from fluoroscopic machine logs. Of 317 cases performed, 22 met or exceeded a RAK of 5 Gy. Of these, 21 were FEVARs and one was an embolization. Dr. Kirkwood reported that the average RAK for all FEVARs was 8 Gy, with a range of 5-11 Gy.
Slightly more than half of patients (52%) had multiple fluoroscopically guided interventions within 6 months of their SRDL event. The average RAK for these patients was 10 Gy (range of 5 - 15). The mean peak skin dose for all FEVARs was 5 Gy (range of 2 - 10 Gy), and the dose index was 0.69. The average peak skin dose for the subset of patients with multiple procedures was 7 Gy (a range of 3 - 9 Gy).
In terms of the follow-up, all 21 FEVAR patients were examined at the 1- or 2-week mark, 81% were examined at 1 month, 52% were examined at 3 months, and 62% were examined at 6 months. No radiation skin injuries were reported. “Based on the published data, we would expect to see all grades of skin injury, especially in the cohort of the 5-10 Gy,” Dr. Kirkwood said.
In the previous study, conducted prior to the new follow-up policy, the dose index for FEVARs was 0.78, “meaning that the peak skin dose that the patient received could be roughly estimated as 78% of the RAK dose displayed on the monitor,” Dr. Kirkwood explained.
“In the current work, the dose index decreased to 60%. This suggests that surgeons in our group have now more appropriately and effectively employed strategies to decrease radiation dose to the patient. However, even when the best operating practice is employed, FEVARs still continue to require high radiation doses in order to complete.”
The present study demonstrated that deterministic skin injuries “are uncommon after FEVAR, even at high RAK levels and regardless of cumulative dose,” she concluded. “Even with more comprehensive patient follow-up, the fact that no skin injuries were reported suggests that skin injuries in this patient cohort are less prevalent than the published guidelines would predict.”
Dr. Kirkwood reported no financial disclosures.
This report is a follow-up of a study by the same group published in the Journal of Vascular Surgery in 2013 (58:715-21) in which they demonstrated that the use of a variety of radiation safety measures including increasing table height, utilizing collimation and angulation, decreasing magnification modes, and maintaining minimal patient-to-detector distance resulted in a 60% reduction in skin dose to their patients when measured as an index of peak skin dose to reference air kerma (PSD/RAK). Unfortunately, skin exposure remained high for FEVAR despite these measures, underscoring the fact that for very complex interventions, even with excellent radiation safety practices, the risk of skin injury remains.
The fact that skin doses as high as 11 Gy did not result in any deterministic injuries is both reassuring and a little surprising. According to the Centers for Disease Control and Prevention, radiation doses of greater than 2 Gy but less than 15 Gy will usually result in erythema within 1-2 days, with a second period of erythema and edema at 2-5 weeks, occasionally resulting in desquamation at 6-7 weeks. Late changes can include mild skin atrophy and some hyperpigmentation. Although complete healing can usually be expected at these doses, squamous skin cancer can still occur, often more than a decade after exposure.

|
Dr. Frank Pomposelli |
So why were no injuries seen? It may be that some were missed since follow-up examinations were not performed in 100% of their patients at any time interval, and it’s not stated whether exams were routinely performed in the first 1-2 days, when I would presume most patients were still hospitalized and the first stage of skin erythema is usually seen. Alternatively, it may be that the surrogate measure of either RAK or the index of PSD/RAK overestimated the true radiation skin dose, which seems highly likely, especially if the time of exposure in any one location was based less on the frequent changes in gantry angle and table position so commonly used in these procedures.
In our hospital, the Massachusetts Department of Public Health regulations require the patient and their physician be notified by letter when the estimated total absorbed radiation dose equals or exceeds 2 Gy. This is based on calculations by our physicist who reviews the details of any case in which the RAK measured equals or exceeds 2 Gy. Like the experiences of the authors, this most commonly occurs with lengthy and complex interventions. In our experience, we have never observed a significant skin injury presumably for the same reason – the exposure in any one location tends to be far less than the total calculated skin dose. Nevertheless, this study should not lull surgeons into a sense of complacency regarding the risk to the patient (and themselves and their staff). As our comfort and expertise with complex interventions increase, it is likely that radiation exposure will continue to increase, placing our patients at increased risk. Understanding the risk of radiation skin injury and how to minimize it is critical for any surgeon performing FEVAR and any other complex intervention utilizing fluoroscopic imaging.
Dr. Frank Pomposelli is an associate professor of surgery at Harvard Medical School. He is also an associate medical editor for Vascular Specialist.
This report is a follow-up of a study by the same group published in the Journal of Vascular Surgery in 2013 (58:715-21) in which they demonstrated that the use of a variety of radiation safety measures including increasing table height, utilizing collimation and angulation, decreasing magnification modes, and maintaining minimal patient-to-detector distance resulted in a 60% reduction in skin dose to their patients when measured as an index of peak skin dose to reference air kerma (PSD/RAK). Unfortunately, skin exposure remained high for FEVAR despite these measures, underscoring the fact that for very complex interventions, even with excellent radiation safety practices, the risk of skin injury remains.
The fact that skin doses as high as 11 Gy did not result in any deterministic injuries is both reassuring and a little surprising. According to the Centers for Disease Control and Prevention, radiation doses of greater than 2 Gy but less than 15 Gy will usually result in erythema within 1-2 days, with a second period of erythema and edema at 2-5 weeks, occasionally resulting in desquamation at 6-7 weeks. Late changes can include mild skin atrophy and some hyperpigmentation. Although complete healing can usually be expected at these doses, squamous skin cancer can still occur, often more than a decade after exposure.

|
Dr. Frank Pomposelli |
So why were no injuries seen? It may be that some were missed since follow-up examinations were not performed in 100% of their patients at any time interval, and it’s not stated whether exams were routinely performed in the first 1-2 days, when I would presume most patients were still hospitalized and the first stage of skin erythema is usually seen. Alternatively, it may be that the surrogate measure of either RAK or the index of PSD/RAK overestimated the true radiation skin dose, which seems highly likely, especially if the time of exposure in any one location was based less on the frequent changes in gantry angle and table position so commonly used in these procedures.
In our hospital, the Massachusetts Department of Public Health regulations require the patient and their physician be notified by letter when the estimated total absorbed radiation dose equals or exceeds 2 Gy. This is based on calculations by our physicist who reviews the details of any case in which the RAK measured equals or exceeds 2 Gy. Like the experiences of the authors, this most commonly occurs with lengthy and complex interventions. In our experience, we have never observed a significant skin injury presumably for the same reason – the exposure in any one location tends to be far less than the total calculated skin dose. Nevertheless, this study should not lull surgeons into a sense of complacency regarding the risk to the patient (and themselves and their staff). As our comfort and expertise with complex interventions increase, it is likely that radiation exposure will continue to increase, placing our patients at increased risk. Understanding the risk of radiation skin injury and how to minimize it is critical for any surgeon performing FEVAR and any other complex intervention utilizing fluoroscopic imaging.
Dr. Frank Pomposelli is an associate professor of surgery at Harvard Medical School. He is also an associate medical editor for Vascular Specialist.
This report is a follow-up of a study by the same group published in the Journal of Vascular Surgery in 2013 (58:715-21) in which they demonstrated that the use of a variety of radiation safety measures including increasing table height, utilizing collimation and angulation, decreasing magnification modes, and maintaining minimal patient-to-detector distance resulted in a 60% reduction in skin dose to their patients when measured as an index of peak skin dose to reference air kerma (PSD/RAK). Unfortunately, skin exposure remained high for FEVAR despite these measures, underscoring the fact that for very complex interventions, even with excellent radiation safety practices, the risk of skin injury remains.
The fact that skin doses as high as 11 Gy did not result in any deterministic injuries is both reassuring and a little surprising. According to the Centers for Disease Control and Prevention, radiation doses of greater than 2 Gy but less than 15 Gy will usually result in erythema within 1-2 days, with a second period of erythema and edema at 2-5 weeks, occasionally resulting in desquamation at 6-7 weeks. Late changes can include mild skin atrophy and some hyperpigmentation. Although complete healing can usually be expected at these doses, squamous skin cancer can still occur, often more than a decade after exposure.

|
Dr. Frank Pomposelli |
So why were no injuries seen? It may be that some were missed since follow-up examinations were not performed in 100% of their patients at any time interval, and it’s not stated whether exams were routinely performed in the first 1-2 days, when I would presume most patients were still hospitalized and the first stage of skin erythema is usually seen. Alternatively, it may be that the surrogate measure of either RAK or the index of PSD/RAK overestimated the true radiation skin dose, which seems highly likely, especially if the time of exposure in any one location was based less on the frequent changes in gantry angle and table position so commonly used in these procedures.
In our hospital, the Massachusetts Department of Public Health regulations require the patient and their physician be notified by letter when the estimated total absorbed radiation dose equals or exceeds 2 Gy. This is based on calculations by our physicist who reviews the details of any case in which the RAK measured equals or exceeds 2 Gy. Like the experiences of the authors, this most commonly occurs with lengthy and complex interventions. In our experience, we have never observed a significant skin injury presumably for the same reason – the exposure in any one location tends to be far less than the total calculated skin dose. Nevertheless, this study should not lull surgeons into a sense of complacency regarding the risk to the patient (and themselves and their staff). As our comfort and expertise with complex interventions increase, it is likely that radiation exposure will continue to increase, placing our patients at increased risk. Understanding the risk of radiation skin injury and how to minimize it is critical for any surgeon performing FEVAR and any other complex intervention utilizing fluoroscopic imaging.
Dr. Frank Pomposelli is an associate professor of surgery at Harvard Medical School. He is also an associate medical editor for Vascular Specialist.
CORONADO, CALIF. – Skin injury following fenestrated endovascular aortic stent grafting is less prevalent than expected, results from a single-center retrospective study showed.
“Radiation-induced skin injury is a serious potential complication of fluoroscopically guided interventions,” Dr. Melissa L. Kirkwood said at the annual meeting of the Western Vascular Society. “These injuries are associated with a threshold radiation dose, above which the severity of injury increases with increasing dose. Instances of these injuries are mostly limited to case reports of coronary interventions, TIPS procedures, and neuroembolizations.”
These radiation-induced skin lesions can be classified as prompt, early, mid-term, or late depending on when they present following the fluoroscopically guided intervention. “The National Cancer Institute has defined four grades of skin injury, with the most frequent being transient erythema, a prompt reaction within the first 24 hours occurring at skin doses as low as 2 Gy,” said Dr. Kirkwood of the division of vascular and endovascular surgery at the University of Texas Southwestern Medical Center, Dallas. “With increasing skin doses, more severe effects present themselves. Atrophy, ulceration, and necrosis are possibilities.”
She went on to note that fenestrated endovascular aneurysm repair often requires high doses of radiation, yet the prevalence of deterministic skin injury following these cases is unknown. In a recent study, Dr. Kirkwood and her associates retrospectively reviewed 61 complex fluoroscopically guided interventions that met substantial radiation dose level (SRDL) criteria, which is defined by the National Council on Radiation and Protection Measurements as a reference air kerma (RAK) greater than or equal to 5 Gy (J. Vasc. Surg. 2014; 60:742-8).
“Despite mean peak skin doses as high as 6.5 Gy, ranging up to 18.5 Gy, we did not detect any skin injuries in this cohort,” Dr. Kirkwood said. “That study, however, was limited by its retrospective design. There was no postoperative protocol in place to ensure that a thorough skin exam was performed on each patient at every follow-up visit. Therefore, we hypothesized that a more thorough postoperative follow-up of patients would detect some skin injury following these cases.”For the current study, she and her associates sought to examine the prevalence of deterministic effects after FEVAR as well as any patient characteristics that may predispose patients to skin injury.
In June 2013, the researchers implemented a new policy regarding the follow-up of FEVAR patients, which involved a full skin exam at postoperative week 2 and 4, and at 3 and 6 months, as well as questioning patients about any skin-related complaints. For the current study, they retrospectively reviewed all FEVARs over a 7-month period after the change in policy and highlighted all the cases that reached a RAK of 5 Gy or greater.
Peak skin dose, a dose index, and simulated skin dose maps were calculated using customized software employing input data from fluoroscopic machine logs. Of 317 cases performed, 22 met or exceeded a RAK of 5 Gy. Of these, 21 were FEVARs and one was an embolization. Dr. Kirkwood reported that the average RAK for all FEVARs was 8 Gy, with a range of 5-11 Gy.
Slightly more than half of patients (52%) had multiple fluoroscopically guided interventions within 6 months of their SRDL event. The average RAK for these patients was 10 Gy (range of 5 - 15). The mean peak skin dose for all FEVARs was 5 Gy (range of 2 - 10 Gy), and the dose index was 0.69. The average peak skin dose for the subset of patients with multiple procedures was 7 Gy (a range of 3 - 9 Gy).
In terms of the follow-up, all 21 FEVAR patients were examined at the 1- or 2-week mark, 81% were examined at 1 month, 52% were examined at 3 months, and 62% were examined at 6 months. No radiation skin injuries were reported. “Based on the published data, we would expect to see all grades of skin injury, especially in the cohort of the 5-10 Gy,” Dr. Kirkwood said.
In the previous study, conducted prior to the new follow-up policy, the dose index for FEVARs was 0.78, “meaning that the peak skin dose that the patient received could be roughly estimated as 78% of the RAK dose displayed on the monitor,” Dr. Kirkwood explained.
“In the current work, the dose index decreased to 60%. This suggests that surgeons in our group have now more appropriately and effectively employed strategies to decrease radiation dose to the patient. However, even when the best operating practice is employed, FEVARs still continue to require high radiation doses in order to complete.”
The present study demonstrated that deterministic skin injuries “are uncommon after FEVAR, even at high RAK levels and regardless of cumulative dose,” she concluded. “Even with more comprehensive patient follow-up, the fact that no skin injuries were reported suggests that skin injuries in this patient cohort are less prevalent than the published guidelines would predict.”
Dr. Kirkwood reported no financial disclosures.
CORONADO, CALIF. – Skin injury following fenestrated endovascular aortic stent grafting is less prevalent than expected, results from a single-center retrospective study showed.
“Radiation-induced skin injury is a serious potential complication of fluoroscopically guided interventions,” Dr. Melissa L. Kirkwood said at the annual meeting of the Western Vascular Society. “These injuries are associated with a threshold radiation dose, above which the severity of injury increases with increasing dose. Instances of these injuries are mostly limited to case reports of coronary interventions, TIPS procedures, and neuroembolizations.”
These radiation-induced skin lesions can be classified as prompt, early, mid-term, or late depending on when they present following the fluoroscopically guided intervention. “The National Cancer Institute has defined four grades of skin injury, with the most frequent being transient erythema, a prompt reaction within the first 24 hours occurring at skin doses as low as 2 Gy,” said Dr. Kirkwood of the division of vascular and endovascular surgery at the University of Texas Southwestern Medical Center, Dallas. “With increasing skin doses, more severe effects present themselves. Atrophy, ulceration, and necrosis are possibilities.”
She went on to note that fenestrated endovascular aneurysm repair often requires high doses of radiation, yet the prevalence of deterministic skin injury following these cases is unknown. In a recent study, Dr. Kirkwood and her associates retrospectively reviewed 61 complex fluoroscopically guided interventions that met substantial radiation dose level (SRDL) criteria, which is defined by the National Council on Radiation and Protection Measurements as a reference air kerma (RAK) greater than or equal to 5 Gy (J. Vasc. Surg. 2014; 60:742-8).
“Despite mean peak skin doses as high as 6.5 Gy, ranging up to 18.5 Gy, we did not detect any skin injuries in this cohort,” Dr. Kirkwood said. “That study, however, was limited by its retrospective design. There was no postoperative protocol in place to ensure that a thorough skin exam was performed on each patient at every follow-up visit. Therefore, we hypothesized that a more thorough postoperative follow-up of patients would detect some skin injury following these cases.”For the current study, she and her associates sought to examine the prevalence of deterministic effects after FEVAR as well as any patient characteristics that may predispose patients to skin injury.
In June 2013, the researchers implemented a new policy regarding the follow-up of FEVAR patients, which involved a full skin exam at postoperative week 2 and 4, and at 3 and 6 months, as well as questioning patients about any skin-related complaints. For the current study, they retrospectively reviewed all FEVARs over a 7-month period after the change in policy and highlighted all the cases that reached a RAK of 5 Gy or greater.
Peak skin dose, a dose index, and simulated skin dose maps were calculated using customized software employing input data from fluoroscopic machine logs. Of 317 cases performed, 22 met or exceeded a RAK of 5 Gy. Of these, 21 were FEVARs and one was an embolization. Dr. Kirkwood reported that the average RAK for all FEVARs was 8 Gy, with a range of 5-11 Gy.
Slightly more than half of patients (52%) had multiple fluoroscopically guided interventions within 6 months of their SRDL event. The average RAK for these patients was 10 Gy (range of 5 - 15). The mean peak skin dose for all FEVARs was 5 Gy (range of 2 - 10 Gy), and the dose index was 0.69. The average peak skin dose for the subset of patients with multiple procedures was 7 Gy (a range of 3 - 9 Gy).
In terms of the follow-up, all 21 FEVAR patients were examined at the 1- or 2-week mark, 81% were examined at 1 month, 52% were examined at 3 months, and 62% were examined at 6 months. No radiation skin injuries were reported. “Based on the published data, we would expect to see all grades of skin injury, especially in the cohort of the 5-10 Gy,” Dr. Kirkwood said.
In the previous study, conducted prior to the new follow-up policy, the dose index for FEVARs was 0.78, “meaning that the peak skin dose that the patient received could be roughly estimated as 78% of the RAK dose displayed on the monitor,” Dr. Kirkwood explained.
“In the current work, the dose index decreased to 60%. This suggests that surgeons in our group have now more appropriately and effectively employed strategies to decrease radiation dose to the patient. However, even when the best operating practice is employed, FEVARs still continue to require high radiation doses in order to complete.”
The present study demonstrated that deterministic skin injuries “are uncommon after FEVAR, even at high RAK levels and regardless of cumulative dose,” she concluded. “Even with more comprehensive patient follow-up, the fact that no skin injuries were reported suggests that skin injuries in this patient cohort are less prevalent than the published guidelines would predict.”
Dr. Kirkwood reported no financial disclosures.
Surgical vs. endovascular repair of popliteal artery aneurysm
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Cigarette smoking rates among U.S. adults hit all-time low
The rate of cigarette smoking among adults in the United States dropped from 20.9% in 2005 to 17.8% in 2013, the lowest it has been since the Centers for Disease Control and Prevention began recording such data in 1965.
The numbers come from the Nov. 28 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2014;63:1108-12), which also states that the percentage of daily smokers who went through 20-29 cigarettes per day dropped from 34.9% in 2005 to 29.3% in 2013. Conversely, the rate of daily smokers who consumed 10 or fewer cigarettes per day increased from 16.4% in 2005 to 23.3% in 2013.
“Though smokers are smoking fewer cigarettes, cutting back by a few cigarettes a day rather than quitting completely does not produce significant health benefits,” Brian King, Ph.D., a senior scientific advisor with the CDC’s Office on Smoking and Health, said in a statement. “Smokers who quit before they’re 40 years old can get back almost all of the 10 years of life expectancy smoking takes away.”
Despite the strides in cutting down overall smoking among American adults, certain demographics continue to struggle. A total of 42.1 million adults remained smokers in 2013. Smoking rates remain especially high among males, younger individuals, those who are multiracial or American Indian/Alaska Native, have less education, live below the federal poverty level, live in the South or Midwest, have a disability or other limitation, and those who are lesbian, gay, or bisexual.
“There is encouraging news in this study, but we still have much more work to do to help people quit,” Dr. Tim McAfee, director of the CDC’s Office on Smoking and Health, noted in the statement. “We can bring down cigarette smoking rates much further, much faster, if strategies proven to work are put in place like funding tobacco control programs at the CDC-recommended levels, increasing prices of tobacco products, implementing and enforcing comprehensive smoke-free laws, and sustaining hard-hitting media campaigns.”
According to the CDC, cigarette smoking is the leading preventable cause of disease and death in the United States, claiming over 480,000 lives annually. Its impact can also be felt economically, with cigarette smoking costing the United States at least $133 billion in direct medical care for adults and more than $156 billion in lost productivity. Meanwhile, the rates of other forms of tobacco consumption, such as cigars and hookahs, are not declining.
Surveys cited by the CDC estimate that 70% of smokers want to quit.
The rate of cigarette smoking among adults in the United States dropped from 20.9% in 2005 to 17.8% in 2013, the lowest it has been since the Centers for Disease Control and Prevention began recording such data in 1965.
The numbers come from the Nov. 28 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2014;63:1108-12), which also states that the percentage of daily smokers who went through 20-29 cigarettes per day dropped from 34.9% in 2005 to 29.3% in 2013. Conversely, the rate of daily smokers who consumed 10 or fewer cigarettes per day increased from 16.4% in 2005 to 23.3% in 2013.
“Though smokers are smoking fewer cigarettes, cutting back by a few cigarettes a day rather than quitting completely does not produce significant health benefits,” Brian King, Ph.D., a senior scientific advisor with the CDC’s Office on Smoking and Health, said in a statement. “Smokers who quit before they’re 40 years old can get back almost all of the 10 years of life expectancy smoking takes away.”
Despite the strides in cutting down overall smoking among American adults, certain demographics continue to struggle. A total of 42.1 million adults remained smokers in 2013. Smoking rates remain especially high among males, younger individuals, those who are multiracial or American Indian/Alaska Native, have less education, live below the federal poverty level, live in the South or Midwest, have a disability or other limitation, and those who are lesbian, gay, or bisexual.
“There is encouraging news in this study, but we still have much more work to do to help people quit,” Dr. Tim McAfee, director of the CDC’s Office on Smoking and Health, noted in the statement. “We can bring down cigarette smoking rates much further, much faster, if strategies proven to work are put in place like funding tobacco control programs at the CDC-recommended levels, increasing prices of tobacco products, implementing and enforcing comprehensive smoke-free laws, and sustaining hard-hitting media campaigns.”
According to the CDC, cigarette smoking is the leading preventable cause of disease and death in the United States, claiming over 480,000 lives annually. Its impact can also be felt economically, with cigarette smoking costing the United States at least $133 billion in direct medical care for adults and more than $156 billion in lost productivity. Meanwhile, the rates of other forms of tobacco consumption, such as cigars and hookahs, are not declining.
Surveys cited by the CDC estimate that 70% of smokers want to quit.
The rate of cigarette smoking among adults in the United States dropped from 20.9% in 2005 to 17.8% in 2013, the lowest it has been since the Centers for Disease Control and Prevention began recording such data in 1965.
The numbers come from the Nov. 28 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2014;63:1108-12), which also states that the percentage of daily smokers who went through 20-29 cigarettes per day dropped from 34.9% in 2005 to 29.3% in 2013. Conversely, the rate of daily smokers who consumed 10 or fewer cigarettes per day increased from 16.4% in 2005 to 23.3% in 2013.
“Though smokers are smoking fewer cigarettes, cutting back by a few cigarettes a day rather than quitting completely does not produce significant health benefits,” Brian King, Ph.D., a senior scientific advisor with the CDC’s Office on Smoking and Health, said in a statement. “Smokers who quit before they’re 40 years old can get back almost all of the 10 years of life expectancy smoking takes away.”
Despite the strides in cutting down overall smoking among American adults, certain demographics continue to struggle. A total of 42.1 million adults remained smokers in 2013. Smoking rates remain especially high among males, younger individuals, those who are multiracial or American Indian/Alaska Native, have less education, live below the federal poverty level, live in the South or Midwest, have a disability or other limitation, and those who are lesbian, gay, or bisexual.
“There is encouraging news in this study, but we still have much more work to do to help people quit,” Dr. Tim McAfee, director of the CDC’s Office on Smoking and Health, noted in the statement. “We can bring down cigarette smoking rates much further, much faster, if strategies proven to work are put in place like funding tobacco control programs at the CDC-recommended levels, increasing prices of tobacco products, implementing and enforcing comprehensive smoke-free laws, and sustaining hard-hitting media campaigns.”
According to the CDC, cigarette smoking is the leading preventable cause of disease and death in the United States, claiming over 480,000 lives annually. Its impact can also be felt economically, with cigarette smoking costing the United States at least $133 billion in direct medical care for adults and more than $156 billion in lost productivity. Meanwhile, the rates of other forms of tobacco consumption, such as cigars and hookahs, are not declining.
Surveys cited by the CDC estimate that 70% of smokers want to quit.
FROM MMWR
Isolated systolic hypertension linked to angina; isolated diastolic hypertension linked to AAA
Isolated high systolic blood pressure is strongly associated with risk for intracerebral hemorrhage, subarachnoid hemorrhage, and stable angina, whereas isolated high diastolic blood pressure is associated with risk for abdominal aortic aneurysm, according to findings published in the Lancet.
The observations debunk widespread assumptions that isolated systolic and diastolic blood pressures are similarly and consistently associated with cardiovascular diseases, wrote Dr. Eleni Rapsomaniki and associates of the Farr Institute of Health Informatics Research in London (Lancet 2014;383:1899-1911).
"Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases," the researchers wrote. These data can be considered when counseling patients.
The results "also emphasize the limitations of existing blood pressure–lowering strategies," they added. Better implementation of existing blood pressure–lowering treatments and better management of other cardiovascular risk factors as part of global risk estimation could help to mitigate excess risk.
The researchers studied 1.25 million patients, aged 30 years or older, who had no previous history of cardiovascular disease. Baseline blood pressures were recorded during primary care consultations. Patients were classified as having hypertension with a baseline blood pressure reading of 140/90 mm Hg or greater, a previous diagnosis of hypertension, or repeat prescriptions for blood pressure–lowering medications.
Isolated systolic hypertension was defined as a value of 140 mm Hg or higher with a diastolic level of lower than 90 mm Hg. Isolated diastolic hypertension was defined as systolic blood pressure of less than 140 mm Hg and a diastolic level of 90 mm Hg or higher.
Endpoints were defined as the initial presentation of any of 12 cardiovascular diseases.
The primary analysis reported associations of outcomes with a 20/10 mm Hg increase in systolic/diastolic blood pressure by age group (30-59 years, 60-79 years, and 80 years or older), and estimated risks and years of life lost associated with hypertension for the index ages of 30, 60, and 80 years. The secondary analysis reported associations of blood pressure with cardiovascular outcomes, after adjusting for smoking status, diabetes, cholesterol, body mass index, and baseline treatment with hypertension drugs.
Isolated systolic hypertension was strongly associated with intracerebral hemorrhage (hazard ratio, 1.44; 95% confidence interval, 1.32-1.58), subarachnoid hemorrhage (HR, 1.43; CI, 1.25-1.63), and stable angina (HR, 1.41; CI, 1.36-1.46).
Isolated diastolic hypertension was associated with abdominal aortic aneurysm (HR, 1.45; 95% CI, 1.34-1.56).
Peripheral arterial disease had the strongest association with pulse pressure (HR, 1.23; CI, 1.20-1.27).
The lifetime risk of total cardiovascular disease at 30 years of age was 63.3% in people with hypertension and 46·1% in those with healthy blood pressure, with stable and unstable angina as the most frequent outcomes. The mean number of cardiovascular disease–free life years lost in those with hypertension was 5 years at 30 years of age, 3.4 years at 60 years, and 1.6 years at 80 years.
The study was funded by the National Institute for Health Research, the Wellcome Trust, the Medical Research Council Prognosis Research Strategy Partnership, and numerous other sources. The researchers had no competing interests.
This study provides important new information to improve risk assessment, patient counseling, and decision making for patients with hypertension.
Factors that can improve drug compliance and treatment persistence to prescribed therapy ought to be better understood than they are at present. Within 2 years, 35% of patients who start antihypertensive drug therapy discontinue treatment. Furthermore, many patients referred for so-called treatment-resistant hypertension do not take their prescribed medication. Additionally, home blood-pressure monitoring and 24-hour ambulatory blood-pressure monitoring would identify patients susceptible to the white coat effect, improve risk stratification, and increase patient engagement.
People with secondary forms of hypertension can often be offered specific treatment and are thus important to identify, in particular those with apparently treatment-resistant disease. Finally, most patients with remaining uncontrolled hypertension can be well controlled when referred to a specialist
The clinical benefit of improved risk assessment and appropriate treatment might be substantial.
Dr. Thomas Kahan is with the Karolinska Institute in Stockholm, Sweden. He made his remarks in an editorial that accompanied the study. Dr. Kahan reported receiving research grants from Celladon, Medtronic, Pfizer, and Servier.
This study provides important new information to improve risk assessment, patient counseling, and decision making for patients with hypertension.
Factors that can improve drug compliance and treatment persistence to prescribed therapy ought to be better understood than they are at present. Within 2 years, 35% of patients who start antihypertensive drug therapy discontinue treatment. Furthermore, many patients referred for so-called treatment-resistant hypertension do not take their prescribed medication. Additionally, home blood-pressure monitoring and 24-hour ambulatory blood-pressure monitoring would identify patients susceptible to the white coat effect, improve risk stratification, and increase patient engagement.
People with secondary forms of hypertension can often be offered specific treatment and are thus important to identify, in particular those with apparently treatment-resistant disease. Finally, most patients with remaining uncontrolled hypertension can be well controlled when referred to a specialist
The clinical benefit of improved risk assessment and appropriate treatment might be substantial.
Dr. Thomas Kahan is with the Karolinska Institute in Stockholm, Sweden. He made his remarks in an editorial that accompanied the study. Dr. Kahan reported receiving research grants from Celladon, Medtronic, Pfizer, and Servier.
This study provides important new information to improve risk assessment, patient counseling, and decision making for patients with hypertension.
Factors that can improve drug compliance and treatment persistence to prescribed therapy ought to be better understood than they are at present. Within 2 years, 35% of patients who start antihypertensive drug therapy discontinue treatment. Furthermore, many patients referred for so-called treatment-resistant hypertension do not take their prescribed medication. Additionally, home blood-pressure monitoring and 24-hour ambulatory blood-pressure monitoring would identify patients susceptible to the white coat effect, improve risk stratification, and increase patient engagement.
People with secondary forms of hypertension can often be offered specific treatment and are thus important to identify, in particular those with apparently treatment-resistant disease. Finally, most patients with remaining uncontrolled hypertension can be well controlled when referred to a specialist
The clinical benefit of improved risk assessment and appropriate treatment might be substantial.
Dr. Thomas Kahan is with the Karolinska Institute in Stockholm, Sweden. He made his remarks in an editorial that accompanied the study. Dr. Kahan reported receiving research grants from Celladon, Medtronic, Pfizer, and Servier.
Isolated high systolic blood pressure is strongly associated with risk for intracerebral hemorrhage, subarachnoid hemorrhage, and stable angina, whereas isolated high diastolic blood pressure is associated with risk for abdominal aortic aneurysm, according to findings published in the Lancet.
The observations debunk widespread assumptions that isolated systolic and diastolic blood pressures are similarly and consistently associated with cardiovascular diseases, wrote Dr. Eleni Rapsomaniki and associates of the Farr Institute of Health Informatics Research in London (Lancet 2014;383:1899-1911).
"Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases," the researchers wrote. These data can be considered when counseling patients.
The results "also emphasize the limitations of existing blood pressure–lowering strategies," they added. Better implementation of existing blood pressure–lowering treatments and better management of other cardiovascular risk factors as part of global risk estimation could help to mitigate excess risk.
The researchers studied 1.25 million patients, aged 30 years or older, who had no previous history of cardiovascular disease. Baseline blood pressures were recorded during primary care consultations. Patients were classified as having hypertension with a baseline blood pressure reading of 140/90 mm Hg or greater, a previous diagnosis of hypertension, or repeat prescriptions for blood pressure–lowering medications.
Isolated systolic hypertension was defined as a value of 140 mm Hg or higher with a diastolic level of lower than 90 mm Hg. Isolated diastolic hypertension was defined as systolic blood pressure of less than 140 mm Hg and a diastolic level of 90 mm Hg or higher.
Endpoints were defined as the initial presentation of any of 12 cardiovascular diseases.
The primary analysis reported associations of outcomes with a 20/10 mm Hg increase in systolic/diastolic blood pressure by age group (30-59 years, 60-79 years, and 80 years or older), and estimated risks and years of life lost associated with hypertension for the index ages of 30, 60, and 80 years. The secondary analysis reported associations of blood pressure with cardiovascular outcomes, after adjusting for smoking status, diabetes, cholesterol, body mass index, and baseline treatment with hypertension drugs.
Isolated systolic hypertension was strongly associated with intracerebral hemorrhage (hazard ratio, 1.44; 95% confidence interval, 1.32-1.58), subarachnoid hemorrhage (HR, 1.43; CI, 1.25-1.63), and stable angina (HR, 1.41; CI, 1.36-1.46).
Isolated diastolic hypertension was associated with abdominal aortic aneurysm (HR, 1.45; 95% CI, 1.34-1.56).
Peripheral arterial disease had the strongest association with pulse pressure (HR, 1.23; CI, 1.20-1.27).
The lifetime risk of total cardiovascular disease at 30 years of age was 63.3% in people with hypertension and 46·1% in those with healthy blood pressure, with stable and unstable angina as the most frequent outcomes. The mean number of cardiovascular disease–free life years lost in those with hypertension was 5 years at 30 years of age, 3.4 years at 60 years, and 1.6 years at 80 years.
The study was funded by the National Institute for Health Research, the Wellcome Trust, the Medical Research Council Prognosis Research Strategy Partnership, and numerous other sources. The researchers had no competing interests.
Isolated high systolic blood pressure is strongly associated with risk for intracerebral hemorrhage, subarachnoid hemorrhage, and stable angina, whereas isolated high diastolic blood pressure is associated with risk for abdominal aortic aneurysm, according to findings published in the Lancet.
The observations debunk widespread assumptions that isolated systolic and diastolic blood pressures are similarly and consistently associated with cardiovascular diseases, wrote Dr. Eleni Rapsomaniki and associates of the Farr Institute of Health Informatics Research in London (Lancet 2014;383:1899-1911).
"Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases," the researchers wrote. These data can be considered when counseling patients.
The results "also emphasize the limitations of existing blood pressure–lowering strategies," they added. Better implementation of existing blood pressure–lowering treatments and better management of other cardiovascular risk factors as part of global risk estimation could help to mitigate excess risk.
The researchers studied 1.25 million patients, aged 30 years or older, who had no previous history of cardiovascular disease. Baseline blood pressures were recorded during primary care consultations. Patients were classified as having hypertension with a baseline blood pressure reading of 140/90 mm Hg or greater, a previous diagnosis of hypertension, or repeat prescriptions for blood pressure–lowering medications.
Isolated systolic hypertension was defined as a value of 140 mm Hg or higher with a diastolic level of lower than 90 mm Hg. Isolated diastolic hypertension was defined as systolic blood pressure of less than 140 mm Hg and a diastolic level of 90 mm Hg or higher.
Endpoints were defined as the initial presentation of any of 12 cardiovascular diseases.
The primary analysis reported associations of outcomes with a 20/10 mm Hg increase in systolic/diastolic blood pressure by age group (30-59 years, 60-79 years, and 80 years or older), and estimated risks and years of life lost associated with hypertension for the index ages of 30, 60, and 80 years. The secondary analysis reported associations of blood pressure with cardiovascular outcomes, after adjusting for smoking status, diabetes, cholesterol, body mass index, and baseline treatment with hypertension drugs.
Isolated systolic hypertension was strongly associated with intracerebral hemorrhage (hazard ratio, 1.44; 95% confidence interval, 1.32-1.58), subarachnoid hemorrhage (HR, 1.43; CI, 1.25-1.63), and stable angina (HR, 1.41; CI, 1.36-1.46).
Isolated diastolic hypertension was associated with abdominal aortic aneurysm (HR, 1.45; 95% CI, 1.34-1.56).
Peripheral arterial disease had the strongest association with pulse pressure (HR, 1.23; CI, 1.20-1.27).
The lifetime risk of total cardiovascular disease at 30 years of age was 63.3% in people with hypertension and 46·1% in those with healthy blood pressure, with stable and unstable angina as the most frequent outcomes. The mean number of cardiovascular disease–free life years lost in those with hypertension was 5 years at 30 years of age, 3.4 years at 60 years, and 1.6 years at 80 years.
The study was funded by the National Institute for Health Research, the Wellcome Trust, the Medical Research Council Prognosis Research Strategy Partnership, and numerous other sources. The researchers had no competing interests.
FROM THE LANCET
Key clinical point: Isolated systolic and diastolic blood pressures are associated with increased risks for different types of cardiovascular disease.
Major finding: High systolic BP was strongly associated with intracerebral hemorrhage (HR, 1.44) and stable angina (HR, 1.41). High diastolic BP was associated with abdominal aortic aneurysm (HR, 1.45).
Data source: A study of the association of blood pressure with 12 cardiovascular diseases in 1.25 million patients in the CALIBER program, 30 years of age and older with no previous diagnosis of CVD.
Disclosures: The study was largely funded by the National Institute for Health Research, the Wellcome Trust, and the Medical Research Council Prognosis Research Strategy Partnership. The researchers declared no competing interests.