User login
FDA extends review period for anticipated Alzheimer’s drug
the drug’s manufacturers have announced. The updated prescription drug user fee act (PDUFA) action date has been pushed forward from March 7 to June 7, 2021.
“As part of the ongoing review, Biogen submitted a response to an information request by the FDA, including additional analyses and clinical data, which the FDA considered a major amendment to the application that will require additional time for review,” Biogen and Eisai said in a statement.
“We are committed to working with the FDA as it completes its review of the aducanumab application. We want to thank the FDA for its continued diligence during the review,” said Biogen CEO Michel Vounatsos.
Biogen submitted the aducanumab application for approval to the FDA in July 2020. The FDA accepted it in August and granted priority review.
Aducanumab is a recombinant human monoclonal antibody targeting beta-amyloid (Abeta). If approved, it would be the first disease-modifying treatment for Alzheimer’s disease.
However, the road to approval has been bumpy. In November, despite high expectations and pleas from patients, caregivers, and advocacy groups, an FDA advisory panel declined to recommend approval of aducanumab.
As previously reported by this news organization, members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee determined that results from Biogen’s one large positive trial did not provide strong enough evidence of efficacy for the treatment of Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
the drug’s manufacturers have announced. The updated prescription drug user fee act (PDUFA) action date has been pushed forward from March 7 to June 7, 2021.
“As part of the ongoing review, Biogen submitted a response to an information request by the FDA, including additional analyses and clinical data, which the FDA considered a major amendment to the application that will require additional time for review,” Biogen and Eisai said in a statement.
“We are committed to working with the FDA as it completes its review of the aducanumab application. We want to thank the FDA for its continued diligence during the review,” said Biogen CEO Michel Vounatsos.
Biogen submitted the aducanumab application for approval to the FDA in July 2020. The FDA accepted it in August and granted priority review.
Aducanumab is a recombinant human monoclonal antibody targeting beta-amyloid (Abeta). If approved, it would be the first disease-modifying treatment for Alzheimer’s disease.
However, the road to approval has been bumpy. In November, despite high expectations and pleas from patients, caregivers, and advocacy groups, an FDA advisory panel declined to recommend approval of aducanumab.
As previously reported by this news organization, members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee determined that results from Biogen’s one large positive trial did not provide strong enough evidence of efficacy for the treatment of Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
the drug’s manufacturers have announced. The updated prescription drug user fee act (PDUFA) action date has been pushed forward from March 7 to June 7, 2021.
“As part of the ongoing review, Biogen submitted a response to an information request by the FDA, including additional analyses and clinical data, which the FDA considered a major amendment to the application that will require additional time for review,” Biogen and Eisai said in a statement.
“We are committed to working with the FDA as it completes its review of the aducanumab application. We want to thank the FDA for its continued diligence during the review,” said Biogen CEO Michel Vounatsos.
Biogen submitted the aducanumab application for approval to the FDA in July 2020. The FDA accepted it in August and granted priority review.
Aducanumab is a recombinant human monoclonal antibody targeting beta-amyloid (Abeta). If approved, it would be the first disease-modifying treatment for Alzheimer’s disease.
However, the road to approval has been bumpy. In November, despite high expectations and pleas from patients, caregivers, and advocacy groups, an FDA advisory panel declined to recommend approval of aducanumab.
As previously reported by this news organization, members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee determined that results from Biogen’s one large positive trial did not provide strong enough evidence of efficacy for the treatment of Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
Cognitive effects seen as transient for Alzheimer’s drug atabecestat
according to follow-up results from a truncated clinical trial.
A blinded, placebo-controlled, manufacturer-sponsored trial that had randomized 557 patients with preclinical Alzheimer’s disease to 25 mg daily oral atabecestat, 5 mg atabecestat, or placebo, was halted in 2018 over concerns about liver toxicity. The main outcome measure of the trial was change on the Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite, while two other scales were used to assess cognitive function and neuropsychological status.
A preliminary analysis found the higher dose of the atabecestat to significantly worsen subjects’ cognition starting at around 3 months of treatment, compared with placebo. Treatment with atabecestat was also seen associated with higher incidence of neuropsychiatric adverse events, including anxiety and depression.
In their follow-up study published Jan. 19, 2021 in JAMA Neurology (doi: 10.1001/jamaneurol.2020.4857), Reisa Sperling, MD, of Brigham and Women’s Hospital, Boston, and colleagues reported that the cognitive worsening and neuropsychiatric adverse effects seen linked to atabecestat treatment reverted to baseline levels within 6 months of halting treatment. Most of the worsening seen in the study was associated with episodic memory tasks, including “list learning, story memory, list recognition, story recall, and figure recall,” Dr. Sperling and colleagues found.
Atabecestat was also associated with “dose-related and duration-related decreases in whole-brain volume, compared with placebo treatment,” the investigators reported. Brain volume loss has been seen in trials of other beta-secretase (BACE) inhibitors and shown with one, umibecestat, to be reversible after stopping treatment.
Dr. Sperling and colleagues acknowledged as a major limitation of their study that just over a third of the cohort received another cognitive composite score after baseline. “The observation that cognitive worsening and neuropsychiatric-related [adverse events] recovered following discontinuation of atabecestat is encouraging but needs replication, given that the observation period after stopping treatment was variable and not preplanned,” the investigators wrote in their analysis. After a median exposure of 21 weeks to the study drug or placebo, subjects were followed off treatment for a median 15 weeks.
Questions surround BACE inhibitors
Development of atabecestat has been discontinued along with others in its class of agents, known as BACE inhibitors, which target an enzyme that initiates production of amyloid-beta, the plaque-forming peptide that is considered a driver of Alzheimer’s disease. In the past few years a number of BACE inhibitors have been shown in trials to worsen cognition in a dose-dependent way, compared with placebo. The reasons for these effects are still unknown.
Dr. Sperling and colleagues concluded that, if BACE investigators like atabecestat are to be studied anew, it must be at low doses, with more modest enzyme inhibition, and alongside careful safety and cognitive monitoring.
While no BACE inhibitor is currently in the pipeline for Alzheimer’s – trials of these agents have been stopped for futility or toxicity –Paul Aisen, MD of the University of Southern California, Los Angeles, and a coauthor of Dr. Sperling and colleagues’ study, commented that it was important that clinical investigation of BACE inhibitors continue.
“This drug class is optimal to correct the metabolic dysregulation that is likely a primary root cause” of Alzheimer’s disease, Dr. Aisen said in an interview. “Evidence from trials such as this suggest that the cognitive toxicity of BACE inhibitors is dose related, nonprogressive, and reversible. We should now focus on establishing the safety of relatively low-dose BACE inhibition so that such regimens can be tested in AD trials.”
Research should continue
Robert Vassar, PhD, of Northwestern University, Chicago, who was not a coauthor on the study, also expressed a desire for BACE inhibitor research to continue.
“It is my view that the cognitive worsening of atabecestat and the other BACE inhibitors was caused by overinhibition of the enzyme related to functions of certain BACE substrates in the brain,” Dr. Vassar commented. “A major question is whether a lower dose of BACE inhibitor – achieving about 30% inhibition – could be safe and lower amyloid-beta enough to delay onset in people still without symptoms. The good news of this study is that the atabecestat-related cognitive worsening is reversible, leaving open the possibility of low-dose prevention trials.”
Dr. Vassar noted that, with both doses of atabecestat, Dr. Sperling and colleagues did not see changes in neurofilament light or total tau, two biomarkers of neurodegeneration, but did report decreases in phosphorylated tau (p181 tau), a marker of disease progression, compared with placebo.
“This indicates that atabecestat did not cause neurodegeneration and in fact moved p181 tau in the beneficial direction for Alzheimer’s disease. Perhaps if it were not for the liver toxicity, the trial may have been completed and other Alzheimer’s disease biomarkers may have changed in the beneficial direction as well,” Dr. Vassar said.
Dr. Sperling and colleagues’ study was sponsored by Janssen, the manufacturer of atabecestat. Dr. Sperling disclosed receiving research funding from Janssen and other drug makers, while nearly all the study’s coauthors reported being directly employed by the sponsor or receiving industry funding. Dr. Aisen disclosed personal fees from several manufacturers and past fees from the sponsor. Dr. Vassar disclosed consulting and other financial relationships with biotechnology companies that did not include this study’s sponsor.
according to follow-up results from a truncated clinical trial.
A blinded, placebo-controlled, manufacturer-sponsored trial that had randomized 557 patients with preclinical Alzheimer’s disease to 25 mg daily oral atabecestat, 5 mg atabecestat, or placebo, was halted in 2018 over concerns about liver toxicity. The main outcome measure of the trial was change on the Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite, while two other scales were used to assess cognitive function and neuropsychological status.
A preliminary analysis found the higher dose of the atabecestat to significantly worsen subjects’ cognition starting at around 3 months of treatment, compared with placebo. Treatment with atabecestat was also seen associated with higher incidence of neuropsychiatric adverse events, including anxiety and depression.
In their follow-up study published Jan. 19, 2021 in JAMA Neurology (doi: 10.1001/jamaneurol.2020.4857), Reisa Sperling, MD, of Brigham and Women’s Hospital, Boston, and colleagues reported that the cognitive worsening and neuropsychiatric adverse effects seen linked to atabecestat treatment reverted to baseline levels within 6 months of halting treatment. Most of the worsening seen in the study was associated with episodic memory tasks, including “list learning, story memory, list recognition, story recall, and figure recall,” Dr. Sperling and colleagues found.
Atabecestat was also associated with “dose-related and duration-related decreases in whole-brain volume, compared with placebo treatment,” the investigators reported. Brain volume loss has been seen in trials of other beta-secretase (BACE) inhibitors and shown with one, umibecestat, to be reversible after stopping treatment.
Dr. Sperling and colleagues acknowledged as a major limitation of their study that just over a third of the cohort received another cognitive composite score after baseline. “The observation that cognitive worsening and neuropsychiatric-related [adverse events] recovered following discontinuation of atabecestat is encouraging but needs replication, given that the observation period after stopping treatment was variable and not preplanned,” the investigators wrote in their analysis. After a median exposure of 21 weeks to the study drug or placebo, subjects were followed off treatment for a median 15 weeks.
Questions surround BACE inhibitors
Development of atabecestat has been discontinued along with others in its class of agents, known as BACE inhibitors, which target an enzyme that initiates production of amyloid-beta, the plaque-forming peptide that is considered a driver of Alzheimer’s disease. In the past few years a number of BACE inhibitors have been shown in trials to worsen cognition in a dose-dependent way, compared with placebo. The reasons for these effects are still unknown.
Dr. Sperling and colleagues concluded that, if BACE investigators like atabecestat are to be studied anew, it must be at low doses, with more modest enzyme inhibition, and alongside careful safety and cognitive monitoring.
While no BACE inhibitor is currently in the pipeline for Alzheimer’s – trials of these agents have been stopped for futility or toxicity –Paul Aisen, MD of the University of Southern California, Los Angeles, and a coauthor of Dr. Sperling and colleagues’ study, commented that it was important that clinical investigation of BACE inhibitors continue.
“This drug class is optimal to correct the metabolic dysregulation that is likely a primary root cause” of Alzheimer’s disease, Dr. Aisen said in an interview. “Evidence from trials such as this suggest that the cognitive toxicity of BACE inhibitors is dose related, nonprogressive, and reversible. We should now focus on establishing the safety of relatively low-dose BACE inhibition so that such regimens can be tested in AD trials.”
Research should continue
Robert Vassar, PhD, of Northwestern University, Chicago, who was not a coauthor on the study, also expressed a desire for BACE inhibitor research to continue.
“It is my view that the cognitive worsening of atabecestat and the other BACE inhibitors was caused by overinhibition of the enzyme related to functions of certain BACE substrates in the brain,” Dr. Vassar commented. “A major question is whether a lower dose of BACE inhibitor – achieving about 30% inhibition – could be safe and lower amyloid-beta enough to delay onset in people still without symptoms. The good news of this study is that the atabecestat-related cognitive worsening is reversible, leaving open the possibility of low-dose prevention trials.”
Dr. Vassar noted that, with both doses of atabecestat, Dr. Sperling and colleagues did not see changes in neurofilament light or total tau, two biomarkers of neurodegeneration, but did report decreases in phosphorylated tau (p181 tau), a marker of disease progression, compared with placebo.
“This indicates that atabecestat did not cause neurodegeneration and in fact moved p181 tau in the beneficial direction for Alzheimer’s disease. Perhaps if it were not for the liver toxicity, the trial may have been completed and other Alzheimer’s disease biomarkers may have changed in the beneficial direction as well,” Dr. Vassar said.
Dr. Sperling and colleagues’ study was sponsored by Janssen, the manufacturer of atabecestat. Dr. Sperling disclosed receiving research funding from Janssen and other drug makers, while nearly all the study’s coauthors reported being directly employed by the sponsor or receiving industry funding. Dr. Aisen disclosed personal fees from several manufacturers and past fees from the sponsor. Dr. Vassar disclosed consulting and other financial relationships with biotechnology companies that did not include this study’s sponsor.
according to follow-up results from a truncated clinical trial.
A blinded, placebo-controlled, manufacturer-sponsored trial that had randomized 557 patients with preclinical Alzheimer’s disease to 25 mg daily oral atabecestat, 5 mg atabecestat, or placebo, was halted in 2018 over concerns about liver toxicity. The main outcome measure of the trial was change on the Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite, while two other scales were used to assess cognitive function and neuropsychological status.
A preliminary analysis found the higher dose of the atabecestat to significantly worsen subjects’ cognition starting at around 3 months of treatment, compared with placebo. Treatment with atabecestat was also seen associated with higher incidence of neuropsychiatric adverse events, including anxiety and depression.
In their follow-up study published Jan. 19, 2021 in JAMA Neurology (doi: 10.1001/jamaneurol.2020.4857), Reisa Sperling, MD, of Brigham and Women’s Hospital, Boston, and colleagues reported that the cognitive worsening and neuropsychiatric adverse effects seen linked to atabecestat treatment reverted to baseline levels within 6 months of halting treatment. Most of the worsening seen in the study was associated with episodic memory tasks, including “list learning, story memory, list recognition, story recall, and figure recall,” Dr. Sperling and colleagues found.
Atabecestat was also associated with “dose-related and duration-related decreases in whole-brain volume, compared with placebo treatment,” the investigators reported. Brain volume loss has been seen in trials of other beta-secretase (BACE) inhibitors and shown with one, umibecestat, to be reversible after stopping treatment.
Dr. Sperling and colleagues acknowledged as a major limitation of their study that just over a third of the cohort received another cognitive composite score after baseline. “The observation that cognitive worsening and neuropsychiatric-related [adverse events] recovered following discontinuation of atabecestat is encouraging but needs replication, given that the observation period after stopping treatment was variable and not preplanned,” the investigators wrote in their analysis. After a median exposure of 21 weeks to the study drug or placebo, subjects were followed off treatment for a median 15 weeks.
Questions surround BACE inhibitors
Development of atabecestat has been discontinued along with others in its class of agents, known as BACE inhibitors, which target an enzyme that initiates production of amyloid-beta, the plaque-forming peptide that is considered a driver of Alzheimer’s disease. In the past few years a number of BACE inhibitors have been shown in trials to worsen cognition in a dose-dependent way, compared with placebo. The reasons for these effects are still unknown.
Dr. Sperling and colleagues concluded that, if BACE investigators like atabecestat are to be studied anew, it must be at low doses, with more modest enzyme inhibition, and alongside careful safety and cognitive monitoring.
While no BACE inhibitor is currently in the pipeline for Alzheimer’s – trials of these agents have been stopped for futility or toxicity –Paul Aisen, MD of the University of Southern California, Los Angeles, and a coauthor of Dr. Sperling and colleagues’ study, commented that it was important that clinical investigation of BACE inhibitors continue.
“This drug class is optimal to correct the metabolic dysregulation that is likely a primary root cause” of Alzheimer’s disease, Dr. Aisen said in an interview. “Evidence from trials such as this suggest that the cognitive toxicity of BACE inhibitors is dose related, nonprogressive, and reversible. We should now focus on establishing the safety of relatively low-dose BACE inhibition so that such regimens can be tested in AD trials.”
Research should continue
Robert Vassar, PhD, of Northwestern University, Chicago, who was not a coauthor on the study, also expressed a desire for BACE inhibitor research to continue.
“It is my view that the cognitive worsening of atabecestat and the other BACE inhibitors was caused by overinhibition of the enzyme related to functions of certain BACE substrates in the brain,” Dr. Vassar commented. “A major question is whether a lower dose of BACE inhibitor – achieving about 30% inhibition – could be safe and lower amyloid-beta enough to delay onset in people still without symptoms. The good news of this study is that the atabecestat-related cognitive worsening is reversible, leaving open the possibility of low-dose prevention trials.”
Dr. Vassar noted that, with both doses of atabecestat, Dr. Sperling and colleagues did not see changes in neurofilament light or total tau, two biomarkers of neurodegeneration, but did report decreases in phosphorylated tau (p181 tau), a marker of disease progression, compared with placebo.
“This indicates that atabecestat did not cause neurodegeneration and in fact moved p181 tau in the beneficial direction for Alzheimer’s disease. Perhaps if it were not for the liver toxicity, the trial may have been completed and other Alzheimer’s disease biomarkers may have changed in the beneficial direction as well,” Dr. Vassar said.
Dr. Sperling and colleagues’ study was sponsored by Janssen, the manufacturer of atabecestat. Dr. Sperling disclosed receiving research funding from Janssen and other drug makers, while nearly all the study’s coauthors reported being directly employed by the sponsor or receiving industry funding. Dr. Aisen disclosed personal fees from several manufacturers and past fees from the sponsor. Dr. Vassar disclosed consulting and other financial relationships with biotechnology companies that did not include this study’s sponsor.
FROM JAMA NEUROLOGY
Afternoon napping associated with better cognition in elderly, study shows
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
Blood biomarker may predict Alzheimer’s disease progression
new research suggests.
In a study of more than 1,000 participants, changes over time in levels of p-tau181 were associated with prospective neurodegeneration and cognitive decline characteristic of Alzheimer’s disease. These results have implications for investigative trials as well as clinical practice, the investigators noted.
Like p-tau181, neurofilament light chain (NfL) is associated with imaging markers of degeneration and cognitive decline; in contrast to the findings related to p-tau181, however, the associations between NfL and these outcomes are not specific to Alzheimer’s disease. Using both biomarkers could improve prediction of outcomes and patient monitoring, according to the researchers.
“These findings demonstrate that p-tau181 and NfL in blood have individual and complementary potential roles in the diagnosis and the monitoring of neurodegenerative disease,” said coinvestigator Michael Schöll, PhD, senior lecturer in psychiatry and neurochemistry at the University of Gothenburg (Sweden).
“With the reservation that we did not assess domain-specific cognitive impairment, p-tau181 was also more strongly associated with cognitive decline than was NfL,” Dr. Schöll added.
The findings were published online Jan. 11 in JAMA Neurology.
Biomarker-tracked neurodegeneration
Monitoring a patient’s neurodegenerative changes is important for tracking Alzheimer’s disease progression. Although clinicians can detect amyloid-beta and tau pathology using PET and cerebrospinal fluid (CSF) biomarkers, the widespread use of the latter has been hampered by cost and limited availability of necessary equipment. The use of blood-based biomarkers is not limited in these ways, and so they could aid in diagnosis and patient monitoring.
Previous studies have suggested that p-tau181 is a marker of Alzheimer’s disease status.
In the current study, investigators examined whether baseline and longitudinal levels of p-tau181 in plasma were associated with progressive neurodegeneration related to the disease. They analyzed data from the Alzheimer’s Disease Neuroimaging Initiative, a multicenter study designed to identify biomarkers for the detection and tracking of Alzheimer’s disease.
The researchers selected data for cognitively unimpaired and cognitively impaired participants who participated in the initiative between Feb. 1, 2007, and June 6, 2016. Participants were eligible for inclusion if plasma p-tau181 and NfL data were available for them and if they had undergone at least one 18fluorodeoxyglucose (FDG)–PET scan or structural T1 MRI at the same study visit. Most had also undergone imaging with 18florbetapir, which detects amyloid-beta.
A single-molecule array was used to analyze concentrations of p-tau181 and NfL in participants’ blood samples. Outliers for p-tau181 and NfL concentrations were excluded from further analysis. Using participants’ FDG-PET scans, the investigators measured glucose hypometabolism characteristic of Alzheimer’s disease. They used T1-weighted MRI scans to measure gray-matter volume.
Cognitively unimpaired participants responded to the Preclinical Alzheimer Cognitive Composite, a measure designed to detect early cognitive changes in cognitively normal patients with Alzheimer’s disease pathology. Cognitively impaired participants underwent the Alzheimer Disease Assessment Scale–Cognitive Subscale with 13 tasks to assess the severity of cognitive impairment.
The researchers included 1,113 participants (54% men; 89% non-Hispanic Whites; mean age, 74 years) in their analysis. In all, 378 participants were cognitively unimpaired, and 735 were cognitively impaired. Of the latter group, 73% had mild cognitive impairment, and 27% had Alzheimer’s disease dementia.
Atrophy predictor
Results showed that higher plasma p-tau181 levels at baseline were associated with more rapid progression of hypometabolism and atrophy in areas vulnerable to Alzheimer’s disease among cognitively impaired participants (FDG-PET standardized uptake value ratio change, r = –0.28; P < .001; gray-matter volume change, r = –0.28; P < .001).
The association with atrophy progression in cognitively impaired participants was stronger for p-tau181 than for NfL.
Plasma p-tau181 levels at baseline also predicted atrophy in temporoparietal regions vulnerable to Alzheimer’s disease among cognitively unimpaired participants (r = –0.11; P = .03). NfL, however, was associated with progressive atrophy in frontal regions among cognitively unimpaired participants.
At baseline, plasma p-tau181 levels were associated with prospective cognitive decline in both the cognitively unimpaired group (r = −0.12; P = .04) and the cognitively impaired group (r = 0.35; P < .001). However, plasma NfL was linked to cognitive decline only among those who were cognitively impaired (r = 0.26; P < .001).
Additional analyses showed that p-tau181, unlike NfL, was associated with hypometabolism and atrophy only in participants with amyloid-beta, regardless of cognitive status.
Between 25% and 45% of the association between baseline p-tau181 level and cognitive decline was mediated by baseline imaging markers of neurodegeneration. This finding suggests that another factor, such as regional tau pathology, might have an independent and direct effect on cognition, Dr. Schöll noted.
Furthermore, changes over time in p-tau181 levels were associated with cognitive decline in the cognitively unimpaired (r = –0.24; P < .001) and cognitively impaired (r = 0.34; P < .001) participants. Longitudinal changes in this biomarker also were associated with a prospective decrease in glucose metabolism in cognitively unimpaired (r = –0.05; P = .48) and cognitively impaired (r = –0.27; P < .001) participants, but the association was only significant in the latter group.
Changes over time in p-tau181 levels were linked to prospective decreases in gray-matter volume in brain regions highly characteristic of Alzheimer’s disease in those who were cognitively unimpaired (r = –0.19; P < .001) and those who were cognitively impaired (r = –0.31, P < .001). However, these associations were obtained only in patients with amyloid-beta.
Dr. Schöll noted that blood-based biomarkers that are sensitive to Alzheimer’s disease could greatly expand patients’ access to a diagnostic workup and could improve screening for clinical trials.
“While the final validation of the existence and the monitoring of potential changes of neuropathology in vivo is likely to be conducted using neuroimaging modalities such as PET, our results suggest that at least a part of these examinations could be replaced by regular blood tests,” Dr. Schöll said.
Lead author Alexis Moscoso, PhD, a postdoctoral researcher in psychiatry and neurochemistry at the University of Gothenburg, reported that the researchers will continue validating blood-based biomarkers, especially against established and well-validated neuroimaging methods. “We are also hoping to be able to compare existing and novel blood-based Alzheimer’s disease biomarkers head to head to establish the individual roles each of these play in the research and diagnosis of Alzheimer’s disease,” Dr. Moscoso said.
‘Outstanding study’
Commenting on the findings, David S. Knopman, MD, professor of neurology at Mayo Clinic, Rochester, Minn., said that this is “an outstanding study” because of its large number of participants and because the investigators are “world leaders in the technology of measuring plasma p-tau and NfL.”
Dr. Knopman, who was not involved with the research, noted that the study had no substantive weaknesses.
“The biggest advantages of a blood-based biomarker over CSF- and PET-based biomarkers of Alzheimer disease are the obvious ones of accessibility, cost, portability, and ease of repeatability,” he said.
“As CSF and PET exams are largely limited to major medical centers, valid blood-based biomarkers of Alzheimer disease that are reasonably specific make large-scale epidemiological studies that investigate dementia etiologies in rural or urban and diverse communities feasible,” he added.
Whereas p-tau181 appears to be specific for plaque and tangle disease, NfL is a nonspecific marker of neurodegeneration.
“Each has a role that could be valuable, depending on the circumstance,” said Dr. Knopman. “Plasma NfL has already proved itself useful in frontotemporal degeneration and chronic traumatic encephalopathy, for example.”
He noted that future studies should examine how closely p-tau181 and NfL align with more granular and direct measures of Alzheimer’s disease–related brain pathologies.
“There has got to be some loss of fidelity in detecting abnormality in going from brain tissue to blood, which might siphon off some time-related and severity-related information,” said Dr. Knopman.
“The exact role that plasma p-tau and NfL will play remains to be seen, because the diagnostic information that these biomarkers provide is contingent on the existence of interventions that require specific or nonspecific information about progressive neurodegeneration due to Alzheimer disease,” he added.
The study was funded by grants from the Spanish Instituto de Salud Carlos III, the Brightfocus Foundation, the Swedish Alzheimer Foundation, and the Swedish Brain Foundation. Dr. Schöll reported serving on a scientific advisory board for Servier on matters unrelated to this study. Dr. Moscoso and Dr. Knopman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a study of more than 1,000 participants, changes over time in levels of p-tau181 were associated with prospective neurodegeneration and cognitive decline characteristic of Alzheimer’s disease. These results have implications for investigative trials as well as clinical practice, the investigators noted.
Like p-tau181, neurofilament light chain (NfL) is associated with imaging markers of degeneration and cognitive decline; in contrast to the findings related to p-tau181, however, the associations between NfL and these outcomes are not specific to Alzheimer’s disease. Using both biomarkers could improve prediction of outcomes and patient monitoring, according to the researchers.
“These findings demonstrate that p-tau181 and NfL in blood have individual and complementary potential roles in the diagnosis and the monitoring of neurodegenerative disease,” said coinvestigator Michael Schöll, PhD, senior lecturer in psychiatry and neurochemistry at the University of Gothenburg (Sweden).
“With the reservation that we did not assess domain-specific cognitive impairment, p-tau181 was also more strongly associated with cognitive decline than was NfL,” Dr. Schöll added.
The findings were published online Jan. 11 in JAMA Neurology.
Biomarker-tracked neurodegeneration
Monitoring a patient’s neurodegenerative changes is important for tracking Alzheimer’s disease progression. Although clinicians can detect amyloid-beta and tau pathology using PET and cerebrospinal fluid (CSF) biomarkers, the widespread use of the latter has been hampered by cost and limited availability of necessary equipment. The use of blood-based biomarkers is not limited in these ways, and so they could aid in diagnosis and patient monitoring.
Previous studies have suggested that p-tau181 is a marker of Alzheimer’s disease status.
In the current study, investigators examined whether baseline and longitudinal levels of p-tau181 in plasma were associated with progressive neurodegeneration related to the disease. They analyzed data from the Alzheimer’s Disease Neuroimaging Initiative, a multicenter study designed to identify biomarkers for the detection and tracking of Alzheimer’s disease.
The researchers selected data for cognitively unimpaired and cognitively impaired participants who participated in the initiative between Feb. 1, 2007, and June 6, 2016. Participants were eligible for inclusion if plasma p-tau181 and NfL data were available for them and if they had undergone at least one 18fluorodeoxyglucose (FDG)–PET scan or structural T1 MRI at the same study visit. Most had also undergone imaging with 18florbetapir, which detects amyloid-beta.
A single-molecule array was used to analyze concentrations of p-tau181 and NfL in participants’ blood samples. Outliers for p-tau181 and NfL concentrations were excluded from further analysis. Using participants’ FDG-PET scans, the investigators measured glucose hypometabolism characteristic of Alzheimer’s disease. They used T1-weighted MRI scans to measure gray-matter volume.
Cognitively unimpaired participants responded to the Preclinical Alzheimer Cognitive Composite, a measure designed to detect early cognitive changes in cognitively normal patients with Alzheimer’s disease pathology. Cognitively impaired participants underwent the Alzheimer Disease Assessment Scale–Cognitive Subscale with 13 tasks to assess the severity of cognitive impairment.
The researchers included 1,113 participants (54% men; 89% non-Hispanic Whites; mean age, 74 years) in their analysis. In all, 378 participants were cognitively unimpaired, and 735 were cognitively impaired. Of the latter group, 73% had mild cognitive impairment, and 27% had Alzheimer’s disease dementia.
Atrophy predictor
Results showed that higher plasma p-tau181 levels at baseline were associated with more rapid progression of hypometabolism and atrophy in areas vulnerable to Alzheimer’s disease among cognitively impaired participants (FDG-PET standardized uptake value ratio change, r = –0.28; P < .001; gray-matter volume change, r = –0.28; P < .001).
The association with atrophy progression in cognitively impaired participants was stronger for p-tau181 than for NfL.
Plasma p-tau181 levels at baseline also predicted atrophy in temporoparietal regions vulnerable to Alzheimer’s disease among cognitively unimpaired participants (r = –0.11; P = .03). NfL, however, was associated with progressive atrophy in frontal regions among cognitively unimpaired participants.
At baseline, plasma p-tau181 levels were associated with prospective cognitive decline in both the cognitively unimpaired group (r = −0.12; P = .04) and the cognitively impaired group (r = 0.35; P < .001). However, plasma NfL was linked to cognitive decline only among those who were cognitively impaired (r = 0.26; P < .001).
Additional analyses showed that p-tau181, unlike NfL, was associated with hypometabolism and atrophy only in participants with amyloid-beta, regardless of cognitive status.
Between 25% and 45% of the association between baseline p-tau181 level and cognitive decline was mediated by baseline imaging markers of neurodegeneration. This finding suggests that another factor, such as regional tau pathology, might have an independent and direct effect on cognition, Dr. Schöll noted.
Furthermore, changes over time in p-tau181 levels were associated with cognitive decline in the cognitively unimpaired (r = –0.24; P < .001) and cognitively impaired (r = 0.34; P < .001) participants. Longitudinal changes in this biomarker also were associated with a prospective decrease in glucose metabolism in cognitively unimpaired (r = –0.05; P = .48) and cognitively impaired (r = –0.27; P < .001) participants, but the association was only significant in the latter group.
Changes over time in p-tau181 levels were linked to prospective decreases in gray-matter volume in brain regions highly characteristic of Alzheimer’s disease in those who were cognitively unimpaired (r = –0.19; P < .001) and those who were cognitively impaired (r = –0.31, P < .001). However, these associations were obtained only in patients with amyloid-beta.
Dr. Schöll noted that blood-based biomarkers that are sensitive to Alzheimer’s disease could greatly expand patients’ access to a diagnostic workup and could improve screening for clinical trials.
“While the final validation of the existence and the monitoring of potential changes of neuropathology in vivo is likely to be conducted using neuroimaging modalities such as PET, our results suggest that at least a part of these examinations could be replaced by regular blood tests,” Dr. Schöll said.
Lead author Alexis Moscoso, PhD, a postdoctoral researcher in psychiatry and neurochemistry at the University of Gothenburg, reported that the researchers will continue validating blood-based biomarkers, especially against established and well-validated neuroimaging methods. “We are also hoping to be able to compare existing and novel blood-based Alzheimer’s disease biomarkers head to head to establish the individual roles each of these play in the research and diagnosis of Alzheimer’s disease,” Dr. Moscoso said.
‘Outstanding study’
Commenting on the findings, David S. Knopman, MD, professor of neurology at Mayo Clinic, Rochester, Minn., said that this is “an outstanding study” because of its large number of participants and because the investigators are “world leaders in the technology of measuring plasma p-tau and NfL.”
Dr. Knopman, who was not involved with the research, noted that the study had no substantive weaknesses.
“The biggest advantages of a blood-based biomarker over CSF- and PET-based biomarkers of Alzheimer disease are the obvious ones of accessibility, cost, portability, and ease of repeatability,” he said.
“As CSF and PET exams are largely limited to major medical centers, valid blood-based biomarkers of Alzheimer disease that are reasonably specific make large-scale epidemiological studies that investigate dementia etiologies in rural or urban and diverse communities feasible,” he added.
Whereas p-tau181 appears to be specific for plaque and tangle disease, NfL is a nonspecific marker of neurodegeneration.
“Each has a role that could be valuable, depending on the circumstance,” said Dr. Knopman. “Plasma NfL has already proved itself useful in frontotemporal degeneration and chronic traumatic encephalopathy, for example.”
He noted that future studies should examine how closely p-tau181 and NfL align with more granular and direct measures of Alzheimer’s disease–related brain pathologies.
“There has got to be some loss of fidelity in detecting abnormality in going from brain tissue to blood, which might siphon off some time-related and severity-related information,” said Dr. Knopman.
“The exact role that plasma p-tau and NfL will play remains to be seen, because the diagnostic information that these biomarkers provide is contingent on the existence of interventions that require specific or nonspecific information about progressive neurodegeneration due to Alzheimer disease,” he added.
The study was funded by grants from the Spanish Instituto de Salud Carlos III, the Brightfocus Foundation, the Swedish Alzheimer Foundation, and the Swedish Brain Foundation. Dr. Schöll reported serving on a scientific advisory board for Servier on matters unrelated to this study. Dr. Moscoso and Dr. Knopman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a study of more than 1,000 participants, changes over time in levels of p-tau181 were associated with prospective neurodegeneration and cognitive decline characteristic of Alzheimer’s disease. These results have implications for investigative trials as well as clinical practice, the investigators noted.
Like p-tau181, neurofilament light chain (NfL) is associated with imaging markers of degeneration and cognitive decline; in contrast to the findings related to p-tau181, however, the associations between NfL and these outcomes are not specific to Alzheimer’s disease. Using both biomarkers could improve prediction of outcomes and patient monitoring, according to the researchers.
“These findings demonstrate that p-tau181 and NfL in blood have individual and complementary potential roles in the diagnosis and the monitoring of neurodegenerative disease,” said coinvestigator Michael Schöll, PhD, senior lecturer in psychiatry and neurochemistry at the University of Gothenburg (Sweden).
“With the reservation that we did not assess domain-specific cognitive impairment, p-tau181 was also more strongly associated with cognitive decline than was NfL,” Dr. Schöll added.
The findings were published online Jan. 11 in JAMA Neurology.
Biomarker-tracked neurodegeneration
Monitoring a patient’s neurodegenerative changes is important for tracking Alzheimer’s disease progression. Although clinicians can detect amyloid-beta and tau pathology using PET and cerebrospinal fluid (CSF) biomarkers, the widespread use of the latter has been hampered by cost and limited availability of necessary equipment. The use of blood-based biomarkers is not limited in these ways, and so they could aid in diagnosis and patient monitoring.
Previous studies have suggested that p-tau181 is a marker of Alzheimer’s disease status.
In the current study, investigators examined whether baseline and longitudinal levels of p-tau181 in plasma were associated with progressive neurodegeneration related to the disease. They analyzed data from the Alzheimer’s Disease Neuroimaging Initiative, a multicenter study designed to identify biomarkers for the detection and tracking of Alzheimer’s disease.
The researchers selected data for cognitively unimpaired and cognitively impaired participants who participated in the initiative between Feb. 1, 2007, and June 6, 2016. Participants were eligible for inclusion if plasma p-tau181 and NfL data were available for them and if they had undergone at least one 18fluorodeoxyglucose (FDG)–PET scan or structural T1 MRI at the same study visit. Most had also undergone imaging with 18florbetapir, which detects amyloid-beta.
A single-molecule array was used to analyze concentrations of p-tau181 and NfL in participants’ blood samples. Outliers for p-tau181 and NfL concentrations were excluded from further analysis. Using participants’ FDG-PET scans, the investigators measured glucose hypometabolism characteristic of Alzheimer’s disease. They used T1-weighted MRI scans to measure gray-matter volume.
Cognitively unimpaired participants responded to the Preclinical Alzheimer Cognitive Composite, a measure designed to detect early cognitive changes in cognitively normal patients with Alzheimer’s disease pathology. Cognitively impaired participants underwent the Alzheimer Disease Assessment Scale–Cognitive Subscale with 13 tasks to assess the severity of cognitive impairment.
The researchers included 1,113 participants (54% men; 89% non-Hispanic Whites; mean age, 74 years) in their analysis. In all, 378 participants were cognitively unimpaired, and 735 were cognitively impaired. Of the latter group, 73% had mild cognitive impairment, and 27% had Alzheimer’s disease dementia.
Atrophy predictor
Results showed that higher plasma p-tau181 levels at baseline were associated with more rapid progression of hypometabolism and atrophy in areas vulnerable to Alzheimer’s disease among cognitively impaired participants (FDG-PET standardized uptake value ratio change, r = –0.28; P < .001; gray-matter volume change, r = –0.28; P < .001).
The association with atrophy progression in cognitively impaired participants was stronger for p-tau181 than for NfL.
Plasma p-tau181 levels at baseline also predicted atrophy in temporoparietal regions vulnerable to Alzheimer’s disease among cognitively unimpaired participants (r = –0.11; P = .03). NfL, however, was associated with progressive atrophy in frontal regions among cognitively unimpaired participants.
At baseline, plasma p-tau181 levels were associated with prospective cognitive decline in both the cognitively unimpaired group (r = −0.12; P = .04) and the cognitively impaired group (r = 0.35; P < .001). However, plasma NfL was linked to cognitive decline only among those who were cognitively impaired (r = 0.26; P < .001).
Additional analyses showed that p-tau181, unlike NfL, was associated with hypometabolism and atrophy only in participants with amyloid-beta, regardless of cognitive status.
Between 25% and 45% of the association between baseline p-tau181 level and cognitive decline was mediated by baseline imaging markers of neurodegeneration. This finding suggests that another factor, such as regional tau pathology, might have an independent and direct effect on cognition, Dr. Schöll noted.
Furthermore, changes over time in p-tau181 levels were associated with cognitive decline in the cognitively unimpaired (r = –0.24; P < .001) and cognitively impaired (r = 0.34; P < .001) participants. Longitudinal changes in this biomarker also were associated with a prospective decrease in glucose metabolism in cognitively unimpaired (r = –0.05; P = .48) and cognitively impaired (r = –0.27; P < .001) participants, but the association was only significant in the latter group.
Changes over time in p-tau181 levels were linked to prospective decreases in gray-matter volume in brain regions highly characteristic of Alzheimer’s disease in those who were cognitively unimpaired (r = –0.19; P < .001) and those who were cognitively impaired (r = –0.31, P < .001). However, these associations were obtained only in patients with amyloid-beta.
Dr. Schöll noted that blood-based biomarkers that are sensitive to Alzheimer’s disease could greatly expand patients’ access to a diagnostic workup and could improve screening for clinical trials.
“While the final validation of the existence and the monitoring of potential changes of neuropathology in vivo is likely to be conducted using neuroimaging modalities such as PET, our results suggest that at least a part of these examinations could be replaced by regular blood tests,” Dr. Schöll said.
Lead author Alexis Moscoso, PhD, a postdoctoral researcher in psychiatry and neurochemistry at the University of Gothenburg, reported that the researchers will continue validating blood-based biomarkers, especially against established and well-validated neuroimaging methods. “We are also hoping to be able to compare existing and novel blood-based Alzheimer’s disease biomarkers head to head to establish the individual roles each of these play in the research and diagnosis of Alzheimer’s disease,” Dr. Moscoso said.
‘Outstanding study’
Commenting on the findings, David S. Knopman, MD, professor of neurology at Mayo Clinic, Rochester, Minn., said that this is “an outstanding study” because of its large number of participants and because the investigators are “world leaders in the technology of measuring plasma p-tau and NfL.”
Dr. Knopman, who was not involved with the research, noted that the study had no substantive weaknesses.
“The biggest advantages of a blood-based biomarker over CSF- and PET-based biomarkers of Alzheimer disease are the obvious ones of accessibility, cost, portability, and ease of repeatability,” he said.
“As CSF and PET exams are largely limited to major medical centers, valid blood-based biomarkers of Alzheimer disease that are reasonably specific make large-scale epidemiological studies that investigate dementia etiologies in rural or urban and diverse communities feasible,” he added.
Whereas p-tau181 appears to be specific for plaque and tangle disease, NfL is a nonspecific marker of neurodegeneration.
“Each has a role that could be valuable, depending on the circumstance,” said Dr. Knopman. “Plasma NfL has already proved itself useful in frontotemporal degeneration and chronic traumatic encephalopathy, for example.”
He noted that future studies should examine how closely p-tau181 and NfL align with more granular and direct measures of Alzheimer’s disease–related brain pathologies.
“There has got to be some loss of fidelity in detecting abnormality in going from brain tissue to blood, which might siphon off some time-related and severity-related information,” said Dr. Knopman.
“The exact role that plasma p-tau and NfL will play remains to be seen, because the diagnostic information that these biomarkers provide is contingent on the existence of interventions that require specific or nonspecific information about progressive neurodegeneration due to Alzheimer disease,” he added.
The study was funded by grants from the Spanish Instituto de Salud Carlos III, the Brightfocus Foundation, the Swedish Alzheimer Foundation, and the Swedish Brain Foundation. Dr. Schöll reported serving on a scientific advisory board for Servier on matters unrelated to this study. Dr. Moscoso and Dr. Knopman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Type of Alzheimer’s disease with intact memory offers new research paths
They also show some differences in neuropathology to typical patients with Alzheimer’s disease, raising hopes of discovering novel mechanisms that might protect against memory loss in typical forms of the disease.
“We are discovering that Alzheimer’s disease has more than one form. While the typical patient with Alzheimer’s disease will have impaired memory, patients with primary progressive aphasia linked to Alzheimer’s disease are quite different. They have problems with language – they know what they want to say but can’t find the words – but their memory is intact,” said lead author Marsel Mesulam, MD.
“We have found that these patients still show the same levels of neurofibrillary tangles which destroy neurons in the memory part of the brain as typical patients with Alzheimer’s disease, but in patients with primary progressive aphasia Alzheimer’s the nondominant side of this part of the brain showed less atrophy,” added Dr. Mesulam, who is director of the Mesulam Center for Cognitive Neurology and Alzheimer’s Disease at Northwestern University, Chicago. “It appears that these patients are more resilient to the effects of the neurofibrillary tangles.”
The researchers also found that two biomarkers that are established risk factors in typical Alzheimer’s disease do not appear to be risk factors for the primary progressive aphasia (PPA) form of the condition.
“These observations suggest that there are mechanisms that may protect the brain from Alzheimer’s-type damage. Studying these patients with this primary progressive aphasia form of Alzheimer’s disease may give us clues as to where to look for these mechanisms that may lead to new treatments for the memory loss associated with typical Alzheimer’s disease,” Dr. Mesulam commented.
The study was published online in the Jan. 13 issue of Neurology.
PPA is diagnosed when language impairment emerges on a background of preserved memory and behavior, with about 40% of cases representing atypical manifestations of Alzheimer’s disease, the researchers explained.
“While we knew that the memories of people with primary progressive aphasia were not affected at first, we did not know if they maintained their memory functioning over years,” Dr. Mesulam noted.
The current study aimed to investigate whether the memory preservation in PPA linked to Alzheimer’s disease is a consistent core feature or a transient finding confined to initial presentation, and to explore the underlying pathology of the condition.
The researchers searched their database to identify patients with PPA with autopsy or biomarker evidence of Alzheimer’s disease, who also had at least two consecutive visits during which language and memory assessment had been obtained with the same tests. The study included 17 patients with the PPA-type Alzheimer’s disease who compared with 14 patients who had typical Alzheimer’s disease with memory loss.
The authors pointed out that characterization of memory in patients with PPA is challenging because most tests use word lists, and thus patients may fail the test because of their language impairments. To address this issue, they included patients with PPA who had undergone memory tests involving recalling pictures of common objects.
Patients with typical Alzheimer’s disease underwent similar tests but used a list of common words.
A second round of tests was conducted in the primary progressive aphasia group an average of 2.4 years later and in the typical Alzheimer’s disease group an average of 1.7 years later.
Brain scans were also available for the patients with PPA, as well as postmortem evaluations for eight of the PPA cases and all the typical Alzheimer’s disease cases.
Results showed that patients with PPA had no decline in their memory skills when they took the tests a second time. At that point, they had been showing symptoms of the disorder for an average of 6 years. In contrast, their language skills declined significantly during the same period. For typical patients with Alzheimer’s disease, verbal memory and language skills declined with equal severity during the study.
Postmortem results showed that the two groups had comparable degrees of Alzheimer’s disease pathology in the medial temporal lobe – the main area of the brain affected in dementia.
However, MRI scans showed that patients with PPA had an asymmetrical atrophy of the dominant (left) hemisphere with sparing of the right sided medial temporal lobe, indicating a lack of neurodegeneration in the nondominant hemisphere, despite the presence of Alzheimer’s disease pathology.
It was also found that the patients with PPA had significantly lower prevalence of two factors strongly linked to Alzheimer’s disease – TDP-43 pathology and APOE ε4 positivity – than the typical patients with Alzheimer’s disease.
The authors concluded that “primary progressive aphasia Alzheimer’s syndrome offers unique opportunities for exploring the biological foundations of these phenomena that interactively modulate the impact of Alzheimer’s disease neuropathology on cognitive function.”
‘Preservation of cognition is the holy grail’
In an accompanying editorial, Seyed Ahmad Sajjadi, MD, University of California, Irvine; Sharon Ash, PhD, University of Pennsylvania, Philadelphia; and Stefano Cappa, MD, University School for Advanced Studies, Pavia, Italy, said these findings have important implications, “as ultimately, preservation of cognition is the holy grail of research in this area.”
They pointed out that the current observations imply “an uncoupling of neurodegeneration and pathology” in patients with PPA-type Alzheimer’s disease, adding that “it seems reasonable to conclude that neurodegeneration, and not mere presence of pathology, is what correlates with clinical presentation in these patients.”
The editorialists noted that the study has some limitations: the sample size is relatively small, not all patients with PPA-type Alzheimer’s disease underwent autopsy, MRI was only available for the aphasia group, and the two groups had different memory tests for comparison of their recognition memory.
But they concluded that this study “provides important insights about the potential reasons for differential vulnerability of the neural substrate of memory in those with different clinical presentations of Alzheimer’s disease pathology.”
The study was supported by the National Institute on Deafness and Communication Disorders, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the Davee Foundation, and the Jeanine Jones Fund.
A version of this article first appeared on Medscape.com.
They also show some differences in neuropathology to typical patients with Alzheimer’s disease, raising hopes of discovering novel mechanisms that might protect against memory loss in typical forms of the disease.
“We are discovering that Alzheimer’s disease has more than one form. While the typical patient with Alzheimer’s disease will have impaired memory, patients with primary progressive aphasia linked to Alzheimer’s disease are quite different. They have problems with language – they know what they want to say but can’t find the words – but their memory is intact,” said lead author Marsel Mesulam, MD.
“We have found that these patients still show the same levels of neurofibrillary tangles which destroy neurons in the memory part of the brain as typical patients with Alzheimer’s disease, but in patients with primary progressive aphasia Alzheimer’s the nondominant side of this part of the brain showed less atrophy,” added Dr. Mesulam, who is director of the Mesulam Center for Cognitive Neurology and Alzheimer’s Disease at Northwestern University, Chicago. “It appears that these patients are more resilient to the effects of the neurofibrillary tangles.”
The researchers also found that two biomarkers that are established risk factors in typical Alzheimer’s disease do not appear to be risk factors for the primary progressive aphasia (PPA) form of the condition.
“These observations suggest that there are mechanisms that may protect the brain from Alzheimer’s-type damage. Studying these patients with this primary progressive aphasia form of Alzheimer’s disease may give us clues as to where to look for these mechanisms that may lead to new treatments for the memory loss associated with typical Alzheimer’s disease,” Dr. Mesulam commented.
The study was published online in the Jan. 13 issue of Neurology.
PPA is diagnosed when language impairment emerges on a background of preserved memory and behavior, with about 40% of cases representing atypical manifestations of Alzheimer’s disease, the researchers explained.
“While we knew that the memories of people with primary progressive aphasia were not affected at first, we did not know if they maintained their memory functioning over years,” Dr. Mesulam noted.
The current study aimed to investigate whether the memory preservation in PPA linked to Alzheimer’s disease is a consistent core feature or a transient finding confined to initial presentation, and to explore the underlying pathology of the condition.
The researchers searched their database to identify patients with PPA with autopsy or biomarker evidence of Alzheimer’s disease, who also had at least two consecutive visits during which language and memory assessment had been obtained with the same tests. The study included 17 patients with the PPA-type Alzheimer’s disease who compared with 14 patients who had typical Alzheimer’s disease with memory loss.
The authors pointed out that characterization of memory in patients with PPA is challenging because most tests use word lists, and thus patients may fail the test because of their language impairments. To address this issue, they included patients with PPA who had undergone memory tests involving recalling pictures of common objects.
Patients with typical Alzheimer’s disease underwent similar tests but used a list of common words.
A second round of tests was conducted in the primary progressive aphasia group an average of 2.4 years later and in the typical Alzheimer’s disease group an average of 1.7 years later.
Brain scans were also available for the patients with PPA, as well as postmortem evaluations for eight of the PPA cases and all the typical Alzheimer’s disease cases.
Results showed that patients with PPA had no decline in their memory skills when they took the tests a second time. At that point, they had been showing symptoms of the disorder for an average of 6 years. In contrast, their language skills declined significantly during the same period. For typical patients with Alzheimer’s disease, verbal memory and language skills declined with equal severity during the study.
Postmortem results showed that the two groups had comparable degrees of Alzheimer’s disease pathology in the medial temporal lobe – the main area of the brain affected in dementia.
However, MRI scans showed that patients with PPA had an asymmetrical atrophy of the dominant (left) hemisphere with sparing of the right sided medial temporal lobe, indicating a lack of neurodegeneration in the nondominant hemisphere, despite the presence of Alzheimer’s disease pathology.
It was also found that the patients with PPA had significantly lower prevalence of two factors strongly linked to Alzheimer’s disease – TDP-43 pathology and APOE ε4 positivity – than the typical patients with Alzheimer’s disease.
The authors concluded that “primary progressive aphasia Alzheimer’s syndrome offers unique opportunities for exploring the biological foundations of these phenomena that interactively modulate the impact of Alzheimer’s disease neuropathology on cognitive function.”
‘Preservation of cognition is the holy grail’
In an accompanying editorial, Seyed Ahmad Sajjadi, MD, University of California, Irvine; Sharon Ash, PhD, University of Pennsylvania, Philadelphia; and Stefano Cappa, MD, University School for Advanced Studies, Pavia, Italy, said these findings have important implications, “as ultimately, preservation of cognition is the holy grail of research in this area.”
They pointed out that the current observations imply “an uncoupling of neurodegeneration and pathology” in patients with PPA-type Alzheimer’s disease, adding that “it seems reasonable to conclude that neurodegeneration, and not mere presence of pathology, is what correlates with clinical presentation in these patients.”
The editorialists noted that the study has some limitations: the sample size is relatively small, not all patients with PPA-type Alzheimer’s disease underwent autopsy, MRI was only available for the aphasia group, and the two groups had different memory tests for comparison of their recognition memory.
But they concluded that this study “provides important insights about the potential reasons for differential vulnerability of the neural substrate of memory in those with different clinical presentations of Alzheimer’s disease pathology.”
The study was supported by the National Institute on Deafness and Communication Disorders, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the Davee Foundation, and the Jeanine Jones Fund.
A version of this article first appeared on Medscape.com.
They also show some differences in neuropathology to typical patients with Alzheimer’s disease, raising hopes of discovering novel mechanisms that might protect against memory loss in typical forms of the disease.
“We are discovering that Alzheimer’s disease has more than one form. While the typical patient with Alzheimer’s disease will have impaired memory, patients with primary progressive aphasia linked to Alzheimer’s disease are quite different. They have problems with language – they know what they want to say but can’t find the words – but their memory is intact,” said lead author Marsel Mesulam, MD.
“We have found that these patients still show the same levels of neurofibrillary tangles which destroy neurons in the memory part of the brain as typical patients with Alzheimer’s disease, but in patients with primary progressive aphasia Alzheimer’s the nondominant side of this part of the brain showed less atrophy,” added Dr. Mesulam, who is director of the Mesulam Center for Cognitive Neurology and Alzheimer’s Disease at Northwestern University, Chicago. “It appears that these patients are more resilient to the effects of the neurofibrillary tangles.”
The researchers also found that two biomarkers that are established risk factors in typical Alzheimer’s disease do not appear to be risk factors for the primary progressive aphasia (PPA) form of the condition.
“These observations suggest that there are mechanisms that may protect the brain from Alzheimer’s-type damage. Studying these patients with this primary progressive aphasia form of Alzheimer’s disease may give us clues as to where to look for these mechanisms that may lead to new treatments for the memory loss associated with typical Alzheimer’s disease,” Dr. Mesulam commented.
The study was published online in the Jan. 13 issue of Neurology.
PPA is diagnosed when language impairment emerges on a background of preserved memory and behavior, with about 40% of cases representing atypical manifestations of Alzheimer’s disease, the researchers explained.
“While we knew that the memories of people with primary progressive aphasia were not affected at first, we did not know if they maintained their memory functioning over years,” Dr. Mesulam noted.
The current study aimed to investigate whether the memory preservation in PPA linked to Alzheimer’s disease is a consistent core feature or a transient finding confined to initial presentation, and to explore the underlying pathology of the condition.
The researchers searched their database to identify patients with PPA with autopsy or biomarker evidence of Alzheimer’s disease, who also had at least two consecutive visits during which language and memory assessment had been obtained with the same tests. The study included 17 patients with the PPA-type Alzheimer’s disease who compared with 14 patients who had typical Alzheimer’s disease with memory loss.
The authors pointed out that characterization of memory in patients with PPA is challenging because most tests use word lists, and thus patients may fail the test because of their language impairments. To address this issue, they included patients with PPA who had undergone memory tests involving recalling pictures of common objects.
Patients with typical Alzheimer’s disease underwent similar tests but used a list of common words.
A second round of tests was conducted in the primary progressive aphasia group an average of 2.4 years later and in the typical Alzheimer’s disease group an average of 1.7 years later.
Brain scans were also available for the patients with PPA, as well as postmortem evaluations for eight of the PPA cases and all the typical Alzheimer’s disease cases.
Results showed that patients with PPA had no decline in their memory skills when they took the tests a second time. At that point, they had been showing symptoms of the disorder for an average of 6 years. In contrast, their language skills declined significantly during the same period. For typical patients with Alzheimer’s disease, verbal memory and language skills declined with equal severity during the study.
Postmortem results showed that the two groups had comparable degrees of Alzheimer’s disease pathology in the medial temporal lobe – the main area of the brain affected in dementia.
However, MRI scans showed that patients with PPA had an asymmetrical atrophy of the dominant (left) hemisphere with sparing of the right sided medial temporal lobe, indicating a lack of neurodegeneration in the nondominant hemisphere, despite the presence of Alzheimer’s disease pathology.
It was also found that the patients with PPA had significantly lower prevalence of two factors strongly linked to Alzheimer’s disease – TDP-43 pathology and APOE ε4 positivity – than the typical patients with Alzheimer’s disease.
The authors concluded that “primary progressive aphasia Alzheimer’s syndrome offers unique opportunities for exploring the biological foundations of these phenomena that interactively modulate the impact of Alzheimer’s disease neuropathology on cognitive function.”
‘Preservation of cognition is the holy grail’
In an accompanying editorial, Seyed Ahmad Sajjadi, MD, University of California, Irvine; Sharon Ash, PhD, University of Pennsylvania, Philadelphia; and Stefano Cappa, MD, University School for Advanced Studies, Pavia, Italy, said these findings have important implications, “as ultimately, preservation of cognition is the holy grail of research in this area.”
They pointed out that the current observations imply “an uncoupling of neurodegeneration and pathology” in patients with PPA-type Alzheimer’s disease, adding that “it seems reasonable to conclude that neurodegeneration, and not mere presence of pathology, is what correlates with clinical presentation in these patients.”
The editorialists noted that the study has some limitations: the sample size is relatively small, not all patients with PPA-type Alzheimer’s disease underwent autopsy, MRI was only available for the aphasia group, and the two groups had different memory tests for comparison of their recognition memory.
But they concluded that this study “provides important insights about the potential reasons for differential vulnerability of the neural substrate of memory in those with different clinical presentations of Alzheimer’s disease pathology.”
The study was supported by the National Institute on Deafness and Communication Disorders, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the Davee Foundation, and the Jeanine Jones Fund.
A version of this article first appeared on Medscape.com.
Overcoming the challenges of COVID-19 for Alzheimer’s patients in long-term care, research
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
Threatening to burn the house down
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.
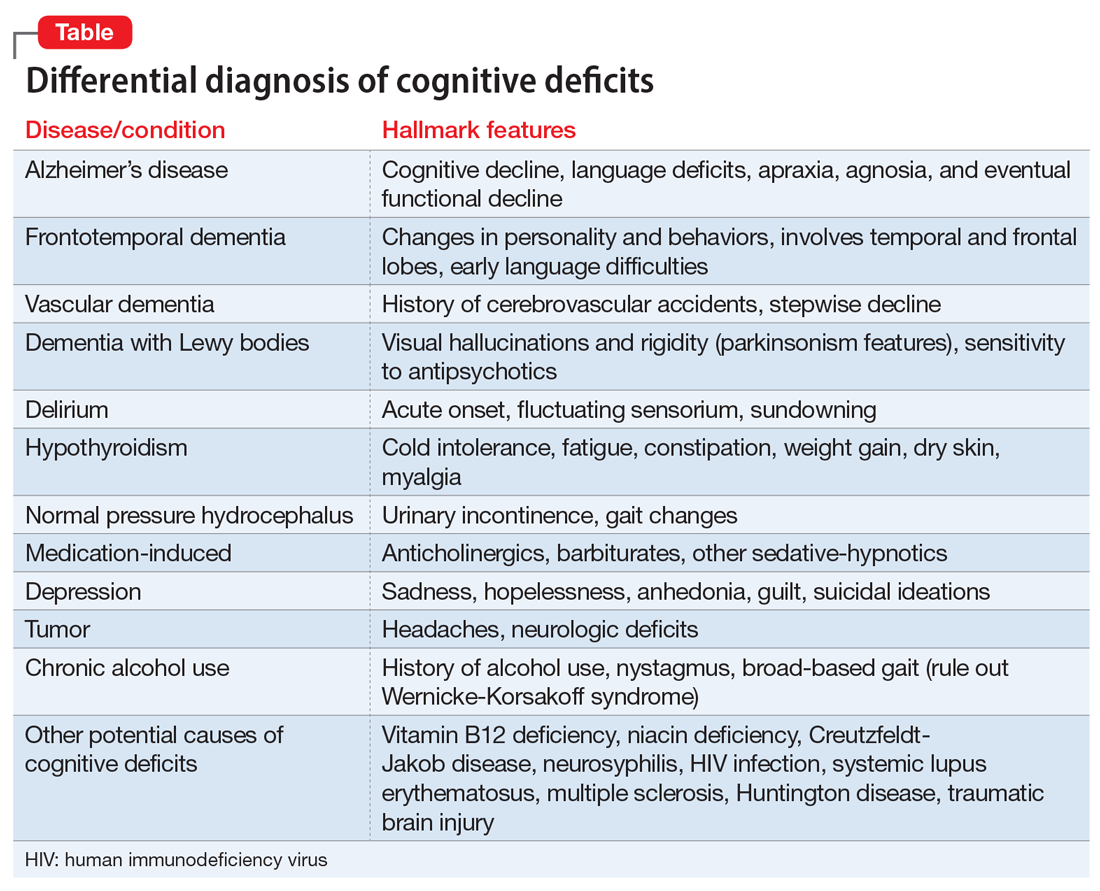
Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
High blood pressure at any age speeds cognitive decline
, new research shows. In a retrospective study of more than 15,000 participants, hypertension during middle age was associated with memory decline, and onset at later ages was linked to worsening memory and global cognition.
The investigators found that prehypertension, defined as systolic pressure of 120-139 mm Hg or diastolic pressure of 80-89 mm Hg, was also linked to accelerated cognitive decline.
Although duration of hypertension was not associated with any marker of cognitive decline, blood pressure control “can substantially reduce hypertension’s deleterious effect on the pace of cognitive decline,” said study investigator Sandhi M. Barreto, MD, PhD, professor of medicine at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
The findings were published online Dec. 14 in Hypertension.
Unanswered questions
Hypertension is an established and highly prevalent risk factor for cognitive decline, but the age at which it begins to affect cognition is unclear. Previous research suggests that onset during middle age is associated with more harmful cognitive effects than onset in later life. One reason for this apparent difference may be that the duration of hypertension influences the magnitude of cognitive decline, the researchers noted.
Other studies have shown that prehypertension is associated with damage to certain organs, but its effects on cognition are uncertain. In addition, the effect of good blood pressure control with antihypertensive medications and the impact on cognition are also unclear.
To investigate, the researchers examined data from the ongoing, multicenter ELSA-Brasil study. ELSA-Brasil follows 15,105 civil servants between the ages of 35 and 74 years. Dr. Barreto and team assessed data from visit 1, which was conducted between 2008 and 2010, and visit 2, which was conducted between 2012 and 2014.
At each visit, participants underwent a memory test, a verbal fluency test, and the Trail Making Test Part B. The investigators calculated Z scores for these tests to derive a global cognitive score.
Blood pressure was measured on the right arm, and hypertension status, age at the time of hypertension diagnosis, duration of hypertension diagnosis, hypertension treatment, and control status were recorded. Other covariables included sex, education, race, smoking status, physical activity, body mass index, and total cholesterol level.
The researchers excluded patients who did not undergo cognitive testing at visit 2, those who had a history of stroke at baseline, and those who initiated antihypertensive medications despite having normotension. After exclusions, the analysis included 7,063 participants (approximately 55% were women, 15% were Black).
At visit 1, the mean age of the group was 58.9 years, and 53.4% of participants had 14 or more years of education. In addition, 22% had prehypertension, and 46.8% had hypertension. The median duration of hypertension was 7 years; 29.8% of participants with hypertension were diagnosed with the condition during middle age.
Of those who reported having hypertension at visit 1, 7.3% were not taking any antihypertensive medication. Among participants with hypertension who were taking antihypertensives, 31.2% had uncontrolled blood pressure.
Independent predictor
Results showed that prehypertension independently predicted a significantly greater decline in verbal fluency (Z score, –0.0095; P < .01) and global cognitive score (Z score, –0.0049; P < .05) compared with normal blood pressure.
At middle age, hypertension was associated with a steeper decline in memory (Z score, –0.0072; P < .05) compared with normal blood pressure. At older ages, hypertension was linked to a steeper decline in both memory (Z score, –0.0151; P < .001) and global cognitive score (Z score, –0.0080; P < .01). Duration of hypertension, however, did not significantly predict changes in cognition (P < .109).
Among those with hypertension who were taking antihypertensive medications, those with uncontrolled blood pressure experienced greater declines in rapid memory (Z score, –0.0126; P < .01) and global cognitive score (Z score, –0.0074; P < .01) than did those with controlled blood pressure.
The investigators noted that the study participants had a comparatively high level of education, which has been shown to “boost cognitive reserve and lessen the speed of age-related cognitive decline,” Dr. Barreto said. However, “our results indicate that the effect of hypertension on cognitive decline affects individuals of all educational levels similarly,” she said.
Dr. Barreto noted that the findings have two major clinical implications. First, “maintaining blood pressure below prehypertension levels is important to preserve cognitive function or delay cognitive decline,” she said. Secondly, “in hypertensive individuals, keeping blood pressure under control is essential to reduce the speed of cognitive decline.”
The researchers plan to conduct further analyses of the data to clarify the observed relationship between memory and verbal fluency. They also plan to examine how hypertension affects long-term executive function.
‘Continuum of risk’
Commenting on the study, Philip B. Gorelick, MD, MPH, adjunct professor of neurology (stroke and neurocritical care) at Northwestern University, Chicago, noted that, so far, research suggests that the risk for stroke associated with blood pressure levels should be understood as representing a continuum rather than as being associated with several discrete points.
“The same may hold true for cognitive decline and dementia. There may be a continuum of risk whereby persons even at so-called elevated but relatively lower levels of blood pressure based on a continuous scale are at risk,” said Dr. Gorelick, who was not involved with the current study.
The investigators relied on a large and well-studied population of civil servants. However, the population’s relative youth and high level of education may limit the generalizability of the findings, he noted. In addition, the follow-up time was relatively short.
“The hard endpoint of dementia was not studied but would be of interest to enhance our understanding of the influence of blood pressure elevation on cognitive decline or dementia during a longer follow-up of the cohort,” Dr. Gorelick said.
The findings also suggest the need to better understand mechanisms that link blood pressure elevation with cognitive decline, he added.
They indicate “the need for additional clinical trials to better elucidate blood pressure lowering targets for cognitive preservation in different groups of persons at risk,” such as those with normal cognition, those with mild cognitive impairment, and those with dementia, said Dr. Gorelick. “For example, is it safe and efficacious to lower blood pressure in persons with more advanced cognitive impairment or dementia?” he asked.
The study was funded by the Brazilian Coordination for the Improvement of Higher Education Personnel. Dr. Barreto has received support from the Research Agency of the State of Minas Gerais. Although Dr. Gorelick was not involved in the ELSA-Brasil cohort study, he serves on a data monitoring committee for a trial of a blood pressure–lowering agent in the preservation of cognition.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 15,000 participants, hypertension during middle age was associated with memory decline, and onset at later ages was linked to worsening memory and global cognition.
The investigators found that prehypertension, defined as systolic pressure of 120-139 mm Hg or diastolic pressure of 80-89 mm Hg, was also linked to accelerated cognitive decline.
Although duration of hypertension was not associated with any marker of cognitive decline, blood pressure control “can substantially reduce hypertension’s deleterious effect on the pace of cognitive decline,” said study investigator Sandhi M. Barreto, MD, PhD, professor of medicine at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
The findings were published online Dec. 14 in Hypertension.
Unanswered questions
Hypertension is an established and highly prevalent risk factor for cognitive decline, but the age at which it begins to affect cognition is unclear. Previous research suggests that onset during middle age is associated with more harmful cognitive effects than onset in later life. One reason for this apparent difference may be that the duration of hypertension influences the magnitude of cognitive decline, the researchers noted.
Other studies have shown that prehypertension is associated with damage to certain organs, but its effects on cognition are uncertain. In addition, the effect of good blood pressure control with antihypertensive medications and the impact on cognition are also unclear.
To investigate, the researchers examined data from the ongoing, multicenter ELSA-Brasil study. ELSA-Brasil follows 15,105 civil servants between the ages of 35 and 74 years. Dr. Barreto and team assessed data from visit 1, which was conducted between 2008 and 2010, and visit 2, which was conducted between 2012 and 2014.
At each visit, participants underwent a memory test, a verbal fluency test, and the Trail Making Test Part B. The investigators calculated Z scores for these tests to derive a global cognitive score.
Blood pressure was measured on the right arm, and hypertension status, age at the time of hypertension diagnosis, duration of hypertension diagnosis, hypertension treatment, and control status were recorded. Other covariables included sex, education, race, smoking status, physical activity, body mass index, and total cholesterol level.
The researchers excluded patients who did not undergo cognitive testing at visit 2, those who had a history of stroke at baseline, and those who initiated antihypertensive medications despite having normotension. After exclusions, the analysis included 7,063 participants (approximately 55% were women, 15% were Black).
At visit 1, the mean age of the group was 58.9 years, and 53.4% of participants had 14 or more years of education. In addition, 22% had prehypertension, and 46.8% had hypertension. The median duration of hypertension was 7 years; 29.8% of participants with hypertension were diagnosed with the condition during middle age.
Of those who reported having hypertension at visit 1, 7.3% were not taking any antihypertensive medication. Among participants with hypertension who were taking antihypertensives, 31.2% had uncontrolled blood pressure.
Independent predictor
Results showed that prehypertension independently predicted a significantly greater decline in verbal fluency (Z score, –0.0095; P < .01) and global cognitive score (Z score, –0.0049; P < .05) compared with normal blood pressure.
At middle age, hypertension was associated with a steeper decline in memory (Z score, –0.0072; P < .05) compared with normal blood pressure. At older ages, hypertension was linked to a steeper decline in both memory (Z score, –0.0151; P < .001) and global cognitive score (Z score, –0.0080; P < .01). Duration of hypertension, however, did not significantly predict changes in cognition (P < .109).
Among those with hypertension who were taking antihypertensive medications, those with uncontrolled blood pressure experienced greater declines in rapid memory (Z score, –0.0126; P < .01) and global cognitive score (Z score, –0.0074; P < .01) than did those with controlled blood pressure.
The investigators noted that the study participants had a comparatively high level of education, which has been shown to “boost cognitive reserve and lessen the speed of age-related cognitive decline,” Dr. Barreto said. However, “our results indicate that the effect of hypertension on cognitive decline affects individuals of all educational levels similarly,” she said.
Dr. Barreto noted that the findings have two major clinical implications. First, “maintaining blood pressure below prehypertension levels is important to preserve cognitive function or delay cognitive decline,” she said. Secondly, “in hypertensive individuals, keeping blood pressure under control is essential to reduce the speed of cognitive decline.”
The researchers plan to conduct further analyses of the data to clarify the observed relationship between memory and verbal fluency. They also plan to examine how hypertension affects long-term executive function.
‘Continuum of risk’
Commenting on the study, Philip B. Gorelick, MD, MPH, adjunct professor of neurology (stroke and neurocritical care) at Northwestern University, Chicago, noted that, so far, research suggests that the risk for stroke associated with blood pressure levels should be understood as representing a continuum rather than as being associated with several discrete points.
“The same may hold true for cognitive decline and dementia. There may be a continuum of risk whereby persons even at so-called elevated but relatively lower levels of blood pressure based on a continuous scale are at risk,” said Dr. Gorelick, who was not involved with the current study.
The investigators relied on a large and well-studied population of civil servants. However, the population’s relative youth and high level of education may limit the generalizability of the findings, he noted. In addition, the follow-up time was relatively short.
“The hard endpoint of dementia was not studied but would be of interest to enhance our understanding of the influence of blood pressure elevation on cognitive decline or dementia during a longer follow-up of the cohort,” Dr. Gorelick said.
The findings also suggest the need to better understand mechanisms that link blood pressure elevation with cognitive decline, he added.
They indicate “the need for additional clinical trials to better elucidate blood pressure lowering targets for cognitive preservation in different groups of persons at risk,” such as those with normal cognition, those with mild cognitive impairment, and those with dementia, said Dr. Gorelick. “For example, is it safe and efficacious to lower blood pressure in persons with more advanced cognitive impairment or dementia?” he asked.
The study was funded by the Brazilian Coordination for the Improvement of Higher Education Personnel. Dr. Barreto has received support from the Research Agency of the State of Minas Gerais. Although Dr. Gorelick was not involved in the ELSA-Brasil cohort study, he serves on a data monitoring committee for a trial of a blood pressure–lowering agent in the preservation of cognition.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 15,000 participants, hypertension during middle age was associated with memory decline, and onset at later ages was linked to worsening memory and global cognition.
The investigators found that prehypertension, defined as systolic pressure of 120-139 mm Hg or diastolic pressure of 80-89 mm Hg, was also linked to accelerated cognitive decline.
Although duration of hypertension was not associated with any marker of cognitive decline, blood pressure control “can substantially reduce hypertension’s deleterious effect on the pace of cognitive decline,” said study investigator Sandhi M. Barreto, MD, PhD, professor of medicine at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
The findings were published online Dec. 14 in Hypertension.
Unanswered questions
Hypertension is an established and highly prevalent risk factor for cognitive decline, but the age at which it begins to affect cognition is unclear. Previous research suggests that onset during middle age is associated with more harmful cognitive effects than onset in later life. One reason for this apparent difference may be that the duration of hypertension influences the magnitude of cognitive decline, the researchers noted.
Other studies have shown that prehypertension is associated with damage to certain organs, but its effects on cognition are uncertain. In addition, the effect of good blood pressure control with antihypertensive medications and the impact on cognition are also unclear.
To investigate, the researchers examined data from the ongoing, multicenter ELSA-Brasil study. ELSA-Brasil follows 15,105 civil servants between the ages of 35 and 74 years. Dr. Barreto and team assessed data from visit 1, which was conducted between 2008 and 2010, and visit 2, which was conducted between 2012 and 2014.
At each visit, participants underwent a memory test, a verbal fluency test, and the Trail Making Test Part B. The investigators calculated Z scores for these tests to derive a global cognitive score.
Blood pressure was measured on the right arm, and hypertension status, age at the time of hypertension diagnosis, duration of hypertension diagnosis, hypertension treatment, and control status were recorded. Other covariables included sex, education, race, smoking status, physical activity, body mass index, and total cholesterol level.
The researchers excluded patients who did not undergo cognitive testing at visit 2, those who had a history of stroke at baseline, and those who initiated antihypertensive medications despite having normotension. After exclusions, the analysis included 7,063 participants (approximately 55% were women, 15% were Black).
At visit 1, the mean age of the group was 58.9 years, and 53.4% of participants had 14 or more years of education. In addition, 22% had prehypertension, and 46.8% had hypertension. The median duration of hypertension was 7 years; 29.8% of participants with hypertension were diagnosed with the condition during middle age.
Of those who reported having hypertension at visit 1, 7.3% were not taking any antihypertensive medication. Among participants with hypertension who were taking antihypertensives, 31.2% had uncontrolled blood pressure.
Independent predictor
Results showed that prehypertension independently predicted a significantly greater decline in verbal fluency (Z score, –0.0095; P < .01) and global cognitive score (Z score, –0.0049; P < .05) compared with normal blood pressure.
At middle age, hypertension was associated with a steeper decline in memory (Z score, –0.0072; P < .05) compared with normal blood pressure. At older ages, hypertension was linked to a steeper decline in both memory (Z score, –0.0151; P < .001) and global cognitive score (Z score, –0.0080; P < .01). Duration of hypertension, however, did not significantly predict changes in cognition (P < .109).
Among those with hypertension who were taking antihypertensive medications, those with uncontrolled blood pressure experienced greater declines in rapid memory (Z score, –0.0126; P < .01) and global cognitive score (Z score, –0.0074; P < .01) than did those with controlled blood pressure.
The investigators noted that the study participants had a comparatively high level of education, which has been shown to “boost cognitive reserve and lessen the speed of age-related cognitive decline,” Dr. Barreto said. However, “our results indicate that the effect of hypertension on cognitive decline affects individuals of all educational levels similarly,” she said.
Dr. Barreto noted that the findings have two major clinical implications. First, “maintaining blood pressure below prehypertension levels is important to preserve cognitive function or delay cognitive decline,” she said. Secondly, “in hypertensive individuals, keeping blood pressure under control is essential to reduce the speed of cognitive decline.”
The researchers plan to conduct further analyses of the data to clarify the observed relationship between memory and verbal fluency. They also plan to examine how hypertension affects long-term executive function.
‘Continuum of risk’
Commenting on the study, Philip B. Gorelick, MD, MPH, adjunct professor of neurology (stroke and neurocritical care) at Northwestern University, Chicago, noted that, so far, research suggests that the risk for stroke associated with blood pressure levels should be understood as representing a continuum rather than as being associated with several discrete points.
“The same may hold true for cognitive decline and dementia. There may be a continuum of risk whereby persons even at so-called elevated but relatively lower levels of blood pressure based on a continuous scale are at risk,” said Dr. Gorelick, who was not involved with the current study.
The investigators relied on a large and well-studied population of civil servants. However, the population’s relative youth and high level of education may limit the generalizability of the findings, he noted. In addition, the follow-up time was relatively short.
“The hard endpoint of dementia was not studied but would be of interest to enhance our understanding of the influence of blood pressure elevation on cognitive decline or dementia during a longer follow-up of the cohort,” Dr. Gorelick said.
The findings also suggest the need to better understand mechanisms that link blood pressure elevation with cognitive decline, he added.
They indicate “the need for additional clinical trials to better elucidate blood pressure lowering targets for cognitive preservation in different groups of persons at risk,” such as those with normal cognition, those with mild cognitive impairment, and those with dementia, said Dr. Gorelick. “For example, is it safe and efficacious to lower blood pressure in persons with more advanced cognitive impairment or dementia?” he asked.
The study was funded by the Brazilian Coordination for the Improvement of Higher Education Personnel. Dr. Barreto has received support from the Research Agency of the State of Minas Gerais. Although Dr. Gorelick was not involved in the ELSA-Brasil cohort study, he serves on a data monitoring committee for a trial of a blood pressure–lowering agent in the preservation of cognition.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
Air pollution linked to brain amyloid pathology
Higher levels of air pollution were associated with an increased risk for amyloid-beta pathology in a new study of older adults with cognitive impairment. “Many studies have now found a link between air pollution and clinical outcomes of dementia or cognitive decline,” said lead author Leonardo Iaccarino, PhD, Weill Institute for Neurosciences, University of California, San Francisco. “But this study is now showing a clear link between air pollution and a biomarker of Alzheimer’s disease: It shows a relationship between bad air quality and pathology in the brain.
“We believe that exposure to air pollution should be considered as one factor in the lifetime risk of developing Alzheimer’s disease,” he added. “We believe it is a significant determinant. Our results suggest that, if we can reduce occupational and residential exposure to air pollution, then this could help reduce the risk of Alzheimer’s disease.”
The study was published online Nov. 30 in JAMA Neurology.
A modifiable risk factor
Dr. Iaccarino explained that it is well known that air pollution is linked to poor health outcomes. “As well as cardiovascular and respiratory disease, there is also growing interest in the relationship between air pollution and brain health,” he said. “The link is becoming more and more convincing, with evidence from laboratory, animal, and human studies suggesting that individuals exposed to poor air quality have an increased risk of cognitive decline and dementia.”
In addition, this year, the Lancet Commission included air pollution in its updated list of modifiable risk factors for dementia.
For the current study, the researchers analyzed data from the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Study, which included more than 18,000 U.S. participants with cognitive impairment who received an amyloid positron-emission tomography scan between 2016 and 2018.
The investigators used data from the IDEAS study to assess the relationship between the air quality at the place of residence of each patient and the likelihood of a positive amyloid PET result. Public records from the U.S. Environmental Protection Agency were used to estimate air quality in individual ZIP-code areas during two periods – 2002-2003 (approximately 14 years before the amyloid PET scan) and 2015-2016 (approximately 1 year before the amyloid PET scan).
Results showed that those living in an area with increased air pollution, as determined using concentrations of predicted fine particulate matter (PM2.5), had a higher probability of a positive amyloid PET scan. This association was dose dependent and statistically significant after adjusting for demographic, lifestyle, and socioeconomic factors as well as medical comorbidities. The association was seen in both periods; the adjusted odds ratio was 1.10 in 2002-2003 and 1.15 in 2015-2016.
“This shows about a 10% increased probability of a positive amyloid test for individuals living in the worst polluted areas, compared with those in the least polluted areas,” Dr. Iaccarino explained.
Every unit increase in PM2.5 in 2002-2003 was associated with an increased probability of positive amyloid findings on PET of 0.5%. Every unit increase in PM2.5 in for the 2015-2016 period was associated with an increased probability of positive amyloid findings on PET of 0.8%.
“This was a very large cohort study, and we adjusted for multiple other factors, so these are pretty robust findings,” Dr. Iaccarino said.
Exposure to higher ozone concentrations was not associated with amyloid positivity on PET scans in either time window.
“These findings suggest that brain amyloid-beta accumulation could be one of the biological pathways in the increased incidence of dementia and cognitive decline associated with exposure to air pollution,” the researchers stated.
A public health concern
“Adverse effects of airborne toxic pollutants associated with amyloid-beta pathology should be considered in public health policy decisions and should inform individual lifetime risk of developing Alzheimer’s disease and dementia,” they concluded.
Dr. Iaccarino noted that, although governments need to take primary action in reducing air pollution, individuals can make some changes to reduce their exposure to poor-quality air.
“Such changes could include not going out or using masks when pollution levels are very high (as happened recently in California with the wildfires) and avoiding areas where the air quality is known to be bad. In addition, there are activities which increase indoor air pollution which can be changed, such as certain types of cooking, cigarette smoking, use of coal fires,” he commented.
“Based on our findings, it would be reasonable to take action on these things, especially for individuals at higher risk of cardiovascular and respiratory disease or Alzheimer’s,” he added.
On a more optimistic note, Dr. Iaccarino pointed out that air quality in the United States has improved significantly in recent years. Meaningful improvements were found between the two periods in this analysis study (2002-2016), “so we are going in the right direction.”
The IDEAS Study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging. Dr. Iaccarino has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Higher levels of air pollution were associated with an increased risk for amyloid-beta pathology in a new study of older adults with cognitive impairment. “Many studies have now found a link between air pollution and clinical outcomes of dementia or cognitive decline,” said lead author Leonardo Iaccarino, PhD, Weill Institute for Neurosciences, University of California, San Francisco. “But this study is now showing a clear link between air pollution and a biomarker of Alzheimer’s disease: It shows a relationship between bad air quality and pathology in the brain.
“We believe that exposure to air pollution should be considered as one factor in the lifetime risk of developing Alzheimer’s disease,” he added. “We believe it is a significant determinant. Our results suggest that, if we can reduce occupational and residential exposure to air pollution, then this could help reduce the risk of Alzheimer’s disease.”
The study was published online Nov. 30 in JAMA Neurology.
A modifiable risk factor
Dr. Iaccarino explained that it is well known that air pollution is linked to poor health outcomes. “As well as cardiovascular and respiratory disease, there is also growing interest in the relationship between air pollution and brain health,” he said. “The link is becoming more and more convincing, with evidence from laboratory, animal, and human studies suggesting that individuals exposed to poor air quality have an increased risk of cognitive decline and dementia.”
In addition, this year, the Lancet Commission included air pollution in its updated list of modifiable risk factors for dementia.
For the current study, the researchers analyzed data from the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Study, which included more than 18,000 U.S. participants with cognitive impairment who received an amyloid positron-emission tomography scan between 2016 and 2018.
The investigators used data from the IDEAS study to assess the relationship between the air quality at the place of residence of each patient and the likelihood of a positive amyloid PET result. Public records from the U.S. Environmental Protection Agency were used to estimate air quality in individual ZIP-code areas during two periods – 2002-2003 (approximately 14 years before the amyloid PET scan) and 2015-2016 (approximately 1 year before the amyloid PET scan).
Results showed that those living in an area with increased air pollution, as determined using concentrations of predicted fine particulate matter (PM2.5), had a higher probability of a positive amyloid PET scan. This association was dose dependent and statistically significant after adjusting for demographic, lifestyle, and socioeconomic factors as well as medical comorbidities. The association was seen in both periods; the adjusted odds ratio was 1.10 in 2002-2003 and 1.15 in 2015-2016.
“This shows about a 10% increased probability of a positive amyloid test for individuals living in the worst polluted areas, compared with those in the least polluted areas,” Dr. Iaccarino explained.
Every unit increase in PM2.5 in 2002-2003 was associated with an increased probability of positive amyloid findings on PET of 0.5%. Every unit increase in PM2.5 in for the 2015-2016 period was associated with an increased probability of positive amyloid findings on PET of 0.8%.
“This was a very large cohort study, and we adjusted for multiple other factors, so these are pretty robust findings,” Dr. Iaccarino said.
Exposure to higher ozone concentrations was not associated with amyloid positivity on PET scans in either time window.
“These findings suggest that brain amyloid-beta accumulation could be one of the biological pathways in the increased incidence of dementia and cognitive decline associated with exposure to air pollution,” the researchers stated.
A public health concern
“Adverse effects of airborne toxic pollutants associated with amyloid-beta pathology should be considered in public health policy decisions and should inform individual lifetime risk of developing Alzheimer’s disease and dementia,” they concluded.
Dr. Iaccarino noted that, although governments need to take primary action in reducing air pollution, individuals can make some changes to reduce their exposure to poor-quality air.
“Such changes could include not going out or using masks when pollution levels are very high (as happened recently in California with the wildfires) and avoiding areas where the air quality is known to be bad. In addition, there are activities which increase indoor air pollution which can be changed, such as certain types of cooking, cigarette smoking, use of coal fires,” he commented.
“Based on our findings, it would be reasonable to take action on these things, especially for individuals at higher risk of cardiovascular and respiratory disease or Alzheimer’s,” he added.
On a more optimistic note, Dr. Iaccarino pointed out that air quality in the United States has improved significantly in recent years. Meaningful improvements were found between the two periods in this analysis study (2002-2016), “so we are going in the right direction.”
The IDEAS Study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging. Dr. Iaccarino has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Higher levels of air pollution were associated with an increased risk for amyloid-beta pathology in a new study of older adults with cognitive impairment. “Many studies have now found a link between air pollution and clinical outcomes of dementia or cognitive decline,” said lead author Leonardo Iaccarino, PhD, Weill Institute for Neurosciences, University of California, San Francisco. “But this study is now showing a clear link between air pollution and a biomarker of Alzheimer’s disease: It shows a relationship between bad air quality and pathology in the brain.
“We believe that exposure to air pollution should be considered as one factor in the lifetime risk of developing Alzheimer’s disease,” he added. “We believe it is a significant determinant. Our results suggest that, if we can reduce occupational and residential exposure to air pollution, then this could help reduce the risk of Alzheimer’s disease.”
The study was published online Nov. 30 in JAMA Neurology.
A modifiable risk factor
Dr. Iaccarino explained that it is well known that air pollution is linked to poor health outcomes. “As well as cardiovascular and respiratory disease, there is also growing interest in the relationship between air pollution and brain health,” he said. “The link is becoming more and more convincing, with evidence from laboratory, animal, and human studies suggesting that individuals exposed to poor air quality have an increased risk of cognitive decline and dementia.”
In addition, this year, the Lancet Commission included air pollution in its updated list of modifiable risk factors for dementia.
For the current study, the researchers analyzed data from the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Study, which included more than 18,000 U.S. participants with cognitive impairment who received an amyloid positron-emission tomography scan between 2016 and 2018.
The investigators used data from the IDEAS study to assess the relationship between the air quality at the place of residence of each patient and the likelihood of a positive amyloid PET result. Public records from the U.S. Environmental Protection Agency were used to estimate air quality in individual ZIP-code areas during two periods – 2002-2003 (approximately 14 years before the amyloid PET scan) and 2015-2016 (approximately 1 year before the amyloid PET scan).
Results showed that those living in an area with increased air pollution, as determined using concentrations of predicted fine particulate matter (PM2.5), had a higher probability of a positive amyloid PET scan. This association was dose dependent and statistically significant after adjusting for demographic, lifestyle, and socioeconomic factors as well as medical comorbidities. The association was seen in both periods; the adjusted odds ratio was 1.10 in 2002-2003 and 1.15 in 2015-2016.
“This shows about a 10% increased probability of a positive amyloid test for individuals living in the worst polluted areas, compared with those in the least polluted areas,” Dr. Iaccarino explained.
Every unit increase in PM2.5 in 2002-2003 was associated with an increased probability of positive amyloid findings on PET of 0.5%. Every unit increase in PM2.5 in for the 2015-2016 period was associated with an increased probability of positive amyloid findings on PET of 0.8%.
“This was a very large cohort study, and we adjusted for multiple other factors, so these are pretty robust findings,” Dr. Iaccarino said.
Exposure to higher ozone concentrations was not associated with amyloid positivity on PET scans in either time window.
“These findings suggest that brain amyloid-beta accumulation could be one of the biological pathways in the increased incidence of dementia and cognitive decline associated with exposure to air pollution,” the researchers stated.
A public health concern
“Adverse effects of airborne toxic pollutants associated with amyloid-beta pathology should be considered in public health policy decisions and should inform individual lifetime risk of developing Alzheimer’s disease and dementia,” they concluded.
Dr. Iaccarino noted that, although governments need to take primary action in reducing air pollution, individuals can make some changes to reduce their exposure to poor-quality air.
“Such changes could include not going out or using masks when pollution levels are very high (as happened recently in California with the wildfires) and avoiding areas where the air quality is known to be bad. In addition, there are activities which increase indoor air pollution which can be changed, such as certain types of cooking, cigarette smoking, use of coal fires,” he commented.
“Based on our findings, it would be reasonable to take action on these things, especially for individuals at higher risk of cardiovascular and respiratory disease or Alzheimer’s,” he added.
On a more optimistic note, Dr. Iaccarino pointed out that air quality in the United States has improved significantly in recent years. Meaningful improvements were found between the two periods in this analysis study (2002-2016), “so we are going in the right direction.”
The IDEAS Study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging. Dr. Iaccarino has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
COVID-19: Hand sanitizer poisonings soar, psych patients at high risk
Cases of poisoning – intentional and unintentional – from ingestion of alcohol-based hand sanitizer have soared during the COVID-19 pandemic.
In the United Kingdom alone, alcohol-based hand sanitizer poisonings reported to the National Poisons Information Service jumped 157% – from 155 between January 1 and September 16, 2019, to 398 between Jan. 1 and Sept. 14, 2020, new research shows.
More needs to be done to protect those at risk of unintentional and intentional swallowing of alcohol-based hand sanitizer, including children, people with dementia/confusion, and those with mental health issues, according to Georgia Richards, DPhil student, Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford (England).
“If providers are supplying alcohol-based hand sanitizers in the community to reduce the spread of SARS-CoV-2, Ms. Richards said in an interview.
The study was published online Dec. 1 in BMJ Evidence-Based Medicine.
European, U.S. poisoning rates soar
In the paper Ms. Richards described two deaths that occurred in hospitals in England.
In one case, a 30-year-old woman, detained in a psychiatric unit who received the antidepressant venlafaxine was found dead in her hospital bed with a container of hand-sanitizing gel beside her.
“The gel was readily accessible to patients on the ward from a communal dispenser, and patients were allowed to fill cups or other containers with it to keep in their rooms,” Ms. Richards reported.
A postmortem analysis found a high level of alcohol in her blood (214 mg of alcohol in 100 mL of blood). The medical cause of death was listed as “ingestion of alcohol and venlafaxine.” The coroner concluded that the combination of these substances suppressed the patient’s breathing, leading to her death.
The other case involved a 76-year-old man who unintentionally swallowed an unknown quantity of alcohol-based hand-sanitizing foam attached to the foot of his hospital bed.
The patient had a history of agitation and depression and was treated with antidepressants. He had become increasingly confused over the preceding 9 months, possibly because of vascular dementia.
His blood ethanol concentration was 463 mg/dL (100 mmol/L) initially and 354 mg/dL (77mmol/L) 10 hours later. He was admitted to the ICU, where he received lorazepam and haloperidol and treated with ventilation, with a plan to allow the alcohol to be naturally metabolized.
The patient developed complications and died 6 days later. The primary causes of death were bronchopneumonia and acute alcohol toxicity, secondary to acute delirium and coronary artery disease.
Since COVID-19 started, alcohol-based hand sanitizers are among the most sought-after commodities around the world. The volume of these products – now found in homes, hospitals, schools, workplaces, and elsewhere – “may be a cause for concern,” Ms. Richards wrote.
Yet, warnings about the toxicity and lethality of intentional or unintentional ingestion of these products have not been widely disseminated, she noted.
To reduce the risk of harm, Ms. Richards suggested educating the public and health care professionals, improving warning labels on products, and increasing the awareness and reporting of such exposures to public health authorities.
“While governments and public health authorities have successfully heightened our awareness of, and need for, better hand hygiene during the COVID-19 outbreak, they must also make the public aware of the potential harms and encourage the reporting of such harms to poisons information centers,” she noted.
Increases in alcohol-based hand sanitizer poisoning during the pandemic have also been reported in the United States.
The American Association of Poison Control Centers reports that data from the National Poison Data System show 32,892 hand sanitizer exposure cases reported to the 55 U.S. poison control centers from Jan. 1 to Nov. 15, 2020 – an increase of 73%, compared with the same time period during the previous year.
An increase in self-harm
Weighing in on this issue, Robert Bassett, DO, associate medical director of the Poison Control Center at Children’s Hospital of Philadelphia, said in an interview that “cleaning agents and disinfectants have been around for eons and their potential for toxicity hasn’t changed.
“Now with COVID, and this hypervigilance when it comes to cleanliness, there is increased access and the exposure risk has gone up,” he said.
“One of the sad casualties of an overstressed health care system and a globally depressed environment is worsening behavioral health emergencies and, as part of that, the risk of self-harm goes up,” Dr. Bassett added.
“The consensus is that there has been an exacerbation of behavioral health emergencies and behavioral health needs since COVID started and hand sanitizers are readily accessible to someone who may be looking to self-harm,” he said.
This research had no specific funding. Ms. Richards is the editorial registrar of BMJ Evidence Based Medicine and is developing a website to track preventable deaths. Dr. Bassett disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Cases of poisoning – intentional and unintentional – from ingestion of alcohol-based hand sanitizer have soared during the COVID-19 pandemic.
In the United Kingdom alone, alcohol-based hand sanitizer poisonings reported to the National Poisons Information Service jumped 157% – from 155 between January 1 and September 16, 2019, to 398 between Jan. 1 and Sept. 14, 2020, new research shows.
More needs to be done to protect those at risk of unintentional and intentional swallowing of alcohol-based hand sanitizer, including children, people with dementia/confusion, and those with mental health issues, according to Georgia Richards, DPhil student, Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford (England).
“If providers are supplying alcohol-based hand sanitizers in the community to reduce the spread of SARS-CoV-2, Ms. Richards said in an interview.
The study was published online Dec. 1 in BMJ Evidence-Based Medicine.
European, U.S. poisoning rates soar
In the paper Ms. Richards described two deaths that occurred in hospitals in England.
In one case, a 30-year-old woman, detained in a psychiatric unit who received the antidepressant venlafaxine was found dead in her hospital bed with a container of hand-sanitizing gel beside her.
“The gel was readily accessible to patients on the ward from a communal dispenser, and patients were allowed to fill cups or other containers with it to keep in their rooms,” Ms. Richards reported.
A postmortem analysis found a high level of alcohol in her blood (214 mg of alcohol in 100 mL of blood). The medical cause of death was listed as “ingestion of alcohol and venlafaxine.” The coroner concluded that the combination of these substances suppressed the patient’s breathing, leading to her death.
The other case involved a 76-year-old man who unintentionally swallowed an unknown quantity of alcohol-based hand-sanitizing foam attached to the foot of his hospital bed.
The patient had a history of agitation and depression and was treated with antidepressants. He had become increasingly confused over the preceding 9 months, possibly because of vascular dementia.
His blood ethanol concentration was 463 mg/dL (100 mmol/L) initially and 354 mg/dL (77mmol/L) 10 hours later. He was admitted to the ICU, where he received lorazepam and haloperidol and treated with ventilation, with a plan to allow the alcohol to be naturally metabolized.
The patient developed complications and died 6 days later. The primary causes of death were bronchopneumonia and acute alcohol toxicity, secondary to acute delirium and coronary artery disease.
Since COVID-19 started, alcohol-based hand sanitizers are among the most sought-after commodities around the world. The volume of these products – now found in homes, hospitals, schools, workplaces, and elsewhere – “may be a cause for concern,” Ms. Richards wrote.
Yet, warnings about the toxicity and lethality of intentional or unintentional ingestion of these products have not been widely disseminated, she noted.
To reduce the risk of harm, Ms. Richards suggested educating the public and health care professionals, improving warning labels on products, and increasing the awareness and reporting of such exposures to public health authorities.
“While governments and public health authorities have successfully heightened our awareness of, and need for, better hand hygiene during the COVID-19 outbreak, they must also make the public aware of the potential harms and encourage the reporting of such harms to poisons information centers,” she noted.
Increases in alcohol-based hand sanitizer poisoning during the pandemic have also been reported in the United States.
The American Association of Poison Control Centers reports that data from the National Poison Data System show 32,892 hand sanitizer exposure cases reported to the 55 U.S. poison control centers from Jan. 1 to Nov. 15, 2020 – an increase of 73%, compared with the same time period during the previous year.
An increase in self-harm
Weighing in on this issue, Robert Bassett, DO, associate medical director of the Poison Control Center at Children’s Hospital of Philadelphia, said in an interview that “cleaning agents and disinfectants have been around for eons and their potential for toxicity hasn’t changed.
“Now with COVID, and this hypervigilance when it comes to cleanliness, there is increased access and the exposure risk has gone up,” he said.
“One of the sad casualties of an overstressed health care system and a globally depressed environment is worsening behavioral health emergencies and, as part of that, the risk of self-harm goes up,” Dr. Bassett added.
“The consensus is that there has been an exacerbation of behavioral health emergencies and behavioral health needs since COVID started and hand sanitizers are readily accessible to someone who may be looking to self-harm,” he said.
This research had no specific funding. Ms. Richards is the editorial registrar of BMJ Evidence Based Medicine and is developing a website to track preventable deaths. Dr. Bassett disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Cases of poisoning – intentional and unintentional – from ingestion of alcohol-based hand sanitizer have soared during the COVID-19 pandemic.
In the United Kingdom alone, alcohol-based hand sanitizer poisonings reported to the National Poisons Information Service jumped 157% – from 155 between January 1 and September 16, 2019, to 398 between Jan. 1 and Sept. 14, 2020, new research shows.
More needs to be done to protect those at risk of unintentional and intentional swallowing of alcohol-based hand sanitizer, including children, people with dementia/confusion, and those with mental health issues, according to Georgia Richards, DPhil student, Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford (England).
“If providers are supplying alcohol-based hand sanitizers in the community to reduce the spread of SARS-CoV-2, Ms. Richards said in an interview.
The study was published online Dec. 1 in BMJ Evidence-Based Medicine.
European, U.S. poisoning rates soar
In the paper Ms. Richards described two deaths that occurred in hospitals in England.
In one case, a 30-year-old woman, detained in a psychiatric unit who received the antidepressant venlafaxine was found dead in her hospital bed with a container of hand-sanitizing gel beside her.
“The gel was readily accessible to patients on the ward from a communal dispenser, and patients were allowed to fill cups or other containers with it to keep in their rooms,” Ms. Richards reported.
A postmortem analysis found a high level of alcohol in her blood (214 mg of alcohol in 100 mL of blood). The medical cause of death was listed as “ingestion of alcohol and venlafaxine.” The coroner concluded that the combination of these substances suppressed the patient’s breathing, leading to her death.
The other case involved a 76-year-old man who unintentionally swallowed an unknown quantity of alcohol-based hand-sanitizing foam attached to the foot of his hospital bed.
The patient had a history of agitation and depression and was treated with antidepressants. He had become increasingly confused over the preceding 9 months, possibly because of vascular dementia.
His blood ethanol concentration was 463 mg/dL (100 mmol/L) initially and 354 mg/dL (77mmol/L) 10 hours later. He was admitted to the ICU, where he received lorazepam and haloperidol and treated with ventilation, with a plan to allow the alcohol to be naturally metabolized.
The patient developed complications and died 6 days later. The primary causes of death were bronchopneumonia and acute alcohol toxicity, secondary to acute delirium and coronary artery disease.
Since COVID-19 started, alcohol-based hand sanitizers are among the most sought-after commodities around the world. The volume of these products – now found in homes, hospitals, schools, workplaces, and elsewhere – “may be a cause for concern,” Ms. Richards wrote.
Yet, warnings about the toxicity and lethality of intentional or unintentional ingestion of these products have not been widely disseminated, she noted.
To reduce the risk of harm, Ms. Richards suggested educating the public and health care professionals, improving warning labels on products, and increasing the awareness and reporting of such exposures to public health authorities.
“While governments and public health authorities have successfully heightened our awareness of, and need for, better hand hygiene during the COVID-19 outbreak, they must also make the public aware of the potential harms and encourage the reporting of such harms to poisons information centers,” she noted.
Increases in alcohol-based hand sanitizer poisoning during the pandemic have also been reported in the United States.
The American Association of Poison Control Centers reports that data from the National Poison Data System show 32,892 hand sanitizer exposure cases reported to the 55 U.S. poison control centers from Jan. 1 to Nov. 15, 2020 – an increase of 73%, compared with the same time period during the previous year.
An increase in self-harm
Weighing in on this issue, Robert Bassett, DO, associate medical director of the Poison Control Center at Children’s Hospital of Philadelphia, said in an interview that “cleaning agents and disinfectants have been around for eons and their potential for toxicity hasn’t changed.
“Now with COVID, and this hypervigilance when it comes to cleanliness, there is increased access and the exposure risk has gone up,” he said.
“One of the sad casualties of an overstressed health care system and a globally depressed environment is worsening behavioral health emergencies and, as part of that, the risk of self-harm goes up,” Dr. Bassett added.
“The consensus is that there has been an exacerbation of behavioral health emergencies and behavioral health needs since COVID started and hand sanitizers are readily accessible to someone who may be looking to self-harm,” he said.
This research had no specific funding. Ms. Richards is the editorial registrar of BMJ Evidence Based Medicine and is developing a website to track preventable deaths. Dr. Bassett disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.







