User login
ADHD med may reduce apathy in Alzheimer’s disease
Methylphenidate is safe and effective for treating apathy in patients with Alzheimer’s disease (AD), new research suggests.
Results from a phase 3 randomized trial showed that, after 6 months of treatment, mean score on the Neuropsychiatric Inventory (NPI) apathy subscale decreased by 4.5 points for patients who received methylphenidate vs. a decrease of 3.1 points for those who received placebo.
In addition, the safety profile showed no significant between-group differences.
“Methylphenidate offers a treatment approach providing a modest but potentially clinically significant benefit for patients and caregivers,” said the investigators, led by Jacobo E. Mintzer, MD, MBA, professor of health studies at the Medical University of South Carolina in Charleston.
The findings were published online Sept. 27 in JAMA Neurology.
Common problem
Apathy, which is common among patients with AD, is associated with increased risk for mortality, financial burden, and caregiver burden. No treatment has proved effective for apathy in this population.
Two trials of methylphenidate, a catecholaminergic agent, have provided preliminary evidence of efficacy. Findings from the Apathy in Dementia Methylphenidate trial (ADMET) suggested the drug was associated with improved cognition and few adverse events. However, both trials had small patient populations and short durations.
The current investigators conducted ADMET 2, a 6-month, phase 3 trial, to investigate methylphenidate further. They recruited 200 patients (mean age, 76 years; 66% men; 90% White) at nine clinical centers that specialized in dementia care in the United States and one in Canada.
Eligible patients had a diagnosis of possible or probable AD and a Mini-Mental State Examination (MMSE) score between 10 and 28. They also had clinically significant apathy for at least 4 weeks and an available caregiver who spent more than 10 hours a week with the patient.
The researchers randomly assigned patients to receive methylphenidate (n = 99) or placebo (n = 101). For 3 days, participants in the active group received 10 mg/day of methylphenidate. After that point, they received 20 mg/day of methylphenidate for the rest of the study.
Patients in both treatment groups were given the same number of identical-appearing capsules each day.
In-person follow-up visits took place monthly for 6 months. Participants also were contacted by telephone at days 15, 45, and 75 after treatment assignment.
Participants underwent cognitive testing at baseline and at 2, 4, and 6 months. The battery of tests included the MMSE, Hopkins Verbal Learning Test, and Wechsler Adult Intelligence Scale – Revised Digit Span.
The trial’s two primary outcomes were mean change in NPI apathy score from baseline to 6 months and the odds of an improved rating on the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change (ADCS-CGIC) between baseline and 6 months.
Significant change on either outcome was to be considered a signal of effective treatment.
Treatment-specific benefit
Ten patients in the methylphenidate group and seven in the placebo group withdrew during the study.
Mean baseline score on the NPI apathy subscale was 8.0 vs. 7.6, respectively.
In an adjusted, longitudinal model, mean between-group difference in change in NPI apathy score at 6 months was –1.25 (P = .002). The mean NPI apathy score decreased by 4.5 points in the methylphenidate group vs. 3.1 points in the placebo group.
The largest change in apathy score occurred during the first 2 months of treatment. At 6 months, 27% of the methylphenidate group vs. 14% of the placebo group had an NPI apathy score of 0.
In addition, 43.8% of the methylphenidate group had improvement on the ADCS-CGIC compared with 35.2% of the placebo group. The odds ratio (OR) for improvement on ADCS-CGIC for methylphenidate vs. placebo was 1.90 (P = .07).
There was also a strong association between score improvement on the NPI apathy subscale and improvement on the ADCS-CGIC subscale (OR, 2.95; P = .002).
“It is important to note that there were no group differences in any of the cognitive measures, suggesting that the effect of the treatment is specific to the treatment of apathy and not a secondary effect of improvement in cognition,” the researchers wrote.
In all, 17 serious adverse events occurred in the methylphenidate group and 10 occurred in the placebo group. However, all events were found to be hospitalizations for events not related to treatment.
‘Enduring effect’
Commenting on the findings, Jeffrey L. Cummings, MD, ScD, professor of brain sciences at the University of Nevada, Las Vegas, noted that the reduction in NPI apathy subscale score of more than 50% was clinically meaningful.
A more robust outcome on the ADCS-CGIC would have been desirable, he added, although that instrument is not designed specifically for apathy.
Methylphenidate’s effect on apathy observed at 2 months and remaining stable throughout the study makes it appear to be “an enduring effect, and not something that the patient accommodates to,” said Dr. Cummings, who was not involved with the research. Such a change may manifest itself in a patient’s greater willingness to help voluntarily with housework or to suggest going for a walk, he noted.
“These are not dramatic changes in cognition, of course, but they are changes in initiative and that is very important,” Dr. Cummings said. Decreased apathy also may improve quality of life for the patient’s caregiver, he added.
Overall, the findings raise the question of whether the Food and Drug Administration should recognize apathy as an indication for which drugs can be approved, said Dr. Cummings.
“For me, that would be the next major step in this line of investigation,” he concluded.
The study was funded by the National Institute on Aging. Dr. Mintzer has served as an adviser to Praxis Bioresearch and Cerevel Therapeutics on matters unrelated to this study. Dr. Cummings is the author of the Neuropsychiatric Inventory but does not receive payments for it from academic trials such as ADMET 2.
A version of this article first appeared on Medscape.com.
Methylphenidate is safe and effective for treating apathy in patients with Alzheimer’s disease (AD), new research suggests.
Results from a phase 3 randomized trial showed that, after 6 months of treatment, mean score on the Neuropsychiatric Inventory (NPI) apathy subscale decreased by 4.5 points for patients who received methylphenidate vs. a decrease of 3.1 points for those who received placebo.
In addition, the safety profile showed no significant between-group differences.
“Methylphenidate offers a treatment approach providing a modest but potentially clinically significant benefit for patients and caregivers,” said the investigators, led by Jacobo E. Mintzer, MD, MBA, professor of health studies at the Medical University of South Carolina in Charleston.
The findings were published online Sept. 27 in JAMA Neurology.
Common problem
Apathy, which is common among patients with AD, is associated with increased risk for mortality, financial burden, and caregiver burden. No treatment has proved effective for apathy in this population.
Two trials of methylphenidate, a catecholaminergic agent, have provided preliminary evidence of efficacy. Findings from the Apathy in Dementia Methylphenidate trial (ADMET) suggested the drug was associated with improved cognition and few adverse events. However, both trials had small patient populations and short durations.
The current investigators conducted ADMET 2, a 6-month, phase 3 trial, to investigate methylphenidate further. They recruited 200 patients (mean age, 76 years; 66% men; 90% White) at nine clinical centers that specialized in dementia care in the United States and one in Canada.
Eligible patients had a diagnosis of possible or probable AD and a Mini-Mental State Examination (MMSE) score between 10 and 28. They also had clinically significant apathy for at least 4 weeks and an available caregiver who spent more than 10 hours a week with the patient.
The researchers randomly assigned patients to receive methylphenidate (n = 99) or placebo (n = 101). For 3 days, participants in the active group received 10 mg/day of methylphenidate. After that point, they received 20 mg/day of methylphenidate for the rest of the study.
Patients in both treatment groups were given the same number of identical-appearing capsules each day.
In-person follow-up visits took place monthly for 6 months. Participants also were contacted by telephone at days 15, 45, and 75 after treatment assignment.
Participants underwent cognitive testing at baseline and at 2, 4, and 6 months. The battery of tests included the MMSE, Hopkins Verbal Learning Test, and Wechsler Adult Intelligence Scale – Revised Digit Span.
The trial’s two primary outcomes were mean change in NPI apathy score from baseline to 6 months and the odds of an improved rating on the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change (ADCS-CGIC) between baseline and 6 months.
Significant change on either outcome was to be considered a signal of effective treatment.
Treatment-specific benefit
Ten patients in the methylphenidate group and seven in the placebo group withdrew during the study.
Mean baseline score on the NPI apathy subscale was 8.0 vs. 7.6, respectively.
In an adjusted, longitudinal model, mean between-group difference in change in NPI apathy score at 6 months was –1.25 (P = .002). The mean NPI apathy score decreased by 4.5 points in the methylphenidate group vs. 3.1 points in the placebo group.
The largest change in apathy score occurred during the first 2 months of treatment. At 6 months, 27% of the methylphenidate group vs. 14% of the placebo group had an NPI apathy score of 0.
In addition, 43.8% of the methylphenidate group had improvement on the ADCS-CGIC compared with 35.2% of the placebo group. The odds ratio (OR) for improvement on ADCS-CGIC for methylphenidate vs. placebo was 1.90 (P = .07).
There was also a strong association between score improvement on the NPI apathy subscale and improvement on the ADCS-CGIC subscale (OR, 2.95; P = .002).
“It is important to note that there were no group differences in any of the cognitive measures, suggesting that the effect of the treatment is specific to the treatment of apathy and not a secondary effect of improvement in cognition,” the researchers wrote.
In all, 17 serious adverse events occurred in the methylphenidate group and 10 occurred in the placebo group. However, all events were found to be hospitalizations for events not related to treatment.
‘Enduring effect’
Commenting on the findings, Jeffrey L. Cummings, MD, ScD, professor of brain sciences at the University of Nevada, Las Vegas, noted that the reduction in NPI apathy subscale score of more than 50% was clinically meaningful.
A more robust outcome on the ADCS-CGIC would have been desirable, he added, although that instrument is not designed specifically for apathy.
Methylphenidate’s effect on apathy observed at 2 months and remaining stable throughout the study makes it appear to be “an enduring effect, and not something that the patient accommodates to,” said Dr. Cummings, who was not involved with the research. Such a change may manifest itself in a patient’s greater willingness to help voluntarily with housework or to suggest going for a walk, he noted.
“These are not dramatic changes in cognition, of course, but they are changes in initiative and that is very important,” Dr. Cummings said. Decreased apathy also may improve quality of life for the patient’s caregiver, he added.
Overall, the findings raise the question of whether the Food and Drug Administration should recognize apathy as an indication for which drugs can be approved, said Dr. Cummings.
“For me, that would be the next major step in this line of investigation,” he concluded.
The study was funded by the National Institute on Aging. Dr. Mintzer has served as an adviser to Praxis Bioresearch and Cerevel Therapeutics on matters unrelated to this study. Dr. Cummings is the author of the Neuropsychiatric Inventory but does not receive payments for it from academic trials such as ADMET 2.
A version of this article first appeared on Medscape.com.
Methylphenidate is safe and effective for treating apathy in patients with Alzheimer’s disease (AD), new research suggests.
Results from a phase 3 randomized trial showed that, after 6 months of treatment, mean score on the Neuropsychiatric Inventory (NPI) apathy subscale decreased by 4.5 points for patients who received methylphenidate vs. a decrease of 3.1 points for those who received placebo.
In addition, the safety profile showed no significant between-group differences.
“Methylphenidate offers a treatment approach providing a modest but potentially clinically significant benefit for patients and caregivers,” said the investigators, led by Jacobo E. Mintzer, MD, MBA, professor of health studies at the Medical University of South Carolina in Charleston.
The findings were published online Sept. 27 in JAMA Neurology.
Common problem
Apathy, which is common among patients with AD, is associated with increased risk for mortality, financial burden, and caregiver burden. No treatment has proved effective for apathy in this population.
Two trials of methylphenidate, a catecholaminergic agent, have provided preliminary evidence of efficacy. Findings from the Apathy in Dementia Methylphenidate trial (ADMET) suggested the drug was associated with improved cognition and few adverse events. However, both trials had small patient populations and short durations.
The current investigators conducted ADMET 2, a 6-month, phase 3 trial, to investigate methylphenidate further. They recruited 200 patients (mean age, 76 years; 66% men; 90% White) at nine clinical centers that specialized in dementia care in the United States and one in Canada.
Eligible patients had a diagnosis of possible or probable AD and a Mini-Mental State Examination (MMSE) score between 10 and 28. They also had clinically significant apathy for at least 4 weeks and an available caregiver who spent more than 10 hours a week with the patient.
The researchers randomly assigned patients to receive methylphenidate (n = 99) or placebo (n = 101). For 3 days, participants in the active group received 10 mg/day of methylphenidate. After that point, they received 20 mg/day of methylphenidate for the rest of the study.
Patients in both treatment groups were given the same number of identical-appearing capsules each day.
In-person follow-up visits took place monthly for 6 months. Participants also were contacted by telephone at days 15, 45, and 75 after treatment assignment.
Participants underwent cognitive testing at baseline and at 2, 4, and 6 months. The battery of tests included the MMSE, Hopkins Verbal Learning Test, and Wechsler Adult Intelligence Scale – Revised Digit Span.
The trial’s two primary outcomes were mean change in NPI apathy score from baseline to 6 months and the odds of an improved rating on the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change (ADCS-CGIC) between baseline and 6 months.
Significant change on either outcome was to be considered a signal of effective treatment.
Treatment-specific benefit
Ten patients in the methylphenidate group and seven in the placebo group withdrew during the study.
Mean baseline score on the NPI apathy subscale was 8.0 vs. 7.6, respectively.
In an adjusted, longitudinal model, mean between-group difference in change in NPI apathy score at 6 months was –1.25 (P = .002). The mean NPI apathy score decreased by 4.5 points in the methylphenidate group vs. 3.1 points in the placebo group.
The largest change in apathy score occurred during the first 2 months of treatment. At 6 months, 27% of the methylphenidate group vs. 14% of the placebo group had an NPI apathy score of 0.
In addition, 43.8% of the methylphenidate group had improvement on the ADCS-CGIC compared with 35.2% of the placebo group. The odds ratio (OR) for improvement on ADCS-CGIC for methylphenidate vs. placebo was 1.90 (P = .07).
There was also a strong association between score improvement on the NPI apathy subscale and improvement on the ADCS-CGIC subscale (OR, 2.95; P = .002).
“It is important to note that there were no group differences in any of the cognitive measures, suggesting that the effect of the treatment is specific to the treatment of apathy and not a secondary effect of improvement in cognition,” the researchers wrote.
In all, 17 serious adverse events occurred in the methylphenidate group and 10 occurred in the placebo group. However, all events were found to be hospitalizations for events not related to treatment.
‘Enduring effect’
Commenting on the findings, Jeffrey L. Cummings, MD, ScD, professor of brain sciences at the University of Nevada, Las Vegas, noted that the reduction in NPI apathy subscale score of more than 50% was clinically meaningful.
A more robust outcome on the ADCS-CGIC would have been desirable, he added, although that instrument is not designed specifically for apathy.
Methylphenidate’s effect on apathy observed at 2 months and remaining stable throughout the study makes it appear to be “an enduring effect, and not something that the patient accommodates to,” said Dr. Cummings, who was not involved with the research. Such a change may manifest itself in a patient’s greater willingness to help voluntarily with housework or to suggest going for a walk, he noted.
“These are not dramatic changes in cognition, of course, but they are changes in initiative and that is very important,” Dr. Cummings said. Decreased apathy also may improve quality of life for the patient’s caregiver, he added.
Overall, the findings raise the question of whether the Food and Drug Administration should recognize apathy as an indication for which drugs can be approved, said Dr. Cummings.
“For me, that would be the next major step in this line of investigation,” he concluded.
The study was funded by the National Institute on Aging. Dr. Mintzer has served as an adviser to Praxis Bioresearch and Cerevel Therapeutics on matters unrelated to this study. Dr. Cummings is the author of the Neuropsychiatric Inventory but does not receive payments for it from academic trials such as ADMET 2.
A version of this article first appeared on Medscape.com.
Persistent altered mental status
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
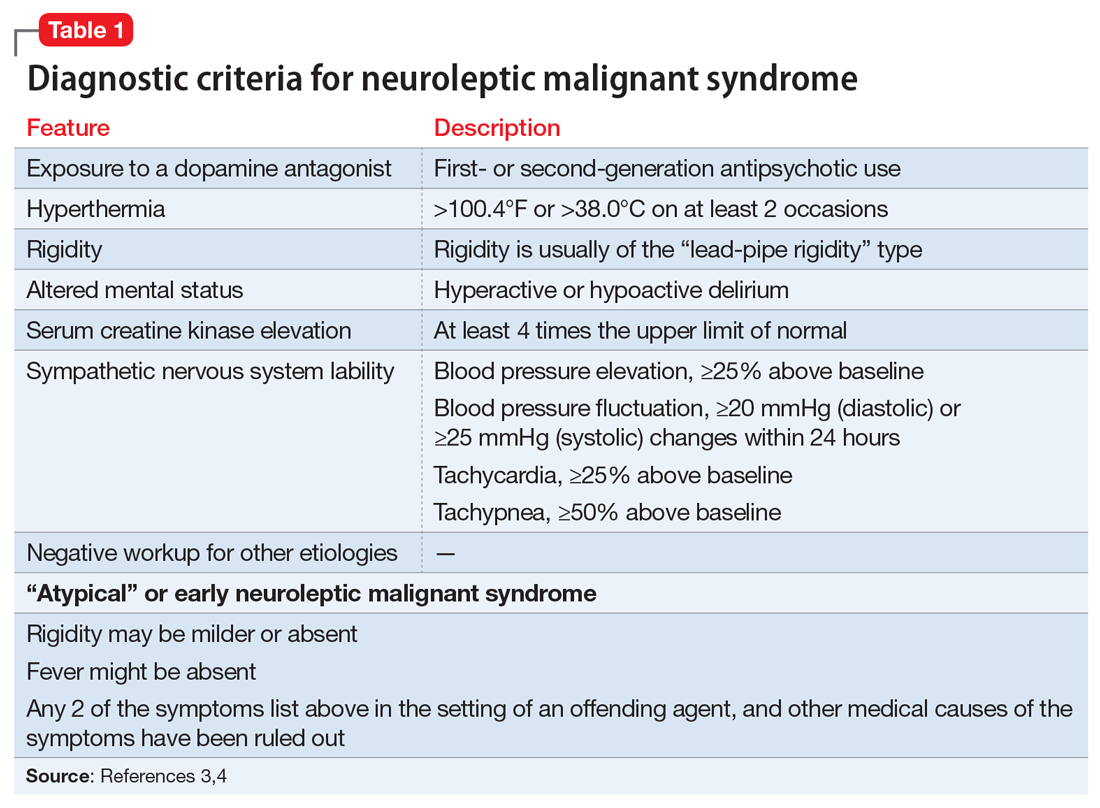
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.
[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
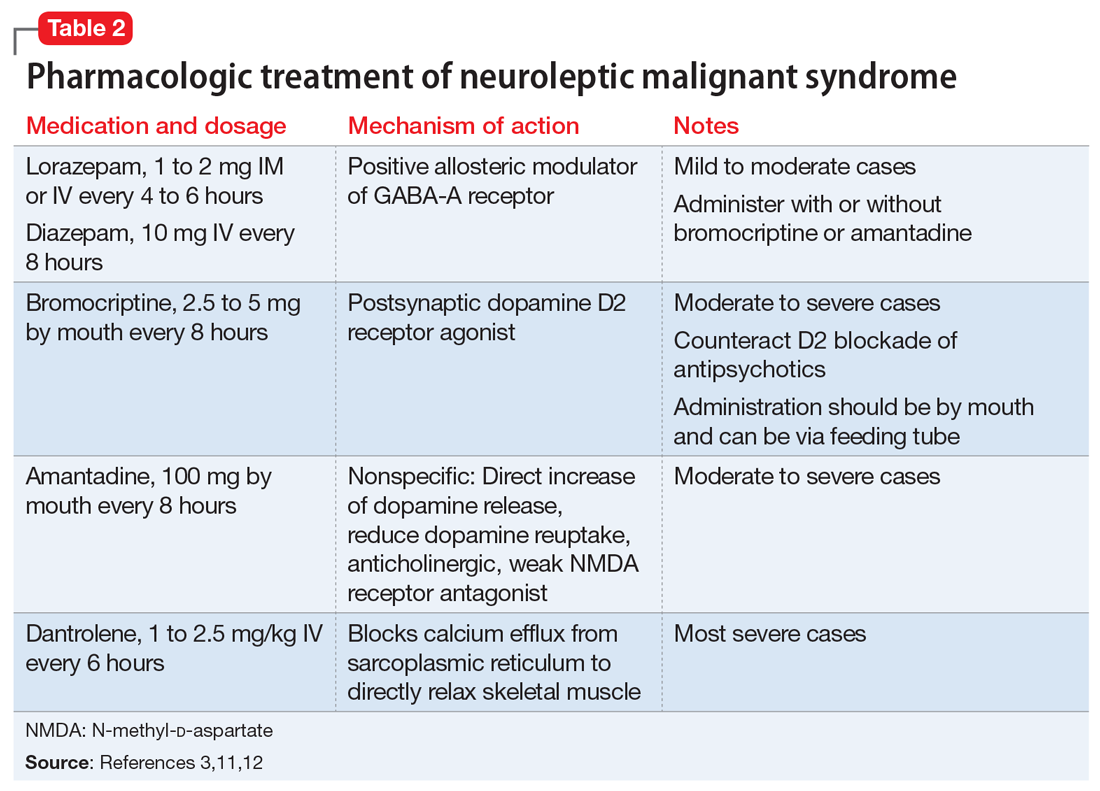
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.
Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.

[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.

Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.

[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.

Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
MIND diet preserves cognition, new data show
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sexual assault in women tied to increased stroke, dementia risk
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADHD a new risk factor for Alzheimer’s?
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Human brain patterns may help build a better AI system
new research suggests. “This work opens new opportunities to discover how the network organization of the brain optimizes cognitive capacity,” wrote researchers from The Neuro (Montreal Neurological Institute–Hospital) and the Quebec Artificial Intelligence Institute.
Senior investigator Bratislav Misic, PhD, said the research has potential clinical application for studying diseases of the brain, which is something his team is actively working on. “For example, using MRI techniques, we can measure different patterns of atrophy in neurodegenerative diseases such as Alzheimer’s disease,” he said.
“We can use these disease patterns from real patients to artificially lesion these connectomes and to ask how a particular disease causes a particular pattern of symptoms and cognitive deficits,” he added.
The findings were published online in Nature Machine Intelligence.
Unique approach
Using brain imaging data, the investigators reconstructed a human brain connectivity pattern and applied it to an artificial neural network. After training, the artificial neural network successfully performed a working memory task more flexibly and efficiently than other “benchmark” AI systems.
The researchers noted that their approach is unique because previous work on brain connectivity, also known as connectomics, has focused on describing brain organization without regard to how it actually functions.
Traditional artificial neural network have arbitrary structures that do not reflect how real brain networks are organized. Integrating brain connectomics into the construction of artificial neural network can reveal how the wiring of the brain supports specific cognitive skills, the investigators wrote.
“Up until now, if you look at how neural networks are constructed, the architectures that are used are very ad hoc and very problem specific,” Dr. Misic said. “But the connectomics revolution that’s happened in neuroscience over the past 20 years or so has given us the ability to really measure and trace out connection patterns in a variety of organisms, including the human brain.”
He noted that the researchers took wiring patterns of the real human brain and implemented it as an artificial neural network. They then “trained that network to perform a very simple cognitive task, and when you compare it to other benchmark architectures, it actually does better.”
This shows that there is “something fundamentally different about how the human brain is wired up and that the design principles that we can see in the human brain could be used to potentially build better artificial networks,” Dr. Misic concluded.
Funding for the research was provided by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative, and by the Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche du Quebec – Santé, the Canadian Institute for Advanced Research, Canada Research Chairs, Fonds de Recherche du Quebec – Nature et Technologies, and the Centre UNIQUE (Union of Neuroscience and Artificial Intelligence). The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. “This work opens new opportunities to discover how the network organization of the brain optimizes cognitive capacity,” wrote researchers from The Neuro (Montreal Neurological Institute–Hospital) and the Quebec Artificial Intelligence Institute.
Senior investigator Bratislav Misic, PhD, said the research has potential clinical application for studying diseases of the brain, which is something his team is actively working on. “For example, using MRI techniques, we can measure different patterns of atrophy in neurodegenerative diseases such as Alzheimer’s disease,” he said.
“We can use these disease patterns from real patients to artificially lesion these connectomes and to ask how a particular disease causes a particular pattern of symptoms and cognitive deficits,” he added.
The findings were published online in Nature Machine Intelligence.
Unique approach
Using brain imaging data, the investigators reconstructed a human brain connectivity pattern and applied it to an artificial neural network. After training, the artificial neural network successfully performed a working memory task more flexibly and efficiently than other “benchmark” AI systems.
The researchers noted that their approach is unique because previous work on brain connectivity, also known as connectomics, has focused on describing brain organization without regard to how it actually functions.
Traditional artificial neural network have arbitrary structures that do not reflect how real brain networks are organized. Integrating brain connectomics into the construction of artificial neural network can reveal how the wiring of the brain supports specific cognitive skills, the investigators wrote.
“Up until now, if you look at how neural networks are constructed, the architectures that are used are very ad hoc and very problem specific,” Dr. Misic said. “But the connectomics revolution that’s happened in neuroscience over the past 20 years or so has given us the ability to really measure and trace out connection patterns in a variety of organisms, including the human brain.”
He noted that the researchers took wiring patterns of the real human brain and implemented it as an artificial neural network. They then “trained that network to perform a very simple cognitive task, and when you compare it to other benchmark architectures, it actually does better.”
This shows that there is “something fundamentally different about how the human brain is wired up and that the design principles that we can see in the human brain could be used to potentially build better artificial networks,” Dr. Misic concluded.
Funding for the research was provided by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative, and by the Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche du Quebec – Santé, the Canadian Institute for Advanced Research, Canada Research Chairs, Fonds de Recherche du Quebec – Nature et Technologies, and the Centre UNIQUE (Union of Neuroscience and Artificial Intelligence). The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. “This work opens new opportunities to discover how the network organization of the brain optimizes cognitive capacity,” wrote researchers from The Neuro (Montreal Neurological Institute–Hospital) and the Quebec Artificial Intelligence Institute.
Senior investigator Bratislav Misic, PhD, said the research has potential clinical application for studying diseases of the brain, which is something his team is actively working on. “For example, using MRI techniques, we can measure different patterns of atrophy in neurodegenerative diseases such as Alzheimer’s disease,” he said.
“We can use these disease patterns from real patients to artificially lesion these connectomes and to ask how a particular disease causes a particular pattern of symptoms and cognitive deficits,” he added.
The findings were published online in Nature Machine Intelligence.
Unique approach
Using brain imaging data, the investigators reconstructed a human brain connectivity pattern and applied it to an artificial neural network. After training, the artificial neural network successfully performed a working memory task more flexibly and efficiently than other “benchmark” AI systems.
The researchers noted that their approach is unique because previous work on brain connectivity, also known as connectomics, has focused on describing brain organization without regard to how it actually functions.
Traditional artificial neural network have arbitrary structures that do not reflect how real brain networks are organized. Integrating brain connectomics into the construction of artificial neural network can reveal how the wiring of the brain supports specific cognitive skills, the investigators wrote.
“Up until now, if you look at how neural networks are constructed, the architectures that are used are very ad hoc and very problem specific,” Dr. Misic said. “But the connectomics revolution that’s happened in neuroscience over the past 20 years or so has given us the ability to really measure and trace out connection patterns in a variety of organisms, including the human brain.”
He noted that the researchers took wiring patterns of the real human brain and implemented it as an artificial neural network. They then “trained that network to perform a very simple cognitive task, and when you compare it to other benchmark architectures, it actually does better.”
This shows that there is “something fundamentally different about how the human brain is wired up and that the design principles that we can see in the human brain could be used to potentially build better artificial networks,” Dr. Misic concluded.
Funding for the research was provided by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative, and by the Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche du Quebec – Santé, the Canadian Institute for Advanced Research, Canada Research Chairs, Fonds de Recherche du Quebec – Nature et Technologies, and the Centre UNIQUE (Union of Neuroscience and Artificial Intelligence). The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MACHINE INTELLIGENCE
Nonmotor symptoms common in Parkinson’s
The hallmark of Parkinson’s disease is the accompanying motor symptoms, but the condition can bring other challenges. Among those are nonmotor symptoms, including depression, dementia, and even psychosis.
The culprit is Lewy bodies, which are also responsible for Lewy body dementia. “What we call Lewy body dementia and Parkinson’s disease are caused by the same pathological process – the formation of Lewy bodies in the brain,” Leslie Citrome, MD, MPH, said in an interview. Dr. Citrome discussed some of the psychiatric comorbidities associated with Parkinson’s disease at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In fact, the association goes both ways. “Many people with Parkinson’s disease develop a dementia. Many people with Lewy body dementia develop motor symptoms that look just like Parkinson’s disease,” said Dr. Citrome, professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, and president of the American Society for Clinical Psychopharmacology.
The motor symptoms of Parkinson’s disease are generally attributable to loss of striatal dopaminergic neurons, while nonmotor symptoms can be traced to loss of neurons in nondopaminergic regions. Nonmotor symptoms – often including sleep disorders, depression, cognitive changes, and psychosis – may occur before motor symptoms. Other problems may include autonomic dysfunction, such as constipation, sexual dysfunction, sweating, or urinary retention.
Patients might not be aware that nonmotor symptoms can occur with Parkinson’s disease and may not even consider mentioning mood changes or hallucinations to their neurologist. Family members may also be unaware.
Sleep problems are common in Parkinson’s disease, including rapid eye-movement sleep behavior disorders, vivid dreams, restless legs syndrome, insomnia, and daytime somnolence. Dopamine agonists may also cause unintended sleep.
Depression is extremely common, affecting up to 90% of Parkinson’s disease patients, and this may be related to dopaminergic losses. Antidepressant medications can worsen Parkinson’s disease symptoms: Tricyclic antidepressants increase risk of adverse events from anticholinergic drugs. Selective serotonin reuptake inhibitors (SSRIs) can exacerbate tremor and may increase risk of serotonin syndrome when combined with MAO‐B inhibitors.
Dr. Citrome was not aware of any antidepressant drugs that have been tested specifically in Parkinson’s disease patients, though “I’d be surprised if there wasn’t,” he said during the Q&A session. “There’s no one perfect antidepressant for people with depression associated with Parkinson’s disease. I would make sure to select one that they would tolerate and be willing to take and that doesn’t interfere with their treatment of their movement disorder, and (I would make sure) that there’s no drug-drug interaction,” he said.
This can include reduced working memory, learning, and planning, and generally does not manifest until at least 1 year after motor symptoms have begun. Rivastigmine is Food and Drug Administration–approved for treatment of cognitive impairment in Parkinson’s disease.
As many as 60% of Parkinson’s disease patients suffer from psychosis at some point, often visual hallucinations or delusions, which can include beliefs of spousal infidelity.
Many clinicians prescribe quetiapine off label, but there are not compelling data to support that it reduces intensity and frequency of hallucinations and delusions, according to Dr. Citrome. However, it is relatively easy to prescribe, requiring no preauthorizations, it is inexpensive, and it may improve sleep.
The FDA approved pimavanserin in 2016 for hallucinations and delusions in Parkinson’s disease, and it doesn’t worsen motor symptoms, Dr. Citrome said. That’s because pimavanserin is a highly selective antagonist of the 5-HT2A receptor, with no effect on dopaminergic, histaminergic, adrenergic, or muscarinic receptors.
The drug improves positive symptoms beginning at days 29 and 43, compared with placebo. An analysis by Dr. Citrome’s group found a number needed to treat (NNT) of 7 to gain a benefit over placebo if the metric is a ≥ 30% reduction in baseline symptom score. The drug had an NNT of 9 to achieve a ≥ 50% reduction, and an NNT of 5 to achieve a score of much improved or very much improved on the Clinical Global Impression–Improvement (CGI-I) scale. In general, an NNT less than 10 suggests that a drug is clinically useful.
In contrast, the number needed to harm (NNH) represents the number of patients who would need to receive a therapy to add one adverse event, compared with placebo. A number greater than 10 indicates that the therapy may be tolerable.
Using various measures, the NNH was well over 10 for pimavanserin. With respect to somnolence, the NNH over placebo was 138, and for a weight gain of 7% or more, the NNH was 594.
Overall, the study found that 4 patients would need to be treated to achieve a benefit over placebo with respect to a ≥ 3–point improvement in the Scale of Positive Symptoms–Parkinson’s Disease (SAPS-PD), while 21 would need to receive the drug to lead to one additional discontinuation because of an adverse event, compared to placebo.
When researchers compared pimavanserin to off-label use of quetiapine, olanzapine, and clozapine, they found a Cohen’s d value of 0.50, which was better than quetiapine and olanzapine, but lower than for clozapine. However, there is no requirement of blood monitoring, and clozapine can potentially worsen motor symptoms.
Dr. Citrome’s presentation should be a reminder to neurologists that psychiatric disorders are an important patient concern, said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience at the University of Cincinnati, who moderated the session.
“I think this serves as a model to recognize that many neurological disorders actually present with numerous psychiatric disorders,” Dr. Nasrallah said during the meeting, presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Dr. Citrome has consulted for AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer-Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular, Janssen, Karuna, Lundbeck, Lyndra, MedAvante-ProPhase, Merck, Neurocrine, Noven, Otsuka, Ovid, Relmada, Sage, Sunovion, and Teva. He has been a speaker for most of those companies, and he holds stock in Bristol Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer.
Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan, Boehringer-Ingelheim, Indivior, Intra-Cellular, Janssen, Neurocrine, Otsuka, Sunovion, and Teva. He has served on a speakers bureau for most of those companies, in addition to that of Noven.
The hallmark of Parkinson’s disease is the accompanying motor symptoms, but the condition can bring other challenges. Among those are nonmotor symptoms, including depression, dementia, and even psychosis.
The culprit is Lewy bodies, which are also responsible for Lewy body dementia. “What we call Lewy body dementia and Parkinson’s disease are caused by the same pathological process – the formation of Lewy bodies in the brain,” Leslie Citrome, MD, MPH, said in an interview. Dr. Citrome discussed some of the psychiatric comorbidities associated with Parkinson’s disease at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In fact, the association goes both ways. “Many people with Parkinson’s disease develop a dementia. Many people with Lewy body dementia develop motor symptoms that look just like Parkinson’s disease,” said Dr. Citrome, professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, and president of the American Society for Clinical Psychopharmacology.
The motor symptoms of Parkinson’s disease are generally attributable to loss of striatal dopaminergic neurons, while nonmotor symptoms can be traced to loss of neurons in nondopaminergic regions. Nonmotor symptoms – often including sleep disorders, depression, cognitive changes, and psychosis – may occur before motor symptoms. Other problems may include autonomic dysfunction, such as constipation, sexual dysfunction, sweating, or urinary retention.
Patients might not be aware that nonmotor symptoms can occur with Parkinson’s disease and may not even consider mentioning mood changes or hallucinations to their neurologist. Family members may also be unaware.
Sleep problems are common in Parkinson’s disease, including rapid eye-movement sleep behavior disorders, vivid dreams, restless legs syndrome, insomnia, and daytime somnolence. Dopamine agonists may also cause unintended sleep.
Depression is extremely common, affecting up to 90% of Parkinson’s disease patients, and this may be related to dopaminergic losses. Antidepressant medications can worsen Parkinson’s disease symptoms: Tricyclic antidepressants increase risk of adverse events from anticholinergic drugs. Selective serotonin reuptake inhibitors (SSRIs) can exacerbate tremor and may increase risk of serotonin syndrome when combined with MAO‐B inhibitors.
Dr. Citrome was not aware of any antidepressant drugs that have been tested specifically in Parkinson’s disease patients, though “I’d be surprised if there wasn’t,” he said during the Q&A session. “There’s no one perfect antidepressant for people with depression associated with Parkinson’s disease. I would make sure to select one that they would tolerate and be willing to take and that doesn’t interfere with their treatment of their movement disorder, and (I would make sure) that there’s no drug-drug interaction,” he said.
This can include reduced working memory, learning, and planning, and generally does not manifest until at least 1 year after motor symptoms have begun. Rivastigmine is Food and Drug Administration–approved for treatment of cognitive impairment in Parkinson’s disease.
As many as 60% of Parkinson’s disease patients suffer from psychosis at some point, often visual hallucinations or delusions, which can include beliefs of spousal infidelity.
Many clinicians prescribe quetiapine off label, but there are not compelling data to support that it reduces intensity and frequency of hallucinations and delusions, according to Dr. Citrome. However, it is relatively easy to prescribe, requiring no preauthorizations, it is inexpensive, and it may improve sleep.
The FDA approved pimavanserin in 2016 for hallucinations and delusions in Parkinson’s disease, and it doesn’t worsen motor symptoms, Dr. Citrome said. That’s because pimavanserin is a highly selective antagonist of the 5-HT2A receptor, with no effect on dopaminergic, histaminergic, adrenergic, or muscarinic receptors.
The drug improves positive symptoms beginning at days 29 and 43, compared with placebo. An analysis by Dr. Citrome’s group found a number needed to treat (NNT) of 7 to gain a benefit over placebo if the metric is a ≥ 30% reduction in baseline symptom score. The drug had an NNT of 9 to achieve a ≥ 50% reduction, and an NNT of 5 to achieve a score of much improved or very much improved on the Clinical Global Impression–Improvement (CGI-I) scale. In general, an NNT less than 10 suggests that a drug is clinically useful.
In contrast, the number needed to harm (NNH) represents the number of patients who would need to receive a therapy to add one adverse event, compared with placebo. A number greater than 10 indicates that the therapy may be tolerable.
Using various measures, the NNH was well over 10 for pimavanserin. With respect to somnolence, the NNH over placebo was 138, and for a weight gain of 7% or more, the NNH was 594.
Overall, the study found that 4 patients would need to be treated to achieve a benefit over placebo with respect to a ≥ 3–point improvement in the Scale of Positive Symptoms–Parkinson’s Disease (SAPS-PD), while 21 would need to receive the drug to lead to one additional discontinuation because of an adverse event, compared to placebo.
When researchers compared pimavanserin to off-label use of quetiapine, olanzapine, and clozapine, they found a Cohen’s d value of 0.50, which was better than quetiapine and olanzapine, but lower than for clozapine. However, there is no requirement of blood monitoring, and clozapine can potentially worsen motor symptoms.
Dr. Citrome’s presentation should be a reminder to neurologists that psychiatric disorders are an important patient concern, said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience at the University of Cincinnati, who moderated the session.
“I think this serves as a model to recognize that many neurological disorders actually present with numerous psychiatric disorders,” Dr. Nasrallah said during the meeting, presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Dr. Citrome has consulted for AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer-Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular, Janssen, Karuna, Lundbeck, Lyndra, MedAvante-ProPhase, Merck, Neurocrine, Noven, Otsuka, Ovid, Relmada, Sage, Sunovion, and Teva. He has been a speaker for most of those companies, and he holds stock in Bristol Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer.
Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan, Boehringer-Ingelheim, Indivior, Intra-Cellular, Janssen, Neurocrine, Otsuka, Sunovion, and Teva. He has served on a speakers bureau for most of those companies, in addition to that of Noven.
The hallmark of Parkinson’s disease is the accompanying motor symptoms, but the condition can bring other challenges. Among those are nonmotor symptoms, including depression, dementia, and even psychosis.
The culprit is Lewy bodies, which are also responsible for Lewy body dementia. “What we call Lewy body dementia and Parkinson’s disease are caused by the same pathological process – the formation of Lewy bodies in the brain,” Leslie Citrome, MD, MPH, said in an interview. Dr. Citrome discussed some of the psychiatric comorbidities associated with Parkinson’s disease at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In fact, the association goes both ways. “Many people with Parkinson’s disease develop a dementia. Many people with Lewy body dementia develop motor symptoms that look just like Parkinson’s disease,” said Dr. Citrome, professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, and president of the American Society for Clinical Psychopharmacology.
The motor symptoms of Parkinson’s disease are generally attributable to loss of striatal dopaminergic neurons, while nonmotor symptoms can be traced to loss of neurons in nondopaminergic regions. Nonmotor symptoms – often including sleep disorders, depression, cognitive changes, and psychosis – may occur before motor symptoms. Other problems may include autonomic dysfunction, such as constipation, sexual dysfunction, sweating, or urinary retention.
Patients might not be aware that nonmotor symptoms can occur with Parkinson’s disease and may not even consider mentioning mood changes or hallucinations to their neurologist. Family members may also be unaware.
Sleep problems are common in Parkinson’s disease, including rapid eye-movement sleep behavior disorders, vivid dreams, restless legs syndrome, insomnia, and daytime somnolence. Dopamine agonists may also cause unintended sleep.
Depression is extremely common, affecting up to 90% of Parkinson’s disease patients, and this may be related to dopaminergic losses. Antidepressant medications can worsen Parkinson’s disease symptoms: Tricyclic antidepressants increase risk of adverse events from anticholinergic drugs. Selective serotonin reuptake inhibitors (SSRIs) can exacerbate tremor and may increase risk of serotonin syndrome when combined with MAO‐B inhibitors.
Dr. Citrome was not aware of any antidepressant drugs that have been tested specifically in Parkinson’s disease patients, though “I’d be surprised if there wasn’t,” he said during the Q&A session. “There’s no one perfect antidepressant for people with depression associated with Parkinson’s disease. I would make sure to select one that they would tolerate and be willing to take and that doesn’t interfere with their treatment of their movement disorder, and (I would make sure) that there’s no drug-drug interaction,” he said.
This can include reduced working memory, learning, and planning, and generally does not manifest until at least 1 year after motor symptoms have begun. Rivastigmine is Food and Drug Administration–approved for treatment of cognitive impairment in Parkinson’s disease.
As many as 60% of Parkinson’s disease patients suffer from psychosis at some point, often visual hallucinations or delusions, which can include beliefs of spousal infidelity.
Many clinicians prescribe quetiapine off label, but there are not compelling data to support that it reduces intensity and frequency of hallucinations and delusions, according to Dr. Citrome. However, it is relatively easy to prescribe, requiring no preauthorizations, it is inexpensive, and it may improve sleep.
The FDA approved pimavanserin in 2016 for hallucinations and delusions in Parkinson’s disease, and it doesn’t worsen motor symptoms, Dr. Citrome said. That’s because pimavanserin is a highly selective antagonist of the 5-HT2A receptor, with no effect on dopaminergic, histaminergic, adrenergic, or muscarinic receptors.
The drug improves positive symptoms beginning at days 29 and 43, compared with placebo. An analysis by Dr. Citrome’s group found a number needed to treat (NNT) of 7 to gain a benefit over placebo if the metric is a ≥ 30% reduction in baseline symptom score. The drug had an NNT of 9 to achieve a ≥ 50% reduction, and an NNT of 5 to achieve a score of much improved or very much improved on the Clinical Global Impression–Improvement (CGI-I) scale. In general, an NNT less than 10 suggests that a drug is clinically useful.
In contrast, the number needed to harm (NNH) represents the number of patients who would need to receive a therapy to add one adverse event, compared with placebo. A number greater than 10 indicates that the therapy may be tolerable.
Using various measures, the NNH was well over 10 for pimavanserin. With respect to somnolence, the NNH over placebo was 138, and for a weight gain of 7% or more, the NNH was 594.
Overall, the study found that 4 patients would need to be treated to achieve a benefit over placebo with respect to a ≥ 3–point improvement in the Scale of Positive Symptoms–Parkinson’s Disease (SAPS-PD), while 21 would need to receive the drug to lead to one additional discontinuation because of an adverse event, compared to placebo.
When researchers compared pimavanserin to off-label use of quetiapine, olanzapine, and clozapine, they found a Cohen’s d value of 0.50, which was better than quetiapine and olanzapine, but lower than for clozapine. However, there is no requirement of blood monitoring, and clozapine can potentially worsen motor symptoms.
Dr. Citrome’s presentation should be a reminder to neurologists that psychiatric disorders are an important patient concern, said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience at the University of Cincinnati, who moderated the session.
“I think this serves as a model to recognize that many neurological disorders actually present with numerous psychiatric disorders,” Dr. Nasrallah said during the meeting, presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Dr. Citrome has consulted for AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer-Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular, Janssen, Karuna, Lundbeck, Lyndra, MedAvante-ProPhase, Merck, Neurocrine, Noven, Otsuka, Ovid, Relmada, Sage, Sunovion, and Teva. He has been a speaker for most of those companies, and he holds stock in Bristol Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer.
Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan, Boehringer-Ingelheim, Indivior, Intra-Cellular, Janssen, Neurocrine, Otsuka, Sunovion, and Teva. He has served on a speakers bureau for most of those companies, in addition to that of Noven.
FROM FOCUS ON NEUROPSYCHIATRY 2021
Stimulating jobs may help stave off dementia onset
Individuals with cognitively stimulating jobs are at a lower risk of developing dementia than their peers with less challenging employment, new research suggests.
Results from a large, multicohort study also showed an association between cognitive stimulation and lower levels of certain plasma proteins, providing possible clues on a protective biological mechanism.
“These new findings support the hypothesis that mental stimulation in adulthood may postpone the onset of dementia,” Mika Kivimäki, PhD, professor and director of the Whitehall II Study, department of epidemiology, University College London, said in an interview.
The results were published online Aug. 19, 2021, in the BMJ.
‘Work fast and hard’
Researchers assessed the association between workplace cognitive stimulation and dementia incidence in seven cohorts that included almost 108,000 men and women (mean age, 44.6 years). All were free of dementia at baseline.
Participants included civil servants, public sector employees, forestry workers, and others from the general working population.
Investigators separated the participants into three categories of workplace cognitive stimulation: “high,” which referred to both high job demand and high job control; “low,” which referred to low demands and low control; and “medium,” which referred to all other combinations of job demand and job control.
“Highly cognitively stimulating jobs require you to work fast and hard, learn new things, be creative, and have a high level of skill,” said Dr. Kivimäki.
The researchers controlled for low education, hypertension, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, and traumatic brain injury. These represent 10 of the 12 dementia risk factors named by the 2020 Lancet Commission on Dementia Prevention as having convincing evidence, Dr. Kivimäki noted.
Although the investigators had no data on the other two risk factors of hearing loss and air pollution, these are unlikely to be confounding factors, he said.
Follow-up for incident dementia varied from 13.7 to 30.1 years, depending on the cohort, and was 16.7 years in the total patient population. The mean age at dementia onset was 71.2 years.
Benefits across the life course
Results showed that incident dementia per 10,000 person years was 7.3 in the low–cognitive stimulation group and 4.8 in the high-stimulation group, for a difference of 2.5.
“These differences were relatively small because the incidence of dementia in this relatively young population was low,” Dr. Kivimäki said.
Compared with those with low stimulation, the adjusted hazard ratio for dementia for this with high stimulation was 0.77 (95% CI, 0.65-0.92).
The results were similar for men and women, and for those younger and older than 60 years. However, the link between workplace cognitive stimulation appeared stronger for Alzheimer’s disease than for other dementias.
There also appeared to be additive effects of higher cognitive stimulation in both childhood, as indicated by higher educational attainment, and adulthood, based on work characteristics, said Dr. Kivimäki.
“These findings support the benefits of cognitive stimulation across the life course, with education leading to higher peak cognitive performance and cognitive stimulation at work lowering age-related cognitive decline,” he added.
The findings don’t seem to be the result of workers with cognitive impairment remaining in unchallenging jobs, he noted. Separate analyses showed lower dementia incidence even when 10 years or more separated the assessment of cognitive stimulation and the dementia diagnosis.
“This suggests that the findings are unlikely to be biased due to reverse causation,” Dr. Kivimäki said.
Possible mechanism
Findings were similar when the researchers assessed effect from job changes. “This is probably because people in highly stimulating jobs are more likely to change to another highly stimulating job than to a low-stimulating job,” said Dr. Kivimäki. “Similarly, people with less stimulating jobs are seldom able to change to a substantially more stimulating job.”
As a dementia risk factor, low workplace stimulation is comparable with high alcohol intake and physical inactivity, but is weaker than education, diabetes, smoking, hypertension, and obesity, Dr. Kivimäki noted.
When asked about individuals with less cognitively stimulating jobs who are enormously stimulated outside work, he said that “previous large-scale studies have failed to find evidence that leisure time cognitive activity would significantly reduce risk of dementia.”
To explore potential underlying mechanisms, the investigators examined almost 5,000 plasma proteins in more than 2,200 individuals from one cohort in the Whitehall II study. They found six proteins were significantly lower among participants with high versus low cognitive stimulation.
In another analysis that included more than 13,500 participants from the Whitehall and another cohort, higher levels of three of these plasma proteins were associated with increased dementia risk – or conversely, lower protein levels with lower dementia risk.
The findings suggest a “novel plausible explanation” for the link between workplace cognitive stimulation and dementia risk, said Dr. Kivimäki.
He noted that higher levels of certain proteins prevent brain cells from forming new connections.
‘Some of the most compelling evidence to date’
In an accompanying editorial, Serhiy Dekhtyar, PhD, assistant professor (Docent), Aging Research Center, Karolinska Institute, Stockholm, noted that the study is “an important piece of work” and “some of the most compelling evidence to date” on the role of occupational cognitive stimulation in dementia risk.
The large-scale investigation in multiple cohorts and contexts has “advanced the field” and could help “explain previously mixed findings in the literature,” Dekhtyar said in an interview.
Importantly, the researchers provide “an indication of biological mechanisms potentially connecting work mental stimulation and dementia,” he added.
However, Dr. Dekhtyar noted that the difference of 2.5 incident cases of dementia per 10,000 person years of follow-up between the low and high mental-stimulation groups “is not especially large” – although it is comparable with other established risk factors for dementia.
He suspects the effect size would have been larger had the follow-up for dementia been longer.
Dr. Dekhtyar also raised the possibility that “innate cognition” might affect both educational and occupational attainment, and the subsequent dementia risk.
“Without taking this into account, we may inadvertently conclude that education or occupational stimulation help differentially preserve cognition into late life – when in reality, it may be initial differences in cognitive ability that are preserved throughout life,” he concluded.
Funding sources for the study included Nordic Research Programme on Health and Welfare (NordForsk), Medical Research Council, Wellcome Trust, Academy of Finland, and Helsinki Institute of Life Science. Dr. Kivimäki has received support from NordForsk, the UK Medical Research Council, the Wellcome Trust, the Academy of Finland, and the Helsinki Institute of Life Science. Dr. Dekhtyar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with cognitively stimulating jobs are at a lower risk of developing dementia than their peers with less challenging employment, new research suggests.
Results from a large, multicohort study also showed an association between cognitive stimulation and lower levels of certain plasma proteins, providing possible clues on a protective biological mechanism.
“These new findings support the hypothesis that mental stimulation in adulthood may postpone the onset of dementia,” Mika Kivimäki, PhD, professor and director of the Whitehall II Study, department of epidemiology, University College London, said in an interview.
The results were published online Aug. 19, 2021, in the BMJ.
‘Work fast and hard’
Researchers assessed the association between workplace cognitive stimulation and dementia incidence in seven cohorts that included almost 108,000 men and women (mean age, 44.6 years). All were free of dementia at baseline.
Participants included civil servants, public sector employees, forestry workers, and others from the general working population.
Investigators separated the participants into three categories of workplace cognitive stimulation: “high,” which referred to both high job demand and high job control; “low,” which referred to low demands and low control; and “medium,” which referred to all other combinations of job demand and job control.
“Highly cognitively stimulating jobs require you to work fast and hard, learn new things, be creative, and have a high level of skill,” said Dr. Kivimäki.
The researchers controlled for low education, hypertension, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, and traumatic brain injury. These represent 10 of the 12 dementia risk factors named by the 2020 Lancet Commission on Dementia Prevention as having convincing evidence, Dr. Kivimäki noted.
Although the investigators had no data on the other two risk factors of hearing loss and air pollution, these are unlikely to be confounding factors, he said.
Follow-up for incident dementia varied from 13.7 to 30.1 years, depending on the cohort, and was 16.7 years in the total patient population. The mean age at dementia onset was 71.2 years.
Benefits across the life course
Results showed that incident dementia per 10,000 person years was 7.3 in the low–cognitive stimulation group and 4.8 in the high-stimulation group, for a difference of 2.5.
“These differences were relatively small because the incidence of dementia in this relatively young population was low,” Dr. Kivimäki said.
Compared with those with low stimulation, the adjusted hazard ratio for dementia for this with high stimulation was 0.77 (95% CI, 0.65-0.92).
The results were similar for men and women, and for those younger and older than 60 years. However, the link between workplace cognitive stimulation appeared stronger for Alzheimer’s disease than for other dementias.
There also appeared to be additive effects of higher cognitive stimulation in both childhood, as indicated by higher educational attainment, and adulthood, based on work characteristics, said Dr. Kivimäki.
“These findings support the benefits of cognitive stimulation across the life course, with education leading to higher peak cognitive performance and cognitive stimulation at work lowering age-related cognitive decline,” he added.
The findings don’t seem to be the result of workers with cognitive impairment remaining in unchallenging jobs, he noted. Separate analyses showed lower dementia incidence even when 10 years or more separated the assessment of cognitive stimulation and the dementia diagnosis.
“This suggests that the findings are unlikely to be biased due to reverse causation,” Dr. Kivimäki said.
Possible mechanism
Findings were similar when the researchers assessed effect from job changes. “This is probably because people in highly stimulating jobs are more likely to change to another highly stimulating job than to a low-stimulating job,” said Dr. Kivimäki. “Similarly, people with less stimulating jobs are seldom able to change to a substantially more stimulating job.”
As a dementia risk factor, low workplace stimulation is comparable with high alcohol intake and physical inactivity, but is weaker than education, diabetes, smoking, hypertension, and obesity, Dr. Kivimäki noted.
When asked about individuals with less cognitively stimulating jobs who are enormously stimulated outside work, he said that “previous large-scale studies have failed to find evidence that leisure time cognitive activity would significantly reduce risk of dementia.”
To explore potential underlying mechanisms, the investigators examined almost 5,000 plasma proteins in more than 2,200 individuals from one cohort in the Whitehall II study. They found six proteins were significantly lower among participants with high versus low cognitive stimulation.
In another analysis that included more than 13,500 participants from the Whitehall and another cohort, higher levels of three of these plasma proteins were associated with increased dementia risk – or conversely, lower protein levels with lower dementia risk.
The findings suggest a “novel plausible explanation” for the link between workplace cognitive stimulation and dementia risk, said Dr. Kivimäki.
He noted that higher levels of certain proteins prevent brain cells from forming new connections.
‘Some of the most compelling evidence to date’
In an accompanying editorial, Serhiy Dekhtyar, PhD, assistant professor (Docent), Aging Research Center, Karolinska Institute, Stockholm, noted that the study is “an important piece of work” and “some of the most compelling evidence to date” on the role of occupational cognitive stimulation in dementia risk.
The large-scale investigation in multiple cohorts and contexts has “advanced the field” and could help “explain previously mixed findings in the literature,” Dekhtyar said in an interview.
Importantly, the researchers provide “an indication of biological mechanisms potentially connecting work mental stimulation and dementia,” he added.
However, Dr. Dekhtyar noted that the difference of 2.5 incident cases of dementia per 10,000 person years of follow-up between the low and high mental-stimulation groups “is not especially large” – although it is comparable with other established risk factors for dementia.
He suspects the effect size would have been larger had the follow-up for dementia been longer.
Dr. Dekhtyar also raised the possibility that “innate cognition” might affect both educational and occupational attainment, and the subsequent dementia risk.
“Without taking this into account, we may inadvertently conclude that education or occupational stimulation help differentially preserve cognition into late life – when in reality, it may be initial differences in cognitive ability that are preserved throughout life,” he concluded.
Funding sources for the study included Nordic Research Programme on Health and Welfare (NordForsk), Medical Research Council, Wellcome Trust, Academy of Finland, and Helsinki Institute of Life Science. Dr. Kivimäki has received support from NordForsk, the UK Medical Research Council, the Wellcome Trust, the Academy of Finland, and the Helsinki Institute of Life Science. Dr. Dekhtyar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with cognitively stimulating jobs are at a lower risk of developing dementia than their peers with less challenging employment, new research suggests.
Results from a large, multicohort study also showed an association between cognitive stimulation and lower levels of certain plasma proteins, providing possible clues on a protective biological mechanism.
“These new findings support the hypothesis that mental stimulation in adulthood may postpone the onset of dementia,” Mika Kivimäki, PhD, professor and director of the Whitehall II Study, department of epidemiology, University College London, said in an interview.
The results were published online Aug. 19, 2021, in the BMJ.
‘Work fast and hard’
Researchers assessed the association between workplace cognitive stimulation and dementia incidence in seven cohorts that included almost 108,000 men and women (mean age, 44.6 years). All were free of dementia at baseline.
Participants included civil servants, public sector employees, forestry workers, and others from the general working population.
Investigators separated the participants into three categories of workplace cognitive stimulation: “high,” which referred to both high job demand and high job control; “low,” which referred to low demands and low control; and “medium,” which referred to all other combinations of job demand and job control.
“Highly cognitively stimulating jobs require you to work fast and hard, learn new things, be creative, and have a high level of skill,” said Dr. Kivimäki.
The researchers controlled for low education, hypertension, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, and traumatic brain injury. These represent 10 of the 12 dementia risk factors named by the 2020 Lancet Commission on Dementia Prevention as having convincing evidence, Dr. Kivimäki noted.
Although the investigators had no data on the other two risk factors of hearing loss and air pollution, these are unlikely to be confounding factors, he said.
Follow-up for incident dementia varied from 13.7 to 30.1 years, depending on the cohort, and was 16.7 years in the total patient population. The mean age at dementia onset was 71.2 years.
Benefits across the life course
Results showed that incident dementia per 10,000 person years was 7.3 in the low–cognitive stimulation group and 4.8 in the high-stimulation group, for a difference of 2.5.
“These differences were relatively small because the incidence of dementia in this relatively young population was low,” Dr. Kivimäki said.
Compared with those with low stimulation, the adjusted hazard ratio for dementia for this with high stimulation was 0.77 (95% CI, 0.65-0.92).
The results were similar for men and women, and for those younger and older than 60 years. However, the link between workplace cognitive stimulation appeared stronger for Alzheimer’s disease than for other dementias.
There also appeared to be additive effects of higher cognitive stimulation in both childhood, as indicated by higher educational attainment, and adulthood, based on work characteristics, said Dr. Kivimäki.
“These findings support the benefits of cognitive stimulation across the life course, with education leading to higher peak cognitive performance and cognitive stimulation at work lowering age-related cognitive decline,” he added.
The findings don’t seem to be the result of workers with cognitive impairment remaining in unchallenging jobs, he noted. Separate analyses showed lower dementia incidence even when 10 years or more separated the assessment of cognitive stimulation and the dementia diagnosis.
“This suggests that the findings are unlikely to be biased due to reverse causation,” Dr. Kivimäki said.
Possible mechanism
Findings were similar when the researchers assessed effect from job changes. “This is probably because people in highly stimulating jobs are more likely to change to another highly stimulating job than to a low-stimulating job,” said Dr. Kivimäki. “Similarly, people with less stimulating jobs are seldom able to change to a substantially more stimulating job.”
As a dementia risk factor, low workplace stimulation is comparable with high alcohol intake and physical inactivity, but is weaker than education, diabetes, smoking, hypertension, and obesity, Dr. Kivimäki noted.
When asked about individuals with less cognitively stimulating jobs who are enormously stimulated outside work, he said that “previous large-scale studies have failed to find evidence that leisure time cognitive activity would significantly reduce risk of dementia.”
To explore potential underlying mechanisms, the investigators examined almost 5,000 plasma proteins in more than 2,200 individuals from one cohort in the Whitehall II study. They found six proteins were significantly lower among participants with high versus low cognitive stimulation.
In another analysis that included more than 13,500 participants from the Whitehall and another cohort, higher levels of three of these plasma proteins were associated with increased dementia risk – or conversely, lower protein levels with lower dementia risk.
The findings suggest a “novel plausible explanation” for the link between workplace cognitive stimulation and dementia risk, said Dr. Kivimäki.
He noted that higher levels of certain proteins prevent brain cells from forming new connections.
‘Some of the most compelling evidence to date’
In an accompanying editorial, Serhiy Dekhtyar, PhD, assistant professor (Docent), Aging Research Center, Karolinska Institute, Stockholm, noted that the study is “an important piece of work” and “some of the most compelling evidence to date” on the role of occupational cognitive stimulation in dementia risk.
The large-scale investigation in multiple cohorts and contexts has “advanced the field” and could help “explain previously mixed findings in the literature,” Dekhtyar said in an interview.
Importantly, the researchers provide “an indication of biological mechanisms potentially connecting work mental stimulation and dementia,” he added.
However, Dr. Dekhtyar noted that the difference of 2.5 incident cases of dementia per 10,000 person years of follow-up between the low and high mental-stimulation groups “is not especially large” – although it is comparable with other established risk factors for dementia.
He suspects the effect size would have been larger had the follow-up for dementia been longer.
Dr. Dekhtyar also raised the possibility that “innate cognition” might affect both educational and occupational attainment, and the subsequent dementia risk.
“Without taking this into account, we may inadvertently conclude that education or occupational stimulation help differentially preserve cognition into late life – when in reality, it may be initial differences in cognitive ability that are preserved throughout life,” he concluded.
Funding sources for the study included Nordic Research Programme on Health and Welfare (NordForsk), Medical Research Council, Wellcome Trust, Academy of Finland, and Helsinki Institute of Life Science. Dr. Kivimäki has received support from NordForsk, the UK Medical Research Council, the Wellcome Trust, the Academy of Finland, and the Helsinki Institute of Life Science. Dr. Dekhtyar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Explosive aggression may be neurologic
Aggression is an underappreciated mental health issue, and biological mechanisms might help explain more extreme forms like intermittent explosive disorder (IED), which is characterized by episodes of sudden impulses and inappropriate aggression, violence, or even verbal outbursts. IED can lead to road rage, domestic abuse, in addition to throwing objects and engaging in other destructive behaviors.
Despite those consequences, aggression hasn’t gained the same level of attention as other psychiatric conditions, according to Emil F. Coccaro, MD, who spoke about the topic at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“People seem to think that aggressive behavior is bad behavior, and therefore people just need an attitude adjustment. So there’s this sort of stigma, and there are no advocacy groups for it. There are no poster children for it. But there’s a whole lot of biology and neuroscience behind it,” said Dr. Coccaro, in an interview. He is a professor and vice chair of research in psychiatry and behavioral health at Ohio State University, Columbus.
, who spoke at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is a general view that psychiatric conditions may lead to increased aggression, but there is little evidence of that. “As a general statement, having a psychological [illness] in and of itself does not really increase the risk of being aggressive. What does is being aggressive in general, and substance use disorder. And the thing with [people who have] substance use disorders is that they only get aggressive when they are aggressive to begin with,” said Dr. Coccaro, noting that the strongest case for the relationship surrounds alcohol abuse.
The DSM-5 criteria for IED include: verbal or physical aggression without destruction, at least twice per week, or three or more episodes of assault or physical destruction within a year. The behavior must be out of proportion to the provocation, cause distress or impairment, and not be accountable by other diagnoses. “If they’re blowing up twice a week, for a few months, and usually they’re doing it for a long time, then that’s different than just blowing up very occasionally. Healthy people, nonaggressive people, maybe they blow up once a year, or even less frequently than that,” Dr. Coccaro said.
Functional magnetic resonance imaging and other imaging studies consistently show differences associated with aggression.
“The IEDs really do distinguish themselves from the psychiatric controls. They also have other stuff going on with them; they have a hostile attribution. And they’re kind of irritable at baseline. They’re not walking around irritable all the time, but the people around them may be walking on eggshells,” Dr. Coccaro said.
The results from these sorts of studies aren’t fully conclusive and can’t be used for diagnosis, in part because of a lack of power. “It’s hard to do these MRI studies and lots and lots of subjects, because they’re kind of expensive,” Dr. Coccaro said. “We’re just not there yet.”
Other, less expensive imaging techniques like near-infrared spectroscopy may improve matters. “That might be something down the road that could lead to something (diagnostic). Right now, most imaging studies are being done to really understand mechanisms,” said Dr. Coccaro.
Those mechanistic studies suggest that the culprit for IED may be a combination of too much drive from subcortical structures like the amygdala and insufficient inhibitor function in the frontal part of the brain. The frontal cortex may suffer a loss of gray matter, according to Dr. Coccaro, and there may be insufficient connectivity, which could weaken signals coming from the frontal areas that might otherwise inhibit lower centers of the brain.
Treatment for IED could be aimed at improving that connectivity and signaling. Ketamine and other anesthetic agents like nitrous oxide may increase connectivity to nerve cells by increasing branching at synaptic dendrites.
Selective serotonin reuptake inhibitors have the potential to treat IED, but their utility is limited because they bind to the presynaptic transporter for serotonin, and more aggressive people have fewer of those transporters. “You only get so much bang for your buck,” Dr. Coccaro said.
Cognitive-behavioral therapy that focuses on anger management and relaxation shows promise. “CBT does help people deal with what’s coming at them. So it’s like, ‘oh, I’m getting angry, I better start doing those relaxation (techniques).’ It teaches them to rethink things.”
During the Q&A session following the presentation, Henry A. Nasrallah, MD, who moderated the session, pointed out that misattribution can occur, leading an affected individual to misread someone’s facial expression and react aggressively, which is a problem also seen in psychosis.
“There are studies showing [that if] you show them a series of faces with different affects, many times paranoid patients read a normal facial expression as threatening. So it may be that it’s the same thing with aggression,” said Dr. Nasrallah, who is a professor of psychiatry, neurology, and neuroscience at the University of Cincinnati.
In the midst of the ongoing COVID-19 pandemic, it’s also possible that mask-wearing could improve or worsen such misunderstandings. “There is expression in the eyes that you can see, but you miss a lot,” Dr. Coccaro said.
For now, the effects of masks remain largely unknown. But that will change. “Sooner or later we will have a bunch of papers coming out about how masks have changed a lot of behaviors,” Dr. Nasrallah said.
Dr. Coccaro has consulted for Avanir, Azevan, and Brackett. Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan Janssen, Otsuka, Indivior, IntraCellular, Neurocrine, Sunovion, Teva, and Boehringer-Ingelheim. Dr. Nasrallah has been on a speaker’s bureau for Acadia, Alkermes, Allergan, Janssen, Otsuka, Indivior, Intracellular, Neurocrine, Noven, Sunovion, and Teva.
Aggression is an underappreciated mental health issue, and biological mechanisms might help explain more extreme forms like intermittent explosive disorder (IED), which is characterized by episodes of sudden impulses and inappropriate aggression, violence, or even verbal outbursts. IED can lead to road rage, domestic abuse, in addition to throwing objects and engaging in other destructive behaviors.
Despite those consequences, aggression hasn’t gained the same level of attention as other psychiatric conditions, according to Emil F. Coccaro, MD, who spoke about the topic at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“People seem to think that aggressive behavior is bad behavior, and therefore people just need an attitude adjustment. So there’s this sort of stigma, and there are no advocacy groups for it. There are no poster children for it. But there’s a whole lot of biology and neuroscience behind it,” said Dr. Coccaro, in an interview. He is a professor and vice chair of research in psychiatry and behavioral health at Ohio State University, Columbus.
, who spoke at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is a general view that psychiatric conditions may lead to increased aggression, but there is little evidence of that. “As a general statement, having a psychological [illness] in and of itself does not really increase the risk of being aggressive. What does is being aggressive in general, and substance use disorder. And the thing with [people who have] substance use disorders is that they only get aggressive when they are aggressive to begin with,” said Dr. Coccaro, noting that the strongest case for the relationship surrounds alcohol abuse.
The DSM-5 criteria for IED include: verbal or physical aggression without destruction, at least twice per week, or three or more episodes of assault or physical destruction within a year. The behavior must be out of proportion to the provocation, cause distress or impairment, and not be accountable by other diagnoses. “If they’re blowing up twice a week, for a few months, and usually they’re doing it for a long time, then that’s different than just blowing up very occasionally. Healthy people, nonaggressive people, maybe they blow up once a year, or even less frequently than that,” Dr. Coccaro said.
Functional magnetic resonance imaging and other imaging studies consistently show differences associated with aggression.
“The IEDs really do distinguish themselves from the psychiatric controls. They also have other stuff going on with them; they have a hostile attribution. And they’re kind of irritable at baseline. They’re not walking around irritable all the time, but the people around them may be walking on eggshells,” Dr. Coccaro said.
The results from these sorts of studies aren’t fully conclusive and can’t be used for diagnosis, in part because of a lack of power. “It’s hard to do these MRI studies and lots and lots of subjects, because they’re kind of expensive,” Dr. Coccaro said. “We’re just not there yet.”
Other, less expensive imaging techniques like near-infrared spectroscopy may improve matters. “That might be something down the road that could lead to something (diagnostic). Right now, most imaging studies are being done to really understand mechanisms,” said Dr. Coccaro.
Those mechanistic studies suggest that the culprit for IED may be a combination of too much drive from subcortical structures like the amygdala and insufficient inhibitor function in the frontal part of the brain. The frontal cortex may suffer a loss of gray matter, according to Dr. Coccaro, and there may be insufficient connectivity, which could weaken signals coming from the frontal areas that might otherwise inhibit lower centers of the brain.
Treatment for IED could be aimed at improving that connectivity and signaling. Ketamine and other anesthetic agents like nitrous oxide may increase connectivity to nerve cells by increasing branching at synaptic dendrites.
Selective serotonin reuptake inhibitors have the potential to treat IED, but their utility is limited because they bind to the presynaptic transporter for serotonin, and more aggressive people have fewer of those transporters. “You only get so much bang for your buck,” Dr. Coccaro said.
Cognitive-behavioral therapy that focuses on anger management and relaxation shows promise. “CBT does help people deal with what’s coming at them. So it’s like, ‘oh, I’m getting angry, I better start doing those relaxation (techniques).’ It teaches them to rethink things.”
During the Q&A session following the presentation, Henry A. Nasrallah, MD, who moderated the session, pointed out that misattribution can occur, leading an affected individual to misread someone’s facial expression and react aggressively, which is a problem also seen in psychosis.
“There are studies showing [that if] you show them a series of faces with different affects, many times paranoid patients read a normal facial expression as threatening. So it may be that it’s the same thing with aggression,” said Dr. Nasrallah, who is a professor of psychiatry, neurology, and neuroscience at the University of Cincinnati.
In the midst of the ongoing COVID-19 pandemic, it’s also possible that mask-wearing could improve or worsen such misunderstandings. “There is expression in the eyes that you can see, but you miss a lot,” Dr. Coccaro said.
For now, the effects of masks remain largely unknown. But that will change. “Sooner or later we will have a bunch of papers coming out about how masks have changed a lot of behaviors,” Dr. Nasrallah said.
Dr. Coccaro has consulted for Avanir, Azevan, and Brackett. Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan Janssen, Otsuka, Indivior, IntraCellular, Neurocrine, Sunovion, Teva, and Boehringer-Ingelheim. Dr. Nasrallah has been on a speaker’s bureau for Acadia, Alkermes, Allergan, Janssen, Otsuka, Indivior, Intracellular, Neurocrine, Noven, Sunovion, and Teva.
Aggression is an underappreciated mental health issue, and biological mechanisms might help explain more extreme forms like intermittent explosive disorder (IED), which is characterized by episodes of sudden impulses and inappropriate aggression, violence, or even verbal outbursts. IED can lead to road rage, domestic abuse, in addition to throwing objects and engaging in other destructive behaviors.
Despite those consequences, aggression hasn’t gained the same level of attention as other psychiatric conditions, according to Emil F. Coccaro, MD, who spoke about the topic at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“People seem to think that aggressive behavior is bad behavior, and therefore people just need an attitude adjustment. So there’s this sort of stigma, and there are no advocacy groups for it. There are no poster children for it. But there’s a whole lot of biology and neuroscience behind it,” said Dr. Coccaro, in an interview. He is a professor and vice chair of research in psychiatry and behavioral health at Ohio State University, Columbus.
, who spoke at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is a general view that psychiatric conditions may lead to increased aggression, but there is little evidence of that. “As a general statement, having a psychological [illness] in and of itself does not really increase the risk of being aggressive. What does is being aggressive in general, and substance use disorder. And the thing with [people who have] substance use disorders is that they only get aggressive when they are aggressive to begin with,” said Dr. Coccaro, noting that the strongest case for the relationship surrounds alcohol abuse.
The DSM-5 criteria for IED include: verbal or physical aggression without destruction, at least twice per week, or three or more episodes of assault or physical destruction within a year. The behavior must be out of proportion to the provocation, cause distress or impairment, and not be accountable by other diagnoses. “If they’re blowing up twice a week, for a few months, and usually they’re doing it for a long time, then that’s different than just blowing up very occasionally. Healthy people, nonaggressive people, maybe they blow up once a year, or even less frequently than that,” Dr. Coccaro said.
Functional magnetic resonance imaging and other imaging studies consistently show differences associated with aggression.
“The IEDs really do distinguish themselves from the psychiatric controls. They also have other stuff going on with them; they have a hostile attribution. And they’re kind of irritable at baseline. They’re not walking around irritable all the time, but the people around them may be walking on eggshells,” Dr. Coccaro said.
The results from these sorts of studies aren’t fully conclusive and can’t be used for diagnosis, in part because of a lack of power. “It’s hard to do these MRI studies and lots and lots of subjects, because they’re kind of expensive,” Dr. Coccaro said. “We’re just not there yet.”
Other, less expensive imaging techniques like near-infrared spectroscopy may improve matters. “That might be something down the road that could lead to something (diagnostic). Right now, most imaging studies are being done to really understand mechanisms,” said Dr. Coccaro.
Those mechanistic studies suggest that the culprit for IED may be a combination of too much drive from subcortical structures like the amygdala and insufficient inhibitor function in the frontal part of the brain. The frontal cortex may suffer a loss of gray matter, according to Dr. Coccaro, and there may be insufficient connectivity, which could weaken signals coming from the frontal areas that might otherwise inhibit lower centers of the brain.
Treatment for IED could be aimed at improving that connectivity and signaling. Ketamine and other anesthetic agents like nitrous oxide may increase connectivity to nerve cells by increasing branching at synaptic dendrites.
Selective serotonin reuptake inhibitors have the potential to treat IED, but their utility is limited because they bind to the presynaptic transporter for serotonin, and more aggressive people have fewer of those transporters. “You only get so much bang for your buck,” Dr. Coccaro said.
Cognitive-behavioral therapy that focuses on anger management and relaxation shows promise. “CBT does help people deal with what’s coming at them. So it’s like, ‘oh, I’m getting angry, I better start doing those relaxation (techniques).’ It teaches them to rethink things.”
During the Q&A session following the presentation, Henry A. Nasrallah, MD, who moderated the session, pointed out that misattribution can occur, leading an affected individual to misread someone’s facial expression and react aggressively, which is a problem also seen in psychosis.
“There are studies showing [that if] you show them a series of faces with different affects, many times paranoid patients read a normal facial expression as threatening. So it may be that it’s the same thing with aggression,” said Dr. Nasrallah, who is a professor of psychiatry, neurology, and neuroscience at the University of Cincinnati.
In the midst of the ongoing COVID-19 pandemic, it’s also possible that mask-wearing could improve or worsen such misunderstandings. “There is expression in the eyes that you can see, but you miss a lot,” Dr. Coccaro said.
For now, the effects of masks remain largely unknown. But that will change. “Sooner or later we will have a bunch of papers coming out about how masks have changed a lot of behaviors,” Dr. Nasrallah said.
Dr. Coccaro has consulted for Avanir, Azevan, and Brackett. Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan Janssen, Otsuka, Indivior, IntraCellular, Neurocrine, Sunovion, Teva, and Boehringer-Ingelheim. Dr. Nasrallah has been on a speaker’s bureau for Acadia, Alkermes, Allergan, Janssen, Otsuka, Indivior, Intracellular, Neurocrine, Noven, Sunovion, and Teva.
REPORTING FROM FOCUS ON NEUROPSYCHIATRY 2021
Wisdom may counter loneliness, burnout in older adults
Wisdom increases with age, and although this personality trait is regarded as nebulous by many, there is evidence that it has biological and neuropsychiatric underpinnings. It could even hold the key to reducing loneliness and burnout among older people.
Those were some of the key messages delivered by Tanya T. Nguyen, PhD, of the department of psychiatry at the University of California, San Diego, who spoke at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“To many people, wisdom remains a fuzzy concept that’s difficult to operationalize and measure. It’s analogous to the concepts of consciousness, emotions, and cognitions, which at one point were considered nonscientific, but today we accept them as biological and scientific entities,” Dr. Nguyen said during her talk at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Interest in quantifying and studying wisdom has picked up in recent years, and Dr. Nguyen gave a definition with six elements that includes prosocial behaviors such as empathy and compassion, as well as emotional regulation, self-reflection, decisiveness, and social decision-making. She also included a spirituality component, though she conceded that this is controversial.
She noted that there are cultural variations in the definition of wisdom, but it has changed little over time, suggesting that it may be biological rather than cultural in nature, and therefore may have a neuropsychiatric underpinning.
Loss of some or all characteristics of wisdom occurs in some behaviors and disorders, including most markedly in the neurodegenerative disorder frontotemporal dementia (FTD), which is characterized by damage only in the prefrontal cortex and anterior temporal lobes. It usually occurs before age 60, and patients exhibit poor social awareness, impulsivity, antisocial behavior, and a lack of insight and empathy.
This and other lines of evidence have led to the suggestion that wisdom may be governed by processes in the prefrontal cortex and the limbic striatum. The prefrontal cortex controls executive functions such as planning, predicting, and anticipating events, as well as managing emotional reactions and impulses. “Thus, wisdom involves parts of the brain that balance cold, hard analytical reasoning with primitive desires and drives, which ultimately leads to self-regulation, social insight, theory of mind, and empathy,” said Dr. Nguyen.
Wisdom has long been associated with age, but age is also linked to cognitive decline. A recent discovery that the brain does not stop evolving at older age may help explain this contradiction. Brains develop in a back to front order, so that the prefrontal cortex is the last to mature. As we age, neural activity shifts from the occipital lobes to the prefrontal cortex and its executive decision-making power.
“The brain may recruit higher-order networks to the prefrontal cortex that are associated with wisdom development,” said Dr. Nguyen. She also pointed out that asymmetry between the left and right hemisphere is reduced with age, as tasks that relied on circuits from one hemisphere or another more often call upon both. “In order to make up for lost synapses and neurons with aging, active older adults use more neuronal networks from both hemispheres to perform the same mental activity,” Dr. Nguyen said.
Some interventions can improve scores in traits associated with wisdom in older adults, and could be an important contributor to improvements in health and longevity, said Dr. Nguyen. Randomized, controlled trials have demonstrated that psychosocial or behavioral interventions can improve elements of wisdom such as prosocial behaviors and emotional regulation, both in people with mental illness and in the general population, with moderate to large effect sizes. But such studies don’t prove an effect on overall wisdom.
The intervention achieved positive results in 89 participants in senior housing communities, though the effect sizes were small, possibly because of high baseline resilience. A subanalysis suggested that reduction in loneliness was mediated by an increase in compassion.
“One of the most striking findings from our research on wisdom is this consistent and very strongly negative correlation between wisdom and loneliness,” Dr. Nguyen said. She highlighted other U.S. nationwide and cross-cultural studies that showed inverse relationships between loneliness and wisdom.
Loneliness is an important topic because it can contribute to burnout and suicide rates.
“Loneliness has a profound effect on how we show up in the workplace, in school, and in our communities. And that leads to anxiety, depression, depersonalization, and emotional fatigue. All are key features of burnout. And together loneliness and burnout have contributed to increased rates of suicide by 30%, and opioid-related deaths almost sixfold since the late 1990s,” Dr. Nguyen said.
Loneliness also is associated with worse physical health, and it may be linked to wisdom. “Loneliness can be conceptualized as being caused and maintained by objective circumstances, such as physical or social distancing, and by thoughts, behaviors, and feelings surrounding those experiences, including biased perceptions of social relations, and a negative assessment of one’s social skills, which then results in a discrepancy between one’s desired and perceived social relationships, which then can contribute to social withdrawal,” Dr. Nguyen said.
Dr. Nguyen highlighted the AARP Foundation’s Experience Corps program, which recruits older adults to act as mentors and tutors for children in kindergarten through third grade. It involves 15 hours per week over an entire school year, with a focus on child literacy, development, and behavioral management skills. A study revealed a significant impact. “It showed improvements in children’s grades and happiness, as well as seniors’ mental and physical health,” Dr. Nguyen said.
Dr. Nguyen concluded that wisdom “may be a vaccine against compassion fatigue and burnout that drive today’s behavioral epidemics of loneliness, opioid abuse, and suicide. It’s a tool for our times. It’s nuanced, flexible, pragmatic, compassionate, and it presents a reasonable framework for getting along in the often messy world that we all share.”
Implications for psychiatrists
Henry A. Nasrallah, MD, who organized the conference, suggested that the benefits of wisdom may not be limited to patients. He pointed out that surgeons often retire at age 60 or 65 because of declining physical skills, while psychiatrists continue to practice.
“We develop more wisdom and better skills, and we can practice into our 60s and 70s. I know psychiatrists who practice sometimes into their 80s. It’s really a wonderful thing to know that what you do in life develops or enhances the neuroplasticity of certain brain regions. In our case, in psychiatry, it is the brain regions involved in wisdom,” commented Dr. Nasrallah, who is a professor of psychiatry, neurology, and neuroscience at the University of Cincinnati.
Dr. Nguyen has no financial disclosures. Dr. Nasrallah has received grants from Abbott, AstraZeneca, Forest, Janssen, Lilly, Pfizer, and Shire, and advises Abbott, AstraZeneca, and Shire.
Wisdom increases with age, and although this personality trait is regarded as nebulous by many, there is evidence that it has biological and neuropsychiatric underpinnings. It could even hold the key to reducing loneliness and burnout among older people.
Those were some of the key messages delivered by Tanya T. Nguyen, PhD, of the department of psychiatry at the University of California, San Diego, who spoke at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“To many people, wisdom remains a fuzzy concept that’s difficult to operationalize and measure. It’s analogous to the concepts of consciousness, emotions, and cognitions, which at one point were considered nonscientific, but today we accept them as biological and scientific entities,” Dr. Nguyen said during her talk at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Interest in quantifying and studying wisdom has picked up in recent years, and Dr. Nguyen gave a definition with six elements that includes prosocial behaviors such as empathy and compassion, as well as emotional regulation, self-reflection, decisiveness, and social decision-making. She also included a spirituality component, though she conceded that this is controversial.
She noted that there are cultural variations in the definition of wisdom, but it has changed little over time, suggesting that it may be biological rather than cultural in nature, and therefore may have a neuropsychiatric underpinning.
Loss of some or all characteristics of wisdom occurs in some behaviors and disorders, including most markedly in the neurodegenerative disorder frontotemporal dementia (FTD), which is characterized by damage only in the prefrontal cortex and anterior temporal lobes. It usually occurs before age 60, and patients exhibit poor social awareness, impulsivity, antisocial behavior, and a lack of insight and empathy.
This and other lines of evidence have led to the suggestion that wisdom may be governed by processes in the prefrontal cortex and the limbic striatum. The prefrontal cortex controls executive functions such as planning, predicting, and anticipating events, as well as managing emotional reactions and impulses. “Thus, wisdom involves parts of the brain that balance cold, hard analytical reasoning with primitive desires and drives, which ultimately leads to self-regulation, social insight, theory of mind, and empathy,” said Dr. Nguyen.
Wisdom has long been associated with age, but age is also linked to cognitive decline. A recent discovery that the brain does not stop evolving at older age may help explain this contradiction. Brains develop in a back to front order, so that the prefrontal cortex is the last to mature. As we age, neural activity shifts from the occipital lobes to the prefrontal cortex and its executive decision-making power.
“The brain may recruit higher-order networks to the prefrontal cortex that are associated with wisdom development,” said Dr. Nguyen. She also pointed out that asymmetry between the left and right hemisphere is reduced with age, as tasks that relied on circuits from one hemisphere or another more often call upon both. “In order to make up for lost synapses and neurons with aging, active older adults use more neuronal networks from both hemispheres to perform the same mental activity,” Dr. Nguyen said.
Some interventions can improve scores in traits associated with wisdom in older adults, and could be an important contributor to improvements in health and longevity, said Dr. Nguyen. Randomized, controlled trials have demonstrated that psychosocial or behavioral interventions can improve elements of wisdom such as prosocial behaviors and emotional regulation, both in people with mental illness and in the general population, with moderate to large effect sizes. But such studies don’t prove an effect on overall wisdom.
The intervention achieved positive results in 89 participants in senior housing communities, though the effect sizes were small, possibly because of high baseline resilience. A subanalysis suggested that reduction in loneliness was mediated by an increase in compassion.
“One of the most striking findings from our research on wisdom is this consistent and very strongly negative correlation between wisdom and loneliness,” Dr. Nguyen said. She highlighted other U.S. nationwide and cross-cultural studies that showed inverse relationships between loneliness and wisdom.
Loneliness is an important topic because it can contribute to burnout and suicide rates.
“Loneliness has a profound effect on how we show up in the workplace, in school, and in our communities. And that leads to anxiety, depression, depersonalization, and emotional fatigue. All are key features of burnout. And together loneliness and burnout have contributed to increased rates of suicide by 30%, and opioid-related deaths almost sixfold since the late 1990s,” Dr. Nguyen said.
Loneliness also is associated with worse physical health, and it may be linked to wisdom. “Loneliness can be conceptualized as being caused and maintained by objective circumstances, such as physical or social distancing, and by thoughts, behaviors, and feelings surrounding those experiences, including biased perceptions of social relations, and a negative assessment of one’s social skills, which then results in a discrepancy between one’s desired and perceived social relationships, which then can contribute to social withdrawal,” Dr. Nguyen said.
Dr. Nguyen highlighted the AARP Foundation’s Experience Corps program, which recruits older adults to act as mentors and tutors for children in kindergarten through third grade. It involves 15 hours per week over an entire school year, with a focus on child literacy, development, and behavioral management skills. A study revealed a significant impact. “It showed improvements in children’s grades and happiness, as well as seniors’ mental and physical health,” Dr. Nguyen said.
Dr. Nguyen concluded that wisdom “may be a vaccine against compassion fatigue and burnout that drive today’s behavioral epidemics of loneliness, opioid abuse, and suicide. It’s a tool for our times. It’s nuanced, flexible, pragmatic, compassionate, and it presents a reasonable framework for getting along in the often messy world that we all share.”
Implications for psychiatrists
Henry A. Nasrallah, MD, who organized the conference, suggested that the benefits of wisdom may not be limited to patients. He pointed out that surgeons often retire at age 60 or 65 because of declining physical skills, while psychiatrists continue to practice.
“We develop more wisdom and better skills, and we can practice into our 60s and 70s. I know psychiatrists who practice sometimes into their 80s. It’s really a wonderful thing to know that what you do in life develops or enhances the neuroplasticity of certain brain regions. In our case, in psychiatry, it is the brain regions involved in wisdom,” commented Dr. Nasrallah, who is a professor of psychiatry, neurology, and neuroscience at the University of Cincinnati.
Dr. Nguyen has no financial disclosures. Dr. Nasrallah has received grants from Abbott, AstraZeneca, Forest, Janssen, Lilly, Pfizer, and Shire, and advises Abbott, AstraZeneca, and Shire.
Wisdom increases with age, and although this personality trait is regarded as nebulous by many, there is evidence that it has biological and neuropsychiatric underpinnings. It could even hold the key to reducing loneliness and burnout among older people.
Those were some of the key messages delivered by Tanya T. Nguyen, PhD, of the department of psychiatry at the University of California, San Diego, who spoke at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“To many people, wisdom remains a fuzzy concept that’s difficult to operationalize and measure. It’s analogous to the concepts of consciousness, emotions, and cognitions, which at one point were considered nonscientific, but today we accept them as biological and scientific entities,” Dr. Nguyen said during her talk at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Interest in quantifying and studying wisdom has picked up in recent years, and Dr. Nguyen gave a definition with six elements that includes prosocial behaviors such as empathy and compassion, as well as emotional regulation, self-reflection, decisiveness, and social decision-making. She also included a spirituality component, though she conceded that this is controversial.
She noted that there are cultural variations in the definition of wisdom, but it has changed little over time, suggesting that it may be biological rather than cultural in nature, and therefore may have a neuropsychiatric underpinning.
Loss of some or all characteristics of wisdom occurs in some behaviors and disorders, including most markedly in the neurodegenerative disorder frontotemporal dementia (FTD), which is characterized by damage only in the prefrontal cortex and anterior temporal lobes. It usually occurs before age 60, and patients exhibit poor social awareness, impulsivity, antisocial behavior, and a lack of insight and empathy.
This and other lines of evidence have led to the suggestion that wisdom may be governed by processes in the prefrontal cortex and the limbic striatum. The prefrontal cortex controls executive functions such as planning, predicting, and anticipating events, as well as managing emotional reactions and impulses. “Thus, wisdom involves parts of the brain that balance cold, hard analytical reasoning with primitive desires and drives, which ultimately leads to self-regulation, social insight, theory of mind, and empathy,” said Dr. Nguyen.
Wisdom has long been associated with age, but age is also linked to cognitive decline. A recent discovery that the brain does not stop evolving at older age may help explain this contradiction. Brains develop in a back to front order, so that the prefrontal cortex is the last to mature. As we age, neural activity shifts from the occipital lobes to the prefrontal cortex and its executive decision-making power.
“The brain may recruit higher-order networks to the prefrontal cortex that are associated with wisdom development,” said Dr. Nguyen. She also pointed out that asymmetry between the left and right hemisphere is reduced with age, as tasks that relied on circuits from one hemisphere or another more often call upon both. “In order to make up for lost synapses and neurons with aging, active older adults use more neuronal networks from both hemispheres to perform the same mental activity,” Dr. Nguyen said.
Some interventions can improve scores in traits associated with wisdom in older adults, and could be an important contributor to improvements in health and longevity, said Dr. Nguyen. Randomized, controlled trials have demonstrated that psychosocial or behavioral interventions can improve elements of wisdom such as prosocial behaviors and emotional regulation, both in people with mental illness and in the general population, with moderate to large effect sizes. But such studies don’t prove an effect on overall wisdom.
The intervention achieved positive results in 89 participants in senior housing communities, though the effect sizes were small, possibly because of high baseline resilience. A subanalysis suggested that reduction in loneliness was mediated by an increase in compassion.
“One of the most striking findings from our research on wisdom is this consistent and very strongly negative correlation between wisdom and loneliness,” Dr. Nguyen said. She highlighted other U.S. nationwide and cross-cultural studies that showed inverse relationships between loneliness and wisdom.
Loneliness is an important topic because it can contribute to burnout and suicide rates.
“Loneliness has a profound effect on how we show up in the workplace, in school, and in our communities. And that leads to anxiety, depression, depersonalization, and emotional fatigue. All are key features of burnout. And together loneliness and burnout have contributed to increased rates of suicide by 30%, and opioid-related deaths almost sixfold since the late 1990s,” Dr. Nguyen said.
Loneliness also is associated with worse physical health, and it may be linked to wisdom. “Loneliness can be conceptualized as being caused and maintained by objective circumstances, such as physical or social distancing, and by thoughts, behaviors, and feelings surrounding those experiences, including biased perceptions of social relations, and a negative assessment of one’s social skills, which then results in a discrepancy between one’s desired and perceived social relationships, which then can contribute to social withdrawal,” Dr. Nguyen said.
Dr. Nguyen highlighted the AARP Foundation’s Experience Corps program, which recruits older adults to act as mentors and tutors for children in kindergarten through third grade. It involves 15 hours per week over an entire school year, with a focus on child literacy, development, and behavioral management skills. A study revealed a significant impact. “It showed improvements in children’s grades and happiness, as well as seniors’ mental and physical health,” Dr. Nguyen said.
Dr. Nguyen concluded that wisdom “may be a vaccine against compassion fatigue and burnout that drive today’s behavioral epidemics of loneliness, opioid abuse, and suicide. It’s a tool for our times. It’s nuanced, flexible, pragmatic, compassionate, and it presents a reasonable framework for getting along in the often messy world that we all share.”
Implications for psychiatrists
Henry A. Nasrallah, MD, who organized the conference, suggested that the benefits of wisdom may not be limited to patients. He pointed out that surgeons often retire at age 60 or 65 because of declining physical skills, while psychiatrists continue to practice.
“We develop more wisdom and better skills, and we can practice into our 60s and 70s. I know psychiatrists who practice sometimes into their 80s. It’s really a wonderful thing to know that what you do in life develops or enhances the neuroplasticity of certain brain regions. In our case, in psychiatry, it is the brain regions involved in wisdom,” commented Dr. Nasrallah, who is a professor of psychiatry, neurology, and neuroscience at the University of Cincinnati.
Dr. Nguyen has no financial disclosures. Dr. Nasrallah has received grants from Abbott, AstraZeneca, Forest, Janssen, Lilly, Pfizer, and Shire, and advises Abbott, AstraZeneca, and Shire.
REPORTING FROM FOCUS ON NEUROPSYCHIATRY 2021













