User login
Choline and prevention of prevalent mental illnesses
Advocating on behalf of the power of prevention in psychiatry has been my life’s work. I ran a world-class community mental health center with a strong wellness component; have taught, researched, written, and spoken extensively about the importance of prevention; and have incorporated preventive ideas into my current clinical practice.
I would like to think that I have been one of the forces that helped start a new movement called “positive psychiatry,” the idea that mental health must encompass more than the reduction or elimination of psychiatric illness. In the new book edited by American Psychiatric Association Past-President Dilip V. Jeste, MD, and Barton W. Palmer, PhD, called “Positive Psychiatry” (Arlington, Va.: American Psychiatric Association Publishing, 2015), I contributed a chapter on the psychosocial factors tied to positive outcomes. In addition, I am part of a group of psychiatrists and researchers affiliated with the World Psychiatric Association who are starting an interest group focusing on positive psychiatry.
Recently, because of the prevalence of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (the American Psychiatric Association’s DSM-5 version of fetal alcohol spectrum disorders) in my community, I have begun to tout this problem as a major public health issue. When we formulated the Institute of Medicine’s 2009 Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities report, we did not include the problem of fetal alcohol exposure – and this was an unfortunate oversight.
However, this area of interest had not yet fully developed, and nearly 8 years later, there have been some confluent developments regarding potential prevention of this problem. They both involve choline.
First, we know that when women drink while pregnant, the alcohol they consume rids their bodies of choline, a nutrient the fetus needs for proper cell construction, neurogenesis, and neurodevelopment. Accordingly, several scientists are exploring using choline both pre- and postnatally to see if the defects on ND-PAE can be ameliorated or prevented. All of the research in this area is new, but it looks very promising.
Recently, I had the good fortune to present an idea during the Andrea Delgado Memorial Lecture at the Black Psychiatrists of America transcultural conference in the Bahamas. I also spoke at a mini-plenary at the 32nd Annual Rosalynn Carter Mental Health Policy Symposium in Atlanta. The core of the presentations were not too deep (to paraphrase a line Morgan Freeman used on Jack Nicholson in the movie “The Bucket List” – ‘I have seen bathtubs that are deeper’), but I think it explicated an essential idea. Jessie Aujla, a 4th-year medical student, and I explored the content of choline in the 25 top prenatal vitamins and found none of them contained the 450-mg daily recommended dose of choline advised by the Institute of Medicine in 1998. In fact, only two contain 50 mg; six others contain less than 30 mg; and the other 17 have no choline whatsoever (this study is in press at the Journal of Family Medicine and Prevention). So we are advocating that the prenatal vitamin manufacturers increase the choline content of their prenatal vitamins, because although women may be getting some choline from their food diets, we found one large study illustrating that 90% of pregnant women are choline deficient.
The other area of interest regarding choline as a preventive agent for mental illness is work published by researchers at the University of Colorado Denver. This research group is proposing that choline may prevent the development of autism, attention-deficit/hyperactivity disorder, and schizophrenia by an epigenetic mechanism involving a nicotinic acetylcholine receptor. This makes perfectly good sense clinically among those of us who are treating patients with ND-PAE. Some of us are starting to think of ND-PAE as a choline deficiency disorder and see symptoms that are extremely similar to autism, ADHD, and schizophrenia in such patients. Many patients with ND-PAE are misdiagnosed with these disorders. Accordingly, there appears to be some common ground between ideas aimed at preventing fetal alcohol exposure and those aimed at preventing autism, ADHD, and schizophrenia – specifically, ensuring that pregnant women get an adequate supply of choline.
There is certainly a great need to do more research to nail down these two potential preventive actions. But until that research is done, it seems to me that the least we can do is to advocate for a position that the manufacturers of prenatal vitamins at least include the daily recommended dose of choline (450 mg/day) pregnant women need per the findings of the Institute of Medicine’s Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline, published in 1998.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago; clinical psychiatrist emeritus, department of psychiatry, at the University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Advocating on behalf of the power of prevention in psychiatry has been my life’s work. I ran a world-class community mental health center with a strong wellness component; have taught, researched, written, and spoken extensively about the importance of prevention; and have incorporated preventive ideas into my current clinical practice.
I would like to think that I have been one of the forces that helped start a new movement called “positive psychiatry,” the idea that mental health must encompass more than the reduction or elimination of psychiatric illness. In the new book edited by American Psychiatric Association Past-President Dilip V. Jeste, MD, and Barton W. Palmer, PhD, called “Positive Psychiatry” (Arlington, Va.: American Psychiatric Association Publishing, 2015), I contributed a chapter on the psychosocial factors tied to positive outcomes. In addition, I am part of a group of psychiatrists and researchers affiliated with the World Psychiatric Association who are starting an interest group focusing on positive psychiatry.
Recently, because of the prevalence of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (the American Psychiatric Association’s DSM-5 version of fetal alcohol spectrum disorders) in my community, I have begun to tout this problem as a major public health issue. When we formulated the Institute of Medicine’s 2009 Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities report, we did not include the problem of fetal alcohol exposure – and this was an unfortunate oversight.
However, this area of interest had not yet fully developed, and nearly 8 years later, there have been some confluent developments regarding potential prevention of this problem. They both involve choline.
First, we know that when women drink while pregnant, the alcohol they consume rids their bodies of choline, a nutrient the fetus needs for proper cell construction, neurogenesis, and neurodevelopment. Accordingly, several scientists are exploring using choline both pre- and postnatally to see if the defects on ND-PAE can be ameliorated or prevented. All of the research in this area is new, but it looks very promising.
Recently, I had the good fortune to present an idea during the Andrea Delgado Memorial Lecture at the Black Psychiatrists of America transcultural conference in the Bahamas. I also spoke at a mini-plenary at the 32nd Annual Rosalynn Carter Mental Health Policy Symposium in Atlanta. The core of the presentations were not too deep (to paraphrase a line Morgan Freeman used on Jack Nicholson in the movie “The Bucket List” – ‘I have seen bathtubs that are deeper’), but I think it explicated an essential idea. Jessie Aujla, a 4th-year medical student, and I explored the content of choline in the 25 top prenatal vitamins and found none of them contained the 450-mg daily recommended dose of choline advised by the Institute of Medicine in 1998. In fact, only two contain 50 mg; six others contain less than 30 mg; and the other 17 have no choline whatsoever (this study is in press at the Journal of Family Medicine and Prevention). So we are advocating that the prenatal vitamin manufacturers increase the choline content of their prenatal vitamins, because although women may be getting some choline from their food diets, we found one large study illustrating that 90% of pregnant women are choline deficient.
The other area of interest regarding choline as a preventive agent for mental illness is work published by researchers at the University of Colorado Denver. This research group is proposing that choline may prevent the development of autism, attention-deficit/hyperactivity disorder, and schizophrenia by an epigenetic mechanism involving a nicotinic acetylcholine receptor. This makes perfectly good sense clinically among those of us who are treating patients with ND-PAE. Some of us are starting to think of ND-PAE as a choline deficiency disorder and see symptoms that are extremely similar to autism, ADHD, and schizophrenia in such patients. Many patients with ND-PAE are misdiagnosed with these disorders. Accordingly, there appears to be some common ground between ideas aimed at preventing fetal alcohol exposure and those aimed at preventing autism, ADHD, and schizophrenia – specifically, ensuring that pregnant women get an adequate supply of choline.
There is certainly a great need to do more research to nail down these two potential preventive actions. But until that research is done, it seems to me that the least we can do is to advocate for a position that the manufacturers of prenatal vitamins at least include the daily recommended dose of choline (450 mg/day) pregnant women need per the findings of the Institute of Medicine’s Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline, published in 1998.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago; clinical psychiatrist emeritus, department of psychiatry, at the University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Advocating on behalf of the power of prevention in psychiatry has been my life’s work. I ran a world-class community mental health center with a strong wellness component; have taught, researched, written, and spoken extensively about the importance of prevention; and have incorporated preventive ideas into my current clinical practice.
I would like to think that I have been one of the forces that helped start a new movement called “positive psychiatry,” the idea that mental health must encompass more than the reduction or elimination of psychiatric illness. In the new book edited by American Psychiatric Association Past-President Dilip V. Jeste, MD, and Barton W. Palmer, PhD, called “Positive Psychiatry” (Arlington, Va.: American Psychiatric Association Publishing, 2015), I contributed a chapter on the psychosocial factors tied to positive outcomes. In addition, I am part of a group of psychiatrists and researchers affiliated with the World Psychiatric Association who are starting an interest group focusing on positive psychiatry.
Recently, because of the prevalence of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (the American Psychiatric Association’s DSM-5 version of fetal alcohol spectrum disorders) in my community, I have begun to tout this problem as a major public health issue. When we formulated the Institute of Medicine’s 2009 Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities report, we did not include the problem of fetal alcohol exposure – and this was an unfortunate oversight.
However, this area of interest had not yet fully developed, and nearly 8 years later, there have been some confluent developments regarding potential prevention of this problem. They both involve choline.
First, we know that when women drink while pregnant, the alcohol they consume rids their bodies of choline, a nutrient the fetus needs for proper cell construction, neurogenesis, and neurodevelopment. Accordingly, several scientists are exploring using choline both pre- and postnatally to see if the defects on ND-PAE can be ameliorated or prevented. All of the research in this area is new, but it looks very promising.
Recently, I had the good fortune to present an idea during the Andrea Delgado Memorial Lecture at the Black Psychiatrists of America transcultural conference in the Bahamas. I also spoke at a mini-plenary at the 32nd Annual Rosalynn Carter Mental Health Policy Symposium in Atlanta. The core of the presentations were not too deep (to paraphrase a line Morgan Freeman used on Jack Nicholson in the movie “The Bucket List” – ‘I have seen bathtubs that are deeper’), but I think it explicated an essential idea. Jessie Aujla, a 4th-year medical student, and I explored the content of choline in the 25 top prenatal vitamins and found none of them contained the 450-mg daily recommended dose of choline advised by the Institute of Medicine in 1998. In fact, only two contain 50 mg; six others contain less than 30 mg; and the other 17 have no choline whatsoever (this study is in press at the Journal of Family Medicine and Prevention). So we are advocating that the prenatal vitamin manufacturers increase the choline content of their prenatal vitamins, because although women may be getting some choline from their food diets, we found one large study illustrating that 90% of pregnant women are choline deficient.
The other area of interest regarding choline as a preventive agent for mental illness is work published by researchers at the University of Colorado Denver. This research group is proposing that choline may prevent the development of autism, attention-deficit/hyperactivity disorder, and schizophrenia by an epigenetic mechanism involving a nicotinic acetylcholine receptor. This makes perfectly good sense clinically among those of us who are treating patients with ND-PAE. Some of us are starting to think of ND-PAE as a choline deficiency disorder and see symptoms that are extremely similar to autism, ADHD, and schizophrenia in such patients. Many patients with ND-PAE are misdiagnosed with these disorders. Accordingly, there appears to be some common ground between ideas aimed at preventing fetal alcohol exposure and those aimed at preventing autism, ADHD, and schizophrenia – specifically, ensuring that pregnant women get an adequate supply of choline.
There is certainly a great need to do more research to nail down these two potential preventive actions. But until that research is done, it seems to me that the least we can do is to advocate for a position that the manufacturers of prenatal vitamins at least include the daily recommended dose of choline (450 mg/day) pregnant women need per the findings of the Institute of Medicine’s Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline, published in 1998.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago; clinical psychiatrist emeritus, department of psychiatry, at the University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Differentiating ADHD and bipolar disorder
Preschool ADHD diagnoses plateaued after 2011 AAP guideline
The introduction of the 2011 American Academy of Pediatrics practice guidelines on attention-deficit/hyperactivity disorder was associated with a leveling off in the number of diagnoses in preschool children.
“In the preguideline period, the trajectory of ADHD diagnosis increased slightly but significantly across practices,” Alexander G. Fiks, MD, from the Children’s Hospital of Philadelphia, and his coinvestigators wrote. “However, the rate of ADHD diagnosis no longer increased significantly after guideline release.”
They found that the rate of ADHD diagnoses was 0.7% before the release of the 2011 guidelines and 0.9% after, while the rate of stimulant prescriptions remained constant at 0.4% across the entire study period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2025).
While the levels of stimulants prescribed remained the same across the period of the analysis, the proportion of children diagnosed with ADHD who were prescribed stimulants had already been in significant decline before the release of the guidelines. After the guidelines, this rate also plateaued, signifying that before – but not after – the guidelines, children were becoming less likely to be prescribed stimulant medication following an ADHD diagnosis.
Commenting on the change in diagnostic and prescribing patterns, the investigators noted that the primary goal of practice guidelines was to standardize care.
“In the case of preschool ADHD, such standardization might have resulted in an increasing trajectory in diagnosis of preschool children if pediatric clinicians had not previously been evaluating ADHD when an evaluation was warranted,” they wrote. “Alternatively, a decrease in diagnosis could have occurred if clinicians were applying more rigorous standards to the diagnosis and therefore excluding certain children who might have previously been diagnosed or no change if a combination of these two patterns was occurring or if there was no change in the standard used.”
They suggested that the observation of a decreasing likelihood of stimulant prescriptions for ADHD before the guidelines may have been driven by the results of the 2006 Preschool ADHD Treatment Study, which showed a lower effect size of stimulant medication in preschool-aged children, compared with school-aged children.
“Alternatively, findings may have resulted from a decrease in the severity of preschool children diagnosed with ADHD as the proportion of all preschoolers diagnosed with ADHD increased,” they wrote.
The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
It is encouraging for those of us who worked on crafting the revised guidelines to find some evidence about the impact of those recommendations. However, as the investigators point out, although they were able to find out that, in preschool-aged children with ADHD, recommended criteria for the use of stimulant medications, specifically methylphenidate, did not result in an increase in its use in this age group, the frequency of behavioral parent training, the first-line recommended treatment, could not be determined.
In addition, to address the issue that was the focus of this study, examining the implementation of evidence into practice, there needs to be greater standardization of assessment and treatment modalities so that we can better examine the outcomes of changes in treatment. Studies of prevalence and treatments of children with ADHD have indicated wide variations across the country. Clarifying those differences will require the improved ability to examine the various factors responsible for these variations, particularly across the systems of care that go beyond just medication use.
Mark L. Wolraich, MD, is from the University of Oklahoma Health Sciences Center, Oklahoma City. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2928). He reported having no financial disclosures.
It is encouraging for those of us who worked on crafting the revised guidelines to find some evidence about the impact of those recommendations. However, as the investigators point out, although they were able to find out that, in preschool-aged children with ADHD, recommended criteria for the use of stimulant medications, specifically methylphenidate, did not result in an increase in its use in this age group, the frequency of behavioral parent training, the first-line recommended treatment, could not be determined.
In addition, to address the issue that was the focus of this study, examining the implementation of evidence into practice, there needs to be greater standardization of assessment and treatment modalities so that we can better examine the outcomes of changes in treatment. Studies of prevalence and treatments of children with ADHD have indicated wide variations across the country. Clarifying those differences will require the improved ability to examine the various factors responsible for these variations, particularly across the systems of care that go beyond just medication use.
Mark L. Wolraich, MD, is from the University of Oklahoma Health Sciences Center, Oklahoma City. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2928). He reported having no financial disclosures.
It is encouraging for those of us who worked on crafting the revised guidelines to find some evidence about the impact of those recommendations. However, as the investigators point out, although they were able to find out that, in preschool-aged children with ADHD, recommended criteria for the use of stimulant medications, specifically methylphenidate, did not result in an increase in its use in this age group, the frequency of behavioral parent training, the first-line recommended treatment, could not be determined.
In addition, to address the issue that was the focus of this study, examining the implementation of evidence into practice, there needs to be greater standardization of assessment and treatment modalities so that we can better examine the outcomes of changes in treatment. Studies of prevalence and treatments of children with ADHD have indicated wide variations across the country. Clarifying those differences will require the improved ability to examine the various factors responsible for these variations, particularly across the systems of care that go beyond just medication use.
Mark L. Wolraich, MD, is from the University of Oklahoma Health Sciences Center, Oklahoma City. These comments are adapted from an accompanying editorial (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2928). He reported having no financial disclosures.
The introduction of the 2011 American Academy of Pediatrics practice guidelines on attention-deficit/hyperactivity disorder was associated with a leveling off in the number of diagnoses in preschool children.
“In the preguideline period, the trajectory of ADHD diagnosis increased slightly but significantly across practices,” Alexander G. Fiks, MD, from the Children’s Hospital of Philadelphia, and his coinvestigators wrote. “However, the rate of ADHD diagnosis no longer increased significantly after guideline release.”
They found that the rate of ADHD diagnoses was 0.7% before the release of the 2011 guidelines and 0.9% after, while the rate of stimulant prescriptions remained constant at 0.4% across the entire study period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2025).
While the levels of stimulants prescribed remained the same across the period of the analysis, the proportion of children diagnosed with ADHD who were prescribed stimulants had already been in significant decline before the release of the guidelines. After the guidelines, this rate also plateaued, signifying that before – but not after – the guidelines, children were becoming less likely to be prescribed stimulant medication following an ADHD diagnosis.
Commenting on the change in diagnostic and prescribing patterns, the investigators noted that the primary goal of practice guidelines was to standardize care.
“In the case of preschool ADHD, such standardization might have resulted in an increasing trajectory in diagnosis of preschool children if pediatric clinicians had not previously been evaluating ADHD when an evaluation was warranted,” they wrote. “Alternatively, a decrease in diagnosis could have occurred if clinicians were applying more rigorous standards to the diagnosis and therefore excluding certain children who might have previously been diagnosed or no change if a combination of these two patterns was occurring or if there was no change in the standard used.”
They suggested that the observation of a decreasing likelihood of stimulant prescriptions for ADHD before the guidelines may have been driven by the results of the 2006 Preschool ADHD Treatment Study, which showed a lower effect size of stimulant medication in preschool-aged children, compared with school-aged children.
“Alternatively, findings may have resulted from a decrease in the severity of preschool children diagnosed with ADHD as the proportion of all preschoolers diagnosed with ADHD increased,” they wrote.
The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
The introduction of the 2011 American Academy of Pediatrics practice guidelines on attention-deficit/hyperactivity disorder was associated with a leveling off in the number of diagnoses in preschool children.
“In the preguideline period, the trajectory of ADHD diagnosis increased slightly but significantly across practices,” Alexander G. Fiks, MD, from the Children’s Hospital of Philadelphia, and his coinvestigators wrote. “However, the rate of ADHD diagnosis no longer increased significantly after guideline release.”
They found that the rate of ADHD diagnoses was 0.7% before the release of the 2011 guidelines and 0.9% after, while the rate of stimulant prescriptions remained constant at 0.4% across the entire study period (Pediatrics. 2016 Nov 15. doi: 10.1542/peds.2016-2025).
While the levels of stimulants prescribed remained the same across the period of the analysis, the proportion of children diagnosed with ADHD who were prescribed stimulants had already been in significant decline before the release of the guidelines. After the guidelines, this rate also plateaued, signifying that before – but not after – the guidelines, children were becoming less likely to be prescribed stimulant medication following an ADHD diagnosis.
Commenting on the change in diagnostic and prescribing patterns, the investigators noted that the primary goal of practice guidelines was to standardize care.
“In the case of preschool ADHD, such standardization might have resulted in an increasing trajectory in diagnosis of preschool children if pediatric clinicians had not previously been evaluating ADHD when an evaluation was warranted,” they wrote. “Alternatively, a decrease in diagnosis could have occurred if clinicians were applying more rigorous standards to the diagnosis and therefore excluding certain children who might have previously been diagnosed or no change if a combination of these two patterns was occurring or if there was no change in the standard used.”
They suggested that the observation of a decreasing likelihood of stimulant prescriptions for ADHD before the guidelines may have been driven by the results of the 2006 Preschool ADHD Treatment Study, which showed a lower effect size of stimulant medication in preschool-aged children, compared with school-aged children.
“Alternatively, findings may have resulted from a decrease in the severity of preschool children diagnosed with ADHD as the proportion of all preschoolers diagnosed with ADHD increased,” they wrote.
The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
Key clinical point:
Major finding: The rate of ADHD diagnoses was 0.7% before the guidelines and 0.9% after, while stimulant prescriptions remained constant at 0.4% across the study period.
Data source: An analysis of electronic health record data from 143,881 children across 63 primary care practice from January 2008 to July 2014.
Disclosures: The study was supported by the U.S. Department of Health & Human Services. Dr. Fiks reported receiving a research grant from Pfizer for work on ADHD unrelated to this study. The other investigators reported having no financial disclosures.
Special Edition: Focus on ADHD
ADHD symptoms are stable, then a sudden relapse
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
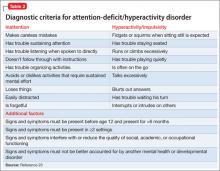
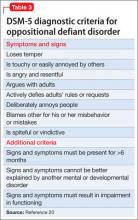
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
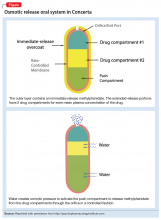
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
Hunting for ‘Woozles’ in the Hundred Acre Wood of ADHD
One fine winter’s day when Piglet was brushing away the snow
in front of his house he happened to look up, and there was
Winnie-the-Pooh. Pooh was walking round and round in a circle,
thinking of something else…
So begins the 1926 Winnie-the-Pooh story.1 In this chapter, the well-meaning yellow bear, Winnie-the-Pooh, has found strange tracks in the snow, which he believes belong to a “Woozle.” Pooh follows the tracks, not realizing that he’s walking in a circle. As such, he begins to notice that the tracks have multiplied, which he interprets as evidence of several Woozles.
This “Woozle Effect” has been well described in research settings and is believed to have resulted in conclusions that are not supported by or are inconsistent with the original data, which are then propagated through successive citations, resulting in a scientific “urban legend.”2
Throughout my training from medical school, through fellowship, and during my tenure as a faculty member, I have found myself, at times, searching for Woozles and often have joined my colleagues on these hunts. Herein, I would like to share with you 3 Woozles that have resulted in current false dogmas related to attention-deficit/hyperactivity disorder (ADHD) and stimulant psychopharmacology.
Stimulants worsen anxiety
FDA-required labeling for stimulants includes strong language noting that these drugs are “contraindicated in marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.”3 However, data from randomized controlled trials and meta-analyses consistently have failed to demonstrate this effect. Moreover, sequenced treatment trials involving adolescents with anxiety disorders and co-occurring ADHD suggest that stimulants actually could reduce anxiety symptoms.
A recent meta-analysis4 that evaluated nearly 2 dozen studies involving approximately 3,000 pediatric patients with ADHD reported that stimulant treatment was associated with a decreased relative risk of anxiety (relative risk: 0.86). The study also observed a dose-response relationship between stimulant dosage and anxiety (Figure, page 6).4 Although the authors note that it is possible that some individuals might experience increased anxiety with stimulants, many patients could show improvement in anxiety symptoms when treated with stimulants, and the authors also advise us, as clinicians, to “consider re-challenging children with ADHD who report … anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.”4
More evidence of a lack of stimulant-induced anxiety comes from a large randomized controlled trial of pediatric patients (age 6 to 17) who met DSM-IV criteria for ADHD and a co-occurring anxiety disorder who were treated with methylphenidate (open-label) and then randomized to fluvoxamine or placebo for treatment of anxiety symptoms.5 However, in this trial >80% of the 32 medication-naïve youth improved after stimulant treatment to the point that they no longer had anxiety symptoms severe enough to be eligible for randomization to adjunctive fluvoxamine or placebo.
Stimulants are contraindicated in patients with tic disorders
The package inserts for most stimulant medications warn clinicians that stimulants are “contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.” This is particularly concerning, especially because of the medicolegal implications of the term “contraindicated” and given that as many as 1 in 5 pediatric patients with ADHD have a tic disorder.6 Therefore, labels that list motor tics as a contraindication to stimulant use potentially eliminate the choice of stimulant pharmacotherapy—the most effective treatment for ADHD—for a large number of patients.
When hunting for the Woozle that linked stimulants and tics and led to this language in the package insert, it is worthwhile to review a recent meta-analysis of 22 studies (involving nearly 2,400 youths with ADHD) that suggested new-onset tics or worsening of tics to be present in 5.7% of patients receiving stimulants and in 6.5% of patients receiving placebo. In addition, in this meta-analysis the class of stimulant, dosage, treatment duration, or patient age did not seem to be associated with onset or worsening of tics.7
Polypharmacy represents a therapeutic failure and is not evidence-based
Although treatment guidelines generally have discouraged combination therapy for treating ADHD, there are—on the basis of efficacy—insufficient data to support this prohibition. Moreover, over the last decade, several studies have suggested benefits for combining ADHD medications that have complimentary mechanisms. In this regard, 2 extended-release formulations of α2 agonists have received FDA approval for as adjunctive treatments in pediatric patients with ADHD (extended-release guanfacine and extended-release clonidine). However, despite these FDA indications as adjunctive treatments, many clinicians remain concerned about combination therapy.
Several months ago, a large, 8-week, National Institutes of Health–sponsored trial shed more light on the use of α2agonist + stimulant combinations. Patients age 7 to 17 (N = 179) were randomized to (1) guanfacine + d-methylphenidate, (2) guanfacine monotherapy, or (3) d-methylphenidate monotherapy.8 In addition to clinical outcomes, the authors evaluated the effects of the medication on background cortical activity. Of interest, monotherapies differed between one another and the combination treatment in their effects on cortical activity. Guanfacine decreased alpha band power and methylphenidate administration was associated with an increase in frontal/central beta power, while combination treatment dampened theta band power and was associated with specific, focal increases in beta power.8 These results, although preliminary, suggest not only that medication results in changes in cortical activity that correlate with symptomatic improvement, but that combination treatment may be associated with a distinct cortical activity pattern that is more than the summation of the effects of the monotherapies. Moreover, these data raise the possibility that this synergistic effect on cortical activity may subtend—or at least—relate to the synergistic clinical effects of the 2 medications.
‘Think it over, think it under’
Having discussed several important Woozles that have inhabited the Hundred Acre Wood of ADHD for decades, it is important to remember there are countless Woozles in the larger “Thousand Acre Wood” of psychiatry and medicine. As we evaluate evidence for our interventions, whether psychopharmacologic or psychotherapeutic, we will do w
1. Milne AA. Winnie-the-Pooh. London, United Kingdom: Methuen & Co. Ltd.; 1926.
2. Strauss MA. Processes Explaining the concealment and distortion of evidence on gender symmetry in partner violence. Eur J Crim Pol Res. 1980;74:227-232.
3. Ritalin LA [package insert]. East Hanover, NJ: Novartis; 2015.
4. Coughlin CG, Cohen SC, Mulqueen JM, et al. Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25(8):611-617.
5. Abikoff H, McGough J, Vitiello B, et al; RUPP ADHD/Anxiety Study Group. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418-427.
6. Bloch MH, Panza KE, Landeros-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
7. Cohen SC, Mulqueen JM, Ferracioli-Oda E, et al. Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(9):728-736.
8. Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1.
One fine winter’s day when Piglet was brushing away the snow
in front of his house he happened to look up, and there was
Winnie-the-Pooh. Pooh was walking round and round in a circle,
thinking of something else…
So begins the 1926 Winnie-the-Pooh story.1 In this chapter, the well-meaning yellow bear, Winnie-the-Pooh, has found strange tracks in the snow, which he believes belong to a “Woozle.” Pooh follows the tracks, not realizing that he’s walking in a circle. As such, he begins to notice that the tracks have multiplied, which he interprets as evidence of several Woozles.
This “Woozle Effect” has been well described in research settings and is believed to have resulted in conclusions that are not supported by or are inconsistent with the original data, which are then propagated through successive citations, resulting in a scientific “urban legend.”2
Throughout my training from medical school, through fellowship, and during my tenure as a faculty member, I have found myself, at times, searching for Woozles and often have joined my colleagues on these hunts. Herein, I would like to share with you 3 Woozles that have resulted in current false dogmas related to attention-deficit/hyperactivity disorder (ADHD) and stimulant psychopharmacology.
Stimulants worsen anxiety
FDA-required labeling for stimulants includes strong language noting that these drugs are “contraindicated in marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.”3 However, data from randomized controlled trials and meta-analyses consistently have failed to demonstrate this effect. Moreover, sequenced treatment trials involving adolescents with anxiety disorders and co-occurring ADHD suggest that stimulants actually could reduce anxiety symptoms.
A recent meta-analysis4 that evaluated nearly 2 dozen studies involving approximately 3,000 pediatric patients with ADHD reported that stimulant treatment was associated with a decreased relative risk of anxiety (relative risk: 0.86). The study also observed a dose-response relationship between stimulant dosage and anxiety (Figure, page 6).4 Although the authors note that it is possible that some individuals might experience increased anxiety with stimulants, many patients could show improvement in anxiety symptoms when treated with stimulants, and the authors also advise us, as clinicians, to “consider re-challenging children with ADHD who report … anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.”4
More evidence of a lack of stimulant-induced anxiety comes from a large randomized controlled trial of pediatric patients (age 6 to 17) who met DSM-IV criteria for ADHD and a co-occurring anxiety disorder who were treated with methylphenidate (open-label) and then randomized to fluvoxamine or placebo for treatment of anxiety symptoms.5 However, in this trial >80% of the 32 medication-naïve youth improved after stimulant treatment to the point that they no longer had anxiety symptoms severe enough to be eligible for randomization to adjunctive fluvoxamine or placebo.
Stimulants are contraindicated in patients with tic disorders
The package inserts for most stimulant medications warn clinicians that stimulants are “contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.” This is particularly concerning, especially because of the medicolegal implications of the term “contraindicated” and given that as many as 1 in 5 pediatric patients with ADHD have a tic disorder.6 Therefore, labels that list motor tics as a contraindication to stimulant use potentially eliminate the choice of stimulant pharmacotherapy—the most effective treatment for ADHD—for a large number of patients.
When hunting for the Woozle that linked stimulants and tics and led to this language in the package insert, it is worthwhile to review a recent meta-analysis of 22 studies (involving nearly 2,400 youths with ADHD) that suggested new-onset tics or worsening of tics to be present in 5.7% of patients receiving stimulants and in 6.5% of patients receiving placebo. In addition, in this meta-analysis the class of stimulant, dosage, treatment duration, or patient age did not seem to be associated with onset or worsening of tics.7
Polypharmacy represents a therapeutic failure and is not evidence-based
Although treatment guidelines generally have discouraged combination therapy for treating ADHD, there are—on the basis of efficacy—insufficient data to support this prohibition. Moreover, over the last decade, several studies have suggested benefits for combining ADHD medications that have complimentary mechanisms. In this regard, 2 extended-release formulations of α2 agonists have received FDA approval for as adjunctive treatments in pediatric patients with ADHD (extended-release guanfacine and extended-release clonidine). However, despite these FDA indications as adjunctive treatments, many clinicians remain concerned about combination therapy.
Several months ago, a large, 8-week, National Institutes of Health–sponsored trial shed more light on the use of α2agonist + stimulant combinations. Patients age 7 to 17 (N = 179) were randomized to (1) guanfacine + d-methylphenidate, (2) guanfacine monotherapy, or (3) d-methylphenidate monotherapy.8 In addition to clinical outcomes, the authors evaluated the effects of the medication on background cortical activity. Of interest, monotherapies differed between one another and the combination treatment in their effects on cortical activity. Guanfacine decreased alpha band power and methylphenidate administration was associated with an increase in frontal/central beta power, while combination treatment dampened theta band power and was associated with specific, focal increases in beta power.8 These results, although preliminary, suggest not only that medication results in changes in cortical activity that correlate with symptomatic improvement, but that combination treatment may be associated with a distinct cortical activity pattern that is more than the summation of the effects of the monotherapies. Moreover, these data raise the possibility that this synergistic effect on cortical activity may subtend—or at least—relate to the synergistic clinical effects of the 2 medications.
‘Think it over, think it under’
Having discussed several important Woozles that have inhabited the Hundred Acre Wood of ADHD for decades, it is important to remember there are countless Woozles in the larger “Thousand Acre Wood” of psychiatry and medicine. As we evaluate evidence for our interventions, whether psychopharmacologic or psychotherapeutic, we will do w
One fine winter’s day when Piglet was brushing away the snow
in front of his house he happened to look up, and there was
Winnie-the-Pooh. Pooh was walking round and round in a circle,
thinking of something else…
So begins the 1926 Winnie-the-Pooh story.1 In this chapter, the well-meaning yellow bear, Winnie-the-Pooh, has found strange tracks in the snow, which he believes belong to a “Woozle.” Pooh follows the tracks, not realizing that he’s walking in a circle. As such, he begins to notice that the tracks have multiplied, which he interprets as evidence of several Woozles.
This “Woozle Effect” has been well described in research settings and is believed to have resulted in conclusions that are not supported by or are inconsistent with the original data, which are then propagated through successive citations, resulting in a scientific “urban legend.”2
Throughout my training from medical school, through fellowship, and during my tenure as a faculty member, I have found myself, at times, searching for Woozles and often have joined my colleagues on these hunts. Herein, I would like to share with you 3 Woozles that have resulted in current false dogmas related to attention-deficit/hyperactivity disorder (ADHD) and stimulant psychopharmacology.
Stimulants worsen anxiety
FDA-required labeling for stimulants includes strong language noting that these drugs are “contraindicated in marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.”3 However, data from randomized controlled trials and meta-analyses consistently have failed to demonstrate this effect. Moreover, sequenced treatment trials involving adolescents with anxiety disorders and co-occurring ADHD suggest that stimulants actually could reduce anxiety symptoms.
A recent meta-analysis4 that evaluated nearly 2 dozen studies involving approximately 3,000 pediatric patients with ADHD reported that stimulant treatment was associated with a decreased relative risk of anxiety (relative risk: 0.86). The study also observed a dose-response relationship between stimulant dosage and anxiety (Figure, page 6).4 Although the authors note that it is possible that some individuals might experience increased anxiety with stimulants, many patients could show improvement in anxiety symptoms when treated with stimulants, and the authors also advise us, as clinicians, to “consider re-challenging children with ADHD who report … anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.”4
More evidence of a lack of stimulant-induced anxiety comes from a large randomized controlled trial of pediatric patients (age 6 to 17) who met DSM-IV criteria for ADHD and a co-occurring anxiety disorder who were treated with methylphenidate (open-label) and then randomized to fluvoxamine or placebo for treatment of anxiety symptoms.5 However, in this trial >80% of the 32 medication-naïve youth improved after stimulant treatment to the point that they no longer had anxiety symptoms severe enough to be eligible for randomization to adjunctive fluvoxamine or placebo.
Stimulants are contraindicated in patients with tic disorders
The package inserts for most stimulant medications warn clinicians that stimulants are “contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.” This is particularly concerning, especially because of the medicolegal implications of the term “contraindicated” and given that as many as 1 in 5 pediatric patients with ADHD have a tic disorder.6 Therefore, labels that list motor tics as a contraindication to stimulant use potentially eliminate the choice of stimulant pharmacotherapy—the most effective treatment for ADHD—for a large number of patients.
When hunting for the Woozle that linked stimulants and tics and led to this language in the package insert, it is worthwhile to review a recent meta-analysis of 22 studies (involving nearly 2,400 youths with ADHD) that suggested new-onset tics or worsening of tics to be present in 5.7% of patients receiving stimulants and in 6.5% of patients receiving placebo. In addition, in this meta-analysis the class of stimulant, dosage, treatment duration, or patient age did not seem to be associated with onset or worsening of tics.7
Polypharmacy represents a therapeutic failure and is not evidence-based
Although treatment guidelines generally have discouraged combination therapy for treating ADHD, there are—on the basis of efficacy—insufficient data to support this prohibition. Moreover, over the last decade, several studies have suggested benefits for combining ADHD medications that have complimentary mechanisms. In this regard, 2 extended-release formulations of α2 agonists have received FDA approval for as adjunctive treatments in pediatric patients with ADHD (extended-release guanfacine and extended-release clonidine). However, despite these FDA indications as adjunctive treatments, many clinicians remain concerned about combination therapy.
Several months ago, a large, 8-week, National Institutes of Health–sponsored trial shed more light on the use of α2agonist + stimulant combinations. Patients age 7 to 17 (N = 179) were randomized to (1) guanfacine + d-methylphenidate, (2) guanfacine monotherapy, or (3) d-methylphenidate monotherapy.8 In addition to clinical outcomes, the authors evaluated the effects of the medication on background cortical activity. Of interest, monotherapies differed between one another and the combination treatment in their effects on cortical activity. Guanfacine decreased alpha band power and methylphenidate administration was associated with an increase in frontal/central beta power, while combination treatment dampened theta band power and was associated with specific, focal increases in beta power.8 These results, although preliminary, suggest not only that medication results in changes in cortical activity that correlate with symptomatic improvement, but that combination treatment may be associated with a distinct cortical activity pattern that is more than the summation of the effects of the monotherapies. Moreover, these data raise the possibility that this synergistic effect on cortical activity may subtend—or at least—relate to the synergistic clinical effects of the 2 medications.
‘Think it over, think it under’
Having discussed several important Woozles that have inhabited the Hundred Acre Wood of ADHD for decades, it is important to remember there are countless Woozles in the larger “Thousand Acre Wood” of psychiatry and medicine. As we evaluate evidence for our interventions, whether psychopharmacologic or psychotherapeutic, we will do w
1. Milne AA. Winnie-the-Pooh. London, United Kingdom: Methuen & Co. Ltd.; 1926.
2. Strauss MA. Processes Explaining the concealment and distortion of evidence on gender symmetry in partner violence. Eur J Crim Pol Res. 1980;74:227-232.
3. Ritalin LA [package insert]. East Hanover, NJ: Novartis; 2015.
4. Coughlin CG, Cohen SC, Mulqueen JM, et al. Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25(8):611-617.
5. Abikoff H, McGough J, Vitiello B, et al; RUPP ADHD/Anxiety Study Group. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418-427.
6. Bloch MH, Panza KE, Landeros-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
7. Cohen SC, Mulqueen JM, Ferracioli-Oda E, et al. Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(9):728-736.
8. Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1.
1. Milne AA. Winnie-the-Pooh. London, United Kingdom: Methuen & Co. Ltd.; 1926.
2. Strauss MA. Processes Explaining the concealment and distortion of evidence on gender symmetry in partner violence. Eur J Crim Pol Res. 1980;74:227-232.
3. Ritalin LA [package insert]. East Hanover, NJ: Novartis; 2015.
4. Coughlin CG, Cohen SC, Mulqueen JM, et al. Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25(8):611-617.
5. Abikoff H, McGough J, Vitiello B, et al; RUPP ADHD/Anxiety Study Group. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418-427.
6. Bloch MH, Panza KE, Landeros-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
7. Cohen SC, Mulqueen JM, Ferracioli-Oda E, et al. Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(9):728-736.
8. Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1.
Rule out these causes of inattention before diagnosing ADHD
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
An irritable, inattentive, and disruptive child: Is it ADHD or bipolar disorder?
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
No more 'stickies'!: Help your patients bring their ‘to-do’ list into the 21st century
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Disordered sleep: Ask the right questions to reveal this hidden confounder
It seems like common sense: Sleeping poorly results in not feeling good. The truth is that many of our patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. The busy life that many patients have does not allow adequate time for sleep. In fact, I have encountered patients who think of sleep as an inconvenience that takes away time from other pursuits.
Sleep deprivation in psychiatric disorders
Sleep deprivation occurs when the duration or quality of sleep is inadequate. Inadequate sleep duration can be caused by insomnia or simply not allowing enough time for sleep (1 aspect of poor sleep hygiene). Poor sleep quality often is caused by sleep-disordered breathing.
Sleep deprivation can result in either sleepiness or fatigue. Sleepiness is a propensity to fall asleep; fatigue is a lack of energy that is not alleviated by additional sleep. Fatigue is more likely to be associated with a psychiatric disorder; sleepiness is more predominant in sleep disorders (although there is significant overlap). For example, patients with a major depressive disorder can experience fatigue as much as patients with sleep deprivation, but the latter also is more likely to result in sleepiness. Trouble concentrating is seen in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and sleep deprivation.1
Insomnia or poor sleep hygiene can be diagnosed with a thorough sleep history. I take special care to consider sleep problems by presenting 5 groups of questions to the patient (Box).
Sleep-disordered breathing
A sleep study is required to accurately diagnose sleep-disordered breathing. Unless this diagnosis is specifically looked for, it remains hidden from both physicians and patients. Clues to the presence of sleep-disordered breathing include snorting, snoring, and gasping for air during sleep; witnessed apnea during sleep; nighttime awakening; daytime fatigue; nocturia; mouth breathing or dry mouth; acid reflux; irritability; morning headache; nighttime sweating; and low libido. Risk factors for sleep-disordered breathing include obesity; smoking; menopause; family history; increasing age; and anatomical factors (eg, deviation of the nasal septum; retrognathia; long face syndrome; high-arched narrow hard palate; large tonsils, uvula, or tongue).2
Measuring sleep quality
Some patients are unaware of the extent to which they are sleepy. The most widely used scale to measure sleepiness is the Epworth Sleepiness Scale.3 Sleep specialists view a score of ≥10 on the Epworth scale as indicative of daytime sleepiness. In addition, a patient’s daily consumption of caffeinated beverages can be a clue to excessive sleepiness or, at least, fatigue. If the degree of sleepiness cannot be determined subjectively, objective measures, such as the Multiple Sleep Latency Test (MSLT), can quantify it. In a randomly selected sample from the general population, 13.4% had excessive daytime sleepiness as measured by the MSLT.4
Adult ADHD and sleep deprivation
In my practice, sleep problems confound both the diagnosis and treatment of psychiatric disorders, especially ADHD. Often, patients who report ADHD symptoms have no clear history of ADHD during childhood. In these cases, I always consider the possibility that their ADHD symptoms are due to sleep deprivation. Sleep deprivation can mimic the poor executive function and difficulty concentrating that is often seen in ADHD, because such deprivation is associated with decreased activity in the prefrontal cortex during wakefulness.5
In patients who provide a clear history of ADHD symptoms during childhood, it is possible that inadequate sleep exacerbates ADHD symptoms as adults. Unless sleep deprivation is diagnosed and treated in these patients, they can end up taking a higher-than-necessary dosage of a stimulant. Also, patients who have ADHD might have a difficult time managing their sleep schedule because of poor executive functioning. This, in turn, can result in additional sleep deprivation, thus worsening their ADHD symptoms, creating a vicious circle.
Psychotropics and sedation
Many psychiatric medications list sedation as a side effect. Patients with untreated sleep problems might be more likely to notice this side effect because sleep problems contribute to their fatigue. I have had patients who were unable to tolerate sedative medications until their sleep apnea was treated.
In conclusion
It is important to consider sleep deprivation in your differential diagnosis of psychiatric patients. This will allow for more accurate diagnosis and treatment and, in some cases, can avoid treatment resistance.
1. Stahl SM. Excessive sleepiness. San Diego, CA: NEI Press; 2005.
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736-747.
3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
4. Drake CL, Roehrs TA, Richardson GS, et al. Epidemiology and morbidity of excessive daytime sleepiness. Sleep. 2002;25:A91-A92.
5. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
It seems like common sense: Sleeping poorly results in not feeling good. The truth is that many of our patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. The busy life that many patients have does not allow adequate time for sleep. In fact, I have encountered patients who think of sleep as an inconvenience that takes away time from other pursuits.
Sleep deprivation in psychiatric disorders
Sleep deprivation occurs when the duration or quality of sleep is inadequate. Inadequate sleep duration can be caused by insomnia or simply not allowing enough time for sleep (1 aspect of poor sleep hygiene). Poor sleep quality often is caused by sleep-disordered breathing.
Sleep deprivation can result in either sleepiness or fatigue. Sleepiness is a propensity to fall asleep; fatigue is a lack of energy that is not alleviated by additional sleep. Fatigue is more likely to be associated with a psychiatric disorder; sleepiness is more predominant in sleep disorders (although there is significant overlap). For example, patients with a major depressive disorder can experience fatigue as much as patients with sleep deprivation, but the latter also is more likely to result in sleepiness. Trouble concentrating is seen in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and sleep deprivation.1
Insomnia or poor sleep hygiene can be diagnosed with a thorough sleep history. I take special care to consider sleep problems by presenting 5 groups of questions to the patient (Box).
Sleep-disordered breathing
A sleep study is required to accurately diagnose sleep-disordered breathing. Unless this diagnosis is specifically looked for, it remains hidden from both physicians and patients. Clues to the presence of sleep-disordered breathing include snorting, snoring, and gasping for air during sleep; witnessed apnea during sleep; nighttime awakening; daytime fatigue; nocturia; mouth breathing or dry mouth; acid reflux; irritability; morning headache; nighttime sweating; and low libido. Risk factors for sleep-disordered breathing include obesity; smoking; menopause; family history; increasing age; and anatomical factors (eg, deviation of the nasal septum; retrognathia; long face syndrome; high-arched narrow hard palate; large tonsils, uvula, or tongue).2
Measuring sleep quality
Some patients are unaware of the extent to which they are sleepy. The most widely used scale to measure sleepiness is the Epworth Sleepiness Scale.3 Sleep specialists view a score of ≥10 on the Epworth scale as indicative of daytime sleepiness. In addition, a patient’s daily consumption of caffeinated beverages can be a clue to excessive sleepiness or, at least, fatigue. If the degree of sleepiness cannot be determined subjectively, objective measures, such as the Multiple Sleep Latency Test (MSLT), can quantify it. In a randomly selected sample from the general population, 13.4% had excessive daytime sleepiness as measured by the MSLT.4
Adult ADHD and sleep deprivation
In my practice, sleep problems confound both the diagnosis and treatment of psychiatric disorders, especially ADHD. Often, patients who report ADHD symptoms have no clear history of ADHD during childhood. In these cases, I always consider the possibility that their ADHD symptoms are due to sleep deprivation. Sleep deprivation can mimic the poor executive function and difficulty concentrating that is often seen in ADHD, because such deprivation is associated with decreased activity in the prefrontal cortex during wakefulness.5
In patients who provide a clear history of ADHD symptoms during childhood, it is possible that inadequate sleep exacerbates ADHD symptoms as adults. Unless sleep deprivation is diagnosed and treated in these patients, they can end up taking a higher-than-necessary dosage of a stimulant. Also, patients who have ADHD might have a difficult time managing their sleep schedule because of poor executive functioning. This, in turn, can result in additional sleep deprivation, thus worsening their ADHD symptoms, creating a vicious circle.
Psychotropics and sedation
Many psychiatric medications list sedation as a side effect. Patients with untreated sleep problems might be more likely to notice this side effect because sleep problems contribute to their fatigue. I have had patients who were unable to tolerate sedative medications until their sleep apnea was treated.
In conclusion
It is important to consider sleep deprivation in your differential diagnosis of psychiatric patients. This will allow for more accurate diagnosis and treatment and, in some cases, can avoid treatment resistance.
It seems like common sense: Sleeping poorly results in not feeling good. The truth is that many of our patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. The busy life that many patients have does not allow adequate time for sleep. In fact, I have encountered patients who think of sleep as an inconvenience that takes away time from other pursuits.
Sleep deprivation in psychiatric disorders
Sleep deprivation occurs when the duration or quality of sleep is inadequate. Inadequate sleep duration can be caused by insomnia or simply not allowing enough time for sleep (1 aspect of poor sleep hygiene). Poor sleep quality often is caused by sleep-disordered breathing.
Sleep deprivation can result in either sleepiness or fatigue. Sleepiness is a propensity to fall asleep; fatigue is a lack of energy that is not alleviated by additional sleep. Fatigue is more likely to be associated with a psychiatric disorder; sleepiness is more predominant in sleep disorders (although there is significant overlap). For example, patients with a major depressive disorder can experience fatigue as much as patients with sleep deprivation, but the latter also is more likely to result in sleepiness. Trouble concentrating is seen in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and sleep deprivation.1
Insomnia or poor sleep hygiene can be diagnosed with a thorough sleep history. I take special care to consider sleep problems by presenting 5 groups of questions to the patient (Box).
Sleep-disordered breathing
A sleep study is required to accurately diagnose sleep-disordered breathing. Unless this diagnosis is specifically looked for, it remains hidden from both physicians and patients. Clues to the presence of sleep-disordered breathing include snorting, snoring, and gasping for air during sleep; witnessed apnea during sleep; nighttime awakening; daytime fatigue; nocturia; mouth breathing or dry mouth; acid reflux; irritability; morning headache; nighttime sweating; and low libido. Risk factors for sleep-disordered breathing include obesity; smoking; menopause; family history; increasing age; and anatomical factors (eg, deviation of the nasal septum; retrognathia; long face syndrome; high-arched narrow hard palate; large tonsils, uvula, or tongue).2
Measuring sleep quality
Some patients are unaware of the extent to which they are sleepy. The most widely used scale to measure sleepiness is the Epworth Sleepiness Scale.3 Sleep specialists view a score of ≥10 on the Epworth scale as indicative of daytime sleepiness. In addition, a patient’s daily consumption of caffeinated beverages can be a clue to excessive sleepiness or, at least, fatigue. If the degree of sleepiness cannot be determined subjectively, objective measures, such as the Multiple Sleep Latency Test (MSLT), can quantify it. In a randomly selected sample from the general population, 13.4% had excessive daytime sleepiness as measured by the MSLT.4
Adult ADHD and sleep deprivation
In my practice, sleep problems confound both the diagnosis and treatment of psychiatric disorders, especially ADHD. Often, patients who report ADHD symptoms have no clear history of ADHD during childhood. In these cases, I always consider the possibility that their ADHD symptoms are due to sleep deprivation. Sleep deprivation can mimic the poor executive function and difficulty concentrating that is often seen in ADHD, because such deprivation is associated with decreased activity in the prefrontal cortex during wakefulness.5
In patients who provide a clear history of ADHD symptoms during childhood, it is possible that inadequate sleep exacerbates ADHD symptoms as adults. Unless sleep deprivation is diagnosed and treated in these patients, they can end up taking a higher-than-necessary dosage of a stimulant. Also, patients who have ADHD might have a difficult time managing their sleep schedule because of poor executive functioning. This, in turn, can result in additional sleep deprivation, thus worsening their ADHD symptoms, creating a vicious circle.
Psychotropics and sedation
Many psychiatric medications list sedation as a side effect. Patients with untreated sleep problems might be more likely to notice this side effect because sleep problems contribute to their fatigue. I have had patients who were unable to tolerate sedative medications until their sleep apnea was treated.
In conclusion
It is important to consider sleep deprivation in your differential diagnosis of psychiatric patients. This will allow for more accurate diagnosis and treatment and, in some cases, can avoid treatment resistance.
1. Stahl SM. Excessive sleepiness. San Diego, CA: NEI Press; 2005.
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736-747.
3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
4. Drake CL, Roehrs TA, Richardson GS, et al. Epidemiology and morbidity of excessive daytime sleepiness. Sleep. 2002;25:A91-A92.
5. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
1. Stahl SM. Excessive sleepiness. San Diego, CA: NEI Press; 2005.
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736-747.
3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
4. Drake CL, Roehrs TA, Richardson GS, et al. Epidemiology and morbidity of excessive daytime sleepiness. Sleep. 2002;25:A91-A92.
5. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.