User login
No spike in overdose deaths from relaxed buprenorphine regulations
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Massive rise in drug overdose deaths driven by opioids
The 376% represents the change in age-adjusted overdose deaths per 100,000 population, which went from 6.9 in 2001 to 32.4 in 2021, as the total number of deaths rose from 19,394 to 106,699 (450%) over that time period, the NCHS said in a recent data brief. That total made 2021 the first year ever with more than 100,000 overdose deaths.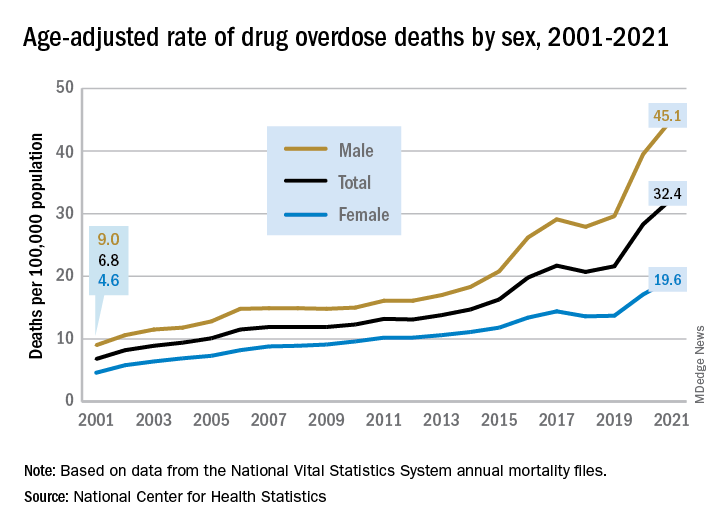
Since the age-adjusted rate stood at 21.6 per 100,000 in 2019, that means 42% of the total increase over 20 years actually occurred in 2020 and 2021. The number of deaths increased by about 36,000 over those 2 years, accounting for 41% of the total annual increase from 2001 to 2021, based on data from the National Vital Statistics System mortality files.
The overdose death rate was significantly higher for males than females for all of the years from 2001 to 2021, with males seeing an increase from 9.0 to 45.1 per 100,000 and females going from 4.6 to 19.6 deaths per 100,000. In the single year from 2020 to 2021, the age-adjusted rate was up by 14% for males and 15% for females, the mortality-file data show.
Analysis by age showed an even larger effect in some groups from 2020 to 2021. Drug overdose deaths jumped 28% among adults aged 65 years and older, more than any other group, and by 21% in those aged 55-64 years, according to the NCHS.
The only age group for which deaths didn’t increase significantly from 2020 to 2021 was 15- to 24-year-olds, whose rate rose by just 3%. The age group with the highest rate in both 2020 and 2021, however, was the 35- to 44-year-olds: 53.9 and 62.0 overdose deaths per 100,000, respectively, for an increase of 15%, the NCHS said in the report.
The drugs now involved in overdose deaths are most often opioids, a change from 2001. That year, opioids were involved in 49% of all overdose deaths, but by 2021 that share had increased to 75%. The trend for opioid-related deaths almost matches that of overall deaths over the 20-year span, and the significantly increasing trend that began for all overdose deaths in 2013 closely follows that of synthetic opioids such as fentanyl and tramadol, the report shows.
Overdose deaths involving cocaine and psychostimulants such as methamphetamine, amphetamine, and methylphenidate also show similar increases. The cocaine-related death rate rose 22% from 2020 to 2021 and is up by 421% since 2012, while the corresponding increases for psychostimulant deaths were 33% and 2,400%, the NCHS said.
The 376% represents the change in age-adjusted overdose deaths per 100,000 population, which went from 6.9 in 2001 to 32.4 in 2021, as the total number of deaths rose from 19,394 to 106,699 (450%) over that time period, the NCHS said in a recent data brief. That total made 2021 the first year ever with more than 100,000 overdose deaths.
Since the age-adjusted rate stood at 21.6 per 100,000 in 2019, that means 42% of the total increase over 20 years actually occurred in 2020 and 2021. The number of deaths increased by about 36,000 over those 2 years, accounting for 41% of the total annual increase from 2001 to 2021, based on data from the National Vital Statistics System mortality files.
The overdose death rate was significantly higher for males than females for all of the years from 2001 to 2021, with males seeing an increase from 9.0 to 45.1 per 100,000 and females going from 4.6 to 19.6 deaths per 100,000. In the single year from 2020 to 2021, the age-adjusted rate was up by 14% for males and 15% for females, the mortality-file data show.
Analysis by age showed an even larger effect in some groups from 2020 to 2021. Drug overdose deaths jumped 28% among adults aged 65 years and older, more than any other group, and by 21% in those aged 55-64 years, according to the NCHS.
The only age group for which deaths didn’t increase significantly from 2020 to 2021 was 15- to 24-year-olds, whose rate rose by just 3%. The age group with the highest rate in both 2020 and 2021, however, was the 35- to 44-year-olds: 53.9 and 62.0 overdose deaths per 100,000, respectively, for an increase of 15%, the NCHS said in the report.
The drugs now involved in overdose deaths are most often opioids, a change from 2001. That year, opioids were involved in 49% of all overdose deaths, but by 2021 that share had increased to 75%. The trend for opioid-related deaths almost matches that of overall deaths over the 20-year span, and the significantly increasing trend that began for all overdose deaths in 2013 closely follows that of synthetic opioids such as fentanyl and tramadol, the report shows.
Overdose deaths involving cocaine and psychostimulants such as methamphetamine, amphetamine, and methylphenidate also show similar increases. The cocaine-related death rate rose 22% from 2020 to 2021 and is up by 421% since 2012, while the corresponding increases for psychostimulant deaths were 33% and 2,400%, the NCHS said.
The 376% represents the change in age-adjusted overdose deaths per 100,000 population, which went from 6.9 in 2001 to 32.4 in 2021, as the total number of deaths rose from 19,394 to 106,699 (450%) over that time period, the NCHS said in a recent data brief. That total made 2021 the first year ever with more than 100,000 overdose deaths.
Since the age-adjusted rate stood at 21.6 per 100,000 in 2019, that means 42% of the total increase over 20 years actually occurred in 2020 and 2021. The number of deaths increased by about 36,000 over those 2 years, accounting for 41% of the total annual increase from 2001 to 2021, based on data from the National Vital Statistics System mortality files.
The overdose death rate was significantly higher for males than females for all of the years from 2001 to 2021, with males seeing an increase from 9.0 to 45.1 per 100,000 and females going from 4.6 to 19.6 deaths per 100,000. In the single year from 2020 to 2021, the age-adjusted rate was up by 14% for males and 15% for females, the mortality-file data show.
Analysis by age showed an even larger effect in some groups from 2020 to 2021. Drug overdose deaths jumped 28% among adults aged 65 years and older, more than any other group, and by 21% in those aged 55-64 years, according to the NCHS.
The only age group for which deaths didn’t increase significantly from 2020 to 2021 was 15- to 24-year-olds, whose rate rose by just 3%. The age group with the highest rate in both 2020 and 2021, however, was the 35- to 44-year-olds: 53.9 and 62.0 overdose deaths per 100,000, respectively, for an increase of 15%, the NCHS said in the report.
The drugs now involved in overdose deaths are most often opioids, a change from 2001. That year, opioids were involved in 49% of all overdose deaths, but by 2021 that share had increased to 75%. The trend for opioid-related deaths almost matches that of overall deaths over the 20-year span, and the significantly increasing trend that began for all overdose deaths in 2013 closely follows that of synthetic opioids such as fentanyl and tramadol, the report shows.
Overdose deaths involving cocaine and psychostimulants such as methamphetamine, amphetamine, and methylphenidate also show similar increases. The cocaine-related death rate rose 22% from 2020 to 2021 and is up by 421% since 2012, while the corresponding increases for psychostimulant deaths were 33% and 2,400%, the NCHS said.
Pandemic pregnancy-linked deaths up 35% from 2019
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
FROM JAMA NETWORK OPEN
Psychiatric illnesses share common brain network
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.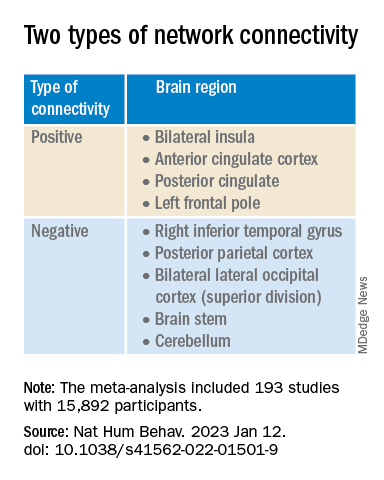
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE HUMAN BEHAVIOR
CDC updates guidance on opioid prescribing in adults
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
Six healthy lifestyle habits linked to slowed memory decline
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.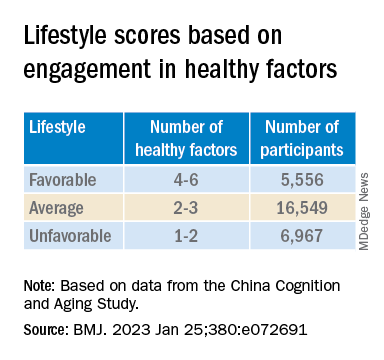
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.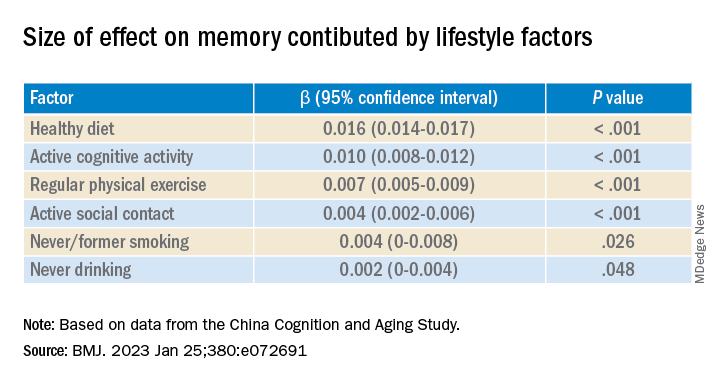
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
Managing patients with comorbid opioid and alcohol use disorders
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.
CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
U.S. ketamine poisonings up 81%
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PSYCHOPHARMACOLOGY
Chronic pain patients swapping opioids for medical cannabis
new research shows.
“That patients report substituting cannabis for pain medicines so much really underscores the need for research on the benefits and risks of using cannabis for chronic pain,” lead author Mark C. Bicket, MD, PhD, assistant professor, department of anesthesiology, and director, Opioid Prescribing Engagement Network, University of Michigan, Ann Arbor, said in an interview.
However, he added, the question is whether they’re turning to cannabis and away from other pain treatments. “What’s not clear and one of the gaps that we wanted to address in the study was if medical cannabis use is changing the use of other treatments for chronic pain,” said Dr. Bicket.
The study was published online in JAMA Network Open.
Decreased opioid use
The survey included a representative sample of 1724 American adults aged 18 years or older with chronic noncancer pain living in areas with a medical cannabis program.
Respondents were asked about their use of three categories of pain treatments. This included medical cannabis; pharmacologic treatments including prescription opioids, nonopioid analgesics, and over-the-counter analgesics; and common nonpharmacologic treatments such as physical therapy, meditation, and cognitive-behavioral therapy (CBT).
Just over 96% of respondents completed the full survey. About 57% of the sample was female and the mean age of the study sample was 52.3 years.
Among study participants, 31% (95% CI, 28.2% - 34.1%) reported having ever used cannabis to manage pain; 25.9% (95% confidence interval, 23.2%-28.8%) reported use in the past 12 months, and 23.2% (95% CI, 20.6%-26%) reported use in the past 30 days.
“This translates into a large number of individuals who are using cannabis in an intended medical way” to treat chronic condition such as low back pain, migraine, and fibromyalgia, said Dr. Bicket.
More than half of survey respondents reported their medical cannabis use led to a decrease in prescription opioid use, prescription nonopioid use and use of over-the-counter medications.
Dr. Bicket noted “almost no one” said medical cannabis use led to higher use of these drugs.
As for nonpharmacologic treatments, 38.7% reported their use of cannabis led to decreased use of physical therapy, 19.1% to lower use of meditation, and 26% to less CBT. At the same time, 5.9%, 23.7% and 17.1%, respectively, reported it led to increased use of physical therapy, meditation, and CBT.
Medical cannabis is regulated at a state level. On a federal level, it’s considered a Schedule I substance, which means it’s deemed not to have a therapeutic use, although some groups are trying to change that categorization, said Dr. Bicket.
As a result, cannabis products “are quite variable” in terms of how they’re used (smoked, eaten etc.) and in their composition, including percentage of cannabidiol and tetrahydrocannabinol.
“We really don’t have a good sense of the relative risks and benefits that could come from cannabis as a treatment for chronic pain,” said Dr. Bicket. “As a physician, it’s difficult to have discussions with patients because I’m not able to understand the products they’re using based on this regulatory environment we have.”
He added clinicians “are operating in an area of uncertainty right now.”
What’s needed is research to determine how safe and effective medical cannabis is for chronic pain, he said.
Pain a leading indication
Commenting on the findings, Jason W. Busse, PhD, professor, department of anesthesia, and associate director, Centre for Medicinal Cannabis Research, McMaster University, Hamilton, Ont., said the study reinforces results of some prior research.
“It gives us current information certainly highlighting the high rate of use of medical cannabis among individuals with chronic pain once it becomes legally available.”
In addition, this high rate of use “means we desperately need information about the benefits and harms” of medical marijuana, he said.
Dr. Busse noted the survey didn’t provide information on the types of cannabis being used or the mode of administration. Oil drops and sprays cause less pulmonary harm than smoked versions, he said. It’s also not clear from the survey if participants are taking formulations with high levels of tetrahydrocannabinol that are associated with greater risk of harm.
He noted cannabis may interact with prescription drugs to make them less effective or, in some cases, to augment their adverse effects.
Dr. Busse pointed out some patients could be using fewer opioids because providers are under “enormous pressure” to reduce prescriptions of these drugs in the wake of spikes in opioid overdoses and deaths.
Chronic pain is “absolutely the leading indication” for medical marijuana, said Dr. Busse. U.S. reimbursement data suggest up to 65% of individuals get cannabis to treat a listed indication for chronic pain.
He said he hopes this new study will increase interest in funding new trials “so we can have better evidence to guide practice to help patients make decisions.”
The study received support from the National Institute on Drug Abuse. Dr. Bicket reported receiving personal fees from Axial Healthcare as well as grants from the National Institutes of Health, the Centers for Disease Control and Prevention, Michigan Department of Health and Human Services, Arnold Foundation, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
“That patients report substituting cannabis for pain medicines so much really underscores the need for research on the benefits and risks of using cannabis for chronic pain,” lead author Mark C. Bicket, MD, PhD, assistant professor, department of anesthesiology, and director, Opioid Prescribing Engagement Network, University of Michigan, Ann Arbor, said in an interview.
However, he added, the question is whether they’re turning to cannabis and away from other pain treatments. “What’s not clear and one of the gaps that we wanted to address in the study was if medical cannabis use is changing the use of other treatments for chronic pain,” said Dr. Bicket.
The study was published online in JAMA Network Open.
Decreased opioid use
The survey included a representative sample of 1724 American adults aged 18 years or older with chronic noncancer pain living in areas with a medical cannabis program.
Respondents were asked about their use of three categories of pain treatments. This included medical cannabis; pharmacologic treatments including prescription opioids, nonopioid analgesics, and over-the-counter analgesics; and common nonpharmacologic treatments such as physical therapy, meditation, and cognitive-behavioral therapy (CBT).
Just over 96% of respondents completed the full survey. About 57% of the sample was female and the mean age of the study sample was 52.3 years.
Among study participants, 31% (95% CI, 28.2% - 34.1%) reported having ever used cannabis to manage pain; 25.9% (95% confidence interval, 23.2%-28.8%) reported use in the past 12 months, and 23.2% (95% CI, 20.6%-26%) reported use in the past 30 days.
“This translates into a large number of individuals who are using cannabis in an intended medical way” to treat chronic condition such as low back pain, migraine, and fibromyalgia, said Dr. Bicket.
More than half of survey respondents reported their medical cannabis use led to a decrease in prescription opioid use, prescription nonopioid use and use of over-the-counter medications.
Dr. Bicket noted “almost no one” said medical cannabis use led to higher use of these drugs.
As for nonpharmacologic treatments, 38.7% reported their use of cannabis led to decreased use of physical therapy, 19.1% to lower use of meditation, and 26% to less CBT. At the same time, 5.9%, 23.7% and 17.1%, respectively, reported it led to increased use of physical therapy, meditation, and CBT.
Medical cannabis is regulated at a state level. On a federal level, it’s considered a Schedule I substance, which means it’s deemed not to have a therapeutic use, although some groups are trying to change that categorization, said Dr. Bicket.
As a result, cannabis products “are quite variable” in terms of how they’re used (smoked, eaten etc.) and in their composition, including percentage of cannabidiol and tetrahydrocannabinol.
“We really don’t have a good sense of the relative risks and benefits that could come from cannabis as a treatment for chronic pain,” said Dr. Bicket. “As a physician, it’s difficult to have discussions with patients because I’m not able to understand the products they’re using based on this regulatory environment we have.”
He added clinicians “are operating in an area of uncertainty right now.”
What’s needed is research to determine how safe and effective medical cannabis is for chronic pain, he said.
Pain a leading indication
Commenting on the findings, Jason W. Busse, PhD, professor, department of anesthesia, and associate director, Centre for Medicinal Cannabis Research, McMaster University, Hamilton, Ont., said the study reinforces results of some prior research.
“It gives us current information certainly highlighting the high rate of use of medical cannabis among individuals with chronic pain once it becomes legally available.”
In addition, this high rate of use “means we desperately need information about the benefits and harms” of medical marijuana, he said.
Dr. Busse noted the survey didn’t provide information on the types of cannabis being used or the mode of administration. Oil drops and sprays cause less pulmonary harm than smoked versions, he said. It’s also not clear from the survey if participants are taking formulations with high levels of tetrahydrocannabinol that are associated with greater risk of harm.
He noted cannabis may interact with prescription drugs to make them less effective or, in some cases, to augment their adverse effects.
Dr. Busse pointed out some patients could be using fewer opioids because providers are under “enormous pressure” to reduce prescriptions of these drugs in the wake of spikes in opioid overdoses and deaths.
Chronic pain is “absolutely the leading indication” for medical marijuana, said Dr. Busse. U.S. reimbursement data suggest up to 65% of individuals get cannabis to treat a listed indication for chronic pain.
He said he hopes this new study will increase interest in funding new trials “so we can have better evidence to guide practice to help patients make decisions.”
The study received support from the National Institute on Drug Abuse. Dr. Bicket reported receiving personal fees from Axial Healthcare as well as grants from the National Institutes of Health, the Centers for Disease Control and Prevention, Michigan Department of Health and Human Services, Arnold Foundation, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
“That patients report substituting cannabis for pain medicines so much really underscores the need for research on the benefits and risks of using cannabis for chronic pain,” lead author Mark C. Bicket, MD, PhD, assistant professor, department of anesthesiology, and director, Opioid Prescribing Engagement Network, University of Michigan, Ann Arbor, said in an interview.
However, he added, the question is whether they’re turning to cannabis and away from other pain treatments. “What’s not clear and one of the gaps that we wanted to address in the study was if medical cannabis use is changing the use of other treatments for chronic pain,” said Dr. Bicket.
The study was published online in JAMA Network Open.
Decreased opioid use
The survey included a representative sample of 1724 American adults aged 18 years or older with chronic noncancer pain living in areas with a medical cannabis program.
Respondents were asked about their use of three categories of pain treatments. This included medical cannabis; pharmacologic treatments including prescription opioids, nonopioid analgesics, and over-the-counter analgesics; and common nonpharmacologic treatments such as physical therapy, meditation, and cognitive-behavioral therapy (CBT).
Just over 96% of respondents completed the full survey. About 57% of the sample was female and the mean age of the study sample was 52.3 years.
Among study participants, 31% (95% CI, 28.2% - 34.1%) reported having ever used cannabis to manage pain; 25.9% (95% confidence interval, 23.2%-28.8%) reported use in the past 12 months, and 23.2% (95% CI, 20.6%-26%) reported use in the past 30 days.
“This translates into a large number of individuals who are using cannabis in an intended medical way” to treat chronic condition such as low back pain, migraine, and fibromyalgia, said Dr. Bicket.
More than half of survey respondents reported their medical cannabis use led to a decrease in prescription opioid use, prescription nonopioid use and use of over-the-counter medications.
Dr. Bicket noted “almost no one” said medical cannabis use led to higher use of these drugs.
As for nonpharmacologic treatments, 38.7% reported their use of cannabis led to decreased use of physical therapy, 19.1% to lower use of meditation, and 26% to less CBT. At the same time, 5.9%, 23.7% and 17.1%, respectively, reported it led to increased use of physical therapy, meditation, and CBT.
Medical cannabis is regulated at a state level. On a federal level, it’s considered a Schedule I substance, which means it’s deemed not to have a therapeutic use, although some groups are trying to change that categorization, said Dr. Bicket.
As a result, cannabis products “are quite variable” in terms of how they’re used (smoked, eaten etc.) and in their composition, including percentage of cannabidiol and tetrahydrocannabinol.
“We really don’t have a good sense of the relative risks and benefits that could come from cannabis as a treatment for chronic pain,” said Dr. Bicket. “As a physician, it’s difficult to have discussions with patients because I’m not able to understand the products they’re using based on this regulatory environment we have.”
He added clinicians “are operating in an area of uncertainty right now.”
What’s needed is research to determine how safe and effective medical cannabis is for chronic pain, he said.
Pain a leading indication
Commenting on the findings, Jason W. Busse, PhD, professor, department of anesthesia, and associate director, Centre for Medicinal Cannabis Research, McMaster University, Hamilton, Ont., said the study reinforces results of some prior research.
“It gives us current information certainly highlighting the high rate of use of medical cannabis among individuals with chronic pain once it becomes legally available.”
In addition, this high rate of use “means we desperately need information about the benefits and harms” of medical marijuana, he said.
Dr. Busse noted the survey didn’t provide information on the types of cannabis being used or the mode of administration. Oil drops and sprays cause less pulmonary harm than smoked versions, he said. It’s also not clear from the survey if participants are taking formulations with high levels of tetrahydrocannabinol that are associated with greater risk of harm.
He noted cannabis may interact with prescription drugs to make them less effective or, in some cases, to augment their adverse effects.
Dr. Busse pointed out some patients could be using fewer opioids because providers are under “enormous pressure” to reduce prescriptions of these drugs in the wake of spikes in opioid overdoses and deaths.
Chronic pain is “absolutely the leading indication” for medical marijuana, said Dr. Busse. U.S. reimbursement data suggest up to 65% of individuals get cannabis to treat a listed indication for chronic pain.
He said he hopes this new study will increase interest in funding new trials “so we can have better evidence to guide practice to help patients make decisions.”
The study received support from the National Institute on Drug Abuse. Dr. Bicket reported receiving personal fees from Axial Healthcare as well as grants from the National Institutes of Health, the Centers for Disease Control and Prevention, Michigan Department of Health and Human Services, Arnold Foundation, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN




