User login
New FDA guidance aims to cut sodium in processed foods
The Food and Drug Administration has issued voluntary, short-term sodium reduction targets for food manufacturers, chain restaurants, and food service operators for processed, packaged, and prepared foods, with an eye toward reducing diet-related conditions such as heart disease and obesity.
The new targets seek to decrease average sodium intake from approximately 3,400 mg/day to 3,000 mg/day, about a 12% reduction, over the next 2.5 years, acting FDA Commissioner Janet Woodcock, MD, and Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said in joint statement.
Although this reduction keeps the average intake above the recommended limit of 2,300 mg/day for individuals 14 years and older as per the Dietary Guidelines for Americans, “we know that even these modest reductions made slowly over the next few years will substantially decrease diet-related diseases,” they added.
The FDA first proposed recommendations for reducing sodium content in draft guidance released in 2016.
Since, then a number of companies in the food industry have already made changes to sodium content in their products, “which is encouraging, but additional support across all types of foods to help consumers meet recommended sodium limits is needed,” Dr. Woodcock and Dr. Mayne said.
They emphasized that the new guidance represents short-term goals that the food industry should work to meet as soon as possible to help optimize public health.
“We will continue our discussions with the food industry as we monitor the sodium content of the food supply to evaluate progress. In the future, we plan to issue revised, subsequent targets to further lower the sodium content incrementally and continue to help reduce sodium intake,” Dr. Woodcock and Dr. Mayne said.
AHA: A good first step that does not go far enough
In a statement, the American Heart Association said the new targets will play “a critical role in helping people across the country achieve healthier levels of sodium and improved well-being overall. These targets will be an important driver to reduce sodium consumption, which can have significant health benefits and lead to lower medical costs.”
“Lowering sodium levels in the food supply would reduce risk of hypertension, heart disease, stroke, heart attack, and death in addition to saving billions of dollars in health care costs over the next decade,” the AHA said.
But the AHA also said lowering sodium intake to 3,000 mg/day is not enough.
“Lowering sodium further to 2,300 mg could prevent an estimated 450,000 cases of cardiovascular disease, gain 2 million quality-adjusted life-years, and save approximately $40 billion in health care costs over a 20-year period,” the AHA said.
The AHA is urging the FDA to “follow [this] action with additional targets to further lower the amount of sodium in the food supply and help people in America attain an appropriate sodium intake.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued voluntary, short-term sodium reduction targets for food manufacturers, chain restaurants, and food service operators for processed, packaged, and prepared foods, with an eye toward reducing diet-related conditions such as heart disease and obesity.
The new targets seek to decrease average sodium intake from approximately 3,400 mg/day to 3,000 mg/day, about a 12% reduction, over the next 2.5 years, acting FDA Commissioner Janet Woodcock, MD, and Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said in joint statement.
Although this reduction keeps the average intake above the recommended limit of 2,300 mg/day for individuals 14 years and older as per the Dietary Guidelines for Americans, “we know that even these modest reductions made slowly over the next few years will substantially decrease diet-related diseases,” they added.
The FDA first proposed recommendations for reducing sodium content in draft guidance released in 2016.
Since, then a number of companies in the food industry have already made changes to sodium content in their products, “which is encouraging, but additional support across all types of foods to help consumers meet recommended sodium limits is needed,” Dr. Woodcock and Dr. Mayne said.
They emphasized that the new guidance represents short-term goals that the food industry should work to meet as soon as possible to help optimize public health.
“We will continue our discussions with the food industry as we monitor the sodium content of the food supply to evaluate progress. In the future, we plan to issue revised, subsequent targets to further lower the sodium content incrementally and continue to help reduce sodium intake,” Dr. Woodcock and Dr. Mayne said.
AHA: A good first step that does not go far enough
In a statement, the American Heart Association said the new targets will play “a critical role in helping people across the country achieve healthier levels of sodium and improved well-being overall. These targets will be an important driver to reduce sodium consumption, which can have significant health benefits and lead to lower medical costs.”
“Lowering sodium levels in the food supply would reduce risk of hypertension, heart disease, stroke, heart attack, and death in addition to saving billions of dollars in health care costs over the next decade,” the AHA said.
But the AHA also said lowering sodium intake to 3,000 mg/day is not enough.
“Lowering sodium further to 2,300 mg could prevent an estimated 450,000 cases of cardiovascular disease, gain 2 million quality-adjusted life-years, and save approximately $40 billion in health care costs over a 20-year period,” the AHA said.
The AHA is urging the FDA to “follow [this] action with additional targets to further lower the amount of sodium in the food supply and help people in America attain an appropriate sodium intake.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued voluntary, short-term sodium reduction targets for food manufacturers, chain restaurants, and food service operators for processed, packaged, and prepared foods, with an eye toward reducing diet-related conditions such as heart disease and obesity.
The new targets seek to decrease average sodium intake from approximately 3,400 mg/day to 3,000 mg/day, about a 12% reduction, over the next 2.5 years, acting FDA Commissioner Janet Woodcock, MD, and Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said in joint statement.
Although this reduction keeps the average intake above the recommended limit of 2,300 mg/day for individuals 14 years and older as per the Dietary Guidelines for Americans, “we know that even these modest reductions made slowly over the next few years will substantially decrease diet-related diseases,” they added.
The FDA first proposed recommendations for reducing sodium content in draft guidance released in 2016.
Since, then a number of companies in the food industry have already made changes to sodium content in their products, “which is encouraging, but additional support across all types of foods to help consumers meet recommended sodium limits is needed,” Dr. Woodcock and Dr. Mayne said.
They emphasized that the new guidance represents short-term goals that the food industry should work to meet as soon as possible to help optimize public health.
“We will continue our discussions with the food industry as we monitor the sodium content of the food supply to evaluate progress. In the future, we plan to issue revised, subsequent targets to further lower the sodium content incrementally and continue to help reduce sodium intake,” Dr. Woodcock and Dr. Mayne said.
AHA: A good first step that does not go far enough
In a statement, the American Heart Association said the new targets will play “a critical role in helping people across the country achieve healthier levels of sodium and improved well-being overall. These targets will be an important driver to reduce sodium consumption, which can have significant health benefits and lead to lower medical costs.”
“Lowering sodium levels in the food supply would reduce risk of hypertension, heart disease, stroke, heart attack, and death in addition to saving billions of dollars in health care costs over the next decade,” the AHA said.
But the AHA also said lowering sodium intake to 3,000 mg/day is not enough.
“Lowering sodium further to 2,300 mg could prevent an estimated 450,000 cases of cardiovascular disease, gain 2 million quality-adjusted life-years, and save approximately $40 billion in health care costs over a 20-year period,” the AHA said.
The AHA is urging the FDA to “follow [this] action with additional targets to further lower the amount of sodium in the food supply and help people in America attain an appropriate sodium intake.”
A version of this article first appeared on Medscape.com.
Circulating post-STEMI ketones elevated, hints at treatment role
Circulating ketone bodies (KBs) are substantially elevated at presentation and 24 hours after ST-segment elevation myocardial infarction (STEMI), according to new research.
The study also showed that greater KB levels measured after 24 hours of reperfusion were associated with larger infarct size and reduced left ventricular ejection fraction (LVEF).
The findings suggest a potential role for ketone metabolism in response to myocardial ischemia, conclude researchers in their report, published in the October 5 issue of the Journal of the American College of Cardiology.
“Ketones serve as an alternative source of energy for the heart,” lead author Marie-Sophie L.Y. de Koning, MD, University Medical Center Groningen, the Netherlands, told this news organization.
“These results might suggest that ketone bodies may be an important fuel for the heart after myocardial ischemia.” The role of KBs in heart failure has been previously studied, but their role in myocardial infarction has not, Dr. De Koning said.
“In heart failure, metabolic changes occur that cause the heart to increasingly rely on ketone bodies as an important energy source. Accordingly, concentrations of circulating ketone bodies are elevated and higher concentrations have been linked with more severe heart failure,” she said.
”This might suggest that upregulation of ketone metabolism is a universal cardiac response to stress,” Dr. De Koning added. “But the role of ketone bodies in myocardial infarction remained largely unknown, and this triggered us to investigate circulating ketone bodies in patients presenting with STEMI.”
She and her team measured circulating KBs in archived plasma samples from 369 participants in the randomized GIPS-III trial. The study had primarily looked at the effect of 4 months of metformin therapy, compared with placebo, on LVEF in nondiabetic patients with a first STEMI.
Blood samples had been taken at baseline before percutaneous coronary intervention (PCI), at 24 hours after reperfusion, and at 4 months.
The current study investigated longitudinal post-STEMI changes in the circulating KBs beta-hydroxybutyrate, acetoacetate, and acetone. It also looked at associations of KBs with infarct size and LVEF, both of which were measured with cardiac magnetic resonance (CMR) imaging 4 months after STEMI.
Circulating KB levels were three times higher at STEMI presentation than at 4 months. At presentation, the median total KB level was 520 μmol/L. It was still higher 24 hours after reperfusion than at 4 months (206 vs. 166 μmol/L; P < .001).
The 24-hour KB elevations were independently and positively associated with larger infarct size (P = .016) and lower LVEF (P = .012), the group reports.
“Our results indicate a possible role for ketone bodies during myocardial infarction,” Dr. De Koning said.
The KB elevations were probably followed by “an increase in cardiac ketone body metabolism, in order to fuel the heart that is energetically depleted.”
But the study didn’t explore cardiac KB consumption, Dr. De Koning cautioned, adding that the next steps in this research should be to investigate post-STEMI cardiac ketone metabolism and its pathophysiologic mechanisms. “This may facilitate future trials to study therapeutic effects of ketone body supplementation during or after STEMI.”
The current findings “form an essential basis for our understanding of the role of KBs in ischemia/reperfusion,” write Salva R. Yurista, MD, PhD, and colleagues, Massachusetts General Hospital and Harvard Medical School, Boston, in an accompanying editorial.
“Although the appeal of enhancing KBs as a therapeutic approach is understandable, additional rigorous preclinical and clinical studies will be required to test this therapeutic hypothesis and determine the mechanisms contributing to any benefits observed,” they note.
”Exposure to cardiac stress, such as ischemia, infarction, or heart failure, will stimulate the release of neurohormones, pro-inflammatory cytokines, and natriuretic peptides, which may play roles in stimulating ketogenesis or the production of ketone bodies,” Dr. Yurista told this news organization.
The increased circulating ketone concentrations and myocardial ketone oxidation that were associated with poor functional outcomes have been reported in other clinical contexts, including heart failure with reduced ejection fraction, heart failure with preserved cardiac function, diabetic cardiomyopathy, and arrhythmogenic cardiomyopathy, he said.
Dr. Yurista agrees that KBs could have therapeutic merit.
“Circulating ketone concentrations determine the contribution of ketones to the cardiac diet,” he said. “Thus, increasing cardiac delivery of ketone bodies through supplementation or other means to the heart undergoing stress, including STEMI and heart failure, could have therapeutic potential.”
The GIPS-III trial was supported by the Netherlands Organization for Health Research and Development (ZonMw). Neither Dr. De Koning nor the other authors report relevant financial relationships. Dr. Yurista and the other editorialists report no relevant relationships.
A version of this article first appeared on Medscape.com.
Circulating ketone bodies (KBs) are substantially elevated at presentation and 24 hours after ST-segment elevation myocardial infarction (STEMI), according to new research.
The study also showed that greater KB levels measured after 24 hours of reperfusion were associated with larger infarct size and reduced left ventricular ejection fraction (LVEF).
The findings suggest a potential role for ketone metabolism in response to myocardial ischemia, conclude researchers in their report, published in the October 5 issue of the Journal of the American College of Cardiology.
“Ketones serve as an alternative source of energy for the heart,” lead author Marie-Sophie L.Y. de Koning, MD, University Medical Center Groningen, the Netherlands, told this news organization.
“These results might suggest that ketone bodies may be an important fuel for the heart after myocardial ischemia.” The role of KBs in heart failure has been previously studied, but their role in myocardial infarction has not, Dr. De Koning said.
“In heart failure, metabolic changes occur that cause the heart to increasingly rely on ketone bodies as an important energy source. Accordingly, concentrations of circulating ketone bodies are elevated and higher concentrations have been linked with more severe heart failure,” she said.
”This might suggest that upregulation of ketone metabolism is a universal cardiac response to stress,” Dr. De Koning added. “But the role of ketone bodies in myocardial infarction remained largely unknown, and this triggered us to investigate circulating ketone bodies in patients presenting with STEMI.”
She and her team measured circulating KBs in archived plasma samples from 369 participants in the randomized GIPS-III trial. The study had primarily looked at the effect of 4 months of metformin therapy, compared with placebo, on LVEF in nondiabetic patients with a first STEMI.
Blood samples had been taken at baseline before percutaneous coronary intervention (PCI), at 24 hours after reperfusion, and at 4 months.
The current study investigated longitudinal post-STEMI changes in the circulating KBs beta-hydroxybutyrate, acetoacetate, and acetone. It also looked at associations of KBs with infarct size and LVEF, both of which were measured with cardiac magnetic resonance (CMR) imaging 4 months after STEMI.
Circulating KB levels were three times higher at STEMI presentation than at 4 months. At presentation, the median total KB level was 520 μmol/L. It was still higher 24 hours after reperfusion than at 4 months (206 vs. 166 μmol/L; P < .001).
The 24-hour KB elevations were independently and positively associated with larger infarct size (P = .016) and lower LVEF (P = .012), the group reports.
“Our results indicate a possible role for ketone bodies during myocardial infarction,” Dr. De Koning said.
The KB elevations were probably followed by “an increase in cardiac ketone body metabolism, in order to fuel the heart that is energetically depleted.”
But the study didn’t explore cardiac KB consumption, Dr. De Koning cautioned, adding that the next steps in this research should be to investigate post-STEMI cardiac ketone metabolism and its pathophysiologic mechanisms. “This may facilitate future trials to study therapeutic effects of ketone body supplementation during or after STEMI.”
The current findings “form an essential basis for our understanding of the role of KBs in ischemia/reperfusion,” write Salva R. Yurista, MD, PhD, and colleagues, Massachusetts General Hospital and Harvard Medical School, Boston, in an accompanying editorial.
“Although the appeal of enhancing KBs as a therapeutic approach is understandable, additional rigorous preclinical and clinical studies will be required to test this therapeutic hypothesis and determine the mechanisms contributing to any benefits observed,” they note.
”Exposure to cardiac stress, such as ischemia, infarction, or heart failure, will stimulate the release of neurohormones, pro-inflammatory cytokines, and natriuretic peptides, which may play roles in stimulating ketogenesis or the production of ketone bodies,” Dr. Yurista told this news organization.
The increased circulating ketone concentrations and myocardial ketone oxidation that were associated with poor functional outcomes have been reported in other clinical contexts, including heart failure with reduced ejection fraction, heart failure with preserved cardiac function, diabetic cardiomyopathy, and arrhythmogenic cardiomyopathy, he said.
Dr. Yurista agrees that KBs could have therapeutic merit.
“Circulating ketone concentrations determine the contribution of ketones to the cardiac diet,” he said. “Thus, increasing cardiac delivery of ketone bodies through supplementation or other means to the heart undergoing stress, including STEMI and heart failure, could have therapeutic potential.”
The GIPS-III trial was supported by the Netherlands Organization for Health Research and Development (ZonMw). Neither Dr. De Koning nor the other authors report relevant financial relationships. Dr. Yurista and the other editorialists report no relevant relationships.
A version of this article first appeared on Medscape.com.
Circulating ketone bodies (KBs) are substantially elevated at presentation and 24 hours after ST-segment elevation myocardial infarction (STEMI), according to new research.
The study also showed that greater KB levels measured after 24 hours of reperfusion were associated with larger infarct size and reduced left ventricular ejection fraction (LVEF).
The findings suggest a potential role for ketone metabolism in response to myocardial ischemia, conclude researchers in their report, published in the October 5 issue of the Journal of the American College of Cardiology.
“Ketones serve as an alternative source of energy for the heart,” lead author Marie-Sophie L.Y. de Koning, MD, University Medical Center Groningen, the Netherlands, told this news organization.
“These results might suggest that ketone bodies may be an important fuel for the heart after myocardial ischemia.” The role of KBs in heart failure has been previously studied, but their role in myocardial infarction has not, Dr. De Koning said.
“In heart failure, metabolic changes occur that cause the heart to increasingly rely on ketone bodies as an important energy source. Accordingly, concentrations of circulating ketone bodies are elevated and higher concentrations have been linked with more severe heart failure,” she said.
”This might suggest that upregulation of ketone metabolism is a universal cardiac response to stress,” Dr. De Koning added. “But the role of ketone bodies in myocardial infarction remained largely unknown, and this triggered us to investigate circulating ketone bodies in patients presenting with STEMI.”
She and her team measured circulating KBs in archived plasma samples from 369 participants in the randomized GIPS-III trial. The study had primarily looked at the effect of 4 months of metformin therapy, compared with placebo, on LVEF in nondiabetic patients with a first STEMI.
Blood samples had been taken at baseline before percutaneous coronary intervention (PCI), at 24 hours after reperfusion, and at 4 months.
The current study investigated longitudinal post-STEMI changes in the circulating KBs beta-hydroxybutyrate, acetoacetate, and acetone. It also looked at associations of KBs with infarct size and LVEF, both of which were measured with cardiac magnetic resonance (CMR) imaging 4 months after STEMI.
Circulating KB levels were three times higher at STEMI presentation than at 4 months. At presentation, the median total KB level was 520 μmol/L. It was still higher 24 hours after reperfusion than at 4 months (206 vs. 166 μmol/L; P < .001).
The 24-hour KB elevations were independently and positively associated with larger infarct size (P = .016) and lower LVEF (P = .012), the group reports.
“Our results indicate a possible role for ketone bodies during myocardial infarction,” Dr. De Koning said.
The KB elevations were probably followed by “an increase in cardiac ketone body metabolism, in order to fuel the heart that is energetically depleted.”
But the study didn’t explore cardiac KB consumption, Dr. De Koning cautioned, adding that the next steps in this research should be to investigate post-STEMI cardiac ketone metabolism and its pathophysiologic mechanisms. “This may facilitate future trials to study therapeutic effects of ketone body supplementation during or after STEMI.”
The current findings “form an essential basis for our understanding of the role of KBs in ischemia/reperfusion,” write Salva R. Yurista, MD, PhD, and colleagues, Massachusetts General Hospital and Harvard Medical School, Boston, in an accompanying editorial.
“Although the appeal of enhancing KBs as a therapeutic approach is understandable, additional rigorous preclinical and clinical studies will be required to test this therapeutic hypothesis and determine the mechanisms contributing to any benefits observed,” they note.
”Exposure to cardiac stress, such as ischemia, infarction, or heart failure, will stimulate the release of neurohormones, pro-inflammatory cytokines, and natriuretic peptides, which may play roles in stimulating ketogenesis or the production of ketone bodies,” Dr. Yurista told this news organization.
The increased circulating ketone concentrations and myocardial ketone oxidation that were associated with poor functional outcomes have been reported in other clinical contexts, including heart failure with reduced ejection fraction, heart failure with preserved cardiac function, diabetic cardiomyopathy, and arrhythmogenic cardiomyopathy, he said.
Dr. Yurista agrees that KBs could have therapeutic merit.
“Circulating ketone concentrations determine the contribution of ketones to the cardiac diet,” he said. “Thus, increasing cardiac delivery of ketone bodies through supplementation or other means to the heart undergoing stress, including STEMI and heart failure, could have therapeutic potential.”
The GIPS-III trial was supported by the Netherlands Organization for Health Research and Development (ZonMw). Neither Dr. De Koning nor the other authors report relevant financial relationships. Dr. Yurista and the other editorialists report no relevant relationships.
A version of this article first appeared on Medscape.com.
Cardiogenic shock teams again tied to lower mortality
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
Weight-loss surgery linked to fewer cardiovascular events, more so with RYGB
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
FROM DIABETES CARE
Case: Patient with statin-associated muscle symptoms
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
STOP-DAPT 2 ACS: 1 month of DAPT proves inadequate for patients with recent ACS
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Bystander rescue breathing CPR in kids tied to better survival
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
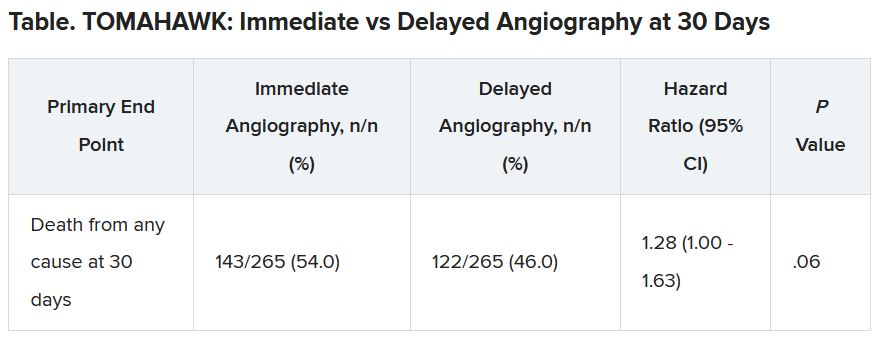
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
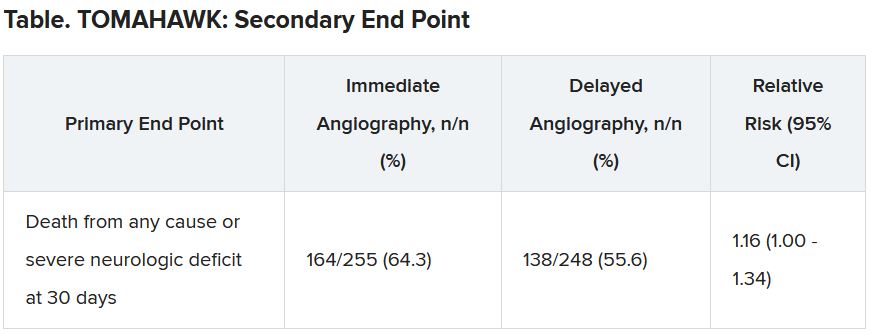
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Although inconclusive, CV safety study of cancer therapy attracts attention
The first global trial to compare the cardiovascular (CV) safety of two therapies for prostate cancer proved inconclusive because of inadequate enrollment and events, but the study is a harbinger of growth in the emerging specialty of cardio-oncology, according to experts.
“Many new cancer agents have extended patient survival, yet some of these agents have significant potential cardiovascular toxicity,” said Renato D. Lopes, MD, in presenting a study at the annual congress of the European Society of Cardiology.
In the context of improving survival in patients with or at risk for both cancer and cardiovascular disease, he suggested that the prostate cancer study he led could be “a model for interdisciplinary collaboration” needed to address the relative and sometimes competing risks of these disease states.
This point was seconded by several pioneers in cardio-oncology who participated in the discussion of the results of the trial, called PRONOUNCE.
“We know many drugs in oncology increase cardiovascular risk, so these are the types of trials we need,” according Thomas M. Suter, MD, who leads the cardio-oncology service at the University Hospital, Berne, Switzerland. He was the ESC-invited discussant for PRONOUNCE.
More than 100 centers in 12 countries involved
In PRONOUNCE, 545 patients with prostate cancer and established atherosclerotic cardiovascular disease were randomized to degarelix, a gonadotropin-releasing hormone antagonist, or leuprolide, a GnRH agonist. The patients were enrolled at 113 participating centers in 12 countries. All of the patients had an indication for an androgen-deprivation therapy (ADT).
In numerous previous studies, “ADT has been associated with higher CV morbidity and mortality, particularly in men with preexisting CV disease,” explained Dr. Lopes, but the relative cardiovascular safety of GnRH agonists relative to GnRH antagonists has been “controversial.”
The PRONOUNCE study was designed to resolve this issue, but the study was terminated early because of slow enrollment (not related to the COVID-19 pandemic). The planned enrollment was 900 patients.
In addition, the rate of major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, or death, was lower over the course of follow-up than anticipated in the study design.
No significant difference on primary endpoint
At the end of 12 months, MACE occurred in 11 (4.1%) of patients randomized to leuprolide and 15 (5.5%) of those randomized to degarelix. The greater hazard ratio for MACE in the degarelix group did not approach statistical significance (hazard ratio, 1.28; P = .53).
As a result, the question of the relative CV safety of these drugs “remains unresolved,” according to Dr. Lopes, professor of medicine at Duke University Medical Center, Durham, N.C.
This does not diminish the need to answer this question. In the addition to the fact that cancer is a malignancy primarily of advancing age when CV disease is prevalent – the mean age in this study was 73 years and 44% were over age 75 – it is often an indolent disease with long periods of survival, according to Dr. Lopes. About half of prostate cancer patients have concomitant CV disease, and about half will receive ADT at some point in their treatment.
In patients receiving ADT, leuprolide is far more commonly used than GnRH antagonists, which are offered in only about 4% of patients, according to data cited by Dr. Lopes. The underlying hypothesis of this study was that leuprolide is associated with greater CV risk, which might have been relevant to a risk-benefit calculation, if the hypothesis had been confirmed.
Cancer drugs can increase CV risk
Based on experimental data, “there is concern the leuprolide is involved in plaque destabilization,” said Dr. Lopes, but he noted that ADTs in general are associated with adverse metabolic changes, including increases in LDL cholesterol, insulin resistance, and body fat, all of which could be relevant to CV risk.
It is the improving rates of survival for prostate cancer as well for other types of cancer that have increased attention to the potential for cancer drugs to increase CV risk, another major cause of early mortality. For these competing risks, objective data are needed to evaluate a relative risk-to-benefit ratio for treatment choices.
This dilemma led the ESC to recently establish its Council on Cardio-Oncology, and many centers around the world are also creating interdisciplinary groups to guide treatment choices for patients with both diseases.
“You will certainly get a lot of referrals,” said Rudolf de Boer, MD, professor of translational cardiology, University Medical Center, Groningen, Netherlands. Basing his remark on his own experience starting a cardio-oncology clinic at his institution, he called this work challenging and agreed that the need for objective data is urgent.
“We need data to provide common ground on which to judge relative risks,” Dr. de Boer said. He also praised the PRONOUNCE investigators for their efforts even if the data failed to answer the question posed.
The PRONOUNCE results were published online in Circulation at the time of Dr. Lopes’s presentation.
The study received funding from Ferring Pharmaceuticals. Dr. Lopes reports financial relationships with Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi. Dr. Suter reports financial relationships with Boehringer Ingelheim, GlaxoSmithKline, and Roche. Dr. de Boer reports financial relationships with AstraZeneca, Abbott, Bristol-Myers Squibb, Novartis, Novo Nordisk, and Roche.
The first global trial to compare the cardiovascular (CV) safety of two therapies for prostate cancer proved inconclusive because of inadequate enrollment and events, but the study is a harbinger of growth in the emerging specialty of cardio-oncology, according to experts.
“Many new cancer agents have extended patient survival, yet some of these agents have significant potential cardiovascular toxicity,” said Renato D. Lopes, MD, in presenting a study at the annual congress of the European Society of Cardiology.
In the context of improving survival in patients with or at risk for both cancer and cardiovascular disease, he suggested that the prostate cancer study he led could be “a model for interdisciplinary collaboration” needed to address the relative and sometimes competing risks of these disease states.
This point was seconded by several pioneers in cardio-oncology who participated in the discussion of the results of the trial, called PRONOUNCE.
“We know many drugs in oncology increase cardiovascular risk, so these are the types of trials we need,” according Thomas M. Suter, MD, who leads the cardio-oncology service at the University Hospital, Berne, Switzerland. He was the ESC-invited discussant for PRONOUNCE.
More than 100 centers in 12 countries involved
In PRONOUNCE, 545 patients with prostate cancer and established atherosclerotic cardiovascular disease were randomized to degarelix, a gonadotropin-releasing hormone antagonist, or leuprolide, a GnRH agonist. The patients were enrolled at 113 participating centers in 12 countries. All of the patients had an indication for an androgen-deprivation therapy (ADT).
In numerous previous studies, “ADT has been associated with higher CV morbidity and mortality, particularly in men with preexisting CV disease,” explained Dr. Lopes, but the relative cardiovascular safety of GnRH agonists relative to GnRH antagonists has been “controversial.”
The PRONOUNCE study was designed to resolve this issue, but the study was terminated early because of slow enrollment (not related to the COVID-19 pandemic). The planned enrollment was 900 patients.
In addition, the rate of major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, or death, was lower over the course of follow-up than anticipated in the study design.
No significant difference on primary endpoint
At the end of 12 months, MACE occurred in 11 (4.1%) of patients randomized to leuprolide and 15 (5.5%) of those randomized to degarelix. The greater hazard ratio for MACE in the degarelix group did not approach statistical significance (hazard ratio, 1.28; P = .53).
As a result, the question of the relative CV safety of these drugs “remains unresolved,” according to Dr. Lopes, professor of medicine at Duke University Medical Center, Durham, N.C.
This does not diminish the need to answer this question. In the addition to the fact that cancer is a malignancy primarily of advancing age when CV disease is prevalent – the mean age in this study was 73 years and 44% were over age 75 – it is often an indolent disease with long periods of survival, according to Dr. Lopes. About half of prostate cancer patients have concomitant CV disease, and about half will receive ADT at some point in their treatment.
In patients receiving ADT, leuprolide is far more commonly used than GnRH antagonists, which are offered in only about 4% of patients, according to data cited by Dr. Lopes. The underlying hypothesis of this study was that leuprolide is associated with greater CV risk, which might have been relevant to a risk-benefit calculation, if the hypothesis had been confirmed.
Cancer drugs can increase CV risk
Based on experimental data, “there is concern the leuprolide is involved in plaque destabilization,” said Dr. Lopes, but he noted that ADTs in general are associated with adverse metabolic changes, including increases in LDL cholesterol, insulin resistance, and body fat, all of which could be relevant to CV risk.
It is the improving rates of survival for prostate cancer as well for other types of cancer that have increased attention to the potential for cancer drugs to increase CV risk, another major cause of early mortality. For these competing risks, objective data are needed to evaluate a relative risk-to-benefit ratio for treatment choices.
This dilemma led the ESC to recently establish its Council on Cardio-Oncology, and many centers around the world are also creating interdisciplinary groups to guide treatment choices for patients with both diseases.
“You will certainly get a lot of referrals,” said Rudolf de Boer, MD, professor of translational cardiology, University Medical Center, Groningen, Netherlands. Basing his remark on his own experience starting a cardio-oncology clinic at his institution, he called this work challenging and agreed that the need for objective data is urgent.
“We need data to provide common ground on which to judge relative risks,” Dr. de Boer said. He also praised the PRONOUNCE investigators for their efforts even if the data failed to answer the question posed.
The PRONOUNCE results were published online in Circulation at the time of Dr. Lopes’s presentation.
The study received funding from Ferring Pharmaceuticals. Dr. Lopes reports financial relationships with Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi. Dr. Suter reports financial relationships with Boehringer Ingelheim, GlaxoSmithKline, and Roche. Dr. de Boer reports financial relationships with AstraZeneca, Abbott, Bristol-Myers Squibb, Novartis, Novo Nordisk, and Roche.
The first global trial to compare the cardiovascular (CV) safety of two therapies for prostate cancer proved inconclusive because of inadequate enrollment and events, but the study is a harbinger of growth in the emerging specialty of cardio-oncology, according to experts.
“Many new cancer agents have extended patient survival, yet some of these agents have significant potential cardiovascular toxicity,” said Renato D. Lopes, MD, in presenting a study at the annual congress of the European Society of Cardiology.
In the context of improving survival in patients with or at risk for both cancer and cardiovascular disease, he suggested that the prostate cancer study he led could be “a model for interdisciplinary collaboration” needed to address the relative and sometimes competing risks of these disease states.
This point was seconded by several pioneers in cardio-oncology who participated in the discussion of the results of the trial, called PRONOUNCE.
“We know many drugs in oncology increase cardiovascular risk, so these are the types of trials we need,” according Thomas M. Suter, MD, who leads the cardio-oncology service at the University Hospital, Berne, Switzerland. He was the ESC-invited discussant for PRONOUNCE.
More than 100 centers in 12 countries involved
In PRONOUNCE, 545 patients with prostate cancer and established atherosclerotic cardiovascular disease were randomized to degarelix, a gonadotropin-releasing hormone antagonist, or leuprolide, a GnRH agonist. The patients were enrolled at 113 participating centers in 12 countries. All of the patients had an indication for an androgen-deprivation therapy (ADT).
In numerous previous studies, “ADT has been associated with higher CV morbidity and mortality, particularly in men with preexisting CV disease,” explained Dr. Lopes, but the relative cardiovascular safety of GnRH agonists relative to GnRH antagonists has been “controversial.”
The PRONOUNCE study was designed to resolve this issue, but the study was terminated early because of slow enrollment (not related to the COVID-19 pandemic). The planned enrollment was 900 patients.
In addition, the rate of major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, or death, was lower over the course of follow-up than anticipated in the study design.
No significant difference on primary endpoint
At the end of 12 months, MACE occurred in 11 (4.1%) of patients randomized to leuprolide and 15 (5.5%) of those randomized to degarelix. The greater hazard ratio for MACE in the degarelix group did not approach statistical significance (hazard ratio, 1.28; P = .53).
As a result, the question of the relative CV safety of these drugs “remains unresolved,” according to Dr. Lopes, professor of medicine at Duke University Medical Center, Durham, N.C.
This does not diminish the need to answer this question. In the addition to the fact that cancer is a malignancy primarily of advancing age when CV disease is prevalent – the mean age in this study was 73 years and 44% were over age 75 – it is often an indolent disease with long periods of survival, according to Dr. Lopes. About half of prostate cancer patients have concomitant CV disease, and about half will receive ADT at some point in their treatment.
In patients receiving ADT, leuprolide is far more commonly used than GnRH antagonists, which are offered in only about 4% of patients, according to data cited by Dr. Lopes. The underlying hypothesis of this study was that leuprolide is associated with greater CV risk, which might have been relevant to a risk-benefit calculation, if the hypothesis had been confirmed.
Cancer drugs can increase CV risk
Based on experimental data, “there is concern the leuprolide is involved in plaque destabilization,” said Dr. Lopes, but he noted that ADTs in general are associated with adverse metabolic changes, including increases in LDL cholesterol, insulin resistance, and body fat, all of which could be relevant to CV risk.
It is the improving rates of survival for prostate cancer as well for other types of cancer that have increased attention to the potential for cancer drugs to increase CV risk, another major cause of early mortality. For these competing risks, objective data are needed to evaluate a relative risk-to-benefit ratio for treatment choices.
This dilemma led the ESC to recently establish its Council on Cardio-Oncology, and many centers around the world are also creating interdisciplinary groups to guide treatment choices for patients with both diseases.
“You will certainly get a lot of referrals,” said Rudolf de Boer, MD, professor of translational cardiology, University Medical Center, Groningen, Netherlands. Basing his remark on his own experience starting a cardio-oncology clinic at his institution, he called this work challenging and agreed that the need for objective data is urgent.
“We need data to provide common ground on which to judge relative risks,” Dr. de Boer said. He also praised the PRONOUNCE investigators for their efforts even if the data failed to answer the question posed.
The PRONOUNCE results were published online in Circulation at the time of Dr. Lopes’s presentation.
The study received funding from Ferring Pharmaceuticals. Dr. Lopes reports financial relationships with Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi. Dr. Suter reports financial relationships with Boehringer Ingelheim, GlaxoSmithKline, and Roche. Dr. de Boer reports financial relationships with AstraZeneca, Abbott, Bristol-Myers Squibb, Novartis, Novo Nordisk, and Roche.
FROM ESC 2021