User login
Expert: Risk of AK Progression to SCC Hard to Predict
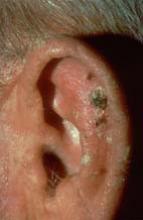
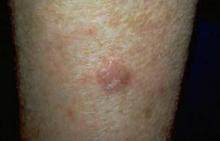
NAPA, CALIF. – Because actinic keratoses are the most common lesions with premalignant potential in the skin, knowing how to predict their malignancy can be life saving, according to Dr. Miriam S. Bettencourt.
Most AKs do not progress to squamous cell carcinoma (SCC), but most invasive SCCs have evidence of preexisting AK, she said. Of the AKs that progress, 10% will metastasize, giving patients a 5-year survival rate, Dr. Bettencourt of the University of Nevada, Las Vegas.
"Major risk factors for AK malignancy include induration and inflammation, a diameter of over 1 cm, rapid enlargement, bleeding, erythema, and ulceration. Minor risk factors include pigmentation changes, palpability, pain, pruritus, and hyperkeratosis.
Hyperkeratotic AKs greater than 1 cm on the hands, wrists, or forearms have a 50% or greater risk for malignancy, she noted.
Other risk factors include age at diagnosis, human papillomavirus status, skin type, sunburns during childhood, proximity to the Equator, amount of outdoor activity, and a history of skin cancer.
Organ-transplant recipients have a 250-fold greater risk for developing AKs, and a 100-fold risk for SCC; 40% of organ-transplant patients with an AK will show progression to SCC, compared with 10% of the average population, she said.
However, the wide range of rates for the risk of AK progression to SCC found in the literature highlights the difficulty of putting risk into perspective, she noted.
In one study, of 169 patients with AKs, 65% developed an SCC from an AK; 97% of the patients had a contiguous AK near the site of the SCC (Dermatol. Surg. 1995;21:184). But a review of 875 studies of AKs found a much lower rate; 11 studies were identified that predicted the risk of malignancy. The review found the rate to be 0.025% to 10% per lesion, per year (Eur. J. Dermatol. 2006;16:335-9).
In another study, 10% of AKs were found to transform into SCC in 2 years (Dermatol. Surg. 2007;33:1099-101).
A more recent report suggests that AKs also may progress to basal cell carcinomas (BCCs), she reported. In a study of 7,784 lesions on the face and ears of 169 patients, 65% of SCCs and 36% of BCCs arose in lesions that were previously diagnosed as AKs (Cancer 2009;115:2523-30). The study also found the risk of progression of AK to SCC to be 0.6% at year 1 and 2.6% at year 4.
It is important to remember that risk applies to each individual AK and that the fate of any one AK is impossible to predict, said Dr. Bettencourt. "Although the risk [of progression] is small, since most [patients] have multiple AKs, the risk of transformation is much greater than the risk of any individual lesion."
She recommended treating all AKs because some may be difficult to distinguish from SCC and lentigo maligna.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – Because actinic keratoses are the most common lesions with premalignant potential in the skin, knowing how to predict their malignancy can be life saving, according to Dr. Miriam S. Bettencourt.
Most AKs do not progress to squamous cell carcinoma (SCC), but most invasive SCCs have evidence of preexisting AK, she said. Of the AKs that progress, 10% will metastasize, giving patients a 5-year survival rate, Dr. Bettencourt of the University of Nevada, Las Vegas.
"Major risk factors for AK malignancy include induration and inflammation, a diameter of over 1 cm, rapid enlargement, bleeding, erythema, and ulceration. Minor risk factors include pigmentation changes, palpability, pain, pruritus, and hyperkeratosis.
Hyperkeratotic AKs greater than 1 cm on the hands, wrists, or forearms have a 50% or greater risk for malignancy, she noted.
Other risk factors include age at diagnosis, human papillomavirus status, skin type, sunburns during childhood, proximity to the Equator, amount of outdoor activity, and a history of skin cancer.
Organ-transplant recipients have a 250-fold greater risk for developing AKs, and a 100-fold risk for SCC; 40% of organ-transplant patients with an AK will show progression to SCC, compared with 10% of the average population, she said.
However, the wide range of rates for the risk of AK progression to SCC found in the literature highlights the difficulty of putting risk into perspective, she noted.
In one study, of 169 patients with AKs, 65% developed an SCC from an AK; 97% of the patients had a contiguous AK near the site of the SCC (Dermatol. Surg. 1995;21:184). But a review of 875 studies of AKs found a much lower rate; 11 studies were identified that predicted the risk of malignancy. The review found the rate to be 0.025% to 10% per lesion, per year (Eur. J. Dermatol. 2006;16:335-9).
In another study, 10% of AKs were found to transform into SCC in 2 years (Dermatol. Surg. 2007;33:1099-101).
A more recent report suggests that AKs also may progress to basal cell carcinomas (BCCs), she reported. In a study of 7,784 lesions on the face and ears of 169 patients, 65% of SCCs and 36% of BCCs arose in lesions that were previously diagnosed as AKs (Cancer 2009;115:2523-30). The study also found the risk of progression of AK to SCC to be 0.6% at year 1 and 2.6% at year 4.
It is important to remember that risk applies to each individual AK and that the fate of any one AK is impossible to predict, said Dr. Bettencourt. "Although the risk [of progression] is small, since most [patients] have multiple AKs, the risk of transformation is much greater than the risk of any individual lesion."
She recommended treating all AKs because some may be difficult to distinguish from SCC and lentigo maligna.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – Because actinic keratoses are the most common lesions with premalignant potential in the skin, knowing how to predict their malignancy can be life saving, according to Dr. Miriam S. Bettencourt.
Most AKs do not progress to squamous cell carcinoma (SCC), but most invasive SCCs have evidence of preexisting AK, she said. Of the AKs that progress, 10% will metastasize, giving patients a 5-year survival rate, Dr. Bettencourt of the University of Nevada, Las Vegas.
"Major risk factors for AK malignancy include induration and inflammation, a diameter of over 1 cm, rapid enlargement, bleeding, erythema, and ulceration. Minor risk factors include pigmentation changes, palpability, pain, pruritus, and hyperkeratosis.
Hyperkeratotic AKs greater than 1 cm on the hands, wrists, or forearms have a 50% or greater risk for malignancy, she noted.
Other risk factors include age at diagnosis, human papillomavirus status, skin type, sunburns during childhood, proximity to the Equator, amount of outdoor activity, and a history of skin cancer.
Organ-transplant recipients have a 250-fold greater risk for developing AKs, and a 100-fold risk for SCC; 40% of organ-transplant patients with an AK will show progression to SCC, compared with 10% of the average population, she said.
However, the wide range of rates for the risk of AK progression to SCC found in the literature highlights the difficulty of putting risk into perspective, she noted.
In one study, of 169 patients with AKs, 65% developed an SCC from an AK; 97% of the patients had a contiguous AK near the site of the SCC (Dermatol. Surg. 1995;21:184). But a review of 875 studies of AKs found a much lower rate; 11 studies were identified that predicted the risk of malignancy. The review found the rate to be 0.025% to 10% per lesion, per year (Eur. J. Dermatol. 2006;16:335-9).
In another study, 10% of AKs were found to transform into SCC in 2 years (Dermatol. Surg. 2007;33:1099-101).
A more recent report suggests that AKs also may progress to basal cell carcinomas (BCCs), she reported. In a study of 7,784 lesions on the face and ears of 169 patients, 65% of SCCs and 36% of BCCs arose in lesions that were previously diagnosed as AKs (Cancer 2009;115:2523-30). The study also found the risk of progression of AK to SCC to be 0.6% at year 1 and 2.6% at year 4.
It is important to remember that risk applies to each individual AK and that the fate of any one AK is impossible to predict, said Dr. Bettencourt. "Although the risk [of progression] is small, since most [patients] have multiple AKs, the risk of transformation is much greater than the risk of any individual lesion."
She recommended treating all AKs because some may be difficult to distinguish from SCC and lentigo maligna.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSISUM
Treat All AKs to Prevent Skin Cancer
NAPA, CALIF. – A preventive approach is needed in the treatment of actinic keratoses to curtail the potential emergence of nonmelanoma skin cancer, according to Dr. Miriam S. Bettencourt.
"In addition to the visible AKs that we see, there’s a lot more in the field area of the AK below the skin surface," she said. "AKs are just the tip of the iceberg."
Because nonmelanoma skin cancer can be easily missed in AK patients, Dr. Bettencourt, of the University of Nevada in Las Vegas, recommended treating all AK lesions.
Many factors should be taken into consideration when determining the best AK treatment for patients, she said, including the number of lesions and location, patient compliance, cosmetic implications, age of patient, history of skin cancer, and insurance coverage.
The treatment of AK involves lesion-directed destructive procedures, topical field-directed therapies, or combination treatment.
Lesion Destruction Therapies
For treating nonspecific lesions, Dr. Bettencourt recommended considering cryosurgery, noting an efficacy rate of 67%-88% and a 1-year recurrence rate of 1.2%-12%. Side effects are mild, the most common being pain, redness, edema, and hypo- or hyperpigmentation.
Chemical peels are an option for extensive AKs, she said, noting an efficacy rate of 75% and a recurrence rate of 25%-35% at 1 year, depending on the agent. Side effects with peels are mild, the most common being pain, inflammation, and pigment alterations.
For single lesions on the face, scalp, or neck, or for full-face resurfacing, CO2 and YAG lasers can be used. While device dependent, 90% of patients achieve lesion remission; however, the recurrence rate is 10%-15% at 3-6 months.
Photodynamic therapy (PDT) offers selective destruction of atypical cells through light activation of a photosensitizer. She noted an efficacy rate of 70%-78% after one treatment session, and a rate of 90% after two sessions. However, there is a risk of photosensitivity and pain from treatment, and it tends to cost more than other therapies, she noted.
Topical Treatment
Topical 5-fluorouracil "inhibits thymidylate synthetase and causes cell death in actively proliferating cells," reported Dr. Bettencourt. A 5% cream or solution is the most popular form of 5-FU, but it is also available in 2%, 1%, and 0.5% formulas.
The 5% cream should be applied twice a day for a month. Common side effects include temporary ulcers, erythema, crusting, and scarring. She highly recommended reviewing the adverse effects with patients before treatment. She noted that she has patients sign the package insert before she begins treatment with 5-FU.
Imiquimod is another topical treatment available for AKs that upregulates a variety of cytokines, she said. "Experimental evidence suggests that patients may develop T-cell memory after treatment, and thus develop less AKs in the future." She recommended Aldara 5%, which is applied 2-3 times per week for up to 4 months, or Zyclara 3.75%, which is applied once a day for two cycles of 2 weeks (with a 2-week free interval in the middle).
The last topical agent she recommended was diclofenac sodium 3% gel (Solaraze), which is a nonsteroidal anti-inflammatory drug that is well tolerated. "The treatment inhibits cyclooxygenase, which is essential for biosynthesis of prostaglandins," she said. Hyaluronic acid delays uptake of the drug, which leads to higher concentrations in the skin. Patients should be treated with the gel twice a day for 3 months. Little to no inflammation may occur during treatment; however, the drug has been known to cause hepatotoxicity.
She noted that diclofenac is her treatment of choice for AKs on the lips.
Combination Therapy
Consider combination, sequential therapy with 0.5% micronized 5-FU before cryotherapy for better results than cryotherapy alone, said Dr. Bettencourt. In a study of 144 patients, 5-FU plus cryotherapy resulted in partial remission in 62% of patients, and complete remission in 17% of patients after 4 weeks of treatment (Arch. Dermatol. 2004;140:813-6).
Low-dose 5-FU plus salicylic acid has also been found to be an effective treatment for AKs, she said. A 2009 study found that 5-FU combined with Jessner’s solution or a 70% glycolic acid peel every other week had an 80% clearance rate and offered photodamage improvement (Int. J. Dermatol. 2009;48:902-7).
PDT followed by treatment with imiquimod 5% was found to reduce lesions by 89.9%, compared with 74.5% with placebo at 12 months (J. Drugs Dermatol. 2009;8:35-9). The randomized vehicle-controlled study enrolled 25 patients (24 completed the study), with a at least 10 AKs. The entire face of each patient was treated with PDT and aminolevulinic acid 20% at baseline and at 1 month; then, at month 2, imiquimod 5% was applied to one-half of each patient’s face and a vehicle to the other half two times a week for 16 weeks. Erythema and flaking were the most serious skin reactions reported during the study.
Positive results have also been seen when imiquimod is combined with cryosurgery, noted Dr. Bettencourt. She also recommended considering diclofenac gel after cryosurgery.
Whatever treatment is deemed best for the AK patient, he or she should be strongly encouraged to avoid the sun whenever possible, to wear protective clothing, to use sunscreen, and to limit outdoor activity between 10 a.m. and 3 p.m., she concluded. A low-fat diet may also lead to a greater resolution of existent AKs and may limit the development of future lesions.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – A preventive approach is needed in the treatment of actinic keratoses to curtail the potential emergence of nonmelanoma skin cancer, according to Dr. Miriam S. Bettencourt.
"In addition to the visible AKs that we see, there’s a lot more in the field area of the AK below the skin surface," she said. "AKs are just the tip of the iceberg."
Because nonmelanoma skin cancer can be easily missed in AK patients, Dr. Bettencourt, of the University of Nevada in Las Vegas, recommended treating all AK lesions.
Many factors should be taken into consideration when determining the best AK treatment for patients, she said, including the number of lesions and location, patient compliance, cosmetic implications, age of patient, history of skin cancer, and insurance coverage.
The treatment of AK involves lesion-directed destructive procedures, topical field-directed therapies, or combination treatment.
Lesion Destruction Therapies
For treating nonspecific lesions, Dr. Bettencourt recommended considering cryosurgery, noting an efficacy rate of 67%-88% and a 1-year recurrence rate of 1.2%-12%. Side effects are mild, the most common being pain, redness, edema, and hypo- or hyperpigmentation.
Chemical peels are an option for extensive AKs, she said, noting an efficacy rate of 75% and a recurrence rate of 25%-35% at 1 year, depending on the agent. Side effects with peels are mild, the most common being pain, inflammation, and pigment alterations.
For single lesions on the face, scalp, or neck, or for full-face resurfacing, CO2 and YAG lasers can be used. While device dependent, 90% of patients achieve lesion remission; however, the recurrence rate is 10%-15% at 3-6 months.
Photodynamic therapy (PDT) offers selective destruction of atypical cells through light activation of a photosensitizer. She noted an efficacy rate of 70%-78% after one treatment session, and a rate of 90% after two sessions. However, there is a risk of photosensitivity and pain from treatment, and it tends to cost more than other therapies, she noted.
Topical Treatment
Topical 5-fluorouracil "inhibits thymidylate synthetase and causes cell death in actively proliferating cells," reported Dr. Bettencourt. A 5% cream or solution is the most popular form of 5-FU, but it is also available in 2%, 1%, and 0.5% formulas.
The 5% cream should be applied twice a day for a month. Common side effects include temporary ulcers, erythema, crusting, and scarring. She highly recommended reviewing the adverse effects with patients before treatment. She noted that she has patients sign the package insert before she begins treatment with 5-FU.
Imiquimod is another topical treatment available for AKs that upregulates a variety of cytokines, she said. "Experimental evidence suggests that patients may develop T-cell memory after treatment, and thus develop less AKs in the future." She recommended Aldara 5%, which is applied 2-3 times per week for up to 4 months, or Zyclara 3.75%, which is applied once a day for two cycles of 2 weeks (with a 2-week free interval in the middle).
The last topical agent she recommended was diclofenac sodium 3% gel (Solaraze), which is a nonsteroidal anti-inflammatory drug that is well tolerated. "The treatment inhibits cyclooxygenase, which is essential for biosynthesis of prostaglandins," she said. Hyaluronic acid delays uptake of the drug, which leads to higher concentrations in the skin. Patients should be treated with the gel twice a day for 3 months. Little to no inflammation may occur during treatment; however, the drug has been known to cause hepatotoxicity.
She noted that diclofenac is her treatment of choice for AKs on the lips.
Combination Therapy
Consider combination, sequential therapy with 0.5% micronized 5-FU before cryotherapy for better results than cryotherapy alone, said Dr. Bettencourt. In a study of 144 patients, 5-FU plus cryotherapy resulted in partial remission in 62% of patients, and complete remission in 17% of patients after 4 weeks of treatment (Arch. Dermatol. 2004;140:813-6).
Low-dose 5-FU plus salicylic acid has also been found to be an effective treatment for AKs, she said. A 2009 study found that 5-FU combined with Jessner’s solution or a 70% glycolic acid peel every other week had an 80% clearance rate and offered photodamage improvement (Int. J. Dermatol. 2009;48:902-7).
PDT followed by treatment with imiquimod 5% was found to reduce lesions by 89.9%, compared with 74.5% with placebo at 12 months (J. Drugs Dermatol. 2009;8:35-9). The randomized vehicle-controlled study enrolled 25 patients (24 completed the study), with a at least 10 AKs. The entire face of each patient was treated with PDT and aminolevulinic acid 20% at baseline and at 1 month; then, at month 2, imiquimod 5% was applied to one-half of each patient’s face and a vehicle to the other half two times a week for 16 weeks. Erythema and flaking were the most serious skin reactions reported during the study.
Positive results have also been seen when imiquimod is combined with cryosurgery, noted Dr. Bettencourt. She also recommended considering diclofenac gel after cryosurgery.
Whatever treatment is deemed best for the AK patient, he or she should be strongly encouraged to avoid the sun whenever possible, to wear protective clothing, to use sunscreen, and to limit outdoor activity between 10 a.m. and 3 p.m., she concluded. A low-fat diet may also lead to a greater resolution of existent AKs and may limit the development of future lesions.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – A preventive approach is needed in the treatment of actinic keratoses to curtail the potential emergence of nonmelanoma skin cancer, according to Dr. Miriam S. Bettencourt.
"In addition to the visible AKs that we see, there’s a lot more in the field area of the AK below the skin surface," she said. "AKs are just the tip of the iceberg."
Because nonmelanoma skin cancer can be easily missed in AK patients, Dr. Bettencourt, of the University of Nevada in Las Vegas, recommended treating all AK lesions.
Many factors should be taken into consideration when determining the best AK treatment for patients, she said, including the number of lesions and location, patient compliance, cosmetic implications, age of patient, history of skin cancer, and insurance coverage.
The treatment of AK involves lesion-directed destructive procedures, topical field-directed therapies, or combination treatment.
Lesion Destruction Therapies
For treating nonspecific lesions, Dr. Bettencourt recommended considering cryosurgery, noting an efficacy rate of 67%-88% and a 1-year recurrence rate of 1.2%-12%. Side effects are mild, the most common being pain, redness, edema, and hypo- or hyperpigmentation.
Chemical peels are an option for extensive AKs, she said, noting an efficacy rate of 75% and a recurrence rate of 25%-35% at 1 year, depending on the agent. Side effects with peels are mild, the most common being pain, inflammation, and pigment alterations.
For single lesions on the face, scalp, or neck, or for full-face resurfacing, CO2 and YAG lasers can be used. While device dependent, 90% of patients achieve lesion remission; however, the recurrence rate is 10%-15% at 3-6 months.
Photodynamic therapy (PDT) offers selective destruction of atypical cells through light activation of a photosensitizer. She noted an efficacy rate of 70%-78% after one treatment session, and a rate of 90% after two sessions. However, there is a risk of photosensitivity and pain from treatment, and it tends to cost more than other therapies, she noted.
Topical Treatment
Topical 5-fluorouracil "inhibits thymidylate synthetase and causes cell death in actively proliferating cells," reported Dr. Bettencourt. A 5% cream or solution is the most popular form of 5-FU, but it is also available in 2%, 1%, and 0.5% formulas.
The 5% cream should be applied twice a day for a month. Common side effects include temporary ulcers, erythema, crusting, and scarring. She highly recommended reviewing the adverse effects with patients before treatment. She noted that she has patients sign the package insert before she begins treatment with 5-FU.
Imiquimod is another topical treatment available for AKs that upregulates a variety of cytokines, she said. "Experimental evidence suggests that patients may develop T-cell memory after treatment, and thus develop less AKs in the future." She recommended Aldara 5%, which is applied 2-3 times per week for up to 4 months, or Zyclara 3.75%, which is applied once a day for two cycles of 2 weeks (with a 2-week free interval in the middle).
The last topical agent she recommended was diclofenac sodium 3% gel (Solaraze), which is a nonsteroidal anti-inflammatory drug that is well tolerated. "The treatment inhibits cyclooxygenase, which is essential for biosynthesis of prostaglandins," she said. Hyaluronic acid delays uptake of the drug, which leads to higher concentrations in the skin. Patients should be treated with the gel twice a day for 3 months. Little to no inflammation may occur during treatment; however, the drug has been known to cause hepatotoxicity.
She noted that diclofenac is her treatment of choice for AKs on the lips.
Combination Therapy
Consider combination, sequential therapy with 0.5% micronized 5-FU before cryotherapy for better results than cryotherapy alone, said Dr. Bettencourt. In a study of 144 patients, 5-FU plus cryotherapy resulted in partial remission in 62% of patients, and complete remission in 17% of patients after 4 weeks of treatment (Arch. Dermatol. 2004;140:813-6).
Low-dose 5-FU plus salicylic acid has also been found to be an effective treatment for AKs, she said. A 2009 study found that 5-FU combined with Jessner’s solution or a 70% glycolic acid peel every other week had an 80% clearance rate and offered photodamage improvement (Int. J. Dermatol. 2009;48:902-7).
PDT followed by treatment with imiquimod 5% was found to reduce lesions by 89.9%, compared with 74.5% with placebo at 12 months (J. Drugs Dermatol. 2009;8:35-9). The randomized vehicle-controlled study enrolled 25 patients (24 completed the study), with a at least 10 AKs. The entire face of each patient was treated with PDT and aminolevulinic acid 20% at baseline and at 1 month; then, at month 2, imiquimod 5% was applied to one-half of each patient’s face and a vehicle to the other half two times a week for 16 weeks. Erythema and flaking were the most serious skin reactions reported during the study.
Positive results have also been seen when imiquimod is combined with cryosurgery, noted Dr. Bettencourt. She also recommended considering diclofenac gel after cryosurgery.
Whatever treatment is deemed best for the AK patient, he or she should be strongly encouraged to avoid the sun whenever possible, to wear protective clothing, to use sunscreen, and to limit outdoor activity between 10 a.m. and 3 p.m., she concluded. A low-fat diet may also lead to a greater resolution of existent AKs and may limit the development of future lesions.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Optimal Treatment for Actinic Keratosis, 0.5% Fluorouracil, Underutilized
NEW YORK – U.S. patients who were treated for actinic keratosis via cryosurgery or fluorouracil failed to receive optimal treatment with these agents, based on a review of more than 16 million patients treated in 2001-2008.
The results "reveal a gap between practice and the evidence in the treatment of AKs [actinic keratoses] by both dermatologists and nondermatologists," reported Dr. Steven R. Feldman and his associates in a poster at the American Academy of Dermatology’s Summer Academy meeting.
"Combination therapy with cryosurgery and topical fluorouracil is underutilized," and when topical fluorouracil is used to treat AKs, "the 0.5% rather than the 5.0% formulation may be preferable to maximize adherence and to minimize cost and adverse events," they wrote.
Their analysis found cryosurgery alone to be the most commonly used treatment in 59% of patients. Neither cryosurgery nor fluorouracil was used in 35% of U.S. patients in 2001-2008. Among the 5% of patients who were treated with a fluorouracil formulation, 3% received the 0.5% formulation, whereas the other 2% received a different formulation (most often the 5.0% strength), reported Dr. Feldman, professor of dermatology and director of the center for dermatology research at Wake Forest University in Winston-Salem, N.C.
Although other treatments for actinic keratosis exist, the researchers noted, "evidence-based medicine supports the use of 0.5% fluorouracil, rather than the 5.0% formulation, for treatment of actinic keratosis. Evidence, including head-to-head clinical trials, indicates that 0.5% fluorouracil has comparable efficacy, greater tolerability, and lower cost, compared with 5.0% fluorouracil. Similar compelling data support the use of combination cryotherapy plus topical fluorouracil rather than either modality alone." (Arch. Dermaol. 2009;145:203-5; Cutis. 2008;81:509-16; J. Drugs Dermatol. 2006;5:133-9). They performed their new analysis "to determine whether current practice reflects the evidence."
They reviewed data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Care Survey. They identified more than 16 million U.S. patients seen with actinic keratosis during the years of 2001-2008 based on ICD-9 coding. Two-thirds of patients received treatment of their actinic keratosis during an office visit.
Dermatologists saw about 90% of these patients. During the period studied, the annual number of patients with AK seen by all physicians and by dermatologists significantly increased, whereas the number seen by nondermatologists did not significantly change.
The data showed that dermatologists did a better job using 0.5% fluorouracil and cryosurgery, compared with nondermatologists. Dermatologists used cryosurgery alone on 62% of their AK patients; they treated 3% with 0.5% fluorouracil, 2% with 5.0% fluorouracil, and 1% with a combination of fluorouracil and cryosurgery.
"Despite the better tolerability and lower cost of the 0.5% fluorouracil formulation, dermatologists prescribed the 5% formulation in over 392,000 patient visits for AK" in 2001-2008. In contrast, nondermatologists used cryosurgery alone on 32% of their AK patients, 0.5% fluorouracil on no patients, 5.0% fluorouracil on 3%, and a combination of fluorouracil and cryosurgery on no patients. Treatments other than cryosurgery or fluorouracil were used by 32% of the dermatologists and by 63% of the nondermatologists.
"Efforts need to be taken to increase physician awareness of the advantages of 0.5% fluorouracil relative to other formulations, and the value of combination therapy," they reported. "Providers who manage AKs should reassess their current treatment practices and adapt them to be consistent with current evidence."
The study was funded by Sanofi-Aventis, which markets a 0.5% formulation of fluorouracil (Carac). Dr. Feldman said that he has received speaking, consulting, or research support from Abbott, Amgen, Astellas, Aventis, Biogen, Centocor, Connetics, Galderma, Genentech, GlaxoSmithKline (Stiefel), Leo, National Biological, Roche, Warner Chilcott, and 3M.
NEW YORK – U.S. patients who were treated for actinic keratosis via cryosurgery or fluorouracil failed to receive optimal treatment with these agents, based on a review of more than 16 million patients treated in 2001-2008.
The results "reveal a gap between practice and the evidence in the treatment of AKs [actinic keratoses] by both dermatologists and nondermatologists," reported Dr. Steven R. Feldman and his associates in a poster at the American Academy of Dermatology’s Summer Academy meeting.
"Combination therapy with cryosurgery and topical fluorouracil is underutilized," and when topical fluorouracil is used to treat AKs, "the 0.5% rather than the 5.0% formulation may be preferable to maximize adherence and to minimize cost and adverse events," they wrote.
Their analysis found cryosurgery alone to be the most commonly used treatment in 59% of patients. Neither cryosurgery nor fluorouracil was used in 35% of U.S. patients in 2001-2008. Among the 5% of patients who were treated with a fluorouracil formulation, 3% received the 0.5% formulation, whereas the other 2% received a different formulation (most often the 5.0% strength), reported Dr. Feldman, professor of dermatology and director of the center for dermatology research at Wake Forest University in Winston-Salem, N.C.
Although other treatments for actinic keratosis exist, the researchers noted, "evidence-based medicine supports the use of 0.5% fluorouracil, rather than the 5.0% formulation, for treatment of actinic keratosis. Evidence, including head-to-head clinical trials, indicates that 0.5% fluorouracil has comparable efficacy, greater tolerability, and lower cost, compared with 5.0% fluorouracil. Similar compelling data support the use of combination cryotherapy plus topical fluorouracil rather than either modality alone." (Arch. Dermaol. 2009;145:203-5; Cutis. 2008;81:509-16; J. Drugs Dermatol. 2006;5:133-9). They performed their new analysis "to determine whether current practice reflects the evidence."
They reviewed data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Care Survey. They identified more than 16 million U.S. patients seen with actinic keratosis during the years of 2001-2008 based on ICD-9 coding. Two-thirds of patients received treatment of their actinic keratosis during an office visit.
Dermatologists saw about 90% of these patients. During the period studied, the annual number of patients with AK seen by all physicians and by dermatologists significantly increased, whereas the number seen by nondermatologists did not significantly change.
The data showed that dermatologists did a better job using 0.5% fluorouracil and cryosurgery, compared with nondermatologists. Dermatologists used cryosurgery alone on 62% of their AK patients; they treated 3% with 0.5% fluorouracil, 2% with 5.0% fluorouracil, and 1% with a combination of fluorouracil and cryosurgery.
"Despite the better tolerability and lower cost of the 0.5% fluorouracil formulation, dermatologists prescribed the 5% formulation in over 392,000 patient visits for AK" in 2001-2008. In contrast, nondermatologists used cryosurgery alone on 32% of their AK patients, 0.5% fluorouracil on no patients, 5.0% fluorouracil on 3%, and a combination of fluorouracil and cryosurgery on no patients. Treatments other than cryosurgery or fluorouracil were used by 32% of the dermatologists and by 63% of the nondermatologists.
"Efforts need to be taken to increase physician awareness of the advantages of 0.5% fluorouracil relative to other formulations, and the value of combination therapy," they reported. "Providers who manage AKs should reassess their current treatment practices and adapt them to be consistent with current evidence."
The study was funded by Sanofi-Aventis, which markets a 0.5% formulation of fluorouracil (Carac). Dr. Feldman said that he has received speaking, consulting, or research support from Abbott, Amgen, Astellas, Aventis, Biogen, Centocor, Connetics, Galderma, Genentech, GlaxoSmithKline (Stiefel), Leo, National Biological, Roche, Warner Chilcott, and 3M.
NEW YORK – U.S. patients who were treated for actinic keratosis via cryosurgery or fluorouracil failed to receive optimal treatment with these agents, based on a review of more than 16 million patients treated in 2001-2008.
The results "reveal a gap between practice and the evidence in the treatment of AKs [actinic keratoses] by both dermatologists and nondermatologists," reported Dr. Steven R. Feldman and his associates in a poster at the American Academy of Dermatology’s Summer Academy meeting.
"Combination therapy with cryosurgery and topical fluorouracil is underutilized," and when topical fluorouracil is used to treat AKs, "the 0.5% rather than the 5.0% formulation may be preferable to maximize adherence and to minimize cost and adverse events," they wrote.
Their analysis found cryosurgery alone to be the most commonly used treatment in 59% of patients. Neither cryosurgery nor fluorouracil was used in 35% of U.S. patients in 2001-2008. Among the 5% of patients who were treated with a fluorouracil formulation, 3% received the 0.5% formulation, whereas the other 2% received a different formulation (most often the 5.0% strength), reported Dr. Feldman, professor of dermatology and director of the center for dermatology research at Wake Forest University in Winston-Salem, N.C.
Although other treatments for actinic keratosis exist, the researchers noted, "evidence-based medicine supports the use of 0.5% fluorouracil, rather than the 5.0% formulation, for treatment of actinic keratosis. Evidence, including head-to-head clinical trials, indicates that 0.5% fluorouracil has comparable efficacy, greater tolerability, and lower cost, compared with 5.0% fluorouracil. Similar compelling data support the use of combination cryotherapy plus topical fluorouracil rather than either modality alone." (Arch. Dermaol. 2009;145:203-5; Cutis. 2008;81:509-16; J. Drugs Dermatol. 2006;5:133-9). They performed their new analysis "to determine whether current practice reflects the evidence."
They reviewed data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Care Survey. They identified more than 16 million U.S. patients seen with actinic keratosis during the years of 2001-2008 based on ICD-9 coding. Two-thirds of patients received treatment of their actinic keratosis during an office visit.
Dermatologists saw about 90% of these patients. During the period studied, the annual number of patients with AK seen by all physicians and by dermatologists significantly increased, whereas the number seen by nondermatologists did not significantly change.
The data showed that dermatologists did a better job using 0.5% fluorouracil and cryosurgery, compared with nondermatologists. Dermatologists used cryosurgery alone on 62% of their AK patients; they treated 3% with 0.5% fluorouracil, 2% with 5.0% fluorouracil, and 1% with a combination of fluorouracil and cryosurgery.
"Despite the better tolerability and lower cost of the 0.5% fluorouracil formulation, dermatologists prescribed the 5% formulation in over 392,000 patient visits for AK" in 2001-2008. In contrast, nondermatologists used cryosurgery alone on 32% of their AK patients, 0.5% fluorouracil on no patients, 5.0% fluorouracil on 3%, and a combination of fluorouracil and cryosurgery on no patients. Treatments other than cryosurgery or fluorouracil were used by 32% of the dermatologists and by 63% of the nondermatologists.
"Efforts need to be taken to increase physician awareness of the advantages of 0.5% fluorouracil relative to other formulations, and the value of combination therapy," they reported. "Providers who manage AKs should reassess their current treatment practices and adapt them to be consistent with current evidence."
The study was funded by Sanofi-Aventis, which markets a 0.5% formulation of fluorouracil (Carac). Dr. Feldman said that he has received speaking, consulting, or research support from Abbott, Amgen, Astellas, Aventis, Biogen, Centocor, Connetics, Galderma, Genentech, GlaxoSmithKline (Stiefel), Leo, National Biological, Roche, Warner Chilcott, and 3M.
FROM THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Major Finding: Among more than 16 million patients treated for actinic keratosis over 8 years, 59% received cryosurgery, 3% received topical treatment with 0.5% fluorouracil, and 2% received topical treatment with a different fluorouracil-strength formulation.
Data Source: The CDC's National Ambulatory Medical Care survey.
Disclosures: The study was funded by Sanofi-Aventis, which markets a 0.5% formulation of fluorouracil (Carac). Dr. Feldman said that he has received speaking, consulting, or research support from Abbott, Amgen, Astellas, Aventis, Biogen, Centocor, Connetics, Galderma, Genentech, GlaxoSmithKline (Stiefel), Leo, National Biological, Roche, Warner Chilcott, and 3M.
Laser Therapy of Nonmelanoma Cancers Continues to Grow
BOCA RATON, FLA. – Experience with various lasers for the treatment and prophylaxis of nonmelanoma skin cancers is increasing, and the outcomes are promising.
One setting where lasers have particular potential is in patients with multiple basal cell carcinomas, Dr. Keyvan Nouri said at the annual meeting of the Florida Society of Dermatology and Dermatologic Surgery.
BCCs tend to have telangiectasias, and by specifically targeting the tumor vasculature, the BCC burden can be decreased or eliminated by laser treatment with little damage to surrounding skin. An ablative approach can also eliminate premalignant cells, said Dr. Nouri, professor of dermatology and otolaryngology and director of Mohs, dermatologic, and laser surgery at the University of Miami.
Studies using this approach have been conducted over the past several years using CO2, Er:YAG, pulsed-dye, and alexandrite lasers.
The 10,600-nm CO2 laser achieves a depth of 15-200 mcm in a single pass, which is adequate for coagulating the epidermis and superficial dermis, and for eliminating squamous cells at risk for evolution to actinic keratosis or squamous cell carcinoma. It also is partially effective for eliminating cells en route to basal cell carcinoma, he said, noting that deeper treatment can be achieved with multiple passes or protocol modifications.
In one study using this CO2 laser, 14 patients with diffuse facial AKs underwent resurfacing and experienced significant long-term reduction in AK burden (Dermatol. Surg. 1997;23:885-9). In another report of two patients with a history of multiple nonmelanoma skin cancer who were treated with two-pass CO2 resurfacing, no new lesions developed in the treated area at 33 and 52 months (Dermatol. Surg. 1999;25:513-16).
In a randomized controlled study of 34 patients, no differences in outcomes were seen at 5 years regardless of whether patients were treated with the CO2 laser, a 30% trichloroacetic acid peel, or 5% fluorouracil cream twice daily for 3 weeks. All of the treatments reduced AK counts by 83%-92%, and reduced the incidence of nonmelanoma skin cancers, compared with control groups (Dermatol. Surg. 1999;25:729-32), Dr. Nouri said.
In three of his patients with basal cell nevus syndrome, the Ultrapulse CO2 laser also proved effective for treatment. Postoperative Mohs micrographic surgical sections verified complete histologic clearance, and the patients had minimal scarring at follow-up (Dermatol. Surg. 2002;28:287-90).
A high-energy pulsed CO2 laser has also been used to treat a squamous cell carcinoma in situ on the nose with one pass (at 250 mJ, 8 W). In that case, no recurrence had occurred at 5 months’ follow-up, and the cosmetic result was excellent, Dr. Nouri said.
In a series of patients with nodular and superficial BCCs, a combination of curettage and one or two passes with the high-energy pulsed CO2 laser was also effective, with no histologic evidence or residual tumor and no known recurrences.
In another study, the pulsed CO2 laser was effective for treating 30 neoplasms, including 17 BCCs and 13 SCCs (Arch. Dermatol. 1998;134:1247-52). Two or three passes were used (at 500 mJ and 2-4 W). Treatment with a 4-mm margin and three passes is recommended for complete vaporization of neoplasms using this laser, Dr. Nouri said. Also, one SCC in situ in that study was incompletely ablated after three passes, so this modality alone is not recommended for thick or keratotic lesions, he said.
More recently, fractional laser resurfacing and pulsed-dye laser treatment have also shown promise for nonmelanoma skin cancers. In a pilot study of 28 patients with mild to moderate actinic damage who were treated with a fractional Er:YAG laser (2,940 nm) with one to four treatments at 4-week intervals, 75% had excellent results and the other 25% had good results. The effects were maintained at up to 9 months (Dermatol. Surg. 2008;34:1048-53).
And the 595-nm pulsed-dye laser has been used successfully to target tumor vasculature in glottal dysplasia and SCCs. Depending on fluence and spot size, the 595-nm pulsed-dye laser can penetrate skin to thicknesses ranging from 0.75 to 1.25 mm, which encompasses most BCCs, Dr. Nouri said.
In one study, a single treatment on seven BCCs resulted in histologic clearance of only one tumor (Lasers Med. Sci. 2003;18:125-6), but in two other studies of patients with superficial BCCs, this laser was associated with no clinical recurrences at a minimum follow-up of 1 year in 16 of 20 BCCs (Lasers Med. Sci. 2005;20:147-8; Dermatol. Ther. 2008; 21:402-5).
In another study, 20 BCCs treated with four 595-nm pulsed-dye laser treatments at 2-week intervals showed that nearly all BCCs less than 1.5 cm responded completely, compared with about 17% of controls. Larger BCCs had a complete response rate of 25% vs. 0% in controls.
Tumors with an incomplete response showed a significant reduction in tumor burden following treatment, with residual histologic tumor burden ranging from less than 1% to 29% of the original clinical tumor diameter, compared with 13%-68% residual tumor burden for the corresponding controls.
The investigators took the study one step further, treating 14 patients with a total of 20 BCCs on the trunk or extremities with four treatments at 3- to 4-week intervals; 19 of 20 BCCs had a complete clinical response, regardless of size and histological subtype (Lasers Surg. Med. 2009; 41:417-22). The investigators concluded that the 595-nm pulsed-dye laser is a "novel, quick, and relatively nonpainful treatment" for BCCs when used in the appropriate clinical setting, Dr. Nouri said.
As for the long-pulse alexandrite laser, it is selective like the pulsed-dye laser, but has deeper penetration, and may be helpful in significantly reducing tumor burden with a single treatment, he said.
In 15 of 18 patients with basal cell nevus syndrome who were treated in one study, a complete clinical response was seen at follow-ups of 2 and 7 months (Lasers Surg. Med. 2010;42:68-71).
No particular laser has emerged as the ideal tool for the treatment or prevention of skin cancer, but work is ongoing. More research is needed to confirm the observations made thus far, as well as to optimize treatment parameters, but the findings to date are encouraging, Dr. Nouri said, noting that he is confident that laser and light sources will eventually be used more for both medical and oncologic purposes.
Dr. Nouri disclosed that he has received grants or research support from Aesthera, CureLight, and Omnilux.
BOCA RATON, FLA. – Experience with various lasers for the treatment and prophylaxis of nonmelanoma skin cancers is increasing, and the outcomes are promising.
One setting where lasers have particular potential is in patients with multiple basal cell carcinomas, Dr. Keyvan Nouri said at the annual meeting of the Florida Society of Dermatology and Dermatologic Surgery.
BCCs tend to have telangiectasias, and by specifically targeting the tumor vasculature, the BCC burden can be decreased or eliminated by laser treatment with little damage to surrounding skin. An ablative approach can also eliminate premalignant cells, said Dr. Nouri, professor of dermatology and otolaryngology and director of Mohs, dermatologic, and laser surgery at the University of Miami.
Studies using this approach have been conducted over the past several years using CO2, Er:YAG, pulsed-dye, and alexandrite lasers.
The 10,600-nm CO2 laser achieves a depth of 15-200 mcm in a single pass, which is adequate for coagulating the epidermis and superficial dermis, and for eliminating squamous cells at risk for evolution to actinic keratosis or squamous cell carcinoma. It also is partially effective for eliminating cells en route to basal cell carcinoma, he said, noting that deeper treatment can be achieved with multiple passes or protocol modifications.
In one study using this CO2 laser, 14 patients with diffuse facial AKs underwent resurfacing and experienced significant long-term reduction in AK burden (Dermatol. Surg. 1997;23:885-9). In another report of two patients with a history of multiple nonmelanoma skin cancer who were treated with two-pass CO2 resurfacing, no new lesions developed in the treated area at 33 and 52 months (Dermatol. Surg. 1999;25:513-16).
In a randomized controlled study of 34 patients, no differences in outcomes were seen at 5 years regardless of whether patients were treated with the CO2 laser, a 30% trichloroacetic acid peel, or 5% fluorouracil cream twice daily for 3 weeks. All of the treatments reduced AK counts by 83%-92%, and reduced the incidence of nonmelanoma skin cancers, compared with control groups (Dermatol. Surg. 1999;25:729-32), Dr. Nouri said.
In three of his patients with basal cell nevus syndrome, the Ultrapulse CO2 laser also proved effective for treatment. Postoperative Mohs micrographic surgical sections verified complete histologic clearance, and the patients had minimal scarring at follow-up (Dermatol. Surg. 2002;28:287-90).
A high-energy pulsed CO2 laser has also been used to treat a squamous cell carcinoma in situ on the nose with one pass (at 250 mJ, 8 W). In that case, no recurrence had occurred at 5 months’ follow-up, and the cosmetic result was excellent, Dr. Nouri said.
In a series of patients with nodular and superficial BCCs, a combination of curettage and one or two passes with the high-energy pulsed CO2 laser was also effective, with no histologic evidence or residual tumor and no known recurrences.
In another study, the pulsed CO2 laser was effective for treating 30 neoplasms, including 17 BCCs and 13 SCCs (Arch. Dermatol. 1998;134:1247-52). Two or three passes were used (at 500 mJ and 2-4 W). Treatment with a 4-mm margin and three passes is recommended for complete vaporization of neoplasms using this laser, Dr. Nouri said. Also, one SCC in situ in that study was incompletely ablated after three passes, so this modality alone is not recommended for thick or keratotic lesions, he said.
More recently, fractional laser resurfacing and pulsed-dye laser treatment have also shown promise for nonmelanoma skin cancers. In a pilot study of 28 patients with mild to moderate actinic damage who were treated with a fractional Er:YAG laser (2,940 nm) with one to four treatments at 4-week intervals, 75% had excellent results and the other 25% had good results. The effects were maintained at up to 9 months (Dermatol. Surg. 2008;34:1048-53).
And the 595-nm pulsed-dye laser has been used successfully to target tumor vasculature in glottal dysplasia and SCCs. Depending on fluence and spot size, the 595-nm pulsed-dye laser can penetrate skin to thicknesses ranging from 0.75 to 1.25 mm, which encompasses most BCCs, Dr. Nouri said.
In one study, a single treatment on seven BCCs resulted in histologic clearance of only one tumor (Lasers Med. Sci. 2003;18:125-6), but in two other studies of patients with superficial BCCs, this laser was associated with no clinical recurrences at a minimum follow-up of 1 year in 16 of 20 BCCs (Lasers Med. Sci. 2005;20:147-8; Dermatol. Ther. 2008; 21:402-5).
In another study, 20 BCCs treated with four 595-nm pulsed-dye laser treatments at 2-week intervals showed that nearly all BCCs less than 1.5 cm responded completely, compared with about 17% of controls. Larger BCCs had a complete response rate of 25% vs. 0% in controls.
Tumors with an incomplete response showed a significant reduction in tumor burden following treatment, with residual histologic tumor burden ranging from less than 1% to 29% of the original clinical tumor diameter, compared with 13%-68% residual tumor burden for the corresponding controls.
The investigators took the study one step further, treating 14 patients with a total of 20 BCCs on the trunk or extremities with four treatments at 3- to 4-week intervals; 19 of 20 BCCs had a complete clinical response, regardless of size and histological subtype (Lasers Surg. Med. 2009; 41:417-22). The investigators concluded that the 595-nm pulsed-dye laser is a "novel, quick, and relatively nonpainful treatment" for BCCs when used in the appropriate clinical setting, Dr. Nouri said.
As for the long-pulse alexandrite laser, it is selective like the pulsed-dye laser, but has deeper penetration, and may be helpful in significantly reducing tumor burden with a single treatment, he said.
In 15 of 18 patients with basal cell nevus syndrome who were treated in one study, a complete clinical response was seen at follow-ups of 2 and 7 months (Lasers Surg. Med. 2010;42:68-71).
No particular laser has emerged as the ideal tool for the treatment or prevention of skin cancer, but work is ongoing. More research is needed to confirm the observations made thus far, as well as to optimize treatment parameters, but the findings to date are encouraging, Dr. Nouri said, noting that he is confident that laser and light sources will eventually be used more for both medical and oncologic purposes.
Dr. Nouri disclosed that he has received grants or research support from Aesthera, CureLight, and Omnilux.
BOCA RATON, FLA. – Experience with various lasers for the treatment and prophylaxis of nonmelanoma skin cancers is increasing, and the outcomes are promising.
One setting where lasers have particular potential is in patients with multiple basal cell carcinomas, Dr. Keyvan Nouri said at the annual meeting of the Florida Society of Dermatology and Dermatologic Surgery.
BCCs tend to have telangiectasias, and by specifically targeting the tumor vasculature, the BCC burden can be decreased or eliminated by laser treatment with little damage to surrounding skin. An ablative approach can also eliminate premalignant cells, said Dr. Nouri, professor of dermatology and otolaryngology and director of Mohs, dermatologic, and laser surgery at the University of Miami.
Studies using this approach have been conducted over the past several years using CO2, Er:YAG, pulsed-dye, and alexandrite lasers.
The 10,600-nm CO2 laser achieves a depth of 15-200 mcm in a single pass, which is adequate for coagulating the epidermis and superficial dermis, and for eliminating squamous cells at risk for evolution to actinic keratosis or squamous cell carcinoma. It also is partially effective for eliminating cells en route to basal cell carcinoma, he said, noting that deeper treatment can be achieved with multiple passes or protocol modifications.
In one study using this CO2 laser, 14 patients with diffuse facial AKs underwent resurfacing and experienced significant long-term reduction in AK burden (Dermatol. Surg. 1997;23:885-9). In another report of two patients with a history of multiple nonmelanoma skin cancer who were treated with two-pass CO2 resurfacing, no new lesions developed in the treated area at 33 and 52 months (Dermatol. Surg. 1999;25:513-16).
In a randomized controlled study of 34 patients, no differences in outcomes were seen at 5 years regardless of whether patients were treated with the CO2 laser, a 30% trichloroacetic acid peel, or 5% fluorouracil cream twice daily for 3 weeks. All of the treatments reduced AK counts by 83%-92%, and reduced the incidence of nonmelanoma skin cancers, compared with control groups (Dermatol. Surg. 1999;25:729-32), Dr. Nouri said.
In three of his patients with basal cell nevus syndrome, the Ultrapulse CO2 laser also proved effective for treatment. Postoperative Mohs micrographic surgical sections verified complete histologic clearance, and the patients had minimal scarring at follow-up (Dermatol. Surg. 2002;28:287-90).
A high-energy pulsed CO2 laser has also been used to treat a squamous cell carcinoma in situ on the nose with one pass (at 250 mJ, 8 W). In that case, no recurrence had occurred at 5 months’ follow-up, and the cosmetic result was excellent, Dr. Nouri said.
In a series of patients with nodular and superficial BCCs, a combination of curettage and one or two passes with the high-energy pulsed CO2 laser was also effective, with no histologic evidence or residual tumor and no known recurrences.
In another study, the pulsed CO2 laser was effective for treating 30 neoplasms, including 17 BCCs and 13 SCCs (Arch. Dermatol. 1998;134:1247-52). Two or three passes were used (at 500 mJ and 2-4 W). Treatment with a 4-mm margin and three passes is recommended for complete vaporization of neoplasms using this laser, Dr. Nouri said. Also, one SCC in situ in that study was incompletely ablated after three passes, so this modality alone is not recommended for thick or keratotic lesions, he said.
More recently, fractional laser resurfacing and pulsed-dye laser treatment have also shown promise for nonmelanoma skin cancers. In a pilot study of 28 patients with mild to moderate actinic damage who were treated with a fractional Er:YAG laser (2,940 nm) with one to four treatments at 4-week intervals, 75% had excellent results and the other 25% had good results. The effects were maintained at up to 9 months (Dermatol. Surg. 2008;34:1048-53).
And the 595-nm pulsed-dye laser has been used successfully to target tumor vasculature in glottal dysplasia and SCCs. Depending on fluence and spot size, the 595-nm pulsed-dye laser can penetrate skin to thicknesses ranging from 0.75 to 1.25 mm, which encompasses most BCCs, Dr. Nouri said.
In one study, a single treatment on seven BCCs resulted in histologic clearance of only one tumor (Lasers Med. Sci. 2003;18:125-6), but in two other studies of patients with superficial BCCs, this laser was associated with no clinical recurrences at a minimum follow-up of 1 year in 16 of 20 BCCs (Lasers Med. Sci. 2005;20:147-8; Dermatol. Ther. 2008; 21:402-5).
In another study, 20 BCCs treated with four 595-nm pulsed-dye laser treatments at 2-week intervals showed that nearly all BCCs less than 1.5 cm responded completely, compared with about 17% of controls. Larger BCCs had a complete response rate of 25% vs. 0% in controls.
Tumors with an incomplete response showed a significant reduction in tumor burden following treatment, with residual histologic tumor burden ranging from less than 1% to 29% of the original clinical tumor diameter, compared with 13%-68% residual tumor burden for the corresponding controls.
The investigators took the study one step further, treating 14 patients with a total of 20 BCCs on the trunk or extremities with four treatments at 3- to 4-week intervals; 19 of 20 BCCs had a complete clinical response, regardless of size and histological subtype (Lasers Surg. Med. 2009; 41:417-22). The investigators concluded that the 595-nm pulsed-dye laser is a "novel, quick, and relatively nonpainful treatment" for BCCs when used in the appropriate clinical setting, Dr. Nouri said.
As for the long-pulse alexandrite laser, it is selective like the pulsed-dye laser, but has deeper penetration, and may be helpful in significantly reducing tumor burden with a single treatment, he said.
In 15 of 18 patients with basal cell nevus syndrome who were treated in one study, a complete clinical response was seen at follow-ups of 2 and 7 months (Lasers Surg. Med. 2010;42:68-71).
No particular laser has emerged as the ideal tool for the treatment or prevention of skin cancer, but work is ongoing. More research is needed to confirm the observations made thus far, as well as to optimize treatment parameters, but the findings to date are encouraging, Dr. Nouri said, noting that he is confident that laser and light sources will eventually be used more for both medical and oncologic purposes.
Dr. Nouri disclosed that he has received grants or research support from Aesthera, CureLight, and Omnilux.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGY AND DERMATOLOGIC SURGERY
What's New in Photodynamic Therapy for Photorejuvenation?
Thulium Laser Yields 'Dramatic' Resolution of AKs
SEOUL, SOUTH KOREA – The nonablative fractionated thulium laser at 1,927-nm wavelength is a promising new noninvasive therapy for actinic keratoses.
The laser was actually developed for superficial skin resurfacing, an application for which it is particularly well suited because the 1,927-nm wavelength minimizes patient discomfort. But while investigating the device for improvement of pigmentation, Dr. Roy G. Geronemus noted incidentally that patients were also achieving "a rather dramatic resolution" of multiple facial actinic keratoses (AKs). So he decided to conduct a formal examination of the laser’s performance for this purpose, he said at the World Congress of Dermatology
To date, in 15 patients followed for 1-6 months after the last of several thulium laser treatment sessions for multiple facial AKs, the mean clearance of the lesions was 84%-91%.
"This compares very favorably to other modalities that are out there, including the topical chemotherapies, immunomodulatory agents, and photodynamic therapy. The advantage of this is not only do you improve the AKs, but you’re also getting the cosmetic benefit simultaneously," said Dr. Geronemus, medical director of the Laser and Skin Surgery Center of New York.
Patients received up to four treatments at 2- to 6-week intervals. The laser setting was 5-20 mJ, with 30%-70% coverage per session. Topical anesthetic was utilized for 1 hour, supplemented as needed by intramuscular ketorolac.
After a single treatment a mean of 63% of AKs were cleared. After two, 84%, and after three, 85%.
The laser therapy was well tolerated. The average pain score during treatment was 2.7 on a 0-9 scale. No scarring or infections have occurred. Mild redness and peeling typically lasted 4-5 days.
Dr. Geronemus said he and his colleagues have also found that the 1,927-nm fractionated thulium laser brings about "dramatic improvement" in actinic cheilitis, and is also highly effective for the thorny problem of enlarged facial pore size.
Dr. Geronemus is a shareholder in Solta Medical, which markets the 1,927-nm fractionated thulium laser. He also is on the advisory boards of numerous dermatologic laser manufacturers.
SEOUL, SOUTH KOREA – The nonablative fractionated thulium laser at 1,927-nm wavelength is a promising new noninvasive therapy for actinic keratoses.
The laser was actually developed for superficial skin resurfacing, an application for which it is particularly well suited because the 1,927-nm wavelength minimizes patient discomfort. But while investigating the device for improvement of pigmentation, Dr. Roy G. Geronemus noted incidentally that patients were also achieving "a rather dramatic resolution" of multiple facial actinic keratoses (AKs). So he decided to conduct a formal examination of the laser’s performance for this purpose, he said at the World Congress of Dermatology
To date, in 15 patients followed for 1-6 months after the last of several thulium laser treatment sessions for multiple facial AKs, the mean clearance of the lesions was 84%-91%.
"This compares very favorably to other modalities that are out there, including the topical chemotherapies, immunomodulatory agents, and photodynamic therapy. The advantage of this is not only do you improve the AKs, but you’re also getting the cosmetic benefit simultaneously," said Dr. Geronemus, medical director of the Laser and Skin Surgery Center of New York.
Patients received up to four treatments at 2- to 6-week intervals. The laser setting was 5-20 mJ, with 30%-70% coverage per session. Topical anesthetic was utilized for 1 hour, supplemented as needed by intramuscular ketorolac.
After a single treatment a mean of 63% of AKs were cleared. After two, 84%, and after three, 85%.
The laser therapy was well tolerated. The average pain score during treatment was 2.7 on a 0-9 scale. No scarring or infections have occurred. Mild redness and peeling typically lasted 4-5 days.
Dr. Geronemus said he and his colleagues have also found that the 1,927-nm fractionated thulium laser brings about "dramatic improvement" in actinic cheilitis, and is also highly effective for the thorny problem of enlarged facial pore size.
Dr. Geronemus is a shareholder in Solta Medical, which markets the 1,927-nm fractionated thulium laser. He also is on the advisory boards of numerous dermatologic laser manufacturers.
SEOUL, SOUTH KOREA – The nonablative fractionated thulium laser at 1,927-nm wavelength is a promising new noninvasive therapy for actinic keratoses.
The laser was actually developed for superficial skin resurfacing, an application for which it is particularly well suited because the 1,927-nm wavelength minimizes patient discomfort. But while investigating the device for improvement of pigmentation, Dr. Roy G. Geronemus noted incidentally that patients were also achieving "a rather dramatic resolution" of multiple facial actinic keratoses (AKs). So he decided to conduct a formal examination of the laser’s performance for this purpose, he said at the World Congress of Dermatology
To date, in 15 patients followed for 1-6 months after the last of several thulium laser treatment sessions for multiple facial AKs, the mean clearance of the lesions was 84%-91%.
"This compares very favorably to other modalities that are out there, including the topical chemotherapies, immunomodulatory agents, and photodynamic therapy. The advantage of this is not only do you improve the AKs, but you’re also getting the cosmetic benefit simultaneously," said Dr. Geronemus, medical director of the Laser and Skin Surgery Center of New York.
Patients received up to four treatments at 2- to 6-week intervals. The laser setting was 5-20 mJ, with 30%-70% coverage per session. Topical anesthetic was utilized for 1 hour, supplemented as needed by intramuscular ketorolac.
After a single treatment a mean of 63% of AKs were cleared. After two, 84%, and after three, 85%.
The laser therapy was well tolerated. The average pain score during treatment was 2.7 on a 0-9 scale. No scarring or infections have occurred. Mild redness and peeling typically lasted 4-5 days.
Dr. Geronemus said he and his colleagues have also found that the 1,927-nm fractionated thulium laser brings about "dramatic improvement" in actinic cheilitis, and is also highly effective for the thorny problem of enlarged facial pore size.
Dr. Geronemus is a shareholder in Solta Medical, which markets the 1,927-nm fractionated thulium laser. He also is on the advisory boards of numerous dermatologic laser manufacturers.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Current Therapies for AK and Precancerous Lesions Subpar
DANA POINT, CALIF. – Despite significant advances in dermatology in recent years, little progress has been made in reducing actinic keratoses and skin cancers, Dr. Mark G. Rubin said at the SDEF Summit in Aesthetic Medicine.
"The take-home message to me in all this is, don’t get actinic keratoses," said Dr. Rubin, who practices dermatology in Beverly Hills, Calif. "We really don’t have a great therapy, so it’s important for your patients and for yourself that you limit the amount of ultraviolet light that you get, because we don’t have a wonderful treatment option for patients at this point."
That message is especially important for immunosuppressed organ transplant patients, who face an incidence of skin cancer 64-250 times higher than that of the general population.
"Immunosuppressed patients grow four times as many squamous cells than basal cells, which is the reverse of the ratio of these cancers in immunocompetent patients," he said. "Those particular tumors are much more aggressive and have a higher incidence of metastasis. Sun protection is tremendously important in these patients."
In a randomized trial of 120 transplant patients, 60 patients applied 2 mg/cm2 of sunscreen with an SPF greater than 50 to the head, neck, forearms, and hands daily for 24 months, while 60 patients in the control group did not apply any sunscreen (Br. J. Derm. 2009;161 [Suppl. 3]:78-84). Both groups of patients had an equal number of AKs at the start of the trial, but at the end of 24 months, 82 new AKs developed in the control group compared with none in the sunscreen treatment group. In addition, eight patients in the control group developed squamous cell carcinoma, compared with none in the sunscreen group, while nine patients in the control group developed basal cell epithelioma, compared with two in the sunscreen group.
While fluorouracil in the form of Efudex 2% and 5%, Fluoroplex 1%, and Carac 0.5% has been a mainstay of AK treatment, imiquimod in the form of Aldara Cream 5% and Zyclara Cream 3.75% "has probably been the most popular recently," said Dr. Rubin, also of the University of California, San Diego. Another treatment option is diclofenac in the form of Solaraze 3%.
"None of these are fun therapies for patients," he said. "It’s hard to get patients to apply 5-FU more than once. They’ll do it once, hate the experience, and ask, ‘What do you have now doc, because I’m never facing that again.’ It’s made some of the other products like Solaraze, Carac, and Aldara more popular because they’re a little less brutal for the patient. But it’s important to realize that any of these products are going to impact your patients’ daily life. They’re all going to cause some redness, swelling, crusting, stinging, and burning that will go on for a period of weeks if not months, depending on the product that you use and the protocol that you follow."
A review of multiple trials suggests that these medical therapies show complete clearing of AKs in 36%-58% of patients within 1-4 months post treatment.
"It’s important to differentiate between reducing the overall bulk of precancer and eradication, or complete clearance," Dr. Rubin added. "You really want to look at complete clearance, because if you just improved it and the keratosis is smaller, 6 months later it will be back and look like you never touched it. Unless you’ve eradicated the lesion, you’re wasting your time."
Systemic retinoids such as Acitretin and Etretinate are another treatment option, yet they are not a long-term solution given their propensity to cause multiple side effects, including chapped lips, dry eyes, headaches, and hyperlipidemia.
"It’s almost unfair to put a patient on these because they’ll love it for awhile, and then you have to take it away, and then they’ll do horribly – unless you’re doing this with a second plan where you put the patient on it for 6 months to stabilize them before moving on to another treatment option, such as photodynamic therapy, which may be reasonable," Dr. Rubin said.
Chemical peels have some value for decreasing AKs in immunocompetent patients, but the results are no better than with topical medications, he said. The deeper the destruction, the better the result.
"Some actinic keratoses and squamous cell in situ go down the hair follicles," Dr. Rubin said. "If you’re not chasing it down the hair follicle you’re leaving the root behind in a lot of these patients, and that’s what creates a lot of these recurrences. Medical therapies are moderately effective if you look at them a couple of months later. But response rates are not wonderful, and the relapse rates are really terrible."
Dr. Rubin disclosed that he has received research support and consulting fees from Medicis. SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Despite significant advances in dermatology in recent years, little progress has been made in reducing actinic keratoses and skin cancers, Dr. Mark G. Rubin said at the SDEF Summit in Aesthetic Medicine.
"The take-home message to me in all this is, don’t get actinic keratoses," said Dr. Rubin, who practices dermatology in Beverly Hills, Calif. "We really don’t have a great therapy, so it’s important for your patients and for yourself that you limit the amount of ultraviolet light that you get, because we don’t have a wonderful treatment option for patients at this point."
That message is especially important for immunosuppressed organ transplant patients, who face an incidence of skin cancer 64-250 times higher than that of the general population.
"Immunosuppressed patients grow four times as many squamous cells than basal cells, which is the reverse of the ratio of these cancers in immunocompetent patients," he said. "Those particular tumors are much more aggressive and have a higher incidence of metastasis. Sun protection is tremendously important in these patients."
In a randomized trial of 120 transplant patients, 60 patients applied 2 mg/cm2 of sunscreen with an SPF greater than 50 to the head, neck, forearms, and hands daily for 24 months, while 60 patients in the control group did not apply any sunscreen (Br. J. Derm. 2009;161 [Suppl. 3]:78-84). Both groups of patients had an equal number of AKs at the start of the trial, but at the end of 24 months, 82 new AKs developed in the control group compared with none in the sunscreen treatment group. In addition, eight patients in the control group developed squamous cell carcinoma, compared with none in the sunscreen group, while nine patients in the control group developed basal cell epithelioma, compared with two in the sunscreen group.
While fluorouracil in the form of Efudex 2% and 5%, Fluoroplex 1%, and Carac 0.5% has been a mainstay of AK treatment, imiquimod in the form of Aldara Cream 5% and Zyclara Cream 3.75% "has probably been the most popular recently," said Dr. Rubin, also of the University of California, San Diego. Another treatment option is diclofenac in the form of Solaraze 3%.
"None of these are fun therapies for patients," he said. "It’s hard to get patients to apply 5-FU more than once. They’ll do it once, hate the experience, and ask, ‘What do you have now doc, because I’m never facing that again.’ It’s made some of the other products like Solaraze, Carac, and Aldara more popular because they’re a little less brutal for the patient. But it’s important to realize that any of these products are going to impact your patients’ daily life. They’re all going to cause some redness, swelling, crusting, stinging, and burning that will go on for a period of weeks if not months, depending on the product that you use and the protocol that you follow."
A review of multiple trials suggests that these medical therapies show complete clearing of AKs in 36%-58% of patients within 1-4 months post treatment.
"It’s important to differentiate between reducing the overall bulk of precancer and eradication, or complete clearance," Dr. Rubin added. "You really want to look at complete clearance, because if you just improved it and the keratosis is smaller, 6 months later it will be back and look like you never touched it. Unless you’ve eradicated the lesion, you’re wasting your time."
Systemic retinoids such as Acitretin and Etretinate are another treatment option, yet they are not a long-term solution given their propensity to cause multiple side effects, including chapped lips, dry eyes, headaches, and hyperlipidemia.
"It’s almost unfair to put a patient on these because they’ll love it for awhile, and then you have to take it away, and then they’ll do horribly – unless you’re doing this with a second plan where you put the patient on it for 6 months to stabilize them before moving on to another treatment option, such as photodynamic therapy, which may be reasonable," Dr. Rubin said.
Chemical peels have some value for decreasing AKs in immunocompetent patients, but the results are no better than with topical medications, he said. The deeper the destruction, the better the result.
"Some actinic keratoses and squamous cell in situ go down the hair follicles," Dr. Rubin said. "If you’re not chasing it down the hair follicle you’re leaving the root behind in a lot of these patients, and that’s what creates a lot of these recurrences. Medical therapies are moderately effective if you look at them a couple of months later. But response rates are not wonderful, and the relapse rates are really terrible."
Dr. Rubin disclosed that he has received research support and consulting fees from Medicis. SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Despite significant advances in dermatology in recent years, little progress has been made in reducing actinic keratoses and skin cancers, Dr. Mark G. Rubin said at the SDEF Summit in Aesthetic Medicine.
"The take-home message to me in all this is, don’t get actinic keratoses," said Dr. Rubin, who practices dermatology in Beverly Hills, Calif. "We really don’t have a great therapy, so it’s important for your patients and for yourself that you limit the amount of ultraviolet light that you get, because we don’t have a wonderful treatment option for patients at this point."
That message is especially important for immunosuppressed organ transplant patients, who face an incidence of skin cancer 64-250 times higher than that of the general population.
"Immunosuppressed patients grow four times as many squamous cells than basal cells, which is the reverse of the ratio of these cancers in immunocompetent patients," he said. "Those particular tumors are much more aggressive and have a higher incidence of metastasis. Sun protection is tremendously important in these patients."
In a randomized trial of 120 transplant patients, 60 patients applied 2 mg/cm2 of sunscreen with an SPF greater than 50 to the head, neck, forearms, and hands daily for 24 months, while 60 patients in the control group did not apply any sunscreen (Br. J. Derm. 2009;161 [Suppl. 3]:78-84). Both groups of patients had an equal number of AKs at the start of the trial, but at the end of 24 months, 82 new AKs developed in the control group compared with none in the sunscreen treatment group. In addition, eight patients in the control group developed squamous cell carcinoma, compared with none in the sunscreen group, while nine patients in the control group developed basal cell epithelioma, compared with two in the sunscreen group.
While fluorouracil in the form of Efudex 2% and 5%, Fluoroplex 1%, and Carac 0.5% has been a mainstay of AK treatment, imiquimod in the form of Aldara Cream 5% and Zyclara Cream 3.75% "has probably been the most popular recently," said Dr. Rubin, also of the University of California, San Diego. Another treatment option is diclofenac in the form of Solaraze 3%.
"None of these are fun therapies for patients," he said. "It’s hard to get patients to apply 5-FU more than once. They’ll do it once, hate the experience, and ask, ‘What do you have now doc, because I’m never facing that again.’ It’s made some of the other products like Solaraze, Carac, and Aldara more popular because they’re a little less brutal for the patient. But it’s important to realize that any of these products are going to impact your patients’ daily life. They’re all going to cause some redness, swelling, crusting, stinging, and burning that will go on for a period of weeks if not months, depending on the product that you use and the protocol that you follow."
A review of multiple trials suggests that these medical therapies show complete clearing of AKs in 36%-58% of patients within 1-4 months post treatment.
"It’s important to differentiate between reducing the overall bulk of precancer and eradication, or complete clearance," Dr. Rubin added. "You really want to look at complete clearance, because if you just improved it and the keratosis is smaller, 6 months later it will be back and look like you never touched it. Unless you’ve eradicated the lesion, you’re wasting your time."
Systemic retinoids such as Acitretin and Etretinate are another treatment option, yet they are not a long-term solution given their propensity to cause multiple side effects, including chapped lips, dry eyes, headaches, and hyperlipidemia.
"It’s almost unfair to put a patient on these because they’ll love it for awhile, and then you have to take it away, and then they’ll do horribly – unless you’re doing this with a second plan where you put the patient on it for 6 months to stabilize them before moving on to another treatment option, such as photodynamic therapy, which may be reasonable," Dr. Rubin said.
Chemical peels have some value for decreasing AKs in immunocompetent patients, but the results are no better than with topical medications, he said. The deeper the destruction, the better the result.
"Some actinic keratoses and squamous cell in situ go down the hair follicles," Dr. Rubin said. "If you’re not chasing it down the hair follicle you’re leaving the root behind in a lot of these patients, and that’s what creates a lot of these recurrences. Medical therapies are moderately effective if you look at them a couple of months later. But response rates are not wonderful, and the relapse rates are really terrible."
Dr. Rubin disclosed that he has received research support and consulting fees from Medicis. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Point/Counterpoint: Is Mohs Surgery Being Overutilized?
Yes - Surgeons Are Softening Criteria for Who Gets Mohs.
By David S. Becker, M.D.
The practice of Mohs micrographic surgery is in some cases drifting away from the classic indications that used to make up most of our practice.
There are different published criteria for cases indicated for Mohs surgery, but these often include patients with recurrent or incompletely excised skin cancers, aggressive histology, or poorly defined margins. Other classic indications include tumors larger than 0.4 cm in high-risk H-zone locations, larger than 1 cm elsewhere on the face, or larger than 2 cm on the trunk or extremities.
Some of these listed criteria would not include a 3-mm basal cell carcinoma on the nose or ear, an 8-mm basal cell carcinoma on the cheek or forehead, or a 1.5-cm lesion on the pretibial skin, but I often have had each of these referred to me for Mohs surgery.
What's driving us to soften these traditional indications for Mohs surgery? Patients, referring dermatologists, dermatopathologists, and Mohs surgeons each may play a role.
Patients love Mohs surgery for many reasons. For one, we do a good job. Perhaps they've had previous Mohs surgery with good results or their friends have, or they have looked online and found that Mohs has the best cure rate. Or, they may have had a bad experience with a prior treatment.
It's not uncommon for patients to do research, and then want to play a role in determining their medical care. If they demand Mohs surgery, and I don't think it's indicated, it can lead to a contentious consultation.
One young man with a squamous cell carcinoma on his chest recently told me that he had researched the cure rates of treatments and he wanted Mohs surgery. He was almost certainly correct that if the sole criteria for therapy were best cure rate, Mohs would be the choice.
There are only two downsides to Mohs versus excision in a case like this: One, the patient has to spend more time in the office for Mohs surgery; and two, it is perhaps overutilization of medical resources. Good luck trying to explain that to patients. They don't care about overutilization of resources.
Who else drives this drift toward enhanced Mohs utilization? Referring dermatologists. They may have an interest in maximizing utilization of a Mohs surgeon in their practice, while some dermatologists have truly been burned, that is, had a patient with a metastatic squamous cell carcinoma or difficult recurrences, and they want the greater certitude of cure from Mohs surgery.
Sometimes the referring dermatologist says the patient is a VIP or relative and asks me to make an exception. If you start making exceptions, pretty soon you're seeing a lot of patients who don't necessarily meet the classic criteria for Mohs surgery.
The next set of people who are driving this drift in overutilization is dermatopathologists. We have dermatopathologists being overly cautious and prudent in ways they may not have been 10-15 years ago. Something that might have been diagnosed as actinic keratosis 20 years ago might now be called actinic keratosis with extension to the base, "squamous cell carcinoma cannot be excluded," or superficially invasive squamous cell carcinoma.
Mohs surgeons also drive the drift in utilization. We do it because that's what we like to do, or because we work for someone and we want to do what they tell us to do, or we have a referring dermatologist whom we want to please. And we want to keep our patients happy too.
It may be time to reconceptualize the indications for Mohs surgery. Should patient demand or anxiety be an indication? Should the referring dermatologist's or Mohs surgeon's instincts about a given lesion be an indication? Should any small lesion on the face be an indication?
Finally, who should decide when to do Mohs surgery? Should it be patients? They want some autonomy and to play a role in managing their own care, and are often educated in doing so.
Should it be the referring dermatologist? They understand the history of a given lesion and know the patient and the response to prior therapies.
Should the Mohs surgeon decide? We have the most experience with cutaneous malignancies, and in many cases we're the best suited to decide who needs Mohs and who doesn't.
Or, finally, should it be the government or insurers? I think that because we have allowed this drift in utilization to go forward, that may be who ultimately is deciding.
Dr. Becker is a Mohs surgeon in private practice in New York. He said he has no relevant conflicts of interest. His comments were presented at the American College of Mohs Surgery meeting.
No - Mohs Surgery Is Being Properly Utilized.
By Gary Monheit, M.D.
Mohs micrographic surgery is not overutilized, it's increasingly utilized.
A recent study reported a twofold increase in the proportion of Mohs surgery being done in Medicare patients from 2001 to 2006, while the rate of excisions has remained stable. So what's driving this increased utilization of Mohs surgery?
Is it greedy surgeons applying Mohs micrographic surgery to actinic keratoses on the arms and trunk? The few surgeons who are utilizing it inappropriately need to be controlled, but I don't believe this is the main reason for the increased utilization.
In most cases, increased Mohs utilization is appropriate. Mohs is a superior and much more cost-effective modality than excision and it has a greater cure rate, which is now being recognized.
The vision of Dr. Frederic E. Mohs was a change in the treatment of skin cancer. He was tired of seeing recurrences with excision. He realized that if things were accurately mapped out, physicians would have a better understanding of how to treat tumors, spare tissue, and reduce costs.
Brilliantly, it was a merger of surgery and histopathology controlled by one practitioner, the Mohs surgeon. Results of surgical treatment approach 100% the first time.
Should this be done for all nonmelanoma skin cancers at all locations? Should patients be cured the first time rather than risk recurrences? Is this cost effective for most tumors?
Twenty years ago, 50% or more of the Mohs surgeries that I did were on patients with tumors that had undergone two surgical excisions with recurrences that were covered up with flaps. I'm very happy to say that now I'm curing patients with a primary basal cell carcinoma the first time I operate.
We are having a cancer epidemic. The annual incidence of nonmelanoma skin cancer is a staggering 3.5 million, according to a recent study involving epidemiologic databases and the National Cancer Institute (Arch. Dermatol. 2010;146:283-7). And, I think the incidence is much higher.
The same study reported a 4.2% annual growth rate. That would predict a 23% growth rate in Mohs micrographic surgery from 2001 to 2006. Instead, we had a 70% growth rate.
What accounts for this? I believe the high growth rate is driven by what I call the "miracle of Mohs surgery." Five-year recurrence rates after Mohs are 1% for primary basal cell carcinomas, compared with 10% for surgical excision in well-controlled studies, and 4% after Mohs surgery for recurrent basal cell carcinomas, as opposed to 17% for excision.
Studies have found that 18%-32% of facial basal cell carcinomas are incompletely excised and require a second procedure. Repeat excisions create larger defects and worse cosmetic outcomes. Complete re-excisions are challenging after flap repairs. But with Mohs, one procedure does it all.
Studies have shown that Mohs surgery is superior financially because it is more cost effective than traditional excision if you include the costs of re-excision and pathology, and re-repair and treatment of recurrences after excisions.
Patients also are twice as satisfied with Mohs surgery, another study found (Dermatol. Surg. 2009;35:1041-9).
We've got an epidemic of cancer, and we have recognition that Mohs is the best technique for treatment. Patients with tumors that would have been excised and recurred are being referred initially for Mohs surgery. Simple primary lesions that are in the right areas should be treated with Mohs.
Does increased utilization of Mohs surgery translate to greater expense in treating skin cancer? The data show that for primary lesions and recurrences, the cost savings is greater with Mohs surgery. Insurance companies are starting to recognize that, but we can't let insurance companies govern simply by cost.
We really don't know for sure what's driving the increase in Mohs micrographic surgery utilization. However, I do believe that the superiority of the technique, which is becoming more recognized and established, and the epidemic of skin cancer have produced this high rate of utilization.
The growth rate reflects recognition of the unparalleled value of Mohs micrographic surgery as we confront the skin cancer epidemic.
Dr. Monheit is a Mohs surgeon in private practice in Birmingham, Ala., and has directed Mohs fellowship programs for 25 years. He said he has no other relevant conflicts of interest. These remarks were presented at the American College of Mohs Surgery meeting.
Yes - Surgeons Are Softening Criteria for Who Gets Mohs.
By David S. Becker, M.D.
The practice of Mohs micrographic surgery is in some cases drifting away from the classic indications that used to make up most of our practice.
There are different published criteria for cases indicated for Mohs surgery, but these often include patients with recurrent or incompletely excised skin cancers, aggressive histology, or poorly defined margins. Other classic indications include tumors larger than 0.4 cm in high-risk H-zone locations, larger than 1 cm elsewhere on the face, or larger than 2 cm on the trunk or extremities.
Some of these listed criteria would not include a 3-mm basal cell carcinoma on the nose or ear, an 8-mm basal cell carcinoma on the cheek or forehead, or a 1.5-cm lesion on the pretibial skin, but I often have had each of these referred to me for Mohs surgery.
What's driving us to soften these traditional indications for Mohs surgery? Patients, referring dermatologists, dermatopathologists, and Mohs surgeons each may play a role.
Patients love Mohs surgery for many reasons. For one, we do a good job. Perhaps they've had previous Mohs surgery with good results or their friends have, or they have looked online and found that Mohs has the best cure rate. Or, they may have had a bad experience with a prior treatment.
It's not uncommon for patients to do research, and then want to play a role in determining their medical care. If they demand Mohs surgery, and I don't think it's indicated, it can lead to a contentious consultation.
One young man with a squamous cell carcinoma on his chest recently told me that he had researched the cure rates of treatments and he wanted Mohs surgery. He was almost certainly correct that if the sole criteria for therapy were best cure rate, Mohs would be the choice.
There are only two downsides to Mohs versus excision in a case like this: One, the patient has to spend more time in the office for Mohs surgery; and two, it is perhaps overutilization of medical resources. Good luck trying to explain that to patients. They don't care about overutilization of resources.
Who else drives this drift toward enhanced Mohs utilization? Referring dermatologists. They may have an interest in maximizing utilization of a Mohs surgeon in their practice, while some dermatologists have truly been burned, that is, had a patient with a metastatic squamous cell carcinoma or difficult recurrences, and they want the greater certitude of cure from Mohs surgery.
Sometimes the referring dermatologist says the patient is a VIP or relative and asks me to make an exception. If you start making exceptions, pretty soon you're seeing a lot of patients who don't necessarily meet the classic criteria for Mohs surgery.
The next set of people who are driving this drift in overutilization is dermatopathologists. We have dermatopathologists being overly cautious and prudent in ways they may not have been 10-15 years ago. Something that might have been diagnosed as actinic keratosis 20 years ago might now be called actinic keratosis with extension to the base, "squamous cell carcinoma cannot be excluded," or superficially invasive squamous cell carcinoma.
Mohs surgeons also drive the drift in utilization. We do it because that's what we like to do, or because we work for someone and we want to do what they tell us to do, or we have a referring dermatologist whom we want to please. And we want to keep our patients happy too.
It may be time to reconceptualize the indications for Mohs surgery. Should patient demand or anxiety be an indication? Should the referring dermatologist's or Mohs surgeon's instincts about a given lesion be an indication? Should any small lesion on the face be an indication?
Finally, who should decide when to do Mohs surgery? Should it be patients? They want some autonomy and to play a role in managing their own care, and are often educated in doing so.
Should it be the referring dermatologist? They understand the history of a given lesion and know the patient and the response to prior therapies.
Should the Mohs surgeon decide? We have the most experience with cutaneous malignancies, and in many cases we're the best suited to decide who needs Mohs and who doesn't.
Or, finally, should it be the government or insurers? I think that because we have allowed this drift in utilization to go forward, that may be who ultimately is deciding.
Dr. Becker is a Mohs surgeon in private practice in New York. He said he has no relevant conflicts of interest. His comments were presented at the American College of Mohs Surgery meeting.
No - Mohs Surgery Is Being Properly Utilized.
By Gary Monheit, M.D.
Mohs micrographic surgery is not overutilized, it's increasingly utilized.
A recent study reported a twofold increase in the proportion of Mohs surgery being done in Medicare patients from 2001 to 2006, while the rate of excisions has remained stable. So what's driving this increased utilization of Mohs surgery?
Is it greedy surgeons applying Mohs micrographic surgery to actinic keratoses on the arms and trunk? The few surgeons who are utilizing it inappropriately need to be controlled, but I don't believe this is the main reason for the increased utilization.
In most cases, increased Mohs utilization is appropriate. Mohs is a superior and much more cost-effective modality than excision and it has a greater cure rate, which is now being recognized.
The vision of Dr. Frederic E. Mohs was a change in the treatment of skin cancer. He was tired of seeing recurrences with excision. He realized that if things were accurately mapped out, physicians would have a better understanding of how to treat tumors, spare tissue, and reduce costs.
Brilliantly, it was a merger of surgery and histopathology controlled by one practitioner, the Mohs surgeon. Results of surgical treatment approach 100% the first time.
Should this be done for all nonmelanoma skin cancers at all locations? Should patients be cured the first time rather than risk recurrences? Is this cost effective for most tumors?
Twenty years ago, 50% or more of the Mohs surgeries that I did were on patients with tumors that had undergone two surgical excisions with recurrences that were covered up with flaps. I'm very happy to say that now I'm curing patients with a primary basal cell carcinoma the first time I operate.
We are having a cancer epidemic. The annual incidence of nonmelanoma skin cancer is a staggering 3.5 million, according to a recent study involving epidemiologic databases and the National Cancer Institute (Arch. Dermatol. 2010;146:283-7). And, I think the incidence is much higher.
The same study reported a 4.2% annual growth rate. That would predict a 23% growth rate in Mohs micrographic surgery from 2001 to 2006. Instead, we had a 70% growth rate.
What accounts for this? I believe the high growth rate is driven by what I call the "miracle of Mohs surgery." Five-year recurrence rates after Mohs are 1% for primary basal cell carcinomas, compared with 10% for surgical excision in well-controlled studies, and 4% after Mohs surgery for recurrent basal cell carcinomas, as opposed to 17% for excision.
Studies have found that 18%-32% of facial basal cell carcinomas are incompletely excised and require a second procedure. Repeat excisions create larger defects and worse cosmetic outcomes. Complete re-excisions are challenging after flap repairs. But with Mohs, one procedure does it all.
Studies have shown that Mohs surgery is superior financially because it is more cost effective than traditional excision if you include the costs of re-excision and pathology, and re-repair and treatment of recurrences after excisions.
Patients also are twice as satisfied with Mohs surgery, another study found (Dermatol. Surg. 2009;35:1041-9).
We've got an epidemic of cancer, and we have recognition that Mohs is the best technique for treatment. Patients with tumors that would have been excised and recurred are being referred initially for Mohs surgery. Simple primary lesions that are in the right areas should be treated with Mohs.
Does increased utilization of Mohs surgery translate to greater expense in treating skin cancer? The data show that for primary lesions and recurrences, the cost savings is greater with Mohs surgery. Insurance companies are starting to recognize that, but we can't let insurance companies govern simply by cost.
We really don't know for sure what's driving the increase in Mohs micrographic surgery utilization. However, I do believe that the superiority of the technique, which is becoming more recognized and established, and the epidemic of skin cancer have produced this high rate of utilization.
The growth rate reflects recognition of the unparalleled value of Mohs micrographic surgery as we confront the skin cancer epidemic.
Dr. Monheit is a Mohs surgeon in private practice in Birmingham, Ala., and has directed Mohs fellowship programs for 25 years. He said he has no other relevant conflicts of interest. These remarks were presented at the American College of Mohs Surgery meeting.
Yes - Surgeons Are Softening Criteria for Who Gets Mohs.
By David S. Becker, M.D.
The practice of Mohs micrographic surgery is in some cases drifting away from the classic indications that used to make up most of our practice.
There are different published criteria for cases indicated for Mohs surgery, but these often include patients with recurrent or incompletely excised skin cancers, aggressive histology, or poorly defined margins. Other classic indications include tumors larger than 0.4 cm in high-risk H-zone locations, larger than 1 cm elsewhere on the face, or larger than 2 cm on the trunk or extremities.
Some of these listed criteria would not include a 3-mm basal cell carcinoma on the nose or ear, an 8-mm basal cell carcinoma on the cheek or forehead, or a 1.5-cm lesion on the pretibial skin, but I often have had each of these referred to me for Mohs surgery.
What's driving us to soften these traditional indications for Mohs surgery? Patients, referring dermatologists, dermatopathologists, and Mohs surgeons each may play a role.
Patients love Mohs surgery for many reasons. For one, we do a good job. Perhaps they've had previous Mohs surgery with good results or their friends have, or they have looked online and found that Mohs has the best cure rate. Or, they may have had a bad experience with a prior treatment.
It's not uncommon for patients to do research, and then want to play a role in determining their medical care. If they demand Mohs surgery, and I don't think it's indicated, it can lead to a contentious consultation.
One young man with a squamous cell carcinoma on his chest recently told me that he had researched the cure rates of treatments and he wanted Mohs surgery. He was almost certainly correct that if the sole criteria for therapy were best cure rate, Mohs would be the choice.
There are only two downsides to Mohs versus excision in a case like this: One, the patient has to spend more time in the office for Mohs surgery; and two, it is perhaps overutilization of medical resources. Good luck trying to explain that to patients. They don't care about overutilization of resources.
Who else drives this drift toward enhanced Mohs utilization? Referring dermatologists. They may have an interest in maximizing utilization of a Mohs surgeon in their practice, while some dermatologists have truly been burned, that is, had a patient with a metastatic squamous cell carcinoma or difficult recurrences, and they want the greater certitude of cure from Mohs surgery.
Sometimes the referring dermatologist says the patient is a VIP or relative and asks me to make an exception. If you start making exceptions, pretty soon you're seeing a lot of patients who don't necessarily meet the classic criteria for Mohs surgery.
The next set of people who are driving this drift in overutilization is dermatopathologists. We have dermatopathologists being overly cautious and prudent in ways they may not have been 10-15 years ago. Something that might have been diagnosed as actinic keratosis 20 years ago might now be called actinic keratosis with extension to the base, "squamous cell carcinoma cannot be excluded," or superficially invasive squamous cell carcinoma.
Mohs surgeons also drive the drift in utilization. We do it because that's what we like to do, or because we work for someone and we want to do what they tell us to do, or we have a referring dermatologist whom we want to please. And we want to keep our patients happy too.
It may be time to reconceptualize the indications for Mohs surgery. Should patient demand or anxiety be an indication? Should the referring dermatologist's or Mohs surgeon's instincts about a given lesion be an indication? Should any small lesion on the face be an indication?
Finally, who should decide when to do Mohs surgery? Should it be patients? They want some autonomy and to play a role in managing their own care, and are often educated in doing so.
Should it be the referring dermatologist? They understand the history of a given lesion and know the patient and the response to prior therapies.
Should the Mohs surgeon decide? We have the most experience with cutaneous malignancies, and in many cases we're the best suited to decide who needs Mohs and who doesn't.
Or, finally, should it be the government or insurers? I think that because we have allowed this drift in utilization to go forward, that may be who ultimately is deciding.
Dr. Becker is a Mohs surgeon in private practice in New York. He said he has no relevant conflicts of interest. His comments were presented at the American College of Mohs Surgery meeting.
No - Mohs Surgery Is Being Properly Utilized.
By Gary Monheit, M.D.
Mohs micrographic surgery is not overutilized, it's increasingly utilized.
A recent study reported a twofold increase in the proportion of Mohs surgery being done in Medicare patients from 2001 to 2006, while the rate of excisions has remained stable. So what's driving this increased utilization of Mohs surgery?
Is it greedy surgeons applying Mohs micrographic surgery to actinic keratoses on the arms and trunk? The few surgeons who are utilizing it inappropriately need to be controlled, but I don't believe this is the main reason for the increased utilization.
In most cases, increased Mohs utilization is appropriate. Mohs is a superior and much more cost-effective modality than excision and it has a greater cure rate, which is now being recognized.
The vision of Dr. Frederic E. Mohs was a change in the treatment of skin cancer. He was tired of seeing recurrences with excision. He realized that if things were accurately mapped out, physicians would have a better understanding of how to treat tumors, spare tissue, and reduce costs.
Brilliantly, it was a merger of surgery and histopathology controlled by one practitioner, the Mohs surgeon. Results of surgical treatment approach 100% the first time.
Should this be done for all nonmelanoma skin cancers at all locations? Should patients be cured the first time rather than risk recurrences? Is this cost effective for most tumors?
Twenty years ago, 50% or more of the Mohs surgeries that I did were on patients with tumors that had undergone two surgical excisions with recurrences that were covered up with flaps. I'm very happy to say that now I'm curing patients with a primary basal cell carcinoma the first time I operate.
We are having a cancer epidemic. The annual incidence of nonmelanoma skin cancer is a staggering 3.5 million, according to a recent study involving epidemiologic databases and the National Cancer Institute (Arch. Dermatol. 2010;146:283-7). And, I think the incidence is much higher.
The same study reported a 4.2% annual growth rate. That would predict a 23% growth rate in Mohs micrographic surgery from 2001 to 2006. Instead, we had a 70% growth rate.
What accounts for this? I believe the high growth rate is driven by what I call the "miracle of Mohs surgery." Five-year recurrence rates after Mohs are 1% for primary basal cell carcinomas, compared with 10% for surgical excision in well-controlled studies, and 4% after Mohs surgery for recurrent basal cell carcinomas, as opposed to 17% for excision.
Studies have found that 18%-32% of facial basal cell carcinomas are incompletely excised and require a second procedure. Repeat excisions create larger defects and worse cosmetic outcomes. Complete re-excisions are challenging after flap repairs. But with Mohs, one procedure does it all.
Studies have shown that Mohs surgery is superior financially because it is more cost effective than traditional excision if you include the costs of re-excision and pathology, and re-repair and treatment of recurrences after excisions.
Patients also are twice as satisfied with Mohs surgery, another study found (Dermatol. Surg. 2009;35:1041-9).
We've got an epidemic of cancer, and we have recognition that Mohs is the best technique for treatment. Patients with tumors that would have been excised and recurred are being referred initially for Mohs surgery. Simple primary lesions that are in the right areas should be treated with Mohs.
Does increased utilization of Mohs surgery translate to greater expense in treating skin cancer? The data show that for primary lesions and recurrences, the cost savings is greater with Mohs surgery. Insurance companies are starting to recognize that, but we can't let insurance companies govern simply by cost.
We really don't know for sure what's driving the increase in Mohs micrographic surgery utilization. However, I do believe that the superiority of the technique, which is becoming more recognized and established, and the epidemic of skin cancer have produced this high rate of utilization.
The growth rate reflects recognition of the unparalleled value of Mohs micrographic surgery as we confront the skin cancer epidemic.
Dr. Monheit is a Mohs surgeon in private practice in Birmingham, Ala., and has directed Mohs fellowship programs for 25 years. He said he has no other relevant conflicts of interest. These remarks were presented at the American College of Mohs Surgery meeting.
Tretinoin, Isotretinoin Found Equally Effective for Photoaging
SEOUL, SOUTH KOREA – Low-dose oral isotretinoin and alternate-day tretinoin proved similarly effective for treatment of photoaging in a prospective randomized trial.
Treatment of photoaged skin is an off-label application for isotretinoin, but there have been a number of published favorable case series (J. Eur. Acad. Dermatol. Venereol. 2009;23:115-23).
Some dermatologists are prescribing it in more severe cases where teratogenicity is a nonissue. So Dr. Edileia Bagatin decided to put the potent oral retinoid to the test in a randomized comparison with tretinoin (Renova), a topical retinoid that does have a regulatory indication for photoaging.
The result of the 12-month study was a draw in terms of efficacy. The two treatment groups showed similar improvements in photoaging based upon blinded dermatologic evaluations, patient self-ratings, quality of life scores, and histologic findings.
And because the topical retinoid doesn't come with the baggage for which isotretinoin is notorious – including dyslipidemia, birth defects, liver dysfunction, depression, and inflammatory bowel disease – tretinoin has the clear advantage in most situations, said Dr. Bagatin at the World Congress of Dermatology.
The trial involved 22 patients, aged 50-75 years, with moderate to advanced photoaging. Nine of 11 subjects in each treatment arm were smokers.
Patients were assigned to either 20 mg/day of isotretinoin for 6 months followed by 0.05% tretinoin cream applied every other day for 6 months, or to alternate-day tretinoin for the full 12 months. All patients were instructed to use a moisturizing sunscreen twice daily.
The number of actinic keratoses on the face was reduced to a similar extent via both therapies: by an average of 74% after 6 months and 54% after 12 months. The number of AKs on the forearms dropped by an average of 42% with either therapy after 6 months, and by 61% after 12 months, according to Dr. Bagatin, of the Federal University of Sao Paulo (Brazil).
The patients themselves rated their wrinkles, skin elasticity, actinic keratoses, and freckles as significantly improved at both 6 and 12 months, with no significant difference between treatment arms. Median Dermatology Life Quality Index scores showed significant improvement over time, again with no difference between the two groups.
On histology, both groups displayed reductions in corneal layer thickness and elastosis, along with increased epidermal thickness; these changes were greater with time. There was also a significant reduction in p53 expression and an increase in collagen I, with both changes being greater at 12 months than 6 months.
The study was funded by the Sao Paulo State Research Foundation. Dr. Bagatin reported having no financial conflicts.
SEOUL, SOUTH KOREA – Low-dose oral isotretinoin and alternate-day tretinoin proved similarly effective for treatment of photoaging in a prospective randomized trial.
Treatment of photoaged skin is an off-label application for isotretinoin, but there have been a number of published favorable case series (J. Eur. Acad. Dermatol. Venereol. 2009;23:115-23).
Some dermatologists are prescribing it in more severe cases where teratogenicity is a nonissue. So Dr. Edileia Bagatin decided to put the potent oral retinoid to the test in a randomized comparison with tretinoin (Renova), a topical retinoid that does have a regulatory indication for photoaging.
The result of the 12-month study was a draw in terms of efficacy. The two treatment groups showed similar improvements in photoaging based upon blinded dermatologic evaluations, patient self-ratings, quality of life scores, and histologic findings.
And because the topical retinoid doesn't come with the baggage for which isotretinoin is notorious – including dyslipidemia, birth defects, liver dysfunction, depression, and inflammatory bowel disease – tretinoin has the clear advantage in most situations, said Dr. Bagatin at the World Congress of Dermatology.
The trial involved 22 patients, aged 50-75 years, with moderate to advanced photoaging. Nine of 11 subjects in each treatment arm were smokers.
Patients were assigned to either 20 mg/day of isotretinoin for 6 months followed by 0.05% tretinoin cream applied every other day for 6 months, or to alternate-day tretinoin for the full 12 months. All patients were instructed to use a moisturizing sunscreen twice daily.
The number of actinic keratoses on the face was reduced to a similar extent via both therapies: by an average of 74% after 6 months and 54% after 12 months. The number of AKs on the forearms dropped by an average of 42% with either therapy after 6 months, and by 61% after 12 months, according to Dr. Bagatin, of the Federal University of Sao Paulo (Brazil).
The patients themselves rated their wrinkles, skin elasticity, actinic keratoses, and freckles as significantly improved at both 6 and 12 months, with no significant difference between treatment arms. Median Dermatology Life Quality Index scores showed significant improvement over time, again with no difference between the two groups.
On histology, both groups displayed reductions in corneal layer thickness and elastosis, along with increased epidermal thickness; these changes were greater with time. There was also a significant reduction in p53 expression and an increase in collagen I, with both changes being greater at 12 months than 6 months.
The study was funded by the Sao Paulo State Research Foundation. Dr. Bagatin reported having no financial conflicts.
SEOUL, SOUTH KOREA – Low-dose oral isotretinoin and alternate-day tretinoin proved similarly effective for treatment of photoaging in a prospective randomized trial.
Treatment of photoaged skin is an off-label application for isotretinoin, but there have been a number of published favorable case series (J. Eur. Acad. Dermatol. Venereol. 2009;23:115-23).
Some dermatologists are prescribing it in more severe cases where teratogenicity is a nonissue. So Dr. Edileia Bagatin decided to put the potent oral retinoid to the test in a randomized comparison with tretinoin (Renova), a topical retinoid that does have a regulatory indication for photoaging.
The result of the 12-month study was a draw in terms of efficacy. The two treatment groups showed similar improvements in photoaging based upon blinded dermatologic evaluations, patient self-ratings, quality of life scores, and histologic findings.
And because the topical retinoid doesn't come with the baggage for which isotretinoin is notorious – including dyslipidemia, birth defects, liver dysfunction, depression, and inflammatory bowel disease – tretinoin has the clear advantage in most situations, said Dr. Bagatin at the World Congress of Dermatology.
The trial involved 22 patients, aged 50-75 years, with moderate to advanced photoaging. Nine of 11 subjects in each treatment arm were smokers.
Patients were assigned to either 20 mg/day of isotretinoin for 6 months followed by 0.05% tretinoin cream applied every other day for 6 months, or to alternate-day tretinoin for the full 12 months. All patients were instructed to use a moisturizing sunscreen twice daily.
The number of actinic keratoses on the face was reduced to a similar extent via both therapies: by an average of 74% after 6 months and 54% after 12 months. The number of AKs on the forearms dropped by an average of 42% with either therapy after 6 months, and by 61% after 12 months, according to Dr. Bagatin, of the Federal University of Sao Paulo (Brazil).
The patients themselves rated their wrinkles, skin elasticity, actinic keratoses, and freckles as significantly improved at both 6 and 12 months, with no significant difference between treatment arms. Median Dermatology Life Quality Index scores showed significant improvement over time, again with no difference between the two groups.
On histology, both groups displayed reductions in corneal layer thickness and elastosis, along with increased epidermal thickness; these changes were greater with time. There was also a significant reduction in p53 expression and an increase in collagen I, with both changes being greater at 12 months than 6 months.
The study was funded by the Sao Paulo State Research Foundation. Dr. Bagatin reported having no financial conflicts.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: Oral isotretinoin and topical tretinoin both reduced facial actinic keratoses by 74% after 6 months and 54% after 12 months.
Data Source: A 1-year randomized trial comparing the efficacy of low-dose oral isotretinoin to alternate-day tretinoin in 22 patients with photoaging.
Disclosures: The study was funded by the Sao Paulo State Research Foundation. Dr. Bagatin reported having no financial conflicts.
Nonablative Fractional Laser Proves Effective for Actinic Cheilitis
GRAPEVINE, TEX. – The nonablative fractional thulium 1927-nm laser effectively treated actinic cheilitis in 15 patients, without subsequent downtime or significant side effects, Dr. Robert Anolik reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Current treatments for actinic cheilitis – including surgery, carbon dioxide/erbium laser ablation, electrodesiccation, and 5-fluorouracil – typically involve significant pain, edema, and other adverse effects, including permanent scarring. The 1927-nm thulium laser, which is effective and well tolerated for superficial resurfacing, has been approved by the Food and Drug Administration for treating actinic keratoses, said Dr. Anolik of the Laser and Skin Surgery Center of New York.
Charts were reviewed for the 15 patients with actinic cheilitis who had been treated with the nonablative fractional 1,927-nm laser at two private laser and skin surgery centers. All were pretreated with topical anesthetic creams and given oral antiviral prophylaxis. Treatment parameters were 10-20 mJ per MTZ, 65%-70% coverage density, and total delivered energy of 0.08-0.1 kJ.
In blinded assessments of before and after photographs using a quartile improvement scale, all 15 patients had improvements of either 76%-100% (9 patients) or 51%-75% (6 patients) after 1-2 treatments. No adverse events occurred, and the only side effects were transient erythema for 1-4 days and edema for 1-3 days. "This is in stark contrast to the wounding, pain, and downtime expected with the other common treatment strategies," Dr. Anolik commented.
Planned next steps include increasing the patient pool, trial treatment of patients with a range of actinic cheilitis severity, assessment of before/after or left/right specimens for molecular features of actinic cheilitis such as p53, and an evaluation of long term benefit, he noted.
Dr. Anolik stated that he has no relevant relationships with industry.
GRAPEVINE, TEX. – The nonablative fractional thulium 1927-nm laser effectively treated actinic cheilitis in 15 patients, without subsequent downtime or significant side effects, Dr. Robert Anolik reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Current treatments for actinic cheilitis – including surgery, carbon dioxide/erbium laser ablation, electrodesiccation, and 5-fluorouracil – typically involve significant pain, edema, and other adverse effects, including permanent scarring. The 1927-nm thulium laser, which is effective and well tolerated for superficial resurfacing, has been approved by the Food and Drug Administration for treating actinic keratoses, said Dr. Anolik of the Laser and Skin Surgery Center of New York.
Charts were reviewed for the 15 patients with actinic cheilitis who had been treated with the nonablative fractional 1,927-nm laser at two private laser and skin surgery centers. All were pretreated with topical anesthetic creams and given oral antiviral prophylaxis. Treatment parameters were 10-20 mJ per MTZ, 65%-70% coverage density, and total delivered energy of 0.08-0.1 kJ.
In blinded assessments of before and after photographs using a quartile improvement scale, all 15 patients had improvements of either 76%-100% (9 patients) or 51%-75% (6 patients) after 1-2 treatments. No adverse events occurred, and the only side effects were transient erythema for 1-4 days and edema for 1-3 days. "This is in stark contrast to the wounding, pain, and downtime expected with the other common treatment strategies," Dr. Anolik commented.
Planned next steps include increasing the patient pool, trial treatment of patients with a range of actinic cheilitis severity, assessment of before/after or left/right specimens for molecular features of actinic cheilitis such as p53, and an evaluation of long term benefit, he noted.
Dr. Anolik stated that he has no relevant relationships with industry.
GRAPEVINE, TEX. – The nonablative fractional thulium 1927-nm laser effectively treated actinic cheilitis in 15 patients, without subsequent downtime or significant side effects, Dr. Robert Anolik reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Current treatments for actinic cheilitis – including surgery, carbon dioxide/erbium laser ablation, electrodesiccation, and 5-fluorouracil – typically involve significant pain, edema, and other adverse effects, including permanent scarring. The 1927-nm thulium laser, which is effective and well tolerated for superficial resurfacing, has been approved by the Food and Drug Administration for treating actinic keratoses, said Dr. Anolik of the Laser and Skin Surgery Center of New York.
Charts were reviewed for the 15 patients with actinic cheilitis who had been treated with the nonablative fractional 1,927-nm laser at two private laser and skin surgery centers. All were pretreated with topical anesthetic creams and given oral antiviral prophylaxis. Treatment parameters were 10-20 mJ per MTZ, 65%-70% coverage density, and total delivered energy of 0.08-0.1 kJ.
In blinded assessments of before and after photographs using a quartile improvement scale, all 15 patients had improvements of either 76%-100% (9 patients) or 51%-75% (6 patients) after 1-2 treatments. No adverse events occurred, and the only side effects were transient erythema for 1-4 days and edema for 1-3 days. "This is in stark contrast to the wounding, pain, and downtime expected with the other common treatment strategies," Dr. Anolik commented.
Planned next steps include increasing the patient pool, trial treatment of patients with a range of actinic cheilitis severity, assessment of before/after or left/right specimens for molecular features of actinic cheilitis such as p53, and an evaluation of long term benefit, he noted.
Dr. Anolik stated that he has no relevant relationships with industry.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: All 15 patients had improvements of either 76%-100% (9) or 51%-75% (6) after one or two treatments with a nonablative fractional thulium 1927-nm laser.
Data Source: Chart review of 15 patients with actinic cheilitis.
Disclosures: Dr. Anolik stated that he has no relevant relationships with industry.