User login
Implementation of a Pharmacist-Led Culture and Susceptibility Review System in Urgent Care and Outpatient Settings
Increasing antibiotic resistance is an urgent threat to public health and establishing a review service for antibiotics could alleviate this problem. As use of antibiotics escalates, the risk of resistance becomes increasingly important. Each year, approximately 269 million antibiotics are dispensed and at least 30% are prescribed inappropriately.1 In addition to inappropriate prescribing, increased antibiotic resistance can be caused by patients not completing an antibiotic course as recommended or inherent bacterial mutations. According to the Centers for Disease Control and Prevention, each year approximately 3 million individuals contract an antibiotic-resistant infection.2 By 2050, it is projected that drug-resistant conditions could cause 300 million deaths and might be as disastrous to the economy as the 2008 global financial crisis.3 Ensuring appropriate use of antibiotic therapy through antimicrobial stewardship can help combat this significant public health issue.
Antimicrobial stewardship promotes appropriate use of antimicrobials to improve patient outcomes, reduce health care costs, and decrease antimicrobial resistance. One study found that nearly 50% of patients discharged from the emergency department with antibiotics required therapy modification after culture and susceptibility results were returned.4 Both the Infectious Disease Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) support incorporating a clinical pharmacist into culture reviews.3 Several institutions have implemented a pharmacist-led culture review service to improve antibiotic usage, which has shown positive results. A retrospective case-control study at University of Rochester Medical Center showed reduced time to positive culture review and to patient or health care provider (HCP) notification when emergency medicine pharmacists were involved in culture review.5 A retrospective study at Carolinas Medical Center-Northeast showed 12% decreased readmission rate using pharmacist-implemented culture review compared with HCP review.6 Results from previous studies showed an overall improvement in patient safety through decreased use of inappropriate agents and reduced time on inappropriate antibiotic therapy.
Establishing a pharmacist-led culture review service at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia, could decrease the time to review of positive culture results, time to patient or HCP notification, and readmission rates. CVVAMC provides outpatient primary care services to about 30,000 veterans in the central and southern regions of Georgia. Our facility has executed an antimicrobial stewardship program based on guidelines published in 2016 by IDSA and SHEA to guide optimal use of antibiotics. Clinical pharmacists play an active role in antimicrobial stewardship throughout the facility. Clinical responsibilities of the antimicrobial stewardship pharmacist include assessing therapy for inappropriate dual anaerobic coverage, evaluating inpatient culture results within 48 hours, dosing and monitoring antibiotic therapy, including vancomycin and aminoglycosides, and implementing IV to by-mouth conversions for appropriate patients. HCPs involved with antimicrobial stewardship could order an array of tests to assess a veteran’s condition, including cultures, when an infection is suspected.
Culture results take about 3 to 5 days, then HCPs evaluate the result to ensure current antibiotic therapy is appropriate. Patients might not receive timely follow-up because HCPs often have many laboratory alerts to sift through every day, and a protocol is not in place for pharmacists to adjust outpatient antimicrobial regimens based on culture results. Before implementing this project, there was no outpatient service for pharmacists to impact culture and susceptibility review. This project was initiated because a lead physician identified difficulty reviewing culture and susceptibility results. HCPs often work on rotating schedules, and there was a concern about possible delay in follow-up of results if a HCP was not scheduled to work for a period of time.
The purpose of this project was to implement an outpatient, pharmacist-managed culture and susceptibility review service to improve patient outcomes, including decreasing and preventing inappropriate antibiotic use. The primary objective was to design and implement a pharmacist-led review service to intervene in cases of mismatched antibiotic bacteria combinations. Secondary objectives included identifying most common culture types and organisms encountered and intervened on at our facility.
Quality Improvement Project
This quality improvement project was approved by the CVVAMC Pharmacy and Therapeutics Committee. Members of the medical review board signed a care coordination agreement between pharmacy and outpatient HCPs to permit pharmacist interventions involving optimization of antibiotic therapy. This agreement allowed pharmacists to make changes to existing antimicrobial regimens within their scope of practice (SOP) without requiring discussion with HCPs. A protocol was also developed to guide pharmacist modification of antimicrobial therapy based on current antimicrobial guidelines.7 This protocol was based on commonly isolated organisms and local resistance patterns and provided guidance for antibiotic treatment based on culture type (ie, skin and soft tissue infection, urine, etc). Computerized Patient Record System (CPRS) note templates were also developed for interventions performed, and patient follow-up after antibiotic regimens were completed (eAppendix 1
Program Inclusion
Veterans were included in this project if they presented to primary care or urgent care clinics for therapy; had positive culture and sensitivity results; and were prescribed an empiric antibiotic. Veterans were not eligible for this project if they were not receiving antibiotic therapy, with or without pending or resulted culture results shown in CPRS.
Implementation
Data gathered through a CPRS dashboard from August 2019 to February 2020 identified patients with pending or completed culture results in urgent care and primary care settings (eAppendix 4). The dashboard was created specifically for this project to show patient details that included initial antibiotic(s) prescribed and preliminary and final culture results. After a mismatched combination was identified, pharmacists contacted patients and assessed symptoms. If a patient was still symptomatic, the pharmacist changed the antibiotic regimen and educated the patient about this change. The pharmacist documented an intervention note in CPRS and added the HCP as a signer so he or she would be aware of the change. The clinical pharmacist followed up after regimens were complete. At this time, the pharmacist assessed patients to ensure the medication was taken as directed (eg, number of days of therapy, how many tablets per day, etc), to discuss any reported adverse effects, and to assess resolution of symptoms. If a patient still had symptoms, the pharmacist contacted the patient’s primary care provider. If the veteran could not be contacted after 3 consecutive attempts via phone, a certified letter was mailed. If patients were asymptomatic at the time of the call, the pharmacist documented the lack of symptoms and added the HCP as a signer for awareness purposes. HCPs continued to practice as usual while this service was implemented.
Observations
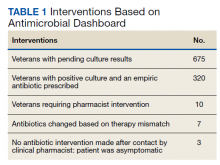
Using the culture and susceptibility dashboard, the pharmacist identified 675 patients as having a pending culture (Table 1). Among these patients, 320 results were positive, and were taking antibiotics empirically. Out of the 320 patients who met inclusion criteria, 10 required pharmacist intervention. After contacting the veterans, 7 required regimen changes because their current antibiotic was not susceptible to the isolated organism. Three additional patients were contacted because of a mismatch between the empiric antibiotic and culture result. Antibiotic therapy was not modified because these patients were asymptomatic at the time the clinical pharmacist contacted them. These patient cases were discussed with the HCP before documenting the intervention to prevent initiation of unwarranted antibiotics.
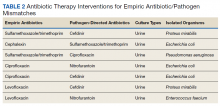
Most of the modified antimicrobial regimens were found in urine cultures from symptomatic patients (Table 2). Of the 7 patients requiring therapy change because of a mismatch antibiotic–bacteria combination, 4 were empirically prescribed fluoroquinolones, 2 received levofloxacin, and 2 were prescribed ciprofloxacin. According to the most recent antibiogram at our facility, some organisms are resistant to fluoroquinolones, specifically Proteus mirabilis (P mirabilis) and Escherichia coli (E coli). These pathogens were the cause of urinary tract infections in 3 of 4 patients with fluoroquinolone prescriptions.
Through the CPRS dashboard, the pharmacist inadvertently identified 4 patients with positive culture results who were not on antibiotic therapy. These patients were contacted by telephone, and antibiotics were initiated for symptomatic patients after consultation with the HCP. The primary culture type intervened on was urine in 12 of 14 cases (86%). The other 2 culture types included oropharynx culture (7%) positive for an acute bacterial respiratory tract infection caused by group C Streptococcus and a stool culture (7%) positive for Pseudomonas aeruginosa (P aeruginosa). E coli (36%) was isolated in 5 cases and was the most commonly isolated organism. P
Discussion
This project was an innovative antimicrobial stewardship endeavor that helped initiate antibiotic interventions quickly and improve patient outcomes. The antimicrobial stewardship pharmacist independently performed interventions for patients without requiring HCP consultation, therefore decreasing HCP burden and possibly reducing time to assessment of culture results.
Limitations
The study results were limited due to its small sample size of antimicrobial interventions. The clinical pharmacist did not contact the patient when the antibiotic prescribed empirically by the HCP was appropriate for the isolated organism. Among the patients contacted, 3 were asymptomatic, did not require further antibiotic therapy, and no intervention was made. Provider education was deemed successful because HCPs did not request further information about the service. However, not all HCPs were provided education because of different shifts and inability to attend educational sessions. Closely working with lead physicians within the facility provided an alternate method for information dissemination.
The care coordination agreement allowed the pharmacist to make changes if patients had a current prescription for an antibiotic. In addition to the changes to antibiotics, this project improved HCP awareness of culture results even in cases of symptomatic patients who were not prescribed therapy. When this occurred, the pharmacist contacted the patient to assess symptoms and then notified the HCP if the patient was symptomatic.
Future Directions
Future endeavors regarding this project include modifying the scope of the service to allow pharmacists to prescribe antibiotics for patients with positive cultures and symptoms without empiric antibiotics in addition to continuing to modify empiric therapy. Additionally, improving dashboard efficiency through changes to include only isolated antibiotic mismatches rather than all antibiotics prescribed and all available cultures would reduce the pharmacists’ time commitment. Expanding to other parts of the medical center, including long-term care facilities and other outpatient clinics, would allow this service to reach more veterans. Integrating this service throughout the medical center will require continued HCP education and modifying care coordination agreements to include these facilities.
On a typical day, 60 to 90 minutes were spent navigating the dashboard and implementing this service. The CPRS dashboard should be modified to streamline patients identified to decrease the daily time commitment. Re-education of HCPs about resistance rates of fluoroquinolones and empirically prescribing these agents also will be completed based on empiric antibiotic interventions made with these agents throughout this project. Discussing HCP viewpoints on this service would be beneficial to ensure HCP satisfaction.
Conclusions
This pharmacy service and antimicrobial stewardship program reduced time patients were on inappropriate antibiotics. Pharmacists reviewed the dashboard daily under the scope of this project, which expedited needed changes and decreased provider burden because pharmacists were able to make changes without interrupting HCPs’ daily tasks, including patient care.
This program may also reduce readmissions. Patients who were still symptomatic were contacted could be given revised medication regimens without the patient returning to the facility for follow-up treatment. An interesting conclusion not included in the current scope of this service was possible reduced time to therapy initiation in cases of positive cultures and symptomatic patients without antibiotic therapy. If this occurred on the dashboard, patient’s symptoms could be assessed, and if symptoms were ongoing, the pharmacist contacted the HCP with a recommended antimicrobial therapy. In these cases, we were able to mail the antibiotic quickly, and many times, on the same day as this intervention through overnight mail. Implementation of a pharmacist-led antimicrobial review service has provided positive results overall for CVVAMC.
Acknowledgment
This material is the result of work supported with resources and the use of the facilities at the Carl Vinson VA Medical Center.
1. Centers for Disease Control and Prevention. Antibiotic use in outpatient settings, 2017: progress and opportunities. Accessed August 19, 2021. https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html
2. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Accessed August 19, 2021. https://www.cdc.gov/drugresistance/index.html
3. Jonas OB, Irwin A, Berthe FCJ, Le Gall FG, Marquez PV. Drug-resistant infections: a threat to our economic future. March 2017. Accessed August 19, 2021. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/323311493396993758/final-report
4. Davis LC, Covey RB, Weston JS, Hu BBY, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5)(suppl 1):S49-S56. doi:10.2146/sp150036
5. Baker SN, Acquisto NM, Ashley ED, Fairbanks RJ, Beamish SE, Haas CE. Pharmacist-managed antimicrobial stewardship program for patients discharged from the emergency department. J Pharm Pract. 2012;25(2):190-194. doi:10.1177/0897190011420160
6 Randolph TC, Parker A, Meyer L, Zeina R. Effect of a pharmacist-managed culture review process on antimicrobial therapy in an emergency department. Am J Health Syst Pharm. 2011;68(10):916-919. doi:10.2146/ajhp090552
7. Infectious Diseases Society of America. Infectious diseases society of America guidelines 2019. Accessed August 24, 2021. https://www.idsociety.org/practice-guideline/practice-guidelines/#/+/0/date_na_dt/desc
Increasing antibiotic resistance is an urgent threat to public health and establishing a review service for antibiotics could alleviate this problem. As use of antibiotics escalates, the risk of resistance becomes increasingly important. Each year, approximately 269 million antibiotics are dispensed and at least 30% are prescribed inappropriately.1 In addition to inappropriate prescribing, increased antibiotic resistance can be caused by patients not completing an antibiotic course as recommended or inherent bacterial mutations. According to the Centers for Disease Control and Prevention, each year approximately 3 million individuals contract an antibiotic-resistant infection.2 By 2050, it is projected that drug-resistant conditions could cause 300 million deaths and might be as disastrous to the economy as the 2008 global financial crisis.3 Ensuring appropriate use of antibiotic therapy through antimicrobial stewardship can help combat this significant public health issue.
Antimicrobial stewardship promotes appropriate use of antimicrobials to improve patient outcomes, reduce health care costs, and decrease antimicrobial resistance. One study found that nearly 50% of patients discharged from the emergency department with antibiotics required therapy modification after culture and susceptibility results were returned.4 Both the Infectious Disease Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) support incorporating a clinical pharmacist into culture reviews.3 Several institutions have implemented a pharmacist-led culture review service to improve antibiotic usage, which has shown positive results. A retrospective case-control study at University of Rochester Medical Center showed reduced time to positive culture review and to patient or health care provider (HCP) notification when emergency medicine pharmacists were involved in culture review.5 A retrospective study at Carolinas Medical Center-Northeast showed 12% decreased readmission rate using pharmacist-implemented culture review compared with HCP review.6 Results from previous studies showed an overall improvement in patient safety through decreased use of inappropriate agents and reduced time on inappropriate antibiotic therapy.
Establishing a pharmacist-led culture review service at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia, could decrease the time to review of positive culture results, time to patient or HCP notification, and readmission rates. CVVAMC provides outpatient primary care services to about 30,000 veterans in the central and southern regions of Georgia. Our facility has executed an antimicrobial stewardship program based on guidelines published in 2016 by IDSA and SHEA to guide optimal use of antibiotics. Clinical pharmacists play an active role in antimicrobial stewardship throughout the facility. Clinical responsibilities of the antimicrobial stewardship pharmacist include assessing therapy for inappropriate dual anaerobic coverage, evaluating inpatient culture results within 48 hours, dosing and monitoring antibiotic therapy, including vancomycin and aminoglycosides, and implementing IV to by-mouth conversions for appropriate patients. HCPs involved with antimicrobial stewardship could order an array of tests to assess a veteran’s condition, including cultures, when an infection is suspected.
Culture results take about 3 to 5 days, then HCPs evaluate the result to ensure current antibiotic therapy is appropriate. Patients might not receive timely follow-up because HCPs often have many laboratory alerts to sift through every day, and a protocol is not in place for pharmacists to adjust outpatient antimicrobial regimens based on culture results. Before implementing this project, there was no outpatient service for pharmacists to impact culture and susceptibility review. This project was initiated because a lead physician identified difficulty reviewing culture and susceptibility results. HCPs often work on rotating schedules, and there was a concern about possible delay in follow-up of results if a HCP was not scheduled to work for a period of time.
The purpose of this project was to implement an outpatient, pharmacist-managed culture and susceptibility review service to improve patient outcomes, including decreasing and preventing inappropriate antibiotic use. The primary objective was to design and implement a pharmacist-led review service to intervene in cases of mismatched antibiotic bacteria combinations. Secondary objectives included identifying most common culture types and organisms encountered and intervened on at our facility.
Quality Improvement Project
This quality improvement project was approved by the CVVAMC Pharmacy and Therapeutics Committee. Members of the medical review board signed a care coordination agreement between pharmacy and outpatient HCPs to permit pharmacist interventions involving optimization of antibiotic therapy. This agreement allowed pharmacists to make changes to existing antimicrobial regimens within their scope of practice (SOP) without requiring discussion with HCPs. A protocol was also developed to guide pharmacist modification of antimicrobial therapy based on current antimicrobial guidelines.7 This protocol was based on commonly isolated organisms and local resistance patterns and provided guidance for antibiotic treatment based on culture type (ie, skin and soft tissue infection, urine, etc). Computerized Patient Record System (CPRS) note templates were also developed for interventions performed, and patient follow-up after antibiotic regimens were completed (eAppendix 1
Program Inclusion
Veterans were included in this project if they presented to primary care or urgent care clinics for therapy; had positive culture and sensitivity results; and were prescribed an empiric antibiotic. Veterans were not eligible for this project if they were not receiving antibiotic therapy, with or without pending or resulted culture results shown in CPRS.
Implementation
Data gathered through a CPRS dashboard from August 2019 to February 2020 identified patients with pending or completed culture results in urgent care and primary care settings (eAppendix 4). The dashboard was created specifically for this project to show patient details that included initial antibiotic(s) prescribed and preliminary and final culture results. After a mismatched combination was identified, pharmacists contacted patients and assessed symptoms. If a patient was still symptomatic, the pharmacist changed the antibiotic regimen and educated the patient about this change. The pharmacist documented an intervention note in CPRS and added the HCP as a signer so he or she would be aware of the change. The clinical pharmacist followed up after regimens were complete. At this time, the pharmacist assessed patients to ensure the medication was taken as directed (eg, number of days of therapy, how many tablets per day, etc), to discuss any reported adverse effects, and to assess resolution of symptoms. If a patient still had symptoms, the pharmacist contacted the patient’s primary care provider. If the veteran could not be contacted after 3 consecutive attempts via phone, a certified letter was mailed. If patients were asymptomatic at the time of the call, the pharmacist documented the lack of symptoms and added the HCP as a signer for awareness purposes. HCPs continued to practice as usual while this service was implemented.
Observations
Using the culture and susceptibility dashboard, the pharmacist identified 675 patients as having a pending culture (Table 1). Among these patients, 320 results were positive, and were taking antibiotics empirically. Out of the 320 patients who met inclusion criteria, 10 required pharmacist intervention. After contacting the veterans, 7 required regimen changes because their current antibiotic was not susceptible to the isolated organism. Three additional patients were contacted because of a mismatch between the empiric antibiotic and culture result. Antibiotic therapy was not modified because these patients were asymptomatic at the time the clinical pharmacist contacted them. These patient cases were discussed with the HCP before documenting the intervention to prevent initiation of unwarranted antibiotics.
Most of the modified antimicrobial regimens were found in urine cultures from symptomatic patients (Table 2). Of the 7 patients requiring therapy change because of a mismatch antibiotic–bacteria combination, 4 were empirically prescribed fluoroquinolones, 2 received levofloxacin, and 2 were prescribed ciprofloxacin. According to the most recent antibiogram at our facility, some organisms are resistant to fluoroquinolones, specifically Proteus mirabilis (P mirabilis) and Escherichia coli (E coli). These pathogens were the cause of urinary tract infections in 3 of 4 patients with fluoroquinolone prescriptions.
Through the CPRS dashboard, the pharmacist inadvertently identified 4 patients with positive culture results who were not on antibiotic therapy. These patients were contacted by telephone, and antibiotics were initiated for symptomatic patients after consultation with the HCP. The primary culture type intervened on was urine in 12 of 14 cases (86%). The other 2 culture types included oropharynx culture (7%) positive for an acute bacterial respiratory tract infection caused by group C Streptococcus and a stool culture (7%) positive for Pseudomonas aeruginosa (P aeruginosa). E coli (36%) was isolated in 5 cases and was the most commonly isolated organism. P
Discussion
This project was an innovative antimicrobial stewardship endeavor that helped initiate antibiotic interventions quickly and improve patient outcomes. The antimicrobial stewardship pharmacist independently performed interventions for patients without requiring HCP consultation, therefore decreasing HCP burden and possibly reducing time to assessment of culture results.
Limitations
The study results were limited due to its small sample size of antimicrobial interventions. The clinical pharmacist did not contact the patient when the antibiotic prescribed empirically by the HCP was appropriate for the isolated organism. Among the patients contacted, 3 were asymptomatic, did not require further antibiotic therapy, and no intervention was made. Provider education was deemed successful because HCPs did not request further information about the service. However, not all HCPs were provided education because of different shifts and inability to attend educational sessions. Closely working with lead physicians within the facility provided an alternate method for information dissemination.
The care coordination agreement allowed the pharmacist to make changes if patients had a current prescription for an antibiotic. In addition to the changes to antibiotics, this project improved HCP awareness of culture results even in cases of symptomatic patients who were not prescribed therapy. When this occurred, the pharmacist contacted the patient to assess symptoms and then notified the HCP if the patient was symptomatic.
Future Directions
Future endeavors regarding this project include modifying the scope of the service to allow pharmacists to prescribe antibiotics for patients with positive cultures and symptoms without empiric antibiotics in addition to continuing to modify empiric therapy. Additionally, improving dashboard efficiency through changes to include only isolated antibiotic mismatches rather than all antibiotics prescribed and all available cultures would reduce the pharmacists’ time commitment. Expanding to other parts of the medical center, including long-term care facilities and other outpatient clinics, would allow this service to reach more veterans. Integrating this service throughout the medical center will require continued HCP education and modifying care coordination agreements to include these facilities.
On a typical day, 60 to 90 minutes were spent navigating the dashboard and implementing this service. The CPRS dashboard should be modified to streamline patients identified to decrease the daily time commitment. Re-education of HCPs about resistance rates of fluoroquinolones and empirically prescribing these agents also will be completed based on empiric antibiotic interventions made with these agents throughout this project. Discussing HCP viewpoints on this service would be beneficial to ensure HCP satisfaction.
Conclusions
This pharmacy service and antimicrobial stewardship program reduced time patients were on inappropriate antibiotics. Pharmacists reviewed the dashboard daily under the scope of this project, which expedited needed changes and decreased provider burden because pharmacists were able to make changes without interrupting HCPs’ daily tasks, including patient care.
This program may also reduce readmissions. Patients who were still symptomatic were contacted could be given revised medication regimens without the patient returning to the facility for follow-up treatment. An interesting conclusion not included in the current scope of this service was possible reduced time to therapy initiation in cases of positive cultures and symptomatic patients without antibiotic therapy. If this occurred on the dashboard, patient’s symptoms could be assessed, and if symptoms were ongoing, the pharmacist contacted the HCP with a recommended antimicrobial therapy. In these cases, we were able to mail the antibiotic quickly, and many times, on the same day as this intervention through overnight mail. Implementation of a pharmacist-led antimicrobial review service has provided positive results overall for CVVAMC.
Acknowledgment
This material is the result of work supported with resources and the use of the facilities at the Carl Vinson VA Medical Center.
Increasing antibiotic resistance is an urgent threat to public health and establishing a review service for antibiotics could alleviate this problem. As use of antibiotics escalates, the risk of resistance becomes increasingly important. Each year, approximately 269 million antibiotics are dispensed and at least 30% are prescribed inappropriately.1 In addition to inappropriate prescribing, increased antibiotic resistance can be caused by patients not completing an antibiotic course as recommended or inherent bacterial mutations. According to the Centers for Disease Control and Prevention, each year approximately 3 million individuals contract an antibiotic-resistant infection.2 By 2050, it is projected that drug-resistant conditions could cause 300 million deaths and might be as disastrous to the economy as the 2008 global financial crisis.3 Ensuring appropriate use of antibiotic therapy through antimicrobial stewardship can help combat this significant public health issue.
Antimicrobial stewardship promotes appropriate use of antimicrobials to improve patient outcomes, reduce health care costs, and decrease antimicrobial resistance. One study found that nearly 50% of patients discharged from the emergency department with antibiotics required therapy modification after culture and susceptibility results were returned.4 Both the Infectious Disease Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) support incorporating a clinical pharmacist into culture reviews.3 Several institutions have implemented a pharmacist-led culture review service to improve antibiotic usage, which has shown positive results. A retrospective case-control study at University of Rochester Medical Center showed reduced time to positive culture review and to patient or health care provider (HCP) notification when emergency medicine pharmacists were involved in culture review.5 A retrospective study at Carolinas Medical Center-Northeast showed 12% decreased readmission rate using pharmacist-implemented culture review compared with HCP review.6 Results from previous studies showed an overall improvement in patient safety through decreased use of inappropriate agents and reduced time on inappropriate antibiotic therapy.
Establishing a pharmacist-led culture review service at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia, could decrease the time to review of positive culture results, time to patient or HCP notification, and readmission rates. CVVAMC provides outpatient primary care services to about 30,000 veterans in the central and southern regions of Georgia. Our facility has executed an antimicrobial stewardship program based on guidelines published in 2016 by IDSA and SHEA to guide optimal use of antibiotics. Clinical pharmacists play an active role in antimicrobial stewardship throughout the facility. Clinical responsibilities of the antimicrobial stewardship pharmacist include assessing therapy for inappropriate dual anaerobic coverage, evaluating inpatient culture results within 48 hours, dosing and monitoring antibiotic therapy, including vancomycin and aminoglycosides, and implementing IV to by-mouth conversions for appropriate patients. HCPs involved with antimicrobial stewardship could order an array of tests to assess a veteran’s condition, including cultures, when an infection is suspected.
Culture results take about 3 to 5 days, then HCPs evaluate the result to ensure current antibiotic therapy is appropriate. Patients might not receive timely follow-up because HCPs often have many laboratory alerts to sift through every day, and a protocol is not in place for pharmacists to adjust outpatient antimicrobial regimens based on culture results. Before implementing this project, there was no outpatient service for pharmacists to impact culture and susceptibility review. This project was initiated because a lead physician identified difficulty reviewing culture and susceptibility results. HCPs often work on rotating schedules, and there was a concern about possible delay in follow-up of results if a HCP was not scheduled to work for a period of time.
The purpose of this project was to implement an outpatient, pharmacist-managed culture and susceptibility review service to improve patient outcomes, including decreasing and preventing inappropriate antibiotic use. The primary objective was to design and implement a pharmacist-led review service to intervene in cases of mismatched antibiotic bacteria combinations. Secondary objectives included identifying most common culture types and organisms encountered and intervened on at our facility.
Quality Improvement Project
This quality improvement project was approved by the CVVAMC Pharmacy and Therapeutics Committee. Members of the medical review board signed a care coordination agreement between pharmacy and outpatient HCPs to permit pharmacist interventions involving optimization of antibiotic therapy. This agreement allowed pharmacists to make changes to existing antimicrobial regimens within their scope of practice (SOP) without requiring discussion with HCPs. A protocol was also developed to guide pharmacist modification of antimicrobial therapy based on current antimicrobial guidelines.7 This protocol was based on commonly isolated organisms and local resistance patterns and provided guidance for antibiotic treatment based on culture type (ie, skin and soft tissue infection, urine, etc). Computerized Patient Record System (CPRS) note templates were also developed for interventions performed, and patient follow-up after antibiotic regimens were completed (eAppendix 1
Program Inclusion
Veterans were included in this project if they presented to primary care or urgent care clinics for therapy; had positive culture and sensitivity results; and were prescribed an empiric antibiotic. Veterans were not eligible for this project if they were not receiving antibiotic therapy, with or without pending or resulted culture results shown in CPRS.
Implementation
Data gathered through a CPRS dashboard from August 2019 to February 2020 identified patients with pending or completed culture results in urgent care and primary care settings (eAppendix 4). The dashboard was created specifically for this project to show patient details that included initial antibiotic(s) prescribed and preliminary and final culture results. After a mismatched combination was identified, pharmacists contacted patients and assessed symptoms. If a patient was still symptomatic, the pharmacist changed the antibiotic regimen and educated the patient about this change. The pharmacist documented an intervention note in CPRS and added the HCP as a signer so he or she would be aware of the change. The clinical pharmacist followed up after regimens were complete. At this time, the pharmacist assessed patients to ensure the medication was taken as directed (eg, number of days of therapy, how many tablets per day, etc), to discuss any reported adverse effects, and to assess resolution of symptoms. If a patient still had symptoms, the pharmacist contacted the patient’s primary care provider. If the veteran could not be contacted after 3 consecutive attempts via phone, a certified letter was mailed. If patients were asymptomatic at the time of the call, the pharmacist documented the lack of symptoms and added the HCP as a signer for awareness purposes. HCPs continued to practice as usual while this service was implemented.
Observations
Using the culture and susceptibility dashboard, the pharmacist identified 675 patients as having a pending culture (Table 1). Among these patients, 320 results were positive, and were taking antibiotics empirically. Out of the 320 patients who met inclusion criteria, 10 required pharmacist intervention. After contacting the veterans, 7 required regimen changes because their current antibiotic was not susceptible to the isolated organism. Three additional patients were contacted because of a mismatch between the empiric antibiotic and culture result. Antibiotic therapy was not modified because these patients were asymptomatic at the time the clinical pharmacist contacted them. These patient cases were discussed with the HCP before documenting the intervention to prevent initiation of unwarranted antibiotics.
Most of the modified antimicrobial regimens were found in urine cultures from symptomatic patients (Table 2). Of the 7 patients requiring therapy change because of a mismatch antibiotic–bacteria combination, 4 were empirically prescribed fluoroquinolones, 2 received levofloxacin, and 2 were prescribed ciprofloxacin. According to the most recent antibiogram at our facility, some organisms are resistant to fluoroquinolones, specifically Proteus mirabilis (P mirabilis) and Escherichia coli (E coli). These pathogens were the cause of urinary tract infections in 3 of 4 patients with fluoroquinolone prescriptions.
Through the CPRS dashboard, the pharmacist inadvertently identified 4 patients with positive culture results who were not on antibiotic therapy. These patients were contacted by telephone, and antibiotics were initiated for symptomatic patients after consultation with the HCP. The primary culture type intervened on was urine in 12 of 14 cases (86%). The other 2 culture types included oropharynx culture (7%) positive for an acute bacterial respiratory tract infection caused by group C Streptococcus and a stool culture (7%) positive for Pseudomonas aeruginosa (P aeruginosa). E coli (36%) was isolated in 5 cases and was the most commonly isolated organism. P
Discussion
This project was an innovative antimicrobial stewardship endeavor that helped initiate antibiotic interventions quickly and improve patient outcomes. The antimicrobial stewardship pharmacist independently performed interventions for patients without requiring HCP consultation, therefore decreasing HCP burden and possibly reducing time to assessment of culture results.
Limitations
The study results were limited due to its small sample size of antimicrobial interventions. The clinical pharmacist did not contact the patient when the antibiotic prescribed empirically by the HCP was appropriate for the isolated organism. Among the patients contacted, 3 were asymptomatic, did not require further antibiotic therapy, and no intervention was made. Provider education was deemed successful because HCPs did not request further information about the service. However, not all HCPs were provided education because of different shifts and inability to attend educational sessions. Closely working with lead physicians within the facility provided an alternate method for information dissemination.
The care coordination agreement allowed the pharmacist to make changes if patients had a current prescription for an antibiotic. In addition to the changes to antibiotics, this project improved HCP awareness of culture results even in cases of symptomatic patients who were not prescribed therapy. When this occurred, the pharmacist contacted the patient to assess symptoms and then notified the HCP if the patient was symptomatic.
Future Directions
Future endeavors regarding this project include modifying the scope of the service to allow pharmacists to prescribe antibiotics for patients with positive cultures and symptoms without empiric antibiotics in addition to continuing to modify empiric therapy. Additionally, improving dashboard efficiency through changes to include only isolated antibiotic mismatches rather than all antibiotics prescribed and all available cultures would reduce the pharmacists’ time commitment. Expanding to other parts of the medical center, including long-term care facilities and other outpatient clinics, would allow this service to reach more veterans. Integrating this service throughout the medical center will require continued HCP education and modifying care coordination agreements to include these facilities.
On a typical day, 60 to 90 minutes were spent navigating the dashboard and implementing this service. The CPRS dashboard should be modified to streamline patients identified to decrease the daily time commitment. Re-education of HCPs about resistance rates of fluoroquinolones and empirically prescribing these agents also will be completed based on empiric antibiotic interventions made with these agents throughout this project. Discussing HCP viewpoints on this service would be beneficial to ensure HCP satisfaction.
Conclusions
This pharmacy service and antimicrobial stewardship program reduced time patients were on inappropriate antibiotics. Pharmacists reviewed the dashboard daily under the scope of this project, which expedited needed changes and decreased provider burden because pharmacists were able to make changes without interrupting HCPs’ daily tasks, including patient care.
This program may also reduce readmissions. Patients who were still symptomatic were contacted could be given revised medication regimens without the patient returning to the facility for follow-up treatment. An interesting conclusion not included in the current scope of this service was possible reduced time to therapy initiation in cases of positive cultures and symptomatic patients without antibiotic therapy. If this occurred on the dashboard, patient’s symptoms could be assessed, and if symptoms were ongoing, the pharmacist contacted the HCP with a recommended antimicrobial therapy. In these cases, we were able to mail the antibiotic quickly, and many times, on the same day as this intervention through overnight mail. Implementation of a pharmacist-led antimicrobial review service has provided positive results overall for CVVAMC.
Acknowledgment
This material is the result of work supported with resources and the use of the facilities at the Carl Vinson VA Medical Center.
1. Centers for Disease Control and Prevention. Antibiotic use in outpatient settings, 2017: progress and opportunities. Accessed August 19, 2021. https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html
2. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Accessed August 19, 2021. https://www.cdc.gov/drugresistance/index.html
3. Jonas OB, Irwin A, Berthe FCJ, Le Gall FG, Marquez PV. Drug-resistant infections: a threat to our economic future. March 2017. Accessed August 19, 2021. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/323311493396993758/final-report
4. Davis LC, Covey RB, Weston JS, Hu BBY, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5)(suppl 1):S49-S56. doi:10.2146/sp150036
5. Baker SN, Acquisto NM, Ashley ED, Fairbanks RJ, Beamish SE, Haas CE. Pharmacist-managed antimicrobial stewardship program for patients discharged from the emergency department. J Pharm Pract. 2012;25(2):190-194. doi:10.1177/0897190011420160
6 Randolph TC, Parker A, Meyer L, Zeina R. Effect of a pharmacist-managed culture review process on antimicrobial therapy in an emergency department. Am J Health Syst Pharm. 2011;68(10):916-919. doi:10.2146/ajhp090552
7. Infectious Diseases Society of America. Infectious diseases society of America guidelines 2019. Accessed August 24, 2021. https://www.idsociety.org/practice-guideline/practice-guidelines/#/+/0/date_na_dt/desc
1. Centers for Disease Control and Prevention. Antibiotic use in outpatient settings, 2017: progress and opportunities. Accessed August 19, 2021. https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html
2. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Accessed August 19, 2021. https://www.cdc.gov/drugresistance/index.html
3. Jonas OB, Irwin A, Berthe FCJ, Le Gall FG, Marquez PV. Drug-resistant infections: a threat to our economic future. March 2017. Accessed August 19, 2021. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/323311493396993758/final-report
4. Davis LC, Covey RB, Weston JS, Hu BBY, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5)(suppl 1):S49-S56. doi:10.2146/sp150036
5. Baker SN, Acquisto NM, Ashley ED, Fairbanks RJ, Beamish SE, Haas CE. Pharmacist-managed antimicrobial stewardship program for patients discharged from the emergency department. J Pharm Pract. 2012;25(2):190-194. doi:10.1177/0897190011420160
6 Randolph TC, Parker A, Meyer L, Zeina R. Effect of a pharmacist-managed culture review process on antimicrobial therapy in an emergency department. Am J Health Syst Pharm. 2011;68(10):916-919. doi:10.2146/ajhp090552
7. Infectious Diseases Society of America. Infectious diseases society of America guidelines 2019. Accessed August 24, 2021. https://www.idsociety.org/practice-guideline/practice-guidelines/#/+/0/date_na_dt/desc
The Expansion of Associated Health Training in the VA
The US Department of Veterans Affairs (VA) is the largest health care delivery system in the United States, comprising 1293 sites of care, including 171 medical centers.1 One of the 4 statutory missions of the VA is to train health care professionals (HCPs) to meet the needs of the VA and the nation.2 Through partnerships with more than 1800 accredited colleges, universities, and training programs, the VA provides training annually to nearly 118,000 health professions trainees (HPTs) across a variety of health care professions, and all of whom provide direct clinical care to veterans.3
In the VA, the Office of Academic Affiliations (OAA) is charged with overseeing health professions training and the VA’s partnership with medical and associated health (AH) professions schools, which was first codified in Policy Memorandum No. 2 in 1946.4,5 Given the scope and breadth of health professions education offered through the VA, OAA is in a unique position to address health care shortage areas as well as influence the educational standards for certain professions.
Many of these health care professions fall under the rubric of AH, which include mental health (MH) specialties, rehabilitative specialties, and others. These professions are critical to address in the expanding world of health care in the United States with its increased specialization and emphasis on coordination of care with interprofessional teams. During the 2019/2020 academic year, the VA provided clinical training to approximately 21,000 AH HPTs from > 40 professions with just over 20% receiving financial support through the OAA. Of the HPTs who train at VA without compensation, most spend shorter amounts of time in clinical rotations in the VA, are in pregraduate-degree education programs where payment for clinical rotations is not expected and may not be eligible for hire immediately on completion of their clinical training experience. The 17 funded professions have been strategically selected by the OAA to ensure a robust pipeline of HCPs to meet the needs of veterans and the nation.
To meet the demands of AH professionals (AHPs), the OAA implemented targeted expansion over the past 10 years. While not exhaustive, this paper describes several expansion efforts based on VA special initiatives, including enhancing clinical access in rural settings and shifting toward postgraduate-degree training and specialization. By aligning expansion with VA priorities as well as trends in health care more broadly, the OAA can ensure that there is a supply of well-trained AHPs who have developed the requisite competencies to contribute to our nation’s health care needs. Further, expansion can help train and recruit health professionals who can be hired into VA positions ready to care for the complex needs of veterans.
Associated Health Professionals
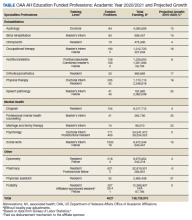
Overseen by the OAA, AH expansion is designed to address the specific needs of the VA and the US health care system. Data from the VA Workforce Management and Consulting (WMC) shows that the VA employment of AHPs has grown from 87,351 AHPs hired in fiscal year (FY) 2010 to 119,120 as of April 2020. This represents an average yearly growth rate of 3.4% and a total growth rate of 36%. The Bureau of Labor Statistics predictions for 2019/2029 suggest that certain AHPs are expected to have a 10-year growth rates of 20% or more to meet the changing health care needs of patients especially as the population ages; the growth rates for many AHPs far surpasses that of physicians, which is anticipated to be 4% (Table).6,7 The VA WMC expects an additional 52,283 AHPs will be hired by the VA by FY 2030 based on the 10-year average growth rate (Kali Clark, Veterans Health Administration Workforce Management and Consulting Office, email communication, May 28, 2020).
One of the driving forces behind the growth rate is the move toward using AHPs to supplement health care for a variety of health conditions.8,9 Examples include the integration of rehabilitation professionals, alternative care professionals (eg, massage therapists, practitioners who offer training in yoga and meditation), chiropractors, MH professionals, and pharmacists in the treatment of chronic pain, the use of a wider range of professionals in the treatment of MH conditions, and the integration of MH professionals into traditional medical settings, such as primary care. This intentional move to a more well-integrated model of interprofessional care is apparent in many other health care systems throughout the United States. Within the VA, this shift may be most evident through the introduction of the Whole Health model of care. The Whole Health model of care uses an interprofessional team to assess and care for veterans, using a personalized health plan addressing medical and MH conditions as well as behavioral, social, or spiritual concerns.10 The Whole Health model of care provides veterans with access to a variety of health care services, including but not limited to MH services, spiritual interventions, exercise-based programs, yoga, meditation, and nutrition counseling.
The OAA and AH education division have focused expansion to meet the increased need for MH and rehabilitation providers, to enhance interprofessional education, and to emphasize postgraduate-degree clinical training. This focus reflects the trends seen in health care training broadly throughout the nation and the intentional pivot is a model of these trends and a model for how to intentionally address these trends. Specific to the VA, focused expansion plans have allowed OAA to address VA strategic initiatives such as pain management and caring for rural veterans.
Funded Training Positions
As a result of recent AH expansion efforts, there has been a 33% increase in stipend-funded positions during the past 10 years, a rate that directly corresponds with the growth of AHPs in the VA. Recent AH expansion efforts can contribute to a particularly positive impact in highly rural and underserved areas where recruiting providers remains challenging.
The OAA launched the Mental Health Education Expansion (MHEE) initiative in 2012, which has now added 782 funded training slots across 10 health professions, 8 of which are psychology, pharmacy, chaplaincy, professional MH counseling, marriage and family therapy (MFT), social work (SW), occupational therapy (OT), and physician assistant (PA). Through the MHEE initiative, the VA has established funded internships for licensed professional mental health counselors and marriage and family therapists, as these professions are targeted for expanding the overall MH workforce in the VA. The OAA currently funds more than 50 total HPT positions for these 2 professions with an aim of increasing their recruitment to the VA MH workforce over the next decade. The MHEE is aligned with specified VA priorities to train a future VA workforce prepared for interprofessional collaboration and clinical care in an increasingly integrated and complex environment. This expansion effort also aligns with an increasing understanding of the importance of addressing the MH needs of our nation by ensuring there is an adequate supply of competent, well-trained clinicians entering the workforce.
The OAA has created and expanded residencies and fellowships in multiple rehabilitation professions, including chiropractic, physical therapy (PT), and OT. With the increased focus on the management of chronic pain in the nation combined with a specific emphasis on this clinical need in the VA, chiropractors have been deemed essential HCPs. In 2014, the VA established 5 chiropractic residency programs while partnering with the Council on Chiropractic Education to develop accreditation standards for residency training. OAA’s efforts have yielded 5 accredited residency programs, the first in the United States. In 2020, the VA doubled the number of available chiropractic residency programs, and future expansion is anticipated. Since 2010, PT residencies have expanded from 1 to 28 programs (42 funded positions) across 4 board certification specialties: cardiovascular-pulmonary, geriatric, neurologic, and orthopedic. Similarly, the VA was one of the first organizations to achieve accreditation for OT fellowships; there are currently 5 accredited OT fellowship programs across 3 areas of practice: assistive technology, MH, and physical rehabilitation. The VA OT fellowship program focused on assistive technology is the only program in the United States at this time.
Interprofessional Education
As one of the primary focus areas for AH expansion, interprofessional education (IPE) has been recognized as increasingly important for the provision of health care and the development of HPT programs. IPE can develop professionals who appreciate the roles of diverse professions and can use teamwork to enhance clinical outcomes for patients.11 There also are a growing number of professional organizations supporting the Interprofessional Education Collaborative with many representing AHPs.12 Collaboration across HCPs is an important way of reducing health care costs by enhancing clinical outcomes, communication, and teamwork.13-16 The VA and the nation’s health care system benefit from the by-products of interprofessional collaboration through investment in targeted training programs. In each phase of the AH expansion, special consideration was given to applicant programs offering unique and innovative clinical and educational experiences consistent with the promotion of interprofessional care. In doing so, increased numbers of AH HPTs have engaged in team-based clinical care.
Pain Management Pharmacy
The efforts of AH to align expansion with high-priority agency-wide efforts has resulted in the growth of pharmacy residency positions focused on pain management. Pharmacy postgraduate year (PGY) 2 residencies focusing on opioid reduction are an example of VA efforts to improve response to managing chronic pain and the long-term risks from opioid use during this national public health crisis.17 These residency programs focus on strategies to reduce the use of opioid medications in the clinical setting and teaching effective clinical interventions for reducing the rates of opioid addiction in veterans while still recognizing the need to identify and treat chronic pain. Before expansion efforts in 2018, there were 6 pharmacy residency programs focused on opioid use reduction in the VA, 8 pharmacy PGY2 residency positions were added in academic year 2019/2020, an additional 5 positions are being added in academic year 2021/2022 with the explicit goal of managing patients with high-risk chronic pain.
Rural Health
The lack of MH providers in rural areas has received much attention and is particularly important in the VA because veterans are more likely to live in less populated areas.18 The VA mandate to address this population was codified by the creation of the Office of Rural Health in 2006 via 38 USC § 7308.19Creating health professions training programs in rural settings provides HPTs the opportunity to learn professional competencies and train with faculty knowledgeable about this population—all of which provide a comprehensive training experience and serve as a recruitment pathway to hire HPTs into staff positions at these sites.19
When MHEE was initiated, not all regions of the country had funded VA psychology training programs, and this geographic gap in psychology training was a contributing factor to recruitment difficulties for psychologists in rural areas. As a result, the request for proposal process in the OAA highlighted and incentivized rurality when considering funding for new training programs. The OAA defined rurality as the number of patients served by the proposed health care facility who lived in a rural or highly rural zip code according to VA Support Service Center Capital Assets data.20 As a result, VA psychology doctoral internships expanded to be available in all states, the District of Columbia, and Puerto Rico. MH training programs were started in the highly rural states of Montana and Wyoming. These expansion efforts promise to be an essential component to addressing the gaps in coverage in rural settings as noted in recent research.21
Pregraduate to Postgraduate Programs
The OAA AH education division supports a significant number of pregraduate-degree and postgraduate-degree training. Some professions, such as psychology, pharmacy, SW, PT, speech pathology, OT, and nutrition/dietetics receive funding at both levels of training. More recent, the OAA has started to move funding from pregraduate to postgraduate-degree positions, specifically within professions where pregraduate funding is uncommon for both federal and nonfederal training positions. The effort is designed to better align stipend-paid training programs with the VA Professional Qualification Standards and the final level of training required for employment in the VA.22This means that HPTs receive stipend support during the highest level of their clinical training before degree conferral, eligibility for VA employment, or while participating in a postgraduate-degree residency or fellowship.
Additionally, this shift in focus and the resulting internal assessment of professions has allowed the OAA to fund more specialized training opportunities, which sometimes go beyond what is required by accrediting bodies or for recruitment into VA positions. For example, the OAA is supporting SW fellowship programs and PA residency positions to allow for greater specialization within these professions; the accrediting agencies for both professions have recently finalized their accreditation standards, and the OAA played a role in moving these standards forward.
While postgraduate residencies and fellowships are not required for all AH HPTs or for employment in the VA, there is a shift in some professions to encourage postgraduate training in advanced competencies in specialized areas. Participation in a residency or fellowship training program affords HPTs additional time and diverse clinical experiences to acquire clinical skills, all while under the supervision of a highly trained practitioner. This additional training also allows for a longitudinal assessment of the HPT to ensure an alignment of the HPTs’ knowledge, abilities, and skills with the expectation should they pursue VA employment.
In academic year 2019/2020, the OAA AH education division in conjunction with the PA national program office transitioned the entirety of the PA pregraduate-degree student positions (415 funded positions) to residency positions, increasing residency positions from 19 to 32 funded positions. This shift in emphasis for funding did not negatively impact the total number of pregraduate PA students receiving training in the VA and has created a pipeline of residency graduates who are ready to enter VA staff positions. To date, the VA has 14 PA residency programs across 3 specialties: emergency medicine (EM), MH, and primary care/geriatrics. Of these tracks, the VA offers 5 EM and 4 MH residencies that position graduates to be eligible for specialty certification. The National Commission on Certification of Physician Assistants established Certificates of Added Qualifications (CAQ) to recognize and document specialty knowledge, skills, and experience. The VA MH residency programs have been established to align with the CAQ expectations, and residents immediately qualify to take the CAQ examination after the completion of training.
Currently, the same process to move pregraduate to postgraduate funding is being implemented for PT and OT. Within the PT profession, there is increased momentum toward residency and fellowship training programs to respond to the changing complexity of the health care systemand reduce the need of complex care to be provided by non-VA providers in the community.23 Both PT and OT have entered the initial phases of transitioning to residency or fellowship-funded positions. The OAA is partnering with these professions to move positions to postgraduate degree within the next 3 years with a commensurate increase in funding. The initial data indicate that 80% of graduated VA PT residents are board-certification eligible, and 89% of those who are eligible passed the examination on their first attempt.
Since 2013, the VA psychology training also has realized a growth in postgraduate-degree residencies. Psychology residency positions have increased 99% to 453 funded positions. This growth represents increased specialization in neuropsychology, geropsychology, rehabilitation psychology, and health psychology. Additionally, postgraduate residencies meet most jurisdictional requirements for postdoctoral supervised experience and better prepare HPTs to enter specialty staff positions that are necessary to care for aging veterans.
Additional professions are being targeted for postgraduate-degree training programs, including dietetics and speech pathology, to align with upcoming changes in the qualification standards for employment. While the process to transition positions to postgraduate-degree training programs can take 3 to 5 years, the outcomes are expected to result in better prepared HPTs who can fill staff vacancies in the VA.
Conclusions
Through its funding and oversight of numerous professions, the OAA is uniquely situated to adapt its portfolio to meet the needs of the VA and the nation. Over the past 10 years, the OAA has expanded its total number of HPT positions to enhance interprofessional care, respond to the VA’s strategic initiatives, address the care needs of rural veterans, and shift positions to postgraduate training programs. The OAA’s investment in high-quality training programs builds a strong health care workforce ready to meet the needs of an increasingly complex and integrated health care environment.
The OAA anticipates future expansion, especially related to promoting rural training opportunities and shifting to postgraduate training programs as a means of promoting advanced health care and health system competencies while continuing to align with workforce projections. Furthermore, while there are data on the percentage of VA staff who participated in OAA training program through the VA All Employee Survey (AES), the range for AH professions is wide. For example, about 37% of rehabilitative staff reported participating in an OAA training program, and 72% of VA psychologists reported having an OAA training experience. To maximize the hiring of HPTs, OAA will continue its partnership with WMC to enact programs aimed at streamlining the hiring process so that veterans have access to HCPs who are specifically trained to work with them.
1. US Department of Veterans Affairs. Providing health care for veterans. Updated April 23, 2021. Accessed July 15, 2021. https://www.va.gov/health
2. Veterans’ Benefits. 38 USC §7301 and §7302 (1991). Accessed May 18, 2020. https://www.govinfo.gov/content/pkg/USCODE-2018-title38/pdf/USCODE-2018-title38-partV-chap73-subchapI-sec7302.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2021. Accessed July 15, 2021. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
4. US Department of Veterans Affairs, VHA Office of Academic Affiliations. VA Policy Memorandum # 2. Policy in association of veterans’ hospitals with medical schools. Published January 30, 1946. Accessed October 13, 2020. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Mission of the office of academic affiliations. Updated September 24, 2019. Accessed July 15, 2021. https://www.va.gov/oaa/oaa_mission.asp
6. US Bureau of Labor Statistics, Office of Occupational Statistics and Employment Projections Occupational Outlook Handbook. Healthcare occupations. Updated May 14, 2021. Accessed July 15, 2021. https://www.bls.gov/ooh/healthcare/home.htm
7. Windmill IM, Freeman BA. Demand for audiology services: 30-yr projections and impact on academic programs. J Am Acad Audiol. 2013;24(5):407-416. doi:10.3766/jaaa.24.5.7
8. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. HRSA health workforce: behavioral health workforce projections, 2017-2030. Accessed July 15, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/bh-workforce-projections-fact-sheet.pdf
9. Centers for Disease Control and Prevention, National Center for Health Statistics. NCHS data brief, No. 325. Use of yoga, meditation, and chiropractors among US adults aged 18 and over. Published November 2018. Accessed September 24, 2020. https://www.cdc.gov/nchs/data/databriefs/db325-h.pdf
10. US Department of Veterans Affairs, Veterans Health Administration Whole Health. Updated July 6, 2021. Accessed July 15, 2021. https://www.va.gov/wholehealth
11. Clark KM. Interprofessional education: making our way out of the silos. Respir Care. 2018;63(5): 637-639. doi:10.4187/respcare.06234
12. Interprofessional Education Collaborative. What is interprofessional education (IPE)? Accessed July 15, 2021. https://www.ipecollaborative.org/about-us
13. Nester J. The importance of interprofessional practice and education in the era of accountable care. N C Med J. 2016;77(2):128-132. doi.10.18043/ncm.77.2.128
14.. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6): 313-319. doi:10.1097/NNA.0000000000000492
15. Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34(2):125-184. doi:10.1016/j.kjms.2017.12.009
16. Ateah CA, Snow W, Wenter P, et al. Stereotyping as a barrier to collaboration: does interprofessional education make a difference? Nurse Educ Today. 2011;31(2):208-213. doi:10.1016/j.nedt.2010.06.004
17. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for Managing Opioid Therapy for Chronic Pain. Published May 7, 1991. Updated February 2017. Accessed July 15, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
18. US Department of Veterans Affairs, Office of Rural Health. VHA office of rural health. Updated March 17, 2021. Accessed July 15, 2021. https://www.ruralhealth.va.gov19. Curran V, Rourke J. The role of medical education in the recruitment and retention of rural physicians. Med Teach. 2004;26(3):265-272. doi:10.1080/0142159042000192055
20. US Department of Veterans Affairs. VHA Support Service Center Capital Assets. Updated December 1, 2020. Accessed July 15, 2021. https://www.data.va.gov/dataset/VHA-Support-Service-Center-Capital-Assets-VSSC-/2fr5-sktm
21. Domino ME, Lin CC, Morrisey JP, et al. Training psychologists for rural practice: exploring opportunities and constraints. J Rural Health. 2019;35(1):35-41. doi:10.1111/jrh.12299
22. US Department of Veterans Affairs. VA Directive 5005: Staffing. Published March 4, 2020. Accessed July 15, 2021. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=1140&FType=2
23. Furze JA, Freeman BA. Physical therapy and fellowship education: reflections on the past, present, and future. Phys Ther. 2016;96(7):949-960. doi:10.2522/ptj.20150473
The US Department of Veterans Affairs (VA) is the largest health care delivery system in the United States, comprising 1293 sites of care, including 171 medical centers.1 One of the 4 statutory missions of the VA is to train health care professionals (HCPs) to meet the needs of the VA and the nation.2 Through partnerships with more than 1800 accredited colleges, universities, and training programs, the VA provides training annually to nearly 118,000 health professions trainees (HPTs) across a variety of health care professions, and all of whom provide direct clinical care to veterans.3
In the VA, the Office of Academic Affiliations (OAA) is charged with overseeing health professions training and the VA’s partnership with medical and associated health (AH) professions schools, which was first codified in Policy Memorandum No. 2 in 1946.4,5 Given the scope and breadth of health professions education offered through the VA, OAA is in a unique position to address health care shortage areas as well as influence the educational standards for certain professions.
Many of these health care professions fall under the rubric of AH, which include mental health (MH) specialties, rehabilitative specialties, and others. These professions are critical to address in the expanding world of health care in the United States with its increased specialization and emphasis on coordination of care with interprofessional teams. During the 2019/2020 academic year, the VA provided clinical training to approximately 21,000 AH HPTs from > 40 professions with just over 20% receiving financial support through the OAA. Of the HPTs who train at VA without compensation, most spend shorter amounts of time in clinical rotations in the VA, are in pregraduate-degree education programs where payment for clinical rotations is not expected and may not be eligible for hire immediately on completion of their clinical training experience. The 17 funded professions have been strategically selected by the OAA to ensure a robust pipeline of HCPs to meet the needs of veterans and the nation.
To meet the demands of AH professionals (AHPs), the OAA implemented targeted expansion over the past 10 years. While not exhaustive, this paper describes several expansion efforts based on VA special initiatives, including enhancing clinical access in rural settings and shifting toward postgraduate-degree training and specialization. By aligning expansion with VA priorities as well as trends in health care more broadly, the OAA can ensure that there is a supply of well-trained AHPs who have developed the requisite competencies to contribute to our nation’s health care needs. Further, expansion can help train and recruit health professionals who can be hired into VA positions ready to care for the complex needs of veterans.
Associated Health Professionals
Overseen by the OAA, AH expansion is designed to address the specific needs of the VA and the US health care system. Data from the VA Workforce Management and Consulting (WMC) shows that the VA employment of AHPs has grown from 87,351 AHPs hired in fiscal year (FY) 2010 to 119,120 as of April 2020. This represents an average yearly growth rate of 3.4% and a total growth rate of 36%. The Bureau of Labor Statistics predictions for 2019/2029 suggest that certain AHPs are expected to have a 10-year growth rates of 20% or more to meet the changing health care needs of patients especially as the population ages; the growth rates for many AHPs far surpasses that of physicians, which is anticipated to be 4% (Table).6,7 The VA WMC expects an additional 52,283 AHPs will be hired by the VA by FY 2030 based on the 10-year average growth rate (Kali Clark, Veterans Health Administration Workforce Management and Consulting Office, email communication, May 28, 2020).
One of the driving forces behind the growth rate is the move toward using AHPs to supplement health care for a variety of health conditions.8,9 Examples include the integration of rehabilitation professionals, alternative care professionals (eg, massage therapists, practitioners who offer training in yoga and meditation), chiropractors, MH professionals, and pharmacists in the treatment of chronic pain, the use of a wider range of professionals in the treatment of MH conditions, and the integration of MH professionals into traditional medical settings, such as primary care. This intentional move to a more well-integrated model of interprofessional care is apparent in many other health care systems throughout the United States. Within the VA, this shift may be most evident through the introduction of the Whole Health model of care. The Whole Health model of care uses an interprofessional team to assess and care for veterans, using a personalized health plan addressing medical and MH conditions as well as behavioral, social, or spiritual concerns.10 The Whole Health model of care provides veterans with access to a variety of health care services, including but not limited to MH services, spiritual interventions, exercise-based programs, yoga, meditation, and nutrition counseling.
The OAA and AH education division have focused expansion to meet the increased need for MH and rehabilitation providers, to enhance interprofessional education, and to emphasize postgraduate-degree clinical training. This focus reflects the trends seen in health care training broadly throughout the nation and the intentional pivot is a model of these trends and a model for how to intentionally address these trends. Specific to the VA, focused expansion plans have allowed OAA to address VA strategic initiatives such as pain management and caring for rural veterans.
Funded Training Positions
As a result of recent AH expansion efforts, there has been a 33% increase in stipend-funded positions during the past 10 years, a rate that directly corresponds with the growth of AHPs in the VA. Recent AH expansion efforts can contribute to a particularly positive impact in highly rural and underserved areas where recruiting providers remains challenging.
The OAA launched the Mental Health Education Expansion (MHEE) initiative in 2012, which has now added 782 funded training slots across 10 health professions, 8 of which are psychology, pharmacy, chaplaincy, professional MH counseling, marriage and family therapy (MFT), social work (SW), occupational therapy (OT), and physician assistant (PA). Through the MHEE initiative, the VA has established funded internships for licensed professional mental health counselors and marriage and family therapists, as these professions are targeted for expanding the overall MH workforce in the VA. The OAA currently funds more than 50 total HPT positions for these 2 professions with an aim of increasing their recruitment to the VA MH workforce over the next decade. The MHEE is aligned with specified VA priorities to train a future VA workforce prepared for interprofessional collaboration and clinical care in an increasingly integrated and complex environment. This expansion effort also aligns with an increasing understanding of the importance of addressing the MH needs of our nation by ensuring there is an adequate supply of competent, well-trained clinicians entering the workforce.
The OAA has created and expanded residencies and fellowships in multiple rehabilitation professions, including chiropractic, physical therapy (PT), and OT. With the increased focus on the management of chronic pain in the nation combined with a specific emphasis on this clinical need in the VA, chiropractors have been deemed essential HCPs. In 2014, the VA established 5 chiropractic residency programs while partnering with the Council on Chiropractic Education to develop accreditation standards for residency training. OAA’s efforts have yielded 5 accredited residency programs, the first in the United States. In 2020, the VA doubled the number of available chiropractic residency programs, and future expansion is anticipated. Since 2010, PT residencies have expanded from 1 to 28 programs (42 funded positions) across 4 board certification specialties: cardiovascular-pulmonary, geriatric, neurologic, and orthopedic. Similarly, the VA was one of the first organizations to achieve accreditation for OT fellowships; there are currently 5 accredited OT fellowship programs across 3 areas of practice: assistive technology, MH, and physical rehabilitation. The VA OT fellowship program focused on assistive technology is the only program in the United States at this time.
Interprofessional Education
As one of the primary focus areas for AH expansion, interprofessional education (IPE) has been recognized as increasingly important for the provision of health care and the development of HPT programs. IPE can develop professionals who appreciate the roles of diverse professions and can use teamwork to enhance clinical outcomes for patients.11 There also are a growing number of professional organizations supporting the Interprofessional Education Collaborative with many representing AHPs.12 Collaboration across HCPs is an important way of reducing health care costs by enhancing clinical outcomes, communication, and teamwork.13-16 The VA and the nation’s health care system benefit from the by-products of interprofessional collaboration through investment in targeted training programs. In each phase of the AH expansion, special consideration was given to applicant programs offering unique and innovative clinical and educational experiences consistent with the promotion of interprofessional care. In doing so, increased numbers of AH HPTs have engaged in team-based clinical care.
Pain Management Pharmacy
The efforts of AH to align expansion with high-priority agency-wide efforts has resulted in the growth of pharmacy residency positions focused on pain management. Pharmacy postgraduate year (PGY) 2 residencies focusing on opioid reduction are an example of VA efforts to improve response to managing chronic pain and the long-term risks from opioid use during this national public health crisis.17 These residency programs focus on strategies to reduce the use of opioid medications in the clinical setting and teaching effective clinical interventions for reducing the rates of opioid addiction in veterans while still recognizing the need to identify and treat chronic pain. Before expansion efforts in 2018, there were 6 pharmacy residency programs focused on opioid use reduction in the VA, 8 pharmacy PGY2 residency positions were added in academic year 2019/2020, an additional 5 positions are being added in academic year 2021/2022 with the explicit goal of managing patients with high-risk chronic pain.
Rural Health
The lack of MH providers in rural areas has received much attention and is particularly important in the VA because veterans are more likely to live in less populated areas.18 The VA mandate to address this population was codified by the creation of the Office of Rural Health in 2006 via 38 USC § 7308.19Creating health professions training programs in rural settings provides HPTs the opportunity to learn professional competencies and train with faculty knowledgeable about this population—all of which provide a comprehensive training experience and serve as a recruitment pathway to hire HPTs into staff positions at these sites.19
When MHEE was initiated, not all regions of the country had funded VA psychology training programs, and this geographic gap in psychology training was a contributing factor to recruitment difficulties for psychologists in rural areas. As a result, the request for proposal process in the OAA highlighted and incentivized rurality when considering funding for new training programs. The OAA defined rurality as the number of patients served by the proposed health care facility who lived in a rural or highly rural zip code according to VA Support Service Center Capital Assets data.20 As a result, VA psychology doctoral internships expanded to be available in all states, the District of Columbia, and Puerto Rico. MH training programs were started in the highly rural states of Montana and Wyoming. These expansion efforts promise to be an essential component to addressing the gaps in coverage in rural settings as noted in recent research.21
Pregraduate to Postgraduate Programs
The OAA AH education division supports a significant number of pregraduate-degree and postgraduate-degree training. Some professions, such as psychology, pharmacy, SW, PT, speech pathology, OT, and nutrition/dietetics receive funding at both levels of training. More recent, the OAA has started to move funding from pregraduate to postgraduate-degree positions, specifically within professions where pregraduate funding is uncommon for both federal and nonfederal training positions. The effort is designed to better align stipend-paid training programs with the VA Professional Qualification Standards and the final level of training required for employment in the VA.22This means that HPTs receive stipend support during the highest level of their clinical training before degree conferral, eligibility for VA employment, or while participating in a postgraduate-degree residency or fellowship.
Additionally, this shift in focus and the resulting internal assessment of professions has allowed the OAA to fund more specialized training opportunities, which sometimes go beyond what is required by accrediting bodies or for recruitment into VA positions. For example, the OAA is supporting SW fellowship programs and PA residency positions to allow for greater specialization within these professions; the accrediting agencies for both professions have recently finalized their accreditation standards, and the OAA played a role in moving these standards forward.
While postgraduate residencies and fellowships are not required for all AH HPTs or for employment in the VA, there is a shift in some professions to encourage postgraduate training in advanced competencies in specialized areas. Participation in a residency or fellowship training program affords HPTs additional time and diverse clinical experiences to acquire clinical skills, all while under the supervision of a highly trained practitioner. This additional training also allows for a longitudinal assessment of the HPT to ensure an alignment of the HPTs’ knowledge, abilities, and skills with the expectation should they pursue VA employment.
In academic year 2019/2020, the OAA AH education division in conjunction with the PA national program office transitioned the entirety of the PA pregraduate-degree student positions (415 funded positions) to residency positions, increasing residency positions from 19 to 32 funded positions. This shift in emphasis for funding did not negatively impact the total number of pregraduate PA students receiving training in the VA and has created a pipeline of residency graduates who are ready to enter VA staff positions. To date, the VA has 14 PA residency programs across 3 specialties: emergency medicine (EM), MH, and primary care/geriatrics. Of these tracks, the VA offers 5 EM and 4 MH residencies that position graduates to be eligible for specialty certification. The National Commission on Certification of Physician Assistants established Certificates of Added Qualifications (CAQ) to recognize and document specialty knowledge, skills, and experience. The VA MH residency programs have been established to align with the CAQ expectations, and residents immediately qualify to take the CAQ examination after the completion of training.
Currently, the same process to move pregraduate to postgraduate funding is being implemented for PT and OT. Within the PT profession, there is increased momentum toward residency and fellowship training programs to respond to the changing complexity of the health care systemand reduce the need of complex care to be provided by non-VA providers in the community.23 Both PT and OT have entered the initial phases of transitioning to residency or fellowship-funded positions. The OAA is partnering with these professions to move positions to postgraduate degree within the next 3 years with a commensurate increase in funding. The initial data indicate that 80% of graduated VA PT residents are board-certification eligible, and 89% of those who are eligible passed the examination on their first attempt.
Since 2013, the VA psychology training also has realized a growth in postgraduate-degree residencies. Psychology residency positions have increased 99% to 453 funded positions. This growth represents increased specialization in neuropsychology, geropsychology, rehabilitation psychology, and health psychology. Additionally, postgraduate residencies meet most jurisdictional requirements for postdoctoral supervised experience and better prepare HPTs to enter specialty staff positions that are necessary to care for aging veterans.
Additional professions are being targeted for postgraduate-degree training programs, including dietetics and speech pathology, to align with upcoming changes in the qualification standards for employment. While the process to transition positions to postgraduate-degree training programs can take 3 to 5 years, the outcomes are expected to result in better prepared HPTs who can fill staff vacancies in the VA.
Conclusions
Through its funding and oversight of numerous professions, the OAA is uniquely situated to adapt its portfolio to meet the needs of the VA and the nation. Over the past 10 years, the OAA has expanded its total number of HPT positions to enhance interprofessional care, respond to the VA’s strategic initiatives, address the care needs of rural veterans, and shift positions to postgraduate training programs. The OAA’s investment in high-quality training programs builds a strong health care workforce ready to meet the needs of an increasingly complex and integrated health care environment.
The OAA anticipates future expansion, especially related to promoting rural training opportunities and shifting to postgraduate training programs as a means of promoting advanced health care and health system competencies while continuing to align with workforce projections. Furthermore, while there are data on the percentage of VA staff who participated in OAA training program through the VA All Employee Survey (AES), the range for AH professions is wide. For example, about 37% of rehabilitative staff reported participating in an OAA training program, and 72% of VA psychologists reported having an OAA training experience. To maximize the hiring of HPTs, OAA will continue its partnership with WMC to enact programs aimed at streamlining the hiring process so that veterans have access to HCPs who are specifically trained to work with them.
The US Department of Veterans Affairs (VA) is the largest health care delivery system in the United States, comprising 1293 sites of care, including 171 medical centers.1 One of the 4 statutory missions of the VA is to train health care professionals (HCPs) to meet the needs of the VA and the nation.2 Through partnerships with more than 1800 accredited colleges, universities, and training programs, the VA provides training annually to nearly 118,000 health professions trainees (HPTs) across a variety of health care professions, and all of whom provide direct clinical care to veterans.3
In the VA, the Office of Academic Affiliations (OAA) is charged with overseeing health professions training and the VA’s partnership with medical and associated health (AH) professions schools, which was first codified in Policy Memorandum No. 2 in 1946.4,5 Given the scope and breadth of health professions education offered through the VA, OAA is in a unique position to address health care shortage areas as well as influence the educational standards for certain professions.
Many of these health care professions fall under the rubric of AH, which include mental health (MH) specialties, rehabilitative specialties, and others. These professions are critical to address in the expanding world of health care in the United States with its increased specialization and emphasis on coordination of care with interprofessional teams. During the 2019/2020 academic year, the VA provided clinical training to approximately 21,000 AH HPTs from > 40 professions with just over 20% receiving financial support through the OAA. Of the HPTs who train at VA without compensation, most spend shorter amounts of time in clinical rotations in the VA, are in pregraduate-degree education programs where payment for clinical rotations is not expected and may not be eligible for hire immediately on completion of their clinical training experience. The 17 funded professions have been strategically selected by the OAA to ensure a robust pipeline of HCPs to meet the needs of veterans and the nation.
To meet the demands of AH professionals (AHPs), the OAA implemented targeted expansion over the past 10 years. While not exhaustive, this paper describes several expansion efforts based on VA special initiatives, including enhancing clinical access in rural settings and shifting toward postgraduate-degree training and specialization. By aligning expansion with VA priorities as well as trends in health care more broadly, the OAA can ensure that there is a supply of well-trained AHPs who have developed the requisite competencies to contribute to our nation’s health care needs. Further, expansion can help train and recruit health professionals who can be hired into VA positions ready to care for the complex needs of veterans.
Associated Health Professionals
Overseen by the OAA, AH expansion is designed to address the specific needs of the VA and the US health care system. Data from the VA Workforce Management and Consulting (WMC) shows that the VA employment of AHPs has grown from 87,351 AHPs hired in fiscal year (FY) 2010 to 119,120 as of April 2020. This represents an average yearly growth rate of 3.4% and a total growth rate of 36%. The Bureau of Labor Statistics predictions for 2019/2029 suggest that certain AHPs are expected to have a 10-year growth rates of 20% or more to meet the changing health care needs of patients especially as the population ages; the growth rates for many AHPs far surpasses that of physicians, which is anticipated to be 4% (Table).6,7 The VA WMC expects an additional 52,283 AHPs will be hired by the VA by FY 2030 based on the 10-year average growth rate (Kali Clark, Veterans Health Administration Workforce Management and Consulting Office, email communication, May 28, 2020).
One of the driving forces behind the growth rate is the move toward using AHPs to supplement health care for a variety of health conditions.8,9 Examples include the integration of rehabilitation professionals, alternative care professionals (eg, massage therapists, practitioners who offer training in yoga and meditation), chiropractors, MH professionals, and pharmacists in the treatment of chronic pain, the use of a wider range of professionals in the treatment of MH conditions, and the integration of MH professionals into traditional medical settings, such as primary care. This intentional move to a more well-integrated model of interprofessional care is apparent in many other health care systems throughout the United States. Within the VA, this shift may be most evident through the introduction of the Whole Health model of care. The Whole Health model of care uses an interprofessional team to assess and care for veterans, using a personalized health plan addressing medical and MH conditions as well as behavioral, social, or spiritual concerns.10 The Whole Health model of care provides veterans with access to a variety of health care services, including but not limited to MH services, spiritual interventions, exercise-based programs, yoga, meditation, and nutrition counseling.
The OAA and AH education division have focused expansion to meet the increased need for MH and rehabilitation providers, to enhance interprofessional education, and to emphasize postgraduate-degree clinical training. This focus reflects the trends seen in health care training broadly throughout the nation and the intentional pivot is a model of these trends and a model for how to intentionally address these trends. Specific to the VA, focused expansion plans have allowed OAA to address VA strategic initiatives such as pain management and caring for rural veterans.
Funded Training Positions
As a result of recent AH expansion efforts, there has been a 33% increase in stipend-funded positions during the past 10 years, a rate that directly corresponds with the growth of AHPs in the VA. Recent AH expansion efforts can contribute to a particularly positive impact in highly rural and underserved areas where recruiting providers remains challenging.
The OAA launched the Mental Health Education Expansion (MHEE) initiative in 2012, which has now added 782 funded training slots across 10 health professions, 8 of which are psychology, pharmacy, chaplaincy, professional MH counseling, marriage and family therapy (MFT), social work (SW), occupational therapy (OT), and physician assistant (PA). Through the MHEE initiative, the VA has established funded internships for licensed professional mental health counselors and marriage and family therapists, as these professions are targeted for expanding the overall MH workforce in the VA. The OAA currently funds more than 50 total HPT positions for these 2 professions with an aim of increasing their recruitment to the VA MH workforce over the next decade. The MHEE is aligned with specified VA priorities to train a future VA workforce prepared for interprofessional collaboration and clinical care in an increasingly integrated and complex environment. This expansion effort also aligns with an increasing understanding of the importance of addressing the MH needs of our nation by ensuring there is an adequate supply of competent, well-trained clinicians entering the workforce.
The OAA has created and expanded residencies and fellowships in multiple rehabilitation professions, including chiropractic, physical therapy (PT), and OT. With the increased focus on the management of chronic pain in the nation combined with a specific emphasis on this clinical need in the VA, chiropractors have been deemed essential HCPs. In 2014, the VA established 5 chiropractic residency programs while partnering with the Council on Chiropractic Education to develop accreditation standards for residency training. OAA’s efforts have yielded 5 accredited residency programs, the first in the United States. In 2020, the VA doubled the number of available chiropractic residency programs, and future expansion is anticipated. Since 2010, PT residencies have expanded from 1 to 28 programs (42 funded positions) across 4 board certification specialties: cardiovascular-pulmonary, geriatric, neurologic, and orthopedic. Similarly, the VA was one of the first organizations to achieve accreditation for OT fellowships; there are currently 5 accredited OT fellowship programs across 3 areas of practice: assistive technology, MH, and physical rehabilitation. The VA OT fellowship program focused on assistive technology is the only program in the United States at this time.
Interprofessional Education
As one of the primary focus areas for AH expansion, interprofessional education (IPE) has been recognized as increasingly important for the provision of health care and the development of HPT programs. IPE can develop professionals who appreciate the roles of diverse professions and can use teamwork to enhance clinical outcomes for patients.11 There also are a growing number of professional organizations supporting the Interprofessional Education Collaborative with many representing AHPs.12 Collaboration across HCPs is an important way of reducing health care costs by enhancing clinical outcomes, communication, and teamwork.13-16 The VA and the nation’s health care system benefit from the by-products of interprofessional collaboration through investment in targeted training programs. In each phase of the AH expansion, special consideration was given to applicant programs offering unique and innovative clinical and educational experiences consistent with the promotion of interprofessional care. In doing so, increased numbers of AH HPTs have engaged in team-based clinical care.
Pain Management Pharmacy
The efforts of AH to align expansion with high-priority agency-wide efforts has resulted in the growth of pharmacy residency positions focused on pain management. Pharmacy postgraduate year (PGY) 2 residencies focusing on opioid reduction are an example of VA efforts to improve response to managing chronic pain and the long-term risks from opioid use during this national public health crisis.17 These residency programs focus on strategies to reduce the use of opioid medications in the clinical setting and teaching effective clinical interventions for reducing the rates of opioid addiction in veterans while still recognizing the need to identify and treat chronic pain. Before expansion efforts in 2018, there were 6 pharmacy residency programs focused on opioid use reduction in the VA, 8 pharmacy PGY2 residency positions were added in academic year 2019/2020, an additional 5 positions are being added in academic year 2021/2022 with the explicit goal of managing patients with high-risk chronic pain.
Rural Health
The lack of MH providers in rural areas has received much attention and is particularly important in the VA because veterans are more likely to live in less populated areas.18 The VA mandate to address this population was codified by the creation of the Office of Rural Health in 2006 via 38 USC § 7308.19Creating health professions training programs in rural settings provides HPTs the opportunity to learn professional competencies and train with faculty knowledgeable about this population—all of which provide a comprehensive training experience and serve as a recruitment pathway to hire HPTs into staff positions at these sites.19
When MHEE was initiated, not all regions of the country had funded VA psychology training programs, and this geographic gap in psychology training was a contributing factor to recruitment difficulties for psychologists in rural areas. As a result, the request for proposal process in the OAA highlighted and incentivized rurality when considering funding for new training programs. The OAA defined rurality as the number of patients served by the proposed health care facility who lived in a rural or highly rural zip code according to VA Support Service Center Capital Assets data.20 As a result, VA psychology doctoral internships expanded to be available in all states, the District of Columbia, and Puerto Rico. MH training programs were started in the highly rural states of Montana and Wyoming. These expansion efforts promise to be an essential component to addressing the gaps in coverage in rural settings as noted in recent research.21
Pregraduate to Postgraduate Programs
The OAA AH education division supports a significant number of pregraduate-degree and postgraduate-degree training. Some professions, such as psychology, pharmacy, SW, PT, speech pathology, OT, and nutrition/dietetics receive funding at both levels of training. More recent, the OAA has started to move funding from pregraduate to postgraduate-degree positions, specifically within professions where pregraduate funding is uncommon for both federal and nonfederal training positions. The effort is designed to better align stipend-paid training programs with the VA Professional Qualification Standards and the final level of training required for employment in the VA.22This means that HPTs receive stipend support during the highest level of their clinical training before degree conferral, eligibility for VA employment, or while participating in a postgraduate-degree residency or fellowship.
Additionally, this shift in focus and the resulting internal assessment of professions has allowed the OAA to fund more specialized training opportunities, which sometimes go beyond what is required by accrediting bodies or for recruitment into VA positions. For example, the OAA is supporting SW fellowship programs and PA residency positions to allow for greater specialization within these professions; the accrediting agencies for both professions have recently finalized their accreditation standards, and the OAA played a role in moving these standards forward.
While postgraduate residencies and fellowships are not required for all AH HPTs or for employment in the VA, there is a shift in some professions to encourage postgraduate training in advanced competencies in specialized areas. Participation in a residency or fellowship training program affords HPTs additional time and diverse clinical experiences to acquire clinical skills, all while under the supervision of a highly trained practitioner. This additional training also allows for a longitudinal assessment of the HPT to ensure an alignment of the HPTs’ knowledge, abilities, and skills with the expectation should they pursue VA employment.
In academic year 2019/2020, the OAA AH education division in conjunction with the PA national program office transitioned the entirety of the PA pregraduate-degree student positions (415 funded positions) to residency positions, increasing residency positions from 19 to 32 funded positions. This shift in emphasis for funding did not negatively impact the total number of pregraduate PA students receiving training in the VA and has created a pipeline of residency graduates who are ready to enter VA staff positions. To date, the VA has 14 PA residency programs across 3 specialties: emergency medicine (EM), MH, and primary care/geriatrics. Of these tracks, the VA offers 5 EM and 4 MH residencies that position graduates to be eligible for specialty certification. The National Commission on Certification of Physician Assistants established Certificates of Added Qualifications (CAQ) to recognize and document specialty knowledge, skills, and experience. The VA MH residency programs have been established to align with the CAQ expectations, and residents immediately qualify to take the CAQ examination after the completion of training.
Currently, the same process to move pregraduate to postgraduate funding is being implemented for PT and OT. Within the PT profession, there is increased momentum toward residency and fellowship training programs to respond to the changing complexity of the health care systemand reduce the need of complex care to be provided by non-VA providers in the community.23 Both PT and OT have entered the initial phases of transitioning to residency or fellowship-funded positions. The OAA is partnering with these professions to move positions to postgraduate degree within the next 3 years with a commensurate increase in funding. The initial data indicate that 80% of graduated VA PT residents are board-certification eligible, and 89% of those who are eligible passed the examination on their first attempt.
Since 2013, the VA psychology training also has realized a growth in postgraduate-degree residencies. Psychology residency positions have increased 99% to 453 funded positions. This growth represents increased specialization in neuropsychology, geropsychology, rehabilitation psychology, and health psychology. Additionally, postgraduate residencies meet most jurisdictional requirements for postdoctoral supervised experience and better prepare HPTs to enter specialty staff positions that are necessary to care for aging veterans.
Additional professions are being targeted for postgraduate-degree training programs, including dietetics and speech pathology, to align with upcoming changes in the qualification standards for employment. While the process to transition positions to postgraduate-degree training programs can take 3 to 5 years, the outcomes are expected to result in better prepared HPTs who can fill staff vacancies in the VA.
Conclusions
Through its funding and oversight of numerous professions, the OAA is uniquely situated to adapt its portfolio to meet the needs of the VA and the nation. Over the past 10 years, the OAA has expanded its total number of HPT positions to enhance interprofessional care, respond to the VA’s strategic initiatives, address the care needs of rural veterans, and shift positions to postgraduate training programs. The OAA’s investment in high-quality training programs builds a strong health care workforce ready to meet the needs of an increasingly complex and integrated health care environment.
The OAA anticipates future expansion, especially related to promoting rural training opportunities and shifting to postgraduate training programs as a means of promoting advanced health care and health system competencies while continuing to align with workforce projections. Furthermore, while there are data on the percentage of VA staff who participated in OAA training program through the VA All Employee Survey (AES), the range for AH professions is wide. For example, about 37% of rehabilitative staff reported participating in an OAA training program, and 72% of VA psychologists reported having an OAA training experience. To maximize the hiring of HPTs, OAA will continue its partnership with WMC to enact programs aimed at streamlining the hiring process so that veterans have access to HCPs who are specifically trained to work with them.
1. US Department of Veterans Affairs. Providing health care for veterans. Updated April 23, 2021. Accessed July 15, 2021. https://www.va.gov/health
2. Veterans’ Benefits. 38 USC §7301 and §7302 (1991). Accessed May 18, 2020. https://www.govinfo.gov/content/pkg/USCODE-2018-title38/pdf/USCODE-2018-title38-partV-chap73-subchapI-sec7302.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2021. Accessed July 15, 2021. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
4. US Department of Veterans Affairs, VHA Office of Academic Affiliations. VA Policy Memorandum # 2. Policy in association of veterans’ hospitals with medical schools. Published January 30, 1946. Accessed October 13, 2020. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Mission of the office of academic affiliations. Updated September 24, 2019. Accessed July 15, 2021. https://www.va.gov/oaa/oaa_mission.asp
6. US Bureau of Labor Statistics, Office of Occupational Statistics and Employment Projections Occupational Outlook Handbook. Healthcare occupations. Updated May 14, 2021. Accessed July 15, 2021. https://www.bls.gov/ooh/healthcare/home.htm
7. Windmill IM, Freeman BA. Demand for audiology services: 30-yr projections and impact on academic programs. J Am Acad Audiol. 2013;24(5):407-416. doi:10.3766/jaaa.24.5.7
8. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. HRSA health workforce: behavioral health workforce projections, 2017-2030. Accessed July 15, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/bh-workforce-projections-fact-sheet.pdf
9. Centers for Disease Control and Prevention, National Center for Health Statistics. NCHS data brief, No. 325. Use of yoga, meditation, and chiropractors among US adults aged 18 and over. Published November 2018. Accessed September 24, 2020. https://www.cdc.gov/nchs/data/databriefs/db325-h.pdf
10. US Department of Veterans Affairs, Veterans Health Administration Whole Health. Updated July 6, 2021. Accessed July 15, 2021. https://www.va.gov/wholehealth
11. Clark KM. Interprofessional education: making our way out of the silos. Respir Care. 2018;63(5): 637-639. doi:10.4187/respcare.06234
12. Interprofessional Education Collaborative. What is interprofessional education (IPE)? Accessed July 15, 2021. https://www.ipecollaborative.org/about-us
13. Nester J. The importance of interprofessional practice and education in the era of accountable care. N C Med J. 2016;77(2):128-132. doi.10.18043/ncm.77.2.128
14.. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6): 313-319. doi:10.1097/NNA.0000000000000492
15. Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34(2):125-184. doi:10.1016/j.kjms.2017.12.009
16. Ateah CA, Snow W, Wenter P, et al. Stereotyping as a barrier to collaboration: does interprofessional education make a difference? Nurse Educ Today. 2011;31(2):208-213. doi:10.1016/j.nedt.2010.06.004
17. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for Managing Opioid Therapy for Chronic Pain. Published May 7, 1991. Updated February 2017. Accessed July 15, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
18. US Department of Veterans Affairs, Office of Rural Health. VHA office of rural health. Updated March 17, 2021. Accessed July 15, 2021. https://www.ruralhealth.va.gov19. Curran V, Rourke J. The role of medical education in the recruitment and retention of rural physicians. Med Teach. 2004;26(3):265-272. doi:10.1080/0142159042000192055
20. US Department of Veterans Affairs. VHA Support Service Center Capital Assets. Updated December 1, 2020. Accessed July 15, 2021. https://www.data.va.gov/dataset/VHA-Support-Service-Center-Capital-Assets-VSSC-/2fr5-sktm
21. Domino ME, Lin CC, Morrisey JP, et al. Training psychologists for rural practice: exploring opportunities and constraints. J Rural Health. 2019;35(1):35-41. doi:10.1111/jrh.12299
22. US Department of Veterans Affairs. VA Directive 5005: Staffing. Published March 4, 2020. Accessed July 15, 2021. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=1140&FType=2
23. Furze JA, Freeman BA. Physical therapy and fellowship education: reflections on the past, present, and future. Phys Ther. 2016;96(7):949-960. doi:10.2522/ptj.20150473
1. US Department of Veterans Affairs. Providing health care for veterans. Updated April 23, 2021. Accessed July 15, 2021. https://www.va.gov/health
2. Veterans’ Benefits. 38 USC §7301 and §7302 (1991). Accessed May 18, 2020. https://www.govinfo.gov/content/pkg/USCODE-2018-title38/pdf/USCODE-2018-title38-partV-chap73-subchapI-sec7302.pdf
3. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2021. Accessed July 15, 2021. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
4. US Department of Veterans Affairs, VHA Office of Academic Affiliations. VA Policy Memorandum # 2. Policy in association of veterans’ hospitals with medical schools. Published January 30, 1946. Accessed October 13, 2020. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Mission of the office of academic affiliations. Updated September 24, 2019. Accessed July 15, 2021. https://www.va.gov/oaa/oaa_mission.asp
6. US Bureau of Labor Statistics, Office of Occupational Statistics and Employment Projections Occupational Outlook Handbook. Healthcare occupations. Updated May 14, 2021. Accessed July 15, 2021. https://www.bls.gov/ooh/healthcare/home.htm
7. Windmill IM, Freeman BA. Demand for audiology services: 30-yr projections and impact on academic programs. J Am Acad Audiol. 2013;24(5):407-416. doi:10.3766/jaaa.24.5.7
8. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. HRSA health workforce: behavioral health workforce projections, 2017-2030. Accessed July 15, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/bh-workforce-projections-fact-sheet.pdf
9. Centers for Disease Control and Prevention, National Center for Health Statistics. NCHS data brief, No. 325. Use of yoga, meditation, and chiropractors among US adults aged 18 and over. Published November 2018. Accessed September 24, 2020. https://www.cdc.gov/nchs/data/databriefs/db325-h.pdf
10. US Department of Veterans Affairs, Veterans Health Administration Whole Health. Updated July 6, 2021. Accessed July 15, 2021. https://www.va.gov/wholehealth
11. Clark KM. Interprofessional education: making our way out of the silos. Respir Care. 2018;63(5): 637-639. doi:10.4187/respcare.06234
12. Interprofessional Education Collaborative. What is interprofessional education (IPE)? Accessed July 15, 2021. https://www.ipecollaborative.org/about-us
13. Nester J. The importance of interprofessional practice and education in the era of accountable care. N C Med J. 2016;77(2):128-132. doi.10.18043/ncm.77.2.128
14.. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6): 313-319. doi:10.1097/NNA.0000000000000492
15. Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34(2):125-184. doi:10.1016/j.kjms.2017.12.009
16. Ateah CA, Snow W, Wenter P, et al. Stereotyping as a barrier to collaboration: does interprofessional education make a difference? Nurse Educ Today. 2011;31(2):208-213. doi:10.1016/j.nedt.2010.06.004
17. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for Managing Opioid Therapy for Chronic Pain. Published May 7, 1991. Updated February 2017. Accessed July 15, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
18. US Department of Veterans Affairs, Office of Rural Health. VHA office of rural health. Updated March 17, 2021. Accessed July 15, 2021. https://www.ruralhealth.va.gov19. Curran V, Rourke J. The role of medical education in the recruitment and retention of rural physicians. Med Teach. 2004;26(3):265-272. doi:10.1080/0142159042000192055
20. US Department of Veterans Affairs. VHA Support Service Center Capital Assets. Updated December 1, 2020. Accessed July 15, 2021. https://www.data.va.gov/dataset/VHA-Support-Service-Center-Capital-Assets-VSSC-/2fr5-sktm
21. Domino ME, Lin CC, Morrisey JP, et al. Training psychologists for rural practice: exploring opportunities and constraints. J Rural Health. 2019;35(1):35-41. doi:10.1111/jrh.12299
22. US Department of Veterans Affairs. VA Directive 5005: Staffing. Published March 4, 2020. Accessed July 15, 2021. https://www.va.gov/vapubs/viewPublication.asp?Pub_ID=1140&FType=2
23. Furze JA, Freeman BA. Physical therapy and fellowship education: reflections on the past, present, and future. Phys Ther. 2016;96(7):949-960. doi:10.2522/ptj.20150473
The Precision Oncology Program for Cancer of the Prostate (POPCaP) Network: A Veterans Affairs/Prostate Cancer Foundation Collaboration(FULL)
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities (FULL)
The US Department of Veterans Affairs (VA) oversees the largest integrated health care system in the nation, administering care to 9 million veterans annually throughout its distributed network of 1,255 medical centers and outpatient facilities. Every year, about 50,000 veterans are diagnosed with and treated for cancer in the VA, representing about 3% of all cancer cases in the US.1 After skin cancer, prostate, colon, and lung cancers are the most common among veterans.1 One way that VA has sought to improve the care of its large cancer patient population is through the adoption of precision oncology, an ever-evolving practice of analyzing an individual patient’s cancer to inform clinical decision making. Most often, the analysis includes conducting genetic testing of the tumor itself. Here, we describe the opportunities and challenges of integrating germline genetics into precision oncology practice.
The Intersection of Precision Oncology and Germline Genetics
Precision oncology typically refers to genetic testing of tumor DNA to identify genetic variants with potential diagnostic, prognostic, or predictive therapeutic implications. It is enabled by a growing body of knowledge that identifies key drivers of cancer development, coupled with advances in tumor analysis by next-generation sequencing and other technologies and by the availability of new and repurposed therapeutic agents.2 Precision oncology has transformed cancer care by targeting both common and rare malignancies with specific therapies that improve clinical outcomes in patients.3
Testing of tumor DNA can reveal both somatic (acquired) and germline (inherited) gene variants. Precision oncology testing strategies can include tumor-only testing with or without subtraction of suspected germline variants, or paired tumor-normal testing with explicit analysis and reporting of genes associated with germline predisposition.2 With tumor-only testing, the germline status of variants may be inferred and follow-up germline testing in normal tissue such as blood or saliva can be considered. Paired tumor-normal testing provides distinct advantages over tumor-only testing, including improvement of the mutation detection rate in tumors and streamlining interpretation of results for both the tumor and germline tests.
Regardless of the strategy used, tumor testing has the potential to uncover clinically relevant germline variation associated with heritable cancer susceptibility and other conditions, as well as carrier status for autosomal recessive disorders (eAppendix
Germline genetic information, independent of somatic variation, can influence the choice of targeted cancer therapies. For example, Mandelker and colleagues identified germline variants that would impact the treatment of 38 (3.7%) of 1,040 patients with cancer.4 Individuals with a germline pathogenic variant in a DNA repair gene (eg, BRCA1, BRCA2, ATM, CHEK2) are candidates for platinum chemotherapy and poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors that target the inability of a tumor to repair double-stranded DNA breaks.5,6 Individuals with a germline pathogenic variant in the MSH2, MLH1, MSH6, PMS2 or EPCAM genes (ie, Lynch syndrome) have tumors that are deficient in mismatch repair, and these tumors are responsive to inhibitors of the programmed death 1 (PD1) pathway.7,8
In addition to changing treatment decisions, identifying pathogenic germline variants can have health, reproductive, and psychosocial implications for the patient and the patient’s family members.9,10 A pathogenic germline variant can imply disease risk for both the patient and his or her relatives. In these cases, it is important to ascertain family history, understand the mode of inheritance, identify at-risk relatives, review the associated phenotype, and discuss management and prevention options for the patient and for family members. For example, a germline pathogenic variant in the BRCA2 gene is associated with increased risk for breast, ovarian, pancreatic, gastric, bile duct, and laryngeal cancer, and melanoma.11 Knowledge of these increased cancer risks could inform cancer prevention and early detection options, such as more frequent and intensive surveillance starting at younger ages compared with that of average-risk individuals, use of chemoprevention treatments, and for those at highest risk, risk-reducing surgical procedures. Therefore, reporting germline test results requires the clinician to take on additional responsibilities beyond those required when reporting only somatic variants.
Because of the complexities inherent in germline genetic testing, it traditionally is offered in the context of a genetic consultation, comprised of genetic evaluation and genetic counseling (Figure). Clinical geneticists are physicians certified by the American Board of Medical Genetics and Genomics (a member board of the American Board of Medical Specialties) who received special training in the diagnosis and management of medical genetic conditions; they are trained to perform all aspects of a genetic consultation across the clinical spectrum and lifespan of a patient.12 In contrast, genetic counselors have a master’s degree in genetic counseling, a communication process that facilitates patient decision making surrounding the genetic evaluation.13 Most work as members of a team to ensure provision of comprehensive clinical genetic services. Genetic counselors are licensed in most states, and licensure in some states sanctions the ordering of genetic tests by genetic counselors. Genetics nurses are licensed professional nurses with special education and training in genetics who function in diverse roles in industry, education, research, and clinical care.14 Genetics nurses in clinical care perform risk assessment based on personal and family history, recognize and identify genetic conditions and predispositions, and discuss the implications of this with patients and their families. Advanced practice nurses (APRNs) have additional training that allows for diagnosis, interpretation of results, and surveillance and management recommendations.15
Germline Genetic Testing Challenges
Integrating germline genetic testing in precision oncology practice presents challenges at the patient, family, health care provider, and health system levels. Due to these challenges, implementation planning is obligatory, as germline testing has become a standard-of-care for certain tumor types and patients.2
On learning of a germline pathogenic variant or variant of uncertain significance, patients may experience distress and anxiety, especially in the short term.16-18 In addition, it can be difficult for patients to share germline genetic test results with their family; parents may feel guilty about the possibility of passing on a predisposition to children, and unaffected siblings may experience survivor guilt. For some veterans, there can be concerns about losing service-connected benefits if a genetic factor is found to contribute to their cancer history. In addition, patients may have concerns about discrimination by employers or insurers, including commercial health insurance or long-term care, disability, and life insurance. Yet there are many state and federal laws that ensure some protection from employment and health insurance discrimination based on genetic information.
For cancer care clinicians, incorporating germline testing requires additional responsibilities that can complicate care. Prior to germline genetic testing, genetic counseling with patients is recommended to review the potential benefits, harms, and limitations of genetic testing. Further, posttest genetic counseling is recommended to help the patient understand how the results may influence future cancer risks, provide recommendations for cancer management and prevention, and discuss implications for family members.9,10 While patients trust their health care providers to help them access and understand their genetic information, most health care providers are unprepared to integrate genetics into their practice; they lack adequate knowledge, skills, and confidence about genetics to effectively deliver genetic services.19-26 This leads to failure to recognize patients with indications for genetic testing, which often is due to insufficient family history collection. Other errors can include offering germline genetic testing to patients without appropriate indications and with inadequate informed consent procedures. When genetic testing is pursued, lack of knowledge about genetic principles and testing methods can lead to misinterpretation and miscommunication of results, contributing to inappropriate management recommendations. These errors can contribute to under-use, overuse, or misuse of genetic testing that can compromise the quality of patient care.27,28 With this in mind, thought must be given at the health care system level to develop effective strategies to deliver genetic services to patients. These strategies must address workforce capacity, organizational structure, and education.
Workforce Capacity
The VA clinical genetics workforce needs to expand to keep pace with increasing demand, which will be accelerated by the precision oncology programs for prostate and lung cancers and the VA Teleoncology initiative. In the US there are 10 to 15 genetics professionals per 1,000,000 residents.29-31 Most genetics professionals work in academic and metropolitan settings, leaving suburban and rural areas underserved. For example, in California, some patients travel up to 386 miles for genetics care (mean, 76.6 miles).32 In the VA, there are only 1 to 2 genetics professionals per 1 million enrollees, about 10-fold fewer than in community care. Meeting clinical needs of patients at the VA is particularly challenging because more than one-third of veterans live in rural areas.33
We recently surveyed genetics professionals in the VA about their practices and capacity to increase patient throughput (Table). Currently in the VA, there are 8 clinical geneticists, not all of whom practice clinical genetics, and 13 genetic counselors. Five VA programs provide clinical genetic services to local and nearby VA facilities near Boston, Massachusetts; Houston, Texas; Los Angeles and San Francisco, California; and Salt Lake City, Utah. These programs, first developed in 2008, typically are staffed by 1 or 2 genetics professionals. Most patients who are referred to the VA genetics programs are evaluated for hereditary cancer syndromes. Multiple modes of delivery may be used, including in-person, telehealth, telephone, and provider-to-provider e-consults in the EHR.
In 2010, in response to increased demand for clinical genetics services, the VA launched the Genomic Medicine Service (GMS), a national program with a centralized team of 9 genetic counselors based in Salt Lake City. GMS provides telehealth genetic counseling services exclusively to veterans onsite and at about 90 VA facilities across the country. More recently, the addition of a clinical geneticist and APRN with genetics expertise has allowed GMS to provide more comprehensive genetic consultative services.
All VA genetics programs are currently at full capacity with long waits for an appointment. To expand clinical genetic services, the VA genetics professionals responding to our survey reported a need for additional support (eg, administrative, care coordination, clinical), resources (eg, clinical space, salary support), and organizational change (eg, division of Medical Genetics at facility level, services provided at the level of the Veterans Integrated Service Network). Given the dearth of genetic care providers in the community, referral to non-VA care is not a viable option in many markets. In addition, avoiding referral outside of the VA could help to ensure continuity of care, more efficient care, and reduce the risk of duplication of testing, and polypharmacy.34-37
As part of its precision oncology initiative, VA is focusing on building clinical genetics services capacity. To increase access to clinical genetic services and appropriate genetic testing, the VA needs more genetics professionals, including clinical geneticists, genetic counselors, and genetic nurses–ideally a workforce study could be performed to inform the right staffing mix needed. To grow the genetics workforce in the long term, the VA could leverage its academic affiliations to train the next generation of genetics professionals. The VA has an important role in training medical professionals. By forming affiliations with medical schools and universities, the VA has become the largest provider of health care training in the US.38
Genetic Health Care Organization in the VA
Understanding a patient’s genetic background increasingly has become more and more important in the clinic, which necessitates a major shift in health care. Unfortunately, on a national scale, the number of clinical genetics professionals has not kept pace with the need-limiting the ability to grow the traditional genetics workforce in the VA in the near term.29-31 Thus, we must look to alternative genetic health care models in which other members of the health care team assume some of the genetic evaluation and counseling activities while caring for their cancer patients with referral to a clinical genetics team, as needed.39
Two genetic health care models have been described.40 Traditionally, clinical genetic services are coordinated between genetics professionals and other clinicians, organized as a regional genetics center and usually affiliated with an academic medical center. By contrast, the nontraditional genetic health care model integrates genetic services within primary and specialty care. Under the new approach, nongeneticists can be assisted by decision support tools in the EHR that help with assessing family history risk, identifying indications for genetic testing, and suggesting management options based on genetic test results.41-43
The VA National Precision Oncology Program (NPOP) is shaped by a commitment to be a high reliability organization (HRO). As such, the goal is to create a system of excellence that integrates precision medicine, implementation science, and the learning health care system to improve the health and health care of veterans with cancer. This initiative is establishing the foundations for best-in-class cancer care to enable veterans access to life-saving therapies through a concerted effort that began with the Cancer Moonshot, development of the NPOP, and collaborations with the VA Office of Research and Development. One of the fundamental objectives of this initiative is to implement strategies that ensure clinical genetic services are available to veterans receiving cancer care at all VA facilities and to extend these services to veterans in remote geographic locations nationwide. The initiative aims to synergize VA Teleoncology services that seek to deliver best-in-class oncology care across the VA enterprise using cutting-edge technologies.
Conclusions
To accomplish the goal of delivering world-class clinical genetic services to veterans and meet the increasing needs of precision oncology and support quality genetic health care, the VA must develop an integrated system of genetic health care that will have a network of clinical genetics that interfaces with other clinical and operational programs, genomics researchers, and educational programs to support quality genetic health care. The VA has highly qualified and dedicated genetics professionals at many sites across the country. Connecting them could create powerful synergies that would benefit patients and strengthen the genetics workforce. The clinical genetics network will enable development and dissemination of evidence-based policies, protocols, and clinical pathways for genomic medicine. This will help to identify, benchmark, and promote best practices for clinical genetic services, and increase access, increase efficiencies, and reduce variability in the care delivered.
The VA is well positioned to achieve successful implementation of genetic services given its investment in genomic medicine and the commitment of the VA NPOP. However, there is a need for structured and targeted implementation strategies for genetic services in the VA, as uptake of this innovation will not occur by passive diffusion.44,45 To keep pace with the demand for germline testing in veterans, VA may want to consider an outsized focus on training genetics professionals, given the high demand for this expertise. Perhaps most importantly, the VA will need to better prepare its frontline clinical workforce to integrate genetics into their practice. This could be facilitated by identifying implementation strategies and educational programs for genomic medicine that help clinicians to think genetically while caring for their patients, performing aspects of family history risk assessment and pre- and posttest genetic counseling as they are able, and referring complex cases to the clinical genetics network when needed.
Much is already known on how best to accomplish this through studies conducted by many talented VA health services researchers.46 Crucially, clinical tools embedded within the VA EHR will be fundamental to these efforts by facilitating identification of patients who can benefit from genetic services and genetic testing at the point of care. Through integration of VA research with clinical genetic services, the VA will become more prepared to realize the promise of genomic medicine for veterans.
Acknowledgments
We thank the members of the Genomic Medicine Program Advisory Committee, Clinical Genetics Subcommittee for providing input and guidance on the topics included in this article.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
2. Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142-1148. doi:10.1038/s41436-020-0783-8
3. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. Published 2020 Jan 14. doi:10.1186/s13073-019-0703-1
4. Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
5. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi:10.1056/NEJMoa1506859
6. Ratta R, Guida A, Scotté F, et al. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review [published online ahead of print, 2020 May 4]. Prostate Cancer Prostatic Dis. 2020;10.1038/s41391-020-0233-3. doi:10.1038/s41391-020-0233-3
7. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. doi:10.1371/journal.pone.0233260
9. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K; American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893-901. doi:10.1200/JCO.2009.27.0660
10. Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21(2):151-161. doi:10.1007/s10897-011-9462-x
11. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993.
12. ACMG Board of Directors. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2015;17(9):e3. doi:10.1038/gim.2015.94
13. National Society of Genetic Counselors’ Definition Task Force, Resta R, Biesecker BB, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77-83. doi:10.1007/s10897-005-9014-3
14. Calzone KA, Cashion A, Feetham S, et al. Nurses transforming health care using genetics and genomics [published correction appears in Nurs Outlook. 2010;58(3):163]. Nurs Outlook. 2010;58(1):26-35. doi:10.1016/j.outlook.2009.05.001
15. US Department of Veterans Affairs, Veterans Health Administration, Office of Nursing Services. 2018 Office of Nursing Services (ONS) Annual Brief. https://www.va.gov/nursing/docs/about/2018_ONS_Annual_Report_Brief.pdf. Accessed July 21, 2020.
16. Lerman C, Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology (Williston Park). 1996;10(2):191-202.
17. Bonadona V, Saltel P, Desseigne F, et al. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11(1):97-104.
18. Murakami Y, Okamura H, Sugano K, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101(2):395-403. doi:10.1002/cncr.20363
19. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413-423.
20. Dhar SU, Cooper HP, Wang T, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(1):221-227. doi:10.1007/s10549-011-1449-7
21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13(2):148-154. doi:10.1097/GIM.0b013e318207f564
22. Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61-66. doi:10.1016/j.amepre.2010.09.027
23. Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367-375. doi:10.1089/gtmb.2012.0381
24. Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48-63. doi:10.1007/s10897-013-9605-3
25. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311-324. doi:10.1007/s10689-013-9695-y
26. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. doi:10.1038/gim.2014.101
27. Scheuner MT, Hilborne L, Brown J, Lubin IM; members of the RAND Molecular Genetic Test Report Advisory Board. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16(7):761-769. doi:10.1089/gtmb.2011.0328
28. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
29. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. doi:10.1097/01.gim.0000172416.35285.9f
30. Institute of Medicine. Roundtable on Translating Genomic-Based Research for Health. Washington, DC: National Academies Press; 2009. https://www.ncbi.nlm.nih.gov/books/NBK26394. Accessed July 22, 2020.
31. Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. doi:10.1007/s10897-017-0158-8
32. Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22(1):227-231. doi:10.1038/s41436-019-0628-5

33. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: US Department of Veterans Affairs; 2011. https://www.hsrd.research.va.gov/publications/esp/ambulatory.cfm. Accessed July 21, 2020.
34. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39-68. doi:10.1111/j.1468-0009.2011.00619.x
35. Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. Published 2010 May 20. doi:10.1186/1472-6963-10-132
36. McDonald KM, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Version 4. Agency for Healthcare Research and Quality Publication No. 14-0037. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed July 22, 2020.
37. Greenwood-Lee J, Jewett L, Woodhouse L, Marshall DA. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18(1):986. Published 2018 Dec 20. doi:10.1186/s12913-018-3745-y
38. US Department of Veterans Affairs, Office of Academic Affiliations. Our medical and dental training program. https://www.va.gov/oaa/gme_default.asp. Updated January 7, 2020. Accessed July 21, 2020.
39. Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16(8):609-619. doi:10.1038/gim.2013.202.
40. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15(1):34-45. doi:10.1159/000328846
41. Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32(2):218-227. doi:10.1080/03014460500074921
42. Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60-69. doi:10.1038/gim.2013.75
43. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
44. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238-245. doi:10.1038/gim.2013.101
45. Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6(2):16. Published 2016 Apr 28. doi:10.3390/jpm6020016
46. US Department of Veterans Affairs, Health Services Research and Development. Genomics. https://www.hsrd.research.va.gov/research/portfolio_description.cfm?Sulu=17. Updated July 21, 2020. Accessed June 22, 2020.
The US Department of Veterans Affairs (VA) oversees the largest integrated health care system in the nation, administering care to 9 million veterans annually throughout its distributed network of 1,255 medical centers and outpatient facilities. Every year, about 50,000 veterans are diagnosed with and treated for cancer in the VA, representing about 3% of all cancer cases in the US.1 After skin cancer, prostate, colon, and lung cancers are the most common among veterans.1 One way that VA has sought to improve the care of its large cancer patient population is through the adoption of precision oncology, an ever-evolving practice of analyzing an individual patient’s cancer to inform clinical decision making. Most often, the analysis includes conducting genetic testing of the tumor itself. Here, we describe the opportunities and challenges of integrating germline genetics into precision oncology practice.
The Intersection of Precision Oncology and Germline Genetics
Precision oncology typically refers to genetic testing of tumor DNA to identify genetic variants with potential diagnostic, prognostic, or predictive therapeutic implications. It is enabled by a growing body of knowledge that identifies key drivers of cancer development, coupled with advances in tumor analysis by next-generation sequencing and other technologies and by the availability of new and repurposed therapeutic agents.2 Precision oncology has transformed cancer care by targeting both common and rare malignancies with specific therapies that improve clinical outcomes in patients.3
Testing of tumor DNA can reveal both somatic (acquired) and germline (inherited) gene variants. Precision oncology testing strategies can include tumor-only testing with or without subtraction of suspected germline variants, or paired tumor-normal testing with explicit analysis and reporting of genes associated with germline predisposition.2 With tumor-only testing, the germline status of variants may be inferred and follow-up germline testing in normal tissue such as blood or saliva can be considered. Paired tumor-normal testing provides distinct advantages over tumor-only testing, including improvement of the mutation detection rate in tumors and streamlining interpretation of results for both the tumor and germline tests.
Regardless of the strategy used, tumor testing has the potential to uncover clinically relevant germline variation associated with heritable cancer susceptibility and other conditions, as well as carrier status for autosomal recessive disorders (eAppendix
Germline genetic information, independent of somatic variation, can influence the choice of targeted cancer therapies. For example, Mandelker and colleagues identified germline variants that would impact the treatment of 38 (3.7%) of 1,040 patients with cancer.4 Individuals with a germline pathogenic variant in a DNA repair gene (eg, BRCA1, BRCA2, ATM, CHEK2) are candidates for platinum chemotherapy and poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors that target the inability of a tumor to repair double-stranded DNA breaks.5,6 Individuals with a germline pathogenic variant in the MSH2, MLH1, MSH6, PMS2 or EPCAM genes (ie, Lynch syndrome) have tumors that are deficient in mismatch repair, and these tumors are responsive to inhibitors of the programmed death 1 (PD1) pathway.7,8
In addition to changing treatment decisions, identifying pathogenic germline variants can have health, reproductive, and psychosocial implications for the patient and the patient’s family members.9,10 A pathogenic germline variant can imply disease risk for both the patient and his or her relatives. In these cases, it is important to ascertain family history, understand the mode of inheritance, identify at-risk relatives, review the associated phenotype, and discuss management and prevention options for the patient and for family members. For example, a germline pathogenic variant in the BRCA2 gene is associated with increased risk for breast, ovarian, pancreatic, gastric, bile duct, and laryngeal cancer, and melanoma.11 Knowledge of these increased cancer risks could inform cancer prevention and early detection options, such as more frequent and intensive surveillance starting at younger ages compared with that of average-risk individuals, use of chemoprevention treatments, and for those at highest risk, risk-reducing surgical procedures. Therefore, reporting germline test results requires the clinician to take on additional responsibilities beyond those required when reporting only somatic variants.
Because of the complexities inherent in germline genetic testing, it traditionally is offered in the context of a genetic consultation, comprised of genetic evaluation and genetic counseling (Figure). Clinical geneticists are physicians certified by the American Board of Medical Genetics and Genomics (a member board of the American Board of Medical Specialties) who received special training in the diagnosis and management of medical genetic conditions; they are trained to perform all aspects of a genetic consultation across the clinical spectrum and lifespan of a patient.12 In contrast, genetic counselors have a master’s degree in genetic counseling, a communication process that facilitates patient decision making surrounding the genetic evaluation.13 Most work as members of a team to ensure provision of comprehensive clinical genetic services. Genetic counselors are licensed in most states, and licensure in some states sanctions the ordering of genetic tests by genetic counselors. Genetics nurses are licensed professional nurses with special education and training in genetics who function in diverse roles in industry, education, research, and clinical care.14 Genetics nurses in clinical care perform risk assessment based on personal and family history, recognize and identify genetic conditions and predispositions, and discuss the implications of this with patients and their families. Advanced practice nurses (APRNs) have additional training that allows for diagnosis, interpretation of results, and surveillance and management recommendations.15
Germline Genetic Testing Challenges
Integrating germline genetic testing in precision oncology practice presents challenges at the patient, family, health care provider, and health system levels. Due to these challenges, implementation planning is obligatory, as germline testing has become a standard-of-care for certain tumor types and patients.2
On learning of a germline pathogenic variant or variant of uncertain significance, patients may experience distress and anxiety, especially in the short term.16-18 In addition, it can be difficult for patients to share germline genetic test results with their family; parents may feel guilty about the possibility of passing on a predisposition to children, and unaffected siblings may experience survivor guilt. For some veterans, there can be concerns about losing service-connected benefits if a genetic factor is found to contribute to their cancer history. In addition, patients may have concerns about discrimination by employers or insurers, including commercial health insurance or long-term care, disability, and life insurance. Yet there are many state and federal laws that ensure some protection from employment and health insurance discrimination based on genetic information.
For cancer care clinicians, incorporating germline testing requires additional responsibilities that can complicate care. Prior to germline genetic testing, genetic counseling with patients is recommended to review the potential benefits, harms, and limitations of genetic testing. Further, posttest genetic counseling is recommended to help the patient understand how the results may influence future cancer risks, provide recommendations for cancer management and prevention, and discuss implications for family members.9,10 While patients trust their health care providers to help them access and understand their genetic information, most health care providers are unprepared to integrate genetics into their practice; they lack adequate knowledge, skills, and confidence about genetics to effectively deliver genetic services.19-26 This leads to failure to recognize patients with indications for genetic testing, which often is due to insufficient family history collection. Other errors can include offering germline genetic testing to patients without appropriate indications and with inadequate informed consent procedures. When genetic testing is pursued, lack of knowledge about genetic principles and testing methods can lead to misinterpretation and miscommunication of results, contributing to inappropriate management recommendations. These errors can contribute to under-use, overuse, or misuse of genetic testing that can compromise the quality of patient care.27,28 With this in mind, thought must be given at the health care system level to develop effective strategies to deliver genetic services to patients. These strategies must address workforce capacity, organizational structure, and education.
Workforce Capacity
The VA clinical genetics workforce needs to expand to keep pace with increasing demand, which will be accelerated by the precision oncology programs for prostate and lung cancers and the VA Teleoncology initiative. In the US there are 10 to 15 genetics professionals per 1,000,000 residents.29-31 Most genetics professionals work in academic and metropolitan settings, leaving suburban and rural areas underserved. For example, in California, some patients travel up to 386 miles for genetics care (mean, 76.6 miles).32 In the VA, there are only 1 to 2 genetics professionals per 1 million enrollees, about 10-fold fewer than in community care. Meeting clinical needs of patients at the VA is particularly challenging because more than one-third of veterans live in rural areas.33
We recently surveyed genetics professionals in the VA about their practices and capacity to increase patient throughput (Table). Currently in the VA, there are 8 clinical geneticists, not all of whom practice clinical genetics, and 13 genetic counselors. Five VA programs provide clinical genetic services to local and nearby VA facilities near Boston, Massachusetts; Houston, Texas; Los Angeles and San Francisco, California; and Salt Lake City, Utah. These programs, first developed in 2008, typically are staffed by 1 or 2 genetics professionals. Most patients who are referred to the VA genetics programs are evaluated for hereditary cancer syndromes. Multiple modes of delivery may be used, including in-person, telehealth, telephone, and provider-to-provider e-consults in the EHR.
In 2010, in response to increased demand for clinical genetics services, the VA launched the Genomic Medicine Service (GMS), a national program with a centralized team of 9 genetic counselors based in Salt Lake City. GMS provides telehealth genetic counseling services exclusively to veterans onsite and at about 90 VA facilities across the country. More recently, the addition of a clinical geneticist and APRN with genetics expertise has allowed GMS to provide more comprehensive genetic consultative services.
All VA genetics programs are currently at full capacity with long waits for an appointment. To expand clinical genetic services, the VA genetics professionals responding to our survey reported a need for additional support (eg, administrative, care coordination, clinical), resources (eg, clinical space, salary support), and organizational change (eg, division of Medical Genetics at facility level, services provided at the level of the Veterans Integrated Service Network). Given the dearth of genetic care providers in the community, referral to non-VA care is not a viable option in many markets. In addition, avoiding referral outside of the VA could help to ensure continuity of care, more efficient care, and reduce the risk of duplication of testing, and polypharmacy.34-37
As part of its precision oncology initiative, VA is focusing on building clinical genetics services capacity. To increase access to clinical genetic services and appropriate genetic testing, the VA needs more genetics professionals, including clinical geneticists, genetic counselors, and genetic nurses–ideally a workforce study could be performed to inform the right staffing mix needed. To grow the genetics workforce in the long term, the VA could leverage its academic affiliations to train the next generation of genetics professionals. The VA has an important role in training medical professionals. By forming affiliations with medical schools and universities, the VA has become the largest provider of health care training in the US.38
Genetic Health Care Organization in the VA
Understanding a patient’s genetic background increasingly has become more and more important in the clinic, which necessitates a major shift in health care. Unfortunately, on a national scale, the number of clinical genetics professionals has not kept pace with the need-limiting the ability to grow the traditional genetics workforce in the VA in the near term.29-31 Thus, we must look to alternative genetic health care models in which other members of the health care team assume some of the genetic evaluation and counseling activities while caring for their cancer patients with referral to a clinical genetics team, as needed.39
Two genetic health care models have been described.40 Traditionally, clinical genetic services are coordinated between genetics professionals and other clinicians, organized as a regional genetics center and usually affiliated with an academic medical center. By contrast, the nontraditional genetic health care model integrates genetic services within primary and specialty care. Under the new approach, nongeneticists can be assisted by decision support tools in the EHR that help with assessing family history risk, identifying indications for genetic testing, and suggesting management options based on genetic test results.41-43
The VA National Precision Oncology Program (NPOP) is shaped by a commitment to be a high reliability organization (HRO). As such, the goal is to create a system of excellence that integrates precision medicine, implementation science, and the learning health care system to improve the health and health care of veterans with cancer. This initiative is establishing the foundations for best-in-class cancer care to enable veterans access to life-saving therapies through a concerted effort that began with the Cancer Moonshot, development of the NPOP, and collaborations with the VA Office of Research and Development. One of the fundamental objectives of this initiative is to implement strategies that ensure clinical genetic services are available to veterans receiving cancer care at all VA facilities and to extend these services to veterans in remote geographic locations nationwide. The initiative aims to synergize VA Teleoncology services that seek to deliver best-in-class oncology care across the VA enterprise using cutting-edge technologies.
Conclusions
To accomplish the goal of delivering world-class clinical genetic services to veterans and meet the increasing needs of precision oncology and support quality genetic health care, the VA must develop an integrated system of genetic health care that will have a network of clinical genetics that interfaces with other clinical and operational programs, genomics researchers, and educational programs to support quality genetic health care. The VA has highly qualified and dedicated genetics professionals at many sites across the country. Connecting them could create powerful synergies that would benefit patients and strengthen the genetics workforce. The clinical genetics network will enable development and dissemination of evidence-based policies, protocols, and clinical pathways for genomic medicine. This will help to identify, benchmark, and promote best practices for clinical genetic services, and increase access, increase efficiencies, and reduce variability in the care delivered.
The VA is well positioned to achieve successful implementation of genetic services given its investment in genomic medicine and the commitment of the VA NPOP. However, there is a need for structured and targeted implementation strategies for genetic services in the VA, as uptake of this innovation will not occur by passive diffusion.44,45 To keep pace with the demand for germline testing in veterans, VA may want to consider an outsized focus on training genetics professionals, given the high demand for this expertise. Perhaps most importantly, the VA will need to better prepare its frontline clinical workforce to integrate genetics into their practice. This could be facilitated by identifying implementation strategies and educational programs for genomic medicine that help clinicians to think genetically while caring for their patients, performing aspects of family history risk assessment and pre- and posttest genetic counseling as they are able, and referring complex cases to the clinical genetics network when needed.
Much is already known on how best to accomplish this through studies conducted by many talented VA health services researchers.46 Crucially, clinical tools embedded within the VA EHR will be fundamental to these efforts by facilitating identification of patients who can benefit from genetic services and genetic testing at the point of care. Through integration of VA research with clinical genetic services, the VA will become more prepared to realize the promise of genomic medicine for veterans.
Acknowledgments
We thank the members of the Genomic Medicine Program Advisory Committee, Clinical Genetics Subcommittee for providing input and guidance on the topics included in this article.
The US Department of Veterans Affairs (VA) oversees the largest integrated health care system in the nation, administering care to 9 million veterans annually throughout its distributed network of 1,255 medical centers and outpatient facilities. Every year, about 50,000 veterans are diagnosed with and treated for cancer in the VA, representing about 3% of all cancer cases in the US.1 After skin cancer, prostate, colon, and lung cancers are the most common among veterans.1 One way that VA has sought to improve the care of its large cancer patient population is through the adoption of precision oncology, an ever-evolving practice of analyzing an individual patient’s cancer to inform clinical decision making. Most often, the analysis includes conducting genetic testing of the tumor itself. Here, we describe the opportunities and challenges of integrating germline genetics into precision oncology practice.
The Intersection of Precision Oncology and Germline Genetics
Precision oncology typically refers to genetic testing of tumor DNA to identify genetic variants with potential diagnostic, prognostic, or predictive therapeutic implications. It is enabled by a growing body of knowledge that identifies key drivers of cancer development, coupled with advances in tumor analysis by next-generation sequencing and other technologies and by the availability of new and repurposed therapeutic agents.2 Precision oncology has transformed cancer care by targeting both common and rare malignancies with specific therapies that improve clinical outcomes in patients.3
Testing of tumor DNA can reveal both somatic (acquired) and germline (inherited) gene variants. Precision oncology testing strategies can include tumor-only testing with or without subtraction of suspected germline variants, or paired tumor-normal testing with explicit analysis and reporting of genes associated with germline predisposition.2 With tumor-only testing, the germline status of variants may be inferred and follow-up germline testing in normal tissue such as blood or saliva can be considered. Paired tumor-normal testing provides distinct advantages over tumor-only testing, including improvement of the mutation detection rate in tumors and streamlining interpretation of results for both the tumor and germline tests.
Regardless of the strategy used, tumor testing has the potential to uncover clinically relevant germline variation associated with heritable cancer susceptibility and other conditions, as well as carrier status for autosomal recessive disorders (eAppendix
Germline genetic information, independent of somatic variation, can influence the choice of targeted cancer therapies. For example, Mandelker and colleagues identified germline variants that would impact the treatment of 38 (3.7%) of 1,040 patients with cancer.4 Individuals with a germline pathogenic variant in a DNA repair gene (eg, BRCA1, BRCA2, ATM, CHEK2) are candidates for platinum chemotherapy and poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors that target the inability of a tumor to repair double-stranded DNA breaks.5,6 Individuals with a germline pathogenic variant in the MSH2, MLH1, MSH6, PMS2 or EPCAM genes (ie, Lynch syndrome) have tumors that are deficient in mismatch repair, and these tumors are responsive to inhibitors of the programmed death 1 (PD1) pathway.7,8
In addition to changing treatment decisions, identifying pathogenic germline variants can have health, reproductive, and psychosocial implications for the patient and the patient’s family members.9,10 A pathogenic germline variant can imply disease risk for both the patient and his or her relatives. In these cases, it is important to ascertain family history, understand the mode of inheritance, identify at-risk relatives, review the associated phenotype, and discuss management and prevention options for the patient and for family members. For example, a germline pathogenic variant in the BRCA2 gene is associated with increased risk for breast, ovarian, pancreatic, gastric, bile duct, and laryngeal cancer, and melanoma.11 Knowledge of these increased cancer risks could inform cancer prevention and early detection options, such as more frequent and intensive surveillance starting at younger ages compared with that of average-risk individuals, use of chemoprevention treatments, and for those at highest risk, risk-reducing surgical procedures. Therefore, reporting germline test results requires the clinician to take on additional responsibilities beyond those required when reporting only somatic variants.
Because of the complexities inherent in germline genetic testing, it traditionally is offered in the context of a genetic consultation, comprised of genetic evaluation and genetic counseling (Figure). Clinical geneticists are physicians certified by the American Board of Medical Genetics and Genomics (a member board of the American Board of Medical Specialties) who received special training in the diagnosis and management of medical genetic conditions; they are trained to perform all aspects of a genetic consultation across the clinical spectrum and lifespan of a patient.12 In contrast, genetic counselors have a master’s degree in genetic counseling, a communication process that facilitates patient decision making surrounding the genetic evaluation.13 Most work as members of a team to ensure provision of comprehensive clinical genetic services. Genetic counselors are licensed in most states, and licensure in some states sanctions the ordering of genetic tests by genetic counselors. Genetics nurses are licensed professional nurses with special education and training in genetics who function in diverse roles in industry, education, research, and clinical care.14 Genetics nurses in clinical care perform risk assessment based on personal and family history, recognize and identify genetic conditions and predispositions, and discuss the implications of this with patients and their families. Advanced practice nurses (APRNs) have additional training that allows for diagnosis, interpretation of results, and surveillance and management recommendations.15
Germline Genetic Testing Challenges
Integrating germline genetic testing in precision oncology practice presents challenges at the patient, family, health care provider, and health system levels. Due to these challenges, implementation planning is obligatory, as germline testing has become a standard-of-care for certain tumor types and patients.2
On learning of a germline pathogenic variant or variant of uncertain significance, patients may experience distress and anxiety, especially in the short term.16-18 In addition, it can be difficult for patients to share germline genetic test results with their family; parents may feel guilty about the possibility of passing on a predisposition to children, and unaffected siblings may experience survivor guilt. For some veterans, there can be concerns about losing service-connected benefits if a genetic factor is found to contribute to their cancer history. In addition, patients may have concerns about discrimination by employers or insurers, including commercial health insurance or long-term care, disability, and life insurance. Yet there are many state and federal laws that ensure some protection from employment and health insurance discrimination based on genetic information.
For cancer care clinicians, incorporating germline testing requires additional responsibilities that can complicate care. Prior to germline genetic testing, genetic counseling with patients is recommended to review the potential benefits, harms, and limitations of genetic testing. Further, posttest genetic counseling is recommended to help the patient understand how the results may influence future cancer risks, provide recommendations for cancer management and prevention, and discuss implications for family members.9,10 While patients trust their health care providers to help them access and understand their genetic information, most health care providers are unprepared to integrate genetics into their practice; they lack adequate knowledge, skills, and confidence about genetics to effectively deliver genetic services.19-26 This leads to failure to recognize patients with indications for genetic testing, which often is due to insufficient family history collection. Other errors can include offering germline genetic testing to patients without appropriate indications and with inadequate informed consent procedures. When genetic testing is pursued, lack of knowledge about genetic principles and testing methods can lead to misinterpretation and miscommunication of results, contributing to inappropriate management recommendations. These errors can contribute to under-use, overuse, or misuse of genetic testing that can compromise the quality of patient care.27,28 With this in mind, thought must be given at the health care system level to develop effective strategies to deliver genetic services to patients. These strategies must address workforce capacity, organizational structure, and education.
Workforce Capacity
The VA clinical genetics workforce needs to expand to keep pace with increasing demand, which will be accelerated by the precision oncology programs for prostate and lung cancers and the VA Teleoncology initiative. In the US there are 10 to 15 genetics professionals per 1,000,000 residents.29-31 Most genetics professionals work in academic and metropolitan settings, leaving suburban and rural areas underserved. For example, in California, some patients travel up to 386 miles for genetics care (mean, 76.6 miles).32 In the VA, there are only 1 to 2 genetics professionals per 1 million enrollees, about 10-fold fewer than in community care. Meeting clinical needs of patients at the VA is particularly challenging because more than one-third of veterans live in rural areas.33
We recently surveyed genetics professionals in the VA about their practices and capacity to increase patient throughput (Table). Currently in the VA, there are 8 clinical geneticists, not all of whom practice clinical genetics, and 13 genetic counselors. Five VA programs provide clinical genetic services to local and nearby VA facilities near Boston, Massachusetts; Houston, Texas; Los Angeles and San Francisco, California; and Salt Lake City, Utah. These programs, first developed in 2008, typically are staffed by 1 or 2 genetics professionals. Most patients who are referred to the VA genetics programs are evaluated for hereditary cancer syndromes. Multiple modes of delivery may be used, including in-person, telehealth, telephone, and provider-to-provider e-consults in the EHR.
In 2010, in response to increased demand for clinical genetics services, the VA launched the Genomic Medicine Service (GMS), a national program with a centralized team of 9 genetic counselors based in Salt Lake City. GMS provides telehealth genetic counseling services exclusively to veterans onsite and at about 90 VA facilities across the country. More recently, the addition of a clinical geneticist and APRN with genetics expertise has allowed GMS to provide more comprehensive genetic consultative services.
All VA genetics programs are currently at full capacity with long waits for an appointment. To expand clinical genetic services, the VA genetics professionals responding to our survey reported a need for additional support (eg, administrative, care coordination, clinical), resources (eg, clinical space, salary support), and organizational change (eg, division of Medical Genetics at facility level, services provided at the level of the Veterans Integrated Service Network). Given the dearth of genetic care providers in the community, referral to non-VA care is not a viable option in many markets. In addition, avoiding referral outside of the VA could help to ensure continuity of care, more efficient care, and reduce the risk of duplication of testing, and polypharmacy.34-37
As part of its precision oncology initiative, VA is focusing on building clinical genetics services capacity. To increase access to clinical genetic services and appropriate genetic testing, the VA needs more genetics professionals, including clinical geneticists, genetic counselors, and genetic nurses–ideally a workforce study could be performed to inform the right staffing mix needed. To grow the genetics workforce in the long term, the VA could leverage its academic affiliations to train the next generation of genetics professionals. The VA has an important role in training medical professionals. By forming affiliations with medical schools and universities, the VA has become the largest provider of health care training in the US.38
Genetic Health Care Organization in the VA
Understanding a patient’s genetic background increasingly has become more and more important in the clinic, which necessitates a major shift in health care. Unfortunately, on a national scale, the number of clinical genetics professionals has not kept pace with the need-limiting the ability to grow the traditional genetics workforce in the VA in the near term.29-31 Thus, we must look to alternative genetic health care models in which other members of the health care team assume some of the genetic evaluation and counseling activities while caring for their cancer patients with referral to a clinical genetics team, as needed.39
Two genetic health care models have been described.40 Traditionally, clinical genetic services are coordinated between genetics professionals and other clinicians, organized as a regional genetics center and usually affiliated with an academic medical center. By contrast, the nontraditional genetic health care model integrates genetic services within primary and specialty care. Under the new approach, nongeneticists can be assisted by decision support tools in the EHR that help with assessing family history risk, identifying indications for genetic testing, and suggesting management options based on genetic test results.41-43
The VA National Precision Oncology Program (NPOP) is shaped by a commitment to be a high reliability organization (HRO). As such, the goal is to create a system of excellence that integrates precision medicine, implementation science, and the learning health care system to improve the health and health care of veterans with cancer. This initiative is establishing the foundations for best-in-class cancer care to enable veterans access to life-saving therapies through a concerted effort that began with the Cancer Moonshot, development of the NPOP, and collaborations with the VA Office of Research and Development. One of the fundamental objectives of this initiative is to implement strategies that ensure clinical genetic services are available to veterans receiving cancer care at all VA facilities and to extend these services to veterans in remote geographic locations nationwide. The initiative aims to synergize VA Teleoncology services that seek to deliver best-in-class oncology care across the VA enterprise using cutting-edge technologies.
Conclusions
To accomplish the goal of delivering world-class clinical genetic services to veterans and meet the increasing needs of precision oncology and support quality genetic health care, the VA must develop an integrated system of genetic health care that will have a network of clinical genetics that interfaces with other clinical and operational programs, genomics researchers, and educational programs to support quality genetic health care. The VA has highly qualified and dedicated genetics professionals at many sites across the country. Connecting them could create powerful synergies that would benefit patients and strengthen the genetics workforce. The clinical genetics network will enable development and dissemination of evidence-based policies, protocols, and clinical pathways for genomic medicine. This will help to identify, benchmark, and promote best practices for clinical genetic services, and increase access, increase efficiencies, and reduce variability in the care delivered.
The VA is well positioned to achieve successful implementation of genetic services given its investment in genomic medicine and the commitment of the VA NPOP. However, there is a need for structured and targeted implementation strategies for genetic services in the VA, as uptake of this innovation will not occur by passive diffusion.44,45 To keep pace with the demand for germline testing in veterans, VA may want to consider an outsized focus on training genetics professionals, given the high demand for this expertise. Perhaps most importantly, the VA will need to better prepare its frontline clinical workforce to integrate genetics into their practice. This could be facilitated by identifying implementation strategies and educational programs for genomic medicine that help clinicians to think genetically while caring for their patients, performing aspects of family history risk assessment and pre- and posttest genetic counseling as they are able, and referring complex cases to the clinical genetics network when needed.
Much is already known on how best to accomplish this through studies conducted by many talented VA health services researchers.46 Crucially, clinical tools embedded within the VA EHR will be fundamental to these efforts by facilitating identification of patients who can benefit from genetic services and genetic testing at the point of care. Through integration of VA research with clinical genetic services, the VA will become more prepared to realize the promise of genomic medicine for veterans.
Acknowledgments
We thank the members of the Genomic Medicine Program Advisory Committee, Clinical Genetics Subcommittee for providing input and guidance on the topics included in this article.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
2. Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142-1148. doi:10.1038/s41436-020-0783-8
3. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. Published 2020 Jan 14. doi:10.1186/s13073-019-0703-1
4. Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
5. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi:10.1056/NEJMoa1506859
6. Ratta R, Guida A, Scotté F, et al. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review [published online ahead of print, 2020 May 4]. Prostate Cancer Prostatic Dis. 2020;10.1038/s41391-020-0233-3. doi:10.1038/s41391-020-0233-3
7. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. doi:10.1371/journal.pone.0233260
9. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K; American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893-901. doi:10.1200/JCO.2009.27.0660
10. Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21(2):151-161. doi:10.1007/s10897-011-9462-x
11. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993.
12. ACMG Board of Directors. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2015;17(9):e3. doi:10.1038/gim.2015.94
13. National Society of Genetic Counselors’ Definition Task Force, Resta R, Biesecker BB, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77-83. doi:10.1007/s10897-005-9014-3
14. Calzone KA, Cashion A, Feetham S, et al. Nurses transforming health care using genetics and genomics [published correction appears in Nurs Outlook. 2010;58(3):163]. Nurs Outlook. 2010;58(1):26-35. doi:10.1016/j.outlook.2009.05.001
15. US Department of Veterans Affairs, Veterans Health Administration, Office of Nursing Services. 2018 Office of Nursing Services (ONS) Annual Brief. https://www.va.gov/nursing/docs/about/2018_ONS_Annual_Report_Brief.pdf. Accessed July 21, 2020.
16. Lerman C, Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology (Williston Park). 1996;10(2):191-202.
17. Bonadona V, Saltel P, Desseigne F, et al. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11(1):97-104.
18. Murakami Y, Okamura H, Sugano K, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101(2):395-403. doi:10.1002/cncr.20363
19. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413-423.
20. Dhar SU, Cooper HP, Wang T, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(1):221-227. doi:10.1007/s10549-011-1449-7
21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13(2):148-154. doi:10.1097/GIM.0b013e318207f564
22. Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61-66. doi:10.1016/j.amepre.2010.09.027
23. Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367-375. doi:10.1089/gtmb.2012.0381
24. Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48-63. doi:10.1007/s10897-013-9605-3
25. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311-324. doi:10.1007/s10689-013-9695-y
26. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. doi:10.1038/gim.2014.101
27. Scheuner MT, Hilborne L, Brown J, Lubin IM; members of the RAND Molecular Genetic Test Report Advisory Board. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16(7):761-769. doi:10.1089/gtmb.2011.0328
28. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
29. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. doi:10.1097/01.gim.0000172416.35285.9f
30. Institute of Medicine. Roundtable on Translating Genomic-Based Research for Health. Washington, DC: National Academies Press; 2009. https://www.ncbi.nlm.nih.gov/books/NBK26394. Accessed July 22, 2020.
31. Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. doi:10.1007/s10897-017-0158-8
32. Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22(1):227-231. doi:10.1038/s41436-019-0628-5

33. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: US Department of Veterans Affairs; 2011. https://www.hsrd.research.va.gov/publications/esp/ambulatory.cfm. Accessed July 21, 2020.
34. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39-68. doi:10.1111/j.1468-0009.2011.00619.x
35. Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. Published 2010 May 20. doi:10.1186/1472-6963-10-132
36. McDonald KM, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Version 4. Agency for Healthcare Research and Quality Publication No. 14-0037. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed July 22, 2020.
37. Greenwood-Lee J, Jewett L, Woodhouse L, Marshall DA. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18(1):986. Published 2018 Dec 20. doi:10.1186/s12913-018-3745-y
38. US Department of Veterans Affairs, Office of Academic Affiliations. Our medical and dental training program. https://www.va.gov/oaa/gme_default.asp. Updated January 7, 2020. Accessed July 21, 2020.
39. Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16(8):609-619. doi:10.1038/gim.2013.202.
40. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15(1):34-45. doi:10.1159/000328846
41. Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32(2):218-227. doi:10.1080/03014460500074921
42. Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60-69. doi:10.1038/gim.2013.75
43. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
44. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238-245. doi:10.1038/gim.2013.101
45. Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6(2):16. Published 2016 Apr 28. doi:10.3390/jpm6020016
46. US Department of Veterans Affairs, Health Services Research and Development. Genomics. https://www.hsrd.research.va.gov/research/portfolio_description.cfm?Sulu=17. Updated July 21, 2020. Accessed June 22, 2020.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
2. Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142-1148. doi:10.1038/s41436-020-0783-8
3. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. Published 2020 Jan 14. doi:10.1186/s13073-019-0703-1
4. Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
5. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi:10.1056/NEJMoa1506859
6. Ratta R, Guida A, Scotté F, et al. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review [published online ahead of print, 2020 May 4]. Prostate Cancer Prostatic Dis. 2020;10.1038/s41391-020-0233-3. doi:10.1038/s41391-020-0233-3
7. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. doi:10.1371/journal.pone.0233260
9. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K; American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893-901. doi:10.1200/JCO.2009.27.0660
10. Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21(2):151-161. doi:10.1007/s10897-011-9462-x
11. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993.
12. ACMG Board of Directors. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2015;17(9):e3. doi:10.1038/gim.2015.94
13. National Society of Genetic Counselors’ Definition Task Force, Resta R, Biesecker BB, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77-83. doi:10.1007/s10897-005-9014-3
14. Calzone KA, Cashion A, Feetham S, et al. Nurses transforming health care using genetics and genomics [published correction appears in Nurs Outlook. 2010;58(3):163]. Nurs Outlook. 2010;58(1):26-35. doi:10.1016/j.outlook.2009.05.001
15. US Department of Veterans Affairs, Veterans Health Administration, Office of Nursing Services. 2018 Office of Nursing Services (ONS) Annual Brief. https://www.va.gov/nursing/docs/about/2018_ONS_Annual_Report_Brief.pdf. Accessed July 21, 2020.
16. Lerman C, Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology (Williston Park). 1996;10(2):191-202.
17. Bonadona V, Saltel P, Desseigne F, et al. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11(1):97-104.
18. Murakami Y, Okamura H, Sugano K, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101(2):395-403. doi:10.1002/cncr.20363
19. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413-423.
20. Dhar SU, Cooper HP, Wang T, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(1):221-227. doi:10.1007/s10549-011-1449-7
21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13(2):148-154. doi:10.1097/GIM.0b013e318207f564
22. Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61-66. doi:10.1016/j.amepre.2010.09.027
23. Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367-375. doi:10.1089/gtmb.2012.0381
24. Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48-63. doi:10.1007/s10897-013-9605-3
25. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311-324. doi:10.1007/s10689-013-9695-y
26. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. doi:10.1038/gim.2014.101
27. Scheuner MT, Hilborne L, Brown J, Lubin IM; members of the RAND Molecular Genetic Test Report Advisory Board. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16(7):761-769. doi:10.1089/gtmb.2011.0328
28. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
29. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. doi:10.1097/01.gim.0000172416.35285.9f
30. Institute of Medicine. Roundtable on Translating Genomic-Based Research for Health. Washington, DC: National Academies Press; 2009. https://www.ncbi.nlm.nih.gov/books/NBK26394. Accessed July 22, 2020.
31. Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. doi:10.1007/s10897-017-0158-8
32. Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22(1):227-231. doi:10.1038/s41436-019-0628-5

33. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: US Department of Veterans Affairs; 2011. https://www.hsrd.research.va.gov/publications/esp/ambulatory.cfm. Accessed July 21, 2020.
34. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39-68. doi:10.1111/j.1468-0009.2011.00619.x
35. Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. Published 2010 May 20. doi:10.1186/1472-6963-10-132
36. McDonald KM, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Version 4. Agency for Healthcare Research and Quality Publication No. 14-0037. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed July 22, 2020.
37. Greenwood-Lee J, Jewett L, Woodhouse L, Marshall DA. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18(1):986. Published 2018 Dec 20. doi:10.1186/s12913-018-3745-y
38. US Department of Veterans Affairs, Office of Academic Affiliations. Our medical and dental training program. https://www.va.gov/oaa/gme_default.asp. Updated January 7, 2020. Accessed July 21, 2020.
39. Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16(8):609-619. doi:10.1038/gim.2013.202.
40. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15(1):34-45. doi:10.1159/000328846
41. Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32(2):218-227. doi:10.1080/03014460500074921
42. Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60-69. doi:10.1038/gim.2013.75
43. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
44. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238-245. doi:10.1038/gim.2013.101
45. Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6(2):16. Published 2016 Apr 28. doi:10.3390/jpm6020016
46. US Department of Veterans Affairs, Health Services Research and Development. Genomics. https://www.hsrd.research.va.gov/research/portfolio_description.cfm?Sulu=17. Updated July 21, 2020. Accessed June 22, 2020.
VA National Precision Oncology Program (FULL)
As the nation’s largest integrated health care system with about 50,000 new cancer diagnoses per year, providing care for over 400,000 veterans with cancer and a robust research portfolio, the US Department of Veterans Affairs (VA) is well positioned to be a leader in both clinical and research in oncology. The VA National Precision Oncology Program (NPOP), which provides tumor sequencing and consultative services, is a key component of VA oncology assets.
Case Presentation
As the mission of the VA is to “care for him who shall have borne the battle,” it is fitting to begin with the story of a US Army veteran in his 40s and the father of 2 young children who developed progressive shortness of breath, cough, and weight loss over a period of 8 months. He was diagnosed with metastatic lung adenocarcinoma in 2016, and standard testing of his tumor showed no alteration of the EGFR and ALK genes. He was treated with whole brain radiation and had begun treatment for carboplatin and pemetrexed chemotherapy with mixed tumor response.
Subsequently, his tumor was tested through NPOP, using a multigene next-generation sequencing (NGS) assay panel, which showed the presence of an abnormal fusion between the EML4 and ALK genes. The chemotherapy was discontinued and oral crizotinib precision therapy was started. The patient had an excellent response in all sites of disease (Figure 1). He was able to return to work and school.
In July 2017, his medication was switched to alectinib for asymptomatic progression in his brain, and there was further response. In September 2019, he was treated with precision intensity-modulated radiotherapy (IMRT), targeting a single brain metastasis as there were no other sites of cancer progression and no cancerrelated symptoms. He finished school and continues to work.
Precision Oncology
Oncology is a relatively young medical field. The early medical treatments for cancer were developed empirically against hematologic malignancies, particularly leukemias. Cytotoxic chemotherapeutic agents as a group have modest effects on most solid tumors, and even modern genomics has had limited ability to predict differential benefit in patients with advanced-stage carcinomas. As a result, the medications are used in a nonprecision manner in which all patients with the same cancer diagnosis and stage receive the same treatment. This is due in part to our limited understanding of both the pathophysiology of cancer and the mechanism of action of cytotoxic agents.
The paradigm of precision oncology, in contrast, utilizes unique, patient-specific molecular characteristics to guide prescribing of antineoplastic agents (Figure 2). These molecular characteristics are frequently tumoral but also may be nontumoral, such as germline genetic variants and even nonhuman, such as the gut microbiome as has been proposed as predictive of response to immune checkpoint inhibitors.1,2
One of the first examples of precision oncology was tumor testing for the estrogen receptor in breast cancer, which distinguishes breast tumors sensitive to hormonal treatments from those that are resistant.3 In 2004, somatically acquired mutation of the EGFR gene was found to be associated with response to EGFR tyrosine kinase inhibitors such as gefitinib and erlotinib, and subsequently it was shown that patients without these mutations derived no benefit from use of these drugs.4 Thus, the precision oncology paradigm is using a molecular diagnostic as part of the indication for an antineoplastic agent, resulting in improved therapeutic efficacy and often reduced toxicity.
By 2015, multiple examples of DNA-based gene alterations that predict drug response were known, including at least 5 in non-small cell lung cancer (NSCLC). The heterogeneity of molecular testing practice patterns and methods of testing in VA along with the increasing number and complexity of molecular tests facilitated launch of a regional precision oncology program based primarily in Veterans Integrated Service Network 1, which provided tumor DNA sequencing through 2 vendors. Advances in DNA sequencing technology, particularly NGS, permit sequencing of multiple genes in clinical tumor samples, using a panel applicable for multiple tumor types. As part of VA contributions to the 2016 White House Cancer Moonshot initiative, the regional program became NPOP with expanded geographic scope, the addition of clinical consultative services, and robust informatics that supports associated research and a learning health care system. NPOP is a component of the VA National Oncology Program Office under the Office of Specialty Care.
Testing
With the launch of NPOP in mid-2016, there was rapid expansion of the number of VA facilities participating, and the number of tumor samples being submitted increased substantially. 5 The expansion was facilitated by both central funding for the tumor DNA sequencing and by NPOP-provided training of pathology laboratory staff and oncologists. Today, NPOP is utilized by almost every oncology practice in VA.
NPOP’s initial focus was on lung cancer, specifically advanced-stage nonsquamous NSCLC, which not only is very common in VA, but also has one of the highest number of mutated genes that result in sensitivity to antineoplastic drugs. Recently, metastatic prostate cancer was added as a second focus tumor type. Dashboards are available on the NPOP website to assist care teams in identifying veterans at their facility with either lung or prostate cancer who may be appropriate for testing. Other solid tumors can be sent for testing through NPOP if patients have advanced stage cancer and are medically appropriate for antineoplastic therapy. To date, NPOP has sequenced > 13,000 samples.
Testing options have been added to NPOP in addition to tumor DNA sequencing. The first addition was the so-called liquid biopsy, more properly known as the cell-free DNA (cfDNA) test, a plasma-based high-sensitivity DNA sequencing assay. cfDNA is shed from dying cells and can be captured and sequenced from a plasma sample obtained by standard venipuncture, using a special-purpose sample collection tube. The test is appropriate for patients who do not have an appropriate archival tumor sample or those who cannot have a new biopsy of tumor tissue. Tumor tissue remains the preferred test sample due to a higher sensitivity than that of cfDNA and less susceptibility to false positives, so consideration of a tumor biopsy is appropriate prior to requesting a cfDNA assay. Therapy can greatly impact the sensitivity of cfDNA testing, so patients should be having disease progression at the time of obtaining a blood sample for cfDNA.
Finally, myeloid leukocytic cells accumulate genetic alterations during aging similar to those found in myelodysplasia and acute myeloid leukemia. These myeloid-associated mutations can be detected in both tumor and cfDNA samples and are known as clonal hyperplasia of indeterminate potential (CHIP). CHIP is much more common in the cfDNA. For lung cancer, CHIP-associated gene variants are readily distinguished from lung cancer-associated variants, but that distinction is much more difficult in many other tumor types.
In partnership with the current DNA sequencing contractor, NPOP provides access to a second gene panel for hematologic malignancies or sarcomas, though neither of these classes of malignancies currently have clear indications for routine NGS multigene panel testing. Given the low rate of finding a gene mutation that would change therapy that could not be found with smaller, less expensive gene panels, NPOP requires prior approval for the use of this panel.
Finally, since early 2019, programmed deathligand 1 (PD-L1) immunohistochemistry analysis is available through NPOP in association with NGS testing of the same sample for those solid tumors with US Food and Drug Administration (FDA)-approved indications that include a PD-L1 companion diagnostic. This service was added to facilitate concurrent testing of PD-L1 and DNA sequencing, which speeds availability of molecular data to the health care provider and veteran.
Determining Clinical Significance
The complexity of tumor NGS gene panel test results is far greater than frequently ordered laboratory or molecular testing due to the near infinite number of possible results and varying degrees of consensus of the significance of the results for therapeutic decision making. That complexity is reflected in the length of the test reports, which are often ≥ 20 pages. Starting from the gene variants identified by the DNA sequencing variant-caller bioinformatics pipeline, there is a 2-step process, referred to as annotation, to interpret the clinical significance that is repeated for each variant.
The first step is to assign a pathogenicity value, also known as oncogenicity, using a 5-point Likert scale from pathogenic to benign with variant of unknown significance (VUS) in the middle of the scale. Only variants that are pathogenic or likely pathogenic are considered further. A VUS is usually communicated to the health care provider but should generally not be acted on, while benign and likely benign variants may or may not be included in the report and should never be acted on. NPOP examined the concordance of pathogenicity calls among 3 annotation services: N-of-One/QCI Precision Insights (qiagen.com), IBM Watson for Genomics (WfG), and OncoKB (www.oncokb.org).6 There was moderate-to-poor concordance, indicating lack of consensus about whether a significant fraction of observed gene variants contributes to the patient’s cancer. This variability likely arises due to differences in algorithms and criteria used to assess pathogenicity.
The second step of annotation is assignment of the actionability of the variant, using a level of evidence (LoE) scale from 1 (on-label indication) to 4 (absence of clinical evidence; ie, only preclinical or theoretical evidence). Initially, NPOP used an adaptation of the LoE scales from WfG and OncoKB but now mostly uses the recently revised OncoKB LoE. Actionability also includes prediction of resistance to a treatment (LoE level R1 and R2). An example of a resistance gene variant is a KRAS mutation in colorectal cancer, which predicts lack of clinical benefit from anti- EGFR antibodies. It is important to note that a determination of actionability requires 3 inputs: gene, variant, and tumor type. A BRAF V600E mutation in melanoma has different medications with level 1 LoE than does the same mutation in colorectal cancer, for example.
Another complexity in annotation for actionability is tumor type ontogeny—the classification system used for cancer types. WfG uses a subset of the National Cancer Institute Thesaurus (ncithesaurus.nci.nih.gov), OncoKB uses the unique OncoTree (oncotree.mskcc.org), and Foundation Medicine (www.foundationmed icine.com), and N-of-One use propriety classification systems. The WfG and OncoKB tumor types have evolved over time, while it is unclear what changes have been made in the FMI and N-of-One tumor type classification systems. Thus, a gene variant observed in a single patient may be annotated differently by these services because of how the tumor type is mapped onto the services’ tumor type ontogeny. NPOP has been assigning WfG diagnoses since 2017, including historic assignment for prior samples back to the pilot project in 2015. In early 2019, NPOP began requiring test requesters to include International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) diagnoses (histology and site codes) with the sample. ICD-O-3 codes are used in all cancer registry data in North America, including the VA Cancer Registry System. The approximately 50,000 possible diagnoses allow fine precision in diagnoses, which is important for less common and rare cancer types; however, the large number of diagnoses adds complexity. NPOP has created a partial translation table for ICD-O-3 to WfG diagnosis that includes all diagnoses seen to date; this table facilitates continuing provision of WfG diagnosis without manual review as was previously required.
NPOP-Provided Genetic Services
Given these complexities in interpretation of NGS multigene panel results, NPOP provides several services to assist health care providers and other members of the care team. First, the NPOP Interfacility Consult (IFC) is a virtual “phone-a-friend” service that provides asynchronous patient-specific expert recommendations in precision oncology. By far the most requested service is assistance with interpretation of a patient’s DNA sequence results. Other requests include advice on whether to perform NGS testing and what molecular testing to perform. The IFC is integral to the VA Computerized Patient Record System electronic health record. Additional requests have been submitted and answered by e-mail.
The Molecular Oncology Tumor Board is a monthly case-based educational conference supported by the VA Employee Education Service, which provides continuing education credits for attendees. NPOP staff coordinate the conference, and a panel of specialists from around the country provide expert commentary.
In 2016, IBM gifted the services of WfG to VA. WfG’s main functionality is annotation of NGS results. About 5,000 samples were processed from 2017 to 2019; sample processing is expected to resume shortly. The availability of WfG annotations early in NPOP operation was very useful to the implementation of NPOP in general and the NPOP consultation services in particular, resulting in improved thoroughness of opinions provided by NPOP staff.
Informatics
Informatics is an essential component of NPOP that facilitates both clinical care and research (Figure 3). Results of NGS gene panels are returned to the facility that submitted the sample for testing as a PDF document. NPOP receives the same PDF report in real time but also structured data of the results including a variant callformat file and XML file. The secondary sequence data in binary alignment map or FASTQ format is received in batches. NPOP program staff extract data from these files and then load it into SQL tables in the VA Corporate Data Warehouse. In partnership with the VA Pharmacy Benefits Management Service, NPOP has constructed user-friendly dashboards that allow users with no technical skills and who have the appropriate data access permissions to view various portions of the NPOP database. There are dashboards to display a list of NPOP samples by facility, find a patient by name or other identifying information, and display a list of patients who have received any antineoplastic drug, among other functions.
The NPOP database has been used to reannotate NGS results, such as when new drugs are approved. For example, when the first neurotrophic tropomyosin receptor kinase (NTRK) inhibitor was approved, NPOP rapidly identified all living patients with NTRK fusions and notified the health care providers of the availability a potential new treatment option for their patient. 7 NPOP is now building a method to allow NPOP dashboard users to similarly identify patients who have not received a corresponding drug for a user-selected LoE annotation. This will facilitate a systems approach to ensure that all patients with EGFR exon 19 deletions, for example, either have received an EGFR inhibitor or are reviewed to determine why they have not. Furthermore, the database will facilitate real-world data analysis in precision oncology. For example, prior to the recent FDA-approval of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors for prostate cancer, 50 veterans already had been treated with one of these agents. These data can help further inform which of the many variants of DNA damage repair genes benefit from PARP inhibitors.
Ensuring Access to Care for All Veterans
With any new medical technology comes an obligation to ensure appropriate equal access so as to not exacerbate health care disparities. Veterans enrolled in VA health care are much more likely to live in rural communities than does the US population as a whole, and there was concern that these veterans would not receive NGS testing at the same rate as urban veterans. NPOP therefore was intentional during implementation to ensure rural veterans were being offered testing. Indeed, there has been nearly equal utilization by rurality. No other disparities in NPOP utilization have been seen.
A majority of veterans who undergo testing in NPOP have at least 1 actionable gene variant reported.5 However, some of these are for lower LoE off-label use of FDA-approved medications, but many are for agents available only through clinical trials. Consideration of treatments available through a clinical trial is part of standard practice for patients with advanced malignancies. NPOP data have helped identify cohorts who are eligible for clinical trials on the basis of their tumor DNA sequencing results. The National Oncology Program Office has been working closely with the VA Office of Research and Development to expand access to cancer clinical trials in VA. Veterans also can be treated on trials outside VA as medically appropriate and with local VA approval.
Conclusions
The VA NPOP is one of the largest clinical DNA sequencing programs in the nation with integrated consultation services and health informatics resources to facilitate patient care, clinical trials, and health outcomes research. The clinical services of NPOP provide cuttingedge oncology services to veterans throughout VA without exacerbating disparities and will be a national resource for research.
Acknowledgments
NPOP was made possible and implemented through the efforts of a number of people in VHA, including the national and regional leaders who supported the program’s vision and implementation, especially Michael Mayo-Smith, David Shulkin, Jennifer S. Lee, and Laurence Meyer, the leaders and staff of the Massachusetts Veterans Epidemiology Research and Information Center who piloted regional NGS testing, and especially my current and former colleagues in the VA National Oncology Program Office, without whom NPOP would not be possible. The contributions of Neil L. Spector who served as inaugural Director of Precision Oncology and Jill E. Duffy in her role as Director of Oncology Operations are particularly noteworthy.
1. Lima ZS, Ghadamzadeh M, Arashloo FT, Amjad G, Ebadi MR, Younesi L. Recent advances of therapeutic targets based on the molecular signature in breast cancer: genetic mutations and implications for current treatment paradigms. J Hematol Oncol. 2019;12(1):38. Published 2019 Apr 11. doi:10.1186/s13045-019-0725-6
2. Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108. Published 2019 Apr 17. doi:10.1186/s40425-019-0574-4
3. Kiang DT, Kennedy BJ. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Intern Med. 1977;87(6):687- 690. doi:10.7326/0003-4819-87-6-687.
4. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497-1500. doi:10.1126/science.1099314
5. Poonnen P, Duffy J, Hintze BJ, et al. Genomic analysis of metastatic solid tumors in veterans: findings from the VHA National Precision Oncology Program. J Clin Oncol. 2019;37(suppl 15):3074. doi:10.1200/JCO.2019.37.15_suppl.3074
6. Katsoulakis E, Duffy JE, Hintze B, Spector NL, Kelley MJ. Comparison of annotation services for nextgeneration sequencing in a large-scale precision oncology program. JCO Precis Oncol. 2020(4):212-221. doi:10.1200/PO.19.00118
7. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
As the nation’s largest integrated health care system with about 50,000 new cancer diagnoses per year, providing care for over 400,000 veterans with cancer and a robust research portfolio, the US Department of Veterans Affairs (VA) is well positioned to be a leader in both clinical and research in oncology. The VA National Precision Oncology Program (NPOP), which provides tumor sequencing and consultative services, is a key component of VA oncology assets.
Case Presentation
As the mission of the VA is to “care for him who shall have borne the battle,” it is fitting to begin with the story of a US Army veteran in his 40s and the father of 2 young children who developed progressive shortness of breath, cough, and weight loss over a period of 8 months. He was diagnosed with metastatic lung adenocarcinoma in 2016, and standard testing of his tumor showed no alteration of the EGFR and ALK genes. He was treated with whole brain radiation and had begun treatment for carboplatin and pemetrexed chemotherapy with mixed tumor response.
Subsequently, his tumor was tested through NPOP, using a multigene next-generation sequencing (NGS) assay panel, which showed the presence of an abnormal fusion between the EML4 and ALK genes. The chemotherapy was discontinued and oral crizotinib precision therapy was started. The patient had an excellent response in all sites of disease (Figure 1). He was able to return to work and school.
In July 2017, his medication was switched to alectinib for asymptomatic progression in his brain, and there was further response. In September 2019, he was treated with precision intensity-modulated radiotherapy (IMRT), targeting a single brain metastasis as there were no other sites of cancer progression and no cancerrelated symptoms. He finished school and continues to work.
Precision Oncology
Oncology is a relatively young medical field. The early medical treatments for cancer were developed empirically against hematologic malignancies, particularly leukemias. Cytotoxic chemotherapeutic agents as a group have modest effects on most solid tumors, and even modern genomics has had limited ability to predict differential benefit in patients with advanced-stage carcinomas. As a result, the medications are used in a nonprecision manner in which all patients with the same cancer diagnosis and stage receive the same treatment. This is due in part to our limited understanding of both the pathophysiology of cancer and the mechanism of action of cytotoxic agents.
The paradigm of precision oncology, in contrast, utilizes unique, patient-specific molecular characteristics to guide prescribing of antineoplastic agents (Figure 2). These molecular characteristics are frequently tumoral but also may be nontumoral, such as germline genetic variants and even nonhuman, such as the gut microbiome as has been proposed as predictive of response to immune checkpoint inhibitors.1,2
One of the first examples of precision oncology was tumor testing for the estrogen receptor in breast cancer, which distinguishes breast tumors sensitive to hormonal treatments from those that are resistant.3 In 2004, somatically acquired mutation of the EGFR gene was found to be associated with response to EGFR tyrosine kinase inhibitors such as gefitinib and erlotinib, and subsequently it was shown that patients without these mutations derived no benefit from use of these drugs.4 Thus, the precision oncology paradigm is using a molecular diagnostic as part of the indication for an antineoplastic agent, resulting in improved therapeutic efficacy and often reduced toxicity.
By 2015, multiple examples of DNA-based gene alterations that predict drug response were known, including at least 5 in non-small cell lung cancer (NSCLC). The heterogeneity of molecular testing practice patterns and methods of testing in VA along with the increasing number and complexity of molecular tests facilitated launch of a regional precision oncology program based primarily in Veterans Integrated Service Network 1, which provided tumor DNA sequencing through 2 vendors. Advances in DNA sequencing technology, particularly NGS, permit sequencing of multiple genes in clinical tumor samples, using a panel applicable for multiple tumor types. As part of VA contributions to the 2016 White House Cancer Moonshot initiative, the regional program became NPOP with expanded geographic scope, the addition of clinical consultative services, and robust informatics that supports associated research and a learning health care system. NPOP is a component of the VA National Oncology Program Office under the Office of Specialty Care.
Testing
With the launch of NPOP in mid-2016, there was rapid expansion of the number of VA facilities participating, and the number of tumor samples being submitted increased substantially. 5 The expansion was facilitated by both central funding for the tumor DNA sequencing and by NPOP-provided training of pathology laboratory staff and oncologists. Today, NPOP is utilized by almost every oncology practice in VA.
NPOP’s initial focus was on lung cancer, specifically advanced-stage nonsquamous NSCLC, which not only is very common in VA, but also has one of the highest number of mutated genes that result in sensitivity to antineoplastic drugs. Recently, metastatic prostate cancer was added as a second focus tumor type. Dashboards are available on the NPOP website to assist care teams in identifying veterans at their facility with either lung or prostate cancer who may be appropriate for testing. Other solid tumors can be sent for testing through NPOP if patients have advanced stage cancer and are medically appropriate for antineoplastic therapy. To date, NPOP has sequenced > 13,000 samples.
Testing options have been added to NPOP in addition to tumor DNA sequencing. The first addition was the so-called liquid biopsy, more properly known as the cell-free DNA (cfDNA) test, a plasma-based high-sensitivity DNA sequencing assay. cfDNA is shed from dying cells and can be captured and sequenced from a plasma sample obtained by standard venipuncture, using a special-purpose sample collection tube. The test is appropriate for patients who do not have an appropriate archival tumor sample or those who cannot have a new biopsy of tumor tissue. Tumor tissue remains the preferred test sample due to a higher sensitivity than that of cfDNA and less susceptibility to false positives, so consideration of a tumor biopsy is appropriate prior to requesting a cfDNA assay. Therapy can greatly impact the sensitivity of cfDNA testing, so patients should be having disease progression at the time of obtaining a blood sample for cfDNA.
Finally, myeloid leukocytic cells accumulate genetic alterations during aging similar to those found in myelodysplasia and acute myeloid leukemia. These myeloid-associated mutations can be detected in both tumor and cfDNA samples and are known as clonal hyperplasia of indeterminate potential (CHIP). CHIP is much more common in the cfDNA. For lung cancer, CHIP-associated gene variants are readily distinguished from lung cancer-associated variants, but that distinction is much more difficult in many other tumor types.
In partnership with the current DNA sequencing contractor, NPOP provides access to a second gene panel for hematologic malignancies or sarcomas, though neither of these classes of malignancies currently have clear indications for routine NGS multigene panel testing. Given the low rate of finding a gene mutation that would change therapy that could not be found with smaller, less expensive gene panels, NPOP requires prior approval for the use of this panel.
Finally, since early 2019, programmed deathligand 1 (PD-L1) immunohistochemistry analysis is available through NPOP in association with NGS testing of the same sample for those solid tumors with US Food and Drug Administration (FDA)-approved indications that include a PD-L1 companion diagnostic. This service was added to facilitate concurrent testing of PD-L1 and DNA sequencing, which speeds availability of molecular data to the health care provider and veteran.
Determining Clinical Significance
The complexity of tumor NGS gene panel test results is far greater than frequently ordered laboratory or molecular testing due to the near infinite number of possible results and varying degrees of consensus of the significance of the results for therapeutic decision making. That complexity is reflected in the length of the test reports, which are often ≥ 20 pages. Starting from the gene variants identified by the DNA sequencing variant-caller bioinformatics pipeline, there is a 2-step process, referred to as annotation, to interpret the clinical significance that is repeated for each variant.
The first step is to assign a pathogenicity value, also known as oncogenicity, using a 5-point Likert scale from pathogenic to benign with variant of unknown significance (VUS) in the middle of the scale. Only variants that are pathogenic or likely pathogenic are considered further. A VUS is usually communicated to the health care provider but should generally not be acted on, while benign and likely benign variants may or may not be included in the report and should never be acted on. NPOP examined the concordance of pathogenicity calls among 3 annotation services: N-of-One/QCI Precision Insights (qiagen.com), IBM Watson for Genomics (WfG), and OncoKB (www.oncokb.org).6 There was moderate-to-poor concordance, indicating lack of consensus about whether a significant fraction of observed gene variants contributes to the patient’s cancer. This variability likely arises due to differences in algorithms and criteria used to assess pathogenicity.
The second step of annotation is assignment of the actionability of the variant, using a level of evidence (LoE) scale from 1 (on-label indication) to 4 (absence of clinical evidence; ie, only preclinical or theoretical evidence). Initially, NPOP used an adaptation of the LoE scales from WfG and OncoKB but now mostly uses the recently revised OncoKB LoE. Actionability also includes prediction of resistance to a treatment (LoE level R1 and R2). An example of a resistance gene variant is a KRAS mutation in colorectal cancer, which predicts lack of clinical benefit from anti- EGFR antibodies. It is important to note that a determination of actionability requires 3 inputs: gene, variant, and tumor type. A BRAF V600E mutation in melanoma has different medications with level 1 LoE than does the same mutation in colorectal cancer, for example.
Another complexity in annotation for actionability is tumor type ontogeny—the classification system used for cancer types. WfG uses a subset of the National Cancer Institute Thesaurus (ncithesaurus.nci.nih.gov), OncoKB uses the unique OncoTree (oncotree.mskcc.org), and Foundation Medicine (www.foundationmed icine.com), and N-of-One use propriety classification systems. The WfG and OncoKB tumor types have evolved over time, while it is unclear what changes have been made in the FMI and N-of-One tumor type classification systems. Thus, a gene variant observed in a single patient may be annotated differently by these services because of how the tumor type is mapped onto the services’ tumor type ontogeny. NPOP has been assigning WfG diagnoses since 2017, including historic assignment for prior samples back to the pilot project in 2015. In early 2019, NPOP began requiring test requesters to include International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) diagnoses (histology and site codes) with the sample. ICD-O-3 codes are used in all cancer registry data in North America, including the VA Cancer Registry System. The approximately 50,000 possible diagnoses allow fine precision in diagnoses, which is important for less common and rare cancer types; however, the large number of diagnoses adds complexity. NPOP has created a partial translation table for ICD-O-3 to WfG diagnosis that includes all diagnoses seen to date; this table facilitates continuing provision of WfG diagnosis without manual review as was previously required.
NPOP-Provided Genetic Services
Given these complexities in interpretation of NGS multigene panel results, NPOP provides several services to assist health care providers and other members of the care team. First, the NPOP Interfacility Consult (IFC) is a virtual “phone-a-friend” service that provides asynchronous patient-specific expert recommendations in precision oncology. By far the most requested service is assistance with interpretation of a patient’s DNA sequence results. Other requests include advice on whether to perform NGS testing and what molecular testing to perform. The IFC is integral to the VA Computerized Patient Record System electronic health record. Additional requests have been submitted and answered by e-mail.
The Molecular Oncology Tumor Board is a monthly case-based educational conference supported by the VA Employee Education Service, which provides continuing education credits for attendees. NPOP staff coordinate the conference, and a panel of specialists from around the country provide expert commentary.
In 2016, IBM gifted the services of WfG to VA. WfG’s main functionality is annotation of NGS results. About 5,000 samples were processed from 2017 to 2019; sample processing is expected to resume shortly. The availability of WfG annotations early in NPOP operation was very useful to the implementation of NPOP in general and the NPOP consultation services in particular, resulting in improved thoroughness of opinions provided by NPOP staff.
Informatics
Informatics is an essential component of NPOP that facilitates both clinical care and research (Figure 3). Results of NGS gene panels are returned to the facility that submitted the sample for testing as a PDF document. NPOP receives the same PDF report in real time but also structured data of the results including a variant callformat file and XML file. The secondary sequence data in binary alignment map or FASTQ format is received in batches. NPOP program staff extract data from these files and then load it into SQL tables in the VA Corporate Data Warehouse. In partnership with the VA Pharmacy Benefits Management Service, NPOP has constructed user-friendly dashboards that allow users with no technical skills and who have the appropriate data access permissions to view various portions of the NPOP database. There are dashboards to display a list of NPOP samples by facility, find a patient by name or other identifying information, and display a list of patients who have received any antineoplastic drug, among other functions.
The NPOP database has been used to reannotate NGS results, such as when new drugs are approved. For example, when the first neurotrophic tropomyosin receptor kinase (NTRK) inhibitor was approved, NPOP rapidly identified all living patients with NTRK fusions and notified the health care providers of the availability a potential new treatment option for their patient. 7 NPOP is now building a method to allow NPOP dashboard users to similarly identify patients who have not received a corresponding drug for a user-selected LoE annotation. This will facilitate a systems approach to ensure that all patients with EGFR exon 19 deletions, for example, either have received an EGFR inhibitor or are reviewed to determine why they have not. Furthermore, the database will facilitate real-world data analysis in precision oncology. For example, prior to the recent FDA-approval of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors for prostate cancer, 50 veterans already had been treated with one of these agents. These data can help further inform which of the many variants of DNA damage repair genes benefit from PARP inhibitors.
Ensuring Access to Care for All Veterans
With any new medical technology comes an obligation to ensure appropriate equal access so as to not exacerbate health care disparities. Veterans enrolled in VA health care are much more likely to live in rural communities than does the US population as a whole, and there was concern that these veterans would not receive NGS testing at the same rate as urban veterans. NPOP therefore was intentional during implementation to ensure rural veterans were being offered testing. Indeed, there has been nearly equal utilization by rurality. No other disparities in NPOP utilization have been seen.
A majority of veterans who undergo testing in NPOP have at least 1 actionable gene variant reported.5 However, some of these are for lower LoE off-label use of FDA-approved medications, but many are for agents available only through clinical trials. Consideration of treatments available through a clinical trial is part of standard practice for patients with advanced malignancies. NPOP data have helped identify cohorts who are eligible for clinical trials on the basis of their tumor DNA sequencing results. The National Oncology Program Office has been working closely with the VA Office of Research and Development to expand access to cancer clinical trials in VA. Veterans also can be treated on trials outside VA as medically appropriate and with local VA approval.
Conclusions
The VA NPOP is one of the largest clinical DNA sequencing programs in the nation with integrated consultation services and health informatics resources to facilitate patient care, clinical trials, and health outcomes research. The clinical services of NPOP provide cuttingedge oncology services to veterans throughout VA without exacerbating disparities and will be a national resource for research.
Acknowledgments
NPOP was made possible and implemented through the efforts of a number of people in VHA, including the national and regional leaders who supported the program’s vision and implementation, especially Michael Mayo-Smith, David Shulkin, Jennifer S. Lee, and Laurence Meyer, the leaders and staff of the Massachusetts Veterans Epidemiology Research and Information Center who piloted regional NGS testing, and especially my current and former colleagues in the VA National Oncology Program Office, without whom NPOP would not be possible. The contributions of Neil L. Spector who served as inaugural Director of Precision Oncology and Jill E. Duffy in her role as Director of Oncology Operations are particularly noteworthy.
As the nation’s largest integrated health care system with about 50,000 new cancer diagnoses per year, providing care for over 400,000 veterans with cancer and a robust research portfolio, the US Department of Veterans Affairs (VA) is well positioned to be a leader in both clinical and research in oncology. The VA National Precision Oncology Program (NPOP), which provides tumor sequencing and consultative services, is a key component of VA oncology assets.
Case Presentation
As the mission of the VA is to “care for him who shall have borne the battle,” it is fitting to begin with the story of a US Army veteran in his 40s and the father of 2 young children who developed progressive shortness of breath, cough, and weight loss over a period of 8 months. He was diagnosed with metastatic lung adenocarcinoma in 2016, and standard testing of his tumor showed no alteration of the EGFR and ALK genes. He was treated with whole brain radiation and had begun treatment for carboplatin and pemetrexed chemotherapy with mixed tumor response.
Subsequently, his tumor was tested through NPOP, using a multigene next-generation sequencing (NGS) assay panel, which showed the presence of an abnormal fusion between the EML4 and ALK genes. The chemotherapy was discontinued and oral crizotinib precision therapy was started. The patient had an excellent response in all sites of disease (Figure 1). He was able to return to work and school.
In July 2017, his medication was switched to alectinib for asymptomatic progression in his brain, and there was further response. In September 2019, he was treated with precision intensity-modulated radiotherapy (IMRT), targeting a single brain metastasis as there were no other sites of cancer progression and no cancerrelated symptoms. He finished school and continues to work.
Precision Oncology
Oncology is a relatively young medical field. The early medical treatments for cancer were developed empirically against hematologic malignancies, particularly leukemias. Cytotoxic chemotherapeutic agents as a group have modest effects on most solid tumors, and even modern genomics has had limited ability to predict differential benefit in patients with advanced-stage carcinomas. As a result, the medications are used in a nonprecision manner in which all patients with the same cancer diagnosis and stage receive the same treatment. This is due in part to our limited understanding of both the pathophysiology of cancer and the mechanism of action of cytotoxic agents.
The paradigm of precision oncology, in contrast, utilizes unique, patient-specific molecular characteristics to guide prescribing of antineoplastic agents (Figure 2). These molecular characteristics are frequently tumoral but also may be nontumoral, such as germline genetic variants and even nonhuman, such as the gut microbiome as has been proposed as predictive of response to immune checkpoint inhibitors.1,2
One of the first examples of precision oncology was tumor testing for the estrogen receptor in breast cancer, which distinguishes breast tumors sensitive to hormonal treatments from those that are resistant.3 In 2004, somatically acquired mutation of the EGFR gene was found to be associated with response to EGFR tyrosine kinase inhibitors such as gefitinib and erlotinib, and subsequently it was shown that patients without these mutations derived no benefit from use of these drugs.4 Thus, the precision oncology paradigm is using a molecular diagnostic as part of the indication for an antineoplastic agent, resulting in improved therapeutic efficacy and often reduced toxicity.
By 2015, multiple examples of DNA-based gene alterations that predict drug response were known, including at least 5 in non-small cell lung cancer (NSCLC). The heterogeneity of molecular testing practice patterns and methods of testing in VA along with the increasing number and complexity of molecular tests facilitated launch of a regional precision oncology program based primarily in Veterans Integrated Service Network 1, which provided tumor DNA sequencing through 2 vendors. Advances in DNA sequencing technology, particularly NGS, permit sequencing of multiple genes in clinical tumor samples, using a panel applicable for multiple tumor types. As part of VA contributions to the 2016 White House Cancer Moonshot initiative, the regional program became NPOP with expanded geographic scope, the addition of clinical consultative services, and robust informatics that supports associated research and a learning health care system. NPOP is a component of the VA National Oncology Program Office under the Office of Specialty Care.
Testing
With the launch of NPOP in mid-2016, there was rapid expansion of the number of VA facilities participating, and the number of tumor samples being submitted increased substantially. 5 The expansion was facilitated by both central funding for the tumor DNA sequencing and by NPOP-provided training of pathology laboratory staff and oncologists. Today, NPOP is utilized by almost every oncology practice in VA.
NPOP’s initial focus was on lung cancer, specifically advanced-stage nonsquamous NSCLC, which not only is very common in VA, but also has one of the highest number of mutated genes that result in sensitivity to antineoplastic drugs. Recently, metastatic prostate cancer was added as a second focus tumor type. Dashboards are available on the NPOP website to assist care teams in identifying veterans at their facility with either lung or prostate cancer who may be appropriate for testing. Other solid tumors can be sent for testing through NPOP if patients have advanced stage cancer and are medically appropriate for antineoplastic therapy. To date, NPOP has sequenced > 13,000 samples.
Testing options have been added to NPOP in addition to tumor DNA sequencing. The first addition was the so-called liquid biopsy, more properly known as the cell-free DNA (cfDNA) test, a plasma-based high-sensitivity DNA sequencing assay. cfDNA is shed from dying cells and can be captured and sequenced from a plasma sample obtained by standard venipuncture, using a special-purpose sample collection tube. The test is appropriate for patients who do not have an appropriate archival tumor sample or those who cannot have a new biopsy of tumor tissue. Tumor tissue remains the preferred test sample due to a higher sensitivity than that of cfDNA and less susceptibility to false positives, so consideration of a tumor biopsy is appropriate prior to requesting a cfDNA assay. Therapy can greatly impact the sensitivity of cfDNA testing, so patients should be having disease progression at the time of obtaining a blood sample for cfDNA.
Finally, myeloid leukocytic cells accumulate genetic alterations during aging similar to those found in myelodysplasia and acute myeloid leukemia. These myeloid-associated mutations can be detected in both tumor and cfDNA samples and are known as clonal hyperplasia of indeterminate potential (CHIP). CHIP is much more common in the cfDNA. For lung cancer, CHIP-associated gene variants are readily distinguished from lung cancer-associated variants, but that distinction is much more difficult in many other tumor types.
In partnership with the current DNA sequencing contractor, NPOP provides access to a second gene panel for hematologic malignancies or sarcomas, though neither of these classes of malignancies currently have clear indications for routine NGS multigene panel testing. Given the low rate of finding a gene mutation that would change therapy that could not be found with smaller, less expensive gene panels, NPOP requires prior approval for the use of this panel.
Finally, since early 2019, programmed deathligand 1 (PD-L1) immunohistochemistry analysis is available through NPOP in association with NGS testing of the same sample for those solid tumors with US Food and Drug Administration (FDA)-approved indications that include a PD-L1 companion diagnostic. This service was added to facilitate concurrent testing of PD-L1 and DNA sequencing, which speeds availability of molecular data to the health care provider and veteran.
Determining Clinical Significance
The complexity of tumor NGS gene panel test results is far greater than frequently ordered laboratory or molecular testing due to the near infinite number of possible results and varying degrees of consensus of the significance of the results for therapeutic decision making. That complexity is reflected in the length of the test reports, which are often ≥ 20 pages. Starting from the gene variants identified by the DNA sequencing variant-caller bioinformatics pipeline, there is a 2-step process, referred to as annotation, to interpret the clinical significance that is repeated for each variant.
The first step is to assign a pathogenicity value, also known as oncogenicity, using a 5-point Likert scale from pathogenic to benign with variant of unknown significance (VUS) in the middle of the scale. Only variants that are pathogenic or likely pathogenic are considered further. A VUS is usually communicated to the health care provider but should generally not be acted on, while benign and likely benign variants may or may not be included in the report and should never be acted on. NPOP examined the concordance of pathogenicity calls among 3 annotation services: N-of-One/QCI Precision Insights (qiagen.com), IBM Watson for Genomics (WfG), and OncoKB (www.oncokb.org).6 There was moderate-to-poor concordance, indicating lack of consensus about whether a significant fraction of observed gene variants contributes to the patient’s cancer. This variability likely arises due to differences in algorithms and criteria used to assess pathogenicity.
The second step of annotation is assignment of the actionability of the variant, using a level of evidence (LoE) scale from 1 (on-label indication) to 4 (absence of clinical evidence; ie, only preclinical or theoretical evidence). Initially, NPOP used an adaptation of the LoE scales from WfG and OncoKB but now mostly uses the recently revised OncoKB LoE. Actionability also includes prediction of resistance to a treatment (LoE level R1 and R2). An example of a resistance gene variant is a KRAS mutation in colorectal cancer, which predicts lack of clinical benefit from anti- EGFR antibodies. It is important to note that a determination of actionability requires 3 inputs: gene, variant, and tumor type. A BRAF V600E mutation in melanoma has different medications with level 1 LoE than does the same mutation in colorectal cancer, for example.
Another complexity in annotation for actionability is tumor type ontogeny—the classification system used for cancer types. WfG uses a subset of the National Cancer Institute Thesaurus (ncithesaurus.nci.nih.gov), OncoKB uses the unique OncoTree (oncotree.mskcc.org), and Foundation Medicine (www.foundationmed icine.com), and N-of-One use propriety classification systems. The WfG and OncoKB tumor types have evolved over time, while it is unclear what changes have been made in the FMI and N-of-One tumor type classification systems. Thus, a gene variant observed in a single patient may be annotated differently by these services because of how the tumor type is mapped onto the services’ tumor type ontogeny. NPOP has been assigning WfG diagnoses since 2017, including historic assignment for prior samples back to the pilot project in 2015. In early 2019, NPOP began requiring test requesters to include International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) diagnoses (histology and site codes) with the sample. ICD-O-3 codes are used in all cancer registry data in North America, including the VA Cancer Registry System. The approximately 50,000 possible diagnoses allow fine precision in diagnoses, which is important for less common and rare cancer types; however, the large number of diagnoses adds complexity. NPOP has created a partial translation table for ICD-O-3 to WfG diagnosis that includes all diagnoses seen to date; this table facilitates continuing provision of WfG diagnosis without manual review as was previously required.
NPOP-Provided Genetic Services
Given these complexities in interpretation of NGS multigene panel results, NPOP provides several services to assist health care providers and other members of the care team. First, the NPOP Interfacility Consult (IFC) is a virtual “phone-a-friend” service that provides asynchronous patient-specific expert recommendations in precision oncology. By far the most requested service is assistance with interpretation of a patient’s DNA sequence results. Other requests include advice on whether to perform NGS testing and what molecular testing to perform. The IFC is integral to the VA Computerized Patient Record System electronic health record. Additional requests have been submitted and answered by e-mail.
The Molecular Oncology Tumor Board is a monthly case-based educational conference supported by the VA Employee Education Service, which provides continuing education credits for attendees. NPOP staff coordinate the conference, and a panel of specialists from around the country provide expert commentary.
In 2016, IBM gifted the services of WfG to VA. WfG’s main functionality is annotation of NGS results. About 5,000 samples were processed from 2017 to 2019; sample processing is expected to resume shortly. The availability of WfG annotations early in NPOP operation was very useful to the implementation of NPOP in general and the NPOP consultation services in particular, resulting in improved thoroughness of opinions provided by NPOP staff.
Informatics
Informatics is an essential component of NPOP that facilitates both clinical care and research (Figure 3). Results of NGS gene panels are returned to the facility that submitted the sample for testing as a PDF document. NPOP receives the same PDF report in real time but also structured data of the results including a variant callformat file and XML file. The secondary sequence data in binary alignment map or FASTQ format is received in batches. NPOP program staff extract data from these files and then load it into SQL tables in the VA Corporate Data Warehouse. In partnership with the VA Pharmacy Benefits Management Service, NPOP has constructed user-friendly dashboards that allow users with no technical skills and who have the appropriate data access permissions to view various portions of the NPOP database. There are dashboards to display a list of NPOP samples by facility, find a patient by name or other identifying information, and display a list of patients who have received any antineoplastic drug, among other functions.
The NPOP database has been used to reannotate NGS results, such as when new drugs are approved. For example, when the first neurotrophic tropomyosin receptor kinase (NTRK) inhibitor was approved, NPOP rapidly identified all living patients with NTRK fusions and notified the health care providers of the availability a potential new treatment option for their patient. 7 NPOP is now building a method to allow NPOP dashboard users to similarly identify patients who have not received a corresponding drug for a user-selected LoE annotation. This will facilitate a systems approach to ensure that all patients with EGFR exon 19 deletions, for example, either have received an EGFR inhibitor or are reviewed to determine why they have not. Furthermore, the database will facilitate real-world data analysis in precision oncology. For example, prior to the recent FDA-approval of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors for prostate cancer, 50 veterans already had been treated with one of these agents. These data can help further inform which of the many variants of DNA damage repair genes benefit from PARP inhibitors.
Ensuring Access to Care for All Veterans
With any new medical technology comes an obligation to ensure appropriate equal access so as to not exacerbate health care disparities. Veterans enrolled in VA health care are much more likely to live in rural communities than does the US population as a whole, and there was concern that these veterans would not receive NGS testing at the same rate as urban veterans. NPOP therefore was intentional during implementation to ensure rural veterans were being offered testing. Indeed, there has been nearly equal utilization by rurality. No other disparities in NPOP utilization have been seen.
A majority of veterans who undergo testing in NPOP have at least 1 actionable gene variant reported.5 However, some of these are for lower LoE off-label use of FDA-approved medications, but many are for agents available only through clinical trials. Consideration of treatments available through a clinical trial is part of standard practice for patients with advanced malignancies. NPOP data have helped identify cohorts who are eligible for clinical trials on the basis of their tumor DNA sequencing results. The National Oncology Program Office has been working closely with the VA Office of Research and Development to expand access to cancer clinical trials in VA. Veterans also can be treated on trials outside VA as medically appropriate and with local VA approval.
Conclusions
The VA NPOP is one of the largest clinical DNA sequencing programs in the nation with integrated consultation services and health informatics resources to facilitate patient care, clinical trials, and health outcomes research. The clinical services of NPOP provide cuttingedge oncology services to veterans throughout VA without exacerbating disparities and will be a national resource for research.
Acknowledgments
NPOP was made possible and implemented through the efforts of a number of people in VHA, including the national and regional leaders who supported the program’s vision and implementation, especially Michael Mayo-Smith, David Shulkin, Jennifer S. Lee, and Laurence Meyer, the leaders and staff of the Massachusetts Veterans Epidemiology Research and Information Center who piloted regional NGS testing, and especially my current and former colleagues in the VA National Oncology Program Office, without whom NPOP would not be possible. The contributions of Neil L. Spector who served as inaugural Director of Precision Oncology and Jill E. Duffy in her role as Director of Oncology Operations are particularly noteworthy.
1. Lima ZS, Ghadamzadeh M, Arashloo FT, Amjad G, Ebadi MR, Younesi L. Recent advances of therapeutic targets based on the molecular signature in breast cancer: genetic mutations and implications for current treatment paradigms. J Hematol Oncol. 2019;12(1):38. Published 2019 Apr 11. doi:10.1186/s13045-019-0725-6
2. Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108. Published 2019 Apr 17. doi:10.1186/s40425-019-0574-4
3. Kiang DT, Kennedy BJ. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Intern Med. 1977;87(6):687- 690. doi:10.7326/0003-4819-87-6-687.
4. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497-1500. doi:10.1126/science.1099314
5. Poonnen P, Duffy J, Hintze BJ, et al. Genomic analysis of metastatic solid tumors in veterans: findings from the VHA National Precision Oncology Program. J Clin Oncol. 2019;37(suppl 15):3074. doi:10.1200/JCO.2019.37.15_suppl.3074
6. Katsoulakis E, Duffy JE, Hintze B, Spector NL, Kelley MJ. Comparison of annotation services for nextgeneration sequencing in a large-scale precision oncology program. JCO Precis Oncol. 2020(4):212-221. doi:10.1200/PO.19.00118
7. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
1. Lima ZS, Ghadamzadeh M, Arashloo FT, Amjad G, Ebadi MR, Younesi L. Recent advances of therapeutic targets based on the molecular signature in breast cancer: genetic mutations and implications for current treatment paradigms. J Hematol Oncol. 2019;12(1):38. Published 2019 Apr 11. doi:10.1186/s13045-019-0725-6
2. Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108. Published 2019 Apr 17. doi:10.1186/s40425-019-0574-4
3. Kiang DT, Kennedy BJ. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Intern Med. 1977;87(6):687- 690. doi:10.7326/0003-4819-87-6-687.
4. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497-1500. doi:10.1126/science.1099314
5. Poonnen P, Duffy J, Hintze BJ, et al. Genomic analysis of metastatic solid tumors in veterans: findings from the VHA National Precision Oncology Program. J Clin Oncol. 2019;37(suppl 15):3074. doi:10.1200/JCO.2019.37.15_suppl.3074
6. Katsoulakis E, Duffy JE, Hintze B, Spector NL, Kelley MJ. Comparison of annotation services for nextgeneration sequencing in a large-scale precision oncology program. JCO Precis Oncol. 2020(4):212-221. doi:10.1200/PO.19.00118
7. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
Strategic Initiatives for Veterans with Lung Cancer (FULL)
The Veterans Health Administration (VHA) facilitates care for > 7,700 veterans with newly diagnosed lung cancer each year.1 This includes comprehensive clinical evaluations and management that are facilitated through interdisciplinary networks of pulmonologists, radiologists, thoracic surgeons, radiation oncologists, and medical oncologists. Veterans with lung cancer have access to advanced medical technologies at US Department of Veterans Affairs (VA) medical centers (VAMCs), including the latest US Food and Drug Administration (FDA)-approved targeted radiation delivery systems and novel immunotherapies, as well as precision oncology-driven clinical trials.2
Despite access to high-quality care, lung cancer remains the leading cause of cancer-related mortality among VHA enrollees as well as the US population.3 About 15 veterans die of lung cancer each day; most are diagnosed with advanced stage III or stage IV disease. To address this issue, VHA launched 3 new initiatives between 2016 and 2017 to improve outcomes for veterans impacted by lung cancer. The VA Partnership to increase Access to Lung Screening (VA-PALS) is a clinical implementation project to increase access to early detection lung screening scans at 10 VAMCs. The Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) is a phase 3 randomized trial that investigates the role of stereotactic body radiation therapy (SBRT) as a potential alternative to surgery for veterans with operable stage I non-small cell lung cancer (NSCLC). The VA Radiation Oncology Quality Surveillance program (VA-ROQS) established national expert-derived benchmarks for the quality assurance of lung cancer therapy.
VA-PALS
The central mission of VA-PALS is to reduce lung cancer mortality among veterans at risk by increasing access to low-dose computed tomography (LDCT) lung screening scans.4,5 The program was developed as a public-private partnership to introduce structured lung cancer screening programs at 10 VAMCs to safely manage large cohorts of veterans undergoing annual screening scans. The VA-PALS project brings together pulmonologists, radiologists, thoracic surgeons, radiation oncologists, medical oncologists, and computer scientists who have experience developing open-source electronic health record systems for VHA networks. The project was launched in 2017 after an earlier clinical demonstration project identified substantial variability and challenges with efforts to implement new lung cancer screening programs in the VA.6
Each of the 10 VA-PALS-designated lung cancer screening programs (Atlanta, Georgia; Phoenix, Arizona; Indianapolis, Indiana; Chicago, Illinois; Nashville, Tennessee; Philadelphia, Pennsylvania; St. Louis, Missouri; Denver, Colorado; Milwaukee, Wisconsin; and Cleveland, Ohio) assumes a major responsibility for ordering and evaluating the results of LDCT scans to ensure appropriate follow-up care of veterans with abnormal radiographic findings. Lung cancer screening programs are supported with a full-time navigator (nurse practitioner or physician assistant) who has received training from the VA-PALS project team with direct supervision by a local site director who is a pulmonologist, thoracic surgeon, or medical oncologist. Lung cancer screening programs establish a centralized approach that aims to reduce the burden on primary care providers for remembering to order annual baseline and repeat LDCT scans. The lung screening programs also manage radiographic findings that usually are benign to facilitate appropriate decisions to minimize the risk of unnecessary tests and procedures. Program implementation across VA-PALS sites includes a strong connection among participants through meetings, newsletters, and attendance at conferences to create a collaborative learning network, which has been shown to improve dissemination of best practices across the VHA.7,8
The International Early Lung Cancer Action Program (I-ELCAP), which pioneered the use of LDCT to reduce lung cancer mortality, is a leading partner for VA-PALS.9 This group has > 25 years of experience overcoming many of the obstacles and challenges that new lung cancer screening programs face.10 The I-ELCAP has successfully implemented new lung cancer screening programs at > 70 health care institutions worldwide. Their implementation processes provide continuous oversight for each center. As a result, the I-ELCAP team has developed a large and detailed lung cancer screening registry with > 75,000 patients enrolled globally, comprising a vast database of clinical data that has produced > 270 scientific publications focusing on improving the quality and safety of lung cancer screening.11,12
These reports have helped guide evidence-based recommendations for lung cancer screening in several countries and include standardized processes for patient counseling and smoking cessation, data acquisition and interpretation of LDCT images, and clinical management of abnormal findings to facilitate timely transition from diagnosis to treatment.13-15 The I-ELCAP management system detects 10% abnormal findings in the baseline screening study, which declines to 6% in subsequent years.12 The scientific findings from this approach have provided additional insights into technical CT scanning errors that can affect tumor nodule measurements.16 The vast amount of clinical data and expertise have helped explore genetic markers.17 The I-ELCAP has facilitated cost-effectiveness investigations to determine the value of screening, and their research portfolio includes investigations into the longer-term outcomes after primary treatment for patients with screen-detected lung cancers.18,19
I-ELCAP gifted its comprehensive clinical software management system that has been in use for the above contributions for use in the VHA through an open source agreement without licensing fees. The I-ELCAP software management system was rewritten in MUMPS, the software programming language that is used by the VA Computerized Patient Record System (CPRS). The newly adapted VA-PALS/I-ELCAP system underwent modifications with VHA clinicians’ input, and was successfully installed at the Phoenix VA Health Care System in Arizona, which has assumed a leading role for the VA-PALS project.
The VA-PALS/I-ELCAP clinical management system currently is under review by the VA Office of Information and Technology for broad distribution across the VHA through the VA Enterprise Cloud. Once in use across the VHA, the VA-PALS/I-ELCAP clinical management system will offer a longitudinal central database that can support numerous quality improvement and quality assurance initiatives, as well as innovative research projects. Research opportunities include: (1) large-scale examination of LDCT images with artificial intelligence and machine learning techniques; (2) epidemiologic investigations of environmental and genetic risk factors to better understand the high percentage of veterans diagnosed with lung cancer who were never smokers or had quit many years ago; and (3) multisite clinical trials that explore early detection blood screening tests that are under development.
The VA-PALS project is sponsored by the VHA Office of Rural Health as an enterprise-wide initiative that focuses on reaching rural veterans at risk. The project received additional support through the VA Secretary’s Center for Strategic Partnerships with a $5.8 million grant from the Bristol-Myers Squibb Foundation. The VistA (Veterans Health Information Systems and Technology Architecture) Expertise Network is an additional key partner that helped adapt the VAPALS-ELCAP system for use on VHA networks.
VALOR Trial
The VA Cooperative Studies Program (CSP) #2005 VALOR study is a randomized phase 3 clinical trial that evaluates optimal treatment for participants with operable early-stage NSCLC.20 The trial is sponsored by the CSP, which is responsible for and provides resources for the planning and conduct of large multicenter surgical and clinical trials in VHA.21 The CSP #2005 VALOR study plans to enroll veterans with stage I NSCLC who will be treated with a surgical lobectomy or SBRT according to random assignment. An alternative surgical approach with a segmentectomy is acceptable, although patients in poor health who are only qualify for a wedge resection will not be enrolled. The CSP will follow each participant for at least 5 years to evaluate which treatment, if either, results in a higher overall survival rate. Secondary outcome measures are quality of life, pulmonary function, health state utilities, patterns of failure, and causes of death.
Although the study design of the VALOR trial is relatively straightforward, recruitment of participants to similar randomized trials of surgery vs SBRT for operable stage I NSCLC outside the VA has historically been very difficult. Three earlier phase 3 trials in the Netherlands and US closed prematurely after collectively enrolling only 4% of planned participants. Although a pooled analysis of 2 of these trials demonstrated a statistically significant difference of 95% vs 79% survival in favor of SBRT at a median follow-up of 40 months, the analysis was underpowered because only 58 of the planned 1,380 participants were enrolled.22,23
The CSP #2005 VALOR study team was keenly aware of these past challenges and addressed many of the obstacles to enrollment by optimizing eligibility criteria and follow-up requirements. Enrollment sites were carefully selected after confirming equipoise between the 2 treatments, and study coordinators at each enrollment site were empowered to provide a leading role with recruitment. Multiple communication channels were established for constant contact to disseminate new best practices for recruitment as they were identified. Furthermore, a veteran-centric educational recruitment video, approved by the VA Central Institutional Review Board, was designed to help study participants better understand the purpose of participating in a clinical trial (www.vacsp.research.va.gov/CSP_2005/CSP_2005.asp).
After the first year of recruitment, researchers identified individual clinician and patient preferences as the predominant difficulty with recruitment, which was not easy to address. The CSP #2005 VALOR study team opted to partner directly with the Qualitative Research Integrated within Trials (QuinteT) team in the United Kingdom to adopt its methods to successfully support randomized clinical trials with serious recruitment challenges.24,25 By working directly with the QuinteT director, the CSP #2005 VALOR team made a major revision to the informed consent forms by shifting focus away from disclosing potential harms of research to an informative document that emphasized the purpose of the study. The work with QuinteT also led to the creation of balanced narratives for study teams to use and for potential participants to read. These provide a more consistent message that describes why the study is important and why clinicians are no longer certain that surgery is the optimal treatment for all patients with operable stage I NSCLC.
The VALOR clinical trial, opened in 2017, remains open at only 9 VAMCs. As of early 2020, it has enrolled more participants than all previous phase 3 trials combined. Once completed, the results from CSP #2005 VALOR study will help clinicians and veterans with operable stage I NSCLC better understand the tradeoffs of surgery vs SBRT as an initial treatment option. Plans are under way to expand the scope of the trial and include investigations of pretreatment radiomic signatures and genetic markers from biopsy tissue and blood samples, to better predict when surgery or SBRT might be the best treatment option for an individual patient.
VA-ROQS
The VA-ROQS was created in 2016 to compare treatment of veterans with lung cancer in the VHA with quality standards recommended by nationally recognized experts in lung cancer care. Partnering with Washington University in St. Louis, Missouri and the American Society for Radiation Oncology, the VHA established a blue-ribbon panel of experts to review clinical trial data and medical literature to provide evidence-based quality metrics for lung cancer therapy. As a result, 26 metrics applicable to each patient’s case were developed, published, and used to assess lung cancer care in each VHA radiation oncology practice.26
By 2019, the resulting data led to a report on 773 lung cancer cases accumulated from all VHA radiation oncology practices. Performance data for each quality metric were compared for each practice within the VHA, which found that VHA practices met > 80% of all 1,278 metrics scored. Quality metrics included those documented within each patient health record and the specific radiation delivery parameters that reflected each health care provider’s treatment. After team investigators visited each center and recorded treatment data, VA-ROQS is now maturing to permit continuous, electronic monitoring of all lung cancer treatment delivered within VHA. As each veteran’s case is planned, the quality of the therapy is monitored, assessed, and reported to the treating physician. Each VHA radiation oncologist will receive up-to-date evaluation of each case compared with these evidence-based quality standards. The quality standards are reviewed by the blue-ribbon panel to keep the process current and valid.
Future of VHA Lung Cancer Care
As VHA continues to prioritize resources to improve and assure optimal outcomes for veterans with lung cancer, it is now looking to create a national network of Lung Cancer Centers of Excellence (LCCE) as described in the VA Budget Submission for fiscal year 2021. If Congress approves funding, LCCEs will soon be developed within the VA regional Veteran Integrated Service Network system to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information, including data on pharmacogenomic profiles. Such a network would create more opportunities to leverage public–private partnerships similar to the VA-PALS project. Creation of LCCEs would help the VA leverage an even stronger learning network to support more research so that all veterans who are impacted by lung cancer have access to personalized care that optimizes safety, quality of life, and overall survival. The lessons learned, networks developed, and partnerships established through VA-PALS, VALOR, and VA-ROQS are instrumental toward achieving these goals.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. 2016;33(suppl 4):18S-22S.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
4. National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
5. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT Screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
6. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi:10.1001/jamainternmed.2016.9022
7. Clancy C. Creating World-class care and service for our nation’s finest: how Veterans Health Administration Diffusion of Excellence Initiative Is innovating and transforming Veterans Affairs health care. Perm J. 2019;23:18.301. doi:10.7812/TPP/18.301
8. Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA health system. JAMA. 2017;317(3):255-256. doi:10.1001/jama.2016.18764
9. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99-105. doi:10.1016/S0140-6736(99)06093-6
10. Mulshine JL, Henschke CI. Lung cancer screening: achieving more by intervening less. Lancet Oncol. 2014;15(12):1284-1285. doi:10.1016/S1470-2045(14)70418-8
11. Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. doi:10.21037/atm.2016.04.06
12. Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246-252. doi:10.7326/0003-4819-158-4-201302190-00004
13. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
14. Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography imaging in a phase II clinical trial in lung cancer. J Med Imaging (Bellingham). 2016;3(3):035505. doi:10.1117/1.JMI.3.3.035505
15. Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP; International Early Lung Cancer Investigators. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201-208. doi:10.1097/CEJ.0000000000000065
16. Armato SG 3rd, McLennan G, Bidaut L, et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys. 2011;38(2):915-931. doi:10.1118/1.3528204
17. Gill RK, Vazquez MF, Kramer A, et al. The use of genetic markers to identify lung cancer in fine needle aspiration samples. Clin Cancer Res. 2008;14(22):7481-7487. doi:10.1158/1078-0432.CCR-07-5242
18. Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272-282.
19. Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37-44. doi:10.12788/jcso.0205
20. Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5(2):183-189. doi:10.21037/tlcr.2016.04.05
21. Bakaeen FG, Reda DJ, Gelijns AC, et al. Department of Veterans Affairs Cooperative Studies Program network of dedicated enrollment sites: implications for surgical trials [published correction appears in JAMA Surg. 2014 Sep;149(9):961]. JAMA Surg. 2014;149(6):507-513. doi:10.1001/jamasurg.2013.4150
22. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials [published correction appears in Lancet Oncol. 2015 Sep;16(9):e427]. Lancet Oncol. 2015;16(6):630-637. doi:10.1016/S1470-2045(15)70168-3
23. Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer. 2017;103:6-10. doi:10.1016/j.lungcan.2016.11.005
24. Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17(1):283. Published 2016 Jun 8. doi:10.1186/s13063-016-1391-4
25. Rooshenas L, Scott LJ, Blazeby JM, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108-120. doi:10.1016/j.jclinepi.2018.10.004
26. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance Program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi:10.1016/j.ijrobp.2019.08.064
The Veterans Health Administration (VHA) facilitates care for > 7,700 veterans with newly diagnosed lung cancer each year.1 This includes comprehensive clinical evaluations and management that are facilitated through interdisciplinary networks of pulmonologists, radiologists, thoracic surgeons, radiation oncologists, and medical oncologists. Veterans with lung cancer have access to advanced medical technologies at US Department of Veterans Affairs (VA) medical centers (VAMCs), including the latest US Food and Drug Administration (FDA)-approved targeted radiation delivery systems and novel immunotherapies, as well as precision oncology-driven clinical trials.2
Despite access to high-quality care, lung cancer remains the leading cause of cancer-related mortality among VHA enrollees as well as the US population.3 About 15 veterans die of lung cancer each day; most are diagnosed with advanced stage III or stage IV disease. To address this issue, VHA launched 3 new initiatives between 2016 and 2017 to improve outcomes for veterans impacted by lung cancer. The VA Partnership to increase Access to Lung Screening (VA-PALS) is a clinical implementation project to increase access to early detection lung screening scans at 10 VAMCs. The Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) is a phase 3 randomized trial that investigates the role of stereotactic body radiation therapy (SBRT) as a potential alternative to surgery for veterans with operable stage I non-small cell lung cancer (NSCLC). The VA Radiation Oncology Quality Surveillance program (VA-ROQS) established national expert-derived benchmarks for the quality assurance of lung cancer therapy.
VA-PALS
The central mission of VA-PALS is to reduce lung cancer mortality among veterans at risk by increasing access to low-dose computed tomography (LDCT) lung screening scans.4,5 The program was developed as a public-private partnership to introduce structured lung cancer screening programs at 10 VAMCs to safely manage large cohorts of veterans undergoing annual screening scans. The VA-PALS project brings together pulmonologists, radiologists, thoracic surgeons, radiation oncologists, medical oncologists, and computer scientists who have experience developing open-source electronic health record systems for VHA networks. The project was launched in 2017 after an earlier clinical demonstration project identified substantial variability and challenges with efforts to implement new lung cancer screening programs in the VA.6
Each of the 10 VA-PALS-designated lung cancer screening programs (Atlanta, Georgia; Phoenix, Arizona; Indianapolis, Indiana; Chicago, Illinois; Nashville, Tennessee; Philadelphia, Pennsylvania; St. Louis, Missouri; Denver, Colorado; Milwaukee, Wisconsin; and Cleveland, Ohio) assumes a major responsibility for ordering and evaluating the results of LDCT scans to ensure appropriate follow-up care of veterans with abnormal radiographic findings. Lung cancer screening programs are supported with a full-time navigator (nurse practitioner or physician assistant) who has received training from the VA-PALS project team with direct supervision by a local site director who is a pulmonologist, thoracic surgeon, or medical oncologist. Lung cancer screening programs establish a centralized approach that aims to reduce the burden on primary care providers for remembering to order annual baseline and repeat LDCT scans. The lung screening programs also manage radiographic findings that usually are benign to facilitate appropriate decisions to minimize the risk of unnecessary tests and procedures. Program implementation across VA-PALS sites includes a strong connection among participants through meetings, newsletters, and attendance at conferences to create a collaborative learning network, which has been shown to improve dissemination of best practices across the VHA.7,8
The International Early Lung Cancer Action Program (I-ELCAP), which pioneered the use of LDCT to reduce lung cancer mortality, is a leading partner for VA-PALS.9 This group has > 25 years of experience overcoming many of the obstacles and challenges that new lung cancer screening programs face.10 The I-ELCAP has successfully implemented new lung cancer screening programs at > 70 health care institutions worldwide. Their implementation processes provide continuous oversight for each center. As a result, the I-ELCAP team has developed a large and detailed lung cancer screening registry with > 75,000 patients enrolled globally, comprising a vast database of clinical data that has produced > 270 scientific publications focusing on improving the quality and safety of lung cancer screening.11,12
These reports have helped guide evidence-based recommendations for lung cancer screening in several countries and include standardized processes for patient counseling and smoking cessation, data acquisition and interpretation of LDCT images, and clinical management of abnormal findings to facilitate timely transition from diagnosis to treatment.13-15 The I-ELCAP management system detects 10% abnormal findings in the baseline screening study, which declines to 6% in subsequent years.12 The scientific findings from this approach have provided additional insights into technical CT scanning errors that can affect tumor nodule measurements.16 The vast amount of clinical data and expertise have helped explore genetic markers.17 The I-ELCAP has facilitated cost-effectiveness investigations to determine the value of screening, and their research portfolio includes investigations into the longer-term outcomes after primary treatment for patients with screen-detected lung cancers.18,19
I-ELCAP gifted its comprehensive clinical software management system that has been in use for the above contributions for use in the VHA through an open source agreement without licensing fees. The I-ELCAP software management system was rewritten in MUMPS, the software programming language that is used by the VA Computerized Patient Record System (CPRS). The newly adapted VA-PALS/I-ELCAP system underwent modifications with VHA clinicians’ input, and was successfully installed at the Phoenix VA Health Care System in Arizona, which has assumed a leading role for the VA-PALS project.
The VA-PALS/I-ELCAP clinical management system currently is under review by the VA Office of Information and Technology for broad distribution across the VHA through the VA Enterprise Cloud. Once in use across the VHA, the VA-PALS/I-ELCAP clinical management system will offer a longitudinal central database that can support numerous quality improvement and quality assurance initiatives, as well as innovative research projects. Research opportunities include: (1) large-scale examination of LDCT images with artificial intelligence and machine learning techniques; (2) epidemiologic investigations of environmental and genetic risk factors to better understand the high percentage of veterans diagnosed with lung cancer who were never smokers or had quit many years ago; and (3) multisite clinical trials that explore early detection blood screening tests that are under development.
The VA-PALS project is sponsored by the VHA Office of Rural Health as an enterprise-wide initiative that focuses on reaching rural veterans at risk. The project received additional support through the VA Secretary’s Center for Strategic Partnerships with a $5.8 million grant from the Bristol-Myers Squibb Foundation. The VistA (Veterans Health Information Systems and Technology Architecture) Expertise Network is an additional key partner that helped adapt the VAPALS-ELCAP system for use on VHA networks.
VALOR Trial
The VA Cooperative Studies Program (CSP) #2005 VALOR study is a randomized phase 3 clinical trial that evaluates optimal treatment for participants with operable early-stage NSCLC.20 The trial is sponsored by the CSP, which is responsible for and provides resources for the planning and conduct of large multicenter surgical and clinical trials in VHA.21 The CSP #2005 VALOR study plans to enroll veterans with stage I NSCLC who will be treated with a surgical lobectomy or SBRT according to random assignment. An alternative surgical approach with a segmentectomy is acceptable, although patients in poor health who are only qualify for a wedge resection will not be enrolled. The CSP will follow each participant for at least 5 years to evaluate which treatment, if either, results in a higher overall survival rate. Secondary outcome measures are quality of life, pulmonary function, health state utilities, patterns of failure, and causes of death.
Although the study design of the VALOR trial is relatively straightforward, recruitment of participants to similar randomized trials of surgery vs SBRT for operable stage I NSCLC outside the VA has historically been very difficult. Three earlier phase 3 trials in the Netherlands and US closed prematurely after collectively enrolling only 4% of planned participants. Although a pooled analysis of 2 of these trials demonstrated a statistically significant difference of 95% vs 79% survival in favor of SBRT at a median follow-up of 40 months, the analysis was underpowered because only 58 of the planned 1,380 participants were enrolled.22,23
The CSP #2005 VALOR study team was keenly aware of these past challenges and addressed many of the obstacles to enrollment by optimizing eligibility criteria and follow-up requirements. Enrollment sites were carefully selected after confirming equipoise between the 2 treatments, and study coordinators at each enrollment site were empowered to provide a leading role with recruitment. Multiple communication channels were established for constant contact to disseminate new best practices for recruitment as they were identified. Furthermore, a veteran-centric educational recruitment video, approved by the VA Central Institutional Review Board, was designed to help study participants better understand the purpose of participating in a clinical trial (www.vacsp.research.va.gov/CSP_2005/CSP_2005.asp).
After the first year of recruitment, researchers identified individual clinician and patient preferences as the predominant difficulty with recruitment, which was not easy to address. The CSP #2005 VALOR study team opted to partner directly with the Qualitative Research Integrated within Trials (QuinteT) team in the United Kingdom to adopt its methods to successfully support randomized clinical trials with serious recruitment challenges.24,25 By working directly with the QuinteT director, the CSP #2005 VALOR team made a major revision to the informed consent forms by shifting focus away from disclosing potential harms of research to an informative document that emphasized the purpose of the study. The work with QuinteT also led to the creation of balanced narratives for study teams to use and for potential participants to read. These provide a more consistent message that describes why the study is important and why clinicians are no longer certain that surgery is the optimal treatment for all patients with operable stage I NSCLC.
The VALOR clinical trial, opened in 2017, remains open at only 9 VAMCs. As of early 2020, it has enrolled more participants than all previous phase 3 trials combined. Once completed, the results from CSP #2005 VALOR study will help clinicians and veterans with operable stage I NSCLC better understand the tradeoffs of surgery vs SBRT as an initial treatment option. Plans are under way to expand the scope of the trial and include investigations of pretreatment radiomic signatures and genetic markers from biopsy tissue and blood samples, to better predict when surgery or SBRT might be the best treatment option for an individual patient.
VA-ROQS
The VA-ROQS was created in 2016 to compare treatment of veterans with lung cancer in the VHA with quality standards recommended by nationally recognized experts in lung cancer care. Partnering with Washington University in St. Louis, Missouri and the American Society for Radiation Oncology, the VHA established a blue-ribbon panel of experts to review clinical trial data and medical literature to provide evidence-based quality metrics for lung cancer therapy. As a result, 26 metrics applicable to each patient’s case were developed, published, and used to assess lung cancer care in each VHA radiation oncology practice.26
By 2019, the resulting data led to a report on 773 lung cancer cases accumulated from all VHA radiation oncology practices. Performance data for each quality metric were compared for each practice within the VHA, which found that VHA practices met > 80% of all 1,278 metrics scored. Quality metrics included those documented within each patient health record and the specific radiation delivery parameters that reflected each health care provider’s treatment. After team investigators visited each center and recorded treatment data, VA-ROQS is now maturing to permit continuous, electronic monitoring of all lung cancer treatment delivered within VHA. As each veteran’s case is planned, the quality of the therapy is monitored, assessed, and reported to the treating physician. Each VHA radiation oncologist will receive up-to-date evaluation of each case compared with these evidence-based quality standards. The quality standards are reviewed by the blue-ribbon panel to keep the process current and valid.
Future of VHA Lung Cancer Care
As VHA continues to prioritize resources to improve and assure optimal outcomes for veterans with lung cancer, it is now looking to create a national network of Lung Cancer Centers of Excellence (LCCE) as described in the VA Budget Submission for fiscal year 2021. If Congress approves funding, LCCEs will soon be developed within the VA regional Veteran Integrated Service Network system to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information, including data on pharmacogenomic profiles. Such a network would create more opportunities to leverage public–private partnerships similar to the VA-PALS project. Creation of LCCEs would help the VA leverage an even stronger learning network to support more research so that all veterans who are impacted by lung cancer have access to personalized care that optimizes safety, quality of life, and overall survival. The lessons learned, networks developed, and partnerships established through VA-PALS, VALOR, and VA-ROQS are instrumental toward achieving these goals.
The Veterans Health Administration (VHA) facilitates care for > 7,700 veterans with newly diagnosed lung cancer each year.1 This includes comprehensive clinical evaluations and management that are facilitated through interdisciplinary networks of pulmonologists, radiologists, thoracic surgeons, radiation oncologists, and medical oncologists. Veterans with lung cancer have access to advanced medical technologies at US Department of Veterans Affairs (VA) medical centers (VAMCs), including the latest US Food and Drug Administration (FDA)-approved targeted radiation delivery systems and novel immunotherapies, as well as precision oncology-driven clinical trials.2
Despite access to high-quality care, lung cancer remains the leading cause of cancer-related mortality among VHA enrollees as well as the US population.3 About 15 veterans die of lung cancer each day; most are diagnosed with advanced stage III or stage IV disease. To address this issue, VHA launched 3 new initiatives between 2016 and 2017 to improve outcomes for veterans impacted by lung cancer. The VA Partnership to increase Access to Lung Screening (VA-PALS) is a clinical implementation project to increase access to early detection lung screening scans at 10 VAMCs. The Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) is a phase 3 randomized trial that investigates the role of stereotactic body radiation therapy (SBRT) as a potential alternative to surgery for veterans with operable stage I non-small cell lung cancer (NSCLC). The VA Radiation Oncology Quality Surveillance program (VA-ROQS) established national expert-derived benchmarks for the quality assurance of lung cancer therapy.
VA-PALS
The central mission of VA-PALS is to reduce lung cancer mortality among veterans at risk by increasing access to low-dose computed tomography (LDCT) lung screening scans.4,5 The program was developed as a public-private partnership to introduce structured lung cancer screening programs at 10 VAMCs to safely manage large cohorts of veterans undergoing annual screening scans. The VA-PALS project brings together pulmonologists, radiologists, thoracic surgeons, radiation oncologists, medical oncologists, and computer scientists who have experience developing open-source electronic health record systems for VHA networks. The project was launched in 2017 after an earlier clinical demonstration project identified substantial variability and challenges with efforts to implement new lung cancer screening programs in the VA.6
Each of the 10 VA-PALS-designated lung cancer screening programs (Atlanta, Georgia; Phoenix, Arizona; Indianapolis, Indiana; Chicago, Illinois; Nashville, Tennessee; Philadelphia, Pennsylvania; St. Louis, Missouri; Denver, Colorado; Milwaukee, Wisconsin; and Cleveland, Ohio) assumes a major responsibility for ordering and evaluating the results of LDCT scans to ensure appropriate follow-up care of veterans with abnormal radiographic findings. Lung cancer screening programs are supported with a full-time navigator (nurse practitioner or physician assistant) who has received training from the VA-PALS project team with direct supervision by a local site director who is a pulmonologist, thoracic surgeon, or medical oncologist. Lung cancer screening programs establish a centralized approach that aims to reduce the burden on primary care providers for remembering to order annual baseline and repeat LDCT scans. The lung screening programs also manage radiographic findings that usually are benign to facilitate appropriate decisions to minimize the risk of unnecessary tests and procedures. Program implementation across VA-PALS sites includes a strong connection among participants through meetings, newsletters, and attendance at conferences to create a collaborative learning network, which has been shown to improve dissemination of best practices across the VHA.7,8
The International Early Lung Cancer Action Program (I-ELCAP), which pioneered the use of LDCT to reduce lung cancer mortality, is a leading partner for VA-PALS.9 This group has > 25 years of experience overcoming many of the obstacles and challenges that new lung cancer screening programs face.10 The I-ELCAP has successfully implemented new lung cancer screening programs at > 70 health care institutions worldwide. Their implementation processes provide continuous oversight for each center. As a result, the I-ELCAP team has developed a large and detailed lung cancer screening registry with > 75,000 patients enrolled globally, comprising a vast database of clinical data that has produced > 270 scientific publications focusing on improving the quality and safety of lung cancer screening.11,12
These reports have helped guide evidence-based recommendations for lung cancer screening in several countries and include standardized processes for patient counseling and smoking cessation, data acquisition and interpretation of LDCT images, and clinical management of abnormal findings to facilitate timely transition from diagnosis to treatment.13-15 The I-ELCAP management system detects 10% abnormal findings in the baseline screening study, which declines to 6% in subsequent years.12 The scientific findings from this approach have provided additional insights into technical CT scanning errors that can affect tumor nodule measurements.16 The vast amount of clinical data and expertise have helped explore genetic markers.17 The I-ELCAP has facilitated cost-effectiveness investigations to determine the value of screening, and their research portfolio includes investigations into the longer-term outcomes after primary treatment for patients with screen-detected lung cancers.18,19
I-ELCAP gifted its comprehensive clinical software management system that has been in use for the above contributions for use in the VHA through an open source agreement without licensing fees. The I-ELCAP software management system was rewritten in MUMPS, the software programming language that is used by the VA Computerized Patient Record System (CPRS). The newly adapted VA-PALS/I-ELCAP system underwent modifications with VHA clinicians’ input, and was successfully installed at the Phoenix VA Health Care System in Arizona, which has assumed a leading role for the VA-PALS project.
The VA-PALS/I-ELCAP clinical management system currently is under review by the VA Office of Information and Technology for broad distribution across the VHA through the VA Enterprise Cloud. Once in use across the VHA, the VA-PALS/I-ELCAP clinical management system will offer a longitudinal central database that can support numerous quality improvement and quality assurance initiatives, as well as innovative research projects. Research opportunities include: (1) large-scale examination of LDCT images with artificial intelligence and machine learning techniques; (2) epidemiologic investigations of environmental and genetic risk factors to better understand the high percentage of veterans diagnosed with lung cancer who were never smokers or had quit many years ago; and (3) multisite clinical trials that explore early detection blood screening tests that are under development.
The VA-PALS project is sponsored by the VHA Office of Rural Health as an enterprise-wide initiative that focuses on reaching rural veterans at risk. The project received additional support through the VA Secretary’s Center for Strategic Partnerships with a $5.8 million grant from the Bristol-Myers Squibb Foundation. The VistA (Veterans Health Information Systems and Technology Architecture) Expertise Network is an additional key partner that helped adapt the VAPALS-ELCAP system for use on VHA networks.
VALOR Trial
The VA Cooperative Studies Program (CSP) #2005 VALOR study is a randomized phase 3 clinical trial that evaluates optimal treatment for participants with operable early-stage NSCLC.20 The trial is sponsored by the CSP, which is responsible for and provides resources for the planning and conduct of large multicenter surgical and clinical trials in VHA.21 The CSP #2005 VALOR study plans to enroll veterans with stage I NSCLC who will be treated with a surgical lobectomy or SBRT according to random assignment. An alternative surgical approach with a segmentectomy is acceptable, although patients in poor health who are only qualify for a wedge resection will not be enrolled. The CSP will follow each participant for at least 5 years to evaluate which treatment, if either, results in a higher overall survival rate. Secondary outcome measures are quality of life, pulmonary function, health state utilities, patterns of failure, and causes of death.
Although the study design of the VALOR trial is relatively straightforward, recruitment of participants to similar randomized trials of surgery vs SBRT for operable stage I NSCLC outside the VA has historically been very difficult. Three earlier phase 3 trials in the Netherlands and US closed prematurely after collectively enrolling only 4% of planned participants. Although a pooled analysis of 2 of these trials demonstrated a statistically significant difference of 95% vs 79% survival in favor of SBRT at a median follow-up of 40 months, the analysis was underpowered because only 58 of the planned 1,380 participants were enrolled.22,23
The CSP #2005 VALOR study team was keenly aware of these past challenges and addressed many of the obstacles to enrollment by optimizing eligibility criteria and follow-up requirements. Enrollment sites were carefully selected after confirming equipoise between the 2 treatments, and study coordinators at each enrollment site were empowered to provide a leading role with recruitment. Multiple communication channels were established for constant contact to disseminate new best practices for recruitment as they were identified. Furthermore, a veteran-centric educational recruitment video, approved by the VA Central Institutional Review Board, was designed to help study participants better understand the purpose of participating in a clinical trial (www.vacsp.research.va.gov/CSP_2005/CSP_2005.asp).
After the first year of recruitment, researchers identified individual clinician and patient preferences as the predominant difficulty with recruitment, which was not easy to address. The CSP #2005 VALOR study team opted to partner directly with the Qualitative Research Integrated within Trials (QuinteT) team in the United Kingdom to adopt its methods to successfully support randomized clinical trials with serious recruitment challenges.24,25 By working directly with the QuinteT director, the CSP #2005 VALOR team made a major revision to the informed consent forms by shifting focus away from disclosing potential harms of research to an informative document that emphasized the purpose of the study. The work with QuinteT also led to the creation of balanced narratives for study teams to use and for potential participants to read. These provide a more consistent message that describes why the study is important and why clinicians are no longer certain that surgery is the optimal treatment for all patients with operable stage I NSCLC.
The VALOR clinical trial, opened in 2017, remains open at only 9 VAMCs. As of early 2020, it has enrolled more participants than all previous phase 3 trials combined. Once completed, the results from CSP #2005 VALOR study will help clinicians and veterans with operable stage I NSCLC better understand the tradeoffs of surgery vs SBRT as an initial treatment option. Plans are under way to expand the scope of the trial and include investigations of pretreatment radiomic signatures and genetic markers from biopsy tissue and blood samples, to better predict when surgery or SBRT might be the best treatment option for an individual patient.
VA-ROQS
The VA-ROQS was created in 2016 to compare treatment of veterans with lung cancer in the VHA with quality standards recommended by nationally recognized experts in lung cancer care. Partnering with Washington University in St. Louis, Missouri and the American Society for Radiation Oncology, the VHA established a blue-ribbon panel of experts to review clinical trial data and medical literature to provide evidence-based quality metrics for lung cancer therapy. As a result, 26 metrics applicable to each patient’s case were developed, published, and used to assess lung cancer care in each VHA radiation oncology practice.26
By 2019, the resulting data led to a report on 773 lung cancer cases accumulated from all VHA radiation oncology practices. Performance data for each quality metric were compared for each practice within the VHA, which found that VHA practices met > 80% of all 1,278 metrics scored. Quality metrics included those documented within each patient health record and the specific radiation delivery parameters that reflected each health care provider’s treatment. After team investigators visited each center and recorded treatment data, VA-ROQS is now maturing to permit continuous, electronic monitoring of all lung cancer treatment delivered within VHA. As each veteran’s case is planned, the quality of the therapy is monitored, assessed, and reported to the treating physician. Each VHA radiation oncologist will receive up-to-date evaluation of each case compared with these evidence-based quality standards. The quality standards are reviewed by the blue-ribbon panel to keep the process current and valid.
Future of VHA Lung Cancer Care
As VHA continues to prioritize resources to improve and assure optimal outcomes for veterans with lung cancer, it is now looking to create a national network of Lung Cancer Centers of Excellence (LCCE) as described in the VA Budget Submission for fiscal year 2021. If Congress approves funding, LCCEs will soon be developed within the VA regional Veteran Integrated Service Network system to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information, including data on pharmacogenomic profiles. Such a network would create more opportunities to leverage public–private partnerships similar to the VA-PALS project. Creation of LCCEs would help the VA leverage an even stronger learning network to support more research so that all veterans who are impacted by lung cancer have access to personalized care that optimizes safety, quality of life, and overall survival. The lessons learned, networks developed, and partnerships established through VA-PALS, VALOR, and VA-ROQS are instrumental toward achieving these goals.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. 2016;33(suppl 4):18S-22S.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
4. National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
5. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT Screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
6. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi:10.1001/jamainternmed.2016.9022
7. Clancy C. Creating World-class care and service for our nation’s finest: how Veterans Health Administration Diffusion of Excellence Initiative Is innovating and transforming Veterans Affairs health care. Perm J. 2019;23:18.301. doi:10.7812/TPP/18.301
8. Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA health system. JAMA. 2017;317(3):255-256. doi:10.1001/jama.2016.18764
9. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99-105. doi:10.1016/S0140-6736(99)06093-6
10. Mulshine JL, Henschke CI. Lung cancer screening: achieving more by intervening less. Lancet Oncol. 2014;15(12):1284-1285. doi:10.1016/S1470-2045(14)70418-8
11. Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. doi:10.21037/atm.2016.04.06
12. Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246-252. doi:10.7326/0003-4819-158-4-201302190-00004
13. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
14. Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography imaging in a phase II clinical trial in lung cancer. J Med Imaging (Bellingham). 2016;3(3):035505. doi:10.1117/1.JMI.3.3.035505
15. Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP; International Early Lung Cancer Investigators. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201-208. doi:10.1097/CEJ.0000000000000065
16. Armato SG 3rd, McLennan G, Bidaut L, et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys. 2011;38(2):915-931. doi:10.1118/1.3528204
17. Gill RK, Vazquez MF, Kramer A, et al. The use of genetic markers to identify lung cancer in fine needle aspiration samples. Clin Cancer Res. 2008;14(22):7481-7487. doi:10.1158/1078-0432.CCR-07-5242
18. Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272-282.
19. Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37-44. doi:10.12788/jcso.0205
20. Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5(2):183-189. doi:10.21037/tlcr.2016.04.05
21. Bakaeen FG, Reda DJ, Gelijns AC, et al. Department of Veterans Affairs Cooperative Studies Program network of dedicated enrollment sites: implications for surgical trials [published correction appears in JAMA Surg. 2014 Sep;149(9):961]. JAMA Surg. 2014;149(6):507-513. doi:10.1001/jamasurg.2013.4150
22. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials [published correction appears in Lancet Oncol. 2015 Sep;16(9):e427]. Lancet Oncol. 2015;16(6):630-637. doi:10.1016/S1470-2045(15)70168-3
23. Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer. 2017;103:6-10. doi:10.1016/j.lungcan.2016.11.005
24. Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17(1):283. Published 2016 Jun 8. doi:10.1186/s13063-016-1391-4
25. Rooshenas L, Scott LJ, Blazeby JM, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108-120. doi:10.1016/j.jclinepi.2018.10.004
26. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance Program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi:10.1016/j.ijrobp.2019.08.064
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. 2016;33(suppl 4):18S-22S.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
4. National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
5. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT Screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
6. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi:10.1001/jamainternmed.2016.9022
7. Clancy C. Creating World-class care and service for our nation’s finest: how Veterans Health Administration Diffusion of Excellence Initiative Is innovating and transforming Veterans Affairs health care. Perm J. 2019;23:18.301. doi:10.7812/TPP/18.301
8. Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA health system. JAMA. 2017;317(3):255-256. doi:10.1001/jama.2016.18764
9. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99-105. doi:10.1016/S0140-6736(99)06093-6
10. Mulshine JL, Henschke CI. Lung cancer screening: achieving more by intervening less. Lancet Oncol. 2014;15(12):1284-1285. doi:10.1016/S1470-2045(14)70418-8
11. Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. doi:10.21037/atm.2016.04.06
12. Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246-252. doi:10.7326/0003-4819-158-4-201302190-00004
13. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
14. Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography imaging in a phase II clinical trial in lung cancer. J Med Imaging (Bellingham). 2016;3(3):035505. doi:10.1117/1.JMI.3.3.035505
15. Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP; International Early Lung Cancer Investigators. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201-208. doi:10.1097/CEJ.0000000000000065
16. Armato SG 3rd, McLennan G, Bidaut L, et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys. 2011;38(2):915-931. doi:10.1118/1.3528204
17. Gill RK, Vazquez MF, Kramer A, et al. The use of genetic markers to identify lung cancer in fine needle aspiration samples. Clin Cancer Res. 2008;14(22):7481-7487. doi:10.1158/1078-0432.CCR-07-5242
18. Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272-282.
19. Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37-44. doi:10.12788/jcso.0205
20. Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5(2):183-189. doi:10.21037/tlcr.2016.04.05
21. Bakaeen FG, Reda DJ, Gelijns AC, et al. Department of Veterans Affairs Cooperative Studies Program network of dedicated enrollment sites: implications for surgical trials [published correction appears in JAMA Surg. 2014 Sep;149(9):961]. JAMA Surg. 2014;149(6):507-513. doi:10.1001/jamasurg.2013.4150
22. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials [published correction appears in Lancet Oncol. 2015 Sep;16(9):e427]. Lancet Oncol. 2015;16(6):630-637. doi:10.1016/S1470-2045(15)70168-3
23. Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer. 2017;103:6-10. doi:10.1016/j.lungcan.2016.11.005
24. Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17(1):283. Published 2016 Jun 8. doi:10.1186/s13063-016-1391-4
25. Rooshenas L, Scott LJ, Blazeby JM, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108-120. doi:10.1016/j.jclinepi.2018.10.004
26. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance Program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi:10.1016/j.ijrobp.2019.08.064
Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology(FULL)
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model of Public-Private Collaboration to Advance Treatment and Care of Invasive Cancers(FULL)
In late 2016, the US Department of Veterans Affairs (VA) and the Prostate Cancer Foundation (PCF) announced a multiyear public-private partnership to deliver precision oncology and best-in-class care to all veterans battling prostate cancer.1 The creation of this partnership was due to several favorable factors. At that time, VA Secretary Robert A. McDonald had created the Secretary’s Center for Strategic Partnerships. This Center provided a mechanism for nonprofit and industry partners to collaborate with the VA, thereby advancing partnerships that served the VA mission of “serving and honoring…America’s veterans.”1,2 Concurrently, Vice President Joseph Biden’s Cancer Moonshot (later renamed the Beau Biden Cancer Moonshot) charged PCF and other cancer-focused organizations with the ambitious goal of making a decade’s worth of advancements in cancer prevention, diagnosis, and treatment in 5 years.3 As such, both organizations were positioned to recognize and address the unique prostate cancer challenges faced by male veterans, which ultimately led to the PCF-VA partnership.
A number of factors have allowed the PCF-VA partnership to scale the Centers of Excellence (COE) program. This article seeks to highlight the strategic organizing and mobilization techniques employed by the PCF-VA partnership, which can inform future public-private hybrid initiatives focused on precision medicine.
Executive Leadership as Patient Advocates
From its creation, the PCF-VA partnership placed as much importance on veteran patient care as it has on making oncologic advances. The fact that this focus came primarily from executive leadership was critical to the partnership’s success. PCF board members emphasized the significance of prioritizing veterans and military families in cancer research efforts.
A notable example is S. Ward “Trip” Casscells, MD, a veteran who was deployed to Iraq in 2006 and subsequently served as US Department of Defense Assistant Secretary of Defense for Health Affairs. He focused much of his leadership on ensuring that veterans and military families, having performed a critical service for the country, were served with this same degree of excellence when it came to health.4 Fellow PCF Board member Lawrence Stupski, spoke publicly about his drug-resistant form of prostate cancer, bringing awareness to the complexity of ending death and suffering from the disease.5 Like Casscells, Stupski has a military service background, and served in Vietnam in 1968 as an officer in the US Navy. Both participated in multiple prostate cancer clinical trials themselves, serving as models of veteran trial participants. This visibility and leadership created a culture where veterans were not just instrumental in advancing cancer research, but also representative of a responsibility to ensure high-quality care for an underserved and at-risk community (Figure 1).
Executive advocacy and visionary philanthropy on behalf of veterans were vital to catalyzing the PCF-VA partnership framework, allowing both organizations to act on shared goals through a joint venture. Stupski’s legacy also jump-started the partnership itself, as the Stupski Foundation provided the crucial initial funding to launch a pilot version of the partnership.
Ultimately, this suggests that entrepreneurial philanthropy, top-level patient-led advocacy, and executive leadership can bolster the success of future health partnerships by advocating for specific missions, thus allowing convergence of goals between public and private entities. Visibility of leaders also encourages participation in the initiative itself, specifically in regard to patients being willing to enroll in clinical trials.
During the Launch Pad: Pathways to Cancer InnoVAtion PCF-VA summit in November 2016, PCF and the VA signed a memorandum of understanding (MOU) that solidified joint goals and accountability practices to create a scalable model for veteran-centered, genomics-based precision oncology care. Special focus was placed upon developing clinical trials for vulnerable veteran populations (Figure 2). PCF dedicated $50 million of funding to this partnership, facilitated largely in part by several philanthropists who stepped up after the MOU was signed, and early, life-extending successes from the pilot were demonstrated. This “snowballing” of funding indicates that the establishment of a public-private health partnership—with clear and compelling goals and early proof-of-concept—galvanizes efforts to further advance the partnership by garnering critical philanthropic investment.
VHA Economy of Scale
Utilizing the vast capacity of the Veterans Health Administration (VHA) for care was integral to the success of the partnership. The VHA serves 9 million veterans each year in 1,255 health care facilities, which include 170 medical centers and 1,075 outpatient clinics.6 As the nation’s largest integrated health care system, the VHA approaches cancer care with a single electronic health record system across all of its facilities, featuring comprehensive clinical outcome documentation.7 The VHA’s systemwide DNA sequence platform, through the National Precision Oncology Program (NPOP), also provided an optimal area for research and standardization of precision oncology practices on a national scale.8
Centers of Excellence: An Adaptable Model
The primary thrust of the partnership centers on the PCF-VA COEs, which form the Precision Oncology Program for Cancer of the Prostate (POPCaP) network. Over the last 4 years, PCF-deployed philanthropy has established 12 PCF-VA COEs, located in the Bronx and Manhattan, New York; Tampa Bay, Florida; Los Angeles, California; Seattle, Washington; Chicago, Illinois; Philadelphia, Pennsylvania; Ann Arbor, Michigan; Durham, North Carolina; Washington, DC; Boston, Massachusetts; and Portland, Oregon. Sites were initially chosen based on strong connections to academic medical centers, National Cancer Institute-designated comprehensive care centers, and physician-scientists who were professionally invested in precision prostate cancer oncology. Drawing on PCF’s existing networks helped to identify these areas, which were already rich in human and technological capital, before expanding to areas that were less resource rich. Future health partnerships may therefore consider capitalizing on existing relationships to spark initial growth, which can provide pathways for scaling.
In collaboration with NPOP, COEs work to sequence genomic and somatic tissue from veterans with metastatic prostate cancer, connect patients to appropriate clinical trials and treatment pathways, and advance guidelines for precision cancer care. Certain aspects of COE operations remain constant across all facilities. Annual progress reports, comprising of a written report, slide deck of accomplishments, and bulleted delineation of challenges and future plans are required of all COE-funded investigators. All COEs also are tasked with hiring a center coordinator, instituting a standardized sequencing and mutation reporting protocol, participating in consortium-wide phase 3 studies, and engaging in monthly conference calls to assess progress. A complete list of requirements is found in the Table.
However, the methods through which these goals must be completed is at the discretion of the COE investigators. Each COE, due to institutional and patient variance, experiences distinctive challenges and must mold its practice to fit existing capacities. For example, certain sites optimized workflow by training coordinators to analyze specimens, thereby improving care speed for veteran patients. Other COEs maximized nearby resources by hiring offsite specialists such as genetic counselors and interventional radiologists. By providing the freedom to design site-specific methodology, the PCF-VA partnership allows each COE to meet the award goals through any appropriate path using the funds provided, increasing efficiency and optimizing progress. This diversity of protocol also helped to expand the capabilities of the POPCaP Network, allowing sites to specialize in areas of interest in precision oncology. This eventually helped to inform future initiatives.
Accelerating Clinical Trials
A critical feature of the POPCaP network is the Prostate Cancer Analysis for Therapy Choice (PATCH) plexus.9 Through this investigative umbrella, veterans who are sequenced at any COE are given access to clinical trials at sites across POPCaP. Funding is available to support veteran travel to these sites, decreasing the chance that a veteran’s location is a barrier to treatment. In this way, the PCF-VA partnership continues to broaden treatment scopes for tens of thousands of veterans while simultaneously advancing clinical knowledge of precision oncology.
Fostering a Scientific Community
The PCF-VA partnership’s COE initiative capitalizes on resources from both nonprofit and public sectors to cultivate dynamic scientific discourse and investigative support. Through monthly meetings of the NPOP Molecular Oncology Tumor Board, COE investigators receive guidance and education to better assist veterans sequenced through their programs. Another example of enriched scientific collaboration are the Dream Team investigators, who were collaboratively funded by PCF, Stand Up 2 Cancer, and the American Association for Cancer Research.10 These teams made significant strides in genomic profiling of advanced prostate cancer and outpatient computed tomography-guided metastatic bone biopsy techniques. Through the PCF-VA partnership, COE researchers benefited from these investigators’ insight and expertise during regular check-in calls with investigators. PCF’s Prescription Pad, also connects all investigators to current therapies and trials, better informing them of future directions for their own work (Figure 3).11,12
The PCF-VA partnership also facilitates peer-to-peer communication through regular inperson and virtual meetings of investigators, coordinators, and other stakeholders. These meetings allow the creation of focused working groups composed of COE leaders across the nation. The working groups seek to improve all aspects of functionality, including operational roadblocks, sequencing and phenotyping protocols, and addressing health service disparities. The VA Puget Sound Health Care System in Seattle, Washington, and the West Los Angeles VA Medical Center in California both are mentorship sites that play instrumental roles in guiding newer sites through challenges, such as obtaining rapid pathology results and navigating the VA system. This interinvestigator communication also helps to recruit new junior and senior investigators to POPCaP, thereby broadening the network’s reach.
Future Pathways
In line with the mission outlined in the MOU of developing treatments for veteran populations, the PCF-VA partnership has actively pursued addressing veteran health inequities. In 2018, a $2.5 million gift from Robert F. Smith, Founder, Chairman, and Chief Executive Officer of Vista Equity Partners, set up the Chicago COE with the express purpose of serving African American veterans, who represent men at highest risk of prostate cancer incidence and mortality.13 A regularly convened health disparities working group explores future efforts. This group, composed of VA investigators, epidemiologists, geneticists, and other field leaders, seeks to advance the most compelling approaches to eliminate inequities in prostate cancer care.
A novel nursing initiative that focuses on the role of nurses in providing genetic services for prostate cancer is being developed. The need for new genetic care models and significant barriers to genetic service delivery have been well-documented for prostate cancer.14 The initiative provides nurses with opportunities to train with POPCaP and VA geneticists, enroll in a City of Hope genetics course, and to join a collaborative of geneticists, medical oncologists, and nurse practitioners.15 By furthering nursing education and leadership, the initiative empowers nurses to fill the gaps in veteran health care, particularly in genomics-based precision oncology.
The COE platform also has provided the foundation for the building of COEs for other cancers relevant to veterans, such as lung cancer. This expansion of COE function helps to further the VA goal of not only creating COEs, but a system of excellence. More recently, COE infrastructure has been leveraged in the fight against COVID-19 through HITCH, a clinical trial investigating the use of temporary androgen suppression in improving clinical outcomes of veterans with COVID-19.16 This expansion of function also provides a mechanism for COEs to continue to be funded in the future: attracting federal capital, private philanthropy, and industrial support is dependent on realized and expanded goals, as well as demonstrable progress in veteran care.
Conclusions
The PCF-VA partnership serves as an example of a public-private health partnership pursuing strategic pathways and bold goals to ensure that every eligible veteran has access to precision oncology. These pathways include advocacy on the part of executive leadership, recognizing existing economies of scale, building compelling narratives to maximize funding, creating flexible requirements, and facilitating a robust, resource-rich scientific network. This partnership already has opened doors to future initiatives and continues to adapt to a rapidly changing health landscape. The discussed strategies have the potential to inform future health initiatives and showcase how a systemic approach to eradicating health inequities can greatly benefit underserved communities.
The success of the PCF-VA partnership represents more than just an efficient partnership model. The partnership’s emphasis on veterans, who exemplify service, highlights the extent to which cancer patients sacrifice to contribute to medical research. This service necessitates a service in kind: all health stakeholders share the responsibility to rapidly advance therapies and care, both to honor the patients who have come before, and to meet the needs of patients with treatment resistant forms of the disease urgently awaiting precision breakthroughs and cures.
1. US Department of Veterans Affairs. Secretary’s Center for Strategic Partnerships (SCSP): about us. https://www.va.gov/scsp/about/. Updated January 22, 2020. Accessed July 27, 2020.
2. US Department of Veterans Affairs. About VA. https://www.va.gov/about_va/mission.asp. Updated August 20, 2015. Accessed July 27, 2020.
3. American Association for Cancer Research. National Cancer Moonshot Initiative. https://www.aacr.org/professionals/policy-and-advocacy/science-policy-government-affairs/national-cancer-moonshot-initiative. Accessed July 30, 2020.
4. Zogby J, Fighting cancer is a Defense Department obligation. https://www.huffpost.com/entry/fighting-cancer-is-our-co_b_837535. Updated May 25, 2011. Accessed July 30, 2020.
5. Colliver V. Lawrence Stupski, former Schwab exec, dies. San Francisco Chronicle June 12, 2013. https://www.sfchronicle.com/bayarea/article/Lawrence-Stupski-former-Schwab-exec-dies-4597329.php. Accessed July 30, 2020.
6. US Department of Veterans Affairs, Veterans Health Administration. About VHA. https://www.va.gov/health/aboutvha.asp. Updated July 14, 2019. Accessed July 27, 2020.
7. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) Network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37 (suppl 4):S48-S53. doi:10.12788/fp.0021
8. US Department of Veterans Affairs, National Oncology Program Office: about us. https://www.cancer.va.gov/CANCER/about.asp. Accessed July 28, 2020.
9. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(8):S62-S67. doi:10.12788/fp.0028
10. Prostate Cancer Foundation. Prostate Cancer Foundation and Stand Up To Cancer announce new dream team [press release]. https://www.pcf.org/news/prostate-cancer-foundation-and-stand-up-to-cancer-announce-new-dream-team/. Published April 1, 2020. Accessed July 30, 2020.
11. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
12. Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer [published correction appears in Nat Genet. 2019 Jul;51(7):1194]. Nat Genet. 2018;50(5):645-651. doi:10.1038/s41588-018-0078-z
13. Prostate Cancer Foundation. $2.5 million gift from Robert Frederick Smith to the Prostate Cancer Foundation is the largest donation ever dedicated to advancing prostate cancer research in African-American men [press release]. https://www.pcf.org/news/robert-frederick-smith-gift/. Published January 14, 2018. Accessed July 27, 2020.
14. Carlo MI, Giri VN, Paller CJ, et al. Evolving intersection between inherited cancer genetics and therapeutic clinical trials in prostate cancer: a white paper from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. JCO Precis Oncol. 2018;2018:10.1200/PO.18.00060. doi:10.1200/PO.18.00060
15. City of Hope. Intensive course in genomic cancer risk assessment. https://www.cityofhope.org/education/health-professional-education/cancer-genomics-education-program/intensive-course-in-cancer-risk-assessment-overview. Accessed July 28, 2020.
16. US National Library of Medicine, Clinicaltrial.gov. Hormonal Intervention for the Treatment in Veterans with COVID-19 Requiring Hospitalization (HITCH): NCT04397718. https://clinicaltrials.gov/ct2/show/NCT04397718. Updated July 23, 2020. Accessed July 30, 2020.
In late 2016, the US Department of Veterans Affairs (VA) and the Prostate Cancer Foundation (PCF) announced a multiyear public-private partnership to deliver precision oncology and best-in-class care to all veterans battling prostate cancer.1 The creation of this partnership was due to several favorable factors. At that time, VA Secretary Robert A. McDonald had created the Secretary’s Center for Strategic Partnerships. This Center provided a mechanism for nonprofit and industry partners to collaborate with the VA, thereby advancing partnerships that served the VA mission of “serving and honoring…America’s veterans.”1,2 Concurrently, Vice President Joseph Biden’s Cancer Moonshot (later renamed the Beau Biden Cancer Moonshot) charged PCF and other cancer-focused organizations with the ambitious goal of making a decade’s worth of advancements in cancer prevention, diagnosis, and treatment in 5 years.3 As such, both organizations were positioned to recognize and address the unique prostate cancer challenges faced by male veterans, which ultimately led to the PCF-VA partnership.
A number of factors have allowed the PCF-VA partnership to scale the Centers of Excellence (COE) program. This article seeks to highlight the strategic organizing and mobilization techniques employed by the PCF-VA partnership, which can inform future public-private hybrid initiatives focused on precision medicine.
Executive Leadership as Patient Advocates
From its creation, the PCF-VA partnership placed as much importance on veteran patient care as it has on making oncologic advances. The fact that this focus came primarily from executive leadership was critical to the partnership’s success. PCF board members emphasized the significance of prioritizing veterans and military families in cancer research efforts.
A notable example is S. Ward “Trip” Casscells, MD, a veteran who was deployed to Iraq in 2006 and subsequently served as US Department of Defense Assistant Secretary of Defense for Health Affairs. He focused much of his leadership on ensuring that veterans and military families, having performed a critical service for the country, were served with this same degree of excellence when it came to health.4 Fellow PCF Board member Lawrence Stupski, spoke publicly about his drug-resistant form of prostate cancer, bringing awareness to the complexity of ending death and suffering from the disease.5 Like Casscells, Stupski has a military service background, and served in Vietnam in 1968 as an officer in the US Navy. Both participated in multiple prostate cancer clinical trials themselves, serving as models of veteran trial participants. This visibility and leadership created a culture where veterans were not just instrumental in advancing cancer research, but also representative of a responsibility to ensure high-quality care for an underserved and at-risk community (Figure 1).
Executive advocacy and visionary philanthropy on behalf of veterans were vital to catalyzing the PCF-VA partnership framework, allowing both organizations to act on shared goals through a joint venture. Stupski’s legacy also jump-started the partnership itself, as the Stupski Foundation provided the crucial initial funding to launch a pilot version of the partnership.
Ultimately, this suggests that entrepreneurial philanthropy, top-level patient-led advocacy, and executive leadership can bolster the success of future health partnerships by advocating for specific missions, thus allowing convergence of goals between public and private entities. Visibility of leaders also encourages participation in the initiative itself, specifically in regard to patients being willing to enroll in clinical trials.
During the Launch Pad: Pathways to Cancer InnoVAtion PCF-VA summit in November 2016, PCF and the VA signed a memorandum of understanding (MOU) that solidified joint goals and accountability practices to create a scalable model for veteran-centered, genomics-based precision oncology care. Special focus was placed upon developing clinical trials for vulnerable veteran populations (Figure 2). PCF dedicated $50 million of funding to this partnership, facilitated largely in part by several philanthropists who stepped up after the MOU was signed, and early, life-extending successes from the pilot were demonstrated. This “snowballing” of funding indicates that the establishment of a public-private health partnership—with clear and compelling goals and early proof-of-concept—galvanizes efforts to further advance the partnership by garnering critical philanthropic investment.
VHA Economy of Scale
Utilizing the vast capacity of the Veterans Health Administration (VHA) for care was integral to the success of the partnership. The VHA serves 9 million veterans each year in 1,255 health care facilities, which include 170 medical centers and 1,075 outpatient clinics.6 As the nation’s largest integrated health care system, the VHA approaches cancer care with a single electronic health record system across all of its facilities, featuring comprehensive clinical outcome documentation.7 The VHA’s systemwide DNA sequence platform, through the National Precision Oncology Program (NPOP), also provided an optimal area for research and standardization of precision oncology practices on a national scale.8
Centers of Excellence: An Adaptable Model
The primary thrust of the partnership centers on the PCF-VA COEs, which form the Precision Oncology Program for Cancer of the Prostate (POPCaP) network. Over the last 4 years, PCF-deployed philanthropy has established 12 PCF-VA COEs, located in the Bronx and Manhattan, New York; Tampa Bay, Florida; Los Angeles, California; Seattle, Washington; Chicago, Illinois; Philadelphia, Pennsylvania; Ann Arbor, Michigan; Durham, North Carolina; Washington, DC; Boston, Massachusetts; and Portland, Oregon. Sites were initially chosen based on strong connections to academic medical centers, National Cancer Institute-designated comprehensive care centers, and physician-scientists who were professionally invested in precision prostate cancer oncology. Drawing on PCF’s existing networks helped to identify these areas, which were already rich in human and technological capital, before expanding to areas that were less resource rich. Future health partnerships may therefore consider capitalizing on existing relationships to spark initial growth, which can provide pathways for scaling.
In collaboration with NPOP, COEs work to sequence genomic and somatic tissue from veterans with metastatic prostate cancer, connect patients to appropriate clinical trials and treatment pathways, and advance guidelines for precision cancer care. Certain aspects of COE operations remain constant across all facilities. Annual progress reports, comprising of a written report, slide deck of accomplishments, and bulleted delineation of challenges and future plans are required of all COE-funded investigators. All COEs also are tasked with hiring a center coordinator, instituting a standardized sequencing and mutation reporting protocol, participating in consortium-wide phase 3 studies, and engaging in monthly conference calls to assess progress. A complete list of requirements is found in the Table.
However, the methods through which these goals must be completed is at the discretion of the COE investigators. Each COE, due to institutional and patient variance, experiences distinctive challenges and must mold its practice to fit existing capacities. For example, certain sites optimized workflow by training coordinators to analyze specimens, thereby improving care speed for veteran patients. Other COEs maximized nearby resources by hiring offsite specialists such as genetic counselors and interventional radiologists. By providing the freedom to design site-specific methodology, the PCF-VA partnership allows each COE to meet the award goals through any appropriate path using the funds provided, increasing efficiency and optimizing progress. This diversity of protocol also helped to expand the capabilities of the POPCaP Network, allowing sites to specialize in areas of interest in precision oncology. This eventually helped to inform future initiatives.
Accelerating Clinical Trials
A critical feature of the POPCaP network is the Prostate Cancer Analysis for Therapy Choice (PATCH) plexus.9 Through this investigative umbrella, veterans who are sequenced at any COE are given access to clinical trials at sites across POPCaP. Funding is available to support veteran travel to these sites, decreasing the chance that a veteran’s location is a barrier to treatment. In this way, the PCF-VA partnership continues to broaden treatment scopes for tens of thousands of veterans while simultaneously advancing clinical knowledge of precision oncology.
Fostering a Scientific Community
The PCF-VA partnership’s COE initiative capitalizes on resources from both nonprofit and public sectors to cultivate dynamic scientific discourse and investigative support. Through monthly meetings of the NPOP Molecular Oncology Tumor Board, COE investigators receive guidance and education to better assist veterans sequenced through their programs. Another example of enriched scientific collaboration are the Dream Team investigators, who were collaboratively funded by PCF, Stand Up 2 Cancer, and the American Association for Cancer Research.10 These teams made significant strides in genomic profiling of advanced prostate cancer and outpatient computed tomography-guided metastatic bone biopsy techniques. Through the PCF-VA partnership, COE researchers benefited from these investigators’ insight and expertise during regular check-in calls with investigators. PCF’s Prescription Pad, also connects all investigators to current therapies and trials, better informing them of future directions for their own work (Figure 3).11,12
The PCF-VA partnership also facilitates peer-to-peer communication through regular inperson and virtual meetings of investigators, coordinators, and other stakeholders. These meetings allow the creation of focused working groups composed of COE leaders across the nation. The working groups seek to improve all aspects of functionality, including operational roadblocks, sequencing and phenotyping protocols, and addressing health service disparities. The VA Puget Sound Health Care System in Seattle, Washington, and the West Los Angeles VA Medical Center in California both are mentorship sites that play instrumental roles in guiding newer sites through challenges, such as obtaining rapid pathology results and navigating the VA system. This interinvestigator communication also helps to recruit new junior and senior investigators to POPCaP, thereby broadening the network’s reach.
Future Pathways
In line with the mission outlined in the MOU of developing treatments for veteran populations, the PCF-VA partnership has actively pursued addressing veteran health inequities. In 2018, a $2.5 million gift from Robert F. Smith, Founder, Chairman, and Chief Executive Officer of Vista Equity Partners, set up the Chicago COE with the express purpose of serving African American veterans, who represent men at highest risk of prostate cancer incidence and mortality.13 A regularly convened health disparities working group explores future efforts. This group, composed of VA investigators, epidemiologists, geneticists, and other field leaders, seeks to advance the most compelling approaches to eliminate inequities in prostate cancer care.
A novel nursing initiative that focuses on the role of nurses in providing genetic services for prostate cancer is being developed. The need for new genetic care models and significant barriers to genetic service delivery have been well-documented for prostate cancer.14 The initiative provides nurses with opportunities to train with POPCaP and VA geneticists, enroll in a City of Hope genetics course, and to join a collaborative of geneticists, medical oncologists, and nurse practitioners.15 By furthering nursing education and leadership, the initiative empowers nurses to fill the gaps in veteran health care, particularly in genomics-based precision oncology.
The COE platform also has provided the foundation for the building of COEs for other cancers relevant to veterans, such as lung cancer. This expansion of COE function helps to further the VA goal of not only creating COEs, but a system of excellence. More recently, COE infrastructure has been leveraged in the fight against COVID-19 through HITCH, a clinical trial investigating the use of temporary androgen suppression in improving clinical outcomes of veterans with COVID-19.16 This expansion of function also provides a mechanism for COEs to continue to be funded in the future: attracting federal capital, private philanthropy, and industrial support is dependent on realized and expanded goals, as well as demonstrable progress in veteran care.
Conclusions
The PCF-VA partnership serves as an example of a public-private health partnership pursuing strategic pathways and bold goals to ensure that every eligible veteran has access to precision oncology. These pathways include advocacy on the part of executive leadership, recognizing existing economies of scale, building compelling narratives to maximize funding, creating flexible requirements, and facilitating a robust, resource-rich scientific network. This partnership already has opened doors to future initiatives and continues to adapt to a rapidly changing health landscape. The discussed strategies have the potential to inform future health initiatives and showcase how a systemic approach to eradicating health inequities can greatly benefit underserved communities.
The success of the PCF-VA partnership represents more than just an efficient partnership model. The partnership’s emphasis on veterans, who exemplify service, highlights the extent to which cancer patients sacrifice to contribute to medical research. This service necessitates a service in kind: all health stakeholders share the responsibility to rapidly advance therapies and care, both to honor the patients who have come before, and to meet the needs of patients with treatment resistant forms of the disease urgently awaiting precision breakthroughs and cures.
In late 2016, the US Department of Veterans Affairs (VA) and the Prostate Cancer Foundation (PCF) announced a multiyear public-private partnership to deliver precision oncology and best-in-class care to all veterans battling prostate cancer.1 The creation of this partnership was due to several favorable factors. At that time, VA Secretary Robert A. McDonald had created the Secretary’s Center for Strategic Partnerships. This Center provided a mechanism for nonprofit and industry partners to collaborate with the VA, thereby advancing partnerships that served the VA mission of “serving and honoring…America’s veterans.”1,2 Concurrently, Vice President Joseph Biden’s Cancer Moonshot (later renamed the Beau Biden Cancer Moonshot) charged PCF and other cancer-focused organizations with the ambitious goal of making a decade’s worth of advancements in cancer prevention, diagnosis, and treatment in 5 years.3 As such, both organizations were positioned to recognize and address the unique prostate cancer challenges faced by male veterans, which ultimately led to the PCF-VA partnership.
A number of factors have allowed the PCF-VA partnership to scale the Centers of Excellence (COE) program. This article seeks to highlight the strategic organizing and mobilization techniques employed by the PCF-VA partnership, which can inform future public-private hybrid initiatives focused on precision medicine.
Executive Leadership as Patient Advocates
From its creation, the PCF-VA partnership placed as much importance on veteran patient care as it has on making oncologic advances. The fact that this focus came primarily from executive leadership was critical to the partnership’s success. PCF board members emphasized the significance of prioritizing veterans and military families in cancer research efforts.
A notable example is S. Ward “Trip” Casscells, MD, a veteran who was deployed to Iraq in 2006 and subsequently served as US Department of Defense Assistant Secretary of Defense for Health Affairs. He focused much of his leadership on ensuring that veterans and military families, having performed a critical service for the country, were served with this same degree of excellence when it came to health.4 Fellow PCF Board member Lawrence Stupski, spoke publicly about his drug-resistant form of prostate cancer, bringing awareness to the complexity of ending death and suffering from the disease.5 Like Casscells, Stupski has a military service background, and served in Vietnam in 1968 as an officer in the US Navy. Both participated in multiple prostate cancer clinical trials themselves, serving as models of veteran trial participants. This visibility and leadership created a culture where veterans were not just instrumental in advancing cancer research, but also representative of a responsibility to ensure high-quality care for an underserved and at-risk community (Figure 1).
Executive advocacy and visionary philanthropy on behalf of veterans were vital to catalyzing the PCF-VA partnership framework, allowing both organizations to act on shared goals through a joint venture. Stupski’s legacy also jump-started the partnership itself, as the Stupski Foundation provided the crucial initial funding to launch a pilot version of the partnership.
Ultimately, this suggests that entrepreneurial philanthropy, top-level patient-led advocacy, and executive leadership can bolster the success of future health partnerships by advocating for specific missions, thus allowing convergence of goals between public and private entities. Visibility of leaders also encourages participation in the initiative itself, specifically in regard to patients being willing to enroll in clinical trials.
During the Launch Pad: Pathways to Cancer InnoVAtion PCF-VA summit in November 2016, PCF and the VA signed a memorandum of understanding (MOU) that solidified joint goals and accountability practices to create a scalable model for veteran-centered, genomics-based precision oncology care. Special focus was placed upon developing clinical trials for vulnerable veteran populations (Figure 2). PCF dedicated $50 million of funding to this partnership, facilitated largely in part by several philanthropists who stepped up after the MOU was signed, and early, life-extending successes from the pilot were demonstrated. This “snowballing” of funding indicates that the establishment of a public-private health partnership—with clear and compelling goals and early proof-of-concept—galvanizes efforts to further advance the partnership by garnering critical philanthropic investment.
VHA Economy of Scale
Utilizing the vast capacity of the Veterans Health Administration (VHA) for care was integral to the success of the partnership. The VHA serves 9 million veterans each year in 1,255 health care facilities, which include 170 medical centers and 1,075 outpatient clinics.6 As the nation’s largest integrated health care system, the VHA approaches cancer care with a single electronic health record system across all of its facilities, featuring comprehensive clinical outcome documentation.7 The VHA’s systemwide DNA sequence platform, through the National Precision Oncology Program (NPOP), also provided an optimal area for research and standardization of precision oncology practices on a national scale.8
Centers of Excellence: An Adaptable Model
The primary thrust of the partnership centers on the PCF-VA COEs, which form the Precision Oncology Program for Cancer of the Prostate (POPCaP) network. Over the last 4 years, PCF-deployed philanthropy has established 12 PCF-VA COEs, located in the Bronx and Manhattan, New York; Tampa Bay, Florida; Los Angeles, California; Seattle, Washington; Chicago, Illinois; Philadelphia, Pennsylvania; Ann Arbor, Michigan; Durham, North Carolina; Washington, DC; Boston, Massachusetts; and Portland, Oregon. Sites were initially chosen based on strong connections to academic medical centers, National Cancer Institute-designated comprehensive care centers, and physician-scientists who were professionally invested in precision prostate cancer oncology. Drawing on PCF’s existing networks helped to identify these areas, which were already rich in human and technological capital, before expanding to areas that were less resource rich. Future health partnerships may therefore consider capitalizing on existing relationships to spark initial growth, which can provide pathways for scaling.
In collaboration with NPOP, COEs work to sequence genomic and somatic tissue from veterans with metastatic prostate cancer, connect patients to appropriate clinical trials and treatment pathways, and advance guidelines for precision cancer care. Certain aspects of COE operations remain constant across all facilities. Annual progress reports, comprising of a written report, slide deck of accomplishments, and bulleted delineation of challenges and future plans are required of all COE-funded investigators. All COEs also are tasked with hiring a center coordinator, instituting a standardized sequencing and mutation reporting protocol, participating in consortium-wide phase 3 studies, and engaging in monthly conference calls to assess progress. A complete list of requirements is found in the Table.
However, the methods through which these goals must be completed is at the discretion of the COE investigators. Each COE, due to institutional and patient variance, experiences distinctive challenges and must mold its practice to fit existing capacities. For example, certain sites optimized workflow by training coordinators to analyze specimens, thereby improving care speed for veteran patients. Other COEs maximized nearby resources by hiring offsite specialists such as genetic counselors and interventional radiologists. By providing the freedom to design site-specific methodology, the PCF-VA partnership allows each COE to meet the award goals through any appropriate path using the funds provided, increasing efficiency and optimizing progress. This diversity of protocol also helped to expand the capabilities of the POPCaP Network, allowing sites to specialize in areas of interest in precision oncology. This eventually helped to inform future initiatives.
Accelerating Clinical Trials
A critical feature of the POPCaP network is the Prostate Cancer Analysis for Therapy Choice (PATCH) plexus.9 Through this investigative umbrella, veterans who are sequenced at any COE are given access to clinical trials at sites across POPCaP. Funding is available to support veteran travel to these sites, decreasing the chance that a veteran’s location is a barrier to treatment. In this way, the PCF-VA partnership continues to broaden treatment scopes for tens of thousands of veterans while simultaneously advancing clinical knowledge of precision oncology.
Fostering a Scientific Community
The PCF-VA partnership’s COE initiative capitalizes on resources from both nonprofit and public sectors to cultivate dynamic scientific discourse and investigative support. Through monthly meetings of the NPOP Molecular Oncology Tumor Board, COE investigators receive guidance and education to better assist veterans sequenced through their programs. Another example of enriched scientific collaboration are the Dream Team investigators, who were collaboratively funded by PCF, Stand Up 2 Cancer, and the American Association for Cancer Research.10 These teams made significant strides in genomic profiling of advanced prostate cancer and outpatient computed tomography-guided metastatic bone biopsy techniques. Through the PCF-VA partnership, COE researchers benefited from these investigators’ insight and expertise during regular check-in calls with investigators. PCF’s Prescription Pad, also connects all investigators to current therapies and trials, better informing them of future directions for their own work (Figure 3).11,12
The PCF-VA partnership also facilitates peer-to-peer communication through regular inperson and virtual meetings of investigators, coordinators, and other stakeholders. These meetings allow the creation of focused working groups composed of COE leaders across the nation. The working groups seek to improve all aspects of functionality, including operational roadblocks, sequencing and phenotyping protocols, and addressing health service disparities. The VA Puget Sound Health Care System in Seattle, Washington, and the West Los Angeles VA Medical Center in California both are mentorship sites that play instrumental roles in guiding newer sites through challenges, such as obtaining rapid pathology results and navigating the VA system. This interinvestigator communication also helps to recruit new junior and senior investigators to POPCaP, thereby broadening the network’s reach.
Future Pathways
In line with the mission outlined in the MOU of developing treatments for veteran populations, the PCF-VA partnership has actively pursued addressing veteran health inequities. In 2018, a $2.5 million gift from Robert F. Smith, Founder, Chairman, and Chief Executive Officer of Vista Equity Partners, set up the Chicago COE with the express purpose of serving African American veterans, who represent men at highest risk of prostate cancer incidence and mortality.13 A regularly convened health disparities working group explores future efforts. This group, composed of VA investigators, epidemiologists, geneticists, and other field leaders, seeks to advance the most compelling approaches to eliminate inequities in prostate cancer care.
A novel nursing initiative that focuses on the role of nurses in providing genetic services for prostate cancer is being developed. The need for new genetic care models and significant barriers to genetic service delivery have been well-documented for prostate cancer.14 The initiative provides nurses with opportunities to train with POPCaP and VA geneticists, enroll in a City of Hope genetics course, and to join a collaborative of geneticists, medical oncologists, and nurse practitioners.15 By furthering nursing education and leadership, the initiative empowers nurses to fill the gaps in veteran health care, particularly in genomics-based precision oncology.
The COE platform also has provided the foundation for the building of COEs for other cancers relevant to veterans, such as lung cancer. This expansion of COE function helps to further the VA goal of not only creating COEs, but a system of excellence. More recently, COE infrastructure has been leveraged in the fight against COVID-19 through HITCH, a clinical trial investigating the use of temporary androgen suppression in improving clinical outcomes of veterans with COVID-19.16 This expansion of function also provides a mechanism for COEs to continue to be funded in the future: attracting federal capital, private philanthropy, and industrial support is dependent on realized and expanded goals, as well as demonstrable progress in veteran care.
Conclusions
The PCF-VA partnership serves as an example of a public-private health partnership pursuing strategic pathways and bold goals to ensure that every eligible veteran has access to precision oncology. These pathways include advocacy on the part of executive leadership, recognizing existing economies of scale, building compelling narratives to maximize funding, creating flexible requirements, and facilitating a robust, resource-rich scientific network. This partnership already has opened doors to future initiatives and continues to adapt to a rapidly changing health landscape. The discussed strategies have the potential to inform future health initiatives and showcase how a systemic approach to eradicating health inequities can greatly benefit underserved communities.
The success of the PCF-VA partnership represents more than just an efficient partnership model. The partnership’s emphasis on veterans, who exemplify service, highlights the extent to which cancer patients sacrifice to contribute to medical research. This service necessitates a service in kind: all health stakeholders share the responsibility to rapidly advance therapies and care, both to honor the patients who have come before, and to meet the needs of patients with treatment resistant forms of the disease urgently awaiting precision breakthroughs and cures.
1. US Department of Veterans Affairs. Secretary’s Center for Strategic Partnerships (SCSP): about us. https://www.va.gov/scsp/about/. Updated January 22, 2020. Accessed July 27, 2020.
2. US Department of Veterans Affairs. About VA. https://www.va.gov/about_va/mission.asp. Updated August 20, 2015. Accessed July 27, 2020.
3. American Association for Cancer Research. National Cancer Moonshot Initiative. https://www.aacr.org/professionals/policy-and-advocacy/science-policy-government-affairs/national-cancer-moonshot-initiative. Accessed July 30, 2020.
4. Zogby J, Fighting cancer is a Defense Department obligation. https://www.huffpost.com/entry/fighting-cancer-is-our-co_b_837535. Updated May 25, 2011. Accessed July 30, 2020.
5. Colliver V. Lawrence Stupski, former Schwab exec, dies. San Francisco Chronicle June 12, 2013. https://www.sfchronicle.com/bayarea/article/Lawrence-Stupski-former-Schwab-exec-dies-4597329.php. Accessed July 30, 2020.
6. US Department of Veterans Affairs, Veterans Health Administration. About VHA. https://www.va.gov/health/aboutvha.asp. Updated July 14, 2019. Accessed July 27, 2020.
7. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) Network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37 (suppl 4):S48-S53. doi:10.12788/fp.0021
8. US Department of Veterans Affairs, National Oncology Program Office: about us. https://www.cancer.va.gov/CANCER/about.asp. Accessed July 28, 2020.
9. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(8):S62-S67. doi:10.12788/fp.0028
10. Prostate Cancer Foundation. Prostate Cancer Foundation and Stand Up To Cancer announce new dream team [press release]. https://www.pcf.org/news/prostate-cancer-foundation-and-stand-up-to-cancer-announce-new-dream-team/. Published April 1, 2020. Accessed July 30, 2020.
11. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
12. Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer [published correction appears in Nat Genet. 2019 Jul;51(7):1194]. Nat Genet. 2018;50(5):645-651. doi:10.1038/s41588-018-0078-z
13. Prostate Cancer Foundation. $2.5 million gift from Robert Frederick Smith to the Prostate Cancer Foundation is the largest donation ever dedicated to advancing prostate cancer research in African-American men [press release]. https://www.pcf.org/news/robert-frederick-smith-gift/. Published January 14, 2018. Accessed July 27, 2020.
14. Carlo MI, Giri VN, Paller CJ, et al. Evolving intersection between inherited cancer genetics and therapeutic clinical trials in prostate cancer: a white paper from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. JCO Precis Oncol. 2018;2018:10.1200/PO.18.00060. doi:10.1200/PO.18.00060
15. City of Hope. Intensive course in genomic cancer risk assessment. https://www.cityofhope.org/education/health-professional-education/cancer-genomics-education-program/intensive-course-in-cancer-risk-assessment-overview. Accessed July 28, 2020.
16. US National Library of Medicine, Clinicaltrial.gov. Hormonal Intervention for the Treatment in Veterans with COVID-19 Requiring Hospitalization (HITCH): NCT04397718. https://clinicaltrials.gov/ct2/show/NCT04397718. Updated July 23, 2020. Accessed July 30, 2020.
1. US Department of Veterans Affairs. Secretary’s Center for Strategic Partnerships (SCSP): about us. https://www.va.gov/scsp/about/. Updated January 22, 2020. Accessed July 27, 2020.
2. US Department of Veterans Affairs. About VA. https://www.va.gov/about_va/mission.asp. Updated August 20, 2015. Accessed July 27, 2020.
3. American Association for Cancer Research. National Cancer Moonshot Initiative. https://www.aacr.org/professionals/policy-and-advocacy/science-policy-government-affairs/national-cancer-moonshot-initiative. Accessed July 30, 2020.
4. Zogby J, Fighting cancer is a Defense Department obligation. https://www.huffpost.com/entry/fighting-cancer-is-our-co_b_837535. Updated May 25, 2011. Accessed July 30, 2020.
5. Colliver V. Lawrence Stupski, former Schwab exec, dies. San Francisco Chronicle June 12, 2013. https://www.sfchronicle.com/bayarea/article/Lawrence-Stupski-former-Schwab-exec-dies-4597329.php. Accessed July 30, 2020.
6. US Department of Veterans Affairs, Veterans Health Administration. About VHA. https://www.va.gov/health/aboutvha.asp. Updated July 14, 2019. Accessed July 27, 2020.
7. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) Network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37 (suppl 4):S48-S53. doi:10.12788/fp.0021
8. US Department of Veterans Affairs, National Oncology Program Office: about us. https://www.cancer.va.gov/CANCER/about.asp. Accessed July 28, 2020.
9. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(8):S62-S67. doi:10.12788/fp.0028
10. Prostate Cancer Foundation. Prostate Cancer Foundation and Stand Up To Cancer announce new dream team [press release]. https://www.pcf.org/news/prostate-cancer-foundation-and-stand-up-to-cancer-announce-new-dream-team/. Published April 1, 2020. Accessed July 30, 2020.
11. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
12. Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer [published correction appears in Nat Genet. 2019 Jul;51(7):1194]. Nat Genet. 2018;50(5):645-651. doi:10.1038/s41588-018-0078-z
13. Prostate Cancer Foundation. $2.5 million gift from Robert Frederick Smith to the Prostate Cancer Foundation is the largest donation ever dedicated to advancing prostate cancer research in African-American men [press release]. https://www.pcf.org/news/robert-frederick-smith-gift/. Published January 14, 2018. Accessed July 27, 2020.
14. Carlo MI, Giri VN, Paller CJ, et al. Evolving intersection between inherited cancer genetics and therapeutic clinical trials in prostate cancer: a white paper from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. JCO Precis Oncol. 2018;2018:10.1200/PO.18.00060. doi:10.1200/PO.18.00060
15. City of Hope. Intensive course in genomic cancer risk assessment. https://www.cityofhope.org/education/health-professional-education/cancer-genomics-education-program/intensive-course-in-cancer-risk-assessment-overview. Accessed July 28, 2020.
16. US National Library of Medicine, Clinicaltrial.gov. Hormonal Intervention for the Treatment in Veterans with COVID-19 Requiring Hospitalization (HITCH): NCT04397718. https://clinicaltrials.gov/ct2/show/NCT04397718. Updated July 23, 2020. Accessed July 30, 2020.
Patient Education After Inadequate Bowel Preparation: Improving Care and Outcomes
Colorectal cancer is the second most common cause of death in the United States. 1 A colonoscopy is the current gold standard for prevention and early detection of colorectal cancers. During a colonoscopy procedure, polyps and lesions are biopsied and removed. The most effective method of colon cleansing for the procedure is achieved by using one of several commercially available colon lavage preparations. Before the colonoscopy, patients are prescribed and instructed to take one of these bowel preparations.
Background
Adequate bowel preparation is defined as sufficient for identification of polyps > 5 mm.2 The impact of inadequate bowel preparation extends beyond the need for additional or repeat procedure(s) and includes potentially missed polyps and cancers. Inferior bowel preparation quality is associated with a significant decrease in the detection of flat or sessile serrated polyps.3 Missed polyps increase the risk of interval colorectal cancers. A high-quality bowel preparation together with the individual skill and experience of the endoscopist are crucial for adequate polyp detection. In addition, other risks of inadequate bowel preparation and repeated colonoscopies reduce adenoma detection rates, undetected carcinomas, and increase the risk of complications, possibly resulting in lawsuits.3
A major difficulty facing the Veterans Health Administration (VHA) medical center gastroenterologists is what to do when a patient is not properly prepared after standard prescreening education and the bowel preparation regimen. Traditionally, the patient is given additional medication and asked to return the next day for a repeat colonoscopy. Alternatively, the patient is given a 2-day bowel preparation to be used prior to a new appointment.
The choice of bowel preparation has been standardized within the US Department of Veterans Affairs (VA) Connecticut Healthcare System in West Haven (
For patients who fail the standard 1-day preparation, the same trained RNs inquire about any difficulties in consuming the preparation and provide the standard 2-day bowel preparation instructions. Multiple factors impact the adherence with preparation directions. Several patient-specific factors, comorbidities, and medications can contribute to inadequate bowel preparation.4 These factors include failing to fast before the procedure; namely, consuming solid foods, not consuming the entire preparation, not taking the preparation as directed, and not consuming adequate amounts of clear liquids or calories. Other reasons for failing the preparation are nausea and vomiting, poor understanding of instructions (including illiteracy), chronic constipation, use of narcotics and psychotropic drugs, and lack of awareness of the consequences of inadequate bowel preparation.
A study by Hautefeuille and colleagues noted that approximately 20% of patients having colonoscopy failure were not adherent to bowel preparation instructions.5 Only 55% of patients were aware of these consequences; whereas 96% of physicians were convinced they had given appropriate and sufficient information.5 As noted earlier, approximately half of patients do not fully comprehend the need to follow all the instructions. Therefore, clear and concise cleansing instructions and patient adherence are key factors that contribute to efficiency and quality of colonoscopy. The preparation failure rate creates a large volume of repeat patients and contributes to reduced efficiency of outpatient endoscopic practice.
A meta-analysis conducted by Chang and colleagues demonstrated that a brief counseling session with patients before colonoscopy ensured better bowel preparation.6 The focus of this article is on using the Colonoscopy Patient Education Bowel Preparation Questionnaire to improve the outcomes of patient education (Table).
As this was part of ongoing care and medication education; the research did not require reviews by a research committee or need institutional review board approval.
Questionnaire
A gastroenterology (GI) advance practice registered nurse (APRN) developed a patient questionnaire after reviewing patient records from 2016 through 2018 and noting information gaps in patient re-education. The information was not clearly and completely documented relating to frequency of bowel movements, constipation, and daily hydration/fluid intake. Several questions were consistently asked of patients who had previously failed 2 bowel preparations to determine the issues preventing a successful bowel cleansing. Notes from the GI and nutrition clinics and the primary care provider (PCP) were reviewed for information on constipation, frequency and quality of bowel movements, average beverage consumption, and hydration status.
The GI APRN conducted the review and used notes from the past year as well as the notes for prior colon preparations documenting bowel preparations and their resulting quality. A review was conducted on each patient who failed the standard 2-day bowel preparation before the GI APRN bowel preparation education session. The review revealed that no single note provided all necessary information. All colonoscopy prescreening education notes contained information from the standard prescreening preparation education class presentation, and any individual patient issues related to preparation consumption. GI and PCP notes included constipation information; however, frequency of bowel movements was seldom mentioned; and no fluid consumption information was provided except for alcohol related to abuse/addiction issues. Of the patients that had been seen by the Nutrition Department staff, their notes included caloric intake, appropriate food/dietary choices, and soda consumption; alcohol use was documented but related only to caloric intake; again, no other fluid intake amounts were documented.
Design
The questionnaire consists of 5 closed-ended, patient-centered questions aimed at accomplishing patient education in a time-efficient manner. It also is a tool to achieve consistency among staff in determining barriers and issues, improve documentation, and then assist the patient in achieving a good-to-excellent quality bowel preparation. The questions elicit information that allow an RN or PCP determine the factors that contributed to bowel preparation failure and allow for a tailored patient-education session. With a clear picture of the patient’s issues and obstacles, the patient-centered prescreening preparation education could focus on solutions to specific barriers, increase patient comprehension and adherence to the instructions, and identify complicating behavioral factors of the prior bowel preparation. For example, question 1 was designed to discover whether the patient failed to consume the preparation and why, such as volume, timing, or taste; question 4 was designed to assist in figuring out whether constipation for any reason may be present, whether currently diagnosed or not; and question 5 determined the risk of dehydration with or without constipation as a key cleansing issue.
The answers to these few questions determined whether the inadequate bowel preparation quality was due to issues of poor understanding, poor following of the directions, or to other complicating factors.
The prescreening bowel preparation education classes are delivered in groups classes, telehealth group classes, and by phone.
Discussion
Following implementation of the questionnaire from 2018 to 2019, a clinical chart review was conducted in 2019 of the first 100 patients who failed the standardized 2-day preparation from 2018 to 2019. These patients were selected by the GI attending physicians based on their multiple prior research studies and the total number of veterans served within VACHS to reflect an adequate test of change. Twenty patients canceled their appointments or refused to obtain an additional colonoscopy. Of the remaining 80 patients, 68 (85%) improved on the bowel preparation screening to an adequate rating.
Within the VACHS, the result of inadequate colon preparation leads to either an aborted colonoscopy or a longer examination duration due to time spent washing the colon mucosa and then suctioning the liquified stool. Using newchoicehealth.com 2021 national data, the colonoscopy average price range was $1800 to $12,500; the national average amount paid is $2750.7 The average screening or diagnostic colonoscopy cost was $4469.8
Using the Colonoscopy Patient-Education Bowel Prep Questionnaire resulted in increased patient satisfaction, better use of current patient appointment slots, increased unique encounters, and direct and indirect fiscal savings. Patient satisfaction resulted from no additional repeat colonoscopies per patient’s statements. The other findings resulted from the reduction in repeat appointments: The appointment slots that would have been taken by repeat colonoscopies were available for new patients, resulting in an increase in unique encounters.
Fiscal savings resulted from avoiding the need for additional bowel preparations for those patients or using the GI staff time (nurses and clerks) to reschedule and educate patients. Prior to the use of the questionnaire, patients who failed preparations would be re-educated, given a new preparation prescription or mailed a new preparation, scheduled, and then mailed the appropriate paperwork, thus, increasing the workload for nurses and clerks.
Conclusions
Use of the questionnaire resulted in increased high-quality bowel preparation, an increase in the number of unique patients served, and improved efficiency. In addition, recovered appointment slots and modest reductions in additional purchases of preparation kits resulted in a potential cost savings for VACHS. Proper cleansing instructions as well as identifying and overcoming barriers to achieving adequate preparation for colonoscopy resulted in improved patient satisfaction, quality care, and cost savings.
Regardless of the type of colon preparation, addressing patient barriers to bowel preparation is translatable to other endoscopy facilities and practices that provide patient education within the VA.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 12, 2021. Accessed May 19, 2021. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. 2020.
2. Clark BT, Protiva P, Nagar A, et al. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology. 2016;150(2):396-405; quiz e14-e15. doi:10.1053/j.gastro.2015.09.041
3. Clark BT, Laine L. High-quality bowel preparation is required for detection of sessile serrated polyps. Clin Gastroenterol Hepatol. 2016;14(8):1155-1162. doi:10.1016/j.cgh.2016.03.044
4. D’Souza SM, Parekh PJ, Johnson DA. The dirty side of colonoscopy: predictors of poor bowel preparation and novel approaches to overcome the shortcomings. Br J Gastroenterology. 2019:1:1.
5. Hautefeuille G, Lapuelle J, Chaussade S, et al. Factors related to bowel cleansing failure before colonoscopy: results of the PACOME study. J United European Gastroenterol J. 2014; 2(1):22-29. doi:10.1177/2050640613518200
6. Chang CW, Shih SC, Wang HY, et al. Meta-analysis: the effect of patient education on bowel preparation for colonoscopy. Endosc Int Open. 2015;3(6):E646-E652. doi:10.1055/s-0034-1392365
7. New Choice Health. How much does a colonoscopy cost? Accessed May 19, 2021. https://www.newchoicehealth.com/colonoscopy/cost
8. MDsave.com. Colonoscopy. Accessed May 19, 2021. https://www.mdsave.com/f/procedure/colonoscopy/06516?q=colonoscopy&type=procedure
Colorectal cancer is the second most common cause of death in the United States. 1 A colonoscopy is the current gold standard for prevention and early detection of colorectal cancers. During a colonoscopy procedure, polyps and lesions are biopsied and removed. The most effective method of colon cleansing for the procedure is achieved by using one of several commercially available colon lavage preparations. Before the colonoscopy, patients are prescribed and instructed to take one of these bowel preparations.
Background
Adequate bowel preparation is defined as sufficient for identification of polyps > 5 mm.2 The impact of inadequate bowel preparation extends beyond the need for additional or repeat procedure(s) and includes potentially missed polyps and cancers. Inferior bowel preparation quality is associated with a significant decrease in the detection of flat or sessile serrated polyps.3 Missed polyps increase the risk of interval colorectal cancers. A high-quality bowel preparation together with the individual skill and experience of the endoscopist are crucial for adequate polyp detection. In addition, other risks of inadequate bowel preparation and repeated colonoscopies reduce adenoma detection rates, undetected carcinomas, and increase the risk of complications, possibly resulting in lawsuits.3
A major difficulty facing the Veterans Health Administration (VHA) medical center gastroenterologists is what to do when a patient is not properly prepared after standard prescreening education and the bowel preparation regimen. Traditionally, the patient is given additional medication and asked to return the next day for a repeat colonoscopy. Alternatively, the patient is given a 2-day bowel preparation to be used prior to a new appointment.
The choice of bowel preparation has been standardized within the US Department of Veterans Affairs (VA) Connecticut Healthcare System in West Haven (
For patients who fail the standard 1-day preparation, the same trained RNs inquire about any difficulties in consuming the preparation and provide the standard 2-day bowel preparation instructions. Multiple factors impact the adherence with preparation directions. Several patient-specific factors, comorbidities, and medications can contribute to inadequate bowel preparation.4 These factors include failing to fast before the procedure; namely, consuming solid foods, not consuming the entire preparation, not taking the preparation as directed, and not consuming adequate amounts of clear liquids or calories. Other reasons for failing the preparation are nausea and vomiting, poor understanding of instructions (including illiteracy), chronic constipation, use of narcotics and psychotropic drugs, and lack of awareness of the consequences of inadequate bowel preparation.
A study by Hautefeuille and colleagues noted that approximately 20% of patients having colonoscopy failure were not adherent to bowel preparation instructions.5 Only 55% of patients were aware of these consequences; whereas 96% of physicians were convinced they had given appropriate and sufficient information.5 As noted earlier, approximately half of patients do not fully comprehend the need to follow all the instructions. Therefore, clear and concise cleansing instructions and patient adherence are key factors that contribute to efficiency and quality of colonoscopy. The preparation failure rate creates a large volume of repeat patients and contributes to reduced efficiency of outpatient endoscopic practice.
A meta-analysis conducted by Chang and colleagues demonstrated that a brief counseling session with patients before colonoscopy ensured better bowel preparation.6 The focus of this article is on using the Colonoscopy Patient Education Bowel Preparation Questionnaire to improve the outcomes of patient education (Table).
As this was part of ongoing care and medication education; the research did not require reviews by a research committee or need institutional review board approval.
Questionnaire
A gastroenterology (GI) advance practice registered nurse (APRN) developed a patient questionnaire after reviewing patient records from 2016 through 2018 and noting information gaps in patient re-education. The information was not clearly and completely documented relating to frequency of bowel movements, constipation, and daily hydration/fluid intake. Several questions were consistently asked of patients who had previously failed 2 bowel preparations to determine the issues preventing a successful bowel cleansing. Notes from the GI and nutrition clinics and the primary care provider (PCP) were reviewed for information on constipation, frequency and quality of bowel movements, average beverage consumption, and hydration status.
The GI APRN conducted the review and used notes from the past year as well as the notes for prior colon preparations documenting bowel preparations and their resulting quality. A review was conducted on each patient who failed the standard 2-day bowel preparation before the GI APRN bowel preparation education session. The review revealed that no single note provided all necessary information. All colonoscopy prescreening education notes contained information from the standard prescreening preparation education class presentation, and any individual patient issues related to preparation consumption. GI and PCP notes included constipation information; however, frequency of bowel movements was seldom mentioned; and no fluid consumption information was provided except for alcohol related to abuse/addiction issues. Of the patients that had been seen by the Nutrition Department staff, their notes included caloric intake, appropriate food/dietary choices, and soda consumption; alcohol use was documented but related only to caloric intake; again, no other fluid intake amounts were documented.
Design
The questionnaire consists of 5 closed-ended, patient-centered questions aimed at accomplishing patient education in a time-efficient manner. It also is a tool to achieve consistency among staff in determining barriers and issues, improve documentation, and then assist the patient in achieving a good-to-excellent quality bowel preparation. The questions elicit information that allow an RN or PCP determine the factors that contributed to bowel preparation failure and allow for a tailored patient-education session. With a clear picture of the patient’s issues and obstacles, the patient-centered prescreening preparation education could focus on solutions to specific barriers, increase patient comprehension and adherence to the instructions, and identify complicating behavioral factors of the prior bowel preparation. For example, question 1 was designed to discover whether the patient failed to consume the preparation and why, such as volume, timing, or taste; question 4 was designed to assist in figuring out whether constipation for any reason may be present, whether currently diagnosed or not; and question 5 determined the risk of dehydration with or without constipation as a key cleansing issue.
The answers to these few questions determined whether the inadequate bowel preparation quality was due to issues of poor understanding, poor following of the directions, or to other complicating factors.
The prescreening bowel preparation education classes are delivered in groups classes, telehealth group classes, and by phone.
Discussion
Following implementation of the questionnaire from 2018 to 2019, a clinical chart review was conducted in 2019 of the first 100 patients who failed the standardized 2-day preparation from 2018 to 2019. These patients were selected by the GI attending physicians based on their multiple prior research studies and the total number of veterans served within VACHS to reflect an adequate test of change. Twenty patients canceled their appointments or refused to obtain an additional colonoscopy. Of the remaining 80 patients, 68 (85%) improved on the bowel preparation screening to an adequate rating.
Within the VACHS, the result of inadequate colon preparation leads to either an aborted colonoscopy or a longer examination duration due to time spent washing the colon mucosa and then suctioning the liquified stool. Using newchoicehealth.com 2021 national data, the colonoscopy average price range was $1800 to $12,500; the national average amount paid is $2750.7 The average screening or diagnostic colonoscopy cost was $4469.8
Using the Colonoscopy Patient-Education Bowel Prep Questionnaire resulted in increased patient satisfaction, better use of current patient appointment slots, increased unique encounters, and direct and indirect fiscal savings. Patient satisfaction resulted from no additional repeat colonoscopies per patient’s statements. The other findings resulted from the reduction in repeat appointments: The appointment slots that would have been taken by repeat colonoscopies were available for new patients, resulting in an increase in unique encounters.
Fiscal savings resulted from avoiding the need for additional bowel preparations for those patients or using the GI staff time (nurses and clerks) to reschedule and educate patients. Prior to the use of the questionnaire, patients who failed preparations would be re-educated, given a new preparation prescription or mailed a new preparation, scheduled, and then mailed the appropriate paperwork, thus, increasing the workload for nurses and clerks.
Conclusions
Use of the questionnaire resulted in increased high-quality bowel preparation, an increase in the number of unique patients served, and improved efficiency. In addition, recovered appointment slots and modest reductions in additional purchases of preparation kits resulted in a potential cost savings for VACHS. Proper cleansing instructions as well as identifying and overcoming barriers to achieving adequate preparation for colonoscopy resulted in improved patient satisfaction, quality care, and cost savings.
Regardless of the type of colon preparation, addressing patient barriers to bowel preparation is translatable to other endoscopy facilities and practices that provide patient education within the VA.
Colorectal cancer is the second most common cause of death in the United States. 1 A colonoscopy is the current gold standard for prevention and early detection of colorectal cancers. During a colonoscopy procedure, polyps and lesions are biopsied and removed. The most effective method of colon cleansing for the procedure is achieved by using one of several commercially available colon lavage preparations. Before the colonoscopy, patients are prescribed and instructed to take one of these bowel preparations.
Background
Adequate bowel preparation is defined as sufficient for identification of polyps > 5 mm.2 The impact of inadequate bowel preparation extends beyond the need for additional or repeat procedure(s) and includes potentially missed polyps and cancers. Inferior bowel preparation quality is associated with a significant decrease in the detection of flat or sessile serrated polyps.3 Missed polyps increase the risk of interval colorectal cancers. A high-quality bowel preparation together with the individual skill and experience of the endoscopist are crucial for adequate polyp detection. In addition, other risks of inadequate bowel preparation and repeated colonoscopies reduce adenoma detection rates, undetected carcinomas, and increase the risk of complications, possibly resulting in lawsuits.3
A major difficulty facing the Veterans Health Administration (VHA) medical center gastroenterologists is what to do when a patient is not properly prepared after standard prescreening education and the bowel preparation regimen. Traditionally, the patient is given additional medication and asked to return the next day for a repeat colonoscopy. Alternatively, the patient is given a 2-day bowel preparation to be used prior to a new appointment.
The choice of bowel preparation has been standardized within the US Department of Veterans Affairs (VA) Connecticut Healthcare System in West Haven (
For patients who fail the standard 1-day preparation, the same trained RNs inquire about any difficulties in consuming the preparation and provide the standard 2-day bowel preparation instructions. Multiple factors impact the adherence with preparation directions. Several patient-specific factors, comorbidities, and medications can contribute to inadequate bowel preparation.4 These factors include failing to fast before the procedure; namely, consuming solid foods, not consuming the entire preparation, not taking the preparation as directed, and not consuming adequate amounts of clear liquids or calories. Other reasons for failing the preparation are nausea and vomiting, poor understanding of instructions (including illiteracy), chronic constipation, use of narcotics and psychotropic drugs, and lack of awareness of the consequences of inadequate bowel preparation.
A study by Hautefeuille and colleagues noted that approximately 20% of patients having colonoscopy failure were not adherent to bowel preparation instructions.5 Only 55% of patients were aware of these consequences; whereas 96% of physicians were convinced they had given appropriate and sufficient information.5 As noted earlier, approximately half of patients do not fully comprehend the need to follow all the instructions. Therefore, clear and concise cleansing instructions and patient adherence are key factors that contribute to efficiency and quality of colonoscopy. The preparation failure rate creates a large volume of repeat patients and contributes to reduced efficiency of outpatient endoscopic practice.
A meta-analysis conducted by Chang and colleagues demonstrated that a brief counseling session with patients before colonoscopy ensured better bowel preparation.6 The focus of this article is on using the Colonoscopy Patient Education Bowel Preparation Questionnaire to improve the outcomes of patient education (Table).
As this was part of ongoing care and medication education; the research did not require reviews by a research committee or need institutional review board approval.
Questionnaire
A gastroenterology (GI) advance practice registered nurse (APRN) developed a patient questionnaire after reviewing patient records from 2016 through 2018 and noting information gaps in patient re-education. The information was not clearly and completely documented relating to frequency of bowel movements, constipation, and daily hydration/fluid intake. Several questions were consistently asked of patients who had previously failed 2 bowel preparations to determine the issues preventing a successful bowel cleansing. Notes from the GI and nutrition clinics and the primary care provider (PCP) were reviewed for information on constipation, frequency and quality of bowel movements, average beverage consumption, and hydration status.
The GI APRN conducted the review and used notes from the past year as well as the notes for prior colon preparations documenting bowel preparations and their resulting quality. A review was conducted on each patient who failed the standard 2-day bowel preparation before the GI APRN bowel preparation education session. The review revealed that no single note provided all necessary information. All colonoscopy prescreening education notes contained information from the standard prescreening preparation education class presentation, and any individual patient issues related to preparation consumption. GI and PCP notes included constipation information; however, frequency of bowel movements was seldom mentioned; and no fluid consumption information was provided except for alcohol related to abuse/addiction issues. Of the patients that had been seen by the Nutrition Department staff, their notes included caloric intake, appropriate food/dietary choices, and soda consumption; alcohol use was documented but related only to caloric intake; again, no other fluid intake amounts were documented.
Design
The questionnaire consists of 5 closed-ended, patient-centered questions aimed at accomplishing patient education in a time-efficient manner. It also is a tool to achieve consistency among staff in determining barriers and issues, improve documentation, and then assist the patient in achieving a good-to-excellent quality bowel preparation. The questions elicit information that allow an RN or PCP determine the factors that contributed to bowel preparation failure and allow for a tailored patient-education session. With a clear picture of the patient’s issues and obstacles, the patient-centered prescreening preparation education could focus on solutions to specific barriers, increase patient comprehension and adherence to the instructions, and identify complicating behavioral factors of the prior bowel preparation. For example, question 1 was designed to discover whether the patient failed to consume the preparation and why, such as volume, timing, or taste; question 4 was designed to assist in figuring out whether constipation for any reason may be present, whether currently diagnosed or not; and question 5 determined the risk of dehydration with or without constipation as a key cleansing issue.
The answers to these few questions determined whether the inadequate bowel preparation quality was due to issues of poor understanding, poor following of the directions, or to other complicating factors.
The prescreening bowel preparation education classes are delivered in groups classes, telehealth group classes, and by phone.
Discussion
Following implementation of the questionnaire from 2018 to 2019, a clinical chart review was conducted in 2019 of the first 100 patients who failed the standardized 2-day preparation from 2018 to 2019. These patients were selected by the GI attending physicians based on their multiple prior research studies and the total number of veterans served within VACHS to reflect an adequate test of change. Twenty patients canceled their appointments or refused to obtain an additional colonoscopy. Of the remaining 80 patients, 68 (85%) improved on the bowel preparation screening to an adequate rating.
Within the VACHS, the result of inadequate colon preparation leads to either an aborted colonoscopy or a longer examination duration due to time spent washing the colon mucosa and then suctioning the liquified stool. Using newchoicehealth.com 2021 national data, the colonoscopy average price range was $1800 to $12,500; the national average amount paid is $2750.7 The average screening or diagnostic colonoscopy cost was $4469.8
Using the Colonoscopy Patient-Education Bowel Prep Questionnaire resulted in increased patient satisfaction, better use of current patient appointment slots, increased unique encounters, and direct and indirect fiscal savings. Patient satisfaction resulted from no additional repeat colonoscopies per patient’s statements. The other findings resulted from the reduction in repeat appointments: The appointment slots that would have been taken by repeat colonoscopies were available for new patients, resulting in an increase in unique encounters.
Fiscal savings resulted from avoiding the need for additional bowel preparations for those patients or using the GI staff time (nurses and clerks) to reschedule and educate patients. Prior to the use of the questionnaire, patients who failed preparations would be re-educated, given a new preparation prescription or mailed a new preparation, scheduled, and then mailed the appropriate paperwork, thus, increasing the workload for nurses and clerks.
Conclusions
Use of the questionnaire resulted in increased high-quality bowel preparation, an increase in the number of unique patients served, and improved efficiency. In addition, recovered appointment slots and modest reductions in additional purchases of preparation kits resulted in a potential cost savings for VACHS. Proper cleansing instructions as well as identifying and overcoming barriers to achieving adequate preparation for colonoscopy resulted in improved patient satisfaction, quality care, and cost savings.
Regardless of the type of colon preparation, addressing patient barriers to bowel preparation is translatable to other endoscopy facilities and practices that provide patient education within the VA.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 12, 2021. Accessed May 19, 2021. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. 2020.
2. Clark BT, Protiva P, Nagar A, et al. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology. 2016;150(2):396-405; quiz e14-e15. doi:10.1053/j.gastro.2015.09.041
3. Clark BT, Laine L. High-quality bowel preparation is required for detection of sessile serrated polyps. Clin Gastroenterol Hepatol. 2016;14(8):1155-1162. doi:10.1016/j.cgh.2016.03.044
4. D’Souza SM, Parekh PJ, Johnson DA. The dirty side of colonoscopy: predictors of poor bowel preparation and novel approaches to overcome the shortcomings. Br J Gastroenterology. 2019:1:1.
5. Hautefeuille G, Lapuelle J, Chaussade S, et al. Factors related to bowel cleansing failure before colonoscopy: results of the PACOME study. J United European Gastroenterol J. 2014; 2(1):22-29. doi:10.1177/2050640613518200
6. Chang CW, Shih SC, Wang HY, et al. Meta-analysis: the effect of patient education on bowel preparation for colonoscopy. Endosc Int Open. 2015;3(6):E646-E652. doi:10.1055/s-0034-1392365
7. New Choice Health. How much does a colonoscopy cost? Accessed May 19, 2021. https://www.newchoicehealth.com/colonoscopy/cost
8. MDsave.com. Colonoscopy. Accessed May 19, 2021. https://www.mdsave.com/f/procedure/colonoscopy/06516?q=colonoscopy&type=procedure
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 12, 2021. Accessed May 19, 2021. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. 2020.
2. Clark BT, Protiva P, Nagar A, et al. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology. 2016;150(2):396-405; quiz e14-e15. doi:10.1053/j.gastro.2015.09.041
3. Clark BT, Laine L. High-quality bowel preparation is required for detection of sessile serrated polyps. Clin Gastroenterol Hepatol. 2016;14(8):1155-1162. doi:10.1016/j.cgh.2016.03.044
4. D’Souza SM, Parekh PJ, Johnson DA. The dirty side of colonoscopy: predictors of poor bowel preparation and novel approaches to overcome the shortcomings. Br J Gastroenterology. 2019:1:1.
5. Hautefeuille G, Lapuelle J, Chaussade S, et al. Factors related to bowel cleansing failure before colonoscopy: results of the PACOME study. J United European Gastroenterol J. 2014; 2(1):22-29. doi:10.1177/2050640613518200
6. Chang CW, Shih SC, Wang HY, et al. Meta-analysis: the effect of patient education on bowel preparation for colonoscopy. Endosc Int Open. 2015;3(6):E646-E652. doi:10.1055/s-0034-1392365
7. New Choice Health. How much does a colonoscopy cost? Accessed May 19, 2021. https://www.newchoicehealth.com/colonoscopy/cost
8. MDsave.com. Colonoscopy. Accessed May 19, 2021. https://www.mdsave.com/f/procedure/colonoscopy/06516?q=colonoscopy&type=procedure
Role of 3D Printing and Modeling to Aid in Neuroradiology Education for Medical Trainees
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010