User login
Effect of Pharmacist Interventions on Hospital Readmissions for Home-Based Primary Care Veterans
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
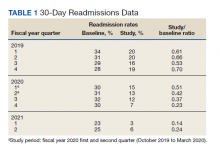
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
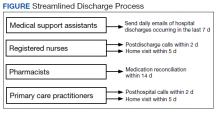
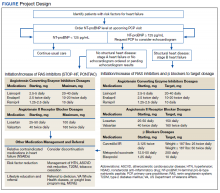
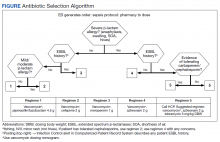
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
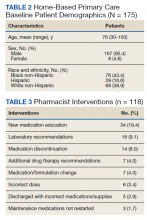
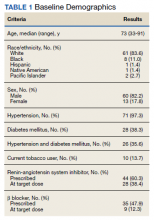
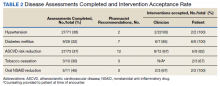
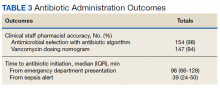
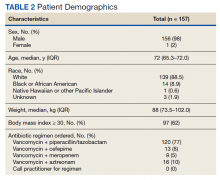
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
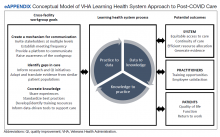
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
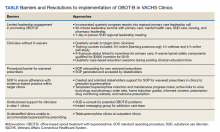
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
Mental Health Support of Frontline Medical Personnel in the Javits New York Medical Station Federal COVID-19 Treatment Center
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
Natriuretic Peptide Screening for Primary Prevention or Early Detection of Heart Failure: A Pharmacist-Driven Team-Based Approach
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
Incorporation of Clinical Staff Pharmacists in the Emergency Department Sepsis Response at a Single Institution
Sepsis is life-threatening organ dysfunction caused by dysregulated host response to an infection that can progress to shock. Sepsis is a major cause of death in the United States, with > 1 million people developing sepsis and > 250,000 people dying from sepsis annually.1 The Surviving Sepsis Campaign (SSC) guidelines recommend treating sepsis as an emergency with timely administration of fluids and antibiotics, as administering antibiotics within the first hour has been found to reduce mortality and disease progression. In addition, empiric antibiotic regimens should be chosen to target the most probable pathogens and dosing should be optimized. To achieve this, the SSC guidelines recommend that hospitals develop quality improvement (QI) programs developed by a multidisciplinary group to improve sepsis recognition and response using a protocolized approach.2
There are several studies describing efforts to improve the sepsis response at facilities, some of which have evaluated the addition of a pharmacist into the sepsis response, particularly in the emergency department (ED). Some studies found improved selection and decreased time to antibiotic administration with the addition of an ED pharmacist.3-7 Despite this, ED pharmacists are not present in all hospitals, with a 2015 national survey reporting the presence of an ED pharmacist in 68.7% of respondents at 187 facilities. Even facilities with ED pharmacists often have limited hours of coverage, with at least 8 hours of coverage in 49.4% of facilities with an ED pharmacist and no weekend coverage at 34.8% of these facilities.8
While many hospitals do not routinely employ ED pharmacists, most hospitals have clinical staff pharmacists (CSPs), and many inpatient hospital pharmacies are staffed with CSPs 24 hours per day, 7 days per week. A 2017 survey conducted by the American Society of Health-System Pharmacists (ASHP) found 43% of all hospital pharmacy departments were staffed by a CSP around the clock, with the prevalence increasing to 56.7 to 100% in hospitals with > 100 beds.9 As a result, CSPs may be a useful resource to assist with the management of patients with sepsis in hospitals without an ED pharmacist.
At the Lexington Veterans Affairs Health Care System (LVAHCS) in Kentucky, the inpatient pharmacy department is staffed with a CSP 24/7 but does not have an ED pharmacist. Therefore, when an interdisciplinary group developed an ED sepsis bundle as part of a QI initiative on sepsis recognition and response, the group took a unique approach of incorporating CSPs into the response team to assist with antimicrobial selection and dosing. An antibiotic selection algorithm and vancomycin dosing nomogram were developed to aid CSPs to select and dose antibiotics (Figure, Table 1). We describe the implementation of this process and evaluate CSPs’ accuracy in antimicrobial selection and vancomycin dosing.
Methods
Lexington VAHCS is a 94-bed hospital that provides services to veterans, including an ED, inpatient medical services, surgical services, acute mental health, progressive care, and intensive care units. This facility has 1 antimicrobial stewardship clinical pharmacy specialist, 2 critical care clinical pharmacy specialists, and 16 full-time CSPs with 24-hour CSP coverage. The annual ED volume at the time of this study was approximately 21,000 patients.
Consistent with the SSC guideline recommendation to develop multidisciplinary QI initiatives on sepsis recognition and response, an Interdisciplinary Sepsis Committee (ISC) was created in 2018 comprised of ED, pulmonary, critical care, and infectious diseases licensed independent practitioners (LIPs), ED nurses, and pharmacists. The ISC developed a comprehensive set of sepsis tools that included a sepsis screening tool used by ED triage nurses to provide early detection of sepsis and an updated electronic order set to decrease time to appropriate treatment. This order set included automatic orders for blood cultures and serum lactate, the initiation of IV crystalloids, as well as a Sepsis Alert order placed by ED LIPs which alerted CSPs to a patient with sepsis in the ED.
To ensure a protocol-based approach by the CSPs responding to the sepsis alert, an antibiotic algorithm and vancomycin dosing nomogram were developed by the ISC based on current guideline recommendations and the local antibiogram. These were subsequently approved by ED practitioners, the pharmacy and therapeutics committee, and the critical care committee. The antibiotic algorithm prompts CSPs to perform a chart review to identify β-lactam allergies, evaluate the severity of the allergy and which agents the patient has tolerated in the past, as well as determine whether the patient has a history of extended spectrum β-lactamase (ESBL)–producing organisms from previous cultures. A decision tree then guides CSPs toward the selection of 1 of 5 empiric antibiotic regimens to cover all likely pathogens. The medication orders are then entered by the CSPs as a telephone order from the ED LIP per protocol. Unless patients had a true vancomycin allergy, all patients received vancomycin as the empiric gram-positive backbone of the regimen. The vancomycin dosing nomogram was created to ensure an appropriate and consistent vancomycin weight-based loading dose was administered.
Prior to implementation, the antimicrobial stewardship pharmacist educated CSPs on the use of these tools, including simulated orders for mock sepsis alerts to ensure competency. A copy of the algorithm and nomogram were emailed to all CSPs and posted in a prominent location in the pharmacy.
As part of continuous performance improvement efforts of the ISC, a retrospective cohort study was conducted through chart review on patients at the Lexington VAHCS with an order for a sepsis alert in the ED from December 3, 2018 to May 31, 2020 to assess the accuracy of the CSPs’ antibiotic selection and dosing. Patients were excluded if they had a vancomycin allergy or if the ED practitioner ordered antibiotics prior to the CSPs placing orders. Patients could be included more than once in the study if they had sepsis alerts placed on different dates.
The primary outcomes were CSPs’ accuracy in antimicrobial selection with the antibiotic selection algorithm and vancomycin dosing nomogram. The antibiotic selection was deemed accurate if the appropriate antibiotic regimen was selected based on allergy status and previous cultures as directed in the algorithm. The vancomycin dose was considered accurate if the dose chosen was appropriate based on the patient’s weight at the time of ED presentation. Secondary outcomes included time to administration of antibiotics from ED presentation as well as time to antibiotics administration from sepsis alert initiation. Time of administration was considered the time the antibiotics were scanned in the bar code medication administration (BCMA) system.
Descriptive statistics were used with data presented as percentages for nominal data and median as IQR for continuous data. In accordance with our facility’s project assessment process, this project was determined not to constitute human subjects research; therefore, this QI project did not require review by the institutional review board.
Results
Between December 3, 2018 and May 31, 2020, 160 sepsis alerts were ordered by ED practitioners. Of the 160 patients, 157 were included in the final data analysis. Two patients were excluded due to vancomycin allergy, and 1 patient because the physician ordered antibiotics prior to pharmacist order entry. The population was largely composed of male patients (98%) with a median age of 72 years (Table 2).
Of 157 sepsis alerts, the antibiotic selection algorithm was used appropriately in 154 (98%) instances (Table 3). Chart reviews were performed in instances of antimicrobial selection different from the algorithm. Of the 3 patients who received antibiotics not consistent with the algorithm, 1 patient without a history of ESBL-producing organisms in their culture history received meropenem instead of piperacillin/tazobactam. Another patient without a penicillin allergy received cefepime (plus metronidazole ordered separately from the ED practitioner) instead of piperacillin/tazobactam, and the third patient received piperacillin/tazobactam instead of meropenem despite a culture history of ESBL-producing organisms. Vancomycin dose was appropriate according to the weight-based nomogram in 147 cases (94%). The median time to administration of first dose antibiotics was 39 minutes after the sepsis alert order was placed and 96 minutes after initial ED presentation.
Discussion
This study found extremely high rates of accuracy among CSPs for both the antibiotic selection algorithm (98%) and the vancomycin dosing nomogram (94%). Moreover, analysis of the 3 patients who received antibiotics that were inconsistent with the algorithm revealed that 2 of these patients arguably still received adequate empiric coverage, increasing the percentage of patients receiving appropriate empiric antibiotics to 99.4%. Similarly, chart review of 10 patients who received vancomycin doses that deviated from the nomogram revealed that in at least 3 cases, patients were likely given correct vancomycin doses based on the patient’s last known weight. However, when actual current weights were recorded soon after admission, the updated weights rendered the initial vancomycin loading dose incorrect when this analysis was performed. Thus, the adherence to the vancomycin dosing nomogram is higher than it appears.
Median time to antibiotic administration from the sepsis alert was 39 minutes—well within SSC recommendations (60 minutes).2 Previous internal analyses at Lexington VAHCS demonstrated the mean time to first dose of antibiotics in the ED has been 39 minutes since about 2015. Thus, this initiative did not necessarily make this process quicker; however it did remove 1 responsibility from LIPs so that they could focus their efforts on other components of sepsis management.
Further studies are needed to evaluate the effects of this initiative on other aspects of the sepsis bundle, such as volume of fluid administered and appropriateness of laboratory tests. It was noted that while the time to first-dose antibiotic administration was < 1 hour from order placement, the median time from ED presentation to antibiotic administration was 96 minutes. This suggests that another focus of the sepsis workgroup should be on speeding recognition of sepsis, triggering the sepsis alert even sooner, and evaluating the feasibility of storing first doses of antibiotics in the automatic dispensing cabinets in the ED.
Limitations
This descriptive study evaluating CSPs’ ability to accurately use the newly developed antibiotic selection algorithm and vancomycin dosing nomogram had no control group for outcome comparison. This study was not designed to evaluate clinical outcomes, such as mortality, so the impact of these interventions need to be further studied. In addition, as veterans receive most of their care at our facility, with their allergies and previous cultures readily available in our electronic health record, this process may not be feasible at other facilities where patients' care is divided among multiple facilities/systems.
Moreover, as the veteran population studied was predominately male patients aged > 60 years, implementation at other hospitals may require the dosing nomograms and treatment algorithms to be adapted for a broader population, such as children and pregnant women. In particular, the ISC chose to implement an algorithm that did not differentiate between suspected source of infections and included anti-Pseudomonal coverage in all regimens based on the most encountered diseases among our veteran population and our local antibiogram; implementation at other facilities would require a thoughtful evaluation of the most appropriate site-specific regimen. Finally, many of the CSPs at our facility are board certified and/or residency trained, so more staff development may be required prior to implementation at other facilities, depending on the experience and comfort level of the CSPs.
Strengths
This study describes an example of a protocolized and multidisciplinary approach to improve sepsis recognition and standardize the response, consistent with SSC guideline recommendations. To the best of our knowledge, this is the first study to demonstrate the incorporation of CSPs into the interdisciplinary sepsis response. This allows for CSPs to practice at the top of their license and contributes to their professional development. Although it was not formally assessed, anecdotally CSPs reported that this process presented a negligible addition to their workload (< 5 minutes was the most reported time requirement), and they expressed satisfaction with their involvement in the sepsis response. Overall, this presents a possible solution to improve the sepsis response in hospitals without a dedicated ED pharmacist.
Conclusions
This study describes the successful incorporation of CSPs into the sepsis response in the ED. As CSPs are more likely than ED pharmacists to be present at a facility, they are arguably an underused resource whose clinical skills can be used to optimize the treatment of patients with sepsis.
1. Centers for Disease Control and Prevention. Sepsis. Accessed March 8, 2022. https://www.cdc.gov/sepsis/what-is-sepsis.html
2. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017 Mar;45(3):486-552. doi:10.1097/CCM.0000000000002255
3. Denny KJ, Gartside JG, Alcorn K, et al. Appropriateness of antibiotic prescribing in the emergency department. J Antimicrob Chemother. 2019 Feb 1;74(2):515-520. doi:10.1093/jac/dky447
4. Laine ME, Flynn JD, Flannery AH. Impact of pharmacist intervention on selection and timing of appropriate antimicrobial therapy in septic shock. J Pharm Pract. 2018 Feb;31(1):46-51. doi:10.1177/0897190017696953
5. Weant KA, Baker SN. Emergency medicine pharmacists and sepsis management. J Pharm Pract. 2013 Aug;26(4):401-5. doi:10.1177/0897190012467211
6. Farmer BM, Hayes BD, Rao R, et al. The role of clinical pharmacists in the emergency department. J Med Toxicol. 2018 Mar;14(1):114-116. doi:10.1007/s13181-017-0634-4
7. Yarbrough N, Bloxam M, Priano J, Louzon Lynch P, Hunt LN, Elfman J. Pharmacist impact on sepsis bundle compliance through participation on an emergency department sepsis alert team. Am J Emerg Med. 2019;37(4):762-763. doi:10.1016/j.ajem.2018.08.00
8. Thomas MC, Acquisto NM, Shirk MB, et al. A national survey of emergency pharmacy practice in the United States. Am J Health Syst Pharm. 2016 Mar 15;73(6):386-94. doi:10.2146/ajhp150321
9. Schneider PJ, Pedersen CA, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: dispensing and administration-2017. Am J Health Syst Pharm. 2018;75(16):1203-1226. doi:10.2146/ajhp180151
Sepsis is life-threatening organ dysfunction caused by dysregulated host response to an infection that can progress to shock. Sepsis is a major cause of death in the United States, with > 1 million people developing sepsis and > 250,000 people dying from sepsis annually.1 The Surviving Sepsis Campaign (SSC) guidelines recommend treating sepsis as an emergency with timely administration of fluids and antibiotics, as administering antibiotics within the first hour has been found to reduce mortality and disease progression. In addition, empiric antibiotic regimens should be chosen to target the most probable pathogens and dosing should be optimized. To achieve this, the SSC guidelines recommend that hospitals develop quality improvement (QI) programs developed by a multidisciplinary group to improve sepsis recognition and response using a protocolized approach.2
There are several studies describing efforts to improve the sepsis response at facilities, some of which have evaluated the addition of a pharmacist into the sepsis response, particularly in the emergency department (ED). Some studies found improved selection and decreased time to antibiotic administration with the addition of an ED pharmacist.3-7 Despite this, ED pharmacists are not present in all hospitals, with a 2015 national survey reporting the presence of an ED pharmacist in 68.7% of respondents at 187 facilities. Even facilities with ED pharmacists often have limited hours of coverage, with at least 8 hours of coverage in 49.4% of facilities with an ED pharmacist and no weekend coverage at 34.8% of these facilities.8
While many hospitals do not routinely employ ED pharmacists, most hospitals have clinical staff pharmacists (CSPs), and many inpatient hospital pharmacies are staffed with CSPs 24 hours per day, 7 days per week. A 2017 survey conducted by the American Society of Health-System Pharmacists (ASHP) found 43% of all hospital pharmacy departments were staffed by a CSP around the clock, with the prevalence increasing to 56.7 to 100% in hospitals with > 100 beds.9 As a result, CSPs may be a useful resource to assist with the management of patients with sepsis in hospitals without an ED pharmacist.
At the Lexington Veterans Affairs Health Care System (LVAHCS) in Kentucky, the inpatient pharmacy department is staffed with a CSP 24/7 but does not have an ED pharmacist. Therefore, when an interdisciplinary group developed an ED sepsis bundle as part of a QI initiative on sepsis recognition and response, the group took a unique approach of incorporating CSPs into the response team to assist with antimicrobial selection and dosing. An antibiotic selection algorithm and vancomycin dosing nomogram were developed to aid CSPs to select and dose antibiotics (Figure, Table 1). We describe the implementation of this process and evaluate CSPs’ accuracy in antimicrobial selection and vancomycin dosing.
Methods
Lexington VAHCS is a 94-bed hospital that provides services to veterans, including an ED, inpatient medical services, surgical services, acute mental health, progressive care, and intensive care units. This facility has 1 antimicrobial stewardship clinical pharmacy specialist, 2 critical care clinical pharmacy specialists, and 16 full-time CSPs with 24-hour CSP coverage. The annual ED volume at the time of this study was approximately 21,000 patients.
Consistent with the SSC guideline recommendation to develop multidisciplinary QI initiatives on sepsis recognition and response, an Interdisciplinary Sepsis Committee (ISC) was created in 2018 comprised of ED, pulmonary, critical care, and infectious diseases licensed independent practitioners (LIPs), ED nurses, and pharmacists. The ISC developed a comprehensive set of sepsis tools that included a sepsis screening tool used by ED triage nurses to provide early detection of sepsis and an updated electronic order set to decrease time to appropriate treatment. This order set included automatic orders for blood cultures and serum lactate, the initiation of IV crystalloids, as well as a Sepsis Alert order placed by ED LIPs which alerted CSPs to a patient with sepsis in the ED.
To ensure a protocol-based approach by the CSPs responding to the sepsis alert, an antibiotic algorithm and vancomycin dosing nomogram were developed by the ISC based on current guideline recommendations and the local antibiogram. These were subsequently approved by ED practitioners, the pharmacy and therapeutics committee, and the critical care committee. The antibiotic algorithm prompts CSPs to perform a chart review to identify β-lactam allergies, evaluate the severity of the allergy and which agents the patient has tolerated in the past, as well as determine whether the patient has a history of extended spectrum β-lactamase (ESBL)–producing organisms from previous cultures. A decision tree then guides CSPs toward the selection of 1 of 5 empiric antibiotic regimens to cover all likely pathogens. The medication orders are then entered by the CSPs as a telephone order from the ED LIP per protocol. Unless patients had a true vancomycin allergy, all patients received vancomycin as the empiric gram-positive backbone of the regimen. The vancomycin dosing nomogram was created to ensure an appropriate and consistent vancomycin weight-based loading dose was administered.
Prior to implementation, the antimicrobial stewardship pharmacist educated CSPs on the use of these tools, including simulated orders for mock sepsis alerts to ensure competency. A copy of the algorithm and nomogram were emailed to all CSPs and posted in a prominent location in the pharmacy.
As part of continuous performance improvement efforts of the ISC, a retrospective cohort study was conducted through chart review on patients at the Lexington VAHCS with an order for a sepsis alert in the ED from December 3, 2018 to May 31, 2020 to assess the accuracy of the CSPs’ antibiotic selection and dosing. Patients were excluded if they had a vancomycin allergy or if the ED practitioner ordered antibiotics prior to the CSPs placing orders. Patients could be included more than once in the study if they had sepsis alerts placed on different dates.
The primary outcomes were CSPs’ accuracy in antimicrobial selection with the antibiotic selection algorithm and vancomycin dosing nomogram. The antibiotic selection was deemed accurate if the appropriate antibiotic regimen was selected based on allergy status and previous cultures as directed in the algorithm. The vancomycin dose was considered accurate if the dose chosen was appropriate based on the patient’s weight at the time of ED presentation. Secondary outcomes included time to administration of antibiotics from ED presentation as well as time to antibiotics administration from sepsis alert initiation. Time of administration was considered the time the antibiotics were scanned in the bar code medication administration (BCMA) system.
Descriptive statistics were used with data presented as percentages for nominal data and median as IQR for continuous data. In accordance with our facility’s project assessment process, this project was determined not to constitute human subjects research; therefore, this QI project did not require review by the institutional review board.
Results
Between December 3, 2018 and May 31, 2020, 160 sepsis alerts were ordered by ED practitioners. Of the 160 patients, 157 were included in the final data analysis. Two patients were excluded due to vancomycin allergy, and 1 patient because the physician ordered antibiotics prior to pharmacist order entry. The population was largely composed of male patients (98%) with a median age of 72 years (Table 2).
Of 157 sepsis alerts, the antibiotic selection algorithm was used appropriately in 154 (98%) instances (Table 3). Chart reviews were performed in instances of antimicrobial selection different from the algorithm. Of the 3 patients who received antibiotics not consistent with the algorithm, 1 patient without a history of ESBL-producing organisms in their culture history received meropenem instead of piperacillin/tazobactam. Another patient without a penicillin allergy received cefepime (plus metronidazole ordered separately from the ED practitioner) instead of piperacillin/tazobactam, and the third patient received piperacillin/tazobactam instead of meropenem despite a culture history of ESBL-producing organisms. Vancomycin dose was appropriate according to the weight-based nomogram in 147 cases (94%). The median time to administration of first dose antibiotics was 39 minutes after the sepsis alert order was placed and 96 minutes after initial ED presentation.
Discussion
This study found extremely high rates of accuracy among CSPs for both the antibiotic selection algorithm (98%) and the vancomycin dosing nomogram (94%). Moreover, analysis of the 3 patients who received antibiotics that were inconsistent with the algorithm revealed that 2 of these patients arguably still received adequate empiric coverage, increasing the percentage of patients receiving appropriate empiric antibiotics to 99.4%. Similarly, chart review of 10 patients who received vancomycin doses that deviated from the nomogram revealed that in at least 3 cases, patients were likely given correct vancomycin doses based on the patient’s last known weight. However, when actual current weights were recorded soon after admission, the updated weights rendered the initial vancomycin loading dose incorrect when this analysis was performed. Thus, the adherence to the vancomycin dosing nomogram is higher than it appears.
Median time to antibiotic administration from the sepsis alert was 39 minutes—well within SSC recommendations (60 minutes).2 Previous internal analyses at Lexington VAHCS demonstrated the mean time to first dose of antibiotics in the ED has been 39 minutes since about 2015. Thus, this initiative did not necessarily make this process quicker; however it did remove 1 responsibility from LIPs so that they could focus their efforts on other components of sepsis management.
Further studies are needed to evaluate the effects of this initiative on other aspects of the sepsis bundle, such as volume of fluid administered and appropriateness of laboratory tests. It was noted that while the time to first-dose antibiotic administration was < 1 hour from order placement, the median time from ED presentation to antibiotic administration was 96 minutes. This suggests that another focus of the sepsis workgroup should be on speeding recognition of sepsis, triggering the sepsis alert even sooner, and evaluating the feasibility of storing first doses of antibiotics in the automatic dispensing cabinets in the ED.
Limitations
This descriptive study evaluating CSPs’ ability to accurately use the newly developed antibiotic selection algorithm and vancomycin dosing nomogram had no control group for outcome comparison. This study was not designed to evaluate clinical outcomes, such as mortality, so the impact of these interventions need to be further studied. In addition, as veterans receive most of their care at our facility, with their allergies and previous cultures readily available in our electronic health record, this process may not be feasible at other facilities where patients' care is divided among multiple facilities/systems.
Moreover, as the veteran population studied was predominately male patients aged > 60 years, implementation at other hospitals may require the dosing nomograms and treatment algorithms to be adapted for a broader population, such as children and pregnant women. In particular, the ISC chose to implement an algorithm that did not differentiate between suspected source of infections and included anti-Pseudomonal coverage in all regimens based on the most encountered diseases among our veteran population and our local antibiogram; implementation at other facilities would require a thoughtful evaluation of the most appropriate site-specific regimen. Finally, many of the CSPs at our facility are board certified and/or residency trained, so more staff development may be required prior to implementation at other facilities, depending on the experience and comfort level of the CSPs.
Strengths
This study describes an example of a protocolized and multidisciplinary approach to improve sepsis recognition and standardize the response, consistent with SSC guideline recommendations. To the best of our knowledge, this is the first study to demonstrate the incorporation of CSPs into the interdisciplinary sepsis response. This allows for CSPs to practice at the top of their license and contributes to their professional development. Although it was not formally assessed, anecdotally CSPs reported that this process presented a negligible addition to their workload (< 5 minutes was the most reported time requirement), and they expressed satisfaction with their involvement in the sepsis response. Overall, this presents a possible solution to improve the sepsis response in hospitals without a dedicated ED pharmacist.
Conclusions
This study describes the successful incorporation of CSPs into the sepsis response in the ED. As CSPs are more likely than ED pharmacists to be present at a facility, they are arguably an underused resource whose clinical skills can be used to optimize the treatment of patients with sepsis.
Sepsis is life-threatening organ dysfunction caused by dysregulated host response to an infection that can progress to shock. Sepsis is a major cause of death in the United States, with > 1 million people developing sepsis and > 250,000 people dying from sepsis annually.1 The Surviving Sepsis Campaign (SSC) guidelines recommend treating sepsis as an emergency with timely administration of fluids and antibiotics, as administering antibiotics within the first hour has been found to reduce mortality and disease progression. In addition, empiric antibiotic regimens should be chosen to target the most probable pathogens and dosing should be optimized. To achieve this, the SSC guidelines recommend that hospitals develop quality improvement (QI) programs developed by a multidisciplinary group to improve sepsis recognition and response using a protocolized approach.2
There are several studies describing efforts to improve the sepsis response at facilities, some of which have evaluated the addition of a pharmacist into the sepsis response, particularly in the emergency department (ED). Some studies found improved selection and decreased time to antibiotic administration with the addition of an ED pharmacist.3-7 Despite this, ED pharmacists are not present in all hospitals, with a 2015 national survey reporting the presence of an ED pharmacist in 68.7% of respondents at 187 facilities. Even facilities with ED pharmacists often have limited hours of coverage, with at least 8 hours of coverage in 49.4% of facilities with an ED pharmacist and no weekend coverage at 34.8% of these facilities.8
While many hospitals do not routinely employ ED pharmacists, most hospitals have clinical staff pharmacists (CSPs), and many inpatient hospital pharmacies are staffed with CSPs 24 hours per day, 7 days per week. A 2017 survey conducted by the American Society of Health-System Pharmacists (ASHP) found 43% of all hospital pharmacy departments were staffed by a CSP around the clock, with the prevalence increasing to 56.7 to 100% in hospitals with > 100 beds.9 As a result, CSPs may be a useful resource to assist with the management of patients with sepsis in hospitals without an ED pharmacist.
At the Lexington Veterans Affairs Health Care System (LVAHCS) in Kentucky, the inpatient pharmacy department is staffed with a CSP 24/7 but does not have an ED pharmacist. Therefore, when an interdisciplinary group developed an ED sepsis bundle as part of a QI initiative on sepsis recognition and response, the group took a unique approach of incorporating CSPs into the response team to assist with antimicrobial selection and dosing. An antibiotic selection algorithm and vancomycin dosing nomogram were developed to aid CSPs to select and dose antibiotics (Figure, Table 1). We describe the implementation of this process and evaluate CSPs’ accuracy in antimicrobial selection and vancomycin dosing.
Methods
Lexington VAHCS is a 94-bed hospital that provides services to veterans, including an ED, inpatient medical services, surgical services, acute mental health, progressive care, and intensive care units. This facility has 1 antimicrobial stewardship clinical pharmacy specialist, 2 critical care clinical pharmacy specialists, and 16 full-time CSPs with 24-hour CSP coverage. The annual ED volume at the time of this study was approximately 21,000 patients.
Consistent with the SSC guideline recommendation to develop multidisciplinary QI initiatives on sepsis recognition and response, an Interdisciplinary Sepsis Committee (ISC) was created in 2018 comprised of ED, pulmonary, critical care, and infectious diseases licensed independent practitioners (LIPs), ED nurses, and pharmacists. The ISC developed a comprehensive set of sepsis tools that included a sepsis screening tool used by ED triage nurses to provide early detection of sepsis and an updated electronic order set to decrease time to appropriate treatment. This order set included automatic orders for blood cultures and serum lactate, the initiation of IV crystalloids, as well as a Sepsis Alert order placed by ED LIPs which alerted CSPs to a patient with sepsis in the ED.
To ensure a protocol-based approach by the CSPs responding to the sepsis alert, an antibiotic algorithm and vancomycin dosing nomogram were developed by the ISC based on current guideline recommendations and the local antibiogram. These were subsequently approved by ED practitioners, the pharmacy and therapeutics committee, and the critical care committee. The antibiotic algorithm prompts CSPs to perform a chart review to identify β-lactam allergies, evaluate the severity of the allergy and which agents the patient has tolerated in the past, as well as determine whether the patient has a history of extended spectrum β-lactamase (ESBL)–producing organisms from previous cultures. A decision tree then guides CSPs toward the selection of 1 of 5 empiric antibiotic regimens to cover all likely pathogens. The medication orders are then entered by the CSPs as a telephone order from the ED LIP per protocol. Unless patients had a true vancomycin allergy, all patients received vancomycin as the empiric gram-positive backbone of the regimen. The vancomycin dosing nomogram was created to ensure an appropriate and consistent vancomycin weight-based loading dose was administered.
Prior to implementation, the antimicrobial stewardship pharmacist educated CSPs on the use of these tools, including simulated orders for mock sepsis alerts to ensure competency. A copy of the algorithm and nomogram were emailed to all CSPs and posted in a prominent location in the pharmacy.
As part of continuous performance improvement efforts of the ISC, a retrospective cohort study was conducted through chart review on patients at the Lexington VAHCS with an order for a sepsis alert in the ED from December 3, 2018 to May 31, 2020 to assess the accuracy of the CSPs’ antibiotic selection and dosing. Patients were excluded if they had a vancomycin allergy or if the ED practitioner ordered antibiotics prior to the CSPs placing orders. Patients could be included more than once in the study if they had sepsis alerts placed on different dates.
The primary outcomes were CSPs’ accuracy in antimicrobial selection with the antibiotic selection algorithm and vancomycin dosing nomogram. The antibiotic selection was deemed accurate if the appropriate antibiotic regimen was selected based on allergy status and previous cultures as directed in the algorithm. The vancomycin dose was considered accurate if the dose chosen was appropriate based on the patient’s weight at the time of ED presentation. Secondary outcomes included time to administration of antibiotics from ED presentation as well as time to antibiotics administration from sepsis alert initiation. Time of administration was considered the time the antibiotics were scanned in the bar code medication administration (BCMA) system.
Descriptive statistics were used with data presented as percentages for nominal data and median as IQR for continuous data. In accordance with our facility’s project assessment process, this project was determined not to constitute human subjects research; therefore, this QI project did not require review by the institutional review board.
Results
Between December 3, 2018 and May 31, 2020, 160 sepsis alerts were ordered by ED practitioners. Of the 160 patients, 157 were included in the final data analysis. Two patients were excluded due to vancomycin allergy, and 1 patient because the physician ordered antibiotics prior to pharmacist order entry. The population was largely composed of male patients (98%) with a median age of 72 years (Table 2).
Of 157 sepsis alerts, the antibiotic selection algorithm was used appropriately in 154 (98%) instances (Table 3). Chart reviews were performed in instances of antimicrobial selection different from the algorithm. Of the 3 patients who received antibiotics not consistent with the algorithm, 1 patient without a history of ESBL-producing organisms in their culture history received meropenem instead of piperacillin/tazobactam. Another patient without a penicillin allergy received cefepime (plus metronidazole ordered separately from the ED practitioner) instead of piperacillin/tazobactam, and the third patient received piperacillin/tazobactam instead of meropenem despite a culture history of ESBL-producing organisms. Vancomycin dose was appropriate according to the weight-based nomogram in 147 cases (94%). The median time to administration of first dose antibiotics was 39 minutes after the sepsis alert order was placed and 96 minutes after initial ED presentation.
Discussion
This study found extremely high rates of accuracy among CSPs for both the antibiotic selection algorithm (98%) and the vancomycin dosing nomogram (94%). Moreover, analysis of the 3 patients who received antibiotics that were inconsistent with the algorithm revealed that 2 of these patients arguably still received adequate empiric coverage, increasing the percentage of patients receiving appropriate empiric antibiotics to 99.4%. Similarly, chart review of 10 patients who received vancomycin doses that deviated from the nomogram revealed that in at least 3 cases, patients were likely given correct vancomycin doses based on the patient’s last known weight. However, when actual current weights were recorded soon after admission, the updated weights rendered the initial vancomycin loading dose incorrect when this analysis was performed. Thus, the adherence to the vancomycin dosing nomogram is higher than it appears.
Median time to antibiotic administration from the sepsis alert was 39 minutes—well within SSC recommendations (60 minutes).2 Previous internal analyses at Lexington VAHCS demonstrated the mean time to first dose of antibiotics in the ED has been 39 minutes since about 2015. Thus, this initiative did not necessarily make this process quicker; however it did remove 1 responsibility from LIPs so that they could focus their efforts on other components of sepsis management.
Further studies are needed to evaluate the effects of this initiative on other aspects of the sepsis bundle, such as volume of fluid administered and appropriateness of laboratory tests. It was noted that while the time to first-dose antibiotic administration was < 1 hour from order placement, the median time from ED presentation to antibiotic administration was 96 minutes. This suggests that another focus of the sepsis workgroup should be on speeding recognition of sepsis, triggering the sepsis alert even sooner, and evaluating the feasibility of storing first doses of antibiotics in the automatic dispensing cabinets in the ED.
Limitations
This descriptive study evaluating CSPs’ ability to accurately use the newly developed antibiotic selection algorithm and vancomycin dosing nomogram had no control group for outcome comparison. This study was not designed to evaluate clinical outcomes, such as mortality, so the impact of these interventions need to be further studied. In addition, as veterans receive most of their care at our facility, with their allergies and previous cultures readily available in our electronic health record, this process may not be feasible at other facilities where patients' care is divided among multiple facilities/systems.
Moreover, as the veteran population studied was predominately male patients aged > 60 years, implementation at other hospitals may require the dosing nomograms and treatment algorithms to be adapted for a broader population, such as children and pregnant women. In particular, the ISC chose to implement an algorithm that did not differentiate between suspected source of infections and included anti-Pseudomonal coverage in all regimens based on the most encountered diseases among our veteran population and our local antibiogram; implementation at other facilities would require a thoughtful evaluation of the most appropriate site-specific regimen. Finally, many of the CSPs at our facility are board certified and/or residency trained, so more staff development may be required prior to implementation at other facilities, depending on the experience and comfort level of the CSPs.
Strengths
This study describes an example of a protocolized and multidisciplinary approach to improve sepsis recognition and standardize the response, consistent with SSC guideline recommendations. To the best of our knowledge, this is the first study to demonstrate the incorporation of CSPs into the interdisciplinary sepsis response. This allows for CSPs to practice at the top of their license and contributes to their professional development. Although it was not formally assessed, anecdotally CSPs reported that this process presented a negligible addition to their workload (< 5 minutes was the most reported time requirement), and they expressed satisfaction with their involvement in the sepsis response. Overall, this presents a possible solution to improve the sepsis response in hospitals without a dedicated ED pharmacist.
Conclusions
This study describes the successful incorporation of CSPs into the sepsis response in the ED. As CSPs are more likely than ED pharmacists to be present at a facility, they are arguably an underused resource whose clinical skills can be used to optimize the treatment of patients with sepsis.
1. Centers for Disease Control and Prevention. Sepsis. Accessed March 8, 2022. https://www.cdc.gov/sepsis/what-is-sepsis.html
2. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017 Mar;45(3):486-552. doi:10.1097/CCM.0000000000002255
3. Denny KJ, Gartside JG, Alcorn K, et al. Appropriateness of antibiotic prescribing in the emergency department. J Antimicrob Chemother. 2019 Feb 1;74(2):515-520. doi:10.1093/jac/dky447
4. Laine ME, Flynn JD, Flannery AH. Impact of pharmacist intervention on selection and timing of appropriate antimicrobial therapy in septic shock. J Pharm Pract. 2018 Feb;31(1):46-51. doi:10.1177/0897190017696953
5. Weant KA, Baker SN. Emergency medicine pharmacists and sepsis management. J Pharm Pract. 2013 Aug;26(4):401-5. doi:10.1177/0897190012467211
6. Farmer BM, Hayes BD, Rao R, et al. The role of clinical pharmacists in the emergency department. J Med Toxicol. 2018 Mar;14(1):114-116. doi:10.1007/s13181-017-0634-4
7. Yarbrough N, Bloxam M, Priano J, Louzon Lynch P, Hunt LN, Elfman J. Pharmacist impact on sepsis bundle compliance through participation on an emergency department sepsis alert team. Am J Emerg Med. 2019;37(4):762-763. doi:10.1016/j.ajem.2018.08.00
8. Thomas MC, Acquisto NM, Shirk MB, et al. A national survey of emergency pharmacy practice in the United States. Am J Health Syst Pharm. 2016 Mar 15;73(6):386-94. doi:10.2146/ajhp150321
9. Schneider PJ, Pedersen CA, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: dispensing and administration-2017. Am J Health Syst Pharm. 2018;75(16):1203-1226. doi:10.2146/ajhp180151
1. Centers for Disease Control and Prevention. Sepsis. Accessed March 8, 2022. https://www.cdc.gov/sepsis/what-is-sepsis.html
2. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017 Mar;45(3):486-552. doi:10.1097/CCM.0000000000002255
3. Denny KJ, Gartside JG, Alcorn K, et al. Appropriateness of antibiotic prescribing in the emergency department. J Antimicrob Chemother. 2019 Feb 1;74(2):515-520. doi:10.1093/jac/dky447
4. Laine ME, Flynn JD, Flannery AH. Impact of pharmacist intervention on selection and timing of appropriate antimicrobial therapy in septic shock. J Pharm Pract. 2018 Feb;31(1):46-51. doi:10.1177/0897190017696953
5. Weant KA, Baker SN. Emergency medicine pharmacists and sepsis management. J Pharm Pract. 2013 Aug;26(4):401-5. doi:10.1177/0897190012467211
6. Farmer BM, Hayes BD, Rao R, et al. The role of clinical pharmacists in the emergency department. J Med Toxicol. 2018 Mar;14(1):114-116. doi:10.1007/s13181-017-0634-4
7. Yarbrough N, Bloxam M, Priano J, Louzon Lynch P, Hunt LN, Elfman J. Pharmacist impact on sepsis bundle compliance through participation on an emergency department sepsis alert team. Am J Emerg Med. 2019;37(4):762-763. doi:10.1016/j.ajem.2018.08.00
8. Thomas MC, Acquisto NM, Shirk MB, et al. A national survey of emergency pharmacy practice in the United States. Am J Health Syst Pharm. 2016 Mar 15;73(6):386-94. doi:10.2146/ajhp150321
9. Schneider PJ, Pedersen CA, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: dispensing and administration-2017. Am J Health Syst Pharm. 2018;75(16):1203-1226. doi:10.2146/ajhp180151
A First Look at the VA MISSION Act Veteran Health Administration Medical School Scholarship and Loan Repayment Programs
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
What Federal Practitioners Need to Know About the National Practitioner Data Bank
Not all federal practitioners know about the National Practitioner Data Bank (NPDB), a federal web-based repository of reports containing information on medical malpractice payments and certain adverse actions related to health care practitioners, providers, and suppliers. This article explains how NPDB statutes and regulations specifically affect federal health care practitioners, which may differ from how the rules affect practitioners in the private sector.1
National Practitioner Data Bank
Established by Congress in 1986, the NPDB contains information health care organizations need to make informed decisions about the health care practitionerss they license, credential, and hire. Federal regulations authorize eligible entities, including government agencies, to report to and query the NPDB. Individuals and organizations that are subjects of these reports have access to their own information. The reports are confidential and not available to the public. The NPDB currently contains > 1.6 million reports.2
Federal Agencies Queries
A query is a search for information in the NPDB regarding a health care practitioners or organization. Some federal agencies are permitted to query the NPDB, and all hospitals, including federal hospitals, are required to query. Agencies administering government health care programs (including private entities administering such programs under contract), federal law enforcement officials and agencies, and federal agencies responsible for the licensing or certification of health care practitioners, health care providers, or health care suppliers may query NPDB. Information received in response to queries includes, among other actions, licensure and certification actions taken by states, medical malpractice payment information, federal licensing and certification actions, and adverse privileging actions.3
Federal Reporting Requirements
Federal government agencies must report exclusions (described below), adjudicated actions, civil judgments, and criminal convictions concerning health care practitioners, providers, or suppliers. The following provides detailed information about the actions federal government agencies are required to report.
Adjudicated Actions or Decisions
Adjudicated actions or decisions are formal or official final actions.3 They include, but are not limited to, personnel-related actions such as suspensions without pay, reductions in pay, reductions in grade for cause, terminations, or other comparable actions. To be reportable, adjudicated actions or decisions must include due process mechanisms. Whether the subject of a report elects not to use the due process mechanism is immaterial as long as such a process is available to the subject before the adjudicated action or decision is made final. In general, if an adjudicated action or decision follows an agency’s established administrative procedures and those procedures ensure that due process is available to the subject, the due process requirement is satisfied. This definition specifically excludes clinical privileging actions taken by federal government agencies, which are described in appropriate memorandums of understanding.
Exclusions
An exclusion is a temporary or permanent debarment of an individual or organization from participation in a federal health-related program, such that items or services furnished by the individual or organization will not be reimbursed under the federal program.3
Civil Judgments and Criminal Convictions
Health care–related civil judgments and settlements must be reported.However, settlements in which no findings of liability have been made are not reportable.3 Health care–related criminal convictions prosecuted by federal government agencies in federal court must be reported to the NPDB. Pleas of guilt and nolo contendere, or no contest, by individuals or organizations also are reportable.3
In addition, final adverse licensure and certification actions are those taken against health care practitioners, providers, or suppliers, regardless of whether the final adverse action is the subject of a pending appeal.3 These must be reported.
Additional Reporting Requirements
Federal hospitals or federal government agencies administering health care services may have additional reporting requirements besides reporting adjudicated actions, exclusions, civil judgments, and criminal convictions. They may include submitting reports under a memorandum of understanding on clinical privileges actions and medical malpractice payments.3 The US Department of Health and Human Services (HHS) has entered into memorandums of understanding with the US Department of Defense and the US Department of Veteran Affairs to ensure their participation in the NPDB system. Federal hospitals should refer to applicable memorandums of understanding and agency-specific policies for guidance on carrying out their reporting responsibilities.4
Responding to a Report
The NPDB sends a letter to health care practitioners when an organization submits a report about the practitioner. The letter has the report number and a password is required to view the report.2 Health care practitioners also can order a self-query online to view any reports on them in the NPDB.
The subject of the report can also add a statement and dispute the report. The statement is an opportunity to provide additional information the subject would like to have included in the report. If the subject disagrees with the accuracy of a report or believes it does not meet NPDB reporting requirements, it can be disputed. The dispute will become part of the report. When the subject adds a statement or dispute, the NPDB notifies the reporting organization and all organizations that received the report within the previous 3 years of the report activity.
Health care practitioners must contact the reporting organization to try to resolve their dispute. If the subject of the report has contacted or tried to contact the reporting organization and could not resolve the dispute after 60 days, or if, within the 60-day period, the organization informs the subject that it will not modify the report, that individual may request dispute resolution.Requesting dispute resolution does not remove the report from the NPDB.
Dispute Resolution
Dispute resolution is a request for the HHS secretary to review the report. The secretary authorizes the Division of Practitioner Data Bank (DPDB) to conduct this review. The DPDB is responsible for oversight of the NPDB. The subject of the report will need to submit relevant supporting documentation to request dispute resolution. This documentation should show that the information in the report is not accurate or that the action is not reportable. Also, proof should be included that the subject contacted or attempted to contact the reporting organization. Submitting large volumes or extraneous documentation can delay the review process.
A dispute resolution manager will review the case and send the reporting organization a request for information if needed. The DPDB will send the subject of the report a courtesy copy of all correspondence. The dispute resolution timeline varies, as the DPDB reviews disputes in the order they are received. It completes a fair and thorough review based on the unique circumstances of each case and will review the case as soon as possible. Once the DPDB receives documentation from the subject and the reporting organization, it reviews the documentation to determine whether the report accurately reflects the record.
The DPDB decides to either maintain the report as is, correct it, or remove it from the NPDB. Once the process is complete, the dispute resolution manager sends a decision letter to the subject of the report and the reporting organization. The dispute resolution decision will appear in the report.
Regulations strictly limit the DPDB’s jurisdiction for reviewing disputed reports. It may only review the following: whether the report was submitted in accordance with reporting requirements, whether the reporting organization was eligible to report the information, and whether the report accurately depicts the action taken by the reporting organization and the basis for the action the reporting organization cited, as shown in the organization’s written record. The subject of the report must resolve any other issues with the reporting organization.
Under the dispute resolution review process, the DPDB cannot conduct an independent review of the merits of the action taken by the reporting organization, review the due process provided by the organization, or substitute its judgment for that of the reporting organization.2 The DPDB does not examine whether the subject of a report was informed of an ongoing investigation. The DPDB does not examine civil rights issues such as claims of discrimination or harassment in the work environment. Practitioners can find additional information at www.npdb.hrsa.gov.
1. US Department of Health and Human Services, National Practitioner Data Bank. NPDB guidebook. Updated October 2018. Accessed December 16, 2021. https://www.npdb.hrsa.gov/resources/aboutGuidebooks.jsp
2. US Department of Health and Human Services, National Practitioner Data Bank. A practitioner’s guide to the NPDB. Updated February 2021. Accessed December 16, 2021. https://www.npdb.hrsa.gov/pract/practGuide.jsp
3. US Department of Health and Human Services, National Practitioner Data Bank. Federal hospitals and federal government agencies. Accessed December 16, 2021. https://www.npdb.hrsa.gov/orgs/federalAgencies.jsp
4. US Department of Health and Human Services, National Practitioner Data Bank. Federal hospitals. Accessed December 16, 2021. https://www.npdb.hrsa.gov/orgs/federalHospitals.jsp
Not all federal practitioners know about the National Practitioner Data Bank (NPDB), a federal web-based repository of reports containing information on medical malpractice payments and certain adverse actions related to health care practitioners, providers, and suppliers. This article explains how NPDB statutes and regulations specifically affect federal health care practitioners, which may differ from how the rules affect practitioners in the private sector.1
National Practitioner Data Bank
Established by Congress in 1986, the NPDB contains information health care organizations need to make informed decisions about the health care practitionerss they license, credential, and hire. Federal regulations authorize eligible entities, including government agencies, to report to and query the NPDB. Individuals and organizations that are subjects of these reports have access to their own information. The reports are confidential and not available to the public. The NPDB currently contains > 1.6 million reports.2
Federal Agencies Queries
A query is a search for information in the NPDB regarding a health care practitioners or organization. Some federal agencies are permitted to query the NPDB, and all hospitals, including federal hospitals, are required to query. Agencies administering government health care programs (including private entities administering such programs under contract), federal law enforcement officials and agencies, and federal agencies responsible for the licensing or certification of health care practitioners, health care providers, or health care suppliers may query NPDB. Information received in response to queries includes, among other actions, licensure and certification actions taken by states, medical malpractice payment information, federal licensing and certification actions, and adverse privileging actions.3
Federal Reporting Requirements
Federal government agencies must report exclusions (described below), adjudicated actions, civil judgments, and criminal convictions concerning health care practitioners, providers, or suppliers. The following provides detailed information about the actions federal government agencies are required to report.
Adjudicated Actions or Decisions
Adjudicated actions or decisions are formal or official final actions.3 They include, but are not limited to, personnel-related actions such as suspensions without pay, reductions in pay, reductions in grade for cause, terminations, or other comparable actions. To be reportable, adjudicated actions or decisions must include due process mechanisms. Whether the subject of a report elects not to use the due process mechanism is immaterial as long as such a process is available to the subject before the adjudicated action or decision is made final. In general, if an adjudicated action or decision follows an agency’s established administrative procedures and those procedures ensure that due process is available to the subject, the due process requirement is satisfied. This definition specifically excludes clinical privileging actions taken by federal government agencies, which are described in appropriate memorandums of understanding.
Exclusions
An exclusion is a temporary or permanent debarment of an individual or organization from participation in a federal health-related program, such that items or services furnished by the individual or organization will not be reimbursed under the federal program.3
Civil Judgments and Criminal Convictions
Health care–related civil judgments and settlements must be reported.However, settlements in which no findings of liability have been made are not reportable.3 Health care–related criminal convictions prosecuted by federal government agencies in federal court must be reported to the NPDB. Pleas of guilt and nolo contendere, or no contest, by individuals or organizations also are reportable.3
In addition, final adverse licensure and certification actions are those taken against health care practitioners, providers, or suppliers, regardless of whether the final adverse action is the subject of a pending appeal.3 These must be reported.
Additional Reporting Requirements
Federal hospitals or federal government agencies administering health care services may have additional reporting requirements besides reporting adjudicated actions, exclusions, civil judgments, and criminal convictions. They may include submitting reports under a memorandum of understanding on clinical privileges actions and medical malpractice payments.3 The US Department of Health and Human Services (HHS) has entered into memorandums of understanding with the US Department of Defense and the US Department of Veteran Affairs to ensure their participation in the NPDB system. Federal hospitals should refer to applicable memorandums of understanding and agency-specific policies for guidance on carrying out their reporting responsibilities.4
Responding to a Report
The NPDB sends a letter to health care practitioners when an organization submits a report about the practitioner. The letter has the report number and a password is required to view the report.2 Health care practitioners also can order a self-query online to view any reports on them in the NPDB.
The subject of the report can also add a statement and dispute the report. The statement is an opportunity to provide additional information the subject would like to have included in the report. If the subject disagrees with the accuracy of a report or believes it does not meet NPDB reporting requirements, it can be disputed. The dispute will become part of the report. When the subject adds a statement or dispute, the NPDB notifies the reporting organization and all organizations that received the report within the previous 3 years of the report activity.
Health care practitioners must contact the reporting organization to try to resolve their dispute. If the subject of the report has contacted or tried to contact the reporting organization and could not resolve the dispute after 60 days, or if, within the 60-day period, the organization informs the subject that it will not modify the report, that individual may request dispute resolution.Requesting dispute resolution does not remove the report from the NPDB.
Dispute Resolution
Dispute resolution is a request for the HHS secretary to review the report. The secretary authorizes the Division of Practitioner Data Bank (DPDB) to conduct this review. The DPDB is responsible for oversight of the NPDB. The subject of the report will need to submit relevant supporting documentation to request dispute resolution. This documentation should show that the information in the report is not accurate or that the action is not reportable. Also, proof should be included that the subject contacted or attempted to contact the reporting organization. Submitting large volumes or extraneous documentation can delay the review process.
A dispute resolution manager will review the case and send the reporting organization a request for information if needed. The DPDB will send the subject of the report a courtesy copy of all correspondence. The dispute resolution timeline varies, as the DPDB reviews disputes in the order they are received. It completes a fair and thorough review based on the unique circumstances of each case and will review the case as soon as possible. Once the DPDB receives documentation from the subject and the reporting organization, it reviews the documentation to determine whether the report accurately reflects the record.
The DPDB decides to either maintain the report as is, correct it, or remove it from the NPDB. Once the process is complete, the dispute resolution manager sends a decision letter to the subject of the report and the reporting organization. The dispute resolution decision will appear in the report.
Regulations strictly limit the DPDB’s jurisdiction for reviewing disputed reports. It may only review the following: whether the report was submitted in accordance with reporting requirements, whether the reporting organization was eligible to report the information, and whether the report accurately depicts the action taken by the reporting organization and the basis for the action the reporting organization cited, as shown in the organization’s written record. The subject of the report must resolve any other issues with the reporting organization.
Under the dispute resolution review process, the DPDB cannot conduct an independent review of the merits of the action taken by the reporting organization, review the due process provided by the organization, or substitute its judgment for that of the reporting organization.2 The DPDB does not examine whether the subject of a report was informed of an ongoing investigation. The DPDB does not examine civil rights issues such as claims of discrimination or harassment in the work environment. Practitioners can find additional information at www.npdb.hrsa.gov.
Not all federal practitioners know about the National Practitioner Data Bank (NPDB), a federal web-based repository of reports containing information on medical malpractice payments and certain adverse actions related to health care practitioners, providers, and suppliers. This article explains how NPDB statutes and regulations specifically affect federal health care practitioners, which may differ from how the rules affect practitioners in the private sector.1
National Practitioner Data Bank
Established by Congress in 1986, the NPDB contains information health care organizations need to make informed decisions about the health care practitionerss they license, credential, and hire. Federal regulations authorize eligible entities, including government agencies, to report to and query the NPDB. Individuals and organizations that are subjects of these reports have access to their own information. The reports are confidential and not available to the public. The NPDB currently contains > 1.6 million reports.2
Federal Agencies Queries
A query is a search for information in the NPDB regarding a health care practitioners or organization. Some federal agencies are permitted to query the NPDB, and all hospitals, including federal hospitals, are required to query. Agencies administering government health care programs (including private entities administering such programs under contract), federal law enforcement officials and agencies, and federal agencies responsible for the licensing or certification of health care practitioners, health care providers, or health care suppliers may query NPDB. Information received in response to queries includes, among other actions, licensure and certification actions taken by states, medical malpractice payment information, federal licensing and certification actions, and adverse privileging actions.3
Federal Reporting Requirements
Federal government agencies must report exclusions (described below), adjudicated actions, civil judgments, and criminal convictions concerning health care practitioners, providers, or suppliers. The following provides detailed information about the actions federal government agencies are required to report.
Adjudicated Actions or Decisions
Adjudicated actions or decisions are formal or official final actions.3 They include, but are not limited to, personnel-related actions such as suspensions without pay, reductions in pay, reductions in grade for cause, terminations, or other comparable actions. To be reportable, adjudicated actions or decisions must include due process mechanisms. Whether the subject of a report elects not to use the due process mechanism is immaterial as long as such a process is available to the subject before the adjudicated action or decision is made final. In general, if an adjudicated action or decision follows an agency’s established administrative procedures and those procedures ensure that due process is available to the subject, the due process requirement is satisfied. This definition specifically excludes clinical privileging actions taken by federal government agencies, which are described in appropriate memorandums of understanding.
Exclusions
An exclusion is a temporary or permanent debarment of an individual or organization from participation in a federal health-related program, such that items or services furnished by the individual or organization will not be reimbursed under the federal program.3
Civil Judgments and Criminal Convictions
Health care–related civil judgments and settlements must be reported.However, settlements in which no findings of liability have been made are not reportable.3 Health care–related criminal convictions prosecuted by federal government agencies in federal court must be reported to the NPDB. Pleas of guilt and nolo contendere, or no contest, by individuals or organizations also are reportable.3
In addition, final adverse licensure and certification actions are those taken against health care practitioners, providers, or suppliers, regardless of whether the final adverse action is the subject of a pending appeal.3 These must be reported.
Additional Reporting Requirements
Federal hospitals or federal government agencies administering health care services may have additional reporting requirements besides reporting adjudicated actions, exclusions, civil judgments, and criminal convictions. They may include submitting reports under a memorandum of understanding on clinical privileges actions and medical malpractice payments.3 The US Department of Health and Human Services (HHS) has entered into memorandums of understanding with the US Department of Defense and the US Department of Veteran Affairs to ensure their participation in the NPDB system. Federal hospitals should refer to applicable memorandums of understanding and agency-specific policies for guidance on carrying out their reporting responsibilities.4
Responding to a Report
The NPDB sends a letter to health care practitioners when an organization submits a report about the practitioner. The letter has the report number and a password is required to view the report.2 Health care practitioners also can order a self-query online to view any reports on them in the NPDB.
The subject of the report can also add a statement and dispute the report. The statement is an opportunity to provide additional information the subject would like to have included in the report. If the subject disagrees with the accuracy of a report or believes it does not meet NPDB reporting requirements, it can be disputed. The dispute will become part of the report. When the subject adds a statement or dispute, the NPDB notifies the reporting organization and all organizations that received the report within the previous 3 years of the report activity.
Health care practitioners must contact the reporting organization to try to resolve their dispute. If the subject of the report has contacted or tried to contact the reporting organization and could not resolve the dispute after 60 days, or if, within the 60-day period, the organization informs the subject that it will not modify the report, that individual may request dispute resolution.Requesting dispute resolution does not remove the report from the NPDB.
Dispute Resolution
Dispute resolution is a request for the HHS secretary to review the report. The secretary authorizes the Division of Practitioner Data Bank (DPDB) to conduct this review. The DPDB is responsible for oversight of the NPDB. The subject of the report will need to submit relevant supporting documentation to request dispute resolution. This documentation should show that the information in the report is not accurate or that the action is not reportable. Also, proof should be included that the subject contacted or attempted to contact the reporting organization. Submitting large volumes or extraneous documentation can delay the review process.
A dispute resolution manager will review the case and send the reporting organization a request for information if needed. The DPDB will send the subject of the report a courtesy copy of all correspondence. The dispute resolution timeline varies, as the DPDB reviews disputes in the order they are received. It completes a fair and thorough review based on the unique circumstances of each case and will review the case as soon as possible. Once the DPDB receives documentation from the subject and the reporting organization, it reviews the documentation to determine whether the report accurately reflects the record.
The DPDB decides to either maintain the report as is, correct it, or remove it from the NPDB. Once the process is complete, the dispute resolution manager sends a decision letter to the subject of the report and the reporting organization. The dispute resolution decision will appear in the report.
Regulations strictly limit the DPDB’s jurisdiction for reviewing disputed reports. It may only review the following: whether the report was submitted in accordance with reporting requirements, whether the reporting organization was eligible to report the information, and whether the report accurately depicts the action taken by the reporting organization and the basis for the action the reporting organization cited, as shown in the organization’s written record. The subject of the report must resolve any other issues with the reporting organization.
Under the dispute resolution review process, the DPDB cannot conduct an independent review of the merits of the action taken by the reporting organization, review the due process provided by the organization, or substitute its judgment for that of the reporting organization.2 The DPDB does not examine whether the subject of a report was informed of an ongoing investigation. The DPDB does not examine civil rights issues such as claims of discrimination or harassment in the work environment. Practitioners can find additional information at www.npdb.hrsa.gov.
1. US Department of Health and Human Services, National Practitioner Data Bank. NPDB guidebook. Updated October 2018. Accessed December 16, 2021. https://www.npdb.hrsa.gov/resources/aboutGuidebooks.jsp
2. US Department of Health and Human Services, National Practitioner Data Bank. A practitioner’s guide to the NPDB. Updated February 2021. Accessed December 16, 2021. https://www.npdb.hrsa.gov/pract/practGuide.jsp
3. US Department of Health and Human Services, National Practitioner Data Bank. Federal hospitals and federal government agencies. Accessed December 16, 2021. https://www.npdb.hrsa.gov/orgs/federalAgencies.jsp
4. US Department of Health and Human Services, National Practitioner Data Bank. Federal hospitals. Accessed December 16, 2021. https://www.npdb.hrsa.gov/orgs/federalHospitals.jsp
1. US Department of Health and Human Services, National Practitioner Data Bank. NPDB guidebook. Updated October 2018. Accessed December 16, 2021. https://www.npdb.hrsa.gov/resources/aboutGuidebooks.jsp
2. US Department of Health and Human Services, National Practitioner Data Bank. A practitioner’s guide to the NPDB. Updated February 2021. Accessed December 16, 2021. https://www.npdb.hrsa.gov/pract/practGuide.jsp
3. US Department of Health and Human Services, National Practitioner Data Bank. Federal hospitals and federal government agencies. Accessed December 16, 2021. https://www.npdb.hrsa.gov/orgs/federalAgencies.jsp
4. US Department of Health and Human Services, National Practitioner Data Bank. Federal hospitals. Accessed December 16, 2021. https://www.npdb.hrsa.gov/orgs/federalHospitals.jsp
The VA My Life My Story Project: Keeping Medical Students and Veterans Socially Connected While Physically Distanced
Narrative competence is the ability to acquire, interpret, and act on the stories of others.1 Developing this skill through guided medical storytelling can improve health care practitioners’ (HCPs) sense of empathy and satisfaction with their work.2 Narrative medicine experiences for medical students can foster a deeper understanding of their patients beyond illness-associated identities.3
Within narrative medicine, the “life story” is a specific technique that allows patients to share experiences through open-ended interviews that are entered into the health record.4,5 By sharing life stories, patients control a narrative encompassing more than their illness and can reinforce a sense of purpose in their lives.6 The US Department of Veterans Affairs (VA) My Life My Story (MLMS) program gives veterans the opportunity to share their narrative with staff and volunteer interviewers. MLMS is well received by veterans, has durable positive effects for HCPs who read the stories, and has been used as a tool to teach patient-centered care to medical trainees.7-9
We created a narrative medicine curriculum at the San Francisco VA Medical Center (SFVAMC) in which medical students interviewed veterans for the MLMS program. Medical students initially collected life stories through in-person conversation. During the COVID-19 pandemic, physical distancing regulations limited direct patient interaction for students and prompted a switch to phone and video interviews. This shift paralleled the widespread adoption of telehealth, which will persist beyond the pandemic and require teachers and learners to develop competency in forming personal connections with patients through videoconferencing.10,11
There are no published studies describing how to guide medical students (or other historians) in generating life stories without in-person patient contact. This article details the design of a medical student curriculum incorporating MLMS and the transition to remote interaction between instructors, students, and veterans during the early COVID-19 pandemic.
MLMS Program Origins
The MLMS project began at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, in 2013 with staff and volunteer interviewers and has expanded to more than 60 VA facilities.7 In January 2020, we initiated a narrative medicine curriculum incorporating MLMS at the SFVAMC as a required component of a third-year internal medicine clerkship for medical students at the University of California San Francisco (UCSF). Fifty-four medical students in 10 cohorts participated in the curriculum in 2020. The primary program objectives were for medical students to develop skills for eliciting and recording a life story and to appreciate the impact of this activity on a veteran’s experience of receiving health care. Secondary objectives were for students to understand the mission of the VA health care system and veteran demographics.
The first cohort of 6 UCSF medical students participated in MLMS during their 8-week VA clerkship. Students attended a 1-hour small group session to introduce the program and build narrative medicine skills. Preparation for this session involved listening to 2 podcast episodes introducing the VA health care system and MLMS.12,13 The session began with a short interactive discussion of veteran demographics with an emphasis on addressing assumptions students might have about the veteran population. Students were taught strategies for engaging in open-ended conversations without emphasizing illness. Each student practiced collecting a life story with a simulated patient portrayed by an instructor and received feedback from classmates and instructors.
Over the following weeks, students selected a hospitalized veteran, typically a patient they were caring for, introduced MLMS, and obtained verbal consent to participate. They conducted a 60- to 90-minute interview, wrote and organized the life story, read it to the veteran, and solicited edits. Once a final version was generated, the student provided the veteran with printed copies and offered to place the story in the Computerized Patient Record System (CPRS).
Near the end of their rotation, students attended a 1-hour small group session in which they shared reflections on the experience of collecting a life story, the impact of veterans’ life experiences on their health and illness, and moments when students confronted their own stereotypes and implicit biases. Students then reviewed narrative medicine skills that are generalizable to all patient interactions.
COVID-19-Related Adaptation
In March 2020, shortly after the second student cohort began, medical students were removed from the clinical setting in response to the COVID-19 pandemic. The 8-week clerkship was converted to a 3-week remote learning rotation. The MLMS experience was preserved by converting small group sessions to videoconferences and expanding the pool of eligible patients to include veterans who students had met on prior rotations, current inpatients, and outpatients from VA primary care clinics. Students contacted veterans after an instructor had introduced MLMS to the veteran and confirmed that the veteran was interested in participating.
Students in the second and third cohorts completed a telephone-based iteration of MLMS in which interviews and life story reviews were conducted over the telephone and printed copies mailed to the veteran. For the fourth, fifth, and sixth cohorts, MLMS was transitioned to a video-based program with inpatients. Instructors collaborated with a volunteer group supplying tablet devices to inpatients to make video calls to their families during the pandemic.14 Clerkship students coordinated with that volunteer group to interview veterans and review their stories through the tablet devices.
From July to December 2020 medical students returned to 4-week on-site clinical rotations at the SFVAMC. The program returned to the original format for cohorts 7 to 10, with students attending in-person small group sessions and conducting in-person interviews with inpatients.
Curriculum Evaluation
Students completed surveys in the week after the curriculum concluded. Survey completion was voluntary, anonymous, and had no bearing on their evaluation or grade (pass/fail only). Likert scale questions (1, strongly disagree; 5, strongly agree) were used to assess the program (eAppendix 1). One-way analysis of variance testing was used to compare means stratified by method of interview (in person, telephone, or video). Surveys also included free-response questions asking students to highlight aspects of the program they valued or would change; responses were summarized by theme. This program evaluation was deemed exempt from review by the UCSF Human Research Protection Program Institutional Review Board.
Sixty-two veteran stories were collected by 54 participating students (one student was unable to complete an interview, while several students completed multiple interviews). Fifty-four (87%) veterans requested their stories be entered into the medical record.
All 54 students completed the survey. Students reported that the MLMS curriculum helped them develop new skills for eliciting and recording a life story (mean [SD] 4.5 [0.7]). Most students strongly agreed that MLMS helped them understand how sharing a life story can impact a veteran’s experience of receiving health care, with a mean (SD) score of 4.8 (0.4). After completing MLMS, students also reported a better understanding of the mission of the VA and veteran demographics with a mean (SD) score of 4.4 (0.7) and 4.3 (0.7), respectively. Stratification of survey responses by method of interview (in person, telephone, or video) revealed no statistically significant differences in evaluations (Table 1).
Fifty-two (96%) students provided responses to free-response survey questions. Students reported that they valued shifting the focus of an interview from medical history to rapport-building and patient engagement, having protected time to focus on the humanistic aspect of doctoring, and redefining healing as a process that occurs in the greater context of a patient’s life. One student reported, “We talk so much about seeing the person instead of the disease, but this is the first time that I really felt like I had the opportunity to wholeheartedly commit myself to that. It was an incredible opportunity and something I wish all medical trainees would have the chance to do.” Another student, after participating in the video version of the project, reported, “I found so much comfort in the time that I just sat and listened to another person’s story firsthand. Not only did this opportunity remind me of why I wanted to work in medicine, but also why I wanted to work with and for other people.” Thirty-three (61%) students provided constructive feedback in response to a free-response question soliciting suggestions for improvement, which guided iterative programmatic changes. For example, 3 students who completed the telephone iteration of MLMS felt that patient engagement suffered due to the lack of nonverbal cues and body language that can enhance the bond between storyteller and interviewer. This prompted a switch to video interviews beginning with the fourth cohort.
The second small group session provided space for students to reflect on their experience. During this session, students frequently referenced the unique connections they developed with veterans. Several students described feeling refreshed by these connections and that MLMS helped them recall their original commitment to become physicians. Students also discovered that the events veterans included in their stories often echoed current societal issues. For example, as social unrest and protests related to racial injustice occurred in the summer of 2020, veterans’ life stories more frequently incorporated examples of prejudice or inequities in the justice system. As the use of force by police moved to the forefront of political discourse, life stories more often included veterans’ experiences working as military and nonmilitary law enforcement. In identifying these common themes, students reported a greater appreciation of the impact of society on patients’ overall health and well-being.
Stories were recorded as CPRS notes titled “My Story,” and completion of a note generated a “My Story” alert on the CPRS landing page at the SFVAMC (eAppendix 2). Physicians and nurses who have discovered the notes reported that patient care has been enhanced by the contextualization provided by a life story. HCPs now frequently contact MLMS instructors inquiring whether students are available to collect life stories for their patients. One physician wrote, “I learned so much from what you documented—much more than I could appreciate in my clinic visits with him. His voice comes shining through. Thank you for highlighting the humanism of medicine in the medical record.” Another physician noted, “The story captured his voice so well. I reread it over the weekend after I got the news that he died, and it helped me celebrate his life. Please tell your students how much their work means to patients, families, and the providers who care for them.”
Discussion
Previous research has demonstrated that a narrative medicine curriculum can help medicine clerkship students develop narrative competence through patient storytelling with a focus on a patient’s illness narrative.15 The VA MLMS program extends the patient narrative beyond health care–related experiences and encompasses their broader life story. This article adds to the MLMS and narrative medicine literature by demonstrating that the efficacy of teaching patient-centered care to medical trainees through direct interviews can be maintained in remote formats.9 The article also provides guidance for MLMS programs that wish to conduct remote veteran interviews.
The widespread adoption of telemedicine will require trainees to develop communication skills to establish therapeutic relationships with patients both face-to-face and through videoconferencing. In order to promote this important skill across varying levels of physical distancing, narrative medicine programs should be adaptable to a virtual learning environment. As we redesigned MLMS for the remote setting, we learned several key lessons that can guide similar curricular and programmatic innovations at other institutions. For example, videoconferencing created stronger connections between the students and veterans than telephone calls. However, tablet-based video interviews also introduced many technological challenges and required on-site personnel (nurses and volunteers) to connect students, veterans, and technology. Solutions for technology and communication challenges related to the basic personnel and infrastructure needed to start and maintain a remote MLMS program are outlined in Table 2.
We are now using this experience to guide the expansion of life story curricula to other affiliated clerkship sites and other medical student rotations. We also are expanding the interviewer pool beyond medical students to VA staff and volunteers, some of whom may be restricted from direct patient contact in the future but who could participate through the remote protocols that we developed.
Limitations
Limitations of this study include the participation of trainees from a single institution and a lack of assessment of the impact of MLMS on veterans. Future research could assess whether life story skills and practices are maintained after the medicine clerkship. In addition, future studies could examine veterans’ perspectives through interviews with qualitative analysis to learn how MLMS affected their experience of receiving health care.
Conclusions
This is the first report of a remote-capable life story curriculum for medical students. Shifting to a virtual MLMS curriculum requires protocols and people to link interviewers, veterans, and technology. Training for in-person interactions while being prepared for remote interviewing is essential to ensure that the MLMS experience remains available to interviewers and veterans who otherwise may never have the chance to connect. The restrictions and isolation of the COVID-19 pandemic will fade, but using MLMS to virtually connect patients, providers, and students will remain an important capability and opportunity as health care shifts to more virtual interaction.
Acknowledgments
The authors thank Emma Levine, MD, for her assistance coordinating video interviews; Thor Ringler, MS, MFA, for his assistance with manuscript review; and the veterans of the San Francisco VA Health Care System for sharing their stories.
1. Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
2. Milota MM, van Thiel GJMW, van Delden JJM. Narrative medicine as a medical education tool: a systematic review. Med Teach. 2019;41(7):802-810. doi:10.1080/0142159X.2019.1584274
3. Garrison D, Lyness JM, Frank JB, Epstein RM. Qualitative analysis of medical student impressions of a narrative exercise in the third-year psychiatry clerkship. Acad Med. 2011;86(1):85-89. doi:10.1097/ACM.0b013e3181ff7a63
4. Divinsky M. Stories for life: introduction to narrative medicine. Can Fam Physician. 2007;53(2):203-211.
5. McAdams DP, McLean KC. Narrative identity. Curr Dir Psychol Sci. 2013;22(3):233-238. doi:10.1177 /0963721413475622
6. Fitchett G, Emanuel L, Handzo G, Boyken L, Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. Published 2015 Mar 21. doi:10.1186/s12904-015-0007-1
7. Ringler T, Ahearn EP, Wise M, Lee ER, Krahn D. Using life stories to connect veterans and providers. Fed Pract. 2015;32(6):8-14.
8. Roberts TJ, Ringler T, Krahn D, Ahearn E. The My Life, My Story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
9. Nathan S, Fiore LL, Saunders S, et al. My Life, My Story: Teaching patient centered care competencies for older adults through life story work [published online ahead of print, 2019 Sep 9] [published correction appears in Gerontol Geriatr Educ. 2019 Oct 15;:1]. Gerontol Geriatr Educ. 2019;1-14. doi:10.1080/02701960.2019.1665038
10. Dorsey ER, Topol EJ. Telemedicine 2020 and the next decade. Lancet. 2020;395(10227):859. doi:10.1016/S0140-6736(20)30424-4
11. Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Nov 13;69(45):1711]. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. Published 2020 Oct 30. doi:10.15585/mmwr.mm6943a3
12. Caputo LV. Across the Street. The VA philosophy: with Dr. Goldberg. July 14, 2019. Accessed November 5, 2021. https://soundcloud.com/user-911014559/the-va-philosophy-with-dr-goldberg-1
13. Sable-Smith B. Storytelling helps hospital staff discover the person within the patient. NPR. Published June 8, 2019. Accessed November 5, 2021. https://www.npr.org/sections/health-shots/2019/06/08/729351842/storytelling-helps-hospital-staff-discover-the-person-within-the-patient
14. Ganeshan S, Hsiang E, Peng T, et al. Enabling patient communication for hospitalised patients during and beyond the COVID-19 pandemic. BMJ Innov. 2021;7(2):316-320. doi:10.1136/bmjinnov-2020-000636
15. Chretien KC, Swenson R, Yoon B, et al. Tell me your story: a pilot narrative medicine curriculum during the medicine clerkship. J Gen Intern Med. 2015;30(7):1025-1028. doi:10.1007/s11606-015-3211-z
Narrative competence is the ability to acquire, interpret, and act on the stories of others.1 Developing this skill through guided medical storytelling can improve health care practitioners’ (HCPs) sense of empathy and satisfaction with their work.2 Narrative medicine experiences for medical students can foster a deeper understanding of their patients beyond illness-associated identities.3
Within narrative medicine, the “life story” is a specific technique that allows patients to share experiences through open-ended interviews that are entered into the health record.4,5 By sharing life stories, patients control a narrative encompassing more than their illness and can reinforce a sense of purpose in their lives.6 The US Department of Veterans Affairs (VA) My Life My Story (MLMS) program gives veterans the opportunity to share their narrative with staff and volunteer interviewers. MLMS is well received by veterans, has durable positive effects for HCPs who read the stories, and has been used as a tool to teach patient-centered care to medical trainees.7-9
We created a narrative medicine curriculum at the San Francisco VA Medical Center (SFVAMC) in which medical students interviewed veterans for the MLMS program. Medical students initially collected life stories through in-person conversation. During the COVID-19 pandemic, physical distancing regulations limited direct patient interaction for students and prompted a switch to phone and video interviews. This shift paralleled the widespread adoption of telehealth, which will persist beyond the pandemic and require teachers and learners to develop competency in forming personal connections with patients through videoconferencing.10,11
There are no published studies describing how to guide medical students (or other historians) in generating life stories without in-person patient contact. This article details the design of a medical student curriculum incorporating MLMS and the transition to remote interaction between instructors, students, and veterans during the early COVID-19 pandemic.
MLMS Program Origins
The MLMS project began at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, in 2013 with staff and volunteer interviewers and has expanded to more than 60 VA facilities.7 In January 2020, we initiated a narrative medicine curriculum incorporating MLMS at the SFVAMC as a required component of a third-year internal medicine clerkship for medical students at the University of California San Francisco (UCSF). Fifty-four medical students in 10 cohorts participated in the curriculum in 2020. The primary program objectives were for medical students to develop skills for eliciting and recording a life story and to appreciate the impact of this activity on a veteran’s experience of receiving health care. Secondary objectives were for students to understand the mission of the VA health care system and veteran demographics.
The first cohort of 6 UCSF medical students participated in MLMS during their 8-week VA clerkship. Students attended a 1-hour small group session to introduce the program and build narrative medicine skills. Preparation for this session involved listening to 2 podcast episodes introducing the VA health care system and MLMS.12,13 The session began with a short interactive discussion of veteran demographics with an emphasis on addressing assumptions students might have about the veteran population. Students were taught strategies for engaging in open-ended conversations without emphasizing illness. Each student practiced collecting a life story with a simulated patient portrayed by an instructor and received feedback from classmates and instructors.
Over the following weeks, students selected a hospitalized veteran, typically a patient they were caring for, introduced MLMS, and obtained verbal consent to participate. They conducted a 60- to 90-minute interview, wrote and organized the life story, read it to the veteran, and solicited edits. Once a final version was generated, the student provided the veteran with printed copies and offered to place the story in the Computerized Patient Record System (CPRS).
Near the end of their rotation, students attended a 1-hour small group session in which they shared reflections on the experience of collecting a life story, the impact of veterans’ life experiences on their health and illness, and moments when students confronted their own stereotypes and implicit biases. Students then reviewed narrative medicine skills that are generalizable to all patient interactions.
COVID-19-Related Adaptation
In March 2020, shortly after the second student cohort began, medical students were removed from the clinical setting in response to the COVID-19 pandemic. The 8-week clerkship was converted to a 3-week remote learning rotation. The MLMS experience was preserved by converting small group sessions to videoconferences and expanding the pool of eligible patients to include veterans who students had met on prior rotations, current inpatients, and outpatients from VA primary care clinics. Students contacted veterans after an instructor had introduced MLMS to the veteran and confirmed that the veteran was interested in participating.
Students in the second and third cohorts completed a telephone-based iteration of MLMS in which interviews and life story reviews were conducted over the telephone and printed copies mailed to the veteran. For the fourth, fifth, and sixth cohorts, MLMS was transitioned to a video-based program with inpatients. Instructors collaborated with a volunteer group supplying tablet devices to inpatients to make video calls to their families during the pandemic.14 Clerkship students coordinated with that volunteer group to interview veterans and review their stories through the tablet devices.
From July to December 2020 medical students returned to 4-week on-site clinical rotations at the SFVAMC. The program returned to the original format for cohorts 7 to 10, with students attending in-person small group sessions and conducting in-person interviews with inpatients.
Curriculum Evaluation
Students completed surveys in the week after the curriculum concluded. Survey completion was voluntary, anonymous, and had no bearing on their evaluation or grade (pass/fail only). Likert scale questions (1, strongly disagree; 5, strongly agree) were used to assess the program (eAppendix 1). One-way analysis of variance testing was used to compare means stratified by method of interview (in person, telephone, or video). Surveys also included free-response questions asking students to highlight aspects of the program they valued or would change; responses were summarized by theme. This program evaluation was deemed exempt from review by the UCSF Human Research Protection Program Institutional Review Board.
Sixty-two veteran stories were collected by 54 participating students (one student was unable to complete an interview, while several students completed multiple interviews). Fifty-four (87%) veterans requested their stories be entered into the medical record.
All 54 students completed the survey. Students reported that the MLMS curriculum helped them develop new skills for eliciting and recording a life story (mean [SD] 4.5 [0.7]). Most students strongly agreed that MLMS helped them understand how sharing a life story can impact a veteran’s experience of receiving health care, with a mean (SD) score of 4.8 (0.4). After completing MLMS, students also reported a better understanding of the mission of the VA and veteran demographics with a mean (SD) score of 4.4 (0.7) and 4.3 (0.7), respectively. Stratification of survey responses by method of interview (in person, telephone, or video) revealed no statistically significant differences in evaluations (Table 1).
Fifty-two (96%) students provided responses to free-response survey questions. Students reported that they valued shifting the focus of an interview from medical history to rapport-building and patient engagement, having protected time to focus on the humanistic aspect of doctoring, and redefining healing as a process that occurs in the greater context of a patient’s life. One student reported, “We talk so much about seeing the person instead of the disease, but this is the first time that I really felt like I had the opportunity to wholeheartedly commit myself to that. It was an incredible opportunity and something I wish all medical trainees would have the chance to do.” Another student, after participating in the video version of the project, reported, “I found so much comfort in the time that I just sat and listened to another person’s story firsthand. Not only did this opportunity remind me of why I wanted to work in medicine, but also why I wanted to work with and for other people.” Thirty-three (61%) students provided constructive feedback in response to a free-response question soliciting suggestions for improvement, which guided iterative programmatic changes. For example, 3 students who completed the telephone iteration of MLMS felt that patient engagement suffered due to the lack of nonverbal cues and body language that can enhance the bond between storyteller and interviewer. This prompted a switch to video interviews beginning with the fourth cohort.
The second small group session provided space for students to reflect on their experience. During this session, students frequently referenced the unique connections they developed with veterans. Several students described feeling refreshed by these connections and that MLMS helped them recall their original commitment to become physicians. Students also discovered that the events veterans included in their stories often echoed current societal issues. For example, as social unrest and protests related to racial injustice occurred in the summer of 2020, veterans’ life stories more frequently incorporated examples of prejudice or inequities in the justice system. As the use of force by police moved to the forefront of political discourse, life stories more often included veterans’ experiences working as military and nonmilitary law enforcement. In identifying these common themes, students reported a greater appreciation of the impact of society on patients’ overall health and well-being.
Stories were recorded as CPRS notes titled “My Story,” and completion of a note generated a “My Story” alert on the CPRS landing page at the SFVAMC (eAppendix 2). Physicians and nurses who have discovered the notes reported that patient care has been enhanced by the contextualization provided by a life story. HCPs now frequently contact MLMS instructors inquiring whether students are available to collect life stories for their patients. One physician wrote, “I learned so much from what you documented—much more than I could appreciate in my clinic visits with him. His voice comes shining through. Thank you for highlighting the humanism of medicine in the medical record.” Another physician noted, “The story captured his voice so well. I reread it over the weekend after I got the news that he died, and it helped me celebrate his life. Please tell your students how much their work means to patients, families, and the providers who care for them.”
Discussion
Previous research has demonstrated that a narrative medicine curriculum can help medicine clerkship students develop narrative competence through patient storytelling with a focus on a patient’s illness narrative.15 The VA MLMS program extends the patient narrative beyond health care–related experiences and encompasses their broader life story. This article adds to the MLMS and narrative medicine literature by demonstrating that the efficacy of teaching patient-centered care to medical trainees through direct interviews can be maintained in remote formats.9 The article also provides guidance for MLMS programs that wish to conduct remote veteran interviews.
The widespread adoption of telemedicine will require trainees to develop communication skills to establish therapeutic relationships with patients both face-to-face and through videoconferencing. In order to promote this important skill across varying levels of physical distancing, narrative medicine programs should be adaptable to a virtual learning environment. As we redesigned MLMS for the remote setting, we learned several key lessons that can guide similar curricular and programmatic innovations at other institutions. For example, videoconferencing created stronger connections between the students and veterans than telephone calls. However, tablet-based video interviews also introduced many technological challenges and required on-site personnel (nurses and volunteers) to connect students, veterans, and technology. Solutions for technology and communication challenges related to the basic personnel and infrastructure needed to start and maintain a remote MLMS program are outlined in Table 2.
We are now using this experience to guide the expansion of life story curricula to other affiliated clerkship sites and other medical student rotations. We also are expanding the interviewer pool beyond medical students to VA staff and volunteers, some of whom may be restricted from direct patient contact in the future but who could participate through the remote protocols that we developed.
Limitations
Limitations of this study include the participation of trainees from a single institution and a lack of assessment of the impact of MLMS on veterans. Future research could assess whether life story skills and practices are maintained after the medicine clerkship. In addition, future studies could examine veterans’ perspectives through interviews with qualitative analysis to learn how MLMS affected their experience of receiving health care.
Conclusions
This is the first report of a remote-capable life story curriculum for medical students. Shifting to a virtual MLMS curriculum requires protocols and people to link interviewers, veterans, and technology. Training for in-person interactions while being prepared for remote interviewing is essential to ensure that the MLMS experience remains available to interviewers and veterans who otherwise may never have the chance to connect. The restrictions and isolation of the COVID-19 pandemic will fade, but using MLMS to virtually connect patients, providers, and students will remain an important capability and opportunity as health care shifts to more virtual interaction.
Acknowledgments
The authors thank Emma Levine, MD, for her assistance coordinating video interviews; Thor Ringler, MS, MFA, for his assistance with manuscript review; and the veterans of the San Francisco VA Health Care System for sharing their stories.
Narrative competence is the ability to acquire, interpret, and act on the stories of others.1 Developing this skill through guided medical storytelling can improve health care practitioners’ (HCPs) sense of empathy and satisfaction with their work.2 Narrative medicine experiences for medical students can foster a deeper understanding of their patients beyond illness-associated identities.3
Within narrative medicine, the “life story” is a specific technique that allows patients to share experiences through open-ended interviews that are entered into the health record.4,5 By sharing life stories, patients control a narrative encompassing more than their illness and can reinforce a sense of purpose in their lives.6 The US Department of Veterans Affairs (VA) My Life My Story (MLMS) program gives veterans the opportunity to share their narrative with staff and volunteer interviewers. MLMS is well received by veterans, has durable positive effects for HCPs who read the stories, and has been used as a tool to teach patient-centered care to medical trainees.7-9
We created a narrative medicine curriculum at the San Francisco VA Medical Center (SFVAMC) in which medical students interviewed veterans for the MLMS program. Medical students initially collected life stories through in-person conversation. During the COVID-19 pandemic, physical distancing regulations limited direct patient interaction for students and prompted a switch to phone and video interviews. This shift paralleled the widespread adoption of telehealth, which will persist beyond the pandemic and require teachers and learners to develop competency in forming personal connections with patients through videoconferencing.10,11
There are no published studies describing how to guide medical students (or other historians) in generating life stories without in-person patient contact. This article details the design of a medical student curriculum incorporating MLMS and the transition to remote interaction between instructors, students, and veterans during the early COVID-19 pandemic.
MLMS Program Origins
The MLMS project began at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, in 2013 with staff and volunteer interviewers and has expanded to more than 60 VA facilities.7 In January 2020, we initiated a narrative medicine curriculum incorporating MLMS at the SFVAMC as a required component of a third-year internal medicine clerkship for medical students at the University of California San Francisco (UCSF). Fifty-four medical students in 10 cohorts participated in the curriculum in 2020. The primary program objectives were for medical students to develop skills for eliciting and recording a life story and to appreciate the impact of this activity on a veteran’s experience of receiving health care. Secondary objectives were for students to understand the mission of the VA health care system and veteran demographics.
The first cohort of 6 UCSF medical students participated in MLMS during their 8-week VA clerkship. Students attended a 1-hour small group session to introduce the program and build narrative medicine skills. Preparation for this session involved listening to 2 podcast episodes introducing the VA health care system and MLMS.12,13 The session began with a short interactive discussion of veteran demographics with an emphasis on addressing assumptions students might have about the veteran population. Students were taught strategies for engaging in open-ended conversations without emphasizing illness. Each student practiced collecting a life story with a simulated patient portrayed by an instructor and received feedback from classmates and instructors.
Over the following weeks, students selected a hospitalized veteran, typically a patient they were caring for, introduced MLMS, and obtained verbal consent to participate. They conducted a 60- to 90-minute interview, wrote and organized the life story, read it to the veteran, and solicited edits. Once a final version was generated, the student provided the veteran with printed copies and offered to place the story in the Computerized Patient Record System (CPRS).
Near the end of their rotation, students attended a 1-hour small group session in which they shared reflections on the experience of collecting a life story, the impact of veterans’ life experiences on their health and illness, and moments when students confronted their own stereotypes and implicit biases. Students then reviewed narrative medicine skills that are generalizable to all patient interactions.
COVID-19-Related Adaptation
In March 2020, shortly after the second student cohort began, medical students were removed from the clinical setting in response to the COVID-19 pandemic. The 8-week clerkship was converted to a 3-week remote learning rotation. The MLMS experience was preserved by converting small group sessions to videoconferences and expanding the pool of eligible patients to include veterans who students had met on prior rotations, current inpatients, and outpatients from VA primary care clinics. Students contacted veterans after an instructor had introduced MLMS to the veteran and confirmed that the veteran was interested in participating.
Students in the second and third cohorts completed a telephone-based iteration of MLMS in which interviews and life story reviews were conducted over the telephone and printed copies mailed to the veteran. For the fourth, fifth, and sixth cohorts, MLMS was transitioned to a video-based program with inpatients. Instructors collaborated with a volunteer group supplying tablet devices to inpatients to make video calls to their families during the pandemic.14 Clerkship students coordinated with that volunteer group to interview veterans and review their stories through the tablet devices.
From July to December 2020 medical students returned to 4-week on-site clinical rotations at the SFVAMC. The program returned to the original format for cohorts 7 to 10, with students attending in-person small group sessions and conducting in-person interviews with inpatients.
Curriculum Evaluation
Students completed surveys in the week after the curriculum concluded. Survey completion was voluntary, anonymous, and had no bearing on their evaluation or grade (pass/fail only). Likert scale questions (1, strongly disagree; 5, strongly agree) were used to assess the program (eAppendix 1). One-way analysis of variance testing was used to compare means stratified by method of interview (in person, telephone, or video). Surveys also included free-response questions asking students to highlight aspects of the program they valued or would change; responses were summarized by theme. This program evaluation was deemed exempt from review by the UCSF Human Research Protection Program Institutional Review Board.
Sixty-two veteran stories were collected by 54 participating students (one student was unable to complete an interview, while several students completed multiple interviews). Fifty-four (87%) veterans requested their stories be entered into the medical record.
All 54 students completed the survey. Students reported that the MLMS curriculum helped them develop new skills for eliciting and recording a life story (mean [SD] 4.5 [0.7]). Most students strongly agreed that MLMS helped them understand how sharing a life story can impact a veteran’s experience of receiving health care, with a mean (SD) score of 4.8 (0.4). After completing MLMS, students also reported a better understanding of the mission of the VA and veteran demographics with a mean (SD) score of 4.4 (0.7) and 4.3 (0.7), respectively. Stratification of survey responses by method of interview (in person, telephone, or video) revealed no statistically significant differences in evaluations (Table 1).
Fifty-two (96%) students provided responses to free-response survey questions. Students reported that they valued shifting the focus of an interview from medical history to rapport-building and patient engagement, having protected time to focus on the humanistic aspect of doctoring, and redefining healing as a process that occurs in the greater context of a patient’s life. One student reported, “We talk so much about seeing the person instead of the disease, but this is the first time that I really felt like I had the opportunity to wholeheartedly commit myself to that. It was an incredible opportunity and something I wish all medical trainees would have the chance to do.” Another student, after participating in the video version of the project, reported, “I found so much comfort in the time that I just sat and listened to another person’s story firsthand. Not only did this opportunity remind me of why I wanted to work in medicine, but also why I wanted to work with and for other people.” Thirty-three (61%) students provided constructive feedback in response to a free-response question soliciting suggestions for improvement, which guided iterative programmatic changes. For example, 3 students who completed the telephone iteration of MLMS felt that patient engagement suffered due to the lack of nonverbal cues and body language that can enhance the bond between storyteller and interviewer. This prompted a switch to video interviews beginning with the fourth cohort.
The second small group session provided space for students to reflect on their experience. During this session, students frequently referenced the unique connections they developed with veterans. Several students described feeling refreshed by these connections and that MLMS helped them recall their original commitment to become physicians. Students also discovered that the events veterans included in their stories often echoed current societal issues. For example, as social unrest and protests related to racial injustice occurred in the summer of 2020, veterans’ life stories more frequently incorporated examples of prejudice or inequities in the justice system. As the use of force by police moved to the forefront of political discourse, life stories more often included veterans’ experiences working as military and nonmilitary law enforcement. In identifying these common themes, students reported a greater appreciation of the impact of society on patients’ overall health and well-being.
Stories were recorded as CPRS notes titled “My Story,” and completion of a note generated a “My Story” alert on the CPRS landing page at the SFVAMC (eAppendix 2). Physicians and nurses who have discovered the notes reported that patient care has been enhanced by the contextualization provided by a life story. HCPs now frequently contact MLMS instructors inquiring whether students are available to collect life stories for their patients. One physician wrote, “I learned so much from what you documented—much more than I could appreciate in my clinic visits with him. His voice comes shining through. Thank you for highlighting the humanism of medicine in the medical record.” Another physician noted, “The story captured his voice so well. I reread it over the weekend after I got the news that he died, and it helped me celebrate his life. Please tell your students how much their work means to patients, families, and the providers who care for them.”
Discussion
Previous research has demonstrated that a narrative medicine curriculum can help medicine clerkship students develop narrative competence through patient storytelling with a focus on a patient’s illness narrative.15 The VA MLMS program extends the patient narrative beyond health care–related experiences and encompasses their broader life story. This article adds to the MLMS and narrative medicine literature by demonstrating that the efficacy of teaching patient-centered care to medical trainees through direct interviews can be maintained in remote formats.9 The article also provides guidance for MLMS programs that wish to conduct remote veteran interviews.
The widespread adoption of telemedicine will require trainees to develop communication skills to establish therapeutic relationships with patients both face-to-face and through videoconferencing. In order to promote this important skill across varying levels of physical distancing, narrative medicine programs should be adaptable to a virtual learning environment. As we redesigned MLMS for the remote setting, we learned several key lessons that can guide similar curricular and programmatic innovations at other institutions. For example, videoconferencing created stronger connections between the students and veterans than telephone calls. However, tablet-based video interviews also introduced many technological challenges and required on-site personnel (nurses and volunteers) to connect students, veterans, and technology. Solutions for technology and communication challenges related to the basic personnel and infrastructure needed to start and maintain a remote MLMS program are outlined in Table 2.
We are now using this experience to guide the expansion of life story curricula to other affiliated clerkship sites and other medical student rotations. We also are expanding the interviewer pool beyond medical students to VA staff and volunteers, some of whom may be restricted from direct patient contact in the future but who could participate through the remote protocols that we developed.
Limitations
Limitations of this study include the participation of trainees from a single institution and a lack of assessment of the impact of MLMS on veterans. Future research could assess whether life story skills and practices are maintained after the medicine clerkship. In addition, future studies could examine veterans’ perspectives through interviews with qualitative analysis to learn how MLMS affected their experience of receiving health care.
Conclusions
This is the first report of a remote-capable life story curriculum for medical students. Shifting to a virtual MLMS curriculum requires protocols and people to link interviewers, veterans, and technology. Training for in-person interactions while being prepared for remote interviewing is essential to ensure that the MLMS experience remains available to interviewers and veterans who otherwise may never have the chance to connect. The restrictions and isolation of the COVID-19 pandemic will fade, but using MLMS to virtually connect patients, providers, and students will remain an important capability and opportunity as health care shifts to more virtual interaction.
Acknowledgments
The authors thank Emma Levine, MD, for her assistance coordinating video interviews; Thor Ringler, MS, MFA, for his assistance with manuscript review; and the veterans of the San Francisco VA Health Care System for sharing their stories.
1. Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
2. Milota MM, van Thiel GJMW, van Delden JJM. Narrative medicine as a medical education tool: a systematic review. Med Teach. 2019;41(7):802-810. doi:10.1080/0142159X.2019.1584274
3. Garrison D, Lyness JM, Frank JB, Epstein RM. Qualitative analysis of medical student impressions of a narrative exercise in the third-year psychiatry clerkship. Acad Med. 2011;86(1):85-89. doi:10.1097/ACM.0b013e3181ff7a63
4. Divinsky M. Stories for life: introduction to narrative medicine. Can Fam Physician. 2007;53(2):203-211.
5. McAdams DP, McLean KC. Narrative identity. Curr Dir Psychol Sci. 2013;22(3):233-238. doi:10.1177 /0963721413475622
6. Fitchett G, Emanuel L, Handzo G, Boyken L, Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. Published 2015 Mar 21. doi:10.1186/s12904-015-0007-1
7. Ringler T, Ahearn EP, Wise M, Lee ER, Krahn D. Using life stories to connect veterans and providers. Fed Pract. 2015;32(6):8-14.
8. Roberts TJ, Ringler T, Krahn D, Ahearn E. The My Life, My Story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
9. Nathan S, Fiore LL, Saunders S, et al. My Life, My Story: Teaching patient centered care competencies for older adults through life story work [published online ahead of print, 2019 Sep 9] [published correction appears in Gerontol Geriatr Educ. 2019 Oct 15;:1]. Gerontol Geriatr Educ. 2019;1-14. doi:10.1080/02701960.2019.1665038
10. Dorsey ER, Topol EJ. Telemedicine 2020 and the next decade. Lancet. 2020;395(10227):859. doi:10.1016/S0140-6736(20)30424-4
11. Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Nov 13;69(45):1711]. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. Published 2020 Oct 30. doi:10.15585/mmwr.mm6943a3
12. Caputo LV. Across the Street. The VA philosophy: with Dr. Goldberg. July 14, 2019. Accessed November 5, 2021. https://soundcloud.com/user-911014559/the-va-philosophy-with-dr-goldberg-1
13. Sable-Smith B. Storytelling helps hospital staff discover the person within the patient. NPR. Published June 8, 2019. Accessed November 5, 2021. https://www.npr.org/sections/health-shots/2019/06/08/729351842/storytelling-helps-hospital-staff-discover-the-person-within-the-patient
14. Ganeshan S, Hsiang E, Peng T, et al. Enabling patient communication for hospitalised patients during and beyond the COVID-19 pandemic. BMJ Innov. 2021;7(2):316-320. doi:10.1136/bmjinnov-2020-000636
15. Chretien KC, Swenson R, Yoon B, et al. Tell me your story: a pilot narrative medicine curriculum during the medicine clerkship. J Gen Intern Med. 2015;30(7):1025-1028. doi:10.1007/s11606-015-3211-z
1. Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
2. Milota MM, van Thiel GJMW, van Delden JJM. Narrative medicine as a medical education tool: a systematic review. Med Teach. 2019;41(7):802-810. doi:10.1080/0142159X.2019.1584274
3. Garrison D, Lyness JM, Frank JB, Epstein RM. Qualitative analysis of medical student impressions of a narrative exercise in the third-year psychiatry clerkship. Acad Med. 2011;86(1):85-89. doi:10.1097/ACM.0b013e3181ff7a63
4. Divinsky M. Stories for life: introduction to narrative medicine. Can Fam Physician. 2007;53(2):203-211.
5. McAdams DP, McLean KC. Narrative identity. Curr Dir Psychol Sci. 2013;22(3):233-238. doi:10.1177 /0963721413475622
6. Fitchett G, Emanuel L, Handzo G, Boyken L, Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. Published 2015 Mar 21. doi:10.1186/s12904-015-0007-1
7. Ringler T, Ahearn EP, Wise M, Lee ER, Krahn D. Using life stories to connect veterans and providers. Fed Pract. 2015;32(6):8-14.
8. Roberts TJ, Ringler T, Krahn D, Ahearn E. The My Life, My Story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
9. Nathan S, Fiore LL, Saunders S, et al. My Life, My Story: Teaching patient centered care competencies for older adults through life story work [published online ahead of print, 2019 Sep 9] [published correction appears in Gerontol Geriatr Educ. 2019 Oct 15;:1]. Gerontol Geriatr Educ. 2019;1-14. doi:10.1080/02701960.2019.1665038
10. Dorsey ER, Topol EJ. Telemedicine 2020 and the next decade. Lancet. 2020;395(10227):859. doi:10.1016/S0140-6736(20)30424-4
11. Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Nov 13;69(45):1711]. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. Published 2020 Oct 30. doi:10.15585/mmwr.mm6943a3
12. Caputo LV. Across the Street. The VA philosophy: with Dr. Goldberg. July 14, 2019. Accessed November 5, 2021. https://soundcloud.com/user-911014559/the-va-philosophy-with-dr-goldberg-1
13. Sable-Smith B. Storytelling helps hospital staff discover the person within the patient. NPR. Published June 8, 2019. Accessed November 5, 2021. https://www.npr.org/sections/health-shots/2019/06/08/729351842/storytelling-helps-hospital-staff-discover-the-person-within-the-patient
14. Ganeshan S, Hsiang E, Peng T, et al. Enabling patient communication for hospitalised patients during and beyond the COVID-19 pandemic. BMJ Innov. 2021;7(2):316-320. doi:10.1136/bmjinnov-2020-000636
15. Chretien KC, Swenson R, Yoon B, et al. Tell me your story: a pilot narrative medicine curriculum during the medicine clerkship. J Gen Intern Med. 2015;30(7):1025-1028. doi:10.1007/s11606-015-3211-z
A Facility-Wide Plan to Increase Access to Medication for Opioid Use Disorder in Primary Care and General Mental Health Settings
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
Evaluating the Impact of a Simulated Hypersensitivity Reaction Case Study for New Fellows and Chemotherapy Nurses in an Outpatient Infusion Clinic
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers