User login
Flu Vaccines to Change After COVID Kills Off One Strain of Virus
The flu vaccine currently in use targets two A strains and two B strains. But the Yamagata/B subtype, which was already in decline, has not been detected worldwide since March 2020, the FDA said. Social distancing and other precautions used to avoid COVID apparently finished it off.
In response to that change, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted on March 5 to recommend the three-strain flu shot.
VRBPAC recommended the egg-based flu vaccines contain an A/Victoria/4897/2022 (H1N1)pdm09-like virus, an A/Thailand/8/2022 (H3N2)-like virus; and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The committee recommended the cell- or recombinant-based flu vaccines contain an A/Wisconsin/67/2022 (H1N1)pdm09-like virus; an A/Massachusetts/18/2022 (H3N2)-like virus; and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The move is no surprise. The World Health Organization and FDA experts had been recommending the change since last year.
Jerry Weir, MD, director of the FDA’s Division of Viral Products, said companies that make flu vaccines should have the trivalent shot ready for the 2024-2025 flu season.
“Each of the U.S. influenza vaccine manufacturers have submitted updated regulatory files related to a trivalent influenza vaccine, and approval of all the necessary regulatory submissions is on track for 2024-25,” he said during the advisory committee’s meeting, according to CNN.
“FDA anticipates that there will be an adequate and diverse supply of approved trivalent seasonal influenza vaccines for the United States in the coming season,” the agency said.
U.S. flu vaccine manufacturers will still make a four-strain vaccine for distribution to overseas markets, CNN said.
A version of this article appeared on WebMD.com.
The flu vaccine currently in use targets two A strains and two B strains. But the Yamagata/B subtype, which was already in decline, has not been detected worldwide since March 2020, the FDA said. Social distancing and other precautions used to avoid COVID apparently finished it off.
In response to that change, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted on March 5 to recommend the three-strain flu shot.
VRBPAC recommended the egg-based flu vaccines contain an A/Victoria/4897/2022 (H1N1)pdm09-like virus, an A/Thailand/8/2022 (H3N2)-like virus; and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The committee recommended the cell- or recombinant-based flu vaccines contain an A/Wisconsin/67/2022 (H1N1)pdm09-like virus; an A/Massachusetts/18/2022 (H3N2)-like virus; and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The move is no surprise. The World Health Organization and FDA experts had been recommending the change since last year.
Jerry Weir, MD, director of the FDA’s Division of Viral Products, said companies that make flu vaccines should have the trivalent shot ready for the 2024-2025 flu season.
“Each of the U.S. influenza vaccine manufacturers have submitted updated regulatory files related to a trivalent influenza vaccine, and approval of all the necessary regulatory submissions is on track for 2024-25,” he said during the advisory committee’s meeting, according to CNN.
“FDA anticipates that there will be an adequate and diverse supply of approved trivalent seasonal influenza vaccines for the United States in the coming season,” the agency said.
U.S. flu vaccine manufacturers will still make a four-strain vaccine for distribution to overseas markets, CNN said.
A version of this article appeared on WebMD.com.
The flu vaccine currently in use targets two A strains and two B strains. But the Yamagata/B subtype, which was already in decline, has not been detected worldwide since March 2020, the FDA said. Social distancing and other precautions used to avoid COVID apparently finished it off.
In response to that change, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted on March 5 to recommend the three-strain flu shot.
VRBPAC recommended the egg-based flu vaccines contain an A/Victoria/4897/2022 (H1N1)pdm09-like virus, an A/Thailand/8/2022 (H3N2)-like virus; and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The committee recommended the cell- or recombinant-based flu vaccines contain an A/Wisconsin/67/2022 (H1N1)pdm09-like virus; an A/Massachusetts/18/2022 (H3N2)-like virus; and a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The move is no surprise. The World Health Organization and FDA experts had been recommending the change since last year.
Jerry Weir, MD, director of the FDA’s Division of Viral Products, said companies that make flu vaccines should have the trivalent shot ready for the 2024-2025 flu season.
“Each of the U.S. influenza vaccine manufacturers have submitted updated regulatory files related to a trivalent influenza vaccine, and approval of all the necessary regulatory submissions is on track for 2024-25,” he said during the advisory committee’s meeting, according to CNN.
“FDA anticipates that there will be an adequate and diverse supply of approved trivalent seasonal influenza vaccines for the United States in the coming season,” the agency said.
U.S. flu vaccine manufacturers will still make a four-strain vaccine for distribution to overseas markets, CNN said.
A version of this article appeared on WebMD.com.
Doxy-PEP Cut STIs in San Francisco in Half
Syphilis and chlamydia infections were reduced by half among men who have sex with men and transgender women 1 year after San Francisco rolled out doxycycline postexposure prophylaxis (doxy-PEP), according to data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) this week.
After a clinical trial showed that doxy-PEP taken after sex reduced the chance of acquiring syphilis, gonorrhea, and chlamydia by about two-thirds, the San Francisco Department of Public Health released the first guidelines in the country in October 2022.
So far, more than 3700 people in San Francisco have been prescribed doxy-PEP, reports Stephanie Cohen, MD, director of HIV and sexually transmitted infection (STI) prevention in the Disease Prevention and Control Branch of Public Health.
Dr. Cohen and her colleagues spent a year monitoring the uptake of doxy-PEP and used a computer model to predict what the rates of sexually transmitted infection would have been without doxy-PEP.
In November 2023, 13 months after the guidelines were introduced, they found that monthly chlamydia and early syphilis infections were 50% and 51% lower, respectively, than what was predicted by the model.
Fewer Infections
The drop in infections is having a tangible effect on patients in San Francisco, and many clinicians are noting that they are seeing far fewer positive tests. “The results that we’re seeing on a city-wide level are absolutely being experienced by individual providers and patients,” Dr. Cohen said.
However, the analysis showed no effect on rates of gonorrhea. It’s not clear why, although Dr. Cohen points out that doxy-PEP was less effective against gonorrhea in the clinical trial. And “there could be other factors in play,” she added. “Adherence might matter more, or it could be affected by the prevalence of tetracycline resistance in the community.”
With rates of STIs, particularly syphilis, quickly rising in recent years, healthcare providers have been scrambling to find effective interventions. So far, doxy-PEP has shown the most promise. “We’ve known for a while that all of the strategies we’ve been employing don’t seem to be working,” noted Chase Cannon, MD, an infectious disease specialist at the University of Washington in Seattle. “That’s why doxy-PEP is important. We haven’t had anything that can deflect the curve in a long time.”
What About the Side Effects?
Some concerns remain, however, about the widespread prophylactic use of antibiotics. There are no long-term safety data on the potential side effects of doxy-PEP, and there is still a lot of stigma around interventions that allow people to have sex the way they want, said Dr. Cannon.
But perhaps, the biggest concern is that doxy-PEP could contribute to antibiotic resistance. Those fears are not misplaced, Dr. Cannon added. The results of one study, presented in a poster at CROI, showed that stool samples from people prescribed doxy-PEP had elevated levels of bacterial genes that can confer resistance to tetracyclines, the class of antibiotics to which doxycycline belongs. There was no change in resistance to other classes of antibiotics and no difference in bacterial diversity over the 6 months of the study.
Dr. Cannon cautioned, however, that we can’t extrapolate these results to clinical outcomes. “We can look for signals [of resistance], but we don’t know if this means someone will fail therapy for chlamydia or syphilis,” he said.
There are still many challenges to overcome before doxy-PEP can be rolled out widely, Dr. Cohen explained. There is a lack of consensus among healthcare professionals about who should be offered doxy-PEP. The clinical trial results and the San Fransisco guidelines only apply to men who have sex with men and to transgender women.
Some clinicians argue that the intervention should be provided to a broader population, whereas others want to see more research to ensure that unnecessary antibiotic use is minimized.
So far just one study has tested doxy-PEP in another population — in women in Kenya — and it was found to not be effective. But the data suggest that adherence to the protocol was poor in that study, so the results may not be reliable, Dr. Cohen said.
“We need effective prevention tools for all genders, especially cis women who bear most of the morbidity,” she said. “It stands to reason that this should work for them, but without high-quality evidence, there is insufficient information to make a recommendation for cis women.”
The US Centers for Disease Control and Prevention is currently reviewing public and expert comments and refining final guidelines for release in the coming months, which should alleviate some of the uncertainty. “Many providers are waiting for that guidance before they will feel confident moving forward,” Dr. Cohen noted.
But despite the risks and uncertainty, doxy-PEP looks set to be a major part of the fight against STIs going forward. “Doxy-PEP is essential for us as a nation to be dealing with the syphilis epidemic,” Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Disease, said in a video introduction to CROI.
A version of this article appeared on Medscape.com.
Syphilis and chlamydia infections were reduced by half among men who have sex with men and transgender women 1 year after San Francisco rolled out doxycycline postexposure prophylaxis (doxy-PEP), according to data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) this week.
After a clinical trial showed that doxy-PEP taken after sex reduced the chance of acquiring syphilis, gonorrhea, and chlamydia by about two-thirds, the San Francisco Department of Public Health released the first guidelines in the country in October 2022.
So far, more than 3700 people in San Francisco have been prescribed doxy-PEP, reports Stephanie Cohen, MD, director of HIV and sexually transmitted infection (STI) prevention in the Disease Prevention and Control Branch of Public Health.
Dr. Cohen and her colleagues spent a year monitoring the uptake of doxy-PEP and used a computer model to predict what the rates of sexually transmitted infection would have been without doxy-PEP.
In November 2023, 13 months after the guidelines were introduced, they found that monthly chlamydia and early syphilis infections were 50% and 51% lower, respectively, than what was predicted by the model.
Fewer Infections
The drop in infections is having a tangible effect on patients in San Francisco, and many clinicians are noting that they are seeing far fewer positive tests. “The results that we’re seeing on a city-wide level are absolutely being experienced by individual providers and patients,” Dr. Cohen said.
However, the analysis showed no effect on rates of gonorrhea. It’s not clear why, although Dr. Cohen points out that doxy-PEP was less effective against gonorrhea in the clinical trial. And “there could be other factors in play,” she added. “Adherence might matter more, or it could be affected by the prevalence of tetracycline resistance in the community.”
With rates of STIs, particularly syphilis, quickly rising in recent years, healthcare providers have been scrambling to find effective interventions. So far, doxy-PEP has shown the most promise. “We’ve known for a while that all of the strategies we’ve been employing don’t seem to be working,” noted Chase Cannon, MD, an infectious disease specialist at the University of Washington in Seattle. “That’s why doxy-PEP is important. We haven’t had anything that can deflect the curve in a long time.”
What About the Side Effects?
Some concerns remain, however, about the widespread prophylactic use of antibiotics. There are no long-term safety data on the potential side effects of doxy-PEP, and there is still a lot of stigma around interventions that allow people to have sex the way they want, said Dr. Cannon.
But perhaps, the biggest concern is that doxy-PEP could contribute to antibiotic resistance. Those fears are not misplaced, Dr. Cannon added. The results of one study, presented in a poster at CROI, showed that stool samples from people prescribed doxy-PEP had elevated levels of bacterial genes that can confer resistance to tetracyclines, the class of antibiotics to which doxycycline belongs. There was no change in resistance to other classes of antibiotics and no difference in bacterial diversity over the 6 months of the study.
Dr. Cannon cautioned, however, that we can’t extrapolate these results to clinical outcomes. “We can look for signals [of resistance], but we don’t know if this means someone will fail therapy for chlamydia or syphilis,” he said.
There are still many challenges to overcome before doxy-PEP can be rolled out widely, Dr. Cohen explained. There is a lack of consensus among healthcare professionals about who should be offered doxy-PEP. The clinical trial results and the San Fransisco guidelines only apply to men who have sex with men and to transgender women.
Some clinicians argue that the intervention should be provided to a broader population, whereas others want to see more research to ensure that unnecessary antibiotic use is minimized.
So far just one study has tested doxy-PEP in another population — in women in Kenya — and it was found to not be effective. But the data suggest that adherence to the protocol was poor in that study, so the results may not be reliable, Dr. Cohen said.
“We need effective prevention tools for all genders, especially cis women who bear most of the morbidity,” she said. “It stands to reason that this should work for them, but without high-quality evidence, there is insufficient information to make a recommendation for cis women.”
The US Centers for Disease Control and Prevention is currently reviewing public and expert comments and refining final guidelines for release in the coming months, which should alleviate some of the uncertainty. “Many providers are waiting for that guidance before they will feel confident moving forward,” Dr. Cohen noted.
But despite the risks and uncertainty, doxy-PEP looks set to be a major part of the fight against STIs going forward. “Doxy-PEP is essential for us as a nation to be dealing with the syphilis epidemic,” Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Disease, said in a video introduction to CROI.
A version of this article appeared on Medscape.com.
Syphilis and chlamydia infections were reduced by half among men who have sex with men and transgender women 1 year after San Francisco rolled out doxycycline postexposure prophylaxis (doxy-PEP), according to data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) this week.
After a clinical trial showed that doxy-PEP taken after sex reduced the chance of acquiring syphilis, gonorrhea, and chlamydia by about two-thirds, the San Francisco Department of Public Health released the first guidelines in the country in October 2022.
So far, more than 3700 people in San Francisco have been prescribed doxy-PEP, reports Stephanie Cohen, MD, director of HIV and sexually transmitted infection (STI) prevention in the Disease Prevention and Control Branch of Public Health.
Dr. Cohen and her colleagues spent a year monitoring the uptake of doxy-PEP and used a computer model to predict what the rates of sexually transmitted infection would have been without doxy-PEP.
In November 2023, 13 months after the guidelines were introduced, they found that monthly chlamydia and early syphilis infections were 50% and 51% lower, respectively, than what was predicted by the model.
Fewer Infections
The drop in infections is having a tangible effect on patients in San Francisco, and many clinicians are noting that they are seeing far fewer positive tests. “The results that we’re seeing on a city-wide level are absolutely being experienced by individual providers and patients,” Dr. Cohen said.
However, the analysis showed no effect on rates of gonorrhea. It’s not clear why, although Dr. Cohen points out that doxy-PEP was less effective against gonorrhea in the clinical trial. And “there could be other factors in play,” she added. “Adherence might matter more, or it could be affected by the prevalence of tetracycline resistance in the community.”
With rates of STIs, particularly syphilis, quickly rising in recent years, healthcare providers have been scrambling to find effective interventions. So far, doxy-PEP has shown the most promise. “We’ve known for a while that all of the strategies we’ve been employing don’t seem to be working,” noted Chase Cannon, MD, an infectious disease specialist at the University of Washington in Seattle. “That’s why doxy-PEP is important. We haven’t had anything that can deflect the curve in a long time.”
What About the Side Effects?
Some concerns remain, however, about the widespread prophylactic use of antibiotics. There are no long-term safety data on the potential side effects of doxy-PEP, and there is still a lot of stigma around interventions that allow people to have sex the way they want, said Dr. Cannon.
But perhaps, the biggest concern is that doxy-PEP could contribute to antibiotic resistance. Those fears are not misplaced, Dr. Cannon added. The results of one study, presented in a poster at CROI, showed that stool samples from people prescribed doxy-PEP had elevated levels of bacterial genes that can confer resistance to tetracyclines, the class of antibiotics to which doxycycline belongs. There was no change in resistance to other classes of antibiotics and no difference in bacterial diversity over the 6 months of the study.
Dr. Cannon cautioned, however, that we can’t extrapolate these results to clinical outcomes. “We can look for signals [of resistance], but we don’t know if this means someone will fail therapy for chlamydia or syphilis,” he said.
There are still many challenges to overcome before doxy-PEP can be rolled out widely, Dr. Cohen explained. There is a lack of consensus among healthcare professionals about who should be offered doxy-PEP. The clinical trial results and the San Fransisco guidelines only apply to men who have sex with men and to transgender women.
Some clinicians argue that the intervention should be provided to a broader population, whereas others want to see more research to ensure that unnecessary antibiotic use is minimized.
So far just one study has tested doxy-PEP in another population — in women in Kenya — and it was found to not be effective. But the data suggest that adherence to the protocol was poor in that study, so the results may not be reliable, Dr. Cohen said.
“We need effective prevention tools for all genders, especially cis women who bear most of the morbidity,” she said. “It stands to reason that this should work for them, but without high-quality evidence, there is insufficient information to make a recommendation for cis women.”
The US Centers for Disease Control and Prevention is currently reviewing public and expert comments and refining final guidelines for release in the coming months, which should alleviate some of the uncertainty. “Many providers are waiting for that guidance before they will feel confident moving forward,” Dr. Cohen noted.
But despite the risks and uncertainty, doxy-PEP looks set to be a major part of the fight against STIs going forward. “Doxy-PEP is essential for us as a nation to be dealing with the syphilis epidemic,” Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Disease, said in a video introduction to CROI.
A version of this article appeared on Medscape.com.
Outside the Guidelines: Denosumab Overuse in Prostate Cancer
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Omalizumab for Food Allergies: What PCPs Should Know
Sandra Hong, MD, chair of allergy and immunology and director of the Food Allergy Center of Excellence at Cleveland Clinic, in Ohio, sees firsthand how situations that feel ordinary to most people strike fear in the hearts of her patients with food allergies.
Not only do some experience reactions to milk when they eat a cheese pizza — they can’t be in the same room with someone enjoying a slice nearby. “That would be terrifying,” Dr. Hong said.
Omalizumab (Xolair), recently approved by the US Food and Drug Administration as monotherapy for the treatment of food allergies, may now bring peace of mind to these patients and their families by reducing their risk of dangerous allergic reactions to accidental exposure.
While the drug does not cure food allergies, a phase 3, placebo-controlled trial found that after 16 weeks of treatment, two thirds of participants were able to tolerate at least 600 mg of peanut protein — equal to about 2.5 peanuts — without experiencing moderate to severe reactions.
An open-label extension trial also found the monoclonal antibody reduced the likelihood of serious reactions to eggs by 67%, milk by 66%, and cashews by 42%. The results of the study were published in The New England Journal of Medicine.
The treatment is approved for children as young as the age of 1 year and is the only treatment approved for multiple food allergies. It does not treat anaphylaxis or other emergency situations.
Patient Selection Key
While 8% of children and 10% of adults in the United States have a true food allergy, Brian Vickery, MD, chief of allergy and immunology and director of the Food Allergy Center at Children’s Healthcare of Atlanta, noted that a significantly higher proportion of the population restricts their diet based on perceived food intolerances.
“Most important for family doctors prior to prescribing the medication will be to be sure that the diagnosis is correct,” Kim said. “We know that allergy blood and skin testing is good but not perfect, and false positive results can occur,” said Edwin Kim, MD, chief of the Division of Pediatric Allergy and Immunology and director of the University of North Carolina Food Allergy Initiative at the University of North Carolina School of Medicine, Chapel Hill, who was a coauthor on the study in the New England Journal of Medicine. “ An allergist can conduct food challenges to confirm the diagnosis if results are unclear.”
Even for patients with confirmed IgE-mediated allergies, Dr. Hong said selecting patients who are good candidates for the therapy has “nuances.”
Patients must be willing and able to commit to injections every 2-4 weeks. Dosing depends on body weight and the total IgE levels of each patient. Patients with IgE levels > 1850 UI/mL likely will be disqualified from treatment since the clinical trial did not enroll patients with total IgE above this level and the appropriate dose in those patients is unknown.
“My recommendation for family physicians who are counseling food-allergic patients interested in omalizumab treatment is to partner with an allergist-immunologist, if at all possible,” Dr. Vickery said. He added that patients should have a comprehensive workup before beginning treatment because starting omalizumab would reduce reactivity and alter the outcome a diagnostic oral food challenge.
Two populations Dr. Hong thinks might particularly benefit from the therapy are college students and preschoolers, who may be unable to completely avoid allergens because of poor impulse control and food sharing in group settings.
“The concerns we have about this age group are whether or not there might be other factors involved that may impede their ability to make good decisions.”
Less control of the environment in dorms or other group living situations also could increase the risk of accidental exposure to a food allergen.
For the right patients, the treatment regimen has significant advantages over oral immunotherapy treatment (OIT), including the fact that it’s not a daily medication and it has the potential to treat allergic asthma at the same time.
“The biggest pro for omalizumab is that it can treat all of your food allergies, whether you have one or many, and do it all in one medication,” Dr. Kim said.
Managing Potential Harms
Omalizumab carries risks both primary care providers and patients must consider. First among them is that the drug carries a “black box” warning for an increased risk of anaphylaxis, Dr. Hong said.
Although patients with multiple food allergies typically already have prescriptions for epinephrine, primary care physicians (PCPs) considering offering omalizumab must be comfortable treating severe systemic reactions and their offices capable of post-dose monitoring, Dr. Hong said.
Anaphylaxis “can occur after the first dose or it can be delayed,” she said. “Typically, allergists will give these in our offices and we’ll actually have people wait for delayed amounts of time, for hours.”
The drug has been available since 2003 as a treatment for allergic asthma and urticaria. In addition to the warning for anaphylaxis, common reactions include joint pain and injection-site reactions. It also increases the risk for parasitic infection, and some studies show an increase in the risk for cancer.
Still, Dr. Kim said omalizumab’s safety profile is reassuring and noted it has advantages over OIT. “Since the patient is not exposing themselves to the food they are allergic to like in OIT, the safety is expected to be far better,” he said.
Lifelong Treatment
Dr. Vickery, Dr. Hong, and Dr. Kim all cautioned that patients should understand that, while omalizumab offers protection against accidental exposure and can meaningfully improve quality of life, it won’t allow them to loosen their allergen-avoidant diets.
Further, maintaining protection requires receiving injections every 2-4 weeks for life. For those without insurance, or whose insurance does not cover the treatment, costs could reach thousands of dollars each month, Dr. Hong said.
Omalizumab “has been well covered by insurance for asthma and chronic hives, but we will have to see what it looks like for food allergy. The range of plans and out-of-pocket deductibles available to patients will also play a big role,” Dr. Kim said.
Other novel approaches to food allergies are currently in clinical trials, and both Dr. Hong and Dr. Vickery are optimistic about potential options in the pipeline.
“We’re just on the brink of really exciting therapies coming forward in the future,” Dr. Hong said.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health; the Claudia and Steve Stange Family Fund; Genentech; and Novartis. Dr. Hong, Dr. Kim, and Dr. Vickery reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sandra Hong, MD, chair of allergy and immunology and director of the Food Allergy Center of Excellence at Cleveland Clinic, in Ohio, sees firsthand how situations that feel ordinary to most people strike fear in the hearts of her patients with food allergies.
Not only do some experience reactions to milk when they eat a cheese pizza — they can’t be in the same room with someone enjoying a slice nearby. “That would be terrifying,” Dr. Hong said.
Omalizumab (Xolair), recently approved by the US Food and Drug Administration as monotherapy for the treatment of food allergies, may now bring peace of mind to these patients and their families by reducing their risk of dangerous allergic reactions to accidental exposure.
While the drug does not cure food allergies, a phase 3, placebo-controlled trial found that after 16 weeks of treatment, two thirds of participants were able to tolerate at least 600 mg of peanut protein — equal to about 2.5 peanuts — without experiencing moderate to severe reactions.
An open-label extension trial also found the monoclonal antibody reduced the likelihood of serious reactions to eggs by 67%, milk by 66%, and cashews by 42%. The results of the study were published in The New England Journal of Medicine.
The treatment is approved for children as young as the age of 1 year and is the only treatment approved for multiple food allergies. It does not treat anaphylaxis or other emergency situations.
Patient Selection Key
While 8% of children and 10% of adults in the United States have a true food allergy, Brian Vickery, MD, chief of allergy and immunology and director of the Food Allergy Center at Children’s Healthcare of Atlanta, noted that a significantly higher proportion of the population restricts their diet based on perceived food intolerances.
“Most important for family doctors prior to prescribing the medication will be to be sure that the diagnosis is correct,” Kim said. “We know that allergy blood and skin testing is good but not perfect, and false positive results can occur,” said Edwin Kim, MD, chief of the Division of Pediatric Allergy and Immunology and director of the University of North Carolina Food Allergy Initiative at the University of North Carolina School of Medicine, Chapel Hill, who was a coauthor on the study in the New England Journal of Medicine. “ An allergist can conduct food challenges to confirm the diagnosis if results are unclear.”
Even for patients with confirmed IgE-mediated allergies, Dr. Hong said selecting patients who are good candidates for the therapy has “nuances.”
Patients must be willing and able to commit to injections every 2-4 weeks. Dosing depends on body weight and the total IgE levels of each patient. Patients with IgE levels > 1850 UI/mL likely will be disqualified from treatment since the clinical trial did not enroll patients with total IgE above this level and the appropriate dose in those patients is unknown.
“My recommendation for family physicians who are counseling food-allergic patients interested in omalizumab treatment is to partner with an allergist-immunologist, if at all possible,” Dr. Vickery said. He added that patients should have a comprehensive workup before beginning treatment because starting omalizumab would reduce reactivity and alter the outcome a diagnostic oral food challenge.
Two populations Dr. Hong thinks might particularly benefit from the therapy are college students and preschoolers, who may be unable to completely avoid allergens because of poor impulse control and food sharing in group settings.
“The concerns we have about this age group are whether or not there might be other factors involved that may impede their ability to make good decisions.”
Less control of the environment in dorms or other group living situations also could increase the risk of accidental exposure to a food allergen.
For the right patients, the treatment regimen has significant advantages over oral immunotherapy treatment (OIT), including the fact that it’s not a daily medication and it has the potential to treat allergic asthma at the same time.
“The biggest pro for omalizumab is that it can treat all of your food allergies, whether you have one or many, and do it all in one medication,” Dr. Kim said.
Managing Potential Harms
Omalizumab carries risks both primary care providers and patients must consider. First among them is that the drug carries a “black box” warning for an increased risk of anaphylaxis, Dr. Hong said.
Although patients with multiple food allergies typically already have prescriptions for epinephrine, primary care physicians (PCPs) considering offering omalizumab must be comfortable treating severe systemic reactions and their offices capable of post-dose monitoring, Dr. Hong said.
Anaphylaxis “can occur after the first dose or it can be delayed,” she said. “Typically, allergists will give these in our offices and we’ll actually have people wait for delayed amounts of time, for hours.”
The drug has been available since 2003 as a treatment for allergic asthma and urticaria. In addition to the warning for anaphylaxis, common reactions include joint pain and injection-site reactions. It also increases the risk for parasitic infection, and some studies show an increase in the risk for cancer.
Still, Dr. Kim said omalizumab’s safety profile is reassuring and noted it has advantages over OIT. “Since the patient is not exposing themselves to the food they are allergic to like in OIT, the safety is expected to be far better,” he said.
Lifelong Treatment
Dr. Vickery, Dr. Hong, and Dr. Kim all cautioned that patients should understand that, while omalizumab offers protection against accidental exposure and can meaningfully improve quality of life, it won’t allow them to loosen their allergen-avoidant diets.
Further, maintaining protection requires receiving injections every 2-4 weeks for life. For those without insurance, or whose insurance does not cover the treatment, costs could reach thousands of dollars each month, Dr. Hong said.
Omalizumab “has been well covered by insurance for asthma and chronic hives, but we will have to see what it looks like for food allergy. The range of plans and out-of-pocket deductibles available to patients will also play a big role,” Dr. Kim said.
Other novel approaches to food allergies are currently in clinical trials, and both Dr. Hong and Dr. Vickery are optimistic about potential options in the pipeline.
“We’re just on the brink of really exciting therapies coming forward in the future,” Dr. Hong said.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health; the Claudia and Steve Stange Family Fund; Genentech; and Novartis. Dr. Hong, Dr. Kim, and Dr. Vickery reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sandra Hong, MD, chair of allergy and immunology and director of the Food Allergy Center of Excellence at Cleveland Clinic, in Ohio, sees firsthand how situations that feel ordinary to most people strike fear in the hearts of her patients with food allergies.
Not only do some experience reactions to milk when they eat a cheese pizza — they can’t be in the same room with someone enjoying a slice nearby. “That would be terrifying,” Dr. Hong said.
Omalizumab (Xolair), recently approved by the US Food and Drug Administration as monotherapy for the treatment of food allergies, may now bring peace of mind to these patients and their families by reducing their risk of dangerous allergic reactions to accidental exposure.
While the drug does not cure food allergies, a phase 3, placebo-controlled trial found that after 16 weeks of treatment, two thirds of participants were able to tolerate at least 600 mg of peanut protein — equal to about 2.5 peanuts — without experiencing moderate to severe reactions.
An open-label extension trial also found the monoclonal antibody reduced the likelihood of serious reactions to eggs by 67%, milk by 66%, and cashews by 42%. The results of the study were published in The New England Journal of Medicine.
The treatment is approved for children as young as the age of 1 year and is the only treatment approved for multiple food allergies. It does not treat anaphylaxis or other emergency situations.
Patient Selection Key
While 8% of children and 10% of adults in the United States have a true food allergy, Brian Vickery, MD, chief of allergy and immunology and director of the Food Allergy Center at Children’s Healthcare of Atlanta, noted that a significantly higher proportion of the population restricts their diet based on perceived food intolerances.
“Most important for family doctors prior to prescribing the medication will be to be sure that the diagnosis is correct,” Kim said. “We know that allergy blood and skin testing is good but not perfect, and false positive results can occur,” said Edwin Kim, MD, chief of the Division of Pediatric Allergy and Immunology and director of the University of North Carolina Food Allergy Initiative at the University of North Carolina School of Medicine, Chapel Hill, who was a coauthor on the study in the New England Journal of Medicine. “ An allergist can conduct food challenges to confirm the diagnosis if results are unclear.”
Even for patients with confirmed IgE-mediated allergies, Dr. Hong said selecting patients who are good candidates for the therapy has “nuances.”
Patients must be willing and able to commit to injections every 2-4 weeks. Dosing depends on body weight and the total IgE levels of each patient. Patients with IgE levels > 1850 UI/mL likely will be disqualified from treatment since the clinical trial did not enroll patients with total IgE above this level and the appropriate dose in those patients is unknown.
“My recommendation for family physicians who are counseling food-allergic patients interested in omalizumab treatment is to partner with an allergist-immunologist, if at all possible,” Dr. Vickery said. He added that patients should have a comprehensive workup before beginning treatment because starting omalizumab would reduce reactivity and alter the outcome a diagnostic oral food challenge.
Two populations Dr. Hong thinks might particularly benefit from the therapy are college students and preschoolers, who may be unable to completely avoid allergens because of poor impulse control and food sharing in group settings.
“The concerns we have about this age group are whether or not there might be other factors involved that may impede their ability to make good decisions.”
Less control of the environment in dorms or other group living situations also could increase the risk of accidental exposure to a food allergen.
For the right patients, the treatment regimen has significant advantages over oral immunotherapy treatment (OIT), including the fact that it’s not a daily medication and it has the potential to treat allergic asthma at the same time.
“The biggest pro for omalizumab is that it can treat all of your food allergies, whether you have one or many, and do it all in one medication,” Dr. Kim said.
Managing Potential Harms
Omalizumab carries risks both primary care providers and patients must consider. First among them is that the drug carries a “black box” warning for an increased risk of anaphylaxis, Dr. Hong said.
Although patients with multiple food allergies typically already have prescriptions for epinephrine, primary care physicians (PCPs) considering offering omalizumab must be comfortable treating severe systemic reactions and their offices capable of post-dose monitoring, Dr. Hong said.
Anaphylaxis “can occur after the first dose or it can be delayed,” she said. “Typically, allergists will give these in our offices and we’ll actually have people wait for delayed amounts of time, for hours.”
The drug has been available since 2003 as a treatment for allergic asthma and urticaria. In addition to the warning for anaphylaxis, common reactions include joint pain and injection-site reactions. It also increases the risk for parasitic infection, and some studies show an increase in the risk for cancer.
Still, Dr. Kim said omalizumab’s safety profile is reassuring and noted it has advantages over OIT. “Since the patient is not exposing themselves to the food they are allergic to like in OIT, the safety is expected to be far better,” he said.
Lifelong Treatment
Dr. Vickery, Dr. Hong, and Dr. Kim all cautioned that patients should understand that, while omalizumab offers protection against accidental exposure and can meaningfully improve quality of life, it won’t allow them to loosen their allergen-avoidant diets.
Further, maintaining protection requires receiving injections every 2-4 weeks for life. For those without insurance, or whose insurance does not cover the treatment, costs could reach thousands of dollars each month, Dr. Hong said.
Omalizumab “has been well covered by insurance for asthma and chronic hives, but we will have to see what it looks like for food allergy. The range of plans and out-of-pocket deductibles available to patients will also play a big role,” Dr. Kim said.
Other novel approaches to food allergies are currently in clinical trials, and both Dr. Hong and Dr. Vickery are optimistic about potential options in the pipeline.
“We’re just on the brink of really exciting therapies coming forward in the future,” Dr. Hong said.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health; the Claudia and Steve Stange Family Fund; Genentech; and Novartis. Dr. Hong, Dr. Kim, and Dr. Vickery reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Clinical Implications of a Formulary Conversion From Budesonide/formoterol to Fluticasone/salmeterol at a VA Medical Center
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
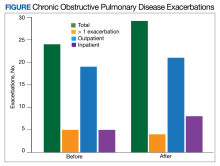
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
Bigfoot, Bermuda Triangle, ‘No Lido With Epi’?
“Fingers, toes, ears, and nose are places where epinephrine never goes,” Thomas Ehlers, DPM, wrote in Podiatry Today. “That is an adage I heard during podiatry school, my clerkships, and from various attendings throughout my training.”
But as Dr. Ehlers added, epinephrine gets a bad rap. The catchy admonition “has been proven a myth time and time again.”
So Although medical trainees across multiple disciplines are taught to fear the practice, citing the potential for gangrene, its reputation for harm is not supported by the evidence.
Lack of Feeling Doesn’t Care About Your Facts
The debate surfaced anew in response to a recent column by Kenny Lin, MD, MPH, family physician and associate director of the Lancaster General Hospital Family Medicine Residency, in Lancaster, Pennsylvania, about the rather pedestrian topic of why he no longer performs surgery to correct ingrown toenails. Dr. Lin’s admission that he used to do the procedure with a combination of epinephrine and lidocaine turned into a major focus of the comments — many of them harshly critical of the practice:
“Epinephrine is not an appropriate drug to use for podiatry or use in any peripheral area. Gangrene?” one commenter posted.
“Leave epi out of lidocaine to fingers, toes, nose, and ear lobes,” another wrote.
“No lido with epi, whether or not it is contraindicated, because: If there’s any adverse outcome, a lawyer will find plenty of references saying it was contraindicated,” a reader chimed in.
Other commenters disagreed, with one saying, “Please, folks, don’t show that you trained 50 years ago and haven’t changed practice since…”
For Dr. Lin, the response was surprising given what he believes to be the lack of evidence supporting the purported dangers.
“When I think about this, it’s something that was taught to me during residency — that they should not be used on certain areas,” Dr. Lin said. “But since then, studies have been published looking at thousands of cases of people using epinephrine with lidocaine and haven’t found any cases of necrosis.”
Many doctors, like Dr. Lin, say they were cautioned against this in their training. Others don’t remember exactly where they’ve heard it but recognize the idea has a nebulous hold on practice.
Combining epinephrine with lidocaine helps make the numbing last longer, stops bleeding, and reduces the use of lidocaine required, all of which improve the chances of an effective and comfortable intervention for the patient, Dr. Lin said. The approach also reduces the use of tourniquets, which come with their own risks including nerve injury.
However, in areas with limited circulation, this vasoconstrictive effect may be more pronounced, potentially leading to complications for patients with complicating factors.
Clinicians who regularly use the combination of epinephrine and lidocaine for surgery do concede that it can pose certain hazards and considerations in areas without robust blood flow.
But the literature largely points to its safety.
In 2001, California-based plastic and reconstructive surgeon Keith Denkler, MD, published a deep dive on the topic starting in the 19th century, including a review of Index Medicus from 1880 to 1966, a computer review of the National Library of Medicine database from 1966 to 2000, and major textbooks from 1900 to 2000.
He found a total of 48 cases of digital gangrene — but most involved the use of cocaine or procaine. Of the 48 cases, 21 involved the use of epinephrine, and 17 used an unknown concentration based on manual dilution.
“Multiple other concurrent conditions (hot soaks, tight tourniquets, and infection) existed in these case reports, making it difficult to determine the exact cause of the tissue insult,” Dr. Denkler wrote.
In a 2010 retrospective review in the Journal of the American Society of Plastic Surgeons, authors examined 1111 cases involving digital and hand surgery. Of the 611 patients who received injections of 1% lidocaine with epinephrine, none experienced digital necrosis.
Another review from 2003 touted the combination’s safety, in hopes to “help dispel the myth that epinephrine has no place in podiatric anesthesia.” But authors noted limitations of use, including “known sensitivity, thyrotoxicosis, and use of either tricyclic antidepressants or monoamine oxidase inhibitors.”
James Christina, DPM, executive director and CEO of the American Podiatric Medical Association, echoed that sentiment. He said he regularly used the combination to correct bunions, hammer toes, and ingrown toenails over his 20 years of practicing but acknowledged the technique is not appropriate for all such patients.
“There’s always been caution when using epinephrine with local anesthetic,” Dr. Christina told this news organization. “You need a healthy patient with normal circulation and no other complications; someone without vascular compromise.”
Marie Hanna, MD, MEHP, chief of regional anesthesia and acute pain management at Johns Hopkins University, Baltimore, counts herself among the cautious. Citing Principles of Office Anesthesia: Part I. Infiltrative Anesthesia, Dr. Hanna said epinephrine should never be used in digital and penile blocks or in skin flaps with marginal viability.
“It is perfectly fine in certain areas, like the wrist or the arm,” Dr. Hanna said. “But specifically for use in end organs like nose, fingers, ears, toes — all of these with tenuous blood supply — it is not good practice.”
The divide among doctors comes down to theoretical concern, rather than empirical basis, said Rebecca Johnson, MD, chair of the American Society of Anesthesiologists committee on Regional Anesthesia and Acute Pain Medicine and a faculty member at Mayo Clinic, in Rochester, Minnesota.
“It’s just one of those myths we have in practice,” she said.
And legally, Dr. Johnson noted, the mere existence of a myth can be enough of a deterrent for medical practitioners: “Like anything, when you’re trying to do the right thing, if a complication would occur for another reason, you’d want to make sure a jury of your peers didn’t bring up that myth.”
The sources in this story reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
“Fingers, toes, ears, and nose are places where epinephrine never goes,” Thomas Ehlers, DPM, wrote in Podiatry Today. “That is an adage I heard during podiatry school, my clerkships, and from various attendings throughout my training.”
But as Dr. Ehlers added, epinephrine gets a bad rap. The catchy admonition “has been proven a myth time and time again.”
So Although medical trainees across multiple disciplines are taught to fear the practice, citing the potential for gangrene, its reputation for harm is not supported by the evidence.
Lack of Feeling Doesn’t Care About Your Facts
The debate surfaced anew in response to a recent column by Kenny Lin, MD, MPH, family physician and associate director of the Lancaster General Hospital Family Medicine Residency, in Lancaster, Pennsylvania, about the rather pedestrian topic of why he no longer performs surgery to correct ingrown toenails. Dr. Lin’s admission that he used to do the procedure with a combination of epinephrine and lidocaine turned into a major focus of the comments — many of them harshly critical of the practice:
“Epinephrine is not an appropriate drug to use for podiatry or use in any peripheral area. Gangrene?” one commenter posted.
“Leave epi out of lidocaine to fingers, toes, nose, and ear lobes,” another wrote.
“No lido with epi, whether or not it is contraindicated, because: If there’s any adverse outcome, a lawyer will find plenty of references saying it was contraindicated,” a reader chimed in.
Other commenters disagreed, with one saying, “Please, folks, don’t show that you trained 50 years ago and haven’t changed practice since…”
For Dr. Lin, the response was surprising given what he believes to be the lack of evidence supporting the purported dangers.
“When I think about this, it’s something that was taught to me during residency — that they should not be used on certain areas,” Dr. Lin said. “But since then, studies have been published looking at thousands of cases of people using epinephrine with lidocaine and haven’t found any cases of necrosis.”
Many doctors, like Dr. Lin, say they were cautioned against this in their training. Others don’t remember exactly where they’ve heard it but recognize the idea has a nebulous hold on practice.
Combining epinephrine with lidocaine helps make the numbing last longer, stops bleeding, and reduces the use of lidocaine required, all of which improve the chances of an effective and comfortable intervention for the patient, Dr. Lin said. The approach also reduces the use of tourniquets, which come with their own risks including nerve injury.
However, in areas with limited circulation, this vasoconstrictive effect may be more pronounced, potentially leading to complications for patients with complicating factors.
Clinicians who regularly use the combination of epinephrine and lidocaine for surgery do concede that it can pose certain hazards and considerations in areas without robust blood flow.
But the literature largely points to its safety.
In 2001, California-based plastic and reconstructive surgeon Keith Denkler, MD, published a deep dive on the topic starting in the 19th century, including a review of Index Medicus from 1880 to 1966, a computer review of the National Library of Medicine database from 1966 to 2000, and major textbooks from 1900 to 2000.
He found a total of 48 cases of digital gangrene — but most involved the use of cocaine or procaine. Of the 48 cases, 21 involved the use of epinephrine, and 17 used an unknown concentration based on manual dilution.
“Multiple other concurrent conditions (hot soaks, tight tourniquets, and infection) existed in these case reports, making it difficult to determine the exact cause of the tissue insult,” Dr. Denkler wrote.
In a 2010 retrospective review in the Journal of the American Society of Plastic Surgeons, authors examined 1111 cases involving digital and hand surgery. Of the 611 patients who received injections of 1% lidocaine with epinephrine, none experienced digital necrosis.
Another review from 2003 touted the combination’s safety, in hopes to “help dispel the myth that epinephrine has no place in podiatric anesthesia.” But authors noted limitations of use, including “known sensitivity, thyrotoxicosis, and use of either tricyclic antidepressants or monoamine oxidase inhibitors.”
James Christina, DPM, executive director and CEO of the American Podiatric Medical Association, echoed that sentiment. He said he regularly used the combination to correct bunions, hammer toes, and ingrown toenails over his 20 years of practicing but acknowledged the technique is not appropriate for all such patients.
“There’s always been caution when using epinephrine with local anesthetic,” Dr. Christina told this news organization. “You need a healthy patient with normal circulation and no other complications; someone without vascular compromise.”
Marie Hanna, MD, MEHP, chief of regional anesthesia and acute pain management at Johns Hopkins University, Baltimore, counts herself among the cautious. Citing Principles of Office Anesthesia: Part I. Infiltrative Anesthesia, Dr. Hanna said epinephrine should never be used in digital and penile blocks or in skin flaps with marginal viability.
“It is perfectly fine in certain areas, like the wrist or the arm,” Dr. Hanna said. “But specifically for use in end organs like nose, fingers, ears, toes — all of these with tenuous blood supply — it is not good practice.”
The divide among doctors comes down to theoretical concern, rather than empirical basis, said Rebecca Johnson, MD, chair of the American Society of Anesthesiologists committee on Regional Anesthesia and Acute Pain Medicine and a faculty member at Mayo Clinic, in Rochester, Minnesota.
“It’s just one of those myths we have in practice,” she said.
And legally, Dr. Johnson noted, the mere existence of a myth can be enough of a deterrent for medical practitioners: “Like anything, when you’re trying to do the right thing, if a complication would occur for another reason, you’d want to make sure a jury of your peers didn’t bring up that myth.”
The sources in this story reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
“Fingers, toes, ears, and nose are places where epinephrine never goes,” Thomas Ehlers, DPM, wrote in Podiatry Today. “That is an adage I heard during podiatry school, my clerkships, and from various attendings throughout my training.”
But as Dr. Ehlers added, epinephrine gets a bad rap. The catchy admonition “has been proven a myth time and time again.”
So Although medical trainees across multiple disciplines are taught to fear the practice, citing the potential for gangrene, its reputation for harm is not supported by the evidence.
Lack of Feeling Doesn’t Care About Your Facts
The debate surfaced anew in response to a recent column by Kenny Lin, MD, MPH, family physician and associate director of the Lancaster General Hospital Family Medicine Residency, in Lancaster, Pennsylvania, about the rather pedestrian topic of why he no longer performs surgery to correct ingrown toenails. Dr. Lin’s admission that he used to do the procedure with a combination of epinephrine and lidocaine turned into a major focus of the comments — many of them harshly critical of the practice:
“Epinephrine is not an appropriate drug to use for podiatry or use in any peripheral area. Gangrene?” one commenter posted.
“Leave epi out of lidocaine to fingers, toes, nose, and ear lobes,” another wrote.
“No lido with epi, whether or not it is contraindicated, because: If there’s any adverse outcome, a lawyer will find plenty of references saying it was contraindicated,” a reader chimed in.
Other commenters disagreed, with one saying, “Please, folks, don’t show that you trained 50 years ago and haven’t changed practice since…”
For Dr. Lin, the response was surprising given what he believes to be the lack of evidence supporting the purported dangers.
“When I think about this, it’s something that was taught to me during residency — that they should not be used on certain areas,” Dr. Lin said. “But since then, studies have been published looking at thousands of cases of people using epinephrine with lidocaine and haven’t found any cases of necrosis.”
Many doctors, like Dr. Lin, say they were cautioned against this in their training. Others don’t remember exactly where they’ve heard it but recognize the idea has a nebulous hold on practice.
Combining epinephrine with lidocaine helps make the numbing last longer, stops bleeding, and reduces the use of lidocaine required, all of which improve the chances of an effective and comfortable intervention for the patient, Dr. Lin said. The approach also reduces the use of tourniquets, which come with their own risks including nerve injury.
However, in areas with limited circulation, this vasoconstrictive effect may be more pronounced, potentially leading to complications for patients with complicating factors.
Clinicians who regularly use the combination of epinephrine and lidocaine for surgery do concede that it can pose certain hazards and considerations in areas without robust blood flow.
But the literature largely points to its safety.
In 2001, California-based plastic and reconstructive surgeon Keith Denkler, MD, published a deep dive on the topic starting in the 19th century, including a review of Index Medicus from 1880 to 1966, a computer review of the National Library of Medicine database from 1966 to 2000, and major textbooks from 1900 to 2000.
He found a total of 48 cases of digital gangrene — but most involved the use of cocaine or procaine. Of the 48 cases, 21 involved the use of epinephrine, and 17 used an unknown concentration based on manual dilution.
“Multiple other concurrent conditions (hot soaks, tight tourniquets, and infection) existed in these case reports, making it difficult to determine the exact cause of the tissue insult,” Dr. Denkler wrote.
In a 2010 retrospective review in the Journal of the American Society of Plastic Surgeons, authors examined 1111 cases involving digital and hand surgery. Of the 611 patients who received injections of 1% lidocaine with epinephrine, none experienced digital necrosis.
Another review from 2003 touted the combination’s safety, in hopes to “help dispel the myth that epinephrine has no place in podiatric anesthesia.” But authors noted limitations of use, including “known sensitivity, thyrotoxicosis, and use of either tricyclic antidepressants or monoamine oxidase inhibitors.”
James Christina, DPM, executive director and CEO of the American Podiatric Medical Association, echoed that sentiment. He said he regularly used the combination to correct bunions, hammer toes, and ingrown toenails over his 20 years of practicing but acknowledged the technique is not appropriate for all such patients.
“There’s always been caution when using epinephrine with local anesthetic,” Dr. Christina told this news organization. “You need a healthy patient with normal circulation and no other complications; someone without vascular compromise.”
Marie Hanna, MD, MEHP, chief of regional anesthesia and acute pain management at Johns Hopkins University, Baltimore, counts herself among the cautious. Citing Principles of Office Anesthesia: Part I. Infiltrative Anesthesia, Dr. Hanna said epinephrine should never be used in digital and penile blocks or in skin flaps with marginal viability.
“It is perfectly fine in certain areas, like the wrist or the arm,” Dr. Hanna said. “But specifically for use in end organs like nose, fingers, ears, toes — all of these with tenuous blood supply — it is not good practice.”
The divide among doctors comes down to theoretical concern, rather than empirical basis, said Rebecca Johnson, MD, chair of the American Society of Anesthesiologists committee on Regional Anesthesia and Acute Pain Medicine and a faculty member at Mayo Clinic, in Rochester, Minnesota.
“It’s just one of those myths we have in practice,” she said.
And legally, Dr. Johnson noted, the mere existence of a myth can be enough of a deterrent for medical practitioners: “Like anything, when you’re trying to do the right thing, if a complication would occur for another reason, you’d want to make sure a jury of your peers didn’t bring up that myth.”
The sources in this story reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Low-Dose Aspirin Associated With Reduced CRC Risk
TOPLINE:
Low-dose aspirin use is associated with a reduced risk for colorectal cancer (CRC), confirms a large-scale cohort study, which also suggests that the risk reduction is greatest for metastatic disease and in individuals who take the drug for at least 5 years.
METHODOLOGY:
- Researchers used several population-based registries to identify individuals aged ≥ 50 years living in Norway between 2014 and 2018, excluding those with a prior history of invasive cancer or who had lived in Norway for less than 6 months before study commencement.
- Sociodemographic information was obtained, as well as low-dose aspirin prescription data to determine the prescription date, number of dispensed packages, and defined daily dose.
- Follow-up began 6 months after entering the cohort and continued until CRC diagnosis, another cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2018.
- CRC cases were categorized by site as well as by clinical stage.
TAKEAWAY:
- Of 2,186,390 individuals included, 38,577 (1.8%) were diagnosed with CRC after a median follow-up of 10.9 years. Low-dose aspirin was used at least once by 579,196 (26.5%) individuals.
- Low-dose aspirin use was more common among males, older individuals, those with a lower education or lower income, those of Norwegian origin, and individuals using other medications, including those targeting cardiovascular conditions.
- Duration of current aspirin use was also associated with the degree of CRC risk, at HRs of 0.91 for < 3 years, 0.85 for ≥ 3 and < 5 years, and 0.84 for ≥ 5 years.
- It was estimated that aspirin use averted 1073 cases of CRC over the study period.
IN PRACTICE:
“We believe that new randomized controlled trials are urgently needed to confirm the potential protective effect of aspirin against CRC and to identify subgroups in the population who might benefit the most from the use of aspirin,” the authors wrote.
SOURCE:
The research, led by Edoardo Botteri, PhD, Department of Research, Cancer Registry of Norway, National Institute of Public Health, Oslo, Norway, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study is limited by its observational nature. Users and nonusers are also “incomparable,” as aspirin is used for the primary prevention of cardiovascular events. Moreover, information was lacking in the registries about “several known risk factors for CRC,” and so the link between aspirin and CRC risk could have been over- or underestimated. Finally, the defined daily dose may not necessarily reflect the dose actually taken by the individual or how often it was taken.
DISCLOSURES:
No relevant financial relationships were declared. The study was funded by the Norwegian Research Council.
A version of this article appeared on Medscape.com.
TOPLINE:
Low-dose aspirin use is associated with a reduced risk for colorectal cancer (CRC), confirms a large-scale cohort study, which also suggests that the risk reduction is greatest for metastatic disease and in individuals who take the drug for at least 5 years.
METHODOLOGY:
- Researchers used several population-based registries to identify individuals aged ≥ 50 years living in Norway between 2014 and 2018, excluding those with a prior history of invasive cancer or who had lived in Norway for less than 6 months before study commencement.
- Sociodemographic information was obtained, as well as low-dose aspirin prescription data to determine the prescription date, number of dispensed packages, and defined daily dose.
- Follow-up began 6 months after entering the cohort and continued until CRC diagnosis, another cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2018.
- CRC cases were categorized by site as well as by clinical stage.
TAKEAWAY:
- Of 2,186,390 individuals included, 38,577 (1.8%) were diagnosed with CRC after a median follow-up of 10.9 years. Low-dose aspirin was used at least once by 579,196 (26.5%) individuals.
- Low-dose aspirin use was more common among males, older individuals, those with a lower education or lower income, those of Norwegian origin, and individuals using other medications, including those targeting cardiovascular conditions.
- Duration of current aspirin use was also associated with the degree of CRC risk, at HRs of 0.91 for < 3 years, 0.85 for ≥ 3 and < 5 years, and 0.84 for ≥ 5 years.
- It was estimated that aspirin use averted 1073 cases of CRC over the study period.
IN PRACTICE:
“We believe that new randomized controlled trials are urgently needed to confirm the potential protective effect of aspirin against CRC and to identify subgroups in the population who might benefit the most from the use of aspirin,” the authors wrote.
SOURCE:
The research, led by Edoardo Botteri, PhD, Department of Research, Cancer Registry of Norway, National Institute of Public Health, Oslo, Norway, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study is limited by its observational nature. Users and nonusers are also “incomparable,” as aspirin is used for the primary prevention of cardiovascular events. Moreover, information was lacking in the registries about “several known risk factors for CRC,” and so the link between aspirin and CRC risk could have been over- or underestimated. Finally, the defined daily dose may not necessarily reflect the dose actually taken by the individual or how often it was taken.
DISCLOSURES:
No relevant financial relationships were declared. The study was funded by the Norwegian Research Council.
A version of this article appeared on Medscape.com.
TOPLINE:
Low-dose aspirin use is associated with a reduced risk for colorectal cancer (CRC), confirms a large-scale cohort study, which also suggests that the risk reduction is greatest for metastatic disease and in individuals who take the drug for at least 5 years.
METHODOLOGY:
- Researchers used several population-based registries to identify individuals aged ≥ 50 years living in Norway between 2014 and 2018, excluding those with a prior history of invasive cancer or who had lived in Norway for less than 6 months before study commencement.
- Sociodemographic information was obtained, as well as low-dose aspirin prescription data to determine the prescription date, number of dispensed packages, and defined daily dose.
- Follow-up began 6 months after entering the cohort and continued until CRC diagnosis, another cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2018.
- CRC cases were categorized by site as well as by clinical stage.
TAKEAWAY:
- Of 2,186,390 individuals included, 38,577 (1.8%) were diagnosed with CRC after a median follow-up of 10.9 years. Low-dose aspirin was used at least once by 579,196 (26.5%) individuals.
- Low-dose aspirin use was more common among males, older individuals, those with a lower education or lower income, those of Norwegian origin, and individuals using other medications, including those targeting cardiovascular conditions.
- Duration of current aspirin use was also associated with the degree of CRC risk, at HRs of 0.91 for < 3 years, 0.85 for ≥ 3 and < 5 years, and 0.84 for ≥ 5 years.
- It was estimated that aspirin use averted 1073 cases of CRC over the study period.
IN PRACTICE:
“We believe that new randomized controlled trials are urgently needed to confirm the potential protective effect of aspirin against CRC and to identify subgroups in the population who might benefit the most from the use of aspirin,” the authors wrote.
SOURCE:
The research, led by Edoardo Botteri, PhD, Department of Research, Cancer Registry of Norway, National Institute of Public Health, Oslo, Norway, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study is limited by its observational nature. Users and nonusers are also “incomparable,” as aspirin is used for the primary prevention of cardiovascular events. Moreover, information was lacking in the registries about “several known risk factors for CRC,” and so the link between aspirin and CRC risk could have been over- or underestimated. Finally, the defined daily dose may not necessarily reflect the dose actually taken by the individual or how often it was taken.
DISCLOSURES:
No relevant financial relationships were declared. The study was funded by the Norwegian Research Council.
A version of this article appeared on Medscape.com.
Galantamine Supplements Found Mislabeled, Contaminated
TOPLINE:
Galantamine purchased as a dietary supplement may be more likely to contain bacterial contaminants and an incorrect amount of the product vs when it is prescribed as a generic drug, new research showed.
METHODOLOGY:
- Galantamine, a plant alkaloid, is approved for treating mild to moderate Alzheimer’s dementia but is also marketed as a dietary supplement for cognitive enhancement.
- In June 2023, researchers purchased all 10 galantamine dietary supplements available on Amazon.com that had a Supplement Facts panel.
- In September 2023, they acquired all 11 generic immediate-release formulations of prescription galantamine available in the United States.
- They analyzed the content of galantamine in each product using ultrahigh-performance liquid chromatography-mass spectrometry and quantified any microorganisms present.
TAKEAWAY:
- Generic galantamine drugs were found to contain 97.5%-104.2% of the labeled content, with no microbial contamination.
- , according to the authors of the study.
IN PRACTICE:
“Clinicians should query patients with memory concerns about the use of dietary supplements and advise patients not to use galantamine supplements,” the researchers wrote.
SOURCE:
The corresponding author of the study was Pieter A. Cohen, MD, with Broadway Clinic, Cambridge Health Alliance, in Somerville, Massachusetts. The paper was published online as a research letter in JAMA.
LIMITATIONS:
The products were purchased at a single point in time and may not reflect current options, the researchers noted. The generalizability of the findings to other supplement ingredients or generic drugs is unknown.
DISCLOSURES:
Dr. Cohen has received grants from the Consumers Union and PEW Charitable Trust and personal fees from UpToDate and the Centers for Disease Control and Prevention. He has been sued by a supplement company in a case where the jury found in his favor.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Galantamine purchased as a dietary supplement may be more likely to contain bacterial contaminants and an incorrect amount of the product vs when it is prescribed as a generic drug, new research showed.
METHODOLOGY:
- Galantamine, a plant alkaloid, is approved for treating mild to moderate Alzheimer’s dementia but is also marketed as a dietary supplement for cognitive enhancement.
- In June 2023, researchers purchased all 10 galantamine dietary supplements available on Amazon.com that had a Supplement Facts panel.
- In September 2023, they acquired all 11 generic immediate-release formulations of prescription galantamine available in the United States.
- They analyzed the content of galantamine in each product using ultrahigh-performance liquid chromatography-mass spectrometry and quantified any microorganisms present.
TAKEAWAY:
- Generic galantamine drugs were found to contain 97.5%-104.2% of the labeled content, with no microbial contamination.
- , according to the authors of the study.
IN PRACTICE:
“Clinicians should query patients with memory concerns about the use of dietary supplements and advise patients not to use galantamine supplements,” the researchers wrote.
SOURCE:
The corresponding author of the study was Pieter A. Cohen, MD, with Broadway Clinic, Cambridge Health Alliance, in Somerville, Massachusetts. The paper was published online as a research letter in JAMA.
LIMITATIONS:
The products were purchased at a single point in time and may not reflect current options, the researchers noted. The generalizability of the findings to other supplement ingredients or generic drugs is unknown.
DISCLOSURES:
Dr. Cohen has received grants from the Consumers Union and PEW Charitable Trust and personal fees from UpToDate and the Centers for Disease Control and Prevention. He has been sued by a supplement company in a case where the jury found in his favor.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Galantamine purchased as a dietary supplement may be more likely to contain bacterial contaminants and an incorrect amount of the product vs when it is prescribed as a generic drug, new research showed.
METHODOLOGY:
- Galantamine, a plant alkaloid, is approved for treating mild to moderate Alzheimer’s dementia but is also marketed as a dietary supplement for cognitive enhancement.
- In June 2023, researchers purchased all 10 galantamine dietary supplements available on Amazon.com that had a Supplement Facts panel.
- In September 2023, they acquired all 11 generic immediate-release formulations of prescription galantamine available in the United States.
- They analyzed the content of galantamine in each product using ultrahigh-performance liquid chromatography-mass spectrometry and quantified any microorganisms present.
TAKEAWAY:
- Generic galantamine drugs were found to contain 97.5%-104.2% of the labeled content, with no microbial contamination.
- , according to the authors of the study.
IN PRACTICE:
“Clinicians should query patients with memory concerns about the use of dietary supplements and advise patients not to use galantamine supplements,” the researchers wrote.
SOURCE:
The corresponding author of the study was Pieter A. Cohen, MD, with Broadway Clinic, Cambridge Health Alliance, in Somerville, Massachusetts. The paper was published online as a research letter in JAMA.
LIMITATIONS:
The products were purchased at a single point in time and may not reflect current options, the researchers noted. The generalizability of the findings to other supplement ingredients or generic drugs is unknown.
DISCLOSURES:
Dr. Cohen has received grants from the Consumers Union and PEW Charitable Trust and personal fees from UpToDate and the Centers for Disease Control and Prevention. He has been sued by a supplement company in a case where the jury found in his favor.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Could EHR Pharmacy Errors Put Veterans at Risk?
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Is Metformin a ‘Drug for All Diseases’?
In 2021 alone, clinicians wrote more than 91 million orders for the medication — up from 40 million 2004.
But is metformin just getting started? Emerging evidence suggests the drug may be effective for a much broader range of conditions beyond managing high blood glucose, including various cancers, obesity, liver disease, cardiovascular, neurodegenerative, and renal diseases. As the evidence for diverse uses accumulates, many trials have launched, with researchers looking to expand metformin’s indications and validate or explore new directions.
Metformin’s long history as a pharmaceutical includes an herbal ancestry, recognition in 1918 for its ability to lower blood glucose, being cast aside because of toxicity fears in the 1930s, rediscovery and synthesis in Europe in the 1940s, the first reported use for diabetes in 1957, and approval in the United States in 1994.
The drug has maintained its place as the preferred first-line treatment for type 2 diabetes since 2011, when it was first included in the World Health Organization’s essential medicines list.
“The focus hitherto has been primarily on its insulin sensitization effects,” Akshay Jain, MD, a clinical and research endocrinologist at TLC Diabetes and Endocrinology, in Surrey, British Columbia, Canada, told this news organization.
“The recent surge of renewed interest is in part related to its postulated effects on multiple other receptors,” he said. “In my mind, the metformin data on coronary artery disease reduction and cancer-protective effects have come farther along than other disease states.”
Cardiovascular Outcomes
Gregory G. Schwartz, MD, PhD, chief of the cardiology section at Rocky Mountain Regional VA Medical Center and professor of medicine at the University of Colorado School of Medicine in Aurora, is leading the VA-IMPACT trial. Despite metformin’s long history and widespread use, he said his study is the first placebo-controlled cardiovascular outcomes trial of the drug.
Launched in 2023, the study tests the hypothesis that metformin reduces the risk for death or nonfatal ischemic cardiovascular events in patients with prediabetes and established coronary, cerebrovascular, or peripheral artery disease, Dr. Schwartz said. The trial is being conducted at roughly 40 VA medical centers, with a planned enrollment of 7410 patients. The estimated completion date is March 2029.
“The principal mechanism of action of metformin is through activation of AMP [adenosine monophosphate]–activated protein kinase, a central pathway in metabolic regulation, cell protection, and survival,” Dr. Schwartz explained. “Experimental data have demonstrated attenuated development of atherosclerosis, reduced myocardial infarct size, improved endothelial function, and antiarrhythmic actions — none of those dependent on the presence of diabetes.”
Dr. Schwartz and his colleagues decided to test their hypothesis in people with prediabetes, rather than diabetes, to create a “true placebo-controlled comparison,” he said.
“If patients with type 2 diabetes had been chosen, there would be potential for confounding because a placebo group would require more treatment with other active antihyperglycemic medications to achieve the same degree of glycemic control as a metformin group,” Dr. Schwartz said.
“If proven efficacious in the VA-IMPACT trial, metformin could provide an inexpensive, generally safe, and well-tolerated approach to reduce cardiovascular morbidity and mortality in a large segment of the population,” Dr. Schwartz added. “Perhaps the old dog can learn some new tricks.”
Other recruiting trials looking at cardiovascular-related outcomes include Met-PEF, LIMIT, and Metformin as an Adjunctive Therapy to Catheter Ablation in Atrial Fibrillation.
Reducing Cancer Risks
Sai Yendamuri, MD, chair of the Department of Thoracic Surgery and director of the Thoracic Surgery Laboratory at Roswell Park Comprehensive Cancer Center in Buffalo, New York, is leading a phase 2 trial exploring whether metformin can prevent lung cancer in people with overweight or obesity who are at a high risk for the malignancy.
The study, which has accrued about 60% of its estimated enrollment, also will assess whether metformin can reprogram participants’ immune systems, with a view toward reducing the activity of regulatory T cells that are linked to development of tumors.
“In our preclinical and retrospective clinical data, we found that metformin had anticancer effects but only if the patients were overweight,” Dr. Yendamuri said. “In mice, we find that obesity increases regulatory T-cell function, which suppresses the immune system of the lungs. This effect is reversed by metformin.” The team is conducting the current study to examine if this happens in patients, as well. Results are expected next year.
Research is underway in other tumor types, including oral and endometrial, and brain cancers.
Preventing Alzheimer’s Disease
Cognitive function — or at least delaying its erosion — represents another front for metformin. José A. Luchsinger, MD, MPH, vice-chair for clinical and epidemiological research and director of the section on geriatrics, gerontology, and aging at Columbia University Irving Medical Center in New York City, is heading a phase 2/3 randomized controlled trial assessing the ability of the drug to prevent Alzheimer›s disease.
The study investigators hope to enroll 326 men and women aged 55-90 years with early and late mild cognitive impairment, overweight or obesity, and no diabetes.
“The hypothesis is that improving insulin and glucose levels can lead to lowering the risk of Alzheimer’s disease,” Dr. Luchsinger said. Recruitment should be complete by the end of 2024 and results are expected in late 2026.
Similar studies are underway in Europe and Asia.
Other areas of investigation, while tantalizing, are mostly in early stages, although bolstered by preclinical and mechanistic studies. The authors of a recent review on the potential mechanisms of action of metformin and existing evidence of the drug›s effectiveness — or lack thereof — in treating diseases other than diabetes, wrote: “Collectively, these data raise the question: Is metformin a drug for all diseases? It remains unclear as to whether all of these putative beneficial effects are secondary to its actions as an antihyperglycemic and insulin-sensitizing drug, or result from other cellular actions, including inhibition of mTOR (mammalian target for rapamycin), or direct antiviral actions.”
Off-Label Uses
Metformin currently is approved by the US Food and Drug Administration only for the treatment of type 2 diabetes, although it is also the only antidiabetic medication for prediabetes currently recommended by the American Diabetes Association.
Some studies currently are looking at its use in a variety of off-label indications, including obesity, gestational diabetes, weight gain from antipsychotics, and polycystic ovary syndrome.
For the most part, metformin is considered a safe drug, but it is not risk-free, Dr. Jain cautioned.
“Although it would certainly be helpful to see if this inexpensive medication that’s universally available can help in disease states, one shouldn’t overlook the potential risk of adverse effects, such as gastrointestinal, potential vitamin B12 deficiency, blunting of skeletal muscle development and the rare risk of lactic acidosis in those with kidney impairment,” he said.
“Similarly, with recent reports of the carcinogenic potential of certain formulations of long-acting metformin that contained NDMA [N-nitrosodimethylamine], it would be imperative that these kinks are removed before we incorporate metformin as the gift that keeps giving.”
Dr. Jain reported financial relationships with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk. Dr. Yendamuri disclosed serving on the scientific advisory board member of Karkinos Healthcare and research funding from Lumeda for the metformin study. Dr. Luchsinger reported receiving donated metformin and matching placebo from EMD Serono, a subsidiary of Merck, for the MAP study. Dr. Schwartz received research support from the US Department of Veterans Affairs as National Chair of the VA-IMPACT trial.
A version of this article appeared on Medscape.com.
In 2021 alone, clinicians wrote more than 91 million orders for the medication — up from 40 million 2004.
But is metformin just getting started? Emerging evidence suggests the drug may be effective for a much broader range of conditions beyond managing high blood glucose, including various cancers, obesity, liver disease, cardiovascular, neurodegenerative, and renal diseases. As the evidence for diverse uses accumulates, many trials have launched, with researchers looking to expand metformin’s indications and validate or explore new directions.
Metformin’s long history as a pharmaceutical includes an herbal ancestry, recognition in 1918 for its ability to lower blood glucose, being cast aside because of toxicity fears in the 1930s, rediscovery and synthesis in Europe in the 1940s, the first reported use for diabetes in 1957, and approval in the United States in 1994.
The drug has maintained its place as the preferred first-line treatment for type 2 diabetes since 2011, when it was first included in the World Health Organization’s essential medicines list.
“The focus hitherto has been primarily on its insulin sensitization effects,” Akshay Jain, MD, a clinical and research endocrinologist at TLC Diabetes and Endocrinology, in Surrey, British Columbia, Canada, told this news organization.
“The recent surge of renewed interest is in part related to its postulated effects on multiple other receptors,” he said. “In my mind, the metformin data on coronary artery disease reduction and cancer-protective effects have come farther along than other disease states.”
Cardiovascular Outcomes
Gregory G. Schwartz, MD, PhD, chief of the cardiology section at Rocky Mountain Regional VA Medical Center and professor of medicine at the University of Colorado School of Medicine in Aurora, is leading the VA-IMPACT trial. Despite metformin’s long history and widespread use, he said his study is the first placebo-controlled cardiovascular outcomes trial of the drug.
Launched in 2023, the study tests the hypothesis that metformin reduces the risk for death or nonfatal ischemic cardiovascular events in patients with prediabetes and established coronary, cerebrovascular, or peripheral artery disease, Dr. Schwartz said. The trial is being conducted at roughly 40 VA medical centers, with a planned enrollment of 7410 patients. The estimated completion date is March 2029.
“The principal mechanism of action of metformin is through activation of AMP [adenosine monophosphate]–activated protein kinase, a central pathway in metabolic regulation, cell protection, and survival,” Dr. Schwartz explained. “Experimental data have demonstrated attenuated development of atherosclerosis, reduced myocardial infarct size, improved endothelial function, and antiarrhythmic actions — none of those dependent on the presence of diabetes.”
Dr. Schwartz and his colleagues decided to test their hypothesis in people with prediabetes, rather than diabetes, to create a “true placebo-controlled comparison,” he said.
“If patients with type 2 diabetes had been chosen, there would be potential for confounding because a placebo group would require more treatment with other active antihyperglycemic medications to achieve the same degree of glycemic control as a metformin group,” Dr. Schwartz said.
“If proven efficacious in the VA-IMPACT trial, metformin could provide an inexpensive, generally safe, and well-tolerated approach to reduce cardiovascular morbidity and mortality in a large segment of the population,” Dr. Schwartz added. “Perhaps the old dog can learn some new tricks.”
Other recruiting trials looking at cardiovascular-related outcomes include Met-PEF, LIMIT, and Metformin as an Adjunctive Therapy to Catheter Ablation in Atrial Fibrillation.
Reducing Cancer Risks
Sai Yendamuri, MD, chair of the Department of Thoracic Surgery and director of the Thoracic Surgery Laboratory at Roswell Park Comprehensive Cancer Center in Buffalo, New York, is leading a phase 2 trial exploring whether metformin can prevent lung cancer in people with overweight or obesity who are at a high risk for the malignancy.
The study, which has accrued about 60% of its estimated enrollment, also will assess whether metformin can reprogram participants’ immune systems, with a view toward reducing the activity of regulatory T cells that are linked to development of tumors.
“In our preclinical and retrospective clinical data, we found that metformin had anticancer effects but only if the patients were overweight,” Dr. Yendamuri said. “In mice, we find that obesity increases regulatory T-cell function, which suppresses the immune system of the lungs. This effect is reversed by metformin.” The team is conducting the current study to examine if this happens in patients, as well. Results are expected next year.
Research is underway in other tumor types, including oral and endometrial, and brain cancers.
Preventing Alzheimer’s Disease
Cognitive function — or at least delaying its erosion — represents another front for metformin. José A. Luchsinger, MD, MPH, vice-chair for clinical and epidemiological research and director of the section on geriatrics, gerontology, and aging at Columbia University Irving Medical Center in New York City, is heading a phase 2/3 randomized controlled trial assessing the ability of the drug to prevent Alzheimer›s disease.
The study investigators hope to enroll 326 men and women aged 55-90 years with early and late mild cognitive impairment, overweight or obesity, and no diabetes.
“The hypothesis is that improving insulin and glucose levels can lead to lowering the risk of Alzheimer’s disease,” Dr. Luchsinger said. Recruitment should be complete by the end of 2024 and results are expected in late 2026.
Similar studies are underway in Europe and Asia.
Other areas of investigation, while tantalizing, are mostly in early stages, although bolstered by preclinical and mechanistic studies. The authors of a recent review on the potential mechanisms of action of metformin and existing evidence of the drug›s effectiveness — or lack thereof — in treating diseases other than diabetes, wrote: “Collectively, these data raise the question: Is metformin a drug for all diseases? It remains unclear as to whether all of these putative beneficial effects are secondary to its actions as an antihyperglycemic and insulin-sensitizing drug, or result from other cellular actions, including inhibition of mTOR (mammalian target for rapamycin), or direct antiviral actions.”
Off-Label Uses
Metformin currently is approved by the US Food and Drug Administration only for the treatment of type 2 diabetes, although it is also the only antidiabetic medication for prediabetes currently recommended by the American Diabetes Association.
Some studies currently are looking at its use in a variety of off-label indications, including obesity, gestational diabetes, weight gain from antipsychotics, and polycystic ovary syndrome.
For the most part, metformin is considered a safe drug, but it is not risk-free, Dr. Jain cautioned.
“Although it would certainly be helpful to see if this inexpensive medication that’s universally available can help in disease states, one shouldn’t overlook the potential risk of adverse effects, such as gastrointestinal, potential vitamin B12 deficiency, blunting of skeletal muscle development and the rare risk of lactic acidosis in those with kidney impairment,” he said.
“Similarly, with recent reports of the carcinogenic potential of certain formulations of long-acting metformin that contained NDMA [N-nitrosodimethylamine], it would be imperative that these kinks are removed before we incorporate metformin as the gift that keeps giving.”
Dr. Jain reported financial relationships with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk. Dr. Yendamuri disclosed serving on the scientific advisory board member of Karkinos Healthcare and research funding from Lumeda for the metformin study. Dr. Luchsinger reported receiving donated metformin and matching placebo from EMD Serono, a subsidiary of Merck, for the MAP study. Dr. Schwartz received research support from the US Department of Veterans Affairs as National Chair of the VA-IMPACT trial.
A version of this article appeared on Medscape.com.
In 2021 alone, clinicians wrote more than 91 million orders for the medication — up from 40 million 2004.
But is metformin just getting started? Emerging evidence suggests the drug may be effective for a much broader range of conditions beyond managing high blood glucose, including various cancers, obesity, liver disease, cardiovascular, neurodegenerative, and renal diseases. As the evidence for diverse uses accumulates, many trials have launched, with researchers looking to expand metformin’s indications and validate or explore new directions.
Metformin’s long history as a pharmaceutical includes an herbal ancestry, recognition in 1918 for its ability to lower blood glucose, being cast aside because of toxicity fears in the 1930s, rediscovery and synthesis in Europe in the 1940s, the first reported use for diabetes in 1957, and approval in the United States in 1994.
The drug has maintained its place as the preferred first-line treatment for type 2 diabetes since 2011, when it was first included in the World Health Organization’s essential medicines list.
“The focus hitherto has been primarily on its insulin sensitization effects,” Akshay Jain, MD, a clinical and research endocrinologist at TLC Diabetes and Endocrinology, in Surrey, British Columbia, Canada, told this news organization.
“The recent surge of renewed interest is in part related to its postulated effects on multiple other receptors,” he said. “In my mind, the metformin data on coronary artery disease reduction and cancer-protective effects have come farther along than other disease states.”
Cardiovascular Outcomes
Gregory G. Schwartz, MD, PhD, chief of the cardiology section at Rocky Mountain Regional VA Medical Center and professor of medicine at the University of Colorado School of Medicine in Aurora, is leading the VA-IMPACT trial. Despite metformin’s long history and widespread use, he said his study is the first placebo-controlled cardiovascular outcomes trial of the drug.
Launched in 2023, the study tests the hypothesis that metformin reduces the risk for death or nonfatal ischemic cardiovascular events in patients with prediabetes and established coronary, cerebrovascular, or peripheral artery disease, Dr. Schwartz said. The trial is being conducted at roughly 40 VA medical centers, with a planned enrollment of 7410 patients. The estimated completion date is March 2029.
“The principal mechanism of action of metformin is through activation of AMP [adenosine monophosphate]–activated protein kinase, a central pathway in metabolic regulation, cell protection, and survival,” Dr. Schwartz explained. “Experimental data have demonstrated attenuated development of atherosclerosis, reduced myocardial infarct size, improved endothelial function, and antiarrhythmic actions — none of those dependent on the presence of diabetes.”
Dr. Schwartz and his colleagues decided to test their hypothesis in people with prediabetes, rather than diabetes, to create a “true placebo-controlled comparison,” he said.
“If patients with type 2 diabetes had been chosen, there would be potential for confounding because a placebo group would require more treatment with other active antihyperglycemic medications to achieve the same degree of glycemic control as a metformin group,” Dr. Schwartz said.
“If proven efficacious in the VA-IMPACT trial, metformin could provide an inexpensive, generally safe, and well-tolerated approach to reduce cardiovascular morbidity and mortality in a large segment of the population,” Dr. Schwartz added. “Perhaps the old dog can learn some new tricks.”
Other recruiting trials looking at cardiovascular-related outcomes include Met-PEF, LIMIT, and Metformin as an Adjunctive Therapy to Catheter Ablation in Atrial Fibrillation.
Reducing Cancer Risks
Sai Yendamuri, MD, chair of the Department of Thoracic Surgery and director of the Thoracic Surgery Laboratory at Roswell Park Comprehensive Cancer Center in Buffalo, New York, is leading a phase 2 trial exploring whether metformin can prevent lung cancer in people with overweight or obesity who are at a high risk for the malignancy.
The study, which has accrued about 60% of its estimated enrollment, also will assess whether metformin can reprogram participants’ immune systems, with a view toward reducing the activity of regulatory T cells that are linked to development of tumors.
“In our preclinical and retrospective clinical data, we found that metformin had anticancer effects but only if the patients were overweight,” Dr. Yendamuri said. “In mice, we find that obesity increases regulatory T-cell function, which suppresses the immune system of the lungs. This effect is reversed by metformin.” The team is conducting the current study to examine if this happens in patients, as well. Results are expected next year.
Research is underway in other tumor types, including oral and endometrial, and brain cancers.
Preventing Alzheimer’s Disease
Cognitive function — or at least delaying its erosion — represents another front for metformin. José A. Luchsinger, MD, MPH, vice-chair for clinical and epidemiological research and director of the section on geriatrics, gerontology, and aging at Columbia University Irving Medical Center in New York City, is heading a phase 2/3 randomized controlled trial assessing the ability of the drug to prevent Alzheimer›s disease.
The study investigators hope to enroll 326 men and women aged 55-90 years with early and late mild cognitive impairment, overweight or obesity, and no diabetes.
“The hypothesis is that improving insulin and glucose levels can lead to lowering the risk of Alzheimer’s disease,” Dr. Luchsinger said. Recruitment should be complete by the end of 2024 and results are expected in late 2026.
Similar studies are underway in Europe and Asia.
Other areas of investigation, while tantalizing, are mostly in early stages, although bolstered by preclinical and mechanistic studies. The authors of a recent review on the potential mechanisms of action of metformin and existing evidence of the drug›s effectiveness — or lack thereof — in treating diseases other than diabetes, wrote: “Collectively, these data raise the question: Is metformin a drug for all diseases? It remains unclear as to whether all of these putative beneficial effects are secondary to its actions as an antihyperglycemic and insulin-sensitizing drug, or result from other cellular actions, including inhibition of mTOR (mammalian target for rapamycin), or direct antiviral actions.”
Off-Label Uses
Metformin currently is approved by the US Food and Drug Administration only for the treatment of type 2 diabetes, although it is also the only antidiabetic medication for prediabetes currently recommended by the American Diabetes Association.
Some studies currently are looking at its use in a variety of off-label indications, including obesity, gestational diabetes, weight gain from antipsychotics, and polycystic ovary syndrome.
For the most part, metformin is considered a safe drug, but it is not risk-free, Dr. Jain cautioned.
“Although it would certainly be helpful to see if this inexpensive medication that’s universally available can help in disease states, one shouldn’t overlook the potential risk of adverse effects, such as gastrointestinal, potential vitamin B12 deficiency, blunting of skeletal muscle development and the rare risk of lactic acidosis in those with kidney impairment,” he said.
“Similarly, with recent reports of the carcinogenic potential of certain formulations of long-acting metformin that contained NDMA [N-nitrosodimethylamine], it would be imperative that these kinks are removed before we incorporate metformin as the gift that keeps giving.”
Dr. Jain reported financial relationships with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk. Dr. Yendamuri disclosed serving on the scientific advisory board member of Karkinos Healthcare and research funding from Lumeda for the metformin study. Dr. Luchsinger reported receiving donated metformin and matching placebo from EMD Serono, a subsidiary of Merck, for the MAP study. Dr. Schwartz received research support from the US Department of Veterans Affairs as National Chair of the VA-IMPACT trial.
A version of this article appeared on Medscape.com.