User login
5 Strategies for managing antipsychotic-induced hyperprolactinemia
There is a well-established relationship between antipsychotic treatment and hyperprolactinemia. Most antipsychotics have been linked to increased prolactin levels, and the risk appears to be dose-related.1 Antipsychotic-induced hyperprolactinemia can be asymptomatic, but it also has been associated with several adverse effects, including menstrual irregularity, osteoporosis, gynecomastia, and sexual dysfunction. Here I discuss what to do before starting a patient on an antipsychotic, and 5 treatment strategies for addressing antipsychotic-induced hyperprolactinemia.
Get a baseline prolactin level
Before starting a patient on an antipsychotic, obtain a baseline prolactin level measurement. If the patient later develops hyperprolactinemia, having a baseline measurement will make it easier to determine if the antipsychotic is a potential cause. Also, it is helpful to gather additional information regarding baseline psychosexual function and menstruation before starting an antipsychotic.
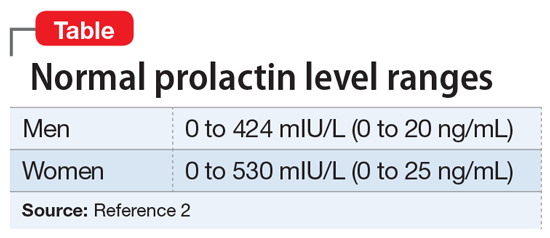
It is critical to determine if a temporal relationship exists between exposure to an antipsychotic and increase in prolactin levels.3 If the time course is unclear, laboratory tests need to be performed, including assessing liver, renal, and thyroid function or imaging of the pituitary gland. Also, hyperprolactinemia should not be diagnosed based on a single blood test result, because emotional and physical stress can elevate prolactin levels.
Continued to: 5 strategies for addressing hyperprolactinemia
5 strategies for addressing hyperprolactinemia
1. Reduce the antipsychotic dose. Because the risk of hyperprolactinemia is dose-dependent, reducing the antipsychotic dose could be helpful for some patients.
2. Switch to a prolactin-sparing antipsychotic, such as clozapine, quetiapine, olanzapine, or ziprasidone. However, it is often difficult to predict positive outcomes because switching antipsychotics may cause new adverse effects or trigger a psychotic relapse.
3. Consider sex hormone replacement therapy. A combined oral contraceptive could prevent osteoporosis and help estrogen deficiency symptoms in women who require antipsychotic medication. However, this treatment approach may worsen galactorrhea.
4. Use a dopamine receptor agonist. Dopamine receptor agonists, such as cabergoline or bromocriptine, have been shown to suppress prolactin secretion. Clinicians should always proceed cautiously because these medications can potentially increase the risk of psychosis.
5. Examine the potential benefits of adding aripiprazole because it can be used for augmentation to reduce prolactin levels in patients receiving other antipsychotics. In some cases, dopamine receptors can be exposed to competition between a partial agonist (aripiprazole) and an antagonist (the current antipsychotic). This competition may decrease the effectiveness of the current antipsychotic.1 Also, adding another antipsychotic could increase overall adverse effects.
1. Montejo ÁL, Arango C, Bernardo M, et al. Multidisciplinary consensus on the therapeutic recommendations for iatrogenic hyperprolactinemia secondary to antipsychotics. Front Neuroendocrinol. 2017;45:25-34.
2. Taylor D, Paton C, Kapur S. Schizophrenia. In: Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in psychiatry. 12th ed. Chichester, UK: Wiley Blackwell; 2015:133-134.
3. Miyamoto BE, Galecki M, Francois D. Guidelines for antipsychotic-induced hyperprolactinemia. Psychiatr Ann. 2015;45(5):266,268,270-272.
There is a well-established relationship between antipsychotic treatment and hyperprolactinemia. Most antipsychotics have been linked to increased prolactin levels, and the risk appears to be dose-related.1 Antipsychotic-induced hyperprolactinemia can be asymptomatic, but it also has been associated with several adverse effects, including menstrual irregularity, osteoporosis, gynecomastia, and sexual dysfunction. Here I discuss what to do before starting a patient on an antipsychotic, and 5 treatment strategies for addressing antipsychotic-induced hyperprolactinemia.
Get a baseline prolactin level
Before starting a patient on an antipsychotic, obtain a baseline prolactin level measurement. If the patient later develops hyperprolactinemia, having a baseline measurement will make it easier to determine if the antipsychotic is a potential cause. Also, it is helpful to gather additional information regarding baseline psychosexual function and menstruation before starting an antipsychotic.

It is critical to determine if a temporal relationship exists between exposure to an antipsychotic and increase in prolactin levels.3 If the time course is unclear, laboratory tests need to be performed, including assessing liver, renal, and thyroid function or imaging of the pituitary gland. Also, hyperprolactinemia should not be diagnosed based on a single blood test result, because emotional and physical stress can elevate prolactin levels.
Continued to: 5 strategies for addressing hyperprolactinemia
5 strategies for addressing hyperprolactinemia
1. Reduce the antipsychotic dose. Because the risk of hyperprolactinemia is dose-dependent, reducing the antipsychotic dose could be helpful for some patients.
2. Switch to a prolactin-sparing antipsychotic, such as clozapine, quetiapine, olanzapine, or ziprasidone. However, it is often difficult to predict positive outcomes because switching antipsychotics may cause new adverse effects or trigger a psychotic relapse.
3. Consider sex hormone replacement therapy. A combined oral contraceptive could prevent osteoporosis and help estrogen deficiency symptoms in women who require antipsychotic medication. However, this treatment approach may worsen galactorrhea.
4. Use a dopamine receptor agonist. Dopamine receptor agonists, such as cabergoline or bromocriptine, have been shown to suppress prolactin secretion. Clinicians should always proceed cautiously because these medications can potentially increase the risk of psychosis.
5. Examine the potential benefits of adding aripiprazole because it can be used for augmentation to reduce prolactin levels in patients receiving other antipsychotics. In some cases, dopamine receptors can be exposed to competition between a partial agonist (aripiprazole) and an antagonist (the current antipsychotic). This competition may decrease the effectiveness of the current antipsychotic.1 Also, adding another antipsychotic could increase overall adverse effects.
There is a well-established relationship between antipsychotic treatment and hyperprolactinemia. Most antipsychotics have been linked to increased prolactin levels, and the risk appears to be dose-related.1 Antipsychotic-induced hyperprolactinemia can be asymptomatic, but it also has been associated with several adverse effects, including menstrual irregularity, osteoporosis, gynecomastia, and sexual dysfunction. Here I discuss what to do before starting a patient on an antipsychotic, and 5 treatment strategies for addressing antipsychotic-induced hyperprolactinemia.
Get a baseline prolactin level
Before starting a patient on an antipsychotic, obtain a baseline prolactin level measurement. If the patient later develops hyperprolactinemia, having a baseline measurement will make it easier to determine if the antipsychotic is a potential cause. Also, it is helpful to gather additional information regarding baseline psychosexual function and menstruation before starting an antipsychotic.

It is critical to determine if a temporal relationship exists between exposure to an antipsychotic and increase in prolactin levels.3 If the time course is unclear, laboratory tests need to be performed, including assessing liver, renal, and thyroid function or imaging of the pituitary gland. Also, hyperprolactinemia should not be diagnosed based on a single blood test result, because emotional and physical stress can elevate prolactin levels.
Continued to: 5 strategies for addressing hyperprolactinemia
5 strategies for addressing hyperprolactinemia
1. Reduce the antipsychotic dose. Because the risk of hyperprolactinemia is dose-dependent, reducing the antipsychotic dose could be helpful for some patients.
2. Switch to a prolactin-sparing antipsychotic, such as clozapine, quetiapine, olanzapine, or ziprasidone. However, it is often difficult to predict positive outcomes because switching antipsychotics may cause new adverse effects or trigger a psychotic relapse.
3. Consider sex hormone replacement therapy. A combined oral contraceptive could prevent osteoporosis and help estrogen deficiency symptoms in women who require antipsychotic medication. However, this treatment approach may worsen galactorrhea.
4. Use a dopamine receptor agonist. Dopamine receptor agonists, such as cabergoline or bromocriptine, have been shown to suppress prolactin secretion. Clinicians should always proceed cautiously because these medications can potentially increase the risk of psychosis.
5. Examine the potential benefits of adding aripiprazole because it can be used for augmentation to reduce prolactin levels in patients receiving other antipsychotics. In some cases, dopamine receptors can be exposed to competition between a partial agonist (aripiprazole) and an antagonist (the current antipsychotic). This competition may decrease the effectiveness of the current antipsychotic.1 Also, adding another antipsychotic could increase overall adverse effects.
1. Montejo ÁL, Arango C, Bernardo M, et al. Multidisciplinary consensus on the therapeutic recommendations for iatrogenic hyperprolactinemia secondary to antipsychotics. Front Neuroendocrinol. 2017;45:25-34.
2. Taylor D, Paton C, Kapur S. Schizophrenia. In: Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in psychiatry. 12th ed. Chichester, UK: Wiley Blackwell; 2015:133-134.
3. Miyamoto BE, Galecki M, Francois D. Guidelines for antipsychotic-induced hyperprolactinemia. Psychiatr Ann. 2015;45(5):266,268,270-272.
1. Montejo ÁL, Arango C, Bernardo M, et al. Multidisciplinary consensus on the therapeutic recommendations for iatrogenic hyperprolactinemia secondary to antipsychotics. Front Neuroendocrinol. 2017;45:25-34.
2. Taylor D, Paton C, Kapur S. Schizophrenia. In: Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in psychiatry. 12th ed. Chichester, UK: Wiley Blackwell; 2015:133-134.
3. Miyamoto BE, Galecki M, Francois D. Guidelines for antipsychotic-induced hyperprolactinemia. Psychiatr Ann. 2015;45(5):266,268,270-272.
Vitamin B6 for tardive dyskinesia?
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
Data-driven prescribing
Computational psychiatry is an emerging field in which artificial intelligence and machine learning are used to find hidden patterns in big data to better understand, predict, and treat mental illness. The field uses various mathematical models to predict the dependent variable y based on the independent variable x. One application of analytics in medicine was the Framingham Heart Study, which used multivariate logistic regression to predict heart disease.1
Analytics could be used to predict the number of bad outcomes associated with different psychiatric medications over time. To demonstrate this, I examined a select data set of 8 psychiatric medications (aripiprazole, ziprasidone, risperidone, olanzapine, sertraline, trazodone, amitriptyline, and lithium) accounting for 59,827 bad outcomes during a 15-year period as reported by U.S. poison control centers,2 and plotted these on the y-axis.
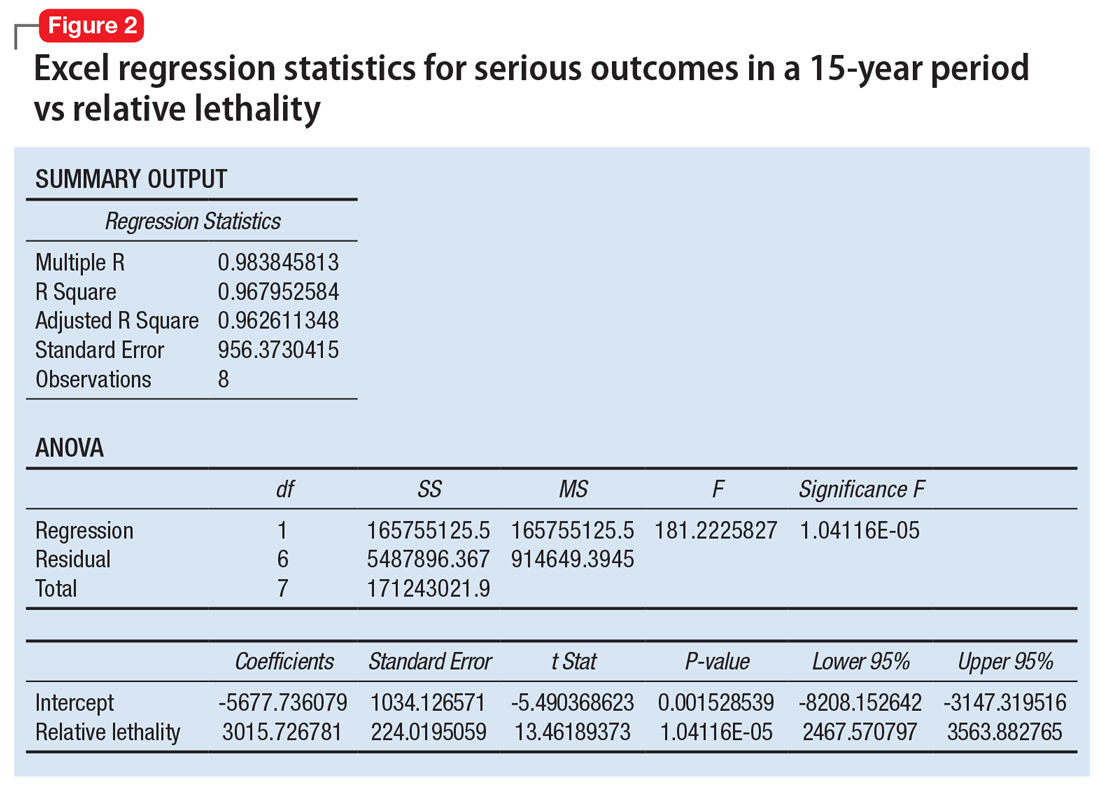
When considering the independent variable to use as a predictor for bad outcomes, I used a composite index derived with the relative lethality (RL) equation, f(x) = 310x /LD50, where x is the daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50.3 I plotted the RL of the 8 medications on the x-axis. Then I attempted to find a mathematical function that would best fit the x and y intersection points (Figure 1). I used the Excel data analysis pack to run a logarithmic regression model (Figure 2).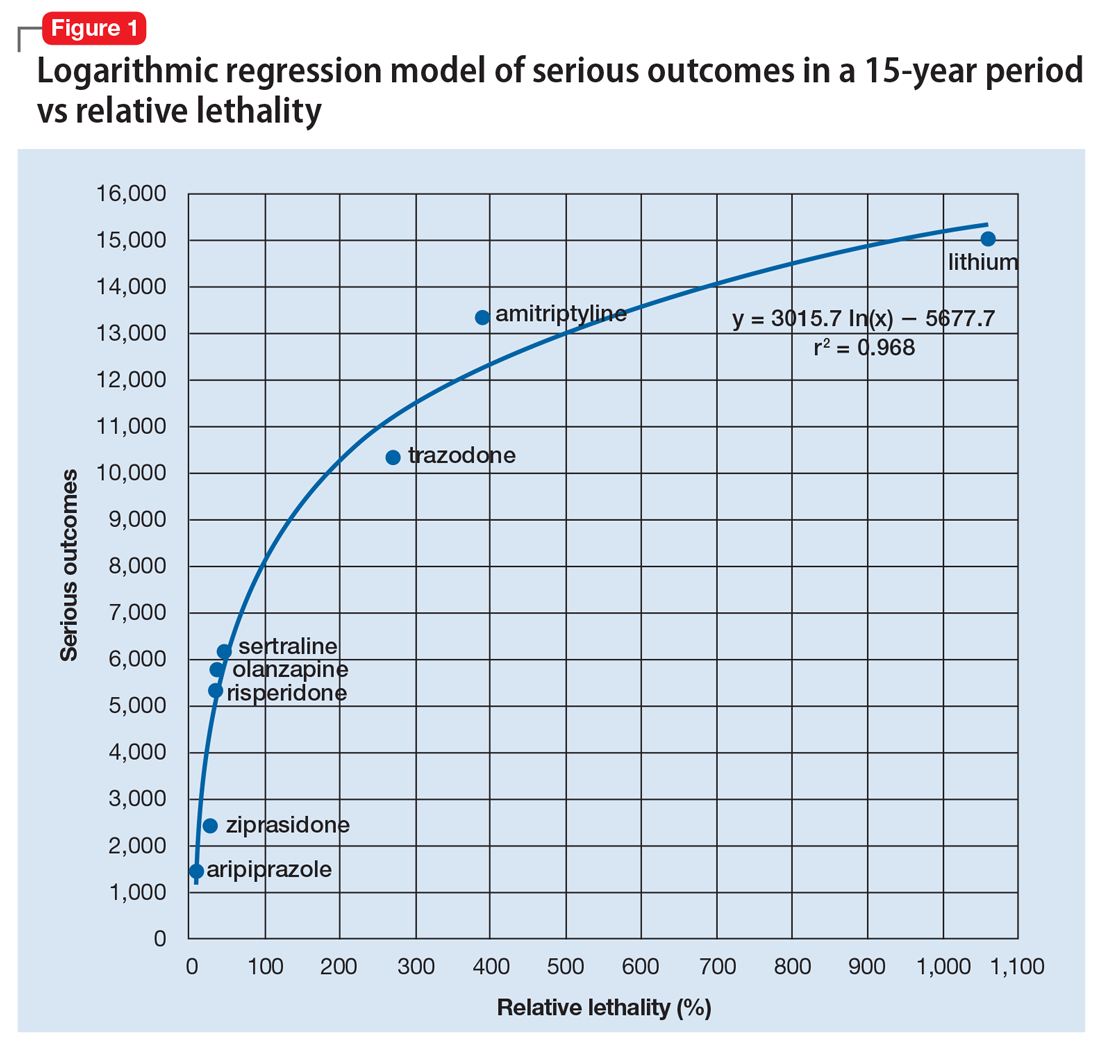
The model predicts that medications with a lower RL will have fewer serious outcomes, including mortality. The coefficient of determination r2 = 0.968, which indicates that 97% of the variation in serious outcomes is attributed to variation in RL, and 3% may be due to other factors, such as the poor quality of U.S. poison control data. This is a very significant correlation, and the causality is self-evident.
Continued to: The distribution of bad outcomes in the model was...
The distribution of bad outcomes in the model was: 1,446 for aripiprazole (RL = 9.76%), 2,387 for ziprasidone (RL = 24.80%), 5,352 for risperidone (RL = 32.63%), 5,798 for olanzapine (RL = 35.03%), 6,120 for sertraline (RL = 46.72%), 10,343 for trazodone (RL = 269.57%), 13,345 for amitriptyline (RL = 387.50%), and 15,036 for lithium (RL = 1,062.86%). The regression equation is: serious outcomes = –5,677.7 + 3,015.7 × ln (RL).
Some doctors may argue that such a data set is too small to make a meaningful model. However, the number of possible ways of ranking the drugs by bad outcomes is 8! = 40,320, so the probability of guessing the right sequence is P = .000024801. To appreciate how small this probability is, imagine trying to find a person of interest in half a football stadium on Superbowl Sunday.
The RL composite index correctly predicted the ranking order of serious outcomes for the 8 medications and may be useful for finding such outcomes in any drug class. For example, with angiotensin-converting enzyme inhibitors (n = 11) the number of possible combinations is 11! = 39,916,800. The probability of guessing the right sequence is like finding a person of interest in Poland. The model predicts the following decreasing sequence: 1) captopril, 2) fosinopril, 3) quinapril, 4) benazepril, 5) enalapril, 6) lisinopril, 7) moexipril, 8) perindopril, 9) cilazapril, 10) ramipril, 11) trandolapril. The predicted number of bad outcomes is highest for captopril, and lowest for trandolapril. The usefulness of the machine learning algorithm becomes immediately apparent.
Data can inform prescribing
Analytics can expose a critical flaw in the academic psychiatry paradigm for prescribing medications. For example, some doctors may regard lithium as the “gold standard” for treating certain mood disorders, but there is evidence that olanzapine is “significantly more effective than lithium in preventing recurrence of manic and mixed episodes.”4 Olanzapine is also 30 times safer than lithium based on its RL index, and had 9,238 fewer bad outcomes based on the 15-year data from U.S. poison control centers.2 A patient who intends to attempt suicide would easily be able to find the lethal dose of lithium from a “suicide” web site, and would quickly be able to figure out that the monthly amount of lithium his or her psychiatrist prescribed, would exceed the lethal dose.
When academia and reality collide, the use of analytics will have the final word by preventing suicide in the short term and reducing the number of bad outcomes in the long term. The irony of data science is that mathematical models can find optimal solutions to complex problems in a fraction of a second, but it may take years for a paradigm shift.
1. Bertsimas D, O’Hair AK, Pulleyblank WR. The analytics edge. Belmont, MA: Dynamic Ideas LLC; 2016.
2. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. poison control centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
4. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):1281-1290.
Computational psychiatry is an emerging field in which artificial intelligence and machine learning are used to find hidden patterns in big data to better understand, predict, and treat mental illness. The field uses various mathematical models to predict the dependent variable y based on the independent variable x. One application of analytics in medicine was the Framingham Heart Study, which used multivariate logistic regression to predict heart disease.1
Analytics could be used to predict the number of bad outcomes associated with different psychiatric medications over time. To demonstrate this, I examined a select data set of 8 psychiatric medications (aripiprazole, ziprasidone, risperidone, olanzapine, sertraline, trazodone, amitriptyline, and lithium) accounting for 59,827 bad outcomes during a 15-year period as reported by U.S. poison control centers,2 and plotted these on the y-axis.
When considering the independent variable to use as a predictor for bad outcomes, I used a composite index derived with the relative lethality (RL) equation, f(x) = 310x /LD50, where x is the daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50.3 I plotted the RL of the 8 medications on the x-axis. Then I attempted to find a mathematical function that would best fit the x and y intersection points (Figure 1). I used the Excel data analysis pack to run a logarithmic regression model (Figure 2).
The model predicts that medications with a lower RL will have fewer serious outcomes, including mortality. The coefficient of determination r2 = 0.968, which indicates that 97% of the variation in serious outcomes is attributed to variation in RL, and 3% may be due to other factors, such as the poor quality of U.S. poison control data. This is a very significant correlation, and the causality is self-evident.

Continued to: The distribution of bad outcomes in the model was...
The distribution of bad outcomes in the model was: 1,446 for aripiprazole (RL = 9.76%), 2,387 for ziprasidone (RL = 24.80%), 5,352 for risperidone (RL = 32.63%), 5,798 for olanzapine (RL = 35.03%), 6,120 for sertraline (RL = 46.72%), 10,343 for trazodone (RL = 269.57%), 13,345 for amitriptyline (RL = 387.50%), and 15,036 for lithium (RL = 1,062.86%). The regression equation is: serious outcomes = –5,677.7 + 3,015.7 × ln (RL).
Some doctors may argue that such a data set is too small to make a meaningful model. However, the number of possible ways of ranking the drugs by bad outcomes is 8! = 40,320, so the probability of guessing the right sequence is P = .000024801. To appreciate how small this probability is, imagine trying to find a person of interest in half a football stadium on Superbowl Sunday.
The RL composite index correctly predicted the ranking order of serious outcomes for the 8 medications and may be useful for finding such outcomes in any drug class. For example, with angiotensin-converting enzyme inhibitors (n = 11) the number of possible combinations is 11! = 39,916,800. The probability of guessing the right sequence is like finding a person of interest in Poland. The model predicts the following decreasing sequence: 1) captopril, 2) fosinopril, 3) quinapril, 4) benazepril, 5) enalapril, 6) lisinopril, 7) moexipril, 8) perindopril, 9) cilazapril, 10) ramipril, 11) trandolapril. The predicted number of bad outcomes is highest for captopril, and lowest for trandolapril. The usefulness of the machine learning algorithm becomes immediately apparent.
Data can inform prescribing
Analytics can expose a critical flaw in the academic psychiatry paradigm for prescribing medications. For example, some doctors may regard lithium as the “gold standard” for treating certain mood disorders, but there is evidence that olanzapine is “significantly more effective than lithium in preventing recurrence of manic and mixed episodes.”4 Olanzapine is also 30 times safer than lithium based on its RL index, and had 9,238 fewer bad outcomes based on the 15-year data from U.S. poison control centers.2 A patient who intends to attempt suicide would easily be able to find the lethal dose of lithium from a “suicide” web site, and would quickly be able to figure out that the monthly amount of lithium his or her psychiatrist prescribed, would exceed the lethal dose.
When academia and reality collide, the use of analytics will have the final word by preventing suicide in the short term and reducing the number of bad outcomes in the long term. The irony of data science is that mathematical models can find optimal solutions to complex problems in a fraction of a second, but it may take years for a paradigm shift.
Computational psychiatry is an emerging field in which artificial intelligence and machine learning are used to find hidden patterns in big data to better understand, predict, and treat mental illness. The field uses various mathematical models to predict the dependent variable y based on the independent variable x. One application of analytics in medicine was the Framingham Heart Study, which used multivariate logistic regression to predict heart disease.1
Analytics could be used to predict the number of bad outcomes associated with different psychiatric medications over time. To demonstrate this, I examined a select data set of 8 psychiatric medications (aripiprazole, ziprasidone, risperidone, olanzapine, sertraline, trazodone, amitriptyline, and lithium) accounting for 59,827 bad outcomes during a 15-year period as reported by U.S. poison control centers,2 and plotted these on the y-axis.
When considering the independent variable to use as a predictor for bad outcomes, I used a composite index derived with the relative lethality (RL) equation, f(x) = 310x /LD50, where x is the daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50.3 I plotted the RL of the 8 medications on the x-axis. Then I attempted to find a mathematical function that would best fit the x and y intersection points (Figure 1). I used the Excel data analysis pack to run a logarithmic regression model (Figure 2).
The model predicts that medications with a lower RL will have fewer serious outcomes, including mortality. The coefficient of determination r2 = 0.968, which indicates that 97% of the variation in serious outcomes is attributed to variation in RL, and 3% may be due to other factors, such as the poor quality of U.S. poison control data. This is a very significant correlation, and the causality is self-evident.

Continued to: The distribution of bad outcomes in the model was...
The distribution of bad outcomes in the model was: 1,446 for aripiprazole (RL = 9.76%), 2,387 for ziprasidone (RL = 24.80%), 5,352 for risperidone (RL = 32.63%), 5,798 for olanzapine (RL = 35.03%), 6,120 for sertraline (RL = 46.72%), 10,343 for trazodone (RL = 269.57%), 13,345 for amitriptyline (RL = 387.50%), and 15,036 for lithium (RL = 1,062.86%). The regression equation is: serious outcomes = –5,677.7 + 3,015.7 × ln (RL).
Some doctors may argue that such a data set is too small to make a meaningful model. However, the number of possible ways of ranking the drugs by bad outcomes is 8! = 40,320, so the probability of guessing the right sequence is P = .000024801. To appreciate how small this probability is, imagine trying to find a person of interest in half a football stadium on Superbowl Sunday.
The RL composite index correctly predicted the ranking order of serious outcomes for the 8 medications and may be useful for finding such outcomes in any drug class. For example, with angiotensin-converting enzyme inhibitors (n = 11) the number of possible combinations is 11! = 39,916,800. The probability of guessing the right sequence is like finding a person of interest in Poland. The model predicts the following decreasing sequence: 1) captopril, 2) fosinopril, 3) quinapril, 4) benazepril, 5) enalapril, 6) lisinopril, 7) moexipril, 8) perindopril, 9) cilazapril, 10) ramipril, 11) trandolapril. The predicted number of bad outcomes is highest for captopril, and lowest for trandolapril. The usefulness of the machine learning algorithm becomes immediately apparent.
Data can inform prescribing
Analytics can expose a critical flaw in the academic psychiatry paradigm for prescribing medications. For example, some doctors may regard lithium as the “gold standard” for treating certain mood disorders, but there is evidence that olanzapine is “significantly more effective than lithium in preventing recurrence of manic and mixed episodes.”4 Olanzapine is also 30 times safer than lithium based on its RL index, and had 9,238 fewer bad outcomes based on the 15-year data from U.S. poison control centers.2 A patient who intends to attempt suicide would easily be able to find the lethal dose of lithium from a “suicide” web site, and would quickly be able to figure out that the monthly amount of lithium his or her psychiatrist prescribed, would exceed the lethal dose.
When academia and reality collide, the use of analytics will have the final word by preventing suicide in the short term and reducing the number of bad outcomes in the long term. The irony of data science is that mathematical models can find optimal solutions to complex problems in a fraction of a second, but it may take years for a paradigm shift.
1. Bertsimas D, O’Hair AK, Pulleyblank WR. The analytics edge. Belmont, MA: Dynamic Ideas LLC; 2016.
2. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. poison control centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
4. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):1281-1290.
1. Bertsimas D, O’Hair AK, Pulleyblank WR. The analytics edge. Belmont, MA: Dynamic Ideas LLC; 2016.
2. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. poison control centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
4. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):1281-1290.
The benzodiazepine dilemma
As clinicians, we are faced with a conflict when deciding whether or not to prescribe a benzodiazepine. If we prescribe one of these agents, we might be putting our patients at risk for dependence and abuse. However, if we do not prescribe them, we risk providing inadequate treatment, especially for patients with panic disorder.
Benzodiazepine dependence and abuse can take many forms. Dependence can be psychological as well as physiologic. While many patients will adhere to their prescribing regimen, some may sell their benzodiazepines, falsely claim that they have “panic attacks,” or take a fatal overdose of an opioid and benzodiazepine combination.
Here I discuss the pros and cons of restricting benzodiazepines use to low doses and/or combination therapy with antidepressants.
_
Weighing the benefits of restricted prescribing
Some double-blind studies referenced in the American Psychiatric Association (APA) 2010 Practice Guideline for the Treatment of Patients with Panic Disorder1 suggest that benzodiazepine duration of treatment and dosages should be severely restricted. These studies found that:
- Although the combination of a selective serotonin reuptake inhibitor (SSRI) and a benzodiazepine initially decreased the number of panic attacks more quickly than SSRI monotherapy, the 2 treatments are equally effective after 4 or 5 weeks.2,3
- For the treatment of panic disorder, a low dosage of a benzodiazepine (clonazepam 1 mg/d or alprazolam 2 mg/d) was as effective as a higher dosage (clonazepam 2 mg/d or alprazolam 6 mg/d).4,5
However, these studies could be misleading. They all excluded patients with a comorbid condition, such as bipolar disorder or depression, that was more severe than their panic disorder. Severe comorbidity is associated with more severe panic symptoms,6,7 which might require an SSRI/benzodiazepine combination or a higher benzodiazepine dosage.
The APA Practice Guideline suggests the following possible options:
- benzodiazepine augmentation if there is a partial response to an SSRI
- substitution with a different SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) if there is no response to an SSRI
- benzodiazepine augmentation or substitution if there is still no therapeutic response.
Continue to: The APA Practice Guideline also states...
The APA Practice Guideline also states that although the highest “usual therapeutic dose” for panic disorder is clonazepam 2 mg/d or alprazolam 4 mg/d, “higher doses are sometimes used for patients who do not respond to the usual therapeutic dose.”1
Presumably, an SSRI/benzodiazepine combination should be considered if an SSRI alleviates major depressive disorder but does not alleviate a comorbid panic disorder. However, the APA Practice Guideline does not include studies that investigated this clinical scenario.
Monitor carefully for dependency/abuse
Restricting benzodiazepine use to low doses over a short period of time may decrease the risk of dependence and abuse. However, this practice may also limit or prevent effective treatment for adherent patients with panic disorder who do not adequately respond to SSRI or SNRI monotherapy.
Therefore, clinicians need to carefully differentiate between patients who are adherent to their prescribed dosages and those who may be at risk for benzodiazepine dependence and abuse. Consider using prescription drug monitoring programs and drug screens to help detect patients who “doctor shop” for benzodiazepines, or who could be abusing opioids, alcohol, marijuana, or other substances while taking a benzodiazepine.
1. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd edition. Washington DC: American Psychiatric Association. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed March 7, 2018.
2. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
3. Pollack MH, Simon NM, Worthington JJ, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003;17(3):276-282.
4. Lydiard RB, Lesser IM, Ballenger JC, et al. A fixed-dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. J Clin Psychopharmacol. 1992;12(2):966-103.
5. Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17(5):390-400.
6. Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70(1):27-33.
7. Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229-235.
As clinicians, we are faced with a conflict when deciding whether or not to prescribe a benzodiazepine. If we prescribe one of these agents, we might be putting our patients at risk for dependence and abuse. However, if we do not prescribe them, we risk providing inadequate treatment, especially for patients with panic disorder.
Benzodiazepine dependence and abuse can take many forms. Dependence can be psychological as well as physiologic. While many patients will adhere to their prescribing regimen, some may sell their benzodiazepines, falsely claim that they have “panic attacks,” or take a fatal overdose of an opioid and benzodiazepine combination.
Here I discuss the pros and cons of restricting benzodiazepines use to low doses and/or combination therapy with antidepressants.
_
Weighing the benefits of restricted prescribing
Some double-blind studies referenced in the American Psychiatric Association (APA) 2010 Practice Guideline for the Treatment of Patients with Panic Disorder1 suggest that benzodiazepine duration of treatment and dosages should be severely restricted. These studies found that:
- Although the combination of a selective serotonin reuptake inhibitor (SSRI) and a benzodiazepine initially decreased the number of panic attacks more quickly than SSRI monotherapy, the 2 treatments are equally effective after 4 or 5 weeks.2,3
- For the treatment of panic disorder, a low dosage of a benzodiazepine (clonazepam 1 mg/d or alprazolam 2 mg/d) was as effective as a higher dosage (clonazepam 2 mg/d or alprazolam 6 mg/d).4,5
However, these studies could be misleading. They all excluded patients with a comorbid condition, such as bipolar disorder or depression, that was more severe than their panic disorder. Severe comorbidity is associated with more severe panic symptoms,6,7 which might require an SSRI/benzodiazepine combination or a higher benzodiazepine dosage.
The APA Practice Guideline suggests the following possible options:
- benzodiazepine augmentation if there is a partial response to an SSRI
- substitution with a different SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) if there is no response to an SSRI
- benzodiazepine augmentation or substitution if there is still no therapeutic response.
Continue to: The APA Practice Guideline also states...
The APA Practice Guideline also states that although the highest “usual therapeutic dose” for panic disorder is clonazepam 2 mg/d or alprazolam 4 mg/d, “higher doses are sometimes used for patients who do not respond to the usual therapeutic dose.”1
Presumably, an SSRI/benzodiazepine combination should be considered if an SSRI alleviates major depressive disorder but does not alleviate a comorbid panic disorder. However, the APA Practice Guideline does not include studies that investigated this clinical scenario.
Monitor carefully for dependency/abuse
Restricting benzodiazepine use to low doses over a short period of time may decrease the risk of dependence and abuse. However, this practice may also limit or prevent effective treatment for adherent patients with panic disorder who do not adequately respond to SSRI or SNRI monotherapy.
Therefore, clinicians need to carefully differentiate between patients who are adherent to their prescribed dosages and those who may be at risk for benzodiazepine dependence and abuse. Consider using prescription drug monitoring programs and drug screens to help detect patients who “doctor shop” for benzodiazepines, or who could be abusing opioids, alcohol, marijuana, or other substances while taking a benzodiazepine.
As clinicians, we are faced with a conflict when deciding whether or not to prescribe a benzodiazepine. If we prescribe one of these agents, we might be putting our patients at risk for dependence and abuse. However, if we do not prescribe them, we risk providing inadequate treatment, especially for patients with panic disorder.
Benzodiazepine dependence and abuse can take many forms. Dependence can be psychological as well as physiologic. While many patients will adhere to their prescribing regimen, some may sell their benzodiazepines, falsely claim that they have “panic attacks,” or take a fatal overdose of an opioid and benzodiazepine combination.
Here I discuss the pros and cons of restricting benzodiazepines use to low doses and/or combination therapy with antidepressants.
_
Weighing the benefits of restricted prescribing
Some double-blind studies referenced in the American Psychiatric Association (APA) 2010 Practice Guideline for the Treatment of Patients with Panic Disorder1 suggest that benzodiazepine duration of treatment and dosages should be severely restricted. These studies found that:
- Although the combination of a selective serotonin reuptake inhibitor (SSRI) and a benzodiazepine initially decreased the number of panic attacks more quickly than SSRI monotherapy, the 2 treatments are equally effective after 4 or 5 weeks.2,3
- For the treatment of panic disorder, a low dosage of a benzodiazepine (clonazepam 1 mg/d or alprazolam 2 mg/d) was as effective as a higher dosage (clonazepam 2 mg/d or alprazolam 6 mg/d).4,5
However, these studies could be misleading. They all excluded patients with a comorbid condition, such as bipolar disorder or depression, that was more severe than their panic disorder. Severe comorbidity is associated with more severe panic symptoms,6,7 which might require an SSRI/benzodiazepine combination or a higher benzodiazepine dosage.
The APA Practice Guideline suggests the following possible options:
- benzodiazepine augmentation if there is a partial response to an SSRI
- substitution with a different SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) if there is no response to an SSRI
- benzodiazepine augmentation or substitution if there is still no therapeutic response.
Continue to: The APA Practice Guideline also states...
The APA Practice Guideline also states that although the highest “usual therapeutic dose” for panic disorder is clonazepam 2 mg/d or alprazolam 4 mg/d, “higher doses are sometimes used for patients who do not respond to the usual therapeutic dose.”1
Presumably, an SSRI/benzodiazepine combination should be considered if an SSRI alleviates major depressive disorder but does not alleviate a comorbid panic disorder. However, the APA Practice Guideline does not include studies that investigated this clinical scenario.
Monitor carefully for dependency/abuse
Restricting benzodiazepine use to low doses over a short period of time may decrease the risk of dependence and abuse. However, this practice may also limit or prevent effective treatment for adherent patients with panic disorder who do not adequately respond to SSRI or SNRI monotherapy.
Therefore, clinicians need to carefully differentiate between patients who are adherent to their prescribed dosages and those who may be at risk for benzodiazepine dependence and abuse. Consider using prescription drug monitoring programs and drug screens to help detect patients who “doctor shop” for benzodiazepines, or who could be abusing opioids, alcohol, marijuana, or other substances while taking a benzodiazepine.
1. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd edition. Washington DC: American Psychiatric Association. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed March 7, 2018.
2. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
3. Pollack MH, Simon NM, Worthington JJ, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003;17(3):276-282.
4. Lydiard RB, Lesser IM, Ballenger JC, et al. A fixed-dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. J Clin Psychopharmacol. 1992;12(2):966-103.
5. Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17(5):390-400.
6. Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70(1):27-33.
7. Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229-235.
1. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd edition. Washington DC: American Psychiatric Association. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed March 7, 2018.
2. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
3. Pollack MH, Simon NM, Worthington JJ, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003;17(3):276-282.
4. Lydiard RB, Lesser IM, Ballenger JC, et al. A fixed-dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. J Clin Psychopharmacol. 1992;12(2):966-103.
5. Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17(5):390-400.
6. Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70(1):27-33.
7. Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229-235.
Providing culturally competent postpartum care for South Asian women
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
How to avoid denied claims
Unless your practice is cash-only, reimbursements from your patients’ health insurance companies are necessary to ensure its survival. Although the reimbursement process appears straightforward (provide a service, submit a claim, and receive a payment), it is actually quite complex, and, if not properly managed, a claim can be denied at any stage of the process.1 In its 2013 National Health Insurer Report Card, the American Medical Association reported that major payers returned 11% to 29% of claim lines with $0 for payment.1,2 This often is the case because patients are responsible for the balance, but it also occurs as the result of claim edits (up to 7%) and other denials (up to 5%).1,2
Claims can be denied for various reasons, including1:
- missed filing deadlines
- billing for non-covered services
- discrepancies between diagnostic codes, procedures codes, modifiers, and clinician documentation
- missing pre-authorization documentation or a signed Advanced Beneficiary Notice of Non-Coverage.
Strategies for avoiding denials
A psychiatric practice requires a practical system to prevent the occurrence of denials, starting from the point of referral. Working through denials is more costly and timeconsuming than preventing them from occurring in the first place. For every 15 denials prevented each month, your practice can save approximately $4,500 per year in costs associated with correcting those claims; by preventing denials, the practice also receives reimbursement sooner.1 You can be guaranteed to leave significant amounts of money on the table if you are not able to prevent or reduce denials.
The following methods can be used to help reduce the likelihood of having a claim denied.1,3
Obtain the patient’s health insurance information at first contact and confirm his or her coverage benefits, deductibles, copay requirements, and exclusions before scheduling the first appointment. Verify this information at each of the patient’s subsequent visits to reduce the chances of having a claim denied due to invalid subscriber information. Also, keep in mind that Medicaid eligibility can change daily.
Employ a digital record system, such as electronic medical records, to track authorizations.
Know the filing deadlines for each of your payers. If you miss a deadline, there is no recourse.
Continue to: Check each claim
Check each claim for accurate coding, diagnosis, and payment (eg, copay, co-insurance, and/or deductible, depending on the health insurance plan) taken before the claim is submitted. If your practice size permits, assign a staff member to confirm this information and keep track of deadlines for submissions, resubmissions, and appeals of denied claims. Using a single gatekeeper can help decrease the chances that a denial will “slip through the cracks.”
Confirm that diagnostic codes, procedures codes, and modifiers are justified by the clinician’s documentation. Have a medical coder compare notes with the clinician to determine if any critical information needed to justify the codes used has been omitted.
Implement an electronic system that can automatically identify any changes and updates to the Centers for Medicare and Medicaid Services (CMS) regulations and guidance, International Classification of Diseases (ICD) versions and codes, and Current Procedural Terminology (CPT) codes and guidelines. To help reduce denied claims, educate all staff (schedulers, coders, billers, nursing staff, and other clinicians) frequently about these changes, and provide regular feedback to those involved in correcting denials.
1. Marting R. The cure for claims denials. Fam Pract Manag. 2015;22(2):7-10.
2. American Medical Association. 2013 National Health Insurer Report Card. Chicago, IL: American Medical Association; 2013.
3. Tohill M. 8 tips for avoiding denials, improving claims reimbursement . RevCycle Intelligence. https://revcycleintelligence.com/news/8-tips-for-avoiding-denials-improving-claims-reimbursement. Published June 6, 2016. Accessed February 19, 2018.
Unless your practice is cash-only, reimbursements from your patients’ health insurance companies are necessary to ensure its survival. Although the reimbursement process appears straightforward (provide a service, submit a claim, and receive a payment), it is actually quite complex, and, if not properly managed, a claim can be denied at any stage of the process.1 In its 2013 National Health Insurer Report Card, the American Medical Association reported that major payers returned 11% to 29% of claim lines with $0 for payment.1,2 This often is the case because patients are responsible for the balance, but it also occurs as the result of claim edits (up to 7%) and other denials (up to 5%).1,2
Claims can be denied for various reasons, including1:
- missed filing deadlines
- billing for non-covered services
- discrepancies between diagnostic codes, procedures codes, modifiers, and clinician documentation
- missing pre-authorization documentation or a signed Advanced Beneficiary Notice of Non-Coverage.
Strategies for avoiding denials
A psychiatric practice requires a practical system to prevent the occurrence of denials, starting from the point of referral. Working through denials is more costly and timeconsuming than preventing them from occurring in the first place. For every 15 denials prevented each month, your practice can save approximately $4,500 per year in costs associated with correcting those claims; by preventing denials, the practice also receives reimbursement sooner.1 You can be guaranteed to leave significant amounts of money on the table if you are not able to prevent or reduce denials.
The following methods can be used to help reduce the likelihood of having a claim denied.1,3
Obtain the patient’s health insurance information at first contact and confirm his or her coverage benefits, deductibles, copay requirements, and exclusions before scheduling the first appointment. Verify this information at each of the patient’s subsequent visits to reduce the chances of having a claim denied due to invalid subscriber information. Also, keep in mind that Medicaid eligibility can change daily.
Employ a digital record system, such as electronic medical records, to track authorizations.
Know the filing deadlines for each of your payers. If you miss a deadline, there is no recourse.
Continue to: Check each claim
Check each claim for accurate coding, diagnosis, and payment (eg, copay, co-insurance, and/or deductible, depending on the health insurance plan) taken before the claim is submitted. If your practice size permits, assign a staff member to confirm this information and keep track of deadlines for submissions, resubmissions, and appeals of denied claims. Using a single gatekeeper can help decrease the chances that a denial will “slip through the cracks.”
Confirm that diagnostic codes, procedures codes, and modifiers are justified by the clinician’s documentation. Have a medical coder compare notes with the clinician to determine if any critical information needed to justify the codes used has been omitted.
Implement an electronic system that can automatically identify any changes and updates to the Centers for Medicare and Medicaid Services (CMS) regulations and guidance, International Classification of Diseases (ICD) versions and codes, and Current Procedural Terminology (CPT) codes and guidelines. To help reduce denied claims, educate all staff (schedulers, coders, billers, nursing staff, and other clinicians) frequently about these changes, and provide regular feedback to those involved in correcting denials.
Unless your practice is cash-only, reimbursements from your patients’ health insurance companies are necessary to ensure its survival. Although the reimbursement process appears straightforward (provide a service, submit a claim, and receive a payment), it is actually quite complex, and, if not properly managed, a claim can be denied at any stage of the process.1 In its 2013 National Health Insurer Report Card, the American Medical Association reported that major payers returned 11% to 29% of claim lines with $0 for payment.1,2 This often is the case because patients are responsible for the balance, but it also occurs as the result of claim edits (up to 7%) and other denials (up to 5%).1,2
Claims can be denied for various reasons, including1:
- missed filing deadlines
- billing for non-covered services
- discrepancies between diagnostic codes, procedures codes, modifiers, and clinician documentation
- missing pre-authorization documentation or a signed Advanced Beneficiary Notice of Non-Coverage.
Strategies for avoiding denials
A psychiatric practice requires a practical system to prevent the occurrence of denials, starting from the point of referral. Working through denials is more costly and timeconsuming than preventing them from occurring in the first place. For every 15 denials prevented each month, your practice can save approximately $4,500 per year in costs associated with correcting those claims; by preventing denials, the practice also receives reimbursement sooner.1 You can be guaranteed to leave significant amounts of money on the table if you are not able to prevent or reduce denials.
The following methods can be used to help reduce the likelihood of having a claim denied.1,3
Obtain the patient’s health insurance information at first contact and confirm his or her coverage benefits, deductibles, copay requirements, and exclusions before scheduling the first appointment. Verify this information at each of the patient’s subsequent visits to reduce the chances of having a claim denied due to invalid subscriber information. Also, keep in mind that Medicaid eligibility can change daily.
Employ a digital record system, such as electronic medical records, to track authorizations.
Know the filing deadlines for each of your payers. If you miss a deadline, there is no recourse.
Continue to: Check each claim
Check each claim for accurate coding, diagnosis, and payment (eg, copay, co-insurance, and/or deductible, depending on the health insurance plan) taken before the claim is submitted. If your practice size permits, assign a staff member to confirm this information and keep track of deadlines for submissions, resubmissions, and appeals of denied claims. Using a single gatekeeper can help decrease the chances that a denial will “slip through the cracks.”
Confirm that diagnostic codes, procedures codes, and modifiers are justified by the clinician’s documentation. Have a medical coder compare notes with the clinician to determine if any critical information needed to justify the codes used has been omitted.
Implement an electronic system that can automatically identify any changes and updates to the Centers for Medicare and Medicaid Services (CMS) regulations and guidance, International Classification of Diseases (ICD) versions and codes, and Current Procedural Terminology (CPT) codes and guidelines. To help reduce denied claims, educate all staff (schedulers, coders, billers, nursing staff, and other clinicians) frequently about these changes, and provide regular feedback to those involved in correcting denials.
1. Marting R. The cure for claims denials. Fam Pract Manag. 2015;22(2):7-10.
2. American Medical Association. 2013 National Health Insurer Report Card. Chicago, IL: American Medical Association; 2013.
3. Tohill M. 8 tips for avoiding denials, improving claims reimbursement . RevCycle Intelligence. https://revcycleintelligence.com/news/8-tips-for-avoiding-denials-improving-claims-reimbursement. Published June 6, 2016. Accessed February 19, 2018.
1. Marting R. The cure for claims denials. Fam Pract Manag. 2015;22(2):7-10.
2. American Medical Association. 2013 National Health Insurer Report Card. Chicago, IL: American Medical Association; 2013.
3. Tohill M. 8 tips for avoiding denials, improving claims reimbursement . RevCycle Intelligence. https://revcycleintelligence.com/news/8-tips-for-avoiding-denials-improving-claims-reimbursement. Published June 6, 2016. Accessed February 19, 2018.
Clozapine-induced GI hypomotility: From constipation to bowel obstruction
Patients who are treated with clozapine—a second-generation antipsychotic approved for treatment-resistant schizophrenia—require monitoring for serious adverse effects. Many of these adverse effects, such as agranulocytosis or seizures, are familiar to clinicians; however, gastrointestinal (GI) hypomotility is not always recognized as a potentially serious adverse effect, even though it is one of the most common causes for hospital admission.1 Its manifestations range from being relatively benign (nausea, vomiting, constipation) to potentially severe (fecal impaction) or even life-threatening (bowel obstruction, ileus, toxic megacolon).2
GI hypomotility is caused by clozapine’s strong anticholinergic properties, which lead to slowed smooth muscle contractions and delayed bowel transit time. It is further compounded by clozapine’s 5-HT3 antagonism, which is also known to slow bowel transit time. To avoid the potentially serious risks associated with GI hypomotility, we offer simple approaches for clinicians to follow when treating patients with clozapine.
Before starting a patient on clozapine, and at all subsequent visits, ask him or her about bowel habits and GI symptoms. Because the onset of GI hypomotility can be subtle, ask patients to pay close attention to their bowel habits and keep a diary to document GI symptoms and bowel movements. Signs of bowel obstruction can include an inability to pass stool, nausea, vomiting, abdominal pain, and a bloated abdomen. Staff who care for patients taking clozapine who live in a supervised setting should be educated about the relevance of a patient’s changing bowel habits or GI complaints. Also, teach patients about simple lifestyle modifications they can make to counteract constipation, including increased physical activity, adequate hydration, and consuming a fiber-rich diet.
If possible, avoid prescribing anticholinergic medications to a patient receiving clozapine because such agents may add to clozapine’s anticholinergic load. Some patients may require medical management of chronic constipation with stool softeners and stimulant laxatives. The Table outlines a typical bowel regimen we use for patients at our clinic. Some clinicians may prefer earlier and more regular use of senna glycoside. Also, patients might need a referral to their primary care physician if prevention has failed and fecal impaction requires enemas or mechanical disimpaction. An urgent referral to the emergency department is needed if a patient has a suspected bowel obstruction.
Acknowledgment
The authors thank Travis Baggett, MD, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, for reviewing the medical management of constipation.
1. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
2. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699-709.
Patients who are treated with clozapine—a second-generation antipsychotic approved for treatment-resistant schizophrenia—require monitoring for serious adverse effects. Many of these adverse effects, such as agranulocytosis or seizures, are familiar to clinicians; however, gastrointestinal (GI) hypomotility is not always recognized as a potentially serious adverse effect, even though it is one of the most common causes for hospital admission.1 Its manifestations range from being relatively benign (nausea, vomiting, constipation) to potentially severe (fecal impaction) or even life-threatening (bowel obstruction, ileus, toxic megacolon).2
GI hypomotility is caused by clozapine’s strong anticholinergic properties, which lead to slowed smooth muscle contractions and delayed bowel transit time. It is further compounded by clozapine’s 5-HT3 antagonism, which is also known to slow bowel transit time. To avoid the potentially serious risks associated with GI hypomotility, we offer simple approaches for clinicians to follow when treating patients with clozapine.
Before starting a patient on clozapine, and at all subsequent visits, ask him or her about bowel habits and GI symptoms. Because the onset of GI hypomotility can be subtle, ask patients to pay close attention to their bowel habits and keep a diary to document GI symptoms and bowel movements. Signs of bowel obstruction can include an inability to pass stool, nausea, vomiting, abdominal pain, and a bloated abdomen. Staff who care for patients taking clozapine who live in a supervised setting should be educated about the relevance of a patient’s changing bowel habits or GI complaints. Also, teach patients about simple lifestyle modifications they can make to counteract constipation, including increased physical activity, adequate hydration, and consuming a fiber-rich diet.
If possible, avoid prescribing anticholinergic medications to a patient receiving clozapine because such agents may add to clozapine’s anticholinergic load. Some patients may require medical management of chronic constipation with stool softeners and stimulant laxatives. The Table outlines a typical bowel regimen we use for patients at our clinic. Some clinicians may prefer earlier and more regular use of senna glycoside. Also, patients might need a referral to their primary care physician if prevention has failed and fecal impaction requires enemas or mechanical disimpaction. An urgent referral to the emergency department is needed if a patient has a suspected bowel obstruction.
Acknowledgment
The authors thank Travis Baggett, MD, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, for reviewing the medical management of constipation.
Patients who are treated with clozapine—a second-generation antipsychotic approved for treatment-resistant schizophrenia—require monitoring for serious adverse effects. Many of these adverse effects, such as agranulocytosis or seizures, are familiar to clinicians; however, gastrointestinal (GI) hypomotility is not always recognized as a potentially serious adverse effect, even though it is one of the most common causes for hospital admission.1 Its manifestations range from being relatively benign (nausea, vomiting, constipation) to potentially severe (fecal impaction) or even life-threatening (bowel obstruction, ileus, toxic megacolon).2
GI hypomotility is caused by clozapine’s strong anticholinergic properties, which lead to slowed smooth muscle contractions and delayed bowel transit time. It is further compounded by clozapine’s 5-HT3 antagonism, which is also known to slow bowel transit time. To avoid the potentially serious risks associated with GI hypomotility, we offer simple approaches for clinicians to follow when treating patients with clozapine.
Before starting a patient on clozapine, and at all subsequent visits, ask him or her about bowel habits and GI symptoms. Because the onset of GI hypomotility can be subtle, ask patients to pay close attention to their bowel habits and keep a diary to document GI symptoms and bowel movements. Signs of bowel obstruction can include an inability to pass stool, nausea, vomiting, abdominal pain, and a bloated abdomen. Staff who care for patients taking clozapine who live in a supervised setting should be educated about the relevance of a patient’s changing bowel habits or GI complaints. Also, teach patients about simple lifestyle modifications they can make to counteract constipation, including increased physical activity, adequate hydration, and consuming a fiber-rich diet.
If possible, avoid prescribing anticholinergic medications to a patient receiving clozapine because such agents may add to clozapine’s anticholinergic load. Some patients may require medical management of chronic constipation with stool softeners and stimulant laxatives. The Table outlines a typical bowel regimen we use for patients at our clinic. Some clinicians may prefer earlier and more regular use of senna glycoside. Also, patients might need a referral to their primary care physician if prevention has failed and fecal impaction requires enemas or mechanical disimpaction. An urgent referral to the emergency department is needed if a patient has a suspected bowel obstruction.
Acknowledgment
The authors thank Travis Baggett, MD, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, for reviewing the medical management of constipation.
1. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
2. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699-709.
1. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
2. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699-709.
10 Myths about ECT
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
