User login
Implementing the VA/DoD Type 2 Diabetes Mellitus Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
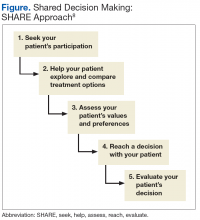
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Implementing the 2017 VA/DoD Diabetes Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Overview and Discussion of the 2017 VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (FULL)
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
Treatment and Management of Patients With Non-Small Cell Lung Cancer (FULL)
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Systems Automation for Cancer Surveillance: A Lean Six Sigma Project for Tracking Care of Patients With Head and Neck Cancer (FULL)
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
VA Nursing Homes Superior to Private-Sector
Data from a VA report on its nursing homesshow that the VA’s 132 community living centers compare closely with 15,487 private-sector nursing homes even though the VA on average cares for sicker patients, with a higher proportion of conditions such as spinal cord injury, PTSD, and combat injury: 25.6% of VA nursing homes rated 5 stars (the highest rating), as did 28.7% of private-sector facilities.
The VA report notes that VA nursing homes do not refuse service to any eligible veteran. The fact that they often house residents with more complex medical needs than private-sector facilities will accept “makes achieving good quality ratings more challenging,” the VA says. VA nursing homes at times rate lower than private-sector facilities on specific metrics such as pain and type of treatment.
But the VA has a significantly lower percentage of 1-star (lowest rated) facilities. Moreover, 60 of the VA’s nursing homes improved their quality score in the past year. The report also says VA nursing homes have a higher staff-to-resident ratio than private-sector facilities, meaning residents in VA facilities get more direct attention
Data from a VA report on its nursing homesshow that the VA’s 132 community living centers compare closely with 15,487 private-sector nursing homes even though the VA on average cares for sicker patients, with a higher proportion of conditions such as spinal cord injury, PTSD, and combat injury: 25.6% of VA nursing homes rated 5 stars (the highest rating), as did 28.7% of private-sector facilities.
The VA report notes that VA nursing homes do not refuse service to any eligible veteran. The fact that they often house residents with more complex medical needs than private-sector facilities will accept “makes achieving good quality ratings more challenging,” the VA says. VA nursing homes at times rate lower than private-sector facilities on specific metrics such as pain and type of treatment.
But the VA has a significantly lower percentage of 1-star (lowest rated) facilities. Moreover, 60 of the VA’s nursing homes improved their quality score in the past year. The report also says VA nursing homes have a higher staff-to-resident ratio than private-sector facilities, meaning residents in VA facilities get more direct attention
Data from a VA report on its nursing homesshow that the VA’s 132 community living centers compare closely with 15,487 private-sector nursing homes even though the VA on average cares for sicker patients, with a higher proportion of conditions such as spinal cord injury, PTSD, and combat injury: 25.6% of VA nursing homes rated 5 stars (the highest rating), as did 28.7% of private-sector facilities.
The VA report notes that VA nursing homes do not refuse service to any eligible veteran. The fact that they often house residents with more complex medical needs than private-sector facilities will accept “makes achieving good quality ratings more challenging,” the VA says. VA nursing homes at times rate lower than private-sector facilities on specific metrics such as pain and type of treatment.
But the VA has a significantly lower percentage of 1-star (lowest rated) facilities. Moreover, 60 of the VA’s nursing homes improved their quality score in the past year. The report also says VA nursing homes have a higher staff-to-resident ratio than private-sector facilities, meaning residents in VA facilities get more direct attention
Strategies to Improve Hepatocellular Carcinoma Surveillance in Veterans With Hepatitis B Infection (FULL)
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
Improving Veteran Access to Treatment for Hepatitis C Virus Infection (FULL)
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
VA Nurses Address Critical Needs
For more than 35 years, the Nurses Organization of Veterans Affairs (NOVA) has been the voice of more than 3,000 Department of Veterans Affairs (VA) nurses caring for veterans. Speaking on behalf of its members, NOVA leaders provide an annual list of its legislative priority goals, which identifies concerns that require either legislation, funding, or implementation at the regulatory level within the VA.
At the top of this list of priorities is the ability to retain, recruit, and hire critical staff. The VA has had difficulty hiring essential staff at many levels within its health care facilities. A VA internal audit found that the need for additional doctors, nurses, and other specialty care was the highest barrier or challenge to providing access to care for veterans. Both congressional VA oversight committees have discussed this issue and included hiring provisions in their respective Choice/Community Care bills that await final action in both chambers.
In its recruitment/staffing goals, NOVA identified the 5 following areas:
- Hire additional human resources (HR) staff and review and streamline policies and procedures to improve the hiring process;
- Review thoroughly downgrades and reclassification of critical positions across the VA;
- Increase training of HR personnel on use of locality pay process in hiring;
- Revise the cap on nurse pay structures and registered nurse pay schedules and reclassification of critical positions so that VA can ensure competitive salaries; and
- Address USAJOBS website problems, including the complexity and excessive time required to complete application and inadequate applications response/feedback.
Addressing Choice/Community Integrated Health Care—Choice 2.0—is another NOVA goal. When the VA cannot provide timely care to veterans, NOVA supports the use of outside provide
Although NOVA supports the addition of community providers as a crucial part of an integrated network designed to provide care where there are shortages, the change has called attention to myriad problems created by outside providers, such as delays in care, the wrong care, or the veteran not being seen at all. Any final Choice/Community Care legislation must include mandatory training for both VA personnel and community providers to improve coordination and timeliness of care and services. The legislation also must hold community providers to the same high standards and quality metrics already in place at the VA.
Last, NOVA addresses information technology (IT) across VHA, which includes supporting an electronic health record for seamless transition of care between DoD and VA, proper funding for all IT stations to improve patient safety, software usability, and standardization of patient health care records across the system. As the VA continues to modernize, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
As an advocate for its members and the patients they serve, NOVA will continue to share its views with Congress, the administration, and VA leadership on how they can work together toward common goals—educating the next generation of nurses, providing innovative health care solutions, or learning how veterans envision their health care. For more information about NOVA, a list of its 2018 Legislative Priority Goals, or to become a member, visit vanurse.org.
For more than 35 years, the Nurses Organization of Veterans Affairs (NOVA) has been the voice of more than 3,000 Department of Veterans Affairs (VA) nurses caring for veterans. Speaking on behalf of its members, NOVA leaders provide an annual list of its legislative priority goals, which identifies concerns that require either legislation, funding, or implementation at the regulatory level within the VA.
At the top of this list of priorities is the ability to retain, recruit, and hire critical staff. The VA has had difficulty hiring essential staff at many levels within its health care facilities. A VA internal audit found that the need for additional doctors, nurses, and other specialty care was the highest barrier or challenge to providing access to care for veterans. Both congressional VA oversight committees have discussed this issue and included hiring provisions in their respective Choice/Community Care bills that await final action in both chambers.
In its recruitment/staffing goals, NOVA identified the 5 following areas:
- Hire additional human resources (HR) staff and review and streamline policies and procedures to improve the hiring process;
- Review thoroughly downgrades and reclassification of critical positions across the VA;
- Increase training of HR personnel on use of locality pay process in hiring;
- Revise the cap on nurse pay structures and registered nurse pay schedules and reclassification of critical positions so that VA can ensure competitive salaries; and
- Address USAJOBS website problems, including the complexity and excessive time required to complete application and inadequate applications response/feedback.
Addressing Choice/Community Integrated Health Care—Choice 2.0—is another NOVA goal. When the VA cannot provide timely care to veterans, NOVA supports the use of outside provide
Although NOVA supports the addition of community providers as a crucial part of an integrated network designed to provide care where there are shortages, the change has called attention to myriad problems created by outside providers, such as delays in care, the wrong care, or the veteran not being seen at all. Any final Choice/Community Care legislation must include mandatory training for both VA personnel and community providers to improve coordination and timeliness of care and services. The legislation also must hold community providers to the same high standards and quality metrics already in place at the VA.
Last, NOVA addresses information technology (IT) across VHA, which includes supporting an electronic health record for seamless transition of care between DoD and VA, proper funding for all IT stations to improve patient safety, software usability, and standardization of patient health care records across the system. As the VA continues to modernize, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
As an advocate for its members and the patients they serve, NOVA will continue to share its views with Congress, the administration, and VA leadership on how they can work together toward common goals—educating the next generation of nurses, providing innovative health care solutions, or learning how veterans envision their health care. For more information about NOVA, a list of its 2018 Legislative Priority Goals, or to become a member, visit vanurse.org.
For more than 35 years, the Nurses Organization of Veterans Affairs (NOVA) has been the voice of more than 3,000 Department of Veterans Affairs (VA) nurses caring for veterans. Speaking on behalf of its members, NOVA leaders provide an annual list of its legislative priority goals, which identifies concerns that require either legislation, funding, or implementation at the regulatory level within the VA.
At the top of this list of priorities is the ability to retain, recruit, and hire critical staff. The VA has had difficulty hiring essential staff at many levels within its health care facilities. A VA internal audit found that the need for additional doctors, nurses, and other specialty care was the highest barrier or challenge to providing access to care for veterans. Both congressional VA oversight committees have discussed this issue and included hiring provisions in their respective Choice/Community Care bills that await final action in both chambers.
In its recruitment/staffing goals, NOVA identified the 5 following areas:
- Hire additional human resources (HR) staff and review and streamline policies and procedures to improve the hiring process;
- Review thoroughly downgrades and reclassification of critical positions across the VA;
- Increase training of HR personnel on use of locality pay process in hiring;
- Revise the cap on nurse pay structures and registered nurse pay schedules and reclassification of critical positions so that VA can ensure competitive salaries; and
- Address USAJOBS website problems, including the complexity and excessive time required to complete application and inadequate applications response/feedback.
Addressing Choice/Community Integrated Health Care—Choice 2.0—is another NOVA goal. When the VA cannot provide timely care to veterans, NOVA supports the use of outside provide
Although NOVA supports the addition of community providers as a crucial part of an integrated network designed to provide care where there are shortages, the change has called attention to myriad problems created by outside providers, such as delays in care, the wrong care, or the veteran not being seen at all. Any final Choice/Community Care legislation must include mandatory training for both VA personnel and community providers to improve coordination and timeliness of care and services. The legislation also must hold community providers to the same high standards and quality metrics already in place at the VA.
Last, NOVA addresses information technology (IT) across VHA, which includes supporting an electronic health record for seamless transition of care between DoD and VA, proper funding for all IT stations to improve patient safety, software usability, and standardization of patient health care records across the system. As the VA continues to modernize, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
As an advocate for its members and the patients they serve, NOVA will continue to share its views with Congress, the administration, and VA leadership on how they can work together toward common goals—educating the next generation of nurses, providing innovative health care solutions, or learning how veterans envision their health care. For more information about NOVA, a list of its 2018 Legislative Priority Goals, or to become a member, visit vanurse.org.
Telehealth for Rural Veterans With Neurologic Disorders (FULL)
Dr. Geppert. I have the pleasure of interviewing Dr. Larry Davis, Distinguished Professor of Neurology at the University of New Mexico School of Medicine in Albuquerque and chief of the NMVAHCS Neurology Service. Welcome, Dr. Davis. Can you describe a teleneurology visit?
Dr. Davis. The NMVAHCS is the only VA located in a large rural state. Location is a real challenge because we treat a lot of veterans who live rurally, and they often have to travel 5 to 6 hours to Albuquerque. When the VA set up its telehealth systems, it was obvious to the NMVAHCS Neurology Service that this was a gold mine. We have many patients who are unable to drive because of their neurologic condition, and they need a caregiver—sometimes a spouse—to drive them to NMVAHCS to see a neurologist, usually in an outpatient setting, often for 30 to 45 minutes, and then drive back 5 hours.
When I get a consult from a rural CBOC, I invite the patient to come to NMVAHCS for the first visit. First, I examine the patient face-to-face so the patient can get to know me. Second, I order special tests or imaging, which are not available in rural areas. Sometimes, for complicated cases, the patient stays overnight.
We discuss the diagnosis with the patient, and make the decision whether the patient is a good candidate for telehealth. If the patient consents and wants to be seen at a local community VA center, we set it up. On a given day, the patient travels—often 15, 20 minutes at most—to the VA facility and goes into a modified examination room. The patient sits in front of a TV screen with a camera focused on him or her. The patient can see me on the screen. In my office I have 2 screens, one has the patient record; the other allows me to see the patient.
Over the years, I have discovered that once I know the patient, it’s just like talking across the table. I can get a history of what has changed either with medications or chronic illness since the last follow-up.
Dr. Geppert. What are the issues and challenges in treating patients with epilepsy, multiple sclerosis, and other neurologic conditions?
Dr. Davis. We have the most difficultly with sensory examinations. I can perform a good motor examination via telehealth, but it is more difficult if I need to look carefully at the patient’s reflexes. We follow individuals with headaches, seizures, multiple sclerosis, Parkinson disease, and a variety of other illnesses. There are not too many we cannot follow that way. If patients have questions, I can look at their medical record and laboratory data while they are on the screen.
The caregiver or spouse can sit next to the patient, so they can be part of the conversation. If the patient is having trouble describing what is going on, the caregiver can offer comments.
Dr. Geppert. If I’m the patient and I’ve had a previous stroke and it looks as though I might have had another, wouldn’t you like to do a neurologic exam but can’t via telehealth?
Dr. Davis. That’s a very good point. When I talk to a patient and I don’t like what I see, I have the ability to ask the patient to come to NMVAHCS. I had one patient who suddenly started getting chest pains during the exam, so we called the CBOC primary care doctor to immediately move the patient to the local hospital. I had another patient who started talking about suicide. I kept the patient on the phone, but we got the primary care doctor in the room. We do have backup.
Dr. Geppert. You mentioned that you often involve family and that you work with local CBOC registered nurses (RNs). Can you tell us how you extend the reach of teleneurology through self-care and family education?
Dr. Davis. We have 2 qualified RN patient educators. One is an expert in Parkinson disease; the other follows up with the patient who has had a stroke, to reduce risk factors for a second stroke.
When I see a patient for a limited time, I deal with the drugs and things like that. I am less focused on what type of chair the patient should sit on, whether the patient might fall, how to safely get up, etc. So I set up a separate appointment with the nurse educator, who goes through all the day-to-day activities that the patient has to be able to do. Patients with Parkinson disease do not like to sit on low sofas, for example. What are the tricks for constipation? If a patient falls, how do you safely get up? The nurse will have the patient get down right in front of the camera and walk them through how to get up.
Dr. Geppert. I know that in many rural areas, especially in our state, there are real shortages of neurologists and psychiatrists. Can you talk about how this helps multiply your ability to care for patients?
Dr. Davis. With the exception of 3 different communities, there are no neurologists within 50 to 100 miles.
Dr. Geppert. Or neuropsychiatrists.
Dr. Davis. Or neuropsychiatrists or even a psychiatry office. So patients are very limited. We have an extremely loyal population of VA patients because it isn’t easy to say, “I’m just going to go down the street and get another doc.”
Dr. Geppert. What type of feedback do you get from patients and family about doing this virtually?
Dr. Davis. We sent a carefully worded satisfaction survey and received 700 responses. We found the following responses: 90% agreed that they received good care during their visit. Ninety-one percent reported that they were able to communicate and 87% would continue their care via teleneurology. Was the teleneurology more convenient than driving here? Yes, 90% said that it was. And did they have overall satisfaction with the visit? Literally 90% reported that they did. It’s a very high satisfaction rate.
We know that patients will say, “Oh, you can see me every 3 months, but I want to come down to see you face-to-face from time-to-time.” When I ask why, the most common answers are either “I live so rurally, there are no stores.” Or “I have family, and I want to come down.” So we then try to set up that face-to-face visit on a Friday.
If they’re traveling, they get their travel pay, but then they get to spend the weekend with a friend or family. There are often secondary reasons. Occasionally, they have to come back for another specialist; we make great efforts to put both those visits on the same day so they don’t have to go back and forth.
Dr. Geppert. So Dr. Davis, you’re a world-famous neurologist, but do you have to have special training or expertise to do teleneurology?
Dr. Davis. It helps to be a good clinician; I’ve been doing this for many years. Because we see patients on a sequence by television, I have to keep track of the time, whereas in the face-to-face clinic if something is really going wrong, I have the ability to shift things around right away and spend more time with that patient. I try to set up longer times for telehealth. I work with a clinical nurse practitioner colleague who takes care of headache patients, and she has a shorter time between visits. I never quite know what’s going to happen, and I want to leave a lot of time for the patient or the spouse to ask questions.
Dr. Geppert. We are both, certainly, senior clinicians and technology is a little different and new. Did you have problems learning to use the teleneurology?
Dr. Davis. Believe it or not, no. The television system is very similar to Skype. It is called Jabber and is encrypted, so it’s safe to transmit. You have to feel comfortable talking to somebody on a TV screen. And the first time I did it, I felt a little awkward. Within a day or two, I was fine.
Dr. Geppert. Federal Practitioner readers may want to start teleneurology or get more involved in it. Are you in touch with other VA and DoD providers?
Dr. Davis. Practitioners can contact me if they want to talk about how we do patient education. We’ve also spent a fair amount of effort developing a handbook that we send out on the ABCs of how to do it.
Dr. Geppert. Do you involve residents in this?
Dr. Davis. No, not at this point. My goal is to do it, but ironically enough, I’m trying to get students who rotate out here to get a feel for what telehealth could be. They don’t do the exam and everything else, but they get a chance to see what telehealth can be. If they want to live in a rural state, they may be involved in it.
Dr. Geppert. If the primary care provider was seeing a patient with multiple sclerosis, which we both know can have symptom flare ups, would they be able to contact you and say, “Hey, Dr. Davis, I’m seeing so and so, and she looks like she’s having some visual problems today.”
Dr. Davis. That’s harder to do because we’re not the only ones using the telehealth system. Telemental health, dietitians, and others are using it all the time, so I have to have a scheduled time to be able to see patients. I’m surprised that more rural states aren’t using it because it really is very enjoyable.
Dr. Geppert. What has the VA learned about telehealth from this program?
Dr. Davis. We know that it actually saves NMVAHCS a lot of money because we don’t pay as much travel pay. The patients like it. The CBOC physicians like it because if I have to, I can type the note right away, and they can read it if the patient is staying there.
Author disclosures
Dr. Hixson has received a collaborative grant from UCB Inc. for research on online epilepsy resources.
Disclaimer
The opinions expressed herein do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Dr. Geppert. I have the pleasure of interviewing Dr. Larry Davis, Distinguished Professor of Neurology at the University of New Mexico School of Medicine in Albuquerque and chief of the NMVAHCS Neurology Service. Welcome, Dr. Davis. Can you describe a teleneurology visit?
Dr. Davis. The NMVAHCS is the only VA located in a large rural state. Location is a real challenge because we treat a lot of veterans who live rurally, and they often have to travel 5 to 6 hours to Albuquerque. When the VA set up its telehealth systems, it was obvious to the NMVAHCS Neurology Service that this was a gold mine. We have many patients who are unable to drive because of their neurologic condition, and they need a caregiver—sometimes a spouse—to drive them to NMVAHCS to see a neurologist, usually in an outpatient setting, often for 30 to 45 minutes, and then drive back 5 hours.
When I get a consult from a rural CBOC, I invite the patient to come to NMVAHCS for the first visit. First, I examine the patient face-to-face so the patient can get to know me. Second, I order special tests or imaging, which are not available in rural areas. Sometimes, for complicated cases, the patient stays overnight.
We discuss the diagnosis with the patient, and make the decision whether the patient is a good candidate for telehealth. If the patient consents and wants to be seen at a local community VA center, we set it up. On a given day, the patient travels—often 15, 20 minutes at most—to the VA facility and goes into a modified examination room. The patient sits in front of a TV screen with a camera focused on him or her. The patient can see me on the screen. In my office I have 2 screens, one has the patient record; the other allows me to see the patient.
Over the years, I have discovered that once I know the patient, it’s just like talking across the table. I can get a history of what has changed either with medications or chronic illness since the last follow-up.
Dr. Geppert. What are the issues and challenges in treating patients with epilepsy, multiple sclerosis, and other neurologic conditions?
Dr. Davis. We have the most difficultly with sensory examinations. I can perform a good motor examination via telehealth, but it is more difficult if I need to look carefully at the patient’s reflexes. We follow individuals with headaches, seizures, multiple sclerosis, Parkinson disease, and a variety of other illnesses. There are not too many we cannot follow that way. If patients have questions, I can look at their medical record and laboratory data while they are on the screen.
The caregiver or spouse can sit next to the patient, so they can be part of the conversation. If the patient is having trouble describing what is going on, the caregiver can offer comments.
Dr. Geppert. If I’m the patient and I’ve had a previous stroke and it looks as though I might have had another, wouldn’t you like to do a neurologic exam but can’t via telehealth?
Dr. Davis. That’s a very good point. When I talk to a patient and I don’t like what I see, I have the ability to ask the patient to come to NMVAHCS. I had one patient who suddenly started getting chest pains during the exam, so we called the CBOC primary care doctor to immediately move the patient to the local hospital. I had another patient who started talking about suicide. I kept the patient on the phone, but we got the primary care doctor in the room. We do have backup.
Dr. Geppert. You mentioned that you often involve family and that you work with local CBOC registered nurses (RNs). Can you tell us how you extend the reach of teleneurology through self-care and family education?
Dr. Davis. We have 2 qualified RN patient educators. One is an expert in Parkinson disease; the other follows up with the patient who has had a stroke, to reduce risk factors for a second stroke.
When I see a patient for a limited time, I deal with the drugs and things like that. I am less focused on what type of chair the patient should sit on, whether the patient might fall, how to safely get up, etc. So I set up a separate appointment with the nurse educator, who goes through all the day-to-day activities that the patient has to be able to do. Patients with Parkinson disease do not like to sit on low sofas, for example. What are the tricks for constipation? If a patient falls, how do you safely get up? The nurse will have the patient get down right in front of the camera and walk them through how to get up.
Dr. Geppert. I know that in many rural areas, especially in our state, there are real shortages of neurologists and psychiatrists. Can you talk about how this helps multiply your ability to care for patients?
Dr. Davis. With the exception of 3 different communities, there are no neurologists within 50 to 100 miles.
Dr. Geppert. Or neuropsychiatrists.
Dr. Davis. Or neuropsychiatrists or even a psychiatry office. So patients are very limited. We have an extremely loyal population of VA patients because it isn’t easy to say, “I’m just going to go down the street and get another doc.”
Dr. Geppert. What type of feedback do you get from patients and family about doing this virtually?
Dr. Davis. We sent a carefully worded satisfaction survey and received 700 responses. We found the following responses: 90% agreed that they received good care during their visit. Ninety-one percent reported that they were able to communicate and 87% would continue their care via teleneurology. Was the teleneurology more convenient than driving here? Yes, 90% said that it was. And did they have overall satisfaction with the visit? Literally 90% reported that they did. It’s a very high satisfaction rate.
We know that patients will say, “Oh, you can see me every 3 months, but I want to come down to see you face-to-face from time-to-time.” When I ask why, the most common answers are either “I live so rurally, there are no stores.” Or “I have family, and I want to come down.” So we then try to set up that face-to-face visit on a Friday.
If they’re traveling, they get their travel pay, but then they get to spend the weekend with a friend or family. There are often secondary reasons. Occasionally, they have to come back for another specialist; we make great efforts to put both those visits on the same day so they don’t have to go back and forth.
Dr. Geppert. So Dr. Davis, you’re a world-famous neurologist, but do you have to have special training or expertise to do teleneurology?
Dr. Davis. It helps to be a good clinician; I’ve been doing this for many years. Because we see patients on a sequence by television, I have to keep track of the time, whereas in the face-to-face clinic if something is really going wrong, I have the ability to shift things around right away and spend more time with that patient. I try to set up longer times for telehealth. I work with a clinical nurse practitioner colleague who takes care of headache patients, and she has a shorter time between visits. I never quite know what’s going to happen, and I want to leave a lot of time for the patient or the spouse to ask questions.
Dr. Geppert. We are both, certainly, senior clinicians and technology is a little different and new. Did you have problems learning to use the teleneurology?
Dr. Davis. Believe it or not, no. The television system is very similar to Skype. It is called Jabber and is encrypted, so it’s safe to transmit. You have to feel comfortable talking to somebody on a TV screen. And the first time I did it, I felt a little awkward. Within a day or two, I was fine.
Dr. Geppert. Federal Practitioner readers may want to start teleneurology or get more involved in it. Are you in touch with other VA and DoD providers?
Dr. Davis. Practitioners can contact me if they want to talk about how we do patient education. We’ve also spent a fair amount of effort developing a handbook that we send out on the ABCs of how to do it.
Dr. Geppert. Do you involve residents in this?
Dr. Davis. No, not at this point. My goal is to do it, but ironically enough, I’m trying to get students who rotate out here to get a feel for what telehealth could be. They don’t do the exam and everything else, but they get a chance to see what telehealth can be. If they want to live in a rural state, they may be involved in it.
Dr. Geppert. If the primary care provider was seeing a patient with multiple sclerosis, which we both know can have symptom flare ups, would they be able to contact you and say, “Hey, Dr. Davis, I’m seeing so and so, and she looks like she’s having some visual problems today.”
Dr. Davis. That’s harder to do because we’re not the only ones using the telehealth system. Telemental health, dietitians, and others are using it all the time, so I have to have a scheduled time to be able to see patients. I’m surprised that more rural states aren’t using it because it really is very enjoyable.
Dr. Geppert. What has the VA learned about telehealth from this program?
Dr. Davis. We know that it actually saves NMVAHCS a lot of money because we don’t pay as much travel pay. The patients like it. The CBOC physicians like it because if I have to, I can type the note right away, and they can read it if the patient is staying there.
Author disclosures
Dr. Hixson has received a collaborative grant from UCB Inc. for research on online epilepsy resources.
Disclaimer
The opinions expressed herein do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Dr. Geppert. I have the pleasure of interviewing Dr. Larry Davis, Distinguished Professor of Neurology at the University of New Mexico School of Medicine in Albuquerque and chief of the NMVAHCS Neurology Service. Welcome, Dr. Davis. Can you describe a teleneurology visit?
Dr. Davis. The NMVAHCS is the only VA located in a large rural state. Location is a real challenge because we treat a lot of veterans who live rurally, and they often have to travel 5 to 6 hours to Albuquerque. When the VA set up its telehealth systems, it was obvious to the NMVAHCS Neurology Service that this was a gold mine. We have many patients who are unable to drive because of their neurologic condition, and they need a caregiver—sometimes a spouse—to drive them to NMVAHCS to see a neurologist, usually in an outpatient setting, often for 30 to 45 minutes, and then drive back 5 hours.
When I get a consult from a rural CBOC, I invite the patient to come to NMVAHCS for the first visit. First, I examine the patient face-to-face so the patient can get to know me. Second, I order special tests or imaging, which are not available in rural areas. Sometimes, for complicated cases, the patient stays overnight.
We discuss the diagnosis with the patient, and make the decision whether the patient is a good candidate for telehealth. If the patient consents and wants to be seen at a local community VA center, we set it up. On a given day, the patient travels—often 15, 20 minutes at most—to the VA facility and goes into a modified examination room. The patient sits in front of a TV screen with a camera focused on him or her. The patient can see me on the screen. In my office I have 2 screens, one has the patient record; the other allows me to see the patient.
Over the years, I have discovered that once I know the patient, it’s just like talking across the table. I can get a history of what has changed either with medications or chronic illness since the last follow-up.
Dr. Geppert. What are the issues and challenges in treating patients with epilepsy, multiple sclerosis, and other neurologic conditions?
Dr. Davis. We have the most difficultly with sensory examinations. I can perform a good motor examination via telehealth, but it is more difficult if I need to look carefully at the patient’s reflexes. We follow individuals with headaches, seizures, multiple sclerosis, Parkinson disease, and a variety of other illnesses. There are not too many we cannot follow that way. If patients have questions, I can look at their medical record and laboratory data while they are on the screen.
The caregiver or spouse can sit next to the patient, so they can be part of the conversation. If the patient is having trouble describing what is going on, the caregiver can offer comments.
Dr. Geppert. If I’m the patient and I’ve had a previous stroke and it looks as though I might have had another, wouldn’t you like to do a neurologic exam but can’t via telehealth?
Dr. Davis. That’s a very good point. When I talk to a patient and I don’t like what I see, I have the ability to ask the patient to come to NMVAHCS. I had one patient who suddenly started getting chest pains during the exam, so we called the CBOC primary care doctor to immediately move the patient to the local hospital. I had another patient who started talking about suicide. I kept the patient on the phone, but we got the primary care doctor in the room. We do have backup.
Dr. Geppert. You mentioned that you often involve family and that you work with local CBOC registered nurses (RNs). Can you tell us how you extend the reach of teleneurology through self-care and family education?
Dr. Davis. We have 2 qualified RN patient educators. One is an expert in Parkinson disease; the other follows up with the patient who has had a stroke, to reduce risk factors for a second stroke.
When I see a patient for a limited time, I deal with the drugs and things like that. I am less focused on what type of chair the patient should sit on, whether the patient might fall, how to safely get up, etc. So I set up a separate appointment with the nurse educator, who goes through all the day-to-day activities that the patient has to be able to do. Patients with Parkinson disease do not like to sit on low sofas, for example. What are the tricks for constipation? If a patient falls, how do you safely get up? The nurse will have the patient get down right in front of the camera and walk them through how to get up.
Dr. Geppert. I know that in many rural areas, especially in our state, there are real shortages of neurologists and psychiatrists. Can you talk about how this helps multiply your ability to care for patients?
Dr. Davis. With the exception of 3 different communities, there are no neurologists within 50 to 100 miles.
Dr. Geppert. Or neuropsychiatrists.
Dr. Davis. Or neuropsychiatrists or even a psychiatry office. So patients are very limited. We have an extremely loyal population of VA patients because it isn’t easy to say, “I’m just going to go down the street and get another doc.”
Dr. Geppert. What type of feedback do you get from patients and family about doing this virtually?
Dr. Davis. We sent a carefully worded satisfaction survey and received 700 responses. We found the following responses: 90% agreed that they received good care during their visit. Ninety-one percent reported that they were able to communicate and 87% would continue their care via teleneurology. Was the teleneurology more convenient than driving here? Yes, 90% said that it was. And did they have overall satisfaction with the visit? Literally 90% reported that they did. It’s a very high satisfaction rate.
We know that patients will say, “Oh, you can see me every 3 months, but I want to come down to see you face-to-face from time-to-time.” When I ask why, the most common answers are either “I live so rurally, there are no stores.” Or “I have family, and I want to come down.” So we then try to set up that face-to-face visit on a Friday.
If they’re traveling, they get their travel pay, but then they get to spend the weekend with a friend or family. There are often secondary reasons. Occasionally, they have to come back for another specialist; we make great efforts to put both those visits on the same day so they don’t have to go back and forth.
Dr. Geppert. So Dr. Davis, you’re a world-famous neurologist, but do you have to have special training or expertise to do teleneurology?
Dr. Davis. It helps to be a good clinician; I’ve been doing this for many years. Because we see patients on a sequence by television, I have to keep track of the time, whereas in the face-to-face clinic if something is really going wrong, I have the ability to shift things around right away and spend more time with that patient. I try to set up longer times for telehealth. I work with a clinical nurse practitioner colleague who takes care of headache patients, and she has a shorter time between visits. I never quite know what’s going to happen, and I want to leave a lot of time for the patient or the spouse to ask questions.
Dr. Geppert. We are both, certainly, senior clinicians and technology is a little different and new. Did you have problems learning to use the teleneurology?
Dr. Davis. Believe it or not, no. The television system is very similar to Skype. It is called Jabber and is encrypted, so it’s safe to transmit. You have to feel comfortable talking to somebody on a TV screen. And the first time I did it, I felt a little awkward. Within a day or two, I was fine.
Dr. Geppert. Federal Practitioner readers may want to start teleneurology or get more involved in it. Are you in touch with other VA and DoD providers?
Dr. Davis. Practitioners can contact me if they want to talk about how we do patient education. We’ve also spent a fair amount of effort developing a handbook that we send out on the ABCs of how to do it.
Dr. Geppert. Do you involve residents in this?
Dr. Davis. No, not at this point. My goal is to do it, but ironically enough, I’m trying to get students who rotate out here to get a feel for what telehealth could be. They don’t do the exam and everything else, but they get a chance to see what telehealth can be. If they want to live in a rural state, they may be involved in it.
Dr. Geppert. If the primary care provider was seeing a patient with multiple sclerosis, which we both know can have symptom flare ups, would they be able to contact you and say, “Hey, Dr. Davis, I’m seeing so and so, and she looks like she’s having some visual problems today.”
Dr. Davis. That’s harder to do because we’re not the only ones using the telehealth system. Telemental health, dietitians, and others are using it all the time, so I have to have a scheduled time to be able to see patients. I’m surprised that more rural states aren’t using it because it really is very enjoyable.
Dr. Geppert. What has the VA learned about telehealth from this program?
Dr. Davis. We know that it actually saves NMVAHCS a lot of money because we don’t pay as much travel pay. The patients like it. The CBOC physicians like it because if I have to, I can type the note right away, and they can read it if the patient is staying there.
Author disclosures
Dr. Hixson has received a collaborative grant from UCB Inc. for research on online epilepsy resources.
Disclaimer
The opinions expressed herein do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.