User login
Inpatient Navigators Reduce Length of Stay without Increasing Readmissions
Clinical question: Does a patient navigator (PN) who facilitates communication between patients and providers impact hospital length of stay (LOS) and readmissions?
Background: Increasing complexity of hospitalization challenges the safety of care transitions. There are few studies about the effectiveness of innovations targeting both communication and transitional care planning.
Study design: Retrospective, cohort study.
Setting: Single academic health center in Canada, 2010-2014.
Synopsis: PNs, dedicated team-based facilitators not responsible for clinical care, served as liaisons between patients and providers on general medicine teams. They rounded with medical teams, tracked action items, expedited tests and consults, and proactively served as direct primary contacts for patients/families during and after hospitalization. PNs had no specified prior training; they underwent on-the-job training with regular feedback.
Researchers matched 7,841 hospitalizations (5,628 with PN; 2,213 without) by case mix, age, and resource intensity. LOS and 30-day readmissions were primary outcomes. Hospitalizations with PNs were 21% shorter (1.3 days; 6.2 v 7.5 days, P<0.001) than those without PNs.
There were no differences in 30-day readmission rates (13.1 v 13.8%, P=0.48). In this single center study in Canada, the impact of PN salaries (the only program cost) relative to savings is unknown.
Bottom line: Inpatient navigators streamline communication and decrease LOS without increasing readmissions. Additional cost-benefit analyses are needed.
Citation: Kwan JL, Morgan MW, Stewart TE, Bell CM. Impact of an innovative patient navigator program on length of stay and 30-day readmission [published online ahead of print August 10, 2015]. J Hosp Med. doi: 10.1002/jhm.2442.
Clinical question: Does a patient navigator (PN) who facilitates communication between patients and providers impact hospital length of stay (LOS) and readmissions?
Background: Increasing complexity of hospitalization challenges the safety of care transitions. There are few studies about the effectiveness of innovations targeting both communication and transitional care planning.
Study design: Retrospective, cohort study.
Setting: Single academic health center in Canada, 2010-2014.
Synopsis: PNs, dedicated team-based facilitators not responsible for clinical care, served as liaisons between patients and providers on general medicine teams. They rounded with medical teams, tracked action items, expedited tests and consults, and proactively served as direct primary contacts for patients/families during and after hospitalization. PNs had no specified prior training; they underwent on-the-job training with regular feedback.
Researchers matched 7,841 hospitalizations (5,628 with PN; 2,213 without) by case mix, age, and resource intensity. LOS and 30-day readmissions were primary outcomes. Hospitalizations with PNs were 21% shorter (1.3 days; 6.2 v 7.5 days, P<0.001) than those without PNs.
There were no differences in 30-day readmission rates (13.1 v 13.8%, P=0.48). In this single center study in Canada, the impact of PN salaries (the only program cost) relative to savings is unknown.
Bottom line: Inpatient navigators streamline communication and decrease LOS without increasing readmissions. Additional cost-benefit analyses are needed.
Citation: Kwan JL, Morgan MW, Stewart TE, Bell CM. Impact of an innovative patient navigator program on length of stay and 30-day readmission [published online ahead of print August 10, 2015]. J Hosp Med. doi: 10.1002/jhm.2442.
Clinical question: Does a patient navigator (PN) who facilitates communication between patients and providers impact hospital length of stay (LOS) and readmissions?
Background: Increasing complexity of hospitalization challenges the safety of care transitions. There are few studies about the effectiveness of innovations targeting both communication and transitional care planning.
Study design: Retrospective, cohort study.
Setting: Single academic health center in Canada, 2010-2014.
Synopsis: PNs, dedicated team-based facilitators not responsible for clinical care, served as liaisons between patients and providers on general medicine teams. They rounded with medical teams, tracked action items, expedited tests and consults, and proactively served as direct primary contacts for patients/families during and after hospitalization. PNs had no specified prior training; they underwent on-the-job training with regular feedback.
Researchers matched 7,841 hospitalizations (5,628 with PN; 2,213 without) by case mix, age, and resource intensity. LOS and 30-day readmissions were primary outcomes. Hospitalizations with PNs were 21% shorter (1.3 days; 6.2 v 7.5 days, P<0.001) than those without PNs.
There were no differences in 30-day readmission rates (13.1 v 13.8%, P=0.48). In this single center study in Canada, the impact of PN salaries (the only program cost) relative to savings is unknown.
Bottom line: Inpatient navigators streamline communication and decrease LOS without increasing readmissions. Additional cost-benefit analyses are needed.
Citation: Kwan JL, Morgan MW, Stewart TE, Bell CM. Impact of an innovative patient navigator program on length of stay and 30-day readmission [published online ahead of print August 10, 2015]. J Hosp Med. doi: 10.1002/jhm.2442.
Dexamethasone Potential Therapy for Asthma Exacerbations in Pediatric Inpatients
Clinical question: In children hospitalized in a non-ICU setting with asthma exacerbation, how effective is dexamethasone compared to prednisone/prednisolone?
Background: Asthma is the second most common reason for hospital admission in childhood.1 National guidelines recommend treatment with systemic corticosteroids in addition to beta-agonists.2 Traditionally, prednisone/prednisolone has been used for asthma exacerbations, but multiple recent studies in ED settings have shown equal efficacy with dexamethasone for mild to moderate exacerbations. Benefits of dexamethasone use include a longer half-life (so single dose or two-day courses can be used), good enteral absorption, general palatability, less emesis, and better adherence. To this point, no studies have compared dexamethasone with prednisone/prednisolone therapy in hospitalized children.
Study design: Multicenter, retrospective cohort study.
Setting: Freestanding, tertiary care children’s hospitals.
Synopsis: The authors used the PHIS (Pediatric Health Information System) database, which includes clinical and billing data from 42 children’s hospitals, to compare children who received dexamethasone to those who were treated with prednisone/prednisolone therapy for asthma exacerbations in the inpatient setting. Patients were included if they were aged four to 17 years, were hospitalized between January 2007 and December 2012 with ICD-9 code for a principal diagnosis of asthma, and received either dexamethasone or prednisone/prednisolone.
Exclusion criteria included:
- Management in the ICU at the time of admission;
- All patient refined diagnosis related groups (APR-DRG) severity level moderate or extreme;
- Complex chronic conditions;
- Secondary diagnosis other than asthma requiring steroids, or treatment with racemic epinephrine;
- Only the first admission was included out of multiple hospitalizations within a 30-day period; and/or
- Patient was treated with both dexamethasone and prednisone/prednisolone.
The primary outcome evaluated was length of stay (LOS); secondary outcomes included readmissions, cost, and transfer to ICU during hospitalization. The authors compared the overall groups, then performed 1:1 propensity score matching to address residual confounding; this statistical technique closely matches patient characteristics between cohorts.
Overall, there were 40,257 hospitalizations, with 1,166 children (2.9%) receiving dexamethasone and 39,091 (97.1%) receiving prednisone/prednisolone. The use of dexamethasone varied greatly between hospitals (35/42 hospitals used dexamethasone, with rates ranging from 0.047% to 77.4%).
In the post-match cohort, 1,284 patients were evaluated, 642 in each group. In this cohort, patients with dexamethasone had significantly shorter LOS (67.4% had LOS less than one day vs. 59.5% in the prednisone/prednisolone group; 6.7% of dexamethasone patients had LOS of more than three days vs. 12% of prednisone/prednisolone patients). Costs were lower for the dexamethasone group, both for the index admission and for episode admission (defined as index admission plus seven-day readmissions). There was no difference in readmissions between the groups, and no patients in this cohort were transferred to the ICU.
There are several limitations to this study. Dexamethasone use varied widely among participating hospitals. The data source did not permit access to dosing, duration, or compliance with therapy and could not compare albuterol use between groups. The findings may not be generalizable to all populations, because it excluded patients with high severity and medical complexity and only evaluated tertiary care children’s hospitals.
Bottom line: Dexamethasone is a potential alternative therapy for asthma exacerbations in the inpatient setting. Further studies are needed to evaluate effectiveness, including dosing, frequency, and duration.
Citation: Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639-644.

References
- Yu H, Wier LM, Elixhauser A. Hospital stays for children, 2009. HCUP statistical brief #118. Agency for Healthcare Research and Quality. August 2011. Accessed November 1, 2015.
- National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma–Summary Report 2007. Accessed November 1, 2015.
Clinical question: In children hospitalized in a non-ICU setting with asthma exacerbation, how effective is dexamethasone compared to prednisone/prednisolone?
Background: Asthma is the second most common reason for hospital admission in childhood.1 National guidelines recommend treatment with systemic corticosteroids in addition to beta-agonists.2 Traditionally, prednisone/prednisolone has been used for asthma exacerbations, but multiple recent studies in ED settings have shown equal efficacy with dexamethasone for mild to moderate exacerbations. Benefits of dexamethasone use include a longer half-life (so single dose or two-day courses can be used), good enteral absorption, general palatability, less emesis, and better adherence. To this point, no studies have compared dexamethasone with prednisone/prednisolone therapy in hospitalized children.
Study design: Multicenter, retrospective cohort study.
Setting: Freestanding, tertiary care children’s hospitals.
Synopsis: The authors used the PHIS (Pediatric Health Information System) database, which includes clinical and billing data from 42 children’s hospitals, to compare children who received dexamethasone to those who were treated with prednisone/prednisolone therapy for asthma exacerbations in the inpatient setting. Patients were included if they were aged four to 17 years, were hospitalized between January 2007 and December 2012 with ICD-9 code for a principal diagnosis of asthma, and received either dexamethasone or prednisone/prednisolone.
Exclusion criteria included:
- Management in the ICU at the time of admission;
- All patient refined diagnosis related groups (APR-DRG) severity level moderate or extreme;
- Complex chronic conditions;
- Secondary diagnosis other than asthma requiring steroids, or treatment with racemic epinephrine;
- Only the first admission was included out of multiple hospitalizations within a 30-day period; and/or
- Patient was treated with both dexamethasone and prednisone/prednisolone.
The primary outcome evaluated was length of stay (LOS); secondary outcomes included readmissions, cost, and transfer to ICU during hospitalization. The authors compared the overall groups, then performed 1:1 propensity score matching to address residual confounding; this statistical technique closely matches patient characteristics between cohorts.
Overall, there were 40,257 hospitalizations, with 1,166 children (2.9%) receiving dexamethasone and 39,091 (97.1%) receiving prednisone/prednisolone. The use of dexamethasone varied greatly between hospitals (35/42 hospitals used dexamethasone, with rates ranging from 0.047% to 77.4%).
In the post-match cohort, 1,284 patients were evaluated, 642 in each group. In this cohort, patients with dexamethasone had significantly shorter LOS (67.4% had LOS less than one day vs. 59.5% in the prednisone/prednisolone group; 6.7% of dexamethasone patients had LOS of more than three days vs. 12% of prednisone/prednisolone patients). Costs were lower for the dexamethasone group, both for the index admission and for episode admission (defined as index admission plus seven-day readmissions). There was no difference in readmissions between the groups, and no patients in this cohort were transferred to the ICU.
There are several limitations to this study. Dexamethasone use varied widely among participating hospitals. The data source did not permit access to dosing, duration, or compliance with therapy and could not compare albuterol use between groups. The findings may not be generalizable to all populations, because it excluded patients with high severity and medical complexity and only evaluated tertiary care children’s hospitals.
Bottom line: Dexamethasone is a potential alternative therapy for asthma exacerbations in the inpatient setting. Further studies are needed to evaluate effectiveness, including dosing, frequency, and duration.
Citation: Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639-644.

References
- Yu H, Wier LM, Elixhauser A. Hospital stays for children, 2009. HCUP statistical brief #118. Agency for Healthcare Research and Quality. August 2011. Accessed November 1, 2015.
- National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma–Summary Report 2007. Accessed November 1, 2015.
Clinical question: In children hospitalized in a non-ICU setting with asthma exacerbation, how effective is dexamethasone compared to prednisone/prednisolone?
Background: Asthma is the second most common reason for hospital admission in childhood.1 National guidelines recommend treatment with systemic corticosteroids in addition to beta-agonists.2 Traditionally, prednisone/prednisolone has been used for asthma exacerbations, but multiple recent studies in ED settings have shown equal efficacy with dexamethasone for mild to moderate exacerbations. Benefits of dexamethasone use include a longer half-life (so single dose or two-day courses can be used), good enteral absorption, general palatability, less emesis, and better adherence. To this point, no studies have compared dexamethasone with prednisone/prednisolone therapy in hospitalized children.
Study design: Multicenter, retrospective cohort study.
Setting: Freestanding, tertiary care children’s hospitals.
Synopsis: The authors used the PHIS (Pediatric Health Information System) database, which includes clinical and billing data from 42 children’s hospitals, to compare children who received dexamethasone to those who were treated with prednisone/prednisolone therapy for asthma exacerbations in the inpatient setting. Patients were included if they were aged four to 17 years, were hospitalized between January 2007 and December 2012 with ICD-9 code for a principal diagnosis of asthma, and received either dexamethasone or prednisone/prednisolone.
Exclusion criteria included:
- Management in the ICU at the time of admission;
- All patient refined diagnosis related groups (APR-DRG) severity level moderate or extreme;
- Complex chronic conditions;
- Secondary diagnosis other than asthma requiring steroids, or treatment with racemic epinephrine;
- Only the first admission was included out of multiple hospitalizations within a 30-day period; and/or
- Patient was treated with both dexamethasone and prednisone/prednisolone.
The primary outcome evaluated was length of stay (LOS); secondary outcomes included readmissions, cost, and transfer to ICU during hospitalization. The authors compared the overall groups, then performed 1:1 propensity score matching to address residual confounding; this statistical technique closely matches patient characteristics between cohorts.
Overall, there were 40,257 hospitalizations, with 1,166 children (2.9%) receiving dexamethasone and 39,091 (97.1%) receiving prednisone/prednisolone. The use of dexamethasone varied greatly between hospitals (35/42 hospitals used dexamethasone, with rates ranging from 0.047% to 77.4%).
In the post-match cohort, 1,284 patients were evaluated, 642 in each group. In this cohort, patients with dexamethasone had significantly shorter LOS (67.4% had LOS less than one day vs. 59.5% in the prednisone/prednisolone group; 6.7% of dexamethasone patients had LOS of more than three days vs. 12% of prednisone/prednisolone patients). Costs were lower for the dexamethasone group, both for the index admission and for episode admission (defined as index admission plus seven-day readmissions). There was no difference in readmissions between the groups, and no patients in this cohort were transferred to the ICU.
There are several limitations to this study. Dexamethasone use varied widely among participating hospitals. The data source did not permit access to dosing, duration, or compliance with therapy and could not compare albuterol use between groups. The findings may not be generalizable to all populations, because it excluded patients with high severity and medical complexity and only evaluated tertiary care children’s hospitals.
Bottom line: Dexamethasone is a potential alternative therapy for asthma exacerbations in the inpatient setting. Further studies are needed to evaluate effectiveness, including dosing, frequency, and duration.
Citation: Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639-644.

References
- Yu H, Wier LM, Elixhauser A. Hospital stays for children, 2009. HCUP statistical brief #118. Agency for Healthcare Research and Quality. August 2011. Accessed November 1, 2015.
- National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma–Summary Report 2007. Accessed November 1, 2015.
What Are the Strategies for Secondary Stroke Prevention after Transient Ischemic Attack?
Case
Mr. G is an 80-year-old man with a pacemaker, peripheral artery disease, atrial fibrillation (AF) on warfarin, and tachy-brady syndrome. He presented after experiencing episodes in which he was unable to speak and had weakness on his right side. He had a normal neurological exam upon arrival to the ED, and his blood pressure was 160/80 mm Hg.
Overview
Transient ischemic attacks (TIAs) are brief interruptions in brain perfusion that do not result in permanent neurologic damage. Up to half a million TIAs occur each year in the U.S., and they account for one third of acute cerebrovascular disease.1 While the term suggests that TIAs are benign, they are in fact an important warning sign of impending stroke and are essentially analogous to unstable angina. Some 10% of TIAs convert to full strokes within 90 days, but growing evidence suggests appropriate interventions can decrease this risk to 3%.2
Unfortunately, the symptoms of TIA have usually resolved by the time patients arrive at the hospital, which makes them challenging to diagnose. This article provides a summary of how to diagnose TIA accurately, using a focused history informed by cerebrovascular localization; how to triage, evaluate, and risk stratify patients; and how to implement preventative strategies.
Review of the Data
Classically, TIAs are defined as lasting less than 24 hours; however, 24 hours is an arbitrary number, and most TIAs last less than one hour.1 Furthermore, this definition has evolved with advances in neuroimaging that reveal that up to 50% of classically defined TIAs have evidence of infarct on MRI.1 There is no absolute temporal cut-off after which infarct is always seen on MRI, but longer duration of symptoms correlates with a higher likelihood of infarct. To reconcile these observations, a recently proposed definition stipulates that a true TIA lasts no more than one hour and does not show evidence of infarct on MRI.3
The causes of TIA are identical to those for ischemic stroke. Cerebral ischemia can result from an embolus, arterial thrombosis, or hypoperfusion due to arterial stenosis. Emboli can be cardiac, most commonly due to AF, or non-cardiac, stemming from a ruptured atherosclerotic plaque in the aortic arch, the carotid or vertebral artery, or an intracranial vessel. Atherosclerotic disease in the carotid arteries or intracranial vessels can also lead to thrombosis and occlusion or flow-related TIAs as a result of severe stenosis.
Risk factors for TIA mirror those for heart disease. Non-modifiable risk factors include older age, black race, male sex, and family history of stroke. Modifiable factors include hypertension, hyperlipidemia, tobacco smoking, diabetes, and AF.4
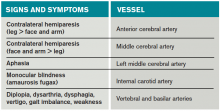
Most of the time, patients’ symptoms will have resolved by the time they are evaluated by a physician. Therefore, the diagnosis of TIA relies almost exclusively on the patient history. Eliciting a good history helps physicians determine whether the episode of transient neurologic dysfunction was caused by cerebral ischemia, as opposed to another mechanism, such as migraine or seizure. This calls for a basic understanding of cerebrovascular anatomy (see Table 1).
Types of Ischemia
Anterior cerebral artery ischemia causes contralateral leg weakness because it supplies the medial frontal and parietal lobes, where the legs in the sensorimotor homunculus are represented. Middle cerebral artery (MCA) ischemia causes contralateral face and arm weakness out of proportion to leg weakness. Ischemia in Broca’s area of the brain, which is supplied by the left MCA, may also cause expressive aphasia. Transient monocular blindness is a TIA of the retina due to atheroemboli originating from the internal carotid artery. Vertebrobasilar TIA is less common than anterior circulation TIA and manifests with brainstem symptoms that include diplopia, dysarthria, dysphagia, vertigo, gait imbalance, and weakness. In general, language and motor symptoms are more specific for cerebral ischemia and therefore more worrisome for TIA than sensory symptoms.5
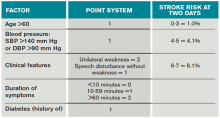
Once a clinical diagnosis of TIA is made, an ABCD2 score (age, blood pressure, clinical features, duration of TIA, presence of diabetes) can be used to predict the short-term risk of subsequent stroke (see Table 2).6,7 A general rule of thumb is to admit patients who present within 72 hours of the event and have an ABCD2 score of three or higher for observation, work-up, and initiation of secondary prevention.1
Although only a small percentage of patients with TIA will have a stroke during the period of observation in the hospital, this approach may be cost effective based on the assumption that hospitalized patients are more likely to receive intravenous tissue plasminogen activator.8 The decision should also be guided by clinical judgment. It is reasonable to admit a patient whose diagnostic workup cannot be rapidly completed.1
The workup for TIA includes routine labs, EKG with cardiac monitoring, and brain imaging. Labs are useful to evaluate for other mimics of TIA such as hyponatremia and glucose abnormalities. In addition, risk factors such as hyperlipidemia and diabetes should be evaluated with fasting lipid panel and blood glucose. The purpose of EKG and telemetry is to identify MI and capture paroxysmal AF. The goal of imaging is to ascertain the presence of vascular disease and to exclude a non-ischemic etiology. While less likely to cause transient neurologic symptoms, a hemorrhagic event must be ruled out, as it would trigger a different management pathway.
Imaging for TIA
There are two primary modes of brain imaging: computed tomography (CT) and MRI. Most patients who are suspected to have had a TIA undergo CT scan, and an infarct is seen about 20% of the time.1 The presence of an infarct usually correlates with the duration of symptoms and has prognostic value. In one study, a new infarct was associated with four times higher risk of stroke in the subsequent 90 days.9 Diffusion-weighted imaging, an MR-based technique, is the preferred modality when it is available because of its higher sensitivity and specificity for identifying acute lesions.1 In an international and multicenter study, incorporating imaging data increased the discriminatory power of stroke prediction.10
Extracranial imaging is mandatory to rule out carotid stenosis as a potential etiology of TIA. The least invasive modality is ultrasound, which can detect carotid stenosis with a sensitivity and specificity approaching 80%.1 While both the intra- and extracranial vasculature can be concurrently assessed using MR- or CT-angiography (CTA), this is not usually necessary in the acute setting, because only detecting carotid stenosis will result in a management change.1
Carotid endarterectomy is standard for symptomatic patients with greater than 70% stenosis and is a consideration for symptomatic patients with greater than 50% stenosis if it is the most probable explanation for the ischemic event.11 Despite a comprehensive workup, about 50% of TIA cases remain cryptogenic.12 In some of these patients, AF can be detected using extended ambulatory cardiac monitoring.12
The goal of admitting high-risk patients is to expedite workup and initiate therapy. Two studies have shown that immediate initiation of preventative treatment significantly reduces the risk of stroke by as much as 80%.13,14 Unless there is a specific indication for anticoagulation, all TIA patients should be started on an antiplatelet agent such as aspirin or clopidogrel. A large randomized trial conducted in China and published in 2013 demonstrated that dual antiplatelet therapy with aspirin and clopidogrel for 21 days, followed by clopidogrel monotherapy, reduced the risk of stroke compared to aspirin monotherapy. An international multicenter trial designed to test the efficacy of short-term dual antiplatelet therapy is ongoing, and if the benefit of this approach is confirmed, this will likely become the standard of care. Evidence-based indications for anticoagulation after TIA are restricted to AF and mural thrombus in the setting of recent MI. Patients with implanted mechanical devices, including left ventricular assist devices and metal heart valves, should also receive anticoagulation.15
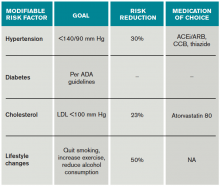
Risk factors should also be targeted in every case. Hypertension should be treated with a goal of lower than 140/90 mm Hg (or 130/80 mm Hg in diabetics and those with renal disease). Studies have shown that patients who are discharged with a blood pressure lower than 140/90 mm Hg are more likely to maintain this blood pressure at one-year follow-up.16 The choice of medication is less well studied, but drugs that act on the renin-angiotensin-aldosterone system and thiazides are generally preferred.15 Treatment with a statin is recommended after cerebrovascular ischemic events, with a goal LDL under 100. This reduces risk of secondary stroke by about 20%.17
At discharge, it is also important to counsel patients on their role in preventing strokes. As with many diseases, making lifestyle changes is key to stroke prevention. Encourage smoking cessation and an increase in physical activity, and discourage heavy alcohol use. The association between smoking and the risk for first stroke is well established. Moderate to high-intensity exercise can reduce secondary stroke risk by as much as 50%18 (see Table 3). While light alcohol consumption can be protective against strokes, heavy use is strongly discouraged. Emerging data suggest obstructive sleep apnea (OSA) may be another modifiable risk factor for stroke and TIA, so screening for potential OSA and referral may be needed.15
Back to the Case
When Mr. G arrived at the ED, his symptoms had resolved. Based on the history of expressive aphasia and right-sided weakness, he most likely had a TIA in the left MCA territory. Hemorrhage was ruled out with a non-contrast head CT. His pacemaker precluded obtaining an MRI. CTA revealed diffuse atherosclerotic disease without evidence of carotid stenosis. His ABCD2 score was six given his age, blood pressure, weakness, and symptom duration, and he was admitted for an expedited workup. His sodium and glucose were within normal limits. His hemoglobin A1c was 6.5%, his LDL was 120, and his international normalized ratio (INR) was therapeutic at 2.1. His TIA may have been due to AF, despite a therapeutic INR, because warfarin does not fully eliminate the stroke risk. It might also have been caused by intracranial atherosclerosis.
Two days later, the patient was discharged on atorvastatin at 80 mg, and his lisinopril was increased for blood pressure control. For his age group, A1c of 6.5% was acceptable, and he was not initiated on glycemic control.
Bottom Line
TIAs are diagnosed based on patient history. Urgent initiation of secondary prevention is important to reduce the short-term risk of stroke and should be implemented by the time of discharge from the hospital.
Dr. Zeng is a hospitalist in the department of internal medicine at Vanderbilt University Medical Center in Nashville, and Dr. Douglas is associate professor in the department of neurology at the University of California at San Francisco.
References
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293.
- Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895, vii.
- Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285.
- Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology. 2005;65(11):1799-1801.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke. 2003;34(12):2894-2898.
- Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010;41(9):1907-1913.
- Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362-387; quiz 367-368.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477.
- Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
- Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46(2):465-470.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026-1039.
Case
Mr. G is an 80-year-old man with a pacemaker, peripheral artery disease, atrial fibrillation (AF) on warfarin, and tachy-brady syndrome. He presented after experiencing episodes in which he was unable to speak and had weakness on his right side. He had a normal neurological exam upon arrival to the ED, and his blood pressure was 160/80 mm Hg.
Overview
Transient ischemic attacks (TIAs) are brief interruptions in brain perfusion that do not result in permanent neurologic damage. Up to half a million TIAs occur each year in the U.S., and they account for one third of acute cerebrovascular disease.1 While the term suggests that TIAs are benign, they are in fact an important warning sign of impending stroke and are essentially analogous to unstable angina. Some 10% of TIAs convert to full strokes within 90 days, but growing evidence suggests appropriate interventions can decrease this risk to 3%.2
Unfortunately, the symptoms of TIA have usually resolved by the time patients arrive at the hospital, which makes them challenging to diagnose. This article provides a summary of how to diagnose TIA accurately, using a focused history informed by cerebrovascular localization; how to triage, evaluate, and risk stratify patients; and how to implement preventative strategies.
Review of the Data
Classically, TIAs are defined as lasting less than 24 hours; however, 24 hours is an arbitrary number, and most TIAs last less than one hour.1 Furthermore, this definition has evolved with advances in neuroimaging that reveal that up to 50% of classically defined TIAs have evidence of infarct on MRI.1 There is no absolute temporal cut-off after which infarct is always seen on MRI, but longer duration of symptoms correlates with a higher likelihood of infarct. To reconcile these observations, a recently proposed definition stipulates that a true TIA lasts no more than one hour and does not show evidence of infarct on MRI.3
The causes of TIA are identical to those for ischemic stroke. Cerebral ischemia can result from an embolus, arterial thrombosis, or hypoperfusion due to arterial stenosis. Emboli can be cardiac, most commonly due to AF, or non-cardiac, stemming from a ruptured atherosclerotic plaque in the aortic arch, the carotid or vertebral artery, or an intracranial vessel. Atherosclerotic disease in the carotid arteries or intracranial vessels can also lead to thrombosis and occlusion or flow-related TIAs as a result of severe stenosis.
Risk factors for TIA mirror those for heart disease. Non-modifiable risk factors include older age, black race, male sex, and family history of stroke. Modifiable factors include hypertension, hyperlipidemia, tobacco smoking, diabetes, and AF.4
Most of the time, patients’ symptoms will have resolved by the time they are evaluated by a physician. Therefore, the diagnosis of TIA relies almost exclusively on the patient history. Eliciting a good history helps physicians determine whether the episode of transient neurologic dysfunction was caused by cerebral ischemia, as opposed to another mechanism, such as migraine or seizure. This calls for a basic understanding of cerebrovascular anatomy (see Table 1).
Types of Ischemia
Anterior cerebral artery ischemia causes contralateral leg weakness because it supplies the medial frontal and parietal lobes, where the legs in the sensorimotor homunculus are represented. Middle cerebral artery (MCA) ischemia causes contralateral face and arm weakness out of proportion to leg weakness. Ischemia in Broca’s area of the brain, which is supplied by the left MCA, may also cause expressive aphasia. Transient monocular blindness is a TIA of the retina due to atheroemboli originating from the internal carotid artery. Vertebrobasilar TIA is less common than anterior circulation TIA and manifests with brainstem symptoms that include diplopia, dysarthria, dysphagia, vertigo, gait imbalance, and weakness. In general, language and motor symptoms are more specific for cerebral ischemia and therefore more worrisome for TIA than sensory symptoms.5
Once a clinical diagnosis of TIA is made, an ABCD2 score (age, blood pressure, clinical features, duration of TIA, presence of diabetes) can be used to predict the short-term risk of subsequent stroke (see Table 2).6,7 A general rule of thumb is to admit patients who present within 72 hours of the event and have an ABCD2 score of three or higher for observation, work-up, and initiation of secondary prevention.1
Although only a small percentage of patients with TIA will have a stroke during the period of observation in the hospital, this approach may be cost effective based on the assumption that hospitalized patients are more likely to receive intravenous tissue plasminogen activator.8 The decision should also be guided by clinical judgment. It is reasonable to admit a patient whose diagnostic workup cannot be rapidly completed.1
The workup for TIA includes routine labs, EKG with cardiac monitoring, and brain imaging. Labs are useful to evaluate for other mimics of TIA such as hyponatremia and glucose abnormalities. In addition, risk factors such as hyperlipidemia and diabetes should be evaluated with fasting lipid panel and blood glucose. The purpose of EKG and telemetry is to identify MI and capture paroxysmal AF. The goal of imaging is to ascertain the presence of vascular disease and to exclude a non-ischemic etiology. While less likely to cause transient neurologic symptoms, a hemorrhagic event must be ruled out, as it would trigger a different management pathway.
Imaging for TIA
There are two primary modes of brain imaging: computed tomography (CT) and MRI. Most patients who are suspected to have had a TIA undergo CT scan, and an infarct is seen about 20% of the time.1 The presence of an infarct usually correlates with the duration of symptoms and has prognostic value. In one study, a new infarct was associated with four times higher risk of stroke in the subsequent 90 days.9 Diffusion-weighted imaging, an MR-based technique, is the preferred modality when it is available because of its higher sensitivity and specificity for identifying acute lesions.1 In an international and multicenter study, incorporating imaging data increased the discriminatory power of stroke prediction.10
Extracranial imaging is mandatory to rule out carotid stenosis as a potential etiology of TIA. The least invasive modality is ultrasound, which can detect carotid stenosis with a sensitivity and specificity approaching 80%.1 While both the intra- and extracranial vasculature can be concurrently assessed using MR- or CT-angiography (CTA), this is not usually necessary in the acute setting, because only detecting carotid stenosis will result in a management change.1
Carotid endarterectomy is standard for symptomatic patients with greater than 70% stenosis and is a consideration for symptomatic patients with greater than 50% stenosis if it is the most probable explanation for the ischemic event.11 Despite a comprehensive workup, about 50% of TIA cases remain cryptogenic.12 In some of these patients, AF can be detected using extended ambulatory cardiac monitoring.12
The goal of admitting high-risk patients is to expedite workup and initiate therapy. Two studies have shown that immediate initiation of preventative treatment significantly reduces the risk of stroke by as much as 80%.13,14 Unless there is a specific indication for anticoagulation, all TIA patients should be started on an antiplatelet agent such as aspirin or clopidogrel. A large randomized trial conducted in China and published in 2013 demonstrated that dual antiplatelet therapy with aspirin and clopidogrel for 21 days, followed by clopidogrel monotherapy, reduced the risk of stroke compared to aspirin monotherapy. An international multicenter trial designed to test the efficacy of short-term dual antiplatelet therapy is ongoing, and if the benefit of this approach is confirmed, this will likely become the standard of care. Evidence-based indications for anticoagulation after TIA are restricted to AF and mural thrombus in the setting of recent MI. Patients with implanted mechanical devices, including left ventricular assist devices and metal heart valves, should also receive anticoagulation.15
Risk factors should also be targeted in every case. Hypertension should be treated with a goal of lower than 140/90 mm Hg (or 130/80 mm Hg in diabetics and those with renal disease). Studies have shown that patients who are discharged with a blood pressure lower than 140/90 mm Hg are more likely to maintain this blood pressure at one-year follow-up.16 The choice of medication is less well studied, but drugs that act on the renin-angiotensin-aldosterone system and thiazides are generally preferred.15 Treatment with a statin is recommended after cerebrovascular ischemic events, with a goal LDL under 100. This reduces risk of secondary stroke by about 20%.17
At discharge, it is also important to counsel patients on their role in preventing strokes. As with many diseases, making lifestyle changes is key to stroke prevention. Encourage smoking cessation and an increase in physical activity, and discourage heavy alcohol use. The association between smoking and the risk for first stroke is well established. Moderate to high-intensity exercise can reduce secondary stroke risk by as much as 50%18 (see Table 3). While light alcohol consumption can be protective against strokes, heavy use is strongly discouraged. Emerging data suggest obstructive sleep apnea (OSA) may be another modifiable risk factor for stroke and TIA, so screening for potential OSA and referral may be needed.15
Back to the Case
When Mr. G arrived at the ED, his symptoms had resolved. Based on the history of expressive aphasia and right-sided weakness, he most likely had a TIA in the left MCA territory. Hemorrhage was ruled out with a non-contrast head CT. His pacemaker precluded obtaining an MRI. CTA revealed diffuse atherosclerotic disease without evidence of carotid stenosis. His ABCD2 score was six given his age, blood pressure, weakness, and symptom duration, and he was admitted for an expedited workup. His sodium and glucose were within normal limits. His hemoglobin A1c was 6.5%, his LDL was 120, and his international normalized ratio (INR) was therapeutic at 2.1. His TIA may have been due to AF, despite a therapeutic INR, because warfarin does not fully eliminate the stroke risk. It might also have been caused by intracranial atherosclerosis.
Two days later, the patient was discharged on atorvastatin at 80 mg, and his lisinopril was increased for blood pressure control. For his age group, A1c of 6.5% was acceptable, and he was not initiated on glycemic control.
Bottom Line
TIAs are diagnosed based on patient history. Urgent initiation of secondary prevention is important to reduce the short-term risk of stroke and should be implemented by the time of discharge from the hospital.
Dr. Zeng is a hospitalist in the department of internal medicine at Vanderbilt University Medical Center in Nashville, and Dr. Douglas is associate professor in the department of neurology at the University of California at San Francisco.
References
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293.
- Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895, vii.
- Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285.
- Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology. 2005;65(11):1799-1801.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke. 2003;34(12):2894-2898.
- Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010;41(9):1907-1913.
- Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362-387; quiz 367-368.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477.
- Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
- Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46(2):465-470.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026-1039.
Case
Mr. G is an 80-year-old man with a pacemaker, peripheral artery disease, atrial fibrillation (AF) on warfarin, and tachy-brady syndrome. He presented after experiencing episodes in which he was unable to speak and had weakness on his right side. He had a normal neurological exam upon arrival to the ED, and his blood pressure was 160/80 mm Hg.
Overview
Transient ischemic attacks (TIAs) are brief interruptions in brain perfusion that do not result in permanent neurologic damage. Up to half a million TIAs occur each year in the U.S., and they account for one third of acute cerebrovascular disease.1 While the term suggests that TIAs are benign, they are in fact an important warning sign of impending stroke and are essentially analogous to unstable angina. Some 10% of TIAs convert to full strokes within 90 days, but growing evidence suggests appropriate interventions can decrease this risk to 3%.2
Unfortunately, the symptoms of TIA have usually resolved by the time patients arrive at the hospital, which makes them challenging to diagnose. This article provides a summary of how to diagnose TIA accurately, using a focused history informed by cerebrovascular localization; how to triage, evaluate, and risk stratify patients; and how to implement preventative strategies.
Review of the Data
Classically, TIAs are defined as lasting less than 24 hours; however, 24 hours is an arbitrary number, and most TIAs last less than one hour.1 Furthermore, this definition has evolved with advances in neuroimaging that reveal that up to 50% of classically defined TIAs have evidence of infarct on MRI.1 There is no absolute temporal cut-off after which infarct is always seen on MRI, but longer duration of symptoms correlates with a higher likelihood of infarct. To reconcile these observations, a recently proposed definition stipulates that a true TIA lasts no more than one hour and does not show evidence of infarct on MRI.3
The causes of TIA are identical to those for ischemic stroke. Cerebral ischemia can result from an embolus, arterial thrombosis, or hypoperfusion due to arterial stenosis. Emboli can be cardiac, most commonly due to AF, or non-cardiac, stemming from a ruptured atherosclerotic plaque in the aortic arch, the carotid or vertebral artery, or an intracranial vessel. Atherosclerotic disease in the carotid arteries or intracranial vessels can also lead to thrombosis and occlusion or flow-related TIAs as a result of severe stenosis.
Risk factors for TIA mirror those for heart disease. Non-modifiable risk factors include older age, black race, male sex, and family history of stroke. Modifiable factors include hypertension, hyperlipidemia, tobacco smoking, diabetes, and AF.4
Most of the time, patients’ symptoms will have resolved by the time they are evaluated by a physician. Therefore, the diagnosis of TIA relies almost exclusively on the patient history. Eliciting a good history helps physicians determine whether the episode of transient neurologic dysfunction was caused by cerebral ischemia, as opposed to another mechanism, such as migraine or seizure. This calls for a basic understanding of cerebrovascular anatomy (see Table 1).
Types of Ischemia
Anterior cerebral artery ischemia causes contralateral leg weakness because it supplies the medial frontal and parietal lobes, where the legs in the sensorimotor homunculus are represented. Middle cerebral artery (MCA) ischemia causes contralateral face and arm weakness out of proportion to leg weakness. Ischemia in Broca’s area of the brain, which is supplied by the left MCA, may also cause expressive aphasia. Transient monocular blindness is a TIA of the retina due to atheroemboli originating from the internal carotid artery. Vertebrobasilar TIA is less common than anterior circulation TIA and manifests with brainstem symptoms that include diplopia, dysarthria, dysphagia, vertigo, gait imbalance, and weakness. In general, language and motor symptoms are more specific for cerebral ischemia and therefore more worrisome for TIA than sensory symptoms.5
Once a clinical diagnosis of TIA is made, an ABCD2 score (age, blood pressure, clinical features, duration of TIA, presence of diabetes) can be used to predict the short-term risk of subsequent stroke (see Table 2).6,7 A general rule of thumb is to admit patients who present within 72 hours of the event and have an ABCD2 score of three or higher for observation, work-up, and initiation of secondary prevention.1
Although only a small percentage of patients with TIA will have a stroke during the period of observation in the hospital, this approach may be cost effective based on the assumption that hospitalized patients are more likely to receive intravenous tissue plasminogen activator.8 The decision should also be guided by clinical judgment. It is reasonable to admit a patient whose diagnostic workup cannot be rapidly completed.1
The workup for TIA includes routine labs, EKG with cardiac monitoring, and brain imaging. Labs are useful to evaluate for other mimics of TIA such as hyponatremia and glucose abnormalities. In addition, risk factors such as hyperlipidemia and diabetes should be evaluated with fasting lipid panel and blood glucose. The purpose of EKG and telemetry is to identify MI and capture paroxysmal AF. The goal of imaging is to ascertain the presence of vascular disease and to exclude a non-ischemic etiology. While less likely to cause transient neurologic symptoms, a hemorrhagic event must be ruled out, as it would trigger a different management pathway.
Imaging for TIA
There are two primary modes of brain imaging: computed tomography (CT) and MRI. Most patients who are suspected to have had a TIA undergo CT scan, and an infarct is seen about 20% of the time.1 The presence of an infarct usually correlates with the duration of symptoms and has prognostic value. In one study, a new infarct was associated with four times higher risk of stroke in the subsequent 90 days.9 Diffusion-weighted imaging, an MR-based technique, is the preferred modality when it is available because of its higher sensitivity and specificity for identifying acute lesions.1 In an international and multicenter study, incorporating imaging data increased the discriminatory power of stroke prediction.10
Extracranial imaging is mandatory to rule out carotid stenosis as a potential etiology of TIA. The least invasive modality is ultrasound, which can detect carotid stenosis with a sensitivity and specificity approaching 80%.1 While both the intra- and extracranial vasculature can be concurrently assessed using MR- or CT-angiography (CTA), this is not usually necessary in the acute setting, because only detecting carotid stenosis will result in a management change.1
Carotid endarterectomy is standard for symptomatic patients with greater than 70% stenosis and is a consideration for symptomatic patients with greater than 50% stenosis if it is the most probable explanation for the ischemic event.11 Despite a comprehensive workup, about 50% of TIA cases remain cryptogenic.12 In some of these patients, AF can be detected using extended ambulatory cardiac monitoring.12
The goal of admitting high-risk patients is to expedite workup and initiate therapy. Two studies have shown that immediate initiation of preventative treatment significantly reduces the risk of stroke by as much as 80%.13,14 Unless there is a specific indication for anticoagulation, all TIA patients should be started on an antiplatelet agent such as aspirin or clopidogrel. A large randomized trial conducted in China and published in 2013 demonstrated that dual antiplatelet therapy with aspirin and clopidogrel for 21 days, followed by clopidogrel monotherapy, reduced the risk of stroke compared to aspirin monotherapy. An international multicenter trial designed to test the efficacy of short-term dual antiplatelet therapy is ongoing, and if the benefit of this approach is confirmed, this will likely become the standard of care. Evidence-based indications for anticoagulation after TIA are restricted to AF and mural thrombus in the setting of recent MI. Patients with implanted mechanical devices, including left ventricular assist devices and metal heart valves, should also receive anticoagulation.15
Risk factors should also be targeted in every case. Hypertension should be treated with a goal of lower than 140/90 mm Hg (or 130/80 mm Hg in diabetics and those with renal disease). Studies have shown that patients who are discharged with a blood pressure lower than 140/90 mm Hg are more likely to maintain this blood pressure at one-year follow-up.16 The choice of medication is less well studied, but drugs that act on the renin-angiotensin-aldosterone system and thiazides are generally preferred.15 Treatment with a statin is recommended after cerebrovascular ischemic events, with a goal LDL under 100. This reduces risk of secondary stroke by about 20%.17
At discharge, it is also important to counsel patients on their role in preventing strokes. As with many diseases, making lifestyle changes is key to stroke prevention. Encourage smoking cessation and an increase in physical activity, and discourage heavy alcohol use. The association between smoking and the risk for first stroke is well established. Moderate to high-intensity exercise can reduce secondary stroke risk by as much as 50%18 (see Table 3). While light alcohol consumption can be protective against strokes, heavy use is strongly discouraged. Emerging data suggest obstructive sleep apnea (OSA) may be another modifiable risk factor for stroke and TIA, so screening for potential OSA and referral may be needed.15
Back to the Case
When Mr. G arrived at the ED, his symptoms had resolved. Based on the history of expressive aphasia and right-sided weakness, he most likely had a TIA in the left MCA territory. Hemorrhage was ruled out with a non-contrast head CT. His pacemaker precluded obtaining an MRI. CTA revealed diffuse atherosclerotic disease without evidence of carotid stenosis. His ABCD2 score was six given his age, blood pressure, weakness, and symptom duration, and he was admitted for an expedited workup. His sodium and glucose were within normal limits. His hemoglobin A1c was 6.5%, his LDL was 120, and his international normalized ratio (INR) was therapeutic at 2.1. His TIA may have been due to AF, despite a therapeutic INR, because warfarin does not fully eliminate the stroke risk. It might also have been caused by intracranial atherosclerosis.
Two days later, the patient was discharged on atorvastatin at 80 mg, and his lisinopril was increased for blood pressure control. For his age group, A1c of 6.5% was acceptable, and he was not initiated on glycemic control.
Bottom Line
TIAs are diagnosed based on patient history. Urgent initiation of secondary prevention is important to reduce the short-term risk of stroke and should be implemented by the time of discharge from the hospital.
Dr. Zeng is a hospitalist in the department of internal medicine at Vanderbilt University Medical Center in Nashville, and Dr. Douglas is associate professor in the department of neurology at the University of California at San Francisco.
References
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293.
- Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895, vii.
- Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285.
- Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology. 2005;65(11):1799-1801.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke. 2003;34(12):2894-2898.
- Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010;41(9):1907-1913.
- Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362-387; quiz 367-368.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477.
- Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
- Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46(2):465-470.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026-1039.
Younger Type 2 Diabetics Face Greater Mortality Risks
NEW YORK - People with type 2 diabetes are 15 percent more likely to die from any cause and 14 percent more likely to die from a cardiovascular cause than non-diabetics at any given time, according to data from several Swedish registries.
The rates are significantly lower than previous estimates. Fifteen years ago, research was suggesting that having diabetes doubled the risk of premature death.
But the new study also found that the risk was dramatically elevated among people whose type 2 diabetes appeared by age 54. The worse their glycemic control and the more evidence of renal problems, the higher the risk.
In contrast, by age 75, type 2 diabetes posed little additional risk for people with good control and no kidney issues, according to the results.
"The overall increased risk of 15 percent among type 2 diabetics in general is a very low figure that has not been found in earlier type 2 diabetes studies," coauthor Dr. Marcus Lind of Uddevalla Hospital said in a telephone interview.
"The other thing that was interesting is that when we looked at patients with good glycemic control and no renal complications, if they were 75 years age, they had a lower risk than those in the general population. That hasn't been shown before," he said.
"What we are seeing is, if you are younger, aggressive management makes a difference," said Dr. Robert Ratner, chief scientific and medical officer of the American Diabetes Association, who was not involved in the research. At age 75, "you don't have to worry about it as much."
The study, published in the October 29 New England Journal of Medicine, is the largest to date to look at premature death in general - and death from cardiovascular causes in particular - among people with type 2 diabetes.
It compared more than 435,000 diabetics who were followed for a mean of 4.6 years with more than 2 million matched controls who were tracked for a mean of 4.8 years. The diabetics had had glucose problems for an average of 5.7 years.
In terms of actual death rates, cardiovascular mortality during the study period was 7.9 percent for diabetics versus 6.1 percent for controls (adjusted hazard ratio, 1.14; 95 percent confidence interval: 1.13-1.15). The respective rates for death from any cause were 17.7 percent and 14.5 percent (aHR, 1.15; 95 percent CI: 1.14-1.16).
For patients under age 55 with glycated hemoglobin levels below 7.0 percent, the risk of death from any cause nearly doubled (aHR, 1.92; 95 percent CI: 1.75-2.11).
"Those who are younger than 55, those who have target glycemic control and no signs of any renal complications, they had a clearly-elevated risk," said Dr. Lind.
But for people over 75, the hazard was actually 5 percent lower than it was for people without diabetes (aHR, 0.95; 95 percent CI: 0.94-0.96).
When the research team factored in people with normoalbuminuria, the risks were slightly mitigated.
Heart attack was the most common cause of death among diabetics.
When glycated hemoglobin levels were at 9.7 percent and higher for people below age 55, the hazard of death from any cause more than quadrupled. The hazard of death from cardiovascular causes rose more than five-fold.
Once again, the danger was far less extreme for people over 75, the researchers found.
"Excess mortality in type 2 diabetes was substantially higher with worsening glycemic control, severe renal complications, impaired renal function, and younger age," they concluded.
Renal function is a key element, Dr. Ratner said.
The study "reinforces the importance of early aggressive management of diabetes in order to prevent premature death and the fact is that the prevention of renal disease is probably the most potent thing we can do to reduce cardiovascular events," he said.
Dr. Lind said the risk may appear lower in the elderly because older people with diabetes are more likely to be getting aggressive treatment for their high blood pressure and high lipid levels, therapy that other people who also have hypertension and high cholesterol levels might not be receiving.
"I think that's the reason the rates are a bit lower" for seniors, he said.
Dr. Ratner said he believes the data for older diabetics simply reflects the fact that "you're seeing the survival cohort. They've made it past the difficult time."
We're all going to die, he said. "The issue is when does it happen? With diabetes, it's happening years ahead of time - in their 50s and early 60s, more so than when they reach 75. The younger you are, the greater that risk. That's when aggressive therapy should be given."
NEW YORK - People with type 2 diabetes are 15 percent more likely to die from any cause and 14 percent more likely to die from a cardiovascular cause than non-diabetics at any given time, according to data from several Swedish registries.
The rates are significantly lower than previous estimates. Fifteen years ago, research was suggesting that having diabetes doubled the risk of premature death.
But the new study also found that the risk was dramatically elevated among people whose type 2 diabetes appeared by age 54. The worse their glycemic control and the more evidence of renal problems, the higher the risk.
In contrast, by age 75, type 2 diabetes posed little additional risk for people with good control and no kidney issues, according to the results.
"The overall increased risk of 15 percent among type 2 diabetics in general is a very low figure that has not been found in earlier type 2 diabetes studies," coauthor Dr. Marcus Lind of Uddevalla Hospital said in a telephone interview.
"The other thing that was interesting is that when we looked at patients with good glycemic control and no renal complications, if they were 75 years age, they had a lower risk than those in the general population. That hasn't been shown before," he said.
"What we are seeing is, if you are younger, aggressive management makes a difference," said Dr. Robert Ratner, chief scientific and medical officer of the American Diabetes Association, who was not involved in the research. At age 75, "you don't have to worry about it as much."
The study, published in the October 29 New England Journal of Medicine, is the largest to date to look at premature death in general - and death from cardiovascular causes in particular - among people with type 2 diabetes.
It compared more than 435,000 diabetics who were followed for a mean of 4.6 years with more than 2 million matched controls who were tracked for a mean of 4.8 years. The diabetics had had glucose problems for an average of 5.7 years.
In terms of actual death rates, cardiovascular mortality during the study period was 7.9 percent for diabetics versus 6.1 percent for controls (adjusted hazard ratio, 1.14; 95 percent confidence interval: 1.13-1.15). The respective rates for death from any cause were 17.7 percent and 14.5 percent (aHR, 1.15; 95 percent CI: 1.14-1.16).
For patients under age 55 with glycated hemoglobin levels below 7.0 percent, the risk of death from any cause nearly doubled (aHR, 1.92; 95 percent CI: 1.75-2.11).
"Those who are younger than 55, those who have target glycemic control and no signs of any renal complications, they had a clearly-elevated risk," said Dr. Lind.
But for people over 75, the hazard was actually 5 percent lower than it was for people without diabetes (aHR, 0.95; 95 percent CI: 0.94-0.96).
When the research team factored in people with normoalbuminuria, the risks were slightly mitigated.
Heart attack was the most common cause of death among diabetics.
When glycated hemoglobin levels were at 9.7 percent and higher for people below age 55, the hazard of death from any cause more than quadrupled. The hazard of death from cardiovascular causes rose more than five-fold.
Once again, the danger was far less extreme for people over 75, the researchers found.
"Excess mortality in type 2 diabetes was substantially higher with worsening glycemic control, severe renal complications, impaired renal function, and younger age," they concluded.
Renal function is a key element, Dr. Ratner said.
The study "reinforces the importance of early aggressive management of diabetes in order to prevent premature death and the fact is that the prevention of renal disease is probably the most potent thing we can do to reduce cardiovascular events," he said.
Dr. Lind said the risk may appear lower in the elderly because older people with diabetes are more likely to be getting aggressive treatment for their high blood pressure and high lipid levels, therapy that other people who also have hypertension and high cholesterol levels might not be receiving.
"I think that's the reason the rates are a bit lower" for seniors, he said.
Dr. Ratner said he believes the data for older diabetics simply reflects the fact that "you're seeing the survival cohort. They've made it past the difficult time."
We're all going to die, he said. "The issue is when does it happen? With diabetes, it's happening years ahead of time - in their 50s and early 60s, more so than when they reach 75. The younger you are, the greater that risk. That's when aggressive therapy should be given."
NEW YORK - People with type 2 diabetes are 15 percent more likely to die from any cause and 14 percent more likely to die from a cardiovascular cause than non-diabetics at any given time, according to data from several Swedish registries.
The rates are significantly lower than previous estimates. Fifteen years ago, research was suggesting that having diabetes doubled the risk of premature death.
But the new study also found that the risk was dramatically elevated among people whose type 2 diabetes appeared by age 54. The worse their glycemic control and the more evidence of renal problems, the higher the risk.
In contrast, by age 75, type 2 diabetes posed little additional risk for people with good control and no kidney issues, according to the results.
"The overall increased risk of 15 percent among type 2 diabetics in general is a very low figure that has not been found in earlier type 2 diabetes studies," coauthor Dr. Marcus Lind of Uddevalla Hospital said in a telephone interview.
"The other thing that was interesting is that when we looked at patients with good glycemic control and no renal complications, if they were 75 years age, they had a lower risk than those in the general population. That hasn't been shown before," he said.
"What we are seeing is, if you are younger, aggressive management makes a difference," said Dr. Robert Ratner, chief scientific and medical officer of the American Diabetes Association, who was not involved in the research. At age 75, "you don't have to worry about it as much."
The study, published in the October 29 New England Journal of Medicine, is the largest to date to look at premature death in general - and death from cardiovascular causes in particular - among people with type 2 diabetes.
It compared more than 435,000 diabetics who were followed for a mean of 4.6 years with more than 2 million matched controls who were tracked for a mean of 4.8 years. The diabetics had had glucose problems for an average of 5.7 years.
In terms of actual death rates, cardiovascular mortality during the study period was 7.9 percent for diabetics versus 6.1 percent for controls (adjusted hazard ratio, 1.14; 95 percent confidence interval: 1.13-1.15). The respective rates for death from any cause were 17.7 percent and 14.5 percent (aHR, 1.15; 95 percent CI: 1.14-1.16).
For patients under age 55 with glycated hemoglobin levels below 7.0 percent, the risk of death from any cause nearly doubled (aHR, 1.92; 95 percent CI: 1.75-2.11).
"Those who are younger than 55, those who have target glycemic control and no signs of any renal complications, they had a clearly-elevated risk," said Dr. Lind.
But for people over 75, the hazard was actually 5 percent lower than it was for people without diabetes (aHR, 0.95; 95 percent CI: 0.94-0.96).
When the research team factored in people with normoalbuminuria, the risks were slightly mitigated.
Heart attack was the most common cause of death among diabetics.
When glycated hemoglobin levels were at 9.7 percent and higher for people below age 55, the hazard of death from any cause more than quadrupled. The hazard of death from cardiovascular causes rose more than five-fold.
Once again, the danger was far less extreme for people over 75, the researchers found.
"Excess mortality in type 2 diabetes was substantially higher with worsening glycemic control, severe renal complications, impaired renal function, and younger age," they concluded.
Renal function is a key element, Dr. Ratner said.
The study "reinforces the importance of early aggressive management of diabetes in order to prevent premature death and the fact is that the prevention of renal disease is probably the most potent thing we can do to reduce cardiovascular events," he said.
Dr. Lind said the risk may appear lower in the elderly because older people with diabetes are more likely to be getting aggressive treatment for their high blood pressure and high lipid levels, therapy that other people who also have hypertension and high cholesterol levels might not be receiving.
"I think that's the reason the rates are a bit lower" for seniors, he said.
Dr. Ratner said he believes the data for older diabetics simply reflects the fact that "you're seeing the survival cohort. They've made it past the difficult time."
We're all going to die, he said. "The issue is when does it happen? With diabetes, it's happening years ahead of time - in their 50s and early 60s, more so than when they reach 75. The younger you are, the greater that risk. That's when aggressive therapy should be given."
Oral Steroids Not Inferior to Intravenous Steroids in Multiple Sclerosis Relapses
Clinical question: Is there any difference between oral and intravenous methylprednisolone for multiple sclerosis relapses?
Background: When relapses of multiple sclerosis occur, studies have shown that intravenous steroids are the treatment of choice. Prior Cochrane meta-analyses have not found any significant difference between intravenous and oral treatments; however, the studies all have had limitations. This study was designed to provide a statistically significant answer.
Study design: Randomized, double-blinded, noninferiority trial.
Setting: Thirteen multiple sclerosis centers in France.
Synopsis: Patients were selected if they had had a relapse within the previous 15 days; the mean time was seven days. One hundred patients were in the oral steroid group, and 99 were in the intravenous steroid group. Each group received 1 g of methylprednisolone daily for three days. In addition, each group received saline infusions or placebo capsules to keep the study blind.
After 28 days, 81% of the oral group and 80% of the intravenous group had improvements of their symptoms. Side effects from the medications were similar as well.
The study was limited by the fixed dosing (1 g daily) that was not bioequivalent. Also, MRIs, although not always used in relapses, could have added more objective information, as everyone was followed clinically using the Kurtzke Functional System Scale.
Bottom line: Consider using oral instead of IV steroids in patients with relapsing multiple sclerosis.
Citation: Le Page E, Veillard D, Laplaud DA, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981.
Clinical question: Is there any difference between oral and intravenous methylprednisolone for multiple sclerosis relapses?
Background: When relapses of multiple sclerosis occur, studies have shown that intravenous steroids are the treatment of choice. Prior Cochrane meta-analyses have not found any significant difference between intravenous and oral treatments; however, the studies all have had limitations. This study was designed to provide a statistically significant answer.
Study design: Randomized, double-blinded, noninferiority trial.
Setting: Thirteen multiple sclerosis centers in France.
Synopsis: Patients were selected if they had had a relapse within the previous 15 days; the mean time was seven days. One hundred patients were in the oral steroid group, and 99 were in the intravenous steroid group. Each group received 1 g of methylprednisolone daily for three days. In addition, each group received saline infusions or placebo capsules to keep the study blind.
After 28 days, 81% of the oral group and 80% of the intravenous group had improvements of their symptoms. Side effects from the medications were similar as well.
The study was limited by the fixed dosing (1 g daily) that was not bioequivalent. Also, MRIs, although not always used in relapses, could have added more objective information, as everyone was followed clinically using the Kurtzke Functional System Scale.
Bottom line: Consider using oral instead of IV steroids in patients with relapsing multiple sclerosis.
Citation: Le Page E, Veillard D, Laplaud DA, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981.
Clinical question: Is there any difference between oral and intravenous methylprednisolone for multiple sclerosis relapses?
Background: When relapses of multiple sclerosis occur, studies have shown that intravenous steroids are the treatment of choice. Prior Cochrane meta-analyses have not found any significant difference between intravenous and oral treatments; however, the studies all have had limitations. This study was designed to provide a statistically significant answer.
Study design: Randomized, double-blinded, noninferiority trial.
Setting: Thirteen multiple sclerosis centers in France.
Synopsis: Patients were selected if they had had a relapse within the previous 15 days; the mean time was seven days. One hundred patients were in the oral steroid group, and 99 were in the intravenous steroid group. Each group received 1 g of methylprednisolone daily for three days. In addition, each group received saline infusions or placebo capsules to keep the study blind.
After 28 days, 81% of the oral group and 80% of the intravenous group had improvements of their symptoms. Side effects from the medications were similar as well.
The study was limited by the fixed dosing (1 g daily) that was not bioequivalent. Also, MRIs, although not always used in relapses, could have added more objective information, as everyone was followed clinically using the Kurtzke Functional System Scale.
Bottom line: Consider using oral instead of IV steroids in patients with relapsing multiple sclerosis.
Citation: Le Page E, Veillard D, Laplaud DA, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981.
Differences in Care, Outcomes for In-Hospital Versus Community-Onset Stroke
Clinical question: Are there any differences in care and outcomes for in-hospital versus community-onset stroke?
Background: In-hospital stroke accounts for 4%-17% of all strokes. Hospitalists and other non-neurological services have to identify and treat subsequent stroke in their patients. There is not much literature detailing the differences between hospitalized stroke patients and those admitted for stroke.
Study design: Prospective cohort study.
Setting: All regional stroke centers in Ontario, Canada.
Synopsis: During a period of nine years, 973 in-hospital and 28,837 community-acquired stroke patients were followed. Compared to community-acquired stroke patients, in-hospital stroke patients had longer time to confirmatory neuroimaging, lower use of thrombolysis, lower use of investigational tests, and longer length of stay, and they were more likely to be disabled or dead at discharge. The two cohorts had similar mortality outcomes after discharge at 30 days and one year, after adjusting for multiple factors. Interestingly, in-hospital stroke patients were more likely to be given the proper medications for secondary prevention at discharge.
The study was limited in that the authors were unable to research why in-hospital patients did not get timely diagnosis and comparable treatment. The admission diagnoses were not enough for the authors to determine if that condition mattered in care. Secondary analysis found that in-hospital stroke patients were older and had more comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and atrial fibrillation). The primary reason in-hospital stroke patients did not get thrombolysis was because of a contraindication.
Bottom line: In-hospital stroke patients have increased lengths of stay and more disability compared to community-onset stroke patients.
Citation: Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in-hospital stroke. JAMA Neurol. 2015;72(7):749-755.
Clinical question: Are there any differences in care and outcomes for in-hospital versus community-onset stroke?
Background: In-hospital stroke accounts for 4%-17% of all strokes. Hospitalists and other non-neurological services have to identify and treat subsequent stroke in their patients. There is not much literature detailing the differences between hospitalized stroke patients and those admitted for stroke.
Study design: Prospective cohort study.
Setting: All regional stroke centers in Ontario, Canada.
Synopsis: During a period of nine years, 973 in-hospital and 28,837 community-acquired stroke patients were followed. Compared to community-acquired stroke patients, in-hospital stroke patients had longer time to confirmatory neuroimaging, lower use of thrombolysis, lower use of investigational tests, and longer length of stay, and they were more likely to be disabled or dead at discharge. The two cohorts had similar mortality outcomes after discharge at 30 days and one year, after adjusting for multiple factors. Interestingly, in-hospital stroke patients were more likely to be given the proper medications for secondary prevention at discharge.
The study was limited in that the authors were unable to research why in-hospital patients did not get timely diagnosis and comparable treatment. The admission diagnoses were not enough for the authors to determine if that condition mattered in care. Secondary analysis found that in-hospital stroke patients were older and had more comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and atrial fibrillation). The primary reason in-hospital stroke patients did not get thrombolysis was because of a contraindication.
Bottom line: In-hospital stroke patients have increased lengths of stay and more disability compared to community-onset stroke patients.
Citation: Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in-hospital stroke. JAMA Neurol. 2015;72(7):749-755.
Clinical question: Are there any differences in care and outcomes for in-hospital versus community-onset stroke?
Background: In-hospital stroke accounts for 4%-17% of all strokes. Hospitalists and other non-neurological services have to identify and treat subsequent stroke in their patients. There is not much literature detailing the differences between hospitalized stroke patients and those admitted for stroke.
Study design: Prospective cohort study.
Setting: All regional stroke centers in Ontario, Canada.
Synopsis: During a period of nine years, 973 in-hospital and 28,837 community-acquired stroke patients were followed. Compared to community-acquired stroke patients, in-hospital stroke patients had longer time to confirmatory neuroimaging, lower use of thrombolysis, lower use of investigational tests, and longer length of stay, and they were more likely to be disabled or dead at discharge. The two cohorts had similar mortality outcomes after discharge at 30 days and one year, after adjusting for multiple factors. Interestingly, in-hospital stroke patients were more likely to be given the proper medications for secondary prevention at discharge.
The study was limited in that the authors were unable to research why in-hospital patients did not get timely diagnosis and comparable treatment. The admission diagnoses were not enough for the authors to determine if that condition mattered in care. Secondary analysis found that in-hospital stroke patients were older and had more comorbidities (i.e., diabetes, hypertension, hyperlipidemia, and atrial fibrillation). The primary reason in-hospital stroke patients did not get thrombolysis was because of a contraindication.
Bottom line: In-hospital stroke patients have increased lengths of stay and more disability compared to community-onset stroke patients.
Citation: Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in-hospital stroke. JAMA Neurol. 2015;72(7):749-755.
When Should Hospitalists Order Continuous Cardiac Monitoring?
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
ED Lung Ultrasound Useful for Differentiating Cardiogenic from Noncardiogenic Dyspnea
Clinical question: Is lung ultrasound a useful tool for helping to diagnose acute decompensated heart failure (ADHF)?
Background: Lung ultrasound is an emerging bedside tool that has been promoted to help evaluate lung water content to help clinicians differentiate ADHF from other causes of dyspnea.
Study design: Prospective, multicenter, observational cohort study.
Setting: Seven EDs in Italy.
Synopsis: A total of 1,005 patients were enrolled in the study. Upon presentation to the ED, patients received a standard workup, including history, physical examination, EKG, and arterial blood gas sampling. Physicians were asked to render a diagnosis of ADHF or noncardiogenic dyspnea. The same physician then performed a lung ultrasound and rendered a revised diagnosis based on the ultrasound findings. A second ED physician and cardiologist, blinded to the ultrasound results, reviewed the medical record and rendered a final diagnosis as to the cause of the patient’s dyspnea.
The ultrasound approach had a higher accuracy than clinical evaluation alone in differentiating ADHF from noncardiac causes of dyspnea (97% vs. 85.3%). The authors also report a higher sensitivity compared to chest X-ray alone (69.5%) and natriuretic peptide testing (85%).
Bottom line: Lung ultrasound combined with clinical evaluation may improve the accuracy of ADHF diagnosis, but its usefulness may be limited by the need for ED physicians to have some degree of expertise in the use of ultrasound.
Citation: Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest. 2015;148(1):202-210.
Clinical question: Is lung ultrasound a useful tool for helping to diagnose acute decompensated heart failure (ADHF)?
Background: Lung ultrasound is an emerging bedside tool that has been promoted to help evaluate lung water content to help clinicians differentiate ADHF from other causes of dyspnea.
Study design: Prospective, multicenter, observational cohort study.
Setting: Seven EDs in Italy.
Synopsis: A total of 1,005 patients were enrolled in the study. Upon presentation to the ED, patients received a standard workup, including history, physical examination, EKG, and arterial blood gas sampling. Physicians were asked to render a diagnosis of ADHF or noncardiogenic dyspnea. The same physician then performed a lung ultrasound and rendered a revised diagnosis based on the ultrasound findings. A second ED physician and cardiologist, blinded to the ultrasound results, reviewed the medical record and rendered a final diagnosis as to the cause of the patient’s dyspnea.
The ultrasound approach had a higher accuracy than clinical evaluation alone in differentiating ADHF from noncardiac causes of dyspnea (97% vs. 85.3%). The authors also report a higher sensitivity compared to chest X-ray alone (69.5%) and natriuretic peptide testing (85%).
Bottom line: Lung ultrasound combined with clinical evaluation may improve the accuracy of ADHF diagnosis, but its usefulness may be limited by the need for ED physicians to have some degree of expertise in the use of ultrasound.
Citation: Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest. 2015;148(1):202-210.
Clinical question: Is lung ultrasound a useful tool for helping to diagnose acute decompensated heart failure (ADHF)?
Background: Lung ultrasound is an emerging bedside tool that has been promoted to help evaluate lung water content to help clinicians differentiate ADHF from other causes of dyspnea.
Study design: Prospective, multicenter, observational cohort study.
Setting: Seven EDs in Italy.
Synopsis: A total of 1,005 patients were enrolled in the study. Upon presentation to the ED, patients received a standard workup, including history, physical examination, EKG, and arterial blood gas sampling. Physicians were asked to render a diagnosis of ADHF or noncardiogenic dyspnea. The same physician then performed a lung ultrasound and rendered a revised diagnosis based on the ultrasound findings. A second ED physician and cardiologist, blinded to the ultrasound results, reviewed the medical record and rendered a final diagnosis as to the cause of the patient’s dyspnea.
The ultrasound approach had a higher accuracy than clinical evaluation alone in differentiating ADHF from noncardiac causes of dyspnea (97% vs. 85.3%). The authors also report a higher sensitivity compared to chest X-ray alone (69.5%) and natriuretic peptide testing (85%).
Bottom line: Lung ultrasound combined with clinical evaluation may improve the accuracy of ADHF diagnosis, but its usefulness may be limited by the need for ED physicians to have some degree of expertise in the use of ultrasound.
Citation: Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest. 2015;148(1):202-210.
Coronary CT Angiography, Perfusion Imaging Effective for Evaluating Patients With Chest Pain
Clinical question: When evaluating the intermediate-risk patient with chest pain, should coronary computed tomography angiography (CCTA) be used instead of myocardial perfusion imaging (MPI)?
Background: CCTA has been shown in prior randomized controlled trials to save time and money compared to other protocols, including stress ECG, echocardiogram, and MPI. Less information is available as to whether CCTA provides a better selection of patients for cardiac catheterization compared to MPI.
Study design: Randomized, controlled, comparative effectiveness trial.
Setting: Telemetry ward of an urban medical center.
Synopsis: Four hundred patients admitted with chest pain and an intermediate, pre-test probability of coronary artery disease were randomized to either CCTA or MPI. Patients were predominantly female, were ethnically varied, and had a mean age of 57 years.
Study results showed no significant difference in rates of cardiac catheterization that did not lead to revascularization at one-year follow-up. Specifically, 7.5% of patients in the CCTA group underwent catheterization not leading to revascularization, compared to 10% in the MPI group.
One limitation of the study is that it was conducted at a single site. Furthermore, the decision to proceed to catheterization was made clinically and not based on a predefined algorithm.
Bottom line: CCTA and MPI are both acceptable choices to determine the need for invasive testing in patients admitted with chest pain.
Citation: Levsky JM, Spevack DM, Travin MI, et al. Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Ann Intern Med. 2015;163(3):174-183.
Clinical question: When evaluating the intermediate-risk patient with chest pain, should coronary computed tomography angiography (CCTA) be used instead of myocardial perfusion imaging (MPI)?
Background: CCTA has been shown in prior randomized controlled trials to save time and money compared to other protocols, including stress ECG, echocardiogram, and MPI. Less information is available as to whether CCTA provides a better selection of patients for cardiac catheterization compared to MPI.
Study design: Randomized, controlled, comparative effectiveness trial.
Setting: Telemetry ward of an urban medical center.
Synopsis: Four hundred patients admitted with chest pain and an intermediate, pre-test probability of coronary artery disease were randomized to either CCTA or MPI. Patients were predominantly female, were ethnically varied, and had a mean age of 57 years.
Study results showed no significant difference in rates of cardiac catheterization that did not lead to revascularization at one-year follow-up. Specifically, 7.5% of patients in the CCTA group underwent catheterization not leading to revascularization, compared to 10% in the MPI group.
One limitation of the study is that it was conducted at a single site. Furthermore, the decision to proceed to catheterization was made clinically and not based on a predefined algorithm.
Bottom line: CCTA and MPI are both acceptable choices to determine the need for invasive testing in patients admitted with chest pain.
Citation: Levsky JM, Spevack DM, Travin MI, et al. Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Ann Intern Med. 2015;163(3):174-183.
Clinical question: When evaluating the intermediate-risk patient with chest pain, should coronary computed tomography angiography (CCTA) be used instead of myocardial perfusion imaging (MPI)?
Background: CCTA has been shown in prior randomized controlled trials to save time and money compared to other protocols, including stress ECG, echocardiogram, and MPI. Less information is available as to whether CCTA provides a better selection of patients for cardiac catheterization compared to MPI.
Study design: Randomized, controlled, comparative effectiveness trial.
Setting: Telemetry ward of an urban medical center.
Synopsis: Four hundred patients admitted with chest pain and an intermediate, pre-test probability of coronary artery disease were randomized to either CCTA or MPI. Patients were predominantly female, were ethnically varied, and had a mean age of 57 years.
Study results showed no significant difference in rates of cardiac catheterization that did not lead to revascularization at one-year follow-up. Specifically, 7.5% of patients in the CCTA group underwent catheterization not leading to revascularization, compared to 10% in the MPI group.
One limitation of the study is that it was conducted at a single site. Furthermore, the decision to proceed to catheterization was made clinically and not based on a predefined algorithm.
Bottom line: CCTA and MPI are both acceptable choices to determine the need for invasive testing in patients admitted with chest pain.
Citation: Levsky JM, Spevack DM, Travin MI, et al. Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Ann Intern Med. 2015;163(3):174-183.
Can Low-Risk Patients with VTE Be Discharged from ED on Rivaroxabon?
Clinical question: Can a low-risk patient newly diagnosed with VTE in the ED be immediately discharged home on a direct factor Xa inhibitor?
Background: Studies have shown that rivaroxaban incurs a risk of 2.1% in VTE recurrence and of 9.4% in clinically relevant major and non-major bleeding (in an average 208 days follow-up). More information is required to determine if similar success can be achieved by discharging low-risk patients from the ED.
Study design: Prospective, observational study.
Setting: EDs at two urban, teaching hospitals.
Synopsis: After fulfilling the criteria for low risk, 106 patients were discharged from the ED with DVT, pulmonary embolism (PE), or both. Most patients were 50 years or younger and had unprovoked VTE. Three of the 106 patients had recurrence of VTE (2.8%, 95% CI=0.6% to 8%) at a mean duration of 389 days. No patient had a major bleeding event.
In this small study, fewer than 80% of patients discharged had at least one clinic follow-up; the majority of these patients (75%) followed up in a clinic staffed by ED physicians. Therefore, ability for close follow-up must be taken into consideration prior to discharge from the ED.
Moreover, one to two days post-discharge, a member of the care team called the patient to confirm their ability to fill the rivaroxaban prescription and to answer other questions related to the new diagnosis.
Bottom line: With close follow-up and confirmation of the ability to fill a rivaroxaban prescription, patients with low-risk VTE may be discharged home from the ED.
Citation: Beam DM, Kahler ZP, Kline JA. Immediate discharge and home treatment with rivaroxaban of low-risk venous thromboembolism diagnosed in two U.S. emergency departments: a one-year preplanned analysis. Acad Emerg Med. 2015;22(7):788-795.
Clinical question: Can a low-risk patient newly diagnosed with VTE in the ED be immediately discharged home on a direct factor Xa inhibitor?
Background: Studies have shown that rivaroxaban incurs a risk of 2.1% in VTE recurrence and of 9.4% in clinically relevant major and non-major bleeding (in an average 208 days follow-up). More information is required to determine if similar success can be achieved by discharging low-risk patients from the ED.
Study design: Prospective, observational study.
Setting: EDs at two urban, teaching hospitals.
Synopsis: After fulfilling the criteria for low risk, 106 patients were discharged from the ED with DVT, pulmonary embolism (PE), or both. Most patients were 50 years or younger and had unprovoked VTE. Three of the 106 patients had recurrence of VTE (2.8%, 95% CI=0.6% to 8%) at a mean duration of 389 days. No patient had a major bleeding event.
In this small study, fewer than 80% of patients discharged had at least one clinic follow-up; the majority of these patients (75%) followed up in a clinic staffed by ED physicians. Therefore, ability for close follow-up must be taken into consideration prior to discharge from the ED.
Moreover, one to two days post-discharge, a member of the care team called the patient to confirm their ability to fill the rivaroxaban prescription and to answer other questions related to the new diagnosis.
Bottom line: With close follow-up and confirmation of the ability to fill a rivaroxaban prescription, patients with low-risk VTE may be discharged home from the ED.
Citation: Beam DM, Kahler ZP, Kline JA. Immediate discharge and home treatment with rivaroxaban of low-risk venous thromboembolism diagnosed in two U.S. emergency departments: a one-year preplanned analysis. Acad Emerg Med. 2015;22(7):788-795.
Clinical question: Can a low-risk patient newly diagnosed with VTE in the ED be immediately discharged home on a direct factor Xa inhibitor?
Background: Studies have shown that rivaroxaban incurs a risk of 2.1% in VTE recurrence and of 9.4% in clinically relevant major and non-major bleeding (in an average 208 days follow-up). More information is required to determine if similar success can be achieved by discharging low-risk patients from the ED.
Study design: Prospective, observational study.
Setting: EDs at two urban, teaching hospitals.
Synopsis: After fulfilling the criteria for low risk, 106 patients were discharged from the ED with DVT, pulmonary embolism (PE), or both. Most patients were 50 years or younger and had unprovoked VTE. Three of the 106 patients had recurrence of VTE (2.8%, 95% CI=0.6% to 8%) at a mean duration of 389 days. No patient had a major bleeding event.
In this small study, fewer than 80% of patients discharged had at least one clinic follow-up; the majority of these patients (75%) followed up in a clinic staffed by ED physicians. Therefore, ability for close follow-up must be taken into consideration prior to discharge from the ED.
Moreover, one to two days post-discharge, a member of the care team called the patient to confirm their ability to fill the rivaroxaban prescription and to answer other questions related to the new diagnosis.
Bottom line: With close follow-up and confirmation of the ability to fill a rivaroxaban prescription, patients with low-risk VTE may be discharged home from the ED.
Citation: Beam DM, Kahler ZP, Kline JA. Immediate discharge and home treatment with rivaroxaban of low-risk venous thromboembolism diagnosed in two U.S. emergency departments: a one-year preplanned analysis. Acad Emerg Med. 2015;22(7):788-795.