User login
High-dose vitamin D and MS relapse: New phase 3 data
, results from a randomized control trial show. However, at least one expert believes the study’s exclusion criteria may have been too broad.
The investigation of vitamin D to prevent relapse of MS is based on older observational studies of people who already had higher blood levels of vitamin D and were less likely to develop MS, said study investigator Ellen Mowry, MD, Richard T. and Frances W. Johnson professor of neurology, Johns Hopkins University, Baltimore.
Later research where participants were given vitamin D as a therapeutic option for MS “were disappointing as the vitamin D had minimal effect,” she said.
“While we were excited by early data suggesting that vitamin D may have an important impact on MS, it’s essential to follow those linkage studies with the gold standard clinical evidence, which we have here,” Dr. Mowry added.
The findings were published online in eClinicalMedicine.
No difference in relapse risk
The multisite, phase 3 Vitamin D to Ameliorate MS (VIDAMS) clinical trial included 172 participants aged 18-50 years with RRMS from 16 neurology clinics between 2012 and 2019.
Inclusion criteria were having one or more clinical episodes of MS in the past year and at least one brain lesion on MRI in the past year or having two or more clinical episodes in the past year. Eligible participants also had to have a score of 4 or less on the Kurtzke Expanded Disability Status Scale.
A total of 83 participants were randomly assigned to receive low-dose vitamin D3 (600 IU/day) and 89 to receive high-dose vitamin D3 (5,000 IU/day). Each participant took the vitamin tablet with glatiramer acetate, a synthetic protein that simulates myelin.
Participants were assessed every 12 weeks to measure serum 25(OH)D levels and every 24 weeks for a number of movement and coordination tests, as well as two 3T clinical brain MRIs to check for lesions.
By the trial’s end at 96 weeks, the researchers found no differences in relapse risk between the high- and low-dose groups (P = .57). In addition, there were no differences in MRI outcomes between the two groups.
Dr. Mowry said that more than a few people have asked her if she is disappointed by the results of the VIDAMS trial. “I tell them that no, I’m not – that we are scientists and clinicians, and it is our job to understand what they can do to fight their disease. And if the answer is not vitamin D, that’s OK – we have many other ideas.”
These include helping patients minimize cardiometabolic comorbidities, such as heart disease and blood pressure, she said.
Exclusion criteria too broad?
Commenting on the findings, Alberto Ascherio, MD, professor of epidemiology and nutrition at Harvard School of Public Health, Boston, said a key principle of recommending vitamin supplements is that they are, generally speaking, only beneficial for individuals with vitamin deficiencies.
He noted that “patients with vitamin D deficiency (25(OH)D < 15 ng/mL, which corresponds to 37.5 nmol/L) were excluded from this study. Most importantly, the baseline mean 25(OH)D levels were about 30 ng/mL (75 nmol/L), which is considered a sufficient level (the IOM considers 20 ng/mL = 50 nmol/L as an adequate level),” with the level further increasing during the trial due to the supplementation.
“It would be a serious mistake to conclude from this trial (or any of the previous trials) that vitamin D supplementation is not important in MS patients,” Dr. Ascherio said.
He added that many individuals with MS have serum vitamin D levels below 20 ng/mL (50 nmol/L) and that this was the median serum value in studies among individuals with MS in Europe.
“These patients would almost certainly benefit from moderate doses of vitamin D supplements or judicious UV light exposure. Most likely even patients with sufficient but suboptimal 25(OH)D levels (between 20 and 30 ng/mL, or 50 and 75 nmol/L) would benefit from an increase,” he said.
The study was funded by the National Multiple Sclerosis Society, Teva Neuroscience, and the National Institute of Health. Dr. Mowry reported grant support from the National MS Society, Biogen, Genentech, and Teva Neuroscience; honoraria from UpToDate; and consulting fees from BeCare Link.
A version of this article first appeared on Medscape.com.
, results from a randomized control trial show. However, at least one expert believes the study’s exclusion criteria may have been too broad.
The investigation of vitamin D to prevent relapse of MS is based on older observational studies of people who already had higher blood levels of vitamin D and were less likely to develop MS, said study investigator Ellen Mowry, MD, Richard T. and Frances W. Johnson professor of neurology, Johns Hopkins University, Baltimore.
Later research where participants were given vitamin D as a therapeutic option for MS “were disappointing as the vitamin D had minimal effect,” she said.
“While we were excited by early data suggesting that vitamin D may have an important impact on MS, it’s essential to follow those linkage studies with the gold standard clinical evidence, which we have here,” Dr. Mowry added.
The findings were published online in eClinicalMedicine.
No difference in relapse risk
The multisite, phase 3 Vitamin D to Ameliorate MS (VIDAMS) clinical trial included 172 participants aged 18-50 years with RRMS from 16 neurology clinics between 2012 and 2019.
Inclusion criteria were having one or more clinical episodes of MS in the past year and at least one brain lesion on MRI in the past year or having two or more clinical episodes in the past year. Eligible participants also had to have a score of 4 or less on the Kurtzke Expanded Disability Status Scale.
A total of 83 participants were randomly assigned to receive low-dose vitamin D3 (600 IU/day) and 89 to receive high-dose vitamin D3 (5,000 IU/day). Each participant took the vitamin tablet with glatiramer acetate, a synthetic protein that simulates myelin.
Participants were assessed every 12 weeks to measure serum 25(OH)D levels and every 24 weeks for a number of movement and coordination tests, as well as two 3T clinical brain MRIs to check for lesions.
By the trial’s end at 96 weeks, the researchers found no differences in relapse risk between the high- and low-dose groups (P = .57). In addition, there were no differences in MRI outcomes between the two groups.
Dr. Mowry said that more than a few people have asked her if she is disappointed by the results of the VIDAMS trial. “I tell them that no, I’m not – that we are scientists and clinicians, and it is our job to understand what they can do to fight their disease. And if the answer is not vitamin D, that’s OK – we have many other ideas.”
These include helping patients minimize cardiometabolic comorbidities, such as heart disease and blood pressure, she said.
Exclusion criteria too broad?
Commenting on the findings, Alberto Ascherio, MD, professor of epidemiology and nutrition at Harvard School of Public Health, Boston, said a key principle of recommending vitamin supplements is that they are, generally speaking, only beneficial for individuals with vitamin deficiencies.
He noted that “patients with vitamin D deficiency (25(OH)D < 15 ng/mL, which corresponds to 37.5 nmol/L) were excluded from this study. Most importantly, the baseline mean 25(OH)D levels were about 30 ng/mL (75 nmol/L), which is considered a sufficient level (the IOM considers 20 ng/mL = 50 nmol/L as an adequate level),” with the level further increasing during the trial due to the supplementation.
“It would be a serious mistake to conclude from this trial (or any of the previous trials) that vitamin D supplementation is not important in MS patients,” Dr. Ascherio said.
He added that many individuals with MS have serum vitamin D levels below 20 ng/mL (50 nmol/L) and that this was the median serum value in studies among individuals with MS in Europe.
“These patients would almost certainly benefit from moderate doses of vitamin D supplements or judicious UV light exposure. Most likely even patients with sufficient but suboptimal 25(OH)D levels (between 20 and 30 ng/mL, or 50 and 75 nmol/L) would benefit from an increase,” he said.
The study was funded by the National Multiple Sclerosis Society, Teva Neuroscience, and the National Institute of Health. Dr. Mowry reported grant support from the National MS Society, Biogen, Genentech, and Teva Neuroscience; honoraria from UpToDate; and consulting fees from BeCare Link.
A version of this article first appeared on Medscape.com.
, results from a randomized control trial show. However, at least one expert believes the study’s exclusion criteria may have been too broad.
The investigation of vitamin D to prevent relapse of MS is based on older observational studies of people who already had higher blood levels of vitamin D and were less likely to develop MS, said study investigator Ellen Mowry, MD, Richard T. and Frances W. Johnson professor of neurology, Johns Hopkins University, Baltimore.
Later research where participants were given vitamin D as a therapeutic option for MS “were disappointing as the vitamin D had minimal effect,” she said.
“While we were excited by early data suggesting that vitamin D may have an important impact on MS, it’s essential to follow those linkage studies with the gold standard clinical evidence, which we have here,” Dr. Mowry added.
The findings were published online in eClinicalMedicine.
No difference in relapse risk
The multisite, phase 3 Vitamin D to Ameliorate MS (VIDAMS) clinical trial included 172 participants aged 18-50 years with RRMS from 16 neurology clinics between 2012 and 2019.
Inclusion criteria were having one or more clinical episodes of MS in the past year and at least one brain lesion on MRI in the past year or having two or more clinical episodes in the past year. Eligible participants also had to have a score of 4 or less on the Kurtzke Expanded Disability Status Scale.
A total of 83 participants were randomly assigned to receive low-dose vitamin D3 (600 IU/day) and 89 to receive high-dose vitamin D3 (5,000 IU/day). Each participant took the vitamin tablet with glatiramer acetate, a synthetic protein that simulates myelin.
Participants were assessed every 12 weeks to measure serum 25(OH)D levels and every 24 weeks for a number of movement and coordination tests, as well as two 3T clinical brain MRIs to check for lesions.
By the trial’s end at 96 weeks, the researchers found no differences in relapse risk between the high- and low-dose groups (P = .57). In addition, there were no differences in MRI outcomes between the two groups.
Dr. Mowry said that more than a few people have asked her if she is disappointed by the results of the VIDAMS trial. “I tell them that no, I’m not – that we are scientists and clinicians, and it is our job to understand what they can do to fight their disease. And if the answer is not vitamin D, that’s OK – we have many other ideas.”
These include helping patients minimize cardiometabolic comorbidities, such as heart disease and blood pressure, she said.
Exclusion criteria too broad?
Commenting on the findings, Alberto Ascherio, MD, professor of epidemiology and nutrition at Harvard School of Public Health, Boston, said a key principle of recommending vitamin supplements is that they are, generally speaking, only beneficial for individuals with vitamin deficiencies.
He noted that “patients with vitamin D deficiency (25(OH)D < 15 ng/mL, which corresponds to 37.5 nmol/L) were excluded from this study. Most importantly, the baseline mean 25(OH)D levels were about 30 ng/mL (75 nmol/L), which is considered a sufficient level (the IOM considers 20 ng/mL = 50 nmol/L as an adequate level),” with the level further increasing during the trial due to the supplementation.
“It would be a serious mistake to conclude from this trial (or any of the previous trials) that vitamin D supplementation is not important in MS patients,” Dr. Ascherio said.
He added that many individuals with MS have serum vitamin D levels below 20 ng/mL (50 nmol/L) and that this was the median serum value in studies among individuals with MS in Europe.
“These patients would almost certainly benefit from moderate doses of vitamin D supplements or judicious UV light exposure. Most likely even patients with sufficient but suboptimal 25(OH)D levels (between 20 and 30 ng/mL, or 50 and 75 nmol/L) would benefit from an increase,” he said.
The study was funded by the National Multiple Sclerosis Society, Teva Neuroscience, and the National Institute of Health. Dr. Mowry reported grant support from the National MS Society, Biogen, Genentech, and Teva Neuroscience; honoraria from UpToDate; and consulting fees from BeCare Link.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
Chronicling gastroenterology’s history
Each May, the gastroenterology community gathers for Digestive Disease Week® to be inspired, meet up with friends and colleagues from across the globe, and learn the latest in scientific advances to inform how we care for our patients in the clinic, on inpatient wards, and in our endoscopy suites. DDW® 2023, held in the Windy City of Chicago, does not disappoint. This year’s conference features a dizzying array of offerings, including 3,500 poster and ePoster presentations and 1,300 abstract lectures, as well as the perennially well-attended AGA Post-Graduate Course and other offerings.
This year’s AGA Presidential Plenary, hosted on May 8 by outgoing AGA President Dr. John M. Carethers, is not to be missed. The session will honor the 125-year history of the AGA and recognizes the barriers overcome in diversifying the practice of gastroenterology. You will learn about individuals such as Alexis St. Martin, MD; Basil Hirschowitz, MD, AGAF; Leonidas Berry, MD; Sadye Curry, MD; and, other barrier-breakers in GI who have been instrumental in shaping the modern practice of gastroenterology. I hope you will join me in attending.
In this month’s issue of GIHN, we introduce the winner of the 2023 AGA Shark Tank innovation competition, which was held during the 2023 AGA Tech Summit. We also report on a landmark phase 4, double-blind randomized trial published in the New England Journal of Medicine demonstrating the effectiveness of vedolizumab in inducing remission in chronic pouchitis, and a new AGA clinical practice update on the role of EUS-guided gallbladder drainage in acute cholecystitis.
The AGA Government Affairs Committee also updates us on their advocacy to reform prior authorization policies affecting GI practice, and explains how you can assist in these efforts. In our Member Spotlight, we introduce you to gastroenterologist Sharmila Anandasabapthy, MD, who shares her passion for global health and the one piece of career advice she’s glad she ignored.
Finally, GIHN Associate Editor Dr. Avi Ketwaroo presents our quarterly Perspectives column highlighting differing approaches to clinical management of pancreatic cystic lesions. We hope you enjoy all of the exciting content featured in this issue and look forward to seeing you in Chicago (or, virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Each May, the gastroenterology community gathers for Digestive Disease Week® to be inspired, meet up with friends and colleagues from across the globe, and learn the latest in scientific advances to inform how we care for our patients in the clinic, on inpatient wards, and in our endoscopy suites. DDW® 2023, held in the Windy City of Chicago, does not disappoint. This year’s conference features a dizzying array of offerings, including 3,500 poster and ePoster presentations and 1,300 abstract lectures, as well as the perennially well-attended AGA Post-Graduate Course and other offerings.
This year’s AGA Presidential Plenary, hosted on May 8 by outgoing AGA President Dr. John M. Carethers, is not to be missed. The session will honor the 125-year history of the AGA and recognizes the barriers overcome in diversifying the practice of gastroenterology. You will learn about individuals such as Alexis St. Martin, MD; Basil Hirschowitz, MD, AGAF; Leonidas Berry, MD; Sadye Curry, MD; and, other barrier-breakers in GI who have been instrumental in shaping the modern practice of gastroenterology. I hope you will join me in attending.
In this month’s issue of GIHN, we introduce the winner of the 2023 AGA Shark Tank innovation competition, which was held during the 2023 AGA Tech Summit. We also report on a landmark phase 4, double-blind randomized trial published in the New England Journal of Medicine demonstrating the effectiveness of vedolizumab in inducing remission in chronic pouchitis, and a new AGA clinical practice update on the role of EUS-guided gallbladder drainage in acute cholecystitis.
The AGA Government Affairs Committee also updates us on their advocacy to reform prior authorization policies affecting GI practice, and explains how you can assist in these efforts. In our Member Spotlight, we introduce you to gastroenterologist Sharmila Anandasabapthy, MD, who shares her passion for global health and the one piece of career advice she’s glad she ignored.
Finally, GIHN Associate Editor Dr. Avi Ketwaroo presents our quarterly Perspectives column highlighting differing approaches to clinical management of pancreatic cystic lesions. We hope you enjoy all of the exciting content featured in this issue and look forward to seeing you in Chicago (or, virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Each May, the gastroenterology community gathers for Digestive Disease Week® to be inspired, meet up with friends and colleagues from across the globe, and learn the latest in scientific advances to inform how we care for our patients in the clinic, on inpatient wards, and in our endoscopy suites. DDW® 2023, held in the Windy City of Chicago, does not disappoint. This year’s conference features a dizzying array of offerings, including 3,500 poster and ePoster presentations and 1,300 abstract lectures, as well as the perennially well-attended AGA Post-Graduate Course and other offerings.
This year’s AGA Presidential Plenary, hosted on May 8 by outgoing AGA President Dr. John M. Carethers, is not to be missed. The session will honor the 125-year history of the AGA and recognizes the barriers overcome in diversifying the practice of gastroenterology. You will learn about individuals such as Alexis St. Martin, MD; Basil Hirschowitz, MD, AGAF; Leonidas Berry, MD; Sadye Curry, MD; and, other barrier-breakers in GI who have been instrumental in shaping the modern practice of gastroenterology. I hope you will join me in attending.
In this month’s issue of GIHN, we introduce the winner of the 2023 AGA Shark Tank innovation competition, which was held during the 2023 AGA Tech Summit. We also report on a landmark phase 4, double-blind randomized trial published in the New England Journal of Medicine demonstrating the effectiveness of vedolizumab in inducing remission in chronic pouchitis, and a new AGA clinical practice update on the role of EUS-guided gallbladder drainage in acute cholecystitis.
The AGA Government Affairs Committee also updates us on their advocacy to reform prior authorization policies affecting GI practice, and explains how you can assist in these efforts. In our Member Spotlight, we introduce you to gastroenterologist Sharmila Anandasabapthy, MD, who shares her passion for global health and the one piece of career advice she’s glad she ignored.
Finally, GIHN Associate Editor Dr. Avi Ketwaroo presents our quarterly Perspectives column highlighting differing approaches to clinical management of pancreatic cystic lesions. We hope you enjoy all of the exciting content featured in this issue and look forward to seeing you in Chicago (or, virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Bariatric surgery cuts risk for obesity-related cancers in half: Study
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).
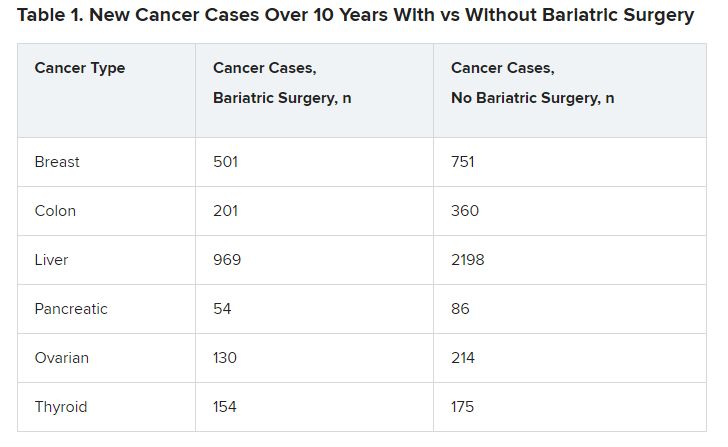
The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
Gut microbiome may guide personalized heart failure therapy
Understanding more about the gut microbiome and how it may affect the development and treatment of heart failure could lead to a more personalized approach to managing the condition, a new review article suggests.
the authors note. “Interactions among the gut microbiome, diet, and medications offer potentially innovative modalities for management of patients with heart failure,” they add.
The review was published online in the Journal of the American College of Cardiology.
“Over the past years we have gathered more understanding about how important the gut microbiome is in relation to how our bodies function overall and even though the cardiovascular system and the heart itself may appear to be quite distant from the gut, we know the gut microbiome affects the cardiovascular system and the physiology of heart failure,” lead author Petra Mamic, MD, Stanford (Calif.) University, told this news organization.
“We’ve also learnt that the microbiome is very personalized. It seems to be affected by a lot of intrinsic and as well as extrinsic factors. For cardiovascular diseases in particular, we always knew that diet and lifestyle were part of the environmental risk, and we now believe that the gut microbiome may be one of the factors that mediates that risk,” she said.
“Studies on the gut microbiome are difficult to do and we are right at the beginning of this type of research. But we have learned that the microbiome is altered or dysregulated in many diseases including many cardiovascular diseases, and many of the changes in the microbiome we see in different cardiovascular diseases seem to overlap,” she added.
Dr. Mamic explained that patients with heart failure have a microbiome that appears different and dysregulated, compared with the microbiome in healthy individuals.
“The difficulty is teasing out whether the microbiome changes are causing heart failure or if they are a consequence of the heart failure and all the medications and comorbidities associated with heart failure,” she commented.
Animal studies have shown that many microbial products, small molecules made by the microbiome, seem to affect how the heart recovers from injury, for example after a myocardial infarction, and how much the heart scars and hypertrophies after an injury, Dr. Mamic reported. These microbiome-derived small molecules can also affect blood pressure, which is dysregulated in heart failure.
Other products of the microbiome can be pro-inflammatory or anti-inflammatory, which can again affect the cardiovascular physiology and the heart, she noted.
High-fiber diet may be beneficial
One area of particular interest at present involves the role of short-chain fatty acids, which are a byproduct of microbes in the gut that digest fiber.
“These short chain fatty acids seem to have positive effects on the host physiology. They are anti-inflammatory; they lower blood pressure; and they seem to protect the heart from scarring and hypertrophy after injury. In heart failure, the gut microbes that make these short-chain fatty acids are significantly depleted,” Dr. Mamic explained.
They are an obvious focus of interest because these short-chain fatty acids are produced when gut bacteria break down dietary fiber, raising the possibility of beneficial effects from eating a high-fiber diet.
Another product of the gut microbiome of interest is trimethylamine N-oxide, formed when gut bacteria break down nutrients such as L-carnitine and phosphatidyl choline, nutrients abundant in foods of animal origin, especially red meat. This metabolite has proatherogenic and prothrombotic effects, and negatively affected cardiac remodeling in a mouse heart failure model, the review notes.
However, though it is too early to make specific dietary recommendations based on these findings, Dr. Mamic points out that a high-fiber diet is thought to be beneficial.
“Nutritional research is very hard to do and the data is limited, but as best as we can summarize things, we know that plant-based diets such as the Mediterranean and DASH diets seem to prevent some of the risk factors for the development of heart failure and seem to slow the progression of heart failure,” she added.
One of the major recommendations in these diets is a high intake of fiber, including whole foods, vegetables, fruits, legumes, and nuts, and less intake of processed food and red meat. “In general, I think everyone should eat like that, but I specifically recommend a plant-based diet with a high amount of fiber to my heart failure patients,” Dr. Mamic said.
Large variation in microbiome composition
The review also explores the idea of personalization of diet or specific treatments dependent on an individual’s gut microbiome composition.
Dr. Mamic explains: “When we look at the microbiome composition between individuals, it is very different. There is very little overlap between individuals, even in people who are related. It seems to be more to do with the environment – people who are living together are more likely to have similarities in their microbiome. We are still trying to understand what drives these differences.”
It is thought that these differences may affect the response to a specific diet or medication. Dr. Mamic gives the example of fiber. “Not all bacteria can digest the same types of fiber, so not everyone responds in the same way to a high-fiber diet. That’s probably because of differences in their microbiome.”
Another example is the response to the heart failure drug digoxin, which is metabolized by one particular strain of bacteria in the gut. The toxicity or effectiveness of digoxin seems to be influenced by levels of this bacterial strain, and this again can be influenced by diet, Dr. Mamic says.
Manipulating the microbiome as a therapeutic strategy
Microbiome-targeting therapies may also become part of future treatment strategies for many conditions, including heart failure, the review authors say.
Probiotics (foods and dietary supplements that contain live microbes) interact with the gut microbiota to alter host physiology beneficially. Certain probiotics may specifically modulate processes dysregulated in heart failure, as was suggested in a rodent heart failure model in which supplementation with Lactobacillus-containing and Bifidobacterium-containing probiotics resulted in markedly improved cardiac function, the authors report.
However, a randomized trial (GutHeart) of probiotic yeast Saccharomyces boulardii in patients with heart failure found no improvement in cardiac function, compared with standard care.
Commenting on this, Dr. Mamic suggested that a more specific approach may be needed.
“Some of our preliminary data have shown people who have heart failure have severely depleted Bifidobacteria,” Dr. Mamic said. These bacteria are commercially available as a probiotic, and the researchers are planning a study to give patients with heart failure these specific probiotics. “We are trying to find practical ways forward and to be guided by the data. These people have very little Bifidobacteria, and we know that probiotics seem to be accepted best by the host where there is a specific need for them, so this seems like a sensible approach.”
Dr. Mamic does not recommend that heart failure patients take general probiotic products at present, but she tells her patients about the study she is doing. “Probiotics are quite different from each other. It is a very unregulated market. A general probiotic product may not contain the specific bacteria needed.”
Include microbiome data in biobanks
The review calls for more research on the subject and a more systematic approach to collecting data on the microbiome.
“At present for medical research, blood samples are collected, stored, and analyzed routinely. I think we should also be collecting stool samples in the same way to analyze the microbiome,” Dr. Mamic suggests.
“If we can combine that with data from blood tests on various metabolites/cytokines and look at how the microbiome changes over time or with medication, or with diet, and how the host responds including clinically relevant data, that would be really important. Given how quickly the field is growing I would think there would be biobanks including the microbiome in a few years’ time.”
“We need to gather this data. We would be looking for which bacteria are there, what their functionality is, how it changes over time, with diet or medication, and even whether we can use the microbiome data to predict who will respond to a specific drug.”
Dr. Mamic believes that in the future, analysis of the microbiome could be a routine part of deciding what people eat for good health and to characterize patients for personalized therapies.
“It is clear that the microbiome can influence health, and a dysregulated microbiome negatively affects the host, but there is lot of work to do. We need to learn a lot more about it, but we shouldn’t miss the opportunity to do this,” she concluded.
Dr. Mamic reported no disclosures.
A version of this article first appeared on Medscape.com.
Understanding more about the gut microbiome and how it may affect the development and treatment of heart failure could lead to a more personalized approach to managing the condition, a new review article suggests.
the authors note. “Interactions among the gut microbiome, diet, and medications offer potentially innovative modalities for management of patients with heart failure,” they add.
The review was published online in the Journal of the American College of Cardiology.
“Over the past years we have gathered more understanding about how important the gut microbiome is in relation to how our bodies function overall and even though the cardiovascular system and the heart itself may appear to be quite distant from the gut, we know the gut microbiome affects the cardiovascular system and the physiology of heart failure,” lead author Petra Mamic, MD, Stanford (Calif.) University, told this news organization.
“We’ve also learnt that the microbiome is very personalized. It seems to be affected by a lot of intrinsic and as well as extrinsic factors. For cardiovascular diseases in particular, we always knew that diet and lifestyle were part of the environmental risk, and we now believe that the gut microbiome may be one of the factors that mediates that risk,” she said.
“Studies on the gut microbiome are difficult to do and we are right at the beginning of this type of research. But we have learned that the microbiome is altered or dysregulated in many diseases including many cardiovascular diseases, and many of the changes in the microbiome we see in different cardiovascular diseases seem to overlap,” she added.
Dr. Mamic explained that patients with heart failure have a microbiome that appears different and dysregulated, compared with the microbiome in healthy individuals.
“The difficulty is teasing out whether the microbiome changes are causing heart failure or if they are a consequence of the heart failure and all the medications and comorbidities associated with heart failure,” she commented.
Animal studies have shown that many microbial products, small molecules made by the microbiome, seem to affect how the heart recovers from injury, for example after a myocardial infarction, and how much the heart scars and hypertrophies after an injury, Dr. Mamic reported. These microbiome-derived small molecules can also affect blood pressure, which is dysregulated in heart failure.
Other products of the microbiome can be pro-inflammatory or anti-inflammatory, which can again affect the cardiovascular physiology and the heart, she noted.
High-fiber diet may be beneficial
One area of particular interest at present involves the role of short-chain fatty acids, which are a byproduct of microbes in the gut that digest fiber.
“These short chain fatty acids seem to have positive effects on the host physiology. They are anti-inflammatory; they lower blood pressure; and they seem to protect the heart from scarring and hypertrophy after injury. In heart failure, the gut microbes that make these short-chain fatty acids are significantly depleted,” Dr. Mamic explained.
They are an obvious focus of interest because these short-chain fatty acids are produced when gut bacteria break down dietary fiber, raising the possibility of beneficial effects from eating a high-fiber diet.
Another product of the gut microbiome of interest is trimethylamine N-oxide, formed when gut bacteria break down nutrients such as L-carnitine and phosphatidyl choline, nutrients abundant in foods of animal origin, especially red meat. This metabolite has proatherogenic and prothrombotic effects, and negatively affected cardiac remodeling in a mouse heart failure model, the review notes.
However, though it is too early to make specific dietary recommendations based on these findings, Dr. Mamic points out that a high-fiber diet is thought to be beneficial.
“Nutritional research is very hard to do and the data is limited, but as best as we can summarize things, we know that plant-based diets such as the Mediterranean and DASH diets seem to prevent some of the risk factors for the development of heart failure and seem to slow the progression of heart failure,” she added.
One of the major recommendations in these diets is a high intake of fiber, including whole foods, vegetables, fruits, legumes, and nuts, and less intake of processed food and red meat. “In general, I think everyone should eat like that, but I specifically recommend a plant-based diet with a high amount of fiber to my heart failure patients,” Dr. Mamic said.
Large variation in microbiome composition
The review also explores the idea of personalization of diet or specific treatments dependent on an individual’s gut microbiome composition.
Dr. Mamic explains: “When we look at the microbiome composition between individuals, it is very different. There is very little overlap between individuals, even in people who are related. It seems to be more to do with the environment – people who are living together are more likely to have similarities in their microbiome. We are still trying to understand what drives these differences.”
It is thought that these differences may affect the response to a specific diet or medication. Dr. Mamic gives the example of fiber. “Not all bacteria can digest the same types of fiber, so not everyone responds in the same way to a high-fiber diet. That’s probably because of differences in their microbiome.”
Another example is the response to the heart failure drug digoxin, which is metabolized by one particular strain of bacteria in the gut. The toxicity or effectiveness of digoxin seems to be influenced by levels of this bacterial strain, and this again can be influenced by diet, Dr. Mamic says.
Manipulating the microbiome as a therapeutic strategy
Microbiome-targeting therapies may also become part of future treatment strategies for many conditions, including heart failure, the review authors say.
Probiotics (foods and dietary supplements that contain live microbes) interact with the gut microbiota to alter host physiology beneficially. Certain probiotics may specifically modulate processes dysregulated in heart failure, as was suggested in a rodent heart failure model in which supplementation with Lactobacillus-containing and Bifidobacterium-containing probiotics resulted in markedly improved cardiac function, the authors report.
However, a randomized trial (GutHeart) of probiotic yeast Saccharomyces boulardii in patients with heart failure found no improvement in cardiac function, compared with standard care.
Commenting on this, Dr. Mamic suggested that a more specific approach may be needed.
“Some of our preliminary data have shown people who have heart failure have severely depleted Bifidobacteria,” Dr. Mamic said. These bacteria are commercially available as a probiotic, and the researchers are planning a study to give patients with heart failure these specific probiotics. “We are trying to find practical ways forward and to be guided by the data. These people have very little Bifidobacteria, and we know that probiotics seem to be accepted best by the host where there is a specific need for them, so this seems like a sensible approach.”
Dr. Mamic does not recommend that heart failure patients take general probiotic products at present, but she tells her patients about the study she is doing. “Probiotics are quite different from each other. It is a very unregulated market. A general probiotic product may not contain the specific bacteria needed.”
Include microbiome data in biobanks
The review calls for more research on the subject and a more systematic approach to collecting data on the microbiome.
“At present for medical research, blood samples are collected, stored, and analyzed routinely. I think we should also be collecting stool samples in the same way to analyze the microbiome,” Dr. Mamic suggests.
“If we can combine that with data from blood tests on various metabolites/cytokines and look at how the microbiome changes over time or with medication, or with diet, and how the host responds including clinically relevant data, that would be really important. Given how quickly the field is growing I would think there would be biobanks including the microbiome in a few years’ time.”
“We need to gather this data. We would be looking for which bacteria are there, what their functionality is, how it changes over time, with diet or medication, and even whether we can use the microbiome data to predict who will respond to a specific drug.”
Dr. Mamic believes that in the future, analysis of the microbiome could be a routine part of deciding what people eat for good health and to characterize patients for personalized therapies.
“It is clear that the microbiome can influence health, and a dysregulated microbiome negatively affects the host, but there is lot of work to do. We need to learn a lot more about it, but we shouldn’t miss the opportunity to do this,” she concluded.
Dr. Mamic reported no disclosures.
A version of this article first appeared on Medscape.com.
Understanding more about the gut microbiome and how it may affect the development and treatment of heart failure could lead to a more personalized approach to managing the condition, a new review article suggests.
the authors note. “Interactions among the gut microbiome, diet, and medications offer potentially innovative modalities for management of patients with heart failure,” they add.
The review was published online in the Journal of the American College of Cardiology.
“Over the past years we have gathered more understanding about how important the gut microbiome is in relation to how our bodies function overall and even though the cardiovascular system and the heart itself may appear to be quite distant from the gut, we know the gut microbiome affects the cardiovascular system and the physiology of heart failure,” lead author Petra Mamic, MD, Stanford (Calif.) University, told this news organization.
“We’ve also learnt that the microbiome is very personalized. It seems to be affected by a lot of intrinsic and as well as extrinsic factors. For cardiovascular diseases in particular, we always knew that diet and lifestyle were part of the environmental risk, and we now believe that the gut microbiome may be one of the factors that mediates that risk,” she said.
“Studies on the gut microbiome are difficult to do and we are right at the beginning of this type of research. But we have learned that the microbiome is altered or dysregulated in many diseases including many cardiovascular diseases, and many of the changes in the microbiome we see in different cardiovascular diseases seem to overlap,” she added.
Dr. Mamic explained that patients with heart failure have a microbiome that appears different and dysregulated, compared with the microbiome in healthy individuals.
“The difficulty is teasing out whether the microbiome changes are causing heart failure or if they are a consequence of the heart failure and all the medications and comorbidities associated with heart failure,” she commented.
Animal studies have shown that many microbial products, small molecules made by the microbiome, seem to affect how the heart recovers from injury, for example after a myocardial infarction, and how much the heart scars and hypertrophies after an injury, Dr. Mamic reported. These microbiome-derived small molecules can also affect blood pressure, which is dysregulated in heart failure.
Other products of the microbiome can be pro-inflammatory or anti-inflammatory, which can again affect the cardiovascular physiology and the heart, she noted.
High-fiber diet may be beneficial
One area of particular interest at present involves the role of short-chain fatty acids, which are a byproduct of microbes in the gut that digest fiber.
“These short chain fatty acids seem to have positive effects on the host physiology. They are anti-inflammatory; they lower blood pressure; and they seem to protect the heart from scarring and hypertrophy after injury. In heart failure, the gut microbes that make these short-chain fatty acids are significantly depleted,” Dr. Mamic explained.
They are an obvious focus of interest because these short-chain fatty acids are produced when gut bacteria break down dietary fiber, raising the possibility of beneficial effects from eating a high-fiber diet.
Another product of the gut microbiome of interest is trimethylamine N-oxide, formed when gut bacteria break down nutrients such as L-carnitine and phosphatidyl choline, nutrients abundant in foods of animal origin, especially red meat. This metabolite has proatherogenic and prothrombotic effects, and negatively affected cardiac remodeling in a mouse heart failure model, the review notes.
However, though it is too early to make specific dietary recommendations based on these findings, Dr. Mamic points out that a high-fiber diet is thought to be beneficial.
“Nutritional research is very hard to do and the data is limited, but as best as we can summarize things, we know that plant-based diets such as the Mediterranean and DASH diets seem to prevent some of the risk factors for the development of heart failure and seem to slow the progression of heart failure,” she added.
One of the major recommendations in these diets is a high intake of fiber, including whole foods, vegetables, fruits, legumes, and nuts, and less intake of processed food and red meat. “In general, I think everyone should eat like that, but I specifically recommend a plant-based diet with a high amount of fiber to my heart failure patients,” Dr. Mamic said.
Large variation in microbiome composition
The review also explores the idea of personalization of diet or specific treatments dependent on an individual’s gut microbiome composition.
Dr. Mamic explains: “When we look at the microbiome composition between individuals, it is very different. There is very little overlap between individuals, even in people who are related. It seems to be more to do with the environment – people who are living together are more likely to have similarities in their microbiome. We are still trying to understand what drives these differences.”
It is thought that these differences may affect the response to a specific diet or medication. Dr. Mamic gives the example of fiber. “Not all bacteria can digest the same types of fiber, so not everyone responds in the same way to a high-fiber diet. That’s probably because of differences in their microbiome.”
Another example is the response to the heart failure drug digoxin, which is metabolized by one particular strain of bacteria in the gut. The toxicity or effectiveness of digoxin seems to be influenced by levels of this bacterial strain, and this again can be influenced by diet, Dr. Mamic says.
Manipulating the microbiome as a therapeutic strategy
Microbiome-targeting therapies may also become part of future treatment strategies for many conditions, including heart failure, the review authors say.
Probiotics (foods and dietary supplements that contain live microbes) interact with the gut microbiota to alter host physiology beneficially. Certain probiotics may specifically modulate processes dysregulated in heart failure, as was suggested in a rodent heart failure model in which supplementation with Lactobacillus-containing and Bifidobacterium-containing probiotics resulted in markedly improved cardiac function, the authors report.
However, a randomized trial (GutHeart) of probiotic yeast Saccharomyces boulardii in patients with heart failure found no improvement in cardiac function, compared with standard care.
Commenting on this, Dr. Mamic suggested that a more specific approach may be needed.
“Some of our preliminary data have shown people who have heart failure have severely depleted Bifidobacteria,” Dr. Mamic said. These bacteria are commercially available as a probiotic, and the researchers are planning a study to give patients with heart failure these specific probiotics. “We are trying to find practical ways forward and to be guided by the data. These people have very little Bifidobacteria, and we know that probiotics seem to be accepted best by the host where there is a specific need for them, so this seems like a sensible approach.”
Dr. Mamic does not recommend that heart failure patients take general probiotic products at present, but she tells her patients about the study she is doing. “Probiotics are quite different from each other. It is a very unregulated market. A general probiotic product may not contain the specific bacteria needed.”
Include microbiome data in biobanks
The review calls for more research on the subject and a more systematic approach to collecting data on the microbiome.
“At present for medical research, blood samples are collected, stored, and analyzed routinely. I think we should also be collecting stool samples in the same way to analyze the microbiome,” Dr. Mamic suggests.
“If we can combine that with data from blood tests on various metabolites/cytokines and look at how the microbiome changes over time or with medication, or with diet, and how the host responds including clinically relevant data, that would be really important. Given how quickly the field is growing I would think there would be biobanks including the microbiome in a few years’ time.”
“We need to gather this data. We would be looking for which bacteria are there, what their functionality is, how it changes over time, with diet or medication, and even whether we can use the microbiome data to predict who will respond to a specific drug.”
Dr. Mamic believes that in the future, analysis of the microbiome could be a routine part of deciding what people eat for good health and to characterize patients for personalized therapies.
“It is clear that the microbiome can influence health, and a dysregulated microbiome negatively affects the host, but there is lot of work to do. We need to learn a lot more about it, but we shouldn’t miss the opportunity to do this,” she concluded.
Dr. Mamic reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FMT in a pill: FDA approves second product to prevent C. diff recurrence
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
Obesity and CRC link ‘may be underestimated’
and so would not reflect prediagnostic weight loss linked to the condition, a new analysis suggests.
Obesity, assessed using BMI, was associated with a twofold higher risk of CRC 8-10 years prior to diagnosis, while weight loss of 2 kg or more within 2 years of diagnosis was associated with a “dramatic” 7.52-fold increased risk of CRC, the researchers said.
The results “illustrate the dramatic change of BMI as a risk factor associated with CRC, depending on whether the period of potential prediagnostic weight loss is accounted for or not,” Hermann Brenner, MD, MPH, of the German Cancer Research Center, Heidelberg, and colleagues conclude.
The study was published online in JAMA Network Open.
Recent evidence suggests that obesity is associated with an estimated 30% greater risk of CRC. But the extent to which excess body weight influences CRC risk may be underestimated because prediagnostic weight loss has historically been overlooked.
To understand how prediagnostic weight loss could affect the associations found between excess weight and CRC risk, the researchers examined weight data on almost 6,500 patients newly diagnosed with CRC and more than 5,400 control persons who were matched for age, sex, and country of residence. The median age of the cohort was 69 years, and 60.3% were men.
At the time of recruitment, 62% of case patients and 66% of control patients were overweight or obese. No association was found between current BMI and CRC risk.
However, when using patients’ weight from 8-10 years before CRC diagnosis, the researchers found a significant positive association between overweight or obesity and CRC risk (adjusted odds ratio, 1.27 for overweight and 2.09 for obesity). The risk for CRC increased significantly for every 5-unit increase in BMI (aOR, 1.35). These results were similar when the patients were stratified by sex and CRC subsites.
The researchers also found that weight loss of 2 kg or more within 2 years of CRC diagnosis or interview was associated with a 7.52-fold increased risk for CRC.
“While we demonstrated that prediagnostic weight loss is a major concern for CRC,” such prediagnostic weight loss “may play a similarly important role for other cancers and noncancer diseases associated with overweight and obesity,” the authors note.
“Most importantly, however, our results emphasize the importance of interventions aimed at preventing and managing overweight and obesity ... and which may factor more substantially into CRC risk and other obesity-related diseases than suggested by existing epidemiological evidence,” they write.
The study was supported in part by grants from the German Research Council and the German Federal Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and so would not reflect prediagnostic weight loss linked to the condition, a new analysis suggests.
Obesity, assessed using BMI, was associated with a twofold higher risk of CRC 8-10 years prior to diagnosis, while weight loss of 2 kg or more within 2 years of diagnosis was associated with a “dramatic” 7.52-fold increased risk of CRC, the researchers said.
The results “illustrate the dramatic change of BMI as a risk factor associated with CRC, depending on whether the period of potential prediagnostic weight loss is accounted for or not,” Hermann Brenner, MD, MPH, of the German Cancer Research Center, Heidelberg, and colleagues conclude.
The study was published online in JAMA Network Open.
Recent evidence suggests that obesity is associated with an estimated 30% greater risk of CRC. But the extent to which excess body weight influences CRC risk may be underestimated because prediagnostic weight loss has historically been overlooked.
To understand how prediagnostic weight loss could affect the associations found between excess weight and CRC risk, the researchers examined weight data on almost 6,500 patients newly diagnosed with CRC and more than 5,400 control persons who were matched for age, sex, and country of residence. The median age of the cohort was 69 years, and 60.3% were men.
At the time of recruitment, 62% of case patients and 66% of control patients were overweight or obese. No association was found between current BMI and CRC risk.
However, when using patients’ weight from 8-10 years before CRC diagnosis, the researchers found a significant positive association between overweight or obesity and CRC risk (adjusted odds ratio, 1.27 for overweight and 2.09 for obesity). The risk for CRC increased significantly for every 5-unit increase in BMI (aOR, 1.35). These results were similar when the patients were stratified by sex and CRC subsites.
The researchers also found that weight loss of 2 kg or more within 2 years of CRC diagnosis or interview was associated with a 7.52-fold increased risk for CRC.
“While we demonstrated that prediagnostic weight loss is a major concern for CRC,” such prediagnostic weight loss “may play a similarly important role for other cancers and noncancer diseases associated with overweight and obesity,” the authors note.
“Most importantly, however, our results emphasize the importance of interventions aimed at preventing and managing overweight and obesity ... and which may factor more substantially into CRC risk and other obesity-related diseases than suggested by existing epidemiological evidence,” they write.
The study was supported in part by grants from the German Research Council and the German Federal Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and so would not reflect prediagnostic weight loss linked to the condition, a new analysis suggests.
Obesity, assessed using BMI, was associated with a twofold higher risk of CRC 8-10 years prior to diagnosis, while weight loss of 2 kg or more within 2 years of diagnosis was associated with a “dramatic” 7.52-fold increased risk of CRC, the researchers said.
The results “illustrate the dramatic change of BMI as a risk factor associated with CRC, depending on whether the period of potential prediagnostic weight loss is accounted for or not,” Hermann Brenner, MD, MPH, of the German Cancer Research Center, Heidelberg, and colleagues conclude.
The study was published online in JAMA Network Open.
Recent evidence suggests that obesity is associated with an estimated 30% greater risk of CRC. But the extent to which excess body weight influences CRC risk may be underestimated because prediagnostic weight loss has historically been overlooked.
To understand how prediagnostic weight loss could affect the associations found between excess weight and CRC risk, the researchers examined weight data on almost 6,500 patients newly diagnosed with CRC and more than 5,400 control persons who were matched for age, sex, and country of residence. The median age of the cohort was 69 years, and 60.3% were men.
At the time of recruitment, 62% of case patients and 66% of control patients were overweight or obese. No association was found between current BMI and CRC risk.
However, when using patients’ weight from 8-10 years before CRC diagnosis, the researchers found a significant positive association between overweight or obesity and CRC risk (adjusted odds ratio, 1.27 for overweight and 2.09 for obesity). The risk for CRC increased significantly for every 5-unit increase in BMI (aOR, 1.35). These results were similar when the patients were stratified by sex and CRC subsites.
The researchers also found that weight loss of 2 kg or more within 2 years of CRC diagnosis or interview was associated with a 7.52-fold increased risk for CRC.
“While we demonstrated that prediagnostic weight loss is a major concern for CRC,” such prediagnostic weight loss “may play a similarly important role for other cancers and noncancer diseases associated with overweight and obesity,” the authors note.
“Most importantly, however, our results emphasize the importance of interventions aimed at preventing and managing overweight and obesity ... and which may factor more substantially into CRC risk and other obesity-related diseases than suggested by existing epidemiological evidence,” they write.
The study was supported in part by grants from the German Research Council and the German Federal Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
New blood pressure thresholds: How do they affect the evaluation and treatment of hypertension?
In a major shift in the definition of hypertension, guidelines published in 2017 reclassified 130/80 mm Hg as high blood pressure, or stage 1 hypertension. Previous guidelines classified 130/80 mm Hg as elevated, and 140/90 mm Hg used to be the threshold for stage 1 hypertension.
“This shift in classification criteria may cause confusion among clinicians caring for patients with hypertension and has a significant impact on how we diagnose and manage hypertension in our practice,” said Shawna D. Nesbitt, MD, professor of internal medicine at the University of Texas Southwestern Medical Center and medical director at Parkland Hypertension Clinic in Dallas. Dr. Nesbitt is an expert in the diagnosis and treatment of hypertension, particularly complex and refractory cases.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for nearly one-quarter of all deaths in men and in women. Hypertension is a key factor contributing to CVD. The hypertension‐related CVD mortality is currently on the rise in many U.S. demographic groups, including younger individuals (35-64 years old), she said.
When asked about the potential causes of this trend, Dr. Nesbitt explained that the epidemics of obesity and overweight are critical contributors to the high prevalence of hypertension.
The new definition means a wider gap in the prevalence of hypertension between men and women, as well as between Black and White people in the United States. The U.S. rates of hypertension and hypertension‐related CVD mortality are much higher in Black than in White people in this country. Hypertension control rates are the lowest in Black, Hispanic, and Asian males, Dr. Nesbitt said.
Accurate measurement of blood pressure is crucial
The changes in classification criteria for hypertension have made accurate measurements of blood pressure important. A key challenge in the evaluation of hypertension in the clinic is the difference in the methods used to measure blood pressure between trials and real-world clinical practice.
“We can’t easily translate data collected in clinical trials into real-life scenarios, and this can have important implications in our expectations of treatment outcome,” Dr. Nesbitt cautioned.
Commenting on the best practices in blood pressure measurements in the office, Dr. Nesbitt said that patients need to be seated with their feet on the floor and their backs and arms supported. In addition, patients need to have at least 5 minutes of rest without talking.
“It is very important to help patients understand what triggers their blood pressure to be elevated and teach them how and when to measure their blood pressure at home using their own devices,” she added.
Another critical question is how to translate the new guidelines into changes in clinical care, she said.
Current treatment landscape of hypertension
Ensuring a healthy diet, weight, and sleep, participating in physical activity, avoiding nicotine, and managing blood pressure, cholesterol, and sugar levels are the new “Life’s Essential 8” strategies proposed by the American Heart Association (AHA) to reduce CVD risk.
“Sleep has recently been added to the AHA guidelines because it modulates many factors contributing to hypertension,” Dr. Nesbitt pointed out. She advised that clinicians should ask patients about their sleep and educate them on healthy sleeping habits.
Some of the evidence used to develop the new AHA guidelines is derived from the SPRINT trial, which showed that controlling blood pressure reduces the risk of major adverse cardiovascular events. “This is our ultimate goal for our patients with hypertension,” Dr. Nesbitt noted.
Regarding the best practice in hypertension management, Dr. Nesbitt explained that with the new blood pressure thresholds, more patients will be diagnosed with stage 1 hypertension and need the nonpharmacological therapy suggested by the AHA. But patients with stage 1 hypertension and with a high CVD risk (at least 10%) also should receive blood pressure-lowering medications, so an accurate assessment of the risk of clinical atherosclerotic cardiovascular disease (ASCVD) or the estimated 10-year CVD risk is crucial. “If we are not careful, we might miss some patients who need to be treated,” she said.
Calcium channel blockers, thiazide diuretics, and ACE inhibitors or angiotensin receptor blockers (ARBs) are the treatment of choice for patients with newly diagnosed hypertension. Although extensively used in the past, beta-blockers are no longer a first-line treatment for hypertension.
When asked why beta-blockers are no longer suitable for routine initial treatment of hypertension, Dr. Nesbitt said that they are effective in controlling palpitations but “other antihypertensive drugs have proven far better in controlling blood pressure.”
Hypertension is multifactorial and often occurs in combination with other conditions, including diabetes and chronic kidney disease. When developing a treatment plan for patients with hypertension, comorbidities need to be considered, because their management may also help control blood pressure, especially for conditions that may contribute to the development of hypertension.
Common conditions that contribute to and often coexist with hypertension include sleep apnea, obesity, anxiety, and depression. However, convincing people to seek mental health support can be very challenging, Dr. Nesbitt said.
She added that hypertension is a complex disease with a strong social component. Understanding its pathophysiology and social determinants is paramount for successfully managing hypertension at the individual level, as well as at the community level.
Identification and management of side effects is key
Dr. Nesbitt also discussed the importance of the identification and management of side effects associated with blood pressure-lowering drugs. She cautioned that, if not managed, side effects can lead to treatment nonadherence and pseudo‐resistance, both of which can jeopardize the successful management of hypertension.
When asked about her approach to managing side effects and convincing patients to continue taking their medications, Dr. Nesbitt noted that “setting realistic expectations and goals is key.”
In an interview after Dr. Nesbitt’s presentation, Jesica Naanous, MD, agreed that having an honest conversation with the patients is the best way to convince them to keep taking their medications. She also explains to patients that the complications of uncontrolled blood pressure are worse than the side effects of the drugs.
“As a last resort, I change a blood pressure-lowering agent to another,” added Dr. Naanous, an internist at the American British Cowdray (ABC) Medical Center in Mexico City. She explained that many antihypertensive drugs have different toxicity profiles, and simply changing to another agent can make treatment more tolerable for the patient.
Dr. Nesbitt reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
In a major shift in the definition of hypertension, guidelines published in 2017 reclassified 130/80 mm Hg as high blood pressure, or stage 1 hypertension. Previous guidelines classified 130/80 mm Hg as elevated, and 140/90 mm Hg used to be the threshold for stage 1 hypertension.
“This shift in classification criteria may cause confusion among clinicians caring for patients with hypertension and has a significant impact on how we diagnose and manage hypertension in our practice,” said Shawna D. Nesbitt, MD, professor of internal medicine at the University of Texas Southwestern Medical Center and medical director at Parkland Hypertension Clinic in Dallas. Dr. Nesbitt is an expert in the diagnosis and treatment of hypertension, particularly complex and refractory cases.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for nearly one-quarter of all deaths in men and in women. Hypertension is a key factor contributing to CVD. The hypertension‐related CVD mortality is currently on the rise in many U.S. demographic groups, including younger individuals (35-64 years old), she said.
When asked about the potential causes of this trend, Dr. Nesbitt explained that the epidemics of obesity and overweight are critical contributors to the high prevalence of hypertension.
The new definition means a wider gap in the prevalence of hypertension between men and women, as well as between Black and White people in the United States. The U.S. rates of hypertension and hypertension‐related CVD mortality are much higher in Black than in White people in this country. Hypertension control rates are the lowest in Black, Hispanic, and Asian males, Dr. Nesbitt said.
Accurate measurement of blood pressure is crucial
The changes in classification criteria for hypertension have made accurate measurements of blood pressure important. A key challenge in the evaluation of hypertension in the clinic is the difference in the methods used to measure blood pressure between trials and real-world clinical practice.
“We can’t easily translate data collected in clinical trials into real-life scenarios, and this can have important implications in our expectations of treatment outcome,” Dr. Nesbitt cautioned.
Commenting on the best practices in blood pressure measurements in the office, Dr. Nesbitt said that patients need to be seated with their feet on the floor and their backs and arms supported. In addition, patients need to have at least 5 minutes of rest without talking.
“It is very important to help patients understand what triggers their blood pressure to be elevated and teach them how and when to measure their blood pressure at home using their own devices,” she added.
Another critical question is how to translate the new guidelines into changes in clinical care, she said.
Current treatment landscape of hypertension
Ensuring a healthy diet, weight, and sleep, participating in physical activity, avoiding nicotine, and managing blood pressure, cholesterol, and sugar levels are the new “Life’s Essential 8” strategies proposed by the American Heart Association (AHA) to reduce CVD risk.
“Sleep has recently been added to the AHA guidelines because it modulates many factors contributing to hypertension,” Dr. Nesbitt pointed out. She advised that clinicians should ask patients about their sleep and educate them on healthy sleeping habits.
Some of the evidence used to develop the new AHA guidelines is derived from the SPRINT trial, which showed that controlling blood pressure reduces the risk of major adverse cardiovascular events. “This is our ultimate goal for our patients with hypertension,” Dr. Nesbitt noted.
Regarding the best practice in hypertension management, Dr. Nesbitt explained that with the new blood pressure thresholds, more patients will be diagnosed with stage 1 hypertension and need the nonpharmacological therapy suggested by the AHA. But patients with stage 1 hypertension and with a high CVD risk (at least 10%) also should receive blood pressure-lowering medications, so an accurate assessment of the risk of clinical atherosclerotic cardiovascular disease (ASCVD) or the estimated 10-year CVD risk is crucial. “If we are not careful, we might miss some patients who need to be treated,” she said.
Calcium channel blockers, thiazide diuretics, and ACE inhibitors or angiotensin receptor blockers (ARBs) are the treatment of choice for patients with newly diagnosed hypertension. Although extensively used in the past, beta-blockers are no longer a first-line treatment for hypertension.
When asked why beta-blockers are no longer suitable for routine initial treatment of hypertension, Dr. Nesbitt said that they are effective in controlling palpitations but “other antihypertensive drugs have proven far better in controlling blood pressure.”
Hypertension is multifactorial and often occurs in combination with other conditions, including diabetes and chronic kidney disease. When developing a treatment plan for patients with hypertension, comorbidities need to be considered, because their management may also help control blood pressure, especially for conditions that may contribute to the development of hypertension.
Common conditions that contribute to and often coexist with hypertension include sleep apnea, obesity, anxiety, and depression. However, convincing people to seek mental health support can be very challenging, Dr. Nesbitt said.
She added that hypertension is a complex disease with a strong social component. Understanding its pathophysiology and social determinants is paramount for successfully managing hypertension at the individual level, as well as at the community level.
Identification and management of side effects is key
Dr. Nesbitt also discussed the importance of the identification and management of side effects associated with blood pressure-lowering drugs. She cautioned that, if not managed, side effects can lead to treatment nonadherence and pseudo‐resistance, both of which can jeopardize the successful management of hypertension.
When asked about her approach to managing side effects and convincing patients to continue taking their medications, Dr. Nesbitt noted that “setting realistic expectations and goals is key.”
In an interview after Dr. Nesbitt’s presentation, Jesica Naanous, MD, agreed that having an honest conversation with the patients is the best way to convince them to keep taking their medications. She also explains to patients that the complications of uncontrolled blood pressure are worse than the side effects of the drugs.
“As a last resort, I change a blood pressure-lowering agent to another,” added Dr. Naanous, an internist at the American British Cowdray (ABC) Medical Center in Mexico City. She explained that many antihypertensive drugs have different toxicity profiles, and simply changing to another agent can make treatment more tolerable for the patient.
Dr. Nesbitt reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
In a major shift in the definition of hypertension, guidelines published in 2017 reclassified 130/80 mm Hg as high blood pressure, or stage 1 hypertension. Previous guidelines classified 130/80 mm Hg as elevated, and 140/90 mm Hg used to be the threshold for stage 1 hypertension.
“This shift in classification criteria may cause confusion among clinicians caring for patients with hypertension and has a significant impact on how we diagnose and manage hypertension in our practice,” said Shawna D. Nesbitt, MD, professor of internal medicine at the University of Texas Southwestern Medical Center and medical director at Parkland Hypertension Clinic in Dallas. Dr. Nesbitt is an expert in the diagnosis and treatment of hypertension, particularly complex and refractory cases.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for nearly one-quarter of all deaths in men and in women. Hypertension is a key factor contributing to CVD. The hypertension‐related CVD mortality is currently on the rise in many U.S. demographic groups, including younger individuals (35-64 years old), she said.
When asked about the potential causes of this trend, Dr. Nesbitt explained that the epidemics of obesity and overweight are critical contributors to the high prevalence of hypertension.
The new definition means a wider gap in the prevalence of hypertension between men and women, as well as between Black and White people in the United States. The U.S. rates of hypertension and hypertension‐related CVD mortality are much higher in Black than in White people in this country. Hypertension control rates are the lowest in Black, Hispanic, and Asian males, Dr. Nesbitt said.
Accurate measurement of blood pressure is crucial
The changes in classification criteria for hypertension have made accurate measurements of blood pressure important. A key challenge in the evaluation of hypertension in the clinic is the difference in the methods used to measure blood pressure between trials and real-world clinical practice.
“We can’t easily translate data collected in clinical trials into real-life scenarios, and this can have important implications in our expectations of treatment outcome,” Dr. Nesbitt cautioned.
Commenting on the best practices in blood pressure measurements in the office, Dr. Nesbitt said that patients need to be seated with their feet on the floor and their backs and arms supported. In addition, patients need to have at least 5 minutes of rest without talking.
“It is very important to help patients understand what triggers their blood pressure to be elevated and teach them how and when to measure their blood pressure at home using their own devices,” she added.
Another critical question is how to translate the new guidelines into changes in clinical care, she said.
Current treatment landscape of hypertension
Ensuring a healthy diet, weight, and sleep, participating in physical activity, avoiding nicotine, and managing blood pressure, cholesterol, and sugar levels are the new “Life’s Essential 8” strategies proposed by the American Heart Association (AHA) to reduce CVD risk.
“Sleep has recently been added to the AHA guidelines because it modulates many factors contributing to hypertension,” Dr. Nesbitt pointed out. She advised that clinicians should ask patients about their sleep and educate them on healthy sleeping habits.
Some of the evidence used to develop the new AHA guidelines is derived from the SPRINT trial, which showed that controlling blood pressure reduces the risk of major adverse cardiovascular events. “This is our ultimate goal for our patients with hypertension,” Dr. Nesbitt noted.
Regarding the best practice in hypertension management, Dr. Nesbitt explained that with the new blood pressure thresholds, more patients will be diagnosed with stage 1 hypertension and need the nonpharmacological therapy suggested by the AHA. But patients with stage 1 hypertension and with a high CVD risk (at least 10%) also should receive blood pressure-lowering medications, so an accurate assessment of the risk of clinical atherosclerotic cardiovascular disease (ASCVD) or the estimated 10-year CVD risk is crucial. “If we are not careful, we might miss some patients who need to be treated,” she said.
Calcium channel blockers, thiazide diuretics, and ACE inhibitors or angiotensin receptor blockers (ARBs) are the treatment of choice for patients with newly diagnosed hypertension. Although extensively used in the past, beta-blockers are no longer a first-line treatment for hypertension.
When asked why beta-blockers are no longer suitable for routine initial treatment of hypertension, Dr. Nesbitt said that they are effective in controlling palpitations but “other antihypertensive drugs have proven far better in controlling blood pressure.”
Hypertension is multifactorial and often occurs in combination with other conditions, including diabetes and chronic kidney disease. When developing a treatment plan for patients with hypertension, comorbidities need to be considered, because their management may also help control blood pressure, especially for conditions that may contribute to the development of hypertension.
Common conditions that contribute to and often coexist with hypertension include sleep apnea, obesity, anxiety, and depression. However, convincing people to seek mental health support can be very challenging, Dr. Nesbitt said.
She added that hypertension is a complex disease with a strong social component. Understanding its pathophysiology and social determinants is paramount for successfully managing hypertension at the individual level, as well as at the community level.
Identification and management of side effects is key
Dr. Nesbitt also discussed the importance of the identification and management of side effects associated with blood pressure-lowering drugs. She cautioned that, if not managed, side effects can lead to treatment nonadherence and pseudo‐resistance, both of which can jeopardize the successful management of hypertension.
When asked about her approach to managing side effects and convincing patients to continue taking their medications, Dr. Nesbitt noted that “setting realistic expectations and goals is key.”
In an interview after Dr. Nesbitt’s presentation, Jesica Naanous, MD, agreed that having an honest conversation with the patients is the best way to convince them to keep taking their medications. She also explains to patients that the complications of uncontrolled blood pressure are worse than the side effects of the drugs.
“As a last resort, I change a blood pressure-lowering agent to another,” added Dr. Naanous, an internist at the American British Cowdray (ABC) Medical Center in Mexico City. She explained that many antihypertensive drugs have different toxicity profiles, and simply changing to another agent can make treatment more tolerable for the patient.
Dr. Nesbitt reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
AT INTERNAL MEDICINE 2023
Remote weight monitoring minimizes office visits for newborns
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT PAS 2023
NSAID use may mask MRI findings in a quarter of spondyloarthritis cases
MANCHESTER, ENGLAND – The use of NSAIDs may mask the true level of inflammation of the sacroiliac joint (SIJ), as seen on MRI, among people with axial spondyloarthritis (axSpA), according to results of the DyNAMISM study.
“We’ve found that in about one in four patients, NSAIDs make a difference to the scan results,” Gareth T. Jones, PhD, said at the annual meeting of the British Society for Rheumatology.
A total of 23% of patients whose MRI results were positive for sacroiliitis when no NSAIDs had been used for a couple of weeks received negative MRI results 6 weeks after the NSAIDs were reinstated.
“This is important in terms of diagnosis, in terms of disease classification, and may be important in terms of future treatment decisions,” added Dr. Jones, professor of epidemiology at the Aberdeen Centre for Arthritis and Musculoskeletal Health at the University of Aberdeen, (Scotland).
“Our recommendation from these results is that if a patient is willing to attempt to wash out [NSAIDs] prior to an MRI, we would recommend that they do so,” Dr. Jones said.
NSAIDs and AxSpA inflammation
“NSAIDs are often used as the first-line treatment for axial spondyloarthritis due to their ability to effectively reduce pain and stiffness associated with the condition,” Denis Poddubnyy, MD, who was not involved in the research, told this news organization.
“However, there is still a question as to whether NSAIDs have a true anti-inflammatory effect on the axial inflammation, as detected by MRI,” added Dr. Poddubnyy, head of rheumatology at Charité–Universitätsmedizin Berlin in Germany.
With an absence of randomized, controlled trials, it remains “uncertain how much of the observed reduction in inflammation is attributable to the natural course of the disease and spontaneous resolution of inflammation rather than the effect of NSAIDs,” Dr. Poddubnyy said.
The DyNAMISM Study
“Sacroiliitis is a painful inflammatory condition. This is investigated looking for the evidence of inflammation on MRI, but many patients are taking anti-inflammatory medication,” Dr. Jones said at the meeting.
“So perhaps patients are taking drugs [that are] hiding the very thing that we’re looking for,” he added. Hence, the DyNAMISM study (Do Nonsteroidal Anti-inflammatory Drugs Mask Inflammation in Spondyloarthritis on MRI) was conceived.
The researchers recruited 311 adults with suspected or established axSpA who were taking daily NSAIDs such as ibuprofen or diclofenac across 34 centers in England and Scotland. Patients taking other anti-inflammatory medications that could not be stopped were excluded, as were patients who were currently taking or had recently taken tumor necrosis factor inhibitors.
The study used a standardized MRI protocol. Two independent readers experienced in scoring SIJ scans were employed; a third was used when the two disagreed. The primary outcome was meeting the Assessment of Spondyloarthritis international Society criteria for a positive result on MRI.
The average age of the study subjects was 42 years, 62% were men, and 87% were White. The median duration of symptoms was 9 years, and the median time since diagnosis was 1 year.
The study design required that patients stop NSAID use over a period of 1-2 weeks before undergoing an MRI scan, which 286 did. Of these, 146 received MRI results that were positive for SIJ inflammation; those patients continued in the study. The 140 patients with negative scans were excluded. Patients could then resume taking NSAIDs before being scanned again around 6 weeks later. In all, 129 patients underwent both MRI scans.
How much might fluctuating inflammation matter?
‘It’s a shame you didn’t scan the negative people, because the natural history is a fluctuating inflammation,” Fraser Birrell, MBChB, PhD, of Newcastle University, Newcastle upon Tyne, England, pointed out in discussion.
“Nonsteroidals are modestly effective and probably made no difference,” he argued. “I would have expected a certain proportion of the negatives are positive.”
The study had a pragmatic design, Dr. Jones countered. “We had enormous debate before the study; it would have been nice to do a sort of a randomized, crossover design, but it would have resulted in a lot of inefficiency.”
Regarding the duration of the NSAID washout period, Dr. Jones noted that they saw little difference between shorter or longer washout periods and that the data showed that “a 2-week washout is a reasonable target.”
Performing the second scan 6 weeks after NSAIDs were reinstated “exceeds the period where clinical benefits should be expected. It may be that if we’d waited longer, the proportion would have gone up. So, we would argue that actually, if anything, that 23% may be an underestimate of the real effect.”
Although some patients may have declined to participate in the study because they did not want to stop taking NSAIDs, Dr. Jones noted that a good proportion did stop taking them, and so the study shows that patients can tolerate washout. Around 45% of patients reported experiencing disease flares during this time, but this did not have any significant effect on validated disease activity or pain measures, Dr. Jones reported.
So, if patients are willing to stop NSAIDs before a scan, “they should be counseled that they may experience a small increase in disease activity and spinal pain, but also to be counseled that the majority of patients can tolerate this,” Dr. Jones suggested.
Trials are needed, Dr. Poddubnyy said: “Future randomized, controlled studies are needed to conclusively determine the efficacy of NSAIDs in reducing inflammation in the axial skeleton of axSpA patients.”
Dr. Poddubnyy added: “It would also be valuable to assess in a randomized setting whether the use of NSAIDs impacts the diagnostic performance of MRI, which takes into account not only inflammatory but also structural changes, which are not influenced by NSAIDs.”
The DyNAMISM study was funded by Arthritis Research UK and was run by the University of Aberdeen in conjunction with NHS Grampian, Scotland. Dr. Jones has disclosed no relevant financial relationships. Dr. Poddubnyy disclosed ties with AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Medscape, Merck Sharp & Dohme, Moonlake, Novartis, PeerVoice, Pfizer, Samsung Bioepis, and UCB.
A version of this article originally appeared on Medscape.com.
MANCHESTER, ENGLAND – The use of NSAIDs may mask the true level of inflammation of the sacroiliac joint (SIJ), as seen on MRI, among people with axial spondyloarthritis (axSpA), according to results of the DyNAMISM study.
“We’ve found that in about one in four patients, NSAIDs make a difference to the scan results,” Gareth T. Jones, PhD, said at the annual meeting of the British Society for Rheumatology.
A total of 23% of patients whose MRI results were positive for sacroiliitis when no NSAIDs had been used for a couple of weeks received negative MRI results 6 weeks after the NSAIDs were reinstated.
“This is important in terms of diagnosis, in terms of disease classification, and may be important in terms of future treatment decisions,” added Dr. Jones, professor of epidemiology at the Aberdeen Centre for Arthritis and Musculoskeletal Health at the University of Aberdeen, (Scotland).
“Our recommendation from these results is that if a patient is willing to attempt to wash out [NSAIDs] prior to an MRI, we would recommend that they do so,” Dr. Jones said.
NSAIDs and AxSpA inflammation
“NSAIDs are often used as the first-line treatment for axial spondyloarthritis due to their ability to effectively reduce pain and stiffness associated with the condition,” Denis Poddubnyy, MD, who was not involved in the research, told this news organization.
“However, there is still a question as to whether NSAIDs have a true anti-inflammatory effect on the axial inflammation, as detected by MRI,” added Dr. Poddubnyy, head of rheumatology at Charité–Universitätsmedizin Berlin in Germany.
With an absence of randomized, controlled trials, it remains “uncertain how much of the observed reduction in inflammation is attributable to the natural course of the disease and spontaneous resolution of inflammation rather than the effect of NSAIDs,” Dr. Poddubnyy said.
The DyNAMISM Study
“Sacroiliitis is a painful inflammatory condition. This is investigated looking for the evidence of inflammation on MRI, but many patients are taking anti-inflammatory medication,” Dr. Jones said at the meeting.
“So perhaps patients are taking drugs [that are] hiding the very thing that we’re looking for,” he added. Hence, the DyNAMISM study (Do Nonsteroidal Anti-inflammatory Drugs Mask Inflammation in Spondyloarthritis on MRI) was conceived.
The researchers recruited 311 adults with suspected or established axSpA who were taking daily NSAIDs such as ibuprofen or diclofenac across 34 centers in England and Scotland. Patients taking other anti-inflammatory medications that could not be stopped were excluded, as were patients who were currently taking or had recently taken tumor necrosis factor inhibitors.
The study used a standardized MRI protocol. Two independent readers experienced in scoring SIJ scans were employed; a third was used when the two disagreed. The primary outcome was meeting the Assessment of Spondyloarthritis international Society criteria for a positive result on MRI.
The average age of the study subjects was 42 years, 62% were men, and 87% were White. The median duration of symptoms was 9 years, and the median time since diagnosis was 1 year.
The study design required that patients stop NSAID use over a period of 1-2 weeks before undergoing an MRI scan, which 286 did. Of these, 146 received MRI results that were positive for SIJ inflammation; those patients continued in the study. The 140 patients with negative scans were excluded. Patients could then resume taking NSAIDs before being scanned again around 6 weeks later. In all, 129 patients underwent both MRI scans.
How much might fluctuating inflammation matter?
‘It’s a shame you didn’t scan the negative people, because the natural history is a fluctuating inflammation,” Fraser Birrell, MBChB, PhD, of Newcastle University, Newcastle upon Tyne, England, pointed out in discussion.
“Nonsteroidals are modestly effective and probably made no difference,” he argued. “I would have expected a certain proportion of the negatives are positive.”
The study had a pragmatic design, Dr. Jones countered. “We had enormous debate before the study; it would have been nice to do a sort of a randomized, crossover design, but it would have resulted in a lot of inefficiency.”
Regarding the duration of the NSAID washout period, Dr. Jones noted that they saw little difference between shorter or longer washout periods and that the data showed that “a 2-week washout is a reasonable target.”
Performing the second scan 6 weeks after NSAIDs were reinstated “exceeds the period where clinical benefits should be expected. It may be that if we’d waited longer, the proportion would have gone up. So, we would argue that actually, if anything, that 23% may be an underestimate of the real effect.”
Although some patients may have declined to participate in the study because they did not want to stop taking NSAIDs, Dr. Jones noted that a good proportion did stop taking them, and so the study shows that patients can tolerate washout. Around 45% of patients reported experiencing disease flares during this time, but this did not have any significant effect on validated disease activity or pain measures, Dr. Jones reported.
So, if patients are willing to stop NSAIDs before a scan, “they should be counseled that they may experience a small increase in disease activity and spinal pain, but also to be counseled that the majority of patients can tolerate this,” Dr. Jones suggested.
Trials are needed, Dr. Poddubnyy said: “Future randomized, controlled studies are needed to conclusively determine the efficacy of NSAIDs in reducing inflammation in the axial skeleton of axSpA patients.”
Dr. Poddubnyy added: “It would also be valuable to assess in a randomized setting whether the use of NSAIDs impacts the diagnostic performance of MRI, which takes into account not only inflammatory but also structural changes, which are not influenced by NSAIDs.”
The DyNAMISM study was funded by Arthritis Research UK and was run by the University of Aberdeen in conjunction with NHS Grampian, Scotland. Dr. Jones has disclosed no relevant financial relationships. Dr. Poddubnyy disclosed ties with AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Medscape, Merck Sharp & Dohme, Moonlake, Novartis, PeerVoice, Pfizer, Samsung Bioepis, and UCB.
A version of this article originally appeared on Medscape.com.
MANCHESTER, ENGLAND – The use of NSAIDs may mask the true level of inflammation of the sacroiliac joint (SIJ), as seen on MRI, among people with axial spondyloarthritis (axSpA), according to results of the DyNAMISM study.
“We’ve found that in about one in four patients, NSAIDs make a difference to the scan results,” Gareth T. Jones, PhD, said at the annual meeting of the British Society for Rheumatology.
A total of 23% of patients whose MRI results were positive for sacroiliitis when no NSAIDs had been used for a couple of weeks received negative MRI results 6 weeks after the NSAIDs were reinstated.
“This is important in terms of diagnosis, in terms of disease classification, and may be important in terms of future treatment decisions,” added Dr. Jones, professor of epidemiology at the Aberdeen Centre for Arthritis and Musculoskeletal Health at the University of Aberdeen, (Scotland).
“Our recommendation from these results is that if a patient is willing to attempt to wash out [NSAIDs] prior to an MRI, we would recommend that they do so,” Dr. Jones said.
NSAIDs and AxSpA inflammation
“NSAIDs are often used as the first-line treatment for axial spondyloarthritis due to their ability to effectively reduce pain and stiffness associated with the condition,” Denis Poddubnyy, MD, who was not involved in the research, told this news organization.
“However, there is still a question as to whether NSAIDs have a true anti-inflammatory effect on the axial inflammation, as detected by MRI,” added Dr. Poddubnyy, head of rheumatology at Charité–Universitätsmedizin Berlin in Germany.
With an absence of randomized, controlled trials, it remains “uncertain how much of the observed reduction in inflammation is attributable to the natural course of the disease and spontaneous resolution of inflammation rather than the effect of NSAIDs,” Dr. Poddubnyy said.
The DyNAMISM Study
“Sacroiliitis is a painful inflammatory condition. This is investigated looking for the evidence of inflammation on MRI, but many patients are taking anti-inflammatory medication,” Dr. Jones said at the meeting.
“So perhaps patients are taking drugs [that are] hiding the very thing that we’re looking for,” he added. Hence, the DyNAMISM study (Do Nonsteroidal Anti-inflammatory Drugs Mask Inflammation in Spondyloarthritis on MRI) was conceived.
The researchers recruited 311 adults with suspected or established axSpA who were taking daily NSAIDs such as ibuprofen or diclofenac across 34 centers in England and Scotland. Patients taking other anti-inflammatory medications that could not be stopped were excluded, as were patients who were currently taking or had recently taken tumor necrosis factor inhibitors.
The study used a standardized MRI protocol. Two independent readers experienced in scoring SIJ scans were employed; a third was used when the two disagreed. The primary outcome was meeting the Assessment of Spondyloarthritis international Society criteria for a positive result on MRI.
The average age of the study subjects was 42 years, 62% were men, and 87% were White. The median duration of symptoms was 9 years, and the median time since diagnosis was 1 year.
The study design required that patients stop NSAID use over a period of 1-2 weeks before undergoing an MRI scan, which 286 did. Of these, 146 received MRI results that were positive for SIJ inflammation; those patients continued in the study. The 140 patients with negative scans were excluded. Patients could then resume taking NSAIDs before being scanned again around 6 weeks later. In all, 129 patients underwent both MRI scans.
How much might fluctuating inflammation matter?
‘It’s a shame you didn’t scan the negative people, because the natural history is a fluctuating inflammation,” Fraser Birrell, MBChB, PhD, of Newcastle University, Newcastle upon Tyne, England, pointed out in discussion.
“Nonsteroidals are modestly effective and probably made no difference,” he argued. “I would have expected a certain proportion of the negatives are positive.”
The study had a pragmatic design, Dr. Jones countered. “We had enormous debate before the study; it would have been nice to do a sort of a randomized, crossover design, but it would have resulted in a lot of inefficiency.”
Regarding the duration of the NSAID washout period, Dr. Jones noted that they saw little difference between shorter or longer washout periods and that the data showed that “a 2-week washout is a reasonable target.”
Performing the second scan 6 weeks after NSAIDs were reinstated “exceeds the period where clinical benefits should be expected. It may be that if we’d waited longer, the proportion would have gone up. So, we would argue that actually, if anything, that 23% may be an underestimate of the real effect.”
Although some patients may have declined to participate in the study because they did not want to stop taking NSAIDs, Dr. Jones noted that a good proportion did stop taking them, and so the study shows that patients can tolerate washout. Around 45% of patients reported experiencing disease flares during this time, but this did not have any significant effect on validated disease activity or pain measures, Dr. Jones reported.
So, if patients are willing to stop NSAIDs before a scan, “they should be counseled that they may experience a small increase in disease activity and spinal pain, but also to be counseled that the majority of patients can tolerate this,” Dr. Jones suggested.
Trials are needed, Dr. Poddubnyy said: “Future randomized, controlled studies are needed to conclusively determine the efficacy of NSAIDs in reducing inflammation in the axial skeleton of axSpA patients.”
Dr. Poddubnyy added: “It would also be valuable to assess in a randomized setting whether the use of NSAIDs impacts the diagnostic performance of MRI, which takes into account not only inflammatory but also structural changes, which are not influenced by NSAIDs.”
The DyNAMISM study was funded by Arthritis Research UK and was run by the University of Aberdeen in conjunction with NHS Grampian, Scotland. Dr. Jones has disclosed no relevant financial relationships. Dr. Poddubnyy disclosed ties with AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Medscape, Merck Sharp & Dohme, Moonlake, Novartis, PeerVoice, Pfizer, Samsung Bioepis, and UCB.
A version of this article originally appeared on Medscape.com.
AT BSR 2023
Can an endoscopic procedure treat type 2 diabetes?
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
FROM DDW 2023












