User login
New Slate of Officers for the CHEST Foundation
The CHEST Foundation has announced Michael E. Nelson, MD, FCCP, as its new President, effective November 1. At CHEST 2016, the annual meeting of the American College of Chest Physicians (CHEST), the CHEST board also confirmed the appointments of Lisa K. Moores, MD, FCCP, as President-Elect of the CHEST Foundation; Doreen J. Addrizzo-Harris, MD, FCCP, as CHEST Foundation President-Designate; and John A. Howington, MD, FCCP, as CHEST Foundation Immediate Past President.
Michael E. Nelson, MD, FCCP, is a private practice pulmonologist at Shawnee Mission Pulmonary Consultants, Kan., specializing in pulmonary diseases, critical care, and sleep medicine. A CHEST Foundation Trustee since 2012, Dr. Nelson has served the last 2 years as Foundation President-Elect, and has been affiliated with the American College of Chest Physicians for almost 22 years, holding a variety of roles. Dr. Nelson is board-certified in internal medicine, pulmonary disease, critical care medicine, and sleep medicine.
Lisa K. Moores, MD, FCCP, is the Associate Dean of Students and Professor of Medicine at F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Md. Currently, Dr. Moores is on active duty with the U.S. Army Medical Corps. She has been a member of the American College of Chest Physicians since 1994, and she has played a very active role in her time with the College, including serving as a CHEST Foundation Trustee-at-Large since 2011. Dr. Moores was awarded the Edward C. Rosenow III, Master FCCP/Master Teacher Honor Lecture Award at CHEST 2013. She is board-certified in critical care medicine, pulmonary disease, and internal medicine.
Doreen J. Addrizzo-Harris, MD, FCCP, is a graduate in medicine from New York University Medical Center, where she currently serves as a Professor of Medicine in the Division of Pulmonary and Critical Care. A CHEST Foundation Trustee-at-Large since 2013, she has been a Fellow of the American College of Chest Physicians for more than 20 years. Dr. Addrizzo-Harris was awarded Teacher of the Year in Pulmonary and Critical Care at NYU in 2000 and then again in 2013, showcasing her outstanding commitment and dedication to teaching.
John A. Howington, MD, FCCP, is a thoracic surgeon oncologist at St. Thomas Health, Nashville, Tenn., specializing in minimally invasive treatment options for thoracic cancer. He serves as the Chairman of Thoracic Surgery and is also the Co-Director of the Thoracic Oncology Program. A CHEST Foundation Trustee since 2012 and CHEST Foundation President for the last 2 years, he has been involved with the American College of Chest Physicians for nearly 20 years. In that time, he has held a variety of roles.
The CHEST Foundation has announced Michael E. Nelson, MD, FCCP, as its new President, effective November 1. At CHEST 2016, the annual meeting of the American College of Chest Physicians (CHEST), the CHEST board also confirmed the appointments of Lisa K. Moores, MD, FCCP, as President-Elect of the CHEST Foundation; Doreen J. Addrizzo-Harris, MD, FCCP, as CHEST Foundation President-Designate; and John A. Howington, MD, FCCP, as CHEST Foundation Immediate Past President.
Michael E. Nelson, MD, FCCP, is a private practice pulmonologist at Shawnee Mission Pulmonary Consultants, Kan., specializing in pulmonary diseases, critical care, and sleep medicine. A CHEST Foundation Trustee since 2012, Dr. Nelson has served the last 2 years as Foundation President-Elect, and has been affiliated with the American College of Chest Physicians for almost 22 years, holding a variety of roles. Dr. Nelson is board-certified in internal medicine, pulmonary disease, critical care medicine, and sleep medicine.
Lisa K. Moores, MD, FCCP, is the Associate Dean of Students and Professor of Medicine at F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Md. Currently, Dr. Moores is on active duty with the U.S. Army Medical Corps. She has been a member of the American College of Chest Physicians since 1994, and she has played a very active role in her time with the College, including serving as a CHEST Foundation Trustee-at-Large since 2011. Dr. Moores was awarded the Edward C. Rosenow III, Master FCCP/Master Teacher Honor Lecture Award at CHEST 2013. She is board-certified in critical care medicine, pulmonary disease, and internal medicine.
Doreen J. Addrizzo-Harris, MD, FCCP, is a graduate in medicine from New York University Medical Center, where she currently serves as a Professor of Medicine in the Division of Pulmonary and Critical Care. A CHEST Foundation Trustee-at-Large since 2013, she has been a Fellow of the American College of Chest Physicians for more than 20 years. Dr. Addrizzo-Harris was awarded Teacher of the Year in Pulmonary and Critical Care at NYU in 2000 and then again in 2013, showcasing her outstanding commitment and dedication to teaching.
John A. Howington, MD, FCCP, is a thoracic surgeon oncologist at St. Thomas Health, Nashville, Tenn., specializing in minimally invasive treatment options for thoracic cancer. He serves as the Chairman of Thoracic Surgery and is also the Co-Director of the Thoracic Oncology Program. A CHEST Foundation Trustee since 2012 and CHEST Foundation President for the last 2 years, he has been involved with the American College of Chest Physicians for nearly 20 years. In that time, he has held a variety of roles.
The CHEST Foundation has announced Michael E. Nelson, MD, FCCP, as its new President, effective November 1. At CHEST 2016, the annual meeting of the American College of Chest Physicians (CHEST), the CHEST board also confirmed the appointments of Lisa K. Moores, MD, FCCP, as President-Elect of the CHEST Foundation; Doreen J. Addrizzo-Harris, MD, FCCP, as CHEST Foundation President-Designate; and John A. Howington, MD, FCCP, as CHEST Foundation Immediate Past President.
Michael E. Nelson, MD, FCCP, is a private practice pulmonologist at Shawnee Mission Pulmonary Consultants, Kan., specializing in pulmonary diseases, critical care, and sleep medicine. A CHEST Foundation Trustee since 2012, Dr. Nelson has served the last 2 years as Foundation President-Elect, and has been affiliated with the American College of Chest Physicians for almost 22 years, holding a variety of roles. Dr. Nelson is board-certified in internal medicine, pulmonary disease, critical care medicine, and sleep medicine.
Lisa K. Moores, MD, FCCP, is the Associate Dean of Students and Professor of Medicine at F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Md. Currently, Dr. Moores is on active duty with the U.S. Army Medical Corps. She has been a member of the American College of Chest Physicians since 1994, and she has played a very active role in her time with the College, including serving as a CHEST Foundation Trustee-at-Large since 2011. Dr. Moores was awarded the Edward C. Rosenow III, Master FCCP/Master Teacher Honor Lecture Award at CHEST 2013. She is board-certified in critical care medicine, pulmonary disease, and internal medicine.
Doreen J. Addrizzo-Harris, MD, FCCP, is a graduate in medicine from New York University Medical Center, where she currently serves as a Professor of Medicine in the Division of Pulmonary and Critical Care. A CHEST Foundation Trustee-at-Large since 2013, she has been a Fellow of the American College of Chest Physicians for more than 20 years. Dr. Addrizzo-Harris was awarded Teacher of the Year in Pulmonary and Critical Care at NYU in 2000 and then again in 2013, showcasing her outstanding commitment and dedication to teaching.
John A. Howington, MD, FCCP, is a thoracic surgeon oncologist at St. Thomas Health, Nashville, Tenn., specializing in minimally invasive treatment options for thoracic cancer. He serves as the Chairman of Thoracic Surgery and is also the Co-Director of the Thoracic Oncology Program. A CHEST Foundation Trustee since 2012 and CHEST Foundation President for the last 2 years, he has been involved with the American College of Chest Physicians for nearly 20 years. In that time, he has held a variety of roles.
Coding Updates
CHEST Physician Editorial Board Member
Confusion in EBUS Coding
There has been some confusion about appropriate coding using the new endobronchial ultrasound codes with some of the other bronchoscopy codes. Notably, when CPT code 31629 bronchoscopy with transbronchial needle aspiration biopsy(s), trachea, main stem and/or lobar bronchus(i) is appropriate to use with code 31652 with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]), one or two mediastinal and/or hilar lymph node stations or structures and code 31653 with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]), 3 or more mediastinal and/or hilar lymph node stations or structures. Both 31652 and 31653 include needle sampling as a part of the work and therefore, if the bronchoscopy involves only one these procedures, it would be inappropriate to include 31629.
However, mediastinal sampling is often done in conjunction with evaluation of a more peripheral lesion. If a bronchoscopy is performed with needle biopsy(ies) of a peripheral lesion and subsequently an EBUS scope is used to sample mediastinal or hilar lymph node stations, one could utilize 31629 and either 31652 or 31653. As an illustrative example, a 75-year-old man is found to have a 2-cm peripheral nodule in the anterior segment of the right-upper lobe with enlarged right hilar and subcarinal lymph nodes on CT scan. Bronchoscopy is performed, and, initially, the patient has a survey bronchoscopy using a non-EBUS scope, and no lesion is visible. A radial ultrasound probe is used to help identify the peripheral lesion, and multiple needle biopsies are performed as are brushings and washings. Subsequently, an EBUS scope is introduced, and right hilar, right paratracheal, and subcarinal needle biopsies were performed. The appropriate codes to utilize to describe the work done in this procedure include 31623, 31629, 31653, and 31654. Had no peripheral needle biopsies been performed, then code 31629 would NOT be used. Hopefully, this clarifies the issue further.
CMS Ceases Use of HCPCS G Codes for Smoking Cessation
Effective on or after October 1, the Centers for Medicare & Medicaid Services (CMS) will no longer allow use of Healthcare Common Procedural Coding System (HCPCS) codes G0436 (Smoking and tobacco cessation counseling visit for the asymptomatic patient; intermediate, greater than 3 minutes, up to 10 minutes) and G0437 (Smoking and tobacco cessation counseling visit for the asymptomatic patient; intensive, greater than 10 minutes). Instead, CMS will utilize the new codes developed for the Current Procedural Terminology (CPT) code set.
CMS has advised its Medicare contractors to replace code G0436 with CPT code 99406 (Smoking and tobacco use cessation counseling visit; intermediate, greater than 3 minutes up to 10 minutes) and code G0437 with CPT code 99407 (Smoking and tobacco use cessation counseling visit; intensive, greater than 10 minutes). According to the Medicare National Coverage Determination Manual, tobacco cessation counseling is covered both for symptomatic and asymptomatic smokers. CMS will allow health-care providers two attempts per 12 months to encourage Medicare patients to cease tobacco use but does not define an attempt. Rather, either of the codes may be used up to four times per attempt; so 99406 and 99407 or a combination of these codes may be used up to 8 times in a 12-month period. These codes may be used either as a stand-alone or with an evaluation and management (E&M) service with appropriate documentation. Remember, however, if one uses these codes during an E&M visit, a 25 modifier will need to be appended to the E&M code.
CHEST Physician Editorial Board Member
Confusion in EBUS Coding
There has been some confusion about appropriate coding using the new endobronchial ultrasound codes with some of the other bronchoscopy codes. Notably, when CPT code 31629 bronchoscopy with transbronchial needle aspiration biopsy(s), trachea, main stem and/or lobar bronchus(i) is appropriate to use with code 31652 with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]), one or two mediastinal and/or hilar lymph node stations or structures and code 31653 with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]), 3 or more mediastinal and/or hilar lymph node stations or structures. Both 31652 and 31653 include needle sampling as a part of the work and therefore, if the bronchoscopy involves only one these procedures, it would be inappropriate to include 31629.
However, mediastinal sampling is often done in conjunction with evaluation of a more peripheral lesion. If a bronchoscopy is performed with needle biopsy(ies) of a peripheral lesion and subsequently an EBUS scope is used to sample mediastinal or hilar lymph node stations, one could utilize 31629 and either 31652 or 31653. As an illustrative example, a 75-year-old man is found to have a 2-cm peripheral nodule in the anterior segment of the right-upper lobe with enlarged right hilar and subcarinal lymph nodes on CT scan. Bronchoscopy is performed, and, initially, the patient has a survey bronchoscopy using a non-EBUS scope, and no lesion is visible. A radial ultrasound probe is used to help identify the peripheral lesion, and multiple needle biopsies are performed as are brushings and washings. Subsequently, an EBUS scope is introduced, and right hilar, right paratracheal, and subcarinal needle biopsies were performed. The appropriate codes to utilize to describe the work done in this procedure include 31623, 31629, 31653, and 31654. Had no peripheral needle biopsies been performed, then code 31629 would NOT be used. Hopefully, this clarifies the issue further.
CMS Ceases Use of HCPCS G Codes for Smoking Cessation
Effective on or after October 1, the Centers for Medicare & Medicaid Services (CMS) will no longer allow use of Healthcare Common Procedural Coding System (HCPCS) codes G0436 (Smoking and tobacco cessation counseling visit for the asymptomatic patient; intermediate, greater than 3 minutes, up to 10 minutes) and G0437 (Smoking and tobacco cessation counseling visit for the asymptomatic patient; intensive, greater than 10 minutes). Instead, CMS will utilize the new codes developed for the Current Procedural Terminology (CPT) code set.
CMS has advised its Medicare contractors to replace code G0436 with CPT code 99406 (Smoking and tobacco use cessation counseling visit; intermediate, greater than 3 minutes up to 10 minutes) and code G0437 with CPT code 99407 (Smoking and tobacco use cessation counseling visit; intensive, greater than 10 minutes). According to the Medicare National Coverage Determination Manual, tobacco cessation counseling is covered both for symptomatic and asymptomatic smokers. CMS will allow health-care providers two attempts per 12 months to encourage Medicare patients to cease tobacco use but does not define an attempt. Rather, either of the codes may be used up to four times per attempt; so 99406 and 99407 or a combination of these codes may be used up to 8 times in a 12-month period. These codes may be used either as a stand-alone or with an evaluation and management (E&M) service with appropriate documentation. Remember, however, if one uses these codes during an E&M visit, a 25 modifier will need to be appended to the E&M code.
CHEST Physician Editorial Board Member
Confusion in EBUS Coding
There has been some confusion about appropriate coding using the new endobronchial ultrasound codes with some of the other bronchoscopy codes. Notably, when CPT code 31629 bronchoscopy with transbronchial needle aspiration biopsy(s), trachea, main stem and/or lobar bronchus(i) is appropriate to use with code 31652 with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]), one or two mediastinal and/or hilar lymph node stations or structures and code 31653 with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]), 3 or more mediastinal and/or hilar lymph node stations or structures. Both 31652 and 31653 include needle sampling as a part of the work and therefore, if the bronchoscopy involves only one these procedures, it would be inappropriate to include 31629.
However, mediastinal sampling is often done in conjunction with evaluation of a more peripheral lesion. If a bronchoscopy is performed with needle biopsy(ies) of a peripheral lesion and subsequently an EBUS scope is used to sample mediastinal or hilar lymph node stations, one could utilize 31629 and either 31652 or 31653. As an illustrative example, a 75-year-old man is found to have a 2-cm peripheral nodule in the anterior segment of the right-upper lobe with enlarged right hilar and subcarinal lymph nodes on CT scan. Bronchoscopy is performed, and, initially, the patient has a survey bronchoscopy using a non-EBUS scope, and no lesion is visible. A radial ultrasound probe is used to help identify the peripheral lesion, and multiple needle biopsies are performed as are brushings and washings. Subsequently, an EBUS scope is introduced, and right hilar, right paratracheal, and subcarinal needle biopsies were performed. The appropriate codes to utilize to describe the work done in this procedure include 31623, 31629, 31653, and 31654. Had no peripheral needle biopsies been performed, then code 31629 would NOT be used. Hopefully, this clarifies the issue further.
CMS Ceases Use of HCPCS G Codes for Smoking Cessation
Effective on or after October 1, the Centers for Medicare & Medicaid Services (CMS) will no longer allow use of Healthcare Common Procedural Coding System (HCPCS) codes G0436 (Smoking and tobacco cessation counseling visit for the asymptomatic patient; intermediate, greater than 3 minutes, up to 10 minutes) and G0437 (Smoking and tobacco cessation counseling visit for the asymptomatic patient; intensive, greater than 10 minutes). Instead, CMS will utilize the new codes developed for the Current Procedural Terminology (CPT) code set.
CMS has advised its Medicare contractors to replace code G0436 with CPT code 99406 (Smoking and tobacco use cessation counseling visit; intermediate, greater than 3 minutes up to 10 minutes) and code G0437 with CPT code 99407 (Smoking and tobacco use cessation counseling visit; intensive, greater than 10 minutes). According to the Medicare National Coverage Determination Manual, tobacco cessation counseling is covered both for symptomatic and asymptomatic smokers. CMS will allow health-care providers two attempts per 12 months to encourage Medicare patients to cease tobacco use but does not define an attempt. Rather, either of the codes may be used up to four times per attempt; so 99406 and 99407 or a combination of these codes may be used up to 8 times in a 12-month period. These codes may be used either as a stand-alone or with an evaluation and management (E&M) service with appropriate documentation. Remember, however, if one uses these codes during an E&M visit, a 25 modifier will need to be appended to the E&M code.
Catching Up With Our Past Presidents
Where are they now? What have they been up to? CHEST’s Past Presidents each forged the way for the many successes of the American College of Chest Physicians (CHEST), leading to enhanced patient care around the globe. Their outstanding leadership and vision are evidenced today in many of CHEST’s current initiatives, and now it is time to check in with these past leaders to give us a look at what’s new.
Paul Stein, MD, FCCP
President 1992-1993
It is now 24 years since I was president of the American College of Chest Physicians (CHEST). Dr. Al Soffer had just retired, and it was Al Lever’s first year. Dr. Roger Bone preceded me as President and Dr. Ron George followed me.
Getting back to pulmonary embolism, with distinguished collaborators throughout the United States, and some in Canada, we did the PIOPED II investigation of the accuracy of multidetector CT pulmonary angiography and PIOPED III, the accuracy of magnetic resonance imaging for the diagnosis of pulmonary embolism. I have written three editions of the book, “Pulmonary Embolism” (1998, 2007, 2016).
So that‘s how I spend most of my time, working and doing research, which I love. I feel blessed to be able to continue being productive at age 82. Janet and I just celebrated our 50th wedding anniversary. We have three children and two grandchildren.
My main hobby is playing the clarinet. I particularly enjoy playing classical sonatas and meet monthly with a superb pianist who had been a professional organist. We are hoping to make a CD. In addition, I play in a “swing band” and a concert band that hopefully will go to a national contest.
Another hobby is collections, which include fossils, old books, rocks, masks, tribal art, and musical instruments, to name a few. It is a thrill to hold the fossil of an animal that lived 400 million years ago or an arrowhead made by someone 10,000 years ago.
Where are they now? What have they been up to? CHEST’s Past Presidents each forged the way for the many successes of the American College of Chest Physicians (CHEST), leading to enhanced patient care around the globe. Their outstanding leadership and vision are evidenced today in many of CHEST’s current initiatives, and now it is time to check in with these past leaders to give us a look at what’s new.
Paul Stein, MD, FCCP
President 1992-1993
It is now 24 years since I was president of the American College of Chest Physicians (CHEST). Dr. Al Soffer had just retired, and it was Al Lever’s first year. Dr. Roger Bone preceded me as President and Dr. Ron George followed me.
Getting back to pulmonary embolism, with distinguished collaborators throughout the United States, and some in Canada, we did the PIOPED II investigation of the accuracy of multidetector CT pulmonary angiography and PIOPED III, the accuracy of magnetic resonance imaging for the diagnosis of pulmonary embolism. I have written three editions of the book, “Pulmonary Embolism” (1998, 2007, 2016).
So that‘s how I spend most of my time, working and doing research, which I love. I feel blessed to be able to continue being productive at age 82. Janet and I just celebrated our 50th wedding anniversary. We have three children and two grandchildren.
My main hobby is playing the clarinet. I particularly enjoy playing classical sonatas and meet monthly with a superb pianist who had been a professional organist. We are hoping to make a CD. In addition, I play in a “swing band” and a concert band that hopefully will go to a national contest.
Another hobby is collections, which include fossils, old books, rocks, masks, tribal art, and musical instruments, to name a few. It is a thrill to hold the fossil of an animal that lived 400 million years ago or an arrowhead made by someone 10,000 years ago.
Where are they now? What have they been up to? CHEST’s Past Presidents each forged the way for the many successes of the American College of Chest Physicians (CHEST), leading to enhanced patient care around the globe. Their outstanding leadership and vision are evidenced today in many of CHEST’s current initiatives, and now it is time to check in with these past leaders to give us a look at what’s new.
Paul Stein, MD, FCCP
President 1992-1993
It is now 24 years since I was president of the American College of Chest Physicians (CHEST). Dr. Al Soffer had just retired, and it was Al Lever’s first year. Dr. Roger Bone preceded me as President and Dr. Ron George followed me.
Getting back to pulmonary embolism, with distinguished collaborators throughout the United States, and some in Canada, we did the PIOPED II investigation of the accuracy of multidetector CT pulmonary angiography and PIOPED III, the accuracy of magnetic resonance imaging for the diagnosis of pulmonary embolism. I have written three editions of the book, “Pulmonary Embolism” (1998, 2007, 2016).
So that‘s how I spend most of my time, working and doing research, which I love. I feel blessed to be able to continue being productive at age 82. Janet and I just celebrated our 50th wedding anniversary. We have three children and two grandchildren.
My main hobby is playing the clarinet. I particularly enjoy playing classical sonatas and meet monthly with a superb pianist who had been a professional organist. We are hoping to make a CD. In addition, I play in a “swing band” and a concert band that hopefully will go to a national contest.
Another hobby is collections, which include fossils, old books, rocks, masks, tribal art, and musical instruments, to name a few. It is a thrill to hold the fossil of an animal that lived 400 million years ago or an arrowhead made by someone 10,000 years ago.
Power of a Summit Award
The American College of Chest Physicians (CHEST) was awarded The Power of a Summit Award from the American Society of Association Executives (ASAE) in October for the China-CHEST Pulmonary and Critical Care Medicine (PCCM) Fellowship Program (Fig 1). CHEST is one of only six associations chosen for this honor.
The goal of the China-CHEST PCCM Fellowship Program is to standardize training and to equip clinicians in China to provide care to those affected by respiratory and critical care illnesses. Through collaboration among multiple international associations, CHEST has been working since 2013 to prepare physicians in the first-ever medical subspecialty of pulmonary and critical care in China.
Since the launch of China-CHEST PCCM Fellowship Program in 2013, 12 participating Chinese institutions started their PCCM training programs. By the end of 2016, 30 programs with 300 fellows and 60 faculty will be participating at institutions throughout China, with the potential to impact the care of thousands of patients. The China-PCCM Fellowship Program welcomed and congratulated its first four graduates in September 2016 (Fig 2).
Li Huang, Xiangya Hospital, Changsha, Hunan (graduate); Chen Wang, MD, FCCP; Xianwen Sun, Ruijin Hospital, Shanghai (graduate); Robb Rabito; Chenjuan Gu, Ruijin (graduate); Renli Qiao, MD, FCCP; and Yingmeng Ni, Ruijing (graduate).
The vast reach and clinical exposure of this program highlights how a CHEST and the China-CHEST PCCM Fellowship Program were recognized at the ASAE’s 17th Annual Summit Awards Dinner in October. Steve Welch, CHEST interim EVP/CEO, accepted the award on behalf of CHEST.
The American College of Chest Physicians (CHEST) was awarded The Power of a Summit Award from the American Society of Association Executives (ASAE) in October for the China-CHEST Pulmonary and Critical Care Medicine (PCCM) Fellowship Program (Fig 1). CHEST is one of only six associations chosen for this honor.
The goal of the China-CHEST PCCM Fellowship Program is to standardize training and to equip clinicians in China to provide care to those affected by respiratory and critical care illnesses. Through collaboration among multiple international associations, CHEST has been working since 2013 to prepare physicians in the first-ever medical subspecialty of pulmonary and critical care in China.
Since the launch of China-CHEST PCCM Fellowship Program in 2013, 12 participating Chinese institutions started their PCCM training programs. By the end of 2016, 30 programs with 300 fellows and 60 faculty will be participating at institutions throughout China, with the potential to impact the care of thousands of patients. The China-PCCM Fellowship Program welcomed and congratulated its first four graduates in September 2016 (Fig 2).
Li Huang, Xiangya Hospital, Changsha, Hunan (graduate); Chen Wang, MD, FCCP; Xianwen Sun, Ruijin Hospital, Shanghai (graduate); Robb Rabito; Chenjuan Gu, Ruijin (graduate); Renli Qiao, MD, FCCP; and Yingmeng Ni, Ruijing (graduate).
The vast reach and clinical exposure of this program highlights how a CHEST and the China-CHEST PCCM Fellowship Program were recognized at the ASAE’s 17th Annual Summit Awards Dinner in October. Steve Welch, CHEST interim EVP/CEO, accepted the award on behalf of CHEST.
The American College of Chest Physicians (CHEST) was awarded The Power of a Summit Award from the American Society of Association Executives (ASAE) in October for the China-CHEST Pulmonary and Critical Care Medicine (PCCM) Fellowship Program (Fig 1). CHEST is one of only six associations chosen for this honor.
The goal of the China-CHEST PCCM Fellowship Program is to standardize training and to equip clinicians in China to provide care to those affected by respiratory and critical care illnesses. Through collaboration among multiple international associations, CHEST has been working since 2013 to prepare physicians in the first-ever medical subspecialty of pulmonary and critical care in China.
Since the launch of China-CHEST PCCM Fellowship Program in 2013, 12 participating Chinese institutions started their PCCM training programs. By the end of 2016, 30 programs with 300 fellows and 60 faculty will be participating at institutions throughout China, with the potential to impact the care of thousands of patients. The China-PCCM Fellowship Program welcomed and congratulated its first four graduates in September 2016 (Fig 2).
Li Huang, Xiangya Hospital, Changsha, Hunan (graduate); Chen Wang, MD, FCCP; Xianwen Sun, Ruijin Hospital, Shanghai (graduate); Robb Rabito; Chenjuan Gu, Ruijin (graduate); Renli Qiao, MD, FCCP; and Yingmeng Ni, Ruijing (graduate).
The vast reach and clinical exposure of this program highlights how a CHEST and the China-CHEST PCCM Fellowship Program were recognized at the ASAE’s 17th Annual Summit Awards Dinner in October. Steve Welch, CHEST interim EVP/CEO, accepted the award on behalf of CHEST.
ABIM Alternative Assessment Model
The American Board of Internal Medicine (ABIM) announced in May 2016 it would be offering an alternate option to the 10-year MOC exam, beginning in January 2018. This announcement came in response to ongoing feedback from physicians and other stakeholders regarding the high-stakes recertification exam every 10 years.
The new option will include shorter, more-frequent assessments that can be completed from a physician’s office or home. These shorter assessments will identify knowledge gaps, so physicians can tailor their continuing education in order to stay current in knowledge and practice. Successful performance on the shorter assessments will allow physicians to opt out of the longer 10-year exam.
Prior to the launch of an alternative assessment model, ABIM has been soliciting input from diplomates through surveys and live conversations at society meetings.
The program will be piloted for internal medicine and select subspecialties, and based on feedback, will be extended to additional subspecialties at a later date. Physicians whose certifications expire prior to the new assessment option becoming available will need to pass the current exam in order to maintain certification, but then will not need to take another assessment for 10 years. Additional details regarding these alternative assessment options will be announced by the end of 2016 and can be tracked via ABIM’s blog. They also have an FAQ document online to address common questions regarding the alternative assessment pathway, as well as other topics related to MOC.
The American Board of Internal Medicine (ABIM) announced in May 2016 it would be offering an alternate option to the 10-year MOC exam, beginning in January 2018. This announcement came in response to ongoing feedback from physicians and other stakeholders regarding the high-stakes recertification exam every 10 years.
The new option will include shorter, more-frequent assessments that can be completed from a physician’s office or home. These shorter assessments will identify knowledge gaps, so physicians can tailor their continuing education in order to stay current in knowledge and practice. Successful performance on the shorter assessments will allow physicians to opt out of the longer 10-year exam.
Prior to the launch of an alternative assessment model, ABIM has been soliciting input from diplomates through surveys and live conversations at society meetings.
The program will be piloted for internal medicine and select subspecialties, and based on feedback, will be extended to additional subspecialties at a later date. Physicians whose certifications expire prior to the new assessment option becoming available will need to pass the current exam in order to maintain certification, but then will not need to take another assessment for 10 years. Additional details regarding these alternative assessment options will be announced by the end of 2016 and can be tracked via ABIM’s blog. They also have an FAQ document online to address common questions regarding the alternative assessment pathway, as well as other topics related to MOC.
The American Board of Internal Medicine (ABIM) announced in May 2016 it would be offering an alternate option to the 10-year MOC exam, beginning in January 2018. This announcement came in response to ongoing feedback from physicians and other stakeholders regarding the high-stakes recertification exam every 10 years.
The new option will include shorter, more-frequent assessments that can be completed from a physician’s office or home. These shorter assessments will identify knowledge gaps, so physicians can tailor their continuing education in order to stay current in knowledge and practice. Successful performance on the shorter assessments will allow physicians to opt out of the longer 10-year exam.
Prior to the launch of an alternative assessment model, ABIM has been soliciting input from diplomates through surveys and live conversations at society meetings.
The program will be piloted for internal medicine and select subspecialties, and based on feedback, will be extended to additional subspecialties at a later date. Physicians whose certifications expire prior to the new assessment option becoming available will need to pass the current exam in order to maintain certification, but then will not need to take another assessment for 10 years. Additional details regarding these alternative assessment options will be announced by the end of 2016 and can be tracked via ABIM’s blog. They also have an FAQ document online to address common questions regarding the alternative assessment pathway, as well as other topics related to MOC.
This Month in CHEST
Editor’s Picks
Giants in Chest Medicine: Bartolome Celli, MD, FCCP. By Dr. G. J. Criner
Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. By Dr. S. Mrozek, et al.
A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. By Dr. S. T. Micek, et al.
Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. By Dr. A. Tremblay, et al.
Editor’s Picks
Giants in Chest Medicine: Bartolome Celli, MD, FCCP. By Dr. G. J. Criner
Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. By Dr. S. Mrozek, et al.
A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. By Dr. S. T. Micek, et al.
Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. By Dr. A. Tremblay, et al.
Editor’s Picks
Giants in Chest Medicine: Bartolome Celli, MD, FCCP. By Dr. G. J. Criner
Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. By Dr. S. Mrozek, et al.
A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. By Dr. S. T. Micek, et al.
Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. By Dr. A. Tremblay, et al.
CHEST Clinical Trials Registry
Are you a clinical trials investigator with unused capacity? Would you like to refer patients to participate in groundbreaking clinical trials?
The CHEST Clinical Trials Registry is a free service that connects physicians to information about clinical trials in respiratory disease conducted by participating pharmaceutical companies.
Ongoing groundbreaking research could have a measurable impact on patient care, but a lack of clinical trial participants is significantly slowing research and threatening the development of new treatments. Recruiting and retaining trial participants are the greatest challenges to developing the next generation of treatment options.
Participation in clinical trials provides an opportunity to advance and accelerate medical research and contribute to an improved health outlook for future generations. Use our registry to get immediate information on how you can be involved in a clinical trial.
Access this site to learn more: www.chestnet.org/Guidelines-and-Resources/Clinical-Trials/Clinical-Trials-Registry.
Are you a clinical trials investigator with unused capacity? Would you like to refer patients to participate in groundbreaking clinical trials?
The CHEST Clinical Trials Registry is a free service that connects physicians to information about clinical trials in respiratory disease conducted by participating pharmaceutical companies.
Ongoing groundbreaking research could have a measurable impact on patient care, but a lack of clinical trial participants is significantly slowing research and threatening the development of new treatments. Recruiting and retaining trial participants are the greatest challenges to developing the next generation of treatment options.
Participation in clinical trials provides an opportunity to advance and accelerate medical research and contribute to an improved health outlook for future generations. Use our registry to get immediate information on how you can be involved in a clinical trial.
Access this site to learn more: www.chestnet.org/Guidelines-and-Resources/Clinical-Trials/Clinical-Trials-Registry.
Are you a clinical trials investigator with unused capacity? Would you like to refer patients to participate in groundbreaking clinical trials?
The CHEST Clinical Trials Registry is a free service that connects physicians to information about clinical trials in respiratory disease conducted by participating pharmaceutical companies.
Ongoing groundbreaking research could have a measurable impact on patient care, but a lack of clinical trial participants is significantly slowing research and threatening the development of new treatments. Recruiting and retaining trial participants are the greatest challenges to developing the next generation of treatment options.
Participation in clinical trials provides an opportunity to advance and accelerate medical research and contribute to an improved health outlook for future generations. Use our registry to get immediate information on how you can be involved in a clinical trial.
Access this site to learn more: www.chestnet.org/Guidelines-and-Resources/Clinical-Trials/Clinical-Trials-Registry.
SUNRISE Program in India
Recently, CHEST completed the SUNRISE (Respiratory Initiative in Scientific Education) live learning program in India. More than 800 physicians attended and gained knowledge in asthma, COPD, ILD, and sleep over a 3-day period in three different cities – Bengaluru, Kolkata, and Delhi. According to the feedback report, more than half of the participants rated the program as highly above average, and approximately 70% will change something in their practice based on what they learned.
Suggestions for next year’s program, including content and speakers, are being considered.
Recently, CHEST completed the SUNRISE (Respiratory Initiative in Scientific Education) live learning program in India. More than 800 physicians attended and gained knowledge in asthma, COPD, ILD, and sleep over a 3-day period in three different cities – Bengaluru, Kolkata, and Delhi. According to the feedback report, more than half of the participants rated the program as highly above average, and approximately 70% will change something in their practice based on what they learned.
Suggestions for next year’s program, including content and speakers, are being considered.
Recently, CHEST completed the SUNRISE (Respiratory Initiative in Scientific Education) live learning program in India. More than 800 physicians attended and gained knowledge in asthma, COPD, ILD, and sleep over a 3-day period in three different cities – Bengaluru, Kolkata, and Delhi. According to the feedback report, more than half of the participants rated the program as highly above average, and approximately 70% will change something in their practice based on what they learned.
Suggestions for next year’s program, including content and speakers, are being considered.
Pulmonary Perspectives® The Sun Should Never Set on an “Un-ultrasound-ed” Pleural Effusion
The adage, “the sun should never set on an untapped pleural effusion,” was instilled in physicians for generations. However, anyone who practices medicine currently knows the sun often rises and sets several times before a pleural effusion is tapped. Why the change in mindset? Since the American Board of Internal Medicine removed the requirement for internal medicine residents to perform a minimum number of bedside procedures for certification, fewer graduating residents feel comfortable performing thoracentesis.
Additionally, the fear of litigation and institutional persecution from a postprocedure complication has caused many frontline clinicians to shy away from performing thoracentesis. Most important, we now appreciate that not all pleural effusions need to be tapped immediately, and the clinical decision making about the timing and technique to drain a pleural effusion is more complex than previously thought.
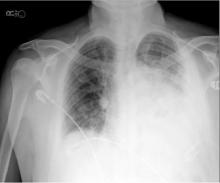
In recent years, the availability of portable ultrasound for bedside diagnostics and procedural guidance has revolutionized the practice of medicine, including the management of pleural effusions. When confronted with an obscured lower lobe on chest radiograph (Figure, left), clinicians were previously relegated to primitive bedside maneuvers, such as percussion or auscultation, to make critical decisions about the clinical management. Now, clinicians are able to look inside the body with point-of-care ultrasound and visually assess a pleural effusion before making any decisions. Point-of-care ultrasound has shifted the paradigm in the management of pleural effusions in several ways.
Ultrasound allows rapid detection and differentiation of pleural effusions from other pathologic findings.
Chest radiographs cannot accurately differentiate a pleural effusion from other common conditions, such as pneumonia, atelectasis, or an elevated hemidiaphragm. Ultrasound is the only bedside diagnostic modality that can rapidly differentiate these conditions within seconds and may reveal unsuspected findings, such as a mass or pericardial effusion.
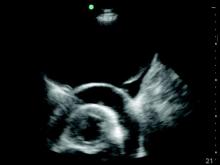
For example, the pleural ultrasound exam of a patient in the confirmed the presence of a large, left-sided pleural effusion (Figure, right) but also revealed an unsuspected large pericardial effusion (asterisk) that was causing hemodynamic compromise. The management of this patient shifted focus from the pleural effusion to the pericardial effusion, and urgent pericardiocentesis was performed. The sensitivity of ultrasound to detect a pleural effusion is proportional to the volume of fluid, reaching 100% with as little as 100 mL of fluid (Kalokairinou-Motogna et al. Med Ultra. 2010;12[1]:12). The diagnostic accuracy of ultrasound for detection of pleural effusions is comparable to CT scans of the chest and superior to portable chest radiographs (Lichtenstein et al. Anesthesiology. 2004;100[1]:9).
Ultrasound characterizes pleural effusions to determine the most appropriate management strategy.
Any clinician with basic ultrasonography skills can learn to evaluate pleural effusions and categorize them as simple or complex based on the sonographic appearance. Visualization of fibrinous stranding or loculations increases the probability of pleural fluid being exudative and often drives the decision to drain the fluid. The density and distribution of loculations can guide decisions about the most appropriate type of drainage procedure – thoracentesis versus tube thoracostomy versus surgical intervention. Use of color flow Doppler ultrasound allows clinicians to assess whether or not pleural fluid is free flowing and amenable to drainage, potentially saving the patient from an unnecessary attempt at drainage.
Ultrasound affords frontline clinicians the ability to streamline consultation with the most appropriate specialist based on the type of drainage procedure indicated and potentially prevent duplicate procedures on the same patient from different specialists.
Ultrasound reduces the risk of postprocedure complications from thoracentesis.
The risk of postthoracentesis pneumothorax was reported to be as high as 20%-39% before the routine use of point-of-care ultrasound (Grogan et al. Arch Int Med. 1990;150:873). Ultrasound guidance has been shown to increase procedural success rates and decrease the risk of postprocedure pneumothorax (2.7%), cost of hospitalization, and length of stay (Mercaldi et al. Chest. 2015;143[2]:532).
Regardless of the chest radiograph or CT scan findings, if the ultrasound exam reveals a scant volume of pleural fluid, or densely loculated pleural fluid, clinicians can avoid unnecessary attempts at bedside drainage, which likely partly accounts for the reduction in postprocedure pneumothorax. Use of ultrasound for needle site selection may prevent up to 10% of potential accidental organ punctures and increases accurate site selection by 26%, compared with chest radiograph and physical examination findings combined (Diacon et al. Chest. 2003;123:436).
Ultrasound facilitates patient-centered care.
Point-of-care ultrasound is the only new technology that has taken clinicians back to the bedside to spend more time with patients. Clinicians can simultaneously perform an ultrasound exam and converse with patients to gather a medical history. The ultrasound image serves as a tool to help patients understand their condition and facilitates shared decision making with clinicians at the bedside.
As more specialties have gained expertise in thoracic ultrasonography, the use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States. Besides pulmonary specialists, several acute care specialists, including hospitalists, intensivists, and emergency medicine physicians, are routinely using point-of-care ultrasound to guide diagnostic decision making and procedures. Over the past 10 years, nearly a dozen procedure services led by internal medicine-trained hospitalists have been created at academic institutions that are routinely performing ultrasound-guided thoracenteses with low complication rates (Franco-Sadud et al. SGIM Forum. 2016;39[5]:13). More important, ultrasound is being used on the front lines to expeditiously evaluate pleural effusions and perform a diagnostic thoracentesis or consult with the appropriate subspecialist. Even though demonstration of competency in bedside procedures is no longer required for board certification in internal medicine, many internal medicine residency programs have incorporated diagnostic and procedural point-of-care ultrasound training into their education curriculum (Schnobrich et al. JGME. 2013;5[3]:498). Further, approximately 62% of medical schools report integrating ultrasound education in their medical student curriculum, and in coming years, most medical students will likely graduate with a basic skill set in point-of-care ultrasonography (Bahner et al. Academic Med. 2014;89[12]:1681). As point-of-care ultrasound education becomes integrated in training of physicians and other health-care providers, use of ultrasound to guide management of pleural effusions could become universally practiced and accepted as the new standard of care. Thus, it is plausible that a day will come in the near future when the sun will not set on an “un-ultrasound-ed” pleural effusion.
Dr. Franco-Sadud is with the section of hospital medicine/division of general internal medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; Dr. Soni is with the section of hospital medicine and the section of pulmonary and critical care medicine, South Texas Veterans Health Care System and University of Texas Health Science Center, San Antonio.
The adage, “the sun should never set on an untapped pleural effusion,” was instilled in physicians for generations. However, anyone who practices medicine currently knows the sun often rises and sets several times before a pleural effusion is tapped. Why the change in mindset? Since the American Board of Internal Medicine removed the requirement for internal medicine residents to perform a minimum number of bedside procedures for certification, fewer graduating residents feel comfortable performing thoracentesis.
Additionally, the fear of litigation and institutional persecution from a postprocedure complication has caused many frontline clinicians to shy away from performing thoracentesis. Most important, we now appreciate that not all pleural effusions need to be tapped immediately, and the clinical decision making about the timing and technique to drain a pleural effusion is more complex than previously thought.
In recent years, the availability of portable ultrasound for bedside diagnostics and procedural guidance has revolutionized the practice of medicine, including the management of pleural effusions. When confronted with an obscured lower lobe on chest radiograph (Figure, left), clinicians were previously relegated to primitive bedside maneuvers, such as percussion or auscultation, to make critical decisions about the clinical management. Now, clinicians are able to look inside the body with point-of-care ultrasound and visually assess a pleural effusion before making any decisions. Point-of-care ultrasound has shifted the paradigm in the management of pleural effusions in several ways.
Ultrasound allows rapid detection and differentiation of pleural effusions from other pathologic findings.
Chest radiographs cannot accurately differentiate a pleural effusion from other common conditions, such as pneumonia, atelectasis, or an elevated hemidiaphragm. Ultrasound is the only bedside diagnostic modality that can rapidly differentiate these conditions within seconds and may reveal unsuspected findings, such as a mass or pericardial effusion.
For example, the pleural ultrasound exam of a patient in the confirmed the presence of a large, left-sided pleural effusion (Figure, right) but also revealed an unsuspected large pericardial effusion (asterisk) that was causing hemodynamic compromise. The management of this patient shifted focus from the pleural effusion to the pericardial effusion, and urgent pericardiocentesis was performed. The sensitivity of ultrasound to detect a pleural effusion is proportional to the volume of fluid, reaching 100% with as little as 100 mL of fluid (Kalokairinou-Motogna et al. Med Ultra. 2010;12[1]:12). The diagnostic accuracy of ultrasound for detection of pleural effusions is comparable to CT scans of the chest and superior to portable chest radiographs (Lichtenstein et al. Anesthesiology. 2004;100[1]:9).
Ultrasound characterizes pleural effusions to determine the most appropriate management strategy.
Any clinician with basic ultrasonography skills can learn to evaluate pleural effusions and categorize them as simple or complex based on the sonographic appearance. Visualization of fibrinous stranding or loculations increases the probability of pleural fluid being exudative and often drives the decision to drain the fluid. The density and distribution of loculations can guide decisions about the most appropriate type of drainage procedure – thoracentesis versus tube thoracostomy versus surgical intervention. Use of color flow Doppler ultrasound allows clinicians to assess whether or not pleural fluid is free flowing and amenable to drainage, potentially saving the patient from an unnecessary attempt at drainage.
Ultrasound affords frontline clinicians the ability to streamline consultation with the most appropriate specialist based on the type of drainage procedure indicated and potentially prevent duplicate procedures on the same patient from different specialists.
Ultrasound reduces the risk of postprocedure complications from thoracentesis.
The risk of postthoracentesis pneumothorax was reported to be as high as 20%-39% before the routine use of point-of-care ultrasound (Grogan et al. Arch Int Med. 1990;150:873). Ultrasound guidance has been shown to increase procedural success rates and decrease the risk of postprocedure pneumothorax (2.7%), cost of hospitalization, and length of stay (Mercaldi et al. Chest. 2015;143[2]:532).
Regardless of the chest radiograph or CT scan findings, if the ultrasound exam reveals a scant volume of pleural fluid, or densely loculated pleural fluid, clinicians can avoid unnecessary attempts at bedside drainage, which likely partly accounts for the reduction in postprocedure pneumothorax. Use of ultrasound for needle site selection may prevent up to 10% of potential accidental organ punctures and increases accurate site selection by 26%, compared with chest radiograph and physical examination findings combined (Diacon et al. Chest. 2003;123:436).
Ultrasound facilitates patient-centered care.
Point-of-care ultrasound is the only new technology that has taken clinicians back to the bedside to spend more time with patients. Clinicians can simultaneously perform an ultrasound exam and converse with patients to gather a medical history. The ultrasound image serves as a tool to help patients understand their condition and facilitates shared decision making with clinicians at the bedside.
As more specialties have gained expertise in thoracic ultrasonography, the use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States. Besides pulmonary specialists, several acute care specialists, including hospitalists, intensivists, and emergency medicine physicians, are routinely using point-of-care ultrasound to guide diagnostic decision making and procedures. Over the past 10 years, nearly a dozen procedure services led by internal medicine-trained hospitalists have been created at academic institutions that are routinely performing ultrasound-guided thoracenteses with low complication rates (Franco-Sadud et al. SGIM Forum. 2016;39[5]:13). More important, ultrasound is being used on the front lines to expeditiously evaluate pleural effusions and perform a diagnostic thoracentesis or consult with the appropriate subspecialist. Even though demonstration of competency in bedside procedures is no longer required for board certification in internal medicine, many internal medicine residency programs have incorporated diagnostic and procedural point-of-care ultrasound training into their education curriculum (Schnobrich et al. JGME. 2013;5[3]:498). Further, approximately 62% of medical schools report integrating ultrasound education in their medical student curriculum, and in coming years, most medical students will likely graduate with a basic skill set in point-of-care ultrasonography (Bahner et al. Academic Med. 2014;89[12]:1681). As point-of-care ultrasound education becomes integrated in training of physicians and other health-care providers, use of ultrasound to guide management of pleural effusions could become universally practiced and accepted as the new standard of care. Thus, it is plausible that a day will come in the near future when the sun will not set on an “un-ultrasound-ed” pleural effusion.
Dr. Franco-Sadud is with the section of hospital medicine/division of general internal medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; Dr. Soni is with the section of hospital medicine and the section of pulmonary and critical care medicine, South Texas Veterans Health Care System and University of Texas Health Science Center, San Antonio.
The adage, “the sun should never set on an untapped pleural effusion,” was instilled in physicians for generations. However, anyone who practices medicine currently knows the sun often rises and sets several times before a pleural effusion is tapped. Why the change in mindset? Since the American Board of Internal Medicine removed the requirement for internal medicine residents to perform a minimum number of bedside procedures for certification, fewer graduating residents feel comfortable performing thoracentesis.
Additionally, the fear of litigation and institutional persecution from a postprocedure complication has caused many frontline clinicians to shy away from performing thoracentesis. Most important, we now appreciate that not all pleural effusions need to be tapped immediately, and the clinical decision making about the timing and technique to drain a pleural effusion is more complex than previously thought.
In recent years, the availability of portable ultrasound for bedside diagnostics and procedural guidance has revolutionized the practice of medicine, including the management of pleural effusions. When confronted with an obscured lower lobe on chest radiograph (Figure, left), clinicians were previously relegated to primitive bedside maneuvers, such as percussion or auscultation, to make critical decisions about the clinical management. Now, clinicians are able to look inside the body with point-of-care ultrasound and visually assess a pleural effusion before making any decisions. Point-of-care ultrasound has shifted the paradigm in the management of pleural effusions in several ways.
Ultrasound allows rapid detection and differentiation of pleural effusions from other pathologic findings.
Chest radiographs cannot accurately differentiate a pleural effusion from other common conditions, such as pneumonia, atelectasis, or an elevated hemidiaphragm. Ultrasound is the only bedside diagnostic modality that can rapidly differentiate these conditions within seconds and may reveal unsuspected findings, such as a mass or pericardial effusion.
For example, the pleural ultrasound exam of a patient in the confirmed the presence of a large, left-sided pleural effusion (Figure, right) but also revealed an unsuspected large pericardial effusion (asterisk) that was causing hemodynamic compromise. The management of this patient shifted focus from the pleural effusion to the pericardial effusion, and urgent pericardiocentesis was performed. The sensitivity of ultrasound to detect a pleural effusion is proportional to the volume of fluid, reaching 100% with as little as 100 mL of fluid (Kalokairinou-Motogna et al. Med Ultra. 2010;12[1]:12). The diagnostic accuracy of ultrasound for detection of pleural effusions is comparable to CT scans of the chest and superior to portable chest radiographs (Lichtenstein et al. Anesthesiology. 2004;100[1]:9).
Ultrasound characterizes pleural effusions to determine the most appropriate management strategy.
Any clinician with basic ultrasonography skills can learn to evaluate pleural effusions and categorize them as simple or complex based on the sonographic appearance. Visualization of fibrinous stranding or loculations increases the probability of pleural fluid being exudative and often drives the decision to drain the fluid. The density and distribution of loculations can guide decisions about the most appropriate type of drainage procedure – thoracentesis versus tube thoracostomy versus surgical intervention. Use of color flow Doppler ultrasound allows clinicians to assess whether or not pleural fluid is free flowing and amenable to drainage, potentially saving the patient from an unnecessary attempt at drainage.
Ultrasound affords frontline clinicians the ability to streamline consultation with the most appropriate specialist based on the type of drainage procedure indicated and potentially prevent duplicate procedures on the same patient from different specialists.
Ultrasound reduces the risk of postprocedure complications from thoracentesis.
The risk of postthoracentesis pneumothorax was reported to be as high as 20%-39% before the routine use of point-of-care ultrasound (Grogan et al. Arch Int Med. 1990;150:873). Ultrasound guidance has been shown to increase procedural success rates and decrease the risk of postprocedure pneumothorax (2.7%), cost of hospitalization, and length of stay (Mercaldi et al. Chest. 2015;143[2]:532).
Regardless of the chest radiograph or CT scan findings, if the ultrasound exam reveals a scant volume of pleural fluid, or densely loculated pleural fluid, clinicians can avoid unnecessary attempts at bedside drainage, which likely partly accounts for the reduction in postprocedure pneumothorax. Use of ultrasound for needle site selection may prevent up to 10% of potential accidental organ punctures and increases accurate site selection by 26%, compared with chest radiograph and physical examination findings combined (Diacon et al. Chest. 2003;123:436).
Ultrasound facilitates patient-centered care.
Point-of-care ultrasound is the only new technology that has taken clinicians back to the bedside to spend more time with patients. Clinicians can simultaneously perform an ultrasound exam and converse with patients to gather a medical history. The ultrasound image serves as a tool to help patients understand their condition and facilitates shared decision making with clinicians at the bedside.
As more specialties have gained expertise in thoracic ultrasonography, the use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States. Besides pulmonary specialists, several acute care specialists, including hospitalists, intensivists, and emergency medicine physicians, are routinely using point-of-care ultrasound to guide diagnostic decision making and procedures. Over the past 10 years, nearly a dozen procedure services led by internal medicine-trained hospitalists have been created at academic institutions that are routinely performing ultrasound-guided thoracenteses with low complication rates (Franco-Sadud et al. SGIM Forum. 2016;39[5]:13). More important, ultrasound is being used on the front lines to expeditiously evaluate pleural effusions and perform a diagnostic thoracentesis or consult with the appropriate subspecialist. Even though demonstration of competency in bedside procedures is no longer required for board certification in internal medicine, many internal medicine residency programs have incorporated diagnostic and procedural point-of-care ultrasound training into their education curriculum (Schnobrich et al. JGME. 2013;5[3]:498). Further, approximately 62% of medical schools report integrating ultrasound education in their medical student curriculum, and in coming years, most medical students will likely graduate with a basic skill set in point-of-care ultrasonography (Bahner et al. Academic Med. 2014;89[12]:1681). As point-of-care ultrasound education becomes integrated in training of physicians and other health-care providers, use of ultrasound to guide management of pleural effusions could become universally practiced and accepted as the new standard of care. Thus, it is plausible that a day will come in the near future when the sun will not set on an “un-ultrasound-ed” pleural effusion.
Dr. Franco-Sadud is with the section of hospital medicine/division of general internal medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; Dr. Soni is with the section of hospital medicine and the section of pulmonary and critical care medicine, South Texas Veterans Health Care System and University of Texas Health Science Center, San Antonio.
Subscribe to the new CHEST SEEK Library
The CHEST SEEK Library includes nearly 1,500 pulmonary, critical care, and sleep medicine questions in a 1-year subscription. Whether preparing for board exams or just wanting a solid review, sharpen your skills with this comprehensive collection for an introductory price of $149 ($199 for nonmembers). This is the best deal ever offered for SEEK, which can now be accessed via Apple, Android, and Web browser. Do not wait. The introductory price will only be offered for a limited time. Learn more at https://www.chestnet.org/Store.
The CHEST SEEK Library includes nearly 1,500 pulmonary, critical care, and sleep medicine questions in a 1-year subscription. Whether preparing for board exams or just wanting a solid review, sharpen your skills with this comprehensive collection for an introductory price of $149 ($199 for nonmembers). This is the best deal ever offered for SEEK, which can now be accessed via Apple, Android, and Web browser. Do not wait. The introductory price will only be offered for a limited time. Learn more at https://www.chestnet.org/Store.
The CHEST SEEK Library includes nearly 1,500 pulmonary, critical care, and sleep medicine questions in a 1-year subscription. Whether preparing for board exams or just wanting a solid review, sharpen your skills with this comprehensive collection for an introductory price of $149 ($199 for nonmembers). This is the best deal ever offered for SEEK, which can now be accessed via Apple, Android, and Web browser. Do not wait. The introductory price will only be offered for a limited time. Learn more at https://www.chestnet.org/Store.