User login
A Practical Guide for Developing a Relationship with the Pharmaceutical, Biotech, and Device Industries
The primary goal of the biotechnology and pharmaceutical industry is to develop medications and medical devices for the treatment of patients, while earning financial gain for investors. An important component of achieving this goal is the role physicians play in the drug and medical device development process. In particular, a physician’s role is to combine their clinical expertise with their knowledge of industry products to better diagnose and treat the ailments of their patients. Thus, medicine and industry have a dependent relationship. In recent times this relationship has been fraught with turmoil as the public, scientific community, and federal government have discovered real and perceived conflicts of interest.
For example, there has been public outrage in the past with reports of doctors receiving gifts, money, and lavish trips in return for prescribing medications or using certain medical devices. Because of this, Congress passed the Sunshine Act, deeming it necessary to report all physician and industry engagements that have any perceived financial value. The passage of this act was in addition to local policies set forth by academic institutions, hospitals, and private practices.
How Do I Get Started?
After checking with your institution, hospital or private practice administrator, the first step is to reach out to a local representative (“rep”) of a pharmaceutical or biotech company in which you are interested. You can accomplish this via the website of the company or by visiting the booth at major gastrointestinal conferences such as Digestive Disease Week (DDW®).
Pharma and device reps are quite knowledgeable about the latest clinical studies regarding their products, disease states, and various competing products in the market. In addition to being a source of valuable medical knowledge and disease-specific practice guidelines, they also can connect you with their medical science liaison (MSL). MSLs often have a background in pharmacy and/or research. Thus, they can provide insights into mechanisms of disease treatments and go beyond discussion of the product label, which pharmaceutical reps adhere to. They also know what therapies or diagnostic tools are in the phases of development and could be available for a clinical trial.
MSLs are also the gatekeepers for Investigator Initiated Studies (IIS). An IIS is a research project that is industry-funded and is solely designed and executed by the clinician. The application process is rigorous but awards may be easier to obtain for non-research-based clinicians who want to develop a disease-specific project that needs funding. Their grant application process can be brief, ideas may not require prior data, and turnaround time to funding may be shorter. IISs often lead to exploratory findings that may facilitate publications or lay groundwork for large-scale grants or even clinical trials. In some instances, you may be granted access to internal data and prescribing patterns, which can answer interesting clinical and research questions.
How Do I Get Started with Clinical Trials?
Being a primary investigator on a clinical trial is a big responsibility. You are responsible to the trial sponsor in addition to your patients. For young clinicians who lack experience with clinical trials, the first thing to do is to find a clinician in your department or another department, who has expertise in performing an industry-sponsored study. These individuals can be invaluable for you in terms of guiding you through the study feasibility process, study startup, and possibly being the lead or co-investigator with you. Partnering with someone with expertise in industry-sponsored clinical trials will help you gain the trust of the industry sponsor, which may be a requirement for some.
There are many additional requirements that need to be fulfilled aside from just having an appropriate and adequate patient population to pull from. You will need to have a coordinator for the study who will help you with patient care, data entry, and study- specific issues. Clinical trials require a significant amount of documentation and reporting that has to be performed within a timely manner. There is no degree prerequisite of the coordinator but it can simplify things for the clinician if they have a RN or LPN degree. Having such a degree will facilitate dual roles of patient care, lab draws, drug administration, medical charting, and other patient care matters.
In addition, you will need to have approval from either your local or central institutional review board (IRB). Also, you will have to review budget and study-specific requirements for equipment and infrastructure with your department manager. You will need to demonstrate adequate ancillary support to process, store, and ship biological specimens. In some instances, you will need a dedicated pharmacist to mix or dispense study drugs.
The process is lengthy and involved, but rewarding in terms of being involved in the drug development process. You will have opportunities to attend meetings at which you can network with other clinicians and provide the sponsor feedback on how the study is going.
How Do I Develop a Consulting Role with Industry?
It is important to check with your institution, hospital, or practice if there are any limitations in becoming a consultant for a pharmaceutical or device company. If it is allowed and will not interfere with your clinical duties, it is important to note that this role takes time to develop. It often comes about after years of experience doing research, clinical and/or basic science, with publications to support expertise. Working on an IIS is a good way to work hand-in-hand with expert industry researchers and facilitate the consulting relationship. Being a primary investigator of clinical trials with successful enrollment of patients and meeting attendance will provide you with insight into the drug development process.
What if None of This Works Out for Me?
Do not give up! Persistence, experience, and hard work are the keys to developing relationships with industry. Remember, industry has a vast network of clinicians and researchers they already work with. The overall pool of companies and experts is limited and can be difficult to break into. But it can be done. Some rely on their research experience, clinical training, and mentors to develop the necessary contacts. Others can develop the contacts via IIS applications. Industry lacks access to the physician-patient experience; this can be your greatest asset and key to your success if leveraged properly. You can consider applying for mentorship with experts in your field via AGA-sponsored events held annually at DDW® to get additional guidance.
Final Thoughts
It is important to remember that all industry relationships require time to develop. They also come at an opportunity cost of time away from your clinical practice and your family, friends and hobbies. However, these relationships also offer a way to increase your insight into new and old treatment and diagnostic paradigms. It is also a way to remain excited about your field and prevent the feeling that your day-to-day clinical practice is becoming routine.
Dr. Nitin Gupta is an Assistant Professor of Medicine, Director of Inflammatory Bowel Disease, and Program Director for the Gastroenterology Fellowship at University of Mississippi Medical Center in Jackson, MS. He has worked in basic science, translational and clinical research and continues projects in these areas. He has experience working with industry via roles of being a primary investigator in several clinical trials and consulting relationships.
The primary goal of the biotechnology and pharmaceutical industry is to develop medications and medical devices for the treatment of patients, while earning financial gain for investors. An important component of achieving this goal is the role physicians play in the drug and medical device development process. In particular, a physician’s role is to combine their clinical expertise with their knowledge of industry products to better diagnose and treat the ailments of their patients. Thus, medicine and industry have a dependent relationship. In recent times this relationship has been fraught with turmoil as the public, scientific community, and federal government have discovered real and perceived conflicts of interest.
For example, there has been public outrage in the past with reports of doctors receiving gifts, money, and lavish trips in return for prescribing medications or using certain medical devices. Because of this, Congress passed the Sunshine Act, deeming it necessary to report all physician and industry engagements that have any perceived financial value. The passage of this act was in addition to local policies set forth by academic institutions, hospitals, and private practices.
How Do I Get Started?
After checking with your institution, hospital or private practice administrator, the first step is to reach out to a local representative (“rep”) of a pharmaceutical or biotech company in which you are interested. You can accomplish this via the website of the company or by visiting the booth at major gastrointestinal conferences such as Digestive Disease Week (DDW®).
Pharma and device reps are quite knowledgeable about the latest clinical studies regarding their products, disease states, and various competing products in the market. In addition to being a source of valuable medical knowledge and disease-specific practice guidelines, they also can connect you with their medical science liaison (MSL). MSLs often have a background in pharmacy and/or research. Thus, they can provide insights into mechanisms of disease treatments and go beyond discussion of the product label, which pharmaceutical reps adhere to. They also know what therapies or diagnostic tools are in the phases of development and could be available for a clinical trial.
MSLs are also the gatekeepers for Investigator Initiated Studies (IIS). An IIS is a research project that is industry-funded and is solely designed and executed by the clinician. The application process is rigorous but awards may be easier to obtain for non-research-based clinicians who want to develop a disease-specific project that needs funding. Their grant application process can be brief, ideas may not require prior data, and turnaround time to funding may be shorter. IISs often lead to exploratory findings that may facilitate publications or lay groundwork for large-scale grants or even clinical trials. In some instances, you may be granted access to internal data and prescribing patterns, which can answer interesting clinical and research questions.
How Do I Get Started with Clinical Trials?
Being a primary investigator on a clinical trial is a big responsibility. You are responsible to the trial sponsor in addition to your patients. For young clinicians who lack experience with clinical trials, the first thing to do is to find a clinician in your department or another department, who has expertise in performing an industry-sponsored study. These individuals can be invaluable for you in terms of guiding you through the study feasibility process, study startup, and possibly being the lead or co-investigator with you. Partnering with someone with expertise in industry-sponsored clinical trials will help you gain the trust of the industry sponsor, which may be a requirement for some.
There are many additional requirements that need to be fulfilled aside from just having an appropriate and adequate patient population to pull from. You will need to have a coordinator for the study who will help you with patient care, data entry, and study- specific issues. Clinical trials require a significant amount of documentation and reporting that has to be performed within a timely manner. There is no degree prerequisite of the coordinator but it can simplify things for the clinician if they have a RN or LPN degree. Having such a degree will facilitate dual roles of patient care, lab draws, drug administration, medical charting, and other patient care matters.
In addition, you will need to have approval from either your local or central institutional review board (IRB). Also, you will have to review budget and study-specific requirements for equipment and infrastructure with your department manager. You will need to demonstrate adequate ancillary support to process, store, and ship biological specimens. In some instances, you will need a dedicated pharmacist to mix or dispense study drugs.
The process is lengthy and involved, but rewarding in terms of being involved in the drug development process. You will have opportunities to attend meetings at which you can network with other clinicians and provide the sponsor feedback on how the study is going.
How Do I Develop a Consulting Role with Industry?
It is important to check with your institution, hospital, or practice if there are any limitations in becoming a consultant for a pharmaceutical or device company. If it is allowed and will not interfere with your clinical duties, it is important to note that this role takes time to develop. It often comes about after years of experience doing research, clinical and/or basic science, with publications to support expertise. Working on an IIS is a good way to work hand-in-hand with expert industry researchers and facilitate the consulting relationship. Being a primary investigator of clinical trials with successful enrollment of patients and meeting attendance will provide you with insight into the drug development process.
What if None of This Works Out for Me?
Do not give up! Persistence, experience, and hard work are the keys to developing relationships with industry. Remember, industry has a vast network of clinicians and researchers they already work with. The overall pool of companies and experts is limited and can be difficult to break into. But it can be done. Some rely on their research experience, clinical training, and mentors to develop the necessary contacts. Others can develop the contacts via IIS applications. Industry lacks access to the physician-patient experience; this can be your greatest asset and key to your success if leveraged properly. You can consider applying for mentorship with experts in your field via AGA-sponsored events held annually at DDW® to get additional guidance.
Final Thoughts
It is important to remember that all industry relationships require time to develop. They also come at an opportunity cost of time away from your clinical practice and your family, friends and hobbies. However, these relationships also offer a way to increase your insight into new and old treatment and diagnostic paradigms. It is also a way to remain excited about your field and prevent the feeling that your day-to-day clinical practice is becoming routine.
Dr. Nitin Gupta is an Assistant Professor of Medicine, Director of Inflammatory Bowel Disease, and Program Director for the Gastroenterology Fellowship at University of Mississippi Medical Center in Jackson, MS. He has worked in basic science, translational and clinical research and continues projects in these areas. He has experience working with industry via roles of being a primary investigator in several clinical trials and consulting relationships.
The primary goal of the biotechnology and pharmaceutical industry is to develop medications and medical devices for the treatment of patients, while earning financial gain for investors. An important component of achieving this goal is the role physicians play in the drug and medical device development process. In particular, a physician’s role is to combine their clinical expertise with their knowledge of industry products to better diagnose and treat the ailments of their patients. Thus, medicine and industry have a dependent relationship. In recent times this relationship has been fraught with turmoil as the public, scientific community, and federal government have discovered real and perceived conflicts of interest.
For example, there has been public outrage in the past with reports of doctors receiving gifts, money, and lavish trips in return for prescribing medications or using certain medical devices. Because of this, Congress passed the Sunshine Act, deeming it necessary to report all physician and industry engagements that have any perceived financial value. The passage of this act was in addition to local policies set forth by academic institutions, hospitals, and private practices.
How Do I Get Started?
After checking with your institution, hospital or private practice administrator, the first step is to reach out to a local representative (“rep”) of a pharmaceutical or biotech company in which you are interested. You can accomplish this via the website of the company or by visiting the booth at major gastrointestinal conferences such as Digestive Disease Week (DDW®).
Pharma and device reps are quite knowledgeable about the latest clinical studies regarding their products, disease states, and various competing products in the market. In addition to being a source of valuable medical knowledge and disease-specific practice guidelines, they also can connect you with their medical science liaison (MSL). MSLs often have a background in pharmacy and/or research. Thus, they can provide insights into mechanisms of disease treatments and go beyond discussion of the product label, which pharmaceutical reps adhere to. They also know what therapies or diagnostic tools are in the phases of development and could be available for a clinical trial.
MSLs are also the gatekeepers for Investigator Initiated Studies (IIS). An IIS is a research project that is industry-funded and is solely designed and executed by the clinician. The application process is rigorous but awards may be easier to obtain for non-research-based clinicians who want to develop a disease-specific project that needs funding. Their grant application process can be brief, ideas may not require prior data, and turnaround time to funding may be shorter. IISs often lead to exploratory findings that may facilitate publications or lay groundwork for large-scale grants or even clinical trials. In some instances, you may be granted access to internal data and prescribing patterns, which can answer interesting clinical and research questions.
How Do I Get Started with Clinical Trials?
Being a primary investigator on a clinical trial is a big responsibility. You are responsible to the trial sponsor in addition to your patients. For young clinicians who lack experience with clinical trials, the first thing to do is to find a clinician in your department or another department, who has expertise in performing an industry-sponsored study. These individuals can be invaluable for you in terms of guiding you through the study feasibility process, study startup, and possibly being the lead or co-investigator with you. Partnering with someone with expertise in industry-sponsored clinical trials will help you gain the trust of the industry sponsor, which may be a requirement for some.
There are many additional requirements that need to be fulfilled aside from just having an appropriate and adequate patient population to pull from. You will need to have a coordinator for the study who will help you with patient care, data entry, and study- specific issues. Clinical trials require a significant amount of documentation and reporting that has to be performed within a timely manner. There is no degree prerequisite of the coordinator but it can simplify things for the clinician if they have a RN or LPN degree. Having such a degree will facilitate dual roles of patient care, lab draws, drug administration, medical charting, and other patient care matters.
In addition, you will need to have approval from either your local or central institutional review board (IRB). Also, you will have to review budget and study-specific requirements for equipment and infrastructure with your department manager. You will need to demonstrate adequate ancillary support to process, store, and ship biological specimens. In some instances, you will need a dedicated pharmacist to mix or dispense study drugs.
The process is lengthy and involved, but rewarding in terms of being involved in the drug development process. You will have opportunities to attend meetings at which you can network with other clinicians and provide the sponsor feedback on how the study is going.
How Do I Develop a Consulting Role with Industry?
It is important to check with your institution, hospital, or practice if there are any limitations in becoming a consultant for a pharmaceutical or device company. If it is allowed and will not interfere with your clinical duties, it is important to note that this role takes time to develop. It often comes about after years of experience doing research, clinical and/or basic science, with publications to support expertise. Working on an IIS is a good way to work hand-in-hand with expert industry researchers and facilitate the consulting relationship. Being a primary investigator of clinical trials with successful enrollment of patients and meeting attendance will provide you with insight into the drug development process.
What if None of This Works Out for Me?
Do not give up! Persistence, experience, and hard work are the keys to developing relationships with industry. Remember, industry has a vast network of clinicians and researchers they already work with. The overall pool of companies and experts is limited and can be difficult to break into. But it can be done. Some rely on their research experience, clinical training, and mentors to develop the necessary contacts. Others can develop the contacts via IIS applications. Industry lacks access to the physician-patient experience; this can be your greatest asset and key to your success if leveraged properly. You can consider applying for mentorship with experts in your field via AGA-sponsored events held annually at DDW® to get additional guidance.
Final Thoughts
It is important to remember that all industry relationships require time to develop. They also come at an opportunity cost of time away from your clinical practice and your family, friends and hobbies. However, these relationships also offer a way to increase your insight into new and old treatment and diagnostic paradigms. It is also a way to remain excited about your field and prevent the feeling that your day-to-day clinical practice is becoming routine.
Dr. Nitin Gupta is an Assistant Professor of Medicine, Director of Inflammatory Bowel Disease, and Program Director for the Gastroenterology Fellowship at University of Mississippi Medical Center in Jackson, MS. He has worked in basic science, translational and clinical research and continues projects in these areas. He has experience working with industry via roles of being a primary investigator in several clinical trials and consulting relationships.
Building and Maintaining a Successful Inflammatory Bowel Disease Practice
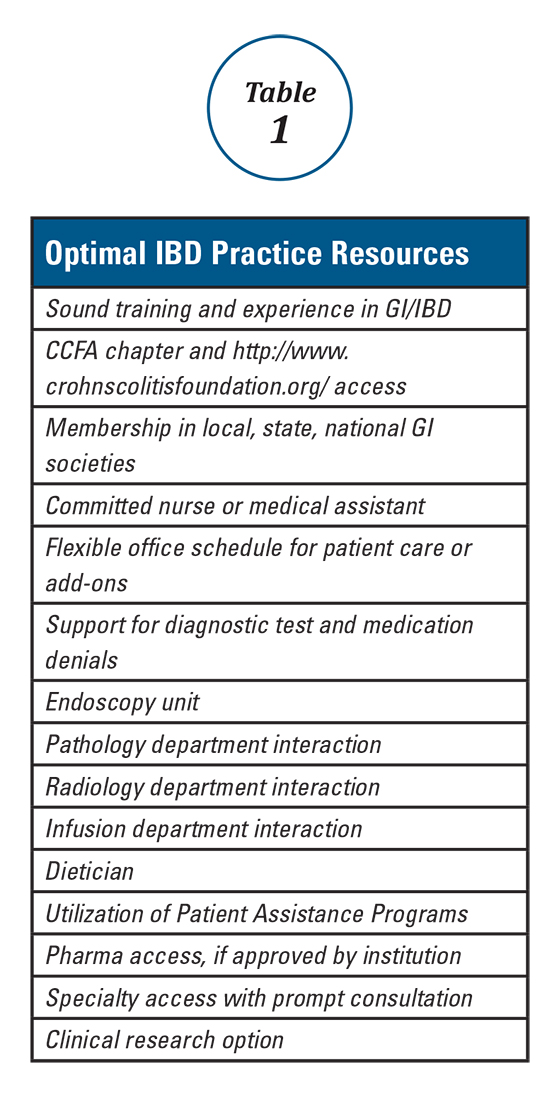
Anyone can build a successful inflammatory bowel disease (IBD) practice. To do so requires commitment and focus in the area of IBD including both Crohn’s disease and ulcerative colitis. It also requires a fundamental knowledge of medicine as well as a desire to excel and learn all that one can in these areas. Given the high number of stakeholders, good interpersonal skills are vital. Establishing an IBD practice provides an opportunity to make a big difference in peoples’ lives and the age range of impact is about the broadest in all of medical practice. The more resources you have, the greater the potential impact of your care. Table 1 lists resources that are useful to provide optimal IBD patient care.
You, the gastroenterologist, is the most important resource for the patient. Medical school, residency, fellowship, and “postgraduate” training serves as the foundation for your wealth of knowledge. Maximizing your training is of value, and this can be done by being part of an academic program, keeping abreast of current literature, and attending meetings and post-graduate courses. AGA offers a variety of publications (http://www.gastro.org/journals-and-publications) and continued training opportunities (http://www.gastro.org/education).
One further point regarding scheduling is that one must be willing and able to see patients urgently, rather than sending them to the emergency room. ERs are appropriate for true emergencies, but are not an ideal place for care when an IBD patient has a flare and requires prompt follow-up. I try to avoid ER visits for my patients unless they are vomiting, have severe abdominal pain, significant bleeding or have clear signs of toxicity. In an ER, abdominal pain equals a CT scan; one should consider seeing these patients in the office and triaging accordingly.
With the increasing requirements of managed care and restrictive medical plans, there has been a similar rise in the frequency of diagnostic test as well as procedure and medication denials. Re-approval and recertification of biologics and other medications have become common, which can add a great deal to your workload and that of your staff. Integration of endoscopy, pathology, and imaging (e.g., ultrasound, CT/CTE) improves response time, dialogue, and can have a positive impact on care. Office infusion allows for a better integration of this service into your practice. There is typically better communication with the infusion nurse(s) and better expedited care as well as fewer cancellations for minor infections. This all helps avoid infusion procedure delays. Infliximab, vedolizumab, ustekinumab, and lyophilized certolizumab pegol as well as intravenous iron administration can also expand services and enhance quality.
Having a medical assistant, nurse, and others in your practice to assist with patient services and care is a must. There will be many phone calls, emails, and other interactions regarding appointments, consults, routine lab testing, radiology testing, standard medications, biologics, and other treatments that necessitate an effective team-approach. For this role, either a nurse or an experienced medical assistant would be well-suited. Additional support staff and services can also aid our IBD patients. A dietitian knowledgeable in IBD and practical dietary options can, in many instances, prove invaluable. Understanding and utilizing pharma-sponsored “Patient Assistance Programs” provides drug access for the 10-20% (or more) of patients who do not have insurance or biologic coverage. Having specialty access and collegiality with colorectal surgeons, general surgeons, OB/GYNs, dermatologists, hematologists, oncologists, and others is important to expedite consults and provide collaborative care. Finally, offering clinical research options improves access for patients with limited and no coverage and also helps provide needed options for all IBD patients.
This brief overview has hopefully given you some insight into how to provide a higher level of evaluation and care for our IBD patients. These approaches have allowed me to build and maintain a successful IBD practice, and I hope that the integration of some or all of these strategies help you to build and sustain a successful IBD practice.
Dr. Wolf is director of IBD research, Atlanta Gastroenterology Associates.
Anyone can build a successful inflammatory bowel disease (IBD) practice. To do so requires commitment and focus in the area of IBD including both Crohn’s disease and ulcerative colitis. It also requires a fundamental knowledge of medicine as well as a desire to excel and learn all that one can in these areas. Given the high number of stakeholders, good interpersonal skills are vital. Establishing an IBD practice provides an opportunity to make a big difference in peoples’ lives and the age range of impact is about the broadest in all of medical practice. The more resources you have, the greater the potential impact of your care. Table 1 lists resources that are useful to provide optimal IBD patient care.
You, the gastroenterologist, is the most important resource for the patient. Medical school, residency, fellowship, and “postgraduate” training serves as the foundation for your wealth of knowledge. Maximizing your training is of value, and this can be done by being part of an academic program, keeping abreast of current literature, and attending meetings and post-graduate courses. AGA offers a variety of publications (http://www.gastro.org/journals-and-publications) and continued training opportunities (http://www.gastro.org/education).

One further point regarding scheduling is that one must be willing and able to see patients urgently, rather than sending them to the emergency room. ERs are appropriate for true emergencies, but are not an ideal place for care when an IBD patient has a flare and requires prompt follow-up. I try to avoid ER visits for my patients unless they are vomiting, have severe abdominal pain, significant bleeding or have clear signs of toxicity. In an ER, abdominal pain equals a CT scan; one should consider seeing these patients in the office and triaging accordingly.
With the increasing requirements of managed care and restrictive medical plans, there has been a similar rise in the frequency of diagnostic test as well as procedure and medication denials. Re-approval and recertification of biologics and other medications have become common, which can add a great deal to your workload and that of your staff. Integration of endoscopy, pathology, and imaging (e.g., ultrasound, CT/CTE) improves response time, dialogue, and can have a positive impact on care. Office infusion allows for a better integration of this service into your practice. There is typically better communication with the infusion nurse(s) and better expedited care as well as fewer cancellations for minor infections. This all helps avoid infusion procedure delays. Infliximab, vedolizumab, ustekinumab, and lyophilized certolizumab pegol as well as intravenous iron administration can also expand services and enhance quality.
Having a medical assistant, nurse, and others in your practice to assist with patient services and care is a must. There will be many phone calls, emails, and other interactions regarding appointments, consults, routine lab testing, radiology testing, standard medications, biologics, and other treatments that necessitate an effective team-approach. For this role, either a nurse or an experienced medical assistant would be well-suited. Additional support staff and services can also aid our IBD patients. A dietitian knowledgeable in IBD and practical dietary options can, in many instances, prove invaluable. Understanding and utilizing pharma-sponsored “Patient Assistance Programs” provides drug access for the 10-20% (or more) of patients who do not have insurance or biologic coverage. Having specialty access and collegiality with colorectal surgeons, general surgeons, OB/GYNs, dermatologists, hematologists, oncologists, and others is important to expedite consults and provide collaborative care. Finally, offering clinical research options improves access for patients with limited and no coverage and also helps provide needed options for all IBD patients.
This brief overview has hopefully given you some insight into how to provide a higher level of evaluation and care for our IBD patients. These approaches have allowed me to build and maintain a successful IBD practice, and I hope that the integration of some or all of these strategies help you to build and sustain a successful IBD practice.
Dr. Wolf is director of IBD research, Atlanta Gastroenterology Associates.
Anyone can build a successful inflammatory bowel disease (IBD) practice. To do so requires commitment and focus in the area of IBD including both Crohn’s disease and ulcerative colitis. It also requires a fundamental knowledge of medicine as well as a desire to excel and learn all that one can in these areas. Given the high number of stakeholders, good interpersonal skills are vital. Establishing an IBD practice provides an opportunity to make a big difference in peoples’ lives and the age range of impact is about the broadest in all of medical practice. The more resources you have, the greater the potential impact of your care. Table 1 lists resources that are useful to provide optimal IBD patient care.
You, the gastroenterologist, is the most important resource for the patient. Medical school, residency, fellowship, and “postgraduate” training serves as the foundation for your wealth of knowledge. Maximizing your training is of value, and this can be done by being part of an academic program, keeping abreast of current literature, and attending meetings and post-graduate courses. AGA offers a variety of publications (http://www.gastro.org/journals-and-publications) and continued training opportunities (http://www.gastro.org/education).

One further point regarding scheduling is that one must be willing and able to see patients urgently, rather than sending them to the emergency room. ERs are appropriate for true emergencies, but are not an ideal place for care when an IBD patient has a flare and requires prompt follow-up. I try to avoid ER visits for my patients unless they are vomiting, have severe abdominal pain, significant bleeding or have clear signs of toxicity. In an ER, abdominal pain equals a CT scan; one should consider seeing these patients in the office and triaging accordingly.
With the increasing requirements of managed care and restrictive medical plans, there has been a similar rise in the frequency of diagnostic test as well as procedure and medication denials. Re-approval and recertification of biologics and other medications have become common, which can add a great deal to your workload and that of your staff. Integration of endoscopy, pathology, and imaging (e.g., ultrasound, CT/CTE) improves response time, dialogue, and can have a positive impact on care. Office infusion allows for a better integration of this service into your practice. There is typically better communication with the infusion nurse(s) and better expedited care as well as fewer cancellations for minor infections. This all helps avoid infusion procedure delays. Infliximab, vedolizumab, ustekinumab, and lyophilized certolizumab pegol as well as intravenous iron administration can also expand services and enhance quality.
Having a medical assistant, nurse, and others in your practice to assist with patient services and care is a must. There will be many phone calls, emails, and other interactions regarding appointments, consults, routine lab testing, radiology testing, standard medications, biologics, and other treatments that necessitate an effective team-approach. For this role, either a nurse or an experienced medical assistant would be well-suited. Additional support staff and services can also aid our IBD patients. A dietitian knowledgeable in IBD and practical dietary options can, in many instances, prove invaluable. Understanding and utilizing pharma-sponsored “Patient Assistance Programs” provides drug access for the 10-20% (or more) of patients who do not have insurance or biologic coverage. Having specialty access and collegiality with colorectal surgeons, general surgeons, OB/GYNs, dermatologists, hematologists, oncologists, and others is important to expedite consults and provide collaborative care. Finally, offering clinical research options improves access for patients with limited and no coverage and also helps provide needed options for all IBD patients.
This brief overview has hopefully given you some insight into how to provide a higher level of evaluation and care for our IBD patients. These approaches have allowed me to build and maintain a successful IBD practice, and I hope that the integration of some or all of these strategies help you to build and sustain a successful IBD practice.
Dr. Wolf is director of IBD research, Atlanta Gastroenterology Associates.
Health Maintenance and Preventive Care in Patients with Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) consists of two chronic inflammatory diseases, Crohn’s disease (CD) and ulcerative colitis (UC), as well as a small category of patients (~10%) who have atypical features called IBD-unclassified (IBD-U) or indeterminate colitis. The prevalence of IBD ranges from 0.3% to 0.5% overall in North America and Europe.1 In North America, the incidences of CD and UC are estimated to be 3.1 to 14.6 per 100,000 person-years and 2.2 to 14.3 cases per 100,000 person-years, respectively; similar rates are seen in Europe.2 However, incidences up to 19.2 and 20.2 per 100,000 for UC and CD, respectively, have been reported in Canada.3,4 The incidences of both UC and CD are increasing over time in Western countries and in rapidly industrializing countries throughout Asia and South America.5-8
Influenza vaccine and pneumococcal vaccine
Influenza A and B outbreaks are commonly seen during the fall and early spring and risk factors for pneumonia and hospitalization include older age, chronic medical conditions, and immunosuppression. The CDC now recommend annual influenza vaccination for all individuals older than six months. For patients on immunosuppression, the vaccine administered should be the inactivated vaccine, as live attenuated vaccines should not be administered to these patients.
In IBD patients, the influenza and pneumococcal vaccines are both well tolerated without an increased rate of adverse effects over the general population and without an increased risk of IBD flares after vaccination.12 A common question for patients on biologic therapy is whether the vaccine should be timed at a specific point in the dose cycle. For infliximab, and likely other biologics, the timing does not change the vaccine immunogenicity and patients should be given these vaccines regardless of where they are in the cycle of administration of their biologic.13 In addition, there is significant response to influenza and pneumococcal vaccines in patients on combination therapy with immunomodulators and anti-TNFs and concerns about a lack of response to vaccines should not discourage vaccination since benefits are still acquired by patients even if immunogenicity is somewhat decreased.14,15
Other vaccinations
In addition to the influenza and pneumococcal vaccines, adult and pediatric patients with IBD should follow the ACIP recommendations for tetanus, diphtheria, pertussis (Tdap), Td boosters, hepatitis A, hepatitis B, human papilloma virus (HPV), and meningococcal vaccinations.16,17
Live vaccines including measles mumps rubella (MMR), varicella, and zoster vaccines are in general contraindicated in immunosuppressed patients on corticosteroids, azathioprine/6-mercaptopurine, methotrexate, anti-TNF, and anti-integrin biologics. An inactive varicella-zoster vaccine will likely be available in the near future and may obviate the need for the live vaccine, which is an important development given the increased risk of zoster in patients with IBD on immunosuppression.18
Osteoporosis screening
Skin cancer screening
Multiple studies have demonstrated that immunosuppression, especially with methotrexate and azathioprine/6-mercaptopurine (6MP) is a risk factor for the development of initial and recurrent non-melanoma skin cancer (NMSC) in IBD patients, the data for biologics are less definitive.23-25 In addition, biologics are associated with increased risk of melanoma in IBD.26 The elevated risk of skin cancer begins in the first year of treatment with thiopurines and may continue after discontinuation. On the basis of this data, screening for melanoma and NMSC is recommended in IBD patients on immunosuppression. Especially for patients on thiopurines it is reasonable for the initial dermatologist visit to occur in the first year of treatment and thereafter with at least annual visits for a full body skin examination. In addition, it is reasonable to recommend regular sunscreen use and protective clothing such as hats.
Cervical cancer screening
A recent meta-analysis shows that women with IBD on immunosuppression have an increased risk of cervical high grade dysplasia and cervical cancer.27 HPV is the major risk factor for cervical cancer and is necessary for its development. The current American College of Gynecology guidelines for women on immunosuppression are to start cervical cancer screening at 21 and annual screening thereafter with Pap and HPV testing.28
Smoking
Smoking has well known associations with poor outcomes in the general population such as increased risk of lung and pancreatic cancers, as well as high risk of cardiovascular disease. In addition, smoking has risks specific to IBD. In CD, smoking is associated with increased disease activity, increased risk of post-operative recurrence, and increased severity of disease.29 Smoking cessation is associated with improved long-term disease outcomes and less risk.30 Making it a point to regularly discuss smoking cessation and partnering with PCPs to offer evidence-based quitting aids may be one of our most significant and beneficial interventions.
Depression and anxiety
Several studies have shown high levels of depression and anxiety in IBD patients and higher levels of depression are associated with increased symptoms, clinical recurrence, poor quality of life and decreased social support.31-33 A recent systematic review of several studies suggested that antidepressants use in IBD patients benefits their mental health and may improve their clinical course as well.34 As such, screening for depression and anxiety regularly and either offering treatment or referral to psychiatrists and psychologists for further management is recommended.10
Conclusion
Patients with IBD frequently develop long-term relationships with their gastroenterologists due to their lifelong chronic disease. It is therefore incumbent on us to be attentive to issues related to IBD patients’ preventive care and collaborate with PCPs to coordinate care for our patients since many of these interventions have both short-term and long-term benefits.
Dr. Chachu is assistant professor and gastroenterologist at Duke University, Durham, N.C.
References
1. Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152(2):313-21.e2.
2. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17.
3. Bernstein CN, Wajda A, Svenson LW, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. The American journal of gastroenterology. 2006;101(7):1559-68.
4. Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn’s disease in Quebec, Canada. Inflammatory bowel diseases. 2009;15(3):429-35.
5. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424-9.
6. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive diseases and sciences. 2013;58(2):519-25.
7. Ng SC, Kaplan G, Banerjee R, et al. 78 Incidence and Phenotype of Inflammatory Bowel Disease From 13 Countries in Asia-Pacific: Results From the Asia-Pacific Crohn’s and Colitis Epidemiologic Study 2011-2013. Gastroenterology.150(4):S21.
8. Parente JML, Coy CSR, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World Journal of Gastroenterology : WJG. 2015;21(4):1197-206.
9. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory bowel diseases. 2008;14(2):253-8.
10. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. The American journal of gastroenterology. 2017;112(2):241-58.
11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. The American journal of gastroenterology. 2013;108(2):240-8.
12. Rahier JF, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-62.
13. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of Influenza Vaccine for Patients with Inflammatory Bowel Disease on Maintenance Infliximab Therapy: A Randomized Trial. Inflammatory bowel diseases. 2016;22(3):638-47.
14. Brezinschek HP, Hofstaetter T, Leeb BF, et al. Immunization of patients with rheumatoid arthritis with antitumor necrosis factor alpha therapy and methotrexate. Current opinion in rheumatology. 2008;20(3):295-9.
15. Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272-9.
16. Kim DK, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):136-8.
17. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134-5.
18. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2392-403.
19. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53(2):251-5.
20. Casals-Seoane F, Chaparro M, Mate J, et al. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflammatory bowel diseases. 2016;22(8):1929-36.
21. Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):32-44.e5.
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359-81.
23. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621-28.e1-5.
24. Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):268-74.
25. Scott FI, Mamtani R, Brensinger CM, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA dermatology. 2016;152(2):164-72.
26. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-9.e1.
27. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015;21(5):1089-97.
28. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016;128(4):e111-30.
29. Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. American journal of surgery. 2004;187(2):219-25.
30. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120(5):1093-9.
31. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697-707.
32. Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. General hospital psychiatry. 1996;18(4):220-9.
33. Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology.14(6):829-35.e1.
34. Macer BJD, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflammatory bowel diseases. 2017;23(4):534-50.
Inflammatory bowel disease (IBD) consists of two chronic inflammatory diseases, Crohn’s disease (CD) and ulcerative colitis (UC), as well as a small category of patients (~10%) who have atypical features called IBD-unclassified (IBD-U) or indeterminate colitis. The prevalence of IBD ranges from 0.3% to 0.5% overall in North America and Europe.1 In North America, the incidences of CD and UC are estimated to be 3.1 to 14.6 per 100,000 person-years and 2.2 to 14.3 cases per 100,000 person-years, respectively; similar rates are seen in Europe.2 However, incidences up to 19.2 and 20.2 per 100,000 for UC and CD, respectively, have been reported in Canada.3,4 The incidences of both UC and CD are increasing over time in Western countries and in rapidly industrializing countries throughout Asia and South America.5-8
Influenza vaccine and pneumococcal vaccine
Influenza A and B outbreaks are commonly seen during the fall and early spring and risk factors for pneumonia and hospitalization include older age, chronic medical conditions, and immunosuppression. The CDC now recommend annual influenza vaccination for all individuals older than six months. For patients on immunosuppression, the vaccine administered should be the inactivated vaccine, as live attenuated vaccines should not be administered to these patients.
In IBD patients, the influenza and pneumococcal vaccines are both well tolerated without an increased rate of adverse effects over the general population and without an increased risk of IBD flares after vaccination.12 A common question for patients on biologic therapy is whether the vaccine should be timed at a specific point in the dose cycle. For infliximab, and likely other biologics, the timing does not change the vaccine immunogenicity and patients should be given these vaccines regardless of where they are in the cycle of administration of their biologic.13 In addition, there is significant response to influenza and pneumococcal vaccines in patients on combination therapy with immunomodulators and anti-TNFs and concerns about a lack of response to vaccines should not discourage vaccination since benefits are still acquired by patients even if immunogenicity is somewhat decreased.14,15
Other vaccinations
In addition to the influenza and pneumococcal vaccines, adult and pediatric patients with IBD should follow the ACIP recommendations for tetanus, diphtheria, pertussis (Tdap), Td boosters, hepatitis A, hepatitis B, human papilloma virus (HPV), and meningococcal vaccinations.16,17
Live vaccines including measles mumps rubella (MMR), varicella, and zoster vaccines are in general contraindicated in immunosuppressed patients on corticosteroids, azathioprine/6-mercaptopurine, methotrexate, anti-TNF, and anti-integrin biologics. An inactive varicella-zoster vaccine will likely be available in the near future and may obviate the need for the live vaccine, which is an important development given the increased risk of zoster in patients with IBD on immunosuppression.18
Osteoporosis screening
Skin cancer screening
Multiple studies have demonstrated that immunosuppression, especially with methotrexate and azathioprine/6-mercaptopurine (6MP) is a risk factor for the development of initial and recurrent non-melanoma skin cancer (NMSC) in IBD patients, the data for biologics are less definitive.23-25 In addition, biologics are associated with increased risk of melanoma in IBD.26 The elevated risk of skin cancer begins in the first year of treatment with thiopurines and may continue after discontinuation. On the basis of this data, screening for melanoma and NMSC is recommended in IBD patients on immunosuppression. Especially for patients on thiopurines it is reasonable for the initial dermatologist visit to occur in the first year of treatment and thereafter with at least annual visits for a full body skin examination. In addition, it is reasonable to recommend regular sunscreen use and protective clothing such as hats.
Cervical cancer screening
A recent meta-analysis shows that women with IBD on immunosuppression have an increased risk of cervical high grade dysplasia and cervical cancer.27 HPV is the major risk factor for cervical cancer and is necessary for its development. The current American College of Gynecology guidelines for women on immunosuppression are to start cervical cancer screening at 21 and annual screening thereafter with Pap and HPV testing.28
Smoking
Smoking has well known associations with poor outcomes in the general population such as increased risk of lung and pancreatic cancers, as well as high risk of cardiovascular disease. In addition, smoking has risks specific to IBD. In CD, smoking is associated with increased disease activity, increased risk of post-operative recurrence, and increased severity of disease.29 Smoking cessation is associated with improved long-term disease outcomes and less risk.30 Making it a point to regularly discuss smoking cessation and partnering with PCPs to offer evidence-based quitting aids may be one of our most significant and beneficial interventions.
Depression and anxiety
Several studies have shown high levels of depression and anxiety in IBD patients and higher levels of depression are associated with increased symptoms, clinical recurrence, poor quality of life and decreased social support.31-33 A recent systematic review of several studies suggested that antidepressants use in IBD patients benefits their mental health and may improve their clinical course as well.34 As such, screening for depression and anxiety regularly and either offering treatment or referral to psychiatrists and psychologists for further management is recommended.10
Conclusion
Patients with IBD frequently develop long-term relationships with their gastroenterologists due to their lifelong chronic disease. It is therefore incumbent on us to be attentive to issues related to IBD patients’ preventive care and collaborate with PCPs to coordinate care for our patients since many of these interventions have both short-term and long-term benefits.
Dr. Chachu is assistant professor and gastroenterologist at Duke University, Durham, N.C.
References
1. Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152(2):313-21.e2.
2. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17.
3. Bernstein CN, Wajda A, Svenson LW, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. The American journal of gastroenterology. 2006;101(7):1559-68.
4. Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn’s disease in Quebec, Canada. Inflammatory bowel diseases. 2009;15(3):429-35.
5. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424-9.
6. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive diseases and sciences. 2013;58(2):519-25.
7. Ng SC, Kaplan G, Banerjee R, et al. 78 Incidence and Phenotype of Inflammatory Bowel Disease From 13 Countries in Asia-Pacific: Results From the Asia-Pacific Crohn’s and Colitis Epidemiologic Study 2011-2013. Gastroenterology.150(4):S21.
8. Parente JML, Coy CSR, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World Journal of Gastroenterology : WJG. 2015;21(4):1197-206.
9. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory bowel diseases. 2008;14(2):253-8.
10. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. The American journal of gastroenterology. 2017;112(2):241-58.
11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. The American journal of gastroenterology. 2013;108(2):240-8.
12. Rahier JF, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-62.
13. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of Influenza Vaccine for Patients with Inflammatory Bowel Disease on Maintenance Infliximab Therapy: A Randomized Trial. Inflammatory bowel diseases. 2016;22(3):638-47.
14. Brezinschek HP, Hofstaetter T, Leeb BF, et al. Immunization of patients with rheumatoid arthritis with antitumor necrosis factor alpha therapy and methotrexate. Current opinion in rheumatology. 2008;20(3):295-9.
15. Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272-9.
16. Kim DK, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):136-8.
17. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134-5.
18. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2392-403.
19. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53(2):251-5.
20. Casals-Seoane F, Chaparro M, Mate J, et al. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflammatory bowel diseases. 2016;22(8):1929-36.
21. Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):32-44.e5.
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359-81.
23. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621-28.e1-5.
24. Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):268-74.
25. Scott FI, Mamtani R, Brensinger CM, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA dermatology. 2016;152(2):164-72.
26. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-9.e1.
27. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015;21(5):1089-97.
28. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016;128(4):e111-30.
29. Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. American journal of surgery. 2004;187(2):219-25.
30. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120(5):1093-9.
31. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697-707.
32. Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. General hospital psychiatry. 1996;18(4):220-9.
33. Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology.14(6):829-35.e1.
34. Macer BJD, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflammatory bowel diseases. 2017;23(4):534-50.
Inflammatory bowel disease (IBD) consists of two chronic inflammatory diseases, Crohn’s disease (CD) and ulcerative colitis (UC), as well as a small category of patients (~10%) who have atypical features called IBD-unclassified (IBD-U) or indeterminate colitis. The prevalence of IBD ranges from 0.3% to 0.5% overall in North America and Europe.1 In North America, the incidences of CD and UC are estimated to be 3.1 to 14.6 per 100,000 person-years and 2.2 to 14.3 cases per 100,000 person-years, respectively; similar rates are seen in Europe.2 However, incidences up to 19.2 and 20.2 per 100,000 for UC and CD, respectively, have been reported in Canada.3,4 The incidences of both UC and CD are increasing over time in Western countries and in rapidly industrializing countries throughout Asia and South America.5-8
Influenza vaccine and pneumococcal vaccine
Influenza A and B outbreaks are commonly seen during the fall and early spring and risk factors for pneumonia and hospitalization include older age, chronic medical conditions, and immunosuppression. The CDC now recommend annual influenza vaccination for all individuals older than six months. For patients on immunosuppression, the vaccine administered should be the inactivated vaccine, as live attenuated vaccines should not be administered to these patients.
In IBD patients, the influenza and pneumococcal vaccines are both well tolerated without an increased rate of adverse effects over the general population and without an increased risk of IBD flares after vaccination.12 A common question for patients on biologic therapy is whether the vaccine should be timed at a specific point in the dose cycle. For infliximab, and likely other biologics, the timing does not change the vaccine immunogenicity and patients should be given these vaccines regardless of where they are in the cycle of administration of their biologic.13 In addition, there is significant response to influenza and pneumococcal vaccines in patients on combination therapy with immunomodulators and anti-TNFs and concerns about a lack of response to vaccines should not discourage vaccination since benefits are still acquired by patients even if immunogenicity is somewhat decreased.14,15
Other vaccinations
In addition to the influenza and pneumococcal vaccines, adult and pediatric patients with IBD should follow the ACIP recommendations for tetanus, diphtheria, pertussis (Tdap), Td boosters, hepatitis A, hepatitis B, human papilloma virus (HPV), and meningococcal vaccinations.16,17
Live vaccines including measles mumps rubella (MMR), varicella, and zoster vaccines are in general contraindicated in immunosuppressed patients on corticosteroids, azathioprine/6-mercaptopurine, methotrexate, anti-TNF, and anti-integrin biologics. An inactive varicella-zoster vaccine will likely be available in the near future and may obviate the need for the live vaccine, which is an important development given the increased risk of zoster in patients with IBD on immunosuppression.18
Osteoporosis screening
Skin cancer screening
Multiple studies have demonstrated that immunosuppression, especially with methotrexate and azathioprine/6-mercaptopurine (6MP) is a risk factor for the development of initial and recurrent non-melanoma skin cancer (NMSC) in IBD patients, the data for biologics are less definitive.23-25 In addition, biologics are associated with increased risk of melanoma in IBD.26 The elevated risk of skin cancer begins in the first year of treatment with thiopurines and may continue after discontinuation. On the basis of this data, screening for melanoma and NMSC is recommended in IBD patients on immunosuppression. Especially for patients on thiopurines it is reasonable for the initial dermatologist visit to occur in the first year of treatment and thereafter with at least annual visits for a full body skin examination. In addition, it is reasonable to recommend regular sunscreen use and protective clothing such as hats.
Cervical cancer screening
A recent meta-analysis shows that women with IBD on immunosuppression have an increased risk of cervical high grade dysplasia and cervical cancer.27 HPV is the major risk factor for cervical cancer and is necessary for its development. The current American College of Gynecology guidelines for women on immunosuppression are to start cervical cancer screening at 21 and annual screening thereafter with Pap and HPV testing.28
Smoking
Smoking has well known associations with poor outcomes in the general population such as increased risk of lung and pancreatic cancers, as well as high risk of cardiovascular disease. In addition, smoking has risks specific to IBD. In CD, smoking is associated with increased disease activity, increased risk of post-operative recurrence, and increased severity of disease.29 Smoking cessation is associated with improved long-term disease outcomes and less risk.30 Making it a point to regularly discuss smoking cessation and partnering with PCPs to offer evidence-based quitting aids may be one of our most significant and beneficial interventions.
Depression and anxiety
Several studies have shown high levels of depression and anxiety in IBD patients and higher levels of depression are associated with increased symptoms, clinical recurrence, poor quality of life and decreased social support.31-33 A recent systematic review of several studies suggested that antidepressants use in IBD patients benefits their mental health and may improve their clinical course as well.34 As such, screening for depression and anxiety regularly and either offering treatment or referral to psychiatrists and psychologists for further management is recommended.10
Conclusion
Patients with IBD frequently develop long-term relationships with their gastroenterologists due to their lifelong chronic disease. It is therefore incumbent on us to be attentive to issues related to IBD patients’ preventive care and collaborate with PCPs to coordinate care for our patients since many of these interventions have both short-term and long-term benefits.
Dr. Chachu is assistant professor and gastroenterologist at Duke University, Durham, N.C.
References
1. Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152(2):313-21.e2.
2. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17.
3. Bernstein CN, Wajda A, Svenson LW, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. The American journal of gastroenterology. 2006;101(7):1559-68.
4. Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn’s disease in Quebec, Canada. Inflammatory bowel diseases. 2009;15(3):429-35.
5. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424-9.
6. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive diseases and sciences. 2013;58(2):519-25.
7. Ng SC, Kaplan G, Banerjee R, et al. 78 Incidence and Phenotype of Inflammatory Bowel Disease From 13 Countries in Asia-Pacific: Results From the Asia-Pacific Crohn’s and Colitis Epidemiologic Study 2011-2013. Gastroenterology.150(4):S21.
8. Parente JML, Coy CSR, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World Journal of Gastroenterology : WJG. 2015;21(4):1197-206.
9. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory bowel diseases. 2008;14(2):253-8.
10. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. The American journal of gastroenterology. 2017;112(2):241-58.
11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. The American journal of gastroenterology. 2013;108(2):240-8.
12. Rahier JF, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-62.
13. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of Influenza Vaccine for Patients with Inflammatory Bowel Disease on Maintenance Infliximab Therapy: A Randomized Trial. Inflammatory bowel diseases. 2016;22(3):638-47.
14. Brezinschek HP, Hofstaetter T, Leeb BF, et al. Immunization of patients with rheumatoid arthritis with antitumor necrosis factor alpha therapy and methotrexate. Current opinion in rheumatology. 2008;20(3):295-9.
15. Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272-9.
16. Kim DK, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):136-8.
17. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134-5.
18. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2392-403.
19. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53(2):251-5.
20. Casals-Seoane F, Chaparro M, Mate J, et al. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflammatory bowel diseases. 2016;22(8):1929-36.
21. Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):32-44.e5.
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359-81.
23. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621-28.e1-5.
24. Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):268-74.
25. Scott FI, Mamtani R, Brensinger CM, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA dermatology. 2016;152(2):164-72.
26. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-9.e1.
27. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015;21(5):1089-97.
28. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016;128(4):e111-30.
29. Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. American journal of surgery. 2004;187(2):219-25.
30. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120(5):1093-9.
31. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697-707.
32. Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. General hospital psychiatry. 1996;18(4):220-9.
33. Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology.14(6):829-35.e1.
34. Macer BJD, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflammatory bowel diseases. 2017;23(4):534-50.
The 'Nuts and Bolts' of Drug Concentration Monitoring in IBD
Introduction
Anti–tumor necrosis factor (anti-TNF) therapy is the cornerstone of inflammatory bowel disease (IBD) treatment.1 Nevertheless, up to 30% of patients show no clinical benefit, considered as primary non-responders, while another 50% lose response over time and need to escalate or discontinue anti-TNF therapy due to either pharmacokinetic (PK) or pharmacodynamic issues.2 Therapeutic drug monitoring (TDM), defined as the assessment of drug concentration and anti-drug antibodies (ADA), is emerging as a new therapeutic strategy to better explain, manage, and hopefully prevent these undesired clinical outcomes.3 Moreover, numerous studies have shown that higher serum anti-TNF drug concentrations both during maintenance and induction therapy are associated with favorable objective therapeutic outcomes, suggesting of a ‘treat-to-trough’ in addition to a ‘treat-to-target’ therapeutic approach.4-6 This concept of TDM is not new in IBD. TDM has also been used for optimizing thiopurines.7 This brief review will discuss a practical approach to the use of TDM in IBD with a focus on its use with anti-TNF therapies.
Reactive TDM of anti-TNF therapy
Reactive TDM more rationally guides therapeutic decisions for dealing with loss of response to anti-TNF therapy in IBD and is actually more cost-effective.8,9 Patients with sub-therapeutic or undetectable drug concentrations without ADA derive more benefit from dose escalation (increasing the dose or decreasing the interval) compared to those switched to another anti-TNF agent. On the other hand, patients with therapeutic or supra-therapeutic drug concentrations have better outcomes when changing to a medication with a different mechanism of action (as their disease is probably no longer TNF-driven).3 A recent study showed that trough concentration of adalimumab >4.5 mcg/mL or infliximab >3.8 mcg/mL at time of loss of response identifies patients who benefit more from alternative therapies rather than dose escalation or switching to another anti-TNF agent.10 In clinical practice, in order to fully optimize the original anti-TNF, we will typically dose optimize patients to drug concentrations of infliximab and adalimumab to >10 mcg/mL before giving up and changing medications. Moreover, patients with high ADA titer have better outcomes when switched to another anti-TNF rather than undergo further dose escalation.3 Vande Casteele et al, showed that antibodies to infliximab (ATI) >9.1 U/mL at time of loss of response resulted in a likelihood ratio of 3.6 for an unsuccessful intervention, defined as the need to initiate corticosteroids, immunomodulators (IMM), or other medications or infliximab discontinuation within two infusions after the intervention (shorten of infusion intervals, dose increase to 10 mg/kg, or a combination of both).11 A proposed treatment algorithm for using reactive TDM for anti-TNF therapy is shown in Figure 1.
Proactive TDM of anti-TNF therapy
TDM of thiopurines
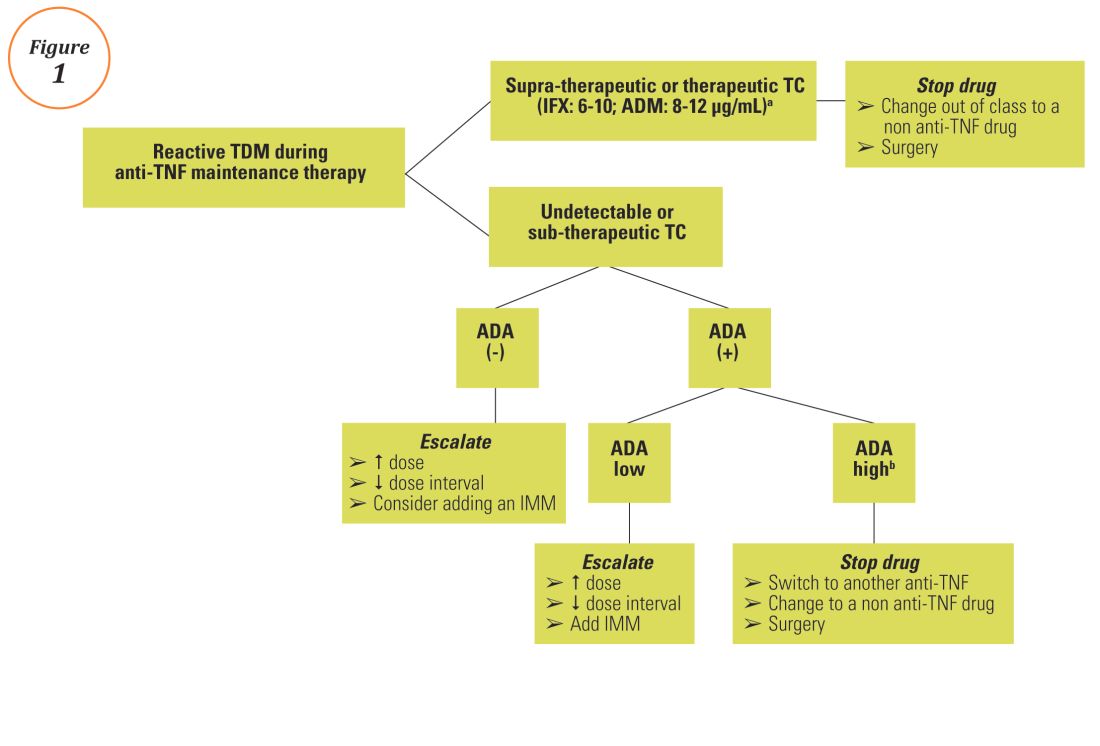
Anti-TNF TDM assays
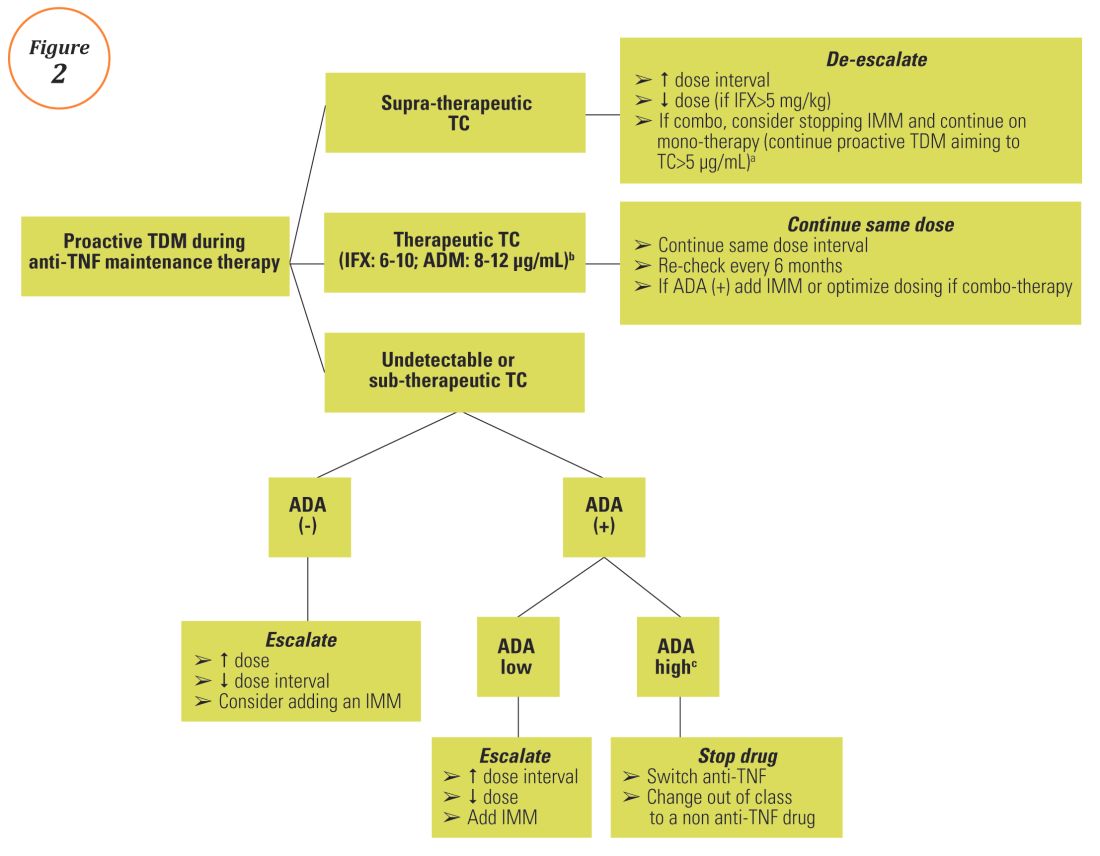
Conclusions
A growing body of evidence demonstrates the clinical utility of TDM of anti-TNF therapy in IBD clinical practice and a move towards personalized medicine, as it is now clear that “one dose does not fit all patients.” Nevertheless, before a TDM-based approach can be widely implemented and emerge as the new standard-of-care for anti-TNF therapy in IBD, several barriers regarding cost issues (insurance coverage and out of pocket expenses), time lag from serum sampling to test results (typically 5 to 10 days), proper interpretation and application of the results, type of assay used, and the optimal timing of serum collection should be overcome. Initiatives are already underway including the development of accurate, easily accessible, and affordable rapid assays that will allow anti-TNF concentration measurement at the point-of-care site and software-decision support tools or ‘dashboards’ that will incorporate a predictive PK model based on patient and disease characteristics.29,30 Additionally, more data from well-designed prospective studies and randomized controlled trials regarding both induction and maintenance treatment and for all available biologics (originators and biosimilars) are urgently needed. A panel consisting of members of the Building Research in Inflammatory Bowel Disease Globally research alliance (www.BRIDGeIBD.com), and recognized leaders in the field of TDM in IBD has recently published recommendations that help clinicians on the appropriate timing and best way to interpret and respond to TDM results depending on the specific clinical scenario.31
Funding: KP received a fellowship grant from the Hellenic Group for the study of IBD.
Potential competing interests: K.P.: nothing to disclose; A.S.C: received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer.
Dr. Papamichail is a research fellow and Dr. Cheifetz is the director of the Center for Inflammatory Bowel Diseases, division of gastroenterology, Beth-Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Papamichail received a fellowship grant from the Hellenic Group for the study of IBD. Dr. Cheifetz received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer.
References
1. Miligkos M, Papamichael K, Casteele NV, et al. Efficacy and safety profile of anti-tumor necrosis factor-alpha versus anti-integrin agents for the treatment of Crohn’s disease: a network meta-analysis of indirect comparisons. Clin Ther. 2016;38(6):1342-1358.e6
2. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182-97
3. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol 2016;7;289-300.
4. Papamichael K, Baert F, Tops S, et al. Post-Induction Adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017;11:53-59
5. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:543-9.
6. Ungar B, Levy I, Yavne Y, et al. Optimizing Anti-TNF-Alpha Therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14:550-557.e2.
7. Singh N, Dubinsky MC. Therapeutic drug monitoring in children and young adults with inflammatory bowel disease: a practical approach. Gastroenterol Hepatol (NY). 2015;11:48-55.
8. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919-27.
9. Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66.
10. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522-30.
11. Casteele NV, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962-71.
12. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996-2003.
13. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320-9.e3.
14. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307.e5.
15. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7.
16. Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:531–8.
17. Singh N, Rosenthal CJ, Melmed GY, et al Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1708-13.
18. Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32.
19. Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016;65:1126–31.
20. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:423-31.e1
21. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014;40:338–53.
22. Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474-81.e2
23. Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 2006;130:1047-53
24. Dassopoulos T, Dubinsky MC, Bentsen JL, et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther 2014;39:163-175.
25. Waljee AK, Joyce JC, Wang S, et al. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol 2010;8:143-150.
26. Yarur A, Kubiliun M, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118-1124.
27. Marini JC, Sendecki J, Cornillie F, et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®.AAPS J. 2016 Sep 6. [Epub ahead of print]. DOI: 10.1208/s12248-016-9981-3
28. Gils A, Vande Casteele N, Poppe R, et al. Development of a universal anti-adalimumab antibody standard for interlaboratory harmonization. Ther Drug Monit. 2014;36:669-673.
29. Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol 2016;7:e206
30. Dubinsky MC, Phan BL, Singh N, et al. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J. 2016 Oct 13. [Epub ahead of print]
31. Melmed GY, Irving PM, Jones J, et al. Appropriateness of testing for anti-tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin Gastroenterol Hepatol 2016;14:1302-9.
32. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601-8.
33. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:514-21.e4.
Introduction
Anti–tumor necrosis factor (anti-TNF) therapy is the cornerstone of inflammatory bowel disease (IBD) treatment.1 Nevertheless, up to 30% of patients show no clinical benefit, considered as primary non-responders, while another 50% lose response over time and need to escalate or discontinue anti-TNF therapy due to either pharmacokinetic (PK) or pharmacodynamic issues.2 Therapeutic drug monitoring (TDM), defined as the assessment of drug concentration and anti-drug antibodies (ADA), is emerging as a new therapeutic strategy to better explain, manage, and hopefully prevent these undesired clinical outcomes.3 Moreover, numerous studies have shown that higher serum anti-TNF drug concentrations both during maintenance and induction therapy are associated with favorable objective therapeutic outcomes, suggesting of a ‘treat-to-trough’ in addition to a ‘treat-to-target’ therapeutic approach.4-6 This concept of TDM is not new in IBD. TDM has also been used for optimizing thiopurines.7 This brief review will discuss a practical approach to the use of TDM in IBD with a focus on its use with anti-TNF therapies.
Reactive TDM of anti-TNF therapy
Reactive TDM more rationally guides therapeutic decisions for dealing with loss of response to anti-TNF therapy in IBD and is actually more cost-effective.8,9 Patients with sub-therapeutic or undetectable drug concentrations without ADA derive more benefit from dose escalation (increasing the dose or decreasing the interval) compared to those switched to another anti-TNF agent. On the other hand, patients with therapeutic or supra-therapeutic drug concentrations have better outcomes when changing to a medication with a different mechanism of action (as their disease is probably no longer TNF-driven).3 A recent study showed that trough concentration of adalimumab >4.5 mcg/mL or infliximab >3.8 mcg/mL at time of loss of response identifies patients who benefit more from alternative therapies rather than dose escalation or switching to another anti-TNF agent.10 In clinical practice, in order to fully optimize the original anti-TNF, we will typically dose optimize patients to drug concentrations of infliximab and adalimumab to >10 mcg/mL before giving up and changing medications. Moreover, patients with high ADA titer have better outcomes when switched to another anti-TNF rather than undergo further dose escalation.3 Vande Casteele et al, showed that antibodies to infliximab (ATI) >9.1 U/mL at time of loss of response resulted in a likelihood ratio of 3.6 for an unsuccessful intervention, defined as the need to initiate corticosteroids, immunomodulators (IMM), or other medications or infliximab discontinuation within two infusions after the intervention (shorten of infusion intervals, dose increase to 10 mg/kg, or a combination of both).11 A proposed treatment algorithm for using reactive TDM for anti-TNF therapy is shown in Figure 1.
Proactive TDM of anti-TNF therapy
TDM of thiopurines

Anti-TNF TDM assays

Conclusions
A growing body of evidence demonstrates the clinical utility of TDM of anti-TNF therapy in IBD clinical practice and a move towards personalized medicine, as it is now clear that “one dose does not fit all patients.” Nevertheless, before a TDM-based approach can be widely implemented and emerge as the new standard-of-care for anti-TNF therapy in IBD, several barriers regarding cost issues (insurance coverage and out of pocket expenses), time lag from serum sampling to test results (typically 5 to 10 days), proper interpretation and application of the results, type of assay used, and the optimal timing of serum collection should be overcome. Initiatives are already underway including the development of accurate, easily accessible, and affordable rapid assays that will allow anti-TNF concentration measurement at the point-of-care site and software-decision support tools or ‘dashboards’ that will incorporate a predictive PK model based on patient and disease characteristics.29,30 Additionally, more data from well-designed prospective studies and randomized controlled trials regarding both induction and maintenance treatment and for all available biologics (originators and biosimilars) are urgently needed. A panel consisting of members of the Building Research in Inflammatory Bowel Disease Globally research alliance (www.BRIDGeIBD.com), and recognized leaders in the field of TDM in IBD has recently published recommendations that help clinicians on the appropriate timing and best way to interpret and respond to TDM results depending on the specific clinical scenario.31
Funding: KP received a fellowship grant from the Hellenic Group for the study of IBD.
Potential competing interests: K.P.: nothing to disclose; A.S.C: received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer.
Dr. Papamichail is a research fellow and Dr. Cheifetz is the director of the Center for Inflammatory Bowel Diseases, division of gastroenterology, Beth-Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Papamichail received a fellowship grant from the Hellenic Group for the study of IBD. Dr. Cheifetz received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer.
References
1. Miligkos M, Papamichael K, Casteele NV, et al. Efficacy and safety profile of anti-tumor necrosis factor-alpha versus anti-integrin agents for the treatment of Crohn’s disease: a network meta-analysis of indirect comparisons. Clin Ther. 2016;38(6):1342-1358.e6
2. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182-97
3. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol 2016;7;289-300.
4. Papamichael K, Baert F, Tops S, et al. Post-Induction Adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017;11:53-59
5. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:543-9.
6. Ungar B, Levy I, Yavne Y, et al. Optimizing Anti-TNF-Alpha Therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14:550-557.e2.
7. Singh N, Dubinsky MC. Therapeutic drug monitoring in children and young adults with inflammatory bowel disease: a practical approach. Gastroenterol Hepatol (NY). 2015;11:48-55.
8. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919-27.
9. Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66.
10. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522-30.
11. Casteele NV, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962-71.
12. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996-2003.
13. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320-9.e3.
14. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307.e5.
15. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7.
16. Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:531–8.
17. Singh N, Rosenthal CJ, Melmed GY, et al Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1708-13.
18. Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32.
19. Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016;65:1126–31.
20. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:423-31.e1
21. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014;40:338–53.
22. Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474-81.e2
23. Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 2006;130:1047-53
24. Dassopoulos T, Dubinsky MC, Bentsen JL, et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther 2014;39:163-175.
25. Waljee AK, Joyce JC, Wang S, et al. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol 2010;8:143-150.
26. Yarur A, Kubiliun M, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118-1124.
27. Marini JC, Sendecki J, Cornillie F, et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®.AAPS J. 2016 Sep 6. [Epub ahead of print]. DOI: 10.1208/s12248-016-9981-3
28. Gils A, Vande Casteele N, Poppe R, et al. Development of a universal anti-adalimumab antibody standard for interlaboratory harmonization. Ther Drug Monit. 2014;36:669-673.
29. Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol 2016;7:e206
30. Dubinsky MC, Phan BL, Singh N, et al. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J. 2016 Oct 13. [Epub ahead of print]
31. Melmed GY, Irving PM, Jones J, et al. Appropriateness of testing for anti-tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin Gastroenterol Hepatol 2016;14:1302-9.
32. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601-8.
33. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:514-21.e4.
Introduction
Anti–tumor necrosis factor (anti-TNF) therapy is the cornerstone of inflammatory bowel disease (IBD) treatment.1 Nevertheless, up to 30% of patients show no clinical benefit, considered as primary non-responders, while another 50% lose response over time and need to escalate or discontinue anti-TNF therapy due to either pharmacokinetic (PK) or pharmacodynamic issues.2 Therapeutic drug monitoring (TDM), defined as the assessment of drug concentration and anti-drug antibodies (ADA), is emerging as a new therapeutic strategy to better explain, manage, and hopefully prevent these undesired clinical outcomes.3 Moreover, numerous studies have shown that higher serum anti-TNF drug concentrations both during maintenance and induction therapy are associated with favorable objective therapeutic outcomes, suggesting of a ‘treat-to-trough’ in addition to a ‘treat-to-target’ therapeutic approach.4-6 This concept of TDM is not new in IBD. TDM has also been used for optimizing thiopurines.7 This brief review will discuss a practical approach to the use of TDM in IBD with a focus on its use with anti-TNF therapies.
Reactive TDM of anti-TNF therapy
Reactive TDM more rationally guides therapeutic decisions for dealing with loss of response to anti-TNF therapy in IBD and is actually more cost-effective.8,9 Patients with sub-therapeutic or undetectable drug concentrations without ADA derive more benefit from dose escalation (increasing the dose or decreasing the interval) compared to those switched to another anti-TNF agent. On the other hand, patients with therapeutic or supra-therapeutic drug concentrations have better outcomes when changing to a medication with a different mechanism of action (as their disease is probably no longer TNF-driven).3 A recent study showed that trough concentration of adalimumab >4.5 mcg/mL or infliximab >3.8 mcg/mL at time of loss of response identifies patients who benefit more from alternative therapies rather than dose escalation or switching to another anti-TNF agent.10 In clinical practice, in order to fully optimize the original anti-TNF, we will typically dose optimize patients to drug concentrations of infliximab and adalimumab to >10 mcg/mL before giving up and changing medications. Moreover, patients with high ADA titer have better outcomes when switched to another anti-TNF rather than undergo further dose escalation.3 Vande Casteele et al, showed that antibodies to infliximab (ATI) >9.1 U/mL at time of loss of response resulted in a likelihood ratio of 3.6 for an unsuccessful intervention, defined as the need to initiate corticosteroids, immunomodulators (IMM), or other medications or infliximab discontinuation within two infusions after the intervention (shorten of infusion intervals, dose increase to 10 mg/kg, or a combination of both).11 A proposed treatment algorithm for using reactive TDM for anti-TNF therapy is shown in Figure 1.
Proactive TDM of anti-TNF therapy
TDM of thiopurines

Anti-TNF TDM assays

Conclusions
A growing body of evidence demonstrates the clinical utility of TDM of anti-TNF therapy in IBD clinical practice and a move towards personalized medicine, as it is now clear that “one dose does not fit all patients.” Nevertheless, before a TDM-based approach can be widely implemented and emerge as the new standard-of-care for anti-TNF therapy in IBD, several barriers regarding cost issues (insurance coverage and out of pocket expenses), time lag from serum sampling to test results (typically 5 to 10 days), proper interpretation and application of the results, type of assay used, and the optimal timing of serum collection should be overcome. Initiatives are already underway including the development of accurate, easily accessible, and affordable rapid assays that will allow anti-TNF concentration measurement at the point-of-care site and software-decision support tools or ‘dashboards’ that will incorporate a predictive PK model based on patient and disease characteristics.29,30 Additionally, more data from well-designed prospective studies and randomized controlled trials regarding both induction and maintenance treatment and for all available biologics (originators and biosimilars) are urgently needed. A panel consisting of members of the Building Research in Inflammatory Bowel Disease Globally research alliance (www.BRIDGeIBD.com), and recognized leaders in the field of TDM in IBD has recently published recommendations that help clinicians on the appropriate timing and best way to interpret and respond to TDM results depending on the specific clinical scenario.31
Funding: KP received a fellowship grant from the Hellenic Group for the study of IBD.
Potential competing interests: K.P.: nothing to disclose; A.S.C: received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer.
Dr. Papamichail is a research fellow and Dr. Cheifetz is the director of the Center for Inflammatory Bowel Diseases, division of gastroenterology, Beth-Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Papamichail received a fellowship grant from the Hellenic Group for the study of IBD. Dr. Cheifetz received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer.
References
1. Miligkos M, Papamichael K, Casteele NV, et al. Efficacy and safety profile of anti-tumor necrosis factor-alpha versus anti-integrin agents for the treatment of Crohn’s disease: a network meta-analysis of indirect comparisons. Clin Ther. 2016;38(6):1342-1358.e6
2. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182-97
3. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol 2016;7;289-300.
4. Papamichael K, Baert F, Tops S, et al. Post-Induction Adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017;11:53-59
5. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:543-9.
6. Ungar B, Levy I, Yavne Y, et al. Optimizing Anti-TNF-Alpha Therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14:550-557.e2.
7. Singh N, Dubinsky MC. Therapeutic drug monitoring in children and young adults with inflammatory bowel disease: a practical approach. Gastroenterol Hepatol (NY). 2015;11:48-55.
8. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919-27.
9. Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66.
10. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522-30.
11. Casteele NV, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962-71.
12. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996-2003.
13. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320-9.e3.
14. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307.e5.
15. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7.
16. Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:531–8.
17. Singh N, Rosenthal CJ, Melmed GY, et al Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1708-13.
18. Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32.
19. Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016;65:1126–31.
20. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:423-31.e1
21. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014;40:338–53.
22. Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474-81.e2
23. Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 2006;130:1047-53
24. Dassopoulos T, Dubinsky MC, Bentsen JL, et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther 2014;39:163-175.
25. Waljee AK, Joyce JC, Wang S, et al. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol 2010;8:143-150.
26. Yarur A, Kubiliun M, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118-1124.
27. Marini JC, Sendecki J, Cornillie F, et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®.AAPS J. 2016 Sep 6. [Epub ahead of print]. DOI: 10.1208/s12248-016-9981-3
28. Gils A, Vande Casteele N, Poppe R, et al. Development of a universal anti-adalimumab antibody standard for interlaboratory harmonization. Ther Drug Monit. 2014;36:669-673.
29. Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol 2016;7:e206
30. Dubinsky MC, Phan BL, Singh N, et al. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J. 2016 Oct 13. [Epub ahead of print]
31. Melmed GY, Irving PM, Jones J, et al. Appropriateness of testing for anti-tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin Gastroenterol Hepatol 2016;14:1302-9.
32. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601-8.
33. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:514-21.e4.
News from the AGA
Introducing a New, Private Community Just for AGA’s Trainee and Early Career Members
Networking is an important part of your career, between connecting with mentors, gaining valuable referrals and tackling that next rung on the career ladder. AGA created the Early Career Group in the AGA Community to help you connect and network through the forum and directory, but also to provide education tools you’re not going to find anywhere else.
In case you haven’t yet taken a tour, the group creates an open dialogue for trainees and early career members up to seven years out of training. Each month will host a new theme and corresponding presentation, webinars, journal articles or tip sheets, as well as other member-only online events, such as forums with leading experts in the field.
Also, the group’s event calendar will help you stay on top of important deadlines, conferences and possibly even local meet-ups.
Visit http://Community.Gastro.org/EarlyCareerGroup/ today to take advantage of this collaboration space created just for you.
18 GIs to Watch: The Newest Class of AGA Future Leaders
AGA has announced the second class of its Future Leaders Program, which was created in 2015 to identify early career gastroenterologists who have the potential to make a significant impact on the specialty. The 18 gastroenterologists selected to participate in the 2017-2018 program stood out for their current achievements, commitment to advancing the field, and potential for future success.
“AGA relies heavily on the engagement and expertise of volunteer leaders to develop programs that continue to advance our specialty and support our members through changes to the health-care delivery landscape,” said Suzanne Rose, MD, MSEd, AGAF, co-program chair for the AGA Future Leaders Program. “The newest class of AGA Future Leaders shows exceptional promise and dedication to the field, and we look forward to working with these rising stars to cultivate the future leaders of AGA and the field of gastroenterology.”
The AGA Future Leaders Program provides a pathway within AGA for selected participants who seek opportunities to support the gastroenterology profession, advance their careers, connect with potential mentors and develop the leadership skills necessary to serve the organization. During this year-long program, participants will receive leadership training and work closely with AGA mentors on projects linked to AGA’s Strategic Plan.
AGA is pleased to announce the second class of the Future Leaders program:
- Arthur Beyder, MD, PhD, Assistant Professor, Mayo Clinic-Rochester
- Brigid S. Boland, MD, Assistant Adjunct Professor of Medicine, University of California, San Diego
- Lea Ann Chen, MD, Assistant Professor of Medicine, New York University School of Medicine, NY
- Bruno P. Chumpitazi, MD, MPH, Director, Neurogastroenterology and Motility Program, Texas Children’s Hospital/Baylor College of Medicine, Houston, TX
- Matthew A. Ciorba, MD, Assistant Professor of Medicine, Washington University in St. Louis, MO
- Katherine S. Garman, MD, Assistant Professor of Medicine, Duke University Medical Center, Durham, NC
- Christina Y. Ha, MD, Assistant Professor of Medicine, University of Los Angeles, David Geffen School of Medicine, CA
- Bryson W. Katona, MD, MS, PhD, Instructor, University of Pennsylvania, Philadelphia
- Peter S. Liang, MD, MPH, Instructor, NYU/Manhattan VA, New York, NY
- Folasade P. May, MD, PhD, MPhil, Assistant Professor of Medicine, David Geffen School of Medicine at the University of California, Los Angeles; Department of Veterans Affairs, Los Angeles, CA
- Marty M. Meyer, MD, Gastroenterologist, The Ohio State University, Columbus, OH
- Susan N. Ramdhaney, MD, AGAF, Gastroenterologist, President Comprehensive Digestive Care, Manhasset, NY
- Jonathan A. Rosenberg, MD, Gastroenterologist, Illinois Gastroenterology Group, Highland Park
- N. Jewel Samadder, MD, Assistant Professor of Medicine, Huntsman Cancer Institute, Salt Lake City, UT
- Siddharth Singh, MD, Assistant Professor of Medicine, University of California, San Diego
- Maria I. Vazquez-Roque, MD, MSc, Gastroenterologist, Mayo Clinic, Jacksonville, FL
- Sachin B. Wani, MD, Associate Professor of Medicine, University of Colorado, Aurora
- Jennifer Weiss, MD, MS, Assistant Professor, University of Wisconsin School of Medicine and Public Health, Madison
Learn more about the AGA Future Leaders program on the AGA website: www.gastro.org.
New AGA Guidelines
AGA recently released new clinical guidelines that provide evidence-based recommendations to help guide your clinical practice decisions based on rigorous systematic reviews of the medical literature.
AGA Institute Guideline on the Management of Crohn’s Disease After Surgical Resection: AGA developed this guideline, technical review and Clinical Decision Support Tool to outline strategies to reduce disease recurrence in Crohn’s disease patients who have achieved remission following bowel resection. Prevention of endoscopic recurrence, a strong surrogate measure of surgical recurrence, was evaluated for the development of the guideline.
The guidelines are intended to reduce practice variation and promote high-value care. The current evidence supports the early prophylactic use of thiopurines and/or anti-TNF therapy in patients who are at higher risk for clinical recurrence. However, some patients at lower risk may opt for close endoscopic monitoring instead. Although all patients should undergo ileocolonoscopy at six to 12 months after surgical resection, surveillance for endoscopic recurrence is most important for patients not on any pharmacological prophylaxis. In general, those with endoscopic recurrence should undergo treatment with anti-TNF and/or thiopurine therapy.
This guideline is available in the January issue of Gastroenterology.
AGA Institute Guidelines for the Diagnosis and Management of Acute Liver Failure: AGA developed this guideline and technical review to provide recommendations about controversial diagnostic and treatment strategies and predictive models for outcome of acute liver failure (ALF), which have arisen since acute liver failure is difficult to study in randomized clinical trials.
Recommendations include a strong recommendation for the use of N-acetyl cysteine (NAC) in patients with ALF related to acetaminophen, but there remains a lack of data to allow recommendations for testing for Wilson’s disease and varicella zoster virus in patients with ALF. Although there are low-quality data, because there are therapies that may be beneficial in patients with ALF, recommendations to test for herpes simplex virus and autoimmune hepatitis are supported, as is hepatitis E virus testing in pregnant women with ALF.
This guideline is available in the February issue of Gastroenterology.
Announcing New Crohn’s & Colitis Congress
AGA and the Crohn’s & Colitis Foundation are partnering to co-sponsor a new annual conference for health-care professionals and researchers. By joining the nation’s leading IBD patient organization with the premier GI professional organization, this will be the must-attend IBD conference, bringing state-of-the-art comprehensive care together with the latest research to advance prevention, treatment and cures for IBD patients.
Save the date – Jan. 18-20, 2018, in Las Vegas. Get ready to expand your knowledge, network with other leaders, and be inspired! Stay tuned for our website launch and more details coming this spring.
Introducing a New, Private Community Just for AGA’s Trainee and Early Career Members
Networking is an important part of your career, between connecting with mentors, gaining valuable referrals and tackling that next rung on the career ladder. AGA created the Early Career Group in the AGA Community to help you connect and network through the forum and directory, but also to provide education tools you’re not going to find anywhere else.
In case you haven’t yet taken a tour, the group creates an open dialogue for trainees and early career members up to seven years out of training. Each month will host a new theme and corresponding presentation, webinars, journal articles or tip sheets, as well as other member-only online events, such as forums with leading experts in the field.
Also, the group’s event calendar will help you stay on top of important deadlines, conferences and possibly even local meet-ups.
Visit http://Community.Gastro.org/EarlyCareerGroup/ today to take advantage of this collaboration space created just for you.
18 GIs to Watch: The Newest Class of AGA Future Leaders
AGA has announced the second class of its Future Leaders Program, which was created in 2015 to identify early career gastroenterologists who have the potential to make a significant impact on the specialty. The 18 gastroenterologists selected to participate in the 2017-2018 program stood out for their current achievements, commitment to advancing the field, and potential for future success.
“AGA relies heavily on the engagement and expertise of volunteer leaders to develop programs that continue to advance our specialty and support our members through changes to the health-care delivery landscape,” said Suzanne Rose, MD, MSEd, AGAF, co-program chair for the AGA Future Leaders Program. “The newest class of AGA Future Leaders shows exceptional promise and dedication to the field, and we look forward to working with these rising stars to cultivate the future leaders of AGA and the field of gastroenterology.”
The AGA Future Leaders Program provides a pathway within AGA for selected participants who seek opportunities to support the gastroenterology profession, advance their careers, connect with potential mentors and develop the leadership skills necessary to serve the organization. During this year-long program, participants will receive leadership training and work closely with AGA mentors on projects linked to AGA’s Strategic Plan.
AGA is pleased to announce the second class of the Future Leaders program:
- Arthur Beyder, MD, PhD, Assistant Professor, Mayo Clinic-Rochester
- Brigid S. Boland, MD, Assistant Adjunct Professor of Medicine, University of California, San Diego
- Lea Ann Chen, MD, Assistant Professor of Medicine, New York University School of Medicine, NY
- Bruno P. Chumpitazi, MD, MPH, Director, Neurogastroenterology and Motility Program, Texas Children’s Hospital/Baylor College of Medicine, Houston, TX
- Matthew A. Ciorba, MD, Assistant Professor of Medicine, Washington University in St. Louis, MO
- Katherine S. Garman, MD, Assistant Professor of Medicine, Duke University Medical Center, Durham, NC
- Christina Y. Ha, MD, Assistant Professor of Medicine, University of Los Angeles, David Geffen School of Medicine, CA
- Bryson W. Katona, MD, MS, PhD, Instructor, University of Pennsylvania, Philadelphia
- Peter S. Liang, MD, MPH, Instructor, NYU/Manhattan VA, New York, NY
- Folasade P. May, MD, PhD, MPhil, Assistant Professor of Medicine, David Geffen School of Medicine at the University of California, Los Angeles; Department of Veterans Affairs, Los Angeles, CA
- Marty M. Meyer, MD, Gastroenterologist, The Ohio State University, Columbus, OH
- Susan N. Ramdhaney, MD, AGAF, Gastroenterologist, President Comprehensive Digestive Care, Manhasset, NY
- Jonathan A. Rosenberg, MD, Gastroenterologist, Illinois Gastroenterology Group, Highland Park
- N. Jewel Samadder, MD, Assistant Professor of Medicine, Huntsman Cancer Institute, Salt Lake City, UT
- Siddharth Singh, MD, Assistant Professor of Medicine, University of California, San Diego
- Maria I. Vazquez-Roque, MD, MSc, Gastroenterologist, Mayo Clinic, Jacksonville, FL
- Sachin B. Wani, MD, Associate Professor of Medicine, University of Colorado, Aurora
- Jennifer Weiss, MD, MS, Assistant Professor, University of Wisconsin School of Medicine and Public Health, Madison
Learn more about the AGA Future Leaders program on the AGA website: www.gastro.org.
New AGA Guidelines
AGA recently released new clinical guidelines that provide evidence-based recommendations to help guide your clinical practice decisions based on rigorous systematic reviews of the medical literature.
AGA Institute Guideline on the Management of Crohn’s Disease After Surgical Resection: AGA developed this guideline, technical review and Clinical Decision Support Tool to outline strategies to reduce disease recurrence in Crohn’s disease patients who have achieved remission following bowel resection. Prevention of endoscopic recurrence, a strong surrogate measure of surgical recurrence, was evaluated for the development of the guideline.
The guidelines are intended to reduce practice variation and promote high-value care. The current evidence supports the early prophylactic use of thiopurines and/or anti-TNF therapy in patients who are at higher risk for clinical recurrence. However, some patients at lower risk may opt for close endoscopic monitoring instead. Although all patients should undergo ileocolonoscopy at six to 12 months after surgical resection, surveillance for endoscopic recurrence is most important for patients not on any pharmacological prophylaxis. In general, those with endoscopic recurrence should undergo treatment with anti-TNF and/or thiopurine therapy.
This guideline is available in the January issue of Gastroenterology.
AGA Institute Guidelines for the Diagnosis and Management of Acute Liver Failure: AGA developed this guideline and technical review to provide recommendations about controversial diagnostic and treatment strategies and predictive models for outcome of acute liver failure (ALF), which have arisen since acute liver failure is difficult to study in randomized clinical trials.
Recommendations include a strong recommendation for the use of N-acetyl cysteine (NAC) in patients with ALF related to acetaminophen, but there remains a lack of data to allow recommendations for testing for Wilson’s disease and varicella zoster virus in patients with ALF. Although there are low-quality data, because there are therapies that may be beneficial in patients with ALF, recommendations to test for herpes simplex virus and autoimmune hepatitis are supported, as is hepatitis E virus testing in pregnant women with ALF.
This guideline is available in the February issue of Gastroenterology.
Announcing New Crohn’s & Colitis Congress
AGA and the Crohn’s & Colitis Foundation are partnering to co-sponsor a new annual conference for health-care professionals and researchers. By joining the nation’s leading IBD patient organization with the premier GI professional organization, this will be the must-attend IBD conference, bringing state-of-the-art comprehensive care together with the latest research to advance prevention, treatment and cures for IBD patients.
Save the date – Jan. 18-20, 2018, in Las Vegas. Get ready to expand your knowledge, network with other leaders, and be inspired! Stay tuned for our website launch and more details coming this spring.
Introducing a New, Private Community Just for AGA’s Trainee and Early Career Members
Networking is an important part of your career, between connecting with mentors, gaining valuable referrals and tackling that next rung on the career ladder. AGA created the Early Career Group in the AGA Community to help you connect and network through the forum and directory, but also to provide education tools you’re not going to find anywhere else.
In case you haven’t yet taken a tour, the group creates an open dialogue for trainees and early career members up to seven years out of training. Each month will host a new theme and corresponding presentation, webinars, journal articles or tip sheets, as well as other member-only online events, such as forums with leading experts in the field.
Also, the group’s event calendar will help you stay on top of important deadlines, conferences and possibly even local meet-ups.
Visit http://Community.Gastro.org/EarlyCareerGroup/ today to take advantage of this collaboration space created just for you.
18 GIs to Watch: The Newest Class of AGA Future Leaders
AGA has announced the second class of its Future Leaders Program, which was created in 2015 to identify early career gastroenterologists who have the potential to make a significant impact on the specialty. The 18 gastroenterologists selected to participate in the 2017-2018 program stood out for their current achievements, commitment to advancing the field, and potential for future success.
“AGA relies heavily on the engagement and expertise of volunteer leaders to develop programs that continue to advance our specialty and support our members through changes to the health-care delivery landscape,” said Suzanne Rose, MD, MSEd, AGAF, co-program chair for the AGA Future Leaders Program. “The newest class of AGA Future Leaders shows exceptional promise and dedication to the field, and we look forward to working with these rising stars to cultivate the future leaders of AGA and the field of gastroenterology.”
The AGA Future Leaders Program provides a pathway within AGA for selected participants who seek opportunities to support the gastroenterology profession, advance their careers, connect with potential mentors and develop the leadership skills necessary to serve the organization. During this year-long program, participants will receive leadership training and work closely with AGA mentors on projects linked to AGA’s Strategic Plan.
AGA is pleased to announce the second class of the Future Leaders program:
- Arthur Beyder, MD, PhD, Assistant Professor, Mayo Clinic-Rochester
- Brigid S. Boland, MD, Assistant Adjunct Professor of Medicine, University of California, San Diego
- Lea Ann Chen, MD, Assistant Professor of Medicine, New York University School of Medicine, NY
- Bruno P. Chumpitazi, MD, MPH, Director, Neurogastroenterology and Motility Program, Texas Children’s Hospital/Baylor College of Medicine, Houston, TX
- Matthew A. Ciorba, MD, Assistant Professor of Medicine, Washington University in St. Louis, MO
- Katherine S. Garman, MD, Assistant Professor of Medicine, Duke University Medical Center, Durham, NC
- Christina Y. Ha, MD, Assistant Professor of Medicine, University of Los Angeles, David Geffen School of Medicine, CA
- Bryson W. Katona, MD, MS, PhD, Instructor, University of Pennsylvania, Philadelphia
- Peter S. Liang, MD, MPH, Instructor, NYU/Manhattan VA, New York, NY
- Folasade P. May, MD, PhD, MPhil, Assistant Professor of Medicine, David Geffen School of Medicine at the University of California, Los Angeles; Department of Veterans Affairs, Los Angeles, CA
- Marty M. Meyer, MD, Gastroenterologist, The Ohio State University, Columbus, OH
- Susan N. Ramdhaney, MD, AGAF, Gastroenterologist, President Comprehensive Digestive Care, Manhasset, NY
- Jonathan A. Rosenberg, MD, Gastroenterologist, Illinois Gastroenterology Group, Highland Park
- N. Jewel Samadder, MD, Assistant Professor of Medicine, Huntsman Cancer Institute, Salt Lake City, UT
- Siddharth Singh, MD, Assistant Professor of Medicine, University of California, San Diego
- Maria I. Vazquez-Roque, MD, MSc, Gastroenterologist, Mayo Clinic, Jacksonville, FL
- Sachin B. Wani, MD, Associate Professor of Medicine, University of Colorado, Aurora
- Jennifer Weiss, MD, MS, Assistant Professor, University of Wisconsin School of Medicine and Public Health, Madison
Learn more about the AGA Future Leaders program on the AGA website: www.gastro.org.
New AGA Guidelines
AGA recently released new clinical guidelines that provide evidence-based recommendations to help guide your clinical practice decisions based on rigorous systematic reviews of the medical literature.
AGA Institute Guideline on the Management of Crohn’s Disease After Surgical Resection: AGA developed this guideline, technical review and Clinical Decision Support Tool to outline strategies to reduce disease recurrence in Crohn’s disease patients who have achieved remission following bowel resection. Prevention of endoscopic recurrence, a strong surrogate measure of surgical recurrence, was evaluated for the development of the guideline.
The guidelines are intended to reduce practice variation and promote high-value care. The current evidence supports the early prophylactic use of thiopurines and/or anti-TNF therapy in patients who are at higher risk for clinical recurrence. However, some patients at lower risk may opt for close endoscopic monitoring instead. Although all patients should undergo ileocolonoscopy at six to 12 months after surgical resection, surveillance for endoscopic recurrence is most important for patients not on any pharmacological prophylaxis. In general, those with endoscopic recurrence should undergo treatment with anti-TNF and/or thiopurine therapy.
This guideline is available in the January issue of Gastroenterology.
AGA Institute Guidelines for the Diagnosis and Management of Acute Liver Failure: AGA developed this guideline and technical review to provide recommendations about controversial diagnostic and treatment strategies and predictive models for outcome of acute liver failure (ALF), which have arisen since acute liver failure is difficult to study in randomized clinical trials.
Recommendations include a strong recommendation for the use of N-acetyl cysteine (NAC) in patients with ALF related to acetaminophen, but there remains a lack of data to allow recommendations for testing for Wilson’s disease and varicella zoster virus in patients with ALF. Although there are low-quality data, because there are therapies that may be beneficial in patients with ALF, recommendations to test for herpes simplex virus and autoimmune hepatitis are supported, as is hepatitis E virus testing in pregnant women with ALF.
This guideline is available in the February issue of Gastroenterology.
Announcing New Crohn’s & Colitis Congress
AGA and the Crohn’s & Colitis Foundation are partnering to co-sponsor a new annual conference for health-care professionals and researchers. By joining the nation’s leading IBD patient organization with the premier GI professional organization, this will be the must-attend IBD conference, bringing state-of-the-art comprehensive care together with the latest research to advance prevention, treatment and cures for IBD patients.
Save the date – Jan. 18-20, 2018, in Las Vegas. Get ready to expand your knowledge, network with other leaders, and be inspired! Stay tuned for our website launch and more details coming this spring.
An 87-Year-Old Woman With Recurrent Dysphagia
The correct answer is C: lymphocytic esophagitis.
References
1. Rubio, C.A., Sjodahl, K., Lagergren, J. Lymphocytic esophagitis: A histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432-7.
2. Cohen, S., Saxena, A., Waljee, A.K., et al. Lymphocytic esophagitis: A diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828-32.
3. Haque, S., Genta, R.M. Lymphocytic oesophagitis: Clinicopathological aspects of an emerging condition. Gut. 2012;61:1108-14.
This article has an accompanying continuing medical education activity, also eligible for MOC credit (see Gastroenterology website for details). Learning Objective: Upon completion of this teaching case and questions, the learners will be able to identify one typical clinical and endoscopic presentation of the entity lymphocytic esophagitis, distinguish its histological pattern from other esophageal disorders and recognize a variety of other clinical presentations of this condition.
The correct answer is C: lymphocytic esophagitis.
References
1. Rubio, C.A., Sjodahl, K., Lagergren, J. Lymphocytic esophagitis: A histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432-7.
2. Cohen, S., Saxena, A., Waljee, A.K., et al. Lymphocytic esophagitis: A diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828-32.
3. Haque, S., Genta, R.M. Lymphocytic oesophagitis: Clinicopathological aspects of an emerging condition. Gut. 2012;61:1108-14.
This article has an accompanying continuing medical education activity, also eligible for MOC credit (see Gastroenterology website for details). Learning Objective: Upon completion of this teaching case and questions, the learners will be able to identify one typical clinical and endoscopic presentation of the entity lymphocytic esophagitis, distinguish its histological pattern from other esophageal disorders and recognize a variety of other clinical presentations of this condition.
The correct answer is C: lymphocytic esophagitis.
References
1. Rubio, C.A., Sjodahl, K., Lagergren, J. Lymphocytic esophagitis: A histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432-7.
2. Cohen, S., Saxena, A., Waljee, A.K., et al. Lymphocytic esophagitis: A diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828-32.
3. Haque, S., Genta, R.M. Lymphocytic oesophagitis: Clinicopathological aspects of an emerging condition. Gut. 2012;61:1108-14.
This article has an accompanying continuing medical education activity, also eligible for MOC credit (see Gastroenterology website for details). Learning Objective: Upon completion of this teaching case and questions, the learners will be able to identify one typical clinical and endoscopic presentation of the entity lymphocytic esophagitis, distinguish its histological pattern from other esophageal disorders and recognize a variety of other clinical presentations of this condition.
Previously Published in Gastroenterology (2016;151:1085-6)
An 87-year-old woman was referred due to dysphagia that had been present for several years. Three years prior to this presentation she had undergone an esophagogastroduodenoscopy (EGD) on the same indication showing a proximal and a distal esophageal benign-appearing stricture but no signs of esophagitis. Both were dilated and biopsied. Histopathology showed infiltration with lymphocytes and neutrophilic granulocytes, and superficially fungal hyphae and spores. No predominance of eosinophilic granulocytes was noted. A proton-pump inhibitor was prescribed and she was scheduled for a control gastroscopy, but was lost to follow-up. She was otherwise healthy without any allergies.
Upon re-presentation, she was under treatment with pantoprazole 40 mg OD. Upon EGD a spiral-shaped proximal esophageal stricture with normal-appearing mucosa only passable with a nasal endoscope was observed. The rest of the esophagus was seen with mucosal concentric rings (Figure A; video). The esophageal mucosa was otherwise endoscopically normal throughout. Biopsies were taken from the distal and proximal esophagus. Balloon dilation of the proximal stricture was performed (CRE, Boston Scientific) to 13.5 mm (video). Subsequently, a standard gastroscope could be passed to the duodenum revealing normal-appearing gastric and duodenal mucosa.
Dr. Havre and Dr. Kalaitzakis are in the Endoscopy Unit of Copenhagen University Hospital/Herlev, University of Copenhagen. Ms. Hallager is in the department of pathology, Copenhagen University Hospital/Herlev. The authors disclose no conflicts.
Special IBD-themed issue
Dear Colleagues,
Inflammatory bowel disease (IBD) is becoming an increasingly important part of GI practice and it is certainly an exciting time to be involved in the field. While new IBD therapeutics often get most of the attention, there are many other issues surrounding IBD care that are important for all of us. This special IBD-themed issue of The New Gastroenterologist provides expert opinions addressing some of these other, important issues that are critical to both the care of IBD patients and the development of an effective IBD practice.
In this issue of The New Gastroenterologist, we also have several articles that will be very helpful to those who either have or are developing a practice with a significant IBD focus. First, Douglas Wolf (Atlanta Gastroenterology Associates) discusses the steps necessary to build a successful IBD practice, and, additionally, Nitin Gupta (University of Mississippi Medical Center) provides some useful tips to help physicians start collaborations with industry.
As MACRA looms over us all, it is only a matter of time before we will all have to firmly understand its intricacies. The implementation of MACRA and MIPS will undoubtedly affect quality measures in IBD and to help all of us understand the complexities of this issue, Ryan McConnell and Fernando Velayos (University of California, San Francisco) provide an overview of quality measures in IBD. Finally, although treatment, monitoring, and quality are all important in the care of IBD patients, so also are the relationships that we develop with our IBD patients. To give us input on this topic from a patient perspective, a group of IBD patients from the Crohn’s and Colitis Foundation of America address what we as physicians can do to enhance our doctor-patient relationships.
If you want to read The New Gastroenterologist “on the go,” please download our free app, or read our electronic version on www.mdedge.com/gihepnews or www.gastro.org. Additionally, if you have other topics you would be interested in reading about, or if you are interested in contributing to future issues, please e-mail me at [email protected] or The New Gastroenterologist’s managing editor Ryan Farrell at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor-In-Chief
Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania
Dear Colleagues,
Inflammatory bowel disease (IBD) is becoming an increasingly important part of GI practice and it is certainly an exciting time to be involved in the field. While new IBD therapeutics often get most of the attention, there are many other issues surrounding IBD care that are important for all of us. This special IBD-themed issue of The New Gastroenterologist provides expert opinions addressing some of these other, important issues that are critical to both the care of IBD patients and the development of an effective IBD practice.
In this issue of The New Gastroenterologist, we also have several articles that will be very helpful to those who either have or are developing a practice with a significant IBD focus. First, Douglas Wolf (Atlanta Gastroenterology Associates) discusses the steps necessary to build a successful IBD practice, and, additionally, Nitin Gupta (University of Mississippi Medical Center) provides some useful tips to help physicians start collaborations with industry.
As MACRA looms over us all, it is only a matter of time before we will all have to firmly understand its intricacies. The implementation of MACRA and MIPS will undoubtedly affect quality measures in IBD and to help all of us understand the complexities of this issue, Ryan McConnell and Fernando Velayos (University of California, San Francisco) provide an overview of quality measures in IBD. Finally, although treatment, monitoring, and quality are all important in the care of IBD patients, so also are the relationships that we develop with our IBD patients. To give us input on this topic from a patient perspective, a group of IBD patients from the Crohn’s and Colitis Foundation of America address what we as physicians can do to enhance our doctor-patient relationships.
If you want to read The New Gastroenterologist “on the go,” please download our free app, or read our electronic version on www.mdedge.com/gihepnews or www.gastro.org. Additionally, if you have other topics you would be interested in reading about, or if you are interested in contributing to future issues, please e-mail me at [email protected] or The New Gastroenterologist’s managing editor Ryan Farrell at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor-In-Chief
Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania
Dear Colleagues,
Inflammatory bowel disease (IBD) is becoming an increasingly important part of GI practice and it is certainly an exciting time to be involved in the field. While new IBD therapeutics often get most of the attention, there are many other issues surrounding IBD care that are important for all of us. This special IBD-themed issue of The New Gastroenterologist provides expert opinions addressing some of these other, important issues that are critical to both the care of IBD patients and the development of an effective IBD practice.
In this issue of The New Gastroenterologist, we also have several articles that will be very helpful to those who either have or are developing a practice with a significant IBD focus. First, Douglas Wolf (Atlanta Gastroenterology Associates) discusses the steps necessary to build a successful IBD practice, and, additionally, Nitin Gupta (University of Mississippi Medical Center) provides some useful tips to help physicians start collaborations with industry.
As MACRA looms over us all, it is only a matter of time before we will all have to firmly understand its intricacies. The implementation of MACRA and MIPS will undoubtedly affect quality measures in IBD and to help all of us understand the complexities of this issue, Ryan McConnell and Fernando Velayos (University of California, San Francisco) provide an overview of quality measures in IBD. Finally, although treatment, monitoring, and quality are all important in the care of IBD patients, so also are the relationships that we develop with our IBD patients. To give us input on this topic from a patient perspective, a group of IBD patients from the Crohn’s and Colitis Foundation of America address what we as physicians can do to enhance our doctor-patient relationships.
If you want to read The New Gastroenterologist “on the go,” please download our free app, or read our electronic version on www.mdedge.com/gihepnews or www.gastro.org. Additionally, if you have other topics you would be interested in reading about, or if you are interested in contributing to future issues, please e-mail me at [email protected] or The New Gastroenterologist’s managing editor Ryan Farrell at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor-In-Chief
Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania
Tales from a GI Hospitalist
What is a GI hospitalist?
A GI hospitalist is a gastroenterologist that primarily provides inpatient care. Their main professional focus is the acute management of gastrointestinal conditions occurring in the hospital setting.
How prevalent are subspecialty hospitalists?
The rise of hospitalists has changed the landscape of medicine. The hospitalist is now the central inpatient provider responsible for patient care and day-to-day housestaff education. From 1995 to 2016, the number of hospitalists increased from 500 to over 50,000.1 While the majority of hospitalists are generalists from the fields of internal medicine, pediatrics, and obstetrics/gynecology, some come in the form of specialists. In a recent survey, up to 10% of internal medicine subspecialists already consider themselves “hospitalists.”2 However, most of these self-described hospitalists only do so part of the time. For example, many group practices have one of their members manage all the hospitalized patients for the group for certain periods of time. It is rare to find full-time subspecialist hospitalists, but there has been an emergence in this new model of GI practice. Many people are unaware of this system of care nor understand how it may influence hospital-based care.
What is the role of a GI hospitalist?
While my primary responsibility is to care for inpatients whom require GI consults, I have outpatient and administrative responsibilities. Generally speaking, I am the de facto consult attending for the year.
How did you decide to become a GI hospitalist?
Upon graduation from my GI fellowship, I wanted an academic job where I could work closely with fellows and manage a wide breadth of complex, high-acuity patients. During fellowship, I enjoyed all areas of gastroenterology and hepatology and did not “sub-subspecialize.” As such, I wanted a job where I would see the full spectrum of GI and liver disease. Additionally, I enjoyed seeing the sickest patients, because I felt I could make the most dramatic differences with my care.
When I was searching for jobs, I spoke with the chief of GI at the hospital where I completed my residency about how I could fill a niche. We conceived of a model that would merge my personal interests and help the division provide consistent teaching for fellows and increase inpatient billing. Prior to my arrival, attendings that staffed the consult service were expected to continue their research and outpatient clinical workload while finding time to come to the hospital. Not surprisingly, attending rounds was erratic. The fellows were left to manage patients independently, scrambled to run cases by whomever happened to be around, or waited until they could reach the attending the next day. Unsurprisingly, billing by attendings was sparse.
What is a typical day like in your life as a GI hospitalist?
My day starts at 7:30 a.m. either with my outpatient office hours, endoscopy session, or GI Grand Rounds. Each week, I have two morning outpatient office sessions, one morning endoscopy session, and one morning session supervising fellows’ endoscopy.
At noon, I round with a team of GI fellows, medical students, and housestaff rotators for 2 hours. After we see the new consults, the remainder of my afternoon is spent seeing the follow-up patients. For two afternoons throughout the week, I have outpatient endoscopy sessions. I typically conclude my day at 5 p.m.
For night coverage, I take emergency calls for my own patients, and share general call duties with the other members of my division. On average, I take calls for one weekday a month and five weekends per year.
Typically, GI hospitalists only cover inpatients during the daytime. All nights and weekends are covered by partners and nonemergent overnight consults are saved until the next day. They have no office work.
What is the most challenging part of being a GI hospitalist?
As the perpetual “GI Consult Attending,” there is the threat of burnout when confronted with a high volume of sick, complex patients. Many of the patients have multiple comorbidities and require a multidisciplinary approach. On average, we have five new consults a day and the number of active follow-up patients is 10. Nonetheless, the nature of the inpatient service makes the volume of work unpredictable. When the service is busy and the census swells, the numbers of patients requiring staffing and notes can become overwhelming.
Importantly, for those without an outpatient practice, one loses the opportunity to develop longitudinal relationships with patients. Additionally, one also loses the ability to provide integrated, comprehensive care for individual patients once they leave the hospital.
How are you paid?
My compensation is based on a base salary with an incentivized system based on my RVUs and collections. For the dedicated hospitalist for a group practice, there is typically a base salary and productivity-based income. Additionally, there should be a path to partnership. Lastly, in balancing the ledger, the diminished inpatient revenue stream is offset by the lack of overhead.
What are the benefits of a GI hospitalist system?
Our system benefits the workflow for the GI fellows. Since I have started, the GI consultation rounds start at a consistent time. During these rounds, we discuss relevant GI literature and make timely plans on all patients. Oftentimes, I am able to supervise the fellows so they can fit in a scope before the end of the workday. Ultimately, the fellows know they can find me and discuss patients throughout the day. The fellows consistently have told me that the since the implementation of the hospitalist system, there has been a dramatic difference. Collectively, they feel both their education and patient care have improved.
In terms of consult efficiency, one study demonstrated that the transition to a GI hospitalist system resulted in a mean decrease in consult to urgent esophagogastroduodenoscopy (EGD) time from approximately 24 to 14 hours.3 However, this occurred in the context of a lower inpatient consult volume and only covered 2 months. Furthermore, the time from admission to EGD did not change. Nonetheless, further studies are needed to examine the impact of this model shift.
In terms of a financial benefit, at our institution the total gross inpatient charges increased more than $850,000 for the year. This was largely attributable to the 79% increase in the gross charges from follow-up notes.
For group practices, the hospitalist system makes more efficient use of physician’s time. Physicians can either focus on outpatients or inpatients without worrying about going between the office, ambulatory surgical center, and the hospital. In general, inpatients require a disproportionate amount of time relative to the revenue collected. Furthermore, by eliminating the need for group physicians to go to the hospital, they can carve out 1-2 hours of office time to increase billing.
When there is one point-person whom handles all inpatient GI, communication is facilitated among primary teams and other services. The GI hospitalist develops working relationships with surgeons, radiologists, anesthesiologists, intensivists, etc. Teams can often just text or call me directly, instead of looking for the covering attending or going through the office phone service.
What are drawbacks to the GI hospitalist model?
Since there is only one gastroenterologist in the hospitalist model, if that person is not doing a good job, it affects the management of GI conditions for the entire hospital.
There is a loss of continuity-of-care. When GI patients get admitted, the gastroenterologists responsible for their care will not be the person with whom they have a long-term relationship. Furthermore, when the patient gets discharged, the primary gastroenterologists will not be fully aware of the inpatient course.
Also, when outpatient and inpatient gastroenterologists become segregated based on hospital setting, they each lose out of learning the intricacies of managing patients in a different context.
What do you like most about being a GI hospitalist?
The GI hospitalist position creates a great opportunity for gastroenterologists to make a remarkable, immediate impact on interesting, high acuity patients. The nature of the job also has the advantage of providing reasonable hours. This may be attractive to many whom want a better work-life balance.
Dr. Wan is assistant professor of medicine, associate program director, GI Fellowship Program, New York Presbyterian/Weill Cornell Medical Center, New York, N.Y.
References
1. Wachter R.M., Goldman L. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Engl J Med. 2016 Sep 15;375[11]:1009-11.
2. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. Analysis in Brief. Available at: https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed May 1st, 2016.
3. Mahadev S., Lebwohl B., Ramirez I., Garcia-Carrasquillo R.J., Freedberg, D.E. Transition to a GI Hospitalist System is Associated with Expedited Upper Endoscopy. Gastroenterology. 2016;150[4]:S639-40.
What is a GI hospitalist?
A GI hospitalist is a gastroenterologist that primarily provides inpatient care. Their main professional focus is the acute management of gastrointestinal conditions occurring in the hospital setting.
How prevalent are subspecialty hospitalists?
The rise of hospitalists has changed the landscape of medicine. The hospitalist is now the central inpatient provider responsible for patient care and day-to-day housestaff education. From 1995 to 2016, the number of hospitalists increased from 500 to over 50,000.1 While the majority of hospitalists are generalists from the fields of internal medicine, pediatrics, and obstetrics/gynecology, some come in the form of specialists. In a recent survey, up to 10% of internal medicine subspecialists already consider themselves “hospitalists.”2 However, most of these self-described hospitalists only do so part of the time. For example, many group practices have one of their members manage all the hospitalized patients for the group for certain periods of time. It is rare to find full-time subspecialist hospitalists, but there has been an emergence in this new model of GI practice. Many people are unaware of this system of care nor understand how it may influence hospital-based care.
What is the role of a GI hospitalist?
While my primary responsibility is to care for inpatients whom require GI consults, I have outpatient and administrative responsibilities. Generally speaking, I am the de facto consult attending for the year.
How did you decide to become a GI hospitalist?
Upon graduation from my GI fellowship, I wanted an academic job where I could work closely with fellows and manage a wide breadth of complex, high-acuity patients. During fellowship, I enjoyed all areas of gastroenterology and hepatology and did not “sub-subspecialize.” As such, I wanted a job where I would see the full spectrum of GI and liver disease. Additionally, I enjoyed seeing the sickest patients, because I felt I could make the most dramatic differences with my care.
When I was searching for jobs, I spoke with the chief of GI at the hospital where I completed my residency about how I could fill a niche. We conceived of a model that would merge my personal interests and help the division provide consistent teaching for fellows and increase inpatient billing. Prior to my arrival, attendings that staffed the consult service were expected to continue their research and outpatient clinical workload while finding time to come to the hospital. Not surprisingly, attending rounds was erratic. The fellows were left to manage patients independently, scrambled to run cases by whomever happened to be around, or waited until they could reach the attending the next day. Unsurprisingly, billing by attendings was sparse.
What is a typical day like in your life as a GI hospitalist?
My day starts at 7:30 a.m. either with my outpatient office hours, endoscopy session, or GI Grand Rounds. Each week, I have two morning outpatient office sessions, one morning endoscopy session, and one morning session supervising fellows’ endoscopy.
At noon, I round with a team of GI fellows, medical students, and housestaff rotators for 2 hours. After we see the new consults, the remainder of my afternoon is spent seeing the follow-up patients. For two afternoons throughout the week, I have outpatient endoscopy sessions. I typically conclude my day at 5 p.m.
For night coverage, I take emergency calls for my own patients, and share general call duties with the other members of my division. On average, I take calls for one weekday a month and five weekends per year.
Typically, GI hospitalists only cover inpatients during the daytime. All nights and weekends are covered by partners and nonemergent overnight consults are saved until the next day. They have no office work.
What is the most challenging part of being a GI hospitalist?
As the perpetual “GI Consult Attending,” there is the threat of burnout when confronted with a high volume of sick, complex patients. Many of the patients have multiple comorbidities and require a multidisciplinary approach. On average, we have five new consults a day and the number of active follow-up patients is 10. Nonetheless, the nature of the inpatient service makes the volume of work unpredictable. When the service is busy and the census swells, the numbers of patients requiring staffing and notes can become overwhelming.
Importantly, for those without an outpatient practice, one loses the opportunity to develop longitudinal relationships with patients. Additionally, one also loses the ability to provide integrated, comprehensive care for individual patients once they leave the hospital.
How are you paid?
My compensation is based on a base salary with an incentivized system based on my RVUs and collections. For the dedicated hospitalist for a group practice, there is typically a base salary and productivity-based income. Additionally, there should be a path to partnership. Lastly, in balancing the ledger, the diminished inpatient revenue stream is offset by the lack of overhead.
What are the benefits of a GI hospitalist system?
Our system benefits the workflow for the GI fellows. Since I have started, the GI consultation rounds start at a consistent time. During these rounds, we discuss relevant GI literature and make timely plans on all patients. Oftentimes, I am able to supervise the fellows so they can fit in a scope before the end of the workday. Ultimately, the fellows know they can find me and discuss patients throughout the day. The fellows consistently have told me that the since the implementation of the hospitalist system, there has been a dramatic difference. Collectively, they feel both their education and patient care have improved.
In terms of consult efficiency, one study demonstrated that the transition to a GI hospitalist system resulted in a mean decrease in consult to urgent esophagogastroduodenoscopy (EGD) time from approximately 24 to 14 hours.3 However, this occurred in the context of a lower inpatient consult volume and only covered 2 months. Furthermore, the time from admission to EGD did not change. Nonetheless, further studies are needed to examine the impact of this model shift.
In terms of a financial benefit, at our institution the total gross inpatient charges increased more than $850,000 for the year. This was largely attributable to the 79% increase in the gross charges from follow-up notes.
For group practices, the hospitalist system makes more efficient use of physician’s time. Physicians can either focus on outpatients or inpatients without worrying about going between the office, ambulatory surgical center, and the hospital. In general, inpatients require a disproportionate amount of time relative to the revenue collected. Furthermore, by eliminating the need for group physicians to go to the hospital, they can carve out 1-2 hours of office time to increase billing.
When there is one point-person whom handles all inpatient GI, communication is facilitated among primary teams and other services. The GI hospitalist develops working relationships with surgeons, radiologists, anesthesiologists, intensivists, etc. Teams can often just text or call me directly, instead of looking for the covering attending or going through the office phone service.
What are drawbacks to the GI hospitalist model?
Since there is only one gastroenterologist in the hospitalist model, if that person is not doing a good job, it affects the management of GI conditions for the entire hospital.
There is a loss of continuity-of-care. When GI patients get admitted, the gastroenterologists responsible for their care will not be the person with whom they have a long-term relationship. Furthermore, when the patient gets discharged, the primary gastroenterologists will not be fully aware of the inpatient course.
Also, when outpatient and inpatient gastroenterologists become segregated based on hospital setting, they each lose out of learning the intricacies of managing patients in a different context.
What do you like most about being a GI hospitalist?
The GI hospitalist position creates a great opportunity for gastroenterologists to make a remarkable, immediate impact on interesting, high acuity patients. The nature of the job also has the advantage of providing reasonable hours. This may be attractive to many whom want a better work-life balance.
Dr. Wan is assistant professor of medicine, associate program director, GI Fellowship Program, New York Presbyterian/Weill Cornell Medical Center, New York, N.Y.
References
1. Wachter R.M., Goldman L. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Engl J Med. 2016 Sep 15;375[11]:1009-11.
2. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. Analysis in Brief. Available at: https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed May 1st, 2016.
3. Mahadev S., Lebwohl B., Ramirez I., Garcia-Carrasquillo R.J., Freedberg, D.E. Transition to a GI Hospitalist System is Associated with Expedited Upper Endoscopy. Gastroenterology. 2016;150[4]:S639-40.
What is a GI hospitalist?
A GI hospitalist is a gastroenterologist that primarily provides inpatient care. Their main professional focus is the acute management of gastrointestinal conditions occurring in the hospital setting.
How prevalent are subspecialty hospitalists?
The rise of hospitalists has changed the landscape of medicine. The hospitalist is now the central inpatient provider responsible for patient care and day-to-day housestaff education. From 1995 to 2016, the number of hospitalists increased from 500 to over 50,000.1 While the majority of hospitalists are generalists from the fields of internal medicine, pediatrics, and obstetrics/gynecology, some come in the form of specialists. In a recent survey, up to 10% of internal medicine subspecialists already consider themselves “hospitalists.”2 However, most of these self-described hospitalists only do so part of the time. For example, many group practices have one of their members manage all the hospitalized patients for the group for certain periods of time. It is rare to find full-time subspecialist hospitalists, but there has been an emergence in this new model of GI practice. Many people are unaware of this system of care nor understand how it may influence hospital-based care.
What is the role of a GI hospitalist?
While my primary responsibility is to care for inpatients whom require GI consults, I have outpatient and administrative responsibilities. Generally speaking, I am the de facto consult attending for the year.
How did you decide to become a GI hospitalist?
Upon graduation from my GI fellowship, I wanted an academic job where I could work closely with fellows and manage a wide breadth of complex, high-acuity patients. During fellowship, I enjoyed all areas of gastroenterology and hepatology and did not “sub-subspecialize.” As such, I wanted a job where I would see the full spectrum of GI and liver disease. Additionally, I enjoyed seeing the sickest patients, because I felt I could make the most dramatic differences with my care.
When I was searching for jobs, I spoke with the chief of GI at the hospital where I completed my residency about how I could fill a niche. We conceived of a model that would merge my personal interests and help the division provide consistent teaching for fellows and increase inpatient billing. Prior to my arrival, attendings that staffed the consult service were expected to continue their research and outpatient clinical workload while finding time to come to the hospital. Not surprisingly, attending rounds was erratic. The fellows were left to manage patients independently, scrambled to run cases by whomever happened to be around, or waited until they could reach the attending the next day. Unsurprisingly, billing by attendings was sparse.
What is a typical day like in your life as a GI hospitalist?
My day starts at 7:30 a.m. either with my outpatient office hours, endoscopy session, or GI Grand Rounds. Each week, I have two morning outpatient office sessions, one morning endoscopy session, and one morning session supervising fellows’ endoscopy.
At noon, I round with a team of GI fellows, medical students, and housestaff rotators for 2 hours. After we see the new consults, the remainder of my afternoon is spent seeing the follow-up patients. For two afternoons throughout the week, I have outpatient endoscopy sessions. I typically conclude my day at 5 p.m.
For night coverage, I take emergency calls for my own patients, and share general call duties with the other members of my division. On average, I take calls for one weekday a month and five weekends per year.
Typically, GI hospitalists only cover inpatients during the daytime. All nights and weekends are covered by partners and nonemergent overnight consults are saved until the next day. They have no office work.
What is the most challenging part of being a GI hospitalist?
As the perpetual “GI Consult Attending,” there is the threat of burnout when confronted with a high volume of sick, complex patients. Many of the patients have multiple comorbidities and require a multidisciplinary approach. On average, we have five new consults a day and the number of active follow-up patients is 10. Nonetheless, the nature of the inpatient service makes the volume of work unpredictable. When the service is busy and the census swells, the numbers of patients requiring staffing and notes can become overwhelming.
Importantly, for those without an outpatient practice, one loses the opportunity to develop longitudinal relationships with patients. Additionally, one also loses the ability to provide integrated, comprehensive care for individual patients once they leave the hospital.
How are you paid?
My compensation is based on a base salary with an incentivized system based on my RVUs and collections. For the dedicated hospitalist for a group practice, there is typically a base salary and productivity-based income. Additionally, there should be a path to partnership. Lastly, in balancing the ledger, the diminished inpatient revenue stream is offset by the lack of overhead.
What are the benefits of a GI hospitalist system?
Our system benefits the workflow for the GI fellows. Since I have started, the GI consultation rounds start at a consistent time. During these rounds, we discuss relevant GI literature and make timely plans on all patients. Oftentimes, I am able to supervise the fellows so they can fit in a scope before the end of the workday. Ultimately, the fellows know they can find me and discuss patients throughout the day. The fellows consistently have told me that the since the implementation of the hospitalist system, there has been a dramatic difference. Collectively, they feel both their education and patient care have improved.
In terms of consult efficiency, one study demonstrated that the transition to a GI hospitalist system resulted in a mean decrease in consult to urgent esophagogastroduodenoscopy (EGD) time from approximately 24 to 14 hours.3 However, this occurred in the context of a lower inpatient consult volume and only covered 2 months. Furthermore, the time from admission to EGD did not change. Nonetheless, further studies are needed to examine the impact of this model shift.
In terms of a financial benefit, at our institution the total gross inpatient charges increased more than $850,000 for the year. This was largely attributable to the 79% increase in the gross charges from follow-up notes.
For group practices, the hospitalist system makes more efficient use of physician’s time. Physicians can either focus on outpatients or inpatients without worrying about going between the office, ambulatory surgical center, and the hospital. In general, inpatients require a disproportionate amount of time relative to the revenue collected. Furthermore, by eliminating the need for group physicians to go to the hospital, they can carve out 1-2 hours of office time to increase billing.
When there is one point-person whom handles all inpatient GI, communication is facilitated among primary teams and other services. The GI hospitalist develops working relationships with surgeons, radiologists, anesthesiologists, intensivists, etc. Teams can often just text or call me directly, instead of looking for the covering attending or going through the office phone service.
What are drawbacks to the GI hospitalist model?
Since there is only one gastroenterologist in the hospitalist model, if that person is not doing a good job, it affects the management of GI conditions for the entire hospital.
There is a loss of continuity-of-care. When GI patients get admitted, the gastroenterologists responsible for their care will not be the person with whom they have a long-term relationship. Furthermore, when the patient gets discharged, the primary gastroenterologists will not be fully aware of the inpatient course.
Also, when outpatient and inpatient gastroenterologists become segregated based on hospital setting, they each lose out of learning the intricacies of managing patients in a different context.
What do you like most about being a GI hospitalist?
The GI hospitalist position creates a great opportunity for gastroenterologists to make a remarkable, immediate impact on interesting, high acuity patients. The nature of the job also has the advantage of providing reasonable hours. This may be attractive to many whom want a better work-life balance.
Dr. Wan is assistant professor of medicine, associate program director, GI Fellowship Program, New York Presbyterian/Weill Cornell Medical Center, New York, N.Y.
References
1. Wachter R.M., Goldman L. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Engl J Med. 2016 Sep 15;375[11]:1009-11.
2. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. Analysis in Brief. Available at: https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed May 1st, 2016.
3. Mahadev S., Lebwohl B., Ramirez I., Garcia-Carrasquillo R.J., Freedberg, D.E. Transition to a GI Hospitalist System is Associated with Expedited Upper Endoscopy. Gastroenterology. 2016;150[4]:S639-40.
Update on the Management of Acute Pancreatitis and Its Complications
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.
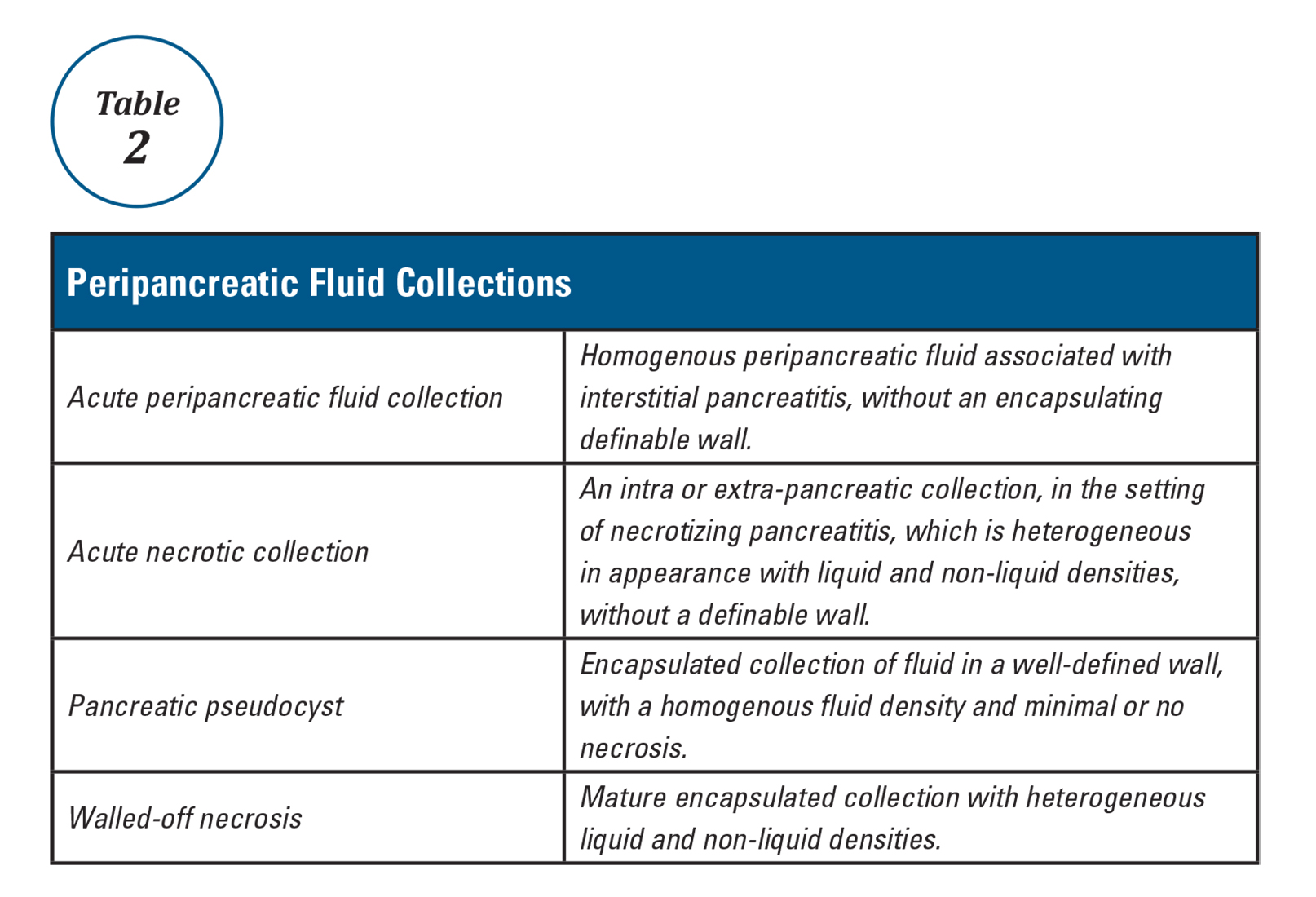
Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.

Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.

Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
The AGA Trainee and Early Career Committee – Shaping the Young GI Experience
AGA’s focus on young GIs
The AGA Trainee and Early Career Committee (formerly Trainee and Young GI Committee) is composed of 12 trainee and early-career AGA members and meets twice a year to develop programs and events specifically targeted to trainees and gastroenterologists (GIs) in their first five years out of fellowship training. The committee was formed by the AGA in February 2013 to address the specific needs of early-career GI professionals and to develop programs to expose younger members to all that the AGA has to offer. The new committee also became a creative space to organize efforts to increase membership among early-career GIs. Trainee and Early Career Committee members are selected for 2-year terms and represent fellowship training programs, universities, and practices from around the nation. Each committee member serves simultaneously on one other AGA committee, which gives young GIs additional opportunities for leadership roles. The committee meets regularly with AGA staff and a governing board liaison to discuss committee goals and the issues most relevant to physicians during and directly after GI fellowship training. The committee also provides feedback to other committees about how programs and initiatives might involve or impact GI fellows and recent graduates. The result is a unique focus group where young GIs from all over the country work collectively to improve the young GI experience through flagship programs like the Regional Practice Skills Workshop, the Young Delegates Program, and Trainee and Early Career events at Digestive Disease Week (DDW)®.
AGA Regional Practice Skills Workshops
The workshop agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiation, health care reform, financial planning, and work-life balance. The program is geared toward second- and third-year fellows, recent fellowship graduates, and those considering a job or career change. All workshops include catered meals and are free to both AGA members and non-members. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops.
The AGA Young Delegates program
The AGA highly values the efforts of our Young Delegates, and the Trainee and Early Career Committee considers them a talent pool from which we can elicit input, select committee members, and find future leaders. More importantly, we hope that the program allows young AGA members to increasingly engage with the AGA to refresh, improve, and strengthen the society. To become a Young Delegate, please visit www.gastro.org/youngdelegates to provide us with your information.
Trainee and early career GIs at DDW
The Trainee and Early Career Committee sponsors several events at DDW to bring together fellows and early-career GIs from all over the country. Each year, our committee hosts a DDW Trainee and Early Career symposium to provide practical advice for early-career GIs from all practice settings. Our DDW 2016 symposium was entitled “Surviving The First Years in Clinical Practice – Roundtable with the Experts,” and featured prominent leaders who shared career perspectives with attendees through formal presentations and more casual discussion. Attendees gained insider tips on how to design and run a fiscally prosperous practice, coding and documentation, and building and maintaining a clinical practice referral base from expert AGA leaders. We are now in the process of planning the DDW 2017 Trainee and Early Career symposium that will focus on “The Road to Leadership in GI.”
Come join us!
The success of the AGA depends on the 16,000 members who volunteer their time for committees, councils, and the governing board. Since its inception, the Trainee and Early Career Committee has allowed young GIs to have a role in the AGA as well as benefit from all of the resources that the AGA has to offer in leadership training, networking, and career preparation. In the past three years, participation of young GIs in the Trainee and Early Career Committee events has been on the rise, which we hope is a reflection of our efforts to address the educational needs of early GIs and the transition from fellowship to practice. We would love to see more fellows and early-career GIs involved!
For more information about the Trainee and Early Career committee, becoming a committee member, and our programs, please visit http://www.gastro.org/trainees. If you have any ideas that you think the committee should consider, please let us know at [email protected].
Dr. Liang is an instructor in the division of gastroenterology, New York University School of Medicine, New York, and an attending physician in the VA New York Harbor Healthcare System, New York. Dr. Kushner is a transplant hepatology fellow in the division of gastroenterology, University of California, San Francisco. Dr. May is assistant professor in the division of digestive diseases, David Geffen School of Medicine, University of California, Los Angeles, and an attending physician in the department of gastroenterology in the VA Greater Los Angeles Healthcare System, Los Angeles.
AGA’s focus on young GIs
The AGA Trainee and Early Career Committee (formerly Trainee and Young GI Committee) is composed of 12 trainee and early-career AGA members and meets twice a year to develop programs and events specifically targeted to trainees and gastroenterologists (GIs) in their first five years out of fellowship training. The committee was formed by the AGA in February 2013 to address the specific needs of early-career GI professionals and to develop programs to expose younger members to all that the AGA has to offer. The new committee also became a creative space to organize efforts to increase membership among early-career GIs. Trainee and Early Career Committee members are selected for 2-year terms and represent fellowship training programs, universities, and practices from around the nation. Each committee member serves simultaneously on one other AGA committee, which gives young GIs additional opportunities for leadership roles. The committee meets regularly with AGA staff and a governing board liaison to discuss committee goals and the issues most relevant to physicians during and directly after GI fellowship training. The committee also provides feedback to other committees about how programs and initiatives might involve or impact GI fellows and recent graduates. The result is a unique focus group where young GIs from all over the country work collectively to improve the young GI experience through flagship programs like the Regional Practice Skills Workshop, the Young Delegates Program, and Trainee and Early Career events at Digestive Disease Week (DDW)®.
AGA Regional Practice Skills Workshops
The workshop agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiation, health care reform, financial planning, and work-life balance. The program is geared toward second- and third-year fellows, recent fellowship graduates, and those considering a job or career change. All workshops include catered meals and are free to both AGA members and non-members. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops.
The AGA Young Delegates program
The AGA highly values the efforts of our Young Delegates, and the Trainee and Early Career Committee considers them a talent pool from which we can elicit input, select committee members, and find future leaders. More importantly, we hope that the program allows young AGA members to increasingly engage with the AGA to refresh, improve, and strengthen the society. To become a Young Delegate, please visit www.gastro.org/youngdelegates to provide us with your information.
Trainee and early career GIs at DDW
The Trainee and Early Career Committee sponsors several events at DDW to bring together fellows and early-career GIs from all over the country. Each year, our committee hosts a DDW Trainee and Early Career symposium to provide practical advice for early-career GIs from all practice settings. Our DDW 2016 symposium was entitled “Surviving The First Years in Clinical Practice – Roundtable with the Experts,” and featured prominent leaders who shared career perspectives with attendees through formal presentations and more casual discussion. Attendees gained insider tips on how to design and run a fiscally prosperous practice, coding and documentation, and building and maintaining a clinical practice referral base from expert AGA leaders. We are now in the process of planning the DDW 2017 Trainee and Early Career symposium that will focus on “The Road to Leadership in GI.”
Come join us!
The success of the AGA depends on the 16,000 members who volunteer their time for committees, councils, and the governing board. Since its inception, the Trainee and Early Career Committee has allowed young GIs to have a role in the AGA as well as benefit from all of the resources that the AGA has to offer in leadership training, networking, and career preparation. In the past three years, participation of young GIs in the Trainee and Early Career Committee events has been on the rise, which we hope is a reflection of our efforts to address the educational needs of early GIs and the transition from fellowship to practice. We would love to see more fellows and early-career GIs involved!
For more information about the Trainee and Early Career committee, becoming a committee member, and our programs, please visit http://www.gastro.org/trainees. If you have any ideas that you think the committee should consider, please let us know at [email protected].
Dr. Liang is an instructor in the division of gastroenterology, New York University School of Medicine, New York, and an attending physician in the VA New York Harbor Healthcare System, New York. Dr. Kushner is a transplant hepatology fellow in the division of gastroenterology, University of California, San Francisco. Dr. May is assistant professor in the division of digestive diseases, David Geffen School of Medicine, University of California, Los Angeles, and an attending physician in the department of gastroenterology in the VA Greater Los Angeles Healthcare System, Los Angeles.
AGA’s focus on young GIs
The AGA Trainee and Early Career Committee (formerly Trainee and Young GI Committee) is composed of 12 trainee and early-career AGA members and meets twice a year to develop programs and events specifically targeted to trainees and gastroenterologists (GIs) in their first five years out of fellowship training. The committee was formed by the AGA in February 2013 to address the specific needs of early-career GI professionals and to develop programs to expose younger members to all that the AGA has to offer. The new committee also became a creative space to organize efforts to increase membership among early-career GIs. Trainee and Early Career Committee members are selected for 2-year terms and represent fellowship training programs, universities, and practices from around the nation. Each committee member serves simultaneously on one other AGA committee, which gives young GIs additional opportunities for leadership roles. The committee meets regularly with AGA staff and a governing board liaison to discuss committee goals and the issues most relevant to physicians during and directly after GI fellowship training. The committee also provides feedback to other committees about how programs and initiatives might involve or impact GI fellows and recent graduates. The result is a unique focus group where young GIs from all over the country work collectively to improve the young GI experience through flagship programs like the Regional Practice Skills Workshop, the Young Delegates Program, and Trainee and Early Career events at Digestive Disease Week (DDW)®.
AGA Regional Practice Skills Workshops
The workshop agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiation, health care reform, financial planning, and work-life balance. The program is geared toward second- and third-year fellows, recent fellowship graduates, and those considering a job or career change. All workshops include catered meals and are free to both AGA members and non-members. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops.
The AGA Young Delegates program
The AGA highly values the efforts of our Young Delegates, and the Trainee and Early Career Committee considers them a talent pool from which we can elicit input, select committee members, and find future leaders. More importantly, we hope that the program allows young AGA members to increasingly engage with the AGA to refresh, improve, and strengthen the society. To become a Young Delegate, please visit www.gastro.org/youngdelegates to provide us with your information.
Trainee and early career GIs at DDW
The Trainee and Early Career Committee sponsors several events at DDW to bring together fellows and early-career GIs from all over the country. Each year, our committee hosts a DDW Trainee and Early Career symposium to provide practical advice for early-career GIs from all practice settings. Our DDW 2016 symposium was entitled “Surviving The First Years in Clinical Practice – Roundtable with the Experts,” and featured prominent leaders who shared career perspectives with attendees through formal presentations and more casual discussion. Attendees gained insider tips on how to design and run a fiscally prosperous practice, coding and documentation, and building and maintaining a clinical practice referral base from expert AGA leaders. We are now in the process of planning the DDW 2017 Trainee and Early Career symposium that will focus on “The Road to Leadership in GI.”
Come join us!
The success of the AGA depends on the 16,000 members who volunteer their time for committees, councils, and the governing board. Since its inception, the Trainee and Early Career Committee has allowed young GIs to have a role in the AGA as well as benefit from all of the resources that the AGA has to offer in leadership training, networking, and career preparation. In the past three years, participation of young GIs in the Trainee and Early Career Committee events has been on the rise, which we hope is a reflection of our efforts to address the educational needs of early GIs and the transition from fellowship to practice. We would love to see more fellows and early-career GIs involved!
For more information about the Trainee and Early Career committee, becoming a committee member, and our programs, please visit http://www.gastro.org/trainees. If you have any ideas that you think the committee should consider, please let us know at [email protected].
Dr. Liang is an instructor in the division of gastroenterology, New York University School of Medicine, New York, and an attending physician in the VA New York Harbor Healthcare System, New York. Dr. Kushner is a transplant hepatology fellow in the division of gastroenterology, University of California, San Francisco. Dr. May is assistant professor in the division of digestive diseases, David Geffen School of Medicine, University of California, Los Angeles, and an attending physician in the department of gastroenterology in the VA Greater Los Angeles Healthcare System, Los Angeles.