User login
Research Committee Chair Reflects
Before Andy Auerbach, MD, MPH, concludes a four-year term as chair of SHM’s Research Committee, I talked with him about his perspective on hospital medicine research. Dr. Auerbach is an associate professor of medicine at the University of California, San Francisco.
He received a career development award from the National Institutes of Health (NIH) early in his career and is the principal investigator of an R01 research project grant from the NHLBI titled “Improving use of perioperative beta-blockers through a multidimensional QI program.”
He is also a co-author of “Outcomes of Patients Treated by Hospitalists, General Internists, and Family Physicians” in the December 2007 New England Journal of Medicine, which found statistically significant differences in length of stay and cost. He received his medical degree from Dartmouth Medical School in Hanover, N.H., and did his residency training in internal medicine at Yale New Haven Hospital in Connecticut. He completed an MPH in clinical epidemiology at the Harvard School of Public Health in Boston in 1998.
Q: So, is academia as glamorous as it sounds?
Dr. Auerbach: Way more glamorous—you should see my office. And yes, we are in a white tower.
Q: How did you get your start in research?
Dr. Auerbach: I actually started out my research fellowship wanting to be a cardiologist and go into the cath lab while developing the skills to participate in and teach research methods. I found I really enjoyed the work, particularly the creative and entrepreneurial aspects of developing a project or grant and seeing it through to completion.
Q: What are the research options for hospitalists practicing in nonteaching settings?
Dr. Auerbach: I think the most straightforward way to participate in research is to partner with a clinical research organization to help enroll patients in their trials. While you don’t get the opportunity to design the study, you do get to get a feel for consent/enrollment and internal review board [IRB] processes.
The next best way to get involved with research is to partner with a researcher—and this need not be a hospitalist—at your site or very near by. Many QI projects are close to being research-ready and may provide an opportunity to make that work count twice. But it will require you to learn about analytic methods.
I’d also be remiss if I didn’t mention the value of other very useful academic products—rigorous reports of a QI intervention (think of both success and failure stories) and patient case reports. If well referenced and used as teaching documents, these can be very useful ways to advance knowledge.
Q: Are there any particular prerequisites in terms of training that you find especially helpful as you conduct your research?
Dr. Auerbach: It is hard to be a capital-R “Researcher” and compete for career development grants and NIH funding without some advanced [degrees] and a clinical research fellowship. I hesitate to call these prerequisites, but they are nearly so.
Q: What do you like best about your career as a hospitalist?
Dr. Auerbach: I really like acute care medicine, but didn’t want to subspecialize—otherwise I’d be wearing lead in a cath lab now. I also like the questions and processes in the hospital a bit more than the clinic setting.
Q: Who are your mentors and how did you find them?
Dr. Auerbach: I’ve had a remarkable set of mentors from fellowship [Mary Beth Hamel, Roger Davis, Russ Phillips] through my early career [Lee Goldman, Bob Wachter, Ralph Gonzales]. Now that I am early-mid career, I’m trying to pass their teaching on.
Q: Any advice for hospitalists interested in research but daunted by the prospect of starting their own studies?
Dr. Auerbach: If you want to do a scholarly/academic project to round out your personal/career satisfaction, I think the daunting nature of research can be overcome with the right questions and right support—and by defining what these are well before you actually dive into a dataset or implementation project. You also have to decide how much satisfaction you will get from the project in the end compared to the incremental nights/weekends you will spend to plan and execute your project—not to mention publish.
If you are thinking of research as a career, be aware of what makes you happy. If you like to write, enjoy the process of hypothesis generating/testing, and take rejection well you may be happy as a researcher. There are still plenty of nights/weekends to be spent, though.
Making a switch from full-time clinical or administrative work to research means making a very big commitment to going back to get the skills as part of a fellowship.
Q: Do researchers interested in quality improvement questions still have to run their work past the IRB?
Dr. Auerbach: Unfortunately this is now an area of uncertainty for people—unnecessarily so. Until recent events, IRBs have not required approval for QI projects that seek to enhance care according to an evidence-based standard, especially if that standard is endorsed by the institution. If you plan to publish your findings—particularly if you talk to or touch patients, or collect personal health information—I think it is nearly always wise to at least call your local IRB to ask for how you can or should conduct the study. This is best done before you start the project, obviously.
If you want to publish your results using deidentified data after the project is done, our IRB would say that is exempt from review [e.g., no need for approval]. But I think even this case would be worth a phone call to ensure your IRB feels similarly.
Whether or not you get IRB approval, be very aware of how and where you store data. TH
Before Andy Auerbach, MD, MPH, concludes a four-year term as chair of SHM’s Research Committee, I talked with him about his perspective on hospital medicine research. Dr. Auerbach is an associate professor of medicine at the University of California, San Francisco.
He received a career development award from the National Institutes of Health (NIH) early in his career and is the principal investigator of an R01 research project grant from the NHLBI titled “Improving use of perioperative beta-blockers through a multidimensional QI program.”
He is also a co-author of “Outcomes of Patients Treated by Hospitalists, General Internists, and Family Physicians” in the December 2007 New England Journal of Medicine, which found statistically significant differences in length of stay and cost. He received his medical degree from Dartmouth Medical School in Hanover, N.H., and did his residency training in internal medicine at Yale New Haven Hospital in Connecticut. He completed an MPH in clinical epidemiology at the Harvard School of Public Health in Boston in 1998.
Q: So, is academia as glamorous as it sounds?
Dr. Auerbach: Way more glamorous—you should see my office. And yes, we are in a white tower.
Q: How did you get your start in research?
Dr. Auerbach: I actually started out my research fellowship wanting to be a cardiologist and go into the cath lab while developing the skills to participate in and teach research methods. I found I really enjoyed the work, particularly the creative and entrepreneurial aspects of developing a project or grant and seeing it through to completion.
Q: What are the research options for hospitalists practicing in nonteaching settings?
Dr. Auerbach: I think the most straightforward way to participate in research is to partner with a clinical research organization to help enroll patients in their trials. While you don’t get the opportunity to design the study, you do get to get a feel for consent/enrollment and internal review board [IRB] processes.
The next best way to get involved with research is to partner with a researcher—and this need not be a hospitalist—at your site or very near by. Many QI projects are close to being research-ready and may provide an opportunity to make that work count twice. But it will require you to learn about analytic methods.
I’d also be remiss if I didn’t mention the value of other very useful academic products—rigorous reports of a QI intervention (think of both success and failure stories) and patient case reports. If well referenced and used as teaching documents, these can be very useful ways to advance knowledge.
Q: Are there any particular prerequisites in terms of training that you find especially helpful as you conduct your research?
Dr. Auerbach: It is hard to be a capital-R “Researcher” and compete for career development grants and NIH funding without some advanced [degrees] and a clinical research fellowship. I hesitate to call these prerequisites, but they are nearly so.
Q: What do you like best about your career as a hospitalist?
Dr. Auerbach: I really like acute care medicine, but didn’t want to subspecialize—otherwise I’d be wearing lead in a cath lab now. I also like the questions and processes in the hospital a bit more than the clinic setting.
Q: Who are your mentors and how did you find them?
Dr. Auerbach: I’ve had a remarkable set of mentors from fellowship [Mary Beth Hamel, Roger Davis, Russ Phillips] through my early career [Lee Goldman, Bob Wachter, Ralph Gonzales]. Now that I am early-mid career, I’m trying to pass their teaching on.
Q: Any advice for hospitalists interested in research but daunted by the prospect of starting their own studies?
Dr. Auerbach: If you want to do a scholarly/academic project to round out your personal/career satisfaction, I think the daunting nature of research can be overcome with the right questions and right support—and by defining what these are well before you actually dive into a dataset or implementation project. You also have to decide how much satisfaction you will get from the project in the end compared to the incremental nights/weekends you will spend to plan and execute your project—not to mention publish.
If you are thinking of research as a career, be aware of what makes you happy. If you like to write, enjoy the process of hypothesis generating/testing, and take rejection well you may be happy as a researcher. There are still plenty of nights/weekends to be spent, though.
Making a switch from full-time clinical or administrative work to research means making a very big commitment to going back to get the skills as part of a fellowship.
Q: Do researchers interested in quality improvement questions still have to run their work past the IRB?
Dr. Auerbach: Unfortunately this is now an area of uncertainty for people—unnecessarily so. Until recent events, IRBs have not required approval for QI projects that seek to enhance care according to an evidence-based standard, especially if that standard is endorsed by the institution. If you plan to publish your findings—particularly if you talk to or touch patients, or collect personal health information—I think it is nearly always wise to at least call your local IRB to ask for how you can or should conduct the study. This is best done before you start the project, obviously.
If you want to publish your results using deidentified data after the project is done, our IRB would say that is exempt from review [e.g., no need for approval]. But I think even this case would be worth a phone call to ensure your IRB feels similarly.
Whether or not you get IRB approval, be very aware of how and where you store data. TH
Before Andy Auerbach, MD, MPH, concludes a four-year term as chair of SHM’s Research Committee, I talked with him about his perspective on hospital medicine research. Dr. Auerbach is an associate professor of medicine at the University of California, San Francisco.
He received a career development award from the National Institutes of Health (NIH) early in his career and is the principal investigator of an R01 research project grant from the NHLBI titled “Improving use of perioperative beta-blockers through a multidimensional QI program.”
He is also a co-author of “Outcomes of Patients Treated by Hospitalists, General Internists, and Family Physicians” in the December 2007 New England Journal of Medicine, which found statistically significant differences in length of stay and cost. He received his medical degree from Dartmouth Medical School in Hanover, N.H., and did his residency training in internal medicine at Yale New Haven Hospital in Connecticut. He completed an MPH in clinical epidemiology at the Harvard School of Public Health in Boston in 1998.
Q: So, is academia as glamorous as it sounds?
Dr. Auerbach: Way more glamorous—you should see my office. And yes, we are in a white tower.
Q: How did you get your start in research?
Dr. Auerbach: I actually started out my research fellowship wanting to be a cardiologist and go into the cath lab while developing the skills to participate in and teach research methods. I found I really enjoyed the work, particularly the creative and entrepreneurial aspects of developing a project or grant and seeing it through to completion.
Q: What are the research options for hospitalists practicing in nonteaching settings?
Dr. Auerbach: I think the most straightforward way to participate in research is to partner with a clinical research organization to help enroll patients in their trials. While you don’t get the opportunity to design the study, you do get to get a feel for consent/enrollment and internal review board [IRB] processes.
The next best way to get involved with research is to partner with a researcher—and this need not be a hospitalist—at your site or very near by. Many QI projects are close to being research-ready and may provide an opportunity to make that work count twice. But it will require you to learn about analytic methods.
I’d also be remiss if I didn’t mention the value of other very useful academic products—rigorous reports of a QI intervention (think of both success and failure stories) and patient case reports. If well referenced and used as teaching documents, these can be very useful ways to advance knowledge.
Q: Are there any particular prerequisites in terms of training that you find especially helpful as you conduct your research?
Dr. Auerbach: It is hard to be a capital-R “Researcher” and compete for career development grants and NIH funding without some advanced [degrees] and a clinical research fellowship. I hesitate to call these prerequisites, but they are nearly so.
Q: What do you like best about your career as a hospitalist?
Dr. Auerbach: I really like acute care medicine, but didn’t want to subspecialize—otherwise I’d be wearing lead in a cath lab now. I also like the questions and processes in the hospital a bit more than the clinic setting.
Q: Who are your mentors and how did you find them?
Dr. Auerbach: I’ve had a remarkable set of mentors from fellowship [Mary Beth Hamel, Roger Davis, Russ Phillips] through my early career [Lee Goldman, Bob Wachter, Ralph Gonzales]. Now that I am early-mid career, I’m trying to pass their teaching on.
Q: Any advice for hospitalists interested in research but daunted by the prospect of starting their own studies?
Dr. Auerbach: If you want to do a scholarly/academic project to round out your personal/career satisfaction, I think the daunting nature of research can be overcome with the right questions and right support—and by defining what these are well before you actually dive into a dataset or implementation project. You also have to decide how much satisfaction you will get from the project in the end compared to the incremental nights/weekends you will spend to plan and execute your project—not to mention publish.
If you are thinking of research as a career, be aware of what makes you happy. If you like to write, enjoy the process of hypothesis generating/testing, and take rejection well you may be happy as a researcher. There are still plenty of nights/weekends to be spent, though.
Making a switch from full-time clinical or administrative work to research means making a very big commitment to going back to get the skills as part of a fellowship.
Q: Do researchers interested in quality improvement questions still have to run their work past the IRB?
Dr. Auerbach: Unfortunately this is now an area of uncertainty for people—unnecessarily so. Until recent events, IRBs have not required approval for QI projects that seek to enhance care according to an evidence-based standard, especially if that standard is endorsed by the institution. If you plan to publish your findings—particularly if you talk to or touch patients, or collect personal health information—I think it is nearly always wise to at least call your local IRB to ask for how you can or should conduct the study. This is best done before you start the project, obviously.
If you want to publish your results using deidentified data after the project is done, our IRB would say that is exempt from review [e.g., no need for approval]. But I think even this case would be worth a phone call to ensure your IRB feels similarly.
Whether or not you get IRB approval, be very aware of how and where you store data. TH
Satisfaction Is Job No. 1
The Career Satisfaction Task Force has focused on two key areas this year to build upon the work that resulted in last year’s white paper “A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction.”
The paper outlined a framework for hospital medicine program leaders and hospitalists to identify important components of matching individuals and programs for the best job fit.
This year, the task force is working to bring the white paper to life and moving it from a conceptual framework to demonstrating how to use it to solve real issues facing programs and individuals.
The first of these projects was a Webinar led by SHM Senior Vice President Joe Miller, Sylvia McKean, MD (course director of Hospital Medicine 2008), and Win Whitcomb, MD (a co-founder of SHM). Each of them has held leadership roles on this task force. About 80 people participated in the December event, and more than three-fourths of attendees rated it highly.
At last year’s Annual Meeting in Dallas, the white paper was presented in a task force workshop. In keeping with our aim to bring the framework to life, this year’s workshop will use real case studies to demonstrate how to use bring the concepts to solutions. The workshop will be facilitated by Chad Whelan, MD, assistant professor of medicine and director of the Hospitalists Scholars Training Program, University of Chicago. Discussing key concepts will be Doug Carlson, MD, associate professor, Pediatrics Division, Washington University School of Medicine in St. Louis, and Tosha Wetterneck, MD, University of Wisconsin Hospital/Clinics, Madison. Drs. Carlson and Wetterneck made significant contributions to the white paper. In this highly interactive workshop, case studies that demonstrate challenges with workload/scheduling and autonomy will be discussed. Drs. Carlson and Wetterneck will lead the participants through discussions aimed at identifying the root causes of struggle and potential solutions for the program.
In the coming months, we hope to develop a series of articles to be published in The Hospitalist addressing the issues of greatest importance for career satisfaction.
The task force realizes there may be opportunities to add knowledge about career satisfaction and provide a valuable service to SHM member. We are in the early stages of developing a survey geared to further clarifying the most important factors in making satisfying career matches as well as providing detailed feedback about programs to their leaders. We are seeking funding to enable us to begin this exciting work.
The Career Satisfaction Task Force has focused on two key areas this year to build upon the work that resulted in last year’s white paper “A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction.”
The paper outlined a framework for hospital medicine program leaders and hospitalists to identify important components of matching individuals and programs for the best job fit.
This year, the task force is working to bring the white paper to life and moving it from a conceptual framework to demonstrating how to use it to solve real issues facing programs and individuals.
The first of these projects was a Webinar led by SHM Senior Vice President Joe Miller, Sylvia McKean, MD (course director of Hospital Medicine 2008), and Win Whitcomb, MD (a co-founder of SHM). Each of them has held leadership roles on this task force. About 80 people participated in the December event, and more than three-fourths of attendees rated it highly.
At last year’s Annual Meeting in Dallas, the white paper was presented in a task force workshop. In keeping with our aim to bring the framework to life, this year’s workshop will use real case studies to demonstrate how to use bring the concepts to solutions. The workshop will be facilitated by Chad Whelan, MD, assistant professor of medicine and director of the Hospitalists Scholars Training Program, University of Chicago. Discussing key concepts will be Doug Carlson, MD, associate professor, Pediatrics Division, Washington University School of Medicine in St. Louis, and Tosha Wetterneck, MD, University of Wisconsin Hospital/Clinics, Madison. Drs. Carlson and Wetterneck made significant contributions to the white paper. In this highly interactive workshop, case studies that demonstrate challenges with workload/scheduling and autonomy will be discussed. Drs. Carlson and Wetterneck will lead the participants through discussions aimed at identifying the root causes of struggle and potential solutions for the program.
In the coming months, we hope to develop a series of articles to be published in The Hospitalist addressing the issues of greatest importance for career satisfaction.
The task force realizes there may be opportunities to add knowledge about career satisfaction and provide a valuable service to SHM member. We are in the early stages of developing a survey geared to further clarifying the most important factors in making satisfying career matches as well as providing detailed feedback about programs to their leaders. We are seeking funding to enable us to begin this exciting work.
The Career Satisfaction Task Force has focused on two key areas this year to build upon the work that resulted in last year’s white paper “A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction.”
The paper outlined a framework for hospital medicine program leaders and hospitalists to identify important components of matching individuals and programs for the best job fit.
This year, the task force is working to bring the white paper to life and moving it from a conceptual framework to demonstrating how to use it to solve real issues facing programs and individuals.
The first of these projects was a Webinar led by SHM Senior Vice President Joe Miller, Sylvia McKean, MD (course director of Hospital Medicine 2008), and Win Whitcomb, MD (a co-founder of SHM). Each of them has held leadership roles on this task force. About 80 people participated in the December event, and more than three-fourths of attendees rated it highly.
At last year’s Annual Meeting in Dallas, the white paper was presented in a task force workshop. In keeping with our aim to bring the framework to life, this year’s workshop will use real case studies to demonstrate how to use bring the concepts to solutions. The workshop will be facilitated by Chad Whelan, MD, assistant professor of medicine and director of the Hospitalists Scholars Training Program, University of Chicago. Discussing key concepts will be Doug Carlson, MD, associate professor, Pediatrics Division, Washington University School of Medicine in St. Louis, and Tosha Wetterneck, MD, University of Wisconsin Hospital/Clinics, Madison. Drs. Carlson and Wetterneck made significant contributions to the white paper. In this highly interactive workshop, case studies that demonstrate challenges with workload/scheduling and autonomy will be discussed. Drs. Carlson and Wetterneck will lead the participants through discussions aimed at identifying the root causes of struggle and potential solutions for the program.
In the coming months, we hope to develop a series of articles to be published in The Hospitalist addressing the issues of greatest importance for career satisfaction.
The task force realizes there may be opportunities to add knowledge about career satisfaction and provide a valuable service to SHM member. We are in the early stages of developing a survey geared to further clarifying the most important factors in making satisfying career matches as well as providing detailed feedback about programs to their leaders. We are seeking funding to enable us to begin this exciting work.
What is the best method of treating acutely worsened chronic pain?
Case
A 69-year-old female with metastatic ovarian cancer and chronic pain syndrome presented to the hospital with seven days of progressively worsening abdominal pain. The pain had been similar to her chronic cancer pain but more severe. She has acute renal failure secondary to volume depletion from poor intake. A CT scan of the abdomen and pelvis reveal progression of her cancer with acute pathology. What is the best method of treating this patient’s pain?
Overview
Pain is pandemic. It is the most common reason patients seek healthcare.1 Almost one-third of Americans will experience severe chronic pain at some point in their lives. Every year, approximately 25 million Americans experience acute pain and 50 million experience chronic pain. Only one in four patients with pain receives appropriate therapy and control of their pain.
Pain is the most common symptom experienced by hospitalized adults.2 Acute or chronic pain can be particularly challenging to treat because these patients are frequently opioid dependent and have many psychosocial factors. No one method of pain control is superior to another. However, one method to gain rapid control of an acute pain crisis in a patient with chronic pain is to use patient-controlled analgesia (PCA).
Review of the Data
The first commercially available PCA pumps became available in 1976.3 They were created after studies in the 1960s demonstrated that small doses of opioids given intravenously provided more effective pain relief than conventional intramuscular injections.
The majority of studies on PCAs are in the postoperative patient, with cancer pain being next most commonly studied. PCAs utilize microprocessor-controlled infusion pumps that deliver a preprogrammed dose of opioid when the patient pushes the demand button. They allow programming of dose (demand dose), time between doses (lockout interval), background infusion rate (basal rate), and nurse-initiated dose (bolus dose).
The PCA paradigm is based on the opioid pharmacologic concept of minimum effective analgesic concentration (MEAC).4,5 The MEAC is the smallest serum opioid concentration at which pain is relieved. The dose-response curve to opioids is sigmoidal such that minimal analgesia is achieved until the MEAC is reached, after which minute increases in opioid concentrations produce analgesia, until further increases produce no significant increased analgesic effect.
PCAs allow individualized dosing and titration to achieve the MEAC, with small incremental doses administered whenever the serum concentration falls below the MEAC. A major goal of PCA technology is to regulate drug delivery to rapidly achieve and maintain the MEAC.
Advantages of PCAs
- More individual dosing and titration of pain medications to account for inter-individual and intra-individual variability in the response to opioids;
- Negative feedback control system, an added safety measure to avoid respiratory depression. As patients become too sedated from opioids, they are no longer able to push the button to receive further opioids;
- Higher patient satisfaction with pain control, a major determinant being personal control over the delivery of pain relief;6-8 and
- Greater analgesic efficacy vs. conventional analgesia.
Disadvantages of PCAs
Select patient populations: Not all patients are able to understand and retain the required instructions necessary to safely or effectively use self-administered opioids (e.g., cognitively impaired patients).
Potential for opioid dosing errors: These are related to equipment factors, medical personnel prescribing or programming errors.
Increased cost: PCAs have been shown to be more expensive in comparison with intramuscular (IM) injections, the prior standard of care.9-10
PCA Prescribing
The parameters programmed into the PCA machine include the basal rate, demand (or incremental) dose, lockout interval, nurse-initiated bolus dose, and choice of opioid.
Basal rate: The continuous infusion of opioid set at an hourly rate. Most studies that compare PCA use with and without basal rates (in postoperative patients) do not show improved pain relief or sleep with basal rates.11 Basal rates have been associated with increased risk of sedation and respiratory depression.12
The routine use of basal rates is not recommended initially, unless a patient is opioid-tolerant (i.e., on chronic opioid therapy). For patients on chronic opioids, their 24-hour total opioid requirement is converted by equianalgesic dosing to the basal rate. Steady state is not achieved for eight to 12 hours of continuous infusion; therefore, it is not recommended to change the basal rate more frequently than every eight hours.13
Demand dose: The dose patients provide themselves by pushing the button. Studies on opioid-naïve patients using morphine PCAs have shown that 1 mg IV morphine was the optimal starting dose, based on good pain relief without respiratory depression. Lower doses, such as 0.5 mg IV morphine, are generally used in the elderly as opioid requirements are known to decrease with patient age.14
For patients with a basal rate, the demand dose is often set at 50% to 100% of the basal rate. The demand dose is the parameter that should be titrated up for acute pain control. World Health Organization guidelines recommend increasing the dose by 25% to 50% for mild to moderate pain, and 50% to 100% for moderate to severe pain.15
Lockout interval: Minimal allowable time between demand doses. This time is based on the time to peak effect of IV opioids and can vary from five to 15 minutes. The effects of varying lockout intervals—seven to 11 minutes for morphine and five to eight minutes for fentanyl—had no effect on pain levels or side effects.16 Ten minutes is a standard lockout interval.
Bolus dose: The nurse-initiated dose that may be given initially to achieve pain control and later to counteract incidental pain (e.g., that caused by physical therapy, dressing changes, or radiology tests). A recommended dose is equivalent to the basal rate or twice the demand dose.
Choice of opioid: Morphine is the standard opioid because of its familiarity, cost, and years of study. Although inter-individual variability exists, there are no major differences in side effects among the different opioids. Renal and hepatic insufficiency can increase the effects of opioids. Morphine is especially troublesome in renal failure because it has an active metabolite—morphine-6-glucuronide—that can accumulate and increase the risk of sedation and respiratory depression.
Other Concerns
PCA complications: The most well-studied adverse effects of PCAs are nausea and respiratory depression. There is no difference between PCAs and conventional analgesia in rates of nausea or respiratory depression.17
Nausea is the most common side effect in postoperative patients on PCAs. Patients rapidly develop tolerance to nausea over a period of days. However, many clinicians are concerned about respiratory depression and the risk of death. The overall incidence of respiratory depression with PCAs is less than 1% (range from 0.1 to 0.8%), similar to conventional analgesia. However, the incidence is significantly higher when basal rates are used, rising to 1.1 to 3.9%. Other factors predisposing a patient to increased risk of respiratory depression are older age, obstructive sleep apnea, hypovolemia, renal failure, and the concurrent use of other sedating medications.18
Medication errors are also common. The overall incidence of medication mishaps with PCAs is 1.2%.19 More than 50% of these occur because of operator-related errors (e.g., improper loading, programming errors, and documentation errors). Equipment malfunction is the next most common error.
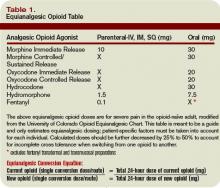
Opioid equianalgesic dosing conversions: The equianalgesic dose ratio is the ratio of the dose of two opioids required to produce the same analgesic effect. (See Table 1, right.) For example, IV morphine is three times as potent as oral morphine, with an equianalgesic dose ratio of 1:3. Equianalgesic dose tables vary somewhat in their values, which have been largely determined by single-dose administration studies.20 The generalizability of these tables to chronic opioid administration is not well studied.
Incomplete cross tolerance: When switching from one opioid to another, lower doses can be used to control pain.21, 22 Tolerance to one opioid does not completely transfer to the new opioid. Starting at half to two-thirds of the new opioid dose is generally recommended to avoid opioid-specific tolerance and inter-individual variability.23,24
Back to the Case
Opioids are the mainstay of pharmacological management of moderate-to-severe cancer pain. Evaluation of the patient reveals that her acute increase in pain is likely due to progression of her cancer. She had been taking morphine (sustained-release, 90 mg oral) twice daily for her pain and had been using approximately five doses per day of immediate-release oral morphine 20 mg for breakthrough pain. This is equivalent to a total 24-hour opioid requirement of 280 mg oral morphine.
She should be started on a PCA for rapid pain control and titration. Hydromorphone (Dilaudid) is a better PCA choice than morphine because she has acute renal failure. The equianalgesic dose ratio of oral morphine to IV hydromorphone is approximately 30:1.5. The total 24-hour opioid dose of 280 mg oral morphine is equivalent to 14 mg IV hydromorphone ([280mg morphine per day ÷ 30] x 1.5 = 14).
After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 8.4 mg IV hydromorphone (14 mg x 0.6 = 8.4 mg). This is approximately equivalent to 0.4 mg IV hydromorphone/hour (8.4 mg ÷ 24 hours), which is her initial basal rate. The demand dose should be set at 0.2 mg (50% the basal rate) with a lockout interval of 10 minutes.
Over a period of several days, the patient’s pain was controlled and her opioid requirements stabilized. She was on a basal rate of 1.4 mg/hour and a demand dose of 1 mg with a 10-minute lockout. Her total 24-hour opioid requirement was 44 mg of IV hydromorphone. As her renal function improved but did not completely normalize, oxycodone was chosen over morphine when converting her back to oral pain medications (less active renal metabolites). The equianalgesic dose ratio of oral oxycodone to IV hydromorphone is approximately 20:1.5. Her total 24-hour opioid dose of 44 mg IV hydromorphone is equivalent to 587 mg oral oxycodone (44 ÷ 1.5) x 20. After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 352 mg oral oxycodone or 180 mg of sustained-release oxycodone twice daily (352 mg ÷ 2 ≈ 180 mg). For breakthrough pain she should receive 40 mg of immediate-release oxycodone every hour as needed (10% to 15% of the 24-hour opioid requirement). TH
Dr. Youngwerth is a hospitalist and instructor of medicine, University of Colorado at Denver, assistant director, Palliative Care Consult Service, associate director, Colorado Palliative Medicine Fellowship Program, and medical director, Hospice of Saint John.
References
- American Pain Society. Pain: Current understanding of assessment, management, and treatments. National Pharmaceutical Council 2006;1-79.
- Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166:1033-1039.
- Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44-S61.
- Etches RC. Patient-controlled analgesia. Surg Clinics N Amer. 1999;79:297-312.
- Nolan MF and Wilson M-C B. Patient-controlled analgesia: A method for the controlled self-administration of opioid pain medications. Phys Ther. 1995;75:374-379.
- Ballantyne JC, Carr DB, Chalmers TC, Dear KBG, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182-193.
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:1-10.
- Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202-209.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
- Manon C, Rittenhouse BE, Perreault S, et al. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Amer Soc Anesth Inc. 1998;89:1377-1388.
- Krenn H, Oczenski W, Jellinek H, Krumpl-Ströher M, Schweitzer E, Fitzgerald RD. Nalbuphine by PCA-pump for analgesia following hysterectomy: Bolus application versus continuous infusion with bolus application. Eur J Pain. 2001;5:219-226.
- Lehmann KA. Recent developments in patient-controlled analgesia. J Pain Symptom Manage. 2005;29:S72-S89.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. 2003:1-73.
- Macintyre PC, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1995;64:357-364.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Adult cancer pain. Version 2.2005:1-30.
- Ginsberg B, Gil KM, Muir M, Sullivan F, Williams DA, Glass PSA. The influence of lockout intervals and drug selection on patient-controlled analgesia following gynecological surgery. Pain. 1995;62:95-100.
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. Acta Anaesthesiol Scand. 2001;45:795-804.
- Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125-132.
- Oswalt KE, Shrewsbury P, Stanton-Hicks M. The incidence of medication mishaps in 3,299 PCA patients. Pain. 1990;S5;S152.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose rations for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-687.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
- Mercandante S. Opioid rotation for cancer pain. Cancer. 1999;86:1856-1866.
- Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharm Sciences. 2001;22:67-70.
Case
A 69-year-old female with metastatic ovarian cancer and chronic pain syndrome presented to the hospital with seven days of progressively worsening abdominal pain. The pain had been similar to her chronic cancer pain but more severe. She has acute renal failure secondary to volume depletion from poor intake. A CT scan of the abdomen and pelvis reveal progression of her cancer with acute pathology. What is the best method of treating this patient’s pain?
Overview
Pain is pandemic. It is the most common reason patients seek healthcare.1 Almost one-third of Americans will experience severe chronic pain at some point in their lives. Every year, approximately 25 million Americans experience acute pain and 50 million experience chronic pain. Only one in four patients with pain receives appropriate therapy and control of their pain.
Pain is the most common symptom experienced by hospitalized adults.2 Acute or chronic pain can be particularly challenging to treat because these patients are frequently opioid dependent and have many psychosocial factors. No one method of pain control is superior to another. However, one method to gain rapid control of an acute pain crisis in a patient with chronic pain is to use patient-controlled analgesia (PCA).
Review of the Data
The first commercially available PCA pumps became available in 1976.3 They were created after studies in the 1960s demonstrated that small doses of opioids given intravenously provided more effective pain relief than conventional intramuscular injections.
The majority of studies on PCAs are in the postoperative patient, with cancer pain being next most commonly studied. PCAs utilize microprocessor-controlled infusion pumps that deliver a preprogrammed dose of opioid when the patient pushes the demand button. They allow programming of dose (demand dose), time between doses (lockout interval), background infusion rate (basal rate), and nurse-initiated dose (bolus dose).
The PCA paradigm is based on the opioid pharmacologic concept of minimum effective analgesic concentration (MEAC).4,5 The MEAC is the smallest serum opioid concentration at which pain is relieved. The dose-response curve to opioids is sigmoidal such that minimal analgesia is achieved until the MEAC is reached, after which minute increases in opioid concentrations produce analgesia, until further increases produce no significant increased analgesic effect.
PCAs allow individualized dosing and titration to achieve the MEAC, with small incremental doses administered whenever the serum concentration falls below the MEAC. A major goal of PCA technology is to regulate drug delivery to rapidly achieve and maintain the MEAC.
Advantages of PCAs
- More individual dosing and titration of pain medications to account for inter-individual and intra-individual variability in the response to opioids;
- Negative feedback control system, an added safety measure to avoid respiratory depression. As patients become too sedated from opioids, they are no longer able to push the button to receive further opioids;
- Higher patient satisfaction with pain control, a major determinant being personal control over the delivery of pain relief;6-8 and
- Greater analgesic efficacy vs. conventional analgesia.
Disadvantages of PCAs
Select patient populations: Not all patients are able to understand and retain the required instructions necessary to safely or effectively use self-administered opioids (e.g., cognitively impaired patients).
Potential for opioid dosing errors: These are related to equipment factors, medical personnel prescribing or programming errors.
Increased cost: PCAs have been shown to be more expensive in comparison with intramuscular (IM) injections, the prior standard of care.9-10
PCA Prescribing
The parameters programmed into the PCA machine include the basal rate, demand (or incremental) dose, lockout interval, nurse-initiated bolus dose, and choice of opioid.
Basal rate: The continuous infusion of opioid set at an hourly rate. Most studies that compare PCA use with and without basal rates (in postoperative patients) do not show improved pain relief or sleep with basal rates.11 Basal rates have been associated with increased risk of sedation and respiratory depression.12
The routine use of basal rates is not recommended initially, unless a patient is opioid-tolerant (i.e., on chronic opioid therapy). For patients on chronic opioids, their 24-hour total opioid requirement is converted by equianalgesic dosing to the basal rate. Steady state is not achieved for eight to 12 hours of continuous infusion; therefore, it is not recommended to change the basal rate more frequently than every eight hours.13
Demand dose: The dose patients provide themselves by pushing the button. Studies on opioid-naïve patients using morphine PCAs have shown that 1 mg IV morphine was the optimal starting dose, based on good pain relief without respiratory depression. Lower doses, such as 0.5 mg IV morphine, are generally used in the elderly as opioid requirements are known to decrease with patient age.14
For patients with a basal rate, the demand dose is often set at 50% to 100% of the basal rate. The demand dose is the parameter that should be titrated up for acute pain control. World Health Organization guidelines recommend increasing the dose by 25% to 50% for mild to moderate pain, and 50% to 100% for moderate to severe pain.15
Lockout interval: Minimal allowable time between demand doses. This time is based on the time to peak effect of IV opioids and can vary from five to 15 minutes. The effects of varying lockout intervals—seven to 11 minutes for morphine and five to eight minutes for fentanyl—had no effect on pain levels or side effects.16 Ten minutes is a standard lockout interval.
Bolus dose: The nurse-initiated dose that may be given initially to achieve pain control and later to counteract incidental pain (e.g., that caused by physical therapy, dressing changes, or radiology tests). A recommended dose is equivalent to the basal rate or twice the demand dose.
Choice of opioid: Morphine is the standard opioid because of its familiarity, cost, and years of study. Although inter-individual variability exists, there are no major differences in side effects among the different opioids. Renal and hepatic insufficiency can increase the effects of opioids. Morphine is especially troublesome in renal failure because it has an active metabolite—morphine-6-glucuronide—that can accumulate and increase the risk of sedation and respiratory depression.
Other Concerns
PCA complications: The most well-studied adverse effects of PCAs are nausea and respiratory depression. There is no difference between PCAs and conventional analgesia in rates of nausea or respiratory depression.17
Nausea is the most common side effect in postoperative patients on PCAs. Patients rapidly develop tolerance to nausea over a period of days. However, many clinicians are concerned about respiratory depression and the risk of death. The overall incidence of respiratory depression with PCAs is less than 1% (range from 0.1 to 0.8%), similar to conventional analgesia. However, the incidence is significantly higher when basal rates are used, rising to 1.1 to 3.9%. Other factors predisposing a patient to increased risk of respiratory depression are older age, obstructive sleep apnea, hypovolemia, renal failure, and the concurrent use of other sedating medications.18
Medication errors are also common. The overall incidence of medication mishaps with PCAs is 1.2%.19 More than 50% of these occur because of operator-related errors (e.g., improper loading, programming errors, and documentation errors). Equipment malfunction is the next most common error.
Opioid equianalgesic dosing conversions: The equianalgesic dose ratio is the ratio of the dose of two opioids required to produce the same analgesic effect. (See Table 1, right.) For example, IV morphine is three times as potent as oral morphine, with an equianalgesic dose ratio of 1:3. Equianalgesic dose tables vary somewhat in their values, which have been largely determined by single-dose administration studies.20 The generalizability of these tables to chronic opioid administration is not well studied.
Incomplete cross tolerance: When switching from one opioid to another, lower doses can be used to control pain.21, 22 Tolerance to one opioid does not completely transfer to the new opioid. Starting at half to two-thirds of the new opioid dose is generally recommended to avoid opioid-specific tolerance and inter-individual variability.23,24
Back to the Case
Opioids are the mainstay of pharmacological management of moderate-to-severe cancer pain. Evaluation of the patient reveals that her acute increase in pain is likely due to progression of her cancer. She had been taking morphine (sustained-release, 90 mg oral) twice daily for her pain and had been using approximately five doses per day of immediate-release oral morphine 20 mg for breakthrough pain. This is equivalent to a total 24-hour opioid requirement of 280 mg oral morphine.
She should be started on a PCA for rapid pain control and titration. Hydromorphone (Dilaudid) is a better PCA choice than morphine because she has acute renal failure. The equianalgesic dose ratio of oral morphine to IV hydromorphone is approximately 30:1.5. The total 24-hour opioid dose of 280 mg oral morphine is equivalent to 14 mg IV hydromorphone ([280mg morphine per day ÷ 30] x 1.5 = 14).
After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 8.4 mg IV hydromorphone (14 mg x 0.6 = 8.4 mg). This is approximately equivalent to 0.4 mg IV hydromorphone/hour (8.4 mg ÷ 24 hours), which is her initial basal rate. The demand dose should be set at 0.2 mg (50% the basal rate) with a lockout interval of 10 minutes.
Over a period of several days, the patient’s pain was controlled and her opioid requirements stabilized. She was on a basal rate of 1.4 mg/hour and a demand dose of 1 mg with a 10-minute lockout. Her total 24-hour opioid requirement was 44 mg of IV hydromorphone. As her renal function improved but did not completely normalize, oxycodone was chosen over morphine when converting her back to oral pain medications (less active renal metabolites). The equianalgesic dose ratio of oral oxycodone to IV hydromorphone is approximately 20:1.5. Her total 24-hour opioid dose of 44 mg IV hydromorphone is equivalent to 587 mg oral oxycodone (44 ÷ 1.5) x 20. After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 352 mg oral oxycodone or 180 mg of sustained-release oxycodone twice daily (352 mg ÷ 2 ≈ 180 mg). For breakthrough pain she should receive 40 mg of immediate-release oxycodone every hour as needed (10% to 15% of the 24-hour opioid requirement). TH
Dr. Youngwerth is a hospitalist and instructor of medicine, University of Colorado at Denver, assistant director, Palliative Care Consult Service, associate director, Colorado Palliative Medicine Fellowship Program, and medical director, Hospice of Saint John.
References
- American Pain Society. Pain: Current understanding of assessment, management, and treatments. National Pharmaceutical Council 2006;1-79.
- Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166:1033-1039.
- Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44-S61.
- Etches RC. Patient-controlled analgesia. Surg Clinics N Amer. 1999;79:297-312.
- Nolan MF and Wilson M-C B. Patient-controlled analgesia: A method for the controlled self-administration of opioid pain medications. Phys Ther. 1995;75:374-379.
- Ballantyne JC, Carr DB, Chalmers TC, Dear KBG, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182-193.
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:1-10.
- Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202-209.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
- Manon C, Rittenhouse BE, Perreault S, et al. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Amer Soc Anesth Inc. 1998;89:1377-1388.
- Krenn H, Oczenski W, Jellinek H, Krumpl-Ströher M, Schweitzer E, Fitzgerald RD. Nalbuphine by PCA-pump for analgesia following hysterectomy: Bolus application versus continuous infusion with bolus application. Eur J Pain. 2001;5:219-226.
- Lehmann KA. Recent developments in patient-controlled analgesia. J Pain Symptom Manage. 2005;29:S72-S89.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. 2003:1-73.
- Macintyre PC, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1995;64:357-364.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Adult cancer pain. Version 2.2005:1-30.
- Ginsberg B, Gil KM, Muir M, Sullivan F, Williams DA, Glass PSA. The influence of lockout intervals and drug selection on patient-controlled analgesia following gynecological surgery. Pain. 1995;62:95-100.
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. Acta Anaesthesiol Scand. 2001;45:795-804.
- Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125-132.
- Oswalt KE, Shrewsbury P, Stanton-Hicks M. The incidence of medication mishaps in 3,299 PCA patients. Pain. 1990;S5;S152.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose rations for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-687.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
- Mercandante S. Opioid rotation for cancer pain. Cancer. 1999;86:1856-1866.
- Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharm Sciences. 2001;22:67-70.
Case
A 69-year-old female with metastatic ovarian cancer and chronic pain syndrome presented to the hospital with seven days of progressively worsening abdominal pain. The pain had been similar to her chronic cancer pain but more severe. She has acute renal failure secondary to volume depletion from poor intake. A CT scan of the abdomen and pelvis reveal progression of her cancer with acute pathology. What is the best method of treating this patient’s pain?
Overview
Pain is pandemic. It is the most common reason patients seek healthcare.1 Almost one-third of Americans will experience severe chronic pain at some point in their lives. Every year, approximately 25 million Americans experience acute pain and 50 million experience chronic pain. Only one in four patients with pain receives appropriate therapy and control of their pain.
Pain is the most common symptom experienced by hospitalized adults.2 Acute or chronic pain can be particularly challenging to treat because these patients are frequently opioid dependent and have many psychosocial factors. No one method of pain control is superior to another. However, one method to gain rapid control of an acute pain crisis in a patient with chronic pain is to use patient-controlled analgesia (PCA).
Review of the Data
The first commercially available PCA pumps became available in 1976.3 They were created after studies in the 1960s demonstrated that small doses of opioids given intravenously provided more effective pain relief than conventional intramuscular injections.
The majority of studies on PCAs are in the postoperative patient, with cancer pain being next most commonly studied. PCAs utilize microprocessor-controlled infusion pumps that deliver a preprogrammed dose of opioid when the patient pushes the demand button. They allow programming of dose (demand dose), time between doses (lockout interval), background infusion rate (basal rate), and nurse-initiated dose (bolus dose).
The PCA paradigm is based on the opioid pharmacologic concept of minimum effective analgesic concentration (MEAC).4,5 The MEAC is the smallest serum opioid concentration at which pain is relieved. The dose-response curve to opioids is sigmoidal such that minimal analgesia is achieved until the MEAC is reached, after which minute increases in opioid concentrations produce analgesia, until further increases produce no significant increased analgesic effect.
PCAs allow individualized dosing and titration to achieve the MEAC, with small incremental doses administered whenever the serum concentration falls below the MEAC. A major goal of PCA technology is to regulate drug delivery to rapidly achieve and maintain the MEAC.
Advantages of PCAs
- More individual dosing and titration of pain medications to account for inter-individual and intra-individual variability in the response to opioids;
- Negative feedback control system, an added safety measure to avoid respiratory depression. As patients become too sedated from opioids, they are no longer able to push the button to receive further opioids;
- Higher patient satisfaction with pain control, a major determinant being personal control over the delivery of pain relief;6-8 and
- Greater analgesic efficacy vs. conventional analgesia.
Disadvantages of PCAs
Select patient populations: Not all patients are able to understand and retain the required instructions necessary to safely or effectively use self-administered opioids (e.g., cognitively impaired patients).
Potential for opioid dosing errors: These are related to equipment factors, medical personnel prescribing or programming errors.
Increased cost: PCAs have been shown to be more expensive in comparison with intramuscular (IM) injections, the prior standard of care.9-10
PCA Prescribing
The parameters programmed into the PCA machine include the basal rate, demand (or incremental) dose, lockout interval, nurse-initiated bolus dose, and choice of opioid.
Basal rate: The continuous infusion of opioid set at an hourly rate. Most studies that compare PCA use with and without basal rates (in postoperative patients) do not show improved pain relief or sleep with basal rates.11 Basal rates have been associated with increased risk of sedation and respiratory depression.12
The routine use of basal rates is not recommended initially, unless a patient is opioid-tolerant (i.e., on chronic opioid therapy). For patients on chronic opioids, their 24-hour total opioid requirement is converted by equianalgesic dosing to the basal rate. Steady state is not achieved for eight to 12 hours of continuous infusion; therefore, it is not recommended to change the basal rate more frequently than every eight hours.13
Demand dose: The dose patients provide themselves by pushing the button. Studies on opioid-naïve patients using morphine PCAs have shown that 1 mg IV morphine was the optimal starting dose, based on good pain relief without respiratory depression. Lower doses, such as 0.5 mg IV morphine, are generally used in the elderly as opioid requirements are known to decrease with patient age.14
For patients with a basal rate, the demand dose is often set at 50% to 100% of the basal rate. The demand dose is the parameter that should be titrated up for acute pain control. World Health Organization guidelines recommend increasing the dose by 25% to 50% for mild to moderate pain, and 50% to 100% for moderate to severe pain.15
Lockout interval: Minimal allowable time between demand doses. This time is based on the time to peak effect of IV opioids and can vary from five to 15 minutes. The effects of varying lockout intervals—seven to 11 minutes for morphine and five to eight minutes for fentanyl—had no effect on pain levels or side effects.16 Ten minutes is a standard lockout interval.
Bolus dose: The nurse-initiated dose that may be given initially to achieve pain control and later to counteract incidental pain (e.g., that caused by physical therapy, dressing changes, or radiology tests). A recommended dose is equivalent to the basal rate or twice the demand dose.
Choice of opioid: Morphine is the standard opioid because of its familiarity, cost, and years of study. Although inter-individual variability exists, there are no major differences in side effects among the different opioids. Renal and hepatic insufficiency can increase the effects of opioids. Morphine is especially troublesome in renal failure because it has an active metabolite—morphine-6-glucuronide—that can accumulate and increase the risk of sedation and respiratory depression.
Other Concerns
PCA complications: The most well-studied adverse effects of PCAs are nausea and respiratory depression. There is no difference between PCAs and conventional analgesia in rates of nausea or respiratory depression.17
Nausea is the most common side effect in postoperative patients on PCAs. Patients rapidly develop tolerance to nausea over a period of days. However, many clinicians are concerned about respiratory depression and the risk of death. The overall incidence of respiratory depression with PCAs is less than 1% (range from 0.1 to 0.8%), similar to conventional analgesia. However, the incidence is significantly higher when basal rates are used, rising to 1.1 to 3.9%. Other factors predisposing a patient to increased risk of respiratory depression are older age, obstructive sleep apnea, hypovolemia, renal failure, and the concurrent use of other sedating medications.18
Medication errors are also common. The overall incidence of medication mishaps with PCAs is 1.2%.19 More than 50% of these occur because of operator-related errors (e.g., improper loading, programming errors, and documentation errors). Equipment malfunction is the next most common error.
Opioid equianalgesic dosing conversions: The equianalgesic dose ratio is the ratio of the dose of two opioids required to produce the same analgesic effect. (See Table 1, right.) For example, IV morphine is three times as potent as oral morphine, with an equianalgesic dose ratio of 1:3. Equianalgesic dose tables vary somewhat in their values, which have been largely determined by single-dose administration studies.20 The generalizability of these tables to chronic opioid administration is not well studied.
Incomplete cross tolerance: When switching from one opioid to another, lower doses can be used to control pain.21, 22 Tolerance to one opioid does not completely transfer to the new opioid. Starting at half to two-thirds of the new opioid dose is generally recommended to avoid opioid-specific tolerance and inter-individual variability.23,24
Back to the Case
Opioids are the mainstay of pharmacological management of moderate-to-severe cancer pain. Evaluation of the patient reveals that her acute increase in pain is likely due to progression of her cancer. She had been taking morphine (sustained-release, 90 mg oral) twice daily for her pain and had been using approximately five doses per day of immediate-release oral morphine 20 mg for breakthrough pain. This is equivalent to a total 24-hour opioid requirement of 280 mg oral morphine.
She should be started on a PCA for rapid pain control and titration. Hydromorphone (Dilaudid) is a better PCA choice than morphine because she has acute renal failure. The equianalgesic dose ratio of oral morphine to IV hydromorphone is approximately 30:1.5. The total 24-hour opioid dose of 280 mg oral morphine is equivalent to 14 mg IV hydromorphone ([280mg morphine per day ÷ 30] x 1.5 = 14).
After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 8.4 mg IV hydromorphone (14 mg x 0.6 = 8.4 mg). This is approximately equivalent to 0.4 mg IV hydromorphone/hour (8.4 mg ÷ 24 hours), which is her initial basal rate. The demand dose should be set at 0.2 mg (50% the basal rate) with a lockout interval of 10 minutes.
Over a period of several days, the patient’s pain was controlled and her opioid requirements stabilized. She was on a basal rate of 1.4 mg/hour and a demand dose of 1 mg with a 10-minute lockout. Her total 24-hour opioid requirement was 44 mg of IV hydromorphone. As her renal function improved but did not completely normalize, oxycodone was chosen over morphine when converting her back to oral pain medications (less active renal metabolites). The equianalgesic dose ratio of oral oxycodone to IV hydromorphone is approximately 20:1.5. Her total 24-hour opioid dose of 44 mg IV hydromorphone is equivalent to 587 mg oral oxycodone (44 ÷ 1.5) x 20. After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 352 mg oral oxycodone or 180 mg of sustained-release oxycodone twice daily (352 mg ÷ 2 ≈ 180 mg). For breakthrough pain she should receive 40 mg of immediate-release oxycodone every hour as needed (10% to 15% of the 24-hour opioid requirement). TH
Dr. Youngwerth is a hospitalist and instructor of medicine, University of Colorado at Denver, assistant director, Palliative Care Consult Service, associate director, Colorado Palliative Medicine Fellowship Program, and medical director, Hospice of Saint John.
References
- American Pain Society. Pain: Current understanding of assessment, management, and treatments. National Pharmaceutical Council 2006;1-79.
- Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166:1033-1039.
- Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44-S61.
- Etches RC. Patient-controlled analgesia. Surg Clinics N Amer. 1999;79:297-312.
- Nolan MF and Wilson M-C B. Patient-controlled analgesia: A method for the controlled self-administration of opioid pain medications. Phys Ther. 1995;75:374-379.
- Ballantyne JC, Carr DB, Chalmers TC, Dear KBG, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182-193.
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:1-10.
- Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202-209.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
- Manon C, Rittenhouse BE, Perreault S, et al. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Amer Soc Anesth Inc. 1998;89:1377-1388.
- Krenn H, Oczenski W, Jellinek H, Krumpl-Ströher M, Schweitzer E, Fitzgerald RD. Nalbuphine by PCA-pump for analgesia following hysterectomy: Bolus application versus continuous infusion with bolus application. Eur J Pain. 2001;5:219-226.
- Lehmann KA. Recent developments in patient-controlled analgesia. J Pain Symptom Manage. 2005;29:S72-S89.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. 2003:1-73.
- Macintyre PC, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1995;64:357-364.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Adult cancer pain. Version 2.2005:1-30.
- Ginsberg B, Gil KM, Muir M, Sullivan F, Williams DA, Glass PSA. The influence of lockout intervals and drug selection on patient-controlled analgesia following gynecological surgery. Pain. 1995;62:95-100.
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. Acta Anaesthesiol Scand. 2001;45:795-804.
- Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125-132.
- Oswalt KE, Shrewsbury P, Stanton-Hicks M. The incidence of medication mishaps in 3,299 PCA patients. Pain. 1990;S5;S152.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose rations for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-687.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
- Mercandante S. Opioid rotation for cancer pain. Cancer. 1999;86:1856-1866.
- Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharm Sciences. 2001;22:67-70.
Malpractice Chronicle
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Oversedated, Unresponsive After Aneurysm Surgery
A 73-year-old man went to a hospital emergency department (ED) with complaints of chest pain, bloating, and blood in his stools. CT revealed an aortic aneurysm, and surgery was performed three days later. In the recovery room, the patient became confused and agitated. He managed to leave the building before several nurses were able to restrain him and return him to his room.
It was determined that the man was experiencing symptoms of alcohol withdrawal, making him uncooperative and potentially violent. He was started on a detoxification protocol, which included lorazepam and haloperidol.
Over the next several days, the patient appeared oversedated and unresponsive, and his lorazepam dosage was reduced. When he developed a rattle in his chest and began to have trouble breathing, no action was taken. He went into respiratory arrest and was intubated; during this procedure, he sustained a punctured lung. Although the man was resuscitated, he was left with anoxic brain injury. He remained in a deep coma until his ventilator was turned off.
Plaintiffs for the decedent claimed that he was oversedated. He developed a mucous plug that he was unable to clear because of physical restraints and a decreased level of consciousness.
According to a published account, a settlement of $107,500 was reached.
Discontinued Monitoring for Abdominal Cyst
The plaintiff child, an infant girl, was delivered in September by an Ob-Gyn from a women's health practice. Her mother had undergone serial fetal ultrasonography to monitor an abdominal cyst that was detected in the unborn child early in the woman's pregnancy.
During the eight weeks following hospital discharge, the child was seen numerous times by physicians at a pediatrics/adolescent medicine group for digestive issues, including nausea, vomiting, diarrhea, and pale-colored stools. In late October, the mother asked one of the pediatricians whether the infant's problems could be related to the cyst; she questioned whether the prenatal ultrasounds had been faxed to the pediatrician's office.
When no follow-up testing was ordered, the parents took the infant to another doctor, who had her admitted. She was diagnosed with a choledochal cyst (a congenital bile duct anomaly) and liver failure. During surgery, associated biliary atresia was also discovered.
In February, the infant underwent a liver transplant. She suffered one episode of liver rejection and will require a lifelong regimen of antirejection medications.
According to the plaintiff, an Ob-Gyn had assured the mother that an order for postpartum ultrasonography would be placed in the chart to ensure follow-up on the infant's cyst, but the test was never ordered. The plaintiffs also claimed that the Ob-Gyn never informed the pediatrics/adolescent medicine group about the cyst and that no information about the cyst was placed in the newborn's charts by hospital nurses. The plaintiff claimed that proper monitoring would have led to early intervention at a time when the infant's liver was salvageable.
The defendants contended that they had communicated properly and that the child would still have needed a liver transplant, even if testing had been conducted earlier.
During the trial, a confidential settlement was reached with the hospital and the pediatrics group. According to a published report, a $16.5 million verdict was returned against the women's health practice.
Abscess Develops Following a Fall
One day at work, a 54-year-old man fell off his chair and landed on his right hip. He did not seek immediate medical attention but was in considerable pain by the time he arrived home. During the night, his pain worsened, and his wife called an ambulance.
When the patient arrived at the ED, he was barely able to walk and reported a 9 on the 10-point pain scale. His skin moistness, color, and temperature were all normal. An IV was started, and the man was given morphine. When his pain persisted, he was given ketorolac tromethamine, meperidine, and hydroxyzine pamoate.
The ED physician made a diagnosis of acute lumbosacral strain and released the man with prescriptions and instructions for hydrocodone/acetaminophen and cyclobenzaprine. He was to be on bed rest for two days, remain home from work for two more days, and see his primary care provider in seven to 10 days if his condition had not resolved.
The man's pain became increasingly severe. He could not walk unaided, and after four days, he was pale or grayish in color, clammy, sweaty, and short of breath. On the fifth day, this US Army veteran kept an appointment at a US Air Force/university hospital–based clinic, where a second-year resident examined him, diagnosed muscle sprain, and prescribed a few more days of rest. The patient was not seen by the supervising physician.
The next day, the man was unable to urinate; the following day, he began to hallucinate. He was transported to an ED, where he was placed on life support. The day after his admission, CT confirmed the presence of a retroperitoneal (psoas) abscess and showed that the abscess was encroaching into the epidural space. By this time, his body had swelled to the point that his skin could no longer expand to accommodate it and had begun to crack.
A surgeon who was consulted diagnosed overwhelming sepsis, making the patient too unstable for surgery. By the time the decision was made to airlift him to the university hospital, the man was technically too heavy for the helicopter guidelines. He died one week later.
The case centered around a dispute regarding what the clinic's second-year resident had told the supervising physician regarding the decedent's symptoms. The court ultimately concluded that there was negligence on the part of both physicians, which led to the patient's death. According to a published report, a bench verdict of $8,265,009 was returned.
Infectious Mononucleosis Misdiagnosed as URI
A 19-year-old college student was treated by the defendant family practitioner, Dr. F., during repeated bouts of sinus infection and upper respiratory infection (URI). When the student's illness recurred in November while he was away at school, he was seen twice by an otolaryngologist. The student received a diagnosis of URI, for which he was given antibiotics and a steroid injection.
During the patient's winter break from school, he was still unwell. He called Dr. F.'s office for an appointment and was seen three days later. No lab work or tests were ordered, but Dr. F. made a diagnosis of strep throat and prescribed antibiotics. The student was also instructed that if his condition persisted or worsened, he should call Dr. F.
Two days later, the patient's mother phoned Dr. F.'s office to report that her son was experiencing severe abdominal pain. Because it was the weekend, the mother spoke with an on-call physician, who ordered a change in antibiotics. The patient's condition improved somewhat, but by the next afternoon, the pain had returned, and he was nauseated and vomiting.
He was taken to an ED, where a diagnosis of infectious mononucleosis was made. Less than an hour later, the patient went into cardiac arrest and died.
An autopsy revealed that the decedent's spleen had become enlarged to 10 times its normal size and ruptured, leading to massive internal bleeding and death.
The plaintiff alleged negligence in Dr. F.'s failure to diagnose infectious mononucleosis, claiming that the physician had not examined the decedent's abdomen during the office visit; had this examination been performed, the decedent's enlarged spleen would have been easily detected. The plaintiff further claimed that Dr. F.'s erroneous diagnosis had discouraged the decedent from seeking ED treatment when his condition first worsened.
The defendant denied any negligence, arguing that the decedent's death was an extremely rare complication of infectious mononucleosis and that the outcome would have been the same, even if the decedent had gone to the ED when his worsening symptoms began.
A defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Oversedated, Unresponsive After Aneurysm Surgery
A 73-year-old man went to a hospital emergency department (ED) with complaints of chest pain, bloating, and blood in his stools. CT revealed an aortic aneurysm, and surgery was performed three days later. In the recovery room, the patient became confused and agitated. He managed to leave the building before several nurses were able to restrain him and return him to his room.
It was determined that the man was experiencing symptoms of alcohol withdrawal, making him uncooperative and potentially violent. He was started on a detoxification protocol, which included lorazepam and haloperidol.
Over the next several days, the patient appeared oversedated and unresponsive, and his lorazepam dosage was reduced. When he developed a rattle in his chest and began to have trouble breathing, no action was taken. He went into respiratory arrest and was intubated; during this procedure, he sustained a punctured lung. Although the man was resuscitated, he was left with anoxic brain injury. He remained in a deep coma until his ventilator was turned off.
Plaintiffs for the decedent claimed that he was oversedated. He developed a mucous plug that he was unable to clear because of physical restraints and a decreased level of consciousness.
According to a published account, a settlement of $107,500 was reached.
Discontinued Monitoring for Abdominal Cyst
The plaintiff child, an infant girl, was delivered in September by an Ob-Gyn from a women's health practice. Her mother had undergone serial fetal ultrasonography to monitor an abdominal cyst that was detected in the unborn child early in the woman's pregnancy.
During the eight weeks following hospital discharge, the child was seen numerous times by physicians at a pediatrics/adolescent medicine group for digestive issues, including nausea, vomiting, diarrhea, and pale-colored stools. In late October, the mother asked one of the pediatricians whether the infant's problems could be related to the cyst; she questioned whether the prenatal ultrasounds had been faxed to the pediatrician's office.
When no follow-up testing was ordered, the parents took the infant to another doctor, who had her admitted. She was diagnosed with a choledochal cyst (a congenital bile duct anomaly) and liver failure. During surgery, associated biliary atresia was also discovered.
In February, the infant underwent a liver transplant. She suffered one episode of liver rejection and will require a lifelong regimen of antirejection medications.
According to the plaintiff, an Ob-Gyn had assured the mother that an order for postpartum ultrasonography would be placed in the chart to ensure follow-up on the infant's cyst, but the test was never ordered. The plaintiffs also claimed that the Ob-Gyn never informed the pediatrics/adolescent medicine group about the cyst and that no information about the cyst was placed in the newborn's charts by hospital nurses. The plaintiff claimed that proper monitoring would have led to early intervention at a time when the infant's liver was salvageable.
The defendants contended that they had communicated properly and that the child would still have needed a liver transplant, even if testing had been conducted earlier.
During the trial, a confidential settlement was reached with the hospital and the pediatrics group. According to a published report, a $16.5 million verdict was returned against the women's health practice.
Abscess Develops Following a Fall
One day at work, a 54-year-old man fell off his chair and landed on his right hip. He did not seek immediate medical attention but was in considerable pain by the time he arrived home. During the night, his pain worsened, and his wife called an ambulance.
When the patient arrived at the ED, he was barely able to walk and reported a 9 on the 10-point pain scale. His skin moistness, color, and temperature were all normal. An IV was started, and the man was given morphine. When his pain persisted, he was given ketorolac tromethamine, meperidine, and hydroxyzine pamoate.
The ED physician made a diagnosis of acute lumbosacral strain and released the man with prescriptions and instructions for hydrocodone/acetaminophen and cyclobenzaprine. He was to be on bed rest for two days, remain home from work for two more days, and see his primary care provider in seven to 10 days if his condition had not resolved.
The man's pain became increasingly severe. He could not walk unaided, and after four days, he was pale or grayish in color, clammy, sweaty, and short of breath. On the fifth day, this US Army veteran kept an appointment at a US Air Force/university hospital–based clinic, where a second-year resident examined him, diagnosed muscle sprain, and prescribed a few more days of rest. The patient was not seen by the supervising physician.
The next day, the man was unable to urinate; the following day, he began to hallucinate. He was transported to an ED, where he was placed on life support. The day after his admission, CT confirmed the presence of a retroperitoneal (psoas) abscess and showed that the abscess was encroaching into the epidural space. By this time, his body had swelled to the point that his skin could no longer expand to accommodate it and had begun to crack.
A surgeon who was consulted diagnosed overwhelming sepsis, making the patient too unstable for surgery. By the time the decision was made to airlift him to the university hospital, the man was technically too heavy for the helicopter guidelines. He died one week later.
The case centered around a dispute regarding what the clinic's second-year resident had told the supervising physician regarding the decedent's symptoms. The court ultimately concluded that there was negligence on the part of both physicians, which led to the patient's death. According to a published report, a bench verdict of $8,265,009 was returned.
Infectious Mononucleosis Misdiagnosed as URI
A 19-year-old college student was treated by the defendant family practitioner, Dr. F., during repeated bouts of sinus infection and upper respiratory infection (URI). When the student's illness recurred in November while he was away at school, he was seen twice by an otolaryngologist. The student received a diagnosis of URI, for which he was given antibiotics and a steroid injection.
During the patient's winter break from school, he was still unwell. He called Dr. F.'s office for an appointment and was seen three days later. No lab work or tests were ordered, but Dr. F. made a diagnosis of strep throat and prescribed antibiotics. The student was also instructed that if his condition persisted or worsened, he should call Dr. F.
Two days later, the patient's mother phoned Dr. F.'s office to report that her son was experiencing severe abdominal pain. Because it was the weekend, the mother spoke with an on-call physician, who ordered a change in antibiotics. The patient's condition improved somewhat, but by the next afternoon, the pain had returned, and he was nauseated and vomiting.
He was taken to an ED, where a diagnosis of infectious mononucleosis was made. Less than an hour later, the patient went into cardiac arrest and died.
An autopsy revealed that the decedent's spleen had become enlarged to 10 times its normal size and ruptured, leading to massive internal bleeding and death.
The plaintiff alleged negligence in Dr. F.'s failure to diagnose infectious mononucleosis, claiming that the physician had not examined the decedent's abdomen during the office visit; had this examination been performed, the decedent's enlarged spleen would have been easily detected. The plaintiff further claimed that Dr. F.'s erroneous diagnosis had discouraged the decedent from seeking ED treatment when his condition first worsened.
The defendant denied any negligence, arguing that the decedent's death was an extremely rare complication of infectious mononucleosis and that the outcome would have been the same, even if the decedent had gone to the ED when his worsening symptoms began.
A defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Oversedated, Unresponsive After Aneurysm Surgery
A 73-year-old man went to a hospital emergency department (ED) with complaints of chest pain, bloating, and blood in his stools. CT revealed an aortic aneurysm, and surgery was performed three days later. In the recovery room, the patient became confused and agitated. He managed to leave the building before several nurses were able to restrain him and return him to his room.
It was determined that the man was experiencing symptoms of alcohol withdrawal, making him uncooperative and potentially violent. He was started on a detoxification protocol, which included lorazepam and haloperidol.
Over the next several days, the patient appeared oversedated and unresponsive, and his lorazepam dosage was reduced. When he developed a rattle in his chest and began to have trouble breathing, no action was taken. He went into respiratory arrest and was intubated; during this procedure, he sustained a punctured lung. Although the man was resuscitated, he was left with anoxic brain injury. He remained in a deep coma until his ventilator was turned off.
Plaintiffs for the decedent claimed that he was oversedated. He developed a mucous plug that he was unable to clear because of physical restraints and a decreased level of consciousness.
According to a published account, a settlement of $107,500 was reached.
Discontinued Monitoring for Abdominal Cyst
The plaintiff child, an infant girl, was delivered in September by an Ob-Gyn from a women's health practice. Her mother had undergone serial fetal ultrasonography to monitor an abdominal cyst that was detected in the unborn child early in the woman's pregnancy.
During the eight weeks following hospital discharge, the child was seen numerous times by physicians at a pediatrics/adolescent medicine group for digestive issues, including nausea, vomiting, diarrhea, and pale-colored stools. In late October, the mother asked one of the pediatricians whether the infant's problems could be related to the cyst; she questioned whether the prenatal ultrasounds had been faxed to the pediatrician's office.
When no follow-up testing was ordered, the parents took the infant to another doctor, who had her admitted. She was diagnosed with a choledochal cyst (a congenital bile duct anomaly) and liver failure. During surgery, associated biliary atresia was also discovered.
In February, the infant underwent a liver transplant. She suffered one episode of liver rejection and will require a lifelong regimen of antirejection medications.
According to the plaintiff, an Ob-Gyn had assured the mother that an order for postpartum ultrasonography would be placed in the chart to ensure follow-up on the infant's cyst, but the test was never ordered. The plaintiffs also claimed that the Ob-Gyn never informed the pediatrics/adolescent medicine group about the cyst and that no information about the cyst was placed in the newborn's charts by hospital nurses. The plaintiff claimed that proper monitoring would have led to early intervention at a time when the infant's liver was salvageable.
The defendants contended that they had communicated properly and that the child would still have needed a liver transplant, even if testing had been conducted earlier.
During the trial, a confidential settlement was reached with the hospital and the pediatrics group. According to a published report, a $16.5 million verdict was returned against the women's health practice.
Abscess Develops Following a Fall
One day at work, a 54-year-old man fell off his chair and landed on his right hip. He did not seek immediate medical attention but was in considerable pain by the time he arrived home. During the night, his pain worsened, and his wife called an ambulance.
When the patient arrived at the ED, he was barely able to walk and reported a 9 on the 10-point pain scale. His skin moistness, color, and temperature were all normal. An IV was started, and the man was given morphine. When his pain persisted, he was given ketorolac tromethamine, meperidine, and hydroxyzine pamoate.
The ED physician made a diagnosis of acute lumbosacral strain and released the man with prescriptions and instructions for hydrocodone/acetaminophen and cyclobenzaprine. He was to be on bed rest for two days, remain home from work for two more days, and see his primary care provider in seven to 10 days if his condition had not resolved.
The man's pain became increasingly severe. He could not walk unaided, and after four days, he was pale or grayish in color, clammy, sweaty, and short of breath. On the fifth day, this US Army veteran kept an appointment at a US Air Force/university hospital–based clinic, where a second-year resident examined him, diagnosed muscle sprain, and prescribed a few more days of rest. The patient was not seen by the supervising physician.
The next day, the man was unable to urinate; the following day, he began to hallucinate. He was transported to an ED, where he was placed on life support. The day after his admission, CT confirmed the presence of a retroperitoneal (psoas) abscess and showed that the abscess was encroaching into the epidural space. By this time, his body had swelled to the point that his skin could no longer expand to accommodate it and had begun to crack.
A surgeon who was consulted diagnosed overwhelming sepsis, making the patient too unstable for surgery. By the time the decision was made to airlift him to the university hospital, the man was technically too heavy for the helicopter guidelines. He died one week later.
The case centered around a dispute regarding what the clinic's second-year resident had told the supervising physician regarding the decedent's symptoms. The court ultimately concluded that there was negligence on the part of both physicians, which led to the patient's death. According to a published report, a bench verdict of $8,265,009 was returned.
Infectious Mononucleosis Misdiagnosed as URI
A 19-year-old college student was treated by the defendant family practitioner, Dr. F., during repeated bouts of sinus infection and upper respiratory infection (URI). When the student's illness recurred in November while he was away at school, he was seen twice by an otolaryngologist. The student received a diagnosis of URI, for which he was given antibiotics and a steroid injection.
During the patient's winter break from school, he was still unwell. He called Dr. F.'s office for an appointment and was seen three days later. No lab work or tests were ordered, but Dr. F. made a diagnosis of strep throat and prescribed antibiotics. The student was also instructed that if his condition persisted or worsened, he should call Dr. F.
Two days later, the patient's mother phoned Dr. F.'s office to report that her son was experiencing severe abdominal pain. Because it was the weekend, the mother spoke with an on-call physician, who ordered a change in antibiotics. The patient's condition improved somewhat, but by the next afternoon, the pain had returned, and he was nauseated and vomiting.
He was taken to an ED, where a diagnosis of infectious mononucleosis was made. Less than an hour later, the patient went into cardiac arrest and died.
An autopsy revealed that the decedent's spleen had become enlarged to 10 times its normal size and ruptured, leading to massive internal bleeding and death.
The plaintiff alleged negligence in Dr. F.'s failure to diagnose infectious mononucleosis, claiming that the physician had not examined the decedent's abdomen during the office visit; had this examination been performed, the decedent's enlarged spleen would have been easily detected. The plaintiff further claimed that Dr. F.'s erroneous diagnosis had discouraged the decedent from seeking ED treatment when his condition first worsened.
The defendant denied any negligence, arguing that the decedent's death was an extremely rare complication of infectious mononucleosis and that the outcome would have been the same, even if the decedent had gone to the ED when his worsening symptoms began.
A defense verdict was returned.
Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
CASE Brachial plexus injury, then a summons
J.L., a 29-year-old primigravida, has gestational diabetes. When she goes into labor at term, she reports to the state-of-the-art hospital where you practice. Delivery is difficult and achieved using forceps. The infant weighs 9 lb 4 oz, and has obvious weakness in his right arm. A neurologist diagnoses Erb’s palsy, and the child undergoes brachial plexus exploration and repair of injured nerves.
Two years later, most arm function has returned. Soon thereafter, you receive a summons from the parents and their attorney demanding $3 million. Do you fight—or settle?
You could say there are two types of physicians: those who have been sued and those who will be.
This is an overstatement, of course, but not by much. In high-risk specialties such as obstetrics, most physicians will receive a summons at some point in their career. In fact, almost nine of every 10 ObGyns report that they have been sued at least once in their career, with an average of 2.6 claims each.Got malpractice distress? You can help yourself survive,” in the February 2008 issue of OBG Mangement, available at www.obgmanagment.comGiven how stressful litigation can be, there are a number of considerations that enter into the calculus of fight or settle. This article will focus on seven of those considerations (see the box above).
How consent-to-settle clauses can protect you
For years, many carriers curried favor with physicians by barring settlement of a case unless the physician agreed to it. If the physician balked, the carrier was obligated to defend the case to the end.
This clause is still found in professional liability policies, but the number of carriers offering such flexibility has decreased considerably. Many carriers now base the decision to settle on both the merits of a case and the cost of defense. If the carrier determines that it would be much less expensive to settle a case for nuisance value than to defend it through trial, the carrier is within its rights to settle. Obviously, this posture has ramifications for the insured physician.
A consent-to-settle clause—or its omission—is usually established contractually at the beginning of coverage. If the ability to demand consent for settling is important to you, look closely for such language when you purchase or renew coverage. State law can also determine whether such a clause is included.
In addition to a standard consent-to-settle provision, some carriers promote a “hammer clause,” by which an insurer’s liability is limited to a recommended settlement. Let’s say the carrier decides to settle a particular case for $100,000, the physician withholds consent, and a judgment of $300,000 is entered. The physician is individually liable for the “overage”—in this case, $200,000.
As if this were not complicated enough, there is also a modified hammer clause, which is a “kinder, gentler” approach. In this scenario, the physician is liable only for a percentage of any judgment above the recommended settlement. In the example just given, if the modified hammer provision were 50%, the carrier would pay its recommended settlement ($100,000) plus 50% of the overage—in this case, another $100,000, for a total of $200,000. The physician would be liable for the remaining $100,000.
Without a consent-to-settle clause, the physician is removed from decision-making. Further, a hammer clause or modified hammer clause should cause a physician to think long and hard before forgoing a recommended settlement.
When personal liability exceeds policy limits
Even if the carrier is bound, through its contract with you, to defend a case to the end, it will generally be limited to a maximum payout. Policy limits depend on the particular policy, with higher limits associated with higher premiums.
Carrying a very high limit can make you a more appealing target for a lawsuit, frivolous or otherwise. Many personal injury attorneys view medical malpractice as little more than a series of insurance transactions. If you have a high coverage limit, you will attract greater attention. This is of particular concern when there are multiple defendants and culpability varies significantly between the actors.
When negligence is proven in states that still allow joint and several liability, even 1% liability can leave you responsible for the entire amount. The solution is to have reasonable—but not excessive—coverage. Many believe this balance lies at $1 million/$3 million limits.
Desire for a payout may persuade a plaintiff to settle for policy limits
If you have coverage of up to $1 million and a court delivers a higher judgment, what happens?
It depends. In theory, you are liable for the overage; the carrier will pay up to the policy limit, and you are responsible for the rest. In reality, however, the situation is more complex.
You often have the right to demand a new trial or appeal the case. You may not prevail, but this approach creates new risks for the plaintiff right after “victory” is tasted. Rather than roll the dice, many plaintiffs, under the advice of their attorney, will reconsider and settle for the policy limit. It is in their interest to lock in a certain figure rather than prolong the case, exposing themselves to increased risk. And if the judgment makes it clear that bankruptcy is an option for the physician, a plaintiff will take pains to prevent that end game. Once bankruptcy is filed, the clock slows, and it may take years for the plaintiff to receive any funds. Even then, the plaintiff may have to wait in line behind more senior creditors.
Consider asset protection
Asset protection prior to litigation can affect the dynamics of posttrial settlement discussions. Asset protection means many things, and there are different degrees of protection. A limited number of attorneys are skilled in asset protection, and plaintiff’s attorneys generally have limited experience breaking through the shield.
With a robust asset-protection program in place, you can come to the table with greater leverage and engage in a more rational discussion about a just settlement in which most, if not all, of the settlement will be within the policy limit.
There are approximately 50,000 to 60,000 medicolegal cases open at any given moment, but the number of physicians involved is much higher because many suits name multiple defendants.3 In 2004, the National Practitioner Data Bank (NPDB) reported entries for more than 200,000 health-care providers since 1990, most of whom had been reported just one time.4 Again that number is low because not every physician who is sued is reported to the NPDB. Reporting is required only if payment is made by settlement or judgment related to a written demand by a plaintiff. If the case against the physician is dismissed, or the physician wins in court, no report is entered. So the 200,000 entries are just the tip of the iceberg. With roughly 700,000 physicians practicing in the United States, the number of physicians affected by liability litigation could be staggering.
When you want to settle, but the carrier doesn’t
Ordinarily, your interests and those of your carrier are aligned. You both want to win—or at least lose less—but there is one scenario in which your interests may diverge. That is when you believe you are at risk for a judgment that will exceed the policy limit. In such a situation, you want your carrier to tender the full limit, but the carrier faces a worst-case scenario: paying the maximum amount on the policy.
If the carrier believes the case is defensible, it may choose to fight, hoping to win or receive a judgment well below the policy limit. If the carrier’s strategy prevails, all parties will be better off. However, if the carrier gambles and loses, you will face the very scenario you hoped to avoid—exposure to a judgment beyond the policy limit.
The law generally provides that a carrier that wants to gamble must do so with its own money. To do otherwise constitutes action “in bad faith.” After judgment, many physicians sue their own insurance company on the basis of exactly that legal theory. It is even more common for a plaintiff, fresh from victory, to join forces with the doctor defendant and take action against the carrier.
This endgame is not automatic, however. If you want to minimize the risk that your pocket will be the only one left to pick after a high-stakes case ends, you must demand in writing that the case be settled up to the policy limit. Under such circumstances, it is best for your personal counsel to deliver that message because the carrier-appointed attorney faces something of a conflict, because she is an advocate for the physician but paid by the carrier.
Avoiding the National Practitioner Data Bank
In 1990, the National Practitioner Data Bank (NPDB) was launched with the goal of keeping dangerous physicians from migrating from state to state to escape accountability. A central database allows licensing agencies to quickly determine whether a doctor has a checkered past.
The NPDB labels a physician “as marked” if money is paid for a malpractice settlement or judgment. The NPDB lists hundreds of thousands of physicians, most of whom have a single entry. Many state licensing agencies have also begun listing physicians who have lost or settled a lawsuit; the only difference is that such information is posted online and is accessible to the public. In contrast, the NPDB remains confidential, accessible only by those who “need to know,” such as credentialing committees, hospitals, and licensing boards.
Even $1 can incur a listing
Many physicians wrongly believe that they will not be reported to the NPDB if they are involved in a case that settles for an amount under $30,000. Low-value settlements are often consummated for nuisance value, meaning that they have no legal merit. However, any payment—even $1—is reportable to the NPDB. It does not matter whether payment is made by settlement or judgment.
A written demand for money, whether as damages for an injury, money to see another physician, or a refund of cash tendered, can sometimes be construed as reportable.
Being joined to a corporate entity can help you
Seasoned plaintiff’s attorneys understand physicians’ deep aversion to being reported. They often take advantage of a well-known exception to reporting: payment made in the name of a corporate entity.
If a physician is employed by a corporation with at least two physicians, and the case is settled in the name of the corporation, the physician can be dismissed from the claim, and no reporting is required. In that case, the physician maintains his clean record in the data bank. That said, this can get complicated if one is obligated to report to state authorities. Because each state is different, these details should be addressed with your attorney.
High-low agreements can avert huge judgments
As discovery progresses, the plaintiff and defendant usually come to a better understanding of their respective risk—but not always. In some situations, the plaintiff may have a strong case in regard to one element of negligence, such as damages, but a weak case in regard to causation. Cerebral palsy cases fit this paradigm. In such cases, the infant has clear-cut medical and rehabilitation needs that can run easily into seven figures, but proving that a physician’s actions or omissions caused the injury can be difficult. Both sides can mitigate risk for one another by embracing a “high-low” agreement—a contract defining how a plaintiff will be paid based on a specific jury verdict.
For example, if the high-low agreement is $500,000/$100,000, the insurer is locked into one of two payments. If the jury returns a verdict for the defense, the carrier pays $100,000; if the verdict is for the plaintiff, the carrier pays $500,000, regardless of the amount of damages awarded by the jury. Without such an agreement, the range of potential judgments is no money at all to almost any amount.
When a high-low agreement is in effect, and the jury returns a verdict for the physician, the settlement is not reported to the NPDB even though the carrier must make a payment.
Why not?
The payment is being made pursuant to a separate agreement between the carrier and the plaintiff. The benefit to the insurer is the limitation of its liability, even if the plaintiff wins at trial and is awarded a higher amount. The benefit to the plaintiff is a guaranteed payment, even if there is no finding of liability against the practitioner.
How a reputation for settling can hurt
If you are so risk-averse that you demand that your carrier settle all cases—even those with no merit—two things will happen:
- Word will spread throughout the plaintiff’s bar that you are an easy target, and the threshold for filing suit against you will decline. And given how little work will be required to net a settlement, attorney’s summons will forever darken your door
- Your medical liability rates will climb—or coverage will be terminated. Settling meritorious cases makes sense, but settling all cases regardless of merit is ill-advised.
Stress is common on both sides of the equation
Lawsuits take a long time to percolate through the system, with an average time from medical event to claim resolution of about 5 years—longer in obstetrics.2
Attorneys are accustomed to this time frame; physicians are not. The lingering effects on doctors include stress, loss of job satisfaction, family strife, depression, substance abuse, and so on.
Because a lawsuit is a major stressful life event, a physician may be only too happy to be done with one. If there were absolutely no consequences to settling, that would be a smart move. But there are consequences, and living with them can also cause stress. The best way to minimize stress on either side of the equation is to think long and hard before settling any case.
1. Wilson N, Strunk A. Overview of the 2006 ACOG Survey on Professional Liability. Available at: www.acog.org/departments/professionalliability/2006surveyNatl.pdf. Accessed March 11, 2008.
2. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354:2024-2033.
3. Mello MM, Studdert DM. The medical malpractice system: structure and performance. In: Sage WM, Kersh R, eds. Medical Malpractice and the U.S. Health Care System: New Century, Different Issues. 1st ed. New York: Cambridge University Press; 2006:11–29.
4. US Department of Health and Human Services. National Practitioner Data Bank. 2004 annual report. Available at: http://www.npdb-hipdb.hrsa.gov/pubs/stats/2004_NPDB_Annual_Report.pdf. Accessed March 11, 2008.
More about professional liability by this author. Prepare your defense of a cerebral palsy claim (and other claims) long beforehand—in the prenatal period. That’s what Dr. Segal advised readers in the July 2007 issue of OBG Mangement. Read his argument for obtaining patient contracts in the “Past Issues” archives at www.obgmanagement.com.
CASE Brachial plexus injury, then a summons
J.L., a 29-year-old primigravida, has gestational diabetes. When she goes into labor at term, she reports to the state-of-the-art hospital where you practice. Delivery is difficult and achieved using forceps. The infant weighs 9 lb 4 oz, and has obvious weakness in his right arm. A neurologist diagnoses Erb’s palsy, and the child undergoes brachial plexus exploration and repair of injured nerves.
Two years later, most arm function has returned. Soon thereafter, you receive a summons from the parents and their attorney demanding $3 million. Do you fight—or settle?
You could say there are two types of physicians: those who have been sued and those who will be.
This is an overstatement, of course, but not by much. In high-risk specialties such as obstetrics, most physicians will receive a summons at some point in their career. In fact, almost nine of every 10 ObGyns report that they have been sued at least once in their career, with an average of 2.6 claims each.Got malpractice distress? You can help yourself survive,” in the February 2008 issue of OBG Mangement, available at www.obgmanagment.comGiven how stressful litigation can be, there are a number of considerations that enter into the calculus of fight or settle. This article will focus on seven of those considerations (see the box above).
How consent-to-settle clauses can protect you
For years, many carriers curried favor with physicians by barring settlement of a case unless the physician agreed to it. If the physician balked, the carrier was obligated to defend the case to the end.
This clause is still found in professional liability policies, but the number of carriers offering such flexibility has decreased considerably. Many carriers now base the decision to settle on both the merits of a case and the cost of defense. If the carrier determines that it would be much less expensive to settle a case for nuisance value than to defend it through trial, the carrier is within its rights to settle. Obviously, this posture has ramifications for the insured physician.
A consent-to-settle clause—or its omission—is usually established contractually at the beginning of coverage. If the ability to demand consent for settling is important to you, look closely for such language when you purchase or renew coverage. State law can also determine whether such a clause is included.
In addition to a standard consent-to-settle provision, some carriers promote a “hammer clause,” by which an insurer’s liability is limited to a recommended settlement. Let’s say the carrier decides to settle a particular case for $100,000, the physician withholds consent, and a judgment of $300,000 is entered. The physician is individually liable for the “overage”—in this case, $200,000.
As if this were not complicated enough, there is also a modified hammer clause, which is a “kinder, gentler” approach. In this scenario, the physician is liable only for a percentage of any judgment above the recommended settlement. In the example just given, if the modified hammer provision were 50%, the carrier would pay its recommended settlement ($100,000) plus 50% of the overage—in this case, another $100,000, for a total of $200,000. The physician would be liable for the remaining $100,000.
Without a consent-to-settle clause, the physician is removed from decision-making. Further, a hammer clause or modified hammer clause should cause a physician to think long and hard before forgoing a recommended settlement.
When personal liability exceeds policy limits
Even if the carrier is bound, through its contract with you, to defend a case to the end, it will generally be limited to a maximum payout. Policy limits depend on the particular policy, with higher limits associated with higher premiums.
Carrying a very high limit can make you a more appealing target for a lawsuit, frivolous or otherwise. Many personal injury attorneys view medical malpractice as little more than a series of insurance transactions. If you have a high coverage limit, you will attract greater attention. This is of particular concern when there are multiple defendants and culpability varies significantly between the actors.
When negligence is proven in states that still allow joint and several liability, even 1% liability can leave you responsible for the entire amount. The solution is to have reasonable—but not excessive—coverage. Many believe this balance lies at $1 million/$3 million limits.
Desire for a payout may persuade a plaintiff to settle for policy limits
If you have coverage of up to $1 million and a court delivers a higher judgment, what happens?
It depends. In theory, you are liable for the overage; the carrier will pay up to the policy limit, and you are responsible for the rest. In reality, however, the situation is more complex.
You often have the right to demand a new trial or appeal the case. You may not prevail, but this approach creates new risks for the plaintiff right after “victory” is tasted. Rather than roll the dice, many plaintiffs, under the advice of their attorney, will reconsider and settle for the policy limit. It is in their interest to lock in a certain figure rather than prolong the case, exposing themselves to increased risk. And if the judgment makes it clear that bankruptcy is an option for the physician, a plaintiff will take pains to prevent that end game. Once bankruptcy is filed, the clock slows, and it may take years for the plaintiff to receive any funds. Even then, the plaintiff may have to wait in line behind more senior creditors.
Consider asset protection
Asset protection prior to litigation can affect the dynamics of posttrial settlement discussions. Asset protection means many things, and there are different degrees of protection. A limited number of attorneys are skilled in asset protection, and plaintiff’s attorneys generally have limited experience breaking through the shield.
With a robust asset-protection program in place, you can come to the table with greater leverage and engage in a more rational discussion about a just settlement in which most, if not all, of the settlement will be within the policy limit.
There are approximately 50,000 to 60,000 medicolegal cases open at any given moment, but the number of physicians involved is much higher because many suits name multiple defendants.3 In 2004, the National Practitioner Data Bank (NPDB) reported entries for more than 200,000 health-care providers since 1990, most of whom had been reported just one time.4 Again that number is low because not every physician who is sued is reported to the NPDB. Reporting is required only if payment is made by settlement or judgment related to a written demand by a plaintiff. If the case against the physician is dismissed, or the physician wins in court, no report is entered. So the 200,000 entries are just the tip of the iceberg. With roughly 700,000 physicians practicing in the United States, the number of physicians affected by liability litigation could be staggering.
When you want to settle, but the carrier doesn’t
Ordinarily, your interests and those of your carrier are aligned. You both want to win—or at least lose less—but there is one scenario in which your interests may diverge. That is when you believe you are at risk for a judgment that will exceed the policy limit. In such a situation, you want your carrier to tender the full limit, but the carrier faces a worst-case scenario: paying the maximum amount on the policy.
If the carrier believes the case is defensible, it may choose to fight, hoping to win or receive a judgment well below the policy limit. If the carrier’s strategy prevails, all parties will be better off. However, if the carrier gambles and loses, you will face the very scenario you hoped to avoid—exposure to a judgment beyond the policy limit.
The law generally provides that a carrier that wants to gamble must do so with its own money. To do otherwise constitutes action “in bad faith.” After judgment, many physicians sue their own insurance company on the basis of exactly that legal theory. It is even more common for a plaintiff, fresh from victory, to join forces with the doctor defendant and take action against the carrier.
This endgame is not automatic, however. If you want to minimize the risk that your pocket will be the only one left to pick after a high-stakes case ends, you must demand in writing that the case be settled up to the policy limit. Under such circumstances, it is best for your personal counsel to deliver that message because the carrier-appointed attorney faces something of a conflict, because she is an advocate for the physician but paid by the carrier.
Avoiding the National Practitioner Data Bank
In 1990, the National Practitioner Data Bank (NPDB) was launched with the goal of keeping dangerous physicians from migrating from state to state to escape accountability. A central database allows licensing agencies to quickly determine whether a doctor has a checkered past.
The NPDB labels a physician “as marked” if money is paid for a malpractice settlement or judgment. The NPDB lists hundreds of thousands of physicians, most of whom have a single entry. Many state licensing agencies have also begun listing physicians who have lost or settled a lawsuit; the only difference is that such information is posted online and is accessible to the public. In contrast, the NPDB remains confidential, accessible only by those who “need to know,” such as credentialing committees, hospitals, and licensing boards.
Even $1 can incur a listing
Many physicians wrongly believe that they will not be reported to the NPDB if they are involved in a case that settles for an amount under $30,000. Low-value settlements are often consummated for nuisance value, meaning that they have no legal merit. However, any payment—even $1—is reportable to the NPDB. It does not matter whether payment is made by settlement or judgment.
A written demand for money, whether as damages for an injury, money to see another physician, or a refund of cash tendered, can sometimes be construed as reportable.
Being joined to a corporate entity can help you
Seasoned plaintiff’s attorneys understand physicians’ deep aversion to being reported. They often take advantage of a well-known exception to reporting: payment made in the name of a corporate entity.
If a physician is employed by a corporation with at least two physicians, and the case is settled in the name of the corporation, the physician can be dismissed from the claim, and no reporting is required. In that case, the physician maintains his clean record in the data bank. That said, this can get complicated if one is obligated to report to state authorities. Because each state is different, these details should be addressed with your attorney.
High-low agreements can avert huge judgments
As discovery progresses, the plaintiff and defendant usually come to a better understanding of their respective risk—but not always. In some situations, the plaintiff may have a strong case in regard to one element of negligence, such as damages, but a weak case in regard to causation. Cerebral palsy cases fit this paradigm. In such cases, the infant has clear-cut medical and rehabilitation needs that can run easily into seven figures, but proving that a physician’s actions or omissions caused the injury can be difficult. Both sides can mitigate risk for one another by embracing a “high-low” agreement—a contract defining how a plaintiff will be paid based on a specific jury verdict.
For example, if the high-low agreement is $500,000/$100,000, the insurer is locked into one of two payments. If the jury returns a verdict for the defense, the carrier pays $100,000; if the verdict is for the plaintiff, the carrier pays $500,000, regardless of the amount of damages awarded by the jury. Without such an agreement, the range of potential judgments is no money at all to almost any amount.
When a high-low agreement is in effect, and the jury returns a verdict for the physician, the settlement is not reported to the NPDB even though the carrier must make a payment.
Why not?
The payment is being made pursuant to a separate agreement between the carrier and the plaintiff. The benefit to the insurer is the limitation of its liability, even if the plaintiff wins at trial and is awarded a higher amount. The benefit to the plaintiff is a guaranteed payment, even if there is no finding of liability against the practitioner.
How a reputation for settling can hurt
If you are so risk-averse that you demand that your carrier settle all cases—even those with no merit—two things will happen:
- Word will spread throughout the plaintiff’s bar that you are an easy target, and the threshold for filing suit against you will decline. And given how little work will be required to net a settlement, attorney’s summons will forever darken your door
- Your medical liability rates will climb—or coverage will be terminated. Settling meritorious cases makes sense, but settling all cases regardless of merit is ill-advised.
Stress is common on both sides of the equation
Lawsuits take a long time to percolate through the system, with an average time from medical event to claim resolution of about 5 years—longer in obstetrics.2
Attorneys are accustomed to this time frame; physicians are not. The lingering effects on doctors include stress, loss of job satisfaction, family strife, depression, substance abuse, and so on.
Because a lawsuit is a major stressful life event, a physician may be only too happy to be done with one. If there were absolutely no consequences to settling, that would be a smart move. But there are consequences, and living with them can also cause stress. The best way to minimize stress on either side of the equation is to think long and hard before settling any case.
CASE Brachial plexus injury, then a summons
J.L., a 29-year-old primigravida, has gestational diabetes. When she goes into labor at term, she reports to the state-of-the-art hospital where you practice. Delivery is difficult and achieved using forceps. The infant weighs 9 lb 4 oz, and has obvious weakness in his right arm. A neurologist diagnoses Erb’s palsy, and the child undergoes brachial plexus exploration and repair of injured nerves.
Two years later, most arm function has returned. Soon thereafter, you receive a summons from the parents and their attorney demanding $3 million. Do you fight—or settle?
You could say there are two types of physicians: those who have been sued and those who will be.
This is an overstatement, of course, but not by much. In high-risk specialties such as obstetrics, most physicians will receive a summons at some point in their career. In fact, almost nine of every 10 ObGyns report that they have been sued at least once in their career, with an average of 2.6 claims each.Got malpractice distress? You can help yourself survive,” in the February 2008 issue of OBG Mangement, available at www.obgmanagment.comGiven how stressful litigation can be, there are a number of considerations that enter into the calculus of fight or settle. This article will focus on seven of those considerations (see the box above).
How consent-to-settle clauses can protect you
For years, many carriers curried favor with physicians by barring settlement of a case unless the physician agreed to it. If the physician balked, the carrier was obligated to defend the case to the end.
This clause is still found in professional liability policies, but the number of carriers offering such flexibility has decreased considerably. Many carriers now base the decision to settle on both the merits of a case and the cost of defense. If the carrier determines that it would be much less expensive to settle a case for nuisance value than to defend it through trial, the carrier is within its rights to settle. Obviously, this posture has ramifications for the insured physician.
A consent-to-settle clause—or its omission—is usually established contractually at the beginning of coverage. If the ability to demand consent for settling is important to you, look closely for such language when you purchase or renew coverage. State law can also determine whether such a clause is included.
In addition to a standard consent-to-settle provision, some carriers promote a “hammer clause,” by which an insurer’s liability is limited to a recommended settlement. Let’s say the carrier decides to settle a particular case for $100,000, the physician withholds consent, and a judgment of $300,000 is entered. The physician is individually liable for the “overage”—in this case, $200,000.
As if this were not complicated enough, there is also a modified hammer clause, which is a “kinder, gentler” approach. In this scenario, the physician is liable only for a percentage of any judgment above the recommended settlement. In the example just given, if the modified hammer provision were 50%, the carrier would pay its recommended settlement ($100,000) plus 50% of the overage—in this case, another $100,000, for a total of $200,000. The physician would be liable for the remaining $100,000.
Without a consent-to-settle clause, the physician is removed from decision-making. Further, a hammer clause or modified hammer clause should cause a physician to think long and hard before forgoing a recommended settlement.
When personal liability exceeds policy limits
Even if the carrier is bound, through its contract with you, to defend a case to the end, it will generally be limited to a maximum payout. Policy limits depend on the particular policy, with higher limits associated with higher premiums.
Carrying a very high limit can make you a more appealing target for a lawsuit, frivolous or otherwise. Many personal injury attorneys view medical malpractice as little more than a series of insurance transactions. If you have a high coverage limit, you will attract greater attention. This is of particular concern when there are multiple defendants and culpability varies significantly between the actors.
When negligence is proven in states that still allow joint and several liability, even 1% liability can leave you responsible for the entire amount. The solution is to have reasonable—but not excessive—coverage. Many believe this balance lies at $1 million/$3 million limits.
Desire for a payout may persuade a plaintiff to settle for policy limits
If you have coverage of up to $1 million and a court delivers a higher judgment, what happens?
It depends. In theory, you are liable for the overage; the carrier will pay up to the policy limit, and you are responsible for the rest. In reality, however, the situation is more complex.
You often have the right to demand a new trial or appeal the case. You may not prevail, but this approach creates new risks for the plaintiff right after “victory” is tasted. Rather than roll the dice, many plaintiffs, under the advice of their attorney, will reconsider and settle for the policy limit. It is in their interest to lock in a certain figure rather than prolong the case, exposing themselves to increased risk. And if the judgment makes it clear that bankruptcy is an option for the physician, a plaintiff will take pains to prevent that end game. Once bankruptcy is filed, the clock slows, and it may take years for the plaintiff to receive any funds. Even then, the plaintiff may have to wait in line behind more senior creditors.
Consider asset protection
Asset protection prior to litigation can affect the dynamics of posttrial settlement discussions. Asset protection means many things, and there are different degrees of protection. A limited number of attorneys are skilled in asset protection, and plaintiff’s attorneys generally have limited experience breaking through the shield.
With a robust asset-protection program in place, you can come to the table with greater leverage and engage in a more rational discussion about a just settlement in which most, if not all, of the settlement will be within the policy limit.
There are approximately 50,000 to 60,000 medicolegal cases open at any given moment, but the number of physicians involved is much higher because many suits name multiple defendants.3 In 2004, the National Practitioner Data Bank (NPDB) reported entries for more than 200,000 health-care providers since 1990, most of whom had been reported just one time.4 Again that number is low because not every physician who is sued is reported to the NPDB. Reporting is required only if payment is made by settlement or judgment related to a written demand by a plaintiff. If the case against the physician is dismissed, or the physician wins in court, no report is entered. So the 200,000 entries are just the tip of the iceberg. With roughly 700,000 physicians practicing in the United States, the number of physicians affected by liability litigation could be staggering.
When you want to settle, but the carrier doesn’t
Ordinarily, your interests and those of your carrier are aligned. You both want to win—or at least lose less—but there is one scenario in which your interests may diverge. That is when you believe you are at risk for a judgment that will exceed the policy limit. In such a situation, you want your carrier to tender the full limit, but the carrier faces a worst-case scenario: paying the maximum amount on the policy.
If the carrier believes the case is defensible, it may choose to fight, hoping to win or receive a judgment well below the policy limit. If the carrier’s strategy prevails, all parties will be better off. However, if the carrier gambles and loses, you will face the very scenario you hoped to avoid—exposure to a judgment beyond the policy limit.
The law generally provides that a carrier that wants to gamble must do so with its own money. To do otherwise constitutes action “in bad faith.” After judgment, many physicians sue their own insurance company on the basis of exactly that legal theory. It is even more common for a plaintiff, fresh from victory, to join forces with the doctor defendant and take action against the carrier.
This endgame is not automatic, however. If you want to minimize the risk that your pocket will be the only one left to pick after a high-stakes case ends, you must demand in writing that the case be settled up to the policy limit. Under such circumstances, it is best for your personal counsel to deliver that message because the carrier-appointed attorney faces something of a conflict, because she is an advocate for the physician but paid by the carrier.
Avoiding the National Practitioner Data Bank
In 1990, the National Practitioner Data Bank (NPDB) was launched with the goal of keeping dangerous physicians from migrating from state to state to escape accountability. A central database allows licensing agencies to quickly determine whether a doctor has a checkered past.
The NPDB labels a physician “as marked” if money is paid for a malpractice settlement or judgment. The NPDB lists hundreds of thousands of physicians, most of whom have a single entry. Many state licensing agencies have also begun listing physicians who have lost or settled a lawsuit; the only difference is that such information is posted online and is accessible to the public. In contrast, the NPDB remains confidential, accessible only by those who “need to know,” such as credentialing committees, hospitals, and licensing boards.
Even $1 can incur a listing
Many physicians wrongly believe that they will not be reported to the NPDB if they are involved in a case that settles for an amount under $30,000. Low-value settlements are often consummated for nuisance value, meaning that they have no legal merit. However, any payment—even $1—is reportable to the NPDB. It does not matter whether payment is made by settlement or judgment.
A written demand for money, whether as damages for an injury, money to see another physician, or a refund of cash tendered, can sometimes be construed as reportable.
Being joined to a corporate entity can help you
Seasoned plaintiff’s attorneys understand physicians’ deep aversion to being reported. They often take advantage of a well-known exception to reporting: payment made in the name of a corporate entity.
If a physician is employed by a corporation with at least two physicians, and the case is settled in the name of the corporation, the physician can be dismissed from the claim, and no reporting is required. In that case, the physician maintains his clean record in the data bank. That said, this can get complicated if one is obligated to report to state authorities. Because each state is different, these details should be addressed with your attorney.
High-low agreements can avert huge judgments
As discovery progresses, the plaintiff and defendant usually come to a better understanding of their respective risk—but not always. In some situations, the plaintiff may have a strong case in regard to one element of negligence, such as damages, but a weak case in regard to causation. Cerebral palsy cases fit this paradigm. In such cases, the infant has clear-cut medical and rehabilitation needs that can run easily into seven figures, but proving that a physician’s actions or omissions caused the injury can be difficult. Both sides can mitigate risk for one another by embracing a “high-low” agreement—a contract defining how a plaintiff will be paid based on a specific jury verdict.
For example, if the high-low agreement is $500,000/$100,000, the insurer is locked into one of two payments. If the jury returns a verdict for the defense, the carrier pays $100,000; if the verdict is for the plaintiff, the carrier pays $500,000, regardless of the amount of damages awarded by the jury. Without such an agreement, the range of potential judgments is no money at all to almost any amount.
When a high-low agreement is in effect, and the jury returns a verdict for the physician, the settlement is not reported to the NPDB even though the carrier must make a payment.
Why not?
The payment is being made pursuant to a separate agreement between the carrier and the plaintiff. The benefit to the insurer is the limitation of its liability, even if the plaintiff wins at trial and is awarded a higher amount. The benefit to the plaintiff is a guaranteed payment, even if there is no finding of liability against the practitioner.
How a reputation for settling can hurt
If you are so risk-averse that you demand that your carrier settle all cases—even those with no merit—two things will happen:
- Word will spread throughout the plaintiff’s bar that you are an easy target, and the threshold for filing suit against you will decline. And given how little work will be required to net a settlement, attorney’s summons will forever darken your door
- Your medical liability rates will climb—or coverage will be terminated. Settling meritorious cases makes sense, but settling all cases regardless of merit is ill-advised.
Stress is common on both sides of the equation
Lawsuits take a long time to percolate through the system, with an average time from medical event to claim resolution of about 5 years—longer in obstetrics.2
Attorneys are accustomed to this time frame; physicians are not. The lingering effects on doctors include stress, loss of job satisfaction, family strife, depression, substance abuse, and so on.
Because a lawsuit is a major stressful life event, a physician may be only too happy to be done with one. If there were absolutely no consequences to settling, that would be a smart move. But there are consequences, and living with them can also cause stress. The best way to minimize stress on either side of the equation is to think long and hard before settling any case.
1. Wilson N, Strunk A. Overview of the 2006 ACOG Survey on Professional Liability. Available at: www.acog.org/departments/professionalliability/2006surveyNatl.pdf. Accessed March 11, 2008.
2. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354:2024-2033.
3. Mello MM, Studdert DM. The medical malpractice system: structure and performance. In: Sage WM, Kersh R, eds. Medical Malpractice and the U.S. Health Care System: New Century, Different Issues. 1st ed. New York: Cambridge University Press; 2006:11–29.
4. US Department of Health and Human Services. National Practitioner Data Bank. 2004 annual report. Available at: http://www.npdb-hipdb.hrsa.gov/pubs/stats/2004_NPDB_Annual_Report.pdf. Accessed March 11, 2008.
More about professional liability by this author. Prepare your defense of a cerebral palsy claim (and other claims) long beforehand—in the prenatal period. That’s what Dr. Segal advised readers in the July 2007 issue of OBG Mangement. Read his argument for obtaining patient contracts in the “Past Issues” archives at www.obgmanagement.com.
1. Wilson N, Strunk A. Overview of the 2006 ACOG Survey on Professional Liability. Available at: www.acog.org/departments/professionalliability/2006surveyNatl.pdf. Accessed March 11, 2008.
2. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354:2024-2033.
3. Mello MM, Studdert DM. The medical malpractice system: structure and performance. In: Sage WM, Kersh R, eds. Medical Malpractice and the U.S. Health Care System: New Century, Different Issues. 1st ed. New York: Cambridge University Press; 2006:11–29.
4. US Department of Health and Human Services. National Practitioner Data Bank. 2004 annual report. Available at: http://www.npdb-hipdb.hrsa.gov/pubs/stats/2004_NPDB_Annual_Report.pdf. Accessed March 11, 2008.
More about professional liability by this author. Prepare your defense of a cerebral palsy claim (and other claims) long beforehand—in the prenatal period. That’s what Dr. Segal advised readers in the July 2007 issue of OBG Mangement. Read his argument for obtaining patient contracts in the “Past Issues” archives at www.obgmanagement.com.
Malpractice Minute
We give you facts of an actual malpractice case. Submit your verdict below and see how your colleagues voted.
Did the patient know the risks of risperidone?
THE PATIENT. A 53-year-old woman hospitalized for depression and suicidal thoughts was prescribed risperidone.
CASE FACTS. The patient developed excessive mouth and tongue movement—including pursed lips, protruding tongue, and biting the inside of her mouth—and uncontrollable urges to move her extremities. She was diagnosed with probable tardive dyskinesia (TD), and risperidone was tapered and discontinued.
THE PATIENT’S CLAIM. The psychiatrist failed to adequately monitor her and recognize early symptoms of TD and did not tell the patient to look for signs of TD.
THE DOCTOR’S DEFENSE. None
Submit your verdict and find out how the court ruled. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
We give you facts of an actual malpractice case. Submit your verdict below and see how your colleagues voted.
Did the patient know the risks of risperidone?
THE PATIENT. A 53-year-old woman hospitalized for depression and suicidal thoughts was prescribed risperidone.
CASE FACTS. The patient developed excessive mouth and tongue movement—including pursed lips, protruding tongue, and biting the inside of her mouth—and uncontrollable urges to move her extremities. She was diagnosed with probable tardive dyskinesia (TD), and risperidone was tapered and discontinued.
THE PATIENT’S CLAIM. The psychiatrist failed to adequately monitor her and recognize early symptoms of TD and did not tell the patient to look for signs of TD.
THE DOCTOR’S DEFENSE. None
Submit your verdict and find out how the court ruled. Click on “Have more to say about this topic?” to comment.
We give you facts of an actual malpractice case. Submit your verdict below and see how your colleagues voted.
Did the patient know the risks of risperidone?
THE PATIENT. A 53-year-old woman hospitalized for depression and suicidal thoughts was prescribed risperidone.
CASE FACTS. The patient developed excessive mouth and tongue movement—including pursed lips, protruding tongue, and biting the inside of her mouth—and uncontrollable urges to move her extremities. She was diagnosed with probable tardive dyskinesia (TD), and risperidone was tapered and discontinued.
THE PATIENT’S CLAIM. The psychiatrist failed to adequately monitor her and recognize early symptoms of TD and did not tell the patient to look for signs of TD.
THE DOCTOR’S DEFENSE. None
Submit your verdict and find out how the court ruled. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Evaluate liability risks in prescribing
Dear Dr. Mossman,
I prescribed topiramate for Mr. B, a patient with no history of kidney stones. Many months later he developed back pain. During the medical workup for a possible kidney stone, Mr. B and I revisited the risk of kidney stones with topiramate, which we had discussed at the beginning of therapy. Mr. B was adamantly opposed to stopping topiramate, even if he had a kidney stone. Testing revealed that Mr. B did not have a stone, but I wasn’t sure how to proceed. I worried that I might be found liable if Mr. B stayed on topiramate and did develop a kidney stone.—Submitted by Dr. A
When a patient develops a medical problem from a drug you prescribed, it is natural to feel responsible—after all, your treatment caused the adverse event. But did you commit malpractice? To answer this, let’s review the concept of “medical negligence.”
Malpractice law applies legal principles of negligence to professional conduct.1 The elements of a negligence case (Table 1) can be summarized as “breach of duty causing damages.” Therefore, when you wonder whether possible harm to a patient might be considered malpractice, ask yourself, “Did I breach my professional duty?”
Physicians have a duty to practice within their specialty’s standard of care, and if they do this, they should not be held liable even if their treatments cause adverse effects. Each jurisdiction defines the standard of care differently, but the general expectation is “that physicians acting within the ambit of their professional work will exercise the skill, knowledge, and care normally possessed and exercised by other members of their profession…in the relevant medical community.”1
It’s impossible to describe all the skills, knowledge, and care a psychiatrist normally employs when prescribing a drug, but elements of good practice include reasonable efforts to:
- make an appropriate diagnosis
- offer appropriate treatment
- monitor effects of treatment.
Further, treatment should occur only when a patient gives informed consent. Let’s examine each of these elements as they apply to Dr. A and Mr. B.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online market-place of risk management publications and resources (www.prms.com).
Table 1
Elements of a successful negligence case
|
| Source: Reference 1 |
Appropriate assessment
Despite the availability of guidelines for psychiatric evaluation,2,3 it is tough to summarize everything psychiatrists do when assessing patients. But—focusing on Dr. A’s question—it is reasonable to ask: Did the psychiatric evaluation provide reasonably good evidence that Mr. B had a condition that topiramate might alleviate? Mr. B’s strong desire to keep taking the drug suggests that the answer is “yes.”
Dr. A also should consider potential interactions between topiramate and any other medications that Mr. B is taking. A prudent clinician must judge whether the potential benefit of topiramate for Mr. B outweighs the risk of adverse effects. If Mr. B actually had developed a kidney stone, Dr. A might seek a nephrologist’s advice about how to minimize the risk of recurrence.
Appropriate treatment
Topiramate is FDA-approved only for treating seizures and for prophylaxis against migraine headaches. However, FDA approval limits only how pharmaceutical companies can promote a medication.4 Physicians may prescribe drugs for unapproved “off-label” uses, and doing so is accepted medical practice. Peer-reviewed publications support using topiramate to treat agitation,5 alcohol dependence,6 binge-eating disorder,7 and other conditions that psychiatrists often manage. A tendency to promote weight loss has made topiramate an attractive add-on medication for patients whose weight problems are causing other health difficulties.8
Assuming that Mr. B is taking topiramate for an off-label purpose, an appropriate question to ask is, “Does professional literature support use of topiramate in Mr. B’s circumstances?” Also, given everything known about Mr. B up to this point, is topiramate a good treatment choice?
Appropriate monitoring
As every clinician knows, medications can cause problems. Monitoring topiramate therapy involves periodic lab testing and assessment of effectiveness. Dr. A should feel reasonably sure that Mr. B—assisted by a family member or close friend, if necessary—can and will cooperate with monitoring requirements. Dr. A also should verify that Mr. B can grasp and follow instructions designed to avert complications—such as ample hydration to reduce risk of nephrolithiasis—and will promptly address problems if they occur.
Informed consent
Informed consent is especially important when a patient receives a treatment that has a known risk. Although the Physician’s Desk Reference does not list previous kidney stones as a contraindication to topiramate therapy, it urges caution under these circumstances.4 Therefore, if Dr. A wishes to prescribe topiramate for a patient with a history of kidney stone, the patient should meaningfully collaborate in the treatment decision.
Informed consent for treatment requires that patients not feel coerced by the doctor or setting and have the mental capacity or competence to give consent. Under the conceptualization developed by Appelbaum and Grisso,9 competent patients can:
- express a consistent choice
- understand medical information provided to them
- appreciate how this information applies to them and their condition
- reason logically about treatment.
What information should patients receive before giving consent? The legal standard varies, but in most U.S. jurisdictions, patients “are entitled to material information about the nature of any proposed medical procedure. For example, patients are entitled to information about the risks of the procedure, its necessity, and alternate procedures that might be preferable.”1 Topiramate’s manufacturer instructs physicians to question and warn patients about the risk of kidney stones—which Dr. A did in Mr. B’s case. When you prescribe a drug off-label, you may want to tell patients this, but explain why the drug is appropriate nonetheless.
Table 2
Evaluating a patient’s capacity to consent to treatment
| Is this patient able to? | Questions to ask |
|---|---|
| Express a clear treatment preference | What treatment have you chosen? |
| Understand basic information communicated by caregivers | Can you tell me in your own words about your condition and the treatment options I have told you about? |
| Appreciate his or her medical condition and how information about treatment applies | What do you think is wrong with your health now? Do you think you need some kind of treatment? What do you think treatment will do for you? |
| Reason logically when choosing treatment options | Why did you choose this treatment? Why is it better than your other treatment options? |
| Source: Adapted and reprinted with permission from Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834-40 | |
1. Dobbs DB. The law of torts. St. Paul, MN: West Group; 2000:269.
2. King RA. Practice parameters for the psychiatric assessment of children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(10 suppl):4S-20S.
3. American Psychiatric Association. Practice guideline for psychiatric evaluation of adults. Am J Psychiatry 1995;152(11 suppl):63-80.
4. Physicians’ Desk Reference. 62 ed. Montvale, NJ: Thomson Healthcare Inc.; 2007.
5. Guay DR. Newer antiepileptic drugs in the management of agitation/aggression in patients with dementia or developmental disability. Consult Pharm 2007;22:1004-34.
6. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for Alcoholism Advisory Board; Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-51.
7. McElroy SL, Arnold LM, Shapira NA, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:255-61.
8. Kirov G, Tredget J. Add-on topiramate reduces weight in overweight patients with affective disorders: a clinical case series. BMC Psychiatry 2005;5(1):19.-
9. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med 1988;319:1635-8.
10. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834-40.
Dear Dr. Mossman,
I prescribed topiramate for Mr. B, a patient with no history of kidney stones. Many months later he developed back pain. During the medical workup for a possible kidney stone, Mr. B and I revisited the risk of kidney stones with topiramate, which we had discussed at the beginning of therapy. Mr. B was adamantly opposed to stopping topiramate, even if he had a kidney stone. Testing revealed that Mr. B did not have a stone, but I wasn’t sure how to proceed. I worried that I might be found liable if Mr. B stayed on topiramate and did develop a kidney stone.—Submitted by Dr. A
When a patient develops a medical problem from a drug you prescribed, it is natural to feel responsible—after all, your treatment caused the adverse event. But did you commit malpractice? To answer this, let’s review the concept of “medical negligence.”
Malpractice law applies legal principles of negligence to professional conduct.1 The elements of a negligence case (Table 1) can be summarized as “breach of duty causing damages.” Therefore, when you wonder whether possible harm to a patient might be considered malpractice, ask yourself, “Did I breach my professional duty?”
Physicians have a duty to practice within their specialty’s standard of care, and if they do this, they should not be held liable even if their treatments cause adverse effects. Each jurisdiction defines the standard of care differently, but the general expectation is “that physicians acting within the ambit of their professional work will exercise the skill, knowledge, and care normally possessed and exercised by other members of their profession…in the relevant medical community.”1
It’s impossible to describe all the skills, knowledge, and care a psychiatrist normally employs when prescribing a drug, but elements of good practice include reasonable efforts to:
- make an appropriate diagnosis
- offer appropriate treatment
- monitor effects of treatment.
Further, treatment should occur only when a patient gives informed consent. Let’s examine each of these elements as they apply to Dr. A and Mr. B.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online market-place of risk management publications and resources (www.prms.com).
Table 1
Elements of a successful negligence case
|
| Source: Reference 1 |
Appropriate assessment
Despite the availability of guidelines for psychiatric evaluation,2,3 it is tough to summarize everything psychiatrists do when assessing patients. But—focusing on Dr. A’s question—it is reasonable to ask: Did the psychiatric evaluation provide reasonably good evidence that Mr. B had a condition that topiramate might alleviate? Mr. B’s strong desire to keep taking the drug suggests that the answer is “yes.”
Dr. A also should consider potential interactions between topiramate and any other medications that Mr. B is taking. A prudent clinician must judge whether the potential benefit of topiramate for Mr. B outweighs the risk of adverse effects. If Mr. B actually had developed a kidney stone, Dr. A might seek a nephrologist’s advice about how to minimize the risk of recurrence.
Appropriate treatment
Topiramate is FDA-approved only for treating seizures and for prophylaxis against migraine headaches. However, FDA approval limits only how pharmaceutical companies can promote a medication.4 Physicians may prescribe drugs for unapproved “off-label” uses, and doing so is accepted medical practice. Peer-reviewed publications support using topiramate to treat agitation,5 alcohol dependence,6 binge-eating disorder,7 and other conditions that psychiatrists often manage. A tendency to promote weight loss has made topiramate an attractive add-on medication for patients whose weight problems are causing other health difficulties.8
Assuming that Mr. B is taking topiramate for an off-label purpose, an appropriate question to ask is, “Does professional literature support use of topiramate in Mr. B’s circumstances?” Also, given everything known about Mr. B up to this point, is topiramate a good treatment choice?
Appropriate monitoring
As every clinician knows, medications can cause problems. Monitoring topiramate therapy involves periodic lab testing and assessment of effectiveness. Dr. A should feel reasonably sure that Mr. B—assisted by a family member or close friend, if necessary—can and will cooperate with monitoring requirements. Dr. A also should verify that Mr. B can grasp and follow instructions designed to avert complications—such as ample hydration to reduce risk of nephrolithiasis—and will promptly address problems if they occur.
Informed consent
Informed consent is especially important when a patient receives a treatment that has a known risk. Although the Physician’s Desk Reference does not list previous kidney stones as a contraindication to topiramate therapy, it urges caution under these circumstances.4 Therefore, if Dr. A wishes to prescribe topiramate for a patient with a history of kidney stone, the patient should meaningfully collaborate in the treatment decision.
Informed consent for treatment requires that patients not feel coerced by the doctor or setting and have the mental capacity or competence to give consent. Under the conceptualization developed by Appelbaum and Grisso,9 competent patients can:
- express a consistent choice
- understand medical information provided to them
- appreciate how this information applies to them and their condition
- reason logically about treatment.
What information should patients receive before giving consent? The legal standard varies, but in most U.S. jurisdictions, patients “are entitled to material information about the nature of any proposed medical procedure. For example, patients are entitled to information about the risks of the procedure, its necessity, and alternate procedures that might be preferable.”1 Topiramate’s manufacturer instructs physicians to question and warn patients about the risk of kidney stones—which Dr. A did in Mr. B’s case. When you prescribe a drug off-label, you may want to tell patients this, but explain why the drug is appropriate nonetheless.
Table 2
Evaluating a patient’s capacity to consent to treatment
| Is this patient able to? | Questions to ask |
|---|---|
| Express a clear treatment preference | What treatment have you chosen? |
| Understand basic information communicated by caregivers | Can you tell me in your own words about your condition and the treatment options I have told you about? |
| Appreciate his or her medical condition and how information about treatment applies | What do you think is wrong with your health now? Do you think you need some kind of treatment? What do you think treatment will do for you? |
| Reason logically when choosing treatment options | Why did you choose this treatment? Why is it better than your other treatment options? |
| Source: Adapted and reprinted with permission from Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834-40 | |
Dear Dr. Mossman,
I prescribed topiramate for Mr. B, a patient with no history of kidney stones. Many months later he developed back pain. During the medical workup for a possible kidney stone, Mr. B and I revisited the risk of kidney stones with topiramate, which we had discussed at the beginning of therapy. Mr. B was adamantly opposed to stopping topiramate, even if he had a kidney stone. Testing revealed that Mr. B did not have a stone, but I wasn’t sure how to proceed. I worried that I might be found liable if Mr. B stayed on topiramate and did develop a kidney stone.—Submitted by Dr. A
When a patient develops a medical problem from a drug you prescribed, it is natural to feel responsible—after all, your treatment caused the adverse event. But did you commit malpractice? To answer this, let’s review the concept of “medical negligence.”
Malpractice law applies legal principles of negligence to professional conduct.1 The elements of a negligence case (Table 1) can be summarized as “breach of duty causing damages.” Therefore, when you wonder whether possible harm to a patient might be considered malpractice, ask yourself, “Did I breach my professional duty?”
Physicians have a duty to practice within their specialty’s standard of care, and if they do this, they should not be held liable even if their treatments cause adverse effects. Each jurisdiction defines the standard of care differently, but the general expectation is “that physicians acting within the ambit of their professional work will exercise the skill, knowledge, and care normally possessed and exercised by other members of their profession…in the relevant medical community.”1
It’s impossible to describe all the skills, knowledge, and care a psychiatrist normally employs when prescribing a drug, but elements of good practice include reasonable efforts to:
- make an appropriate diagnosis
- offer appropriate treatment
- monitor effects of treatment.
Further, treatment should occur only when a patient gives informed consent. Let’s examine each of these elements as they apply to Dr. A and Mr. B.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online market-place of risk management publications and resources (www.prms.com).
Table 1
Elements of a successful negligence case
|
| Source: Reference 1 |
Appropriate assessment
Despite the availability of guidelines for psychiatric evaluation,2,3 it is tough to summarize everything psychiatrists do when assessing patients. But—focusing on Dr. A’s question—it is reasonable to ask: Did the psychiatric evaluation provide reasonably good evidence that Mr. B had a condition that topiramate might alleviate? Mr. B’s strong desire to keep taking the drug suggests that the answer is “yes.”
Dr. A also should consider potential interactions between topiramate and any other medications that Mr. B is taking. A prudent clinician must judge whether the potential benefit of topiramate for Mr. B outweighs the risk of adverse effects. If Mr. B actually had developed a kidney stone, Dr. A might seek a nephrologist’s advice about how to minimize the risk of recurrence.
Appropriate treatment
Topiramate is FDA-approved only for treating seizures and for prophylaxis against migraine headaches. However, FDA approval limits only how pharmaceutical companies can promote a medication.4 Physicians may prescribe drugs for unapproved “off-label” uses, and doing so is accepted medical practice. Peer-reviewed publications support using topiramate to treat agitation,5 alcohol dependence,6 binge-eating disorder,7 and other conditions that psychiatrists often manage. A tendency to promote weight loss has made topiramate an attractive add-on medication for patients whose weight problems are causing other health difficulties.8
Assuming that Mr. B is taking topiramate for an off-label purpose, an appropriate question to ask is, “Does professional literature support use of topiramate in Mr. B’s circumstances?” Also, given everything known about Mr. B up to this point, is topiramate a good treatment choice?
Appropriate monitoring
As every clinician knows, medications can cause problems. Monitoring topiramate therapy involves periodic lab testing and assessment of effectiveness. Dr. A should feel reasonably sure that Mr. B—assisted by a family member or close friend, if necessary—can and will cooperate with monitoring requirements. Dr. A also should verify that Mr. B can grasp and follow instructions designed to avert complications—such as ample hydration to reduce risk of nephrolithiasis—and will promptly address problems if they occur.
Informed consent
Informed consent is especially important when a patient receives a treatment that has a known risk. Although the Physician’s Desk Reference does not list previous kidney stones as a contraindication to topiramate therapy, it urges caution under these circumstances.4 Therefore, if Dr. A wishes to prescribe topiramate for a patient with a history of kidney stone, the patient should meaningfully collaborate in the treatment decision.
Informed consent for treatment requires that patients not feel coerced by the doctor or setting and have the mental capacity or competence to give consent. Under the conceptualization developed by Appelbaum and Grisso,9 competent patients can:
- express a consistent choice
- understand medical information provided to them
- appreciate how this information applies to them and their condition
- reason logically about treatment.
What information should patients receive before giving consent? The legal standard varies, but in most U.S. jurisdictions, patients “are entitled to material information about the nature of any proposed medical procedure. For example, patients are entitled to information about the risks of the procedure, its necessity, and alternate procedures that might be preferable.”1 Topiramate’s manufacturer instructs physicians to question and warn patients about the risk of kidney stones—which Dr. A did in Mr. B’s case. When you prescribe a drug off-label, you may want to tell patients this, but explain why the drug is appropriate nonetheless.
Table 2
Evaluating a patient’s capacity to consent to treatment
| Is this patient able to? | Questions to ask |
|---|---|
| Express a clear treatment preference | What treatment have you chosen? |
| Understand basic information communicated by caregivers | Can you tell me in your own words about your condition and the treatment options I have told you about? |
| Appreciate his or her medical condition and how information about treatment applies | What do you think is wrong with your health now? Do you think you need some kind of treatment? What do you think treatment will do for you? |
| Reason logically when choosing treatment options | Why did you choose this treatment? Why is it better than your other treatment options? |
| Source: Adapted and reprinted with permission from Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834-40 | |
1. Dobbs DB. The law of torts. St. Paul, MN: West Group; 2000:269.
2. King RA. Practice parameters for the psychiatric assessment of children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(10 suppl):4S-20S.
3. American Psychiatric Association. Practice guideline for psychiatric evaluation of adults. Am J Psychiatry 1995;152(11 suppl):63-80.
4. Physicians’ Desk Reference. 62 ed. Montvale, NJ: Thomson Healthcare Inc.; 2007.
5. Guay DR. Newer antiepileptic drugs in the management of agitation/aggression in patients with dementia or developmental disability. Consult Pharm 2007;22:1004-34.
6. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for Alcoholism Advisory Board; Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-51.
7. McElroy SL, Arnold LM, Shapira NA, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:255-61.
8. Kirov G, Tredget J. Add-on topiramate reduces weight in overweight patients with affective disorders: a clinical case series. BMC Psychiatry 2005;5(1):19.-
9. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med 1988;319:1635-8.
10. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834-40.
1. Dobbs DB. The law of torts. St. Paul, MN: West Group; 2000:269.
2. King RA. Practice parameters for the psychiatric assessment of children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(10 suppl):4S-20S.
3. American Psychiatric Association. Practice guideline for psychiatric evaluation of adults. Am J Psychiatry 1995;152(11 suppl):63-80.
4. Physicians’ Desk Reference. 62 ed. Montvale, NJ: Thomson Healthcare Inc.; 2007.
5. Guay DR. Newer antiepileptic drugs in the management of agitation/aggression in patients with dementia or developmental disability. Consult Pharm 2007;22:1004-34.
6. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for Alcoholism Advisory Board; Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-51.
7. McElroy SL, Arnold LM, Shapira NA, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:255-61.
8. Kirov G, Tredget J. Add-on topiramate reduces weight in overweight patients with affective disorders: a clinical case series. BMC Psychiatry 2005;5(1):19.-
9. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med 1988;319:1635-8.
10. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834-40.
Nocturnal Economics
In my previous column, I reviewed different strategies for providing hospitalist practice night coverage based on the size of the group (February 2008, p. 61). I suggested that dedicated nocturnists are a valuable though expensive asset that any practice larger than about six to eight full-time equivalents (FTE) should consider.
This month, I offer additional thoughts about compensation for nocturnists. I’ll demonstrate why adding dedicated night coverage—in which the doctor working at night doesn’t work during the daytime hours the day before or after the night shift—may not increase practice workload significantly.
What follows is adapted from a new book I co-wrote with Joe Miller, senior vice president of SHM, and Win Whitcomb, MD, a hospitalist at Mercy Medical Center in Springfield, Mass., and co-founder of SHM.1
Compensation
If all hospitalists provide an equal amount of night coverage in rotation (e.g., each member of a four-person group works 61 nights annually), it’s not necessary to adjust the compensation scheme to reflect night work. A night-shift differential in this situation will not influence a doctor’s annual income relative to that of his partner hospitalists.
However, if the hospitalist program seeks more flexibility, it may be advisable to pay more for a night of work than a day of work. Under this scheme, hospitalists may trade day and night work among themselves, leading to enhanced satisfaction. For example, Dr. McCartney is willing to work some of Dr. Lennon’s nights because of the income benefit. Dr. Lennon may or may not work some of Dr. McCartney’s days in return.
If the practice has one or more dedicated nocturnists, they will need to realize some benefit to working only nights. This benefit can take many forms:
- The night hospitalist works less often than day doctors (e.g., day doctors work 220 days annually, night doctors work 182);
- The night hospitalist has a lighter patient load (e.g., a night hospitalist in a small practice typically sleeps three to six hours per night shift while the day doctors typically work a busy eight-to-12-hour shift);
- The night doctor earns more than the day doctors; or
- The night doctor has a higher priority in time-off scheduling.
It is common to combine these benefits. For example a night hospitalist might work less often than day doctors, have a lighter patient load, and earn the same annual income. Anecdotal experience shows that having more income or fewer workdays than day doctors is valued more than a reduced patient load.
For most practices, compensating hospitalists based significantly or entirely on their production can be a good idea but might be problematic for a night doctor. It could lead the night doctor to encourage marginal admissions, some of whom would need to be discharged by the daytime hospitalists hours later. In effect, the night hospitalist could say: “I’ll admit anyone I can get my hands on because my income will increase. I’ll leave it for the day doctors to sort out what to do with all these patients tomorrow.”
An Example
A traditional system of night call (such as pager call from home while also working days) is usually cheaper than dedicated night shifts. And while there are many benefits to having dedicated night shifts, increased patient capacity may not be one of them. Consider the following example:
- On any given day, a five-FTE hospitalist practice has three doctors working, one of whom will be on-call that night by pager;
- That will mean 219 worked days per year for each doctor, one-third of which (73) will be on-call. Each hospitalist gets 146 days off per year;
- The practice decides to switch to dedicated night shifts in which the doctors do not work the day before or after a night shift. The practice wants to retain the 146 days off for each hospitalist. This new coverage arrangement is equivalent to adding 365 shifts annually (one for each night); and
- This will require an additional 1.67 FTE hospitalists (1.67 hospitalists at 219 shifts/year=365).
In this example, by switching from on-call coverage to on-site coverage, the practice increased from five FTEs to 6.67 FTEs. If the daytime work was already enough to keep all three doctors busy, adding 1.67 FTEs for dedicated night shifts may not increase practice productivity or revenue significantly. The practice looks much less productive per FTE (6.67 FTEs are now seeing the volume previously handled by five FTEs) and much costlier.
Changing from traditional night call to dedicated night coverage can be expensive because it may require adding staff yet doesn’t usually increase practice capacity significantly. But it offers other benefits such as those listed in Table 1 (see p. TK). Some practices find they must provide dedicated night coverage to recruit hospitalists. Other institutions choose to support it believing it leads to more timely, efficient, higher-quality care. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Reference
- Miller J, Nelson J, Whitcomb W. Hospitalists: A Guide to Building and Sustaining a Successful Program. Chicago:Health Administration Press;2007:149-150.
In my previous column, I reviewed different strategies for providing hospitalist practice night coverage based on the size of the group (February 2008, p. 61). I suggested that dedicated nocturnists are a valuable though expensive asset that any practice larger than about six to eight full-time equivalents (FTE) should consider.
This month, I offer additional thoughts about compensation for nocturnists. I’ll demonstrate why adding dedicated night coverage—in which the doctor working at night doesn’t work during the daytime hours the day before or after the night shift—may not increase practice workload significantly.
What follows is adapted from a new book I co-wrote with Joe Miller, senior vice president of SHM, and Win Whitcomb, MD, a hospitalist at Mercy Medical Center in Springfield, Mass., and co-founder of SHM.1
Compensation
If all hospitalists provide an equal amount of night coverage in rotation (e.g., each member of a four-person group works 61 nights annually), it’s not necessary to adjust the compensation scheme to reflect night work. A night-shift differential in this situation will not influence a doctor’s annual income relative to that of his partner hospitalists.
However, if the hospitalist program seeks more flexibility, it may be advisable to pay more for a night of work than a day of work. Under this scheme, hospitalists may trade day and night work among themselves, leading to enhanced satisfaction. For example, Dr. McCartney is willing to work some of Dr. Lennon’s nights because of the income benefit. Dr. Lennon may or may not work some of Dr. McCartney’s days in return.
If the practice has one or more dedicated nocturnists, they will need to realize some benefit to working only nights. This benefit can take many forms:
- The night hospitalist works less often than day doctors (e.g., day doctors work 220 days annually, night doctors work 182);
- The night hospitalist has a lighter patient load (e.g., a night hospitalist in a small practice typically sleeps three to six hours per night shift while the day doctors typically work a busy eight-to-12-hour shift);
- The night doctor earns more than the day doctors; or
- The night doctor has a higher priority in time-off scheduling.
It is common to combine these benefits. For example a night hospitalist might work less often than day doctors, have a lighter patient load, and earn the same annual income. Anecdotal experience shows that having more income or fewer workdays than day doctors is valued more than a reduced patient load.
For most practices, compensating hospitalists based significantly or entirely on their production can be a good idea but might be problematic for a night doctor. It could lead the night doctor to encourage marginal admissions, some of whom would need to be discharged by the daytime hospitalists hours later. In effect, the night hospitalist could say: “I’ll admit anyone I can get my hands on because my income will increase. I’ll leave it for the day doctors to sort out what to do with all these patients tomorrow.”
An Example
A traditional system of night call (such as pager call from home while also working days) is usually cheaper than dedicated night shifts. And while there are many benefits to having dedicated night shifts, increased patient capacity may not be one of them. Consider the following example:
- On any given day, a five-FTE hospitalist practice has three doctors working, one of whom will be on-call that night by pager;
- That will mean 219 worked days per year for each doctor, one-third of which (73) will be on-call. Each hospitalist gets 146 days off per year;
- The practice decides to switch to dedicated night shifts in which the doctors do not work the day before or after a night shift. The practice wants to retain the 146 days off for each hospitalist. This new coverage arrangement is equivalent to adding 365 shifts annually (one for each night); and
- This will require an additional 1.67 FTE hospitalists (1.67 hospitalists at 219 shifts/year=365).
In this example, by switching from on-call coverage to on-site coverage, the practice increased from five FTEs to 6.67 FTEs. If the daytime work was already enough to keep all three doctors busy, adding 1.67 FTEs for dedicated night shifts may not increase practice productivity or revenue significantly. The practice looks much less productive per FTE (6.67 FTEs are now seeing the volume previously handled by five FTEs) and much costlier.
Changing from traditional night call to dedicated night coverage can be expensive because it may require adding staff yet doesn’t usually increase practice capacity significantly. But it offers other benefits such as those listed in Table 1 (see p. TK). Some practices find they must provide dedicated night coverage to recruit hospitalists. Other institutions choose to support it believing it leads to more timely, efficient, higher-quality care. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Reference
- Miller J, Nelson J, Whitcomb W. Hospitalists: A Guide to Building and Sustaining a Successful Program. Chicago:Health Administration Press;2007:149-150.
In my previous column, I reviewed different strategies for providing hospitalist practice night coverage based on the size of the group (February 2008, p. 61). I suggested that dedicated nocturnists are a valuable though expensive asset that any practice larger than about six to eight full-time equivalents (FTE) should consider.
This month, I offer additional thoughts about compensation for nocturnists. I’ll demonstrate why adding dedicated night coverage—in which the doctor working at night doesn’t work during the daytime hours the day before or after the night shift—may not increase practice workload significantly.
What follows is adapted from a new book I co-wrote with Joe Miller, senior vice president of SHM, and Win Whitcomb, MD, a hospitalist at Mercy Medical Center in Springfield, Mass., and co-founder of SHM.1
Compensation
If all hospitalists provide an equal amount of night coverage in rotation (e.g., each member of a four-person group works 61 nights annually), it’s not necessary to adjust the compensation scheme to reflect night work. A night-shift differential in this situation will not influence a doctor’s annual income relative to that of his partner hospitalists.
However, if the hospitalist program seeks more flexibility, it may be advisable to pay more for a night of work than a day of work. Under this scheme, hospitalists may trade day and night work among themselves, leading to enhanced satisfaction. For example, Dr. McCartney is willing to work some of Dr. Lennon’s nights because of the income benefit. Dr. Lennon may or may not work some of Dr. McCartney’s days in return.
If the practice has one or more dedicated nocturnists, they will need to realize some benefit to working only nights. This benefit can take many forms:
- The night hospitalist works less often than day doctors (e.g., day doctors work 220 days annually, night doctors work 182);
- The night hospitalist has a lighter patient load (e.g., a night hospitalist in a small practice typically sleeps three to six hours per night shift while the day doctors typically work a busy eight-to-12-hour shift);
- The night doctor earns more than the day doctors; or
- The night doctor has a higher priority in time-off scheduling.
It is common to combine these benefits. For example a night hospitalist might work less often than day doctors, have a lighter patient load, and earn the same annual income. Anecdotal experience shows that having more income or fewer workdays than day doctors is valued more than a reduced patient load.
For most practices, compensating hospitalists based significantly or entirely on their production can be a good idea but might be problematic for a night doctor. It could lead the night doctor to encourage marginal admissions, some of whom would need to be discharged by the daytime hospitalists hours later. In effect, the night hospitalist could say: “I’ll admit anyone I can get my hands on because my income will increase. I’ll leave it for the day doctors to sort out what to do with all these patients tomorrow.”
An Example
A traditional system of night call (such as pager call from home while also working days) is usually cheaper than dedicated night shifts. And while there are many benefits to having dedicated night shifts, increased patient capacity may not be one of them. Consider the following example:
- On any given day, a five-FTE hospitalist practice has three doctors working, one of whom will be on-call that night by pager;
- That will mean 219 worked days per year for each doctor, one-third of which (73) will be on-call. Each hospitalist gets 146 days off per year;
- The practice decides to switch to dedicated night shifts in which the doctors do not work the day before or after a night shift. The practice wants to retain the 146 days off for each hospitalist. This new coverage arrangement is equivalent to adding 365 shifts annually (one for each night); and
- This will require an additional 1.67 FTE hospitalists (1.67 hospitalists at 219 shifts/year=365).
In this example, by switching from on-call coverage to on-site coverage, the practice increased from five FTEs to 6.67 FTEs. If the daytime work was already enough to keep all three doctors busy, adding 1.67 FTEs for dedicated night shifts may not increase practice productivity or revenue significantly. The practice looks much less productive per FTE (6.67 FTEs are now seeing the volume previously handled by five FTEs) and much costlier.
Changing from traditional night call to dedicated night coverage can be expensive because it may require adding staff yet doesn’t usually increase practice capacity significantly. But it offers other benefits such as those listed in Table 1 (see p. TK). Some practices find they must provide dedicated night coverage to recruit hospitalists. Other institutions choose to support it believing it leads to more timely, efficient, higher-quality care. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Reference
- Miller J, Nelson J, Whitcomb W. Hospitalists: A Guide to Building and Sustaining a Successful Program. Chicago:Health Administration Press;2007:149-150.
What is the target blood glucose for noncritical care patients?
Case
A 65-year-old obese (100 kg) man with type 2 diabetes, hypertension, and a pack-a-day smoking habit is admitted with moderately severe bilobar pneumonia. His condition is manifest by fever, cough, chills, leukocytosis, and a modest oxygen requirement. You order oxygen, intravenous (IV) fluids, diet, and appropriate antibiotics while continuing the history and chart review. The patient uses metformin and glyburide, and his home glucose readings are generally in the 160 to 180 mg/dL range. An HbA1c level performed three months ago was 9.8, leading to an increased dose of glyburide. As you finish the history, the nurse reports a glucose reading of 198 mg/dL. What is the target blood glucose for noncritical care adult inpatients?
Overview
Diabetes mellitus is an epidemic in the United States. At least 9.3% of adults older than 20 (more than 20 million people) have diabetes. Approximately 30% are unaware they have diabetes.1 Concurrent with the increasing prevalence of diabetes in the U.S. from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled between 1980 and 2003. These trends are expected to accelerate.2 Studies suggest 26% of inpatients have diabetes and 12% have pre-diabetes, previously undiagnosed diabetes, or stress hyperglycemia.3
Review of the Data
A full review of the evidence is beyond the scope of this article. What follows is a sampling of the most representative or influential critical care studies.
Physiology
Fluid and electrolyte balance, left ventricular (LV) function, leukocyte action, wound healing, endothelial function, and immunoglobulin function are all impaired with hyperglycemia.
A prothrombotic state and enhanced platelet aggregation have been demonstrated with even mild elevations of blood glucose.
The mechanisms are multifactorial and complex and involve metabolic derangements leading to oxidative stress, release of free fatty acids, and counter-regulatory hormones.4-6
Observational Studies
A strong and consistent association with hyperglycemia and adverse outcomes is seen in a wide variety of critical care and peri-operative settings. Trauma survival, stroke survival and function, and the incidence of post-operative infections are all adversely affected by hyperglycemia.7-10 Acute myocardial infarction (MI) mortality, acute MI infarct size, and LV dysfunction are also consistently adversely affected in these studies.11-13
This association is typically present in hyperglycemic patients whether they have a diagnosis of diabetes or not, and the association is often even stronger in those lacking a pre-existing diagnosis. Dysfunction typically is detectable at only modest elevations of blood glucose and becomes more marked in a dose response relationship.
Uncontrolled Interventional Studies
The Portland Diabetic Project is a prospective, non-randomized, observational study of 5,510 consecutive diabetic cardiac surgery patients.14-15 The three-day blood glucose average (3-BG) has been progressively reduced for the population through the use of continuous insulin infusion (CII).
The last reported glycemic target is less than 130 mg/dL, and the current glycemic target is less than 110 mg/dL. Both CII for three days and a favorable 3-BG were independently associated with improved mortality, deep sternal-wound infection rates, and length of stay. Mortality and deep sternal-wound infection rates for diabetic patients with well-controlled glucose levels are equal to patients without diabetes.
Another study compared 800 mixed medical-surgical ICU patients with tight glycemic control (mean BG 130.7 mg/dL) to historical controls with a mean glucose of 152.3 mg/dL. The insulin infusion group had associated significant reductions in mortality and median length of ICU stay.16
Randomized Controlled Trials and Meta-Analyses
In the first Diabetes and Insulin-Glucose study (DIGAMI 1), patients with acute MI received IV insulin therapy for 24 hours, followed by multiple daily injections for three months or longer. The insulin group had lower glucose values and a 29% reduction in mortality at one year and 28% reduction at 3.4 years compared with the control group.17-18
In the most influential study to date, van den Berghe, et al., randomized 1,548 surgical intensive-care unit (ICU) patients to either intensive (IT) or conventional (CT) insulin therapy.19 The glycemic target in the IT arm was 80 to 110 mg/dL (mean glucose attained was 103 mg/dL), while the CT arm had a mean glucose level of 153 mg/dL. The IT group enjoyed substantial reductions in both ICU and total in-hospital mortality, as well as reductions in blood stream infections, acute renal failure, transfusions and the duration of mechanical ventilation (p<0.01 for all).
While a similar study in a medical ICU did not achieve statistical significance in the overall intention-to-treat analysis for mortality, it did demonstrate reductions in mortality in patients with at least three days of ICU treatment and significant reductions in morbidity.20
A meta-analysis of these two studies demonstrated a relative risk reduction in mortality (23.6 to 20.4%) and morbidity in all patients treated with intensive insulin therapy.21
A separate meta-analysis of 35 clinical trials evaluating the effect of intensive insulin infusion therapy on mortality in critically ill inpatients revealed a 15% reduction in short-term mortality.22
Noncritical Care Settings
There are no randomized controlled trials establishing the optimal glycemic target for noncritical care inpatients. There are a number of observational and pilot studies that reinforce the studies performed in critical care settings.
In a retrospective review of almost 1,900 general medical-surgical admissions, Umpierrez, et al., reported an 18-fold increase in mortality in hyperglycemic patients without prior history of diabetes and a 2.5-fold increase in mortality in patients with known diabetes compared to controls. These associations persisted with adjustment for severity of illness.23
A variety of observational and pilot studies associate hyperglycemia with poor outcomes in community acquired pneumonia, renal transplant, and the durability of remission in acute lymphocytic leukemia.24-25
Guidelines and Recommendations
Spurred by the emerging controlled trial evidence, the American Association of Clinical Endocrinologists (AACE) convened a consensus conference involving nine organizations, including SHM. Recommendations for the management of inpatient hyperglycemia included stringent glycemic targets for critical care and noncritical care areas.26 The American Diabetes Association (ADA) produced an excellent technical review on inpatient diabetes that provided the basis for ADA Clinical Practice Guideline glycemic targets.27 The glycemic targets recommended are shown in Table 1 (above).
Caveats
Before accepting these recommended glycemic targets, review the shortcomings of the literature supporting them and consider institutional and individual patient factors that might modify the glycemic target.
Insulin has beneficial vascular and anti-inflammatory effects in its own right, making it difficult in some studies to distinguish the benefit of glucose lowering from the benefit of the insulin used to attain improved control.
The majority of studies supporting inpatient glycemic targets are observational or non-randomized. Some use admission blood glucose concentrations as the sole measure of glucose control.
While most of these studies used valid methods to control for severity of illness and co-morbidities, these methods are not perfect. In some cases, hyperglycemia may have been a marker of a more stressed and sick patient rather than an independent source of adverse outcome.
The dramatic results from the first van den Berghe study have proved difficult to replicate, in part because other investigators have had difficulty achieving stringent glycemic targets safely. Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia, but the studies have not yet been published in final form.28-29
Finally, it bears repeating that the proposed glucose targets for noncritically ill patients are based on essentially no clinical trial data in that population. In part, the glycemic targets reflect the evidence derived from landmark outpatient randomized trials.30-31 In the outpatient setting, insulin requirements and nutritional intake are far more reliable than the inpatient setting, where the iatrogenic induction of excessive hypoglycemia is a valid concern.
Safe Glycemic Control
Rapidly fluctuating nutritional status, changing insulin requirements, varied levels of expertise, and hand-offs between geographic locations and providers are all common in the inpatient setting.
Aggressively pursuing glycemic targets without having systems and safeguards in place could lead to net harm.
The AACE recently identified these barriers and recommended a multidisciplinary team approach, reliable metrics, and a standardized method for insulin protocols, orders, and hypoglycemia prevention and treatment techniques.32
Both the AACE and SHM have produced toolkits to assist institutions to safely achieve improved glycemic control and care.33-34 The SHM Glycemic Control Task Force recently summarized key concepts to emphasize in formulating protocols and order sets in the noncritical care setting (see Table 2, left).
Stringent glycemic targets recommended by the ADA and the AACE may be appropriately moderated in centers that do not yet have the systems in place to achieve those goals safely.
Your glycemic target need not be identical to the ADA and AACE glycemic targets but should be similar to them. Examples of glycemic targets for noncritically ill inpatients are shown in Table 3 (see p. 48).
The glycemic target should be actionable, in that some institutionally endorsed action should result when a patient’s glycemic target is consistently not met.
Back to the Case
Your patient has an active infection, a glucose of 198 mg/dL and an elevated HbA1c. You hold the oral agents and start a basal bolus insulin regimen.
You generate an estimate for a safe insulin total daily dose of 60 units (100 kg x 0.6 units per kg for an obese, type 2 diabetes patient), and administer half as basal insulin, with the remaining 30 units distributed as rapid acting insulin in three divided doses. Your orders include routine glucose monitoring, and you plan to adjust the insulin daily as needed to adhere to the institutional glycemic target for noncritically ill patients of 90 to 150 mg/dL. TH
Dr. Maynard is the division chief for hospital medicine at the University of California, San Diego. He is the leader of SHM’s Glycemic Control Task Force and a leader of the VTE Prevention Collaborative.
References
- Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263-1268.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, 2005. Available at www.cdc.gov/diabetes/pubs/factsheet05.htm. Last accessed September 18, 2007.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982.
- Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB, the Diabetes In Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals (Technical Review). Diabetes Care. 2004;27:553–591.
- Zarich SW. Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness. Rev Cardiovasc Med. 2006;7 (Suppl 2):S35-43.
- Hansen T, Thiel S, Wouters P, Christiansen J, VandenBerghe B. Intensive insulin therapy exerts anti-inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-gind lectin levels. J Clin Endocrinol Metab. 2003;88:1082-1088.
- Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33-38.
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein H. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–
- 2432.
- Bruno A, Williams LS, Kent TA. How important is hyperglycemia during acute brain infarction? Neurologist 2004;10(4):195-200.
- Pomposelli JJ, Baxter JK, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr.1998;22(2):77-81.
- Ainla T, Baburin A, Teesalu R, Rahu M. The association between hyperglycaemia on admission and 180-day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med. 2005;22(10):1321-1325.
- Timmer JR, van der Horst IC, Ottervanger JP, et al. Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am Heart J. 2004;148:399-404.
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778.
- Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006:12 (Suppl 3): 22-26.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–1021.
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478.
- Malmberg K. Prospective randomised study of intensive insulin treatment on long-term survival after acute myocardial infarction inpatients with diabetes mellitus. BMJ. 1997;314:1512–1515.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-461.
- Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151-3159.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164(18):2005-2011.
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-815.
- Thomas M, Mathew T, Russ G, Rao M, Moran J. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation. 2001;72(7):1321-1324.
- Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100(6):1179-1185.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al., American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10:77–82.
- Standards of medical care in diabetes-2006. Diabetes Care. 2006;29 (Suppl. 1):S4-S42.
- Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132(1):268-278.
- Devos P, Preiser JC. Current controversies around tight glucose control in critically ill patients. Curr Opin Clin Nutr Metab Care. 2007;10(2):206-209.
- The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action. Diabetes Care. 2006;29(8):1955-1962.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center 2007. Available at http://resources.aace.com/index.asp. Last accessed December 18, 2007.
- Society of Hospital Medicine Glycemic Control Resource Room. Available at www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Last accessed Nov. 25, 2007.
Case
A 65-year-old obese (100 kg) man with type 2 diabetes, hypertension, and a pack-a-day smoking habit is admitted with moderately severe bilobar pneumonia. His condition is manifest by fever, cough, chills, leukocytosis, and a modest oxygen requirement. You order oxygen, intravenous (IV) fluids, diet, and appropriate antibiotics while continuing the history and chart review. The patient uses metformin and glyburide, and his home glucose readings are generally in the 160 to 180 mg/dL range. An HbA1c level performed three months ago was 9.8, leading to an increased dose of glyburide. As you finish the history, the nurse reports a glucose reading of 198 mg/dL. What is the target blood glucose for noncritical care adult inpatients?
Overview
Diabetes mellitus is an epidemic in the United States. At least 9.3% of adults older than 20 (more than 20 million people) have diabetes. Approximately 30% are unaware they have diabetes.1 Concurrent with the increasing prevalence of diabetes in the U.S. from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled between 1980 and 2003. These trends are expected to accelerate.2 Studies suggest 26% of inpatients have diabetes and 12% have pre-diabetes, previously undiagnosed diabetes, or stress hyperglycemia.3
Review of the Data
A full review of the evidence is beyond the scope of this article. What follows is a sampling of the most representative or influential critical care studies.
Physiology
Fluid and electrolyte balance, left ventricular (LV) function, leukocyte action, wound healing, endothelial function, and immunoglobulin function are all impaired with hyperglycemia.
A prothrombotic state and enhanced platelet aggregation have been demonstrated with even mild elevations of blood glucose.
The mechanisms are multifactorial and complex and involve metabolic derangements leading to oxidative stress, release of free fatty acids, and counter-regulatory hormones.4-6
Observational Studies
A strong and consistent association with hyperglycemia and adverse outcomes is seen in a wide variety of critical care and peri-operative settings. Trauma survival, stroke survival and function, and the incidence of post-operative infections are all adversely affected by hyperglycemia.7-10 Acute myocardial infarction (MI) mortality, acute MI infarct size, and LV dysfunction are also consistently adversely affected in these studies.11-13
This association is typically present in hyperglycemic patients whether they have a diagnosis of diabetes or not, and the association is often even stronger in those lacking a pre-existing diagnosis. Dysfunction typically is detectable at only modest elevations of blood glucose and becomes more marked in a dose response relationship.
Uncontrolled Interventional Studies
The Portland Diabetic Project is a prospective, non-randomized, observational study of 5,510 consecutive diabetic cardiac surgery patients.14-15 The three-day blood glucose average (3-BG) has been progressively reduced for the population through the use of continuous insulin infusion (CII).
The last reported glycemic target is less than 130 mg/dL, and the current glycemic target is less than 110 mg/dL. Both CII for three days and a favorable 3-BG were independently associated with improved mortality, deep sternal-wound infection rates, and length of stay. Mortality and deep sternal-wound infection rates for diabetic patients with well-controlled glucose levels are equal to patients without diabetes.
Another study compared 800 mixed medical-surgical ICU patients with tight glycemic control (mean BG 130.7 mg/dL) to historical controls with a mean glucose of 152.3 mg/dL. The insulin infusion group had associated significant reductions in mortality and median length of ICU stay.16
Randomized Controlled Trials and Meta-Analyses
In the first Diabetes and Insulin-Glucose study (DIGAMI 1), patients with acute MI received IV insulin therapy for 24 hours, followed by multiple daily injections for three months or longer. The insulin group had lower glucose values and a 29% reduction in mortality at one year and 28% reduction at 3.4 years compared with the control group.17-18
In the most influential study to date, van den Berghe, et al., randomized 1,548 surgical intensive-care unit (ICU) patients to either intensive (IT) or conventional (CT) insulin therapy.19 The glycemic target in the IT arm was 80 to 110 mg/dL (mean glucose attained was 103 mg/dL), while the CT arm had a mean glucose level of 153 mg/dL. The IT group enjoyed substantial reductions in both ICU and total in-hospital mortality, as well as reductions in blood stream infections, acute renal failure, transfusions and the duration of mechanical ventilation (p<0.01 for all).
While a similar study in a medical ICU did not achieve statistical significance in the overall intention-to-treat analysis for mortality, it did demonstrate reductions in mortality in patients with at least three days of ICU treatment and significant reductions in morbidity.20
A meta-analysis of these two studies demonstrated a relative risk reduction in mortality (23.6 to 20.4%) and morbidity in all patients treated with intensive insulin therapy.21
A separate meta-analysis of 35 clinical trials evaluating the effect of intensive insulin infusion therapy on mortality in critically ill inpatients revealed a 15% reduction in short-term mortality.22
Noncritical Care Settings
There are no randomized controlled trials establishing the optimal glycemic target for noncritical care inpatients. There are a number of observational and pilot studies that reinforce the studies performed in critical care settings.
In a retrospective review of almost 1,900 general medical-surgical admissions, Umpierrez, et al., reported an 18-fold increase in mortality in hyperglycemic patients without prior history of diabetes and a 2.5-fold increase in mortality in patients with known diabetes compared to controls. These associations persisted with adjustment for severity of illness.23
A variety of observational and pilot studies associate hyperglycemia with poor outcomes in community acquired pneumonia, renal transplant, and the durability of remission in acute lymphocytic leukemia.24-25
Guidelines and Recommendations
Spurred by the emerging controlled trial evidence, the American Association of Clinical Endocrinologists (AACE) convened a consensus conference involving nine organizations, including SHM. Recommendations for the management of inpatient hyperglycemia included stringent glycemic targets for critical care and noncritical care areas.26 The American Diabetes Association (ADA) produced an excellent technical review on inpatient diabetes that provided the basis for ADA Clinical Practice Guideline glycemic targets.27 The glycemic targets recommended are shown in Table 1 (above).
Caveats
Before accepting these recommended glycemic targets, review the shortcomings of the literature supporting them and consider institutional and individual patient factors that might modify the glycemic target.
Insulin has beneficial vascular and anti-inflammatory effects in its own right, making it difficult in some studies to distinguish the benefit of glucose lowering from the benefit of the insulin used to attain improved control.
The majority of studies supporting inpatient glycemic targets are observational or non-randomized. Some use admission blood glucose concentrations as the sole measure of glucose control.
While most of these studies used valid methods to control for severity of illness and co-morbidities, these methods are not perfect. In some cases, hyperglycemia may have been a marker of a more stressed and sick patient rather than an independent source of adverse outcome.
The dramatic results from the first van den Berghe study have proved difficult to replicate, in part because other investigators have had difficulty achieving stringent glycemic targets safely. Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia, but the studies have not yet been published in final form.28-29
Finally, it bears repeating that the proposed glucose targets for noncritically ill patients are based on essentially no clinical trial data in that population. In part, the glycemic targets reflect the evidence derived from landmark outpatient randomized trials.30-31 In the outpatient setting, insulin requirements and nutritional intake are far more reliable than the inpatient setting, where the iatrogenic induction of excessive hypoglycemia is a valid concern.
Safe Glycemic Control
Rapidly fluctuating nutritional status, changing insulin requirements, varied levels of expertise, and hand-offs between geographic locations and providers are all common in the inpatient setting.
Aggressively pursuing glycemic targets without having systems and safeguards in place could lead to net harm.
The AACE recently identified these barriers and recommended a multidisciplinary team approach, reliable metrics, and a standardized method for insulin protocols, orders, and hypoglycemia prevention and treatment techniques.32
Both the AACE and SHM have produced toolkits to assist institutions to safely achieve improved glycemic control and care.33-34 The SHM Glycemic Control Task Force recently summarized key concepts to emphasize in formulating protocols and order sets in the noncritical care setting (see Table 2, left).
Stringent glycemic targets recommended by the ADA and the AACE may be appropriately moderated in centers that do not yet have the systems in place to achieve those goals safely.
Your glycemic target need not be identical to the ADA and AACE glycemic targets but should be similar to them. Examples of glycemic targets for noncritically ill inpatients are shown in Table 3 (see p. 48).
The glycemic target should be actionable, in that some institutionally endorsed action should result when a patient’s glycemic target is consistently not met.
Back to the Case
Your patient has an active infection, a glucose of 198 mg/dL and an elevated HbA1c. You hold the oral agents and start a basal bolus insulin regimen.
You generate an estimate for a safe insulin total daily dose of 60 units (100 kg x 0.6 units per kg for an obese, type 2 diabetes patient), and administer half as basal insulin, with the remaining 30 units distributed as rapid acting insulin in three divided doses. Your orders include routine glucose monitoring, and you plan to adjust the insulin daily as needed to adhere to the institutional glycemic target for noncritically ill patients of 90 to 150 mg/dL. TH
Dr. Maynard is the division chief for hospital medicine at the University of California, San Diego. He is the leader of SHM’s Glycemic Control Task Force and a leader of the VTE Prevention Collaborative.
References
- Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263-1268.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, 2005. Available at www.cdc.gov/diabetes/pubs/factsheet05.htm. Last accessed September 18, 2007.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982.
- Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB, the Diabetes In Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals (Technical Review). Diabetes Care. 2004;27:553–591.
- Zarich SW. Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness. Rev Cardiovasc Med. 2006;7 (Suppl 2):S35-43.
- Hansen T, Thiel S, Wouters P, Christiansen J, VandenBerghe B. Intensive insulin therapy exerts anti-inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-gind lectin levels. J Clin Endocrinol Metab. 2003;88:1082-1088.
- Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33-38.
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein H. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–
- 2432.
- Bruno A, Williams LS, Kent TA. How important is hyperglycemia during acute brain infarction? Neurologist 2004;10(4):195-200.
- Pomposelli JJ, Baxter JK, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr.1998;22(2):77-81.
- Ainla T, Baburin A, Teesalu R, Rahu M. The association between hyperglycaemia on admission and 180-day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med. 2005;22(10):1321-1325.
- Timmer JR, van der Horst IC, Ottervanger JP, et al. Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am Heart J. 2004;148:399-404.
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778.
- Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006:12 (Suppl 3): 22-26.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–1021.
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478.
- Malmberg K. Prospective randomised study of intensive insulin treatment on long-term survival after acute myocardial infarction inpatients with diabetes mellitus. BMJ. 1997;314:1512–1515.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-461.
- Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151-3159.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164(18):2005-2011.
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-815.
- Thomas M, Mathew T, Russ G, Rao M, Moran J. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation. 2001;72(7):1321-1324.
- Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100(6):1179-1185.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al., American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10:77–82.
- Standards of medical care in diabetes-2006. Diabetes Care. 2006;29 (Suppl. 1):S4-S42.
- Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132(1):268-278.
- Devos P, Preiser JC. Current controversies around tight glucose control in critically ill patients. Curr Opin Clin Nutr Metab Care. 2007;10(2):206-209.
- The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action. Diabetes Care. 2006;29(8):1955-1962.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center 2007. Available at http://resources.aace.com/index.asp. Last accessed December 18, 2007.
- Society of Hospital Medicine Glycemic Control Resource Room. Available at www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Last accessed Nov. 25, 2007.
Case
A 65-year-old obese (100 kg) man with type 2 diabetes, hypertension, and a pack-a-day smoking habit is admitted with moderately severe bilobar pneumonia. His condition is manifest by fever, cough, chills, leukocytosis, and a modest oxygen requirement. You order oxygen, intravenous (IV) fluids, diet, and appropriate antibiotics while continuing the history and chart review. The patient uses metformin and glyburide, and his home glucose readings are generally in the 160 to 180 mg/dL range. An HbA1c level performed three months ago was 9.8, leading to an increased dose of glyburide. As you finish the history, the nurse reports a glucose reading of 198 mg/dL. What is the target blood glucose for noncritical care adult inpatients?
Overview
Diabetes mellitus is an epidemic in the United States. At least 9.3% of adults older than 20 (more than 20 million people) have diabetes. Approximately 30% are unaware they have diabetes.1 Concurrent with the increasing prevalence of diabetes in the U.S. from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled between 1980 and 2003. These trends are expected to accelerate.2 Studies suggest 26% of inpatients have diabetes and 12% have pre-diabetes, previously undiagnosed diabetes, or stress hyperglycemia.3
Review of the Data
A full review of the evidence is beyond the scope of this article. What follows is a sampling of the most representative or influential critical care studies.
Physiology
Fluid and electrolyte balance, left ventricular (LV) function, leukocyte action, wound healing, endothelial function, and immunoglobulin function are all impaired with hyperglycemia.
A prothrombotic state and enhanced platelet aggregation have been demonstrated with even mild elevations of blood glucose.
The mechanisms are multifactorial and complex and involve metabolic derangements leading to oxidative stress, release of free fatty acids, and counter-regulatory hormones.4-6
Observational Studies
A strong and consistent association with hyperglycemia and adverse outcomes is seen in a wide variety of critical care and peri-operative settings. Trauma survival, stroke survival and function, and the incidence of post-operative infections are all adversely affected by hyperglycemia.7-10 Acute myocardial infarction (MI) mortality, acute MI infarct size, and LV dysfunction are also consistently adversely affected in these studies.11-13
This association is typically present in hyperglycemic patients whether they have a diagnosis of diabetes or not, and the association is often even stronger in those lacking a pre-existing diagnosis. Dysfunction typically is detectable at only modest elevations of blood glucose and becomes more marked in a dose response relationship.
Uncontrolled Interventional Studies
The Portland Diabetic Project is a prospective, non-randomized, observational study of 5,510 consecutive diabetic cardiac surgery patients.14-15 The three-day blood glucose average (3-BG) has been progressively reduced for the population through the use of continuous insulin infusion (CII).
The last reported glycemic target is less than 130 mg/dL, and the current glycemic target is less than 110 mg/dL. Both CII for three days and a favorable 3-BG were independently associated with improved mortality, deep sternal-wound infection rates, and length of stay. Mortality and deep sternal-wound infection rates for diabetic patients with well-controlled glucose levels are equal to patients without diabetes.
Another study compared 800 mixed medical-surgical ICU patients with tight glycemic control (mean BG 130.7 mg/dL) to historical controls with a mean glucose of 152.3 mg/dL. The insulin infusion group had associated significant reductions in mortality and median length of ICU stay.16
Randomized Controlled Trials and Meta-Analyses
In the first Diabetes and Insulin-Glucose study (DIGAMI 1), patients with acute MI received IV insulin therapy for 24 hours, followed by multiple daily injections for three months or longer. The insulin group had lower glucose values and a 29% reduction in mortality at one year and 28% reduction at 3.4 years compared with the control group.17-18
In the most influential study to date, van den Berghe, et al., randomized 1,548 surgical intensive-care unit (ICU) patients to either intensive (IT) or conventional (CT) insulin therapy.19 The glycemic target in the IT arm was 80 to 110 mg/dL (mean glucose attained was 103 mg/dL), while the CT arm had a mean glucose level of 153 mg/dL. The IT group enjoyed substantial reductions in both ICU and total in-hospital mortality, as well as reductions in blood stream infections, acute renal failure, transfusions and the duration of mechanical ventilation (p<0.01 for all).
While a similar study in a medical ICU did not achieve statistical significance in the overall intention-to-treat analysis for mortality, it did demonstrate reductions in mortality in patients with at least three days of ICU treatment and significant reductions in morbidity.20
A meta-analysis of these two studies demonstrated a relative risk reduction in mortality (23.6 to 20.4%) and morbidity in all patients treated with intensive insulin therapy.21
A separate meta-analysis of 35 clinical trials evaluating the effect of intensive insulin infusion therapy on mortality in critically ill inpatients revealed a 15% reduction in short-term mortality.22
Noncritical Care Settings
There are no randomized controlled trials establishing the optimal glycemic target for noncritical care inpatients. There are a number of observational and pilot studies that reinforce the studies performed in critical care settings.
In a retrospective review of almost 1,900 general medical-surgical admissions, Umpierrez, et al., reported an 18-fold increase in mortality in hyperglycemic patients without prior history of diabetes and a 2.5-fold increase in mortality in patients with known diabetes compared to controls. These associations persisted with adjustment for severity of illness.23
A variety of observational and pilot studies associate hyperglycemia with poor outcomes in community acquired pneumonia, renal transplant, and the durability of remission in acute lymphocytic leukemia.24-25
Guidelines and Recommendations
Spurred by the emerging controlled trial evidence, the American Association of Clinical Endocrinologists (AACE) convened a consensus conference involving nine organizations, including SHM. Recommendations for the management of inpatient hyperglycemia included stringent glycemic targets for critical care and noncritical care areas.26 The American Diabetes Association (ADA) produced an excellent technical review on inpatient diabetes that provided the basis for ADA Clinical Practice Guideline glycemic targets.27 The glycemic targets recommended are shown in Table 1 (above).
Caveats
Before accepting these recommended glycemic targets, review the shortcomings of the literature supporting them and consider institutional and individual patient factors that might modify the glycemic target.
Insulin has beneficial vascular and anti-inflammatory effects in its own right, making it difficult in some studies to distinguish the benefit of glucose lowering from the benefit of the insulin used to attain improved control.
The majority of studies supporting inpatient glycemic targets are observational or non-randomized. Some use admission blood glucose concentrations as the sole measure of glucose control.
While most of these studies used valid methods to control for severity of illness and co-morbidities, these methods are not perfect. In some cases, hyperglycemia may have been a marker of a more stressed and sick patient rather than an independent source of adverse outcome.
The dramatic results from the first van den Berghe study have proved difficult to replicate, in part because other investigators have had difficulty achieving stringent glycemic targets safely. Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia, but the studies have not yet been published in final form.28-29
Finally, it bears repeating that the proposed glucose targets for noncritically ill patients are based on essentially no clinical trial data in that population. In part, the glycemic targets reflect the evidence derived from landmark outpatient randomized trials.30-31 In the outpatient setting, insulin requirements and nutritional intake are far more reliable than the inpatient setting, where the iatrogenic induction of excessive hypoglycemia is a valid concern.
Safe Glycemic Control
Rapidly fluctuating nutritional status, changing insulin requirements, varied levels of expertise, and hand-offs between geographic locations and providers are all common in the inpatient setting.
Aggressively pursuing glycemic targets without having systems and safeguards in place could lead to net harm.
The AACE recently identified these barriers and recommended a multidisciplinary team approach, reliable metrics, and a standardized method for insulin protocols, orders, and hypoglycemia prevention and treatment techniques.32
Both the AACE and SHM have produced toolkits to assist institutions to safely achieve improved glycemic control and care.33-34 The SHM Glycemic Control Task Force recently summarized key concepts to emphasize in formulating protocols and order sets in the noncritical care setting (see Table 2, left).
Stringent glycemic targets recommended by the ADA and the AACE may be appropriately moderated in centers that do not yet have the systems in place to achieve those goals safely.
Your glycemic target need not be identical to the ADA and AACE glycemic targets but should be similar to them. Examples of glycemic targets for noncritically ill inpatients are shown in Table 3 (see p. 48).
The glycemic target should be actionable, in that some institutionally endorsed action should result when a patient’s glycemic target is consistently not met.
Back to the Case
Your patient has an active infection, a glucose of 198 mg/dL and an elevated HbA1c. You hold the oral agents and start a basal bolus insulin regimen.
You generate an estimate for a safe insulin total daily dose of 60 units (100 kg x 0.6 units per kg for an obese, type 2 diabetes patient), and administer half as basal insulin, with the remaining 30 units distributed as rapid acting insulin in three divided doses. Your orders include routine glucose monitoring, and you plan to adjust the insulin daily as needed to adhere to the institutional glycemic target for noncritically ill patients of 90 to 150 mg/dL. TH
Dr. Maynard is the division chief for hospital medicine at the University of California, San Diego. He is the leader of SHM’s Glycemic Control Task Force and a leader of the VTE Prevention Collaborative.
References
- Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263-1268.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, 2005. Available at www.cdc.gov/diabetes/pubs/factsheet05.htm. Last accessed September 18, 2007.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982.
- Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB, the Diabetes In Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals (Technical Review). Diabetes Care. 2004;27:553–591.
- Zarich SW. Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness. Rev Cardiovasc Med. 2006;7 (Suppl 2):S35-43.
- Hansen T, Thiel S, Wouters P, Christiansen J, VandenBerghe B. Intensive insulin therapy exerts anti-inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-gind lectin levels. J Clin Endocrinol Metab. 2003;88:1082-1088.
- Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33-38.
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein H. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–
- 2432.
- Bruno A, Williams LS, Kent TA. How important is hyperglycemia during acute brain infarction? Neurologist 2004;10(4):195-200.
- Pomposelli JJ, Baxter JK, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr.1998;22(2):77-81.
- Ainla T, Baburin A, Teesalu R, Rahu M. The association between hyperglycaemia on admission and 180-day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med. 2005;22(10):1321-1325.
- Timmer JR, van der Horst IC, Ottervanger JP, et al. Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am Heart J. 2004;148:399-404.
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778.
- Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006:12 (Suppl 3): 22-26.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–1021.
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478.
- Malmberg K. Prospective randomised study of intensive insulin treatment on long-term survival after acute myocardial infarction inpatients with diabetes mellitus. BMJ. 1997;314:1512–1515.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-461.
- Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151-3159.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164(18):2005-2011.
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-815.
- Thomas M, Mathew T, Russ G, Rao M, Moran J. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation. 2001;72(7):1321-1324.
- Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100(6):1179-1185.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al., American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10:77–82.
- Standards of medical care in diabetes-2006. Diabetes Care. 2006;29 (Suppl. 1):S4-S42.
- Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132(1):268-278.
- Devos P, Preiser JC. Current controversies around tight glucose control in critically ill patients. Curr Opin Clin Nutr Metab Care. 2007;10(2):206-209.
- The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action. Diabetes Care. 2006;29(8):1955-1962.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center 2007. Available at http://resources.aace.com/index.asp. Last accessed December 18, 2007.
- Society of Hospital Medicine Glycemic Control Resource Room. Available at www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Last accessed Nov. 25, 2007.
Subtle Skills
Hospitalists with a hunger for taking on administrative roles often pursue an advanced degree. But whether it’s to assume a leadership role or just do a better job, it’s the not-altogether-obvious skills that can help hospitalists improve their careers and job satisfaction.
By refining communication styles, being receptive to mentoring, or learning how to influence decision-makers, hospitalists can convey competence to their peers and superiors. Intangible strengths such as these will help the hospitalist who wishes to carve a niche as a quality-improvement researcher, director of a medical education clerkship, patient safety officer, or medical director.
Who Needs What
The administrative skills hospitalists need depend on their career goals. Those who reflect on their career goals, identify their core values, and consider what is feasible at different stages in their lives can more quickly build the abilities they’ll need. This self-awareness is perhaps the first skill to develop.
The setting and practice model hospitalists work in also influences which skills they may need.
“Although the skills needed in different settings may be fundamentally the same, the politics differ between a community hospital and a teaching hospital,” says Sayeed Khan, MD, director of the hospitalist program of Lakeside Medical Group. “Communication skills may be even more crucial in a community hospital, where it’s less understood what a hospitalist is.” Such ability to educate people in Hospitalist 101 is yet another skill a savvy administrator or administrator-to-be should hone.
Hospitalists also need to understand quality control and other measures—and what the numbers mean.
For example, says Dr. Khan, it’s valuable to know:
- What it means to have good bed days at the end of the month and an average length of stay of 3.3 days;
- How that compares with other groups in other hospitals; and
- The implications of those measures in terms of outcomes, dollar costs, and savings to the hospital as well as the group—and how that translates for the patient.
“Those are the types of figures that many hospitalists don’t really understand,” says Dr. Khan.
But hospitalists can learn by observing and studying. “I’m a good example of that in that I do not have a formal business background,” Dr. Khan says. “Along with the literature, networking with other people, particularly at the SHM annual meeting, can help hospitalists gain a better understanding of what these numbers mean and what the benchmarks are.”
He believes administrative skills can be divided into two categories: those related to metrics (the math behind what hospitalists do) and those related to patient care.
In regard to patient care, effective committee participation is an administrative ability that can influence the standard of care. For example, Dr. Khan is participating in committee work in the area of maintaining patients’ glycemic control.
“Historically, that issue was not well addressed,” he says. It is now recognized that patients who have tight glycemic control do much better while hospitalized, irrespective of whether they have diabetes. “But it’s difficult to get other clinicians to change their practice styles,” says Dr. Khan. “You can implement change in your own practice, and others can learn by example. But if you are on a committee that designs new protocols and those get implemented, then you’ve directly changed how medicine is practiced at that hospital.”
Being able to win buy-in for your ideas makes that possible. “Purely speaking, committee participation is not an administrative role,” says Dr. Khan. “But it is an administrative skill in that it is outside the scope of what’s normally required for a hospitalist.”
Get Help
Honing one’s receptivity to mentorship is another vital ability for the upwardly mobile hospitalist. Mentors can direct inexperienced physicians to resources that may help them develop proficiency. A mentor who has grappled with the same issues can help open doors to opportunities hospitalists may not know about.
As she reflects on her early career, when she had no mentors and no administrative experience, Sylvia C.W. McKean, MD, realizes she could have used guidance and advocacy. Effective mentorship helps hospitalists reach their goals faster with fewer impediments, she says.
“Mentorship is critical,” says Dr. McKean, medical director, Brigham and Women’s Hospital/Faulkner Hospitalist Service of Brigham and Women’s Hospital in Boston. “But knowing how to receive the information you’re getting and how to apply it to your own specific professional goals can really help you develop the skills that will help you move your career forward. Informal mentorship is one area where there has been less opportunity for women in the past, resulting in more promotions for men.”
Other skills women may especially need are learning the written and unwritten rules of promotion, being more assertive in finding out what they are, and developing diplomacy—including learning to say no with finesse.
“The reality is that if you are in an environment that has predominantly male leadership, it is important for [a woman] to have male advocates to support whatever it is that you are trying to do,” says Dr. McKean. “In some instances they may have to speak for you.”
Efficiency and setting priorities are also important skills.
“I learned very early on that efficiency was critical to managing several roles—administrative, patient care, and raising three boys,” says Dr. McKean. “There were some things, however, that in retrospect I did not need to do. For example, I did my own home-improvement tasks instead of hiring someone else to do them. For women in particular, you don’t have to be a super everything. At different phases in your life your priorities will vary. Get help so that you’re not spending time doing tasks that don’t further your goals.”
Communication
Facility with communication, of course, is paramount in every aspect of medicine. Being poised, articulate, concise, and persuasive to get your message across, says Dr. McKean, goes a long way toward advancing one’s career.
“It took me a long time to realize this,” says Dr. McKean. “For example, whenever I generated reports I tried to have as much information in there as possible because I thought it would look like I was very knowledgeable. A one-page document that summarizes the key points is often more effective in getting people’s attention.”
Another subset of communication is skill at public speaking, which may lead to being invited to give lectures.
Dr. Khan believes shy, less-articulate clinicians can begin to improve their public speaking by serving on committees. “Unless the committee is a committee of two, that is the appropriate forum to begin voicing your opinions and expertise on a particular matter,” he says. “There’s a certain comfort level built into that because you’re not necessarily speaking on a topic you are unfamiliar with.”
Another intangible administrative skill, he says, is the ability to deal with people from different walks of life. Some highly placed hospital administrators don’t have clinical backgrounds and will require explanations of clinical situations that mesh with their business understanding.
Time Management
Organization is a critical administrative skill no matter what career path a hospitalist follows.
“As hospitalists we are typically juggling more than one thing at one time,” Dr. Khan says. “As a hospitalist who is involved in administrative tasks, if you’re not organized, that is a path to failure.”
Strive to hire the right people for clerical and administrative staff positions. They will fill in the weak spots to keep you on track and present a good image as your front person. But having a good clerical or administrative assistant doesn’t let you off the hook; you, too, must demonstrate solid time management. Make sure you take good notes at committees, quickly access data or documentation, and research and report back well.
The Interpersonal
Robert L. Benak, MD, a hospitalist and medical director of Champlain Valley Physicians Hospital (CVPH) Medical Center, a 341-bed acute care hospital and 54-bed skilled nursing facility in Plattsburgh, N.Y., thinks the most important intangible skills involve managing relationships.
Again, self-reflection helps. “Understand what your personality is like on a calm day and what it is like on a stressful day,” he says.
He says it’s critical to be able to negotiate with others. “Understanding what lies underneath, what common and different interests the two negotiating partners have helps you focus on getting the best compromise of conflicting interests to resolve a disagreement in an amicable and effective way,” he says.
Dr. Benak, who joined SHM around the time his group started in October 2006, thanks the SHM Leadership Academy for strengthening his interpersonal skills. He has tried to bring home what he learned to his group of five hospitalists.
Recently, he had to determine whether to designate a patient with abdominal pain as a surgical or medical patient. Dr. Benak invoked his “ability to sit down with the orthopedic surgeon and general surgeon, recognizing that they’ve got legitimate interests and concerns, as do I, and figuring out what works well for us, and more importantly, what works best for the patient.”
That ability to compromise is indispensable to growth as a hospitalist, he says.
“I was a chemistry major in college and loved working with concrete, though sometimes complicated, problems where you’re either right or you’re wrong,” he says. “I’ve come to see that giving up being right, and giving up any sense of entitlement I may feel in having the principal position, are skills. Even if you think the other person is being unreasonable, you have to accept that as a fact and figure out how to cope with that in a way that is a credit to yourself and your program.” TH
Andrea Sattinger is a medical writer based in North Carolina.
Hospitalists with a hunger for taking on administrative roles often pursue an advanced degree. But whether it’s to assume a leadership role or just do a better job, it’s the not-altogether-obvious skills that can help hospitalists improve their careers and job satisfaction.
By refining communication styles, being receptive to mentoring, or learning how to influence decision-makers, hospitalists can convey competence to their peers and superiors. Intangible strengths such as these will help the hospitalist who wishes to carve a niche as a quality-improvement researcher, director of a medical education clerkship, patient safety officer, or medical director.
Who Needs What
The administrative skills hospitalists need depend on their career goals. Those who reflect on their career goals, identify their core values, and consider what is feasible at different stages in their lives can more quickly build the abilities they’ll need. This self-awareness is perhaps the first skill to develop.
The setting and practice model hospitalists work in also influences which skills they may need.
“Although the skills needed in different settings may be fundamentally the same, the politics differ between a community hospital and a teaching hospital,” says Sayeed Khan, MD, director of the hospitalist program of Lakeside Medical Group. “Communication skills may be even more crucial in a community hospital, where it’s less understood what a hospitalist is.” Such ability to educate people in Hospitalist 101 is yet another skill a savvy administrator or administrator-to-be should hone.
Hospitalists also need to understand quality control and other measures—and what the numbers mean.
For example, says Dr. Khan, it’s valuable to know:
- What it means to have good bed days at the end of the month and an average length of stay of 3.3 days;
- How that compares with other groups in other hospitals; and
- The implications of those measures in terms of outcomes, dollar costs, and savings to the hospital as well as the group—and how that translates for the patient.
“Those are the types of figures that many hospitalists don’t really understand,” says Dr. Khan.
But hospitalists can learn by observing and studying. “I’m a good example of that in that I do not have a formal business background,” Dr. Khan says. “Along with the literature, networking with other people, particularly at the SHM annual meeting, can help hospitalists gain a better understanding of what these numbers mean and what the benchmarks are.”
He believes administrative skills can be divided into two categories: those related to metrics (the math behind what hospitalists do) and those related to patient care.
In regard to patient care, effective committee participation is an administrative ability that can influence the standard of care. For example, Dr. Khan is participating in committee work in the area of maintaining patients’ glycemic control.
“Historically, that issue was not well addressed,” he says. It is now recognized that patients who have tight glycemic control do much better while hospitalized, irrespective of whether they have diabetes. “But it’s difficult to get other clinicians to change their practice styles,” says Dr. Khan. “You can implement change in your own practice, and others can learn by example. But if you are on a committee that designs new protocols and those get implemented, then you’ve directly changed how medicine is practiced at that hospital.”
Being able to win buy-in for your ideas makes that possible. “Purely speaking, committee participation is not an administrative role,” says Dr. Khan. “But it is an administrative skill in that it is outside the scope of what’s normally required for a hospitalist.”
Get Help
Honing one’s receptivity to mentorship is another vital ability for the upwardly mobile hospitalist. Mentors can direct inexperienced physicians to resources that may help them develop proficiency. A mentor who has grappled with the same issues can help open doors to opportunities hospitalists may not know about.
As she reflects on her early career, when she had no mentors and no administrative experience, Sylvia C.W. McKean, MD, realizes she could have used guidance and advocacy. Effective mentorship helps hospitalists reach their goals faster with fewer impediments, she says.
“Mentorship is critical,” says Dr. McKean, medical director, Brigham and Women’s Hospital/Faulkner Hospitalist Service of Brigham and Women’s Hospital in Boston. “But knowing how to receive the information you’re getting and how to apply it to your own specific professional goals can really help you develop the skills that will help you move your career forward. Informal mentorship is one area where there has been less opportunity for women in the past, resulting in more promotions for men.”
Other skills women may especially need are learning the written and unwritten rules of promotion, being more assertive in finding out what they are, and developing diplomacy—including learning to say no with finesse.
“The reality is that if you are in an environment that has predominantly male leadership, it is important for [a woman] to have male advocates to support whatever it is that you are trying to do,” says Dr. McKean. “In some instances they may have to speak for you.”
Efficiency and setting priorities are also important skills.
“I learned very early on that efficiency was critical to managing several roles—administrative, patient care, and raising three boys,” says Dr. McKean. “There were some things, however, that in retrospect I did not need to do. For example, I did my own home-improvement tasks instead of hiring someone else to do them. For women in particular, you don’t have to be a super everything. At different phases in your life your priorities will vary. Get help so that you’re not spending time doing tasks that don’t further your goals.”
Communication
Facility with communication, of course, is paramount in every aspect of medicine. Being poised, articulate, concise, and persuasive to get your message across, says Dr. McKean, goes a long way toward advancing one’s career.
“It took me a long time to realize this,” says Dr. McKean. “For example, whenever I generated reports I tried to have as much information in there as possible because I thought it would look like I was very knowledgeable. A one-page document that summarizes the key points is often more effective in getting people’s attention.”
Another subset of communication is skill at public speaking, which may lead to being invited to give lectures.
Dr. Khan believes shy, less-articulate clinicians can begin to improve their public speaking by serving on committees. “Unless the committee is a committee of two, that is the appropriate forum to begin voicing your opinions and expertise on a particular matter,” he says. “There’s a certain comfort level built into that because you’re not necessarily speaking on a topic you are unfamiliar with.”
Another intangible administrative skill, he says, is the ability to deal with people from different walks of life. Some highly placed hospital administrators don’t have clinical backgrounds and will require explanations of clinical situations that mesh with their business understanding.
Time Management
Organization is a critical administrative skill no matter what career path a hospitalist follows.
“As hospitalists we are typically juggling more than one thing at one time,” Dr. Khan says. “As a hospitalist who is involved in administrative tasks, if you’re not organized, that is a path to failure.”
Strive to hire the right people for clerical and administrative staff positions. They will fill in the weak spots to keep you on track and present a good image as your front person. But having a good clerical or administrative assistant doesn’t let you off the hook; you, too, must demonstrate solid time management. Make sure you take good notes at committees, quickly access data or documentation, and research and report back well.
The Interpersonal
Robert L. Benak, MD, a hospitalist and medical director of Champlain Valley Physicians Hospital (CVPH) Medical Center, a 341-bed acute care hospital and 54-bed skilled nursing facility in Plattsburgh, N.Y., thinks the most important intangible skills involve managing relationships.
Again, self-reflection helps. “Understand what your personality is like on a calm day and what it is like on a stressful day,” he says.
He says it’s critical to be able to negotiate with others. “Understanding what lies underneath, what common and different interests the two negotiating partners have helps you focus on getting the best compromise of conflicting interests to resolve a disagreement in an amicable and effective way,” he says.
Dr. Benak, who joined SHM around the time his group started in October 2006, thanks the SHM Leadership Academy for strengthening his interpersonal skills. He has tried to bring home what he learned to his group of five hospitalists.
Recently, he had to determine whether to designate a patient with abdominal pain as a surgical or medical patient. Dr. Benak invoked his “ability to sit down with the orthopedic surgeon and general surgeon, recognizing that they’ve got legitimate interests and concerns, as do I, and figuring out what works well for us, and more importantly, what works best for the patient.”
That ability to compromise is indispensable to growth as a hospitalist, he says.
“I was a chemistry major in college and loved working with concrete, though sometimes complicated, problems where you’re either right or you’re wrong,” he says. “I’ve come to see that giving up being right, and giving up any sense of entitlement I may feel in having the principal position, are skills. Even if you think the other person is being unreasonable, you have to accept that as a fact and figure out how to cope with that in a way that is a credit to yourself and your program.” TH
Andrea Sattinger is a medical writer based in North Carolina.
Hospitalists with a hunger for taking on administrative roles often pursue an advanced degree. But whether it’s to assume a leadership role or just do a better job, it’s the not-altogether-obvious skills that can help hospitalists improve their careers and job satisfaction.
By refining communication styles, being receptive to mentoring, or learning how to influence decision-makers, hospitalists can convey competence to their peers and superiors. Intangible strengths such as these will help the hospitalist who wishes to carve a niche as a quality-improvement researcher, director of a medical education clerkship, patient safety officer, or medical director.
Who Needs What
The administrative skills hospitalists need depend on their career goals. Those who reflect on their career goals, identify their core values, and consider what is feasible at different stages in their lives can more quickly build the abilities they’ll need. This self-awareness is perhaps the first skill to develop.
The setting and practice model hospitalists work in also influences which skills they may need.
“Although the skills needed in different settings may be fundamentally the same, the politics differ between a community hospital and a teaching hospital,” says Sayeed Khan, MD, director of the hospitalist program of Lakeside Medical Group. “Communication skills may be even more crucial in a community hospital, where it’s less understood what a hospitalist is.” Such ability to educate people in Hospitalist 101 is yet another skill a savvy administrator or administrator-to-be should hone.
Hospitalists also need to understand quality control and other measures—and what the numbers mean.
For example, says Dr. Khan, it’s valuable to know:
- What it means to have good bed days at the end of the month and an average length of stay of 3.3 days;
- How that compares with other groups in other hospitals; and
- The implications of those measures in terms of outcomes, dollar costs, and savings to the hospital as well as the group—and how that translates for the patient.
“Those are the types of figures that many hospitalists don’t really understand,” says Dr. Khan.
But hospitalists can learn by observing and studying. “I’m a good example of that in that I do not have a formal business background,” Dr. Khan says. “Along with the literature, networking with other people, particularly at the SHM annual meeting, can help hospitalists gain a better understanding of what these numbers mean and what the benchmarks are.”
He believes administrative skills can be divided into two categories: those related to metrics (the math behind what hospitalists do) and those related to patient care.
In regard to patient care, effective committee participation is an administrative ability that can influence the standard of care. For example, Dr. Khan is participating in committee work in the area of maintaining patients’ glycemic control.
“Historically, that issue was not well addressed,” he says. It is now recognized that patients who have tight glycemic control do much better while hospitalized, irrespective of whether they have diabetes. “But it’s difficult to get other clinicians to change their practice styles,” says Dr. Khan. “You can implement change in your own practice, and others can learn by example. But if you are on a committee that designs new protocols and those get implemented, then you’ve directly changed how medicine is practiced at that hospital.”
Being able to win buy-in for your ideas makes that possible. “Purely speaking, committee participation is not an administrative role,” says Dr. Khan. “But it is an administrative skill in that it is outside the scope of what’s normally required for a hospitalist.”
Get Help
Honing one’s receptivity to mentorship is another vital ability for the upwardly mobile hospitalist. Mentors can direct inexperienced physicians to resources that may help them develop proficiency. A mentor who has grappled with the same issues can help open doors to opportunities hospitalists may not know about.
As she reflects on her early career, when she had no mentors and no administrative experience, Sylvia C.W. McKean, MD, realizes she could have used guidance and advocacy. Effective mentorship helps hospitalists reach their goals faster with fewer impediments, she says.
“Mentorship is critical,” says Dr. McKean, medical director, Brigham and Women’s Hospital/Faulkner Hospitalist Service of Brigham and Women’s Hospital in Boston. “But knowing how to receive the information you’re getting and how to apply it to your own specific professional goals can really help you develop the skills that will help you move your career forward. Informal mentorship is one area where there has been less opportunity for women in the past, resulting in more promotions for men.”
Other skills women may especially need are learning the written and unwritten rules of promotion, being more assertive in finding out what they are, and developing diplomacy—including learning to say no with finesse.
“The reality is that if you are in an environment that has predominantly male leadership, it is important for [a woman] to have male advocates to support whatever it is that you are trying to do,” says Dr. McKean. “In some instances they may have to speak for you.”
Efficiency and setting priorities are also important skills.
“I learned very early on that efficiency was critical to managing several roles—administrative, patient care, and raising three boys,” says Dr. McKean. “There were some things, however, that in retrospect I did not need to do. For example, I did my own home-improvement tasks instead of hiring someone else to do them. For women in particular, you don’t have to be a super everything. At different phases in your life your priorities will vary. Get help so that you’re not spending time doing tasks that don’t further your goals.”
Communication
Facility with communication, of course, is paramount in every aspect of medicine. Being poised, articulate, concise, and persuasive to get your message across, says Dr. McKean, goes a long way toward advancing one’s career.
“It took me a long time to realize this,” says Dr. McKean. “For example, whenever I generated reports I tried to have as much information in there as possible because I thought it would look like I was very knowledgeable. A one-page document that summarizes the key points is often more effective in getting people’s attention.”
Another subset of communication is skill at public speaking, which may lead to being invited to give lectures.
Dr. Khan believes shy, less-articulate clinicians can begin to improve their public speaking by serving on committees. “Unless the committee is a committee of two, that is the appropriate forum to begin voicing your opinions and expertise on a particular matter,” he says. “There’s a certain comfort level built into that because you’re not necessarily speaking on a topic you are unfamiliar with.”
Another intangible administrative skill, he says, is the ability to deal with people from different walks of life. Some highly placed hospital administrators don’t have clinical backgrounds and will require explanations of clinical situations that mesh with their business understanding.
Time Management
Organization is a critical administrative skill no matter what career path a hospitalist follows.
“As hospitalists we are typically juggling more than one thing at one time,” Dr. Khan says. “As a hospitalist who is involved in administrative tasks, if you’re not organized, that is a path to failure.”
Strive to hire the right people for clerical and administrative staff positions. They will fill in the weak spots to keep you on track and present a good image as your front person. But having a good clerical or administrative assistant doesn’t let you off the hook; you, too, must demonstrate solid time management. Make sure you take good notes at committees, quickly access data or documentation, and research and report back well.
The Interpersonal
Robert L. Benak, MD, a hospitalist and medical director of Champlain Valley Physicians Hospital (CVPH) Medical Center, a 341-bed acute care hospital and 54-bed skilled nursing facility in Plattsburgh, N.Y., thinks the most important intangible skills involve managing relationships.
Again, self-reflection helps. “Understand what your personality is like on a calm day and what it is like on a stressful day,” he says.
He says it’s critical to be able to negotiate with others. “Understanding what lies underneath, what common and different interests the two negotiating partners have helps you focus on getting the best compromise of conflicting interests to resolve a disagreement in an amicable and effective way,” he says.
Dr. Benak, who joined SHM around the time his group started in October 2006, thanks the SHM Leadership Academy for strengthening his interpersonal skills. He has tried to bring home what he learned to his group of five hospitalists.
Recently, he had to determine whether to designate a patient with abdominal pain as a surgical or medical patient. Dr. Benak invoked his “ability to sit down with the orthopedic surgeon and general surgeon, recognizing that they’ve got legitimate interests and concerns, as do I, and figuring out what works well for us, and more importantly, what works best for the patient.”
That ability to compromise is indispensable to growth as a hospitalist, he says.
“I was a chemistry major in college and loved working with concrete, though sometimes complicated, problems where you’re either right or you’re wrong,” he says. “I’ve come to see that giving up being right, and giving up any sense of entitlement I may feel in having the principal position, are skills. Even if you think the other person is being unreasonable, you have to accept that as a fact and figure out how to cope with that in a way that is a credit to yourself and your program.” TH
Andrea Sattinger is a medical writer based in North Carolina.