User login
In the Literature
Literature at a Glance
- Drug-eluting stents decrease the need for revascularization.
- Case volume is related to hospital performance assessment.
- Prolonged QRS duration in patients with CHF is associated with increased morbidity and mortality.
- For out-of-hospital ACLS, vasopressin plus epinephrine is not better than vasopressin alone.
- Oral rivaroxaban is more efficacious than enoxaparin for VTE prophylaxis after total hip replacement.
- LMWH and UFH offer similar perioperative VTE prophylaxis benefit in patients with cancer.
- Salmeterol added to inhaled corticosteroids decreases severe asthma exacerbations.
- Early invasive strategy has unclear benefit in low-risk women with unstable angina or NSTEMI.
- Strategies to prevent contrast-induced acute kidney injury are not uniform.
- Hyperglycemia in hospitalized children is common and associated with ICU admission.
Do drug-eluting stents improve outcomes after ST-elevation myocardial infarction (STEMI)?
Background: Drug-eluting stents reduce restenosis rates compared to bare-metal stents. However, there is concern drug-eluting stents increase the risk of stent thrombosis leading to MI and death. Prior studies compared patients who received bare-metal versus those who received drug-eluting stents. Outcomes on a population level might provide new insight.
Study design: Observational study.
Setting: 100% national sample of patients 65 and older who received a coronary stent from 2002-05 enrolled in the traditional fee-for-service Medicare program.
Synopsis: 38,917 patients in the pre-drug-eluting-stent era from October 2002 to March 2003 received bare-metal stents. Nearly 62% of 28,086 patients studied from September to December 2003 received drug-eluting stents. The remaining 38.5% received bare-metal stents. Outcomes of percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), STEMI, and death were observed through December 31, 2005.
Patients in the drug-eluting-stent era had a lower two-year risk for repeat revascularization compared to patients in the bare-metal-stent era. In the drug-eluting versus bare-metal eras, repeat PCI was 17.1% versus 20.0% (p<0.001) and need for CABG was 2.7% versus 4.2% (p<0.01). Comparing adjusted outcomes for death, or STEMI, at two years, the two groups appeared similar.
The study did have limitations: the data only reflect sirolimus stents, the authors could not assess dual-antiplatelet therapy or obtain information on coronary anatomy or procedure details to account for selection bias in stent utilization, and the patients were all Medicare beneficiaries.
Bottom line: Drug-eluting stents are associated with fewer repeat revascularization procedures than bare-metal stents, but have not shown a significant improvement in the subsequent risk of STEMI or death.
Citation: Malenka DJ, Kaplan AV, Lucas FL, Sharp SM, Skinner JA. Outcomes following coronary stenting in the era of bare-metal vs. the era of drug-eluting stents. JAMA 2008;299(24):2868-2877.
Does case volume affect hospital performance for publicly reported process measures?
Background: Hospitals are increasingly graded and compared to one another. “Top medical centers” are defined as those within the top 10% of hospitals in specified performance measures. Hospitals with large and small case volumes might not be compared evenly and fairly.
Study design: Eight publicly reported process measures for acute myocardial infarction (AMI) were compared to hospital case volume, process performance, and label as “top hospital.”
Setting: Data were analyzed from the Hospital Quality Alliance for 3,761 hospitals from January to December 2005.
Synopsis: Hospitals with large case volume overall had better process performance. For example, looking at use of beta-blockers in patients with AMI on arrival to a hospital, small-volume hospitals (<10 AMI cases) averaged 72% while large volume (>100 AMI cases) averaged 80% (p<0.001). However, hospitals with small case volumes were more likely to receive “top hospital” rating even when hospitals with very low case volumes were excluded.
Hospital quality reporting that does not account for case volume is misleading to hospitals and consumers. In this study, larger-volume hospitals appeared to perform better in process measures, but were less likely to receive “top hospital” rating.
Bottom line: Hospitals with large and small case volumes can easily be compared to one another for process measures in AMI.
Citation: O’Brien SM, DeLong ER, Peterson ED. Impact of case volume on hospital performance assessment. Arch Intern Med. 2008;168(12):1277-1284.
What is the predictive value of QRS duration in patients hospitalized with worsening CHF?
Background: In outpatients, a prolonged QRS duration (greater than 120 ms) is associated with increased mortality. Its value in the inpatient setting is unclear. For patients hospitalized with CHF exacerbations, establishing the value of QRS duration may allow for tailored management.
Study design: Retrospective post hoc analysis from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST).
Setting: 4,133 patients were enrolled from North American, South American, and European sites.
Synopsis: Of 2,962 patients included in the final post hoc analysis, 1,321 (44.6%) had a prolonged QRS duration. During a median follow up of 9.9 months, the all-cause mortality rate was 18.7% for patients with a normal baseline QRS duration and 28.1% for patients with a prolonged baseline QRS.
After adjusting for confounding variables, patients with a prolonged baseline QRS had a 24% increased risk of all-cause mortality and a 28% increased risk for a composite endpoint of cardiac mortality or hospitalization for heart failure exacerbation.
The retrospective nature of the analysis represents the major limitation of this study. In addition, most of the enrolled patients were white, which limits the studies generalizability to other ethnic groups.
Bottom Line: A prolonged QRS duration for patients admitted with decompensated left ventricular heart failure is common and may be associated with increased morbidity and mortality.
Citation: Wang NC, Maggioni AP, Konstam MA, Zannad F, Drasa HB, Burnett JC, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299(22):2656-2666.
For patients with out-of-hospital cardiac arrest, does the addition of vasopressin to epinephrine in a protocol for ACLS improve outcomes?
Background: The outcome for patients experiencing cardiac arrest who require vasopressors remains extremely poor. Despite disappointing data on vasopressin as an alternative treatment during cardiac arrest, a recent subgroup analysis suggested patients who received epinephrine and vasopressin together had superior clinical outcomes.
Study Design: Prospective multicenter randomized double-blind controlled trial.
Setting: 31 emergency medical service organizations in France.
Synopsis: Of the 2,894 patients, 20.7% of those receiving combination treatment (vasopressin plus epinephrine) survived to hospital admission versus 21.3% of those in the epinephrine-only group. For those same groups, 1.7% of combination and 2.3% of epinephrine-only patients survived to hospital discharge. No significant outcome differences were found in any group or subgroup analysis.
The study had lower-than-expected overall survival to hospital discharge, which may have handicapped its effort to find a true difference in treatment arms.
Bottom line: The addition of vasopressin to epinephrine in the treatment of out-of-hospital cardiac arrest does not improve outcomes.
Citation: Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien P, Mauriaucourt P, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359(1):21-30.
Is oral rivaroxaban more efficacious than subcutaneous enoxaparin in preventing VTE after hip-replacement surgery?
Background: Venous thromboembolism (VTE) prophylaxis after total hip replacement (THR) is important but can be cumbersome because the most commonly used anticoagulants are either subcutaneous or require frequent monitoring. Rivaroxaban, an oral direct inhibitor of factor Xa may provide more convenient anticoagulation postoperatively. However, its efficacy and safety are unknown.
Study design: Randomized double-blind trial.
Setting: Multicenter study performed in 27 countries.
Synopsis: Patients undergoing THR surgery were randomized to oral rivaroxaban (10mg once daily without monitoring, started six to eight hours after surgery) or subcutaneous enoxaparin (40mg once daily, started 12 hours prior to surgery). After surgery, prophylaxis was administered for 35 days. The primary outcome was a composite of asymptomatic deep venous thrombosis (DVT), symptomatic DVT or pulmonary embolism (PE), or death from any cause at 36 days after surgery.
In the enoxaparin group, 3.7% of patients experienced the primary outcome. This decreased to 1.1% in the rivaroxaban group. Approximately one-third of events were symptomatic. Major bleeding occurred in 0.1% and 0.3% (p=NS) of patients in the enoxaparin and rivaroxaban groups, respectively.
The study is limited by the exclusion of 1,388 of the 4,541 patients (30.6%) randomized, primarily due to having inadequate venography. Also, because the majority of thromboembolic events were asymptomatic, the primary outcome overemphasizes the clinical difference.
Bottom line: Oral rivaroxaban without monitoring is more efficacious than, and as safe as, subcutaneous enoxaparin when used for VTE prophylaxis for THR.
Citation: Eriksson B, Borris LC, Friedman RJ, Hass S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-75.
Is LMWH more efficacious than UFH in preventing postoperative VTE in cancer patients?
Background: Patients with cancer are at increased risk for VTE and require prophylaxis to prevent this complication postoperatively. Low molecular weight heparin (LMWH) has proven more efficacious than subcutaneous unfractionated heparin (UFH) in other settings (e.g., DVT treatment). However, it is still unknown whether LMWH offers better prophylaxis compared to UFH for cancer patients undergoing surgery.
Study design: Systematic review and meta-analysis.
Setting: 14 randomized controlled trials.
Synopsis: Eleven trials exclusively examined patients with cancer (n=4006) and three trials reported data for cancer patients as subgroups (n=1816). There were in differences in mortality, pulmonary embolism, and symptomatic DVT rates between the two groups.
LMWH was associated with a decrease in total (asymptomatic or symptomatic) DVT (RR, 0.72; 95% CI, 0.55-0.94). Rates of major bleeding, minor bleeding, and intraoperative blood loss were similar between the two treatments.
This meta-analysis is limited because 12 remaining trials (n=3185) also enrolled cancer patients but did not provide specific data for the cancer patient subgroup. The study also is limited by the heterogeneity of the original trials, including utilizing varying LMWHs and dosing regimens, numerous types of surgeries, and a wide range of neoplasms.
Bottom line: LMWH does not decrease mortality, pulmonary embolism, or symptomatic DVT compared to UFH in cancer patients undergoing surgery.
Citation: Akl EA, Terrenato I, Barba M, Sperati F, Sempos EV, Muti P, et al. Low-molecular-weight heparin vs. unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Arch Intern Med. 2008;168:1261-9.
Does salmeterol added to inhaled corticosteroids improve severe asthma-related events?
Background: Asthma is a chronic disease causing major morbidity and mortality worldwide. Disease guidelines recommend all patients with persistent asthma be treated with inhaled corticosteroids. These same guidelines recommend adding a long-acting beta-agonist for patients whose symptoms persist. However, the safety of this practice has come under scrutiny.
Study design: Meta-analysis.
Setting: Sixty-six randomized, controlled trials conducted worldwide.
Synopsis: Analysis included 66 GlaxoSmithKline trials with a total of 20,966 patients with persistent asthma. Patients used either salmeterol (50mcg twice daily) plus inhaled corticosteroid (10,400 patients) or inhaled corticosteroid alone (10,566 patients).
Results showed no differences in asthma-related hospitalizations, asthma-related intubations, or deaths between the two groups. However, due to the low number of events, definitive conclusions are difficult to make. Severe asthma exacerbations requiring systemic corticosteroids significantly decreased in the inhaled corticosteroid plus salmeterol group.
The study is limited by it inclusion of only those trials sponsored by GlaxoSmithKline and by the short duration of most of the studies. Additionally, the studies included in the analysis used clinical outcomes as secondary endpoints.
Bottom line: Adding salmeterol to inhaled corticosteroid decreases severe asthma exacerbations and is likely safe, but does not have an effect on asthma-related hospitalization or death.
Citation: Bateman E, Nelson H, Bousquet J, Kral K, Sutton L, Ortega H, et.al. Meta-analysis: Effects of adding salmeterol to inhaled corticosteroids on serious asthma-related events. Annals Intern Med. 2008;149:33-42.
Is an early invasive strategy effective in women with unstable angina or NSTEMI?
Background: Despite many trials showing the value of an early invasive strategy for patients with non-ST-segment elevation acute coronary syndrome (NSTE ACS), data from several trials question this benefit in women. Some trials show higher risk of death and myocardial infarction (MI) in subgroup analysis of women.
Study Design: Meta-analysis.
Setting: Eight randomized, controlled trials conducted worldwide.
Synopsis: Analysis included eight trials with 10,412 patients (3,075 women) with NSTE ACS. The invasive group (5,083 patients) was defined as those referred for coronary angiography with subsequent intervention as needed. The composite endpoint of death, MI, or rehospitalization within 12 months with ACS occurred in 21.1% of the invasive group and 25.9% of the medically managed group (OR, 0.78; CI, 0.61-0.98).
The subgroup, including only women, had a non-statistically significant OR of 0.81 (CI, 0.65-1.01), including no effect on all-cause mortality, nonfatal MI, or the composite of death and MI. However, women with high-risk features (elevated biomarkers) undergoing the invasive strategy had a significant reduction in the composite endpoint (OR, 0.67; CI, 0.50-0.88).
The study is limited by the use of subgroup analysis, secondary endpoints, heterogeneity between trials, and possible publication bias.
Bottom line: Early invasive strategy is effective in men and high-risk women with NSTE ACS, but not in low-risk women.
Citation: O’Donoghue M, Boden W, Braunwald E, Cannon CP, Clayton TC, Winter RJ, et.al. Early invasive vs. conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2008;300:71-80.
What strategies are used to prevent contrast-induced acute kidney injury?
Background: Contrast-induced acute kidney injury (CIAKI) is a condition potentially amenable to preventive care. Several trials have identified intravenous hydration, N-acetylcysteine, and withdrawal of NSAIDS as interventions that reduce the possibility of CIAKI in high-risk patients. Little is known about whether healthcare providers routinely use these strategies.
Study design: Prospective observational cohort study.
Setting: Veterans Affairs (VA) Pittsburgh Healthcare System.
Synopsis: 11,410 patients scheduled for radiographic procedures were screened. After exclusion criteria and eligibility, 660 patients with an estimated glomerular filtration rate less than 60ml/min/1.73m2 were identified. Usage of intravenous fluids, N-acetylcysteine, and discontinuation of NSAIDS were recorded. Serum creatinine (SCr) was measured 48 to 96 hours post-procedure. CIAKI was defined as relative increase in SCr from baseline (≥25%, ≥50% and ≥100%) and absolute increase in SCr levels from baseline (≥0.25, ≥0.5, and ≥1.0). CIAKI association with adverse outcomes was evaluated by tracking 30-day mortality, need for dialysis, and hospitalization.
The incidence of CIAKI was less common in patients undergoing CT scans versus those having angiograms. Adverse 30-day outcomes were uncommon. Pre- and post-procedure intravenous hydration was administered to 40% of study patients, more commonly with coronary angiogram than with computed tomography (91.2% vs. 16%, p<0.0001). N-acetylcysteine was administered to 39.2%. Only 6.8% of those taking NSAIDS reported being told to discontinue the medication.
Study limitations include the small sample size and the single site location, both limiting generalizability.
Bottom line: Clinically significant CIAKI is uncommon, and preventive care is not uniformly implemented in patients undergoing contrast-enhanced radiographic procedures.
Citation: Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168(12):1325-1332.
How does hyperglycemia affect morbidity and mortality in children admitted to a community pediatric hospital?
Background: Inpatient hyperglycemia in adult patients is a predictor of poor clinical outcomes. The association of hyperglycemia and clinical outcomes in children admitted to a general community hospital has not been studied.
Study design: Retrospective observational cohort study.
Setting: A community pediatric hospital in Atlanta, Ga.
Synopsis: Review of medical records of 903 consecutive pediatric patients admitted to critical and non-critical areas took place. Of these, 542 patients constituted the study population. The study excluded 342 patients who didn’t have a blood glucose measurement. Hyperglycemia was defined as an admission or in-hospital blood glucose greater than 120mg/dl.
One-fourth of the children admitted to the hospital had hyperglycemia, most without a prior history of diabetes. The presence of hyperglycemia on admission was not associated with increased length of stay (LOS) or increased mortality. Children with hyperglycemia were more likely to be admitted to the ICU and had longer ICU LOS.
This was a retrospective study conducted at a single site whose demographics and disease spectrum may differ from those of other institutions. There were an insufficient number of deaths to make any conclusions regarding the impact of hyperglycemia on mortality. Prospective, randomized, multicenter trials are needed to better elucidate the effects of in-patient hyperglycemia.
Bottom line: Hyperglycemia is common in children with or without diabetes admitted to the hospital, and is associated with increased ICU admissions and ICU length of stay. Its connection to mortality is inconclusive.
Citation: Palaio A, Smiley D, Ceron M, Klein R, Cho IS, Mejia R, et al. Prevalence and clinical outcome of inpatient hyperglycemia in a community pediatric hospital. J Hosp Med.2008;3(3):212-217.
Literature at a Glance
- Drug-eluting stents decrease the need for revascularization.
- Case volume is related to hospital performance assessment.
- Prolonged QRS duration in patients with CHF is associated with increased morbidity and mortality.
- For out-of-hospital ACLS, vasopressin plus epinephrine is not better than vasopressin alone.
- Oral rivaroxaban is more efficacious than enoxaparin for VTE prophylaxis after total hip replacement.
- LMWH and UFH offer similar perioperative VTE prophylaxis benefit in patients with cancer.
- Salmeterol added to inhaled corticosteroids decreases severe asthma exacerbations.
- Early invasive strategy has unclear benefit in low-risk women with unstable angina or NSTEMI.
- Strategies to prevent contrast-induced acute kidney injury are not uniform.
- Hyperglycemia in hospitalized children is common and associated with ICU admission.
Do drug-eluting stents improve outcomes after ST-elevation myocardial infarction (STEMI)?
Background: Drug-eluting stents reduce restenosis rates compared to bare-metal stents. However, there is concern drug-eluting stents increase the risk of stent thrombosis leading to MI and death. Prior studies compared patients who received bare-metal versus those who received drug-eluting stents. Outcomes on a population level might provide new insight.
Study design: Observational study.
Setting: 100% national sample of patients 65 and older who received a coronary stent from 2002-05 enrolled in the traditional fee-for-service Medicare program.
Synopsis: 38,917 patients in the pre-drug-eluting-stent era from October 2002 to March 2003 received bare-metal stents. Nearly 62% of 28,086 patients studied from September to December 2003 received drug-eluting stents. The remaining 38.5% received bare-metal stents. Outcomes of percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), STEMI, and death were observed through December 31, 2005.
Patients in the drug-eluting-stent era had a lower two-year risk for repeat revascularization compared to patients in the bare-metal-stent era. In the drug-eluting versus bare-metal eras, repeat PCI was 17.1% versus 20.0% (p<0.001) and need for CABG was 2.7% versus 4.2% (p<0.01). Comparing adjusted outcomes for death, or STEMI, at two years, the two groups appeared similar.
The study did have limitations: the data only reflect sirolimus stents, the authors could not assess dual-antiplatelet therapy or obtain information on coronary anatomy or procedure details to account for selection bias in stent utilization, and the patients were all Medicare beneficiaries.
Bottom line: Drug-eluting stents are associated with fewer repeat revascularization procedures than bare-metal stents, but have not shown a significant improvement in the subsequent risk of STEMI or death.
Citation: Malenka DJ, Kaplan AV, Lucas FL, Sharp SM, Skinner JA. Outcomes following coronary stenting in the era of bare-metal vs. the era of drug-eluting stents. JAMA 2008;299(24):2868-2877.
Does case volume affect hospital performance for publicly reported process measures?
Background: Hospitals are increasingly graded and compared to one another. “Top medical centers” are defined as those within the top 10% of hospitals in specified performance measures. Hospitals with large and small case volumes might not be compared evenly and fairly.
Study design: Eight publicly reported process measures for acute myocardial infarction (AMI) were compared to hospital case volume, process performance, and label as “top hospital.”
Setting: Data were analyzed from the Hospital Quality Alliance for 3,761 hospitals from January to December 2005.
Synopsis: Hospitals with large case volume overall had better process performance. For example, looking at use of beta-blockers in patients with AMI on arrival to a hospital, small-volume hospitals (<10 AMI cases) averaged 72% while large volume (>100 AMI cases) averaged 80% (p<0.001). However, hospitals with small case volumes were more likely to receive “top hospital” rating even when hospitals with very low case volumes were excluded.
Hospital quality reporting that does not account for case volume is misleading to hospitals and consumers. In this study, larger-volume hospitals appeared to perform better in process measures, but were less likely to receive “top hospital” rating.
Bottom line: Hospitals with large and small case volumes can easily be compared to one another for process measures in AMI.
Citation: O’Brien SM, DeLong ER, Peterson ED. Impact of case volume on hospital performance assessment. Arch Intern Med. 2008;168(12):1277-1284.
What is the predictive value of QRS duration in patients hospitalized with worsening CHF?
Background: In outpatients, a prolonged QRS duration (greater than 120 ms) is associated with increased mortality. Its value in the inpatient setting is unclear. For patients hospitalized with CHF exacerbations, establishing the value of QRS duration may allow for tailored management.
Study design: Retrospective post hoc analysis from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST).
Setting: 4,133 patients were enrolled from North American, South American, and European sites.
Synopsis: Of 2,962 patients included in the final post hoc analysis, 1,321 (44.6%) had a prolonged QRS duration. During a median follow up of 9.9 months, the all-cause mortality rate was 18.7% for patients with a normal baseline QRS duration and 28.1% for patients with a prolonged baseline QRS.
After adjusting for confounding variables, patients with a prolonged baseline QRS had a 24% increased risk of all-cause mortality and a 28% increased risk for a composite endpoint of cardiac mortality or hospitalization for heart failure exacerbation.
The retrospective nature of the analysis represents the major limitation of this study. In addition, most of the enrolled patients were white, which limits the studies generalizability to other ethnic groups.
Bottom Line: A prolonged QRS duration for patients admitted with decompensated left ventricular heart failure is common and may be associated with increased morbidity and mortality.
Citation: Wang NC, Maggioni AP, Konstam MA, Zannad F, Drasa HB, Burnett JC, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299(22):2656-2666.
For patients with out-of-hospital cardiac arrest, does the addition of vasopressin to epinephrine in a protocol for ACLS improve outcomes?
Background: The outcome for patients experiencing cardiac arrest who require vasopressors remains extremely poor. Despite disappointing data on vasopressin as an alternative treatment during cardiac arrest, a recent subgroup analysis suggested patients who received epinephrine and vasopressin together had superior clinical outcomes.
Study Design: Prospective multicenter randomized double-blind controlled trial.
Setting: 31 emergency medical service organizations in France.
Synopsis: Of the 2,894 patients, 20.7% of those receiving combination treatment (vasopressin plus epinephrine) survived to hospital admission versus 21.3% of those in the epinephrine-only group. For those same groups, 1.7% of combination and 2.3% of epinephrine-only patients survived to hospital discharge. No significant outcome differences were found in any group or subgroup analysis.
The study had lower-than-expected overall survival to hospital discharge, which may have handicapped its effort to find a true difference in treatment arms.
Bottom line: The addition of vasopressin to epinephrine in the treatment of out-of-hospital cardiac arrest does not improve outcomes.
Citation: Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien P, Mauriaucourt P, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359(1):21-30.
Is oral rivaroxaban more efficacious than subcutaneous enoxaparin in preventing VTE after hip-replacement surgery?
Background: Venous thromboembolism (VTE) prophylaxis after total hip replacement (THR) is important but can be cumbersome because the most commonly used anticoagulants are either subcutaneous or require frequent monitoring. Rivaroxaban, an oral direct inhibitor of factor Xa may provide more convenient anticoagulation postoperatively. However, its efficacy and safety are unknown.
Study design: Randomized double-blind trial.
Setting: Multicenter study performed in 27 countries.
Synopsis: Patients undergoing THR surgery were randomized to oral rivaroxaban (10mg once daily without monitoring, started six to eight hours after surgery) or subcutaneous enoxaparin (40mg once daily, started 12 hours prior to surgery). After surgery, prophylaxis was administered for 35 days. The primary outcome was a composite of asymptomatic deep venous thrombosis (DVT), symptomatic DVT or pulmonary embolism (PE), or death from any cause at 36 days after surgery.
In the enoxaparin group, 3.7% of patients experienced the primary outcome. This decreased to 1.1% in the rivaroxaban group. Approximately one-third of events were symptomatic. Major bleeding occurred in 0.1% and 0.3% (p=NS) of patients in the enoxaparin and rivaroxaban groups, respectively.
The study is limited by the exclusion of 1,388 of the 4,541 patients (30.6%) randomized, primarily due to having inadequate venography. Also, because the majority of thromboembolic events were asymptomatic, the primary outcome overemphasizes the clinical difference.
Bottom line: Oral rivaroxaban without monitoring is more efficacious than, and as safe as, subcutaneous enoxaparin when used for VTE prophylaxis for THR.
Citation: Eriksson B, Borris LC, Friedman RJ, Hass S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-75.
Is LMWH more efficacious than UFH in preventing postoperative VTE in cancer patients?
Background: Patients with cancer are at increased risk for VTE and require prophylaxis to prevent this complication postoperatively. Low molecular weight heparin (LMWH) has proven more efficacious than subcutaneous unfractionated heparin (UFH) in other settings (e.g., DVT treatment). However, it is still unknown whether LMWH offers better prophylaxis compared to UFH for cancer patients undergoing surgery.
Study design: Systematic review and meta-analysis.
Setting: 14 randomized controlled trials.
Synopsis: Eleven trials exclusively examined patients with cancer (n=4006) and three trials reported data for cancer patients as subgroups (n=1816). There were in differences in mortality, pulmonary embolism, and symptomatic DVT rates between the two groups.
LMWH was associated with a decrease in total (asymptomatic or symptomatic) DVT (RR, 0.72; 95% CI, 0.55-0.94). Rates of major bleeding, minor bleeding, and intraoperative blood loss were similar between the two treatments.
This meta-analysis is limited because 12 remaining trials (n=3185) also enrolled cancer patients but did not provide specific data for the cancer patient subgroup. The study also is limited by the heterogeneity of the original trials, including utilizing varying LMWHs and dosing regimens, numerous types of surgeries, and a wide range of neoplasms.
Bottom line: LMWH does not decrease mortality, pulmonary embolism, or symptomatic DVT compared to UFH in cancer patients undergoing surgery.
Citation: Akl EA, Terrenato I, Barba M, Sperati F, Sempos EV, Muti P, et al. Low-molecular-weight heparin vs. unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Arch Intern Med. 2008;168:1261-9.
Does salmeterol added to inhaled corticosteroids improve severe asthma-related events?
Background: Asthma is a chronic disease causing major morbidity and mortality worldwide. Disease guidelines recommend all patients with persistent asthma be treated with inhaled corticosteroids. These same guidelines recommend adding a long-acting beta-agonist for patients whose symptoms persist. However, the safety of this practice has come under scrutiny.
Study design: Meta-analysis.
Setting: Sixty-six randomized, controlled trials conducted worldwide.
Synopsis: Analysis included 66 GlaxoSmithKline trials with a total of 20,966 patients with persistent asthma. Patients used either salmeterol (50mcg twice daily) plus inhaled corticosteroid (10,400 patients) or inhaled corticosteroid alone (10,566 patients).
Results showed no differences in asthma-related hospitalizations, asthma-related intubations, or deaths between the two groups. However, due to the low number of events, definitive conclusions are difficult to make. Severe asthma exacerbations requiring systemic corticosteroids significantly decreased in the inhaled corticosteroid plus salmeterol group.
The study is limited by it inclusion of only those trials sponsored by GlaxoSmithKline and by the short duration of most of the studies. Additionally, the studies included in the analysis used clinical outcomes as secondary endpoints.
Bottom line: Adding salmeterol to inhaled corticosteroid decreases severe asthma exacerbations and is likely safe, but does not have an effect on asthma-related hospitalization or death.
Citation: Bateman E, Nelson H, Bousquet J, Kral K, Sutton L, Ortega H, et.al. Meta-analysis: Effects of adding salmeterol to inhaled corticosteroids on serious asthma-related events. Annals Intern Med. 2008;149:33-42.
Is an early invasive strategy effective in women with unstable angina or NSTEMI?
Background: Despite many trials showing the value of an early invasive strategy for patients with non-ST-segment elevation acute coronary syndrome (NSTE ACS), data from several trials question this benefit in women. Some trials show higher risk of death and myocardial infarction (MI) in subgroup analysis of women.
Study Design: Meta-analysis.
Setting: Eight randomized, controlled trials conducted worldwide.
Synopsis: Analysis included eight trials with 10,412 patients (3,075 women) with NSTE ACS. The invasive group (5,083 patients) was defined as those referred for coronary angiography with subsequent intervention as needed. The composite endpoint of death, MI, or rehospitalization within 12 months with ACS occurred in 21.1% of the invasive group and 25.9% of the medically managed group (OR, 0.78; CI, 0.61-0.98).
The subgroup, including only women, had a non-statistically significant OR of 0.81 (CI, 0.65-1.01), including no effect on all-cause mortality, nonfatal MI, or the composite of death and MI. However, women with high-risk features (elevated biomarkers) undergoing the invasive strategy had a significant reduction in the composite endpoint (OR, 0.67; CI, 0.50-0.88).
The study is limited by the use of subgroup analysis, secondary endpoints, heterogeneity between trials, and possible publication bias.
Bottom line: Early invasive strategy is effective in men and high-risk women with NSTE ACS, but not in low-risk women.
Citation: O’Donoghue M, Boden W, Braunwald E, Cannon CP, Clayton TC, Winter RJ, et.al. Early invasive vs. conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2008;300:71-80.
What strategies are used to prevent contrast-induced acute kidney injury?
Background: Contrast-induced acute kidney injury (CIAKI) is a condition potentially amenable to preventive care. Several trials have identified intravenous hydration, N-acetylcysteine, and withdrawal of NSAIDS as interventions that reduce the possibility of CIAKI in high-risk patients. Little is known about whether healthcare providers routinely use these strategies.
Study design: Prospective observational cohort study.
Setting: Veterans Affairs (VA) Pittsburgh Healthcare System.
Synopsis: 11,410 patients scheduled for radiographic procedures were screened. After exclusion criteria and eligibility, 660 patients with an estimated glomerular filtration rate less than 60ml/min/1.73m2 were identified. Usage of intravenous fluids, N-acetylcysteine, and discontinuation of NSAIDS were recorded. Serum creatinine (SCr) was measured 48 to 96 hours post-procedure. CIAKI was defined as relative increase in SCr from baseline (≥25%, ≥50% and ≥100%) and absolute increase in SCr levels from baseline (≥0.25, ≥0.5, and ≥1.0). CIAKI association with adverse outcomes was evaluated by tracking 30-day mortality, need for dialysis, and hospitalization.
The incidence of CIAKI was less common in patients undergoing CT scans versus those having angiograms. Adverse 30-day outcomes were uncommon. Pre- and post-procedure intravenous hydration was administered to 40% of study patients, more commonly with coronary angiogram than with computed tomography (91.2% vs. 16%, p<0.0001). N-acetylcysteine was administered to 39.2%. Only 6.8% of those taking NSAIDS reported being told to discontinue the medication.
Study limitations include the small sample size and the single site location, both limiting generalizability.
Bottom line: Clinically significant CIAKI is uncommon, and preventive care is not uniformly implemented in patients undergoing contrast-enhanced radiographic procedures.
Citation: Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168(12):1325-1332.
How does hyperglycemia affect morbidity and mortality in children admitted to a community pediatric hospital?
Background: Inpatient hyperglycemia in adult patients is a predictor of poor clinical outcomes. The association of hyperglycemia and clinical outcomes in children admitted to a general community hospital has not been studied.
Study design: Retrospective observational cohort study.
Setting: A community pediatric hospital in Atlanta, Ga.
Synopsis: Review of medical records of 903 consecutive pediatric patients admitted to critical and non-critical areas took place. Of these, 542 patients constituted the study population. The study excluded 342 patients who didn’t have a blood glucose measurement. Hyperglycemia was defined as an admission or in-hospital blood glucose greater than 120mg/dl.
One-fourth of the children admitted to the hospital had hyperglycemia, most without a prior history of diabetes. The presence of hyperglycemia on admission was not associated with increased length of stay (LOS) or increased mortality. Children with hyperglycemia were more likely to be admitted to the ICU and had longer ICU LOS.
This was a retrospective study conducted at a single site whose demographics and disease spectrum may differ from those of other institutions. There were an insufficient number of deaths to make any conclusions regarding the impact of hyperglycemia on mortality. Prospective, randomized, multicenter trials are needed to better elucidate the effects of in-patient hyperglycemia.
Bottom line: Hyperglycemia is common in children with or without diabetes admitted to the hospital, and is associated with increased ICU admissions and ICU length of stay. Its connection to mortality is inconclusive.
Citation: Palaio A, Smiley D, Ceron M, Klein R, Cho IS, Mejia R, et al. Prevalence and clinical outcome of inpatient hyperglycemia in a community pediatric hospital. J Hosp Med.2008;3(3):212-217.
Literature at a Glance
- Drug-eluting stents decrease the need for revascularization.
- Case volume is related to hospital performance assessment.
- Prolonged QRS duration in patients with CHF is associated with increased morbidity and mortality.
- For out-of-hospital ACLS, vasopressin plus epinephrine is not better than vasopressin alone.
- Oral rivaroxaban is more efficacious than enoxaparin for VTE prophylaxis after total hip replacement.
- LMWH and UFH offer similar perioperative VTE prophylaxis benefit in patients with cancer.
- Salmeterol added to inhaled corticosteroids decreases severe asthma exacerbations.
- Early invasive strategy has unclear benefit in low-risk women with unstable angina or NSTEMI.
- Strategies to prevent contrast-induced acute kidney injury are not uniform.
- Hyperglycemia in hospitalized children is common and associated with ICU admission.
Do drug-eluting stents improve outcomes after ST-elevation myocardial infarction (STEMI)?
Background: Drug-eluting stents reduce restenosis rates compared to bare-metal stents. However, there is concern drug-eluting stents increase the risk of stent thrombosis leading to MI and death. Prior studies compared patients who received bare-metal versus those who received drug-eluting stents. Outcomes on a population level might provide new insight.
Study design: Observational study.
Setting: 100% national sample of patients 65 and older who received a coronary stent from 2002-05 enrolled in the traditional fee-for-service Medicare program.
Synopsis: 38,917 patients in the pre-drug-eluting-stent era from October 2002 to March 2003 received bare-metal stents. Nearly 62% of 28,086 patients studied from September to December 2003 received drug-eluting stents. The remaining 38.5% received bare-metal stents. Outcomes of percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), STEMI, and death were observed through December 31, 2005.
Patients in the drug-eluting-stent era had a lower two-year risk for repeat revascularization compared to patients in the bare-metal-stent era. In the drug-eluting versus bare-metal eras, repeat PCI was 17.1% versus 20.0% (p<0.001) and need for CABG was 2.7% versus 4.2% (p<0.01). Comparing adjusted outcomes for death, or STEMI, at two years, the two groups appeared similar.
The study did have limitations: the data only reflect sirolimus stents, the authors could not assess dual-antiplatelet therapy or obtain information on coronary anatomy or procedure details to account for selection bias in stent utilization, and the patients were all Medicare beneficiaries.
Bottom line: Drug-eluting stents are associated with fewer repeat revascularization procedures than bare-metal stents, but have not shown a significant improvement in the subsequent risk of STEMI or death.
Citation: Malenka DJ, Kaplan AV, Lucas FL, Sharp SM, Skinner JA. Outcomes following coronary stenting in the era of bare-metal vs. the era of drug-eluting stents. JAMA 2008;299(24):2868-2877.
Does case volume affect hospital performance for publicly reported process measures?
Background: Hospitals are increasingly graded and compared to one another. “Top medical centers” are defined as those within the top 10% of hospitals in specified performance measures. Hospitals with large and small case volumes might not be compared evenly and fairly.
Study design: Eight publicly reported process measures for acute myocardial infarction (AMI) were compared to hospital case volume, process performance, and label as “top hospital.”
Setting: Data were analyzed from the Hospital Quality Alliance for 3,761 hospitals from January to December 2005.
Synopsis: Hospitals with large case volume overall had better process performance. For example, looking at use of beta-blockers in patients with AMI on arrival to a hospital, small-volume hospitals (<10 AMI cases) averaged 72% while large volume (>100 AMI cases) averaged 80% (p<0.001). However, hospitals with small case volumes were more likely to receive “top hospital” rating even when hospitals with very low case volumes were excluded.
Hospital quality reporting that does not account for case volume is misleading to hospitals and consumers. In this study, larger-volume hospitals appeared to perform better in process measures, but were less likely to receive “top hospital” rating.
Bottom line: Hospitals with large and small case volumes can easily be compared to one another for process measures in AMI.
Citation: O’Brien SM, DeLong ER, Peterson ED. Impact of case volume on hospital performance assessment. Arch Intern Med. 2008;168(12):1277-1284.
What is the predictive value of QRS duration in patients hospitalized with worsening CHF?
Background: In outpatients, a prolonged QRS duration (greater than 120 ms) is associated with increased mortality. Its value in the inpatient setting is unclear. For patients hospitalized with CHF exacerbations, establishing the value of QRS duration may allow for tailored management.
Study design: Retrospective post hoc analysis from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST).
Setting: 4,133 patients were enrolled from North American, South American, and European sites.
Synopsis: Of 2,962 patients included in the final post hoc analysis, 1,321 (44.6%) had a prolonged QRS duration. During a median follow up of 9.9 months, the all-cause mortality rate was 18.7% for patients with a normal baseline QRS duration and 28.1% for patients with a prolonged baseline QRS.
After adjusting for confounding variables, patients with a prolonged baseline QRS had a 24% increased risk of all-cause mortality and a 28% increased risk for a composite endpoint of cardiac mortality or hospitalization for heart failure exacerbation.
The retrospective nature of the analysis represents the major limitation of this study. In addition, most of the enrolled patients were white, which limits the studies generalizability to other ethnic groups.
Bottom Line: A prolonged QRS duration for patients admitted with decompensated left ventricular heart failure is common and may be associated with increased morbidity and mortality.
Citation: Wang NC, Maggioni AP, Konstam MA, Zannad F, Drasa HB, Burnett JC, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299(22):2656-2666.
For patients with out-of-hospital cardiac arrest, does the addition of vasopressin to epinephrine in a protocol for ACLS improve outcomes?
Background: The outcome for patients experiencing cardiac arrest who require vasopressors remains extremely poor. Despite disappointing data on vasopressin as an alternative treatment during cardiac arrest, a recent subgroup analysis suggested patients who received epinephrine and vasopressin together had superior clinical outcomes.
Study Design: Prospective multicenter randomized double-blind controlled trial.
Setting: 31 emergency medical service organizations in France.
Synopsis: Of the 2,894 patients, 20.7% of those receiving combination treatment (vasopressin plus epinephrine) survived to hospital admission versus 21.3% of those in the epinephrine-only group. For those same groups, 1.7% of combination and 2.3% of epinephrine-only patients survived to hospital discharge. No significant outcome differences were found in any group or subgroup analysis.
The study had lower-than-expected overall survival to hospital discharge, which may have handicapped its effort to find a true difference in treatment arms.
Bottom line: The addition of vasopressin to epinephrine in the treatment of out-of-hospital cardiac arrest does not improve outcomes.
Citation: Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien P, Mauriaucourt P, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359(1):21-30.
Is oral rivaroxaban more efficacious than subcutaneous enoxaparin in preventing VTE after hip-replacement surgery?
Background: Venous thromboembolism (VTE) prophylaxis after total hip replacement (THR) is important but can be cumbersome because the most commonly used anticoagulants are either subcutaneous or require frequent monitoring. Rivaroxaban, an oral direct inhibitor of factor Xa may provide more convenient anticoagulation postoperatively. However, its efficacy and safety are unknown.
Study design: Randomized double-blind trial.
Setting: Multicenter study performed in 27 countries.
Synopsis: Patients undergoing THR surgery were randomized to oral rivaroxaban (10mg once daily without monitoring, started six to eight hours after surgery) or subcutaneous enoxaparin (40mg once daily, started 12 hours prior to surgery). After surgery, prophylaxis was administered for 35 days. The primary outcome was a composite of asymptomatic deep venous thrombosis (DVT), symptomatic DVT or pulmonary embolism (PE), or death from any cause at 36 days after surgery.
In the enoxaparin group, 3.7% of patients experienced the primary outcome. This decreased to 1.1% in the rivaroxaban group. Approximately one-third of events were symptomatic. Major bleeding occurred in 0.1% and 0.3% (p=NS) of patients in the enoxaparin and rivaroxaban groups, respectively.
The study is limited by the exclusion of 1,388 of the 4,541 patients (30.6%) randomized, primarily due to having inadequate venography. Also, because the majority of thromboembolic events were asymptomatic, the primary outcome overemphasizes the clinical difference.
Bottom line: Oral rivaroxaban without monitoring is more efficacious than, and as safe as, subcutaneous enoxaparin when used for VTE prophylaxis for THR.
Citation: Eriksson B, Borris LC, Friedman RJ, Hass S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-75.
Is LMWH more efficacious than UFH in preventing postoperative VTE in cancer patients?
Background: Patients with cancer are at increased risk for VTE and require prophylaxis to prevent this complication postoperatively. Low molecular weight heparin (LMWH) has proven more efficacious than subcutaneous unfractionated heparin (UFH) in other settings (e.g., DVT treatment). However, it is still unknown whether LMWH offers better prophylaxis compared to UFH for cancer patients undergoing surgery.
Study design: Systematic review and meta-analysis.
Setting: 14 randomized controlled trials.
Synopsis: Eleven trials exclusively examined patients with cancer (n=4006) and three trials reported data for cancer patients as subgroups (n=1816). There were in differences in mortality, pulmonary embolism, and symptomatic DVT rates between the two groups.
LMWH was associated with a decrease in total (asymptomatic or symptomatic) DVT (RR, 0.72; 95% CI, 0.55-0.94). Rates of major bleeding, minor bleeding, and intraoperative blood loss were similar between the two treatments.
This meta-analysis is limited because 12 remaining trials (n=3185) also enrolled cancer patients but did not provide specific data for the cancer patient subgroup. The study also is limited by the heterogeneity of the original trials, including utilizing varying LMWHs and dosing regimens, numerous types of surgeries, and a wide range of neoplasms.
Bottom line: LMWH does not decrease mortality, pulmonary embolism, or symptomatic DVT compared to UFH in cancer patients undergoing surgery.
Citation: Akl EA, Terrenato I, Barba M, Sperati F, Sempos EV, Muti P, et al. Low-molecular-weight heparin vs. unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Arch Intern Med. 2008;168:1261-9.
Does salmeterol added to inhaled corticosteroids improve severe asthma-related events?
Background: Asthma is a chronic disease causing major morbidity and mortality worldwide. Disease guidelines recommend all patients with persistent asthma be treated with inhaled corticosteroids. These same guidelines recommend adding a long-acting beta-agonist for patients whose symptoms persist. However, the safety of this practice has come under scrutiny.
Study design: Meta-analysis.
Setting: Sixty-six randomized, controlled trials conducted worldwide.
Synopsis: Analysis included 66 GlaxoSmithKline trials with a total of 20,966 patients with persistent asthma. Patients used either salmeterol (50mcg twice daily) plus inhaled corticosteroid (10,400 patients) or inhaled corticosteroid alone (10,566 patients).
Results showed no differences in asthma-related hospitalizations, asthma-related intubations, or deaths between the two groups. However, due to the low number of events, definitive conclusions are difficult to make. Severe asthma exacerbations requiring systemic corticosteroids significantly decreased in the inhaled corticosteroid plus salmeterol group.
The study is limited by it inclusion of only those trials sponsored by GlaxoSmithKline and by the short duration of most of the studies. Additionally, the studies included in the analysis used clinical outcomes as secondary endpoints.
Bottom line: Adding salmeterol to inhaled corticosteroid decreases severe asthma exacerbations and is likely safe, but does not have an effect on asthma-related hospitalization or death.
Citation: Bateman E, Nelson H, Bousquet J, Kral K, Sutton L, Ortega H, et.al. Meta-analysis: Effects of adding salmeterol to inhaled corticosteroids on serious asthma-related events. Annals Intern Med. 2008;149:33-42.
Is an early invasive strategy effective in women with unstable angina or NSTEMI?
Background: Despite many trials showing the value of an early invasive strategy for patients with non-ST-segment elevation acute coronary syndrome (NSTE ACS), data from several trials question this benefit in women. Some trials show higher risk of death and myocardial infarction (MI) in subgroup analysis of women.
Study Design: Meta-analysis.
Setting: Eight randomized, controlled trials conducted worldwide.
Synopsis: Analysis included eight trials with 10,412 patients (3,075 women) with NSTE ACS. The invasive group (5,083 patients) was defined as those referred for coronary angiography with subsequent intervention as needed. The composite endpoint of death, MI, or rehospitalization within 12 months with ACS occurred in 21.1% of the invasive group and 25.9% of the medically managed group (OR, 0.78; CI, 0.61-0.98).
The subgroup, including only women, had a non-statistically significant OR of 0.81 (CI, 0.65-1.01), including no effect on all-cause mortality, nonfatal MI, or the composite of death and MI. However, women with high-risk features (elevated biomarkers) undergoing the invasive strategy had a significant reduction in the composite endpoint (OR, 0.67; CI, 0.50-0.88).
The study is limited by the use of subgroup analysis, secondary endpoints, heterogeneity between trials, and possible publication bias.
Bottom line: Early invasive strategy is effective in men and high-risk women with NSTE ACS, but not in low-risk women.
Citation: O’Donoghue M, Boden W, Braunwald E, Cannon CP, Clayton TC, Winter RJ, et.al. Early invasive vs. conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2008;300:71-80.
What strategies are used to prevent contrast-induced acute kidney injury?
Background: Contrast-induced acute kidney injury (CIAKI) is a condition potentially amenable to preventive care. Several trials have identified intravenous hydration, N-acetylcysteine, and withdrawal of NSAIDS as interventions that reduce the possibility of CIAKI in high-risk patients. Little is known about whether healthcare providers routinely use these strategies.
Study design: Prospective observational cohort study.
Setting: Veterans Affairs (VA) Pittsburgh Healthcare System.
Synopsis: 11,410 patients scheduled for radiographic procedures were screened. After exclusion criteria and eligibility, 660 patients with an estimated glomerular filtration rate less than 60ml/min/1.73m2 were identified. Usage of intravenous fluids, N-acetylcysteine, and discontinuation of NSAIDS were recorded. Serum creatinine (SCr) was measured 48 to 96 hours post-procedure. CIAKI was defined as relative increase in SCr from baseline (≥25%, ≥50% and ≥100%) and absolute increase in SCr levels from baseline (≥0.25, ≥0.5, and ≥1.0). CIAKI association with adverse outcomes was evaluated by tracking 30-day mortality, need for dialysis, and hospitalization.
The incidence of CIAKI was less common in patients undergoing CT scans versus those having angiograms. Adverse 30-day outcomes were uncommon. Pre- and post-procedure intravenous hydration was administered to 40% of study patients, more commonly with coronary angiogram than with computed tomography (91.2% vs. 16%, p<0.0001). N-acetylcysteine was administered to 39.2%. Only 6.8% of those taking NSAIDS reported being told to discontinue the medication.
Study limitations include the small sample size and the single site location, both limiting generalizability.
Bottom line: Clinically significant CIAKI is uncommon, and preventive care is not uniformly implemented in patients undergoing contrast-enhanced radiographic procedures.
Citation: Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168(12):1325-1332.
How does hyperglycemia affect morbidity and mortality in children admitted to a community pediatric hospital?
Background: Inpatient hyperglycemia in adult patients is a predictor of poor clinical outcomes. The association of hyperglycemia and clinical outcomes in children admitted to a general community hospital has not been studied.
Study design: Retrospective observational cohort study.
Setting: A community pediatric hospital in Atlanta, Ga.
Synopsis: Review of medical records of 903 consecutive pediatric patients admitted to critical and non-critical areas took place. Of these, 542 patients constituted the study population. The study excluded 342 patients who didn’t have a blood glucose measurement. Hyperglycemia was defined as an admission or in-hospital blood glucose greater than 120mg/dl.
One-fourth of the children admitted to the hospital had hyperglycemia, most without a prior history of diabetes. The presence of hyperglycemia on admission was not associated with increased length of stay (LOS) or increased mortality. Children with hyperglycemia were more likely to be admitted to the ICU and had longer ICU LOS.
This was a retrospective study conducted at a single site whose demographics and disease spectrum may differ from those of other institutions. There were an insufficient number of deaths to make any conclusions regarding the impact of hyperglycemia on mortality. Prospective, randomized, multicenter trials are needed to better elucidate the effects of in-patient hyperglycemia.
Bottom line: Hyperglycemia is common in children with or without diabetes admitted to the hospital, and is associated with increased ICU admissions and ICU length of stay. Its connection to mortality is inconclusive.
Citation: Palaio A, Smiley D, Ceron M, Klein R, Cho IS, Mejia R, et al. Prevalence and clinical outcome of inpatient hyperglycemia in a community pediatric hospital. J Hosp Med.2008;3(3):212-217.
Neal R. Axon, MD
Ed note: This article is the second in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Ever consider working as an academic hospitalist? Here to give you the scoop on what it’s like is “Team Hospitalist” member R. Neal Axon, MD, assistant professor of internal medicine and pediatrics at the Medical University of South Carolina (MUSC) in Charleston.
Dr. Axon completed his residency at Duke University Medical Center and received his medical degree from the University of Alabama School of Medicine in 2000.
Why is it important to conduct research in hospital medicine?
We haven’t perfected medicine just yet, and until we do we have to work to make it better. Even though hospital medicine research is different from clinical medicine, we need to have people who are working to make the systems of care better.
What attracted you to academic medicine?
I love teaching residents and medical students, and I missed doing it when I entered in private practice. I just completed my master’s at MUSC in clinical research. My department was very supportive and even paid my tuition.
Is it difficult to balance research work with shift work?
It’s definitely a challenge. Fortunately for me, my group does not have shift work in the traditional sense. We do have a night shift, but it’s something we do on an infrequent basis. It would be extremely difficult to do in a seven-on, seven-off schedule that most hospitalists have.
What type of research are you working on?
I’m currently doing some work with hypertension. One of the projects is doing survey work where we access the attitudes of providers (doctors and house staff) on what to do with patients who have hypertension. My observation has been that, in many cases, when patients are admitted to a hospital, they also have high blood pressure that may equate with hypertension in the outpatient setting. It’s not clear when that should be addressed--or how. This survey would help us understand that.
What do you like about what you do?
I worry more about what the department chief thinks than what the CEO of the hospital thinks. At community and non-teaching hospitals, the focus is much more on the bottom line.
So is it impossible to do research if you work at a non-teaching hospital?
I think it’s likely to be more difficult--in that setting--to be a pure clinical researcher, but I do think there are opportunities out there for every day hospitalists to participate in research. This is one of the things I’m currently working on as a member of the SHM Research Committee. One deliverable we’re excited about is the fact that there will be sessions at the [2009] annual meeting in Chicago that will specifically address how hospitalists can do research.
Another thing I hope can evolve is practice-based research networks, which exist in the primary care setting, but not so much in hospital medicine. These networks include groups of community doctors who band together to do clinical research projects. Central leadership helps the members of the group come up with research questions. This is something I’m working on in my state to develop, but this type of setup does exist in other areas.
What advice do you have for hospitalists who are interested in research?
The most important piece of advice is to find a good mentor.
The second thing is that most medical schools have master’s degree programs that teach you the skills that will get you started in clinical research. I went to medical school, but didn’t learn anything about biostatistics or trial design. TH
Ed note: This article is the second in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Ever consider working as an academic hospitalist? Here to give you the scoop on what it’s like is “Team Hospitalist” member R. Neal Axon, MD, assistant professor of internal medicine and pediatrics at the Medical University of South Carolina (MUSC) in Charleston.
Dr. Axon completed his residency at Duke University Medical Center and received his medical degree from the University of Alabama School of Medicine in 2000.
Why is it important to conduct research in hospital medicine?
We haven’t perfected medicine just yet, and until we do we have to work to make it better. Even though hospital medicine research is different from clinical medicine, we need to have people who are working to make the systems of care better.
What attracted you to academic medicine?
I love teaching residents and medical students, and I missed doing it when I entered in private practice. I just completed my master’s at MUSC in clinical research. My department was very supportive and even paid my tuition.
Is it difficult to balance research work with shift work?
It’s definitely a challenge. Fortunately for me, my group does not have shift work in the traditional sense. We do have a night shift, but it’s something we do on an infrequent basis. It would be extremely difficult to do in a seven-on, seven-off schedule that most hospitalists have.
What type of research are you working on?
I’m currently doing some work with hypertension. One of the projects is doing survey work where we access the attitudes of providers (doctors and house staff) on what to do with patients who have hypertension. My observation has been that, in many cases, when patients are admitted to a hospital, they also have high blood pressure that may equate with hypertension in the outpatient setting. It’s not clear when that should be addressed--or how. This survey would help us understand that.
What do you like about what you do?
I worry more about what the department chief thinks than what the CEO of the hospital thinks. At community and non-teaching hospitals, the focus is much more on the bottom line.
So is it impossible to do research if you work at a non-teaching hospital?
I think it’s likely to be more difficult--in that setting--to be a pure clinical researcher, but I do think there are opportunities out there for every day hospitalists to participate in research. This is one of the things I’m currently working on as a member of the SHM Research Committee. One deliverable we’re excited about is the fact that there will be sessions at the [2009] annual meeting in Chicago that will specifically address how hospitalists can do research.
Another thing I hope can evolve is practice-based research networks, which exist in the primary care setting, but not so much in hospital medicine. These networks include groups of community doctors who band together to do clinical research projects. Central leadership helps the members of the group come up with research questions. This is something I’m working on in my state to develop, but this type of setup does exist in other areas.
What advice do you have for hospitalists who are interested in research?
The most important piece of advice is to find a good mentor.
The second thing is that most medical schools have master’s degree programs that teach you the skills that will get you started in clinical research. I went to medical school, but didn’t learn anything about biostatistics or trial design. TH
Ed note: This article is the second in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Ever consider working as an academic hospitalist? Here to give you the scoop on what it’s like is “Team Hospitalist” member R. Neal Axon, MD, assistant professor of internal medicine and pediatrics at the Medical University of South Carolina (MUSC) in Charleston.
Dr. Axon completed his residency at Duke University Medical Center and received his medical degree from the University of Alabama School of Medicine in 2000.
Why is it important to conduct research in hospital medicine?
We haven’t perfected medicine just yet, and until we do we have to work to make it better. Even though hospital medicine research is different from clinical medicine, we need to have people who are working to make the systems of care better.
What attracted you to academic medicine?
I love teaching residents and medical students, and I missed doing it when I entered in private practice. I just completed my master’s at MUSC in clinical research. My department was very supportive and even paid my tuition.
Is it difficult to balance research work with shift work?
It’s definitely a challenge. Fortunately for me, my group does not have shift work in the traditional sense. We do have a night shift, but it’s something we do on an infrequent basis. It would be extremely difficult to do in a seven-on, seven-off schedule that most hospitalists have.
What type of research are you working on?
I’m currently doing some work with hypertension. One of the projects is doing survey work where we access the attitudes of providers (doctors and house staff) on what to do with patients who have hypertension. My observation has been that, in many cases, when patients are admitted to a hospital, they also have high blood pressure that may equate with hypertension in the outpatient setting. It’s not clear when that should be addressed--or how. This survey would help us understand that.
What do you like about what you do?
I worry more about what the department chief thinks than what the CEO of the hospital thinks. At community and non-teaching hospitals, the focus is much more on the bottom line.
So is it impossible to do research if you work at a non-teaching hospital?
I think it’s likely to be more difficult--in that setting--to be a pure clinical researcher, but I do think there are opportunities out there for every day hospitalists to participate in research. This is one of the things I’m currently working on as a member of the SHM Research Committee. One deliverable we’re excited about is the fact that there will be sessions at the [2009] annual meeting in Chicago that will specifically address how hospitalists can do research.
Another thing I hope can evolve is practice-based research networks, which exist in the primary care setting, but not so much in hospital medicine. These networks include groups of community doctors who band together to do clinical research projects. Central leadership helps the members of the group come up with research questions. This is something I’m working on in my state to develop, but this type of setup does exist in other areas.
What advice do you have for hospitalists who are interested in research?
The most important piece of advice is to find a good mentor.
The second thing is that most medical schools have master’s degree programs that teach you the skills that will get you started in clinical research. I went to medical school, but didn’t learn anything about biostatistics or trial design. TH
SHM Invests in 'Champions' and SHM's Future
Not content with the status quo, SHM has taken significant steps to ensure the society’s reach grows in line with the exponential expansion of hospital medicine. These investments put SHM’s premier educational content into the hands of more hospitalists and increase the society’s ability to hear firsthand—and more quickly react to—the challenges of the field.
One key initiative is SHM’s Champions Program, which identifies hospitalists on the ground to serve as information conduits. These “champions” share feedback with SHM’s staff and leadership about the state of hospital medicine locally, and react to SHM plans and proposals. They also help SHM disseminate news and resources to hospitalists in their communities.
In the Champions Program’s short tenure, it already has proven its value. Champions in 26 key markets are engaged and providing valuable feedback in a wide variety of areas, through participation on conference calls, individual surveys, and even a private breakfast with SHM CEO Larry Wellikson.
On top of that, the program has enabled SHM to expand its pool of identified hospitalists by nearly 60%. This expansion not only means SHM’s education and quality improvement resources will reach more communities, but that the society will gain clout and credibility in all facets of healthcare from divergent groups, such as MedPAC and Congress.
Champions are leaders in their communities, dedicated to making a difference in hospital medicine. If this describes you, consider becoming a Champion. Committing to this vital program means helping to steer the course of hospital medicine locally and globally. If you aspire to become a leader within the society, the Champions Program is a great way to begin.
If you’re interested in becoming a Champion or want to suggest someone in your community who would be a good fit, call Cathy Peduzzi, SHM’s manager of membership outreach programs at (215) 351-2584 or e-mail her at [email protected].
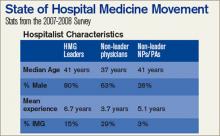
The SHM Bi-Annual Survey on the State of the Hospital Medicine Movement has quickly become a must-have resource for hospital medicine group leaders, administrators, and hospitalists. This survey provides baseline information on topics such as hospital medicine group financial support, compensation and employment models, and productivity.
Whether you’re an experienced hospitalist or you are just starting out in the specialty, the survey has information that will benefit you. Here’s a sampling of the frequently asked questions that can be answered using information found in the survey:
Q: I’m recruiting hospitalists for a hospital medicine group on the East Coast. What’s the average salary for a hospitalist in my area?
A: The average salary for the hospitalist on the East Coast is $189,400, compared to the national average of $193,300.
Q: As a hospital medicine group leader, should I expect financial support for my program?
A: According to the survey, 91% of HMGs receive some kind of financial backing for their program, with an average of $97,275 in support per FTE physician.
Q: How do hospital medicine groups handle night coverage?
A: Currently, 53% of HMGs have an on-site provider, 27% have a hospitalist on-call at home, 16% have a combination of on-site and on-call coverage, and 3% have no night coverage at all.
To purchase the “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement,” visit www.hospital medicine.org/survey.
Not content with the status quo, SHM has taken significant steps to ensure the society’s reach grows in line with the exponential expansion of hospital medicine. These investments put SHM’s premier educational content into the hands of more hospitalists and increase the society’s ability to hear firsthand—and more quickly react to—the challenges of the field.
One key initiative is SHM’s Champions Program, which identifies hospitalists on the ground to serve as information conduits. These “champions” share feedback with SHM’s staff and leadership about the state of hospital medicine locally, and react to SHM plans and proposals. They also help SHM disseminate news and resources to hospitalists in their communities.
In the Champions Program’s short tenure, it already has proven its value. Champions in 26 key markets are engaged and providing valuable feedback in a wide variety of areas, through participation on conference calls, individual surveys, and even a private breakfast with SHM CEO Larry Wellikson.
On top of that, the program has enabled SHM to expand its pool of identified hospitalists by nearly 60%. This expansion not only means SHM’s education and quality improvement resources will reach more communities, but that the society will gain clout and credibility in all facets of healthcare from divergent groups, such as MedPAC and Congress.
Champions are leaders in their communities, dedicated to making a difference in hospital medicine. If this describes you, consider becoming a Champion. Committing to this vital program means helping to steer the course of hospital medicine locally and globally. If you aspire to become a leader within the society, the Champions Program is a great way to begin.
If you’re interested in becoming a Champion or want to suggest someone in your community who would be a good fit, call Cathy Peduzzi, SHM’s manager of membership outreach programs at (215) 351-2584 or e-mail her at [email protected].
The SHM Bi-Annual Survey on the State of the Hospital Medicine Movement has quickly become a must-have resource for hospital medicine group leaders, administrators, and hospitalists. This survey provides baseline information on topics such as hospital medicine group financial support, compensation and employment models, and productivity.
Whether you’re an experienced hospitalist or you are just starting out in the specialty, the survey has information that will benefit you. Here’s a sampling of the frequently asked questions that can be answered using information found in the survey:
Q: I’m recruiting hospitalists for a hospital medicine group on the East Coast. What’s the average salary for a hospitalist in my area?
A: The average salary for the hospitalist on the East Coast is $189,400, compared to the national average of $193,300.
Q: As a hospital medicine group leader, should I expect financial support for my program?
A: According to the survey, 91% of HMGs receive some kind of financial backing for their program, with an average of $97,275 in support per FTE physician.
Q: How do hospital medicine groups handle night coverage?
A: Currently, 53% of HMGs have an on-site provider, 27% have a hospitalist on-call at home, 16% have a combination of on-site and on-call coverage, and 3% have no night coverage at all.
To purchase the “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement,” visit www.hospital medicine.org/survey.
Not content with the status quo, SHM has taken significant steps to ensure the society’s reach grows in line with the exponential expansion of hospital medicine. These investments put SHM’s premier educational content into the hands of more hospitalists and increase the society’s ability to hear firsthand—and more quickly react to—the challenges of the field.
One key initiative is SHM’s Champions Program, which identifies hospitalists on the ground to serve as information conduits. These “champions” share feedback with SHM’s staff and leadership about the state of hospital medicine locally, and react to SHM plans and proposals. They also help SHM disseminate news and resources to hospitalists in their communities.
In the Champions Program’s short tenure, it already has proven its value. Champions in 26 key markets are engaged and providing valuable feedback in a wide variety of areas, through participation on conference calls, individual surveys, and even a private breakfast with SHM CEO Larry Wellikson.
On top of that, the program has enabled SHM to expand its pool of identified hospitalists by nearly 60%. This expansion not only means SHM’s education and quality improvement resources will reach more communities, but that the society will gain clout and credibility in all facets of healthcare from divergent groups, such as MedPAC and Congress.
Champions are leaders in their communities, dedicated to making a difference in hospital medicine. If this describes you, consider becoming a Champion. Committing to this vital program means helping to steer the course of hospital medicine locally and globally. If you aspire to become a leader within the society, the Champions Program is a great way to begin.
If you’re interested in becoming a Champion or want to suggest someone in your community who would be a good fit, call Cathy Peduzzi, SHM’s manager of membership outreach programs at (215) 351-2584 or e-mail her at [email protected].
The SHM Bi-Annual Survey on the State of the Hospital Medicine Movement has quickly become a must-have resource for hospital medicine group leaders, administrators, and hospitalists. This survey provides baseline information on topics such as hospital medicine group financial support, compensation and employment models, and productivity.
Whether you’re an experienced hospitalist or you are just starting out in the specialty, the survey has information that will benefit you. Here’s a sampling of the frequently asked questions that can be answered using information found in the survey:
Q: I’m recruiting hospitalists for a hospital medicine group on the East Coast. What’s the average salary for a hospitalist in my area?
A: The average salary for the hospitalist on the East Coast is $189,400, compared to the national average of $193,300.
Q: As a hospital medicine group leader, should I expect financial support for my program?
A: According to the survey, 91% of HMGs receive some kind of financial backing for their program, with an average of $97,275 in support per FTE physician.
Q: How do hospital medicine groups handle night coverage?
A: Currently, 53% of HMGs have an on-site provider, 27% have a hospitalist on-call at home, 16% have a combination of on-site and on-call coverage, and 3% have no night coverage at all.
To purchase the “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement,” visit www.hospital medicine.org/survey.
Malpractice Chronicle
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Insufficient Monitoring for Cancer Patient With Pneumonia
A 53-year-old woman who was undergoing chemotherapy for breast cancer received a diagnosis of pneumonia from her oncologist, who prescribed antibiotics for her to take at home. When the patient had no response to the antibiotics, her oncologist admitted her to the hospital under the care of the defendant internist. The oncologist and the internist agreed to call in a pulmonologist to provide assistance in treating the woman’s pneumonia.
She did not respond well to the antibiotics administered in the hospital and experienced respiratory distress. The defendant internist called in and ordered a nonrebreather oxygen mask. The nurses contacted the pulmonologist, who stated that since the patient was at 100% oxygenation, there was no need for him to come in to see her.
The internist called in again and was told by staff that the patient’s condition was stable. Her spouse visited with her until 10 pm. Staff nurses checked on her at 2 am.
At 4 am, the decedent was found dead on the bathroom floor. An autopsy confirmed that she had died of bronchopneumonia.
The plaintiff claimed that the defendant violated the standard of care by not seeing the decedent after her respiratory crisis and by not transferring her to the ICU for oxygen monitoring. The defendant argued that he had ordered oxygen monitoring but claimed that the nursing staff failed to follow his order. The defendant further contended that he had requested the pulmonologist to examine the decedent after her respiratory crisis.
The plaintiff settled with the hospital for an undisclosed amount prior to trial. According to published reports, a defense verdict was entered.
Complications From Laparoscopy—or Preexisting Condition?
A woman in her 30s had a long history of abdominal surgeries, beginning with a ruptured appendix and serious infection at age 8. She complained of abdominal pain throughout her teens and underwent additional surgeries in 1991 and 2002.
The plaintiff was seen by the defendant surgeon in March 2003 regarding her abdominal complaints. A decision was made to perform a laparoscopic cholecystectomy in April. Shortly after the surgery, the defendant left town.
The next morning, the plaintiff experienced “bileish” discharge. The defendant returned that evening and advised her that a second surgery would be needed to repair a suspected bowel perforation. The surgery was performed that night.
The plaintiff was discharged a week after the initial surgery but continued to have problems. She was hospitalized a few days later, then transferred to another hospital under the care of other physicians, at her husband’s request. She was left with a nonhealing abdominal wound and lesion.
The plaintiff claimed that she was not an appropriate candidate for surgery and that the procedure should not have been performed laparoscopically. Further, the defendant’s medical license had been placed under a mandatory restriction, and she charged that this fact should have been disclosed to her.
The defendant claimed that the plaintiff had insisted on a laparoscopic procedure and argued that bowel perforations and other complications are known risks of such an operation. The defendant claimed that the plaintiff’s current problems were related to her preexisting history of abdominal problems.
According to a published report, a defense verdict was returned.
Impaired Blood Flow to Injured Leg Goes Untreated
A few days after being struck by an automobile in a parking lot, a Massachusetts woman presented to her primary care physician’s office, where she was seen by the defendant nurse practitioner. The NP noted a large abrasion on the patient’s left leg with some purulent drainage, as well as a large bruise on her back. The woman reported that her left leg felt cooler to the touch than her right leg.
She was instructed to go to the emergency department (ED) if she experienced any worsening pain, numbness, or tingling. Two days later, the patient was seen again by the defendant NP, during which visit she repeated her complaint of coolness in her left leg. She also told the NP that her leg was becoming progressively colder toward her foot and toes and that her toes were slightly discolored.
The woman was given an appointment to see the primary care physician the following week. When he examined her, the physician noted some swelling in the plaintiff’s left lower leg and found that her left great toe and second toe were dusky in color. She had palpable but diminished pulses in the left leg, compared with the right. The patient was given an appointment to see a surgeon.
The next day, the defendant surgeon examined the woman and noted that the toes on her left foot were bluish and felt cold, and that the plaintiff had faintly palpable bilateral femoral pulses but no palpable pulses in the popliteal or posterior tibial or dorsalis pedal area. Doppler ultrasonography showed intact posterior tibial pulses bilaterally but no dorsalis pedal pulse on the left.
The surgeon developed a plan that included repeat ultrasonography of the woman’s left leg in two weeks. He also told her that it would be fine for her to leave the next day for a vacation in Maine.
The following day, because her condition was worsening, the patient went to another surgeon with severe left foot pain and discoloration. She was hospitalized immediately and underwent an angiogram. The imaging revealed a thrombus in the proximal superficial femoral artery with a complete occlusion of the popliteal artery above the knee.
She was operated on twice during the next two days in an attempt to restore meaningful blood flow to her leg. The surgeries were unsuccessful, and she was transferred to another hospital for possible leg salvage. Attempts were unsuccessful, and the woman underwent amputation of her left leg above the knee.
The plaintiff alleged negligence by the NP in failing to admit her to the hospital when she reported the cool leg and foot, and by the first surgeon in failing to hospitalize her when she had an obvious lack of blood flow to the leg and foot. The plaintiff claimed that her foot and lower leg could have been saved if she had undergone surgery at any time before the day she was hospitalized.
The defendants denied any negligence and claimed that clots forming in the small vessels of the leg made saving the leg impossible.
A $2 million settlement was reached.
No Investigation of Elevated Hemoglobin and Hematocrit
Over the course of several years’ treatment of an elderly man with a medical history that included colon cancer, hypertension, type 2 diabetes mellitus, and Parkinson’s disease, the defendant primary care physician had ordered various tests. On several occasions, the patient’s hemoglobin and hematocrit values were elevated. The defendant physician ordered no further testing to investigate these findings.
When the man presented to the defendant with cyanosis in one of his fingers, he was referred to a vascular surgeon to be assessed for possible peripheral vascular disease. The surgeon ordered a number of tests, including a transesophageal echocardiogram, and started the patient on warfarin. During the procedure, his blood pressure became extremely elevated. He was admitted to the ICU, where he was noted to have difficulty speaking and swallowing. CT showed that he had experienced a cerebral infarct.
It was determined during the man’s hospitalization that he had polycythemia vera, which causes the blood to be abnormally thick and poses an increased risk of stroke. CT performed the day after this discovery revealed a hemorrhage in the brain stem. The patient’s condition deteriorated, and he died a few weeks later.
The plaintiff claimed that the decedent’s elevated hemoglobin and hematocrit should have been investigated earlier. The defendant claimed that the decedent’s stroke was unrelated to the elevated hematocrit or to polycythemia vera and that earlier treatment of the blood disorder would not have prevented the vascular injury that occurred.
According to a published account, a $250,000 settlement was reached.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Insufficient Monitoring for Cancer Patient With Pneumonia
A 53-year-old woman who was undergoing chemotherapy for breast cancer received a diagnosis of pneumonia from her oncologist, who prescribed antibiotics for her to take at home. When the patient had no response to the antibiotics, her oncologist admitted her to the hospital under the care of the defendant internist. The oncologist and the internist agreed to call in a pulmonologist to provide assistance in treating the woman’s pneumonia.
She did not respond well to the antibiotics administered in the hospital and experienced respiratory distress. The defendant internist called in and ordered a nonrebreather oxygen mask. The nurses contacted the pulmonologist, who stated that since the patient was at 100% oxygenation, there was no need for him to come in to see her.
The internist called in again and was told by staff that the patient’s condition was stable. Her spouse visited with her until 10 pm. Staff nurses checked on her at 2 am.
At 4 am, the decedent was found dead on the bathroom floor. An autopsy confirmed that she had died of bronchopneumonia.
The plaintiff claimed that the defendant violated the standard of care by not seeing the decedent after her respiratory crisis and by not transferring her to the ICU for oxygen monitoring. The defendant argued that he had ordered oxygen monitoring but claimed that the nursing staff failed to follow his order. The defendant further contended that he had requested the pulmonologist to examine the decedent after her respiratory crisis.
The plaintiff settled with the hospital for an undisclosed amount prior to trial. According to published reports, a defense verdict was entered.
Complications From Laparoscopy—or Preexisting Condition?
A woman in her 30s had a long history of abdominal surgeries, beginning with a ruptured appendix and serious infection at age 8. She complained of abdominal pain throughout her teens and underwent additional surgeries in 1991 and 2002.
The plaintiff was seen by the defendant surgeon in March 2003 regarding her abdominal complaints. A decision was made to perform a laparoscopic cholecystectomy in April. Shortly after the surgery, the defendant left town.
The next morning, the plaintiff experienced “bileish” discharge. The defendant returned that evening and advised her that a second surgery would be needed to repair a suspected bowel perforation. The surgery was performed that night.
The plaintiff was discharged a week after the initial surgery but continued to have problems. She was hospitalized a few days later, then transferred to another hospital under the care of other physicians, at her husband’s request. She was left with a nonhealing abdominal wound and lesion.
The plaintiff claimed that she was not an appropriate candidate for surgery and that the procedure should not have been performed laparoscopically. Further, the defendant’s medical license had been placed under a mandatory restriction, and she charged that this fact should have been disclosed to her.
The defendant claimed that the plaintiff had insisted on a laparoscopic procedure and argued that bowel perforations and other complications are known risks of such an operation. The defendant claimed that the plaintiff’s current problems were related to her preexisting history of abdominal problems.
According to a published report, a defense verdict was returned.
Impaired Blood Flow to Injured Leg Goes Untreated
A few days after being struck by an automobile in a parking lot, a Massachusetts woman presented to her primary care physician’s office, where she was seen by the defendant nurse practitioner. The NP noted a large abrasion on the patient’s left leg with some purulent drainage, as well as a large bruise on her back. The woman reported that her left leg felt cooler to the touch than her right leg.
She was instructed to go to the emergency department (ED) if she experienced any worsening pain, numbness, or tingling. Two days later, the patient was seen again by the defendant NP, during which visit she repeated her complaint of coolness in her left leg. She also told the NP that her leg was becoming progressively colder toward her foot and toes and that her toes were slightly discolored.
The woman was given an appointment to see the primary care physician the following week. When he examined her, the physician noted some swelling in the plaintiff’s left lower leg and found that her left great toe and second toe were dusky in color. She had palpable but diminished pulses in the left leg, compared with the right. The patient was given an appointment to see a surgeon.
The next day, the defendant surgeon examined the woman and noted that the toes on her left foot were bluish and felt cold, and that the plaintiff had faintly palpable bilateral femoral pulses but no palpable pulses in the popliteal or posterior tibial or dorsalis pedal area. Doppler ultrasonography showed intact posterior tibial pulses bilaterally but no dorsalis pedal pulse on the left.
The surgeon developed a plan that included repeat ultrasonography of the woman’s left leg in two weeks. He also told her that it would be fine for her to leave the next day for a vacation in Maine.
The following day, because her condition was worsening, the patient went to another surgeon with severe left foot pain and discoloration. She was hospitalized immediately and underwent an angiogram. The imaging revealed a thrombus in the proximal superficial femoral artery with a complete occlusion of the popliteal artery above the knee.
She was operated on twice during the next two days in an attempt to restore meaningful blood flow to her leg. The surgeries were unsuccessful, and she was transferred to another hospital for possible leg salvage. Attempts were unsuccessful, and the woman underwent amputation of her left leg above the knee.
The plaintiff alleged negligence by the NP in failing to admit her to the hospital when she reported the cool leg and foot, and by the first surgeon in failing to hospitalize her when she had an obvious lack of blood flow to the leg and foot. The plaintiff claimed that her foot and lower leg could have been saved if she had undergone surgery at any time before the day she was hospitalized.
The defendants denied any negligence and claimed that clots forming in the small vessels of the leg made saving the leg impossible.
A $2 million settlement was reached.
No Investigation of Elevated Hemoglobin and Hematocrit
Over the course of several years’ treatment of an elderly man with a medical history that included colon cancer, hypertension, type 2 diabetes mellitus, and Parkinson’s disease, the defendant primary care physician had ordered various tests. On several occasions, the patient’s hemoglobin and hematocrit values were elevated. The defendant physician ordered no further testing to investigate these findings.
When the man presented to the defendant with cyanosis in one of his fingers, he was referred to a vascular surgeon to be assessed for possible peripheral vascular disease. The surgeon ordered a number of tests, including a transesophageal echocardiogram, and started the patient on warfarin. During the procedure, his blood pressure became extremely elevated. He was admitted to the ICU, where he was noted to have difficulty speaking and swallowing. CT showed that he had experienced a cerebral infarct.
It was determined during the man’s hospitalization that he had polycythemia vera, which causes the blood to be abnormally thick and poses an increased risk of stroke. CT performed the day after this discovery revealed a hemorrhage in the brain stem. The patient’s condition deteriorated, and he died a few weeks later.
The plaintiff claimed that the decedent’s elevated hemoglobin and hematocrit should have been investigated earlier. The defendant claimed that the decedent’s stroke was unrelated to the elevated hematocrit or to polycythemia vera and that earlier treatment of the blood disorder would not have prevented the vascular injury that occurred.
According to a published account, a $250,000 settlement was reached.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Insufficient Monitoring for Cancer Patient With Pneumonia
A 53-year-old woman who was undergoing chemotherapy for breast cancer received a diagnosis of pneumonia from her oncologist, who prescribed antibiotics for her to take at home. When the patient had no response to the antibiotics, her oncologist admitted her to the hospital under the care of the defendant internist. The oncologist and the internist agreed to call in a pulmonologist to provide assistance in treating the woman’s pneumonia.
She did not respond well to the antibiotics administered in the hospital and experienced respiratory distress. The defendant internist called in and ordered a nonrebreather oxygen mask. The nurses contacted the pulmonologist, who stated that since the patient was at 100% oxygenation, there was no need for him to come in to see her.
The internist called in again and was told by staff that the patient’s condition was stable. Her spouse visited with her until 10 pm. Staff nurses checked on her at 2 am.
At 4 am, the decedent was found dead on the bathroom floor. An autopsy confirmed that she had died of bronchopneumonia.
The plaintiff claimed that the defendant violated the standard of care by not seeing the decedent after her respiratory crisis and by not transferring her to the ICU for oxygen monitoring. The defendant argued that he had ordered oxygen monitoring but claimed that the nursing staff failed to follow his order. The defendant further contended that he had requested the pulmonologist to examine the decedent after her respiratory crisis.
The plaintiff settled with the hospital for an undisclosed amount prior to trial. According to published reports, a defense verdict was entered.
Complications From Laparoscopy—or Preexisting Condition?
A woman in her 30s had a long history of abdominal surgeries, beginning with a ruptured appendix and serious infection at age 8. She complained of abdominal pain throughout her teens and underwent additional surgeries in 1991 and 2002.
The plaintiff was seen by the defendant surgeon in March 2003 regarding her abdominal complaints. A decision was made to perform a laparoscopic cholecystectomy in April. Shortly after the surgery, the defendant left town.
The next morning, the plaintiff experienced “bileish” discharge. The defendant returned that evening and advised her that a second surgery would be needed to repair a suspected bowel perforation. The surgery was performed that night.
The plaintiff was discharged a week after the initial surgery but continued to have problems. She was hospitalized a few days later, then transferred to another hospital under the care of other physicians, at her husband’s request. She was left with a nonhealing abdominal wound and lesion.
The plaintiff claimed that she was not an appropriate candidate for surgery and that the procedure should not have been performed laparoscopically. Further, the defendant’s medical license had been placed under a mandatory restriction, and she charged that this fact should have been disclosed to her.
The defendant claimed that the plaintiff had insisted on a laparoscopic procedure and argued that bowel perforations and other complications are known risks of such an operation. The defendant claimed that the plaintiff’s current problems were related to her preexisting history of abdominal problems.
According to a published report, a defense verdict was returned.
Impaired Blood Flow to Injured Leg Goes Untreated
A few days after being struck by an automobile in a parking lot, a Massachusetts woman presented to her primary care physician’s office, where she was seen by the defendant nurse practitioner. The NP noted a large abrasion on the patient’s left leg with some purulent drainage, as well as a large bruise on her back. The woman reported that her left leg felt cooler to the touch than her right leg.
She was instructed to go to the emergency department (ED) if she experienced any worsening pain, numbness, or tingling. Two days later, the patient was seen again by the defendant NP, during which visit she repeated her complaint of coolness in her left leg. She also told the NP that her leg was becoming progressively colder toward her foot and toes and that her toes were slightly discolored.
The woman was given an appointment to see the primary care physician the following week. When he examined her, the physician noted some swelling in the plaintiff’s left lower leg and found that her left great toe and second toe were dusky in color. She had palpable but diminished pulses in the left leg, compared with the right. The patient was given an appointment to see a surgeon.
The next day, the defendant surgeon examined the woman and noted that the toes on her left foot were bluish and felt cold, and that the plaintiff had faintly palpable bilateral femoral pulses but no palpable pulses in the popliteal or posterior tibial or dorsalis pedal area. Doppler ultrasonography showed intact posterior tibial pulses bilaterally but no dorsalis pedal pulse on the left.
The surgeon developed a plan that included repeat ultrasonography of the woman’s left leg in two weeks. He also told her that it would be fine for her to leave the next day for a vacation in Maine.
The following day, because her condition was worsening, the patient went to another surgeon with severe left foot pain and discoloration. She was hospitalized immediately and underwent an angiogram. The imaging revealed a thrombus in the proximal superficial femoral artery with a complete occlusion of the popliteal artery above the knee.
She was operated on twice during the next two days in an attempt to restore meaningful blood flow to her leg. The surgeries were unsuccessful, and she was transferred to another hospital for possible leg salvage. Attempts were unsuccessful, and the woman underwent amputation of her left leg above the knee.
The plaintiff alleged negligence by the NP in failing to admit her to the hospital when she reported the cool leg and foot, and by the first surgeon in failing to hospitalize her when she had an obvious lack of blood flow to the leg and foot. The plaintiff claimed that her foot and lower leg could have been saved if she had undergone surgery at any time before the day she was hospitalized.
The defendants denied any negligence and claimed that clots forming in the small vessels of the leg made saving the leg impossible.
A $2 million settlement was reached.
No Investigation of Elevated Hemoglobin and Hematocrit
Over the course of several years’ treatment of an elderly man with a medical history that included colon cancer, hypertension, type 2 diabetes mellitus, and Parkinson’s disease, the defendant primary care physician had ordered various tests. On several occasions, the patient’s hemoglobin and hematocrit values were elevated. The defendant physician ordered no further testing to investigate these findings.
When the man presented to the defendant with cyanosis in one of his fingers, he was referred to a vascular surgeon to be assessed for possible peripheral vascular disease. The surgeon ordered a number of tests, including a transesophageal echocardiogram, and started the patient on warfarin. During the procedure, his blood pressure became extremely elevated. He was admitted to the ICU, where he was noted to have difficulty speaking and swallowing. CT showed that he had experienced a cerebral infarct.
It was determined during the man’s hospitalization that he had polycythemia vera, which causes the blood to be abnormally thick and poses an increased risk of stroke. CT performed the day after this discovery revealed a hemorrhage in the brain stem. The patient’s condition deteriorated, and he died a few weeks later.
The plaintiff claimed that the decedent’s elevated hemoglobin and hematocrit should have been investigated earlier. The defendant claimed that the decedent’s stroke was unrelated to the elevated hematocrit or to polycythemia vera and that earlier treatment of the blood disorder would not have prevented the vascular injury that occurred.
According to a published account, a $250,000 settlement was reached.
Malpractice minute
Could a child’s suicide have been prevented?
THE PATIENT. A 9-year-old boy was undergoing psychiatric treatment.
CASE FACTS. A psychiatrist prescribed bupropion. The child committed suicide.
THE PARENTS’ CLAIM. The psychiatrist was negligent because he did not diagnose suicidal behavior during the initial visit and prescribed bupropion without proper warnings and follow-up.
THE DOCTOR’S DEFENSE. He did not receive information from the patient’s family that would have indicated suicidal behavior, bupropion was an appropriate treatment and was unrelated to the suicide, the family received proper warnings about the drug, and the suicide was unforeseeable.
Submit your verdict and find out how the court ruled and see how your colleagues voted in August’s Malpractice Minute. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Could a child’s suicide have been prevented?
THE PATIENT. A 9-year-old boy was undergoing psychiatric treatment.
CASE FACTS. A psychiatrist prescribed bupropion. The child committed suicide.
THE PARENTS’ CLAIM. The psychiatrist was negligent because he did not diagnose suicidal behavior during the initial visit and prescribed bupropion without proper warnings and follow-up.
THE DOCTOR’S DEFENSE. He did not receive information from the patient’s family that would have indicated suicidal behavior, bupropion was an appropriate treatment and was unrelated to the suicide, the family received proper warnings about the drug, and the suicide was unforeseeable.
Submit your verdict and find out how the court ruled and see how your colleagues voted in August’s Malpractice Minute. Click on “Have more to say about this topic?” to comment.
Could a child’s suicide have been prevented?
THE PATIENT. A 9-year-old boy was undergoing psychiatric treatment.
CASE FACTS. A psychiatrist prescribed bupropion. The child committed suicide.
THE PARENTS’ CLAIM. The psychiatrist was negligent because he did not diagnose suicidal behavior during the initial visit and prescribed bupropion without proper warnings and follow-up.
THE DOCTOR’S DEFENSE. He did not receive information from the patient’s family that would have indicated suicidal behavior, bupropion was an appropriate treatment and was unrelated to the suicide, the family received proper warnings about the drug, and the suicide was unforeseeable.
Submit your verdict and find out how the court ruled and see how your colleagues voted in August’s Malpractice Minute. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Going outside your area of expertise: How far is too far?
Dear Dr. Mossman:
I am an adult psychiatrist practicing in a geographically isolated area. I am working with the family of 10-year-old “Bobby” who is struggling with attention problems. Top notch neuropsychologic testing recommends a stimulant trial, but the local pediatrician is too busy to give Bobby adequate follow-up and attention.
I am an experienced psychopharmacologist but have not prescribed medication to children since residency. My relationship with the family is excellent, and the local pediatrician said that she would supervise me. If I choose to treat Bobby, what are the possible liability issues I should be aware of, and how can I address them?—Submitted by “Dr. F”
Dr. F’s question raises issues that come up whenever patients need treatment for conditions outside the few with which you are highly familiar. Although you can’t be an expert on every aspect of every patient’s treatment, psychiatrists shouldn’t practice outside their area of competence.
Thus, the main liability-related issue that Dr. F should ask herself is, “Can I treat Bobby competently?” Of course, whenever you decide to treat any patient, you should be able to answer “yes” to this question. When thinking about potential liability related to treating Bobby, Dr. F might also ask, “If a lawsuit occurred, how would my treatment of Bobby appear?” This article discusses key issues that arise when general psychiatrists treat children and the steps general psychiatrists can take to show that they are practicing prudently.
Problem: Not enough clinicians
Child and adolescent psychiatrists (CAPs) are in short supply.1,2 In 2001 the United States had 8.67 CAPs per 100,000 youths and 1.6 CAPs for every 1,000 youths with severe mental disorders.1 Studies suggest that the United States needs nearly twice that many CAPs.3 The shortage is especially severe in rural areas, but approximately one-half of metropolitan counties with populations of >250,000 have no CAPs.1 In much of the nation, finding CAPs who are accepting new patients is difficult, and child and adolescent psychiatric treatment often is delivered by pediatricians, family practitioners, psychiatric nurse practitioners, and general adult psychiatrists.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Children’s special medical issues
General psychiatrists know that children aren’t just little adults. CAPs develop skills and thinking styles during their 2 years of subspecialty fellowship training that are quite different from those used by their general psychiatric colleagues.
Communication. Children and adolescents who need psychiatric care often have limited verbal abilities. Working and communicating with these patients requires a different interactive style.
Information sources. CAPs learn to seek and assimilate clinically important information from many settings—especially a child’s home—where their patients interact with others.
Caution. Only a small subset of psychotropic medications that adult psychiatrists prescribe are FDA-approved for use in children (Table 1).4 Because we don’t know how psychotropic drugs affect brain development, CAPs sometimes are leery of giving kids the same medications that adult psychiatrists readily prescribe.
Table 1
FDA-approved drugs and dosages for ADHD in children and adults
| Brand name | Generic name | Drug class | Dosing forms (mg) | Dosage range | Age range |
|---|---|---|---|---|---|
| Adderall | Amphetamine-dextroamphetamine | IR stimulant | 5, 7.5, 10, 12.5, 15, 20, 30 | 5 to 40 mg | 3 to 18 years |
| Adderall XR | Amphetamine-dextroamphetamine | ER stimulant | 5, 10, 15, 20, 25, 30 | 5 to 30 mg | 3 years to adult |
| Concerta | Methylphenidate | ER stimulant | 18, 27, 36, 54 | 18 to 72 mg | 6 years to adult |
| Daytrana | Methylphenidate transdermal | Stimulant | 10, 15, 20, 30 (patch) | 10 to 30 mg | 6 to 18 years |
| Focalin | Dexmethylphenidate | IR stimulant | 2.5, 5, 10 | 2.5 to 10 mg bid | 6 to 17 years |
| Focalin XR | Dexmethylphenidate | ER stimulant | 5, 10, 15, 20 | 5 to 20 mg | 6 years to adult |
| Metadate CD | Methylphenidate | ER stimulant | 10, 20, 30, 50, 60 | 10 to 60 mg | 6 to 18 years |
| Ritalin | Methylphenidate | IR stimulant | 5, 10, 20 | 5 to 20 mg bid or tid | 6 to 18 years |
| Ritalin LA | Methylphenidate | ER stimulant | 10, 20, 30, 40 | 10 to 60 mg | 6 to 18 years |
| Strattera | Atomoxetine | SNRI | 10, 18, 25, 40, 60, 80, 100 | 10 to 100 mg | 6 years to adult |
| Vyvanse | Lisdexamfetamine dimesylate | ER stimulant (precursor) | 30, 50, 70 | 30 to 70 mg | 6 years to adult |
| LA: long acting; CD: controlled delivery; ER, XR: extended release; IR: immediate release; SNRI: selective norepinephrine reuptake inhibitor | |||||
| Source: Adapted from references 2,3 | |||||
Different drugs. Some medications commonly taken by children are not often prescribed for adults, although this is changing as attention-deficit/hyperactivity disorder (ADHD) is better recognized in adults.5,6
Dosages. Dosing psychotropics in adults is fairly standardized, but in children and adolescents dosages vary with age, body weight, and physical maturity.
Adverse effects. The side effects kids experience and the way they report them can differ markedly from adults and will vary with age and developmental maturity. Some issues related to monitoring children—such as appropriate cardiac screening before starting stimulants—are controversial and remain unsettled.7,8
Consider alternatives
Dr. F may be tempted to treat Bobby because of her preexisting, positive relationship with the child’s family and a laudable desire to help. But Dr. F needs to ask, “Is there really no other workable alternative for Bobby?” Some possibilities include:
- Refer Bobby to a CAP in another community for initiation of treatment. Dr. F or Bobby’s pediatrician might safely continue care once a CAP establishes an effective treatment regimen.
- Find another pediatrician who might have more time to provide the follow-up that Dr. F feels is necessary.
- Decline to treat Bobby. Before doing this, Dr. F should consider what effect this refusal might have on her relationship with the family and the consequences for Bobby if his problems go untreated.
- Consult a CAP from another community, describing the situation and clinical factors in detail without naming or identifying the patient, and then ask, “Is this really the best thing to do?”
From a liability standpoint, this last point may be crucial. If the CAP answers “yes,” Dr. F can document the alternatives she has considered and her consultation and discussion with the CAP colleague as evidence of prudent practice. Dr. F can also document any advice that she has received and her plans to follow it.
If you choose to treat
Presumably, Dr. F would not perform thoracic surgery or provide any treatment that is far outside a general psychiatrist’s competence except under the most dire circumstances. General psychiatrists receive child psychiatry training during residency, and treating children is within their scope of practice. Similarly, most elderly patients are treated by general psychiatrists, rather than graduates of geropsychiatry fellowships. Prescribing medication for Bobby is not grossly different from Dr. F’s other duties, and she might provide services that a pediatrician might not.
Ask yourself 4 questions to determine if you are competent to provide medical treatment outside your usual area of expertise (Table 2). In Bobby’s case, Dr. F can consider these additional questions:
- Am I comfortable doing this? Would I be comfortable with this scenario if Bobby were my child?
- How extensive was my general residency training in child psychiatry?
- How long ago was my last CAP experience?
- Have I treated ADHD in adults, and am I familiar with stimulant medications?
- What kind of supervision could I arrange, such as regular phone consultation with a CAP or pediatrician?
- How helpful are other information sources, such as recent texts, journals, and medical Web sites?
- What is my relationship with the family, and how would treating Bobby affect it?
Table 2
Should you provide treatment? 4 questions to ask yourself
| How sure am I that I know what I don’t know? |
| How will I know when I should ask for help? |
| Do I have colleagues readily available for consultation if I need help? |
| Do I have a good track record for seeking consultation when I need it? |
Advantages and benefits
So far, we’ve emphasized cautions, but Dr. F also should remember that she may offer patients services that general psychiatrists provide but that pediatricians might not do routinely. Among the possibilities:
- Performing a diagnostic assessment that incorporates biopsychosocial factors.
- Taking time to foster a strong doctor-patient relationship with the family.
- Reserving time for medication-related psychoeducation.
- Scheduling longer visits to discuss a child’s psychiatric problems and explore solutions.
- Utilizing knowledge of and existing relationships with nonphysician therapists who could provide additional psychotherapy.
1. Thomas CR, Holzer CE, III. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006;45:1023-31.
2. U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville, MD: National Institute of Mental Health; 1999. Available at: http://www.surgeongeneral.gov/library/mentalhealth/home.html. Accessed August 20, 2008.
3. Levin A. Rural counties suffer from child psychiatry shortage. Psychiatr News 2006;42(14):4-41.
4. National Institute of Mental Health. Treatment of children with mental disorders. 2004. Available at: http://www.nimh.nih.gov/publicat/childqa.cfm. Accessed August 2, 2008.
5. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med 2002;53:113-31.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S.
7. Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 2008;122:451-3.
8. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008;117:2407-23.
Dear Dr. Mossman:
I am an adult psychiatrist practicing in a geographically isolated area. I am working with the family of 10-year-old “Bobby” who is struggling with attention problems. Top notch neuropsychologic testing recommends a stimulant trial, but the local pediatrician is too busy to give Bobby adequate follow-up and attention.
I am an experienced psychopharmacologist but have not prescribed medication to children since residency. My relationship with the family is excellent, and the local pediatrician said that she would supervise me. If I choose to treat Bobby, what are the possible liability issues I should be aware of, and how can I address them?—Submitted by “Dr. F”
Dr. F’s question raises issues that come up whenever patients need treatment for conditions outside the few with which you are highly familiar. Although you can’t be an expert on every aspect of every patient’s treatment, psychiatrists shouldn’t practice outside their area of competence.
Thus, the main liability-related issue that Dr. F should ask herself is, “Can I treat Bobby competently?” Of course, whenever you decide to treat any patient, you should be able to answer “yes” to this question. When thinking about potential liability related to treating Bobby, Dr. F might also ask, “If a lawsuit occurred, how would my treatment of Bobby appear?” This article discusses key issues that arise when general psychiatrists treat children and the steps general psychiatrists can take to show that they are practicing prudently.
Problem: Not enough clinicians
Child and adolescent psychiatrists (CAPs) are in short supply.1,2 In 2001 the United States had 8.67 CAPs per 100,000 youths and 1.6 CAPs for every 1,000 youths with severe mental disorders.1 Studies suggest that the United States needs nearly twice that many CAPs.3 The shortage is especially severe in rural areas, but approximately one-half of metropolitan counties with populations of >250,000 have no CAPs.1 In much of the nation, finding CAPs who are accepting new patients is difficult, and child and adolescent psychiatric treatment often is delivered by pediatricians, family practitioners, psychiatric nurse practitioners, and general adult psychiatrists.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Children’s special medical issues
General psychiatrists know that children aren’t just little adults. CAPs develop skills and thinking styles during their 2 years of subspecialty fellowship training that are quite different from those used by their general psychiatric colleagues.
Communication. Children and adolescents who need psychiatric care often have limited verbal abilities. Working and communicating with these patients requires a different interactive style.
Information sources. CAPs learn to seek and assimilate clinically important information from many settings—especially a child’s home—where their patients interact with others.
Caution. Only a small subset of psychotropic medications that adult psychiatrists prescribe are FDA-approved for use in children (Table 1).4 Because we don’t know how psychotropic drugs affect brain development, CAPs sometimes are leery of giving kids the same medications that adult psychiatrists readily prescribe.
Table 1
FDA-approved drugs and dosages for ADHD in children and adults
| Brand name | Generic name | Drug class | Dosing forms (mg) | Dosage range | Age range |
|---|---|---|---|---|---|
| Adderall | Amphetamine-dextroamphetamine | IR stimulant | 5, 7.5, 10, 12.5, 15, 20, 30 | 5 to 40 mg | 3 to 18 years |
| Adderall XR | Amphetamine-dextroamphetamine | ER stimulant | 5, 10, 15, 20, 25, 30 | 5 to 30 mg | 3 years to adult |
| Concerta | Methylphenidate | ER stimulant | 18, 27, 36, 54 | 18 to 72 mg | 6 years to adult |
| Daytrana | Methylphenidate transdermal | Stimulant | 10, 15, 20, 30 (patch) | 10 to 30 mg | 6 to 18 years |
| Focalin | Dexmethylphenidate | IR stimulant | 2.5, 5, 10 | 2.5 to 10 mg bid | 6 to 17 years |
| Focalin XR | Dexmethylphenidate | ER stimulant | 5, 10, 15, 20 | 5 to 20 mg | 6 years to adult |
| Metadate CD | Methylphenidate | ER stimulant | 10, 20, 30, 50, 60 | 10 to 60 mg | 6 to 18 years |
| Ritalin | Methylphenidate | IR stimulant | 5, 10, 20 | 5 to 20 mg bid or tid | 6 to 18 years |
| Ritalin LA | Methylphenidate | ER stimulant | 10, 20, 30, 40 | 10 to 60 mg | 6 to 18 years |
| Strattera | Atomoxetine | SNRI | 10, 18, 25, 40, 60, 80, 100 | 10 to 100 mg | 6 years to adult |
| Vyvanse | Lisdexamfetamine dimesylate | ER stimulant (precursor) | 30, 50, 70 | 30 to 70 mg | 6 years to adult |
| LA: long acting; CD: controlled delivery; ER, XR: extended release; IR: immediate release; SNRI: selective norepinephrine reuptake inhibitor | |||||
| Source: Adapted from references 2,3 | |||||
Different drugs. Some medications commonly taken by children are not often prescribed for adults, although this is changing as attention-deficit/hyperactivity disorder (ADHD) is better recognized in adults.5,6
Dosages. Dosing psychotropics in adults is fairly standardized, but in children and adolescents dosages vary with age, body weight, and physical maturity.
Adverse effects. The side effects kids experience and the way they report them can differ markedly from adults and will vary with age and developmental maturity. Some issues related to monitoring children—such as appropriate cardiac screening before starting stimulants—are controversial and remain unsettled.7,8
Consider alternatives
Dr. F may be tempted to treat Bobby because of her preexisting, positive relationship with the child’s family and a laudable desire to help. But Dr. F needs to ask, “Is there really no other workable alternative for Bobby?” Some possibilities include:
- Refer Bobby to a CAP in another community for initiation of treatment. Dr. F or Bobby’s pediatrician might safely continue care once a CAP establishes an effective treatment regimen.
- Find another pediatrician who might have more time to provide the follow-up that Dr. F feels is necessary.
- Decline to treat Bobby. Before doing this, Dr. F should consider what effect this refusal might have on her relationship with the family and the consequences for Bobby if his problems go untreated.
- Consult a CAP from another community, describing the situation and clinical factors in detail without naming or identifying the patient, and then ask, “Is this really the best thing to do?”
From a liability standpoint, this last point may be crucial. If the CAP answers “yes,” Dr. F can document the alternatives she has considered and her consultation and discussion with the CAP colleague as evidence of prudent practice. Dr. F can also document any advice that she has received and her plans to follow it.
If you choose to treat
Presumably, Dr. F would not perform thoracic surgery or provide any treatment that is far outside a general psychiatrist’s competence except under the most dire circumstances. General psychiatrists receive child psychiatry training during residency, and treating children is within their scope of practice. Similarly, most elderly patients are treated by general psychiatrists, rather than graduates of geropsychiatry fellowships. Prescribing medication for Bobby is not grossly different from Dr. F’s other duties, and she might provide services that a pediatrician might not.
Ask yourself 4 questions to determine if you are competent to provide medical treatment outside your usual area of expertise (Table 2). In Bobby’s case, Dr. F can consider these additional questions:
- Am I comfortable doing this? Would I be comfortable with this scenario if Bobby were my child?
- How extensive was my general residency training in child psychiatry?
- How long ago was my last CAP experience?
- Have I treated ADHD in adults, and am I familiar with stimulant medications?
- What kind of supervision could I arrange, such as regular phone consultation with a CAP or pediatrician?
- How helpful are other information sources, such as recent texts, journals, and medical Web sites?
- What is my relationship with the family, and how would treating Bobby affect it?
Table 2
Should you provide treatment? 4 questions to ask yourself
| How sure am I that I know what I don’t know? |
| How will I know when I should ask for help? |
| Do I have colleagues readily available for consultation if I need help? |
| Do I have a good track record for seeking consultation when I need it? |
Advantages and benefits
So far, we’ve emphasized cautions, but Dr. F also should remember that she may offer patients services that general psychiatrists provide but that pediatricians might not do routinely. Among the possibilities:
- Performing a diagnostic assessment that incorporates biopsychosocial factors.
- Taking time to foster a strong doctor-patient relationship with the family.
- Reserving time for medication-related psychoeducation.
- Scheduling longer visits to discuss a child’s psychiatric problems and explore solutions.
- Utilizing knowledge of and existing relationships with nonphysician therapists who could provide additional psychotherapy.
Dear Dr. Mossman:
I am an adult psychiatrist practicing in a geographically isolated area. I am working with the family of 10-year-old “Bobby” who is struggling with attention problems. Top notch neuropsychologic testing recommends a stimulant trial, but the local pediatrician is too busy to give Bobby adequate follow-up and attention.
I am an experienced psychopharmacologist but have not prescribed medication to children since residency. My relationship with the family is excellent, and the local pediatrician said that she would supervise me. If I choose to treat Bobby, what are the possible liability issues I should be aware of, and how can I address them?—Submitted by “Dr. F”
Dr. F’s question raises issues that come up whenever patients need treatment for conditions outside the few with which you are highly familiar. Although you can’t be an expert on every aspect of every patient’s treatment, psychiatrists shouldn’t practice outside their area of competence.
Thus, the main liability-related issue that Dr. F should ask herself is, “Can I treat Bobby competently?” Of course, whenever you decide to treat any patient, you should be able to answer “yes” to this question. When thinking about potential liability related to treating Bobby, Dr. F might also ask, “If a lawsuit occurred, how would my treatment of Bobby appear?” This article discusses key issues that arise when general psychiatrists treat children and the steps general psychiatrists can take to show that they are practicing prudently.
Problem: Not enough clinicians
Child and adolescent psychiatrists (CAPs) are in short supply.1,2 In 2001 the United States had 8.67 CAPs per 100,000 youths and 1.6 CAPs for every 1,000 youths with severe mental disorders.1 Studies suggest that the United States needs nearly twice that many CAPs.3 The shortage is especially severe in rural areas, but approximately one-half of metropolitan counties with populations of >250,000 have no CAPs.1 In much of the nation, finding CAPs who are accepting new patients is difficult, and child and adolescent psychiatric treatment often is delivered by pediatricians, family practitioners, psychiatric nurse practitioners, and general adult psychiatrists.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Children’s special medical issues
General psychiatrists know that children aren’t just little adults. CAPs develop skills and thinking styles during their 2 years of subspecialty fellowship training that are quite different from those used by their general psychiatric colleagues.
Communication. Children and adolescents who need psychiatric care often have limited verbal abilities. Working and communicating with these patients requires a different interactive style.
Information sources. CAPs learn to seek and assimilate clinically important information from many settings—especially a child’s home—where their patients interact with others.
Caution. Only a small subset of psychotropic medications that adult psychiatrists prescribe are FDA-approved for use in children (Table 1).4 Because we don’t know how psychotropic drugs affect brain development, CAPs sometimes are leery of giving kids the same medications that adult psychiatrists readily prescribe.
Table 1
FDA-approved drugs and dosages for ADHD in children and adults
| Brand name | Generic name | Drug class | Dosing forms (mg) | Dosage range | Age range |
|---|---|---|---|---|---|
| Adderall | Amphetamine-dextroamphetamine | IR stimulant | 5, 7.5, 10, 12.5, 15, 20, 30 | 5 to 40 mg | 3 to 18 years |
| Adderall XR | Amphetamine-dextroamphetamine | ER stimulant | 5, 10, 15, 20, 25, 30 | 5 to 30 mg | 3 years to adult |
| Concerta | Methylphenidate | ER stimulant | 18, 27, 36, 54 | 18 to 72 mg | 6 years to adult |
| Daytrana | Methylphenidate transdermal | Stimulant | 10, 15, 20, 30 (patch) | 10 to 30 mg | 6 to 18 years |
| Focalin | Dexmethylphenidate | IR stimulant | 2.5, 5, 10 | 2.5 to 10 mg bid | 6 to 17 years |
| Focalin XR | Dexmethylphenidate | ER stimulant | 5, 10, 15, 20 | 5 to 20 mg | 6 years to adult |
| Metadate CD | Methylphenidate | ER stimulant | 10, 20, 30, 50, 60 | 10 to 60 mg | 6 to 18 years |
| Ritalin | Methylphenidate | IR stimulant | 5, 10, 20 | 5 to 20 mg bid or tid | 6 to 18 years |
| Ritalin LA | Methylphenidate | ER stimulant | 10, 20, 30, 40 | 10 to 60 mg | 6 to 18 years |
| Strattera | Atomoxetine | SNRI | 10, 18, 25, 40, 60, 80, 100 | 10 to 100 mg | 6 years to adult |
| Vyvanse | Lisdexamfetamine dimesylate | ER stimulant (precursor) | 30, 50, 70 | 30 to 70 mg | 6 years to adult |
| LA: long acting; CD: controlled delivery; ER, XR: extended release; IR: immediate release; SNRI: selective norepinephrine reuptake inhibitor | |||||
| Source: Adapted from references 2,3 | |||||
Different drugs. Some medications commonly taken by children are not often prescribed for adults, although this is changing as attention-deficit/hyperactivity disorder (ADHD) is better recognized in adults.5,6
Dosages. Dosing psychotropics in adults is fairly standardized, but in children and adolescents dosages vary with age, body weight, and physical maturity.
Adverse effects. The side effects kids experience and the way they report them can differ markedly from adults and will vary with age and developmental maturity. Some issues related to monitoring children—such as appropriate cardiac screening before starting stimulants—are controversial and remain unsettled.7,8
Consider alternatives
Dr. F may be tempted to treat Bobby because of her preexisting, positive relationship with the child’s family and a laudable desire to help. But Dr. F needs to ask, “Is there really no other workable alternative for Bobby?” Some possibilities include:
- Refer Bobby to a CAP in another community for initiation of treatment. Dr. F or Bobby’s pediatrician might safely continue care once a CAP establishes an effective treatment regimen.
- Find another pediatrician who might have more time to provide the follow-up that Dr. F feels is necessary.
- Decline to treat Bobby. Before doing this, Dr. F should consider what effect this refusal might have on her relationship with the family and the consequences for Bobby if his problems go untreated.
- Consult a CAP from another community, describing the situation and clinical factors in detail without naming or identifying the patient, and then ask, “Is this really the best thing to do?”
From a liability standpoint, this last point may be crucial. If the CAP answers “yes,” Dr. F can document the alternatives she has considered and her consultation and discussion with the CAP colleague as evidence of prudent practice. Dr. F can also document any advice that she has received and her plans to follow it.
If you choose to treat
Presumably, Dr. F would not perform thoracic surgery or provide any treatment that is far outside a general psychiatrist’s competence except under the most dire circumstances. General psychiatrists receive child psychiatry training during residency, and treating children is within their scope of practice. Similarly, most elderly patients are treated by general psychiatrists, rather than graduates of geropsychiatry fellowships. Prescribing medication for Bobby is not grossly different from Dr. F’s other duties, and she might provide services that a pediatrician might not.
Ask yourself 4 questions to determine if you are competent to provide medical treatment outside your usual area of expertise (Table 2). In Bobby’s case, Dr. F can consider these additional questions:
- Am I comfortable doing this? Would I be comfortable with this scenario if Bobby were my child?
- How extensive was my general residency training in child psychiatry?
- How long ago was my last CAP experience?
- Have I treated ADHD in adults, and am I familiar with stimulant medications?
- What kind of supervision could I arrange, such as regular phone consultation with a CAP or pediatrician?
- How helpful are other information sources, such as recent texts, journals, and medical Web sites?
- What is my relationship with the family, and how would treating Bobby affect it?
Table 2
Should you provide treatment? 4 questions to ask yourself
| How sure am I that I know what I don’t know? |
| How will I know when I should ask for help? |
| Do I have colleagues readily available for consultation if I need help? |
| Do I have a good track record for seeking consultation when I need it? |
Advantages and benefits
So far, we’ve emphasized cautions, but Dr. F also should remember that she may offer patients services that general psychiatrists provide but that pediatricians might not do routinely. Among the possibilities:
- Performing a diagnostic assessment that incorporates biopsychosocial factors.
- Taking time to foster a strong doctor-patient relationship with the family.
- Reserving time for medication-related psychoeducation.
- Scheduling longer visits to discuss a child’s psychiatric problems and explore solutions.
- Utilizing knowledge of and existing relationships with nonphysician therapists who could provide additional psychotherapy.
1. Thomas CR, Holzer CE, III. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006;45:1023-31.
2. U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville, MD: National Institute of Mental Health; 1999. Available at: http://www.surgeongeneral.gov/library/mentalhealth/home.html. Accessed August 20, 2008.
3. Levin A. Rural counties suffer from child psychiatry shortage. Psychiatr News 2006;42(14):4-41.
4. National Institute of Mental Health. Treatment of children with mental disorders. 2004. Available at: http://www.nimh.nih.gov/publicat/childqa.cfm. Accessed August 2, 2008.
5. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med 2002;53:113-31.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S.
7. Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 2008;122:451-3.
8. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008;117:2407-23.
1. Thomas CR, Holzer CE, III. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006;45:1023-31.
2. U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville, MD: National Institute of Mental Health; 1999. Available at: http://www.surgeongeneral.gov/library/mentalhealth/home.html. Accessed August 20, 2008.
3. Levin A. Rural counties suffer from child psychiatry shortage. Psychiatr News 2006;42(14):4-41.
4. National Institute of Mental Health. Treatment of children with mental disorders. 2004. Available at: http://www.nimh.nih.gov/publicat/childqa.cfm. Accessed August 2, 2008.
5. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med 2002;53:113-31.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S.
7. Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 2008;122:451-3.
8. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008;117:2407-23.
Raajev Alexander, MD
Ed note: This article is the first in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Raajev Alexander, MD, is one busy hospitalist. For the past three years, he has been the lead hospitalist for the Oregon Medical Group, a group that caters to McKenzie-Willamette Medical Center in Springfield, Ore., and Sacred Heart Medical Center in Eugene, Ore. In addition to seeing about 15 patients a day, Dr. Alexander’s expertise in systems development has made him an attractive local expert. He serves on about five hospital committees (“I’ve lost track.”) and often attends meetings on his days off.
Dr. Alexander graduated from the University of Utah School of Medicine in 1995. After completing an internship and residency at Legacy Portland Hospital’s Internal Medicine program in 1998, he was recruited into the Oregon Medical Group.
He recently spoke with The Hospitalist about what he likes about his job, but why he also feels hospitalists should be compensated for the extra duties they undertake.
What attracted you to hospital medicine?
There is this kind of patient acuity where the sort of problems you’re solving seem important. Patients can have serious illnesses so you’re using your skills as an internist. I also like that there is a discreet arch to the hospitalization: There is the beginning of the hospitalization, the middle, the end, and then you’re sort of done. And I like that there is an interdisciplinary aspect; you work with nurses, care management, speech therapists, physical therapists, and ancillary therapists.
What are the challenges of leading a hospitalist group?
I do more than the full number of shifts per year. In addition to that, I go to meetings and deal with everything from a nurse calls and complaints about a hospitalist, to administration of the group. The CEO [of Oregon Medical Group] and I talk about staffing plans and how we can better serve the two hospitals in our area. I also sit on several hospital committees where I contribute my opinions on how to deploy pharmacists to how to redesign the case management program. My group finally decided to compensate me for certain meetings, but I still don’t get paid for half the meetings I go to.
Is this an issue other groups have?
I’m almost positive this is an ongoing issue for all hospitalist groups—at least I think it ought to be.
Hospitalists provide quality improvement on two different levels. One level is that, because we are in the hospital every day, we get to know the nurses, case managers, unit managers, lead respiratory therapists, and physical therapists. So, we effect change just by standing in the hallway.
The cross-education of pharmacists, nurses, and doctors is getting better every day. This is different from the way it used to be when a doctor had to run to the hospital at noon to see two patients, then run back to the office. Another way we improve quality is through committees. For all of the committees I sit on, the hospital gets get all my knowledge and ideas about systems, medications, and cross-reactions of drugs for free. But there isn’t enough time in my day to see patients, do nurse education and respiratory therapy education, to create protocols, and to sit on committees.
What’s the solution?
There are certain business models in hospital medicine that don’t make it possible to last as a hospitalist for 25 to 30 years. For example, there are some models where you get a bonus if you hit 18 to 20 patient encounters a day—even though those numbers are outside the SHM guidelines. If you’re seeing that many patients, you’re not providing optimal patient care.
A good business model is one where you can have 12 encounters per day and make a good living. Or see eight encounters per day and do administrative work, and still make a good living. The way to get there is for the specialty to better identify its mission and who its constituents are. TH
Ed note: This article is the first in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Raajev Alexander, MD, is one busy hospitalist. For the past three years, he has been the lead hospitalist for the Oregon Medical Group, a group that caters to McKenzie-Willamette Medical Center in Springfield, Ore., and Sacred Heart Medical Center in Eugene, Ore. In addition to seeing about 15 patients a day, Dr. Alexander’s expertise in systems development has made him an attractive local expert. He serves on about five hospital committees (“I’ve lost track.”) and often attends meetings on his days off.
Dr. Alexander graduated from the University of Utah School of Medicine in 1995. After completing an internship and residency at Legacy Portland Hospital’s Internal Medicine program in 1998, he was recruited into the Oregon Medical Group.
He recently spoke with The Hospitalist about what he likes about his job, but why he also feels hospitalists should be compensated for the extra duties they undertake.
What attracted you to hospital medicine?
There is this kind of patient acuity where the sort of problems you’re solving seem important. Patients can have serious illnesses so you’re using your skills as an internist. I also like that there is a discreet arch to the hospitalization: There is the beginning of the hospitalization, the middle, the end, and then you’re sort of done. And I like that there is an interdisciplinary aspect; you work with nurses, care management, speech therapists, physical therapists, and ancillary therapists.
What are the challenges of leading a hospitalist group?
I do more than the full number of shifts per year. In addition to that, I go to meetings and deal with everything from a nurse calls and complaints about a hospitalist, to administration of the group. The CEO [of Oregon Medical Group] and I talk about staffing plans and how we can better serve the two hospitals in our area. I also sit on several hospital committees where I contribute my opinions on how to deploy pharmacists to how to redesign the case management program. My group finally decided to compensate me for certain meetings, but I still don’t get paid for half the meetings I go to.
Is this an issue other groups have?
I’m almost positive this is an ongoing issue for all hospitalist groups—at least I think it ought to be.
Hospitalists provide quality improvement on two different levels. One level is that, because we are in the hospital every day, we get to know the nurses, case managers, unit managers, lead respiratory therapists, and physical therapists. So, we effect change just by standing in the hallway.
The cross-education of pharmacists, nurses, and doctors is getting better every day. This is different from the way it used to be when a doctor had to run to the hospital at noon to see two patients, then run back to the office. Another way we improve quality is through committees. For all of the committees I sit on, the hospital gets get all my knowledge and ideas about systems, medications, and cross-reactions of drugs for free. But there isn’t enough time in my day to see patients, do nurse education and respiratory therapy education, to create protocols, and to sit on committees.
What’s the solution?
There are certain business models in hospital medicine that don’t make it possible to last as a hospitalist for 25 to 30 years. For example, there are some models where you get a bonus if you hit 18 to 20 patient encounters a day—even though those numbers are outside the SHM guidelines. If you’re seeing that many patients, you’re not providing optimal patient care.
A good business model is one where you can have 12 encounters per day and make a good living. Or see eight encounters per day and do administrative work, and still make a good living. The way to get there is for the specialty to better identify its mission and who its constituents are. TH
Ed note: This article is the first in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Raajev Alexander, MD, is one busy hospitalist. For the past three years, he has been the lead hospitalist for the Oregon Medical Group, a group that caters to McKenzie-Willamette Medical Center in Springfield, Ore., and Sacred Heart Medical Center in Eugene, Ore. In addition to seeing about 15 patients a day, Dr. Alexander’s expertise in systems development has made him an attractive local expert. He serves on about five hospital committees (“I’ve lost track.”) and often attends meetings on his days off.
Dr. Alexander graduated from the University of Utah School of Medicine in 1995. After completing an internship and residency at Legacy Portland Hospital’s Internal Medicine program in 1998, he was recruited into the Oregon Medical Group.
He recently spoke with The Hospitalist about what he likes about his job, but why he also feels hospitalists should be compensated for the extra duties they undertake.
What attracted you to hospital medicine?
There is this kind of patient acuity where the sort of problems you’re solving seem important. Patients can have serious illnesses so you’re using your skills as an internist. I also like that there is a discreet arch to the hospitalization: There is the beginning of the hospitalization, the middle, the end, and then you’re sort of done. And I like that there is an interdisciplinary aspect; you work with nurses, care management, speech therapists, physical therapists, and ancillary therapists.
What are the challenges of leading a hospitalist group?
I do more than the full number of shifts per year. In addition to that, I go to meetings and deal with everything from a nurse calls and complaints about a hospitalist, to administration of the group. The CEO [of Oregon Medical Group] and I talk about staffing plans and how we can better serve the two hospitals in our area. I also sit on several hospital committees where I contribute my opinions on how to deploy pharmacists to how to redesign the case management program. My group finally decided to compensate me for certain meetings, but I still don’t get paid for half the meetings I go to.
Is this an issue other groups have?
I’m almost positive this is an ongoing issue for all hospitalist groups—at least I think it ought to be.
Hospitalists provide quality improvement on two different levels. One level is that, because we are in the hospital every day, we get to know the nurses, case managers, unit managers, lead respiratory therapists, and physical therapists. So, we effect change just by standing in the hallway.
The cross-education of pharmacists, nurses, and doctors is getting better every day. This is different from the way it used to be when a doctor had to run to the hospital at noon to see two patients, then run back to the office. Another way we improve quality is through committees. For all of the committees I sit on, the hospital gets get all my knowledge and ideas about systems, medications, and cross-reactions of drugs for free. But there isn’t enough time in my day to see patients, do nurse education and respiratory therapy education, to create protocols, and to sit on committees.
What’s the solution?
There are certain business models in hospital medicine that don’t make it possible to last as a hospitalist for 25 to 30 years. For example, there are some models where you get a bonus if you hit 18 to 20 patient encounters a day—even though those numbers are outside the SHM guidelines. If you’re seeing that many patients, you’re not providing optimal patient care.
A good business model is one where you can have 12 encounters per day and make a good living. Or see eight encounters per day and do administrative work, and still make a good living. The way to get there is for the specialty to better identify its mission and who its constituents are. TH
How can a patient with a hip fracture reduce the risk of repeat fractures?
Case
A 66-year-old female with a pack-a-day smoking habit is admitted to orthopedics with a hip fracture following a fall in her home. You are consulted to perform a pre-operative risk assessment and manage her heart failure. The following day, she undergoes an open reduction and internal fixation and does well following the surgery. She is scheduled to be discharged for rehabilitation in two days. She will continue taking her cardiac medications and the narcotics (as needed) for pain. What else can you recommend to reduce her chances of suffering another hip fracture?
Overview
Approximately 300,000 hip fractures occur each year in the United States.¹ The lifetime risk of sustaining a hip fracture is 18% for a woman and 6% for a man.2 One-year mortality after a hip fracture is 20% to 25%, and up to half of patients who live independently prior to their fracture cannot gain independence afterward.
In the late 1990s, inpatient care, nursing home care, and outpatient services associated with hip fractures totaled approximately $14 billion annually. These costs are predicted to reach $50 billion by the year 2040.3 Not surprisingly, second hip fractures are common, with up to 12% of patients suffering another fracture within one year of follow up.1 Risk of morbidity and mortality are even higher after a second hip fracture.
In most experts’ opinions, a fragility fracture indicates osteoporosis and warrants treatment—regardless of bone densitometry findings. Still, multiple studies have shown patients who sustain a hip fracture frequently are not diagnosed, evaluated, or treated for osteoporosis.4 This is analogous to treating an acute coronary syndrome without initiating treatment for a patient’s hypertension and hyperlipidemia prior to discharge. As such, providers clearly are missing an opportunity to begin effective measures at a critical stage in the disease.
Data Review
Physiology of bone strength: Bone minerals—in particular calcium hydroxyapatite—contribute to bone strength by making bone a hard tissue. Collagen adds flexibility and gives bone the ability to absorb energy. The degree of bone mineralization and the number of collagen crosslinks help determine how much stress a bone can tolerate before it breaks. Further, in response to daily stressors, bone accumulates microcracks. Remodeling is then accomplished by bone resorption and formation.5
Estrogen plays an important role in normal remodeling by controlling osteoclast action. Thus, estrogen deficiency leads to prolonged osteoclast activity and increased rates of bone resorption. This explains why bone remodeling typically favors bone resorption later in life and why women are at greatest risk for fracture.5
Vitamin D and calcium: Vitamin D, produced by the skin or ingested, is transported in the circulation by a binding protein to the liver, where it is converted to 25-hydroxyvitamin D. This form is inactive and must be converted by the kidneys to the active form, 1,25-dihydroxyvitamin D. The active form is needed for absorption of renal and intestinal calcium.6
Without vitamin D only 10% to 15% of dietary calcium is absorbed. In one study, serum levels of 25-hydroxyvitamin D directly were related to bone mineral density. When the level was 30 ng/mL or less, there was a significant decrease in intestinal calcium absorption and bone mineral density.6
Diagnostic evaluation: The “gold standard” for diagnosis of osteoporosis is bone mineral density (BMD) testing. The National Osteoporosis Foundation (NOF), the American Association of Clinical Endocrinologists (AACE), and the North American Menopause Society (NAMS) all agree, however, that the history of fragility fracture is diagnostic for osteoporosis, and all recommend initiating pharmacologic therapy in patients with this type of fracture. BMD testing is then used to track a patient’s response to therapy rather than as a diagnostic test.7 An osteoporosis diagnosis should always trigger a history, physical, and evaluation to identify the underlying cause.
Laboratory testing: All patients with osteoporosis should receive laboratory testing. As a baseline obtain chemistry studies, glucose, liver enzymes, albumin, total protein, alkaline phosphatase, and a complete blood count. Also, obtain a 25-hydroxyvitamin D level to help direct the immediate treatment.
Management
Patients with previous fractures related to osteoporosis require aggressive nonpharmacologic and pharmacologic therapy. Physicians should encourage lifestyle changes that include regular weight-bearing exercise, fall prevention, and discontinuation of tobacco products. Minimizing alcohol ingestion and sedating medications also is recommended. Physical therapy should evaluate gait and balance prior to discharge. Hip protectors may be beneficial, although the data to support this practice is sparse. It also is helpful to arrange a home nurse/therapy visit to assess for hazards in the home that might contribute to falls.
In addition, patients should have adequate calcium and vitamin D intake. The Women’s Health Initiative study showed that calcium with vitamin D use lead to a statistically significant improvement in hip bone density and a 29% reduction in the risk of hip fracture.3 The NOF recommends adults 50 and older have a daily intake of 1,200 mg of calcium and 800 to 1,000 IU of vitamin D. While no definitive data exist to guide the doses of vitamin D and calcium for osteoporosis treatment, it’s reasonable to tailor treatment to the patient’s 25-hydroxyvitamin level.
Specifically, initiate bisphosphonates along with calcium and vitamin D in patients with mild vitamin D deficiency (levels 10 to 30 ng/mL). Patients with severe vitamin D deficiency (<10 ng/mL) should have two to three months of aggressive vitamin D replacement prior to beginning a bisphosphonate. Vitamin D deficiency often is associated with impaired bone mineralization, which potentially could worsen with a bisphosphonate.
Some of the FDA-approved pharmacologic therapies for osteoporosis include antiresorptive bisphosphonates, such as alendronate, risedronate, ibandronate, zoledronic acid, and raloxifene, as well as the human parathyroid hormone teriparatide. Morin et al., performed a population-based, retrospective cohort study using administrative databases to identify patients hospitalized for a hip fracture. They found patients exposed to antiresorptives had a 26% reduction in the rate of recurrent fractures.8
Bisphosphonates are the current first-line treatment of choice unless the clinical situation warrants otherwise. Do not prescribe oral bisphosphonates for patients with hypocalcemia, creatinine clearance lower than 30mL/min, esophageal stricture, or for those who cannot remain upright for 30 minutes.7
Recently, the use of the IV bisphosphonate zolendronic acid within three months of a hip fracture was evaluated. The study randomized approximately 2,100 patients to zolendronic acid 5 mg IV or placebo annually and followed them for a median of 1.9 years. Both groups received vitamin D and calcium supplementation. Those patients using zolendronic acid saw a statistically significant reduction in overall fracture (13.9% vs. 8.6%) and mortality (13.3% vs. 9.6%) rates. While these data support the timely use of bisphosphonate therapy, it is notable that only patients who refused or couldn’t tolerate oral bisphosphonate therapy received the drug, and it was generally not started in the hospital. Still, it’s reasonable to suspect that these beneficial effects would occur even if started in the hospital, as long as the vitamin D and calcium levels did not contraindicate commencement.9
Physicians Don’t Recognize Osteoporosis
In 2000, Kamel et al. retrospectively studied the charts of 170 patients age 65 and older who were hospitalized with a hip fracture, and found that fewer than 5% had been diagnosed with or treated for osteoporosis.7 Follin et al., noted similar results in 2003, reporting that only 14% of the patients were diagnosed with osteoporosis prior to discharge and 75% of patients received no therapy.10
Follin et al., also noted patients who received a diagnosis of osteoporosis prior to discharge were more likely to receive therapy. Sixty-five percent of patients diagnosed with osteoporosis received treatment as opposed to 20% of those not diagnosed. They surmised the lack of treatment may relate to the lack of recognition that a fragility fracture often means osteoporosis.10
Hospitalist Consult, Treatment of Osteoporosis in Hip Fracture Patients
A 2003 retrospective analysis from a university-based academic hospital aimed to determine whether hospitalist consultation during admission for a hip fracture resulted in improved treatment of osteoporosis. The results indicated 29% of patients received treatment for osteoporosis at the time of discharge. Twenty percent received calcium, and only 7% received a bisphosphonate. Those who received hospitalist consultation did not have a significant improvement in osteoporosis treatment, thus representing a huge missed opportunity.11
Back to the Case
You recognize that, because your patient has sustained a fragility fracture, she has osteoporosis and you wish to initiate treatment before she leaves the hospital. Her 25-hydroxyvitamin D level is 18 ng/mL. You commence 50,000 units of vitamin D once weekly and advise that she have her vitamin D level checked again in three months by her primary care provider. She has no contraindications, thus you also initiate a bisphosphonate and remind her to take 1,200 mg of calcium daily.
You encourage smoking cessation, decreased alcohol use, a simplified medication regimen, and weight-bearing exercises in the future. In addition, you ensure she has the proper gait stability items at discharge. You arrange a visiting nurse/therapist to assess her home for fall risks. Lastly, you schedule an outpatient bone mineral density scan and arrange a follow-up with her primary care provider. TH
Dr. Baker is a hospitalist at Ohio State University. Dr McDermott is professor of medicine and clinical pharmacy and endocrinology and diabetes practice director, University of Colorado Denver.
References
- Berry SD, Samelson EJ, Hannan MT, et al. Second hip fracture in older men and women. The Framingham Study. Arch Intern Med. 2007;167(18):1971-1976.
- Juby AG, De Gues-Wenceslau CM. Evaluation of osteoporosis treatment in seniors after hip fracture. Osteoporosis Int. 2002;13:205-210.
- Gardner MJ, Brophy RH, Demetrakopoulos D, et al. Interventions to improve osteoporosis treatment following hip fracture. The Journal of Bone and Joint Surgery. 2005;87-A(1):3-7.
- Gardner MJ, Flik KR, Mooar P, Lane JM.Improve-ment in the undertreatment of osteoporosis following hip fracture. The Journal of Bone and Joint Surgery. 2002;84-A(8):1342-1348.
- Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250-2261.
- Holick, MF. Vitamin D Deficiency. N Engl J Med. 2007;357(3):266-281.
- Glauser T. Practical strategies for managing osteoporosis: An evidence-based approach to risk assessment and treatment. Dialogues in Clinical Practice. 2007.
- Morin S, Rahme E, Behlouli H, Tenenhouse A, Goltzman D, Pilote L. Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporosis Int. 2007;18:1625-1632.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zolendronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
- Follin SL, Black JN, McDermott MT. Lack of diagnosis and treatment of osteoporosis in men and women after hip fracture. Pharmacotherapy.2003;23(2):190-198.
- Jachna CM, Whittle J, Lukert B, Graves L, Bhargava T. Effect of hospitalist consultation on treatment of osteoporosis in hip fracture patients. Osteoporosis Int. 2003;14:665-671.
Case
A 66-year-old female with a pack-a-day smoking habit is admitted to orthopedics with a hip fracture following a fall in her home. You are consulted to perform a pre-operative risk assessment and manage her heart failure. The following day, she undergoes an open reduction and internal fixation and does well following the surgery. She is scheduled to be discharged for rehabilitation in two days. She will continue taking her cardiac medications and the narcotics (as needed) for pain. What else can you recommend to reduce her chances of suffering another hip fracture?
Overview
Approximately 300,000 hip fractures occur each year in the United States.¹ The lifetime risk of sustaining a hip fracture is 18% for a woman and 6% for a man.2 One-year mortality after a hip fracture is 20% to 25%, and up to half of patients who live independently prior to their fracture cannot gain independence afterward.
In the late 1990s, inpatient care, nursing home care, and outpatient services associated with hip fractures totaled approximately $14 billion annually. These costs are predicted to reach $50 billion by the year 2040.3 Not surprisingly, second hip fractures are common, with up to 12% of patients suffering another fracture within one year of follow up.1 Risk of morbidity and mortality are even higher after a second hip fracture.
In most experts’ opinions, a fragility fracture indicates osteoporosis and warrants treatment—regardless of bone densitometry findings. Still, multiple studies have shown patients who sustain a hip fracture frequently are not diagnosed, evaluated, or treated for osteoporosis.4 This is analogous to treating an acute coronary syndrome without initiating treatment for a patient’s hypertension and hyperlipidemia prior to discharge. As such, providers clearly are missing an opportunity to begin effective measures at a critical stage in the disease.
Data Review
Physiology of bone strength: Bone minerals—in particular calcium hydroxyapatite—contribute to bone strength by making bone a hard tissue. Collagen adds flexibility and gives bone the ability to absorb energy. The degree of bone mineralization and the number of collagen crosslinks help determine how much stress a bone can tolerate before it breaks. Further, in response to daily stressors, bone accumulates microcracks. Remodeling is then accomplished by bone resorption and formation.5
Estrogen plays an important role in normal remodeling by controlling osteoclast action. Thus, estrogen deficiency leads to prolonged osteoclast activity and increased rates of bone resorption. This explains why bone remodeling typically favors bone resorption later in life and why women are at greatest risk for fracture.5
Vitamin D and calcium: Vitamin D, produced by the skin or ingested, is transported in the circulation by a binding protein to the liver, where it is converted to 25-hydroxyvitamin D. This form is inactive and must be converted by the kidneys to the active form, 1,25-dihydroxyvitamin D. The active form is needed for absorption of renal and intestinal calcium.6
Without vitamin D only 10% to 15% of dietary calcium is absorbed. In one study, serum levels of 25-hydroxyvitamin D directly were related to bone mineral density. When the level was 30 ng/mL or less, there was a significant decrease in intestinal calcium absorption and bone mineral density.6
Diagnostic evaluation: The “gold standard” for diagnosis of osteoporosis is bone mineral density (BMD) testing. The National Osteoporosis Foundation (NOF), the American Association of Clinical Endocrinologists (AACE), and the North American Menopause Society (NAMS) all agree, however, that the history of fragility fracture is diagnostic for osteoporosis, and all recommend initiating pharmacologic therapy in patients with this type of fracture. BMD testing is then used to track a patient’s response to therapy rather than as a diagnostic test.7 An osteoporosis diagnosis should always trigger a history, physical, and evaluation to identify the underlying cause.
Laboratory testing: All patients with osteoporosis should receive laboratory testing. As a baseline obtain chemistry studies, glucose, liver enzymes, albumin, total protein, alkaline phosphatase, and a complete blood count. Also, obtain a 25-hydroxyvitamin D level to help direct the immediate treatment.
Management
Patients with previous fractures related to osteoporosis require aggressive nonpharmacologic and pharmacologic therapy. Physicians should encourage lifestyle changes that include regular weight-bearing exercise, fall prevention, and discontinuation of tobacco products. Minimizing alcohol ingestion and sedating medications also is recommended. Physical therapy should evaluate gait and balance prior to discharge. Hip protectors may be beneficial, although the data to support this practice is sparse. It also is helpful to arrange a home nurse/therapy visit to assess for hazards in the home that might contribute to falls.
In addition, patients should have adequate calcium and vitamin D intake. The Women’s Health Initiative study showed that calcium with vitamin D use lead to a statistically significant improvement in hip bone density and a 29% reduction in the risk of hip fracture.3 The NOF recommends adults 50 and older have a daily intake of 1,200 mg of calcium and 800 to 1,000 IU of vitamin D. While no definitive data exist to guide the doses of vitamin D and calcium for osteoporosis treatment, it’s reasonable to tailor treatment to the patient’s 25-hydroxyvitamin level.
Specifically, initiate bisphosphonates along with calcium and vitamin D in patients with mild vitamin D deficiency (levels 10 to 30 ng/mL). Patients with severe vitamin D deficiency (<10 ng/mL) should have two to three months of aggressive vitamin D replacement prior to beginning a bisphosphonate. Vitamin D deficiency often is associated with impaired bone mineralization, which potentially could worsen with a bisphosphonate.
Some of the FDA-approved pharmacologic therapies for osteoporosis include antiresorptive bisphosphonates, such as alendronate, risedronate, ibandronate, zoledronic acid, and raloxifene, as well as the human parathyroid hormone teriparatide. Morin et al., performed a population-based, retrospective cohort study using administrative databases to identify patients hospitalized for a hip fracture. They found patients exposed to antiresorptives had a 26% reduction in the rate of recurrent fractures.8
Bisphosphonates are the current first-line treatment of choice unless the clinical situation warrants otherwise. Do not prescribe oral bisphosphonates for patients with hypocalcemia, creatinine clearance lower than 30mL/min, esophageal stricture, or for those who cannot remain upright for 30 minutes.7
Recently, the use of the IV bisphosphonate zolendronic acid within three months of a hip fracture was evaluated. The study randomized approximately 2,100 patients to zolendronic acid 5 mg IV or placebo annually and followed them for a median of 1.9 years. Both groups received vitamin D and calcium supplementation. Those patients using zolendronic acid saw a statistically significant reduction in overall fracture (13.9% vs. 8.6%) and mortality (13.3% vs. 9.6%) rates. While these data support the timely use of bisphosphonate therapy, it is notable that only patients who refused or couldn’t tolerate oral bisphosphonate therapy received the drug, and it was generally not started in the hospital. Still, it’s reasonable to suspect that these beneficial effects would occur even if started in the hospital, as long as the vitamin D and calcium levels did not contraindicate commencement.9
Physicians Don’t Recognize Osteoporosis
In 2000, Kamel et al. retrospectively studied the charts of 170 patients age 65 and older who were hospitalized with a hip fracture, and found that fewer than 5% had been diagnosed with or treated for osteoporosis.7 Follin et al., noted similar results in 2003, reporting that only 14% of the patients were diagnosed with osteoporosis prior to discharge and 75% of patients received no therapy.10
Follin et al., also noted patients who received a diagnosis of osteoporosis prior to discharge were more likely to receive therapy. Sixty-five percent of patients diagnosed with osteoporosis received treatment as opposed to 20% of those not diagnosed. They surmised the lack of treatment may relate to the lack of recognition that a fragility fracture often means osteoporosis.10
Hospitalist Consult, Treatment of Osteoporosis in Hip Fracture Patients
A 2003 retrospective analysis from a university-based academic hospital aimed to determine whether hospitalist consultation during admission for a hip fracture resulted in improved treatment of osteoporosis. The results indicated 29% of patients received treatment for osteoporosis at the time of discharge. Twenty percent received calcium, and only 7% received a bisphosphonate. Those who received hospitalist consultation did not have a significant improvement in osteoporosis treatment, thus representing a huge missed opportunity.11
Back to the Case
You recognize that, because your patient has sustained a fragility fracture, she has osteoporosis and you wish to initiate treatment before she leaves the hospital. Her 25-hydroxyvitamin D level is 18 ng/mL. You commence 50,000 units of vitamin D once weekly and advise that she have her vitamin D level checked again in three months by her primary care provider. She has no contraindications, thus you also initiate a bisphosphonate and remind her to take 1,200 mg of calcium daily.
You encourage smoking cessation, decreased alcohol use, a simplified medication regimen, and weight-bearing exercises in the future. In addition, you ensure she has the proper gait stability items at discharge. You arrange a visiting nurse/therapist to assess her home for fall risks. Lastly, you schedule an outpatient bone mineral density scan and arrange a follow-up with her primary care provider. TH
Dr. Baker is a hospitalist at Ohio State University. Dr McDermott is professor of medicine and clinical pharmacy and endocrinology and diabetes practice director, University of Colorado Denver.
References
- Berry SD, Samelson EJ, Hannan MT, et al. Second hip fracture in older men and women. The Framingham Study. Arch Intern Med. 2007;167(18):1971-1976.
- Juby AG, De Gues-Wenceslau CM. Evaluation of osteoporosis treatment in seniors after hip fracture. Osteoporosis Int. 2002;13:205-210.
- Gardner MJ, Brophy RH, Demetrakopoulos D, et al. Interventions to improve osteoporosis treatment following hip fracture. The Journal of Bone and Joint Surgery. 2005;87-A(1):3-7.
- Gardner MJ, Flik KR, Mooar P, Lane JM.Improve-ment in the undertreatment of osteoporosis following hip fracture. The Journal of Bone and Joint Surgery. 2002;84-A(8):1342-1348.
- Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250-2261.
- Holick, MF. Vitamin D Deficiency. N Engl J Med. 2007;357(3):266-281.
- Glauser T. Practical strategies for managing osteoporosis: An evidence-based approach to risk assessment and treatment. Dialogues in Clinical Practice. 2007.
- Morin S, Rahme E, Behlouli H, Tenenhouse A, Goltzman D, Pilote L. Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporosis Int. 2007;18:1625-1632.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zolendronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
- Follin SL, Black JN, McDermott MT. Lack of diagnosis and treatment of osteoporosis in men and women after hip fracture. Pharmacotherapy.2003;23(2):190-198.
- Jachna CM, Whittle J, Lukert B, Graves L, Bhargava T. Effect of hospitalist consultation on treatment of osteoporosis in hip fracture patients. Osteoporosis Int. 2003;14:665-671.
Case
A 66-year-old female with a pack-a-day smoking habit is admitted to orthopedics with a hip fracture following a fall in her home. You are consulted to perform a pre-operative risk assessment and manage her heart failure. The following day, she undergoes an open reduction and internal fixation and does well following the surgery. She is scheduled to be discharged for rehabilitation in two days. She will continue taking her cardiac medications and the narcotics (as needed) for pain. What else can you recommend to reduce her chances of suffering another hip fracture?
Overview
Approximately 300,000 hip fractures occur each year in the United States.¹ The lifetime risk of sustaining a hip fracture is 18% for a woman and 6% for a man.2 One-year mortality after a hip fracture is 20% to 25%, and up to half of patients who live independently prior to their fracture cannot gain independence afterward.
In the late 1990s, inpatient care, nursing home care, and outpatient services associated with hip fractures totaled approximately $14 billion annually. These costs are predicted to reach $50 billion by the year 2040.3 Not surprisingly, second hip fractures are common, with up to 12% of patients suffering another fracture within one year of follow up.1 Risk of morbidity and mortality are even higher after a second hip fracture.
In most experts’ opinions, a fragility fracture indicates osteoporosis and warrants treatment—regardless of bone densitometry findings. Still, multiple studies have shown patients who sustain a hip fracture frequently are not diagnosed, evaluated, or treated for osteoporosis.4 This is analogous to treating an acute coronary syndrome without initiating treatment for a patient’s hypertension and hyperlipidemia prior to discharge. As such, providers clearly are missing an opportunity to begin effective measures at a critical stage in the disease.
Data Review
Physiology of bone strength: Bone minerals—in particular calcium hydroxyapatite—contribute to bone strength by making bone a hard tissue. Collagen adds flexibility and gives bone the ability to absorb energy. The degree of bone mineralization and the number of collagen crosslinks help determine how much stress a bone can tolerate before it breaks. Further, in response to daily stressors, bone accumulates microcracks. Remodeling is then accomplished by bone resorption and formation.5
Estrogen plays an important role in normal remodeling by controlling osteoclast action. Thus, estrogen deficiency leads to prolonged osteoclast activity and increased rates of bone resorption. This explains why bone remodeling typically favors bone resorption later in life and why women are at greatest risk for fracture.5
Vitamin D and calcium: Vitamin D, produced by the skin or ingested, is transported in the circulation by a binding protein to the liver, where it is converted to 25-hydroxyvitamin D. This form is inactive and must be converted by the kidneys to the active form, 1,25-dihydroxyvitamin D. The active form is needed for absorption of renal and intestinal calcium.6
Without vitamin D only 10% to 15% of dietary calcium is absorbed. In one study, serum levels of 25-hydroxyvitamin D directly were related to bone mineral density. When the level was 30 ng/mL or less, there was a significant decrease in intestinal calcium absorption and bone mineral density.6
Diagnostic evaluation: The “gold standard” for diagnosis of osteoporosis is bone mineral density (BMD) testing. The National Osteoporosis Foundation (NOF), the American Association of Clinical Endocrinologists (AACE), and the North American Menopause Society (NAMS) all agree, however, that the history of fragility fracture is diagnostic for osteoporosis, and all recommend initiating pharmacologic therapy in patients with this type of fracture. BMD testing is then used to track a patient’s response to therapy rather than as a diagnostic test.7 An osteoporosis diagnosis should always trigger a history, physical, and evaluation to identify the underlying cause.
Laboratory testing: All patients with osteoporosis should receive laboratory testing. As a baseline obtain chemistry studies, glucose, liver enzymes, albumin, total protein, alkaline phosphatase, and a complete blood count. Also, obtain a 25-hydroxyvitamin D level to help direct the immediate treatment.
Management
Patients with previous fractures related to osteoporosis require aggressive nonpharmacologic and pharmacologic therapy. Physicians should encourage lifestyle changes that include regular weight-bearing exercise, fall prevention, and discontinuation of tobacco products. Minimizing alcohol ingestion and sedating medications also is recommended. Physical therapy should evaluate gait and balance prior to discharge. Hip protectors may be beneficial, although the data to support this practice is sparse. It also is helpful to arrange a home nurse/therapy visit to assess for hazards in the home that might contribute to falls.
In addition, patients should have adequate calcium and vitamin D intake. The Women’s Health Initiative study showed that calcium with vitamin D use lead to a statistically significant improvement in hip bone density and a 29% reduction in the risk of hip fracture.3 The NOF recommends adults 50 and older have a daily intake of 1,200 mg of calcium and 800 to 1,000 IU of vitamin D. While no definitive data exist to guide the doses of vitamin D and calcium for osteoporosis treatment, it’s reasonable to tailor treatment to the patient’s 25-hydroxyvitamin level.
Specifically, initiate bisphosphonates along with calcium and vitamin D in patients with mild vitamin D deficiency (levels 10 to 30 ng/mL). Patients with severe vitamin D deficiency (<10 ng/mL) should have two to three months of aggressive vitamin D replacement prior to beginning a bisphosphonate. Vitamin D deficiency often is associated with impaired bone mineralization, which potentially could worsen with a bisphosphonate.
Some of the FDA-approved pharmacologic therapies for osteoporosis include antiresorptive bisphosphonates, such as alendronate, risedronate, ibandronate, zoledronic acid, and raloxifene, as well as the human parathyroid hormone teriparatide. Morin et al., performed a population-based, retrospective cohort study using administrative databases to identify patients hospitalized for a hip fracture. They found patients exposed to antiresorptives had a 26% reduction in the rate of recurrent fractures.8
Bisphosphonates are the current first-line treatment of choice unless the clinical situation warrants otherwise. Do not prescribe oral bisphosphonates for patients with hypocalcemia, creatinine clearance lower than 30mL/min, esophageal stricture, or for those who cannot remain upright for 30 minutes.7
Recently, the use of the IV bisphosphonate zolendronic acid within three months of a hip fracture was evaluated. The study randomized approximately 2,100 patients to zolendronic acid 5 mg IV or placebo annually and followed them for a median of 1.9 years. Both groups received vitamin D and calcium supplementation. Those patients using zolendronic acid saw a statistically significant reduction in overall fracture (13.9% vs. 8.6%) and mortality (13.3% vs. 9.6%) rates. While these data support the timely use of bisphosphonate therapy, it is notable that only patients who refused or couldn’t tolerate oral bisphosphonate therapy received the drug, and it was generally not started in the hospital. Still, it’s reasonable to suspect that these beneficial effects would occur even if started in the hospital, as long as the vitamin D and calcium levels did not contraindicate commencement.9
Physicians Don’t Recognize Osteoporosis
In 2000, Kamel et al. retrospectively studied the charts of 170 patients age 65 and older who were hospitalized with a hip fracture, and found that fewer than 5% had been diagnosed with or treated for osteoporosis.7 Follin et al., noted similar results in 2003, reporting that only 14% of the patients were diagnosed with osteoporosis prior to discharge and 75% of patients received no therapy.10
Follin et al., also noted patients who received a diagnosis of osteoporosis prior to discharge were more likely to receive therapy. Sixty-five percent of patients diagnosed with osteoporosis received treatment as opposed to 20% of those not diagnosed. They surmised the lack of treatment may relate to the lack of recognition that a fragility fracture often means osteoporosis.10
Hospitalist Consult, Treatment of Osteoporosis in Hip Fracture Patients
A 2003 retrospective analysis from a university-based academic hospital aimed to determine whether hospitalist consultation during admission for a hip fracture resulted in improved treatment of osteoporosis. The results indicated 29% of patients received treatment for osteoporosis at the time of discharge. Twenty percent received calcium, and only 7% received a bisphosphonate. Those who received hospitalist consultation did not have a significant improvement in osteoporosis treatment, thus representing a huge missed opportunity.11
Back to the Case
You recognize that, because your patient has sustained a fragility fracture, she has osteoporosis and you wish to initiate treatment before she leaves the hospital. Her 25-hydroxyvitamin D level is 18 ng/mL. You commence 50,000 units of vitamin D once weekly and advise that she have her vitamin D level checked again in three months by her primary care provider. She has no contraindications, thus you also initiate a bisphosphonate and remind her to take 1,200 mg of calcium daily.
You encourage smoking cessation, decreased alcohol use, a simplified medication regimen, and weight-bearing exercises in the future. In addition, you ensure she has the proper gait stability items at discharge. You arrange a visiting nurse/therapist to assess her home for fall risks. Lastly, you schedule an outpatient bone mineral density scan and arrange a follow-up with her primary care provider. TH
Dr. Baker is a hospitalist at Ohio State University. Dr McDermott is professor of medicine and clinical pharmacy and endocrinology and diabetes practice director, University of Colorado Denver.
References
- Berry SD, Samelson EJ, Hannan MT, et al. Second hip fracture in older men and women. The Framingham Study. Arch Intern Med. 2007;167(18):1971-1976.
- Juby AG, De Gues-Wenceslau CM. Evaluation of osteoporosis treatment in seniors after hip fracture. Osteoporosis Int. 2002;13:205-210.
- Gardner MJ, Brophy RH, Demetrakopoulos D, et al. Interventions to improve osteoporosis treatment following hip fracture. The Journal of Bone and Joint Surgery. 2005;87-A(1):3-7.
- Gardner MJ, Flik KR, Mooar P, Lane JM.Improve-ment in the undertreatment of osteoporosis following hip fracture. The Journal of Bone and Joint Surgery. 2002;84-A(8):1342-1348.
- Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250-2261.
- Holick, MF. Vitamin D Deficiency. N Engl J Med. 2007;357(3):266-281.
- Glauser T. Practical strategies for managing osteoporosis: An evidence-based approach to risk assessment and treatment. Dialogues in Clinical Practice. 2007.
- Morin S, Rahme E, Behlouli H, Tenenhouse A, Goltzman D, Pilote L. Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporosis Int. 2007;18:1625-1632.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zolendronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
- Follin SL, Black JN, McDermott MT. Lack of diagnosis and treatment of osteoporosis in men and women after hip fracture. Pharmacotherapy.2003;23(2):190-198.
- Jachna CM, Whittle J, Lukert B, Graves L, Bhargava T. Effect of hospitalist consultation on treatment of osteoporosis in hip fracture patients. Osteoporosis Int. 2003;14:665-671.
The Observation Deck
Observation care provides a mechanism to evaluate and treat patients without the resource utilization and financial responsibility associated with an inpatient admission. Hospitalists may not understand the billing compliance risk and corresponding revenue implications when observation services (OBS) are not captured correctly.
Are OBS best reported with observation care codes (99218-99220, 99234-99236), office visit codes (99201-99215), or initial hospital care codes (99221-99223)? Code selection depends upon the patient’s registered status, the nature of the provided service, and the length of stay. Review the following information before reporting OBS to ensure an accurate claim submission.
Attending Physician Responsibilities
The physician-documented reason for observation substantiates the medical necessity for the OBS admission. Contractors often evaluate medical records to determine the consistency between the physician order (physician intent), the services actually provided (inpatient or outpatient), and the medical necessity of those services, including the medical appropriateness of the inpatient or observation stay.
Certain diagnoses and procedures generally do not support an inpatient admission and fall within the definitions of outpatient observation. Uncomplicated presentations of chest pain (rule out MI), mild asthma/COPD, mild CHF, syncope and decreased responsiveness, atrial arrhythmias, and renal colic all frequently are associated with the expectation of a brief (less than 24-hour) stay unless serious pathology is uncovered.2 Situations that do not meet the criteria for observation care are considered “not medically necessary” and separate payment is not permitted. Examples of circumstances that lack medical necessity include:
- Outpatient blood administration;
- Lack of/delay in patient transportation;
- Provision of a medical exam for patients who do not require skilled support;
- Routine preparation prior to and recovery after diagnostic testing;
- Routine recovery and post-operative care after ambulatory surgery;
- When used for the convenience of the physician, patient or patient’s family;
- While awaiting transfer to another facility;
- Duration of care exceeding 48 hours;
- When an overnight stay is planned prior to diagnostic testing;
- Standing orders following outpatient surgery;
- Services that would normally require inpatient stay;
- No physicians order to admit to observation;
- Observation following an uncomplicated treatment or procedure;
- Services that are not reasonable and necessary for care of the patient;
- Services provided concurrently with chemotherapy; and
- Inpatients discharged to outpatient observation status.3
The attending physician of record assumes responsibility for the patient’s admission to observation and is permitted to report observation care codes. In addition to the reason for admission, a medical record involving the observation stay must include dated and timed physician admitting orders outlining the care plan, physician progress notes, and discharge orders. This documentation must be added to any other record prepared as a result of an emergency department or outpatient clinic encounter. If physicians other than the admitting physician/group (i.e., physicians in different specialties) provide services to the patient during observation, they must use the appropriate outpatient visit (e.g., 99214) or consultation code (e.g., 99244).
Length of Stay4
In general, the duration of observation care services typically does not exceed 24 hours, although in some circumstances patients may require a second day. Observation care for greater than 48 hours without inpatient admission is not considered medically necessary but may be payable after medical review. When the stay spans two calendar days, physician billing is straightforward: Select an initial observation care code (99218-99220) for calendar day one and the observation discharge code (99217) for day two. Only the admitting physician/group may report the discharge service, when applicable. Documentation must demonstrate a face-to-face encounter by the physician for each date of service.
Should the stay only constitute one calendar day, the duration of care becomes a crucial factor in determining the code category. Standard OBS codes (99218-99220) are applicable if the patient stay is less than eight hours on any given date. The OBS discharge code (99217) is not reported in this instance, although the documentation should reflect the attending physician’s written order and appropriate discharge plan. Alternately, same day admit/discharge codes (99234-99236) apply to single-day stays lasting more than eight hours. The OBS discharge code (99217) also is not reported in this instance. Documentation must identify, at a minimum:
- Duration of the stay;
- Presence by the billing physician; and
- Physician performance of each service (i.e., both an admission and discharge note).
Inpatient Admission1,4-5
Sometimes the patient requires inpatient admission after initially being placed in observation. If the inpatient admission occurs on the same day as the OBS admission, only one service is reported (e.g., 99222). The physician need not redocument a complete history and physical (H&P) but merely write the new order for admission and update the OBS assessment with any relevant, new information.
Should the inpatient admission occur on the second calendar day of the OBS stay, the physician is able to report the initial observation care code (e.g., 99219) on day one, and the initial inpatient care code (e.g., 99223) on day two. However, the physician must meet the documentation guidelines for initial hospital care and redocument the H&P associated with the reported visit level. In the case of 99223, the physician must document a comprehensive history (only referring to the previous review of systems and histories, while rewriting the history of present illness) and high complexity decision making. If the physician chooses not to document to this extent, a subsequent hospital care code (99231-99233) is reasonable because the episode of care is a continuation from the observation phase.
Beware that some insurers may change the patient’s status for the entire episode of care. In other words, the conversion to inpatient status occurs on day two of the patient stay, but the insurer may convert the entire stay, including day one, to an inpatient status. Should this happen, the physician is responsible for reporting the visit category that corresponds with the patient’s status. Inpatient services codes are required for claim submission when the patient stay qualifies as an inpatient admission. Because these conversions occur with some frequency, it is advisable to hold claims intended for observation patients until the correct patient status can be confirmed by the utilization review team, and communicated to the physician. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
References
- 1. American Medical Association. cpt 2008, Current Procedural Terminology Professional Edition. Chicago, IL: American Medical Association, 2007; 9-16 CPT codes, descriptions and other data only are copyright 2007 American Medical Association (AMA). All Rights Reserved (or such other date of publication of CPT). CPT is a trademark of the AMA.
- 2. Highmark Medicare Services. Local Coverage Determination L27548 Acute Care: Inpatient, Observation and Treatment Room Services. Available at www.highmarkmedicareservices.com/policy/mac-ab/127548.html. Accessed July 14, 2008.
- 3. Cigna. Healthcare Coverage Position: Observation Care. Available at www.cigna.com/customer_care/healthcare_ professional/coverage_positions/medical/mm_0411_coveragepositioncriteria_observation_care.pdf. Accessed July 12, 2008.
- 4. Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual: Chapter 12, Section 30.6.8. Available at www.cms.hhs.gov/manuals/downloads/ clm104c12.pdf. Accessed July 13, 2008.
- 5. Pohlig C. Evaluation & Management Services: An Overview. Coding for Chest Medicine 2008. Northbrook, IL: American College of Chest Physicians, 2008; 57-69.
- 6. Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual: Chapter 1, Section 50.3. Available at www.cms.hhs.gov/manuals/downloads/ clm104c01.pdf. Accessed July 13, 2008.
Observation care provides a mechanism to evaluate and treat patients without the resource utilization and financial responsibility associated with an inpatient admission. Hospitalists may not understand the billing compliance risk and corresponding revenue implications when observation services (OBS) are not captured correctly.
Are OBS best reported with observation care codes (99218-99220, 99234-99236), office visit codes (99201-99215), or initial hospital care codes (99221-99223)? Code selection depends upon the patient’s registered status, the nature of the provided service, and the length of stay. Review the following information before reporting OBS to ensure an accurate claim submission.
Attending Physician Responsibilities
The physician-documented reason for observation substantiates the medical necessity for the OBS admission. Contractors often evaluate medical records to determine the consistency between the physician order (physician intent), the services actually provided (inpatient or outpatient), and the medical necessity of those services, including the medical appropriateness of the inpatient or observation stay.
Certain diagnoses and procedures generally do not support an inpatient admission and fall within the definitions of outpatient observation. Uncomplicated presentations of chest pain (rule out MI), mild asthma/COPD, mild CHF, syncope and decreased responsiveness, atrial arrhythmias, and renal colic all frequently are associated with the expectation of a brief (less than 24-hour) stay unless serious pathology is uncovered.2 Situations that do not meet the criteria for observation care are considered “not medically necessary” and separate payment is not permitted. Examples of circumstances that lack medical necessity include:
- Outpatient blood administration;
- Lack of/delay in patient transportation;
- Provision of a medical exam for patients who do not require skilled support;
- Routine preparation prior to and recovery after diagnostic testing;
- Routine recovery and post-operative care after ambulatory surgery;
- When used for the convenience of the physician, patient or patient’s family;
- While awaiting transfer to another facility;
- Duration of care exceeding 48 hours;
- When an overnight stay is planned prior to diagnostic testing;
- Standing orders following outpatient surgery;
- Services that would normally require inpatient stay;
- No physicians order to admit to observation;
- Observation following an uncomplicated treatment or procedure;
- Services that are not reasonable and necessary for care of the patient;
- Services provided concurrently with chemotherapy; and
- Inpatients discharged to outpatient observation status.3
The attending physician of record assumes responsibility for the patient’s admission to observation and is permitted to report observation care codes. In addition to the reason for admission, a medical record involving the observation stay must include dated and timed physician admitting orders outlining the care plan, physician progress notes, and discharge orders. This documentation must be added to any other record prepared as a result of an emergency department or outpatient clinic encounter. If physicians other than the admitting physician/group (i.e., physicians in different specialties) provide services to the patient during observation, they must use the appropriate outpatient visit (e.g., 99214) or consultation code (e.g., 99244).
Length of Stay4
In general, the duration of observation care services typically does not exceed 24 hours, although in some circumstances patients may require a second day. Observation care for greater than 48 hours without inpatient admission is not considered medically necessary but may be payable after medical review. When the stay spans two calendar days, physician billing is straightforward: Select an initial observation care code (99218-99220) for calendar day one and the observation discharge code (99217) for day two. Only the admitting physician/group may report the discharge service, when applicable. Documentation must demonstrate a face-to-face encounter by the physician for each date of service.
Should the stay only constitute one calendar day, the duration of care becomes a crucial factor in determining the code category. Standard OBS codes (99218-99220) are applicable if the patient stay is less than eight hours on any given date. The OBS discharge code (99217) is not reported in this instance, although the documentation should reflect the attending physician’s written order and appropriate discharge plan. Alternately, same day admit/discharge codes (99234-99236) apply to single-day stays lasting more than eight hours. The OBS discharge code (99217) also is not reported in this instance. Documentation must identify, at a minimum:
- Duration of the stay;
- Presence by the billing physician; and
- Physician performance of each service (i.e., both an admission and discharge note).
Inpatient Admission1,4-5
Sometimes the patient requires inpatient admission after initially being placed in observation. If the inpatient admission occurs on the same day as the OBS admission, only one service is reported (e.g., 99222). The physician need not redocument a complete history and physical (H&P) but merely write the new order for admission and update the OBS assessment with any relevant, new information.
Should the inpatient admission occur on the second calendar day of the OBS stay, the physician is able to report the initial observation care code (e.g., 99219) on day one, and the initial inpatient care code (e.g., 99223) on day two. However, the physician must meet the documentation guidelines for initial hospital care and redocument the H&P associated with the reported visit level. In the case of 99223, the physician must document a comprehensive history (only referring to the previous review of systems and histories, while rewriting the history of present illness) and high complexity decision making. If the physician chooses not to document to this extent, a subsequent hospital care code (99231-99233) is reasonable because the episode of care is a continuation from the observation phase.
Beware that some insurers may change the patient’s status for the entire episode of care. In other words, the conversion to inpatient status occurs on day two of the patient stay, but the insurer may convert the entire stay, including day one, to an inpatient status. Should this happen, the physician is responsible for reporting the visit category that corresponds with the patient’s status. Inpatient services codes are required for claim submission when the patient stay qualifies as an inpatient admission. Because these conversions occur with some frequency, it is advisable to hold claims intended for observation patients until the correct patient status can be confirmed by the utilization review team, and communicated to the physician. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
References
- 1. American Medical Association. cpt 2008, Current Procedural Terminology Professional Edition. Chicago, IL: American Medical Association, 2007; 9-16 CPT codes, descriptions and other data only are copyright 2007 American Medical Association (AMA). All Rights Reserved (or such other date of publication of CPT). CPT is a trademark of the AMA.
- 2. Highmark Medicare Services. Local Coverage Determination L27548 Acute Care: Inpatient, Observation and Treatment Room Services. Available at www.highmarkmedicareservices.com/policy/mac-ab/127548.html. Accessed July 14, 2008.
- 3. Cigna. Healthcare Coverage Position: Observation Care. Available at www.cigna.com/customer_care/healthcare_ professional/coverage_positions/medical/mm_0411_coveragepositioncriteria_observation_care.pdf. Accessed July 12, 2008.
- 4. Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual: Chapter 12, Section 30.6.8. Available at www.cms.hhs.gov/manuals/downloads/ clm104c12.pdf. Accessed July 13, 2008.
- 5. Pohlig C. Evaluation & Management Services: An Overview. Coding for Chest Medicine 2008. Northbrook, IL: American College of Chest Physicians, 2008; 57-69.
- 6. Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual: Chapter 1, Section 50.3. Available at www.cms.hhs.gov/manuals/downloads/ clm104c01.pdf. Accessed July 13, 2008.
Observation care provides a mechanism to evaluate and treat patients without the resource utilization and financial responsibility associated with an inpatient admission. Hospitalists may not understand the billing compliance risk and corresponding revenue implications when observation services (OBS) are not captured correctly.
Are OBS best reported with observation care codes (99218-99220, 99234-99236), office visit codes (99201-99215), or initial hospital care codes (99221-99223)? Code selection depends upon the patient’s registered status, the nature of the provided service, and the length of stay. Review the following information before reporting OBS to ensure an accurate claim submission.
Attending Physician Responsibilities
The physician-documented reason for observation substantiates the medical necessity for the OBS admission. Contractors often evaluate medical records to determine the consistency between the physician order (physician intent), the services actually provided (inpatient or outpatient), and the medical necessity of those services, including the medical appropriateness of the inpatient or observation stay.
Certain diagnoses and procedures generally do not support an inpatient admission and fall within the definitions of outpatient observation. Uncomplicated presentations of chest pain (rule out MI), mild asthma/COPD, mild CHF, syncope and decreased responsiveness, atrial arrhythmias, and renal colic all frequently are associated with the expectation of a brief (less than 24-hour) stay unless serious pathology is uncovered.2 Situations that do not meet the criteria for observation care are considered “not medically necessary” and separate payment is not permitted. Examples of circumstances that lack medical necessity include:
- Outpatient blood administration;
- Lack of/delay in patient transportation;
- Provision of a medical exam for patients who do not require skilled support;
- Routine preparation prior to and recovery after diagnostic testing;
- Routine recovery and post-operative care after ambulatory surgery;
- When used for the convenience of the physician, patient or patient’s family;
- While awaiting transfer to another facility;
- Duration of care exceeding 48 hours;
- When an overnight stay is planned prior to diagnostic testing;
- Standing orders following outpatient surgery;
- Services that would normally require inpatient stay;
- No physicians order to admit to observation;
- Observation following an uncomplicated treatment or procedure;
- Services that are not reasonable and necessary for care of the patient;
- Services provided concurrently with chemotherapy; and
- Inpatients discharged to outpatient observation status.3
The attending physician of record assumes responsibility for the patient’s admission to observation and is permitted to report observation care codes. In addition to the reason for admission, a medical record involving the observation stay must include dated and timed physician admitting orders outlining the care plan, physician progress notes, and discharge orders. This documentation must be added to any other record prepared as a result of an emergency department or outpatient clinic encounter. If physicians other than the admitting physician/group (i.e., physicians in different specialties) provide services to the patient during observation, they must use the appropriate outpatient visit (e.g., 99214) or consultation code (e.g., 99244).
Length of Stay4
In general, the duration of observation care services typically does not exceed 24 hours, although in some circumstances patients may require a second day. Observation care for greater than 48 hours without inpatient admission is not considered medically necessary but may be payable after medical review. When the stay spans two calendar days, physician billing is straightforward: Select an initial observation care code (99218-99220) for calendar day one and the observation discharge code (99217) for day two. Only the admitting physician/group may report the discharge service, when applicable. Documentation must demonstrate a face-to-face encounter by the physician for each date of service.
Should the stay only constitute one calendar day, the duration of care becomes a crucial factor in determining the code category. Standard OBS codes (99218-99220) are applicable if the patient stay is less than eight hours on any given date. The OBS discharge code (99217) is not reported in this instance, although the documentation should reflect the attending physician’s written order and appropriate discharge plan. Alternately, same day admit/discharge codes (99234-99236) apply to single-day stays lasting more than eight hours. The OBS discharge code (99217) also is not reported in this instance. Documentation must identify, at a minimum:
- Duration of the stay;
- Presence by the billing physician; and
- Physician performance of each service (i.e., both an admission and discharge note).
Inpatient Admission1,4-5
Sometimes the patient requires inpatient admission after initially being placed in observation. If the inpatient admission occurs on the same day as the OBS admission, only one service is reported (e.g., 99222). The physician need not redocument a complete history and physical (H&P) but merely write the new order for admission and update the OBS assessment with any relevant, new information.
Should the inpatient admission occur on the second calendar day of the OBS stay, the physician is able to report the initial observation care code (e.g., 99219) on day one, and the initial inpatient care code (e.g., 99223) on day two. However, the physician must meet the documentation guidelines for initial hospital care and redocument the H&P associated with the reported visit level. In the case of 99223, the physician must document a comprehensive history (only referring to the previous review of systems and histories, while rewriting the history of present illness) and high complexity decision making. If the physician chooses not to document to this extent, a subsequent hospital care code (99231-99233) is reasonable because the episode of care is a continuation from the observation phase.
Beware that some insurers may change the patient’s status for the entire episode of care. In other words, the conversion to inpatient status occurs on day two of the patient stay, but the insurer may convert the entire stay, including day one, to an inpatient status. Should this happen, the physician is responsible for reporting the visit category that corresponds with the patient’s status. Inpatient services codes are required for claim submission when the patient stay qualifies as an inpatient admission. Because these conversions occur with some frequency, it is advisable to hold claims intended for observation patients until the correct patient status can be confirmed by the utilization review team, and communicated to the physician. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
References
- 1. American Medical Association. cpt 2008, Current Procedural Terminology Professional Edition. Chicago, IL: American Medical Association, 2007; 9-16 CPT codes, descriptions and other data only are copyright 2007 American Medical Association (AMA). All Rights Reserved (or such other date of publication of CPT). CPT is a trademark of the AMA.
- 2. Highmark Medicare Services. Local Coverage Determination L27548 Acute Care: Inpatient, Observation and Treatment Room Services. Available at www.highmarkmedicareservices.com/policy/mac-ab/127548.html. Accessed July 14, 2008.
- 3. Cigna. Healthcare Coverage Position: Observation Care. Available at www.cigna.com/customer_care/healthcare_ professional/coverage_positions/medical/mm_0411_coveragepositioncriteria_observation_care.pdf. Accessed July 12, 2008.
- 4. Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual: Chapter 12, Section 30.6.8. Available at www.cms.hhs.gov/manuals/downloads/ clm104c12.pdf. Accessed July 13, 2008.
- 5. Pohlig C. Evaluation & Management Services: An Overview. Coding for Chest Medicine 2008. Northbrook, IL: American College of Chest Physicians, 2008; 57-69.
- 6. Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual: Chapter 1, Section 50.3. Available at www.cms.hhs.gov/manuals/downloads/ clm104c01.pdf. Accessed July 13, 2008.
e-Prescription for Success?
CMS has taken up the e-prescribing torch. In July, the agency announced a preliminary program to promote widespread adoption of electronic prescribing.
E-prescribing is a natural goal for CMS; it has been proven to improve quality of care, reduce medication errors, increase efficiency, and lower administrative costs. Kerry Weems, the acting CMS administrator, says an all-electronic prescribing system could save Medicare as much as $156 million over five years—largely through improved quality care.
Though details on the e-prescribing plan are not yet decided, CMS has revealed that beginning in 2009 (and for the next four years) it will provide incentive payments to physicians who are “successful electronic prescribers.”
Details to Be Determined
The e-prescribing plan will be included in the Physician Quality Reporting Initiative (PQRI), with guidelines included in the 2009 PQRI. (How the new plan will work with the current PQRI e-prescribing measure is one of the unknown details.)
Weems says CMS will use its standard rule-making process to shape the e-prescribing plan. Therefore, details of the incentives program will not be available until this fall, when Medicare releases its final rule on the 2009 physician fee schedule. According to Weems, the 2009 fee schedule and PQRI will clarify some murkiness. “They will be specific about what constitutes e-prescribing, including the extent and reporting of what needs to be done through PQRI,” he says.
Rewards, Then Possible Punishments
Physicians can start reporting on e-prescribing Jan. 1, and those who do will reap the benefits. Patrick Conway, MD, MSc, a hospitalist, an assistant professor at Cincinnati Children’s Hospital Medical Center, and a 2007-2008 White House Fellow working in the Department of Health and Human Services (HHS), says initial discussions about promoting e-prescribing included talk of an incentive-based plan.
“It’s my opinion that, for physicians, it’s beneficial to start with a reward or carrot rather than a punishment,” he says. “And generally, CMS has approached physician programs with this method—like the PQRI.”
The current plan’s outlines indicates that in 2009 and 2010, physicians who successfully report on e-prescribing will receive an incentive payment of up to 2% of their total Medicare allowed charges, matching the maximum bonus they can earn under the regular PQRI. Payment will be additive, so a physician can earn up to 4% (2% for PQRI and 2% for e-prescribing.)
The e-prescribing incentive will drop to 1% in 2011 and 2012, and to 0.5% incentive payment in 2013. After 2013, the carrot is replaced with a stick, and those who do not use e-prescribing will suffer a reduction in payment.
Cost Concerns
CMS estimates the cost of adopting e-prescribing will be approximately $3,000 per individual prescriber. This includes equipment, training, and program maintenance. That can add up to a sizeable expense—particularly for small groups. For that reason, the agency promises a built-in hardship exemption for small practices and others who prove they cannot afford to adopt e-prescribing.
Also, some funding is available: Dr. Conway says CMS has a financial-incentive program for electronic health records, many of which include e-prescribing. “The CMS Electronic Health Records Demonstration is a $150 million program that will provide funds to 1,200 physician practices to adopt this technology,” he says. “They’re currently recruiting practices.” Details on the demonstration are available at www.cms.hhs.gov/DemoProjectsEvalRpts/.
—Patrick Conway, MD, MSc
Will Hospitalists Participate?
Until details of the e-prescribing program are published, no one can say whether the plan will encompass hospitalists. However, Dr. Conway says, “I think this plan is conceptually relevant to hospitalists: It’s possible that hospitalists will be able to participate in the current plan. We don’t know yet. But CMS will continue to push forward on initiatives that increase quality and decrease costs, including e-prescribing. They’ll support electronic health records, whether this particular initiative applies to hospitals or not.”
Even if it turns out hospital medicine groups can’t reap incentive payments from the new plan, Dr. Conway hopes they still will adopt the technology. “Computerized physician order entry (CPOE) and e-prescribing have the potential to decrease errors and increase the quality of care,” he says. “Therefore, I would encourage hospitals and hospitalists to implement electronic health records with computerized order entry and e-prescribing when possible.”
He says the real benefit to hospitals seeking to improve quality and reduce error is not the electronic transmission of prescriptions to the pharmacy, but CPOE. “Most evidence of increased quality is around computerized physician order entry, which includes decision support at the time of the order,” he points out. “One could argue that you could have an incentive for hospitals that utilize CPOE, but I have no idea if CMS will pursue that.”
Next Steps
On Oct. 6-7 CMS will host a conference on the complete e-prescribing plan for pharmacists and physicians in Boston. For details, check the CMS site at www.cms.hhs.gov/eprescribing or www.cms.hhs.gov/pqri.
Dr. Conway thinks the meeting is a good next step for CMS. “I believe it’s very important to engage frontline providers and stakeholders, so the concept of holding a conference to ensure the design of the program is understood, and to get buy-in from the people participating, is a wise choice,” he says.
In the next few months, physicians likely will be inundated with information on e-prescribing processes under the CMS plan. Stay abreast of the latest information through the CMS Web site and, if it turns out, hospitalists can actively participate in the plan, through the SHM Web site at www.hospitalmedicine.org. TH
Jane Jerrard is a medical writer based in Chicago.
CMS has taken up the e-prescribing torch. In July, the agency announced a preliminary program to promote widespread adoption of electronic prescribing.
E-prescribing is a natural goal for CMS; it has been proven to improve quality of care, reduce medication errors, increase efficiency, and lower administrative costs. Kerry Weems, the acting CMS administrator, says an all-electronic prescribing system could save Medicare as much as $156 million over five years—largely through improved quality care.
Though details on the e-prescribing plan are not yet decided, CMS has revealed that beginning in 2009 (and for the next four years) it will provide incentive payments to physicians who are “successful electronic prescribers.”
Details to Be Determined
The e-prescribing plan will be included in the Physician Quality Reporting Initiative (PQRI), with guidelines included in the 2009 PQRI. (How the new plan will work with the current PQRI e-prescribing measure is one of the unknown details.)
Weems says CMS will use its standard rule-making process to shape the e-prescribing plan. Therefore, details of the incentives program will not be available until this fall, when Medicare releases its final rule on the 2009 physician fee schedule. According to Weems, the 2009 fee schedule and PQRI will clarify some murkiness. “They will be specific about what constitutes e-prescribing, including the extent and reporting of what needs to be done through PQRI,” he says.
Rewards, Then Possible Punishments
Physicians can start reporting on e-prescribing Jan. 1, and those who do will reap the benefits. Patrick Conway, MD, MSc, a hospitalist, an assistant professor at Cincinnati Children’s Hospital Medical Center, and a 2007-2008 White House Fellow working in the Department of Health and Human Services (HHS), says initial discussions about promoting e-prescribing included talk of an incentive-based plan.
“It’s my opinion that, for physicians, it’s beneficial to start with a reward or carrot rather than a punishment,” he says. “And generally, CMS has approached physician programs with this method—like the PQRI.”
The current plan’s outlines indicates that in 2009 and 2010, physicians who successfully report on e-prescribing will receive an incentive payment of up to 2% of their total Medicare allowed charges, matching the maximum bonus they can earn under the regular PQRI. Payment will be additive, so a physician can earn up to 4% (2% for PQRI and 2% for e-prescribing.)
The e-prescribing incentive will drop to 1% in 2011 and 2012, and to 0.5% incentive payment in 2013. After 2013, the carrot is replaced with a stick, and those who do not use e-prescribing will suffer a reduction in payment.
Cost Concerns
CMS estimates the cost of adopting e-prescribing will be approximately $3,000 per individual prescriber. This includes equipment, training, and program maintenance. That can add up to a sizeable expense—particularly for small groups. For that reason, the agency promises a built-in hardship exemption for small practices and others who prove they cannot afford to adopt e-prescribing.
Also, some funding is available: Dr. Conway says CMS has a financial-incentive program for electronic health records, many of which include e-prescribing. “The CMS Electronic Health Records Demonstration is a $150 million program that will provide funds to 1,200 physician practices to adopt this technology,” he says. “They’re currently recruiting practices.” Details on the demonstration are available at www.cms.hhs.gov/DemoProjectsEvalRpts/.
—Patrick Conway, MD, MSc
Will Hospitalists Participate?
Until details of the e-prescribing program are published, no one can say whether the plan will encompass hospitalists. However, Dr. Conway says, “I think this plan is conceptually relevant to hospitalists: It’s possible that hospitalists will be able to participate in the current plan. We don’t know yet. But CMS will continue to push forward on initiatives that increase quality and decrease costs, including e-prescribing. They’ll support electronic health records, whether this particular initiative applies to hospitals or not.”
Even if it turns out hospital medicine groups can’t reap incentive payments from the new plan, Dr. Conway hopes they still will adopt the technology. “Computerized physician order entry (CPOE) and e-prescribing have the potential to decrease errors and increase the quality of care,” he says. “Therefore, I would encourage hospitals and hospitalists to implement electronic health records with computerized order entry and e-prescribing when possible.”
He says the real benefit to hospitals seeking to improve quality and reduce error is not the electronic transmission of prescriptions to the pharmacy, but CPOE. “Most evidence of increased quality is around computerized physician order entry, which includes decision support at the time of the order,” he points out. “One could argue that you could have an incentive for hospitals that utilize CPOE, but I have no idea if CMS will pursue that.”
Next Steps
On Oct. 6-7 CMS will host a conference on the complete e-prescribing plan for pharmacists and physicians in Boston. For details, check the CMS site at www.cms.hhs.gov/eprescribing or www.cms.hhs.gov/pqri.
Dr. Conway thinks the meeting is a good next step for CMS. “I believe it’s very important to engage frontline providers and stakeholders, so the concept of holding a conference to ensure the design of the program is understood, and to get buy-in from the people participating, is a wise choice,” he says.
In the next few months, physicians likely will be inundated with information on e-prescribing processes under the CMS plan. Stay abreast of the latest information through the CMS Web site and, if it turns out, hospitalists can actively participate in the plan, through the SHM Web site at www.hospitalmedicine.org. TH
Jane Jerrard is a medical writer based in Chicago.
CMS has taken up the e-prescribing torch. In July, the agency announced a preliminary program to promote widespread adoption of electronic prescribing.
E-prescribing is a natural goal for CMS; it has been proven to improve quality of care, reduce medication errors, increase efficiency, and lower administrative costs. Kerry Weems, the acting CMS administrator, says an all-electronic prescribing system could save Medicare as much as $156 million over five years—largely through improved quality care.
Though details on the e-prescribing plan are not yet decided, CMS has revealed that beginning in 2009 (and for the next four years) it will provide incentive payments to physicians who are “successful electronic prescribers.”
Details to Be Determined
The e-prescribing plan will be included in the Physician Quality Reporting Initiative (PQRI), with guidelines included in the 2009 PQRI. (How the new plan will work with the current PQRI e-prescribing measure is one of the unknown details.)
Weems says CMS will use its standard rule-making process to shape the e-prescribing plan. Therefore, details of the incentives program will not be available until this fall, when Medicare releases its final rule on the 2009 physician fee schedule. According to Weems, the 2009 fee schedule and PQRI will clarify some murkiness. “They will be specific about what constitutes e-prescribing, including the extent and reporting of what needs to be done through PQRI,” he says.
Rewards, Then Possible Punishments
Physicians can start reporting on e-prescribing Jan. 1, and those who do will reap the benefits. Patrick Conway, MD, MSc, a hospitalist, an assistant professor at Cincinnati Children’s Hospital Medical Center, and a 2007-2008 White House Fellow working in the Department of Health and Human Services (HHS), says initial discussions about promoting e-prescribing included talk of an incentive-based plan.
“It’s my opinion that, for physicians, it’s beneficial to start with a reward or carrot rather than a punishment,” he says. “And generally, CMS has approached physician programs with this method—like the PQRI.”
The current plan’s outlines indicates that in 2009 and 2010, physicians who successfully report on e-prescribing will receive an incentive payment of up to 2% of their total Medicare allowed charges, matching the maximum bonus they can earn under the regular PQRI. Payment will be additive, so a physician can earn up to 4% (2% for PQRI and 2% for e-prescribing.)
The e-prescribing incentive will drop to 1% in 2011 and 2012, and to 0.5% incentive payment in 2013. After 2013, the carrot is replaced with a stick, and those who do not use e-prescribing will suffer a reduction in payment.
Cost Concerns
CMS estimates the cost of adopting e-prescribing will be approximately $3,000 per individual prescriber. This includes equipment, training, and program maintenance. That can add up to a sizeable expense—particularly for small groups. For that reason, the agency promises a built-in hardship exemption for small practices and others who prove they cannot afford to adopt e-prescribing.
Also, some funding is available: Dr. Conway says CMS has a financial-incentive program for electronic health records, many of which include e-prescribing. “The CMS Electronic Health Records Demonstration is a $150 million program that will provide funds to 1,200 physician practices to adopt this technology,” he says. “They’re currently recruiting practices.” Details on the demonstration are available at www.cms.hhs.gov/DemoProjectsEvalRpts/.
—Patrick Conway, MD, MSc
Will Hospitalists Participate?
Until details of the e-prescribing program are published, no one can say whether the plan will encompass hospitalists. However, Dr. Conway says, “I think this plan is conceptually relevant to hospitalists: It’s possible that hospitalists will be able to participate in the current plan. We don’t know yet. But CMS will continue to push forward on initiatives that increase quality and decrease costs, including e-prescribing. They’ll support electronic health records, whether this particular initiative applies to hospitals or not.”
Even if it turns out hospital medicine groups can’t reap incentive payments from the new plan, Dr. Conway hopes they still will adopt the technology. “Computerized physician order entry (CPOE) and e-prescribing have the potential to decrease errors and increase the quality of care,” he says. “Therefore, I would encourage hospitals and hospitalists to implement electronic health records with computerized order entry and e-prescribing when possible.”
He says the real benefit to hospitals seeking to improve quality and reduce error is not the electronic transmission of prescriptions to the pharmacy, but CPOE. “Most evidence of increased quality is around computerized physician order entry, which includes decision support at the time of the order,” he points out. “One could argue that you could have an incentive for hospitals that utilize CPOE, but I have no idea if CMS will pursue that.”
Next Steps
On Oct. 6-7 CMS will host a conference on the complete e-prescribing plan for pharmacists and physicians in Boston. For details, check the CMS site at www.cms.hhs.gov/eprescribing or www.cms.hhs.gov/pqri.
Dr. Conway thinks the meeting is a good next step for CMS. “I believe it’s very important to engage frontline providers and stakeholders, so the concept of holding a conference to ensure the design of the program is understood, and to get buy-in from the people participating, is a wise choice,” he says.
In the next few months, physicians likely will be inundated with information on e-prescribing processes under the CMS plan. Stay abreast of the latest information through the CMS Web site and, if it turns out, hospitalists can actively participate in the plan, through the SHM Web site at www.hospitalmedicine.org. TH
Jane Jerrard is a medical writer based in Chicago.