User login
What is the best antibiotic treatment for C.difficile-associated diarrhea?
Case
An 84-year-old woman presents with watery diarrhea. She recently received a fluoroquinolone antibiotic during a hospitalization for pneumonia. Her temperature is 101 degrees, her heart rate is 110 beats per minute, and her respiratory rate is 22 breaths per minute. Her abdominal exam is significant for mild distention, hyperactive bowel sounds, and diffuse, mild tenderness without rebound or guarding. Her white blood cell count is 18,200 cells/mm3. You suspect C. difficile infection. Should you treat empirically with antibiotics and, if so, which antibiotic should you prescribe?
Overview
C. difficile is an anaerobic gram-positive bacillus that produces spores and toxins. In 1978, C. difficile was identified as the causative agent for antibiotic-associated diarrhea.1 The portal of entry is via the fecal-oral route.
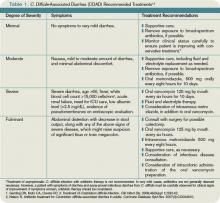
Some patients carry C. difficile in their intestinal flora and show no signs of infection. Patients who develop symptoms commonly present with profuse, watery diarrhea. Nausea, vomiting, and abdominal pain also can be seen. Severe cases of C. difficile-associated diarrhea (CDAD) can present with significant abdominal pain and multisystem organ failure, with toxic megacolon resulting from toxin production and ileus.2 In severe cases due to ileus, diarrhea may be absent. Risk of mortality in severe cases is high, with some reviews citing death rates of 57% in patients requiring total colectomy.3 Risk factors for developing CDAD include the prior or current use of antibiotics, advanced age, hospitalization, and prior gastrointestinal surgery or procedures.4
The initial CDAD treatment involves removal of the agent that incited the infection. In most cases, this means discontinuation of an antimicrobial agent. Removal of the inciting agent allows restoration of the normal bowel flora. In mild CDAD cases, this may be sufficient therapy. However, most CDAD cases require treatment. Although many antimicrobial and probiotic agents have been used in CDAD treatment, metronidazole and vancomycin are the most commonly prescribed agents. There is an ongoing debate as to which should be considered the first-line agent.
Review of the Data
Metronidazole and vancomycin have the longest histories of use and are the most studied agents in CDAD. Metronidazole is prescribed 250 mg four times daily (or 500 mg twice daily) for 14 days. It is reasonably tolerated, although it can cause a metallic taste in the mouth. Vancomycin is given 125 mg four times daily (or 500 mg three times daily) for 10 to 14 days. Unlike metronidazole, which can be given by mouth or intravenously, only oral vancomycin is effective in CDAD.
Historically, metronidazole has been prescribed more frequently as the first-line agent in CDAD. Proponents of the drug tout its low cost and the importance of minimizing the development of vancomycin-resistant enteric pathogens. There are two small, prospective, randomized studies comparing the efficacy of the agents against one another in the treatment of C. difficile infection, with similar efficacy demonstrated in both studies. In the early 1980s, Teasley and colleagues randomized 94 patients with C. difficile infection to either metronidazole or vancomycin.5 All the patients receiving vancomycin resolved their disease; 95% of patients receiving metronidazole were cured. The differences were not statistically significant.
In the mid-1990s, Wenisch and colleagues randomized patients with C. difficile infection to receive vancomycin, metronidazole, fusidic acid, or teicoplanin therapy.6 Ninety-four percent of patients in both the vancomycin and metronidazole groups were cured.
However, since 2000, investigators have reported higher failure rates with metronidazole therapy in C. difficile infections. For example, in 2005, Pepin and colleagues reviewed cases of C. difficile infections at a hospital in Quebec.7 They determined the number of patients with C. difficile infection initially treated with metronidazole who required additional therapy had markedly increased. Between 1991 and 2002, 9.6% of patients who initially were treated with metronidazole required a switch to vancomycin (or the addition of vancomycin) because of a disappointing response. This figure doubled to 25.7% in 2003-2004. The 60-day probability of recurrence also increased in the 2003-2004 test group (47.2%), compared with the 1991-2002 group (20.8%). Both results were statistically significant. Such data contributed to the debate regarding whether metronidazole or vancomycin is the superior agent in the treatment of C. difficile infections.
In 2007, Zar and colleagues studied the efficacy of metronidazole and vancomycin in the treatment of CDAD patients, but the study stratified patients according to disease severity.8 This allowed the authors to investigate whether one agent was superior in treating mild or severe CDAD. They determined disease severity by assigning points to individual patient characteristics. Patients with two or more points were deemed to have “severe” CDAD.
The investigators assigned one point for each of the following patient characteristics: temperature >38.3 degrees Celsius, age >60 years, albumin level <2.5 mg/dL, and white blood cell count >15,000 cells/mm3 within 48 hours of enrolling in the study. Any patient with endoscopic evidence of pseudomembrane formation or admission to the intensive-care unit (ICU) for CDAD treatment was considered to have severe disease.
This was a prospective, randomized controlled trial of 150 patients. Patients were randomly prescribed 500 mg metronidazole by mouth three times daily or 125 mg of vancomycin by mouth four times daily. Patients with mild CDAD had similar cure rates: 90% metronidazole versus 98% vancomycin (P=0.36). However, patients with severe CDAD fared statistically better when treated with oral vancomycin. Ninety-seven percent of severe CDAD patients treated with oral vancomycin had a clinical cure, while only 76% of those treated with metronidazole were cured (P=0.02). Recurrence of the disease was similar in each treatment group.
Based on this study, metronidazole and vancomycin appear equally effective in the treatment of mild CDAD, but vancomycin is the superior agent in the treatment of patients with severe CDAD.
Back to the Case
Our patient had several risk factors predisposing her to developing CDAD. She was of advanced age and took a fluoroquinolone antibiotic during a recent hospitalization. She also presented with signs consistent with a severe case of CDAD. She had a fever, a white blood cell count >15,000 cells/mm3, and was older than 60. Thus, she should be treated with supportive care, placed on contact precautions, and administered oral vancomycin 125 mg by mouth every six hours for 10 days as empiric therapy for CDAD. Stool cultures should be sent to confirm the presence of the C. difficile toxin.
Bottom Line
The appropriate antibiotic choice to treat CDAD in any patient depends upon the clinical severity of the disease. Treat patients with mild CDAD with metronidazole; prescribe oral vancomycin for patients with severe CDAD. TH
Dr. Mattison, instructor of medicine at Harvard Medical School, is a hospitalist and co-director of the Inpatient Geriatrics Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston. Dr. Li, assistant professor of medicine at Harvard Medical School, is director of hospital medicine and associate chief of BIDMC’s Division of General Medicine and Primary Care.
References
1.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of C. difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778-782.
2.Poutanen SM, Simor AE. C. difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
3.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant C. difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372.
4.Bartlett JG. Narrative review: the new epidemic of C. difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for C. difficile-associated diarrhea and colitis. Lancet. 1983;2:1043-1046.
6.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of C. difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
7.Pepin J, Alary M, Valiquette L, et al. Increasing risk of relapse after treatment of C. difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of C. difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
Case
An 84-year-old woman presents with watery diarrhea. She recently received a fluoroquinolone antibiotic during a hospitalization for pneumonia. Her temperature is 101 degrees, her heart rate is 110 beats per minute, and her respiratory rate is 22 breaths per minute. Her abdominal exam is significant for mild distention, hyperactive bowel sounds, and diffuse, mild tenderness without rebound or guarding. Her white blood cell count is 18,200 cells/mm3. You suspect C. difficile infection. Should you treat empirically with antibiotics and, if so, which antibiotic should you prescribe?
Overview
C. difficile is an anaerobic gram-positive bacillus that produces spores and toxins. In 1978, C. difficile was identified as the causative agent for antibiotic-associated diarrhea.1 The portal of entry is via the fecal-oral route.
Some patients carry C. difficile in their intestinal flora and show no signs of infection. Patients who develop symptoms commonly present with profuse, watery diarrhea. Nausea, vomiting, and abdominal pain also can be seen. Severe cases of C. difficile-associated diarrhea (CDAD) can present with significant abdominal pain and multisystem organ failure, with toxic megacolon resulting from toxin production and ileus.2 In severe cases due to ileus, diarrhea may be absent. Risk of mortality in severe cases is high, with some reviews citing death rates of 57% in patients requiring total colectomy.3 Risk factors for developing CDAD include the prior or current use of antibiotics, advanced age, hospitalization, and prior gastrointestinal surgery or procedures.4
The initial CDAD treatment involves removal of the agent that incited the infection. In most cases, this means discontinuation of an antimicrobial agent. Removal of the inciting agent allows restoration of the normal bowel flora. In mild CDAD cases, this may be sufficient therapy. However, most CDAD cases require treatment. Although many antimicrobial and probiotic agents have been used in CDAD treatment, metronidazole and vancomycin are the most commonly prescribed agents. There is an ongoing debate as to which should be considered the first-line agent.
Review of the Data
Metronidazole and vancomycin have the longest histories of use and are the most studied agents in CDAD. Metronidazole is prescribed 250 mg four times daily (or 500 mg twice daily) for 14 days. It is reasonably tolerated, although it can cause a metallic taste in the mouth. Vancomycin is given 125 mg four times daily (or 500 mg three times daily) for 10 to 14 days. Unlike metronidazole, which can be given by mouth or intravenously, only oral vancomycin is effective in CDAD.
Historically, metronidazole has been prescribed more frequently as the first-line agent in CDAD. Proponents of the drug tout its low cost and the importance of minimizing the development of vancomycin-resistant enteric pathogens. There are two small, prospective, randomized studies comparing the efficacy of the agents against one another in the treatment of C. difficile infection, with similar efficacy demonstrated in both studies. In the early 1980s, Teasley and colleagues randomized 94 patients with C. difficile infection to either metronidazole or vancomycin.5 All the patients receiving vancomycin resolved their disease; 95% of patients receiving metronidazole were cured. The differences were not statistically significant.
In the mid-1990s, Wenisch and colleagues randomized patients with C. difficile infection to receive vancomycin, metronidazole, fusidic acid, or teicoplanin therapy.6 Ninety-four percent of patients in both the vancomycin and metronidazole groups were cured.
However, since 2000, investigators have reported higher failure rates with metronidazole therapy in C. difficile infections. For example, in 2005, Pepin and colleagues reviewed cases of C. difficile infections at a hospital in Quebec.7 They determined the number of patients with C. difficile infection initially treated with metronidazole who required additional therapy had markedly increased. Between 1991 and 2002, 9.6% of patients who initially were treated with metronidazole required a switch to vancomycin (or the addition of vancomycin) because of a disappointing response. This figure doubled to 25.7% in 2003-2004. The 60-day probability of recurrence also increased in the 2003-2004 test group (47.2%), compared with the 1991-2002 group (20.8%). Both results were statistically significant. Such data contributed to the debate regarding whether metronidazole or vancomycin is the superior agent in the treatment of C. difficile infections.
In 2007, Zar and colleagues studied the efficacy of metronidazole and vancomycin in the treatment of CDAD patients, but the study stratified patients according to disease severity.8 This allowed the authors to investigate whether one agent was superior in treating mild or severe CDAD. They determined disease severity by assigning points to individual patient characteristics. Patients with two or more points were deemed to have “severe” CDAD.
The investigators assigned one point for each of the following patient characteristics: temperature >38.3 degrees Celsius, age >60 years, albumin level <2.5 mg/dL, and white blood cell count >15,000 cells/mm3 within 48 hours of enrolling in the study. Any patient with endoscopic evidence of pseudomembrane formation or admission to the intensive-care unit (ICU) for CDAD treatment was considered to have severe disease.
This was a prospective, randomized controlled trial of 150 patients. Patients were randomly prescribed 500 mg metronidazole by mouth three times daily or 125 mg of vancomycin by mouth four times daily. Patients with mild CDAD had similar cure rates: 90% metronidazole versus 98% vancomycin (P=0.36). However, patients with severe CDAD fared statistically better when treated with oral vancomycin. Ninety-seven percent of severe CDAD patients treated with oral vancomycin had a clinical cure, while only 76% of those treated with metronidazole were cured (P=0.02). Recurrence of the disease was similar in each treatment group.
Based on this study, metronidazole and vancomycin appear equally effective in the treatment of mild CDAD, but vancomycin is the superior agent in the treatment of patients with severe CDAD.
Back to the Case
Our patient had several risk factors predisposing her to developing CDAD. She was of advanced age and took a fluoroquinolone antibiotic during a recent hospitalization. She also presented with signs consistent with a severe case of CDAD. She had a fever, a white blood cell count >15,000 cells/mm3, and was older than 60. Thus, she should be treated with supportive care, placed on contact precautions, and administered oral vancomycin 125 mg by mouth every six hours for 10 days as empiric therapy for CDAD. Stool cultures should be sent to confirm the presence of the C. difficile toxin.
Bottom Line
The appropriate antibiotic choice to treat CDAD in any patient depends upon the clinical severity of the disease. Treat patients with mild CDAD with metronidazole; prescribe oral vancomycin for patients with severe CDAD. TH
Dr. Mattison, instructor of medicine at Harvard Medical School, is a hospitalist and co-director of the Inpatient Geriatrics Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston. Dr. Li, assistant professor of medicine at Harvard Medical School, is director of hospital medicine and associate chief of BIDMC’s Division of General Medicine and Primary Care.
References
1.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of C. difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778-782.
2.Poutanen SM, Simor AE. C. difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
3.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant C. difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372.
4.Bartlett JG. Narrative review: the new epidemic of C. difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for C. difficile-associated diarrhea and colitis. Lancet. 1983;2:1043-1046.
6.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of C. difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
7.Pepin J, Alary M, Valiquette L, et al. Increasing risk of relapse after treatment of C. difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of C. difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
Case
An 84-year-old woman presents with watery diarrhea. She recently received a fluoroquinolone antibiotic during a hospitalization for pneumonia. Her temperature is 101 degrees, her heart rate is 110 beats per minute, and her respiratory rate is 22 breaths per minute. Her abdominal exam is significant for mild distention, hyperactive bowel sounds, and diffuse, mild tenderness without rebound or guarding. Her white blood cell count is 18,200 cells/mm3. You suspect C. difficile infection. Should you treat empirically with antibiotics and, if so, which antibiotic should you prescribe?
Overview
C. difficile is an anaerobic gram-positive bacillus that produces spores and toxins. In 1978, C. difficile was identified as the causative agent for antibiotic-associated diarrhea.1 The portal of entry is via the fecal-oral route.
Some patients carry C. difficile in their intestinal flora and show no signs of infection. Patients who develop symptoms commonly present with profuse, watery diarrhea. Nausea, vomiting, and abdominal pain also can be seen. Severe cases of C. difficile-associated diarrhea (CDAD) can present with significant abdominal pain and multisystem organ failure, with toxic megacolon resulting from toxin production and ileus.2 In severe cases due to ileus, diarrhea may be absent. Risk of mortality in severe cases is high, with some reviews citing death rates of 57% in patients requiring total colectomy.3 Risk factors for developing CDAD include the prior or current use of antibiotics, advanced age, hospitalization, and prior gastrointestinal surgery or procedures.4
The initial CDAD treatment involves removal of the agent that incited the infection. In most cases, this means discontinuation of an antimicrobial agent. Removal of the inciting agent allows restoration of the normal bowel flora. In mild CDAD cases, this may be sufficient therapy. However, most CDAD cases require treatment. Although many antimicrobial and probiotic agents have been used in CDAD treatment, metronidazole and vancomycin are the most commonly prescribed agents. There is an ongoing debate as to which should be considered the first-line agent.
Review of the Data
Metronidazole and vancomycin have the longest histories of use and are the most studied agents in CDAD. Metronidazole is prescribed 250 mg four times daily (or 500 mg twice daily) for 14 days. It is reasonably tolerated, although it can cause a metallic taste in the mouth. Vancomycin is given 125 mg four times daily (or 500 mg three times daily) for 10 to 14 days. Unlike metronidazole, which can be given by mouth or intravenously, only oral vancomycin is effective in CDAD.
Historically, metronidazole has been prescribed more frequently as the first-line agent in CDAD. Proponents of the drug tout its low cost and the importance of minimizing the development of vancomycin-resistant enteric pathogens. There are two small, prospective, randomized studies comparing the efficacy of the agents against one another in the treatment of C. difficile infection, with similar efficacy demonstrated in both studies. In the early 1980s, Teasley and colleagues randomized 94 patients with C. difficile infection to either metronidazole or vancomycin.5 All the patients receiving vancomycin resolved their disease; 95% of patients receiving metronidazole were cured. The differences were not statistically significant.
In the mid-1990s, Wenisch and colleagues randomized patients with C. difficile infection to receive vancomycin, metronidazole, fusidic acid, or teicoplanin therapy.6 Ninety-four percent of patients in both the vancomycin and metronidazole groups were cured.
However, since 2000, investigators have reported higher failure rates with metronidazole therapy in C. difficile infections. For example, in 2005, Pepin and colleagues reviewed cases of C. difficile infections at a hospital in Quebec.7 They determined the number of patients with C. difficile infection initially treated with metronidazole who required additional therapy had markedly increased. Between 1991 and 2002, 9.6% of patients who initially were treated with metronidazole required a switch to vancomycin (or the addition of vancomycin) because of a disappointing response. This figure doubled to 25.7% in 2003-2004. The 60-day probability of recurrence also increased in the 2003-2004 test group (47.2%), compared with the 1991-2002 group (20.8%). Both results were statistically significant. Such data contributed to the debate regarding whether metronidazole or vancomycin is the superior agent in the treatment of C. difficile infections.
In 2007, Zar and colleagues studied the efficacy of metronidazole and vancomycin in the treatment of CDAD patients, but the study stratified patients according to disease severity.8 This allowed the authors to investigate whether one agent was superior in treating mild or severe CDAD. They determined disease severity by assigning points to individual patient characteristics. Patients with two or more points were deemed to have “severe” CDAD.
The investigators assigned one point for each of the following patient characteristics: temperature >38.3 degrees Celsius, age >60 years, albumin level <2.5 mg/dL, and white blood cell count >15,000 cells/mm3 within 48 hours of enrolling in the study. Any patient with endoscopic evidence of pseudomembrane formation or admission to the intensive-care unit (ICU) for CDAD treatment was considered to have severe disease.
This was a prospective, randomized controlled trial of 150 patients. Patients were randomly prescribed 500 mg metronidazole by mouth three times daily or 125 mg of vancomycin by mouth four times daily. Patients with mild CDAD had similar cure rates: 90% metronidazole versus 98% vancomycin (P=0.36). However, patients with severe CDAD fared statistically better when treated with oral vancomycin. Ninety-seven percent of severe CDAD patients treated with oral vancomycin had a clinical cure, while only 76% of those treated with metronidazole were cured (P=0.02). Recurrence of the disease was similar in each treatment group.
Based on this study, metronidazole and vancomycin appear equally effective in the treatment of mild CDAD, but vancomycin is the superior agent in the treatment of patients with severe CDAD.
Back to the Case
Our patient had several risk factors predisposing her to developing CDAD. She was of advanced age and took a fluoroquinolone antibiotic during a recent hospitalization. She also presented with signs consistent with a severe case of CDAD. She had a fever, a white blood cell count >15,000 cells/mm3, and was older than 60. Thus, she should be treated with supportive care, placed on contact precautions, and administered oral vancomycin 125 mg by mouth every six hours for 10 days as empiric therapy for CDAD. Stool cultures should be sent to confirm the presence of the C. difficile toxin.
Bottom Line
The appropriate antibiotic choice to treat CDAD in any patient depends upon the clinical severity of the disease. Treat patients with mild CDAD with metronidazole; prescribe oral vancomycin for patients with severe CDAD. TH
Dr. Mattison, instructor of medicine at Harvard Medical School, is a hospitalist and co-director of the Inpatient Geriatrics Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston. Dr. Li, assistant professor of medicine at Harvard Medical School, is director of hospital medicine and associate chief of BIDMC’s Division of General Medicine and Primary Care.
References
1.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of C. difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778-782.
2.Poutanen SM, Simor AE. C. difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
3.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant C. difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363-372.
4.Bartlett JG. Narrative review: the new epidemic of C. difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for C. difficile-associated diarrhea and colitis. Lancet. 1983;2:1043-1046.
6.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of C. difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
7.Pepin J, Alary M, Valiquette L, et al. Increasing risk of relapse after treatment of C. difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of C. difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
Why off-label isn’t off base
This article is adapted from the February 2009 issue of Current Psychiatry, a Quadrant HealthCom Inc. publication.
The author reports no financial relationships relevant to this article.
CASE
A physician speaking with a colleague expressed his anxiety and uncertainty over off-label prescribing:
“When I was a resident, attending physicians occasionally cited journal articles in their consultation notes to substantiate their treatment choices. Since then, I’ve done this at times when I’ve prescribed a drug off label.
“Recently, I mentioned this practice to a physician who is trained as a lawyer. He thought citing articles in a patient’s chart was a bad idea, because by doing so I was automatically making the referred-to article the ‘expert witness.’ If a lawsuit occurred, I might be called upon to justify the article’s validity, statistical details, methodology, etc. My intent is to show that I have a detailed, well-thought-out justification for my treatment choice.
“Am I placing myself at greater risk of incurring liability should a lawsuit occur?”
This physician wants to know how he can minimize malpractice risk when prescribing a medication off label. He wonders if citing an article in a patient’s chart is a good—or bad—idea.
In law school, attorneys-in-training learn to answer very general legal questions with “It depends.” There’s little certainty about how to avoid successful malpractice litigation because few, if any, strategies have been tested systematically. However, this article will explain and, I hope, help you avoid the medicolegal pitfalls of off-label prescribing.
Limited testing for safety and effectiveness. Experiences such as the fen-phen (weight loss) controversy1 and estrogens for preventing vascular disease in postmenopausal women2 remind physicians that some untested treatments may do more harm than good.
Commercial influence. Pharmaceutical companies have used advisory boards, consultant meetings, and continuing medical education events to promote unproven off-label indications for drugs.3,4 Many studies that were, ostensibly, designed and proposed by researchers show evidence of so-called ghost authorship by commercial concerns.5
Study bias. Even peer-reviewed, double-blind studies that are published in the medical literature might not sufficiently support off-label prescribing practices because sponsors of such studies can structure them or use statistical analyses to make results look favorable. Former editors of the British Medical Journal and The Lancet have acknowledged that their publications unwittingly served as “an extension of the marketing arm” or “laundering operations” for drug manufacturers.6,7 Even for FDA-approved indications, a selective, positive-result publication bias and nonreporting of negative results may make drugs seem more effective than the full range of studies would justify.8
Legal use of labeling. Although off-label prescribing is accepted medical practice, doctors “may be found negligent if their decision to use a drug off label is sufficiently careless, imprudent, or unprofessional.”9 During a malpractice lawsuit, plaintiff’s counsel could try to use FDA-approved labeling or prescribing information to establish a presumptive standard of care. Such evidence usually is admissible if it is supported by expert testimony. The burden of proof is then placed on the defendant physician to show how an off-label use met the standard of care.10
References
1. Connolly H, Crary J, McGoon M, et al. Vascular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-588.
2. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
3. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286.-
4. Steinman MA, Bero L, Chren M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145:284-293.
5. Gøtzsche PC, Hróbjartsson A, Johansen H, et al. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4(1):e19.-
6. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.-
7. Horton R. The dawn of McScience. New York Rev Books. 2004;51(4):7-9.
8. Turner EH, Matthews A, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
9. Richardson v Miller. 44 SW3d 1 (Tenn Ct App 2000).
10. Henry V. Off-label prescribing. Legal implications. J Leg Med. 1999;20:365-383.
Off-label: Accepted and necessary
Off-label prescribing occurs when a physician prescribes a medication or uses a medical device outside the scope of FDA-approved labeling. Most commonly, off-label use involves prescribing a medication for something other than its FDA-approved indication. An example is sildenafil [Viagra] for women who have antidepressant-induced sexual dysfunction.1
Other examples are prescribing a drug:
- at an unapproved dose
- in an unapproved format (e.g., mixing capsule contents with applesauce)
- outside the approved age group
- for longer than the approved interval
- at a different dose schedule (e.g., qhs instead of bid or tid).
Typically, it takes years for a new drug to gain FDA approval and additional time for an already-approved drug to gain approval for a new indication. In the meantime, clinicians treat their patients with available drugs prescribed off label.
Off-label prescribing is legal. FDA approval means drugs may be sold and marketed in specific ways. But the FDA does not tell physicians how they can use approved drugs. As each edition of the Physicians’ Desk Reference explains, “Once a product has been approved for marketing, a physician may prescribe it for uses or in treatment regimens or patient populations that are not included in approved labeling.”2 Federal statutes state that FDA approval does not “limit or interfere with the authority of a health care practitioner to prescribe” approved drugs or devices “for any condition or disease.”3
- Know why an article applies to your patient. If you are sued for malpractice, you can use an article to support your treatment choice by explaining how this information contributed to your decision-making.
- Tell your patient that the proposed treatment is an off-label use when you obtain consent, even though case law says you don’t have to do this. Telling your patient helps him understand your reasoning and prevents surprises that may give offense.
- Engage in ongoing informed consent. Uncertainty is part of medical practice and is heightened when physicians prescribe off label. Ongoing discussions help patients understand, accept, and share that uncertainty.
- Document informed consent. This will show—if it becomes necessary—that you and your patient made collaborative, conscientious decisions about treatment.1
Reference
1. Royal College of Psychiatrists. CR142. Use of unlicensed medicine for unlicensed applications in psychiatric practice. Available at: http://www.rcpsych.ac.uk/publications/collegereports/cr/cr142.aspx. Accessed March 4, 2009.
Does off-label constitute malpractice?
Off-label use is not only legal—it’s often wise medical practice. Many drug uses that now have FDA approval were off label just a few years ago. Examples include using selective serotonin reuptake inhibitors (SSRIs) to treat panic disorder and obsessive–compulsive disorder. Fluoxetine is the only FDA-approved drug for treating depression in adolescents, but other SSRIs may also have a favorable risk–benefit profile.6
The practice is common—we know that
Numerous studies have shown that off-label prescribing is common in, for example, psychiatry7 and other specialties.8,9 Because the practice is so common, the mere fact that a drug is not FDA-approved for a particular use does not imply that the drug was prescribed negligently.
Some commentators have suggested that off-label prescribing amounts to human experimentation.10 Without FDA approval, they say, physicians lack hard evidence, so to speak, that a product is safe and effective—making off-label prescribing a small-scale clinical trial based on the doctor’s educated guesses.
If this reasoning is correct, off-label prescribing would require the same human subject protections used in research, including institutional review board approval and special consent forms.
Although this argument sounds plausible, off-label prescribing is not experimentation or research (see “4 reasons why off-label prescribing can be controversial”).4,11-19 Researchers investigate hypotheses to obtain generalizable knowledge, whereas medical therapy aims to benefit individual patients. This experimentation–therapy distinction is not perfect because successful off-label treatment of one patient might imply beneficial effects for others.10 When courts have looked at this matter, though, they have found that “off-label use… by a physician seeking an optimal treatment for his or her patient is not necessarily…research or an investigational or experimental treatment when the use is customarily followed by physicians.”4
Courts also have said that off-label use does not require special informed consent. Just because a drug is prescribed off label doesn’t mean it’s risky. FDA approval “is not a material risk inherently involved in a proposed therapy which a physician should have disclosed to a patient prior to the therapy.”20 In other words, a physician is not required to discuss FDA regulatory status—such as off-label uses of a medication—to comply with standards of informed consent. FDA regulatory status has nothing to do with the risks or benefits of a medication and it does not provide information about treatment alternatives.21
What should you do?
For advice on protecting yourself when you prescribe off label, see the box above.
In addition, you should keep abreast of news and scientific evidence concerning drug uses, effects, interactions, and adverse effects, especially when prescribing for uses that are different from the manufacturer’s intended purposes.22
Last, collect articles on off-label uses, but keep them separate from your patients’ files. Good attorneys are highly skilled at using documents to score legal points, and opposing counsel will prepare questions to focus on the articles’ faults or limitations in isolation.
1. Nurnberg HG, Hensley PL, Heiman JR, et al. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
2. Physicians’ Desk Reference. 62nd ed. Montvale, NJ: Thomson Healthcare, Inc.; 2007.
3. Food, Drug and Cosmetic Act, 21USC §396
4. Richardson v Miller. 44 SW3d 1 (Tenn Ct App 2000).
5. Buckman Co. v Plaintiffs’ Legal Comm., 531 US 341 (2001).
6. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696.
7. Baldwin DS, Kosky N. Off-label prescribing in psychiatric practice. Adv Psychiatr Treat. 2007;13:414-422.
8. Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320:79-82.
9. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021-1026.
10. Mehlman MJ. Off-label prescribing. Available at: http://www.thedoctorwillseeyounow.com/articles/bioethics/offlabel_11. Accessed October 21, 2008.
11. Connolly H, Crary J, McGoon M, et al. Vascular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-588.
12. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
13. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286.-
14. Steinman MA, Bero L, Chren M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145:284-293.
15. Gøtzsche PC, Hróbjartsson A, Johansen H, et al. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4(1):e19.-
16. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.-
17. Horton R. The dawn of McScience. New York Rev Books. 2004;51(4):7-9.
18. Turner EH, Matthews A, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
19. Henry V. Off-label prescribing. Legal implications. J Leg Med. 1999;20:365-383.
20. Klein v Biscup. 673 NE2d 225 (Ohio App 1996).
21. Beck JM, Azari ED. FDA, off-label use, and informed consent: debunking myths and misconceptions. Food Drug Law J. 1998;53:71-104.
22. Shajnfeld A, Krueger RB. Reforming (purportedly) non-punitive responses to sexual offending. Dev Mental Health Law. 2006;25:81-99.
This article is adapted from the February 2009 issue of Current Psychiatry, a Quadrant HealthCom Inc. publication.
The author reports no financial relationships relevant to this article.
CASE
A physician speaking with a colleague expressed his anxiety and uncertainty over off-label prescribing:
“When I was a resident, attending physicians occasionally cited journal articles in their consultation notes to substantiate their treatment choices. Since then, I’ve done this at times when I’ve prescribed a drug off label.
“Recently, I mentioned this practice to a physician who is trained as a lawyer. He thought citing articles in a patient’s chart was a bad idea, because by doing so I was automatically making the referred-to article the ‘expert witness.’ If a lawsuit occurred, I might be called upon to justify the article’s validity, statistical details, methodology, etc. My intent is to show that I have a detailed, well-thought-out justification for my treatment choice.
“Am I placing myself at greater risk of incurring liability should a lawsuit occur?”
This physician wants to know how he can minimize malpractice risk when prescribing a medication off label. He wonders if citing an article in a patient’s chart is a good—or bad—idea.
In law school, attorneys-in-training learn to answer very general legal questions with “It depends.” There’s little certainty about how to avoid successful malpractice litigation because few, if any, strategies have been tested systematically. However, this article will explain and, I hope, help you avoid the medicolegal pitfalls of off-label prescribing.
Limited testing for safety and effectiveness. Experiences such as the fen-phen (weight loss) controversy1 and estrogens for preventing vascular disease in postmenopausal women2 remind physicians that some untested treatments may do more harm than good.
Commercial influence. Pharmaceutical companies have used advisory boards, consultant meetings, and continuing medical education events to promote unproven off-label indications for drugs.3,4 Many studies that were, ostensibly, designed and proposed by researchers show evidence of so-called ghost authorship by commercial concerns.5
Study bias. Even peer-reviewed, double-blind studies that are published in the medical literature might not sufficiently support off-label prescribing practices because sponsors of such studies can structure them or use statistical analyses to make results look favorable. Former editors of the British Medical Journal and The Lancet have acknowledged that their publications unwittingly served as “an extension of the marketing arm” or “laundering operations” for drug manufacturers.6,7 Even for FDA-approved indications, a selective, positive-result publication bias and nonreporting of negative results may make drugs seem more effective than the full range of studies would justify.8
Legal use of labeling. Although off-label prescribing is accepted medical practice, doctors “may be found negligent if their decision to use a drug off label is sufficiently careless, imprudent, or unprofessional.”9 During a malpractice lawsuit, plaintiff’s counsel could try to use FDA-approved labeling or prescribing information to establish a presumptive standard of care. Such evidence usually is admissible if it is supported by expert testimony. The burden of proof is then placed on the defendant physician to show how an off-label use met the standard of care.10
References
1. Connolly H, Crary J, McGoon M, et al. Vascular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-588.
2. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
3. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286.-
4. Steinman MA, Bero L, Chren M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145:284-293.
5. Gøtzsche PC, Hróbjartsson A, Johansen H, et al. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4(1):e19.-
6. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.-
7. Horton R. The dawn of McScience. New York Rev Books. 2004;51(4):7-9.
8. Turner EH, Matthews A, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
9. Richardson v Miller. 44 SW3d 1 (Tenn Ct App 2000).
10. Henry V. Off-label prescribing. Legal implications. J Leg Med. 1999;20:365-383.
Off-label: Accepted and necessary
Off-label prescribing occurs when a physician prescribes a medication or uses a medical device outside the scope of FDA-approved labeling. Most commonly, off-label use involves prescribing a medication for something other than its FDA-approved indication. An example is sildenafil [Viagra] for women who have antidepressant-induced sexual dysfunction.1
Other examples are prescribing a drug:
- at an unapproved dose
- in an unapproved format (e.g., mixing capsule contents with applesauce)
- outside the approved age group
- for longer than the approved interval
- at a different dose schedule (e.g., qhs instead of bid or tid).
Typically, it takes years for a new drug to gain FDA approval and additional time for an already-approved drug to gain approval for a new indication. In the meantime, clinicians treat their patients with available drugs prescribed off label.
Off-label prescribing is legal. FDA approval means drugs may be sold and marketed in specific ways. But the FDA does not tell physicians how they can use approved drugs. As each edition of the Physicians’ Desk Reference explains, “Once a product has been approved for marketing, a physician may prescribe it for uses or in treatment regimens or patient populations that are not included in approved labeling.”2 Federal statutes state that FDA approval does not “limit or interfere with the authority of a health care practitioner to prescribe” approved drugs or devices “for any condition or disease.”3
- Know why an article applies to your patient. If you are sued for malpractice, you can use an article to support your treatment choice by explaining how this information contributed to your decision-making.
- Tell your patient that the proposed treatment is an off-label use when you obtain consent, even though case law says you don’t have to do this. Telling your patient helps him understand your reasoning and prevents surprises that may give offense.
- Engage in ongoing informed consent. Uncertainty is part of medical practice and is heightened when physicians prescribe off label. Ongoing discussions help patients understand, accept, and share that uncertainty.
- Document informed consent. This will show—if it becomes necessary—that you and your patient made collaborative, conscientious decisions about treatment.1
Reference
1. Royal College of Psychiatrists. CR142. Use of unlicensed medicine for unlicensed applications in psychiatric practice. Available at: http://www.rcpsych.ac.uk/publications/collegereports/cr/cr142.aspx. Accessed March 4, 2009.
Does off-label constitute malpractice?
Off-label use is not only legal—it’s often wise medical practice. Many drug uses that now have FDA approval were off label just a few years ago. Examples include using selective serotonin reuptake inhibitors (SSRIs) to treat panic disorder and obsessive–compulsive disorder. Fluoxetine is the only FDA-approved drug for treating depression in adolescents, but other SSRIs may also have a favorable risk–benefit profile.6
The practice is common—we know that
Numerous studies have shown that off-label prescribing is common in, for example, psychiatry7 and other specialties.8,9 Because the practice is so common, the mere fact that a drug is not FDA-approved for a particular use does not imply that the drug was prescribed negligently.
Some commentators have suggested that off-label prescribing amounts to human experimentation.10 Without FDA approval, they say, physicians lack hard evidence, so to speak, that a product is safe and effective—making off-label prescribing a small-scale clinical trial based on the doctor’s educated guesses.
If this reasoning is correct, off-label prescribing would require the same human subject protections used in research, including institutional review board approval and special consent forms.
Although this argument sounds plausible, off-label prescribing is not experimentation or research (see “4 reasons why off-label prescribing can be controversial”).4,11-19 Researchers investigate hypotheses to obtain generalizable knowledge, whereas medical therapy aims to benefit individual patients. This experimentation–therapy distinction is not perfect because successful off-label treatment of one patient might imply beneficial effects for others.10 When courts have looked at this matter, though, they have found that “off-label use… by a physician seeking an optimal treatment for his or her patient is not necessarily…research or an investigational or experimental treatment when the use is customarily followed by physicians.”4
Courts also have said that off-label use does not require special informed consent. Just because a drug is prescribed off label doesn’t mean it’s risky. FDA approval “is not a material risk inherently involved in a proposed therapy which a physician should have disclosed to a patient prior to the therapy.”20 In other words, a physician is not required to discuss FDA regulatory status—such as off-label uses of a medication—to comply with standards of informed consent. FDA regulatory status has nothing to do with the risks or benefits of a medication and it does not provide information about treatment alternatives.21
What should you do?
For advice on protecting yourself when you prescribe off label, see the box above.
In addition, you should keep abreast of news and scientific evidence concerning drug uses, effects, interactions, and adverse effects, especially when prescribing for uses that are different from the manufacturer’s intended purposes.22
Last, collect articles on off-label uses, but keep them separate from your patients’ files. Good attorneys are highly skilled at using documents to score legal points, and opposing counsel will prepare questions to focus on the articles’ faults or limitations in isolation.
This article is adapted from the February 2009 issue of Current Psychiatry, a Quadrant HealthCom Inc. publication.
The author reports no financial relationships relevant to this article.
CASE
A physician speaking with a colleague expressed his anxiety and uncertainty over off-label prescribing:
“When I was a resident, attending physicians occasionally cited journal articles in their consultation notes to substantiate their treatment choices. Since then, I’ve done this at times when I’ve prescribed a drug off label.
“Recently, I mentioned this practice to a physician who is trained as a lawyer. He thought citing articles in a patient’s chart was a bad idea, because by doing so I was automatically making the referred-to article the ‘expert witness.’ If a lawsuit occurred, I might be called upon to justify the article’s validity, statistical details, methodology, etc. My intent is to show that I have a detailed, well-thought-out justification for my treatment choice.
“Am I placing myself at greater risk of incurring liability should a lawsuit occur?”
This physician wants to know how he can minimize malpractice risk when prescribing a medication off label. He wonders if citing an article in a patient’s chart is a good—or bad—idea.
In law school, attorneys-in-training learn to answer very general legal questions with “It depends.” There’s little certainty about how to avoid successful malpractice litigation because few, if any, strategies have been tested systematically. However, this article will explain and, I hope, help you avoid the medicolegal pitfalls of off-label prescribing.
Limited testing for safety and effectiveness. Experiences such as the fen-phen (weight loss) controversy1 and estrogens for preventing vascular disease in postmenopausal women2 remind physicians that some untested treatments may do more harm than good.
Commercial influence. Pharmaceutical companies have used advisory boards, consultant meetings, and continuing medical education events to promote unproven off-label indications for drugs.3,4 Many studies that were, ostensibly, designed and proposed by researchers show evidence of so-called ghost authorship by commercial concerns.5
Study bias. Even peer-reviewed, double-blind studies that are published in the medical literature might not sufficiently support off-label prescribing practices because sponsors of such studies can structure them or use statistical analyses to make results look favorable. Former editors of the British Medical Journal and The Lancet have acknowledged that their publications unwittingly served as “an extension of the marketing arm” or “laundering operations” for drug manufacturers.6,7 Even for FDA-approved indications, a selective, positive-result publication bias and nonreporting of negative results may make drugs seem more effective than the full range of studies would justify.8
Legal use of labeling. Although off-label prescribing is accepted medical practice, doctors “may be found negligent if their decision to use a drug off label is sufficiently careless, imprudent, or unprofessional.”9 During a malpractice lawsuit, plaintiff’s counsel could try to use FDA-approved labeling or prescribing information to establish a presumptive standard of care. Such evidence usually is admissible if it is supported by expert testimony. The burden of proof is then placed on the defendant physician to show how an off-label use met the standard of care.10
References
1. Connolly H, Crary J, McGoon M, et al. Vascular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-588.
2. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
3. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286.-
4. Steinman MA, Bero L, Chren M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145:284-293.
5. Gøtzsche PC, Hróbjartsson A, Johansen H, et al. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4(1):e19.-
6. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.-
7. Horton R. The dawn of McScience. New York Rev Books. 2004;51(4):7-9.
8. Turner EH, Matthews A, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
9. Richardson v Miller. 44 SW3d 1 (Tenn Ct App 2000).
10. Henry V. Off-label prescribing. Legal implications. J Leg Med. 1999;20:365-383.
Off-label: Accepted and necessary
Off-label prescribing occurs when a physician prescribes a medication or uses a medical device outside the scope of FDA-approved labeling. Most commonly, off-label use involves prescribing a medication for something other than its FDA-approved indication. An example is sildenafil [Viagra] for women who have antidepressant-induced sexual dysfunction.1
Other examples are prescribing a drug:
- at an unapproved dose
- in an unapproved format (e.g., mixing capsule contents with applesauce)
- outside the approved age group
- for longer than the approved interval
- at a different dose schedule (e.g., qhs instead of bid or tid).
Typically, it takes years for a new drug to gain FDA approval and additional time for an already-approved drug to gain approval for a new indication. In the meantime, clinicians treat their patients with available drugs prescribed off label.
Off-label prescribing is legal. FDA approval means drugs may be sold and marketed in specific ways. But the FDA does not tell physicians how they can use approved drugs. As each edition of the Physicians’ Desk Reference explains, “Once a product has been approved for marketing, a physician may prescribe it for uses or in treatment regimens or patient populations that are not included in approved labeling.”2 Federal statutes state that FDA approval does not “limit or interfere with the authority of a health care practitioner to prescribe” approved drugs or devices “for any condition or disease.”3
- Know why an article applies to your patient. If you are sued for malpractice, you can use an article to support your treatment choice by explaining how this information contributed to your decision-making.
- Tell your patient that the proposed treatment is an off-label use when you obtain consent, even though case law says you don’t have to do this. Telling your patient helps him understand your reasoning and prevents surprises that may give offense.
- Engage in ongoing informed consent. Uncertainty is part of medical practice and is heightened when physicians prescribe off label. Ongoing discussions help patients understand, accept, and share that uncertainty.
- Document informed consent. This will show—if it becomes necessary—that you and your patient made collaborative, conscientious decisions about treatment.1
Reference
1. Royal College of Psychiatrists. CR142. Use of unlicensed medicine for unlicensed applications in psychiatric practice. Available at: http://www.rcpsych.ac.uk/publications/collegereports/cr/cr142.aspx. Accessed March 4, 2009.
Does off-label constitute malpractice?
Off-label use is not only legal—it’s often wise medical practice. Many drug uses that now have FDA approval were off label just a few years ago. Examples include using selective serotonin reuptake inhibitors (SSRIs) to treat panic disorder and obsessive–compulsive disorder. Fluoxetine is the only FDA-approved drug for treating depression in adolescents, but other SSRIs may also have a favorable risk–benefit profile.6
The practice is common—we know that
Numerous studies have shown that off-label prescribing is common in, for example, psychiatry7 and other specialties.8,9 Because the practice is so common, the mere fact that a drug is not FDA-approved for a particular use does not imply that the drug was prescribed negligently.
Some commentators have suggested that off-label prescribing amounts to human experimentation.10 Without FDA approval, they say, physicians lack hard evidence, so to speak, that a product is safe and effective—making off-label prescribing a small-scale clinical trial based on the doctor’s educated guesses.
If this reasoning is correct, off-label prescribing would require the same human subject protections used in research, including institutional review board approval and special consent forms.
Although this argument sounds plausible, off-label prescribing is not experimentation or research (see “4 reasons why off-label prescribing can be controversial”).4,11-19 Researchers investigate hypotheses to obtain generalizable knowledge, whereas medical therapy aims to benefit individual patients. This experimentation–therapy distinction is not perfect because successful off-label treatment of one patient might imply beneficial effects for others.10 When courts have looked at this matter, though, they have found that “off-label use… by a physician seeking an optimal treatment for his or her patient is not necessarily…research or an investigational or experimental treatment when the use is customarily followed by physicians.”4
Courts also have said that off-label use does not require special informed consent. Just because a drug is prescribed off label doesn’t mean it’s risky. FDA approval “is not a material risk inherently involved in a proposed therapy which a physician should have disclosed to a patient prior to the therapy.”20 In other words, a physician is not required to discuss FDA regulatory status—such as off-label uses of a medication—to comply with standards of informed consent. FDA regulatory status has nothing to do with the risks or benefits of a medication and it does not provide information about treatment alternatives.21
What should you do?
For advice on protecting yourself when you prescribe off label, see the box above.
In addition, you should keep abreast of news and scientific evidence concerning drug uses, effects, interactions, and adverse effects, especially when prescribing for uses that are different from the manufacturer’s intended purposes.22
Last, collect articles on off-label uses, but keep them separate from your patients’ files. Good attorneys are highly skilled at using documents to score legal points, and opposing counsel will prepare questions to focus on the articles’ faults or limitations in isolation.
1. Nurnberg HG, Hensley PL, Heiman JR, et al. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
2. Physicians’ Desk Reference. 62nd ed. Montvale, NJ: Thomson Healthcare, Inc.; 2007.
3. Food, Drug and Cosmetic Act, 21USC §396
4. Richardson v Miller. 44 SW3d 1 (Tenn Ct App 2000).
5. Buckman Co. v Plaintiffs’ Legal Comm., 531 US 341 (2001).
6. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696.
7. Baldwin DS, Kosky N. Off-label prescribing in psychiatric practice. Adv Psychiatr Treat. 2007;13:414-422.
8. Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320:79-82.
9. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021-1026.
10. Mehlman MJ. Off-label prescribing. Available at: http://www.thedoctorwillseeyounow.com/articles/bioethics/offlabel_11. Accessed October 21, 2008.
11. Connolly H, Crary J, McGoon M, et al. Vascular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-588.
12. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
13. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286.-
14. Steinman MA, Bero L, Chren M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145:284-293.
15. Gøtzsche PC, Hróbjartsson A, Johansen H, et al. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4(1):e19.-
16. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.-
17. Horton R. The dawn of McScience. New York Rev Books. 2004;51(4):7-9.
18. Turner EH, Matthews A, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
19. Henry V. Off-label prescribing. Legal implications. J Leg Med. 1999;20:365-383.
20. Klein v Biscup. 673 NE2d 225 (Ohio App 1996).
21. Beck JM, Azari ED. FDA, off-label use, and informed consent: debunking myths and misconceptions. Food Drug Law J. 1998;53:71-104.
22. Shajnfeld A, Krueger RB. Reforming (purportedly) non-punitive responses to sexual offending. Dev Mental Health Law. 2006;25:81-99.
1. Nurnberg HG, Hensley PL, Heiman JR, et al. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395-404.
2. Physicians’ Desk Reference. 62nd ed. Montvale, NJ: Thomson Healthcare, Inc.; 2007.
3. Food, Drug and Cosmetic Act, 21USC §396
4. Richardson v Miller. 44 SW3d 1 (Tenn Ct App 2000).
5. Buckman Co. v Plaintiffs’ Legal Comm., 531 US 341 (2001).
6. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696.
7. Baldwin DS, Kosky N. Off-label prescribing in psychiatric practice. Adv Psychiatr Treat. 2007;13:414-422.
8. Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320:79-82.
9. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021-1026.
10. Mehlman MJ. Off-label prescribing. Available at: http://www.thedoctorwillseeyounow.com/articles/bioethics/offlabel_11. Accessed October 21, 2008.
11. Connolly H, Crary J, McGoon M, et al. Vascular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-588.
12. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
13. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286.-
14. Steinman MA, Bero L, Chren M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145:284-293.
15. Gøtzsche PC, Hróbjartsson A, Johansen H, et al. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007;4(1):e19.-
16. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.-
17. Horton R. The dawn of McScience. New York Rev Books. 2004;51(4):7-9.
18. Turner EH, Matthews A, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
19. Henry V. Off-label prescribing. Legal implications. J Leg Med. 1999;20:365-383.
20. Klein v Biscup. 673 NE2d 225 (Ohio App 1996).
21. Beck JM, Azari ED. FDA, off-label use, and informed consent: debunking myths and misconceptions. Food Drug Law J. 1998;53:71-104.
22. Shajnfeld A, Krueger RB. Reforming (purportedly) non-punitive responses to sexual offending. Dev Mental Health Law. 2006;25:81-99.
Bonus-Pay Bonanza
Although there is a lot of debate about the effectiveness of pay-for-performance (P4P) plans, I think the plans are only going to increase in the foreseeable future.
We need more research to tell us the relative impact of public reporting of performance data and P4P programs. Most importantly, the details of how these plans are set up, how and what they measure, and the dollar amount involved will have everything to do with whether they are successful in improving the value of care we provide.
SHM’S Practice Management Committee conducted a mini-survey of hospitalist group leaders in 2006. Here are some of the key findings.
P4P Prevalence
Forty-one percent (60 out of 146) of hospital medicine group (HMG) leaders reported their groups have a quality-incentive program. Of those HMG leaders more likely to report participation in a quality-incentive program:
- 60% were at hospitals participating in a P4P program;
- 50% were at multispecialty/PCP medical groups; and
- 50% were in the Southern region.
Of those HMG leaders less likely to report participation in P4P programs, 28% were at academic programs and 31% were at local hospitalist-only groups.
Group vs. Individual Incentives
Of the HMG leaders participating in a quality-incentive program:
- 43% reported it was an individual incentive;
- 35% reported it was a group incentive;
- 10% reported the plan had elements of both individual and group incentives; and
- 12% were not sure if their plans had individual or group incentives.
Basis of Quality Targets
Of the HMG leaders reporting that they participate in a quality-incentive program (respondents could indicate one or more answers):
- 60% of the programs have targets based on national benchmarks;
- 23% have targets based on local or regional benchmarks;
- 37% have targets based on their hospital’s previous experience; and
- 47% have targets based on improvement over a baseline.
Maximum Impact of Incentives
Of the HMG leaders reporting that they participate in a quality-incentive program:
- 16% report the maximum impact is less than 3%;
- 24% report the maximum impact is from 3% to 7%;
- 35% report the maximum impact is from 8% to 10%;
- 17% report the maximum impact is from 11% to 20%;
- 3% report the maximum impact is more than 20%; and
- 5% report they do not know the maximum impact.
Group vs. Individual Incentives
Of the HMG leaders reporting that they participate in a quality-incentive program:
- 61% said they have received an incentive payment;
- 37% have not received an incentive payment; and
- 2% were unsure if they have received an incentive payment.
Quality Metrics
The most common metrics used in P4P programs, based on 29 responses to the SHM survey:
- 93% of HM programs have metrics based on The Joint Commission’s (JCAHO) heart failure measures;
- 86% have metrics based on JCAHO pneumonia measures;
- 79% have metrics based on JCAHO myocardial infarction measures;
- 28% have metrics based on a measure of medication reconciliation;
- 24% have metrics based on avoidance of unapproved abbreviations;
- 24% have metrics based on 100,000 Lives Campaign measures;
- 21% have metrics based on patient satisfaction measures;
- 17% have metrics based on transitions-of-care measures;
- 10% have metrics based on throughput measures;
- 7% have metrics based on end-of-life measures;
- 7% have metrics based on “good citizenship” measures;
- 7% have metrics based on mortality rate measures; and
- 7% have metrics based on readmission rate measures.
The most common metrics used in quality-incentive programs, based on 45 responses to SHM’s survey:
- 73% of programs use JCAHO heart failure measures;
- 73% use “good citizenship” measures;
- 73% use patient satisfaction measures;
- 67% use JCAHO pneumonia measures;
- 51% use transitions-of-care measures;
- 44% use JCAHO M.I. measures;
- 31% use throughput measures;
- 27% use avoidance of unapproved abbreviations;
- 24% use a measure based on medication reconciliation;
- 11% use 100,000 Lives Campaign measures;
- 9% use readmission rate measures;
- 7% use mortality rate measures; and
- 2% use end-of-life measures.
Recommendations
The prevalence of hospitalist quality-based compensation plans is continuing to grow rapidly, but the details of the plans’ structure will govern whether they benefit our patients, improve the overall value of the care we provide, and serve as a meaningful component of our compensation. I suggest each practice consider implementing plans with the following attributes:
A total dollar amount available for performance that is enough to influence hospitalist behavior. I think quality incentives should compose as much as 15% to 20% of a hospitalist’s annual income. Plans connecting quality performance to equal to or less than 7% of annual compensation (the case for 40% of groups in the above survey) rarely are effective.
Money vs. metrics. It usually is better to establish a plan based on a sliding scale of improved performance rather than a single threshold. For example, if all of the bonus money is available for a 10% improvement in performance, consider providing 10% of the total available money for each 1% improvement in performance.
Degree of difficulty. Performance thresholds should be set so that hospitalists need to change their practices to achieve them, but not so far out of reach that hospitalists give up on them. This can get tricky. Many practices set thresholds that are very easy to reach (e.g., they may be near the current level of performance).
Metrics for which trusted data is readily available. In most cases, this means using data already being collected. Avoid hard-to-track metrics, as they are likely to lead to disagreements about their accuracy.
Group vs. individual measures. Most performance metrics can’t be clearly attributed to one hospitalist as compared to another. For example, who gets the credit or blame for Ms. Smith getting or not getting a pneumovax? The majority of performance metrics are best measured and paid on a group basis. Some metrics, such as documenting medicine reconciliation on admission and discharge, can be effectively attributed to a single hospitalist and could be paid on an individual basis.
Small number of metrics, A meaningfully large amount of money should be connected to each one. Don’t make the mistake of having a $10,000 per doctor annual quality bonus pool divided among 20 metrics (each metric would pay a maximum of $500 per year).
Rotating metrics. Consider an annual meeting with members of your hospital’s administration to jointly establish the metrics used in the hospitalist quality incentive for that year. It is reasonable to change the metrics periodically.
It seems to me P4P programs are in their infancy, and will continue to evolve rapidly. Plans that fail to improve outcomes enough to justify the complexity of implementing, tracking, and paying for them will disappear slowly. (I wonder if payment for pneumovax administration during the hospital stay will be in this category.) And new, more effective, and more valuable programs will be developed.
Hospitalist practices will need to be nimble to keep pace with all of this change. Although SHM can alert you to how new P4P initiatives might affect your practice, and even recommend methods to improve your performance, you and your hospitalist colleagues still will have a lot of work to operationalize these programs in your practice. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Although there is a lot of debate about the effectiveness of pay-for-performance (P4P) plans, I think the plans are only going to increase in the foreseeable future.
We need more research to tell us the relative impact of public reporting of performance data and P4P programs. Most importantly, the details of how these plans are set up, how and what they measure, and the dollar amount involved will have everything to do with whether they are successful in improving the value of care we provide.
SHM’S Practice Management Committee conducted a mini-survey of hospitalist group leaders in 2006. Here are some of the key findings.
P4P Prevalence
Forty-one percent (60 out of 146) of hospital medicine group (HMG) leaders reported their groups have a quality-incentive program. Of those HMG leaders more likely to report participation in a quality-incentive program:
- 60% were at hospitals participating in a P4P program;
- 50% were at multispecialty/PCP medical groups; and
- 50% were in the Southern region.
Of those HMG leaders less likely to report participation in P4P programs, 28% were at academic programs and 31% were at local hospitalist-only groups.
Group vs. Individual Incentives
Of the HMG leaders participating in a quality-incentive program:
- 43% reported it was an individual incentive;
- 35% reported it was a group incentive;
- 10% reported the plan had elements of both individual and group incentives; and
- 12% were not sure if their plans had individual or group incentives.
Basis of Quality Targets
Of the HMG leaders reporting that they participate in a quality-incentive program (respondents could indicate one or more answers):
- 60% of the programs have targets based on national benchmarks;
- 23% have targets based on local or regional benchmarks;
- 37% have targets based on their hospital’s previous experience; and
- 47% have targets based on improvement over a baseline.
Maximum Impact of Incentives
Of the HMG leaders reporting that they participate in a quality-incentive program:
- 16% report the maximum impact is less than 3%;
- 24% report the maximum impact is from 3% to 7%;
- 35% report the maximum impact is from 8% to 10%;
- 17% report the maximum impact is from 11% to 20%;
- 3% report the maximum impact is more than 20%; and
- 5% report they do not know the maximum impact.
Group vs. Individual Incentives
Of the HMG leaders reporting that they participate in a quality-incentive program:
- 61% said they have received an incentive payment;
- 37% have not received an incentive payment; and
- 2% were unsure if they have received an incentive payment.
Quality Metrics
The most common metrics used in P4P programs, based on 29 responses to the SHM survey:
- 93% of HM programs have metrics based on The Joint Commission’s (JCAHO) heart failure measures;
- 86% have metrics based on JCAHO pneumonia measures;
- 79% have metrics based on JCAHO myocardial infarction measures;
- 28% have metrics based on a measure of medication reconciliation;
- 24% have metrics based on avoidance of unapproved abbreviations;
- 24% have metrics based on 100,000 Lives Campaign measures;
- 21% have metrics based on patient satisfaction measures;
- 17% have metrics based on transitions-of-care measures;
- 10% have metrics based on throughput measures;
- 7% have metrics based on end-of-life measures;
- 7% have metrics based on “good citizenship” measures;
- 7% have metrics based on mortality rate measures; and
- 7% have metrics based on readmission rate measures.
The most common metrics used in quality-incentive programs, based on 45 responses to SHM’s survey:
- 73% of programs use JCAHO heart failure measures;
- 73% use “good citizenship” measures;
- 73% use patient satisfaction measures;
- 67% use JCAHO pneumonia measures;
- 51% use transitions-of-care measures;
- 44% use JCAHO M.I. measures;
- 31% use throughput measures;
- 27% use avoidance of unapproved abbreviations;
- 24% use a measure based on medication reconciliation;
- 11% use 100,000 Lives Campaign measures;
- 9% use readmission rate measures;
- 7% use mortality rate measures; and
- 2% use end-of-life measures.
Recommendations
The prevalence of hospitalist quality-based compensation plans is continuing to grow rapidly, but the details of the plans’ structure will govern whether they benefit our patients, improve the overall value of the care we provide, and serve as a meaningful component of our compensation. I suggest each practice consider implementing plans with the following attributes:
A total dollar amount available for performance that is enough to influence hospitalist behavior. I think quality incentives should compose as much as 15% to 20% of a hospitalist’s annual income. Plans connecting quality performance to equal to or less than 7% of annual compensation (the case for 40% of groups in the above survey) rarely are effective.
Money vs. metrics. It usually is better to establish a plan based on a sliding scale of improved performance rather than a single threshold. For example, if all of the bonus money is available for a 10% improvement in performance, consider providing 10% of the total available money for each 1% improvement in performance.
Degree of difficulty. Performance thresholds should be set so that hospitalists need to change their practices to achieve them, but not so far out of reach that hospitalists give up on them. This can get tricky. Many practices set thresholds that are very easy to reach (e.g., they may be near the current level of performance).
Metrics for which trusted data is readily available. In most cases, this means using data already being collected. Avoid hard-to-track metrics, as they are likely to lead to disagreements about their accuracy.
Group vs. individual measures. Most performance metrics can’t be clearly attributed to one hospitalist as compared to another. For example, who gets the credit or blame for Ms. Smith getting or not getting a pneumovax? The majority of performance metrics are best measured and paid on a group basis. Some metrics, such as documenting medicine reconciliation on admission and discharge, can be effectively attributed to a single hospitalist and could be paid on an individual basis.
Small number of metrics, A meaningfully large amount of money should be connected to each one. Don’t make the mistake of having a $10,000 per doctor annual quality bonus pool divided among 20 metrics (each metric would pay a maximum of $500 per year).
Rotating metrics. Consider an annual meeting with members of your hospital’s administration to jointly establish the metrics used in the hospitalist quality incentive for that year. It is reasonable to change the metrics periodically.
It seems to me P4P programs are in their infancy, and will continue to evolve rapidly. Plans that fail to improve outcomes enough to justify the complexity of implementing, tracking, and paying for them will disappear slowly. (I wonder if payment for pneumovax administration during the hospital stay will be in this category.) And new, more effective, and more valuable programs will be developed.
Hospitalist practices will need to be nimble to keep pace with all of this change. Although SHM can alert you to how new P4P initiatives might affect your practice, and even recommend methods to improve your performance, you and your hospitalist colleagues still will have a lot of work to operationalize these programs in your practice. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Although there is a lot of debate about the effectiveness of pay-for-performance (P4P) plans, I think the plans are only going to increase in the foreseeable future.
We need more research to tell us the relative impact of public reporting of performance data and P4P programs. Most importantly, the details of how these plans are set up, how and what they measure, and the dollar amount involved will have everything to do with whether they are successful in improving the value of care we provide.
SHM’S Practice Management Committee conducted a mini-survey of hospitalist group leaders in 2006. Here are some of the key findings.
P4P Prevalence
Forty-one percent (60 out of 146) of hospital medicine group (HMG) leaders reported their groups have a quality-incentive program. Of those HMG leaders more likely to report participation in a quality-incentive program:
- 60% were at hospitals participating in a P4P program;
- 50% were at multispecialty/PCP medical groups; and
- 50% were in the Southern region.
Of those HMG leaders less likely to report participation in P4P programs, 28% were at academic programs and 31% were at local hospitalist-only groups.
Group vs. Individual Incentives
Of the HMG leaders participating in a quality-incentive program:
- 43% reported it was an individual incentive;
- 35% reported it was a group incentive;
- 10% reported the plan had elements of both individual and group incentives; and
- 12% were not sure if their plans had individual or group incentives.
Basis of Quality Targets
Of the HMG leaders reporting that they participate in a quality-incentive program (respondents could indicate one or more answers):
- 60% of the programs have targets based on national benchmarks;
- 23% have targets based on local or regional benchmarks;
- 37% have targets based on their hospital’s previous experience; and
- 47% have targets based on improvement over a baseline.
Maximum Impact of Incentives
Of the HMG leaders reporting that they participate in a quality-incentive program:
- 16% report the maximum impact is less than 3%;
- 24% report the maximum impact is from 3% to 7%;
- 35% report the maximum impact is from 8% to 10%;
- 17% report the maximum impact is from 11% to 20%;
- 3% report the maximum impact is more than 20%; and
- 5% report they do not know the maximum impact.
Group vs. Individual Incentives
Of the HMG leaders reporting that they participate in a quality-incentive program:
- 61% said they have received an incentive payment;
- 37% have not received an incentive payment; and
- 2% were unsure if they have received an incentive payment.
Quality Metrics
The most common metrics used in P4P programs, based on 29 responses to the SHM survey:
- 93% of HM programs have metrics based on The Joint Commission’s (JCAHO) heart failure measures;
- 86% have metrics based on JCAHO pneumonia measures;
- 79% have metrics based on JCAHO myocardial infarction measures;
- 28% have metrics based on a measure of medication reconciliation;
- 24% have metrics based on avoidance of unapproved abbreviations;
- 24% have metrics based on 100,000 Lives Campaign measures;
- 21% have metrics based on patient satisfaction measures;
- 17% have metrics based on transitions-of-care measures;
- 10% have metrics based on throughput measures;
- 7% have metrics based on end-of-life measures;
- 7% have metrics based on “good citizenship” measures;
- 7% have metrics based on mortality rate measures; and
- 7% have metrics based on readmission rate measures.
The most common metrics used in quality-incentive programs, based on 45 responses to SHM’s survey:
- 73% of programs use JCAHO heart failure measures;
- 73% use “good citizenship” measures;
- 73% use patient satisfaction measures;
- 67% use JCAHO pneumonia measures;
- 51% use transitions-of-care measures;
- 44% use JCAHO M.I. measures;
- 31% use throughput measures;
- 27% use avoidance of unapproved abbreviations;
- 24% use a measure based on medication reconciliation;
- 11% use 100,000 Lives Campaign measures;
- 9% use readmission rate measures;
- 7% use mortality rate measures; and
- 2% use end-of-life measures.
Recommendations
The prevalence of hospitalist quality-based compensation plans is continuing to grow rapidly, but the details of the plans’ structure will govern whether they benefit our patients, improve the overall value of the care we provide, and serve as a meaningful component of our compensation. I suggest each practice consider implementing plans with the following attributes:
A total dollar amount available for performance that is enough to influence hospitalist behavior. I think quality incentives should compose as much as 15% to 20% of a hospitalist’s annual income. Plans connecting quality performance to equal to or less than 7% of annual compensation (the case for 40% of groups in the above survey) rarely are effective.
Money vs. metrics. It usually is better to establish a plan based on a sliding scale of improved performance rather than a single threshold. For example, if all of the bonus money is available for a 10% improvement in performance, consider providing 10% of the total available money for each 1% improvement in performance.
Degree of difficulty. Performance thresholds should be set so that hospitalists need to change their practices to achieve them, but not so far out of reach that hospitalists give up on them. This can get tricky. Many practices set thresholds that are very easy to reach (e.g., they may be near the current level of performance).
Metrics for which trusted data is readily available. In most cases, this means using data already being collected. Avoid hard-to-track metrics, as they are likely to lead to disagreements about their accuracy.
Group vs. individual measures. Most performance metrics can’t be clearly attributed to one hospitalist as compared to another. For example, who gets the credit or blame for Ms. Smith getting or not getting a pneumovax? The majority of performance metrics are best measured and paid on a group basis. Some metrics, such as documenting medicine reconciliation on admission and discharge, can be effectively attributed to a single hospitalist and could be paid on an individual basis.
Small number of metrics, A meaningfully large amount of money should be connected to each one. Don’t make the mistake of having a $10,000 per doctor annual quality bonus pool divided among 20 metrics (each metric would pay a maximum of $500 per year).
Rotating metrics. Consider an annual meeting with members of your hospital’s administration to jointly establish the metrics used in the hospitalist quality incentive for that year. It is reasonable to change the metrics periodically.
It seems to me P4P programs are in their infancy, and will continue to evolve rapidly. Plans that fail to improve outcomes enough to justify the complexity of implementing, tracking, and paying for them will disappear slowly. (I wonder if payment for pneumovax administration during the hospital stay will be in this category.) And new, more effective, and more valuable programs will be developed.
Hospitalist practices will need to be nimble to keep pace with all of this change. Although SHM can alert you to how new P4P initiatives might affect your practice, and even recommend methods to improve your performance, you and your hospitalist colleagues still will have a lot of work to operationalize these programs in your practice. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
What is the proper duration of antibiotic treatment in adults hospitalized with community-acquired pneumonia?
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Safety in Numbers
Patient safety organizations, commonly referred to as PSOs, are about to take off. And when they do, PSOs should provide hospitalists with invaluable data on improving patient safety.
“PSOs are a great concept, but even though it’s been around since 2005, I haven’t seen it clinically,” says Janet Nagamine, RN, MD, hospitalist at Kaiser Permanente in Santa Clara, Calif., and chair of SHM’s Hospital Quality and Patient Safety Committee.
This calendar year, Nagamine and the rest of hospital medicine should start to see some movement—“PSO 1.0,” if you will.
Background
PSOs are public and private organizations approved by the Agency for Healthcare Research and Quality (AHRQ); they include such groups as Health Watch Inc., Human Performance Technology Group, and the Institute for Safe Medication Practices, which will collect, aggregate, and analyze data on patient safety events. Hospitals and other healthcare providers will voluntarily and confidentially report data. The ultimate goal is to advance changes in culture, processes, and systems to enhance patient safety.
PSOs grew out of the Patient Safety and Quality Improvement Act of 2005, which was a response to the Institute of Medicine’s landmark report “To Err Is Human: Building a Safer Health System.” But it wasn’t until last year that the U.S. Department of Health and Human Services issued a final rule outlining PSO requirements and procedures. The rule became effective Jan. 19, 2009.
AHRQ is responsible for coordinating the development of a set of common definitions and reporting formats, called common formats, for collecting the data. Eventually, AHRQ will create a network of patient safety databases to which PSOs, providers, and others can voluntarily contribute non-identifiable patient safety information. This network will serve as an interactive evidence-based management resource for providers, PSOs, and other entities. AHRQ will use data from the network to analyze national and regional statistics regarding patient safety events. Findings will be made public and will be included in AHRQ’s annual National Healthcare Quality Report.
IT Example
To date, the only comparable data-collection system is MedMarx, which compiles information on medication errors. The Joint Commission requires providers to supply a root-cause analysis on every Level 1 incident, “but that’s just scratching the surface of what occurs,” Dr. Nagamine says. “There are far many more Level 2 and Level 3 events with the same precursors, and that information would be very valuable.”
By collecting nationwide data on patient safety events, PSOs will be able to bridge the gaps in the reporting system and provide crucial patient safety information to the healthcare industry. “In general, the concept of aggregate information that allows us to compare events is incredibly important,” Dr. Nagamine says. “A hospitalist working in one hospital has only the information about events in that hospital, but 5,000 hospitals can provide more specific and actionable information. We just haven’t seen this operationalized yet.”
Dr. Nagamine uses a technology example to show the value PSOs could have in identifying patterns or problems that threaten patient safety: “Every hospital has a horror story of implementing a new information technology (IT) system, and we’re getting some very interesting feedback from hospitals about unintended consequences,” she says. “We’re hearing that patients are being hurt because of mistakes in systems—the use of dropdown menus (on computer screens) that don’t drop down far enough to reveal all options, or a screen where it’s easy to click the wrong item.”
Current systems might not allow problems like these to be highlighted. Even if staff knows of a problem, their hospital’s coding system might not allow them to detail it. “It may fall under ‘communications’ or ‘physician computerized order entry’ or something vague, so the data won’t show the specifics of what happened,” Dr. Nagamine points out. “If we had aggregate data on issues like this, we could address it. Right now, we just have word of mouth.”
An isolated event at a hospital is one thing, but similar data from around the nation is significant. “Drug companies or IT vendors confronted with (patterns) might make some changes,” she says. “That kind of data is powerful.”
Hospital Medicine on Board
When hospitals start reporting data to PSOs, where will hospitalists fit into the process? Hospitalists likely will be interviewed to answer some of the PSO’s questions, but they will not be the ones filling out the forms, Dr. Nagamine says. Hospitalists also will be among the ranks of healthcare professionals eagerly awaiting the release of the data. “The way that PSOs approach patient safety and quality—what’s near and dear to our hearts—is it gives us more data,” Dr. Nagamine says. This is crucial for hospitalists leading quality-improvement projects and similar tasks. “Without that data, it’s hard to get traction and movement. That data will help convince someone to invest more time and money in a particular problem area.”
Phase One: Participation
AHRQ has established a comprehensive Web site (www.pso.ahrq.gov) that includes information on the first draft of common formats for use with hospital inpatients. These are found on downloadable paper forms, available at the PSO Privacy Protection Center (PPC) Web site at www.psoppc.org/ web/patientsafety/paperforms.
“The forms are a first step,” Dr. Nagamine explains. “If we had these data points on every incident at every hospital, we’d know a lot more than we do now. We’d be able to harness that information.”
It will be a while before healthcare providers can search the data for patterns and possible solutions in patient safety, but the wait should be worth it. “You’ve got to start somewhere, and it’s not going to happen in one sweep,” Dr. Nagamine says. “This is simply a start. Hopefully, in a decade, we’ll have a lot more actionable information.” TH
Jane Jerrard is a medical writer based in Chicago.
Patient safety organizations, commonly referred to as PSOs, are about to take off. And when they do, PSOs should provide hospitalists with invaluable data on improving patient safety.
“PSOs are a great concept, but even though it’s been around since 2005, I haven’t seen it clinically,” says Janet Nagamine, RN, MD, hospitalist at Kaiser Permanente in Santa Clara, Calif., and chair of SHM’s Hospital Quality and Patient Safety Committee.
This calendar year, Nagamine and the rest of hospital medicine should start to see some movement—“PSO 1.0,” if you will.
Background
PSOs are public and private organizations approved by the Agency for Healthcare Research and Quality (AHRQ); they include such groups as Health Watch Inc., Human Performance Technology Group, and the Institute for Safe Medication Practices, which will collect, aggregate, and analyze data on patient safety events. Hospitals and other healthcare providers will voluntarily and confidentially report data. The ultimate goal is to advance changes in culture, processes, and systems to enhance patient safety.
PSOs grew out of the Patient Safety and Quality Improvement Act of 2005, which was a response to the Institute of Medicine’s landmark report “To Err Is Human: Building a Safer Health System.” But it wasn’t until last year that the U.S. Department of Health and Human Services issued a final rule outlining PSO requirements and procedures. The rule became effective Jan. 19, 2009.
AHRQ is responsible for coordinating the development of a set of common definitions and reporting formats, called common formats, for collecting the data. Eventually, AHRQ will create a network of patient safety databases to which PSOs, providers, and others can voluntarily contribute non-identifiable patient safety information. This network will serve as an interactive evidence-based management resource for providers, PSOs, and other entities. AHRQ will use data from the network to analyze national and regional statistics regarding patient safety events. Findings will be made public and will be included in AHRQ’s annual National Healthcare Quality Report.
IT Example
To date, the only comparable data-collection system is MedMarx, which compiles information on medication errors. The Joint Commission requires providers to supply a root-cause analysis on every Level 1 incident, “but that’s just scratching the surface of what occurs,” Dr. Nagamine says. “There are far many more Level 2 and Level 3 events with the same precursors, and that information would be very valuable.”
By collecting nationwide data on patient safety events, PSOs will be able to bridge the gaps in the reporting system and provide crucial patient safety information to the healthcare industry. “In general, the concept of aggregate information that allows us to compare events is incredibly important,” Dr. Nagamine says. “A hospitalist working in one hospital has only the information about events in that hospital, but 5,000 hospitals can provide more specific and actionable information. We just haven’t seen this operationalized yet.”
Dr. Nagamine uses a technology example to show the value PSOs could have in identifying patterns or problems that threaten patient safety: “Every hospital has a horror story of implementing a new information technology (IT) system, and we’re getting some very interesting feedback from hospitals about unintended consequences,” she says. “We’re hearing that patients are being hurt because of mistakes in systems—the use of dropdown menus (on computer screens) that don’t drop down far enough to reveal all options, or a screen where it’s easy to click the wrong item.”
Current systems might not allow problems like these to be highlighted. Even if staff knows of a problem, their hospital’s coding system might not allow them to detail it. “It may fall under ‘communications’ or ‘physician computerized order entry’ or something vague, so the data won’t show the specifics of what happened,” Dr. Nagamine points out. “If we had aggregate data on issues like this, we could address it. Right now, we just have word of mouth.”
An isolated event at a hospital is one thing, but similar data from around the nation is significant. “Drug companies or IT vendors confronted with (patterns) might make some changes,” she says. “That kind of data is powerful.”
Hospital Medicine on Board
When hospitals start reporting data to PSOs, where will hospitalists fit into the process? Hospitalists likely will be interviewed to answer some of the PSO’s questions, but they will not be the ones filling out the forms, Dr. Nagamine says. Hospitalists also will be among the ranks of healthcare professionals eagerly awaiting the release of the data. “The way that PSOs approach patient safety and quality—what’s near and dear to our hearts—is it gives us more data,” Dr. Nagamine says. This is crucial for hospitalists leading quality-improvement projects and similar tasks. “Without that data, it’s hard to get traction and movement. That data will help convince someone to invest more time and money in a particular problem area.”
Phase One: Participation
AHRQ has established a comprehensive Web site (www.pso.ahrq.gov) that includes information on the first draft of common formats for use with hospital inpatients. These are found on downloadable paper forms, available at the PSO Privacy Protection Center (PPC) Web site at www.psoppc.org/ web/patientsafety/paperforms.
“The forms are a first step,” Dr. Nagamine explains. “If we had these data points on every incident at every hospital, we’d know a lot more than we do now. We’d be able to harness that information.”
It will be a while before healthcare providers can search the data for patterns and possible solutions in patient safety, but the wait should be worth it. “You’ve got to start somewhere, and it’s not going to happen in one sweep,” Dr. Nagamine says. “This is simply a start. Hopefully, in a decade, we’ll have a lot more actionable information.” TH
Jane Jerrard is a medical writer based in Chicago.
Patient safety organizations, commonly referred to as PSOs, are about to take off. And when they do, PSOs should provide hospitalists with invaluable data on improving patient safety.
“PSOs are a great concept, but even though it’s been around since 2005, I haven’t seen it clinically,” says Janet Nagamine, RN, MD, hospitalist at Kaiser Permanente in Santa Clara, Calif., and chair of SHM’s Hospital Quality and Patient Safety Committee.
This calendar year, Nagamine and the rest of hospital medicine should start to see some movement—“PSO 1.0,” if you will.
Background
PSOs are public and private organizations approved by the Agency for Healthcare Research and Quality (AHRQ); they include such groups as Health Watch Inc., Human Performance Technology Group, and the Institute for Safe Medication Practices, which will collect, aggregate, and analyze data on patient safety events. Hospitals and other healthcare providers will voluntarily and confidentially report data. The ultimate goal is to advance changes in culture, processes, and systems to enhance patient safety.
PSOs grew out of the Patient Safety and Quality Improvement Act of 2005, which was a response to the Institute of Medicine’s landmark report “To Err Is Human: Building a Safer Health System.” But it wasn’t until last year that the U.S. Department of Health and Human Services issued a final rule outlining PSO requirements and procedures. The rule became effective Jan. 19, 2009.
AHRQ is responsible for coordinating the development of a set of common definitions and reporting formats, called common formats, for collecting the data. Eventually, AHRQ will create a network of patient safety databases to which PSOs, providers, and others can voluntarily contribute non-identifiable patient safety information. This network will serve as an interactive evidence-based management resource for providers, PSOs, and other entities. AHRQ will use data from the network to analyze national and regional statistics regarding patient safety events. Findings will be made public and will be included in AHRQ’s annual National Healthcare Quality Report.
IT Example
To date, the only comparable data-collection system is MedMarx, which compiles information on medication errors. The Joint Commission requires providers to supply a root-cause analysis on every Level 1 incident, “but that’s just scratching the surface of what occurs,” Dr. Nagamine says. “There are far many more Level 2 and Level 3 events with the same precursors, and that information would be very valuable.”
By collecting nationwide data on patient safety events, PSOs will be able to bridge the gaps in the reporting system and provide crucial patient safety information to the healthcare industry. “In general, the concept of aggregate information that allows us to compare events is incredibly important,” Dr. Nagamine says. “A hospitalist working in one hospital has only the information about events in that hospital, but 5,000 hospitals can provide more specific and actionable information. We just haven’t seen this operationalized yet.”
Dr. Nagamine uses a technology example to show the value PSOs could have in identifying patterns or problems that threaten patient safety: “Every hospital has a horror story of implementing a new information technology (IT) system, and we’re getting some very interesting feedback from hospitals about unintended consequences,” she says. “We’re hearing that patients are being hurt because of mistakes in systems—the use of dropdown menus (on computer screens) that don’t drop down far enough to reveal all options, or a screen where it’s easy to click the wrong item.”
Current systems might not allow problems like these to be highlighted. Even if staff knows of a problem, their hospital’s coding system might not allow them to detail it. “It may fall under ‘communications’ or ‘physician computerized order entry’ or something vague, so the data won’t show the specifics of what happened,” Dr. Nagamine points out. “If we had aggregate data on issues like this, we could address it. Right now, we just have word of mouth.”
An isolated event at a hospital is one thing, but similar data from around the nation is significant. “Drug companies or IT vendors confronted with (patterns) might make some changes,” she says. “That kind of data is powerful.”
Hospital Medicine on Board
When hospitals start reporting data to PSOs, where will hospitalists fit into the process? Hospitalists likely will be interviewed to answer some of the PSO’s questions, but they will not be the ones filling out the forms, Dr. Nagamine says. Hospitalists also will be among the ranks of healthcare professionals eagerly awaiting the release of the data. “The way that PSOs approach patient safety and quality—what’s near and dear to our hearts—is it gives us more data,” Dr. Nagamine says. This is crucial for hospitalists leading quality-improvement projects and similar tasks. “Without that data, it’s hard to get traction and movement. That data will help convince someone to invest more time and money in a particular problem area.”
Phase One: Participation
AHRQ has established a comprehensive Web site (www.pso.ahrq.gov) that includes information on the first draft of common formats for use with hospital inpatients. These are found on downloadable paper forms, available at the PSO Privacy Protection Center (PPC) Web site at www.psoppc.org/ web/patientsafety/paperforms.
“The forms are a first step,” Dr. Nagamine explains. “If we had these data points on every incident at every hospital, we’d know a lot more than we do now. We’d be able to harness that information.”
It will be a while before healthcare providers can search the data for patterns and possible solutions in patient safety, but the wait should be worth it. “You’ve got to start somewhere, and it’s not going to happen in one sweep,” Dr. Nagamine says. “This is simply a start. Hopefully, in a decade, we’ll have a lot more actionable information.” TH
Jane Jerrard is a medical writer based in Chicago.
Converse Like a Leader
Communication is an integral part of a hospitalist’s job: from admission interviews to conveying orders to nursing staff, communicating clearly and precisely is part of numerous best practices. When a hospitalist assumes a leadership role, however, the types and styles of communication change. A committee chair or department head must be aware of the messages they send—both literally and in the most general sense of the term. This transition to leadership can be tough.
“Physician communication is focused on clinical outcome. That’s easy for someone trained in medicine. But in leadership communication, there may not be a defined outcome,” says Timothy J. Keogh, PhD, assistant professor at The Citadel School of Business Administration in Charleston, S.C. “That’s a difficult switch from clinician to leader; maybe half of the problems a leader faces can’t be solved.”
Dr. Keogh and William F. Martin, PsyD, MPH, summarized their research data in “Managing Medical Groups: 21st Century Challenges and the Impact of Physician Leadership Styles,” published in the September-October 2004 issue of Journal of Medical Practice Management.
The Basics
The most basic communication skill a hospitalist leader should practice, according to Dr. Keogh, is “being less direct than [he or she] would like to be.” Dr. Keogh, who teaches communication skills as part of SHM’s Leadership Academy, says, “Data shows that physicians prefer to be more precise and cover topics quickly. In a leadership role, the initial part of the communication or conversation needs to be chattier. Some physicians believe that this uses up too much time, but, in fact, it doesn’t take that long and it’s a necessary step.” Acknowledge others’ need for connection by making eye contact, pausing, and exchanging quick pleasantries. “Leaders need to be able to say things in passing, greet people, et cetera,” Dr. Keogh stresses.
But what about in-depth communication?
Management Topics
If you supervise hospitalists, you can condense discussions of your expectations—at least compared with managers in business fields. “Physicians are skilled, well-trained individuals, so you don’t have to do so much of this,” Dr. Keogh says. “They have an internal sense of quality and you don’t really need to motivate them. It’s a matter of adjusting the edges.”
A hospitalist supervisor might need to address a situation in which a physician on the team has been disruptive or needs a disciplinary talk. In these instances, Dr. Keogh says, “The leader has to somehow collect data on what the hospitalist has displayed that doesn’t fit in with teamwork. The hard part is that the data is likely to be hearsay—what was said by whom, when. That’s management.”
For example, you might receive complaints from nursing staff of abrupt or rude behavior from a hospitalist. “Physicians may not think of this as data, but it is. What someone said or did, or gestured,” Dr. Keogh points out. “You have to be able to say, ‘Here’s what we know happened on this date. Help me understand what happened, because we have to change this. This behavior is not acceptable.’”
The key to all official management communication is to carefully consider how to frame the conversation and keep it flowing for both parties: Speak with hospitalists, not to them. Here is an example: “In a performance appraisal, one suggestion we make is to have a conversation about data,” Dr. Keogh explains. “Look at some numbers on quality assurance, patient load, or whatever you’re discussing. Do your homework and allow the other person to have a look at the data before you sit down with them. That’s a sign of respect.”
Styles of Communication
Dr. Keogh’s training for physician executives—including what he teaches at the Leadership Academy—is based on the personality profile system developed by Carlson Learning Company (now Inscape Publishing).1 The DiSC model outlines behavior or characteristic leadership preferences in four dimensions:
- Dominance (D): People who score high in this category have behavioral characteristics that include being motivated by control over tasks and work environment, directing others, and achieving specific stretch goals. In general, physician managers who score high on dominance tend to be results-focused, fast-paced, and value autonomy.
- Influence (I): People who score high in this category are motivated by interacting with others, giving and receiving immediate feedback, and acknowledging emotions as well as facts.
- Steadiness (S): People who score high in this category are motivated by job security, predictability, and clearly defined expectations.
- Conscientiousness (C): People who score high in this category are motivated by needing to be right, working alone, and preferring to work on tasks rather than dealing with people.
“This model provides groundwork for seeing that other people have different ways, different preferences of communicating,” Dr. Keogh says. In his own research, he says, he has found “nearly half of all physicians are some combination of time-sensitive and perfectionist,” meaning they fit the dominance style.
The trick to effective communication is learning to modify your style when necessary. Task-focused individuals must practice taking a minute for a greeting or a pleasantry, even if it initially goes against their routine. “Someone who is extremely outgoing and open can have trouble, too,” Dr. Keogh points out. “You can’t start a conversation with a lot of chit-chat, if you’re addressing someone who is direct. … That won’t work, either.”
The solution is to practice stepping outside of your normal communication style. “You can learn how to adjust your style, how to flex to others’ styles,” Dr. Keogh says. “Depending on whom you’re communicating with, you can mirror the style of the other person.” This helps to ensure that what you’re saying is received and understood.
Practicing new ways to communicate means making a fundamental shift in your behavior. It sounds difficult, but Dr. Keogh promises it’s not.
“The transition is not as hard as you think, because hospitalists have been trained to do patient interviews,” he says. “They’re skilled at observation and listening. In the transition to leadership, they sometimes forget that they have these skills and can use these to be a great leader.”” TH
Jane Jerrard is a medical writer based in Chicago. She also writes “Public Policy” for The Hospitalist.
Reference
1. Straw J. The 4-Dimensional Manager: DiSC Strategies for Managing Different People in the Best Ways. San Francisco: Berrett-Koehler Publishers, Inc.; 2002.
Communication is an integral part of a hospitalist’s job: from admission interviews to conveying orders to nursing staff, communicating clearly and precisely is part of numerous best practices. When a hospitalist assumes a leadership role, however, the types and styles of communication change. A committee chair or department head must be aware of the messages they send—both literally and in the most general sense of the term. This transition to leadership can be tough.
“Physician communication is focused on clinical outcome. That’s easy for someone trained in medicine. But in leadership communication, there may not be a defined outcome,” says Timothy J. Keogh, PhD, assistant professor at The Citadel School of Business Administration in Charleston, S.C. “That’s a difficult switch from clinician to leader; maybe half of the problems a leader faces can’t be solved.”
Dr. Keogh and William F. Martin, PsyD, MPH, summarized their research data in “Managing Medical Groups: 21st Century Challenges and the Impact of Physician Leadership Styles,” published in the September-October 2004 issue of Journal of Medical Practice Management.
The Basics
The most basic communication skill a hospitalist leader should practice, according to Dr. Keogh, is “being less direct than [he or she] would like to be.” Dr. Keogh, who teaches communication skills as part of SHM’s Leadership Academy, says, “Data shows that physicians prefer to be more precise and cover topics quickly. In a leadership role, the initial part of the communication or conversation needs to be chattier. Some physicians believe that this uses up too much time, but, in fact, it doesn’t take that long and it’s a necessary step.” Acknowledge others’ need for connection by making eye contact, pausing, and exchanging quick pleasantries. “Leaders need to be able to say things in passing, greet people, et cetera,” Dr. Keogh stresses.
But what about in-depth communication?
Management Topics
If you supervise hospitalists, you can condense discussions of your expectations—at least compared with managers in business fields. “Physicians are skilled, well-trained individuals, so you don’t have to do so much of this,” Dr. Keogh says. “They have an internal sense of quality and you don’t really need to motivate them. It’s a matter of adjusting the edges.”
A hospitalist supervisor might need to address a situation in which a physician on the team has been disruptive or needs a disciplinary talk. In these instances, Dr. Keogh says, “The leader has to somehow collect data on what the hospitalist has displayed that doesn’t fit in with teamwork. The hard part is that the data is likely to be hearsay—what was said by whom, when. That’s management.”
For example, you might receive complaints from nursing staff of abrupt or rude behavior from a hospitalist. “Physicians may not think of this as data, but it is. What someone said or did, or gestured,” Dr. Keogh points out. “You have to be able to say, ‘Here’s what we know happened on this date. Help me understand what happened, because we have to change this. This behavior is not acceptable.’”
The key to all official management communication is to carefully consider how to frame the conversation and keep it flowing for both parties: Speak with hospitalists, not to them. Here is an example: “In a performance appraisal, one suggestion we make is to have a conversation about data,” Dr. Keogh explains. “Look at some numbers on quality assurance, patient load, or whatever you’re discussing. Do your homework and allow the other person to have a look at the data before you sit down with them. That’s a sign of respect.”
Styles of Communication
Dr. Keogh’s training for physician executives—including what he teaches at the Leadership Academy—is based on the personality profile system developed by Carlson Learning Company (now Inscape Publishing).1 The DiSC model outlines behavior or characteristic leadership preferences in four dimensions:
- Dominance (D): People who score high in this category have behavioral characteristics that include being motivated by control over tasks and work environment, directing others, and achieving specific stretch goals. In general, physician managers who score high on dominance tend to be results-focused, fast-paced, and value autonomy.
- Influence (I): People who score high in this category are motivated by interacting with others, giving and receiving immediate feedback, and acknowledging emotions as well as facts.
- Steadiness (S): People who score high in this category are motivated by job security, predictability, and clearly defined expectations.
- Conscientiousness (C): People who score high in this category are motivated by needing to be right, working alone, and preferring to work on tasks rather than dealing with people.
“This model provides groundwork for seeing that other people have different ways, different preferences of communicating,” Dr. Keogh says. In his own research, he says, he has found “nearly half of all physicians are some combination of time-sensitive and perfectionist,” meaning they fit the dominance style.
The trick to effective communication is learning to modify your style when necessary. Task-focused individuals must practice taking a minute for a greeting or a pleasantry, even if it initially goes against their routine. “Someone who is extremely outgoing and open can have trouble, too,” Dr. Keogh points out. “You can’t start a conversation with a lot of chit-chat, if you’re addressing someone who is direct. … That won’t work, either.”
The solution is to practice stepping outside of your normal communication style. “You can learn how to adjust your style, how to flex to others’ styles,” Dr. Keogh says. “Depending on whom you’re communicating with, you can mirror the style of the other person.” This helps to ensure that what you’re saying is received and understood.
Practicing new ways to communicate means making a fundamental shift in your behavior. It sounds difficult, but Dr. Keogh promises it’s not.
“The transition is not as hard as you think, because hospitalists have been trained to do patient interviews,” he says. “They’re skilled at observation and listening. In the transition to leadership, they sometimes forget that they have these skills and can use these to be a great leader.”” TH
Jane Jerrard is a medical writer based in Chicago. She also writes “Public Policy” for The Hospitalist.
Reference
1. Straw J. The 4-Dimensional Manager: DiSC Strategies for Managing Different People in the Best Ways. San Francisco: Berrett-Koehler Publishers, Inc.; 2002.
Communication is an integral part of a hospitalist’s job: from admission interviews to conveying orders to nursing staff, communicating clearly and precisely is part of numerous best practices. When a hospitalist assumes a leadership role, however, the types and styles of communication change. A committee chair or department head must be aware of the messages they send—both literally and in the most general sense of the term. This transition to leadership can be tough.
“Physician communication is focused on clinical outcome. That’s easy for someone trained in medicine. But in leadership communication, there may not be a defined outcome,” says Timothy J. Keogh, PhD, assistant professor at The Citadel School of Business Administration in Charleston, S.C. “That’s a difficult switch from clinician to leader; maybe half of the problems a leader faces can’t be solved.”
Dr. Keogh and William F. Martin, PsyD, MPH, summarized their research data in “Managing Medical Groups: 21st Century Challenges and the Impact of Physician Leadership Styles,” published in the September-October 2004 issue of Journal of Medical Practice Management.
The Basics
The most basic communication skill a hospitalist leader should practice, according to Dr. Keogh, is “being less direct than [he or she] would like to be.” Dr. Keogh, who teaches communication skills as part of SHM’s Leadership Academy, says, “Data shows that physicians prefer to be more precise and cover topics quickly. In a leadership role, the initial part of the communication or conversation needs to be chattier. Some physicians believe that this uses up too much time, but, in fact, it doesn’t take that long and it’s a necessary step.” Acknowledge others’ need for connection by making eye contact, pausing, and exchanging quick pleasantries. “Leaders need to be able to say things in passing, greet people, et cetera,” Dr. Keogh stresses.
But what about in-depth communication?
Management Topics
If you supervise hospitalists, you can condense discussions of your expectations—at least compared with managers in business fields. “Physicians are skilled, well-trained individuals, so you don’t have to do so much of this,” Dr. Keogh says. “They have an internal sense of quality and you don’t really need to motivate them. It’s a matter of adjusting the edges.”
A hospitalist supervisor might need to address a situation in which a physician on the team has been disruptive or needs a disciplinary talk. In these instances, Dr. Keogh says, “The leader has to somehow collect data on what the hospitalist has displayed that doesn’t fit in with teamwork. The hard part is that the data is likely to be hearsay—what was said by whom, when. That’s management.”
For example, you might receive complaints from nursing staff of abrupt or rude behavior from a hospitalist. “Physicians may not think of this as data, but it is. What someone said or did, or gestured,” Dr. Keogh points out. “You have to be able to say, ‘Here’s what we know happened on this date. Help me understand what happened, because we have to change this. This behavior is not acceptable.’”
The key to all official management communication is to carefully consider how to frame the conversation and keep it flowing for both parties: Speak with hospitalists, not to them. Here is an example: “In a performance appraisal, one suggestion we make is to have a conversation about data,” Dr. Keogh explains. “Look at some numbers on quality assurance, patient load, or whatever you’re discussing. Do your homework and allow the other person to have a look at the data before you sit down with them. That’s a sign of respect.”
Styles of Communication
Dr. Keogh’s training for physician executives—including what he teaches at the Leadership Academy—is based on the personality profile system developed by Carlson Learning Company (now Inscape Publishing).1 The DiSC model outlines behavior or characteristic leadership preferences in four dimensions:
- Dominance (D): People who score high in this category have behavioral characteristics that include being motivated by control over tasks and work environment, directing others, and achieving specific stretch goals. In general, physician managers who score high on dominance tend to be results-focused, fast-paced, and value autonomy.
- Influence (I): People who score high in this category are motivated by interacting with others, giving and receiving immediate feedback, and acknowledging emotions as well as facts.
- Steadiness (S): People who score high in this category are motivated by job security, predictability, and clearly defined expectations.
- Conscientiousness (C): People who score high in this category are motivated by needing to be right, working alone, and preferring to work on tasks rather than dealing with people.
“This model provides groundwork for seeing that other people have different ways, different preferences of communicating,” Dr. Keogh says. In his own research, he says, he has found “nearly half of all physicians are some combination of time-sensitive and perfectionist,” meaning they fit the dominance style.
The trick to effective communication is learning to modify your style when necessary. Task-focused individuals must practice taking a minute for a greeting or a pleasantry, even if it initially goes against their routine. “Someone who is extremely outgoing and open can have trouble, too,” Dr. Keogh points out. “You can’t start a conversation with a lot of chit-chat, if you’re addressing someone who is direct. … That won’t work, either.”
The solution is to practice stepping outside of your normal communication style. “You can learn how to adjust your style, how to flex to others’ styles,” Dr. Keogh says. “Depending on whom you’re communicating with, you can mirror the style of the other person.” This helps to ensure that what you’re saying is received and understood.
Practicing new ways to communicate means making a fundamental shift in your behavior. It sounds difficult, but Dr. Keogh promises it’s not.
“The transition is not as hard as you think, because hospitalists have been trained to do patient interviews,” he says. “They’re skilled at observation and listening. In the transition to leadership, they sometimes forget that they have these skills and can use these to be a great leader.”” TH
Jane Jerrard is a medical writer based in Chicago. She also writes “Public Policy” for The Hospitalist.
Reference
1. Straw J. The 4-Dimensional Manager: DiSC Strategies for Managing Different People in the Best Ways. San Francisco: Berrett-Koehler Publishers, Inc.; 2002.
Never-Event Implications
The Center for Medicare and Medicaid Services (CMS) recently announced federal payor programs no longer reimburses for medical services rendered to treat certain complications of care. Although CMS chose the majority of these complications because they are “reasonably preventable by following evidence-based guidelines,” the national media and patient advocacy groups have adopted the term “never events” to describe them.
Aside from the payment implications, CMS’ new policy affects the liability risk of any person providing inpatient care, regardless of whether a federal payor is involved.
In its press release announcing the new payment policy, CMS stated, “when you enter the hospital for treatment of one medical problem, you don’t expect to leave with additional injuries, infections, or serious conditions that occur during the course of your stay.” Recognizing “some of these complications may not be avoidable,” CMS found “too often patients suffer from injuries or illnesses that could have been prevented if the hospital had taken proper precautions.”
Consequently, “as part of its commitment to improve the quality of care [patients] receive during a hospital stay,” CMS policy is targeted at reducing “hospital-acquired conditions like certain infections, advanced bed sores, or fractures;” and “preventable medical errors, like performing surgery on the wrong side of the body, that should never happen.”
The list of “never events” covered under the CMS payment policy can be organized into three categories: surgical events, medical products and devices, and case management. The following breaks down each category:
Case Management
- Stage III and Stage IV pressure ulcers;
- Air embolism;
- Manifestations of poor control of blood sugar levels; and
- Fracture, burns, joint dislocations, and other injuries occurring from falls or other trauma suffered while an inpatient.
Surgical Events
- Surgery on wrong body part;
- Surgery on the wrong patient;
- Wrong surgery on a patient;
- Retention of a foreign object, such as a sponge or needle, inadvertently left in a patient after surgery;
- Surgical site infection following a coronary artery bypass graft;
- Surgical site infection following bariatric surgery;
- Surgical site infection following certain orthopedic procedures; and
- Deep vein thrombosis or pulmonary embolism following certain orthopedic procedures.
Medical Products and Devices
- Transfusion of wrong blood type;
- Catheter associated urinary tract infection; and
- Vascular catheter associated infections.
It’s easy to see why some of the complications made the list. Wrong-side surgery or surgery on the wrong patient are the quintessential cases where liability is generally uncontested. There is not much one can do to satisfactorily explain to a patient, or a jury, why a surgeon and surgical team operated on the wrong body part.
In other cases, however, such as fatal pulmonary embolus, death can occur even when a patient has been appropriately managed. In fact, medical literature demonstrates a small percentage of patients will develop deep vein thrombosis or pulmonary embolus even after having received therapeutic doses of heparin.
Reasonable Expectations
In any case involving a “never event,” we expect plaintiffs’ attorneys to argue CMS’ reimbursement determination is tantamount to a finding of substandard care. In other words, plaintiffs’ attorneys will argue a physician acted negligently simply because the patient incurred one of the proscribed complications. It’s a compelling argument because the federal government has essentially concluded these complications do not occur if physicians and hospitals pay attention while providing care.
You may have heard the Latin phrase res ipsa loquitur; it translates to “the thing speaks for itself.” Legally, res ipsa loquitur states a rule of law where a jury must presume a defendant was negligent when a certain type of injury occurs. The burden then shifts to the defendant to prove the injury occurred in the absence of negligence. The res ipsa rule originated in 1863 when a plaintiff was struck by a barrel of flour falling from a second-story window. The barrel caused the judge hearing the case to remark, “It is the duty of persons who keep barrels in a warehouse to take care that they do not roll out, and I think that such a case would, beyond all doubt, afford prima facie evidence of negligence. A barrel could not roll out of a warehouse without some negligence. … [I]f there are any facts inconsistent with negligence, it is for the defendant to prove them.” Thus, res ipsa is grounded in the notion everyone knows barrels aren’t supposed to fall from second-floor windows.
Traditionally, res ipsa applied only in a small class of medical malpractice cases, such as retained objects following surgery. In such cases, jurors are just as capable as medical professionals in understanding someone was negligent. For example, it does not take expert testimony to establish there has been negligence when a surgical instrument is left in a patient. There’s simply no compelling medical reason for a surgeon to leave an instrument in a patient’s abdomen.
In contrast, res ipsa generally has not applied in cases involving pulmonary embolus because the process of thromboembolic disease is beyond the average juror’s understanding and death by pulmonary embolus would not give rise to a presumption of negligence.
Where res ipsa applies, it’s a powerful concept. If res ipsa were found to apply to pulmonary embolus cases, the jury would be instructed it is the duty of a physician caring for a post-surgical patient to take care that the patient does not develop pulmonary embolus. Thus, a jury would begin with the presumption a patient would not develop pulmonary embolus absent negligence. The physician would then be left with the burden to prove otherwise. Given such a charge, it is foreseeable a jury could return a verdict against a physician, even if the physician managed the patient’s care appropriately and ordered appropriate prophylaxis.
Take Extra Precaution
To prevent CMS' reimbursement decisions from becoming the functional equivalent of a res ipsa instruction, physicians need to raise the level of precaution they employ against “never event” complications. At the heart of CMS' decision is its statement “never event” complications are “reasonably preventable by following evidence-based guidelines.” When a condition is only “reasonably preventable,” instead of “absolutely preventable,” a defense lawyer retains the ability to argue some patients will develop the condition even when the care was entirely appropriate.
We believe most jurors understand the inherent difficulties of caring for sick patients, and the risks that exist every time a patient undergoes a surgical procedure. The defense lawyer’s challenge is convincing a jury the patient received appropriate care, notwithstanding the complication.
Because CMS refers to “evidence-based guidelines,” physicians must know and follow the guidelines. The first step is becoming familiar with the complications CMS will deny reimbursement, and then regularly review the available guidelines to identify practices to reduce or eliminate the complication. Re-evaluate and update your practice whenever new information becomes available.
A consistent cycle of evaluating and responding to complications will afford the defense lawyer the ability to argue the physician and hospital complied with “evidence-based guidelines” and the patient’s case represents one of the unfortunate incidents where a patient suffers a complication despite receiving the highest-level of care.
In our experience, many providers initially create good systems, but run into trouble in the follow up. Make sure you respond to new or additional information or methods of practice. Without this follow up, CMS’ reimbursement decisions have the potential to create malpractice liabilities for all inpatient providers. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado Denver.
The Center for Medicare and Medicaid Services (CMS) recently announced federal payor programs no longer reimburses for medical services rendered to treat certain complications of care. Although CMS chose the majority of these complications because they are “reasonably preventable by following evidence-based guidelines,” the national media and patient advocacy groups have adopted the term “never events” to describe them.
Aside from the payment implications, CMS’ new policy affects the liability risk of any person providing inpatient care, regardless of whether a federal payor is involved.
In its press release announcing the new payment policy, CMS stated, “when you enter the hospital for treatment of one medical problem, you don’t expect to leave with additional injuries, infections, or serious conditions that occur during the course of your stay.” Recognizing “some of these complications may not be avoidable,” CMS found “too often patients suffer from injuries or illnesses that could have been prevented if the hospital had taken proper precautions.”
Consequently, “as part of its commitment to improve the quality of care [patients] receive during a hospital stay,” CMS policy is targeted at reducing “hospital-acquired conditions like certain infections, advanced bed sores, or fractures;” and “preventable medical errors, like performing surgery on the wrong side of the body, that should never happen.”
The list of “never events” covered under the CMS payment policy can be organized into three categories: surgical events, medical products and devices, and case management. The following breaks down each category:
Case Management
- Stage III and Stage IV pressure ulcers;
- Air embolism;
- Manifestations of poor control of blood sugar levels; and
- Fracture, burns, joint dislocations, and other injuries occurring from falls or other trauma suffered while an inpatient.
Surgical Events
- Surgery on wrong body part;
- Surgery on the wrong patient;
- Wrong surgery on a patient;
- Retention of a foreign object, such as a sponge or needle, inadvertently left in a patient after surgery;
- Surgical site infection following a coronary artery bypass graft;
- Surgical site infection following bariatric surgery;
- Surgical site infection following certain orthopedic procedures; and
- Deep vein thrombosis or pulmonary embolism following certain orthopedic procedures.
Medical Products and Devices
- Transfusion of wrong blood type;
- Catheter associated urinary tract infection; and
- Vascular catheter associated infections.
It’s easy to see why some of the complications made the list. Wrong-side surgery or surgery on the wrong patient are the quintessential cases where liability is generally uncontested. There is not much one can do to satisfactorily explain to a patient, or a jury, why a surgeon and surgical team operated on the wrong body part.
In other cases, however, such as fatal pulmonary embolus, death can occur even when a patient has been appropriately managed. In fact, medical literature demonstrates a small percentage of patients will develop deep vein thrombosis or pulmonary embolus even after having received therapeutic doses of heparin.
Reasonable Expectations
In any case involving a “never event,” we expect plaintiffs’ attorneys to argue CMS’ reimbursement determination is tantamount to a finding of substandard care. In other words, plaintiffs’ attorneys will argue a physician acted negligently simply because the patient incurred one of the proscribed complications. It’s a compelling argument because the federal government has essentially concluded these complications do not occur if physicians and hospitals pay attention while providing care.
You may have heard the Latin phrase res ipsa loquitur; it translates to “the thing speaks for itself.” Legally, res ipsa loquitur states a rule of law where a jury must presume a defendant was negligent when a certain type of injury occurs. The burden then shifts to the defendant to prove the injury occurred in the absence of negligence. The res ipsa rule originated in 1863 when a plaintiff was struck by a barrel of flour falling from a second-story window. The barrel caused the judge hearing the case to remark, “It is the duty of persons who keep barrels in a warehouse to take care that they do not roll out, and I think that such a case would, beyond all doubt, afford prima facie evidence of negligence. A barrel could not roll out of a warehouse without some negligence. … [I]f there are any facts inconsistent with negligence, it is for the defendant to prove them.” Thus, res ipsa is grounded in the notion everyone knows barrels aren’t supposed to fall from second-floor windows.
Traditionally, res ipsa applied only in a small class of medical malpractice cases, such as retained objects following surgery. In such cases, jurors are just as capable as medical professionals in understanding someone was negligent. For example, it does not take expert testimony to establish there has been negligence when a surgical instrument is left in a patient. There’s simply no compelling medical reason for a surgeon to leave an instrument in a patient’s abdomen.
In contrast, res ipsa generally has not applied in cases involving pulmonary embolus because the process of thromboembolic disease is beyond the average juror’s understanding and death by pulmonary embolus would not give rise to a presumption of negligence.
Where res ipsa applies, it’s a powerful concept. If res ipsa were found to apply to pulmonary embolus cases, the jury would be instructed it is the duty of a physician caring for a post-surgical patient to take care that the patient does not develop pulmonary embolus. Thus, a jury would begin with the presumption a patient would not develop pulmonary embolus absent negligence. The physician would then be left with the burden to prove otherwise. Given such a charge, it is foreseeable a jury could return a verdict against a physician, even if the physician managed the patient’s care appropriately and ordered appropriate prophylaxis.
Take Extra Precaution
To prevent CMS' reimbursement decisions from becoming the functional equivalent of a res ipsa instruction, physicians need to raise the level of precaution they employ against “never event” complications. At the heart of CMS' decision is its statement “never event” complications are “reasonably preventable by following evidence-based guidelines.” When a condition is only “reasonably preventable,” instead of “absolutely preventable,” a defense lawyer retains the ability to argue some patients will develop the condition even when the care was entirely appropriate.
We believe most jurors understand the inherent difficulties of caring for sick patients, and the risks that exist every time a patient undergoes a surgical procedure. The defense lawyer’s challenge is convincing a jury the patient received appropriate care, notwithstanding the complication.
Because CMS refers to “evidence-based guidelines,” physicians must know and follow the guidelines. The first step is becoming familiar with the complications CMS will deny reimbursement, and then regularly review the available guidelines to identify practices to reduce or eliminate the complication. Re-evaluate and update your practice whenever new information becomes available.
A consistent cycle of evaluating and responding to complications will afford the defense lawyer the ability to argue the physician and hospital complied with “evidence-based guidelines” and the patient’s case represents one of the unfortunate incidents where a patient suffers a complication despite receiving the highest-level of care.
In our experience, many providers initially create good systems, but run into trouble in the follow up. Make sure you respond to new or additional information or methods of practice. Without this follow up, CMS’ reimbursement decisions have the potential to create malpractice liabilities for all inpatient providers. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado Denver.
The Center for Medicare and Medicaid Services (CMS) recently announced federal payor programs no longer reimburses for medical services rendered to treat certain complications of care. Although CMS chose the majority of these complications because they are “reasonably preventable by following evidence-based guidelines,” the national media and patient advocacy groups have adopted the term “never events” to describe them.
Aside from the payment implications, CMS’ new policy affects the liability risk of any person providing inpatient care, regardless of whether a federal payor is involved.
In its press release announcing the new payment policy, CMS stated, “when you enter the hospital for treatment of one medical problem, you don’t expect to leave with additional injuries, infections, or serious conditions that occur during the course of your stay.” Recognizing “some of these complications may not be avoidable,” CMS found “too often patients suffer from injuries or illnesses that could have been prevented if the hospital had taken proper precautions.”
Consequently, “as part of its commitment to improve the quality of care [patients] receive during a hospital stay,” CMS policy is targeted at reducing “hospital-acquired conditions like certain infections, advanced bed sores, or fractures;” and “preventable medical errors, like performing surgery on the wrong side of the body, that should never happen.”
The list of “never events” covered under the CMS payment policy can be organized into three categories: surgical events, medical products and devices, and case management. The following breaks down each category:
Case Management
- Stage III and Stage IV pressure ulcers;
- Air embolism;
- Manifestations of poor control of blood sugar levels; and
- Fracture, burns, joint dislocations, and other injuries occurring from falls or other trauma suffered while an inpatient.
Surgical Events
- Surgery on wrong body part;
- Surgery on the wrong patient;
- Wrong surgery on a patient;
- Retention of a foreign object, such as a sponge or needle, inadvertently left in a patient after surgery;
- Surgical site infection following a coronary artery bypass graft;
- Surgical site infection following bariatric surgery;
- Surgical site infection following certain orthopedic procedures; and
- Deep vein thrombosis or pulmonary embolism following certain orthopedic procedures.
Medical Products and Devices
- Transfusion of wrong blood type;
- Catheter associated urinary tract infection; and
- Vascular catheter associated infections.
It’s easy to see why some of the complications made the list. Wrong-side surgery or surgery on the wrong patient are the quintessential cases where liability is generally uncontested. There is not much one can do to satisfactorily explain to a patient, or a jury, why a surgeon and surgical team operated on the wrong body part.
In other cases, however, such as fatal pulmonary embolus, death can occur even when a patient has been appropriately managed. In fact, medical literature demonstrates a small percentage of patients will develop deep vein thrombosis or pulmonary embolus even after having received therapeutic doses of heparin.
Reasonable Expectations
In any case involving a “never event,” we expect plaintiffs’ attorneys to argue CMS’ reimbursement determination is tantamount to a finding of substandard care. In other words, plaintiffs’ attorneys will argue a physician acted negligently simply because the patient incurred one of the proscribed complications. It’s a compelling argument because the federal government has essentially concluded these complications do not occur if physicians and hospitals pay attention while providing care.
You may have heard the Latin phrase res ipsa loquitur; it translates to “the thing speaks for itself.” Legally, res ipsa loquitur states a rule of law where a jury must presume a defendant was negligent when a certain type of injury occurs. The burden then shifts to the defendant to prove the injury occurred in the absence of negligence. The res ipsa rule originated in 1863 when a plaintiff was struck by a barrel of flour falling from a second-story window. The barrel caused the judge hearing the case to remark, “It is the duty of persons who keep barrels in a warehouse to take care that they do not roll out, and I think that such a case would, beyond all doubt, afford prima facie evidence of negligence. A barrel could not roll out of a warehouse without some negligence. … [I]f there are any facts inconsistent with negligence, it is for the defendant to prove them.” Thus, res ipsa is grounded in the notion everyone knows barrels aren’t supposed to fall from second-floor windows.
Traditionally, res ipsa applied only in a small class of medical malpractice cases, such as retained objects following surgery. In such cases, jurors are just as capable as medical professionals in understanding someone was negligent. For example, it does not take expert testimony to establish there has been negligence when a surgical instrument is left in a patient. There’s simply no compelling medical reason for a surgeon to leave an instrument in a patient’s abdomen.
In contrast, res ipsa generally has not applied in cases involving pulmonary embolus because the process of thromboembolic disease is beyond the average juror’s understanding and death by pulmonary embolus would not give rise to a presumption of negligence.
Where res ipsa applies, it’s a powerful concept. If res ipsa were found to apply to pulmonary embolus cases, the jury would be instructed it is the duty of a physician caring for a post-surgical patient to take care that the patient does not develop pulmonary embolus. Thus, a jury would begin with the presumption a patient would not develop pulmonary embolus absent negligence. The physician would then be left with the burden to prove otherwise. Given such a charge, it is foreseeable a jury could return a verdict against a physician, even if the physician managed the patient’s care appropriately and ordered appropriate prophylaxis.
Take Extra Precaution
To prevent CMS' reimbursement decisions from becoming the functional equivalent of a res ipsa instruction, physicians need to raise the level of precaution they employ against “never event” complications. At the heart of CMS' decision is its statement “never event” complications are “reasonably preventable by following evidence-based guidelines.” When a condition is only “reasonably preventable,” instead of “absolutely preventable,” a defense lawyer retains the ability to argue some patients will develop the condition even when the care was entirely appropriate.
We believe most jurors understand the inherent difficulties of caring for sick patients, and the risks that exist every time a patient undergoes a surgical procedure. The defense lawyer’s challenge is convincing a jury the patient received appropriate care, notwithstanding the complication.
Because CMS refers to “evidence-based guidelines,” physicians must know and follow the guidelines. The first step is becoming familiar with the complications CMS will deny reimbursement, and then regularly review the available guidelines to identify practices to reduce or eliminate the complication. Re-evaluate and update your practice whenever new information becomes available.
A consistent cycle of evaluating and responding to complications will afford the defense lawyer the ability to argue the physician and hospital complied with “evidence-based guidelines” and the patient’s case represents one of the unfortunate incidents where a patient suffers a complication despite receiving the highest-level of care.
In our experience, many providers initially create good systems, but run into trouble in the follow up. Make sure you respond to new or additional information or methods of practice. Without this follow up, CMS’ reimbursement decisions have the potential to create malpractice liabilities for all inpatient providers. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado Denver.
Focused on Patient Care
Julia S. Wright, MD, spent 10 years in traditional practice before joining the University of Wisconsin School of Medicine and Public Health in Madison. Now a clinical associate professor and section head of hospital medicine, the title has changed and the professional demands have intensified. The mission, however, remains the same.
Every decision she’s made in her career—whether it be medical, managerial, financial, or even her seemingly curious choice of college majors—has been guided by one basic question: What will this mean for the quality of patient care? “It may sound cliché, but that’s always the bottom line,” Dr. Wright says. “It’s why I do what I do.”
You spent 10 years in traditional practice before becoming a hospitalist. How did that prepare you for what you’re doing now?
Answer: That experience is a tremendous asset. I made the switch to inpatient medicine because that was the arena I enjoyed the most. But having had experience in the outpatient setting gives me a better understanding of the continuum of care, and my clinical skills have been honed by that experience. I also have an understanding of the strengths of the primary care physicians (PCP), as well as the breadth of the problems they are able to take on.
How does that help you?
A: It really helps in the patient transition, especially on the return side, when patients go back to their PCP after hospitalization. Having an understanding of what resources are available to PCPs helps to determine an appropriate time to discharge a patient. Someone without that experience may have a tendency to think every loose end has to be tied up, or they may make changes that wind up making things more difficult for PCP. But if you have an understanding of a PCP’s capabilities, maybe the patient can be discharged a little earlier.
The University of Wisconsin’s hospitalist program started in 2005. You were named director in 2006. How exciting is it to guide a new program and help it develop?
A: It’s been a tremendous experience. When I joined, we had six hospitalists. Now we have 19. But it was strategic growth; it wasn’t haphazard.
What do you mean by strategic growth?
A: We didn’t simply say, ‘We need more people.’ Every time we brought somebody on, we really made sure it was going to be someone who would add value to the program and be beneficial to the hospital. We were worried, as we got bigger, that it’d be hard to keep the same cohesiveness and that same espirit de corps. During the interviews and the search process, we’re not just asking, ‘Are they qualified?’ We’re asking, ‘Are they a really good fit?’ And everybody has different areas of interest within the group. Some are more research-inclined; one developed a curriculum; some are more clinically oriented. That allows each hospitalist to have an identity in the group. That translated into a good expansion, and it’s been a win-win for us.
What makes someone a good fit for your program?
A: It sounds cliché, but I think the key to a strong developing program is to have a strong sense of what the group values. For us, we value the academic experience. For people going into academic medicine, the struggle is to be able to provide value to the hospital and yet stay academic. Our hospitalists are very clear—that’s our vision. With the support of the hospital and the department, we were able to do both at the same time.
Can you pinpoint one experience that you’ve had that made you realize you’re doing what you’re meant to do?
A: The biggest reward is always patient care. I enjoy the direct patient experience as much as I always have, and that’s what keeps me in the game.
What have you enjoyed most about your transition to a leadership role?
A: I really enjoy the position, not because of the hierarchy, but because of the opportunities afforded by it. I get to interact with hospital medicine staff and the department of medicine chair, and the vice chairs. I’ve been able to interact with others in hospital medicine across the country, and that has been a great experience.
Some hospitalists enjoy what they do because they don’t have to handle the business side of operations or deal with the administrative hassles that private physicians face. In your role, though, you do have to face those challenges. Is that a drawback?
A: I do have to pay attention to the numbers. That’s the bottom line. But it’s something I actually really enjoy. When it comes to awareness of the balance sheet, there’s a division between the leadership level and the clinician level. It’s hard to bridge that chasm of, ‘I’m here for patient care and I don’t necessarily focus on the numbers.’
How do you bridge the chasm?
A: It’s something we should be emphasizing more in hospital medicine. Some people may think it’s distasteful to think about, but it’s something hospitals do need to care about. There’s not enough of that trickling down. This is a huge area for potential growth. It’s important to have an understanding of the importance of the bottom line without feeling too much like it’s threatening the quality of the practice or getting in the way of what we want to be doing.
What other changes are in store for hospital medicine?
A: If you look at the traditional role of a hospitalist, you do a few things on the side of quality, but basically you’re seeing patients. The theory is there could be market saturation, because there are only a certain number of patients you can see in a hospital. But now hospitalists are seen as a physician resource that didn’t exist before. You have a group of doctors that understand patient care very well and are available to make changes and implement initiatives within a hospital. That’s going to lead to more roles besides direct patient care role. Hospitalists are going to be in charge of a number of administrative duties or assume administrative positions within hospitals. Because we’re branching out into other areas of hospital-based care, we’ll see more growth and still see high demand.
One of your primary medical interests is healthcare for Spanish-speaking families. Why is that so important to you?
A: My interest in working with the Latino population comes from my own background. I was a Spanish literature major at Northwestern University, and I’ve had a lot of opportunities to travel. When I started practicing, a large number of the patients were Latino. It became clear how important it is for us to understand what’s happening in our communities. We need to know what patients are coming in, what their demographics are, what their experiences have been, and what their needs are. Everything we do in a hospital translates to what’s happening outside the hospital.
Hospital medicine is quite a switch from Spanish literature. How’d that come about?
A: Actually, it was planned. I always knew I was going to medical school, but I really enjoy linguistics and language. I kept that balance. I didn’t want to be too science-oriented. It was one of those left brain-right brain things.
What is the biggest challenge facing hospital medicine?
A: The challenge is going to be retaining hospitalists and trying to avoid burnout.
How do you address the retention issue?
A: A big part of retention is making people feel happy in their field. It’s about allowing them to feel like they’re contributing in their particular area of physician interest, making them feel like their contributions are valued, making them feel like they can effect change, and making them feel like they’re really part of a team. We’ve been able to keep those as priorities.
How about the risk of burnout?
A: We have to find ways to balance our professions with our personal lives. Everybody’s looking for that balance. I have a husband and three children, so I want it, too. We really have to be reasonable. An important part of being good doctors is being good human beings.
What’s next for you personally?
A: Expanding research and developing a fellowship are two of the primary areas of focus. We also want to focus on triage and flow, and improving the throughput. Beyond that, it’s going to be program expansion, just like it’s been. That’s going to keep me busy. TH
Mark Leiser is a freelance writer in New Jersey.
Julia S. Wright, MD, spent 10 years in traditional practice before joining the University of Wisconsin School of Medicine and Public Health in Madison. Now a clinical associate professor and section head of hospital medicine, the title has changed and the professional demands have intensified. The mission, however, remains the same.
Every decision she’s made in her career—whether it be medical, managerial, financial, or even her seemingly curious choice of college majors—has been guided by one basic question: What will this mean for the quality of patient care? “It may sound cliché, but that’s always the bottom line,” Dr. Wright says. “It’s why I do what I do.”
You spent 10 years in traditional practice before becoming a hospitalist. How did that prepare you for what you’re doing now?
Answer: That experience is a tremendous asset. I made the switch to inpatient medicine because that was the arena I enjoyed the most. But having had experience in the outpatient setting gives me a better understanding of the continuum of care, and my clinical skills have been honed by that experience. I also have an understanding of the strengths of the primary care physicians (PCP), as well as the breadth of the problems they are able to take on.
How does that help you?
A: It really helps in the patient transition, especially on the return side, when patients go back to their PCP after hospitalization. Having an understanding of what resources are available to PCPs helps to determine an appropriate time to discharge a patient. Someone without that experience may have a tendency to think every loose end has to be tied up, or they may make changes that wind up making things more difficult for PCP. But if you have an understanding of a PCP’s capabilities, maybe the patient can be discharged a little earlier.
The University of Wisconsin’s hospitalist program started in 2005. You were named director in 2006. How exciting is it to guide a new program and help it develop?
A: It’s been a tremendous experience. When I joined, we had six hospitalists. Now we have 19. But it was strategic growth; it wasn’t haphazard.
What do you mean by strategic growth?
A: We didn’t simply say, ‘We need more people.’ Every time we brought somebody on, we really made sure it was going to be someone who would add value to the program and be beneficial to the hospital. We were worried, as we got bigger, that it’d be hard to keep the same cohesiveness and that same espirit de corps. During the interviews and the search process, we’re not just asking, ‘Are they qualified?’ We’re asking, ‘Are they a really good fit?’ And everybody has different areas of interest within the group. Some are more research-inclined; one developed a curriculum; some are more clinically oriented. That allows each hospitalist to have an identity in the group. That translated into a good expansion, and it’s been a win-win for us.
What makes someone a good fit for your program?
A: It sounds cliché, but I think the key to a strong developing program is to have a strong sense of what the group values. For us, we value the academic experience. For people going into academic medicine, the struggle is to be able to provide value to the hospital and yet stay academic. Our hospitalists are very clear—that’s our vision. With the support of the hospital and the department, we were able to do both at the same time.
Can you pinpoint one experience that you’ve had that made you realize you’re doing what you’re meant to do?
A: The biggest reward is always patient care. I enjoy the direct patient experience as much as I always have, and that’s what keeps me in the game.
What have you enjoyed most about your transition to a leadership role?
A: I really enjoy the position, not because of the hierarchy, but because of the opportunities afforded by it. I get to interact with hospital medicine staff and the department of medicine chair, and the vice chairs. I’ve been able to interact with others in hospital medicine across the country, and that has been a great experience.
Some hospitalists enjoy what they do because they don’t have to handle the business side of operations or deal with the administrative hassles that private physicians face. In your role, though, you do have to face those challenges. Is that a drawback?
A: I do have to pay attention to the numbers. That’s the bottom line. But it’s something I actually really enjoy. When it comes to awareness of the balance sheet, there’s a division between the leadership level and the clinician level. It’s hard to bridge that chasm of, ‘I’m here for patient care and I don’t necessarily focus on the numbers.’
How do you bridge the chasm?
A: It’s something we should be emphasizing more in hospital medicine. Some people may think it’s distasteful to think about, but it’s something hospitals do need to care about. There’s not enough of that trickling down. This is a huge area for potential growth. It’s important to have an understanding of the importance of the bottom line without feeling too much like it’s threatening the quality of the practice or getting in the way of what we want to be doing.
What other changes are in store for hospital medicine?
A: If you look at the traditional role of a hospitalist, you do a few things on the side of quality, but basically you’re seeing patients. The theory is there could be market saturation, because there are only a certain number of patients you can see in a hospital. But now hospitalists are seen as a physician resource that didn’t exist before. You have a group of doctors that understand patient care very well and are available to make changes and implement initiatives within a hospital. That’s going to lead to more roles besides direct patient care role. Hospitalists are going to be in charge of a number of administrative duties or assume administrative positions within hospitals. Because we’re branching out into other areas of hospital-based care, we’ll see more growth and still see high demand.
One of your primary medical interests is healthcare for Spanish-speaking families. Why is that so important to you?
A: My interest in working with the Latino population comes from my own background. I was a Spanish literature major at Northwestern University, and I’ve had a lot of opportunities to travel. When I started practicing, a large number of the patients were Latino. It became clear how important it is for us to understand what’s happening in our communities. We need to know what patients are coming in, what their demographics are, what their experiences have been, and what their needs are. Everything we do in a hospital translates to what’s happening outside the hospital.
Hospital medicine is quite a switch from Spanish literature. How’d that come about?
A: Actually, it was planned. I always knew I was going to medical school, but I really enjoy linguistics and language. I kept that balance. I didn’t want to be too science-oriented. It was one of those left brain-right brain things.
What is the biggest challenge facing hospital medicine?
A: The challenge is going to be retaining hospitalists and trying to avoid burnout.
How do you address the retention issue?
A: A big part of retention is making people feel happy in their field. It’s about allowing them to feel like they’re contributing in their particular area of physician interest, making them feel like their contributions are valued, making them feel like they can effect change, and making them feel like they’re really part of a team. We’ve been able to keep those as priorities.
How about the risk of burnout?
A: We have to find ways to balance our professions with our personal lives. Everybody’s looking for that balance. I have a husband and three children, so I want it, too. We really have to be reasonable. An important part of being good doctors is being good human beings.
What’s next for you personally?
A: Expanding research and developing a fellowship are two of the primary areas of focus. We also want to focus on triage and flow, and improving the throughput. Beyond that, it’s going to be program expansion, just like it’s been. That’s going to keep me busy. TH
Mark Leiser is a freelance writer in New Jersey.
Julia S. Wright, MD, spent 10 years in traditional practice before joining the University of Wisconsin School of Medicine and Public Health in Madison. Now a clinical associate professor and section head of hospital medicine, the title has changed and the professional demands have intensified. The mission, however, remains the same.
Every decision she’s made in her career—whether it be medical, managerial, financial, or even her seemingly curious choice of college majors—has been guided by one basic question: What will this mean for the quality of patient care? “It may sound cliché, but that’s always the bottom line,” Dr. Wright says. “It’s why I do what I do.”
You spent 10 years in traditional practice before becoming a hospitalist. How did that prepare you for what you’re doing now?
Answer: That experience is a tremendous asset. I made the switch to inpatient medicine because that was the arena I enjoyed the most. But having had experience in the outpatient setting gives me a better understanding of the continuum of care, and my clinical skills have been honed by that experience. I also have an understanding of the strengths of the primary care physicians (PCP), as well as the breadth of the problems they are able to take on.
How does that help you?
A: It really helps in the patient transition, especially on the return side, when patients go back to their PCP after hospitalization. Having an understanding of what resources are available to PCPs helps to determine an appropriate time to discharge a patient. Someone without that experience may have a tendency to think every loose end has to be tied up, or they may make changes that wind up making things more difficult for PCP. But if you have an understanding of a PCP’s capabilities, maybe the patient can be discharged a little earlier.
The University of Wisconsin’s hospitalist program started in 2005. You were named director in 2006. How exciting is it to guide a new program and help it develop?
A: It’s been a tremendous experience. When I joined, we had six hospitalists. Now we have 19. But it was strategic growth; it wasn’t haphazard.
What do you mean by strategic growth?
A: We didn’t simply say, ‘We need more people.’ Every time we brought somebody on, we really made sure it was going to be someone who would add value to the program and be beneficial to the hospital. We were worried, as we got bigger, that it’d be hard to keep the same cohesiveness and that same espirit de corps. During the interviews and the search process, we’re not just asking, ‘Are they qualified?’ We’re asking, ‘Are they a really good fit?’ And everybody has different areas of interest within the group. Some are more research-inclined; one developed a curriculum; some are more clinically oriented. That allows each hospitalist to have an identity in the group. That translated into a good expansion, and it’s been a win-win for us.
What makes someone a good fit for your program?
A: It sounds cliché, but I think the key to a strong developing program is to have a strong sense of what the group values. For us, we value the academic experience. For people going into academic medicine, the struggle is to be able to provide value to the hospital and yet stay academic. Our hospitalists are very clear—that’s our vision. With the support of the hospital and the department, we were able to do both at the same time.
Can you pinpoint one experience that you’ve had that made you realize you’re doing what you’re meant to do?
A: The biggest reward is always patient care. I enjoy the direct patient experience as much as I always have, and that’s what keeps me in the game.
What have you enjoyed most about your transition to a leadership role?
A: I really enjoy the position, not because of the hierarchy, but because of the opportunities afforded by it. I get to interact with hospital medicine staff and the department of medicine chair, and the vice chairs. I’ve been able to interact with others in hospital medicine across the country, and that has been a great experience.
Some hospitalists enjoy what they do because they don’t have to handle the business side of operations or deal with the administrative hassles that private physicians face. In your role, though, you do have to face those challenges. Is that a drawback?
A: I do have to pay attention to the numbers. That’s the bottom line. But it’s something I actually really enjoy. When it comes to awareness of the balance sheet, there’s a division between the leadership level and the clinician level. It’s hard to bridge that chasm of, ‘I’m here for patient care and I don’t necessarily focus on the numbers.’
How do you bridge the chasm?
A: It’s something we should be emphasizing more in hospital medicine. Some people may think it’s distasteful to think about, but it’s something hospitals do need to care about. There’s not enough of that trickling down. This is a huge area for potential growth. It’s important to have an understanding of the importance of the bottom line without feeling too much like it’s threatening the quality of the practice or getting in the way of what we want to be doing.
What other changes are in store for hospital medicine?
A: If you look at the traditional role of a hospitalist, you do a few things on the side of quality, but basically you’re seeing patients. The theory is there could be market saturation, because there are only a certain number of patients you can see in a hospital. But now hospitalists are seen as a physician resource that didn’t exist before. You have a group of doctors that understand patient care very well and are available to make changes and implement initiatives within a hospital. That’s going to lead to more roles besides direct patient care role. Hospitalists are going to be in charge of a number of administrative duties or assume administrative positions within hospitals. Because we’re branching out into other areas of hospital-based care, we’ll see more growth and still see high demand.
One of your primary medical interests is healthcare for Spanish-speaking families. Why is that so important to you?
A: My interest in working with the Latino population comes from my own background. I was a Spanish literature major at Northwestern University, and I’ve had a lot of opportunities to travel. When I started practicing, a large number of the patients were Latino. It became clear how important it is for us to understand what’s happening in our communities. We need to know what patients are coming in, what their demographics are, what their experiences have been, and what their needs are. Everything we do in a hospital translates to what’s happening outside the hospital.
Hospital medicine is quite a switch from Spanish literature. How’d that come about?
A: Actually, it was planned. I always knew I was going to medical school, but I really enjoy linguistics and language. I kept that balance. I didn’t want to be too science-oriented. It was one of those left brain-right brain things.
What is the biggest challenge facing hospital medicine?
A: The challenge is going to be retaining hospitalists and trying to avoid burnout.
How do you address the retention issue?
A: A big part of retention is making people feel happy in their field. It’s about allowing them to feel like they’re contributing in their particular area of physician interest, making them feel like their contributions are valued, making them feel like they can effect change, and making them feel like they’re really part of a team. We’ve been able to keep those as priorities.
How about the risk of burnout?
A: We have to find ways to balance our professions with our personal lives. Everybody’s looking for that balance. I have a husband and three children, so I want it, too. We really have to be reasonable. An important part of being good doctors is being good human beings.
What’s next for you personally?
A: Expanding research and developing a fellowship are two of the primary areas of focus. We also want to focus on triage and flow, and improving the throughput. Beyond that, it’s going to be program expansion, just like it’s been. That’s going to keep me busy. TH
Mark Leiser is a freelance writer in New Jersey.
“Caregiver Culture” and End-of-Life Discussions
When it comes to discussing a patient’s wishes for code status care, practices at the institution play a more important role than almost any other factor, according to a new study, “Factors Associated with Discussion of Care Plans and Code Status at the Time of Hospital Admission: Results from the Multicenter Hospitalist Study,” in the Journal of Hospital Medicine.
“What was most surprising to me was how variable the discussion rate was,” lead author Andrew Auerbach, MD, tells The Hospitalist. “It had little or nothing to do with how sick the patient was, or with the type of institution in which the discussion took place.”
Hospitalists are no more or less likely to document such discussions than doctors in any other specialty, says Dr. Auerbach, a hospitalist and associate professor of medicine at the University of California San Francisco. He and his colleagues analyzed data from patients admitted to the general medicine services at six academic medical centers as part of the Multicenter Hospitalist Study. Each site complied with requirements established by the Patient Self-Determination Act (PSDA), which says patients must be informed of their right to create an advance directive.
None of the hospitals in the study had established guidelines or formal policies regarding physician-patient discussions about code status or end-of-life care. Patients were interviewed immediately after informed consent was obtained, usually within 24 hours of admission. In each case, the authors determined whether or not the patient had had a care discussion, defined as a documented discussion “between patients (or family) and at least one physician … during the first 24 hours of hospitalization,” the authors write. “Care discussions needed to specify that the person who wrote the note had actually spoken with the patient or their family for the purposes of determining preferences for care, and that this discussion resulted in a specific care plan.”
A notation, such as “the patient continues to want full efforts,” qualified as a care discussion. Less-specific comments, such as “DNR/DNI” or “spoke with family, questions answered,” did not qualify. “These were all really, really sick people, and it was important to assess their wishes for care,” Dr. Auerbach says. Individually, PSDA or durable power of attorney may not adequately convey a patient’s true wishes, because often times the measures involve nothing more than having the patient or family complete a form. “The true marker is a conversation,” he says.
—Andrew Auerbach, MD, University of California San Francisco
By the Numbers
Of 17,097 patients interviewed, only 1,776 (10.4%) had a documented care discussion within 24 hours of hospital admission. The frequency of discussions varied from a low of 2.8% at one institution, to a high of 24.9% at another.
On unadjusted analysis, patients with documented care discussions were more likely to have living wills, durable powers of attorney, or the names of surrogate decision-makers in their charts (P<0.0001 for all categories). These patients were older, more likely to be white, and more likely to be on Medicare, compared to patients without documented care discussions.
The unadjusted analysis also showed patients with care discussions were more likely to be married, but less likely to be living in their own home or apartment, and, not surprisingly, more likely to have been hospitalized at least once within the previous 12 months. Overall, the general health of patients with care discussions was poorer than those without. Patients with documented discussions were more likely to report needing help within the past month with chores or bathing or dressing themselves, than were patients who did not have care discussions. Cancer, depression, and a history of stroke were common among patients with care discussions. Compared to patients without documented discussions, those who did have the discussion appear to want more of a say in their care: they were less likely to agree with the study statements, “I prefer my doctor give me choices regarding my care,” and “I prefer to leave care decisions to my physician.”
The authors found multivariate analysis showed many of these factors turn out to have only a moderate association with a documented care discussion, with adjusted odds ratios of less than 2.0. The strongest predictors were the existence of informal notations describing pre-hospital care wishes, with odds ratios ranging from 3.22 to 11.32, compared to people with no such documentation, and site of enrollment, with odds ratios of 1.74 to 5.14.
The Caregiver Culture
These findings suggest the “caregiver culture” at any given institution is a stronger determinant of a patient participating in a documented care discussion than other, more intuitive factors, such as medical condition or socioeconomic characteristics, or even whether or not the patient has a pre-existing advance directive or durable power of attorney, Dr. Auerbach explains. “It may just be a part of what some hospitals do. It’s driven by what your peers are doing and by local practices.”
Based on the results of the study, Dr. Auerbach and his co-authors suggest simply establishing mandates to document code status on admission probably will not encourage more conversations of this nature, “unless sites also develop an approach to using this newly documented information as a prompt for subsequent discussions.” The Patient Self-Determination Act went into effect in 1991, but its effect on improving communication around end-of-life care remains uncertain, the authors note. It might be why California passed a new law, effective as of January 2009, requiring physicians and health care organizations in the state to provide terminally ill patients who ask about their end-of-life care options with comprehensive information and counseling. Such discussions must cover advance directives, hospice care, and the right to receive palliative care. The new law is the first of its kind in the nation, but proponents of the legislation hope it will serve as a model for other states to adopt.1
“Documentation has an impact on quality of care. Lots of errors are driven by code status,” Dr. Auerbach points out. “If a patient is admitted in the middle of the night, when the doctor checks in on him the next day, he can look at the chart and see that his partner documented the care discussion the night before. That is incredibly helpful.” TH
Norra MacReady is a medical writer based in California.
Reference
1. O’Reilly KB. California law mandates discussing end-of-life options. Am Med News Web site. Available at amaassn.org/amednews/2008/11/10/prsc1110.htm. Last accessed November 6, 2008.
When it comes to discussing a patient’s wishes for code status care, practices at the institution play a more important role than almost any other factor, according to a new study, “Factors Associated with Discussion of Care Plans and Code Status at the Time of Hospital Admission: Results from the Multicenter Hospitalist Study,” in the Journal of Hospital Medicine.
“What was most surprising to me was how variable the discussion rate was,” lead author Andrew Auerbach, MD, tells The Hospitalist. “It had little or nothing to do with how sick the patient was, or with the type of institution in which the discussion took place.”
Hospitalists are no more or less likely to document such discussions than doctors in any other specialty, says Dr. Auerbach, a hospitalist and associate professor of medicine at the University of California San Francisco. He and his colleagues analyzed data from patients admitted to the general medicine services at six academic medical centers as part of the Multicenter Hospitalist Study. Each site complied with requirements established by the Patient Self-Determination Act (PSDA), which says patients must be informed of their right to create an advance directive.
None of the hospitals in the study had established guidelines or formal policies regarding physician-patient discussions about code status or end-of-life care. Patients were interviewed immediately after informed consent was obtained, usually within 24 hours of admission. In each case, the authors determined whether or not the patient had had a care discussion, defined as a documented discussion “between patients (or family) and at least one physician … during the first 24 hours of hospitalization,” the authors write. “Care discussions needed to specify that the person who wrote the note had actually spoken with the patient or their family for the purposes of determining preferences for care, and that this discussion resulted in a specific care plan.”
A notation, such as “the patient continues to want full efforts,” qualified as a care discussion. Less-specific comments, such as “DNR/DNI” or “spoke with family, questions answered,” did not qualify. “These were all really, really sick people, and it was important to assess their wishes for care,” Dr. Auerbach says. Individually, PSDA or durable power of attorney may not adequately convey a patient’s true wishes, because often times the measures involve nothing more than having the patient or family complete a form. “The true marker is a conversation,” he says.
—Andrew Auerbach, MD, University of California San Francisco
By the Numbers
Of 17,097 patients interviewed, only 1,776 (10.4%) had a documented care discussion within 24 hours of hospital admission. The frequency of discussions varied from a low of 2.8% at one institution, to a high of 24.9% at another.
On unadjusted analysis, patients with documented care discussions were more likely to have living wills, durable powers of attorney, or the names of surrogate decision-makers in their charts (P<0.0001 for all categories). These patients were older, more likely to be white, and more likely to be on Medicare, compared to patients without documented care discussions.
The unadjusted analysis also showed patients with care discussions were more likely to be married, but less likely to be living in their own home or apartment, and, not surprisingly, more likely to have been hospitalized at least once within the previous 12 months. Overall, the general health of patients with care discussions was poorer than those without. Patients with documented discussions were more likely to report needing help within the past month with chores or bathing or dressing themselves, than were patients who did not have care discussions. Cancer, depression, and a history of stroke were common among patients with care discussions. Compared to patients without documented discussions, those who did have the discussion appear to want more of a say in their care: they were less likely to agree with the study statements, “I prefer my doctor give me choices regarding my care,” and “I prefer to leave care decisions to my physician.”
The authors found multivariate analysis showed many of these factors turn out to have only a moderate association with a documented care discussion, with adjusted odds ratios of less than 2.0. The strongest predictors were the existence of informal notations describing pre-hospital care wishes, with odds ratios ranging from 3.22 to 11.32, compared to people with no such documentation, and site of enrollment, with odds ratios of 1.74 to 5.14.
The Caregiver Culture
These findings suggest the “caregiver culture” at any given institution is a stronger determinant of a patient participating in a documented care discussion than other, more intuitive factors, such as medical condition or socioeconomic characteristics, or even whether or not the patient has a pre-existing advance directive or durable power of attorney, Dr. Auerbach explains. “It may just be a part of what some hospitals do. It’s driven by what your peers are doing and by local practices.”
Based on the results of the study, Dr. Auerbach and his co-authors suggest simply establishing mandates to document code status on admission probably will not encourage more conversations of this nature, “unless sites also develop an approach to using this newly documented information as a prompt for subsequent discussions.” The Patient Self-Determination Act went into effect in 1991, but its effect on improving communication around end-of-life care remains uncertain, the authors note. It might be why California passed a new law, effective as of January 2009, requiring physicians and health care organizations in the state to provide terminally ill patients who ask about their end-of-life care options with comprehensive information and counseling. Such discussions must cover advance directives, hospice care, and the right to receive palliative care. The new law is the first of its kind in the nation, but proponents of the legislation hope it will serve as a model for other states to adopt.1
“Documentation has an impact on quality of care. Lots of errors are driven by code status,” Dr. Auerbach points out. “If a patient is admitted in the middle of the night, when the doctor checks in on him the next day, he can look at the chart and see that his partner documented the care discussion the night before. That is incredibly helpful.” TH
Norra MacReady is a medical writer based in California.
Reference
1. O’Reilly KB. California law mandates discussing end-of-life options. Am Med News Web site. Available at amaassn.org/amednews/2008/11/10/prsc1110.htm. Last accessed November 6, 2008.
When it comes to discussing a patient’s wishes for code status care, practices at the institution play a more important role than almost any other factor, according to a new study, “Factors Associated with Discussion of Care Plans and Code Status at the Time of Hospital Admission: Results from the Multicenter Hospitalist Study,” in the Journal of Hospital Medicine.
“What was most surprising to me was how variable the discussion rate was,” lead author Andrew Auerbach, MD, tells The Hospitalist. “It had little or nothing to do with how sick the patient was, or with the type of institution in which the discussion took place.”
Hospitalists are no more or less likely to document such discussions than doctors in any other specialty, says Dr. Auerbach, a hospitalist and associate professor of medicine at the University of California San Francisco. He and his colleagues analyzed data from patients admitted to the general medicine services at six academic medical centers as part of the Multicenter Hospitalist Study. Each site complied with requirements established by the Patient Self-Determination Act (PSDA), which says patients must be informed of their right to create an advance directive.
None of the hospitals in the study had established guidelines or formal policies regarding physician-patient discussions about code status or end-of-life care. Patients were interviewed immediately after informed consent was obtained, usually within 24 hours of admission. In each case, the authors determined whether or not the patient had had a care discussion, defined as a documented discussion “between patients (or family) and at least one physician … during the first 24 hours of hospitalization,” the authors write. “Care discussions needed to specify that the person who wrote the note had actually spoken with the patient or their family for the purposes of determining preferences for care, and that this discussion resulted in a specific care plan.”
A notation, such as “the patient continues to want full efforts,” qualified as a care discussion. Less-specific comments, such as “DNR/DNI” or “spoke with family, questions answered,” did not qualify. “These were all really, really sick people, and it was important to assess their wishes for care,” Dr. Auerbach says. Individually, PSDA or durable power of attorney may not adequately convey a patient’s true wishes, because often times the measures involve nothing more than having the patient or family complete a form. “The true marker is a conversation,” he says.
—Andrew Auerbach, MD, University of California San Francisco
By the Numbers
Of 17,097 patients interviewed, only 1,776 (10.4%) had a documented care discussion within 24 hours of hospital admission. The frequency of discussions varied from a low of 2.8% at one institution, to a high of 24.9% at another.
On unadjusted analysis, patients with documented care discussions were more likely to have living wills, durable powers of attorney, or the names of surrogate decision-makers in their charts (P<0.0001 for all categories). These patients were older, more likely to be white, and more likely to be on Medicare, compared to patients without documented care discussions.
The unadjusted analysis also showed patients with care discussions were more likely to be married, but less likely to be living in their own home or apartment, and, not surprisingly, more likely to have been hospitalized at least once within the previous 12 months. Overall, the general health of patients with care discussions was poorer than those without. Patients with documented discussions were more likely to report needing help within the past month with chores or bathing or dressing themselves, than were patients who did not have care discussions. Cancer, depression, and a history of stroke were common among patients with care discussions. Compared to patients without documented discussions, those who did have the discussion appear to want more of a say in their care: they were less likely to agree with the study statements, “I prefer my doctor give me choices regarding my care,” and “I prefer to leave care decisions to my physician.”
The authors found multivariate analysis showed many of these factors turn out to have only a moderate association with a documented care discussion, with adjusted odds ratios of less than 2.0. The strongest predictors were the existence of informal notations describing pre-hospital care wishes, with odds ratios ranging from 3.22 to 11.32, compared to people with no such documentation, and site of enrollment, with odds ratios of 1.74 to 5.14.
The Caregiver Culture
These findings suggest the “caregiver culture” at any given institution is a stronger determinant of a patient participating in a documented care discussion than other, more intuitive factors, such as medical condition or socioeconomic characteristics, or even whether or not the patient has a pre-existing advance directive or durable power of attorney, Dr. Auerbach explains. “It may just be a part of what some hospitals do. It’s driven by what your peers are doing and by local practices.”
Based on the results of the study, Dr. Auerbach and his co-authors suggest simply establishing mandates to document code status on admission probably will not encourage more conversations of this nature, “unless sites also develop an approach to using this newly documented information as a prompt for subsequent discussions.” The Patient Self-Determination Act went into effect in 1991, but its effect on improving communication around end-of-life care remains uncertain, the authors note. It might be why California passed a new law, effective as of January 2009, requiring physicians and health care organizations in the state to provide terminally ill patients who ask about their end-of-life care options with comprehensive information and counseling. Such discussions must cover advance directives, hospice care, and the right to receive palliative care. The new law is the first of its kind in the nation, but proponents of the legislation hope it will serve as a model for other states to adopt.1
“Documentation has an impact on quality of care. Lots of errors are driven by code status,” Dr. Auerbach points out. “If a patient is admitted in the middle of the night, when the doctor checks in on him the next day, he can look at the chart and see that his partner documented the care discussion the night before. That is incredibly helpful.” TH
Norra MacReady is a medical writer based in California.
Reference
1. O’Reilly KB. California law mandates discussing end-of-life options. Am Med News Web site. Available at amaassn.org/amednews/2008/11/10/prsc1110.htm. Last accessed November 6, 2008.
QTc Interval Prolongation
Terfenadine, cisapride, astemizole … do you remember these drugs? They all were removed from the U.S. market subsequent to adverse outcomes related to QTc interval prolongation, including ventricular arrhythmias.1-3 Many drugs prolong the QTc interval, particularly if a drug is combined with others that affect its metabolism.
QTc interval prolongation can lead to torsades de pointes (TdP). Certain individuals are particularly predisposed to developing TdP, including: women, people with hypokalemia or hypomagnesemia, and those with a history of congenital or idiopathic QTc syndrome, cardiac arrest, syncope, congestive heart failure, bradycardia, baseline QT prolongation, renal failure, or cardiac failure.4 Some agents can prolong the QTc interval by five to 10 milliseconds and cause TdP, while others require a 50-millisecond increase or more.
Drugs that confer a risk of ventricular arrhythmias include: disopyramide, dofetilide, ibutilide, procainamide, quinidine, sotalol, and amiodarone (antiarrhythmic agents); clarithromycin, erythromycin, levofloxacin, gatifloxacin, gemifloxacin, moxifloxacin, telithromycin (anti-infectives); domperidone and droperidol antiemetics; chlorpromazine, haloperidol, mesoridazine, thioridazine, and pimozide (antipsychotics); amitriptyline, desipramine, doxepin, fluoxetine, imipramine, sertraline, and venlafaxine (antidepressants); fluconazole, itraconazole, and ketoconazole (antifungals); naratriptan, sumatriptan, and zolmitriptan; and methadone.4-8 Other related agents, such as voriconazole and ondansetron, have been reported to cause QTc prolongation.
Drugs of special concern are those that frequently inhibit the metabolism of other agents, including erythromycin, clarithromycin, ketoconazole, itraconazole, amiodarone, and quinidine, and many antidepressants and antiretroviral agents. Of the deaths associated with drug-induced QTc prolongation related to the prokinetic agent cisapride, many were due to drug interactions with an imidazole or macrolide antibiotic. In these cases, increased serum concentrations of cisapride occurred due to inhibition of the cytochrome P450 CYP3A4 isoenzyme.9
If treatment with a drug that has the potential for causing QTc prolongation is begun, tell your patient to report any “potential cardiac” symptoms, such as palpitations, syncope, or near-syncope with or without palpitations, to a member of the healthcare team. Always be on the lookout for any concomitant conditions or treatments that can cause hypokalemia (e.g., diuretic use, gastroenteritis, diarrhea, excessive vomiting), or other agents that inhibit drug metabolism.
Obtaining a complete medication history, including the use of herbal products and over-the-counter medications, can help identify and prevent QTc prolongation from a drug interaction. A routine, 12-lead electrocardiogram (EKG) should be utilized during treatment to detect asymptomatic QTc prolongation or abnormal postectopic QTc intervals. Additionally, any patient predisposed to QTc prolongation should have an EKG performed before commencing treatment as well as after treatment is complete. If a drug prolongs the QTc interval beyond normal limits, the benefit of continuing the drug should be weighed against the risk of serious adverse cardiac events.10 TH
Michele B Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
1. Kupec IF. Seldane and generic terfenadine withdrawn from market. Food and Drug Administration Web site. Available at: www.fda.gov/bbs/topics/answers/ ans00853.html. Accessed Nov. 7, 2008.
2. Zalewski JM. Cisapride withdrawal requires alternate therapy. Cleveland Clinic Web site. Available at: www.clevelandclinicmeded.com/medicalpubs/pharmacy/mayjune2000/cisapride.htm. Accessed Nov. 7, 2008.
3. Drugs removed from or restricted in the U.S. market because of drug interactions. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/drugReactions/CERT%20Educational%20Module%201/sld013.htm. Updated Dec. 22, 2008. Accessed Nov. 7, 2008.
4. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-1022.
5. Pham CP, de Feiter PW, van der Kuy PHM, van Mook WN. Long QTc interval and torsades de pointes caused by fluconazole. Ann Pharmacother. 2006;40:1456-1461.
6. Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 1005;39:543-546.
7. Philips JA, Marty FM, Stone RM et al. Torsades de pointes associated with voriconazole use. Transpl Infect Dis. 2007;9:33-36.
8. Charbit B, Alvarez JC, Dasque E, Abe E, Démolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation. Anesthesiol. 2008;109:206-212.
9. Yap YG, Camm AJ. Drug induced qt prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
10. Jayasinghe R, Registrar S, Kovoor P. Drugs and the QTc interval. Aust Prescr. 2002;25:63-65.
11. Atacand HCT 32/25 mg gives patients and physicians more treatment flexibility. Available at: www.pharmacitelink.com/news/2008/08/14_az.pdf. Accessed Nov. 4, 2008.
12. FDA approves astellas’ vaprisol (conivaptan hydrochloride injection) premixed in 5% dextrose for the treatment of hyponatremia. Sandoz Web site. Available at: sandoz.yellowbrix.com/pages/sandoz/Story.nsp?story_id=122559939. Accessed Nov. 4, 2008.
13. U.S. FDA drug shortages. Available at: www.fda.gov/cder/drug/shortages/default.htm#Foscavir. Accessed Nov. 3, 2008.
14. FDA Drug Shortages. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/ shortages/discontinuation.pdf. Accessed Nov. 6, 2008.
15. Waknine Y. FDA safety changes: mirena, zyvox, orencia. Medscape Web site. Available at: www.medscape.com/viewarticle/580101. Accessed Nov. 3, 2008.
16. MannKind and Pfizer announce collaboration for certain exubera patients to transition to Mannkind’s inhaled insulin therapy. Drugs.com Web site. Available at: www.drugs.com/news/mannkind-pfizer-announce-collaboration-certain-exubera-patients-transition-mannkind-s-inhaled-13677.html. Accessed Nov. 3, 2008.
17. MannKind reports positive data from a phase 3 clinical study of technosphere insulin in Type 1 diabetics. Drugs.com Web site. Available at: www.drugs.com/ clinical_trials/mannkind-reports-positive-data-phase-3-clinical-study-technosphere-insulin-type-1-diabetes-5554.html. Accessed Nov. 3, 2008.
18. Bratulic A. Sanofi-aventis to halt all Acomplia trials. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=34DAB2DC3D7A48939A1D24AB97204CB4&logRowId=263560. Accessed Nov. 6, 2008.
Terfenadine, cisapride, astemizole … do you remember these drugs? They all were removed from the U.S. market subsequent to adverse outcomes related to QTc interval prolongation, including ventricular arrhythmias.1-3 Many drugs prolong the QTc interval, particularly if a drug is combined with others that affect its metabolism.
QTc interval prolongation can lead to torsades de pointes (TdP). Certain individuals are particularly predisposed to developing TdP, including: women, people with hypokalemia or hypomagnesemia, and those with a history of congenital or idiopathic QTc syndrome, cardiac arrest, syncope, congestive heart failure, bradycardia, baseline QT prolongation, renal failure, or cardiac failure.4 Some agents can prolong the QTc interval by five to 10 milliseconds and cause TdP, while others require a 50-millisecond increase or more.
Drugs that confer a risk of ventricular arrhythmias include: disopyramide, dofetilide, ibutilide, procainamide, quinidine, sotalol, and amiodarone (antiarrhythmic agents); clarithromycin, erythromycin, levofloxacin, gatifloxacin, gemifloxacin, moxifloxacin, telithromycin (anti-infectives); domperidone and droperidol antiemetics; chlorpromazine, haloperidol, mesoridazine, thioridazine, and pimozide (antipsychotics); amitriptyline, desipramine, doxepin, fluoxetine, imipramine, sertraline, and venlafaxine (antidepressants); fluconazole, itraconazole, and ketoconazole (antifungals); naratriptan, sumatriptan, and zolmitriptan; and methadone.4-8 Other related agents, such as voriconazole and ondansetron, have been reported to cause QTc prolongation.
Drugs of special concern are those that frequently inhibit the metabolism of other agents, including erythromycin, clarithromycin, ketoconazole, itraconazole, amiodarone, and quinidine, and many antidepressants and antiretroviral agents. Of the deaths associated with drug-induced QTc prolongation related to the prokinetic agent cisapride, many were due to drug interactions with an imidazole or macrolide antibiotic. In these cases, increased serum concentrations of cisapride occurred due to inhibition of the cytochrome P450 CYP3A4 isoenzyme.9
If treatment with a drug that has the potential for causing QTc prolongation is begun, tell your patient to report any “potential cardiac” symptoms, such as palpitations, syncope, or near-syncope with or without palpitations, to a member of the healthcare team. Always be on the lookout for any concomitant conditions or treatments that can cause hypokalemia (e.g., diuretic use, gastroenteritis, diarrhea, excessive vomiting), or other agents that inhibit drug metabolism.
Obtaining a complete medication history, including the use of herbal products and over-the-counter medications, can help identify and prevent QTc prolongation from a drug interaction. A routine, 12-lead electrocardiogram (EKG) should be utilized during treatment to detect asymptomatic QTc prolongation or abnormal postectopic QTc intervals. Additionally, any patient predisposed to QTc prolongation should have an EKG performed before commencing treatment as well as after treatment is complete. If a drug prolongs the QTc interval beyond normal limits, the benefit of continuing the drug should be weighed against the risk of serious adverse cardiac events.10 TH
Michele B Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
1. Kupec IF. Seldane and generic terfenadine withdrawn from market. Food and Drug Administration Web site. Available at: www.fda.gov/bbs/topics/answers/ ans00853.html. Accessed Nov. 7, 2008.
2. Zalewski JM. Cisapride withdrawal requires alternate therapy. Cleveland Clinic Web site. Available at: www.clevelandclinicmeded.com/medicalpubs/pharmacy/mayjune2000/cisapride.htm. Accessed Nov. 7, 2008.
3. Drugs removed from or restricted in the U.S. market because of drug interactions. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/drugReactions/CERT%20Educational%20Module%201/sld013.htm. Updated Dec. 22, 2008. Accessed Nov. 7, 2008.
4. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-1022.
5. Pham CP, de Feiter PW, van der Kuy PHM, van Mook WN. Long QTc interval and torsades de pointes caused by fluconazole. Ann Pharmacother. 2006;40:1456-1461.
6. Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 1005;39:543-546.
7. Philips JA, Marty FM, Stone RM et al. Torsades de pointes associated with voriconazole use. Transpl Infect Dis. 2007;9:33-36.
8. Charbit B, Alvarez JC, Dasque E, Abe E, Démolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation. Anesthesiol. 2008;109:206-212.
9. Yap YG, Camm AJ. Drug induced qt prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
10. Jayasinghe R, Registrar S, Kovoor P. Drugs and the QTc interval. Aust Prescr. 2002;25:63-65.
11. Atacand HCT 32/25 mg gives patients and physicians more treatment flexibility. Available at: www.pharmacitelink.com/news/2008/08/14_az.pdf. Accessed Nov. 4, 2008.
12. FDA approves astellas’ vaprisol (conivaptan hydrochloride injection) premixed in 5% dextrose for the treatment of hyponatremia. Sandoz Web site. Available at: sandoz.yellowbrix.com/pages/sandoz/Story.nsp?story_id=122559939. Accessed Nov. 4, 2008.
13. U.S. FDA drug shortages. Available at: www.fda.gov/cder/drug/shortages/default.htm#Foscavir. Accessed Nov. 3, 2008.
14. FDA Drug Shortages. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/ shortages/discontinuation.pdf. Accessed Nov. 6, 2008.
15. Waknine Y. FDA safety changes: mirena, zyvox, orencia. Medscape Web site. Available at: www.medscape.com/viewarticle/580101. Accessed Nov. 3, 2008.
16. MannKind and Pfizer announce collaboration for certain exubera patients to transition to Mannkind’s inhaled insulin therapy. Drugs.com Web site. Available at: www.drugs.com/news/mannkind-pfizer-announce-collaboration-certain-exubera-patients-transition-mannkind-s-inhaled-13677.html. Accessed Nov. 3, 2008.
17. MannKind reports positive data from a phase 3 clinical study of technosphere insulin in Type 1 diabetics. Drugs.com Web site. Available at: www.drugs.com/ clinical_trials/mannkind-reports-positive-data-phase-3-clinical-study-technosphere-insulin-type-1-diabetes-5554.html. Accessed Nov. 3, 2008.
18. Bratulic A. Sanofi-aventis to halt all Acomplia trials. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=34DAB2DC3D7A48939A1D24AB97204CB4&logRowId=263560. Accessed Nov. 6, 2008.
Terfenadine, cisapride, astemizole … do you remember these drugs? They all were removed from the U.S. market subsequent to adverse outcomes related to QTc interval prolongation, including ventricular arrhythmias.1-3 Many drugs prolong the QTc interval, particularly if a drug is combined with others that affect its metabolism.
QTc interval prolongation can lead to torsades de pointes (TdP). Certain individuals are particularly predisposed to developing TdP, including: women, people with hypokalemia or hypomagnesemia, and those with a history of congenital or idiopathic QTc syndrome, cardiac arrest, syncope, congestive heart failure, bradycardia, baseline QT prolongation, renal failure, or cardiac failure.4 Some agents can prolong the QTc interval by five to 10 milliseconds and cause TdP, while others require a 50-millisecond increase or more.
Drugs that confer a risk of ventricular arrhythmias include: disopyramide, dofetilide, ibutilide, procainamide, quinidine, sotalol, and amiodarone (antiarrhythmic agents); clarithromycin, erythromycin, levofloxacin, gatifloxacin, gemifloxacin, moxifloxacin, telithromycin (anti-infectives); domperidone and droperidol antiemetics; chlorpromazine, haloperidol, mesoridazine, thioridazine, and pimozide (antipsychotics); amitriptyline, desipramine, doxepin, fluoxetine, imipramine, sertraline, and venlafaxine (antidepressants); fluconazole, itraconazole, and ketoconazole (antifungals); naratriptan, sumatriptan, and zolmitriptan; and methadone.4-8 Other related agents, such as voriconazole and ondansetron, have been reported to cause QTc prolongation.
Drugs of special concern are those that frequently inhibit the metabolism of other agents, including erythromycin, clarithromycin, ketoconazole, itraconazole, amiodarone, and quinidine, and many antidepressants and antiretroviral agents. Of the deaths associated with drug-induced QTc prolongation related to the prokinetic agent cisapride, many were due to drug interactions with an imidazole or macrolide antibiotic. In these cases, increased serum concentrations of cisapride occurred due to inhibition of the cytochrome P450 CYP3A4 isoenzyme.9
If treatment with a drug that has the potential for causing QTc prolongation is begun, tell your patient to report any “potential cardiac” symptoms, such as palpitations, syncope, or near-syncope with or without palpitations, to a member of the healthcare team. Always be on the lookout for any concomitant conditions or treatments that can cause hypokalemia (e.g., diuretic use, gastroenteritis, diarrhea, excessive vomiting), or other agents that inhibit drug metabolism.
Obtaining a complete medication history, including the use of herbal products and over-the-counter medications, can help identify and prevent QTc prolongation from a drug interaction. A routine, 12-lead electrocardiogram (EKG) should be utilized during treatment to detect asymptomatic QTc prolongation or abnormal postectopic QTc intervals. Additionally, any patient predisposed to QTc prolongation should have an EKG performed before commencing treatment as well as after treatment is complete. If a drug prolongs the QTc interval beyond normal limits, the benefit of continuing the drug should be weighed against the risk of serious adverse cardiac events.10 TH
Michele B Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
1. Kupec IF. Seldane and generic terfenadine withdrawn from market. Food and Drug Administration Web site. Available at: www.fda.gov/bbs/topics/answers/ ans00853.html. Accessed Nov. 7, 2008.
2. Zalewski JM. Cisapride withdrawal requires alternate therapy. Cleveland Clinic Web site. Available at: www.clevelandclinicmeded.com/medicalpubs/pharmacy/mayjune2000/cisapride.htm. Accessed Nov. 7, 2008.
3. Drugs removed from or restricted in the U.S. market because of drug interactions. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/drugReactions/CERT%20Educational%20Module%201/sld013.htm. Updated Dec. 22, 2008. Accessed Nov. 7, 2008.
4. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-1022.
5. Pham CP, de Feiter PW, van der Kuy PHM, van Mook WN. Long QTc interval and torsades de pointes caused by fluconazole. Ann Pharmacother. 2006;40:1456-1461.
6. Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 1005;39:543-546.
7. Philips JA, Marty FM, Stone RM et al. Torsades de pointes associated with voriconazole use. Transpl Infect Dis. 2007;9:33-36.
8. Charbit B, Alvarez JC, Dasque E, Abe E, Démolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation. Anesthesiol. 2008;109:206-212.
9. Yap YG, Camm AJ. Drug induced qt prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
10. Jayasinghe R, Registrar S, Kovoor P. Drugs and the QTc interval. Aust Prescr. 2002;25:63-65.
11. Atacand HCT 32/25 mg gives patients and physicians more treatment flexibility. Available at: www.pharmacitelink.com/news/2008/08/14_az.pdf. Accessed Nov. 4, 2008.
12. FDA approves astellas’ vaprisol (conivaptan hydrochloride injection) premixed in 5% dextrose for the treatment of hyponatremia. Sandoz Web site. Available at: sandoz.yellowbrix.com/pages/sandoz/Story.nsp?story_id=122559939. Accessed Nov. 4, 2008.
13. U.S. FDA drug shortages. Available at: www.fda.gov/cder/drug/shortages/default.htm#Foscavir. Accessed Nov. 3, 2008.
14. FDA Drug Shortages. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/ shortages/discontinuation.pdf. Accessed Nov. 6, 2008.
15. Waknine Y. FDA safety changes: mirena, zyvox, orencia. Medscape Web site. Available at: www.medscape.com/viewarticle/580101. Accessed Nov. 3, 2008.
16. MannKind and Pfizer announce collaboration for certain exubera patients to transition to Mannkind’s inhaled insulin therapy. Drugs.com Web site. Available at: www.drugs.com/news/mannkind-pfizer-announce-collaboration-certain-exubera-patients-transition-mannkind-s-inhaled-13677.html. Accessed Nov. 3, 2008.
17. MannKind reports positive data from a phase 3 clinical study of technosphere insulin in Type 1 diabetics. Drugs.com Web site. Available at: www.drugs.com/ clinical_trials/mannkind-reports-positive-data-phase-3-clinical-study-technosphere-insulin-type-1-diabetes-5554.html. Accessed Nov. 3, 2008.
18. Bratulic A. Sanofi-aventis to halt all Acomplia trials. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=34DAB2DC3D7A48939A1D24AB97204CB4&logRowId=263560. Accessed Nov. 6, 2008.