User login
Criteria for Appropriate Use of Peripherally Inserted Central Catheters
Clinical question: What are criteria for appropriate and inappropriate use of PICCs?
Background: PICCs are commonly used in medical care in a variety of clinical contexts; however, criteria defining the appropriate use of PICCs and practices related to PICC placement have not been previously established.
Study design: A multispecialty panel classified indications for PICC use as appropriate or inappropriate using the RAND/UCLA Appropriateness Method.
Synopsis: Selected appropriate PICC uses include:
- Infusion of peripherally compatible infusates, intermittent infusions, or infrequent phlebotomy in patients with poor or difficult venous access when the expected duration of use is at least six days;
- Phlebotomy at least every eight hours when the expected duration of use is at least six days; and
- Invasive hemodynamic monitoring in a critically ill patient only if the duration of use is expected to exceed 15 days.
Selected appropriate PICC-related practices:
- Verify PICC tip position using a chest radiograph only after non-ECG or non-fluoroscopically guided PICC insertion;
- Provide an interval without a PICC to allow resolution of bacteremia when managing PICC-related bloodstream infections; and
- For PICC-related DVT, provide at least three months of systemic anticoagulation if not otherwise contraindicated.
Selected inappropriate PICC-related practices:
- Adjustment of PICC tips that reside in the lower third of the superior vena cava, cavoatrial junction, or right atrium; and
- Removal or replacement of PICCs that are clinically necessary, well positioned, and functional in the setting of PICC-related DVT or without evidence of catheter-associated bloodstream infection.
Bottom line: A multispecialty expert panel provides guidance for appropriate use of PICCs and PICC-related practices.
Citation: Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6):S1-S40.
Clinical question: What are criteria for appropriate and inappropriate use of PICCs?
Background: PICCs are commonly used in medical care in a variety of clinical contexts; however, criteria defining the appropriate use of PICCs and practices related to PICC placement have not been previously established.
Study design: A multispecialty panel classified indications for PICC use as appropriate or inappropriate using the RAND/UCLA Appropriateness Method.
Synopsis: Selected appropriate PICC uses include:
- Infusion of peripherally compatible infusates, intermittent infusions, or infrequent phlebotomy in patients with poor or difficult venous access when the expected duration of use is at least six days;
- Phlebotomy at least every eight hours when the expected duration of use is at least six days; and
- Invasive hemodynamic monitoring in a critically ill patient only if the duration of use is expected to exceed 15 days.
Selected appropriate PICC-related practices:
- Verify PICC tip position using a chest radiograph only after non-ECG or non-fluoroscopically guided PICC insertion;
- Provide an interval without a PICC to allow resolution of bacteremia when managing PICC-related bloodstream infections; and
- For PICC-related DVT, provide at least three months of systemic anticoagulation if not otherwise contraindicated.
Selected inappropriate PICC-related practices:
- Adjustment of PICC tips that reside in the lower third of the superior vena cava, cavoatrial junction, or right atrium; and
- Removal or replacement of PICCs that are clinically necessary, well positioned, and functional in the setting of PICC-related DVT or without evidence of catheter-associated bloodstream infection.
Bottom line: A multispecialty expert panel provides guidance for appropriate use of PICCs and PICC-related practices.
Citation: Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6):S1-S40.
Clinical question: What are criteria for appropriate and inappropriate use of PICCs?
Background: PICCs are commonly used in medical care in a variety of clinical contexts; however, criteria defining the appropriate use of PICCs and practices related to PICC placement have not been previously established.
Study design: A multispecialty panel classified indications for PICC use as appropriate or inappropriate using the RAND/UCLA Appropriateness Method.
Synopsis: Selected appropriate PICC uses include:
- Infusion of peripherally compatible infusates, intermittent infusions, or infrequent phlebotomy in patients with poor or difficult venous access when the expected duration of use is at least six days;
- Phlebotomy at least every eight hours when the expected duration of use is at least six days; and
- Invasive hemodynamic monitoring in a critically ill patient only if the duration of use is expected to exceed 15 days.
Selected appropriate PICC-related practices:
- Verify PICC tip position using a chest radiograph only after non-ECG or non-fluoroscopically guided PICC insertion;
- Provide an interval without a PICC to allow resolution of bacteremia when managing PICC-related bloodstream infections; and
- For PICC-related DVT, provide at least three months of systemic anticoagulation if not otherwise contraindicated.
Selected inappropriate PICC-related practices:
- Adjustment of PICC tips that reside in the lower third of the superior vena cava, cavoatrial junction, or right atrium; and
- Removal or replacement of PICCs that are clinically necessary, well positioned, and functional in the setting of PICC-related DVT or without evidence of catheter-associated bloodstream infection.
Bottom line: A multispecialty expert panel provides guidance for appropriate use of PICCs and PICC-related practices.
Citation: Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6):S1-S40.
ACP Guidelines for Evaluation of Suspected Pulmonary Embolism
Clinical question: What are best practices for evaluating patients with suspected acute pulmonary embolism (PE)?
Background: Use of CT in the evaluation of PE has increased across all clinical settings without improving mortality. Contrast CT carries the risks of radiation exposure, contrast-induced nephropathy, and incidental findings that require further investigation. The authors highlight evidence-based strategies for evaluation of PE, focusing on delivering high-value care.
Study design: Clinical guideline.
Setting: Literature review of studies across all adult clinical settings.
Synopsis: The clinical guidelines committee of the American College of Physicians conducted a literature search surrounding evaluation of suspected acute PE. From their review, they concluded:
- Pretest probability should initially be determined based on validated prediction tools (Wells score, Revised Geneva);
- In patients found to have low pretest probability and meeting the pulmonary embolism rule-out criteria (PERC), clinicians can forego d-dimer testing;
- In those with intermediate pretest probability or those with low pre-test probability who do not pass PERC, d-dimer measurement should be obtained;
- The d-dimer threshold should be age adjusted and imaging should not be pursued in patients whose d-dimer level falls below this cutoff, while those with positive d-dimers should receive CT pulmonary angiography (CTPA); and
- Patients with high pretest probability should undergo CTPA (or V/Q scan if CTPA is contraindicated) without d-dimer testing.
Bottom line: In suspected acute PE, first determine pretest probability using Wells and Revised Geneva, and then use this probability in conjunction with the PERC and d-dimer (as indicated) to guide decisions about imaging.
Citation: Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
Clinical question: What are best practices for evaluating patients with suspected acute pulmonary embolism (PE)?
Background: Use of CT in the evaluation of PE has increased across all clinical settings without improving mortality. Contrast CT carries the risks of radiation exposure, contrast-induced nephropathy, and incidental findings that require further investigation. The authors highlight evidence-based strategies for evaluation of PE, focusing on delivering high-value care.
Study design: Clinical guideline.
Setting: Literature review of studies across all adult clinical settings.
Synopsis: The clinical guidelines committee of the American College of Physicians conducted a literature search surrounding evaluation of suspected acute PE. From their review, they concluded:
- Pretest probability should initially be determined based on validated prediction tools (Wells score, Revised Geneva);
- In patients found to have low pretest probability and meeting the pulmonary embolism rule-out criteria (PERC), clinicians can forego d-dimer testing;
- In those with intermediate pretest probability or those with low pre-test probability who do not pass PERC, d-dimer measurement should be obtained;
- The d-dimer threshold should be age adjusted and imaging should not be pursued in patients whose d-dimer level falls below this cutoff, while those with positive d-dimers should receive CT pulmonary angiography (CTPA); and
- Patients with high pretest probability should undergo CTPA (or V/Q scan if CTPA is contraindicated) without d-dimer testing.
Bottom line: In suspected acute PE, first determine pretest probability using Wells and Revised Geneva, and then use this probability in conjunction with the PERC and d-dimer (as indicated) to guide decisions about imaging.
Citation: Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
Clinical question: What are best practices for evaluating patients with suspected acute pulmonary embolism (PE)?
Background: Use of CT in the evaluation of PE has increased across all clinical settings without improving mortality. Contrast CT carries the risks of radiation exposure, contrast-induced nephropathy, and incidental findings that require further investigation. The authors highlight evidence-based strategies for evaluation of PE, focusing on delivering high-value care.
Study design: Clinical guideline.
Setting: Literature review of studies across all adult clinical settings.
Synopsis: The clinical guidelines committee of the American College of Physicians conducted a literature search surrounding evaluation of suspected acute PE. From their review, they concluded:
- Pretest probability should initially be determined based on validated prediction tools (Wells score, Revised Geneva);
- In patients found to have low pretest probability and meeting the pulmonary embolism rule-out criteria (PERC), clinicians can forego d-dimer testing;
- In those with intermediate pretest probability or those with low pre-test probability who do not pass PERC, d-dimer measurement should be obtained;
- The d-dimer threshold should be age adjusted and imaging should not be pursued in patients whose d-dimer level falls below this cutoff, while those with positive d-dimers should receive CT pulmonary angiography (CTPA); and
- Patients with high pretest probability should undergo CTPA (or V/Q scan if CTPA is contraindicated) without d-dimer testing.
Bottom line: In suspected acute PE, first determine pretest probability using Wells and Revised Geneva, and then use this probability in conjunction with the PERC and d-dimer (as indicated) to guide decisions about imaging.
Citation: Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
When Should Hospitalists Order Continuous Cardiac Monitoring?
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
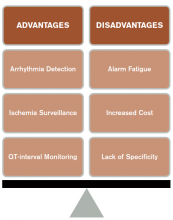
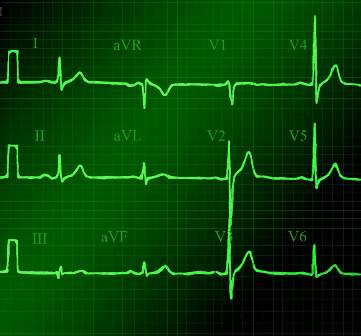
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
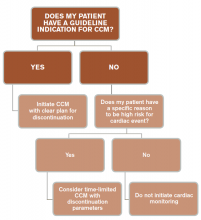
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
Case
Two patients on continuous cardiac monitoring (CCM) are admitted to the hospital. One is a 56-year-old man with hemodynamically stable sepsis secondary to pneumonia. There is no sign of arrhythmia on initial evaluation. The second patient is a 67-year-old man with a history of coronary artery disease (CAD) admitted with chest pain. Should these patients be admitted with CCM?
Overview
CCM was first introduced in hospitals in the early 1960s for heart rate and rhythm monitoring in coronary ICUs. Since that time, CCM has been widely used in the hospital setting among critically and noncritically ill patients. Some hospitals have a limited capacity for monitoring, which is dictated by bed or technology availability. Other hospitals have the ability to monitor any patient.
Guidelines from the American College of Cardiology (ACC) in 1991 and the American Heart Association (AHA) in 2004 guide inpatient use of CCM. These guidelines make recommendations based on the likelihood of patient benefit—will likely benefit, may benefit, unlikely to benefit—and are primarily based on expert opinion; rigorous clinical trial data is not available.1,2 Based on these guidelines, patients with primary cardiac diagnoses, including acute coronary syndrome (ACS), post-cardiac surgery, and arrhythmia, are the most likely to benefit from monitoring.2,3
In practical use, many hospitalists use CCM to detect signs of hemodynamic instability.3 Currently there is no data to support the idea that CCM is a safe or equivalent method of detecting hemodynamic instability compared to close clinical evaluation and frequent vital sign measurement. In fact, physicians overestimate the utility of CCM in guiding management decisions, and witnessed clinical deterioration is a more frequent factor in the decision to escalate the level of care of a patient.3,4
Guideline Recommendations
CCM is intended to identify life-threatening arrhythmias, ischemia, and QT prolongation (see Figure 1). The AHA guidelines address which patients will benefit from CCM; the main indications include an acute cardiac diagnosis or critical illness.1
In addition, the AHA guidelines provide recommendations for the duration of monitoring. These recommendations vary from time-limited monitoring (e.g. unexplained syncope) to a therapeutic-based recommendation (e.g. high-grade atrioventricular block requiring pacemaker placement).
The guidelines also identify a subset of patients who are unlikely to benefit from monitoring (Class III), including low-risk post-operative patients, patients with rate-controlled atrial fibrillation, and patients undergoing hemodialysis without other indications for monitoring.
Several studies have examined the frequency of CCM use. In one study of 236 admissions to a community hospital general ward population, approximately 50% of the 745 monitoring days were not indicated by ACC/AHA guidelines.5 In this study, only 5% of telemetry events occurred in patients without indications, and none of these events required any specific therapy.5 Thus, improved adherence to the ACC/AHA guidelines can decrease CCM use in patients who are unlikely to benefit.
Life-threatening arrhythmia detection. Cleverley and colleagues reported that patients who suffered a cardiac arrest on noncritical care units had a higher survival to hospital discharge if they were on CCM during the event.6 However, a similar study recently showed no benefit to cardiac monitoring for in-hospital arrest if patients were monitored remotely.7 Patients who experience a cardiac arrest in a noncritical care area may benefit from direct cardiac monitoring, though larger studies are needed to assess all potential confounding effects, including nurse-to-patient ratios, location of monitoring (remote or unit-based), advanced cardiac life support response times, and whether the event was witnessed.
Bottom line: AHA guidelines recommend use of CCM in patients with a higher likelihood of developing a life-threatening arrhythmia, including those with an ACS, those experiencing post-cardiac arrest, or those who are critically ill. Medical ward patients who should be monitored include those with acute or subacute congestive heart failure, syncope of unknown etiology, and uncontrolled atrial fibrillation.1
Ischemia surveillance. Computerized ST-segment monitoring has been available for high-risk post-operative patients and those with acute cardiac events since the mid-1980s. When properly used, it offers the ability to detect “silent” ischemia, which is associated with increased in-hospital complications and worse patient outcomes.
Computerized ST-segment monitoring is often associated with a high rate of false positive alarms, however, and has not been universally adopted. Recommendations for its use are based on expert opinion, because no randomized trial has shown that increasing the sensitivity of ischemia detection improves patient outcomes.
Bottom line: AHA guidelines recommend ST-segment monitoring in patients with early ACS and post-acute MI as well as in patients at high risk for silent ischemia, including high-risk post-operative patients.1
QT-interval monitoring. A corrected QT-interval (QTc) greater than 0.50 milliseconds correlates with a higher risk for torsades de pointes and is associated with higher mortality. In critically ill patients in a large academic medical center, guideline-based QT-interval monitoring showed poor specificity for predicting the development of QTc prolongation; however, the risk of QTc prolongation increased with the presence of multiple risk factors.8
Bottom line: AHA guidelines recommend QT-interval monitoring in patients with risk factors for QTc-prolongation, including those starting QTc-prolonging drugs, those with overdose of pro-arrhythmic drugs, those with new-onset bradyarrhythmias, those with severe hypokalemia or hypomagnesemia, and those who have experienced acute neurologic events.1
Recommendations Outside of Guidelines
Patients admitted to medical services for noncardiac diagnoses have a high rate of telemetry use and a perceived benefit associated with cardiac monitoring.3 Although guidelines for noncardiac patients to direct hospitalists on when to use this technology are lacking, there may be some utility in monitoring certain subsets of inpatients.
Sepsis. Patients with hemodynamically stable sepsis develop atrial fibrillation at a higher rate than patients without sepsis and have higher in-hospital mortality. Patients at highest risk are those who are elderly or have severe sepsis.7 CCM can identify atrial fibrillation in real time, which may allow for earlier intervention; however, it is important to consider that other modalities, such as patient symptoms, physical exam, and standard EKG, are potentially as effective at detecting atrial fibrillation as CCM.
Bottom line: Our recommendation is to use CCM in patients who are at higher risk, including elderly patients and those with severe sepsis, until sepsis has resolved and/or the patient is hemodynamically stable for 24 hours.
Alcohol withdrawal. Patients with severe alcohol withdrawal have an increased incidence of arrhythmia and ischemia during the detoxification process. Specifically, patients with delirium tremens and seizures are at higher risk for significant QTc prolongation and tachyarrhythmias.9
Bottom line: Our recommendation is to use CCM in patients with severe alcohol withdrawal and to discontinue monitoring once withdrawal has resolved.
COPD. Patients with COPD exacerbations have a high risk of in-hospital and long-term mortality. The highest risk for mortality appears to be in patients presenting with atrial or ventricular arrhythmias and those over 65 years old.10 There is no clear evidence that beta-agonist use in COPD exacerbations increases arrhythmias other than sinus tachycardia or is associated with worse outcomes.11
Bottom line: Our recommendation is to use CCM only in patients with COPD exacerbation who have other indications as described in the AHA guidelines.
CCM Disadvantages
Alarm fatigue. Alarm fatigue is defined as the desensitization of a clinician to an alarm stimulus, resulting from sensory overload and causing the response of an alarm to be delayed or dismissed.12 In 2014, the Emergency Care Research Institute named alarm hazards as the number one health technology hazard, noting that numerous alarms on a daily basis can lead to desensitization and “alarm fatigue.”
CCM, and the overuse of CCM in particular, contribute to alarm fatigue, which can lead to patient safety issues, including delays in treatment, medication errors, and potentially death.
Increased cost. Because telemetry requires specialized equipment and trained monitoring staff, cost can be significant. In addition to equipment, cost includes time spent by providers, nurses, and technicians interpreting the images and discussing findings with consultants, as well as the additional studies obtained as a result of identified arrhythmias.
Studies on CCM cost vary widely, with conservative estimates of approximately $53 to as much as $1,400 per patient per day in some hospitals.13
Lack of specificity. Because of the high sensitivity and low specificity of CCM, use of CCM in low-risk patients without indications increases the risk of misinterpreting false-positive findings as clinically significant. This can lead to errors in management, including overtesting, unnecessary consultation with subspecialists, and the potential for inappropriate invasive procedures.1
High-Value CCM Use
Because of the low value associated with cardiac monitoring in many patients and the high sensitivity of the guidelines to capture patients at high risk for cardiac events, many hospitals have sought to limit the overuse of this technology. The most successful interventions have targeted the electronic ordering system by requiring an indication and hardwiring an order duration based on guideline recommendations. In a recent study, this intervention led to a 70% decrease in usage and reported $4.8 million cost savings without increasing the rate of in-hospital rapid response or cardiac arrest.14
Systems-level interventions to decrease inappropriate initiation and facilitate discontinuation of cardiac monitoring are a proven way to increase compliance with guidelines and decrease the overuse of CCM.
Back to the Case
According to AHA guidelines, the only patient who has an indication for CCM is the 67-year-old man with known CAD and chest pain, and, accordingly, the patient was placed on CCM. The patient underwent evaluation for ACS, and monitoring was discontinued after 24 hours when ACS was ruled out. The 56-year-old man with sepsis responded to treatment of pneumonia and was not placed on CCM.
In general, patients admitted with acute cardiac-related diseases should be placed on CCM. Guidelines are lacking with respect to many noncardiac diseases, and we recommend a time-limited duration (typically 24 hours) if CCM is ordered for a patient with a special circumstance outside of guidelines (see Figure 3).
Key Takeaway
Hospitalists should use continuous cardiac monitoring for specific indications and not routinely for all patients.
Drs. Lacy and Rendon are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Davis is a resident in internal medicine at UNM, and Dr. Tolstrup is a cardiologist at UNM.
References
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. doi:10.1161/01.CIR.0000145144.56673.59.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol. 1991;18(6):1431-1433.
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med. 2012;172(17):1349-1350. doi:10.1001/archinternmed.2012.3163.
- Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol. 1995;76(12):960-965.
- Curry JP, Hanson CW III, Russell MW, Hanna C, Devine G, Ochroch EA. The use and effectiveness of electrocardiographic telemetry monitoring in a community hospital general care setting. Anesth Analg. 2003;97(5):1483-1487.
- Cleverley K, Mousavi N, Stronger L, et al. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation. 2013;84(7):878-882. doi:10.1016/j.resuscitation.2013.01.038.
- Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3. doi:10.1016/j.ahj.2013.03.020.
- Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Drew BJ. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol. 2010;43(6):572-576. doi:10.1016/j.jelectrocard.2010.05.016.
- Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227. doi:2006/13/smw-11319.
- Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272-277.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321.
- McCartney PR. Clinical alarm management. MCN Am J Matern Child Nurs. 2012;37(3):202. doi:10.1097/NMC.0b013e31824c5b4a.
- Benjamin EM, Klugman RA, Luckmann R, Fairchild DG, Abookire SA. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care. 2013;19(6):e225-e232.
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. doi:10.1001/jamainternmed.2014.4491.
COPD Exacerbation Prevention: April 2015 CHEST Guidelines
Background
The CHEST guidelines for the prevention of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) were developed through a collaboration between the American College of Chest Physicians (CHEST) and the Canadian Thoracic Society (CTS). They are the first evidence-based guidelines dedicated entirely to the prevention of AECOPD and largely exclude material related to the treatment of symptomatic disease.1
Patients with AECOPD are commonly cared for by hospitalists, so we fill an important role in the longitudinal treatment of this disease. Acute exacerbations and hospitalizations for COPD account for 50% of all COPD-related expenses.2,3 Further, the Agency for Healthcare Research and Quality showed a 20% readmission rate nationally for AECOPD, far higher than the rate for most other diagnoses.4 Consequently, COPD readmission has been added to Medicare’s Hospital Readmissions Reduction Program for fiscal year 2015.5
Hospitalization, when the patient is a captive audience and many of the necessary resources are available, may be a time to initiate preventative strategies.
Guideline Updates
The guidelines for non-pharmacologic interventions start with vaccination and continue with behavioral modification. They support the use of the 23-valent polysaccharide pneumococcal vaccine and annual influenza vaccination, noting that only influenza vaccination has been shown to decrease AECOPD.
Pulmonary rehabilitation is recommended for patients with a recent (fewer than four weeks) exacerbation. Several recommendations favor combining social work interventions with education, adding that face-to-face verbal education is superior to written educational materials. Interestingly, smoking cessation interventions received a weak recommendation, based upon lack of literature specifically focusing on the prevention of AECOPD. Despite this recommendation, smoking cessation intervention is strongly encouraged by the authors, given evidence of a marked reduction in morbidity, mortality, and healthcare utilization among smokers with COPD who quit.6 Finally, telemonitoring is not considered to be superior to usual care.
The guidelines concerning inhaled therapies fall into three major drug classes, including short- and long-acting inhaled muscarinic antagonists (anticholinergic agents), short- and long-acting inhaled beta-agonists, and inhaled corticosteroids.
Long-acting medications are generally considered more effective in preventing exacerbations than those that are short acting. Long-acting muscarinic antagonists (LAMAs) are highlighted for their efficacy, and combination inhaled long-acting beta-agonists (LABAs) and inhaled corticosteroids are preferred over monotherapy with either agent alone. LAMAs are preferred to inhaled corticosteroids or LABAs when given as monotherapy.
Short-acting agents are rated as inferior at preventing exacerbations compared to their long-acting analogs, but short-acting medications are better than placebo when combined with long-acting agents from other drug classes. Triple drug therapy (inhaled LAMAs, LABAs, and corticosteroids) can be considered based on current evidence.
The final recommendations address the use of oral medications. A potentially practice-changing guideline is the recommendation for long-term use of N-acetylcysteine tablets twice daily for patients who have experienced more than two exacerbations within two years. A more intuitive recommendation in this group is that treating an AECOPD with oral or IV steroids decreases the chance of recurrent exacerbations in the future.
The remaining recommendations include daily macrolide therapy, the phosphodiesterate-4 inhibitor roflumilast for those with chronic bronchitis and a recent exacerbation, and slow-release theophylline for stable disease. These guidelines also point out that statins do not have a role in AECOPD prevention. An expert consensus also recommends carbocysteine for patients who have failed “maximal” therapy.1
Established Guideline Analysis
Prior guidelines that address stable COPD do exist, most notably from Quaseem and colleagues in the 2011 Annals of Internal Medicine (AIM) and the 2015 GOLD guidelines.7,8 The prior guidelines published in AIM offered limited recommendations on the preventative interventions of bronchodilator use, pulmonary rehabilitation, and oxygen use.
The recommendations made in AIM are similar to those in the CHEST guidelines; the lack of breadth in the AIM report reflects new data generated over the last half decade. They include preventing causative exposures (e.g. tobacco, occupational), recommending bronchodilator use (with or without inhaled corticosteroids), possibly using phosphodiesterase-4 inhibitors (PD-4 inhibitors), administering appropriate vaccines, and providing education; however, GOLD does not actually present or rate the evidence associated with those recommendations. GOLD does specifically state that statins have no role in AECOPD prevention, a position that is updated from more recent literature.8,9
The National Guideline Clearinghouse (NGC) also includes some references to prevention of AECOPD but has no sections explicitly dedicated to prevention. Of note, the NGC still endorses statin use and does not appear to have incorporated data from newer studies.8,10
Hospitalist Takeaways
Given the high rate of COPD readmissions and its broad impact on morbidity and healthcare costs, measures to prevent COPD exacerbations cannot remain out of scope of care for inpatient physicians. It is important to initiate pulmonary rehab within four weeks of an exacerbation of COPD to prevent future exacerbations. Systems should be put in place to assure that all patients who qualify are vaccinated for influenza and patients who continue to smoke receive cessation counseling.
Today, hospitalists are comfortable with these non-pharmacologic interventions, as well as medications that include inhaled bronchodilators, nebulized medications, macrolide maintenance therapy, and oral steroids; however, other oral medications, such as phosphodiesterase inhibitors, theophylline, and N-acetylcysteine, may be appropriate for select patients, and hospitalists should become more familiar with their utility.11,12,13,14
Finally, it is important to note that both short- and long-acting inhaled muscarinic antagonists have come to the forefront of pharmacologic interventions for COPD exacerbation prevention.
Dr. Lampman, MD, is a hospitalist, consulting provider, and physician leader of the physician advisor program at Duke Regional Hospital in Durham, N.C. Dr. Lovins is a hospitalist, associate chief medical informatics officer, and assistant professor of medicine at Duke University and Duke Regional Hospital.
References
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894-942.
- Miravitlles M, Garcia-Polo C, Domenech A, Villegas G, Conget F, de la Roza C. Clinical outcomes and cost analysis of exacerbations in chronic obstructive pulmonary disease. Lung. 2013;191(5):523-530.
- Miravitlles M, Murio C, Guerrero T, Gisbert R; DAFNE Study Group. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121(5):1449-1455.
- Elixhauser A, Au DH, Podulka J. Readmissions for Chronic Obstructive Pulmonary Disease, 2008: Statistical Brief #121.In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, Md.: Agency for Health Care Policy and Research: 2006.
- Readmissions Reduction Program. Centers for Medicare and Medicaid Services website. Accessed September 8, 2015.
- Sicras-Mainar A, Rejas-Gutiérrez J, Navarro-Artieda R, Ibáñez-Nolla J. The effect of quitting smoking on costs and healthcare utilization in patients with chronic obstructive pulmonary disease: a comparison of current smokers versus ex-smokers in routine clinical practice. Lung. 2014;192(4):505-518.
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Global Strategy for the Diagnosis, Management, and Prevention of COPD. Accessed September 8, 2015.
- Criner GJ, Connett JE, Aaron SK, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201-2210.
- Agency for Healthcare Research and Quality. National Guideline Clearinghouse. COPD – chronic obstructive pulmonary disease. In: Pulmonary (acute & chronic). Accessed September 8, 2015.
- Cazzola M, Matera MG. N-acetylcysteine in COPD may be beneficial, but for whom? Lancet Respir Med. 2014;2(3):166-167.
- Turner RD Bothamley. N-acetylcysteine for COPD: the evidence remains inconclusive. Lancet Respir Med. 2014;2(4):e3.
- Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2(3):187-194.
- Amazon.com. Amazon Search. 2015 06/1/2015].
Background
The CHEST guidelines for the prevention of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) were developed through a collaboration between the American College of Chest Physicians (CHEST) and the Canadian Thoracic Society (CTS). They are the first evidence-based guidelines dedicated entirely to the prevention of AECOPD and largely exclude material related to the treatment of symptomatic disease.1
Patients with AECOPD are commonly cared for by hospitalists, so we fill an important role in the longitudinal treatment of this disease. Acute exacerbations and hospitalizations for COPD account for 50% of all COPD-related expenses.2,3 Further, the Agency for Healthcare Research and Quality showed a 20% readmission rate nationally for AECOPD, far higher than the rate for most other diagnoses.4 Consequently, COPD readmission has been added to Medicare’s Hospital Readmissions Reduction Program for fiscal year 2015.5
Hospitalization, when the patient is a captive audience and many of the necessary resources are available, may be a time to initiate preventative strategies.
Guideline Updates
The guidelines for non-pharmacologic interventions start with vaccination and continue with behavioral modification. They support the use of the 23-valent polysaccharide pneumococcal vaccine and annual influenza vaccination, noting that only influenza vaccination has been shown to decrease AECOPD.
Pulmonary rehabilitation is recommended for patients with a recent (fewer than four weeks) exacerbation. Several recommendations favor combining social work interventions with education, adding that face-to-face verbal education is superior to written educational materials. Interestingly, smoking cessation interventions received a weak recommendation, based upon lack of literature specifically focusing on the prevention of AECOPD. Despite this recommendation, smoking cessation intervention is strongly encouraged by the authors, given evidence of a marked reduction in morbidity, mortality, and healthcare utilization among smokers with COPD who quit.6 Finally, telemonitoring is not considered to be superior to usual care.
The guidelines concerning inhaled therapies fall into three major drug classes, including short- and long-acting inhaled muscarinic antagonists (anticholinergic agents), short- and long-acting inhaled beta-agonists, and inhaled corticosteroids.
Long-acting medications are generally considered more effective in preventing exacerbations than those that are short acting. Long-acting muscarinic antagonists (LAMAs) are highlighted for their efficacy, and combination inhaled long-acting beta-agonists (LABAs) and inhaled corticosteroids are preferred over monotherapy with either agent alone. LAMAs are preferred to inhaled corticosteroids or LABAs when given as monotherapy.
Short-acting agents are rated as inferior at preventing exacerbations compared to their long-acting analogs, but short-acting medications are better than placebo when combined with long-acting agents from other drug classes. Triple drug therapy (inhaled LAMAs, LABAs, and corticosteroids) can be considered based on current evidence.
The final recommendations address the use of oral medications. A potentially practice-changing guideline is the recommendation for long-term use of N-acetylcysteine tablets twice daily for patients who have experienced more than two exacerbations within two years. A more intuitive recommendation in this group is that treating an AECOPD with oral or IV steroids decreases the chance of recurrent exacerbations in the future.
The remaining recommendations include daily macrolide therapy, the phosphodiesterate-4 inhibitor roflumilast for those with chronic bronchitis and a recent exacerbation, and slow-release theophylline for stable disease. These guidelines also point out that statins do not have a role in AECOPD prevention. An expert consensus also recommends carbocysteine for patients who have failed “maximal” therapy.1
Established Guideline Analysis
Prior guidelines that address stable COPD do exist, most notably from Quaseem and colleagues in the 2011 Annals of Internal Medicine (AIM) and the 2015 GOLD guidelines.7,8 The prior guidelines published in AIM offered limited recommendations on the preventative interventions of bronchodilator use, pulmonary rehabilitation, and oxygen use.
The recommendations made in AIM are similar to those in the CHEST guidelines; the lack of breadth in the AIM report reflects new data generated over the last half decade. They include preventing causative exposures (e.g. tobacco, occupational), recommending bronchodilator use (with or without inhaled corticosteroids), possibly using phosphodiesterase-4 inhibitors (PD-4 inhibitors), administering appropriate vaccines, and providing education; however, GOLD does not actually present or rate the evidence associated with those recommendations. GOLD does specifically state that statins have no role in AECOPD prevention, a position that is updated from more recent literature.8,9
The National Guideline Clearinghouse (NGC) also includes some references to prevention of AECOPD but has no sections explicitly dedicated to prevention. Of note, the NGC still endorses statin use and does not appear to have incorporated data from newer studies.8,10
Hospitalist Takeaways
Given the high rate of COPD readmissions and its broad impact on morbidity and healthcare costs, measures to prevent COPD exacerbations cannot remain out of scope of care for inpatient physicians. It is important to initiate pulmonary rehab within four weeks of an exacerbation of COPD to prevent future exacerbations. Systems should be put in place to assure that all patients who qualify are vaccinated for influenza and patients who continue to smoke receive cessation counseling.
Today, hospitalists are comfortable with these non-pharmacologic interventions, as well as medications that include inhaled bronchodilators, nebulized medications, macrolide maintenance therapy, and oral steroids; however, other oral medications, such as phosphodiesterase inhibitors, theophylline, and N-acetylcysteine, may be appropriate for select patients, and hospitalists should become more familiar with their utility.11,12,13,14
Finally, it is important to note that both short- and long-acting inhaled muscarinic antagonists have come to the forefront of pharmacologic interventions for COPD exacerbation prevention.
Dr. Lampman, MD, is a hospitalist, consulting provider, and physician leader of the physician advisor program at Duke Regional Hospital in Durham, N.C. Dr. Lovins is a hospitalist, associate chief medical informatics officer, and assistant professor of medicine at Duke University and Duke Regional Hospital.
References
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894-942.
- Miravitlles M, Garcia-Polo C, Domenech A, Villegas G, Conget F, de la Roza C. Clinical outcomes and cost analysis of exacerbations in chronic obstructive pulmonary disease. Lung. 2013;191(5):523-530.
- Miravitlles M, Murio C, Guerrero T, Gisbert R; DAFNE Study Group. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121(5):1449-1455.
- Elixhauser A, Au DH, Podulka J. Readmissions for Chronic Obstructive Pulmonary Disease, 2008: Statistical Brief #121.In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, Md.: Agency for Health Care Policy and Research: 2006.
- Readmissions Reduction Program. Centers for Medicare and Medicaid Services website. Accessed September 8, 2015.
- Sicras-Mainar A, Rejas-Gutiérrez J, Navarro-Artieda R, Ibáñez-Nolla J. The effect of quitting smoking on costs and healthcare utilization in patients with chronic obstructive pulmonary disease: a comparison of current smokers versus ex-smokers in routine clinical practice. Lung. 2014;192(4):505-518.
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Global Strategy for the Diagnosis, Management, and Prevention of COPD. Accessed September 8, 2015.
- Criner GJ, Connett JE, Aaron SK, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201-2210.
- Agency for Healthcare Research and Quality. National Guideline Clearinghouse. COPD – chronic obstructive pulmonary disease. In: Pulmonary (acute & chronic). Accessed September 8, 2015.
- Cazzola M, Matera MG. N-acetylcysteine in COPD may be beneficial, but for whom? Lancet Respir Med. 2014;2(3):166-167.
- Turner RD Bothamley. N-acetylcysteine for COPD: the evidence remains inconclusive. Lancet Respir Med. 2014;2(4):e3.
- Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2(3):187-194.
- Amazon.com. Amazon Search. 2015 06/1/2015].
Background
The CHEST guidelines for the prevention of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) were developed through a collaboration between the American College of Chest Physicians (CHEST) and the Canadian Thoracic Society (CTS). They are the first evidence-based guidelines dedicated entirely to the prevention of AECOPD and largely exclude material related to the treatment of symptomatic disease.1
Patients with AECOPD are commonly cared for by hospitalists, so we fill an important role in the longitudinal treatment of this disease. Acute exacerbations and hospitalizations for COPD account for 50% of all COPD-related expenses.2,3 Further, the Agency for Healthcare Research and Quality showed a 20% readmission rate nationally for AECOPD, far higher than the rate for most other diagnoses.4 Consequently, COPD readmission has been added to Medicare’s Hospital Readmissions Reduction Program for fiscal year 2015.5
Hospitalization, when the patient is a captive audience and many of the necessary resources are available, may be a time to initiate preventative strategies.
Guideline Updates
The guidelines for non-pharmacologic interventions start with vaccination and continue with behavioral modification. They support the use of the 23-valent polysaccharide pneumococcal vaccine and annual influenza vaccination, noting that only influenza vaccination has been shown to decrease AECOPD.
Pulmonary rehabilitation is recommended for patients with a recent (fewer than four weeks) exacerbation. Several recommendations favor combining social work interventions with education, adding that face-to-face verbal education is superior to written educational materials. Interestingly, smoking cessation interventions received a weak recommendation, based upon lack of literature specifically focusing on the prevention of AECOPD. Despite this recommendation, smoking cessation intervention is strongly encouraged by the authors, given evidence of a marked reduction in morbidity, mortality, and healthcare utilization among smokers with COPD who quit.6 Finally, telemonitoring is not considered to be superior to usual care.
The guidelines concerning inhaled therapies fall into three major drug classes, including short- and long-acting inhaled muscarinic antagonists (anticholinergic agents), short- and long-acting inhaled beta-agonists, and inhaled corticosteroids.
Long-acting medications are generally considered more effective in preventing exacerbations than those that are short acting. Long-acting muscarinic antagonists (LAMAs) are highlighted for their efficacy, and combination inhaled long-acting beta-agonists (LABAs) and inhaled corticosteroids are preferred over monotherapy with either agent alone. LAMAs are preferred to inhaled corticosteroids or LABAs when given as monotherapy.
Short-acting agents are rated as inferior at preventing exacerbations compared to their long-acting analogs, but short-acting medications are better than placebo when combined with long-acting agents from other drug classes. Triple drug therapy (inhaled LAMAs, LABAs, and corticosteroids) can be considered based on current evidence.
The final recommendations address the use of oral medications. A potentially practice-changing guideline is the recommendation for long-term use of N-acetylcysteine tablets twice daily for patients who have experienced more than two exacerbations within two years. A more intuitive recommendation in this group is that treating an AECOPD with oral or IV steroids decreases the chance of recurrent exacerbations in the future.
The remaining recommendations include daily macrolide therapy, the phosphodiesterate-4 inhibitor roflumilast for those with chronic bronchitis and a recent exacerbation, and slow-release theophylline for stable disease. These guidelines also point out that statins do not have a role in AECOPD prevention. An expert consensus also recommends carbocysteine for patients who have failed “maximal” therapy.1
Established Guideline Analysis
Prior guidelines that address stable COPD do exist, most notably from Quaseem and colleagues in the 2011 Annals of Internal Medicine (AIM) and the 2015 GOLD guidelines.7,8 The prior guidelines published in AIM offered limited recommendations on the preventative interventions of bronchodilator use, pulmonary rehabilitation, and oxygen use.
The recommendations made in AIM are similar to those in the CHEST guidelines; the lack of breadth in the AIM report reflects new data generated over the last half decade. They include preventing causative exposures (e.g. tobacco, occupational), recommending bronchodilator use (with or without inhaled corticosteroids), possibly using phosphodiesterase-4 inhibitors (PD-4 inhibitors), administering appropriate vaccines, and providing education; however, GOLD does not actually present or rate the evidence associated with those recommendations. GOLD does specifically state that statins have no role in AECOPD prevention, a position that is updated from more recent literature.8,9
The National Guideline Clearinghouse (NGC) also includes some references to prevention of AECOPD but has no sections explicitly dedicated to prevention. Of note, the NGC still endorses statin use and does not appear to have incorporated data from newer studies.8,10
Hospitalist Takeaways
Given the high rate of COPD readmissions and its broad impact on morbidity and healthcare costs, measures to prevent COPD exacerbations cannot remain out of scope of care for inpatient physicians. It is important to initiate pulmonary rehab within four weeks of an exacerbation of COPD to prevent future exacerbations. Systems should be put in place to assure that all patients who qualify are vaccinated for influenza and patients who continue to smoke receive cessation counseling.
Today, hospitalists are comfortable with these non-pharmacologic interventions, as well as medications that include inhaled bronchodilators, nebulized medications, macrolide maintenance therapy, and oral steroids; however, other oral medications, such as phosphodiesterase inhibitors, theophylline, and N-acetylcysteine, may be appropriate for select patients, and hospitalists should become more familiar with their utility.11,12,13,14
Finally, it is important to note that both short- and long-acting inhaled muscarinic antagonists have come to the forefront of pharmacologic interventions for COPD exacerbation prevention.
Dr. Lampman, MD, is a hospitalist, consulting provider, and physician leader of the physician advisor program at Duke Regional Hospital in Durham, N.C. Dr. Lovins is a hospitalist, associate chief medical informatics officer, and assistant professor of medicine at Duke University and Duke Regional Hospital.
References
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894-942.
- Miravitlles M, Garcia-Polo C, Domenech A, Villegas G, Conget F, de la Roza C. Clinical outcomes and cost analysis of exacerbations in chronic obstructive pulmonary disease. Lung. 2013;191(5):523-530.
- Miravitlles M, Murio C, Guerrero T, Gisbert R; DAFNE Study Group. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121(5):1449-1455.
- Elixhauser A, Au DH, Podulka J. Readmissions for Chronic Obstructive Pulmonary Disease, 2008: Statistical Brief #121.In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, Md.: Agency for Health Care Policy and Research: 2006.
- Readmissions Reduction Program. Centers for Medicare and Medicaid Services website. Accessed September 8, 2015.
- Sicras-Mainar A, Rejas-Gutiérrez J, Navarro-Artieda R, Ibáñez-Nolla J. The effect of quitting smoking on costs and healthcare utilization in patients with chronic obstructive pulmonary disease: a comparison of current smokers versus ex-smokers in routine clinical practice. Lung. 2014;192(4):505-518.
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Global Strategy for the Diagnosis, Management, and Prevention of COPD. Accessed September 8, 2015.
- Criner GJ, Connett JE, Aaron SK, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201-2210.
- Agency for Healthcare Research and Quality. National Guideline Clearinghouse. COPD – chronic obstructive pulmonary disease. In: Pulmonary (acute & chronic). Accessed September 8, 2015.
- Cazzola M, Matera MG. N-acetylcysteine in COPD may be beneficial, but for whom? Lancet Respir Med. 2014;2(3):166-167.
- Turner RD Bothamley. N-acetylcysteine for COPD: the evidence remains inconclusive. Lancet Respir Med. 2014;2(4):e3.
- Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2(3):187-194.
- Amazon.com. Amazon Search. 2015 06/1/2015].
Antibiotic Stewardship and Hospitalists: How to Educate Patients on Antibiotics
Editor’s note: This article originally appeared on SHM’s official blog, “The Hospital Leader,” in June 2015.
“Tell me what you know about antibiotics.”
That’s the discussion I start with hospitalized patients all the time, right after they ask me to prescribe antibiotics for their simple cough or other viral-like illness.
And, from their perspective, asking for antibiotics makes sense. After all, antibiotics have been the physician’s knee-jerk reaction to a number of patient symptoms for decades, especially for a cough or upper respiratory infection. We have inadvertently trained our patients that there is an easy solution to almost any common medical problem.
But patients often answer my question with something like “not much,” coupled with a little surprise that I haven’t already started ordering the prescription.
That’s when I talk about the potential harms of antibiotics. And that’s also when their eyebrows go up. I start with the easy harms, like the fact that many antibiotics can cause diarrhea, a symptom nobody wants to deal with along with their runny nose. Then I move on to the big ones: Use of antibiotics today could make the patient resistant to antibiotics later in life, when they might really need them, and using antibiotics can lead to other painful and even fatal conditions, like Clostridium difficile.
After that, every patient agrees with my recommendations that we hold off on antibiotics for certain, particularly viral-like, ailments.
Change the Conversation. Change the Approach.
It’s a longer conversation, but it’s worth it. Overuse of antibiotics affects not only the patient in front of me, but also entire communities. By creating antibiotic-resistant bacteria, we make everyone more vulnerable to the very diseases the antibiotics were originally intended to treat, like tuberculosis, staph infections, and numerous others.
That’s why the hospitalists in my hospital at Johns Hopkins Bayview teamed up with the infectious diseases division to improve our approach to cellulitis and antibiotic use.
In short, cellulitis is a bacterial skin infection. The most feared bacterial skin infection is MRSA (methicillin-resistant Staphylococcus aureus), a “super bug” that requires highly selective antibiotics like vancomycin; however, other more common and less virulent bacteria also cause cellulitis, and they don’t need super bug fighter medications. Some types of skin ailments, like those caused by poor circulation in the legs, are not infectious at all but can look like cellulitis, even to experienced doctors.
Thanks to the collaboration between infectious disease doctors and hospitalists, the hospitalists are much less likely to prescribe inappropriate antibiotics. That’s a triple-win: It reduces the length of stay for the patient, the incidence of C. diff, and costs.
The Front Line
This concern isn’t limited to a single hospital. There are now more than 44,000 hospitalists nationwide, and every one of us plays an important role in antibiotic stewardship. The bedside is the front line of the fight against antibiotic resistance.
tuberculosis, staph infections, and numerous others.
—Eric Howell, MD, SFHM
There are more than 5,000 hospitals across the country, and hospitalists in every one of them must take responsibility for the appropriate use of antibiotics for their patients.
Announcing SHM’s National Commitment to Antibiotic Stewardship
SHM was proud to join more than 150 major organizations at the White House Forum on Antibiotic Stewardship to announce commitments to implement changes over the next five years that will slow the emergence of antibiotic-resistant bacteria, detect resistant strains, preserve the efficacy of our existing antibiotics, and prevent the spread of resistant infections.
Specifically, SHM has committed to three national initiatives that are aligned with our organizational goal of providing the best possible care for the hospitalized patient and the federal government’s dedication to this important issue:
- Enhance hospitalists’ awareness of key antimicrobial stewardship best practices and ask them to formally commit to at least two behavior changes to reduce inappropriate antimicrobial use and antimicrobial resistance;
- Support national initiatives that advocate for the appropriate use of antimicrobials and promote strategies to reduce antimicrobial resistance; and
- Identify partnerships and other opportunities to support the development of a comprehensive program to implement antimicrobial stewardship best practices in America’s hospitals.
These commitments, which I shared with White House Forum participants, play to the strengths of hospitalists in healthcare: advocacy on behalf of patients and quality improvement and collaboration with others.
What Hospitalists Can Do Now
I also know, however, that you aren’t the kind of people to wait for official pronouncements and campaigns to start a program that will improve the care of hospitalized patients. That’s why SHM and I are recommending that all hospitalists begin to take steps immediately to address this national healthcare crisis:
- Start the conversation with your patients. It’s easy to prescribe antibiotics, but it can also be harmful. Talk with your patients about when antibiotics are medically appropriate and the potential harms they may cause.
- Prescribe antibiotics for specific diagnoses. Prescribing “just in case” is a prescription for antibiotic resistance. Make sure you understand the signs and symptoms of the conditions for which you’re prescribing antibiotics. As we learned at our hospital, cellulitis and venous insufficiency can look similar, but only one responds to antibiotic treatment.
- Work with your infectious disease colleagues. They can help you create systems and diagnose patients to help improve your hospital’s antibiotic stewardship.
After all, we are on the front lines, protecting our current and future patients. And we can’t afford to wait.
Dr. Howell is a veteran hospitalist at Johns Hopkins Bayview Hospital in Baltimore and a past president of SHM.
Editor’s note: This article originally appeared on SHM’s official blog, “The Hospital Leader,” in June 2015.
“Tell me what you know about antibiotics.”
That’s the discussion I start with hospitalized patients all the time, right after they ask me to prescribe antibiotics for their simple cough or other viral-like illness.
And, from their perspective, asking for antibiotics makes sense. After all, antibiotics have been the physician’s knee-jerk reaction to a number of patient symptoms for decades, especially for a cough or upper respiratory infection. We have inadvertently trained our patients that there is an easy solution to almost any common medical problem.
But patients often answer my question with something like “not much,” coupled with a little surprise that I haven’t already started ordering the prescription.
That’s when I talk about the potential harms of antibiotics. And that’s also when their eyebrows go up. I start with the easy harms, like the fact that many antibiotics can cause diarrhea, a symptom nobody wants to deal with along with their runny nose. Then I move on to the big ones: Use of antibiotics today could make the patient resistant to antibiotics later in life, when they might really need them, and using antibiotics can lead to other painful and even fatal conditions, like Clostridium difficile.
After that, every patient agrees with my recommendations that we hold off on antibiotics for certain, particularly viral-like, ailments.
Change the Conversation. Change the Approach.
It’s a longer conversation, but it’s worth it. Overuse of antibiotics affects not only the patient in front of me, but also entire communities. By creating antibiotic-resistant bacteria, we make everyone more vulnerable to the very diseases the antibiotics were originally intended to treat, like tuberculosis, staph infections, and numerous others.
That’s why the hospitalists in my hospital at Johns Hopkins Bayview teamed up with the infectious diseases division to improve our approach to cellulitis and antibiotic use.
In short, cellulitis is a bacterial skin infection. The most feared bacterial skin infection is MRSA (methicillin-resistant Staphylococcus aureus), a “super bug” that requires highly selective antibiotics like vancomycin; however, other more common and less virulent bacteria also cause cellulitis, and they don’t need super bug fighter medications. Some types of skin ailments, like those caused by poor circulation in the legs, are not infectious at all but can look like cellulitis, even to experienced doctors.
Thanks to the collaboration between infectious disease doctors and hospitalists, the hospitalists are much less likely to prescribe inappropriate antibiotics. That’s a triple-win: It reduces the length of stay for the patient, the incidence of C. diff, and costs.
The Front Line
This concern isn’t limited to a single hospital. There are now more than 44,000 hospitalists nationwide, and every one of us plays an important role in antibiotic stewardship. The bedside is the front line of the fight against antibiotic resistance.
tuberculosis, staph infections, and numerous others.
—Eric Howell, MD, SFHM
There are more than 5,000 hospitals across the country, and hospitalists in every one of them must take responsibility for the appropriate use of antibiotics for their patients.
Announcing SHM’s National Commitment to Antibiotic Stewardship
SHM was proud to join more than 150 major organizations at the White House Forum on Antibiotic Stewardship to announce commitments to implement changes over the next five years that will slow the emergence of antibiotic-resistant bacteria, detect resistant strains, preserve the efficacy of our existing antibiotics, and prevent the spread of resistant infections.
Specifically, SHM has committed to three national initiatives that are aligned with our organizational goal of providing the best possible care for the hospitalized patient and the federal government’s dedication to this important issue:
- Enhance hospitalists’ awareness of key antimicrobial stewardship best practices and ask them to formally commit to at least two behavior changes to reduce inappropriate antimicrobial use and antimicrobial resistance;
- Support national initiatives that advocate for the appropriate use of antimicrobials and promote strategies to reduce antimicrobial resistance; and
- Identify partnerships and other opportunities to support the development of a comprehensive program to implement antimicrobial stewardship best practices in America’s hospitals.
These commitments, which I shared with White House Forum participants, play to the strengths of hospitalists in healthcare: advocacy on behalf of patients and quality improvement and collaboration with others.
What Hospitalists Can Do Now
I also know, however, that you aren’t the kind of people to wait for official pronouncements and campaigns to start a program that will improve the care of hospitalized patients. That’s why SHM and I are recommending that all hospitalists begin to take steps immediately to address this national healthcare crisis:
- Start the conversation with your patients. It’s easy to prescribe antibiotics, but it can also be harmful. Talk with your patients about when antibiotics are medically appropriate and the potential harms they may cause.
- Prescribe antibiotics for specific diagnoses. Prescribing “just in case” is a prescription for antibiotic resistance. Make sure you understand the signs and symptoms of the conditions for which you’re prescribing antibiotics. As we learned at our hospital, cellulitis and venous insufficiency can look similar, but only one responds to antibiotic treatment.
- Work with your infectious disease colleagues. They can help you create systems and diagnose patients to help improve your hospital’s antibiotic stewardship.
After all, we are on the front lines, protecting our current and future patients. And we can’t afford to wait.
Dr. Howell is a veteran hospitalist at Johns Hopkins Bayview Hospital in Baltimore and a past president of SHM.
Editor’s note: This article originally appeared on SHM’s official blog, “The Hospital Leader,” in June 2015.
“Tell me what you know about antibiotics.”
That’s the discussion I start with hospitalized patients all the time, right after they ask me to prescribe antibiotics for their simple cough or other viral-like illness.
And, from their perspective, asking for antibiotics makes sense. After all, antibiotics have been the physician’s knee-jerk reaction to a number of patient symptoms for decades, especially for a cough or upper respiratory infection. We have inadvertently trained our patients that there is an easy solution to almost any common medical problem.
But patients often answer my question with something like “not much,” coupled with a little surprise that I haven’t already started ordering the prescription.
That’s when I talk about the potential harms of antibiotics. And that’s also when their eyebrows go up. I start with the easy harms, like the fact that many antibiotics can cause diarrhea, a symptom nobody wants to deal with along with their runny nose. Then I move on to the big ones: Use of antibiotics today could make the patient resistant to antibiotics later in life, when they might really need them, and using antibiotics can lead to other painful and even fatal conditions, like Clostridium difficile.
After that, every patient agrees with my recommendations that we hold off on antibiotics for certain, particularly viral-like, ailments.
Change the Conversation. Change the Approach.
It’s a longer conversation, but it’s worth it. Overuse of antibiotics affects not only the patient in front of me, but also entire communities. By creating antibiotic-resistant bacteria, we make everyone more vulnerable to the very diseases the antibiotics were originally intended to treat, like tuberculosis, staph infections, and numerous others.
That’s why the hospitalists in my hospital at Johns Hopkins Bayview teamed up with the infectious diseases division to improve our approach to cellulitis and antibiotic use.
In short, cellulitis is a bacterial skin infection. The most feared bacterial skin infection is MRSA (methicillin-resistant Staphylococcus aureus), a “super bug” that requires highly selective antibiotics like vancomycin; however, other more common and less virulent bacteria also cause cellulitis, and they don’t need super bug fighter medications. Some types of skin ailments, like those caused by poor circulation in the legs, are not infectious at all but can look like cellulitis, even to experienced doctors.
Thanks to the collaboration between infectious disease doctors and hospitalists, the hospitalists are much less likely to prescribe inappropriate antibiotics. That’s a triple-win: It reduces the length of stay for the patient, the incidence of C. diff, and costs.
The Front Line
This concern isn’t limited to a single hospital. There are now more than 44,000 hospitalists nationwide, and every one of us plays an important role in antibiotic stewardship. The bedside is the front line of the fight against antibiotic resistance.
tuberculosis, staph infections, and numerous others.
—Eric Howell, MD, SFHM
There are more than 5,000 hospitals across the country, and hospitalists in every one of them must take responsibility for the appropriate use of antibiotics for their patients.
Announcing SHM’s National Commitment to Antibiotic Stewardship
SHM was proud to join more than 150 major organizations at the White House Forum on Antibiotic Stewardship to announce commitments to implement changes over the next five years that will slow the emergence of antibiotic-resistant bacteria, detect resistant strains, preserve the efficacy of our existing antibiotics, and prevent the spread of resistant infections.
Specifically, SHM has committed to three national initiatives that are aligned with our organizational goal of providing the best possible care for the hospitalized patient and the federal government’s dedication to this important issue:
- Enhance hospitalists’ awareness of key antimicrobial stewardship best practices and ask them to formally commit to at least two behavior changes to reduce inappropriate antimicrobial use and antimicrobial resistance;
- Support national initiatives that advocate for the appropriate use of antimicrobials and promote strategies to reduce antimicrobial resistance; and
- Identify partnerships and other opportunities to support the development of a comprehensive program to implement antimicrobial stewardship best practices in America’s hospitals.
These commitments, which I shared with White House Forum participants, play to the strengths of hospitalists in healthcare: advocacy on behalf of patients and quality improvement and collaboration with others.
What Hospitalists Can Do Now
I also know, however, that you aren’t the kind of people to wait for official pronouncements and campaigns to start a program that will improve the care of hospitalized patients. That’s why SHM and I are recommending that all hospitalists begin to take steps immediately to address this national healthcare crisis:
- Start the conversation with your patients. It’s easy to prescribe antibiotics, but it can also be harmful. Talk with your patients about when antibiotics are medically appropriate and the potential harms they may cause.
- Prescribe antibiotics for specific diagnoses. Prescribing “just in case” is a prescription for antibiotic resistance. Make sure you understand the signs and symptoms of the conditions for which you’re prescribing antibiotics. As we learned at our hospital, cellulitis and venous insufficiency can look similar, but only one responds to antibiotic treatment.
- Work with your infectious disease colleagues. They can help you create systems and diagnose patients to help improve your hospital’s antibiotic stewardship.
After all, we are on the front lines, protecting our current and future patients. And we can’t afford to wait.
Dr. Howell is a veteran hospitalist at Johns Hopkins Bayview Hospital in Baltimore and a past president of SHM.
Study establishes protocol for perioperative dabigatran discontinuation
TORONTO – In atrial fibrillation (AF) patients who must discontinue dabigatran for elective surgery, the risk of both stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting anticoagulation, according to results of a prospective study presented at the International Society on Thrombosis and Haemostasis Congress.
Among key findings presented at a press conference at the ISTH 2015 Congress, no strokes were recorded in more than 500 patients managed with the protocol, and the major bleeding rate was less than 2%, reported the study’s principal investigator, Dr. Sam Schulman, professor of hematology and thromboembolism, McMaster University, Hamilton, Ont.
Data from this study (Circulation 2015) were reported at the press conference alongside a second study of perioperative warfarin management. Both studies are potentially practice changing, because they supply evidence-based guidance for anticoagulation in patients with AF.
Based on the findings from these two studies, “it is important to get this message out” that there are now data available on which to base clinical decisions, reported Dr. Schulman, who is also president of the ISTH 2015 Congress. His data were presented alongside a study that found no benefit from heparin bridging in AF patients when warfarin was stopped 5 days in advance of surgery.
In the study presented by Dr. Schulman, 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment. The protocol provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
The primary outcome evaluated in the study was major bleeding in the first 30 days. Other outcomes of interest included thromboembolic complications, death and minor bleeding.
Major bleeding was observed in 1.8% of patients, a rate that Dr. Schulman characterized as “low and acceptable” in the context of expected background bleeding rates. There were four deaths, but all were unrelated to either bleeding or arterial thromboembolism. The only thromboembolic complication was a single transient ischemic attack. Minor bleeding occurred in 5.2%.
On the basis of the protocol, about half of the patients discontinued dabigatran 24 hours before surgery. No patient discontinued therapy more than 96 hours prior to surgery. The median time to resumption of dabigatran after surgery was 1 day, but the point at which it was restarted ranged between hours and 2 days. Bridging, which describes the injection of heparin for short-term anticoagulation, was not employed preoperatively but was used in 1.7% of cases postoperatively.
At the press conference, data also were reported from the BRIDGE study. That study, published online in the New England Journal of Medicine (2015 June 22; epub ahead of print ), found that bridging was not an effective strategy in AF patients who discontinue warfarin prior to elective surgery. In the press conference, Dr. Thomas L. Ortel, hematology/oncology division, Duke University Medical Center, Durham, N.C., agreed with Dr. Schulman that this is an area where evidence is needed to guide care.
In the absence of data, “physicians do whatever they think is best,” Dr. Schulman noted at the press conference. Referring to strategies for stopping anticoagulants for surgery in patients with AF, Dr. Schulman said, “some of them stop the blood thinner too early because they are afraid that the patient is going to bleed during surgery and instead the patient can have a stroke. Some stop too late, and the patient can have bleeding.”
The data presented at the meeting provide an evidence base for clinical decisions. Dr. Schulman suggested that these data are meaningful for guiding care.
Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
TORONTO – In atrial fibrillation (AF) patients who must discontinue dabigatran for elective surgery, the risk of both stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting anticoagulation, according to results of a prospective study presented at the International Society on Thrombosis and Haemostasis Congress.
Among key findings presented at a press conference at the ISTH 2015 Congress, no strokes were recorded in more than 500 patients managed with the protocol, and the major bleeding rate was less than 2%, reported the study’s principal investigator, Dr. Sam Schulman, professor of hematology and thromboembolism, McMaster University, Hamilton, Ont.
Data from this study (Circulation 2015) were reported at the press conference alongside a second study of perioperative warfarin management. Both studies are potentially practice changing, because they supply evidence-based guidance for anticoagulation in patients with AF.
Based on the findings from these two studies, “it is important to get this message out” that there are now data available on which to base clinical decisions, reported Dr. Schulman, who is also president of the ISTH 2015 Congress. His data were presented alongside a study that found no benefit from heparin bridging in AF patients when warfarin was stopped 5 days in advance of surgery.
In the study presented by Dr. Schulman, 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment. The protocol provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
The primary outcome evaluated in the study was major bleeding in the first 30 days. Other outcomes of interest included thromboembolic complications, death and minor bleeding.
Major bleeding was observed in 1.8% of patients, a rate that Dr. Schulman characterized as “low and acceptable” in the context of expected background bleeding rates. There were four deaths, but all were unrelated to either bleeding or arterial thromboembolism. The only thromboembolic complication was a single transient ischemic attack. Minor bleeding occurred in 5.2%.
On the basis of the protocol, about half of the patients discontinued dabigatran 24 hours before surgery. No patient discontinued therapy more than 96 hours prior to surgery. The median time to resumption of dabigatran after surgery was 1 day, but the point at which it was restarted ranged between hours and 2 days. Bridging, which describes the injection of heparin for short-term anticoagulation, was not employed preoperatively but was used in 1.7% of cases postoperatively.
At the press conference, data also were reported from the BRIDGE study. That study, published online in the New England Journal of Medicine (2015 June 22; epub ahead of print ), found that bridging was not an effective strategy in AF patients who discontinue warfarin prior to elective surgery. In the press conference, Dr. Thomas L. Ortel, hematology/oncology division, Duke University Medical Center, Durham, N.C., agreed with Dr. Schulman that this is an area where evidence is needed to guide care.
In the absence of data, “physicians do whatever they think is best,” Dr. Schulman noted at the press conference. Referring to strategies for stopping anticoagulants for surgery in patients with AF, Dr. Schulman said, “some of them stop the blood thinner too early because they are afraid that the patient is going to bleed during surgery and instead the patient can have a stroke. Some stop too late, and the patient can have bleeding.”
The data presented at the meeting provide an evidence base for clinical decisions. Dr. Schulman suggested that these data are meaningful for guiding care.
Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
TORONTO – In atrial fibrillation (AF) patients who must discontinue dabigatran for elective surgery, the risk of both stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting anticoagulation, according to results of a prospective study presented at the International Society on Thrombosis and Haemostasis Congress.
Among key findings presented at a press conference at the ISTH 2015 Congress, no strokes were recorded in more than 500 patients managed with the protocol, and the major bleeding rate was less than 2%, reported the study’s principal investigator, Dr. Sam Schulman, professor of hematology and thromboembolism, McMaster University, Hamilton, Ont.
Data from this study (Circulation 2015) were reported at the press conference alongside a second study of perioperative warfarin management. Both studies are potentially practice changing, because they supply evidence-based guidance for anticoagulation in patients with AF.
Based on the findings from these two studies, “it is important to get this message out” that there are now data available on which to base clinical decisions, reported Dr. Schulman, who is also president of the ISTH 2015 Congress. His data were presented alongside a study that found no benefit from heparin bridging in AF patients when warfarin was stopped 5 days in advance of surgery.
In the study presented by Dr. Schulman, 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment. The protocol provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
The primary outcome evaluated in the study was major bleeding in the first 30 days. Other outcomes of interest included thromboembolic complications, death and minor bleeding.
Major bleeding was observed in 1.8% of patients, a rate that Dr. Schulman characterized as “low and acceptable” in the context of expected background bleeding rates. There were four deaths, but all were unrelated to either bleeding or arterial thromboembolism. The only thromboembolic complication was a single transient ischemic attack. Minor bleeding occurred in 5.2%.
On the basis of the protocol, about half of the patients discontinued dabigatran 24 hours before surgery. No patient discontinued therapy more than 96 hours prior to surgery. The median time to resumption of dabigatran after surgery was 1 day, but the point at which it was restarted ranged between hours and 2 days. Bridging, which describes the injection of heparin for short-term anticoagulation, was not employed preoperatively but was used in 1.7% of cases postoperatively.
At the press conference, data also were reported from the BRIDGE study. That study, published online in the New England Journal of Medicine (2015 June 22; epub ahead of print ), found that bridging was not an effective strategy in AF patients who discontinue warfarin prior to elective surgery. In the press conference, Dr. Thomas L. Ortel, hematology/oncology division, Duke University Medical Center, Durham, N.C., agreed with Dr. Schulman that this is an area where evidence is needed to guide care.
In the absence of data, “physicians do whatever they think is best,” Dr. Schulman noted at the press conference. Referring to strategies for stopping anticoagulants for surgery in patients with AF, Dr. Schulman said, “some of them stop the blood thinner too early because they are afraid that the patient is going to bleed during surgery and instead the patient can have a stroke. Some stop too late, and the patient can have bleeding.”
The data presented at the meeting provide an evidence base for clinical decisions. Dr. Schulman suggested that these data are meaningful for guiding care.
Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
AT 2015 ISTH CONGRESS
Key clinical point: The risk of stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting dabigatran.
Major finding: The protocol developed provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
Data source: 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment.
Disclosures: Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
Hospital Management of Patients Presenting with ALTE: An Evidence-Based Approach
In a presentation on guidelines for ALTE, Jack Percelay, SHM representative to the AAP Subcommittee, provided further insight to the work that has been done for the clinical entity known as apparent life-threatening events (ALTE) since a consensus statement was put forward by the NIH in 1986. The original statement emphasized 4 possible features to constitute ALTE: apnea, color change, change in tone or gagging. The imprecise nature of the definition, along with both provider and caretaker anxiety related to the diagnosis, have lead to a cascade of diagnostic testing and treatments for what is a symptom complex, not a disease.
Subsequent work in the field has clarified that an ALTE is not a risk factor for SIDS. Of the myriad of etiologies that can cause an ALTE, many will have a readily identifiable etiology that a good history and physical exam will diagnose. Most other diseases, if not diagnosed at initial presentation, will become apparent subsequently without any significant consequences (for example epilepsy). Two diagnoses, which if missed, may have significant consequences include child abuse and a cardiac arrhythmia.
In an effort to synthesize new data along with expert opinion, the American Academy of Pediatrics has convened a Subcommittee on the Guideline for ALTE, lead by Joel Tieder, to develop a new practice guideline. This guideline is still in development with certain areas not ready for broad dissemination. The highlight of the new guideline will be a proposal for a name change for ALTEs. Dr Percelay reports the proposed new name would be BRUE (pronounced “brew”), Brief Resolved Unexplained Event. He anticipates further information to be published that will offer a framework to specify which infants to consider at low risk of recurrence versus higher risk for significant pathology. For those infants identified as low risk, the guideline will offer specific evaluation and treatment recommendations. An anticipated key point of the new guideline will be that a careful history and physical is the cornerstone of the initial evaluation and that in the absence of specific historical or exam findings, diagnostic testing of well-appearing infants is of low value.
In a presentation on guidelines for ALTE, Jack Percelay, SHM representative to the AAP Subcommittee, provided further insight to the work that has been done for the clinical entity known as apparent life-threatening events (ALTE) since a consensus statement was put forward by the NIH in 1986. The original statement emphasized 4 possible features to constitute ALTE: apnea, color change, change in tone or gagging. The imprecise nature of the definition, along with both provider and caretaker anxiety related to the diagnosis, have lead to a cascade of diagnostic testing and treatments for what is a symptom complex, not a disease.
Subsequent work in the field has clarified that an ALTE is not a risk factor for SIDS. Of the myriad of etiologies that can cause an ALTE, many will have a readily identifiable etiology that a good history and physical exam will diagnose. Most other diseases, if not diagnosed at initial presentation, will become apparent subsequently without any significant consequences (for example epilepsy). Two diagnoses, which if missed, may have significant consequences include child abuse and a cardiac arrhythmia.
In an effort to synthesize new data along with expert opinion, the American Academy of Pediatrics has convened a Subcommittee on the Guideline for ALTE, lead by Joel Tieder, to develop a new practice guideline. This guideline is still in development with certain areas not ready for broad dissemination. The highlight of the new guideline will be a proposal for a name change for ALTEs. Dr Percelay reports the proposed new name would be BRUE (pronounced “brew”), Brief Resolved Unexplained Event. He anticipates further information to be published that will offer a framework to specify which infants to consider at low risk of recurrence versus higher risk for significant pathology. For those infants identified as low risk, the guideline will offer specific evaluation and treatment recommendations. An anticipated key point of the new guideline will be that a careful history and physical is the cornerstone of the initial evaluation and that in the absence of specific historical or exam findings, diagnostic testing of well-appearing infants is of low value.
In a presentation on guidelines for ALTE, Jack Percelay, SHM representative to the AAP Subcommittee, provided further insight to the work that has been done for the clinical entity known as apparent life-threatening events (ALTE) since a consensus statement was put forward by the NIH in 1986. The original statement emphasized 4 possible features to constitute ALTE: apnea, color change, change in tone or gagging. The imprecise nature of the definition, along with both provider and caretaker anxiety related to the diagnosis, have lead to a cascade of diagnostic testing and treatments for what is a symptom complex, not a disease.
Subsequent work in the field has clarified that an ALTE is not a risk factor for SIDS. Of the myriad of etiologies that can cause an ALTE, many will have a readily identifiable etiology that a good history and physical exam will diagnose. Most other diseases, if not diagnosed at initial presentation, will become apparent subsequently without any significant consequences (for example epilepsy). Two diagnoses, which if missed, may have significant consequences include child abuse and a cardiac arrhythmia.
In an effort to synthesize new data along with expert opinion, the American Academy of Pediatrics has convened a Subcommittee on the Guideline for ALTE, lead by Joel Tieder, to develop a new practice guideline. This guideline is still in development with certain areas not ready for broad dissemination. The highlight of the new guideline will be a proposal for a name change for ALTEs. Dr Percelay reports the proposed new name would be BRUE (pronounced “brew”), Brief Resolved Unexplained Event. He anticipates further information to be published that will offer a framework to specify which infants to consider at low risk of recurrence versus higher risk for significant pathology. For those infants identified as low risk, the guideline will offer specific evaluation and treatment recommendations. An anticipated key point of the new guideline will be that a careful history and physical is the cornerstone of the initial evaluation and that in the absence of specific historical or exam findings, diagnostic testing of well-appearing infants is of low value.
AHA/ACC updates hypertension guidelines for CAD patients
The first update to U.S. guidelines for managing hypertension in adult patients with coronary artery disease in 8 years reset the target blood pressure for most of these patients to less than 140/90 mm Hg, and highlighted beta-blockers, renin-angiotensin-aldosterone system blockers, and thiazide diuretics as the mainstays of drug treatment for these patients.
The main messages in the new scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension, released on March 31 in an article published online (Hypertension 2015 [doi:10.116/HYP.0000000000000018]) are the blood pressure targets set for patients with coronary artery disease (CAD) and the designations of the preferred drugs to use to achieve the blood pressure goals when lifestyle measures alone prove inadequate, said Dr. Clive Rosendorff, chair of the panel that wrote the new statement.
But the statement also highlighted that a blood pressure target of less than 130/80 mm Hg “could be considered” and was reasonable for selected CAD patients whom physicians judge capable of achieving this lower blood pressure level safely and who are at especially high risk for cerebrovascular events.
“We felt the best evidence [to prevent future cardiovascular events] was to reduce pressure below 140/90 mm Hg, but a goal pressure of less than 130/80 mm Hg may be appropriate in some cases; we left it to the discretion of physicians to decide which blood pressure target to choose,” said Dr. Rosendorff, professor of medicine at Mount Sinai Hospital in New York.
The default blood pressure goal of less than 140/90 for most CAD patients represented an increase from the less than 130/80 mm Hg goal set by the prior edition of this guideline, issued in 2007 (Circulation 2007;115:2761-88). Current evidence for the lower blood pressure target of less than 130/80 mm Hg “was not as strong,” Dr. Rosendorff said in an interview. He suggested that physicians consider using the lower target for patients who are younger, reasonably healthy, able to tolerate a regimen that brings them to a lower blood pressure without an increase in angina or other significant effects caused by the drugs themselves, do not experience compromised renal function with reduced blood pressure, and have an increased risk for cerebrovascular events.
“These guidelines are not rigid, and should involve a discussion with the patient of the benefits and risks,” he said.
The new statement targets a blood pressure goal of less than 150/90 mm Hg for CAD patients who are more than 80 years old.
The new target for CAD patients represents something of a response to the blood pressure target of less than 150/90 mm Hg for people at least 60 years old recommended last year in recommendations made by the panel originally assembled as the Eighth Joint National Committee (JNC 8) (JAMA 2014;311:507-20). Although the JNC 8 recommendations aimed at the general population in a primary prevention setting, as opposed to CAD patients for whom secondary prevention is the goal, the target of less than 150/90 mm Hg became “highly controversial” and was a factor in composing the new recommendation, Dr. Rosendorff said. He also stressed that the AHA, ACC, and ASH have assembled a group that is formulating new recommendations for diagnosing and managing hypertension for the general population in a primary prevention setting that will come out sometime in the future.
The new hypertension guideline for CAD patients and the 2014 statement from the JNC 8 panel should be seen as distinct recommendations because they targeted different patient populations and because they were based on different ground rules for evidence, said Dr. Suzanne Oparil, one of three people who served on both writing groups. The JNC 8 group focused exclusively on findings from randomized, controlled trials that used hard cardiovascular disease endpoints. The writing committee for the new guidelines targeted specifically at CAD patients also considered evidence from epidemiologic studies. In addition, the new guidelines is targeted at primarily a cardiologist audience, while the 2014 JNC 8 guidelines were written primarily for primary care physicians, she said in an interview.
“I do not believe that the new CAD guidelines will change practice. They reflect what most cardiologists already do,” said Dr. Oparil, professor of medicine and director of the vascular biology and hypertension program at the University of Alabama, Birmingham.
Regarding antihypertensive drug selection the new statement endorses a focus on treating hypertensive patients with established CAD with a beta-blocker, a renin-angiotensin-aldosterone system blocker such as an ACE inhibitor or angiotensin-receptor blocker, and a thiazide or thiazide-like diuretic. Hypertensive patients with CAD should immediately start on all three drug classes, Dr. Rosendorff said.
“For patients with established CAD, a treatment with a beta-blocker moves from the limbo they are in for treating uncomplicated hypertension to center stage,” he said. The statement gives more detailed guidance on which specific drugs from the beta-blocker class have the best evidence for efficacy in various types of patients with CAD.
Dr. Rosendorff had no disclosures. Dr. Oparil has been a consultant to Bayer, Daiichi Sankyo, and Pfizer, and has received research grants from Medtronic, Merck, Novartis, and Takeda.
On Twitter @mitchelzoler
The new statement on treating hypertension in patients with established coronary artery disease clears up what had been a confusing situation for U.S. physicians during the past year.
In early 2014, the panel that had originally been assembled as the Eighth Joint National Committee (JNC 8) issued a statement that called for a blood pressure target of less than 150/90 mm Hg for people 60 years or older (JAMA 2014;311:507-20). People were very confused about that, and may have erroneously believed that this recommendation applied to patients with CAD. I and many of my colleagues believe that having a recommendation to treat to just less than 150/90 mm Hg potentially put millions of CAD patients at risk, especially at risk for stroke. The new statement highlights the high risk faced by CAD patients who need special attention to their blood pressure.

|
| Mitchel L. Zoler/Fronbtline Medical News Dr. Elliott M. Antman |
The epidemiologic evidence clearly shows that increased blood pressure relates to an increased risk for cardiovascular events across a blood pressure range from 115/75 mm Hg to 185/115 mm Hg.
The new recommendations for CAD patients also say that a target blood pressure of less than 130/80 mm Hg may be preferred for selected patients, although the statement does not offer clear steps on how to identify these patients. Physicians must use their clinical judgment.
In my practice, I make sure not to drop a patient’s creatinine clearance to an unacceptably low level, and I would especially consider the lower target for patients with a history of heart failure or left ventricular dilatation or hypertrophy. I believe that in the past, physicians have been too conservative about blood pressure reduction in CAD patients, in part out of a concern about reducing perfusion pressure too much. I believe that if a CAD patient can tolerate a lower blood pressure and the treatment it takes to achieve it, then it is better to be more aggressive.
We also must always remember that the lifestyle modifications, including less dietary sodium, weight loss, and exercise, are the first steps to reducing blood pressure.
Dr. Elliott M. Antman is professor of medicine at Harvard University in Boston and president of the American Heart Association. He had no relevant disclosures. He made these comments in an interview.
The new statement on treating hypertension in patients with established coronary artery disease clears up what had been a confusing situation for U.S. physicians during the past year.
In early 2014, the panel that had originally been assembled as the Eighth Joint National Committee (JNC 8) issued a statement that called for a blood pressure target of less than 150/90 mm Hg for people 60 years or older (JAMA 2014;311:507-20). People were very confused about that, and may have erroneously believed that this recommendation applied to patients with CAD. I and many of my colleagues believe that having a recommendation to treat to just less than 150/90 mm Hg potentially put millions of CAD patients at risk, especially at risk for stroke. The new statement highlights the high risk faced by CAD patients who need special attention to their blood pressure.

|
| Mitchel L. Zoler/Fronbtline Medical News Dr. Elliott M. Antman |
The epidemiologic evidence clearly shows that increased blood pressure relates to an increased risk for cardiovascular events across a blood pressure range from 115/75 mm Hg to 185/115 mm Hg.
The new recommendations for CAD patients also say that a target blood pressure of less than 130/80 mm Hg may be preferred for selected patients, although the statement does not offer clear steps on how to identify these patients. Physicians must use their clinical judgment.
In my practice, I make sure not to drop a patient’s creatinine clearance to an unacceptably low level, and I would especially consider the lower target for patients with a history of heart failure or left ventricular dilatation or hypertrophy. I believe that in the past, physicians have been too conservative about blood pressure reduction in CAD patients, in part out of a concern about reducing perfusion pressure too much. I believe that if a CAD patient can tolerate a lower blood pressure and the treatment it takes to achieve it, then it is better to be more aggressive.
We also must always remember that the lifestyle modifications, including less dietary sodium, weight loss, and exercise, are the first steps to reducing blood pressure.
Dr. Elliott M. Antman is professor of medicine at Harvard University in Boston and president of the American Heart Association. He had no relevant disclosures. He made these comments in an interview.
The new statement on treating hypertension in patients with established coronary artery disease clears up what had been a confusing situation for U.S. physicians during the past year.
In early 2014, the panel that had originally been assembled as the Eighth Joint National Committee (JNC 8) issued a statement that called for a blood pressure target of less than 150/90 mm Hg for people 60 years or older (JAMA 2014;311:507-20). People were very confused about that, and may have erroneously believed that this recommendation applied to patients with CAD. I and many of my colleagues believe that having a recommendation to treat to just less than 150/90 mm Hg potentially put millions of CAD patients at risk, especially at risk for stroke. The new statement highlights the high risk faced by CAD patients who need special attention to their blood pressure.

|
| Mitchel L. Zoler/Fronbtline Medical News Dr. Elliott M. Antman |
The epidemiologic evidence clearly shows that increased blood pressure relates to an increased risk for cardiovascular events across a blood pressure range from 115/75 mm Hg to 185/115 mm Hg.
The new recommendations for CAD patients also say that a target blood pressure of less than 130/80 mm Hg may be preferred for selected patients, although the statement does not offer clear steps on how to identify these patients. Physicians must use their clinical judgment.
In my practice, I make sure not to drop a patient’s creatinine clearance to an unacceptably low level, and I would especially consider the lower target for patients with a history of heart failure or left ventricular dilatation or hypertrophy. I believe that in the past, physicians have been too conservative about blood pressure reduction in CAD patients, in part out of a concern about reducing perfusion pressure too much. I believe that if a CAD patient can tolerate a lower blood pressure and the treatment it takes to achieve it, then it is better to be more aggressive.
We also must always remember that the lifestyle modifications, including less dietary sodium, weight loss, and exercise, are the first steps to reducing blood pressure.
Dr. Elliott M. Antman is professor of medicine at Harvard University in Boston and president of the American Heart Association. He had no relevant disclosures. He made these comments in an interview.
The first update to U.S. guidelines for managing hypertension in adult patients with coronary artery disease in 8 years reset the target blood pressure for most of these patients to less than 140/90 mm Hg, and highlighted beta-blockers, renin-angiotensin-aldosterone system blockers, and thiazide diuretics as the mainstays of drug treatment for these patients.
The main messages in the new scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension, released on March 31 in an article published online (Hypertension 2015 [doi:10.116/HYP.0000000000000018]) are the blood pressure targets set for patients with coronary artery disease (CAD) and the designations of the preferred drugs to use to achieve the blood pressure goals when lifestyle measures alone prove inadequate, said Dr. Clive Rosendorff, chair of the panel that wrote the new statement.
But the statement also highlighted that a blood pressure target of less than 130/80 mm Hg “could be considered” and was reasonable for selected CAD patients whom physicians judge capable of achieving this lower blood pressure level safely and who are at especially high risk for cerebrovascular events.
“We felt the best evidence [to prevent future cardiovascular events] was to reduce pressure below 140/90 mm Hg, but a goal pressure of less than 130/80 mm Hg may be appropriate in some cases; we left it to the discretion of physicians to decide which blood pressure target to choose,” said Dr. Rosendorff, professor of medicine at Mount Sinai Hospital in New York.
The default blood pressure goal of less than 140/90 for most CAD patients represented an increase from the less than 130/80 mm Hg goal set by the prior edition of this guideline, issued in 2007 (Circulation 2007;115:2761-88). Current evidence for the lower blood pressure target of less than 130/80 mm Hg “was not as strong,” Dr. Rosendorff said in an interview. He suggested that physicians consider using the lower target for patients who are younger, reasonably healthy, able to tolerate a regimen that brings them to a lower blood pressure without an increase in angina or other significant effects caused by the drugs themselves, do not experience compromised renal function with reduced blood pressure, and have an increased risk for cerebrovascular events.
“These guidelines are not rigid, and should involve a discussion with the patient of the benefits and risks,” he said.
The new statement targets a blood pressure goal of less than 150/90 mm Hg for CAD patients who are more than 80 years old.
The new target for CAD patients represents something of a response to the blood pressure target of less than 150/90 mm Hg for people at least 60 years old recommended last year in recommendations made by the panel originally assembled as the Eighth Joint National Committee (JNC 8) (JAMA 2014;311:507-20). Although the JNC 8 recommendations aimed at the general population in a primary prevention setting, as opposed to CAD patients for whom secondary prevention is the goal, the target of less than 150/90 mm Hg became “highly controversial” and was a factor in composing the new recommendation, Dr. Rosendorff said. He also stressed that the AHA, ACC, and ASH have assembled a group that is formulating new recommendations for diagnosing and managing hypertension for the general population in a primary prevention setting that will come out sometime in the future.
The new hypertension guideline for CAD patients and the 2014 statement from the JNC 8 panel should be seen as distinct recommendations because they targeted different patient populations and because they were based on different ground rules for evidence, said Dr. Suzanne Oparil, one of three people who served on both writing groups. The JNC 8 group focused exclusively on findings from randomized, controlled trials that used hard cardiovascular disease endpoints. The writing committee for the new guidelines targeted specifically at CAD patients also considered evidence from epidemiologic studies. In addition, the new guidelines is targeted at primarily a cardiologist audience, while the 2014 JNC 8 guidelines were written primarily for primary care physicians, she said in an interview.
“I do not believe that the new CAD guidelines will change practice. They reflect what most cardiologists already do,” said Dr. Oparil, professor of medicine and director of the vascular biology and hypertension program at the University of Alabama, Birmingham.
Regarding antihypertensive drug selection the new statement endorses a focus on treating hypertensive patients with established CAD with a beta-blocker, a renin-angiotensin-aldosterone system blocker such as an ACE inhibitor or angiotensin-receptor blocker, and a thiazide or thiazide-like diuretic. Hypertensive patients with CAD should immediately start on all three drug classes, Dr. Rosendorff said.
“For patients with established CAD, a treatment with a beta-blocker moves from the limbo they are in for treating uncomplicated hypertension to center stage,” he said. The statement gives more detailed guidance on which specific drugs from the beta-blocker class have the best evidence for efficacy in various types of patients with CAD.
Dr. Rosendorff had no disclosures. Dr. Oparil has been a consultant to Bayer, Daiichi Sankyo, and Pfizer, and has received research grants from Medtronic, Merck, Novartis, and Takeda.
On Twitter @mitchelzoler
The first update to U.S. guidelines for managing hypertension in adult patients with coronary artery disease in 8 years reset the target blood pressure for most of these patients to less than 140/90 mm Hg, and highlighted beta-blockers, renin-angiotensin-aldosterone system blockers, and thiazide diuretics as the mainstays of drug treatment for these patients.
The main messages in the new scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension, released on March 31 in an article published online (Hypertension 2015 [doi:10.116/HYP.0000000000000018]) are the blood pressure targets set for patients with coronary artery disease (CAD) and the designations of the preferred drugs to use to achieve the blood pressure goals when lifestyle measures alone prove inadequate, said Dr. Clive Rosendorff, chair of the panel that wrote the new statement.
But the statement also highlighted that a blood pressure target of less than 130/80 mm Hg “could be considered” and was reasonable for selected CAD patients whom physicians judge capable of achieving this lower blood pressure level safely and who are at especially high risk for cerebrovascular events.
“We felt the best evidence [to prevent future cardiovascular events] was to reduce pressure below 140/90 mm Hg, but a goal pressure of less than 130/80 mm Hg may be appropriate in some cases; we left it to the discretion of physicians to decide which blood pressure target to choose,” said Dr. Rosendorff, professor of medicine at Mount Sinai Hospital in New York.
The default blood pressure goal of less than 140/90 for most CAD patients represented an increase from the less than 130/80 mm Hg goal set by the prior edition of this guideline, issued in 2007 (Circulation 2007;115:2761-88). Current evidence for the lower blood pressure target of less than 130/80 mm Hg “was not as strong,” Dr. Rosendorff said in an interview. He suggested that physicians consider using the lower target for patients who are younger, reasonably healthy, able to tolerate a regimen that brings them to a lower blood pressure without an increase in angina or other significant effects caused by the drugs themselves, do not experience compromised renal function with reduced blood pressure, and have an increased risk for cerebrovascular events.
“These guidelines are not rigid, and should involve a discussion with the patient of the benefits and risks,” he said.
The new statement targets a blood pressure goal of less than 150/90 mm Hg for CAD patients who are more than 80 years old.
The new target for CAD patients represents something of a response to the blood pressure target of less than 150/90 mm Hg for people at least 60 years old recommended last year in recommendations made by the panel originally assembled as the Eighth Joint National Committee (JNC 8) (JAMA 2014;311:507-20). Although the JNC 8 recommendations aimed at the general population in a primary prevention setting, as opposed to CAD patients for whom secondary prevention is the goal, the target of less than 150/90 mm Hg became “highly controversial” and was a factor in composing the new recommendation, Dr. Rosendorff said. He also stressed that the AHA, ACC, and ASH have assembled a group that is formulating new recommendations for diagnosing and managing hypertension for the general population in a primary prevention setting that will come out sometime in the future.
The new hypertension guideline for CAD patients and the 2014 statement from the JNC 8 panel should be seen as distinct recommendations because they targeted different patient populations and because they were based on different ground rules for evidence, said Dr. Suzanne Oparil, one of three people who served on both writing groups. The JNC 8 group focused exclusively on findings from randomized, controlled trials that used hard cardiovascular disease endpoints. The writing committee for the new guidelines targeted specifically at CAD patients also considered evidence from epidemiologic studies. In addition, the new guidelines is targeted at primarily a cardiologist audience, while the 2014 JNC 8 guidelines were written primarily for primary care physicians, she said in an interview.
“I do not believe that the new CAD guidelines will change practice. They reflect what most cardiologists already do,” said Dr. Oparil, professor of medicine and director of the vascular biology and hypertension program at the University of Alabama, Birmingham.
Regarding antihypertensive drug selection the new statement endorses a focus on treating hypertensive patients with established CAD with a beta-blocker, a renin-angiotensin-aldosterone system blocker such as an ACE inhibitor or angiotensin-receptor blocker, and a thiazide or thiazide-like diuretic. Hypertensive patients with CAD should immediately start on all three drug classes, Dr. Rosendorff said.
“For patients with established CAD, a treatment with a beta-blocker moves from the limbo they are in for treating uncomplicated hypertension to center stage,” he said. The statement gives more detailed guidance on which specific drugs from the beta-blocker class have the best evidence for efficacy in various types of patients with CAD.
Dr. Rosendorff had no disclosures. Dr. Oparil has been a consultant to Bayer, Daiichi Sankyo, and Pfizer, and has received research grants from Medtronic, Merck, Novartis, and Takeda.
On Twitter @mitchelzoler
FROM HYPERTENSION
ACP: Avoid ECG, MPI cardiac screening in low-risk patients
Clinicians should not screen for cardiac disease in asymptomatic, low-risk adults using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging , according to new guidelines from the American College of Physicians.
“There is no evidence that cardiac screening of low-risk adults with resting or stress ECG, stress echocardiography, or stress MPI improves outcomes, but it is associated with increased costs and potential harms,” wrote the guideline’s author, Dr. Roger Chou, associate professor of medicine at Oregon Health & Science University, Portland.
The recommendation is based on a systematic literature review, recommendations from the U.S. Preventive Services Task Force, and American College of Cardiology guidelines. The new ACP clinical guideline was published March 17 in Annals of Internal Medicine (doi: 10.7326/M14-1225).
“What we are saying here is that, as physicians, we have responsibility to understand what the pretest probability is, and what the likelihood is that someone actually has disease – and if it’s low enough, then doing the screening test is going to cause a lot more false positives than true positives,” Dr. Robert Centor, regional dean of the Huntsville Medical Campus of the University of Alabama at Birmingham, explained in an interview.
“Even if it is a true positive, there is no evidence that we can find that finding that heart disease will do anything other than lead someone to do a procedure that we have no evidence will improve their outcomes,” added Dr. Centor, chair of the ACP Board of Regents.
Despite existing recommendations to the contrary, physicians are increasingly performing these tests on low-risk patients, the ACP cautioned.
For example, a Consumer Reports survey found that “39% of asymptomatic adults without high blood pressure or a high cholesterol level reported having ECG within the past 5 years, and 12% reported undergoing exercise ECG,” Dr. Chou wrote in his report on behalf of the ACP High Value Care Task Force. More than half of those patients said their physicians recommended the tests as part of their routine health care.
The rise in the use of such tests is likely the result of a combination of factors, Dr. Centor said. Those factors include money (patients see no out-of-pocket cost and thus don’t consider the cost of tests in their decision making), direct-to-consumer advertising, fear on behalf of physicians that they might miss a diagnosis, and a lack of understanding by patients on the adverse effects of screening if they are at low risk for heart disease.
Dr. Chou identified a number of potential harms related to unnecessary screenings, including sudden death or hospitalization during stress tests; adverse events from pharmacologics used to induce stress; radiation exposure from myocardial perfusion imaging; false positive results that, in turn, lead to anxiety by the patient and additional unnecessary tests and treatments; disease labeling; and downstream harms from follow-up testing and interventions.
“To be most effective, efforts to reduce the use of imaging should be multifocal and should address clinician behavior, patient expectations, direct-to-consumer screening programs, and financial incentives,” Dr. Chou explained.
In low-risk patients, physicians instead should “focus on treating modifiable risk factors (such as smoking, diabetes, hypertension, hyperlipidemia, and overweight) and encouraging healthy levels of exercise,” according to the guideline.
Clinicians should not screen for cardiac disease in asymptomatic, low-risk adults using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging , according to new guidelines from the American College of Physicians.
“There is no evidence that cardiac screening of low-risk adults with resting or stress ECG, stress echocardiography, or stress MPI improves outcomes, but it is associated with increased costs and potential harms,” wrote the guideline’s author, Dr. Roger Chou, associate professor of medicine at Oregon Health & Science University, Portland.
The recommendation is based on a systematic literature review, recommendations from the U.S. Preventive Services Task Force, and American College of Cardiology guidelines. The new ACP clinical guideline was published March 17 in Annals of Internal Medicine (doi: 10.7326/M14-1225).
“What we are saying here is that, as physicians, we have responsibility to understand what the pretest probability is, and what the likelihood is that someone actually has disease – and if it’s low enough, then doing the screening test is going to cause a lot more false positives than true positives,” Dr. Robert Centor, regional dean of the Huntsville Medical Campus of the University of Alabama at Birmingham, explained in an interview.
“Even if it is a true positive, there is no evidence that we can find that finding that heart disease will do anything other than lead someone to do a procedure that we have no evidence will improve their outcomes,” added Dr. Centor, chair of the ACP Board of Regents.
Despite existing recommendations to the contrary, physicians are increasingly performing these tests on low-risk patients, the ACP cautioned.
For example, a Consumer Reports survey found that “39% of asymptomatic adults without high blood pressure or a high cholesterol level reported having ECG within the past 5 years, and 12% reported undergoing exercise ECG,” Dr. Chou wrote in his report on behalf of the ACP High Value Care Task Force. More than half of those patients said their physicians recommended the tests as part of their routine health care.
The rise in the use of such tests is likely the result of a combination of factors, Dr. Centor said. Those factors include money (patients see no out-of-pocket cost and thus don’t consider the cost of tests in their decision making), direct-to-consumer advertising, fear on behalf of physicians that they might miss a diagnosis, and a lack of understanding by patients on the adverse effects of screening if they are at low risk for heart disease.
Dr. Chou identified a number of potential harms related to unnecessary screenings, including sudden death or hospitalization during stress tests; adverse events from pharmacologics used to induce stress; radiation exposure from myocardial perfusion imaging; false positive results that, in turn, lead to anxiety by the patient and additional unnecessary tests and treatments; disease labeling; and downstream harms from follow-up testing and interventions.
“To be most effective, efforts to reduce the use of imaging should be multifocal and should address clinician behavior, patient expectations, direct-to-consumer screening programs, and financial incentives,” Dr. Chou explained.
In low-risk patients, physicians instead should “focus on treating modifiable risk factors (such as smoking, diabetes, hypertension, hyperlipidemia, and overweight) and encouraging healthy levels of exercise,” according to the guideline.
Clinicians should not screen for cardiac disease in asymptomatic, low-risk adults using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging , according to new guidelines from the American College of Physicians.
“There is no evidence that cardiac screening of low-risk adults with resting or stress ECG, stress echocardiography, or stress MPI improves outcomes, but it is associated with increased costs and potential harms,” wrote the guideline’s author, Dr. Roger Chou, associate professor of medicine at Oregon Health & Science University, Portland.
The recommendation is based on a systematic literature review, recommendations from the U.S. Preventive Services Task Force, and American College of Cardiology guidelines. The new ACP clinical guideline was published March 17 in Annals of Internal Medicine (doi: 10.7326/M14-1225).
“What we are saying here is that, as physicians, we have responsibility to understand what the pretest probability is, and what the likelihood is that someone actually has disease – and if it’s low enough, then doing the screening test is going to cause a lot more false positives than true positives,” Dr. Robert Centor, regional dean of the Huntsville Medical Campus of the University of Alabama at Birmingham, explained in an interview.
“Even if it is a true positive, there is no evidence that we can find that finding that heart disease will do anything other than lead someone to do a procedure that we have no evidence will improve their outcomes,” added Dr. Centor, chair of the ACP Board of Regents.
Despite existing recommendations to the contrary, physicians are increasingly performing these tests on low-risk patients, the ACP cautioned.
For example, a Consumer Reports survey found that “39% of asymptomatic adults without high blood pressure or a high cholesterol level reported having ECG within the past 5 years, and 12% reported undergoing exercise ECG,” Dr. Chou wrote in his report on behalf of the ACP High Value Care Task Force. More than half of those patients said their physicians recommended the tests as part of their routine health care.
The rise in the use of such tests is likely the result of a combination of factors, Dr. Centor said. Those factors include money (patients see no out-of-pocket cost and thus don’t consider the cost of tests in their decision making), direct-to-consumer advertising, fear on behalf of physicians that they might miss a diagnosis, and a lack of understanding by patients on the adverse effects of screening if they are at low risk for heart disease.
Dr. Chou identified a number of potential harms related to unnecessary screenings, including sudden death or hospitalization during stress tests; adverse events from pharmacologics used to induce stress; radiation exposure from myocardial perfusion imaging; false positive results that, in turn, lead to anxiety by the patient and additional unnecessary tests and treatments; disease labeling; and downstream harms from follow-up testing and interventions.
“To be most effective, efforts to reduce the use of imaging should be multifocal and should address clinician behavior, patient expectations, direct-to-consumer screening programs, and financial incentives,” Dr. Chou explained.
In low-risk patients, physicians instead should “focus on treating modifiable risk factors (such as smoking, diabetes, hypertension, hyperlipidemia, and overweight) and encouraging healthy levels of exercise,” according to the guideline.
FROM ANNALS OF INTERNAL MEDICINE
FDA guidance focuses on infections with reusable devices, including duodenoscopes
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.