User login
Biden administration nixes buprenorphine waiver, docs disappointed
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
Feds look to retrofit factories to increase COVID vaccine production
The Biden administration is exploring whether factories can be retrofitted to produce more of the Pfizer/BioNTech and Moderna COVID-19 mRNA vaccines to speed up vaccination of the vast majority of Americans.
The announcement comes as the nation is on track to see 479,000-514,000 deaths by the end of February, said Rochelle Walensky, MD, the director of the Centers for Disease Control and Prevention.
Dr. Walensky, speaking to reporters Wednesday in the first briefing from the White House COVID-19 Response Team, said that 1.6 million COVID-19 shots had been administered each day over the past week and that 3.4 million Americans have been fully vaccinated with two doses.
More than 500 million doses will be needed to vaccinate every American older than 16 years, Andy Slavitt, the senior advisor to the COVID-19 response team, told reporters. Pfizer and Moderna are due to deliver an additional 200 million doses near the end of March, and President Biden is seeking to purchase another 200 million doses from the companies, said Mr. Slavitt.
But it may not be enough. Whether companies can retrofit factories to produce vaccines is “something that’s under active exploration,” Mr. Slavitt said.
“This is a national emergency,” said Jeff Zients, the White House COVID-19 response coordinator. “Everything is on the table across the whole supply chain,” he said. He noted that the administration was also buying low-dead-space syringes to help extract an additional sixth dose from every Pfizer vial.
Mr. Slavitt said the team had identified 12 areas in which Mr. Biden was authorized to use the Defense Production Act to spur the manufacture of items such as masks and COVID-19 diagnostics.
More sequencing needed
As new variants emerge, vaccine makers and the CDC are racing to stay a step ahead. “RNA viruses mutate all the time – that’s what they do, that’s their business,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Mr. Biden’s chief medical adviser, in the briefing.
Three concerning variants have emerged: the B117, which is circulating widely in the United Kingdom; the B1.351 in South Africa; and the P.1 in Brazil. As of Jan. 26, no cases involving the B1.351 variant have been detected in the United States; one person with the P.1 variant was identified in Minnesota. The CDC has identified 308 cases of the U.K. variant in 26 states, said Dr. Walensky.
The United States is dismally behind in surveillance and sequencing of variants, said Zients. “We are 43rd in the world at genomic sequencing,” which he said was “totally unacceptable.”
Dr. Walensky said the CDC is working on improving data collection and sequencing, but she said more money is needed to “do the amount of sequencing and surveillance that we need in order to be able to detect these when they first start to emerge.”
Both she and Mr. Zients called on Congress to pass Mr. Biden’s proposed American Rescue package, which includes more money for sequencing.
Dr. Fauci said the National Institutes of Health was collaborating with the CDC to determine whether other newly emerging variants pose any threat – such as increased transmissibility or lethality or some other functional characteristic. Scientists will also monitor “in real-time” whether current vaccines continue to make neutralizing antibodies against these mutants.
“With the U.K. variant, what we’re seeing is a very slight, if at all, impact on vaccine-induced antibodies and very little impact on anything else,” he said. With the South African variant, there is “a multifold diminution in the in vitro neutralization by vaccine-induced antibodies,” but “it still is well within the cushion of protection” for the current vaccines.
But, he added, “we have to be concerned looking forward of what the further evolution of this might be.” The anti-COVID monoclonal antibodies – bamlanivimab and the combination of casirivimab and imdevimab – are “more seriously inhibited by this South African strain,” which is spurring development of new monoclonals.
Dr. Fauci also noted that the Johnson & Johnson/Janssen vaccine that is in development – for which phase 3 data may be released within days – was tested in South Africa and Brazil in addition to the United States. The comparative data could help researchers and clinicians make better-informed decisions about what vaccine to use if the South African variant “seeds itself in the U.S.”
A version of this article first appeared on Medscape.com.
The Biden administration is exploring whether factories can be retrofitted to produce more of the Pfizer/BioNTech and Moderna COVID-19 mRNA vaccines to speed up vaccination of the vast majority of Americans.
The announcement comes as the nation is on track to see 479,000-514,000 deaths by the end of February, said Rochelle Walensky, MD, the director of the Centers for Disease Control and Prevention.
Dr. Walensky, speaking to reporters Wednesday in the first briefing from the White House COVID-19 Response Team, said that 1.6 million COVID-19 shots had been administered each day over the past week and that 3.4 million Americans have been fully vaccinated with two doses.
More than 500 million doses will be needed to vaccinate every American older than 16 years, Andy Slavitt, the senior advisor to the COVID-19 response team, told reporters. Pfizer and Moderna are due to deliver an additional 200 million doses near the end of March, and President Biden is seeking to purchase another 200 million doses from the companies, said Mr. Slavitt.
But it may not be enough. Whether companies can retrofit factories to produce vaccines is “something that’s under active exploration,” Mr. Slavitt said.
“This is a national emergency,” said Jeff Zients, the White House COVID-19 response coordinator. “Everything is on the table across the whole supply chain,” he said. He noted that the administration was also buying low-dead-space syringes to help extract an additional sixth dose from every Pfizer vial.
Mr. Slavitt said the team had identified 12 areas in which Mr. Biden was authorized to use the Defense Production Act to spur the manufacture of items such as masks and COVID-19 diagnostics.
More sequencing needed
As new variants emerge, vaccine makers and the CDC are racing to stay a step ahead. “RNA viruses mutate all the time – that’s what they do, that’s their business,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Mr. Biden’s chief medical adviser, in the briefing.
Three concerning variants have emerged: the B117, which is circulating widely in the United Kingdom; the B1.351 in South Africa; and the P.1 in Brazil. As of Jan. 26, no cases involving the B1.351 variant have been detected in the United States; one person with the P.1 variant was identified in Minnesota. The CDC has identified 308 cases of the U.K. variant in 26 states, said Dr. Walensky.
The United States is dismally behind in surveillance and sequencing of variants, said Zients. “We are 43rd in the world at genomic sequencing,” which he said was “totally unacceptable.”
Dr. Walensky said the CDC is working on improving data collection and sequencing, but she said more money is needed to “do the amount of sequencing and surveillance that we need in order to be able to detect these when they first start to emerge.”
Both she and Mr. Zients called on Congress to pass Mr. Biden’s proposed American Rescue package, which includes more money for sequencing.
Dr. Fauci said the National Institutes of Health was collaborating with the CDC to determine whether other newly emerging variants pose any threat – such as increased transmissibility or lethality or some other functional characteristic. Scientists will also monitor “in real-time” whether current vaccines continue to make neutralizing antibodies against these mutants.
“With the U.K. variant, what we’re seeing is a very slight, if at all, impact on vaccine-induced antibodies and very little impact on anything else,” he said. With the South African variant, there is “a multifold diminution in the in vitro neutralization by vaccine-induced antibodies,” but “it still is well within the cushion of protection” for the current vaccines.
But, he added, “we have to be concerned looking forward of what the further evolution of this might be.” The anti-COVID monoclonal antibodies – bamlanivimab and the combination of casirivimab and imdevimab – are “more seriously inhibited by this South African strain,” which is spurring development of new monoclonals.
Dr. Fauci also noted that the Johnson & Johnson/Janssen vaccine that is in development – for which phase 3 data may be released within days – was tested in South Africa and Brazil in addition to the United States. The comparative data could help researchers and clinicians make better-informed decisions about what vaccine to use if the South African variant “seeds itself in the U.S.”
A version of this article first appeared on Medscape.com.
The Biden administration is exploring whether factories can be retrofitted to produce more of the Pfizer/BioNTech and Moderna COVID-19 mRNA vaccines to speed up vaccination of the vast majority of Americans.
The announcement comes as the nation is on track to see 479,000-514,000 deaths by the end of February, said Rochelle Walensky, MD, the director of the Centers for Disease Control and Prevention.
Dr. Walensky, speaking to reporters Wednesday in the first briefing from the White House COVID-19 Response Team, said that 1.6 million COVID-19 shots had been administered each day over the past week and that 3.4 million Americans have been fully vaccinated with two doses.
More than 500 million doses will be needed to vaccinate every American older than 16 years, Andy Slavitt, the senior advisor to the COVID-19 response team, told reporters. Pfizer and Moderna are due to deliver an additional 200 million doses near the end of March, and President Biden is seeking to purchase another 200 million doses from the companies, said Mr. Slavitt.
But it may not be enough. Whether companies can retrofit factories to produce vaccines is “something that’s under active exploration,” Mr. Slavitt said.
“This is a national emergency,” said Jeff Zients, the White House COVID-19 response coordinator. “Everything is on the table across the whole supply chain,” he said. He noted that the administration was also buying low-dead-space syringes to help extract an additional sixth dose from every Pfizer vial.
Mr. Slavitt said the team had identified 12 areas in which Mr. Biden was authorized to use the Defense Production Act to spur the manufacture of items such as masks and COVID-19 diagnostics.
More sequencing needed
As new variants emerge, vaccine makers and the CDC are racing to stay a step ahead. “RNA viruses mutate all the time – that’s what they do, that’s their business,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Mr. Biden’s chief medical adviser, in the briefing.
Three concerning variants have emerged: the B117, which is circulating widely in the United Kingdom; the B1.351 in South Africa; and the P.1 in Brazil. As of Jan. 26, no cases involving the B1.351 variant have been detected in the United States; one person with the P.1 variant was identified in Minnesota. The CDC has identified 308 cases of the U.K. variant in 26 states, said Dr. Walensky.
The United States is dismally behind in surveillance and sequencing of variants, said Zients. “We are 43rd in the world at genomic sequencing,” which he said was “totally unacceptable.”
Dr. Walensky said the CDC is working on improving data collection and sequencing, but she said more money is needed to “do the amount of sequencing and surveillance that we need in order to be able to detect these when they first start to emerge.”
Both she and Mr. Zients called on Congress to pass Mr. Biden’s proposed American Rescue package, which includes more money for sequencing.
Dr. Fauci said the National Institutes of Health was collaborating with the CDC to determine whether other newly emerging variants pose any threat – such as increased transmissibility or lethality or some other functional characteristic. Scientists will also monitor “in real-time” whether current vaccines continue to make neutralizing antibodies against these mutants.
“With the U.K. variant, what we’re seeing is a very slight, if at all, impact on vaccine-induced antibodies and very little impact on anything else,” he said. With the South African variant, there is “a multifold diminution in the in vitro neutralization by vaccine-induced antibodies,” but “it still is well within the cushion of protection” for the current vaccines.
But, he added, “we have to be concerned looking forward of what the further evolution of this might be.” The anti-COVID monoclonal antibodies – bamlanivimab and the combination of casirivimab and imdevimab – are “more seriously inhibited by this South African strain,” which is spurring development of new monoclonals.
Dr. Fauci also noted that the Johnson & Johnson/Janssen vaccine that is in development – for which phase 3 data may be released within days – was tested in South Africa and Brazil in addition to the United States. The comparative data could help researchers and clinicians make better-informed decisions about what vaccine to use if the South African variant “seeds itself in the U.S.”
A version of this article first appeared on Medscape.com.
Seven ways President Biden could now change health care
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Biden kicks off health agenda with COVID actions, WHO outreach
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
Biden’s COVID-19 challenge: 100 million vaccinations in the first 100 days. It won’t be easy.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
HHS will drop buprenorphine waiver rule for most physicians
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Eliminating hepatitis by 2030: HHS releases new strategic plan
In an effort to counteract alarming trends in rising hepatitis infections, the U.S. Department of Health and Human Services has developed and released its Viral Hepatitis National Strategic Plan 2021-2025, which aims to eliminate viral hepatitis infection in the United States by 2030.
An estimated 3.3 million people in the United States were chronically infected with hepatitis B (HBV) and hepatitis C (HCV) as of 2016. In addition, the country “is currently facing unprecedented hepatitis A (HAV) outbreaks, while progress in preventing hepatitis B has stalled, and hepatitis C rates nearly tripled from 2011 to 2018,” according to the HHS.
The new plan, “A Roadmap to Elimination for the United States,” builds upon previous initiatives the HHS has made to tackle the diseases and was coordinated by the Office of the Assistant Secretary for Health through the Office of Infectious Disease and HIV/AIDS Policy.
The plan focuses on HAV, HBV, and HCV, which have the largest impact on the health of the nation, according to the HHS. The plan addresses populations with the highest burden of viral hepatitis based on nationwide data so that resources can be focused there to achieve the greatest impact. Persons who inject drugs are a priority population for all three hepatitis viruses. HAV efforts will also include a focus on the homeless population. HBV efforts will also focus on Asian and Pacific Islander and the Black, non-Hispanic populations, while HCV efforts will include a focus on Black, non-Hispanic people, people born during 1945-1965, people with HIV, and the American Indian/Alaska Native population.
Goal-setting
There are five main goals outlined in the plan, according to the HHS:
- Prevent new hepatitis infections.
- Improve hepatitis-related health outcomes of people with viral hepatitis.
- Reduce hepatitis-related disparities and health inequities.
- Improve hepatitis surveillance and data use.
- Achieve integrated, coordinated efforts that address the viral hepatitis epidemics among all partners and stakeholders.
“The United States will be a place where new viral hepatitis infections are prevented, every person knows their status, and every person with viral hepatitis has high-quality health care and treatment and lives free from stigma and discrimination. This vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” according to the HHS vision statement.
In an effort to counteract alarming trends in rising hepatitis infections, the U.S. Department of Health and Human Services has developed and released its Viral Hepatitis National Strategic Plan 2021-2025, which aims to eliminate viral hepatitis infection in the United States by 2030.
An estimated 3.3 million people in the United States were chronically infected with hepatitis B (HBV) and hepatitis C (HCV) as of 2016. In addition, the country “is currently facing unprecedented hepatitis A (HAV) outbreaks, while progress in preventing hepatitis B has stalled, and hepatitis C rates nearly tripled from 2011 to 2018,” according to the HHS.
The new plan, “A Roadmap to Elimination for the United States,” builds upon previous initiatives the HHS has made to tackle the diseases and was coordinated by the Office of the Assistant Secretary for Health through the Office of Infectious Disease and HIV/AIDS Policy.
The plan focuses on HAV, HBV, and HCV, which have the largest impact on the health of the nation, according to the HHS. The plan addresses populations with the highest burden of viral hepatitis based on nationwide data so that resources can be focused there to achieve the greatest impact. Persons who inject drugs are a priority population for all three hepatitis viruses. HAV efforts will also include a focus on the homeless population. HBV efforts will also focus on Asian and Pacific Islander and the Black, non-Hispanic populations, while HCV efforts will include a focus on Black, non-Hispanic people, people born during 1945-1965, people with HIV, and the American Indian/Alaska Native population.
Goal-setting
There are five main goals outlined in the plan, according to the HHS:
- Prevent new hepatitis infections.
- Improve hepatitis-related health outcomes of people with viral hepatitis.
- Reduce hepatitis-related disparities and health inequities.
- Improve hepatitis surveillance and data use.
- Achieve integrated, coordinated efforts that address the viral hepatitis epidemics among all partners and stakeholders.
“The United States will be a place where new viral hepatitis infections are prevented, every person knows their status, and every person with viral hepatitis has high-quality health care and treatment and lives free from stigma and discrimination. This vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” according to the HHS vision statement.
In an effort to counteract alarming trends in rising hepatitis infections, the U.S. Department of Health and Human Services has developed and released its Viral Hepatitis National Strategic Plan 2021-2025, which aims to eliminate viral hepatitis infection in the United States by 2030.
An estimated 3.3 million people in the United States were chronically infected with hepatitis B (HBV) and hepatitis C (HCV) as of 2016. In addition, the country “is currently facing unprecedented hepatitis A (HAV) outbreaks, while progress in preventing hepatitis B has stalled, and hepatitis C rates nearly tripled from 2011 to 2018,” according to the HHS.
The new plan, “A Roadmap to Elimination for the United States,” builds upon previous initiatives the HHS has made to tackle the diseases and was coordinated by the Office of the Assistant Secretary for Health through the Office of Infectious Disease and HIV/AIDS Policy.
The plan focuses on HAV, HBV, and HCV, which have the largest impact on the health of the nation, according to the HHS. The plan addresses populations with the highest burden of viral hepatitis based on nationwide data so that resources can be focused there to achieve the greatest impact. Persons who inject drugs are a priority population for all three hepatitis viruses. HAV efforts will also include a focus on the homeless population. HBV efforts will also focus on Asian and Pacific Islander and the Black, non-Hispanic populations, while HCV efforts will include a focus on Black, non-Hispanic people, people born during 1945-1965, people with HIV, and the American Indian/Alaska Native population.
Goal-setting
There are five main goals outlined in the plan, according to the HHS:
- Prevent new hepatitis infections.
- Improve hepatitis-related health outcomes of people with viral hepatitis.
- Reduce hepatitis-related disparities and health inequities.
- Improve hepatitis surveillance and data use.
- Achieve integrated, coordinated efforts that address the viral hepatitis epidemics among all partners and stakeholders.
“The United States will be a place where new viral hepatitis infections are prevented, every person knows their status, and every person with viral hepatitis has high-quality health care and treatment and lives free from stigma and discrimination. This vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” according to the HHS vision statement.
Pressure builds on CDC to prioritize both diabetes types for vaccine
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
VA Ramps up Vaccinations as COVID-19 Cases Continue to Rise
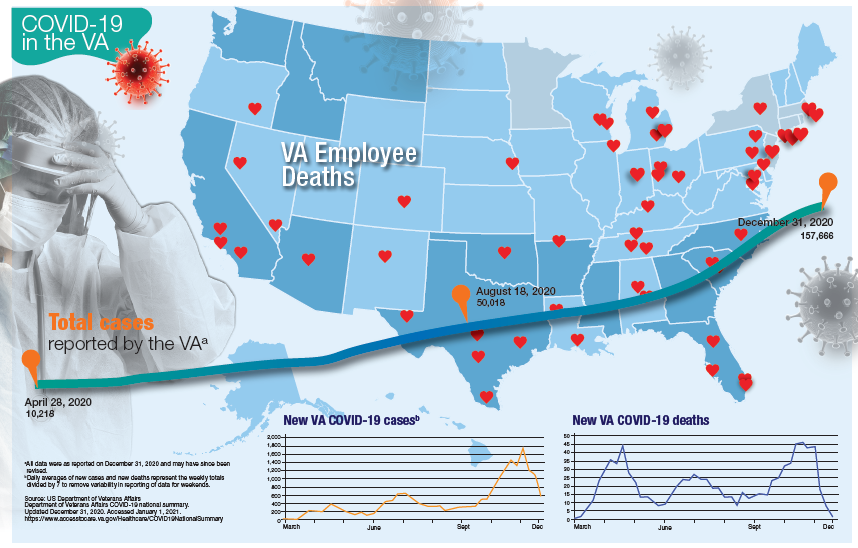
Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.
New dietary guidelines omit recommended cuts to sugar, alcohol intake
Although the new guidelines were informed by an advisory committee’s scientific report, officials omitted certain recommendations that would have reduced allowances for added sugars and alcohol intake.
The 2020-2025 Dietary Guidelines for Americans “carried forward the committee’s emphasis on limiting these dietary components, but did not include changes to quantitative recommendations, as there was not a preponderance of evidence in the material the committee reviewed to support specific changes, as required by law,” the agencies said in a news release.
The guidelines encourage Americans to “Make Every Bite Count” through four overarching suggestions:
- Follow a healthy dietary pattern at every life stage.
- Customize nutrient-dense food and beverage choices to reflect preferences, cultural traditions, and budgets.
- Focus on meeting dietary needs from five food groups – vegetables, fruits, grains, dairy and fortified soy alternatives, and proteins – and stay within calorie limits.
- Limit foods and beverages that are higher in added sugars, saturated fat, and sodium, and limit alcoholic beverages.
The guidance “can help all Americans lead healthier lives by making every bite count,” Secretary of Agriculture Sonny Perdue said.
Proposed cutoffs rejected
The guidelines omit a recommendation from the advisory committee’s scientific report to reduce intake of added sugars from less than 10% of calories to less than 6% of calories.
It also omits a recommendation that men and women who drink alcohol limit themselves to one drink per day. It maintains guidance from the 2015-2020 edition that allows two drinks per day for men.
The agencies published a document explaining why they omitted the advisory committee›s conclusions.
The American Heart Association in July had praised the suggestion to reduce added sugars. The proposed change would have helped “steer the public toward a more heart-healthy path in their daily diets,” Mitchell S.V. Elkind, MD, president of the AHA, said at the time. The association would “strongly oppose any efforts to weaken these recommendations,” he added.
In its response to the new guidelines, Dr. Elkind praised the emphasis on a healthy diet “at every life stage” but called out a missed opportunity.
“We are disappointed that USDA and HHS did not accept all of the Dietary Guidelines Advisory Committee’s science-based recommendations in the final guidelines for 2020, including the recommendation to lower added sugars consumption to less than 6% of calories,” he said in a prepared statement.
Guidance for infants and toddlers
The guidelines advise that for about the first 6 months of life, infants should exclusively receive breast milk. Infants should continue to receive breast milk through at least the first year of life, and longer if desired. Infants should be fed iron-fortified infant formula during the first year of life when breast milk is unavailable, and infants should receive supplemental vitamin D soon after birth, the guidelines advise.
At about 6 months, infants should be introduced to a variety of nutrient-dense complementary foods, including potentially allergenic foods. Infants should eat foods that are rich in iron and zinc, particularly if they are fed breast milk.
The guidelines also include dietary and caloric advice for pregnant and lactating women with daily or weekly amounts of food from different groups and subgroups.
Dr. Elkind highlighted the significance of these additions.
“We are pleased that for the first time, the guidelines provide recommendations for pregnant and breastfeeding women as well as infants and toddlers, underscoring the importance of maternal health and proper nutrition across the lifespan,” he said.
For all ages
From 12 months through older adulthood, people should follow a healthy dietary pattern to meet nutrient needs, help achieve a healthy body weight, and reduce the risk of chronic disease.
According to the guidelines, core elements of a healthy diet include:
- Vegetables of all types (dark green; red and orange; beans, peas, and lentils; starchy; and other types).
- Fruits (especially whole fruit).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and lactose-free versions; and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
The guidelines spell out limits to added sugars, sodium, saturated fat, and alcohol. The recommendation to limit added sugars to less than 10% of calories per day starts at age 2 years. Before age 2, foods and beverages with added sugars should be avoided.
Saturated fat should be limited to less than 10% of calories per day starting at age 2. And sodium intake should be limited to 2,300 mg/day for those age 14 and older, but just 1,200 mg/day for toddlers, 1,500 mg/day for children aged 4-8, and 1,800 mg/day for children 9-13.
“Adults of legal drinking age can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women, when alcohol is consumed,” the agencies said. “Drinking less is better for health than drinking more. There are some adults who should not drink alcohol, such as women who are pregnant.”
An appendix includes estimated calorie needs based on a person’s age, sex, height, weight, and level of physical activity. A need to lose, maintain, or gain weight are among the factors that influence how many calories should be consumed, the guidelines note.
The guidelines are designed for use by health care professionals and policymakers. The USDA has launched a new MyPlate website to help consumers incorporate the dietary guidance.
A version of this article first appeared on Medscape.com.
Although the new guidelines were informed by an advisory committee’s scientific report, officials omitted certain recommendations that would have reduced allowances for added sugars and alcohol intake.
The 2020-2025 Dietary Guidelines for Americans “carried forward the committee’s emphasis on limiting these dietary components, but did not include changes to quantitative recommendations, as there was not a preponderance of evidence in the material the committee reviewed to support specific changes, as required by law,” the agencies said in a news release.
The guidelines encourage Americans to “Make Every Bite Count” through four overarching suggestions:
- Follow a healthy dietary pattern at every life stage.
- Customize nutrient-dense food and beverage choices to reflect preferences, cultural traditions, and budgets.
- Focus on meeting dietary needs from five food groups – vegetables, fruits, grains, dairy and fortified soy alternatives, and proteins – and stay within calorie limits.
- Limit foods and beverages that are higher in added sugars, saturated fat, and sodium, and limit alcoholic beverages.
The guidance “can help all Americans lead healthier lives by making every bite count,” Secretary of Agriculture Sonny Perdue said.
Proposed cutoffs rejected
The guidelines omit a recommendation from the advisory committee’s scientific report to reduce intake of added sugars from less than 10% of calories to less than 6% of calories.
It also omits a recommendation that men and women who drink alcohol limit themselves to one drink per day. It maintains guidance from the 2015-2020 edition that allows two drinks per day for men.
The agencies published a document explaining why they omitted the advisory committee›s conclusions.
The American Heart Association in July had praised the suggestion to reduce added sugars. The proposed change would have helped “steer the public toward a more heart-healthy path in their daily diets,” Mitchell S.V. Elkind, MD, president of the AHA, said at the time. The association would “strongly oppose any efforts to weaken these recommendations,” he added.
In its response to the new guidelines, Dr. Elkind praised the emphasis on a healthy diet “at every life stage” but called out a missed opportunity.
“We are disappointed that USDA and HHS did not accept all of the Dietary Guidelines Advisory Committee’s science-based recommendations in the final guidelines for 2020, including the recommendation to lower added sugars consumption to less than 6% of calories,” he said in a prepared statement.
Guidance for infants and toddlers
The guidelines advise that for about the first 6 months of life, infants should exclusively receive breast milk. Infants should continue to receive breast milk through at least the first year of life, and longer if desired. Infants should be fed iron-fortified infant formula during the first year of life when breast milk is unavailable, and infants should receive supplemental vitamin D soon after birth, the guidelines advise.
At about 6 months, infants should be introduced to a variety of nutrient-dense complementary foods, including potentially allergenic foods. Infants should eat foods that are rich in iron and zinc, particularly if they are fed breast milk.
The guidelines also include dietary and caloric advice for pregnant and lactating women with daily or weekly amounts of food from different groups and subgroups.
Dr. Elkind highlighted the significance of these additions.
“We are pleased that for the first time, the guidelines provide recommendations for pregnant and breastfeeding women as well as infants and toddlers, underscoring the importance of maternal health and proper nutrition across the lifespan,” he said.
For all ages
From 12 months through older adulthood, people should follow a healthy dietary pattern to meet nutrient needs, help achieve a healthy body weight, and reduce the risk of chronic disease.
According to the guidelines, core elements of a healthy diet include:
- Vegetables of all types (dark green; red and orange; beans, peas, and lentils; starchy; and other types).
- Fruits (especially whole fruit).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and lactose-free versions; and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
The guidelines spell out limits to added sugars, sodium, saturated fat, and alcohol. The recommendation to limit added sugars to less than 10% of calories per day starts at age 2 years. Before age 2, foods and beverages with added sugars should be avoided.
Saturated fat should be limited to less than 10% of calories per day starting at age 2. And sodium intake should be limited to 2,300 mg/day for those age 14 and older, but just 1,200 mg/day for toddlers, 1,500 mg/day for children aged 4-8, and 1,800 mg/day for children 9-13.
“Adults of legal drinking age can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women, when alcohol is consumed,” the agencies said. “Drinking less is better for health than drinking more. There are some adults who should not drink alcohol, such as women who are pregnant.”
An appendix includes estimated calorie needs based on a person’s age, sex, height, weight, and level of physical activity. A need to lose, maintain, or gain weight are among the factors that influence how many calories should be consumed, the guidelines note.
The guidelines are designed for use by health care professionals and policymakers. The USDA has launched a new MyPlate website to help consumers incorporate the dietary guidance.
A version of this article first appeared on Medscape.com.
Although the new guidelines were informed by an advisory committee’s scientific report, officials omitted certain recommendations that would have reduced allowances for added sugars and alcohol intake.
The 2020-2025 Dietary Guidelines for Americans “carried forward the committee’s emphasis on limiting these dietary components, but did not include changes to quantitative recommendations, as there was not a preponderance of evidence in the material the committee reviewed to support specific changes, as required by law,” the agencies said in a news release.
The guidelines encourage Americans to “Make Every Bite Count” through four overarching suggestions:
- Follow a healthy dietary pattern at every life stage.
- Customize nutrient-dense food and beverage choices to reflect preferences, cultural traditions, and budgets.
- Focus on meeting dietary needs from five food groups – vegetables, fruits, grains, dairy and fortified soy alternatives, and proteins – and stay within calorie limits.
- Limit foods and beverages that are higher in added sugars, saturated fat, and sodium, and limit alcoholic beverages.
The guidance “can help all Americans lead healthier lives by making every bite count,” Secretary of Agriculture Sonny Perdue said.
Proposed cutoffs rejected
The guidelines omit a recommendation from the advisory committee’s scientific report to reduce intake of added sugars from less than 10% of calories to less than 6% of calories.
It also omits a recommendation that men and women who drink alcohol limit themselves to one drink per day. It maintains guidance from the 2015-2020 edition that allows two drinks per day for men.
The agencies published a document explaining why they omitted the advisory committee›s conclusions.
The American Heart Association in July had praised the suggestion to reduce added sugars. The proposed change would have helped “steer the public toward a more heart-healthy path in their daily diets,” Mitchell S.V. Elkind, MD, president of the AHA, said at the time. The association would “strongly oppose any efforts to weaken these recommendations,” he added.
In its response to the new guidelines, Dr. Elkind praised the emphasis on a healthy diet “at every life stage” but called out a missed opportunity.
“We are disappointed that USDA and HHS did not accept all of the Dietary Guidelines Advisory Committee’s science-based recommendations in the final guidelines for 2020, including the recommendation to lower added sugars consumption to less than 6% of calories,” he said in a prepared statement.
Guidance for infants and toddlers
The guidelines advise that for about the first 6 months of life, infants should exclusively receive breast milk. Infants should continue to receive breast milk through at least the first year of life, and longer if desired. Infants should be fed iron-fortified infant formula during the first year of life when breast milk is unavailable, and infants should receive supplemental vitamin D soon after birth, the guidelines advise.
At about 6 months, infants should be introduced to a variety of nutrient-dense complementary foods, including potentially allergenic foods. Infants should eat foods that are rich in iron and zinc, particularly if they are fed breast milk.
The guidelines also include dietary and caloric advice for pregnant and lactating women with daily or weekly amounts of food from different groups and subgroups.
Dr. Elkind highlighted the significance of these additions.
“We are pleased that for the first time, the guidelines provide recommendations for pregnant and breastfeeding women as well as infants and toddlers, underscoring the importance of maternal health and proper nutrition across the lifespan,” he said.
For all ages
From 12 months through older adulthood, people should follow a healthy dietary pattern to meet nutrient needs, help achieve a healthy body weight, and reduce the risk of chronic disease.
According to the guidelines, core elements of a healthy diet include:
- Vegetables of all types (dark green; red and orange; beans, peas, and lentils; starchy; and other types).
- Fruits (especially whole fruit).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and lactose-free versions; and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
The guidelines spell out limits to added sugars, sodium, saturated fat, and alcohol. The recommendation to limit added sugars to less than 10% of calories per day starts at age 2 years. Before age 2, foods and beverages with added sugars should be avoided.
Saturated fat should be limited to less than 10% of calories per day starting at age 2. And sodium intake should be limited to 2,300 mg/day for those age 14 and older, but just 1,200 mg/day for toddlers, 1,500 mg/day for children aged 4-8, and 1,800 mg/day for children 9-13.
“Adults of legal drinking age can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women, when alcohol is consumed,” the agencies said. “Drinking less is better for health than drinking more. There are some adults who should not drink alcohol, such as women who are pregnant.”
An appendix includes estimated calorie needs based on a person’s age, sex, height, weight, and level of physical activity. A need to lose, maintain, or gain weight are among the factors that influence how many calories should be consumed, the guidelines note.
The guidelines are designed for use by health care professionals and policymakers. The USDA has launched a new MyPlate website to help consumers incorporate the dietary guidance.
A version of this article first appeared on Medscape.com.