User login
What Is the Best Way to Manage Axial Spondyloarthritis in Primary Care?
When axial spondyloarthritis (SpA) is suspected, a “prompt referral to a rheumatologist” is in order. But with the referral possibly taking several weeks, if not months in some parts of the world, how can primary care practitioners manage patients with this type of chronic back pain in the meantime? And what is the long-term role of the primary care practitioner in managing someone diagnosed with the condition? This news organization asked rheumatologist Marina Magrey, MD, and general internal medicine physician Debra Leizman, MD, for their expert advice.
Steps to Manage Suspected Axial SpA
“As [primary care practitioners] identify patients who they suspect may have axial spondyloarthritis, the first thing they should do is a prompt referral to a rheumatologist so that there is a timely diagnosis,” said Dr. Magrey, who heads up the division of rheumatology at University Hospitals Cleveland Medical Center and is professor of medicine at Case Western Reserve University School of Medicine in Cleveland, Ohio.
Importantly, the referral should “explicitly say that they’re suspecting axial spondyloarthritis” and not just chronic back pain, Dr. Magrey added, otherwise it may not “hit the radar” that patients need to be seen as soon as possible. Results of lab tests such as C-reactive protein, erythrocyte sedimentation rate, and human leukocyte antigen B27, along with basic pelvic imaging results, are useful to note on the referral. “If the patient comes with that information, it makes it much easier for the rheumatologist,” she said.
Additionally,
First-Line Treatment Options
“The goal is to improve the quality of life for our patients: To reduce pain, fatigue, inflammation,” Dr. Magrey noted. “So, starting a nonsteroidal anti-inflammatory drug [NSAID] with physical therapy is very useful” in primary care, she added. These remain the “cornerstone” of treatment for axial SpA even in secondary care.
Dr. Leizman agreed that her “go to” treatment for suspected axial SpA is physical therapy alongside one of the many NSAIDs available, such as naproxen or celecoxib. She may also use topical treatments such as lidocaine or diclofenac.
“I’m not going to start any biologics; I leave that for my rheumatologist,” said Dr. Leizman, who is a senior attending physician in the division of general internal medicine at University Hospitals Cleveland Medical Center and associate professor of medicine at Case Western Reserve University.
“If I think it’s a possibility that the patient will be going on to a biologic; however, I will try to check their TB status, immunizations, and vaccination titers, making sure that the patient is up to date and as healthy otherwise as possible so that they will be primed and ready, hopefully, to go on to the biologics,” she added.
Dr. Magrey cautioned that disease-modifying antirheumatic drugs, such as methotrexate and sulfasalazine, and systemic steroids such as oral prednisone “do not work in axial spondyloarthritis, so they are not recommended.”
Does the Choice of NSAID Matter?
The choice of NSAID is really down to the personal choice of the physician in agreement with the patient, and of course whether the medical insurance will cover it, Dr. Magrey observed. There appears to be little difference between the available NSAIDs, and it doesn’t appear to matter whether they are long-acting and taken once a day — which may be a convenient option for some patients — or short-acting and taken twice a day. The important point is that patients are taking these drugs continuously and not on demand and that they are being given at full dose.
“Start with one NSAID at the maximum strength, and then you try that for 2-4 weeks. If that doesn’t work, switch to another one,” Dr. Magrey advised.
American College of Rheumatology (ACR) guidelines for axial SpA recommend that a trial of at least two NSAIDs is undertaken before any biologic treatment is considered, but because the presentation of axial SpA is so heterogeneous, the decision to escalate treatment — usually to a tumor necrosis factor inhibitor first — is best left until after the referral and the diagnosis had been confirmed, she suggested.
What Type of Physical Therapy Works?
Physical therapy and nonpharmacologic ways to help people are integral to optimal patient management. But these still need to be prescribed and administered by a qualified physiotherapist, which means another, separate referral that can also take time, as it’s important to match the patient to the right physiotherapist, Dr. Leizman observed.
Patients need to be informed about the benefits of regular exercise, and suggesting low-impact exercises for the back can be helpful, Dr. Magrey noted.
“Supervised physical therapy is preferred over unsupervised back exercises,” Dr. Magrey said, summarizing current ACR recommendations, which also suggest that land-based activities are preferred over water-based exercises and group physical therapy rather than home-based exercises, according to the available evidence, although it is of low-to-moderate quality.
What type of physical therapy to recommend really boils down to what services are available, what facilities the patient has access to, and what they feel they are capable of doing or are willing to do.
Back pain can be frustrating for patients, said Dr. Leizman, because they hurt when they move, and there’s not a simple solution of “do this or that and you’ll get better.”
“If it’s possible for a patient to do aqua therapy, that has been a good option for many of my patients who are unable to get moving on land without pain,” she said, and “I’ve had some great success with some yoga therapists who work with my patients.”
Long-term Role of the Primary Care Practitioner
Once referred, patients with axial SpA will usually be seen by their rheumatologists at least twice a year to monitor their response to treatment. Primary care practitioners will also continue to see these people for other reasons and can help monitor for drug toxicity by performing blood and liver function tests, as well as looking for signs of associated conditions such as uveitis, psoriasis, and inflammatory bowel disease and referring patients on to other specialists as required.
Treating the inflammatory back pain may sometimes help treat the related conditions and vice versa, but not always, noted Dr. Leizman. Communication between professionals is thus very important to ensure that everyone is on the same page, and regular updates help enormously.
Dr. Leizman tries to see all her patients regularly, at least once a year, but it can be once or twice a year, depending on their age, how healthy they are, and what underlying conditions they may have that she is also managing along with the inflammatory back condition. It is a balancing act to prevent too many appointments, she said, but also helps patients manage the multiple recommendations.
At these appointments, she’ll not only check on patients’ progress and ensure that they have had all the tests that they should have, but she’ll also discuss general measures that may help with patients’ general health, such as weight control, their ability to manage disease processes with other daily activities of living, and other creative coping mechanisms.
“The weight discussion is never easy, but it is helpful to address the impact of weight if it may be contributing to their discomfort,” Dr. Leizman said. “I also think that there are diets patients can choose that are less inflammatory and that can be beneficial.”
Ultimately, “I want my patients to be on the least amount of medicine possible,” Dr. Leizman said. “If they need medications, I support my rheumatologists’ recommendations. I help my patients as they try whatever works to make them feel better, both the nonpharmaceutical options and the medications,” she said.
“Importantly, I am there for support as a resource and a partner,” Leizman added. “I’m the main quarterback for my patients.”
Key Takeaways
- Prompt referral to a rheumatologist remains key.
- The treatment goal is to improve patients’ quality of life by reducing symptoms such as pain and fatigue.
- Physical therapy and NSAIDs remain first-line treatment in primary care.
- NSAID treatment should be at the full recommended dose and given continuously, not as needed.
- The choice of NSAID does not matter; try switching the NSAID if no effects are seen.
- Physical therapy such as water-based activities and yoga may be beneficial, but exercise programs should be prescribed by a qualified therapist.
- Remember general health advice regarding diet and nutrition can be helpful.
A version of this article appeared on Medscape.com.
When axial spondyloarthritis (SpA) is suspected, a “prompt referral to a rheumatologist” is in order. But with the referral possibly taking several weeks, if not months in some parts of the world, how can primary care practitioners manage patients with this type of chronic back pain in the meantime? And what is the long-term role of the primary care practitioner in managing someone diagnosed with the condition? This news organization asked rheumatologist Marina Magrey, MD, and general internal medicine physician Debra Leizman, MD, for their expert advice.
Steps to Manage Suspected Axial SpA
“As [primary care practitioners] identify patients who they suspect may have axial spondyloarthritis, the first thing they should do is a prompt referral to a rheumatologist so that there is a timely diagnosis,” said Dr. Magrey, who heads up the division of rheumatology at University Hospitals Cleveland Medical Center and is professor of medicine at Case Western Reserve University School of Medicine in Cleveland, Ohio.
Importantly, the referral should “explicitly say that they’re suspecting axial spondyloarthritis” and not just chronic back pain, Dr. Magrey added, otherwise it may not “hit the radar” that patients need to be seen as soon as possible. Results of lab tests such as C-reactive protein, erythrocyte sedimentation rate, and human leukocyte antigen B27, along with basic pelvic imaging results, are useful to note on the referral. “If the patient comes with that information, it makes it much easier for the rheumatologist,” she said.
Additionally,
First-Line Treatment Options
“The goal is to improve the quality of life for our patients: To reduce pain, fatigue, inflammation,” Dr. Magrey noted. “So, starting a nonsteroidal anti-inflammatory drug [NSAID] with physical therapy is very useful” in primary care, she added. These remain the “cornerstone” of treatment for axial SpA even in secondary care.
Dr. Leizman agreed that her “go to” treatment for suspected axial SpA is physical therapy alongside one of the many NSAIDs available, such as naproxen or celecoxib. She may also use topical treatments such as lidocaine or diclofenac.
“I’m not going to start any biologics; I leave that for my rheumatologist,” said Dr. Leizman, who is a senior attending physician in the division of general internal medicine at University Hospitals Cleveland Medical Center and associate professor of medicine at Case Western Reserve University.
“If I think it’s a possibility that the patient will be going on to a biologic; however, I will try to check their TB status, immunizations, and vaccination titers, making sure that the patient is up to date and as healthy otherwise as possible so that they will be primed and ready, hopefully, to go on to the biologics,” she added.
Dr. Magrey cautioned that disease-modifying antirheumatic drugs, such as methotrexate and sulfasalazine, and systemic steroids such as oral prednisone “do not work in axial spondyloarthritis, so they are not recommended.”
Does the Choice of NSAID Matter?
The choice of NSAID is really down to the personal choice of the physician in agreement with the patient, and of course whether the medical insurance will cover it, Dr. Magrey observed. There appears to be little difference between the available NSAIDs, and it doesn’t appear to matter whether they are long-acting and taken once a day — which may be a convenient option for some patients — or short-acting and taken twice a day. The important point is that patients are taking these drugs continuously and not on demand and that they are being given at full dose.
“Start with one NSAID at the maximum strength, and then you try that for 2-4 weeks. If that doesn’t work, switch to another one,” Dr. Magrey advised.
American College of Rheumatology (ACR) guidelines for axial SpA recommend that a trial of at least two NSAIDs is undertaken before any biologic treatment is considered, but because the presentation of axial SpA is so heterogeneous, the decision to escalate treatment — usually to a tumor necrosis factor inhibitor first — is best left until after the referral and the diagnosis had been confirmed, she suggested.
What Type of Physical Therapy Works?
Physical therapy and nonpharmacologic ways to help people are integral to optimal patient management. But these still need to be prescribed and administered by a qualified physiotherapist, which means another, separate referral that can also take time, as it’s important to match the patient to the right physiotherapist, Dr. Leizman observed.
Patients need to be informed about the benefits of regular exercise, and suggesting low-impact exercises for the back can be helpful, Dr. Magrey noted.
“Supervised physical therapy is preferred over unsupervised back exercises,” Dr. Magrey said, summarizing current ACR recommendations, which also suggest that land-based activities are preferred over water-based exercises and group physical therapy rather than home-based exercises, according to the available evidence, although it is of low-to-moderate quality.
What type of physical therapy to recommend really boils down to what services are available, what facilities the patient has access to, and what they feel they are capable of doing or are willing to do.
Back pain can be frustrating for patients, said Dr. Leizman, because they hurt when they move, and there’s not a simple solution of “do this or that and you’ll get better.”
“If it’s possible for a patient to do aqua therapy, that has been a good option for many of my patients who are unable to get moving on land without pain,” she said, and “I’ve had some great success with some yoga therapists who work with my patients.”
Long-term Role of the Primary Care Practitioner
Once referred, patients with axial SpA will usually be seen by their rheumatologists at least twice a year to monitor their response to treatment. Primary care practitioners will also continue to see these people for other reasons and can help monitor for drug toxicity by performing blood and liver function tests, as well as looking for signs of associated conditions such as uveitis, psoriasis, and inflammatory bowel disease and referring patients on to other specialists as required.
Treating the inflammatory back pain may sometimes help treat the related conditions and vice versa, but not always, noted Dr. Leizman. Communication between professionals is thus very important to ensure that everyone is on the same page, and regular updates help enormously.
Dr. Leizman tries to see all her patients regularly, at least once a year, but it can be once or twice a year, depending on their age, how healthy they are, and what underlying conditions they may have that she is also managing along with the inflammatory back condition. It is a balancing act to prevent too many appointments, she said, but also helps patients manage the multiple recommendations.
At these appointments, she’ll not only check on patients’ progress and ensure that they have had all the tests that they should have, but she’ll also discuss general measures that may help with patients’ general health, such as weight control, their ability to manage disease processes with other daily activities of living, and other creative coping mechanisms.
“The weight discussion is never easy, but it is helpful to address the impact of weight if it may be contributing to their discomfort,” Dr. Leizman said. “I also think that there are diets patients can choose that are less inflammatory and that can be beneficial.”
Ultimately, “I want my patients to be on the least amount of medicine possible,” Dr. Leizman said. “If they need medications, I support my rheumatologists’ recommendations. I help my patients as they try whatever works to make them feel better, both the nonpharmaceutical options and the medications,” she said.
“Importantly, I am there for support as a resource and a partner,” Leizman added. “I’m the main quarterback for my patients.”
Key Takeaways
- Prompt referral to a rheumatologist remains key.
- The treatment goal is to improve patients’ quality of life by reducing symptoms such as pain and fatigue.
- Physical therapy and NSAIDs remain first-line treatment in primary care.
- NSAID treatment should be at the full recommended dose and given continuously, not as needed.
- The choice of NSAID does not matter; try switching the NSAID if no effects are seen.
- Physical therapy such as water-based activities and yoga may be beneficial, but exercise programs should be prescribed by a qualified therapist.
- Remember general health advice regarding diet and nutrition can be helpful.
A version of this article appeared on Medscape.com.
When axial spondyloarthritis (SpA) is suspected, a “prompt referral to a rheumatologist” is in order. But with the referral possibly taking several weeks, if not months in some parts of the world, how can primary care practitioners manage patients with this type of chronic back pain in the meantime? And what is the long-term role of the primary care practitioner in managing someone diagnosed with the condition? This news organization asked rheumatologist Marina Magrey, MD, and general internal medicine physician Debra Leizman, MD, for their expert advice.
Steps to Manage Suspected Axial SpA
“As [primary care practitioners] identify patients who they suspect may have axial spondyloarthritis, the first thing they should do is a prompt referral to a rheumatologist so that there is a timely diagnosis,” said Dr. Magrey, who heads up the division of rheumatology at University Hospitals Cleveland Medical Center and is professor of medicine at Case Western Reserve University School of Medicine in Cleveland, Ohio.
Importantly, the referral should “explicitly say that they’re suspecting axial spondyloarthritis” and not just chronic back pain, Dr. Magrey added, otherwise it may not “hit the radar” that patients need to be seen as soon as possible. Results of lab tests such as C-reactive protein, erythrocyte sedimentation rate, and human leukocyte antigen B27, along with basic pelvic imaging results, are useful to note on the referral. “If the patient comes with that information, it makes it much easier for the rheumatologist,” she said.
Additionally,
First-Line Treatment Options
“The goal is to improve the quality of life for our patients: To reduce pain, fatigue, inflammation,” Dr. Magrey noted. “So, starting a nonsteroidal anti-inflammatory drug [NSAID] with physical therapy is very useful” in primary care, she added. These remain the “cornerstone” of treatment for axial SpA even in secondary care.
Dr. Leizman agreed that her “go to” treatment for suspected axial SpA is physical therapy alongside one of the many NSAIDs available, such as naproxen or celecoxib. She may also use topical treatments such as lidocaine or diclofenac.
“I’m not going to start any biologics; I leave that for my rheumatologist,” said Dr. Leizman, who is a senior attending physician in the division of general internal medicine at University Hospitals Cleveland Medical Center and associate professor of medicine at Case Western Reserve University.
“If I think it’s a possibility that the patient will be going on to a biologic; however, I will try to check their TB status, immunizations, and vaccination titers, making sure that the patient is up to date and as healthy otherwise as possible so that they will be primed and ready, hopefully, to go on to the biologics,” she added.
Dr. Magrey cautioned that disease-modifying antirheumatic drugs, such as methotrexate and sulfasalazine, and systemic steroids such as oral prednisone “do not work in axial spondyloarthritis, so they are not recommended.”
Does the Choice of NSAID Matter?
The choice of NSAID is really down to the personal choice of the physician in agreement with the patient, and of course whether the medical insurance will cover it, Dr. Magrey observed. There appears to be little difference between the available NSAIDs, and it doesn’t appear to matter whether they are long-acting and taken once a day — which may be a convenient option for some patients — or short-acting and taken twice a day. The important point is that patients are taking these drugs continuously and not on demand and that they are being given at full dose.
“Start with one NSAID at the maximum strength, and then you try that for 2-4 weeks. If that doesn’t work, switch to another one,” Dr. Magrey advised.
American College of Rheumatology (ACR) guidelines for axial SpA recommend that a trial of at least two NSAIDs is undertaken before any biologic treatment is considered, but because the presentation of axial SpA is so heterogeneous, the decision to escalate treatment — usually to a tumor necrosis factor inhibitor first — is best left until after the referral and the diagnosis had been confirmed, she suggested.
What Type of Physical Therapy Works?
Physical therapy and nonpharmacologic ways to help people are integral to optimal patient management. But these still need to be prescribed and administered by a qualified physiotherapist, which means another, separate referral that can also take time, as it’s important to match the patient to the right physiotherapist, Dr. Leizman observed.
Patients need to be informed about the benefits of regular exercise, and suggesting low-impact exercises for the back can be helpful, Dr. Magrey noted.
“Supervised physical therapy is preferred over unsupervised back exercises,” Dr. Magrey said, summarizing current ACR recommendations, which also suggest that land-based activities are preferred over water-based exercises and group physical therapy rather than home-based exercises, according to the available evidence, although it is of low-to-moderate quality.
What type of physical therapy to recommend really boils down to what services are available, what facilities the patient has access to, and what they feel they are capable of doing or are willing to do.
Back pain can be frustrating for patients, said Dr. Leizman, because they hurt when they move, and there’s not a simple solution of “do this or that and you’ll get better.”
“If it’s possible for a patient to do aqua therapy, that has been a good option for many of my patients who are unable to get moving on land without pain,” she said, and “I’ve had some great success with some yoga therapists who work with my patients.”
Long-term Role of the Primary Care Practitioner
Once referred, patients with axial SpA will usually be seen by their rheumatologists at least twice a year to monitor their response to treatment. Primary care practitioners will also continue to see these people for other reasons and can help monitor for drug toxicity by performing blood and liver function tests, as well as looking for signs of associated conditions such as uveitis, psoriasis, and inflammatory bowel disease and referring patients on to other specialists as required.
Treating the inflammatory back pain may sometimes help treat the related conditions and vice versa, but not always, noted Dr. Leizman. Communication between professionals is thus very important to ensure that everyone is on the same page, and regular updates help enormously.
Dr. Leizman tries to see all her patients regularly, at least once a year, but it can be once or twice a year, depending on their age, how healthy they are, and what underlying conditions they may have that she is also managing along with the inflammatory back condition. It is a balancing act to prevent too many appointments, she said, but also helps patients manage the multiple recommendations.
At these appointments, she’ll not only check on patients’ progress and ensure that they have had all the tests that they should have, but she’ll also discuss general measures that may help with patients’ general health, such as weight control, their ability to manage disease processes with other daily activities of living, and other creative coping mechanisms.
“The weight discussion is never easy, but it is helpful to address the impact of weight if it may be contributing to their discomfort,” Dr. Leizman said. “I also think that there are diets patients can choose that are less inflammatory and that can be beneficial.”
Ultimately, “I want my patients to be on the least amount of medicine possible,” Dr. Leizman said. “If they need medications, I support my rheumatologists’ recommendations. I help my patients as they try whatever works to make them feel better, both the nonpharmaceutical options and the medications,” she said.
“Importantly, I am there for support as a resource and a partner,” Leizman added. “I’m the main quarterback for my patients.”
Key Takeaways
- Prompt referral to a rheumatologist remains key.
- The treatment goal is to improve patients’ quality of life by reducing symptoms such as pain and fatigue.
- Physical therapy and NSAIDs remain first-line treatment in primary care.
- NSAID treatment should be at the full recommended dose and given continuously, not as needed.
- The choice of NSAID does not matter; try switching the NSAID if no effects are seen.
- Physical therapy such as water-based activities and yoga may be beneficial, but exercise programs should be prescribed by a qualified therapist.
- Remember general health advice regarding diet and nutrition can be helpful.
A version of this article appeared on Medscape.com.
Should Physicians Offer Patients Medical Credit Cards?
With healthcare costs rising and payer reimbursements dwindling, many physicians are focusing even more on collecting outstanding patient balances.
Medical credit cards can be a popular choice to fill this gap because doctors get reimbursed upfront while patients receive special financing offers and the care they seek or need.
But, in recent months, federal officials have questioned whether these arrangements are genuinely win-win or if the cards prey on low-income and vulnerable individuals and warrant tighter regulatory oversight.
In July, the Consumer Financial Protection Bureau (CFPB), the US Department of Health and Human Services, and the US Department of Treasury announced an inquiry into medical credit cards. The agencies sought public comments from patients and providers to determine how much they are used.
Medical credit cards typically offer 0% or low-interest terms ranging from 6 to 24 months. Minimum monthly payments are required, often as low as $30 and not usually enough to pay the balance by the end of the promotional period.
After the introductory rate, card issuers may charge interest rates approaching 30% — not just on the remaining balance but on the original amount financed, adding considerably to total out-of-pocket costs.
Ophthalmologist Michael A. Brusco, MD, FACS, specializes in laser-assisted in situ keratomileuses and vision correction at his practice in the greater Washington, DC, area. He told this news organization that nearly all his patients are self-paying, and just under half utilize one of two medical credit cards he offers through third-party vendors, CareCredit and Alphaeon Credit.
“We are clear with our patients that it is interest-free only if they make all payments on time, and if they don’t, then the penalties and fees skyrocket,” Dr. Brusco said.
Patients pay no interest if they make the minimum monthly payments and pay the entire balance by the end of the term. Brusco said those who qualify and abide by those conditions can benefit from spreading healthcare expenses over several months and reducing the stress and financial strain associated with a larger, one-time payment.
He acknowledged that deferred interest can be problematic if patients are caught unaware but said his staff has received training from both vendors on clearly explaining the plans to patients. If someone doesn’t think they can pay off the balance in the timeframe, he suggests they pursue an alternative payment method.
Community Catalyst, a nonprofit health advocacy organization, has joined 60 other groups urging the Biden Administration to ban deferred interest medical credit cards.
They say that patients don’t understand what they are signing up for due to comments like these:
“Even though I’ve made monthly automatic payments on my account, as long as I have any balance on my account by [the end of the promotion], I’d be charged a 26.99% interest rate on the whole medical bill of [$2700].”
“I had nearly [$700] of interest that had accumulated within 4 months…based on one [$2000] charge. The employees at medical offices are selling a product they know little about without fully disclosing the terms and conditions to their patients.”
Historically, patients who apply for the cards have tended to use them to finance cosmetic or other lifestyle medicine procedures, but the CFPB said patients increasingly rely on them for routine and emergency care, which may contribute to growing medical debts and collections balances.
Federal authorities have expressed concerns that doctors may direct patients toward these financial arrangements instead of properly screening them for assistance programs or pursuing the sometimes arduous claims process to capture reimbursement from payers.
Growth of Medical Credit Card Market
One of the most widely used cards, CareCredit, is owned by Synchrony Bank and accepted at over 260,000 locations. Beyond private practices, the vendor has multiyear deals with over 300 hospitals, including Kaiser Permanente and the Cleveland Clinic.
Despite growing popularity and acceptance within the medical community, the cards may work well for some, but not all, patients.
According to a CFPB report released earlier this year, deferred interest medical credit cards were used to pay nearly $23 billion in healthcare expenses from 2018 to 2020. Individuals unable to stick to the terms paid $1 billion in deferred interest payments during that period. Three quarters of CareCredit consumers pay no interest, the organization reported.
Healthcare costs are likely driving demand for medical credit cards. In a recent survey by the Commonwealth Fund, almost half of respondents said it was very or somewhat difficult to afford care even when having insurance coverage through an employer, individual, or government plan. Consumers in the survey cited the high costs as a reason why they delayed or skipped care and prescription medication in the past year, including 29% of those with employer coverage and 42% with Medicare.
These dynamics can leave doctors between a rock and a hard place, said Alan P. Sager, PhD, a professor of health law, policy, and management at Boston University School of Public Health. He told this news organization that medical credit cards can keep cash flowing for doctors and provide elective and necessary care for patients, but the double-digit interest rates outside of the promotional periods can put patients at risk of bankruptcy. He views them as a short-term solution to a more significant problem.
“What doctors need and deserve is patients who have full coverage so that there are no medical debts and no need for medical credit cards,” said Dr. Sager.
Doctor Groups Weigh In
The Medical Group Management Association (MGMA), representing more than 15,000 medical groups, said in its public comments that Medicare cuts and staffing and inflation challenges have made running a profitable practice challenging, particularly for rural and less-resourced offices.
The organization said medical credit cards with transparent terms and conditions can help patients afford care and keep practice doors open amid rising operational costs. However, MGMA worries that the CFPB’s inquiry could “perpetuate the notion that it is acceptable for payment not to be rendered immediately after clinical services are provided, and it’s ok that payments are often subject to significant delays.”
Meanwhile, the American Society of Plastic Surgeons (ASPS) has endorsed CareCredit for over 20 years. In response to the CFPB’s request for information, the association said it supports medical credit cards that offer promotional low- or no-interest terms.
Steven Williams, MD, ASPS president, told this news organization that patients appreciate multiple payment options and the flexibility to move forward with care on short notice. Still, he said that it requires due diligence on everyone’s part.
“Lenders have a responsibility to educate their customers, and it’s critical that lending products have full disclosure in plain and clear language. And with any substantial purchase, patients need to analyze how much it adds to the bottom line,” he said.
A version of this article appeared on Medscape.com.
With healthcare costs rising and payer reimbursements dwindling, many physicians are focusing even more on collecting outstanding patient balances.
Medical credit cards can be a popular choice to fill this gap because doctors get reimbursed upfront while patients receive special financing offers and the care they seek or need.
But, in recent months, federal officials have questioned whether these arrangements are genuinely win-win or if the cards prey on low-income and vulnerable individuals and warrant tighter regulatory oversight.
In July, the Consumer Financial Protection Bureau (CFPB), the US Department of Health and Human Services, and the US Department of Treasury announced an inquiry into medical credit cards. The agencies sought public comments from patients and providers to determine how much they are used.
Medical credit cards typically offer 0% or low-interest terms ranging from 6 to 24 months. Minimum monthly payments are required, often as low as $30 and not usually enough to pay the balance by the end of the promotional period.
After the introductory rate, card issuers may charge interest rates approaching 30% — not just on the remaining balance but on the original amount financed, adding considerably to total out-of-pocket costs.
Ophthalmologist Michael A. Brusco, MD, FACS, specializes in laser-assisted in situ keratomileuses and vision correction at his practice in the greater Washington, DC, area. He told this news organization that nearly all his patients are self-paying, and just under half utilize one of two medical credit cards he offers through third-party vendors, CareCredit and Alphaeon Credit.
“We are clear with our patients that it is interest-free only if they make all payments on time, and if they don’t, then the penalties and fees skyrocket,” Dr. Brusco said.
Patients pay no interest if they make the minimum monthly payments and pay the entire balance by the end of the term. Brusco said those who qualify and abide by those conditions can benefit from spreading healthcare expenses over several months and reducing the stress and financial strain associated with a larger, one-time payment.
He acknowledged that deferred interest can be problematic if patients are caught unaware but said his staff has received training from both vendors on clearly explaining the plans to patients. If someone doesn’t think they can pay off the balance in the timeframe, he suggests they pursue an alternative payment method.
Community Catalyst, a nonprofit health advocacy organization, has joined 60 other groups urging the Biden Administration to ban deferred interest medical credit cards.
They say that patients don’t understand what they are signing up for due to comments like these:
“Even though I’ve made monthly automatic payments on my account, as long as I have any balance on my account by [the end of the promotion], I’d be charged a 26.99% interest rate on the whole medical bill of [$2700].”
“I had nearly [$700] of interest that had accumulated within 4 months…based on one [$2000] charge. The employees at medical offices are selling a product they know little about without fully disclosing the terms and conditions to their patients.”
Historically, patients who apply for the cards have tended to use them to finance cosmetic or other lifestyle medicine procedures, but the CFPB said patients increasingly rely on them for routine and emergency care, which may contribute to growing medical debts and collections balances.
Federal authorities have expressed concerns that doctors may direct patients toward these financial arrangements instead of properly screening them for assistance programs or pursuing the sometimes arduous claims process to capture reimbursement from payers.
Growth of Medical Credit Card Market
One of the most widely used cards, CareCredit, is owned by Synchrony Bank and accepted at over 260,000 locations. Beyond private practices, the vendor has multiyear deals with over 300 hospitals, including Kaiser Permanente and the Cleveland Clinic.
Despite growing popularity and acceptance within the medical community, the cards may work well for some, but not all, patients.
According to a CFPB report released earlier this year, deferred interest medical credit cards were used to pay nearly $23 billion in healthcare expenses from 2018 to 2020. Individuals unable to stick to the terms paid $1 billion in deferred interest payments during that period. Three quarters of CareCredit consumers pay no interest, the organization reported.
Healthcare costs are likely driving demand for medical credit cards. In a recent survey by the Commonwealth Fund, almost half of respondents said it was very or somewhat difficult to afford care even when having insurance coverage through an employer, individual, or government plan. Consumers in the survey cited the high costs as a reason why they delayed or skipped care and prescription medication in the past year, including 29% of those with employer coverage and 42% with Medicare.
These dynamics can leave doctors between a rock and a hard place, said Alan P. Sager, PhD, a professor of health law, policy, and management at Boston University School of Public Health. He told this news organization that medical credit cards can keep cash flowing for doctors and provide elective and necessary care for patients, but the double-digit interest rates outside of the promotional periods can put patients at risk of bankruptcy. He views them as a short-term solution to a more significant problem.
“What doctors need and deserve is patients who have full coverage so that there are no medical debts and no need for medical credit cards,” said Dr. Sager.
Doctor Groups Weigh In
The Medical Group Management Association (MGMA), representing more than 15,000 medical groups, said in its public comments that Medicare cuts and staffing and inflation challenges have made running a profitable practice challenging, particularly for rural and less-resourced offices.
The organization said medical credit cards with transparent terms and conditions can help patients afford care and keep practice doors open amid rising operational costs. However, MGMA worries that the CFPB’s inquiry could “perpetuate the notion that it is acceptable for payment not to be rendered immediately after clinical services are provided, and it’s ok that payments are often subject to significant delays.”
Meanwhile, the American Society of Plastic Surgeons (ASPS) has endorsed CareCredit for over 20 years. In response to the CFPB’s request for information, the association said it supports medical credit cards that offer promotional low- or no-interest terms.
Steven Williams, MD, ASPS president, told this news organization that patients appreciate multiple payment options and the flexibility to move forward with care on short notice. Still, he said that it requires due diligence on everyone’s part.
“Lenders have a responsibility to educate their customers, and it’s critical that lending products have full disclosure in plain and clear language. And with any substantial purchase, patients need to analyze how much it adds to the bottom line,” he said.
A version of this article appeared on Medscape.com.
With healthcare costs rising and payer reimbursements dwindling, many physicians are focusing even more on collecting outstanding patient balances.
Medical credit cards can be a popular choice to fill this gap because doctors get reimbursed upfront while patients receive special financing offers and the care they seek or need.
But, in recent months, federal officials have questioned whether these arrangements are genuinely win-win or if the cards prey on low-income and vulnerable individuals and warrant tighter regulatory oversight.
In July, the Consumer Financial Protection Bureau (CFPB), the US Department of Health and Human Services, and the US Department of Treasury announced an inquiry into medical credit cards. The agencies sought public comments from patients and providers to determine how much they are used.
Medical credit cards typically offer 0% or low-interest terms ranging from 6 to 24 months. Minimum monthly payments are required, often as low as $30 and not usually enough to pay the balance by the end of the promotional period.
After the introductory rate, card issuers may charge interest rates approaching 30% — not just on the remaining balance but on the original amount financed, adding considerably to total out-of-pocket costs.
Ophthalmologist Michael A. Brusco, MD, FACS, specializes in laser-assisted in situ keratomileuses and vision correction at his practice in the greater Washington, DC, area. He told this news organization that nearly all his patients are self-paying, and just under half utilize one of two medical credit cards he offers through third-party vendors, CareCredit and Alphaeon Credit.
“We are clear with our patients that it is interest-free only if they make all payments on time, and if they don’t, then the penalties and fees skyrocket,” Dr. Brusco said.
Patients pay no interest if they make the minimum monthly payments and pay the entire balance by the end of the term. Brusco said those who qualify and abide by those conditions can benefit from spreading healthcare expenses over several months and reducing the stress and financial strain associated with a larger, one-time payment.
He acknowledged that deferred interest can be problematic if patients are caught unaware but said his staff has received training from both vendors on clearly explaining the plans to patients. If someone doesn’t think they can pay off the balance in the timeframe, he suggests they pursue an alternative payment method.
Community Catalyst, a nonprofit health advocacy organization, has joined 60 other groups urging the Biden Administration to ban deferred interest medical credit cards.
They say that patients don’t understand what they are signing up for due to comments like these:
“Even though I’ve made monthly automatic payments on my account, as long as I have any balance on my account by [the end of the promotion], I’d be charged a 26.99% interest rate on the whole medical bill of [$2700].”
“I had nearly [$700] of interest that had accumulated within 4 months…based on one [$2000] charge. The employees at medical offices are selling a product they know little about without fully disclosing the terms and conditions to their patients.”
Historically, patients who apply for the cards have tended to use them to finance cosmetic or other lifestyle medicine procedures, but the CFPB said patients increasingly rely on them for routine and emergency care, which may contribute to growing medical debts and collections balances.
Federal authorities have expressed concerns that doctors may direct patients toward these financial arrangements instead of properly screening them for assistance programs or pursuing the sometimes arduous claims process to capture reimbursement from payers.
Growth of Medical Credit Card Market
One of the most widely used cards, CareCredit, is owned by Synchrony Bank and accepted at over 260,000 locations. Beyond private practices, the vendor has multiyear deals with over 300 hospitals, including Kaiser Permanente and the Cleveland Clinic.
Despite growing popularity and acceptance within the medical community, the cards may work well for some, but not all, patients.
According to a CFPB report released earlier this year, deferred interest medical credit cards were used to pay nearly $23 billion in healthcare expenses from 2018 to 2020. Individuals unable to stick to the terms paid $1 billion in deferred interest payments during that period. Three quarters of CareCredit consumers pay no interest, the organization reported.
Healthcare costs are likely driving demand for medical credit cards. In a recent survey by the Commonwealth Fund, almost half of respondents said it was very or somewhat difficult to afford care even when having insurance coverage through an employer, individual, or government plan. Consumers in the survey cited the high costs as a reason why they delayed or skipped care and prescription medication in the past year, including 29% of those with employer coverage and 42% with Medicare.
These dynamics can leave doctors between a rock and a hard place, said Alan P. Sager, PhD, a professor of health law, policy, and management at Boston University School of Public Health. He told this news organization that medical credit cards can keep cash flowing for doctors and provide elective and necessary care for patients, but the double-digit interest rates outside of the promotional periods can put patients at risk of bankruptcy. He views them as a short-term solution to a more significant problem.
“What doctors need and deserve is patients who have full coverage so that there are no medical debts and no need for medical credit cards,” said Dr. Sager.
Doctor Groups Weigh In
The Medical Group Management Association (MGMA), representing more than 15,000 medical groups, said in its public comments that Medicare cuts and staffing and inflation challenges have made running a profitable practice challenging, particularly for rural and less-resourced offices.
The organization said medical credit cards with transparent terms and conditions can help patients afford care and keep practice doors open amid rising operational costs. However, MGMA worries that the CFPB’s inquiry could “perpetuate the notion that it is acceptable for payment not to be rendered immediately after clinical services are provided, and it’s ok that payments are often subject to significant delays.”
Meanwhile, the American Society of Plastic Surgeons (ASPS) has endorsed CareCredit for over 20 years. In response to the CFPB’s request for information, the association said it supports medical credit cards that offer promotional low- or no-interest terms.
Steven Williams, MD, ASPS president, told this news organization that patients appreciate multiple payment options and the flexibility to move forward with care on short notice. Still, he said that it requires due diligence on everyone’s part.
“Lenders have a responsibility to educate their customers, and it’s critical that lending products have full disclosure in plain and clear language. And with any substantial purchase, patients need to analyze how much it adds to the bottom line,” he said.
A version of this article appeared on Medscape.com.
Older Adults Want Medicare, Insurance to Cover Obesity Drugs
Weight-loss drugs should be covered by Medicare and by other health insurance, according to a poll of US adults aged 50-80 years.
Among more than 2600 polled, 83% say that health insurance should cover prescription weight-loss drugs that have been approved by the US Food and Drug Administration (FDA), and 76% say Medicare should cover such drugs. However, only 30% would be willing to pay higher Medicare premiums to have these medications covered.
Among the 27% of respondents who say they are overweight, 63% are interested in taking such medications, as are 45% of those with diabetes, regardless of weight.
The University of Michigan (U-M) National Poll on Healthy Aging was published online on December 13, 2023.
High Awareness
The findings come at a time when injectable glucagon-like peptide 1 receptor agonists (GLP-1 RAs), such as Ozempic, Wegovy, Zepbound, and Mounjaro, are receiving a lot of public attention, the university noted.
Overall, 64% of survey respondents had heard of at least one prescription medication used for weight management.
By brand name, 61% had heard of Ozempic, approved for the treatment of type 2 diabetes but prescribed off label for weight loss; 18% had heard of Wegovy; and 13% had heard of the anorexiant drug phentermine .
Very few respondents (3% for each) had heard of the GLP-1 RA Saxenda, Qsymia (phentermine plus the anticonvulsant topiramate ), and the opiate antagonist Contrave.
Zepbound, the obesity -specific form of the diabetes drug Mounjaro, received FDA approval after the poll was taken and was not included in survey questions.
Among respondents who had heard of at least one prescription medication used for weight management, 58% had heard about them through the news (eg, TV, magazines, newspapers) and 53% had heard about them from an advertisement on TV, the Internet, or radio. Only 11% heard about them from their healthcare providers.
Respondents more likely to be interested in taking a prescription medication for weight management included women, those aged 50-64 years, Black persons, Hispanic persons, those with household incomes of less than $60,000 annually, those with lower levels of education, those in fair or poor physical or mental health, and those with a health problem or disability limiting their daily activities.
Spotty Coverage
The GLP-1 RAs can cost more than $12,000 a year for people who pay out of pocket, the university noted.
A Medicare Part D law passed in 2003 prohibits Medicare from covering medications for weight loss, although currently it can cover such drugs to help people with type 2 diabetes manage their weight.
Medicaid covers the cost of antiobesity drugs in some states.
Most private plans and the Veterans Health Administration cover them, but with restrictions due to high monthly costs for the newer medications.
The American Medical Association recently called on insurers to cover evidence-based weight-loss medications.
The strong demand for these medications, including for off-label purposes by people willing to pay full price, has created major shortages, the university noted.
“As these medications grow in awareness and use, and insurers make decisions about coverage, it’s crucial for patients who have obesity or diabetes, or who are overweight with other health problems, to talk with their healthcare providers about their options,” said poll director Jeffrey Kullgren, MD, MPH, MS, a primary care physician at the VA Ann Arbor Healthcare System and associate professor of internal medicine at U-M.
Other weight-management strategies that respondents think should be covered by health insurance include sessions with a registered dietitian or nutritionist (85%); weight-loss surgery (73%); gym or fitness facility memberships (65%); apps or online programs to track diet, exercise, and/or behavior change (58%); and sessions with a personal trainer (53%).
The randomly selected nationally representative household survey of 2657 adults was conducted from July 17 to August 7, 2023, by NORC at the University of Chicago for the U-M Institute for Healthcare Policy and Innovation. The sample was subsequently weighted to reflect population figures from the US Census Bureau. The completion rate was 50% among those contacted to participate. The margin of error is ±1 to 5 percentage points for questions asked of the full sample and higher among subgroups.
The poll is based at the U-M Institute for Healthcare Policy and Innovation and supported by AARP and Michigan Medicine, the University of Michigan’s academic medical center.
A version of this article appeared on Medscape.com.
Weight-loss drugs should be covered by Medicare and by other health insurance, according to a poll of US adults aged 50-80 years.
Among more than 2600 polled, 83% say that health insurance should cover prescription weight-loss drugs that have been approved by the US Food and Drug Administration (FDA), and 76% say Medicare should cover such drugs. However, only 30% would be willing to pay higher Medicare premiums to have these medications covered.
Among the 27% of respondents who say they are overweight, 63% are interested in taking such medications, as are 45% of those with diabetes, regardless of weight.
The University of Michigan (U-M) National Poll on Healthy Aging was published online on December 13, 2023.
High Awareness
The findings come at a time when injectable glucagon-like peptide 1 receptor agonists (GLP-1 RAs), such as Ozempic, Wegovy, Zepbound, and Mounjaro, are receiving a lot of public attention, the university noted.
Overall, 64% of survey respondents had heard of at least one prescription medication used for weight management.
By brand name, 61% had heard of Ozempic, approved for the treatment of type 2 diabetes but prescribed off label for weight loss; 18% had heard of Wegovy; and 13% had heard of the anorexiant drug phentermine .
Very few respondents (3% for each) had heard of the GLP-1 RA Saxenda, Qsymia (phentermine plus the anticonvulsant topiramate ), and the opiate antagonist Contrave.
Zepbound, the obesity -specific form of the diabetes drug Mounjaro, received FDA approval after the poll was taken and was not included in survey questions.
Among respondents who had heard of at least one prescription medication used for weight management, 58% had heard about them through the news (eg, TV, magazines, newspapers) and 53% had heard about them from an advertisement on TV, the Internet, or radio. Only 11% heard about them from their healthcare providers.
Respondents more likely to be interested in taking a prescription medication for weight management included women, those aged 50-64 years, Black persons, Hispanic persons, those with household incomes of less than $60,000 annually, those with lower levels of education, those in fair or poor physical or mental health, and those with a health problem or disability limiting their daily activities.
Spotty Coverage
The GLP-1 RAs can cost more than $12,000 a year for people who pay out of pocket, the university noted.
A Medicare Part D law passed in 2003 prohibits Medicare from covering medications for weight loss, although currently it can cover such drugs to help people with type 2 diabetes manage their weight.
Medicaid covers the cost of antiobesity drugs in some states.
Most private plans and the Veterans Health Administration cover them, but with restrictions due to high monthly costs for the newer medications.
The American Medical Association recently called on insurers to cover evidence-based weight-loss medications.
The strong demand for these medications, including for off-label purposes by people willing to pay full price, has created major shortages, the university noted.
“As these medications grow in awareness and use, and insurers make decisions about coverage, it’s crucial for patients who have obesity or diabetes, or who are overweight with other health problems, to talk with their healthcare providers about their options,” said poll director Jeffrey Kullgren, MD, MPH, MS, a primary care physician at the VA Ann Arbor Healthcare System and associate professor of internal medicine at U-M.
Other weight-management strategies that respondents think should be covered by health insurance include sessions with a registered dietitian or nutritionist (85%); weight-loss surgery (73%); gym or fitness facility memberships (65%); apps or online programs to track diet, exercise, and/or behavior change (58%); and sessions with a personal trainer (53%).
The randomly selected nationally representative household survey of 2657 adults was conducted from July 17 to August 7, 2023, by NORC at the University of Chicago for the U-M Institute for Healthcare Policy and Innovation. The sample was subsequently weighted to reflect population figures from the US Census Bureau. The completion rate was 50% among those contacted to participate. The margin of error is ±1 to 5 percentage points for questions asked of the full sample and higher among subgroups.
The poll is based at the U-M Institute for Healthcare Policy and Innovation and supported by AARP and Michigan Medicine, the University of Michigan’s academic medical center.
A version of this article appeared on Medscape.com.
Weight-loss drugs should be covered by Medicare and by other health insurance, according to a poll of US adults aged 50-80 years.
Among more than 2600 polled, 83% say that health insurance should cover prescription weight-loss drugs that have been approved by the US Food and Drug Administration (FDA), and 76% say Medicare should cover such drugs. However, only 30% would be willing to pay higher Medicare premiums to have these medications covered.
Among the 27% of respondents who say they are overweight, 63% are interested in taking such medications, as are 45% of those with diabetes, regardless of weight.
The University of Michigan (U-M) National Poll on Healthy Aging was published online on December 13, 2023.
High Awareness
The findings come at a time when injectable glucagon-like peptide 1 receptor agonists (GLP-1 RAs), such as Ozempic, Wegovy, Zepbound, and Mounjaro, are receiving a lot of public attention, the university noted.
Overall, 64% of survey respondents had heard of at least one prescription medication used for weight management.
By brand name, 61% had heard of Ozempic, approved for the treatment of type 2 diabetes but prescribed off label for weight loss; 18% had heard of Wegovy; and 13% had heard of the anorexiant drug phentermine .
Very few respondents (3% for each) had heard of the GLP-1 RA Saxenda, Qsymia (phentermine plus the anticonvulsant topiramate ), and the opiate antagonist Contrave.
Zepbound, the obesity -specific form of the diabetes drug Mounjaro, received FDA approval after the poll was taken and was not included in survey questions.
Among respondents who had heard of at least one prescription medication used for weight management, 58% had heard about them through the news (eg, TV, magazines, newspapers) and 53% had heard about them from an advertisement on TV, the Internet, or radio. Only 11% heard about them from their healthcare providers.
Respondents more likely to be interested in taking a prescription medication for weight management included women, those aged 50-64 years, Black persons, Hispanic persons, those with household incomes of less than $60,000 annually, those with lower levels of education, those in fair or poor physical or mental health, and those with a health problem or disability limiting their daily activities.
Spotty Coverage
The GLP-1 RAs can cost more than $12,000 a year for people who pay out of pocket, the university noted.
A Medicare Part D law passed in 2003 prohibits Medicare from covering medications for weight loss, although currently it can cover such drugs to help people with type 2 diabetes manage their weight.
Medicaid covers the cost of antiobesity drugs in some states.
Most private plans and the Veterans Health Administration cover them, but with restrictions due to high monthly costs for the newer medications.
The American Medical Association recently called on insurers to cover evidence-based weight-loss medications.
The strong demand for these medications, including for off-label purposes by people willing to pay full price, has created major shortages, the university noted.
“As these medications grow in awareness and use, and insurers make decisions about coverage, it’s crucial for patients who have obesity or diabetes, or who are overweight with other health problems, to talk with their healthcare providers about their options,” said poll director Jeffrey Kullgren, MD, MPH, MS, a primary care physician at the VA Ann Arbor Healthcare System and associate professor of internal medicine at U-M.
Other weight-management strategies that respondents think should be covered by health insurance include sessions with a registered dietitian or nutritionist (85%); weight-loss surgery (73%); gym or fitness facility memberships (65%); apps or online programs to track diet, exercise, and/or behavior change (58%); and sessions with a personal trainer (53%).
The randomly selected nationally representative household survey of 2657 adults was conducted from July 17 to August 7, 2023, by NORC at the University of Chicago for the U-M Institute for Healthcare Policy and Innovation. The sample was subsequently weighted to reflect population figures from the US Census Bureau. The completion rate was 50% among those contacted to participate. The margin of error is ±1 to 5 percentage points for questions asked of the full sample and higher among subgroups.
The poll is based at the U-M Institute for Healthcare Policy and Innovation and supported by AARP and Michigan Medicine, the University of Michigan’s academic medical center.
A version of this article appeared on Medscape.com.
Male Surgeons Linked With Higher Subsequent Healthcare Costs
, data suggested.
A retrospective, population-based cohort study that included more than 1 million adults undergoing any of 25 common surgical procedures found that total healthcare costs assessed at 1 year following surgery were more than $6000 higher when the surgery was performed by a male surgeon. Costs were also higher at 30 and 90 days for patients treated by male surgeons.
“As a male surgeon, I think our results should cause me and my colleagues to pause and consider why this may be,” said lead author Christopher J. D. Wallis, MD, PhD, assistant professor of surgery at the University of Toronto.
“None of us believe that the presence of a Y chromosome in surgeons means there are worse outcomes, it’s just that generally speaking, men and women, as we have known for decades, practice medicine a little differently. Things like communication style, time they spend with their patients, and even things like guideline adherence are different, and understanding how those differences translate into patient outcomes is the goal of this whole body of work,” said Wallis.
The study was published online November 29 in JAMA Surgery.
Explanation Is Elusive
In earlier work, Dr. Wallis and his team reported that patients treated by female surgeons had a small but statistically significant decrease in 30-day mortality, were less likely to be readmitted to the hospital, and had fewer complications than those treated by male surgeons. In another study, they found worse outcomes among female patients treated by male surgeons.
In the current study, the researchers examined the association between surgeon sex and healthcare costs among patients undergoing various surgical procedures, including coronary artery bypass grafting, appendectomy, hysterectomy, anterior spinal decompression, and knee replacement. They included all adult patients who underwent these procedures at hospitals in Ontario, Canada, between January 2007 and December 2019 in their analysis.
The study sample included 1,165,711 patients. Of this group, 151,054 patients were treated by a female surgeon, and 1,014,657 were treated by a male surgeon.
After adjusting for patient-, surgeon-, anesthesiologist-, and hospital-related factors, they found that 1-year total healthcare costs were $24,882 for patients treated by male surgeons vs $18,517 for patients treated by female surgeons. Healthcare costs were also higher at 30 days (adjusted absolute difference, $3115) and at 90 days (adjusted absolute difference, $4228).
“This translates into a 9%-10% higher risk of costs with male surgeons compared with women surgeons at these time points,” said Dr. Wallis.
“This study cannot provide a specific answer as to why these differences are occurring,” Dr. Wallis said.
“We are currently undertaking more research to better understand the reasons. Our previous studies have shown that patients treated by male physicians have higher rates of death, readmission, and complications. Managing these adverse postoperative events is costly and likely contributes to these differences. Given the size of our study and similar training pathways, we do not think there are technical differences between male and female surgeons. Rather, we are hypothesizing that there may be differences in how physicians practice, make decisions, and consult with patients,” he said.
Ultimately, Dr. Wallis said he would like his research to prompt “a moment of introspection” among his surgical colleagues.
“Hopefully, these data will provide the impetus for further efforts to make surgery, and medicine in general, a field that is welcoming to women,” he said.
Potential Confounding Factors
This study expands the evidence suggesting significant practice differences between male and female surgeons, Ursula Adams, MD, a resident; Caprice C. Greenberg, MD, MPH, chair; and Jared Gallaher, MD, MPH, adjunct assistant professor, all from the Department of Surgery at the University of North Carolina in Chapel Hill, wrote in an accompanying editorial.
They cautioned, however, that “there are many potential confounding factors and possible explanatory mechanisms associated with surgeon sex that make it challenging to untangle influences on costs. Sex may be an easily captured data point, but is understanding the mechanism by which it affects cost the right next step? Surgeons control how and where they practice; they do not have control over their own demographics.”
The editorialists added that while recruiting and retaining women in surgery is important, it is not a solution to controlling costs.
“We must provide surgeons with better data to understand how practice approach and decisions affect cost and support for practice improvement. Only with these insights will we ensure patients of male surgeons receive care that is just as cost-effective as that provided by female surgeons, while also helping to bend the cost curve and improve the quality of surgical care,” they concluded.
‘Admirable’ Data Use
Commenting on the findings, Oluwadamilola “Lola” Fayanju, MD, chief of breast surgery at Penn Medicine in Philadelphia, said, “It is interesting that the study was performed in Canada with its different healthcare system.” Dr. Fayanju did not participate in the study.
“They used administrative data from a national database, and it is admirable that they were able to do that. These data allow us to make large-scale geographical assessments, although they are subject to errors and unmeasured confounders,” said Dr. Fayanju.
Women surgeons may do things that result in better outcomes, she suggested. “In this study, the women were younger and so perhaps were more up to date. They might have optimized management of their patients in the pre-op phase, including better patient selection, which led to better costs. Or in the post-op phase, they might have made themselves readily accessible. For instance, I remove all barriers about getting in touch with me, and I tell my students to make sure the patient can reach you easily,” said Dr. Fayanju.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care, and the Data Sciences Institute at the University of Toronto. Dr. Wallis, Dr. Adams, Dr. Greenberg, Dr. Gallaher, and Dr. Fayanju reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, data suggested.
A retrospective, population-based cohort study that included more than 1 million adults undergoing any of 25 common surgical procedures found that total healthcare costs assessed at 1 year following surgery were more than $6000 higher when the surgery was performed by a male surgeon. Costs were also higher at 30 and 90 days for patients treated by male surgeons.
“As a male surgeon, I think our results should cause me and my colleagues to pause and consider why this may be,” said lead author Christopher J. D. Wallis, MD, PhD, assistant professor of surgery at the University of Toronto.
“None of us believe that the presence of a Y chromosome in surgeons means there are worse outcomes, it’s just that generally speaking, men and women, as we have known for decades, practice medicine a little differently. Things like communication style, time they spend with their patients, and even things like guideline adherence are different, and understanding how those differences translate into patient outcomes is the goal of this whole body of work,” said Wallis.
The study was published online November 29 in JAMA Surgery.
Explanation Is Elusive
In earlier work, Dr. Wallis and his team reported that patients treated by female surgeons had a small but statistically significant decrease in 30-day mortality, were less likely to be readmitted to the hospital, and had fewer complications than those treated by male surgeons. In another study, they found worse outcomes among female patients treated by male surgeons.
In the current study, the researchers examined the association between surgeon sex and healthcare costs among patients undergoing various surgical procedures, including coronary artery bypass grafting, appendectomy, hysterectomy, anterior spinal decompression, and knee replacement. They included all adult patients who underwent these procedures at hospitals in Ontario, Canada, between January 2007 and December 2019 in their analysis.
The study sample included 1,165,711 patients. Of this group, 151,054 patients were treated by a female surgeon, and 1,014,657 were treated by a male surgeon.
After adjusting for patient-, surgeon-, anesthesiologist-, and hospital-related factors, they found that 1-year total healthcare costs were $24,882 for patients treated by male surgeons vs $18,517 for patients treated by female surgeons. Healthcare costs were also higher at 30 days (adjusted absolute difference, $3115) and at 90 days (adjusted absolute difference, $4228).
“This translates into a 9%-10% higher risk of costs with male surgeons compared with women surgeons at these time points,” said Dr. Wallis.
“This study cannot provide a specific answer as to why these differences are occurring,” Dr. Wallis said.
“We are currently undertaking more research to better understand the reasons. Our previous studies have shown that patients treated by male physicians have higher rates of death, readmission, and complications. Managing these adverse postoperative events is costly and likely contributes to these differences. Given the size of our study and similar training pathways, we do not think there are technical differences between male and female surgeons. Rather, we are hypothesizing that there may be differences in how physicians practice, make decisions, and consult with patients,” he said.
Ultimately, Dr. Wallis said he would like his research to prompt “a moment of introspection” among his surgical colleagues.
“Hopefully, these data will provide the impetus for further efforts to make surgery, and medicine in general, a field that is welcoming to women,” he said.
Potential Confounding Factors
This study expands the evidence suggesting significant practice differences between male and female surgeons, Ursula Adams, MD, a resident; Caprice C. Greenberg, MD, MPH, chair; and Jared Gallaher, MD, MPH, adjunct assistant professor, all from the Department of Surgery at the University of North Carolina in Chapel Hill, wrote in an accompanying editorial.
They cautioned, however, that “there are many potential confounding factors and possible explanatory mechanisms associated with surgeon sex that make it challenging to untangle influences on costs. Sex may be an easily captured data point, but is understanding the mechanism by which it affects cost the right next step? Surgeons control how and where they practice; they do not have control over their own demographics.”
The editorialists added that while recruiting and retaining women in surgery is important, it is not a solution to controlling costs.
“We must provide surgeons with better data to understand how practice approach and decisions affect cost and support for practice improvement. Only with these insights will we ensure patients of male surgeons receive care that is just as cost-effective as that provided by female surgeons, while also helping to bend the cost curve and improve the quality of surgical care,” they concluded.
‘Admirable’ Data Use
Commenting on the findings, Oluwadamilola “Lola” Fayanju, MD, chief of breast surgery at Penn Medicine in Philadelphia, said, “It is interesting that the study was performed in Canada with its different healthcare system.” Dr. Fayanju did not participate in the study.
“They used administrative data from a national database, and it is admirable that they were able to do that. These data allow us to make large-scale geographical assessments, although they are subject to errors and unmeasured confounders,” said Dr. Fayanju.
Women surgeons may do things that result in better outcomes, she suggested. “In this study, the women were younger and so perhaps were more up to date. They might have optimized management of their patients in the pre-op phase, including better patient selection, which led to better costs. Or in the post-op phase, they might have made themselves readily accessible. For instance, I remove all barriers about getting in touch with me, and I tell my students to make sure the patient can reach you easily,” said Dr. Fayanju.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care, and the Data Sciences Institute at the University of Toronto. Dr. Wallis, Dr. Adams, Dr. Greenberg, Dr. Gallaher, and Dr. Fayanju reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, data suggested.
A retrospective, population-based cohort study that included more than 1 million adults undergoing any of 25 common surgical procedures found that total healthcare costs assessed at 1 year following surgery were more than $6000 higher when the surgery was performed by a male surgeon. Costs were also higher at 30 and 90 days for patients treated by male surgeons.
“As a male surgeon, I think our results should cause me and my colleagues to pause and consider why this may be,” said lead author Christopher J. D. Wallis, MD, PhD, assistant professor of surgery at the University of Toronto.
“None of us believe that the presence of a Y chromosome in surgeons means there are worse outcomes, it’s just that generally speaking, men and women, as we have known for decades, practice medicine a little differently. Things like communication style, time they spend with their patients, and even things like guideline adherence are different, and understanding how those differences translate into patient outcomes is the goal of this whole body of work,” said Wallis.
The study was published online November 29 in JAMA Surgery.
Explanation Is Elusive
In earlier work, Dr. Wallis and his team reported that patients treated by female surgeons had a small but statistically significant decrease in 30-day mortality, were less likely to be readmitted to the hospital, and had fewer complications than those treated by male surgeons. In another study, they found worse outcomes among female patients treated by male surgeons.
In the current study, the researchers examined the association between surgeon sex and healthcare costs among patients undergoing various surgical procedures, including coronary artery bypass grafting, appendectomy, hysterectomy, anterior spinal decompression, and knee replacement. They included all adult patients who underwent these procedures at hospitals in Ontario, Canada, between January 2007 and December 2019 in their analysis.
The study sample included 1,165,711 patients. Of this group, 151,054 patients were treated by a female surgeon, and 1,014,657 were treated by a male surgeon.
After adjusting for patient-, surgeon-, anesthesiologist-, and hospital-related factors, they found that 1-year total healthcare costs were $24,882 for patients treated by male surgeons vs $18,517 for patients treated by female surgeons. Healthcare costs were also higher at 30 days (adjusted absolute difference, $3115) and at 90 days (adjusted absolute difference, $4228).
“This translates into a 9%-10% higher risk of costs with male surgeons compared with women surgeons at these time points,” said Dr. Wallis.
“This study cannot provide a specific answer as to why these differences are occurring,” Dr. Wallis said.
“We are currently undertaking more research to better understand the reasons. Our previous studies have shown that patients treated by male physicians have higher rates of death, readmission, and complications. Managing these adverse postoperative events is costly and likely contributes to these differences. Given the size of our study and similar training pathways, we do not think there are technical differences between male and female surgeons. Rather, we are hypothesizing that there may be differences in how physicians practice, make decisions, and consult with patients,” he said.
Ultimately, Dr. Wallis said he would like his research to prompt “a moment of introspection” among his surgical colleagues.
“Hopefully, these data will provide the impetus for further efforts to make surgery, and medicine in general, a field that is welcoming to women,” he said.
Potential Confounding Factors
This study expands the evidence suggesting significant practice differences between male and female surgeons, Ursula Adams, MD, a resident; Caprice C. Greenberg, MD, MPH, chair; and Jared Gallaher, MD, MPH, adjunct assistant professor, all from the Department of Surgery at the University of North Carolina in Chapel Hill, wrote in an accompanying editorial.
They cautioned, however, that “there are many potential confounding factors and possible explanatory mechanisms associated with surgeon sex that make it challenging to untangle influences on costs. Sex may be an easily captured data point, but is understanding the mechanism by which it affects cost the right next step? Surgeons control how and where they practice; they do not have control over their own demographics.”
The editorialists added that while recruiting and retaining women in surgery is important, it is not a solution to controlling costs.
“We must provide surgeons with better data to understand how practice approach and decisions affect cost and support for practice improvement. Only with these insights will we ensure patients of male surgeons receive care that is just as cost-effective as that provided by female surgeons, while also helping to bend the cost curve and improve the quality of surgical care,” they concluded.
‘Admirable’ Data Use
Commenting on the findings, Oluwadamilola “Lola” Fayanju, MD, chief of breast surgery at Penn Medicine in Philadelphia, said, “It is interesting that the study was performed in Canada with its different healthcare system.” Dr. Fayanju did not participate in the study.
“They used administrative data from a national database, and it is admirable that they were able to do that. These data allow us to make large-scale geographical assessments, although they are subject to errors and unmeasured confounders,” said Dr. Fayanju.
Women surgeons may do things that result in better outcomes, she suggested. “In this study, the women were younger and so perhaps were more up to date. They might have optimized management of their patients in the pre-op phase, including better patient selection, which led to better costs. Or in the post-op phase, they might have made themselves readily accessible. For instance, I remove all barriers about getting in touch with me, and I tell my students to make sure the patient can reach you easily,” said Dr. Fayanju.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care, and the Data Sciences Institute at the University of Toronto. Dr. Wallis, Dr. Adams, Dr. Greenberg, Dr. Gallaher, and Dr. Fayanju reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Long COVID: New Info on Who Is Most Likely to Get It
The COVID-19 pandemic may no longer be a global public health emergency, but millions continue to struggle with the aftermath: Long COVID. New research and clinical anecdotes suggest that certain individuals are more likely to be afflicted by the condition, nearly 4 years after the virus emerged.
, said doctors who specialize in treating the condition.
Many patients with long COVID struggle with debilitating fatigue, brain fog, and cognitive impairment. The condition is also characterized by a catalog of other symptoms that may be difficult to recognize as long COVID, experts said. That’s especially true when patients may not mention seemingly unrelated information, such as underlying health conditions that might make them more vulnerable. This makes screening for certain conditions and investigating every symptom especially important.
The severity of a patient’s initial infection is not the only determining factor for developing long COVID, experts said.
“Don’t judge the person based on how sick they were initially,” said Mark Bayley, MD, medical director of the Toronto Rehabilitation Institute at University Health Network and a professor with the Temerty Faculty of Medicine at the University of Toronto. “You have to evaluate every symptom as best you can to make sure you’re not missing anything else.”
Someone who only had a bad cough or felt really unwell for just a few days and recovered but started feeling rotten again later — “that’s the person that we are seeing for long COVID,” said Dr. Bayley.
While patients who become severely sick and require hospitalization have a higher risk of developing long COVID, this group size is small compared with the much larger number of people infected overall. As a result, despite the lower risk, those who only become mild to moderately sick make up the vast majority of patients in long COVID clinics.
A small Northwestern Medicine study found that 41% of patients with long COVID never tested positive for COVID-19 but were found to have antibodies that indicated exposure to the virus.
Doctors treating patients with long COVID should consider several risk factors, specialists said. They include:
- A history of asthma, eczema, or allergies
- Signs of autonomic nervous system dysfunction
- Preexisting immune system issues
- Chronic infections
- Diabetes
- Being slightly overweight
- A preexisting history of anxiety or depression
- Joint hypermobility ( being “double-jointed” with pain and other symptoms)
Screening for Allergies
Alba Azola, MD, assistant professor of Physical Medicine and Rehabilitation at Johns Hopkins Medicine, said a history of asthma, allergies, and eczema and an onset of new food allergies may be an important factor in long COVID that doctors should consider when evaluating at-risk patients.
It is important to identify this subgroup of patients because they respond to antihistamines and mast cell stabilizers, which not only relieve their allergy symptoms but may also help improve overall fatigue and their tolerance for basic activities like standing, Dr. Azola said.
A recently published systemic review of prospective cohort studies on long COVID also found that patients with preexisting allergic conditions like asthma or rhinitis may be linked to a higher risk of developing long COVID. The authors cautioned, however, that the evidence for the link is uncertain and more rigorous research is needed.
“It stands to reason that if your immune system tends to be a bit hyperactive that triggering it with a virus will make it worse,” said Dr. Bayley.
Signs of Dysautonomia, Joint Hypermobility
Patients should also be screened for signs and symptoms of dysautonomia, or autonomic nervous system disorder, such as postural orthostatic tachycardia syndrome (POTS) or another type of autonomic dysfunction, doctors said.
“There’s a whole list because the autonomic nervous system involves every part of your body, every system,” Dr. Azola said.
Issues with standing, vision, digestion, urination, and bowel movement, for example, appear to be multisystemic problems but may all be linked to autonomic dysfunction, she explained.
Patients who have POTS usually experience a worsening of symptoms after COVID infection, Dr. Azola said, adding that some patients may have even assumed their pre-COVID symptoms of POTS were normal.
She also screens for joint hypermobility or hypermobile Ehlers-Danlos syndrome, which affects connective tissue. Research has long shown a relationship between autonomic dysfunction, mast cell activation syndrome (repeated severe allergy symptoms that affect multiple systems), and the presence of hypermobility, Dr. Azola said. She added that gentle physical therapy can be helpful for patients with hypermobility issues.
Previous studies before and during the pandemic have also found that a substantial subset of patients with myalgic encephalomyelitis/chronic fatigue syndrome, which shares many similarities with long COVID, also have connective tissue/hypermobility disorders.
Depression, Anxiety, and Female Patients
People with a preexisting history of anxiety or depression also appear to be at a higher risk for long COVID, Dr. Bayley said, noting that patients with these conditions appear more vulnerable to brain fog and other difficulties brought on by COVID infection. Earlier research found biochemical evidence of brain inflammation that correlates with symptoms of anxiety in patients with long COVID.
“We know that depression is related to neurotransmitters like adrenaline and serotonin,” Dr. Bayley said. “The chronic inflammation that’s associated with COVID — this will make people feel more depressed because they’re not getting the neurotransmitters in their brain releasing at the right times.”
It may also put patients at a risk for anxiety due to fears of post-exertional malaise (PEM), where symptoms worsen after even very minor physical or mental exertion and can last days or weeks.
“You can see how that leads to a bit of a vicious cycle,” said Dr. Bayley, explaining that the cycle of fear and avoidance makes patients less active and deconditioned. But he added that learning to manage their activity can actually help mitigate PEM due to the anti-inflammatory effects of exercise, its positive impact on mood, and benefits to the immune and cardiovascular systems.
Meanwhile, a number of epidemiologic studies have found a higher prevalence of long COVID among women. Perimenopausal and menopausal women in particular appeared more prone, and at least one study reported that women under 50 years were five times more likely to develop post-COVID symptoms than men.
A recent small UK study that focused on COVID-19 hospitalizations found that women who had lower levels of inflammatory biomarkers at admission were more likely to experience certain long-term symptoms like muscle ache, low mood and anxiety, adding to earlier research linking female patients, long COVID, and neuropsychiatric symptoms.
History of Immune Dysfunction, Diabetes, Elevated Body Mass Index (BMI)
Immune dysfunction, a history of recurrent infections, or chronic sinus infections are also common among patients under Dr. Azola and her team’s care. Those who have arthritis or other autoimmune diseases such as lupus also appear more vulnerable, Dr. Bayley said, along with patients who have diabetes or a little overweight.
Recent research out of the University of Queensland found that being overweight can negatively affect the body’s immune response to the SARS-CoV-2 virus. Blood samples collected 13 months after infection, for example, found that individuals with a higher BMI had lower antibody activity and a reduced percentage of relevant B cells that help build antibodies to fight the virus. Being overweight did not affect the antibody response to the COVID-19 vaccines, however, giving further support for vaccination over infection-induced immunity as an important protective factor, researchers said.
Narrowing the Information Gap
The latest Centers for Centers for Disease Control and Prevention’s Household Pulse Survey estimates that 14% of all American adults have had long COVID at some point, with more than 5% of the entire adult population currently experiencing long COVID. With millions of Americans affected, experts and advocates highlight the importance of bridging the knowledge gap with primary care doctors.
Long COVID specialists said understanding these connections helps guide treatment plans and manage symptoms, such as finding the right medications, improving tolerance, optimizing sleep, applying cognitive strategies for brain fog, dietary changes, respiratory exercises to help with shortness of breath, and finding the fine line between what causes PEM and what doesn’t.
“Whenever you see a disease like this one, you always have to ask yourself, is there an alternative way of looking at this that might explain what we’re seeing?” said Dr. Bayley. “It remains to be said that all bets are still open and that we need to continue to be very broad thinking about this.”
A version of this article appeared on Medscape.com.
The COVID-19 pandemic may no longer be a global public health emergency, but millions continue to struggle with the aftermath: Long COVID. New research and clinical anecdotes suggest that certain individuals are more likely to be afflicted by the condition, nearly 4 years after the virus emerged.
, said doctors who specialize in treating the condition.
Many patients with long COVID struggle with debilitating fatigue, brain fog, and cognitive impairment. The condition is also characterized by a catalog of other symptoms that may be difficult to recognize as long COVID, experts said. That’s especially true when patients may not mention seemingly unrelated information, such as underlying health conditions that might make them more vulnerable. This makes screening for certain conditions and investigating every symptom especially important.
The severity of a patient’s initial infection is not the only determining factor for developing long COVID, experts said.
“Don’t judge the person based on how sick they were initially,” said Mark Bayley, MD, medical director of the Toronto Rehabilitation Institute at University Health Network and a professor with the Temerty Faculty of Medicine at the University of Toronto. “You have to evaluate every symptom as best you can to make sure you’re not missing anything else.”
Someone who only had a bad cough or felt really unwell for just a few days and recovered but started feeling rotten again later — “that’s the person that we are seeing for long COVID,” said Dr. Bayley.
While patients who become severely sick and require hospitalization have a higher risk of developing long COVID, this group size is small compared with the much larger number of people infected overall. As a result, despite the lower risk, those who only become mild to moderately sick make up the vast majority of patients in long COVID clinics.
A small Northwestern Medicine study found that 41% of patients with long COVID never tested positive for COVID-19 but were found to have antibodies that indicated exposure to the virus.
Doctors treating patients with long COVID should consider several risk factors, specialists said. They include:
- A history of asthma, eczema, or allergies
- Signs of autonomic nervous system dysfunction
- Preexisting immune system issues
- Chronic infections
- Diabetes
- Being slightly overweight
- A preexisting history of anxiety or depression
- Joint hypermobility ( being “double-jointed” with pain and other symptoms)
Screening for Allergies
Alba Azola, MD, assistant professor of Physical Medicine and Rehabilitation at Johns Hopkins Medicine, said a history of asthma, allergies, and eczema and an onset of new food allergies may be an important factor in long COVID that doctors should consider when evaluating at-risk patients.
It is important to identify this subgroup of patients because they respond to antihistamines and mast cell stabilizers, which not only relieve their allergy symptoms but may also help improve overall fatigue and their tolerance for basic activities like standing, Dr. Azola said.
A recently published systemic review of prospective cohort studies on long COVID also found that patients with preexisting allergic conditions like asthma or rhinitis may be linked to a higher risk of developing long COVID. The authors cautioned, however, that the evidence for the link is uncertain and more rigorous research is needed.
“It stands to reason that if your immune system tends to be a bit hyperactive that triggering it with a virus will make it worse,” said Dr. Bayley.
Signs of Dysautonomia, Joint Hypermobility
Patients should also be screened for signs and symptoms of dysautonomia, or autonomic nervous system disorder, such as postural orthostatic tachycardia syndrome (POTS) or another type of autonomic dysfunction, doctors said.
“There’s a whole list because the autonomic nervous system involves every part of your body, every system,” Dr. Azola said.
Issues with standing, vision, digestion, urination, and bowel movement, for example, appear to be multisystemic problems but may all be linked to autonomic dysfunction, she explained.
Patients who have POTS usually experience a worsening of symptoms after COVID infection, Dr. Azola said, adding that some patients may have even assumed their pre-COVID symptoms of POTS were normal.
She also screens for joint hypermobility or hypermobile Ehlers-Danlos syndrome, which affects connective tissue. Research has long shown a relationship between autonomic dysfunction, mast cell activation syndrome (repeated severe allergy symptoms that affect multiple systems), and the presence of hypermobility, Dr. Azola said. She added that gentle physical therapy can be helpful for patients with hypermobility issues.
Previous studies before and during the pandemic have also found that a substantial subset of patients with myalgic encephalomyelitis/chronic fatigue syndrome, which shares many similarities with long COVID, also have connective tissue/hypermobility disorders.
Depression, Anxiety, and Female Patients
People with a preexisting history of anxiety or depression also appear to be at a higher risk for long COVID, Dr. Bayley said, noting that patients with these conditions appear more vulnerable to brain fog and other difficulties brought on by COVID infection. Earlier research found biochemical evidence of brain inflammation that correlates with symptoms of anxiety in patients with long COVID.
“We know that depression is related to neurotransmitters like adrenaline and serotonin,” Dr. Bayley said. “The chronic inflammation that’s associated with COVID — this will make people feel more depressed because they’re not getting the neurotransmitters in their brain releasing at the right times.”
It may also put patients at a risk for anxiety due to fears of post-exertional malaise (PEM), where symptoms worsen after even very minor physical or mental exertion and can last days or weeks.
“You can see how that leads to a bit of a vicious cycle,” said Dr. Bayley, explaining that the cycle of fear and avoidance makes patients less active and deconditioned. But he added that learning to manage their activity can actually help mitigate PEM due to the anti-inflammatory effects of exercise, its positive impact on mood, and benefits to the immune and cardiovascular systems.
Meanwhile, a number of epidemiologic studies have found a higher prevalence of long COVID among women. Perimenopausal and menopausal women in particular appeared more prone, and at least one study reported that women under 50 years were five times more likely to develop post-COVID symptoms than men.
A recent small UK study that focused on COVID-19 hospitalizations found that women who had lower levels of inflammatory biomarkers at admission were more likely to experience certain long-term symptoms like muscle ache, low mood and anxiety, adding to earlier research linking female patients, long COVID, and neuropsychiatric symptoms.
History of Immune Dysfunction, Diabetes, Elevated Body Mass Index (BMI)
Immune dysfunction, a history of recurrent infections, or chronic sinus infections are also common among patients under Dr. Azola and her team’s care. Those who have arthritis or other autoimmune diseases such as lupus also appear more vulnerable, Dr. Bayley said, along with patients who have diabetes or a little overweight.
Recent research out of the University of Queensland found that being overweight can negatively affect the body’s immune response to the SARS-CoV-2 virus. Blood samples collected 13 months after infection, for example, found that individuals with a higher BMI had lower antibody activity and a reduced percentage of relevant B cells that help build antibodies to fight the virus. Being overweight did not affect the antibody response to the COVID-19 vaccines, however, giving further support for vaccination over infection-induced immunity as an important protective factor, researchers said.
Narrowing the Information Gap
The latest Centers for Centers for Disease Control and Prevention’s Household Pulse Survey estimates that 14% of all American adults have had long COVID at some point, with more than 5% of the entire adult population currently experiencing long COVID. With millions of Americans affected, experts and advocates highlight the importance of bridging the knowledge gap with primary care doctors.
Long COVID specialists said understanding these connections helps guide treatment plans and manage symptoms, such as finding the right medications, improving tolerance, optimizing sleep, applying cognitive strategies for brain fog, dietary changes, respiratory exercises to help with shortness of breath, and finding the fine line between what causes PEM and what doesn’t.
“Whenever you see a disease like this one, you always have to ask yourself, is there an alternative way of looking at this that might explain what we’re seeing?” said Dr. Bayley. “It remains to be said that all bets are still open and that we need to continue to be very broad thinking about this.”
A version of this article appeared on Medscape.com.
The COVID-19 pandemic may no longer be a global public health emergency, but millions continue to struggle with the aftermath: Long COVID. New research and clinical anecdotes suggest that certain individuals are more likely to be afflicted by the condition, nearly 4 years after the virus emerged.
, said doctors who specialize in treating the condition.
Many patients with long COVID struggle with debilitating fatigue, brain fog, and cognitive impairment. The condition is also characterized by a catalog of other symptoms that may be difficult to recognize as long COVID, experts said. That’s especially true when patients may not mention seemingly unrelated information, such as underlying health conditions that might make them more vulnerable. This makes screening for certain conditions and investigating every symptom especially important.
The severity of a patient’s initial infection is not the only determining factor for developing long COVID, experts said.
“Don’t judge the person based on how sick they were initially,” said Mark Bayley, MD, medical director of the Toronto Rehabilitation Institute at University Health Network and a professor with the Temerty Faculty of Medicine at the University of Toronto. “You have to evaluate every symptom as best you can to make sure you’re not missing anything else.”
Someone who only had a bad cough or felt really unwell for just a few days and recovered but started feeling rotten again later — “that’s the person that we are seeing for long COVID,” said Dr. Bayley.
While patients who become severely sick and require hospitalization have a higher risk of developing long COVID, this group size is small compared with the much larger number of people infected overall. As a result, despite the lower risk, those who only become mild to moderately sick make up the vast majority of patients in long COVID clinics.
A small Northwestern Medicine study found that 41% of patients with long COVID never tested positive for COVID-19 but were found to have antibodies that indicated exposure to the virus.
Doctors treating patients with long COVID should consider several risk factors, specialists said. They include:
- A history of asthma, eczema, or allergies
- Signs of autonomic nervous system dysfunction
- Preexisting immune system issues
- Chronic infections
- Diabetes
- Being slightly overweight
- A preexisting history of anxiety or depression
- Joint hypermobility ( being “double-jointed” with pain and other symptoms)
Screening for Allergies
Alba Azola, MD, assistant professor of Physical Medicine and Rehabilitation at Johns Hopkins Medicine, said a history of asthma, allergies, and eczema and an onset of new food allergies may be an important factor in long COVID that doctors should consider when evaluating at-risk patients.
It is important to identify this subgroup of patients because they respond to antihistamines and mast cell stabilizers, which not only relieve their allergy symptoms but may also help improve overall fatigue and their tolerance for basic activities like standing, Dr. Azola said.
A recently published systemic review of prospective cohort studies on long COVID also found that patients with preexisting allergic conditions like asthma or rhinitis may be linked to a higher risk of developing long COVID. The authors cautioned, however, that the evidence for the link is uncertain and more rigorous research is needed.
“It stands to reason that if your immune system tends to be a bit hyperactive that triggering it with a virus will make it worse,” said Dr. Bayley.
Signs of Dysautonomia, Joint Hypermobility
Patients should also be screened for signs and symptoms of dysautonomia, or autonomic nervous system disorder, such as postural orthostatic tachycardia syndrome (POTS) or another type of autonomic dysfunction, doctors said.
“There’s a whole list because the autonomic nervous system involves every part of your body, every system,” Dr. Azola said.
Issues with standing, vision, digestion, urination, and bowel movement, for example, appear to be multisystemic problems but may all be linked to autonomic dysfunction, she explained.
Patients who have POTS usually experience a worsening of symptoms after COVID infection, Dr. Azola said, adding that some patients may have even assumed their pre-COVID symptoms of POTS were normal.
She also screens for joint hypermobility or hypermobile Ehlers-Danlos syndrome, which affects connective tissue. Research has long shown a relationship between autonomic dysfunction, mast cell activation syndrome (repeated severe allergy symptoms that affect multiple systems), and the presence of hypermobility, Dr. Azola said. She added that gentle physical therapy can be helpful for patients with hypermobility issues.
Previous studies before and during the pandemic have also found that a substantial subset of patients with myalgic encephalomyelitis/chronic fatigue syndrome, which shares many similarities with long COVID, also have connective tissue/hypermobility disorders.
Depression, Anxiety, and Female Patients
People with a preexisting history of anxiety or depression also appear to be at a higher risk for long COVID, Dr. Bayley said, noting that patients with these conditions appear more vulnerable to brain fog and other difficulties brought on by COVID infection. Earlier research found biochemical evidence of brain inflammation that correlates with symptoms of anxiety in patients with long COVID.
“We know that depression is related to neurotransmitters like adrenaline and serotonin,” Dr. Bayley said. “The chronic inflammation that’s associated with COVID — this will make people feel more depressed because they’re not getting the neurotransmitters in their brain releasing at the right times.”
It may also put patients at a risk for anxiety due to fears of post-exertional malaise (PEM), where symptoms worsen after even very minor physical or mental exertion and can last days or weeks.
“You can see how that leads to a bit of a vicious cycle,” said Dr. Bayley, explaining that the cycle of fear and avoidance makes patients less active and deconditioned. But he added that learning to manage their activity can actually help mitigate PEM due to the anti-inflammatory effects of exercise, its positive impact on mood, and benefits to the immune and cardiovascular systems.
Meanwhile, a number of epidemiologic studies have found a higher prevalence of long COVID among women. Perimenopausal and menopausal women in particular appeared more prone, and at least one study reported that women under 50 years were five times more likely to develop post-COVID symptoms than men.
A recent small UK study that focused on COVID-19 hospitalizations found that women who had lower levels of inflammatory biomarkers at admission were more likely to experience certain long-term symptoms like muscle ache, low mood and anxiety, adding to earlier research linking female patients, long COVID, and neuropsychiatric symptoms.
History of Immune Dysfunction, Diabetes, Elevated Body Mass Index (BMI)
Immune dysfunction, a history of recurrent infections, or chronic sinus infections are also common among patients under Dr. Azola and her team’s care. Those who have arthritis or other autoimmune diseases such as lupus also appear more vulnerable, Dr. Bayley said, along with patients who have diabetes or a little overweight.
Recent research out of the University of Queensland found that being overweight can negatively affect the body’s immune response to the SARS-CoV-2 virus. Blood samples collected 13 months after infection, for example, found that individuals with a higher BMI had lower antibody activity and a reduced percentage of relevant B cells that help build antibodies to fight the virus. Being overweight did not affect the antibody response to the COVID-19 vaccines, however, giving further support for vaccination over infection-induced immunity as an important protective factor, researchers said.
Narrowing the Information Gap
The latest Centers for Centers for Disease Control and Prevention’s Household Pulse Survey estimates that 14% of all American adults have had long COVID at some point, with more than 5% of the entire adult population currently experiencing long COVID. With millions of Americans affected, experts and advocates highlight the importance of bridging the knowledge gap with primary care doctors.
Long COVID specialists said understanding these connections helps guide treatment plans and manage symptoms, such as finding the right medications, improving tolerance, optimizing sleep, applying cognitive strategies for brain fog, dietary changes, respiratory exercises to help with shortness of breath, and finding the fine line between what causes PEM and what doesn’t.
“Whenever you see a disease like this one, you always have to ask yourself, is there an alternative way of looking at this that might explain what we’re seeing?” said Dr. Bayley. “It remains to be said that all bets are still open and that we need to continue to be very broad thinking about this.”
A version of this article appeared on Medscape.com.
Spending the Holidays With GLP-1 Receptor Agonists: 5 Things to Know
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
A New Test Could Save Arthritis Patients Time, Money, and Pain. But Will It Be Used?
Erinn Maury, MD, knew Remicade wasn’t the right drug for Patti Schulte, a patient with rheumatoid arthritis the physician saw at her Millersville, Maryland, practice. Schulte’s swollen, painful joints hadn’t responded to Enbrel or Humira, two drugs in the same class.
But the insurer insisted, so Schulte went on Remicade. It didn’t work either.
What’s more, Schulte suffered a severe allergic reaction to the infusion therapy, requiring a heavy dose of prednisone, a steroid with grave side effects if used at high doses for too long.
After 18 months, her insurer finally approved Maury’s drug of choice, Orencia. By then, Schulte’s vertebrae, weakened by prednisone, had started cracking. She was only 60.
It’s also a story of how doctors are steered by pharmacy benefit managers — the middlemen of the drug market — as well as by insurers.
Once people with inflammatory conditions such as rheumatoid arthritis reach a certain stage, the first prescription offered is typically Humira, the best-selling drug in history, and part of a class known as tumor necrosis factor inhibitors, or TNFis, which fail to significantly help about half of the patients who take it.
“We practice rheumatology without any help,” said Vibeke Strand, a rheumatologist and adjunct clinical professor at Stanford. She bemoaned the lack of tools available to choose the right drug while bristling at corporate intervention in the decision. “We are told by the insurer what to prescribe to the patient. After they fail methotrexate, it’s a TNF inhibitor, almost always Humira. And that’s not OK.”
If there’s a shred of hope in this story, it’s that a blood test, PrismRA, may herald an era of improved care for patients with rheumatoid arthritis and other autoimmune conditions. But first, it must be embraced by insurers.
PrismRA employs a predictive model that combines clinical factors, blood tests, and 19 gene patterns to identify the roughly 60% of patients who are very unlikely to respond to a TNFi drug.
Over the past 25 years, drug companies have introduced five new classes of autoimmune drugs. TNFis were the first to market, starting in the late 1990s.
Some 1.3 million Americans have rheumatoid arthritis, a disease in which a person’s immune system attacks their joints, causing crippling pain and, if improperly treated, disfigurement. The newer drugs, mostly so-called biologics, are also used by some of the 25 million or more Americans with other autoimmune diseases, such as lupus, Crohn’s disease, and psoriasis. Typically costing tens of thousands of dollars annually, the drugs are prescribed after a patient fails to respond to older, cheaper drugs like methotrexate.
Until recently, rheumatologists have had few ways to predict which of the new drugs would work best on which patients. Often, “it’s a coin flip whether I prescribe drug A or B,” said Jeffrey Curtis, MD, a rheumatology professor at the University of Alabama-Birmingham.
Yet about 90% of the patients who are given one of these advanced drugs start on a TNFi, although there’s often no reason to think a TNFi will work better than another type.
Under these puzzling circumstances, it’s often the insurer rather than the doctor who chooses the patient’s drug. Insurers lean toward TNFis such as adalimumab, commonly sold as brand name Humira, in part because they get large rebates from manufacturers for using them. Although the size of such payments is a trade secret, AbbVie is said to be offering rebates to insurers of up to 60% of Humira’s price. That has enabled it to control 98.5% of the US adalimumab market, even though it has eight biosimilar competitors.
PrismRA’s developer, Scipher Medicine, has provided more than 26,000 test results, rarely covered by insurance. But on October 15, the Centers for Medicare & Medicaid began reimbursing for the test, and its use is expected to rise. At least two other companies are developing drug-matching tests for patients with rheumatoid arthritis.
Although critics say PrismRA is not always useful, it is likely to be the first in a series of diagnostics anticipated over the next decade that could reduce the time that patients with autoimmune disease suffer on the wrong drug.
Academics, small biotechs, and large pharmaceutical companies are investing in methods to distinguish the biological pathways involved in these diseases and the best way to treat each one. This approach, called precision medicine, has existed for years in cancer medicine, in which it’s routine to test the genetics of patients’ tumors to determine the appropriate drug treatment.
“You wouldn’t give Herceptin to a breast cancer patient without knowing whether her tumor was HER2-positive,” said Costantino Pitzalis, MD, a rheumatology professor at the William Harvey Research Institute in London, England. He was speaking before a well-attended session at an American College of Rheumatology conference in San Diego in November. “Why do we not use biopsies or seek molecular markers in rheumatoid arthritis?”
It’s not only patients and doctors who have a stake in which drugs work best for a given person.
When Remicade failed and Schulte waited for the insurer to approve Orencia, she insisted on keeping her job as an accountant. But as her prednisone-related spinal problems worsened, Schulte was forced to retire, go on Medicaid, and seek disability, something she had always sworn to avoid.
Now taxpayers, rather than the insurer, are covering Schulte’s medical bills, Dr. Maury noted.
Precision medicine hasn’t seemed like a priority for large makers of autoimmune drugs, which presumably have some knowledge of which patients are most likely to benefit from their drugs, because they have tested and sold millions of doses over the years. By offering rebate incentives to insurers, companies like AbbVie, which makes Humira, can guarantee theirs are the drugs of choice with insurers.
“If you were AbbVie,” Dr. Curtis said, “why would you ever want to publish data showing who’s not going to do well on your drug, if, in the absence of the test, everyone will start with your drug first?”
What Testing Could Do
Medicare and commercial insurers haven’t yet set a price for PrismRA, but it could save insurers thousands of dollars a year for each patient it helps, according to Krishna Patel, PharmD, Scipher’s associate director of medical affairs.
“If the test cost $750, I still only need it once, and it costs less than a month of whatever drug is not going to work very well for you,” said Dr. Curtis, a coauthor of some studies of the test. “The economics of a biomarker that’s anything but worthless is pretty favorable because our biologics and targeted drugs are so expensive.”
Patients are enthusiastic about the test because so many have had to take TNFis that didn’t work. Many insurers require patients to try a second TNFi and sometimes a third.
Jen Weaver, a patient advocate and mother of three, got little benefit from hydroxychloroquine, sulfasalazine, methotrexate, and Orencia, a non-TNFi biologic therapy, before finding some relief in another, Actemra. But she was taken off that drug when her white blood cells plunged, and the next three drugs she tried — all TNFis — caused allergic reactions, culminating with an outbreak of pus-filled sores. Another drug, Otezla, eventually seemed to help heal the sores, and she’s been stable on it since in combination with methotrexate, Ms. Weaver said.
“What is needed is to substantially shorten this trial-and-error period for patients,” said Shilpa Venkatachalam, PhD, herself a patient and the director of research operations at the Global Healthy Living Foundation. “There’s a lot of anxiety and frustration, weeks in pain wondering whether a drug is going to work for you and what to do if it doesn’t.” A survey by her group found that 91% of patients worried their medications would stop working. And there is evidence that the longer it takes to resolve arthritis symptoms, the less chance they will ever stop.
How insurers will respond to the availability of tests isn’t clear, partly because the arrival of new biosimilar drugs — essentially generic versions — is making TNFis cheaper for insurance plans. While Humira still dominates, AbbVie has increased rebates to insurers, in effect lowering its cost. Lower prices make the PrismRA test less appealing to insurers because widespread use of the test could cut TNFi prescriptions by up to a third.
However, rheumatologist John B. Boone, MD, in Louisville, Kentucky, found to his surprise that insurers mostly accepted alternative prescriptions for 41 patients whom the test showed unlikely to respond to TNFis as part of a clinical trial. Dr. Boone receives consulting fees from Scipher.
Although the test didn’t guarantee good outcomes, he said, the few patients given TNFis despite the test results almost all did poorly on that regimen.
Scientists from AbbVie, which makes several rheumatology drugs in addition to Humira, presented a study at the San Diego conference examining biomarkers that might show which patients would respond to Rinvoq, a new immune-suppressing drug in a class known as the JAK inhibitors. When asked about its use of precision medicine, AbbVie declined to comment.
Over two decades, Humira has been a blockbuster drug for AbbVie. The company sold more than $3.5 billion worth of Humira in the third quarter of 2023, 36% less than a year ago. Sales of Rinvoq, which AbbVie is marketing as a treatment for patients failed by Humira and its class, jumped 60% to $1.1 billion.
What Patients Want
Shannan O’Hara-Levi, a 38-year-old in Monroe, New York, has been on scores of drugs and supplements since being diagnosed with juvenile arthritis at age 3. She’s been nauseated, fatigued, and short of breath and has suffered allergic reactions, but she says the worst part of it was finding a drug that worked and then losing access because of insurance. This happened shortly after she gave birth to a daughter in 2022 and then endured intense joint pain.
“If I could take a blood test that tells me not to waste months or years of my life — absolutely,” she said. “If I could have started my current drug last fall and saved many months of not being able to engage with my baby on the floor — absolutely.”
This article originally appeared on KFFHealthNews.org. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
Erinn Maury, MD, knew Remicade wasn’t the right drug for Patti Schulte, a patient with rheumatoid arthritis the physician saw at her Millersville, Maryland, practice. Schulte’s swollen, painful joints hadn’t responded to Enbrel or Humira, two drugs in the same class.
But the insurer insisted, so Schulte went on Remicade. It didn’t work either.
What’s more, Schulte suffered a severe allergic reaction to the infusion therapy, requiring a heavy dose of prednisone, a steroid with grave side effects if used at high doses for too long.
After 18 months, her insurer finally approved Maury’s drug of choice, Orencia. By then, Schulte’s vertebrae, weakened by prednisone, had started cracking. She was only 60.
It’s also a story of how doctors are steered by pharmacy benefit managers — the middlemen of the drug market — as well as by insurers.
Once people with inflammatory conditions such as rheumatoid arthritis reach a certain stage, the first prescription offered is typically Humira, the best-selling drug in history, and part of a class known as tumor necrosis factor inhibitors, or TNFis, which fail to significantly help about half of the patients who take it.
“We practice rheumatology without any help,” said Vibeke Strand, a rheumatologist and adjunct clinical professor at Stanford. She bemoaned the lack of tools available to choose the right drug while bristling at corporate intervention in the decision. “We are told by the insurer what to prescribe to the patient. After they fail methotrexate, it’s a TNF inhibitor, almost always Humira. And that’s not OK.”
If there’s a shred of hope in this story, it’s that a blood test, PrismRA, may herald an era of improved care for patients with rheumatoid arthritis and other autoimmune conditions. But first, it must be embraced by insurers.
PrismRA employs a predictive model that combines clinical factors, blood tests, and 19 gene patterns to identify the roughly 60% of patients who are very unlikely to respond to a TNFi drug.
Over the past 25 years, drug companies have introduced five new classes of autoimmune drugs. TNFis were the first to market, starting in the late 1990s.
Some 1.3 million Americans have rheumatoid arthritis, a disease in which a person’s immune system attacks their joints, causing crippling pain and, if improperly treated, disfigurement. The newer drugs, mostly so-called biologics, are also used by some of the 25 million or more Americans with other autoimmune diseases, such as lupus, Crohn’s disease, and psoriasis. Typically costing tens of thousands of dollars annually, the drugs are prescribed after a patient fails to respond to older, cheaper drugs like methotrexate.
Until recently, rheumatologists have had few ways to predict which of the new drugs would work best on which patients. Often, “it’s a coin flip whether I prescribe drug A or B,” said Jeffrey Curtis, MD, a rheumatology professor at the University of Alabama-Birmingham.
Yet about 90% of the patients who are given one of these advanced drugs start on a TNFi, although there’s often no reason to think a TNFi will work better than another type.
Under these puzzling circumstances, it’s often the insurer rather than the doctor who chooses the patient’s drug. Insurers lean toward TNFis such as adalimumab, commonly sold as brand name Humira, in part because they get large rebates from manufacturers for using them. Although the size of such payments is a trade secret, AbbVie is said to be offering rebates to insurers of up to 60% of Humira’s price. That has enabled it to control 98.5% of the US adalimumab market, even though it has eight biosimilar competitors.
PrismRA’s developer, Scipher Medicine, has provided more than 26,000 test results, rarely covered by insurance. But on October 15, the Centers for Medicare & Medicaid began reimbursing for the test, and its use is expected to rise. At least two other companies are developing drug-matching tests for patients with rheumatoid arthritis.
Although critics say PrismRA is not always useful, it is likely to be the first in a series of diagnostics anticipated over the next decade that could reduce the time that patients with autoimmune disease suffer on the wrong drug.
Academics, small biotechs, and large pharmaceutical companies are investing in methods to distinguish the biological pathways involved in these diseases and the best way to treat each one. This approach, called precision medicine, has existed for years in cancer medicine, in which it’s routine to test the genetics of patients’ tumors to determine the appropriate drug treatment.
“You wouldn’t give Herceptin to a breast cancer patient without knowing whether her tumor was HER2-positive,” said Costantino Pitzalis, MD, a rheumatology professor at the William Harvey Research Institute in London, England. He was speaking before a well-attended session at an American College of Rheumatology conference in San Diego in November. “Why do we not use biopsies or seek molecular markers in rheumatoid arthritis?”
It’s not only patients and doctors who have a stake in which drugs work best for a given person.
When Remicade failed and Schulte waited for the insurer to approve Orencia, she insisted on keeping her job as an accountant. But as her prednisone-related spinal problems worsened, Schulte was forced to retire, go on Medicaid, and seek disability, something she had always sworn to avoid.
Now taxpayers, rather than the insurer, are covering Schulte’s medical bills, Dr. Maury noted.
Precision medicine hasn’t seemed like a priority for large makers of autoimmune drugs, which presumably have some knowledge of which patients are most likely to benefit from their drugs, because they have tested and sold millions of doses over the years. By offering rebate incentives to insurers, companies like AbbVie, which makes Humira, can guarantee theirs are the drugs of choice with insurers.
“If you were AbbVie,” Dr. Curtis said, “why would you ever want to publish data showing who’s not going to do well on your drug, if, in the absence of the test, everyone will start with your drug first?”
What Testing Could Do
Medicare and commercial insurers haven’t yet set a price for PrismRA, but it could save insurers thousands of dollars a year for each patient it helps, according to Krishna Patel, PharmD, Scipher’s associate director of medical affairs.
“If the test cost $750, I still only need it once, and it costs less than a month of whatever drug is not going to work very well for you,” said Dr. Curtis, a coauthor of some studies of the test. “The economics of a biomarker that’s anything but worthless is pretty favorable because our biologics and targeted drugs are so expensive.”
Patients are enthusiastic about the test because so many have had to take TNFis that didn’t work. Many insurers require patients to try a second TNFi and sometimes a third.
Jen Weaver, a patient advocate and mother of three, got little benefit from hydroxychloroquine, sulfasalazine, methotrexate, and Orencia, a non-TNFi biologic therapy, before finding some relief in another, Actemra. But she was taken off that drug when her white blood cells plunged, and the next three drugs she tried — all TNFis — caused allergic reactions, culminating with an outbreak of pus-filled sores. Another drug, Otezla, eventually seemed to help heal the sores, and she’s been stable on it since in combination with methotrexate, Ms. Weaver said.
“What is needed is to substantially shorten this trial-and-error period for patients,” said Shilpa Venkatachalam, PhD, herself a patient and the director of research operations at the Global Healthy Living Foundation. “There’s a lot of anxiety and frustration, weeks in pain wondering whether a drug is going to work for you and what to do if it doesn’t.” A survey by her group found that 91% of patients worried their medications would stop working. And there is evidence that the longer it takes to resolve arthritis symptoms, the less chance they will ever stop.
How insurers will respond to the availability of tests isn’t clear, partly because the arrival of new biosimilar drugs — essentially generic versions — is making TNFis cheaper for insurance plans. While Humira still dominates, AbbVie has increased rebates to insurers, in effect lowering its cost. Lower prices make the PrismRA test less appealing to insurers because widespread use of the test could cut TNFi prescriptions by up to a third.
However, rheumatologist John B. Boone, MD, in Louisville, Kentucky, found to his surprise that insurers mostly accepted alternative prescriptions for 41 patients whom the test showed unlikely to respond to TNFis as part of a clinical trial. Dr. Boone receives consulting fees from Scipher.
Although the test didn’t guarantee good outcomes, he said, the few patients given TNFis despite the test results almost all did poorly on that regimen.
Scientists from AbbVie, which makes several rheumatology drugs in addition to Humira, presented a study at the San Diego conference examining biomarkers that might show which patients would respond to Rinvoq, a new immune-suppressing drug in a class known as the JAK inhibitors. When asked about its use of precision medicine, AbbVie declined to comment.
Over two decades, Humira has been a blockbuster drug for AbbVie. The company sold more than $3.5 billion worth of Humira in the third quarter of 2023, 36% less than a year ago. Sales of Rinvoq, which AbbVie is marketing as a treatment for patients failed by Humira and its class, jumped 60% to $1.1 billion.
What Patients Want
Shannan O’Hara-Levi, a 38-year-old in Monroe, New York, has been on scores of drugs and supplements since being diagnosed with juvenile arthritis at age 3. She’s been nauseated, fatigued, and short of breath and has suffered allergic reactions, but she says the worst part of it was finding a drug that worked and then losing access because of insurance. This happened shortly after she gave birth to a daughter in 2022 and then endured intense joint pain.
“If I could take a blood test that tells me not to waste months or years of my life — absolutely,” she said. “If I could have started my current drug last fall and saved many months of not being able to engage with my baby on the floor — absolutely.”
This article originally appeared on KFFHealthNews.org. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
Erinn Maury, MD, knew Remicade wasn’t the right drug for Patti Schulte, a patient with rheumatoid arthritis the physician saw at her Millersville, Maryland, practice. Schulte’s swollen, painful joints hadn’t responded to Enbrel or Humira, two drugs in the same class.
But the insurer insisted, so Schulte went on Remicade. It didn’t work either.
What’s more, Schulte suffered a severe allergic reaction to the infusion therapy, requiring a heavy dose of prednisone, a steroid with grave side effects if used at high doses for too long.
After 18 months, her insurer finally approved Maury’s drug of choice, Orencia. By then, Schulte’s vertebrae, weakened by prednisone, had started cracking. She was only 60.
It’s also a story of how doctors are steered by pharmacy benefit managers — the middlemen of the drug market — as well as by insurers.
Once people with inflammatory conditions such as rheumatoid arthritis reach a certain stage, the first prescription offered is typically Humira, the best-selling drug in history, and part of a class known as tumor necrosis factor inhibitors, or TNFis, which fail to significantly help about half of the patients who take it.
“We practice rheumatology without any help,” said Vibeke Strand, a rheumatologist and adjunct clinical professor at Stanford. She bemoaned the lack of tools available to choose the right drug while bristling at corporate intervention in the decision. “We are told by the insurer what to prescribe to the patient. After they fail methotrexate, it’s a TNF inhibitor, almost always Humira. And that’s not OK.”
If there’s a shred of hope in this story, it’s that a blood test, PrismRA, may herald an era of improved care for patients with rheumatoid arthritis and other autoimmune conditions. But first, it must be embraced by insurers.
PrismRA employs a predictive model that combines clinical factors, blood tests, and 19 gene patterns to identify the roughly 60% of patients who are very unlikely to respond to a TNFi drug.
Over the past 25 years, drug companies have introduced five new classes of autoimmune drugs. TNFis were the first to market, starting in the late 1990s.
Some 1.3 million Americans have rheumatoid arthritis, a disease in which a person’s immune system attacks their joints, causing crippling pain and, if improperly treated, disfigurement. The newer drugs, mostly so-called biologics, are also used by some of the 25 million or more Americans with other autoimmune diseases, such as lupus, Crohn’s disease, and psoriasis. Typically costing tens of thousands of dollars annually, the drugs are prescribed after a patient fails to respond to older, cheaper drugs like methotrexate.
Until recently, rheumatologists have had few ways to predict which of the new drugs would work best on which patients. Often, “it’s a coin flip whether I prescribe drug A or B,” said Jeffrey Curtis, MD, a rheumatology professor at the University of Alabama-Birmingham.
Yet about 90% of the patients who are given one of these advanced drugs start on a TNFi, although there’s often no reason to think a TNFi will work better than another type.
Under these puzzling circumstances, it’s often the insurer rather than the doctor who chooses the patient’s drug. Insurers lean toward TNFis such as adalimumab, commonly sold as brand name Humira, in part because they get large rebates from manufacturers for using them. Although the size of such payments is a trade secret, AbbVie is said to be offering rebates to insurers of up to 60% of Humira’s price. That has enabled it to control 98.5% of the US adalimumab market, even though it has eight biosimilar competitors.
PrismRA’s developer, Scipher Medicine, has provided more than 26,000 test results, rarely covered by insurance. But on October 15, the Centers for Medicare & Medicaid began reimbursing for the test, and its use is expected to rise. At least two other companies are developing drug-matching tests for patients with rheumatoid arthritis.
Although critics say PrismRA is not always useful, it is likely to be the first in a series of diagnostics anticipated over the next decade that could reduce the time that patients with autoimmune disease suffer on the wrong drug.
Academics, small biotechs, and large pharmaceutical companies are investing in methods to distinguish the biological pathways involved in these diseases and the best way to treat each one. This approach, called precision medicine, has existed for years in cancer medicine, in which it’s routine to test the genetics of patients’ tumors to determine the appropriate drug treatment.
“You wouldn’t give Herceptin to a breast cancer patient without knowing whether her tumor was HER2-positive,” said Costantino Pitzalis, MD, a rheumatology professor at the William Harvey Research Institute in London, England. He was speaking before a well-attended session at an American College of Rheumatology conference in San Diego in November. “Why do we not use biopsies or seek molecular markers in rheumatoid arthritis?”
It’s not only patients and doctors who have a stake in which drugs work best for a given person.
When Remicade failed and Schulte waited for the insurer to approve Orencia, she insisted on keeping her job as an accountant. But as her prednisone-related spinal problems worsened, Schulte was forced to retire, go on Medicaid, and seek disability, something she had always sworn to avoid.
Now taxpayers, rather than the insurer, are covering Schulte’s medical bills, Dr. Maury noted.
Precision medicine hasn’t seemed like a priority for large makers of autoimmune drugs, which presumably have some knowledge of which patients are most likely to benefit from their drugs, because they have tested and sold millions of doses over the years. By offering rebate incentives to insurers, companies like AbbVie, which makes Humira, can guarantee theirs are the drugs of choice with insurers.
“If you were AbbVie,” Dr. Curtis said, “why would you ever want to publish data showing who’s not going to do well on your drug, if, in the absence of the test, everyone will start with your drug first?”
What Testing Could Do
Medicare and commercial insurers haven’t yet set a price for PrismRA, but it could save insurers thousands of dollars a year for each patient it helps, according to Krishna Patel, PharmD, Scipher’s associate director of medical affairs.
“If the test cost $750, I still only need it once, and it costs less than a month of whatever drug is not going to work very well for you,” said Dr. Curtis, a coauthor of some studies of the test. “The economics of a biomarker that’s anything but worthless is pretty favorable because our biologics and targeted drugs are so expensive.”
Patients are enthusiastic about the test because so many have had to take TNFis that didn’t work. Many insurers require patients to try a second TNFi and sometimes a third.
Jen Weaver, a patient advocate and mother of three, got little benefit from hydroxychloroquine, sulfasalazine, methotrexate, and Orencia, a non-TNFi biologic therapy, before finding some relief in another, Actemra. But she was taken off that drug when her white blood cells plunged, and the next three drugs she tried — all TNFis — caused allergic reactions, culminating with an outbreak of pus-filled sores. Another drug, Otezla, eventually seemed to help heal the sores, and she’s been stable on it since in combination with methotrexate, Ms. Weaver said.
“What is needed is to substantially shorten this trial-and-error period for patients,” said Shilpa Venkatachalam, PhD, herself a patient and the director of research operations at the Global Healthy Living Foundation. “There’s a lot of anxiety and frustration, weeks in pain wondering whether a drug is going to work for you and what to do if it doesn’t.” A survey by her group found that 91% of patients worried their medications would stop working. And there is evidence that the longer it takes to resolve arthritis symptoms, the less chance they will ever stop.
How insurers will respond to the availability of tests isn’t clear, partly because the arrival of new biosimilar drugs — essentially generic versions — is making TNFis cheaper for insurance plans. While Humira still dominates, AbbVie has increased rebates to insurers, in effect lowering its cost. Lower prices make the PrismRA test less appealing to insurers because widespread use of the test could cut TNFi prescriptions by up to a third.
However, rheumatologist John B. Boone, MD, in Louisville, Kentucky, found to his surprise that insurers mostly accepted alternative prescriptions for 41 patients whom the test showed unlikely to respond to TNFis as part of a clinical trial. Dr. Boone receives consulting fees from Scipher.
Although the test didn’t guarantee good outcomes, he said, the few patients given TNFis despite the test results almost all did poorly on that regimen.
Scientists from AbbVie, which makes several rheumatology drugs in addition to Humira, presented a study at the San Diego conference examining biomarkers that might show which patients would respond to Rinvoq, a new immune-suppressing drug in a class known as the JAK inhibitors. When asked about its use of precision medicine, AbbVie declined to comment.
Over two decades, Humira has been a blockbuster drug for AbbVie. The company sold more than $3.5 billion worth of Humira in the third quarter of 2023, 36% less than a year ago. Sales of Rinvoq, which AbbVie is marketing as a treatment for patients failed by Humira and its class, jumped 60% to $1.1 billion.
What Patients Want
Shannan O’Hara-Levi, a 38-year-old in Monroe, New York, has been on scores of drugs and supplements since being diagnosed with juvenile arthritis at age 3. She’s been nauseated, fatigued, and short of breath and has suffered allergic reactions, but she says the worst part of it was finding a drug that worked and then losing access because of insurance. This happened shortly after she gave birth to a daughter in 2022 and then endured intense joint pain.
“If I could take a blood test that tells me not to waste months or years of my life — absolutely,” she said. “If I could have started my current drug last fall and saved many months of not being able to engage with my baby on the floor — absolutely.”
This article originally appeared on KFFHealthNews.org. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
Monitoring Tech for Pulmonary Disorders Moving Beyond Wearables
, but, as with any emergent technology, pitfalls come along with the promise and potential.
Meanwhile, technology to remotely monitor respiratory diseases is advancing into other modalities. In recent months, researchers have reported on an artificial intelligence–aided home stethoscope to monitor asthma exacerbations and an ingestible electronic capsule, which has shown some facility for continuous, remote monitoring of sleep apnea and opioid induced respiratory depression.
“Smartphones and wearable technology in health care are here to stay,” Mariam Louis, MD, pulmonologist and sleep medicine physician at the University of Florida Health and chair of the nonrespiratory sleep section of the Sleep Medicine Network with the American College of Chest Physicians, said in an interview.
“It is an exciting field, as it encourages patients to be actively involved in their medical care and can potentially offer more real-time feedback regarding the patient’s medical conditions,” she said. “There are currently many apps that are being used to monitor sleep and other diseases. However, the technology is still rudimentary, and much more research is needed to see if these apps are accurate and dependable.”
Studies in the past few months have reported on the accuracy of 18 wearable sleep-tracker devices, finding they overestimated sleep duration by 19 minutes on average (Sleep. 2023 Nov 8. doi: 10.1093/sleep/zsad288). Researchers in the United States also recently reported on the first human trial of an ingestible pill for monitoring sleep apnea that sends data to a receiving device up to six feet away (Device. 2023 Nov 17. doi: 10.1016/j.device.2023.100125), and a group in Poland reported than an AI-aided home stethoscope provided reliable information on asthma exacerbations in 149 patients (Ann Fam Med. 2023;21:517-25).
Targeting Challenges With Polysomnography
All of these technologies aim to overcome challenges with traditional devices, such as polysomnography (PSG) for evaluating sleep. Jaques Reifman, PhD, a senior research scientist at the U.S. Army Medical Research and Development Command in Fort Detrick, Maryland, led the study of 18 wearable sleep trackers. “Both polysomnography and sleep tracking devices in a sense are attempting to reach the same goal: they’re trying to estimate certain sleep parameters,” Dr. Reifman said in an interview.
“But they use very different signals,” he added, noting that PSG uses electroencephalography (EEG) to measure electrical signals in the skull whereas most sleep trackers used an accelerometer to measure body movement. “As your wrist moves around, it determines if you are moving or not,” Dr. Reifman said.
“Each of them have their plusses and minuses,” he added. PSG, while it’s considered the gold standard for measuring sleep, isn’t a consumer product. “It generally requires a very sophisticated data acquisition system; they are laden with motion artifacts and you have to have software to remove them before you analyze the data,” Dr. Reifman said. “They generally require an expert to interpret the results, although lately there are a few AI-based algorithms that you can provide the EEG signals to and it does score those stages for you”
Sleep trackers, on the other hand, are consumer products. “They can be used outside the lab, and you can use them to record for long periods of time, which is not really possible with PSG,” Dr. Reifman said. “They are low cost, they are easy to use, small size, and folks have developed algorithms that can directly tell the consumer you slept seven hours last night.
“In that sense, they’re comfortable to use as opposed to using an almost-like shower cap with the EEG and face sensors as part of the PSG montage.”
However, what sleep trackers offer in convenience, they lack in accuracy. “There are things they just cannot do based on the limitations of the signals that they use,” Dr. Reifman said.
The study was actually a meta-analysis of 14 different studies that evaluated 18 different sleep-tracking devices in 364 patients. The meta-analysis found wide variability in accuracy between devices; for example, a 75-minute overestimation of sleep with one device and a one-minute overestimation with another.
And different studies reported variations with the same tracker or different models of a tracker. The Fitbit Charge 2, for example, was found to underestimate sleep by 12 minutes in one study and overestimate sleep by 9 minutes in another, while the Fitbit HR Charge was found to overestimate sleep by 52 minutes in a third study.
The meta-analysis found while sleep trackers have high sensitivity (>90%), they had a relatively low specificity (<50%), Dr. Reifman noted.
“Because they are mainly based on the acceleration of your wrist, if you are laying down in bed and motionless after a few minutes the device is going to think you’re asleep when in reality you’re just motionless, daydreaming or trying to go to sleep but not sleeping, so the specificity to sleep is not that high,” he said.
These types of devices still have obstacles to overcome before they’re more widely used, Dr. Louis said. “All of these technologies are proprietary,” she said. “As such, little is known about the algorithms used to come up with the diagnosis or other conclusions. In addition, the majority of the data cannot be analyzed independently by the providers, limiting some of the usage of these devices for now.”
Early Study of Ingestible Capsule
To overcome some of those challenges with collecting data from wearables, researchers from the Massachusetts Institute of Technology and West Virginia University have worked with Celero Systems to develop a pill-sized capsule the patient swallows and which then collects vitals data from inside the gastrointestinal tract. The first in-human study evaluated the device, called the vitals-monitoring (VM) pill, in 10 patients. The study reported the data captured by the pill aligned with that gathered with standard sleep metrics and that it could detect sleep apnea episodes.
The study described the pill as a wireless device that uses a custom configuration of four off-the shelf integrated circuits — a microcontroller, accelerometer, memory component and radio signal — and electronic sensors for ballistic measurements from within the GI tract. The accelerometer measures movement of the abdomen during breathing.
Ingestible devices have actually been around for a couple of decades. The most common, the PillCam, is mostly used by gastroenterologists to capture images of the small intestine.
In the VM pill study, 3 of the 10 human volunteers had a diagnosis of either central or obstructive sleep apnea and wore a continuous positive airway pressure device during the study. The patients also had PSG. The study found that the heart rate accuracy of the VM pill was within 2.5 beats per minute of the PSG measure. The study found no significant difference in the ability of the VM pill to accurately measure respiratory rate with or without CPAP.
Since study completion, the device has been evaluated in another 10 patients, Ben Pless, CEO of Celero Systems, the company developing the VM pill and a coauthor of the study, said in an interview. All patients passed the capsule without any adverse events, he said.
The capsule carries the advantages of an implantable device without the surgery, Mr. Pless said. “In addition to the product being inside body, it is very good at measuring core temperature and, of course, there are diurnal variations in core temperature,” he said. “Even though this was not in the paper, we found the combination of monitoring respiration and core temperature is a very powerful way to do sleep staging in a completely unobtrusive and discrete way.”
The first study evaluated the overnight use of the VM pill, but future studies will evaluate longer duration of the device, first up to a week and then extending out to a month, with the goal of collecting data through the entire duration, Mr. Pless said.
“If you want to do ongoing monitoring for events that may have a low incidence, for example COPD exacerbations or some asthma which does not occur every day and you want to do long-term monitoring, an ingestible format where you ultimately take one capsule and you’re monitored for a month in a completely unobtrusive way would be a great way to do patient monitoring,” he said.
This platform could also collect multinight data for sleep studies, he added.
“While this is an exciting technology, there is much more to diagnosing sleep apnea than just heart rate and breathing,” Dr. Louis said. “During a sleep study, we look at oxygen levels, snoring, and many other variables.”
AI-Aided Stethoscope
The AI-aided stethoscope demonstrated an ability to collect reliable information on asthma exacerbations, the study in Poland found. “The parameters provided are effective for children, especially those younger than 5 years of age,” the study authors wrote.
The study enrolled patients of various ages with asthma, using the AI-aided stethoscope to monitor asthma-related physiologic parameters at home for six months. The stethoscope recorded auscultatory sounds from standard chest point and sent them to a dedicated mobile phone application in which an AI module automatically analyzed the recordings and displayed the results. The researchers trained the AI module using more than 10,000 recordings of respiratory sounds.
The study showed that a host of parameters — wheezes, rhonchi, coarse and fine crackles, heart rate, respiratory rate and inspiration-to-expiration duration ration — measured with the AI-aided stethoscope can detect asthma exacerbations without the need for obtaining peak expiratory flow measurements. It also showed a potential to make asthma diagnosis more straightforward in younger children.
“As we learn more and refine these technologies, we will be able to offer more patient centered and precise medicine to our patients, tailored specifically to their needs,” Dr. Louis said. “AI will certainly play a part in the future.”
Dr. Louis and Dr. Reifman have no relevant relationships to disclose. Mr. Pless is CEO of Celero Systems, a privately held company in Lincoln, Mass.
, but, as with any emergent technology, pitfalls come along with the promise and potential.
Meanwhile, technology to remotely monitor respiratory diseases is advancing into other modalities. In recent months, researchers have reported on an artificial intelligence–aided home stethoscope to monitor asthma exacerbations and an ingestible electronic capsule, which has shown some facility for continuous, remote monitoring of sleep apnea and opioid induced respiratory depression.
“Smartphones and wearable technology in health care are here to stay,” Mariam Louis, MD, pulmonologist and sleep medicine physician at the University of Florida Health and chair of the nonrespiratory sleep section of the Sleep Medicine Network with the American College of Chest Physicians, said in an interview.
“It is an exciting field, as it encourages patients to be actively involved in their medical care and can potentially offer more real-time feedback regarding the patient’s medical conditions,” she said. “There are currently many apps that are being used to monitor sleep and other diseases. However, the technology is still rudimentary, and much more research is needed to see if these apps are accurate and dependable.”
Studies in the past few months have reported on the accuracy of 18 wearable sleep-tracker devices, finding they overestimated sleep duration by 19 minutes on average (Sleep. 2023 Nov 8. doi: 10.1093/sleep/zsad288). Researchers in the United States also recently reported on the first human trial of an ingestible pill for monitoring sleep apnea that sends data to a receiving device up to six feet away (Device. 2023 Nov 17. doi: 10.1016/j.device.2023.100125), and a group in Poland reported than an AI-aided home stethoscope provided reliable information on asthma exacerbations in 149 patients (Ann Fam Med. 2023;21:517-25).
Targeting Challenges With Polysomnography
All of these technologies aim to overcome challenges with traditional devices, such as polysomnography (PSG) for evaluating sleep. Jaques Reifman, PhD, a senior research scientist at the U.S. Army Medical Research and Development Command in Fort Detrick, Maryland, led the study of 18 wearable sleep trackers. “Both polysomnography and sleep tracking devices in a sense are attempting to reach the same goal: they’re trying to estimate certain sleep parameters,” Dr. Reifman said in an interview.
“But they use very different signals,” he added, noting that PSG uses electroencephalography (EEG) to measure electrical signals in the skull whereas most sleep trackers used an accelerometer to measure body movement. “As your wrist moves around, it determines if you are moving or not,” Dr. Reifman said.
“Each of them have their plusses and minuses,” he added. PSG, while it’s considered the gold standard for measuring sleep, isn’t a consumer product. “It generally requires a very sophisticated data acquisition system; they are laden with motion artifacts and you have to have software to remove them before you analyze the data,” Dr. Reifman said. “They generally require an expert to interpret the results, although lately there are a few AI-based algorithms that you can provide the EEG signals to and it does score those stages for you”
Sleep trackers, on the other hand, are consumer products. “They can be used outside the lab, and you can use them to record for long periods of time, which is not really possible with PSG,” Dr. Reifman said. “They are low cost, they are easy to use, small size, and folks have developed algorithms that can directly tell the consumer you slept seven hours last night.
“In that sense, they’re comfortable to use as opposed to using an almost-like shower cap with the EEG and face sensors as part of the PSG montage.”
However, what sleep trackers offer in convenience, they lack in accuracy. “There are things they just cannot do based on the limitations of the signals that they use,” Dr. Reifman said.
The study was actually a meta-analysis of 14 different studies that evaluated 18 different sleep-tracking devices in 364 patients. The meta-analysis found wide variability in accuracy between devices; for example, a 75-minute overestimation of sleep with one device and a one-minute overestimation with another.
And different studies reported variations with the same tracker or different models of a tracker. The Fitbit Charge 2, for example, was found to underestimate sleep by 12 minutes in one study and overestimate sleep by 9 minutes in another, while the Fitbit HR Charge was found to overestimate sleep by 52 minutes in a third study.
The meta-analysis found while sleep trackers have high sensitivity (>90%), they had a relatively low specificity (<50%), Dr. Reifman noted.
“Because they are mainly based on the acceleration of your wrist, if you are laying down in bed and motionless after a few minutes the device is going to think you’re asleep when in reality you’re just motionless, daydreaming or trying to go to sleep but not sleeping, so the specificity to sleep is not that high,” he said.
These types of devices still have obstacles to overcome before they’re more widely used, Dr. Louis said. “All of these technologies are proprietary,” she said. “As such, little is known about the algorithms used to come up with the diagnosis or other conclusions. In addition, the majority of the data cannot be analyzed independently by the providers, limiting some of the usage of these devices for now.”
Early Study of Ingestible Capsule
To overcome some of those challenges with collecting data from wearables, researchers from the Massachusetts Institute of Technology and West Virginia University have worked with Celero Systems to develop a pill-sized capsule the patient swallows and which then collects vitals data from inside the gastrointestinal tract. The first in-human study evaluated the device, called the vitals-monitoring (VM) pill, in 10 patients. The study reported the data captured by the pill aligned with that gathered with standard sleep metrics and that it could detect sleep apnea episodes.
The study described the pill as a wireless device that uses a custom configuration of four off-the shelf integrated circuits — a microcontroller, accelerometer, memory component and radio signal — and electronic sensors for ballistic measurements from within the GI tract. The accelerometer measures movement of the abdomen during breathing.
Ingestible devices have actually been around for a couple of decades. The most common, the PillCam, is mostly used by gastroenterologists to capture images of the small intestine.
In the VM pill study, 3 of the 10 human volunteers had a diagnosis of either central or obstructive sleep apnea and wore a continuous positive airway pressure device during the study. The patients also had PSG. The study found that the heart rate accuracy of the VM pill was within 2.5 beats per minute of the PSG measure. The study found no significant difference in the ability of the VM pill to accurately measure respiratory rate with or without CPAP.
Since study completion, the device has been evaluated in another 10 patients, Ben Pless, CEO of Celero Systems, the company developing the VM pill and a coauthor of the study, said in an interview. All patients passed the capsule without any adverse events, he said.
The capsule carries the advantages of an implantable device without the surgery, Mr. Pless said. “In addition to the product being inside body, it is very good at measuring core temperature and, of course, there are diurnal variations in core temperature,” he said. “Even though this was not in the paper, we found the combination of monitoring respiration and core temperature is a very powerful way to do sleep staging in a completely unobtrusive and discrete way.”
The first study evaluated the overnight use of the VM pill, but future studies will evaluate longer duration of the device, first up to a week and then extending out to a month, with the goal of collecting data through the entire duration, Mr. Pless said.
“If you want to do ongoing monitoring for events that may have a low incidence, for example COPD exacerbations or some asthma which does not occur every day and you want to do long-term monitoring, an ingestible format where you ultimately take one capsule and you’re monitored for a month in a completely unobtrusive way would be a great way to do patient monitoring,” he said.
This platform could also collect multinight data for sleep studies, he added.
“While this is an exciting technology, there is much more to diagnosing sleep apnea than just heart rate and breathing,” Dr. Louis said. “During a sleep study, we look at oxygen levels, snoring, and many other variables.”
AI-Aided Stethoscope
The AI-aided stethoscope demonstrated an ability to collect reliable information on asthma exacerbations, the study in Poland found. “The parameters provided are effective for children, especially those younger than 5 years of age,” the study authors wrote.
The study enrolled patients of various ages with asthma, using the AI-aided stethoscope to monitor asthma-related physiologic parameters at home for six months. The stethoscope recorded auscultatory sounds from standard chest point and sent them to a dedicated mobile phone application in which an AI module automatically analyzed the recordings and displayed the results. The researchers trained the AI module using more than 10,000 recordings of respiratory sounds.
The study showed that a host of parameters — wheezes, rhonchi, coarse and fine crackles, heart rate, respiratory rate and inspiration-to-expiration duration ration — measured with the AI-aided stethoscope can detect asthma exacerbations without the need for obtaining peak expiratory flow measurements. It also showed a potential to make asthma diagnosis more straightforward in younger children.
“As we learn more and refine these technologies, we will be able to offer more patient centered and precise medicine to our patients, tailored specifically to their needs,” Dr. Louis said. “AI will certainly play a part in the future.”
Dr. Louis and Dr. Reifman have no relevant relationships to disclose. Mr. Pless is CEO of Celero Systems, a privately held company in Lincoln, Mass.
, but, as with any emergent technology, pitfalls come along with the promise and potential.
Meanwhile, technology to remotely monitor respiratory diseases is advancing into other modalities. In recent months, researchers have reported on an artificial intelligence–aided home stethoscope to monitor asthma exacerbations and an ingestible electronic capsule, which has shown some facility for continuous, remote monitoring of sleep apnea and opioid induced respiratory depression.
“Smartphones and wearable technology in health care are here to stay,” Mariam Louis, MD, pulmonologist and sleep medicine physician at the University of Florida Health and chair of the nonrespiratory sleep section of the Sleep Medicine Network with the American College of Chest Physicians, said in an interview.
“It is an exciting field, as it encourages patients to be actively involved in their medical care and can potentially offer more real-time feedback regarding the patient’s medical conditions,” she said. “There are currently many apps that are being used to monitor sleep and other diseases. However, the technology is still rudimentary, and much more research is needed to see if these apps are accurate and dependable.”
Studies in the past few months have reported on the accuracy of 18 wearable sleep-tracker devices, finding they overestimated sleep duration by 19 minutes on average (Sleep. 2023 Nov 8. doi: 10.1093/sleep/zsad288). Researchers in the United States also recently reported on the first human trial of an ingestible pill for monitoring sleep apnea that sends data to a receiving device up to six feet away (Device. 2023 Nov 17. doi: 10.1016/j.device.2023.100125), and a group in Poland reported than an AI-aided home stethoscope provided reliable information on asthma exacerbations in 149 patients (Ann Fam Med. 2023;21:517-25).
Targeting Challenges With Polysomnography
All of these technologies aim to overcome challenges with traditional devices, such as polysomnography (PSG) for evaluating sleep. Jaques Reifman, PhD, a senior research scientist at the U.S. Army Medical Research and Development Command in Fort Detrick, Maryland, led the study of 18 wearable sleep trackers. “Both polysomnography and sleep tracking devices in a sense are attempting to reach the same goal: they’re trying to estimate certain sleep parameters,” Dr. Reifman said in an interview.
“But they use very different signals,” he added, noting that PSG uses electroencephalography (EEG) to measure electrical signals in the skull whereas most sleep trackers used an accelerometer to measure body movement. “As your wrist moves around, it determines if you are moving or not,” Dr. Reifman said.
“Each of them have their plusses and minuses,” he added. PSG, while it’s considered the gold standard for measuring sleep, isn’t a consumer product. “It generally requires a very sophisticated data acquisition system; they are laden with motion artifacts and you have to have software to remove them before you analyze the data,” Dr. Reifman said. “They generally require an expert to interpret the results, although lately there are a few AI-based algorithms that you can provide the EEG signals to and it does score those stages for you”
Sleep trackers, on the other hand, are consumer products. “They can be used outside the lab, and you can use them to record for long periods of time, which is not really possible with PSG,” Dr. Reifman said. “They are low cost, they are easy to use, small size, and folks have developed algorithms that can directly tell the consumer you slept seven hours last night.
“In that sense, they’re comfortable to use as opposed to using an almost-like shower cap with the EEG and face sensors as part of the PSG montage.”
However, what sleep trackers offer in convenience, they lack in accuracy. “There are things they just cannot do based on the limitations of the signals that they use,” Dr. Reifman said.
The study was actually a meta-analysis of 14 different studies that evaluated 18 different sleep-tracking devices in 364 patients. The meta-analysis found wide variability in accuracy between devices; for example, a 75-minute overestimation of sleep with one device and a one-minute overestimation with another.
And different studies reported variations with the same tracker or different models of a tracker. The Fitbit Charge 2, for example, was found to underestimate sleep by 12 minutes in one study and overestimate sleep by 9 minutes in another, while the Fitbit HR Charge was found to overestimate sleep by 52 minutes in a third study.
The meta-analysis found while sleep trackers have high sensitivity (>90%), they had a relatively low specificity (<50%), Dr. Reifman noted.
“Because they are mainly based on the acceleration of your wrist, if you are laying down in bed and motionless after a few minutes the device is going to think you’re asleep when in reality you’re just motionless, daydreaming or trying to go to sleep but not sleeping, so the specificity to sleep is not that high,” he said.
These types of devices still have obstacles to overcome before they’re more widely used, Dr. Louis said. “All of these technologies are proprietary,” she said. “As such, little is known about the algorithms used to come up with the diagnosis or other conclusions. In addition, the majority of the data cannot be analyzed independently by the providers, limiting some of the usage of these devices for now.”
Early Study of Ingestible Capsule
To overcome some of those challenges with collecting data from wearables, researchers from the Massachusetts Institute of Technology and West Virginia University have worked with Celero Systems to develop a pill-sized capsule the patient swallows and which then collects vitals data from inside the gastrointestinal tract. The first in-human study evaluated the device, called the vitals-monitoring (VM) pill, in 10 patients. The study reported the data captured by the pill aligned with that gathered with standard sleep metrics and that it could detect sleep apnea episodes.
The study described the pill as a wireless device that uses a custom configuration of four off-the shelf integrated circuits — a microcontroller, accelerometer, memory component and radio signal — and electronic sensors for ballistic measurements from within the GI tract. The accelerometer measures movement of the abdomen during breathing.
Ingestible devices have actually been around for a couple of decades. The most common, the PillCam, is mostly used by gastroenterologists to capture images of the small intestine.
In the VM pill study, 3 of the 10 human volunteers had a diagnosis of either central or obstructive sleep apnea and wore a continuous positive airway pressure device during the study. The patients also had PSG. The study found that the heart rate accuracy of the VM pill was within 2.5 beats per minute of the PSG measure. The study found no significant difference in the ability of the VM pill to accurately measure respiratory rate with or without CPAP.
Since study completion, the device has been evaluated in another 10 patients, Ben Pless, CEO of Celero Systems, the company developing the VM pill and a coauthor of the study, said in an interview. All patients passed the capsule without any adverse events, he said.
The capsule carries the advantages of an implantable device without the surgery, Mr. Pless said. “In addition to the product being inside body, it is very good at measuring core temperature and, of course, there are diurnal variations in core temperature,” he said. “Even though this was not in the paper, we found the combination of monitoring respiration and core temperature is a very powerful way to do sleep staging in a completely unobtrusive and discrete way.”
The first study evaluated the overnight use of the VM pill, but future studies will evaluate longer duration of the device, first up to a week and then extending out to a month, with the goal of collecting data through the entire duration, Mr. Pless said.
“If you want to do ongoing monitoring for events that may have a low incidence, for example COPD exacerbations or some asthma which does not occur every day and you want to do long-term monitoring, an ingestible format where you ultimately take one capsule and you’re monitored for a month in a completely unobtrusive way would be a great way to do patient monitoring,” he said.
This platform could also collect multinight data for sleep studies, he added.
“While this is an exciting technology, there is much more to diagnosing sleep apnea than just heart rate and breathing,” Dr. Louis said. “During a sleep study, we look at oxygen levels, snoring, and many other variables.”
AI-Aided Stethoscope
The AI-aided stethoscope demonstrated an ability to collect reliable information on asthma exacerbations, the study in Poland found. “The parameters provided are effective for children, especially those younger than 5 years of age,” the study authors wrote.
The study enrolled patients of various ages with asthma, using the AI-aided stethoscope to monitor asthma-related physiologic parameters at home for six months. The stethoscope recorded auscultatory sounds from standard chest point and sent them to a dedicated mobile phone application in which an AI module automatically analyzed the recordings and displayed the results. The researchers trained the AI module using more than 10,000 recordings of respiratory sounds.
The study showed that a host of parameters — wheezes, rhonchi, coarse and fine crackles, heart rate, respiratory rate and inspiration-to-expiration duration ration — measured with the AI-aided stethoscope can detect asthma exacerbations without the need for obtaining peak expiratory flow measurements. It also showed a potential to make asthma diagnosis more straightforward in younger children.
“As we learn more and refine these technologies, we will be able to offer more patient centered and precise medicine to our patients, tailored specifically to their needs,” Dr. Louis said. “AI will certainly play a part in the future.”
Dr. Louis and Dr. Reifman have no relevant relationships to disclose. Mr. Pless is CEO of Celero Systems, a privately held company in Lincoln, Mass.
Where Is the ‘Microbiome Revolution’ Headed Next?
Human microbiome research has progressed in leaps and bounds over the past decades, from pivotal studies begun in the 1970s to the launch of the Human Microbiome Project in 2007. Breakthroughs have laid the groundwork for more recent clinical applications, such as fecal microbiota transplantation (FMT), and advanced techniques to explore new therapeutic pathways. Yet the “microbiome revolution” is just getting started, according to professor Martin J. Blaser, MD, one of the field’s pioneers.
, says Dr. Blaser, who holds the Henry Rutgers Chair of the Human Microbiome and is director of the Center for Advanced Biotechnology and Medicine at Rutgers University in New Brunswick, New Jersey.
Dr. Blaser is the author of Missing Microbes: How the Overuse of Antibiotics Is Fueling Our Modern Plagues, serves as chair of the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria and is a member of the scientific advisory board of the biotech startup Micronoma.
In this interview, which has been condensed and edited for clarity, Dr. Blaser discusses where we’re at now and where he sees the microbiome field evolving in the coming years.
Highlighting the Most Promising Applications
Which recent studies on the link between the human microbiome and disease have you found particularly promising?
There have been a number of studies, including our own, focusing on the gut-kidney axis. The gut microbiome produces, or detoxifies, metabolites that are toxic to the kidney: for example, those involved in the formation of kidney stones and in the worsening of uremia.
Altering the microbiome to reduce the uremic toxins and the nidus for stone formation is a very promising field of research.
What other disease states may be amenable to microbiome-based interventions?
There are diseases that are caused by known genetic mutations. Yet, for nearly all of them, there is great variation in clinical outcomes, which might be classed as genes multiplied by environment interactions.
It seems likely to me that microbiome variation could account for some proportion of those differences for some genetic diseases.
It’s now well established that altering the microbiome with FMT is a successful intervention for recurrent Clostridioides difficile infections. What do you see as the next disease states where FMT could prove successful?
If you go to ClinicalTrials.gov, you will find that that there are 471 trials registered using FMT. This is across a broad range of illnesses, including metabolic, immunological, autoimmune, inflammatory, degenerative, and neoplastic diseases.
Which will be the next condition showing marked efficacy is anyone’s guess. That is why we must do clinical trials to assess what works and what does not, regardless of specific illness.
The donor’s microbiome appears to be vital to engraftment success, with “superdonors” even being identified. What factors do you think primarily influence microbiome engraftment?
There is an emerging science about this question, driven in part by classical ecological theory.
Right now, we are using FMT as if one size fits all. But this probably would not provide optimal treatment for all. Just as we type blood donors and recipients before the blood transfusion, one could easily imagine a parallel kind of procedure.
Are there any diseases where it’s just too far-fetched to think altering the microbiome could make a difference?
The link between the microbiome and human health is so pervasive that there are few conditions that are out of the realm of possibility. It really is a frontier.
Not that the microbiome causes everything, but by understanding and manipulating the microbiome, we could at least palliate, or slow down, particular pathologic processes.
For all the major causes of death in the United States — cardiovascular disease, cancer, dementia and neurogenerative diseases, diabetes, and lung, liver, and kidney diseases — there is ongoing investigation of the microbiome. A greater promise would be to prevent or cure these illnesses.
Predicting the Next Stages of the ‘Microbiome Revolution’
Do you believe we are at a turning point with the microbiome in terms of being able to manipulate or engineer it?
The microbiome is a scientific frontier that has an impact across the biosphere. It is a broad frontier involving human and veterinary medicine, agriculture, and the environment. Knowledge is increasing incrementally, as expected.
Are we at the point yet where doctors should be incorporating microbiome-related lifestyle changes for people with or at risk for cancer, heart disease, Alzheimer’s disease, or other chronic conditions?
Although we are still in the early stages of the “microbiome revolution,” which I first wrote about in EMBO Reports in 2006 and then again in the Journal of Clinical Investigation in 2014, I think important advances for all of these conditions are coming our way in the next 5-10 years.
How are prebiotics, probiotics, and postbiotics being used to shape the microbiome?
This is a very important and active area in clinical investigation, which needs to be ramped up.
Tens of millions of people are using probiotics and prebiotics every day for vague indications, and which have only infrequently been tested in robust clinical trials. So, there is a disconnect between what’s being claimed with the bulk of the probiotics at present and what we’ll actually know in the future.
How do you think the microbiome will stack up to other factors influencing health, such as genetics, exercise, and nutrition?
All are important, but unlike genetics, the microbiome is tractable, like diet and exercise.
It is essentially impossible to change one’s genome, but that might become more likely before too long. However, we can easily change someone’s microbiome through dietary means, for example. Once we know the ground rules, there will be many options. Right now, it is mostly one-offs, but as the scientific basis broadens, much more will be possible.
In the future, do you think we’ll be able to look at a person’s microbiome and tell what his or her risk of developing disease is, similar to the way we use gene panels now?
Yes, but we will need scientific advances to teach us what are the important biomarkers in general and in particular people. This will be one area of precision medicine.
Lessons From Decades at the Forefront
You’ve been involved in this research for over 30 years, and the majority has focused on the human microbiome and its role in disease. When did it become apparent to you that this research had unique therapeutic promise?
From the very start, there was always the potential to harness the microbiome to improve human health. In fact, I wrote a perspective in PNAS on that theme in 2010.
The key is to understand the biology of the microbiome, and from the scientific study comes new preventives and new treatments. Right now, there are many “probiotic” products on the market. Probiotics have a great future, but most of what is out there has not been rigorously tested for effectiveness.
Was there a particular series of studies that occurred before the launch of the Human Microbiome Project and brought us to the current era?
The studies in the 1970s-1980s by Carl Woese using 16S rRNA genes to understand phylogeny and evolution opened up the field of DNA sequencing to consider bacterial evolution and issues of ancestry.
A key subject of your research and the focus of your book is antibiotic-resistant bacteria. What did this work teach you about describing the science of antibiotic resistance to the general public?
People don’t care very much about antibiotic resistance. They think that affects other people, mostly. In contrast, they care about their own health and their children’s health.
The more that the data show that using antibiotics can be harmful to health in some circumstances, the more that use will diminish. We need more transparency about benefits and costs.
Are there any common misconceptions about the microbiome that you hear from the general public, or even clinicians, that you would like to see greater efforts to dispel?
The public and the medical profession are in love with probiotics, buying them by the tens of millions. But as stated before, they are very diverse and mostly untested for efficacy.
The next step is to test specific formulations to see which ones work, and for whom, and which ones don’t. That would be a big advance.
A version of this article appeared on Medscape.com.
Human microbiome research has progressed in leaps and bounds over the past decades, from pivotal studies begun in the 1970s to the launch of the Human Microbiome Project in 2007. Breakthroughs have laid the groundwork for more recent clinical applications, such as fecal microbiota transplantation (FMT), and advanced techniques to explore new therapeutic pathways. Yet the “microbiome revolution” is just getting started, according to professor Martin J. Blaser, MD, one of the field’s pioneers.
, says Dr. Blaser, who holds the Henry Rutgers Chair of the Human Microbiome and is director of the Center for Advanced Biotechnology and Medicine at Rutgers University in New Brunswick, New Jersey.
Dr. Blaser is the author of Missing Microbes: How the Overuse of Antibiotics Is Fueling Our Modern Plagues, serves as chair of the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria and is a member of the scientific advisory board of the biotech startup Micronoma.
In this interview, which has been condensed and edited for clarity, Dr. Blaser discusses where we’re at now and where he sees the microbiome field evolving in the coming years.
Highlighting the Most Promising Applications
Which recent studies on the link between the human microbiome and disease have you found particularly promising?
There have been a number of studies, including our own, focusing on the gut-kidney axis. The gut microbiome produces, or detoxifies, metabolites that are toxic to the kidney: for example, those involved in the formation of kidney stones and in the worsening of uremia.
Altering the microbiome to reduce the uremic toxins and the nidus for stone formation is a very promising field of research.
What other disease states may be amenable to microbiome-based interventions?
There are diseases that are caused by known genetic mutations. Yet, for nearly all of them, there is great variation in clinical outcomes, which might be classed as genes multiplied by environment interactions.
It seems likely to me that microbiome variation could account for some proportion of those differences for some genetic diseases.
It’s now well established that altering the microbiome with FMT is a successful intervention for recurrent Clostridioides difficile infections. What do you see as the next disease states where FMT could prove successful?
If you go to ClinicalTrials.gov, you will find that that there are 471 trials registered using FMT. This is across a broad range of illnesses, including metabolic, immunological, autoimmune, inflammatory, degenerative, and neoplastic diseases.
Which will be the next condition showing marked efficacy is anyone’s guess. That is why we must do clinical trials to assess what works and what does not, regardless of specific illness.
The donor’s microbiome appears to be vital to engraftment success, with “superdonors” even being identified. What factors do you think primarily influence microbiome engraftment?
There is an emerging science about this question, driven in part by classical ecological theory.
Right now, we are using FMT as if one size fits all. But this probably would not provide optimal treatment for all. Just as we type blood donors and recipients before the blood transfusion, one could easily imagine a parallel kind of procedure.
Are there any diseases where it’s just too far-fetched to think altering the microbiome could make a difference?
The link between the microbiome and human health is so pervasive that there are few conditions that are out of the realm of possibility. It really is a frontier.
Not that the microbiome causes everything, but by understanding and manipulating the microbiome, we could at least palliate, or slow down, particular pathologic processes.
For all the major causes of death in the United States — cardiovascular disease, cancer, dementia and neurogenerative diseases, diabetes, and lung, liver, and kidney diseases — there is ongoing investigation of the microbiome. A greater promise would be to prevent or cure these illnesses.
Predicting the Next Stages of the ‘Microbiome Revolution’
Do you believe we are at a turning point with the microbiome in terms of being able to manipulate or engineer it?
The microbiome is a scientific frontier that has an impact across the biosphere. It is a broad frontier involving human and veterinary medicine, agriculture, and the environment. Knowledge is increasing incrementally, as expected.
Are we at the point yet where doctors should be incorporating microbiome-related lifestyle changes for people with or at risk for cancer, heart disease, Alzheimer’s disease, or other chronic conditions?
Although we are still in the early stages of the “microbiome revolution,” which I first wrote about in EMBO Reports in 2006 and then again in the Journal of Clinical Investigation in 2014, I think important advances for all of these conditions are coming our way in the next 5-10 years.
How are prebiotics, probiotics, and postbiotics being used to shape the microbiome?
This is a very important and active area in clinical investigation, which needs to be ramped up.
Tens of millions of people are using probiotics and prebiotics every day for vague indications, and which have only infrequently been tested in robust clinical trials. So, there is a disconnect between what’s being claimed with the bulk of the probiotics at present and what we’ll actually know in the future.
How do you think the microbiome will stack up to other factors influencing health, such as genetics, exercise, and nutrition?
All are important, but unlike genetics, the microbiome is tractable, like diet and exercise.
It is essentially impossible to change one’s genome, but that might become more likely before too long. However, we can easily change someone’s microbiome through dietary means, for example. Once we know the ground rules, there will be many options. Right now, it is mostly one-offs, but as the scientific basis broadens, much more will be possible.
In the future, do you think we’ll be able to look at a person’s microbiome and tell what his or her risk of developing disease is, similar to the way we use gene panels now?
Yes, but we will need scientific advances to teach us what are the important biomarkers in general and in particular people. This will be one area of precision medicine.
Lessons From Decades at the Forefront
You’ve been involved in this research for over 30 years, and the majority has focused on the human microbiome and its role in disease. When did it become apparent to you that this research had unique therapeutic promise?
From the very start, there was always the potential to harness the microbiome to improve human health. In fact, I wrote a perspective in PNAS on that theme in 2010.
The key is to understand the biology of the microbiome, and from the scientific study comes new preventives and new treatments. Right now, there are many “probiotic” products on the market. Probiotics have a great future, but most of what is out there has not been rigorously tested for effectiveness.
Was there a particular series of studies that occurred before the launch of the Human Microbiome Project and brought us to the current era?
The studies in the 1970s-1980s by Carl Woese using 16S rRNA genes to understand phylogeny and evolution opened up the field of DNA sequencing to consider bacterial evolution and issues of ancestry.
A key subject of your research and the focus of your book is antibiotic-resistant bacteria. What did this work teach you about describing the science of antibiotic resistance to the general public?
People don’t care very much about antibiotic resistance. They think that affects other people, mostly. In contrast, they care about their own health and their children’s health.
The more that the data show that using antibiotics can be harmful to health in some circumstances, the more that use will diminish. We need more transparency about benefits and costs.
Are there any common misconceptions about the microbiome that you hear from the general public, or even clinicians, that you would like to see greater efforts to dispel?
The public and the medical profession are in love with probiotics, buying them by the tens of millions. But as stated before, they are very diverse and mostly untested for efficacy.
The next step is to test specific formulations to see which ones work, and for whom, and which ones don’t. That would be a big advance.
A version of this article appeared on Medscape.com.
Human microbiome research has progressed in leaps and bounds over the past decades, from pivotal studies begun in the 1970s to the launch of the Human Microbiome Project in 2007. Breakthroughs have laid the groundwork for more recent clinical applications, such as fecal microbiota transplantation (FMT), and advanced techniques to explore new therapeutic pathways. Yet the “microbiome revolution” is just getting started, according to professor Martin J. Blaser, MD, one of the field’s pioneers.
, says Dr. Blaser, who holds the Henry Rutgers Chair of the Human Microbiome and is director of the Center for Advanced Biotechnology and Medicine at Rutgers University in New Brunswick, New Jersey.
Dr. Blaser is the author of Missing Microbes: How the Overuse of Antibiotics Is Fueling Our Modern Plagues, serves as chair of the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria and is a member of the scientific advisory board of the biotech startup Micronoma.
In this interview, which has been condensed and edited for clarity, Dr. Blaser discusses where we’re at now and where he sees the microbiome field evolving in the coming years.
Highlighting the Most Promising Applications
Which recent studies on the link between the human microbiome and disease have you found particularly promising?
There have been a number of studies, including our own, focusing on the gut-kidney axis. The gut microbiome produces, or detoxifies, metabolites that are toxic to the kidney: for example, those involved in the formation of kidney stones and in the worsening of uremia.
Altering the microbiome to reduce the uremic toxins and the nidus for stone formation is a very promising field of research.
What other disease states may be amenable to microbiome-based interventions?
There are diseases that are caused by known genetic mutations. Yet, for nearly all of them, there is great variation in clinical outcomes, which might be classed as genes multiplied by environment interactions.
It seems likely to me that microbiome variation could account for some proportion of those differences for some genetic diseases.
It’s now well established that altering the microbiome with FMT is a successful intervention for recurrent Clostridioides difficile infections. What do you see as the next disease states where FMT could prove successful?
If you go to ClinicalTrials.gov, you will find that that there are 471 trials registered using FMT. This is across a broad range of illnesses, including metabolic, immunological, autoimmune, inflammatory, degenerative, and neoplastic diseases.
Which will be the next condition showing marked efficacy is anyone’s guess. That is why we must do clinical trials to assess what works and what does not, regardless of specific illness.
The donor’s microbiome appears to be vital to engraftment success, with “superdonors” even being identified. What factors do you think primarily influence microbiome engraftment?
There is an emerging science about this question, driven in part by classical ecological theory.
Right now, we are using FMT as if one size fits all. But this probably would not provide optimal treatment for all. Just as we type blood donors and recipients before the blood transfusion, one could easily imagine a parallel kind of procedure.
Are there any diseases where it’s just too far-fetched to think altering the microbiome could make a difference?
The link between the microbiome and human health is so pervasive that there are few conditions that are out of the realm of possibility. It really is a frontier.
Not that the microbiome causes everything, but by understanding and manipulating the microbiome, we could at least palliate, or slow down, particular pathologic processes.
For all the major causes of death in the United States — cardiovascular disease, cancer, dementia and neurogenerative diseases, diabetes, and lung, liver, and kidney diseases — there is ongoing investigation of the microbiome. A greater promise would be to prevent or cure these illnesses.
Predicting the Next Stages of the ‘Microbiome Revolution’
Do you believe we are at a turning point with the microbiome in terms of being able to manipulate or engineer it?
The microbiome is a scientific frontier that has an impact across the biosphere. It is a broad frontier involving human and veterinary medicine, agriculture, and the environment. Knowledge is increasing incrementally, as expected.
Are we at the point yet where doctors should be incorporating microbiome-related lifestyle changes for people with or at risk for cancer, heart disease, Alzheimer’s disease, or other chronic conditions?
Although we are still in the early stages of the “microbiome revolution,” which I first wrote about in EMBO Reports in 2006 and then again in the Journal of Clinical Investigation in 2014, I think important advances for all of these conditions are coming our way in the next 5-10 years.
How are prebiotics, probiotics, and postbiotics being used to shape the microbiome?
This is a very important and active area in clinical investigation, which needs to be ramped up.
Tens of millions of people are using probiotics and prebiotics every day for vague indications, and which have only infrequently been tested in robust clinical trials. So, there is a disconnect between what’s being claimed with the bulk of the probiotics at present and what we’ll actually know in the future.
How do you think the microbiome will stack up to other factors influencing health, such as genetics, exercise, and nutrition?
All are important, but unlike genetics, the microbiome is tractable, like diet and exercise.
It is essentially impossible to change one’s genome, but that might become more likely before too long. However, we can easily change someone’s microbiome through dietary means, for example. Once we know the ground rules, there will be many options. Right now, it is mostly one-offs, but as the scientific basis broadens, much more will be possible.
In the future, do you think we’ll be able to look at a person’s microbiome and tell what his or her risk of developing disease is, similar to the way we use gene panels now?
Yes, but we will need scientific advances to teach us what are the important biomarkers in general and in particular people. This will be one area of precision medicine.
Lessons From Decades at the Forefront
You’ve been involved in this research for over 30 years, and the majority has focused on the human microbiome and its role in disease. When did it become apparent to you that this research had unique therapeutic promise?
From the very start, there was always the potential to harness the microbiome to improve human health. In fact, I wrote a perspective in PNAS on that theme in 2010.
The key is to understand the biology of the microbiome, and from the scientific study comes new preventives and new treatments. Right now, there are many “probiotic” products on the market. Probiotics have a great future, but most of what is out there has not been rigorously tested for effectiveness.
Was there a particular series of studies that occurred before the launch of the Human Microbiome Project and brought us to the current era?
The studies in the 1970s-1980s by Carl Woese using 16S rRNA genes to understand phylogeny and evolution opened up the field of DNA sequencing to consider bacterial evolution and issues of ancestry.
A key subject of your research and the focus of your book is antibiotic-resistant bacteria. What did this work teach you about describing the science of antibiotic resistance to the general public?
People don’t care very much about antibiotic resistance. They think that affects other people, mostly. In contrast, they care about their own health and their children’s health.
The more that the data show that using antibiotics can be harmful to health in some circumstances, the more that use will diminish. We need more transparency about benefits and costs.
Are there any common misconceptions about the microbiome that you hear from the general public, or even clinicians, that you would like to see greater efforts to dispel?
The public and the medical profession are in love with probiotics, buying them by the tens of millions. But as stated before, they are very diverse and mostly untested for efficacy.
The next step is to test specific formulations to see which ones work, and for whom, and which ones don’t. That would be a big advance.
A version of this article appeared on Medscape.com.
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.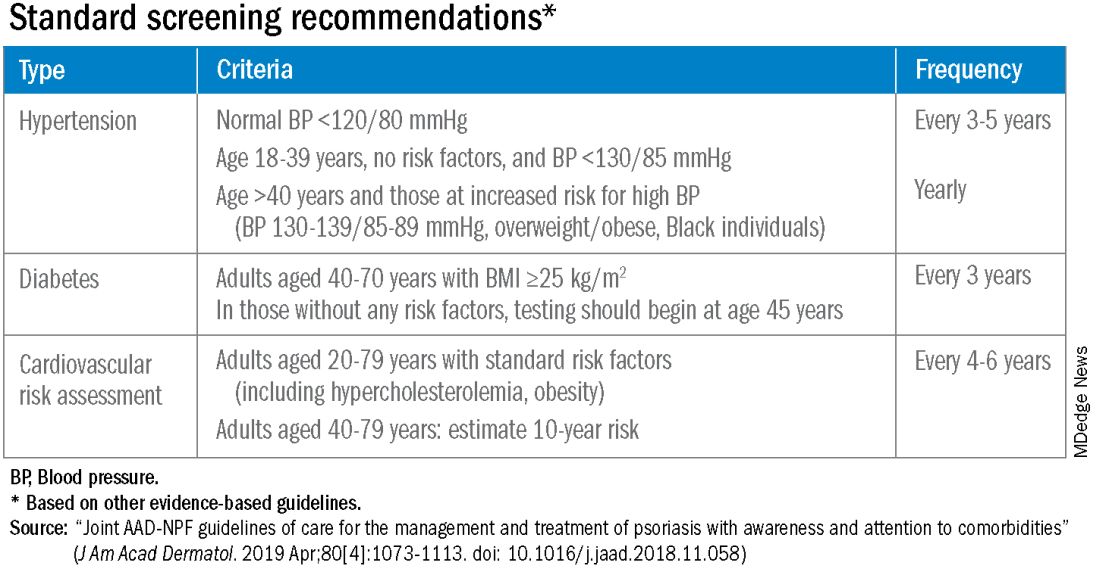
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.








