User login
Your patient’s brain is different at every visit
Unlike other organs in the human body, the brain is constantly changing. The main driver for this ongoing re-engineering across various neural circuits is “experiential neuroplasticity,” which creates billions of new synapses and dendrite spines as well as new connections. And as the brain reinvents itself from day to day, the mind evolves as well.
The neurobiologic re-sculpting of the brain’s complex innards continuously encodes memories of what we learn and experience during waking hours, including all that we see, hear, feel, think, contemplate, plan, and decide. However, in addition to the ongoing intrinsic neuroplasticity that records life’s experiences within neural circuits, there are many extrinsic factors that can further modify the brain and the “psyche” it generates via electrical, neurochemical, and physiological mechanisms. That’s why every patient a psychiatrist sees at follow-up visits will have a brain that will be different from the previous encounter.
Consider the following factors that can modify a patient’s brain (for better or worse) between sessions:
- Psychotherapy that the patient received at the last session will biologically modify his or her brain. Creating new insights and understanding of one’s behavior and “connecting the dots” of the past and present emotions and reactions are all associated with neuroplastic changes within the brain.
- Mood or psychotic episodes. Depressive, manic, or psychotic episodes are associated with neuroinflammation, oxidative stress, and apoptotic effects, which can disrupt the brain’s cytoarchitecture. That’s why psychiatrists must inquire about such episodes between visits and document the possible effects on the patient’s mental status.
- Psychotropic medications all bind to one or more brain receptors to exert therapeutic or adverse effects, both of which are associated with changes in neurotransmitter pathways. A key component of every follow-up visit is to gauge the risks and benefits of the pharmacotherapy prescribed at the prior visit.
- Nonpsychiatric prescription medications are often associated with iatrogenic effects on the brain apart from their intended target organs. These iatrogenic effects include anxiety, depression, mania, psychosis, and cognitive changes. That’s why during each visit, the physician or nurse practitioner must review all prescription medications and consider their potential effects on the patient’s mental status.
- Over-the-counter drugs and supplements may exert neurologic effects via histaminergic, muscarinic, glutamatergic, adrenergic, or serotonergic effects—all of which can alter brain chemistry and contribute to mental status changes. They can also inhibit or induce cytochrome enzymes and induce adverse effects or loss of efficacy of the primary psychotropic medication the patient takes.
- Medical illness, even as simple as an upper respiratory viral infection, can alter brain function due to illness-induced physiological aberrations, including pain and peripheral inflammation, with neurologic consequences. Common metabolic disorders such as diabetes, hyperlipidemia, and hypertension can exert mental status changes.
- Alcohol and drugs of abuse alter brain structure and function and can induce psychological and cognitive changes. Inquiring about the amount and frequency of alcohol and recreational drug use must be done in detail at every visit.
- Stressful events. It is almost impossible for a psychiatric patient not to encounter stressful life events between visits. Coping with any mental disorder can be quite stressful and challenging due to its social, vocational, or personal consequences. Stress increases cortisol, which is associated with deleterious inflammatory effects on the brain. Persistent stress can lead to hippocampal atrophy because of the abundance of glucocorticoid receptors in the hippocampus. Inquiry about stressors must be part of every psychiatric follow-up visit. Multiple psychological, physiological, and behavioral effects are well known to be generated by stress, especially in individuals already impaired by mental illness.
- Diet. What a patient eats (or avoids eating) can affect the brain. High-fat diets can be inflammatory, while a diet rich in fruits, vegetables, and nuts can be neuroprotective. The microbiota and the enteric brain—both in the gastrointestinal tract—have been reported to influence mood and behavior. (For more on this, see “Gut microbiota and its implications for psychiatry: A review of 3 studies” on page 40 and “It takes guts to be mentally ill: Microbiota and psychopathology,” From the Editor,
Current Psychiatry , September 2018, p. 4-6.) - Obesity is associated with brain atrophy as well as depression. Weight should be assessed at every visit and coupled with counseling about diet and exercise.
- Exercise, or the lack of it, can alter the brain in good or bad ways. Many studies have shown that regular exercise can induce hippocampal neurogenesis and sharpen memory and cognition. On the other hand, a sedentary lifestyle can be detrimental to the heart, bones, and brain, with an elevation in cerebrovascular and cardiovascular risks, both of which can progressively alter brain structure and function.
- Concussion, contusions, and traumatic brain injury obviously can activate the microglia and trigger neurologic sequelae and mental repercussions. At every visit, patients should be asked if they have experienced a mild or severe head injury, whether it is accidental or sports-related.
- Dehydration, especially on the day of the visit, can alter mental status in subtle ways. Cerebral ventricular volume has been shown to change with dehydration. Asking a patient about daily fluid intake should be a standard question, especially for older patients, who may experience hypotension and mental status changes due to hypovolemia.
- Sleep, whether too much or too little, is associated with brain effects and can impact cognition and behavior. Asking patients about sleep is important because it can affect the brain, and also can be a symptom of unresolved psychiatric disorders. Chronic sleep disorders are associated with neuroinflammation.
- Menstrual cycle. Various neurotransmitters fluctuate during a woman’s menstrual cycle. Her cognition becomes sharper around ovulation, and that may influence her mental status and perhaps the neuroplasticity of her brain.
- Pregnancy and its major hormone changes can change brain structure and function. Estrogen, progesterone, and prolactin have different structural effects on the brain that can help the future mother care for her dependent baby. Asking about missed periods and pregnancy during childbearing years can be useful during psychiatric encounters.
Continue to: In summary...
In summary, numerous variables can affect the patient’s brain between visits, influencing his or her mental status. The ever-changing brain can be challenging to assess, especially in brief 15- to 20-minute follow-up sessions that have become more common in psychiatry. Perhaps patients should help their psychiatrists or nurse practitioners by completing a checklist with all the above variables, either online on the day of their appointment or on a form in the waiting room immediately prior to the visit. This might also increase patients’ awareness of the importance of participating in monitoring themselves.
And finally, let’s not forget that the psychiatrist’s brain also changes continuously due to his or her own daily experiences, stresses, diet, lifestyle, medical illness, or medications. Thus, at every psychiatric session, the brains of both patient and psychiatrist are very different from the previous encounter!
To comment on this editorial or other topics of interest: [email protected].
Unlike other organs in the human body, the brain is constantly changing. The main driver for this ongoing re-engineering across various neural circuits is “experiential neuroplasticity,” which creates billions of new synapses and dendrite spines as well as new connections. And as the brain reinvents itself from day to day, the mind evolves as well.
The neurobiologic re-sculpting of the brain’s complex innards continuously encodes memories of what we learn and experience during waking hours, including all that we see, hear, feel, think, contemplate, plan, and decide. However, in addition to the ongoing intrinsic neuroplasticity that records life’s experiences within neural circuits, there are many extrinsic factors that can further modify the brain and the “psyche” it generates via electrical, neurochemical, and physiological mechanisms. That’s why every patient a psychiatrist sees at follow-up visits will have a brain that will be different from the previous encounter.
Consider the following factors that can modify a patient’s brain (for better or worse) between sessions:
- Psychotherapy that the patient received at the last session will biologically modify his or her brain. Creating new insights and understanding of one’s behavior and “connecting the dots” of the past and present emotions and reactions are all associated with neuroplastic changes within the brain.
- Mood or psychotic episodes. Depressive, manic, or psychotic episodes are associated with neuroinflammation, oxidative stress, and apoptotic effects, which can disrupt the brain’s cytoarchitecture. That’s why psychiatrists must inquire about such episodes between visits and document the possible effects on the patient’s mental status.
- Psychotropic medications all bind to one or more brain receptors to exert therapeutic or adverse effects, both of which are associated with changes in neurotransmitter pathways. A key component of every follow-up visit is to gauge the risks and benefits of the pharmacotherapy prescribed at the prior visit.
- Nonpsychiatric prescription medications are often associated with iatrogenic effects on the brain apart from their intended target organs. These iatrogenic effects include anxiety, depression, mania, psychosis, and cognitive changes. That’s why during each visit, the physician or nurse practitioner must review all prescription medications and consider their potential effects on the patient’s mental status.
- Over-the-counter drugs and supplements may exert neurologic effects via histaminergic, muscarinic, glutamatergic, adrenergic, or serotonergic effects—all of which can alter brain chemistry and contribute to mental status changes. They can also inhibit or induce cytochrome enzymes and induce adverse effects or loss of efficacy of the primary psychotropic medication the patient takes.
- Medical illness, even as simple as an upper respiratory viral infection, can alter brain function due to illness-induced physiological aberrations, including pain and peripheral inflammation, with neurologic consequences. Common metabolic disorders such as diabetes, hyperlipidemia, and hypertension can exert mental status changes.
- Alcohol and drugs of abuse alter brain structure and function and can induce psychological and cognitive changes. Inquiring about the amount and frequency of alcohol and recreational drug use must be done in detail at every visit.
- Stressful events. It is almost impossible for a psychiatric patient not to encounter stressful life events between visits. Coping with any mental disorder can be quite stressful and challenging due to its social, vocational, or personal consequences. Stress increases cortisol, which is associated with deleterious inflammatory effects on the brain. Persistent stress can lead to hippocampal atrophy because of the abundance of glucocorticoid receptors in the hippocampus. Inquiry about stressors must be part of every psychiatric follow-up visit. Multiple psychological, physiological, and behavioral effects are well known to be generated by stress, especially in individuals already impaired by mental illness.
- Diet. What a patient eats (or avoids eating) can affect the brain. High-fat diets can be inflammatory, while a diet rich in fruits, vegetables, and nuts can be neuroprotective. The microbiota and the enteric brain—both in the gastrointestinal tract—have been reported to influence mood and behavior. (For more on this, see “Gut microbiota and its implications for psychiatry: A review of 3 studies” on page 40 and “It takes guts to be mentally ill: Microbiota and psychopathology,” From the Editor,
Current Psychiatry , September 2018, p. 4-6.) - Obesity is associated with brain atrophy as well as depression. Weight should be assessed at every visit and coupled with counseling about diet and exercise.
- Exercise, or the lack of it, can alter the brain in good or bad ways. Many studies have shown that regular exercise can induce hippocampal neurogenesis and sharpen memory and cognition. On the other hand, a sedentary lifestyle can be detrimental to the heart, bones, and brain, with an elevation in cerebrovascular and cardiovascular risks, both of which can progressively alter brain structure and function.
- Concussion, contusions, and traumatic brain injury obviously can activate the microglia and trigger neurologic sequelae and mental repercussions. At every visit, patients should be asked if they have experienced a mild or severe head injury, whether it is accidental or sports-related.
- Dehydration, especially on the day of the visit, can alter mental status in subtle ways. Cerebral ventricular volume has been shown to change with dehydration. Asking a patient about daily fluid intake should be a standard question, especially for older patients, who may experience hypotension and mental status changes due to hypovolemia.
- Sleep, whether too much or too little, is associated with brain effects and can impact cognition and behavior. Asking patients about sleep is important because it can affect the brain, and also can be a symptom of unresolved psychiatric disorders. Chronic sleep disorders are associated with neuroinflammation.
- Menstrual cycle. Various neurotransmitters fluctuate during a woman’s menstrual cycle. Her cognition becomes sharper around ovulation, and that may influence her mental status and perhaps the neuroplasticity of her brain.
- Pregnancy and its major hormone changes can change brain structure and function. Estrogen, progesterone, and prolactin have different structural effects on the brain that can help the future mother care for her dependent baby. Asking about missed periods and pregnancy during childbearing years can be useful during psychiatric encounters.
Continue to: In summary...
In summary, numerous variables can affect the patient’s brain between visits, influencing his or her mental status. The ever-changing brain can be challenging to assess, especially in brief 15- to 20-minute follow-up sessions that have become more common in psychiatry. Perhaps patients should help their psychiatrists or nurse practitioners by completing a checklist with all the above variables, either online on the day of their appointment or on a form in the waiting room immediately prior to the visit. This might also increase patients’ awareness of the importance of participating in monitoring themselves.
And finally, let’s not forget that the psychiatrist’s brain also changes continuously due to his or her own daily experiences, stresses, diet, lifestyle, medical illness, or medications. Thus, at every psychiatric session, the brains of both patient and psychiatrist are very different from the previous encounter!
To comment on this editorial or other topics of interest: [email protected].
Unlike other organs in the human body, the brain is constantly changing. The main driver for this ongoing re-engineering across various neural circuits is “experiential neuroplasticity,” which creates billions of new synapses and dendrite spines as well as new connections. And as the brain reinvents itself from day to day, the mind evolves as well.
The neurobiologic re-sculpting of the brain’s complex innards continuously encodes memories of what we learn and experience during waking hours, including all that we see, hear, feel, think, contemplate, plan, and decide. However, in addition to the ongoing intrinsic neuroplasticity that records life’s experiences within neural circuits, there are many extrinsic factors that can further modify the brain and the “psyche” it generates via electrical, neurochemical, and physiological mechanisms. That’s why every patient a psychiatrist sees at follow-up visits will have a brain that will be different from the previous encounter.
Consider the following factors that can modify a patient’s brain (for better or worse) between sessions:
- Psychotherapy that the patient received at the last session will biologically modify his or her brain. Creating new insights and understanding of one’s behavior and “connecting the dots” of the past and present emotions and reactions are all associated with neuroplastic changes within the brain.
- Mood or psychotic episodes. Depressive, manic, or psychotic episodes are associated with neuroinflammation, oxidative stress, and apoptotic effects, which can disrupt the brain’s cytoarchitecture. That’s why psychiatrists must inquire about such episodes between visits and document the possible effects on the patient’s mental status.
- Psychotropic medications all bind to one or more brain receptors to exert therapeutic or adverse effects, both of which are associated with changes in neurotransmitter pathways. A key component of every follow-up visit is to gauge the risks and benefits of the pharmacotherapy prescribed at the prior visit.
- Nonpsychiatric prescription medications are often associated with iatrogenic effects on the brain apart from their intended target organs. These iatrogenic effects include anxiety, depression, mania, psychosis, and cognitive changes. That’s why during each visit, the physician or nurse practitioner must review all prescription medications and consider their potential effects on the patient’s mental status.
- Over-the-counter drugs and supplements may exert neurologic effects via histaminergic, muscarinic, glutamatergic, adrenergic, or serotonergic effects—all of which can alter brain chemistry and contribute to mental status changes. They can also inhibit or induce cytochrome enzymes and induce adverse effects or loss of efficacy of the primary psychotropic medication the patient takes.
- Medical illness, even as simple as an upper respiratory viral infection, can alter brain function due to illness-induced physiological aberrations, including pain and peripheral inflammation, with neurologic consequences. Common metabolic disorders such as diabetes, hyperlipidemia, and hypertension can exert mental status changes.
- Alcohol and drugs of abuse alter brain structure and function and can induce psychological and cognitive changes. Inquiring about the amount and frequency of alcohol and recreational drug use must be done in detail at every visit.
- Stressful events. It is almost impossible for a psychiatric patient not to encounter stressful life events between visits. Coping with any mental disorder can be quite stressful and challenging due to its social, vocational, or personal consequences. Stress increases cortisol, which is associated with deleterious inflammatory effects on the brain. Persistent stress can lead to hippocampal atrophy because of the abundance of glucocorticoid receptors in the hippocampus. Inquiry about stressors must be part of every psychiatric follow-up visit. Multiple psychological, physiological, and behavioral effects are well known to be generated by stress, especially in individuals already impaired by mental illness.
- Diet. What a patient eats (or avoids eating) can affect the brain. High-fat diets can be inflammatory, while a diet rich in fruits, vegetables, and nuts can be neuroprotective. The microbiota and the enteric brain—both in the gastrointestinal tract—have been reported to influence mood and behavior. (For more on this, see “Gut microbiota and its implications for psychiatry: A review of 3 studies” on page 40 and “It takes guts to be mentally ill: Microbiota and psychopathology,” From the Editor,
Current Psychiatry , September 2018, p. 4-6.) - Obesity is associated with brain atrophy as well as depression. Weight should be assessed at every visit and coupled with counseling about diet and exercise.
- Exercise, or the lack of it, can alter the brain in good or bad ways. Many studies have shown that regular exercise can induce hippocampal neurogenesis and sharpen memory and cognition. On the other hand, a sedentary lifestyle can be detrimental to the heart, bones, and brain, with an elevation in cerebrovascular and cardiovascular risks, both of which can progressively alter brain structure and function.
- Concussion, contusions, and traumatic brain injury obviously can activate the microglia and trigger neurologic sequelae and mental repercussions. At every visit, patients should be asked if they have experienced a mild or severe head injury, whether it is accidental or sports-related.
- Dehydration, especially on the day of the visit, can alter mental status in subtle ways. Cerebral ventricular volume has been shown to change with dehydration. Asking a patient about daily fluid intake should be a standard question, especially for older patients, who may experience hypotension and mental status changes due to hypovolemia.
- Sleep, whether too much or too little, is associated with brain effects and can impact cognition and behavior. Asking patients about sleep is important because it can affect the brain, and also can be a symptom of unresolved psychiatric disorders. Chronic sleep disorders are associated with neuroinflammation.
- Menstrual cycle. Various neurotransmitters fluctuate during a woman’s menstrual cycle. Her cognition becomes sharper around ovulation, and that may influence her mental status and perhaps the neuroplasticity of her brain.
- Pregnancy and its major hormone changes can change brain structure and function. Estrogen, progesterone, and prolactin have different structural effects on the brain that can help the future mother care for her dependent baby. Asking about missed periods and pregnancy during childbearing years can be useful during psychiatric encounters.
Continue to: In summary...
In summary, numerous variables can affect the patient’s brain between visits, influencing his or her mental status. The ever-changing brain can be challenging to assess, especially in brief 15- to 20-minute follow-up sessions that have become more common in psychiatry. Perhaps patients should help their psychiatrists or nurse practitioners by completing a checklist with all the above variables, either online on the day of their appointment or on a form in the waiting room immediately prior to the visit. This might also increase patients’ awareness of the importance of participating in monitoring themselves.
And finally, let’s not forget that the psychiatrist’s brain also changes continuously due to his or her own daily experiences, stresses, diet, lifestyle, medical illness, or medications. Thus, at every psychiatric session, the brains of both patient and psychiatrist are very different from the previous encounter!
To comment on this editorial or other topics of interest: [email protected].
Screening and counseling interventions to prevent peripartum depression: A practical approach
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
Can a humanities background prevent physician burnout?
At the extreme, this discontent sears our professional being and results in early retirement, change of profession, and, for many, searching for ways to limit clinical practice time—while often saying how much they wish they could “just practice medicine.” Such are some of the manifestations of burnout.
Studies indicate that contributors to burnout are many. And as in all observational studies, the establishment of cause, effect, and degree of codependency is difficult if not impossible to ascertain. Many major changes have temporally coincided with the rise in physician dissatisfaction. One is the increasing corporatization of medicine. In 2016, in some parts of the country, over 40% of physicians were employed by hospitals.1 Surveys indicate that these employed physicians have a modestly higher degree of dissatisfaction than those in “independent” practices, often citing loss of control of their practice style and increased regulatory demands as contributors to their misery—which is ironic, since the reason many physicians join large hospital-employed groups is to minimize external financial and regulatory pressures.
Astute corporate medical leaders have recognized the burnout issue and are struggling to diminish its negative impact on the healthcare system, patient care, and individual physicians. But many initial approaches have been aimed at soothing the already singed. Health days, yoga sessions, mindfulness classes, and various ways to soften the impact of the EMR on our lives have all been offered up along with other creative and well-intentioned balms. It is not clear to me that any of these address the primary issues contributing to the growing challenge of professional and personal discontent. Some of these approaches may take root and improve a few physicians’ ability to cope. But will that be sufficient to save a generation of skilled and experienced but increasingly disconnected physicians and clinical faculty?
On this landscape, Mangione and Kahn in this issue of the Journal argue for the humanities as part of the solution for what ails us. They cite Sir William Osler, the titan of internal medicine, who a century ago urged physicians to cultivate a strong background in the humanities as a counterweight to the objective science that he also so strongly endorsed and inculcated into the culture at Johns Hopkins. Mangione and Kahn present nascent data suggesting that students who choose to have extra interactions with the arts and humanities exhibit greater resilience, tolerance of ambiguity, and more of the empathetic traits that we desire in physicians, and they posit that these traits will decrease the sense of professional burnout.
We don’t know whether it is the impact of extra exposure to the humanities or the personality of those students who choose to partake of these programs that is the major contributor to the behavioral outcomes, though I suspect it is both. The real question is this: even if we can enhance through greater exposure to the humanities the desired attitudes in our medical students, residents, and young physicians, can we slow the rate of professional dissatisfaction and burnout in them?
To answer this, we need a deeper understanding of the burnout process and whether it will affect younger physicians and physicians currently in training the same way it has affected an older generation of physicians, many of whom have had to face the challenges of coping with the new digital world that our younger colleagues have grown up with. Many of us also have needed to change our practice patterns and expectations. Our younger colleagues may not be faced with the same contextual dissonance that we have had to adjust to in reconciling our (idealistic) image of clinical practice with the pragmatic business of medicine. Their expectations for both are, and will likely remain, quite different.
The next generation of physicians will undoubtedly have their own challenges. They are well familiarized with the digital and virtual world and will likely accept avatar medicine to a far greater degree than we have. But I think the study of the humanities will be of great value to them as well, not necessarily to imbue them with a greater sense of resilience in coping with the digital and science aspects of medicine, but to provide reminders of what Bruce Springsteen has called the “human touch.” Studying the humanities may provide the conceptual reminder of the value of humanness—as we physicians evolve into the world of providing an increasing amount of care via advanced-care providers, shortened real visits, and telemedicine and other virtual consultative visits.
Hopefully, we can indeed find a way to nurture within us Osler’s conceptual tree of medicine that harbors on the same stem the “twin berries” of “the Humanities and Science.”
- Haefner M. Hospitals employed 42% of physicians in 2016: 5 study findings. Becker’s Hospital Review. March 15, 2018. https://www.beckershospitalreview.com/hospital-physician-relationships/hospitals-employed-42-of-physicians-in-2016-5-study-findings.html. Accessed March 19, 2018.
At the extreme, this discontent sears our professional being and results in early retirement, change of profession, and, for many, searching for ways to limit clinical practice time—while often saying how much they wish they could “just practice medicine.” Such are some of the manifestations of burnout.
Studies indicate that contributors to burnout are many. And as in all observational studies, the establishment of cause, effect, and degree of codependency is difficult if not impossible to ascertain. Many major changes have temporally coincided with the rise in physician dissatisfaction. One is the increasing corporatization of medicine. In 2016, in some parts of the country, over 40% of physicians were employed by hospitals.1 Surveys indicate that these employed physicians have a modestly higher degree of dissatisfaction than those in “independent” practices, often citing loss of control of their practice style and increased regulatory demands as contributors to their misery—which is ironic, since the reason many physicians join large hospital-employed groups is to minimize external financial and regulatory pressures.
Astute corporate medical leaders have recognized the burnout issue and are struggling to diminish its negative impact on the healthcare system, patient care, and individual physicians. But many initial approaches have been aimed at soothing the already singed. Health days, yoga sessions, mindfulness classes, and various ways to soften the impact of the EMR on our lives have all been offered up along with other creative and well-intentioned balms. It is not clear to me that any of these address the primary issues contributing to the growing challenge of professional and personal discontent. Some of these approaches may take root and improve a few physicians’ ability to cope. But will that be sufficient to save a generation of skilled and experienced but increasingly disconnected physicians and clinical faculty?
On this landscape, Mangione and Kahn in this issue of the Journal argue for the humanities as part of the solution for what ails us. They cite Sir William Osler, the titan of internal medicine, who a century ago urged physicians to cultivate a strong background in the humanities as a counterweight to the objective science that he also so strongly endorsed and inculcated into the culture at Johns Hopkins. Mangione and Kahn present nascent data suggesting that students who choose to have extra interactions with the arts and humanities exhibit greater resilience, tolerance of ambiguity, and more of the empathetic traits that we desire in physicians, and they posit that these traits will decrease the sense of professional burnout.
We don’t know whether it is the impact of extra exposure to the humanities or the personality of those students who choose to partake of these programs that is the major contributor to the behavioral outcomes, though I suspect it is both. The real question is this: even if we can enhance through greater exposure to the humanities the desired attitudes in our medical students, residents, and young physicians, can we slow the rate of professional dissatisfaction and burnout in them?
To answer this, we need a deeper understanding of the burnout process and whether it will affect younger physicians and physicians currently in training the same way it has affected an older generation of physicians, many of whom have had to face the challenges of coping with the new digital world that our younger colleagues have grown up with. Many of us also have needed to change our practice patterns and expectations. Our younger colleagues may not be faced with the same contextual dissonance that we have had to adjust to in reconciling our (idealistic) image of clinical practice with the pragmatic business of medicine. Their expectations for both are, and will likely remain, quite different.
The next generation of physicians will undoubtedly have their own challenges. They are well familiarized with the digital and virtual world and will likely accept avatar medicine to a far greater degree than we have. But I think the study of the humanities will be of great value to them as well, not necessarily to imbue them with a greater sense of resilience in coping with the digital and science aspects of medicine, but to provide reminders of what Bruce Springsteen has called the “human touch.” Studying the humanities may provide the conceptual reminder of the value of humanness—as we physicians evolve into the world of providing an increasing amount of care via advanced-care providers, shortened real visits, and telemedicine and other virtual consultative visits.
Hopefully, we can indeed find a way to nurture within us Osler’s conceptual tree of medicine that harbors on the same stem the “twin berries” of “the Humanities and Science.”
At the extreme, this discontent sears our professional being and results in early retirement, change of profession, and, for many, searching for ways to limit clinical practice time—while often saying how much they wish they could “just practice medicine.” Such are some of the manifestations of burnout.
Studies indicate that contributors to burnout are many. And as in all observational studies, the establishment of cause, effect, and degree of codependency is difficult if not impossible to ascertain. Many major changes have temporally coincided with the rise in physician dissatisfaction. One is the increasing corporatization of medicine. In 2016, in some parts of the country, over 40% of physicians were employed by hospitals.1 Surveys indicate that these employed physicians have a modestly higher degree of dissatisfaction than those in “independent” practices, often citing loss of control of their practice style and increased regulatory demands as contributors to their misery—which is ironic, since the reason many physicians join large hospital-employed groups is to minimize external financial and regulatory pressures.
Astute corporate medical leaders have recognized the burnout issue and are struggling to diminish its negative impact on the healthcare system, patient care, and individual physicians. But many initial approaches have been aimed at soothing the already singed. Health days, yoga sessions, mindfulness classes, and various ways to soften the impact of the EMR on our lives have all been offered up along with other creative and well-intentioned balms. It is not clear to me that any of these address the primary issues contributing to the growing challenge of professional and personal discontent. Some of these approaches may take root and improve a few physicians’ ability to cope. But will that be sufficient to save a generation of skilled and experienced but increasingly disconnected physicians and clinical faculty?
On this landscape, Mangione and Kahn in this issue of the Journal argue for the humanities as part of the solution for what ails us. They cite Sir William Osler, the titan of internal medicine, who a century ago urged physicians to cultivate a strong background in the humanities as a counterweight to the objective science that he also so strongly endorsed and inculcated into the culture at Johns Hopkins. Mangione and Kahn present nascent data suggesting that students who choose to have extra interactions with the arts and humanities exhibit greater resilience, tolerance of ambiguity, and more of the empathetic traits that we desire in physicians, and they posit that these traits will decrease the sense of professional burnout.
We don’t know whether it is the impact of extra exposure to the humanities or the personality of those students who choose to partake of these programs that is the major contributor to the behavioral outcomes, though I suspect it is both. The real question is this: even if we can enhance through greater exposure to the humanities the desired attitudes in our medical students, residents, and young physicians, can we slow the rate of professional dissatisfaction and burnout in them?
To answer this, we need a deeper understanding of the burnout process and whether it will affect younger physicians and physicians currently in training the same way it has affected an older generation of physicians, many of whom have had to face the challenges of coping with the new digital world that our younger colleagues have grown up with. Many of us also have needed to change our practice patterns and expectations. Our younger colleagues may not be faced with the same contextual dissonance that we have had to adjust to in reconciling our (idealistic) image of clinical practice with the pragmatic business of medicine. Their expectations for both are, and will likely remain, quite different.
The next generation of physicians will undoubtedly have their own challenges. They are well familiarized with the digital and virtual world and will likely accept avatar medicine to a far greater degree than we have. But I think the study of the humanities will be of great value to them as well, not necessarily to imbue them with a greater sense of resilience in coping with the digital and science aspects of medicine, but to provide reminders of what Bruce Springsteen has called the “human touch.” Studying the humanities may provide the conceptual reminder of the value of humanness—as we physicians evolve into the world of providing an increasing amount of care via advanced-care providers, shortened real visits, and telemedicine and other virtual consultative visits.
Hopefully, we can indeed find a way to nurture within us Osler’s conceptual tree of medicine that harbors on the same stem the “twin berries” of “the Humanities and Science.”
- Haefner M. Hospitals employed 42% of physicians in 2016: 5 study findings. Becker’s Hospital Review. March 15, 2018. https://www.beckershospitalreview.com/hospital-physician-relationships/hospitals-employed-42-of-physicians-in-2016-5-study-findings.html. Accessed March 19, 2018.
- Haefner M. Hospitals employed 42% of physicians in 2016: 5 study findings. Becker’s Hospital Review. March 15, 2018. https://www.beckershospitalreview.com/hospital-physician-relationships/hospitals-employed-42-of-physicians-in-2016-5-study-findings.html. Accessed March 19, 2018.
Pimavanserin: A potentially safer alternative to clozapine for refractory hallucinations and delusions
Up to 30% of patients with schizophrenia do not respond to dopamine antagonists, which include all first- and second-generation antipsychotics. They are labeled as “treatment-resistant” if they have a partial response, or “treatment-refractory” if their hallucinations and/or delusions do not improve at all despite multiple trials of antipsychotics.
That’s why clozapine is considered a “lifesaver” for such patients, a last-resort medication that unshackles patients with refractory psychotic symptoms from the tyranny of auditory and/or visual hallucinations and the reality distortion of fixed false beliefs such as paranoid delusions.
Many long-suffering patients with refractory psychosis recover and return to their baseline, thanks to clozapine. In a past editorial, I discussed how one of my patients, Bethany, who had dropped out of college and became homeless for 4 years with refractory delusions and hallucinations, recovered completely when she received clozapine.1 She then returned to college, graduated with honors, and authored a book about her journey of recovery.2 She and I later established a nonprofit foundation we called CURESZ (Comprehensive Understanding via Research and Education in Schizophrenia), and assembled a panel of 80 clozapine experts across the country to provide access to clozapine for the hundreds of thousands of individuals with refractory psychosis who never received a trial of clozapine from their psychiatrists or psychiatric nurse practitioners. (Visit CURESZ.org for details.)
Bethany was very lucky to respond and recover completely, because only 40% of patients with refractory psychosis respond to clozapine. She does not mind having her blood drawn every week to measure her white blood cell count for early detection of potentially fatal agranulocytosis. Many refractory, often homeless patients with chronic schizophrenia refuse to have weekly phlebotomy and therefore are not treated with clozapine. Bethany was also fortunate to experience only 1 adverse effect of clozapine: extreme sedation that forced her to sleep up to 15 hours a day (this was reduced to 9 to 10 hours a day with adjunctive modafinil). Fortunately, she was spared the multiple other serious adverse effects of clozapine, which include excessive salivation, extreme weight gain, diabetes, hyperlipidemia, cardiomyopathy, pancreatitis, seizures, and ileus.3 Clozapine is also associated with sudden death more than any other antipsychotic agent.4
So, what can be done for patients with refractory hallucinations and delusions who are among the 60% who fail to respond to clozapine, or who experience intolerable adverse effects or safety problems, or who refuse to take clozapine and have their blood drawn every week? This is a desperately ill and seriously disabled group of patients who are deemed to be beyond the reach of medical intervention by psychiatry. They are often treated with various off-label medications as adjunctive therapy to clozapine, to which they failed to respond. This includes adding lamotrigine5 or benzoate,6 but none have been approved as an efficacious and safe monotherapy alternative to clozapine. So, what can be done for patients with refractory illness?
Enter pimavanserin. This new medication is an inverse agonist of serotonin 5-HT2A receptors and (to a lesser extent) serotonin 5-HT2C receptors. It was recently FDA-approved for treating the hallucinations and delusions of Parkinson’s disease psychosis,7 which is estimated to develop in up to 50% of individuals with Parkinson’s disease. It does not have any affinity to any dopamine receptors, which makes it an ideal antipsychotic for Parkinson’s disease, where any dopamine antagonism can worsen the motor symptoms (rigidity, hypokinesia, and tremors) associated with that movement disorder. Thus, pimavanserin became the first ever non-dopaminergic antipsychotic in the world and is indicated only for Parkinson’s disease psychosis.
Our clinical team made a serendipitous discovery about the efficacy of pimavanserin in patients with schizophrenia who failed to respond to clozapine therapy after several months at clinically adequate doses. Our findings were published online last month in the highly respected journal Schizophrenia Research.8 We reported the successful treatment with pimavanserin in 2 groups:
- patients who had not responded to clozapine received pimavanserin as an add-on to clozapine in doses of 34 mg/d, the same dose recommended for patients with Parkinson’s disease hallucinations and/or delusions.
- patients who had hallucinations and delusions that failed to respond to several non-clozapine antipsychotics received pimavanserin monotherapy instead of clozapine to avoid blood draws and serious adverse effects.
Continue to: Pimavanserin successfully treated...
Pimavanserin successfully treated the hallucinations and delusions of all 10 patients in both groups. Remission occurred within 1 month in most cases, and after 2 months in 1 patient. Those patients no longer required hospitalization as they did prior to taking pimavanserin, and they maintained their response for several months of follow-up. We were also pleased to note that most patients became more sociable and affable, with improved mood and affect, after their hallucinations and delusions disappeared with pimavanserin. We did have a few patients who did not respond to 34 mg/d of pimavanserin, and some who responded for several months but then showed signs of recurrence. We are considering increasing the dose to 68 mg/d in such patients because it is possible that a higher dose may be needed in some patients with refractory illness, who may vary in symptom severity or biology.
We are now planning to apply for a research grant to conduct a controlled trial to confirm our very encouraging clinical findings, and we hope other investigators will also conduct clinical trials in patients with refractory psychosis comparing pimavanserin with placebo or pimavanserin with clozapine in double-blind studies.
As a disclosure, our clinical findings were obtained without any knowledge of, or funding from, the company that makes pimavanserin (Acadia Pharmaceuticals Inc.). The company was informed of our findings only after our article was accepted for publication.
I hope this important finding of a potentially safer alternative to clozapine may address a major unmet need in psychiatry, involving the treatment of hundreds of thousands of patients with treatment-resistant or treatment-refractory psychosis, which includes patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21, 24-25.
2. Yeiser B. Mind estranged: my journey from schizophrenia and homelessness to recovery. Seattle, WA: Amazon; 2014.
3. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf. 2014;9(3):163-195.
4. Manu P, Kane JM, Corell CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936-941.
5. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2009;109(1-3):10-14.
6. Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2017;84(6):422-432.
7. Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in p atients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
8. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist [Epub ahead of print March 2, 2019]. Schizophrenia Res. 2019. doi: 10.1016/j.schres.2019.02.018.
Up to 30% of patients with schizophrenia do not respond to dopamine antagonists, which include all first- and second-generation antipsychotics. They are labeled as “treatment-resistant” if they have a partial response, or “treatment-refractory” if their hallucinations and/or delusions do not improve at all despite multiple trials of antipsychotics.
That’s why clozapine is considered a “lifesaver” for such patients, a last-resort medication that unshackles patients with refractory psychotic symptoms from the tyranny of auditory and/or visual hallucinations and the reality distortion of fixed false beliefs such as paranoid delusions.
Many long-suffering patients with refractory psychosis recover and return to their baseline, thanks to clozapine. In a past editorial, I discussed how one of my patients, Bethany, who had dropped out of college and became homeless for 4 years with refractory delusions and hallucinations, recovered completely when she received clozapine.1 She then returned to college, graduated with honors, and authored a book about her journey of recovery.2 She and I later established a nonprofit foundation we called CURESZ (Comprehensive Understanding via Research and Education in Schizophrenia), and assembled a panel of 80 clozapine experts across the country to provide access to clozapine for the hundreds of thousands of individuals with refractory psychosis who never received a trial of clozapine from their psychiatrists or psychiatric nurse practitioners. (Visit CURESZ.org for details.)
Bethany was very lucky to respond and recover completely, because only 40% of patients with refractory psychosis respond to clozapine. She does not mind having her blood drawn every week to measure her white blood cell count for early detection of potentially fatal agranulocytosis. Many refractory, often homeless patients with chronic schizophrenia refuse to have weekly phlebotomy and therefore are not treated with clozapine. Bethany was also fortunate to experience only 1 adverse effect of clozapine: extreme sedation that forced her to sleep up to 15 hours a day (this was reduced to 9 to 10 hours a day with adjunctive modafinil). Fortunately, she was spared the multiple other serious adverse effects of clozapine, which include excessive salivation, extreme weight gain, diabetes, hyperlipidemia, cardiomyopathy, pancreatitis, seizures, and ileus.3 Clozapine is also associated with sudden death more than any other antipsychotic agent.4
So, what can be done for patients with refractory hallucinations and delusions who are among the 60% who fail to respond to clozapine, or who experience intolerable adverse effects or safety problems, or who refuse to take clozapine and have their blood drawn every week? This is a desperately ill and seriously disabled group of patients who are deemed to be beyond the reach of medical intervention by psychiatry. They are often treated with various off-label medications as adjunctive therapy to clozapine, to which they failed to respond. This includes adding lamotrigine5 or benzoate,6 but none have been approved as an efficacious and safe monotherapy alternative to clozapine. So, what can be done for patients with refractory illness?
Enter pimavanserin. This new medication is an inverse agonist of serotonin 5-HT2A receptors and (to a lesser extent) serotonin 5-HT2C receptors. It was recently FDA-approved for treating the hallucinations and delusions of Parkinson’s disease psychosis,7 which is estimated to develop in up to 50% of individuals with Parkinson’s disease. It does not have any affinity to any dopamine receptors, which makes it an ideal antipsychotic for Parkinson’s disease, where any dopamine antagonism can worsen the motor symptoms (rigidity, hypokinesia, and tremors) associated with that movement disorder. Thus, pimavanserin became the first ever non-dopaminergic antipsychotic in the world and is indicated only for Parkinson’s disease psychosis.
Our clinical team made a serendipitous discovery about the efficacy of pimavanserin in patients with schizophrenia who failed to respond to clozapine therapy after several months at clinically adequate doses. Our findings were published online last month in the highly respected journal Schizophrenia Research.8 We reported the successful treatment with pimavanserin in 2 groups:
- patients who had not responded to clozapine received pimavanserin as an add-on to clozapine in doses of 34 mg/d, the same dose recommended for patients with Parkinson’s disease hallucinations and/or delusions.
- patients who had hallucinations and delusions that failed to respond to several non-clozapine antipsychotics received pimavanserin monotherapy instead of clozapine to avoid blood draws and serious adverse effects.
Continue to: Pimavanserin successfully treated...
Pimavanserin successfully treated the hallucinations and delusions of all 10 patients in both groups. Remission occurred within 1 month in most cases, and after 2 months in 1 patient. Those patients no longer required hospitalization as they did prior to taking pimavanserin, and they maintained their response for several months of follow-up. We were also pleased to note that most patients became more sociable and affable, with improved mood and affect, after their hallucinations and delusions disappeared with pimavanserin. We did have a few patients who did not respond to 34 mg/d of pimavanserin, and some who responded for several months but then showed signs of recurrence. We are considering increasing the dose to 68 mg/d in such patients because it is possible that a higher dose may be needed in some patients with refractory illness, who may vary in symptom severity or biology.
We are now planning to apply for a research grant to conduct a controlled trial to confirm our very encouraging clinical findings, and we hope other investigators will also conduct clinical trials in patients with refractory psychosis comparing pimavanserin with placebo or pimavanserin with clozapine in double-blind studies.
As a disclosure, our clinical findings were obtained without any knowledge of, or funding from, the company that makes pimavanserin (Acadia Pharmaceuticals Inc.). The company was informed of our findings only after our article was accepted for publication.
I hope this important finding of a potentially safer alternative to clozapine may address a major unmet need in psychiatry, involving the treatment of hundreds of thousands of patients with treatment-resistant or treatment-refractory psychosis, which includes patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder.
To comment on this editorial or other topics of interest: [email protected].
Up to 30% of patients with schizophrenia do not respond to dopamine antagonists, which include all first- and second-generation antipsychotics. They are labeled as “treatment-resistant” if they have a partial response, or “treatment-refractory” if their hallucinations and/or delusions do not improve at all despite multiple trials of antipsychotics.
That’s why clozapine is considered a “lifesaver” for such patients, a last-resort medication that unshackles patients with refractory psychotic symptoms from the tyranny of auditory and/or visual hallucinations and the reality distortion of fixed false beliefs such as paranoid delusions.
Many long-suffering patients with refractory psychosis recover and return to their baseline, thanks to clozapine. In a past editorial, I discussed how one of my patients, Bethany, who had dropped out of college and became homeless for 4 years with refractory delusions and hallucinations, recovered completely when she received clozapine.1 She then returned to college, graduated with honors, and authored a book about her journey of recovery.2 She and I later established a nonprofit foundation we called CURESZ (Comprehensive Understanding via Research and Education in Schizophrenia), and assembled a panel of 80 clozapine experts across the country to provide access to clozapine for the hundreds of thousands of individuals with refractory psychosis who never received a trial of clozapine from their psychiatrists or psychiatric nurse practitioners. (Visit CURESZ.org for details.)
Bethany was very lucky to respond and recover completely, because only 40% of patients with refractory psychosis respond to clozapine. She does not mind having her blood drawn every week to measure her white blood cell count for early detection of potentially fatal agranulocytosis. Many refractory, often homeless patients with chronic schizophrenia refuse to have weekly phlebotomy and therefore are not treated with clozapine. Bethany was also fortunate to experience only 1 adverse effect of clozapine: extreme sedation that forced her to sleep up to 15 hours a day (this was reduced to 9 to 10 hours a day with adjunctive modafinil). Fortunately, she was spared the multiple other serious adverse effects of clozapine, which include excessive salivation, extreme weight gain, diabetes, hyperlipidemia, cardiomyopathy, pancreatitis, seizures, and ileus.3 Clozapine is also associated with sudden death more than any other antipsychotic agent.4
So, what can be done for patients with refractory hallucinations and delusions who are among the 60% who fail to respond to clozapine, or who experience intolerable adverse effects or safety problems, or who refuse to take clozapine and have their blood drawn every week? This is a desperately ill and seriously disabled group of patients who are deemed to be beyond the reach of medical intervention by psychiatry. They are often treated with various off-label medications as adjunctive therapy to clozapine, to which they failed to respond. This includes adding lamotrigine5 or benzoate,6 but none have been approved as an efficacious and safe monotherapy alternative to clozapine. So, what can be done for patients with refractory illness?
Enter pimavanserin. This new medication is an inverse agonist of serotonin 5-HT2A receptors and (to a lesser extent) serotonin 5-HT2C receptors. It was recently FDA-approved for treating the hallucinations and delusions of Parkinson’s disease psychosis,7 which is estimated to develop in up to 50% of individuals with Parkinson’s disease. It does not have any affinity to any dopamine receptors, which makes it an ideal antipsychotic for Parkinson’s disease, where any dopamine antagonism can worsen the motor symptoms (rigidity, hypokinesia, and tremors) associated with that movement disorder. Thus, pimavanserin became the first ever non-dopaminergic antipsychotic in the world and is indicated only for Parkinson’s disease psychosis.
Our clinical team made a serendipitous discovery about the efficacy of pimavanserin in patients with schizophrenia who failed to respond to clozapine therapy after several months at clinically adequate doses. Our findings were published online last month in the highly respected journal Schizophrenia Research.8 We reported the successful treatment with pimavanserin in 2 groups:
- patients who had not responded to clozapine received pimavanserin as an add-on to clozapine in doses of 34 mg/d, the same dose recommended for patients with Parkinson’s disease hallucinations and/or delusions.
- patients who had hallucinations and delusions that failed to respond to several non-clozapine antipsychotics received pimavanserin monotherapy instead of clozapine to avoid blood draws and serious adverse effects.
Continue to: Pimavanserin successfully treated...
Pimavanserin successfully treated the hallucinations and delusions of all 10 patients in both groups. Remission occurred within 1 month in most cases, and after 2 months in 1 patient. Those patients no longer required hospitalization as they did prior to taking pimavanserin, and they maintained their response for several months of follow-up. We were also pleased to note that most patients became more sociable and affable, with improved mood and affect, after their hallucinations and delusions disappeared with pimavanserin. We did have a few patients who did not respond to 34 mg/d of pimavanserin, and some who responded for several months but then showed signs of recurrence. We are considering increasing the dose to 68 mg/d in such patients because it is possible that a higher dose may be needed in some patients with refractory illness, who may vary in symptom severity or biology.
We are now planning to apply for a research grant to conduct a controlled trial to confirm our very encouraging clinical findings, and we hope other investigators will also conduct clinical trials in patients with refractory psychosis comparing pimavanserin with placebo or pimavanserin with clozapine in double-blind studies.
As a disclosure, our clinical findings were obtained without any knowledge of, or funding from, the company that makes pimavanserin (Acadia Pharmaceuticals Inc.). The company was informed of our findings only after our article was accepted for publication.
I hope this important finding of a potentially safer alternative to clozapine may address a major unmet need in psychiatry, involving the treatment of hundreds of thousands of patients with treatment-resistant or treatment-refractory psychosis, which includes patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21, 24-25.
2. Yeiser B. Mind estranged: my journey from schizophrenia and homelessness to recovery. Seattle, WA: Amazon; 2014.
3. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf. 2014;9(3):163-195.
4. Manu P, Kane JM, Corell CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936-941.
5. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2009;109(1-3):10-14.
6. Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2017;84(6):422-432.
7. Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in p atients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
8. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist [Epub ahead of print March 2, 2019]. Schizophrenia Res. 2019. doi: 10.1016/j.schres.2019.02.018.
1. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21, 24-25.
2. Yeiser B. Mind estranged: my journey from schizophrenia and homelessness to recovery. Seattle, WA: Amazon; 2014.
3. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf. 2014;9(3):163-195.
4. Manu P, Kane JM, Corell CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936-941.
5. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2009;109(1-3):10-14.
6. Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2017;84(6):422-432.
7. Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in p atients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
8. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist [Epub ahead of print March 2, 2019]. Schizophrenia Res. 2019. doi: 10.1016/j.schres.2019.02.018.
The paclitaxel paradox
As medical editor of Vascular Specialist, it has always been my hope to use our excellent reporters and rapid production schedule to keep readers abreast of the latest news in vascular surgery. While my colleagues at the Journal of Vascular Surgery publish studies that will drive treatment, my goal is to drive discussion.
With topics like burnout, workforce shortages, and electronic medical records, I feel we have been successful. The downside of staying current is we sometimes find ourselves publishing contradictory stories. This has been the case with paclitaxel. Let’s take a break from the fray and review where we are, and where we might go from here.
In 2012, the Zilver PTX became the first drug-eluting stent (DES) to gain Food and Drug Administration approval for the treatment of peripheral vascular disease. Two years later, the FDA approved the Lutonix 035 as the first drug-coated balloon (DCB) for use in the femoral-popliteal arteries. The Lutonix would also gain a second indication for failing dialysis fistulas. Medtronic and Spectranetics received authorizations for their DCBs in 2015 and 2017, respectively.
While the safety of paclitaxel-coated devices in the coronary system had previously been called into question, the drug was generally considered safe and effective in the peripheral arterial system. The controversy began in December 2018, when Katsanos et al.1 published a meta-analysis of 28 randomized, controlled trials (RCTs) investigating paclitaxel-coated devices in the femoral-popliteal arteries. While all-cause patient mortality was similar at 1 year between paclitaxel-coated devices and controls (2.3% in each), at 2 years the risk of death was significantly higher in those treated with paclitaxel (7.2% vs. 3.8%). The 5-year data were available for three trials where there was a continued significantly increased risk of mortality with paclitaxel (14.7% vs. 8.1%).
Opposition to these findings was prompt from both physicians and industry. Weaknesses of the analysis, both perceived and real, were hammered. The meta-analysis did not include individual patient data, and the actual cause of death was unknown in most of the included trials. The study was not adequately powered to eliminate the risk of type 1 error when comparing mortality after 2 years. Individuals assigned to the control group may have received paclitaxel treatment at some point in their follow-up. The DCB and DES treatment groups were combined. The methods employed by the authors, however, stood up reasonably well to scrutiny.
On Jan. 17, 2019, the FDA issued their first response stating, “the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”2
Later that month, Peter Schneider, MD, and associates published a patient-level meta-analysis in the Journal of the American College of Cardiology.3 The study included 1,980 patients and found no statistically significant difference in all-cause mortality between DCB (9.3%) and percutaneous transluminal angioplasty (PTA) (11.2%) through 5 years. Shortly after that, however, a correction was issued.
On Feb. 15, 2019, Medtronic reported an error in the 2- and 3-year follow-up periods for the IN.PACT Global postmarket study. The company stated, “Due to a programming error, mortality data were inadvertently omitted from the summary tables included in the statistical analysis.” The mortality in the DCB cohort was corrected from 9.30% to 15.12%. The authors stated that this new mortality rate was still not significantly higher than the PTA group (P = .09).4
Less than 1 week later, another device company issued a correction. And once again, the error had been made in favor of the paclitaxel-treated group. In 2016, the 5-year data from Cook Medical’s Zilver PTX trial were published in Circulation. The study reported a mortality of 10.2% in the DES group and 16.9% in the PTA cohort. Regrettably, these numbers were reversed and significantly higher in the paclitaxel-treated group (16.9% vs. 10.2%, P = .03).5
On Feb. 12, 2019, another response to the Katsanos meta-analysis was published in JAMA Cardiology.6 In this study, Secemsky et al. analyzed patient-level data from a Medicare database. The authors reported finding no evidence of paclitaxel- related deaths in 16,560 patients. Unfortunately, the mean follow-up time was only 389 days, which may have been insufficient to detect the late mortality reported in the Katsanos meta-analysis.
On March 15, 2019, the FDA issued a second statement, this time with a much stronger tone.7 The agency reported an ongoing analysis of the long-term survival data from the pivotal randomized trials. In the three studies with 5-year data available, each showed a significantly higher mortality in the paclitaxel group.
When pooled, there were 975 patients, and the risk of death was 20.1% in the paclitaxel group versus 13.4 % in the controls. The FDA recommended discussing the increased risk of mortality with all patients receiving paclitaxel therapy as part of the informed consent process. They also stated that for most patients alternative options should generally be used until additional analysis of the mortality risk is performed.
Industry bristled at this new, strongly worded statement. Becton Dickinson, makers of the Lutonix balloon, asserted that the FDA recommendation was based on “a limited review of data from less than 1,000 patients.”8 The company noted that its LEVANT 2 trial did not see a signal of increased mortality at 5 years. Although they did acknowledge that, among the randomized patients, there was a significantly higher mortality at 5 years for those treated with paclitaxel.
How do we make sense of this? Pac-litaxel is a cytotoxic drug. Its pharmacokinetics vary significantly based on the preparation and administration. The FDA label for the injectable form (Taxol) warns of anaphylaxis and severe hypersensitivity reactions, but there is no mention of long-term mortality. In the coronary vessels, paclitaxel-coated devices have been associated with myocardial infarction and death. Obviously it is easy to comprehend how local vessel effects in the coronary system can lead to increased mortality. The pathway is less clear with femoral-popliteal interventions. If the association of paclitaxel with death is truly causation there must be some systemic effects. The dose delivered with femoral- popliteal interventions is much higher than that seen with coronary devices.
The mortality may be associated with the platform used or even the formulation (crystalline formularies have a longer half-life). Could it be something more benign? Paclitaxel-treated patients see less recurrence of their femoral-popliteal disease. Are the control group patients with more recurrences seeing their interventionalist more often and therefore receiving more frequent reminders to comply with medical therapy?
At this point, we have few answers. After an all-day town hall at the recent Cardiovascular Research Technologies conference,9 one moderator said, “I came in with uncertainty and now I’m going away with uncertainty, but we made tremendous progress.” His comoderator added, “I know I don’t know.” Well then, glad we cleared that up!
In any event, changes are coming. The BASIL-3 trial has suspended recruitment. Physicians using paclitaxel-coated devices are now advised by the FDA to inform patients of the increased risk of death and to use alternatives in most cases. Therefore, if you employ these devices routinely in the femoral-popliteal vessels you are seemingly doing so in opposition to the recommendations of the FDA. Legal peril may follow.
The time for nitpicking the Katsanos analysis has ended. Our industry partners must be compelled to supply the data and finances needed to settle this issue. The signal seems real and it is time to find answers. Research initiatives are underway through the SVS, the VIVA group, the UK Medicines and Healthcare Products Regulatory Agency, and the FDA.
Going forward, the SVS has formed a Paclitaxel Safety Task Force under the leadership of President-elect Kim Hodgson. Their mission is to facilitate the performance and interpretation of an Individual Patient Data meta-analysis using patient-level RCT data from industry partners. The task force states: “We remain troubled by the recent reports of reanalysis of existing datasets, pooled analyses of RCTs, and other ‘series’, as we believe that the findings of these statistically inferior analyses bring no additional clarity, cannot be relied upon for guidance, and distract us from the analysis that needs to be performed.”
References
1. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.
2. www.fda.gov/medicaldevices/safety/letterstohealthcareproviders/ucm629589.htm.
3. J Am Coll Cardiol. Jan 2019. doi: 10.1016/j.jacc.2019.01.013.
4. Circulation. 2019;139:e42.
5. https://evtoday.com/2019/02/20/zilver-ptx-trial-5-year-mortality-data-corrected-in-circulation.
6. JAMA Cardiol. 2019 Feb 12. doi:10.1001/jamacardio.2019.0325.
7. www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm.
8. www.med-technews.com/news/bud-defends-safety-of-drug-coated-device-following-fda-warnin/.
9. www.crtonline.org/news-detail/paclitaxel-device-safety-thoroughly-discussed-at-c.
As medical editor of Vascular Specialist, it has always been my hope to use our excellent reporters and rapid production schedule to keep readers abreast of the latest news in vascular surgery. While my colleagues at the Journal of Vascular Surgery publish studies that will drive treatment, my goal is to drive discussion.
With topics like burnout, workforce shortages, and electronic medical records, I feel we have been successful. The downside of staying current is we sometimes find ourselves publishing contradictory stories. This has been the case with paclitaxel. Let’s take a break from the fray and review where we are, and where we might go from here.
In 2012, the Zilver PTX became the first drug-eluting stent (DES) to gain Food and Drug Administration approval for the treatment of peripheral vascular disease. Two years later, the FDA approved the Lutonix 035 as the first drug-coated balloon (DCB) for use in the femoral-popliteal arteries. The Lutonix would also gain a second indication for failing dialysis fistulas. Medtronic and Spectranetics received authorizations for their DCBs in 2015 and 2017, respectively.
While the safety of paclitaxel-coated devices in the coronary system had previously been called into question, the drug was generally considered safe and effective in the peripheral arterial system. The controversy began in December 2018, when Katsanos et al.1 published a meta-analysis of 28 randomized, controlled trials (RCTs) investigating paclitaxel-coated devices in the femoral-popliteal arteries. While all-cause patient mortality was similar at 1 year between paclitaxel-coated devices and controls (2.3% in each), at 2 years the risk of death was significantly higher in those treated with paclitaxel (7.2% vs. 3.8%). The 5-year data were available for three trials where there was a continued significantly increased risk of mortality with paclitaxel (14.7% vs. 8.1%).
Opposition to these findings was prompt from both physicians and industry. Weaknesses of the analysis, both perceived and real, were hammered. The meta-analysis did not include individual patient data, and the actual cause of death was unknown in most of the included trials. The study was not adequately powered to eliminate the risk of type 1 error when comparing mortality after 2 years. Individuals assigned to the control group may have received paclitaxel treatment at some point in their follow-up. The DCB and DES treatment groups were combined. The methods employed by the authors, however, stood up reasonably well to scrutiny.
On Jan. 17, 2019, the FDA issued their first response stating, “the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”2
Later that month, Peter Schneider, MD, and associates published a patient-level meta-analysis in the Journal of the American College of Cardiology.3 The study included 1,980 patients and found no statistically significant difference in all-cause mortality between DCB (9.3%) and percutaneous transluminal angioplasty (PTA) (11.2%) through 5 years. Shortly after that, however, a correction was issued.
On Feb. 15, 2019, Medtronic reported an error in the 2- and 3-year follow-up periods for the IN.PACT Global postmarket study. The company stated, “Due to a programming error, mortality data were inadvertently omitted from the summary tables included in the statistical analysis.” The mortality in the DCB cohort was corrected from 9.30% to 15.12%. The authors stated that this new mortality rate was still not significantly higher than the PTA group (P = .09).4
Less than 1 week later, another device company issued a correction. And once again, the error had been made in favor of the paclitaxel-treated group. In 2016, the 5-year data from Cook Medical’s Zilver PTX trial were published in Circulation. The study reported a mortality of 10.2% in the DES group and 16.9% in the PTA cohort. Regrettably, these numbers were reversed and significantly higher in the paclitaxel-treated group (16.9% vs. 10.2%, P = .03).5
On Feb. 12, 2019, another response to the Katsanos meta-analysis was published in JAMA Cardiology.6 In this study, Secemsky et al. analyzed patient-level data from a Medicare database. The authors reported finding no evidence of paclitaxel- related deaths in 16,560 patients. Unfortunately, the mean follow-up time was only 389 days, which may have been insufficient to detect the late mortality reported in the Katsanos meta-analysis.
On March 15, 2019, the FDA issued a second statement, this time with a much stronger tone.7 The agency reported an ongoing analysis of the long-term survival data from the pivotal randomized trials. In the three studies with 5-year data available, each showed a significantly higher mortality in the paclitaxel group.
When pooled, there were 975 patients, and the risk of death was 20.1% in the paclitaxel group versus 13.4 % in the controls. The FDA recommended discussing the increased risk of mortality with all patients receiving paclitaxel therapy as part of the informed consent process. They also stated that for most patients alternative options should generally be used until additional analysis of the mortality risk is performed.
Industry bristled at this new, strongly worded statement. Becton Dickinson, makers of the Lutonix balloon, asserted that the FDA recommendation was based on “a limited review of data from less than 1,000 patients.”8 The company noted that its LEVANT 2 trial did not see a signal of increased mortality at 5 years. Although they did acknowledge that, among the randomized patients, there was a significantly higher mortality at 5 years for those treated with paclitaxel.
How do we make sense of this? Pac-litaxel is a cytotoxic drug. Its pharmacokinetics vary significantly based on the preparation and administration. The FDA label for the injectable form (Taxol) warns of anaphylaxis and severe hypersensitivity reactions, but there is no mention of long-term mortality. In the coronary vessels, paclitaxel-coated devices have been associated with myocardial infarction and death. Obviously it is easy to comprehend how local vessel effects in the coronary system can lead to increased mortality. The pathway is less clear with femoral-popliteal interventions. If the association of paclitaxel with death is truly causation there must be some systemic effects. The dose delivered with femoral- popliteal interventions is much higher than that seen with coronary devices.
The mortality may be associated with the platform used or even the formulation (crystalline formularies have a longer half-life). Could it be something more benign? Paclitaxel-treated patients see less recurrence of their femoral-popliteal disease. Are the control group patients with more recurrences seeing their interventionalist more often and therefore receiving more frequent reminders to comply with medical therapy?
At this point, we have few answers. After an all-day town hall at the recent Cardiovascular Research Technologies conference,9 one moderator said, “I came in with uncertainty and now I’m going away with uncertainty, but we made tremendous progress.” His comoderator added, “I know I don’t know.” Well then, glad we cleared that up!
In any event, changes are coming. The BASIL-3 trial has suspended recruitment. Physicians using paclitaxel-coated devices are now advised by the FDA to inform patients of the increased risk of death and to use alternatives in most cases. Therefore, if you employ these devices routinely in the femoral-popliteal vessels you are seemingly doing so in opposition to the recommendations of the FDA. Legal peril may follow.
The time for nitpicking the Katsanos analysis has ended. Our industry partners must be compelled to supply the data and finances needed to settle this issue. The signal seems real and it is time to find answers. Research initiatives are underway through the SVS, the VIVA group, the UK Medicines and Healthcare Products Regulatory Agency, and the FDA.
Going forward, the SVS has formed a Paclitaxel Safety Task Force under the leadership of President-elect Kim Hodgson. Their mission is to facilitate the performance and interpretation of an Individual Patient Data meta-analysis using patient-level RCT data from industry partners. The task force states: “We remain troubled by the recent reports of reanalysis of existing datasets, pooled analyses of RCTs, and other ‘series’, as we believe that the findings of these statistically inferior analyses bring no additional clarity, cannot be relied upon for guidance, and distract us from the analysis that needs to be performed.”
References
1. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.
2. www.fda.gov/medicaldevices/safety/letterstohealthcareproviders/ucm629589.htm.
3. J Am Coll Cardiol. Jan 2019. doi: 10.1016/j.jacc.2019.01.013.
4. Circulation. 2019;139:e42.
5. https://evtoday.com/2019/02/20/zilver-ptx-trial-5-year-mortality-data-corrected-in-circulation.
6. JAMA Cardiol. 2019 Feb 12. doi:10.1001/jamacardio.2019.0325.
7. www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm.
8. www.med-technews.com/news/bud-defends-safety-of-drug-coated-device-following-fda-warnin/.
9. www.crtonline.org/news-detail/paclitaxel-device-safety-thoroughly-discussed-at-c.
As medical editor of Vascular Specialist, it has always been my hope to use our excellent reporters and rapid production schedule to keep readers abreast of the latest news in vascular surgery. While my colleagues at the Journal of Vascular Surgery publish studies that will drive treatment, my goal is to drive discussion.
With topics like burnout, workforce shortages, and electronic medical records, I feel we have been successful. The downside of staying current is we sometimes find ourselves publishing contradictory stories. This has been the case with paclitaxel. Let’s take a break from the fray and review where we are, and where we might go from here.
In 2012, the Zilver PTX became the first drug-eluting stent (DES) to gain Food and Drug Administration approval for the treatment of peripheral vascular disease. Two years later, the FDA approved the Lutonix 035 as the first drug-coated balloon (DCB) for use in the femoral-popliteal arteries. The Lutonix would also gain a second indication for failing dialysis fistulas. Medtronic and Spectranetics received authorizations for their DCBs in 2015 and 2017, respectively.
While the safety of paclitaxel-coated devices in the coronary system had previously been called into question, the drug was generally considered safe and effective in the peripheral arterial system. The controversy began in December 2018, when Katsanos et al.1 published a meta-analysis of 28 randomized, controlled trials (RCTs) investigating paclitaxel-coated devices in the femoral-popliteal arteries. While all-cause patient mortality was similar at 1 year between paclitaxel-coated devices and controls (2.3% in each), at 2 years the risk of death was significantly higher in those treated with paclitaxel (7.2% vs. 3.8%). The 5-year data were available for three trials where there was a continued significantly increased risk of mortality with paclitaxel (14.7% vs. 8.1%).
Opposition to these findings was prompt from both physicians and industry. Weaknesses of the analysis, both perceived and real, were hammered. The meta-analysis did not include individual patient data, and the actual cause of death was unknown in most of the included trials. The study was not adequately powered to eliminate the risk of type 1 error when comparing mortality after 2 years. Individuals assigned to the control group may have received paclitaxel treatment at some point in their follow-up. The DCB and DES treatment groups were combined. The methods employed by the authors, however, stood up reasonably well to scrutiny.
On Jan. 17, 2019, the FDA issued their first response stating, “the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”2
Later that month, Peter Schneider, MD, and associates published a patient-level meta-analysis in the Journal of the American College of Cardiology.3 The study included 1,980 patients and found no statistically significant difference in all-cause mortality between DCB (9.3%) and percutaneous transluminal angioplasty (PTA) (11.2%) through 5 years. Shortly after that, however, a correction was issued.
On Feb. 15, 2019, Medtronic reported an error in the 2- and 3-year follow-up periods for the IN.PACT Global postmarket study. The company stated, “Due to a programming error, mortality data were inadvertently omitted from the summary tables included in the statistical analysis.” The mortality in the DCB cohort was corrected from 9.30% to 15.12%. The authors stated that this new mortality rate was still not significantly higher than the PTA group (P = .09).4
Less than 1 week later, another device company issued a correction. And once again, the error had been made in favor of the paclitaxel-treated group. In 2016, the 5-year data from Cook Medical’s Zilver PTX trial were published in Circulation. The study reported a mortality of 10.2% in the DES group and 16.9% in the PTA cohort. Regrettably, these numbers were reversed and significantly higher in the paclitaxel-treated group (16.9% vs. 10.2%, P = .03).5
On Feb. 12, 2019, another response to the Katsanos meta-analysis was published in JAMA Cardiology.6 In this study, Secemsky et al. analyzed patient-level data from a Medicare database. The authors reported finding no evidence of paclitaxel- related deaths in 16,560 patients. Unfortunately, the mean follow-up time was only 389 days, which may have been insufficient to detect the late mortality reported in the Katsanos meta-analysis.
On March 15, 2019, the FDA issued a second statement, this time with a much stronger tone.7 The agency reported an ongoing analysis of the long-term survival data from the pivotal randomized trials. In the three studies with 5-year data available, each showed a significantly higher mortality in the paclitaxel group.
When pooled, there were 975 patients, and the risk of death was 20.1% in the paclitaxel group versus 13.4 % in the controls. The FDA recommended discussing the increased risk of mortality with all patients receiving paclitaxel therapy as part of the informed consent process. They also stated that for most patients alternative options should generally be used until additional analysis of the mortality risk is performed.
Industry bristled at this new, strongly worded statement. Becton Dickinson, makers of the Lutonix balloon, asserted that the FDA recommendation was based on “a limited review of data from less than 1,000 patients.”8 The company noted that its LEVANT 2 trial did not see a signal of increased mortality at 5 years. Although they did acknowledge that, among the randomized patients, there was a significantly higher mortality at 5 years for those treated with paclitaxel.
How do we make sense of this? Pac-litaxel is a cytotoxic drug. Its pharmacokinetics vary significantly based on the preparation and administration. The FDA label for the injectable form (Taxol) warns of anaphylaxis and severe hypersensitivity reactions, but there is no mention of long-term mortality. In the coronary vessels, paclitaxel-coated devices have been associated with myocardial infarction and death. Obviously it is easy to comprehend how local vessel effects in the coronary system can lead to increased mortality. The pathway is less clear with femoral-popliteal interventions. If the association of paclitaxel with death is truly causation there must be some systemic effects. The dose delivered with femoral- popliteal interventions is much higher than that seen with coronary devices.
The mortality may be associated with the platform used or even the formulation (crystalline formularies have a longer half-life). Could it be something more benign? Paclitaxel-treated patients see less recurrence of their femoral-popliteal disease. Are the control group patients with more recurrences seeing their interventionalist more often and therefore receiving more frequent reminders to comply with medical therapy?
At this point, we have few answers. After an all-day town hall at the recent Cardiovascular Research Technologies conference,9 one moderator said, “I came in with uncertainty and now I’m going away with uncertainty, but we made tremendous progress.” His comoderator added, “I know I don’t know.” Well then, glad we cleared that up!
In any event, changes are coming. The BASIL-3 trial has suspended recruitment. Physicians using paclitaxel-coated devices are now advised by the FDA to inform patients of the increased risk of death and to use alternatives in most cases. Therefore, if you employ these devices routinely in the femoral-popliteal vessels you are seemingly doing so in opposition to the recommendations of the FDA. Legal peril may follow.
The time for nitpicking the Katsanos analysis has ended. Our industry partners must be compelled to supply the data and finances needed to settle this issue. The signal seems real and it is time to find answers. Research initiatives are underway through the SVS, the VIVA group, the UK Medicines and Healthcare Products Regulatory Agency, and the FDA.
Going forward, the SVS has formed a Paclitaxel Safety Task Force under the leadership of President-elect Kim Hodgson. Their mission is to facilitate the performance and interpretation of an Individual Patient Data meta-analysis using patient-level RCT data from industry partners. The task force states: “We remain troubled by the recent reports of reanalysis of existing datasets, pooled analyses of RCTs, and other ‘series’, as we believe that the findings of these statistically inferior analyses bring no additional clarity, cannot be relied upon for guidance, and distract us from the analysis that needs to be performed.”
References
1. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.
2. www.fda.gov/medicaldevices/safety/letterstohealthcareproviders/ucm629589.htm.
3. J Am Coll Cardiol. Jan 2019. doi: 10.1016/j.jacc.2019.01.013.
4. Circulation. 2019;139:e42.
5. https://evtoday.com/2019/02/20/zilver-ptx-trial-5-year-mortality-data-corrected-in-circulation.
6. JAMA Cardiol. 2019 Feb 12. doi:10.1001/jamacardio.2019.0325.
7. www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm.
8. www.med-technews.com/news/bud-defends-safety-of-drug-coated-device-following-fda-warnin/.
9. www.crtonline.org/news-detail/paclitaxel-device-safety-thoroughly-discussed-at-c.
The Sarcoma Journal enters its second full year with renewed commitment and energy
We begin this second full year publishing The Sarcoma Journal—Official Journal of the Sarcoma Foundation of AmericaTM—with renewed energy and dedication to its founding principle of communicating authoritative and comprehensive scientific information on the diagnosis and treatment of sarcomas and sarcoma subtypes.
Despite advances in treatment and management options for our patients, the need still exists for more effective treatment strategies, new treatment paradigms, and improved understanding of disease biology. This journal, along with its online platform, plays an important role in the dissemination of reliable, peer-reviewed content to the sarcoma community and aims to bridge the gap between bench and bedside.
To this end, we have again recruited an outstanding advisory board, many of whom have long-standing affiliations with the Sarcoma Foundation of America. With their help, the editorial staff and I will continue to build the journal and make it the number one academic and practice resource for the sarcoma community.
We invite you and your colleagues to submit original research, review articles, case reports, opinion pieces, and meeting summaries for publication. In this way we will create the robust forum we all desire.
We begin this second full year publishing The Sarcoma Journal—Official Journal of the Sarcoma Foundation of AmericaTM—with renewed energy and dedication to its founding principle of communicating authoritative and comprehensive scientific information on the diagnosis and treatment of sarcomas and sarcoma subtypes.
Despite advances in treatment and management options for our patients, the need still exists for more effective treatment strategies, new treatment paradigms, and improved understanding of disease biology. This journal, along with its online platform, plays an important role in the dissemination of reliable, peer-reviewed content to the sarcoma community and aims to bridge the gap between bench and bedside.
To this end, we have again recruited an outstanding advisory board, many of whom have long-standing affiliations with the Sarcoma Foundation of America. With their help, the editorial staff and I will continue to build the journal and make it the number one academic and practice resource for the sarcoma community.
We invite you and your colleagues to submit original research, review articles, case reports, opinion pieces, and meeting summaries for publication. In this way we will create the robust forum we all desire.
We begin this second full year publishing The Sarcoma Journal—Official Journal of the Sarcoma Foundation of AmericaTM—with renewed energy and dedication to its founding principle of communicating authoritative and comprehensive scientific information on the diagnosis and treatment of sarcomas and sarcoma subtypes.
Despite advances in treatment and management options for our patients, the need still exists for more effective treatment strategies, new treatment paradigms, and improved understanding of disease biology. This journal, along with its online platform, plays an important role in the dissemination of reliable, peer-reviewed content to the sarcoma community and aims to bridge the gap between bench and bedside.
To this end, we have again recruited an outstanding advisory board, many of whom have long-standing affiliations with the Sarcoma Foundation of America. With their help, the editorial staff and I will continue to build the journal and make it the number one academic and practice resource for the sarcoma community.
We invite you and your colleagues to submit original research, review articles, case reports, opinion pieces, and meeting summaries for publication. In this way we will create the robust forum we all desire.
What is your approach to the persistent occiput posterior malposition?
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.
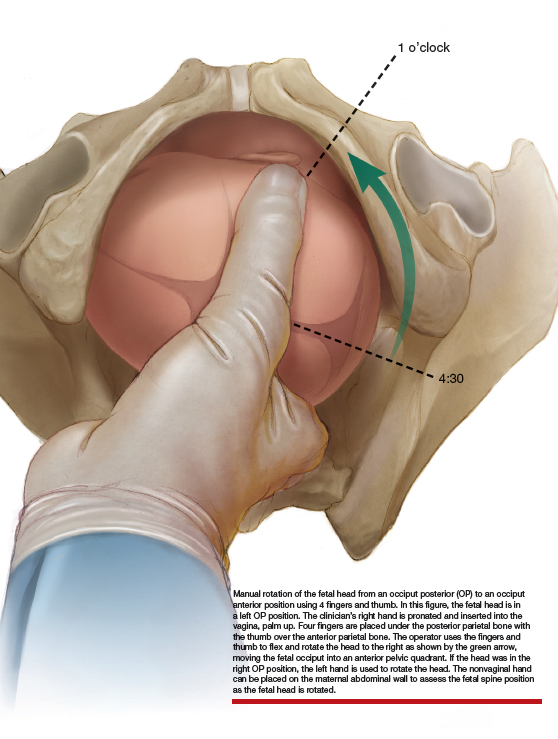
Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.