User login
Use, Interpretation of SpO2 Treatment for Pediatric Bronchiolitis Is Questioned
Clinical question: Does artificial elevation of pulse oximetry measurement in bronchiolitis patients during ED evaluations affect hospitalization rates?
Background: Bronchiolitis is the leading cause of hospitalization for infants younger than one year, leading to direct medical costs in the U.S. of $543 million in 2002. Compromised oxyhemoglobin saturation in bronchiolitis often leads to hospitalization and is assessed by pulse oximetry (SpO2) more commonly than by arterial blood gas (SaO2) due to ease, cost, and comfort considerations.
SpO2 can vary due to fever, acidosis, hemoglobinopathies, underperfusion, and poor probe placement. An American Academy of Pediatrics clinical practice guideline published in 2006 recommended supplemental oxygen if SpO2 drops below 90% in previously healthy infants, but the data supporting this cutoff are sparse.
Recommendations for supplemental oxygen, and thus hospitalization, are variable, with recommended minimum SpO2 ranging from 90 to 95%.
Study design: Single-center randomized, double-blind, parallel-group trial.
Setting: ED at 370-bed, urban, tertiary care children’s hospital.
Synopsis: Over a 50-month period, previously healthy infants aged four weeks to 12 months who were diagnosed with bronchiolitis in the ED and had initial triage SpO2 above 88% were enrolled by investigators. Subjects were excluded if severe respiratory distress, as measured by the Respiratory Distress Assessment Instrument (RDAI), or impending respiratory failure was present. Subjects were randomized to two groups: The control group had their true SpO2 displayed during their ED stay, and the intervention group had an SpO2 that was three points higher displayed. ED physicians were not aware of the primary hypothesis being tested. All subjects underwent concealed continuous oximetry for safety reasons, with monitors alarming if SpO2 dropped below 92%. Study nurses telephoned participants discharged home 72 hours after enrollment.
The primary outcome of hospitalization was defined as admission to an inpatient ward, hospital care provided for more than six hours in the ED if no hospital beds were available, or hospitalization after discharge if within 72 hours of enrollment. Secondary outcomes included supplemental oxygen administration, length of stay in the ED, and unscheduled return visits for bronchiolitis within 72 hours. Exploratory outcomes included delayed hospitalizations within 72 hours, active hospital treatment for more than six hours (with inhaled bronchodilators, oxygen, or intravenous fluids), and hospitalization at the index visit.
Of 1,812 patients assessed, 213 were randomized after exclusion criteria and consent. The “true” group and the “altered” group were similar in initial RDAI (8.0 vs. 8.3 respectively); 41% of children in the “true” oximetry group were hospitalized within 72 hours, compared with 25% in the “high” oximetry group (P 0.005). There were no significant differences in the secondary outcomes. The only exploratory outcome to show a significant difference was treatment for longer than six hours, with 37% of the “true group” receiving treatment for longer than six hours, compared to 20% of the “altered” group (P 0.01).
Bottom line: Perception of improved oxygenation based on falsely elevated SpO2 alone can reduce the inclination of a clinician to admit children with bronchiolitis. This brings into question the use and interpretation of SpO2 in treating children with bronchiolitis.
Citation: Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-728.

Clinical question: Does artificial elevation of pulse oximetry measurement in bronchiolitis patients during ED evaluations affect hospitalization rates?
Background: Bronchiolitis is the leading cause of hospitalization for infants younger than one year, leading to direct medical costs in the U.S. of $543 million in 2002. Compromised oxyhemoglobin saturation in bronchiolitis often leads to hospitalization and is assessed by pulse oximetry (SpO2) more commonly than by arterial blood gas (SaO2) due to ease, cost, and comfort considerations.
SpO2 can vary due to fever, acidosis, hemoglobinopathies, underperfusion, and poor probe placement. An American Academy of Pediatrics clinical practice guideline published in 2006 recommended supplemental oxygen if SpO2 drops below 90% in previously healthy infants, but the data supporting this cutoff are sparse.
Recommendations for supplemental oxygen, and thus hospitalization, are variable, with recommended minimum SpO2 ranging from 90 to 95%.
Study design: Single-center randomized, double-blind, parallel-group trial.
Setting: ED at 370-bed, urban, tertiary care children’s hospital.
Synopsis: Over a 50-month period, previously healthy infants aged four weeks to 12 months who were diagnosed with bronchiolitis in the ED and had initial triage SpO2 above 88% were enrolled by investigators. Subjects were excluded if severe respiratory distress, as measured by the Respiratory Distress Assessment Instrument (RDAI), or impending respiratory failure was present. Subjects were randomized to two groups: The control group had their true SpO2 displayed during their ED stay, and the intervention group had an SpO2 that was three points higher displayed. ED physicians were not aware of the primary hypothesis being tested. All subjects underwent concealed continuous oximetry for safety reasons, with monitors alarming if SpO2 dropped below 92%. Study nurses telephoned participants discharged home 72 hours after enrollment.
The primary outcome of hospitalization was defined as admission to an inpatient ward, hospital care provided for more than six hours in the ED if no hospital beds were available, or hospitalization after discharge if within 72 hours of enrollment. Secondary outcomes included supplemental oxygen administration, length of stay in the ED, and unscheduled return visits for bronchiolitis within 72 hours. Exploratory outcomes included delayed hospitalizations within 72 hours, active hospital treatment for more than six hours (with inhaled bronchodilators, oxygen, or intravenous fluids), and hospitalization at the index visit.
Of 1,812 patients assessed, 213 were randomized after exclusion criteria and consent. The “true” group and the “altered” group were similar in initial RDAI (8.0 vs. 8.3 respectively); 41% of children in the “true” oximetry group were hospitalized within 72 hours, compared with 25% in the “high” oximetry group (P 0.005). There were no significant differences in the secondary outcomes. The only exploratory outcome to show a significant difference was treatment for longer than six hours, with 37% of the “true group” receiving treatment for longer than six hours, compared to 20% of the “altered” group (P 0.01).
Bottom line: Perception of improved oxygenation based on falsely elevated SpO2 alone can reduce the inclination of a clinician to admit children with bronchiolitis. This brings into question the use and interpretation of SpO2 in treating children with bronchiolitis.
Citation: Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-728.

Clinical question: Does artificial elevation of pulse oximetry measurement in bronchiolitis patients during ED evaluations affect hospitalization rates?
Background: Bronchiolitis is the leading cause of hospitalization for infants younger than one year, leading to direct medical costs in the U.S. of $543 million in 2002. Compromised oxyhemoglobin saturation in bronchiolitis often leads to hospitalization and is assessed by pulse oximetry (SpO2) more commonly than by arterial blood gas (SaO2) due to ease, cost, and comfort considerations.
SpO2 can vary due to fever, acidosis, hemoglobinopathies, underperfusion, and poor probe placement. An American Academy of Pediatrics clinical practice guideline published in 2006 recommended supplemental oxygen if SpO2 drops below 90% in previously healthy infants, but the data supporting this cutoff are sparse.
Recommendations for supplemental oxygen, and thus hospitalization, are variable, with recommended minimum SpO2 ranging from 90 to 95%.
Study design: Single-center randomized, double-blind, parallel-group trial.
Setting: ED at 370-bed, urban, tertiary care children’s hospital.
Synopsis: Over a 50-month period, previously healthy infants aged four weeks to 12 months who were diagnosed with bronchiolitis in the ED and had initial triage SpO2 above 88% were enrolled by investigators. Subjects were excluded if severe respiratory distress, as measured by the Respiratory Distress Assessment Instrument (RDAI), or impending respiratory failure was present. Subjects were randomized to two groups: The control group had their true SpO2 displayed during their ED stay, and the intervention group had an SpO2 that was three points higher displayed. ED physicians were not aware of the primary hypothesis being tested. All subjects underwent concealed continuous oximetry for safety reasons, with monitors alarming if SpO2 dropped below 92%. Study nurses telephoned participants discharged home 72 hours after enrollment.
The primary outcome of hospitalization was defined as admission to an inpatient ward, hospital care provided for more than six hours in the ED if no hospital beds were available, or hospitalization after discharge if within 72 hours of enrollment. Secondary outcomes included supplemental oxygen administration, length of stay in the ED, and unscheduled return visits for bronchiolitis within 72 hours. Exploratory outcomes included delayed hospitalizations within 72 hours, active hospital treatment for more than six hours (with inhaled bronchodilators, oxygen, or intravenous fluids), and hospitalization at the index visit.
Of 1,812 patients assessed, 213 were randomized after exclusion criteria and consent. The “true” group and the “altered” group were similar in initial RDAI (8.0 vs. 8.3 respectively); 41% of children in the “true” oximetry group were hospitalized within 72 hours, compared with 25% in the “high” oximetry group (P 0.005). There were no significant differences in the secondary outcomes. The only exploratory outcome to show a significant difference was treatment for longer than six hours, with 37% of the “true group” receiving treatment for longer than six hours, compared to 20% of the “altered” group (P 0.01).
Bottom line: Perception of improved oxygenation based on falsely elevated SpO2 alone can reduce the inclination of a clinician to admit children with bronchiolitis. This brings into question the use and interpretation of SpO2 in treating children with bronchiolitis.
Citation: Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-728.

69%: hospitals with perfect hand-hygiene compliance
69%: the percentage of hospitals that had perfect compliance with the Leapfrog Group employer coalition’s safe practices for hand hygiene in its 2013 annual quality survey of 1,437 U.S. hospitals.
The CDC estimates 2 million patients annually acquire hospital-acquired infections (HAIs), often spread by contaminated hands of healthcare workers.
Urban hospitals performed better than rural hospitals in compliance with Leapfrog’s standard.
69%: the percentage of hospitals that had perfect compliance with the Leapfrog Group employer coalition’s safe practices for hand hygiene in its 2013 annual quality survey of 1,437 U.S. hospitals.
The CDC estimates 2 million patients annually acquire hospital-acquired infections (HAIs), often spread by contaminated hands of healthcare workers.
Urban hospitals performed better than rural hospitals in compliance with Leapfrog’s standard.
69%: the percentage of hospitals that had perfect compliance with the Leapfrog Group employer coalition’s safe practices for hand hygiene in its 2013 annual quality survey of 1,437 U.S. hospitals.
The CDC estimates 2 million patients annually acquire hospital-acquired infections (HAIs), often spread by contaminated hands of healthcare workers.
Urban hospitals performed better than rural hospitals in compliance with Leapfrog’s standard.
LISTEN NOW: Steve Pantilat, MD, SFHM, explains hospitalists' role in palliative care
LISTEN NOW: M.D. Anderson hospitalists discuss caring for cancer patients
Josiah Halm, MD, and Sahitya Gadiraju, DO, assistant professors of general internal medicine at M.D. Anderson Cancer Center in Houston, discuss the breadth of care provided to cancer patients, a risk assessment being developed there on readmission risk, and factors in care that go beyond the medical.
Josiah Halm, MD, and Sahitya Gadiraju, DO, assistant professors of general internal medicine at M.D. Anderson Cancer Center in Houston, discuss the breadth of care provided to cancer patients, a risk assessment being developed there on readmission risk, and factors in care that go beyond the medical.
Josiah Halm, MD, and Sahitya Gadiraju, DO, assistant professors of general internal medicine at M.D. Anderson Cancer Center in Houston, discuss the breadth of care provided to cancer patients, a risk assessment being developed there on readmission risk, and factors in care that go beyond the medical.
10 Things Oncologists Think Hospitalists Need to Know
Things you need to know
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
COMING UP: 10 Things Endocrinologists Want HM to Know Archived: @the-hospitalist.org
- 10 Things Infectious Disease
- 12 Things Cardiology
- 12 Things Nephrology
- 12 Things Billing & Coding
Cancer patients can be some of the most complicated and high-stakes patients who come into a hospitalist’s care.
The issues faced by such patients are three-pronged: Besides the effects of the cancer itself, these often elderly patients also grapple with the side effects of treatment and other medical issues.
The Hospitalist sought tips for caring for hospitalized cancer patients from a half-dozen experts in hematology and oncology. Here are the 10 most common pieces of advice they had for hospitalists caring for cancer patients.
1 Know the History
This includes the subtleties of the patient history, which can be quite involved, says Fadlo R. Khuri, MD, FACP, deputy director of the Winship Cancer Institute of Emory University and chair of hematology and medical oncology at the Emory University School of Medicine in Atlanta.
“Part of that history may be obtained from the patient and the patient’s family, but if the treatment has been evolving over time, you need to get in touch with the treating physician or at least have access to the records of the patient’s treatment,” he says. “The arsenal of drugs that we use against cancer has expanded dramatically and in different directions. Now we have tremendous technological innovations with very focused radiation or very refined surgery, and not just novel chemotherapy but also targeted therapies that can target a specific Achilles heel of cancer.”
Basically, it is important for hospitalists to know exactly “what you are dealing with.”
“That’s a lot of information that the hospitalist needs to know. Whom do I contact? Whom do I need to access, not just on the web, but in person, to understand what this patient is going through?” he adds.
With many patients, time is of the essence. This is part of the reason why it’s so important to get a complete history and full picture of a patient’s treatment right away, Dr. Khuri says.
“The patient with cancer often presents in worse shape than patients with other diseases,” he says. “Therefore, with patients with cancer or patients with other really life-threatening illness, you generally have less time to figure out what is going on.”
2 Communication Is Paramount
“The reason that communication is important is to convey the right message to the patient,” says Suresh Ramalingam, MD, professor and director of medical oncology and the lung cancer program at the Emory School of Medicine. “An oncologist who’s been following a patient for a year and a half…I would think has some insight that he or she can provide the hospitalist to manage the acute illness that the patient is admitted with.
“The other thing is many times a patient comes in the hospital and the first question they have is, ‘Does this mean my cancer is getting worse? What is the next option for me? And am I going to die right away?’ And they’re going to ask this question of whomever they see first. Having the oncologist’s thoughts on the patient’s overall status of cancer is important to address such issues.”
Dr. Ramalingam says that a situation that used to occur, but is now less frequent, is frantic calls from a patient in a hospital bed saying, “The hospitalist just walked in, and he said I’m going to die in three weeks. You never told me about that.”
When that happens, “we have to go back and talk to the patient and reassure the patient that that’s not the case,” Dr. Ramalingam says.
3 Treating Cancer Is More Than Treating Cancer
At the MD Anderson Cancer Center in Houston, where a pilot hospitalist program that began six years ago has grown into a permanent part of the center, treatment comes from all angles, not just medical, says Josiah Halm, MD, MS, FACP, FHM, CMQ, and Sahitya Gadiraju, DO, assistant professors of general internal medicine at the center.
“I think the biggest thing is to understand that a cancer patient is very complex and there’s much more than the physical component,” says Dr. Gadiraju, one of nine hospitalists at MD Anderson. “There’s an emotional component. There’s a mental component. There’s the family that’s involved.
“One of the biggest things that we do is not just support the patient physically and medically but also emotionally and mentally. And we provide very good family support working as part of an interdisciplinary team.”
4 Know the Baseline
Dr. Khuri says hospitalists should start by seeking answers to some simple questions.
“What kind of situation were they in when they began to deteriorate? Was this patient walking, talking, healthy, eating, working? And is this an acute deterioration, or is this a gradual deterioration?” he says.
The hospitalist caring for a patient with an acute decline might play a major role in the outcome.
“Some of these acute, precipitating events may be treatable, and the hospitalist may be—forgive my language—Johnny-on-the-spot—and may be able to make a major difference in turning that patient around,” he says.
5 Fight for DVT prophylaxis
When patients should be given prophylaxis for DVT, do not be deterred from doing so by the treating oncologist, says Efrén Manjarrez, MD, SFHM, assistant professor of medicine and interim chief of the division of hospital medicine and patient safety officer for the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine. For patients undergoing chemotherapy, oncologists might be concerned about the potential for bleeding events, but it’s important to “get with the guidelines,” Dr. Manjarrez says.
“Oftentimes, hospitalists can be undermined by the oncologists that they’re managing their patients with,” he says. “Make sure that you stick to your guns and make sure that you’re strong about giving DVT prophylaxis to these patients, unless they truly meet exclusion criteria for that prophylaxis.
“Sometimes, hematologists or oncologists might actually cancel your order.”
6 ‘More Is Better’ for Genome Analysis
With a fine-needle biopsy, there might not be enough specimen left for molecular analysis, Dr. Ramalingam explains.
“The purpose of the biopsy is no longer just diagnostic; it has significant therapeutic implications. Therefore, getting as much tissue [as possible] during that initial diagnostic biopsy is very helpful, because we conduct detailed molecular studies on these specimens,” he says. “If you don’t get enough specimen in the first biopsy, but you just have enough to make a diagnosis of the type of cancer, then you have to resort to a second biopsy. So, more is better when it comes to tissue.”
7 Consider Pediatric Test Tubes for Pancytopenic Patients
Using smaller test tubes will lower the potential for anemia caused by frequent blood draws, Dr. Manjarrez says. Recent evidence suggests that hospital-acquired anemia prolongs hospital costs, length of stay, and mortality risk—all directly proportional to the level of anemia.1
“We’re causing [patients] to be more anemic with blood draws,” he says. “When you have cancer patients who get chemotherapy, their bone marrow is wiped out by the chemotherapy. So what happens is that you end up in the cycle where you have to keep transfusing these patients. The more blood draws that you get from them, the more we’re exacerbating it.”
8 Respect Your Turf, Their Turf
Dr. Manjarrez says the best way to ensure the hem-onc specialists respect the hospitalist’s turf, and vice versa, is to discuss the treatment parameters ahead of time.
“Try and negotiate comanagement deals with your hematologist-oncologist colleagues before you enter into comanagement relationships with them,” he says.
One particularly sticky situation is when a patient is admitted with the expectation that the hospitalist will be caring for acute issues like infection or cancer-related pain, but then the hospitalization is extended because the oncologist wants to start chemotherapy.
“That can be a problem,” he says. “Agree with your hematology-oncology colleagues what you’re going to do in advance, as much as you can.”

“Oftentimes, hospitalists can be undermined by the oncologists that they’re managing their patients with. Make sure that you stick to your guns and make sure that you’re strong about giving DVT prophylaxis to these patients, unless they truly meet exclusion criteria for that prophylaxis.”
—Efrén Manjarrez, MD, SFHM, assistant professor of medicine, interim chief, division of hospital medicine, patient safety officer, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
9 Be Cautious in Using Granulocyte Colony-Stimulating Factor (GCSF)
The medication is used to stimulate the body to produce more white blood cells, which sometimes is needed after chemotherapy. They are good for certain situations but should be handled with care, says Lowell Schnipper, MD, clinical director of the Beth Israel Deaconess Medical Center Cancer Center in Boston.
“Because it’s unnecessary and very expensive,” says Dr. Schnipper, who is chair of the American Society of Clinical Oncology’s Value of Care Task Force. “If this is a chemotherapy regimen that has a risk of fever and neutropenia in the context of the chemotherapy, [and] the odds of having that complication are 20% percent or higher with a chemotherapy regimen, we suggest using GCSF.”
If not, then GCSF should be avoided, he says.
Such decisions likely will fall to the treating oncologist, but Dr. Schnipper says it is a topic with which hospitalists should be familiar.
10 Rethink Imaging
“If you get a PET scan in the hospital and a patient is admitted for a different diagnosis, there’s a good likelihood that it’s not going to be reimbursed,” Dr. Ramalingam says.
Plus, he says, a scan done in the hospital could cloud the radiographic findings used to make decisions.
“For instance, for someone with pneumonia, the infiltrate might be difficult to differentiate from cancer,” he says.
Tom Collins is a freelance author in South Florida and longtime contributor to The Hospitalist.
Reference
Things you need to know
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
COMING UP: 10 Things Endocrinologists Want HM to Know Archived: @the-hospitalist.org
- 10 Things Infectious Disease
- 12 Things Cardiology
- 12 Things Nephrology
- 12 Things Billing & Coding
Cancer patients can be some of the most complicated and high-stakes patients who come into a hospitalist’s care.
The issues faced by such patients are three-pronged: Besides the effects of the cancer itself, these often elderly patients also grapple with the side effects of treatment and other medical issues.
The Hospitalist sought tips for caring for hospitalized cancer patients from a half-dozen experts in hematology and oncology. Here are the 10 most common pieces of advice they had for hospitalists caring for cancer patients.
1 Know the History
This includes the subtleties of the patient history, which can be quite involved, says Fadlo R. Khuri, MD, FACP, deputy director of the Winship Cancer Institute of Emory University and chair of hematology and medical oncology at the Emory University School of Medicine in Atlanta.
“Part of that history may be obtained from the patient and the patient’s family, but if the treatment has been evolving over time, you need to get in touch with the treating physician or at least have access to the records of the patient’s treatment,” he says. “The arsenal of drugs that we use against cancer has expanded dramatically and in different directions. Now we have tremendous technological innovations with very focused radiation or very refined surgery, and not just novel chemotherapy but also targeted therapies that can target a specific Achilles heel of cancer.”
Basically, it is important for hospitalists to know exactly “what you are dealing with.”
“That’s a lot of information that the hospitalist needs to know. Whom do I contact? Whom do I need to access, not just on the web, but in person, to understand what this patient is going through?” he adds.
With many patients, time is of the essence. This is part of the reason why it’s so important to get a complete history and full picture of a patient’s treatment right away, Dr. Khuri says.
“The patient with cancer often presents in worse shape than patients with other diseases,” he says. “Therefore, with patients with cancer or patients with other really life-threatening illness, you generally have less time to figure out what is going on.”
2 Communication Is Paramount
“The reason that communication is important is to convey the right message to the patient,” says Suresh Ramalingam, MD, professor and director of medical oncology and the lung cancer program at the Emory School of Medicine. “An oncologist who’s been following a patient for a year and a half…I would think has some insight that he or she can provide the hospitalist to manage the acute illness that the patient is admitted with.
“The other thing is many times a patient comes in the hospital and the first question they have is, ‘Does this mean my cancer is getting worse? What is the next option for me? And am I going to die right away?’ And they’re going to ask this question of whomever they see first. Having the oncologist’s thoughts on the patient’s overall status of cancer is important to address such issues.”
Dr. Ramalingam says that a situation that used to occur, but is now less frequent, is frantic calls from a patient in a hospital bed saying, “The hospitalist just walked in, and he said I’m going to die in three weeks. You never told me about that.”
When that happens, “we have to go back and talk to the patient and reassure the patient that that’s not the case,” Dr. Ramalingam says.
3 Treating Cancer Is More Than Treating Cancer
At the MD Anderson Cancer Center in Houston, where a pilot hospitalist program that began six years ago has grown into a permanent part of the center, treatment comes from all angles, not just medical, says Josiah Halm, MD, MS, FACP, FHM, CMQ, and Sahitya Gadiraju, DO, assistant professors of general internal medicine at the center.
“I think the biggest thing is to understand that a cancer patient is very complex and there’s much more than the physical component,” says Dr. Gadiraju, one of nine hospitalists at MD Anderson. “There’s an emotional component. There’s a mental component. There’s the family that’s involved.
“One of the biggest things that we do is not just support the patient physically and medically but also emotionally and mentally. And we provide very good family support working as part of an interdisciplinary team.”
4 Know the Baseline
Dr. Khuri says hospitalists should start by seeking answers to some simple questions.
“What kind of situation were they in when they began to deteriorate? Was this patient walking, talking, healthy, eating, working? And is this an acute deterioration, or is this a gradual deterioration?” he says.
The hospitalist caring for a patient with an acute decline might play a major role in the outcome.
“Some of these acute, precipitating events may be treatable, and the hospitalist may be—forgive my language—Johnny-on-the-spot—and may be able to make a major difference in turning that patient around,” he says.
5 Fight for DVT prophylaxis
When patients should be given prophylaxis for DVT, do not be deterred from doing so by the treating oncologist, says Efrén Manjarrez, MD, SFHM, assistant professor of medicine and interim chief of the division of hospital medicine and patient safety officer for the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine. For patients undergoing chemotherapy, oncologists might be concerned about the potential for bleeding events, but it’s important to “get with the guidelines,” Dr. Manjarrez says.
“Oftentimes, hospitalists can be undermined by the oncologists that they’re managing their patients with,” he says. “Make sure that you stick to your guns and make sure that you’re strong about giving DVT prophylaxis to these patients, unless they truly meet exclusion criteria for that prophylaxis.
“Sometimes, hematologists or oncologists might actually cancel your order.”
6 ‘More Is Better’ for Genome Analysis
With a fine-needle biopsy, there might not be enough specimen left for molecular analysis, Dr. Ramalingam explains.
“The purpose of the biopsy is no longer just diagnostic; it has significant therapeutic implications. Therefore, getting as much tissue [as possible] during that initial diagnostic biopsy is very helpful, because we conduct detailed molecular studies on these specimens,” he says. “If you don’t get enough specimen in the first biopsy, but you just have enough to make a diagnosis of the type of cancer, then you have to resort to a second biopsy. So, more is better when it comes to tissue.”
7 Consider Pediatric Test Tubes for Pancytopenic Patients
Using smaller test tubes will lower the potential for anemia caused by frequent blood draws, Dr. Manjarrez says. Recent evidence suggests that hospital-acquired anemia prolongs hospital costs, length of stay, and mortality risk—all directly proportional to the level of anemia.1
“We’re causing [patients] to be more anemic with blood draws,” he says. “When you have cancer patients who get chemotherapy, their bone marrow is wiped out by the chemotherapy. So what happens is that you end up in the cycle where you have to keep transfusing these patients. The more blood draws that you get from them, the more we’re exacerbating it.”
8 Respect Your Turf, Their Turf
Dr. Manjarrez says the best way to ensure the hem-onc specialists respect the hospitalist’s turf, and vice versa, is to discuss the treatment parameters ahead of time.
“Try and negotiate comanagement deals with your hematologist-oncologist colleagues before you enter into comanagement relationships with them,” he says.
One particularly sticky situation is when a patient is admitted with the expectation that the hospitalist will be caring for acute issues like infection or cancer-related pain, but then the hospitalization is extended because the oncologist wants to start chemotherapy.
“That can be a problem,” he says. “Agree with your hematology-oncology colleagues what you’re going to do in advance, as much as you can.”

“Oftentimes, hospitalists can be undermined by the oncologists that they’re managing their patients with. Make sure that you stick to your guns and make sure that you’re strong about giving DVT prophylaxis to these patients, unless they truly meet exclusion criteria for that prophylaxis.”
—Efrén Manjarrez, MD, SFHM, assistant professor of medicine, interim chief, division of hospital medicine, patient safety officer, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
9 Be Cautious in Using Granulocyte Colony-Stimulating Factor (GCSF)
The medication is used to stimulate the body to produce more white blood cells, which sometimes is needed after chemotherapy. They are good for certain situations but should be handled with care, says Lowell Schnipper, MD, clinical director of the Beth Israel Deaconess Medical Center Cancer Center in Boston.
“Because it’s unnecessary and very expensive,” says Dr. Schnipper, who is chair of the American Society of Clinical Oncology’s Value of Care Task Force. “If this is a chemotherapy regimen that has a risk of fever and neutropenia in the context of the chemotherapy, [and] the odds of having that complication are 20% percent or higher with a chemotherapy regimen, we suggest using GCSF.”
If not, then GCSF should be avoided, he says.
Such decisions likely will fall to the treating oncologist, but Dr. Schnipper says it is a topic with which hospitalists should be familiar.
10 Rethink Imaging
“If you get a PET scan in the hospital and a patient is admitted for a different diagnosis, there’s a good likelihood that it’s not going to be reimbursed,” Dr. Ramalingam says.
Plus, he says, a scan done in the hospital could cloud the radiographic findings used to make decisions.
“For instance, for someone with pneumonia, the infiltrate might be difficult to differentiate from cancer,” he says.
Tom Collins is a freelance author in South Florida and longtime contributor to The Hospitalist.
Reference
Things you need to know
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
COMING UP: 10 Things Endocrinologists Want HM to Know Archived: @the-hospitalist.org
- 10 Things Infectious Disease
- 12 Things Cardiology
- 12 Things Nephrology
- 12 Things Billing & Coding
Cancer patients can be some of the most complicated and high-stakes patients who come into a hospitalist’s care.
The issues faced by such patients are three-pronged: Besides the effects of the cancer itself, these often elderly patients also grapple with the side effects of treatment and other medical issues.
The Hospitalist sought tips for caring for hospitalized cancer patients from a half-dozen experts in hematology and oncology. Here are the 10 most common pieces of advice they had for hospitalists caring for cancer patients.
1 Know the History
This includes the subtleties of the patient history, which can be quite involved, says Fadlo R. Khuri, MD, FACP, deputy director of the Winship Cancer Institute of Emory University and chair of hematology and medical oncology at the Emory University School of Medicine in Atlanta.
“Part of that history may be obtained from the patient and the patient’s family, but if the treatment has been evolving over time, you need to get in touch with the treating physician or at least have access to the records of the patient’s treatment,” he says. “The arsenal of drugs that we use against cancer has expanded dramatically and in different directions. Now we have tremendous technological innovations with very focused radiation or very refined surgery, and not just novel chemotherapy but also targeted therapies that can target a specific Achilles heel of cancer.”
Basically, it is important for hospitalists to know exactly “what you are dealing with.”
“That’s a lot of information that the hospitalist needs to know. Whom do I contact? Whom do I need to access, not just on the web, but in person, to understand what this patient is going through?” he adds.
With many patients, time is of the essence. This is part of the reason why it’s so important to get a complete history and full picture of a patient’s treatment right away, Dr. Khuri says.
“The patient with cancer often presents in worse shape than patients with other diseases,” he says. “Therefore, with patients with cancer or patients with other really life-threatening illness, you generally have less time to figure out what is going on.”
2 Communication Is Paramount
“The reason that communication is important is to convey the right message to the patient,” says Suresh Ramalingam, MD, professor and director of medical oncology and the lung cancer program at the Emory School of Medicine. “An oncologist who’s been following a patient for a year and a half…I would think has some insight that he or she can provide the hospitalist to manage the acute illness that the patient is admitted with.
“The other thing is many times a patient comes in the hospital and the first question they have is, ‘Does this mean my cancer is getting worse? What is the next option for me? And am I going to die right away?’ And they’re going to ask this question of whomever they see first. Having the oncologist’s thoughts on the patient’s overall status of cancer is important to address such issues.”
Dr. Ramalingam says that a situation that used to occur, but is now less frequent, is frantic calls from a patient in a hospital bed saying, “The hospitalist just walked in, and he said I’m going to die in three weeks. You never told me about that.”
When that happens, “we have to go back and talk to the patient and reassure the patient that that’s not the case,” Dr. Ramalingam says.
3 Treating Cancer Is More Than Treating Cancer
At the MD Anderson Cancer Center in Houston, where a pilot hospitalist program that began six years ago has grown into a permanent part of the center, treatment comes from all angles, not just medical, says Josiah Halm, MD, MS, FACP, FHM, CMQ, and Sahitya Gadiraju, DO, assistant professors of general internal medicine at the center.
“I think the biggest thing is to understand that a cancer patient is very complex and there’s much more than the physical component,” says Dr. Gadiraju, one of nine hospitalists at MD Anderson. “There’s an emotional component. There’s a mental component. There’s the family that’s involved.
“One of the biggest things that we do is not just support the patient physically and medically but also emotionally and mentally. And we provide very good family support working as part of an interdisciplinary team.”
4 Know the Baseline
Dr. Khuri says hospitalists should start by seeking answers to some simple questions.
“What kind of situation were they in when they began to deteriorate? Was this patient walking, talking, healthy, eating, working? And is this an acute deterioration, or is this a gradual deterioration?” he says.
The hospitalist caring for a patient with an acute decline might play a major role in the outcome.
“Some of these acute, precipitating events may be treatable, and the hospitalist may be—forgive my language—Johnny-on-the-spot—and may be able to make a major difference in turning that patient around,” he says.
5 Fight for DVT prophylaxis
When patients should be given prophylaxis for DVT, do not be deterred from doing so by the treating oncologist, says Efrén Manjarrez, MD, SFHM, assistant professor of medicine and interim chief of the division of hospital medicine and patient safety officer for the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine. For patients undergoing chemotherapy, oncologists might be concerned about the potential for bleeding events, but it’s important to “get with the guidelines,” Dr. Manjarrez says.
“Oftentimes, hospitalists can be undermined by the oncologists that they’re managing their patients with,” he says. “Make sure that you stick to your guns and make sure that you’re strong about giving DVT prophylaxis to these patients, unless they truly meet exclusion criteria for that prophylaxis.
“Sometimes, hematologists or oncologists might actually cancel your order.”
6 ‘More Is Better’ for Genome Analysis
With a fine-needle biopsy, there might not be enough specimen left for molecular analysis, Dr. Ramalingam explains.
“The purpose of the biopsy is no longer just diagnostic; it has significant therapeutic implications. Therefore, getting as much tissue [as possible] during that initial diagnostic biopsy is very helpful, because we conduct detailed molecular studies on these specimens,” he says. “If you don’t get enough specimen in the first biopsy, but you just have enough to make a diagnosis of the type of cancer, then you have to resort to a second biopsy. So, more is better when it comes to tissue.”
7 Consider Pediatric Test Tubes for Pancytopenic Patients
Using smaller test tubes will lower the potential for anemia caused by frequent blood draws, Dr. Manjarrez says. Recent evidence suggests that hospital-acquired anemia prolongs hospital costs, length of stay, and mortality risk—all directly proportional to the level of anemia.1
“We’re causing [patients] to be more anemic with blood draws,” he says. “When you have cancer patients who get chemotherapy, their bone marrow is wiped out by the chemotherapy. So what happens is that you end up in the cycle where you have to keep transfusing these patients. The more blood draws that you get from them, the more we’re exacerbating it.”
8 Respect Your Turf, Their Turf
Dr. Manjarrez says the best way to ensure the hem-onc specialists respect the hospitalist’s turf, and vice versa, is to discuss the treatment parameters ahead of time.
“Try and negotiate comanagement deals with your hematologist-oncologist colleagues before you enter into comanagement relationships with them,” he says.
One particularly sticky situation is when a patient is admitted with the expectation that the hospitalist will be caring for acute issues like infection or cancer-related pain, but then the hospitalization is extended because the oncologist wants to start chemotherapy.
“That can be a problem,” he says. “Agree with your hematology-oncology colleagues what you’re going to do in advance, as much as you can.”

“Oftentimes, hospitalists can be undermined by the oncologists that they’re managing their patients with. Make sure that you stick to your guns and make sure that you’re strong about giving DVT prophylaxis to these patients, unless they truly meet exclusion criteria for that prophylaxis.”
—Efrén Manjarrez, MD, SFHM, assistant professor of medicine, interim chief, division of hospital medicine, patient safety officer, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
9 Be Cautious in Using Granulocyte Colony-Stimulating Factor (GCSF)
The medication is used to stimulate the body to produce more white blood cells, which sometimes is needed after chemotherapy. They are good for certain situations but should be handled with care, says Lowell Schnipper, MD, clinical director of the Beth Israel Deaconess Medical Center Cancer Center in Boston.
“Because it’s unnecessary and very expensive,” says Dr. Schnipper, who is chair of the American Society of Clinical Oncology’s Value of Care Task Force. “If this is a chemotherapy regimen that has a risk of fever and neutropenia in the context of the chemotherapy, [and] the odds of having that complication are 20% percent or higher with a chemotherapy regimen, we suggest using GCSF.”
If not, then GCSF should be avoided, he says.
Such decisions likely will fall to the treating oncologist, but Dr. Schnipper says it is a topic with which hospitalists should be familiar.
10 Rethink Imaging
“If you get a PET scan in the hospital and a patient is admitted for a different diagnosis, there’s a good likelihood that it’s not going to be reimbursed,” Dr. Ramalingam says.
Plus, he says, a scan done in the hospital could cloud the radiographic findings used to make decisions.
“For instance, for someone with pneumonia, the infiltrate might be difficult to differentiate from cancer,” he says.
Tom Collins is a freelance author in South Florida and longtime contributor to The Hospitalist.
Reference
Should Patients with an Unprovoked VTE Be Screened for Malignancy or a Hypercoagulable State?
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
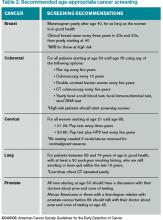
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
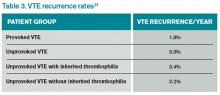
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Early Warning System Boosts Sepsis Detection, Care
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
LISTEN NOW: Emergency Medicine and Hospitalist Collaboration
The focus for emergency physicians, says Dr. Heinrich, is triage and disposition. Differing incentives for hospitalists and emergency physicians can cause stress between the groups, and dialogue is needed to defray the tension, he notes. Dr. Epstein says he thinks that collaboration can be an effective tactic against becoming a “30 day readmission rule” statistic. Shared metrics, developed in partnership, can also improve patient care, he adds.
For more features, visit The Hospitalist's podcast archive.
The focus for emergency physicians, says Dr. Heinrich, is triage and disposition. Differing incentives for hospitalists and emergency physicians can cause stress between the groups, and dialogue is needed to defray the tension, he notes. Dr. Epstein says he thinks that collaboration can be an effective tactic against becoming a “30 day readmission rule” statistic. Shared metrics, developed in partnership, can also improve patient care, he adds.
For more features, visit The Hospitalist's podcast archive.
The focus for emergency physicians, says Dr. Heinrich, is triage and disposition. Differing incentives for hospitalists and emergency physicians can cause stress between the groups, and dialogue is needed to defray the tension, he notes. Dr. Epstein says he thinks that collaboration can be an effective tactic against becoming a “30 day readmission rule” statistic. Shared metrics, developed in partnership, can also improve patient care, he adds.
For more features, visit The Hospitalist's podcast archive.
Hospitalists Channel Osler, Pioneer in Bedside Exams
Hands-on workshop helps hospitalists gain confidence in fundamentals, learn to teach physical exam skills better
Hands-on workshop helps hospitalists gain confidence in fundamentals, learn to teach physical exam skills better
Hands-on workshop helps hospitalists gain confidence in fundamentals, learn to teach physical exam skills better
LISTEN NOW: Dr. Kendall Rogers, MD, SFHM, Encourages Hospitalists to Work as Part of Quality Teams to Achieve Glycemic Control
As SHM's glycemic control lead mentor and a hospitalist at the University of New Mexico in Albuquerque, Kendall Rogers, MD, CPE, FACP, SFHM, offers advice to hospitalists when working as part of a quality team in achieving glycemic control.
As SHM's glycemic control lead mentor and a hospitalist at the University of New Mexico in Albuquerque, Kendall Rogers, MD, CPE, FACP, SFHM, offers advice to hospitalists when working as part of a quality team in achieving glycemic control.
As SHM's glycemic control lead mentor and a hospitalist at the University of New Mexico in Albuquerque, Kendall Rogers, MD, CPE, FACP, SFHM, offers advice to hospitalists when working as part of a quality team in achieving glycemic control.