User login
Dabigatran crushes warfarin for AF ablation
Washington – Uninterrupted dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation proved far superior to uninterrupted warfarin – the current standard – in the randomized multicenter RE-CIRCUIT trial, Hugh Calkins, MD, reported at the annual meeting of the American College of Cardiology.
The primary study endpoint – the incidence of major bleeding events from the time of the first femoral puncture at the procedure’s start through the subsequent 8 weeks – occurred in 1.6% of the dabigatran (Pradaxa) group and 6.9% of the warfarin group, for an absolute 5.9% reduction in risk and a 77% relative risk reduction favoring the novel anticoagulant.
“This trial will definitely affect my own practice, and I think it will quickly affect the practices of electrophysiologists around the world,” declared Dr. Calkins, professor of cardiology and medicine and director of the clinical electrophysiology laboratory and the arrhythmia service at Johns Hopkins University, Baltimore.
RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy) was a multicenter, prospective, international trial conducted in 635 atrial fibrillation (AF) patients who underwent catheter ablation at 104 sites. The trial was of necessity open label because of the need for frequent adjustments of warfarin dosing; however, outcome assessment was carried out by a blinded panel of six cardiologists and three neurologists.
Standard practice among AF ablationists is to continue oral anticoagulation periprocedurally because prior studies have convincingly shown that periprocedural interruption of warfarin in an effort to reduce bleeding results in a sharply increased risk of periprocedural stroke. So participants in RE-CIRCUIT were randomized to 4-8 weeks of uninterrupted anticoagulation with either dabigatran at 150 mg b.i.d. or warfarin with a target international normalized ratio (INR) of 2.0-3.0 prior to the ablation procedure, during it, and for 8 weeks afterward, at which time an individualized decision was made as to whether to stop or continue the drug.
Major bleeding events were defined by the International Society on Thrombosis and Hemostatis criteria. Most of these bleeds occurred within the first day or two after the procedure. Pericardial tamponades and groin hematomas were significantly less common with dabigatran.
The incidence of minor bleeding events was similar, at around 18% in the two treatment arms. No strokes or systemic embolisms occurred in the study. One patient on warfarin experienced a transient ischemic attack.
Dr. Calkins elaborated on why RE-CIRCUIT will change clinical practice: “A stroke is a terrible thing during an AF procedure and cardiac tamponade is the most common cause of death from the procedure. And now we have high-quality data showing that if you perform this procedure on uninterrupted dabigatran, the risk of stroke and other systemic embolic events is extremely low, and the rate of major bleeding was 77% less.
“Plus, the logistics of warfarin are a pain,” he continued. “If the patient presents on the day of ablation with an INR that’s too high, the procedure is canceled, and if they present with an INR that’s too low and the procedure is carried out, it’s done so with an increased stroke risk.”
Dr. Calkins said he suspects the sharp reduction in major bleeding events during and after AF catheter ablation is a class effect shared by the other NOACs. Studies with those agents are ongoing. But for now, the unique availability of an immediate reversal agent in the form of idarucizumab (Praxbind) for dabigatran in the event of uncontrolled major bleeding is a source of reassurance for operators and patients alike. The antidote was never required in RE-CIRCUIT, though, the cardiologist noted.
Discussant William G. Stevenson, MD, called the trial “informative and helpful.”
“Something we’ve all been struggling with was that some concern was earlier raised that dabigatran might be associated with more thromboembolic events in this scenario. This study clearly refutes that concern,” observed Dr. Stevenson, director of the clinical cardiac electrophysiology program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
Dr. Stevenson wondered whether the outcome differences between the two study groups could be explained by differences in operator techniques and tools. That’s highly unlikely, Dr. Calkins replied. Randomization was done patient by patient, not center by center.
Then why the big difference in major bleeding complications? Dr. Stevenson asked.
“It may be that, if you poke a hole when a patient is on a more forgiving anticoagulant like dabigatran, the bleeding doesn’t persist and turn into a tamponade, whereas if you poke a hole on warfarin it turns into a bigger problem,” Dr. Calkins responded. “When you think about it, warfarin really impacts the whole coagulation cascade through factors VII, IX, and X, so multiple coagulation factors are rendered impotent, whereas dabigatran is a direct thrombin inhibitor, so you’re selectively knocking out just one component of the coagulation cascade. It provides more leeway in preventing a small hole from turning into a big effusion,” he said.
The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Simultaneously with Dr. Calkins’ presentation at ACC 17, the RE-CIRCUIT study was published online (N Engl J Med. 2017 Mar 19. doi: 10.1056/NEJMoa1701005).
Washington – Uninterrupted dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation proved far superior to uninterrupted warfarin – the current standard – in the randomized multicenter RE-CIRCUIT trial, Hugh Calkins, MD, reported at the annual meeting of the American College of Cardiology.
The primary study endpoint – the incidence of major bleeding events from the time of the first femoral puncture at the procedure’s start through the subsequent 8 weeks – occurred in 1.6% of the dabigatran (Pradaxa) group and 6.9% of the warfarin group, for an absolute 5.9% reduction in risk and a 77% relative risk reduction favoring the novel anticoagulant.
“This trial will definitely affect my own practice, and I think it will quickly affect the practices of electrophysiologists around the world,” declared Dr. Calkins, professor of cardiology and medicine and director of the clinical electrophysiology laboratory and the arrhythmia service at Johns Hopkins University, Baltimore.
RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy) was a multicenter, prospective, international trial conducted in 635 atrial fibrillation (AF) patients who underwent catheter ablation at 104 sites. The trial was of necessity open label because of the need for frequent adjustments of warfarin dosing; however, outcome assessment was carried out by a blinded panel of six cardiologists and three neurologists.
Standard practice among AF ablationists is to continue oral anticoagulation periprocedurally because prior studies have convincingly shown that periprocedural interruption of warfarin in an effort to reduce bleeding results in a sharply increased risk of periprocedural stroke. So participants in RE-CIRCUIT were randomized to 4-8 weeks of uninterrupted anticoagulation with either dabigatran at 150 mg b.i.d. or warfarin with a target international normalized ratio (INR) of 2.0-3.0 prior to the ablation procedure, during it, and for 8 weeks afterward, at which time an individualized decision was made as to whether to stop or continue the drug.
Major bleeding events were defined by the International Society on Thrombosis and Hemostatis criteria. Most of these bleeds occurred within the first day or two after the procedure. Pericardial tamponades and groin hematomas were significantly less common with dabigatran.
The incidence of minor bleeding events was similar, at around 18% in the two treatment arms. No strokes or systemic embolisms occurred in the study. One patient on warfarin experienced a transient ischemic attack.
Dr. Calkins elaborated on why RE-CIRCUIT will change clinical practice: “A stroke is a terrible thing during an AF procedure and cardiac tamponade is the most common cause of death from the procedure. And now we have high-quality data showing that if you perform this procedure on uninterrupted dabigatran, the risk of stroke and other systemic embolic events is extremely low, and the rate of major bleeding was 77% less.
“Plus, the logistics of warfarin are a pain,” he continued. “If the patient presents on the day of ablation with an INR that’s too high, the procedure is canceled, and if they present with an INR that’s too low and the procedure is carried out, it’s done so with an increased stroke risk.”
Dr. Calkins said he suspects the sharp reduction in major bleeding events during and after AF catheter ablation is a class effect shared by the other NOACs. Studies with those agents are ongoing. But for now, the unique availability of an immediate reversal agent in the form of idarucizumab (Praxbind) for dabigatran in the event of uncontrolled major bleeding is a source of reassurance for operators and patients alike. The antidote was never required in RE-CIRCUIT, though, the cardiologist noted.
Discussant William G. Stevenson, MD, called the trial “informative and helpful.”
“Something we’ve all been struggling with was that some concern was earlier raised that dabigatran might be associated with more thromboembolic events in this scenario. This study clearly refutes that concern,” observed Dr. Stevenson, director of the clinical cardiac electrophysiology program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
Dr. Stevenson wondered whether the outcome differences between the two study groups could be explained by differences in operator techniques and tools. That’s highly unlikely, Dr. Calkins replied. Randomization was done patient by patient, not center by center.
Then why the big difference in major bleeding complications? Dr. Stevenson asked.
“It may be that, if you poke a hole when a patient is on a more forgiving anticoagulant like dabigatran, the bleeding doesn’t persist and turn into a tamponade, whereas if you poke a hole on warfarin it turns into a bigger problem,” Dr. Calkins responded. “When you think about it, warfarin really impacts the whole coagulation cascade through factors VII, IX, and X, so multiple coagulation factors are rendered impotent, whereas dabigatran is a direct thrombin inhibitor, so you’re selectively knocking out just one component of the coagulation cascade. It provides more leeway in preventing a small hole from turning into a big effusion,” he said.
The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Simultaneously with Dr. Calkins’ presentation at ACC 17, the RE-CIRCUIT study was published online (N Engl J Med. 2017 Mar 19. doi: 10.1056/NEJMoa1701005).
Washington – Uninterrupted dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation proved far superior to uninterrupted warfarin – the current standard – in the randomized multicenter RE-CIRCUIT trial, Hugh Calkins, MD, reported at the annual meeting of the American College of Cardiology.
The primary study endpoint – the incidence of major bleeding events from the time of the first femoral puncture at the procedure’s start through the subsequent 8 weeks – occurred in 1.6% of the dabigatran (Pradaxa) group and 6.9% of the warfarin group, for an absolute 5.9% reduction in risk and a 77% relative risk reduction favoring the novel anticoagulant.
“This trial will definitely affect my own practice, and I think it will quickly affect the practices of electrophysiologists around the world,” declared Dr. Calkins, professor of cardiology and medicine and director of the clinical electrophysiology laboratory and the arrhythmia service at Johns Hopkins University, Baltimore.
RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy) was a multicenter, prospective, international trial conducted in 635 atrial fibrillation (AF) patients who underwent catheter ablation at 104 sites. The trial was of necessity open label because of the need for frequent adjustments of warfarin dosing; however, outcome assessment was carried out by a blinded panel of six cardiologists and three neurologists.
Standard practice among AF ablationists is to continue oral anticoagulation periprocedurally because prior studies have convincingly shown that periprocedural interruption of warfarin in an effort to reduce bleeding results in a sharply increased risk of periprocedural stroke. So participants in RE-CIRCUIT were randomized to 4-8 weeks of uninterrupted anticoagulation with either dabigatran at 150 mg b.i.d. or warfarin with a target international normalized ratio (INR) of 2.0-3.0 prior to the ablation procedure, during it, and for 8 weeks afterward, at which time an individualized decision was made as to whether to stop or continue the drug.
Major bleeding events were defined by the International Society on Thrombosis and Hemostatis criteria. Most of these bleeds occurred within the first day or two after the procedure. Pericardial tamponades and groin hematomas were significantly less common with dabigatran.
The incidence of minor bleeding events was similar, at around 18% in the two treatment arms. No strokes or systemic embolisms occurred in the study. One patient on warfarin experienced a transient ischemic attack.
Dr. Calkins elaborated on why RE-CIRCUIT will change clinical practice: “A stroke is a terrible thing during an AF procedure and cardiac tamponade is the most common cause of death from the procedure. And now we have high-quality data showing that if you perform this procedure on uninterrupted dabigatran, the risk of stroke and other systemic embolic events is extremely low, and the rate of major bleeding was 77% less.
“Plus, the logistics of warfarin are a pain,” he continued. “If the patient presents on the day of ablation with an INR that’s too high, the procedure is canceled, and if they present with an INR that’s too low and the procedure is carried out, it’s done so with an increased stroke risk.”
Dr. Calkins said he suspects the sharp reduction in major bleeding events during and after AF catheter ablation is a class effect shared by the other NOACs. Studies with those agents are ongoing. But for now, the unique availability of an immediate reversal agent in the form of idarucizumab (Praxbind) for dabigatran in the event of uncontrolled major bleeding is a source of reassurance for operators and patients alike. The antidote was never required in RE-CIRCUIT, though, the cardiologist noted.
Discussant William G. Stevenson, MD, called the trial “informative and helpful.”
“Something we’ve all been struggling with was that some concern was earlier raised that dabigatran might be associated with more thromboembolic events in this scenario. This study clearly refutes that concern,” observed Dr. Stevenson, director of the clinical cardiac electrophysiology program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
Dr. Stevenson wondered whether the outcome differences between the two study groups could be explained by differences in operator techniques and tools. That’s highly unlikely, Dr. Calkins replied. Randomization was done patient by patient, not center by center.
Then why the big difference in major bleeding complications? Dr. Stevenson asked.
“It may be that, if you poke a hole when a patient is on a more forgiving anticoagulant like dabigatran, the bleeding doesn’t persist and turn into a tamponade, whereas if you poke a hole on warfarin it turns into a bigger problem,” Dr. Calkins responded. “When you think about it, warfarin really impacts the whole coagulation cascade through factors VII, IX, and X, so multiple coagulation factors are rendered impotent, whereas dabigatran is a direct thrombin inhibitor, so you’re selectively knocking out just one component of the coagulation cascade. It provides more leeway in preventing a small hole from turning into a big effusion,” he said.
The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Simultaneously with Dr. Calkins’ presentation at ACC 17, the RE-CIRCUIT study was published online (N Engl J Med. 2017 Mar 19. doi: 10.1056/NEJMoa1701005).
At ACC 17
Key clinical point:
Major finding: The incidence of major bleeding events in conjunction with catheter ablation of atrial fibrillation was reduced by 77% in patients on periprocedural dabigatran compared with those on warfarin.
Data source: A randomized multicenter prospective international trial of 635 patients who underwent catheter ablation for atrial fibrillation supported by uninterrupted oral anticoagulation.
Disclosures: The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis
Clinical Question: What are the benefits and harms of perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery?
Background: Both cancer and surgery increase the risk of venous thromboembolism (VTE). In postsurgical patients with cancer, the benefits and harms of anticoagulation remain unknown.
Synopsis: Thirty-nine trials were deemed eligible for inclusion in the meta-analysis. Twenty-five of these were prospective and 14 were retrospective. The overall incidence of deep venous thrombosis (DVT) and pulmonary embolism was 0.9% (across 20 studies) and 0.3% (across 19 studies), respectively. Pharmacologic prophylaxis overall reduced DVT incidence (0.5% vs. 1.2%; relative risk, 0.51; P = .03). Subgroup analysis demonstrated this was significant for abdominal/pelvic surgeries and with low molecular weight heparin. Six studies compared duration of standard prophylaxis (10 days) with extended prophylaxis (4 weeks), with a lower VTE rate in the extended group. Bleeding events were noted in 13 studies and pharmacologic prophylaxis significantly increased bleeding risk (2.7% vs. 8%; RR, 2.51; P less than .0001).
Bottom Line: Perioperative pharmacologic prophylaxis reduces DVT risk in patients with cancer, with greatest risk reduction seen in patients undergoing abdominal/pelvic surgeries. This comes at the cost of increased bleeding complications.
Citations: Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Ann Surg. 2016 Nov. doi: 10.1097/SLA.0000000000002074.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: What are the benefits and harms of perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery?
Background: Both cancer and surgery increase the risk of venous thromboembolism (VTE). In postsurgical patients with cancer, the benefits and harms of anticoagulation remain unknown.
Synopsis: Thirty-nine trials were deemed eligible for inclusion in the meta-analysis. Twenty-five of these were prospective and 14 were retrospective. The overall incidence of deep venous thrombosis (DVT) and pulmonary embolism was 0.9% (across 20 studies) and 0.3% (across 19 studies), respectively. Pharmacologic prophylaxis overall reduced DVT incidence (0.5% vs. 1.2%; relative risk, 0.51; P = .03). Subgroup analysis demonstrated this was significant for abdominal/pelvic surgeries and with low molecular weight heparin. Six studies compared duration of standard prophylaxis (10 days) with extended prophylaxis (4 weeks), with a lower VTE rate in the extended group. Bleeding events were noted in 13 studies and pharmacologic prophylaxis significantly increased bleeding risk (2.7% vs. 8%; RR, 2.51; P less than .0001).
Bottom Line: Perioperative pharmacologic prophylaxis reduces DVT risk in patients with cancer, with greatest risk reduction seen in patients undergoing abdominal/pelvic surgeries. This comes at the cost of increased bleeding complications.
Citations: Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Ann Surg. 2016 Nov. doi: 10.1097/SLA.0000000000002074.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: What are the benefits and harms of perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery?
Background: Both cancer and surgery increase the risk of venous thromboembolism (VTE). In postsurgical patients with cancer, the benefits and harms of anticoagulation remain unknown.
Synopsis: Thirty-nine trials were deemed eligible for inclusion in the meta-analysis. Twenty-five of these were prospective and 14 were retrospective. The overall incidence of deep venous thrombosis (DVT) and pulmonary embolism was 0.9% (across 20 studies) and 0.3% (across 19 studies), respectively. Pharmacologic prophylaxis overall reduced DVT incidence (0.5% vs. 1.2%; relative risk, 0.51; P = .03). Subgroup analysis demonstrated this was significant for abdominal/pelvic surgeries and with low molecular weight heparin. Six studies compared duration of standard prophylaxis (10 days) with extended prophylaxis (4 weeks), with a lower VTE rate in the extended group. Bleeding events were noted in 13 studies and pharmacologic prophylaxis significantly increased bleeding risk (2.7% vs. 8%; RR, 2.51; P less than .0001).
Bottom Line: Perioperative pharmacologic prophylaxis reduces DVT risk in patients with cancer, with greatest risk reduction seen in patients undergoing abdominal/pelvic surgeries. This comes at the cost of increased bleeding complications.
Citations: Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Ann Surg. 2016 Nov. doi: 10.1097/SLA.0000000000002074.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Readmission rates after passage of the hospital readmissions reduction program
Clinical Question: Did hospitals receiving the highest penalties for readmissions have accelerated improvement in this metric after passage of Medicare Hospital Readmissions Reduction Program (HRRP)?
Background: Medicare passed the HRRP to incentivize reductions in readmission rates. The impact of penalties on relative hospital improvement rates remains unknown.
Setting: Query of national Medicare Provider Analysis and Review files.
Synopsis: 2,868 hospitals were identified as candidates for analysis and were stratified into four risk groups based on penalty size under HRRP: highest-performing, average-performing, low-performing, and lowest-performing. The primary outcomes were hospital-specific, 30-day, all-cause risk-standardized readmission rates (RSRRs) for patients discharged with acute MI, HF, or pneumonia. The investigators separated data into a pre-law period and post-law period. They fitted a logistic regression model to pre-law RSRRs and developed a piecewise linear model on post-law RSRRs with pre-law data as the dependent variable. All hospital groups had reductions in RSRRs, with the lowest quartile demonstrating greatest improvement.
Bottom Line: HRRP has resulted in reductions in RSRRs with greatest improvement in hospitals with lowest pre-law performance.
Citations: Wasfy JH, Zigler CM, Choirat C, et al. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017 Mar;166(5):324-31.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: Did hospitals receiving the highest penalties for readmissions have accelerated improvement in this metric after passage of Medicare Hospital Readmissions Reduction Program (HRRP)?
Background: Medicare passed the HRRP to incentivize reductions in readmission rates. The impact of penalties on relative hospital improvement rates remains unknown.
Setting: Query of national Medicare Provider Analysis and Review files.
Synopsis: 2,868 hospitals were identified as candidates for analysis and were stratified into four risk groups based on penalty size under HRRP: highest-performing, average-performing, low-performing, and lowest-performing. The primary outcomes were hospital-specific, 30-day, all-cause risk-standardized readmission rates (RSRRs) for patients discharged with acute MI, HF, or pneumonia. The investigators separated data into a pre-law period and post-law period. They fitted a logistic regression model to pre-law RSRRs and developed a piecewise linear model on post-law RSRRs with pre-law data as the dependent variable. All hospital groups had reductions in RSRRs, with the lowest quartile demonstrating greatest improvement.
Bottom Line: HRRP has resulted in reductions in RSRRs with greatest improvement in hospitals with lowest pre-law performance.
Citations: Wasfy JH, Zigler CM, Choirat C, et al. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017 Mar;166(5):324-31.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: Did hospitals receiving the highest penalties for readmissions have accelerated improvement in this metric after passage of Medicare Hospital Readmissions Reduction Program (HRRP)?
Background: Medicare passed the HRRP to incentivize reductions in readmission rates. The impact of penalties on relative hospital improvement rates remains unknown.
Setting: Query of national Medicare Provider Analysis and Review files.
Synopsis: 2,868 hospitals were identified as candidates for analysis and were stratified into four risk groups based on penalty size under HRRP: highest-performing, average-performing, low-performing, and lowest-performing. The primary outcomes were hospital-specific, 30-day, all-cause risk-standardized readmission rates (RSRRs) for patients discharged with acute MI, HF, or pneumonia. The investigators separated data into a pre-law period and post-law period. They fitted a logistic regression model to pre-law RSRRs and developed a piecewise linear model on post-law RSRRs with pre-law data as the dependent variable. All hospital groups had reductions in RSRRs, with the lowest quartile demonstrating greatest improvement.
Bottom Line: HRRP has resulted in reductions in RSRRs with greatest improvement in hospitals with lowest pre-law performance.
Citations: Wasfy JH, Zigler CM, Choirat C, et al. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017 Mar;166(5):324-31.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Assessment of goals of care in nursing home reduces hospitalization for patients with dementia
CLINICAL QUESTION: For patients with advanced dementia, does a goals-of-care intervention improve communication and care outcomes?
BACKGROUND: Patients with advanced dementia are frequently admitted from nursing homes for acute conditions. Prior research demonstrates deficits in documentation of advanced directives.
STUDY DESIGN: Single-blind cluster randomized trial.
SETTING: Twenty-two nursing homes in North Carolina.
BOTTOM LINE: Goals of care discussions for patients with advanced dementia appears to reduce hospitalizations.
CITATIONS: Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:24-31.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: For patients with advanced dementia, does a goals-of-care intervention improve communication and care outcomes?
BACKGROUND: Patients with advanced dementia are frequently admitted from nursing homes for acute conditions. Prior research demonstrates deficits in documentation of advanced directives.
STUDY DESIGN: Single-blind cluster randomized trial.
SETTING: Twenty-two nursing homes in North Carolina.
BOTTOM LINE: Goals of care discussions for patients with advanced dementia appears to reduce hospitalizations.
CITATIONS: Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:24-31.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: For patients with advanced dementia, does a goals-of-care intervention improve communication and care outcomes?
BACKGROUND: Patients with advanced dementia are frequently admitted from nursing homes for acute conditions. Prior research demonstrates deficits in documentation of advanced directives.
STUDY DESIGN: Single-blind cluster randomized trial.
SETTING: Twenty-two nursing homes in North Carolina.
BOTTOM LINE: Goals of care discussions for patients with advanced dementia appears to reduce hospitalizations.
CITATIONS: Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:24-31.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Antipsychotics ineffective for symptoms of delirium in palliative care
CLINICAL QUESTION: Do antipsychotics provide symptomatic benefit for delirium in palliative care?
BACKGROUND: Antipsychotics are frequently used for the treatment of delirium and guideline recommended for delirium-associated distress. However, a 2016 meta-analysis found antipsychotics are not associated with change in delirium duration or severity. Antipsychotics for palliative management of delirium at end of life is not well studied.
STUDY DESIGN: Double-blind randomized controlled trial with placebo, haloperidol, and risperidone arms.
SETTING: Eleven Australian inpatient hospice or palliative care services.
SYNOPSIS: 247 patients (mean age, 74.9 years; 88.3% with cancer) with advanced incurable disease and active delirium were studied. Most had mild-moderate severity delirium. All received nonpharmacological measures and plan to address reversible precipitants. Patients were randomized to placebo (84), haloperidol (81), or risperidone (82) for 72 hours. Dose titration was allowed based on delirium symptoms. In intention to treat analysis the delirium severity scores were statistically higher in haloperidol and risperidone arms, compared with placebo. This reached statistical significance although less than the minimum clinically significant difference. Mortality, use of rescue medicines, and extrapyramidal symptoms were higher in antipsychotic groups.
BOTTOM LINE: Antipsychotics cause side effects without efficacy in palliation of symptoms of delirium.
CITATIONS: Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:34-42.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: Do antipsychotics provide symptomatic benefit for delirium in palliative care?
BACKGROUND: Antipsychotics are frequently used for the treatment of delirium and guideline recommended for delirium-associated distress. However, a 2016 meta-analysis found antipsychotics are not associated with change in delirium duration or severity. Antipsychotics for palliative management of delirium at end of life is not well studied.
STUDY DESIGN: Double-blind randomized controlled trial with placebo, haloperidol, and risperidone arms.
SETTING: Eleven Australian inpatient hospice or palliative care services.
SYNOPSIS: 247 patients (mean age, 74.9 years; 88.3% with cancer) with advanced incurable disease and active delirium were studied. Most had mild-moderate severity delirium. All received nonpharmacological measures and plan to address reversible precipitants. Patients were randomized to placebo (84), haloperidol (81), or risperidone (82) for 72 hours. Dose titration was allowed based on delirium symptoms. In intention to treat analysis the delirium severity scores were statistically higher in haloperidol and risperidone arms, compared with placebo. This reached statistical significance although less than the minimum clinically significant difference. Mortality, use of rescue medicines, and extrapyramidal symptoms were higher in antipsychotic groups.
BOTTOM LINE: Antipsychotics cause side effects without efficacy in palliation of symptoms of delirium.
CITATIONS: Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:34-42.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: Do antipsychotics provide symptomatic benefit for delirium in palliative care?
BACKGROUND: Antipsychotics are frequently used for the treatment of delirium and guideline recommended for delirium-associated distress. However, a 2016 meta-analysis found antipsychotics are not associated with change in delirium duration or severity. Antipsychotics for palliative management of delirium at end of life is not well studied.
STUDY DESIGN: Double-blind randomized controlled trial with placebo, haloperidol, and risperidone arms.
SETTING: Eleven Australian inpatient hospice or palliative care services.
SYNOPSIS: 247 patients (mean age, 74.9 years; 88.3% with cancer) with advanced incurable disease and active delirium were studied. Most had mild-moderate severity delirium. All received nonpharmacological measures and plan to address reversible precipitants. Patients were randomized to placebo (84), haloperidol (81), or risperidone (82) for 72 hours. Dose titration was allowed based on delirium symptoms. In intention to treat analysis the delirium severity scores were statistically higher in haloperidol and risperidone arms, compared with placebo. This reached statistical significance although less than the minimum clinically significant difference. Mortality, use of rescue medicines, and extrapyramidal symptoms were higher in antipsychotic groups.
BOTTOM LINE: Antipsychotics cause side effects without efficacy in palliation of symptoms of delirium.
CITATIONS: Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:34-42.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
A little rivaroxaban goes a long way
In patients with venous thromboembolism at equipoise for further anticoagulation therapy, treatment with a 10-mg dose of rivaroxaban (Xarelto) had comparable efficacy to a 20-mg dose, with both leading to fewer recurrences than treatment with aspirin. There were no statistically significant differences in clinically relevant nonmajor bleeding between the three groups.
The study’s conclusions are limited to relatively healthy patients such as the ones who were selected for the study.
The findings were presented at the annual meeting of the American College of Cardiology and published simultaneously in the New England Journal of Medicine (N Engl J Med 2017 March 18. doi: 10.1056/NEJMoa1700518).
Anticoagulants are the primary treatment for prevention of venous thromboembolism recurrences, but the medications are often stopped after 6-12 months because of concerns about bleeding. To counter that issue, physicians may prescribe lower doses of anticoagulants such as rivaroxaban, or substitute aspirin.
The work follows another recent study of apixaban (Eliquis), which showed that a 2.5-mg twice-daily dose performed the same as did a 5.0-mg twice-daily dose in the prevention of venous thromboembolism recurrence (N Engl J Med. 2013;368[8]:699-708).
“I think the story is kind of the same here,” said David Garcia, MD, professor of hematology at the University of Washington, Seattle, who was one of the principal investigators in the trial.
Patients with unprompted venous thromboembolism are increasingly being offered anticoagulant therapy to prevent recurrences. Those drugs have inherent bleeding risks, but the newer drugs and even warfarin are becoming safer. Even so, “as we embark on that, one has to remember that the risk of anticoagulants is cumulative. It may only carry a risk of 1% per year of major hemorrhage, but if the patient has to take it for 10 or 20 or 30 years, it’s a nontrivial risk of major bleeding over that time,” said Dr. Garcia.
The researchers conducted the Reduced-Dose Rivaroxaban in the Long-Term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN CHOICE) trial, in which 3,365 patients from 24 sites were randomized to receive 20 mg rivaroxaban, 10 mg rivaroxaban, or 100 mg aspirin for up to 1 year following an initial 6-12 months of treatment with anticoagulation therapy.
During a median follow-up of 1 year, 4.4% of patients on aspirin experienced a recurrence, compared with 1.5% of patients in the 20-mg rivaroxaban group (hazard ratio versus aspirin, 0.34; 95% confidence interval, 0.20-0.59; P less than .001), and 1.2% in the 10-mg rivaroxaban group (HR versus aspirin, 0.26; 95% CI, 0.14-0.47; P less than .001). There was no statistical significance between the two doses of rivaroxaban.
The rates of fatal thromboembolism were similar, at 0.2% in the 20-mg rivaroxaban group, 0% in the 10-mg group, and 0.2% in the aspirin group.
Major bleeding occurred in 0.5% of patients in the 20-mg rivaroxaban group, in 0.4% of the 10-mg rivaroxaban group, and 0.3% in the aspirin group. Nonmajor, clinically relevant bleeding was also similar between groups, at 2.7% in the 20-mg group, 2.0% in the 10-mg group, and 1.8% in the aspirin group. These differences were not statistically significant.
The study is good news for clinicians as they help patients decide whether to undergo preventive therapy. “Even before the newer agents arrived on the market, we had moved the needle a lot in terms of maximizing the safety of warfarin. I think these drugs take it to yet another level,” said Dr. Garcia.
Bayer Pharmaceuticals funded the study. Dr. Garcia has received honoraria from Bristol-Meyers Squibb, Pfizer, and Boehringer Ingelheim.
“Given the protection from recurrent venous thromboembolism afforded by reduced-dose rivaroxaban, extending treatment beyond 3 months could be considered in patients with provoked venous thromboembolism who are at average risk for bleeding and who are strongly averse to having another episode of venous thromboembolism. In light of the safety profile of low-dose rivaroxaban, the benefit of this strategy does not need to be large in order to justify the extension of therapy.
“This trial suggests that it would be helpful to evaluate the effects of reduced doses of rivaroxaban within 6 months after an episode of venous thromboembolism.
“For patients without cancer, the use of direct oral anticoagulant agents might be considered as first-line treatment for those with acute venous thromboembolism. Full-dose treatment could be continued for a minimum of 3-6 months. In patients in whom there is equipoise with respect to continuing anticoagulant therapy beyond this period, the use of a reduced-intensity direct oral anticoagulant agent might be considered. Clinicians who choose this strategy can be confident of excellent efficacy and low bleeding risk similar to that observed with aspirin or placebo” (N Engl J Med 2017 March 18. doi: 10.1056/NEJMe1701628).
Mark Crowther, MD, is professor of medicine at McMaster University, Hamilton, Ont. Adam Cuker is assistant professor of medicine at the hospital of the University of Pennsylvania, Philadelphia.
“Given the protection from recurrent venous thromboembolism afforded by reduced-dose rivaroxaban, extending treatment beyond 3 months could be considered in patients with provoked venous thromboembolism who are at average risk for bleeding and who are strongly averse to having another episode of venous thromboembolism. In light of the safety profile of low-dose rivaroxaban, the benefit of this strategy does not need to be large in order to justify the extension of therapy.
“This trial suggests that it would be helpful to evaluate the effects of reduced doses of rivaroxaban within 6 months after an episode of venous thromboembolism.
“For patients without cancer, the use of direct oral anticoagulant agents might be considered as first-line treatment for those with acute venous thromboembolism. Full-dose treatment could be continued for a minimum of 3-6 months. In patients in whom there is equipoise with respect to continuing anticoagulant therapy beyond this period, the use of a reduced-intensity direct oral anticoagulant agent might be considered. Clinicians who choose this strategy can be confident of excellent efficacy and low bleeding risk similar to that observed with aspirin or placebo” (N Engl J Med 2017 March 18. doi: 10.1056/NEJMe1701628).
Mark Crowther, MD, is professor of medicine at McMaster University, Hamilton, Ont. Adam Cuker is assistant professor of medicine at the hospital of the University of Pennsylvania, Philadelphia.
“Given the protection from recurrent venous thromboembolism afforded by reduced-dose rivaroxaban, extending treatment beyond 3 months could be considered in patients with provoked venous thromboembolism who are at average risk for bleeding and who are strongly averse to having another episode of venous thromboembolism. In light of the safety profile of low-dose rivaroxaban, the benefit of this strategy does not need to be large in order to justify the extension of therapy.
“This trial suggests that it would be helpful to evaluate the effects of reduced doses of rivaroxaban within 6 months after an episode of venous thromboembolism.
“For patients without cancer, the use of direct oral anticoagulant agents might be considered as first-line treatment for those with acute venous thromboembolism. Full-dose treatment could be continued for a minimum of 3-6 months. In patients in whom there is equipoise with respect to continuing anticoagulant therapy beyond this period, the use of a reduced-intensity direct oral anticoagulant agent might be considered. Clinicians who choose this strategy can be confident of excellent efficacy and low bleeding risk similar to that observed with aspirin or placebo” (N Engl J Med 2017 March 18. doi: 10.1056/NEJMe1701628).
Mark Crowther, MD, is professor of medicine at McMaster University, Hamilton, Ont. Adam Cuker is assistant professor of medicine at the hospital of the University of Pennsylvania, Philadelphia.
In patients with venous thromboembolism at equipoise for further anticoagulation therapy, treatment with a 10-mg dose of rivaroxaban (Xarelto) had comparable efficacy to a 20-mg dose, with both leading to fewer recurrences than treatment with aspirin. There were no statistically significant differences in clinically relevant nonmajor bleeding between the three groups.
The study’s conclusions are limited to relatively healthy patients such as the ones who were selected for the study.
The findings were presented at the annual meeting of the American College of Cardiology and published simultaneously in the New England Journal of Medicine (N Engl J Med 2017 March 18. doi: 10.1056/NEJMoa1700518).
Anticoagulants are the primary treatment for prevention of venous thromboembolism recurrences, but the medications are often stopped after 6-12 months because of concerns about bleeding. To counter that issue, physicians may prescribe lower doses of anticoagulants such as rivaroxaban, or substitute aspirin.
The work follows another recent study of apixaban (Eliquis), which showed that a 2.5-mg twice-daily dose performed the same as did a 5.0-mg twice-daily dose in the prevention of venous thromboembolism recurrence (N Engl J Med. 2013;368[8]:699-708).
“I think the story is kind of the same here,” said David Garcia, MD, professor of hematology at the University of Washington, Seattle, who was one of the principal investigators in the trial.
Patients with unprompted venous thromboembolism are increasingly being offered anticoagulant therapy to prevent recurrences. Those drugs have inherent bleeding risks, but the newer drugs and even warfarin are becoming safer. Even so, “as we embark on that, one has to remember that the risk of anticoagulants is cumulative. It may only carry a risk of 1% per year of major hemorrhage, but if the patient has to take it for 10 or 20 or 30 years, it’s a nontrivial risk of major bleeding over that time,” said Dr. Garcia.
The researchers conducted the Reduced-Dose Rivaroxaban in the Long-Term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN CHOICE) trial, in which 3,365 patients from 24 sites were randomized to receive 20 mg rivaroxaban, 10 mg rivaroxaban, or 100 mg aspirin for up to 1 year following an initial 6-12 months of treatment with anticoagulation therapy.
During a median follow-up of 1 year, 4.4% of patients on aspirin experienced a recurrence, compared with 1.5% of patients in the 20-mg rivaroxaban group (hazard ratio versus aspirin, 0.34; 95% confidence interval, 0.20-0.59; P less than .001), and 1.2% in the 10-mg rivaroxaban group (HR versus aspirin, 0.26; 95% CI, 0.14-0.47; P less than .001). There was no statistical significance between the two doses of rivaroxaban.
The rates of fatal thromboembolism were similar, at 0.2% in the 20-mg rivaroxaban group, 0% in the 10-mg group, and 0.2% in the aspirin group.
Major bleeding occurred in 0.5% of patients in the 20-mg rivaroxaban group, in 0.4% of the 10-mg rivaroxaban group, and 0.3% in the aspirin group. Nonmajor, clinically relevant bleeding was also similar between groups, at 2.7% in the 20-mg group, 2.0% in the 10-mg group, and 1.8% in the aspirin group. These differences were not statistically significant.
The study is good news for clinicians as they help patients decide whether to undergo preventive therapy. “Even before the newer agents arrived on the market, we had moved the needle a lot in terms of maximizing the safety of warfarin. I think these drugs take it to yet another level,” said Dr. Garcia.
Bayer Pharmaceuticals funded the study. Dr. Garcia has received honoraria from Bristol-Meyers Squibb, Pfizer, and Boehringer Ingelheim.
In patients with venous thromboembolism at equipoise for further anticoagulation therapy, treatment with a 10-mg dose of rivaroxaban (Xarelto) had comparable efficacy to a 20-mg dose, with both leading to fewer recurrences than treatment with aspirin. There were no statistically significant differences in clinically relevant nonmajor bleeding between the three groups.
The study’s conclusions are limited to relatively healthy patients such as the ones who were selected for the study.
The findings were presented at the annual meeting of the American College of Cardiology and published simultaneously in the New England Journal of Medicine (N Engl J Med 2017 March 18. doi: 10.1056/NEJMoa1700518).
Anticoagulants are the primary treatment for prevention of venous thromboembolism recurrences, but the medications are often stopped after 6-12 months because of concerns about bleeding. To counter that issue, physicians may prescribe lower doses of anticoagulants such as rivaroxaban, or substitute aspirin.
The work follows another recent study of apixaban (Eliquis), which showed that a 2.5-mg twice-daily dose performed the same as did a 5.0-mg twice-daily dose in the prevention of venous thromboembolism recurrence (N Engl J Med. 2013;368[8]:699-708).
“I think the story is kind of the same here,” said David Garcia, MD, professor of hematology at the University of Washington, Seattle, who was one of the principal investigators in the trial.
Patients with unprompted venous thromboembolism are increasingly being offered anticoagulant therapy to prevent recurrences. Those drugs have inherent bleeding risks, but the newer drugs and even warfarin are becoming safer. Even so, “as we embark on that, one has to remember that the risk of anticoagulants is cumulative. It may only carry a risk of 1% per year of major hemorrhage, but if the patient has to take it for 10 or 20 or 30 years, it’s a nontrivial risk of major bleeding over that time,” said Dr. Garcia.
The researchers conducted the Reduced-Dose Rivaroxaban in the Long-Term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN CHOICE) trial, in which 3,365 patients from 24 sites were randomized to receive 20 mg rivaroxaban, 10 mg rivaroxaban, or 100 mg aspirin for up to 1 year following an initial 6-12 months of treatment with anticoagulation therapy.
During a median follow-up of 1 year, 4.4% of patients on aspirin experienced a recurrence, compared with 1.5% of patients in the 20-mg rivaroxaban group (hazard ratio versus aspirin, 0.34; 95% confidence interval, 0.20-0.59; P less than .001), and 1.2% in the 10-mg rivaroxaban group (HR versus aspirin, 0.26; 95% CI, 0.14-0.47; P less than .001). There was no statistical significance between the two doses of rivaroxaban.
The rates of fatal thromboembolism were similar, at 0.2% in the 20-mg rivaroxaban group, 0% in the 10-mg group, and 0.2% in the aspirin group.
Major bleeding occurred in 0.5% of patients in the 20-mg rivaroxaban group, in 0.4% of the 10-mg rivaroxaban group, and 0.3% in the aspirin group. Nonmajor, clinically relevant bleeding was also similar between groups, at 2.7% in the 20-mg group, 2.0% in the 10-mg group, and 1.8% in the aspirin group. These differences were not statistically significant.
The study is good news for clinicians as they help patients decide whether to undergo preventive therapy. “Even before the newer agents arrived on the market, we had moved the needle a lot in terms of maximizing the safety of warfarin. I think these drugs take it to yet another level,” said Dr. Garcia.
Bayer Pharmaceuticals funded the study. Dr. Garcia has received honoraria from Bristol-Meyers Squibb, Pfizer, and Boehringer Ingelheim.
FROM ACC 17
Key clinical point: In venous thromboembolism prevention, a 10-mg dose matched 20 mg.
Major finding: The recurrence rates were 1.2% at 10 mg versus 4.4% with aspirin.
Data source: Randomized comparison trial of 3,365 patients.
Disclosures: Bayer Pharmaceuticals funded the study. Dr. Garcia has received honoraria from Bristol-Meyers Squibb, Pfizer, and Boehringer Ingelheim.
What are indications, complications of acute blood transfusions in sickle cell anemia? Key Points Additional Reading
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2
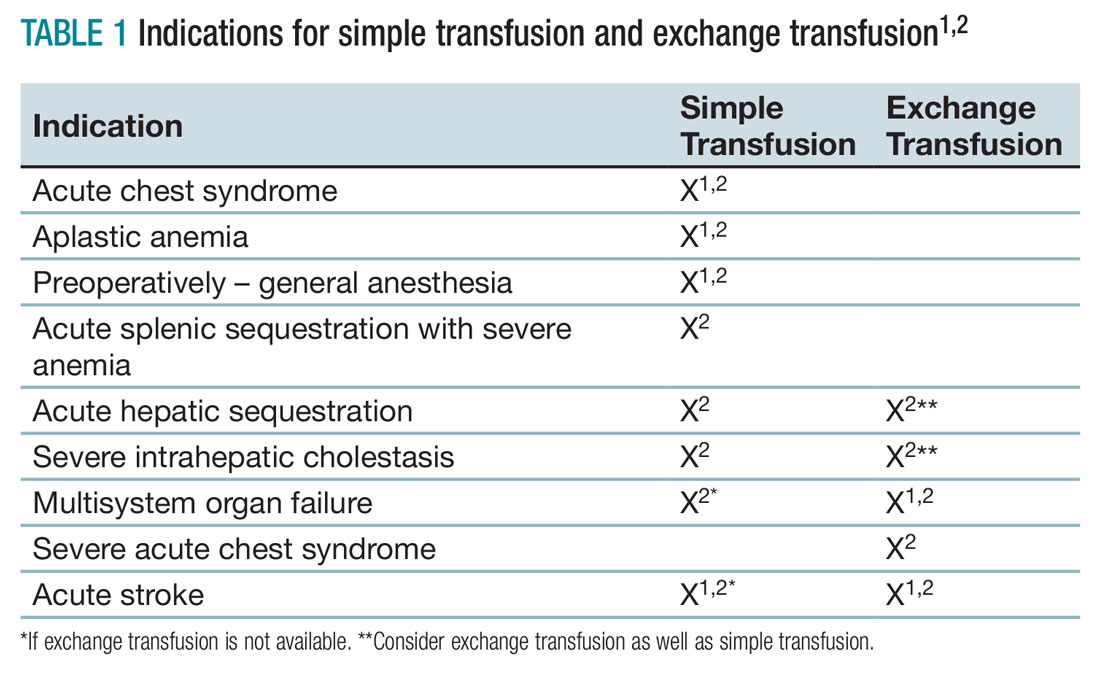
Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2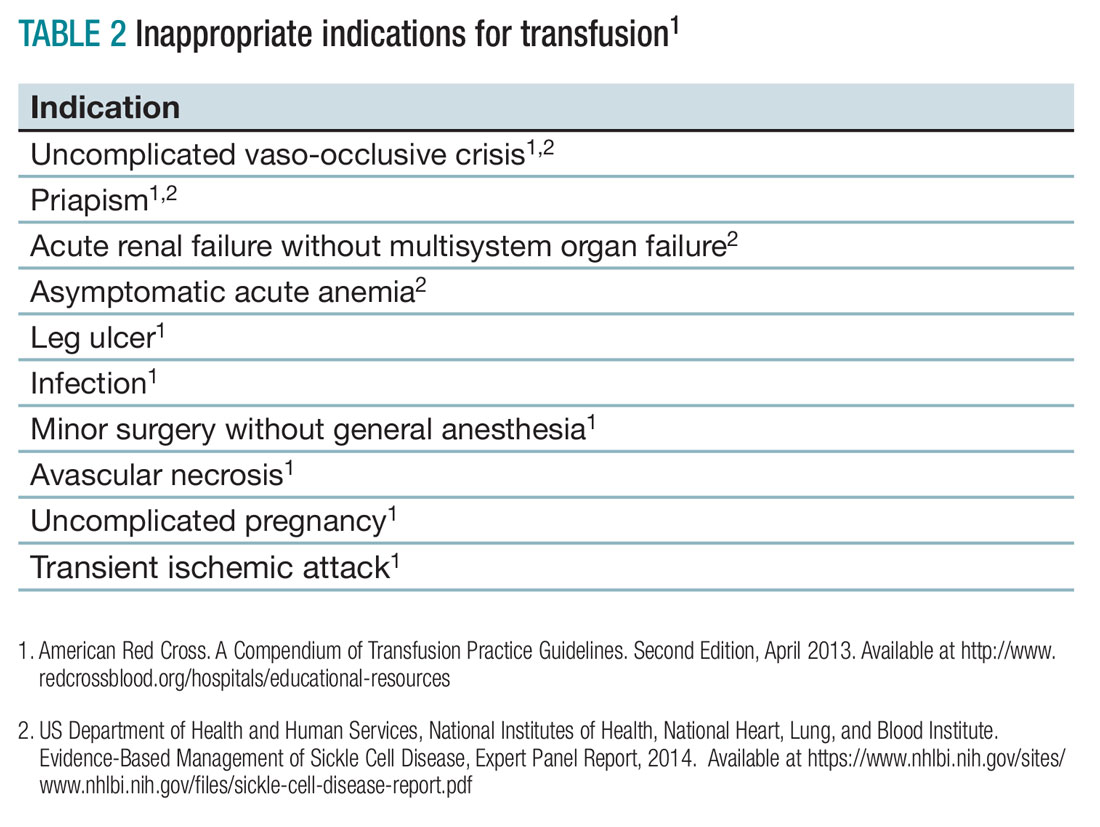
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2

Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2

Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2016 Updates to AASLD Guidance Document on gastroesophageal bleeding in decompensated cirrhosis
Clinical question: What is appropriate inpatient management of a cirrhotic patient with acute esophageal or gastric variceal bleeding?
Study design: Guidance document developed by expert panel based on literature review, consensus conferences and authors’ clinical experience.
Background: Practice guidelines for the diagnosis and treatment of gastroesophageal hemorrhage were last published in 2007 and endorsed by several major professional societies. Since then, there have been a number of randomized controlled trials (RCTs) and consensus conferences. The American Association for the Study of Liver Diseases (AASLD) published updated practice guidelines in 2016 that encompass pathophysiology, monitoring, diagnosis, and treatment of gastroesophageal hemorrhage in cirrhotic patients. This summary will focus on inpatient management for active gastroesophageal hemorrhage.
Synopsis of Inpatient Management for Esophageal Variceal Hemorrhage: The authors suggest that all VH requires ICU admission with the goal of acute control of bleeding, prevention of early recurrence, and reduction in 6-week mortality. Imaging to rule out portal vein thrombosis and HCC should be considered. Hepatic-Venous Pressure Gradient (HVPG) greater than 20 mm Hg is the strongest predictor of early rebleeding and death. However, catheter measurements of portal pressure are not available at most centers. As with any critically ill patient, stabilization of respiratory status and ensuring hemodynamic stability with volume resuscitation is paramount. RCTs evaluating transfusion goals suggest that a restrictive transfusion goal of HgB 7 g/dL is superior to a liberal goal of 9 g/dL. The authors hypothesize this may be related to lower HVPG observed with lower transfusion thresholds. In terms of treating coagulopathy, RCTs evaluating recombinant VIIa have not shown clear benefit. Correction of INR with FFPs similarly not recommended. No recommendations are made regarding utility of platelet transfusions. Vasoactive drugs should be administered when VH is suspected with the goal of decreasing splanchnic blood flow. Octreotide is the only vasoactive drug available in the United States. RCTs show that antibiotics administered prophylactically decrease infections, recurrent hemorrhage, and death. Ceftriaxone 1 g daily is the drug of choice in the United States and should be given up to a maximum of 7 days. A reasonable strategy is discontinuation of prophylaxis concurrently with discontinuation of vasoactive agents. After stabilization of hemodynamics, patients should proceed to endoscopy no more than 12 hours after presentation. Endoscopic Variceal Ligation (EVL) should be done if signs of active or recent variceal bleeding are found. After EVL, select patients at high risk of rebleeding (Child-Pugh B with active bleeding seen on endoscopy or Child-Pugh C patients) may benefit from TIPS within 72 hours. If TIPS is done, vasoactive agents can be discontinued. Otherwise, vasoactive agents should continue for 2-5 days with subsequent transition to nonselective beta blockers (NSBB) such as nadolol or propranolol. For secondary prophylaxis of esophageal bleeding, combination EVL and NSBB is first-line therapy. If recurrent hemorrhage occurs while on secondary prophylaxis, rescue TIPS is recommended.
Synopsis of Inpatient Management for Gastric Variceal Hemorrhage: Management of Gastric Variceal Hemorrhage is similar to Esophageal Variceal (EV) Hemorrhage and encompasses volume resuscitation, vasoactive drugs, and antibiotics with endoscopy shortly thereafter. Balloon tamponade can be used as a bridge to endoscopy in massive bleeds. In addition to the above, anatomic location of Gastric Varices (GV) affects choice of intervention. GOV1 varices extend from the gastric cardia to the lesser curvature and represent 75% of GV. If these are small, they can be managed with EVL. Otherwise these can be managed with injection of cyanoacrylate glue. GOV2 varices extend from the gastric cardia into the fundus. Isolated GV type 1 varices (IGV1) are located entirely in the fundus and have the highest propensity for bleeding. For these latter two types of “cardio-fundal varices” TIPS is the preferred intervention to control acute bleeding. Data on the efficacy of secondary prophylaxis for GV bleeding is limited. A combination of NSBB, cyanoacrylate injection, or TIPS can be considered. Balloon Occluded Retrograde Transvenous Obliteration (BRTO) can be considered if fundal varices are associated with a large gastrorenal or splenorenal collateral. However, no RCTs have compared BRTO with other strategies. Isolated GV type 2 (IGV2) varices are not localized to the esophageal or gastric cardio-fundal region and are rare in cirrhotic patients but tend to occur in pre-hepatic portal hypertension. Management requires multidisciplinary input from endoscopists, hepatologists, interventional radiologists, and surgeons.
Bottom line: For esophageal variceal bleeding related to cirrhosis: volume resuscitation, antibiotic prophylaxis, and vasoactive agents are mainstays of therapy to stabilize patient for endoscopic intervention within 12 hours. This should be followed by early TIPS within 72 hours in high risk patients.
A similar approach applies to gastric variceal bleeding, but interventional management is dependent on the anatomic location of the varices in question.
Citations: Garcia-Tsao G et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis and management – 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017 Jan;65[1]:310-35.
Dr. Lu is a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: What is appropriate inpatient management of a cirrhotic patient with acute esophageal or gastric variceal bleeding?
Study design: Guidance document developed by expert panel based on literature review, consensus conferences and authors’ clinical experience.
Background: Practice guidelines for the diagnosis and treatment of gastroesophageal hemorrhage were last published in 2007 and endorsed by several major professional societies. Since then, there have been a number of randomized controlled trials (RCTs) and consensus conferences. The American Association for the Study of Liver Diseases (AASLD) published updated practice guidelines in 2016 that encompass pathophysiology, monitoring, diagnosis, and treatment of gastroesophageal hemorrhage in cirrhotic patients. This summary will focus on inpatient management for active gastroesophageal hemorrhage.
Synopsis of Inpatient Management for Esophageal Variceal Hemorrhage: The authors suggest that all VH requires ICU admission with the goal of acute control of bleeding, prevention of early recurrence, and reduction in 6-week mortality. Imaging to rule out portal vein thrombosis and HCC should be considered. Hepatic-Venous Pressure Gradient (HVPG) greater than 20 mm Hg is the strongest predictor of early rebleeding and death. However, catheter measurements of portal pressure are not available at most centers. As with any critically ill patient, stabilization of respiratory status and ensuring hemodynamic stability with volume resuscitation is paramount. RCTs evaluating transfusion goals suggest that a restrictive transfusion goal of HgB 7 g/dL is superior to a liberal goal of 9 g/dL. The authors hypothesize this may be related to lower HVPG observed with lower transfusion thresholds. In terms of treating coagulopathy, RCTs evaluating recombinant VIIa have not shown clear benefit. Correction of INR with FFPs similarly not recommended. No recommendations are made regarding utility of platelet transfusions. Vasoactive drugs should be administered when VH is suspected with the goal of decreasing splanchnic blood flow. Octreotide is the only vasoactive drug available in the United States. RCTs show that antibiotics administered prophylactically decrease infections, recurrent hemorrhage, and death. Ceftriaxone 1 g daily is the drug of choice in the United States and should be given up to a maximum of 7 days. A reasonable strategy is discontinuation of prophylaxis concurrently with discontinuation of vasoactive agents. After stabilization of hemodynamics, patients should proceed to endoscopy no more than 12 hours after presentation. Endoscopic Variceal Ligation (EVL) should be done if signs of active or recent variceal bleeding are found. After EVL, select patients at high risk of rebleeding (Child-Pugh B with active bleeding seen on endoscopy or Child-Pugh C patients) may benefit from TIPS within 72 hours. If TIPS is done, vasoactive agents can be discontinued. Otherwise, vasoactive agents should continue for 2-5 days with subsequent transition to nonselective beta blockers (NSBB) such as nadolol or propranolol. For secondary prophylaxis of esophageal bleeding, combination EVL and NSBB is first-line therapy. If recurrent hemorrhage occurs while on secondary prophylaxis, rescue TIPS is recommended.
Synopsis of Inpatient Management for Gastric Variceal Hemorrhage: Management of Gastric Variceal Hemorrhage is similar to Esophageal Variceal (EV) Hemorrhage and encompasses volume resuscitation, vasoactive drugs, and antibiotics with endoscopy shortly thereafter. Balloon tamponade can be used as a bridge to endoscopy in massive bleeds. In addition to the above, anatomic location of Gastric Varices (GV) affects choice of intervention. GOV1 varices extend from the gastric cardia to the lesser curvature and represent 75% of GV. If these are small, they can be managed with EVL. Otherwise these can be managed with injection of cyanoacrylate glue. GOV2 varices extend from the gastric cardia into the fundus. Isolated GV type 1 varices (IGV1) are located entirely in the fundus and have the highest propensity for bleeding. For these latter two types of “cardio-fundal varices” TIPS is the preferred intervention to control acute bleeding. Data on the efficacy of secondary prophylaxis for GV bleeding is limited. A combination of NSBB, cyanoacrylate injection, or TIPS can be considered. Balloon Occluded Retrograde Transvenous Obliteration (BRTO) can be considered if fundal varices are associated with a large gastrorenal or splenorenal collateral. However, no RCTs have compared BRTO with other strategies. Isolated GV type 2 (IGV2) varices are not localized to the esophageal or gastric cardio-fundal region and are rare in cirrhotic patients but tend to occur in pre-hepatic portal hypertension. Management requires multidisciplinary input from endoscopists, hepatologists, interventional radiologists, and surgeons.
Bottom line: For esophageal variceal bleeding related to cirrhosis: volume resuscitation, antibiotic prophylaxis, and vasoactive agents are mainstays of therapy to stabilize patient for endoscopic intervention within 12 hours. This should be followed by early TIPS within 72 hours in high risk patients.
A similar approach applies to gastric variceal bleeding, but interventional management is dependent on the anatomic location of the varices in question.
Citations: Garcia-Tsao G et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis and management – 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017 Jan;65[1]:310-35.
Dr. Lu is a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: What is appropriate inpatient management of a cirrhotic patient with acute esophageal or gastric variceal bleeding?
Study design: Guidance document developed by expert panel based on literature review, consensus conferences and authors’ clinical experience.
Background: Practice guidelines for the diagnosis and treatment of gastroesophageal hemorrhage were last published in 2007 and endorsed by several major professional societies. Since then, there have been a number of randomized controlled trials (RCTs) and consensus conferences. The American Association for the Study of Liver Diseases (AASLD) published updated practice guidelines in 2016 that encompass pathophysiology, monitoring, diagnosis, and treatment of gastroesophageal hemorrhage in cirrhotic patients. This summary will focus on inpatient management for active gastroesophageal hemorrhage.
Synopsis of Inpatient Management for Esophageal Variceal Hemorrhage: The authors suggest that all VH requires ICU admission with the goal of acute control of bleeding, prevention of early recurrence, and reduction in 6-week mortality. Imaging to rule out portal vein thrombosis and HCC should be considered. Hepatic-Venous Pressure Gradient (HVPG) greater than 20 mm Hg is the strongest predictor of early rebleeding and death. However, catheter measurements of portal pressure are not available at most centers. As with any critically ill patient, stabilization of respiratory status and ensuring hemodynamic stability with volume resuscitation is paramount. RCTs evaluating transfusion goals suggest that a restrictive transfusion goal of HgB 7 g/dL is superior to a liberal goal of 9 g/dL. The authors hypothesize this may be related to lower HVPG observed with lower transfusion thresholds. In terms of treating coagulopathy, RCTs evaluating recombinant VIIa have not shown clear benefit. Correction of INR with FFPs similarly not recommended. No recommendations are made regarding utility of platelet transfusions. Vasoactive drugs should be administered when VH is suspected with the goal of decreasing splanchnic blood flow. Octreotide is the only vasoactive drug available in the United States. RCTs show that antibiotics administered prophylactically decrease infections, recurrent hemorrhage, and death. Ceftriaxone 1 g daily is the drug of choice in the United States and should be given up to a maximum of 7 days. A reasonable strategy is discontinuation of prophylaxis concurrently with discontinuation of vasoactive agents. After stabilization of hemodynamics, patients should proceed to endoscopy no more than 12 hours after presentation. Endoscopic Variceal Ligation (EVL) should be done if signs of active or recent variceal bleeding are found. After EVL, select patients at high risk of rebleeding (Child-Pugh B with active bleeding seen on endoscopy or Child-Pugh C patients) may benefit from TIPS within 72 hours. If TIPS is done, vasoactive agents can be discontinued. Otherwise, vasoactive agents should continue for 2-5 days with subsequent transition to nonselective beta blockers (NSBB) such as nadolol or propranolol. For secondary prophylaxis of esophageal bleeding, combination EVL and NSBB is first-line therapy. If recurrent hemorrhage occurs while on secondary prophylaxis, rescue TIPS is recommended.
Synopsis of Inpatient Management for Gastric Variceal Hemorrhage: Management of Gastric Variceal Hemorrhage is similar to Esophageal Variceal (EV) Hemorrhage and encompasses volume resuscitation, vasoactive drugs, and antibiotics with endoscopy shortly thereafter. Balloon tamponade can be used as a bridge to endoscopy in massive bleeds. In addition to the above, anatomic location of Gastric Varices (GV) affects choice of intervention. GOV1 varices extend from the gastric cardia to the lesser curvature and represent 75% of GV. If these are small, they can be managed with EVL. Otherwise these can be managed with injection of cyanoacrylate glue. GOV2 varices extend from the gastric cardia into the fundus. Isolated GV type 1 varices (IGV1) are located entirely in the fundus and have the highest propensity for bleeding. For these latter two types of “cardio-fundal varices” TIPS is the preferred intervention to control acute bleeding. Data on the efficacy of secondary prophylaxis for GV bleeding is limited. A combination of NSBB, cyanoacrylate injection, or TIPS can be considered. Balloon Occluded Retrograde Transvenous Obliteration (BRTO) can be considered if fundal varices are associated with a large gastrorenal or splenorenal collateral. However, no RCTs have compared BRTO with other strategies. Isolated GV type 2 (IGV2) varices are not localized to the esophageal or gastric cardio-fundal region and are rare in cirrhotic patients but tend to occur in pre-hepatic portal hypertension. Management requires multidisciplinary input from endoscopists, hepatologists, interventional radiologists, and surgeons.
Bottom line: For esophageal variceal bleeding related to cirrhosis: volume resuscitation, antibiotic prophylaxis, and vasoactive agents are mainstays of therapy to stabilize patient for endoscopic intervention within 12 hours. This should be followed by early TIPS within 72 hours in high risk patients.
A similar approach applies to gastric variceal bleeding, but interventional management is dependent on the anatomic location of the varices in question.
Citations: Garcia-Tsao G et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis and management – 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017 Jan;65[1]:310-35.
Dr. Lu is a hospitalist at Cooper University Hospital in Camden, N.J.
Recent increase in subdural hematoma may be linked to antithrombotics
according to data published online Feb. 28 in JAMA.
A retrospective case-control study of 10,010 patients aged 20-89 years with a first-time subdural hematoma, matched by age, sex, and year to 400,380 general controls, showed treatment with a vitamin K antagonist was associated with a 3.69 greater risk of subdural hematoma, compared with controls, and a fourfold increase in risk when used concurrently with an antiplatelet drug (JAMA. 2017;317:836-46. doi: 10.1001/jama.2017.0639).
Low-dose aspirin alone was associated with a 24% increase in the risk of subdural hematoma; clopidogrel was associated with an 87% increase; and a direct oral anticoagulant such as dabigatran etexilate, rivaroxaban, or apixaban was associated with a 73% increase in risk.
Antithrombotic drugs were also associated with an increased risk of death from a subdural hematoma within 30 days after discharge for the hematoma’s diagnosis, an effect most evident with a direct oral anticoagulant or vitamin K antagonist.
Over the course of the Danish population-based study, which covered 2000-2015, the prevalence of antithrombotic drug use more than doubled, from 31 individuals per 1,000 to 76.9 per 1,000.
At the same time, the incidence of subdural hematoma nearly doubled (10.9 per 100,000 person-years to 19 per 100,000 person-years). The increase in subdural hematoma was greatest among older patients, from 55.1 per 100,000 person-years to 99.7 per 100,000 person-years.
“Although use of antithrombotic drugs has long been recognized as a risk factor for subdural hematoma, previous studies were either based exclusively on patients with subdural hematoma (i.e.,with no comparison group) or focused exclusively on patients treated with an anticoagulant,” wrote David Gaist, MD, PhD, of Odense University Hospital in Denmark and coauthors.
While the risk of subdural hematoma was greatest for the shortest duration of treatment with low-dose aspirin, the risk remained steady across all durations of treatment with clopidogrel and did not vary significantly for direct oral anticoagulants or vitamin K antagonists.
Women were more likely to show an increased risk of subdural hematoma with low-dose aspirin or vitamin K antagonist than men.
The analysis also showed that the association between low-dose aspirin and subdural hematoma was significantly higher for individuals aged 75-89 years than for those aged 20-64 years.
“Furthermore, the present results emphasize that the major shifts in patterns of antithrombotic drug treatment for older individuals, and the increasing use of more aggressive antithrombotic regimens, have already had a major effect on subdural hematoma incidence,” the authors wrote.
Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
according to data published online Feb. 28 in JAMA.
A retrospective case-control study of 10,010 patients aged 20-89 years with a first-time subdural hematoma, matched by age, sex, and year to 400,380 general controls, showed treatment with a vitamin K antagonist was associated with a 3.69 greater risk of subdural hematoma, compared with controls, and a fourfold increase in risk when used concurrently with an antiplatelet drug (JAMA. 2017;317:836-46. doi: 10.1001/jama.2017.0639).
Low-dose aspirin alone was associated with a 24% increase in the risk of subdural hematoma; clopidogrel was associated with an 87% increase; and a direct oral anticoagulant such as dabigatran etexilate, rivaroxaban, or apixaban was associated with a 73% increase in risk.
Antithrombotic drugs were also associated with an increased risk of death from a subdural hematoma within 30 days after discharge for the hematoma’s diagnosis, an effect most evident with a direct oral anticoagulant or vitamin K antagonist.
Over the course of the Danish population-based study, which covered 2000-2015, the prevalence of antithrombotic drug use more than doubled, from 31 individuals per 1,000 to 76.9 per 1,000.
At the same time, the incidence of subdural hematoma nearly doubled (10.9 per 100,000 person-years to 19 per 100,000 person-years). The increase in subdural hematoma was greatest among older patients, from 55.1 per 100,000 person-years to 99.7 per 100,000 person-years.
“Although use of antithrombotic drugs has long been recognized as a risk factor for subdural hematoma, previous studies were either based exclusively on patients with subdural hematoma (i.e.,with no comparison group) or focused exclusively on patients treated with an anticoagulant,” wrote David Gaist, MD, PhD, of Odense University Hospital in Denmark and coauthors.
While the risk of subdural hematoma was greatest for the shortest duration of treatment with low-dose aspirin, the risk remained steady across all durations of treatment with clopidogrel and did not vary significantly for direct oral anticoagulants or vitamin K antagonists.
Women were more likely to show an increased risk of subdural hematoma with low-dose aspirin or vitamin K antagonist than men.
The analysis also showed that the association between low-dose aspirin and subdural hematoma was significantly higher for individuals aged 75-89 years than for those aged 20-64 years.
“Furthermore, the present results emphasize that the major shifts in patterns of antithrombotic drug treatment for older individuals, and the increasing use of more aggressive antithrombotic regimens, have already had a major effect on subdural hematoma incidence,” the authors wrote.
Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
according to data published online Feb. 28 in JAMA.
A retrospective case-control study of 10,010 patients aged 20-89 years with a first-time subdural hematoma, matched by age, sex, and year to 400,380 general controls, showed treatment with a vitamin K antagonist was associated with a 3.69 greater risk of subdural hematoma, compared with controls, and a fourfold increase in risk when used concurrently with an antiplatelet drug (JAMA. 2017;317:836-46. doi: 10.1001/jama.2017.0639).
Low-dose aspirin alone was associated with a 24% increase in the risk of subdural hematoma; clopidogrel was associated with an 87% increase; and a direct oral anticoagulant such as dabigatran etexilate, rivaroxaban, or apixaban was associated with a 73% increase in risk.
Antithrombotic drugs were also associated with an increased risk of death from a subdural hematoma within 30 days after discharge for the hematoma’s diagnosis, an effect most evident with a direct oral anticoagulant or vitamin K antagonist.
Over the course of the Danish population-based study, which covered 2000-2015, the prevalence of antithrombotic drug use more than doubled, from 31 individuals per 1,000 to 76.9 per 1,000.
At the same time, the incidence of subdural hematoma nearly doubled (10.9 per 100,000 person-years to 19 per 100,000 person-years). The increase in subdural hematoma was greatest among older patients, from 55.1 per 100,000 person-years to 99.7 per 100,000 person-years.
“Although use of antithrombotic drugs has long been recognized as a risk factor for subdural hematoma, previous studies were either based exclusively on patients with subdural hematoma (i.e.,with no comparison group) or focused exclusively on patients treated with an anticoagulant,” wrote David Gaist, MD, PhD, of Odense University Hospital in Denmark and coauthors.
While the risk of subdural hematoma was greatest for the shortest duration of treatment with low-dose aspirin, the risk remained steady across all durations of treatment with clopidogrel and did not vary significantly for direct oral anticoagulants or vitamin K antagonists.
Women were more likely to show an increased risk of subdural hematoma with low-dose aspirin or vitamin K antagonist than men.
The analysis also showed that the association between low-dose aspirin and subdural hematoma was significantly higher for individuals aged 75-89 years than for those aged 20-64 years.
“Furthermore, the present results emphasize that the major shifts in patterns of antithrombotic drug treatment for older individuals, and the increasing use of more aggressive antithrombotic regimens, have already had a major effect on subdural hematoma incidence,” the authors wrote.
Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
FROM JAMA
Key clinical point: The increasing incidence of subdural hematoma may be linked more common use of antithrombotics.
Major finding: Antithrombotic medication is associated with as much as a fourfold increase in the risk of subdural hematoma.
Data source: A retrospective case-control study of 10,010 patients with a first-ever subdural hematoma.
Disclosures: Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
Prediction: LVADs will rule end-stage heart failure
SNOWMASS, COLO. – Multifaceted progress in mechanical circulatory support as long-term therapy in end-stage heart failure is happening at a brisk pace, Y. Joseph C. Woo, MD, reported at the Annual Cardiovascular Conference at Snowmass.
declared Dr. Woo, professor and chair of the department of cardiothoracic surgery at Stanford (Calif.) University.
That’s quite a prediction, especially considering the source: Stanford is where the late Dr. Norman Shumway – widely considered “the father of heart transplantation” – performed the first adult heart transplant in the United States in 1968.
Dr. Woo was coauthor of an American Heart Association policy statement on the future of cardiovascular disease in the United States, which forecast a 25% increase in heart failure between 2010 and 2030 (Circulation. 2011 Mar 1;123[8]:933-44). There is simply no way that heart transplantation can begin to meet the projected growing need for effective therapy in patients with end-stage disease.
Here’s what Dr. Woo sees as the future of MCS:
Minimally invasive implantation
At Stanford, LVAD implantations are now routinely done off-pump on a beating heart.
“We clamp only when there is a sound reason, like the presence of left ventricular thrombus, where you run the risk of embolization without the cross clamp,” the surgeon said.
Concomitant valvular surgery
At Stanford and other centers of excellence, surgeons perform additional procedures as warranted while they implant an LVAD, including atrial fibrillation ablation, revascularization of the right heart coronaries, patent foramen ovale closure, and repair of the tricuspid, pulmonic, or aortic valves.
Enhanced right ventricular management
Survival is greatly impaired if a patient with an LVAD later requires the addition of a right ventricular assist device. This realization has led to the development of multiple preoperative risk scoring systems by the Stanford group (Ann Thorac Surg. 2013 Sep;96[3]:857-63) and others, including investigators at the Deutsche Herzzentrum Berlin, the world’s busiest heart transplant center. The purpose is to identify upfront those patients who are likely to later develop right heart failure so they can receive biventricular MCS from the start.
Adjunctive biologic therapies
Intramyocardial injection of 25 million allogeneic mesenchymal precursor cells during LVAD implantation appeared to be safe and showed a promising efficacy signal in a 30-patient, multicenter, double-blind, placebo-controlled, National Institutes of Health–sponsored proof of concept study in which Dr. Woo was a coinvestigator (Circulation. 2014 Jun 3;129[22]:2287-96).
The goal of this research effort is to provide a cell therapy assist to the LVAD as a bridge to recovery of left ventricular function such that the device might eventually no longer be needed, he explained.
These cells are immune privileged. They can be transplanted into recipients without need for immunosuppressive therapy or HLA matching, basically as an off the shelf product. Rather than transforming into cardiomyocytes, it appears that the mechanism by which the donor cells enhance cardiac performance in heart failure is via secretion of a shower of growth and angiogenic factors.
Based upon the encouraging results of the initial study, a 90-patient, phase II, double-blind clinical trial is underway. In order to better evaluate efficacy, this time the patients will receive 150 million mesenchymal precursor cells rather than 25 million.
New technologies
The developmental pipeline is chock full of MCS devices. The trend is to go smaller and simpler. HeartWare is developing a miniaturized version of its approved continuous flow centrifugal force LVAD. The ReliantHeart aVAD, an intraventricular device less than 2.5 cm in diameter, is approved in Europe and under study in the U.S. The Thoratec HeartMate III is a smaller version of the HeartMate II, which is FDA-approved as destination therapy. And the Circulite Synergy micropump, designed to provide partial circulatory support to patients who don’t require a full-force LVAD, is the size of a AA battery.
Dr. Woo reported having no financial conflicts.
[email protected]
SNOWMASS, COLO. – Multifaceted progress in mechanical circulatory support as long-term therapy in end-stage heart failure is happening at a brisk pace, Y. Joseph C. Woo, MD, reported at the Annual Cardiovascular Conference at Snowmass.
declared Dr. Woo, professor and chair of the department of cardiothoracic surgery at Stanford (Calif.) University.
That’s quite a prediction, especially considering the source: Stanford is where the late Dr. Norman Shumway – widely considered “the father of heart transplantation” – performed the first adult heart transplant in the United States in 1968.
Dr. Woo was coauthor of an American Heart Association policy statement on the future of cardiovascular disease in the United States, which forecast a 25% increase in heart failure between 2010 and 2030 (Circulation. 2011 Mar 1;123[8]:933-44). There is simply no way that heart transplantation can begin to meet the projected growing need for effective therapy in patients with end-stage disease.
Here’s what Dr. Woo sees as the future of MCS:
Minimally invasive implantation
At Stanford, LVAD implantations are now routinely done off-pump on a beating heart.
“We clamp only when there is a sound reason, like the presence of left ventricular thrombus, where you run the risk of embolization without the cross clamp,” the surgeon said.
Concomitant valvular surgery
At Stanford and other centers of excellence, surgeons perform additional procedures as warranted while they implant an LVAD, including atrial fibrillation ablation, revascularization of the right heart coronaries, patent foramen ovale closure, and repair of the tricuspid, pulmonic, or aortic valves.
Enhanced right ventricular management
Survival is greatly impaired if a patient with an LVAD later requires the addition of a right ventricular assist device. This realization has led to the development of multiple preoperative risk scoring systems by the Stanford group (Ann Thorac Surg. 2013 Sep;96[3]:857-63) and others, including investigators at the Deutsche Herzzentrum Berlin, the world’s busiest heart transplant center. The purpose is to identify upfront those patients who are likely to later develop right heart failure so they can receive biventricular MCS from the start.
Adjunctive biologic therapies
Intramyocardial injection of 25 million allogeneic mesenchymal precursor cells during LVAD implantation appeared to be safe and showed a promising efficacy signal in a 30-patient, multicenter, double-blind, placebo-controlled, National Institutes of Health–sponsored proof of concept study in which Dr. Woo was a coinvestigator (Circulation. 2014 Jun 3;129[22]:2287-96).
The goal of this research effort is to provide a cell therapy assist to the LVAD as a bridge to recovery of left ventricular function such that the device might eventually no longer be needed, he explained.
These cells are immune privileged. They can be transplanted into recipients without need for immunosuppressive therapy or HLA matching, basically as an off the shelf product. Rather than transforming into cardiomyocytes, it appears that the mechanism by which the donor cells enhance cardiac performance in heart failure is via secretion of a shower of growth and angiogenic factors.
Based upon the encouraging results of the initial study, a 90-patient, phase II, double-blind clinical trial is underway. In order to better evaluate efficacy, this time the patients will receive 150 million mesenchymal precursor cells rather than 25 million.
New technologies
The developmental pipeline is chock full of MCS devices. The trend is to go smaller and simpler. HeartWare is developing a miniaturized version of its approved continuous flow centrifugal force LVAD. The ReliantHeart aVAD, an intraventricular device less than 2.5 cm in diameter, is approved in Europe and under study in the U.S. The Thoratec HeartMate III is a smaller version of the HeartMate II, which is FDA-approved as destination therapy. And the Circulite Synergy micropump, designed to provide partial circulatory support to patients who don’t require a full-force LVAD, is the size of a AA battery.
Dr. Woo reported having no financial conflicts.
[email protected]
SNOWMASS, COLO. – Multifaceted progress in mechanical circulatory support as long-term therapy in end-stage heart failure is happening at a brisk pace, Y. Joseph C. Woo, MD, reported at the Annual Cardiovascular Conference at Snowmass.
declared Dr. Woo, professor and chair of the department of cardiothoracic surgery at Stanford (Calif.) University.
That’s quite a prediction, especially considering the source: Stanford is where the late Dr. Norman Shumway – widely considered “the father of heart transplantation” – performed the first adult heart transplant in the United States in 1968.
Dr. Woo was coauthor of an American Heart Association policy statement on the future of cardiovascular disease in the United States, which forecast a 25% increase in heart failure between 2010 and 2030 (Circulation. 2011 Mar 1;123[8]:933-44). There is simply no way that heart transplantation can begin to meet the projected growing need for effective therapy in patients with end-stage disease.
Here’s what Dr. Woo sees as the future of MCS:
Minimally invasive implantation
At Stanford, LVAD implantations are now routinely done off-pump on a beating heart.
“We clamp only when there is a sound reason, like the presence of left ventricular thrombus, where you run the risk of embolization without the cross clamp,” the surgeon said.
Concomitant valvular surgery
At Stanford and other centers of excellence, surgeons perform additional procedures as warranted while they implant an LVAD, including atrial fibrillation ablation, revascularization of the right heart coronaries, patent foramen ovale closure, and repair of the tricuspid, pulmonic, or aortic valves.
Enhanced right ventricular management
Survival is greatly impaired if a patient with an LVAD later requires the addition of a right ventricular assist device. This realization has led to the development of multiple preoperative risk scoring systems by the Stanford group (Ann Thorac Surg. 2013 Sep;96[3]:857-63) and others, including investigators at the Deutsche Herzzentrum Berlin, the world’s busiest heart transplant center. The purpose is to identify upfront those patients who are likely to later develop right heart failure so they can receive biventricular MCS from the start.
Adjunctive biologic therapies
Intramyocardial injection of 25 million allogeneic mesenchymal precursor cells during LVAD implantation appeared to be safe and showed a promising efficacy signal in a 30-patient, multicenter, double-blind, placebo-controlled, National Institutes of Health–sponsored proof of concept study in which Dr. Woo was a coinvestigator (Circulation. 2014 Jun 3;129[22]:2287-96).
The goal of this research effort is to provide a cell therapy assist to the LVAD as a bridge to recovery of left ventricular function such that the device might eventually no longer be needed, he explained.
These cells are immune privileged. They can be transplanted into recipients without need for immunosuppressive therapy or HLA matching, basically as an off the shelf product. Rather than transforming into cardiomyocytes, it appears that the mechanism by which the donor cells enhance cardiac performance in heart failure is via secretion of a shower of growth and angiogenic factors.
Based upon the encouraging results of the initial study, a 90-patient, phase II, double-blind clinical trial is underway. In order to better evaluate efficacy, this time the patients will receive 150 million mesenchymal precursor cells rather than 25 million.
New technologies
The developmental pipeline is chock full of MCS devices. The trend is to go smaller and simpler. HeartWare is developing a miniaturized version of its approved continuous flow centrifugal force LVAD. The ReliantHeart aVAD, an intraventricular device less than 2.5 cm in diameter, is approved in Europe and under study in the U.S. The Thoratec HeartMate III is a smaller version of the HeartMate II, which is FDA-approved as destination therapy. And the Circulite Synergy micropump, designed to provide partial circulatory support to patients who don’t require a full-force LVAD, is the size of a AA battery.
Dr. Woo reported having no financial conflicts.
[email protected]
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS