User login
Hospital-led interventions cut pediatric asthma hospitalizations
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
FROM JAMA PEDIATRICS
Key clinical point: A hospital-driven intervention to improve management of asthma in children has achieved significant reductions in asthma-related hospitalizations and emergency department visits and increased medication uptake.
Major finding: A multifactorial intervention to improve asthma management in children was associated with a 41.8% relative reduction in asthma-related hospitalizations and a 42.4% reduction in emergency department visits.
Data source: A hospital-based intervention.
Disclosures: The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
VIDEO: What’s new in AAP’s pediatric hypertension guidelines
SAN FRANCISCO – The American Academy of Pediatrics recently released new hypertension guidelines for children and adolescents.
Some of the advice is similar to the group’s last effort in 2004, but there are a few key changes that clinicians need to know, according to lead author Joseph Flynn, MD, professor of pediatrics and chief of nephrology at Seattle Children’s Hospital. He explained what they are, and the reasons behind them, in an interview at the joint hypertension scientific sessions sponsored by the American Heart Association and the American Society of Hypertension (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
The prevalence of pediatric hypertension, he said, now rivals asthma.
SAN FRANCISCO – The American Academy of Pediatrics recently released new hypertension guidelines for children and adolescents.
Some of the advice is similar to the group’s last effort in 2004, but there are a few key changes that clinicians need to know, according to lead author Joseph Flynn, MD, professor of pediatrics and chief of nephrology at Seattle Children’s Hospital. He explained what they are, and the reasons behind them, in an interview at the joint hypertension scientific sessions sponsored by the American Heart Association and the American Society of Hypertension (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
The prevalence of pediatric hypertension, he said, now rivals asthma.
SAN FRANCISCO – The American Academy of Pediatrics recently released new hypertension guidelines for children and adolescents.
Some of the advice is similar to the group’s last effort in 2004, but there are a few key changes that clinicians need to know, according to lead author Joseph Flynn, MD, professor of pediatrics and chief of nephrology at Seattle Children’s Hospital. He explained what they are, and the reasons behind them, in an interview at the joint hypertension scientific sessions sponsored by the American Heart Association and the American Society of Hypertension (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
The prevalence of pediatric hypertension, he said, now rivals asthma.
EXPERT ANALYSIS FROM THE AHA/ASH JOINT SCIENTIFIC SESSIONS
Increase in sepsis incidence stable from 2009 to 2014
The trend for sepsis incidence from 2009 to 2014, “calculated relative to the observed 2014 rates,” was a stable increase of 0.6% per year using the more accurate of two forms of analysis, investigators reported.
The incidence of sepsis was an adjusted 5.9% among hospitalized adults in 2014, with in-hospital mortality of 15%, according to a retrospective cohort study published online Sept. 13 in JAMA.
“Most studies [of sepsis incidence] have used claims data, but increasing clinical awareness, changes in diagnosis and coding practices, and variable definitions have led to uncertainty about the accuracy of reported trends,” wrote Chanu Rhee, MD, of Harvard Medical School, Boston, and his associates (JAMA. 2017 Sep 13. doi: 10.1001/jama.2017.13836).
They used two methods – one involving claims-based estimates using ICD-9-CM codes and the other based on clinical data from electronic health records (EHRs) – to analyze data for more than 2.9 million adults admitted to 409 U.S. academic, community, and federal acute-care hospitals in 2014. The claims-based “explicit-codes” approach used discharge diagnoses of severe sepsis (995.92) or septic shock (785.52), while the EHR-based, clinical-criteria method included blood cultures, antibiotics, and concurrent organ dysfunction with or without the criterion of a lactate level of 2.0 mmol/L or greater, the investigators said.
The explicit-codes approach produced an increase of 10.3% per year in sepsis incidence from 2009 to 2014, compared with 0.6% per year for the clinical-criteria approach, while in-hospital mortality declined by 7% a year using explicit codes and 3.3% using clinical criteria, Dr. Rhee and his associates reported.
“EHR-based criteria were more sensitive than explicit sepsis codes on medical record review, with comparable [positive predictive value]; EHR-based criteria had similar sensitivity to implicit or explicit codes combined but higher [positive predictive value],” they said.
The estimates provided by Dr. Rhee and his associates provide “a clearer understanding of trends in the incidence and mortality of sepsis in the United States but also a better understanding of the challenges in improving ICD coding to accurately document the global burden of sepsis,” Kristina E. Rudd, MD, of the University of Washington, Seattle, and her associates said in an editorial (JAMA 2017 Sep 13. doi: 10.1001/jama.2017.13697).
The study was funded by the Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, National Institutes of Health, Department of Veterans Affairs, National Institutes of Health Clinical Center, and National Institute of Allergy and Infectious Diseases. Three of Dr. Rhee’s associates reported receiving personal fees from private companies or serving on advisory boards or as consultants. No other authors reported disclosures. Dr. Rudd and her associates had no conflicts of interest to report.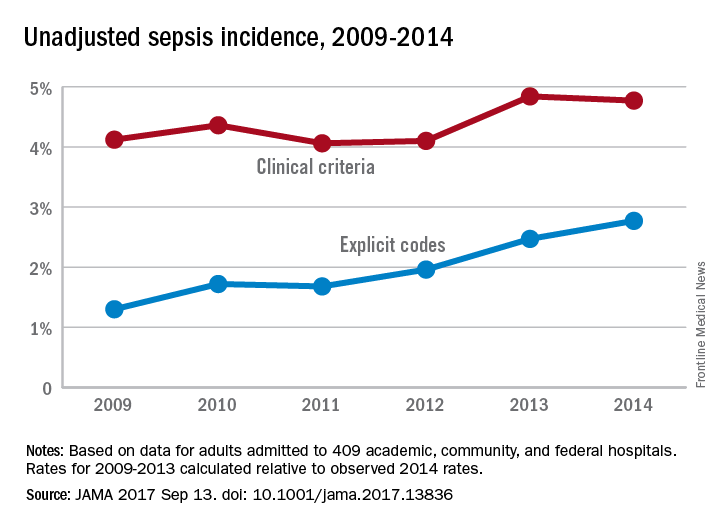
The trend for sepsis incidence from 2009 to 2014, “calculated relative to the observed 2014 rates,” was a stable increase of 0.6% per year using the more accurate of two forms of analysis, investigators reported.
The incidence of sepsis was an adjusted 5.9% among hospitalized adults in 2014, with in-hospital mortality of 15%, according to a retrospective cohort study published online Sept. 13 in JAMA.
“Most studies [of sepsis incidence] have used claims data, but increasing clinical awareness, changes in diagnosis and coding practices, and variable definitions have led to uncertainty about the accuracy of reported trends,” wrote Chanu Rhee, MD, of Harvard Medical School, Boston, and his associates (JAMA. 2017 Sep 13. doi: 10.1001/jama.2017.13836).
They used two methods – one involving claims-based estimates using ICD-9-CM codes and the other based on clinical data from electronic health records (EHRs) – to analyze data for more than 2.9 million adults admitted to 409 U.S. academic, community, and federal acute-care hospitals in 2014. The claims-based “explicit-codes” approach used discharge diagnoses of severe sepsis (995.92) or septic shock (785.52), while the EHR-based, clinical-criteria method included blood cultures, antibiotics, and concurrent organ dysfunction with or without the criterion of a lactate level of 2.0 mmol/L or greater, the investigators said.
The explicit-codes approach produced an increase of 10.3% per year in sepsis incidence from 2009 to 2014, compared with 0.6% per year for the clinical-criteria approach, while in-hospital mortality declined by 7% a year using explicit codes and 3.3% using clinical criteria, Dr. Rhee and his associates reported.
“EHR-based criteria were more sensitive than explicit sepsis codes on medical record review, with comparable [positive predictive value]; EHR-based criteria had similar sensitivity to implicit or explicit codes combined but higher [positive predictive value],” they said.
The estimates provided by Dr. Rhee and his associates provide “a clearer understanding of trends in the incidence and mortality of sepsis in the United States but also a better understanding of the challenges in improving ICD coding to accurately document the global burden of sepsis,” Kristina E. Rudd, MD, of the University of Washington, Seattle, and her associates said in an editorial (JAMA 2017 Sep 13. doi: 10.1001/jama.2017.13697).
The study was funded by the Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, National Institutes of Health, Department of Veterans Affairs, National Institutes of Health Clinical Center, and National Institute of Allergy and Infectious Diseases. Three of Dr. Rhee’s associates reported receiving personal fees from private companies or serving on advisory boards or as consultants. No other authors reported disclosures. Dr. Rudd and her associates had no conflicts of interest to report.
The trend for sepsis incidence from 2009 to 2014, “calculated relative to the observed 2014 rates,” was a stable increase of 0.6% per year using the more accurate of two forms of analysis, investigators reported.
The incidence of sepsis was an adjusted 5.9% among hospitalized adults in 2014, with in-hospital mortality of 15%, according to a retrospective cohort study published online Sept. 13 in JAMA.
“Most studies [of sepsis incidence] have used claims data, but increasing clinical awareness, changes in diagnosis and coding practices, and variable definitions have led to uncertainty about the accuracy of reported trends,” wrote Chanu Rhee, MD, of Harvard Medical School, Boston, and his associates (JAMA. 2017 Sep 13. doi: 10.1001/jama.2017.13836).
They used two methods – one involving claims-based estimates using ICD-9-CM codes and the other based on clinical data from electronic health records (EHRs) – to analyze data for more than 2.9 million adults admitted to 409 U.S. academic, community, and federal acute-care hospitals in 2014. The claims-based “explicit-codes” approach used discharge diagnoses of severe sepsis (995.92) or septic shock (785.52), while the EHR-based, clinical-criteria method included blood cultures, antibiotics, and concurrent organ dysfunction with or without the criterion of a lactate level of 2.0 mmol/L or greater, the investigators said.
The explicit-codes approach produced an increase of 10.3% per year in sepsis incidence from 2009 to 2014, compared with 0.6% per year for the clinical-criteria approach, while in-hospital mortality declined by 7% a year using explicit codes and 3.3% using clinical criteria, Dr. Rhee and his associates reported.
“EHR-based criteria were more sensitive than explicit sepsis codes on medical record review, with comparable [positive predictive value]; EHR-based criteria had similar sensitivity to implicit or explicit codes combined but higher [positive predictive value],” they said.
The estimates provided by Dr. Rhee and his associates provide “a clearer understanding of trends in the incidence and mortality of sepsis in the United States but also a better understanding of the challenges in improving ICD coding to accurately document the global burden of sepsis,” Kristina E. Rudd, MD, of the University of Washington, Seattle, and her associates said in an editorial (JAMA 2017 Sep 13. doi: 10.1001/jama.2017.13697).
The study was funded by the Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, National Institutes of Health, Department of Veterans Affairs, National Institutes of Health Clinical Center, and National Institute of Allergy and Infectious Diseases. Three of Dr. Rhee’s associates reported receiving personal fees from private companies or serving on advisory boards or as consultants. No other authors reported disclosures. Dr. Rudd and her associates had no conflicts of interest to report.
FROM JAMA
Bezlotoxumab may lower risk of C. difficile readmissions
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: A total of 27 of 530 (5%) bezlotoxumab patients were readmitted within 30 days of discharge compared with 58 of 520 (11%) placebo patients.
Data source: Randomized, double-blind, placebo-controlled, multicenter, global phase 3 trials conducted from November 2011-May 2015 at 322 sites in 30 countries.
Disclosures: All investigators report employment or financial support with Merck and have individually reported financial ties to similar companies like Astellas, AstraZeneca, Pfizer, and others.
Alcohol misuse universal screening effective and efficient
Universal screening for alcohol misuse in acute medical admissions is feasible and can reduce readmissions for liver disease, according to a new study.
Detecting patients’ alcohol misuse early can help treat or prevent alcohol-related liver disease, such as cirrhosis; however, screening is not being used in a routine, effective way, according to Greta Westwood, PhD, head of Nursing, Midwifery, and AHP Research at Portsmouth (England) Hospitals and her fellow investigators.
“In primary care, screening is highly variable, and treatment rates are low, often focusing on patients who already have advanced psychiatric or physical illness,” Dr. Westwood and her colleagues wrote. “In addition, many patients with alcohol use disorders do not fully engage with primary care services for a variety of reasons, often leading to excessive use of the hospital ED as the first point of contact.”
Investigators conducted a retrospective, observational study of 53,165 patients who were admitted to the acute medical unit at Queen Alexandra Hospital, Portsmouth, England, between July 2011 and March 2014 (J Hepatol. 2017 Sep;67[3]:559-67).
More than half of patients were male (52%), the average patient age was 67 years, and the patients had an average of three previous hospital admissions.
Of the patients observed, 48,211 (90.68%) completed the screening test, while the remaining 4,934 (9.32%) did not.
Those who were not screened had a higher mortality rate than did those who were (8.30% vs 6.17%; P less than .001), were more likely to be discharged the same day (3.37% vs. 1.87%; P less than .001), and were more likely to discharge themselves (29.67% vs. 13.31%; P less than .001).
The screening process, an electronic modified version of the Paddington Alcohol Test, consisted of the nurses’ asking a series of questions about types of alcohol consumed, frequency and maximum daily amount, whether the admission was considered alcohol related, and they documented signs of alcohol withdrawal.
Patients were then given a score based on how the answers compared with the healthy level of alcohol consumption, with 0-2 points considered “low risk,” 3-5 points considered “increasing risk,” and 6-10 points considered “high risk.”
Those assigned a low-risk status were not referred to intervention, but doctors recommended increasing-risk patients attend a community alcohol intervention team for brief intervention, while high-risk patients were automatically referred to an Alcohol Specialist Nursing Service (ASNS).
Of those screened, there were 1,135 patients (2.33%) considered at increasing risk of alcohol misuse and 1,921 (3.98%) at high risk.
While 68.5% of patients with a high-risk score were referred to the ASNS, all those who were referred completed the medical detoxification course, according to investigators.
High-risk patients were found to have had, on average, more hospital visits than increasing- and low-risk patients – 4.74 visits, compared with 2.92 and 3.00, respectively; they also reported more ED trips – 7.68 visits, compared with 3.81 and 2.64, respectively.
Dr. Westwood and her colleagues found that, when using the screening tool, investigators were more likely to find signs of alcohol-related liver disease among those with higher scores.
Liver, pancreatic, and digestive disorders accounted for 22.1% of primary admission codes of high-risk patients, compared with 3.2% of low-risk patients.
Investigators wrote that this tool can help doctors identify at-risk patients early and attack the problem of alcohol misuse head on and in a timely manner.
“It is vital that patients with cirrhosis who continue to drink are identified and referred to dedicated hospital alcohol care teams,” Dr. Westwood and her colleagues wrote. “Screening can identify patients at an increased risk of alcohol-related harm whose range of diagnoses is not dissimilar to lower-risk patients and whose misuse of alcohol might otherwise have not been identified.”
Investigators did not account for decreased scores or testing effectiveness in patients readmitted and retested. Additionally, the long-term impact of ASNS care is still being studied.
Two investigators reported affiliations with the Learning Clinic, which created and licensed the analysis program that is part of the screening tool. All other investigators, including Dr. Westwood, reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
Universal screening for alcohol misuse in acute medical admissions is feasible and can reduce readmissions for liver disease, according to a new study.
Detecting patients’ alcohol misuse early can help treat or prevent alcohol-related liver disease, such as cirrhosis; however, screening is not being used in a routine, effective way, according to Greta Westwood, PhD, head of Nursing, Midwifery, and AHP Research at Portsmouth (England) Hospitals and her fellow investigators.
“In primary care, screening is highly variable, and treatment rates are low, often focusing on patients who already have advanced psychiatric or physical illness,” Dr. Westwood and her colleagues wrote. “In addition, many patients with alcohol use disorders do not fully engage with primary care services for a variety of reasons, often leading to excessive use of the hospital ED as the first point of contact.”
Investigators conducted a retrospective, observational study of 53,165 patients who were admitted to the acute medical unit at Queen Alexandra Hospital, Portsmouth, England, between July 2011 and March 2014 (J Hepatol. 2017 Sep;67[3]:559-67).
More than half of patients were male (52%), the average patient age was 67 years, and the patients had an average of three previous hospital admissions.
Of the patients observed, 48,211 (90.68%) completed the screening test, while the remaining 4,934 (9.32%) did not.
Those who were not screened had a higher mortality rate than did those who were (8.30% vs 6.17%; P less than .001), were more likely to be discharged the same day (3.37% vs. 1.87%; P less than .001), and were more likely to discharge themselves (29.67% vs. 13.31%; P less than .001).
The screening process, an electronic modified version of the Paddington Alcohol Test, consisted of the nurses’ asking a series of questions about types of alcohol consumed, frequency and maximum daily amount, whether the admission was considered alcohol related, and they documented signs of alcohol withdrawal.
Patients were then given a score based on how the answers compared with the healthy level of alcohol consumption, with 0-2 points considered “low risk,” 3-5 points considered “increasing risk,” and 6-10 points considered “high risk.”
Those assigned a low-risk status were not referred to intervention, but doctors recommended increasing-risk patients attend a community alcohol intervention team for brief intervention, while high-risk patients were automatically referred to an Alcohol Specialist Nursing Service (ASNS).
Of those screened, there were 1,135 patients (2.33%) considered at increasing risk of alcohol misuse and 1,921 (3.98%) at high risk.
While 68.5% of patients with a high-risk score were referred to the ASNS, all those who were referred completed the medical detoxification course, according to investigators.
High-risk patients were found to have had, on average, more hospital visits than increasing- and low-risk patients – 4.74 visits, compared with 2.92 and 3.00, respectively; they also reported more ED trips – 7.68 visits, compared with 3.81 and 2.64, respectively.
Dr. Westwood and her colleagues found that, when using the screening tool, investigators were more likely to find signs of alcohol-related liver disease among those with higher scores.
Liver, pancreatic, and digestive disorders accounted for 22.1% of primary admission codes of high-risk patients, compared with 3.2% of low-risk patients.
Investigators wrote that this tool can help doctors identify at-risk patients early and attack the problem of alcohol misuse head on and in a timely manner.
“It is vital that patients with cirrhosis who continue to drink are identified and referred to dedicated hospital alcohol care teams,” Dr. Westwood and her colleagues wrote. “Screening can identify patients at an increased risk of alcohol-related harm whose range of diagnoses is not dissimilar to lower-risk patients and whose misuse of alcohol might otherwise have not been identified.”
Investigators did not account for decreased scores or testing effectiveness in patients readmitted and retested. Additionally, the long-term impact of ASNS care is still being studied.
Two investigators reported affiliations with the Learning Clinic, which created and licensed the analysis program that is part of the screening tool. All other investigators, including Dr. Westwood, reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
Universal screening for alcohol misuse in acute medical admissions is feasible and can reduce readmissions for liver disease, according to a new study.
Detecting patients’ alcohol misuse early can help treat or prevent alcohol-related liver disease, such as cirrhosis; however, screening is not being used in a routine, effective way, according to Greta Westwood, PhD, head of Nursing, Midwifery, and AHP Research at Portsmouth (England) Hospitals and her fellow investigators.
“In primary care, screening is highly variable, and treatment rates are low, often focusing on patients who already have advanced psychiatric or physical illness,” Dr. Westwood and her colleagues wrote. “In addition, many patients with alcohol use disorders do not fully engage with primary care services for a variety of reasons, often leading to excessive use of the hospital ED as the first point of contact.”
Investigators conducted a retrospective, observational study of 53,165 patients who were admitted to the acute medical unit at Queen Alexandra Hospital, Portsmouth, England, between July 2011 and March 2014 (J Hepatol. 2017 Sep;67[3]:559-67).
More than half of patients were male (52%), the average patient age was 67 years, and the patients had an average of three previous hospital admissions.
Of the patients observed, 48,211 (90.68%) completed the screening test, while the remaining 4,934 (9.32%) did not.
Those who were not screened had a higher mortality rate than did those who were (8.30% vs 6.17%; P less than .001), were more likely to be discharged the same day (3.37% vs. 1.87%; P less than .001), and were more likely to discharge themselves (29.67% vs. 13.31%; P less than .001).
The screening process, an electronic modified version of the Paddington Alcohol Test, consisted of the nurses’ asking a series of questions about types of alcohol consumed, frequency and maximum daily amount, whether the admission was considered alcohol related, and they documented signs of alcohol withdrawal.
Patients were then given a score based on how the answers compared with the healthy level of alcohol consumption, with 0-2 points considered “low risk,” 3-5 points considered “increasing risk,” and 6-10 points considered “high risk.”
Those assigned a low-risk status were not referred to intervention, but doctors recommended increasing-risk patients attend a community alcohol intervention team for brief intervention, while high-risk patients were automatically referred to an Alcohol Specialist Nursing Service (ASNS).
Of those screened, there were 1,135 patients (2.33%) considered at increasing risk of alcohol misuse and 1,921 (3.98%) at high risk.
While 68.5% of patients with a high-risk score were referred to the ASNS, all those who were referred completed the medical detoxification course, according to investigators.
High-risk patients were found to have had, on average, more hospital visits than increasing- and low-risk patients – 4.74 visits, compared with 2.92 and 3.00, respectively; they also reported more ED trips – 7.68 visits, compared with 3.81 and 2.64, respectively.
Dr. Westwood and her colleagues found that, when using the screening tool, investigators were more likely to find signs of alcohol-related liver disease among those with higher scores.
Liver, pancreatic, and digestive disorders accounted for 22.1% of primary admission codes of high-risk patients, compared with 3.2% of low-risk patients.
Investigators wrote that this tool can help doctors identify at-risk patients early and attack the problem of alcohol misuse head on and in a timely manner.
“It is vital that patients with cirrhosis who continue to drink are identified and referred to dedicated hospital alcohol care teams,” Dr. Westwood and her colleagues wrote. “Screening can identify patients at an increased risk of alcohol-related harm whose range of diagnoses is not dissimilar to lower-risk patients and whose misuse of alcohol might otherwise have not been identified.”
Investigators did not account for decreased scores or testing effectiveness in patients readmitted and retested. Additionally, the long-term impact of ASNS care is still being studied.
Two investigators reported affiliations with the Learning Clinic, which created and licensed the analysis program that is part of the screening tool. All other investigators, including Dr. Westwood, reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
FROM JOURNAL OF HEPATOLOGY
Key clinical point:
Major finding: Patients who were admitted and were not screened for alcohol misuse risk had a higher mortality rate, compared with those who were screened (8.3% vs. 6.17%; P less than .001).
Data source: Retrospective observational study of 53,165 patients admitted to the acute medical clinic of the Queen Alexandra Hospital, Portsmouth, England, between July 2011 and March 2014.
Disclosures: Two investigators reported affiliations with the Learning Clinic, which created and licensed the analysis program that is part of the screening tool. All other investigators, including Dr. Westwood, reported no relevant financial disclosures.
Study: Don’t separate NAS infants from moms
NASHVILLE, TENN. – When newborns withdrawing from opioids stay with their mothers after delivery instead of going to the NICU, they are far less likely to receive morphine and other drugs and leave the hospital days sooner; they also are more likely to go home with their mother, a meta-analysis showed.
The analysis likely is the first to pool results from studies of rooming-in for infants with neonatal abstinence syndrome (NAS). A strong case has been building in the literature for several years that newborns do better with rooming-in, instead of the traditional approach for NAS – NICU housing and opioid dosing based on a symptom checklist.
“We found consistent emerging evidence that rooming-in is more effective than standard care in the NICU for infants with NAS. Based on these findings, we believe rooming-in should be established as the new evidence-based standard of care for this patient population,” said investigator Kanak Verma, a medical student at Dartmouth College, Hanover, N.H.
Rooming-in was associated with a 63% reduction in the need for pharmacotherapy, a decrease in hospital length of stay by more than 10 days, and a substantial, statistically significant decrease in cost from – in one study – a mean of almost $45,000 per NAS infant stay to just over $10,000.
“We were worried that by rooming-in we would be undertreating infants with NAS, and that they would be at increased risk for readmission, but there was no statistically significant increase in readmission rates for infants rooming in with their mothers,” Ms. Verma said at the Pediatric Hospital Medical annual meeting.
Infants also were more likely to go home with their mother or a family member. “Mothers who use opioid replacements have decreased ability to bond” with their infants. Rooming-in helps create that bond, and probably made discharge with a family member more likely, said coinvestigator Cassandra Rendon, also a Dartmouth medical student.
It’s unclear what exactly accounts for the better results, but “having a baby stay with [its] mom creates an opportunity for a lot of things that we know are effective,” including skin-to-skin contact, breastfeeding, and involvement of mothers in the care and monitoring of their infants, Ms. Rendon said.
Also, “we know that in babies with NAS, a low-stimulation environment is ideal,” Ms. Verma said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association. That’s a challenge in a busy NICU, but “we can create that in an isolated room with just the mother,” she added.
At least one of the studies used a new, more holistic approach to assess the need for pharmacologic management in NAS. Symptom scores still are considered, but how well the infant is eating, sleeping, and able to be consoled are considered as well. With the traditional symptom checklist, “we end up just treating the number, instead of treating the baby. What Dartmouth and other facilities are doing is looking at” how well the baby is doing overall, Ms. Rendon said.
If the baby is otherwise doing well, providers are less likely to give opioids for a little jitteriness or sweating. The decreased use of opioids leads, in turn, to shorter hospital stays.
Dartmouth is collaborating with Yale University in New Haven , Conn., and the Boston Medical Center to integrate the new treatment model into standard practice. For other centers interested in doing the same, Ms. Verma noted that nursery staff buy-in is essential. Nurses and others have to be comfortable “taking these patients out of the NICU” and treating them in a new way.
The investigators had no relevant financial disclosures.
NASHVILLE, TENN. – When newborns withdrawing from opioids stay with their mothers after delivery instead of going to the NICU, they are far less likely to receive morphine and other drugs and leave the hospital days sooner; they also are more likely to go home with their mother, a meta-analysis showed.
The analysis likely is the first to pool results from studies of rooming-in for infants with neonatal abstinence syndrome (NAS). A strong case has been building in the literature for several years that newborns do better with rooming-in, instead of the traditional approach for NAS – NICU housing and opioid dosing based on a symptom checklist.
“We found consistent emerging evidence that rooming-in is more effective than standard care in the NICU for infants with NAS. Based on these findings, we believe rooming-in should be established as the new evidence-based standard of care for this patient population,” said investigator Kanak Verma, a medical student at Dartmouth College, Hanover, N.H.
Rooming-in was associated with a 63% reduction in the need for pharmacotherapy, a decrease in hospital length of stay by more than 10 days, and a substantial, statistically significant decrease in cost from – in one study – a mean of almost $45,000 per NAS infant stay to just over $10,000.
“We were worried that by rooming-in we would be undertreating infants with NAS, and that they would be at increased risk for readmission, but there was no statistically significant increase in readmission rates for infants rooming in with their mothers,” Ms. Verma said at the Pediatric Hospital Medical annual meeting.
Infants also were more likely to go home with their mother or a family member. “Mothers who use opioid replacements have decreased ability to bond” with their infants. Rooming-in helps create that bond, and probably made discharge with a family member more likely, said coinvestigator Cassandra Rendon, also a Dartmouth medical student.
It’s unclear what exactly accounts for the better results, but “having a baby stay with [its] mom creates an opportunity for a lot of things that we know are effective,” including skin-to-skin contact, breastfeeding, and involvement of mothers in the care and monitoring of their infants, Ms. Rendon said.
Also, “we know that in babies with NAS, a low-stimulation environment is ideal,” Ms. Verma said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association. That’s a challenge in a busy NICU, but “we can create that in an isolated room with just the mother,” she added.
At least one of the studies used a new, more holistic approach to assess the need for pharmacologic management in NAS. Symptom scores still are considered, but how well the infant is eating, sleeping, and able to be consoled are considered as well. With the traditional symptom checklist, “we end up just treating the number, instead of treating the baby. What Dartmouth and other facilities are doing is looking at” how well the baby is doing overall, Ms. Rendon said.
If the baby is otherwise doing well, providers are less likely to give opioids for a little jitteriness or sweating. The decreased use of opioids leads, in turn, to shorter hospital stays.
Dartmouth is collaborating with Yale University in New Haven , Conn., and the Boston Medical Center to integrate the new treatment model into standard practice. For other centers interested in doing the same, Ms. Verma noted that nursery staff buy-in is essential. Nurses and others have to be comfortable “taking these patients out of the NICU” and treating them in a new way.
The investigators had no relevant financial disclosures.
NASHVILLE, TENN. – When newborns withdrawing from opioids stay with their mothers after delivery instead of going to the NICU, they are far less likely to receive morphine and other drugs and leave the hospital days sooner; they also are more likely to go home with their mother, a meta-analysis showed.
The analysis likely is the first to pool results from studies of rooming-in for infants with neonatal abstinence syndrome (NAS). A strong case has been building in the literature for several years that newborns do better with rooming-in, instead of the traditional approach for NAS – NICU housing and opioid dosing based on a symptom checklist.
“We found consistent emerging evidence that rooming-in is more effective than standard care in the NICU for infants with NAS. Based on these findings, we believe rooming-in should be established as the new evidence-based standard of care for this patient population,” said investigator Kanak Verma, a medical student at Dartmouth College, Hanover, N.H.
Rooming-in was associated with a 63% reduction in the need for pharmacotherapy, a decrease in hospital length of stay by more than 10 days, and a substantial, statistically significant decrease in cost from – in one study – a mean of almost $45,000 per NAS infant stay to just over $10,000.
“We were worried that by rooming-in we would be undertreating infants with NAS, and that they would be at increased risk for readmission, but there was no statistically significant increase in readmission rates for infants rooming in with their mothers,” Ms. Verma said at the Pediatric Hospital Medical annual meeting.
Infants also were more likely to go home with their mother or a family member. “Mothers who use opioid replacements have decreased ability to bond” with their infants. Rooming-in helps create that bond, and probably made discharge with a family member more likely, said coinvestigator Cassandra Rendon, also a Dartmouth medical student.
It’s unclear what exactly accounts for the better results, but “having a baby stay with [its] mom creates an opportunity for a lot of things that we know are effective,” including skin-to-skin contact, breastfeeding, and involvement of mothers in the care and monitoring of their infants, Ms. Rendon said.
Also, “we know that in babies with NAS, a low-stimulation environment is ideal,” Ms. Verma said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association. That’s a challenge in a busy NICU, but “we can create that in an isolated room with just the mother,” she added.
At least one of the studies used a new, more holistic approach to assess the need for pharmacologic management in NAS. Symptom scores still are considered, but how well the infant is eating, sleeping, and able to be consoled are considered as well. With the traditional symptom checklist, “we end up just treating the number, instead of treating the baby. What Dartmouth and other facilities are doing is looking at” how well the baby is doing overall, Ms. Rendon said.
If the baby is otherwise doing well, providers are less likely to give opioids for a little jitteriness or sweating. The decreased use of opioids leads, in turn, to shorter hospital stays.
Dartmouth is collaborating with Yale University in New Haven , Conn., and the Boston Medical Center to integrate the new treatment model into standard practice. For other centers interested in doing the same, Ms. Verma noted that nursery staff buy-in is essential. Nurses and others have to be comfortable “taking these patients out of the NICU” and treating them in a new way.
The investigators had no relevant financial disclosures.
AT PHM 2017
Key clinical point:
Major finding: Rooming-in was associated with a 63% reduction in the need for pharmacotherapy, a decrease in hospital length of stay by more than 10 days, and a substantial, statistically significant decrease in cost from, in one study, a mean of almost $45,000 per NAS infant stay to just over $10,000.
Data source: A meta-analysis of six studies.
Disclosures: The investigators had no relevant financial disclosures.
Ivabradine cut mortality in HFrEF patients not on beta-blocker
BARCELONA – The time is right for a placebo-controlled, randomized trial of ivabradine in patients with heart failure with reduced ejection fraction who are unwilling or unable to take a beta-blocker as recommended in the guidelines, John G.F. Cleland, MD, asserted at the annual congress of the European Society of Cardiology.
He cited as the rationale for such a study a new post-hoc analysis of data from the SHIFT trial showing that ivabradine (Corlanor) significantly reduced both cardiovascular and all-cause mortality, as well as hospitalizations for heart failure, in the subset of study participants who weren’t on beta-blocker therapy.
“I think there would be ethical equipoise,” he added. “If patients are unwilling or unable to take a beta-blocker, or their cardiologist feels it’s not in their best interest, then I certainly think a placebo-controlled trial would not only be appropriate, but there’s also an onus on the cardiology community to do such a trial.”
Ivabradine slows heart rate by a unique mechanism that doesn’t involve blockade of adrenergic receptors. In the SHIFT trial (Lancet. 2010 Sep 11;376[9744]:875-85), more than 6,500 patients with heart failure with reduced ejection fraction (HFrEF) in sinus rhythm and with a heart rate greater than 70 bpm were randomized to ivabradine or placebo on top of guideline-directed medical therapy for heart failure. During a median 23 months of follow-up, heart failure hospitalizations were significantly reduced by 26% in the ivabradine group, although cardiovascular deaths were not significantly affected.
As a result of the SHIFT findings, the drug was approved with an indication for use only in combination with a beta-blocker in patients with HFrEF whose on-treatment heart rate exceeds 70 bpm. Ivabradine is not currently recommended as an alternative to beta-blocker therapy. However, in real-world clinical practice a large number of heart failure patients are not managed with a beta-blocker, the cardiologist noted.
His post-hoc analysis focused on the 685 SHIFT participants who were not on a beta-blocker at randomization. During follow-up, there were 93 deaths among patients who were on placebo and only 71 in those randomized to ivabradine, for a statistically significant 30% reduction in all-cause mortality. Cardiovascular mortality was reduced to a similar extent. These hazard ratios remained similar after adjusting for differences in heart rate and other clinical characteristics.
“Beta-blockers are a highly effective therapy for heart failure with reduced ejection fraction, but the mechanism of benefit remains uncertain. It might simply be due to heart rate reduction. And I would point out that we have no evidence of a dose response for beta-blockers: It may well be that you get most of the effect of a beta-blocker with the lowest dose. Titrating to the full dose of a beta-blocker might only be helpful in that it lowers your heart rate. I would argue that 6.25 mg/day of carvedilol plus ivabradine might be as good as 50 mg twice daily of carvedilol but with much higher patient acceptability. We don’t know,” said Dr. Cleland.
“This is an interesting, hypothesis-generating analysis, and we need confirmation now that ivabradine reduces mortality in heart failure patients who are unwilling or unable to take a beta-blocker,” he concluded.
The SHIFT trial was sponsored by Servier. Dr. Cleland reported serving as a consultant to and receiving research funding from that company and others.
BARCELONA – The time is right for a placebo-controlled, randomized trial of ivabradine in patients with heart failure with reduced ejection fraction who are unwilling or unable to take a beta-blocker as recommended in the guidelines, John G.F. Cleland, MD, asserted at the annual congress of the European Society of Cardiology.
He cited as the rationale for such a study a new post-hoc analysis of data from the SHIFT trial showing that ivabradine (Corlanor) significantly reduced both cardiovascular and all-cause mortality, as well as hospitalizations for heart failure, in the subset of study participants who weren’t on beta-blocker therapy.
“I think there would be ethical equipoise,” he added. “If patients are unwilling or unable to take a beta-blocker, or their cardiologist feels it’s not in their best interest, then I certainly think a placebo-controlled trial would not only be appropriate, but there’s also an onus on the cardiology community to do such a trial.”
Ivabradine slows heart rate by a unique mechanism that doesn’t involve blockade of adrenergic receptors. In the SHIFT trial (Lancet. 2010 Sep 11;376[9744]:875-85), more than 6,500 patients with heart failure with reduced ejection fraction (HFrEF) in sinus rhythm and with a heart rate greater than 70 bpm were randomized to ivabradine or placebo on top of guideline-directed medical therapy for heart failure. During a median 23 months of follow-up, heart failure hospitalizations were significantly reduced by 26% in the ivabradine group, although cardiovascular deaths were not significantly affected.
As a result of the SHIFT findings, the drug was approved with an indication for use only in combination with a beta-blocker in patients with HFrEF whose on-treatment heart rate exceeds 70 bpm. Ivabradine is not currently recommended as an alternative to beta-blocker therapy. However, in real-world clinical practice a large number of heart failure patients are not managed with a beta-blocker, the cardiologist noted.
His post-hoc analysis focused on the 685 SHIFT participants who were not on a beta-blocker at randomization. During follow-up, there were 93 deaths among patients who were on placebo and only 71 in those randomized to ivabradine, for a statistically significant 30% reduction in all-cause mortality. Cardiovascular mortality was reduced to a similar extent. These hazard ratios remained similar after adjusting for differences in heart rate and other clinical characteristics.
“Beta-blockers are a highly effective therapy for heart failure with reduced ejection fraction, but the mechanism of benefit remains uncertain. It might simply be due to heart rate reduction. And I would point out that we have no evidence of a dose response for beta-blockers: It may well be that you get most of the effect of a beta-blocker with the lowest dose. Titrating to the full dose of a beta-blocker might only be helpful in that it lowers your heart rate. I would argue that 6.25 mg/day of carvedilol plus ivabradine might be as good as 50 mg twice daily of carvedilol but with much higher patient acceptability. We don’t know,” said Dr. Cleland.
“This is an interesting, hypothesis-generating analysis, and we need confirmation now that ivabradine reduces mortality in heart failure patients who are unwilling or unable to take a beta-blocker,” he concluded.
The SHIFT trial was sponsored by Servier. Dr. Cleland reported serving as a consultant to and receiving research funding from that company and others.
BARCELONA – The time is right for a placebo-controlled, randomized trial of ivabradine in patients with heart failure with reduced ejection fraction who are unwilling or unable to take a beta-blocker as recommended in the guidelines, John G.F. Cleland, MD, asserted at the annual congress of the European Society of Cardiology.
He cited as the rationale for such a study a new post-hoc analysis of data from the SHIFT trial showing that ivabradine (Corlanor) significantly reduced both cardiovascular and all-cause mortality, as well as hospitalizations for heart failure, in the subset of study participants who weren’t on beta-blocker therapy.
“I think there would be ethical equipoise,” he added. “If patients are unwilling or unable to take a beta-blocker, or their cardiologist feels it’s not in their best interest, then I certainly think a placebo-controlled trial would not only be appropriate, but there’s also an onus on the cardiology community to do such a trial.”
Ivabradine slows heart rate by a unique mechanism that doesn’t involve blockade of adrenergic receptors. In the SHIFT trial (Lancet. 2010 Sep 11;376[9744]:875-85), more than 6,500 patients with heart failure with reduced ejection fraction (HFrEF) in sinus rhythm and with a heart rate greater than 70 bpm were randomized to ivabradine or placebo on top of guideline-directed medical therapy for heart failure. During a median 23 months of follow-up, heart failure hospitalizations were significantly reduced by 26% in the ivabradine group, although cardiovascular deaths were not significantly affected.
As a result of the SHIFT findings, the drug was approved with an indication for use only in combination with a beta-blocker in patients with HFrEF whose on-treatment heart rate exceeds 70 bpm. Ivabradine is not currently recommended as an alternative to beta-blocker therapy. However, in real-world clinical practice a large number of heart failure patients are not managed with a beta-blocker, the cardiologist noted.
His post-hoc analysis focused on the 685 SHIFT participants who were not on a beta-blocker at randomization. During follow-up, there were 93 deaths among patients who were on placebo and only 71 in those randomized to ivabradine, for a statistically significant 30% reduction in all-cause mortality. Cardiovascular mortality was reduced to a similar extent. These hazard ratios remained similar after adjusting for differences in heart rate and other clinical characteristics.
“Beta-blockers are a highly effective therapy for heart failure with reduced ejection fraction, but the mechanism of benefit remains uncertain. It might simply be due to heart rate reduction. And I would point out that we have no evidence of a dose response for beta-blockers: It may well be that you get most of the effect of a beta-blocker with the lowest dose. Titrating to the full dose of a beta-blocker might only be helpful in that it lowers your heart rate. I would argue that 6.25 mg/day of carvedilol plus ivabradine might be as good as 50 mg twice daily of carvedilol but with much higher patient acceptability. We don’t know,” said Dr. Cleland.
“This is an interesting, hypothesis-generating analysis, and we need confirmation now that ivabradine reduces mortality in heart failure patients who are unwilling or unable to take a beta-blocker,” he concluded.
The SHIFT trial was sponsored by Servier. Dr. Cleland reported serving as a consultant to and receiving research funding from that company and others.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: All-cause mortality was reduced by 30%, compared with placebo, in ivabradine-treated patients with heart failure with reduced ejection fraction who were not on a beta-blocker.
Data source: A post-hoc analysis of the 685 patients in a much larger randomized, placebo-controlled clinical trial of ivabradine in patients with heart failure with reduced ejection fraction.
Disclosures: The SHIFT trial was funded by Servier. The presenter reported serving as a consultant to and recipient of research grants from that and other companies.
Short Takes
Hospitalized-patient one-year mortality risk (HOMR) score an excellent prognostic tool
The HOMR score, derived from administrative data, accurately predicts mortality. This study derived the score from medical records which providers can access and found it still accurately determines 1-year mortality.
Citation: Casey G, van Walraven C. Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record. J Hosp Med. 2017 April; 12(4):224-30.
New drug for C. difficile recurrence
Bezlotoxumab is now approved to reduce recurrence of Clostridium difficile. This is an injectable human monoclonal antibody to C. difficile toxin and must be used in conjunction with antibiotics.
Citation: U.S. Food and Drug Administration. Drug Label. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 May 2017.
Hospitalized-patient one-year mortality risk (HOMR) score an excellent prognostic tool
The HOMR score, derived from administrative data, accurately predicts mortality. This study derived the score from medical records which providers can access and found it still accurately determines 1-year mortality.
Citation: Casey G, van Walraven C. Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record. J Hosp Med. 2017 April; 12(4):224-30.
New drug for C. difficile recurrence
Bezlotoxumab is now approved to reduce recurrence of Clostridium difficile. This is an injectable human monoclonal antibody to C. difficile toxin and must be used in conjunction with antibiotics.
Citation: U.S. Food and Drug Administration. Drug Label. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 May 2017.
Hospitalized-patient one-year mortality risk (HOMR) score an excellent prognostic tool
The HOMR score, derived from administrative data, accurately predicts mortality. This study derived the score from medical records which providers can access and found it still accurately determines 1-year mortality.
Citation: Casey G, van Walraven C. Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record. J Hosp Med. 2017 April; 12(4):224-30.
New drug for C. difficile recurrence
Bezlotoxumab is now approved to reduce recurrence of Clostridium difficile. This is an injectable human monoclonal antibody to C. difficile toxin and must be used in conjunction with antibiotics.
Citation: U.S. Food and Drug Administration. Drug Label. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 May 2017.
Heart failure guidelines updated
Clinical Question: What new evidence is available to guide heart failure (HF) management?
Background: New data has become available since the 2013 HF guidelines.
Study Design: A focused update.
Setting: Ongoing review of HF literature.
Synopsis: Beta-natriuretic peptide (BNP) is recommended to screen at risk patients (IIaB), on admission (IA), and prior to discharge (IIaB). The combination of ARB and neprilysin inhibitor (ARB-NI) is recommended in symptomatic patients with HF with reduced ejection fraction (HFrEF) who are tolerant of ACE inhibition (IB). For these patients, transitioning from ACE-inhibitor to the ARB-NI combination, valsartan-sacubitril significantly reduced hospitalization and mortality. Optimal dose and titration strategies remain unclear. ARB-NIs should not be used in patients with a history of angioedema (IIIC) or within 36 hours of receiving ACE-inhibitors (IIIB). Ivabradine, a selective inhibitor of the If current in the sinoatrial node, is recommended to reduce hospitalizations for patients with HFrEF with stable symptoms with resting sinus heart rate greater than or equal to 70 despite maximally-tolerated beta-blockade (IIaB). Intravenous iron replacement is recommended to improve function and quality of life for patients with symptomatic HF and iron deficiency (IIbB).
Bottom Line: Updates support use of BNP, ARB-NIs, ivabradine, and IV iron for HFrEF.
Citation: Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Published online, 2017 Apr 28. Circulation. doi: 10.1161/CIR.0000000000000509.
Dr. Sweigart is an assistant professor in the University of Kentucky division of hospital medicine and Lexington VA Medical Center.
Clinical Question: What new evidence is available to guide heart failure (HF) management?
Background: New data has become available since the 2013 HF guidelines.
Study Design: A focused update.
Setting: Ongoing review of HF literature.
Synopsis: Beta-natriuretic peptide (BNP) is recommended to screen at risk patients (IIaB), on admission (IA), and prior to discharge (IIaB). The combination of ARB and neprilysin inhibitor (ARB-NI) is recommended in symptomatic patients with HF with reduced ejection fraction (HFrEF) who are tolerant of ACE inhibition (IB). For these patients, transitioning from ACE-inhibitor to the ARB-NI combination, valsartan-sacubitril significantly reduced hospitalization and mortality. Optimal dose and titration strategies remain unclear. ARB-NIs should not be used in patients with a history of angioedema (IIIC) or within 36 hours of receiving ACE-inhibitors (IIIB). Ivabradine, a selective inhibitor of the If current in the sinoatrial node, is recommended to reduce hospitalizations for patients with HFrEF with stable symptoms with resting sinus heart rate greater than or equal to 70 despite maximally-tolerated beta-blockade (IIaB). Intravenous iron replacement is recommended to improve function and quality of life for patients with symptomatic HF and iron deficiency (IIbB).
Bottom Line: Updates support use of BNP, ARB-NIs, ivabradine, and IV iron for HFrEF.
Citation: Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Published online, 2017 Apr 28. Circulation. doi: 10.1161/CIR.0000000000000509.
Dr. Sweigart is an assistant professor in the University of Kentucky division of hospital medicine and Lexington VA Medical Center.
Clinical Question: What new evidence is available to guide heart failure (HF) management?
Background: New data has become available since the 2013 HF guidelines.
Study Design: A focused update.
Setting: Ongoing review of HF literature.
Synopsis: Beta-natriuretic peptide (BNP) is recommended to screen at risk patients (IIaB), on admission (IA), and prior to discharge (IIaB). The combination of ARB and neprilysin inhibitor (ARB-NI) is recommended in symptomatic patients with HF with reduced ejection fraction (HFrEF) who are tolerant of ACE inhibition (IB). For these patients, transitioning from ACE-inhibitor to the ARB-NI combination, valsartan-sacubitril significantly reduced hospitalization and mortality. Optimal dose and titration strategies remain unclear. ARB-NIs should not be used in patients with a history of angioedema (IIIC) or within 36 hours of receiving ACE-inhibitors (IIIB). Ivabradine, a selective inhibitor of the If current in the sinoatrial node, is recommended to reduce hospitalizations for patients with HFrEF with stable symptoms with resting sinus heart rate greater than or equal to 70 despite maximally-tolerated beta-blockade (IIaB). Intravenous iron replacement is recommended to improve function and quality of life for patients with symptomatic HF and iron deficiency (IIbB).
Bottom Line: Updates support use of BNP, ARB-NIs, ivabradine, and IV iron for HFrEF.
Citation: Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Published online, 2017 Apr 28. Circulation. doi: 10.1161/CIR.0000000000000509.
Dr. Sweigart is an assistant professor in the University of Kentucky division of hospital medicine and Lexington VA Medical Center.
Triple therapy reduces exacerbations in patients with symptomatic COPD
Clinical Question: Does triple therapy (long-acting beta2-agonist, long-acting muscarinic antagonist, and inhaled corticosteroid) reduce exacerbations in patients with symptomatic chronic obstructive pulmonary disease (COPD)?
Background: Guidelines from GOLD and NICE recommend considering a step-up to triple therapy for patients with refractory COPD symptoms or exacerbations. However, it is unknown if this reduces the long term risk of exacerbations.
Study Design: A randomized controlled trial.
Synopsis: This study enrolled 2,691 patients with COPD, severe airflow restriction (FEV1 less than 50%), significant symptoms (CAT score greater than or equal to 10), and at least one exacerbation in the past year. Participants were randomized to a novel three-agent inhaler (containing an extrafine formulation of beclomethasone, formoterol, and glycopyrronium), an “open triple” regimen including beclomethasone/formoterol plus tiotropium, or to tiotropium alone.
During 52 weeks of treatment, the triple therapy regimens significantly reduced moderate to severe COPD exacerbations, compared with tiotropium alone, with annualized exacerbation rates of 0.46 (95% confidence interval, 0.41-0.51), 0.45 (0.39-0.52), and 0.57 (0.52-0.63), respectively. Rates of adverse events were similar between all three groups.
Bottom Line: Triple therapy was superior to tiotropium alone for reducing exacerbations in patients with symptomatic COPD. The two triple therapy regimens studied did not significantly differ in efficacy.
Citation: Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
Dr. Troy is assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Does triple therapy (long-acting beta2-agonist, long-acting muscarinic antagonist, and inhaled corticosteroid) reduce exacerbations in patients with symptomatic chronic obstructive pulmonary disease (COPD)?
Background: Guidelines from GOLD and NICE recommend considering a step-up to triple therapy for patients with refractory COPD symptoms or exacerbations. However, it is unknown if this reduces the long term risk of exacerbations.
Study Design: A randomized controlled trial.
Synopsis: This study enrolled 2,691 patients with COPD, severe airflow restriction (FEV1 less than 50%), significant symptoms (CAT score greater than or equal to 10), and at least one exacerbation in the past year. Participants were randomized to a novel three-agent inhaler (containing an extrafine formulation of beclomethasone, formoterol, and glycopyrronium), an “open triple” regimen including beclomethasone/formoterol plus tiotropium, or to tiotropium alone.
During 52 weeks of treatment, the triple therapy regimens significantly reduced moderate to severe COPD exacerbations, compared with tiotropium alone, with annualized exacerbation rates of 0.46 (95% confidence interval, 0.41-0.51), 0.45 (0.39-0.52), and 0.57 (0.52-0.63), respectively. Rates of adverse events were similar between all three groups.
Bottom Line: Triple therapy was superior to tiotropium alone for reducing exacerbations in patients with symptomatic COPD. The two triple therapy regimens studied did not significantly differ in efficacy.
Citation: Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
Dr. Troy is assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Does triple therapy (long-acting beta2-agonist, long-acting muscarinic antagonist, and inhaled corticosteroid) reduce exacerbations in patients with symptomatic chronic obstructive pulmonary disease (COPD)?
Background: Guidelines from GOLD and NICE recommend considering a step-up to triple therapy for patients with refractory COPD symptoms or exacerbations. However, it is unknown if this reduces the long term risk of exacerbations.
Study Design: A randomized controlled trial.
Synopsis: This study enrolled 2,691 patients with COPD, severe airflow restriction (FEV1 less than 50%), significant symptoms (CAT score greater than or equal to 10), and at least one exacerbation in the past year. Participants were randomized to a novel three-agent inhaler (containing an extrafine formulation of beclomethasone, formoterol, and glycopyrronium), an “open triple” regimen including beclomethasone/formoterol plus tiotropium, or to tiotropium alone.
During 52 weeks of treatment, the triple therapy regimens significantly reduced moderate to severe COPD exacerbations, compared with tiotropium alone, with annualized exacerbation rates of 0.46 (95% confidence interval, 0.41-0.51), 0.45 (0.39-0.52), and 0.57 (0.52-0.63), respectively. Rates of adverse events were similar between all three groups.
Bottom Line: Triple therapy was superior to tiotropium alone for reducing exacerbations in patients with symptomatic COPD. The two triple therapy regimens studied did not significantly differ in efficacy.
Citation: Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
Dr. Troy is assistant professor in the University of Kentucky division of hospital medicine.














