User login
Better communication with pharmacists can improve postop pain control
LAS VEGAS – . Watch out for overlapping medication orders. Beware of gabapentin mishaps, and embrace Tylenol – but not always.
April Smith, PharmD, associate professor of pharmacy practice at Creighton University, Omaha, offered these tips about postoperative care to surgeons at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“We’re probably one of the most underutilized professions you have on your team,” she said, adding that “we have to know what you’re doing to help you.”
As she explained, “if you’re going to have a new order set, let us know that, so we can be your allies in helping nurses and other people understand why we’re doing what we’re doing. I’m on the same floor, and the nurses are coming up to me and asking me questions. If I can explain to them why we’re doing these things, they’ll get on board a lot faster and save you a lot of phone calls. I know you’re surgeons and you hate that [phone calls].”
Better communication with pharmacists can also boost the stocking of enhanced-recovery medications in automatic dispensing machines, she said, so they’re ready when patients need them.
Dr. Smith offered these tips about specific postsurgery medications:
- Scopolamine is a “great drug for post-op vomiting and nausea,” Dr. Smith said. But do not use it in patients over 65, and it’s contraindicated in glaucoma. Beware of these notable side effects: Blurry vision, constipation, and urinary retention. Dexamethasone and ondansetron can be used as an alternative, she said.
- Use of the blood thinner enoxaparin after discharge may become more common as surgical stays become shorter, Dr. Smith said. She urged surgeons to keep its cost in mind: a 10-day course can be as little as $2 with Medicaid or as much as $140 (a cash price for patients without coverage).
- Make sure to adjust medications based on preoperative or intraoperative doses, she said, to avoid endangering patients by inadvertently doubling up on doses. And watch out for previous use of gabapentin, which is part of enhanced-recovery protocols. Patients who take the drug at home should be put back on their typical dose.
- Also, she warned, “don’t give gabapentin to someone who’s never had it before plus an opioid.” This, she said, can cause delirium.
- Consider starting liquids the night of surgery so patients can begin taking their home medications such as sleep, chronic pain, and psychiatric drugs. Patients will be more stable and satisfied, Dr. Smith said.
- Don’t prescribe hard-to-find medications like oxycodone oral solution or oral ketorolac. These drugs will send patients from pharmacy to pharmacy in search of them, Dr. Smith said.
- Embrace a “Meds to Beds” program if possible. These programs enlist on-site pharmacies to deliver medications to bedside for patients to take home.
- Consider Tylenol as a postoperative painkiller with scheduled doses and be aware that you can prescribe the over-the-counter adult liquid form. However, Dr. Smith cautioned that Tylenol is “not great” on an as-needed basis. Gabapentin and celecoxib (unless contraindicated) are also helpful for postop pain relief, and they’re inexpensive, she said. Three to five days should be enough in most minimally invasive surgeries.
- Don’t overprescribe opioids. “The more we prescribe, the more they will consume,” Dr. Smith said. Check the American College of Surgeons guidelines regarding the ideal number of postsurgery, 5-mg doses of oxycodone to prescribe to opioid-naive patients at discharge. No more than 10 or 15 pills are recommended for several types of general surgery (J Amer Coll Surg. 2018;227:411-8).
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Smith reports no relevant disclosures.
LAS VEGAS – . Watch out for overlapping medication orders. Beware of gabapentin mishaps, and embrace Tylenol – but not always.
April Smith, PharmD, associate professor of pharmacy practice at Creighton University, Omaha, offered these tips about postoperative care to surgeons at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“We’re probably one of the most underutilized professions you have on your team,” she said, adding that “we have to know what you’re doing to help you.”
As she explained, “if you’re going to have a new order set, let us know that, so we can be your allies in helping nurses and other people understand why we’re doing what we’re doing. I’m on the same floor, and the nurses are coming up to me and asking me questions. If I can explain to them why we’re doing these things, they’ll get on board a lot faster and save you a lot of phone calls. I know you’re surgeons and you hate that [phone calls].”
Better communication with pharmacists can also boost the stocking of enhanced-recovery medications in automatic dispensing machines, she said, so they’re ready when patients need them.
Dr. Smith offered these tips about specific postsurgery medications:
- Scopolamine is a “great drug for post-op vomiting and nausea,” Dr. Smith said. But do not use it in patients over 65, and it’s contraindicated in glaucoma. Beware of these notable side effects: Blurry vision, constipation, and urinary retention. Dexamethasone and ondansetron can be used as an alternative, she said.
- Use of the blood thinner enoxaparin after discharge may become more common as surgical stays become shorter, Dr. Smith said. She urged surgeons to keep its cost in mind: a 10-day course can be as little as $2 with Medicaid or as much as $140 (a cash price for patients without coverage).
- Make sure to adjust medications based on preoperative or intraoperative doses, she said, to avoid endangering patients by inadvertently doubling up on doses. And watch out for previous use of gabapentin, which is part of enhanced-recovery protocols. Patients who take the drug at home should be put back on their typical dose.
- Also, she warned, “don’t give gabapentin to someone who’s never had it before plus an opioid.” This, she said, can cause delirium.
- Consider starting liquids the night of surgery so patients can begin taking their home medications such as sleep, chronic pain, and psychiatric drugs. Patients will be more stable and satisfied, Dr. Smith said.
- Don’t prescribe hard-to-find medications like oxycodone oral solution or oral ketorolac. These drugs will send patients from pharmacy to pharmacy in search of them, Dr. Smith said.
- Embrace a “Meds to Beds” program if possible. These programs enlist on-site pharmacies to deliver medications to bedside for patients to take home.
- Consider Tylenol as a postoperative painkiller with scheduled doses and be aware that you can prescribe the over-the-counter adult liquid form. However, Dr. Smith cautioned that Tylenol is “not great” on an as-needed basis. Gabapentin and celecoxib (unless contraindicated) are also helpful for postop pain relief, and they’re inexpensive, she said. Three to five days should be enough in most minimally invasive surgeries.
- Don’t overprescribe opioids. “The more we prescribe, the more they will consume,” Dr. Smith said. Check the American College of Surgeons guidelines regarding the ideal number of postsurgery, 5-mg doses of oxycodone to prescribe to opioid-naive patients at discharge. No more than 10 or 15 pills are recommended for several types of general surgery (J Amer Coll Surg. 2018;227:411-8).
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Smith reports no relevant disclosures.
LAS VEGAS – . Watch out for overlapping medication orders. Beware of gabapentin mishaps, and embrace Tylenol – but not always.
April Smith, PharmD, associate professor of pharmacy practice at Creighton University, Omaha, offered these tips about postoperative care to surgeons at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“We’re probably one of the most underutilized professions you have on your team,” she said, adding that “we have to know what you’re doing to help you.”
As she explained, “if you’re going to have a new order set, let us know that, so we can be your allies in helping nurses and other people understand why we’re doing what we’re doing. I’m on the same floor, and the nurses are coming up to me and asking me questions. If I can explain to them why we’re doing these things, they’ll get on board a lot faster and save you a lot of phone calls. I know you’re surgeons and you hate that [phone calls].”
Better communication with pharmacists can also boost the stocking of enhanced-recovery medications in automatic dispensing machines, she said, so they’re ready when patients need them.
Dr. Smith offered these tips about specific postsurgery medications:
- Scopolamine is a “great drug for post-op vomiting and nausea,” Dr. Smith said. But do not use it in patients over 65, and it’s contraindicated in glaucoma. Beware of these notable side effects: Blurry vision, constipation, and urinary retention. Dexamethasone and ondansetron can be used as an alternative, she said.
- Use of the blood thinner enoxaparin after discharge may become more common as surgical stays become shorter, Dr. Smith said. She urged surgeons to keep its cost in mind: a 10-day course can be as little as $2 with Medicaid or as much as $140 (a cash price for patients without coverage).
- Make sure to adjust medications based on preoperative or intraoperative doses, she said, to avoid endangering patients by inadvertently doubling up on doses. And watch out for previous use of gabapentin, which is part of enhanced-recovery protocols. Patients who take the drug at home should be put back on their typical dose.
- Also, she warned, “don’t give gabapentin to someone who’s never had it before plus an opioid.” This, she said, can cause delirium.
- Consider starting liquids the night of surgery so patients can begin taking their home medications such as sleep, chronic pain, and psychiatric drugs. Patients will be more stable and satisfied, Dr. Smith said.
- Don’t prescribe hard-to-find medications like oxycodone oral solution or oral ketorolac. These drugs will send patients from pharmacy to pharmacy in search of them, Dr. Smith said.
- Embrace a “Meds to Beds” program if possible. These programs enlist on-site pharmacies to deliver medications to bedside for patients to take home.
- Consider Tylenol as a postoperative painkiller with scheduled doses and be aware that you can prescribe the over-the-counter adult liquid form. However, Dr. Smith cautioned that Tylenol is “not great” on an as-needed basis. Gabapentin and celecoxib (unless contraindicated) are also helpful for postop pain relief, and they’re inexpensive, she said. Three to five days should be enough in most minimally invasive surgeries.
- Don’t overprescribe opioids. “The more we prescribe, the more they will consume,” Dr. Smith said. Check the American College of Surgeons guidelines regarding the ideal number of postsurgery, 5-mg doses of oxycodone to prescribe to opioid-naive patients at discharge. No more than 10 or 15 pills are recommended for several types of general surgery (J Amer Coll Surg. 2018;227:411-8).
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Smith reports no relevant disclosures.
EXPERT ANALYSIS FROM MISS
Surge of gabapentinoids for pain lacks supporting evidence
Many clinicians are prescribing the gabapentinoid drugs pregabalin (Lyrica) and gabapentin (Neurontin) for off-label treatment of pain, despite a lack of supporting data or approval from the Food and Drug Administration, according to investigators.
Over the past 15 years, use of gabapentinoids has tripled, a level of growth that cannot be explained by prescriptions for approved indications, reported coauthors Christopher W. Goodman, MD, and Allan S. Brett, MD, of the University of South Carolina, Columbia. Instead, clinicians are turning to gabapentinoids, partly as an option to substitute for opioids, which now have greater prescribing restrictions as a result of the current opioid crisis.
Although clinicians may cite guidelines that support off-label use of gabapentinoids for pain, the investigators warned that many of these recommendations stand on shaky ground.
“Clinicians who prescribe gabapentinoids off-label for pain should be aware of the limited evidence and should acknowledge to patients that potential benefits are uncertain for most off-label uses,” the investigators wrote in a clinical review published online March 25 in JAMA Internal Medicine.
The investigators narrowed down 677 publications to 84 papers describing the use of gabapentinoids for outpatient noncancer pain syndromes for which they are not FDA approved; 54 for gabapentin and 30 for pregabalin. In the domain of analgesia, both agents are currently FDA-approved for postherpetic neuralgia, while pregabalin is additionally approved for pain associated with fibromyalgia and neuropathic pain from diabetic neuropathy and spinal cord injury. Indications in reviewed studies ranged broadly, from conditions somewhat related to those currently approved, such as unspecified neuropathy, to dissimilar conditions, such as chronic pancreatitis and burn injury.
The investigators summarized findings from randomized clinical trials while using case studies to illustrate potential problems with off-label use. In addition, they reviewed the history of gabapentinoids and sources of recommendations for off-label use, such as guidelines and previous review articles.
Six major findings were reported: (1) evidence supporting gabapentin for diabetic neuropathy pain is “mixed at best”; (2) evidence supporting gabapentin for nondiabetic neuropathies is very limited; (3) evidence does not support gabapentinoids for radiculopathy or low back pain; (4) gabapentin has minimal benefit for fibromyalgia pain, based on minimal evidence; (5) evidence does not support gabapentinoids for acute herpes zoster pain; and (6) in almost all studies for other painful indications, gabapentinoids were ineffective or “associated with small analgesic effects that were statistically significant but of questionable clinical importance.”
Case studies complemented this overview, highlighting related clinical dilemmas that the investigators encounter “repeatedly” during inpatient and outpatient care. Along with off-label use, such as gabapentinoid prescriptions for acute sciatica, the investigators reported cases in which neuropathy was diagnosed in place of nonspecific lower body pain to facilitate gabapentin prescription. They also described apparent disregard for risks of polypharmacy in prescriptions for elderly patients and rote use of gabapentinoids in patients with diabetic neuropathy who did not have sufficient discomfort to warrant prescription.
The investigators also cited a number of problems with the language of reviews and guidelines involving gabapentinoids.
“The wording in many guidelines and review articles reinforces an inflated view of gabapentinoid effectiveness or fails to distinguish carefully between evidence-based and non–evidence-based recommendations,” they wrote, adding that clinicians may have misconceptions about neuropathic pain. “One unintended effect of the broad definition [of neuropathic pain] might be to create a mistaken perception that an effective drug for one type of neuropathic pain is effective for all neuropathic pain, regardless of underlying etiology or mechanism,” the investigators suggested.
Another facet of prescribing behavior could be explained in economic terms. Pregabalin, sold under the brand name Lyrica, is considerably more expensive than gabapentin; however, the investigators warned that the similarity of these agents does not equate with interchangeability, noting differences in bioavailability and rate of absorption.
“Unfortunately, published direct comparisons between the 2 drugs in double-blind studies of patients with chronic noncancer pain are virtually nonexistent,” the investigators wrote.
In addition to questionable effectiveness of gabapentinoids for off-label chronic noncancer pain syndromes, Dr. Goodman and Dr. Brett noted that the drugs produce a “substantial incidence of dizziness, somnolence, and gait disturbance.”
They also described a new trend of gabapentinoid abuse and diversion, which may not be surprising, considering that gabapentinoids are reported to augment opioid-induced euphoria.
“Evidence of misuse of gabapentinoids is accumulating and likely related to the opioid epidemic. A recent review article reported an overall population prevalence of gabapentinoid ‘misuse and abuse’ as high as 1%, with substantially higher prevalence noted among patients with opioid use disorders,” the investigators wrote. “This trend is troubling, particularly because concomitant use of opioids and gabapentinoids is associated with increased odds of opioid-related death. Whether these concerns apply to patients receiving long-term prescribed opioid therapy is unclear.”
In the era of the opioid crisis, the investigators acknowledged that many clinicians have serious concerns about adequately treating chronic noncancer pain.
“Comprehensive management of pain in primary care settings is difficult. It requires time and resources that are frequently unavailable,” the investigators wrote. “Many patients with chronic pain have limited or no access to high-quality pain practices or to nonpharmacologic interventions, such as cognitive behavior therapy.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Goodman CW et al. JAMA Intern Med. 2019 Mar 25. doi: 10.1001/jamainternmed.2019.0086
Many clinicians are prescribing the gabapentinoid drugs pregabalin (Lyrica) and gabapentin (Neurontin) for off-label treatment of pain, despite a lack of supporting data or approval from the Food and Drug Administration, according to investigators.
Over the past 15 years, use of gabapentinoids has tripled, a level of growth that cannot be explained by prescriptions for approved indications, reported coauthors Christopher W. Goodman, MD, and Allan S. Brett, MD, of the University of South Carolina, Columbia. Instead, clinicians are turning to gabapentinoids, partly as an option to substitute for opioids, which now have greater prescribing restrictions as a result of the current opioid crisis.
Although clinicians may cite guidelines that support off-label use of gabapentinoids for pain, the investigators warned that many of these recommendations stand on shaky ground.
“Clinicians who prescribe gabapentinoids off-label for pain should be aware of the limited evidence and should acknowledge to patients that potential benefits are uncertain for most off-label uses,” the investigators wrote in a clinical review published online March 25 in JAMA Internal Medicine.
The investigators narrowed down 677 publications to 84 papers describing the use of gabapentinoids for outpatient noncancer pain syndromes for which they are not FDA approved; 54 for gabapentin and 30 for pregabalin. In the domain of analgesia, both agents are currently FDA-approved for postherpetic neuralgia, while pregabalin is additionally approved for pain associated with fibromyalgia and neuropathic pain from diabetic neuropathy and spinal cord injury. Indications in reviewed studies ranged broadly, from conditions somewhat related to those currently approved, such as unspecified neuropathy, to dissimilar conditions, such as chronic pancreatitis and burn injury.
The investigators summarized findings from randomized clinical trials while using case studies to illustrate potential problems with off-label use. In addition, they reviewed the history of gabapentinoids and sources of recommendations for off-label use, such as guidelines and previous review articles.
Six major findings were reported: (1) evidence supporting gabapentin for diabetic neuropathy pain is “mixed at best”; (2) evidence supporting gabapentin for nondiabetic neuropathies is very limited; (3) evidence does not support gabapentinoids for radiculopathy or low back pain; (4) gabapentin has minimal benefit for fibromyalgia pain, based on minimal evidence; (5) evidence does not support gabapentinoids for acute herpes zoster pain; and (6) in almost all studies for other painful indications, gabapentinoids were ineffective or “associated with small analgesic effects that were statistically significant but of questionable clinical importance.”
Case studies complemented this overview, highlighting related clinical dilemmas that the investigators encounter “repeatedly” during inpatient and outpatient care. Along with off-label use, such as gabapentinoid prescriptions for acute sciatica, the investigators reported cases in which neuropathy was diagnosed in place of nonspecific lower body pain to facilitate gabapentin prescription. They also described apparent disregard for risks of polypharmacy in prescriptions for elderly patients and rote use of gabapentinoids in patients with diabetic neuropathy who did not have sufficient discomfort to warrant prescription.
The investigators also cited a number of problems with the language of reviews and guidelines involving gabapentinoids.
“The wording in many guidelines and review articles reinforces an inflated view of gabapentinoid effectiveness or fails to distinguish carefully between evidence-based and non–evidence-based recommendations,” they wrote, adding that clinicians may have misconceptions about neuropathic pain. “One unintended effect of the broad definition [of neuropathic pain] might be to create a mistaken perception that an effective drug for one type of neuropathic pain is effective for all neuropathic pain, regardless of underlying etiology or mechanism,” the investigators suggested.
Another facet of prescribing behavior could be explained in economic terms. Pregabalin, sold under the brand name Lyrica, is considerably more expensive than gabapentin; however, the investigators warned that the similarity of these agents does not equate with interchangeability, noting differences in bioavailability and rate of absorption.
“Unfortunately, published direct comparisons between the 2 drugs in double-blind studies of patients with chronic noncancer pain are virtually nonexistent,” the investigators wrote.
In addition to questionable effectiveness of gabapentinoids for off-label chronic noncancer pain syndromes, Dr. Goodman and Dr. Brett noted that the drugs produce a “substantial incidence of dizziness, somnolence, and gait disturbance.”
They also described a new trend of gabapentinoid abuse and diversion, which may not be surprising, considering that gabapentinoids are reported to augment opioid-induced euphoria.
“Evidence of misuse of gabapentinoids is accumulating and likely related to the opioid epidemic. A recent review article reported an overall population prevalence of gabapentinoid ‘misuse and abuse’ as high as 1%, with substantially higher prevalence noted among patients with opioid use disorders,” the investigators wrote. “This trend is troubling, particularly because concomitant use of opioids and gabapentinoids is associated with increased odds of opioid-related death. Whether these concerns apply to patients receiving long-term prescribed opioid therapy is unclear.”
In the era of the opioid crisis, the investigators acknowledged that many clinicians have serious concerns about adequately treating chronic noncancer pain.
“Comprehensive management of pain in primary care settings is difficult. It requires time and resources that are frequently unavailable,” the investigators wrote. “Many patients with chronic pain have limited or no access to high-quality pain practices or to nonpharmacologic interventions, such as cognitive behavior therapy.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Goodman CW et al. JAMA Intern Med. 2019 Mar 25. doi: 10.1001/jamainternmed.2019.0086
Many clinicians are prescribing the gabapentinoid drugs pregabalin (Lyrica) and gabapentin (Neurontin) for off-label treatment of pain, despite a lack of supporting data or approval from the Food and Drug Administration, according to investigators.
Over the past 15 years, use of gabapentinoids has tripled, a level of growth that cannot be explained by prescriptions for approved indications, reported coauthors Christopher W. Goodman, MD, and Allan S. Brett, MD, of the University of South Carolina, Columbia. Instead, clinicians are turning to gabapentinoids, partly as an option to substitute for opioids, which now have greater prescribing restrictions as a result of the current opioid crisis.
Although clinicians may cite guidelines that support off-label use of gabapentinoids for pain, the investigators warned that many of these recommendations stand on shaky ground.
“Clinicians who prescribe gabapentinoids off-label for pain should be aware of the limited evidence and should acknowledge to patients that potential benefits are uncertain for most off-label uses,” the investigators wrote in a clinical review published online March 25 in JAMA Internal Medicine.
The investigators narrowed down 677 publications to 84 papers describing the use of gabapentinoids for outpatient noncancer pain syndromes for which they are not FDA approved; 54 for gabapentin and 30 for pregabalin. In the domain of analgesia, both agents are currently FDA-approved for postherpetic neuralgia, while pregabalin is additionally approved for pain associated with fibromyalgia and neuropathic pain from diabetic neuropathy and spinal cord injury. Indications in reviewed studies ranged broadly, from conditions somewhat related to those currently approved, such as unspecified neuropathy, to dissimilar conditions, such as chronic pancreatitis and burn injury.
The investigators summarized findings from randomized clinical trials while using case studies to illustrate potential problems with off-label use. In addition, they reviewed the history of gabapentinoids and sources of recommendations for off-label use, such as guidelines and previous review articles.
Six major findings were reported: (1) evidence supporting gabapentin for diabetic neuropathy pain is “mixed at best”; (2) evidence supporting gabapentin for nondiabetic neuropathies is very limited; (3) evidence does not support gabapentinoids for radiculopathy or low back pain; (4) gabapentin has minimal benefit for fibromyalgia pain, based on minimal evidence; (5) evidence does not support gabapentinoids for acute herpes zoster pain; and (6) in almost all studies for other painful indications, gabapentinoids were ineffective or “associated with small analgesic effects that were statistically significant but of questionable clinical importance.”
Case studies complemented this overview, highlighting related clinical dilemmas that the investigators encounter “repeatedly” during inpatient and outpatient care. Along with off-label use, such as gabapentinoid prescriptions for acute sciatica, the investigators reported cases in which neuropathy was diagnosed in place of nonspecific lower body pain to facilitate gabapentin prescription. They also described apparent disregard for risks of polypharmacy in prescriptions for elderly patients and rote use of gabapentinoids in patients with diabetic neuropathy who did not have sufficient discomfort to warrant prescription.
The investigators also cited a number of problems with the language of reviews and guidelines involving gabapentinoids.
“The wording in many guidelines and review articles reinforces an inflated view of gabapentinoid effectiveness or fails to distinguish carefully between evidence-based and non–evidence-based recommendations,” they wrote, adding that clinicians may have misconceptions about neuropathic pain. “One unintended effect of the broad definition [of neuropathic pain] might be to create a mistaken perception that an effective drug for one type of neuropathic pain is effective for all neuropathic pain, regardless of underlying etiology or mechanism,” the investigators suggested.
Another facet of prescribing behavior could be explained in economic terms. Pregabalin, sold under the brand name Lyrica, is considerably more expensive than gabapentin; however, the investigators warned that the similarity of these agents does not equate with interchangeability, noting differences in bioavailability and rate of absorption.
“Unfortunately, published direct comparisons between the 2 drugs in double-blind studies of patients with chronic noncancer pain are virtually nonexistent,” the investigators wrote.
In addition to questionable effectiveness of gabapentinoids for off-label chronic noncancer pain syndromes, Dr. Goodman and Dr. Brett noted that the drugs produce a “substantial incidence of dizziness, somnolence, and gait disturbance.”
They also described a new trend of gabapentinoid abuse and diversion, which may not be surprising, considering that gabapentinoids are reported to augment opioid-induced euphoria.
“Evidence of misuse of gabapentinoids is accumulating and likely related to the opioid epidemic. A recent review article reported an overall population prevalence of gabapentinoid ‘misuse and abuse’ as high as 1%, with substantially higher prevalence noted among patients with opioid use disorders,” the investigators wrote. “This trend is troubling, particularly because concomitant use of opioids and gabapentinoids is associated with increased odds of opioid-related death. Whether these concerns apply to patients receiving long-term prescribed opioid therapy is unclear.”
In the era of the opioid crisis, the investigators acknowledged that many clinicians have serious concerns about adequately treating chronic noncancer pain.
“Comprehensive management of pain in primary care settings is difficult. It requires time and resources that are frequently unavailable,” the investigators wrote. “Many patients with chronic pain have limited or no access to high-quality pain practices or to nonpharmacologic interventions, such as cognitive behavior therapy.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Goodman CW et al. JAMA Intern Med. 2019 Mar 25. doi: 10.1001/jamainternmed.2019.0086
FROM JAMA INTERNAL MEDICINE
Hands-on critical care lessons provided at HM19
As the hospitalist tried to position the portable video laryngoscope properly in the airway of the critically ill “patient,” HM19 faculty moderator Brian Kaufman, MD, professor of medicine, anesthesiology, and neurology at New York University (NYU) School of Medicine, issued a word of caution: Rotating it into position should be done gently or there’s a risk of tearing tissue.
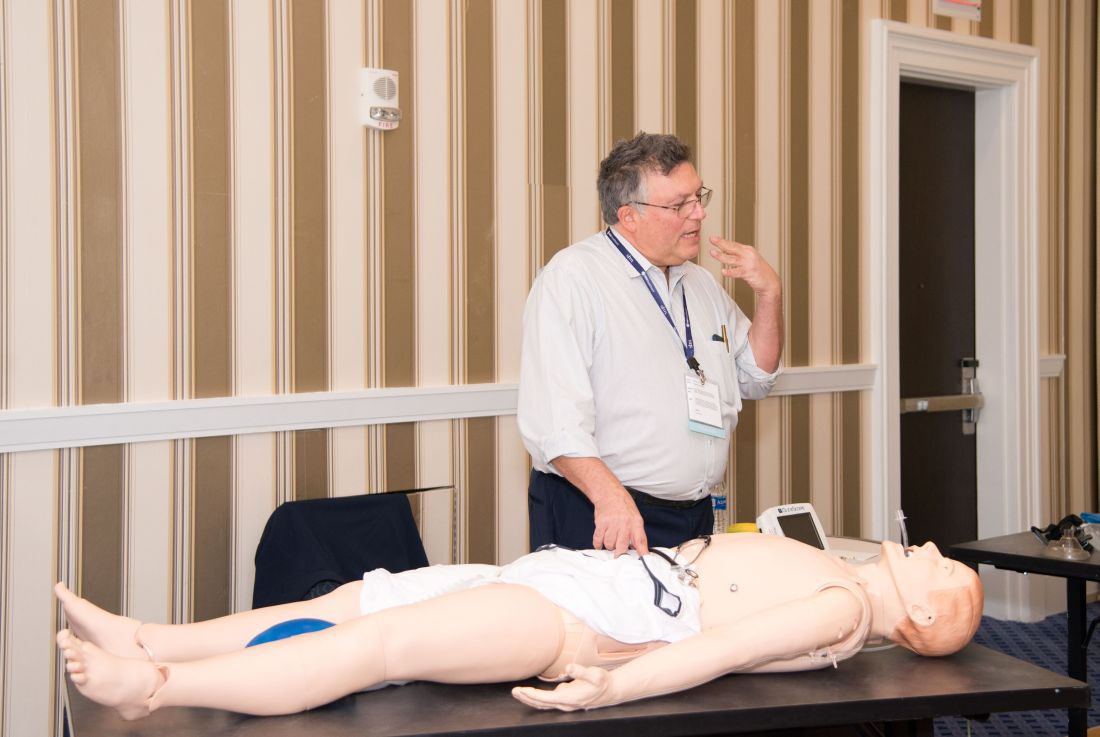
One step at a time, hospitalists attending the session grew more confident and knowledgeable in handling urgent matters involving patients who are critically ill, including cases of shock, mechanical ventilation, overdoses, and ultrasound.
Kevin Felner, MD, associate professor of medicine at NYU School of Medicine, said there’s a growing need for more exposure to caring for the critically ill, including intubation.
“There are a lot of hospitalists who are intubating, and they’re not formally trained in it because medicine residencies don’t typically train people to manage airways,” he said. “We’ve met hospitalists who’ve said, ‘I was hired and was told I had to manage an airway.’”
“It might massage some of the things you’re doing, make you afraid of things you should be afraid of, make you think about something that’s easy to do that you’re not doing, and make things safer,” Dr. Felner said.
In a simulation room, James Horowitz, MD, clinical assistant professor and cardiologist at NYU School of Medicine, demonstrated how to use a laryngeal mask airway (LMA), a simpler alternative to intubating the trachea for keeping an airway open. Dr. Kaufman, standing next to him, clarified how important a skill this is, especially when someone needs air in the next minute or is at risk of death.
“Knowing how to put an LMA in can be life-saving,” Dr. Kaufman said.
In a lecture on shock in the critically ill, Dr. Felner said it’s important to be nimble in handling this common problem –quickly identifying the cause, whether it’s a cardiogenic issue, a low-volume circulation problem, a question of vasodilation, or an obstructive problem. He said guidelines – such as aiming for a mean arterial pressure of 65 mm Hg –are helpful generally, but individuals routinely call for making exceptions to guidelines.
Anthony Andriotis, MD, a pulmonologist at NYU who specializes in critical care, offered an array of key points when managing patients with a ventilator. For instance, when you need to prolong a patient’s expiratory time so they can exhale air more effectively to get rid of entrapped air in their lungs, lowering their respiratory rate is far more effective than decreasing the time it takes them to breathe in or increasing the flow rate of the air they’re breathing.
Some basic points – such as remembering that it’s important to be aware of the pressure when volume control has been imposed and to be aware of volume control when the pressure has been set – are crucial, he said.
The idea behind the pre-course, Dr. Felner said, was to give hospitalists a chance to enter tricky situations with everything to gain, but nothing to lose. He described it as giving students “learning scars” – those times you made a serious error that left you with a lesson you’ll never forget.
“We’re trying to create learning scars, but in a safe scenario.”
As the hospitalist tried to position the portable video laryngoscope properly in the airway of the critically ill “patient,” HM19 faculty moderator Brian Kaufman, MD, professor of medicine, anesthesiology, and neurology at New York University (NYU) School of Medicine, issued a word of caution: Rotating it into position should be done gently or there’s a risk of tearing tissue.

One step at a time, hospitalists attending the session grew more confident and knowledgeable in handling urgent matters involving patients who are critically ill, including cases of shock, mechanical ventilation, overdoses, and ultrasound.
Kevin Felner, MD, associate professor of medicine at NYU School of Medicine, said there’s a growing need for more exposure to caring for the critically ill, including intubation.
“There are a lot of hospitalists who are intubating, and they’re not formally trained in it because medicine residencies don’t typically train people to manage airways,” he said. “We’ve met hospitalists who’ve said, ‘I was hired and was told I had to manage an airway.’”
“It might massage some of the things you’re doing, make you afraid of things you should be afraid of, make you think about something that’s easy to do that you’re not doing, and make things safer,” Dr. Felner said.
In a simulation room, James Horowitz, MD, clinical assistant professor and cardiologist at NYU School of Medicine, demonstrated how to use a laryngeal mask airway (LMA), a simpler alternative to intubating the trachea for keeping an airway open. Dr. Kaufman, standing next to him, clarified how important a skill this is, especially when someone needs air in the next minute or is at risk of death.
“Knowing how to put an LMA in can be life-saving,” Dr. Kaufman said.
In a lecture on shock in the critically ill, Dr. Felner said it’s important to be nimble in handling this common problem –quickly identifying the cause, whether it’s a cardiogenic issue, a low-volume circulation problem, a question of vasodilation, or an obstructive problem. He said guidelines – such as aiming for a mean arterial pressure of 65 mm Hg –are helpful generally, but individuals routinely call for making exceptions to guidelines.
Anthony Andriotis, MD, a pulmonologist at NYU who specializes in critical care, offered an array of key points when managing patients with a ventilator. For instance, when you need to prolong a patient’s expiratory time so they can exhale air more effectively to get rid of entrapped air in their lungs, lowering their respiratory rate is far more effective than decreasing the time it takes them to breathe in or increasing the flow rate of the air they’re breathing.
Some basic points – such as remembering that it’s important to be aware of the pressure when volume control has been imposed and to be aware of volume control when the pressure has been set – are crucial, he said.
The idea behind the pre-course, Dr. Felner said, was to give hospitalists a chance to enter tricky situations with everything to gain, but nothing to lose. He described it as giving students “learning scars” – those times you made a serious error that left you with a lesson you’ll never forget.
“We’re trying to create learning scars, but in a safe scenario.”
As the hospitalist tried to position the portable video laryngoscope properly in the airway of the critically ill “patient,” HM19 faculty moderator Brian Kaufman, MD, professor of medicine, anesthesiology, and neurology at New York University (NYU) School of Medicine, issued a word of caution: Rotating it into position should be done gently or there’s a risk of tearing tissue.

One step at a time, hospitalists attending the session grew more confident and knowledgeable in handling urgent matters involving patients who are critically ill, including cases of shock, mechanical ventilation, overdoses, and ultrasound.
Kevin Felner, MD, associate professor of medicine at NYU School of Medicine, said there’s a growing need for more exposure to caring for the critically ill, including intubation.
“There are a lot of hospitalists who are intubating, and they’re not formally trained in it because medicine residencies don’t typically train people to manage airways,” he said. “We’ve met hospitalists who’ve said, ‘I was hired and was told I had to manage an airway.’”
“It might massage some of the things you’re doing, make you afraid of things you should be afraid of, make you think about something that’s easy to do that you’re not doing, and make things safer,” Dr. Felner said.
In a simulation room, James Horowitz, MD, clinical assistant professor and cardiologist at NYU School of Medicine, demonstrated how to use a laryngeal mask airway (LMA), a simpler alternative to intubating the trachea for keeping an airway open. Dr. Kaufman, standing next to him, clarified how important a skill this is, especially when someone needs air in the next minute or is at risk of death.
“Knowing how to put an LMA in can be life-saving,” Dr. Kaufman said.
In a lecture on shock in the critically ill, Dr. Felner said it’s important to be nimble in handling this common problem –quickly identifying the cause, whether it’s a cardiogenic issue, a low-volume circulation problem, a question of vasodilation, or an obstructive problem. He said guidelines – such as aiming for a mean arterial pressure of 65 mm Hg –are helpful generally, but individuals routinely call for making exceptions to guidelines.
Anthony Andriotis, MD, a pulmonologist at NYU who specializes in critical care, offered an array of key points when managing patients with a ventilator. For instance, when you need to prolong a patient’s expiratory time so they can exhale air more effectively to get rid of entrapped air in their lungs, lowering their respiratory rate is far more effective than decreasing the time it takes them to breathe in or increasing the flow rate of the air they’re breathing.
Some basic points – such as remembering that it’s important to be aware of the pressure when volume control has been imposed and to be aware of volume control when the pressure has been set – are crucial, he said.
The idea behind the pre-course, Dr. Felner said, was to give hospitalists a chance to enter tricky situations with everything to gain, but nothing to lose. He described it as giving students “learning scars” – those times you made a serious error that left you with a lesson you’ll never forget.
“We’re trying to create learning scars, but in a safe scenario.”
Occurrence of pulmonary embolisms in hospitalized patients nearly doubled during 2004-2015
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
REPORTING FROM ACC 2019
Hospitalists and PTs: Building strong relationships
Optimizing discharge disposition and longitudinal recovery
Sanctimonious, self-righteous, discharge saboteurs. These are just a few descriptors I’ve heard hospitalists use to describe my physical therapy (PT) colleagues.
These charged comments come mostly after a hospitalist reads therapy notes and encounters a contradiction to their chosen discharge location for a patient.
I recently met with hospitalists from four different hospitals. They echoed the frustrations of their physician colleagues across the country. The PTs they work with write “the patient requires 24-hour supervision and 3 hours of therapy a day,” or “the patient is unsafe to go home and needs continued therapy at an inpatient rehabilitation center.” The hospitalists in turn want to know “If I discharge the patient home am I liable if the patient falls or has some other negative outcome?” The frustration hospitalists experience is palpable and understandable as their attempts to support a home recovery are often contradicted.
Outside the four walls
The transition from fee-for-service to value-based care now calls upon hospitalists to be innovators in managing patients in alternative payment models, such as accountable care organizations, bundled payment programs, and Medicare Advantage plans. Each model looks to support a home recovery whenever possible and prevent readmissions.
Case managers for Medicare Advantage programs routinely review PT notes to inform hospital discharge disposition and post-acute authorization for skilled nursing facility (SNF) admissions and days in SNF. Hospitalists, working with care managers, can follow suit to succeed in alternative payment models. They have the advantage of in-person access to PT colleagues for elaboration and push-back as necessary. For hospitalists, working collaboratively with PTs is crucial to improving the value of care provided as patients transition beyond the four walls of the hospital.
The evolution of PT in acute care
Prior to diagnosis-related groups (DRGs), PTs were profit centers for hospitals – rehabilitation departments were well staffed and easily accommodated consults and requests for mobility.
With the advent of DRGs, physical therapy became a cost center, and rehabilitation staffs were reduced. PTs became overextended, were less available for consultations for mobilization, and patients suffered the deleterious effects of immobility. With reduced staffing and a rush to get patients out of the hospital, acute PT practice morphed into evaluating functional status and determining discharge destination.
Now, as members of an aligned health care team, PTs need to facilitate a safe home discharge whenever possible and determine what skilled services a patient needs post-acute stay, not where they should receive them.
Discharge disposition and longitudinal recovery
PTs, as experts in function, have a series of “special tests” at their disposal beyond pain, range of motion, and strength assessments. These include: Activity Measure for Post-Acute Care (AM-PAC) or “6-Clicks” Mobility Score, Timed Up and Go, Six-Minute Walk Test, Tinetti, Berg Balance Scale, Modified Barthel Index, Five Times Chair Rise, and Thirty-Second Chair Rise. These are all objective measures of function that can be used to inform discharge disposition and guide longitudinal recovery.
To elaborate on one tool, the 6-Clicks Mobility Score is a validated test that allows PTs to assess basic mobility.1,2 It rates six functional tasks (hence 6 clicks) that include: turning over in bed, moving from lying to sitting, moving to/from bed to chair, transitioning from sitting to standing from a chair, walking in a hospital room, and climbing three to five steps. These functional tasks are scored based on the amount of assistance needed. The scores, in turn, have been shown to support discharge destination planning.1 In addition to informing discharge destination decisions, hospitalists and the rest of the health care team can use 6-Clicks to estimate prolonged hospital stays, readmissions, and emergency department (ED) visits.3
Of course, discharge disposition is influenced by many factors in addition to functional status. Hospitalists are the obvious choice to lead the health care team in interpreting relevant data and test results, and to communicate these results to patients and caregivers so together they can decide the most appropriate discharge destination.
I envision a conversation between a fully informed hospitalist and a patient as follows: “Based on your past history, your living situation, all of your test results including labs, x-rays and the functional tests performed by your PT, your potential for a full recovery is good. You have a moderate decline in function with a high likelihood of returning home in the next 7-10 days. I recommend you go to a SNF for high-intensity rehabilitation for 7 days and that the SNF order PT and OT twice a day and walks with nursing every evening.”
This fully informed conversation can only take place if hospitalists are provided clear, concise documentation, including results of objective functional testing, by their physical therapy colleagues.
In conclusion, PTs working in the acute setting need to use validated tests to objectively assess function and educate their hospitalist colleagues on the meaning of these tests. Hospitalists in turn can incorporate these assessments into a discussion of discharge disposition and longitudinal recovery with patients. In this way, hospitalists and physical therapists can work together to achieve patient-centered, high-value care during and following a hospitalization.
Ms. Tammany is SVP of clinical strategy & innovation for Remedy Partners, Norwalk, Conn.
References
1. Jette DU et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014 Sep;94(9):1252-61.
2. Jette DU et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014 Mar;94(3):379-91.
3. Menendez ME et al. Does “6-Clicks” Day 1 Postoperative Mobility Score Predict Discharge Disposition After Total Hip and Knee Arthroplasties?” J Arthroplasty. 2016 Sep;31(9):1916-20.
Optimizing discharge disposition and longitudinal recovery
Optimizing discharge disposition and longitudinal recovery
Sanctimonious, self-righteous, discharge saboteurs. These are just a few descriptors I’ve heard hospitalists use to describe my physical therapy (PT) colleagues.
These charged comments come mostly after a hospitalist reads therapy notes and encounters a contradiction to their chosen discharge location for a patient.
I recently met with hospitalists from four different hospitals. They echoed the frustrations of their physician colleagues across the country. The PTs they work with write “the patient requires 24-hour supervision and 3 hours of therapy a day,” or “the patient is unsafe to go home and needs continued therapy at an inpatient rehabilitation center.” The hospitalists in turn want to know “If I discharge the patient home am I liable if the patient falls or has some other negative outcome?” The frustration hospitalists experience is palpable and understandable as their attempts to support a home recovery are often contradicted.
Outside the four walls
The transition from fee-for-service to value-based care now calls upon hospitalists to be innovators in managing patients in alternative payment models, such as accountable care organizations, bundled payment programs, and Medicare Advantage plans. Each model looks to support a home recovery whenever possible and prevent readmissions.
Case managers for Medicare Advantage programs routinely review PT notes to inform hospital discharge disposition and post-acute authorization for skilled nursing facility (SNF) admissions and days in SNF. Hospitalists, working with care managers, can follow suit to succeed in alternative payment models. They have the advantage of in-person access to PT colleagues for elaboration and push-back as necessary. For hospitalists, working collaboratively with PTs is crucial to improving the value of care provided as patients transition beyond the four walls of the hospital.
The evolution of PT in acute care
Prior to diagnosis-related groups (DRGs), PTs were profit centers for hospitals – rehabilitation departments were well staffed and easily accommodated consults and requests for mobility.
With the advent of DRGs, physical therapy became a cost center, and rehabilitation staffs were reduced. PTs became overextended, were less available for consultations for mobilization, and patients suffered the deleterious effects of immobility. With reduced staffing and a rush to get patients out of the hospital, acute PT practice morphed into evaluating functional status and determining discharge destination.
Now, as members of an aligned health care team, PTs need to facilitate a safe home discharge whenever possible and determine what skilled services a patient needs post-acute stay, not where they should receive them.
Discharge disposition and longitudinal recovery
PTs, as experts in function, have a series of “special tests” at their disposal beyond pain, range of motion, and strength assessments. These include: Activity Measure for Post-Acute Care (AM-PAC) or “6-Clicks” Mobility Score, Timed Up and Go, Six-Minute Walk Test, Tinetti, Berg Balance Scale, Modified Barthel Index, Five Times Chair Rise, and Thirty-Second Chair Rise. These are all objective measures of function that can be used to inform discharge disposition and guide longitudinal recovery.
To elaborate on one tool, the 6-Clicks Mobility Score is a validated test that allows PTs to assess basic mobility.1,2 It rates six functional tasks (hence 6 clicks) that include: turning over in bed, moving from lying to sitting, moving to/from bed to chair, transitioning from sitting to standing from a chair, walking in a hospital room, and climbing three to five steps. These functional tasks are scored based on the amount of assistance needed. The scores, in turn, have been shown to support discharge destination planning.1 In addition to informing discharge destination decisions, hospitalists and the rest of the health care team can use 6-Clicks to estimate prolonged hospital stays, readmissions, and emergency department (ED) visits.3
Of course, discharge disposition is influenced by many factors in addition to functional status. Hospitalists are the obvious choice to lead the health care team in interpreting relevant data and test results, and to communicate these results to patients and caregivers so together they can decide the most appropriate discharge destination.
I envision a conversation between a fully informed hospitalist and a patient as follows: “Based on your past history, your living situation, all of your test results including labs, x-rays and the functional tests performed by your PT, your potential for a full recovery is good. You have a moderate decline in function with a high likelihood of returning home in the next 7-10 days. I recommend you go to a SNF for high-intensity rehabilitation for 7 days and that the SNF order PT and OT twice a day and walks with nursing every evening.”
This fully informed conversation can only take place if hospitalists are provided clear, concise documentation, including results of objective functional testing, by their physical therapy colleagues.
In conclusion, PTs working in the acute setting need to use validated tests to objectively assess function and educate their hospitalist colleagues on the meaning of these tests. Hospitalists in turn can incorporate these assessments into a discussion of discharge disposition and longitudinal recovery with patients. In this way, hospitalists and physical therapists can work together to achieve patient-centered, high-value care during and following a hospitalization.
Ms. Tammany is SVP of clinical strategy & innovation for Remedy Partners, Norwalk, Conn.
References
1. Jette DU et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014 Sep;94(9):1252-61.
2. Jette DU et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014 Mar;94(3):379-91.
3. Menendez ME et al. Does “6-Clicks” Day 1 Postoperative Mobility Score Predict Discharge Disposition After Total Hip and Knee Arthroplasties?” J Arthroplasty. 2016 Sep;31(9):1916-20.
Sanctimonious, self-righteous, discharge saboteurs. These are just a few descriptors I’ve heard hospitalists use to describe my physical therapy (PT) colleagues.
These charged comments come mostly after a hospitalist reads therapy notes and encounters a contradiction to their chosen discharge location for a patient.
I recently met with hospitalists from four different hospitals. They echoed the frustrations of their physician colleagues across the country. The PTs they work with write “the patient requires 24-hour supervision and 3 hours of therapy a day,” or “the patient is unsafe to go home and needs continued therapy at an inpatient rehabilitation center.” The hospitalists in turn want to know “If I discharge the patient home am I liable if the patient falls or has some other negative outcome?” The frustration hospitalists experience is palpable and understandable as their attempts to support a home recovery are often contradicted.
Outside the four walls
The transition from fee-for-service to value-based care now calls upon hospitalists to be innovators in managing patients in alternative payment models, such as accountable care organizations, bundled payment programs, and Medicare Advantage plans. Each model looks to support a home recovery whenever possible and prevent readmissions.
Case managers for Medicare Advantage programs routinely review PT notes to inform hospital discharge disposition and post-acute authorization for skilled nursing facility (SNF) admissions and days in SNF. Hospitalists, working with care managers, can follow suit to succeed in alternative payment models. They have the advantage of in-person access to PT colleagues for elaboration and push-back as necessary. For hospitalists, working collaboratively with PTs is crucial to improving the value of care provided as patients transition beyond the four walls of the hospital.
The evolution of PT in acute care
Prior to diagnosis-related groups (DRGs), PTs were profit centers for hospitals – rehabilitation departments were well staffed and easily accommodated consults and requests for mobility.
With the advent of DRGs, physical therapy became a cost center, and rehabilitation staffs were reduced. PTs became overextended, were less available for consultations for mobilization, and patients suffered the deleterious effects of immobility. With reduced staffing and a rush to get patients out of the hospital, acute PT practice morphed into evaluating functional status and determining discharge destination.
Now, as members of an aligned health care team, PTs need to facilitate a safe home discharge whenever possible and determine what skilled services a patient needs post-acute stay, not where they should receive them.
Discharge disposition and longitudinal recovery
PTs, as experts in function, have a series of “special tests” at their disposal beyond pain, range of motion, and strength assessments. These include: Activity Measure for Post-Acute Care (AM-PAC) or “6-Clicks” Mobility Score, Timed Up and Go, Six-Minute Walk Test, Tinetti, Berg Balance Scale, Modified Barthel Index, Five Times Chair Rise, and Thirty-Second Chair Rise. These are all objective measures of function that can be used to inform discharge disposition and guide longitudinal recovery.
To elaborate on one tool, the 6-Clicks Mobility Score is a validated test that allows PTs to assess basic mobility.1,2 It rates six functional tasks (hence 6 clicks) that include: turning over in bed, moving from lying to sitting, moving to/from bed to chair, transitioning from sitting to standing from a chair, walking in a hospital room, and climbing three to five steps. These functional tasks are scored based on the amount of assistance needed. The scores, in turn, have been shown to support discharge destination planning.1 In addition to informing discharge destination decisions, hospitalists and the rest of the health care team can use 6-Clicks to estimate prolonged hospital stays, readmissions, and emergency department (ED) visits.3
Of course, discharge disposition is influenced by many factors in addition to functional status. Hospitalists are the obvious choice to lead the health care team in interpreting relevant data and test results, and to communicate these results to patients and caregivers so together they can decide the most appropriate discharge destination.
I envision a conversation between a fully informed hospitalist and a patient as follows: “Based on your past history, your living situation, all of your test results including labs, x-rays and the functional tests performed by your PT, your potential for a full recovery is good. You have a moderate decline in function with a high likelihood of returning home in the next 7-10 days. I recommend you go to a SNF for high-intensity rehabilitation for 7 days and that the SNF order PT and OT twice a day and walks with nursing every evening.”
This fully informed conversation can only take place if hospitalists are provided clear, concise documentation, including results of objective functional testing, by their physical therapy colleagues.
In conclusion, PTs working in the acute setting need to use validated tests to objectively assess function and educate their hospitalist colleagues on the meaning of these tests. Hospitalists in turn can incorporate these assessments into a discussion of discharge disposition and longitudinal recovery with patients. In this way, hospitalists and physical therapists can work together to achieve patient-centered, high-value care during and following a hospitalization.
Ms. Tammany is SVP of clinical strategy & innovation for Remedy Partners, Norwalk, Conn.
References
1. Jette DU et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014 Sep;94(9):1252-61.
2. Jette DU et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014 Mar;94(3):379-91.
3. Menendez ME et al. Does “6-Clicks” Day 1 Postoperative Mobility Score Predict Discharge Disposition After Total Hip and Knee Arthroplasties?” J Arthroplasty. 2016 Sep;31(9):1916-20.
Quick Byte: Trauma care
Innovating quickly
The U.S. military has completely transformed trauma care over the past 17 years, and that success offers lessons for civilian medicine.
In the civilian world, it takes an average of 17 years for a new discovery to change medical practice, but the military has developed or significantly expanded more than 27 major innovations, such as redesigned tourniquets and new transport procedures, in about a decade. As a result, the death rate from battlefield wounds has decreased by half.
Reference
Kellermann A et al. How the US military reinvented trauma care and what this means for US medicine. Health Aff. 2018 Jul 3. doi: 10.1377/hblog20180628.431867.
Innovating quickly
Innovating quickly
The U.S. military has completely transformed trauma care over the past 17 years, and that success offers lessons for civilian medicine.
In the civilian world, it takes an average of 17 years for a new discovery to change medical practice, but the military has developed or significantly expanded more than 27 major innovations, such as redesigned tourniquets and new transport procedures, in about a decade. As a result, the death rate from battlefield wounds has decreased by half.
Reference
Kellermann A et al. How the US military reinvented trauma care and what this means for US medicine. Health Aff. 2018 Jul 3. doi: 10.1377/hblog20180628.431867.
The U.S. military has completely transformed trauma care over the past 17 years, and that success offers lessons for civilian medicine.
In the civilian world, it takes an average of 17 years for a new discovery to change medical practice, but the military has developed or significantly expanded more than 27 major innovations, such as redesigned tourniquets and new transport procedures, in about a decade. As a result, the death rate from battlefield wounds has decreased by half.
Reference
Kellermann A et al. How the US military reinvented trauma care and what this means for US medicine. Health Aff. 2018 Jul 3. doi: 10.1377/hblog20180628.431867.
Intensive blood pressure lowering may not reduce risk of recurrent stroke
HONOLULU – according to research presented at the International Stroke Conference sponsored by the American Heart Association.
Combined with data from previous trials, these results support a target systolic blood pressure of less than 130 mm Hg and a diastolic blood pressure of less than 80 mm Hg for secondary stroke prevention, said Kazuo Kitagawa, MD, PhD.
Lowering blood pressure reduces the risk of recurrent stroke, but investigators have not identified the best target blood pressure for this indication. The Secondary Prevention of Small Subcortical Strokes Trial (SPS3) examined the efficacy of intensive blood pressure treatment for secondary stroke prevention. The investigators randomized more than 3,000 patients with recent lacunar stroke to intensive or standard blood pressure treatment. Intensive treatment (a target systolic blood pressure of less than 130 mm Hg) conferred a nonsignificant reduction of the risk of recurrent stroke. A 2018 meta-analysis of SPS3 and two smaller randomized controlled trials also showed that intensive treatment did not significantly reduce the risk of recurrent stroke.
A new multicenter trial
Dr. Kitagawa, of Tokyo Women’s Medical University, and colleagues conducted a new trial to evaluate whether intensive blood pressure reduction significantly reduced the risk of recurrent stroke, compared with standard treatment (a systolic target of less than 140 mm Hg and a diastolic target of less than 90 mm Hg). Between 2010 and 2016, they enrolled patients with a history of stroke within the previous 3 years at 140 hospitals in Japan. Participants were randomized to standard blood pressure treatment or intensive blood pressure treatment (defined in this study as a systolic target of less than 120 mm Hg and a diastolic target of less than 80 mm Hg). The primary end point was recurrent stroke.
Both treatment regimens were based on stepwise multidrug rationing. Step 1 was an angiotensin II receptor blockade (ARB), step 2 was the addition of diuretics, step 3 was the addition of calcium channel blockers, step 4 was an increase of the ARB, step 5 was increase of the calcium channel blocker, and step 6 was the addition of spironolactone.
This trial was stopped at the end of 2016 because of slow recruitment and funding cessation. Investigators randomized 1,280 patients out of a planned 2,000. Seventeen patients were excluded from analysis. At baseline, participants’ mean age was 67 years, and mean systolic blood pressure was 145 mm Hg. The qualifying event was ischemic stroke for 85% of patients and intracerebral hemorrhage for 15%. Mean follow-up duration was 3.9 years.
Intensive treatment reduced blood pressure
At 1 year, the mean systolic blood pressure was 132.0 mm Hg in the standard-treatment group and 123.7 mm Hg in the intensive-treatment group. Mean diastolic blood pressure was 77.5 mm Hg in the standard-treatment group and 72.8 mm Hg in the intensive-treatment group. The investigators observed a significant difference in blood pressure between the groups throughout the study period.
The annual rate of stroke recurrence was 2.26% in the standard-treatment group and 1.65% in the intensive-treatment group. Intensive treatment tended to reduce stroke recurrence (hazard ratio, 0.73), but the result was not statistically significant. “The nonsignificant finding might be due to early termination or the modest difference in blood pressure level [between groups],” said Dr. Kitagawa.
Subgroup analyses did not indicate any interaction between treatment group and age, sex, qualifying event, mean systolic blood pressure at baseline, or diabetes. The rate of ischemic stroke was similar between the two groups, but the rate of intracerebral hemorrhage was lower in the intensive treatment group than in the standard treatment group. The rate of serious adverse events was similar between treatment groups.
When Dr. Kitagawa and colleagues pooled their data with those examined in the 2018 meta-analysis, they found that intensive treatment significantly reduced the risk of recurrent stroke (hazard ratio, 0.68), compared with standard treatment.
This study was sponsored by Biomedis International.
SOURCE: Kitagawa K et al. ISC 2019, Abstract LB10.
HONOLULU – according to research presented at the International Stroke Conference sponsored by the American Heart Association.
Combined with data from previous trials, these results support a target systolic blood pressure of less than 130 mm Hg and a diastolic blood pressure of less than 80 mm Hg for secondary stroke prevention, said Kazuo Kitagawa, MD, PhD.
Lowering blood pressure reduces the risk of recurrent stroke, but investigators have not identified the best target blood pressure for this indication. The Secondary Prevention of Small Subcortical Strokes Trial (SPS3) examined the efficacy of intensive blood pressure treatment for secondary stroke prevention. The investigators randomized more than 3,000 patients with recent lacunar stroke to intensive or standard blood pressure treatment. Intensive treatment (a target systolic blood pressure of less than 130 mm Hg) conferred a nonsignificant reduction of the risk of recurrent stroke. A 2018 meta-analysis of SPS3 and two smaller randomized controlled trials also showed that intensive treatment did not significantly reduce the risk of recurrent stroke.
A new multicenter trial
Dr. Kitagawa, of Tokyo Women’s Medical University, and colleagues conducted a new trial to evaluate whether intensive blood pressure reduction significantly reduced the risk of recurrent stroke, compared with standard treatment (a systolic target of less than 140 mm Hg and a diastolic target of less than 90 mm Hg). Between 2010 and 2016, they enrolled patients with a history of stroke within the previous 3 years at 140 hospitals in Japan. Participants were randomized to standard blood pressure treatment or intensive blood pressure treatment (defined in this study as a systolic target of less than 120 mm Hg and a diastolic target of less than 80 mm Hg). The primary end point was recurrent stroke.
Both treatment regimens were based on stepwise multidrug rationing. Step 1 was an angiotensin II receptor blockade (ARB), step 2 was the addition of diuretics, step 3 was the addition of calcium channel blockers, step 4 was an increase of the ARB, step 5 was increase of the calcium channel blocker, and step 6 was the addition of spironolactone.
This trial was stopped at the end of 2016 because of slow recruitment and funding cessation. Investigators randomized 1,280 patients out of a planned 2,000. Seventeen patients were excluded from analysis. At baseline, participants’ mean age was 67 years, and mean systolic blood pressure was 145 mm Hg. The qualifying event was ischemic stroke for 85% of patients and intracerebral hemorrhage for 15%. Mean follow-up duration was 3.9 years.
Intensive treatment reduced blood pressure
At 1 year, the mean systolic blood pressure was 132.0 mm Hg in the standard-treatment group and 123.7 mm Hg in the intensive-treatment group. Mean diastolic blood pressure was 77.5 mm Hg in the standard-treatment group and 72.8 mm Hg in the intensive-treatment group. The investigators observed a significant difference in blood pressure between the groups throughout the study period.
The annual rate of stroke recurrence was 2.26% in the standard-treatment group and 1.65% in the intensive-treatment group. Intensive treatment tended to reduce stroke recurrence (hazard ratio, 0.73), but the result was not statistically significant. “The nonsignificant finding might be due to early termination or the modest difference in blood pressure level [between groups],” said Dr. Kitagawa.
Subgroup analyses did not indicate any interaction between treatment group and age, sex, qualifying event, mean systolic blood pressure at baseline, or diabetes. The rate of ischemic stroke was similar between the two groups, but the rate of intracerebral hemorrhage was lower in the intensive treatment group than in the standard treatment group. The rate of serious adverse events was similar between treatment groups.
When Dr. Kitagawa and colleagues pooled their data with those examined in the 2018 meta-analysis, they found that intensive treatment significantly reduced the risk of recurrent stroke (hazard ratio, 0.68), compared with standard treatment.
This study was sponsored by Biomedis International.
SOURCE: Kitagawa K et al. ISC 2019, Abstract LB10.
HONOLULU – according to research presented at the International Stroke Conference sponsored by the American Heart Association.
Combined with data from previous trials, these results support a target systolic blood pressure of less than 130 mm Hg and a diastolic blood pressure of less than 80 mm Hg for secondary stroke prevention, said Kazuo Kitagawa, MD, PhD.
Lowering blood pressure reduces the risk of recurrent stroke, but investigators have not identified the best target blood pressure for this indication. The Secondary Prevention of Small Subcortical Strokes Trial (SPS3) examined the efficacy of intensive blood pressure treatment for secondary stroke prevention. The investigators randomized more than 3,000 patients with recent lacunar stroke to intensive or standard blood pressure treatment. Intensive treatment (a target systolic blood pressure of less than 130 mm Hg) conferred a nonsignificant reduction of the risk of recurrent stroke. A 2018 meta-analysis of SPS3 and two smaller randomized controlled trials also showed that intensive treatment did not significantly reduce the risk of recurrent stroke.
A new multicenter trial
Dr. Kitagawa, of Tokyo Women’s Medical University, and colleagues conducted a new trial to evaluate whether intensive blood pressure reduction significantly reduced the risk of recurrent stroke, compared with standard treatment (a systolic target of less than 140 mm Hg and a diastolic target of less than 90 mm Hg). Between 2010 and 2016, they enrolled patients with a history of stroke within the previous 3 years at 140 hospitals in Japan. Participants were randomized to standard blood pressure treatment or intensive blood pressure treatment (defined in this study as a systolic target of less than 120 mm Hg and a diastolic target of less than 80 mm Hg). The primary end point was recurrent stroke.
Both treatment regimens were based on stepwise multidrug rationing. Step 1 was an angiotensin II receptor blockade (ARB), step 2 was the addition of diuretics, step 3 was the addition of calcium channel blockers, step 4 was an increase of the ARB, step 5 was increase of the calcium channel blocker, and step 6 was the addition of spironolactone.
This trial was stopped at the end of 2016 because of slow recruitment and funding cessation. Investigators randomized 1,280 patients out of a planned 2,000. Seventeen patients were excluded from analysis. At baseline, participants’ mean age was 67 years, and mean systolic blood pressure was 145 mm Hg. The qualifying event was ischemic stroke for 85% of patients and intracerebral hemorrhage for 15%. Mean follow-up duration was 3.9 years.
Intensive treatment reduced blood pressure
At 1 year, the mean systolic blood pressure was 132.0 mm Hg in the standard-treatment group and 123.7 mm Hg in the intensive-treatment group. Mean diastolic blood pressure was 77.5 mm Hg in the standard-treatment group and 72.8 mm Hg in the intensive-treatment group. The investigators observed a significant difference in blood pressure between the groups throughout the study period.
The annual rate of stroke recurrence was 2.26% in the standard-treatment group and 1.65% in the intensive-treatment group. Intensive treatment tended to reduce stroke recurrence (hazard ratio, 0.73), but the result was not statistically significant. “The nonsignificant finding might be due to early termination or the modest difference in blood pressure level [between groups],” said Dr. Kitagawa.
Subgroup analyses did not indicate any interaction between treatment group and age, sex, qualifying event, mean systolic blood pressure at baseline, or diabetes. The rate of ischemic stroke was similar between the two groups, but the rate of intracerebral hemorrhage was lower in the intensive treatment group than in the standard treatment group. The rate of serious adverse events was similar between treatment groups.
When Dr. Kitagawa and colleagues pooled their data with those examined in the 2018 meta-analysis, they found that intensive treatment significantly reduced the risk of recurrent stroke (hazard ratio, 0.68), compared with standard treatment.
This study was sponsored by Biomedis International.
SOURCE: Kitagawa K et al. ISC 2019, Abstract LB10.
REPORTING FROM ISC 2019
Improving research dissemination among hospitalists
Social media a great platform
Medical journals and societies are trying to figure out ways to use social media to connect with hospitalists and others interested in their subject matter, says Charlie Wray, DO, MS, lead author of a paper proposing a way they can do that: implementing a journal-sponsored club on Twitter.
“At the Journal of Hospital Medicine (JHM), we noticed that there was a large community of hospitalists on Twitter who were looking for a community to engage in hospital medicine topics,” Dr. Wray said. “We created #JHMChat to bring the hospital medicine community together on a regular basis to talk about pertinent research, medical education philosophies, and value-based care interventions. Our ultimate goal was to increase engagement, networking, and communication among this community, while highlighting the work that is being published in JHM.”
A study of #JHMChat showed that social media is a great platform for large organizations to reach out, connect, and create a community around, he added. “We were very surprised by both the Twitter metrics (i.e., number of participants and overall impressions), which showed very large dissemination numbers, in addition to the external dissemination metrics (i.e., page views and altmetrics scores), which showed that each chat basically corresponded to a release of a new issue. This could be informative to other journals as they look for ways to increase their web traffic or disseminate their work to their respective audiences.”
Dr. Wray hopes the study alerts hospitalists to the fact that there is a large and ever-growing community available within social media.
“Second, we know that careers in hospital medicine can be tough, regardless of whether you’re at a community hospital or a large academic center. Knowing that there is a community with which you can connect to is both comforting and reassuring.”
Reference
Wray C et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018 Nov;13(11):764-9.
Social media a great platform
Social media a great platform
Medical journals and societies are trying to figure out ways to use social media to connect with hospitalists and others interested in their subject matter, says Charlie Wray, DO, MS, lead author of a paper proposing a way they can do that: implementing a journal-sponsored club on Twitter.
“At the Journal of Hospital Medicine (JHM), we noticed that there was a large community of hospitalists on Twitter who were looking for a community to engage in hospital medicine topics,” Dr. Wray said. “We created #JHMChat to bring the hospital medicine community together on a regular basis to talk about pertinent research, medical education philosophies, and value-based care interventions. Our ultimate goal was to increase engagement, networking, and communication among this community, while highlighting the work that is being published in JHM.”
A study of #JHMChat showed that social media is a great platform for large organizations to reach out, connect, and create a community around, he added. “We were very surprised by both the Twitter metrics (i.e., number of participants and overall impressions), which showed very large dissemination numbers, in addition to the external dissemination metrics (i.e., page views and altmetrics scores), which showed that each chat basically corresponded to a release of a new issue. This could be informative to other journals as they look for ways to increase their web traffic or disseminate their work to their respective audiences.”
Dr. Wray hopes the study alerts hospitalists to the fact that there is a large and ever-growing community available within social media.
“Second, we know that careers in hospital medicine can be tough, regardless of whether you’re at a community hospital or a large academic center. Knowing that there is a community with which you can connect to is both comforting and reassuring.”
Reference
Wray C et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018 Nov;13(11):764-9.
Medical journals and societies are trying to figure out ways to use social media to connect with hospitalists and others interested in their subject matter, says Charlie Wray, DO, MS, lead author of a paper proposing a way they can do that: implementing a journal-sponsored club on Twitter.
“At the Journal of Hospital Medicine (JHM), we noticed that there was a large community of hospitalists on Twitter who were looking for a community to engage in hospital medicine topics,” Dr. Wray said. “We created #JHMChat to bring the hospital medicine community together on a regular basis to talk about pertinent research, medical education philosophies, and value-based care interventions. Our ultimate goal was to increase engagement, networking, and communication among this community, while highlighting the work that is being published in JHM.”
A study of #JHMChat showed that social media is a great platform for large organizations to reach out, connect, and create a community around, he added. “We were very surprised by both the Twitter metrics (i.e., number of participants and overall impressions), which showed very large dissemination numbers, in addition to the external dissemination metrics (i.e., page views and altmetrics scores), which showed that each chat basically corresponded to a release of a new issue. This could be informative to other journals as they look for ways to increase their web traffic or disseminate their work to their respective audiences.”
Dr. Wray hopes the study alerts hospitalists to the fact that there is a large and ever-growing community available within social media.
“Second, we know that careers in hospital medicine can be tough, regardless of whether you’re at a community hospital or a large academic center. Knowing that there is a community with which you can connect to is both comforting and reassuring.”
Reference
Wray C et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018 Nov;13(11):764-9.
Andexanet alfa effectively reverses factor Xa inhibition
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
REPORTING FROM ISC 2019
Is a telehospitalist service right for you and your group?
Telemedicine “ripe for adoption” by hospitalists
For medical inpatients, the advent of virtual care began decades ago with telephones and the ability of physicians to give “verbal orders” while outside the hospital. It evolved into widespread adoption of pagers and is now ubiquitous through smart phones, texting, and HIPPA-compliant applications. In the past few years, inpatient telemedicine programs have been developed and studied including tele-ICU, telestroke, and now the telehospitalist.
Telemedicine is not new and has seen rapid adoption in the outpatient setting over the past decade,1 especially since the passing of telemedicine parity laws in 35 states to support equal reimbursement with face-to-face visits.2 In addition, 24 states have joined the Interstate Medical Licensure Compact (IMLC).3 This voluntary program provides an expedited pathway to licensure for qualified physicians who practice in multiple states. The goal is to increase access to care for patients in underserved and rural areas and to allow easier consultation through telemedicine. Combined, these two federal initiatives have lowered two major barriers to entry for telemedicine: reimbursement and credentialing.
Only a handful of papers have been published on the telehospitalist model with one of the first in 2007 in The Hospitalist reporting on the intersection between tele-ICU and telehospitalist care.4 More recent work describes the implementation of a telehospitalist program between a large university hospitalist program and a rural, critical access hospital.5 A key goal of this program, developed by Dr. Ethan Kuperman and colleagues at the University of Iowa, was to keep patients at the critical access hospital that previously would have been transferred. This has obvious benefits for patients, the critical access hospital, and the local community. It also benefited the tertiary care referral center, which was dealing with high occupancy rates. Keeping lower acuity patients at the critical access hospital helps maintain access for more complex patients at the referral center. This same principle has applied to the use of the tele-ICU where lower acuity ICU patients could remain in the small, rural ICU, and only those patients who the intensivist believes would benefit from a higher level of care in a tertiary center would be transferred.
As this study and others have shown, telemedicine is ripe for adoption by hospitalists. The bigger question is how should it fit into the current model of hospital medicine? There are several different applications we are familiar with and each has unique considerations. The first model, as applied in the Kuperman paper, is for a larger hospitalist program to provide a telehospitalist service to a smaller, unaffiliated hospital (for example, critical access hospitals) that employs nurse practitioners or physician assistants on site but can’t recruit or retain full-time hospitalist coverage. In this collaborative model of care, the local provider performs the physical exam but provides care under the guidance and supervision of a hospital medicine specialist. This is expected to improve outcomes and bring the benefits of hospital medicine, including improved outcomes and decreased hospital spending, to smaller communities.6 In this model, the critical access hospital pays a fee for the service and retains the billing to third party payers.
A variation on that model would provide telehospitalist services to other hospitals within an existing health care network (such as Kaiser Permanente, Intermountain Healthcare, government hospitals) that have different financial models with incentives to collaborate. The Veterans Health Administration is embarking on a pilot through the VA Office of Rural Health to provide a telehospitalist service to small rural VA hospitals using the consultative model during the day with a nurse practitioner at the local site and physician backup from the emergency department. Although existing night cross-coverage will be maintained by a physician on call, this telehospitalist service may also evolve into providing cross-coverage on nights and weekends.
A third would be like a locum tenens model in which telehospitalist services are contracted for short periods of time when coverage is needed for vacations or staff shortages. A fourth model of telehospitalist care would be to international areas in need of hospitalist expertise, like a medical mission model but without the expense or time required to travel. Other models will likely evolve based on the demand for services, supply of hospitalists, changes in regulations, and reimbursement.
Another important consideration is how this will evolve for the practicing hospitalist. Will we have dedicated virtual hospitalists, akin to the “nocturnist” who covers nights and weekends? Or will working on the telehospitalist service be in the rotation of duties like many programs have with teaching and “nonteaching” services, medical consultation, and even transition clinics and emergency department triage responsibilities? It could serve as a lower-intensity service that can be staffed during office-based time that would include scholarly work, quality improvement, and administrative duties. If financially viable, it could be mutually beneficial for both the provider and recipient sides of telehospitalist care.
For any of these models to work, technical aspects must be ironed-out. It is indispensable for the provider to have remote access to the electronic health record for data review, documentation, and placing orders if needed. Adequate broadband for effective video connection, accompanied by the appropriate HIPPA-compliant software and hardware must be in place. Although highly specialized hardware has been developed, including remote stethoscopes and otoscopes, the key component is a good camera and video screen on each end of the interaction. Based upon prior experience with telemedicine programs, establishment of trusting relationships with the receiving hospital staff, physicians, and nurse practitioners is also critical. Optimally, the telehospitalist would have an opportunity to travel to the remote site to meet with the local care team and learn about the local resources and community. Many other operational and logistical issues need to be considered and will be supported by the Society of Hospital Medicine through publications, online resources, and national and regional meeting educational content on telehospitalist programs.
As hospital medicine adopts the telehospitalist model, it brings with it important considerations. First, is how we embrace the concept of the medical virtualist, a term used to describe physicians who spend the majority or all of their time caring for patients using a virtual medium.7 We find it difficult to imagine spending all or the majority of our time as a virtual hospitalist, but years ago many could not imagine someone being a full-time hospitalist or nocturnist. Some individuals will see this as a career opportunity that allows them to work as a hospitalist regardless of where they live or where the hospital is located. That has obvious advantages for both career choice and the provision of hospital medicine expertise to low-resourced or low-volume settings, such as rural or international locations and nights and weekends.
Second, the telehospitalist model will require professional standards, training, reimbursement and coding adjustments, hardware and software development, and managing patient expectations for care.
Lastly, hospitals, health care systems, hospitalist groups, and even individual hospitalists will have to determine how best to take advantage of this innovative model of care to provide the highest possible quality, in a cost-efficient manner, that supports professional satisfaction and development.
Dr. Kaboli and Dr. Gutierrez are based at the Center for Access and Delivery Research and Evaluation (CADRE) at the Iowa City VA Healthcare System, the Veterans Rural Health Resource Center-Iowa City, VA Office of Rural Health, and the department of internal medicine, University of Iowa, both in Iowa City.
References
1. Barnett ML et al. Trends in telemedicine use in a large commercially insured population, 2005-2017. JAMA. 2018;320(20):2147-9.
2. American Telemedicine Association State Policy Resource Center. 2018; http://www.americantelemed.org/main/policy-page/state-policy-resource-center. Accessed 2018 Dec 14.
3. Interstate Medical Licensure Compact 2018; https://imlcc.org/. Accessed 2018 Dec 14.
4. Hengehold D. The telehospitalist. The Hospitalist. 2007;7(July). https://www.the-hospitalist.org/hospitalist/article/123381/telehospitalist. Accessed 2018 Dec 14.
5. Kuperman EF et al. The virtual hospitalist: A single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-63.
6. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic proceedings. 2009;84(3):248-54.
7. Nochomovitz M, Sharma R. Is it time for a new medical specialty?: The medical virtualist. JAMA. 2018;319(5):437-8.
Telemedicine “ripe for adoption” by hospitalists
Telemedicine “ripe for adoption” by hospitalists
For medical inpatients, the advent of virtual care began decades ago with telephones and the ability of physicians to give “verbal orders” while outside the hospital. It evolved into widespread adoption of pagers and is now ubiquitous through smart phones, texting, and HIPPA-compliant applications. In the past few years, inpatient telemedicine programs have been developed and studied including tele-ICU, telestroke, and now the telehospitalist.
Telemedicine is not new and has seen rapid adoption in the outpatient setting over the past decade,1 especially since the passing of telemedicine parity laws in 35 states to support equal reimbursement with face-to-face visits.2 In addition, 24 states have joined the Interstate Medical Licensure Compact (IMLC).3 This voluntary program provides an expedited pathway to licensure for qualified physicians who practice in multiple states. The goal is to increase access to care for patients in underserved and rural areas and to allow easier consultation through telemedicine. Combined, these two federal initiatives have lowered two major barriers to entry for telemedicine: reimbursement and credentialing.
Only a handful of papers have been published on the telehospitalist model with one of the first in 2007 in The Hospitalist reporting on the intersection between tele-ICU and telehospitalist care.4 More recent work describes the implementation of a telehospitalist program between a large university hospitalist program and a rural, critical access hospital.5 A key goal of this program, developed by Dr. Ethan Kuperman and colleagues at the University of Iowa, was to keep patients at the critical access hospital that previously would have been transferred. This has obvious benefits for patients, the critical access hospital, and the local community. It also benefited the tertiary care referral center, which was dealing with high occupancy rates. Keeping lower acuity patients at the critical access hospital helps maintain access for more complex patients at the referral center. This same principle has applied to the use of the tele-ICU where lower acuity ICU patients could remain in the small, rural ICU, and only those patients who the intensivist believes would benefit from a higher level of care in a tertiary center would be transferred.
As this study and others have shown, telemedicine is ripe for adoption by hospitalists. The bigger question is how should it fit into the current model of hospital medicine? There are several different applications we are familiar with and each has unique considerations. The first model, as applied in the Kuperman paper, is for a larger hospitalist program to provide a telehospitalist service to a smaller, unaffiliated hospital (for example, critical access hospitals) that employs nurse practitioners or physician assistants on site but can’t recruit or retain full-time hospitalist coverage. In this collaborative model of care, the local provider performs the physical exam but provides care under the guidance and supervision of a hospital medicine specialist. This is expected to improve outcomes and bring the benefits of hospital medicine, including improved outcomes and decreased hospital spending, to smaller communities.6 In this model, the critical access hospital pays a fee for the service and retains the billing to third party payers.
A variation on that model would provide telehospitalist services to other hospitals within an existing health care network (such as Kaiser Permanente, Intermountain Healthcare, government hospitals) that have different financial models with incentives to collaborate. The Veterans Health Administration is embarking on a pilot through the VA Office of Rural Health to provide a telehospitalist service to small rural VA hospitals using the consultative model during the day with a nurse practitioner at the local site and physician backup from the emergency department. Although existing night cross-coverage will be maintained by a physician on call, this telehospitalist service may also evolve into providing cross-coverage on nights and weekends.
A third would be like a locum tenens model in which telehospitalist services are contracted for short periods of time when coverage is needed for vacations or staff shortages. A fourth model of telehospitalist care would be to international areas in need of hospitalist expertise, like a medical mission model but without the expense or time required to travel. Other models will likely evolve based on the demand for services, supply of hospitalists, changes in regulations, and reimbursement.
Another important consideration is how this will evolve for the practicing hospitalist. Will we have dedicated virtual hospitalists, akin to the “nocturnist” who covers nights and weekends? Or will working on the telehospitalist service be in the rotation of duties like many programs have with teaching and “nonteaching” services, medical consultation, and even transition clinics and emergency department triage responsibilities? It could serve as a lower-intensity service that can be staffed during office-based time that would include scholarly work, quality improvement, and administrative duties. If financially viable, it could be mutually beneficial for both the provider and recipient sides of telehospitalist care.
For any of these models to work, technical aspects must be ironed-out. It is indispensable for the provider to have remote access to the electronic health record for data review, documentation, and placing orders if needed. Adequate broadband for effective video connection, accompanied by the appropriate HIPPA-compliant software and hardware must be in place. Although highly specialized hardware has been developed, including remote stethoscopes and otoscopes, the key component is a good camera and video screen on each end of the interaction. Based upon prior experience with telemedicine programs, establishment of trusting relationships with the receiving hospital staff, physicians, and nurse practitioners is also critical. Optimally, the telehospitalist would have an opportunity to travel to the remote site to meet with the local care team and learn about the local resources and community. Many other operational and logistical issues need to be considered and will be supported by the Society of Hospital Medicine through publications, online resources, and national and regional meeting educational content on telehospitalist programs.
As hospital medicine adopts the telehospitalist model, it brings with it important considerations. First, is how we embrace the concept of the medical virtualist, a term used to describe physicians who spend the majority or all of their time caring for patients using a virtual medium.7 We find it difficult to imagine spending all or the majority of our time as a virtual hospitalist, but years ago many could not imagine someone being a full-time hospitalist or nocturnist. Some individuals will see this as a career opportunity that allows them to work as a hospitalist regardless of where they live or where the hospital is located. That has obvious advantages for both career choice and the provision of hospital medicine expertise to low-resourced or low-volume settings, such as rural or international locations and nights and weekends.
Second, the telehospitalist model will require professional standards, training, reimbursement and coding adjustments, hardware and software development, and managing patient expectations for care.
Lastly, hospitals, health care systems, hospitalist groups, and even individual hospitalists will have to determine how best to take advantage of this innovative model of care to provide the highest possible quality, in a cost-efficient manner, that supports professional satisfaction and development.
Dr. Kaboli and Dr. Gutierrez are based at the Center for Access and Delivery Research and Evaluation (CADRE) at the Iowa City VA Healthcare System, the Veterans Rural Health Resource Center-Iowa City, VA Office of Rural Health, and the department of internal medicine, University of Iowa, both in Iowa City.
References
1. Barnett ML et al. Trends in telemedicine use in a large commercially insured population, 2005-2017. JAMA. 2018;320(20):2147-9.
2. American Telemedicine Association State Policy Resource Center. 2018; http://www.americantelemed.org/main/policy-page/state-policy-resource-center. Accessed 2018 Dec 14.
3. Interstate Medical Licensure Compact 2018; https://imlcc.org/. Accessed 2018 Dec 14.
4. Hengehold D. The telehospitalist. The Hospitalist. 2007;7(July). https://www.the-hospitalist.org/hospitalist/article/123381/telehospitalist. Accessed 2018 Dec 14.
5. Kuperman EF et al. The virtual hospitalist: A single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-63.
6. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic proceedings. 2009;84(3):248-54.
7. Nochomovitz M, Sharma R. Is it time for a new medical specialty?: The medical virtualist. JAMA. 2018;319(5):437-8.
For medical inpatients, the advent of virtual care began decades ago with telephones and the ability of physicians to give “verbal orders” while outside the hospital. It evolved into widespread adoption of pagers and is now ubiquitous through smart phones, texting, and HIPPA-compliant applications. In the past few years, inpatient telemedicine programs have been developed and studied including tele-ICU, telestroke, and now the telehospitalist.
Telemedicine is not new and has seen rapid adoption in the outpatient setting over the past decade,1 especially since the passing of telemedicine parity laws in 35 states to support equal reimbursement with face-to-face visits.2 In addition, 24 states have joined the Interstate Medical Licensure Compact (IMLC).3 This voluntary program provides an expedited pathway to licensure for qualified physicians who practice in multiple states. The goal is to increase access to care for patients in underserved and rural areas and to allow easier consultation through telemedicine. Combined, these two federal initiatives have lowered two major barriers to entry for telemedicine: reimbursement and credentialing.
Only a handful of papers have been published on the telehospitalist model with one of the first in 2007 in The Hospitalist reporting on the intersection between tele-ICU and telehospitalist care.4 More recent work describes the implementation of a telehospitalist program between a large university hospitalist program and a rural, critical access hospital.5 A key goal of this program, developed by Dr. Ethan Kuperman and colleagues at the University of Iowa, was to keep patients at the critical access hospital that previously would have been transferred. This has obvious benefits for patients, the critical access hospital, and the local community. It also benefited the tertiary care referral center, which was dealing with high occupancy rates. Keeping lower acuity patients at the critical access hospital helps maintain access for more complex patients at the referral center. This same principle has applied to the use of the tele-ICU where lower acuity ICU patients could remain in the small, rural ICU, and only those patients who the intensivist believes would benefit from a higher level of care in a tertiary center would be transferred.
As this study and others have shown, telemedicine is ripe for adoption by hospitalists. The bigger question is how should it fit into the current model of hospital medicine? There are several different applications we are familiar with and each has unique considerations. The first model, as applied in the Kuperman paper, is for a larger hospitalist program to provide a telehospitalist service to a smaller, unaffiliated hospital (for example, critical access hospitals) that employs nurse practitioners or physician assistants on site but can’t recruit or retain full-time hospitalist coverage. In this collaborative model of care, the local provider performs the physical exam but provides care under the guidance and supervision of a hospital medicine specialist. This is expected to improve outcomes and bring the benefits of hospital medicine, including improved outcomes and decreased hospital spending, to smaller communities.6 In this model, the critical access hospital pays a fee for the service and retains the billing to third party payers.
A variation on that model would provide telehospitalist services to other hospitals within an existing health care network (such as Kaiser Permanente, Intermountain Healthcare, government hospitals) that have different financial models with incentives to collaborate. The Veterans Health Administration is embarking on a pilot through the VA Office of Rural Health to provide a telehospitalist service to small rural VA hospitals using the consultative model during the day with a nurse practitioner at the local site and physician backup from the emergency department. Although existing night cross-coverage will be maintained by a physician on call, this telehospitalist service may also evolve into providing cross-coverage on nights and weekends.
A third would be like a locum tenens model in which telehospitalist services are contracted for short periods of time when coverage is needed for vacations or staff shortages. A fourth model of telehospitalist care would be to international areas in need of hospitalist expertise, like a medical mission model but without the expense or time required to travel. Other models will likely evolve based on the demand for services, supply of hospitalists, changes in regulations, and reimbursement.
Another important consideration is how this will evolve for the practicing hospitalist. Will we have dedicated virtual hospitalists, akin to the “nocturnist” who covers nights and weekends? Or will working on the telehospitalist service be in the rotation of duties like many programs have with teaching and “nonteaching” services, medical consultation, and even transition clinics and emergency department triage responsibilities? It could serve as a lower-intensity service that can be staffed during office-based time that would include scholarly work, quality improvement, and administrative duties. If financially viable, it could be mutually beneficial for both the provider and recipient sides of telehospitalist care.
For any of these models to work, technical aspects must be ironed-out. It is indispensable for the provider to have remote access to the electronic health record for data review, documentation, and placing orders if needed. Adequate broadband for effective video connection, accompanied by the appropriate HIPPA-compliant software and hardware must be in place. Although highly specialized hardware has been developed, including remote stethoscopes and otoscopes, the key component is a good camera and video screen on each end of the interaction. Based upon prior experience with telemedicine programs, establishment of trusting relationships with the receiving hospital staff, physicians, and nurse practitioners is also critical. Optimally, the telehospitalist would have an opportunity to travel to the remote site to meet with the local care team and learn about the local resources and community. Many other operational and logistical issues need to be considered and will be supported by the Society of Hospital Medicine through publications, online resources, and national and regional meeting educational content on telehospitalist programs.
As hospital medicine adopts the telehospitalist model, it brings with it important considerations. First, is how we embrace the concept of the medical virtualist, a term used to describe physicians who spend the majority or all of their time caring for patients using a virtual medium.7 We find it difficult to imagine spending all or the majority of our time as a virtual hospitalist, but years ago many could not imagine someone being a full-time hospitalist or nocturnist. Some individuals will see this as a career opportunity that allows them to work as a hospitalist regardless of where they live or where the hospital is located. That has obvious advantages for both career choice and the provision of hospital medicine expertise to low-resourced or low-volume settings, such as rural or international locations and nights and weekends.
Second, the telehospitalist model will require professional standards, training, reimbursement and coding adjustments, hardware and software development, and managing patient expectations for care.
Lastly, hospitals, health care systems, hospitalist groups, and even individual hospitalists will have to determine how best to take advantage of this innovative model of care to provide the highest possible quality, in a cost-efficient manner, that supports professional satisfaction and development.
Dr. Kaboli and Dr. Gutierrez are based at the Center for Access and Delivery Research and Evaluation (CADRE) at the Iowa City VA Healthcare System, the Veterans Rural Health Resource Center-Iowa City, VA Office of Rural Health, and the department of internal medicine, University of Iowa, both in Iowa City.
References
1. Barnett ML et al. Trends in telemedicine use in a large commercially insured population, 2005-2017. JAMA. 2018;320(20):2147-9.
2. American Telemedicine Association State Policy Resource Center. 2018; http://www.americantelemed.org/main/policy-page/state-policy-resource-center. Accessed 2018 Dec 14.
3. Interstate Medical Licensure Compact 2018; https://imlcc.org/. Accessed 2018 Dec 14.
4. Hengehold D. The telehospitalist. The Hospitalist. 2007;7(July). https://www.the-hospitalist.org/hospitalist/article/123381/telehospitalist. Accessed 2018 Dec 14.
5. Kuperman EF et al. The virtual hospitalist: A single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-63.
6. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clinic proceedings. 2009;84(3):248-54.
7. Nochomovitz M, Sharma R. Is it time for a new medical specialty?: The medical virtualist. JAMA. 2018;319(5):437-8.