User login
Acne and Pregnancy: A Clinical Review and Practice Pearls
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.
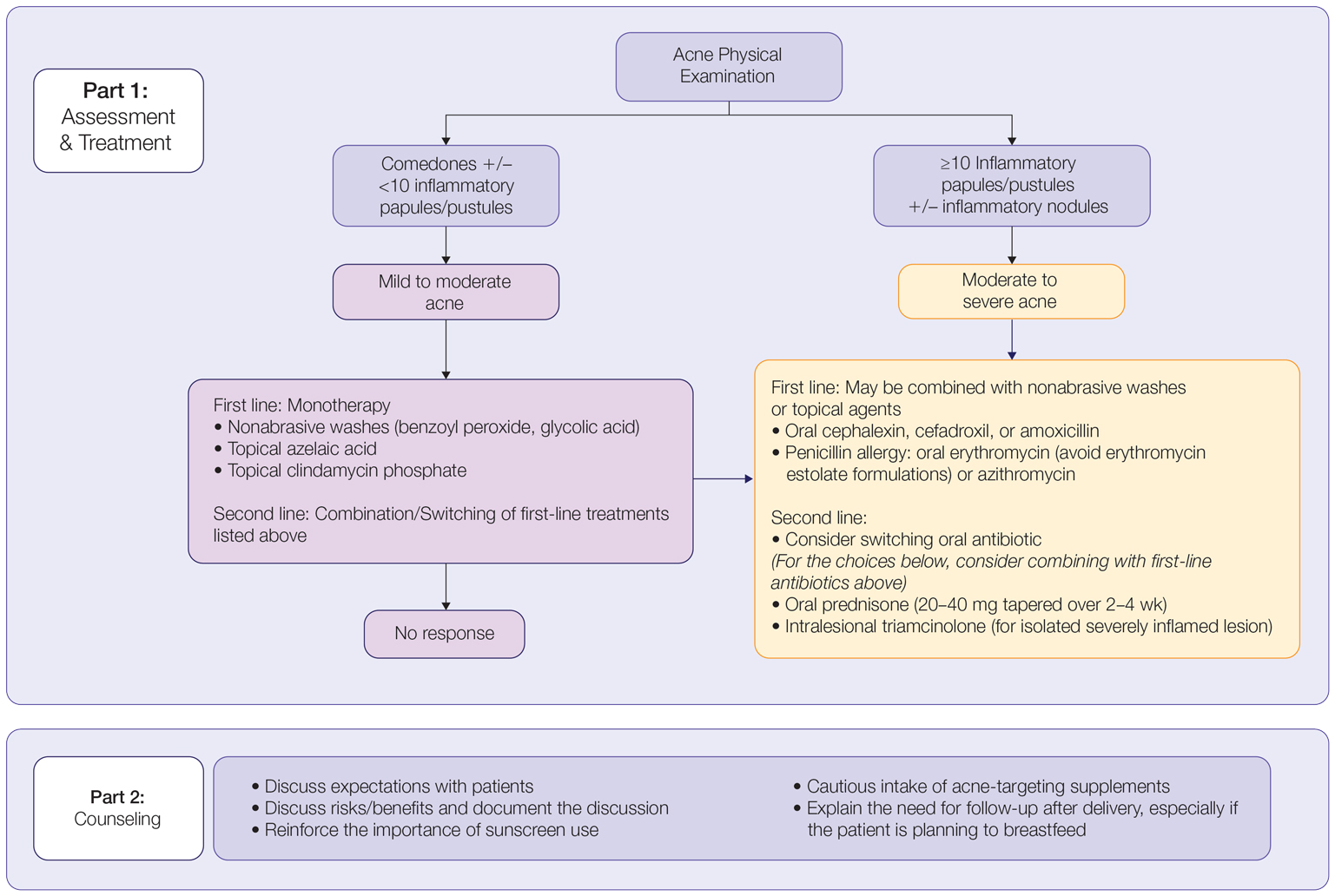
In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Practice Points
- The management of acne in pregnancy requires careful consideration of therapeutic choices to guarantee the safety of both the mother and the developing fetus.
- The use of topicals should be observed as first-line therapy, but consideration for systemic therapy in cases of treatment failure or more severe disease is warranted.
- Discussion of patient expectations and involving them in decision-making for therapeutic choice is crucial.
Preventing ASCVD Events: Using Coronary Artery Calcification Scores to Personalize Risk and Guide Statin Therapy
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
Axillary Contact Dermatitis: An Update on Potential Allergens and Management
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34
Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58
Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65
Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92
Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Practice Points
- The differential diagnosis of axillary dermatitis is broad. Contact dermatitis—both irritant and allergic—represents common etiologies.
- Understanding the clinical features and range of potential sources in axillary contact dermatitis allows for efficient recognition and elimination of causative exposure.
- For cases of suspected allergic contact dermatitis, patch testing and subsequent allergen avoidance are paramount in the management of axillary eruptions.
Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10
Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28
In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
PRACTICE POINTS
- Squamous cell carcinoma can develop within chronic inflammatory dermatoses.
- Orolabial discoid lupus erythematosus (DLE), oral lichen planus, and lichen sclerosus can lead to relatively rapid tumorigenesis. Squamous cell carcinoma arising in cutaneous DLE, hidradenitis suppurativa (HS), necrobiosis lipoidica, chronic wounds, and hypertrophic lichen planus tends to appear after decades of inflammation.
- Be especially mindful of new orolabial DLE cases and chronic cases of HS and Marjolin ulcer because malignancies in these settings are particularly aggressive.
Low-Carbohydrate and Ketogenic Dietary Patterns for Type 2 Diabetes Management
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent studyshowed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent studyshowed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent studyshowed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
The Role of Toluidine Blue in Mohs Micrographic Surgery: A Systematic Review
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.
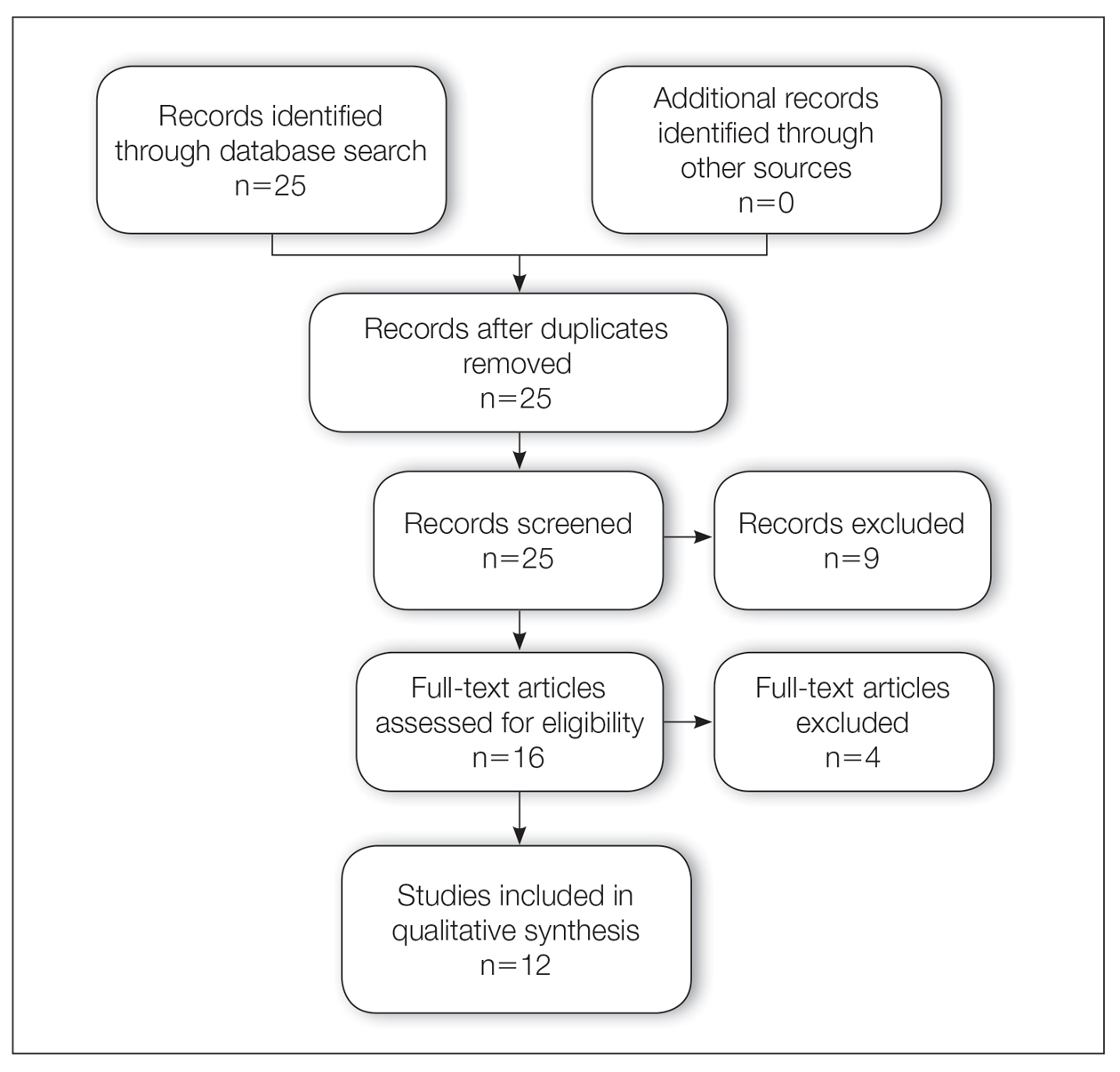
Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).
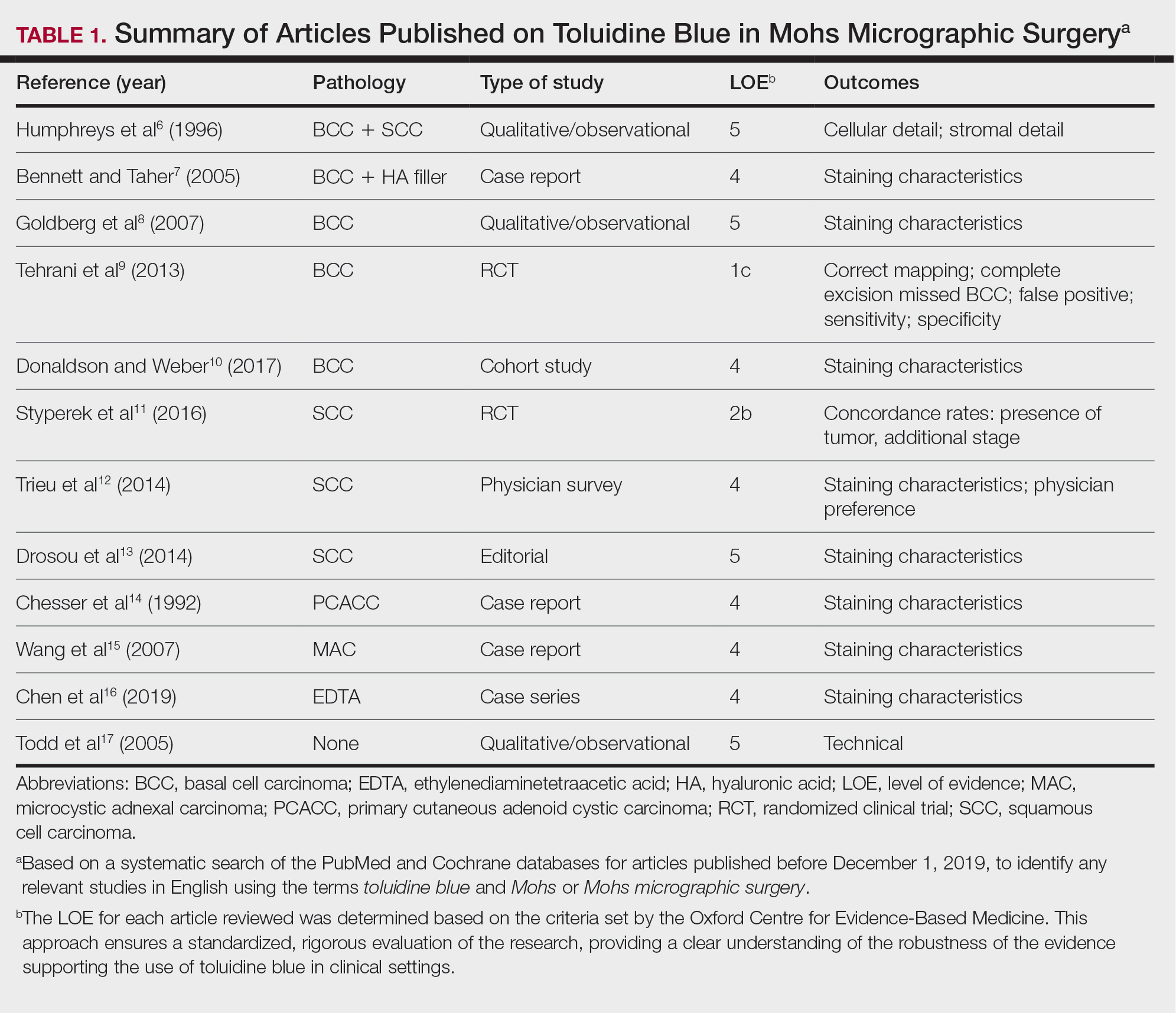
A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.
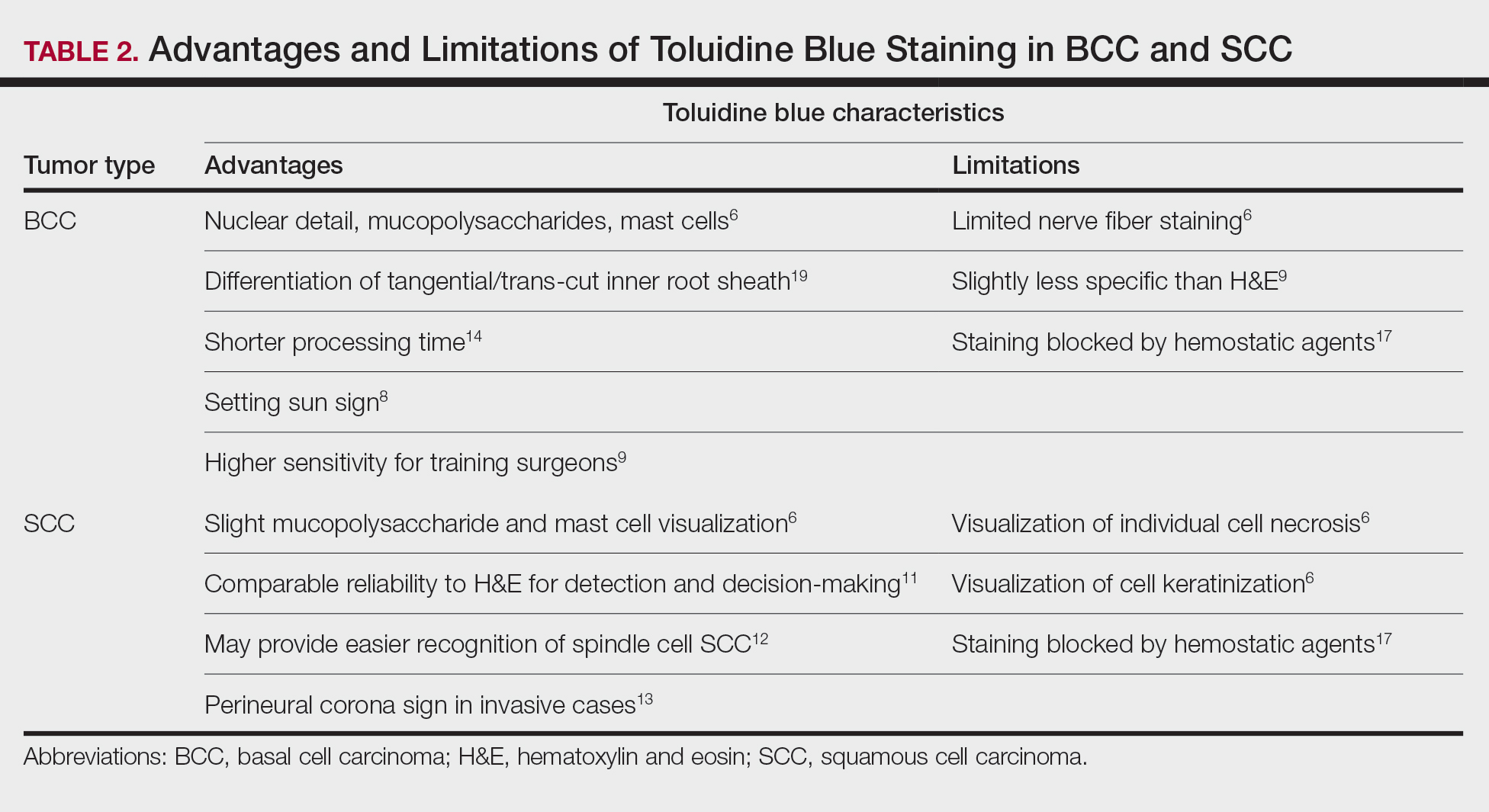
Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6
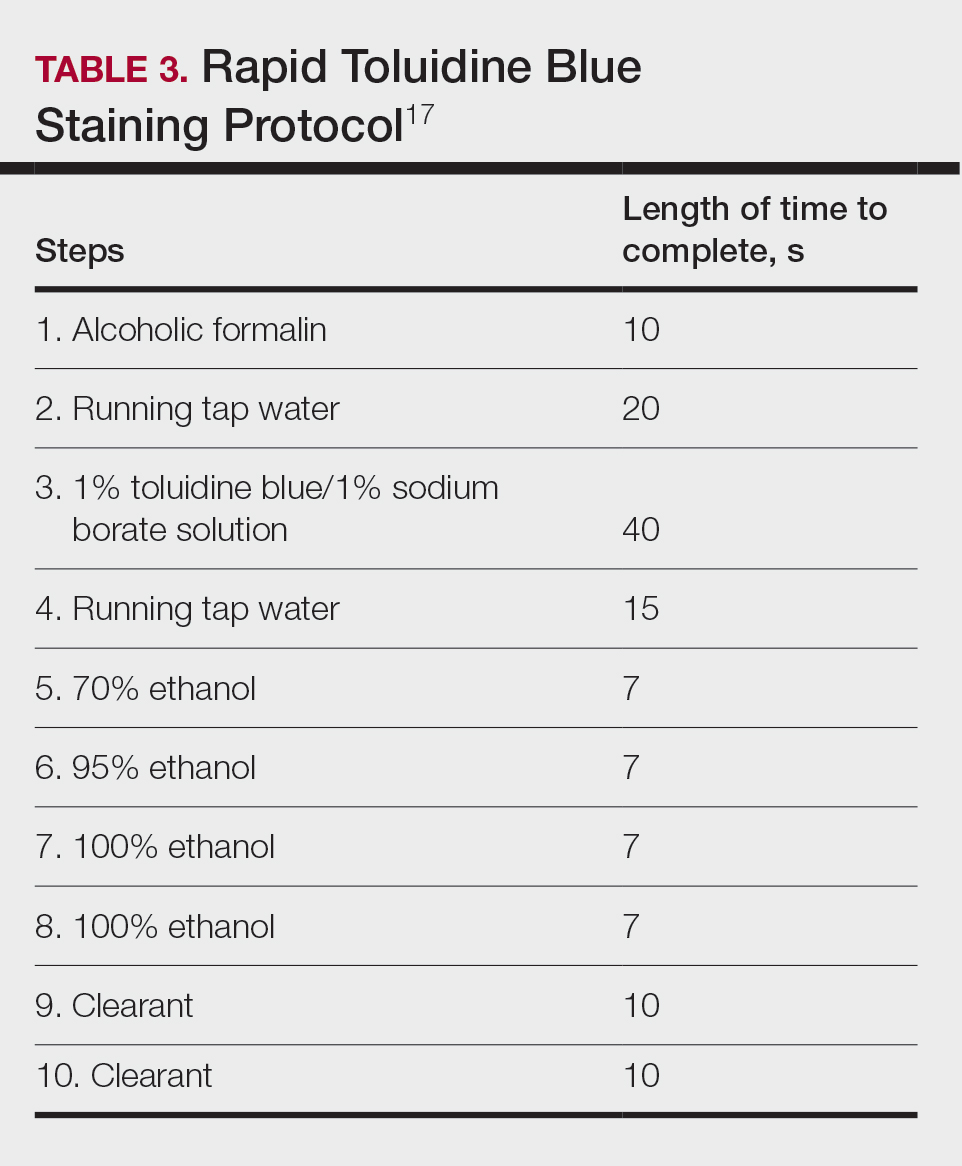
Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Practice Points
- Toluidine blue (TB) staining can be integrated into Mohs micrographic surgery (MMS) for enhanced diagnosis of cutaneous tumors. Its metachromatic properties can aid in differentiating tumor cells from surrounding tissues, especially in basal cell carcinomas and squamous cell carcinomas.
- It is important to develop expertise in interpreting TB-stained sections, as it may offer clearer visualization of nuclear details and stromal components, potentially leading to more accurate diagnosis and effective tumor margin identification.
- Toluidine blue staining can be incorporated into routine MMS practice considering its quick staining process and low disruption to workflow. This can potentially improve diagnostic efficiency without significantly lengthening surgery time.
2023 Update on minimally invasive gynecologic surgery
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15
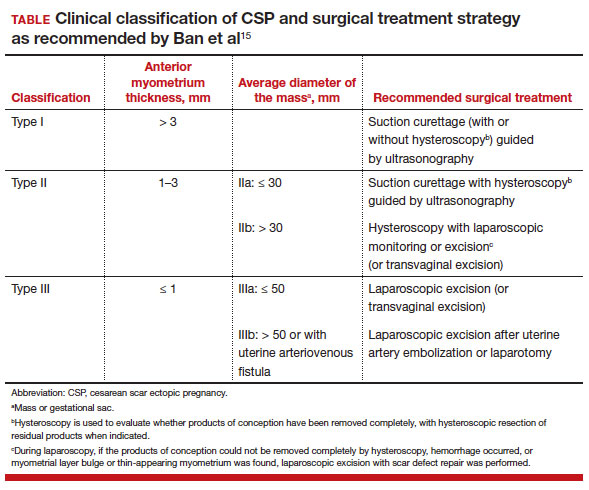
Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
Norgestrel for nonprescription contraception: What you and your patients need to know
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.
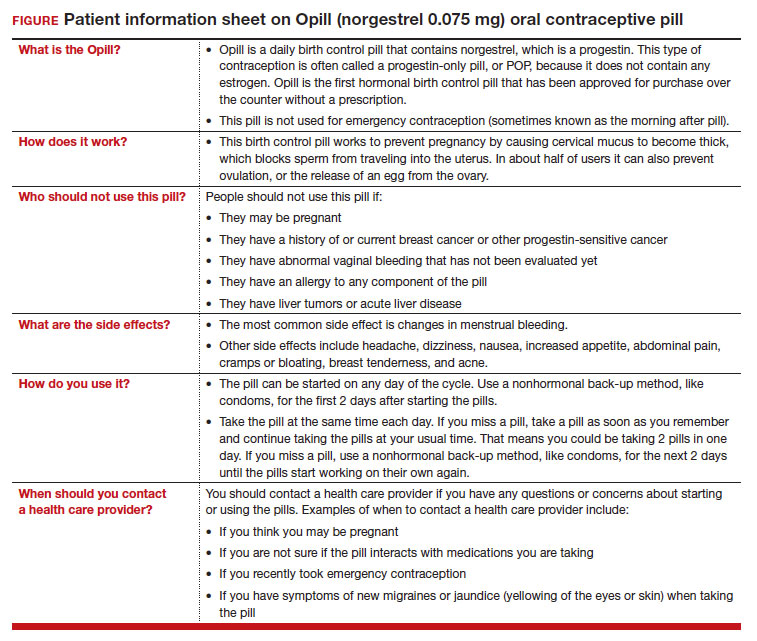
How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8
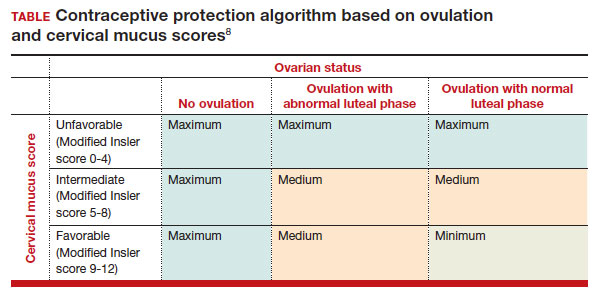
In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.

How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8

In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.

How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8

In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
Recruiting ObGyns: Starting salary considerations
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4
Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4


Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4


Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
Patient counseling for breast cancer screening: Taking changes to USPSTF recommendations into account
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.