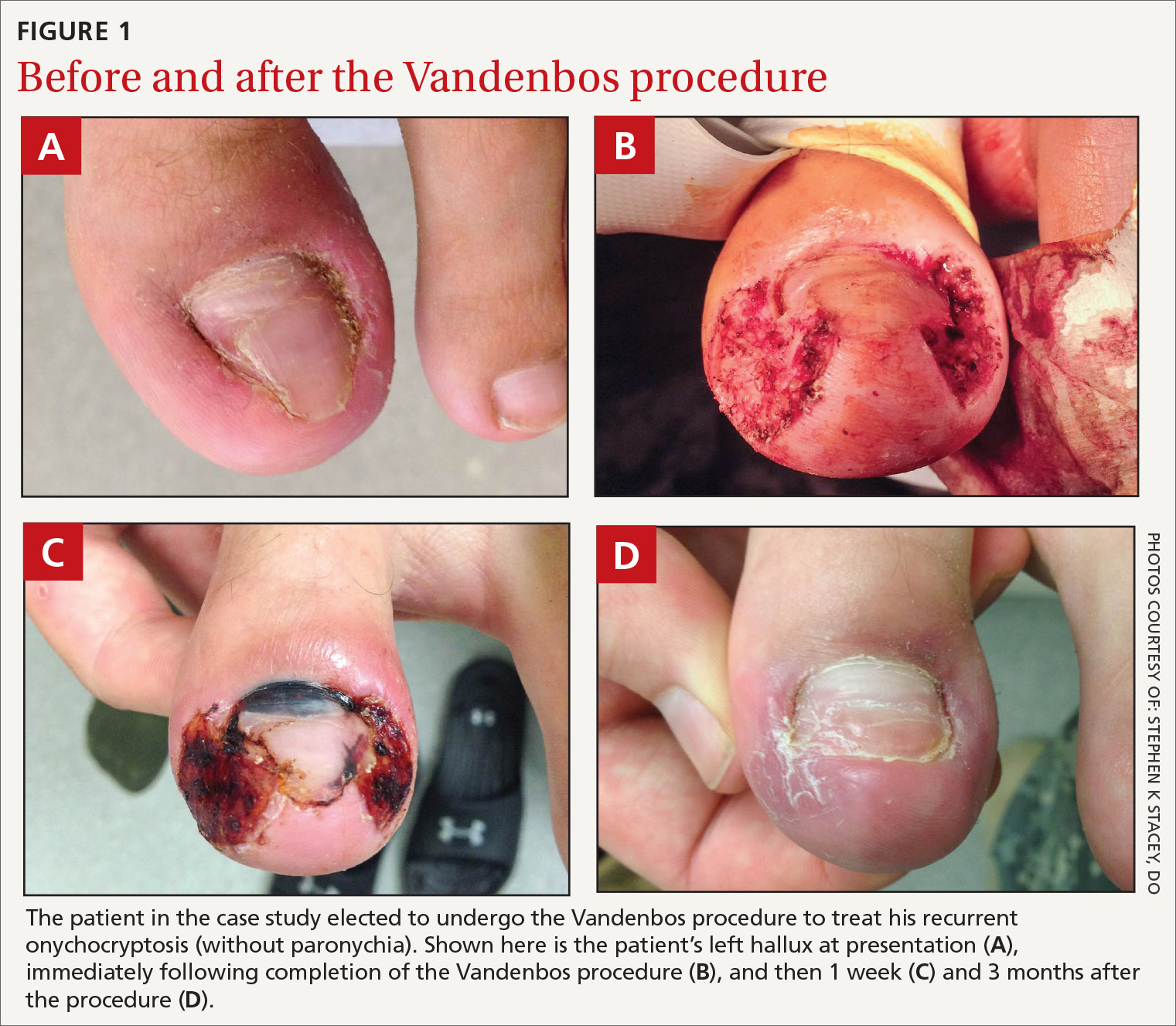
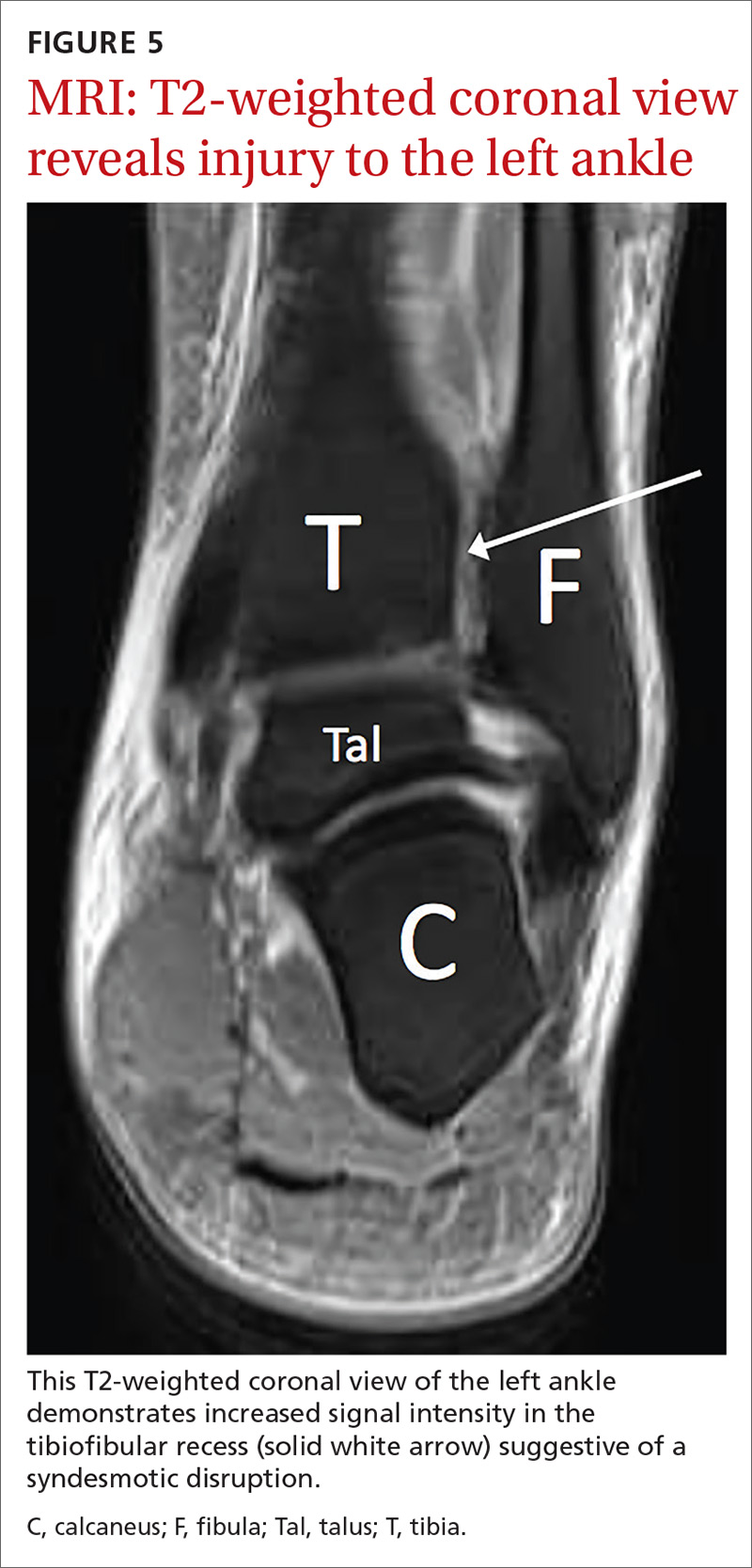
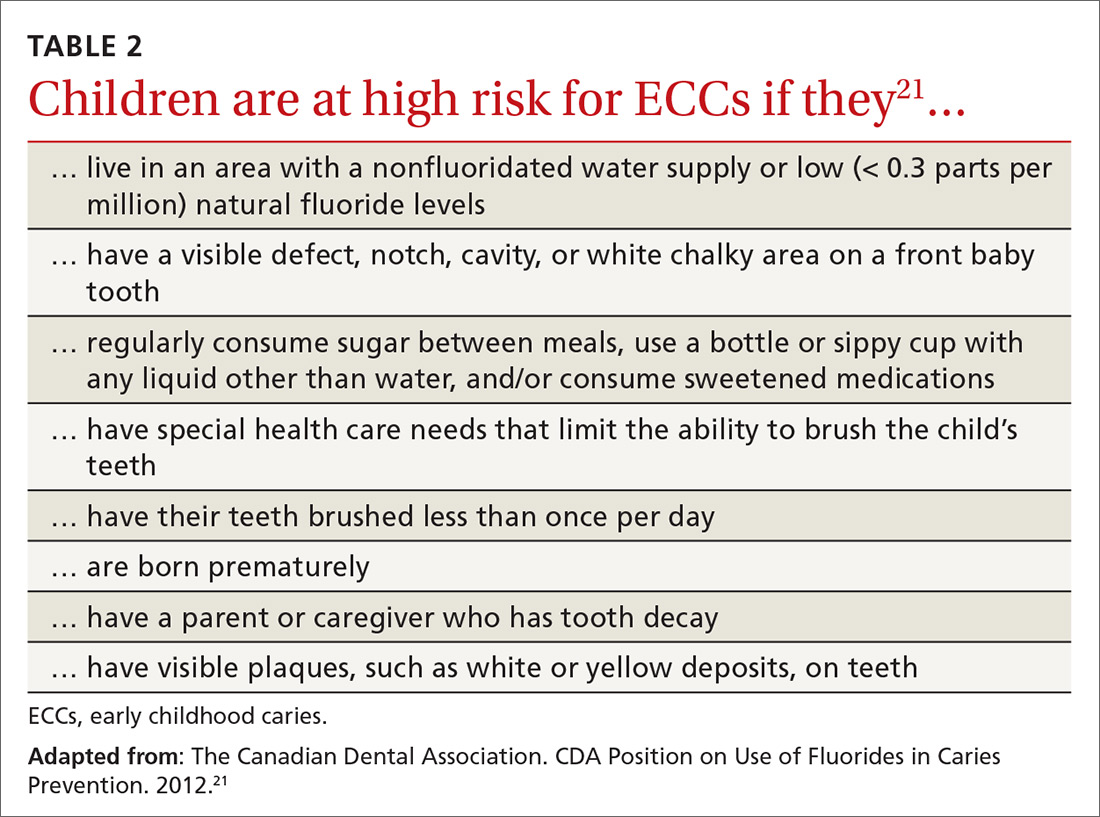
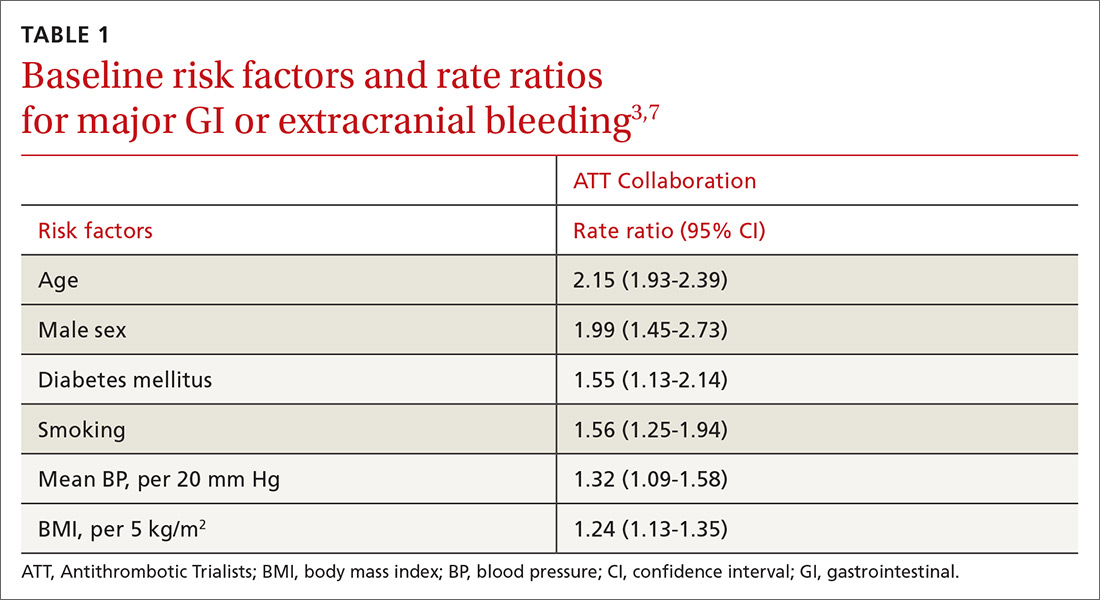
User login
How to incorporate HIV PrEP into your practice
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
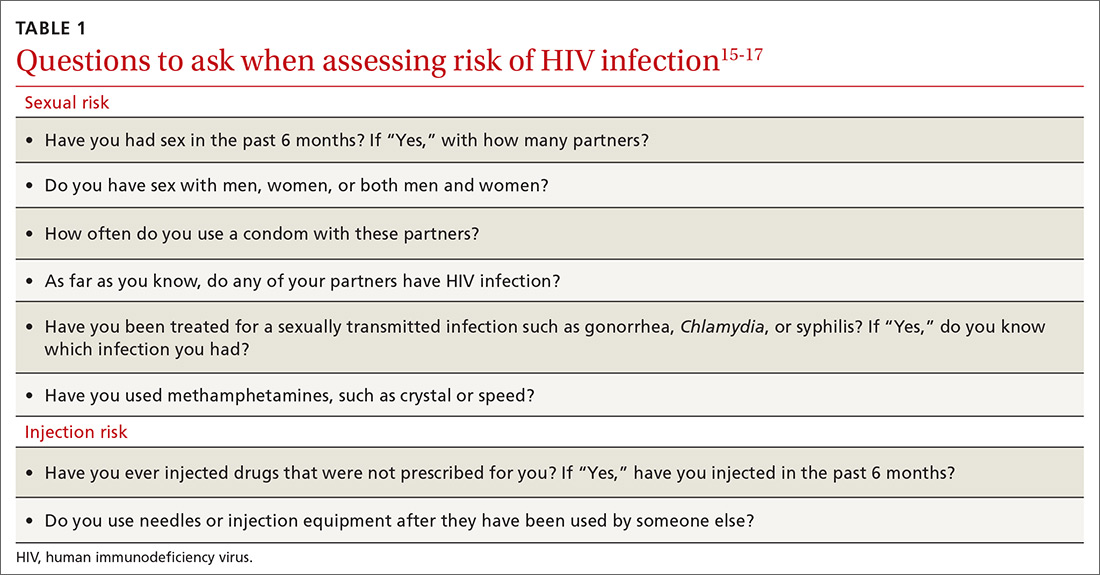
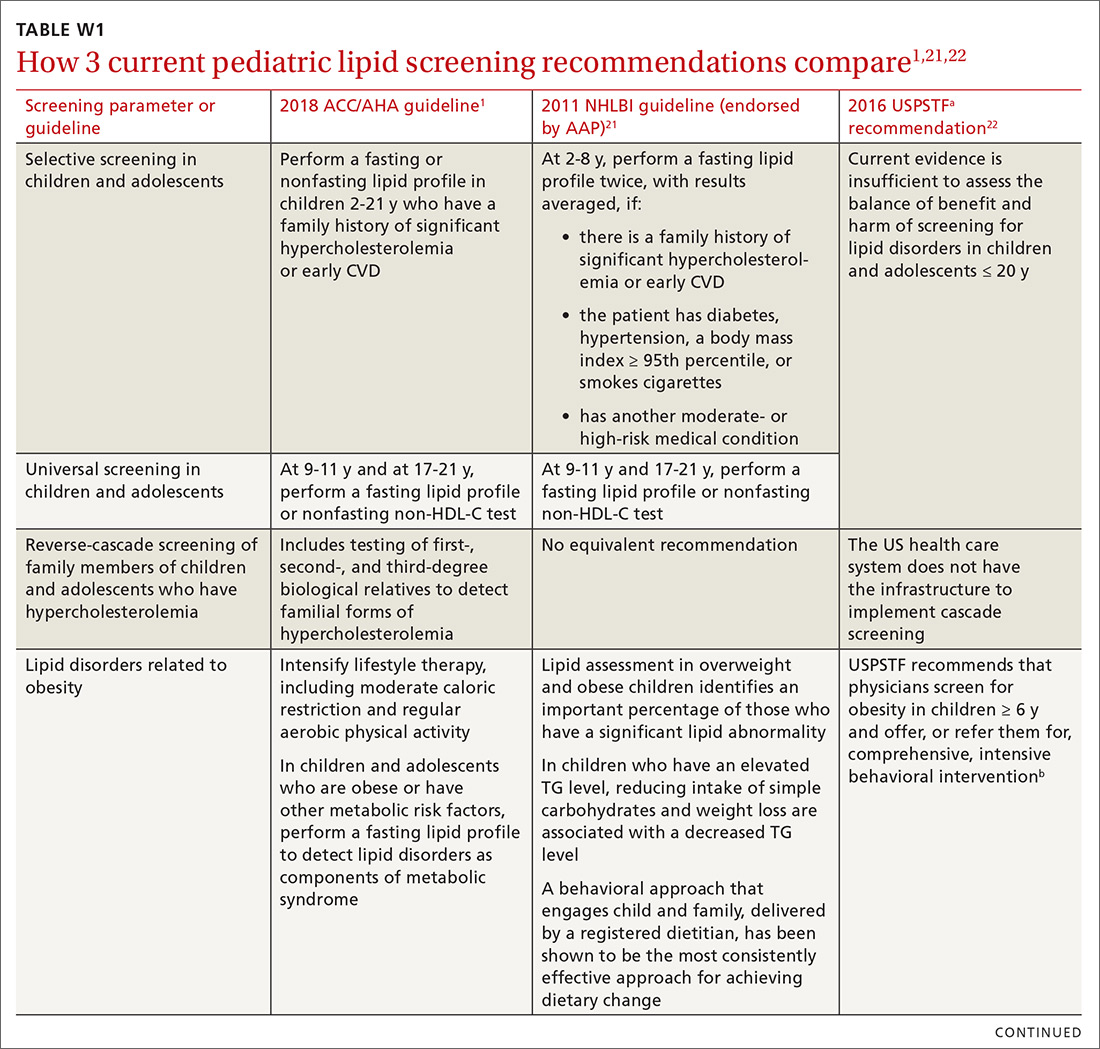
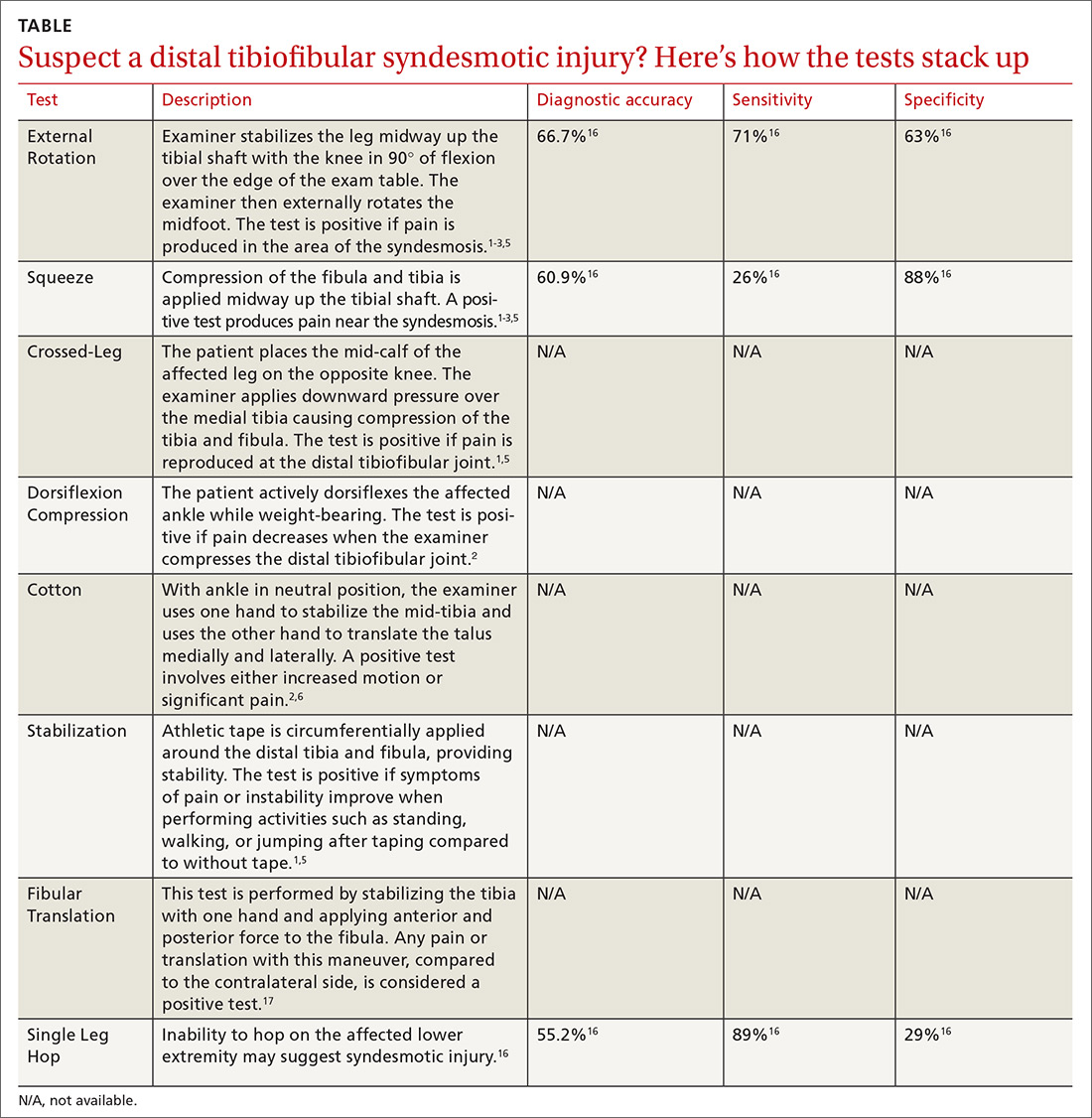
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
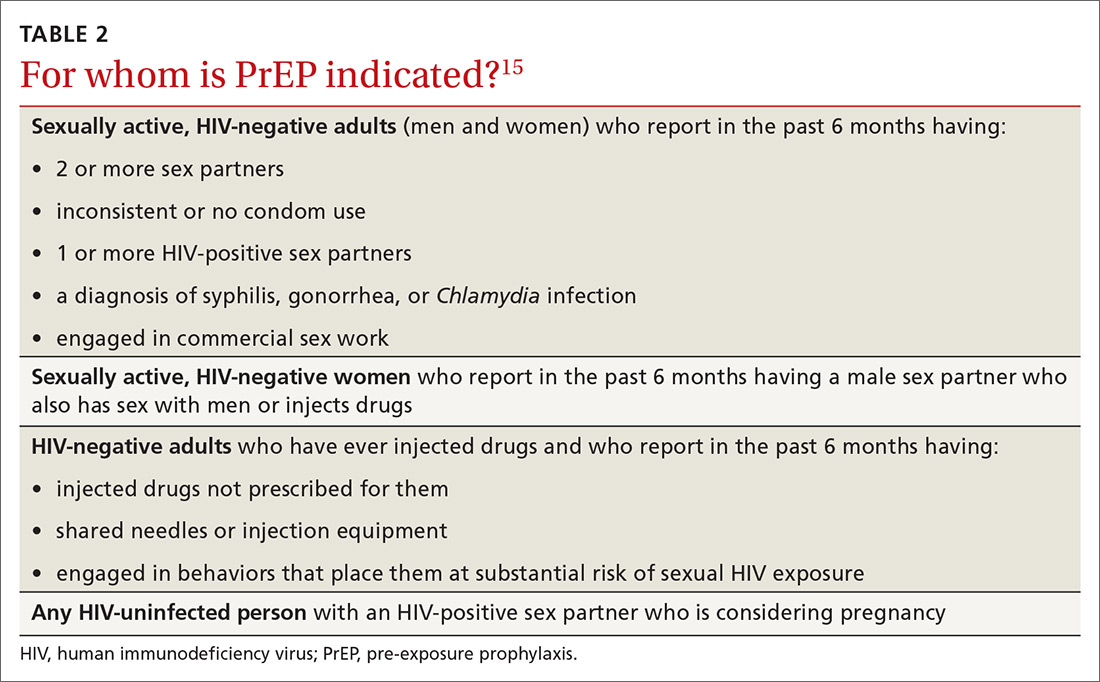
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
What steps should be taken before and after initiating PrEP?
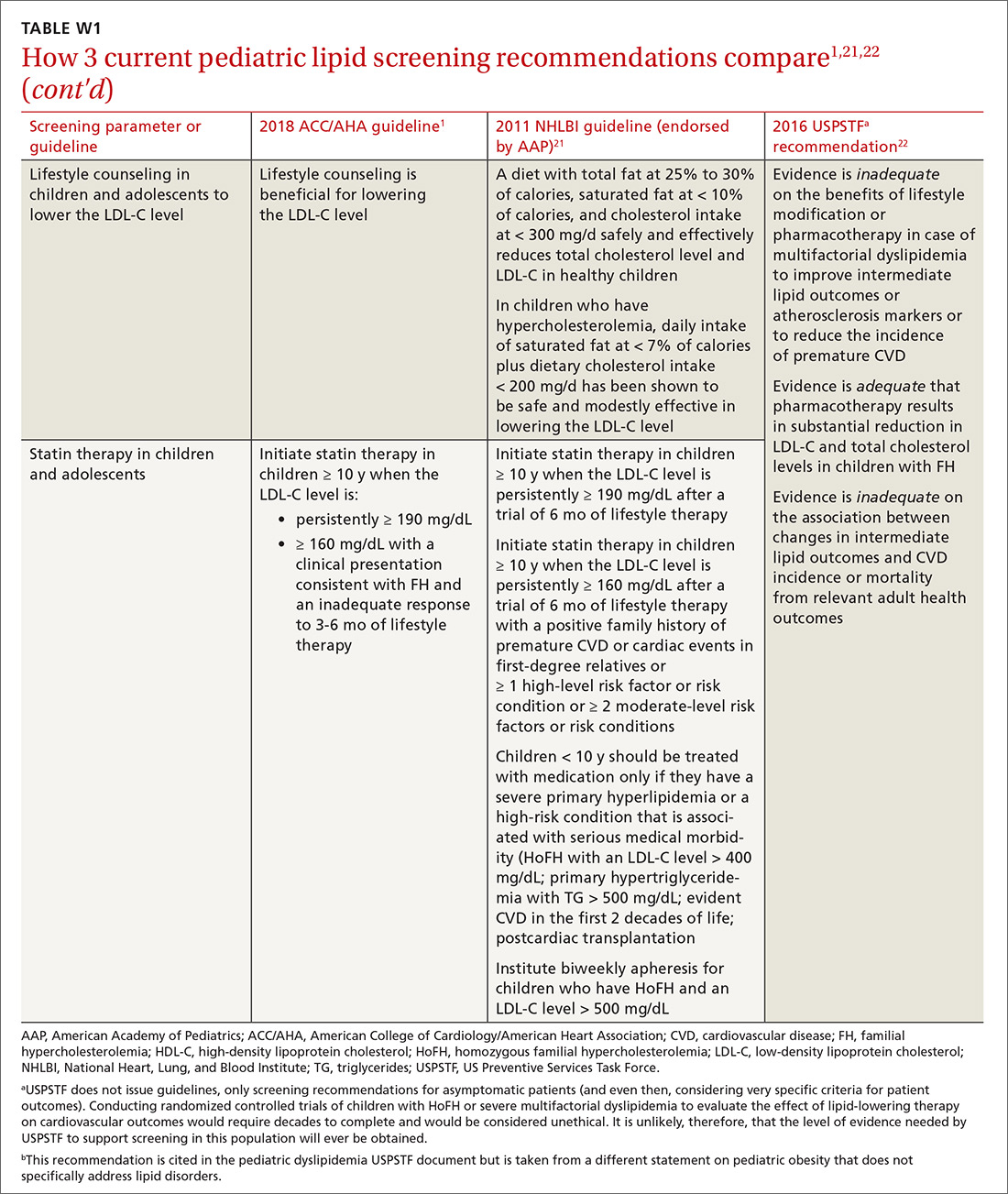
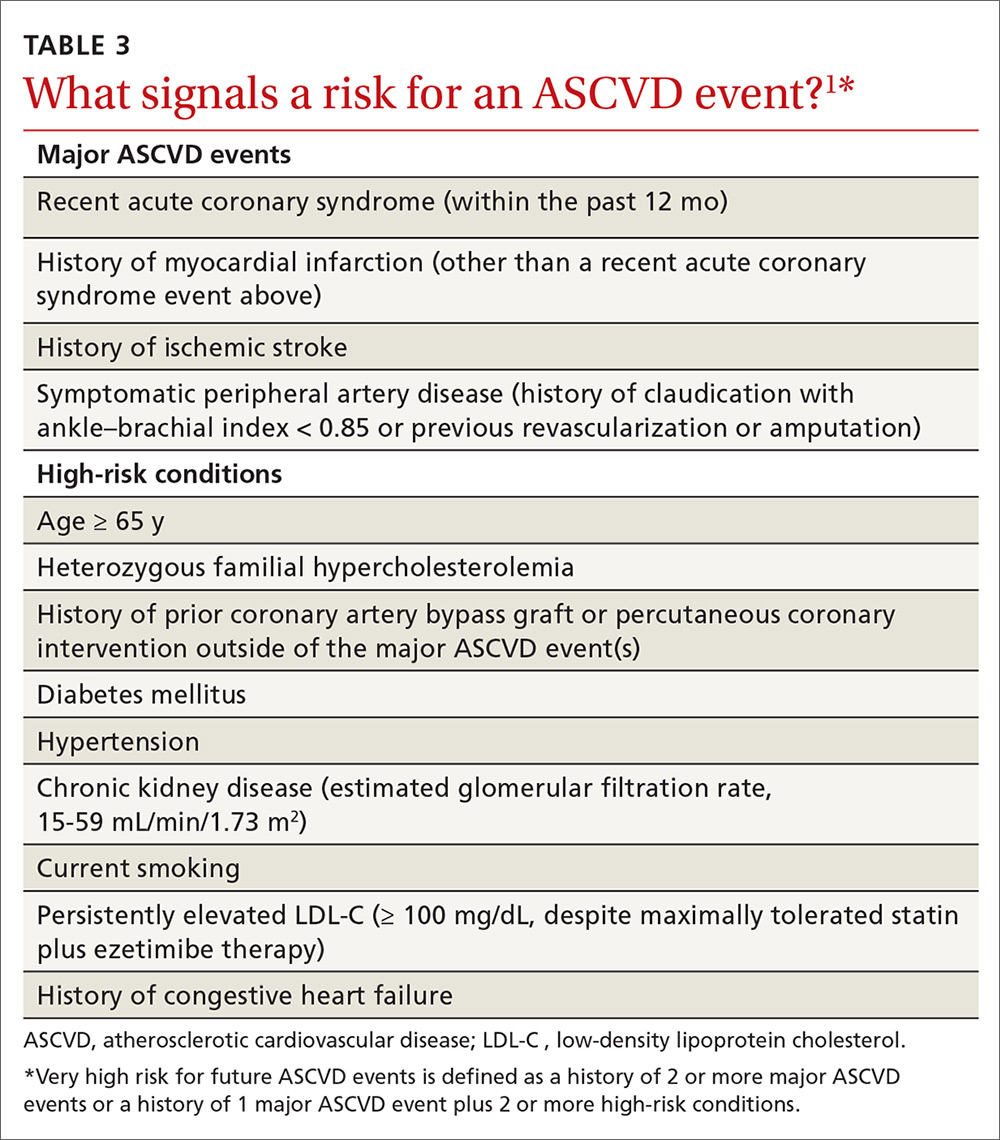
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.
Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
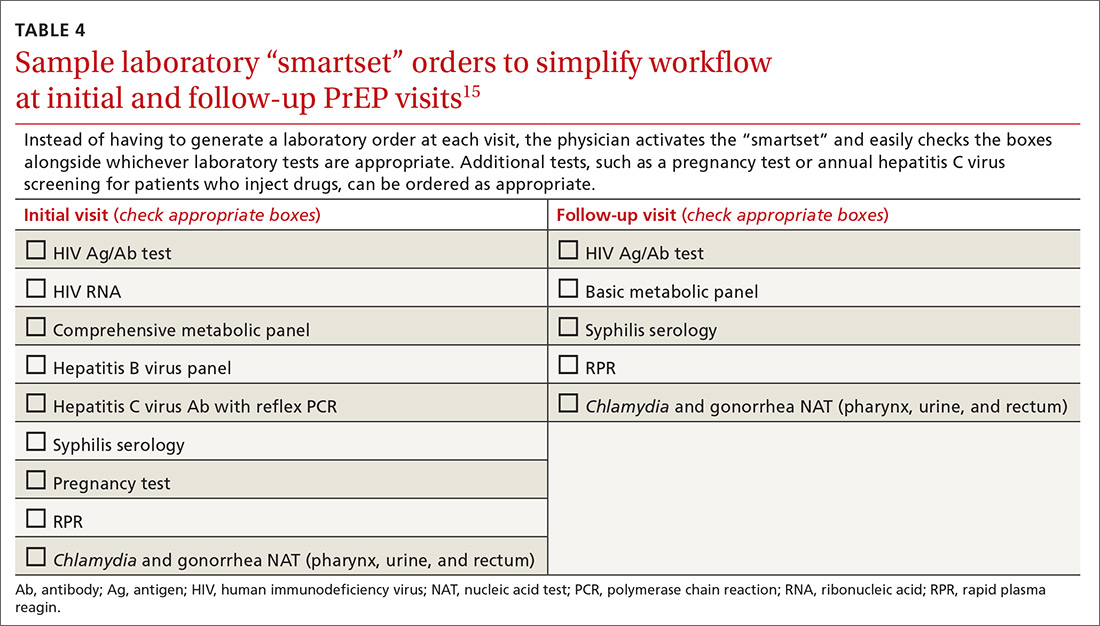
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
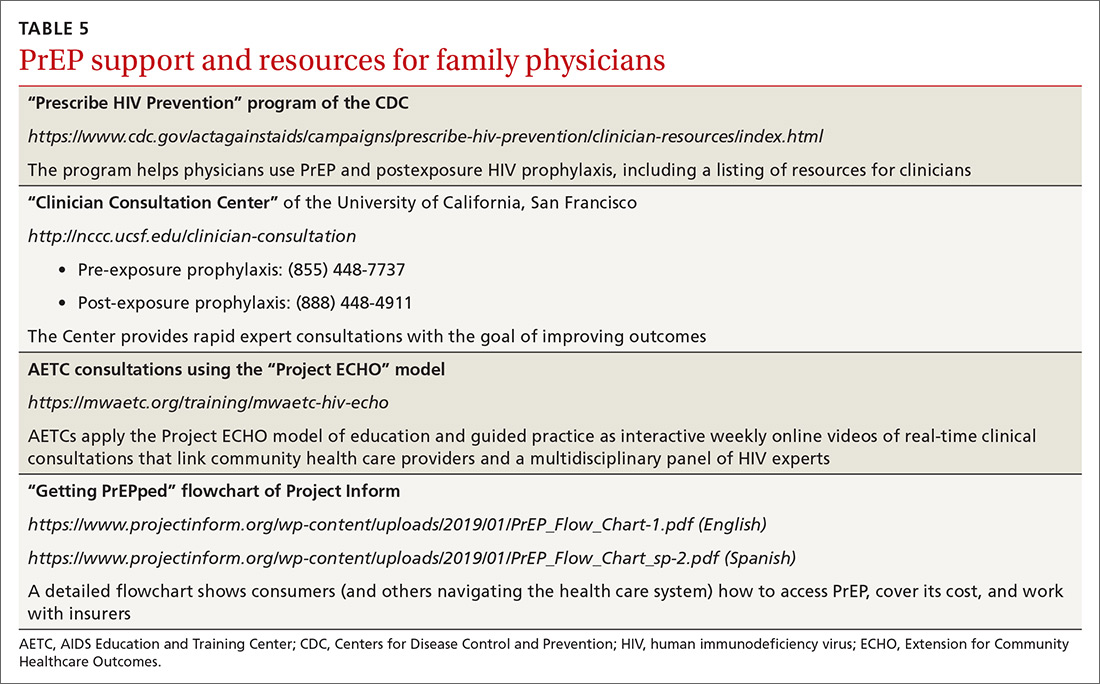
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.
Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.
Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
PRACTICE RECOMMENDATIONS
› Actively screen and identify HIV-negative patients who are a candidate for pre-exposure prophylaxis (PrEP); commit to talking to the most easily identifiable subsets of these patients, such as men who have sex with men and transgender patients. B
› Recognize that PrEP is indicated for patients who: are sexually active with inconsistent condom use and multiple recent sex partners; have recently been given a diagnosis of a sexually transmitted infection; or have a sexual or injection partner known to be HIV-infected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Allergy immunotherapy: Who, what, when … and how safe?
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.
Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
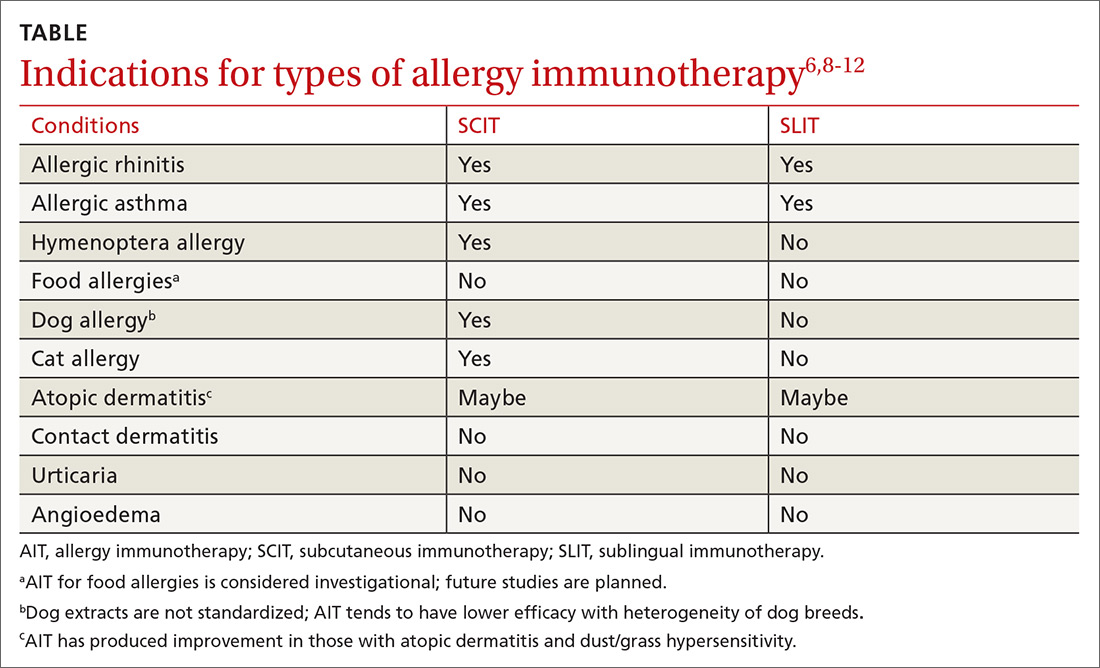
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
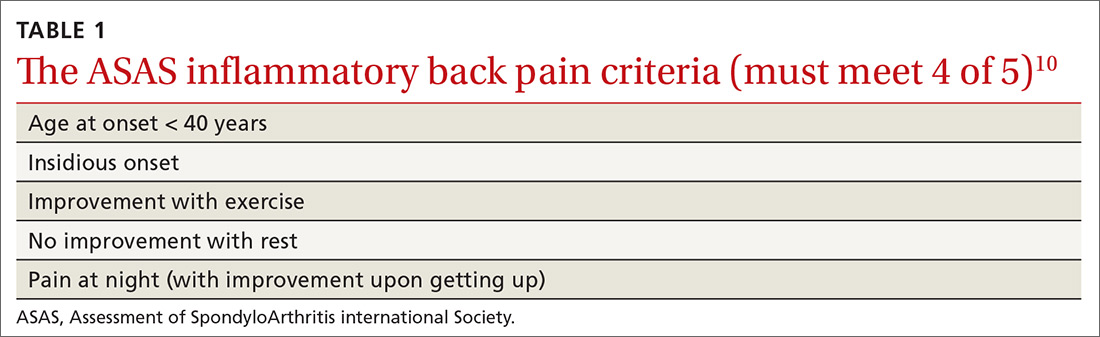
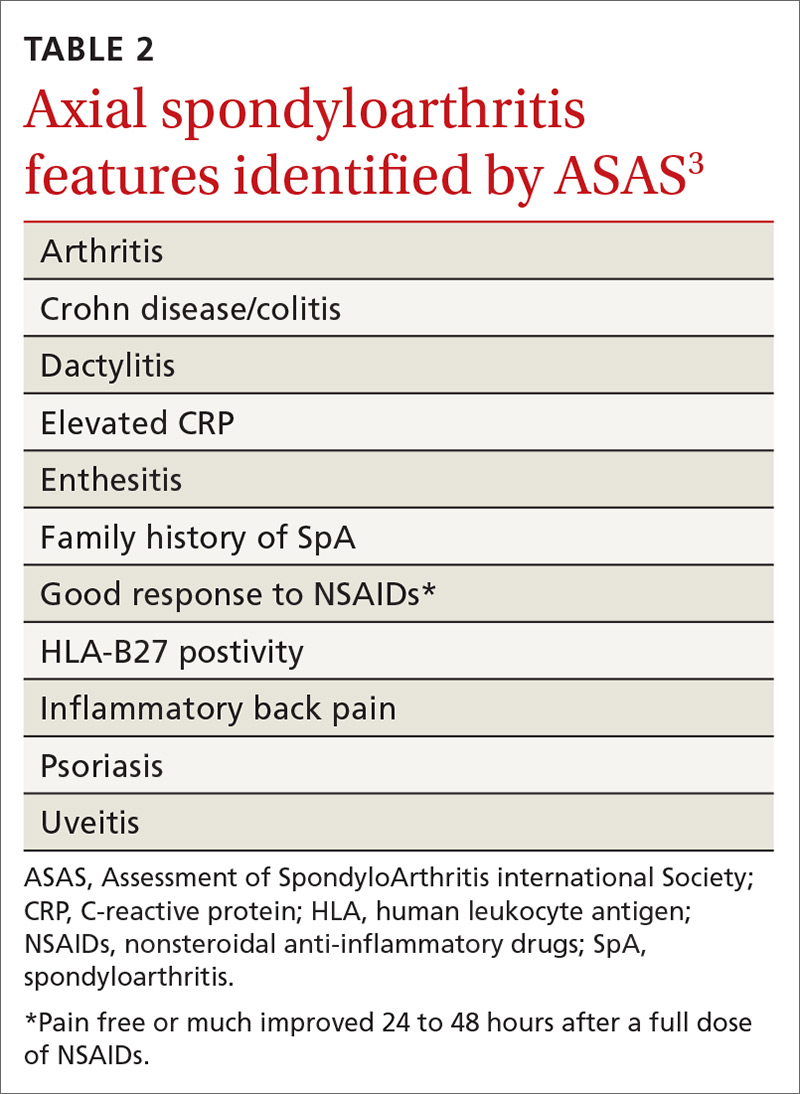
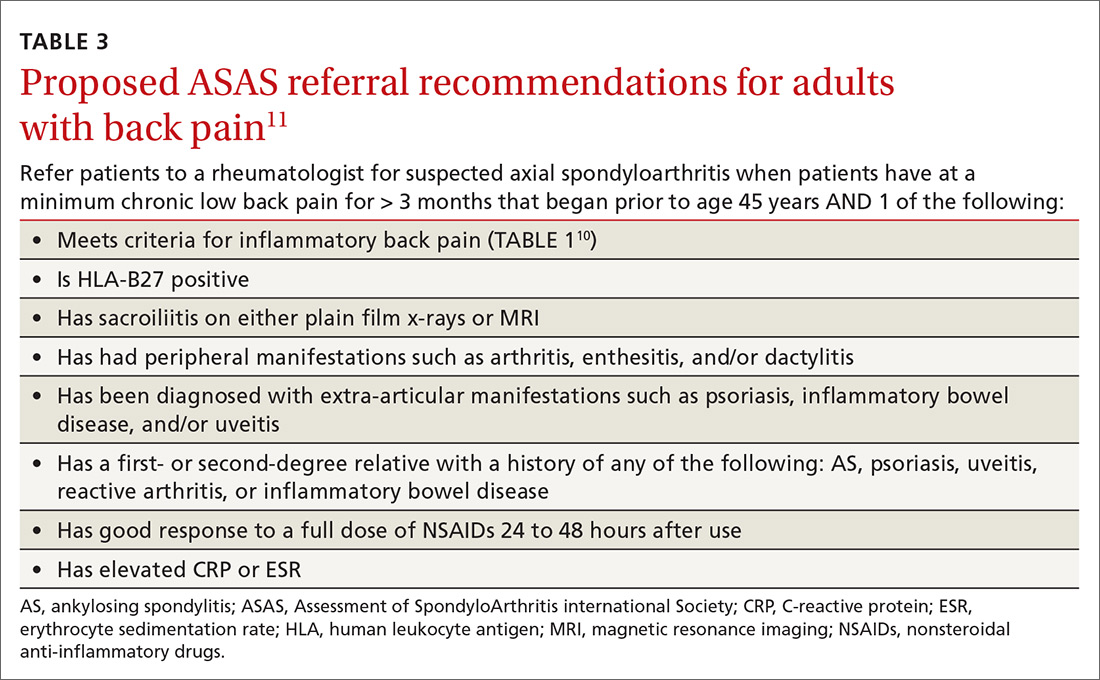
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.
Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.
Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
PRACTICE RECOMMENDATIONS
› Diagnose allergies that are amenable to allergy immunotherapy (AIT) using skin prick/puncture allergy testing in conjunction with clinical symptoms, triggers, and exposure. A
› Do not use AIT for urticaria, angioedema, drug hypersensitivity, or latex allergy. A
› Do not initiate AIT during pregnancy or in patients with acquired immune deficiency syndrome or severe asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Could that back pain be caused by ankylosing spondylitis?
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; [email protected]
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
PRACTICE RECOMMENDATIONS
› Evaluate all patients with back pain lasting > 3 months for inflammatory back pain features. C
› Treat all patients with confirmed or suspected axial spondyloarthritis with a trial of nonsteroidal anti-inflammatory drugs. A
› Recommend that all patients with back pain—including those with suspected axial spondyloarthritis—start an exercise program that includes both strength and aerobic activities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A practical guide to the care of ingrown toenails
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.
How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.
How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.
How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
Translating AHA/ACC cholesterol guidelines into meaningful risk reduction
A new cholesterol guideline1 builds on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol guidelines,2 which were a major paradigm shift in the evaluation and management of blood cholesterol levels and risk for atherosclerotic cardiovascular disease (ASCVD). The work was presented (and simultaneously published) on November 10, 2018, at the annual AHA Scientific Sessions in Chicago. Full text,1 an executive summary,3 and accompanying systematic review of evidence4 are available online.
The 2018 AHA/ACC cholesterol guideline represents a step forward in ASCVD prevention—especially in primary prevention, where it provides guidance for risk refinement and personalization. In this article, we mine the details of what has changed and what is new in this guideline so that you can prepare to adopt the recommendations in your practice.
2013 and 2018 guidelines: Similarities, differences
As in earlier iterations, the 2018 guideline emphasizes healthy lifestyle across the life-course as the basis of ASCVD prevention—as elaborated in the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.5 In contrast to the 2013 guidelines,2 the 2018 guideline is more comprehensive and more personalized, focusing on risk assessment for individual patients, rather than simply providing population-based approaches. Moreover, the guideline isn’t limited to adults: It makes recommendations pertaining to children and adolescents.1
TABLE 11,2 compares the most important differences between the 2013 and 2018 guidelines.
The 2013 ACC/AHA guidelines eliminated low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C)a goals of therapy and replaced them with the concept of 4 “statin benefit groups”—that is, patient populations for which clear evidence supports the role of statin therapy.4 In the 2018 guideline, statin benefit groups have been maintained, although without explicit use of this term.1
Primary prevention. Although no major changes in statin indications are made for patients with (1) established ASCVD (ie, for secondary prevention), (2) diabetes mellitus (DM) and who are 40 to 75 years of age, or (3) a primary LDL-C elevation ≥ 190 mg/dL, significant changes were made for primary prevention patients ages 40 to 75 years.1 ASCVD risk calculation using the 2013 pooled cohort equations (PCE) is still recommended4; however, risk estimation is refined by the use of specific so-called risk-enhancing factors (TABLE 21). In cases in which the risk decision remains uncertain, obtaining the coronary artery calcium (CAC) score (which we’ll describe shortly) using specialized computed tomography (CT) is advised to facilitate the shared physician–patient decision-making process.1
LDL-C and non-HDL-C thresholds. Although LDL-C and non-HDL-C goals are not overtly brought back from the 2002 National Cholesterol Education Program/Adult Treatment Panel guidelines,6 the new guideline does introduce LDL-C and non-HDL-C thresholds—levels at which adding nonstatin therapy can be considered, in contrast to previous goals to which therapy was titrated. Definitions of statin intensity remain the same: Moderate-intensity statin therapy is expected to reduce the LDL-C level by 30% to 50%; high-intensity statin therapy, by ≥ 50%.1 The intensity of statin therapy has been de-escalated in the intermediate-risk group, where previous guidelines advised high-intensity statin therapy,4 and replaced with moderate-intensity statin therapy (similar to 2016 US Preventive Services Task Force [USPSTF] recommendations7).
[polldaddy:10312157]
Continue to: Fasting vs nonfasting lipid profiles
Fasting vs nonfasting lipid profiles. In contrast to previous guidelines,2,8 which used fasting lipid profiles, nonfasting lipid profiles are now recommended for establishing a baseline LDL-C level and for ASCVD risk estimation for most patients—as long as the triglycerides (TG) level is < 400 mg/dL. When the calculated LDL-C level is < 70 mg/dL using the standard Friedewald formula, obtaining a direct LDL-C or a modified LDL-C estimate9 is deemed reasonable to improve accuracy. (The modified LDL-C can be estimated using The Johns Hopkins Hospital’s free “LDL Cholesterol Calculator” [www.hopkinsmedicine.org/apps/all-apps/ldl-cholesterol-calculator]).
A fasting lipid profile is still preferred for patients who have a family history of a lipid disorder. The definition of hypertriglyceridemia has been revised from a fasting TG level ≥ 150 mg/dL to a nonfasting or fasting TG level ≥ 175 mg/dL.1
Nonstatin add-on therapy. The new guideline supports the addition of nonstatin therapies to maximally tolerated statin therapy in patients who have established ASCVD or a primary LDL-C elevation ≥ 190 mg/dL when (1) the LDL-C level has not been reduced by the expected percentage (≥ 50% for high-intensity statin therapy) or (2) explicit LDL-C level thresholds have been met.1
The principal 2 groups of recommended nonstatins for which there is randomized, controlled trial evidence of cardiovascular benefit are (1) the cholesterol-absorbing agent ezetimibe10 and (2) the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab11 and alirocumab.12
AAFP’s guarded positions on the 2013 and 2018 guidelines
The American Academy of Family Physicians (AAFP) welcomed the patient-centered and outcome-oriented aspects of the 2013 ACC/AHA guidelines, endorsing them with 3 qualifications.13
- Many of the recommendations were based on expert opinion, not rigorous research results—in particular, not on the findings of randomized controlled trials (although key points are based on high-quality evidence).
- There were conflicts of interest disclosed for 15 members of the guidelines panel, including a vice chair.
- Validation of the PCE risk estimation tool was lacking.
Continue to: AAFP announced...
AAFP announced in March that it does not endorse the 2018 AHA/ACC guideline, asserting that (1) only a small portion of the recommendations, primarily focused on the addition of nonstatin therapy, were addressed by an independent systematic review and (2) many of the guideline recommendations are based on low-quality or insufficient evidence. AAFP nevertheless bestowed an “affirmation of value” designation on the guideline—meaning that it provides some benefit for family physicians’ practice without fulfilling all criteria for full endorsement.14
Detailed recommendations from the 2018 guideline
Lifestyle modification
When talking about ASCVD risk with patients, it is important to review current lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). Subsequent to that conversation, a healthy lifestyle should be endorsed and relevant advice provided. In addition, patient-directed materials (eg, ACC’s CardioSmart [www.cardiosmart.org]; AHA’s Life’s Simple 7 [www.heart.org/en/professional/workplace-health/lifes-simple-7]; and the National Lipid Association’s Patient Tear Sheets [www.lipid.org/practicetools/tools/tearsheets] and Clinicians’ Lifestyle Modification Toolbox [www.lipid.org/CLMT]) and referrals (eg, to cardiac rehabilitation, a dietitian, a smoking-cessation program) should be provided.1
Primary prevention of ASCVD
Risk assessment for primary prevention is now approached as a process, rather than the simple risk calculation used in the 2013 ACC/AHA guidelines.2 Assessment involves risk estimation followed by risk personalization, which, in some cases, is followed by risk reclassification using CAC scoring.1
Patients are classified into 1 of 4 risk groups, based on the PCE1:
- low (< 5%)
- borderline (5%-7.5%)
- intermediate (7.5%-19.9%)
- high (≥ 20%).
However, the PCE-based risk score is a population-based tool, which might not reflect the actual risk of individual patients. In some populations, PCE underestimates ASCVD risk; in others, it overestimates risk. A central tenet of the new guideline is personalization of risk, taking into account the unique circumstances of each patient. Moreover, the new guideline provides guidance on how to interpret the PCE risk score for several different ethnic and racial groups.1
Continue to: Medical therapy
Medical therapy. The decision to start lipid-lowering therapy should be made after a physician–patient discussion that considers costs of therapy as well as patient preferences and values in the context of shared decision-making. Discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, and the LDL-C level), the PCE risk score, the presence of risk-enhancing factors (TABLE 21), potential benefits of lifestyle changes and statin therapy, and the potential for adverse drug effects and drug–drug interactions.1
If the estimated ASCVD risk is 7.5%-19.9%, starting moderate-intensity statin therapy is recommended. Risk-enhancing factors favor initiation of statin therapy, even in patients at borderline risk (5%-7.5%). If risk is uncertain, the CAC score can be used to facilitate shared decision-making.1 The use of CAC is in agreement with the USPSTF statement that CAC can moderately improve discrimination and reclassification, but has an unclear effect on downstream health care utilization.15 Importantly, CAC should not be measured routinely in patients already taking a statin because its primary role is to facilitate shared decision-making regarding initiation of statin therapy.16
If the 10-year ASCVD risk is ≥ 20%, high-intensity statin therapy is advised, without need to obtain the CAC score. If high-intensity statin therapy is advisable but not acceptable to, or tolerated by, the patient, it might be reasonable to add a nonstatin drug (ezetimibe or a bile-acid sequestrant) to moderate-intensity statin therapy.1
Risk-enhancing factors (TABLE 21) apply to intermediate- and borderline-risk patients. Importantly, these factors include membership in specific ethnic groups, conditions specific to females, and male–female distinctions in risk. Risk-enhancing factors also incorporate biomarkers that are often measured by lipid specialists, such as lipoprotein(a) (Lp[a]) and apolipoprotein B (ApoB).1
Lp(a) is an atherogenic particle, akin to an LDL particle, that consists of a molecule of apolipoprotein (a) (a nonfunctional mimic of a portion of plasminogen) covalently bound to ApoB, like the one found on the LDL particle. Lp(a) is proportionally associated with an increased risk for ASCVD and aortic stenosis at a level > 50 mg/dL.17 A family history of premature ASCVD is a relative indication for measuring Lp(a).1
Continue to: When and why to measure CAC
When and why to measure CAC
If the decision to initiate statin therapy is still uncertain after risk estimation and personalization, or when a patient is undecided about committing to lifelong lipid-lowering therapy, the new guideline recommends obtaining a CAC score to inform the shared decision-making process.1,18 Measurement of CAC is obtained by noncontrast, electrocardiographic-gated CT that can be performed in 10 to 15 minutes, requiring approximately 1 millisievert of radiation (equivalent of the approximate dose absorbed during 2 mammograms). Although measurement of the CAC score is generally not covered by insurance, its cost ($50-$450) nationwide makes it accessible.19
CAC measures the presence (or absence) of subclinical atherosclerosis by detecting calcified plaque in coronary arteries. The absolute CAC score is expressed in Agatston units; an age–gender population percentile is also provided. Keep in mind that the presence of any CAC (ie, a score > 0) is abnormal and demonstrates the presence of subclinical coronary artery disease. The prevalence of CAC > 0 increases with age, but a significant percentage of older people have a CAC score = 0. When CAC > 0, additional information is provided by the distribution of plaque burden among the different coronary arteries.20
Among intermediate-risk patients, 50% have CAC = 0 and, therefore, a very low event rate over the ensuing 10 years, which allows statin therapy to be safely deferred unless certain risk factors are present (eg, family history, smoking, DM).1,18 It is reasonable to repeat CAC testing in 5 to 10 years to assess whether subclinical atherosclerosis has developed. The 2018 guideline emphasizes that, when the CAC score is > 0 but < 100 Agatston units, statin therapy is favored, especially in patients > 55 years of age; when the CAC score is ≥ 100 Agatston units or at the ≥ 75th percentile, statin therapy is indicated regardless of age.1
Patients who might benefit from knowing their CAC score include those who are:
- reluctant to initiate statin therapy but who want to understand their risk and potential for benefit more precisely
- concerned about the need to reinstitute statin therapy after discontinuing it because of statin-associated adverse effects
- older (men, 55-80 years; women, 60-80 years) who have a low burden of risk factors and who question whether they would benefit from statin therapy
- middle-aged (40-55 years) and who have a PCE-calculated risk of 5% to < 7.5% for ASCVD and factors that increase their risk for ASCVD, even though they are in a borderline-risk group.1
Primary prevention in special populations
Older patients. In adults ≥ 75 years who have an LDL-C level 70 to 189 mg/dL, initiating a moderate-intensity statin might be reasonable; however, it might also be reasonable to stop treatment in this population when physical or cognitive decline, multiple morbidities, frailty, or reduced life expectancy limits the potential benefit of statin therapy. It might be reasonable to use the CAC score in adults 76 to 80 years of age who have an LDL-C level of 70 to 189 mg/dL to reclassify those whose CAC score = 0, so that they can avoid statin therapy.1
Continue to: Children and adolescents
Children and adolescents. In alignment with current pediatric guidelines,21 but in contrast to USPSTF reccomendations,22 the 2018 ACC/AHA guideline endorses universal lipid screening for pediatric patients (see TABLE W11,21,22). It is reasonable to obtain a fasting lipid profile or nonfasting non-HDL-C in all children and adolescents who have neither cardiovascular risk factors nor a family history of early cardiovascular disease to detect moderate-to-severe lipid abnormalities. Screening should be done once at 9 to 11 years of age and again at 17 to 21 years.1
A screening test as early as 2 years of age to detect familial hypercholesterolemia (FH) is reasonable when a family history of either early CVD or significant hypercholesterolemia is present. The guideline endorses reverse cascade screening for detection of FH in family members of children and adolescents who have severe hypercholesterolemia.1
In children and adolescents with a lipid abnormality, especially when associated with the metabolic syndrome, lifestyle counseling is beneficial for lowering the LDL-C level. In children and adolescents ≥ 10 years of age with (1) an LDL-C level persistently ≥ 190 mg/dL or (2) an LDL level ≥ 160 mg/dL plus a clinical presentation consistent with FH, it is reasonable to initiate statin therapy if they do not respond adequately to 3 to 6 months of lifestyle therapy.1
Ethnicity as a risk-modifying factor. The PCE distinguishes between US adults of European ancestry and African ancestry, but no other ethnic groups are distinguished.4 The new guideline advocates for the use of PCE in other populations; however, it states that, for clinical decision-making purposes, it is reasonable, in adults of different races and ethnicities, for the physician to review racial and ethnic features that can influence ASCVD risk to allow adjustment of the choice of statin or intensity of treatment. Specifically, South Asian ancestry is now treated as a risk-enhancing factor, given the high prevalence of premature and extensive ASCVD in this patient population.1
Concerns specific to women. Considering conditions specific to women as potential risk-enhancing factors is advised when discussing lifestyle intervention and the potential for benefit from statin therapy—in particular, (1) in the setting of premature menopause (< 40 years) and (2) when there is a history of a pregnancy-associated disorder (eg, hypertension, preeclampsia, gestational DM, a small-for-gestational-age infant, and preterm delivery). If the decision is made to initiate statin therapy in women of childbearing age who are sexually active, there is a guideline mandate to counsel patients on using reliable contraception. When pregnancy is planned, statin therapy should be discontinued 1 to 2 months before pregnancy is attempted; when pregnancy occurs while a patient is taking a statin, therapy should be stopped as soon as the pregnancy is discovered.1
Continue to: Adults with chronic kidney disease
Adults with chronic kidney disease. Chronic kidney disease that is not treated with dialysis or kidney transplantation is considered a risk-enhancing factor; initiation of a moderate-intensity statin or a moderate-intensity statin plus ezetimibe can be useful in patients with chronic kidney disease who are 40 to 75 years of age and have an LDL-C level of 70 to 189 mg/dL and a PCE-calculated risk ≥ 7.5%. In adults with advanced kidney disease that requires dialysis who are already taking a statin, it may be reasonable to continue the statin; however, initiation of a statin in adults with advanced kidney disease who require dialysis is not recommended because of an apparent lack of benefit.1
Adults with a chronic inflammatory disorder or human immunodeficiency virus infection. Any of these conditions are treated as risk-enhancing factors; in a risk discussion with affected patients, therefore, moderate-intensity statin therapy or high-intensity statin therapy is favored for those 40 to 75 years of age who have an LDL-C level of 70 to 189 mg/dL and PCE-calculated risk ≥ 7.5%. A fasting lipid profile and assessment of ASCVD risk factors for these patients can be useful (1) as a guide to the potential benefit of statin therapy and (2) for monitoring or adjusting lipid-lowering drug therapy before, and 4 to 12 weeks after, starting inflammatory disease-modifying therapy or antiretroviral therapy.
In adults with rheumatoid arthritis who undergo ASCVD risk assessment with a lipid profile, it can be useful to recheck lipid values and other major ASCVD risk factors 2 to 4 months after the inflammatory disease has been controlled.1
Primary hypercholesterolemia
The diagnosis and management of heterozygous or homozygous familial hypercholesterolemia (HeFH or HoFH) is beyond the scope of the 2018 ACC/AHA cholesterol guidelines; instead, the 2015 AHA Scientific Statement, “The Agenda for Familial Hypercholesterolemia,” provides a contemporary review of these topics.23 However, the 2018 cholesterol guideline does acknowledge that an LDL-C level ≥ 190 mg/dL often corresponds to primary (ie, genetic) hypercholesterolemia.
In patients 20 to 75 years of age who have a primary elevation of LDL-C level ≥ 190 mg/dL, the guideline recommends initiation of high-intensity statin therapy without calculating ASCVD risk using the PCE. If a > 50% LDL-C reduction is not achieved, or if the LDL-C level on maximally tolerated statin therapy remains ≥ 100 mg/dL, adding ezetimibe is considered reasonable. If there is < 50% reduction in the LDL-C level while taking maximally tolerated statin and ezetimibe therapy, adding a bile-acid sequestrant can be considered, as long as the TG level is not > 300 mg/dL (ie, bile-acid sequestrants can elevate the TG level significantly).
Continue to: In patients 30 to 75 years of age...
In patients 30 to 75 years of age who have a diagnosis of HeFH and an LDL-C level ≥ 100 mg/dL while taking maximally tolerated statin and ezetimibe therapy, the addition of a PCSK9 inhibitor can be considered. Regardless of whether there is a diagnosis of HeFH, addition of a PCSK9 inhibitor can be considered in patients 40 to 75 years of age who have a baseline LDL-C level ≥ 220 mg/dL and who achieve an on-treatment LDL-C level ≥ 130 mg/dL while receiving maximally tolerated statin therapy and ezetimibe.1
Diabetes mellitus
In patients with DM who are 40 to 75 years of age, moderate-intensity statin therapy is recommended without calculating the 10-year ASCVD risk. When the LDL-C level is 70 to 189 mg/dL, however, it is reasonable to use the PCE to assess 10-year ASCVD risk to facilitate risk stratification.
In patients with DM who are at higher risk, especially those who have multiple risk factors or are 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥ 50 %. In adults > 75 years of age with DM who are already on statin therapy, it is reasonable to continue statin therapy; for those that age who are not on statin therapy, it might be reasonable to initiate statin therapy after a physician–patient discussion of potential benefits and risks.
In adults with DM and PCE-calculated risk ≥ 20%, it might be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce the LDL-C level by ≥ 50%. In adults 20 to 39 years of age with DM of long duration (≥ 10 years of type 2 DM, ≥ 20 years of type 1 DM), albuminuria (≥ 30 μg of albumin/mg creatinine), estimated glomerular filtration rate < 60 mL/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index < 0.9, it might be reasonable to initiate statin therapy.1
Secondary prevention
Presence of clinical ASCVD. In patients with clinical ASCVD who are ≤ 75 years of age, high-intensity statin therapy should be initiated or continued, with the aim of achieving ≥ 50% reduction in the LDL-C level. When high-intensity statin therapy is contraindicated or if a patient experiences statin-associated adverse effects, moderate-intensity statin therapy should be initiated or continued with the aim of achieving a 30% to 49% reduction in the LDL-C level.
Continue to: In patients...
In patients > 75 years of age with clinical ASCVD, it is reasonable to initiate or continue moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preference.1
Very high risk. In patients at very high risk (this includes a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions), maximally tolerated LDL-C-lowering therapy should include maximally tolerated statin therapy and ezetimibe before considering a PCSK9 inhibitor. An LDL-C level ≥ 70 mg/dL or a non-HDL-C level ≥ 100 mg/dL is considered a reasonable threshold for adding a PCSK9 inhibitor to background lipid-lowering therapy1 (TABLE 31).
Heart failure. In patients with heart failure who have (1) a reduced ejection fraction attributable to ischemic heart disease, (2) a reasonable life expectancy (3-5 years), and (3) are not already on a statin because of ASCVD, consider initiating moderate-intensity statin therapy to reduce the risk for an ASCVD event.1
Reduction of elevated triglycerides
The guideline defines moderate hypertriglyceridemia as a nonfasting or fasting TG level of 175 to 499 mg/dL. Such a finding is considered a risk-enhancing factor and is 1 of 5 components of the metabolic syndrome. Three independent measurements are advised to diagnose primary moderate hypertriglyceridemia. Severe hypertriglyceridemia is diagnosed when the fasting TG level is ≥ 500 mg/dL.1
In moderate hypertriglyceridemia, most TGs are carried in very-low-density lipoprotein particles; in severe hypertriglyceridemia, on the other hand, chylomicrons predominate, raising the risk for pancreatitis. In adults with severe hypertriglyceridemia, therefore—especially when the fasting TG level is ≥ 1000 mg/dL—it is reasonable to identify and address other causes of hypertriglyceridemia. If TGs are persistently elevated or increasing, levels should be reduced to prevent acute pancreatitis with a very low-fat diet and by avoiding refined carbohydrates and alcohol; consuming omega-3 fatty acids; and, if necessary, taking a fibrate.1
Continue to: In adults...
In adults ≥ 20 years of age with moderate hypertriglyceridemia, lifestyle factors (eg, obesity, metabolic syndrome), secondary factors (eg, DM, chronic liver or kidney disease, nephrotic syndrome, hypothyroidism), and medications that increase the TG level need to be addressed first. In adults 40 to 75 years of age with moderate or severe hypertriglyceridemia and a PCE-calculated ASCVD risk ≥ 7.5%, it is reasonable to reevaluate risk after lifestyle and secondary factors are addressed and to consider a persistently elevated TG level as a factor favoring initiation or intensification of statin therapy. In adults 40 to 75 years of age with severe hypertriglyceridemia and ASCVD risk ≥ 7.5%, it is reasonable to address reversible causes of a high TG level and to initiate statin therapy.1
Other considerations in cholesterol management
Tools to assess adherence
The response to lifestyle and statin therapy should be evaluated by the percentage reduction in the LDL-C level compared with baseline, not by assessment of the absolute LDL-C level. When seeing a patient whose treatment is ongoing, a baseline level can be estimated using a desktop LDL-calculator app.
Adherence and percentage response to LDL-C–lowering medications and lifestyle changes should be evaluated with repeat lipid measurement 4 to 12 weeks after either a statin is initiated or the dosage is adjusted, and repeated every 3 to 12 months as needed. In patients with established ASCVD who are at very high risk, triggers for adding nonstatin therapy are defined by a threshold LDL-C level ≥ 70 mg/dL on maximal statin therapy.1
Interventions focused on improving adherence to prescribed therapy are recommended for management of adults with an elevated cholesterol level. These interventions include telephone reminders, calendar reminders, integrated multidisciplinary educational activities, and pharmacist-led interventions, such as simplification of the medication regimen to once-daily dosing.1
Statin safety and associated adverse effects
A physician–patient risk discussion is recommended before initiating statin therapy to review net clinical benefit, during which the 2 parties weigh the potential for ASCVD risk reduction against the potential for statin-associated adverse effects, statin–drug interactions, and safety, with the physician emphasizing that adverse effects can be addressed successfully.
Continue to: Statins are one of...
Statins are one of the safest classes of medication, with an excellent risk-benefit ratio. However, there are myriad confusing media reports regarding potential adverse effects and safety of the statin class—reports that often lead patients to discontinue or refuse statins.
Statin-associated adverse effects include the common statin-associated muscle symptoms (SAMS), new-onset DM, cognitive effects, and hepatic injury. The frequency of new-onset DM depends on the population exposed to statins, with a higher incidence of new-onset DM found in patients who are already predisposed, such as those with obesity, prediabetes, and metabolic syndrome. Cognitive effects are rare and difficult to interpret; they were not reported in the large statin mega-trials but have been described in case reports. Significant transaminase elevations > 3 times the upper limit of normal are infrequent; hepatic failure with statins is extremely rare and found at the same incidence in the general population.1
SAMS include (in order of decreasing prevalence)24:
- myalgias with a normal creatine kinase (CK) level
- conditions such as myositis or myopathy (elevated CK level)
- rhabdomyolysis (CK level > 10 times the upper limit of normal, plus renal injury)
- extremely rare statin-associated autoimmune myopathy, with detectable 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase antibodies.
In patients with SAMS, thorough assessment of symptoms is recommended, in addition to evaluation for nonstatin causes and predisposing factors. Identification of potential SAMS-predisposing factors is recommended before initiation of treatment, including demographics (eg, East-Asian ancestry), comorbid conditions (eg, hypothyroidism and vitamin D deficiency), and use of medications adversely affecting statin metabolism (eg, cyclosporine).
In patients with statin-associated adverse effects that are not severe, it is recommended to reassess and rechallenge to achieve a maximal lowering of the LDL-C level by a modified dosing regimen or an alternate statin or by combining a statin with nonstatin therapy. In patients with increased risk for DM or new-onset DM, it is recommended to continue statin therapy.
Continue to: Routine CK and liver function testing...
Routine CK and liver function testing is not useful in patients treated with statins; however, it is recommended that CK be measured in patients with severe SAMS or objective muscle weakness, or both, and to measure liver function if symptoms suggest hepatotoxicity. In patients at increased risk for ASCVD who have chronic, stable liver disease (including non-alcoholic fatty liver disease), it is reasonable, when appropriately indicated, to use statins after obtaining baseline measurements and determining a schedule of monitoring and safety checks.
In patients at increased risk for ASCVD who have severe or recurrent SAMS after appropriate statin rechallenge, it is reasonable to use nonstatin therapy that is likely to provide net clinical benefit. The guideline does not recommend routine use of coenzyme Q10 supplementation for the treatment or prevention of SAMS.1
Guideline criticism
Guideline development is challenging on multiple levels, including balancing perspectives from multiple stakeholders. Nevertheless, the 2018 AHA/ACC cholesterol guideline builds nicely on progress made since its 2013 predecessor was released.4 This document was developed with the participation of representatives from 10 professional societies in addition to the ACC and AHA—notably, the National Lipid Association and American Society for Preventive Cardiology.1
To refine risk estimation and facilitate shared decision-making, the new guideline introduced so-called risk-enhancing factors and use of the CAC.1 However, some potential risk-enhancing factors were left out: erectile dysfunction, for example, often a marker of increased cardiovascular risk in men < 50 years of age.25 In addition, although pretreatment ApoB was introduced as a risk-enhancing factor,1 no recommendation is given to measure ApoB after initiation of therapy for evaluation of residual cardiovascular risk, as endorsed in other guidelines.26,27
Moreover, the guideline does not include the “extreme risk” category in the guideline developed by the American Association of Clinical Endocrinologists (AACE).28 Although the 2018 AHA/ACC guideline introduces < 70 mg/dL and < 100 mg/dL LDL-C thresholds,1 the < 55 mg/dL LDL-C threshold used for patients in the AACE/American College of Endocrinology extreme-risk category is not mentioned.26 This omission might leave patients who are at extreme ASCVD risk without optimal lipid-lowering therapy. Similarly, the guideline does not elaborate on the diagnosis and treatment of HoFH and HeFH.1 The age cutoff of 30 years for the recommendation to consider PCSK9 inhibitors in patients with HeFH appears arbitrary and excludes younger FH patients who have an extreme LDL-C elevation from potentially important therapy.23
Continue to: Guidelines are dynamic instruments...
Guidelines are dynamic instruments that require constant updating, given the production of new evidence. In fact, the results of the Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) were presented at the same meeting at which this guideline was unveiled.29 REDUCE-IT demonstrated an astonishing highly significant 25% reduction in the composite primary major adverse cardiovascular event outcome in patients with an LDL-C level of 44 to 100 mg/dL on statin therapy, who had a TG level of 135 to 499 mg/dL and had been treated for a median of 4.9 years with 4 g of pure eicosapentaenoic acid.
In addition, the guideline’s value statements, which address the need to consider the cost of drugs in determining most appropriate treatment, are no longer accurate because the price of PCSK9 inhibitors has dropped by more than half since the guideline was issued.30
An upward climb to clinical payoff
Even after close study of the 2018 AHA/ACC cholesterol guideline, implementing it in practice might remain a challenge to clinicians who are inexperienced in ordering lipid markers such as Lp(a) and interpreting the CAC score. Moreover, initiating and monitoring nonstatin therapies will be a demanding task—especially with PCSK9 inhibitors, which present access difficulties because they are relatively expensive (even after the recent price cut). That’s why, when there is doubt in the mind of the physician or other provider, we will likely see more referrals to specialists in lipid management and ASCVD risk estimation to optimize preventive therapy.31
CORRESPONDENCE
Cezary Wójcik, MD, PhD, FNLA, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; [email protected]
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. pii: S0735-1097(18)39034-X. [Epub ahead of print]
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-S45.
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39033-8. [Epub ahead of print]
4. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39035-1. [Epub ahead of print]
5. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
6. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III): final report. Circulation. 2002;106:3143-3421.
7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997-2007.
8. National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel II). Circulation. 1994;89:1333-1445.
9. Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749-753.
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
12. Szarek M, White HD, Schwartz GG, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387-396.
13. Crawford C. AAFP endorses ACC/AHA cholesterol management guidelines with qualifications. Leawood, KS: American Academy of Family Physicians; 2014 June 18. www.aafp.org/news/health-of-the-public/20140618cholesterolgdlnendorse.html. Accessed March 20, 2019.
14. Crawford C. AAFP News. AAFP affirms value of new cholesterol management guideline. March 20, 2019. www.aafp.org/news/health-of-the-public/20190320acc-ahacholguidln.html?cmpid=em_AP_20190320. Accessed April 1, 2019.
15. Lin JS, Evans CV, Johnson E, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Evidence Synthesis, No. 166. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 Jul. Report No.: 17-05225-EF-1.
16. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273-1282.
17. Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553-1560.
18. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92:1831-1841.
19. MDsave. Cardiac CT calcium scoring. www.mdsave.com/procedures/cardiac-ct-calcium-scoring/d785f4cf. Accessed Aprl 1, 2019.
20. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407-1416.
21. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213-S256.
22. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633.
23. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167-2192.
24. Newman CB, Preiss D, Tobert JA, et al; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38-e81.
25. Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sex Med Rev. 2018 Jan 27. pii: S2050-0521(18)30009-X. [Epub ahead of print]
26. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
27. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282.
28. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
29. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
30. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC Managed Markets Network. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed April 12, 2019.
31. Kaufman TM, Duell PB, Purnell JQ, et al. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499-501.
A new cholesterol guideline1 builds on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol guidelines,2 which were a major paradigm shift in the evaluation and management of blood cholesterol levels and risk for atherosclerotic cardiovascular disease (ASCVD). The work was presented (and simultaneously published) on November 10, 2018, at the annual AHA Scientific Sessions in Chicago. Full text,1 an executive summary,3 and accompanying systematic review of evidence4 are available online.
The 2018 AHA/ACC cholesterol guideline represents a step forward in ASCVD prevention—especially in primary prevention, where it provides guidance for risk refinement and personalization. In this article, we mine the details of what has changed and what is new in this guideline so that you can prepare to adopt the recommendations in your practice.
2013 and 2018 guidelines: Similarities, differences
As in earlier iterations, the 2018 guideline emphasizes healthy lifestyle across the life-course as the basis of ASCVD prevention—as elaborated in the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.5 In contrast to the 2013 guidelines,2 the 2018 guideline is more comprehensive and more personalized, focusing on risk assessment for individual patients, rather than simply providing population-based approaches. Moreover, the guideline isn’t limited to adults: It makes recommendations pertaining to children and adolescents.1
TABLE 11,2 compares the most important differences between the 2013 and 2018 guidelines.
The 2013 ACC/AHA guidelines eliminated low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C)a goals of therapy and replaced them with the concept of 4 “statin benefit groups”—that is, patient populations for which clear evidence supports the role of statin therapy.4 In the 2018 guideline, statin benefit groups have been maintained, although without explicit use of this term.1
Primary prevention. Although no major changes in statin indications are made for patients with (1) established ASCVD (ie, for secondary prevention), (2) diabetes mellitus (DM) and who are 40 to 75 years of age, or (3) a primary LDL-C elevation ≥ 190 mg/dL, significant changes were made for primary prevention patients ages 40 to 75 years.1 ASCVD risk calculation using the 2013 pooled cohort equations (PCE) is still recommended4; however, risk estimation is refined by the use of specific so-called risk-enhancing factors (TABLE 21). In cases in which the risk decision remains uncertain, obtaining the coronary artery calcium (CAC) score (which we’ll describe shortly) using specialized computed tomography (CT) is advised to facilitate the shared physician–patient decision-making process.1
LDL-C and non-HDL-C thresholds. Although LDL-C and non-HDL-C goals are not overtly brought back from the 2002 National Cholesterol Education Program/Adult Treatment Panel guidelines,6 the new guideline does introduce LDL-C and non-HDL-C thresholds—levels at which adding nonstatin therapy can be considered, in contrast to previous goals to which therapy was titrated. Definitions of statin intensity remain the same: Moderate-intensity statin therapy is expected to reduce the LDL-C level by 30% to 50%; high-intensity statin therapy, by ≥ 50%.1 The intensity of statin therapy has been de-escalated in the intermediate-risk group, where previous guidelines advised high-intensity statin therapy,4 and replaced with moderate-intensity statin therapy (similar to 2016 US Preventive Services Task Force [USPSTF] recommendations7).
[polldaddy:10312157]
Continue to: Fasting vs nonfasting lipid profiles
Fasting vs nonfasting lipid profiles. In contrast to previous guidelines,2,8 which used fasting lipid profiles, nonfasting lipid profiles are now recommended for establishing a baseline LDL-C level and for ASCVD risk estimation for most patients—as long as the triglycerides (TG) level is < 400 mg/dL. When the calculated LDL-C level is < 70 mg/dL using the standard Friedewald formula, obtaining a direct LDL-C or a modified LDL-C estimate9 is deemed reasonable to improve accuracy. (The modified LDL-C can be estimated using The Johns Hopkins Hospital’s free “LDL Cholesterol Calculator” [www.hopkinsmedicine.org/apps/all-apps/ldl-cholesterol-calculator]).
A fasting lipid profile is still preferred for patients who have a family history of a lipid disorder. The definition of hypertriglyceridemia has been revised from a fasting TG level ≥ 150 mg/dL to a nonfasting or fasting TG level ≥ 175 mg/dL.1
Nonstatin add-on therapy. The new guideline supports the addition of nonstatin therapies to maximally tolerated statin therapy in patients who have established ASCVD or a primary LDL-C elevation ≥ 190 mg/dL when (1) the LDL-C level has not been reduced by the expected percentage (≥ 50% for high-intensity statin therapy) or (2) explicit LDL-C level thresholds have been met.1
The principal 2 groups of recommended nonstatins for which there is randomized, controlled trial evidence of cardiovascular benefit are (1) the cholesterol-absorbing agent ezetimibe10 and (2) the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab11 and alirocumab.12
AAFP’s guarded positions on the 2013 and 2018 guidelines
The American Academy of Family Physicians (AAFP) welcomed the patient-centered and outcome-oriented aspects of the 2013 ACC/AHA guidelines, endorsing them with 3 qualifications.13
- Many of the recommendations were based on expert opinion, not rigorous research results—in particular, not on the findings of randomized controlled trials (although key points are based on high-quality evidence).
- There were conflicts of interest disclosed for 15 members of the guidelines panel, including a vice chair.
- Validation of the PCE risk estimation tool was lacking.
Continue to: AAFP announced...
AAFP announced in March that it does not endorse the 2018 AHA/ACC guideline, asserting that (1) only a small portion of the recommendations, primarily focused on the addition of nonstatin therapy, were addressed by an independent systematic review and (2) many of the guideline recommendations are based on low-quality or insufficient evidence. AAFP nevertheless bestowed an “affirmation of value” designation on the guideline—meaning that it provides some benefit for family physicians’ practice without fulfilling all criteria for full endorsement.14
Detailed recommendations from the 2018 guideline
Lifestyle modification
When talking about ASCVD risk with patients, it is important to review current lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). Subsequent to that conversation, a healthy lifestyle should be endorsed and relevant advice provided. In addition, patient-directed materials (eg, ACC’s CardioSmart [www.cardiosmart.org]; AHA’s Life’s Simple 7 [www.heart.org/en/professional/workplace-health/lifes-simple-7]; and the National Lipid Association’s Patient Tear Sheets [www.lipid.org/practicetools/tools/tearsheets] and Clinicians’ Lifestyle Modification Toolbox [www.lipid.org/CLMT]) and referrals (eg, to cardiac rehabilitation, a dietitian, a smoking-cessation program) should be provided.1
Primary prevention of ASCVD
Risk assessment for primary prevention is now approached as a process, rather than the simple risk calculation used in the 2013 ACC/AHA guidelines.2 Assessment involves risk estimation followed by risk personalization, which, in some cases, is followed by risk reclassification using CAC scoring.1
Patients are classified into 1 of 4 risk groups, based on the PCE1:
- low (< 5%)
- borderline (5%-7.5%)
- intermediate (7.5%-19.9%)
- high (≥ 20%).
However, the PCE-based risk score is a population-based tool, which might not reflect the actual risk of individual patients. In some populations, PCE underestimates ASCVD risk; in others, it overestimates risk. A central tenet of the new guideline is personalization of risk, taking into account the unique circumstances of each patient. Moreover, the new guideline provides guidance on how to interpret the PCE risk score for several different ethnic and racial groups.1
Continue to: Medical therapy
Medical therapy. The decision to start lipid-lowering therapy should be made after a physician–patient discussion that considers costs of therapy as well as patient preferences and values in the context of shared decision-making. Discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, and the LDL-C level), the PCE risk score, the presence of risk-enhancing factors (TABLE 21), potential benefits of lifestyle changes and statin therapy, and the potential for adverse drug effects and drug–drug interactions.1
If the estimated ASCVD risk is 7.5%-19.9%, starting moderate-intensity statin therapy is recommended. Risk-enhancing factors favor initiation of statin therapy, even in patients at borderline risk (5%-7.5%). If risk is uncertain, the CAC score can be used to facilitate shared decision-making.1 The use of CAC is in agreement with the USPSTF statement that CAC can moderately improve discrimination and reclassification, but has an unclear effect on downstream health care utilization.15 Importantly, CAC should not be measured routinely in patients already taking a statin because its primary role is to facilitate shared decision-making regarding initiation of statin therapy.16
If the 10-year ASCVD risk is ≥ 20%, high-intensity statin therapy is advised, without need to obtain the CAC score. If high-intensity statin therapy is advisable but not acceptable to, or tolerated by, the patient, it might be reasonable to add a nonstatin drug (ezetimibe or a bile-acid sequestrant) to moderate-intensity statin therapy.1
Risk-enhancing factors (TABLE 21) apply to intermediate- and borderline-risk patients. Importantly, these factors include membership in specific ethnic groups, conditions specific to females, and male–female distinctions in risk. Risk-enhancing factors also incorporate biomarkers that are often measured by lipid specialists, such as lipoprotein(a) (Lp[a]) and apolipoprotein B (ApoB).1
Lp(a) is an atherogenic particle, akin to an LDL particle, that consists of a molecule of apolipoprotein (a) (a nonfunctional mimic of a portion of plasminogen) covalently bound to ApoB, like the one found on the LDL particle. Lp(a) is proportionally associated with an increased risk for ASCVD and aortic stenosis at a level > 50 mg/dL.17 A family history of premature ASCVD is a relative indication for measuring Lp(a).1
Continue to: When and why to measure CAC
When and why to measure CAC
If the decision to initiate statin therapy is still uncertain after risk estimation and personalization, or when a patient is undecided about committing to lifelong lipid-lowering therapy, the new guideline recommends obtaining a CAC score to inform the shared decision-making process.1,18 Measurement of CAC is obtained by noncontrast, electrocardiographic-gated CT that can be performed in 10 to 15 minutes, requiring approximately 1 millisievert of radiation (equivalent of the approximate dose absorbed during 2 mammograms). Although measurement of the CAC score is generally not covered by insurance, its cost ($50-$450) nationwide makes it accessible.19
CAC measures the presence (or absence) of subclinical atherosclerosis by detecting calcified plaque in coronary arteries. The absolute CAC score is expressed in Agatston units; an age–gender population percentile is also provided. Keep in mind that the presence of any CAC (ie, a score > 0) is abnormal and demonstrates the presence of subclinical coronary artery disease. The prevalence of CAC > 0 increases with age, but a significant percentage of older people have a CAC score = 0. When CAC > 0, additional information is provided by the distribution of plaque burden among the different coronary arteries.20
Among intermediate-risk patients, 50% have CAC = 0 and, therefore, a very low event rate over the ensuing 10 years, which allows statin therapy to be safely deferred unless certain risk factors are present (eg, family history, smoking, DM).1,18 It is reasonable to repeat CAC testing in 5 to 10 years to assess whether subclinical atherosclerosis has developed. The 2018 guideline emphasizes that, when the CAC score is > 0 but < 100 Agatston units, statin therapy is favored, especially in patients > 55 years of age; when the CAC score is ≥ 100 Agatston units or at the ≥ 75th percentile, statin therapy is indicated regardless of age.1
Patients who might benefit from knowing their CAC score include those who are:
- reluctant to initiate statin therapy but who want to understand their risk and potential for benefit more precisely
- concerned about the need to reinstitute statin therapy after discontinuing it because of statin-associated adverse effects
- older (men, 55-80 years; women, 60-80 years) who have a low burden of risk factors and who question whether they would benefit from statin therapy
- middle-aged (40-55 years) and who have a PCE-calculated risk of 5% to < 7.5% for ASCVD and factors that increase their risk for ASCVD, even though they are in a borderline-risk group.1
Primary prevention in special populations
Older patients. In adults ≥ 75 years who have an LDL-C level 70 to 189 mg/dL, initiating a moderate-intensity statin might be reasonable; however, it might also be reasonable to stop treatment in this population when physical or cognitive decline, multiple morbidities, frailty, or reduced life expectancy limits the potential benefit of statin therapy. It might be reasonable to use the CAC score in adults 76 to 80 years of age who have an LDL-C level of 70 to 189 mg/dL to reclassify those whose CAC score = 0, so that they can avoid statin therapy.1
Continue to: Children and adolescents
Children and adolescents. In alignment with current pediatric guidelines,21 but in contrast to USPSTF reccomendations,22 the 2018 ACC/AHA guideline endorses universal lipid screening for pediatric patients (see TABLE W11,21,22). It is reasonable to obtain a fasting lipid profile or nonfasting non-HDL-C in all children and adolescents who have neither cardiovascular risk factors nor a family history of early cardiovascular disease to detect moderate-to-severe lipid abnormalities. Screening should be done once at 9 to 11 years of age and again at 17 to 21 years.1
A screening test as early as 2 years of age to detect familial hypercholesterolemia (FH) is reasonable when a family history of either early CVD or significant hypercholesterolemia is present. The guideline endorses reverse cascade screening for detection of FH in family members of children and adolescents who have severe hypercholesterolemia.1
In children and adolescents with a lipid abnormality, especially when associated with the metabolic syndrome, lifestyle counseling is beneficial for lowering the LDL-C level. In children and adolescents ≥ 10 years of age with (1) an LDL-C level persistently ≥ 190 mg/dL or (2) an LDL level ≥ 160 mg/dL plus a clinical presentation consistent with FH, it is reasonable to initiate statin therapy if they do not respond adequately to 3 to 6 months of lifestyle therapy.1
Ethnicity as a risk-modifying factor. The PCE distinguishes between US adults of European ancestry and African ancestry, but no other ethnic groups are distinguished.4 The new guideline advocates for the use of PCE in other populations; however, it states that, for clinical decision-making purposes, it is reasonable, in adults of different races and ethnicities, for the physician to review racial and ethnic features that can influence ASCVD risk to allow adjustment of the choice of statin or intensity of treatment. Specifically, South Asian ancestry is now treated as a risk-enhancing factor, given the high prevalence of premature and extensive ASCVD in this patient population.1
Concerns specific to women. Considering conditions specific to women as potential risk-enhancing factors is advised when discussing lifestyle intervention and the potential for benefit from statin therapy—in particular, (1) in the setting of premature menopause (< 40 years) and (2) when there is a history of a pregnancy-associated disorder (eg, hypertension, preeclampsia, gestational DM, a small-for-gestational-age infant, and preterm delivery). If the decision is made to initiate statin therapy in women of childbearing age who are sexually active, there is a guideline mandate to counsel patients on using reliable contraception. When pregnancy is planned, statin therapy should be discontinued 1 to 2 months before pregnancy is attempted; when pregnancy occurs while a patient is taking a statin, therapy should be stopped as soon as the pregnancy is discovered.1
Continue to: Adults with chronic kidney disease
Adults with chronic kidney disease. Chronic kidney disease that is not treated with dialysis or kidney transplantation is considered a risk-enhancing factor; initiation of a moderate-intensity statin or a moderate-intensity statin plus ezetimibe can be useful in patients with chronic kidney disease who are 40 to 75 years of age and have an LDL-C level of 70 to 189 mg/dL and a PCE-calculated risk ≥ 7.5%. In adults with advanced kidney disease that requires dialysis who are already taking a statin, it may be reasonable to continue the statin; however, initiation of a statin in adults with advanced kidney disease who require dialysis is not recommended because of an apparent lack of benefit.1
Adults with a chronic inflammatory disorder or human immunodeficiency virus infection. Any of these conditions are treated as risk-enhancing factors; in a risk discussion with affected patients, therefore, moderate-intensity statin therapy or high-intensity statin therapy is favored for those 40 to 75 years of age who have an LDL-C level of 70 to 189 mg/dL and PCE-calculated risk ≥ 7.5%. A fasting lipid profile and assessment of ASCVD risk factors for these patients can be useful (1) as a guide to the potential benefit of statin therapy and (2) for monitoring or adjusting lipid-lowering drug therapy before, and 4 to 12 weeks after, starting inflammatory disease-modifying therapy or antiretroviral therapy.
In adults with rheumatoid arthritis who undergo ASCVD risk assessment with a lipid profile, it can be useful to recheck lipid values and other major ASCVD risk factors 2 to 4 months after the inflammatory disease has been controlled.1
Primary hypercholesterolemia
The diagnosis and management of heterozygous or homozygous familial hypercholesterolemia (HeFH or HoFH) is beyond the scope of the 2018 ACC/AHA cholesterol guidelines; instead, the 2015 AHA Scientific Statement, “The Agenda for Familial Hypercholesterolemia,” provides a contemporary review of these topics.23 However, the 2018 cholesterol guideline does acknowledge that an LDL-C level ≥ 190 mg/dL often corresponds to primary (ie, genetic) hypercholesterolemia.
In patients 20 to 75 years of age who have a primary elevation of LDL-C level ≥ 190 mg/dL, the guideline recommends initiation of high-intensity statin therapy without calculating ASCVD risk using the PCE. If a > 50% LDL-C reduction is not achieved, or if the LDL-C level on maximally tolerated statin therapy remains ≥ 100 mg/dL, adding ezetimibe is considered reasonable. If there is < 50% reduction in the LDL-C level while taking maximally tolerated statin and ezetimibe therapy, adding a bile-acid sequestrant can be considered, as long as the TG level is not > 300 mg/dL (ie, bile-acid sequestrants can elevate the TG level significantly).
Continue to: In patients 30 to 75 years of age...
In patients 30 to 75 years of age who have a diagnosis of HeFH and an LDL-C level ≥ 100 mg/dL while taking maximally tolerated statin and ezetimibe therapy, the addition of a PCSK9 inhibitor can be considered. Regardless of whether there is a diagnosis of HeFH, addition of a PCSK9 inhibitor can be considered in patients 40 to 75 years of age who have a baseline LDL-C level ≥ 220 mg/dL and who achieve an on-treatment LDL-C level ≥ 130 mg/dL while receiving maximally tolerated statin therapy and ezetimibe.1
Diabetes mellitus
In patients with DM who are 40 to 75 years of age, moderate-intensity statin therapy is recommended without calculating the 10-year ASCVD risk. When the LDL-C level is 70 to 189 mg/dL, however, it is reasonable to use the PCE to assess 10-year ASCVD risk to facilitate risk stratification.
In patients with DM who are at higher risk, especially those who have multiple risk factors or are 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥ 50 %. In adults > 75 years of age with DM who are already on statin therapy, it is reasonable to continue statin therapy; for those that age who are not on statin therapy, it might be reasonable to initiate statin therapy after a physician–patient discussion of potential benefits and risks.
In adults with DM and PCE-calculated risk ≥ 20%, it might be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce the LDL-C level by ≥ 50%. In adults 20 to 39 years of age with DM of long duration (≥ 10 years of type 2 DM, ≥ 20 years of type 1 DM), albuminuria (≥ 30 μg of albumin/mg creatinine), estimated glomerular filtration rate < 60 mL/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index < 0.9, it might be reasonable to initiate statin therapy.1
Secondary prevention
Presence of clinical ASCVD. In patients with clinical ASCVD who are ≤ 75 years of age, high-intensity statin therapy should be initiated or continued, with the aim of achieving ≥ 50% reduction in the LDL-C level. When high-intensity statin therapy is contraindicated or if a patient experiences statin-associated adverse effects, moderate-intensity statin therapy should be initiated or continued with the aim of achieving a 30% to 49% reduction in the LDL-C level.
Continue to: In patients...
In patients > 75 years of age with clinical ASCVD, it is reasonable to initiate or continue moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preference.1
Very high risk. In patients at very high risk (this includes a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions), maximally tolerated LDL-C-lowering therapy should include maximally tolerated statin therapy and ezetimibe before considering a PCSK9 inhibitor. An LDL-C level ≥ 70 mg/dL or a non-HDL-C level ≥ 100 mg/dL is considered a reasonable threshold for adding a PCSK9 inhibitor to background lipid-lowering therapy1 (TABLE 31).
Heart failure. In patients with heart failure who have (1) a reduced ejection fraction attributable to ischemic heart disease, (2) a reasonable life expectancy (3-5 years), and (3) are not already on a statin because of ASCVD, consider initiating moderate-intensity statin therapy to reduce the risk for an ASCVD event.1
Reduction of elevated triglycerides
The guideline defines moderate hypertriglyceridemia as a nonfasting or fasting TG level of 175 to 499 mg/dL. Such a finding is considered a risk-enhancing factor and is 1 of 5 components of the metabolic syndrome. Three independent measurements are advised to diagnose primary moderate hypertriglyceridemia. Severe hypertriglyceridemia is diagnosed when the fasting TG level is ≥ 500 mg/dL.1
In moderate hypertriglyceridemia, most TGs are carried in very-low-density lipoprotein particles; in severe hypertriglyceridemia, on the other hand, chylomicrons predominate, raising the risk for pancreatitis. In adults with severe hypertriglyceridemia, therefore—especially when the fasting TG level is ≥ 1000 mg/dL—it is reasonable to identify and address other causes of hypertriglyceridemia. If TGs are persistently elevated or increasing, levels should be reduced to prevent acute pancreatitis with a very low-fat diet and by avoiding refined carbohydrates and alcohol; consuming omega-3 fatty acids; and, if necessary, taking a fibrate.1
Continue to: In adults...
In adults ≥ 20 years of age with moderate hypertriglyceridemia, lifestyle factors (eg, obesity, metabolic syndrome), secondary factors (eg, DM, chronic liver or kidney disease, nephrotic syndrome, hypothyroidism), and medications that increase the TG level need to be addressed first. In adults 40 to 75 years of age with moderate or severe hypertriglyceridemia and a PCE-calculated ASCVD risk ≥ 7.5%, it is reasonable to reevaluate risk after lifestyle and secondary factors are addressed and to consider a persistently elevated TG level as a factor favoring initiation or intensification of statin therapy. In adults 40 to 75 years of age with severe hypertriglyceridemia and ASCVD risk ≥ 7.5%, it is reasonable to address reversible causes of a high TG level and to initiate statin therapy.1
Other considerations in cholesterol management
Tools to assess adherence
The response to lifestyle and statin therapy should be evaluated by the percentage reduction in the LDL-C level compared with baseline, not by assessment of the absolute LDL-C level. When seeing a patient whose treatment is ongoing, a baseline level can be estimated using a desktop LDL-calculator app.
Adherence and percentage response to LDL-C–lowering medications and lifestyle changes should be evaluated with repeat lipid measurement 4 to 12 weeks after either a statin is initiated or the dosage is adjusted, and repeated every 3 to 12 months as needed. In patients with established ASCVD who are at very high risk, triggers for adding nonstatin therapy are defined by a threshold LDL-C level ≥ 70 mg/dL on maximal statin therapy.1
Interventions focused on improving adherence to prescribed therapy are recommended for management of adults with an elevated cholesterol level. These interventions include telephone reminders, calendar reminders, integrated multidisciplinary educational activities, and pharmacist-led interventions, such as simplification of the medication regimen to once-daily dosing.1
Statin safety and associated adverse effects
A physician–patient risk discussion is recommended before initiating statin therapy to review net clinical benefit, during which the 2 parties weigh the potential for ASCVD risk reduction against the potential for statin-associated adverse effects, statin–drug interactions, and safety, with the physician emphasizing that adverse effects can be addressed successfully.
Continue to: Statins are one of...
Statins are one of the safest classes of medication, with an excellent risk-benefit ratio. However, there are myriad confusing media reports regarding potential adverse effects and safety of the statin class—reports that often lead patients to discontinue or refuse statins.
Statin-associated adverse effects include the common statin-associated muscle symptoms (SAMS), new-onset DM, cognitive effects, and hepatic injury. The frequency of new-onset DM depends on the population exposed to statins, with a higher incidence of new-onset DM found in patients who are already predisposed, such as those with obesity, prediabetes, and metabolic syndrome. Cognitive effects are rare and difficult to interpret; they were not reported in the large statin mega-trials but have been described in case reports. Significant transaminase elevations > 3 times the upper limit of normal are infrequent; hepatic failure with statins is extremely rare and found at the same incidence in the general population.1
SAMS include (in order of decreasing prevalence)24:
- myalgias with a normal creatine kinase (CK) level
- conditions such as myositis or myopathy (elevated CK level)
- rhabdomyolysis (CK level > 10 times the upper limit of normal, plus renal injury)
- extremely rare statin-associated autoimmune myopathy, with detectable 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase antibodies.
In patients with SAMS, thorough assessment of symptoms is recommended, in addition to evaluation for nonstatin causes and predisposing factors. Identification of potential SAMS-predisposing factors is recommended before initiation of treatment, including demographics (eg, East-Asian ancestry), comorbid conditions (eg, hypothyroidism and vitamin D deficiency), and use of medications adversely affecting statin metabolism (eg, cyclosporine).
In patients with statin-associated adverse effects that are not severe, it is recommended to reassess and rechallenge to achieve a maximal lowering of the LDL-C level by a modified dosing regimen or an alternate statin or by combining a statin with nonstatin therapy. In patients with increased risk for DM or new-onset DM, it is recommended to continue statin therapy.
Continue to: Routine CK and liver function testing...
Routine CK and liver function testing is not useful in patients treated with statins; however, it is recommended that CK be measured in patients with severe SAMS or objective muscle weakness, or both, and to measure liver function if symptoms suggest hepatotoxicity. In patients at increased risk for ASCVD who have chronic, stable liver disease (including non-alcoholic fatty liver disease), it is reasonable, when appropriately indicated, to use statins after obtaining baseline measurements and determining a schedule of monitoring and safety checks.
In patients at increased risk for ASCVD who have severe or recurrent SAMS after appropriate statin rechallenge, it is reasonable to use nonstatin therapy that is likely to provide net clinical benefit. The guideline does not recommend routine use of coenzyme Q10 supplementation for the treatment or prevention of SAMS.1
Guideline criticism
Guideline development is challenging on multiple levels, including balancing perspectives from multiple stakeholders. Nevertheless, the 2018 AHA/ACC cholesterol guideline builds nicely on progress made since its 2013 predecessor was released.4 This document was developed with the participation of representatives from 10 professional societies in addition to the ACC and AHA—notably, the National Lipid Association and American Society for Preventive Cardiology.1
To refine risk estimation and facilitate shared decision-making, the new guideline introduced so-called risk-enhancing factors and use of the CAC.1 However, some potential risk-enhancing factors were left out: erectile dysfunction, for example, often a marker of increased cardiovascular risk in men < 50 years of age.25 In addition, although pretreatment ApoB was introduced as a risk-enhancing factor,1 no recommendation is given to measure ApoB after initiation of therapy for evaluation of residual cardiovascular risk, as endorsed in other guidelines.26,27
Moreover, the guideline does not include the “extreme risk” category in the guideline developed by the American Association of Clinical Endocrinologists (AACE).28 Although the 2018 AHA/ACC guideline introduces < 70 mg/dL and < 100 mg/dL LDL-C thresholds,1 the < 55 mg/dL LDL-C threshold used for patients in the AACE/American College of Endocrinology extreme-risk category is not mentioned.26 This omission might leave patients who are at extreme ASCVD risk without optimal lipid-lowering therapy. Similarly, the guideline does not elaborate on the diagnosis and treatment of HoFH and HeFH.1 The age cutoff of 30 years for the recommendation to consider PCSK9 inhibitors in patients with HeFH appears arbitrary and excludes younger FH patients who have an extreme LDL-C elevation from potentially important therapy.23
Continue to: Guidelines are dynamic instruments...
Guidelines are dynamic instruments that require constant updating, given the production of new evidence. In fact, the results of the Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) were presented at the same meeting at which this guideline was unveiled.29 REDUCE-IT demonstrated an astonishing highly significant 25% reduction in the composite primary major adverse cardiovascular event outcome in patients with an LDL-C level of 44 to 100 mg/dL on statin therapy, who had a TG level of 135 to 499 mg/dL and had been treated for a median of 4.9 years with 4 g of pure eicosapentaenoic acid.
In addition, the guideline’s value statements, which address the need to consider the cost of drugs in determining most appropriate treatment, are no longer accurate because the price of PCSK9 inhibitors has dropped by more than half since the guideline was issued.30
An upward climb to clinical payoff
Even after close study of the 2018 AHA/ACC cholesterol guideline, implementing it in practice might remain a challenge to clinicians who are inexperienced in ordering lipid markers such as Lp(a) and interpreting the CAC score. Moreover, initiating and monitoring nonstatin therapies will be a demanding task—especially with PCSK9 inhibitors, which present access difficulties because they are relatively expensive (even after the recent price cut). That’s why, when there is doubt in the mind of the physician or other provider, we will likely see more referrals to specialists in lipid management and ASCVD risk estimation to optimize preventive therapy.31
CORRESPONDENCE
Cezary Wójcik, MD, PhD, FNLA, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; [email protected]
A new cholesterol guideline1 builds on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol guidelines,2 which were a major paradigm shift in the evaluation and management of blood cholesterol levels and risk for atherosclerotic cardiovascular disease (ASCVD). The work was presented (and simultaneously published) on November 10, 2018, at the annual AHA Scientific Sessions in Chicago. Full text,1 an executive summary,3 and accompanying systematic review of evidence4 are available online.
The 2018 AHA/ACC cholesterol guideline represents a step forward in ASCVD prevention—especially in primary prevention, where it provides guidance for risk refinement and personalization. In this article, we mine the details of what has changed and what is new in this guideline so that you can prepare to adopt the recommendations in your practice.
2013 and 2018 guidelines: Similarities, differences
As in earlier iterations, the 2018 guideline emphasizes healthy lifestyle across the life-course as the basis of ASCVD prevention—as elaborated in the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.5 In contrast to the 2013 guidelines,2 the 2018 guideline is more comprehensive and more personalized, focusing on risk assessment for individual patients, rather than simply providing population-based approaches. Moreover, the guideline isn’t limited to adults: It makes recommendations pertaining to children and adolescents.1
TABLE 11,2 compares the most important differences between the 2013 and 2018 guidelines.
The 2013 ACC/AHA guidelines eliminated low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C)a goals of therapy and replaced them with the concept of 4 “statin benefit groups”—that is, patient populations for which clear evidence supports the role of statin therapy.4 In the 2018 guideline, statin benefit groups have been maintained, although without explicit use of this term.1
Primary prevention. Although no major changes in statin indications are made for patients with (1) established ASCVD (ie, for secondary prevention), (2) diabetes mellitus (DM) and who are 40 to 75 years of age, or (3) a primary LDL-C elevation ≥ 190 mg/dL, significant changes were made for primary prevention patients ages 40 to 75 years.1 ASCVD risk calculation using the 2013 pooled cohort equations (PCE) is still recommended4; however, risk estimation is refined by the use of specific so-called risk-enhancing factors (TABLE 21). In cases in which the risk decision remains uncertain, obtaining the coronary artery calcium (CAC) score (which we’ll describe shortly) using specialized computed tomography (CT) is advised to facilitate the shared physician–patient decision-making process.1
LDL-C and non-HDL-C thresholds. Although LDL-C and non-HDL-C goals are not overtly brought back from the 2002 National Cholesterol Education Program/Adult Treatment Panel guidelines,6 the new guideline does introduce LDL-C and non-HDL-C thresholds—levels at which adding nonstatin therapy can be considered, in contrast to previous goals to which therapy was titrated. Definitions of statin intensity remain the same: Moderate-intensity statin therapy is expected to reduce the LDL-C level by 30% to 50%; high-intensity statin therapy, by ≥ 50%.1 The intensity of statin therapy has been de-escalated in the intermediate-risk group, where previous guidelines advised high-intensity statin therapy,4 and replaced with moderate-intensity statin therapy (similar to 2016 US Preventive Services Task Force [USPSTF] recommendations7).
[polldaddy:10312157]
Continue to: Fasting vs nonfasting lipid profiles
Fasting vs nonfasting lipid profiles. In contrast to previous guidelines,2,8 which used fasting lipid profiles, nonfasting lipid profiles are now recommended for establishing a baseline LDL-C level and for ASCVD risk estimation for most patients—as long as the triglycerides (TG) level is < 400 mg/dL. When the calculated LDL-C level is < 70 mg/dL using the standard Friedewald formula, obtaining a direct LDL-C or a modified LDL-C estimate9 is deemed reasonable to improve accuracy. (The modified LDL-C can be estimated using The Johns Hopkins Hospital’s free “LDL Cholesterol Calculator” [www.hopkinsmedicine.org/apps/all-apps/ldl-cholesterol-calculator]).
A fasting lipid profile is still preferred for patients who have a family history of a lipid disorder. The definition of hypertriglyceridemia has been revised from a fasting TG level ≥ 150 mg/dL to a nonfasting or fasting TG level ≥ 175 mg/dL.1
Nonstatin add-on therapy. The new guideline supports the addition of nonstatin therapies to maximally tolerated statin therapy in patients who have established ASCVD or a primary LDL-C elevation ≥ 190 mg/dL when (1) the LDL-C level has not been reduced by the expected percentage (≥ 50% for high-intensity statin therapy) or (2) explicit LDL-C level thresholds have been met.1
The principal 2 groups of recommended nonstatins for which there is randomized, controlled trial evidence of cardiovascular benefit are (1) the cholesterol-absorbing agent ezetimibe10 and (2) the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab11 and alirocumab.12
AAFP’s guarded positions on the 2013 and 2018 guidelines
The American Academy of Family Physicians (AAFP) welcomed the patient-centered and outcome-oriented aspects of the 2013 ACC/AHA guidelines, endorsing them with 3 qualifications.13
- Many of the recommendations were based on expert opinion, not rigorous research results—in particular, not on the findings of randomized controlled trials (although key points are based on high-quality evidence).
- There were conflicts of interest disclosed for 15 members of the guidelines panel, including a vice chair.
- Validation of the PCE risk estimation tool was lacking.
Continue to: AAFP announced...
AAFP announced in March that it does not endorse the 2018 AHA/ACC guideline, asserting that (1) only a small portion of the recommendations, primarily focused on the addition of nonstatin therapy, were addressed by an independent systematic review and (2) many of the guideline recommendations are based on low-quality or insufficient evidence. AAFP nevertheless bestowed an “affirmation of value” designation on the guideline—meaning that it provides some benefit for family physicians’ practice without fulfilling all criteria for full endorsement.14
Detailed recommendations from the 2018 guideline
Lifestyle modification
When talking about ASCVD risk with patients, it is important to review current lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). Subsequent to that conversation, a healthy lifestyle should be endorsed and relevant advice provided. In addition, patient-directed materials (eg, ACC’s CardioSmart [www.cardiosmart.org]; AHA’s Life’s Simple 7 [www.heart.org/en/professional/workplace-health/lifes-simple-7]; and the National Lipid Association’s Patient Tear Sheets [www.lipid.org/practicetools/tools/tearsheets] and Clinicians’ Lifestyle Modification Toolbox [www.lipid.org/CLMT]) and referrals (eg, to cardiac rehabilitation, a dietitian, a smoking-cessation program) should be provided.1
Primary prevention of ASCVD
Risk assessment for primary prevention is now approached as a process, rather than the simple risk calculation used in the 2013 ACC/AHA guidelines.2 Assessment involves risk estimation followed by risk personalization, which, in some cases, is followed by risk reclassification using CAC scoring.1
Patients are classified into 1 of 4 risk groups, based on the PCE1:
- low (< 5%)
- borderline (5%-7.5%)
- intermediate (7.5%-19.9%)
- high (≥ 20%).
However, the PCE-based risk score is a population-based tool, which might not reflect the actual risk of individual patients. In some populations, PCE underestimates ASCVD risk; in others, it overestimates risk. A central tenet of the new guideline is personalization of risk, taking into account the unique circumstances of each patient. Moreover, the new guideline provides guidance on how to interpret the PCE risk score for several different ethnic and racial groups.1
Continue to: Medical therapy
Medical therapy. The decision to start lipid-lowering therapy should be made after a physician–patient discussion that considers costs of therapy as well as patient preferences and values in the context of shared decision-making. Discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, and the LDL-C level), the PCE risk score, the presence of risk-enhancing factors (TABLE 21), potential benefits of lifestyle changes and statin therapy, and the potential for adverse drug effects and drug–drug interactions.1
If the estimated ASCVD risk is 7.5%-19.9%, starting moderate-intensity statin therapy is recommended. Risk-enhancing factors favor initiation of statin therapy, even in patients at borderline risk (5%-7.5%). If risk is uncertain, the CAC score can be used to facilitate shared decision-making.1 The use of CAC is in agreement with the USPSTF statement that CAC can moderately improve discrimination and reclassification, but has an unclear effect on downstream health care utilization.15 Importantly, CAC should not be measured routinely in patients already taking a statin because its primary role is to facilitate shared decision-making regarding initiation of statin therapy.16
If the 10-year ASCVD risk is ≥ 20%, high-intensity statin therapy is advised, without need to obtain the CAC score. If high-intensity statin therapy is advisable but not acceptable to, or tolerated by, the patient, it might be reasonable to add a nonstatin drug (ezetimibe or a bile-acid sequestrant) to moderate-intensity statin therapy.1
Risk-enhancing factors (TABLE 21) apply to intermediate- and borderline-risk patients. Importantly, these factors include membership in specific ethnic groups, conditions specific to females, and male–female distinctions in risk. Risk-enhancing factors also incorporate biomarkers that are often measured by lipid specialists, such as lipoprotein(a) (Lp[a]) and apolipoprotein B (ApoB).1
Lp(a) is an atherogenic particle, akin to an LDL particle, that consists of a molecule of apolipoprotein (a) (a nonfunctional mimic of a portion of plasminogen) covalently bound to ApoB, like the one found on the LDL particle. Lp(a) is proportionally associated with an increased risk for ASCVD and aortic stenosis at a level > 50 mg/dL.17 A family history of premature ASCVD is a relative indication for measuring Lp(a).1
Continue to: When and why to measure CAC
When and why to measure CAC
If the decision to initiate statin therapy is still uncertain after risk estimation and personalization, or when a patient is undecided about committing to lifelong lipid-lowering therapy, the new guideline recommends obtaining a CAC score to inform the shared decision-making process.1,18 Measurement of CAC is obtained by noncontrast, electrocardiographic-gated CT that can be performed in 10 to 15 minutes, requiring approximately 1 millisievert of radiation (equivalent of the approximate dose absorbed during 2 mammograms). Although measurement of the CAC score is generally not covered by insurance, its cost ($50-$450) nationwide makes it accessible.19
CAC measures the presence (or absence) of subclinical atherosclerosis by detecting calcified plaque in coronary arteries. The absolute CAC score is expressed in Agatston units; an age–gender population percentile is also provided. Keep in mind that the presence of any CAC (ie, a score > 0) is abnormal and demonstrates the presence of subclinical coronary artery disease. The prevalence of CAC > 0 increases with age, but a significant percentage of older people have a CAC score = 0. When CAC > 0, additional information is provided by the distribution of plaque burden among the different coronary arteries.20
Among intermediate-risk patients, 50% have CAC = 0 and, therefore, a very low event rate over the ensuing 10 years, which allows statin therapy to be safely deferred unless certain risk factors are present (eg, family history, smoking, DM).1,18 It is reasonable to repeat CAC testing in 5 to 10 years to assess whether subclinical atherosclerosis has developed. The 2018 guideline emphasizes that, when the CAC score is > 0 but < 100 Agatston units, statin therapy is favored, especially in patients > 55 years of age; when the CAC score is ≥ 100 Agatston units or at the ≥ 75th percentile, statin therapy is indicated regardless of age.1
Patients who might benefit from knowing their CAC score include those who are:
- reluctant to initiate statin therapy but who want to understand their risk and potential for benefit more precisely
- concerned about the need to reinstitute statin therapy after discontinuing it because of statin-associated adverse effects
- older (men, 55-80 years; women, 60-80 years) who have a low burden of risk factors and who question whether they would benefit from statin therapy
- middle-aged (40-55 years) and who have a PCE-calculated risk of 5% to < 7.5% for ASCVD and factors that increase their risk for ASCVD, even though they are in a borderline-risk group.1
Primary prevention in special populations
Older patients. In adults ≥ 75 years who have an LDL-C level 70 to 189 mg/dL, initiating a moderate-intensity statin might be reasonable; however, it might also be reasonable to stop treatment in this population when physical or cognitive decline, multiple morbidities, frailty, or reduced life expectancy limits the potential benefit of statin therapy. It might be reasonable to use the CAC score in adults 76 to 80 years of age who have an LDL-C level of 70 to 189 mg/dL to reclassify those whose CAC score = 0, so that they can avoid statin therapy.1
Continue to: Children and adolescents
Children and adolescents. In alignment with current pediatric guidelines,21 but in contrast to USPSTF reccomendations,22 the 2018 ACC/AHA guideline endorses universal lipid screening for pediatric patients (see TABLE W11,21,22). It is reasonable to obtain a fasting lipid profile or nonfasting non-HDL-C in all children and adolescents who have neither cardiovascular risk factors nor a family history of early cardiovascular disease to detect moderate-to-severe lipid abnormalities. Screening should be done once at 9 to 11 years of age and again at 17 to 21 years.1
A screening test as early as 2 years of age to detect familial hypercholesterolemia (FH) is reasonable when a family history of either early CVD or significant hypercholesterolemia is present. The guideline endorses reverse cascade screening for detection of FH in family members of children and adolescents who have severe hypercholesterolemia.1
In children and adolescents with a lipid abnormality, especially when associated with the metabolic syndrome, lifestyle counseling is beneficial for lowering the LDL-C level. In children and adolescents ≥ 10 years of age with (1) an LDL-C level persistently ≥ 190 mg/dL or (2) an LDL level ≥ 160 mg/dL plus a clinical presentation consistent with FH, it is reasonable to initiate statin therapy if they do not respond adequately to 3 to 6 months of lifestyle therapy.1
Ethnicity as a risk-modifying factor. The PCE distinguishes between US adults of European ancestry and African ancestry, but no other ethnic groups are distinguished.4 The new guideline advocates for the use of PCE in other populations; however, it states that, for clinical decision-making purposes, it is reasonable, in adults of different races and ethnicities, for the physician to review racial and ethnic features that can influence ASCVD risk to allow adjustment of the choice of statin or intensity of treatment. Specifically, South Asian ancestry is now treated as a risk-enhancing factor, given the high prevalence of premature and extensive ASCVD in this patient population.1
Concerns specific to women. Considering conditions specific to women as potential risk-enhancing factors is advised when discussing lifestyle intervention and the potential for benefit from statin therapy—in particular, (1) in the setting of premature menopause (< 40 years) and (2) when there is a history of a pregnancy-associated disorder (eg, hypertension, preeclampsia, gestational DM, a small-for-gestational-age infant, and preterm delivery). If the decision is made to initiate statin therapy in women of childbearing age who are sexually active, there is a guideline mandate to counsel patients on using reliable contraception. When pregnancy is planned, statin therapy should be discontinued 1 to 2 months before pregnancy is attempted; when pregnancy occurs while a patient is taking a statin, therapy should be stopped as soon as the pregnancy is discovered.1
Continue to: Adults with chronic kidney disease
Adults with chronic kidney disease. Chronic kidney disease that is not treated with dialysis or kidney transplantation is considered a risk-enhancing factor; initiation of a moderate-intensity statin or a moderate-intensity statin plus ezetimibe can be useful in patients with chronic kidney disease who are 40 to 75 years of age and have an LDL-C level of 70 to 189 mg/dL and a PCE-calculated risk ≥ 7.5%. In adults with advanced kidney disease that requires dialysis who are already taking a statin, it may be reasonable to continue the statin; however, initiation of a statin in adults with advanced kidney disease who require dialysis is not recommended because of an apparent lack of benefit.1
Adults with a chronic inflammatory disorder or human immunodeficiency virus infection. Any of these conditions are treated as risk-enhancing factors; in a risk discussion with affected patients, therefore, moderate-intensity statin therapy or high-intensity statin therapy is favored for those 40 to 75 years of age who have an LDL-C level of 70 to 189 mg/dL and PCE-calculated risk ≥ 7.5%. A fasting lipid profile and assessment of ASCVD risk factors for these patients can be useful (1) as a guide to the potential benefit of statin therapy and (2) for monitoring or adjusting lipid-lowering drug therapy before, and 4 to 12 weeks after, starting inflammatory disease-modifying therapy or antiretroviral therapy.
In adults with rheumatoid arthritis who undergo ASCVD risk assessment with a lipid profile, it can be useful to recheck lipid values and other major ASCVD risk factors 2 to 4 months after the inflammatory disease has been controlled.1
Primary hypercholesterolemia
The diagnosis and management of heterozygous or homozygous familial hypercholesterolemia (HeFH or HoFH) is beyond the scope of the 2018 ACC/AHA cholesterol guidelines; instead, the 2015 AHA Scientific Statement, “The Agenda for Familial Hypercholesterolemia,” provides a contemporary review of these topics.23 However, the 2018 cholesterol guideline does acknowledge that an LDL-C level ≥ 190 mg/dL often corresponds to primary (ie, genetic) hypercholesterolemia.
In patients 20 to 75 years of age who have a primary elevation of LDL-C level ≥ 190 mg/dL, the guideline recommends initiation of high-intensity statin therapy without calculating ASCVD risk using the PCE. If a > 50% LDL-C reduction is not achieved, or if the LDL-C level on maximally tolerated statin therapy remains ≥ 100 mg/dL, adding ezetimibe is considered reasonable. If there is < 50% reduction in the LDL-C level while taking maximally tolerated statin and ezetimibe therapy, adding a bile-acid sequestrant can be considered, as long as the TG level is not > 300 mg/dL (ie, bile-acid sequestrants can elevate the TG level significantly).
Continue to: In patients 30 to 75 years of age...
In patients 30 to 75 years of age who have a diagnosis of HeFH and an LDL-C level ≥ 100 mg/dL while taking maximally tolerated statin and ezetimibe therapy, the addition of a PCSK9 inhibitor can be considered. Regardless of whether there is a diagnosis of HeFH, addition of a PCSK9 inhibitor can be considered in patients 40 to 75 years of age who have a baseline LDL-C level ≥ 220 mg/dL and who achieve an on-treatment LDL-C level ≥ 130 mg/dL while receiving maximally tolerated statin therapy and ezetimibe.1
Diabetes mellitus
In patients with DM who are 40 to 75 years of age, moderate-intensity statin therapy is recommended without calculating the 10-year ASCVD risk. When the LDL-C level is 70 to 189 mg/dL, however, it is reasonable to use the PCE to assess 10-year ASCVD risk to facilitate risk stratification.
In patients with DM who are at higher risk, especially those who have multiple risk factors or are 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥ 50 %. In adults > 75 years of age with DM who are already on statin therapy, it is reasonable to continue statin therapy; for those that age who are not on statin therapy, it might be reasonable to initiate statin therapy after a physician–patient discussion of potential benefits and risks.
In adults with DM and PCE-calculated risk ≥ 20%, it might be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce the LDL-C level by ≥ 50%. In adults 20 to 39 years of age with DM of long duration (≥ 10 years of type 2 DM, ≥ 20 years of type 1 DM), albuminuria (≥ 30 μg of albumin/mg creatinine), estimated glomerular filtration rate < 60 mL/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index < 0.9, it might be reasonable to initiate statin therapy.1
Secondary prevention
Presence of clinical ASCVD. In patients with clinical ASCVD who are ≤ 75 years of age, high-intensity statin therapy should be initiated or continued, with the aim of achieving ≥ 50% reduction in the LDL-C level. When high-intensity statin therapy is contraindicated or if a patient experiences statin-associated adverse effects, moderate-intensity statin therapy should be initiated or continued with the aim of achieving a 30% to 49% reduction in the LDL-C level.
Continue to: In patients...
In patients > 75 years of age with clinical ASCVD, it is reasonable to initiate or continue moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preference.1
Very high risk. In patients at very high risk (this includes a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions), maximally tolerated LDL-C-lowering therapy should include maximally tolerated statin therapy and ezetimibe before considering a PCSK9 inhibitor. An LDL-C level ≥ 70 mg/dL or a non-HDL-C level ≥ 100 mg/dL is considered a reasonable threshold for adding a PCSK9 inhibitor to background lipid-lowering therapy1 (TABLE 31).
Heart failure. In patients with heart failure who have (1) a reduced ejection fraction attributable to ischemic heart disease, (2) a reasonable life expectancy (3-5 years), and (3) are not already on a statin because of ASCVD, consider initiating moderate-intensity statin therapy to reduce the risk for an ASCVD event.1
Reduction of elevated triglycerides
The guideline defines moderate hypertriglyceridemia as a nonfasting or fasting TG level of 175 to 499 mg/dL. Such a finding is considered a risk-enhancing factor and is 1 of 5 components of the metabolic syndrome. Three independent measurements are advised to diagnose primary moderate hypertriglyceridemia. Severe hypertriglyceridemia is diagnosed when the fasting TG level is ≥ 500 mg/dL.1
In moderate hypertriglyceridemia, most TGs are carried in very-low-density lipoprotein particles; in severe hypertriglyceridemia, on the other hand, chylomicrons predominate, raising the risk for pancreatitis. In adults with severe hypertriglyceridemia, therefore—especially when the fasting TG level is ≥ 1000 mg/dL—it is reasonable to identify and address other causes of hypertriglyceridemia. If TGs are persistently elevated or increasing, levels should be reduced to prevent acute pancreatitis with a very low-fat diet and by avoiding refined carbohydrates and alcohol; consuming omega-3 fatty acids; and, if necessary, taking a fibrate.1
Continue to: In adults...
In adults ≥ 20 years of age with moderate hypertriglyceridemia, lifestyle factors (eg, obesity, metabolic syndrome), secondary factors (eg, DM, chronic liver or kidney disease, nephrotic syndrome, hypothyroidism), and medications that increase the TG level need to be addressed first. In adults 40 to 75 years of age with moderate or severe hypertriglyceridemia and a PCE-calculated ASCVD risk ≥ 7.5%, it is reasonable to reevaluate risk after lifestyle and secondary factors are addressed and to consider a persistently elevated TG level as a factor favoring initiation or intensification of statin therapy. In adults 40 to 75 years of age with severe hypertriglyceridemia and ASCVD risk ≥ 7.5%, it is reasonable to address reversible causes of a high TG level and to initiate statin therapy.1
Other considerations in cholesterol management
Tools to assess adherence
The response to lifestyle and statin therapy should be evaluated by the percentage reduction in the LDL-C level compared with baseline, not by assessment of the absolute LDL-C level. When seeing a patient whose treatment is ongoing, a baseline level can be estimated using a desktop LDL-calculator app.
Adherence and percentage response to LDL-C–lowering medications and lifestyle changes should be evaluated with repeat lipid measurement 4 to 12 weeks after either a statin is initiated or the dosage is adjusted, and repeated every 3 to 12 months as needed. In patients with established ASCVD who are at very high risk, triggers for adding nonstatin therapy are defined by a threshold LDL-C level ≥ 70 mg/dL on maximal statin therapy.1
Interventions focused on improving adherence to prescribed therapy are recommended for management of adults with an elevated cholesterol level. These interventions include telephone reminders, calendar reminders, integrated multidisciplinary educational activities, and pharmacist-led interventions, such as simplification of the medication regimen to once-daily dosing.1
Statin safety and associated adverse effects
A physician–patient risk discussion is recommended before initiating statin therapy to review net clinical benefit, during which the 2 parties weigh the potential for ASCVD risk reduction against the potential for statin-associated adverse effects, statin–drug interactions, and safety, with the physician emphasizing that adverse effects can be addressed successfully.
Continue to: Statins are one of...
Statins are one of the safest classes of medication, with an excellent risk-benefit ratio. However, there are myriad confusing media reports regarding potential adverse effects and safety of the statin class—reports that often lead patients to discontinue or refuse statins.
Statin-associated adverse effects include the common statin-associated muscle symptoms (SAMS), new-onset DM, cognitive effects, and hepatic injury. The frequency of new-onset DM depends on the population exposed to statins, with a higher incidence of new-onset DM found in patients who are already predisposed, such as those with obesity, prediabetes, and metabolic syndrome. Cognitive effects are rare and difficult to interpret; they were not reported in the large statin mega-trials but have been described in case reports. Significant transaminase elevations > 3 times the upper limit of normal are infrequent; hepatic failure with statins is extremely rare and found at the same incidence in the general population.1
SAMS include (in order of decreasing prevalence)24:
- myalgias with a normal creatine kinase (CK) level
- conditions such as myositis or myopathy (elevated CK level)
- rhabdomyolysis (CK level > 10 times the upper limit of normal, plus renal injury)
- extremely rare statin-associated autoimmune myopathy, with detectable 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase antibodies.
In patients with SAMS, thorough assessment of symptoms is recommended, in addition to evaluation for nonstatin causes and predisposing factors. Identification of potential SAMS-predisposing factors is recommended before initiation of treatment, including demographics (eg, East-Asian ancestry), comorbid conditions (eg, hypothyroidism and vitamin D deficiency), and use of medications adversely affecting statin metabolism (eg, cyclosporine).
In patients with statin-associated adverse effects that are not severe, it is recommended to reassess and rechallenge to achieve a maximal lowering of the LDL-C level by a modified dosing regimen or an alternate statin or by combining a statin with nonstatin therapy. In patients with increased risk for DM or new-onset DM, it is recommended to continue statin therapy.
Continue to: Routine CK and liver function testing...
Routine CK and liver function testing is not useful in patients treated with statins; however, it is recommended that CK be measured in patients with severe SAMS or objective muscle weakness, or both, and to measure liver function if symptoms suggest hepatotoxicity. In patients at increased risk for ASCVD who have chronic, stable liver disease (including non-alcoholic fatty liver disease), it is reasonable, when appropriately indicated, to use statins after obtaining baseline measurements and determining a schedule of monitoring and safety checks.
In patients at increased risk for ASCVD who have severe or recurrent SAMS after appropriate statin rechallenge, it is reasonable to use nonstatin therapy that is likely to provide net clinical benefit. The guideline does not recommend routine use of coenzyme Q10 supplementation for the treatment or prevention of SAMS.1
Guideline criticism
Guideline development is challenging on multiple levels, including balancing perspectives from multiple stakeholders. Nevertheless, the 2018 AHA/ACC cholesterol guideline builds nicely on progress made since its 2013 predecessor was released.4 This document was developed with the participation of representatives from 10 professional societies in addition to the ACC and AHA—notably, the National Lipid Association and American Society for Preventive Cardiology.1
To refine risk estimation and facilitate shared decision-making, the new guideline introduced so-called risk-enhancing factors and use of the CAC.1 However, some potential risk-enhancing factors were left out: erectile dysfunction, for example, often a marker of increased cardiovascular risk in men < 50 years of age.25 In addition, although pretreatment ApoB was introduced as a risk-enhancing factor,1 no recommendation is given to measure ApoB after initiation of therapy for evaluation of residual cardiovascular risk, as endorsed in other guidelines.26,27
Moreover, the guideline does not include the “extreme risk” category in the guideline developed by the American Association of Clinical Endocrinologists (AACE).28 Although the 2018 AHA/ACC guideline introduces < 70 mg/dL and < 100 mg/dL LDL-C thresholds,1 the < 55 mg/dL LDL-C threshold used for patients in the AACE/American College of Endocrinology extreme-risk category is not mentioned.26 This omission might leave patients who are at extreme ASCVD risk without optimal lipid-lowering therapy. Similarly, the guideline does not elaborate on the diagnosis and treatment of HoFH and HeFH.1 The age cutoff of 30 years for the recommendation to consider PCSK9 inhibitors in patients with HeFH appears arbitrary and excludes younger FH patients who have an extreme LDL-C elevation from potentially important therapy.23
Continue to: Guidelines are dynamic instruments...
Guidelines are dynamic instruments that require constant updating, given the production of new evidence. In fact, the results of the Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) were presented at the same meeting at which this guideline was unveiled.29 REDUCE-IT demonstrated an astonishing highly significant 25% reduction in the composite primary major adverse cardiovascular event outcome in patients with an LDL-C level of 44 to 100 mg/dL on statin therapy, who had a TG level of 135 to 499 mg/dL and had been treated for a median of 4.9 years with 4 g of pure eicosapentaenoic acid.
In addition, the guideline’s value statements, which address the need to consider the cost of drugs in determining most appropriate treatment, are no longer accurate because the price of PCSK9 inhibitors has dropped by more than half since the guideline was issued.30
An upward climb to clinical payoff
Even after close study of the 2018 AHA/ACC cholesterol guideline, implementing it in practice might remain a challenge to clinicians who are inexperienced in ordering lipid markers such as Lp(a) and interpreting the CAC score. Moreover, initiating and monitoring nonstatin therapies will be a demanding task—especially with PCSK9 inhibitors, which present access difficulties because they are relatively expensive (even after the recent price cut). That’s why, when there is doubt in the mind of the physician or other provider, we will likely see more referrals to specialists in lipid management and ASCVD risk estimation to optimize preventive therapy.31
CORRESPONDENCE
Cezary Wójcik, MD, PhD, FNLA, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; [email protected]
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. pii: S0735-1097(18)39034-X. [Epub ahead of print]
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-S45.
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39033-8. [Epub ahead of print]
4. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39035-1. [Epub ahead of print]
5. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
6. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III): final report. Circulation. 2002;106:3143-3421.
7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997-2007.
8. National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel II). Circulation. 1994;89:1333-1445.
9. Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749-753.
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
12. Szarek M, White HD, Schwartz GG, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387-396.
13. Crawford C. AAFP endorses ACC/AHA cholesterol management guidelines with qualifications. Leawood, KS: American Academy of Family Physicians; 2014 June 18. www.aafp.org/news/health-of-the-public/20140618cholesterolgdlnendorse.html. Accessed March 20, 2019.
14. Crawford C. AAFP News. AAFP affirms value of new cholesterol management guideline. March 20, 2019. www.aafp.org/news/health-of-the-public/20190320acc-ahacholguidln.html?cmpid=em_AP_20190320. Accessed April 1, 2019.
15. Lin JS, Evans CV, Johnson E, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Evidence Synthesis, No. 166. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 Jul. Report No.: 17-05225-EF-1.
16. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273-1282.
17. Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553-1560.
18. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92:1831-1841.
19. MDsave. Cardiac CT calcium scoring. www.mdsave.com/procedures/cardiac-ct-calcium-scoring/d785f4cf. Accessed Aprl 1, 2019.
20. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407-1416.
21. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213-S256.
22. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633.
23. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167-2192.
24. Newman CB, Preiss D, Tobert JA, et al; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38-e81.
25. Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sex Med Rev. 2018 Jan 27. pii: S2050-0521(18)30009-X. [Epub ahead of print]
26. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
27. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282.
28. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
29. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
30. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC Managed Markets Network. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed April 12, 2019.
31. Kaufman TM, Duell PB, Purnell JQ, et al. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499-501.
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. pii: S0735-1097(18)39034-X. [Epub ahead of print]
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-S45.
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39033-8. [Epub ahead of print]
4. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39035-1. [Epub ahead of print]
5. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
6. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III): final report. Circulation. 2002;106:3143-3421.
7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997-2007.
8. National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel II). Circulation. 1994;89:1333-1445.
9. Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749-753.
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
12. Szarek M, White HD, Schwartz GG, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387-396.
13. Crawford C. AAFP endorses ACC/AHA cholesterol management guidelines with qualifications. Leawood, KS: American Academy of Family Physicians; 2014 June 18. www.aafp.org/news/health-of-the-public/20140618cholesterolgdlnendorse.html. Accessed March 20, 2019.
14. Crawford C. AAFP News. AAFP affirms value of new cholesterol management guideline. March 20, 2019. www.aafp.org/news/health-of-the-public/20190320acc-ahacholguidln.html?cmpid=em_AP_20190320. Accessed April 1, 2019.
15. Lin JS, Evans CV, Johnson E, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Evidence Synthesis, No. 166. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 Jul. Report No.: 17-05225-EF-1.
16. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273-1282.
17. Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553-1560.
18. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92:1831-1841.
19. MDsave. Cardiac CT calcium scoring. www.mdsave.com/procedures/cardiac-ct-calcium-scoring/d785f4cf. Accessed Aprl 1, 2019.
20. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407-1416.
21. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213-S256.
22. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633.
23. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167-2192.
24. Newman CB, Preiss D, Tobert JA, et al; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38-e81.
25. Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sex Med Rev. 2018 Jan 27. pii: S2050-0521(18)30009-X. [Epub ahead of print]
26. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
27. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282.
28. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
29. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
30. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC Managed Markets Network. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed April 12, 2019.
31. Kaufman TM, Duell PB, Purnell JQ, et al. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499-501.
PRACTICE RECOMMENDATIONS
› Reduce the low-density lipoprotein cholesterol (LDL-C) level in patients with clinical atherosclerotic cardiovascular disease (ASCVD) using high-intensity statin therapy or maximally tolerated statin therapy. A
› Use an LDL-C threshold of 70 mg/dL to prompt consideration of adding nonstatin therapy in patients who have very high-risk ASCVD. A
› Start high-intensity statin therapy in patients who have primary hypercholesterolemia (LDL-C level ≥ 190 mg/dL) without calculating the 10-year ASCVD risk. A
› Begin moderate-intensity statin therapy in patients 40 to 75 years of age who have diabetes mellitus and an LDL-C level ≥ 70 mg/dL without calculating 10-year ASCVD risk. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
High ankle sprains: Easy to miss, so follow these tips
CASE
A 19-year-old college football player presents to your outpatient family practice clinic after suffering a right ankle injury during a football game over the weekend. He reports having his right ankle planted on the turf with his foot externally rotated when an opponent fell onto his posterior right lower extremity. He reports having felt immediate pain in the area of the right ankle and requiring assistance off of the field, as he had difficulty walking. The patient was taken to the emergency department where x-rays of the right foot and ankle did not show any signs of acute fracture or dislocation. The patient was diagnosed with a lateral ankle sprain, placed in a pneumatic ankle walking brace, and given crutches.
A high ankle sprain, or distal tibiofibular syndesmotic injury, can be an elusive diagnosis and is often mistaken for the more common lateral ankle sprain. Syndesmotic injuries have been documented to occur in approximately 1% to 10% of all ankle sprains.1-3 The highest number of these injuries occurs between the ages of 18 and 34 years, and they are more frequently seen in athletes than in nonathletes, particularly those who play collision sports, such as football, ice hockey, rugby, wrestling, and lacrosse.1-9 In one study by Hunt et al,10 syndesmotic injuries accounted for 24.6% of all ankle injuries in National Collegiate Athletic Association (NCAA) football players. Incidence continues to grow as recognition of high ankle sprains increases among medical professionals.1,5 Identification of syndesmotic injury is critical, as lack of detection can lead to extensive time missed from athletic participation and chronic ankle dysfunction, including pain and instability.2,4,6,11
Back to basics: A brief anatomy review
Stability in the distal tibiofibular joint is maintained by the syndesmotic ligaments, which include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the transverse ligament, and the interosseous ligament.3-6,8 This complex of ligaments stabilizes the fibula within the incisura of the tibia and maintains a stable ankle mortise.1,4,5,11 The deep portion of the deltoid ligament also adds stability to the syndesmosis and may be disrupted by a syndesmotic injury.2,5-7,11
Mechanisms of injury: From most common to less likely
The distal tibiofibular syndesmosis is disrupted when an injury forces apart the distal tibiofibular joint. The most commonly reported means of injury is external rotation with hyper-dorsiflexion of the ankle.1-3,5,6,11 With excessive external rotation of the forefoot, the talus is forced against the medial aspect of the fibula, resulting in separation of the distal tibia and fibula and injury to the syndesmotic ligaments.2,3,5,6 Injuries associated with external rotation are commonly seen in sports that immobilize the ankle within a rigid boot, such as skiing and ice hockey.1,2,5 Some authors have suggested that a planovalgus foot alignment may place athletes at inherent risk for an external rotation ankle injury.5,6
Syndesmotic injury may also occur with hyper-dorsiflexion, as the anterior, widest portion of the talus rotates into the ankle mortise, wedging the tibia and fibula apart.2,3,5 There have also been reports of syndesmotic injuries associated with internal rotation, plantar flexion, inversion, and eversion.3,5,11 Therefore, physicians should maintain a high index of suspicion for injury to the distal tibiofibular joint, regardless of the mechanism of injury.
Presentation and evaluation
Observation of the patient and visualization of the affected ankle can provide many clues. Many patients will have difficulty walking after suffering a syndesmotic injury and may require the use of an assistive device.5 The inability to bear weight after an ankle injury points to a more severe diagnosis, such as an ankle fracture or syndesmotic injury, as opposed to a simple lateral ankle sprain. Patients may report anterior ankle pain, a sensation of instability with weight bearing on the affected ankle, or have persistent symptoms despite a course of conservative treatment. Also, they can have a variable amount of edema and ecchymosis associated with their injury; a minimal extent of swelling or ecchymosis does not exclude syndesmotic injury.3
A large percentage of patients will present with a concomitant sprain of the lateral ligaments associated with lateral swelling and bruising. One study found that 91% of syndesmotic injuries involved at least 1 of the lateral collateral ligaments (anterior talofibular ligament [ATFL], calcaneofibular ligament [CFL], or posterior talofibular ligament [PTFL]).12 Patients may have pain or a sensation of instability when pushing off with the toes,5 and patients with syndesmotic injuries often have tenderness to palpation over the distal anterolateral ankle or syndesmotic ligaments.7
Continue to: A thorough examination...
A thorough examination of the ankle, including palpation of common fracture sites, is important. Employ the Ottawa Ankle Rules (see http://www.theottawarules.ca/ankle_rules) to investigate for: tenderness to palpation over the posterior 6 cm of the posterior aspects of the distal medial and lateral malleoli; tenderness over the navicular; tenderness over the base of the fifth metatarsal; and/or the inability to bear weight on the affected lower extremity immediately after injury or upon evaluation in the physician’s office. Any of these findings should raise concern for a possible fracture (see “Adult foot fractures: A guide”) and require an x-ray(s) for further evaluation.13
Perform range-of-motion and strength testing with regard to ankle dorsiflexion, plantar flexion, abduction, adduction, inversion, and eversion. Palpate the ATFL, CFL, and PTFL for tenderness, as these structures may be involved to varying degrees in lateral ankle sprains. An anterior drawer test (see https://www.youtube.com/watch?v=vAcBEYZKcto) may be positive with injury to the ATFL. This test is performed by stabilizing the distal tibia with one hand and using the other hand to grasp the posterior aspect of the calcaneus and apply an anterior force. The test is positive if the talus translates forward, which correlates with laxity or rupture of the ATFL.13 The examiner should also palpate the Achilles tendon, peroneal tendons just posterior to the lateral malleolus, and the tibialis posterior tendon just posterior to the medial malleolus to inspect for tenderness or defects that may be signs of injury to these tendons.
An associated Weber B or C fracture? Trauma causing ankle syndesmosis injuries may be associated with Weber B or Weber C distal fibula fractures.7 Weber B fractures occur in the distal fibula at the level of the ankle joint (see FIGURE 1). These types of fractures are typically associated with external rotation injuries and are usually not associated with disruption of the interosseous membrane.
Weber C fractures are distal fibular fractures occurring above the level of the ankle joint. These fractures are also typically associated with external ankle rotation injuries and include disruption of the syndesmosis and deltoid ligament.14
Also pay special attention to the proximal fibula, as syndesmotic injuries are commonly associated with a Maisonneuve fracture, which is a proximal fibula fracture associated with external rotation forces of the ankle (see FIGURE 1).1,2,4,11,14,15 Further workup should occur in any patient with the possibility of a Weber- or Maisonneuve-type fracture.
Continue to: Multiple tests...
Multiple tests are available to investigate the possibility of a syndesmotic injury and to assess return-to-sport readiness, including the External Rotation Test, the Squeeze Test, the Crossed-Leg Test, the Dorsiflexion Compression Test, the Cotton Test, the Stabilization Test, the Fibular Translation Test, and the Single Leg Hop Test (see TABLE1-3,5,6,16,17). The External Rotation Test is noted by some authors to have the highest interobserver reliability, and is our preferred test.2 The Squeeze Test also has moderate interobserver reliability.2 There is a significant degree of variation among the sensitivity and specificity of these diagnostic tests, and no single test is sufficiently reliable or accurate to diagnose a syndesmotic ankle injury. Therefore, it is recommended to use multiple physical exam maneuvers, the history and mechanism of injury, and findings on imaging studies in conjunction to make the diagnosis of a syndesmotic injury.1,16
Imaging: Which modes and when?
The initial workup should include ankle x-rays when evaluating for the possibility of a distal tibiofibular syndesmosis injury. While the Ottawa Ankle Rules are helpful in providing guidance with regard to x-rays, suspicion of a syndesmotic injury mandates x-rays to determine the stability of the joint and rule out fracture. The European Society of Sports Traumatology, Knee Surgery and Arthroscopy–European Foot and Ankle Associates (ESSKA-AFAS) recommend, at a minimum, obtaining anteroposterior (AP)- and mortise-view ankle x-rays to investigate the tibiofibular clear space, medial clear space, and tibiofibular overlap.7 Most physicians also include a lateral ankle x-ray.
If possible, images should be performed while the patient is bearing weight to further evaluate stability. Radiographic findings that support the diagnosis of syndesmotic injury include a tibiofibular clear space > 6 mm on AP view, medial clear space > 4 mm on mortise view, or tibiofibular overlap < 6 mm on AP view or < 1 mm on mortise view (see FIGURES 2 and 3).1,3,5,8 Additionally, if you suspect a proximal fibular fracture, obtain an x-ray series of the proximal tibia and fibula to investigate the possibility of a Maisonneuve injury.1,2,4,11
If you continue to suspect a syndesmotic injury despite normal x-rays, obtain stress x-rays, in addition to the AP and mortise views, to ensure stability. These x-rays include AP and mortise ankle views with manual external rotation of the ankle joint, which may demonstrate abnormalities not seen on standard x-rays. Bilateral imaging can also be useful to further assess when mild abnormalities vs symmetric anatomic variants are in question.1,7
If there is concern for an unstable injury, refer the patient to a foot and ankle surgeon, who may pursue magnetic resonance imaging (MRI) or standing computed tomography (CT).1,2,5,7 MRI is the recommended choice for further evaluation of a syndesmotic injury, as it is proven to be accurate in evaluating the integrity of the syndesmotic ligaments (see FIGURES 4 and 5).18 MRI has demonstrated 100% sensitivity for detecting AITFL and PITFL injuries, as well as 93% and 100% specificity for AITFL and PITFL tears, respectively.8 A weight-bearing CT scan, particularly axial views, can also be a useful adjunct, as it is more sensitive than standard x-rays for assessing for mild diastasis. Although CT can provide an assessment of bony structures, it is not able to evaluate soft tissue structures, limiting its utility in evaluation of syndesmotic injuries.1,7
Continue to: Although not the standard of care...
Although not the standard of care, ultrasonography (US) is gaining traction as a means of investigating the integrity of the syndesmotic ligaments. US is inexpensive, readily available in many clinics, allows for dynamic testing, and avoids radiation exposure.7 However, US requires a skilled sonographer with experience in the ankle joint for an accurate diagnosis. If the workup with advanced imaging is inconclusive, but a high degree of suspicion remains for an unstable syndesmotic injury, consider arthroscopy to directly visualize and assess the syndesmotic structures.1,2,5,7,8
Grading the severity of the injury and pursuing appropriate Tx
Typically, the severity of a syndesmotic injury is classified as fitting into 1 of 3 categories: Grade I and II injuries are the most common, each accounting for 40% of syndesmotic injuries, while 20% of high ankle sprains are classified as Grade III.12
A Grade I injury consists of a stable syndesmotic joint without abnormal radiographic findings. There may be associated tenderness to palpation over the distal tibiofibular joint, and provocative testing may be subtle or normal. These injuries are often minor and able to be treated conservatively.
A Grade II injury is associated with a partial syndesmotic disruption, typically with partial tearing of the AITFL and interosseous ligament. These injuries may be stable or accompanied by mild instability, and provocative testing is usually positive. X-rays are typically normal with Grade II injuries, but may display subtle radiographic findings suggestive of a syndesmotic injury. Treatment of Grade II injuries is somewhat controversial and should be an individualized decision based upon the patient’s age, activity level, clinical exam, and imaging findings. Therefore, treatment of Grade II syndesmotic injuries may include a trial of conservative management or surgical intervention.
A Grade III injury represents inherent instability of the distal tibiofibular joint with complete disruption of all syndesmotic ligaments, with or without involvement of the deltoid ligament. X-rays will be positive in Grade III syndesmotic injuries because of the complete disruption of syndesmotic ligaments. All Grade III injuries require surgical intervention with a syndesmotic screw or other stabilization procedure.1,6-8,15
Continue to: A 3-stage rehabilitation protocol
A 3-stage rehabilitation protocol
When conservative management is deemed appropriate for a stable syndesmotic sprain, a 3-stage rehabilitation protocol is typically utilized.
The acute phase focuses on protection, pain control, and decreasing inflammation. The patient’s ankle is often immobilized in a cast or controlled ankle movement (CAM) boot. The patient is typically allowed to bear weight in the immobilizer during this phase as long as he/she is pain-free. If pain is present with weight bearing despite immobilization, non-weight bearing is recommended. The patient is instructed to elevate the lower extremity, take anti-inflammatory medication, and ice the affected ankle. Additionally, physical therapy modalities may be utilized to help with edema and pain. Joint immobilization is typically employed for 1 to 3 weeks post-injury. In the acute phase, the patient may also work with a physical therapist or athletic trainer on passive range of motion (ROM), progressing to active ROM as tolerated.1,5,7,8,19
The patient can transition from the CAM boot to a lace-up ankle brace when he/she is able to bear full weight and can navigate stairs without pain, which typically occurs around 3 to 6 weeks post-injury.1,5,7 A pneumatic walking brace may also be used as a transition device to provide added stabilization.
In the sub-acute phase, rehabilitation may progress to increase ankle mobility, strengthening, neuromuscular control, and to allow the patient to perform activities of daily living.5-7
The advanced training phase includes continued neuromuscular control, increased strengthening, plyometrics, agility, and sports-specific drills.5 Athletes are allowed to return to full participation when they have regained full ROM, are able to perform sport-specific agility drills without pain or instability, and have near-normal strength.5-7 Some authors also advocate that a Single Leg Hop Test should be included in the physical exam, and that it should be pain free prior to allowing an athlete to return to competition.20 Both progression in physical rehabilitation and return to sport should be individualized based upon injury severity, patient functionality, and physical exam findings.
Continue to: Outcomes forecast
Outcomes forecast: Variable
The resolution of symptoms and return to competition after a syndesmotic injury is variable. In one cohort study of cadets (N = 614) at the United States Military Academy, the average time lost from a syndesmotic ankle sprain was 9.82 days (range 3-21 days).9 In a retrospective review of National Hockey League players, average time to return to competition after a syndesmotic ankle injury sprain (n = 14) was 45 days (range 6-137 days) vs 1.4 days (range 0-6 days) for lateral ankle sprains (n = 5).21 In another study, National Football League players with syndesmotic sprains (n = 36) had a mean time loss from play of 15.4 days (± 11.1 days) vs 6.5 days (± 6.5 days) of time loss from play in those with lateral ankle sprains (n = 53).22
Although there is a fair amount of variability among studies, most authors agree that the average athlete can expect to return to sport 4 to 8 weeks post-injury with conservative management.19 At least 1 study suggests that the average time to return to sport in patients with Grade III syndesmotic injuries who undergo surgical treatment with a syndesmotic screw is 41 days (range 32-48).23 The differences in return to sport may be related to severity of injury and/or type of activity.
Persistent symptoms are relatively common after conservative management of syndesmotic injuries. One case series found that 36% of patients treated conservatively had complaints of persistent mild-to-moderate ankle stiffness, 23% had mild-to-moderate pain, and 18% had mild-to-moderate ankle swelling.24 Despite these symptoms, 86% of the patients rated their ankle function as good after conservative treatment.24 In patients with persistent symptoms, other possible etiologies should be considered including neurologic injury, complex regional pain syndrome, osteochondral defect, loose body, or other sources that may be contributing to pain, swelling, or delayed recovery.
At least 1 randomized controlled trial (RCT) investigated the utility of platelet rich plasma (PRP) injections around the injured AITFL in the setting of an acute syndesmotic injury. The study showed promising results, including quicker return to play, restabilization of the syndesmotic joint, and less residual pain;25 however, the study population was relatively small (N = 16), and the authors believed that more research is required on the benefits of PRP therapy in syndesmotic injuries before recommendations can be made.
An ounce of preventionis worth a pound of cure
Although injury is not always avoidable, there are measures that can help prevent ankle sprains and facilitate return to play after injury. As previously mentioned, athletes should be able to demonstrate the ability to run, cut, jump, and perform sport-specific activities without limitations prior to being allowed to return to sport after injury.5-7,26 Additionally, issues with biomechanics and functional deficits should be analyzed and addressed. By targeting specific strength deficits, focusing on proprioceptive awareness, and working on neuromuscular control, injury rates and recurrent injuries can be minimized. One RCT showed a 35% reduction in the recurrence rate of lateral ankle sprains with the use of an unsupervised home-based proprioceptive training program.27
Continue to: Strength training...
Strength training, proprioceptive and neuromuscular control activities, and low-risk activities such as jogging, biking, and swimming do not necessarily require the use of prophylactic bracing. However, because syndesmotic injuries are associated with recurrent ankle injuries, prophylactic bracing should be used during high-risk activities that involve agility maneuvers and jumping. Substantial evidence demonstrates that the use of ankle taping or ankle bracing decreases the incidence of ankle injuries, particularly in those who have had previous ankle injuries.26 In one study (N = 450), only 3% of athletes with a history of prior ankle injuries suffered a recurrent ankle sprain when using an ankle orthosis compared with a 17% injury rate in the control group.28
More recently, 2 separate studies by McGuine et al demonstrated that the use of lace-up ankle braces led to a reduction in the incidence of acute ankle injuries by 61% among 2081 high-school football players, and resulted in a significant reduction in acute ankle injuries in a study of 1460 male and female high-school basketball players, compared with the control groups.29,30
CASE
Ten days after injuring himself, the patient returns for a follow-up exam. Despite using the walking brace and crutches, he is still having significant difficulty bearing weight. He reports a sensation of instability in the right ankle. On exam, you note visible edema of the right ankle and ecchymosis over the lateral ankle, as well as moderate tenderness to palpation over the area of the ATFL and deltoid ligament. Tenderness over the medial malleolus, lateral malleolus, fifth metatarsal, and navicular is absent. Pain is reproducible with external rotation, and a Squeeze Test is positive. There is no tenderness over the proximal tibia or fibula. The patient is neurovascularly intact.
You order stress x-rays, which show widening of the medial clear space. The patient is placed in a CAM boot, instructed to continue non–weight-bearing on the ankle, and referred to a local foot and ankle surgeon for consideration of surgical fixation.
CORRESPONDENCE
John T. Nickless, MD, Division of Primary Care Sports Medicine, Department of Orthopedic Surgery, Rush University Medical Center, 1611 W. Harrison Street, Suite 200, Chicago, IL, 60612; [email protected].
1. Switaj PJ, Mendoza M, Kadakia AR. Acute and chronic Injuries to the syndesmosis. Clin Sports Med. 2015;34:643-677.
2. Scheyerer MJ, Helfet DL, Wirth S, et al. Diagnostics in suspicion of ankle syndesmotic injury. Am J Orthop. 2011;40:192-197.
3. Smith KM, Kovacich-Smith KJ, Witt M. Evaluation and management of high ankle sprains. Clin Podiatr Med Surg. 2001;18:443-456.
4. Reissig J, Bitterman A, Lee S. Common foot and ankle injuries: what not to miss and how best to manage. J Am Osteopath Assoc. 2017;117:98-104.
5. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2:460-470.
6. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35:1197-1207.
7. Vopat ML, Vopat BG, Lubberts B, et al. Current trends in the diagnosis and management of syndesmotic injury. Curr Rev Musculoskelet Med. 2017;10:94-103.
8. Mak MF, Gartner L, Pearce CJ. Management of syndesmosis injuries in the elite athlete. Foot Ankle Clin. 2013;18:195-214.
9. Waterman BR, Belmont PJ, Cameron KL, et al. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38:797-803.
10. Hunt KJ, George E, Harris AHS, et al. Epidemiology of syndesmosis injuries in intercollegiate football: incidence and risk factors from National Collegiate Athletic Association injury surveillance system data from 2004-2005 to 2008-2009. Clin J Sport Med. 2013;23:278-282.
11. Schnetzke M, Vetter SY, Beisemann N, et al. Management of syndesmotic injuries: what is the evidence? World J Orthop. 2016;7:718-725.
12. de César PC, Ávila EM, de Abreu MR. Comparison of magnetic resonance imaging to physical examination for syndesmotic injury after lateral ankle sprain. Foot Ankle Int. 2011;32:1110-1114.
13. Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720.
14. Porter D, Rund A, Barnes AF, et al. Optimal management of ankle syndesmosis injuries. Open Access J Sports Med. 2014;5:173-182.
15. Press CM, Gupta A, Hutchinson MR. Management of ankle syndesmosis injuries in the athlete. Curr Sports Med Rep. 2009;8:228-233.
16. Sman AD, Hiller CE, Rae K, et al. Diagnostic accuracy of clinical tests for ankle syndesmosis injury. Br J Sports Med. 2015;49:323-329.
17. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14:232-236.
18. Hunt KJ. Syndesmosis injuries. Curr Rev Musculoskelet Med. 2013;6:304-312.
19. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
20. Miller BS, Downie BK, Johnson PD, et al. Time to return to play after high ankle sprains in collegiate football players: a prediction model. Sports Health. 2012;4:504-509.
21. Wright RW, Barile RJ, Surprenant DA, et al. Ankle syndesmosis sprains in National Hockey League players. Am J Sports Med. 2016;32:1941-1945.
22. Osbahr DC, Drakos MC, O’Loughlin PF, et al. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378-e1384.
23. Taylor DC, Tenuta JJ, Uhorchak JM, et al. Aggressive surgical treatment and early return to sports in athletes with grade III syndesmosis sprains. Am J Sports Med. 2007;35:1133-1138.
24. Taylor DC, Englehardt DL, Bassett FH. Syndesmosis sprains of the ankle: the influence of heterotopic ossification. Am J Sports Med. 1992;20:146-150.
25. Laver L, Carmont MR, McConkey MO, et al. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: a randomized control trial. Knee Surg Sports Traumatol Arthrosc. 2014;23:3383-3392.
26. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
27. Hupperets MDW, Verhagen EALM, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684.
28. Tropp H, Askling C, Gillquist J. Prevention of ankle sprains. Am J Sports Med. 1985;13:259-262.
29. McGuine TA, Brooks A, Hetzel S. The effect of lace-up ankle braces on injury rates in high school basketball players. Am J Sports Med. 2011;39:1840-1848.
30. McGuine TA, Hetzel S, Wilson J, et al. The effect of lace-up ankle braces on injury rates in high school football players. Am J Sports Med. 2012;40:49-57.
CASE
A 19-year-old college football player presents to your outpatient family practice clinic after suffering a right ankle injury during a football game over the weekend. He reports having his right ankle planted on the turf with his foot externally rotated when an opponent fell onto his posterior right lower extremity. He reports having felt immediate pain in the area of the right ankle and requiring assistance off of the field, as he had difficulty walking. The patient was taken to the emergency department where x-rays of the right foot and ankle did not show any signs of acute fracture or dislocation. The patient was diagnosed with a lateral ankle sprain, placed in a pneumatic ankle walking brace, and given crutches.
A high ankle sprain, or distal tibiofibular syndesmotic injury, can be an elusive diagnosis and is often mistaken for the more common lateral ankle sprain. Syndesmotic injuries have been documented to occur in approximately 1% to 10% of all ankle sprains.1-3 The highest number of these injuries occurs between the ages of 18 and 34 years, and they are more frequently seen in athletes than in nonathletes, particularly those who play collision sports, such as football, ice hockey, rugby, wrestling, and lacrosse.1-9 In one study by Hunt et al,10 syndesmotic injuries accounted for 24.6% of all ankle injuries in National Collegiate Athletic Association (NCAA) football players. Incidence continues to grow as recognition of high ankle sprains increases among medical professionals.1,5 Identification of syndesmotic injury is critical, as lack of detection can lead to extensive time missed from athletic participation and chronic ankle dysfunction, including pain and instability.2,4,6,11
Back to basics: A brief anatomy review
Stability in the distal tibiofibular joint is maintained by the syndesmotic ligaments, which include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the transverse ligament, and the interosseous ligament.3-6,8 This complex of ligaments stabilizes the fibula within the incisura of the tibia and maintains a stable ankle mortise.1,4,5,11 The deep portion of the deltoid ligament also adds stability to the syndesmosis and may be disrupted by a syndesmotic injury.2,5-7,11
Mechanisms of injury: From most common to less likely
The distal tibiofibular syndesmosis is disrupted when an injury forces apart the distal tibiofibular joint. The most commonly reported means of injury is external rotation with hyper-dorsiflexion of the ankle.1-3,5,6,11 With excessive external rotation of the forefoot, the talus is forced against the medial aspect of the fibula, resulting in separation of the distal tibia and fibula and injury to the syndesmotic ligaments.2,3,5,6 Injuries associated with external rotation are commonly seen in sports that immobilize the ankle within a rigid boot, such as skiing and ice hockey.1,2,5 Some authors have suggested that a planovalgus foot alignment may place athletes at inherent risk for an external rotation ankle injury.5,6
Syndesmotic injury may also occur with hyper-dorsiflexion, as the anterior, widest portion of the talus rotates into the ankle mortise, wedging the tibia and fibula apart.2,3,5 There have also been reports of syndesmotic injuries associated with internal rotation, plantar flexion, inversion, and eversion.3,5,11 Therefore, physicians should maintain a high index of suspicion for injury to the distal tibiofibular joint, regardless of the mechanism of injury.
Presentation and evaluation
Observation of the patient and visualization of the affected ankle can provide many clues. Many patients will have difficulty walking after suffering a syndesmotic injury and may require the use of an assistive device.5 The inability to bear weight after an ankle injury points to a more severe diagnosis, such as an ankle fracture or syndesmotic injury, as opposed to a simple lateral ankle sprain. Patients may report anterior ankle pain, a sensation of instability with weight bearing on the affected ankle, or have persistent symptoms despite a course of conservative treatment. Also, they can have a variable amount of edema and ecchymosis associated with their injury; a minimal extent of swelling or ecchymosis does not exclude syndesmotic injury.3
A large percentage of patients will present with a concomitant sprain of the lateral ligaments associated with lateral swelling and bruising. One study found that 91% of syndesmotic injuries involved at least 1 of the lateral collateral ligaments (anterior talofibular ligament [ATFL], calcaneofibular ligament [CFL], or posterior talofibular ligament [PTFL]).12 Patients may have pain or a sensation of instability when pushing off with the toes,5 and patients with syndesmotic injuries often have tenderness to palpation over the distal anterolateral ankle or syndesmotic ligaments.7
Continue to: A thorough examination...
A thorough examination of the ankle, including palpation of common fracture sites, is important. Employ the Ottawa Ankle Rules (see http://www.theottawarules.ca/ankle_rules) to investigate for: tenderness to palpation over the posterior 6 cm of the posterior aspects of the distal medial and lateral malleoli; tenderness over the navicular; tenderness over the base of the fifth metatarsal; and/or the inability to bear weight on the affected lower extremity immediately after injury or upon evaluation in the physician’s office. Any of these findings should raise concern for a possible fracture (see “Adult foot fractures: A guide”) and require an x-ray(s) for further evaluation.13
Perform range-of-motion and strength testing with regard to ankle dorsiflexion, plantar flexion, abduction, adduction, inversion, and eversion. Palpate the ATFL, CFL, and PTFL for tenderness, as these structures may be involved to varying degrees in lateral ankle sprains. An anterior drawer test (see https://www.youtube.com/watch?v=vAcBEYZKcto) may be positive with injury to the ATFL. This test is performed by stabilizing the distal tibia with one hand and using the other hand to grasp the posterior aspect of the calcaneus and apply an anterior force. The test is positive if the talus translates forward, which correlates with laxity or rupture of the ATFL.13 The examiner should also palpate the Achilles tendon, peroneal tendons just posterior to the lateral malleolus, and the tibialis posterior tendon just posterior to the medial malleolus to inspect for tenderness or defects that may be signs of injury to these tendons.
An associated Weber B or C fracture? Trauma causing ankle syndesmosis injuries may be associated with Weber B or Weber C distal fibula fractures.7 Weber B fractures occur in the distal fibula at the level of the ankle joint (see FIGURE 1). These types of fractures are typically associated with external rotation injuries and are usually not associated with disruption of the interosseous membrane.
Weber C fractures are distal fibular fractures occurring above the level of the ankle joint. These fractures are also typically associated with external ankle rotation injuries and include disruption of the syndesmosis and deltoid ligament.14
Also pay special attention to the proximal fibula, as syndesmotic injuries are commonly associated with a Maisonneuve fracture, which is a proximal fibula fracture associated with external rotation forces of the ankle (see FIGURE 1).1,2,4,11,14,15 Further workup should occur in any patient with the possibility of a Weber- or Maisonneuve-type fracture.
Continue to: Multiple tests...
Multiple tests are available to investigate the possibility of a syndesmotic injury and to assess return-to-sport readiness, including the External Rotation Test, the Squeeze Test, the Crossed-Leg Test, the Dorsiflexion Compression Test, the Cotton Test, the Stabilization Test, the Fibular Translation Test, and the Single Leg Hop Test (see TABLE1-3,5,6,16,17). The External Rotation Test is noted by some authors to have the highest interobserver reliability, and is our preferred test.2 The Squeeze Test also has moderate interobserver reliability.2 There is a significant degree of variation among the sensitivity and specificity of these diagnostic tests, and no single test is sufficiently reliable or accurate to diagnose a syndesmotic ankle injury. Therefore, it is recommended to use multiple physical exam maneuvers, the history and mechanism of injury, and findings on imaging studies in conjunction to make the diagnosis of a syndesmotic injury.1,16
Imaging: Which modes and when?
The initial workup should include ankle x-rays when evaluating for the possibility of a distal tibiofibular syndesmosis injury. While the Ottawa Ankle Rules are helpful in providing guidance with regard to x-rays, suspicion of a syndesmotic injury mandates x-rays to determine the stability of the joint and rule out fracture. The European Society of Sports Traumatology, Knee Surgery and Arthroscopy–European Foot and Ankle Associates (ESSKA-AFAS) recommend, at a minimum, obtaining anteroposterior (AP)- and mortise-view ankle x-rays to investigate the tibiofibular clear space, medial clear space, and tibiofibular overlap.7 Most physicians also include a lateral ankle x-ray.
If possible, images should be performed while the patient is bearing weight to further evaluate stability. Radiographic findings that support the diagnosis of syndesmotic injury include a tibiofibular clear space > 6 mm on AP view, medial clear space > 4 mm on mortise view, or tibiofibular overlap < 6 mm on AP view or < 1 mm on mortise view (see FIGURES 2 and 3).1,3,5,8 Additionally, if you suspect a proximal fibular fracture, obtain an x-ray series of the proximal tibia and fibula to investigate the possibility of a Maisonneuve injury.1,2,4,11
If you continue to suspect a syndesmotic injury despite normal x-rays, obtain stress x-rays, in addition to the AP and mortise views, to ensure stability. These x-rays include AP and mortise ankle views with manual external rotation of the ankle joint, which may demonstrate abnormalities not seen on standard x-rays. Bilateral imaging can also be useful to further assess when mild abnormalities vs symmetric anatomic variants are in question.1,7
If there is concern for an unstable injury, refer the patient to a foot and ankle surgeon, who may pursue magnetic resonance imaging (MRI) or standing computed tomography (CT).1,2,5,7 MRI is the recommended choice for further evaluation of a syndesmotic injury, as it is proven to be accurate in evaluating the integrity of the syndesmotic ligaments (see FIGURES 4 and 5).18 MRI has demonstrated 100% sensitivity for detecting AITFL and PITFL injuries, as well as 93% and 100% specificity for AITFL and PITFL tears, respectively.8 A weight-bearing CT scan, particularly axial views, can also be a useful adjunct, as it is more sensitive than standard x-rays for assessing for mild diastasis. Although CT can provide an assessment of bony structures, it is not able to evaluate soft tissue structures, limiting its utility in evaluation of syndesmotic injuries.1,7
Continue to: Although not the standard of care...
Although not the standard of care, ultrasonography (US) is gaining traction as a means of investigating the integrity of the syndesmotic ligaments. US is inexpensive, readily available in many clinics, allows for dynamic testing, and avoids radiation exposure.7 However, US requires a skilled sonographer with experience in the ankle joint for an accurate diagnosis. If the workup with advanced imaging is inconclusive, but a high degree of suspicion remains for an unstable syndesmotic injury, consider arthroscopy to directly visualize and assess the syndesmotic structures.1,2,5,7,8
Grading the severity of the injury and pursuing appropriate Tx
Typically, the severity of a syndesmotic injury is classified as fitting into 1 of 3 categories: Grade I and II injuries are the most common, each accounting for 40% of syndesmotic injuries, while 20% of high ankle sprains are classified as Grade III.12
A Grade I injury consists of a stable syndesmotic joint without abnormal radiographic findings. There may be associated tenderness to palpation over the distal tibiofibular joint, and provocative testing may be subtle or normal. These injuries are often minor and able to be treated conservatively.
A Grade II injury is associated with a partial syndesmotic disruption, typically with partial tearing of the AITFL and interosseous ligament. These injuries may be stable or accompanied by mild instability, and provocative testing is usually positive. X-rays are typically normal with Grade II injuries, but may display subtle radiographic findings suggestive of a syndesmotic injury. Treatment of Grade II injuries is somewhat controversial and should be an individualized decision based upon the patient’s age, activity level, clinical exam, and imaging findings. Therefore, treatment of Grade II syndesmotic injuries may include a trial of conservative management or surgical intervention.
A Grade III injury represents inherent instability of the distal tibiofibular joint with complete disruption of all syndesmotic ligaments, with or without involvement of the deltoid ligament. X-rays will be positive in Grade III syndesmotic injuries because of the complete disruption of syndesmotic ligaments. All Grade III injuries require surgical intervention with a syndesmotic screw or other stabilization procedure.1,6-8,15
Continue to: A 3-stage rehabilitation protocol
A 3-stage rehabilitation protocol
When conservative management is deemed appropriate for a stable syndesmotic sprain, a 3-stage rehabilitation protocol is typically utilized.
The acute phase focuses on protection, pain control, and decreasing inflammation. The patient’s ankle is often immobilized in a cast or controlled ankle movement (CAM) boot. The patient is typically allowed to bear weight in the immobilizer during this phase as long as he/she is pain-free. If pain is present with weight bearing despite immobilization, non-weight bearing is recommended. The patient is instructed to elevate the lower extremity, take anti-inflammatory medication, and ice the affected ankle. Additionally, physical therapy modalities may be utilized to help with edema and pain. Joint immobilization is typically employed for 1 to 3 weeks post-injury. In the acute phase, the patient may also work with a physical therapist or athletic trainer on passive range of motion (ROM), progressing to active ROM as tolerated.1,5,7,8,19
The patient can transition from the CAM boot to a lace-up ankle brace when he/she is able to bear full weight and can navigate stairs without pain, which typically occurs around 3 to 6 weeks post-injury.1,5,7 A pneumatic walking brace may also be used as a transition device to provide added stabilization.
In the sub-acute phase, rehabilitation may progress to increase ankle mobility, strengthening, neuromuscular control, and to allow the patient to perform activities of daily living.5-7
The advanced training phase includes continued neuromuscular control, increased strengthening, plyometrics, agility, and sports-specific drills.5 Athletes are allowed to return to full participation when they have regained full ROM, are able to perform sport-specific agility drills without pain or instability, and have near-normal strength.5-7 Some authors also advocate that a Single Leg Hop Test should be included in the physical exam, and that it should be pain free prior to allowing an athlete to return to competition.20 Both progression in physical rehabilitation and return to sport should be individualized based upon injury severity, patient functionality, and physical exam findings.
Continue to: Outcomes forecast
Outcomes forecast: Variable
The resolution of symptoms and return to competition after a syndesmotic injury is variable. In one cohort study of cadets (N = 614) at the United States Military Academy, the average time lost from a syndesmotic ankle sprain was 9.82 days (range 3-21 days).9 In a retrospective review of National Hockey League players, average time to return to competition after a syndesmotic ankle injury sprain (n = 14) was 45 days (range 6-137 days) vs 1.4 days (range 0-6 days) for lateral ankle sprains (n = 5).21 In another study, National Football League players with syndesmotic sprains (n = 36) had a mean time loss from play of 15.4 days (± 11.1 days) vs 6.5 days (± 6.5 days) of time loss from play in those with lateral ankle sprains (n = 53).22
Although there is a fair amount of variability among studies, most authors agree that the average athlete can expect to return to sport 4 to 8 weeks post-injury with conservative management.19 At least 1 study suggests that the average time to return to sport in patients with Grade III syndesmotic injuries who undergo surgical treatment with a syndesmotic screw is 41 days (range 32-48).23 The differences in return to sport may be related to severity of injury and/or type of activity.
Persistent symptoms are relatively common after conservative management of syndesmotic injuries. One case series found that 36% of patients treated conservatively had complaints of persistent mild-to-moderate ankle stiffness, 23% had mild-to-moderate pain, and 18% had mild-to-moderate ankle swelling.24 Despite these symptoms, 86% of the patients rated their ankle function as good after conservative treatment.24 In patients with persistent symptoms, other possible etiologies should be considered including neurologic injury, complex regional pain syndrome, osteochondral defect, loose body, or other sources that may be contributing to pain, swelling, or delayed recovery.
At least 1 randomized controlled trial (RCT) investigated the utility of platelet rich plasma (PRP) injections around the injured AITFL in the setting of an acute syndesmotic injury. The study showed promising results, including quicker return to play, restabilization of the syndesmotic joint, and less residual pain;25 however, the study population was relatively small (N = 16), and the authors believed that more research is required on the benefits of PRP therapy in syndesmotic injuries before recommendations can be made.
An ounce of preventionis worth a pound of cure
Although injury is not always avoidable, there are measures that can help prevent ankle sprains and facilitate return to play after injury. As previously mentioned, athletes should be able to demonstrate the ability to run, cut, jump, and perform sport-specific activities without limitations prior to being allowed to return to sport after injury.5-7,26 Additionally, issues with biomechanics and functional deficits should be analyzed and addressed. By targeting specific strength deficits, focusing on proprioceptive awareness, and working on neuromuscular control, injury rates and recurrent injuries can be minimized. One RCT showed a 35% reduction in the recurrence rate of lateral ankle sprains with the use of an unsupervised home-based proprioceptive training program.27
Continue to: Strength training...
Strength training, proprioceptive and neuromuscular control activities, and low-risk activities such as jogging, biking, and swimming do not necessarily require the use of prophylactic bracing. However, because syndesmotic injuries are associated with recurrent ankle injuries, prophylactic bracing should be used during high-risk activities that involve agility maneuvers and jumping. Substantial evidence demonstrates that the use of ankle taping or ankle bracing decreases the incidence of ankle injuries, particularly in those who have had previous ankle injuries.26 In one study (N = 450), only 3% of athletes with a history of prior ankle injuries suffered a recurrent ankle sprain when using an ankle orthosis compared with a 17% injury rate in the control group.28
More recently, 2 separate studies by McGuine et al demonstrated that the use of lace-up ankle braces led to a reduction in the incidence of acute ankle injuries by 61% among 2081 high-school football players, and resulted in a significant reduction in acute ankle injuries in a study of 1460 male and female high-school basketball players, compared with the control groups.29,30
CASE
Ten days after injuring himself, the patient returns for a follow-up exam. Despite using the walking brace and crutches, he is still having significant difficulty bearing weight. He reports a sensation of instability in the right ankle. On exam, you note visible edema of the right ankle and ecchymosis over the lateral ankle, as well as moderate tenderness to palpation over the area of the ATFL and deltoid ligament. Tenderness over the medial malleolus, lateral malleolus, fifth metatarsal, and navicular is absent. Pain is reproducible with external rotation, and a Squeeze Test is positive. There is no tenderness over the proximal tibia or fibula. The patient is neurovascularly intact.
You order stress x-rays, which show widening of the medial clear space. The patient is placed in a CAM boot, instructed to continue non–weight-bearing on the ankle, and referred to a local foot and ankle surgeon for consideration of surgical fixation.
CORRESPONDENCE
John T. Nickless, MD, Division of Primary Care Sports Medicine, Department of Orthopedic Surgery, Rush University Medical Center, 1611 W. Harrison Street, Suite 200, Chicago, IL, 60612; [email protected].
CASE
A 19-year-old college football player presents to your outpatient family practice clinic after suffering a right ankle injury during a football game over the weekend. He reports having his right ankle planted on the turf with his foot externally rotated when an opponent fell onto his posterior right lower extremity. He reports having felt immediate pain in the area of the right ankle and requiring assistance off of the field, as he had difficulty walking. The patient was taken to the emergency department where x-rays of the right foot and ankle did not show any signs of acute fracture or dislocation. The patient was diagnosed with a lateral ankle sprain, placed in a pneumatic ankle walking brace, and given crutches.
A high ankle sprain, or distal tibiofibular syndesmotic injury, can be an elusive diagnosis and is often mistaken for the more common lateral ankle sprain. Syndesmotic injuries have been documented to occur in approximately 1% to 10% of all ankle sprains.1-3 The highest number of these injuries occurs between the ages of 18 and 34 years, and they are more frequently seen in athletes than in nonathletes, particularly those who play collision sports, such as football, ice hockey, rugby, wrestling, and lacrosse.1-9 In one study by Hunt et al,10 syndesmotic injuries accounted for 24.6% of all ankle injuries in National Collegiate Athletic Association (NCAA) football players. Incidence continues to grow as recognition of high ankle sprains increases among medical professionals.1,5 Identification of syndesmotic injury is critical, as lack of detection can lead to extensive time missed from athletic participation and chronic ankle dysfunction, including pain and instability.2,4,6,11
Back to basics: A brief anatomy review
Stability in the distal tibiofibular joint is maintained by the syndesmotic ligaments, which include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the transverse ligament, and the interosseous ligament.3-6,8 This complex of ligaments stabilizes the fibula within the incisura of the tibia and maintains a stable ankle mortise.1,4,5,11 The deep portion of the deltoid ligament also adds stability to the syndesmosis and may be disrupted by a syndesmotic injury.2,5-7,11
Mechanisms of injury: From most common to less likely
The distal tibiofibular syndesmosis is disrupted when an injury forces apart the distal tibiofibular joint. The most commonly reported means of injury is external rotation with hyper-dorsiflexion of the ankle.1-3,5,6,11 With excessive external rotation of the forefoot, the talus is forced against the medial aspect of the fibula, resulting in separation of the distal tibia and fibula and injury to the syndesmotic ligaments.2,3,5,6 Injuries associated with external rotation are commonly seen in sports that immobilize the ankle within a rigid boot, such as skiing and ice hockey.1,2,5 Some authors have suggested that a planovalgus foot alignment may place athletes at inherent risk for an external rotation ankle injury.5,6
Syndesmotic injury may also occur with hyper-dorsiflexion, as the anterior, widest portion of the talus rotates into the ankle mortise, wedging the tibia and fibula apart.2,3,5 There have also been reports of syndesmotic injuries associated with internal rotation, plantar flexion, inversion, and eversion.3,5,11 Therefore, physicians should maintain a high index of suspicion for injury to the distal tibiofibular joint, regardless of the mechanism of injury.
Presentation and evaluation
Observation of the patient and visualization of the affected ankle can provide many clues. Many patients will have difficulty walking after suffering a syndesmotic injury and may require the use of an assistive device.5 The inability to bear weight after an ankle injury points to a more severe diagnosis, such as an ankle fracture or syndesmotic injury, as opposed to a simple lateral ankle sprain. Patients may report anterior ankle pain, a sensation of instability with weight bearing on the affected ankle, or have persistent symptoms despite a course of conservative treatment. Also, they can have a variable amount of edema and ecchymosis associated with their injury; a minimal extent of swelling or ecchymosis does not exclude syndesmotic injury.3
A large percentage of patients will present with a concomitant sprain of the lateral ligaments associated with lateral swelling and bruising. One study found that 91% of syndesmotic injuries involved at least 1 of the lateral collateral ligaments (anterior talofibular ligament [ATFL], calcaneofibular ligament [CFL], or posterior talofibular ligament [PTFL]).12 Patients may have pain or a sensation of instability when pushing off with the toes,5 and patients with syndesmotic injuries often have tenderness to palpation over the distal anterolateral ankle or syndesmotic ligaments.7
Continue to: A thorough examination...
A thorough examination of the ankle, including palpation of common fracture sites, is important. Employ the Ottawa Ankle Rules (see http://www.theottawarules.ca/ankle_rules) to investigate for: tenderness to palpation over the posterior 6 cm of the posterior aspects of the distal medial and lateral malleoli; tenderness over the navicular; tenderness over the base of the fifth metatarsal; and/or the inability to bear weight on the affected lower extremity immediately after injury or upon evaluation in the physician’s office. Any of these findings should raise concern for a possible fracture (see “Adult foot fractures: A guide”) and require an x-ray(s) for further evaluation.13
Perform range-of-motion and strength testing with regard to ankle dorsiflexion, plantar flexion, abduction, adduction, inversion, and eversion. Palpate the ATFL, CFL, and PTFL for tenderness, as these structures may be involved to varying degrees in lateral ankle sprains. An anterior drawer test (see https://www.youtube.com/watch?v=vAcBEYZKcto) may be positive with injury to the ATFL. This test is performed by stabilizing the distal tibia with one hand and using the other hand to grasp the posterior aspect of the calcaneus and apply an anterior force. The test is positive if the talus translates forward, which correlates with laxity or rupture of the ATFL.13 The examiner should also palpate the Achilles tendon, peroneal tendons just posterior to the lateral malleolus, and the tibialis posterior tendon just posterior to the medial malleolus to inspect for tenderness or defects that may be signs of injury to these tendons.
An associated Weber B or C fracture? Trauma causing ankle syndesmosis injuries may be associated with Weber B or Weber C distal fibula fractures.7 Weber B fractures occur in the distal fibula at the level of the ankle joint (see FIGURE 1). These types of fractures are typically associated with external rotation injuries and are usually not associated with disruption of the interosseous membrane.
Weber C fractures are distal fibular fractures occurring above the level of the ankle joint. These fractures are also typically associated with external ankle rotation injuries and include disruption of the syndesmosis and deltoid ligament.14
Also pay special attention to the proximal fibula, as syndesmotic injuries are commonly associated with a Maisonneuve fracture, which is a proximal fibula fracture associated with external rotation forces of the ankle (see FIGURE 1).1,2,4,11,14,15 Further workup should occur in any patient with the possibility of a Weber- or Maisonneuve-type fracture.
Continue to: Multiple tests...
Multiple tests are available to investigate the possibility of a syndesmotic injury and to assess return-to-sport readiness, including the External Rotation Test, the Squeeze Test, the Crossed-Leg Test, the Dorsiflexion Compression Test, the Cotton Test, the Stabilization Test, the Fibular Translation Test, and the Single Leg Hop Test (see TABLE1-3,5,6,16,17). The External Rotation Test is noted by some authors to have the highest interobserver reliability, and is our preferred test.2 The Squeeze Test also has moderate interobserver reliability.2 There is a significant degree of variation among the sensitivity and specificity of these diagnostic tests, and no single test is sufficiently reliable or accurate to diagnose a syndesmotic ankle injury. Therefore, it is recommended to use multiple physical exam maneuvers, the history and mechanism of injury, and findings on imaging studies in conjunction to make the diagnosis of a syndesmotic injury.1,16
Imaging: Which modes and when?
The initial workup should include ankle x-rays when evaluating for the possibility of a distal tibiofibular syndesmosis injury. While the Ottawa Ankle Rules are helpful in providing guidance with regard to x-rays, suspicion of a syndesmotic injury mandates x-rays to determine the stability of the joint and rule out fracture. The European Society of Sports Traumatology, Knee Surgery and Arthroscopy–European Foot and Ankle Associates (ESSKA-AFAS) recommend, at a minimum, obtaining anteroposterior (AP)- and mortise-view ankle x-rays to investigate the tibiofibular clear space, medial clear space, and tibiofibular overlap.7 Most physicians also include a lateral ankle x-ray.
If possible, images should be performed while the patient is bearing weight to further evaluate stability. Radiographic findings that support the diagnosis of syndesmotic injury include a tibiofibular clear space > 6 mm on AP view, medial clear space > 4 mm on mortise view, or tibiofibular overlap < 6 mm on AP view or < 1 mm on mortise view (see FIGURES 2 and 3).1,3,5,8 Additionally, if you suspect a proximal fibular fracture, obtain an x-ray series of the proximal tibia and fibula to investigate the possibility of a Maisonneuve injury.1,2,4,11
If you continue to suspect a syndesmotic injury despite normal x-rays, obtain stress x-rays, in addition to the AP and mortise views, to ensure stability. These x-rays include AP and mortise ankle views with manual external rotation of the ankle joint, which may demonstrate abnormalities not seen on standard x-rays. Bilateral imaging can also be useful to further assess when mild abnormalities vs symmetric anatomic variants are in question.1,7
If there is concern for an unstable injury, refer the patient to a foot and ankle surgeon, who may pursue magnetic resonance imaging (MRI) or standing computed tomography (CT).1,2,5,7 MRI is the recommended choice for further evaluation of a syndesmotic injury, as it is proven to be accurate in evaluating the integrity of the syndesmotic ligaments (see FIGURES 4 and 5).18 MRI has demonstrated 100% sensitivity for detecting AITFL and PITFL injuries, as well as 93% and 100% specificity for AITFL and PITFL tears, respectively.8 A weight-bearing CT scan, particularly axial views, can also be a useful adjunct, as it is more sensitive than standard x-rays for assessing for mild diastasis. Although CT can provide an assessment of bony structures, it is not able to evaluate soft tissue structures, limiting its utility in evaluation of syndesmotic injuries.1,7
Continue to: Although not the standard of care...
Although not the standard of care, ultrasonography (US) is gaining traction as a means of investigating the integrity of the syndesmotic ligaments. US is inexpensive, readily available in many clinics, allows for dynamic testing, and avoids radiation exposure.7 However, US requires a skilled sonographer with experience in the ankle joint for an accurate diagnosis. If the workup with advanced imaging is inconclusive, but a high degree of suspicion remains for an unstable syndesmotic injury, consider arthroscopy to directly visualize and assess the syndesmotic structures.1,2,5,7,8
Grading the severity of the injury and pursuing appropriate Tx
Typically, the severity of a syndesmotic injury is classified as fitting into 1 of 3 categories: Grade I and II injuries are the most common, each accounting for 40% of syndesmotic injuries, while 20% of high ankle sprains are classified as Grade III.12
A Grade I injury consists of a stable syndesmotic joint without abnormal radiographic findings. There may be associated tenderness to palpation over the distal tibiofibular joint, and provocative testing may be subtle or normal. These injuries are often minor and able to be treated conservatively.
A Grade II injury is associated with a partial syndesmotic disruption, typically with partial tearing of the AITFL and interosseous ligament. These injuries may be stable or accompanied by mild instability, and provocative testing is usually positive. X-rays are typically normal with Grade II injuries, but may display subtle radiographic findings suggestive of a syndesmotic injury. Treatment of Grade II injuries is somewhat controversial and should be an individualized decision based upon the patient’s age, activity level, clinical exam, and imaging findings. Therefore, treatment of Grade II syndesmotic injuries may include a trial of conservative management or surgical intervention.
A Grade III injury represents inherent instability of the distal tibiofibular joint with complete disruption of all syndesmotic ligaments, with or without involvement of the deltoid ligament. X-rays will be positive in Grade III syndesmotic injuries because of the complete disruption of syndesmotic ligaments. All Grade III injuries require surgical intervention with a syndesmotic screw or other stabilization procedure.1,6-8,15
Continue to: A 3-stage rehabilitation protocol
A 3-stage rehabilitation protocol
When conservative management is deemed appropriate for a stable syndesmotic sprain, a 3-stage rehabilitation protocol is typically utilized.
The acute phase focuses on protection, pain control, and decreasing inflammation. The patient’s ankle is often immobilized in a cast or controlled ankle movement (CAM) boot. The patient is typically allowed to bear weight in the immobilizer during this phase as long as he/she is pain-free. If pain is present with weight bearing despite immobilization, non-weight bearing is recommended. The patient is instructed to elevate the lower extremity, take anti-inflammatory medication, and ice the affected ankle. Additionally, physical therapy modalities may be utilized to help with edema and pain. Joint immobilization is typically employed for 1 to 3 weeks post-injury. In the acute phase, the patient may also work with a physical therapist or athletic trainer on passive range of motion (ROM), progressing to active ROM as tolerated.1,5,7,8,19
The patient can transition from the CAM boot to a lace-up ankle brace when he/she is able to bear full weight and can navigate stairs without pain, which typically occurs around 3 to 6 weeks post-injury.1,5,7 A pneumatic walking brace may also be used as a transition device to provide added stabilization.
In the sub-acute phase, rehabilitation may progress to increase ankle mobility, strengthening, neuromuscular control, and to allow the patient to perform activities of daily living.5-7
The advanced training phase includes continued neuromuscular control, increased strengthening, plyometrics, agility, and sports-specific drills.5 Athletes are allowed to return to full participation when they have regained full ROM, are able to perform sport-specific agility drills without pain or instability, and have near-normal strength.5-7 Some authors also advocate that a Single Leg Hop Test should be included in the physical exam, and that it should be pain free prior to allowing an athlete to return to competition.20 Both progression in physical rehabilitation and return to sport should be individualized based upon injury severity, patient functionality, and physical exam findings.
Continue to: Outcomes forecast
Outcomes forecast: Variable
The resolution of symptoms and return to competition after a syndesmotic injury is variable. In one cohort study of cadets (N = 614) at the United States Military Academy, the average time lost from a syndesmotic ankle sprain was 9.82 days (range 3-21 days).9 In a retrospective review of National Hockey League players, average time to return to competition after a syndesmotic ankle injury sprain (n = 14) was 45 days (range 6-137 days) vs 1.4 days (range 0-6 days) for lateral ankle sprains (n = 5).21 In another study, National Football League players with syndesmotic sprains (n = 36) had a mean time loss from play of 15.4 days (± 11.1 days) vs 6.5 days (± 6.5 days) of time loss from play in those with lateral ankle sprains (n = 53).22
Although there is a fair amount of variability among studies, most authors agree that the average athlete can expect to return to sport 4 to 8 weeks post-injury with conservative management.19 At least 1 study suggests that the average time to return to sport in patients with Grade III syndesmotic injuries who undergo surgical treatment with a syndesmotic screw is 41 days (range 32-48).23 The differences in return to sport may be related to severity of injury and/or type of activity.
Persistent symptoms are relatively common after conservative management of syndesmotic injuries. One case series found that 36% of patients treated conservatively had complaints of persistent mild-to-moderate ankle stiffness, 23% had mild-to-moderate pain, and 18% had mild-to-moderate ankle swelling.24 Despite these symptoms, 86% of the patients rated their ankle function as good after conservative treatment.24 In patients with persistent symptoms, other possible etiologies should be considered including neurologic injury, complex regional pain syndrome, osteochondral defect, loose body, or other sources that may be contributing to pain, swelling, or delayed recovery.
At least 1 randomized controlled trial (RCT) investigated the utility of platelet rich plasma (PRP) injections around the injured AITFL in the setting of an acute syndesmotic injury. The study showed promising results, including quicker return to play, restabilization of the syndesmotic joint, and less residual pain;25 however, the study population was relatively small (N = 16), and the authors believed that more research is required on the benefits of PRP therapy in syndesmotic injuries before recommendations can be made.
An ounce of preventionis worth a pound of cure
Although injury is not always avoidable, there are measures that can help prevent ankle sprains and facilitate return to play after injury. As previously mentioned, athletes should be able to demonstrate the ability to run, cut, jump, and perform sport-specific activities without limitations prior to being allowed to return to sport after injury.5-7,26 Additionally, issues with biomechanics and functional deficits should be analyzed and addressed. By targeting specific strength deficits, focusing on proprioceptive awareness, and working on neuromuscular control, injury rates and recurrent injuries can be minimized. One RCT showed a 35% reduction in the recurrence rate of lateral ankle sprains with the use of an unsupervised home-based proprioceptive training program.27
Continue to: Strength training...
Strength training, proprioceptive and neuromuscular control activities, and low-risk activities such as jogging, biking, and swimming do not necessarily require the use of prophylactic bracing. However, because syndesmotic injuries are associated with recurrent ankle injuries, prophylactic bracing should be used during high-risk activities that involve agility maneuvers and jumping. Substantial evidence demonstrates that the use of ankle taping or ankle bracing decreases the incidence of ankle injuries, particularly in those who have had previous ankle injuries.26 In one study (N = 450), only 3% of athletes with a history of prior ankle injuries suffered a recurrent ankle sprain when using an ankle orthosis compared with a 17% injury rate in the control group.28
More recently, 2 separate studies by McGuine et al demonstrated that the use of lace-up ankle braces led to a reduction in the incidence of acute ankle injuries by 61% among 2081 high-school football players, and resulted in a significant reduction in acute ankle injuries in a study of 1460 male and female high-school basketball players, compared with the control groups.29,30
CASE
Ten days after injuring himself, the patient returns for a follow-up exam. Despite using the walking brace and crutches, he is still having significant difficulty bearing weight. He reports a sensation of instability in the right ankle. On exam, you note visible edema of the right ankle and ecchymosis over the lateral ankle, as well as moderate tenderness to palpation over the area of the ATFL and deltoid ligament. Tenderness over the medial malleolus, lateral malleolus, fifth metatarsal, and navicular is absent. Pain is reproducible with external rotation, and a Squeeze Test is positive. There is no tenderness over the proximal tibia or fibula. The patient is neurovascularly intact.
You order stress x-rays, which show widening of the medial clear space. The patient is placed in a CAM boot, instructed to continue non–weight-bearing on the ankle, and referred to a local foot and ankle surgeon for consideration of surgical fixation.
CORRESPONDENCE
John T. Nickless, MD, Division of Primary Care Sports Medicine, Department of Orthopedic Surgery, Rush University Medical Center, 1611 W. Harrison Street, Suite 200, Chicago, IL, 60612; [email protected].
1. Switaj PJ, Mendoza M, Kadakia AR. Acute and chronic Injuries to the syndesmosis. Clin Sports Med. 2015;34:643-677.
2. Scheyerer MJ, Helfet DL, Wirth S, et al. Diagnostics in suspicion of ankle syndesmotic injury. Am J Orthop. 2011;40:192-197.
3. Smith KM, Kovacich-Smith KJ, Witt M. Evaluation and management of high ankle sprains. Clin Podiatr Med Surg. 2001;18:443-456.
4. Reissig J, Bitterman A, Lee S. Common foot and ankle injuries: what not to miss and how best to manage. J Am Osteopath Assoc. 2017;117:98-104.
5. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2:460-470.
6. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35:1197-1207.
7. Vopat ML, Vopat BG, Lubberts B, et al. Current trends in the diagnosis and management of syndesmotic injury. Curr Rev Musculoskelet Med. 2017;10:94-103.
8. Mak MF, Gartner L, Pearce CJ. Management of syndesmosis injuries in the elite athlete. Foot Ankle Clin. 2013;18:195-214.
9. Waterman BR, Belmont PJ, Cameron KL, et al. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38:797-803.
10. Hunt KJ, George E, Harris AHS, et al. Epidemiology of syndesmosis injuries in intercollegiate football: incidence and risk factors from National Collegiate Athletic Association injury surveillance system data from 2004-2005 to 2008-2009. Clin J Sport Med. 2013;23:278-282.
11. Schnetzke M, Vetter SY, Beisemann N, et al. Management of syndesmotic injuries: what is the evidence? World J Orthop. 2016;7:718-725.
12. de César PC, Ávila EM, de Abreu MR. Comparison of magnetic resonance imaging to physical examination for syndesmotic injury after lateral ankle sprain. Foot Ankle Int. 2011;32:1110-1114.
13. Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720.
14. Porter D, Rund A, Barnes AF, et al. Optimal management of ankle syndesmosis injuries. Open Access J Sports Med. 2014;5:173-182.
15. Press CM, Gupta A, Hutchinson MR. Management of ankle syndesmosis injuries in the athlete. Curr Sports Med Rep. 2009;8:228-233.
16. Sman AD, Hiller CE, Rae K, et al. Diagnostic accuracy of clinical tests for ankle syndesmosis injury. Br J Sports Med. 2015;49:323-329.
17. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14:232-236.
18. Hunt KJ. Syndesmosis injuries. Curr Rev Musculoskelet Med. 2013;6:304-312.
19. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
20. Miller BS, Downie BK, Johnson PD, et al. Time to return to play after high ankle sprains in collegiate football players: a prediction model. Sports Health. 2012;4:504-509.
21. Wright RW, Barile RJ, Surprenant DA, et al. Ankle syndesmosis sprains in National Hockey League players. Am J Sports Med. 2016;32:1941-1945.
22. Osbahr DC, Drakos MC, O’Loughlin PF, et al. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378-e1384.
23. Taylor DC, Tenuta JJ, Uhorchak JM, et al. Aggressive surgical treatment and early return to sports in athletes with grade III syndesmosis sprains. Am J Sports Med. 2007;35:1133-1138.
24. Taylor DC, Englehardt DL, Bassett FH. Syndesmosis sprains of the ankle: the influence of heterotopic ossification. Am J Sports Med. 1992;20:146-150.
25. Laver L, Carmont MR, McConkey MO, et al. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: a randomized control trial. Knee Surg Sports Traumatol Arthrosc. 2014;23:3383-3392.
26. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
27. Hupperets MDW, Verhagen EALM, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684.
28. Tropp H, Askling C, Gillquist J. Prevention of ankle sprains. Am J Sports Med. 1985;13:259-262.
29. McGuine TA, Brooks A, Hetzel S. The effect of lace-up ankle braces on injury rates in high school basketball players. Am J Sports Med. 2011;39:1840-1848.
30. McGuine TA, Hetzel S, Wilson J, et al. The effect of lace-up ankle braces on injury rates in high school football players. Am J Sports Med. 2012;40:49-57.
1. Switaj PJ, Mendoza M, Kadakia AR. Acute and chronic Injuries to the syndesmosis. Clin Sports Med. 2015;34:643-677.
2. Scheyerer MJ, Helfet DL, Wirth S, et al. Diagnostics in suspicion of ankle syndesmotic injury. Am J Orthop. 2011;40:192-197.
3. Smith KM, Kovacich-Smith KJ, Witt M. Evaluation and management of high ankle sprains. Clin Podiatr Med Surg. 2001;18:443-456.
4. Reissig J, Bitterman A, Lee S. Common foot and ankle injuries: what not to miss and how best to manage. J Am Osteopath Assoc. 2017;117:98-104.
5. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2:460-470.
6. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35:1197-1207.
7. Vopat ML, Vopat BG, Lubberts B, et al. Current trends in the diagnosis and management of syndesmotic injury. Curr Rev Musculoskelet Med. 2017;10:94-103.
8. Mak MF, Gartner L, Pearce CJ. Management of syndesmosis injuries in the elite athlete. Foot Ankle Clin. 2013;18:195-214.
9. Waterman BR, Belmont PJ, Cameron KL, et al. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38:797-803.
10. Hunt KJ, George E, Harris AHS, et al. Epidemiology of syndesmosis injuries in intercollegiate football: incidence and risk factors from National Collegiate Athletic Association injury surveillance system data from 2004-2005 to 2008-2009. Clin J Sport Med. 2013;23:278-282.
11. Schnetzke M, Vetter SY, Beisemann N, et al. Management of syndesmotic injuries: what is the evidence? World J Orthop. 2016;7:718-725.
12. de César PC, Ávila EM, de Abreu MR. Comparison of magnetic resonance imaging to physical examination for syndesmotic injury after lateral ankle sprain. Foot Ankle Int. 2011;32:1110-1114.
13. Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720.
14. Porter D, Rund A, Barnes AF, et al. Optimal management of ankle syndesmosis injuries. Open Access J Sports Med. 2014;5:173-182.
15. Press CM, Gupta A, Hutchinson MR. Management of ankle syndesmosis injuries in the athlete. Curr Sports Med Rep. 2009;8:228-233.
16. Sman AD, Hiller CE, Rae K, et al. Diagnostic accuracy of clinical tests for ankle syndesmosis injury. Br J Sports Med. 2015;49:323-329.
17. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14:232-236.
18. Hunt KJ. Syndesmosis injuries. Curr Rev Musculoskelet Med. 2013;6:304-312.
19. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
20. Miller BS, Downie BK, Johnson PD, et al. Time to return to play after high ankle sprains in collegiate football players: a prediction model. Sports Health. 2012;4:504-509.
21. Wright RW, Barile RJ, Surprenant DA, et al. Ankle syndesmosis sprains in National Hockey League players. Am J Sports Med. 2016;32:1941-1945.
22. Osbahr DC, Drakos MC, O’Loughlin PF, et al. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378-e1384.
23. Taylor DC, Tenuta JJ, Uhorchak JM, et al. Aggressive surgical treatment and early return to sports in athletes with grade III syndesmosis sprains. Am J Sports Med. 2007;35:1133-1138.
24. Taylor DC, Englehardt DL, Bassett FH. Syndesmosis sprains of the ankle: the influence of heterotopic ossification. Am J Sports Med. 1992;20:146-150.
25. Laver L, Carmont MR, McConkey MO, et al. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: a randomized control trial. Knee Surg Sports Traumatol Arthrosc. 2014;23:3383-3392.
26. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
27. Hupperets MDW, Verhagen EALM, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684.
28. Tropp H, Askling C, Gillquist J. Prevention of ankle sprains. Am J Sports Med. 1985;13:259-262.
29. McGuine TA, Brooks A, Hetzel S. The effect of lace-up ankle braces on injury rates in high school basketball players. Am J Sports Med. 2011;39:1840-1848.
30. McGuine TA, Hetzel S, Wilson J, et al. The effect of lace-up ankle braces on injury rates in high school football players. Am J Sports Med. 2012;40:49-57.
PRACTICE RECOMMENDATIONS
› Maintain a high level of suspicion for syndesmotic injury in any athlete describing an external rotation or hyper-dorsiflexion ankle injury. A
› Obtain weight-bearing anteroposterior- and mortise-view ankle x-rays in all cases of suspected syndesmotic injuries. A
› Consider stress x-rays of the affected ankle, contralateral ankle x-rays for comparison views, or advanced imaging with magnetic resonance imaging (MRI) or computed tomography if initial x-rays are unrevealing. A
› Treat stable syndesmotic injuries with conservative measures and rehabilitation. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keeping caries at bay in breastfeeding babies
Early childhood caries (ECCs) are a preventable public health challenge. Breastfeeding may provide early protection from ECCs. In addition, oral hygiene that begins in infancy, regular dental care visits, and a healthy diet can minimize ECC risk.
In this article we review the critical role of the family physician (FP) in reducing ECCs by promoting breastfeeding and infant oral health and addressing dental health concerns.
How ECCs develop
ECCs represent decayed, missing, or filled areas in the primary dentition of the tooth surface. The bacteria that cause them (most often Streptococcus mutans1) strongly adhere to teeth and produce acids as waste products of fermentable carbohydrate metabolism that demineralize tooth enamel and progress into the dentin. Weakened enamel and dentin can result in cavitation (ie, a dental cavity). Left untreated, caries can extend to the pulp and destroy the entire tooth. ECCs are a risk factor not only for dental caries in primary teeth, but in permanent dentition as well.
ECCs are the most common chronic disease affecting young children.1 Dental disease may begin soon after tooth eruption with detrimental effects on oral development. Almost half of children have dental caries by 5 years of age.2
ECCs represent a complex and multifactorial disease that is impacted by biomedical factors and unmet social needs. Children who are most at risk include those with low socioeconomic status, a high-sugar diet, exposure to household smoke, and limited dental care access.3 In addition, women with low education, poor oral health, and/or a lack of fluoride exposure are more likely to have children with ECCs.3 This is partly because of vertical transmission of cariogenic bacteria from caregiver to child. Horizontal transmission in daycare settings can also occur. Paternal and child oral health have not been linked.
Support breastfeeding; keep oral microbiome changes in mind
The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life, a combination of breastfeeding and complementary foods until 12 months of age, and continued breastfeeding for as long as mutually desired by mother and baby.4 The World Health Organization (WHO) recommends continued breastfeeding until 2 years of age or beyond.5 In fact, the WHO global nutrition targets for 2025 include increasing the rate of exclusive breastfeeding in the first 6 months of life to at least 50%.6
In addition to maternal, financial, and societal benefits, human milk offers nutritional and other health-related advantages for children that optimize growth and development into adulthood.4 Breastfed infants may benefit from reduction in infections and diseases, including asthma, diabetes mellitus, childhood cancer, and obesity.7 Improved neurocognitive development, intelligence, and education attainment in adulthood have also been described.8 And the rich microbiome of human milk helps to establish oral and intestinal floras9 and may mediate protection from ECCs.3
Continue to: However, as a child's oral microbiome changes...
However, as a child’s oral microbiome changes with the emergence of primary teeth and exposure to more and varied bacteria and dietary sugars, the natural sugars in human milk may become the substrate for cariogenic bacteria.3 ECCs can develop and progress rapidly. Importantly, both the practice of breastfeeding and ECC risk are modified by socioeconomic status, maternal oral health and education, and exposure to household smoking.3,7 Understanding these relationships may help you better target risk assessment and counseling efforts.
What the research tells us about breastfeeding and ECCs
Breastfeeding is hypothesized to be one of many factors that influence ECC development. However, studies on this association have had conflicting results and have not adequately controlled for major confounders, such as dietary composition, maternal and infant oral hygiene, and maternal oral health status.
So here is what we know.
Breastfeeding during the first year. In one meta-analysis involving children who breastfed for up to 12 months, those who breastfed longer within the 12-month period had a reduced risk of ECCs compared with those who breastfed for a shorter period of time,3 which implies that breast milk may be protective in the first year of life.3
Further, a 2014 study with about 500 participants found that children were more likely to have caries by 5 years of age if they breastfed for <6 months than if they breastfed for at least 6 months.10
Continue to: After the first year
After the first year. A Canadian study found an increased risk of ECCs associated with breastfeeding for longer periods of time. The study of healthy urban children reported that breastfeeding for >24 months was associated with a 2- to 3-fold increased odds of ECCs compared with shorter breastfeeding duration.11
No relationship? Lastly, a US study using National Health and Nutrition Examination Survey data found there was no evidence to suggest that breastfeeding duration was an independent risk factor for ECCs.12
A possible explanation for a link
An initial protective effect of breastfeeding against ECCs may be related to breast milk’s immunomodulatory factors and rich microbiome. Breast milk contains Lactobacilli and substances, including human casein and secretory IgA, that inhibit growth and attachment of bacteria,9 particularly the caries pathogen S mutans. Early defense against ECCs may be mediated through the establishment of a healthy oral and gut microbiome that results from exposure to breastfeeding and contact with skin, gut, and breast milk microbiomes. Later on, the child’s oral microbiome changes with the emergence of teeth and the introduction of complementary foods andother drinks.
A look at the role vitamin D plays
Vitamin D status may influence childhood dental health.13 Low maternal vitamin D levels have been associated with ECCs,14 and mothers with higher prenatal vitamin D intakes were more likely to report that their children were caries-free compared with women who had lower vitamin D intake.15 Additionally, children with severe ECCs were found to have lower vitamin D levels than cavity-free children.16 Unfortunately, only a minority of infants who are predominantly breastfed for > 6 months receive vitamin D supplementation.17
Other factors at work: Carbohydrate exposure, nocturnal feedings
Exposure to carbohydrates—the essential substrate for cariogenic bacteria—is a key factor in ECC development. Refined sugars contribute considerably to tooth decay. Frequency of feeding and feeding practices, such as prolonged nocturnal feeding (either breast or bottle) may increase ECC risk.3 Further, a major determinant of ECC risk is colonization of the infant’s mouth by cariogenic bacteria. Finally, ECC risk depends on socioeconomic status, oral hygiene, exposure to fluoride, and the mother’s oral health, education, and smoking status.3 Even birth order plays a role, with those born first having lower risk than subsequent children.18
Continue to: Breastfeeding and another area of oral health...
Breastfeeding and another area of oral health: Malocclusion
In addition to its relationship with ECCs, breastfeeding promotes adequate development of craniofacial structures (comprising the tongue, facial muscles, and jaw), which are important for smiling, emotion, and social contact. Breastfeeding may prevent the development of malocclusion (ie, a misalignment of the teeth) in primary dentition, which is a risk factor for malocclusion in adulthood.7 Although previous studies had conflicting results, a large prospective study found that breastfeeding significantly reduced the risk of moderate and severe malocclusion; however, this effect was nullified by nonnutritive sucking and pacifier use.19
Oral health recommendations: The FP’s role
ECCs are theoretically preventable. To optimize the benefits of breastfeeding and minimize ECC risk, parents should follow recommendations for their children regarding proper oral hygiene, appropriate fluoride exposure, regular dental visits, and a healthy diet.1
Be sure to advise parents to:
- avoid saliva-sharing behaviors (eg, sharing utensils with their children or cleaning a pacifier with their mouth), as these may increase early colonization of S mutans in infants;
- seek regular preventive dental care and attend to caries—both for their children and themselves; and
- use antimicrobial oral care products including xylitol-containing chewing gum to lower levels of cariogenic microorganisms in themselves and, in turn, reduce mother–child vertical transmission of S mutans.1
In addition, make sure your prenatal counseling includes a discussion of the importance of good maternal oral health and diet—including an adequate vitamin D intake—to prevent ECCs in their children.
It’s never too early to start
Providing guidance on children’s oral health can start with the first well-infant visit. FPs should perform an oral health risk assessment by 6 months of age (see the AAP’s Oral Health Risk Assessment Tool at https://www.aap.org/en-us/Documents/oralhealth_RiskAssessmentTool.pdf) and evaluate fluoride exposure. Advise parents to establish a dental home by the time the child is 12 months of age; to clean their children’s mouths after feedings (before teeth arrive) with a clean, wet, soft washcloth; and to brush their children’s teeth, once they erupt, twice daily using a soft toothbrush (TABLE 120).
Continue to: Talk to parents about...
Talk to parents about how to provide optimal exposure to fluoride, which is known to be safe and effective for the prevention of ECCs.1 Use of fluoridated toothpaste in small amounts provides the benefits of fluoride without increasing the risk of fluorosis, especially for children at risk for caries (see TABLE 221).
The US Preventive Services Task Force recommends that primary care practitioners apply fluoride varnish biannually for at least 2 years to the primary teeth of all children up to 5 years of age (Grade B evidence).22 This is particularly important for high-risk children, such as those with low-income or minority status. However, practitioners should also take into account that high cumulative fluoride intake can lead to dental fluorosis.1 Finally, tell parents to avoid giving their children sugar-containing snacks and drinks to reduce ECC risk.
CORRESPONDENCE
Peter D. Wong, MD, 303-89 Humber College Boulevard, Toronto, Ontario, Canada M9V 4B8; [email protected].
1. American Academy of Pediatric Dentistry. Guideline on infant oral health care. Pediatr Dent. 2012;34:e148-e152.
2. Dye BA, Thornton-Evans G, Li X, et al. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. NCHS Data Brief. No. 191, March 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db191.pdf. Accessed January 25, 2019.
3. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
4. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk (section on breastfeeding). Pediatrics. 2012;129:e827-e841.
5. World Health Organization. 55th World Health Assembly. Agenda Item 13.10: Infant and young child nutrition. https://www.who.int/nutrition/topics/WHA55.25_iycn_en.pdf?ua=1. May 18, 2002. Accessed January 25, 2019.
6. World Health Organization. Global Nutrition Targets 2025 Breastfeeding Policy Brief 5. http://apps.who.int/iris/bitstream/10665/149022/1/WHO_NMH_NHD_14.7_eng.pdf?ua=1. Accessed April 1, 2019.
7. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490.
8. Victora CG, Horta BL, de Mola CL, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199-e205.
9. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14-25.
10. Hong L, Levy SM, Warren JJ, et al. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36:342-347.
11. Wong PD, Birken CS, Parkin PC, et al. Total breast-feeding duration and dental caries in healthy urban children. Acad Pediatr. 2017;17:310-315.
12. Iida H, Auinger P, Billings RJ, et al. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120:e944-e952.
13. Schroth R, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res. 2016;95:173-179.
14. Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:e1277-e1284.
15. Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25:620-625.
16. Schroth RJ, Halchuk S, Star L. Prevalence and risk factors of caregiver reported severe early childhood caries in Manitoba First Nations children: results from the RHS Phase 2 (2008-2010). Int J Circumpolar Health. 2013;72.
17. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105-111.
18. Nicolau B, Marcenes W, Bartley M, et al. A life course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319-326.
19. Peres KG, Cascaes AM, Peres MA, et al. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 2015;136:e60-e67.
20. Tinanoff N, Reisin S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396-403.
21. Canadian Dental Association. CDA Position on Use of Fluorides in Caries Prevention. 2012. https://www.cda-adc.ca/_files/position_statements/fluoride.pdf. Accessed January 25, 2019.
22. US Preventive Services Task Force. USPSTF A and B Recommendations. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed April 1, 2019.
Early childhood caries (ECCs) are a preventable public health challenge. Breastfeeding may provide early protection from ECCs. In addition, oral hygiene that begins in infancy, regular dental care visits, and a healthy diet can minimize ECC risk.
In this article we review the critical role of the family physician (FP) in reducing ECCs by promoting breastfeeding and infant oral health and addressing dental health concerns.
How ECCs develop
ECCs represent decayed, missing, or filled areas in the primary dentition of the tooth surface. The bacteria that cause them (most often Streptococcus mutans1) strongly adhere to teeth and produce acids as waste products of fermentable carbohydrate metabolism that demineralize tooth enamel and progress into the dentin. Weakened enamel and dentin can result in cavitation (ie, a dental cavity). Left untreated, caries can extend to the pulp and destroy the entire tooth. ECCs are a risk factor not only for dental caries in primary teeth, but in permanent dentition as well.
ECCs are the most common chronic disease affecting young children.1 Dental disease may begin soon after tooth eruption with detrimental effects on oral development. Almost half of children have dental caries by 5 years of age.2
ECCs represent a complex and multifactorial disease that is impacted by biomedical factors and unmet social needs. Children who are most at risk include those with low socioeconomic status, a high-sugar diet, exposure to household smoke, and limited dental care access.3 In addition, women with low education, poor oral health, and/or a lack of fluoride exposure are more likely to have children with ECCs.3 This is partly because of vertical transmission of cariogenic bacteria from caregiver to child. Horizontal transmission in daycare settings can also occur. Paternal and child oral health have not been linked.
Support breastfeeding; keep oral microbiome changes in mind
The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life, a combination of breastfeeding and complementary foods until 12 months of age, and continued breastfeeding for as long as mutually desired by mother and baby.4 The World Health Organization (WHO) recommends continued breastfeeding until 2 years of age or beyond.5 In fact, the WHO global nutrition targets for 2025 include increasing the rate of exclusive breastfeeding in the first 6 months of life to at least 50%.6
In addition to maternal, financial, and societal benefits, human milk offers nutritional and other health-related advantages for children that optimize growth and development into adulthood.4 Breastfed infants may benefit from reduction in infections and diseases, including asthma, diabetes mellitus, childhood cancer, and obesity.7 Improved neurocognitive development, intelligence, and education attainment in adulthood have also been described.8 And the rich microbiome of human milk helps to establish oral and intestinal floras9 and may mediate protection from ECCs.3
Continue to: However, as a child's oral microbiome changes...
However, as a child’s oral microbiome changes with the emergence of primary teeth and exposure to more and varied bacteria and dietary sugars, the natural sugars in human milk may become the substrate for cariogenic bacteria.3 ECCs can develop and progress rapidly. Importantly, both the practice of breastfeeding and ECC risk are modified by socioeconomic status, maternal oral health and education, and exposure to household smoking.3,7 Understanding these relationships may help you better target risk assessment and counseling efforts.
What the research tells us about breastfeeding and ECCs
Breastfeeding is hypothesized to be one of many factors that influence ECC development. However, studies on this association have had conflicting results and have not adequately controlled for major confounders, such as dietary composition, maternal and infant oral hygiene, and maternal oral health status.
So here is what we know.
Breastfeeding during the first year. In one meta-analysis involving children who breastfed for up to 12 months, those who breastfed longer within the 12-month period had a reduced risk of ECCs compared with those who breastfed for a shorter period of time,3 which implies that breast milk may be protective in the first year of life.3
Further, a 2014 study with about 500 participants found that children were more likely to have caries by 5 years of age if they breastfed for <6 months than if they breastfed for at least 6 months.10
Continue to: After the first year
After the first year. A Canadian study found an increased risk of ECCs associated with breastfeeding for longer periods of time. The study of healthy urban children reported that breastfeeding for >24 months was associated with a 2- to 3-fold increased odds of ECCs compared with shorter breastfeeding duration.11
No relationship? Lastly, a US study using National Health and Nutrition Examination Survey data found there was no evidence to suggest that breastfeeding duration was an independent risk factor for ECCs.12
A possible explanation for a link
An initial protective effect of breastfeeding against ECCs may be related to breast milk’s immunomodulatory factors and rich microbiome. Breast milk contains Lactobacilli and substances, including human casein and secretory IgA, that inhibit growth and attachment of bacteria,9 particularly the caries pathogen S mutans. Early defense against ECCs may be mediated through the establishment of a healthy oral and gut microbiome that results from exposure to breastfeeding and contact with skin, gut, and breast milk microbiomes. Later on, the child’s oral microbiome changes with the emergence of teeth and the introduction of complementary foods andother drinks.
A look at the role vitamin D plays
Vitamin D status may influence childhood dental health.13 Low maternal vitamin D levels have been associated with ECCs,14 and mothers with higher prenatal vitamin D intakes were more likely to report that their children were caries-free compared with women who had lower vitamin D intake.15 Additionally, children with severe ECCs were found to have lower vitamin D levels than cavity-free children.16 Unfortunately, only a minority of infants who are predominantly breastfed for > 6 months receive vitamin D supplementation.17
Other factors at work: Carbohydrate exposure, nocturnal feedings
Exposure to carbohydrates—the essential substrate for cariogenic bacteria—is a key factor in ECC development. Refined sugars contribute considerably to tooth decay. Frequency of feeding and feeding practices, such as prolonged nocturnal feeding (either breast or bottle) may increase ECC risk.3 Further, a major determinant of ECC risk is colonization of the infant’s mouth by cariogenic bacteria. Finally, ECC risk depends on socioeconomic status, oral hygiene, exposure to fluoride, and the mother’s oral health, education, and smoking status.3 Even birth order plays a role, with those born first having lower risk than subsequent children.18
Continue to: Breastfeeding and another area of oral health...
Breastfeeding and another area of oral health: Malocclusion
In addition to its relationship with ECCs, breastfeeding promotes adequate development of craniofacial structures (comprising the tongue, facial muscles, and jaw), which are important for smiling, emotion, and social contact. Breastfeeding may prevent the development of malocclusion (ie, a misalignment of the teeth) in primary dentition, which is a risk factor for malocclusion in adulthood.7 Although previous studies had conflicting results, a large prospective study found that breastfeeding significantly reduced the risk of moderate and severe malocclusion; however, this effect was nullified by nonnutritive sucking and pacifier use.19
Oral health recommendations: The FP’s role
ECCs are theoretically preventable. To optimize the benefits of breastfeeding and minimize ECC risk, parents should follow recommendations for their children regarding proper oral hygiene, appropriate fluoride exposure, regular dental visits, and a healthy diet.1
Be sure to advise parents to:
- avoid saliva-sharing behaviors (eg, sharing utensils with their children or cleaning a pacifier with their mouth), as these may increase early colonization of S mutans in infants;
- seek regular preventive dental care and attend to caries—both for their children and themselves; and
- use antimicrobial oral care products including xylitol-containing chewing gum to lower levels of cariogenic microorganisms in themselves and, in turn, reduce mother–child vertical transmission of S mutans.1
In addition, make sure your prenatal counseling includes a discussion of the importance of good maternal oral health and diet—including an adequate vitamin D intake—to prevent ECCs in their children.
It’s never too early to start
Providing guidance on children’s oral health can start with the first well-infant visit. FPs should perform an oral health risk assessment by 6 months of age (see the AAP’s Oral Health Risk Assessment Tool at https://www.aap.org/en-us/Documents/oralhealth_RiskAssessmentTool.pdf) and evaluate fluoride exposure. Advise parents to establish a dental home by the time the child is 12 months of age; to clean their children’s mouths after feedings (before teeth arrive) with a clean, wet, soft washcloth; and to brush their children’s teeth, once they erupt, twice daily using a soft toothbrush (TABLE 120).
Continue to: Talk to parents about...
Talk to parents about how to provide optimal exposure to fluoride, which is known to be safe and effective for the prevention of ECCs.1 Use of fluoridated toothpaste in small amounts provides the benefits of fluoride without increasing the risk of fluorosis, especially for children at risk for caries (see TABLE 221).
The US Preventive Services Task Force recommends that primary care practitioners apply fluoride varnish biannually for at least 2 years to the primary teeth of all children up to 5 years of age (Grade B evidence).22 This is particularly important for high-risk children, such as those with low-income or minority status. However, practitioners should also take into account that high cumulative fluoride intake can lead to dental fluorosis.1 Finally, tell parents to avoid giving their children sugar-containing snacks and drinks to reduce ECC risk.
CORRESPONDENCE
Peter D. Wong, MD, 303-89 Humber College Boulevard, Toronto, Ontario, Canada M9V 4B8; [email protected].
Early childhood caries (ECCs) are a preventable public health challenge. Breastfeeding may provide early protection from ECCs. In addition, oral hygiene that begins in infancy, regular dental care visits, and a healthy diet can minimize ECC risk.
In this article we review the critical role of the family physician (FP) in reducing ECCs by promoting breastfeeding and infant oral health and addressing dental health concerns.
How ECCs develop
ECCs represent decayed, missing, or filled areas in the primary dentition of the tooth surface. The bacteria that cause them (most often Streptococcus mutans1) strongly adhere to teeth and produce acids as waste products of fermentable carbohydrate metabolism that demineralize tooth enamel and progress into the dentin. Weakened enamel and dentin can result in cavitation (ie, a dental cavity). Left untreated, caries can extend to the pulp and destroy the entire tooth. ECCs are a risk factor not only for dental caries in primary teeth, but in permanent dentition as well.
ECCs are the most common chronic disease affecting young children.1 Dental disease may begin soon after tooth eruption with detrimental effects on oral development. Almost half of children have dental caries by 5 years of age.2
ECCs represent a complex and multifactorial disease that is impacted by biomedical factors and unmet social needs. Children who are most at risk include those with low socioeconomic status, a high-sugar diet, exposure to household smoke, and limited dental care access.3 In addition, women with low education, poor oral health, and/or a lack of fluoride exposure are more likely to have children with ECCs.3 This is partly because of vertical transmission of cariogenic bacteria from caregiver to child. Horizontal transmission in daycare settings can also occur. Paternal and child oral health have not been linked.
Support breastfeeding; keep oral microbiome changes in mind
The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life, a combination of breastfeeding and complementary foods until 12 months of age, and continued breastfeeding for as long as mutually desired by mother and baby.4 The World Health Organization (WHO) recommends continued breastfeeding until 2 years of age or beyond.5 In fact, the WHO global nutrition targets for 2025 include increasing the rate of exclusive breastfeeding in the first 6 months of life to at least 50%.6
In addition to maternal, financial, and societal benefits, human milk offers nutritional and other health-related advantages for children that optimize growth and development into adulthood.4 Breastfed infants may benefit from reduction in infections and diseases, including asthma, diabetes mellitus, childhood cancer, and obesity.7 Improved neurocognitive development, intelligence, and education attainment in adulthood have also been described.8 And the rich microbiome of human milk helps to establish oral and intestinal floras9 and may mediate protection from ECCs.3
Continue to: However, as a child's oral microbiome changes...
However, as a child’s oral microbiome changes with the emergence of primary teeth and exposure to more and varied bacteria and dietary sugars, the natural sugars in human milk may become the substrate for cariogenic bacteria.3 ECCs can develop and progress rapidly. Importantly, both the practice of breastfeeding and ECC risk are modified by socioeconomic status, maternal oral health and education, and exposure to household smoking.3,7 Understanding these relationships may help you better target risk assessment and counseling efforts.
What the research tells us about breastfeeding and ECCs
Breastfeeding is hypothesized to be one of many factors that influence ECC development. However, studies on this association have had conflicting results and have not adequately controlled for major confounders, such as dietary composition, maternal and infant oral hygiene, and maternal oral health status.
So here is what we know.
Breastfeeding during the first year. In one meta-analysis involving children who breastfed for up to 12 months, those who breastfed longer within the 12-month period had a reduced risk of ECCs compared with those who breastfed for a shorter period of time,3 which implies that breast milk may be protective in the first year of life.3
Further, a 2014 study with about 500 participants found that children were more likely to have caries by 5 years of age if they breastfed for <6 months than if they breastfed for at least 6 months.10
Continue to: After the first year
After the first year. A Canadian study found an increased risk of ECCs associated with breastfeeding for longer periods of time. The study of healthy urban children reported that breastfeeding for >24 months was associated with a 2- to 3-fold increased odds of ECCs compared with shorter breastfeeding duration.11
No relationship? Lastly, a US study using National Health and Nutrition Examination Survey data found there was no evidence to suggest that breastfeeding duration was an independent risk factor for ECCs.12
A possible explanation for a link
An initial protective effect of breastfeeding against ECCs may be related to breast milk’s immunomodulatory factors and rich microbiome. Breast milk contains Lactobacilli and substances, including human casein and secretory IgA, that inhibit growth and attachment of bacteria,9 particularly the caries pathogen S mutans. Early defense against ECCs may be mediated through the establishment of a healthy oral and gut microbiome that results from exposure to breastfeeding and contact with skin, gut, and breast milk microbiomes. Later on, the child’s oral microbiome changes with the emergence of teeth and the introduction of complementary foods andother drinks.
A look at the role vitamin D plays
Vitamin D status may influence childhood dental health.13 Low maternal vitamin D levels have been associated with ECCs,14 and mothers with higher prenatal vitamin D intakes were more likely to report that their children were caries-free compared with women who had lower vitamin D intake.15 Additionally, children with severe ECCs were found to have lower vitamin D levels than cavity-free children.16 Unfortunately, only a minority of infants who are predominantly breastfed for > 6 months receive vitamin D supplementation.17
Other factors at work: Carbohydrate exposure, nocturnal feedings
Exposure to carbohydrates—the essential substrate for cariogenic bacteria—is a key factor in ECC development. Refined sugars contribute considerably to tooth decay. Frequency of feeding and feeding practices, such as prolonged nocturnal feeding (either breast or bottle) may increase ECC risk.3 Further, a major determinant of ECC risk is colonization of the infant’s mouth by cariogenic bacteria. Finally, ECC risk depends on socioeconomic status, oral hygiene, exposure to fluoride, and the mother’s oral health, education, and smoking status.3 Even birth order plays a role, with those born first having lower risk than subsequent children.18
Continue to: Breastfeeding and another area of oral health...
Breastfeeding and another area of oral health: Malocclusion
In addition to its relationship with ECCs, breastfeeding promotes adequate development of craniofacial structures (comprising the tongue, facial muscles, and jaw), which are important for smiling, emotion, and social contact. Breastfeeding may prevent the development of malocclusion (ie, a misalignment of the teeth) in primary dentition, which is a risk factor for malocclusion in adulthood.7 Although previous studies had conflicting results, a large prospective study found that breastfeeding significantly reduced the risk of moderate and severe malocclusion; however, this effect was nullified by nonnutritive sucking and pacifier use.19
Oral health recommendations: The FP’s role
ECCs are theoretically preventable. To optimize the benefits of breastfeeding and minimize ECC risk, parents should follow recommendations for their children regarding proper oral hygiene, appropriate fluoride exposure, regular dental visits, and a healthy diet.1
Be sure to advise parents to:
- avoid saliva-sharing behaviors (eg, sharing utensils with their children or cleaning a pacifier with their mouth), as these may increase early colonization of S mutans in infants;
- seek regular preventive dental care and attend to caries—both for their children and themselves; and
- use antimicrobial oral care products including xylitol-containing chewing gum to lower levels of cariogenic microorganisms in themselves and, in turn, reduce mother–child vertical transmission of S mutans.1
In addition, make sure your prenatal counseling includes a discussion of the importance of good maternal oral health and diet—including an adequate vitamin D intake—to prevent ECCs in their children.
It’s never too early to start
Providing guidance on children’s oral health can start with the first well-infant visit. FPs should perform an oral health risk assessment by 6 months of age (see the AAP’s Oral Health Risk Assessment Tool at https://www.aap.org/en-us/Documents/oralhealth_RiskAssessmentTool.pdf) and evaluate fluoride exposure. Advise parents to establish a dental home by the time the child is 12 months of age; to clean their children’s mouths after feedings (before teeth arrive) with a clean, wet, soft washcloth; and to brush their children’s teeth, once they erupt, twice daily using a soft toothbrush (TABLE 120).
Continue to: Talk to parents about...
Talk to parents about how to provide optimal exposure to fluoride, which is known to be safe and effective for the prevention of ECCs.1 Use of fluoridated toothpaste in small amounts provides the benefits of fluoride without increasing the risk of fluorosis, especially for children at risk for caries (see TABLE 221).
The US Preventive Services Task Force recommends that primary care practitioners apply fluoride varnish biannually for at least 2 years to the primary teeth of all children up to 5 years of age (Grade B evidence).22 This is particularly important for high-risk children, such as those with low-income or minority status. However, practitioners should also take into account that high cumulative fluoride intake can lead to dental fluorosis.1 Finally, tell parents to avoid giving their children sugar-containing snacks and drinks to reduce ECC risk.
CORRESPONDENCE
Peter D. Wong, MD, 303-89 Humber College Boulevard, Toronto, Ontario, Canada M9V 4B8; [email protected].
1. American Academy of Pediatric Dentistry. Guideline on infant oral health care. Pediatr Dent. 2012;34:e148-e152.
2. Dye BA, Thornton-Evans G, Li X, et al. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. NCHS Data Brief. No. 191, March 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db191.pdf. Accessed January 25, 2019.
3. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
4. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk (section on breastfeeding). Pediatrics. 2012;129:e827-e841.
5. World Health Organization. 55th World Health Assembly. Agenda Item 13.10: Infant and young child nutrition. https://www.who.int/nutrition/topics/WHA55.25_iycn_en.pdf?ua=1. May 18, 2002. Accessed January 25, 2019.
6. World Health Organization. Global Nutrition Targets 2025 Breastfeeding Policy Brief 5. http://apps.who.int/iris/bitstream/10665/149022/1/WHO_NMH_NHD_14.7_eng.pdf?ua=1. Accessed April 1, 2019.
7. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490.
8. Victora CG, Horta BL, de Mola CL, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199-e205.
9. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14-25.
10. Hong L, Levy SM, Warren JJ, et al. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36:342-347.
11. Wong PD, Birken CS, Parkin PC, et al. Total breast-feeding duration and dental caries in healthy urban children. Acad Pediatr. 2017;17:310-315.
12. Iida H, Auinger P, Billings RJ, et al. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120:e944-e952.
13. Schroth R, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res. 2016;95:173-179.
14. Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:e1277-e1284.
15. Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25:620-625.
16. Schroth RJ, Halchuk S, Star L. Prevalence and risk factors of caregiver reported severe early childhood caries in Manitoba First Nations children: results from the RHS Phase 2 (2008-2010). Int J Circumpolar Health. 2013;72.
17. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105-111.
18. Nicolau B, Marcenes W, Bartley M, et al. A life course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319-326.
19. Peres KG, Cascaes AM, Peres MA, et al. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 2015;136:e60-e67.
20. Tinanoff N, Reisin S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396-403.
21. Canadian Dental Association. CDA Position on Use of Fluorides in Caries Prevention. 2012. https://www.cda-adc.ca/_files/position_statements/fluoride.pdf. Accessed January 25, 2019.
22. US Preventive Services Task Force. USPSTF A and B Recommendations. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed April 1, 2019.
1. American Academy of Pediatric Dentistry. Guideline on infant oral health care. Pediatr Dent. 2012;34:e148-e152.
2. Dye BA, Thornton-Evans G, Li X, et al. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. NCHS Data Brief. No. 191, March 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db191.pdf. Accessed January 25, 2019.
3. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
4. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk (section on breastfeeding). Pediatrics. 2012;129:e827-e841.
5. World Health Organization. 55th World Health Assembly. Agenda Item 13.10: Infant and young child nutrition. https://www.who.int/nutrition/topics/WHA55.25_iycn_en.pdf?ua=1. May 18, 2002. Accessed January 25, 2019.
6. World Health Organization. Global Nutrition Targets 2025 Breastfeeding Policy Brief 5. http://apps.who.int/iris/bitstream/10665/149022/1/WHO_NMH_NHD_14.7_eng.pdf?ua=1. Accessed April 1, 2019.
7. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490.
8. Victora CG, Horta BL, de Mola CL, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199-e205.
9. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14-25.
10. Hong L, Levy SM, Warren JJ, et al. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36:342-347.
11. Wong PD, Birken CS, Parkin PC, et al. Total breast-feeding duration and dental caries in healthy urban children. Acad Pediatr. 2017;17:310-315.
12. Iida H, Auinger P, Billings RJ, et al. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120:e944-e952.
13. Schroth R, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res. 2016;95:173-179.
14. Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:e1277-e1284.
15. Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25:620-625.
16. Schroth RJ, Halchuk S, Star L. Prevalence and risk factors of caregiver reported severe early childhood caries in Manitoba First Nations children: results from the RHS Phase 2 (2008-2010). Int J Circumpolar Health. 2013;72.
17. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105-111.
18. Nicolau B, Marcenes W, Bartley M, et al. A life course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319-326.
19. Peres KG, Cascaes AM, Peres MA, et al. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 2015;136:e60-e67.
20. Tinanoff N, Reisin S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396-403.
21. Canadian Dental Association. CDA Position on Use of Fluorides in Caries Prevention. 2012. https://www.cda-adc.ca/_files/position_statements/fluoride.pdf. Accessed January 25, 2019.
22. US Preventive Services Task Force. USPSTF A and B Recommendations. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed April 1, 2019.
PRACTICE RECOMMENDATIONS
› Promote breastfeeding as the preferred method of feeding infants. A
› Optimize pediatric oral health by reducing risk factors for dental disease and by providing parents with anticipatory guidance to prevent early childhood caries. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Aspirin for primary prevention: USPSTF recommendations for CVD and colorectal cancer
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; [email protected].
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; [email protected].
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; [email protected].
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
PRACTICE RECOMMENDATIONS
› Consider aspirin for patients 50 to 59 years of age who have a 10-year cardiovascular disease (CVD) risk of ≥ 10% and low bleeding risk. C
› Discuss prophylactic aspirin (using a shared decision-making model) with patients 60 to 69 years of age who have a 10-year CVD risk of ≥ 10% and low bleeding risk. C
› Avoid using aspirin for primary prevention in patients ≥ 70 years of age. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s time to start asking all patients about intimate partner violence
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
PRACTICE RECOMMENDATIONS
› Perform annual screening for intimate partner violence of all female patients of childbearing age; strongly consider a pilot program of universal screening (all male and female patients, across the lifespan). B
› Establish a protocol for intimate partner violence screening and referral—possibly the most effective means of identifying intimate partner violence at early and severe stages. B
› Collaborate with the patient in the safety planning and referral process; benefits include improved likelihood that the patient will adhere to a safety plan and follow through with the referral. B
› Utilize online resources to 1) ease the process of establishing relationships with local intimate partner violence referrals and 2) facilitate warm handoffs to increase the likelihood of patient engagement. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series