User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Risk for herpes zoster in RA higher with tsDMARDs/bDMARDs vs csDMARDs
Key clinical point: In patients with rheumatoid arthritis (RA), treatment with targeted synthetic and biologic disease-modifying antirheumatic drugs (tsDMARDs/bDMARDs) vs conventional synthetic DMARDs (csDMARDs) was associated with a significantly higher risk for herpes zoster (HZ).
Major finding: Compared with csDMARDs, Janus kinase inhibitors (adjusted hazard ratio [aHR], 3.66; P less than .0001), monoclonal anti-tumor necrosis factor antibodies (aHR, 1.63; P = .0042), and B-cell targeted treatment (aHR, 1.57; P = .0355) were associated with a significantly higher risk for HZ.
Study details: The data come from an analysis of 13,991 patients with RA from the German RABBIT register, which included patients who initiated tsDMARD/bDMARD or csDMARD treatment after at least 1 prior DMARD.
Disclosures: RABBIT is supported by a joint, unconditional grant from AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Hexal, Lilly, MSD, Viatris, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. A Strangfeld, A Zink, GR Burmester, and J Braun declared receiving speaker fees, honoraria, consulting, and/or grants from various sources.
Source: Redeker I et al. Ann Rheum Dis. 2021 Jul 28. doi: 10.1136/annrheumdis-2021-220651.
Key clinical point: In patients with rheumatoid arthritis (RA), treatment with targeted synthetic and biologic disease-modifying antirheumatic drugs (tsDMARDs/bDMARDs) vs conventional synthetic DMARDs (csDMARDs) was associated with a significantly higher risk for herpes zoster (HZ).
Major finding: Compared with csDMARDs, Janus kinase inhibitors (adjusted hazard ratio [aHR], 3.66; P less than .0001), monoclonal anti-tumor necrosis factor antibodies (aHR, 1.63; P = .0042), and B-cell targeted treatment (aHR, 1.57; P = .0355) were associated with a significantly higher risk for HZ.
Study details: The data come from an analysis of 13,991 patients with RA from the German RABBIT register, which included patients who initiated tsDMARD/bDMARD or csDMARD treatment after at least 1 prior DMARD.
Disclosures: RABBIT is supported by a joint, unconditional grant from AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Hexal, Lilly, MSD, Viatris, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. A Strangfeld, A Zink, GR Burmester, and J Braun declared receiving speaker fees, honoraria, consulting, and/or grants from various sources.
Source: Redeker I et al. Ann Rheum Dis. 2021 Jul 28. doi: 10.1136/annrheumdis-2021-220651.
Key clinical point: In patients with rheumatoid arthritis (RA), treatment with targeted synthetic and biologic disease-modifying antirheumatic drugs (tsDMARDs/bDMARDs) vs conventional synthetic DMARDs (csDMARDs) was associated with a significantly higher risk for herpes zoster (HZ).
Major finding: Compared with csDMARDs, Janus kinase inhibitors (adjusted hazard ratio [aHR], 3.66; P less than .0001), monoclonal anti-tumor necrosis factor antibodies (aHR, 1.63; P = .0042), and B-cell targeted treatment (aHR, 1.57; P = .0355) were associated with a significantly higher risk for HZ.
Study details: The data come from an analysis of 13,991 patients with RA from the German RABBIT register, which included patients who initiated tsDMARD/bDMARD or csDMARD treatment after at least 1 prior DMARD.
Disclosures: RABBIT is supported by a joint, unconditional grant from AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Hexal, Lilly, MSD, Viatris, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. A Strangfeld, A Zink, GR Burmester, and J Braun declared receiving speaker fees, honoraria, consulting, and/or grants from various sources.
Source: Redeker I et al. Ann Rheum Dis. 2021 Jul 28. doi: 10.1136/annrheumdis-2021-220651.
Higher anti-PC autoantibodies indicate lower risk for cardiovascular events in early RA
Key clinical point: Higher baseline levels of immunoglobulin M (IgM) anti-phosphorylcholine (anti-PC) autoantibodies in early rheumatoid arthritis (RA) showed benefits in the reduction of cardiovascular risk in younger patients, men, and those at risk for cardiovascular event.
Major finding: Baseline IgM anti-PC autoantibody level above vs below median was associated with lower risk of cardiovascular outcome in patients below 55 years of age at inclusion (adjusted hazard ratio [aHR], 0.360; P = .032), male patients (aHR, 0.558; P = .034), those with a body mass index greater than 30 kg/m2 (aHR, 0.235; P = .026), and those who did not achieve Disease Activity Score for 28 joints remission at 1 year (aHR, 0.592; P = .021).
Study details: Findings are from an analysis of 653 patients with early RA without prevalent cardiovascular disease from the prospective, observational BARFOT cohort.
Disclosures: The Swedish Rheumatism Association, the King Gustav V 80 year’s Foundation, and the Swedish Heart-Lung Foundation funded this research. J Frostegård declared being a registered patent holder for anti-PC.
Source: Ajeganova S et al. Arthritis Res Ther. 2021 Jul 27. doi: 10.1186/s13075-021-02581-0.
Key clinical point: Higher baseline levels of immunoglobulin M (IgM) anti-phosphorylcholine (anti-PC) autoantibodies in early rheumatoid arthritis (RA) showed benefits in the reduction of cardiovascular risk in younger patients, men, and those at risk for cardiovascular event.
Major finding: Baseline IgM anti-PC autoantibody level above vs below median was associated with lower risk of cardiovascular outcome in patients below 55 years of age at inclusion (adjusted hazard ratio [aHR], 0.360; P = .032), male patients (aHR, 0.558; P = .034), those with a body mass index greater than 30 kg/m2 (aHR, 0.235; P = .026), and those who did not achieve Disease Activity Score for 28 joints remission at 1 year (aHR, 0.592; P = .021).
Study details: Findings are from an analysis of 653 patients with early RA without prevalent cardiovascular disease from the prospective, observational BARFOT cohort.
Disclosures: The Swedish Rheumatism Association, the King Gustav V 80 year’s Foundation, and the Swedish Heart-Lung Foundation funded this research. J Frostegård declared being a registered patent holder for anti-PC.
Source: Ajeganova S et al. Arthritis Res Ther. 2021 Jul 27. doi: 10.1186/s13075-021-02581-0.
Key clinical point: Higher baseline levels of immunoglobulin M (IgM) anti-phosphorylcholine (anti-PC) autoantibodies in early rheumatoid arthritis (RA) showed benefits in the reduction of cardiovascular risk in younger patients, men, and those at risk for cardiovascular event.
Major finding: Baseline IgM anti-PC autoantibody level above vs below median was associated with lower risk of cardiovascular outcome in patients below 55 years of age at inclusion (adjusted hazard ratio [aHR], 0.360; P = .032), male patients (aHR, 0.558; P = .034), those with a body mass index greater than 30 kg/m2 (aHR, 0.235; P = .026), and those who did not achieve Disease Activity Score for 28 joints remission at 1 year (aHR, 0.592; P = .021).
Study details: Findings are from an analysis of 653 patients with early RA without prevalent cardiovascular disease from the prospective, observational BARFOT cohort.
Disclosures: The Swedish Rheumatism Association, the King Gustav V 80 year’s Foundation, and the Swedish Heart-Lung Foundation funded this research. J Frostegård declared being a registered patent holder for anti-PC.
Source: Ajeganova S et al. Arthritis Res Ther. 2021 Jul 27. doi: 10.1186/s13075-021-02581-0.
Real-world comparative assessment of tofacitinib and baricitinib in RA
Key clinical point: In real medical practice, tofacitinib and baricitinib demonstrated comparable efficacies and similar safety profiles in patients with rheumatoid arthritis (RA); however, predictive factors contributing to their treatment responses were different.
Major finding: At 24 weeks, clinical response (adjusted mean difference, −0.04; 95% confidence interval, −0.35 to 0.28) and adverse events (P = .866) were not significantly different between the 2 groups. Factors independently associated with the achievement of disease activity score-low disease activity were concomitant oral steroid use (odds ratio [OR], 0.470) in the tofacitinib group and the number of previous use of biological and/or targeted synthetic disease-modifying antirheumatic drugs (OR, 0.700) in the baricitinib group (both P < .05).
Study details: Findings are from the analysis of a real-world cohort of 242 patients with RA who were treated with tofacitinib (n=161) between August 2013 and October 2019 or baricitinib (n=81) between January 2018 and February 2020.
Disclosures: This study did not receive any funding. The authors declared no conflict of interests.
Source: Iwamoto N et al. Arthritis Res Ther. 2021 Jul 23. doi: 10.1186/s13075-021-02582-z.
Key clinical point: In real medical practice, tofacitinib and baricitinib demonstrated comparable efficacies and similar safety profiles in patients with rheumatoid arthritis (RA); however, predictive factors contributing to their treatment responses were different.
Major finding: At 24 weeks, clinical response (adjusted mean difference, −0.04; 95% confidence interval, −0.35 to 0.28) and adverse events (P = .866) were not significantly different between the 2 groups. Factors independently associated with the achievement of disease activity score-low disease activity were concomitant oral steroid use (odds ratio [OR], 0.470) in the tofacitinib group and the number of previous use of biological and/or targeted synthetic disease-modifying antirheumatic drugs (OR, 0.700) in the baricitinib group (both P < .05).
Study details: Findings are from the analysis of a real-world cohort of 242 patients with RA who were treated with tofacitinib (n=161) between August 2013 and October 2019 or baricitinib (n=81) between January 2018 and February 2020.
Disclosures: This study did not receive any funding. The authors declared no conflict of interests.
Source: Iwamoto N et al. Arthritis Res Ther. 2021 Jul 23. doi: 10.1186/s13075-021-02582-z.
Key clinical point: In real medical practice, tofacitinib and baricitinib demonstrated comparable efficacies and similar safety profiles in patients with rheumatoid arthritis (RA); however, predictive factors contributing to their treatment responses were different.
Major finding: At 24 weeks, clinical response (adjusted mean difference, −0.04; 95% confidence interval, −0.35 to 0.28) and adverse events (P = .866) were not significantly different between the 2 groups. Factors independently associated with the achievement of disease activity score-low disease activity were concomitant oral steroid use (odds ratio [OR], 0.470) in the tofacitinib group and the number of previous use of biological and/or targeted synthetic disease-modifying antirheumatic drugs (OR, 0.700) in the baricitinib group (both P < .05).
Study details: Findings are from the analysis of a real-world cohort of 242 patients with RA who were treated with tofacitinib (n=161) between August 2013 and October 2019 or baricitinib (n=81) between January 2018 and February 2020.
Disclosures: This study did not receive any funding. The authors declared no conflict of interests.
Source: Iwamoto N et al. Arthritis Res Ther. 2021 Jul 23. doi: 10.1186/s13075-021-02582-z.
Increased risk for vertebral fractures persists even with low-dose oral glucocorticoids for RA
Key clinical point: Patients with rheumatoid arthritis (RA) currently using low-dose oral glucocorticoid (7.5 mg or lower of prednisolone equivalent dose/day) were at a 59% increased risk of sustaining clinical vertebral fracture compared with past users.
Major finding: Although the overall risk for osteoporotic fractures was not different between past and current users of low-dose oral glucocorticoids (adjusted hazard ratio [aHR], 1.14; 95% confidence interval [CI], 0.98-1.33), the risk for clinical vertebral fracture was significantly higher with current use (aHR, 1.59; 95% CI, 1.11-2.29).
Study details: This was a retrospective cohort study of 15,123 adults aged 50 years or older from the Clinical Practice Research Datalink who were diagnosed with RA between 1997 and 2017.
Disclosures: No specific funding was received for this study. AM Burden and JP van den Bergh reported receiving grants and lectures/advisory board meetings fees from various sources. FD Vries supervised 3 PhD students who were employed with F. Hoffmann La Roche Ltd. All the other authors declared no conflict of interests.
Source: Abtahi S et al. Rheumatology (Oxford). 2021 Jul 13. doi: 10.1093/rheumatology/keab548.
Key clinical point: Patients with rheumatoid arthritis (RA) currently using low-dose oral glucocorticoid (7.5 mg or lower of prednisolone equivalent dose/day) were at a 59% increased risk of sustaining clinical vertebral fracture compared with past users.
Major finding: Although the overall risk for osteoporotic fractures was not different between past and current users of low-dose oral glucocorticoids (adjusted hazard ratio [aHR], 1.14; 95% confidence interval [CI], 0.98-1.33), the risk for clinical vertebral fracture was significantly higher with current use (aHR, 1.59; 95% CI, 1.11-2.29).
Study details: This was a retrospective cohort study of 15,123 adults aged 50 years or older from the Clinical Practice Research Datalink who were diagnosed with RA between 1997 and 2017.
Disclosures: No specific funding was received for this study. AM Burden and JP van den Bergh reported receiving grants and lectures/advisory board meetings fees from various sources. FD Vries supervised 3 PhD students who were employed with F. Hoffmann La Roche Ltd. All the other authors declared no conflict of interests.
Source: Abtahi S et al. Rheumatology (Oxford). 2021 Jul 13. doi: 10.1093/rheumatology/keab548.
Key clinical point: Patients with rheumatoid arthritis (RA) currently using low-dose oral glucocorticoid (7.5 mg or lower of prednisolone equivalent dose/day) were at a 59% increased risk of sustaining clinical vertebral fracture compared with past users.
Major finding: Although the overall risk for osteoporotic fractures was not different between past and current users of low-dose oral glucocorticoids (adjusted hazard ratio [aHR], 1.14; 95% confidence interval [CI], 0.98-1.33), the risk for clinical vertebral fracture was significantly higher with current use (aHR, 1.59; 95% CI, 1.11-2.29).
Study details: This was a retrospective cohort study of 15,123 adults aged 50 years or older from the Clinical Practice Research Datalink who were diagnosed with RA between 1997 and 2017.
Disclosures: No specific funding was received for this study. AM Burden and JP van den Bergh reported receiving grants and lectures/advisory board meetings fees from various sources. FD Vries supervised 3 PhD students who were employed with F. Hoffmann La Roche Ltd. All the other authors declared no conflict of interests.
Source: Abtahi S et al. Rheumatology (Oxford). 2021 Jul 13. doi: 10.1093/rheumatology/keab548.
Rheumatoid arthritis: Olokizumab shows promise in phase 3
Key clinical point: Olokizumab significantly improved signs and symptoms of rheumatoid arthritis (RA) in patients with inadequate response to methotrexate and was reasonably well tolerated over a period of 24 weeks.
Major finding: At 12 weeks, the proportion of patients who achieved American College of Rheumatology 20% response was significantly higher with olokizumab every 2 (63.6%) and 4 (70.4%) weeks vs placebo (25.9%; both P < .0001). Most treatment-emergent adverse events were mild to moderate.
Study details: Findings are from CREDO 1, a phase 3 trial of 428 patients with active RA despite treatment with methotrexate who were randomly assigned to receive subcutaneous olokizumab 64 mg once every 2 or 4 weeks or placebo with continuation of background methotrexate.
Disclosures: This study was funded by CJSC R-Pharm. E Korneva and M Samsonov reported being employees of R-Pharm. Some of the authors declared receiving consulting fees and/or research grants from, being on speakers’ bureau for, being consultant and/or employee of, and/or holding stocks for various sources.
Source: Nasonov E et al. Ann Rheum Dis. 2021 Aug 3. doi: 10.1136/annrheumdis-2021-219876.
Key clinical point: Olokizumab significantly improved signs and symptoms of rheumatoid arthritis (RA) in patients with inadequate response to methotrexate and was reasonably well tolerated over a period of 24 weeks.
Major finding: At 12 weeks, the proportion of patients who achieved American College of Rheumatology 20% response was significantly higher with olokizumab every 2 (63.6%) and 4 (70.4%) weeks vs placebo (25.9%; both P < .0001). Most treatment-emergent adverse events were mild to moderate.
Study details: Findings are from CREDO 1, a phase 3 trial of 428 patients with active RA despite treatment with methotrexate who were randomly assigned to receive subcutaneous olokizumab 64 mg once every 2 or 4 weeks or placebo with continuation of background methotrexate.
Disclosures: This study was funded by CJSC R-Pharm. E Korneva and M Samsonov reported being employees of R-Pharm. Some of the authors declared receiving consulting fees and/or research grants from, being on speakers’ bureau for, being consultant and/or employee of, and/or holding stocks for various sources.
Source: Nasonov E et al. Ann Rheum Dis. 2021 Aug 3. doi: 10.1136/annrheumdis-2021-219876.
Key clinical point: Olokizumab significantly improved signs and symptoms of rheumatoid arthritis (RA) in patients with inadequate response to methotrexate and was reasonably well tolerated over a period of 24 weeks.
Major finding: At 12 weeks, the proportion of patients who achieved American College of Rheumatology 20% response was significantly higher with olokizumab every 2 (63.6%) and 4 (70.4%) weeks vs placebo (25.9%; both P < .0001). Most treatment-emergent adverse events were mild to moderate.
Study details: Findings are from CREDO 1, a phase 3 trial of 428 patients with active RA despite treatment with methotrexate who were randomly assigned to receive subcutaneous olokizumab 64 mg once every 2 or 4 weeks or placebo with continuation of background methotrexate.
Disclosures: This study was funded by CJSC R-Pharm. E Korneva and M Samsonov reported being employees of R-Pharm. Some of the authors declared receiving consulting fees and/or research grants from, being on speakers’ bureau for, being consultant and/or employee of, and/or holding stocks for various sources.
Source: Nasonov E et al. Ann Rheum Dis. 2021 Aug 3. doi: 10.1136/annrheumdis-2021-219876.
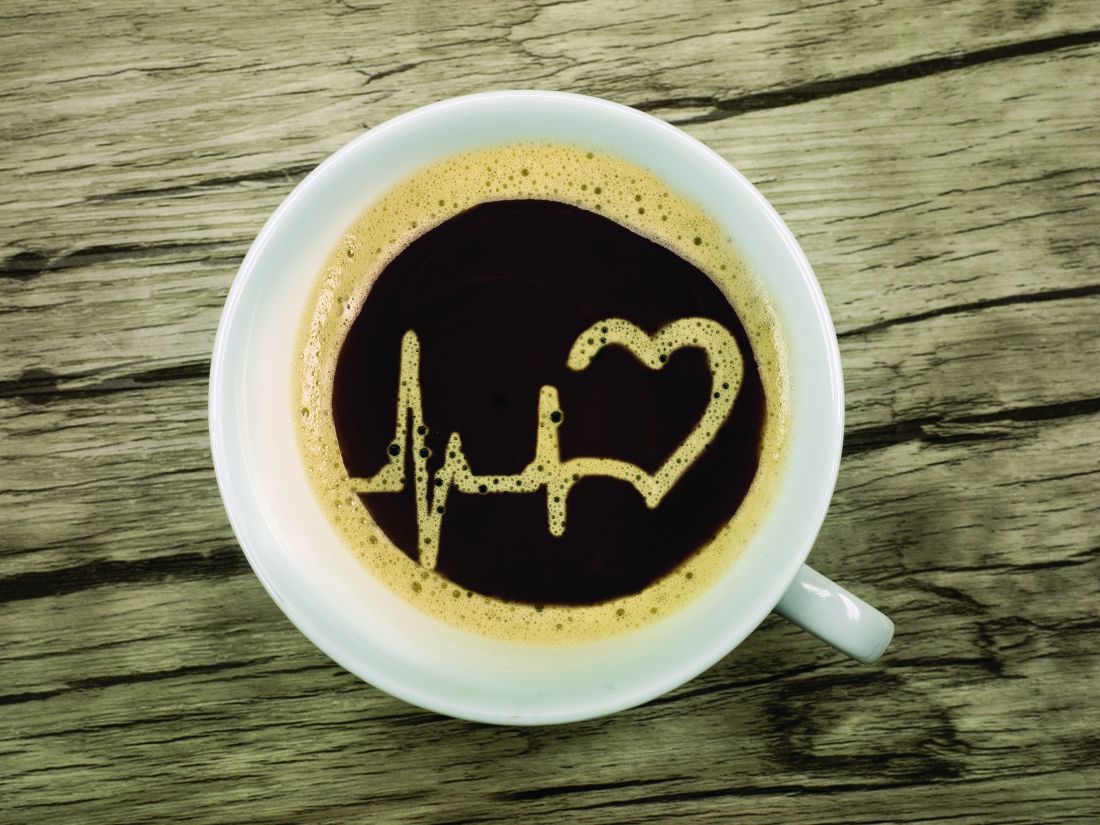
Coffee drinking in midlife tied to heart benefits
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Circulating miR-140 and serum leptin could help discriminate PsA from RA
Key clinical point: Increased expression levels of circulating microRNA (miRNA) like miR-140 and serum levels of few adipokines like leptin could serve as biomarkers for psoriatic arthritis (PsA) and could be particularly helpful in the differential diagnosis of peripheral PsA from other rheumatic diseases like rheumatoid arthritis (RA).
Major finding: MiR-140 and serum leptin levels were significantly higher in patients with PsA vs those with RA and healthy controls (HCs; both P < .001) and served as predictors of RA vs PsA (area under the receiver-operating characteristic curve, 0.91 and 0.83, respectively).
Study details: Findings are from a case-control study including 50 patients with peripheral PsA, 50 patients with RA, and 50 HCs.
Disclosures: No specific funding was received for this study. The authors declared no conflict of interests.
Source: Cheleschi S et al. Transl Res. 2021 Aug 8. doi: 10.1016/j.trsl.2021.08.001.
Key clinical point: Increased expression levels of circulating microRNA (miRNA) like miR-140 and serum levels of few adipokines like leptin could serve as biomarkers for psoriatic arthritis (PsA) and could be particularly helpful in the differential diagnosis of peripheral PsA from other rheumatic diseases like rheumatoid arthritis (RA).
Major finding: MiR-140 and serum leptin levels were significantly higher in patients with PsA vs those with RA and healthy controls (HCs; both P < .001) and served as predictors of RA vs PsA (area under the receiver-operating characteristic curve, 0.91 and 0.83, respectively).
Study details: Findings are from a case-control study including 50 patients with peripheral PsA, 50 patients with RA, and 50 HCs.
Disclosures: No specific funding was received for this study. The authors declared no conflict of interests.
Source: Cheleschi S et al. Transl Res. 2021 Aug 8. doi: 10.1016/j.trsl.2021.08.001.
Key clinical point: Increased expression levels of circulating microRNA (miRNA) like miR-140 and serum levels of few adipokines like leptin could serve as biomarkers for psoriatic arthritis (PsA) and could be particularly helpful in the differential diagnosis of peripheral PsA from other rheumatic diseases like rheumatoid arthritis (RA).
Major finding: MiR-140 and serum leptin levels were significantly higher in patients with PsA vs those with RA and healthy controls (HCs; both P < .001) and served as predictors of RA vs PsA (area under the receiver-operating characteristic curve, 0.91 and 0.83, respectively).
Study details: Findings are from a case-control study including 50 patients with peripheral PsA, 50 patients with RA, and 50 HCs.
Disclosures: No specific funding was received for this study. The authors declared no conflict of interests.
Source: Cheleschi S et al. Transl Res. 2021 Aug 8. doi: 10.1016/j.trsl.2021.08.001.
Identifying potential risks factors for PsA
Key clinical point: A previous diagnosis of gout, uveitis, metabolic and lifestyle factors like obesity, alcohol consumption, and infections like pharyngitis and skin infections were potential risk factors for psoriatic arthritis (PsA), whereas statin use showed a negative association with PsA.
Major finding: A previous diagnosis of gout (odds ratio [OR], 2.19), uveitis (OR, 3.79), alcohol use (OR, 1.67), obesity (OR, 1.64), pharyngitis (OR, 1.23), and skin infection (OR, 1.37; all P < .001) were significant risk factors for PsA. The use of statin was negatively associated with PsA (OR, 0.53; P < .001).
Study details: This was a set of 4 separate case-control studies conducted in parallel and included cases of incident PsA (n=7,594), psoriasis (n=111,375), rheumatoid arthritis (RA; n=28,341), and ankylosing spondylitis (AS; n=3,253) matched to control participants (PsA, n=75,930; psoriasis, n=1113,345; RA, n=283,226; and AS, n=32,530).
Disclosures: This work was supported by grants from the National Institute of Health and internal grants from the University of Pennsylvania. Dr. Gelfand, Dr. Love, and Dr. Ogdie declared receiving research grants, honoraria, and reimbursement from and/or serving as a consultant for various sources.
Source: Meer E et al. J Rheumatol. 2021 Aug 1. doi: 10.3899/jrheum.210006.
Key clinical point: A previous diagnosis of gout, uveitis, metabolic and lifestyle factors like obesity, alcohol consumption, and infections like pharyngitis and skin infections were potential risk factors for psoriatic arthritis (PsA), whereas statin use showed a negative association with PsA.
Major finding: A previous diagnosis of gout (odds ratio [OR], 2.19), uveitis (OR, 3.79), alcohol use (OR, 1.67), obesity (OR, 1.64), pharyngitis (OR, 1.23), and skin infection (OR, 1.37; all P < .001) were significant risk factors for PsA. The use of statin was negatively associated with PsA (OR, 0.53; P < .001).
Study details: This was a set of 4 separate case-control studies conducted in parallel and included cases of incident PsA (n=7,594), psoriasis (n=111,375), rheumatoid arthritis (RA; n=28,341), and ankylosing spondylitis (AS; n=3,253) matched to control participants (PsA, n=75,930; psoriasis, n=1113,345; RA, n=283,226; and AS, n=32,530).
Disclosures: This work was supported by grants from the National Institute of Health and internal grants from the University of Pennsylvania. Dr. Gelfand, Dr. Love, and Dr. Ogdie declared receiving research grants, honoraria, and reimbursement from and/or serving as a consultant for various sources.
Source: Meer E et al. J Rheumatol. 2021 Aug 1. doi: 10.3899/jrheum.210006.
Key clinical point: A previous diagnosis of gout, uveitis, metabolic and lifestyle factors like obesity, alcohol consumption, and infections like pharyngitis and skin infections were potential risk factors for psoriatic arthritis (PsA), whereas statin use showed a negative association with PsA.
Major finding: A previous diagnosis of gout (odds ratio [OR], 2.19), uveitis (OR, 3.79), alcohol use (OR, 1.67), obesity (OR, 1.64), pharyngitis (OR, 1.23), and skin infection (OR, 1.37; all P < .001) were significant risk factors for PsA. The use of statin was negatively associated with PsA (OR, 0.53; P < .001).
Study details: This was a set of 4 separate case-control studies conducted in parallel and included cases of incident PsA (n=7,594), psoriasis (n=111,375), rheumatoid arthritis (RA; n=28,341), and ankylosing spondylitis (AS; n=3,253) matched to control participants (PsA, n=75,930; psoriasis, n=1113,345; RA, n=283,226; and AS, n=32,530).
Disclosures: This work was supported by grants from the National Institute of Health and internal grants from the University of Pennsylvania. Dr. Gelfand, Dr. Love, and Dr. Ogdie declared receiving research grants, honoraria, and reimbursement from and/or serving as a consultant for various sources.
Source: Meer E et al. J Rheumatol. 2021 Aug 1. doi: 10.3899/jrheum.210006.
No clinically relevant increase in mortality in patients with PsA
Key clinical point: Psoriatic arthritis (PsA) was not associated with a significant increase in risk for all-cause mortality in the more recent era between 2003 and 2018. Moreover, the major causes of death in PsA were not different than those commonly reported in the general population.
Major finding: The number of deaths was similar in PsA and control groups (8.9% and 7.9%, respectively), and the association between PsA and a higher risk for all-cause mortality was not significant (hazard ratio, 1.02; 95% confidence interval, 0.90-1.15). In the general population, malignancy (26.0%) and ischemic heart disease (15.8%) were the leading causes of death in patients with PsA.
Study details: Findings are from an analysis of a cohort of 5,275 adults newly diagnosed with PsA between 2003 and 2018, matched with 21,011 control participants and followed for 7.2±4.4 years.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Haddad A et al. J Rheumatol. 2021 Jul 15. doi: 10.3899/jrheum.210159.
Key clinical point: Psoriatic arthritis (PsA) was not associated with a significant increase in risk for all-cause mortality in the more recent era between 2003 and 2018. Moreover, the major causes of death in PsA were not different than those commonly reported in the general population.
Major finding: The number of deaths was similar in PsA and control groups (8.9% and 7.9%, respectively), and the association between PsA and a higher risk for all-cause mortality was not significant (hazard ratio, 1.02; 95% confidence interval, 0.90-1.15). In the general population, malignancy (26.0%) and ischemic heart disease (15.8%) were the leading causes of death in patients with PsA.
Study details: Findings are from an analysis of a cohort of 5,275 adults newly diagnosed with PsA between 2003 and 2018, matched with 21,011 control participants and followed for 7.2±4.4 years.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Haddad A et al. J Rheumatol. 2021 Jul 15. doi: 10.3899/jrheum.210159.
Key clinical point: Psoriatic arthritis (PsA) was not associated with a significant increase in risk for all-cause mortality in the more recent era between 2003 and 2018. Moreover, the major causes of death in PsA were not different than those commonly reported in the general population.
Major finding: The number of deaths was similar in PsA and control groups (8.9% and 7.9%, respectively), and the association between PsA and a higher risk for all-cause mortality was not significant (hazard ratio, 1.02; 95% confidence interval, 0.90-1.15). In the general population, malignancy (26.0%) and ischemic heart disease (15.8%) were the leading causes of death in patients with PsA.
Study details: Findings are from an analysis of a cohort of 5,275 adults newly diagnosed with PsA between 2003 and 2018, matched with 21,011 control participants and followed for 7.2±4.4 years.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Haddad A et al. J Rheumatol. 2021 Jul 15. doi: 10.3899/jrheum.210159.
Oligoarticular PsA similar to polyarticular PsA with few exceptions
Key clinical point: Oligoarticular psoriatic arthritis (PsA) is similar to polyarticular disease. However, involvement of lower extremity small joint had the highest chances of polyarticular presentation, whereas low Short Form-36 (SF-36) Mental Component Summary (MCS) score was significantly associated with progression from oligoarthritis to polyarthritis.
Major finding: Demographics and clinical characteristics were similar between the oligoarticular and polyarticular PsA groups. However, polyarticular PsA was associated with higher odds of lower extremity small joints (odds ratio, 17.15; P < .001). Among patients with oligoarticular PsA, 39% developed polyarticular PsA with lower SF-36 MCS being the only predictor for progression (hazard ratio, 0.97; P = .01).
Study details: Findings are from a longitudinal study including 407 patients with PsA who entered the University of Toronto PsA clinic within 12 months of diagnosis between 1978 and 2018, of which 47% presented with oligoarthritis and 53% with polyarthritis.
Disclosures: The University of Toronto PsA program was funded by the Krembil Foundation. The authors declared no conflict of interests.
Source: Gladman DD et al. J Rheumatol. 2021 Aug 1. doi: 10.3899/jrheum.210434.
Key clinical point: Oligoarticular psoriatic arthritis (PsA) is similar to polyarticular disease. However, involvement of lower extremity small joint had the highest chances of polyarticular presentation, whereas low Short Form-36 (SF-36) Mental Component Summary (MCS) score was significantly associated with progression from oligoarthritis to polyarthritis.
Major finding: Demographics and clinical characteristics were similar between the oligoarticular and polyarticular PsA groups. However, polyarticular PsA was associated with higher odds of lower extremity small joints (odds ratio, 17.15; P < .001). Among patients with oligoarticular PsA, 39% developed polyarticular PsA with lower SF-36 MCS being the only predictor for progression (hazard ratio, 0.97; P = .01).
Study details: Findings are from a longitudinal study including 407 patients with PsA who entered the University of Toronto PsA clinic within 12 months of diagnosis between 1978 and 2018, of which 47% presented with oligoarthritis and 53% with polyarthritis.
Disclosures: The University of Toronto PsA program was funded by the Krembil Foundation. The authors declared no conflict of interests.
Source: Gladman DD et al. J Rheumatol. 2021 Aug 1. doi: 10.3899/jrheum.210434.
Key clinical point: Oligoarticular psoriatic arthritis (PsA) is similar to polyarticular disease. However, involvement of lower extremity small joint had the highest chances of polyarticular presentation, whereas low Short Form-36 (SF-36) Mental Component Summary (MCS) score was significantly associated with progression from oligoarthritis to polyarthritis.
Major finding: Demographics and clinical characteristics were similar between the oligoarticular and polyarticular PsA groups. However, polyarticular PsA was associated with higher odds of lower extremity small joints (odds ratio, 17.15; P < .001). Among patients with oligoarticular PsA, 39% developed polyarticular PsA with lower SF-36 MCS being the only predictor for progression (hazard ratio, 0.97; P = .01).
Study details: Findings are from a longitudinal study including 407 patients with PsA who entered the University of Toronto PsA clinic within 12 months of diagnosis between 1978 and 2018, of which 47% presented with oligoarthritis and 53% with polyarthritis.
Disclosures: The University of Toronto PsA program was funded by the Krembil Foundation. The authors declared no conflict of interests.
Source: Gladman DD et al. J Rheumatol. 2021 Aug 1. doi: 10.3899/jrheum.210434.