User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
SCAMP: Assessing body-focused repetitive behaviors
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
Are we failing to diagnose and treat the many faces of catatonia?
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
How do digital technologies affect young people’s mental health?
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
Is anosognosia a delusion, a negative symptom, or a cognitive deficit?
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
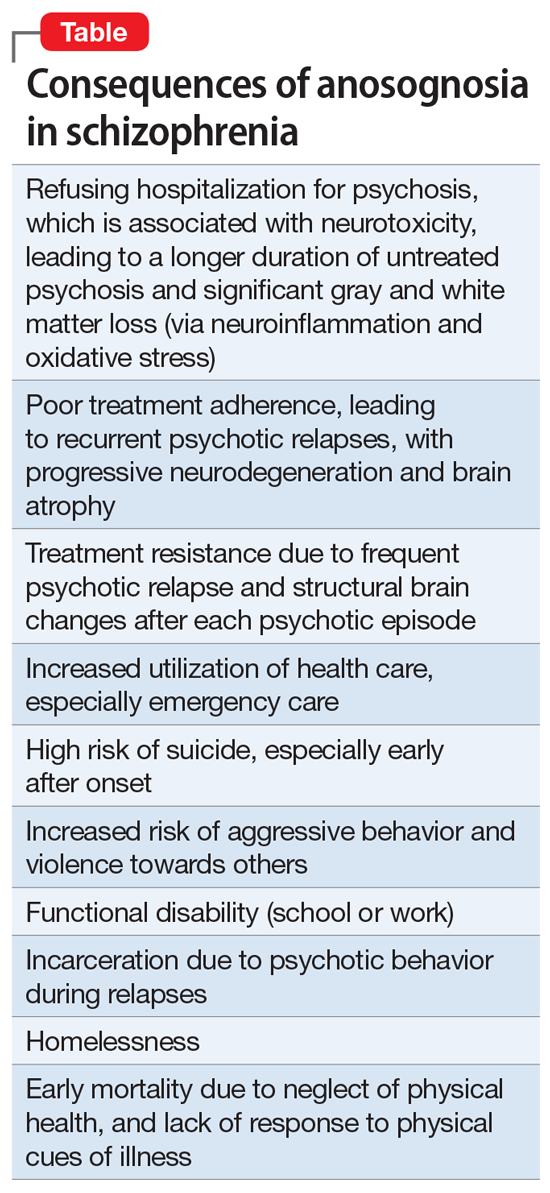
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.
Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Formaldehyde exposure tied to cognitive impairment
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
New studies suggest Omicron infections are less severe than Delta ones
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
Last call? Moderate alcohol’s health benefits look increasingly doubtful
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
FDA authorizes Pfizer antiviral pill for COVID-19
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
Transcranial magnetic stimulation shows promise for alcohol addiction
Deep, repetitive transcranial magnetic stimulation (TMS) is safe and effective in decreasing symptoms of alcohol addiction and brain reactivity, new research suggests.
In a randomized, double-blind, sham-controlled trial, participants who received TMS targeting the medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC) for 3 weeks showed significantly reduced heavy drinking days, compared with a group who received a sham treatment.
and showed less functional connectivity on MRI in areas of the brain that can trigger craving and relapse.
Clinicians should “keep their eyes open” in the wake of this phase 2 trial, cocorresponding author Markus Heilig, MD, PhD, professor of psychiatry and director at the Center for Social and Affective Neuroscience, department of biomedical and clinical sciences, Linköping (Sweden) University, said in an interview.
“If and when this replicates in the equivalent of a phase 3 study, we will actually have a completely novel treatment available for this difficult to treat and very impactful disease,” Dr. Heilig said.
The findings were published online Dec. 5, 2021, in Biological Psychiatry.
Proof of concept
In the proof-of-concept trial, researchers enrolled and randomly assigned 51 treatment-seeking adults with moderate to severe alcohol dependence to receive active or sham treatment. Before treatment, participants completed “craving induction,” which included holding and smelling but not consuming an alcoholic beverage.
Dr. Heilig noted that, before stimulating the brain, “you want to make it as malleable as possible;” and brain networks tend to be more malleable when they are active.
During the 3-week treatment phase, active or sham stimulations were delivered in five 30-minute sessions per week. During the sessions, all participants wore a helmet with a deep TMS coil produced by BrainsWay.
In the active-stimulation group, each session included 100 trains of 30 pulses at 10 Hz (3 seconds) with 15-second intervals, for a total of 3,000 pulses. The sham stimulation produced the same acoustic artifact and generated skin sensations mimicking those of the active stimulation, but it did not involve a magnetic field.
Participants, operators, and raters were blinded to the type of coil used.
Five participants relapsed during the first 3 weeks of treatment and were excluded from the analysis. The mean age of those completing treatment (n = 23 in each group) was 43 years, and two-thirds were men.
The gender makeup of the study reflects “what a real treatment-seeking group looks like,” Dr. Heilig said.
During the 12-week follow-up phase, five additional participants dropped out.
‘Pretty robust’ treatment effect
The primary outcome was reduction in percentage of heavy drinking days (pHDD), defined as consuming at least five drinks of 12 grams of alcohol per day for men and at least four such drinks for women.
Initially, pHDD dropped in both groups, which is something generally seen in alcohol studies, said Dr. Heilig. “The moment people decide to participate in a study, everybody drops their consumption, [which] biases a study like this against picking up an effect.”
However, heavy drinking days increased during follow-up in the sham group but remained low in the active-treatment group. The mean pHDD was significantly lower in the active versus sham groups (2.9% vs. 10.6%, P = .037).
“So despite the bias, a treatment effect does emerge,” and was “pretty robust,” Dr. Heilig said.
This was supported by a significant group difference in weekly alcohol consumption and a trend-level difference in percentage of alcohol-positive urine samples.
A secondary outcome was change in alcohol craving, assessed with the Penn Alcohol Craving Scale; PACS scores decreased in both groups during treatment but was more steeply reduced in the active group. During follow-up, craving levels increased to a lesser extent in the active group.
MRI scans showed reduced connectivity from the mPFC to the subgenual ACC, an area involved in negative emotions that can trigger craving and relapse, said Dr. Heilig. There was also reduced connectivity between the dorsal ACC and caudate, a circuit involved in the reward system.
In treatment trials, researchers look for a biomarker of target engagement. However, “to date, there has been no study using TMS that has actually demonstrated the intervention had a measurable effect on brain activity. So to me, this is a biomarker; it did something to the brain,” Dr. Heilig said.
Delving deeper into the brain
The results underline the importance of stimulating deeper parts of the brain, cocorresponding author Abraham Zangen, PhD, head of the Brain Stimulation and Behavior Lab and chair of the psychobiology brain program, Ben Gurion University, Be’er Sheva, Israel, said in an interview.
Early TMS studies, which involved superficial brain stimulation, reduced cigarette consumption but was not associated with quitting, Dr. Zangen said. “It was only when we targeted deeper parts of the prefrontal cortex that we were able to induce smoking cessation.”
It was this research that led to approval by the Food and Drug Administration of deep TMS for smoking cessation.
This same deep-brain approach was used in the current study. “So the emphasis on the technology that allows penetration into deeper parts of the brain and targeting the relevant pathological circuitry of addiction is a key complement of the success of this study,” Dr. Zangen said.
Results also showed no serious adverse events. Only a few participants reported transient headaches, which all resolved spontaneously; and frequency did not differ between groups.
Dr. Heilig now hopes to carry out a multisite phase 3 study of the intervention and would suggest it involve 4 (instead of 3) weeks of initial treatment and then a weekly booster session. “There are biological reasons to believe that might be more efficient, although we don’t have the data,” he said.
On the other hand, he noted, the longer the trial, the more difficult it might be to recruit patients.
Clinically significant?
Commenting on the study, Derek Blevins, MD, assistant professor of clinical psychiatry at Columbia University and research psychiatrist, division on substance use disorders, New York State Psychiatric Institute, both in New York, called the research “really exciting.”
To date, most TMS studies have been relatively small and looked at a target such as craving. Although these studies did show some effect, the clinical significance of that effect was unclear, said Dr. Blevins, who was not involved with the current research.
“I think this new study actually demonstrated a clinically significant effect of a noninvasive treatment for a disease that’s very difficult to treat,” he said.
A potential limitation of the study, however, was it required abstinence, Dr. Blevins noted. It would be “really helpful” to understand how TMS might aid individuals such as those who relapsed during the study, “because they’re the more treatment-refractory individuals we see in clinical practice.”
If a multicenter trial is launched, Dr. Blevins said he would also like it to include an ethnically and racially diverse population.
The study was supported by grants from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council. Dr. Heilig reported having received consulting fees, research support, or other compensation from Indivior, Camurus, BrainsWay, Aelis Farma, and Janssen Pharmaceuticals. Dr. Zangen is an inventor of deep TMS coils and has financial interest in BrainsWay, which produces and markets these coils. Dr. Blevins reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deep, repetitive transcranial magnetic stimulation (TMS) is safe and effective in decreasing symptoms of alcohol addiction and brain reactivity, new research suggests.
In a randomized, double-blind, sham-controlled trial, participants who received TMS targeting the medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC) for 3 weeks showed significantly reduced heavy drinking days, compared with a group who received a sham treatment.
and showed less functional connectivity on MRI in areas of the brain that can trigger craving and relapse.
Clinicians should “keep their eyes open” in the wake of this phase 2 trial, cocorresponding author Markus Heilig, MD, PhD, professor of psychiatry and director at the Center for Social and Affective Neuroscience, department of biomedical and clinical sciences, Linköping (Sweden) University, said in an interview.
“If and when this replicates in the equivalent of a phase 3 study, we will actually have a completely novel treatment available for this difficult to treat and very impactful disease,” Dr. Heilig said.
The findings were published online Dec. 5, 2021, in Biological Psychiatry.
Proof of concept
In the proof-of-concept trial, researchers enrolled and randomly assigned 51 treatment-seeking adults with moderate to severe alcohol dependence to receive active or sham treatment. Before treatment, participants completed “craving induction,” which included holding and smelling but not consuming an alcoholic beverage.
Dr. Heilig noted that, before stimulating the brain, “you want to make it as malleable as possible;” and brain networks tend to be more malleable when they are active.
During the 3-week treatment phase, active or sham stimulations were delivered in five 30-minute sessions per week. During the sessions, all participants wore a helmet with a deep TMS coil produced by BrainsWay.
In the active-stimulation group, each session included 100 trains of 30 pulses at 10 Hz (3 seconds) with 15-second intervals, for a total of 3,000 pulses. The sham stimulation produced the same acoustic artifact and generated skin sensations mimicking those of the active stimulation, but it did not involve a magnetic field.
Participants, operators, and raters were blinded to the type of coil used.
Five participants relapsed during the first 3 weeks of treatment and were excluded from the analysis. The mean age of those completing treatment (n = 23 in each group) was 43 years, and two-thirds were men.
The gender makeup of the study reflects “what a real treatment-seeking group looks like,” Dr. Heilig said.
During the 12-week follow-up phase, five additional participants dropped out.
‘Pretty robust’ treatment effect
The primary outcome was reduction in percentage of heavy drinking days (pHDD), defined as consuming at least five drinks of 12 grams of alcohol per day for men and at least four such drinks for women.
Initially, pHDD dropped in both groups, which is something generally seen in alcohol studies, said Dr. Heilig. “The moment people decide to participate in a study, everybody drops their consumption, [which] biases a study like this against picking up an effect.”
However, heavy drinking days increased during follow-up in the sham group but remained low in the active-treatment group. The mean pHDD was significantly lower in the active versus sham groups (2.9% vs. 10.6%, P = .037).
“So despite the bias, a treatment effect does emerge,” and was “pretty robust,” Dr. Heilig said.
This was supported by a significant group difference in weekly alcohol consumption and a trend-level difference in percentage of alcohol-positive urine samples.
A secondary outcome was change in alcohol craving, assessed with the Penn Alcohol Craving Scale; PACS scores decreased in both groups during treatment but was more steeply reduced in the active group. During follow-up, craving levels increased to a lesser extent in the active group.
MRI scans showed reduced connectivity from the mPFC to the subgenual ACC, an area involved in negative emotions that can trigger craving and relapse, said Dr. Heilig. There was also reduced connectivity between the dorsal ACC and caudate, a circuit involved in the reward system.
In treatment trials, researchers look for a biomarker of target engagement. However, “to date, there has been no study using TMS that has actually demonstrated the intervention had a measurable effect on brain activity. So to me, this is a biomarker; it did something to the brain,” Dr. Heilig said.
Delving deeper into the brain
The results underline the importance of stimulating deeper parts of the brain, cocorresponding author Abraham Zangen, PhD, head of the Brain Stimulation and Behavior Lab and chair of the psychobiology brain program, Ben Gurion University, Be’er Sheva, Israel, said in an interview.
Early TMS studies, which involved superficial brain stimulation, reduced cigarette consumption but was not associated with quitting, Dr. Zangen said. “It was only when we targeted deeper parts of the prefrontal cortex that we were able to induce smoking cessation.”
It was this research that led to approval by the Food and Drug Administration of deep TMS for smoking cessation.
This same deep-brain approach was used in the current study. “So the emphasis on the technology that allows penetration into deeper parts of the brain and targeting the relevant pathological circuitry of addiction is a key complement of the success of this study,” Dr. Zangen said.
Results also showed no serious adverse events. Only a few participants reported transient headaches, which all resolved spontaneously; and frequency did not differ between groups.
Dr. Heilig now hopes to carry out a multisite phase 3 study of the intervention and would suggest it involve 4 (instead of 3) weeks of initial treatment and then a weekly booster session. “There are biological reasons to believe that might be more efficient, although we don’t have the data,” he said.
On the other hand, he noted, the longer the trial, the more difficult it might be to recruit patients.
Clinically significant?
Commenting on the study, Derek Blevins, MD, assistant professor of clinical psychiatry at Columbia University and research psychiatrist, division on substance use disorders, New York State Psychiatric Institute, both in New York, called the research “really exciting.”
To date, most TMS studies have been relatively small and looked at a target such as craving. Although these studies did show some effect, the clinical significance of that effect was unclear, said Dr. Blevins, who was not involved with the current research.
“I think this new study actually demonstrated a clinically significant effect of a noninvasive treatment for a disease that’s very difficult to treat,” he said.
A potential limitation of the study, however, was it required abstinence, Dr. Blevins noted. It would be “really helpful” to understand how TMS might aid individuals such as those who relapsed during the study, “because they’re the more treatment-refractory individuals we see in clinical practice.”
If a multicenter trial is launched, Dr. Blevins said he would also like it to include an ethnically and racially diverse population.
The study was supported by grants from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council. Dr. Heilig reported having received consulting fees, research support, or other compensation from Indivior, Camurus, BrainsWay, Aelis Farma, and Janssen Pharmaceuticals. Dr. Zangen is an inventor of deep TMS coils and has financial interest in BrainsWay, which produces and markets these coils. Dr. Blevins reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deep, repetitive transcranial magnetic stimulation (TMS) is safe and effective in decreasing symptoms of alcohol addiction and brain reactivity, new research suggests.
In a randomized, double-blind, sham-controlled trial, participants who received TMS targeting the medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC) for 3 weeks showed significantly reduced heavy drinking days, compared with a group who received a sham treatment.
and showed less functional connectivity on MRI in areas of the brain that can trigger craving and relapse.
Clinicians should “keep their eyes open” in the wake of this phase 2 trial, cocorresponding author Markus Heilig, MD, PhD, professor of psychiatry and director at the Center for Social and Affective Neuroscience, department of biomedical and clinical sciences, Linköping (Sweden) University, said in an interview.
“If and when this replicates in the equivalent of a phase 3 study, we will actually have a completely novel treatment available for this difficult to treat and very impactful disease,” Dr. Heilig said.
The findings were published online Dec. 5, 2021, in Biological Psychiatry.
Proof of concept
In the proof-of-concept trial, researchers enrolled and randomly assigned 51 treatment-seeking adults with moderate to severe alcohol dependence to receive active or sham treatment. Before treatment, participants completed “craving induction,” which included holding and smelling but not consuming an alcoholic beverage.
Dr. Heilig noted that, before stimulating the brain, “you want to make it as malleable as possible;” and brain networks tend to be more malleable when they are active.
During the 3-week treatment phase, active or sham stimulations were delivered in five 30-minute sessions per week. During the sessions, all participants wore a helmet with a deep TMS coil produced by BrainsWay.
In the active-stimulation group, each session included 100 trains of 30 pulses at 10 Hz (3 seconds) with 15-second intervals, for a total of 3,000 pulses. The sham stimulation produced the same acoustic artifact and generated skin sensations mimicking those of the active stimulation, but it did not involve a magnetic field.
Participants, operators, and raters were blinded to the type of coil used.
Five participants relapsed during the first 3 weeks of treatment and were excluded from the analysis. The mean age of those completing treatment (n = 23 in each group) was 43 years, and two-thirds were men.
The gender makeup of the study reflects “what a real treatment-seeking group looks like,” Dr. Heilig said.
During the 12-week follow-up phase, five additional participants dropped out.
‘Pretty robust’ treatment effect
The primary outcome was reduction in percentage of heavy drinking days (pHDD), defined as consuming at least five drinks of 12 grams of alcohol per day for men and at least four such drinks for women.
Initially, pHDD dropped in both groups, which is something generally seen in alcohol studies, said Dr. Heilig. “The moment people decide to participate in a study, everybody drops their consumption, [which] biases a study like this against picking up an effect.”
However, heavy drinking days increased during follow-up in the sham group but remained low in the active-treatment group. The mean pHDD was significantly lower in the active versus sham groups (2.9% vs. 10.6%, P = .037).
“So despite the bias, a treatment effect does emerge,” and was “pretty robust,” Dr. Heilig said.
This was supported by a significant group difference in weekly alcohol consumption and a trend-level difference in percentage of alcohol-positive urine samples.
A secondary outcome was change in alcohol craving, assessed with the Penn Alcohol Craving Scale; PACS scores decreased in both groups during treatment but was more steeply reduced in the active group. During follow-up, craving levels increased to a lesser extent in the active group.
MRI scans showed reduced connectivity from the mPFC to the subgenual ACC, an area involved in negative emotions that can trigger craving and relapse, said Dr. Heilig. There was also reduced connectivity between the dorsal ACC and caudate, a circuit involved in the reward system.
In treatment trials, researchers look for a biomarker of target engagement. However, “to date, there has been no study using TMS that has actually demonstrated the intervention had a measurable effect on brain activity. So to me, this is a biomarker; it did something to the brain,” Dr. Heilig said.
Delving deeper into the brain
The results underline the importance of stimulating deeper parts of the brain, cocorresponding author Abraham Zangen, PhD, head of the Brain Stimulation and Behavior Lab and chair of the psychobiology brain program, Ben Gurion University, Be’er Sheva, Israel, said in an interview.
Early TMS studies, which involved superficial brain stimulation, reduced cigarette consumption but was not associated with quitting, Dr. Zangen said. “It was only when we targeted deeper parts of the prefrontal cortex that we were able to induce smoking cessation.”
It was this research that led to approval by the Food and Drug Administration of deep TMS for smoking cessation.
This same deep-brain approach was used in the current study. “So the emphasis on the technology that allows penetration into deeper parts of the brain and targeting the relevant pathological circuitry of addiction is a key complement of the success of this study,” Dr. Zangen said.
Results also showed no serious adverse events. Only a few participants reported transient headaches, which all resolved spontaneously; and frequency did not differ between groups.
Dr. Heilig now hopes to carry out a multisite phase 3 study of the intervention and would suggest it involve 4 (instead of 3) weeks of initial treatment and then a weekly booster session. “There are biological reasons to believe that might be more efficient, although we don’t have the data,” he said.
On the other hand, he noted, the longer the trial, the more difficult it might be to recruit patients.
Clinically significant?
Commenting on the study, Derek Blevins, MD, assistant professor of clinical psychiatry at Columbia University and research psychiatrist, division on substance use disorders, New York State Psychiatric Institute, both in New York, called the research “really exciting.”
To date, most TMS studies have been relatively small and looked at a target such as craving. Although these studies did show some effect, the clinical significance of that effect was unclear, said Dr. Blevins, who was not involved with the current research.
“I think this new study actually demonstrated a clinically significant effect of a noninvasive treatment for a disease that’s very difficult to treat,” he said.
A potential limitation of the study, however, was it required abstinence, Dr. Blevins noted. It would be “really helpful” to understand how TMS might aid individuals such as those who relapsed during the study, “because they’re the more treatment-refractory individuals we see in clinical practice.”
If a multicenter trial is launched, Dr. Blevins said he would also like it to include an ethnically and racially diverse population.
The study was supported by grants from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council. Dr. Heilig reported having received consulting fees, research support, or other compensation from Indivior, Camurus, BrainsWay, Aelis Farma, and Janssen Pharmaceuticals. Dr. Zangen is an inventor of deep TMS coils and has financial interest in BrainsWay, which produces and markets these coils. Dr. Blevins reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BIOLOGICAL PSYCHIATRY
The mess that is matching in psychiatry
The day I interviewed at Johns Hopkins in Baltimore, like every other day of residency interviews, was a very long and draining day.
I started by meeting alone with Philip Slavney, MD, the residency director, who spoke with me about the program and gave me a schedule to follow. I was to meet with residents and psychiatrists, some of whom had graduated from my medical school, and was sent to the Bayview campus a few miles away to have lunch and attend a few meetings. By the time I boarded an Amtrak train at Baltimore Penn Station, I was tired but I liked what I had seen. By the end of the interview season, I had crossed four programs off my list and had decided to rank only three.
In 1987, there were 987 residency positions in psychiatry in the United States, and 83.6% of those positions filled with a combination of U.S. and international medical graduates. Still, this was a risky move; the programs that I decided to rank would fill, but I was matching separately for an internship year in internal medicine in New York and decided that I would rather reapply in a year than risk matching at a program I didn’t want to go to.
I wasn’t quite sure where I wanted to rank Hopkins on my list, so I called Dr. Slavney and said I wanted to come back and meet more members of the department. He did not hide his surprise and was quick to tell me that no one had ever requested a second set of interviews. I mentioned specific people I wanted to meet with, and he was kind enough to accommodate my request and set up a second day of interviews for me.
Needless to say, the residency match felt very personal – at least to me – and although I felt vulnerable, I also felt empowered. Because of the low pay, patients with stigmatized illnesses, and the rampant belief that psychiatry was not “real” medicine and the patients never got better, psychiatry was not a desired specialty.
The residency application process in psychiatry (and every other specialty) has become a much different process. In 2006, the Association of American Medical Colleges called on medical schools to increase their enrollments to address the national shortage of physicians. Soon, there were more medical schools, bigger classes, and more doctors being minted, but the Balanced Budget Act of 1997 prevented a proportional increase in residency positions.
Len Marquez, senior director of government relations at the AAMC noted: “The Resident Physician Shortage Reduction Act of 2021 (S. 834), sponsored by Sen. Robert Menendez (D-N.J.), Sen. John Boozman (R-Ark.), and Majority Leader Sen. Charles Schumer (D-N.Y.), would support 2,000 additional Medicare-supported residency positions each year for 7 years, but Congress has not yet acted on the legislation. We were very pleased that last year, Congress passed the first increase in Medicare-supported graduate medical education in 25 years by including 1,000 new slots as part of the Consolidated Appropriations Act, 2021.”
In addition, the Build Back Better Act, which is currently being debated in Congress, would provide 4,000 more graduate medical education slots, including a specific requirement that 15% of them go to “psychiatry-related residencies,” he added.
Over 90% of graduates from U.S. medical schools currently match into a residency position. That statistic for international medical graduates is notably lower, with perhaps as few as 50% of all applicants matching.
Since 2014, the number of applicants to psychiatry residencies has nearly doubled. For the 2021 match, there were 2,486 applicants applying for 1,858 positions in psychiatry – so 1.34 applicants for each slot. Of the 1,117 senior medical students at U.S. schools who applied to psychiatry residencies, 129 did not match. Overall, 99.8% of residency positions in psychiatry filled.
“It used to be less competitive,” said Kaz J. Nelson, MD, the vice chair for education at the University of Minnesota’s department of psychiatry and behavioral sciences in Minneapolis, adding that interest in psychiatry has increased over the years.
“Interest has skyrocketed as the word has gotten out about how great a field it is. It helps that reimbursements are better, that there is less bias and discrimination against patients with psychiatric issues, and that psychiatric care is seen as a legitimate part of medicine. It has been exciting to watch!” Dr. Nelson said.
The numbers are only one part of the story, however.
Application submission now involves a centralized, electronic process, and it has become easier for applicants to apply to a lot of programs indiscriminately. It’s not unusual for applicants to apply to 70 or more programs. The factors that have limited applications include the cost: Electronic Residency Application Services (ERAS) charges for each application package they send to a program, and applicants traditionally pay to travel to the programs where they interview. This all changed with the 2021 cycle when in-person interviews were halted for the pandemic and interviews became virtual. While I recall applying to 7 residency programs, this year the average number of applications was 54.7 per applicant.
“It used to be that the cap on interviewing was financial,” Dr. Nelson said. “It was discriminatory and favored those who had more money to travel to interviews. There are still the ERAS fees, but COVID has been an equalizer and we are getting more applicants, and interviewing more who are not from Minnesota or the Midwest. We have been working to make our program attractive in terms of diversity, equity, inclusion, and justice. Our hospital is located a mile from where George Floyd was murdered, and it’s our responsibility to lead the effort to ensure the psychiatry workforce is diverse, and inclusive, as possible.”
Daniel E. Gih, MD, is the program director for a new psychiatry residency at the University of Nebraska, Omaha. When the program started in 2019, there were spots for four residents and the program had 588 applications. In 2020, the program grew to five positions and this year there were 553 applicants. Dr. Gih attributed the high number of applications to his program’s strong social media presence.
“Going through the applications and meeting the students are some of the most enjoyable parts of my work,” Dr. Gih said. “I feel guilty though, that I’m likely going to miss a great applicant. Each application averages 35 pages and it’s inevitable that programs have to take shortcuts. Applicants worry that they’ll be ranked by board scores. While we certainly don’t do that here, students might feel ruled out of a program if their numbers aren’t high enough. Furthermore, wealthy students can apply to more programs. The pandemic has really highlighted the inequity issues.”
Dr. Gih noted that the Zoom interview process has not been disappointing: “Two of the people we matched had never been to Omaha, and many expressed concerns about what it is like here. Of course, on Zoom you don’t catch subtle interpersonal issues, but we have been pleasantly surprised that the people we matched were consistent with what we expected. It is exciting to meet the people who will eventually replace us as psychiatrists, they will be here to deal with future challenges!” His enthusiasm was tangible.
While the program directors remain optimistic, the system is not without its stresses, as many programs receive over 1,000 applications.
“This is difficult,” Dr. Nelson said.” It’s wonderful for the programs, but for the medical students, not matching is experienced by them as being catastrophic, so they apply to a lot of programs. Getting this many applications is a challenge, yet I don’t want to interview someone if they are going to rank our program No. 80 on their list!”
Residencies have dealt with the deluge of applicants in a number of ways. Some specialties started a “signal” protocol wherein candidates and programs receive a certain number of tokens to indicate that each would rank the other highly, but psychiatry has not done this. Early on in the Zoom process, multiple applicants would be offered interviews simultaneously, and the interview would be given to the candidate who responded first. Students vented their frustrations on Twitter when they lost interview spots at their coveted programs because they hadn’t checked their email in time or had gone to take a shower.
“The American Association of Directors of Psychiatry Residency Training Programs issued guidelines saying that it is unacceptable to offer interview spots without allowing a reasonable time for the applicant to respond, and that it is not appropriate to offer multiple candidates one spot on a first-come, first-serve basis,” Dr. Nelson explained.
Her program has managed some of the application chaos by using a software program called Scutmonkey, codeveloped by David Ross, MD, PhD, the associate program director of the Yale Adult Psychiatry Residency Program.
“It lets us screen applications for candidates who specifically are interested in being here, and for those who qualify as part of the mission we are trying to fulfill.”
One fourth-year student at a mid-Atlantic medical school who is applying in psychiatry – who I’ll call Sacha to protect his anonymity – applied to 73 psychiatry programs and to date, has interviewed at 6. He describes a stressful, roller coaster experience:
“I got those six interviews right away and that was an amazing start, but then I didn’t get any more. The interviews I had went well, but it has been disappointing not to have more. Some were all-day interviews, while other programs had me meet with residents and attendings for 20 minutes each and it was all done after 2 hours.”
He has mixed opinions about not seeing the schools in person. “There are very heavy pros and cons. I’ve saved thousands of dollars in travel expenses that would have limited my applications, so logistically it’s a dream. On the other hand, I’ve interviewed in cities I have never been to, it’s hard to get a sense of the intangibles of a program, and the shorter interviews feel very impersonal.”
Sacha expressed anxieties about the process. “With so many applicants, it’s difficult for someone with a nontraditional story to get a spot and it’s easier for the programs to toss applications. it’s common enough and everyone has seen someone who has gone through this. At times, we feel powerless; we have no real agency or control. We send stuff out and then we sit in the prayer position and wait.”
I think back on my own application process with a sense of gratitude. I certainly didn’t feel powerless, and in today’s world, postinterview communications with program directors are regulated for both parties. Dr. Slavney was kind enough to humor my request, but I don’t believe this would be feasible in the current environment.
Even though it is wonderful that more doctors have figured out that careers in psychiatry are rewarding, the current situation is overwhelming for both the applicants and the programs. With over 100 applicants for every position – many of whom will have no interest in going to some of the programs they apply to – qualified candidates who go unmatched, and a roulette wheel which requires heavily indebted students to pay to apply, this is simply not sustainable in a country with a shortage of physicians – psychiatrists in particular.
We hear that mid-level practitioners are the answer to our shortages, but perhaps we need to create a system with enough residency positions to accommodate highly trained and qualified physicians in a more inviting and targeted way.
Dinah Miller, MD, is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. A version of this article first appeared on Medscape.com.
The day I interviewed at Johns Hopkins in Baltimore, like every other day of residency interviews, was a very long and draining day.
I started by meeting alone with Philip Slavney, MD, the residency director, who spoke with me about the program and gave me a schedule to follow. I was to meet with residents and psychiatrists, some of whom had graduated from my medical school, and was sent to the Bayview campus a few miles away to have lunch and attend a few meetings. By the time I boarded an Amtrak train at Baltimore Penn Station, I was tired but I liked what I had seen. By the end of the interview season, I had crossed four programs off my list and had decided to rank only three.
In 1987, there were 987 residency positions in psychiatry in the United States, and 83.6% of those positions filled with a combination of U.S. and international medical graduates. Still, this was a risky move; the programs that I decided to rank would fill, but I was matching separately for an internship year in internal medicine in New York and decided that I would rather reapply in a year than risk matching at a program I didn’t want to go to.
I wasn’t quite sure where I wanted to rank Hopkins on my list, so I called Dr. Slavney and said I wanted to come back and meet more members of the department. He did not hide his surprise and was quick to tell me that no one had ever requested a second set of interviews. I mentioned specific people I wanted to meet with, and he was kind enough to accommodate my request and set up a second day of interviews for me.
Needless to say, the residency match felt very personal – at least to me – and although I felt vulnerable, I also felt empowered. Because of the low pay, patients with stigmatized illnesses, and the rampant belief that psychiatry was not “real” medicine and the patients never got better, psychiatry was not a desired specialty.
The residency application process in psychiatry (and every other specialty) has become a much different process. In 2006, the Association of American Medical Colleges called on medical schools to increase their enrollments to address the national shortage of physicians. Soon, there were more medical schools, bigger classes, and more doctors being minted, but the Balanced Budget Act of 1997 prevented a proportional increase in residency positions.
Len Marquez, senior director of government relations at the AAMC noted: “The Resident Physician Shortage Reduction Act of 2021 (S. 834), sponsored by Sen. Robert Menendez (D-N.J.), Sen. John Boozman (R-Ark.), and Majority Leader Sen. Charles Schumer (D-N.Y.), would support 2,000 additional Medicare-supported residency positions each year for 7 years, but Congress has not yet acted on the legislation. We were very pleased that last year, Congress passed the first increase in Medicare-supported graduate medical education in 25 years by including 1,000 new slots as part of the Consolidated Appropriations Act, 2021.”
In addition, the Build Back Better Act, which is currently being debated in Congress, would provide 4,000 more graduate medical education slots, including a specific requirement that 15% of them go to “psychiatry-related residencies,” he added.
Over 90% of graduates from U.S. medical schools currently match into a residency position. That statistic for international medical graduates is notably lower, with perhaps as few as 50% of all applicants matching.
Since 2014, the number of applicants to psychiatry residencies has nearly doubled. For the 2021 match, there were 2,486 applicants applying for 1,858 positions in psychiatry – so 1.34 applicants for each slot. Of the 1,117 senior medical students at U.S. schools who applied to psychiatry residencies, 129 did not match. Overall, 99.8% of residency positions in psychiatry filled.
“It used to be less competitive,” said Kaz J. Nelson, MD, the vice chair for education at the University of Minnesota’s department of psychiatry and behavioral sciences in Minneapolis, adding that interest in psychiatry has increased over the years.
“Interest has skyrocketed as the word has gotten out about how great a field it is. It helps that reimbursements are better, that there is less bias and discrimination against patients with psychiatric issues, and that psychiatric care is seen as a legitimate part of medicine. It has been exciting to watch!” Dr. Nelson said.
The numbers are only one part of the story, however.
Application submission now involves a centralized, electronic process, and it has become easier for applicants to apply to a lot of programs indiscriminately. It’s not unusual for applicants to apply to 70 or more programs. The factors that have limited applications include the cost: Electronic Residency Application Services (ERAS) charges for each application package they send to a program, and applicants traditionally pay to travel to the programs where they interview. This all changed with the 2021 cycle when in-person interviews were halted for the pandemic and interviews became virtual. While I recall applying to 7 residency programs, this year the average number of applications was 54.7 per applicant.
“It used to be that the cap on interviewing was financial,” Dr. Nelson said. “It was discriminatory and favored those who had more money to travel to interviews. There are still the ERAS fees, but COVID has been an equalizer and we are getting more applicants, and interviewing more who are not from Minnesota or the Midwest. We have been working to make our program attractive in terms of diversity, equity, inclusion, and justice. Our hospital is located a mile from where George Floyd was murdered, and it’s our responsibility to lead the effort to ensure the psychiatry workforce is diverse, and inclusive, as possible.”
Daniel E. Gih, MD, is the program director for a new psychiatry residency at the University of Nebraska, Omaha. When the program started in 2019, there were spots for four residents and the program had 588 applications. In 2020, the program grew to five positions and this year there were 553 applicants. Dr. Gih attributed the high number of applications to his program’s strong social media presence.
“Going through the applications and meeting the students are some of the most enjoyable parts of my work,” Dr. Gih said. “I feel guilty though, that I’m likely going to miss a great applicant. Each application averages 35 pages and it’s inevitable that programs have to take shortcuts. Applicants worry that they’ll be ranked by board scores. While we certainly don’t do that here, students might feel ruled out of a program if their numbers aren’t high enough. Furthermore, wealthy students can apply to more programs. The pandemic has really highlighted the inequity issues.”
Dr. Gih noted that the Zoom interview process has not been disappointing: “Two of the people we matched had never been to Omaha, and many expressed concerns about what it is like here. Of course, on Zoom you don’t catch subtle interpersonal issues, but we have been pleasantly surprised that the people we matched were consistent with what we expected. It is exciting to meet the people who will eventually replace us as psychiatrists, they will be here to deal with future challenges!” His enthusiasm was tangible.
While the program directors remain optimistic, the system is not without its stresses, as many programs receive over 1,000 applications.
“This is difficult,” Dr. Nelson said.” It’s wonderful for the programs, but for the medical students, not matching is experienced by them as being catastrophic, so they apply to a lot of programs. Getting this many applications is a challenge, yet I don’t want to interview someone if they are going to rank our program No. 80 on their list!”
Residencies have dealt with the deluge of applicants in a number of ways. Some specialties started a “signal” protocol wherein candidates and programs receive a certain number of tokens to indicate that each would rank the other highly, but psychiatry has not done this. Early on in the Zoom process, multiple applicants would be offered interviews simultaneously, and the interview would be given to the candidate who responded first. Students vented their frustrations on Twitter when they lost interview spots at their coveted programs because they hadn’t checked their email in time or had gone to take a shower.
“The American Association of Directors of Psychiatry Residency Training Programs issued guidelines saying that it is unacceptable to offer interview spots without allowing a reasonable time for the applicant to respond, and that it is not appropriate to offer multiple candidates one spot on a first-come, first-serve basis,” Dr. Nelson explained.
Her program has managed some of the application chaos by using a software program called Scutmonkey, codeveloped by David Ross, MD, PhD, the associate program director of the Yale Adult Psychiatry Residency Program.
“It lets us screen applications for candidates who specifically are interested in being here, and for those who qualify as part of the mission we are trying to fulfill.”
One fourth-year student at a mid-Atlantic medical school who is applying in psychiatry – who I’ll call Sacha to protect his anonymity – applied to 73 psychiatry programs and to date, has interviewed at 6. He describes a stressful, roller coaster experience:
“I got those six interviews right away and that was an amazing start, but then I didn’t get any more. The interviews I had went well, but it has been disappointing not to have more. Some were all-day interviews, while other programs had me meet with residents and attendings for 20 minutes each and it was all done after 2 hours.”
He has mixed opinions about not seeing the schools in person. “There are very heavy pros and cons. I’ve saved thousands of dollars in travel expenses that would have limited my applications, so logistically it’s a dream. On the other hand, I’ve interviewed in cities I have never been to, it’s hard to get a sense of the intangibles of a program, and the shorter interviews feel very impersonal.”
Sacha expressed anxieties about the process. “With so many applicants, it’s difficult for someone with a nontraditional story to get a spot and it’s easier for the programs to toss applications. it’s common enough and everyone has seen someone who has gone through this. At times, we feel powerless; we have no real agency or control. We send stuff out and then we sit in the prayer position and wait.”
I think back on my own application process with a sense of gratitude. I certainly didn’t feel powerless, and in today’s world, postinterview communications with program directors are regulated for both parties. Dr. Slavney was kind enough to humor my request, but I don’t believe this would be feasible in the current environment.
Even though it is wonderful that more doctors have figured out that careers in psychiatry are rewarding, the current situation is overwhelming for both the applicants and the programs. With over 100 applicants for every position – many of whom will have no interest in going to some of the programs they apply to – qualified candidates who go unmatched, and a roulette wheel which requires heavily indebted students to pay to apply, this is simply not sustainable in a country with a shortage of physicians – psychiatrists in particular.
We hear that mid-level practitioners are the answer to our shortages, but perhaps we need to create a system with enough residency positions to accommodate highly trained and qualified physicians in a more inviting and targeted way.
Dinah Miller, MD, is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. A version of this article first appeared on Medscape.com.
The day I interviewed at Johns Hopkins in Baltimore, like every other day of residency interviews, was a very long and draining day.
I started by meeting alone with Philip Slavney, MD, the residency director, who spoke with me about the program and gave me a schedule to follow. I was to meet with residents and psychiatrists, some of whom had graduated from my medical school, and was sent to the Bayview campus a few miles away to have lunch and attend a few meetings. By the time I boarded an Amtrak train at Baltimore Penn Station, I was tired but I liked what I had seen. By the end of the interview season, I had crossed four programs off my list and had decided to rank only three.
In 1987, there were 987 residency positions in psychiatry in the United States, and 83.6% of those positions filled with a combination of U.S. and international medical graduates. Still, this was a risky move; the programs that I decided to rank would fill, but I was matching separately for an internship year in internal medicine in New York and decided that I would rather reapply in a year than risk matching at a program I didn’t want to go to.
I wasn’t quite sure where I wanted to rank Hopkins on my list, so I called Dr. Slavney and said I wanted to come back and meet more members of the department. He did not hide his surprise and was quick to tell me that no one had ever requested a second set of interviews. I mentioned specific people I wanted to meet with, and he was kind enough to accommodate my request and set up a second day of interviews for me.
Needless to say, the residency match felt very personal – at least to me – and although I felt vulnerable, I also felt empowered. Because of the low pay, patients with stigmatized illnesses, and the rampant belief that psychiatry was not “real” medicine and the patients never got better, psychiatry was not a desired specialty.
The residency application process in psychiatry (and every other specialty) has become a much different process. In 2006, the Association of American Medical Colleges called on medical schools to increase their enrollments to address the national shortage of physicians. Soon, there were more medical schools, bigger classes, and more doctors being minted, but the Balanced Budget Act of 1997 prevented a proportional increase in residency positions.
Len Marquez, senior director of government relations at the AAMC noted: “The Resident Physician Shortage Reduction Act of 2021 (S. 834), sponsored by Sen. Robert Menendez (D-N.J.), Sen. John Boozman (R-Ark.), and Majority Leader Sen. Charles Schumer (D-N.Y.), would support 2,000 additional Medicare-supported residency positions each year for 7 years, but Congress has not yet acted on the legislation. We were very pleased that last year, Congress passed the first increase in Medicare-supported graduate medical education in 25 years by including 1,000 new slots as part of the Consolidated Appropriations Act, 2021.”
In addition, the Build Back Better Act, which is currently being debated in Congress, would provide 4,000 more graduate medical education slots, including a specific requirement that 15% of them go to “psychiatry-related residencies,” he added.
Over 90% of graduates from U.S. medical schools currently match into a residency position. That statistic for international medical graduates is notably lower, with perhaps as few as 50% of all applicants matching.
Since 2014, the number of applicants to psychiatry residencies has nearly doubled. For the 2021 match, there were 2,486 applicants applying for 1,858 positions in psychiatry – so 1.34 applicants for each slot. Of the 1,117 senior medical students at U.S. schools who applied to psychiatry residencies, 129 did not match. Overall, 99.8% of residency positions in psychiatry filled.
“It used to be less competitive,” said Kaz J. Nelson, MD, the vice chair for education at the University of Minnesota’s department of psychiatry and behavioral sciences in Minneapolis, adding that interest in psychiatry has increased over the years.
“Interest has skyrocketed as the word has gotten out about how great a field it is. It helps that reimbursements are better, that there is less bias and discrimination against patients with psychiatric issues, and that psychiatric care is seen as a legitimate part of medicine. It has been exciting to watch!” Dr. Nelson said.
The numbers are only one part of the story, however.
Application submission now involves a centralized, electronic process, and it has become easier for applicants to apply to a lot of programs indiscriminately. It’s not unusual for applicants to apply to 70 or more programs. The factors that have limited applications include the cost: Electronic Residency Application Services (ERAS) charges for each application package they send to a program, and applicants traditionally pay to travel to the programs where they interview. This all changed with the 2021 cycle when in-person interviews were halted for the pandemic and interviews became virtual. While I recall applying to 7 residency programs, this year the average number of applications was 54.7 per applicant.
“It used to be that the cap on interviewing was financial,” Dr. Nelson said. “It was discriminatory and favored those who had more money to travel to interviews. There are still the ERAS fees, but COVID has been an equalizer and we are getting more applicants, and interviewing more who are not from Minnesota or the Midwest. We have been working to make our program attractive in terms of diversity, equity, inclusion, and justice. Our hospital is located a mile from where George Floyd was murdered, and it’s our responsibility to lead the effort to ensure the psychiatry workforce is diverse, and inclusive, as possible.”
Daniel E. Gih, MD, is the program director for a new psychiatry residency at the University of Nebraska, Omaha. When the program started in 2019, there were spots for four residents and the program had 588 applications. In 2020, the program grew to five positions and this year there were 553 applicants. Dr. Gih attributed the high number of applications to his program’s strong social media presence.
“Going through the applications and meeting the students are some of the most enjoyable parts of my work,” Dr. Gih said. “I feel guilty though, that I’m likely going to miss a great applicant. Each application averages 35 pages and it’s inevitable that programs have to take shortcuts. Applicants worry that they’ll be ranked by board scores. While we certainly don’t do that here, students might feel ruled out of a program if their numbers aren’t high enough. Furthermore, wealthy students can apply to more programs. The pandemic has really highlighted the inequity issues.”
Dr. Gih noted that the Zoom interview process has not been disappointing: “Two of the people we matched had never been to Omaha, and many expressed concerns about what it is like here. Of course, on Zoom you don’t catch subtle interpersonal issues, but we have been pleasantly surprised that the people we matched were consistent with what we expected. It is exciting to meet the people who will eventually replace us as psychiatrists, they will be here to deal with future challenges!” His enthusiasm was tangible.
While the program directors remain optimistic, the system is not without its stresses, as many programs receive over 1,000 applications.
“This is difficult,” Dr. Nelson said.” It’s wonderful for the programs, but for the medical students, not matching is experienced by them as being catastrophic, so they apply to a lot of programs. Getting this many applications is a challenge, yet I don’t want to interview someone if they are going to rank our program No. 80 on their list!”
Residencies have dealt with the deluge of applicants in a number of ways. Some specialties started a “signal” protocol wherein candidates and programs receive a certain number of tokens to indicate that each would rank the other highly, but psychiatry has not done this. Early on in the Zoom process, multiple applicants would be offered interviews simultaneously, and the interview would be given to the candidate who responded first. Students vented their frustrations on Twitter when they lost interview spots at their coveted programs because they hadn’t checked their email in time or had gone to take a shower.
“The American Association of Directors of Psychiatry Residency Training Programs issued guidelines saying that it is unacceptable to offer interview spots without allowing a reasonable time for the applicant to respond, and that it is not appropriate to offer multiple candidates one spot on a first-come, first-serve basis,” Dr. Nelson explained.
Her program has managed some of the application chaos by using a software program called Scutmonkey, codeveloped by David Ross, MD, PhD, the associate program director of the Yale Adult Psychiatry Residency Program.
“It lets us screen applications for candidates who specifically are interested in being here, and for those who qualify as part of the mission we are trying to fulfill.”
One fourth-year student at a mid-Atlantic medical school who is applying in psychiatry – who I’ll call Sacha to protect his anonymity – applied to 73 psychiatry programs and to date, has interviewed at 6. He describes a stressful, roller coaster experience:
“I got those six interviews right away and that was an amazing start, but then I didn’t get any more. The interviews I had went well, but it has been disappointing not to have more. Some were all-day interviews, while other programs had me meet with residents and attendings for 20 minutes each and it was all done after 2 hours.”
He has mixed opinions about not seeing the schools in person. “There are very heavy pros and cons. I’ve saved thousands of dollars in travel expenses that would have limited my applications, so logistically it’s a dream. On the other hand, I’ve interviewed in cities I have never been to, it’s hard to get a sense of the intangibles of a program, and the shorter interviews feel very impersonal.”
Sacha expressed anxieties about the process. “With so many applicants, it’s difficult for someone with a nontraditional story to get a spot and it’s easier for the programs to toss applications. it’s common enough and everyone has seen someone who has gone through this. At times, we feel powerless; we have no real agency or control. We send stuff out and then we sit in the prayer position and wait.”
I think back on my own application process with a sense of gratitude. I certainly didn’t feel powerless, and in today’s world, postinterview communications with program directors are regulated for both parties. Dr. Slavney was kind enough to humor my request, but I don’t believe this would be feasible in the current environment.
Even though it is wonderful that more doctors have figured out that careers in psychiatry are rewarding, the current situation is overwhelming for both the applicants and the programs. With over 100 applicants for every position – many of whom will have no interest in going to some of the programs they apply to – qualified candidates who go unmatched, and a roulette wheel which requires heavily indebted students to pay to apply, this is simply not sustainable in a country with a shortage of physicians – psychiatrists in particular.
We hear that mid-level practitioners are the answer to our shortages, but perhaps we need to create a system with enough residency positions to accommodate highly trained and qualified physicians in a more inviting and targeted way.
Dinah Miller, MD, is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. A version of this article first appeared on Medscape.com.







