User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Charcoal could be the cure for the common high-fat diet
Charcoal won’t let high-fat diet weigh you down
Do you want to be the funniest person alive? Of course you do. It’s really simple too, just one joke can make you the greatest comedian of all time. All you have to do is go camping and cook food over a roaring campfire. When someone drops food into the fire (which they always will), get ready. Once they fish out the offending food, which is almost certainly coated in hot coals, tell them: “Ah, eat it anyway. A little texture never hurt!” Trust us, most hilarious and original gag of all time.
But before your hapless friend brushes off his hot dog and forces a laugh, consider this: Japanese researchers have found that a charcoal supplement can prevent weight gain in mice consuming a high-fat diet. Charcoal is actually quite the helpful substance, and not just for grilling. It’s been used as medicine for hundreds of years and even today is used as a treatment for drug overdose and excess gas and flatulence.
The study involved two groups of mice: One was fed a normal diet, the other a high-fat diet. After 12 weeks, the high-fat diet mice had gained weight. At that point, edible activated charcoal was added to their diet. From that point, weight gain was similar between the two groups, and the amount of bile acid, cholesterol, triglyceride, and fatty acid excreted by the high-fat mice increased by two to four times.
The researchers supported the notion that consuming an activated charcoal supplement before or while eating fatty food could prevent weight gain from said fatty food. Which works out well for the classic American barbecue, which is traditionally both high in fat and charcoal. All you have to do is buy some extra charcoal briquettes to pass around and munch on with your friends. Now that’s a party we can get behind.
There’s awake, and then there’s neurologically awake
Time to toss another urban legend onto the trash heap of history. Say goodbye to the benefits of uninterrupted sleep. It’s a fraud, a fake, a myth, a hit or myth, a swing and a myth, an old wives’ tale. You can stuff it and put it on a shelf next to Bigfoot, the Slender Man, and Twinkies.
We all thought we needed 8 hours of uninterrupted sleep every night, but guess who we forgot to tell? Our brains. They’ve been doing exactly the opposite all along, laughing at us the whole time. Smug SOBs.
To straighten out this mess, let’s bring in a scientist, Celia Kjaerby of the Center for Translational Neuromedicine at the University of Copenhagen: “You may think that sleep is a constant state that you are in, and then you wake up. But there is a lot more to sleep than meets the eye. We have learned that noradrenaline causes you to wake up more than 100 times a night. And that is during perfectly normal sleep.”
Those 100 or so sleep interruptions are so brief that we don’t even notice, but they are very important, according to a study conducted at the university. Those tiny little wake-up calls are “the essence for the part of sleep that makes us wake up rested and which enables us to remember what we learned the day before. ... The very short awakenings are created by waves of norepinephrine [and they] reset the brain so that it is ready to store memory when you dive back into sleep,” lead author Maiken Nedergaard, MD, explained.
The investigators compared the level of noradrenaline in sleeping mice with their electrical activity and found that the hormone constantly increased and decreased in a wavelike pattern. A high level meant that the animal was neurologically awake. Deeper valleys between the high points meant better sleep, and the mice with the “highest number of deep noradrenaline valleys were also the ones with the best memory,” the team said in their written statement.
Not just the best memory, they said, but “super memory.” That, of course, was enough to get the attention of Marvel Comics, so the next Disney superhero blockbuster will feature Nocturna, the queen of the night. Her power? Never forgets. Her archnemesis? The Insomniac. Her catchphrase? “Let me sleep on it.”
Words can hurt, literally
Growing up, we’re sure you heard the “sticks and stones” rhyme. Maybe you’ve even recited it once or twice to defend yourself. Well, forget it, because words can hurt and your brain knows it.
In a new study published in Frontiers in Communication, Marijn Struiksma, PhD, of Utrecht University, and colleagues incorporated the use of electroencephalography (EEG) and skin conductance on 79 women to see how words (specifically insults) actually affect the human body.
Each subject was asked to read three different types of statements: an insult, a compliment, and something factual but neutral. Half of the statements contained the subject’s name and half used somebody else’s. The participants were told that these statements were collected from three men.
Nobody interacted with each other, and the setting was completely clinical, yet the results were unmistakable. The EEG showed an effect in P2 amplitude with repetitive insults, no matter who it was about. Even though the insults weren’t real and the participants were aware of it, the brain still recognized them as hurtful, coming across as “mini slaps in the face,” Dr. Struiksma noted in a written statement.
The researchers noted that more needs to be done to better understand the long-term effects that insults can have and create a deeper understanding between words and emotion, but studying the effects of insults in a real-life setting is ethically tricky. This study is a start.
So, yeah, sticks and stones can break your bones, but words will actually hurt you.
This article was updated 7/21/22.
Charcoal won’t let high-fat diet weigh you down
Do you want to be the funniest person alive? Of course you do. It’s really simple too, just one joke can make you the greatest comedian of all time. All you have to do is go camping and cook food over a roaring campfire. When someone drops food into the fire (which they always will), get ready. Once they fish out the offending food, which is almost certainly coated in hot coals, tell them: “Ah, eat it anyway. A little texture never hurt!” Trust us, most hilarious and original gag of all time.
But before your hapless friend brushes off his hot dog and forces a laugh, consider this: Japanese researchers have found that a charcoal supplement can prevent weight gain in mice consuming a high-fat diet. Charcoal is actually quite the helpful substance, and not just for grilling. It’s been used as medicine for hundreds of years and even today is used as a treatment for drug overdose and excess gas and flatulence.
The study involved two groups of mice: One was fed a normal diet, the other a high-fat diet. After 12 weeks, the high-fat diet mice had gained weight. At that point, edible activated charcoal was added to their diet. From that point, weight gain was similar between the two groups, and the amount of bile acid, cholesterol, triglyceride, and fatty acid excreted by the high-fat mice increased by two to four times.
The researchers supported the notion that consuming an activated charcoal supplement before or while eating fatty food could prevent weight gain from said fatty food. Which works out well for the classic American barbecue, which is traditionally both high in fat and charcoal. All you have to do is buy some extra charcoal briquettes to pass around and munch on with your friends. Now that’s a party we can get behind.
There’s awake, and then there’s neurologically awake
Time to toss another urban legend onto the trash heap of history. Say goodbye to the benefits of uninterrupted sleep. It’s a fraud, a fake, a myth, a hit or myth, a swing and a myth, an old wives’ tale. You can stuff it and put it on a shelf next to Bigfoot, the Slender Man, and Twinkies.
We all thought we needed 8 hours of uninterrupted sleep every night, but guess who we forgot to tell? Our brains. They’ve been doing exactly the opposite all along, laughing at us the whole time. Smug SOBs.
To straighten out this mess, let’s bring in a scientist, Celia Kjaerby of the Center for Translational Neuromedicine at the University of Copenhagen: “You may think that sleep is a constant state that you are in, and then you wake up. But there is a lot more to sleep than meets the eye. We have learned that noradrenaline causes you to wake up more than 100 times a night. And that is during perfectly normal sleep.”
Those 100 or so sleep interruptions are so brief that we don’t even notice, but they are very important, according to a study conducted at the university. Those tiny little wake-up calls are “the essence for the part of sleep that makes us wake up rested and which enables us to remember what we learned the day before. ... The very short awakenings are created by waves of norepinephrine [and they] reset the brain so that it is ready to store memory when you dive back into sleep,” lead author Maiken Nedergaard, MD, explained.
The investigators compared the level of noradrenaline in sleeping mice with their electrical activity and found that the hormone constantly increased and decreased in a wavelike pattern. A high level meant that the animal was neurologically awake. Deeper valleys between the high points meant better sleep, and the mice with the “highest number of deep noradrenaline valleys were also the ones with the best memory,” the team said in their written statement.
Not just the best memory, they said, but “super memory.” That, of course, was enough to get the attention of Marvel Comics, so the next Disney superhero blockbuster will feature Nocturna, the queen of the night. Her power? Never forgets. Her archnemesis? The Insomniac. Her catchphrase? “Let me sleep on it.”
Words can hurt, literally
Growing up, we’re sure you heard the “sticks and stones” rhyme. Maybe you’ve even recited it once or twice to defend yourself. Well, forget it, because words can hurt and your brain knows it.
In a new study published in Frontiers in Communication, Marijn Struiksma, PhD, of Utrecht University, and colleagues incorporated the use of electroencephalography (EEG) and skin conductance on 79 women to see how words (specifically insults) actually affect the human body.
Each subject was asked to read three different types of statements: an insult, a compliment, and something factual but neutral. Half of the statements contained the subject’s name and half used somebody else’s. The participants were told that these statements were collected from three men.
Nobody interacted with each other, and the setting was completely clinical, yet the results were unmistakable. The EEG showed an effect in P2 amplitude with repetitive insults, no matter who it was about. Even though the insults weren’t real and the participants were aware of it, the brain still recognized them as hurtful, coming across as “mini slaps in the face,” Dr. Struiksma noted in a written statement.
The researchers noted that more needs to be done to better understand the long-term effects that insults can have and create a deeper understanding between words and emotion, but studying the effects of insults in a real-life setting is ethically tricky. This study is a start.
So, yeah, sticks and stones can break your bones, but words will actually hurt you.
This article was updated 7/21/22.
Charcoal won’t let high-fat diet weigh you down
Do you want to be the funniest person alive? Of course you do. It’s really simple too, just one joke can make you the greatest comedian of all time. All you have to do is go camping and cook food over a roaring campfire. When someone drops food into the fire (which they always will), get ready. Once they fish out the offending food, which is almost certainly coated in hot coals, tell them: “Ah, eat it anyway. A little texture never hurt!” Trust us, most hilarious and original gag of all time.
But before your hapless friend brushes off his hot dog and forces a laugh, consider this: Japanese researchers have found that a charcoal supplement can prevent weight gain in mice consuming a high-fat diet. Charcoal is actually quite the helpful substance, and not just for grilling. It’s been used as medicine for hundreds of years and even today is used as a treatment for drug overdose and excess gas and flatulence.
The study involved two groups of mice: One was fed a normal diet, the other a high-fat diet. After 12 weeks, the high-fat diet mice had gained weight. At that point, edible activated charcoal was added to their diet. From that point, weight gain was similar between the two groups, and the amount of bile acid, cholesterol, triglyceride, and fatty acid excreted by the high-fat mice increased by two to four times.
The researchers supported the notion that consuming an activated charcoal supplement before or while eating fatty food could prevent weight gain from said fatty food. Which works out well for the classic American barbecue, which is traditionally both high in fat and charcoal. All you have to do is buy some extra charcoal briquettes to pass around and munch on with your friends. Now that’s a party we can get behind.
There’s awake, and then there’s neurologically awake
Time to toss another urban legend onto the trash heap of history. Say goodbye to the benefits of uninterrupted sleep. It’s a fraud, a fake, a myth, a hit or myth, a swing and a myth, an old wives’ tale. You can stuff it and put it on a shelf next to Bigfoot, the Slender Man, and Twinkies.
We all thought we needed 8 hours of uninterrupted sleep every night, but guess who we forgot to tell? Our brains. They’ve been doing exactly the opposite all along, laughing at us the whole time. Smug SOBs.
To straighten out this mess, let’s bring in a scientist, Celia Kjaerby of the Center for Translational Neuromedicine at the University of Copenhagen: “You may think that sleep is a constant state that you are in, and then you wake up. But there is a lot more to sleep than meets the eye. We have learned that noradrenaline causes you to wake up more than 100 times a night. And that is during perfectly normal sleep.”
Those 100 or so sleep interruptions are so brief that we don’t even notice, but they are very important, according to a study conducted at the university. Those tiny little wake-up calls are “the essence for the part of sleep that makes us wake up rested and which enables us to remember what we learned the day before. ... The very short awakenings are created by waves of norepinephrine [and they] reset the brain so that it is ready to store memory when you dive back into sleep,” lead author Maiken Nedergaard, MD, explained.
The investigators compared the level of noradrenaline in sleeping mice with their electrical activity and found that the hormone constantly increased and decreased in a wavelike pattern. A high level meant that the animal was neurologically awake. Deeper valleys between the high points meant better sleep, and the mice with the “highest number of deep noradrenaline valleys were also the ones with the best memory,” the team said in their written statement.
Not just the best memory, they said, but “super memory.” That, of course, was enough to get the attention of Marvel Comics, so the next Disney superhero blockbuster will feature Nocturna, the queen of the night. Her power? Never forgets. Her archnemesis? The Insomniac. Her catchphrase? “Let me sleep on it.”
Words can hurt, literally
Growing up, we’re sure you heard the “sticks and stones” rhyme. Maybe you’ve even recited it once or twice to defend yourself. Well, forget it, because words can hurt and your brain knows it.
In a new study published in Frontiers in Communication, Marijn Struiksma, PhD, of Utrecht University, and colleagues incorporated the use of electroencephalography (EEG) and skin conductance on 79 women to see how words (specifically insults) actually affect the human body.
Each subject was asked to read three different types of statements: an insult, a compliment, and something factual but neutral. Half of the statements contained the subject’s name and half used somebody else’s. The participants were told that these statements were collected from three men.
Nobody interacted with each other, and the setting was completely clinical, yet the results were unmistakable. The EEG showed an effect in P2 amplitude with repetitive insults, no matter who it was about. Even though the insults weren’t real and the participants were aware of it, the brain still recognized them as hurtful, coming across as “mini slaps in the face,” Dr. Struiksma noted in a written statement.
The researchers noted that more needs to be done to better understand the long-term effects that insults can have and create a deeper understanding between words and emotion, but studying the effects of insults in a real-life setting is ethically tricky. This study is a start.
So, yeah, sticks and stones can break your bones, but words will actually hurt you.
This article was updated 7/21/22.
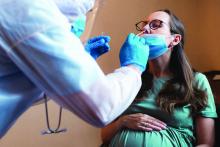
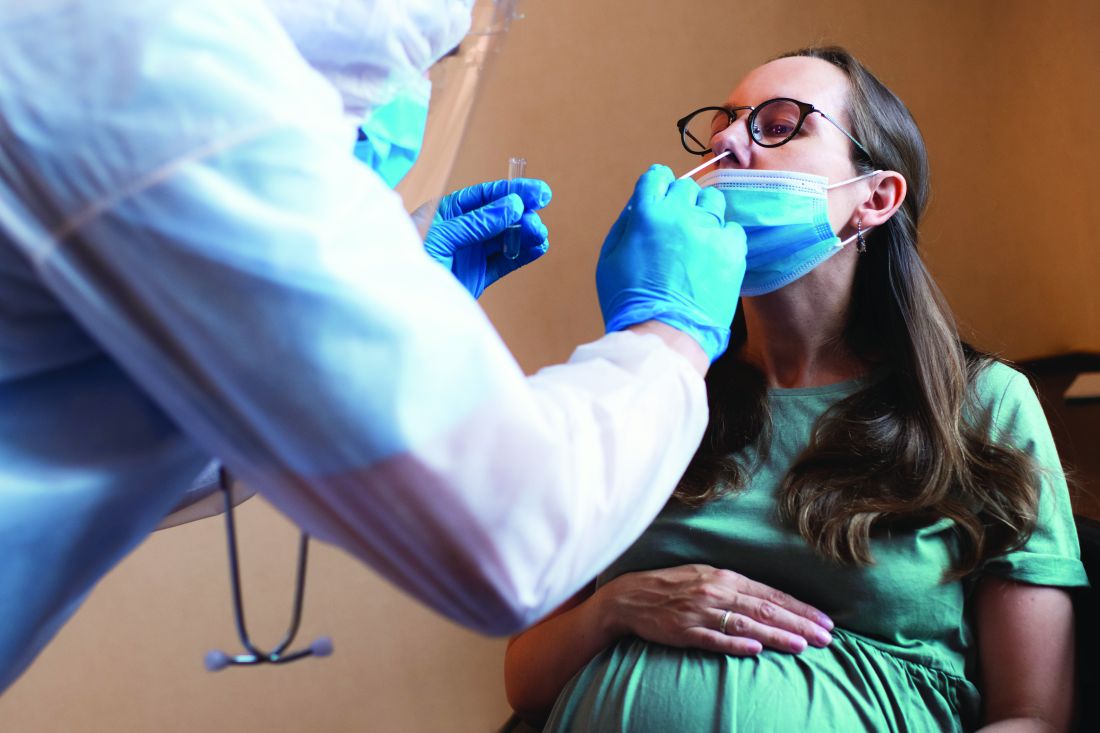
COVID-19 infection late in pregnancy linked to sevenfold risk of preterm birth
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
FROM PLOS ONE
Pediatric obesity treatment options: Beyond lifestyle modification
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Our role in preventing postpartum depression
Tragic, embarrassing, criminal ... Choose your own adjective. The maternal mortality rate in this country is the worst of any developed nation in the world. And the numbers are getting worse with an increase of 14% over the previous year. One-third of these deaths occur weeks or months after the delivery.
In a recent issue of Harvard Public Health, researchers at the T.H. Chan School of Public Health discuss some of the possible remedies for what they describe as a crisis. While some of the solutions they list will require major restructuring of how we deliver health care to mothers, others could take advantage of our current systems by employing a slight shift in emphasis. And here is where those of us on the frontline of care delivery can make a difference.
The researchers point out that “More than 90% of maternal deaths could be prevented if women had access to quality care.” They also observe that most mothers have a single postpartum check with the ob.gyn. facility that delivered the baby and then are often left to navigate the health system because transfer to their primary care and/or mental health professional is haphazard or lacking in follow-up.
As I read through the article it struck me that as pediatricians we could and should be playing a larger role in this critical postpartum period when so many women seem to be falling through the cracks in our health care nonsystem. This is not a great “Ah-ha” moment for which I deserve any credit. In 2010 the American Academy of Pediatrics recommended that mothers be screened for depression at the 1-, 2-, and 4-month visits using either a validated 10-question screening instrument or a more direct 2-question tool (Pediatrics 2010;126[5]:1032-9). However, a periodic survey of AAP members 3 years later revealed that less than a third of the respondents were screening regularly for postpartum depression. In 2019 the academy reemphasized the important role that pediatric primary care givers can play in the detection and early management of the condition.
The reasons for the disappointing response include the list of usual suspects of inadequate training, workload demands, reimbursement, liability concerns, and the difficulty in finding and establishing effective referral networks. Unfortunately, these factors continue to exist, and many cases have multiplied in the wake of the pandemic.
In some states, educational outreach, funding, and changes in the reimbursement structure have resulted in improved outcomes. Not all of us are fortunate enough to live in a state that has made postpartum depression detection and management a priority. However, simply making it our own professional priority can save lives, ease suffering, and improve postpartum outcomes. Here I am talking about first caring and then inquiring about a mother’s mental health. Asking how much sleep she is getting. And then spending the time to give personalized advice on feeding and sleep schedules. Even, if this means ignoring half of the topics on the recommended health maintenance. It doesn’t take but a few minutes to convince yourself that the baby is healthy, and you know that 90% of them are.
However, a new mother who is sleep deprived and already has one foot on the spiral staircase down into postpartum depression represents an emergency. And, you should have the skills to turn it around. But, you have to care about the problem and make it your own priority – high enough on the list to make a follow-up appointment or call in a week instead of waiting a month or 2 until the next visit.
Unfortunately, even with your best efforts there are some families who need services beyond the scope of your practice. Making the necessary referrals can be frustrating and time consuming but not dropping ball until it lands in the appropriate place may save a life.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Tragic, embarrassing, criminal ... Choose your own adjective. The maternal mortality rate in this country is the worst of any developed nation in the world. And the numbers are getting worse with an increase of 14% over the previous year. One-third of these deaths occur weeks or months after the delivery.
In a recent issue of Harvard Public Health, researchers at the T.H. Chan School of Public Health discuss some of the possible remedies for what they describe as a crisis. While some of the solutions they list will require major restructuring of how we deliver health care to mothers, others could take advantage of our current systems by employing a slight shift in emphasis. And here is where those of us on the frontline of care delivery can make a difference.
The researchers point out that “More than 90% of maternal deaths could be prevented if women had access to quality care.” They also observe that most mothers have a single postpartum check with the ob.gyn. facility that delivered the baby and then are often left to navigate the health system because transfer to their primary care and/or mental health professional is haphazard or lacking in follow-up.
As I read through the article it struck me that as pediatricians we could and should be playing a larger role in this critical postpartum period when so many women seem to be falling through the cracks in our health care nonsystem. This is not a great “Ah-ha” moment for which I deserve any credit. In 2010 the American Academy of Pediatrics recommended that mothers be screened for depression at the 1-, 2-, and 4-month visits using either a validated 10-question screening instrument or a more direct 2-question tool (Pediatrics 2010;126[5]:1032-9). However, a periodic survey of AAP members 3 years later revealed that less than a third of the respondents were screening regularly for postpartum depression. In 2019 the academy reemphasized the important role that pediatric primary care givers can play in the detection and early management of the condition.
The reasons for the disappointing response include the list of usual suspects of inadequate training, workload demands, reimbursement, liability concerns, and the difficulty in finding and establishing effective referral networks. Unfortunately, these factors continue to exist, and many cases have multiplied in the wake of the pandemic.
In some states, educational outreach, funding, and changes in the reimbursement structure have resulted in improved outcomes. Not all of us are fortunate enough to live in a state that has made postpartum depression detection and management a priority. However, simply making it our own professional priority can save lives, ease suffering, and improve postpartum outcomes. Here I am talking about first caring and then inquiring about a mother’s mental health. Asking how much sleep she is getting. And then spending the time to give personalized advice on feeding and sleep schedules. Even, if this means ignoring half of the topics on the recommended health maintenance. It doesn’t take but a few minutes to convince yourself that the baby is healthy, and you know that 90% of them are.
However, a new mother who is sleep deprived and already has one foot on the spiral staircase down into postpartum depression represents an emergency. And, you should have the skills to turn it around. But, you have to care about the problem and make it your own priority – high enough on the list to make a follow-up appointment or call in a week instead of waiting a month or 2 until the next visit.
Unfortunately, even with your best efforts there are some families who need services beyond the scope of your practice. Making the necessary referrals can be frustrating and time consuming but not dropping ball until it lands in the appropriate place may save a life.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Tragic, embarrassing, criminal ... Choose your own adjective. The maternal mortality rate in this country is the worst of any developed nation in the world. And the numbers are getting worse with an increase of 14% over the previous year. One-third of these deaths occur weeks or months after the delivery.
In a recent issue of Harvard Public Health, researchers at the T.H. Chan School of Public Health discuss some of the possible remedies for what they describe as a crisis. While some of the solutions they list will require major restructuring of how we deliver health care to mothers, others could take advantage of our current systems by employing a slight shift in emphasis. And here is where those of us on the frontline of care delivery can make a difference.
The researchers point out that “More than 90% of maternal deaths could be prevented if women had access to quality care.” They also observe that most mothers have a single postpartum check with the ob.gyn. facility that delivered the baby and then are often left to navigate the health system because transfer to their primary care and/or mental health professional is haphazard or lacking in follow-up.
As I read through the article it struck me that as pediatricians we could and should be playing a larger role in this critical postpartum period when so many women seem to be falling through the cracks in our health care nonsystem. This is not a great “Ah-ha” moment for which I deserve any credit. In 2010 the American Academy of Pediatrics recommended that mothers be screened for depression at the 1-, 2-, and 4-month visits using either a validated 10-question screening instrument or a more direct 2-question tool (Pediatrics 2010;126[5]:1032-9). However, a periodic survey of AAP members 3 years later revealed that less than a third of the respondents were screening regularly for postpartum depression. In 2019 the academy reemphasized the important role that pediatric primary care givers can play in the detection and early management of the condition.
The reasons for the disappointing response include the list of usual suspects of inadequate training, workload demands, reimbursement, liability concerns, and the difficulty in finding and establishing effective referral networks. Unfortunately, these factors continue to exist, and many cases have multiplied in the wake of the pandemic.
In some states, educational outreach, funding, and changes in the reimbursement structure have resulted in improved outcomes. Not all of us are fortunate enough to live in a state that has made postpartum depression detection and management a priority. However, simply making it our own professional priority can save lives, ease suffering, and improve postpartum outcomes. Here I am talking about first caring and then inquiring about a mother’s mental health. Asking how much sleep she is getting. And then spending the time to give personalized advice on feeding and sleep schedules. Even, if this means ignoring half of the topics on the recommended health maintenance. It doesn’t take but a few minutes to convince yourself that the baby is healthy, and you know that 90% of them are.
However, a new mother who is sleep deprived and already has one foot on the spiral staircase down into postpartum depression represents an emergency. And, you should have the skills to turn it around. But, you have to care about the problem and make it your own priority – high enough on the list to make a follow-up appointment or call in a week instead of waiting a month or 2 until the next visit.
Unfortunately, even with your best efforts there are some families who need services beyond the scope of your practice. Making the necessary referrals can be frustrating and time consuming but not dropping ball until it lands in the appropriate place may save a life.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Steroids no cure for obstructive sleep apnea in children
Children with obstructive sleep apnea syndrome (OSAS) who undergo treatment with intranasal corticosteroids (INCS) did not experience significant improvement in polysomnographic, neurobehavioral, and other symptoms at 3 and 12 months of treatment. At 12 months of INCS treatment, there was a statistically significant but not clinically relevant reduction in the obstructive apnea hypopnea index (OHAI).
“Previous studies were done in children with OSA with an obstructive apnea hypopnea index of less than 5, so they had very mild OSA,” Ignacio Tapia, MD, associate professor of pediatrics, University of Pennsylvania, Philadelphia, said in an interview.
“But then people started using the INCS for a whole range of OSA, so this is why we wanted to do the trial, to make sure that these drugs were being used correctly,” he added.
“The main message from this paper is that I think INCS may still have a role to play in OSA to treat some of the symptoms, like snoring, and in our study, quality of life indices also improved, ,” Dr. Tapia emphasized.
The study was published online in the journal Chest.
3 months of INCS
A total of 134 children between 5 and 12 years of age were randomly assigned to receive INCS for 3 months or placebo. Children in the original INCS arm were then reassigned to receive 9 more months of the same treatment or placebo. Symptoms as well as polysomnographic and neurobehavioral findings were measured at baseline, at 3 months, and again at 12 months.
“The primary outcome was OAHI change at 3 months, available for 122 children,” the authors explained. The OSAS was defined as an OAHI of between two and three events per hour. The median age of the children at baseline was 7.9 years, and the median OAHI baseline score was 5.8/hr (95% confidence interval, 3.6-9.7/hr). The total daily dose of the INCS used was 110 mcg.
At 3 months, the mean change in the OAHI from baseline was –1.73/hr (95% CI, –3.91 to 1.92/hr), while at 12 months, the mean change in the same index was –1.21 (–4.22 to 1.71/hr). These changes were not significantly different from OAHI changes observed among control participants. “OSAS symptoms and neurobehavioral results were not different [either] between the INCS and placebo groups at 3 and12 months,” the authors added.
However, among those children who received INCS treatment for the entire 12 months, the OAHI decreased significantly from 7.2/hr (95% CI, 3.62-9.88/hr) at baseline to 3.71/hr (95% CI, 1.56-6.4/hr; P = .039), although the OAHI did not normalize, the authors noted. Asked to clarify whether this change was not significant, Dr. Tapia said that it did meet statistical significance, but clinically, it meant that the children still needed some form of treatment, because they still had OSA in the range needing treatment.
The placebo group had more asthma exacerbations, upper respiratory tract infections, and exacerbations of OSAS symptoms, compared with children in the INCS group. It is possible that INCS provided a certain degree of protection from asthma exacerbation, the authors suggested.
However, recent guidelines from the American Academy of Pediatrics suggest that clinicians may prescribe these agents for children with mild OSAS in whom adenotonsillectomy is contraindicated; for those with mild postoperative OSAS, adenotonsillectomy remains the treatment of choice for childhood OSA. “The low level of enthusiasm for INCS in these guidelines is based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data,” the authors wrote.
“The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS,” they wrote.
Complex issue
In a comment, Rakesh Bhattacharjee, MD, associate professor of pediatrics, University of California, San Diego, noted that he does prescribe INCS for children with mild OSA but not for all children. “We based our decisions on polysomnography, which we use to categorize OSA as mild, moderate, or severe.
“But we certainly do offer this treatment for children with mild sleep apnea as a way to avoid surgical treatment,” Dr. Bhattacharjee added. He also uses INCS for residual sleep apnea that some children experience following adenotonsillectomy. As the current study suggests, many people are treating sleep apnea empirically without confirming the severity of the disorder by a sleep study.
“If a sleep study is not done, we don’t know how severe it is, so this would advocate for the utility of a sleep study so that you can quantify the severity of symptoms and target your therapy to children who might be appropriate for INCS therapy,” Dr. Bhattacharjee said.
On the other hand, surgery is not always relevant even if a child has enlarged adenoids and tonsils, as, for example, a child with obesity. In these children, physicians need to think about other treatments, such as continuous positive airways pressure. “CPAP is not perfect,” Dr. Bhattacharjee observed. “And as pediatricians, we need to do a lot of work to improve the use of CPAP, but, that said, there are children for whom INCS and surgery might be a waste of time, and this is where CPAP might be an alternative.”
Dr. Bhattacharjee previously was the lead author of a large study of children who underwent treatment with CPAP. While findings suggested that adherence to treatment is lower in children than it is for adults, the authors also showed that numerous actionable factors could used to improve adherence to CPAP among children who might otherwise benefit from it.
The authors disclosed no relevant financial relationships. Dr. Bhattacharjee has served as a scientific adviser for Jazz Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Children with obstructive sleep apnea syndrome (OSAS) who undergo treatment with intranasal corticosteroids (INCS) did not experience significant improvement in polysomnographic, neurobehavioral, and other symptoms at 3 and 12 months of treatment. At 12 months of INCS treatment, there was a statistically significant but not clinically relevant reduction in the obstructive apnea hypopnea index (OHAI).
“Previous studies were done in children with OSA with an obstructive apnea hypopnea index of less than 5, so they had very mild OSA,” Ignacio Tapia, MD, associate professor of pediatrics, University of Pennsylvania, Philadelphia, said in an interview.
“But then people started using the INCS for a whole range of OSA, so this is why we wanted to do the trial, to make sure that these drugs were being used correctly,” he added.
“The main message from this paper is that I think INCS may still have a role to play in OSA to treat some of the symptoms, like snoring, and in our study, quality of life indices also improved, ,” Dr. Tapia emphasized.
The study was published online in the journal Chest.
3 months of INCS
A total of 134 children between 5 and 12 years of age were randomly assigned to receive INCS for 3 months or placebo. Children in the original INCS arm were then reassigned to receive 9 more months of the same treatment or placebo. Symptoms as well as polysomnographic and neurobehavioral findings were measured at baseline, at 3 months, and again at 12 months.
“The primary outcome was OAHI change at 3 months, available for 122 children,” the authors explained. The OSAS was defined as an OAHI of between two and three events per hour. The median age of the children at baseline was 7.9 years, and the median OAHI baseline score was 5.8/hr (95% confidence interval, 3.6-9.7/hr). The total daily dose of the INCS used was 110 mcg.
At 3 months, the mean change in the OAHI from baseline was –1.73/hr (95% CI, –3.91 to 1.92/hr), while at 12 months, the mean change in the same index was –1.21 (–4.22 to 1.71/hr). These changes were not significantly different from OAHI changes observed among control participants. “OSAS symptoms and neurobehavioral results were not different [either] between the INCS and placebo groups at 3 and12 months,” the authors added.
However, among those children who received INCS treatment for the entire 12 months, the OAHI decreased significantly from 7.2/hr (95% CI, 3.62-9.88/hr) at baseline to 3.71/hr (95% CI, 1.56-6.4/hr; P = .039), although the OAHI did not normalize, the authors noted. Asked to clarify whether this change was not significant, Dr. Tapia said that it did meet statistical significance, but clinically, it meant that the children still needed some form of treatment, because they still had OSA in the range needing treatment.
The placebo group had more asthma exacerbations, upper respiratory tract infections, and exacerbations of OSAS symptoms, compared with children in the INCS group. It is possible that INCS provided a certain degree of protection from asthma exacerbation, the authors suggested.
However, recent guidelines from the American Academy of Pediatrics suggest that clinicians may prescribe these agents for children with mild OSAS in whom adenotonsillectomy is contraindicated; for those with mild postoperative OSAS, adenotonsillectomy remains the treatment of choice for childhood OSA. “The low level of enthusiasm for INCS in these guidelines is based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data,” the authors wrote.
“The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS,” they wrote.
Complex issue
In a comment, Rakesh Bhattacharjee, MD, associate professor of pediatrics, University of California, San Diego, noted that he does prescribe INCS for children with mild OSA but not for all children. “We based our decisions on polysomnography, which we use to categorize OSA as mild, moderate, or severe.
“But we certainly do offer this treatment for children with mild sleep apnea as a way to avoid surgical treatment,” Dr. Bhattacharjee added. He also uses INCS for residual sleep apnea that some children experience following adenotonsillectomy. As the current study suggests, many people are treating sleep apnea empirically without confirming the severity of the disorder by a sleep study.
“If a sleep study is not done, we don’t know how severe it is, so this would advocate for the utility of a sleep study so that you can quantify the severity of symptoms and target your therapy to children who might be appropriate for INCS therapy,” Dr. Bhattacharjee said.
On the other hand, surgery is not always relevant even if a child has enlarged adenoids and tonsils, as, for example, a child with obesity. In these children, physicians need to think about other treatments, such as continuous positive airways pressure. “CPAP is not perfect,” Dr. Bhattacharjee observed. “And as pediatricians, we need to do a lot of work to improve the use of CPAP, but, that said, there are children for whom INCS and surgery might be a waste of time, and this is where CPAP might be an alternative.”
Dr. Bhattacharjee previously was the lead author of a large study of children who underwent treatment with CPAP. While findings suggested that adherence to treatment is lower in children than it is for adults, the authors also showed that numerous actionable factors could used to improve adherence to CPAP among children who might otherwise benefit from it.
The authors disclosed no relevant financial relationships. Dr. Bhattacharjee has served as a scientific adviser for Jazz Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Children with obstructive sleep apnea syndrome (OSAS) who undergo treatment with intranasal corticosteroids (INCS) did not experience significant improvement in polysomnographic, neurobehavioral, and other symptoms at 3 and 12 months of treatment. At 12 months of INCS treatment, there was a statistically significant but not clinically relevant reduction in the obstructive apnea hypopnea index (OHAI).
“Previous studies were done in children with OSA with an obstructive apnea hypopnea index of less than 5, so they had very mild OSA,” Ignacio Tapia, MD, associate professor of pediatrics, University of Pennsylvania, Philadelphia, said in an interview.
“But then people started using the INCS for a whole range of OSA, so this is why we wanted to do the trial, to make sure that these drugs were being used correctly,” he added.
“The main message from this paper is that I think INCS may still have a role to play in OSA to treat some of the symptoms, like snoring, and in our study, quality of life indices also improved, ,” Dr. Tapia emphasized.
The study was published online in the journal Chest.
3 months of INCS
A total of 134 children between 5 and 12 years of age were randomly assigned to receive INCS for 3 months or placebo. Children in the original INCS arm were then reassigned to receive 9 more months of the same treatment or placebo. Symptoms as well as polysomnographic and neurobehavioral findings were measured at baseline, at 3 months, and again at 12 months.
“The primary outcome was OAHI change at 3 months, available for 122 children,” the authors explained. The OSAS was defined as an OAHI of between two and three events per hour. The median age of the children at baseline was 7.9 years, and the median OAHI baseline score was 5.8/hr (95% confidence interval, 3.6-9.7/hr). The total daily dose of the INCS used was 110 mcg.
At 3 months, the mean change in the OAHI from baseline was –1.73/hr (95% CI, –3.91 to 1.92/hr), while at 12 months, the mean change in the same index was –1.21 (–4.22 to 1.71/hr). These changes were not significantly different from OAHI changes observed among control participants. “OSAS symptoms and neurobehavioral results were not different [either] between the INCS and placebo groups at 3 and12 months,” the authors added.
However, among those children who received INCS treatment for the entire 12 months, the OAHI decreased significantly from 7.2/hr (95% CI, 3.62-9.88/hr) at baseline to 3.71/hr (95% CI, 1.56-6.4/hr; P = .039), although the OAHI did not normalize, the authors noted. Asked to clarify whether this change was not significant, Dr. Tapia said that it did meet statistical significance, but clinically, it meant that the children still needed some form of treatment, because they still had OSA in the range needing treatment.
The placebo group had more asthma exacerbations, upper respiratory tract infections, and exacerbations of OSAS symptoms, compared with children in the INCS group. It is possible that INCS provided a certain degree of protection from asthma exacerbation, the authors suggested.
However, recent guidelines from the American Academy of Pediatrics suggest that clinicians may prescribe these agents for children with mild OSAS in whom adenotonsillectomy is contraindicated; for those with mild postoperative OSAS, adenotonsillectomy remains the treatment of choice for childhood OSA. “The low level of enthusiasm for INCS in these guidelines is based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data,” the authors wrote.
“The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS,” they wrote.
Complex issue
In a comment, Rakesh Bhattacharjee, MD, associate professor of pediatrics, University of California, San Diego, noted that he does prescribe INCS for children with mild OSA but not for all children. “We based our decisions on polysomnography, which we use to categorize OSA as mild, moderate, or severe.
“But we certainly do offer this treatment for children with mild sleep apnea as a way to avoid surgical treatment,” Dr. Bhattacharjee added. He also uses INCS for residual sleep apnea that some children experience following adenotonsillectomy. As the current study suggests, many people are treating sleep apnea empirically without confirming the severity of the disorder by a sleep study.
“If a sleep study is not done, we don’t know how severe it is, so this would advocate for the utility of a sleep study so that you can quantify the severity of symptoms and target your therapy to children who might be appropriate for INCS therapy,” Dr. Bhattacharjee said.
On the other hand, surgery is not always relevant even if a child has enlarged adenoids and tonsils, as, for example, a child with obesity. In these children, physicians need to think about other treatments, such as continuous positive airways pressure. “CPAP is not perfect,” Dr. Bhattacharjee observed. “And as pediatricians, we need to do a lot of work to improve the use of CPAP, but, that said, there are children for whom INCS and surgery might be a waste of time, and this is where CPAP might be an alternative.”
Dr. Bhattacharjee previously was the lead author of a large study of children who underwent treatment with CPAP. While findings suggested that adherence to treatment is lower in children than it is for adults, the authors also showed that numerous actionable factors could used to improve adherence to CPAP among children who might otherwise benefit from it.
The authors disclosed no relevant financial relationships. Dr. Bhattacharjee has served as a scientific adviser for Jazz Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM CHEST
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.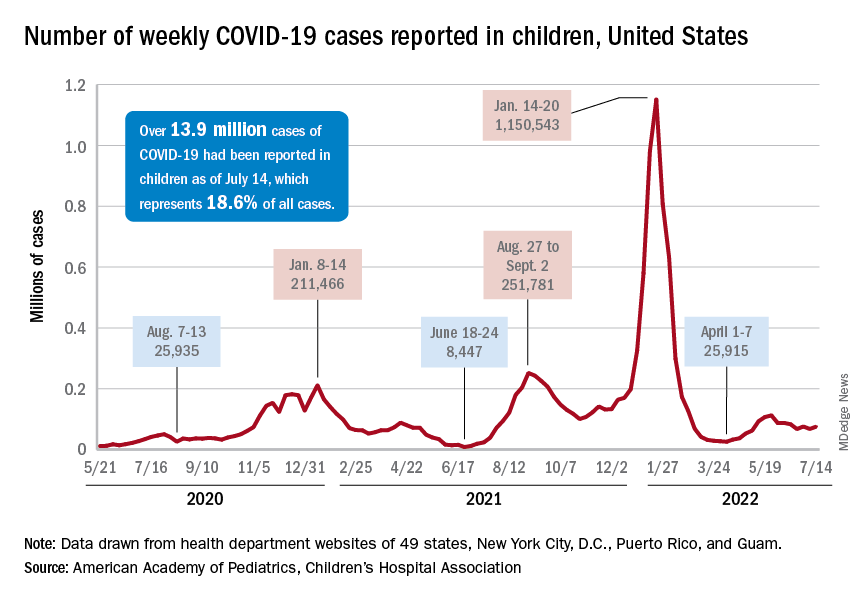
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.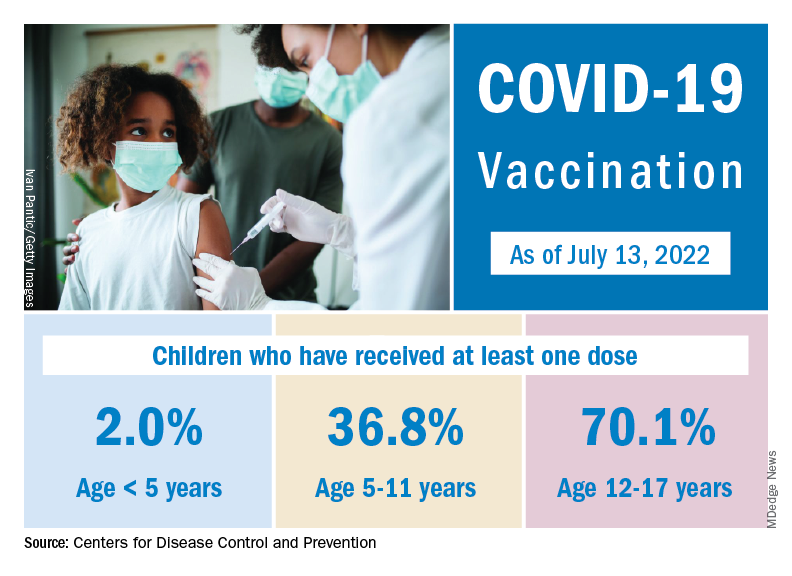
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
Anxiety spreads from mother to daughter, father to son
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
CDC warns about potentially deadly virus in infants
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
Commentary: Reversal of Roe v. Wade affects adolescents
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
Think of pediatric morphea as a systemic, chronic disease, expert advises
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
AT SPD 2022