User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Why do GI symptoms persist in some children with celiac disease?
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
Guidelines recommend CBT alone for mild acute depression, more options for more severe cases
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
AHA scientific statement on rapid evaluation for suspected TIA
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.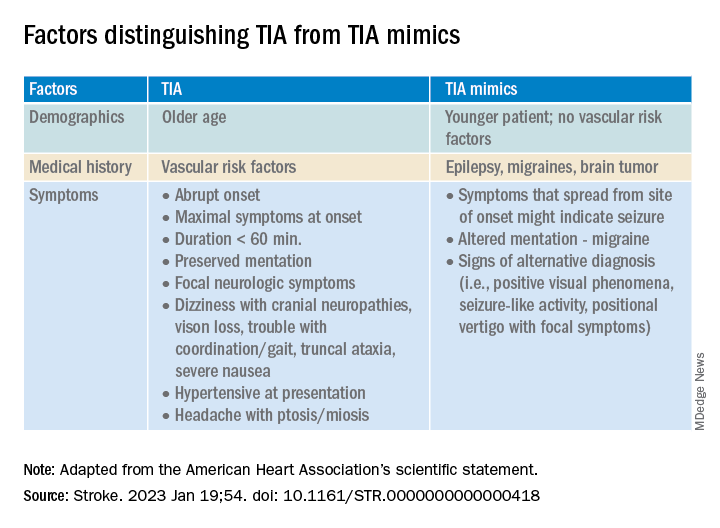
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
FROM STROKE
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
Hope for catching infants with CP early
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Should pediatricians fret over their falling board scores?
Few pediatricians have warm, fuzzy memories about taking their initial board exam.
But many reacted strongly when they read a recent post on Twitter by Bryan Carmody, MD, who noted that the passing rate for first-time test takers had dipped to its lowest level in 5 years – hitting 81% in 2021, down from 91% 3 years earlier.
“It’s literally an awfully written exam,” replied one person who posted. Another asked: “At what point is the exam just not reflective of clinical practice?” And, inevitably, the question of the effect of COVID-19 surfaced: “Is any of this attributable to pulling early career physicians into the pandemic?”
But Dr. Carmody, an associate professor of pediatrics at the University of Eastern Virginia Medical School, Norfolk, isn’t buying that explanation. He researched board scores for internal medicine, general surgery, and family medicine for 2021. All were stable during the same period, he said, leading him to dismiss the idea that the pandemic drove the decline. “It’s not really clear to me why other specialties wouldn’t have seen similar drops,” Dr. Carmody said.
The slip has caught the attention of the American Board of Pediatrics, according to Judy Schaechter, MD, MBA, who was chair of the department of pediatrics at the University of Miami before taking her post as president and CEO of the American Board of Pediatrics in 2021.
“So, our first question was, was this within the range of what one might expect?” Dr. Schaechter said. “Were there other factors that might have come into play?”
The board performs an extensive analysis every year before releasing scores, and it didn’t uncover any changes in the difficulty or content of the test in 2021, nor did the score that was needed to pass increase. Dr. Schaechter pointed out that the passing rate that year was not unprecedented – in 2016, it also dipped to 81%.
Dr. Schaechter said COVID-19 might have affected test takers. “Remember, pediatrics was different from any other specialty during the pandemic,” she said. The census in pediatric wards around the country dropped dramatically in the first two winters of the pandemic, leaving residents with less hands-on experience with patients and mentorship from attendings – both of which can help test-takers pass the exam.
Eyal Ben-Isaac, MD, an associate professor of the department of pediatrics at the Keck School of Medicine at the University of Southern California, Los Angeles, said residents likely suffered during the pandemic, when noon lectures and grand rounds became virtual events.
“I’m sure that clearly affected a person’s ability to sit and listen and really learn the material, as opposed to either doing it hands on or learning the material from a faculty member face to face,” Dr. Ben-Isaac said.
But how much do the didactic experiences of residency programs contribute to residents’ readiness to take the boards? Thomas Welch, MD, professor and chair emeritus of the department of pediatrics at SUNY Upstate Medical University, Syracuse, credits his own success in advancing through college, medical school, pediatric residency, and nephrology fellowship to his skill as a test taker.
He confirmed his suspicions by conducting a study that evaluated correlations between residents’ performance on the United States Medical Licensing Exam (USMLE) taken during medical school and their board scores after completing residency.
Dr. Welch said he wasn’t surprised to find that “the best predictor of one’s passing the pediatric boards was not the training program in which one worked. It was their performance on Step 2 [taken during the fourth year of medical school] of the USMLE.”
Although Dr. Ben-Isaac felt that changes in residency training opportunities might have partially explained the drop in passing rates, he agreed that other factors contribute to success on boards. As director of the pediatric residency program at Children’s Hospital of Los Angeles from 1994 to 2019, one of his first goals was to increase the pass rate of graduates. He developed a board review course for residents, revising it over time on the basis of resident feedback and adding individual coaching for residents who wanted more help.
“Without a question, it raised our board pass rate to being one of the highest in the country,” he said.
Dr. Welch said that while “being up all night with a sick child teaches you a lot about medicine and certainly makes you a better doctor, it doesn’t do anything to improve your board scores.”
None of the pediatricians was too worried about a 1-year drop in scores, and the consensus was that supporting residents with review courses and coaching on how to take multiple choice tests would raise passing rates.
“There are definitely people who are amazing clinicians who did not pass the boards on their first attempt,” Dr. Ben-Isaac said.
But Dr. Schaechter defended the importance of the examination. “Our first obligation is really to the public,” she said. The ABP’s role is to ensure that pediatricians “provide the care that parents want their kids to have.”
As Dr. Welch put it, “Would I trust someone who didn’t pass the board exam to take care of my own kid? Probably not.”
A version of this article first appeared on Medscape.com.
Few pediatricians have warm, fuzzy memories about taking their initial board exam.
But many reacted strongly when they read a recent post on Twitter by Bryan Carmody, MD, who noted that the passing rate for first-time test takers had dipped to its lowest level in 5 years – hitting 81% in 2021, down from 91% 3 years earlier.
“It’s literally an awfully written exam,” replied one person who posted. Another asked: “At what point is the exam just not reflective of clinical practice?” And, inevitably, the question of the effect of COVID-19 surfaced: “Is any of this attributable to pulling early career physicians into the pandemic?”
But Dr. Carmody, an associate professor of pediatrics at the University of Eastern Virginia Medical School, Norfolk, isn’t buying that explanation. He researched board scores for internal medicine, general surgery, and family medicine for 2021. All were stable during the same period, he said, leading him to dismiss the idea that the pandemic drove the decline. “It’s not really clear to me why other specialties wouldn’t have seen similar drops,” Dr. Carmody said.
The slip has caught the attention of the American Board of Pediatrics, according to Judy Schaechter, MD, MBA, who was chair of the department of pediatrics at the University of Miami before taking her post as president and CEO of the American Board of Pediatrics in 2021.
“So, our first question was, was this within the range of what one might expect?” Dr. Schaechter said. “Were there other factors that might have come into play?”
The board performs an extensive analysis every year before releasing scores, and it didn’t uncover any changes in the difficulty or content of the test in 2021, nor did the score that was needed to pass increase. Dr. Schaechter pointed out that the passing rate that year was not unprecedented – in 2016, it also dipped to 81%.
Dr. Schaechter said COVID-19 might have affected test takers. “Remember, pediatrics was different from any other specialty during the pandemic,” she said. The census in pediatric wards around the country dropped dramatically in the first two winters of the pandemic, leaving residents with less hands-on experience with patients and mentorship from attendings – both of which can help test-takers pass the exam.
Eyal Ben-Isaac, MD, an associate professor of the department of pediatrics at the Keck School of Medicine at the University of Southern California, Los Angeles, said residents likely suffered during the pandemic, when noon lectures and grand rounds became virtual events.
“I’m sure that clearly affected a person’s ability to sit and listen and really learn the material, as opposed to either doing it hands on or learning the material from a faculty member face to face,” Dr. Ben-Isaac said.
But how much do the didactic experiences of residency programs contribute to residents’ readiness to take the boards? Thomas Welch, MD, professor and chair emeritus of the department of pediatrics at SUNY Upstate Medical University, Syracuse, credits his own success in advancing through college, medical school, pediatric residency, and nephrology fellowship to his skill as a test taker.
He confirmed his suspicions by conducting a study that evaluated correlations between residents’ performance on the United States Medical Licensing Exam (USMLE) taken during medical school and their board scores after completing residency.
Dr. Welch said he wasn’t surprised to find that “the best predictor of one’s passing the pediatric boards was not the training program in which one worked. It was their performance on Step 2 [taken during the fourth year of medical school] of the USMLE.”
Although Dr. Ben-Isaac felt that changes in residency training opportunities might have partially explained the drop in passing rates, he agreed that other factors contribute to success on boards. As director of the pediatric residency program at Children’s Hospital of Los Angeles from 1994 to 2019, one of his first goals was to increase the pass rate of graduates. He developed a board review course for residents, revising it over time on the basis of resident feedback and adding individual coaching for residents who wanted more help.
“Without a question, it raised our board pass rate to being one of the highest in the country,” he said.
Dr. Welch said that while “being up all night with a sick child teaches you a lot about medicine and certainly makes you a better doctor, it doesn’t do anything to improve your board scores.”
None of the pediatricians was too worried about a 1-year drop in scores, and the consensus was that supporting residents with review courses and coaching on how to take multiple choice tests would raise passing rates.
“There are definitely people who are amazing clinicians who did not pass the boards on their first attempt,” Dr. Ben-Isaac said.
But Dr. Schaechter defended the importance of the examination. “Our first obligation is really to the public,” she said. The ABP’s role is to ensure that pediatricians “provide the care that parents want their kids to have.”
As Dr. Welch put it, “Would I trust someone who didn’t pass the board exam to take care of my own kid? Probably not.”
A version of this article first appeared on Medscape.com.
Few pediatricians have warm, fuzzy memories about taking their initial board exam.
But many reacted strongly when they read a recent post on Twitter by Bryan Carmody, MD, who noted that the passing rate for first-time test takers had dipped to its lowest level in 5 years – hitting 81% in 2021, down from 91% 3 years earlier.
“It’s literally an awfully written exam,” replied one person who posted. Another asked: “At what point is the exam just not reflective of clinical practice?” And, inevitably, the question of the effect of COVID-19 surfaced: “Is any of this attributable to pulling early career physicians into the pandemic?”
But Dr. Carmody, an associate professor of pediatrics at the University of Eastern Virginia Medical School, Norfolk, isn’t buying that explanation. He researched board scores for internal medicine, general surgery, and family medicine for 2021. All were stable during the same period, he said, leading him to dismiss the idea that the pandemic drove the decline. “It’s not really clear to me why other specialties wouldn’t have seen similar drops,” Dr. Carmody said.
The slip has caught the attention of the American Board of Pediatrics, according to Judy Schaechter, MD, MBA, who was chair of the department of pediatrics at the University of Miami before taking her post as president and CEO of the American Board of Pediatrics in 2021.
“So, our first question was, was this within the range of what one might expect?” Dr. Schaechter said. “Were there other factors that might have come into play?”
The board performs an extensive analysis every year before releasing scores, and it didn’t uncover any changes in the difficulty or content of the test in 2021, nor did the score that was needed to pass increase. Dr. Schaechter pointed out that the passing rate that year was not unprecedented – in 2016, it also dipped to 81%.
Dr. Schaechter said COVID-19 might have affected test takers. “Remember, pediatrics was different from any other specialty during the pandemic,” she said. The census in pediatric wards around the country dropped dramatically in the first two winters of the pandemic, leaving residents with less hands-on experience with patients and mentorship from attendings – both of which can help test-takers pass the exam.
Eyal Ben-Isaac, MD, an associate professor of the department of pediatrics at the Keck School of Medicine at the University of Southern California, Los Angeles, said residents likely suffered during the pandemic, when noon lectures and grand rounds became virtual events.
“I’m sure that clearly affected a person’s ability to sit and listen and really learn the material, as opposed to either doing it hands on or learning the material from a faculty member face to face,” Dr. Ben-Isaac said.
But how much do the didactic experiences of residency programs contribute to residents’ readiness to take the boards? Thomas Welch, MD, professor and chair emeritus of the department of pediatrics at SUNY Upstate Medical University, Syracuse, credits his own success in advancing through college, medical school, pediatric residency, and nephrology fellowship to his skill as a test taker.
He confirmed his suspicions by conducting a study that evaluated correlations between residents’ performance on the United States Medical Licensing Exam (USMLE) taken during medical school and their board scores after completing residency.
Dr. Welch said he wasn’t surprised to find that “the best predictor of one’s passing the pediatric boards was not the training program in which one worked. It was their performance on Step 2 [taken during the fourth year of medical school] of the USMLE.”
Although Dr. Ben-Isaac felt that changes in residency training opportunities might have partially explained the drop in passing rates, he agreed that other factors contribute to success on boards. As director of the pediatric residency program at Children’s Hospital of Los Angeles from 1994 to 2019, one of his first goals was to increase the pass rate of graduates. He developed a board review course for residents, revising it over time on the basis of resident feedback and adding individual coaching for residents who wanted more help.
“Without a question, it raised our board pass rate to being one of the highest in the country,” he said.
Dr. Welch said that while “being up all night with a sick child teaches you a lot about medicine and certainly makes you a better doctor, it doesn’t do anything to improve your board scores.”
None of the pediatricians was too worried about a 1-year drop in scores, and the consensus was that supporting residents with review courses and coaching on how to take multiple choice tests would raise passing rates.
“There are definitely people who are amazing clinicians who did not pass the boards on their first attempt,” Dr. Ben-Isaac said.
But Dr. Schaechter defended the importance of the examination. “Our first obligation is really to the public,” she said. The ABP’s role is to ensure that pediatricians “provide the care that parents want their kids to have.”
As Dr. Welch put it, “Would I trust someone who didn’t pass the board exam to take care of my own kid? Probably not.”
A version of this article first appeared on Medscape.com.
A freak impalement by a model rocket has this doctor scrambling
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
U.S. ketamine poisonings up 81%
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PSYCHOPHARMACOLOGY
Age-related atopic dermatitis phenotypes evaluated in study
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
FROM JAAD INTERNATIONAL