User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Supreme Court sets date for case that challenges Roe v. Wade
The Supreme Court will hear arguments in a major Mississippi abortion case on Dec. 1, which could challenge the landmark Roe v. Wade decision that guarantees a woman’s right to an abortion.
On Sept. 20, the court issued its calendar for arguments that will be heard in late November and early December, The Associated Press reported.
The Mississippi case, Dobbs v. Jackson Women’s Health Organization, is seeking to overturn Roe v. Wade by asking the Supreme Court to uphold a ban on most abortions after the 15th week of pregnancy. The state also said the court should overrule the 1992 decision in Planned Parenthood v. Casey that prevents states from banning abortion before viability, which is around 24 weeks of pregnancy.
Earlier in September, the Supreme Court allowed a Texas law to take effect that bans abortions after cardiac activity can be detected, which is around 6 weeks of pregnancy and often before many women know they’re pregnant. The court, which was split 5-4, didn’t rule on the constitutional nature of the law, instead declining to block its enforcement.
Hundreds of legal briefs have been filed on both sides of the case, the AP reported. On Sept. 20, more than 500 women athletes, including members of the Women’s National Basketball Players Association, the National Women’s Soccer League Players Association, and Olympic medalists, filed a brief that said an abortion ban would be devastating for female athletes.
The Mississippi law was enacted in 2018 but was blocked after a challenge at the federal court level. The state’s only abortion clinic, Jackson Women’s Health Organization, remains open and offers abortions up to 16 weeks of pregnancy, the AP reported. About 100 abortions a year are completed after 15 weeks, the organization said.
More than 90% of abortions in the United States occur in the first 13 weeks of pregnancy, the AP said.
The Supreme Court justices will return to the courtroom in October to hear arguments now that all of them have been vaccinated, the AP reported. The justices had been hearing cases by phone during the pandemic.
The public won’t be able to attend sessions, but the court will allow live audio of the session.
A version of this article first appeared on WebMD.com.
The Supreme Court will hear arguments in a major Mississippi abortion case on Dec. 1, which could challenge the landmark Roe v. Wade decision that guarantees a woman’s right to an abortion.
On Sept. 20, the court issued its calendar for arguments that will be heard in late November and early December, The Associated Press reported.
The Mississippi case, Dobbs v. Jackson Women’s Health Organization, is seeking to overturn Roe v. Wade by asking the Supreme Court to uphold a ban on most abortions after the 15th week of pregnancy. The state also said the court should overrule the 1992 decision in Planned Parenthood v. Casey that prevents states from banning abortion before viability, which is around 24 weeks of pregnancy.
Earlier in September, the Supreme Court allowed a Texas law to take effect that bans abortions after cardiac activity can be detected, which is around 6 weeks of pregnancy and often before many women know they’re pregnant. The court, which was split 5-4, didn’t rule on the constitutional nature of the law, instead declining to block its enforcement.
Hundreds of legal briefs have been filed on both sides of the case, the AP reported. On Sept. 20, more than 500 women athletes, including members of the Women’s National Basketball Players Association, the National Women’s Soccer League Players Association, and Olympic medalists, filed a brief that said an abortion ban would be devastating for female athletes.
The Mississippi law was enacted in 2018 but was blocked after a challenge at the federal court level. The state’s only abortion clinic, Jackson Women’s Health Organization, remains open and offers abortions up to 16 weeks of pregnancy, the AP reported. About 100 abortions a year are completed after 15 weeks, the organization said.
More than 90% of abortions in the United States occur in the first 13 weeks of pregnancy, the AP said.
The Supreme Court justices will return to the courtroom in October to hear arguments now that all of them have been vaccinated, the AP reported. The justices had been hearing cases by phone during the pandemic.
The public won’t be able to attend sessions, but the court will allow live audio of the session.
A version of this article first appeared on WebMD.com.
The Supreme Court will hear arguments in a major Mississippi abortion case on Dec. 1, which could challenge the landmark Roe v. Wade decision that guarantees a woman’s right to an abortion.
On Sept. 20, the court issued its calendar for arguments that will be heard in late November and early December, The Associated Press reported.
The Mississippi case, Dobbs v. Jackson Women’s Health Organization, is seeking to overturn Roe v. Wade by asking the Supreme Court to uphold a ban on most abortions after the 15th week of pregnancy. The state also said the court should overrule the 1992 decision in Planned Parenthood v. Casey that prevents states from banning abortion before viability, which is around 24 weeks of pregnancy.
Earlier in September, the Supreme Court allowed a Texas law to take effect that bans abortions after cardiac activity can be detected, which is around 6 weeks of pregnancy and often before many women know they’re pregnant. The court, which was split 5-4, didn’t rule on the constitutional nature of the law, instead declining to block its enforcement.
Hundreds of legal briefs have been filed on both sides of the case, the AP reported. On Sept. 20, more than 500 women athletes, including members of the Women’s National Basketball Players Association, the National Women’s Soccer League Players Association, and Olympic medalists, filed a brief that said an abortion ban would be devastating for female athletes.
The Mississippi law was enacted in 2018 but was blocked after a challenge at the federal court level. The state’s only abortion clinic, Jackson Women’s Health Organization, remains open and offers abortions up to 16 weeks of pregnancy, the AP reported. About 100 abortions a year are completed after 15 weeks, the organization said.
More than 90% of abortions in the United States occur in the first 13 weeks of pregnancy, the AP said.
The Supreme Court justices will return to the courtroom in October to hear arguments now that all of them have been vaccinated, the AP reported. The justices had been hearing cases by phone during the pandemic.
The public won’t be able to attend sessions, but the court will allow live audio of the session.
A version of this article first appeared on WebMD.com.
Texas doctor admits to violating abortion ban
A Texas doctor revealed in a Washington Post op-ed Sept. 18 that he violated the state ban on abortions performed beyond 6 weeks -- a move he knows could come with legal consequences.
San Antonio doctor Alan Braid, MD, said the new statewide restrictions reminded him of darker days during his 1972 obstetrics and gynecology residency, when he saw three teenagers die from illegal abortions.
“For me, it is 1972 all over again,” he wrote. “And that is why, on the morning of Sept. 6, I provided an abortion to a woman who, though still in her first trimester, was beyond the state’s new limit. I acted because I had a duty of care to this patient, as I do for all patients, and because she has a fundamental right to receive this care.”
“I fully understood that there could be legal consequences -- but I wanted to make sure that Texas didn’t get away with its bid to prevent this blatantly unconstitutional law from being tested,” he continued.
According to The Washington Post, Dr. Braid’s wish may come true. Two lawsuits against were filed Sept. 20. In one, a prisoner in Arkansas said he filed the suit in part because he could receive $10,000 if successful, according to the Post. The second was filed by a man in Chicago who wants the law struck down.
Dr. Braid’s op-ed is the first public admission to violating a Texas state law that took effect Sept. 1 banning abortion once a fetal heartbeat is detected. The controversial policy gives private citizens the right to bring civil litigation -- resulting in at least $10,000 in damages -- against providers and anyone else involved in the process.
Since the law went into effect, most patients seeking abortions are too far along to qualify, Dr. Braid wrote.
“I tell them that we can offer services only if we cannot see the presence of cardiac activity on an ultrasound, which usually occurs at about six weeks, before most people know they are pregnant. The tension is unbearable as they lie there, waiting to hear their fate,” he wrote.
“I understand that by providing an abortion beyond the new legal limit, I am taking a personal risk, but it’s something I believe in strongly,” he continued. “Represented by the Center for Reproductive Rights, my clinics are among the plaintiffs in an ongoing federal lawsuit to stop S.B. 8.”
A version of this article first appeared on WebMD.com .
A Texas doctor revealed in a Washington Post op-ed Sept. 18 that he violated the state ban on abortions performed beyond 6 weeks -- a move he knows could come with legal consequences.
San Antonio doctor Alan Braid, MD, said the new statewide restrictions reminded him of darker days during his 1972 obstetrics and gynecology residency, when he saw three teenagers die from illegal abortions.
“For me, it is 1972 all over again,” he wrote. “And that is why, on the morning of Sept. 6, I provided an abortion to a woman who, though still in her first trimester, was beyond the state’s new limit. I acted because I had a duty of care to this patient, as I do for all patients, and because she has a fundamental right to receive this care.”
“I fully understood that there could be legal consequences -- but I wanted to make sure that Texas didn’t get away with its bid to prevent this blatantly unconstitutional law from being tested,” he continued.
According to The Washington Post, Dr. Braid’s wish may come true. Two lawsuits against were filed Sept. 20. In one, a prisoner in Arkansas said he filed the suit in part because he could receive $10,000 if successful, according to the Post. The second was filed by a man in Chicago who wants the law struck down.
Dr. Braid’s op-ed is the first public admission to violating a Texas state law that took effect Sept. 1 banning abortion once a fetal heartbeat is detected. The controversial policy gives private citizens the right to bring civil litigation -- resulting in at least $10,000 in damages -- against providers and anyone else involved in the process.
Since the law went into effect, most patients seeking abortions are too far along to qualify, Dr. Braid wrote.
“I tell them that we can offer services only if we cannot see the presence of cardiac activity on an ultrasound, which usually occurs at about six weeks, before most people know they are pregnant. The tension is unbearable as they lie there, waiting to hear their fate,” he wrote.
“I understand that by providing an abortion beyond the new legal limit, I am taking a personal risk, but it’s something I believe in strongly,” he continued. “Represented by the Center for Reproductive Rights, my clinics are among the plaintiffs in an ongoing federal lawsuit to stop S.B. 8.”
A version of this article first appeared on WebMD.com .
A Texas doctor revealed in a Washington Post op-ed Sept. 18 that he violated the state ban on abortions performed beyond 6 weeks -- a move he knows could come with legal consequences.
San Antonio doctor Alan Braid, MD, said the new statewide restrictions reminded him of darker days during his 1972 obstetrics and gynecology residency, when he saw three teenagers die from illegal abortions.
“For me, it is 1972 all over again,” he wrote. “And that is why, on the morning of Sept. 6, I provided an abortion to a woman who, though still in her first trimester, was beyond the state’s new limit. I acted because I had a duty of care to this patient, as I do for all patients, and because she has a fundamental right to receive this care.”
“I fully understood that there could be legal consequences -- but I wanted to make sure that Texas didn’t get away with its bid to prevent this blatantly unconstitutional law from being tested,” he continued.
According to The Washington Post, Dr. Braid’s wish may come true. Two lawsuits against were filed Sept. 20. In one, a prisoner in Arkansas said he filed the suit in part because he could receive $10,000 if successful, according to the Post. The second was filed by a man in Chicago who wants the law struck down.
Dr. Braid’s op-ed is the first public admission to violating a Texas state law that took effect Sept. 1 banning abortion once a fetal heartbeat is detected. The controversial policy gives private citizens the right to bring civil litigation -- resulting in at least $10,000 in damages -- against providers and anyone else involved in the process.
Since the law went into effect, most patients seeking abortions are too far along to qualify, Dr. Braid wrote.
“I tell them that we can offer services only if we cannot see the presence of cardiac activity on an ultrasound, which usually occurs at about six weeks, before most people know they are pregnant. The tension is unbearable as they lie there, waiting to hear their fate,” he wrote.
“I understand that by providing an abortion beyond the new legal limit, I am taking a personal risk, but it’s something I believe in strongly,” he continued. “Represented by the Center for Reproductive Rights, my clinics are among the plaintiffs in an ongoing federal lawsuit to stop S.B. 8.”
A version of this article first appeared on WebMD.com .
Moderate alcohol intake may curb subsequent diabetes after gestational diabetes
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
COVID-19 claims more than 675,000 U.S. lives, surpassing the 1918 flu
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
Guideline gives weak support to trying oral medical cannabis for chronic pain
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
FROM THE BMJ
Survey: Nursing shortages affect safety during labor and delivery
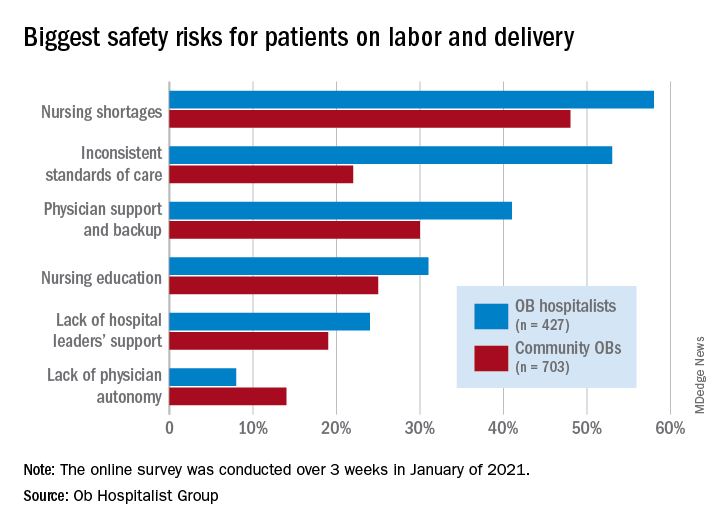
Just over 58% of the 1,130 respondents put nursing shortages ahead of physician support and backup (39.0%), inconsistent standards of care (38.5%), and nursing education (31.1%) as the most important challenge to patient safety, the Ob Hospitalist Group (OBHG) said in a new report.
“The survey reveals some startling gaps in physician and patient support all along the care continuum in obstetrics and OB hospitalist programs filling them,” said Lenny Castiglione, the CEO of OBHG, a national network of over 1,000 obstetric hospitalists. “As pressure builds on ob. units to improve care and reduce costs, and as clinical resources are stretched in the continuing battle against COVID-19 and its variants, health systems need to take serious measures to fill these gaps through staff recruitment, retention, and training.”
The national shortage of nurses is expected to get worse over the coming years as Baby Boomers’ need for health care increases and the large population (over 1 million) of older registered nurses retires by 2030, the OBHG said based on projections from the American Association of Colleges of Nursing.
Ob. hospitalists were somewhat more likely to see the nursing shortage as a major problem, compared with community-based Obs (58% vs. 48%), but the biggest difference in perception of safety risk between the two groups (53% for hospitalists vs. 22% for community physicians) involved inconsistent standards of care. “This is likely due to the ob. hospitalists’ 24/7 presence on the unit, and their visibility into the care provided across the unit,” the report noted.
Priorities for the future
Participants also were asked to rank each of seven focus areas on a scale of 0 (lowest priority) to 3 (highest) by its importance in the next 5 years. Maternal mortality was identified as the highest priority by 59.2% of physicians, followed by gaps in access to care between patient populations (38.0%), rural health care (26.5%), and ob.gyn. shortage (26.4%), the OBHG said.
A number of respondents noted the increase in high-risk patients, many of whom are obese and/or older and have comorbidities. “We know that the risk of C-sections increases relative to maternal weight. We need to focus on maternal morbidity and mortality,” one physician wrote in the open-ended response section.
When compared with the community obs., the hospitalists were much more likely to assign top priority over the next 5 years to maternal mortality (73% vs. 50%) and to gaps in access between patient populations (51% vs. 30%), according to the OBHG survey, which was conducted in January of 2021.
How will practice change in 5 years?
As for changes coming to obstetrical care over the next 5 years, respondents gave their strongest prediction to increased use of telemedicine, with 81.2% saying it would increase and just 14.4% predicting no change. The focus on subspecialization is expected to increase by 79.4% of participants (16.5% said no change), and 75.7% said that use of mid-level providers would rise (20.6% said no change), the survey data show.
The move toward mid-level providers was noted in many of the open-ended responses. “There is nothing mid-level about the midwifery care my colleagues provide our patients. They are experts in their field,” one physician wrote, but another said, “just because I foresee a shift toward increasing utilization of mid-levels and primary care practitioners for women’s health does not mean I endorse this trend.”

Just over 58% of the 1,130 respondents put nursing shortages ahead of physician support and backup (39.0%), inconsistent standards of care (38.5%), and nursing education (31.1%) as the most important challenge to patient safety, the Ob Hospitalist Group (OBHG) said in a new report.
“The survey reveals some startling gaps in physician and patient support all along the care continuum in obstetrics and OB hospitalist programs filling them,” said Lenny Castiglione, the CEO of OBHG, a national network of over 1,000 obstetric hospitalists. “As pressure builds on ob. units to improve care and reduce costs, and as clinical resources are stretched in the continuing battle against COVID-19 and its variants, health systems need to take serious measures to fill these gaps through staff recruitment, retention, and training.”
The national shortage of nurses is expected to get worse over the coming years as Baby Boomers’ need for health care increases and the large population (over 1 million) of older registered nurses retires by 2030, the OBHG said based on projections from the American Association of Colleges of Nursing.
Ob. hospitalists were somewhat more likely to see the nursing shortage as a major problem, compared with community-based Obs (58% vs. 48%), but the biggest difference in perception of safety risk between the two groups (53% for hospitalists vs. 22% for community physicians) involved inconsistent standards of care. “This is likely due to the ob. hospitalists’ 24/7 presence on the unit, and their visibility into the care provided across the unit,” the report noted.
Priorities for the future
Participants also were asked to rank each of seven focus areas on a scale of 0 (lowest priority) to 3 (highest) by its importance in the next 5 years. Maternal mortality was identified as the highest priority by 59.2% of physicians, followed by gaps in access to care between patient populations (38.0%), rural health care (26.5%), and ob.gyn. shortage (26.4%), the OBHG said.
A number of respondents noted the increase in high-risk patients, many of whom are obese and/or older and have comorbidities. “We know that the risk of C-sections increases relative to maternal weight. We need to focus on maternal morbidity and mortality,” one physician wrote in the open-ended response section.
When compared with the community obs., the hospitalists were much more likely to assign top priority over the next 5 years to maternal mortality (73% vs. 50%) and to gaps in access between patient populations (51% vs. 30%), according to the OBHG survey, which was conducted in January of 2021.
How will practice change in 5 years?
As for changes coming to obstetrical care over the next 5 years, respondents gave their strongest prediction to increased use of telemedicine, with 81.2% saying it would increase and just 14.4% predicting no change. The focus on subspecialization is expected to increase by 79.4% of participants (16.5% said no change), and 75.7% said that use of mid-level providers would rise (20.6% said no change), the survey data show.
The move toward mid-level providers was noted in many of the open-ended responses. “There is nothing mid-level about the midwifery care my colleagues provide our patients. They are experts in their field,” one physician wrote, but another said, “just because I foresee a shift toward increasing utilization of mid-levels and primary care practitioners for women’s health does not mean I endorse this trend.”

Just over 58% of the 1,130 respondents put nursing shortages ahead of physician support and backup (39.0%), inconsistent standards of care (38.5%), and nursing education (31.1%) as the most important challenge to patient safety, the Ob Hospitalist Group (OBHG) said in a new report.
“The survey reveals some startling gaps in physician and patient support all along the care continuum in obstetrics and OB hospitalist programs filling them,” said Lenny Castiglione, the CEO of OBHG, a national network of over 1,000 obstetric hospitalists. “As pressure builds on ob. units to improve care and reduce costs, and as clinical resources are stretched in the continuing battle against COVID-19 and its variants, health systems need to take serious measures to fill these gaps through staff recruitment, retention, and training.”
The national shortage of nurses is expected to get worse over the coming years as Baby Boomers’ need for health care increases and the large population (over 1 million) of older registered nurses retires by 2030, the OBHG said based on projections from the American Association of Colleges of Nursing.
Ob. hospitalists were somewhat more likely to see the nursing shortage as a major problem, compared with community-based Obs (58% vs. 48%), but the biggest difference in perception of safety risk between the two groups (53% for hospitalists vs. 22% for community physicians) involved inconsistent standards of care. “This is likely due to the ob. hospitalists’ 24/7 presence on the unit, and their visibility into the care provided across the unit,” the report noted.
Priorities for the future
Participants also were asked to rank each of seven focus areas on a scale of 0 (lowest priority) to 3 (highest) by its importance in the next 5 years. Maternal mortality was identified as the highest priority by 59.2% of physicians, followed by gaps in access to care between patient populations (38.0%), rural health care (26.5%), and ob.gyn. shortage (26.4%), the OBHG said.
A number of respondents noted the increase in high-risk patients, many of whom are obese and/or older and have comorbidities. “We know that the risk of C-sections increases relative to maternal weight. We need to focus on maternal morbidity and mortality,” one physician wrote in the open-ended response section.
When compared with the community obs., the hospitalists were much more likely to assign top priority over the next 5 years to maternal mortality (73% vs. 50%) and to gaps in access between patient populations (51% vs. 30%), according to the OBHG survey, which was conducted in January of 2021.
How will practice change in 5 years?
As for changes coming to obstetrical care over the next 5 years, respondents gave their strongest prediction to increased use of telemedicine, with 81.2% saying it would increase and just 14.4% predicting no change. The focus on subspecialization is expected to increase by 79.4% of participants (16.5% said no change), and 75.7% said that use of mid-level providers would rise (20.6% said no change), the survey data show.
The move toward mid-level providers was noted in many of the open-ended responses. “There is nothing mid-level about the midwifery care my colleagues provide our patients. They are experts in their field,” one physician wrote, but another said, “just because I foresee a shift toward increasing utilization of mid-levels and primary care practitioners for women’s health does not mean I endorse this trend.”
Maryland reduces cesarean delivery rates
A statewide educational initiative in Maryland was associated with a significant reduction in cesarean delivery rates over 30 months, although program implementation was widely variable across participating hospitals, according to investigators.
Cesarean deliveries dropped 1.6% among nulliparous, term, singleton, vertex births, falling short of the 3.2% reduction recently achieved by a similar program in California, reported lead author Jennifer A. Callaghan-Koru, PhD, of the University of Maryland, Baltimore County, and colleagues.
“Although cesarean delivery can be lifesaving, evidence suggests that there is no benefit to maternal health when national cesarean delivery rates are higher than 20 per 100 live births,” the investigators wrote in Obstetrics & Gynecology.
They noted that cesarean delivery rates in the United States rose dramatically between 1996 and 2006, from 20.7% to 32.9%, before falling back to 31.7% in 2019.
According to the investigators, high cesarean delivery rates have drawn action from a roster of stakeholders, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), the Department of Health and Human Services, the Joint Commission, and the Council on Patient Safety in Women’s Health Care.
The latter group created an evidence-based obstetric patient safety bundle that was used in the present study. The investigators recruited 31 out of 32 birthing hospitals in Maryland, and over the course of 30 months, educated the participating hospitals on the practices recommended by the bundle, via in-person and remote training.
To measure implementation and associated outcomes, the investigators used a data portal provided by the ACOG Alliance for Innovation in Maternal Health (AIM) program, which supports adoption of the council’s safety bundle nationwide. Data included number of births; number of cesarean births; overall cesarean delivery rates; cesarean rates among nulliparous, term, singleton, vertex births; cesarean rates among nulliparous, term, singleton, vertex inductions; as well as severe maternal morbidity rates.
Among the 26 safety practices in the bundle, hospitals reported current use of 7 (median) before the program began, with a range from 0 to 23. During the 30-month collaboration, hospitals added a median of 4 practices, ranging from 0 to 17.
Concurrently, cesarean delivery rates for nulliparous, term, singleton, vertex births dropped from 26.9% to 28.5% (P = .011), while rates for inductions dropped to a greater degree, from 36.1% to 31.3% (P less than .001). Further analysis showed that greater reductions in rates of cesarean delivery were associated with adoption of practices in the “Response” domain.
“The Response domain has the largest number of practices that standardize clinical care, including induction scheduling, diagnosis and treatment of labor dystocia and failed induction, and interpretation of fetal heart rate patterns,” the investigators wrote. “The important role of standardizing care is consistent with a Cochrane review that found moderately strong evidence that the implementation of clinical practice guidelines, alongside feedback to clinicians (e.g., second opinions, audit and feedback of rates), can reduce cesarean delivery.”
Dr. Callaghan-Koru and colleagues noted the high variability of implementation among hospitals, which could explain why statewide reductions weren’t more dramatic.
“Other evaluations of perinatal quality improvement collaboratives have also found that some hospitals get left behind in these efforts, without making considerable progress and improving outcomes,” they wrote. “Given that work by state perinatal quality improvement collaboratives represents a primary national strategy for reducing maternal morbidity and mortality, it is critically important to conduct further implementation research to identify determinants of success and strategies to support all participating hospitals to make improvements.”
According to Iris Krishna, MD, of Emory University, Atlanta, each state’s starting point may predict how successful similar programs will be.
“The safe reduction in the cesarean delivery rate will vary by state,” Dr. Krishna said in a written comment. “States that have a well-established perinatal quality collaborative that have the support on the provider, hospital, and statewide level are more likely to successfully implement strategies and see a statistically significant decrease.”
She went on to emphasize the importance of collaboration across multiple levels of organization, and across a variety of health care providers and administrators.
“Successful implementation requires a multidisciplinary team (physicians, nurses, quality improvement officers) and a multifaceted approach (statewide policies),” Dr. Krishna said. “Key stakeholders will need to ‘buy in’ or be willing to support the policy and practice change to ensure its success. To address obstacles, initiatives to support vaginal birth are important, such as provider training on labor and support techniques, criteria for diagnosis of and management of labor dystocia and arrest disorders, standard responses to abnormal fetal heart rate patterns, and availability of expertise to lessen the need for cesarean delivery, such as breech version, instrumented delivery, and twin delivery protocols. It is also important for hospitals to a have a mentor model of quality improvement and shared learning strategies that work.”
Dr. Krishna agreed with the investigators that more work is necessary to determine best strategies for future intervention.
“Research is needed in identifying determinants of success and sustainment,” she said.
Dr. Burke received funding from her employer, Trinity Health, to conduct a pilot study concerning blood loss.
A statewide educational initiative in Maryland was associated with a significant reduction in cesarean delivery rates over 30 months, although program implementation was widely variable across participating hospitals, according to investigators.
Cesarean deliveries dropped 1.6% among nulliparous, term, singleton, vertex births, falling short of the 3.2% reduction recently achieved by a similar program in California, reported lead author Jennifer A. Callaghan-Koru, PhD, of the University of Maryland, Baltimore County, and colleagues.
“Although cesarean delivery can be lifesaving, evidence suggests that there is no benefit to maternal health when national cesarean delivery rates are higher than 20 per 100 live births,” the investigators wrote in Obstetrics & Gynecology.
They noted that cesarean delivery rates in the United States rose dramatically between 1996 and 2006, from 20.7% to 32.9%, before falling back to 31.7% in 2019.
According to the investigators, high cesarean delivery rates have drawn action from a roster of stakeholders, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), the Department of Health and Human Services, the Joint Commission, and the Council on Patient Safety in Women’s Health Care.
The latter group created an evidence-based obstetric patient safety bundle that was used in the present study. The investigators recruited 31 out of 32 birthing hospitals in Maryland, and over the course of 30 months, educated the participating hospitals on the practices recommended by the bundle, via in-person and remote training.
To measure implementation and associated outcomes, the investigators used a data portal provided by the ACOG Alliance for Innovation in Maternal Health (AIM) program, which supports adoption of the council’s safety bundle nationwide. Data included number of births; number of cesarean births; overall cesarean delivery rates; cesarean rates among nulliparous, term, singleton, vertex births; cesarean rates among nulliparous, term, singleton, vertex inductions; as well as severe maternal morbidity rates.
Among the 26 safety practices in the bundle, hospitals reported current use of 7 (median) before the program began, with a range from 0 to 23. During the 30-month collaboration, hospitals added a median of 4 practices, ranging from 0 to 17.
Concurrently, cesarean delivery rates for nulliparous, term, singleton, vertex births dropped from 26.9% to 28.5% (P = .011), while rates for inductions dropped to a greater degree, from 36.1% to 31.3% (P less than .001). Further analysis showed that greater reductions in rates of cesarean delivery were associated with adoption of practices in the “Response” domain.
“The Response domain has the largest number of practices that standardize clinical care, including induction scheduling, diagnosis and treatment of labor dystocia and failed induction, and interpretation of fetal heart rate patterns,” the investigators wrote. “The important role of standardizing care is consistent with a Cochrane review that found moderately strong evidence that the implementation of clinical practice guidelines, alongside feedback to clinicians (e.g., second opinions, audit and feedback of rates), can reduce cesarean delivery.”
Dr. Callaghan-Koru and colleagues noted the high variability of implementation among hospitals, which could explain why statewide reductions weren’t more dramatic.
“Other evaluations of perinatal quality improvement collaboratives have also found that some hospitals get left behind in these efforts, without making considerable progress and improving outcomes,” they wrote. “Given that work by state perinatal quality improvement collaboratives represents a primary national strategy for reducing maternal morbidity and mortality, it is critically important to conduct further implementation research to identify determinants of success and strategies to support all participating hospitals to make improvements.”
According to Iris Krishna, MD, of Emory University, Atlanta, each state’s starting point may predict how successful similar programs will be.
“The safe reduction in the cesarean delivery rate will vary by state,” Dr. Krishna said in a written comment. “States that have a well-established perinatal quality collaborative that have the support on the provider, hospital, and statewide level are more likely to successfully implement strategies and see a statistically significant decrease.”
She went on to emphasize the importance of collaboration across multiple levels of organization, and across a variety of health care providers and administrators.
“Successful implementation requires a multidisciplinary team (physicians, nurses, quality improvement officers) and a multifaceted approach (statewide policies),” Dr. Krishna said. “Key stakeholders will need to ‘buy in’ or be willing to support the policy and practice change to ensure its success. To address obstacles, initiatives to support vaginal birth are important, such as provider training on labor and support techniques, criteria for diagnosis of and management of labor dystocia and arrest disorders, standard responses to abnormal fetal heart rate patterns, and availability of expertise to lessen the need for cesarean delivery, such as breech version, instrumented delivery, and twin delivery protocols. It is also important for hospitals to a have a mentor model of quality improvement and shared learning strategies that work.”
Dr. Krishna agreed with the investigators that more work is necessary to determine best strategies for future intervention.
“Research is needed in identifying determinants of success and sustainment,” she said.
Dr. Burke received funding from her employer, Trinity Health, to conduct a pilot study concerning blood loss.
A statewide educational initiative in Maryland was associated with a significant reduction in cesarean delivery rates over 30 months, although program implementation was widely variable across participating hospitals, according to investigators.
Cesarean deliveries dropped 1.6% among nulliparous, term, singleton, vertex births, falling short of the 3.2% reduction recently achieved by a similar program in California, reported lead author Jennifer A. Callaghan-Koru, PhD, of the University of Maryland, Baltimore County, and colleagues.
“Although cesarean delivery can be lifesaving, evidence suggests that there is no benefit to maternal health when national cesarean delivery rates are higher than 20 per 100 live births,” the investigators wrote in Obstetrics & Gynecology.
They noted that cesarean delivery rates in the United States rose dramatically between 1996 and 2006, from 20.7% to 32.9%, before falling back to 31.7% in 2019.
According to the investigators, high cesarean delivery rates have drawn action from a roster of stakeholders, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), the Department of Health and Human Services, the Joint Commission, and the Council on Patient Safety in Women’s Health Care.
The latter group created an evidence-based obstetric patient safety bundle that was used in the present study. The investigators recruited 31 out of 32 birthing hospitals in Maryland, and over the course of 30 months, educated the participating hospitals on the practices recommended by the bundle, via in-person and remote training.
To measure implementation and associated outcomes, the investigators used a data portal provided by the ACOG Alliance for Innovation in Maternal Health (AIM) program, which supports adoption of the council’s safety bundle nationwide. Data included number of births; number of cesarean births; overall cesarean delivery rates; cesarean rates among nulliparous, term, singleton, vertex births; cesarean rates among nulliparous, term, singleton, vertex inductions; as well as severe maternal morbidity rates.
Among the 26 safety practices in the bundle, hospitals reported current use of 7 (median) before the program began, with a range from 0 to 23. During the 30-month collaboration, hospitals added a median of 4 practices, ranging from 0 to 17.
Concurrently, cesarean delivery rates for nulliparous, term, singleton, vertex births dropped from 26.9% to 28.5% (P = .011), while rates for inductions dropped to a greater degree, from 36.1% to 31.3% (P less than .001). Further analysis showed that greater reductions in rates of cesarean delivery were associated with adoption of practices in the “Response” domain.
“The Response domain has the largest number of practices that standardize clinical care, including induction scheduling, diagnosis and treatment of labor dystocia and failed induction, and interpretation of fetal heart rate patterns,” the investigators wrote. “The important role of standardizing care is consistent with a Cochrane review that found moderately strong evidence that the implementation of clinical practice guidelines, alongside feedback to clinicians (e.g., second opinions, audit and feedback of rates), can reduce cesarean delivery.”
Dr. Callaghan-Koru and colleagues noted the high variability of implementation among hospitals, which could explain why statewide reductions weren’t more dramatic.
“Other evaluations of perinatal quality improvement collaboratives have also found that some hospitals get left behind in these efforts, without making considerable progress and improving outcomes,” they wrote. “Given that work by state perinatal quality improvement collaboratives represents a primary national strategy for reducing maternal morbidity and mortality, it is critically important to conduct further implementation research to identify determinants of success and strategies to support all participating hospitals to make improvements.”
According to Iris Krishna, MD, of Emory University, Atlanta, each state’s starting point may predict how successful similar programs will be.
“The safe reduction in the cesarean delivery rate will vary by state,” Dr. Krishna said in a written comment. “States that have a well-established perinatal quality collaborative that have the support on the provider, hospital, and statewide level are more likely to successfully implement strategies and see a statistically significant decrease.”
She went on to emphasize the importance of collaboration across multiple levels of organization, and across a variety of health care providers and administrators.
“Successful implementation requires a multidisciplinary team (physicians, nurses, quality improvement officers) and a multifaceted approach (statewide policies),” Dr. Krishna said. “Key stakeholders will need to ‘buy in’ or be willing to support the policy and practice change to ensure its success. To address obstacles, initiatives to support vaginal birth are important, such as provider training on labor and support techniques, criteria for diagnosis of and management of labor dystocia and arrest disorders, standard responses to abnormal fetal heart rate patterns, and availability of expertise to lessen the need for cesarean delivery, such as breech version, instrumented delivery, and twin delivery protocols. It is also important for hospitals to a have a mentor model of quality improvement and shared learning strategies that work.”
Dr. Krishna agreed with the investigators that more work is necessary to determine best strategies for future intervention.
“Research is needed in identifying determinants of success and sustainment,” she said.
Dr. Burke received funding from her employer, Trinity Health, to conduct a pilot study concerning blood loss.
FROM OBSTETRICS & GYNECOLOGY
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
How knowledgeable are ObGyns about dense breasts?
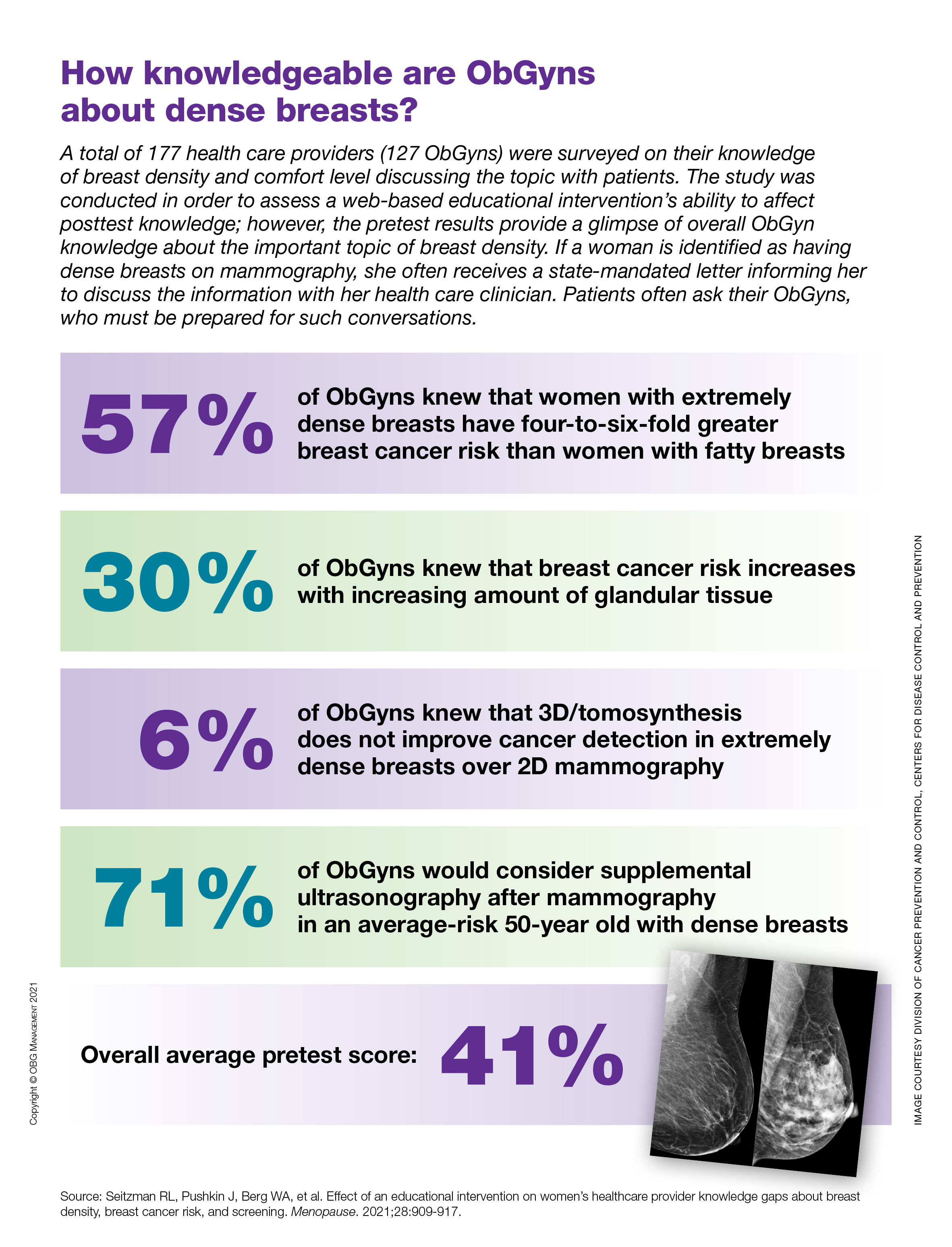


‘New first-line standard of care’ in cervical cancer
That declaration was made by Raza Mirza, MD, chief oncologist at Copenhagen University Hospital in Denmark, who was invited to discuss the pros and cons of the KEYNOTE-826 trial at the European Society for Medical Oncology (ESMO) Congress 2021.
The trial showed that adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard chemotherapy — with or without bevacizumab — resulted in about a one third reduction in the risk for both disease progression and death compared with chemotherapy alone.
The benefit of adding pembrolizumab was seen both in the overall study population and in patients with higher levels of programmed death ligand-1 (PD-L1), but not in those with biomarker-negative tumors, reported investigator Nicoletta Colombo, MD, PhD, from the University of Milan-Bicocca, Italy.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus platinum-based chemotherapy with or without bevacizumab may be a new first-line standard of care,” she said in a late-breaking oral abstract presentation. The study was also simultaneously published online in The New England Journal of Medicine.
Since 2014, the standard of care for treating patients with recurrent, persistent, or metastatic cervical cancer has been chemotherapy with a platinum compound, paclitaxel, plus bevacizumab, based on the results of the GOG 240 study.
Immunotherapy with PD-1 inhibitors have shown efficacy as monotherapy in second- or later-line therapy for women with cervical cancer, but until now no data about the addition of these agents to chemotherapy were available, Dr. Colombo noted.
Dr. Mirza noted that there is sound rationale for using checkpoint inhibitors targeted against PD-1 in patients with cervical cancer, because PD-L1 has been shown to be a consistent biomarker for infection of the cervix with human papillomavirus (HPV), which is responsible for more than 90% of cervical cancers.
“PD-L1 is significantly upregulated in cervical cancer and detectable by immunohistochemistry,” he said. “PD-L1 expression reduces the immune response since it is able to bind to PD-1 on T-cell lymphocytes, thereby inhibiting their function. These findings suggest that targeting the PD-1/PD-L1 pathway may be therapeutically effective and should be considered in the treatment of cervical cancer.”
KEYNOTE-826 details
This was a double-blind trial conducted in 617 patients stratified by metastatic disease status at diagnosis; PD-L1 combined positive score (CPS) either < 1, 1 to < 10, or ≥ 10. They were randomized in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus platinum-based chemotherapy, with bevacizumab added at the investigator’s discretion.
The dual primary endpoints of progression-free survival (PFS) and overall survival (OS) were each tested sequentially in patients with a PD-L1 CPS ≥ 1 in both the intention-to-treat (ITT) or “all-comers” population, and in patients with a PD-L1 CPS ≥ 10.
Patient characteristics were generally well balanced between the treatment groups, except for a slightly higher proportion of patients with squamous cell histology in the pembrolizumab versus the placebo group (76.3% vs 68.3%).
PFS and OS results
The addition of pembrolizumab was associated with improved PFS across most protocol-specified subgroups, Dr. Colombo and colleagues noted.
After a median follow-up of 22 months, the 12-month PFS rate in the biomarker-selected population (all patients with a PD-L1 CPS ≥ 1) was 45.5% for patients in the pembrolizumab group versus 34.1% in the placebo group. This translated into a hazard ratio (HR) for progression on pembrolizumab of 0.62 (P < .001).
The respective PFS rates in the ITT population were 44.7% and 33.5%, with an HR for progression of 0.65 (P < .001) with the checkpoint inhibitor.
In patients with PD-L1 CPS ≥ 10, the respective rates of PFS and the HR were 44.6%, 33.5%, and 0.58 (P < .001).
OS rates were also significantly improved, he noted.
The 12-month and 24-month OS rates in all patients with PD-L1 CPS ≥ 1 were 75.3% and 53%, respectively, for patients assigned to pembrolizumab versus 63.1% and 41.7% in patients assigned to placebo, translating to an HR for death with pembrolizumab in this group of 0.64 (P < .001).
In the all-comers (ITT) population, respective 12- and 24-month OS rates were 74.8% and 50.4% with pembrolizumab versus 63.6% and 40.4% with placebo. This difference translated into an HR for death with anti-PD-1 of 0.67 (P < .001).
Among patients with the higher PD-L1 levels (≥ CPS 10), the respective OS rates were 75.7% and 54.4% with pembrolizumab versus 61.5% and 44.6% with placebo (HR 0.61, P < .001).
Dr. Mirza emphasized that “we did not see any efficacy of pembrolizumab in the biomarker-negative population,” with an HR for PFS of 0.94 and HR for OS of 1.0 in this subgroup.
The most common grade ≥ 3 adverse events were anemia, which occurred in 30.3% of patients assigned to pembrolizumab compared with 26.9% in the placebo group, and neutropenias, which occurred in 12.4% and 9.7% of patients, respectively. One patient in the pembrolizumab group died from an immune-related event, encephalitis.
Despite his enthusiasm for the regimen, Dr. Mirza tempered it by pointing out that there was an imbalance in the sample sizes regarding histology, and a potential bias introduced by the failure to stratify by tumor histology.
He noted that in other studies checkpoint inhibitors have had only modest activity against adenocarcinomas, which were more frequent in the placebo group in KEYNOTE-826, resulting in a potential positive bias in favor of pembrolizumab.
KEYNOTE-826 is funded by MSD. Dr. Colombo has disclosed consultant, research, and promotional speaking activities for multiple companies. Dr. Mirza has disclosed personal financial interests with Merck and other companies.
A version of this article was first published on Medscape.com.
That declaration was made by Raza Mirza, MD, chief oncologist at Copenhagen University Hospital in Denmark, who was invited to discuss the pros and cons of the KEYNOTE-826 trial at the European Society for Medical Oncology (ESMO) Congress 2021.
The trial showed that adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard chemotherapy — with or without bevacizumab — resulted in about a one third reduction in the risk for both disease progression and death compared with chemotherapy alone.
The benefit of adding pembrolizumab was seen both in the overall study population and in patients with higher levels of programmed death ligand-1 (PD-L1), but not in those with biomarker-negative tumors, reported investigator Nicoletta Colombo, MD, PhD, from the University of Milan-Bicocca, Italy.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus platinum-based chemotherapy with or without bevacizumab may be a new first-line standard of care,” she said in a late-breaking oral abstract presentation. The study was also simultaneously published online in The New England Journal of Medicine.
Since 2014, the standard of care for treating patients with recurrent, persistent, or metastatic cervical cancer has been chemotherapy with a platinum compound, paclitaxel, plus bevacizumab, based on the results of the GOG 240 study.
Immunotherapy with PD-1 inhibitors have shown efficacy as monotherapy in second- or later-line therapy for women with cervical cancer, but until now no data about the addition of these agents to chemotherapy were available, Dr. Colombo noted.
Dr. Mirza noted that there is sound rationale for using checkpoint inhibitors targeted against PD-1 in patients with cervical cancer, because PD-L1 has been shown to be a consistent biomarker for infection of the cervix with human papillomavirus (HPV), which is responsible for more than 90% of cervical cancers.
“PD-L1 is significantly upregulated in cervical cancer and detectable by immunohistochemistry,” he said. “PD-L1 expression reduces the immune response since it is able to bind to PD-1 on T-cell lymphocytes, thereby inhibiting their function. These findings suggest that targeting the PD-1/PD-L1 pathway may be therapeutically effective and should be considered in the treatment of cervical cancer.”
KEYNOTE-826 details
This was a double-blind trial conducted in 617 patients stratified by metastatic disease status at diagnosis; PD-L1 combined positive score (CPS) either < 1, 1 to < 10, or ≥ 10. They were randomized in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus platinum-based chemotherapy, with bevacizumab added at the investigator’s discretion.
The dual primary endpoints of progression-free survival (PFS) and overall survival (OS) were each tested sequentially in patients with a PD-L1 CPS ≥ 1 in both the intention-to-treat (ITT) or “all-comers” population, and in patients with a PD-L1 CPS ≥ 10.
Patient characteristics were generally well balanced between the treatment groups, except for a slightly higher proportion of patients with squamous cell histology in the pembrolizumab versus the placebo group (76.3% vs 68.3%).
PFS and OS results
The addition of pembrolizumab was associated with improved PFS across most protocol-specified subgroups, Dr. Colombo and colleagues noted.
After a median follow-up of 22 months, the 12-month PFS rate in the biomarker-selected population (all patients with a PD-L1 CPS ≥ 1) was 45.5% for patients in the pembrolizumab group versus 34.1% in the placebo group. This translated into a hazard ratio (HR) for progression on pembrolizumab of 0.62 (P < .001).
The respective PFS rates in the ITT population were 44.7% and 33.5%, with an HR for progression of 0.65 (P < .001) with the checkpoint inhibitor.
In patients with PD-L1 CPS ≥ 10, the respective rates of PFS and the HR were 44.6%, 33.5%, and 0.58 (P < .001).
OS rates were also significantly improved, he noted.
The 12-month and 24-month OS rates in all patients with PD-L1 CPS ≥ 1 were 75.3% and 53%, respectively, for patients assigned to pembrolizumab versus 63.1% and 41.7% in patients assigned to placebo, translating to an HR for death with pembrolizumab in this group of 0.64 (P < .001).
In the all-comers (ITT) population, respective 12- and 24-month OS rates were 74.8% and 50.4% with pembrolizumab versus 63.6% and 40.4% with placebo. This difference translated into an HR for death with anti-PD-1 of 0.67 (P < .001).
Among patients with the higher PD-L1 levels (≥ CPS 10), the respective OS rates were 75.7% and 54.4% with pembrolizumab versus 61.5% and 44.6% with placebo (HR 0.61, P < .001).
Dr. Mirza emphasized that “we did not see any efficacy of pembrolizumab in the biomarker-negative population,” with an HR for PFS of 0.94 and HR for OS of 1.0 in this subgroup.
The most common grade ≥ 3 adverse events were anemia, which occurred in 30.3% of patients assigned to pembrolizumab compared with 26.9% in the placebo group, and neutropenias, which occurred in 12.4% and 9.7% of patients, respectively. One patient in the pembrolizumab group died from an immune-related event, encephalitis.
Despite his enthusiasm for the regimen, Dr. Mirza tempered it by pointing out that there was an imbalance in the sample sizes regarding histology, and a potential bias introduced by the failure to stratify by tumor histology.
He noted that in other studies checkpoint inhibitors have had only modest activity against adenocarcinomas, which were more frequent in the placebo group in KEYNOTE-826, resulting in a potential positive bias in favor of pembrolizumab.
KEYNOTE-826 is funded by MSD. Dr. Colombo has disclosed consultant, research, and promotional speaking activities for multiple companies. Dr. Mirza has disclosed personal financial interests with Merck and other companies.
A version of this article was first published on Medscape.com.
That declaration was made by Raza Mirza, MD, chief oncologist at Copenhagen University Hospital in Denmark, who was invited to discuss the pros and cons of the KEYNOTE-826 trial at the European Society for Medical Oncology (ESMO) Congress 2021.
The trial showed that adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard chemotherapy — with or without bevacizumab — resulted in about a one third reduction in the risk for both disease progression and death compared with chemotherapy alone.
The benefit of adding pembrolizumab was seen both in the overall study population and in patients with higher levels of programmed death ligand-1 (PD-L1), but not in those with biomarker-negative tumors, reported investigator Nicoletta Colombo, MD, PhD, from the University of Milan-Bicocca, Italy.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus platinum-based chemotherapy with or without bevacizumab may be a new first-line standard of care,” she said in a late-breaking oral abstract presentation. The study was also simultaneously published online in The New England Journal of Medicine.
Since 2014, the standard of care for treating patients with recurrent, persistent, or metastatic cervical cancer has been chemotherapy with a platinum compound, paclitaxel, plus bevacizumab, based on the results of the GOG 240 study.
Immunotherapy with PD-1 inhibitors have shown efficacy as monotherapy in second- or later-line therapy for women with cervical cancer, but until now no data about the addition of these agents to chemotherapy were available, Dr. Colombo noted.
Dr. Mirza noted that there is sound rationale for using checkpoint inhibitors targeted against PD-1 in patients with cervical cancer, because PD-L1 has been shown to be a consistent biomarker for infection of the cervix with human papillomavirus (HPV), which is responsible for more than 90% of cervical cancers.
“PD-L1 is significantly upregulated in cervical cancer and detectable by immunohistochemistry,” he said. “PD-L1 expression reduces the immune response since it is able to bind to PD-1 on T-cell lymphocytes, thereby inhibiting their function. These findings suggest that targeting the PD-1/PD-L1 pathway may be therapeutically effective and should be considered in the treatment of cervical cancer.”
KEYNOTE-826 details
This was a double-blind trial conducted in 617 patients stratified by metastatic disease status at diagnosis; PD-L1 combined positive score (CPS) either < 1, 1 to < 10, or ≥ 10. They were randomized in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus platinum-based chemotherapy, with bevacizumab added at the investigator’s discretion.
The dual primary endpoints of progression-free survival (PFS) and overall survival (OS) were each tested sequentially in patients with a PD-L1 CPS ≥ 1 in both the intention-to-treat (ITT) or “all-comers” population, and in patients with a PD-L1 CPS ≥ 10.
Patient characteristics were generally well balanced between the treatment groups, except for a slightly higher proportion of patients with squamous cell histology in the pembrolizumab versus the placebo group (76.3% vs 68.3%).
PFS and OS results
The addition of pembrolizumab was associated with improved PFS across most protocol-specified subgroups, Dr. Colombo and colleagues noted.
After a median follow-up of 22 months, the 12-month PFS rate in the biomarker-selected population (all patients with a PD-L1 CPS ≥ 1) was 45.5% for patients in the pembrolizumab group versus 34.1% in the placebo group. This translated into a hazard ratio (HR) for progression on pembrolizumab of 0.62 (P < .001).
The respective PFS rates in the ITT population were 44.7% and 33.5%, with an HR for progression of 0.65 (P < .001) with the checkpoint inhibitor.
In patients with PD-L1 CPS ≥ 10, the respective rates of PFS and the HR were 44.6%, 33.5%, and 0.58 (P < .001).
OS rates were also significantly improved, he noted.
The 12-month and 24-month OS rates in all patients with PD-L1 CPS ≥ 1 were 75.3% and 53%, respectively, for patients assigned to pembrolizumab versus 63.1% and 41.7% in patients assigned to placebo, translating to an HR for death with pembrolizumab in this group of 0.64 (P < .001).
In the all-comers (ITT) population, respective 12- and 24-month OS rates were 74.8% and 50.4% with pembrolizumab versus 63.6% and 40.4% with placebo. This difference translated into an HR for death with anti-PD-1 of 0.67 (P < .001).
Among patients with the higher PD-L1 levels (≥ CPS 10), the respective OS rates were 75.7% and 54.4% with pembrolizumab versus 61.5% and 44.6% with placebo (HR 0.61, P < .001).
Dr. Mirza emphasized that “we did not see any efficacy of pembrolizumab in the biomarker-negative population,” with an HR for PFS of 0.94 and HR for OS of 1.0 in this subgroup.
The most common grade ≥ 3 adverse events were anemia, which occurred in 30.3% of patients assigned to pembrolizumab compared with 26.9% in the placebo group, and neutropenias, which occurred in 12.4% and 9.7% of patients, respectively. One patient in the pembrolizumab group died from an immune-related event, encephalitis.
Despite his enthusiasm for the regimen, Dr. Mirza tempered it by pointing out that there was an imbalance in the sample sizes regarding histology, and a potential bias introduced by the failure to stratify by tumor histology.
He noted that in other studies checkpoint inhibitors have had only modest activity against adenocarcinomas, which were more frequent in the placebo group in KEYNOTE-826, resulting in a potential positive bias in favor of pembrolizumab.
KEYNOTE-826 is funded by MSD. Dr. Colombo has disclosed consultant, research, and promotional speaking activities for multiple companies. Dr. Mirza has disclosed personal financial interests with Merck and other companies.
A version of this article was first published on Medscape.com.



