User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Commonly used antibiotics in ObGyn practice
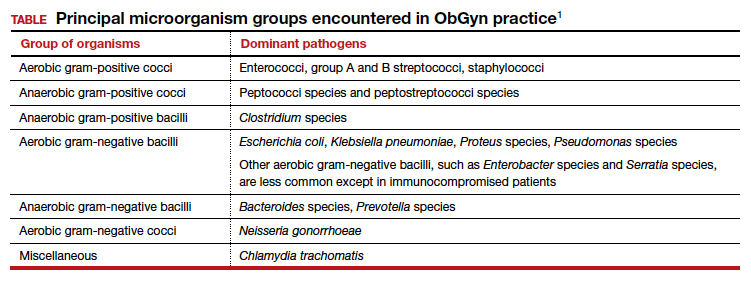
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.
Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.

Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.

Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
Can US “pattern recognition” of classic adnexal lesions reduce surgery, and even referrals for other imaging, in average-risk women?
Gupta A, Jha P, Baran TM, et al. Ovarian cancer detection in average-risk women: classic- versus nonclassic-appearing adnexal lesions at US. Radiology. 2022;212338. doi: 10.1148/radiol.212338.
Expert commentary
Gupta and colleagues conducted a multicenter, retrospective review of 970 adnexal lesions among 878 women—75% were premenopausal and 25% were postmenopausal.
Imaging details
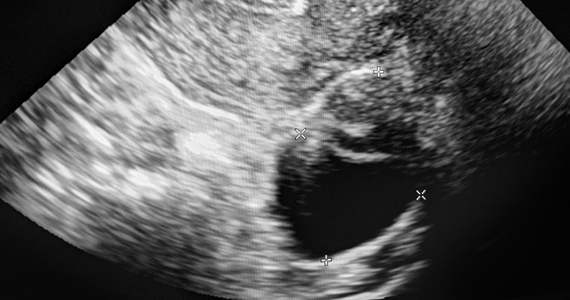
The lesions were characterized by pattern recognition as “classic” (simple cysts, endometriomas, hemorrhagic cysts, or dermoids) or “nonclassic.” Out of 673 classic lesions, there were 4 malignancies (0.6%), of which 1 was an endometrioma and 3 were classified as simple cysts. However, out of 297 nonclassic lesions (multilocular, unilocular with solid areas or wall irregularity, or mostly solid), 32% (33/103) were malignant when vascularity was present, while 8% (16/184) were malignant when no intralesional vascularity was appreciated.
The authors pointed out that, especially because their study was retrospective, there was no standardization of scan technique or equipment employed. However, this point adds credibility to the “real world” nature of such imaging.
Other data corroborate findings
Other studies have looked at pattern recognition in efforts to optimize a conservative approach to benign masses and referral to oncology for suspected malignant masses, as described above. This was the main cornerstone of the International Consensus Conference,2 which also identified next steps for indeterminate masses, including evidence-based risk assessment algorithms and referral (to an expert imager or gynecologic oncologist). A multicenter trial in Europe3 found that ultrasound experience substantially impacts on diagnostic performance when adnexal masses are classified using pattern recognition. This occurred in a stepwise fashion with increasing accuracy directly related to the level of expertise. Shetty and colleagues4 found that pattern recognition performed better than the risk of malignancy index (sensitivities of 95% and 79%, respectively). ●
While the concept of pattern recognition for some “classic” benign ovarian masses has been around for some time, this is the first time a large United States–based study (albeit retrospective) has corroborated that when ultrasonography reveals a classic, or “almost certainly benign” finding, patients can be reassured that the lesion is benign, thereby avoiding extensive further workup. When a lesion is “nonclassic” in appearance and without any blood flow, further imaging with follow-up magnetic resonance imaging or repeat ultrasound could be considered. In women with a nonclassic lesion with blood flow, particularly in older women, referral to a gynecologic oncologic surgeon will help ensure expeditious treatment of possible ovarian cancer.
- Boll D, Geomini PM, Brölmann HA. The pre-operative assessment of the adnexal mass: the accuracy of clinical estimates versus clinical prediction rules. BJOG. 2003;110:519-523.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36:849-863. doi: 10.1002/jum.14197.
- Van Holsbeke C, Daemen A, Yazbek J, et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest. 2010;69:160-168. doi: 10.1159/000265012.
- Shetty J, Reddy G, Pandey D. Role of sonographic grayscale pattern recognition in the diagnosis of adnexal masses. J Clin Diagn Res. 2017;11:QC12-QC15. doi: 10.7860 /JCDR/2017/28533.10614.
Gupta A, Jha P, Baran TM, et al. Ovarian cancer detection in average-risk women: classic- versus nonclassic-appearing adnexal lesions at US. Radiology. 2022;212338. doi: 10.1148/radiol.212338.
Expert commentary
Gupta and colleagues conducted a multicenter, retrospective review of 970 adnexal lesions among 878 women—75% were premenopausal and 25% were postmenopausal.
Imaging details
The lesions were characterized by pattern recognition as “classic” (simple cysts, endometriomas, hemorrhagic cysts, or dermoids) or “nonclassic.” Out of 673 classic lesions, there were 4 malignancies (0.6%), of which 1 was an endometrioma and 3 were classified as simple cysts. However, out of 297 nonclassic lesions (multilocular, unilocular with solid areas or wall irregularity, or mostly solid), 32% (33/103) were malignant when vascularity was present, while 8% (16/184) were malignant when no intralesional vascularity was appreciated.
The authors pointed out that, especially because their study was retrospective, there was no standardization of scan technique or equipment employed. However, this point adds credibility to the “real world” nature of such imaging.
Other data corroborate findings
Other studies have looked at pattern recognition in efforts to optimize a conservative approach to benign masses and referral to oncology for suspected malignant masses, as described above. This was the main cornerstone of the International Consensus Conference,2 which also identified next steps for indeterminate masses, including evidence-based risk assessment algorithms and referral (to an expert imager or gynecologic oncologist). A multicenter trial in Europe3 found that ultrasound experience substantially impacts on diagnostic performance when adnexal masses are classified using pattern recognition. This occurred in a stepwise fashion with increasing accuracy directly related to the level of expertise. Shetty and colleagues4 found that pattern recognition performed better than the risk of malignancy index (sensitivities of 95% and 79%, respectively). ●
While the concept of pattern recognition for some “classic” benign ovarian masses has been around for some time, this is the first time a large United States–based study (albeit retrospective) has corroborated that when ultrasonography reveals a classic, or “almost certainly benign” finding, patients can be reassured that the lesion is benign, thereby avoiding extensive further workup. When a lesion is “nonclassic” in appearance and without any blood flow, further imaging with follow-up magnetic resonance imaging or repeat ultrasound could be considered. In women with a nonclassic lesion with blood flow, particularly in older women, referral to a gynecologic oncologic surgeon will help ensure expeditious treatment of possible ovarian cancer.
Gupta A, Jha P, Baran TM, et al. Ovarian cancer detection in average-risk women: classic- versus nonclassic-appearing adnexal lesions at US. Radiology. 2022;212338. doi: 10.1148/radiol.212338.
Expert commentary
Gupta and colleagues conducted a multicenter, retrospective review of 970 adnexal lesions among 878 women—75% were premenopausal and 25% were postmenopausal.
Imaging details
The lesions were characterized by pattern recognition as “classic” (simple cysts, endometriomas, hemorrhagic cysts, or dermoids) or “nonclassic.” Out of 673 classic lesions, there were 4 malignancies (0.6%), of which 1 was an endometrioma and 3 were classified as simple cysts. However, out of 297 nonclassic lesions (multilocular, unilocular with solid areas or wall irregularity, or mostly solid), 32% (33/103) were malignant when vascularity was present, while 8% (16/184) were malignant when no intralesional vascularity was appreciated.
The authors pointed out that, especially because their study was retrospective, there was no standardization of scan technique or equipment employed. However, this point adds credibility to the “real world” nature of such imaging.
Other data corroborate findings
Other studies have looked at pattern recognition in efforts to optimize a conservative approach to benign masses and referral to oncology for suspected malignant masses, as described above. This was the main cornerstone of the International Consensus Conference,2 which also identified next steps for indeterminate masses, including evidence-based risk assessment algorithms and referral (to an expert imager or gynecologic oncologist). A multicenter trial in Europe3 found that ultrasound experience substantially impacts on diagnostic performance when adnexal masses are classified using pattern recognition. This occurred in a stepwise fashion with increasing accuracy directly related to the level of expertise. Shetty and colleagues4 found that pattern recognition performed better than the risk of malignancy index (sensitivities of 95% and 79%, respectively). ●
While the concept of pattern recognition for some “classic” benign ovarian masses has been around for some time, this is the first time a large United States–based study (albeit retrospective) has corroborated that when ultrasonography reveals a classic, or “almost certainly benign” finding, patients can be reassured that the lesion is benign, thereby avoiding extensive further workup. When a lesion is “nonclassic” in appearance and without any blood flow, further imaging with follow-up magnetic resonance imaging or repeat ultrasound could be considered. In women with a nonclassic lesion with blood flow, particularly in older women, referral to a gynecologic oncologic surgeon will help ensure expeditious treatment of possible ovarian cancer.
- Boll D, Geomini PM, Brölmann HA. The pre-operative assessment of the adnexal mass: the accuracy of clinical estimates versus clinical prediction rules. BJOG. 2003;110:519-523.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36:849-863. doi: 10.1002/jum.14197.
- Van Holsbeke C, Daemen A, Yazbek J, et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest. 2010;69:160-168. doi: 10.1159/000265012.
- Shetty J, Reddy G, Pandey D. Role of sonographic grayscale pattern recognition in the diagnosis of adnexal masses. J Clin Diagn Res. 2017;11:QC12-QC15. doi: 10.7860 /JCDR/2017/28533.10614.
- Boll D, Geomini PM, Brölmann HA. The pre-operative assessment of the adnexal mass: the accuracy of clinical estimates versus clinical prediction rules. BJOG. 2003;110:519-523.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36:849-863. doi: 10.1002/jum.14197.
- Van Holsbeke C, Daemen A, Yazbek J, et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest. 2010;69:160-168. doi: 10.1159/000265012.
- Shetty J, Reddy G, Pandey D. Role of sonographic grayscale pattern recognition in the diagnosis of adnexal masses. J Clin Diagn Res. 2017;11:QC12-QC15. doi: 10.7860 /JCDR/2017/28533.10614.
Optimize detection and treatment of iron deficiency in pregnancy
During pregnancy, anemia and iron deficiency are prevalent because the fetus depletes maternal iron stores. Iron deficiency and iron deficiency anemia are not synonymous. Effective screening for iron deficiency in the first trimester of pregnancy requires the measurement of a sensitive and specific biomarker of iron deficiency, such as ferritin. Limiting the measurement of ferritin to the subset of patients with anemia will result in missing many cases of iron deficiency. By the time iron deficiency causes anemia, a severe deficiency is present. Detecting iron deficiency in pregnancy and promptly treating the deficiency will reduce the number of women with anemia in the third trimester and at birth.
Diagnosis of anemia
Anemia in pregnancy is diagnosed by a hemoglobin level and hematocrit concentration below 11 g/dL and 33%, respectively, in the first and third trimesters and below 10.5 g/dL and 32%, respectively, in the second trimester.1 The prevalence of anemia in the first, second, and third trimesters is approximately 3%, 2%, and 11%, respectively.2 At a hemoglobin concentration <11 g/dL, severe maternal morbidity rises significantly.3 The laboratory evaluation of pregnant women with anemia may require assessment of iron stores, measurement of folate and cobalamin (vitamin B12), and hemoglobin electrophoresis, if indicated.
Diagnosis of iron deficiency
Iron deficiency anemia is diagnosed by a ferritin level below 30 ng/mL.4,5 Normal iron stores and iron insufficiency are indicated by ferritin levels 45 to 150 ng/mL and 30 to 44 ng/mL, respectively.4,5 Ferritin is an acute phase reactant, and patients with inflammation or chronic illnesses may have iron deficiency and a normal ferritin level. For these patients, a transferrin saturation (TSAT) <16% would support a diagnosis of iron deficiency.6 TSAT is calculated from measurement of serum iron and total iron binding capacity. TSAT saturation may be elevated by iron supplements, which increase serum iron. If measurement of TSAT is necessary, interference with the measurement accuracy can be minimized by not taking an iron supplement on the day of testing.
Iron deficiency is present in approximately 50% of pregnant women.7,8 The greatest prevalence of iron deficiency in pregnancy is observed in non-Hispanic Black females, followed by Hispanic females. Non-Hispanic White females had the lowest prevalence of iron deficiency.2
Fetal needs for iron often cause the depletion of maternal iron stores. Many pregnant women who have a normal ferritin level in the first trimester will develop iron deficiency in the third trimester, even with the usual recommended daily oral iron supplementation. We recommend measuring ferritin and hemoglobin at the first prenatal visit and again between 24 and 28 weeks’ gestation.
Impact of maternal anemia on maternal and newborn health
Iron plays a critical role in maternal health and fetal development independent of its role in red blood cell formation. Many proteins critical to maternal health and fetal development contain iron, including hemoglobin, myoglobin, cytochromes, ribonucleotide reductase, peroxidases, lipoxygenases, and cyclooxygenases. In the fetus, iron plays an important role in myelination of nerves, dendrite arborization, and synthesis of monoamine neurotransmitters.9
Many studies report that maternal anemia is associated with severe maternal morbidity and adverse newborn outcomes. The current literature must be interpreted with caution because socioeconomic factors influence iron stores. Iron deficiency and anemia is more common among economically and socially disadvantaged populations.10-12 It is possible that repleting iron stores, alone, without addressing social determinants of health, including food and housing insecurity, may be insufficient to improve maternal and newborn health.
Maternal anemia is a risk factor for severe maternal morbidity and adverse newborn outcomes.3,13-18 In a study of 515,270 live births in British Columbia between 2004 and 2016, maternal anemia was diagnosed in 12.8% of mothers.15 Maternal morbidity at birth was increased among patients with mild anemia (hemoglobin concentration of 9 to 10.9 g/dL), including higher rates of intrapartum transfusion (adjusted odds ratio [OR], 2.45; 95% confidence interval [CI], 1.74-3.45), cesarean birth (aOR, 1.17; 95% CI, 1.14-1.19), and chorioamnionitis (aOR, 1.35; 95% CI, 1.27-1.44). Newborn morbidity was also increased among newborns of mothers with mild anemia (hemoglobin concentrations of 9 to 10.9 g/dL), including birth before 37 weeks’ gestation (aOR, 1.09; 95% CI, 1.05-1.12), birth before 32 weeks’ gestation (aOR, 1.30; 95% CI, 1.21-1.39), admission to the intensive care unit (aOR, 1.21; 95% CI, 1.17-1.25), and respiratory distress syndrome (aOR, 1.35; 95% CI, 1.24-1.46).15 Adverse maternal and newborn outcomes were more prevalent among mothers with moderate (hemoglobin concentrations of 7 to 8.9 g/dL) or severe anemia (hemoglobin concentrations of <7 g/dL), compared with mild anemia. For example, compared with mothers with no anemia, mothers with moderate anemia had an increased risk of birth <37 weeks (aOR, 2.26) and birth <32 weeks (aOR, 3.95).15
In a study of 166,566 US pregnant patients, 6.1% were diagnosed with anemia.18 Patients with anemia were more likely to have antepartum thrombosis, preeclampsia, eclampsia, a cesarean birth, postpartum hemorrhage, a blood transfusion, and postpartum thrombosis.18 In this study, the newborns of mothers with anemia were more likely to have a diagnosis of antenatal or intrapartum fetal distress, a 5-minute Apgar score <7, and an admission to the neonatal intensive care unit.
Continue to: Maternal anemia and neurodevelopmental disorders in children...
Maternal anemia and neurodevelopmental disorders in children
Some experts, but not all, believe that iron deficiency during pregnancy may adversely impact fetal neurodevelopment and result in childhood behavior issues. All experts agree that more research is needed to understand if maternal anemia causes mental health issues in newborns. In one meta-analysis, among 20 studies of the association of maternal iron deficiency and newborn neurodevelopment, approximately half the studies reported that low maternal ferritin levels were associated with lower childhood performance on standardized tests of cognitive, motor, verbal, and memory function.19 Another systematic review concluded that the evidence linking maternal iron deficiency and child neurodevelopment is equivocal.20
In a study of 532,232 nonadoptive children born in Sweden from 1987 to 2010, maternal anemia was associated with an increased risk of autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and intellectual disability (ID).21 In Sweden maternal hemoglobin concentration is measured at 10, 25, and 37 weeks of gestation, permitting comparisons of anemia diagnosed early and late in pregnancy with neurodevelopmental outcomes. The association between anemia and neurodevelopmental disorders was greatest if anemia was diagnosed within the first 30 weeks of pregnancy. Compared with mothers without anemia, maternal anemia diagnosed within the first 30 weeks of pregnancy was associated with higher childhood rates of ASD (4.9% vs 3.5%), ADHD (9.3% vs 7.1%), and ID (3.1% vs 1.3%).21 The differences persisted in analyses that controlled for socioeconomic, maternal, and pregnancy-related factors. In a matched sibling comparison, the diagnosis of maternal anemia within the first 30 weeks of gestation was associated with an increased risk of ASD (OR, 2.25; 95% CI, 1.24-4.11) and ID (OR, 2.59; 95% CI, 1.08-6.22) but not ADHD.21 Other studies have also reported a relationship between maternal anemia and intellectual disability.22,23
Measurement of hemoglobin will identify anemia, but hemoglobin measurement is not sufficiently sensitive to identify most cases of iron deficiency. Measuring ferritin can help to identify cases of iron deficiency before the onset of anemia, permitting early treatment of the nutrient deficiency. In pregnancy, iron deficiency is the prelude to developing anemia. Waiting until anemia occurs to diagnose and treat iron deficiency is suboptimal and may miss a critical window of fetal development that is dependent on maternal iron stores. During pregnancy, ferritin levels decrease as much as 80% between the first and third trimesters, as the fetus utilizes maternal iron stores for its growth.24 We recommend the measurement of ferritin and hemoglobin at the first prenatal visit and again at 24 to 28 weeks’ gestation to optimize early detection and treatment of iron deficiency and reduce the frequency of anemia prior to birth. ●
- American College of Obstetricians and Gynecologists. Anemia in pregnancy. ACOG Practice Bulletin No 233. Obstet Gynecol. 2021;138:e55-64.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
- Ray JG, Davidson AJF, Berger H, et al. Haemoglobin levels in early pregnancy and severe maternal morbidity: population-based cohort study. BJOG. 2020;127:1154-1164.
- Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45-51.
- Parvord S, Daru J, Prasannan N, et al. UK Guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2020;188:819-830.
- Camaschell C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832-1843.
- Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
- Teichman J, Nisenbaum R, Lausman A, et al. Suboptimal iron deficiency screening in pregnancy and the impact of socioeconomic status in high-resource setting. Blood Adv. 2021;5:4666-4673.
- Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43-S48.
- Bodnar LM, Scanlon KS, Freedman DS, et al. High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185:438-443.
- Dondi A, PIccinno V, Morigi F, et al. Food insecurity and major diet-related morbidities in migrating children: a systematic review. Nutrients. 2020;12:379.
- Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298-2302.
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman S, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Smith C, Teng F, Branch E, et al. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134:1234-1244.
- Parks S, Hoffman MK, Goudar SS, et al. Maternal anaemia and maternal, fetal and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126:737-743.
- Guignard J, Deneux-Tharaux C, Seco A, et al. Gestational anemia and severe acute maternal morbidity: a population based study. Anesthesia. 2021;76:61-71.
- Harrison RK, Lauhon SR, Colvin ZA, et al. Maternal anemia and severe maternal mortality in a US cohort. Am J Obstet Gynecol MFM. 2021;3:100395.
- Quesada-Pinedo HG, Cassel F, Duijts L, et al. Maternal iron status in pregnancy and child health outcomes after birth: a systematic review and meta-analysis. Nutrients. 2021;13:2221.
- McCann S, Perapoch Amado M, Moore SE. The role of iron in brain development: a systematic review. Nutrients. 2020;12:2001.
- Wiegersma AM, Dalman C, Lee BK, et al. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. 2019;76:1294-1304.
- Leonard H, de Klerk N, Bourke J, et al. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol. 2006;16:448-454.
- Drassinower D, Lavery JA, Friedman AM, et al. The effect of maternal hematocrit on offspring IQ at 4 and 7 years of age: a secondary analysis. BJOG. 2016;123:2087-2093.
- Horton KD, Adetona O, Aguilar-Villalobos M, et al. Changes in the concentration of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru 2004-2005. Nutrition J. 2013;12:80.
During pregnancy, anemia and iron deficiency are prevalent because the fetus depletes maternal iron stores. Iron deficiency and iron deficiency anemia are not synonymous. Effective screening for iron deficiency in the first trimester of pregnancy requires the measurement of a sensitive and specific biomarker of iron deficiency, such as ferritin. Limiting the measurement of ferritin to the subset of patients with anemia will result in missing many cases of iron deficiency. By the time iron deficiency causes anemia, a severe deficiency is present. Detecting iron deficiency in pregnancy and promptly treating the deficiency will reduce the number of women with anemia in the third trimester and at birth.
Diagnosis of anemia
Anemia in pregnancy is diagnosed by a hemoglobin level and hematocrit concentration below 11 g/dL and 33%, respectively, in the first and third trimesters and below 10.5 g/dL and 32%, respectively, in the second trimester.1 The prevalence of anemia in the first, second, and third trimesters is approximately 3%, 2%, and 11%, respectively.2 At a hemoglobin concentration <11 g/dL, severe maternal morbidity rises significantly.3 The laboratory evaluation of pregnant women with anemia may require assessment of iron stores, measurement of folate and cobalamin (vitamin B12), and hemoglobin electrophoresis, if indicated.
Diagnosis of iron deficiency
Iron deficiency anemia is diagnosed by a ferritin level below 30 ng/mL.4,5 Normal iron stores and iron insufficiency are indicated by ferritin levels 45 to 150 ng/mL and 30 to 44 ng/mL, respectively.4,5 Ferritin is an acute phase reactant, and patients with inflammation or chronic illnesses may have iron deficiency and a normal ferritin level. For these patients, a transferrin saturation (TSAT) <16% would support a diagnosis of iron deficiency.6 TSAT is calculated from measurement of serum iron and total iron binding capacity. TSAT saturation may be elevated by iron supplements, which increase serum iron. If measurement of TSAT is necessary, interference with the measurement accuracy can be minimized by not taking an iron supplement on the day of testing.
Iron deficiency is present in approximately 50% of pregnant women.7,8 The greatest prevalence of iron deficiency in pregnancy is observed in non-Hispanic Black females, followed by Hispanic females. Non-Hispanic White females had the lowest prevalence of iron deficiency.2
Fetal needs for iron often cause the depletion of maternal iron stores. Many pregnant women who have a normal ferritin level in the first trimester will develop iron deficiency in the third trimester, even with the usual recommended daily oral iron supplementation. We recommend measuring ferritin and hemoglobin at the first prenatal visit and again between 24 and 28 weeks’ gestation.
Impact of maternal anemia on maternal and newborn health
Iron plays a critical role in maternal health and fetal development independent of its role in red blood cell formation. Many proteins critical to maternal health and fetal development contain iron, including hemoglobin, myoglobin, cytochromes, ribonucleotide reductase, peroxidases, lipoxygenases, and cyclooxygenases. In the fetus, iron plays an important role in myelination of nerves, dendrite arborization, and synthesis of monoamine neurotransmitters.9
Many studies report that maternal anemia is associated with severe maternal morbidity and adverse newborn outcomes. The current literature must be interpreted with caution because socioeconomic factors influence iron stores. Iron deficiency and anemia is more common among economically and socially disadvantaged populations.10-12 It is possible that repleting iron stores, alone, without addressing social determinants of health, including food and housing insecurity, may be insufficient to improve maternal and newborn health.
Maternal anemia is a risk factor for severe maternal morbidity and adverse newborn outcomes.3,13-18 In a study of 515,270 live births in British Columbia between 2004 and 2016, maternal anemia was diagnosed in 12.8% of mothers.15 Maternal morbidity at birth was increased among patients with mild anemia (hemoglobin concentration of 9 to 10.9 g/dL), including higher rates of intrapartum transfusion (adjusted odds ratio [OR], 2.45; 95% confidence interval [CI], 1.74-3.45), cesarean birth (aOR, 1.17; 95% CI, 1.14-1.19), and chorioamnionitis (aOR, 1.35; 95% CI, 1.27-1.44). Newborn morbidity was also increased among newborns of mothers with mild anemia (hemoglobin concentrations of 9 to 10.9 g/dL), including birth before 37 weeks’ gestation (aOR, 1.09; 95% CI, 1.05-1.12), birth before 32 weeks’ gestation (aOR, 1.30; 95% CI, 1.21-1.39), admission to the intensive care unit (aOR, 1.21; 95% CI, 1.17-1.25), and respiratory distress syndrome (aOR, 1.35; 95% CI, 1.24-1.46).15 Adverse maternal and newborn outcomes were more prevalent among mothers with moderate (hemoglobin concentrations of 7 to 8.9 g/dL) or severe anemia (hemoglobin concentrations of <7 g/dL), compared with mild anemia. For example, compared with mothers with no anemia, mothers with moderate anemia had an increased risk of birth <37 weeks (aOR, 2.26) and birth <32 weeks (aOR, 3.95).15
In a study of 166,566 US pregnant patients, 6.1% were diagnosed with anemia.18 Patients with anemia were more likely to have antepartum thrombosis, preeclampsia, eclampsia, a cesarean birth, postpartum hemorrhage, a blood transfusion, and postpartum thrombosis.18 In this study, the newborns of mothers with anemia were more likely to have a diagnosis of antenatal or intrapartum fetal distress, a 5-minute Apgar score <7, and an admission to the neonatal intensive care unit.
Continue to: Maternal anemia and neurodevelopmental disorders in children...
Maternal anemia and neurodevelopmental disorders in children
Some experts, but not all, believe that iron deficiency during pregnancy may adversely impact fetal neurodevelopment and result in childhood behavior issues. All experts agree that more research is needed to understand if maternal anemia causes mental health issues in newborns. In one meta-analysis, among 20 studies of the association of maternal iron deficiency and newborn neurodevelopment, approximately half the studies reported that low maternal ferritin levels were associated with lower childhood performance on standardized tests of cognitive, motor, verbal, and memory function.19 Another systematic review concluded that the evidence linking maternal iron deficiency and child neurodevelopment is equivocal.20
In a study of 532,232 nonadoptive children born in Sweden from 1987 to 2010, maternal anemia was associated with an increased risk of autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and intellectual disability (ID).21 In Sweden maternal hemoglobin concentration is measured at 10, 25, and 37 weeks of gestation, permitting comparisons of anemia diagnosed early and late in pregnancy with neurodevelopmental outcomes. The association between anemia and neurodevelopmental disorders was greatest if anemia was diagnosed within the first 30 weeks of pregnancy. Compared with mothers without anemia, maternal anemia diagnosed within the first 30 weeks of pregnancy was associated with higher childhood rates of ASD (4.9% vs 3.5%), ADHD (9.3% vs 7.1%), and ID (3.1% vs 1.3%).21 The differences persisted in analyses that controlled for socioeconomic, maternal, and pregnancy-related factors. In a matched sibling comparison, the diagnosis of maternal anemia within the first 30 weeks of gestation was associated with an increased risk of ASD (OR, 2.25; 95% CI, 1.24-4.11) and ID (OR, 2.59; 95% CI, 1.08-6.22) but not ADHD.21 Other studies have also reported a relationship between maternal anemia and intellectual disability.22,23
Measurement of hemoglobin will identify anemia, but hemoglobin measurement is not sufficiently sensitive to identify most cases of iron deficiency. Measuring ferritin can help to identify cases of iron deficiency before the onset of anemia, permitting early treatment of the nutrient deficiency. In pregnancy, iron deficiency is the prelude to developing anemia. Waiting until anemia occurs to diagnose and treat iron deficiency is suboptimal and may miss a critical window of fetal development that is dependent on maternal iron stores. During pregnancy, ferritin levels decrease as much as 80% between the first and third trimesters, as the fetus utilizes maternal iron stores for its growth.24 We recommend the measurement of ferritin and hemoglobin at the first prenatal visit and again at 24 to 28 weeks’ gestation to optimize early detection and treatment of iron deficiency and reduce the frequency of anemia prior to birth. ●
During pregnancy, anemia and iron deficiency are prevalent because the fetus depletes maternal iron stores. Iron deficiency and iron deficiency anemia are not synonymous. Effective screening for iron deficiency in the first trimester of pregnancy requires the measurement of a sensitive and specific biomarker of iron deficiency, such as ferritin. Limiting the measurement of ferritin to the subset of patients with anemia will result in missing many cases of iron deficiency. By the time iron deficiency causes anemia, a severe deficiency is present. Detecting iron deficiency in pregnancy and promptly treating the deficiency will reduce the number of women with anemia in the third trimester and at birth.
Diagnosis of anemia
Anemia in pregnancy is diagnosed by a hemoglobin level and hematocrit concentration below 11 g/dL and 33%, respectively, in the first and third trimesters and below 10.5 g/dL and 32%, respectively, in the second trimester.1 The prevalence of anemia in the first, second, and third trimesters is approximately 3%, 2%, and 11%, respectively.2 At a hemoglobin concentration <11 g/dL, severe maternal morbidity rises significantly.3 The laboratory evaluation of pregnant women with anemia may require assessment of iron stores, measurement of folate and cobalamin (vitamin B12), and hemoglobin electrophoresis, if indicated.
Diagnosis of iron deficiency
Iron deficiency anemia is diagnosed by a ferritin level below 30 ng/mL.4,5 Normal iron stores and iron insufficiency are indicated by ferritin levels 45 to 150 ng/mL and 30 to 44 ng/mL, respectively.4,5 Ferritin is an acute phase reactant, and patients with inflammation or chronic illnesses may have iron deficiency and a normal ferritin level. For these patients, a transferrin saturation (TSAT) <16% would support a diagnosis of iron deficiency.6 TSAT is calculated from measurement of serum iron and total iron binding capacity. TSAT saturation may be elevated by iron supplements, which increase serum iron. If measurement of TSAT is necessary, interference with the measurement accuracy can be minimized by not taking an iron supplement on the day of testing.
Iron deficiency is present in approximately 50% of pregnant women.7,8 The greatest prevalence of iron deficiency in pregnancy is observed in non-Hispanic Black females, followed by Hispanic females. Non-Hispanic White females had the lowest prevalence of iron deficiency.2
Fetal needs for iron often cause the depletion of maternal iron stores. Many pregnant women who have a normal ferritin level in the first trimester will develop iron deficiency in the third trimester, even with the usual recommended daily oral iron supplementation. We recommend measuring ferritin and hemoglobin at the first prenatal visit and again between 24 and 28 weeks’ gestation.
Impact of maternal anemia on maternal and newborn health
Iron plays a critical role in maternal health and fetal development independent of its role in red blood cell formation. Many proteins critical to maternal health and fetal development contain iron, including hemoglobin, myoglobin, cytochromes, ribonucleotide reductase, peroxidases, lipoxygenases, and cyclooxygenases. In the fetus, iron plays an important role in myelination of nerves, dendrite arborization, and synthesis of monoamine neurotransmitters.9
Many studies report that maternal anemia is associated with severe maternal morbidity and adverse newborn outcomes. The current literature must be interpreted with caution because socioeconomic factors influence iron stores. Iron deficiency and anemia is more common among economically and socially disadvantaged populations.10-12 It is possible that repleting iron stores, alone, without addressing social determinants of health, including food and housing insecurity, may be insufficient to improve maternal and newborn health.
Maternal anemia is a risk factor for severe maternal morbidity and adverse newborn outcomes.3,13-18 In a study of 515,270 live births in British Columbia between 2004 and 2016, maternal anemia was diagnosed in 12.8% of mothers.15 Maternal morbidity at birth was increased among patients with mild anemia (hemoglobin concentration of 9 to 10.9 g/dL), including higher rates of intrapartum transfusion (adjusted odds ratio [OR], 2.45; 95% confidence interval [CI], 1.74-3.45), cesarean birth (aOR, 1.17; 95% CI, 1.14-1.19), and chorioamnionitis (aOR, 1.35; 95% CI, 1.27-1.44). Newborn morbidity was also increased among newborns of mothers with mild anemia (hemoglobin concentrations of 9 to 10.9 g/dL), including birth before 37 weeks’ gestation (aOR, 1.09; 95% CI, 1.05-1.12), birth before 32 weeks’ gestation (aOR, 1.30; 95% CI, 1.21-1.39), admission to the intensive care unit (aOR, 1.21; 95% CI, 1.17-1.25), and respiratory distress syndrome (aOR, 1.35; 95% CI, 1.24-1.46).15 Adverse maternal and newborn outcomes were more prevalent among mothers with moderate (hemoglobin concentrations of 7 to 8.9 g/dL) or severe anemia (hemoglobin concentrations of <7 g/dL), compared with mild anemia. For example, compared with mothers with no anemia, mothers with moderate anemia had an increased risk of birth <37 weeks (aOR, 2.26) and birth <32 weeks (aOR, 3.95).15
In a study of 166,566 US pregnant patients, 6.1% were diagnosed with anemia.18 Patients with anemia were more likely to have antepartum thrombosis, preeclampsia, eclampsia, a cesarean birth, postpartum hemorrhage, a blood transfusion, and postpartum thrombosis.18 In this study, the newborns of mothers with anemia were more likely to have a diagnosis of antenatal or intrapartum fetal distress, a 5-minute Apgar score <7, and an admission to the neonatal intensive care unit.
Continue to: Maternal anemia and neurodevelopmental disorders in children...
Maternal anemia and neurodevelopmental disorders in children
Some experts, but not all, believe that iron deficiency during pregnancy may adversely impact fetal neurodevelopment and result in childhood behavior issues. All experts agree that more research is needed to understand if maternal anemia causes mental health issues in newborns. In one meta-analysis, among 20 studies of the association of maternal iron deficiency and newborn neurodevelopment, approximately half the studies reported that low maternal ferritin levels were associated with lower childhood performance on standardized tests of cognitive, motor, verbal, and memory function.19 Another systematic review concluded that the evidence linking maternal iron deficiency and child neurodevelopment is equivocal.20
In a study of 532,232 nonadoptive children born in Sweden from 1987 to 2010, maternal anemia was associated with an increased risk of autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and intellectual disability (ID).21 In Sweden maternal hemoglobin concentration is measured at 10, 25, and 37 weeks of gestation, permitting comparisons of anemia diagnosed early and late in pregnancy with neurodevelopmental outcomes. The association between anemia and neurodevelopmental disorders was greatest if anemia was diagnosed within the first 30 weeks of pregnancy. Compared with mothers without anemia, maternal anemia diagnosed within the first 30 weeks of pregnancy was associated with higher childhood rates of ASD (4.9% vs 3.5%), ADHD (9.3% vs 7.1%), and ID (3.1% vs 1.3%).21 The differences persisted in analyses that controlled for socioeconomic, maternal, and pregnancy-related factors. In a matched sibling comparison, the diagnosis of maternal anemia within the first 30 weeks of gestation was associated with an increased risk of ASD (OR, 2.25; 95% CI, 1.24-4.11) and ID (OR, 2.59; 95% CI, 1.08-6.22) but not ADHD.21 Other studies have also reported a relationship between maternal anemia and intellectual disability.22,23
Measurement of hemoglobin will identify anemia, but hemoglobin measurement is not sufficiently sensitive to identify most cases of iron deficiency. Measuring ferritin can help to identify cases of iron deficiency before the onset of anemia, permitting early treatment of the nutrient deficiency. In pregnancy, iron deficiency is the prelude to developing anemia. Waiting until anemia occurs to diagnose and treat iron deficiency is suboptimal and may miss a critical window of fetal development that is dependent on maternal iron stores. During pregnancy, ferritin levels decrease as much as 80% between the first and third trimesters, as the fetus utilizes maternal iron stores for its growth.24 We recommend the measurement of ferritin and hemoglobin at the first prenatal visit and again at 24 to 28 weeks’ gestation to optimize early detection and treatment of iron deficiency and reduce the frequency of anemia prior to birth. ●
- American College of Obstetricians and Gynecologists. Anemia in pregnancy. ACOG Practice Bulletin No 233. Obstet Gynecol. 2021;138:e55-64.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
- Ray JG, Davidson AJF, Berger H, et al. Haemoglobin levels in early pregnancy and severe maternal morbidity: population-based cohort study. BJOG. 2020;127:1154-1164.
- Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45-51.
- Parvord S, Daru J, Prasannan N, et al. UK Guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2020;188:819-830.
- Camaschell C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832-1843.
- Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
- Teichman J, Nisenbaum R, Lausman A, et al. Suboptimal iron deficiency screening in pregnancy and the impact of socioeconomic status in high-resource setting. Blood Adv. 2021;5:4666-4673.
- Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43-S48.
- Bodnar LM, Scanlon KS, Freedman DS, et al. High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185:438-443.
- Dondi A, PIccinno V, Morigi F, et al. Food insecurity and major diet-related morbidities in migrating children: a systematic review. Nutrients. 2020;12:379.
- Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298-2302.
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman S, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Smith C, Teng F, Branch E, et al. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134:1234-1244.
- Parks S, Hoffman MK, Goudar SS, et al. Maternal anaemia and maternal, fetal and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126:737-743.
- Guignard J, Deneux-Tharaux C, Seco A, et al. Gestational anemia and severe acute maternal morbidity: a population based study. Anesthesia. 2021;76:61-71.
- Harrison RK, Lauhon SR, Colvin ZA, et al. Maternal anemia and severe maternal mortality in a US cohort. Am J Obstet Gynecol MFM. 2021;3:100395.
- Quesada-Pinedo HG, Cassel F, Duijts L, et al. Maternal iron status in pregnancy and child health outcomes after birth: a systematic review and meta-analysis. Nutrients. 2021;13:2221.
- McCann S, Perapoch Amado M, Moore SE. The role of iron in brain development: a systematic review. Nutrients. 2020;12:2001.
- Wiegersma AM, Dalman C, Lee BK, et al. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. 2019;76:1294-1304.
- Leonard H, de Klerk N, Bourke J, et al. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol. 2006;16:448-454.
- Drassinower D, Lavery JA, Friedman AM, et al. The effect of maternal hematocrit on offspring IQ at 4 and 7 years of age: a secondary analysis. BJOG. 2016;123:2087-2093.
- Horton KD, Adetona O, Aguilar-Villalobos M, et al. Changes in the concentration of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru 2004-2005. Nutrition J. 2013;12:80.
- American College of Obstetricians and Gynecologists. Anemia in pregnancy. ACOG Practice Bulletin No 233. Obstet Gynecol. 2021;138:e55-64.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
- Ray JG, Davidson AJF, Berger H, et al. Haemoglobin levels in early pregnancy and severe maternal morbidity: population-based cohort study. BJOG. 2020;127:1154-1164.
- Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45-51.
- Parvord S, Daru J, Prasannan N, et al. UK Guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2020;188:819-830.
- Camaschell C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832-1843.
- Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
- Teichman J, Nisenbaum R, Lausman A, et al. Suboptimal iron deficiency screening in pregnancy and the impact of socioeconomic status in high-resource setting. Blood Adv. 2021;5:4666-4673.
- Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43-S48.
- Bodnar LM, Scanlon KS, Freedman DS, et al. High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185:438-443.
- Dondi A, PIccinno V, Morigi F, et al. Food insecurity and major diet-related morbidities in migrating children: a systematic review. Nutrients. 2020;12:379.
- Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298-2302.
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman S, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Smith C, Teng F, Branch E, et al. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134:1234-1244.
- Parks S, Hoffman MK, Goudar SS, et al. Maternal anaemia and maternal, fetal and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126:737-743.
- Guignard J, Deneux-Tharaux C, Seco A, et al. Gestational anemia and severe acute maternal morbidity: a population based study. Anesthesia. 2021;76:61-71.
- Harrison RK, Lauhon SR, Colvin ZA, et al. Maternal anemia and severe maternal mortality in a US cohort. Am J Obstet Gynecol MFM. 2021;3:100395.
- Quesada-Pinedo HG, Cassel F, Duijts L, et al. Maternal iron status in pregnancy and child health outcomes after birth: a systematic review and meta-analysis. Nutrients. 2021;13:2221.
- McCann S, Perapoch Amado M, Moore SE. The role of iron in brain development: a systematic review. Nutrients. 2020;12:2001.
- Wiegersma AM, Dalman C, Lee BK, et al. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. 2019;76:1294-1304.
- Leonard H, de Klerk N, Bourke J, et al. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol. 2006;16:448-454.
- Drassinower D, Lavery JA, Friedman AM, et al. The effect of maternal hematocrit on offspring IQ at 4 and 7 years of age: a secondary analysis. BJOG. 2016;123:2087-2093.
- Horton KD, Adetona O, Aguilar-Villalobos M, et al. Changes in the concentration of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru 2004-2005. Nutrition J. 2013;12:80.
Small bowel entrapment during vaginal reconstructive surgery
1931 state law makes abortion a felony if Roe falls, warns Michigan Attorney General
When Stephanie Mejia Arciñiega drove her friend to a Planned Parenthood clinic in Ann Arbor, Mich., they were surrounded by anti-abortion protesters as soon as they tried to park.
“They come up to your car super fast,” Ms. Mejia Arciñiega said. “You don’t want to run their feet over, so we had to stop and be like, ‘OK, no thank you.’ But then they started throwing a bunch of papers and resources at us. We tried to go inside, but we couldn’t.”
The clinic, which offers abortion care as well as birth control, cancer screenings, and STD treatment, has long been the target of anti-abortion protesters. Protesters’ efforts to limit abortions in the state may soon get a huge boost, if the Supreme Court strikes down Roe v. Wade.
In Michigan, this would have an immediate impact. Overnight, nearly all abortions would become a felony carrying a penalty of up to 4 years, even in cases of rape and incest. That’s because an old state law, last updated in 1931, was never repealed, even after Roe made it unenforceable in 1973.
Michigan Attorney General Dana Nessel, a Democrat, said she won’t enforce the law if it springs back into effect. But Michigan has 83 local county prosecutors, and Ms. Nessel said they could enforce the 1931 law. “I don’t think that I have the authority to tell the duly elected county prosecutors what they can and what they cannot charge,” Ms. Nessel told journalists.
Ms. Mejia Arciñiega, 18, who drove her friend to the Ann Arbor clinic, said she never imagined a world where abortion is illegal. “You wouldn’t think that in 2022, we’d be worrying about women’s rights, reproduction rights,” she said. “You wouldn’t want someone young that isn’t ready [to] have to have a baby because the law says ‘No.’ It’s not fair.”
The way the old state law is written, Ms. Nessel said, it’s possible that prosecutors could go after anyone who provides an abortion, as well as those who take medications to end their own pregnancies.
That could “create a scenario where if a woman has self-aborted and she seeks medical care after that, will the doctor then have to report that to law enforcement?”
Speaking to reporters, Ms. Nessel also discussed the abortion she had years ago – one that would be illegal in the state if Roe falls. She was pregnant with triplets and doctors told her the embryos weren’t growing in utero, she said.
“And I was told very, very specifically that there was no way that all three would make it to term. But if I aborted one, that it was possible that the other two might live,” Ms. Nessel said. “I took my doctor’s advice … And you know what? It turned out that he was right. And now I have two beautiful sons.”
The 1931 law allows just one exemption: Abortions “to preserve the life” of the woman. Yet doctors say they have no idea how to interpret that. Consider a woman who has severe heart disease with a 20%-30% chance of dying during pregnancy.
“Is that enough of a chance?” asked Dr. Lisa Harris, a University of Michigan professor and ob.gyn., speaking on Michigan Radio’s Stateside. “I hate to even put it that way, but is that enough of a chance of dying that that person would qualify under Michigan’s ban for a lifesaving abortion? Or would their risk of dying need to be 50% or 100%?”
Or what if a pregnant person has cancer and needs to end the pregnancy to begin chemotherapy? “There’s not an imminent risk of dying, but there might be a risk of dying years later if they didn’t have chemotherapy,” Dr. Harris said. “So these are the kind of situations doctors are wondering about.”
It’s also unclear whether a woman whose pregnancy would become life-threatening only in its later stages would be required to delay termination until then.
“We see people with things like kidney disease or other problems, where they’re actually OK during early pregnancy. But if the pregnancy were to continue and they were to give birth, then they would have a very high chance of dying,” Dr. Harris explained.
The state legislature is controlled by Republicans, but Michigan Gov. Gretchen Whitmer, a Democrat, filed a preemptive lawsuit seeking to block the 1931 law from taking effect. Planned Parenthood filed a similar suit as well. And a campaign to collect enough signatures is underway to put abortion on the ballot in November. But that would be months after the U.S. Supreme Court makes its final ruling on Roe, which is expected in late June or early July.
In the meantime, the confusion and uncertainty caused by the 1931 law could be enough for some health care professionals to stop offering abortions, Ms. Nessel said.
“I think that this will have the kind of chilling effect that doctors just simply will not perform this procedure really under any set of circumstances, because they don’t want to get dragged into court,” she said. “They don’t want to face the possibility of being prosecuted and the possibility of going to jail or prison. So I think that, honestly, you’ll have doctors that really have to violate their Hippocratic oath and just say, ‘I’m sorry, I can’t help you.’ ”
This story is part of a partnership that includes Michigan Radio, NPR and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. Kate Wells is a reporter with Michigan Radio.
When Stephanie Mejia Arciñiega drove her friend to a Planned Parenthood clinic in Ann Arbor, Mich., they were surrounded by anti-abortion protesters as soon as they tried to park.
“They come up to your car super fast,” Ms. Mejia Arciñiega said. “You don’t want to run their feet over, so we had to stop and be like, ‘OK, no thank you.’ But then they started throwing a bunch of papers and resources at us. We tried to go inside, but we couldn’t.”
The clinic, which offers abortion care as well as birth control, cancer screenings, and STD treatment, has long been the target of anti-abortion protesters. Protesters’ efforts to limit abortions in the state may soon get a huge boost, if the Supreme Court strikes down Roe v. Wade.
In Michigan, this would have an immediate impact. Overnight, nearly all abortions would become a felony carrying a penalty of up to 4 years, even in cases of rape and incest. That’s because an old state law, last updated in 1931, was never repealed, even after Roe made it unenforceable in 1973.
Michigan Attorney General Dana Nessel, a Democrat, said she won’t enforce the law if it springs back into effect. But Michigan has 83 local county prosecutors, and Ms. Nessel said they could enforce the 1931 law. “I don’t think that I have the authority to tell the duly elected county prosecutors what they can and what they cannot charge,” Ms. Nessel told journalists.
Ms. Mejia Arciñiega, 18, who drove her friend to the Ann Arbor clinic, said she never imagined a world where abortion is illegal. “You wouldn’t think that in 2022, we’d be worrying about women’s rights, reproduction rights,” she said. “You wouldn’t want someone young that isn’t ready [to] have to have a baby because the law says ‘No.’ It’s not fair.”
The way the old state law is written, Ms. Nessel said, it’s possible that prosecutors could go after anyone who provides an abortion, as well as those who take medications to end their own pregnancies.
That could “create a scenario where if a woman has self-aborted and she seeks medical care after that, will the doctor then have to report that to law enforcement?”
Speaking to reporters, Ms. Nessel also discussed the abortion she had years ago – one that would be illegal in the state if Roe falls. She was pregnant with triplets and doctors told her the embryos weren’t growing in utero, she said.
“And I was told very, very specifically that there was no way that all three would make it to term. But if I aborted one, that it was possible that the other two might live,” Ms. Nessel said. “I took my doctor’s advice … And you know what? It turned out that he was right. And now I have two beautiful sons.”
The 1931 law allows just one exemption: Abortions “to preserve the life” of the woman. Yet doctors say they have no idea how to interpret that. Consider a woman who has severe heart disease with a 20%-30% chance of dying during pregnancy.
“Is that enough of a chance?” asked Dr. Lisa Harris, a University of Michigan professor and ob.gyn., speaking on Michigan Radio’s Stateside. “I hate to even put it that way, but is that enough of a chance of dying that that person would qualify under Michigan’s ban for a lifesaving abortion? Or would their risk of dying need to be 50% or 100%?”
Or what if a pregnant person has cancer and needs to end the pregnancy to begin chemotherapy? “There’s not an imminent risk of dying, but there might be a risk of dying years later if they didn’t have chemotherapy,” Dr. Harris said. “So these are the kind of situations doctors are wondering about.”
It’s also unclear whether a woman whose pregnancy would become life-threatening only in its later stages would be required to delay termination until then.
“We see people with things like kidney disease or other problems, where they’re actually OK during early pregnancy. But if the pregnancy were to continue and they were to give birth, then they would have a very high chance of dying,” Dr. Harris explained.
The state legislature is controlled by Republicans, but Michigan Gov. Gretchen Whitmer, a Democrat, filed a preemptive lawsuit seeking to block the 1931 law from taking effect. Planned Parenthood filed a similar suit as well. And a campaign to collect enough signatures is underway to put abortion on the ballot in November. But that would be months after the U.S. Supreme Court makes its final ruling on Roe, which is expected in late June or early July.
In the meantime, the confusion and uncertainty caused by the 1931 law could be enough for some health care professionals to stop offering abortions, Ms. Nessel said.
“I think that this will have the kind of chilling effect that doctors just simply will not perform this procedure really under any set of circumstances, because they don’t want to get dragged into court,” she said. “They don’t want to face the possibility of being prosecuted and the possibility of going to jail or prison. So I think that, honestly, you’ll have doctors that really have to violate their Hippocratic oath and just say, ‘I’m sorry, I can’t help you.’ ”
This story is part of a partnership that includes Michigan Radio, NPR and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. Kate Wells is a reporter with Michigan Radio.
When Stephanie Mejia Arciñiega drove her friend to a Planned Parenthood clinic in Ann Arbor, Mich., they were surrounded by anti-abortion protesters as soon as they tried to park.
“They come up to your car super fast,” Ms. Mejia Arciñiega said. “You don’t want to run their feet over, so we had to stop and be like, ‘OK, no thank you.’ But then they started throwing a bunch of papers and resources at us. We tried to go inside, but we couldn’t.”
The clinic, which offers abortion care as well as birth control, cancer screenings, and STD treatment, has long been the target of anti-abortion protesters. Protesters’ efforts to limit abortions in the state may soon get a huge boost, if the Supreme Court strikes down Roe v. Wade.
In Michigan, this would have an immediate impact. Overnight, nearly all abortions would become a felony carrying a penalty of up to 4 years, even in cases of rape and incest. That’s because an old state law, last updated in 1931, was never repealed, even after Roe made it unenforceable in 1973.
Michigan Attorney General Dana Nessel, a Democrat, said she won’t enforce the law if it springs back into effect. But Michigan has 83 local county prosecutors, and Ms. Nessel said they could enforce the 1931 law. “I don’t think that I have the authority to tell the duly elected county prosecutors what they can and what they cannot charge,” Ms. Nessel told journalists.
Ms. Mejia Arciñiega, 18, who drove her friend to the Ann Arbor clinic, said she never imagined a world where abortion is illegal. “You wouldn’t think that in 2022, we’d be worrying about women’s rights, reproduction rights,” she said. “You wouldn’t want someone young that isn’t ready [to] have to have a baby because the law says ‘No.’ It’s not fair.”
The way the old state law is written, Ms. Nessel said, it’s possible that prosecutors could go after anyone who provides an abortion, as well as those who take medications to end their own pregnancies.
That could “create a scenario where if a woman has self-aborted and she seeks medical care after that, will the doctor then have to report that to law enforcement?”
Speaking to reporters, Ms. Nessel also discussed the abortion she had years ago – one that would be illegal in the state if Roe falls. She was pregnant with triplets and doctors told her the embryos weren’t growing in utero, she said.
“And I was told very, very specifically that there was no way that all three would make it to term. But if I aborted one, that it was possible that the other two might live,” Ms. Nessel said. “I took my doctor’s advice … And you know what? It turned out that he was right. And now I have two beautiful sons.”
The 1931 law allows just one exemption: Abortions “to preserve the life” of the woman. Yet doctors say they have no idea how to interpret that. Consider a woman who has severe heart disease with a 20%-30% chance of dying during pregnancy.
“Is that enough of a chance?” asked Dr. Lisa Harris, a University of Michigan professor and ob.gyn., speaking on Michigan Radio’s Stateside. “I hate to even put it that way, but is that enough of a chance of dying that that person would qualify under Michigan’s ban for a lifesaving abortion? Or would their risk of dying need to be 50% or 100%?”
Or what if a pregnant person has cancer and needs to end the pregnancy to begin chemotherapy? “There’s not an imminent risk of dying, but there might be a risk of dying years later if they didn’t have chemotherapy,” Dr. Harris said. “So these are the kind of situations doctors are wondering about.”
It’s also unclear whether a woman whose pregnancy would become life-threatening only in its later stages would be required to delay termination until then.
“We see people with things like kidney disease or other problems, where they’re actually OK during early pregnancy. But if the pregnancy were to continue and they were to give birth, then they would have a very high chance of dying,” Dr. Harris explained.
The state legislature is controlled by Republicans, but Michigan Gov. Gretchen Whitmer, a Democrat, filed a preemptive lawsuit seeking to block the 1931 law from taking effect. Planned Parenthood filed a similar suit as well. And a campaign to collect enough signatures is underway to put abortion on the ballot in November. But that would be months after the U.S. Supreme Court makes its final ruling on Roe, which is expected in late June or early July.
In the meantime, the confusion and uncertainty caused by the 1931 law could be enough for some health care professionals to stop offering abortions, Ms. Nessel said.
“I think that this will have the kind of chilling effect that doctors just simply will not perform this procedure really under any set of circumstances, because they don’t want to get dragged into court,” she said. “They don’t want to face the possibility of being prosecuted and the possibility of going to jail or prison. So I think that, honestly, you’ll have doctors that really have to violate their Hippocratic oath and just say, ‘I’m sorry, I can’t help you.’ ”
This story is part of a partnership that includes Michigan Radio, NPR and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. Kate Wells is a reporter with Michigan Radio.
‘Goodie bag’ pill mill doctor sentenced to 2 decades in prison
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
ED staff speak out about workplace violence, ask for mitigation
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
Colorado law would lift veil of secrecy on sperm donations
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
TikTok challenge hits Taco Bell right in its ‘Stuft Nacho’
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Headache in pregnancy: New ACOG guidelines offer insight
SAN DIEGO – If a medical professional is trying to figure out the best medical treatment for a pregnant woman with headache, it may be helpful to review data from randomized clinical trials (RCTs). Well, make that data from the RCT. There’s just been one, Northwestern Medicine obstetrician-gynecologist Catherine Stika, MD, told colleagues at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Only a single efficacy RCT has examined headache in pregnancy, said Dr. Stika. “Overall, we have very limited data in pregnancy to tell us exactly what to do,” she added.
But ob.gyns. aren’t entirely in the dark, according to medical specialists who spoke at the session. Expert opinion and fetal safety data offer insight into the best treatments, as does a new ACOG clinical practice guideline on headaches during pregnancy and post partum that was coauthored by the speakers.
And there’s some good news: Pregnancy itself is often a good treatment for headaches.
Pregnant women often find relief from one kind of headache – migraine – as their estradiol levels rise, said Laura Mercer, MD, an ob.gyn. at the University of Arizona, Phoenix. “About half of patients will report that migraines are getting better as early as the first trimester, and upwards of 83% will say that their migraines are better by the time they’re in their third trimester,” she said. “What this means for us as obstetricians is that oftentimes we can actually discontinue preventative therapies for patients during pregnancy.”
But simply discontinuing every headache treatment during pregnancy may not be the right approach, Dr. Mercer said. Instead, she said, consider the benefits and risks.
Divalproex sodium (Depakote) and topiramate (Topamax) must be avoided because of fetal risk, she said. “In fact, we will prefer that people stop these medications before they discontinue their contraception if they’re planning on getting pregnant,” she said.
Other medications, such as ACE inhibitors and the herbal remedy feverfew, should not be used at any time during pregnancy, she said.
On the other hand, calcium channel blockers and antihistamines are alright to use in pregnancy, she said. “These two should be considered first-line because there’s no known risks for them.”
Beta-blockers also may be used “with some consideration to the known risks that we’re familiar with when we use them for other indications,” she said.
There are questions about the safety of oral magnesium in pregnancy, although it’s generally considered safe, she added, and “nerve blocks and nerve stimulators seem very promising and have little known risks.”
Dr. Mercer recommended gradually tapering most medications prior to conception. But it’s crucial to stop higher-risk drugs immediately once pregnancy is confirmed, she said.
In regard to acute headache, Dr. Stika urged caution if a patient reports taking a headache medication more than twice a week. “All the medications we use for the treatment of migraine, both in and outside of pregnancy, carry the risk of what’s called medication overuse” that can lead to rebound headaches, she said.
Excedrin Tension Headache may be used for headaches in pregnancy, she said, but not Excedrin Migraine since it includes aspirin. Triptans are not recommended as first-line therapy, she added, and they “should absolutely not be used in any pregnant patient with a history of known cardiac disease or hypertension.”
Dr. Stika added that ACOG advises against the use of drugs that contain butalbital, a barbiturate that’s combined with other agents to treat headache. “Butalbital is the drug that’s most closely associated with getting people into this medication overuse headache,” she said. “It’s even worse than opioids.”
Unlike multiple other countries and the entire European Union, the United States has not banned compounds that contain butalbital, she said.
In some cases, she said, patients may present to triage with vomiting, an inability to keep food down, and persistent headache despite treatment. “This is a really classic presentation.”
The ACOG clinical practice guideline offers a flow chart about what to do, she said. Hydration is key, she said, and various treatment options can help. A referral to neurology may be needed in extreme cases, she said. But “most of the time, you’re able to get rid of her headache.”
Dr. Mercer and Dr. Stika report no disclosures.
SAN DIEGO – If a medical professional is trying to figure out the best medical treatment for a pregnant woman with headache, it may be helpful to review data from randomized clinical trials (RCTs). Well, make that data from the RCT. There’s just been one, Northwestern Medicine obstetrician-gynecologist Catherine Stika, MD, told colleagues at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Only a single efficacy RCT has examined headache in pregnancy, said Dr. Stika. “Overall, we have very limited data in pregnancy to tell us exactly what to do,” she added.
But ob.gyns. aren’t entirely in the dark, according to medical specialists who spoke at the session. Expert opinion and fetal safety data offer insight into the best treatments, as does a new ACOG clinical practice guideline on headaches during pregnancy and post partum that was coauthored by the speakers.
And there’s some good news: Pregnancy itself is often a good treatment for headaches.
Pregnant women often find relief from one kind of headache – migraine – as their estradiol levels rise, said Laura Mercer, MD, an ob.gyn. at the University of Arizona, Phoenix. “About half of patients will report that migraines are getting better as early as the first trimester, and upwards of 83% will say that their migraines are better by the time they’re in their third trimester,” she said. “What this means for us as obstetricians is that oftentimes we can actually discontinue preventative therapies for patients during pregnancy.”
But simply discontinuing every headache treatment during pregnancy may not be the right approach, Dr. Mercer said. Instead, she said, consider the benefits and risks.
Divalproex sodium (Depakote) and topiramate (Topamax) must be avoided because of fetal risk, she said. “In fact, we will prefer that people stop these medications before they discontinue their contraception if they’re planning on getting pregnant,” she said.
Other medications, such as ACE inhibitors and the herbal remedy feverfew, should not be used at any time during pregnancy, she said.
On the other hand, calcium channel blockers and antihistamines are alright to use in pregnancy, she said. “These two should be considered first-line because there’s no known risks for them.”
Beta-blockers also may be used “with some consideration to the known risks that we’re familiar with when we use them for other indications,” she said.
There are questions about the safety of oral magnesium in pregnancy, although it’s generally considered safe, she added, and “nerve blocks and nerve stimulators seem very promising and have little known risks.”
Dr. Mercer recommended gradually tapering most medications prior to conception. But it’s crucial to stop higher-risk drugs immediately once pregnancy is confirmed, she said.
In regard to acute headache, Dr. Stika urged caution if a patient reports taking a headache medication more than twice a week. “All the medications we use for the treatment of migraine, both in and outside of pregnancy, carry the risk of what’s called medication overuse” that can lead to rebound headaches, she said.
Excedrin Tension Headache may be used for headaches in pregnancy, she said, but not Excedrin Migraine since it includes aspirin. Triptans are not recommended as first-line therapy, she added, and they “should absolutely not be used in any pregnant patient with a history of known cardiac disease or hypertension.”
Dr. Stika added that ACOG advises against the use of drugs that contain butalbital, a barbiturate that’s combined with other agents to treat headache. “Butalbital is the drug that’s most closely associated with getting people into this medication overuse headache,” she said. “It’s even worse than opioids.”
Unlike multiple other countries and the entire European Union, the United States has not banned compounds that contain butalbital, she said.
In some cases, she said, patients may present to triage with vomiting, an inability to keep food down, and persistent headache despite treatment. “This is a really classic presentation.”
The ACOG clinical practice guideline offers a flow chart about what to do, she said. Hydration is key, she said, and various treatment options can help. A referral to neurology may be needed in extreme cases, she said. But “most of the time, you’re able to get rid of her headache.”
Dr. Mercer and Dr. Stika report no disclosures.
SAN DIEGO – If a medical professional is trying to figure out the best medical treatment for a pregnant woman with headache, it may be helpful to review data from randomized clinical trials (RCTs). Well, make that data from the RCT. There’s just been one, Northwestern Medicine obstetrician-gynecologist Catherine Stika, MD, told colleagues at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Only a single efficacy RCT has examined headache in pregnancy, said Dr. Stika. “Overall, we have very limited data in pregnancy to tell us exactly what to do,” she added.
But ob.gyns. aren’t entirely in the dark, according to medical specialists who spoke at the session. Expert opinion and fetal safety data offer insight into the best treatments, as does a new ACOG clinical practice guideline on headaches during pregnancy and post partum that was coauthored by the speakers.
And there’s some good news: Pregnancy itself is often a good treatment for headaches.
Pregnant women often find relief from one kind of headache – migraine – as their estradiol levels rise, said Laura Mercer, MD, an ob.gyn. at the University of Arizona, Phoenix. “About half of patients will report that migraines are getting better as early as the first trimester, and upwards of 83% will say that their migraines are better by the time they’re in their third trimester,” she said. “What this means for us as obstetricians is that oftentimes we can actually discontinue preventative therapies for patients during pregnancy.”
But simply discontinuing every headache treatment during pregnancy may not be the right approach, Dr. Mercer said. Instead, she said, consider the benefits and risks.
Divalproex sodium (Depakote) and topiramate (Topamax) must be avoided because of fetal risk, she said. “In fact, we will prefer that people stop these medications before they discontinue their contraception if they’re planning on getting pregnant,” she said.
Other medications, such as ACE inhibitors and the herbal remedy feverfew, should not be used at any time during pregnancy, she said.
On the other hand, calcium channel blockers and antihistamines are alright to use in pregnancy, she said. “These two should be considered first-line because there’s no known risks for them.”
Beta-blockers also may be used “with some consideration to the known risks that we’re familiar with when we use them for other indications,” she said.
There are questions about the safety of oral magnesium in pregnancy, although it’s generally considered safe, she added, and “nerve blocks and nerve stimulators seem very promising and have little known risks.”
Dr. Mercer recommended gradually tapering most medications prior to conception. But it’s crucial to stop higher-risk drugs immediately once pregnancy is confirmed, she said.
In regard to acute headache, Dr. Stika urged caution if a patient reports taking a headache medication more than twice a week. “All the medications we use for the treatment of migraine, both in and outside of pregnancy, carry the risk of what’s called medication overuse” that can lead to rebound headaches, she said.
Excedrin Tension Headache may be used for headaches in pregnancy, she said, but not Excedrin Migraine since it includes aspirin. Triptans are not recommended as first-line therapy, she added, and they “should absolutely not be used in any pregnant patient with a history of known cardiac disease or hypertension.”
Dr. Stika added that ACOG advises against the use of drugs that contain butalbital, a barbiturate that’s combined with other agents to treat headache. “Butalbital is the drug that’s most closely associated with getting people into this medication overuse headache,” she said. “It’s even worse than opioids.”
Unlike multiple other countries and the entire European Union, the United States has not banned compounds that contain butalbital, she said.
In some cases, she said, patients may present to triage with vomiting, an inability to keep food down, and persistent headache despite treatment. “This is a really classic presentation.”
The ACOG clinical practice guideline offers a flow chart about what to do, she said. Hydration is key, she said, and various treatment options can help. A referral to neurology may be needed in extreme cases, she said. But “most of the time, you’re able to get rid of her headache.”
Dr. Mercer and Dr. Stika report no disclosures.
AT ACOG 2022