User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Germline genetic testing: Why it matters and where we are failing
Historically, the role of genetic testing has been to identify familial cancer syndromes and initiate cascade testing. If a germline pathogenic variant is found in an individual, cascade testing involves genetic counseling and testing of blood relatives, starting with those closest in relation to the proband, to identify other family members at high hereditary cancer risk. Once testing identifies those family members at higher cancer risk, these individuals can be referred for risk-reducing procedures. They can undergo screening tests starting at an earlier age and/or increased frequency to help prevent invasive cancer or diagnose it at an earlier stage.
Genetic testing can also inform prognosis. While women with a BRCA1 or BRCA2 mutation are at higher risk of developing ovarian cancer compared with the baseline population, the presence of a germline BRCA mutation has been shown to confer improved survival compared with no BRCA mutation (BRCA wild type). However, more recent data have shown that when long-term survival was analyzed, the prognostic benefit seen in patients with a germline BRCA mutation was lost. The initial survival advantage seen in this population may be related to increased sensitivity to treatment. There appears to be improved response to platinum therapy, which is the standard of care for upfront treatment, in germline BRCA mutation carriers.
Most recently, genetic testing has been used to guide treatment decisions in gynecologic cancers. In 2014, the first poly ADP-ribose polymerase (PARP) inhibitor, olaparib, received Food and Drug Administration approval for the treatment of recurrent ovarian cancer in the presence of a germline BRCA mutation. Now there are multiple PARP inhibitors that have FDA approval for ovarian cancer treatment, some as frontline treatment.
Previous data indicate that 13%-18% of women with ovarian cancer have a germline BRCA mutation that places them at increased risk of hereditary ovarian cancer.1 Current guidelines from the American Society of Clinical Oncology, the U.S. Preventive Services Task Force, the National Comprehensive Cancer Network, the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists recommend universal genetic counseling and testing for patients diagnosed with epithelial ovarian cancer. Despite these guidelines, rates of referral for genetic counseling and completion of genetic testing are low.
There has been improvement for both referrals and testing since the publication of the 2014 SGO clinical practice statement on genetic testing for ovarian cancer patients, which recommended that all women, even those without any significant family history, should receive genetic counseling and be offered genetic testing.2 When including only studies that collected data after the publication of the 2014 SGO clinical practice statement on genetic testing, a recent systematic review found that 64% of patients were referred for genetic counseling and 63% underwent testing.3
Clinical interventions to target genetic evaluation appear to improve uptake of both counseling and testing. These interventions include using telemedicine to deliver genetic counseling services, mainstreaming (counseling and testing are provided in an oncology clinic by nongenetics specialists), having a genetic counselor within the clinic, and performing reflex testing. With limited numbers of genetic counselors (and even further limited numbers of cancer-specific genetic counselors),4 referral for genetic counseling before testing is often challenging and may not be feasible. There is continued need for strategies to help overcome the barrier to accessing genetic counseling.
While the data are limited, there appear to be significant disparities in rates of genetic testing. Genetic counseling and testing were completed by White (43% and 40%) patients more frequently than by either Black (24% and 26%) or Asian (23% and 14%) patients.4 Uninsured patients were about half as likely (23% vs. 47%) to complete genetic testing as were those with private insurance.4
Genetic testing is an important tool to help identify individuals and families at risk of having hereditary cancer syndromes. This identification allows us to prevent many cancers and identify others while still early stage, significantly decreasing the health care and financial burden on our society and improving outcomes for patients. While we have seen improvement in rates of referral for genetic counseling and testing, we are still falling short. Given the shortage of genetic counselors, it is imperative that we find solutions to ensure continued and improved access to genetic testing for our patients.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Norquist BM et al. JAMA Oncol. 2016;2(4):482-90.
2. SGO Clinical Practice Statement. 2014 Oct 1.
3. Lin J et al. Gynecol Oncol. 2021;162(2):506-16.
4. American Society of Clinical Oncology. J Oncol Pract. 2016 Apr;12(4):339-83.
Historically, the role of genetic testing has been to identify familial cancer syndromes and initiate cascade testing. If a germline pathogenic variant is found in an individual, cascade testing involves genetic counseling and testing of blood relatives, starting with those closest in relation to the proband, to identify other family members at high hereditary cancer risk. Once testing identifies those family members at higher cancer risk, these individuals can be referred for risk-reducing procedures. They can undergo screening tests starting at an earlier age and/or increased frequency to help prevent invasive cancer or diagnose it at an earlier stage.
Genetic testing can also inform prognosis. While women with a BRCA1 or BRCA2 mutation are at higher risk of developing ovarian cancer compared with the baseline population, the presence of a germline BRCA mutation has been shown to confer improved survival compared with no BRCA mutation (BRCA wild type). However, more recent data have shown that when long-term survival was analyzed, the prognostic benefit seen in patients with a germline BRCA mutation was lost. The initial survival advantage seen in this population may be related to increased sensitivity to treatment. There appears to be improved response to platinum therapy, which is the standard of care for upfront treatment, in germline BRCA mutation carriers.
Most recently, genetic testing has been used to guide treatment decisions in gynecologic cancers. In 2014, the first poly ADP-ribose polymerase (PARP) inhibitor, olaparib, received Food and Drug Administration approval for the treatment of recurrent ovarian cancer in the presence of a germline BRCA mutation. Now there are multiple PARP inhibitors that have FDA approval for ovarian cancer treatment, some as frontline treatment.
Previous data indicate that 13%-18% of women with ovarian cancer have a germline BRCA mutation that places them at increased risk of hereditary ovarian cancer.1 Current guidelines from the American Society of Clinical Oncology, the U.S. Preventive Services Task Force, the National Comprehensive Cancer Network, the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists recommend universal genetic counseling and testing for patients diagnosed with epithelial ovarian cancer. Despite these guidelines, rates of referral for genetic counseling and completion of genetic testing are low.
There has been improvement for both referrals and testing since the publication of the 2014 SGO clinical practice statement on genetic testing for ovarian cancer patients, which recommended that all women, even those without any significant family history, should receive genetic counseling and be offered genetic testing.2 When including only studies that collected data after the publication of the 2014 SGO clinical practice statement on genetic testing, a recent systematic review found that 64% of patients were referred for genetic counseling and 63% underwent testing.3
Clinical interventions to target genetic evaluation appear to improve uptake of both counseling and testing. These interventions include using telemedicine to deliver genetic counseling services, mainstreaming (counseling and testing are provided in an oncology clinic by nongenetics specialists), having a genetic counselor within the clinic, and performing reflex testing. With limited numbers of genetic counselors (and even further limited numbers of cancer-specific genetic counselors),4 referral for genetic counseling before testing is often challenging and may not be feasible. There is continued need for strategies to help overcome the barrier to accessing genetic counseling.
While the data are limited, there appear to be significant disparities in rates of genetic testing. Genetic counseling and testing were completed by White (43% and 40%) patients more frequently than by either Black (24% and 26%) or Asian (23% and 14%) patients.4 Uninsured patients were about half as likely (23% vs. 47%) to complete genetic testing as were those with private insurance.4
Genetic testing is an important tool to help identify individuals and families at risk of having hereditary cancer syndromes. This identification allows us to prevent many cancers and identify others while still early stage, significantly decreasing the health care and financial burden on our society and improving outcomes for patients. While we have seen improvement in rates of referral for genetic counseling and testing, we are still falling short. Given the shortage of genetic counselors, it is imperative that we find solutions to ensure continued and improved access to genetic testing for our patients.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Norquist BM et al. JAMA Oncol. 2016;2(4):482-90.
2. SGO Clinical Practice Statement. 2014 Oct 1.
3. Lin J et al. Gynecol Oncol. 2021;162(2):506-16.
4. American Society of Clinical Oncology. J Oncol Pract. 2016 Apr;12(4):339-83.
Historically, the role of genetic testing has been to identify familial cancer syndromes and initiate cascade testing. If a germline pathogenic variant is found in an individual, cascade testing involves genetic counseling and testing of blood relatives, starting with those closest in relation to the proband, to identify other family members at high hereditary cancer risk. Once testing identifies those family members at higher cancer risk, these individuals can be referred for risk-reducing procedures. They can undergo screening tests starting at an earlier age and/or increased frequency to help prevent invasive cancer or diagnose it at an earlier stage.
Genetic testing can also inform prognosis. While women with a BRCA1 or BRCA2 mutation are at higher risk of developing ovarian cancer compared with the baseline population, the presence of a germline BRCA mutation has been shown to confer improved survival compared with no BRCA mutation (BRCA wild type). However, more recent data have shown that when long-term survival was analyzed, the prognostic benefit seen in patients with a germline BRCA mutation was lost. The initial survival advantage seen in this population may be related to increased sensitivity to treatment. There appears to be improved response to platinum therapy, which is the standard of care for upfront treatment, in germline BRCA mutation carriers.
Most recently, genetic testing has been used to guide treatment decisions in gynecologic cancers. In 2014, the first poly ADP-ribose polymerase (PARP) inhibitor, olaparib, received Food and Drug Administration approval for the treatment of recurrent ovarian cancer in the presence of a germline BRCA mutation. Now there are multiple PARP inhibitors that have FDA approval for ovarian cancer treatment, some as frontline treatment.
Previous data indicate that 13%-18% of women with ovarian cancer have a germline BRCA mutation that places them at increased risk of hereditary ovarian cancer.1 Current guidelines from the American Society of Clinical Oncology, the U.S. Preventive Services Task Force, the National Comprehensive Cancer Network, the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists recommend universal genetic counseling and testing for patients diagnosed with epithelial ovarian cancer. Despite these guidelines, rates of referral for genetic counseling and completion of genetic testing are low.
There has been improvement for both referrals and testing since the publication of the 2014 SGO clinical practice statement on genetic testing for ovarian cancer patients, which recommended that all women, even those without any significant family history, should receive genetic counseling and be offered genetic testing.2 When including only studies that collected data after the publication of the 2014 SGO clinical practice statement on genetic testing, a recent systematic review found that 64% of patients were referred for genetic counseling and 63% underwent testing.3
Clinical interventions to target genetic evaluation appear to improve uptake of both counseling and testing. These interventions include using telemedicine to deliver genetic counseling services, mainstreaming (counseling and testing are provided in an oncology clinic by nongenetics specialists), having a genetic counselor within the clinic, and performing reflex testing. With limited numbers of genetic counselors (and even further limited numbers of cancer-specific genetic counselors),4 referral for genetic counseling before testing is often challenging and may not be feasible. There is continued need for strategies to help overcome the barrier to accessing genetic counseling.
While the data are limited, there appear to be significant disparities in rates of genetic testing. Genetic counseling and testing were completed by White (43% and 40%) patients more frequently than by either Black (24% and 26%) or Asian (23% and 14%) patients.4 Uninsured patients were about half as likely (23% vs. 47%) to complete genetic testing as were those with private insurance.4
Genetic testing is an important tool to help identify individuals and families at risk of having hereditary cancer syndromes. This identification allows us to prevent many cancers and identify others while still early stage, significantly decreasing the health care and financial burden on our society and improving outcomes for patients. While we have seen improvement in rates of referral for genetic counseling and testing, we are still falling short. Given the shortage of genetic counselors, it is imperative that we find solutions to ensure continued and improved access to genetic testing for our patients.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Norquist BM et al. JAMA Oncol. 2016;2(4):482-90.
2. SGO Clinical Practice Statement. 2014 Oct 1.
3. Lin J et al. Gynecol Oncol. 2021;162(2):506-16.
4. American Society of Clinical Oncology. J Oncol Pract. 2016 Apr;12(4):339-83.
Pregnancy termination counseling for lung disease requires new caution
NASHVILLE, TENN. – In a growing number of states, , according to a panel of experts assembled for a special session at the annual meeting of the American College of Chest Physicians.
Following the June 24 decision by the U.S. Supreme Court to overturn Roe v. Wade, several states were swift to enact tight restrictions on abortion. These restrictions include bans on elective abortions for almost any reason. Worded in various ways, the new laws typically include exceptions when the health of the mother is threatened, but these exceptions must be navigated carefully.
As a general rule, “there is no clear and specific definition of when the mother’s life is at risk. These laws are vague on purpose,” said Rebecca Cohen, MD, division chief, Complex Family Planning, University of Colorado at Denver, Aurora.
The remarks were relevant to any clinician who advises women regarding pregnancy termination, but Dr. Cohen’s advice was tailored to pulmonologists. Advances have reduced the proportion of women with severe lung diseases, such as pulmonary arterial hypertension or interstitial lung disease, that make pregnancy untenable, but serious risks persist.
Clinicians need to assume a defensive posture, and the first step is to understand the laws, according to Dr. Cohen. For this, she recommended the nongovernmental Guttmacher Institute as a resource. With a focus on sexual and reproductive health, this research institute maintains a state-by-state summary of laws that govern pregnancy termination. The laws are being reconsidered across the country, and Dr. Cohen said the website updates its summaries accordingly.
In states with the most rigorous restrictions, the risks to physicians are substantial. Pulmonologists need to recognize that they might face legal consequences from merely advising a patient to terminate her pregnancy if the medical need is ambiguous or unclear, according to Dr. Cohen.
“If the advice is interpreted as aiding and abetting an elective abortion, it is a felony offense in some states,” Dr. Cohen said.
In states with restrictive laws, pregnancy prevention is the safest approach for women of childbearing age who face life-threatening complications in the event of pregnancy, according to Dr. Cohen. This might reasonably include a step beyond standard contraception. Dr. Cohen mentioned such approaches as period tracking to double down.
In addition, for women of childbearing age with health problems that might result in complications in the event of a pregnancy, it is appropriate to establish this fact in the medical record. This history could prove useful for maximizing options when making decisions in the best interest of the mother’s health in the event of contraception failure.
In addition, pulmonologists who counsel women about the potential for pregnancy termination should consider establishing a relationship with the legal department at the institution where they work, according to Dr. Cohen. In specific cases in which termination is recommended, she further advised building documentation with participation from additional medical specialists, such as an obstetrician who manages high-risk pregnancies.
“There is no guarantee that any given documentation is adequate,” Dr. Cohen warned. She indicated that consensus from multiple clinicians can strengthen the legal defense if one is necessary.
For some serious lung conditions that are incompatible with pregnancy, the threat to the mother’s life can occur early, according to Deborah Jo Levine, MD, a clinical instructor in the division of pulmonary, allergy, and critical care medicine, Stanford (Calif.) University.
As a result, “you need to identify at-risk patients early and develop a plan promptly,” said Dr. Levine, who joined Dr. Cohen on the special panel at the CHEST 2022 meeting. Even when termination is medically appropriate, restrictive laws are making these services harder to find.
In the case of a pregnancy likely to pose a high risk of complications owing to the patient’s having lung disease, “it is important to involve a high-risk ob quickly,” Dr. Levine warned. “In some cases, termination poses less risk if performed early.”
Sunjay R. Devarajan, MD, assistant professor of pulmonary medicine and critical care, Baylor College of Medicine, Houston, has faced this issue in a state that has some of the most restrictive laws. Even when there is no debate about the necessity of a medically indicated abortion, he cautioned that abortion services are becoming harder to find.
“A recent patient who had a complicated unintentional pregnancy on our service had to go out of state for pregnancy termination,” Dr. Devarajan said. He noted that this option is not available to all women, particularly in states such as his own in which most bordering states also now have highly restrictive abortion laws.
On the basis of this experience, he is thinking more defensively. Now that clinicians can be drawn into legal proceedings even when pregnancy termination is indicated, he agreed that clinicians must become familiar with the local laws.
“We are doing better in managing pregnancies in women with serious lung diseases, but termination is still the prudent approach in some cases,” Dr. Devarajan said. He indicated that he considered the advice offered by Dr. Cohen helpful in avoiding complications for the patient and the physician.
Dr. Cohen, Dr. Levine, and Dr. Devarajan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – In a growing number of states, , according to a panel of experts assembled for a special session at the annual meeting of the American College of Chest Physicians.
Following the June 24 decision by the U.S. Supreme Court to overturn Roe v. Wade, several states were swift to enact tight restrictions on abortion. These restrictions include bans on elective abortions for almost any reason. Worded in various ways, the new laws typically include exceptions when the health of the mother is threatened, but these exceptions must be navigated carefully.
As a general rule, “there is no clear and specific definition of when the mother’s life is at risk. These laws are vague on purpose,” said Rebecca Cohen, MD, division chief, Complex Family Planning, University of Colorado at Denver, Aurora.
The remarks were relevant to any clinician who advises women regarding pregnancy termination, but Dr. Cohen’s advice was tailored to pulmonologists. Advances have reduced the proportion of women with severe lung diseases, such as pulmonary arterial hypertension or interstitial lung disease, that make pregnancy untenable, but serious risks persist.
Clinicians need to assume a defensive posture, and the first step is to understand the laws, according to Dr. Cohen. For this, she recommended the nongovernmental Guttmacher Institute as a resource. With a focus on sexual and reproductive health, this research institute maintains a state-by-state summary of laws that govern pregnancy termination. The laws are being reconsidered across the country, and Dr. Cohen said the website updates its summaries accordingly.
In states with the most rigorous restrictions, the risks to physicians are substantial. Pulmonologists need to recognize that they might face legal consequences from merely advising a patient to terminate her pregnancy if the medical need is ambiguous or unclear, according to Dr. Cohen.
“If the advice is interpreted as aiding and abetting an elective abortion, it is a felony offense in some states,” Dr. Cohen said.
In states with restrictive laws, pregnancy prevention is the safest approach for women of childbearing age who face life-threatening complications in the event of pregnancy, according to Dr. Cohen. This might reasonably include a step beyond standard contraception. Dr. Cohen mentioned such approaches as period tracking to double down.
In addition, for women of childbearing age with health problems that might result in complications in the event of a pregnancy, it is appropriate to establish this fact in the medical record. This history could prove useful for maximizing options when making decisions in the best interest of the mother’s health in the event of contraception failure.
In addition, pulmonologists who counsel women about the potential for pregnancy termination should consider establishing a relationship with the legal department at the institution where they work, according to Dr. Cohen. In specific cases in which termination is recommended, she further advised building documentation with participation from additional medical specialists, such as an obstetrician who manages high-risk pregnancies.
“There is no guarantee that any given documentation is adequate,” Dr. Cohen warned. She indicated that consensus from multiple clinicians can strengthen the legal defense if one is necessary.
For some serious lung conditions that are incompatible with pregnancy, the threat to the mother’s life can occur early, according to Deborah Jo Levine, MD, a clinical instructor in the division of pulmonary, allergy, and critical care medicine, Stanford (Calif.) University.
As a result, “you need to identify at-risk patients early and develop a plan promptly,” said Dr. Levine, who joined Dr. Cohen on the special panel at the CHEST 2022 meeting. Even when termination is medically appropriate, restrictive laws are making these services harder to find.
In the case of a pregnancy likely to pose a high risk of complications owing to the patient’s having lung disease, “it is important to involve a high-risk ob quickly,” Dr. Levine warned. “In some cases, termination poses less risk if performed early.”
Sunjay R. Devarajan, MD, assistant professor of pulmonary medicine and critical care, Baylor College of Medicine, Houston, has faced this issue in a state that has some of the most restrictive laws. Even when there is no debate about the necessity of a medically indicated abortion, he cautioned that abortion services are becoming harder to find.
“A recent patient who had a complicated unintentional pregnancy on our service had to go out of state for pregnancy termination,” Dr. Devarajan said. He noted that this option is not available to all women, particularly in states such as his own in which most bordering states also now have highly restrictive abortion laws.
On the basis of this experience, he is thinking more defensively. Now that clinicians can be drawn into legal proceedings even when pregnancy termination is indicated, he agreed that clinicians must become familiar with the local laws.
“We are doing better in managing pregnancies in women with serious lung diseases, but termination is still the prudent approach in some cases,” Dr. Devarajan said. He indicated that he considered the advice offered by Dr. Cohen helpful in avoiding complications for the patient and the physician.
Dr. Cohen, Dr. Levine, and Dr. Devarajan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – In a growing number of states, , according to a panel of experts assembled for a special session at the annual meeting of the American College of Chest Physicians.
Following the June 24 decision by the U.S. Supreme Court to overturn Roe v. Wade, several states were swift to enact tight restrictions on abortion. These restrictions include bans on elective abortions for almost any reason. Worded in various ways, the new laws typically include exceptions when the health of the mother is threatened, but these exceptions must be navigated carefully.
As a general rule, “there is no clear and specific definition of when the mother’s life is at risk. These laws are vague on purpose,” said Rebecca Cohen, MD, division chief, Complex Family Planning, University of Colorado at Denver, Aurora.
The remarks were relevant to any clinician who advises women regarding pregnancy termination, but Dr. Cohen’s advice was tailored to pulmonologists. Advances have reduced the proportion of women with severe lung diseases, such as pulmonary arterial hypertension or interstitial lung disease, that make pregnancy untenable, but serious risks persist.
Clinicians need to assume a defensive posture, and the first step is to understand the laws, according to Dr. Cohen. For this, she recommended the nongovernmental Guttmacher Institute as a resource. With a focus on sexual and reproductive health, this research institute maintains a state-by-state summary of laws that govern pregnancy termination. The laws are being reconsidered across the country, and Dr. Cohen said the website updates its summaries accordingly.
In states with the most rigorous restrictions, the risks to physicians are substantial. Pulmonologists need to recognize that they might face legal consequences from merely advising a patient to terminate her pregnancy if the medical need is ambiguous or unclear, according to Dr. Cohen.
“If the advice is interpreted as aiding and abetting an elective abortion, it is a felony offense in some states,” Dr. Cohen said.
In states with restrictive laws, pregnancy prevention is the safest approach for women of childbearing age who face life-threatening complications in the event of pregnancy, according to Dr. Cohen. This might reasonably include a step beyond standard contraception. Dr. Cohen mentioned such approaches as period tracking to double down.
In addition, for women of childbearing age with health problems that might result in complications in the event of a pregnancy, it is appropriate to establish this fact in the medical record. This history could prove useful for maximizing options when making decisions in the best interest of the mother’s health in the event of contraception failure.
In addition, pulmonologists who counsel women about the potential for pregnancy termination should consider establishing a relationship with the legal department at the institution where they work, according to Dr. Cohen. In specific cases in which termination is recommended, she further advised building documentation with participation from additional medical specialists, such as an obstetrician who manages high-risk pregnancies.
“There is no guarantee that any given documentation is adequate,” Dr. Cohen warned. She indicated that consensus from multiple clinicians can strengthen the legal defense if one is necessary.
For some serious lung conditions that are incompatible with pregnancy, the threat to the mother’s life can occur early, according to Deborah Jo Levine, MD, a clinical instructor in the division of pulmonary, allergy, and critical care medicine, Stanford (Calif.) University.
As a result, “you need to identify at-risk patients early and develop a plan promptly,” said Dr. Levine, who joined Dr. Cohen on the special panel at the CHEST 2022 meeting. Even when termination is medically appropriate, restrictive laws are making these services harder to find.
In the case of a pregnancy likely to pose a high risk of complications owing to the patient’s having lung disease, “it is important to involve a high-risk ob quickly,” Dr. Levine warned. “In some cases, termination poses less risk if performed early.”
Sunjay R. Devarajan, MD, assistant professor of pulmonary medicine and critical care, Baylor College of Medicine, Houston, has faced this issue in a state that has some of the most restrictive laws. Even when there is no debate about the necessity of a medically indicated abortion, he cautioned that abortion services are becoming harder to find.
“A recent patient who had a complicated unintentional pregnancy on our service had to go out of state for pregnancy termination,” Dr. Devarajan said. He noted that this option is not available to all women, particularly in states such as his own in which most bordering states also now have highly restrictive abortion laws.
On the basis of this experience, he is thinking more defensively. Now that clinicians can be drawn into legal proceedings even when pregnancy termination is indicated, he agreed that clinicians must become familiar with the local laws.
“We are doing better in managing pregnancies in women with serious lung diseases, but termination is still the prudent approach in some cases,” Dr. Devarajan said. He indicated that he considered the advice offered by Dr. Cohen helpful in avoiding complications for the patient and the physician.
Dr. Cohen, Dr. Levine, and Dr. Devarajan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Reminder that COVID-19 and cancer can be a deadly combo
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JMIR CANCER
This brain surgery was BYOS: Bring your own saxophone
Tumor vs. saxophone: The surgical grudge match
Brain surgery is a notoriously difficult task. There’s a reason we say, “Well, at least it’s not brain surgery” when we’re trying to convince someone that a task isn’t that tough. Make one wrong incision, cut the wrong neuron, and it’s goodbye higher cognitive function. And most people appreciate thinking. Crazy, right?
One would imagine that the act of brain surgery would become even more difficult when the patient brings his saxophone and plays it randomly throughout the operation. It’s a hospital, after all, not a jazz club. Patients don’t get to play musical instruments during other surgeries. Why should brain surgery patients get special treatment?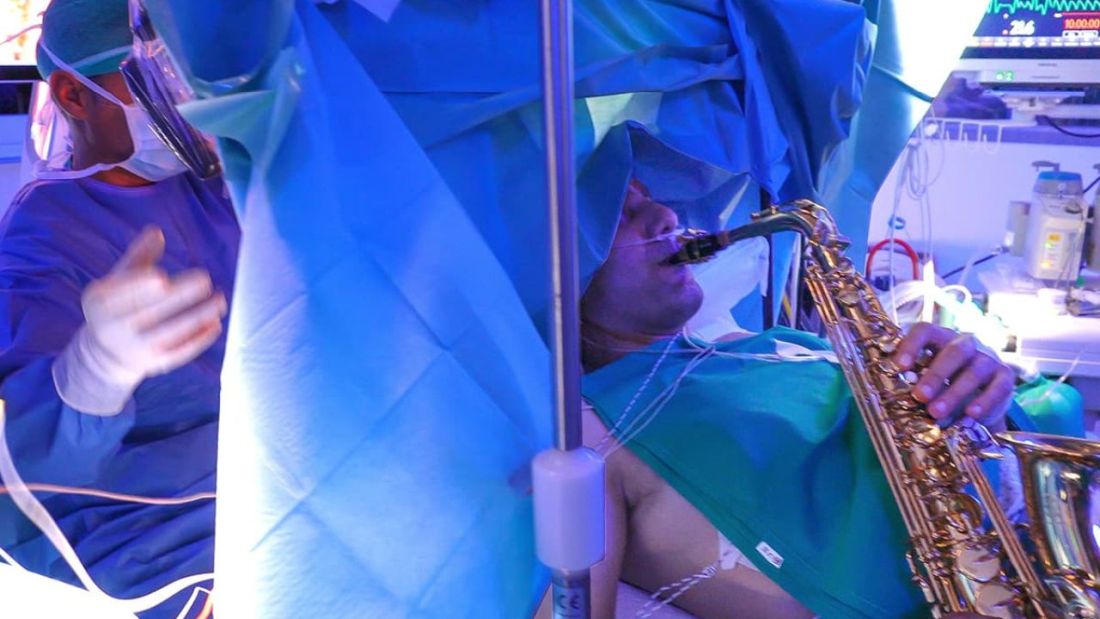
As it turns out, the musical performance was actually quite helpful. A man in Italy had a brain tumor in a particularly complex area, and he’s left-handed, which apparently makes the brain’s neural pathways much more complicated. Plus, he insisted that he retain his musical ability after the surgery. So he and his medical team had a crazy thought: Why not play the saxophone throughout the surgery? After all, according to head surgeon Christian Brogna, MD, playing an instrument means you understand music, which tests many higher cognitive functions such as coordination, mathematics, and memory.
And so, at various points throughout the 9-hour surgery, the patient played his saxophone for his doctors. Doing so allowed the surgeons to map the patient’s brain in a more complete and personalized fashion. With that extra knowledge, they were able to successfully remove the tumor while maintaining the patient’s musical ability, and the patient was discharged on Oct. 13, just 3 days after his operation.
While we’re happy the patient recovered, we do have to question his choice of music. During the surgery, he played the theme to the 1970 movie “Love Story” and the Italian national anthem. Perfectly fine pieces, no doubt, but the saxophone solo in “Jungleland” exists. And we could listen to that for 9 hours straight. In fact, we do that every Friday in the LOTME office.
Basketball has the Big Dance. Mosquitoes get the Big Sniff
In this week’s installment of our seemingly never-ending series, “Mosquitoes and the scientists who love them,” we visit The Rockefeller University in New York, where the olfactory capabilities of Aedes Aegypti – the primary vector species for Zika, dengue, yellow fever, and chikungunya – became the subject of a round robin–style tournament.
First things first, though. If you’re going to test mosquito noses, you have to give them something to smell. The researchers enrolled eight humans who were willing to wear nylon stockings on their forearms for 6 hours a day for multiple days. “Over the next few years, the researchers tested the nylons against each other in all possible pairings,” Leslie B. Vosshall, PhD, and associates said in a statement from the university. In other words, mosquito March Madness.
Nylons from different participants were hooked up in pairs to an olfactometer assay consisting of a plexiglass chamber divided into two tubes, each ending in a box that held a stocking. The mosquitoes were placed in the main chamber and observed as they flew down the tubes toward one stocking or the other.
Eventually, the “winner” of the “tournament” was Subject 33. And no, we don’t know why there was a Subject 33 since the study involved only eight participants. We do know that the nylons worn by Subject 33 were “four times more attractive to the mosquitoes than the next most-attractive study participant, and an astonishing 100 times more appealing than the least attractive, Subject 19,” according to the written statement.
Chemical analysis identified 50 molecular compounds that were elevated in the sebum of the high-attracting participants, and eventually the investigators discovered that mosquito magnets produced carboxylic acids at much higher levels than the less-attractive volunteers.
We could go on about the research team genetically engineering mosquitoes without odor receptors, but we have to save something for later. Tune in again next week for another exciting episode of “Mosquitoes and the scientists who love them.”
Are women better with words?
Men vs. Women is probably the oldest argument in the book, but there may now be movement. Researchers have been able not only to shift the advantage toward women, but also to use that knowledge to medical advantage.
When it comes to the matter of words and remembering them, women apparently have men beat. The margin is small, said lead author Marco Hirnstein, PhD, of the University of Bergen, Norway, but, after performing a meta-analysis of 168 published studies and PhD theses involving more than 350,000 participants, it’s pretty clear. The research supports women’s advantage over men in recall, verbal fluency (categorical and phonemic), and recognition.
So how is this information useful from a medical standpoint?
Dr. Hirnstein and colleagues suggested that this information can help in interpreting diagnostic assessment results. The example given was dementia diagnosis. Since women are underdiagnosed because their baseline exceeds average while men are overdiagnosed, taking gender and performance into account could clear up or catch cases that might otherwise slip through the cracks.
Now, let’s just put this part of the debate to rest and take this not only as a win for women but for science as well.
Tumor vs. saxophone: The surgical grudge match
Brain surgery is a notoriously difficult task. There’s a reason we say, “Well, at least it’s not brain surgery” when we’re trying to convince someone that a task isn’t that tough. Make one wrong incision, cut the wrong neuron, and it’s goodbye higher cognitive function. And most people appreciate thinking. Crazy, right?
One would imagine that the act of brain surgery would become even more difficult when the patient brings his saxophone and plays it randomly throughout the operation. It’s a hospital, after all, not a jazz club. Patients don’t get to play musical instruments during other surgeries. Why should brain surgery patients get special treatment?
As it turns out, the musical performance was actually quite helpful. A man in Italy had a brain tumor in a particularly complex area, and he’s left-handed, which apparently makes the brain’s neural pathways much more complicated. Plus, he insisted that he retain his musical ability after the surgery. So he and his medical team had a crazy thought: Why not play the saxophone throughout the surgery? After all, according to head surgeon Christian Brogna, MD, playing an instrument means you understand music, which tests many higher cognitive functions such as coordination, mathematics, and memory.
And so, at various points throughout the 9-hour surgery, the patient played his saxophone for his doctors. Doing so allowed the surgeons to map the patient’s brain in a more complete and personalized fashion. With that extra knowledge, they were able to successfully remove the tumor while maintaining the patient’s musical ability, and the patient was discharged on Oct. 13, just 3 days after his operation.
While we’re happy the patient recovered, we do have to question his choice of music. During the surgery, he played the theme to the 1970 movie “Love Story” and the Italian national anthem. Perfectly fine pieces, no doubt, but the saxophone solo in “Jungleland” exists. And we could listen to that for 9 hours straight. In fact, we do that every Friday in the LOTME office.
Basketball has the Big Dance. Mosquitoes get the Big Sniff
In this week’s installment of our seemingly never-ending series, “Mosquitoes and the scientists who love them,” we visit The Rockefeller University in New York, where the olfactory capabilities of Aedes Aegypti – the primary vector species for Zika, dengue, yellow fever, and chikungunya – became the subject of a round robin–style tournament.
First things first, though. If you’re going to test mosquito noses, you have to give them something to smell. The researchers enrolled eight humans who were willing to wear nylon stockings on their forearms for 6 hours a day for multiple days. “Over the next few years, the researchers tested the nylons against each other in all possible pairings,” Leslie B. Vosshall, PhD, and associates said in a statement from the university. In other words, mosquito March Madness.
Nylons from different participants were hooked up in pairs to an olfactometer assay consisting of a plexiglass chamber divided into two tubes, each ending in a box that held a stocking. The mosquitoes were placed in the main chamber and observed as they flew down the tubes toward one stocking or the other.
Eventually, the “winner” of the “tournament” was Subject 33. And no, we don’t know why there was a Subject 33 since the study involved only eight participants. We do know that the nylons worn by Subject 33 were “four times more attractive to the mosquitoes than the next most-attractive study participant, and an astonishing 100 times more appealing than the least attractive, Subject 19,” according to the written statement.
Chemical analysis identified 50 molecular compounds that were elevated in the sebum of the high-attracting participants, and eventually the investigators discovered that mosquito magnets produced carboxylic acids at much higher levels than the less-attractive volunteers.
We could go on about the research team genetically engineering mosquitoes without odor receptors, but we have to save something for later. Tune in again next week for another exciting episode of “Mosquitoes and the scientists who love them.”
Are women better with words?
Men vs. Women is probably the oldest argument in the book, but there may now be movement. Researchers have been able not only to shift the advantage toward women, but also to use that knowledge to medical advantage.
When it comes to the matter of words and remembering them, women apparently have men beat. The margin is small, said lead author Marco Hirnstein, PhD, of the University of Bergen, Norway, but, after performing a meta-analysis of 168 published studies and PhD theses involving more than 350,000 participants, it’s pretty clear. The research supports women’s advantage over men in recall, verbal fluency (categorical and phonemic), and recognition.
So how is this information useful from a medical standpoint?
Dr. Hirnstein and colleagues suggested that this information can help in interpreting diagnostic assessment results. The example given was dementia diagnosis. Since women are underdiagnosed because their baseline exceeds average while men are overdiagnosed, taking gender and performance into account could clear up or catch cases that might otherwise slip through the cracks.
Now, let’s just put this part of the debate to rest and take this not only as a win for women but for science as well.
Tumor vs. saxophone: The surgical grudge match
Brain surgery is a notoriously difficult task. There’s a reason we say, “Well, at least it’s not brain surgery” when we’re trying to convince someone that a task isn’t that tough. Make one wrong incision, cut the wrong neuron, and it’s goodbye higher cognitive function. And most people appreciate thinking. Crazy, right?
One would imagine that the act of brain surgery would become even more difficult when the patient brings his saxophone and plays it randomly throughout the operation. It’s a hospital, after all, not a jazz club. Patients don’t get to play musical instruments during other surgeries. Why should brain surgery patients get special treatment?
As it turns out, the musical performance was actually quite helpful. A man in Italy had a brain tumor in a particularly complex area, and he’s left-handed, which apparently makes the brain’s neural pathways much more complicated. Plus, he insisted that he retain his musical ability after the surgery. So he and his medical team had a crazy thought: Why not play the saxophone throughout the surgery? After all, according to head surgeon Christian Brogna, MD, playing an instrument means you understand music, which tests many higher cognitive functions such as coordination, mathematics, and memory.
And so, at various points throughout the 9-hour surgery, the patient played his saxophone for his doctors. Doing so allowed the surgeons to map the patient’s brain in a more complete and personalized fashion. With that extra knowledge, they were able to successfully remove the tumor while maintaining the patient’s musical ability, and the patient was discharged on Oct. 13, just 3 days after his operation.
While we’re happy the patient recovered, we do have to question his choice of music. During the surgery, he played the theme to the 1970 movie “Love Story” and the Italian national anthem. Perfectly fine pieces, no doubt, but the saxophone solo in “Jungleland” exists. And we could listen to that for 9 hours straight. In fact, we do that every Friday in the LOTME office.
Basketball has the Big Dance. Mosquitoes get the Big Sniff
In this week’s installment of our seemingly never-ending series, “Mosquitoes and the scientists who love them,” we visit The Rockefeller University in New York, where the olfactory capabilities of Aedes Aegypti – the primary vector species for Zika, dengue, yellow fever, and chikungunya – became the subject of a round robin–style tournament.
First things first, though. If you’re going to test mosquito noses, you have to give them something to smell. The researchers enrolled eight humans who were willing to wear nylon stockings on their forearms for 6 hours a day for multiple days. “Over the next few years, the researchers tested the nylons against each other in all possible pairings,” Leslie B. Vosshall, PhD, and associates said in a statement from the university. In other words, mosquito March Madness.
Nylons from different participants were hooked up in pairs to an olfactometer assay consisting of a plexiglass chamber divided into two tubes, each ending in a box that held a stocking. The mosquitoes were placed in the main chamber and observed as they flew down the tubes toward one stocking or the other.
Eventually, the “winner” of the “tournament” was Subject 33. And no, we don’t know why there was a Subject 33 since the study involved only eight participants. We do know that the nylons worn by Subject 33 were “four times more attractive to the mosquitoes than the next most-attractive study participant, and an astonishing 100 times more appealing than the least attractive, Subject 19,” according to the written statement.
Chemical analysis identified 50 molecular compounds that were elevated in the sebum of the high-attracting participants, and eventually the investigators discovered that mosquito magnets produced carboxylic acids at much higher levels than the less-attractive volunteers.
We could go on about the research team genetically engineering mosquitoes without odor receptors, but we have to save something for later. Tune in again next week for another exciting episode of “Mosquitoes and the scientists who love them.”
Are women better with words?
Men vs. Women is probably the oldest argument in the book, but there may now be movement. Researchers have been able not only to shift the advantage toward women, but also to use that knowledge to medical advantage.
When it comes to the matter of words and remembering them, women apparently have men beat. The margin is small, said lead author Marco Hirnstein, PhD, of the University of Bergen, Norway, but, after performing a meta-analysis of 168 published studies and PhD theses involving more than 350,000 participants, it’s pretty clear. The research supports women’s advantage over men in recall, verbal fluency (categorical and phonemic), and recognition.
So how is this information useful from a medical standpoint?
Dr. Hirnstein and colleagues suggested that this information can help in interpreting diagnostic assessment results. The example given was dementia diagnosis. Since women are underdiagnosed because their baseline exceeds average while men are overdiagnosed, taking gender and performance into account could clear up or catch cases that might otherwise slip through the cracks.
Now, let’s just put this part of the debate to rest and take this not only as a win for women but for science as well.
FDA panel recommends withdrawal of Makena for preterm birth
A federal advisory panel recommended the United States withdraw from the market an injection given to women at risk for giving birth prematurely. Many of its members argued this step is needed to allow further testing to see if this drug actually works.
The Food and Drug Administration has been seeking to pull the approval of hydroxyprogesterone caproate (17P) injection (Makena, Covis) since 2020, after the drug failed to show a benefit in the PROLONG study. This study was meant as a confirmatory trial for the accelerated approval the FDA granted Makena in 2011 based on promising results from an earlier small study, known as the Meis trial. The manufacturer, Covis, contends that the flaws in the PROLONG study made Makena appear ineffective.
The FDA asked its Obstetrics, Reproductive and Urologic Drugs Advisory Committee to review the evidence gathered to date on Makena at a hearing that ran from Oct. 17 to Oct. 19. At the conclusion, the FDA asked the committee to vote on whether the agency should allow Makena to remain on the market while an appropriate confirmatory study is designed and conducted.
The vote was 14-1 against this plan.
There needs to be another study as a “tiebreaker” to determine which of the previous Makena trials was correct, said FDA panelist Michael K. Lindsay, MD, MPH, who is also director of the division of maternal-fetal medicine for Grady and Emory University Hospital Midtown, Atlanta.
“I think there needs to be another trial,” Dr. Lindsay said. “If you can do the trial without the medication being FDA approved, then I am supportive of that.”
Members of the FDA panel noted the difficulties that would ensue if Covis attempted further study of Makena with the drug still approved, including difficulties in recruiting patients. Indeed, there were delays in recruiting patients for the PROLONG trial in part because Makena was perceived as the standard of care for pregnant women who had a prior spontaneous preterm birth. That led to efforts to enroll patients outside of the United States, particularly in Eastern European countries.
Panelist Cassandra E. Henderson, MD, of the New York-based Garden OB/GYN practice, was the dissenter in the 14-1 vote.
Withdrawing the approval of Makena may lead to increased use of pharmacy-compounded versions of this medicine, as women look for options to try to extend their pregnancies, she said.
“They may seek it in other ways and get something that we don’t have any control over, and we don’t know what the fetus may be exposed to,” Dr. Henderson said.
Dr. Henderson also said there should be greater discussion with patients about questions of potential “intergenerational risk” because of fetal exposure to the medicine. Covis could add a registry similar to the University of Chicago’s DES Program to its research program for Makena, she said.
Race-based argument
Covis has been fighting to keep the Makena approval by offering theories for why the PROLONG study failed to show a benefit for the drug.
Covis emphasizes the different racial make-up of patients in the two trials. Black women composed 59% of the Meis study population, compared with only 6.7% for the PROLONG study, Covis said in its briefing document for the hearing. The Luxembourg-based company also says that there may have been unreliable estimates of the gestational age in the PROLONG trial, which enrolled many subjects in Ukraine and Russia.
During deliberations among panelists on Oct. 19, Dr. Henderson emphasized a need to consider other factors that may have been involved and encouraged continued study of the drug in Black women. She dismissed the idea of a race-based difference being the explanation for the difference between the two trials, but instead stressed that race serves as a marker for inequities, which are known to increase risk for preterm birth.
“Targeting a population that is at risk, particularly Black women in the United States, may show something that would be beneficial” from Makena, Dr. Henderson said.
Other physicians have argued that this approach would actually put Black women and children at greater risk of an ineffective drug with potential side effects.
“The drug is not proven to work so keeping it on the market to be injected into Black women to see what subgroups it might work in essentially amounts to experimentation,” said Adam Urato, MD, chief of maternal-fetal medicine at MetroWest Medical Center in Framingham, Mass., during the public comment session of the hearing.
The vote marks the second time that the FDA’s advisers on reproductive health have told the agency that the evidence gathered on the drug does not support its use. An advisory committee also cast votes against the drug at a 2019 meeting.
The rate of preterm birth in Black women in 2020 was 14.4%, significantly higher than the rate of preterm birth in White or Hispanic women, 9.1% and 9.8%, respectively, according to the Centers for Disease Control and Prevention. The potential for harm to children from premature birth led the FDA to clear Makena through the accelerated approval pathway, said Patrizia Cavazzoni, MD, the director of FDA’s Center for Drug Evaluation and Research, in the opening session of the hearing.
“We once thought Makena was likely to be part of the answer to that problem,” Dr. Cavazzoni said. “Unfortunately we no longer do, based on the evidence available.”
A federal advisory panel recommended the United States withdraw from the market an injection given to women at risk for giving birth prematurely. Many of its members argued this step is needed to allow further testing to see if this drug actually works.
The Food and Drug Administration has been seeking to pull the approval of hydroxyprogesterone caproate (17P) injection (Makena, Covis) since 2020, after the drug failed to show a benefit in the PROLONG study. This study was meant as a confirmatory trial for the accelerated approval the FDA granted Makena in 2011 based on promising results from an earlier small study, known as the Meis trial. The manufacturer, Covis, contends that the flaws in the PROLONG study made Makena appear ineffective.
The FDA asked its Obstetrics, Reproductive and Urologic Drugs Advisory Committee to review the evidence gathered to date on Makena at a hearing that ran from Oct. 17 to Oct. 19. At the conclusion, the FDA asked the committee to vote on whether the agency should allow Makena to remain on the market while an appropriate confirmatory study is designed and conducted.
The vote was 14-1 against this plan.
There needs to be another study as a “tiebreaker” to determine which of the previous Makena trials was correct, said FDA panelist Michael K. Lindsay, MD, MPH, who is also director of the division of maternal-fetal medicine for Grady and Emory University Hospital Midtown, Atlanta.
“I think there needs to be another trial,” Dr. Lindsay said. “If you can do the trial without the medication being FDA approved, then I am supportive of that.”
Members of the FDA panel noted the difficulties that would ensue if Covis attempted further study of Makena with the drug still approved, including difficulties in recruiting patients. Indeed, there were delays in recruiting patients for the PROLONG trial in part because Makena was perceived as the standard of care for pregnant women who had a prior spontaneous preterm birth. That led to efforts to enroll patients outside of the United States, particularly in Eastern European countries.
Panelist Cassandra E. Henderson, MD, of the New York-based Garden OB/GYN practice, was the dissenter in the 14-1 vote.
Withdrawing the approval of Makena may lead to increased use of pharmacy-compounded versions of this medicine, as women look for options to try to extend their pregnancies, she said.
“They may seek it in other ways and get something that we don’t have any control over, and we don’t know what the fetus may be exposed to,” Dr. Henderson said.
Dr. Henderson also said there should be greater discussion with patients about questions of potential “intergenerational risk” because of fetal exposure to the medicine. Covis could add a registry similar to the University of Chicago’s DES Program to its research program for Makena, she said.
Race-based argument
Covis has been fighting to keep the Makena approval by offering theories for why the PROLONG study failed to show a benefit for the drug.
Covis emphasizes the different racial make-up of patients in the two trials. Black women composed 59% of the Meis study population, compared with only 6.7% for the PROLONG study, Covis said in its briefing document for the hearing. The Luxembourg-based company also says that there may have been unreliable estimates of the gestational age in the PROLONG trial, which enrolled many subjects in Ukraine and Russia.
During deliberations among panelists on Oct. 19, Dr. Henderson emphasized a need to consider other factors that may have been involved and encouraged continued study of the drug in Black women. She dismissed the idea of a race-based difference being the explanation for the difference between the two trials, but instead stressed that race serves as a marker for inequities, which are known to increase risk for preterm birth.
“Targeting a population that is at risk, particularly Black women in the United States, may show something that would be beneficial” from Makena, Dr. Henderson said.
Other physicians have argued that this approach would actually put Black women and children at greater risk of an ineffective drug with potential side effects.
“The drug is not proven to work so keeping it on the market to be injected into Black women to see what subgroups it might work in essentially amounts to experimentation,” said Adam Urato, MD, chief of maternal-fetal medicine at MetroWest Medical Center in Framingham, Mass., during the public comment session of the hearing.
The vote marks the second time that the FDA’s advisers on reproductive health have told the agency that the evidence gathered on the drug does not support its use. An advisory committee also cast votes against the drug at a 2019 meeting.
The rate of preterm birth in Black women in 2020 was 14.4%, significantly higher than the rate of preterm birth in White or Hispanic women, 9.1% and 9.8%, respectively, according to the Centers for Disease Control and Prevention. The potential for harm to children from premature birth led the FDA to clear Makena through the accelerated approval pathway, said Patrizia Cavazzoni, MD, the director of FDA’s Center for Drug Evaluation and Research, in the opening session of the hearing.
“We once thought Makena was likely to be part of the answer to that problem,” Dr. Cavazzoni said. “Unfortunately we no longer do, based on the evidence available.”
A federal advisory panel recommended the United States withdraw from the market an injection given to women at risk for giving birth prematurely. Many of its members argued this step is needed to allow further testing to see if this drug actually works.
The Food and Drug Administration has been seeking to pull the approval of hydroxyprogesterone caproate (17P) injection (Makena, Covis) since 2020, after the drug failed to show a benefit in the PROLONG study. This study was meant as a confirmatory trial for the accelerated approval the FDA granted Makena in 2011 based on promising results from an earlier small study, known as the Meis trial. The manufacturer, Covis, contends that the flaws in the PROLONG study made Makena appear ineffective.
The FDA asked its Obstetrics, Reproductive and Urologic Drugs Advisory Committee to review the evidence gathered to date on Makena at a hearing that ran from Oct. 17 to Oct. 19. At the conclusion, the FDA asked the committee to vote on whether the agency should allow Makena to remain on the market while an appropriate confirmatory study is designed and conducted.
The vote was 14-1 against this plan.
There needs to be another study as a “tiebreaker” to determine which of the previous Makena trials was correct, said FDA panelist Michael K. Lindsay, MD, MPH, who is also director of the division of maternal-fetal medicine for Grady and Emory University Hospital Midtown, Atlanta.
“I think there needs to be another trial,” Dr. Lindsay said. “If you can do the trial without the medication being FDA approved, then I am supportive of that.”
Members of the FDA panel noted the difficulties that would ensue if Covis attempted further study of Makena with the drug still approved, including difficulties in recruiting patients. Indeed, there were delays in recruiting patients for the PROLONG trial in part because Makena was perceived as the standard of care for pregnant women who had a prior spontaneous preterm birth. That led to efforts to enroll patients outside of the United States, particularly in Eastern European countries.
Panelist Cassandra E. Henderson, MD, of the New York-based Garden OB/GYN practice, was the dissenter in the 14-1 vote.
Withdrawing the approval of Makena may lead to increased use of pharmacy-compounded versions of this medicine, as women look for options to try to extend their pregnancies, she said.
“They may seek it in other ways and get something that we don’t have any control over, and we don’t know what the fetus may be exposed to,” Dr. Henderson said.
Dr. Henderson also said there should be greater discussion with patients about questions of potential “intergenerational risk” because of fetal exposure to the medicine. Covis could add a registry similar to the University of Chicago’s DES Program to its research program for Makena, she said.
Race-based argument
Covis has been fighting to keep the Makena approval by offering theories for why the PROLONG study failed to show a benefit for the drug.
Covis emphasizes the different racial make-up of patients in the two trials. Black women composed 59% of the Meis study population, compared with only 6.7% for the PROLONG study, Covis said in its briefing document for the hearing. The Luxembourg-based company also says that there may have been unreliable estimates of the gestational age in the PROLONG trial, which enrolled many subjects in Ukraine and Russia.
During deliberations among panelists on Oct. 19, Dr. Henderson emphasized a need to consider other factors that may have been involved and encouraged continued study of the drug in Black women. She dismissed the idea of a race-based difference being the explanation for the difference between the two trials, but instead stressed that race serves as a marker for inequities, which are known to increase risk for preterm birth.
“Targeting a population that is at risk, particularly Black women in the United States, may show something that would be beneficial” from Makena, Dr. Henderson said.
Other physicians have argued that this approach would actually put Black women and children at greater risk of an ineffective drug with potential side effects.
“The drug is not proven to work so keeping it on the market to be injected into Black women to see what subgroups it might work in essentially amounts to experimentation,” said Adam Urato, MD, chief of maternal-fetal medicine at MetroWest Medical Center in Framingham, Mass., during the public comment session of the hearing.
The vote marks the second time that the FDA’s advisers on reproductive health have told the agency that the evidence gathered on the drug does not support its use. An advisory committee also cast votes against the drug at a 2019 meeting.
The rate of preterm birth in Black women in 2020 was 14.4%, significantly higher than the rate of preterm birth in White or Hispanic women, 9.1% and 9.8%, respectively, according to the Centers for Disease Control and Prevention. The potential for harm to children from premature birth led the FDA to clear Makena through the accelerated approval pathway, said Patrizia Cavazzoni, MD, the director of FDA’s Center for Drug Evaluation and Research, in the opening session of the hearing.
“We once thought Makena was likely to be part of the answer to that problem,” Dr. Cavazzoni said. “Unfortunately we no longer do, based on the evidence available.”
Nonhormonal drug fezolinetant found safe for hot flashes in yearlong study
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
FROM NAMS 2022
Menopause symptoms negatively affect women’s work
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
FROM NAMS 2022
Poor evidence for vaginal laser therapy
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
FROM NAMS 2022
Chest reconstruction surgeries up nearly fourfold among adolescents
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Commentary: Postpartum hemorrhage and acute chest pain obstetric emergencies, October 2022
The three PPH articles examine the use of preventive B-Lynch suture, risk factors for failure of intrauterine tamponade, and trend changes in risk factors for PPH. Kuwabara and colleagues looked at the effectiveness of preventative B-Lynch sutures in patients at high risk for PPH. Their retrospective observational study included 38 of 663 patients who underwent cesarean section (CS) who received the B-Lynch procedure at their tertiary perinatal medical center in Gifu, Japan, between January 2019 and May 2021. Overall, 92% of patients who received the B-Lynch suture showed no apparent postoperative bleeding within 2 hours after the CS. A total of 24 patients required blood transfusion, none required hysterectomy, and only one patient with a twin pregnancy required additional treatment because of secondary PPH 5 days after the CS. This suggests that earlier use of B-Lynch sutures could be considered in patients at high risk for atony.
Gibier and colleagues examined risk factors for uterine tamponade failure in women with PPH. This was a population-based retrospective cohort study of 1761 women with deliveries complicated by PPH who underwent intrauterine tamponade within 24 hours of PPH to manage persistent bleeding. They noted that the intrauterine tamponade failure rate was 11.1%. Risk for intrauterine tamponade failure was higher in women with CS (adjusted odds ratio [aOR] 4.2; 95% CI 2.9-6.0), preeclampsia (aOR 2.3; 95% CI 1.3-3.9), and uterine rupture (aOR 14.1; 95% CI 2.4-83.0). They concluded that CS, preeclampsia, and uterine rupture were significant risk factors for failures in this procedure.
Sade and colleagues examined trend changes in the individual contribution of risk factors for PPH over more than two decades. Their population-based, retrospective, nested, case-control study included 285,992 pregnancies and suggested that, in their hospital setting in Israel, risks from perineal or vaginal tears were increasing while large-for-gestational-age fetuses decreased and other risk factors remained stable.
Finally, Wu and colleagues examined incidence and outcomes of AHRCP diseases during pregnancy and the puerperium. This observational analysis examined 41,174,101 patients hospitalized for pregnancy and during the puerperium in the National Inpatient Sample (NIS) database from January 1, 2008, to December 31, 2017. The study noted that 40,285 patients were diagnosed with AHRCP diseases during this period. The NIS is the largest publicly available all-payer database in the United States. The investigators found that the incidence of AHRCP diseases increased significantly between 2002 and 2017, especially pulmonary embolism in the puerperium. Although mortality showed a downward trend, it is still at a high level. They suggested that we should strengthen monitoring and management of AHRCP in pregnancy and puerperium, especially for Black women, those in the lowest-income households, and parturients over 35 years of age.
The three PPH articles examine the use of preventive B-Lynch suture, risk factors for failure of intrauterine tamponade, and trend changes in risk factors for PPH. Kuwabara and colleagues looked at the effectiveness of preventative B-Lynch sutures in patients at high risk for PPH. Their retrospective observational study included 38 of 663 patients who underwent cesarean section (CS) who received the B-Lynch procedure at their tertiary perinatal medical center in Gifu, Japan, between January 2019 and May 2021. Overall, 92% of patients who received the B-Lynch suture showed no apparent postoperative bleeding within 2 hours after the CS. A total of 24 patients required blood transfusion, none required hysterectomy, and only one patient with a twin pregnancy required additional treatment because of secondary PPH 5 days after the CS. This suggests that earlier use of B-Lynch sutures could be considered in patients at high risk for atony.
Gibier and colleagues examined risk factors for uterine tamponade failure in women with PPH. This was a population-based retrospective cohort study of 1761 women with deliveries complicated by PPH who underwent intrauterine tamponade within 24 hours of PPH to manage persistent bleeding. They noted that the intrauterine tamponade failure rate was 11.1%. Risk for intrauterine tamponade failure was higher in women with CS (adjusted odds ratio [aOR] 4.2; 95% CI 2.9-6.0), preeclampsia (aOR 2.3; 95% CI 1.3-3.9), and uterine rupture (aOR 14.1; 95% CI 2.4-83.0). They concluded that CS, preeclampsia, and uterine rupture were significant risk factors for failures in this procedure.
Sade and colleagues examined trend changes in the individual contribution of risk factors for PPH over more than two decades. Their population-based, retrospective, nested, case-control study included 285,992 pregnancies and suggested that, in their hospital setting in Israel, risks from perineal or vaginal tears were increasing while large-for-gestational-age fetuses decreased and other risk factors remained stable.
Finally, Wu and colleagues examined incidence and outcomes of AHRCP diseases during pregnancy and the puerperium. This observational analysis examined 41,174,101 patients hospitalized for pregnancy and during the puerperium in the National Inpatient Sample (NIS) database from January 1, 2008, to December 31, 2017. The study noted that 40,285 patients were diagnosed with AHRCP diseases during this period. The NIS is the largest publicly available all-payer database in the United States. The investigators found that the incidence of AHRCP diseases increased significantly between 2002 and 2017, especially pulmonary embolism in the puerperium. Although mortality showed a downward trend, it is still at a high level. They suggested that we should strengthen monitoring and management of AHRCP in pregnancy and puerperium, especially for Black women, those in the lowest-income households, and parturients over 35 years of age.
The three PPH articles examine the use of preventive B-Lynch suture, risk factors for failure of intrauterine tamponade, and trend changes in risk factors for PPH. Kuwabara and colleagues looked at the effectiveness of preventative B-Lynch sutures in patients at high risk for PPH. Their retrospective observational study included 38 of 663 patients who underwent cesarean section (CS) who received the B-Lynch procedure at their tertiary perinatal medical center in Gifu, Japan, between January 2019 and May 2021. Overall, 92% of patients who received the B-Lynch suture showed no apparent postoperative bleeding within 2 hours after the CS. A total of 24 patients required blood transfusion, none required hysterectomy, and only one patient with a twin pregnancy required additional treatment because of secondary PPH 5 days after the CS. This suggests that earlier use of B-Lynch sutures could be considered in patients at high risk for atony.
Gibier and colleagues examined risk factors for uterine tamponade failure in women with PPH. This was a population-based retrospective cohort study of 1761 women with deliveries complicated by PPH who underwent intrauterine tamponade within 24 hours of PPH to manage persistent bleeding. They noted that the intrauterine tamponade failure rate was 11.1%. Risk for intrauterine tamponade failure was higher in women with CS (adjusted odds ratio [aOR] 4.2; 95% CI 2.9-6.0), preeclampsia (aOR 2.3; 95% CI 1.3-3.9), and uterine rupture (aOR 14.1; 95% CI 2.4-83.0). They concluded that CS, preeclampsia, and uterine rupture were significant risk factors for failures in this procedure.
Sade and colleagues examined trend changes in the individual contribution of risk factors for PPH over more than two decades. Their population-based, retrospective, nested, case-control study included 285,992 pregnancies and suggested that, in their hospital setting in Israel, risks from perineal or vaginal tears were increasing while large-for-gestational-age fetuses decreased and other risk factors remained stable.
Finally, Wu and colleagues examined incidence and outcomes of AHRCP diseases during pregnancy and the puerperium. This observational analysis examined 41,174,101 patients hospitalized for pregnancy and during the puerperium in the National Inpatient Sample (NIS) database from January 1, 2008, to December 31, 2017. The study noted that 40,285 patients were diagnosed with AHRCP diseases during this period. The NIS is the largest publicly available all-payer database in the United States. The investigators found that the incidence of AHRCP diseases increased significantly between 2002 and 2017, especially pulmonary embolism in the puerperium. Although mortality showed a downward trend, it is still at a high level. They suggested that we should strengthen monitoring and management of AHRCP in pregnancy and puerperium, especially for Black women, those in the lowest-income households, and parturients over 35 years of age.













